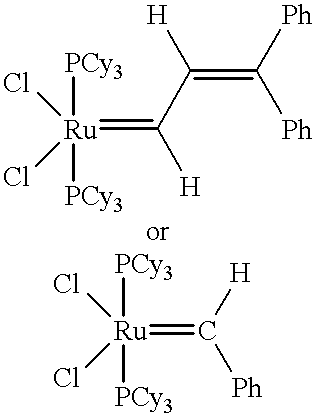
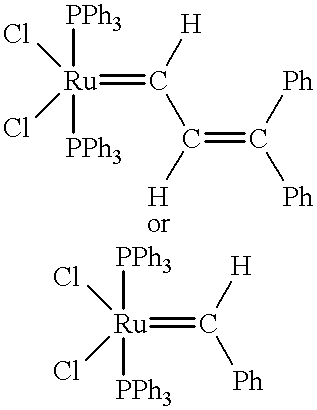
Patents
Literature
3524 results about "Polymeric matrix" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
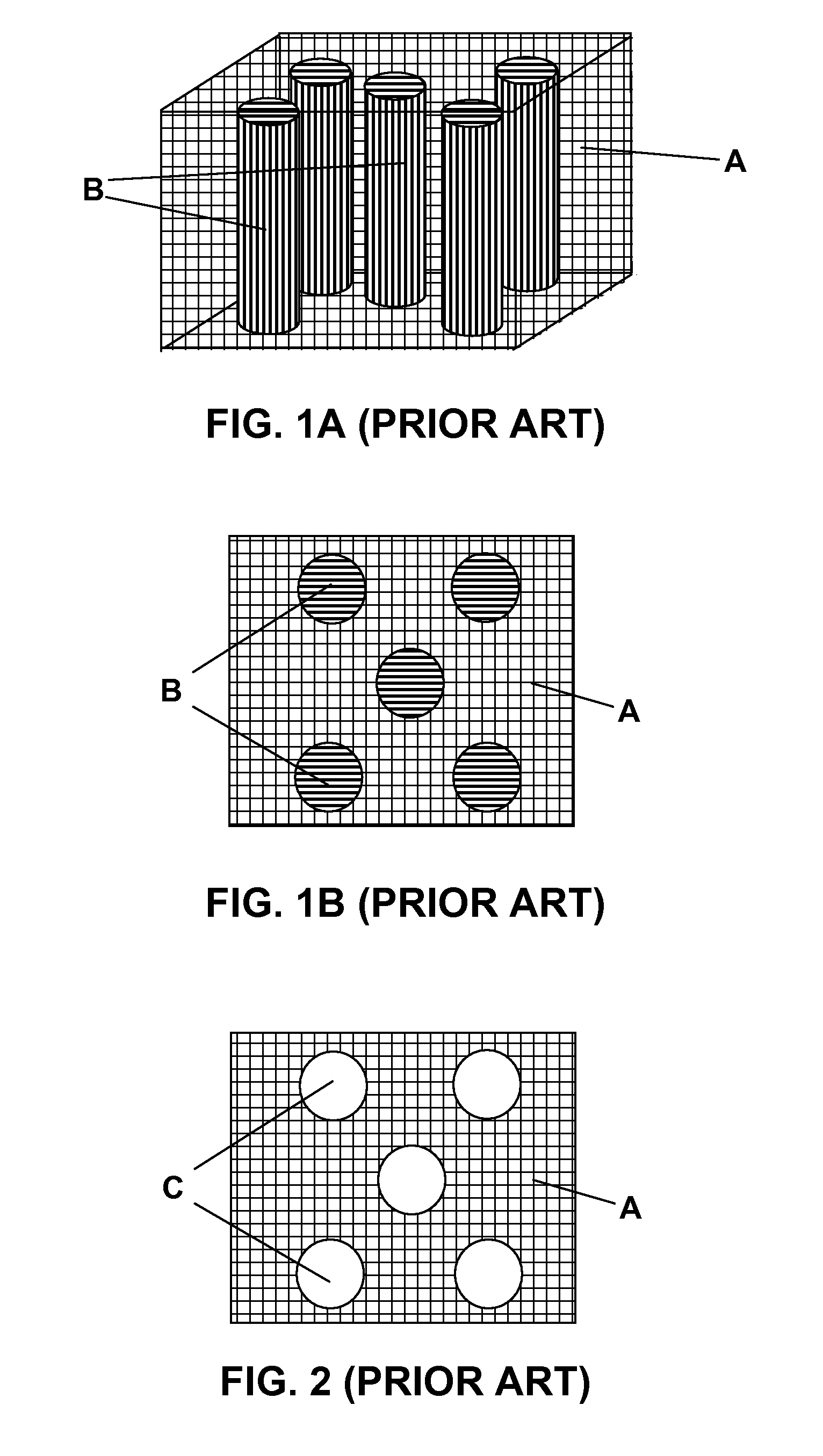
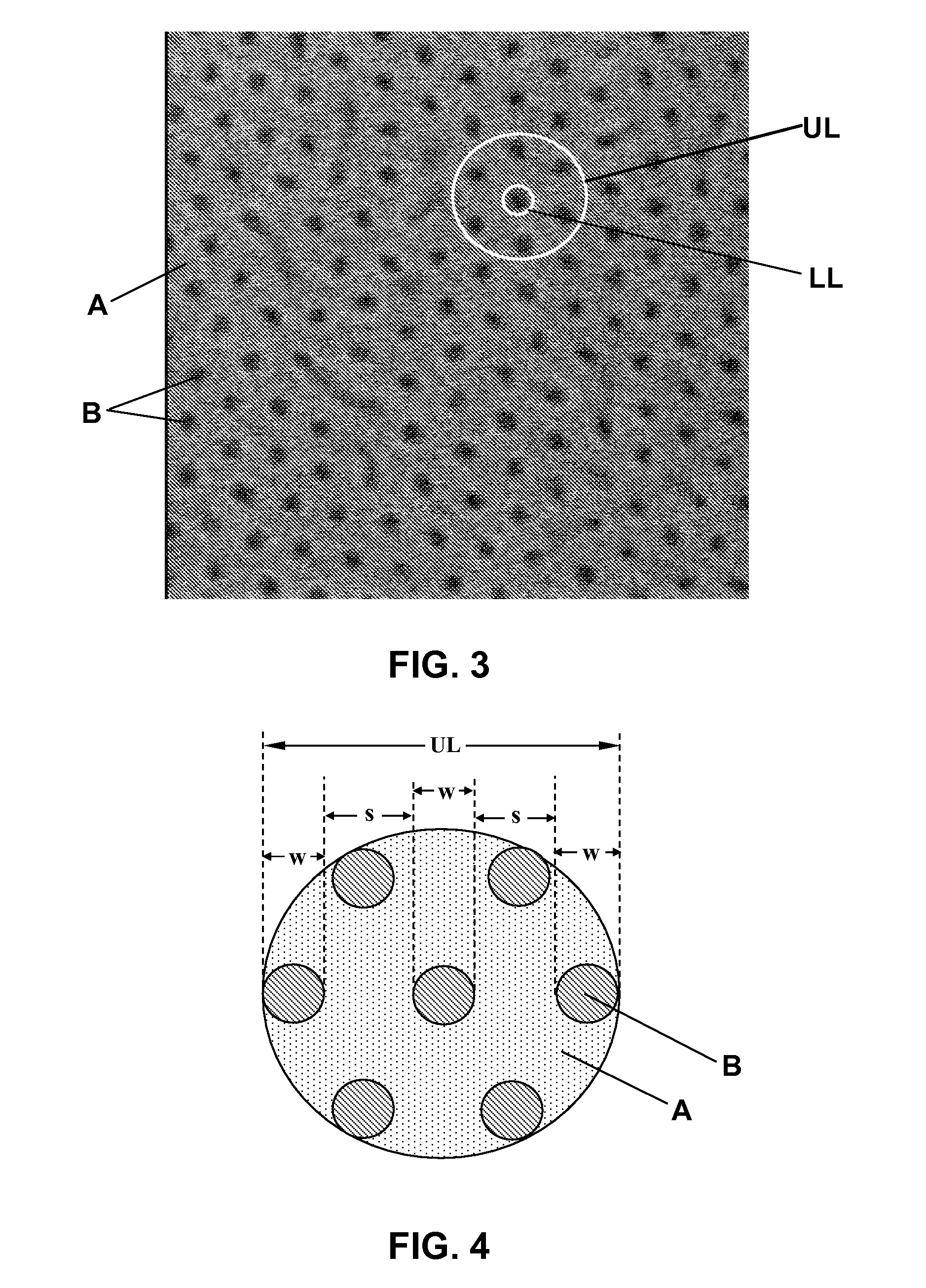
The term polymer-matrix composite is applied to a number of plastic-based materials in which several phases are present. It is often used to describe systems in which a continuous phase (the matrix) is polymeric and another phase (the reinforcement) has at least one long dimension.
Composite material for implantable device
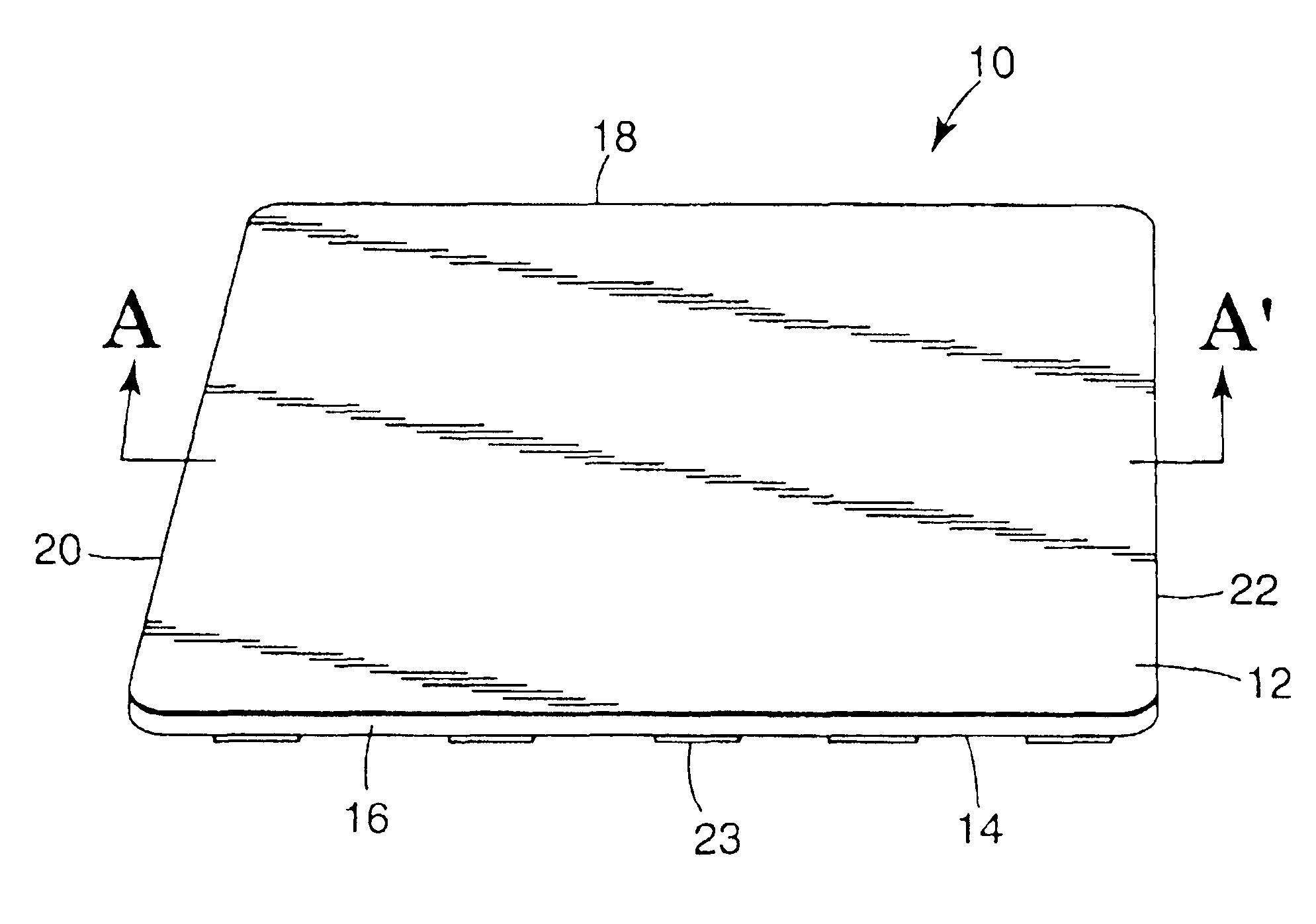
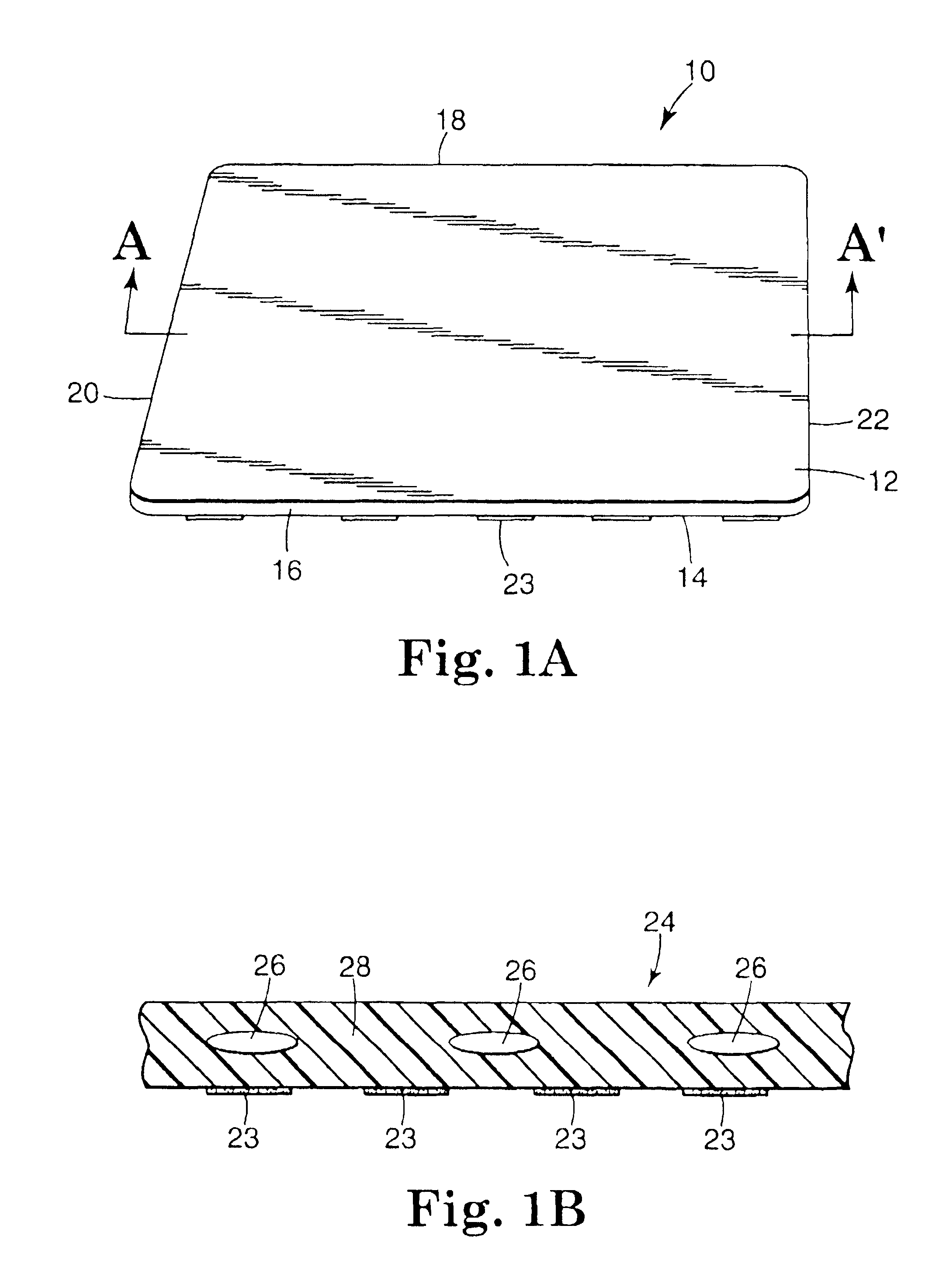
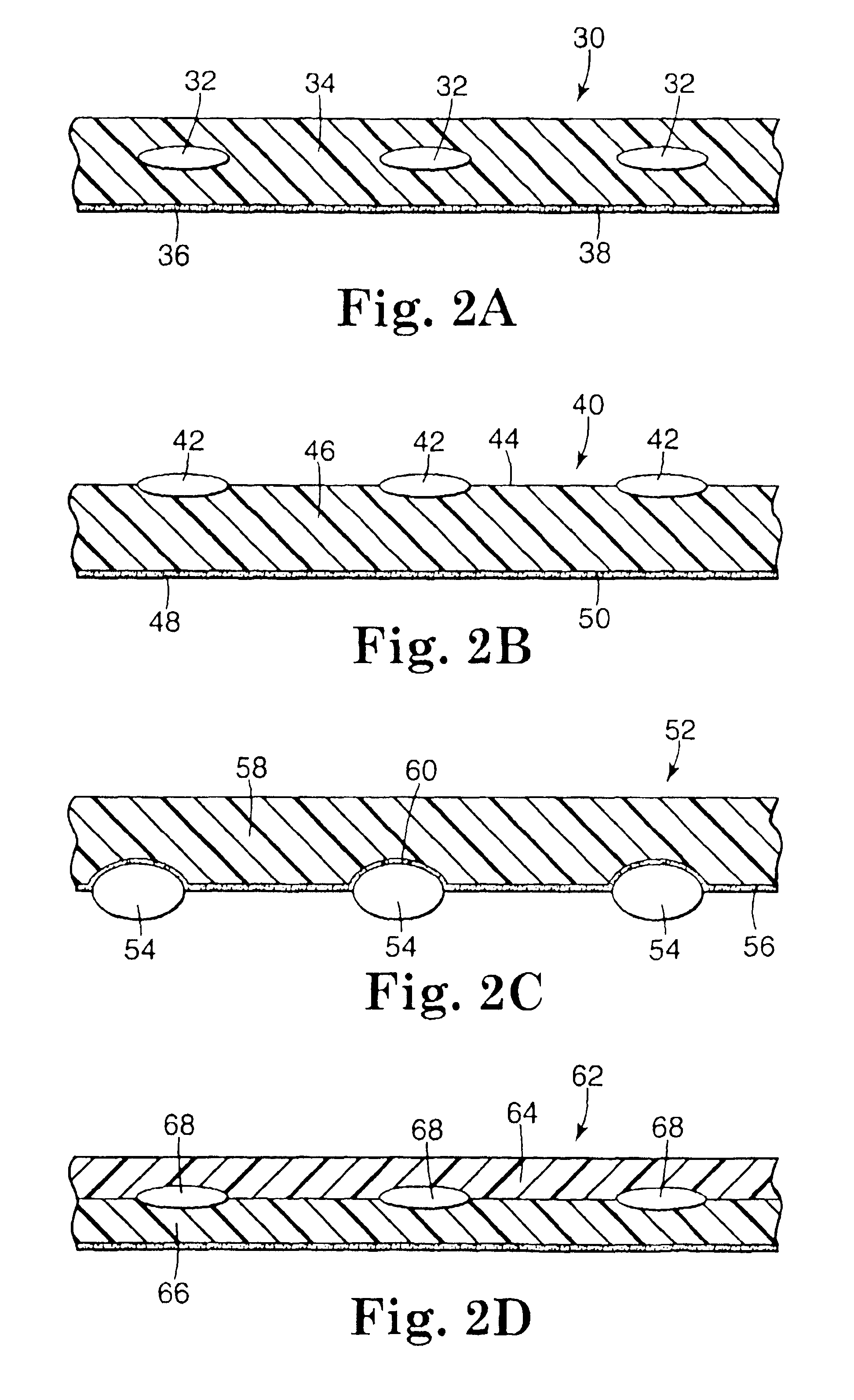
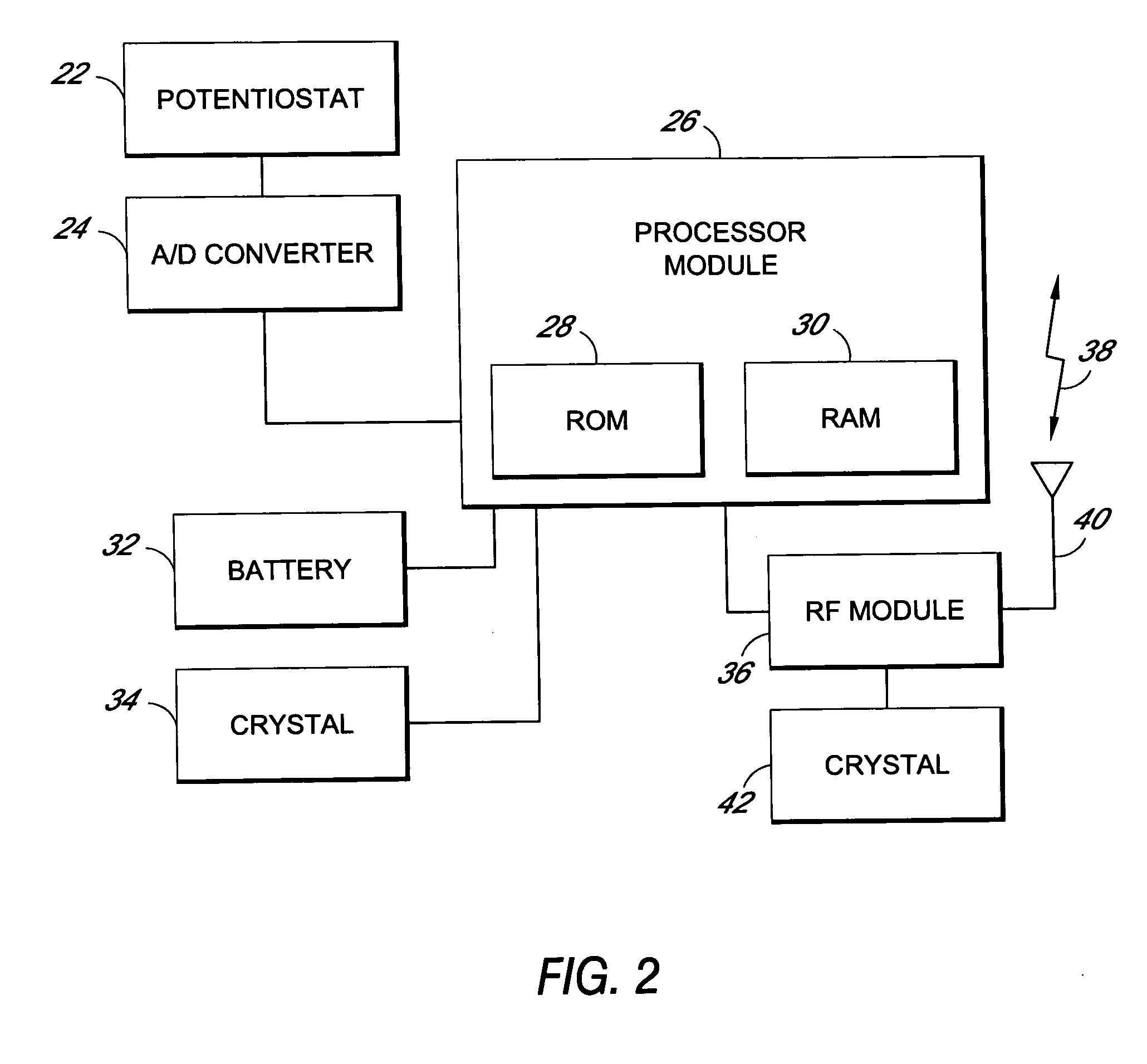
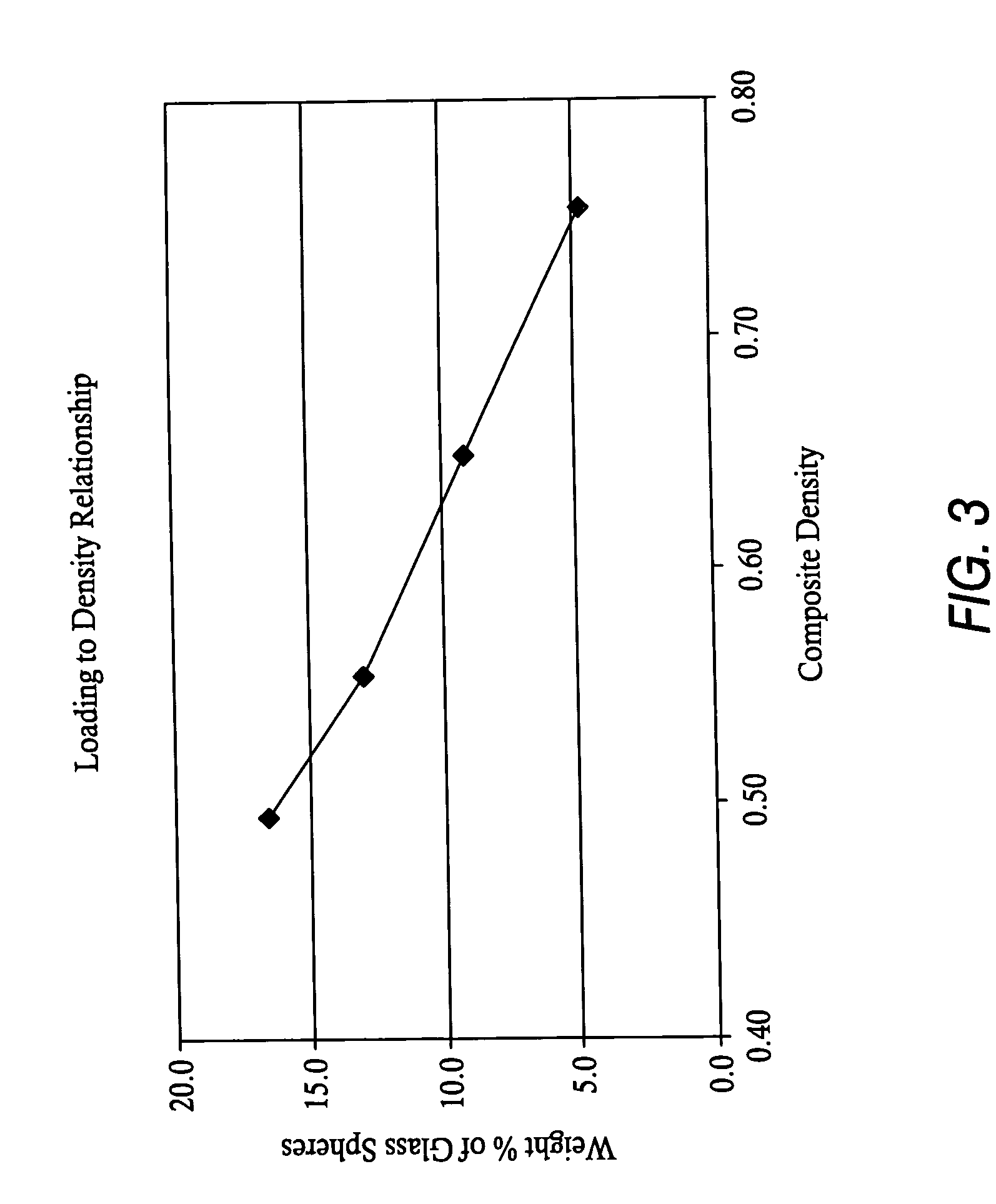
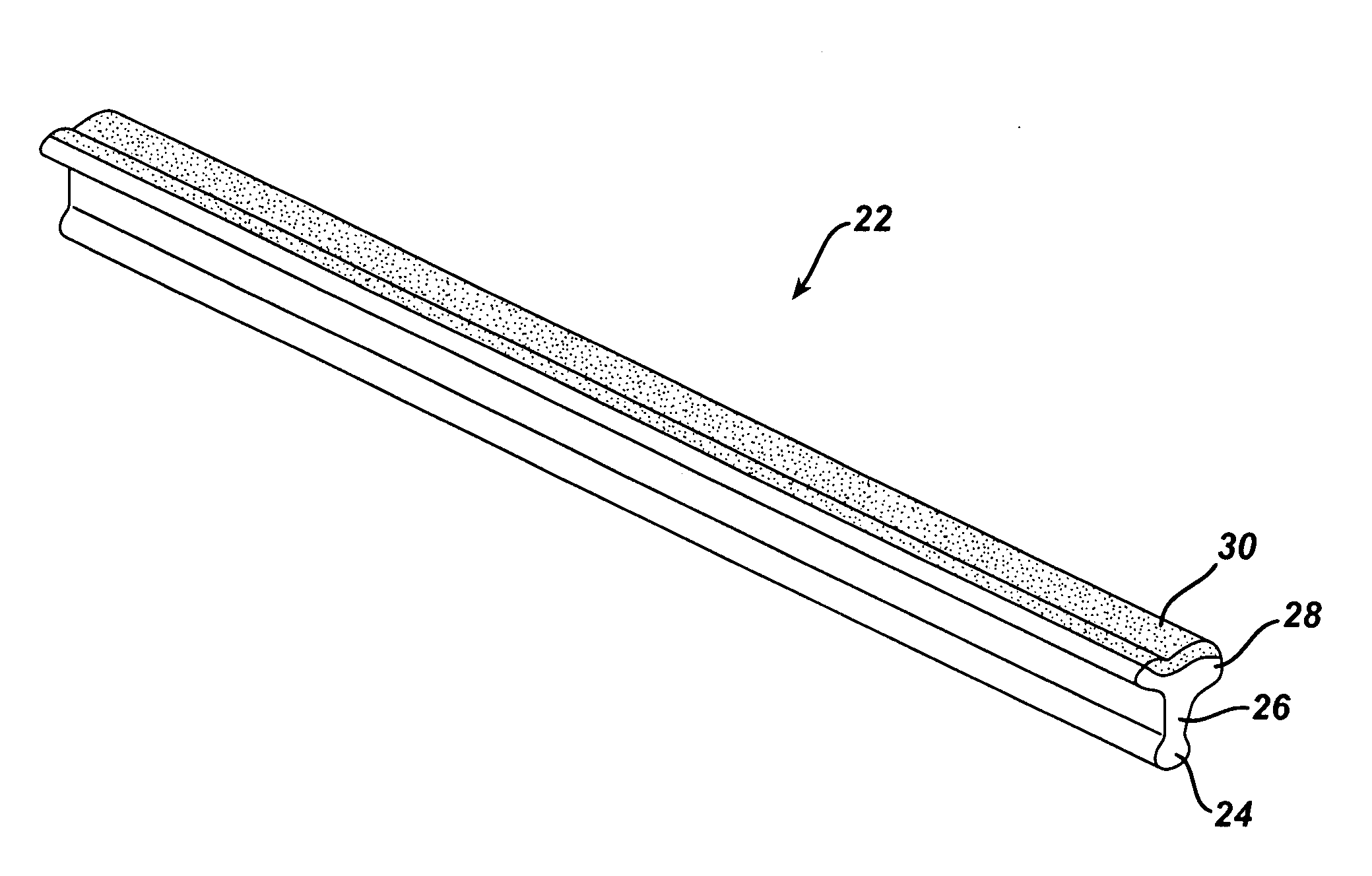
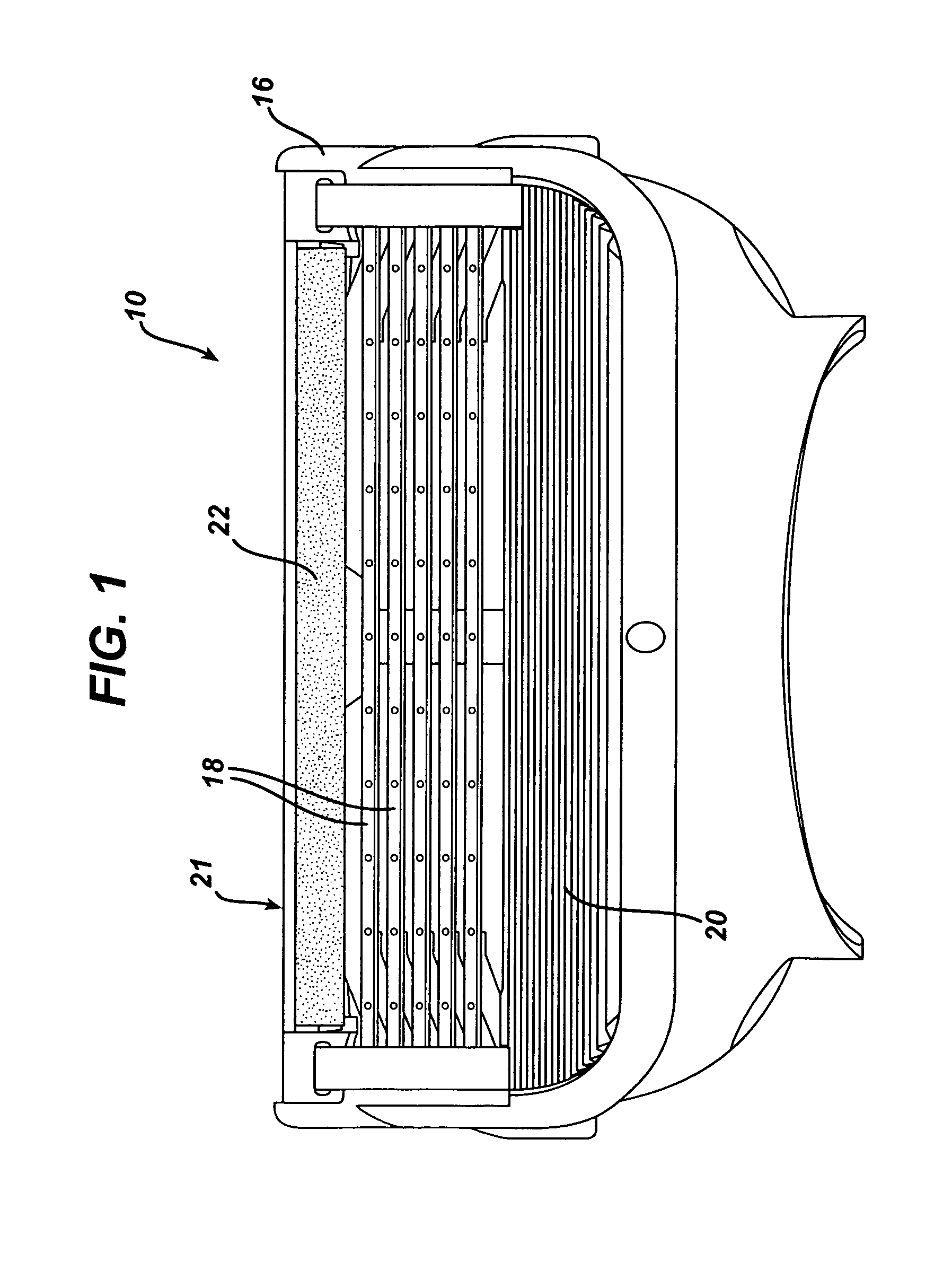
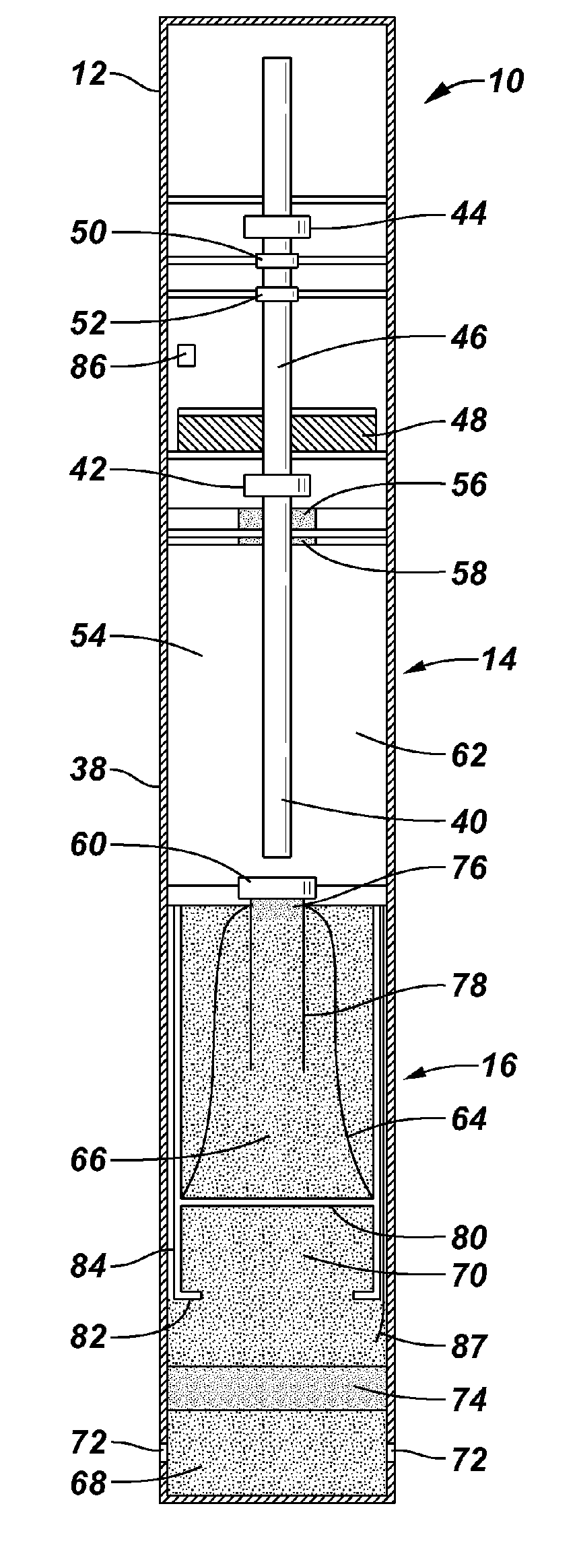
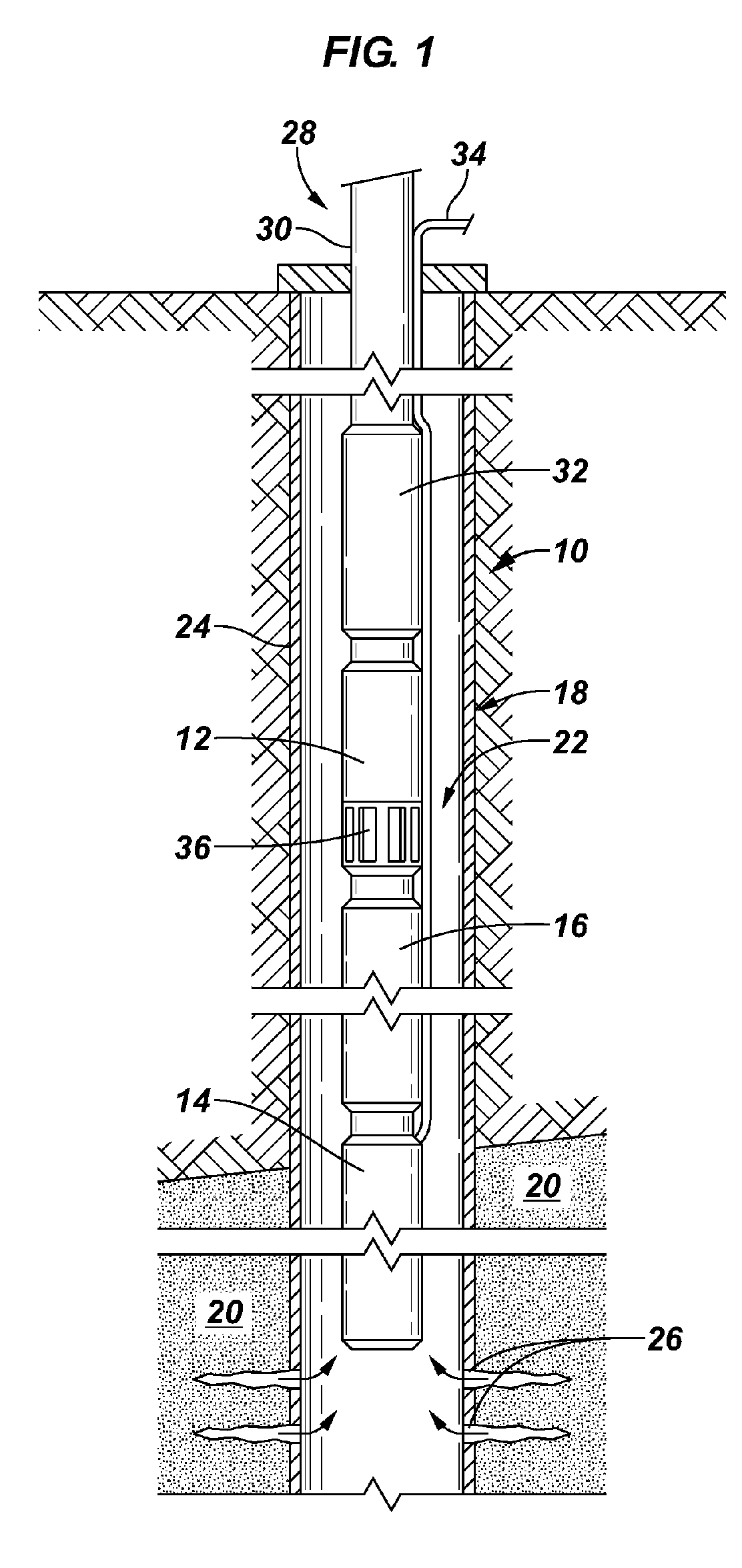
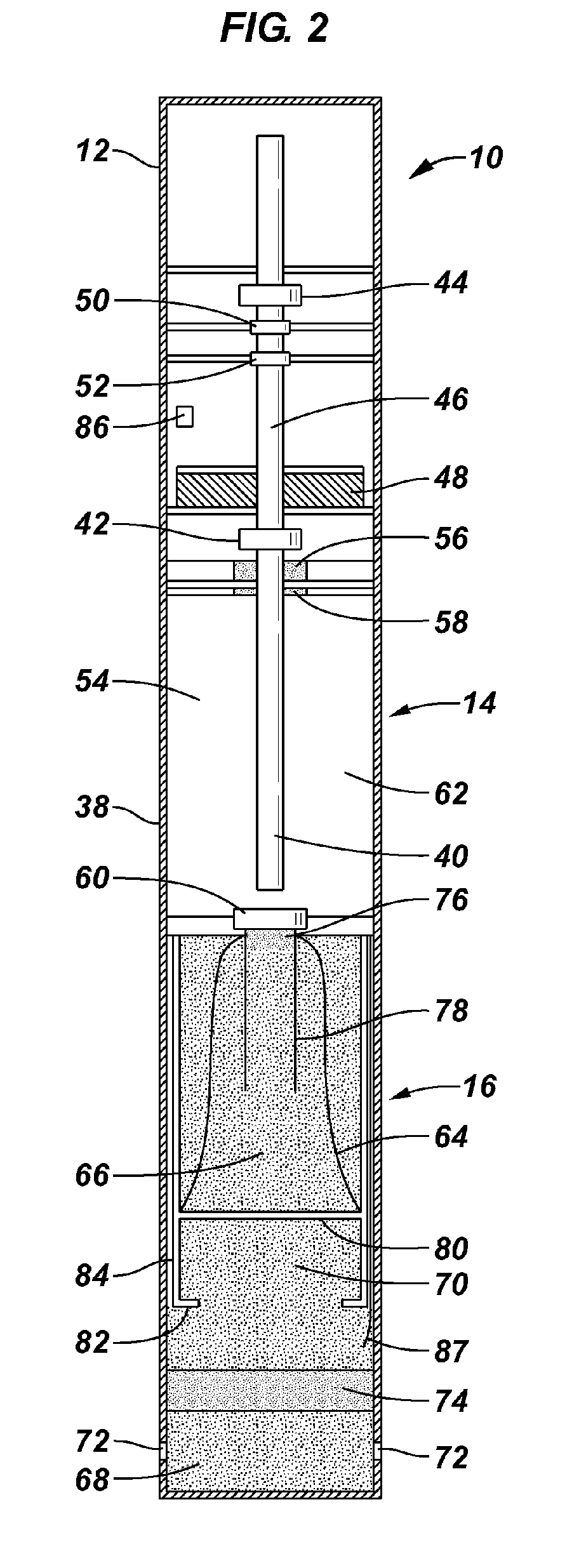
Devices suitable for implantation in a body of a host and systems and methods for their manufacture are provided. The implantable devices include a composite material formed at least from a matrix material and hollow gas-filled beads. In preferred embodiments, the composite material includes a polymeric matrix mixed with hollow air-filled glass beads, which are mixed and cured to form at least a portion of the body of the implantable device. Implantable devices including this composite material have decreased weight and / or overall density as compared to implantable devices without the beads incorporated therein, which is believed to improve the acceptance and function of the implantable device in vivo. Additionally, implantable devices concerned with transmitting and receiving via RF are believed to achieve improved RF performance due to a reduced dielectric constant provided by the incorporation of beads within the composite material.
Owner:DEXCOM INC
Coating composition for multiple hydrophilic applications
InactiveUS20030203991A1Tough and durable and printable surfaceImprove wettabilityOther chemical processesSynthetic resin layered productsColloidWear resistance
A coating composition is disclosed which comprises an aqueous polymeric matrix, a hydrophilic polymer, a colloidal metal oxide and a crosslinker. The coating composition when applied on medical devices is hydrophilic, shows improved lubricity, abrasion resistance and substrate adhesion on metallic or plastic substrates. The coating also shows improved water sheeting thus providing the coated substrates with anti-fog properties. The coating absorbs aqueous dye or stain solutions making the substrate suitable for printing.
Owner:HYDROMER INC
Therapeutic treatment and prevention of infections with a bioactive materials encapsulated within a biodegradable-biocompatible polymeric matrix
InactiveUS6309669B1Sustained release of active agent over timeEfficient and effective usePowder deliveryPeptide/protein ingredientsAdjuvantEnd-group
Novel burst-free, sustained release biocompatible and biodegrable microcapsules which can be programmed to release their active core for variable durations ranging from 1-100 days in an aqueous physiological environment. The microcapsules are comprised of a core of polypeptide or other biologically active agent encapsulated in a matrix of poly(lactide / glycolide) copolymer, which may contain a pharmaceutically-acceptable adjuvant, as a blend of upcapped free carboxyl end group and end-capped forms ranging in ratios from 100 / 0 to 1 / 99.
Owner:ARMY GOVERNMENT OF THE UNITED STATES AS REPRESENTED BY THE SEC OF THE
Protein stabilized pharmacologically active agents, methods for the preparation thereof and methods for the use thereof
InactiveUS6749868B1Low toxicityLong half-lifePowder deliveryEchographic/ultrasound-imaging preparationsSuspended particlesFree protein
In accordance with the present invention, there are provided compositions and methods useful for the in vivo delivery of substantially water insoluble pharmacologically active agents (such as the anticancer drug paclitaxel) in which the pharmacologically active agent is delivered in the form of suspended particles coated with protein (which acts as a stabilizing agent). In particular, protein and pharmacologically active agent in a biocompatible dispersing medium are subjected to high shear, in the absence of any conventional surfactants, and also in the absence of any polymeric core material for the particles. The procedure yields particles with a diameter of less than about 1 micron. The use of specific composition and preparation conditions (e.g., addition of a polar solvent to the organic phase), and careful election of the proper organic phase and phase fraction, enables the reproducible production of unusually small nanoparticles of less than 200 nm diameter, which can be sterile-filtered. The particulate system produced according to the invention can be converted into a redispersible dry powder comprising nanoparticles of water-insoluble drug coated with a protein, and free protein to which molecules of the pharmacological agent are bound. This results in a unique delivery system, in which part of the pharmacologically active agent is readily bioavailable (in the form of molecules bound to the protein), and part of the agent is present within particles without any polymeric matrix therein.
Owner:ABRAXIS BIOSCI LLC
Porous polymeric matrices made of natural polymers and synthetic polymers and optionally at least one cation and methods of making
A porous polymeric matrix containing at least one natural polymer and at least one synthetic polymer and optionally at least one cation. Furthermore, a method of making a porous polymeric matrix involving mixing at least one natural polymer and inorganic salts with a solution comprising at least one solvent and at least one synthetic polymer to form a slurry, casting the slurry in a mold and removing the solvent to form solid matrices, immersing the solid matrices in deionized water to allow natural polymer cross-linking and pore creation to occur simultaneously, and drying the matrices to create a porous polymeric matrix; wherein the matrix contains a cation. Also, a method of making a porous polymeric matrix, involving mixing at least one natural polymer in an aqueous solvent and mixing at least one synthetic polymer in an organic solvent, combining the mixtures and casting in a mold, and separately removing said aqueous solvent and said organic solvent to form a porous polymeric matrix; wherein the porous polymeric matrix does not contain a cation.
Owner:US SEC AGRI
Coating composition for multiple hydrophilic applications
InactiveUS7008979B2Improve adhesionImprove the lubrication effectSurgerySynthetic resin layered productsColloidWear resistance
Owner:HYDROMER INC
Adhesive composite having distinct phases
InactiveUS6927315B1Increase stiffnessHigh tensile strengthPlastersAdhesive dressingsMoisture vapor transmission rateEngineering
A conformable adhesive article for use as a sterile medical dressing is described. The article includes a breathable polymeric matrix, a plurality of phases, and an adhesive composition positioned on the polymeric matrix. The plurality of phases preferably provide reinforcement and stiffness to the article. The article permits transport of moisture across the breathable polymeric matrix, preferably at an Inverted water moisture vapor transmission rate of at least 300 g / m2 / 24 hours.
Owner:3M INNOVATIVE PROPERTIES CO
Composite material for implantable device
Devices suitable for implantation in a body of a host and systems and methods for their manufacture are provided. The implantable devices include a composite material formed at least from a matrix material and hollow gas-filled beads. In preferred embodiments, the composite material includes a polymeric matrix mixed with hollow air-filled glass beads, which are mixed and cured to form at least a portion of the body of the implantable device. Implantable devices including this composite material have decreased weight and / or overall density as compared to implantable devices without the beads incorporated therein, which is believed to improve the acceptance and function of the implantable device in vivo. Additionally, implantable devices concerned with transmitting and receiving via RF are believed to achieve improved RF performance due to a reduced dielectric constant provided by the incorporation of beads within the composite material.
Owner:DEXCOM
Preparation method of polymer/graphene composite material through in situ reduction
ActiveCN101864098AEvenly dispersedQuality improvementSpecial tyresNon-conductive material with dispersed conductive materialElectrical conductorVulcanization
The invention relates to a preparation method of a polymer / graphene composite material through in situ reduction, which is characterized by comprising the following steps: adopting ultrasonic wave or grinding to evenly disperse the graphite oxide prepared by a Hummers method into polymer dispersion; introducing reducing agent into the polymer dispersion for in situ reduction, enabling the graphite oxide to be reduced into the grapheme so as to obtain stable polymer / graphene composite emulsion; carrying out demulsification, agglomeration and drying to obtain the composite polymer / grapheme composite master batch; adding the dried polymer / grapheme composite master batch and various assistants into the polymeric matrix according to a certain ratio; and carrying out double-roller mixing, vulcanization, melt extrusion or injection molding to obtain the polymer / graphene composite material with excellent physical and mechanical properties.
Owner:成都创威新材料有限公司
Wet shaving system including a mineral oil coated shaving aid
InactiveUS20080060201A1Easy to glideInhibit swellingMetal working apparatusWater insolubleWater soluble
A wet shaving system is disclosed including a blade member and a skin-engaging portion in proximity to said blade member, the skin-engaging portion comprising a solid polymeric shaving aid composite including an exposed portion containing a water-soluble shaving aid dispersed in a water-insoluble polymeric matrix, the water soluble shaving aid being coated with mineral oil.
Owner:THE GILLETTE CO
Design of poly(ester amides) for the control of agent-release from polymeric compositions
The present invention generally encompasses a medical article, such as a medical device or coating comprising an agent or combination of agents, wherein the agent is distributed throughout a polymeric matrix. The polymeric matrix comprises an agent and a poly(ester amide) having a design that was preselected to provide a predetermined release rate of the combination of agents from the medical article.
Owner:ABBOTT CARDIOVASCULAR
Polymeric Composites, Oilfield Elements Comprising Same, and Methods of Using Same in Oilfield Applications
InactiveUS20070142547A1Improve barrier propertiesImprove mechanical propertiesNon-metal conductorsLayered productsSubject matterEngineering
Oilfield elements and assemblies are described comprising a polymeric matrix formed into an oilfield element, and a plurality of expanded graphitic nanoflakes and / or nanoplatelets dispersed in the polymeric matrix. Methods of using the oilfield elements and assemblies including same in oilfield operations are also described. This abstract allows a searcher or other reader to quickly ascertain the subject matter of the disclosure. It will not be used to interpret or limit the scope or meaning of the claims. 37 CFR 1.72(b).
Owner:SCHLUMBERGER TECH CORP
Coatings on ophthalmic lenses
This invention is directed toward surface treatment of a device. The surface treatment comprises the attachment of terminal functionalized surfactants to the surface of the substrate by means of reactive functionalities of the terminal functionalized surfactant material reacting with complementary surface reactive functionalities in monomeric units along the polymer substrate. The present invention is also directed to a surface modified medical device, examples of which include contact lenses, intraocular lenses, vascular stents, phakic intraocular lenses, aphakic intraocular lenses, corneal implants, catheters, implants, and the like, comprising a surface made by such a method.
Owner:BAUSCH & LOMB INC
Compositions comprising lipophilic active compounds and method for their preparation
ActiveUS20090098200A1Quick releaseImprove bioavailabilityPowder deliveryBiocideHydrophilic polymersCompound (substance)
Compositions are provided comprising a lipophilic active compound, e.g., a human or veterinary drug or a nutraceutical, interwoven with a polymeric matrix formed by two or more polymers, wherein one of the polymers is an amphiphilic polymer and the other polymer is either an amphiphilic polymer with a different hydrophobic-hydrophilic balance or a hydrophilic polymer, and the active lipophilic compound has modified physicochemical properties. The composition forms colloidal nanodispersion upon contact with aqueous media.
Owner:SOLUBEST
Multifunctional medical articles
ActiveUS20070003588A1Minimize timeMaximum flexibilityOrganic active ingredientsPowder deliveryMedicineBiocompatibility Testing
The invention provides medical articles that include more than one biocompatibility-promoting function. In one aspect, the invention provides a medical articles that include a polymeric matrix including more than one biocompatible agent, wherein each biocompatible agent is provided at a distinct portion of the medical article surface. Methods of making medical articles, as well as methods of using the same, are also described.
Owner:SURMODICS INC
Porous coatings for drug release from medical devices
InactiveUS20050079199A1Increase ratingsIncrease load capacityWound drainsSurgeryPorous coatingSurface layer
Extravascular implantable medical devices are described. The devices include a polymeric layer comprising a polymeric matrix and pores. Therapeutic agent is loaded in the matrix, in the pores, or in the matrix and the pores. The devices include a structural surface layer. Additional therapeutic agent may be loaded in or on the surface layer. The devices may also include one or more intermediate layer, into or onto which additional therapeutic agent may be loaded.
Owner:MEDTRONIC INC
Polymeric composites including dicyclopentadiene and related monomers
InactiveUS6310121B1Group 8/9/10/18 element organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsFiberMonomer composition
Compositions including fibers, fillers, reinforcing agents or other property-enhancing agents added to a polymeric matrix formed from DCPD monomer or related compounds such as norbornene and norbornadiene are disclosed. The composition has a mechanically forgiving matrix due to the polymer with the inherent strength and other physical properties of the enhancement agent according to the invention. Enhancement agents according to the invention generally include inorganic, organic and metallic substances in a variety of forms.
Owner:CYMETECH
Smart hydrogel particles for biomarker harvesting
ActiveUS20090148961A1Bioreactor/fermenter combinationsBiological substance pretreatmentsAnalyteSmart hydrogels
Capture particles for harvesting analytes from solution and methods for using them are described. The capture particles are made up of a polymeric matrix having pore size that allows for the analytes to enter the capture particles. The pore size of the capture particles may be changeable upon application of a stimulus to the particles, allowing the pore size of the particles to be changed so that analytes of interest remain sequestered inside the particles. The polymeric matrix of the capture particles may be made of co-polymeric materials having a structural monomer and an affinity monomer, the affinity monomer having properties that attract the analyte to the capture particle. The capture particles may be used to isolate and identify analytes present in a mixture. They may also be used to protect analytes which are typically subject to degradation upon harvesting and to concentrate low an analyte in low abundance in a fluid.
Owner:INST SUPERIORE DI SANITA +1
Sensing system for monitoring the structural health of composite structures
ActiveUS20050284232A1Easy to stickImprove signal-to-noise ratioUsing optical meansElectrical/magnetic solid deformation measurementGrid patternState of health
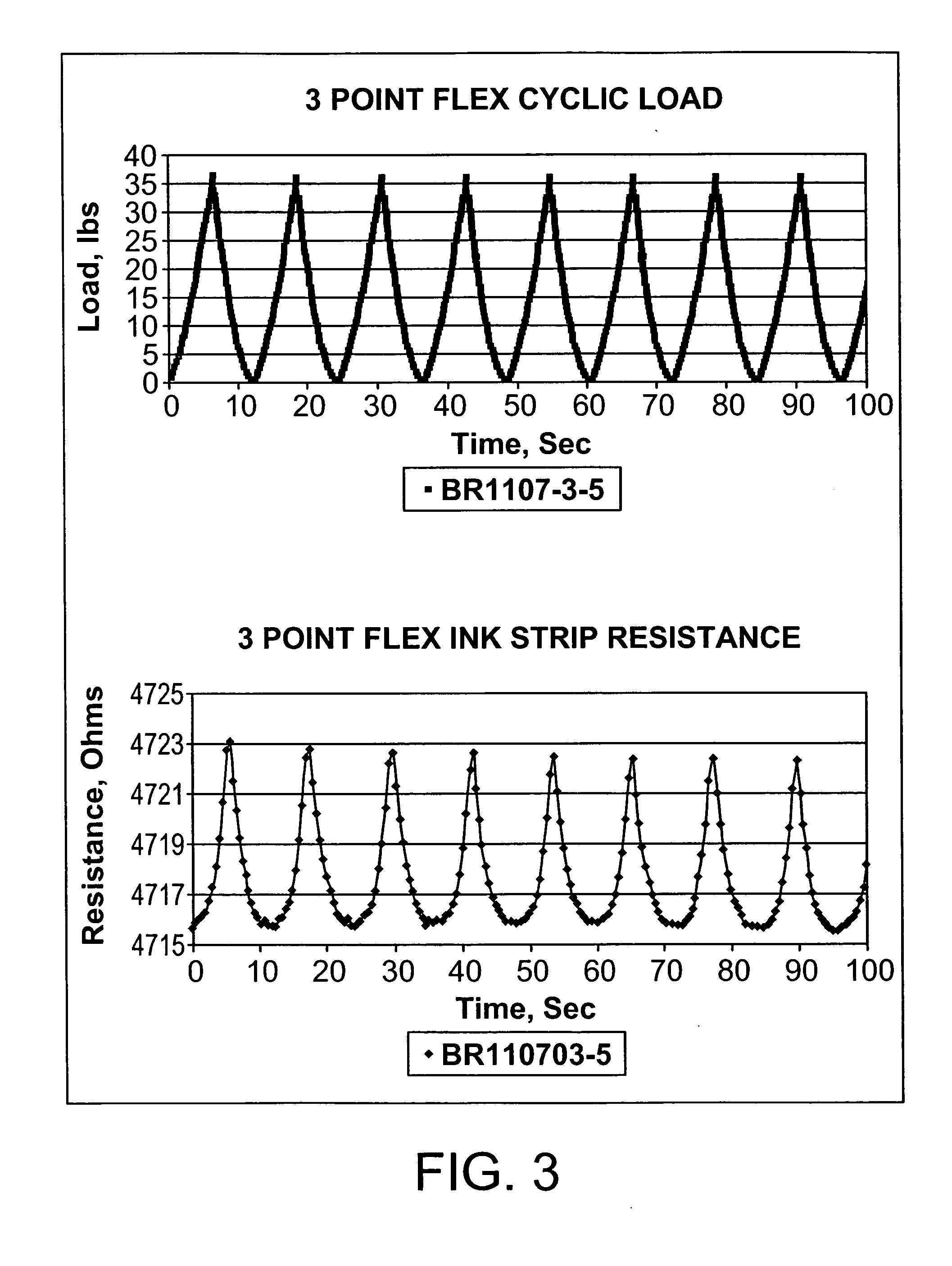
A sensing system for use in monitoring the structural health of a structure such as a polymeric matrix composite structure is provided. The system includes a sensor formed from a conductive ink containing carbon nanofibers and a polymeric resin, and a data acquisition system for acquiring and evaluating data from the sensor. The conductive ink may be applied directly to the structure to be monitored in the form of a grid pattern. Damage to the structure may be detected by measuring changes in resistance values detected from the sensor.
Owner:UNIV OF DAYTON
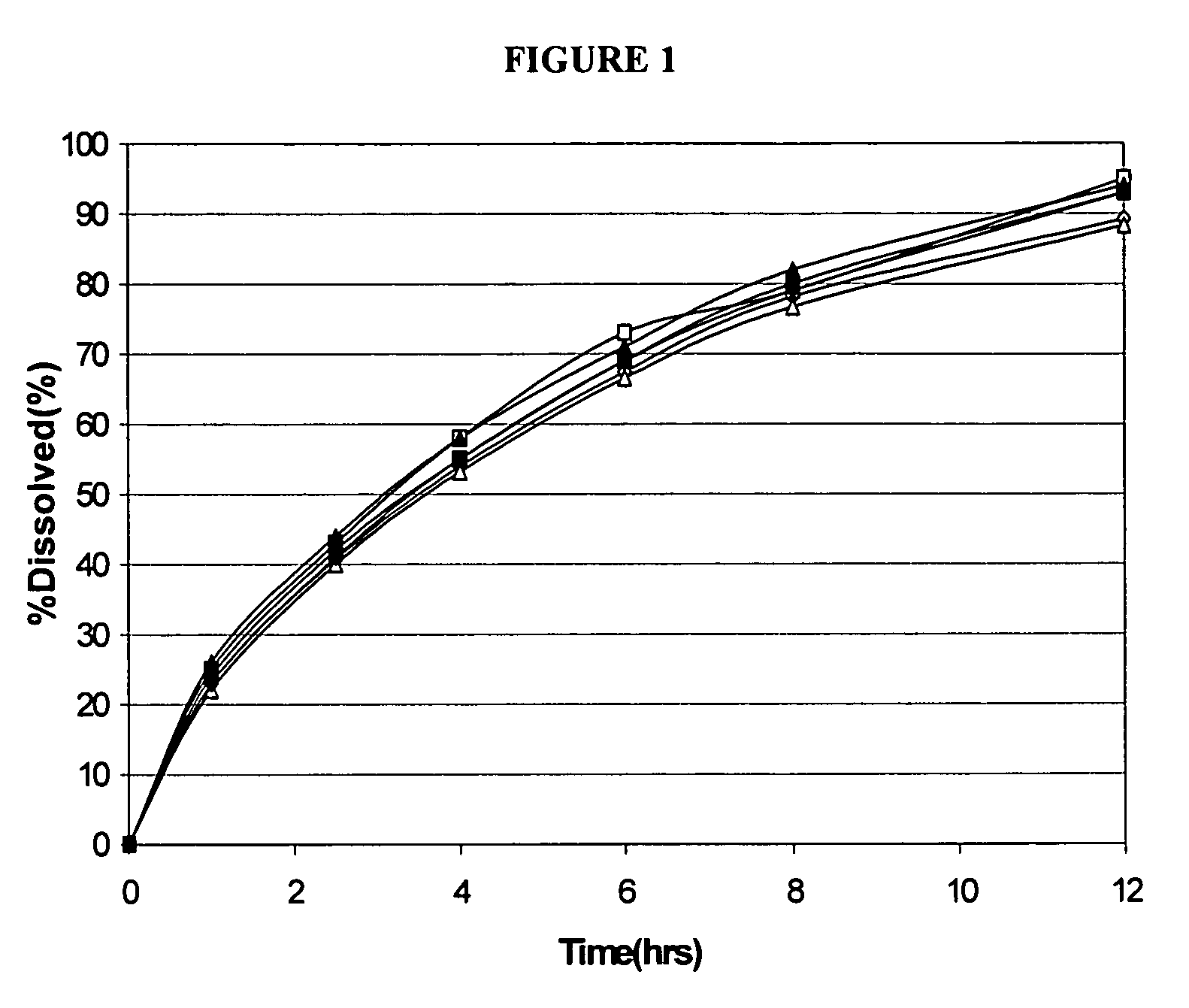
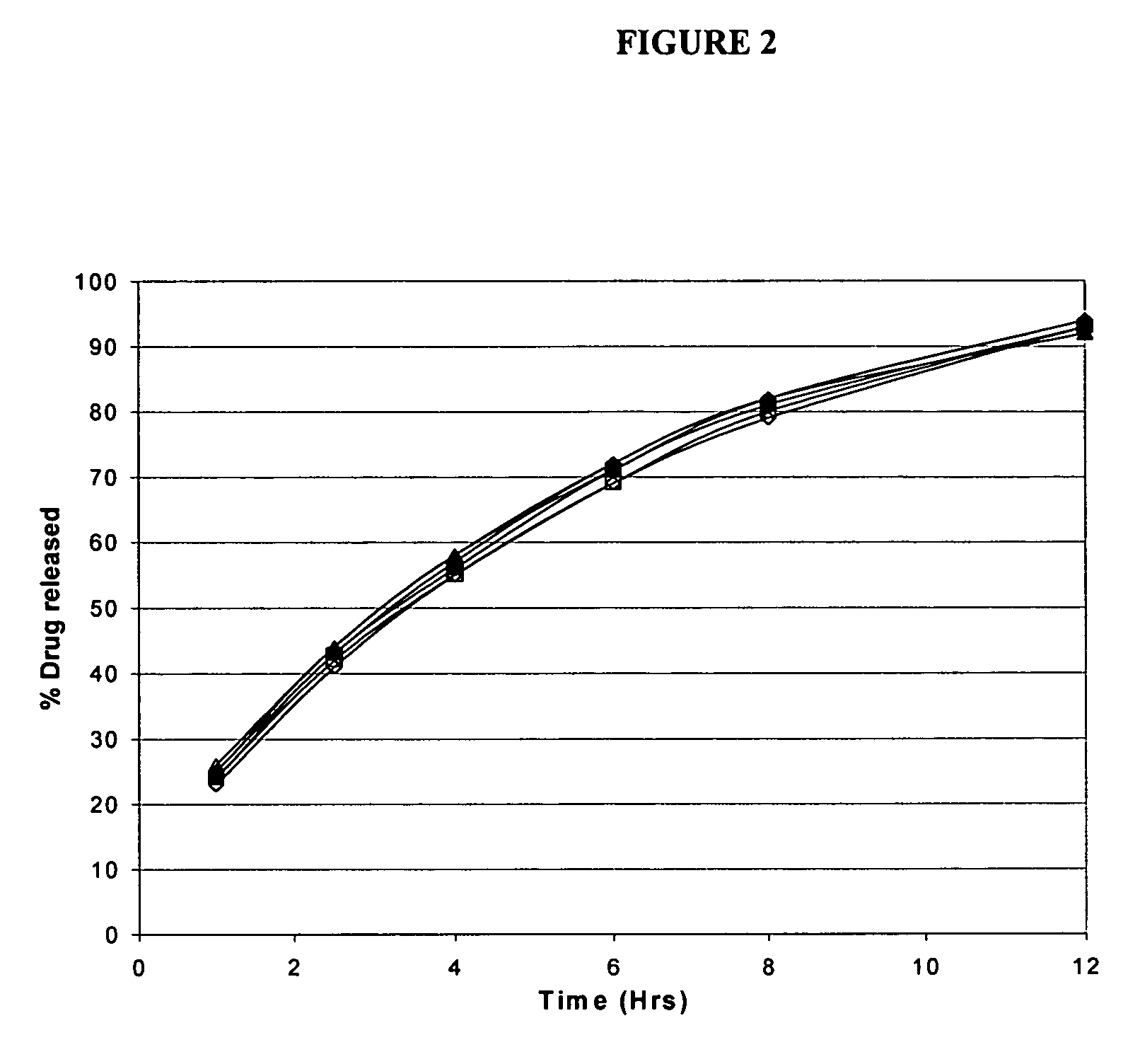
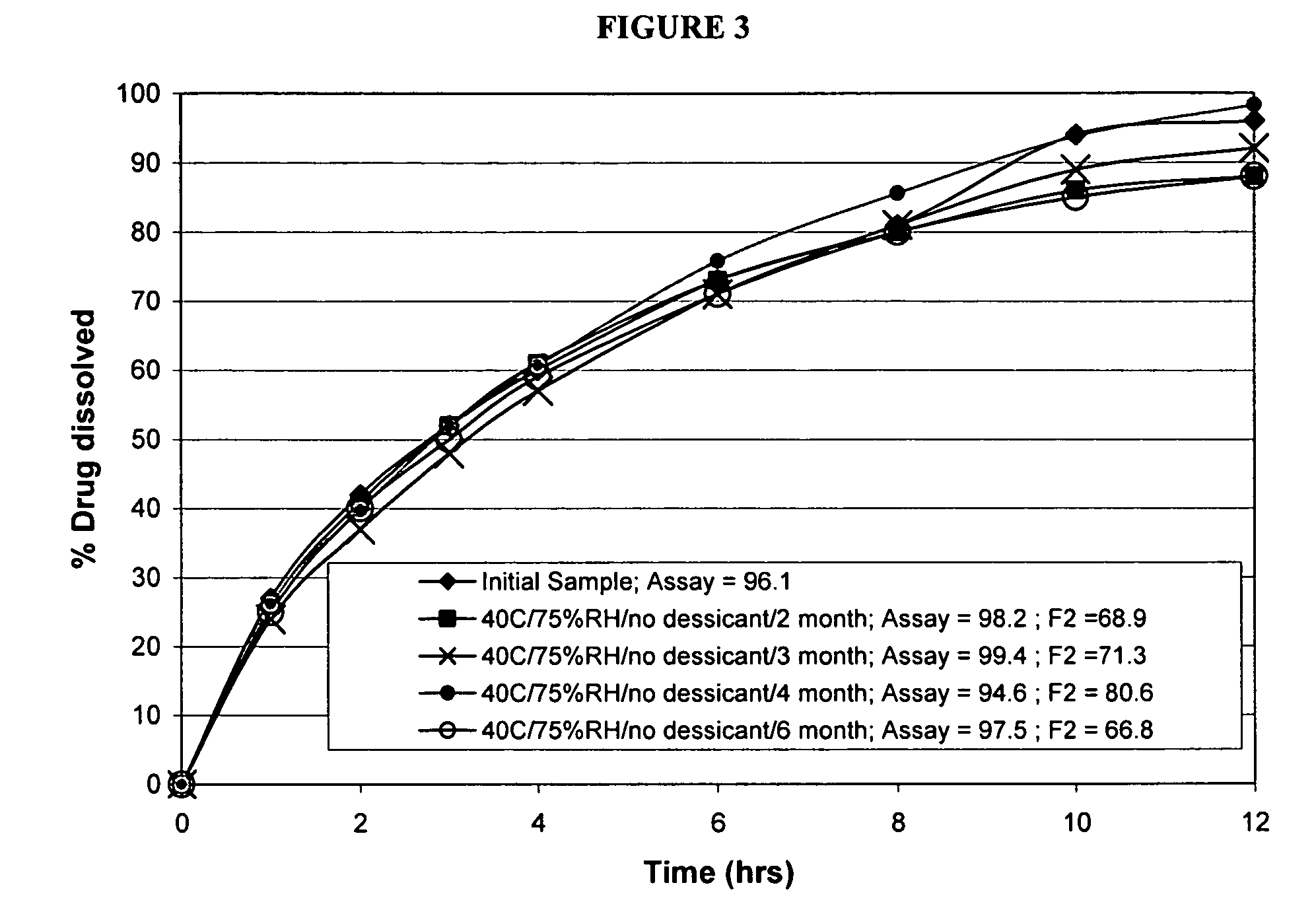
Modified release formulations of memantine oral dosage forms
The present invention provides pharmaceutical compositions given once daily containing at least one therapeutically active ingredient selected from the group consisting of memantine and a pharmaceutically acceptable salt of memantine, and a pharmaceutically acceptable polymeric matrix carrier. The dosage forms of the invention sustain the release of the therapeutically active agent from about 4 to about 24 hours when said dosage form is exposed to aqueous solutions. following entry of said form into a use environment, wherein said dosage form has a dissolution rate of more than about 80% after passage of about 6 hours to about 12 hours following said entry into said use environment.
Owner:FOREST LAB HLDG LTD
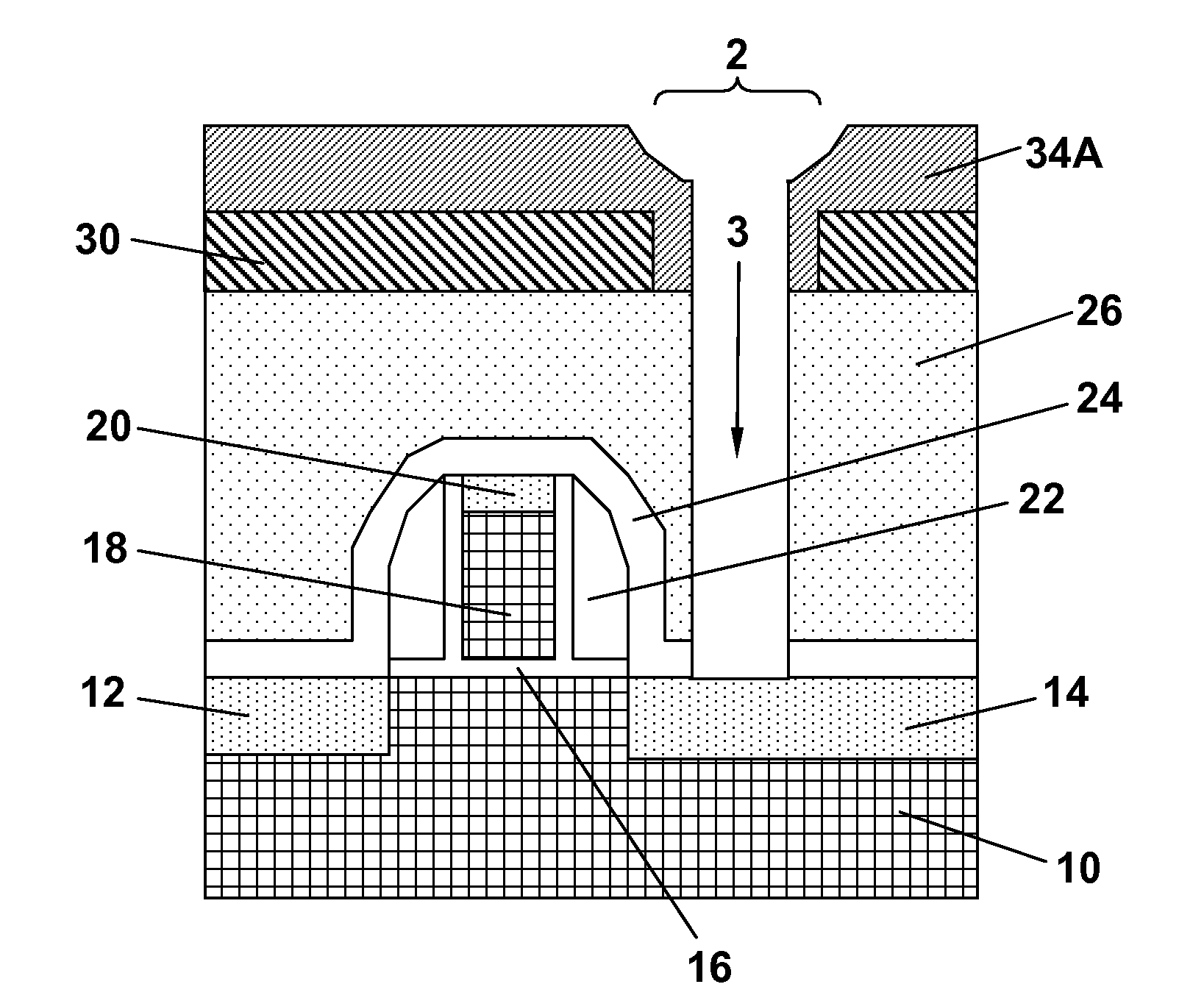
Sub-lithographic feature patterning using self-aligned self-assembly polymers
ActiveUS20070293041A1Semiconductor/solid-state device detailsPhotomechanical apparatusLithographic artistEngineering
A method for conducting sub-lithography feature patterning of a device structure is provided. First, a lithographically patterned mask layer that contains one or more mask openings of a diameter d is formed by lithography and etching over an upper surface of the device structure. Next, a layer of a self-assembling block copolymer is applied over the lithographically patterned mask layer and then annealed to form a single unit polymer block of a diameter w inside each of the mask openings, provided that w<d. Each single unit polymer block of the present invention is embedded in a polymeric matrix and can be selectively removed against the polymeric matrix to form a single opening of the diameter w in the polymeric matrix inside each of the mask openings. Sub-lithography feature patterning can then be conducted in the device structure using the single openings of diameter w.
Owner:IBM CORP
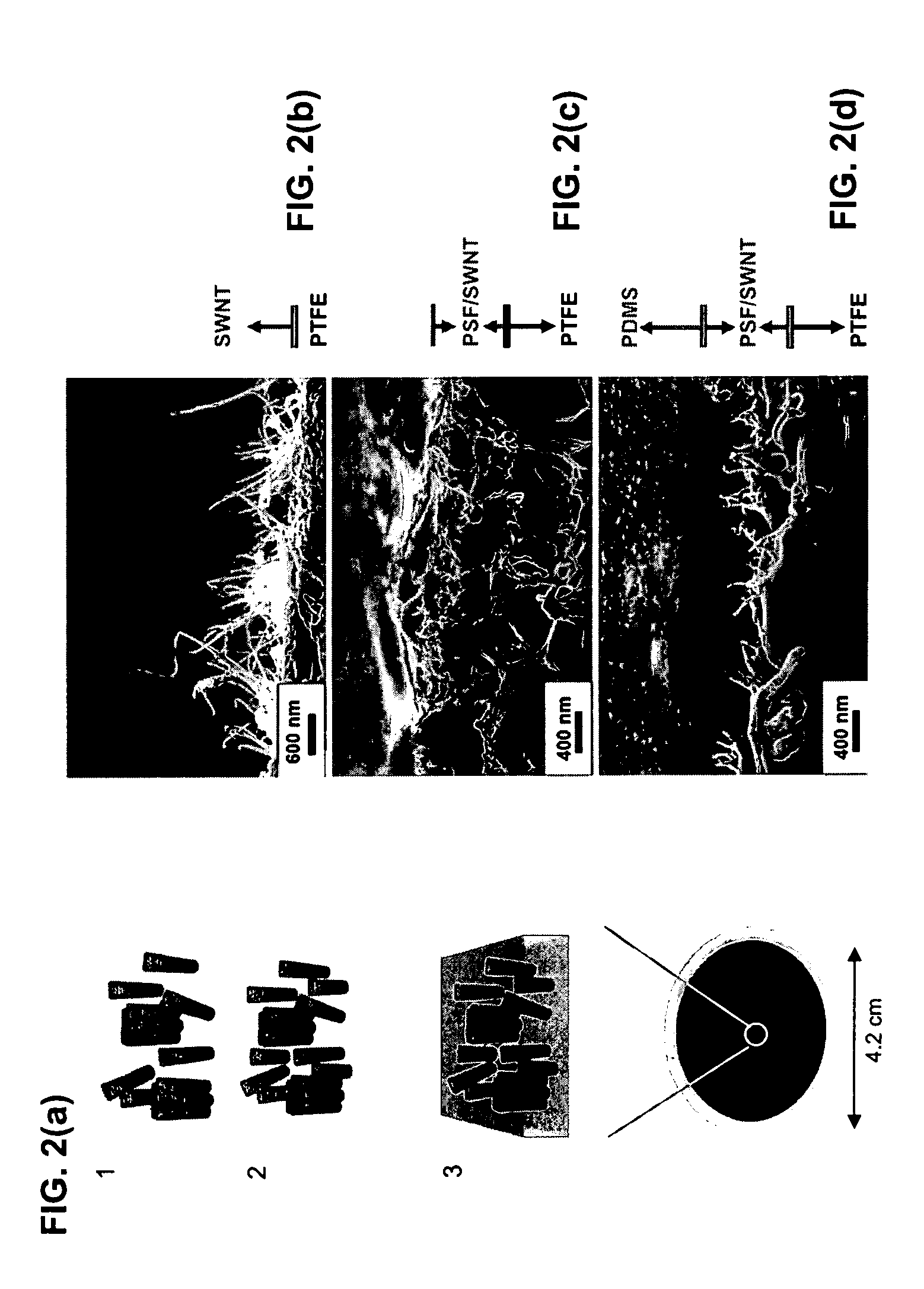
Composite materials comprising polar polymers and single-wall carbon nanotubes
InactiveUS6936653B2Improve conductivityMaterial nanotechnologyIndividual molecule manipulationPolyesterPolymer science
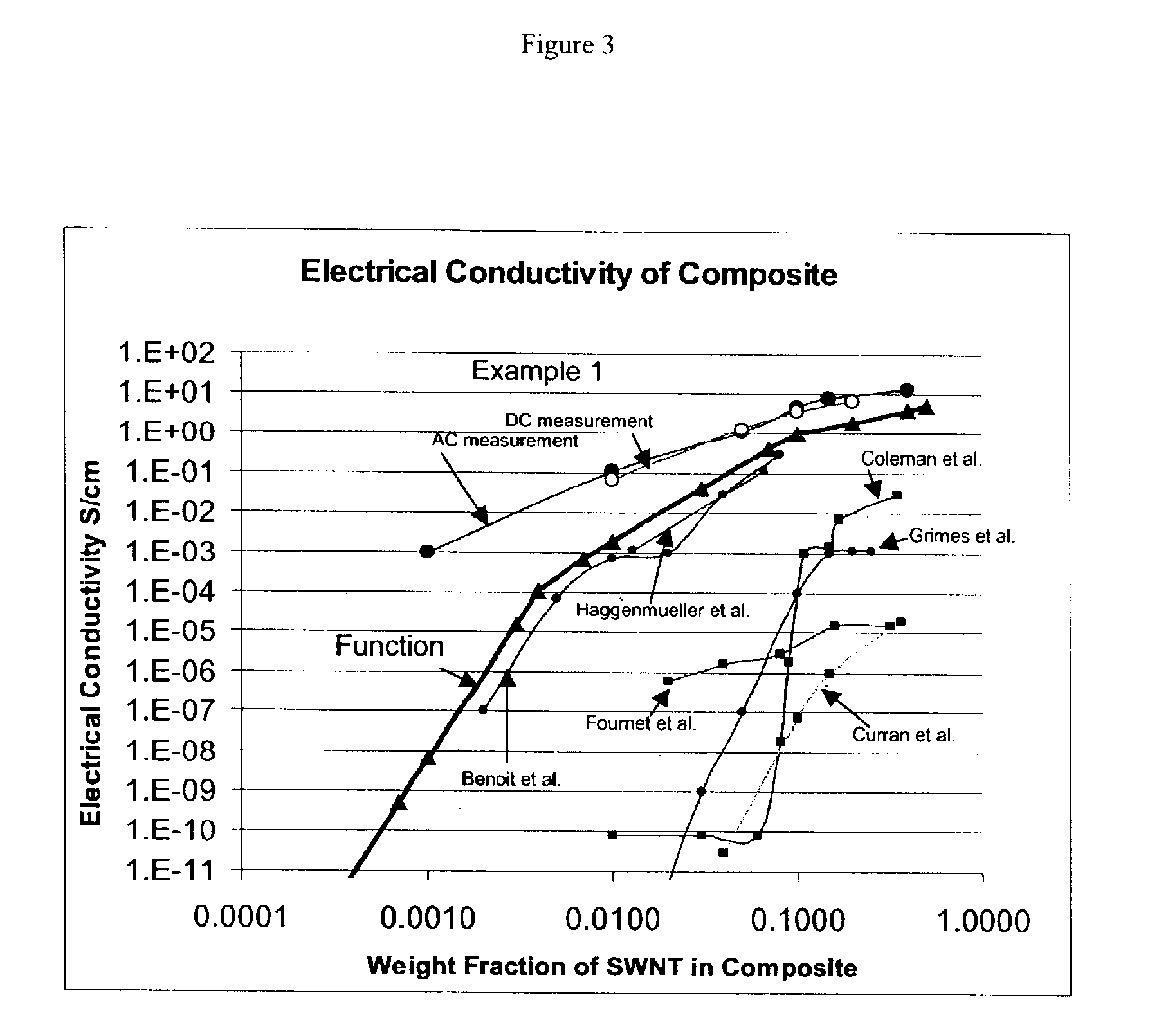
The invention relates to a composite comprising a weight fraction of single-wall carbon nanotubes and at least one polar polymer wherein the composite has an electrical and / or thermal conductivity enhanced over that of the polymer alone. The invention also comprises a method for making this polymer composition. The present application provides composite compositions that, over a wide range of single-wall carbon nanotube loading, have electrical conductivities exceeding those known in the art by more than one order of magnitude. The electrical conductivity enhancement depends on the weight fraction (F) of the single-wall carbon nanotubes in the composite. The electrical conductivity of the composite of this invention is at least 5 Siemens per centimeter (S / cm) at (F) of 0.5 (i.e. where single-wall carbon nanotube loading weight represents half of the total composite weight), at least 1 S / cm at a F of 0.1, at least 1×10−4 S / cm at (F) of 0.004, at least 6×10−9 S / cm at (F) of 0.001 and at least 3×10−16 S / cm (F) plus the intrinsic conductivity of the polymer matrix material at of 0.0001. The thermal conductivity enhancement is in excess of 1 Watt / m-° K. The polar polymer can be polycarbonate, poly(acrylic acid), poly(acrylic acid), poly(methacrylic acid), polyoxide, polysulfide, polysulfone, polyamides, polyester, polyurethane, polyimide, poly(vinyl acetate), poly(vinyl alcohol), poly(vinyl chloride), poly(vinyl pyridine), poly(vinyl pyrrolidone), copolymers thereof and combinations thereof. The composite can further comprise a nonpolar polymer, such as, a polyolefin polymer, polyethylene, polypropylene, polybutene, polyisobutene, polyisoprene, polystyrene, copolymers thereof and combinations thereof.
Owner:SAMSUNG ELECTRONICS CO LTD
pH-triggered microparticles
InactiveUS20050123596A1Efficient deliveryGood biocompatibilityPowder deliveryMicroencapsulation basedPolyesterLipid formation
Microparticles that are designed to release their payload when exposed to acidic conditions are provided as a vehicle for drug delivery. Any therapeutic, diagnostic, or prophylatic agent may be encapsulated in a lipid-protein-sugar or polymeric matrix including a pH triggering agent to form pH triggerable microparticles. Preferably the diameter of the pH triggered microparticles ranges from 50 nm to 10 micrometers. The matrix of the particles may be prepared using any known lipid (e.g., DPPC), protein (e.g., albumin), or sugar (e.g., lactose). The matrix of the particles may also be prepared using any synthetic polymers such as polyesters. Methods of preparing and administering the particles are provided. Methods of immunization, transfection, and gene therapy are also provided by administering pH triggerable microparticles.
Owner:DANA FARBER CANCER INST INC +2
Collagen matrix for soft tissue augmentation
InactiveUS20050142161A1Minimize patient discomfortMinimize side effectsOrganic active ingredientsBiocideWrinkle skinTissue defect
The present invention includes methods and materials for soft tissue implant formed from biologically-compatible polymeric matrixes. The matrixes may have pores sized for in-growth of soft tissue. The material may be utilized with collagen or other matrix materials. This material may be used in a method of reforming soft tissues by implanting the material within soft body tissues to modify soft tissue defects such as wrinkles or biopsy tissue defects and to reshape soft tissue.
Owner:ETHICON ENDO SURGERY INC
Zero-order sustained release delivery system for carbamazephine derivatives
A zero-order sustained-release delivery system for delivery of carbamazepine or a derivative thereof. A polymeric matrix formulation of carbamazepine comprises hydrophilic polymer or hydrophilic / hydropholic polymer mixture which permits carbamazepine or carbamezepine derivative to be released from the polymer matrix in zero-order release kinetics.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
Biocompatible carrier containing L-arginine
L-arginine conjugated to a polymeric matrix is disclosed. The matrix can be used as, for example, a coating for an implantable medical device such as a stent.
Owner:ABBOTT CARDIOVASCULAR
Hemostatic wound dressing and method of making same
The present invention is directed to wound dressings that contain a fabric made from biocompatible polymeric fibers and having flexibility, strength and porosity effective for use as a hemostat, and a porous, polymeric matrix prepared from a biocompatible, water-soluble or water-swellable polymer dispersed through the fabric; and to methods of making such wound dressings.
Owner:ETHICON INC
Polymeric drug delivery system for hydrophobic drugs
InactiveUS20050249799A1Low oral bioavailabilityStable against aggregationAntibacterial agentsPowder deliveryHydrophobic polymerImmediate release
An oral delivery system for Class II drugs that have low oral bioavailability due to their insolubility in water and slow dissolution kinetics and method for making such a drug delivery system are disclosed herein. The formulation may be a controlled release or immediate release formulation. The immediate release formulation contains a Class II drug, together with a hydrophobic polymer, preferably a bioadhesive polymer. In one embodiment, the drug and polymer are co-dissolved in a common solvent. The solution is formed into small solid particles by any convenient method, particularly by spray drying. The resulting particles contain drug dispersed as small particles in a polymeric matrix. The particles are stable against aggregation, and can be put into capsules or tableted for administration. The controlled release formulations contain a BCS Class II drug and a bioadhesive polymer. The controlled release formulations may be in the form of a tablet, capsules, mini-tab, microparticulate, or osmotic pump. Enhancement of oral uptake of the drug from use of bioadhesive polymers occurs through (1) increased dissolution kinetics due to stable micronization of the drug, (2) rapid release of the drug from the polymer in the GI tract; and (3) prolonged GI transit due to bioadhesive properties of the polymers. The combination of these effects allows the preparation of a compact, stable dosage form suitable for oral administration of many class II drugs.
Owner:SPHERICS
Method for making oriented single-walled carbon nanotube/;polymer nano-composite membranes
ActiveUS20080290020A1Rapid of compoundRapid transportSemi-permeable membranesNanotechFiltrationCarbon nanotube
Nano-composite membranes and methods for making them are described. The nano-composite membranes a made from a layer of oriented carbon nanotubes fixed in a polymeric matrix. Methods for efficient, facile, and inexpensive fabrication of the nano-composite membranes using a filtration method are also described. The carbon nanotubes may also be modified with chemical functional groups to promote their orientation in the carbon nanotube layer or to confer to them other properties.
Owner:VIRGINIA TECH INTPROP INC
Synthetic, bioabsorbable polymer materials and implants
Owner:BIORETEC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com