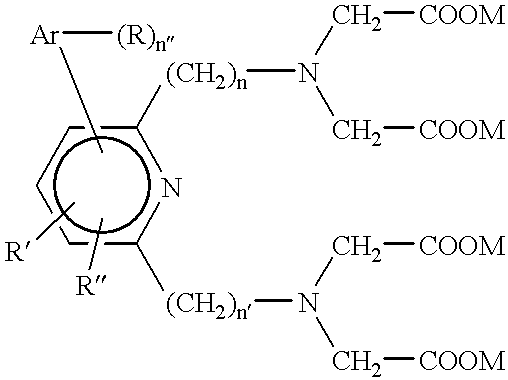
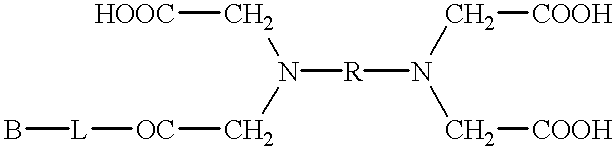
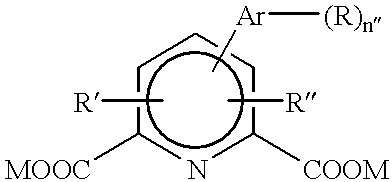
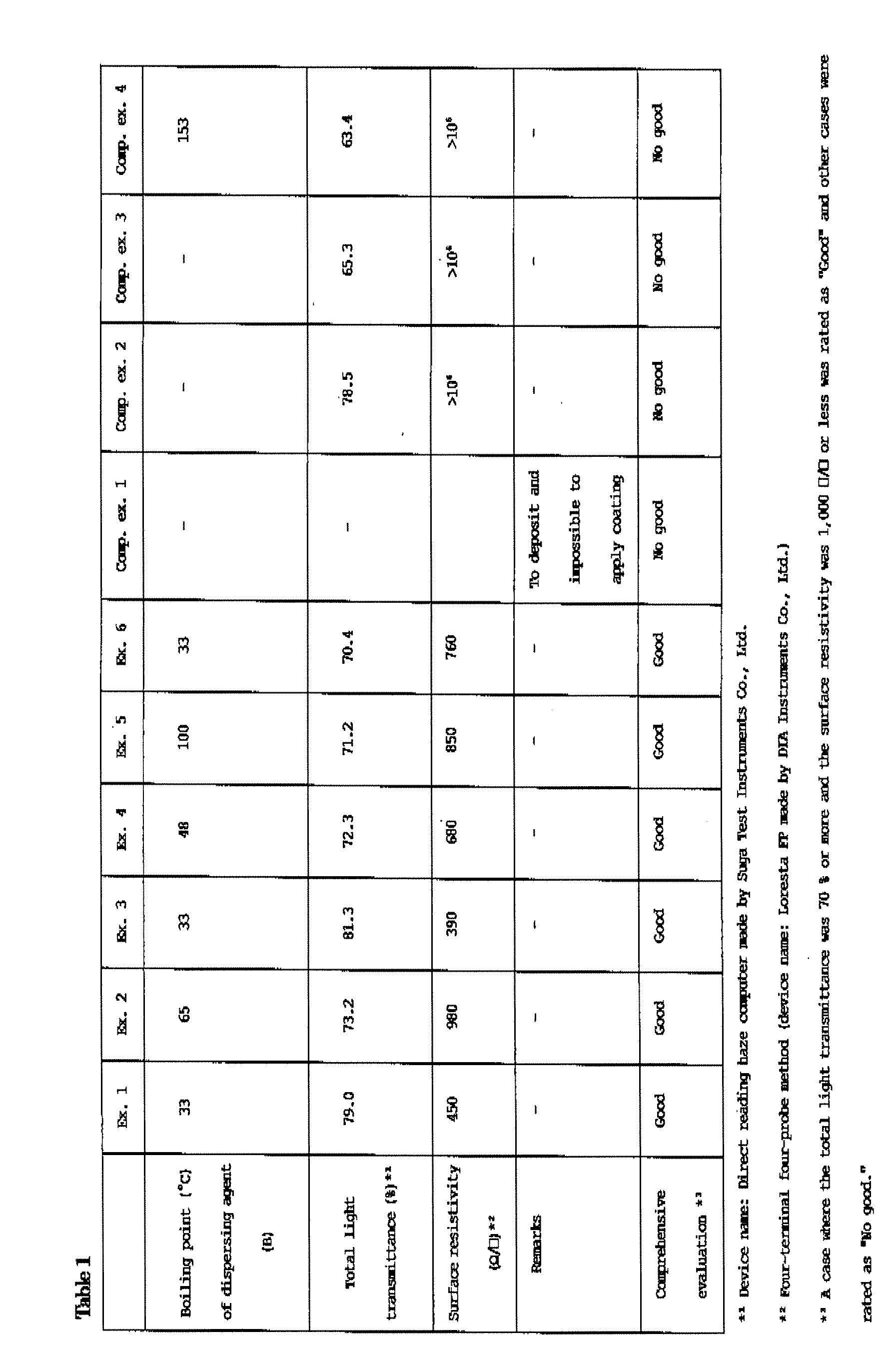
Patents
Literature
2043results about "Non-metal conductors" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
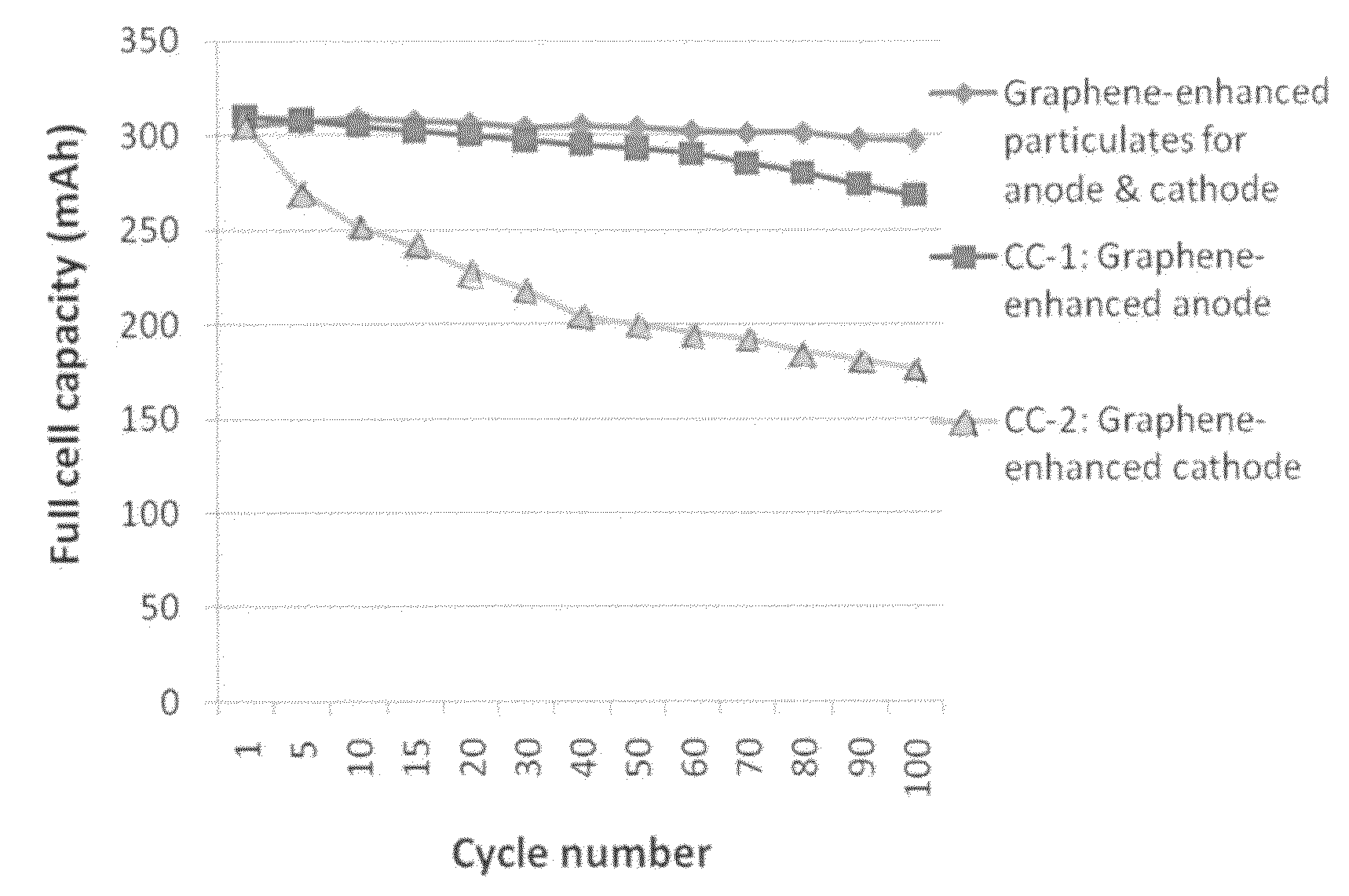
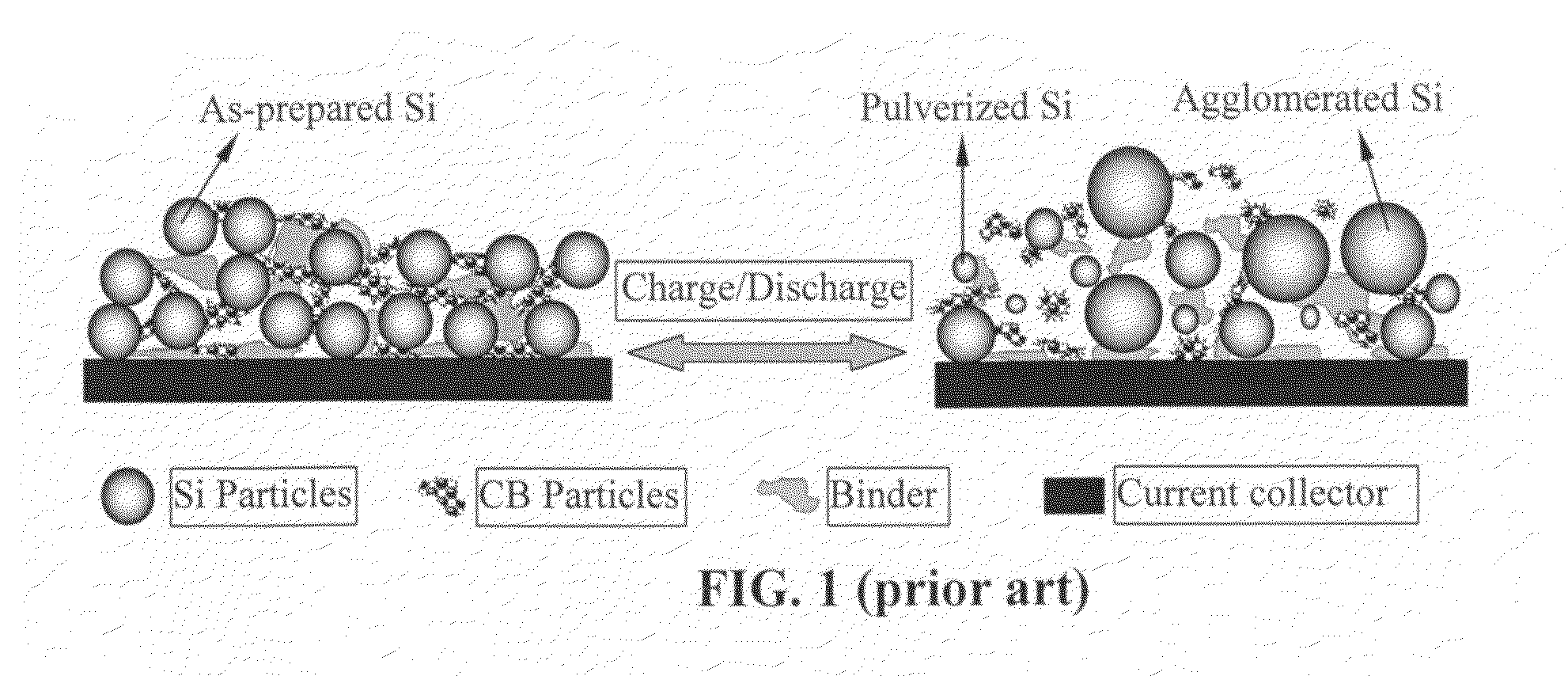
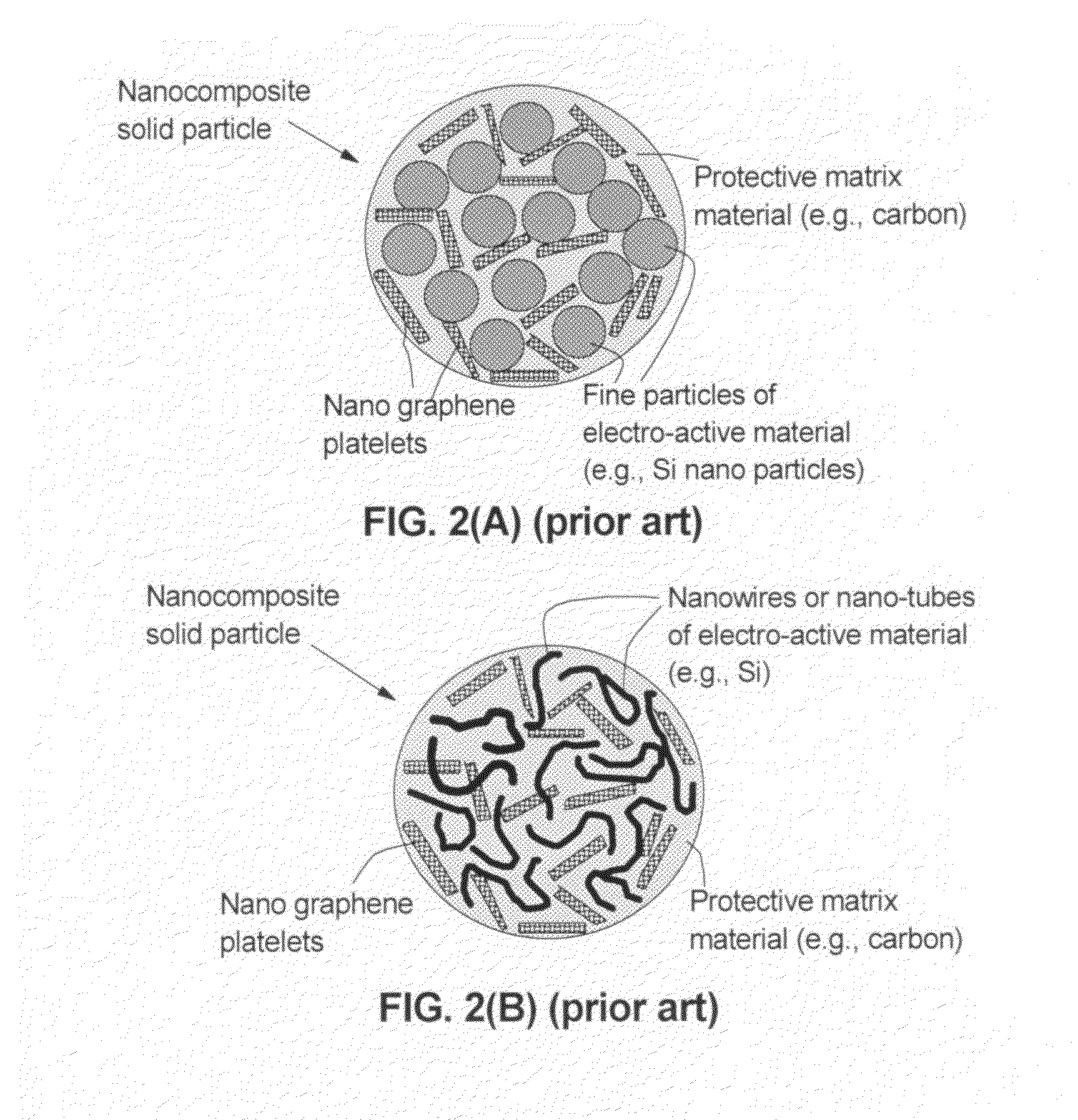
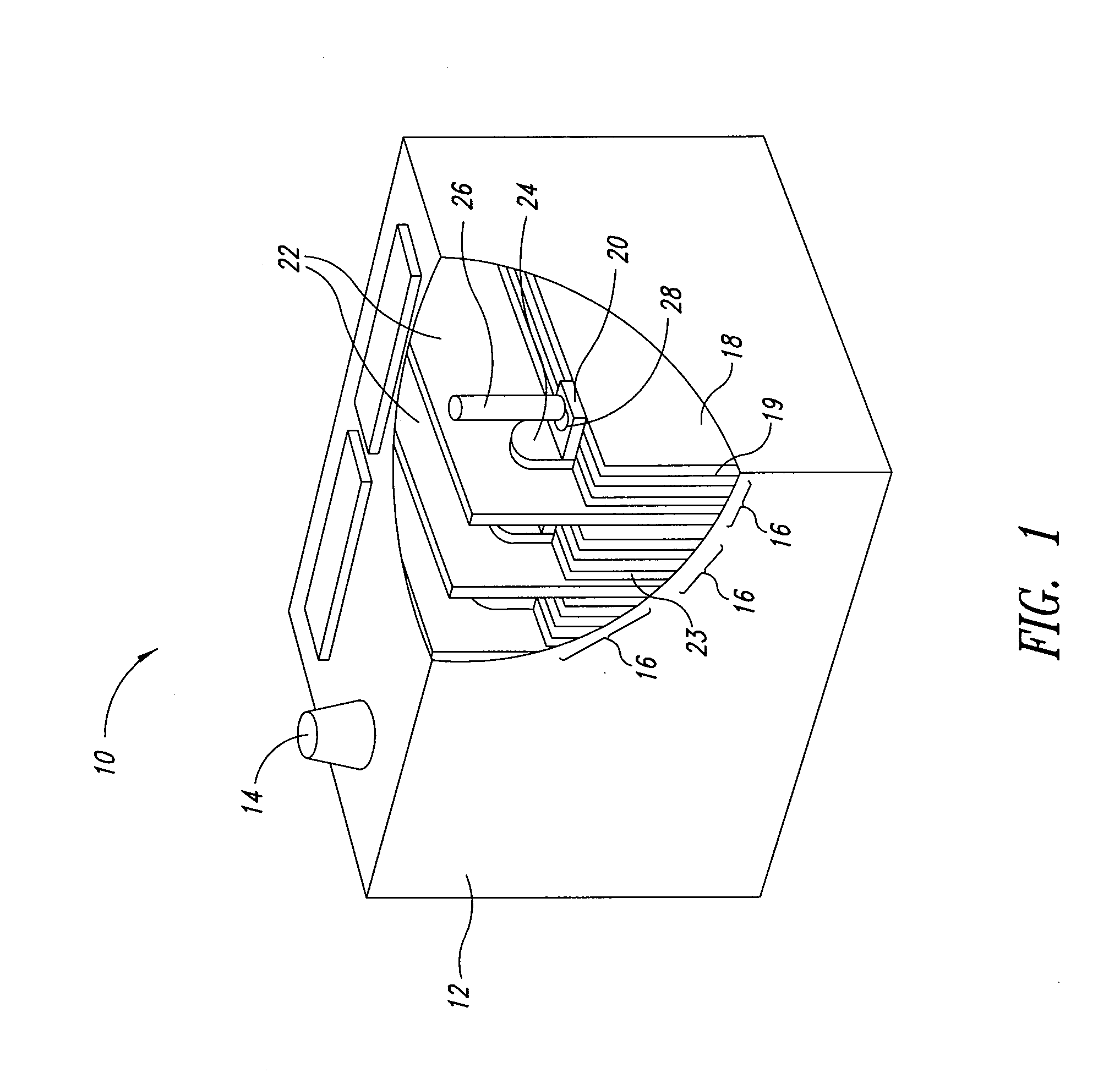
Graphene-enhanced anode particulates for lithium ion batteries
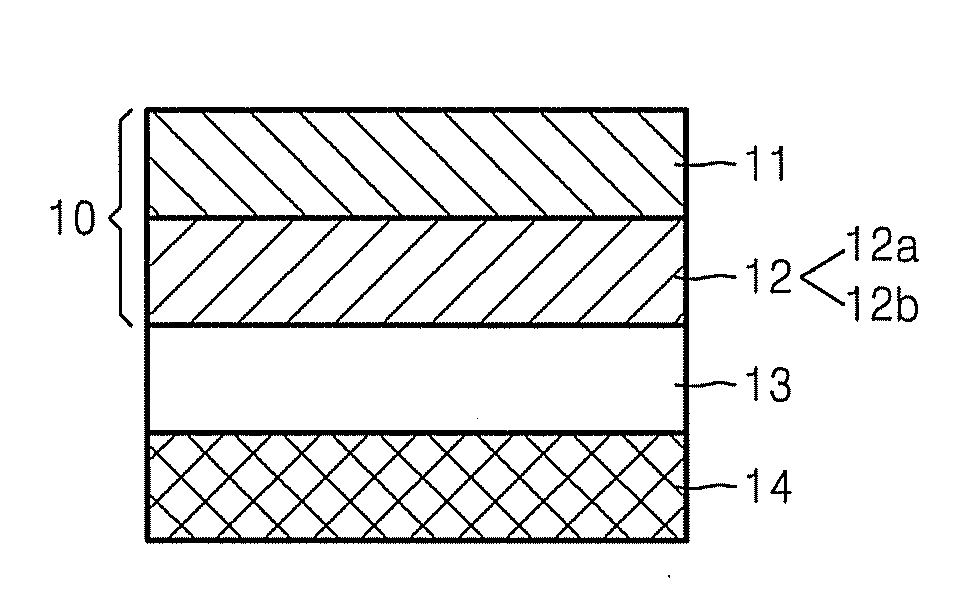
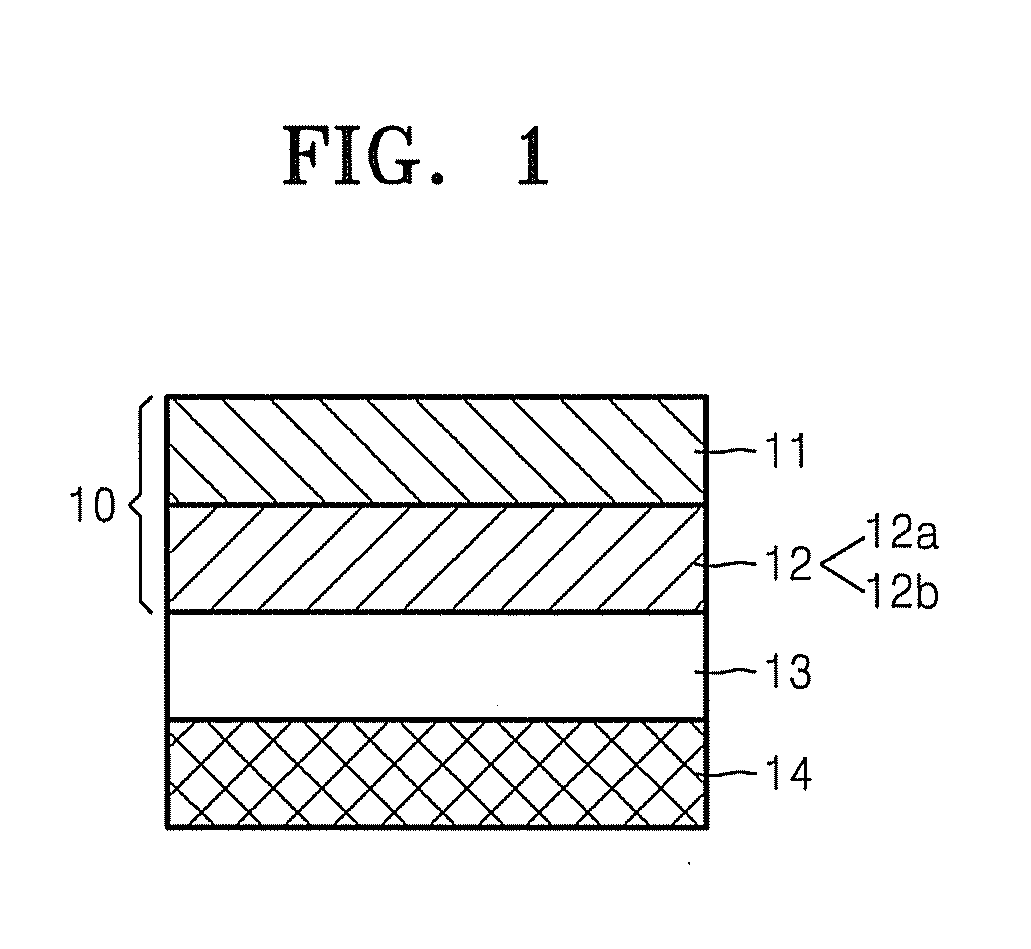
ActiveUS20120064409A1Enhanced Li-ion insertionIncrease capacityNon-metal conductorsMaterial nanotechnologyParticulatesMicroparticle
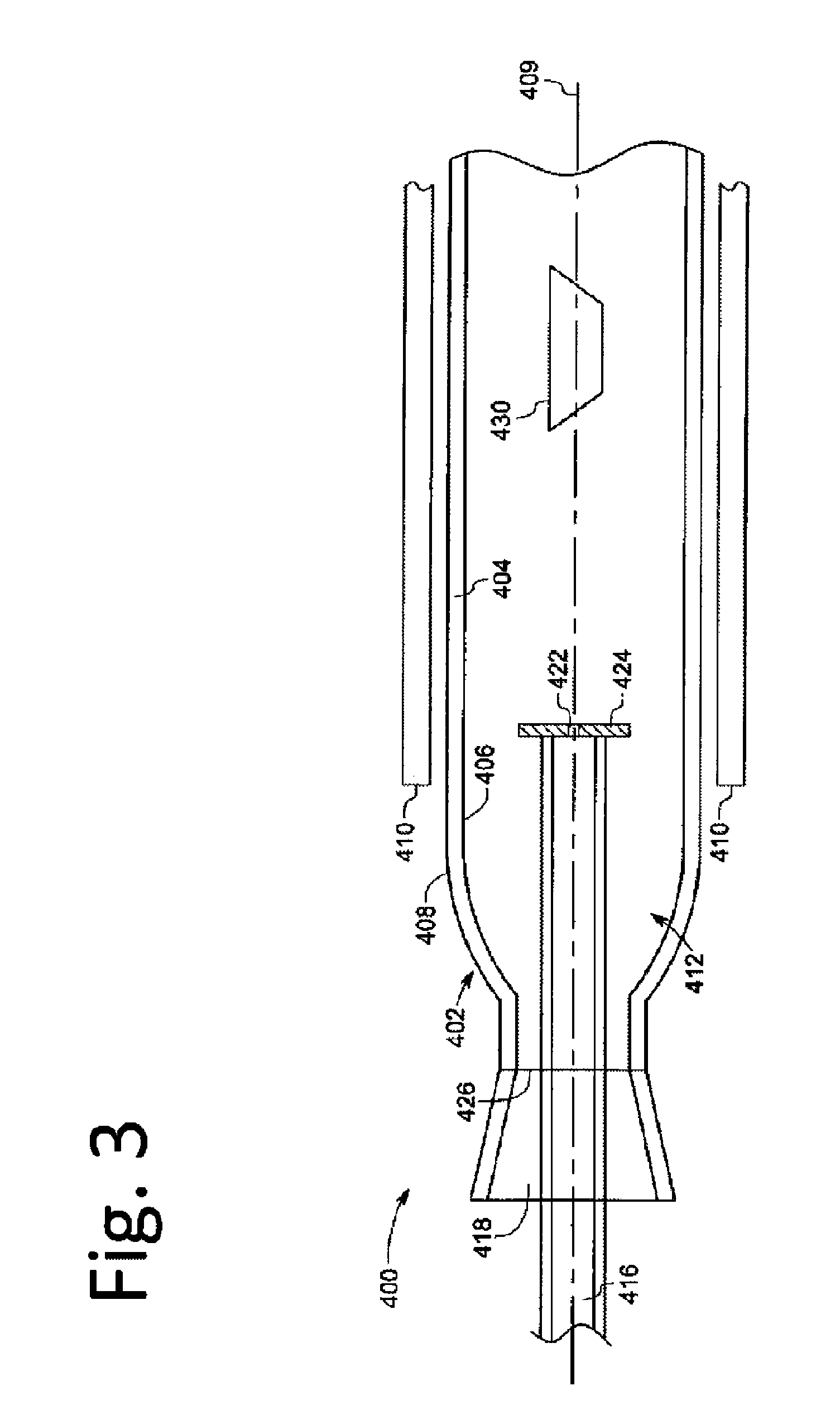
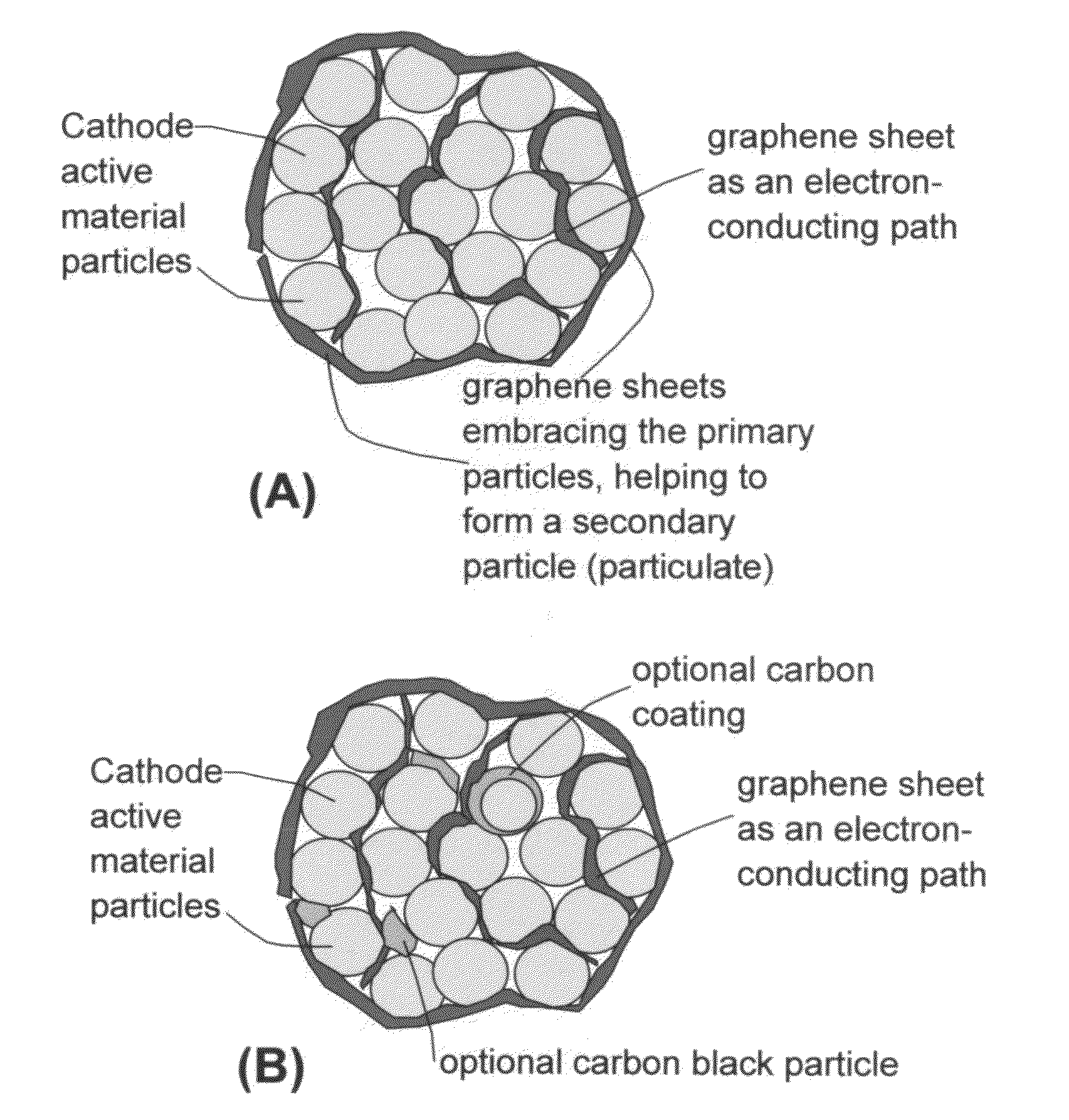
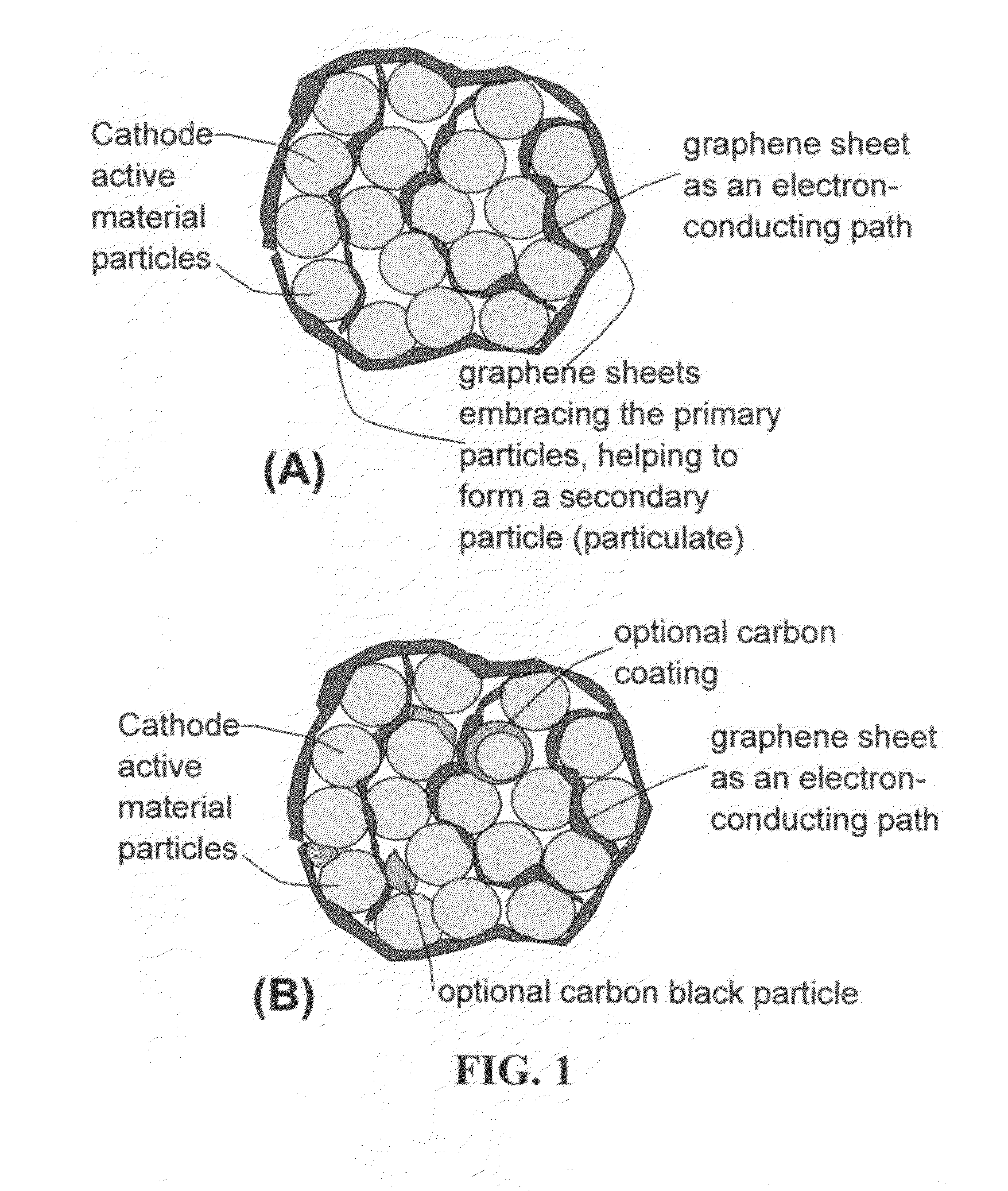
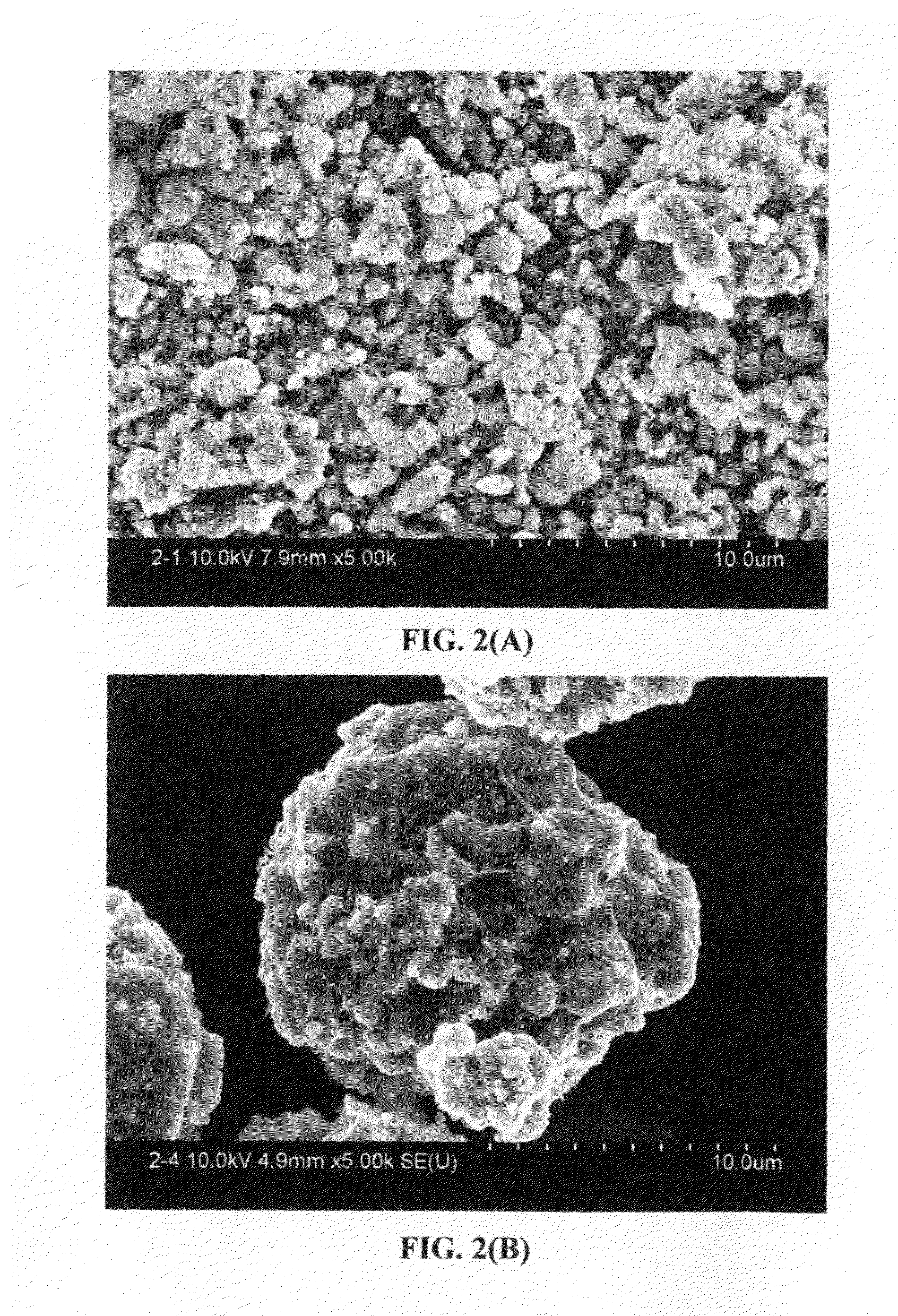
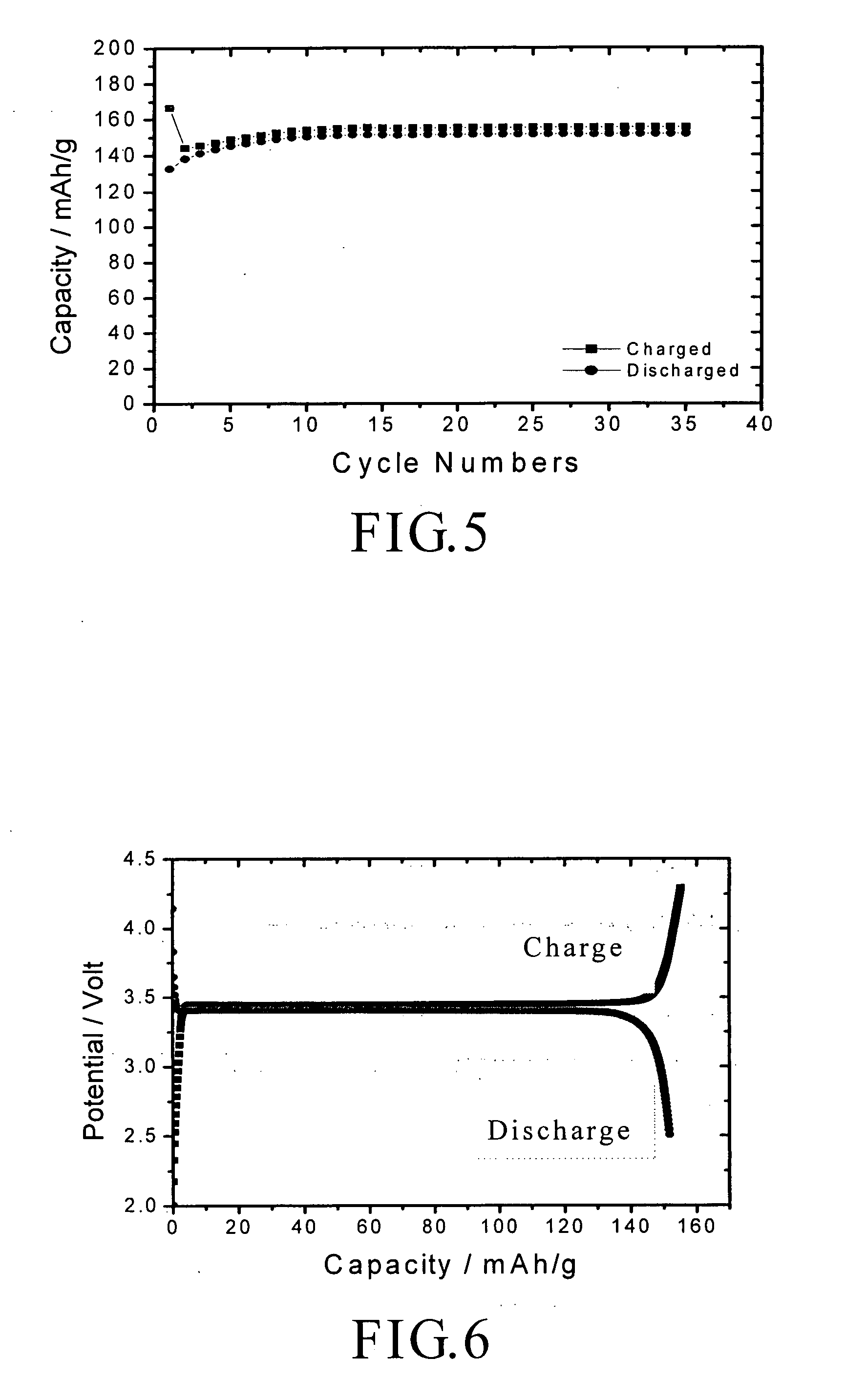
A nano graphene-enhanced particulate for use as a lithium-ion battery anode active material, wherein the particulate is formed of a single sheet of graphene or a plurality of graphene sheets and a plurality of fine anode active material particles with a size smaller than 10 μm. The graphene sheets and the particles are mutually bonded or agglomerated into the particulate with at least a graphene sheet embracing the anode active material particles. The amount of graphene is at least 0.01% by weight and the amount of the anode active material is at least 0.1% by weight, all based on the total weight of the particulate. A lithium-ion battery having an anode containing these graphene-enhanced particulates exhibits a stable charge and discharge cycling response, a high specific capacity per unit mass, a high first-cycle efficiency, a high capacity per electrode volume, and a long cycle life.
Owner:SAMSUNG ELECTRONICS CO LTD +1
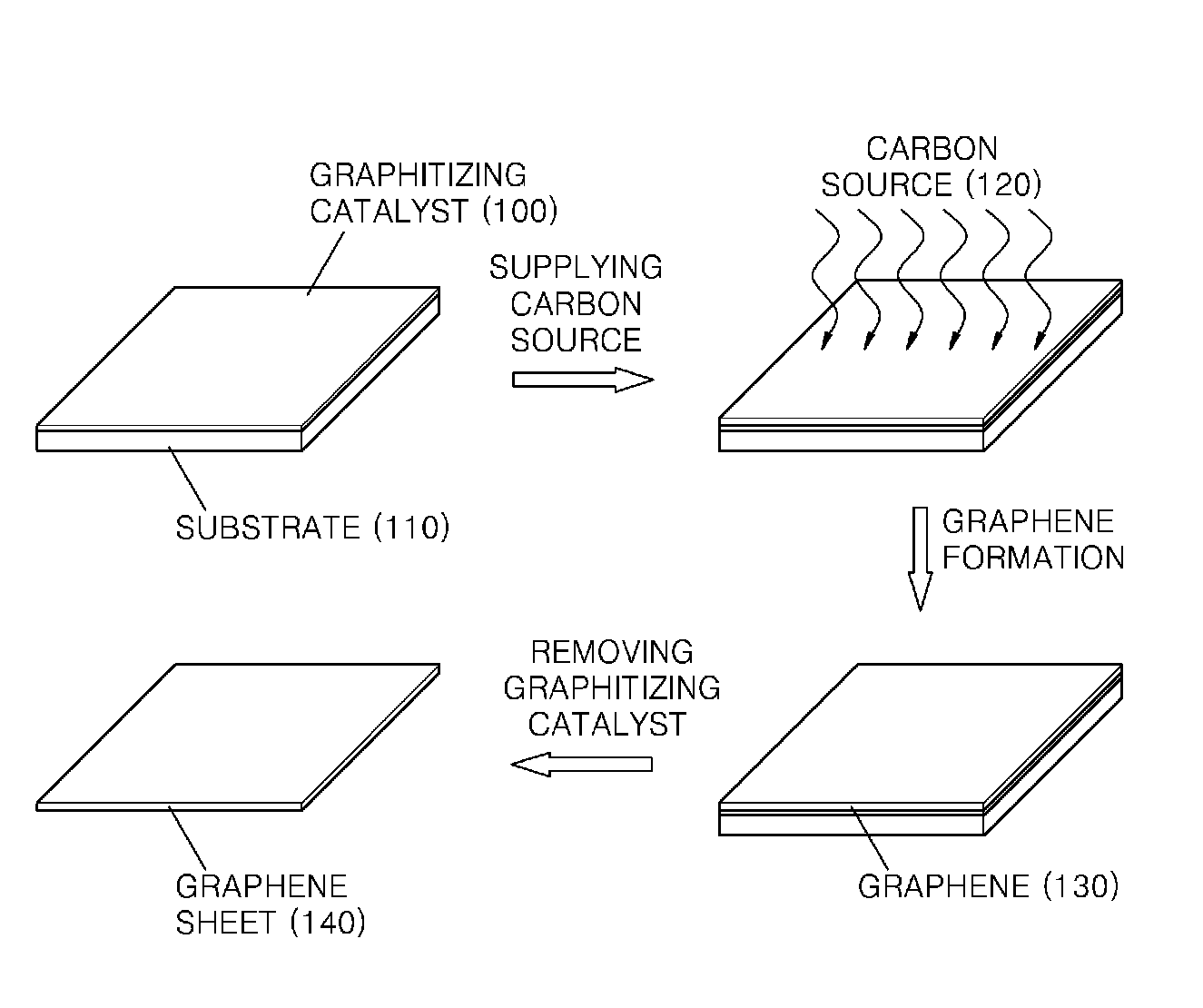
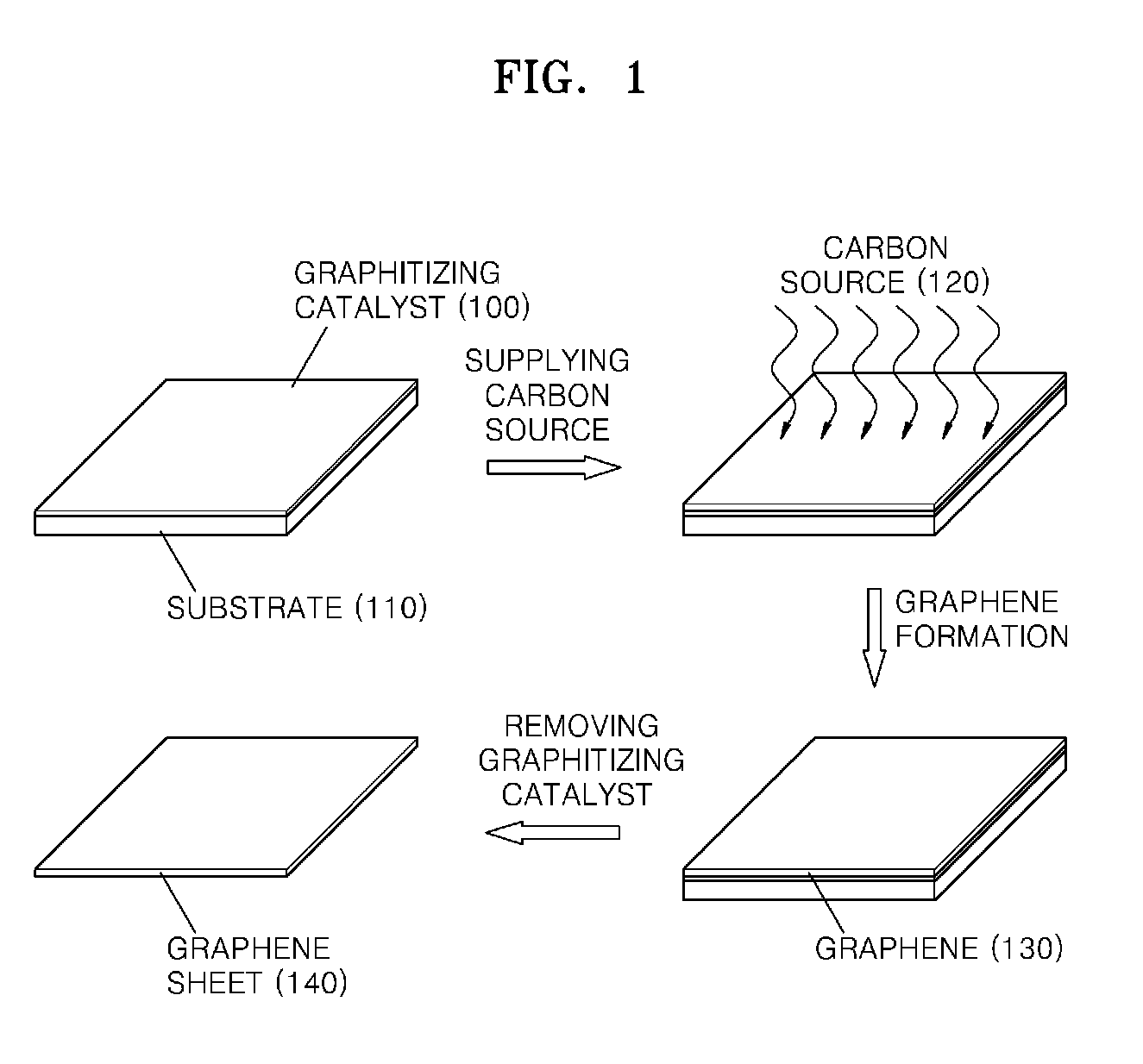
Graphene sheet and method of preparing the same
An economical method of preparing a large-sized graphene sheet having a desired thickness includes forming a film, the film comprising a graphitizing catalyst; heat-treating a gaseous carbon source in the presence of the graphitizing catalyst to form graphene; and cooling the graphene to form a graphene sheet. A graphene sheet prepared according to the disclosed method is also described.
Owner:SAMSUNG ELECTRONICS CO LTD
Polycrystalline group iii metal nitride with getter and method of making
ActiveUS20100151194A1Simple and cost-effective to manufactureCost-effectiveConductive materialRecord information storageNitrogenNitride
A gettered polycrystalline group III metal nitride is formed by heating a group III metal with an added getter in a nitrogen-containing gas. Most of the residual oxygen in the gettered polycrystalline nitride is chemically bound by the getter. The gettered polycrystalline group III metal nitride is useful as a raw material for ammonothermal growth of bulk group III nitride crystals.
Owner:SLT TECH
Graphene-Enhanced cathode materials for lithium batteries
ActiveUS20120058397A1Short timeEasy dischargeNon-metal conductorsMaterial nanotechnologyParticulatesCvd graphene
A nano graphene-enhanced particulate for use as a lithium battery cathode active material, wherein the particulate is formed of a single or a plurality of graphene sheets and a plurality of fine cathode active material particles with a size smaller than 10 μm (preferably sub-micron or nano-scaled), and the graphene sheets and the particles are mutually bonded or agglomerated into an individual discrete particulate with at least a graphene sheet embracing the cathode active material particles, and wherein the particulate has an electrical conductivity no less than 10−4 S / cm and the graphene is in an amount of from 0.01% to 30% by weight based on the total weight of graphene and the cathode active material combined.
Owner:GLOBAL GRAPHENE GRP INC
Powder material, electrode structure using the powder material, and energy storage device having the electrode structure
InactiveUS20080003503A1Fast chargingIncrease energy densityNon-metal conductorsElectrode manufacturing processesElectrical conductorHigh energy
A powder material which can electrochemically store and release lithium ions rapidly in a large amount is provided. In addition, an electrode structure for an energy storage device which can provide a high energy density and a high power density and has a long life, and an energy storage device using the electrode structure are provided. In a powder material which can electrochemically store and release lithium ions, the surface of particles of one of silicon metal and tin metal and an alloy of any thereof is coated by an oxide including a transition metal element selected from the group consisting of W, Ti, Mo, Nb, and V as a main component. The electrode structure includes the powder material. The battery device includes a negative electrode having the electrode structure, a lithium ion conductor, and a positive electrode, and utilizes an oxidation reaction of lithium and a reduction reaction of lithium ion.
Owner:CANON KK
Polymeric Composites, Oilfield Elements Comprising Same, and Methods of Using Same in Oilfield Applications
InactiveUS20070142547A1Improve barrier propertiesImprove mechanical propertiesNon-metal conductorsLayered productsSubject matterEngineering
Oilfield elements and assemblies are described comprising a polymeric matrix formed into an oilfield element, and a plurality of expanded graphitic nanoflakes and / or nanoplatelets dispersed in the polymeric matrix. Methods of using the oilfield elements and assemblies including same in oilfield operations are also described. This abstract allows a searcher or other reader to quickly ascertain the subject matter of the disclosure. It will not be used to interpret or limit the scope or meaning of the claims. 37 CFR 1.72(b).
Owner:SCHLUMBERGER TECH CORP
Proton-selective conducting membranes
InactiveUS20020127474A1Easy to operateSelective operationIon-exchanger regenerationSolid electrolyte cellsHydrophobic polymerProton
A membrane comprising: (a) a hydrophobic matrix polymer, and (b) a hydrophilic non-ionic polymer, wherein the hydrophobic polymer and the hydrophilic polymer are disposed so as to form a dense selectively proton-conducting membrane. The microstructure of such a membrane can be tailored to specific functionality requirements, such as proton conductivity vs. proton selectivity, and selectivity to particular species.
Owner:E C R ELECTRO CHEM RES
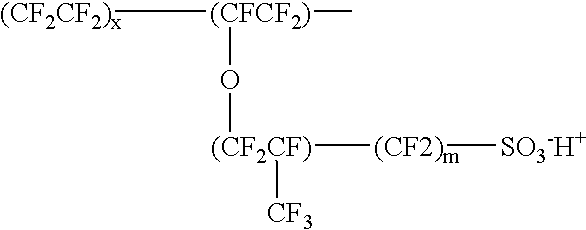
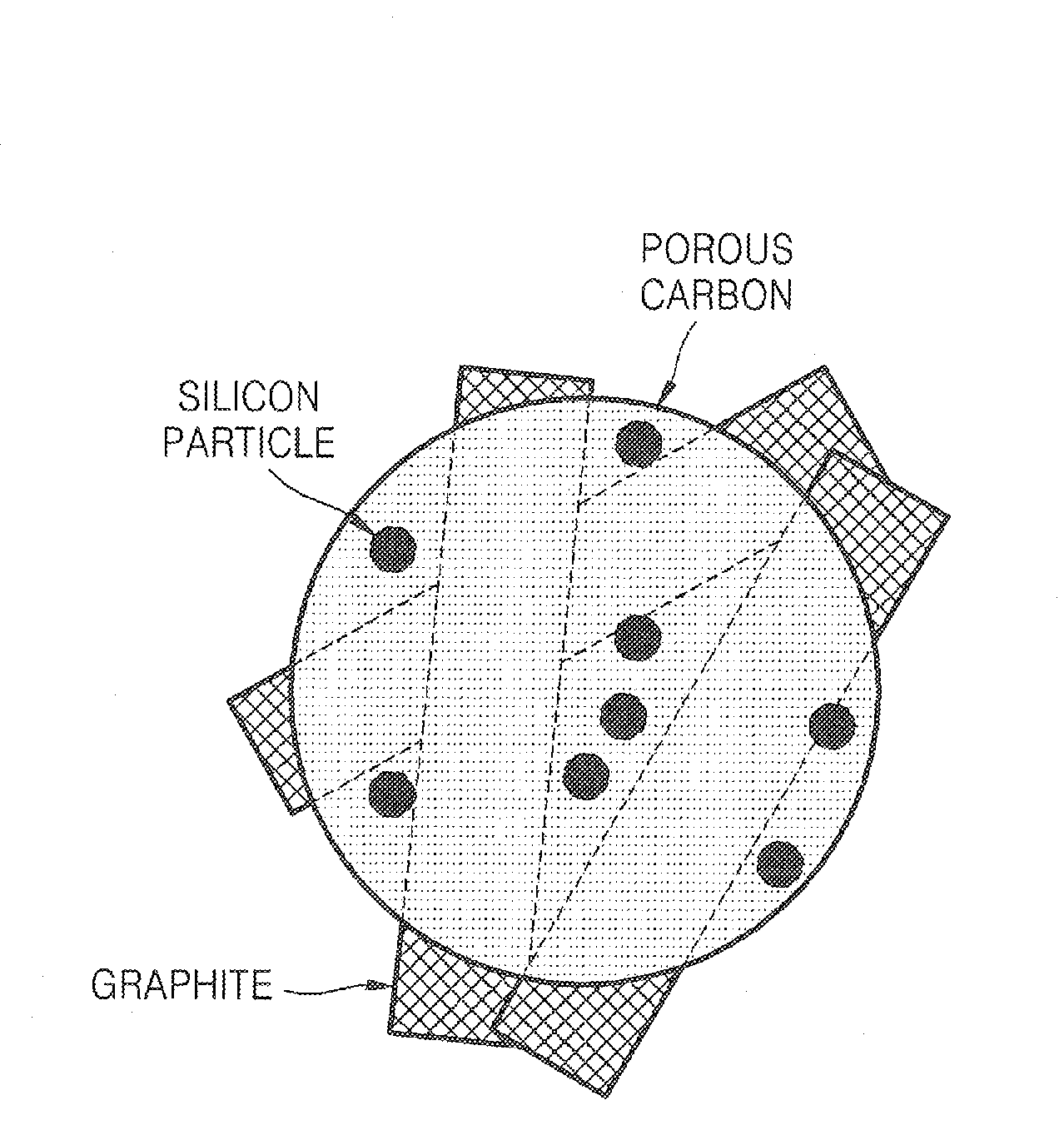
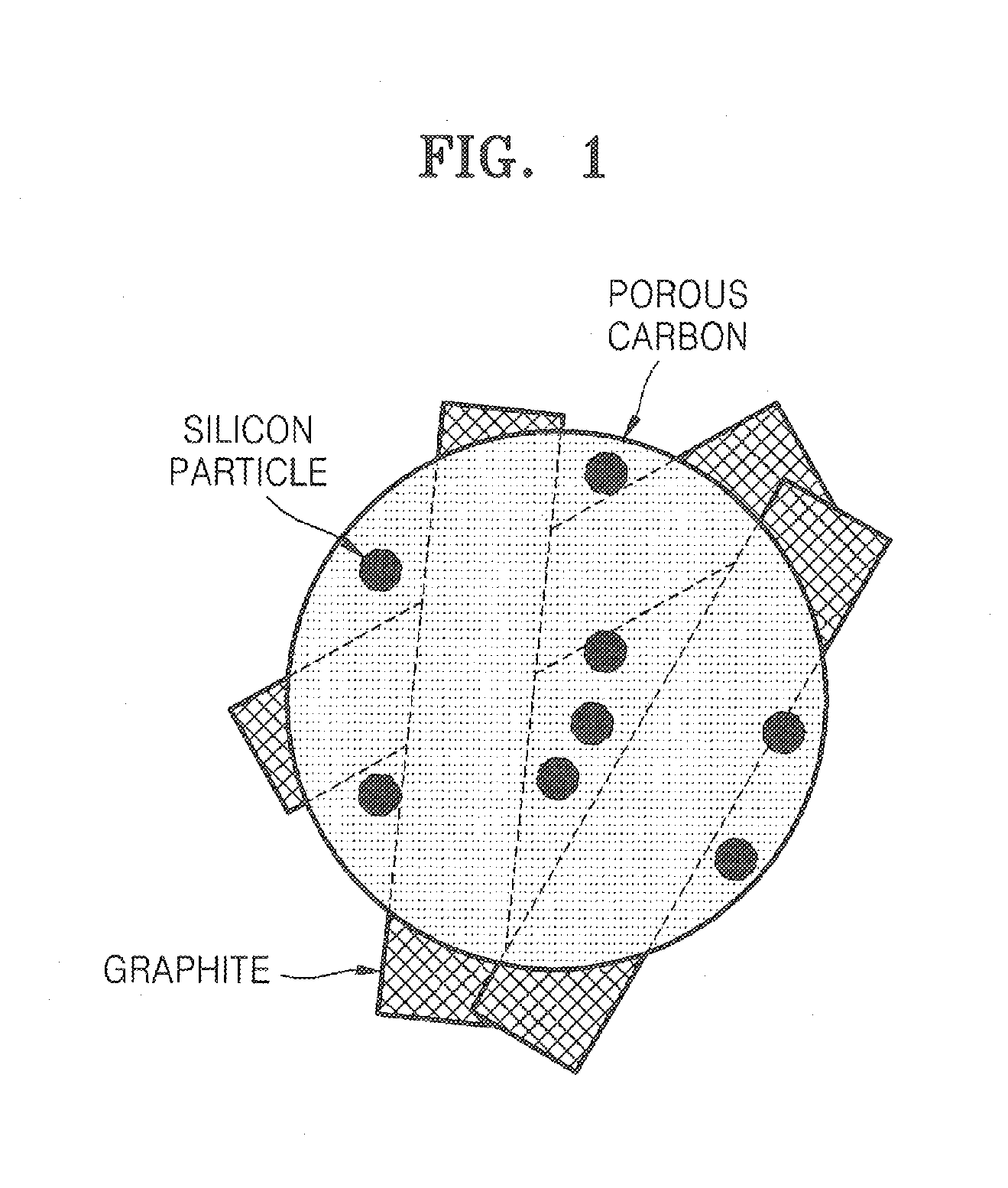
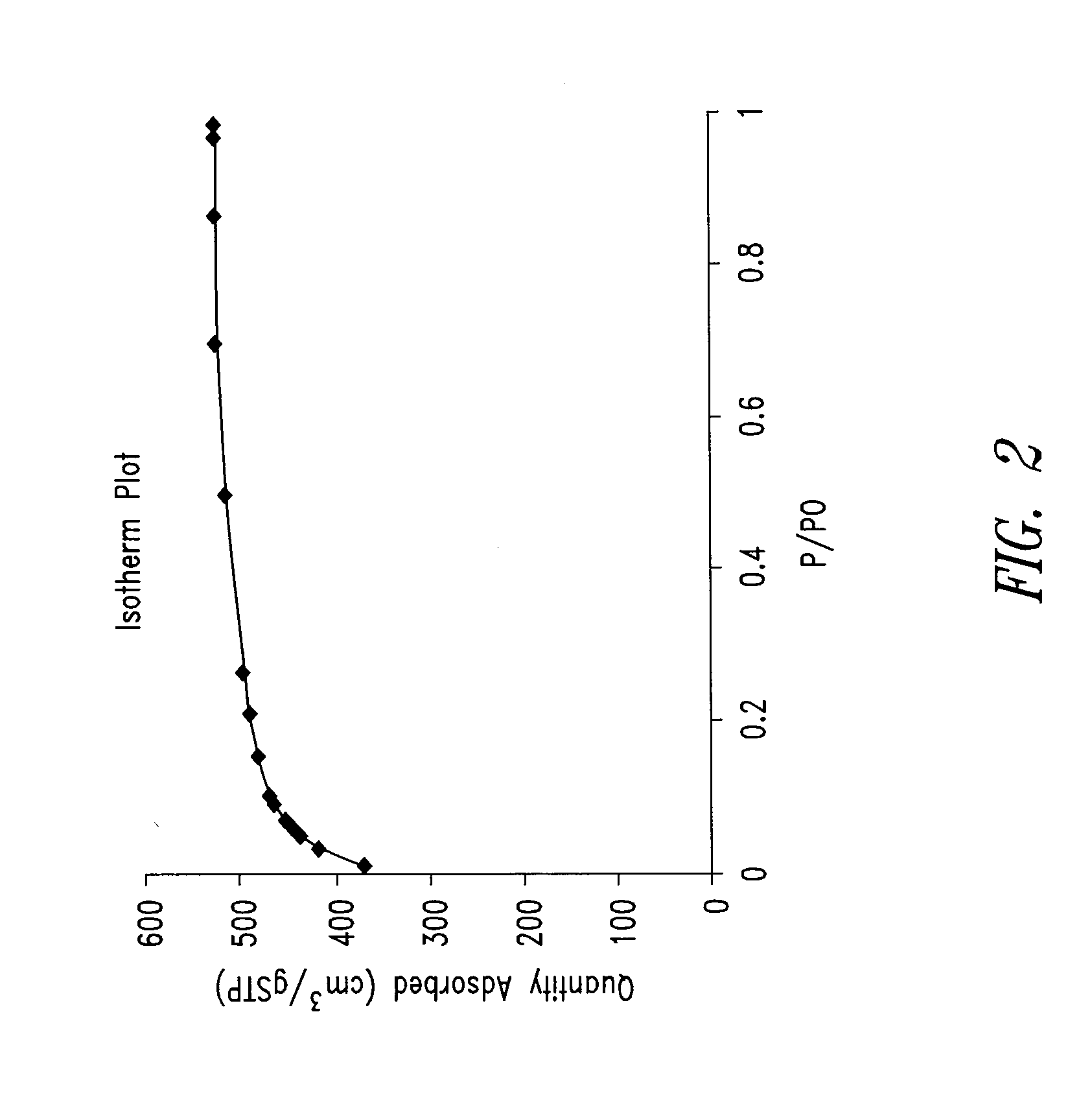
Porous anode active material, method of preparing the same, and anode and lithium battery employing the same
ActiveUS20080145757A1Efficiently remove stressExcellent charge and discharge characteristicsSilver accumulatorsMaterial nanotechnologyAlloyPore diameter
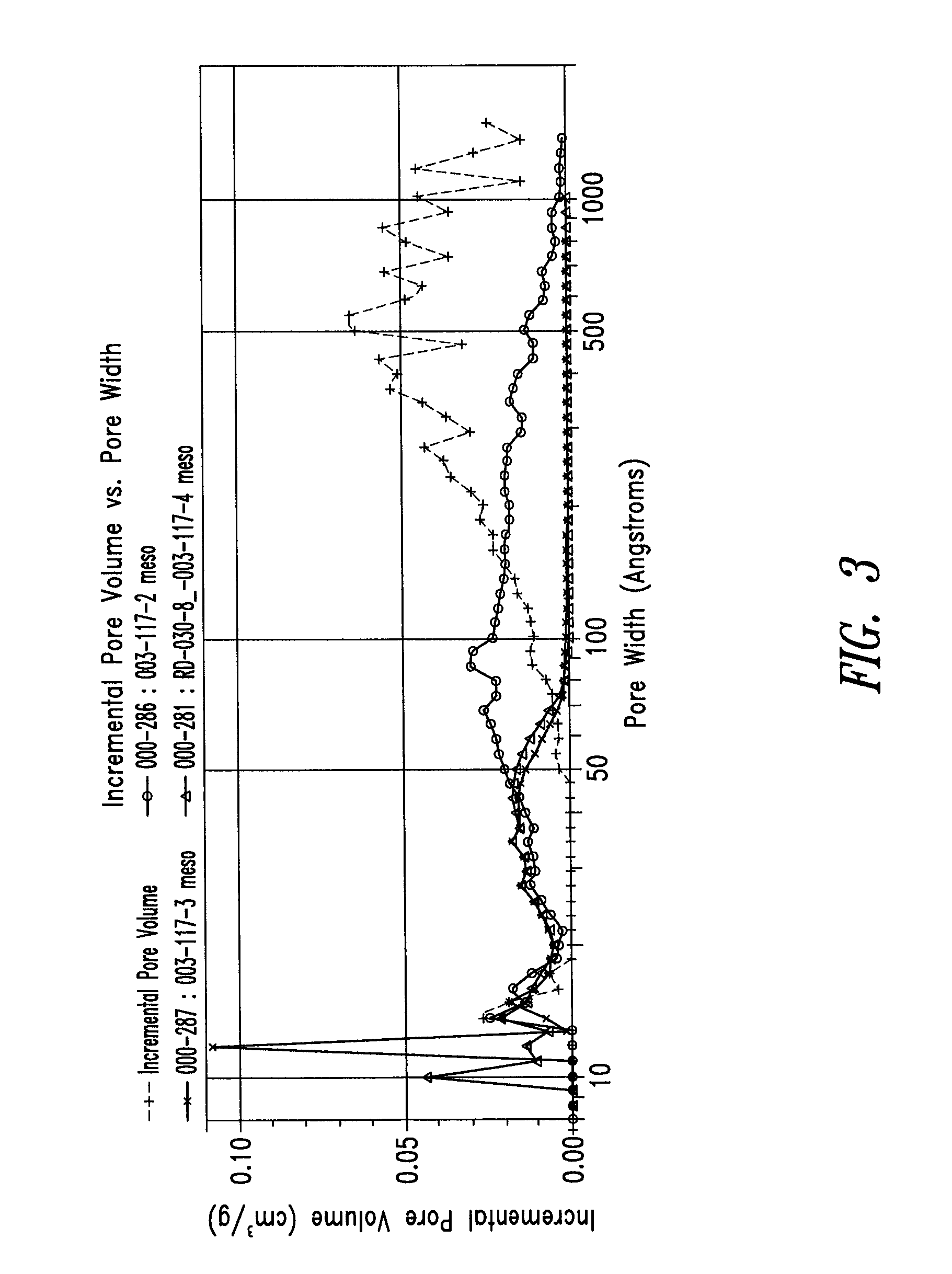
Provided are a porous anode active material, a method of preparing the same, and an anode and a lithium battery employing the same. The porous anode active material includes fine particles of metallic substance capable of forming a lithium alloy; a crystalline carboneous substance; and a porous carboneous material coating and attaching to the fine particles of metallic substance and the crystalline carboneous substance, the porous anode active material having pores exhibiting a bimodal size distribution with two pore diameter peaks as measured by a Barrett-Joyner-Halenda (BJH) pore size distribution from a nitrogen adsorption. The porous anode active material has the pores having a bimodal size distribution, and thus may efficiently remove a stress occurring due to a difference of expansion between a carboneous material and a metallic active material during charging and discharging. Further, the anode electrode and the lithium battery comprising the anode active material have excellent charge / discharge characteristics.
Owner:SAMSUNG SDI CO LTD
Metal-containing compounds
The invention relates to a novel solid state process for the preparation of metal-containing compounds comprising the steps i) forming a reaction mixture comprising one or more metal-containing precursor compounds and optionally one or more non-metal-containing reactants, and ii) using one or more hypophosphite-containing materials as a reducing agent; wherein one or more of the hypophosphite-containing materials is used as an agent to reduce one or more of the metal-containing precursor compounds; and further wherein the process is performed in the absence of an oxidizing atmosphere. Materials made by such a process are useful, for example, as electrode materials in alkali metal-ion battery applications.
Owner:LITHIUM WERKS TECH BV
Composite solid polymer elecrolyte membranes
InactiveUS20020045085A1Optimize swellingOptimize fuel crossover resistanceElectrolyte holding meansFinal product manufacturePolymer electrolytesPolymer science
The present invention relates to composite solid polymer electrolyte membranes (SPEMs) which include a porous polymer substrate interpenetrated with an ion-conducting material. SPEMs of the present invention are useful in electrochemical applications, including fuel cells and electrodialysis.
Owner:FOSTER-MILLER
Nano-scaled graphene plate films and articles
ActiveUS20080248275A1Improve thermal conductivityNon-metal conductorsMaterial nanotechnologyLightning strikePlatelet
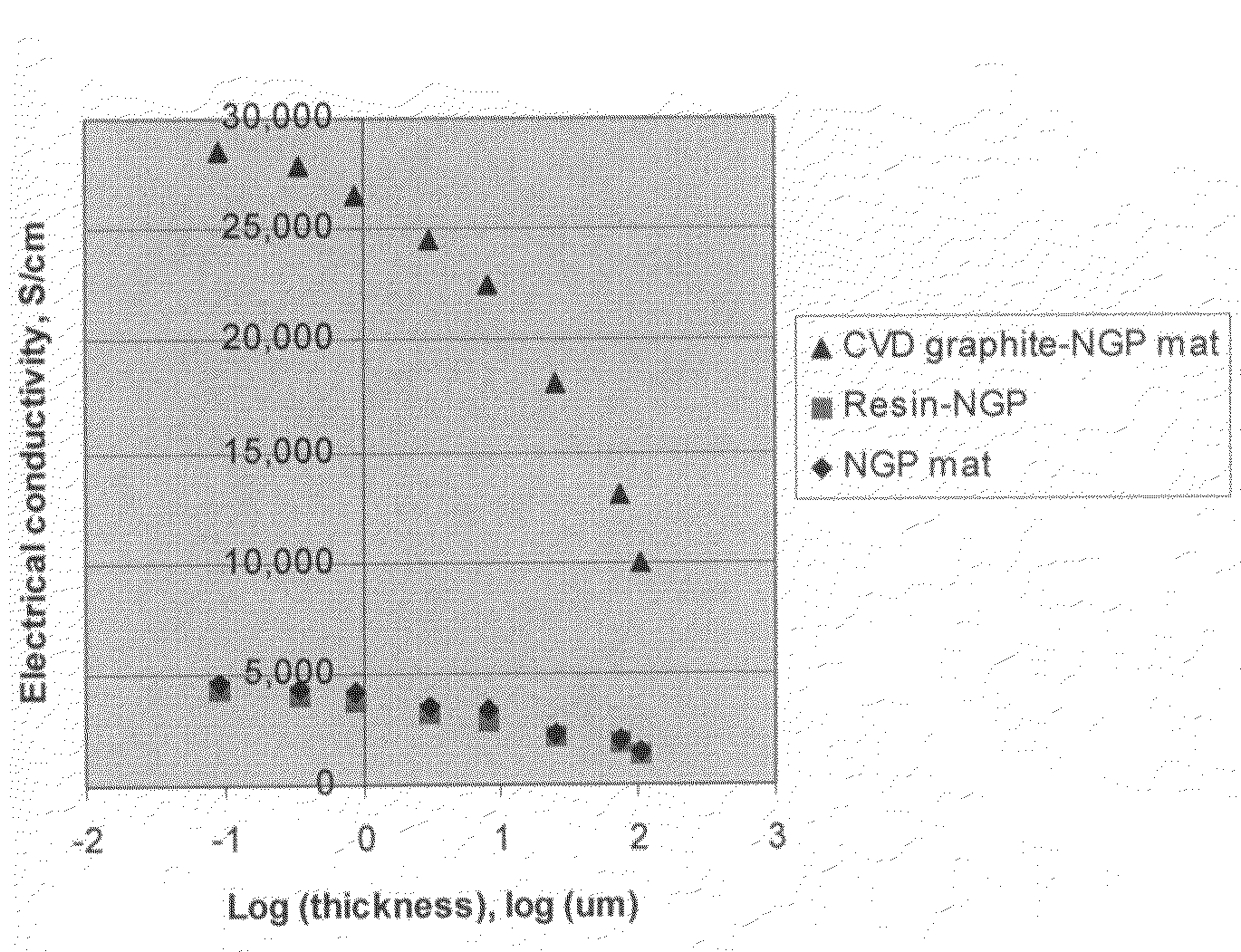
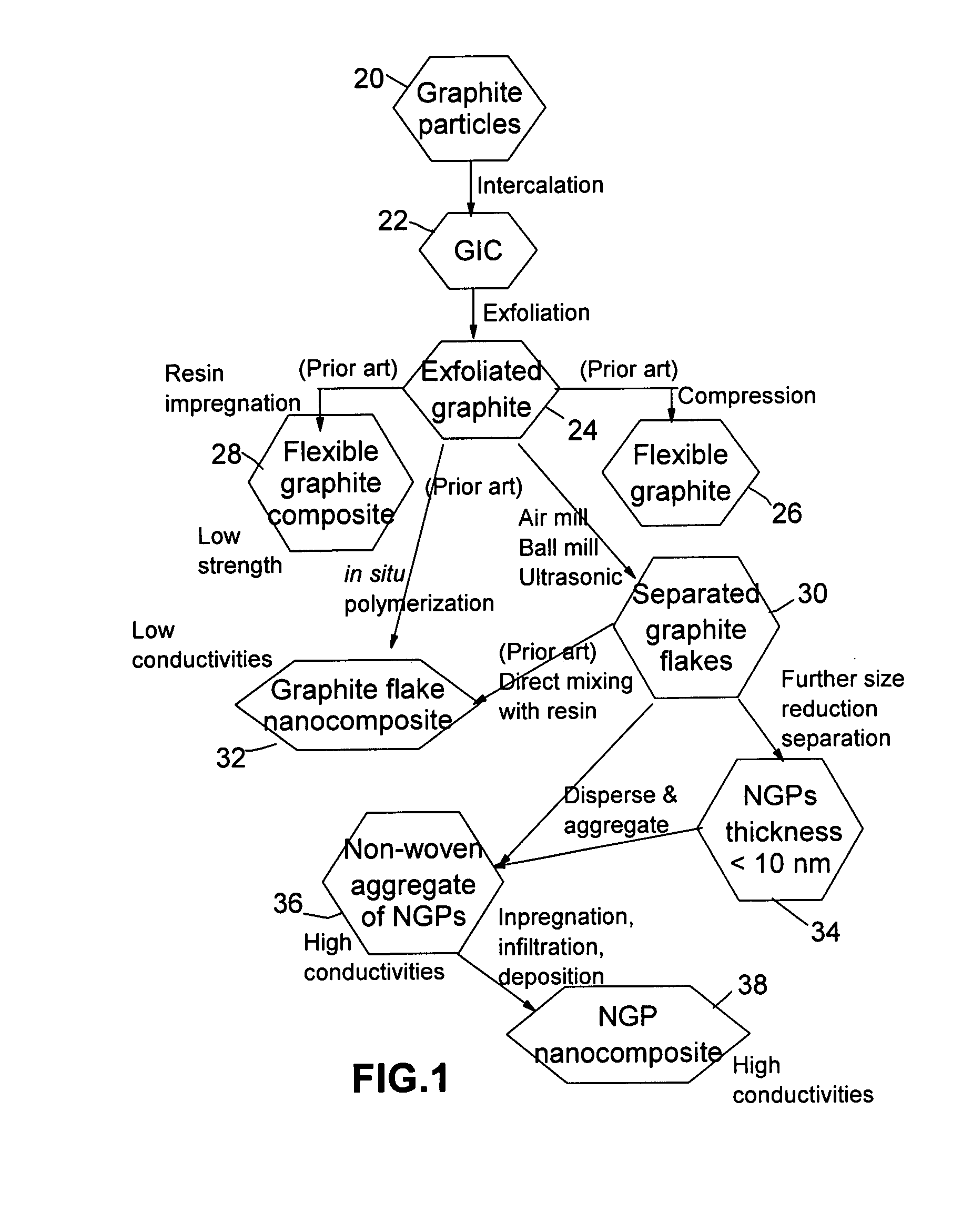
Disclosed is a nano-scaled graphene article comprising a non-woven aggregate of nano-scaled graphene platelets wherein each of the platelets comprises a graphene sheet or multiple graphene sheets and the platelets have a thickness no greater than 100 nm (preferably smaller than 10 nm) and platelets contact other platelets to define a plurality of conductive pathways along the article. The article has an exceptional thermal conductivity (typically greater than 500 Wm−1K−1) and excellent electrical conductivity (typically greater than 1,000 S / cm). Thin-film articles of the present invention can be used for thermal management in micro-electronic devices and for current-dissipating on an aircraft skin against lightning strikes.
Owner:GLOBAL GRAPHENE GRP INC
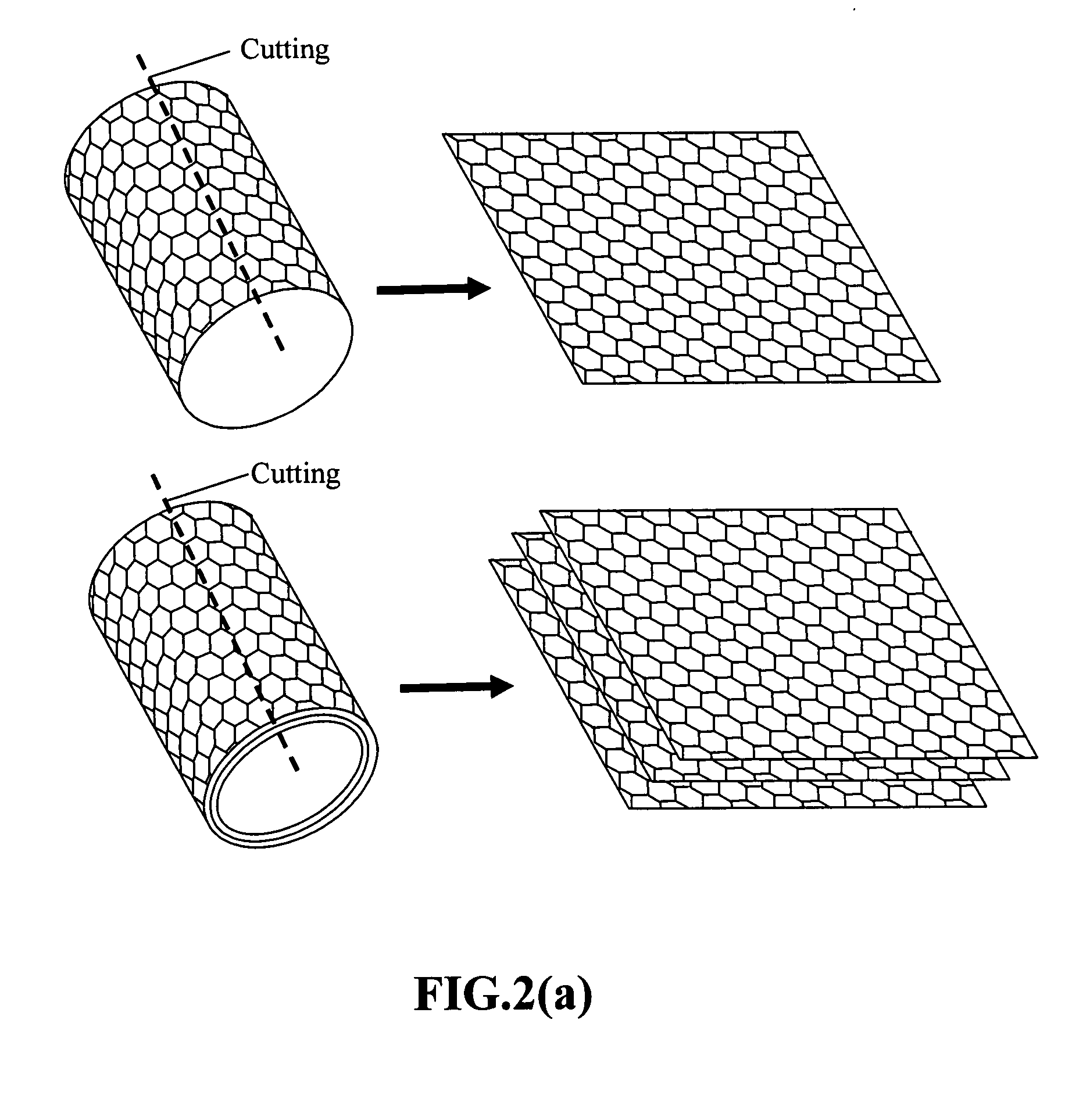
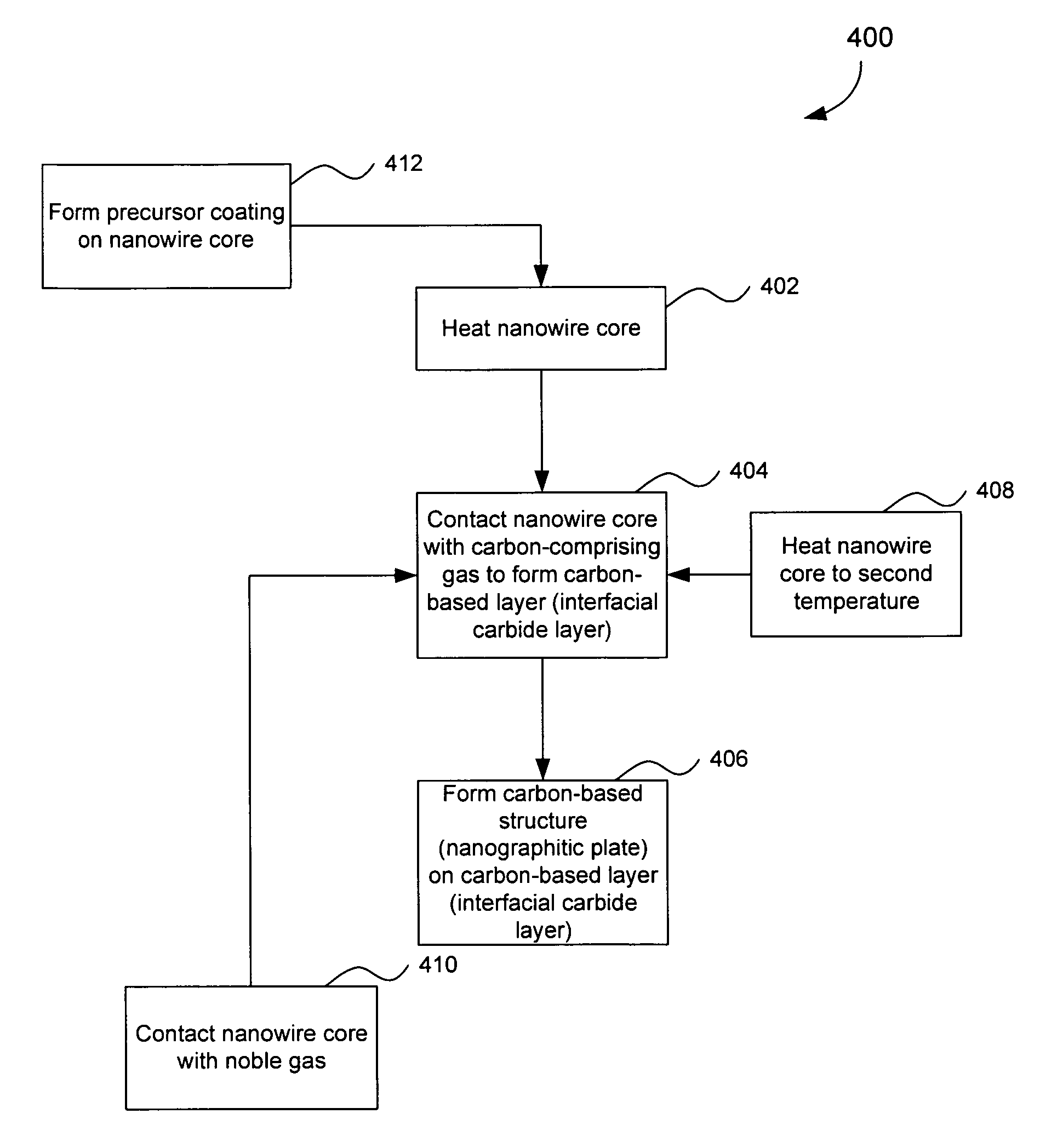
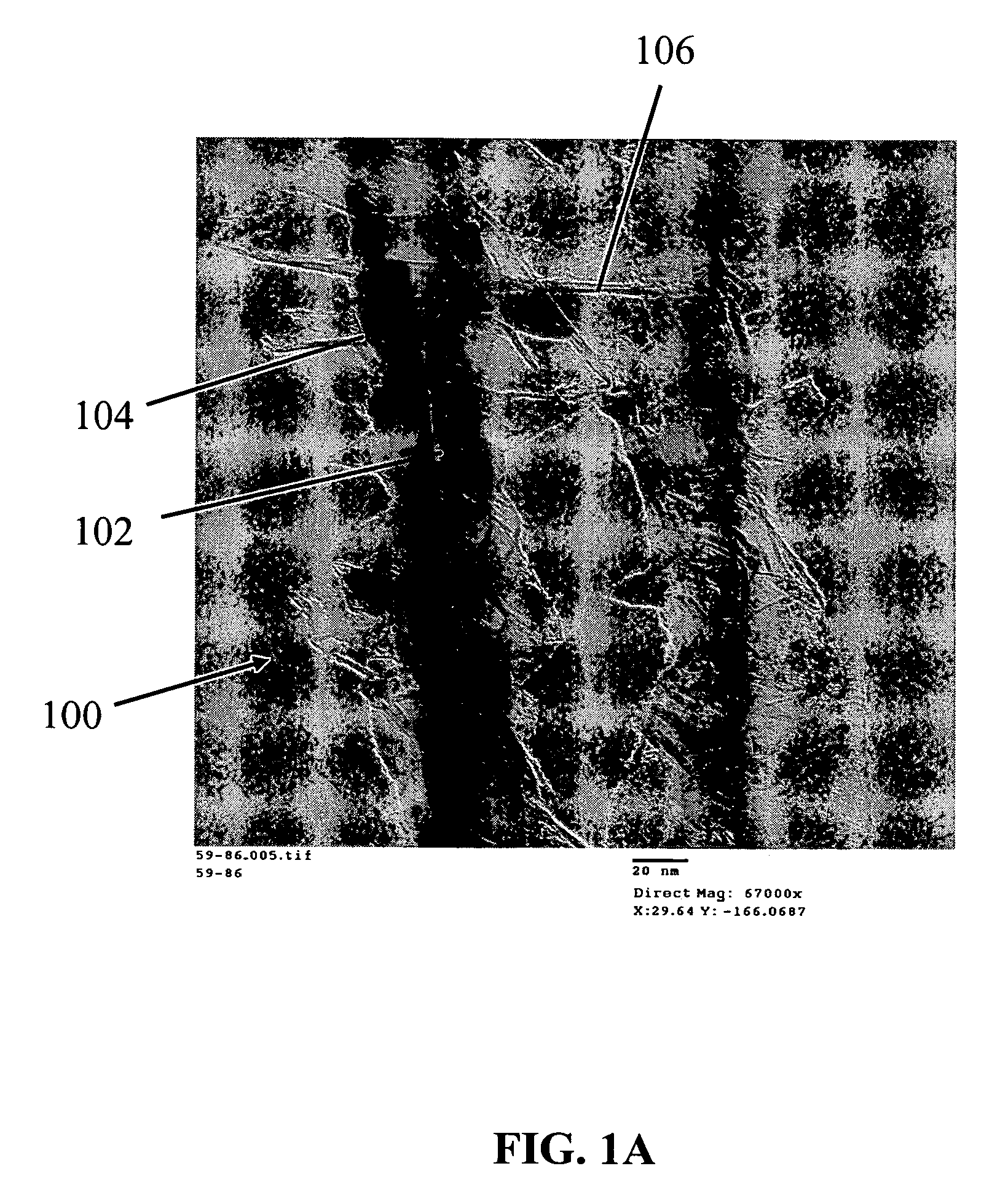
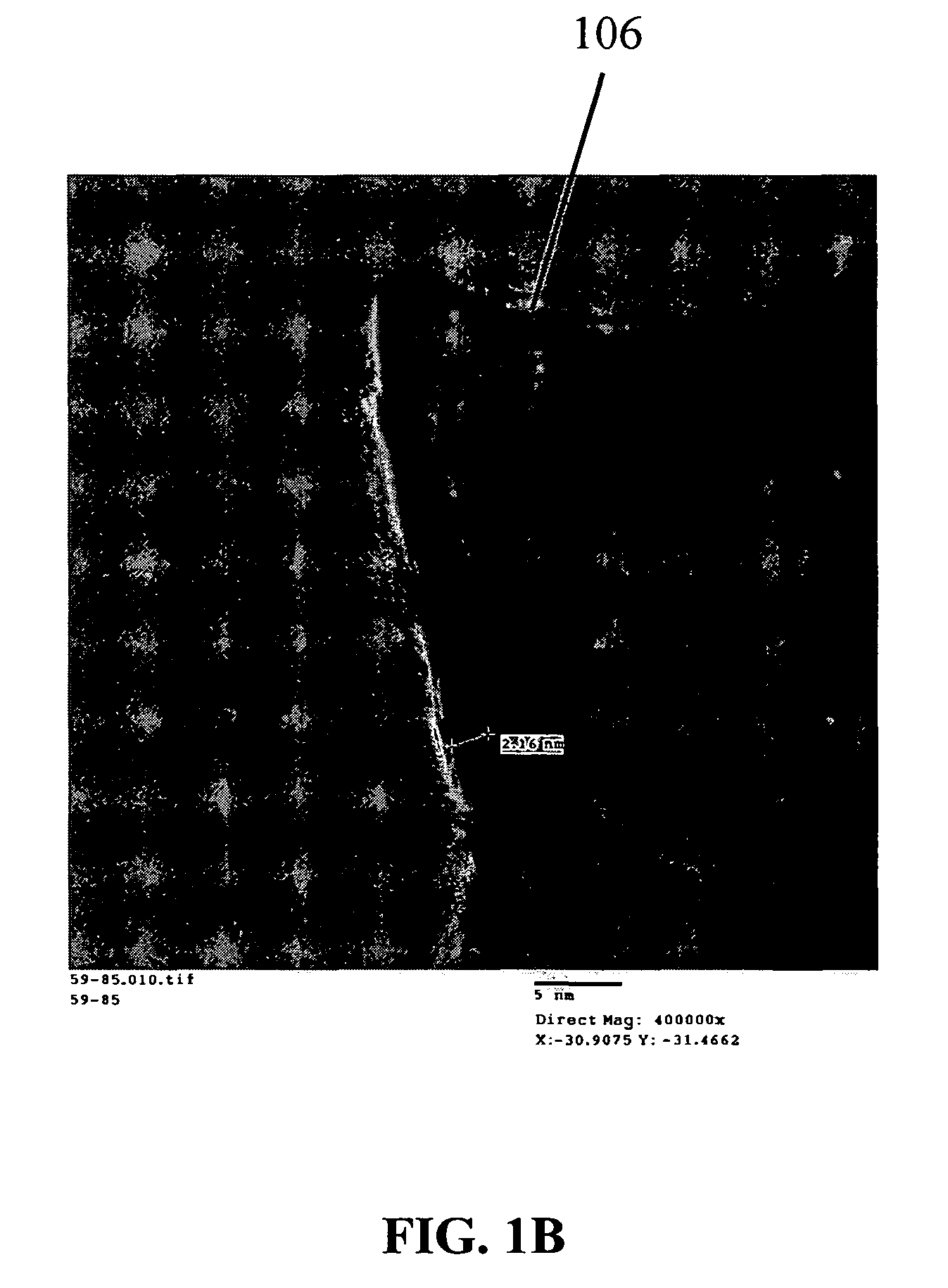
Nanowire structures comprising carbon
ActiveUS7939218B2High rateLow costElectric discharge heatingFuel cells groupingField emission deviceNanowire
The present invention is directed to nanowire structures and interconnected nanowire networks comprising such structures, as well as methods for their production. The nanowire structures comprise a nanowire core, a carbon-based layer, and in additional embodiments, carbon-based structures such as nanographitic plates consisting of graphenes formed on the nanowire cores, interconnecting the nanowire structures in the networks. The networks are porous structures that can be formed into membranes or particles. The nanowire structures and the networks formed using them are useful in catalyst and electrode applications, including fuel cells, as well as field emission devices, support substrates and chromatographic applications.
Owner:ONED MATERIAL INC
Reduced graphene oxide doped with dopant, thin layer and transparent electrode
InactiveUS20090146111A1Improve electrical performanceMaterial nanotechnologyNon-metal conductorsDopantDisplay device
Disclosed herein is a reduced graphene oxide doped with a dopant, and a thin layer, a transparent electrode, a display device and a solar cell including the reduced graphene oxide. The reduced graphene oxide doped with a dopant includes an organic dopant and / or an inorganic dopant.
Owner:SAMSUNG ELECTRONICS CO LTD
Low density lightning strike protection for use in airplanes
ActiveUS20090227162A1Minimize micro-crackingWeight optimizationConductive materialWarp knittingFiberEpoxy
Surface films, paints, or primers can be used in preparing aircraft structural composites that may be exposed to lightning strikes. Methods for making and using these films, paints or primers are also disclosed. The surface film can include a thermoset resin or polymer, e.g., an epoxy resin and / or a thermoplastic polymer, which can be cured, bonded, or painted on the composite structure. Low-density electrically conductive materials are disclosed, such as carbon nanofiber, copper powder, metal coated microspheres, metal-coated carbon nanotubes, single wall carbon nanotubes, graphite nanoplatelets and the like, that can be uniformly dispersed throughout or on the film. Low density conductive materials can include metal screens, optionally in combination with carbon nanofibers.
Owner:ROHR INC +1
Anisotropic conductive adhesive and method for preparation thereof and an electronic apparatus using said adhesive
InactiveUS6039896AReduce weightEasy to manufactureNon-macromolecular adhesive additivesDigital data processing detailsEpoxyPhosphoric Acid Esters
An anisotropic conductive adhesive contains conductive particles dispersed in a resin composition, wherein the resin composition includes a radical polymerization resin (A), an organic peroxide (B), a thermoplastic elastomer (C) and a phosphoric ester (D). The resin composition can further contain an epoxy silane coupling agent (E) represented by formula (2) or (3). The resin composition is mixed with other components after the radical polymerization resin (A), the thermoplastic elastomer (C), the phosphoric ester (D) and the epoxy silane coupling agent (E) are reacted. It is also possible to preliminarily react only the phosphoric ester (D) and the epoxy silane coupling agent (E) and to react the product of the preliminary reaction with the radical polymerization resin (A) and the thermoplastic elastomer (C), and then to add other components. The anisotropic conductive adhesive of the present invention can be used for electrical joining of electronic or electric parts of electrical apparatus.
Owner:SUMITOMO BAKELITE CO LTD
Composite binder containing carbon nanotube and lithium secondary battery employing the same
ActiveUS20070202403A1Improve mechanical propertiesGood strength propertiesNon-metal conductorsMaterial nanotechnologyLithiumCarbon nanotube
Provided is a nanocomposite binder for an electrode mix of a secondary battery, comprising carbon nanotubes in a photo- and / or thermo-polymerizable material or polymer, or a mixture thereof; and a lithium secondary battery comprising the same. The carbon nanotube-containing composite binder according to the present invention and a lithium secondary battery comprising the same employs a novel nanocomposite, prepared by combination of carbon nanotubes with a conventional binder material, as a binder of an anode. As a result, the present invention provides advantages such as improved conductivity of the anode due to decreased electrical resistance of the binder, and enhanced mechanical properties of the binder and thereby being capable of preventing the separation of an anode active material layer from a current collector due to volume changes occurring upon charge / discharge cycles.
Owner:LG CHEM LTD
Homogeneous fluorassay methods employing fluorescent background rejection and water-soluble rare earth metal chelates
InactiveUS6242268B1High binding constantNon-metal conductorsGlass making apparatusHalf-lifeMetal chelate
Homogeneous assays for determining quantitatively the extent of a specific binding reaction can be carried out effectively on very dilute solutions using measurements of fluorescence if a fluorescence measurement scheme that is capable of rejecting short-lived background fluorescence is employed and if the fluorescent group being measured has the following properties: a. the group being measured must be a rare earth metal chelate complex combination; b. the chelate must be water-soluble; c. the complex combination must also be stable in extremely dilute aqueous solutions, that is, the measured chelate must have at least one ligand having a metal-to-ligand binding constant of at least about 1013M-1 or greater and it must have a fluorescent emission that is long-lived compared to the longest decay lifetime of ambient substances and have a half life of from 0.01 to 50 msec.
Owner:EG&G WALLAC
Carbon nanotube dispersion liquid and transparent conductive film using same
Disclosed is a carbon nanotube dispersion liquid which enables to easily form a transparent conductive film. Also disclosed is a transparent conductive film obtained by using such a carbon nanotube dispersion liquid. Specifically disclosed is a carbon nanotube dispersion liquid containing a carbon nanotube (A), a dispersing agent (B) composed of an organic compound containing one of a carboxyl group, epoxy group, amino group and sulfonyl group and having a boiling point of not less than 30˚C and not more than 150˚C, and a solvent (C). Also disclosed are a transparent conductive film containing a layer composed of a solid component of such a dispersion liquid, and a method for producing such a transparent conductive film.
Owner:KURARAY CO LTD
Lithium ion polymer electrolytes
InactiveUS6413676B1Prevent kinkingAvoid deformationElectrode manufacturing processesFinal product manufacturePorosityCross-link
A dimensionally stable, highly resilient, hybrid copolymer solid-solution electrolyte-retention film for use in a lithium ion battery in one preferred embodiment has a predominantly amorphous structure and mechanical strength despite contact with liquid solvent electrolyte. The film is a thinned (stretched), cast film of a homogeneous blend of two or more polymers, one of which is selected for its pronounced solvent retention properties. A very high surface area inorganic filler dispersed in the blend during formation thereof serves to increase the porosity of the film and thereby enhance electrolyte retention. The film is soaked in a solution of liquid polymer with liquid organic solvent electrolyte and lithium salt, for absorption thereof. Use of a cross-linked liquid polymer enhances trapping of molecules of the electrolyte into pores of the film. The electrolyte film is sandwiched between flexible active anode and cathode layers to form the lithium ion battery. Novel methods are provided for forming the electrodes, the polymer substrate, and other elements of the battery.
Owner:LITHIUM POWER TECH
Method for making a lithium mixed metal compound having an olivine structure
ActiveUS20070207080A1Mitigate such drawbackNon-metal conductorsLiquid surface applicatorsLithiumCarbon layer
Owner:AQUIRE ENERGY CO LTD
Crystalline composition, wafer, and semi-conductor structure
ActiveUS20080008855A1Reduce defect levelConductive materialSolid-state devicesCrystallographySemiconductor structure
A crystalline composition is provided. The crystalline composition may include gallium and nitrogen; and the crystalline composition may have an infrared absorption peak at about 3175 cm−1, with an absorbance per unit thickness of greater than about 0.01 cm−1.
Owner:SLT TECH
Electrode composite, battery electrode formed from said composite, and lithium battery comprising such an electrode
InactiveUS20110163274A1Improve cycle stabilityGood dispersionMaterial nanotechnologyNon-metal conductorsElectrical batteryCarbon nanofiber
An electrode composite and to its manufacturing process. The composite includes an active element, i.e. one exhibiting electrochemical activity, a conductive additive and a binder. The conductive additive is a mixture of conductive additives containing at least carbon nanofibres (CNFs) and at least carbon nanotubes (CNTs). Also, the negative electrodes for electrochemical devices of the lithium battery type including said composite and to the secondary (Li-ion) batteries provided with such a negative electrode.
Owner:ARKEMA FRANCE SA +1
Coatings containing functionalized graphene sheets and articles coated therewith
Coatings comprising functionalized graphene sheets and at least one binder. In one embodiment, the coatings are electrically conductive.
Owner:THE TRUSTEES FOR PRINCETON UNIV +1
Ultrapure synthetic carbon materials
ActiveUS20110002086A1Short lifeEasy to operateNon-insulated conductorsElectrolytic capacitorsCapacitancePorosity
The present application is generally directed to ultrapure synthetic carbon materials having both high surface area and high porosity, ultrapure polymer gels and devices containing the same. The disclosed ultrapure synthetic carbon materials find utility in any number of devices, for example, in electric double layer capacitance devices and batteries. Methods for making ultrapure synthetic carbon materials and ultrapure polymer gels are also disclosed.
Owner:BASF AG
Ultrapure synthetic carbon materials
ActiveUS8404384B2Short lifeEasy to operateNon-insulated conductorsElectrolytic capacitorsPorosityPolymer science
The present application is generally directed to ultrapure synthetic carbon materials having both high surface area and high porosity, ultrapure polymer gels and devices containing the same. The disclosed ultrapure synthetic carbon materials find utility in any number of devices, for example, in electric double layer capacitance devices and batteries. Methods for making ultrapure synthetic carbon materials and ultrapure polymer gels are also disclosed.
Owner:BASF SE
Cable semiconducting shields
A semiconducting composition comprising (i) an olefinic polymer and (ii) about 25 to about 45 percent by weight, based on the weight of the composition, of a carbon black having the following properties: (a) a particle size of at least about 29 nanometers; (b) a tint strength of less than about 100 percent; (c) a loss of volatiles at 950 degrees C in a nitrogen atmosphere of less than about 1 weight percent based on the weight of the carbon black; (d) a DBP oil absorption of about 80 to about 300 cubic centimeters per 100 grams; (e) a nitrogen surface adsorption area of about 30 to about 300 square meters per gram or an iodine adsorption number of about 30 to about 300 grams per kilogram; (f) a CTAB surface area of about 30 to about 150 square meters per gram; and (g) a ratio of property (e) to property (f) of greater than about 1.1.
Owner:UNION CARBIDE CHEM & PLASTICS TECH CORP
Manufacturing methods of catalysts for carbon fiber composition and carbon material compound, manufacturing methods of carbon fiber and catalyst material for fuel cell, and catalyst material for fuel cell
The carbon fibers of this invention is characterized in that irreducible inorganic material particles in a mean primary particle size below 500 nm and reducible inorganic material particles in a mean primary particle size below 500 nm were mixed by pulverizing and then, the mixture was heat treated under the reducing atmosphere and metal particles in a mean particle size below 1 μm were obtained, and the mixed powder of the thus obtained metal particles with the irreducible inorganic material particles are included in the carbon fibers.
Owner:KK TOSHIBA
Stable electrolyte counteranions for electrochemical devices
InactiveUS20070048605A1Desirable chemical stabilityDesirable thermal stabilityConductive materialOrganic electrolyte cellsArylHydrogen
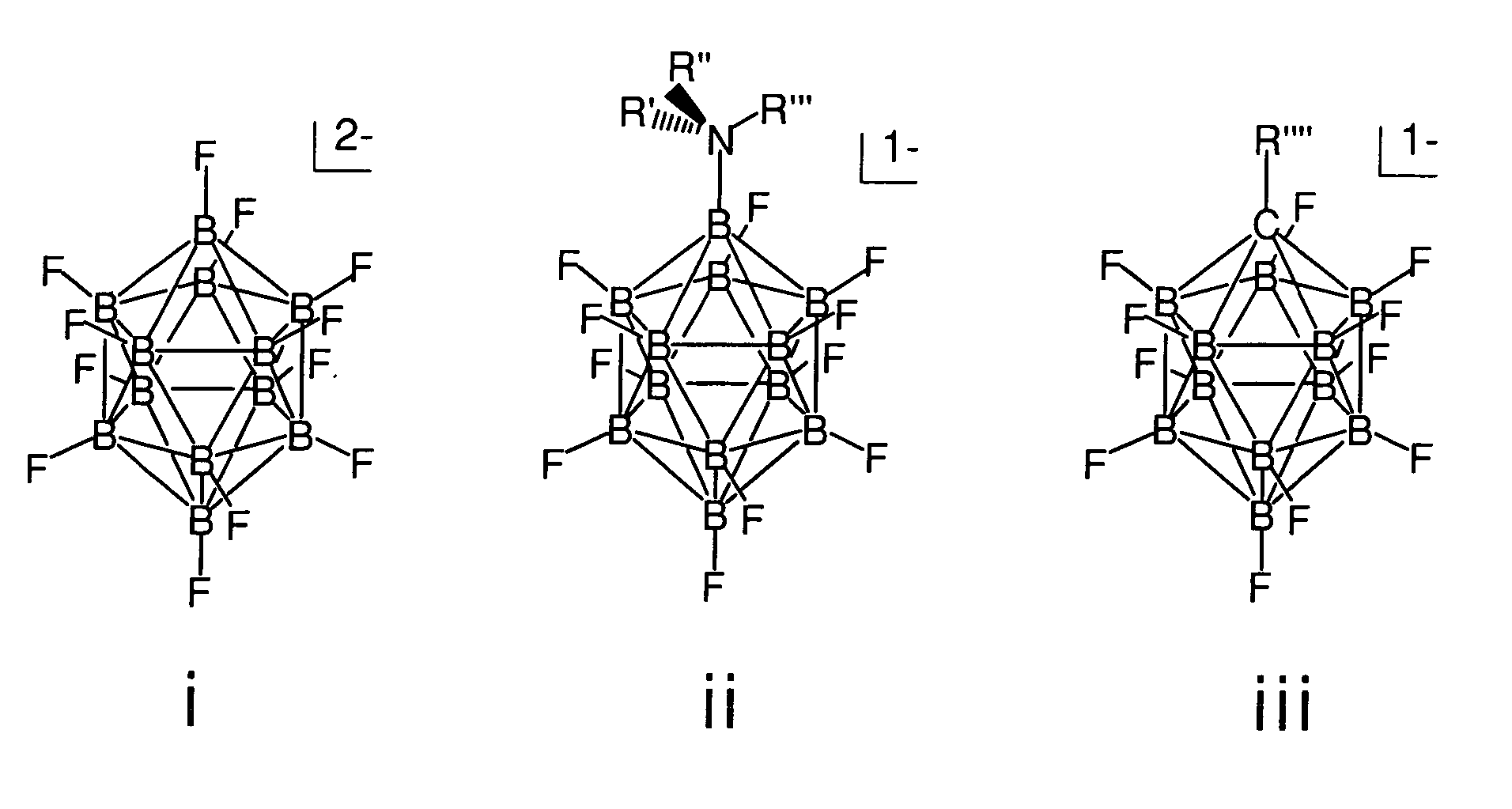
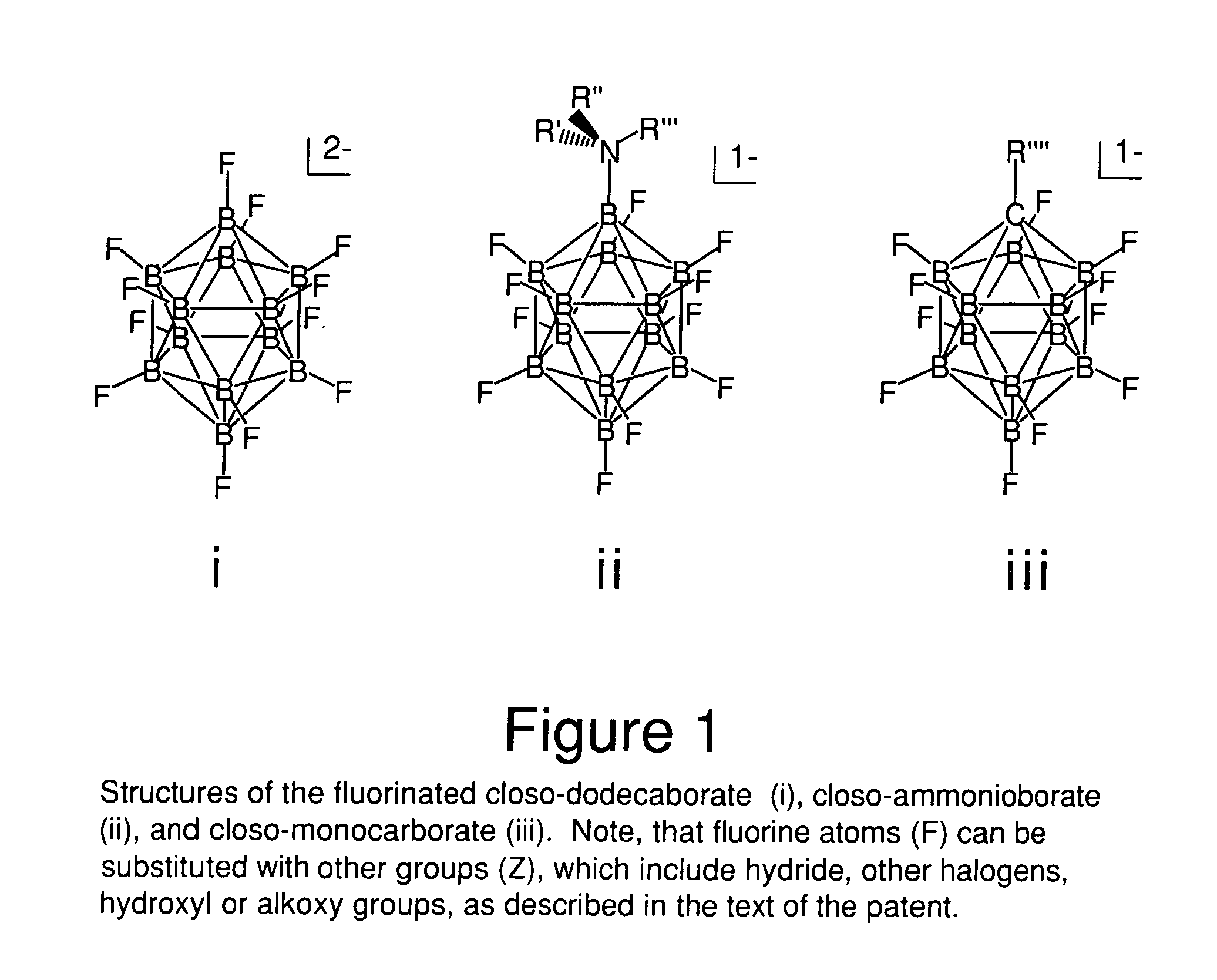
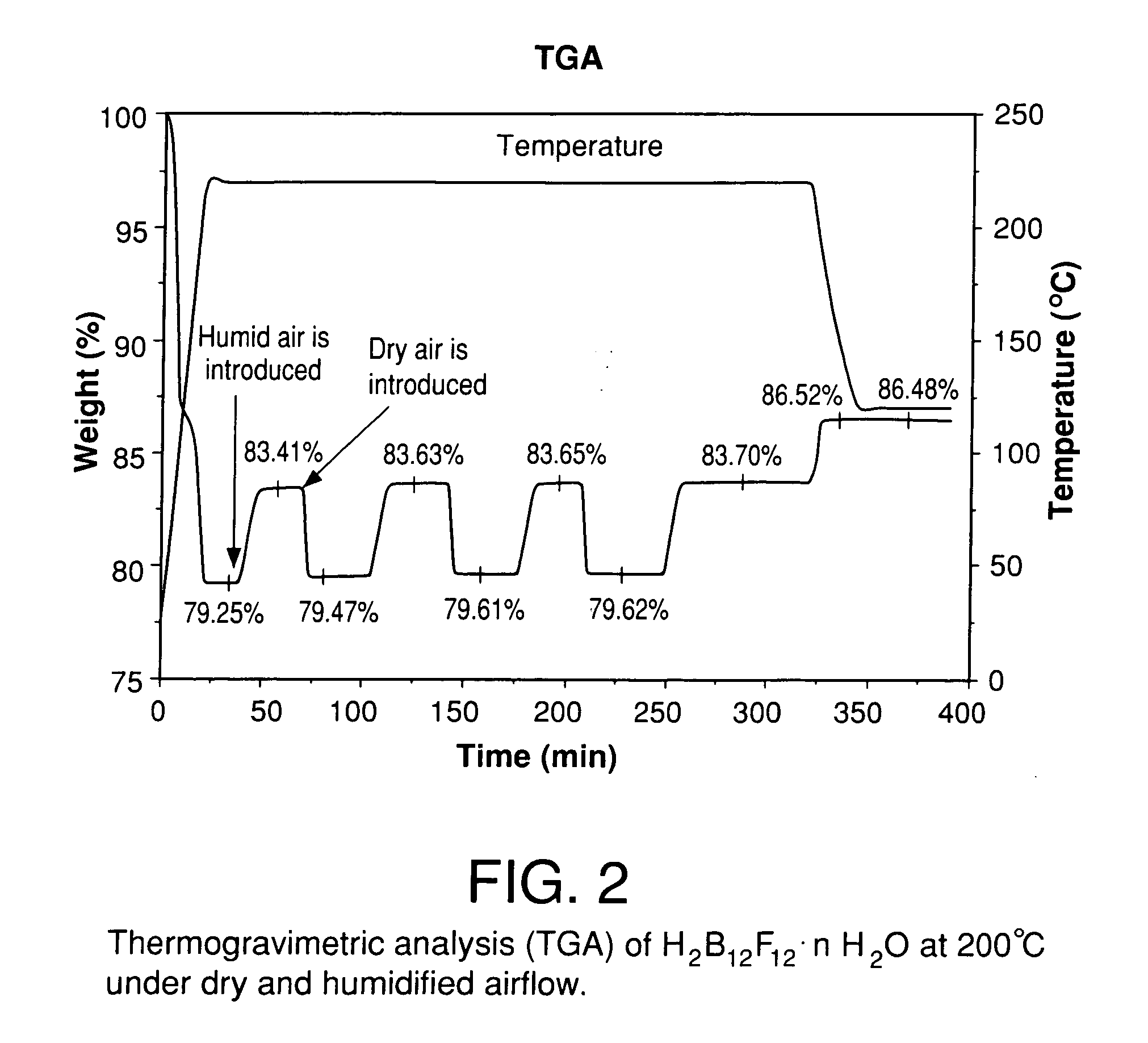
The invention relates to electrolyte salts for electrochemical devices of improved physical, chemical and electrochemical stability. The improvement resides in the use of anions of salts of the formula comprising: i) (B12FxZ12-x)2− wherein Z comprises at least one of H, Cl, Br or OR; R comprises at least one of H, alkyl or fluoroalkyl, or at least one polymer and x is at least 3 on an average basis but not more than 12; ii) ((R′R″R′″)NB12FxZ(11-x))−, wherein N is bonded to B and each of R′, R″, R′″ comprise a member independently selected from the group consisting of hydrogen, alkyl, cycloalkyl, aryl and a polymer; Z comprises H, Cl, Br, or OR, where R comprises H, alkyl or perfluoroalkyl or a polymer, and x is an integer from 0 to 11; or iii) (R″″CB11FxZ(11-x))−, wherein R″″ is bonded to C and comprises a member selected from the group consisting of hydrogen, alkyl, cycloalkyl, aryl, and a polymer, Z comprises H, Cl, Br, or OR, wherein R comprises H, alkyl or perfluoroalkyl or a polymer, and x is an integer from 0 to 11.
Owner:AIR PROD & CHEM INC
Carbon materials comprising an electrochemical modifier
InactiveUS20110159375A1Prolong lifeImprove stabilitySilver accumulatorsConductive materialElectrical devicesMaterials science
The present application is directed to carbon materials comprising an electrochemical modifier. The carbon materials find utility in any number of electrical devices, for example, in lead acid batteries. Methods for making the disclosed carbon materials are also disclosed.
Owner:BASF AG
Popular searches
Electrode thermal treatment Secondary cells Non-conductive material with dispersed conductive material Non-aqueous electrolyte accumulator electrodes Carbon-silicon compound conductors Single layer graphene Synthetic resin layered products Fibre chemical features Chemical vapor deposition coating Carbonsing rags
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com