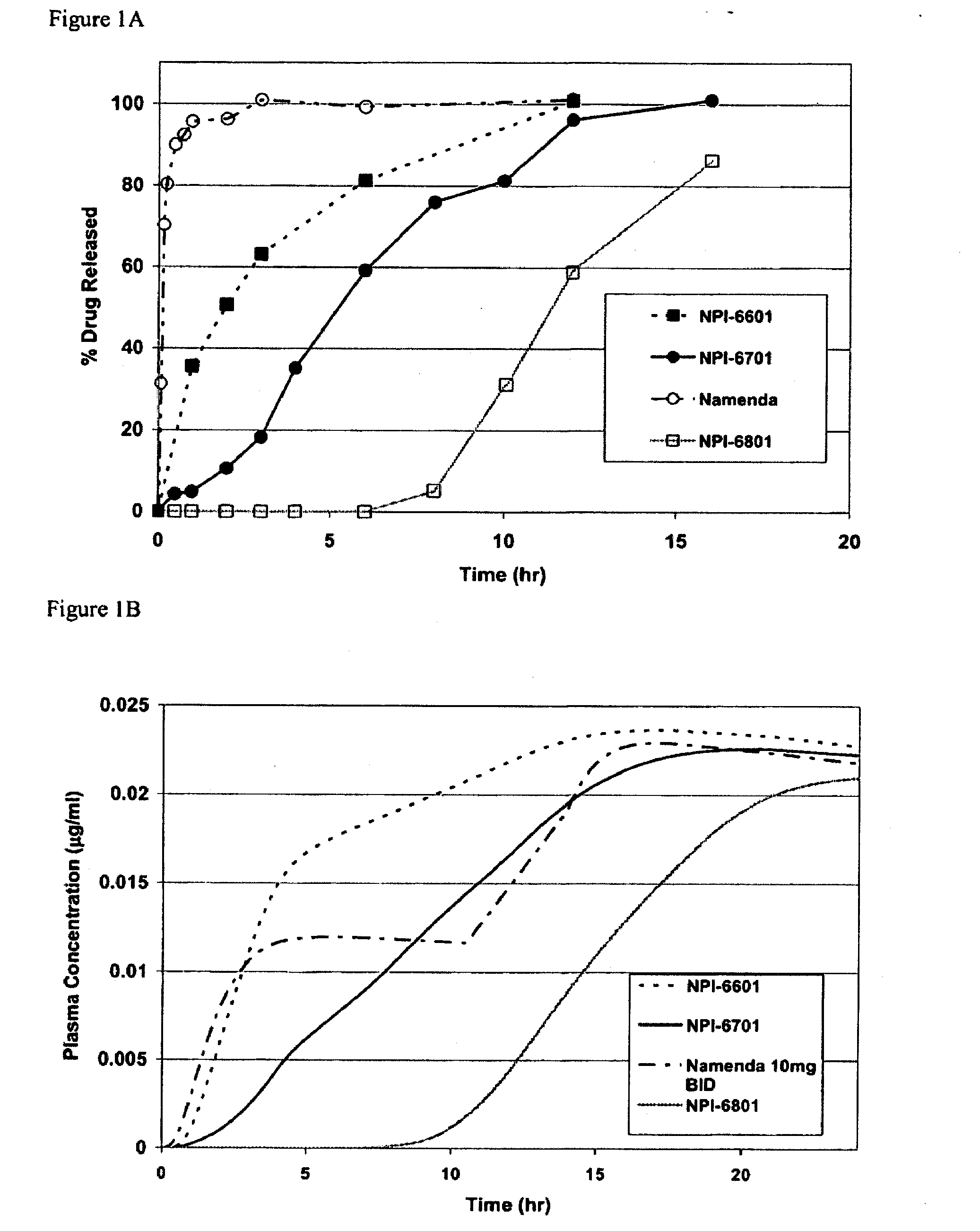
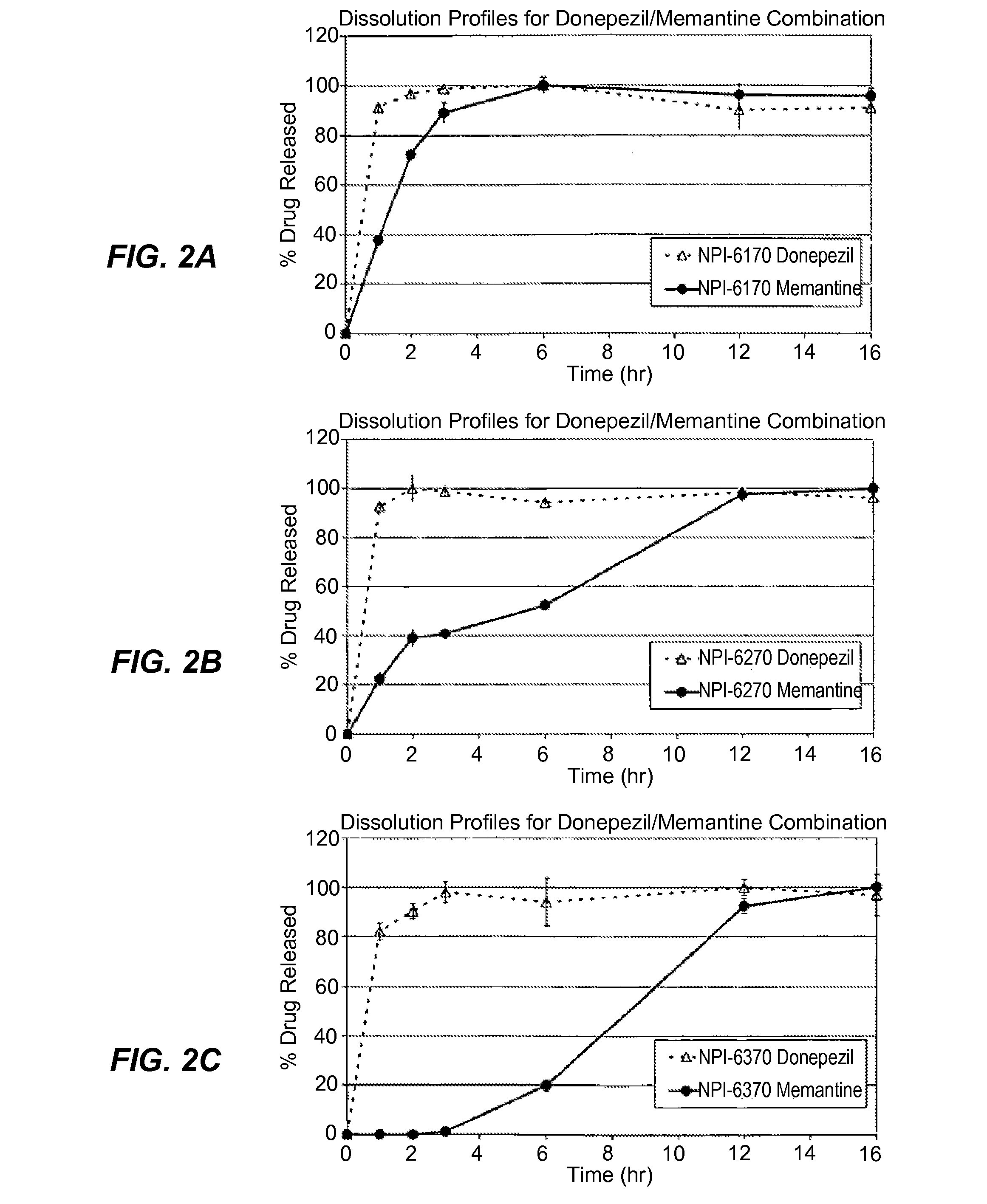
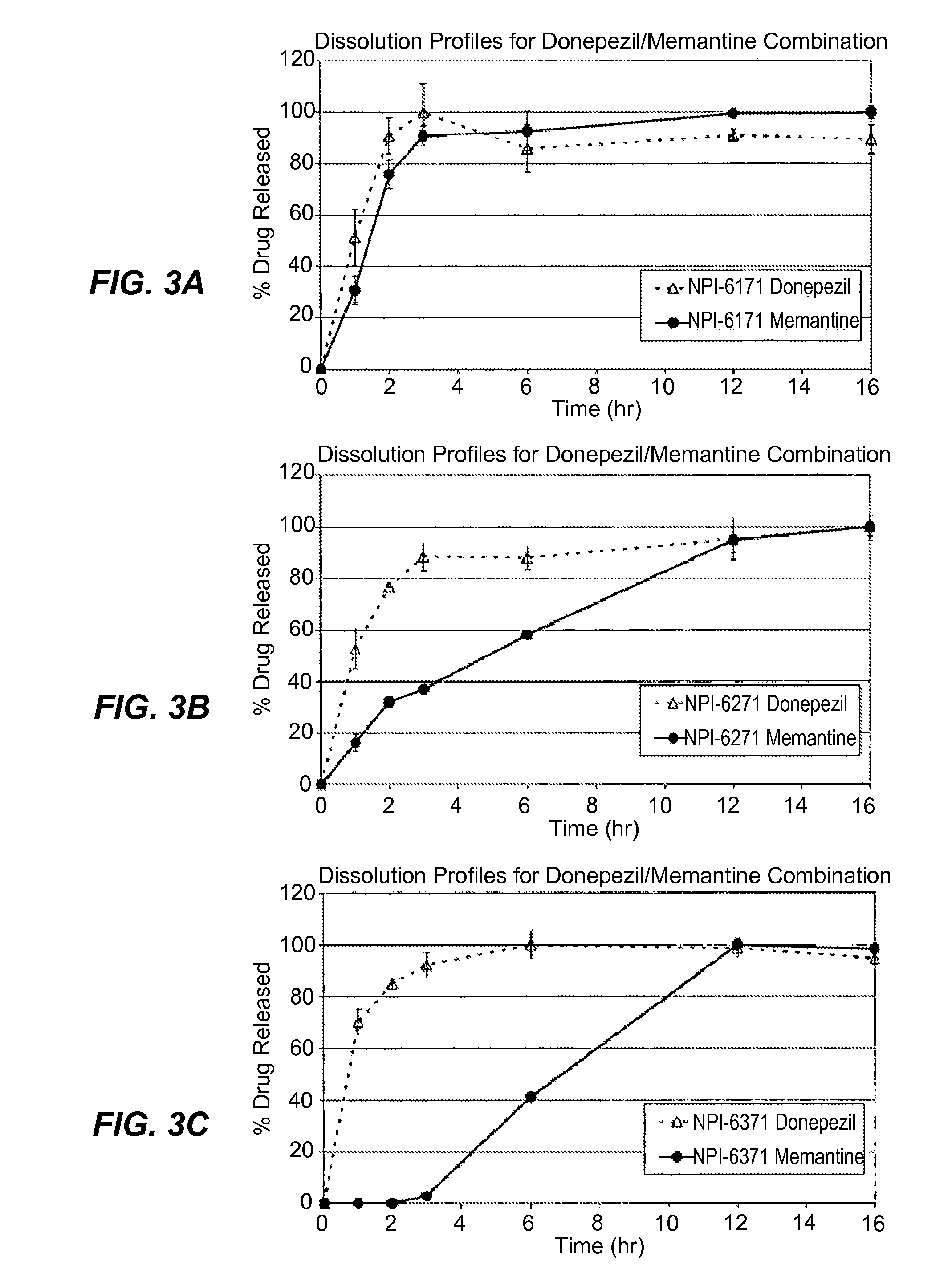
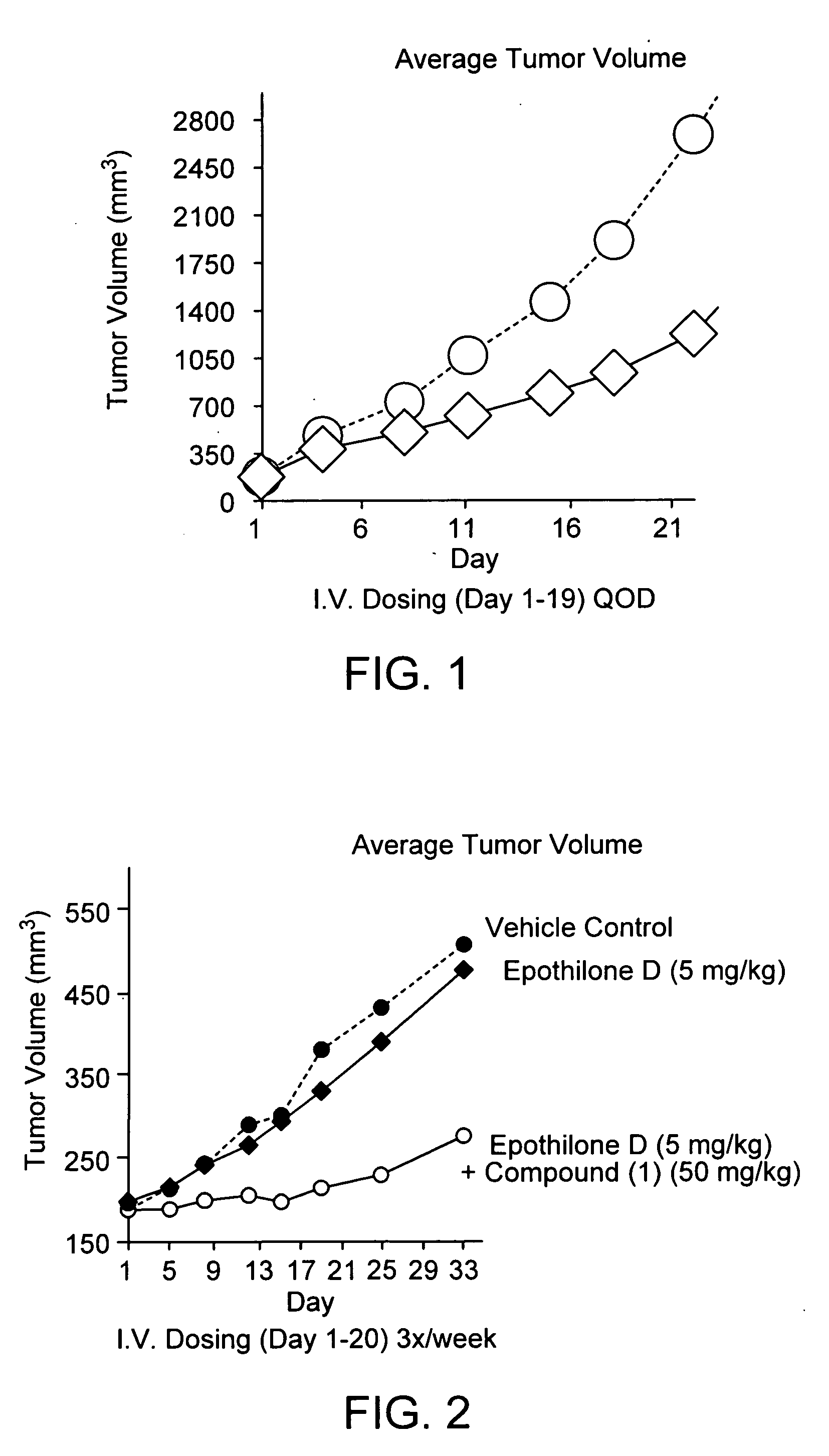
Patents
Literature
412results about How to "Minimize side effects" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
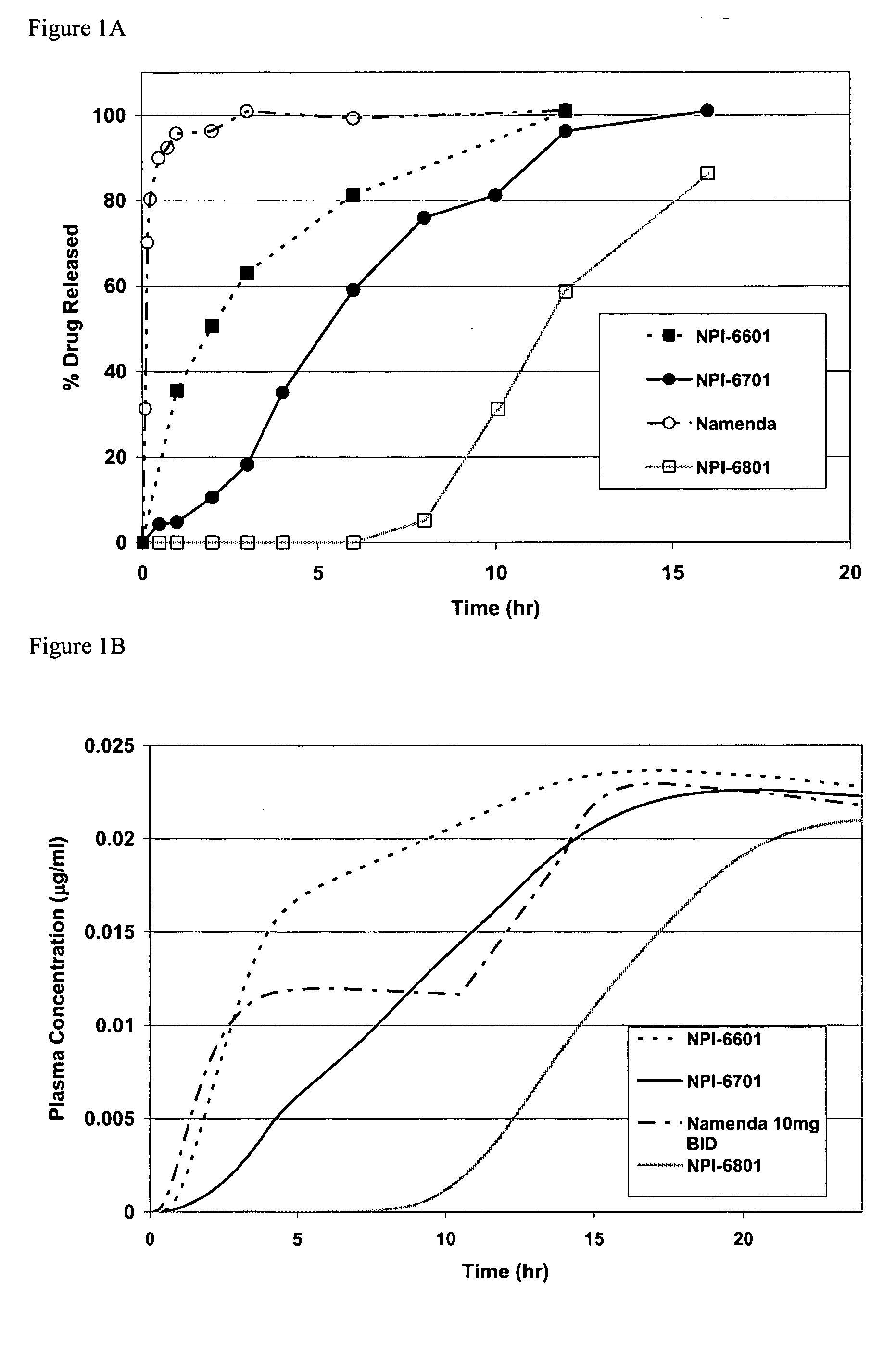
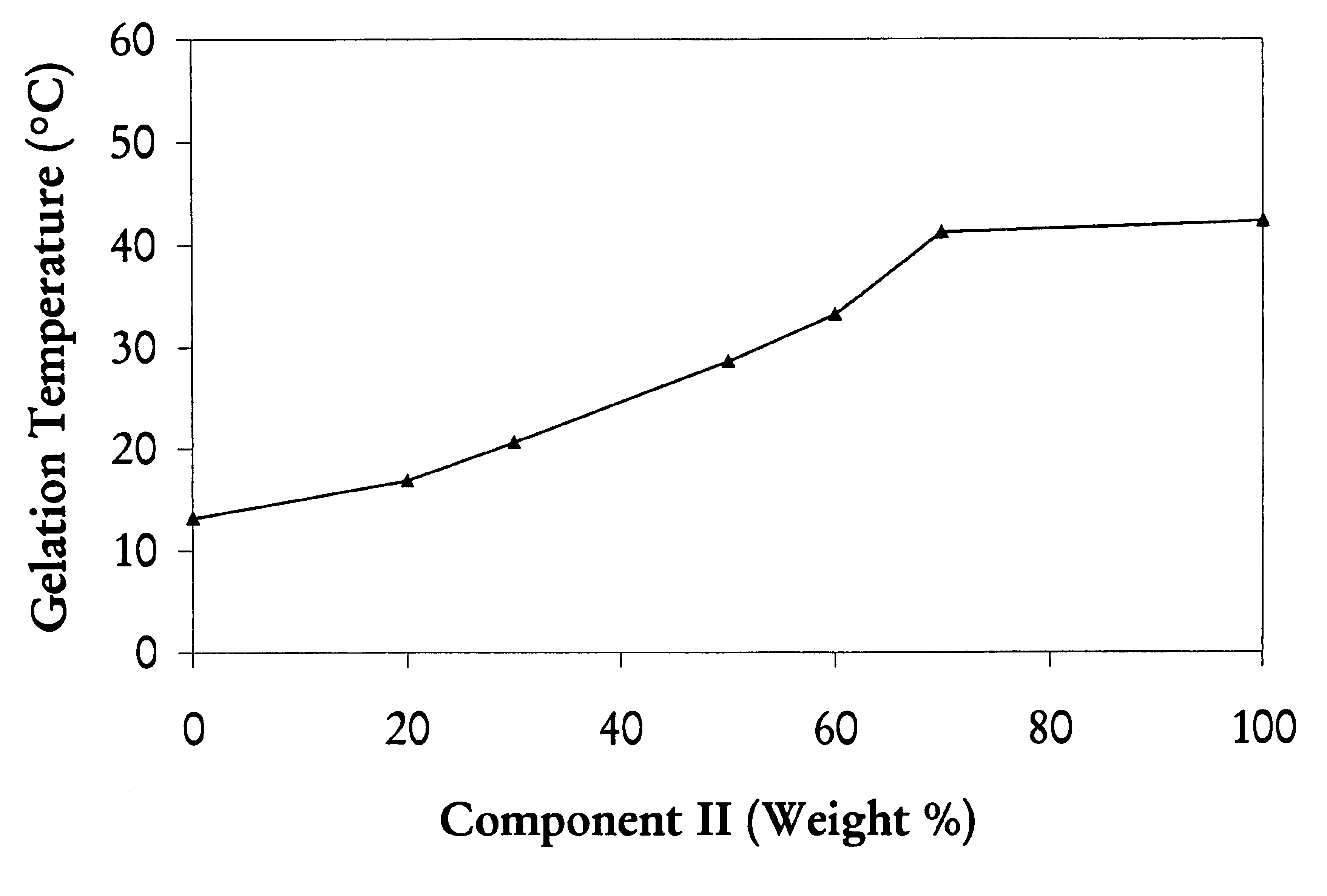
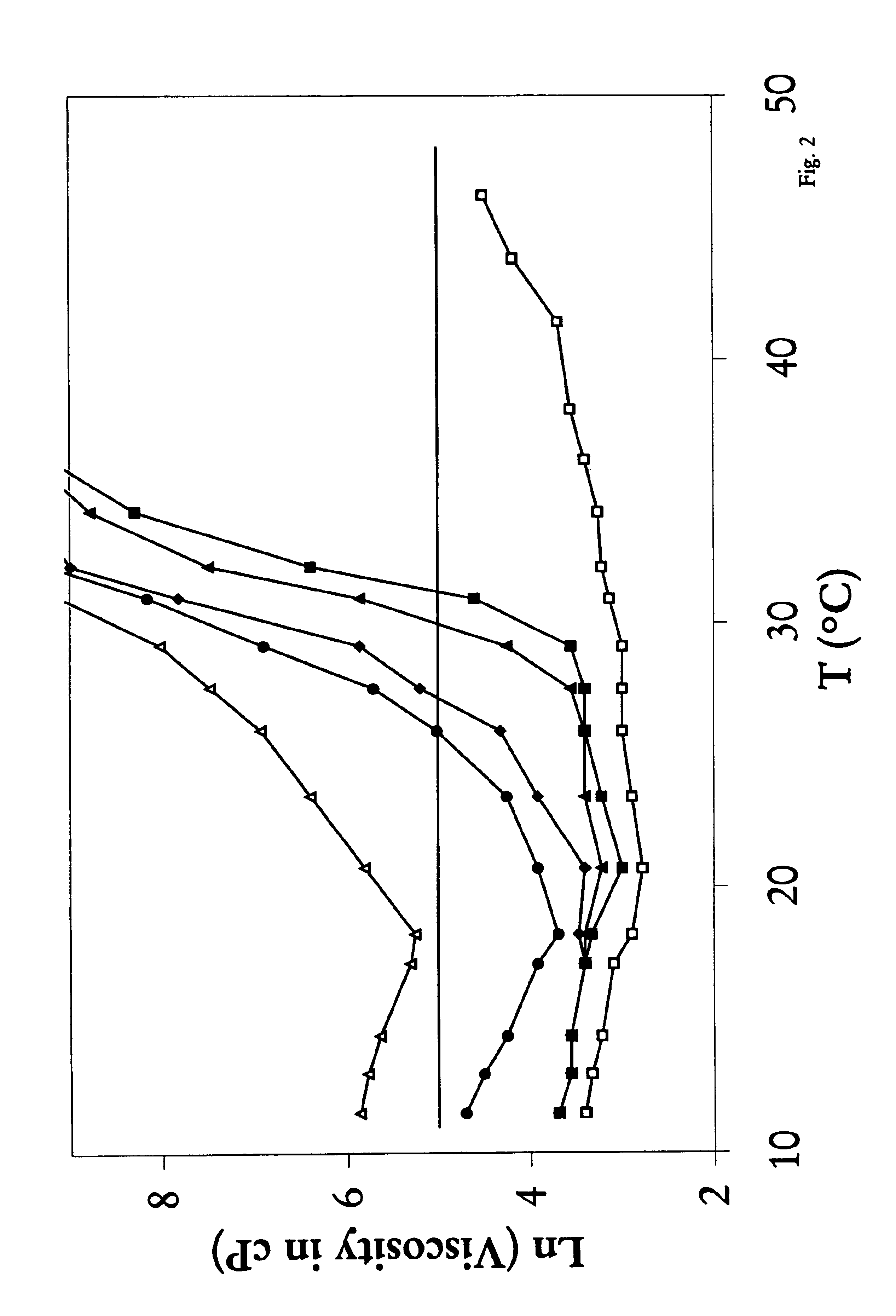
Biodegradable low molecular weight triblock poly (lactide-co-glycolide) polyethylene glycol copolymers having reverse thermal gelation properties
InactiveUS6117949AReduce solubilityReduced stabilityPowder deliveryPeptide/protein ingredientsSolubilityPolymer science
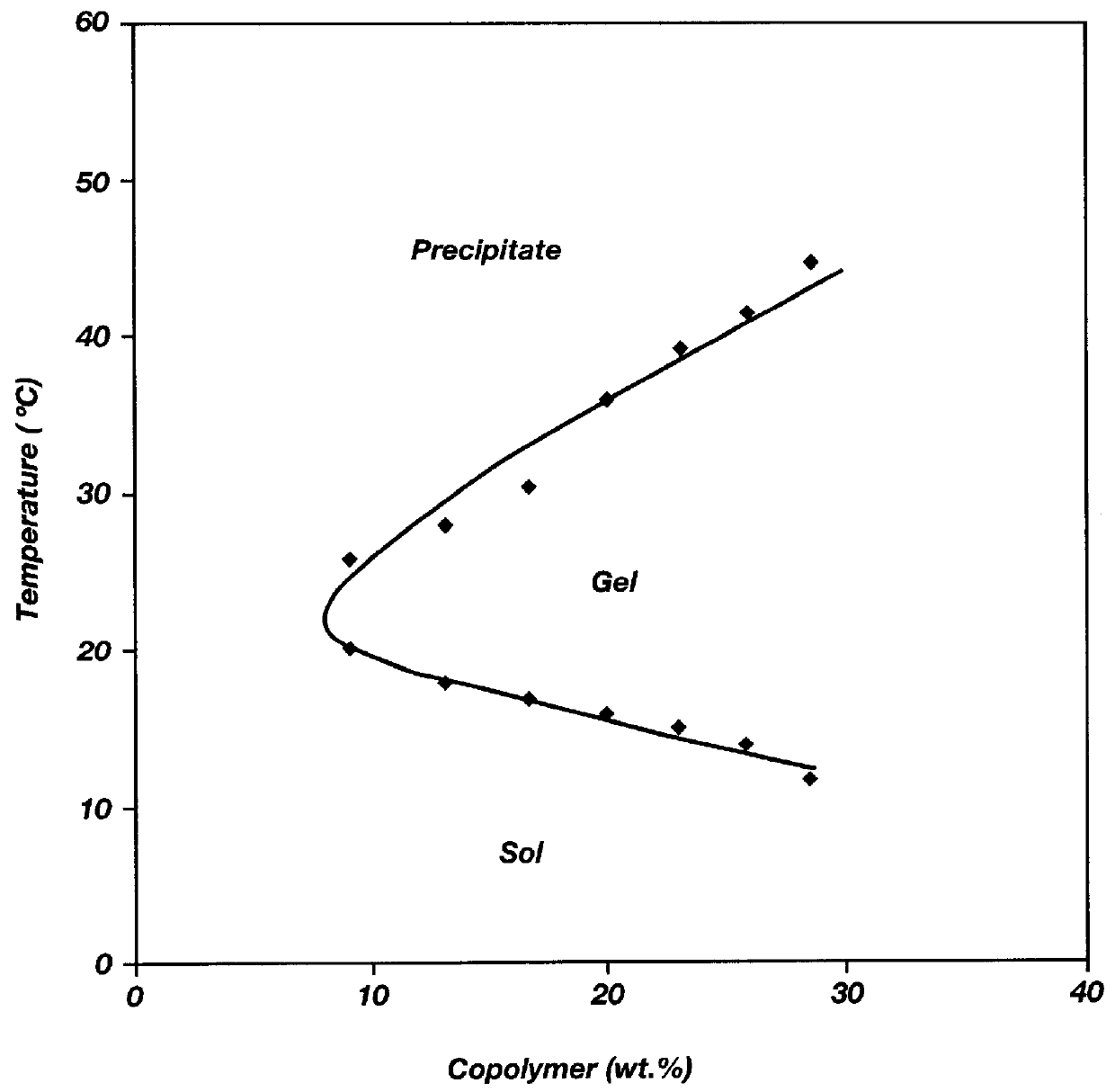
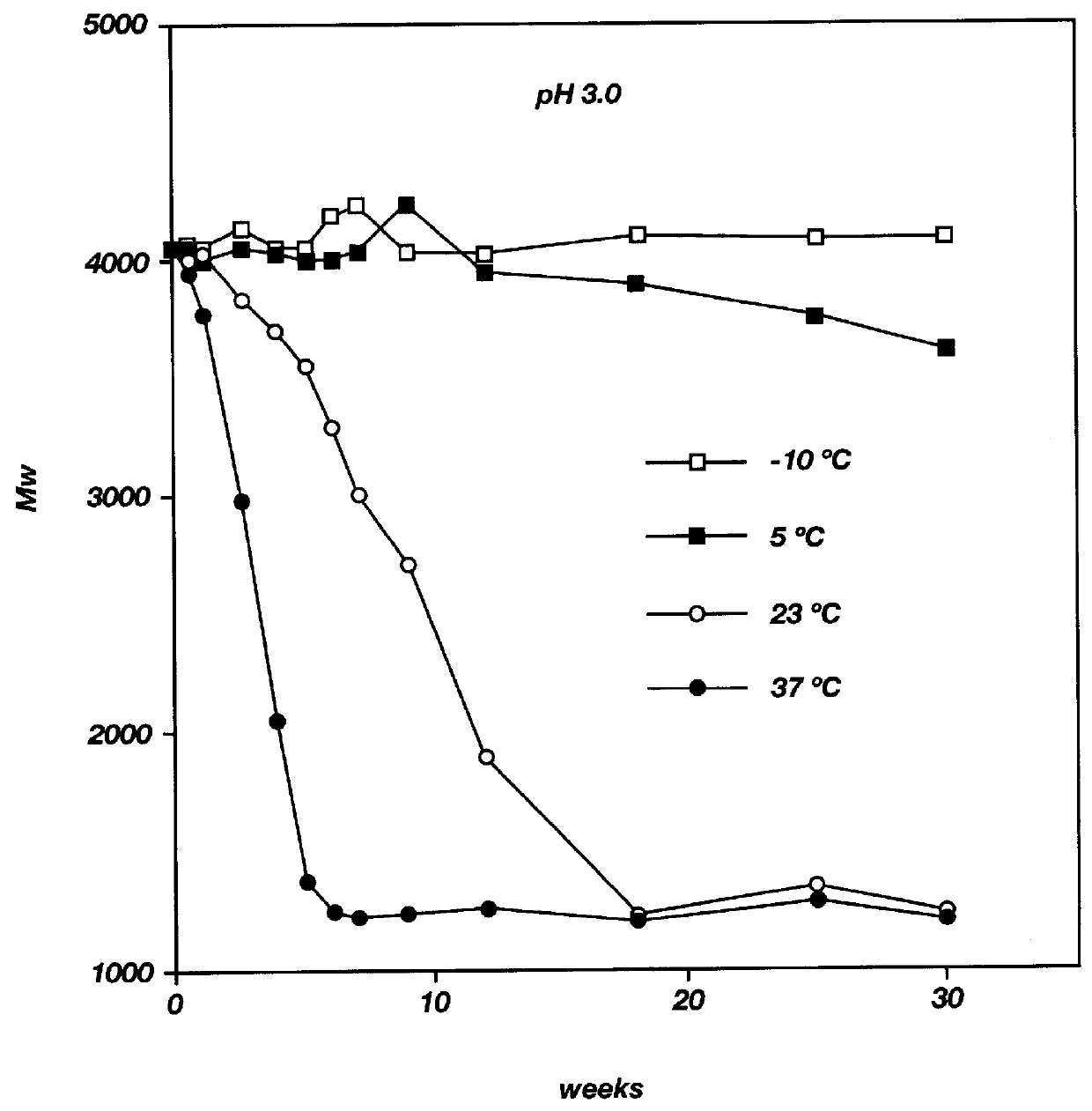
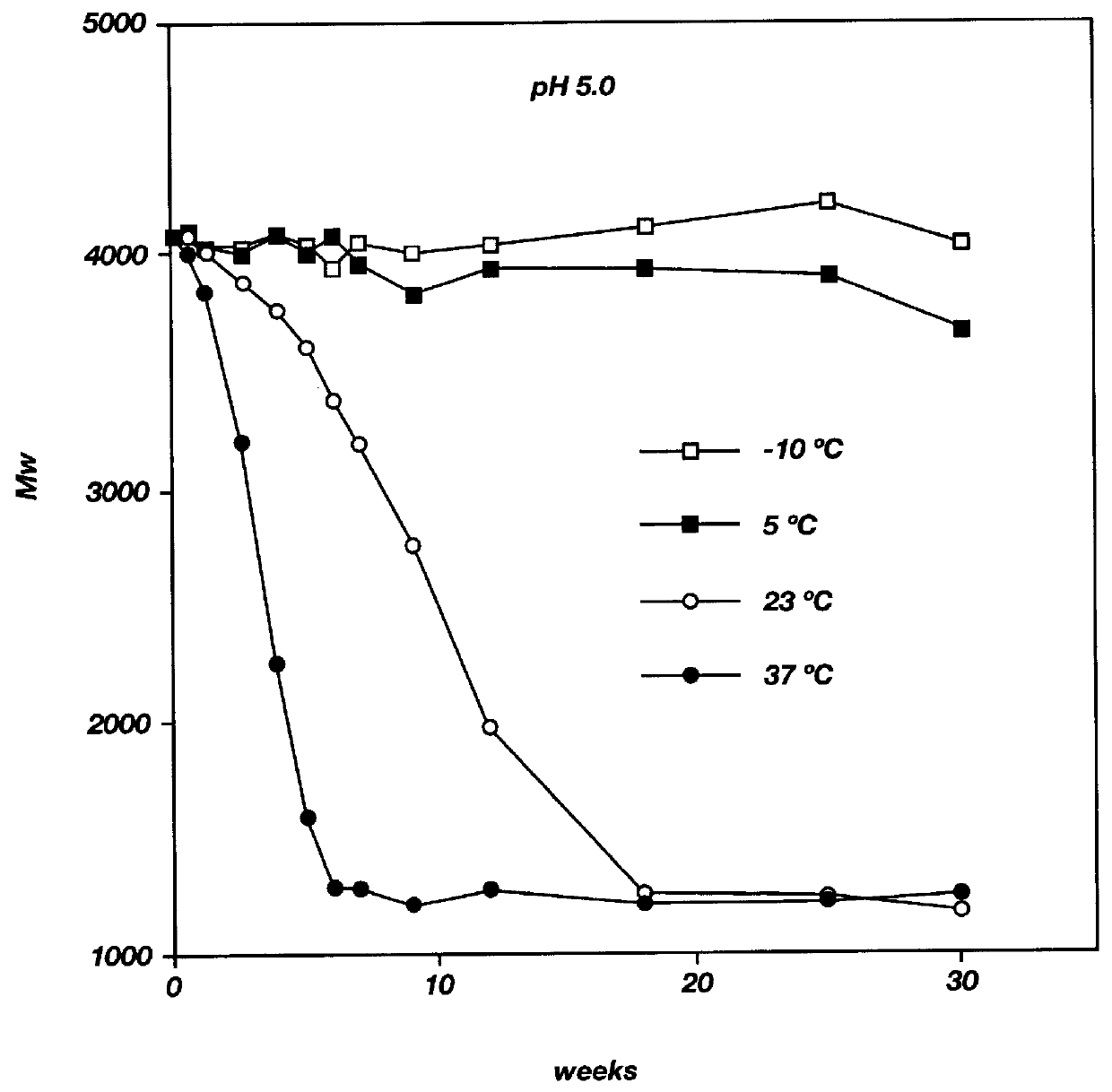
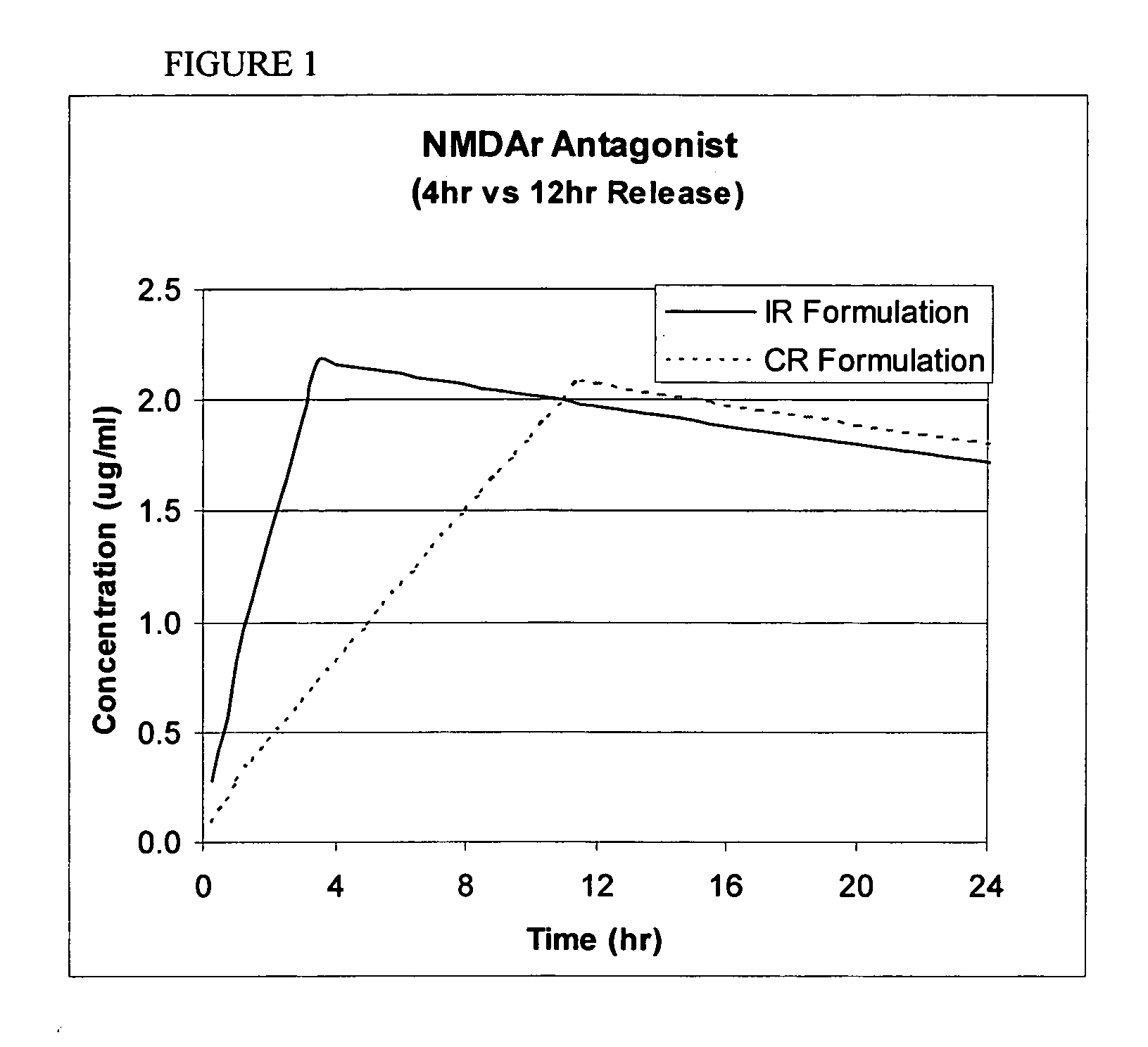
A water soluble biodegradable ABA- or BAB-type triblock polymer is disclosed that is made up of a major amount of a hydrophobic polymer made of a poly(lactide-co-glycolide) copolymer or poly(lactide) polymer as the A-blocks and a minor amount of a hydrophilic polyethylene glycol polymer B-block, having an overall weight average molecular weight of between about 2000 and 4990, and that possesses reverse thermal gelation properties. Effective concentrations of the triblock polymer and a drug may be uniformly contained in an aqueous phase to form a drug delivery composition. At temperatures below the gelation temperature of the triblock polymer the composition is a liquid and at temperatures at or above the gelation temperature the composition is a gel or semi-solid. The composition may be administered to a warm-blooded animal as a liquid by parenteral, ocular, topical, inhalation, transdermal, vaginal, transurethral, rectal, nasal, oral, pulmonary or aural delivery means and is a gel at body temperature. The composition may also be administered as a gel. The drug is released at a controlled rate from the gel which biodegrades into non-toxic products. The release rate of the drug may be adjusted by changing various parameters such as hydrophobic / hydrophilic componenet content, polymer concentration, molecular weight and polydispersity of the triblock polymer. Because the triblock polymer is amphiphilic, it functions to increase the solubility and / or stability of drugs in the composition.
Owner:BTG INT LTD +2
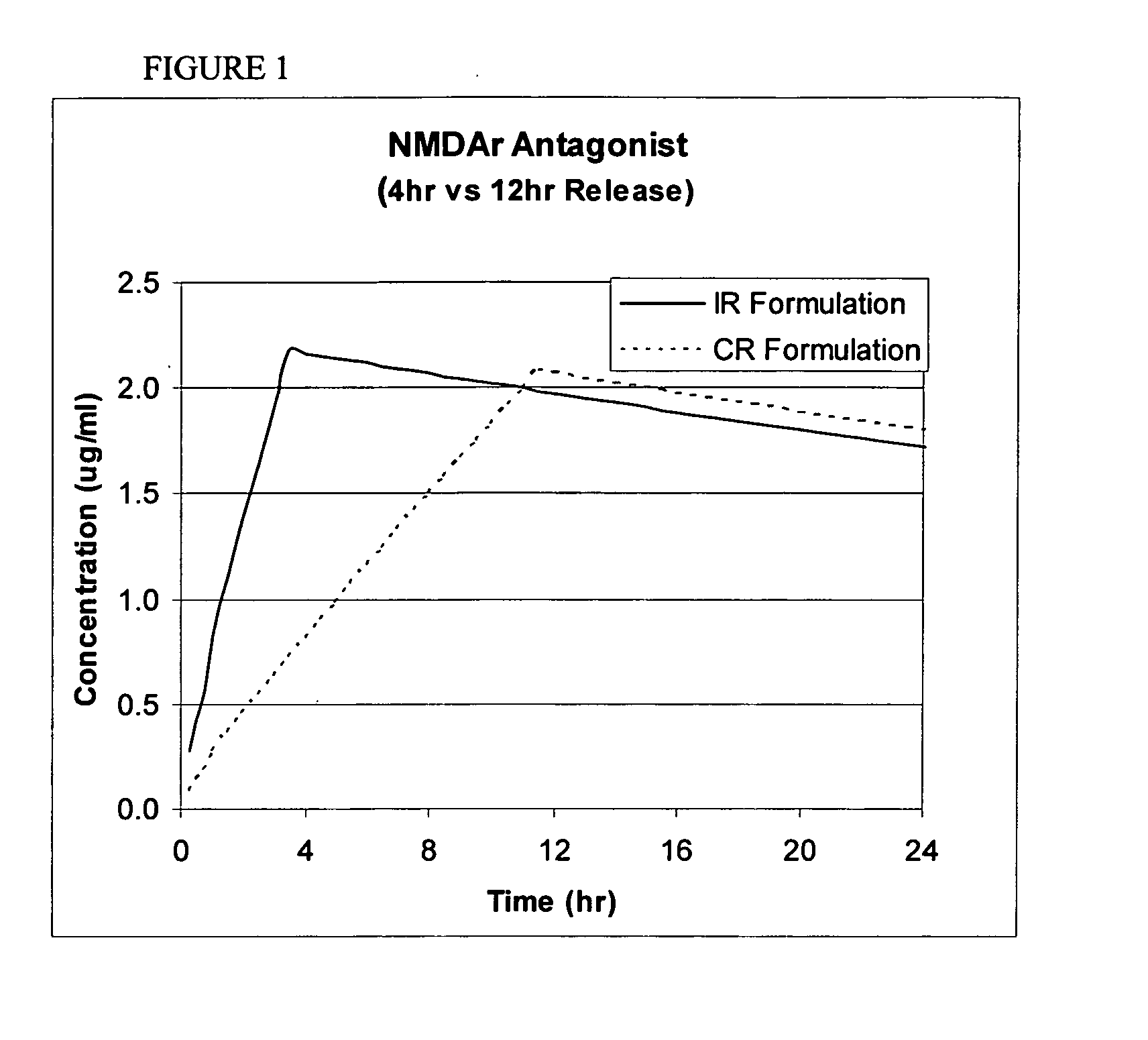
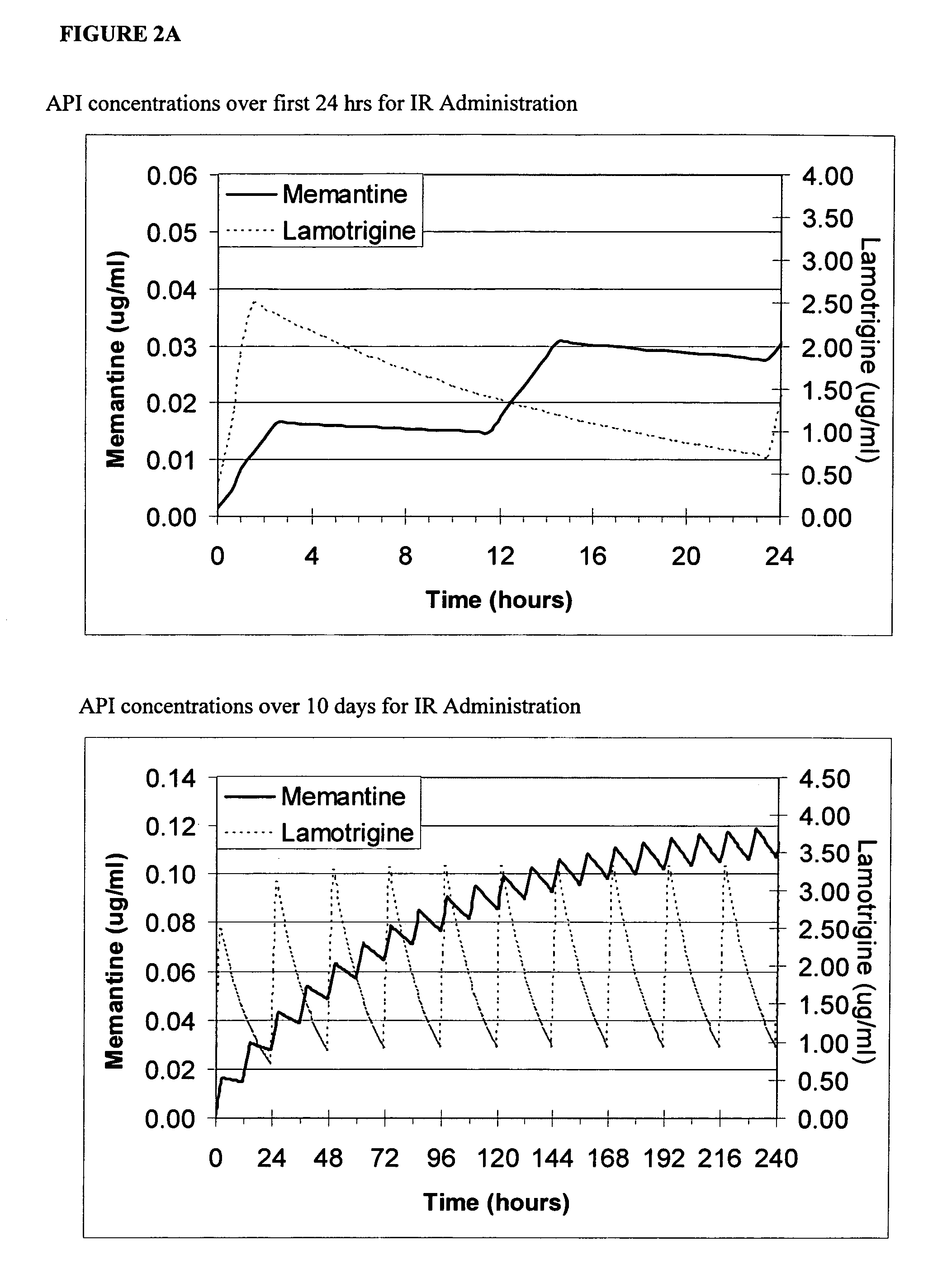
Methods and compositions for the treatment of psychiatric conditions
InactiveUS20050209218A1Low variabilityMaximizes therapeutic benefitBiocideNervous disorderDiseasePsychogenic disease
Owner:ADAMAS PHARMA INC
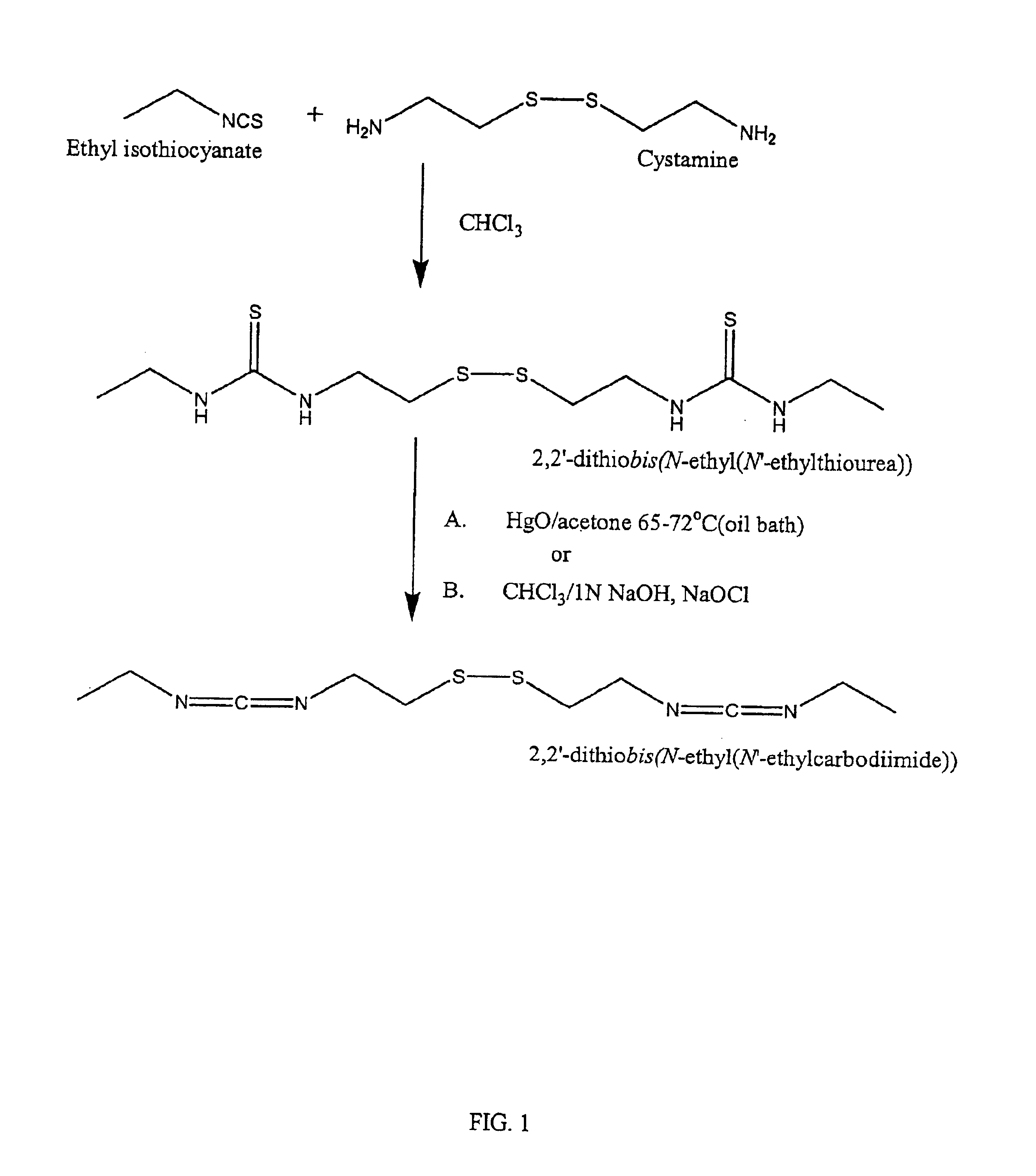
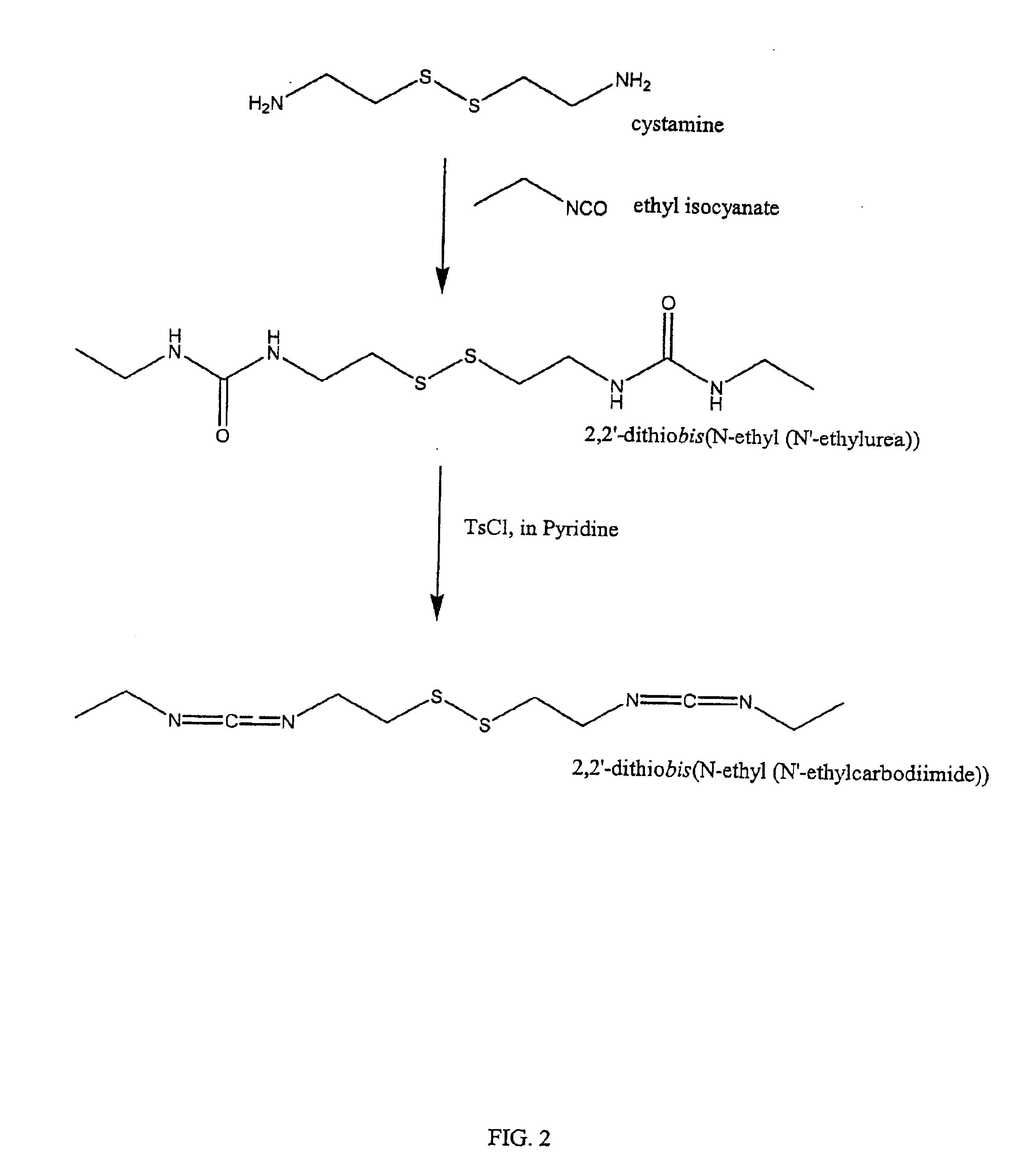
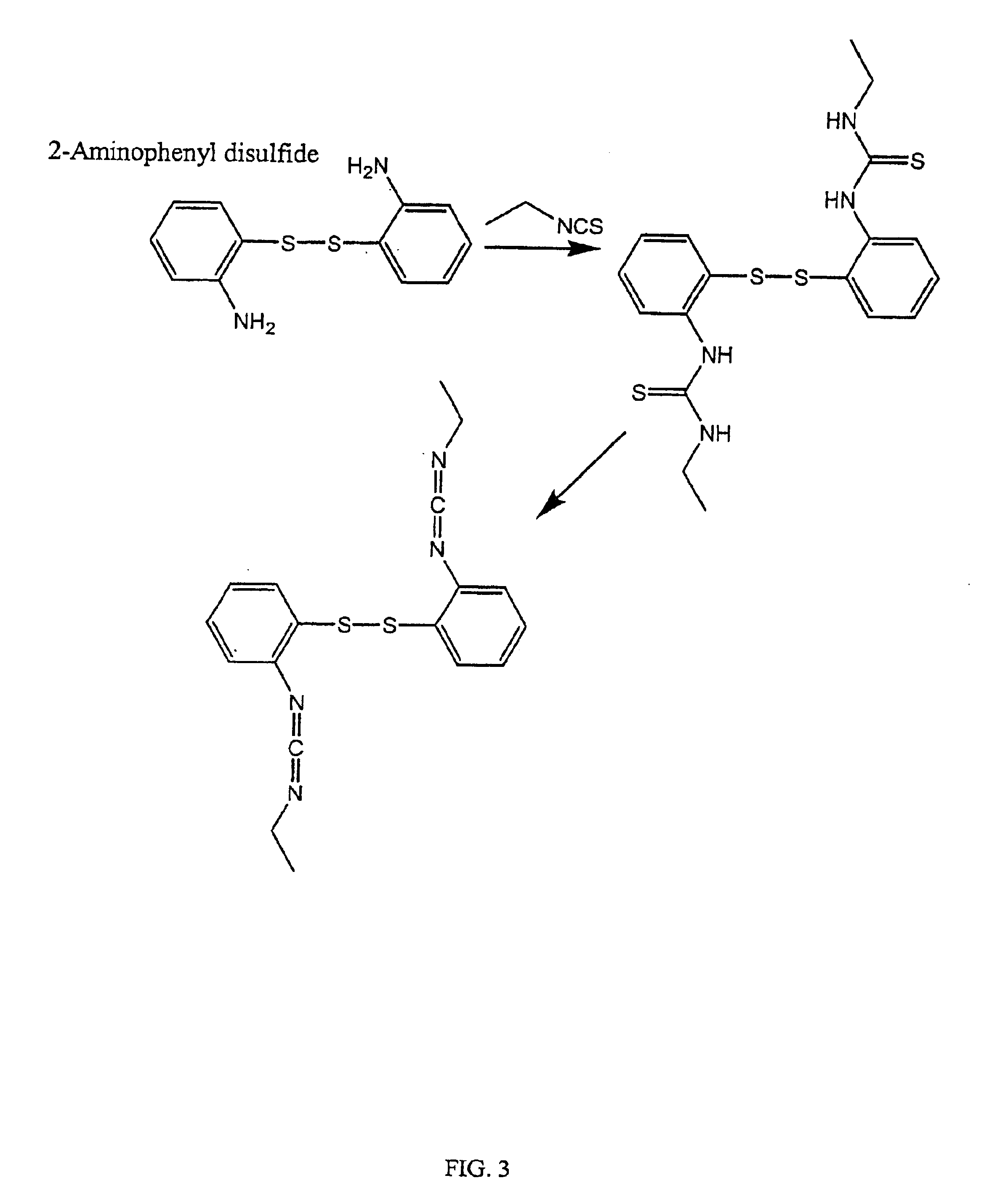
Thiol-modified hyaluronan
InactiveUS6884788B2High activityControl moreBiocideOrganic active ingredientsUrea derivativesCross-link
The present invention relates to biscarbodiimides, thiourea derivatives, urea derivatives, and cross-linked hyaluronan derivatives having at least one intramolecular disulfide bond, and methods of preparation thereof. The invention also includes thiolated hyaluronan derivatives and salts thereof having at least one pendant thiol group or a modified pendant thiol group, and methods of preparation thereof. An example of a modified pendant thiol group is a sulfhydryl group linked to a small molecule such as a bioactive agent, for example a drug or pharmaceutically active moiety. A hyaluronan derivative having a sulfhydryl group linked to a pharmaceutically active moiety is useful as a sustained or controlled release drug delivery vehicle. Compositions containing the hyaluronan derivatives of the invention can reversibly viscosify in vivo or in vitro, in response to mild changes in condition, and are thus useful in ophthalmic surgery and in tissue engineering.
Owner:ANIKA THERAPEUTICS INC
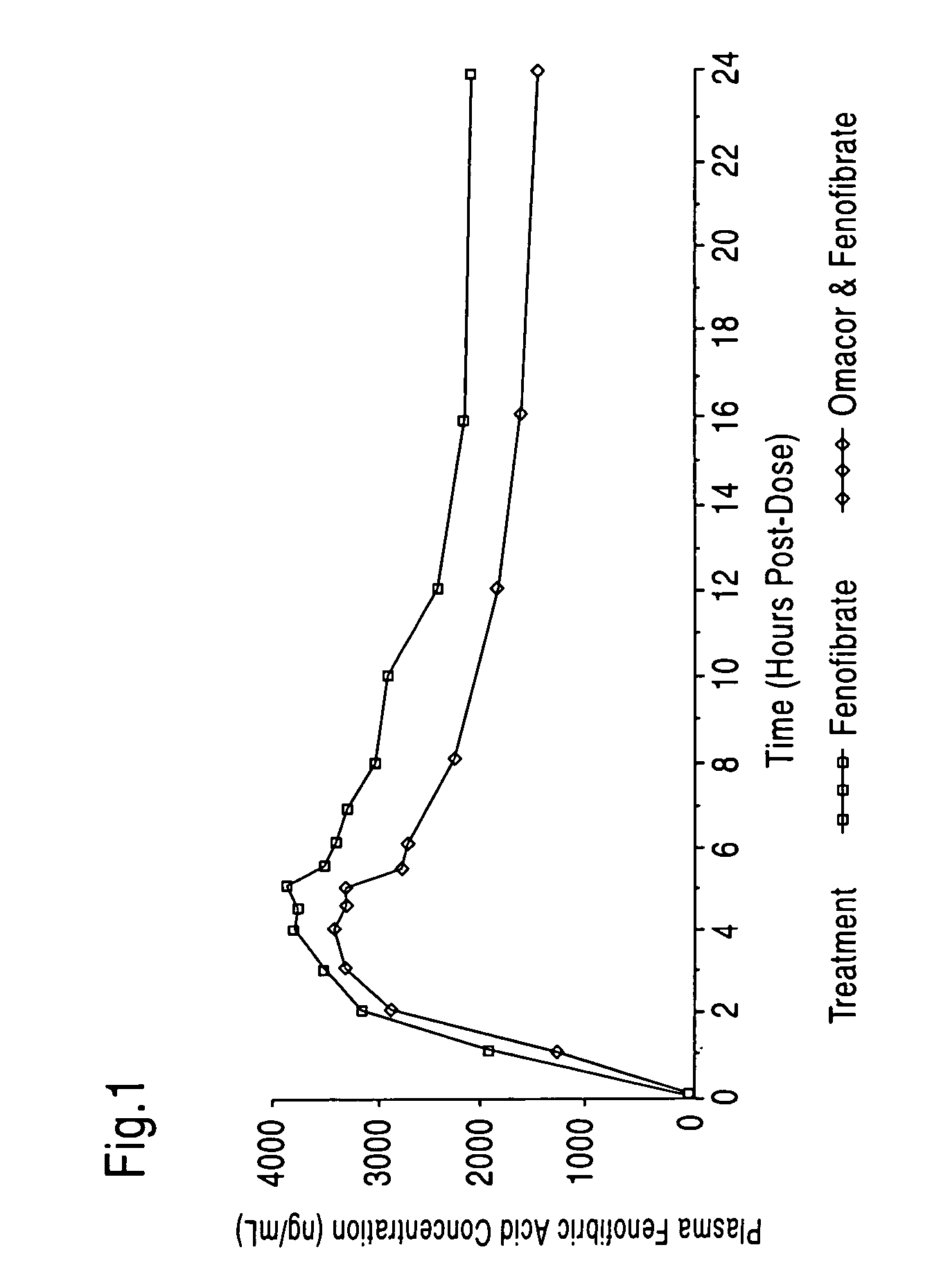
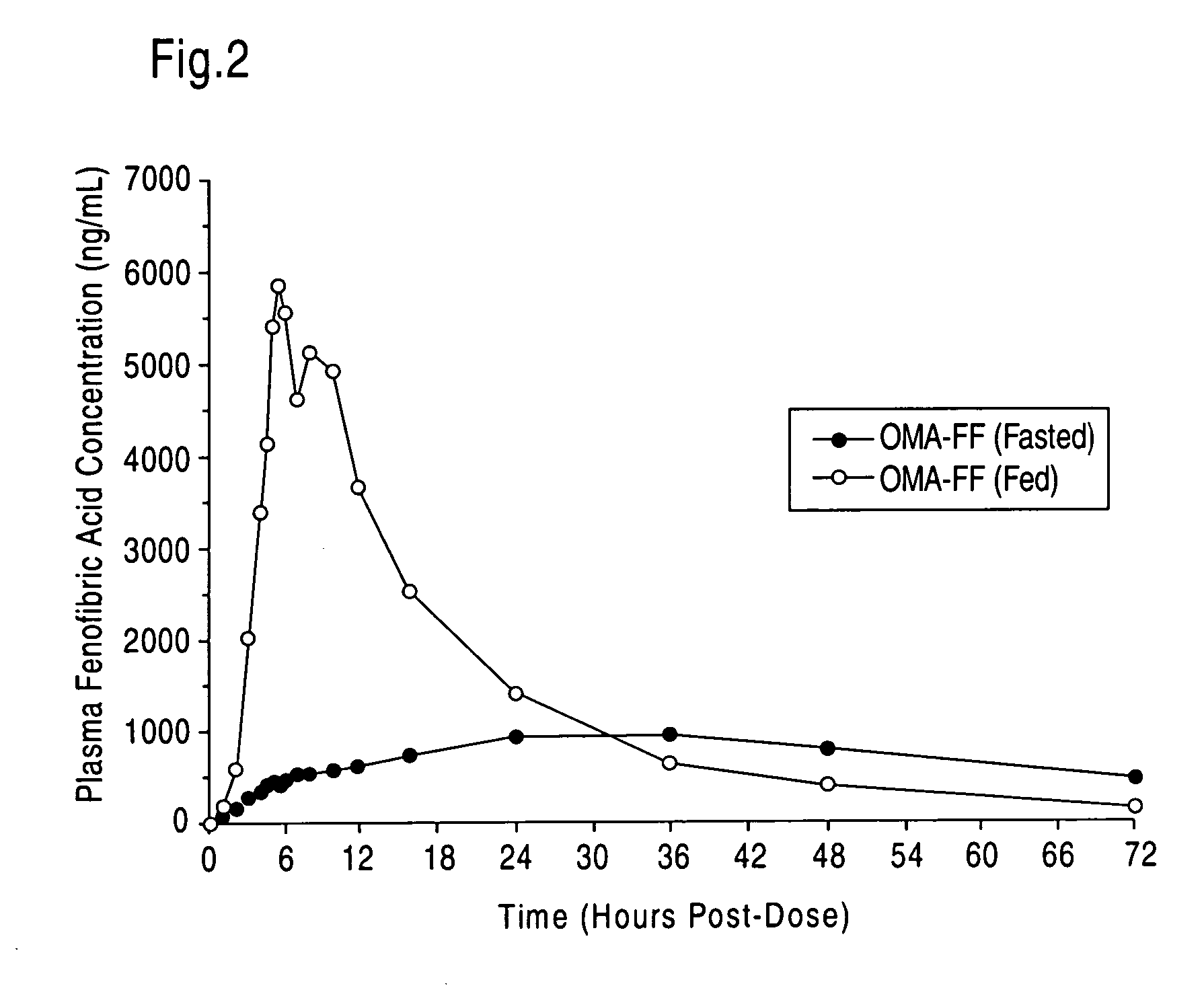
Treatment and prevention of cardiovascular disease in patients with chronic kidney disease by administering Omega-3 Fatty Acids
InactiveUS20080125490A1Effective pharmaceutical treatmentMinimize side effectsBiocideAnimal repellantsDyslipidemiaCoronary event
Compositions comprising omega-3 fatty acids are provided, where the compositions are useful for treating cardiovascular disease in patients suffering from chronic kidney disease (CKD), preventing its further progression, and treating underlying risk factors such as hypertension, dyslipidemia, obesity and / or diabetes. Also provided are methods of using the compositions to reduce the occurrence of or prevent major coronary events, including myocardial infarctions, in patients with CKD.
Owner:PRONOVA BIOCARE AS
Treatment and prevention of major adverse cardiovascular events or major coronary evens by administering Omega-3 fatty acids
InactiveUS20080306154A1Effective treatmentReduce generationBiocideMetabolism disorderDyslipidemiaCoronary event
Omega-3 fatty acid compositions comprising eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are provided, where the compositions are useful for treating, reducing the occurrence of, or preventing major adverse cardiovascular events or major coronary events in patients who have established cardiovascular disease without prior myocardial infarction, preventing their further progression, and treating underlying risk factors for CVD such as hypertension, dyslipidemia, obesity and / or diabetes.
Owner:PRONOVA BIOCARE AS
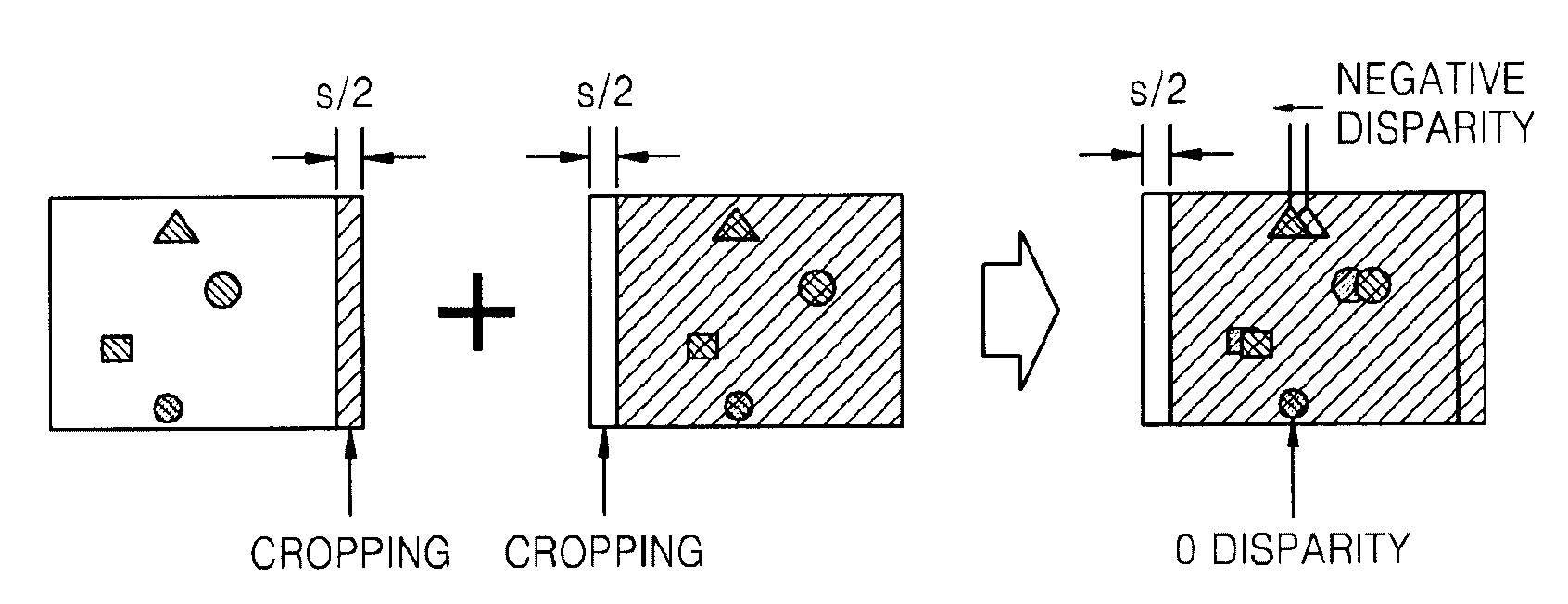
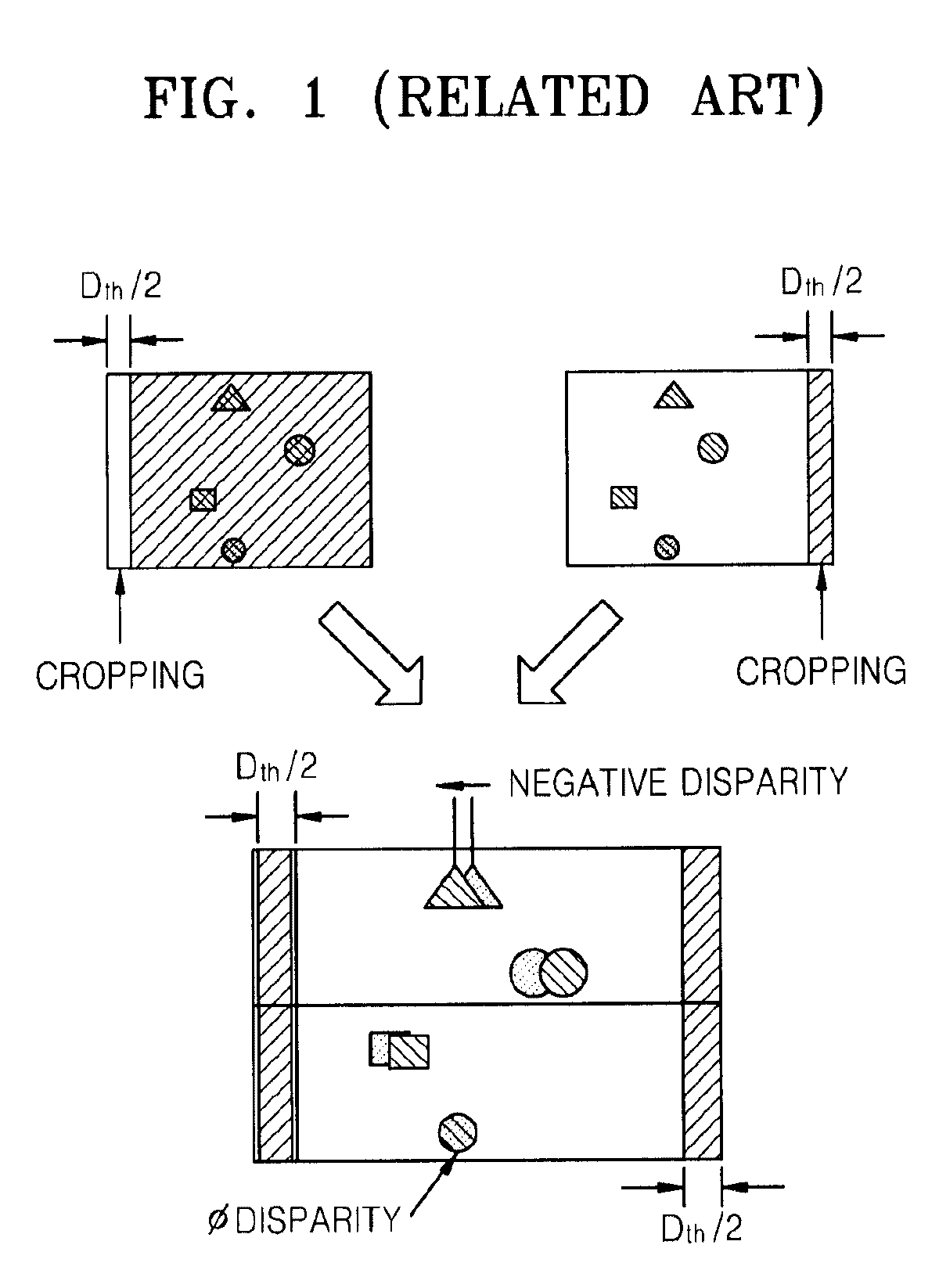
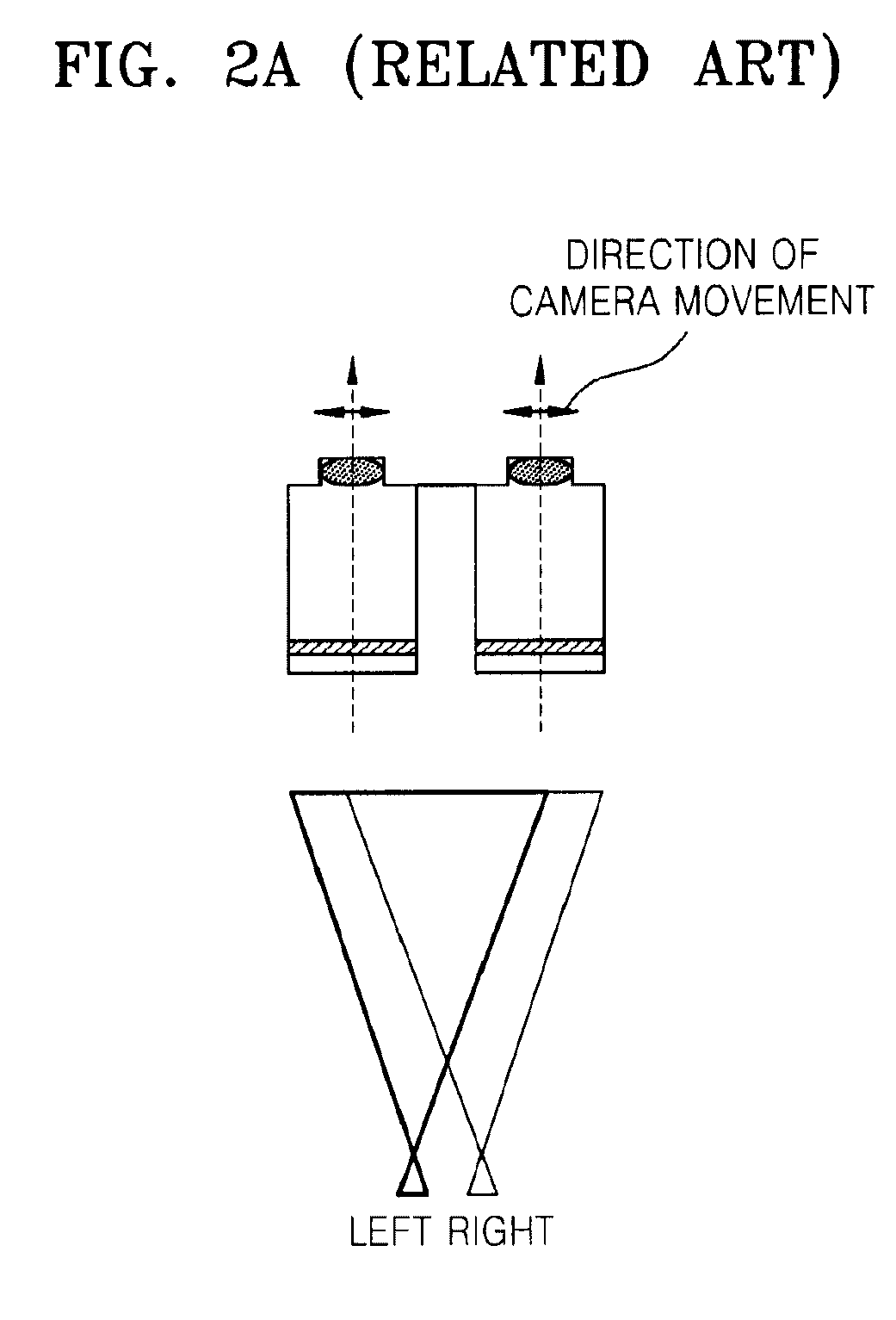
Method and apparatus for controlling dynamic depth of stereo-view or multi-view sequence images
A method and an apparatus for controlling a dynamic depth of stereo-view or multi-view images. The method includes receiving stereo-view or multi-view images, generating a disparity histogram by estimating the disparity of two images corresponding to the received images and measuring the frequency of the estimated disparity, determining the disparity control amount of the stereo-view or multi-view images by convoluting the generated disparity histogram and a characteristic function, and rearranging stereo-view or multi-view input images by controlling parallax according to the control amount of parity.
Owner:SAMSUNG ELECTRONICS CO LTD
Treatment with omega-3 fatty acids and PPAR agonist and/or antagonist and a combination product thereof
InactiveUS20060211749A1Reduced dosages of PPAR agonistEffective treatmentBiocideMetabolism disorderDyslipidemiaFasting glucose
A method and composition for blood lipid therapy that comprises administering to the subject an effective amount of a PPAR agonist and / or antagonist and an omega-3 fatty acid. The methods and compositions include combination products or concomitant therapy for the treatment of subjects with hypertriglyceridemia, hypercholesteremia, mixed dyslipidemia, vascular disease, artherosclerotic disease and related conditions, obesity, the prevention or reduction of cardiovascular and vascular events, the reduction of insulin resistance, fasting glucose levels and postprandial glucose levels, and / or the reduction of incidence and / or the delay of onset of diabetes.
Owner:RELIANT PHARMACEUTICALS INC
Methods and Compositions for Treating Migraine Pain
InactiveUS20100029665A1Benefit maximizationEliminate side effectsBiocideNervous disorderHeadache severeVascular headache
The present invention provides novel methods and compositions for the treatment and prevention of headaches, vascular headaches, migraine headaches, cluster headaches, and migraine. One of the headaches, vascular headaches, migraine headaches, cluster headaches, and migraine treated by the methods and compositions of the invention is migraine.
Owner:MEYERSON LAURENCE R +3
External addition of pulses to fluid channels of body to release or suppress endothelial mediators and to determine effectiveness of such intervention
ActiveUS20020103454A1Regulates the endothelial releaseImprove bioavailabilityElectrotherapyPneumatic massageShear stressReciprocating motion
Methods of medical treatment and diagnosis using mediators released by endothelial cells stimulated by external addition of pulses to the circulation are disclosed. The external pulses produce circumferential shear stress in body fluid channels that subsequently stimulates the endothelial cells to produce mediators that become available for therapeutic and diagnostic purposes. The preferred means of adding external pulses is the mechanical inducement of periodic acceleration of the body or parts of the body by a reciprocating motion platform.
Owner:NON INVASIVE MONITORING SYST INC
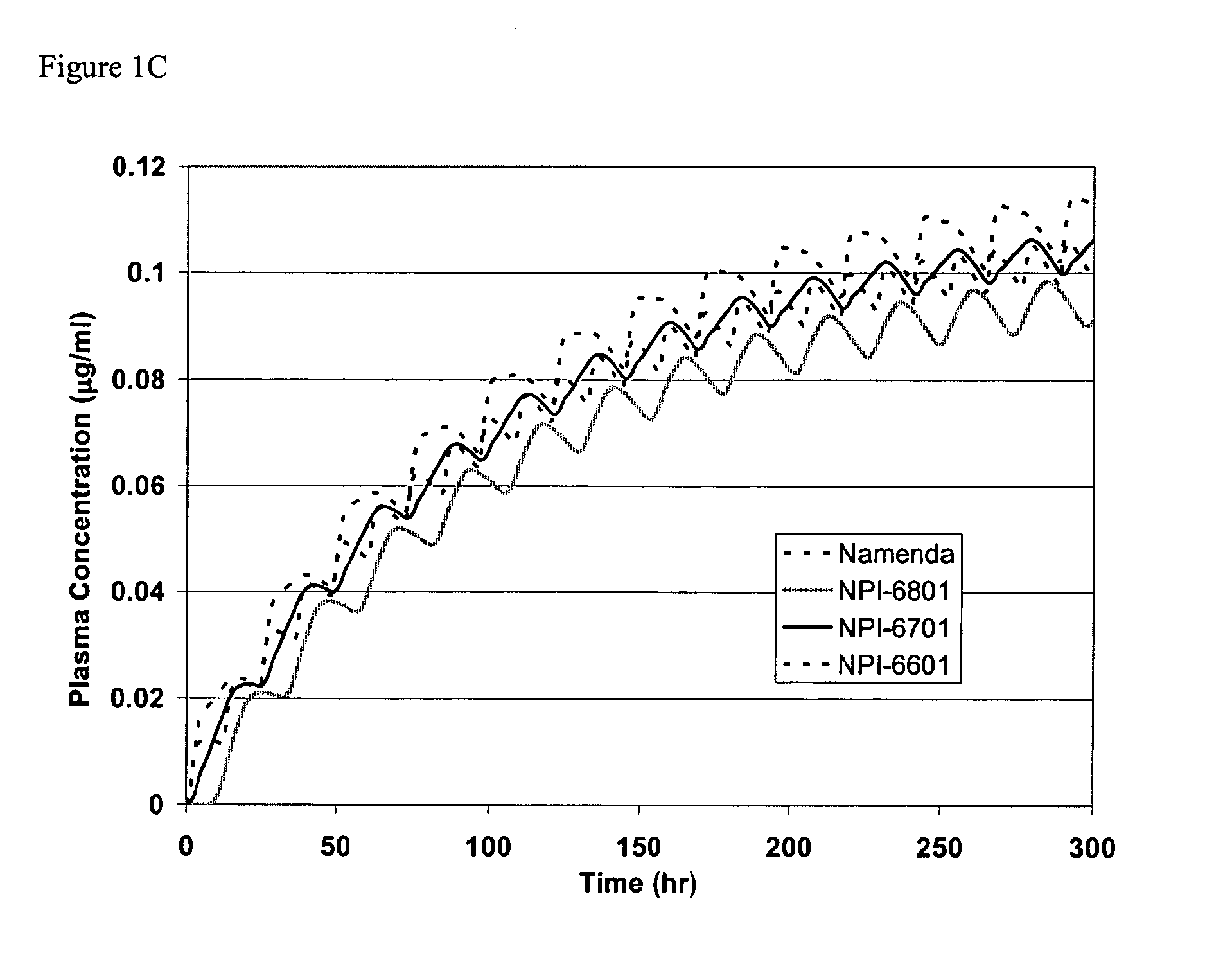
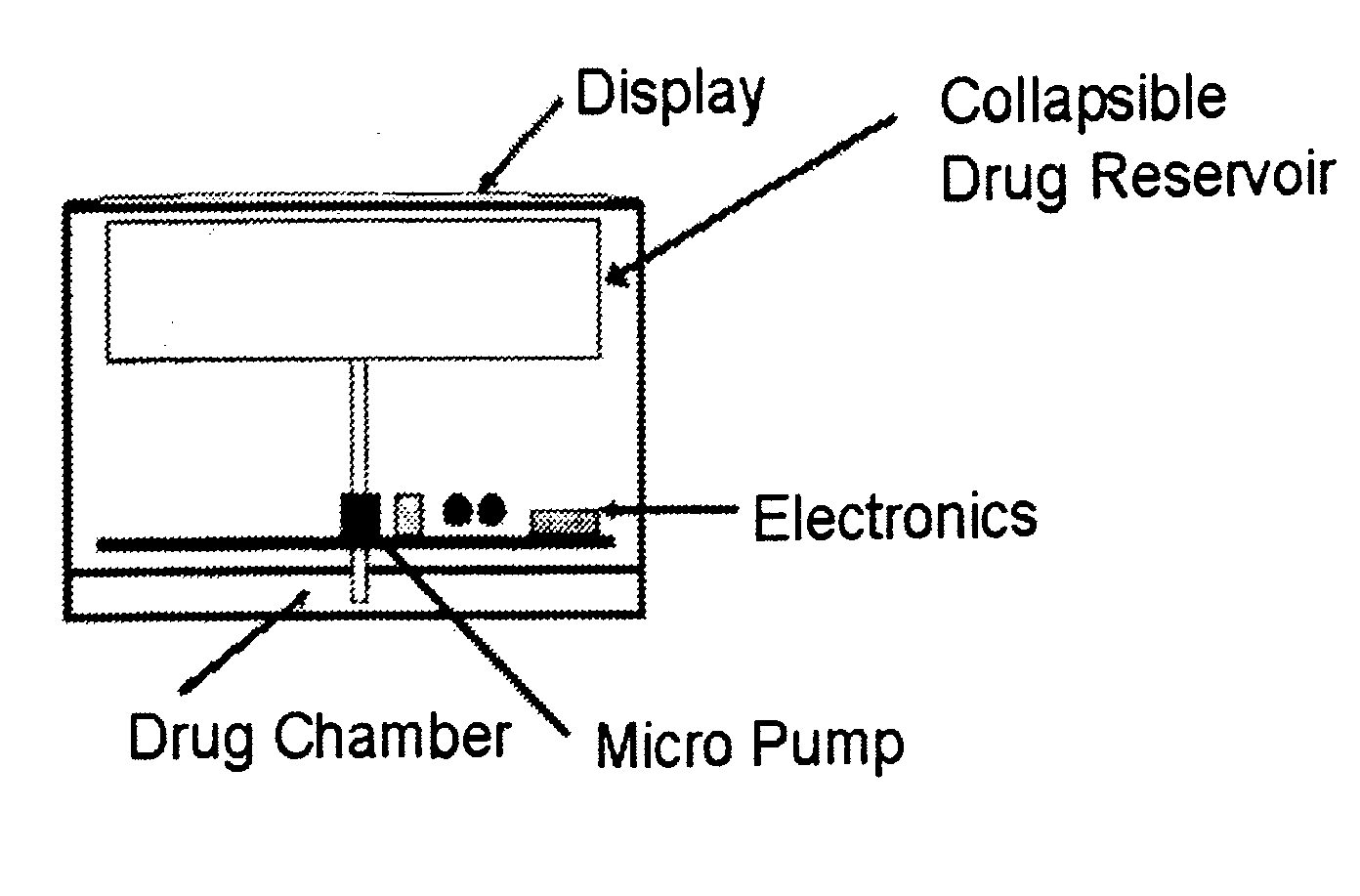
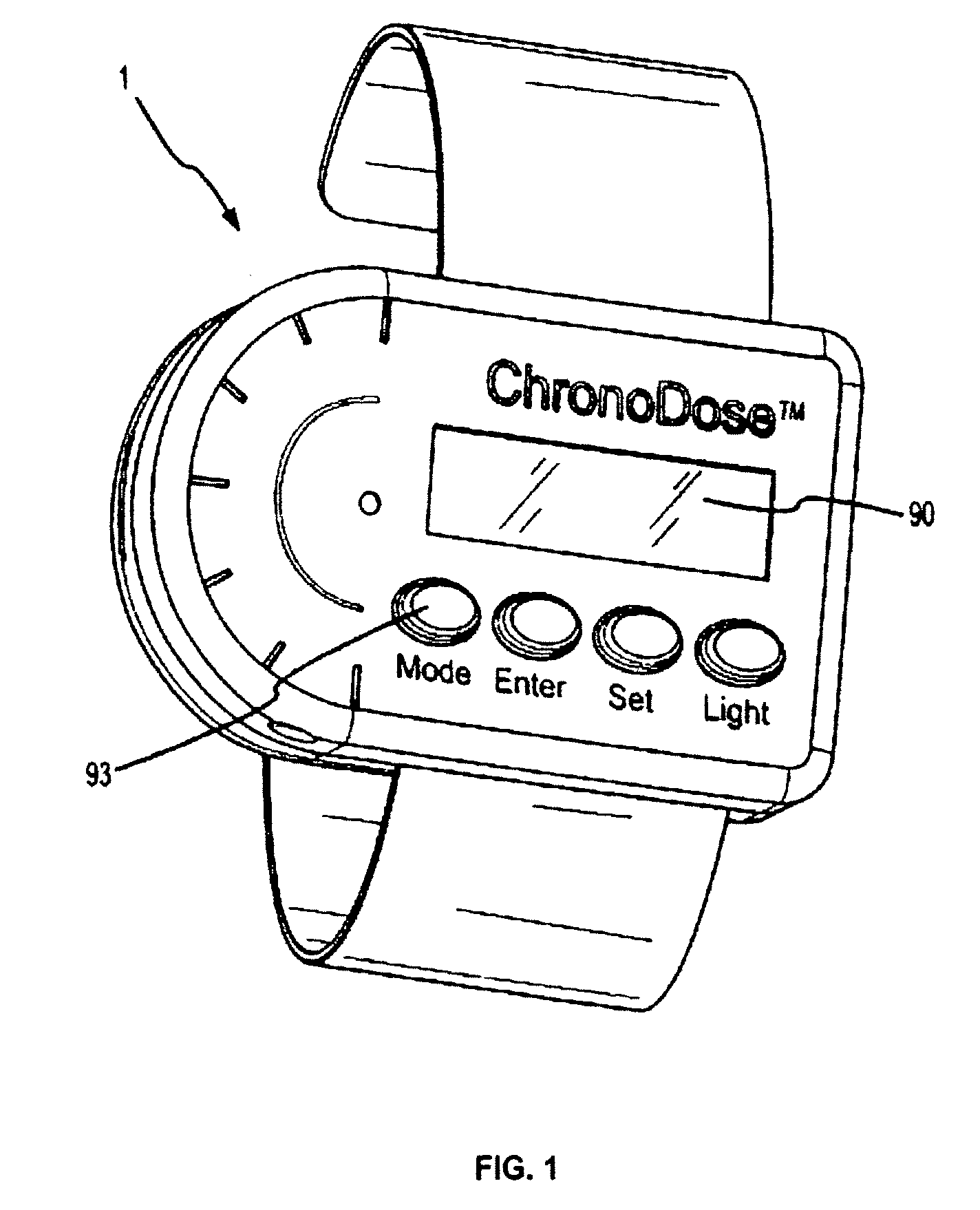
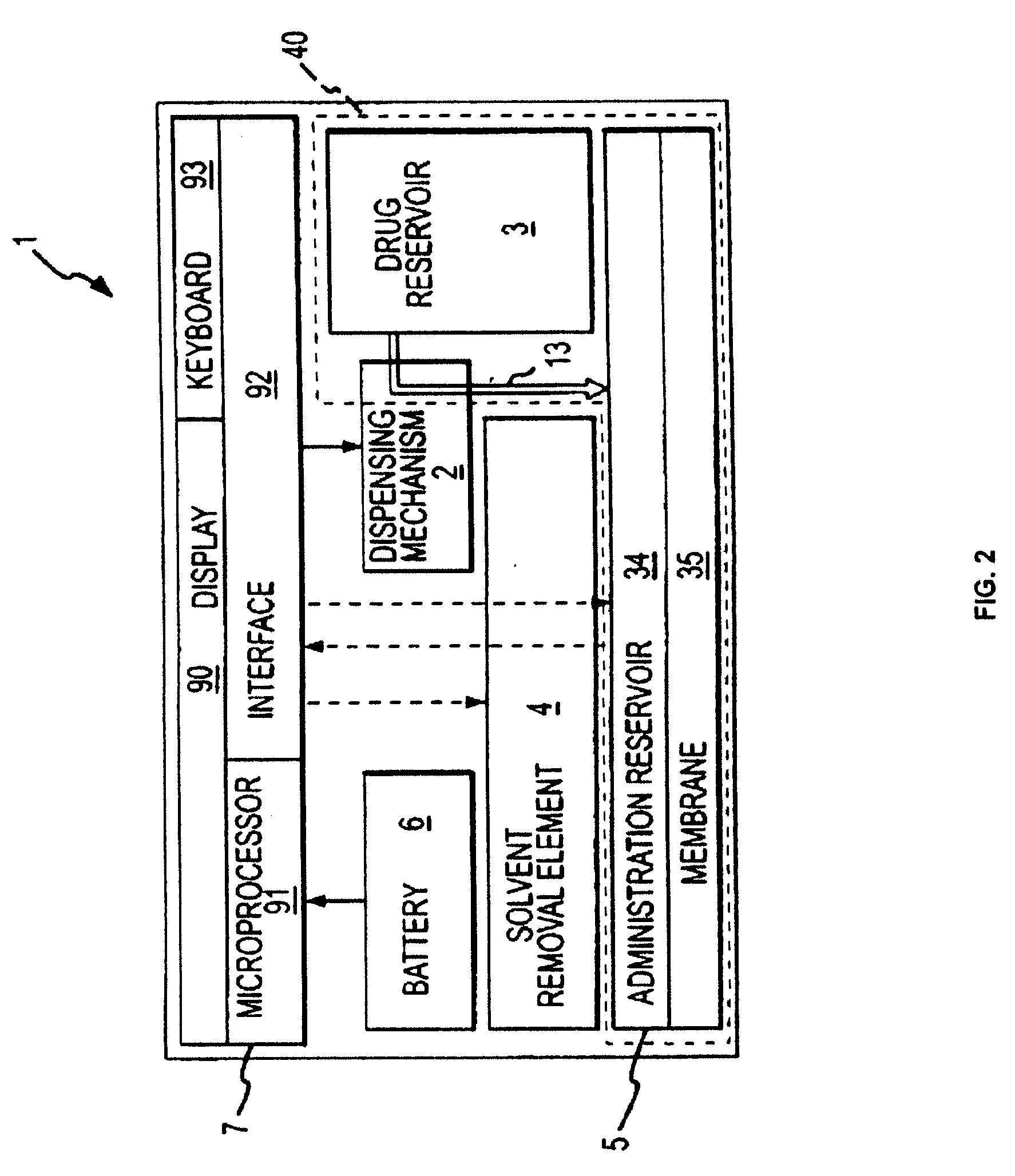
Biosynchronous transdermal drug delivery for longevity, anti-aging, fatigue management, obesity, weight loss, weight management, delivery of nutraceuticals, and the treatment of hyperglycemia, alzheimer's disease, sleep disorders, parkinson's disease, aids, epilepsy, attention deficit disorder, nicotine addiction, cancer, headache and pain control, asthma, angina, hypertension, depression, cold, flu and the like
ActiveUS20080220092A1Improve performanceReduce the amount requiredHeavy metal active ingredientsBiocidePhytochemicalAntioxidant
Systems and methods for longevity, anti-aging, fatigue management, obesity, weight loss, weight management, delivery of nutraceuticals, and treating hyperglycemia, Alzheimer's disease, sleep disorders, Parkinson's disease, Attention Deficit Disorder and nicotine addiction involve synchronizing and tailoring the administration of nutraceuticals, medications and other substances (for example, stimulants) in accordance with the body's natural circadian rhythms, meal times and other factors. Improved control of blood glucose levels, extended alertness, and weight control, and counteracting of disease symptoms when they are at their worst are possible. An automated, pre-programmable transdermal administration system is used to provide pulsed doses of medications, pharmaceuticals, hormones, neuropeptides, anorexigens, pro-drugs, stimulants, plant extracts, botanicals, nutraceuticals, cosmeceuticals, phytochemicals, phytonutrients, enzymes, antioxidants, essential oils, fatty acids, minerals, vitamins, amino acids, coenzymes, or other physiological active ingredient or precursor. The system can utilize a pump, pressurized reservoir, a system for removing depleted carrier solution, or other modulated dispensing actuator, in conjunction with porous membranes or micro-fabricated structures.
Owner:MORNINGSIDE VENTURE INVESTMENTS
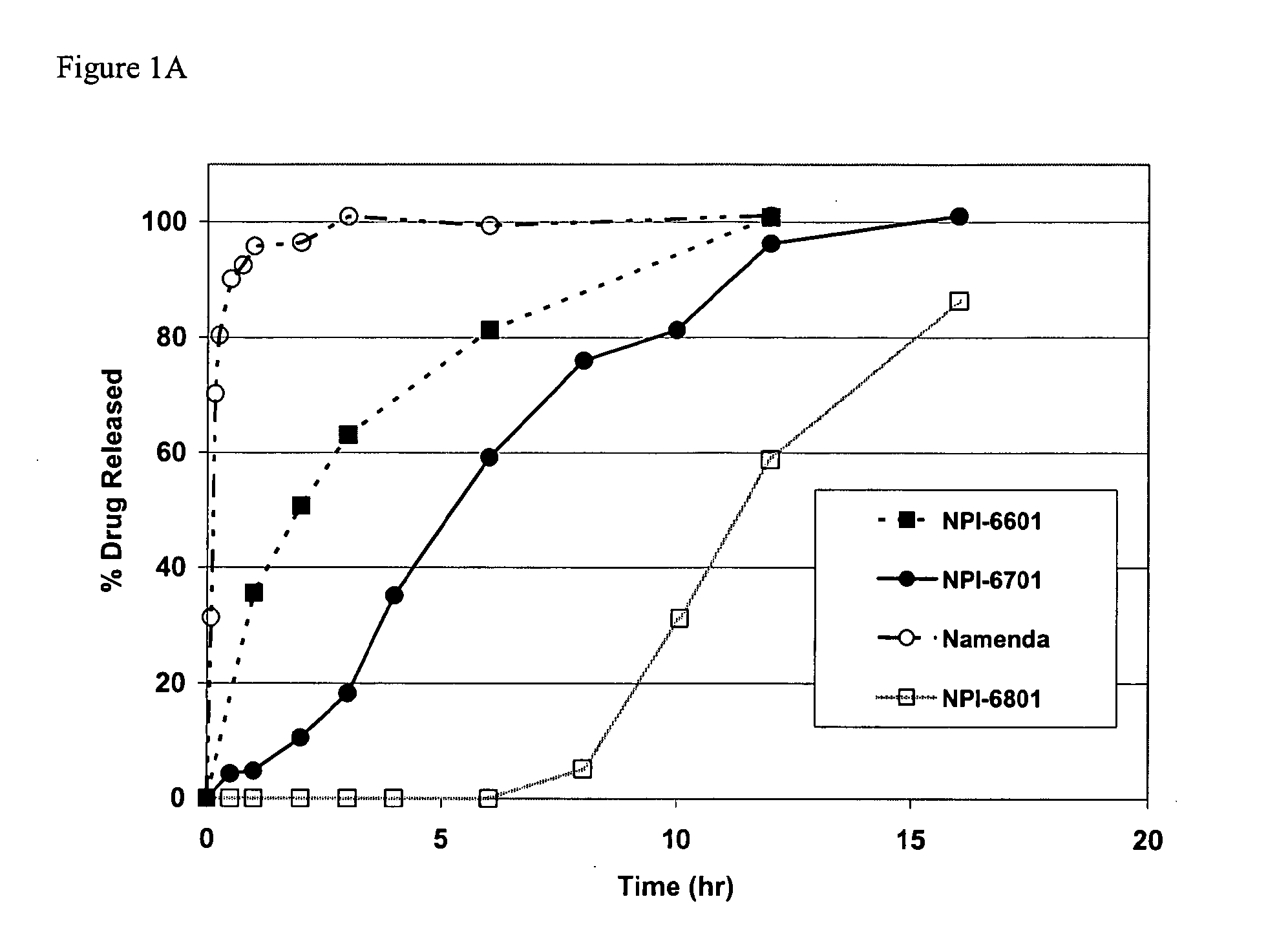
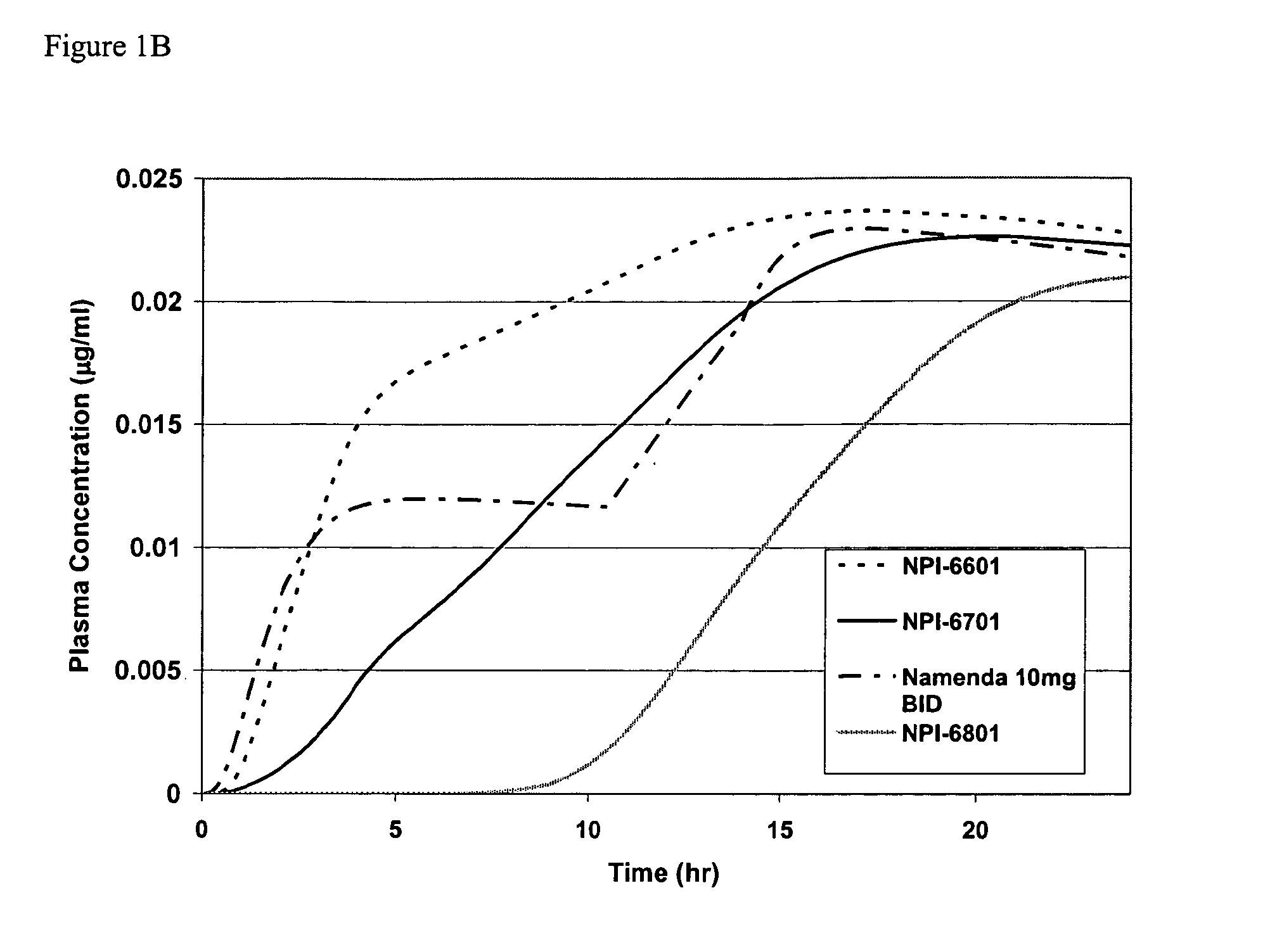
Methods and compositions for the treatment of CNS-related conditions
ActiveUS20060252788A1Reduce and even minimize variationMinimizing CratioBiocideNervous disorderDiseasePsychiatry
The present invention provides novel methods and compositions for the treatment and prevention of CNS-related conditions. One of the CNS-related conditions treated by the methods and compositions of the invention is Alzheimer's disease.
Owner:ADAMAS PHARMA LLC
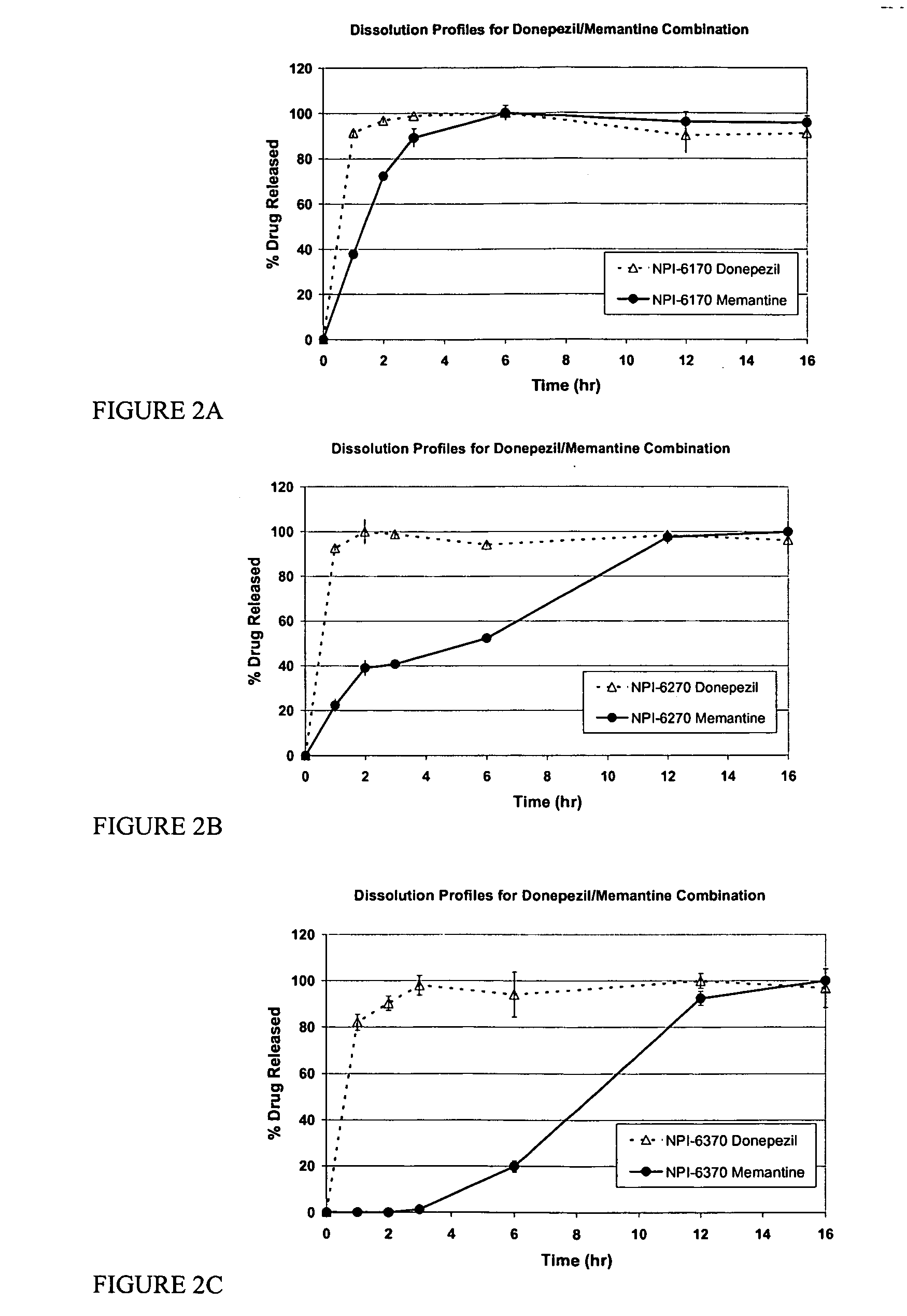
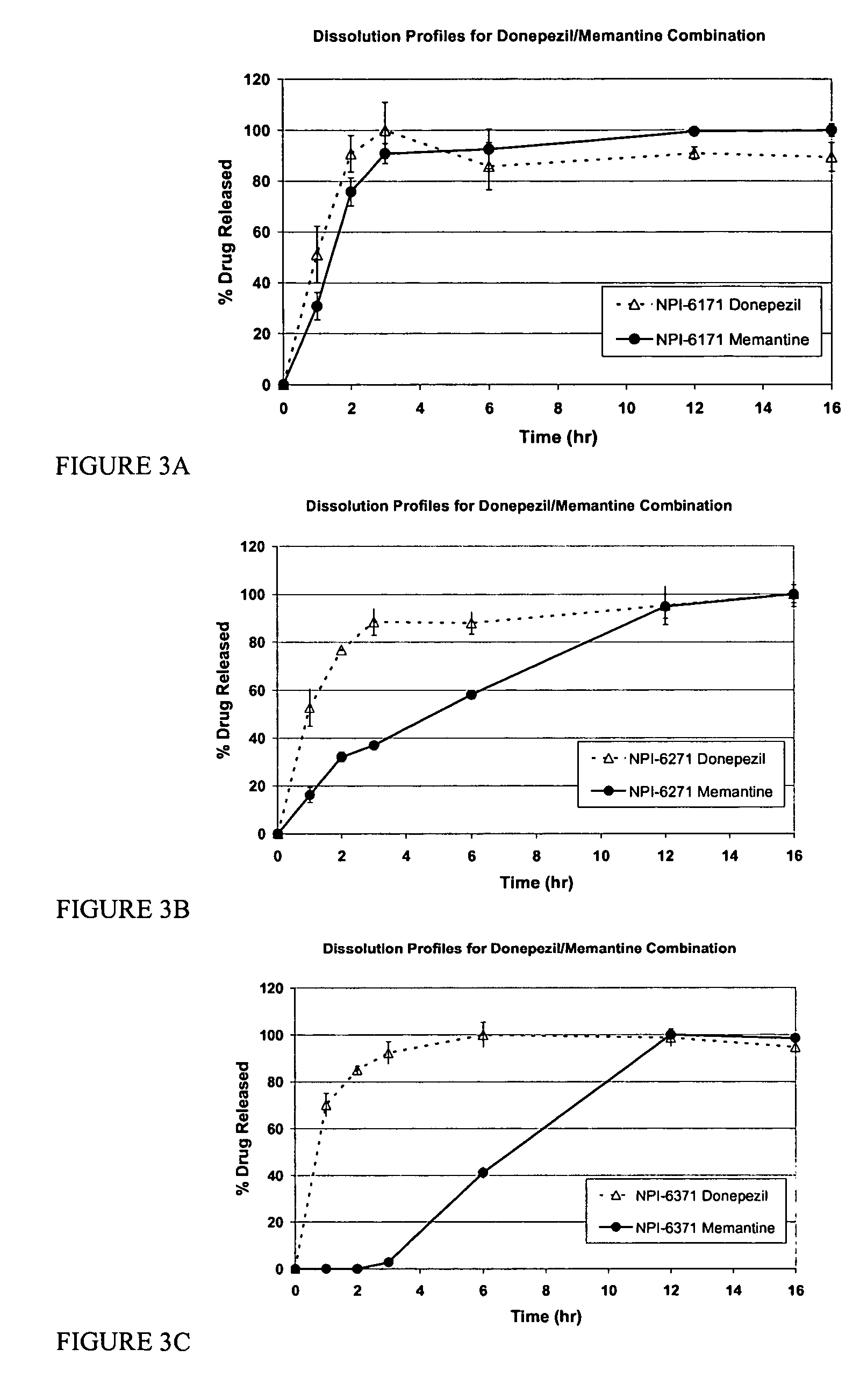
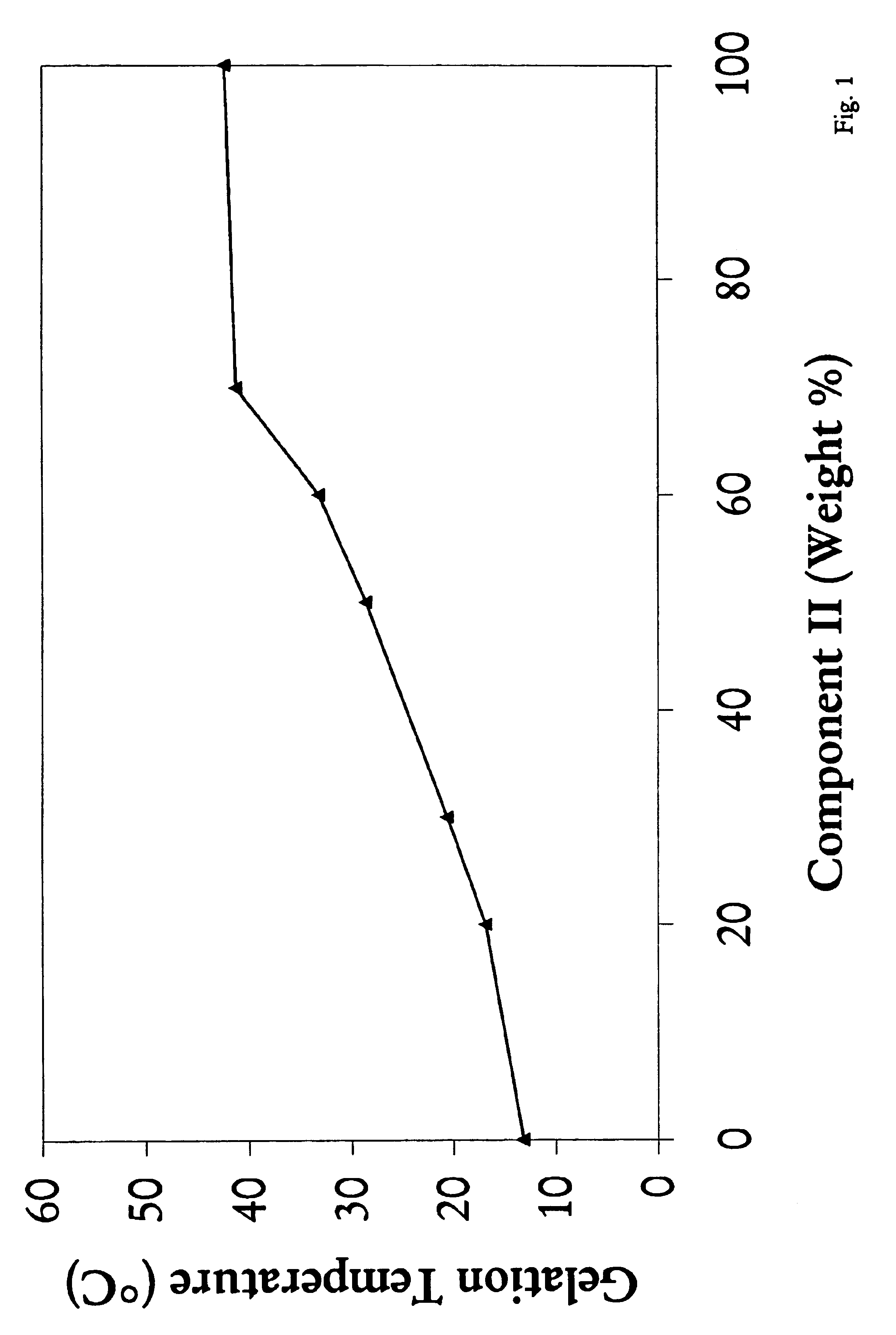
Mixtures of various triblock polyester polyethylene glycol copolymers having improved gel properties
InactiveUS7018645B1Good curative effectMinimize side effectsPowder deliveryPeptide/protein ingredientsPolymer scienceErosion rate
A water soluble, biodegradable reverse thermal gelation system comprising a mixture of at least two types of tri-block copolymer components is disclosed. The tri-block copolymer components are made of a hydrophobic biodegradable polyester A-polymer block and a hydrophilic polyethylene glycol B-polymer block. The drug release and gel matrix erosion rates of the biodegradable reverse thermal gelation system may be modulated by various parameters such as the hydrophobic / hydrophilic component contents, polymer block concentrations, molecular weights and gelation temperatures, and weight ratios of the tri-block copolymer components in the mixture.
Owner:BTG INT LTD
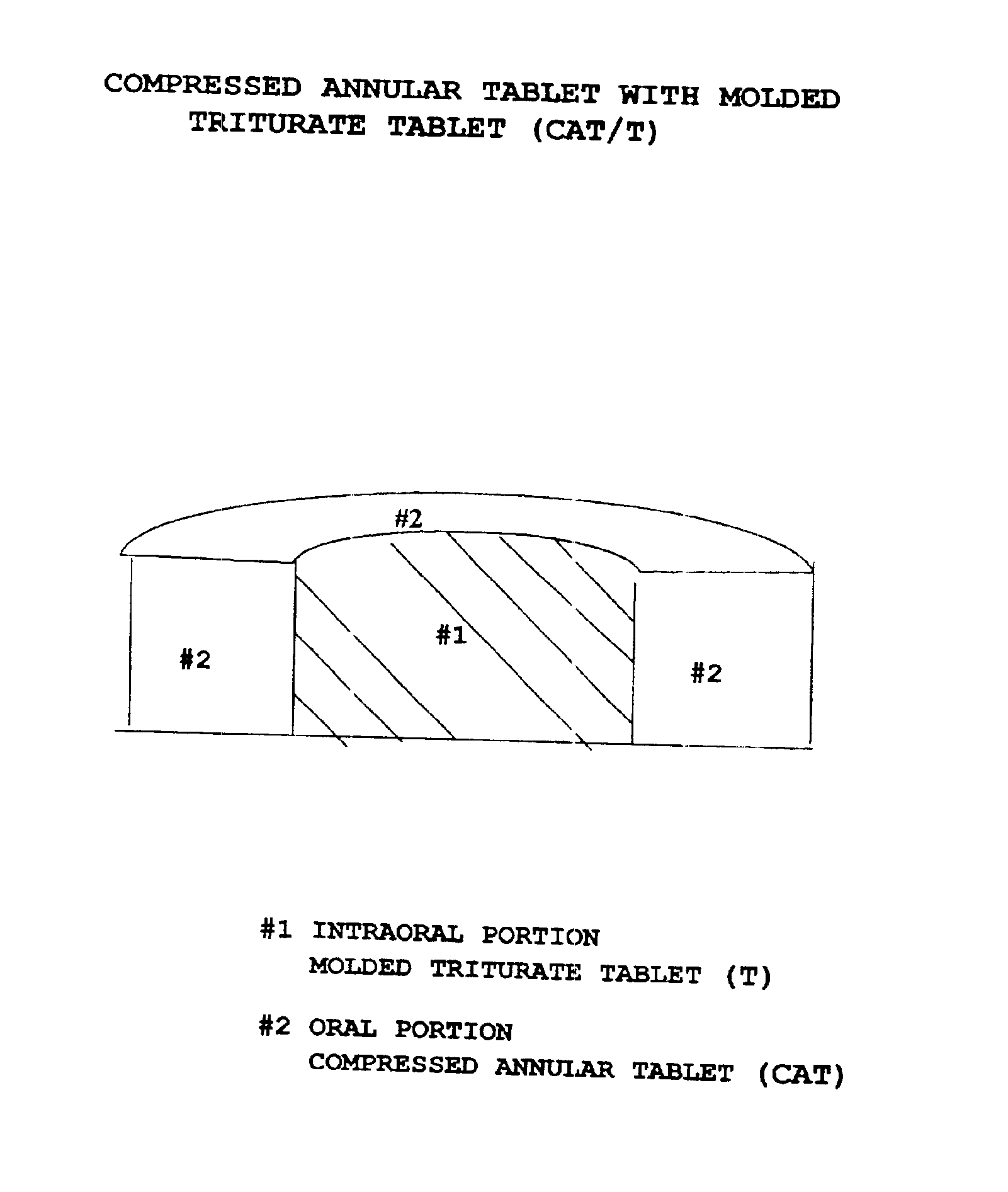
Pharmaceutical composition for compressed annular tablet with molded triturate tablet for both intraoral and oral administration
InactiveUS6863901B2Easily administrable to a patientMaximize the effect of treatmentNervous disorderSkeletal disorderOral medicationPharmaceutical drug
New pharmaceutical compositions in unit dosage form are disclosed for both intraoral and oral administration to a patient, said unit dosage form configured to be placed intraorally of said patient, which comprises:(a) as a first portion, at least one discrete molded triturate tablet comprising a therapeutically effective amount of at least one pharmaceutically active ingredient capable of intraoral administration; and(b) as a second portion located around the said first portion, a therapeutically effective amount of at least one pharmaceutically active ingredient capable of oral administration and which is releasable and orally ingestible by the patient after the molded triturate tablet has disintegrated or has dissolved intraorally.
Owner:HIRSH JANE +1
Methods and systems for glucose regulation
ActiveUS20090254143A1Improve blood sugar controlStimulate insulin releaseSpinal electrodesIntravenous devicesBlocking nerveSplanchnic
Owner:RESHAPE LIFESCIENCES INC
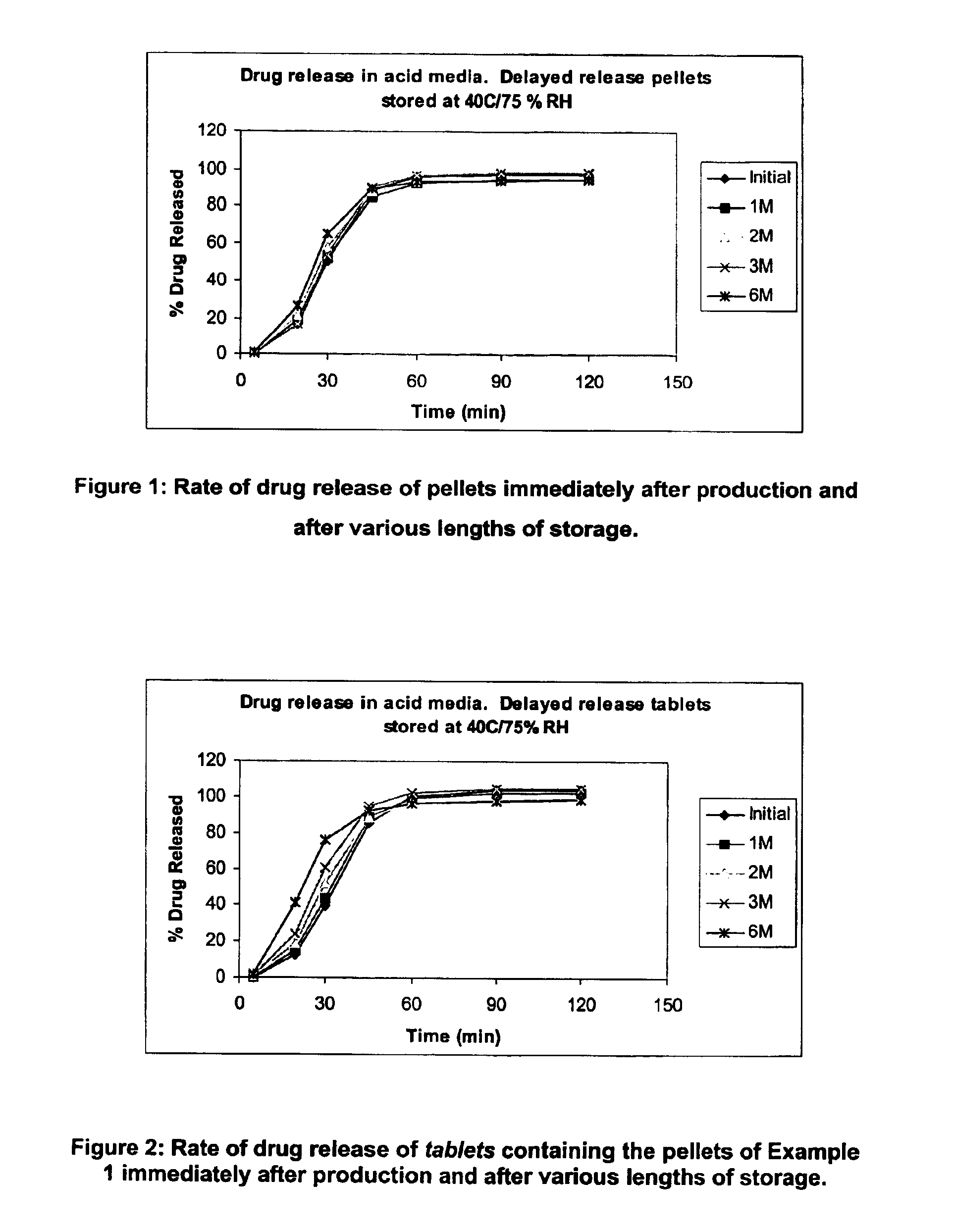
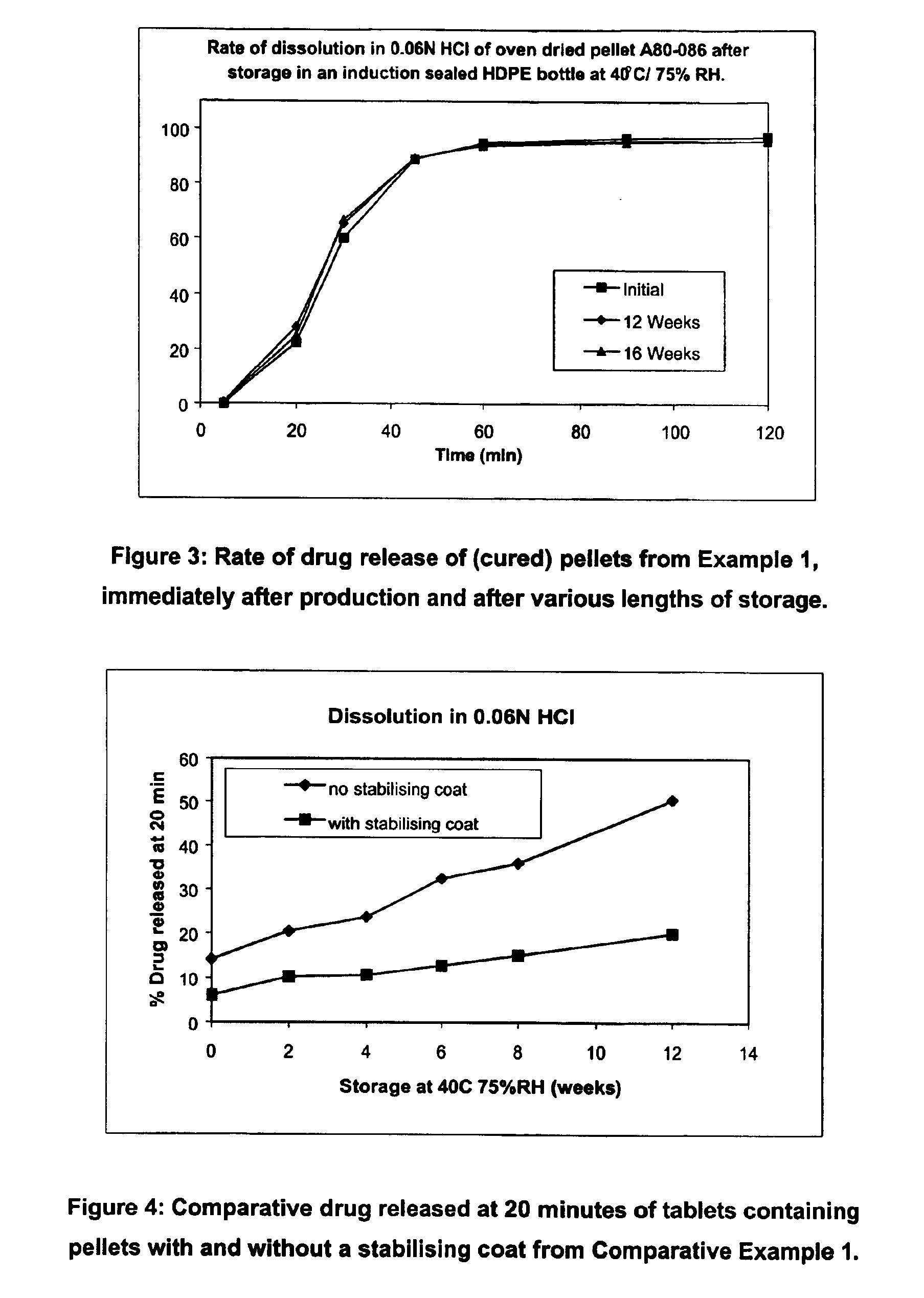
Modified release coated drug preparation
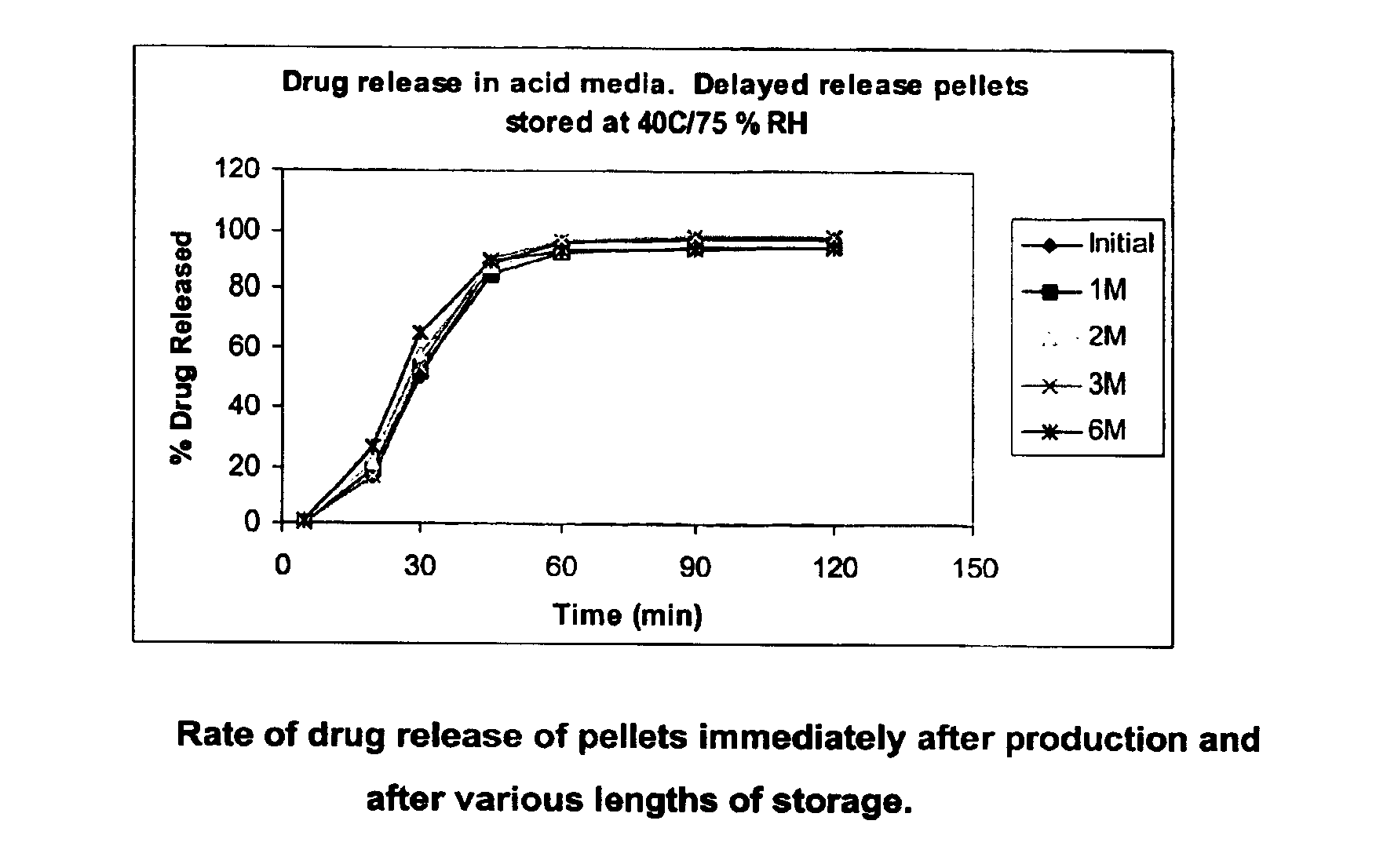
InactiveUS6958161B2Extension of timeImprove trustBiocideTetracycline active ingredientsDissolutionBULK ACTIVE INGREDIENT
A modified release preparation having one or more coated core elements, each core element including an active ingredient and having a modified release coating, wherein a stabilising coat is provided between each core element and its modified release coating so that, upon in vitro dissolution testing, the amount of active ingredient released at any time on a post-storage dissolution profile is within 40 percentage points of the amount of active ingredient released at any time on a pre-storage dissolution profile.
Owner:MAYNE PHARMA INT
Methods and compositions for the treatment of epilepsy, seizure disorders, and other CNS disorders
InactiveUS20050245460A1Benefit maximizationReducing unwanted side effectBiocideNervous disorderDiseaseEpileptic disorder
Owner:NEUROMOLECULAR INC
Medical devices employing novel polymers
InactiveUS20060188546A1Faster and more targeted reliefMinimize side effectsSurgeryCatheterMedicineActive agent
Medical devices with at least one surface comprising a polymer or polymers on the surface are provided. The polymer or polymers are capable of breaking down (e.g., including, but not limited to, hydrolyzing) in the physiologic milieu to form an active agent or agents under physiologic conditions, and can contain other active agents dispersed within or appended to the polymer matrix. Methods of delivering an active agent to an interior surface of a vein or artery are also provided.
Owner:XENOGENICS
Method and synergistic composition for treating attention deficit/hyperactivity disorder
InactiveUS6541043B2Minimize side effectsBiocideHydroxy compound active ingredientsBeta-CaroteneBetaine
A composition and method for treating Attention Deficit / Hyperactivity Disorder (ADHD) is provided which can be used both with and without ethical drugs now used to treat ADHD. The composition contains dimethylaminoethanol (DMAE), omega 3-fatty acids, betaine, oligomeric proanthocyanidins (OPC), folic acid, vitamins C, E, B12, B6, B5 and beta-carotene and minerals (calcium, magnesium, zinc and selenium). Ethical drugs such as amphetamines, methylphenidate HCl and pemoline are known to control ADHD, but each has significant side effects when used in their therapeutic dose. When combining the composition with such ethical drugs, the amount of the ethical drug can be lowered below a level which causes undesirable side effects which is an important feature. Preferred compositions contain one or more of lecithin, choline, 5-hydroxytryptophan, tyrosine, Reishi Extract, Kava Extract, Gingko, Ginseng and St. John's Wort.
Owner:PHILIP C LANG
Methods, systems and devices for neuromodulating spinal anatomy
ActiveUS20100292769A1Minimizing complicationMinimize side effectsSpinal electrodesExternal electrodesSide effectSpinal anatomy
Devices, systems and methods for treating pain or other conditions while minimizing possible complications and side effects. Treatment typically includes electrical stimulation and / or delivery of pharmacological or other agents with the use of a lead or catheter. The devices, systems and methods provide improved anchoring which reduces migration of the lead yet allows for easy repositioning or removal of the lead if desired. The devices, systems and methods also provide for simultaneous treatment of multiple targeted anatomies. This shortens procedure time and allows for less access points, such as needle sticks to the epidural space, which in turn reduces complications, such as cerebral spinal fluid leaks, patient soreness and recovery time. Other possible complications related to the placement of multiple devices are also reduced.
Owner:TC1 LLC
Treatment of refractory human tumors with epidermal growth factor receptor and HER1 mitogenic ligand (EGFRML) antagonists
InactiveUS20030202973A1Reduce HAMA responseMinimize side effectsImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsHuman tumorHuman epidermal growth factor receptor
A method of inhibiting the growth of refractory tumors that are stimulated by mitogenic ligands of epidermal growth factor receptor in human patients, comprising treating the human patients with an effective amount of a mitogenic ligand antagonist.
Owner:DR GEORGE PIECZENIK
Synergistic use of thiazolidinediones with glucagon-like peptide-1 and agonists thereof to treat metabolic instability associated with non-insulin dependent diabetes
InactiveUS7223728B2Lower blood sugar levelsIncrease secretionPeptide/protein ingredientsMetabolism disorderInsulin dependent diabetesSide effect
Thiazolidinedione (TZD) and its pharmacologically active derivatives can be used, in combination with agonists of glucagon-like peptide-1 (GLP-1), to treat non-insulin dependent diabetes mellitus, optionally with other therapies, by improving glycemic control while minimizing side effects, such as heart hypertrophy and elevated fed-state plasma glucose, which are associate with both TZD and GLP-1 monotherapies. Thus, the co-administration of TZD and GLP-1 helps regulate glucose homeostasis in Type II diabetic patients.
Owner:ELI LILLY & CO
Dry Powder Aerosolized Inhaler
InactiveUS20090025720A1Increase inhalation rateEffective wayRespiratorsLiquid surface applicatorsSide effectInhalation
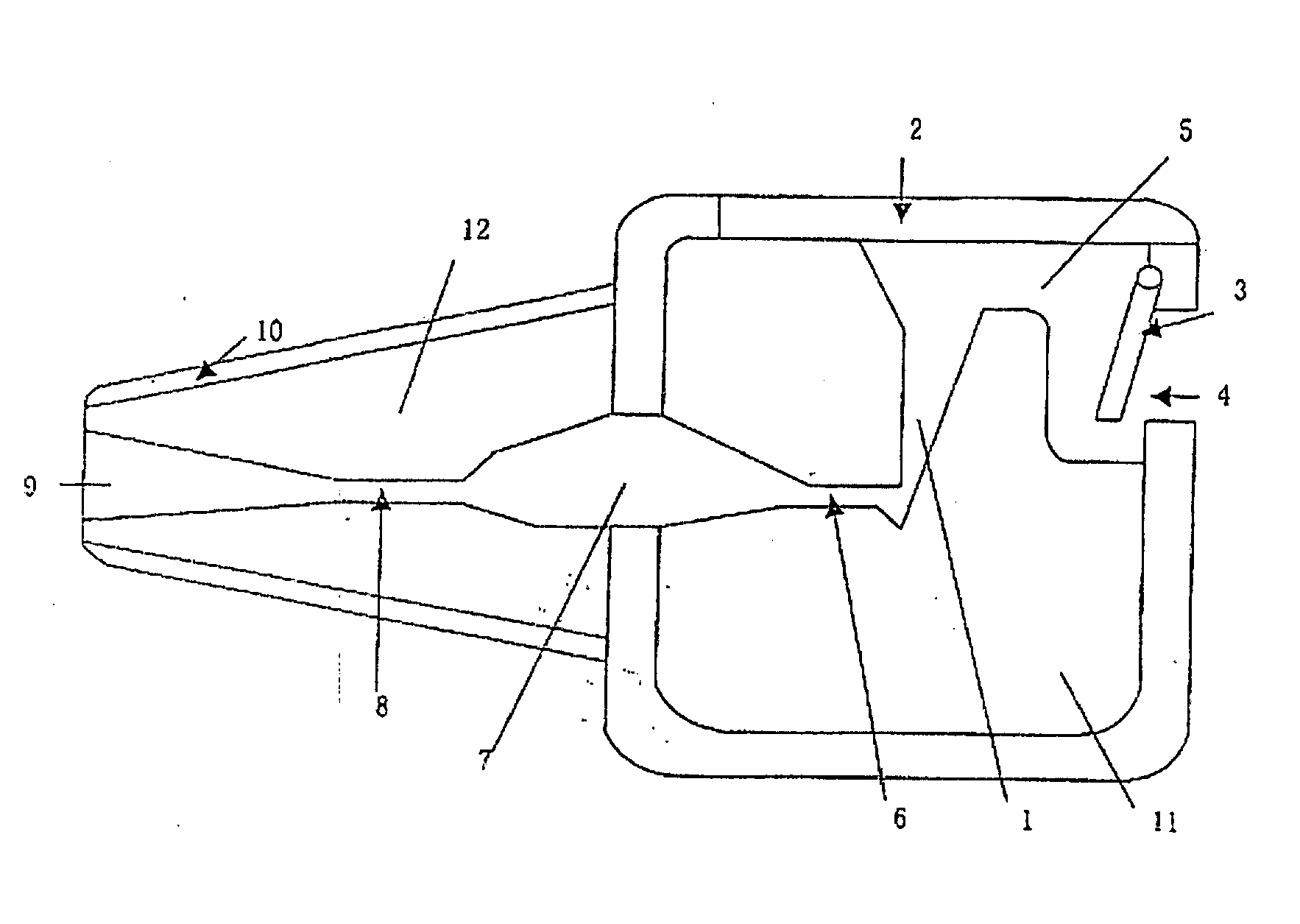
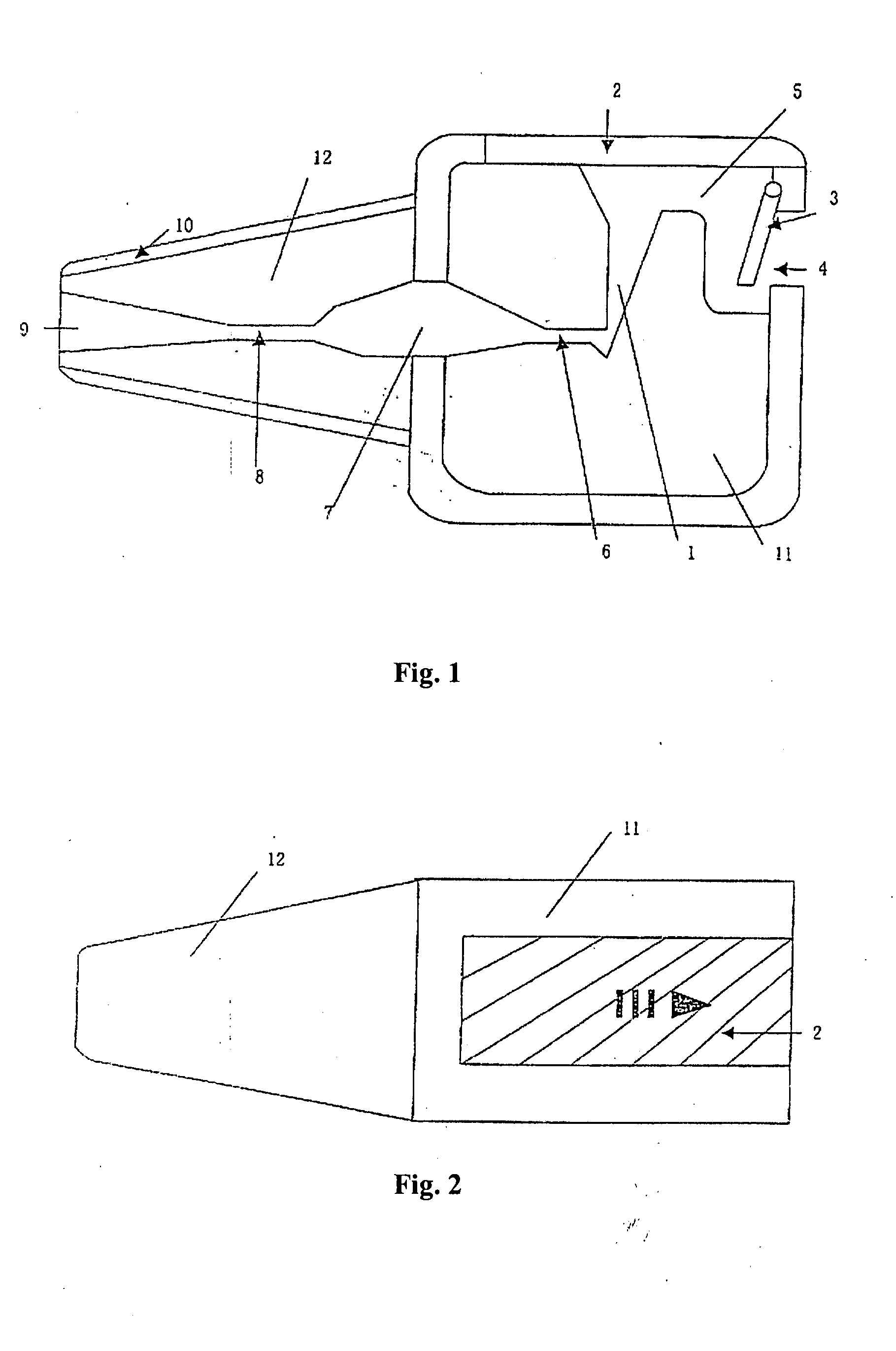
This invention provides an aerosolized inhaler which enables the drug powder to aerosolize within the inhaler by breathing in the air, comprising the main body (11) and the mouthpiece connector (12) connected thereupon, wherein the inhaler the main body (11) has drug holding opening (1), which connects with the vortex passage, and the vortex passage connects with the mouthpiece (9) on the mouthpiece connector (12). It has many obvious advantages as increasing the inhalation rate, pushing more drug granule to reach to the lower respiratory tract and even into the alveolus; and meanwhile, keeping the drug from being blown out of the inhaler when the users breathes out, preventing from cross inflection and minimizing the side effect of the drug to the whole body, and obviously lightening the burden on the users.
Owner:CHEN QINGTANG +1
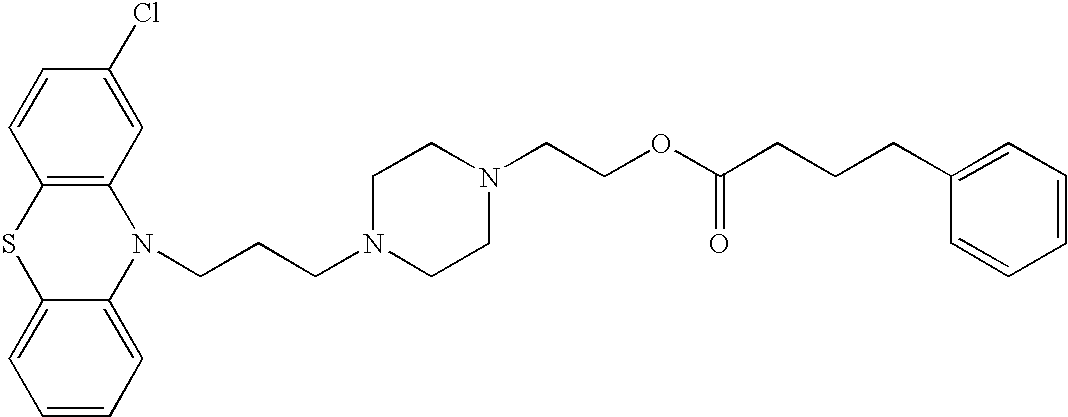
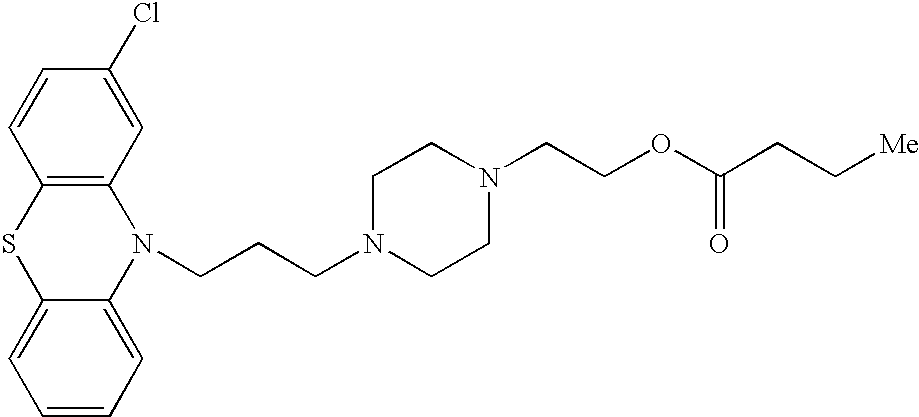
Conjugated psychotropic drugs and uses thereof
Owner:BAR ILAN UNIV +1
Glycemic control for prediabetes and/or diabetes Type II using docosahexaenoic acid
InactiveUS20040092590A1Improve blood sugar controlReduce drug side effectsBiocidePeptide/protein ingredientsDocosahexaenoic acidPrediabetes
This invention is directed to methods of treating patients with metabolic syndrome, prediabetes and / or Type II diabetes mellitus by administering docosahexaenoic acid (DHA) alone or in combination with diabetes-related medications.
Owner:MARTEK BIOSCIENCES CORP
Compositions and methods for prevention and treatment of amyloid-beta peptide-related disorders
ActiveUS20040028673A1Modulate it functionImprove effectivenessOrganic active ingredientsNervous disorderDiseasePharmaceutical drug
The present invention provides methods and compositions for modulating levels of amyloid-beta peptide (Abeta) exhibited by cells or tissues. The invention also provides pharmaceutical compositions and methods of screening for compounds that modulate Abeta levels. The invention also provides modulation of Abeta levels via selective modulation (e.g., inhibition) of ATP-dependent gamma-secretase activity. The invention also provides methods of preventing, treating or ameliorating the symptoms of a disorder, including but not limited to an Abeta-related disorder, by administering a modulator of gamma-secretase, including, but not limited to, a selective inhibitor of ATP-dependent gamma-secretase activity or an agent that decreases the formation of active (or optimally active) gamma-secretase. The invention also provides the use of inhibitors of ATP-dependent gamma-secretase activity to prevent, treat or ameliorate the symptoms of Alzheimer's disease.
Owner:THE ROCKEFELLER UNIV
Medical devices employing novel polymers
Medical devices with at least one surface comprising a polymer or polymers on the surface are provided. The polymer or polymers are capable of breaking down (e.g., including, but not limited to, hydrolyzing) in the physiologic milieu to form an active agent or agents under physiologic conditions, and can contain other active agents dispersed within or appended to the polymer matrix. Methods of delivering an active agent to an interior surface of a vein or artery are also provided.
Owner:XENOGENICS
Methods and Compositions for the Treatment of CNS-Related Conditions
ActiveUS20100311697A1Benefit maximizationEliminate side effectsBiocideNervous disorderDiseaseMedicine
Owner:ADAMAS PHARMA LLC
Bis(thio-hydrazide amides) for treatment of hyperplasia
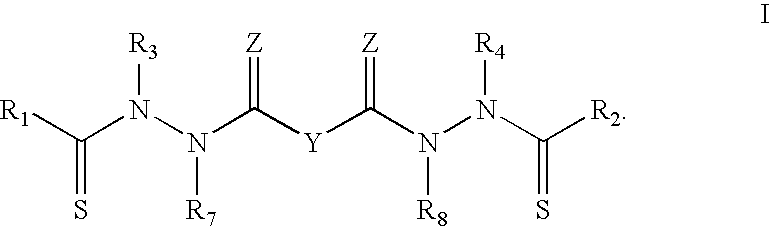
InactiveUS20060142393A1Good treatment effectMinimize side effectsBiocideAmide active ingredientsThio-Percent Diameter Stenosis
Methods and medical devices for treating a proliferative disorder in a subject, e.g., restenosis in a blood vessel that has been implanted with a stent, employ a bis(thio-hydrazide amide) represented by Structural Formula I or a pharmaceutically acceptable salt or solvate thereof. Y is a covalent bond or an optionally substituted straight chained hydrocarbyl group, or, Y, taken together with both >C=Z groups to which it is bonded, is an optionally substituted aromatic group. R1-R4 are independently —H, an optionally substituted aliphatic group, an optionally substituted aryl group, or R1 and R3 taken together with the carbon and nitrogen atoms to which they are bonded, and / or R2 and R4 taken together with the carbon and nitrogen atoms to which they are bonded, form a non-aromatic heterocyclic ring optionally fused to an aromatic ring. R7-R8 are independently —H, an optionally substituted aliphatic group, or an optionally substituted aryl group. Z is O or S.
Owner:SYNTA PHARMA CORP
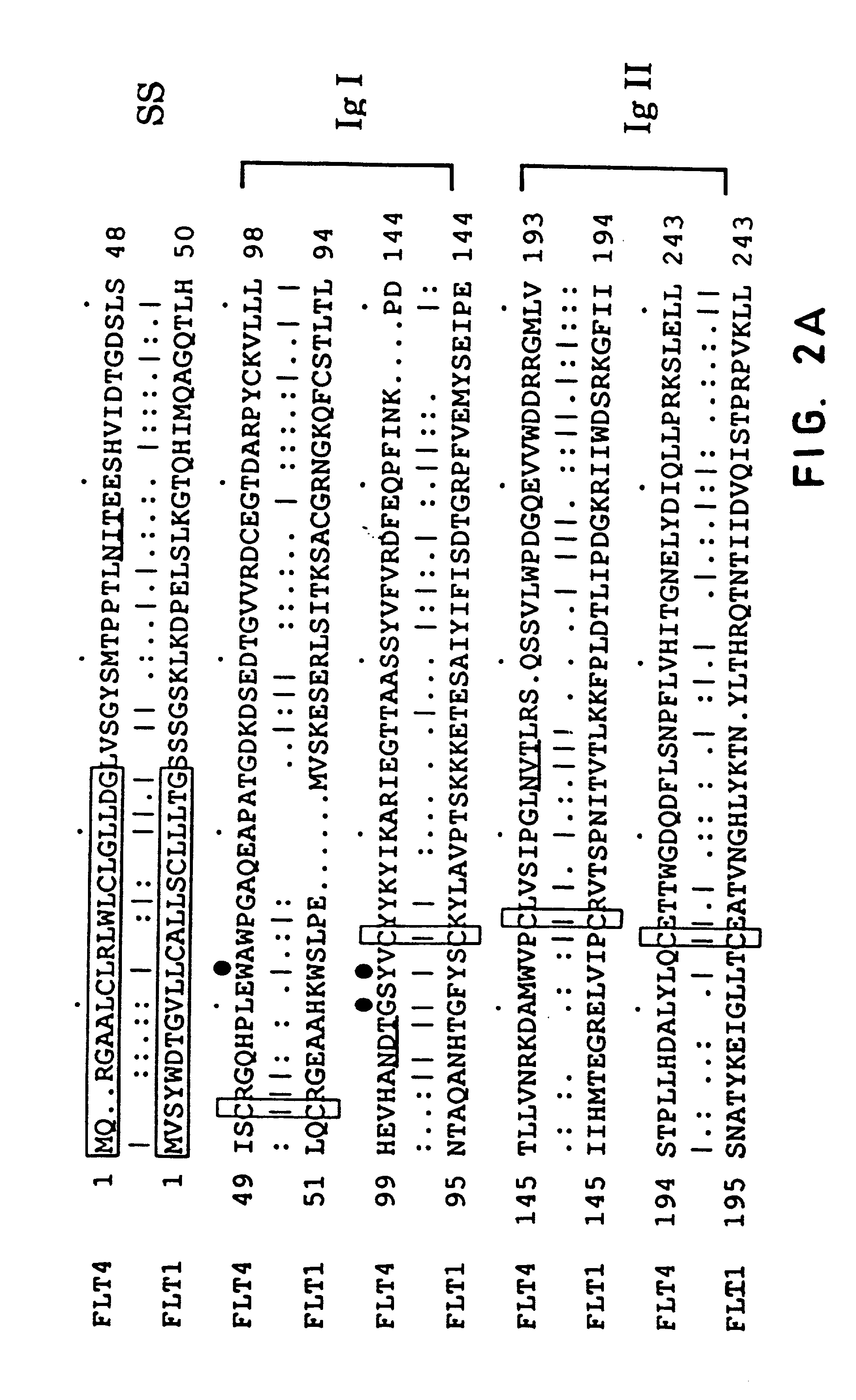
FLT4 (VEGFR-3) as a target for tumor imaging and anti-tumor therapy
InactiveUS7034105B2Minimize side effectsQuick distinctionPeptide/protein ingredientsAntibody mimetics/scaffoldsReceptor tyrosine kinaseWilms' tumor
Owner:VEGENICS PTY LTD
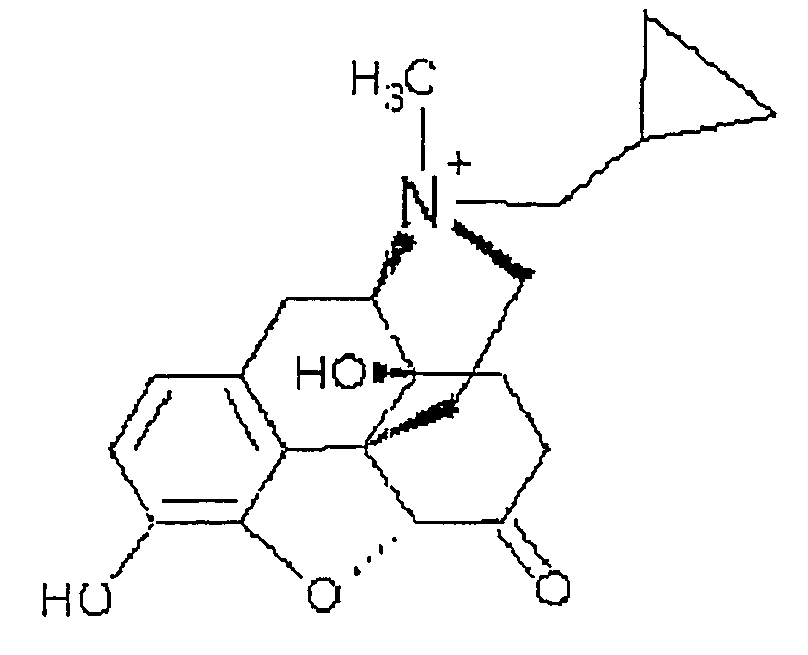
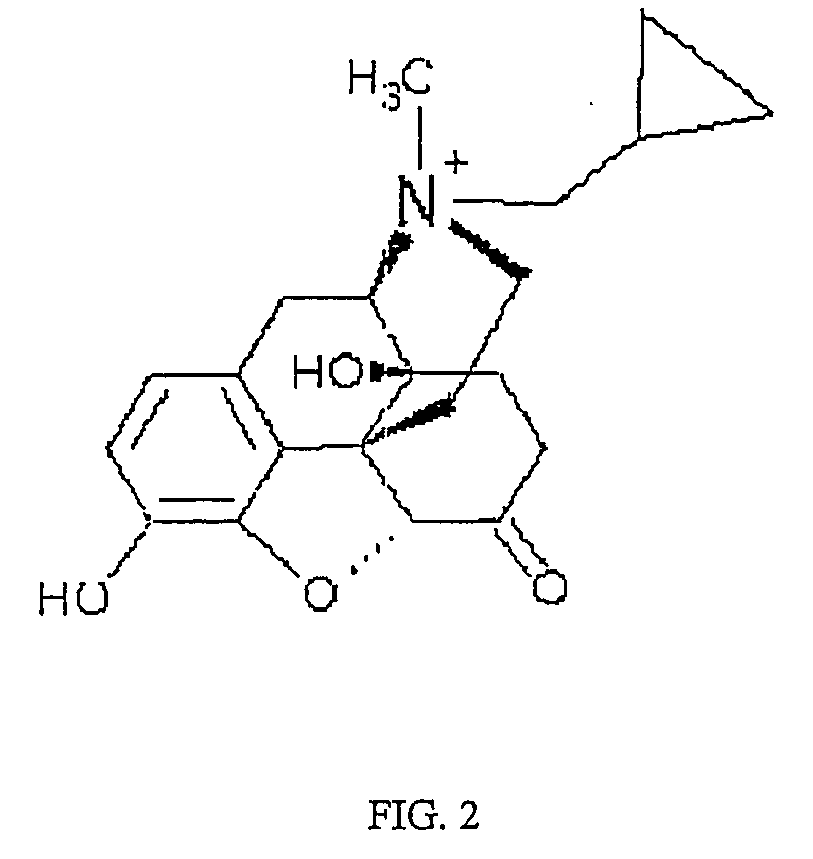
Use of methylnaltrexone in treating gastrointestinal dysfunction in equines
InactiveUS20050011468A1Relieving inhibition of gastrointestinal motilityMaintaining pain-reducing effectOrganic active ingredientsOther apparatusMotilityEquine Species
Systems and methods are described for using methylnaltrexone to treat or prevent inhibition of gastrointestinal motility in equines. A method for preventing or treating opioid-induced and non-opioid-induced gastrointestinal dysfunction includes administering a quaternary derivative of noroxymorphone, preferably methylnaltrexone, to an equine before or after the onset of the gastrointestinal dysfunction.
Owner:PROGENICS PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com