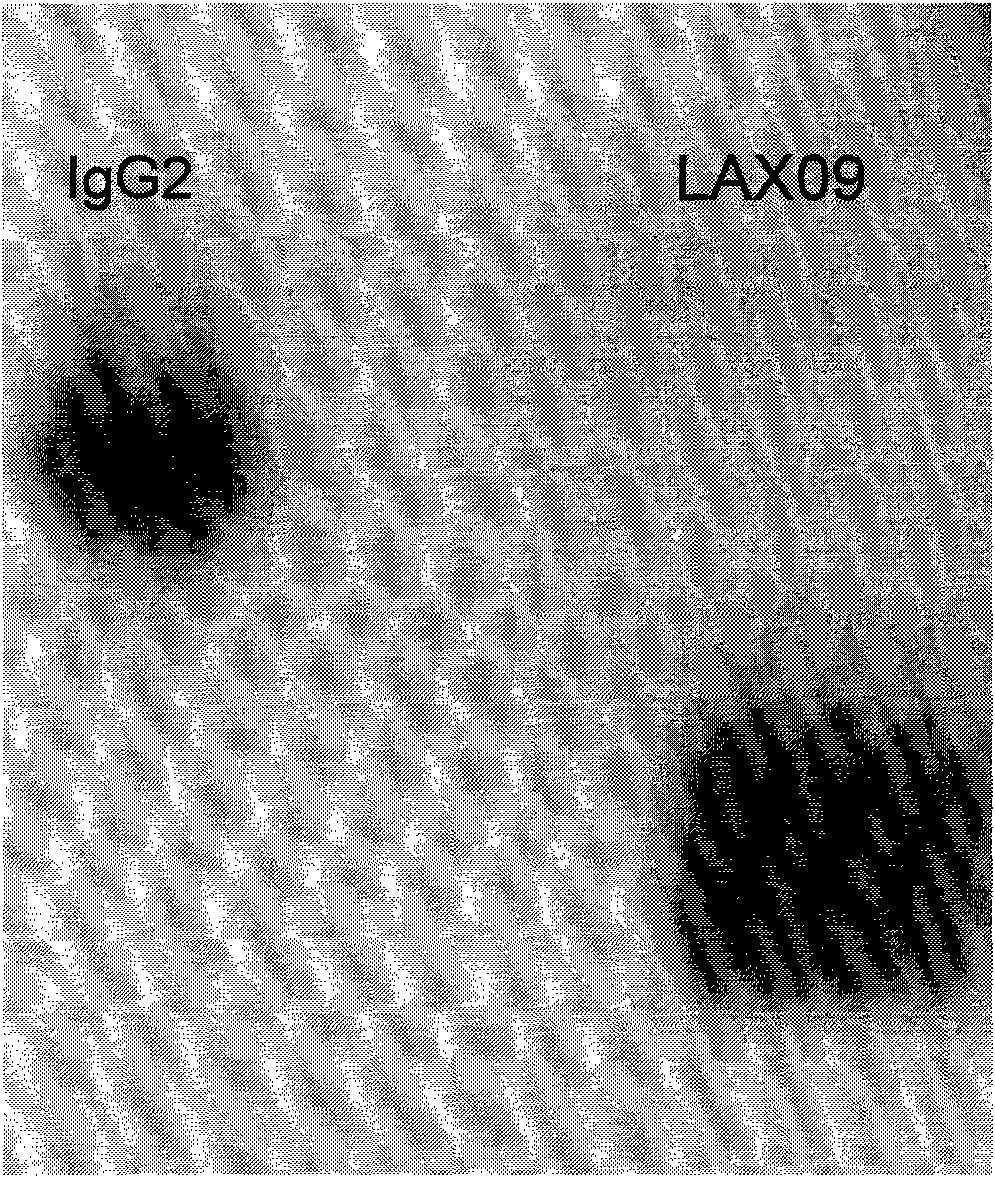Patents
Literature
884 results about "Motility" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
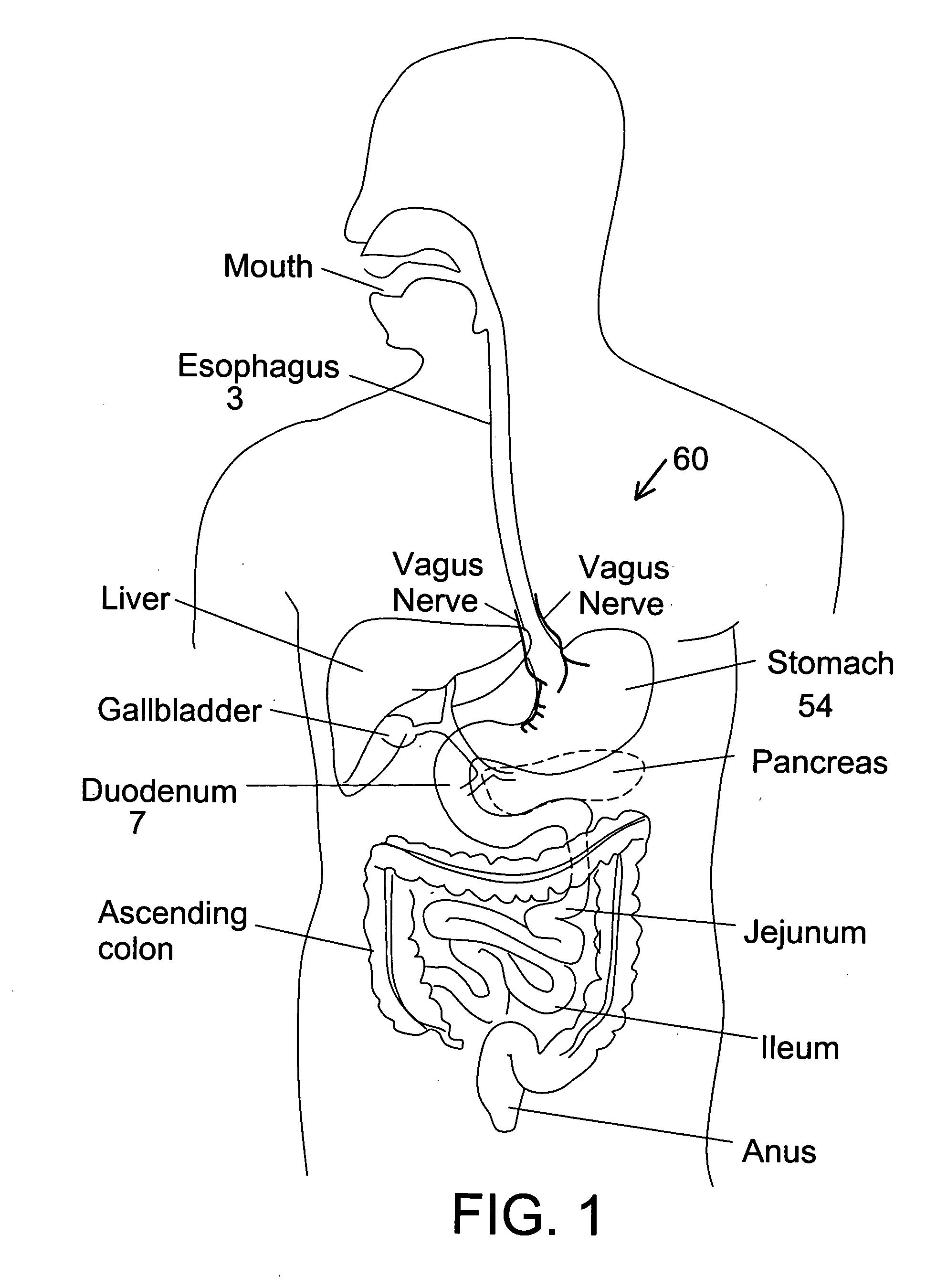
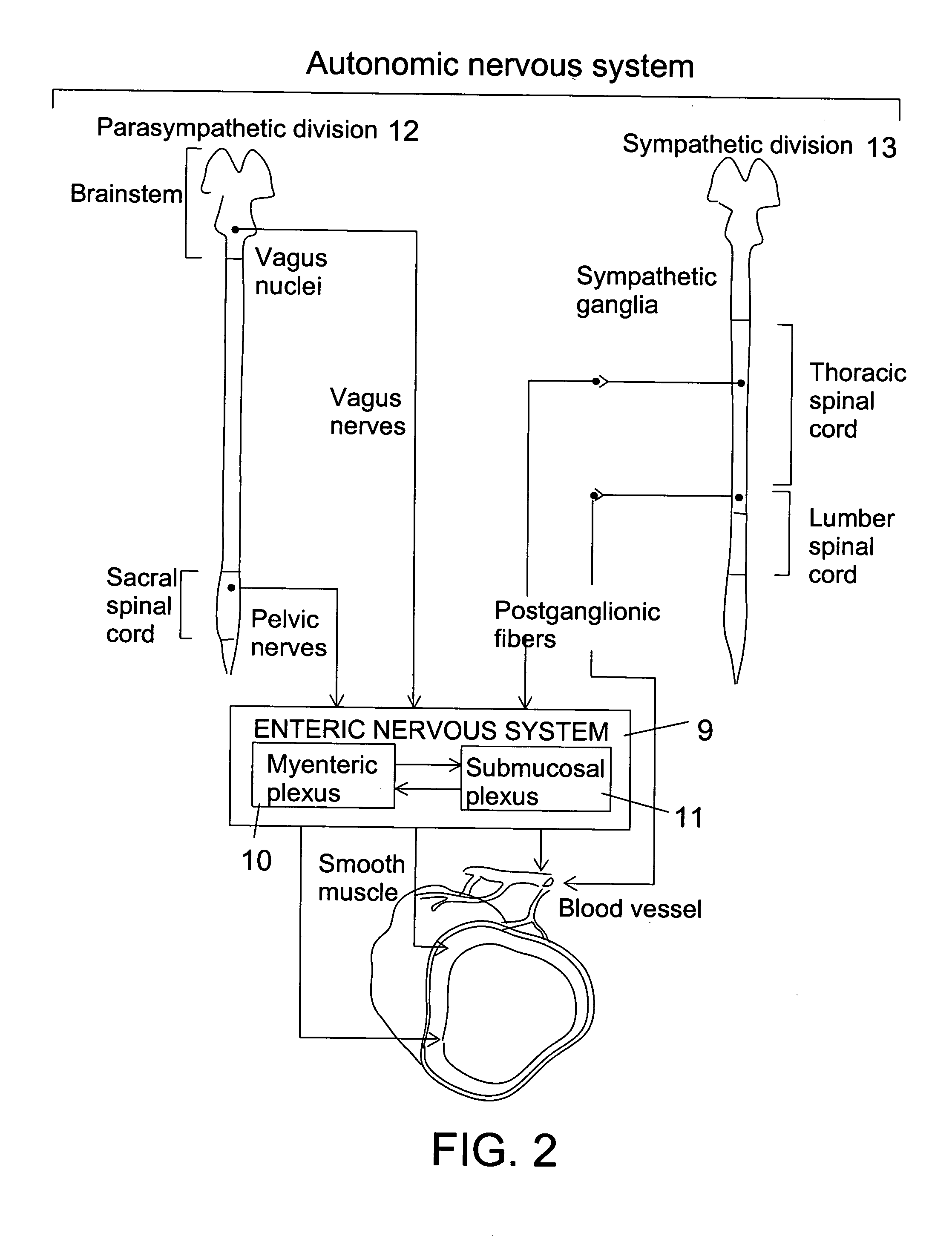
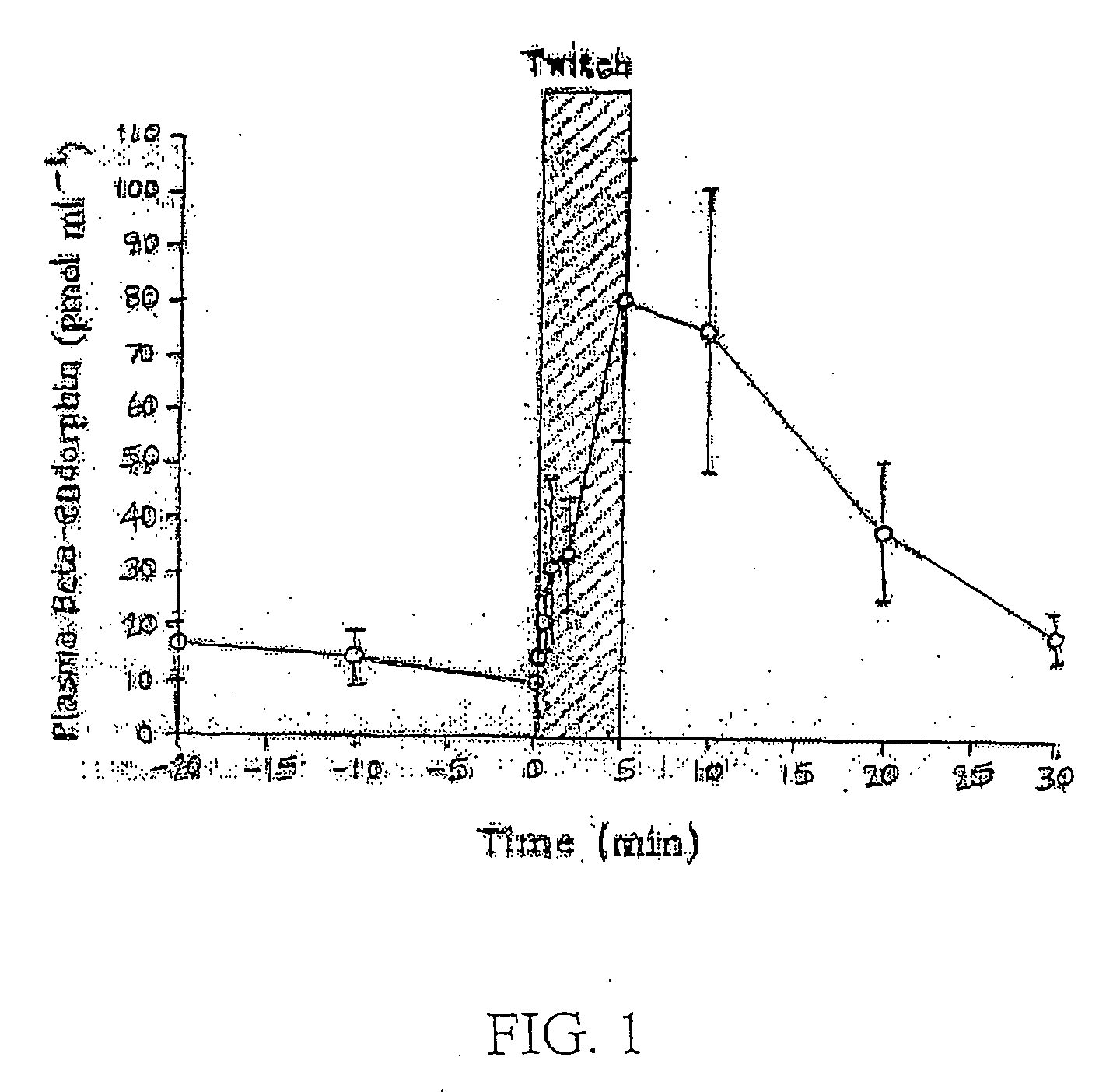
Motility is the ability of an organism to move independently, using metabolic energy. This is in contrast to mobility, which describes the ability of an object to be moved. Motility is genetically determined, but may be affected by environmental factors. For instance, muscles give animals motility but the consumption of hydrogen cyanide (the environmental factor in this case) would adversely affect muscle physiology, causing them to stiffen, leading to rigor mortis. In addition to animal locomotion, most animals are motile (some move by passive locomotion). The term applies to bacteria and other microorganisms, and to some multicellular organisms, as well as to some mechanisms of fluid flow in multicellular organs and tissue. Motile marine animals are commonly called free-swimming, and motile non-parasitic organisms are called free-living.
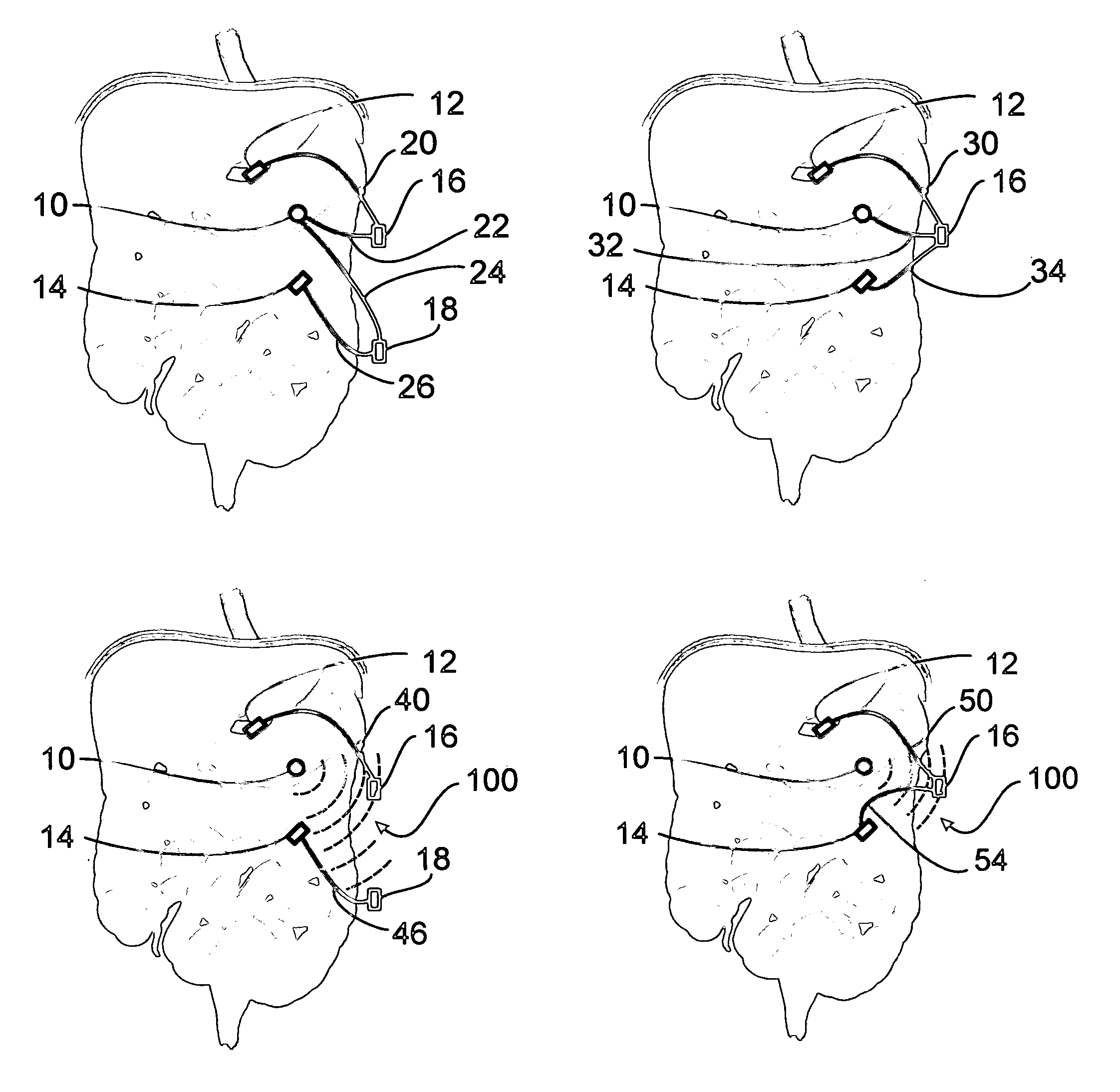
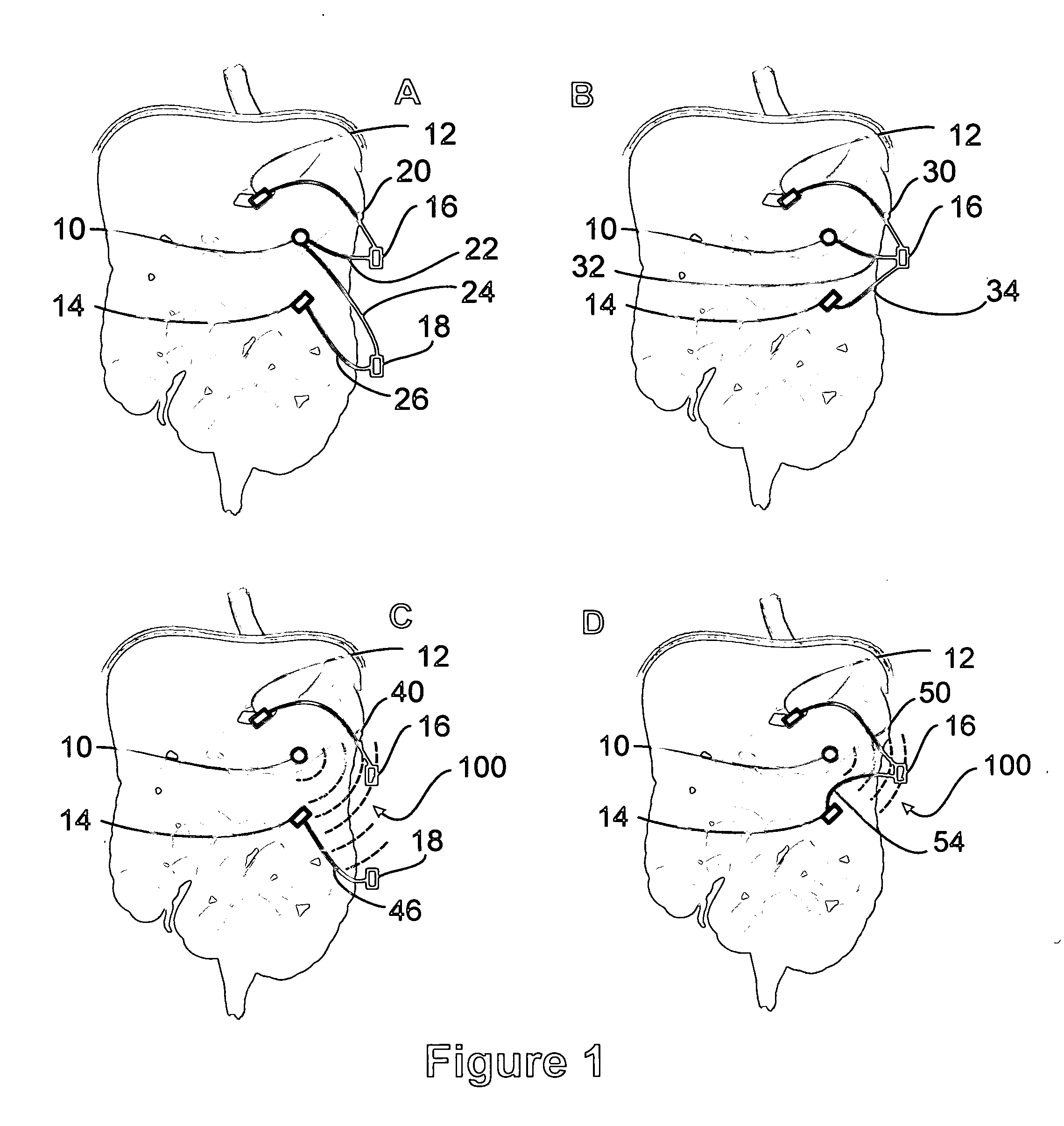
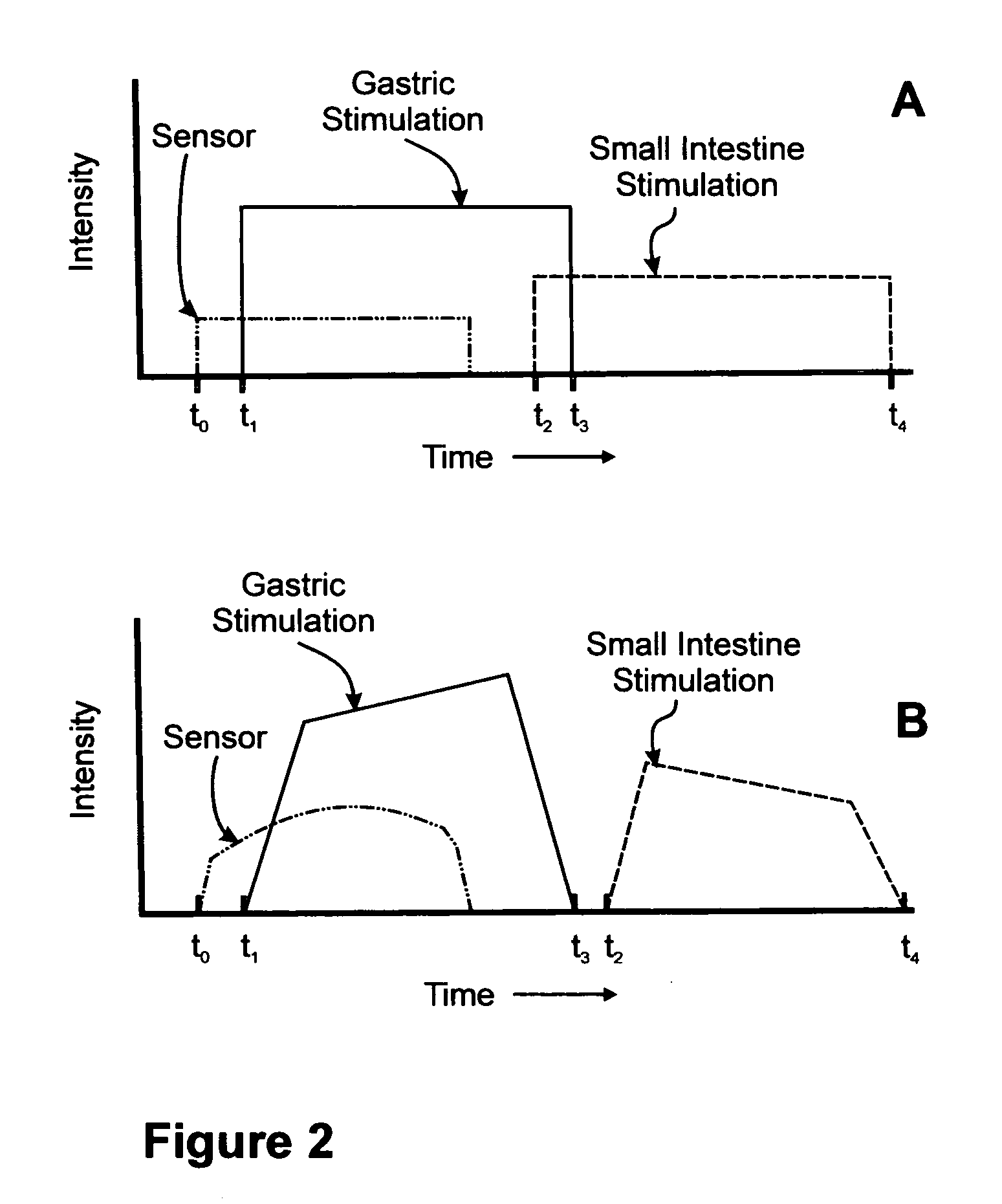
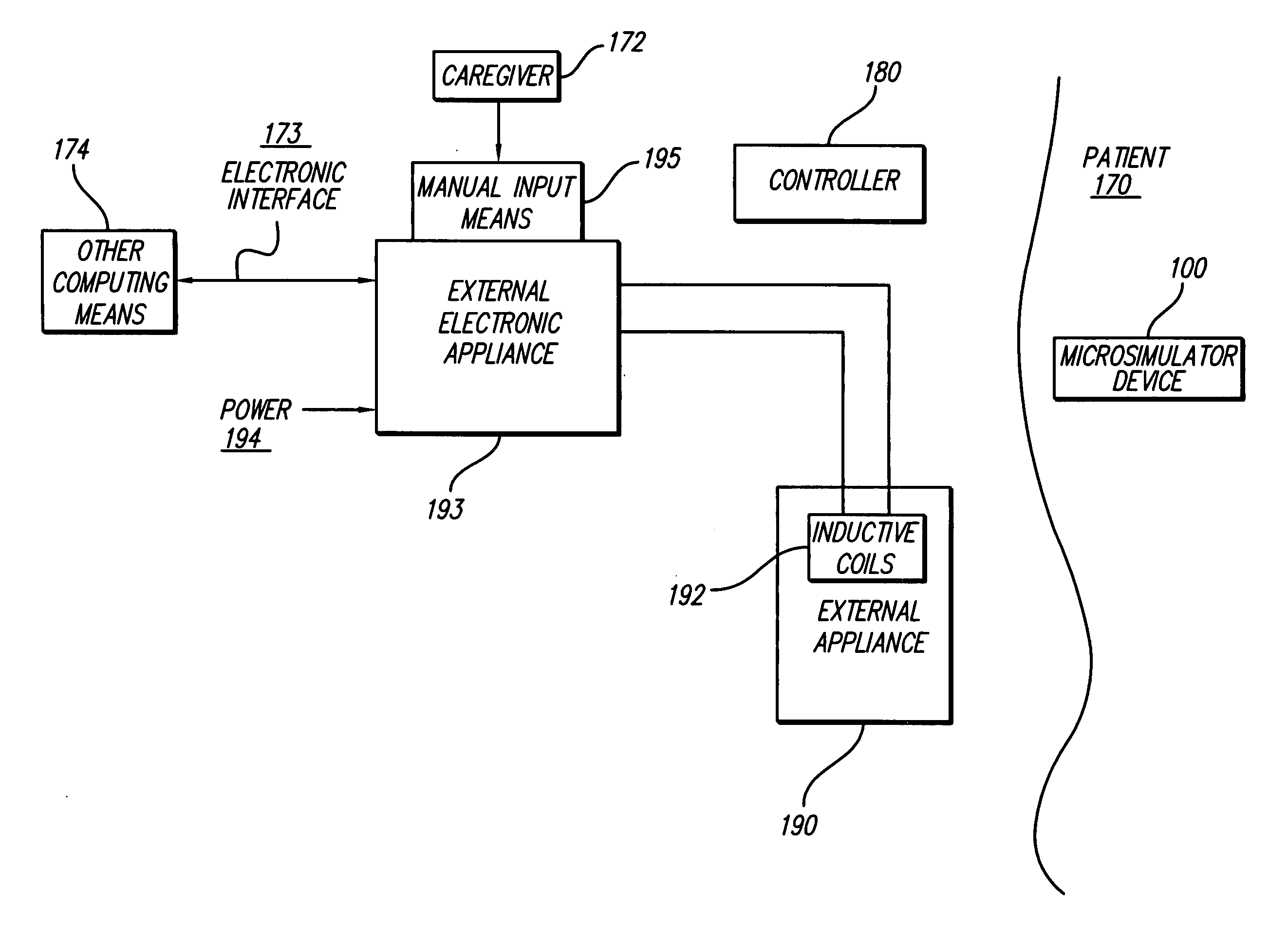
Sensor based gastrointestinal electrical stimulation for the treatment of obesity or motility disorders
InactiveUS20050222638A1Reduce riskAvoid stimulationElectrotherapyArtificial respirationGastroparesisMotility
A method for treatment of obesity, especially morbid obesity, gastroparesis and other syndromes related to motor disorders of the stomach. The method of this invention utilizes a sensor to detect food entering the patient's stomach, thereby the sensor communicates with and activates at least one electrical stimulation device attached to either the stomach or the small intestine.
Owner:MEDTRONIC TRANSNEURONIX
Methods for treating gastrointestinal disorders
A small implantable stimulator(s) having at least two electrodes is implanted adjacent to a gastrointestinal nerve and / or muscle for the stimulation treatment of gastrointestinal disorders, including gastrointestinal motility, sphincteric disorders, and / or eating disorders. The stimulator provides a means of stimulating tissue at a stimulation site when desired, and may be implanted via a minimal surgical procedure.
Owner:BOSTON SCI NEUROMODULATION CORP
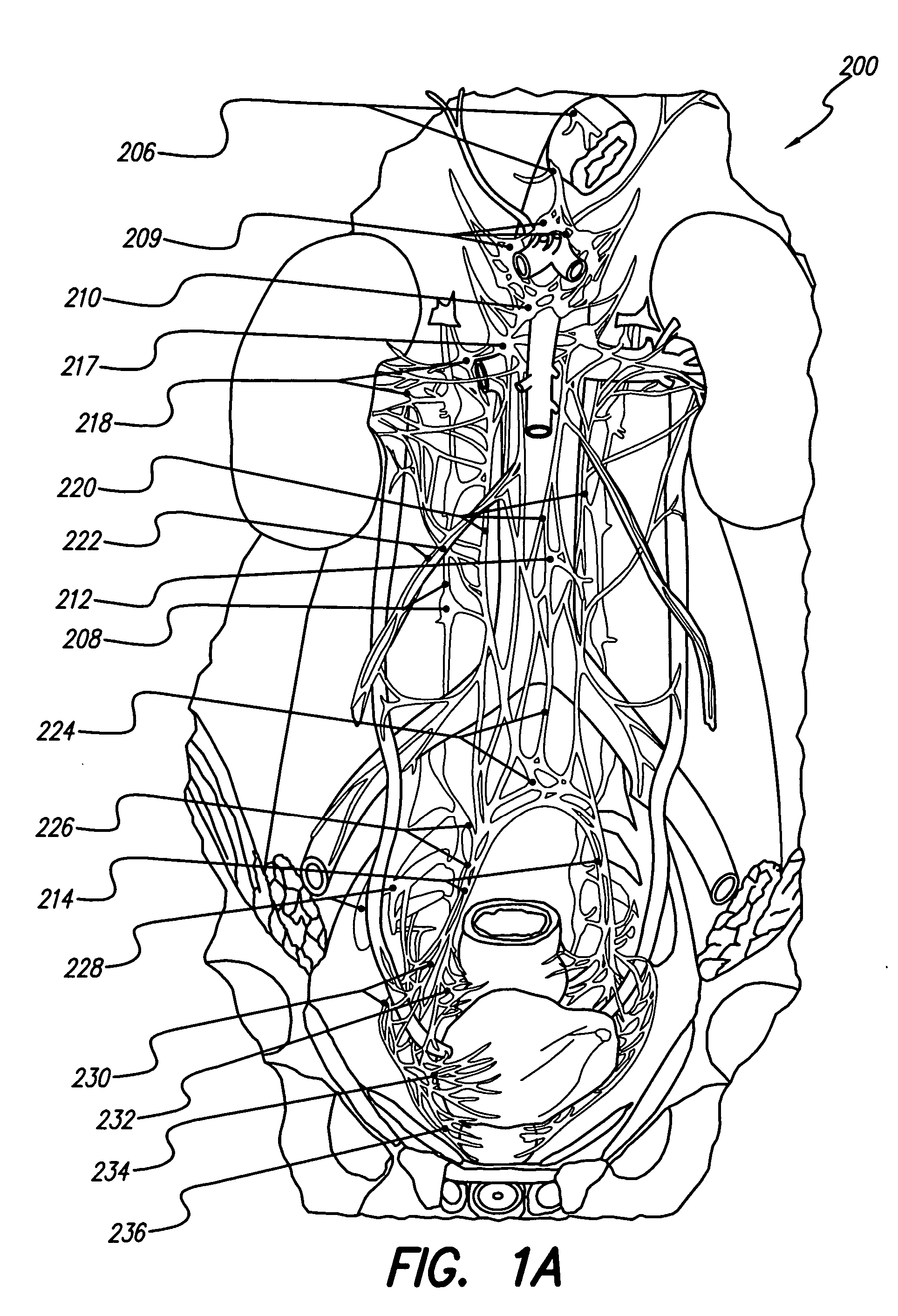
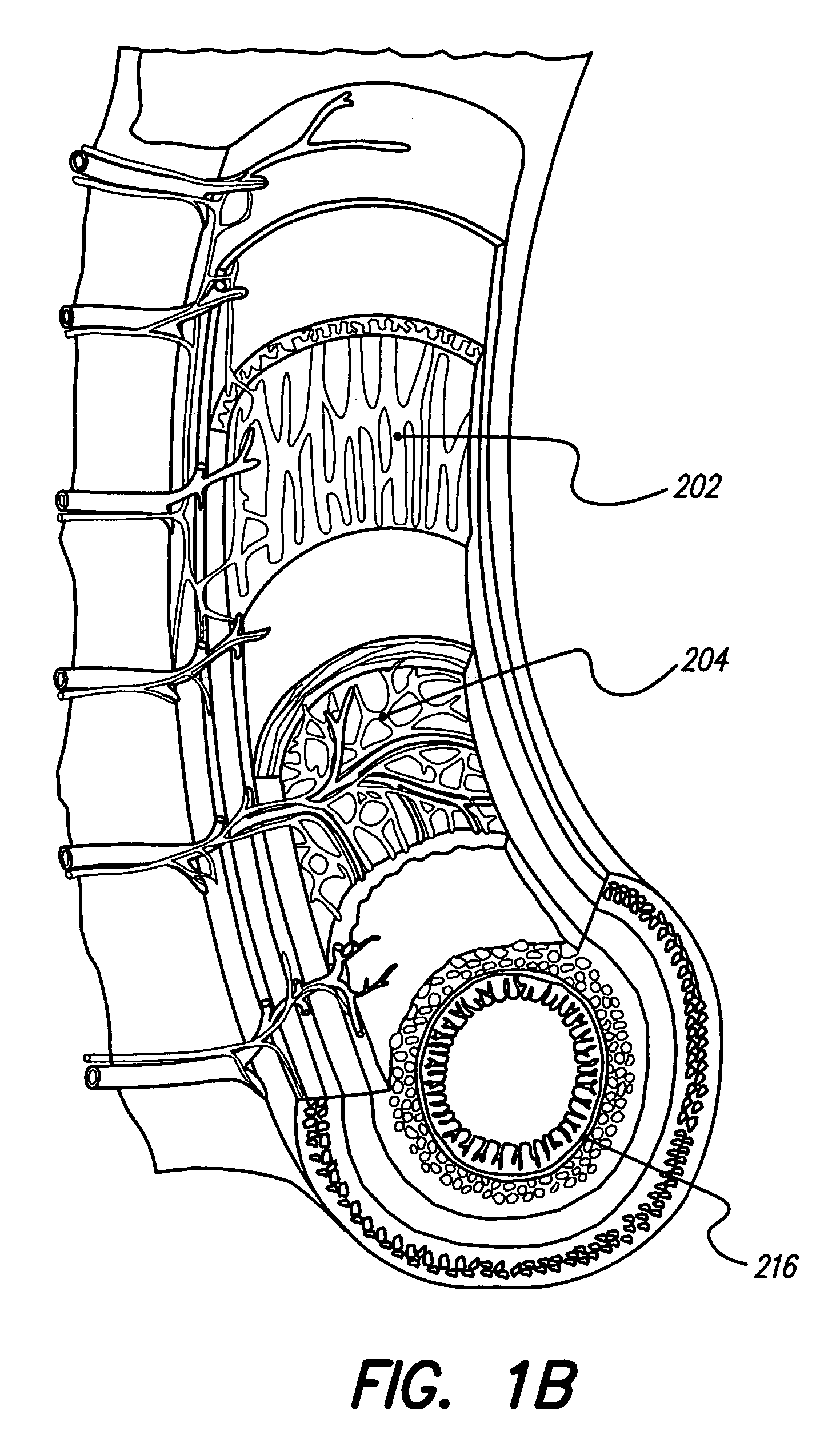
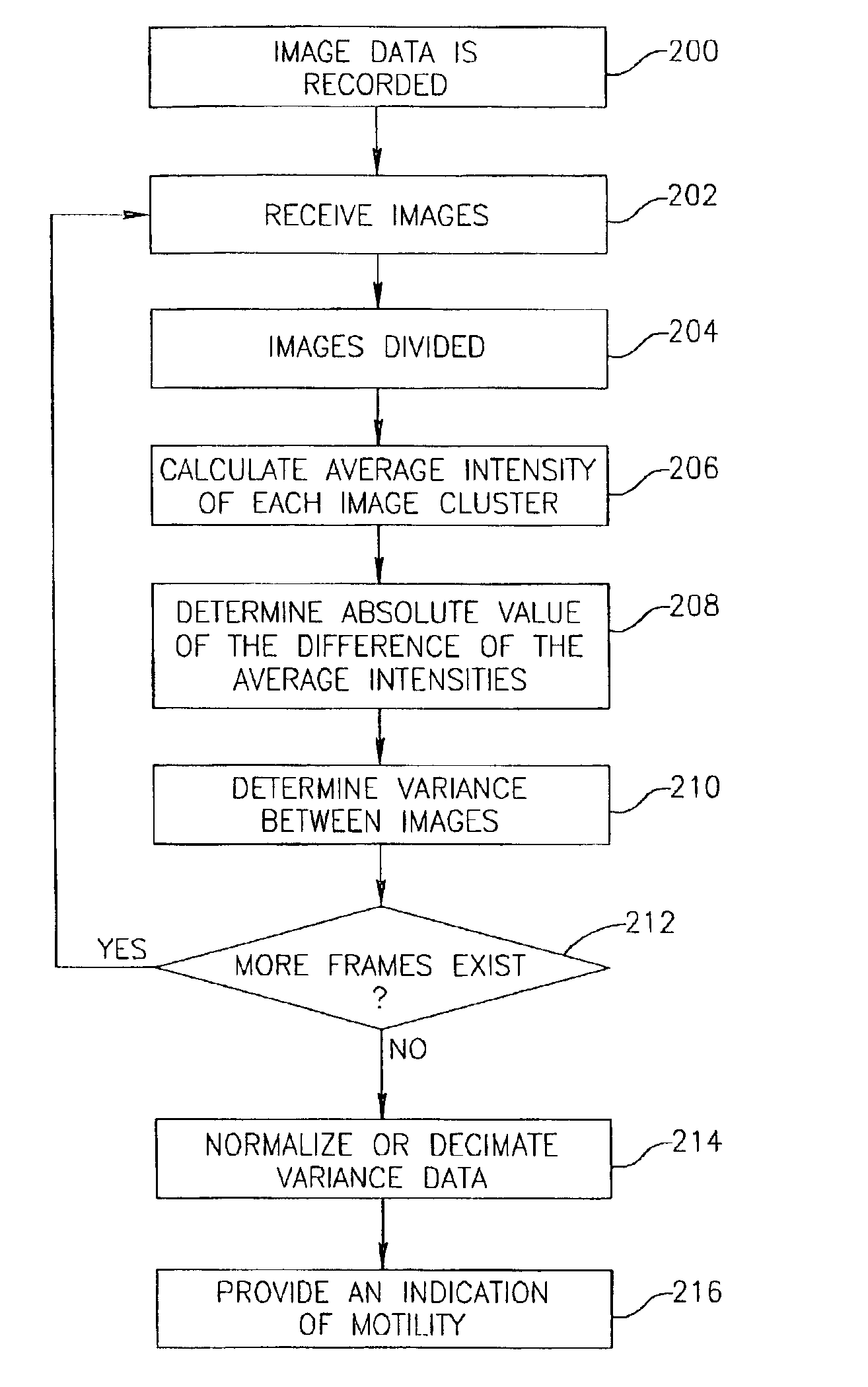
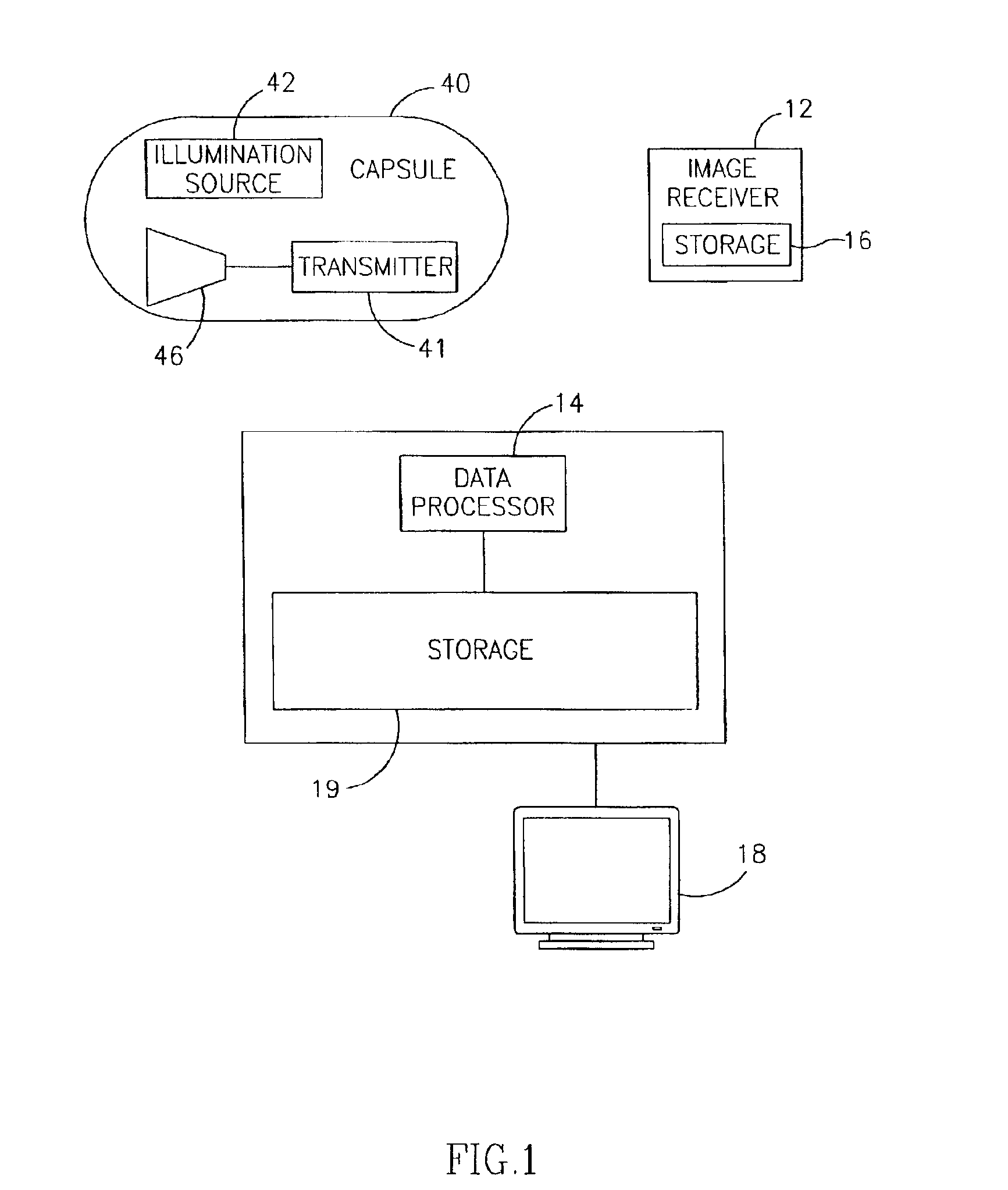
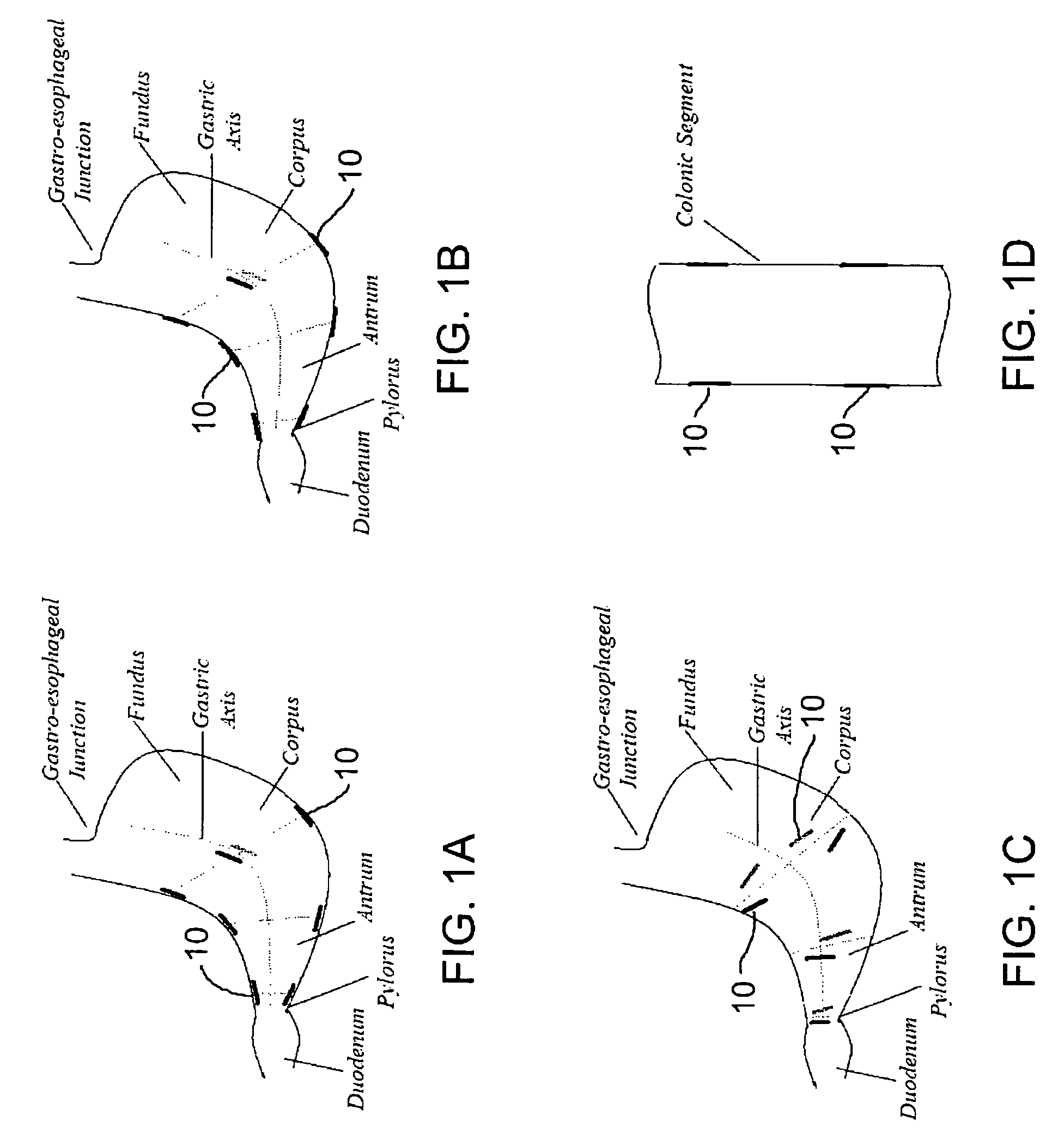
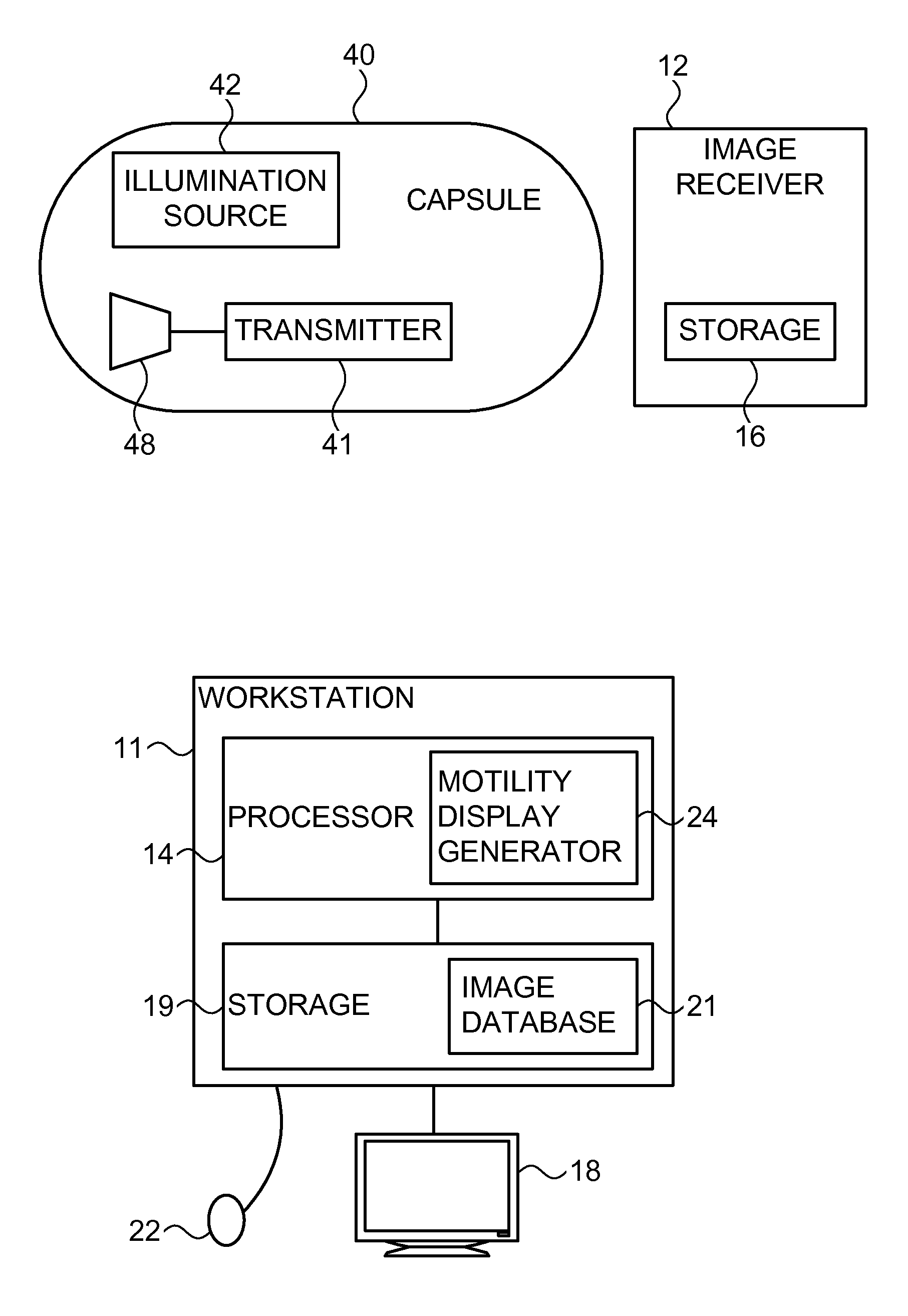
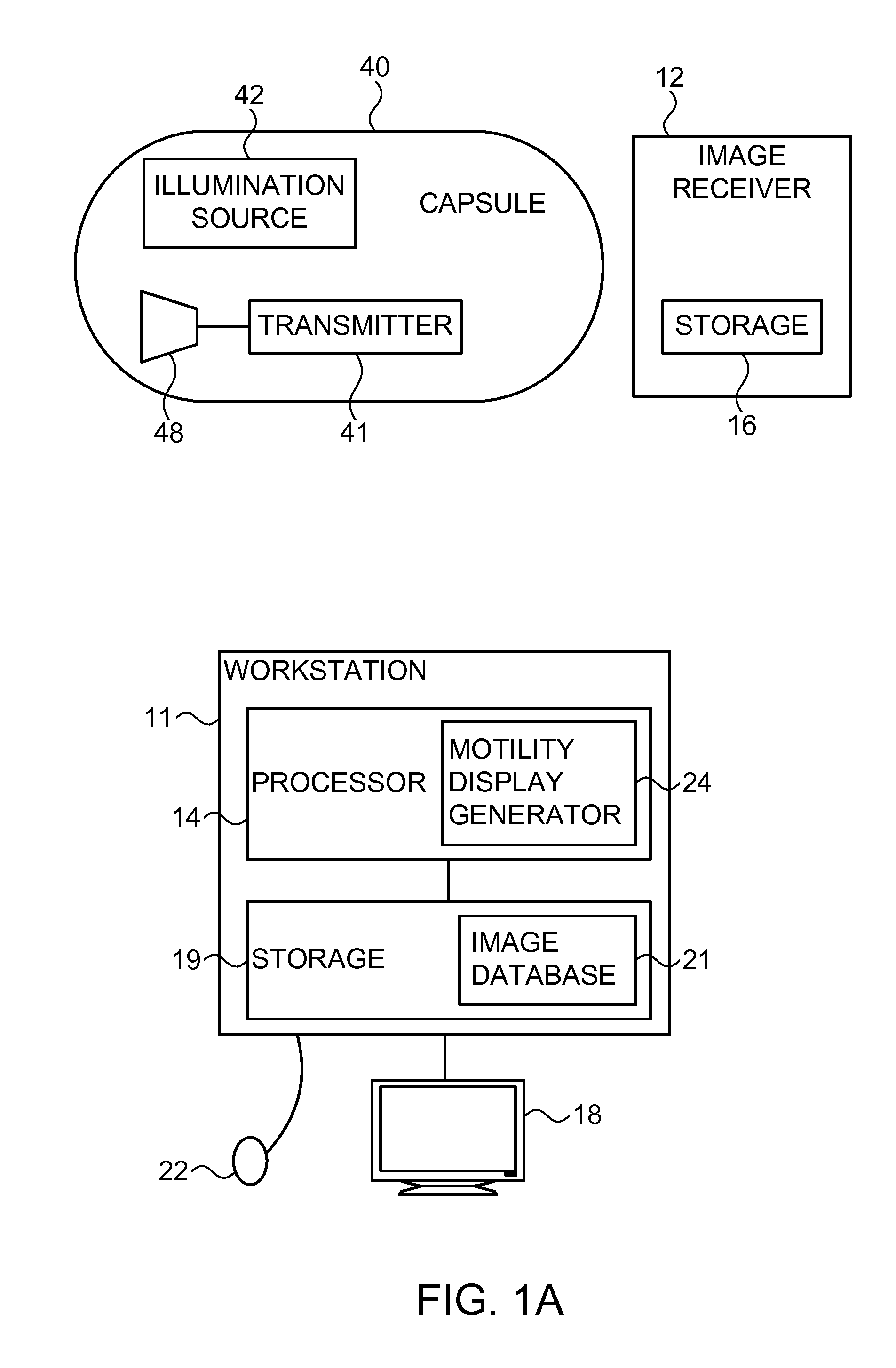
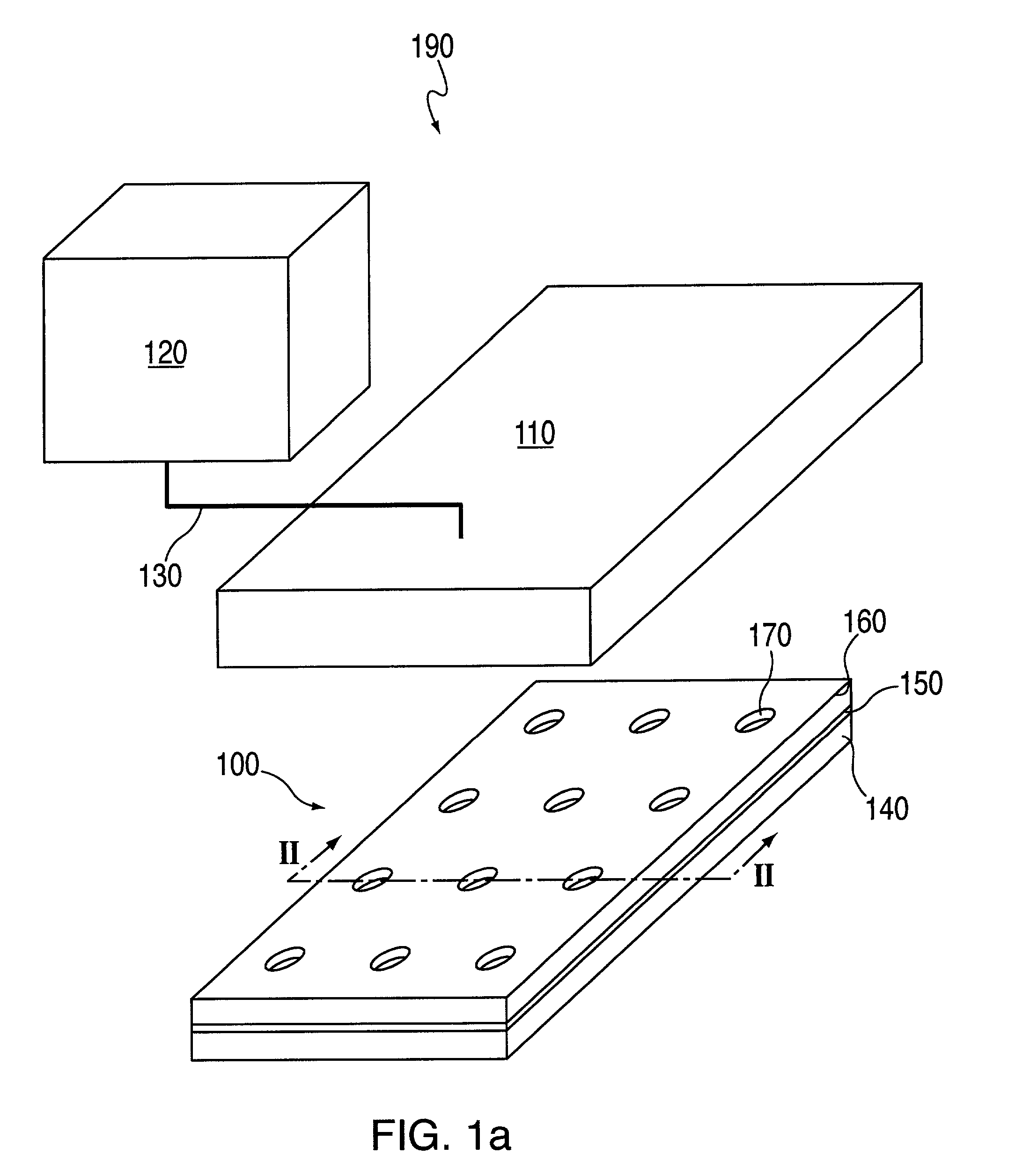
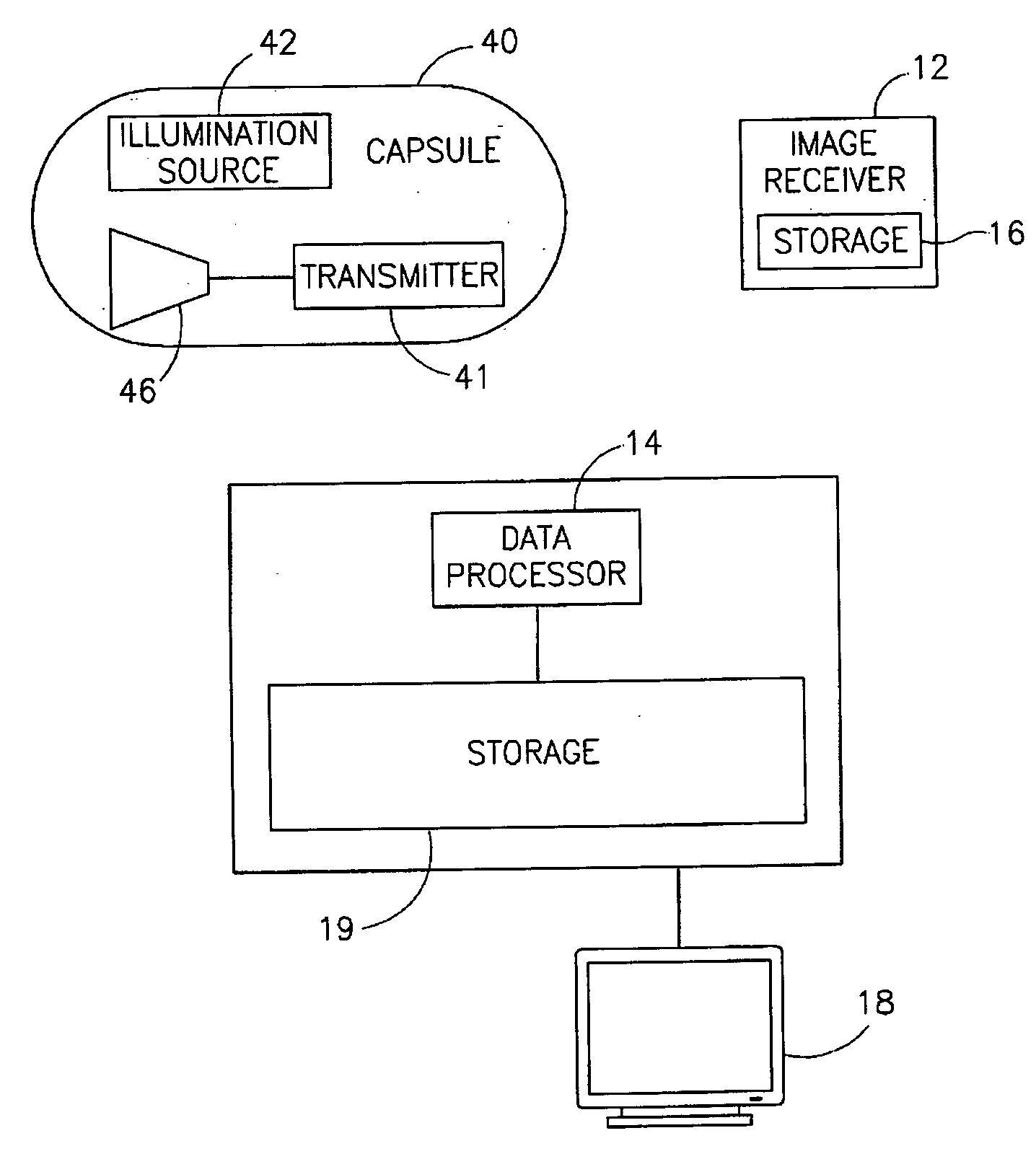
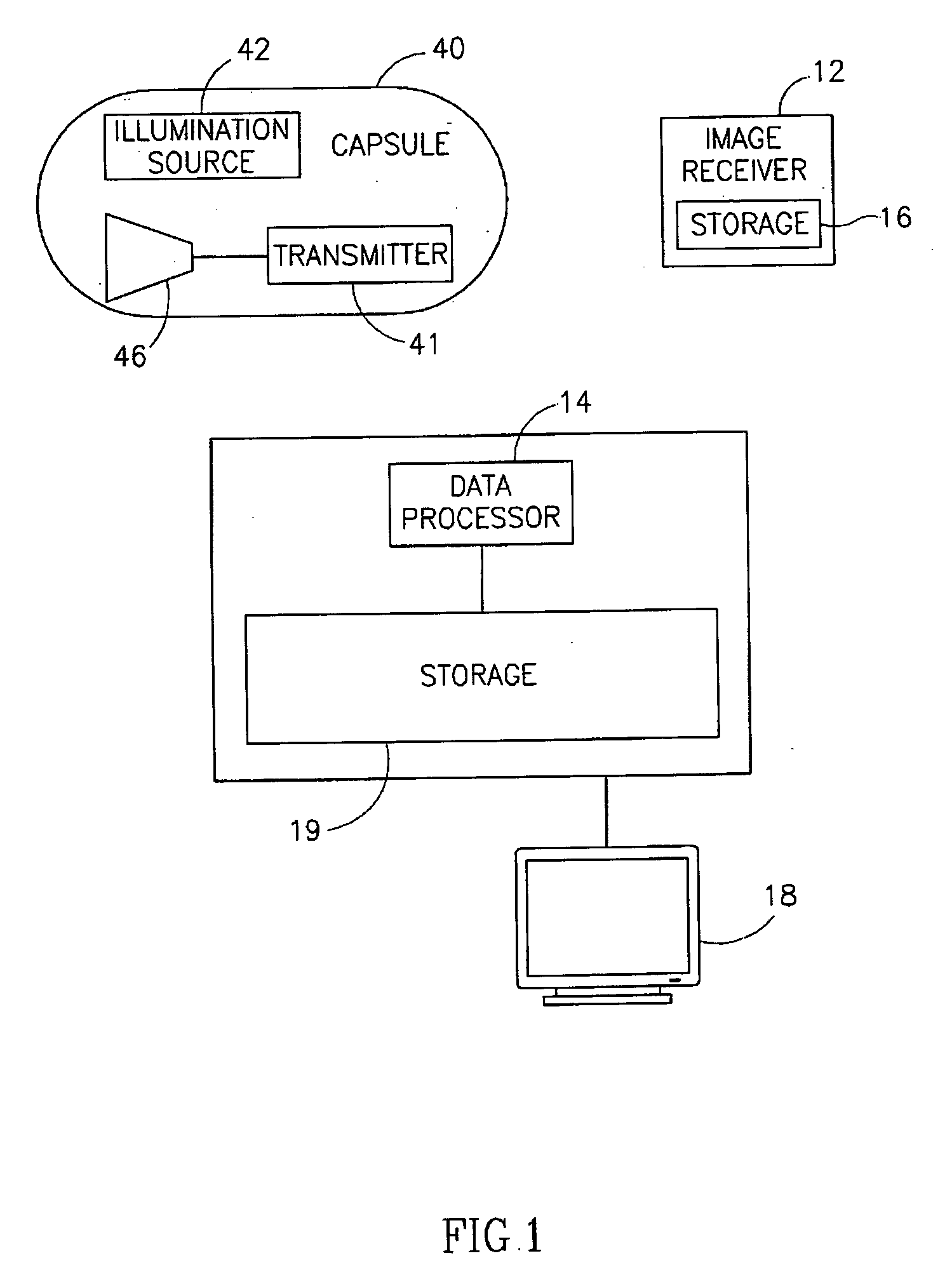
Motility analysis within a gastrointestinal tract
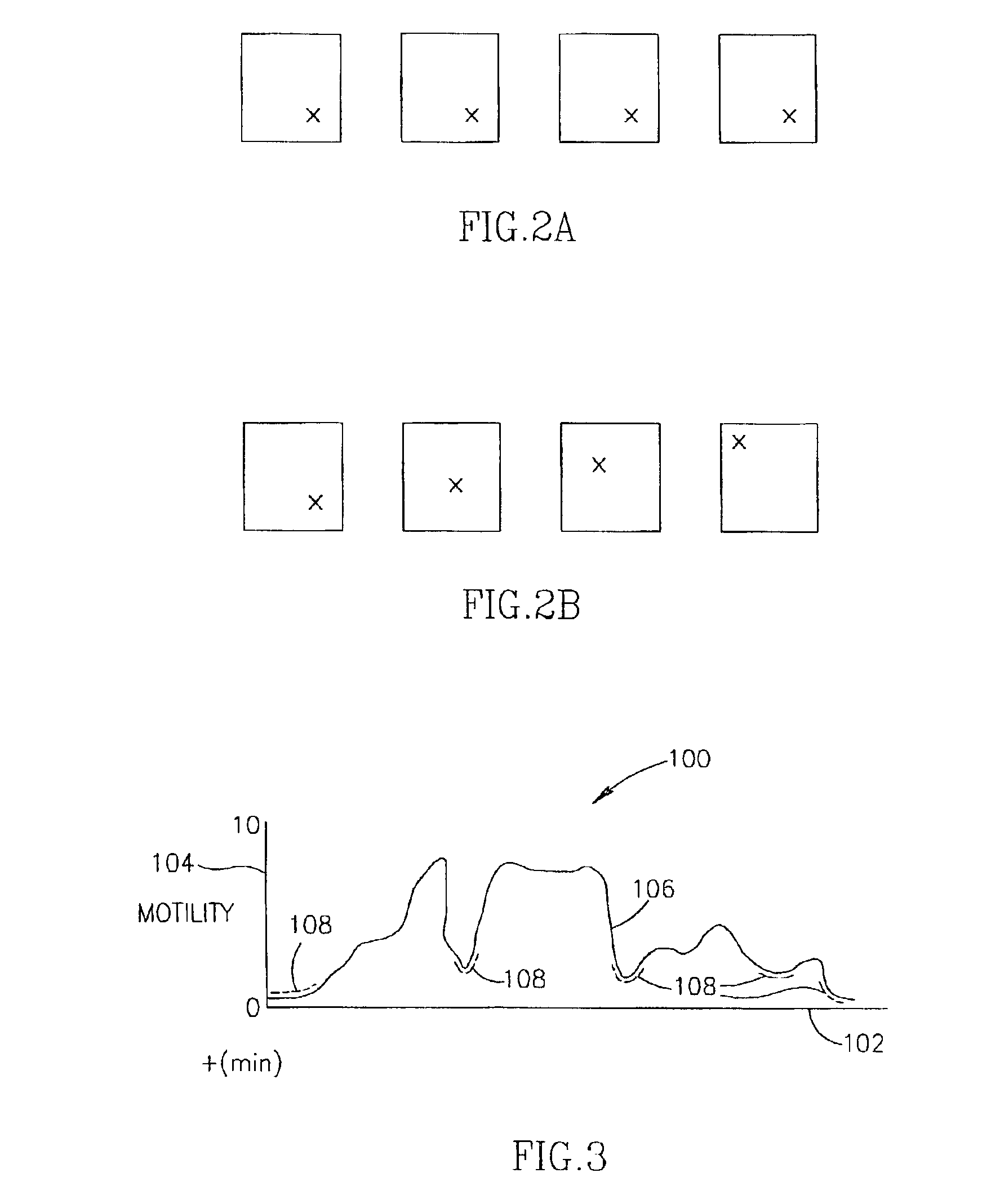
A system and method for measuring and analyzing motility within a body lumen such as the gastrointestinal (GI) tract, where an in vivo imaging device such as a capsule captures images and transmits the images to a processor, which calculates the motility of the device based on comparison of the images. Preferably, the processor compares the intensity of pairs of images or of elements of pairs of images, generates a variance for the compared images, and calculates the motility of the imaging device from the variances. The motility data may be presented to a user in various manners; for example, a plot of motility over time may be generated, or indications of low motility may be presented to the user of the system.
Owner:GIVEN IMAGING LTD
Optical Hydrology Arrays and System and Method for Monitoring Water Displacement During Treatment of Patient Tissue
ActiveUS20110251605A1Accurate measurementSurgical needlesDiagnostics using spectroscopyFiberTissue hydration
A system that monitors water displacement in tissue during patient therapy includes a generator supplying electrosurgical energy to tissue, a spectrometer operably coupled to the generator, and a processor communicating with the generator and with the spectrometer having a light source for exposing tissue to light and a light sensor. The light sensor is configured to sense changes in light through tissue in response to tissue treatment and communicate the changes to the processor to determine tissue hydration levels and motility. A plurality of optical fibers may be configured in an array to communicate light between the generator and tissue. An optical temperature monitor may communicate with the processor and be coupled to an optical fiber. The optical fibers may have an optic fiber distance between adjacent optical fibers. The system may be incorporated within an electrosurgical pencil or a forceps. A corresponding method of detecting hydration is also disclosed.
Owner:TYCO HEALTHCARE GRP LP
Anti-diabetic peptides
InactiveUS6087334AInhibiting gastric emptyingSmall sizeHormone peptidesPeptide/protein ingredientsMetabolic derangementMammal
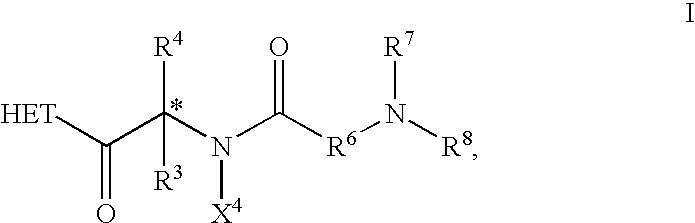
Compounds of formula I which act as amylin agonists with respect to certain desired amylin activities and as calcitonin agonists with respect to certain desired calcitonin activities are provided. Such compounds are useful in treating disturbances in fuel metabolism in mammals, including, but not limited to diabetes mellitus, including Type I diabetes and Type II diabetes. The present invention also relates to methods of treating Type I diabetes, treating Type II diabetes and to methods of beneficially regulating gastrointestinal motility comprising administration of a therapeutically effective among of one of the compounds. Also provided are pharmaceutical composition comprising a compound of formula I and a pharmaceutically acceptable carrier.
Owner:ASTRAZENECA PHARMA LP
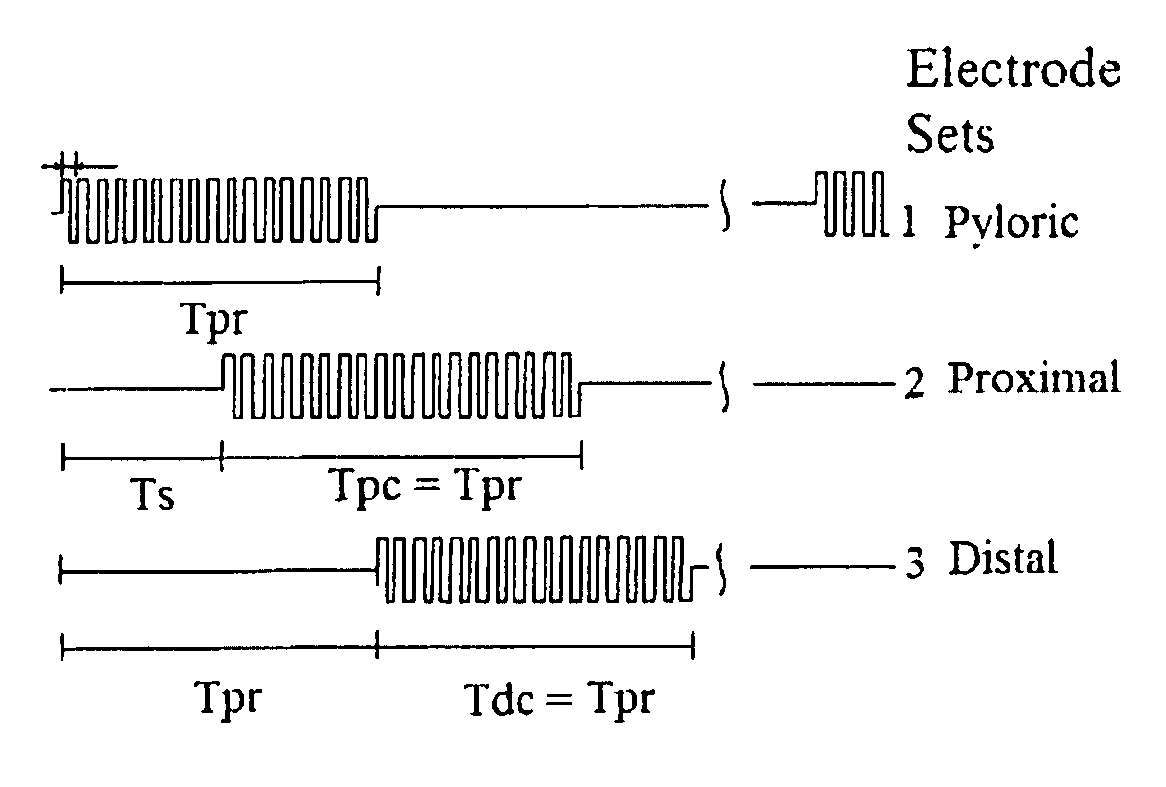
Gastrointestinal motility control
A method and a multichannel implantable device are described for partial or complete restoration of impaired gastrointestinal motility, or for disturbing and / or partially or completely blocking normal gastrointestinal motility using one or multiple microsystem-controlled channels of circumferentially arranged sets of two or more electrodes which provide externally-invoked synchronized electrical signals to the smooth muscles via the neural pathways.
Owner:UTI LLP
Method and system for gastric ablation and gastric pacing to provide therapy for obesity, motility disorders, or to induce weight loss
InactiveUS20050240239A1Avoid side effectsProviding therapyUltrasound therapyInternal electrodesCardiac pacemaker electrodeMotility
Method and system to provide therapy for obesity, gastric motility, or to induce weight loss comprises ablating the gastric tissue around the “pacemaker” region of the stomach, and electrically pacing the stomach with a pulse generator / stimulator to control the electrical activity of the gastric muscle. The ablation to the gastric tissue may be from the epigastric side, or may be from inside the stomach. The ablation may be performed utilizing any one of: radiofrequency catheter ablation; radiofrequency catheter ablation using an irrigated tip catheter; microwave ablation; cryoablation; high intensity focused ultrasound (HIFU) ablation; and laser ablation. The ablation of the “pacemaker” region of the stomach may be partial or complete. A gastric pulse generator / stimulator is implanted to provide electrical pulses to the stomach. The function of the gastric stimulator after complete ablation of the pacemaker region, is to provide a basic electrical rhythm (BER) to regulate and control electrical activity of the stomach. Alternatively, if partial ablation is performed the function of the gastric pulse generator / stimulator is to enhance the residual basic electrical rhythm (BER), or to interfere with the residual basic electrical rhythm (BER).
Owner:BOVEJA BIRINDER R +1
Compounds as rearranged during transfection (RET) inhibitors
This invention relates to novel compounds which are inhibitors of the Rearranged during Transfection (RET) kinase, to pharmaceutical compositions containing them, to processes for their preparation, and to their use in therapy, alone or in combination, for the normalization of gastrointestinal sensitivity, motility and / or secretion and / or abdominal disorders or diseases and / or treatment related to diseases related to RET dysfunction or where modulation of RET activity may have therapeutic benefit including but not limited to all classifications of irritable bowel syndrome (IBS) including diarrhea-predominant, constipation-predominant or alternating stool pattern, functional bloating, functional constipation, functional diarrhea, unspecified functional bowel disorder, functional abdominal pain syndrome, chronic idiopathic constipation, functional esophageal disorders, functional gastroduodenal disorders, functional anorectal pain, inflammatory bowel disease, proliferative diseases such as non-small cell lung cancer, hepatocellular carcinoma, colorectal cancer, medullary thyroid cancer, follicular thyroid cancer, anaplastic thyroid cancer, papillary thyroid cancer, brain tumors, peritoneal cavity cancer, solid tumors, other lung cancer, head and neck cancer, gliomas, neuroblastomas, Von Hippel-Lindau Syndrome and kidney tumors, breast cancer, fallopian tube cancer, ovarian cancer, transitional cell cancer, prostate cancer, cancer of the esophagus and gastroesophageal junction, biliary cancer, adenocarcinoma, and any malignancy with increased RET kinase activity.
Owner:GLAXOSMITHKLINE INTPROP DEV LTD
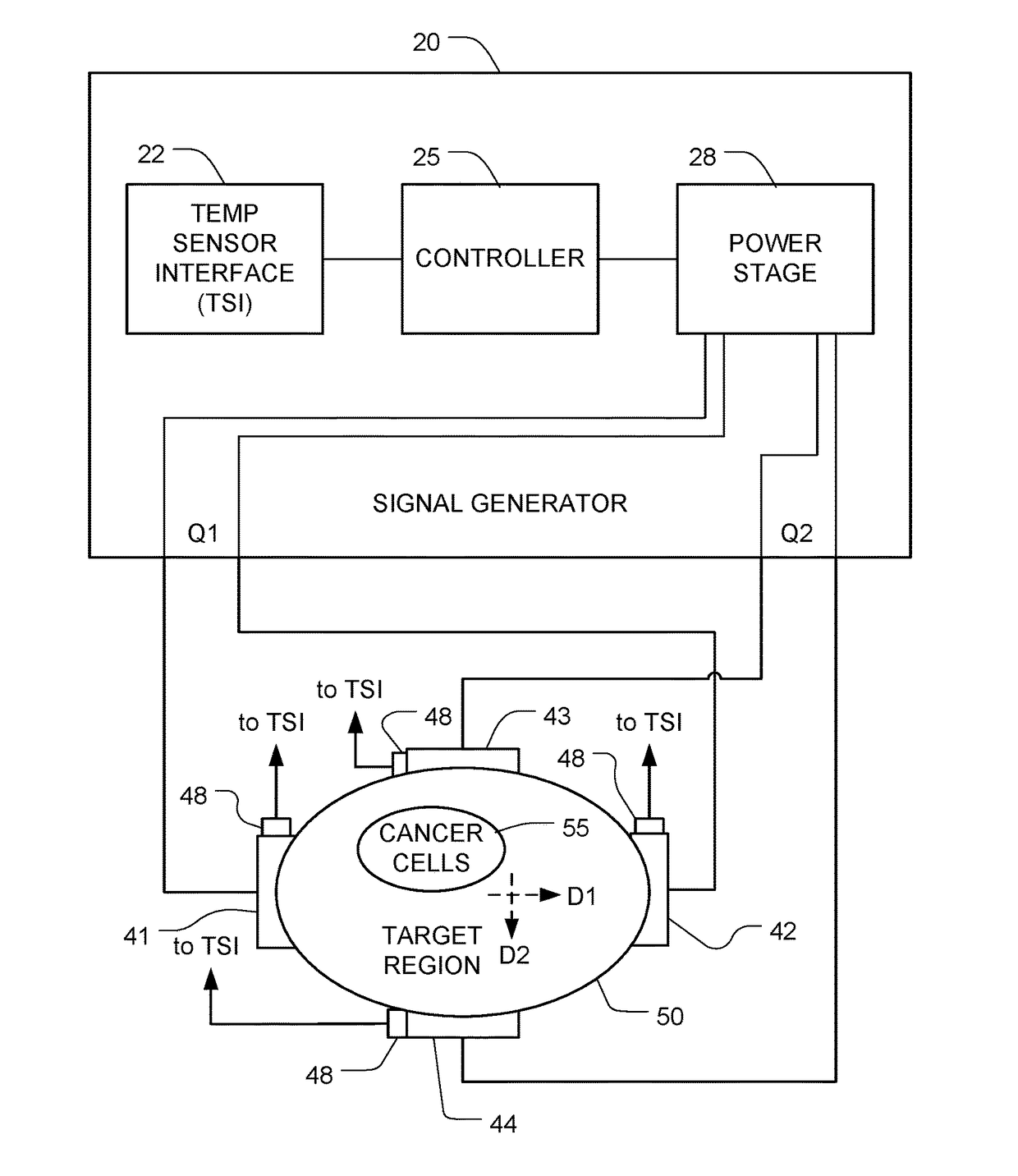
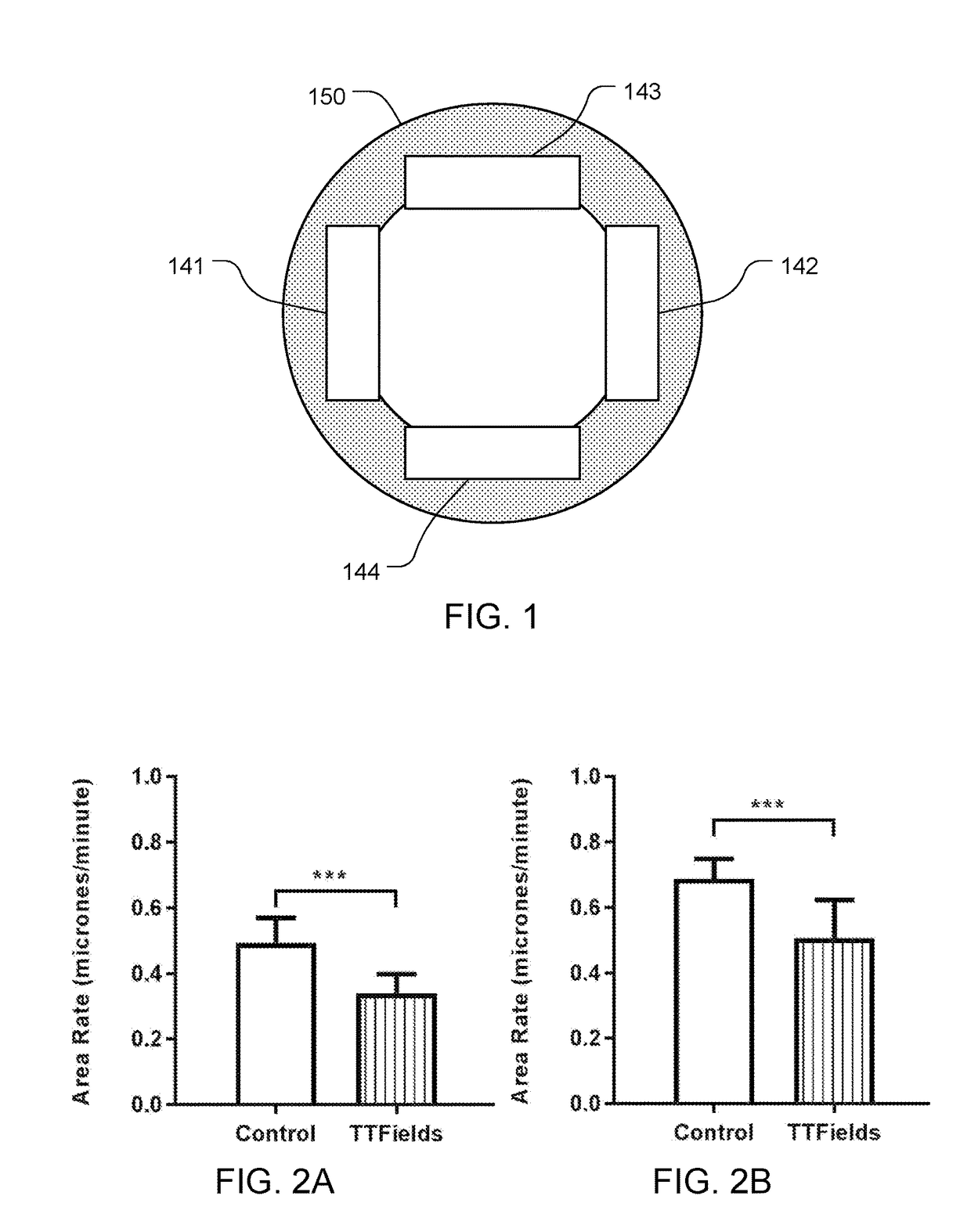
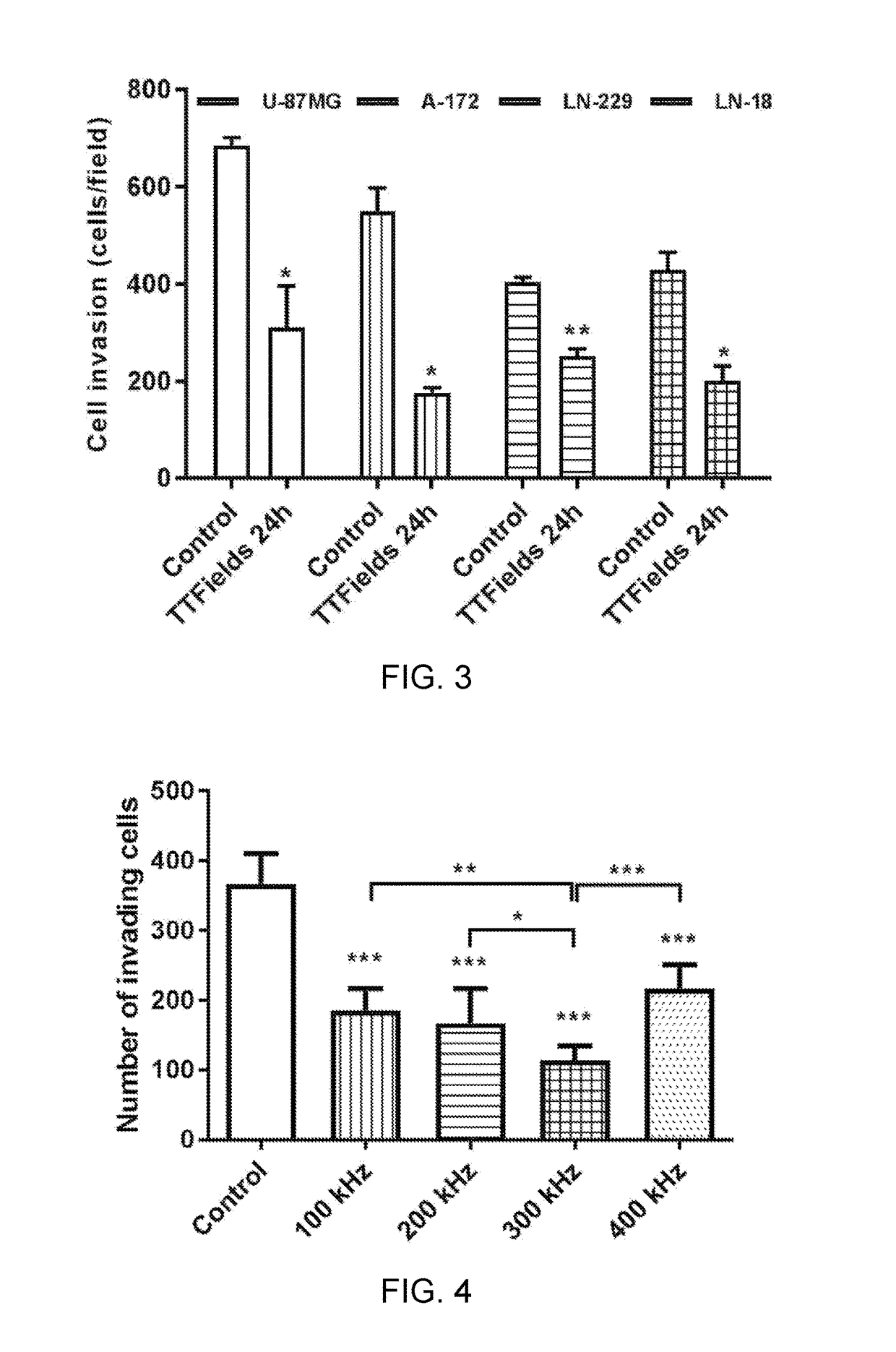
Reducing Motility of Cancer Cells Using Tumor Treating Fields (TTFields)
ActiveUS20170281934A1Reduce exerciseAvoid spreadingOrganic active ingredientsElectrotherapyCancer cellMotility
The spreading of cancer cells in a target region can be inhibited by imposing a first AC electric field in the target region for a first interval of time, with a frequency and amplitude selected to disrupt mitosis of the cancer cells; and imposing a second AC electric field in the target region for a second interval of time, with a frequency and the amplitude selected to reduce motility of the cancer cells. The amplitude of the second AC electric field is lower than the amplitude of the first AC electric field.
Owner:NOVOCURE GMBH
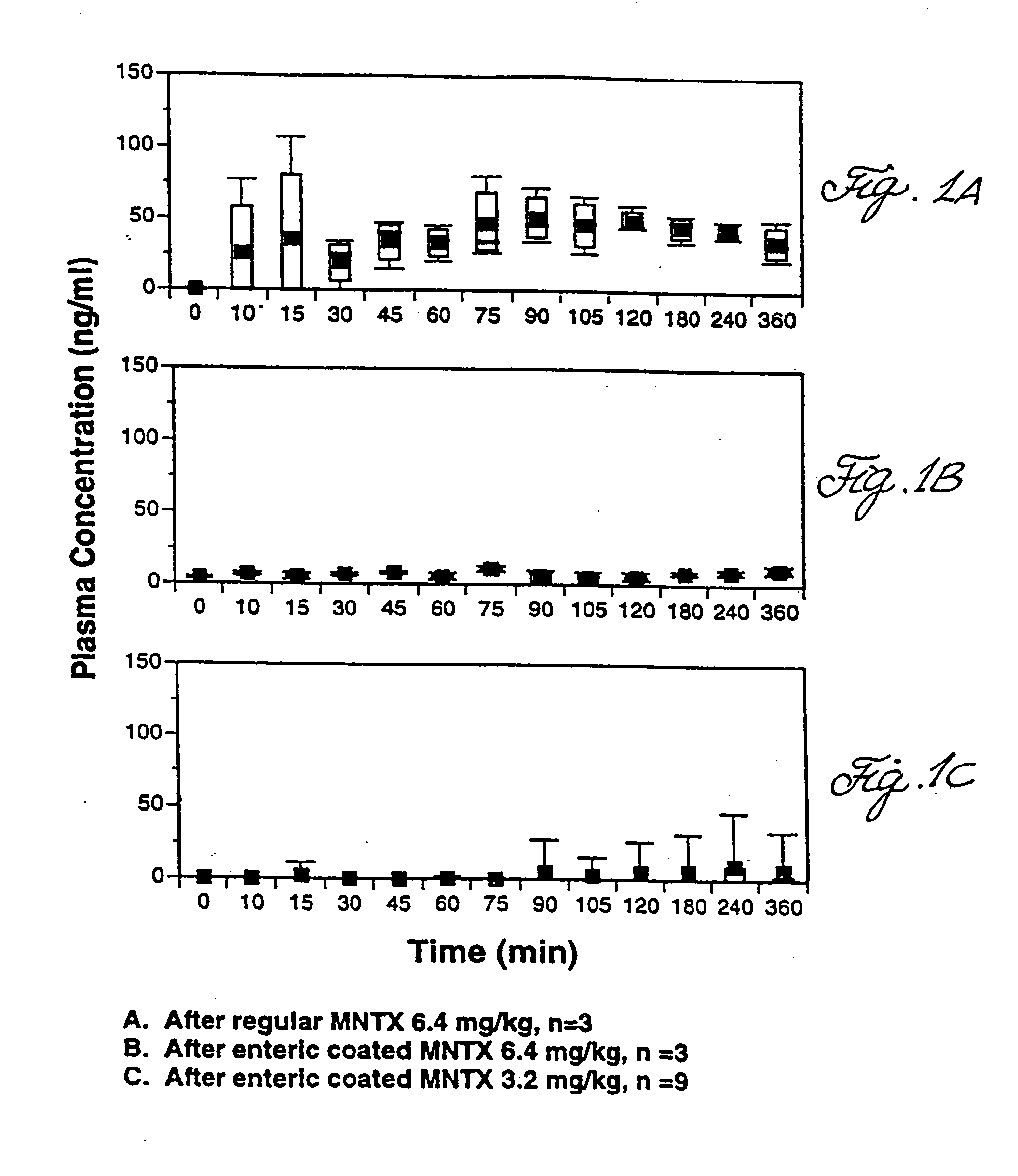
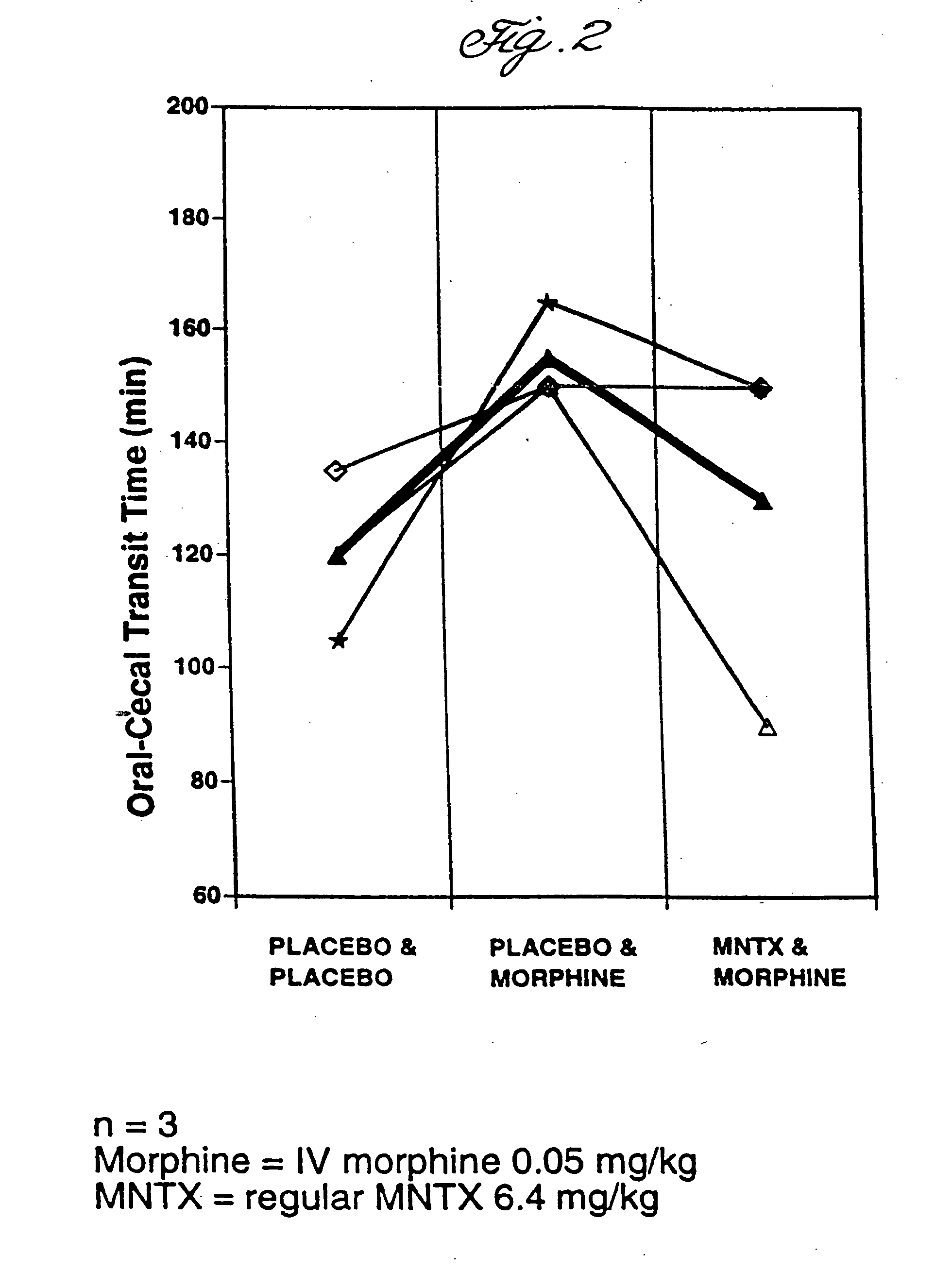
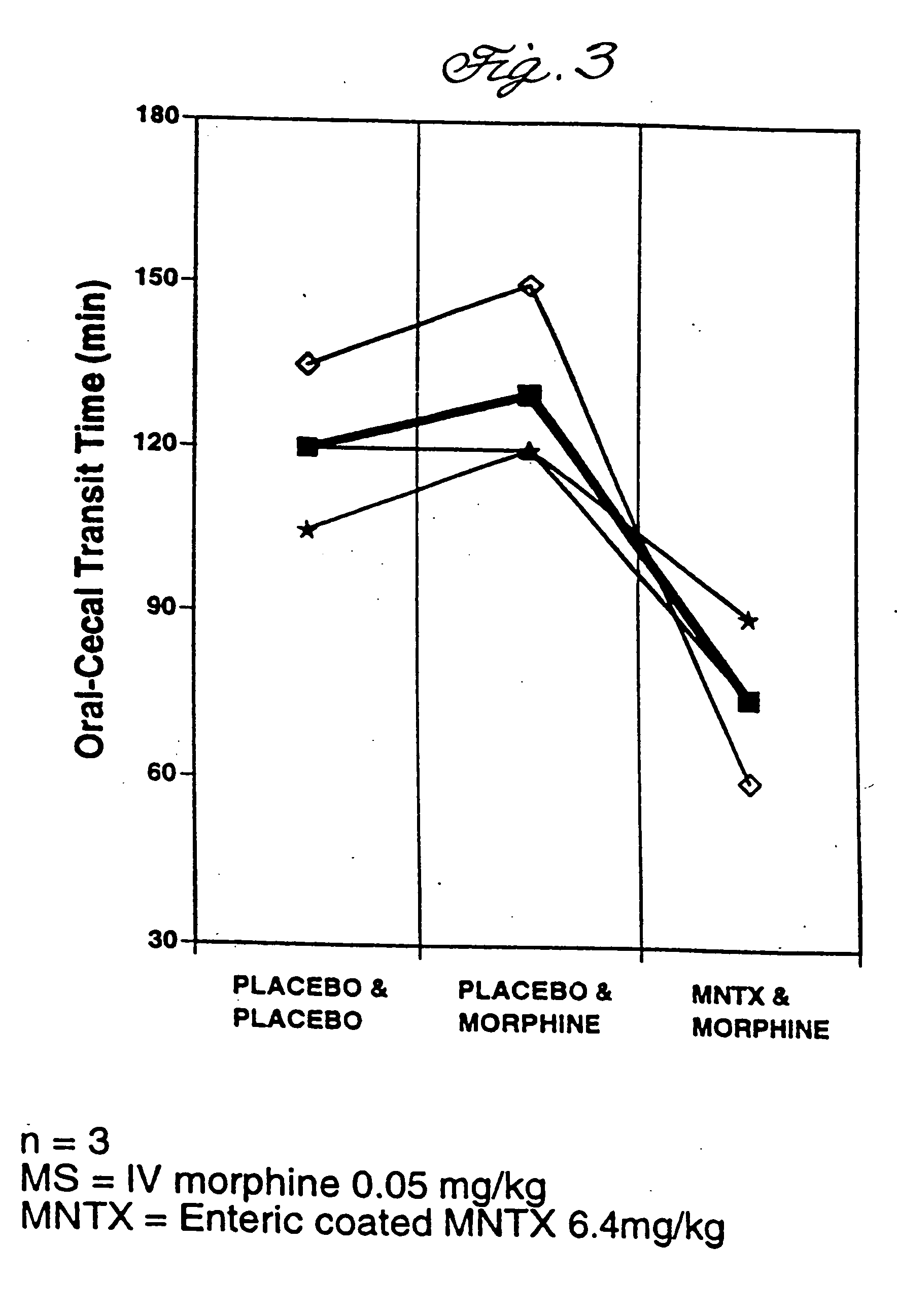
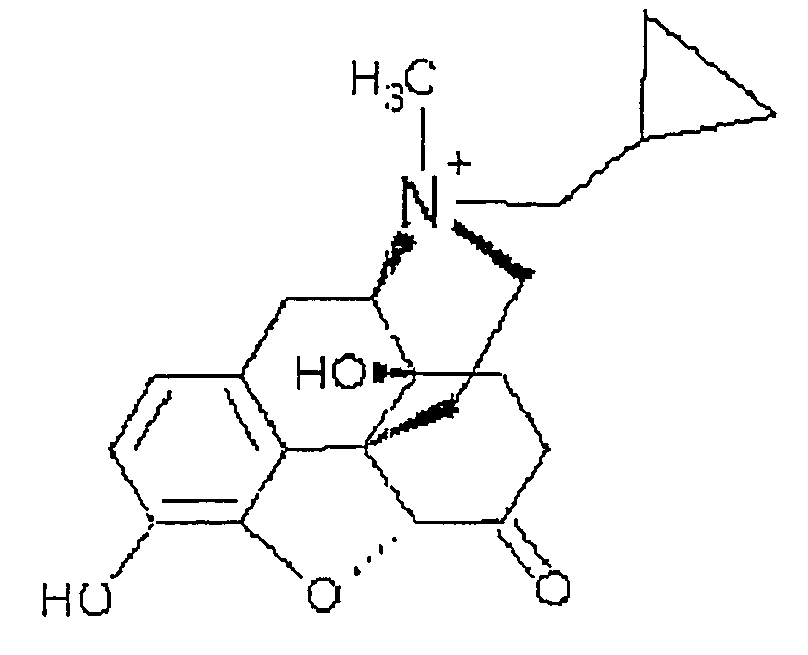
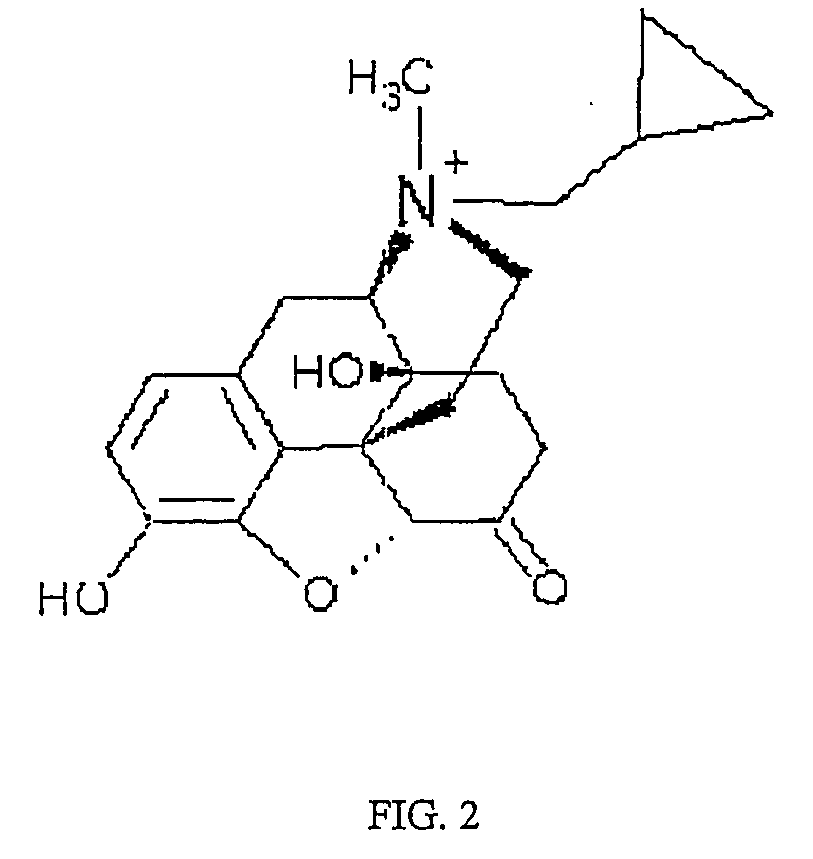
Use of methylnaltrexone and related compounds
A composition for preventing or treating the opioid induced side effect, inhibition of gastrointestinal motility is disclosed. The composition comprises methylnaltrexone or another quaternary derivative of noroxymorphone administered to a patient prior to the administration of an opioid or after the onset of side effects induced by the administration of an opioid, wherein the methylnaltrexone or quaternary derivative is administered orally in an enterically coated form.
Owner:PROGENICS PHARMA NEVADA
Gastrointestinal motility control
A method and a multichannel implantable device are described for partial or complete restoration of impaired gastrointestinal motility, or for disturbing and / or partially or completely blocking normal gastrointestinal motility using one or multiple microsystem-controlled channels of circumferentially arranged sets of two or more electrodes which provide externally-invoked synchronized electrical signals to the smooth muscles via the neural pathways.
Owner:UTI LLP
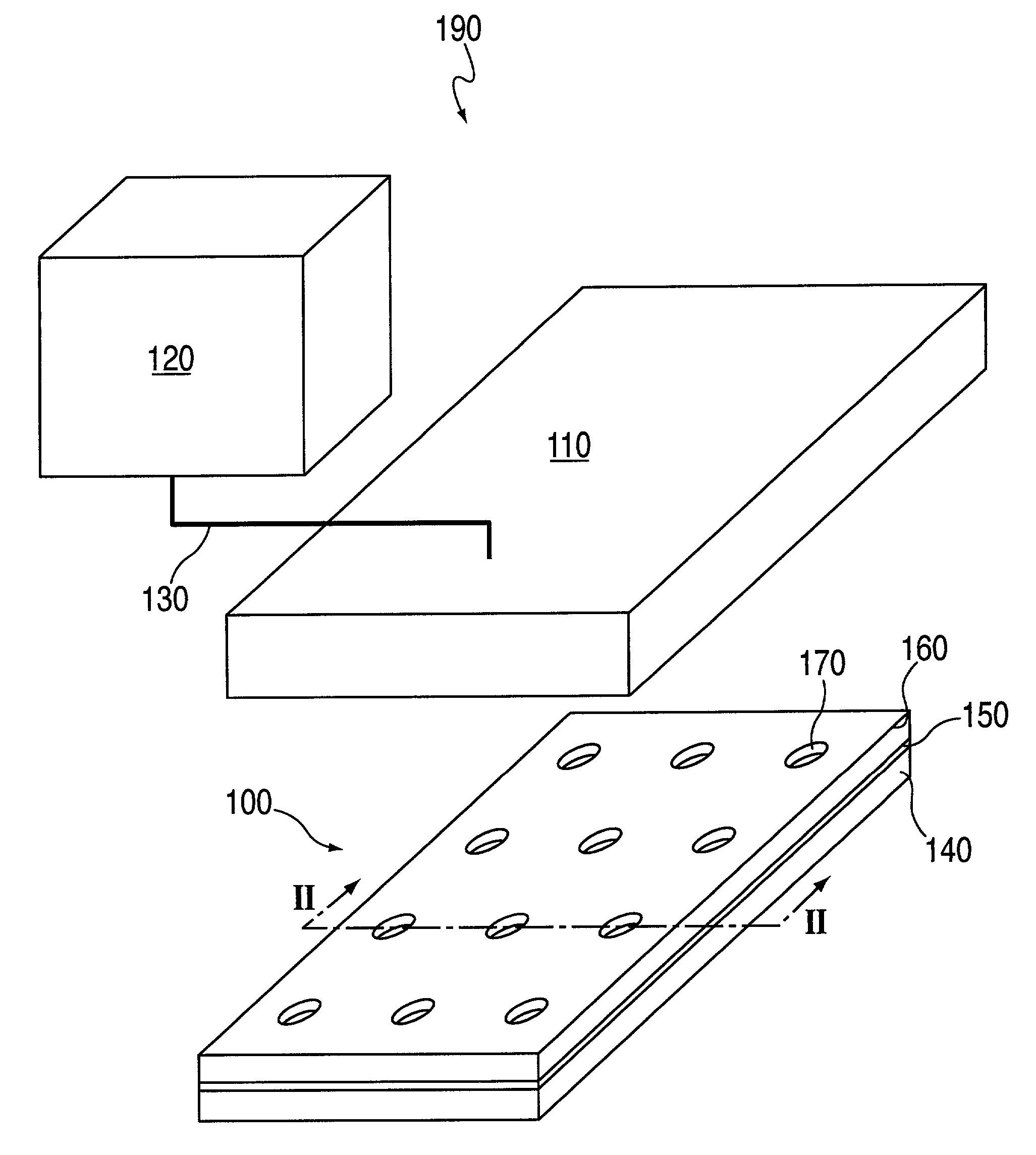
Device for monitoring cell motility in real-time
The invention relates to devices, devices for arraying biomolecules, including cells, methods for arraying biomolecules, assays for monitoring cellular movement, and systems for monitoring cellular movement. The devices include a support; a first layer configured to be placed in fluid-tight contact with the support, the first layer having an upper surface and defining a pattern of micro-orifices, each micro-orifice of the pattern of micro-orifices having walls and defining a micro-region on the support when the first layer is placed in fluid-tight contact with the support such that the walls of said each micro-orifice and the micro-region on the support together define a micro-well; and a second layer configured to be placed in fluid-tight contact with the upper surface of the first layer, the second layer defining a pattern of macro-orifices, each macro-orifice of the pattern of macro-orifices having walls and defining a macro-region when the first layer is placed in fluid-tight contact with the support and the second layer is placed in fluid-tight contact with the first layer such that the walls of the macro-orifice and the macro-region together define a macro-well.
Owner:SURFACE LOGIX INC
Reduction of sperm sensitivity to chilling
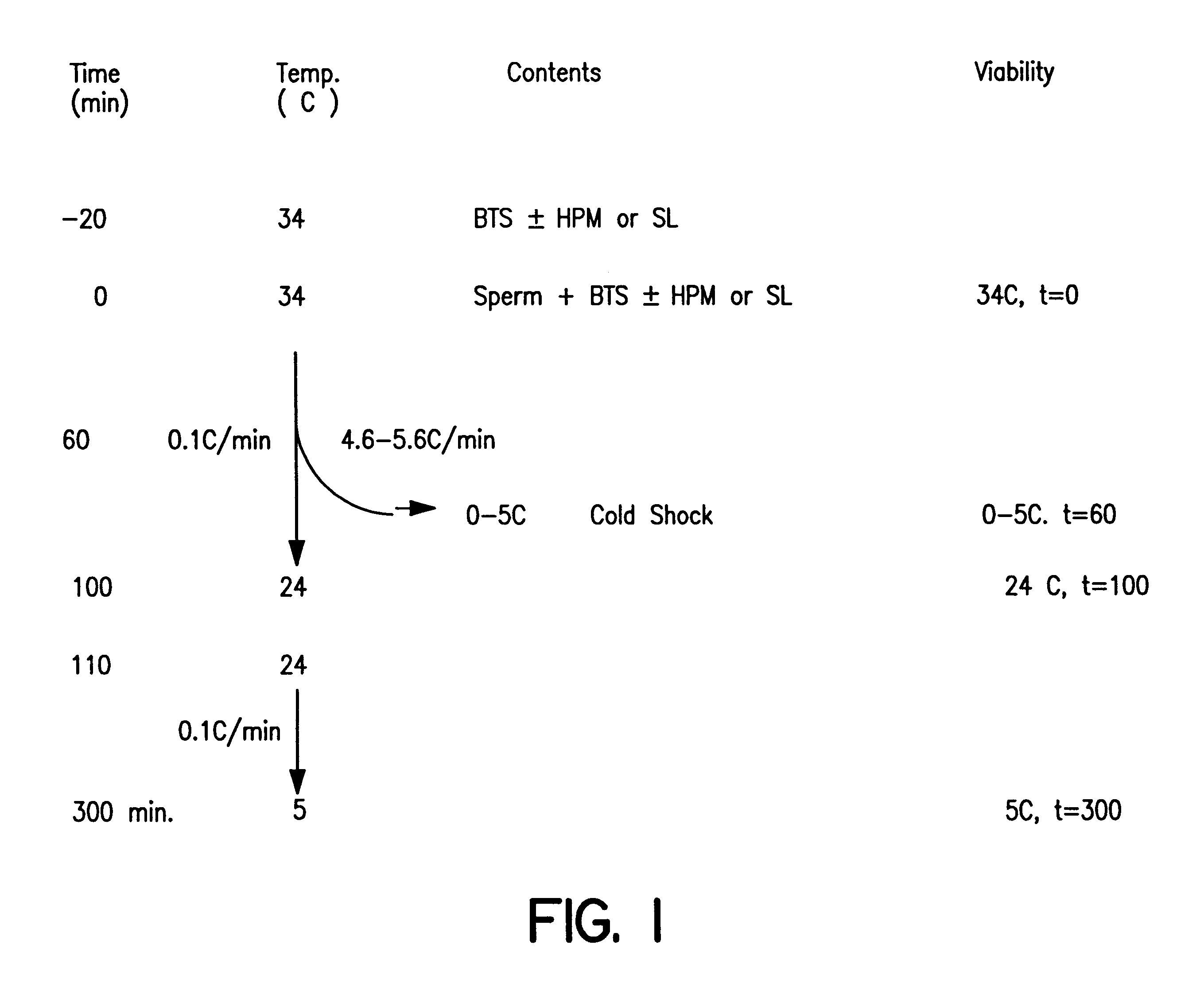
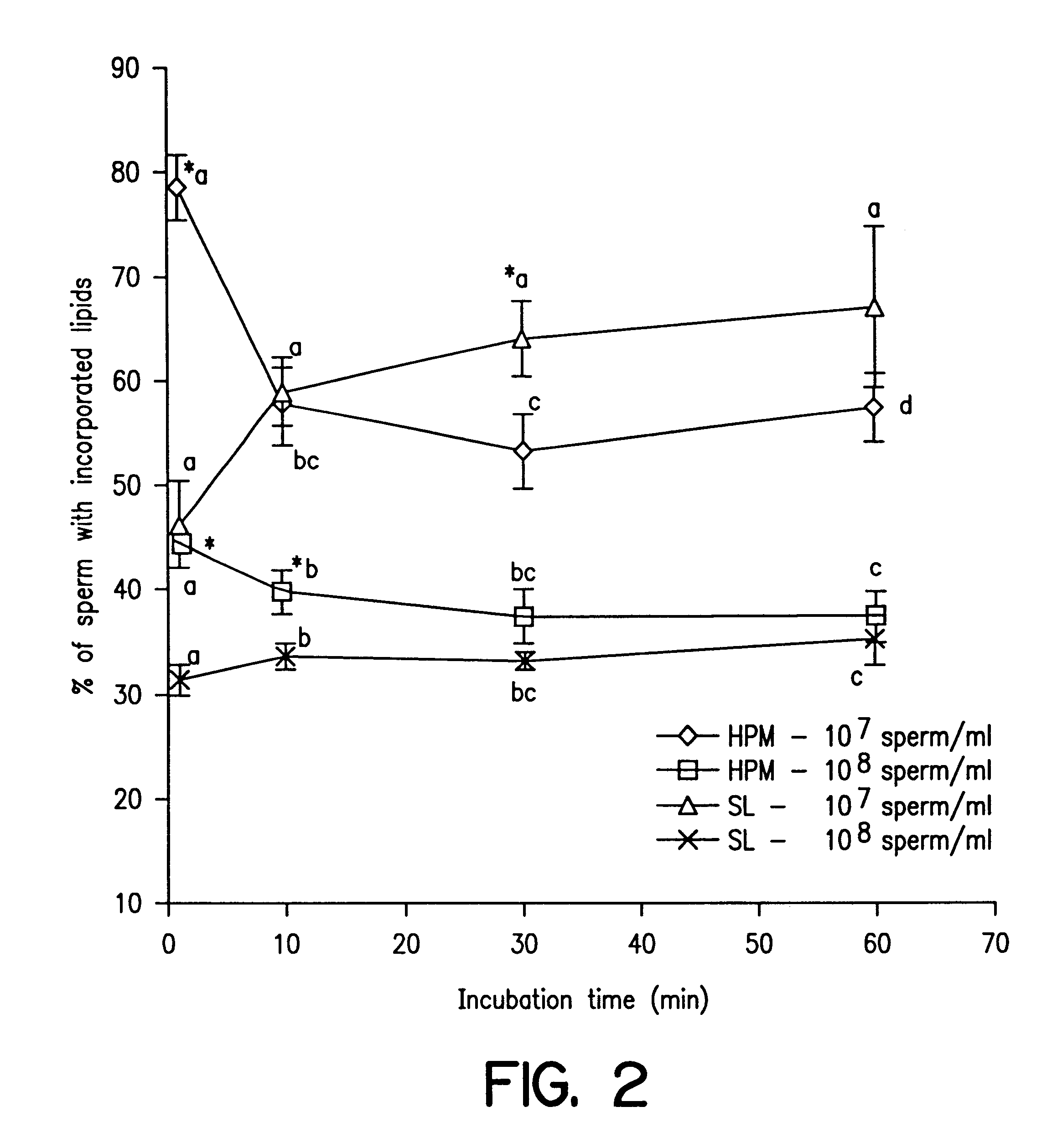
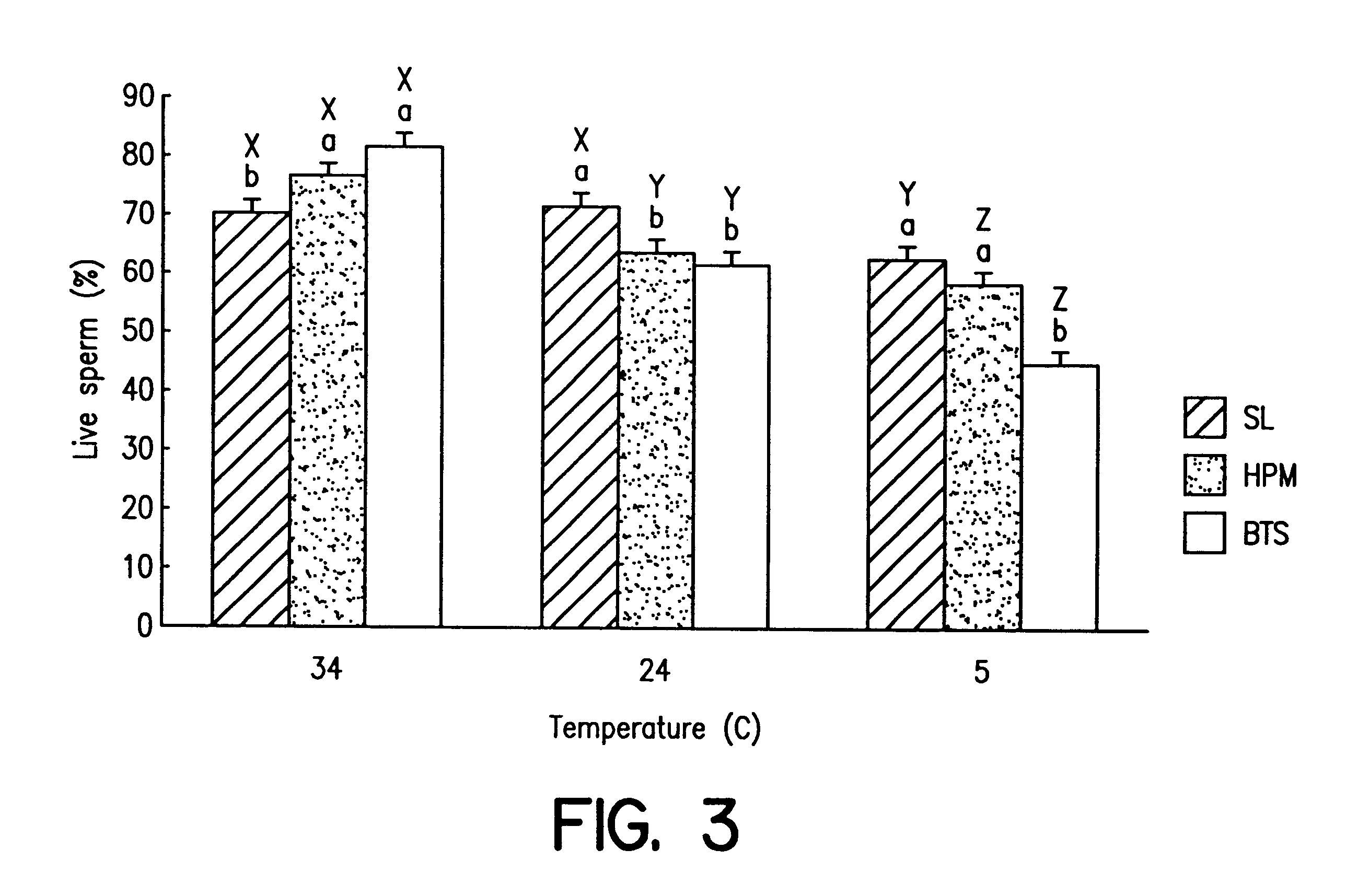
Cryopreserved boar spermatozoa is much less fertile than that of other species, which could be due to damage to sperm membrane lipids during the cryopreservation process. Incorporation of selected lipids improves the survival of boar spermatozoa following cryopreservation.Liposomes were made from lipids extracted from head plasma membrane (HPM) of boar spematozoa or from selected lipids (SL) which contained specific phospholipids. At a fixed lipid concentration, fusion efficiency with spermatozoa as measured by flow cytometry and R18 dequenching was affected by lipid type, sperm concentration and incubation time.SL and HPM improved sperm viability (SYBR-14 and propidium iodide) and motility during cooling to 5C., with SL±egg yolk better than or equal to HPM (P<0.05). Post-thaw, egg yolk showed a strong cryoprotective effect. Compared to HPM, SL-treated sperm had higher post-thaw viability, progressive motility and total motility in the extender including egg yolk and higher viability in the extender excluding egg yolk (P<0.05).
Owner:GUELPH UNIV OF
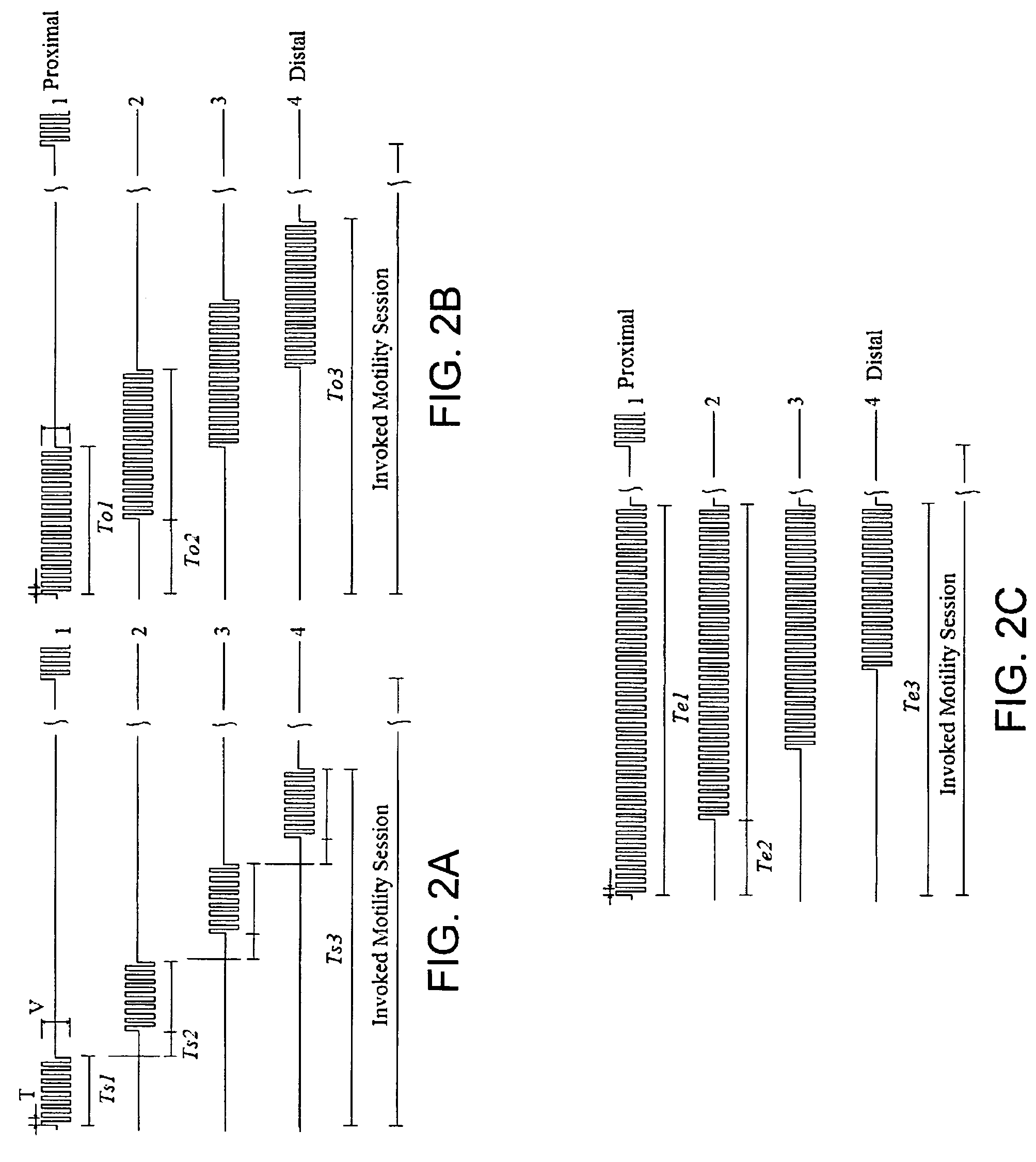
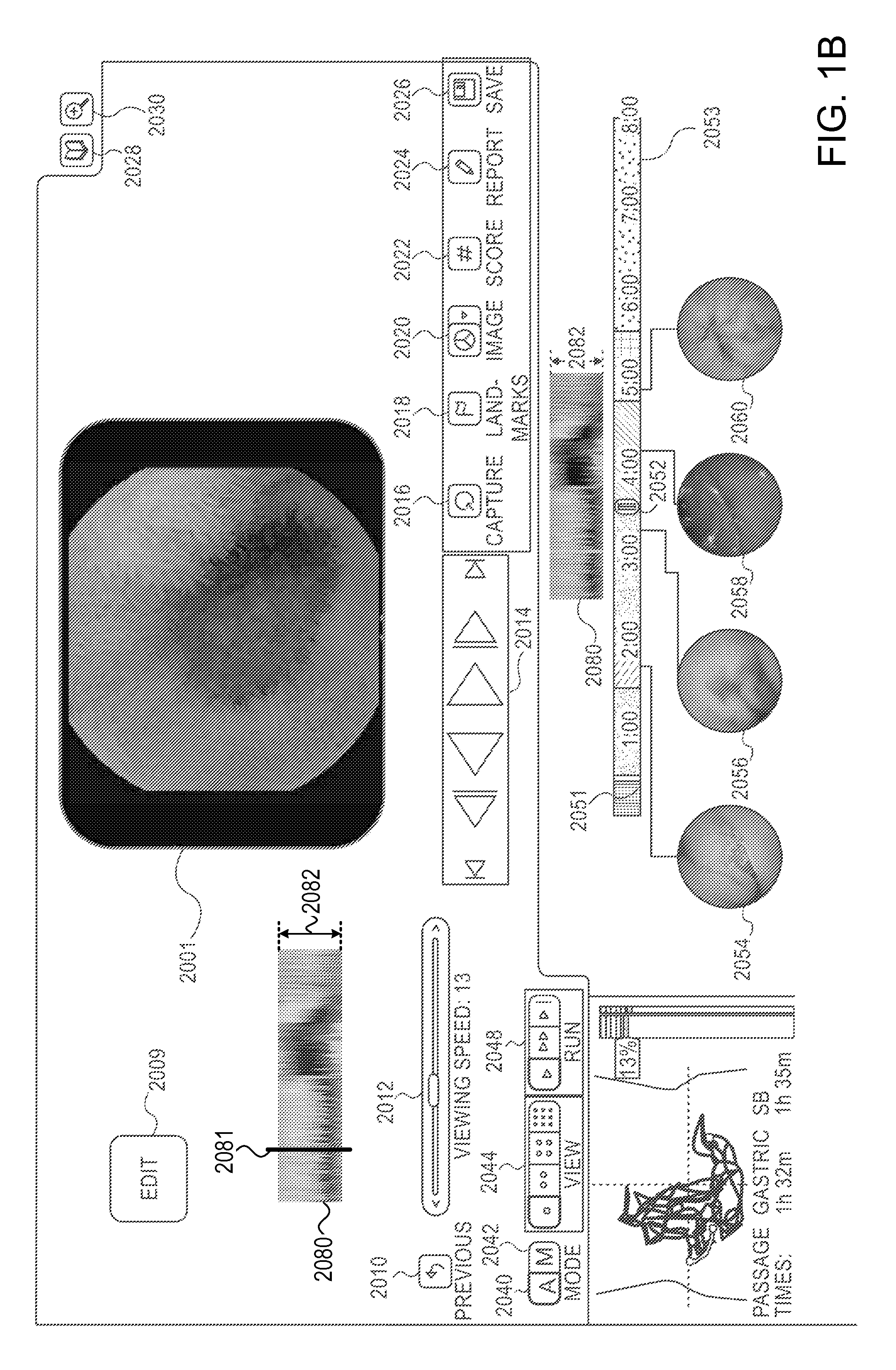
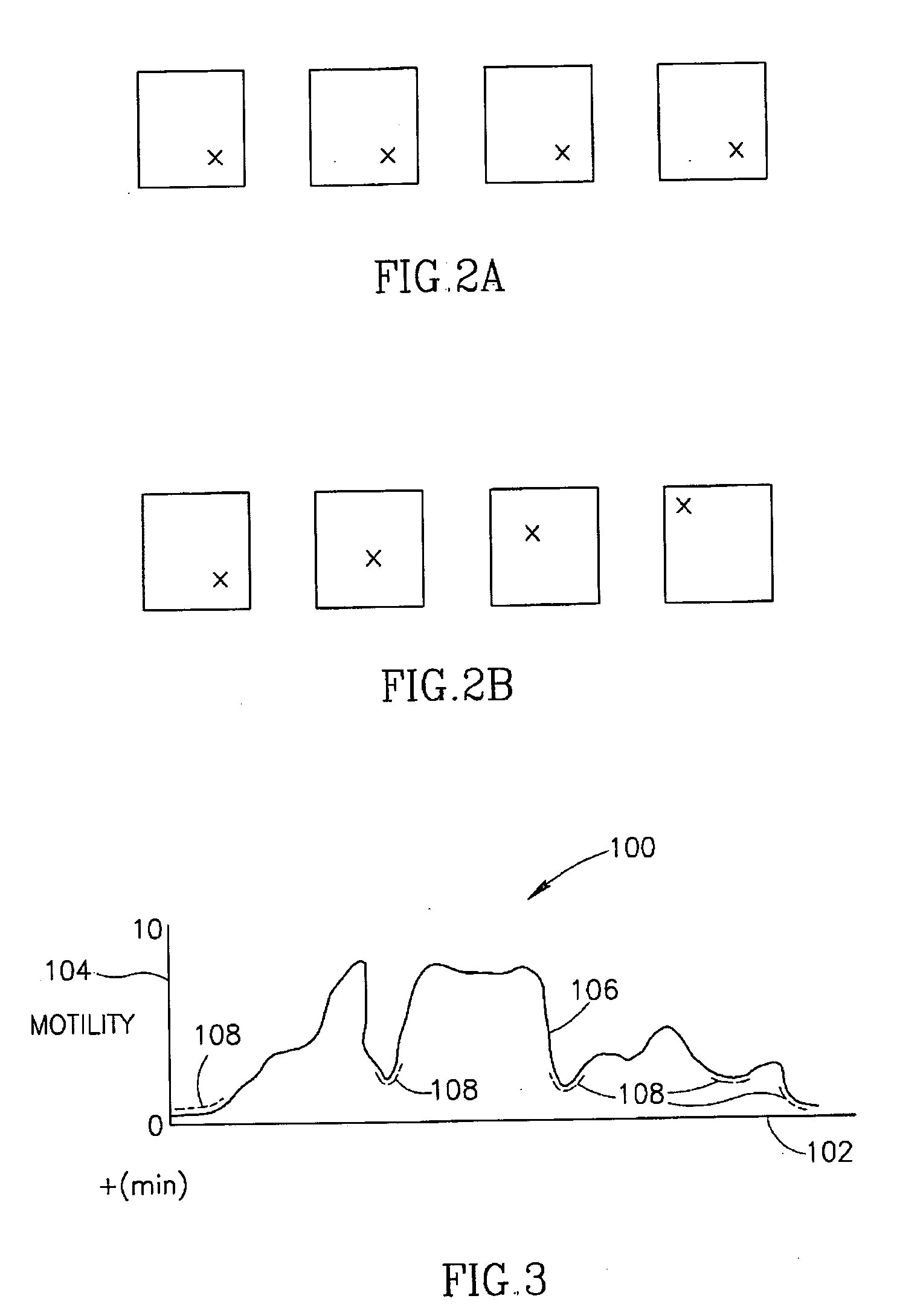
System and method for displaying motility events in an in vivo image stream
ActiveUS20150016700A1Easy to analyzeDiagnosing intestinal motility disordersImage enhancementImage analysisStream captureMotility
A system and method may analyse and display intestinal motility events, based on an image stream captured by an in vivo imaging device. According to some embodiments, the system includes a storage unit to store image frames from the image stream, a processor to select a strip of pixels from a plurality of image frames of the image stream and to align the selected strips adjacently to form a motility events bar, and a visual display unit for displaying the motility events bar to a user.
Owner:GIVEN IMAGING LTD
System for monitoring cell motility in real-time
The invention relates to devices, devices for arraying biomolecules, including cells, methods for arraying biomolecules, assays for monitoring cellular movement, and systems for monitoring cellular movement. The devices include a support; a first layer configured to be placed in fluid-tight contact with the support, the first layer having an upper surface and defining a pattern of micro-orifices, each micro-orifice of the pattern of micro-orifices having walls and defining a micro-region on the support when the first layer is placed in fluid-tight contact with the support such that the walls of said each micro-orifice and the micro-region on the support together define a micro-well; and a second layer configured to be placed in fluid-tight contact with the upper surface of the first layer, the second layer defining a pattern of macro-orifices, each macro-orifice of the pattern of macro-orifices having walls and defining a macro-region when the first layer is placed in fluid-tight contact with the support and the second layer is placed in fluid-tight contact with the first layer such that the walls of the macro-orifice and the macro-region together define a macro-well.
Owner:SURFACE LOGIX INC
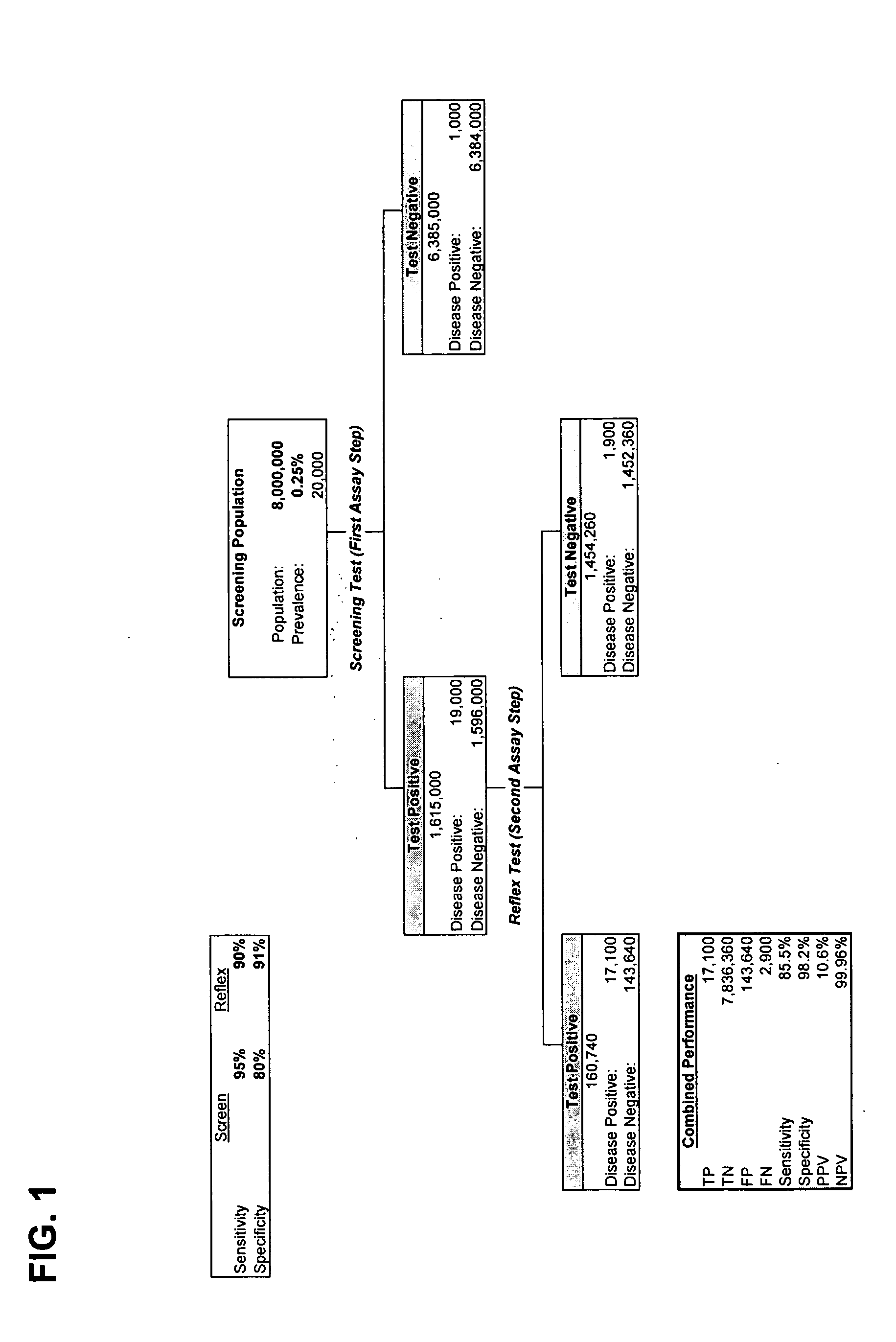
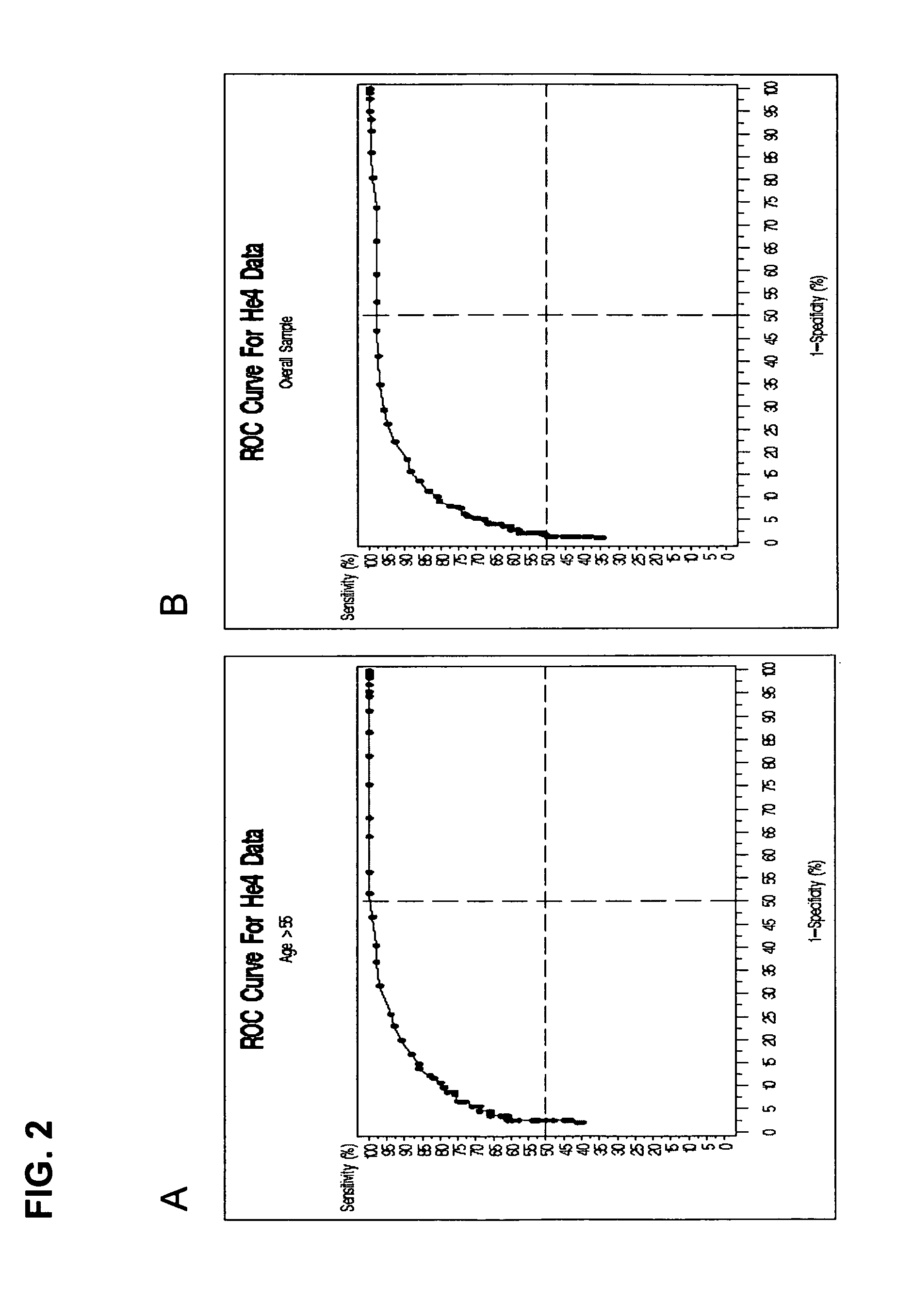
Methods for identifying patients with an increased likelihood of having ovarian cancer and compositions therefor
InactiveUS20070212721A1Easy to detectRaise the possibilityMicrobiological testing/measurementBiological testingCancer cellMotility
Screening methods for identifying patients with an increased likelihood of having ovarian cancer are provided. The screening methods involve the detection of expression of a plurality of biomarkers in a body sample, wherein overexpression of the biomarkers is indicative of an increased likelihood of having ovarian cancer. The screening methods may further comprise a two-step analysis. Biomarkers of interest include genes and proteins that are, for example, involved in defects in DNA replication / cell cycle control, cell growth and proliferation, escape from apoptosis, angiogenesis or lymphogenesis, or the mechanisms of cancer cell motility and invasion. In some aspects of the invention, expression of a biomarker is detected at the protein level using a biomarker-specific antibody or at the nucleic acid level using nucleic acid hybridization techniques. Methods for detecting ovarian cancer in patients are further disclosed herein. Kits for practicing the methods of the invention are further provided.
Owner:TRIPATH IMAGING INC
Methods and Systems for Submucosal Implantation of a Device for Diagnosis and Treatment of a Body
Instruments, systems and methods are provided for performing submucosal medical procedures in a desired area of the digestive tract using endoscopy. Instruments include a safe access needle injection instrument, a submucosal tunneling instrument, a submucosal dissection instrument, and a mucosal resection device. Systems include a combination of one or more of such instruments with or without injectable agents. Embodiments of various methods for performing the procedures are also provided. In accordance with one aspect there is provided a submucosal implant device for diagnosing and treating disorders of the body. The submucosal implant device may take the form of a gastric stimulator in which signals are supplied to the muscular wall of a mammal to treat motility disorders. In accordance with yet another aspect there is provided a method for performing a submucosal medical procedure to deploy a submucosal implant device in the digestive tract of a mammal.
Owner:APOLLO ENDOSURGERY INC
Use of methylnaltrexone in treating gastrointestinal dysfunction in equines
InactiveUS20050011468A1Relieving inhibition of gastrointestinal motilityMaintaining pain-reducing effectOrganic active ingredientsOther apparatusMotilityEquine Species
Systems and methods are described for using methylnaltrexone to treat or prevent inhibition of gastrointestinal motility in equines. A method for preventing or treating opioid-induced and non-opioid-induced gastrointestinal dysfunction includes administering a quaternary derivative of noroxymorphone, preferably methylnaltrexone, to an equine before or after the onset of the gastrointestinal dysfunction.
Owner:PROGENICS PHARMA INC
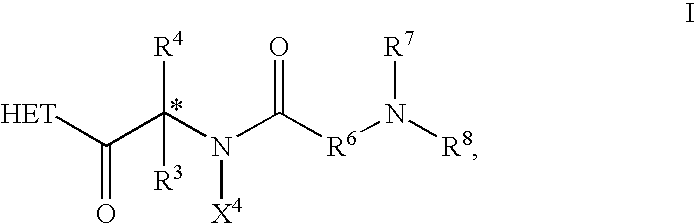
Compositions and methods for stimulating gastrointestinal mobility
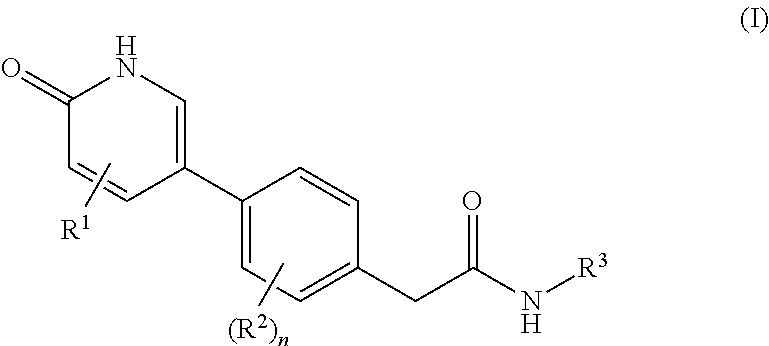
The present invention is directed to methods for stimulating the motility of the gastrointestinal system in a patient which comprises administering a growth hormone secretagogue, a prodrug thereof or a pharmaceutically acceptable salt of said secretagogue or said prodrug. More particularly, the present invention provides methods for stimulating the motility of the gastrointestinal system in a patient which comprises administering a compound of Formula I: a prodrug thereof or a pharmaceutically acceptable salt of said secretagogue or said prodrug.
Owner:RAQUALIA PHARMA INC
Methods of inhibiting and treating bacterial biofilms by metal chelators
The invention presented herein provides methods and compositions for the prevention and treatment of bacterial infections. The methods are based on the discovery that depletion of bioavailable iron stimulates surface motility in bacteria thus inhibiting the ability of a bacterial population to develop into a biofilm.
Owner:UNIV OF IOWA RES FOUND
Compositions and methods for bowel care in individuals with chronic intestinal pseudo-obstruction
ActiveUS7635709B2Shorten the construction periodDifficult to administerBiocideAmine active ingredientsBowel careSide effect
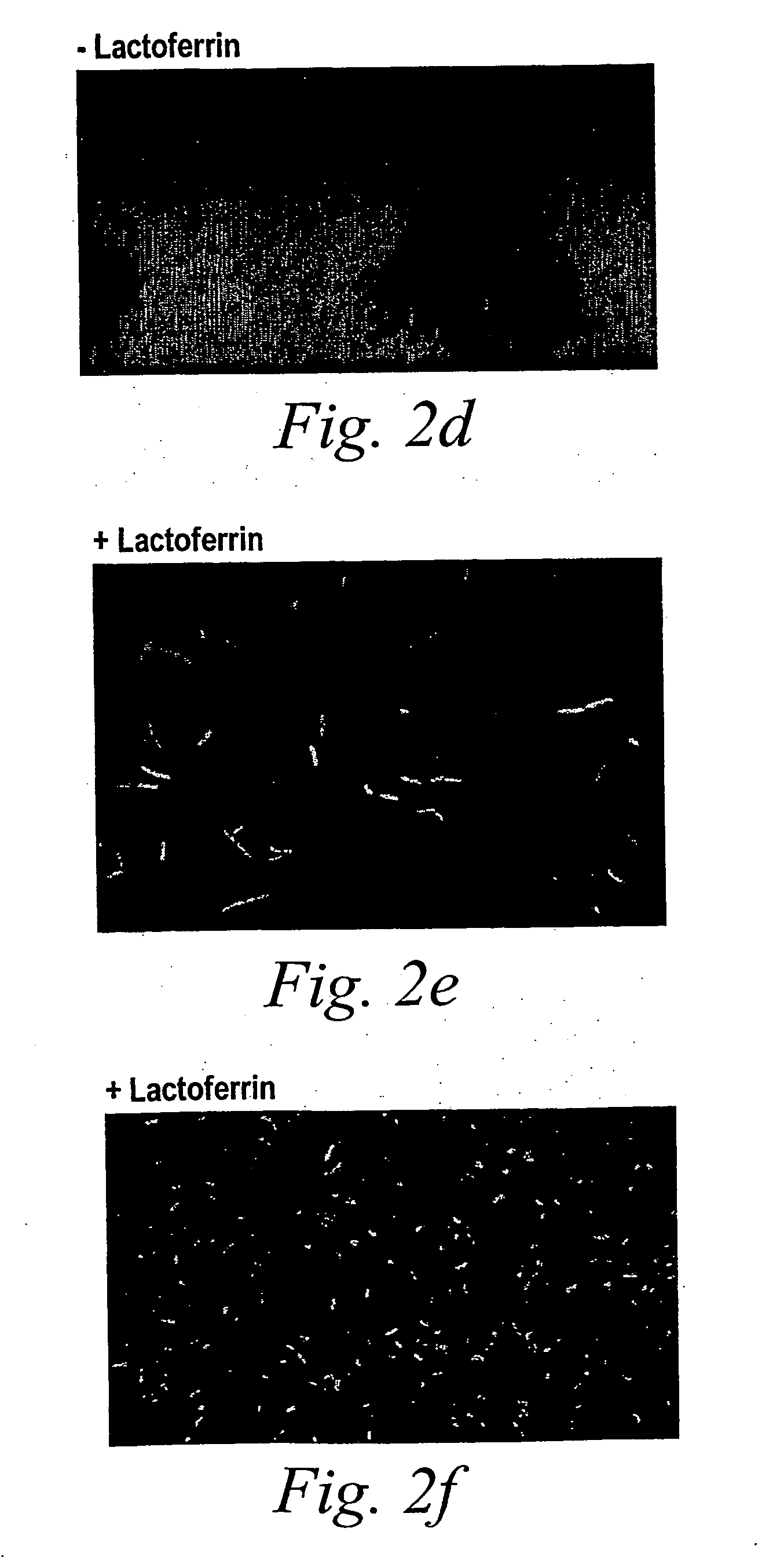
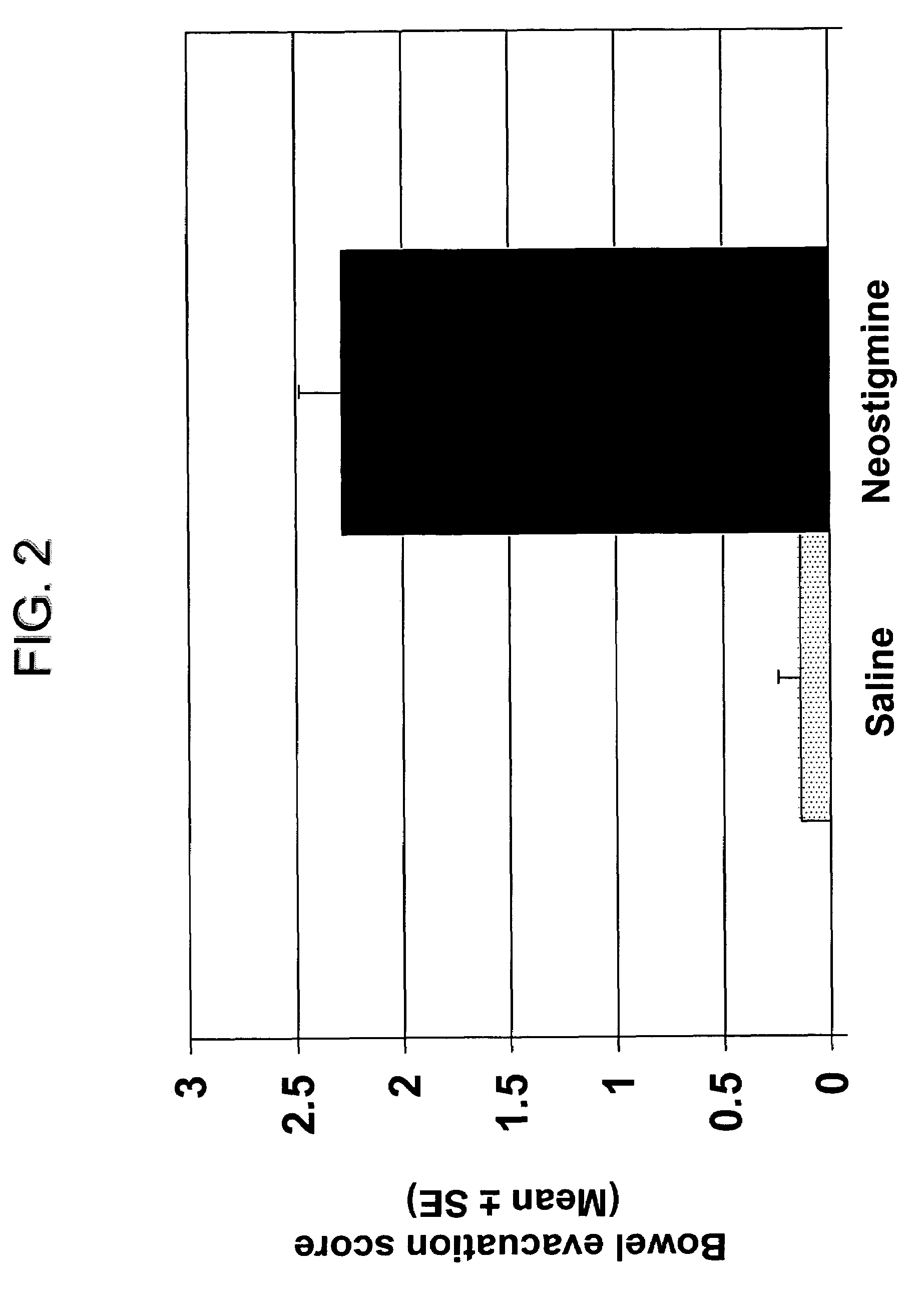
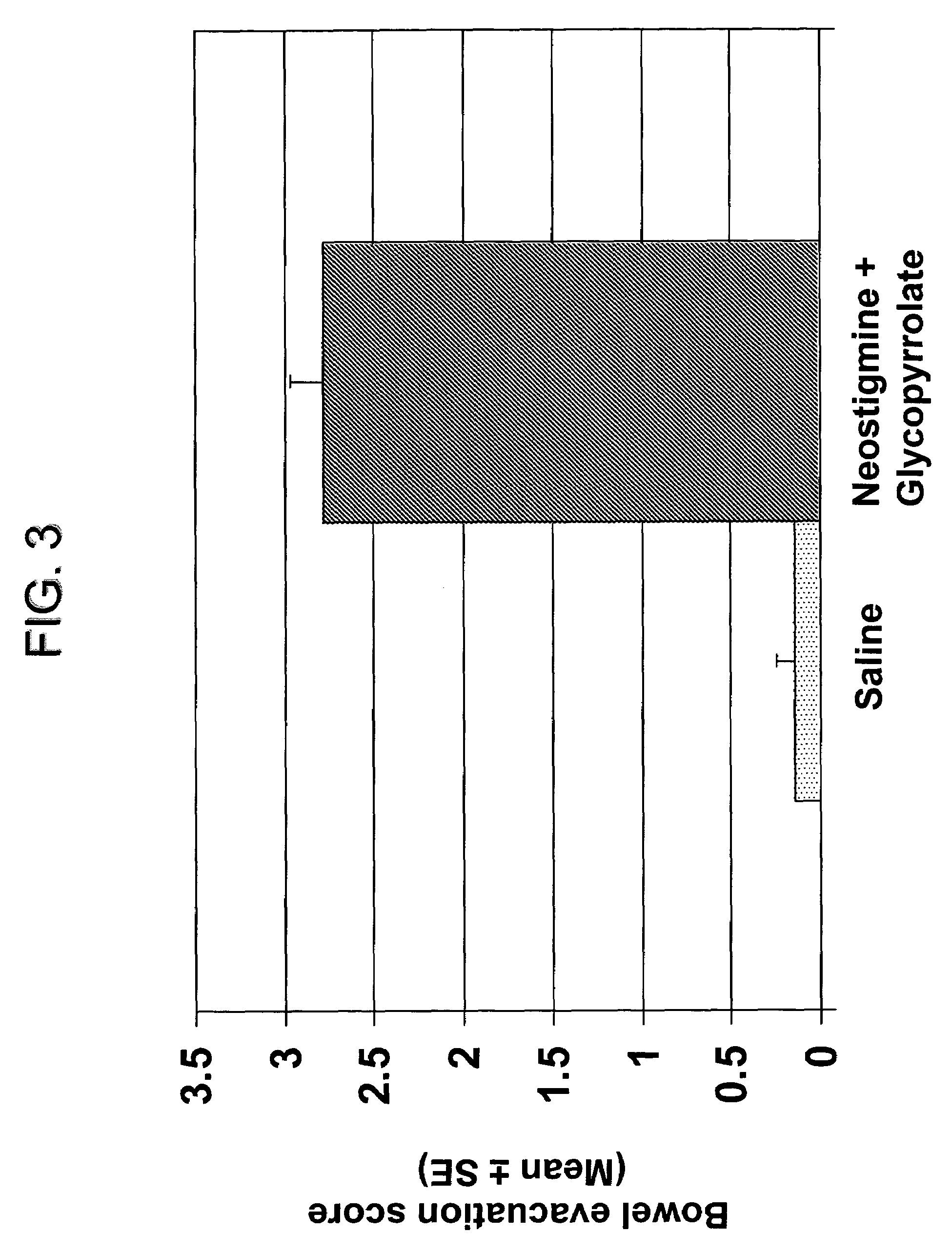
The present disclosure provides compositions and methods for on-going bowel care for persons with chronic intestinal pseudo-obstruction. The compositions and methods can be administered in a non-clinical setting. The compositions comprise acetylcholinesterase inhibitors for stimulating motility of the bowel in combination with anti-cholinergic agents to counteract the potentially dangerous cardiac side effects of the acetylcholinesterase inhibitor. In some examples, the acetylcholinesterase inhibitor, neostigmine, and the anti-cholinergic agent, glycopyrrolate, are combined in a pharmaceutical composition. Certain examples also provide the frequency and duration of administration of the disclosed drug combinations.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
Motility analysis within a gastrointestinal tract
Owner:GIVEN IMAGING LTD
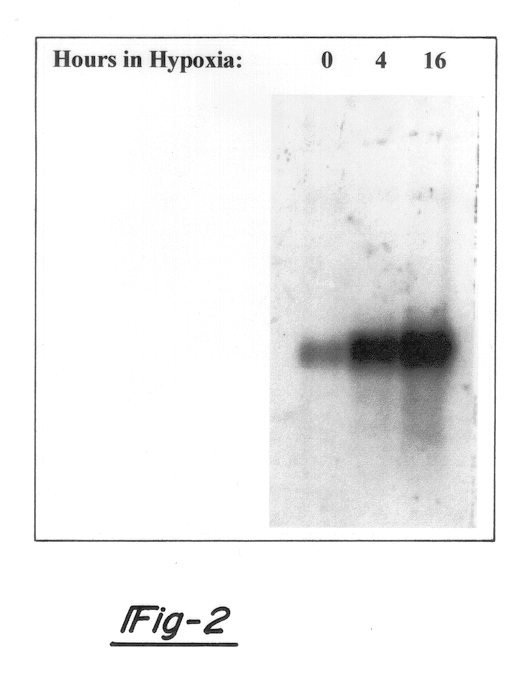
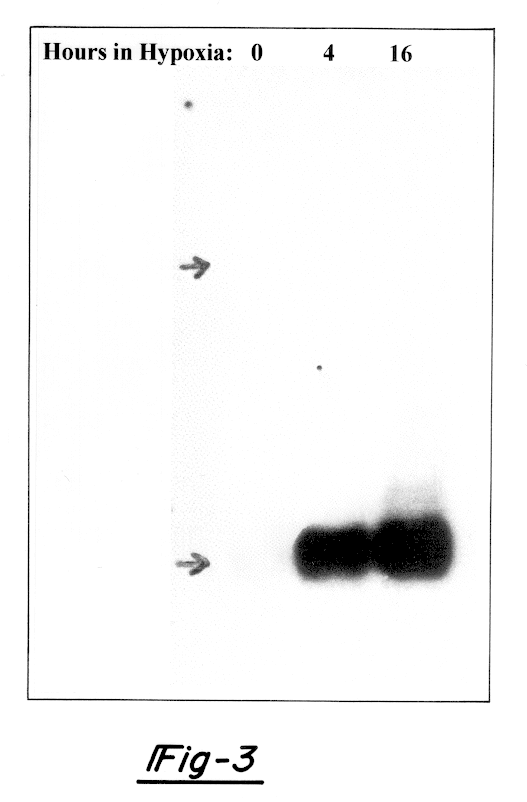
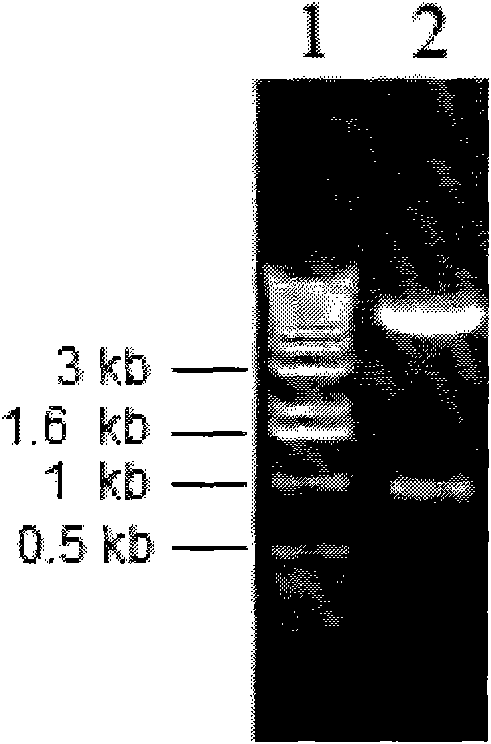
Hypoxia-regulated genes
Owner:QUARK FARMACUITIKALS INC
Exendin-4 and analog fusion protein thereof
ActiveCN101891823APromote regenerationPromote repairPeptide/protein ingredientsAntibody mimetics/scaffoldsDiseaseMotility
The invention discloses exendin-4, an analog fusion protein thereof, the corresponding polynucleotide sequence, carrier, host cell and pharmaceutical composite and a preparation method and applications of the fusion protein. The fusion protein is prepared by fusing exendin-4 and analog thereof with human immunoglobulin IgG2-Fc through special connecting peptide and has better stability and loner half-life in vivo. The fusion protein can be administered by performing local delivery, using aerosol and using injection. The fusion protein can promote the regeneration and repair of islet beta cells, increase islet beta cells, promote the secretion of insulin and improve the sensitivity of organism to insulin. The fusion protein is used to cure diabetes, adiposity and other diseases which can be benefited by reducing plasma glucose and inhibiting gastrointestinal motility and gastric emptying.
Owner:BEIJING DONGFANG BIOTECH
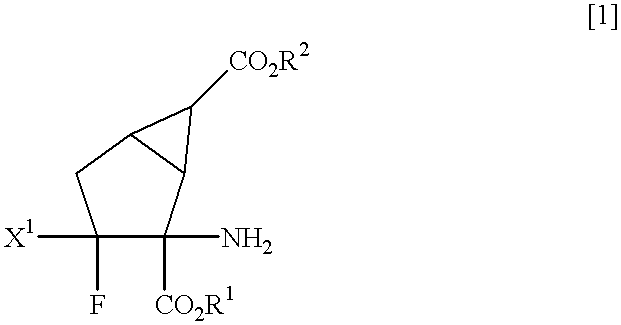
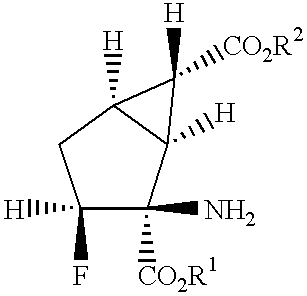
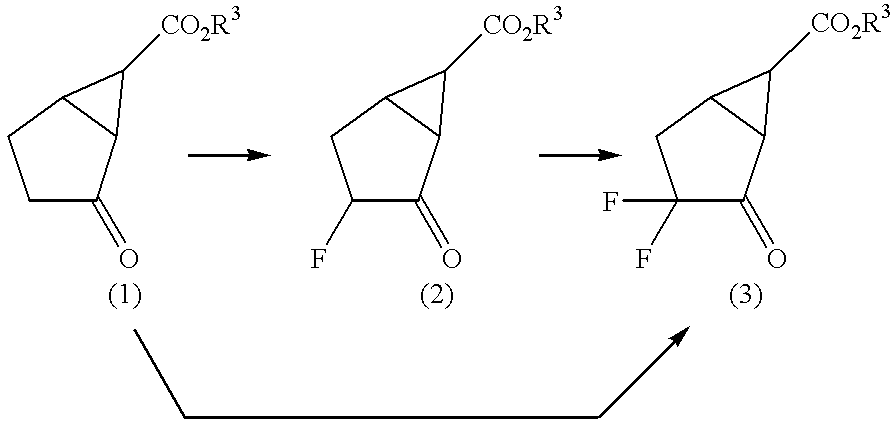
Fluorine-containing amino acid derivatives
Fluorine-containing amino acid derivatives represented general formula (I), pharmaceutically acceptable salts thereof or hydrates of the same, wherein X1 represents hydrogen or fluorine; and R1 and R2 are the same or different and each represents hydrogen or lower C1-10 alkyl. These compounds are useful as drugs, in particular, group 2 metabotropic glutamate receptor agonists for treating and preventing psychiatric disorders such as schizophrenia, anxiety and associated diseases, depression, bipolar disturbance and epilepsy, and neurological diseases such as drug addiction, cognition disorder, Alzheimer's disease, Huntington's chorea, Parkinson's disease, motility disturbance associating muscular stiffness, cerebral ischemia, cerebral insufficiency, spinal cord lesion and head disturbance.
Owner:TAISHO PHARMACEUTICAL CO LTD
Mushroom dried bean curd preparation method
InactiveCN102894104AImprove immunityFavorable peristalsisCheese manufactureFood scienceMotilityShiitake mushrooms
The invention discloses a mushroom dried bean curd preparation method, which is prepared by using the following raw materials in parts by weight: 1000-1200 parts of soybean, 10-12 parts of Gracilaria lemaneiformis, 10-12 parts of Brasenia schreberi, 30-40 parts of mushrooms, 10-15 parts of Salvia miltiorrhiza, 10-12 parts of Equisetum hiemale, 10-12 parts of Petasites tatewakianus Kitam, 10-12 parts of angelica roots, 10-12 parts of cresses, 10-12 parts of pumpkin flowers, 5-8 parts of Potentilla chinensis, 20-30 parts of hot peppers, 5-8 parts of pitaya flowers, 10-12 of green tea, 10-12 parts of white fungi, 10-12 parts of o red tangerine peels and 10-12 parts of Chinese chives. Since various wild vegetables and medicine-food homologous traditional Chinese medicine healthcare components are added into dried bean curd raw materials and the mushrooms have an effect of improving immunity, can prevent and resist cancers and are beneficial to health, the mushroom dried bean curd does not have a side effect when the mushroom dried bean curd is eaten for a long term. Besides, since a multiple-time grinding process is adopted, bean dregs are reduced, dietary fibers in the bean dregs are added into soymilk, the gastrointestinal motility of human bodies is improved and the defecation is facilitated; and since brine contains various traditional spices and also contains components such as American ginseng, the human body immunity is improved and the life is prolonged.
Owner:HEFEI FENGLUOHE BEAN FOOD
Novel compounds as rearranged during transfection (RET) inhibitors
This invention relates to novel compounds which are inhibitors of the Rearranged during Transfection (RET) kinase, to pharmaceutical compositions containing them, to processes for their preparation, and to their use in therapy, alone or in combination, for the normalization of gastrointestinal sensitivity, motility and / or secretion and / or abdominal disorders or diseases and / or treatment related to diseases related to RET dysfunction or where modulation of RET activity may have therapeutic benefit including but not limited to all classifications of irritable bowel syndrome (IBS) including diarrhea-predominant, constipation-predominant or alternating stool pattern, functional bloating, functional constipation, functional diarrhea, unspecified functional bowel disorder, functional abdominal pain syndrome, chronic idiopathic constipation, functional esophageal disorders, functional gastroduodenal disorders, functional anorectal pain, inflammatory bowel disease, proliferative diseases such as non-small cell lung cancer, hepatocellular carcinoma, colorectal cancer, medullary thyroid cancer, follicular thyroid cancer, anaplastic thyroid cancer, papillary thyroid cancer, brain tumors, peritoneal cavity cancer, solid tumors, other lung cancer, head and neck cancer, gliomas, neuroblastomas, Von Hippel-Lindau Syndrome and kidney tumors, breast cancer, fallopian tube cancer, ovarian cancer, transitional cell cancer, prostate cancer, cancer of the esophagus and gastroesophageal junction, biliary cancer, adenocarcinoma, and any malignancy with increased RET kinase activity.
Owner:GLAXOSMITHKLINE INTPROP DEV LTD
Method and composition for improving fertility health in female and male animals and humans
InactiveUS7045151B2Increasing for conceptionFree from damageHeavy metal active ingredientsBiocideToxicantArginine
In a new pharmaceutical combination, the herb, Vitex agnus-castus (chasteberry), enhances hormone balance by increasing progesterone release and, therefore, ovulation frequency. The antioxidants, green tea, vitamin E, and selenium, improve overall reproductive health. L-arginine, an amino acid, stimulates the reproductive organs by improving circulation. Folic acid, vitamins B6 and B12, iron, zinc and magnesium help promote womens' fertility. Sperms are highly susceptible to free radical or oxidative damage from environmental toxicants and natural aging. Vitamins C and E, coenzyme Q10 and selenium are all potent antioxidants that help improve sperm counts and quality. Ferulic acid, an antioxidant found in Dong quai, also improves sperm quality. Zinc and B vitamins (B6, B12 and folate) are critical nutrients in male reproductive systems for hormone metabolism, sperm formation and motility. The amino acid, L-carnitine, promotes formation of healthy sperm.
Owner:THE DAILY WELLNESS
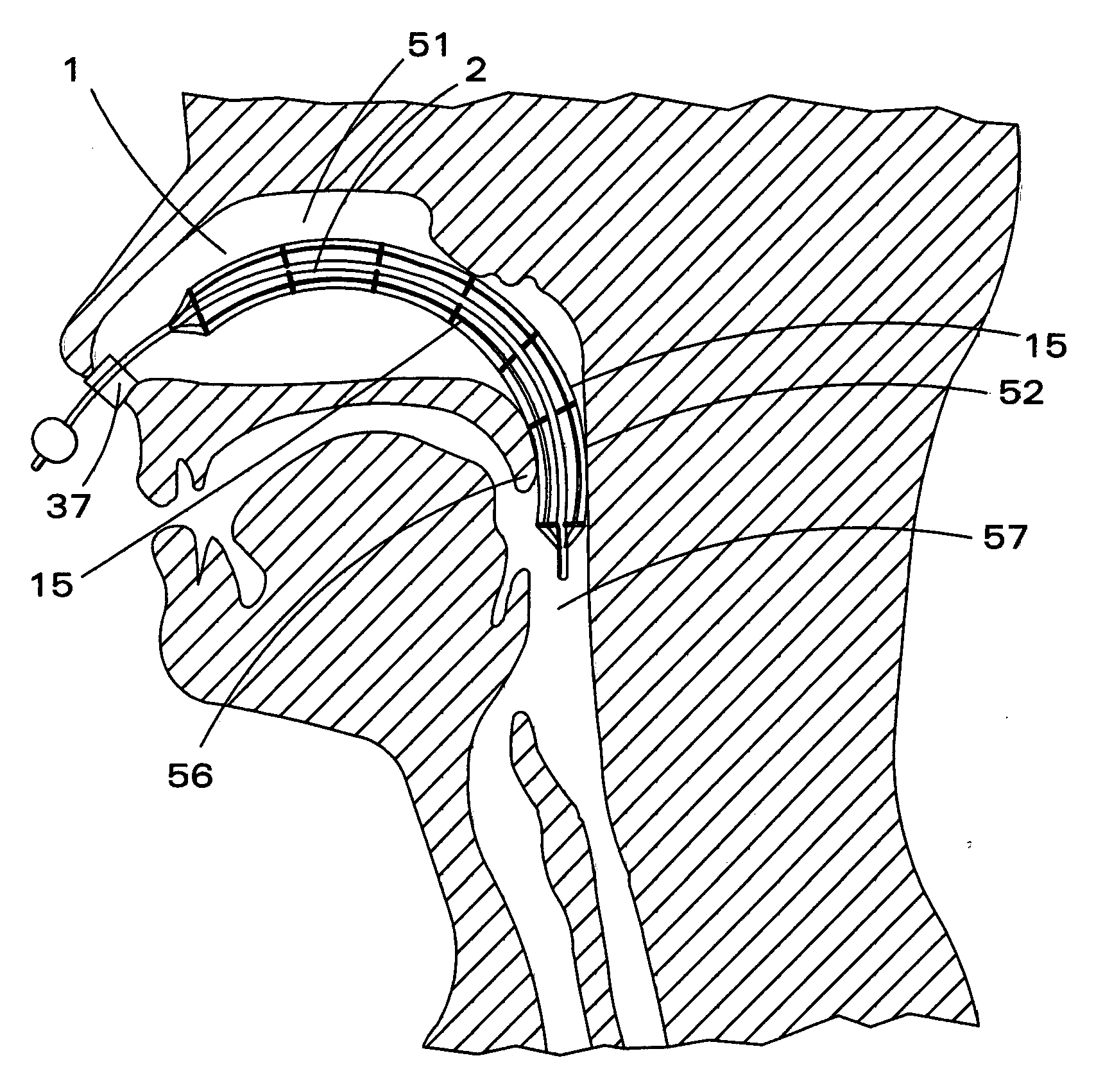
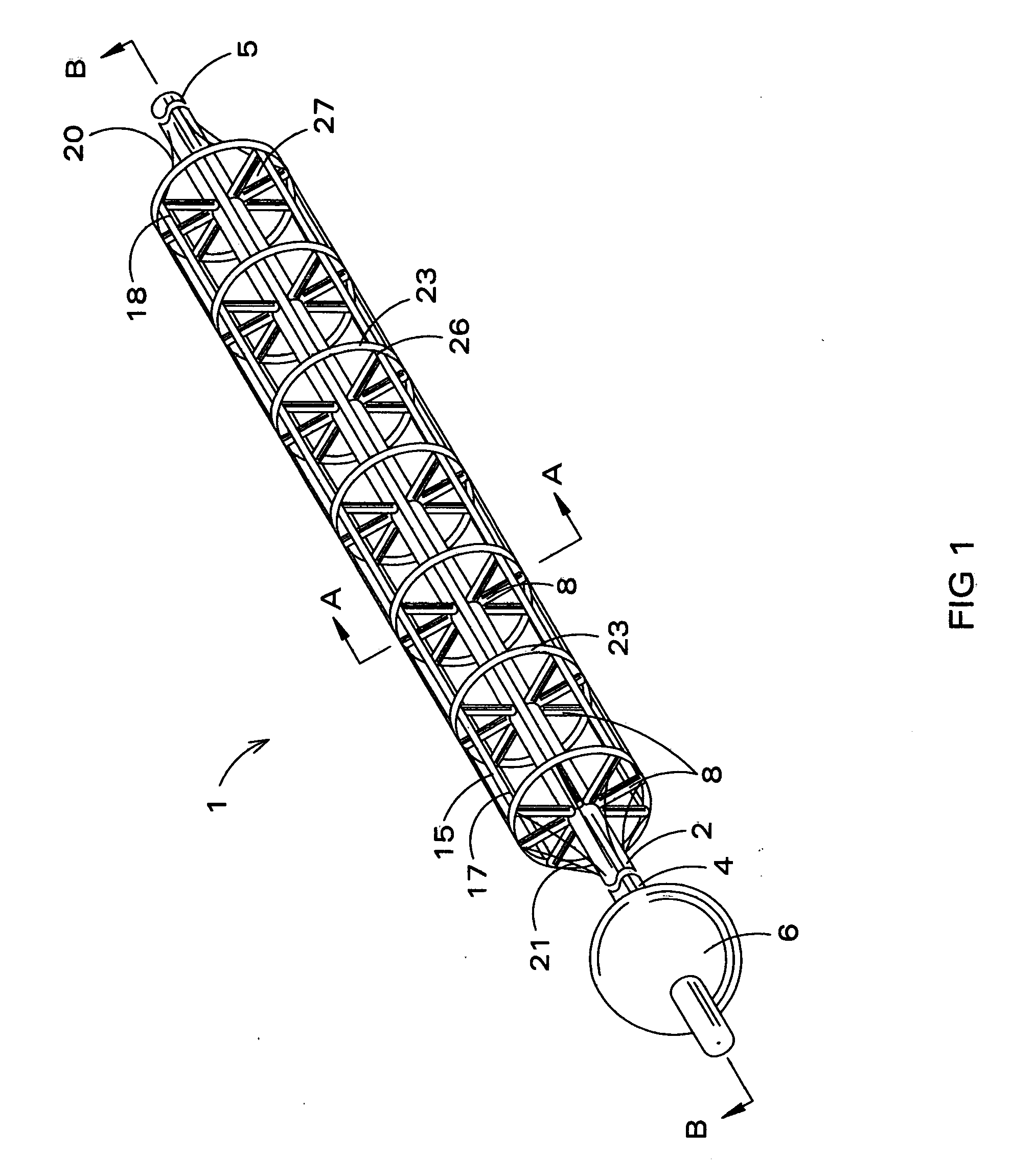
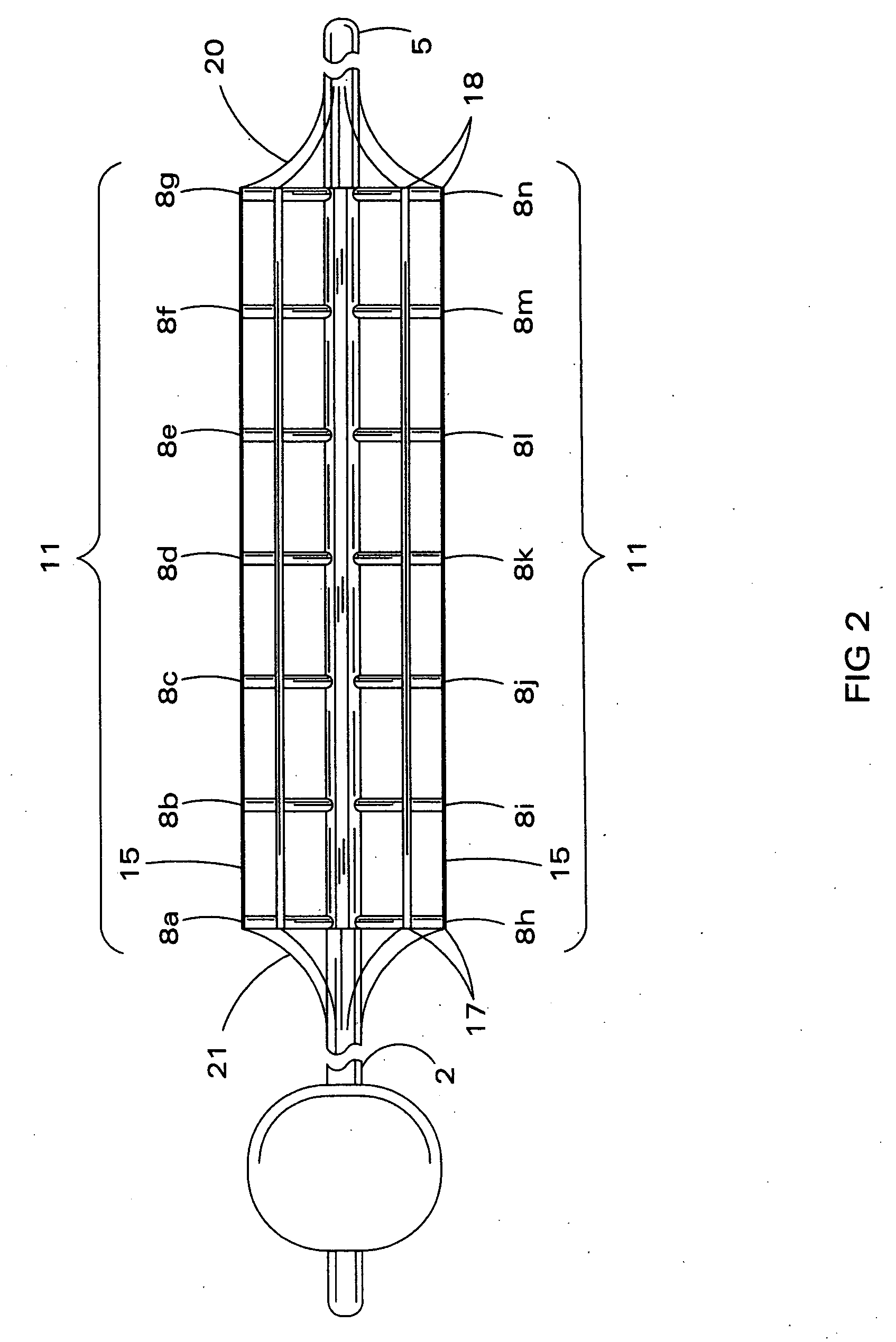
Inflatable nasopharyngeal stent
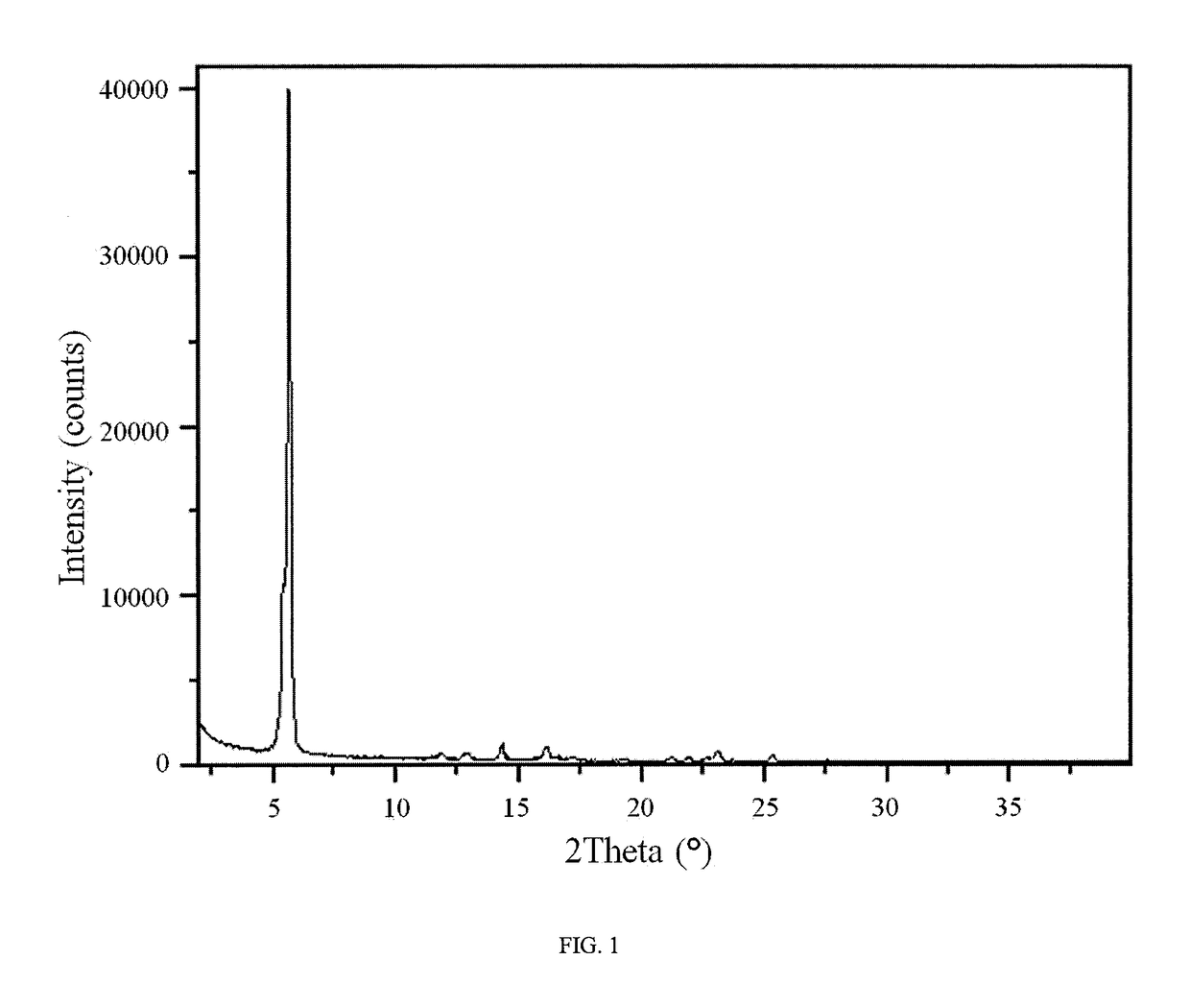
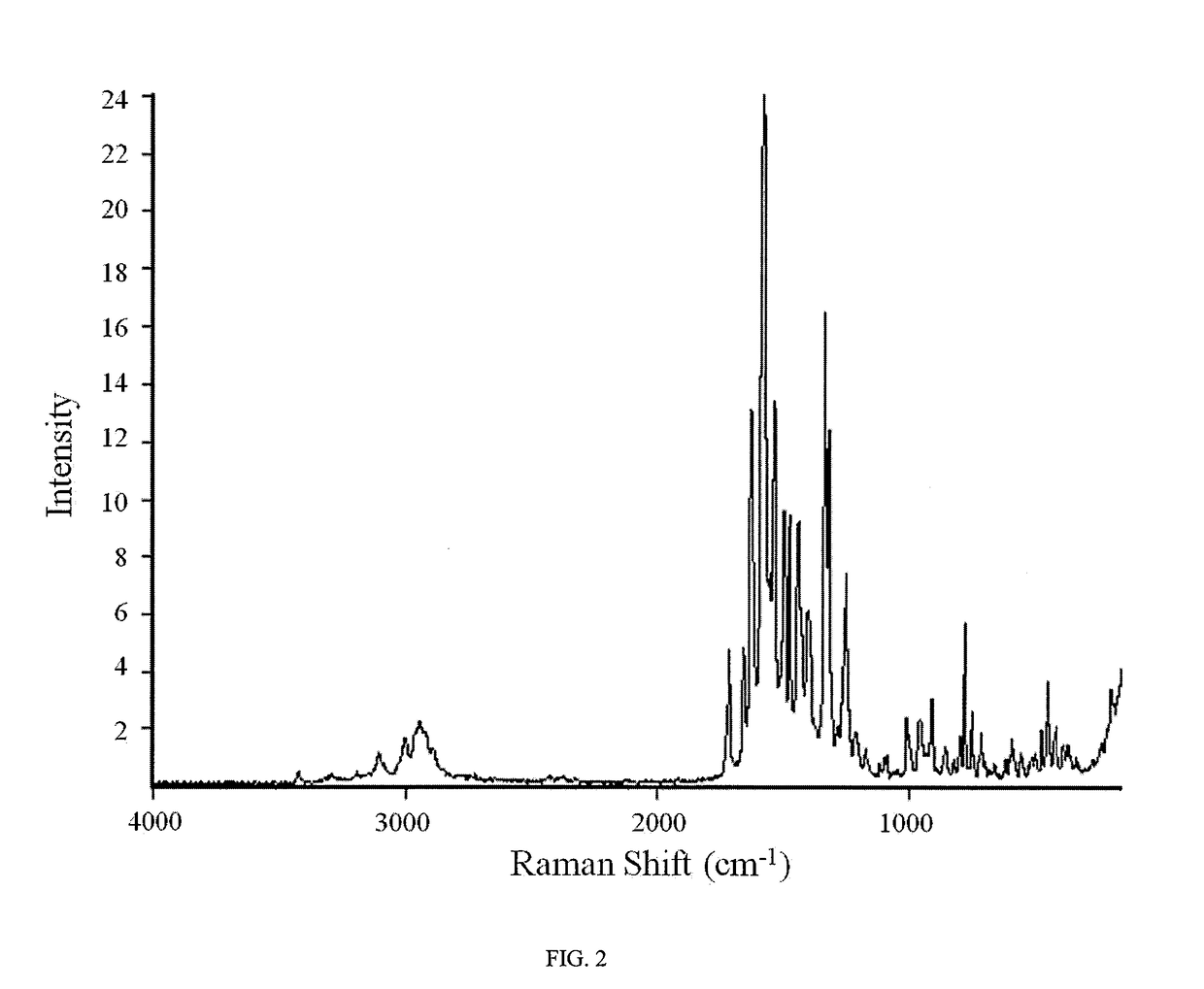
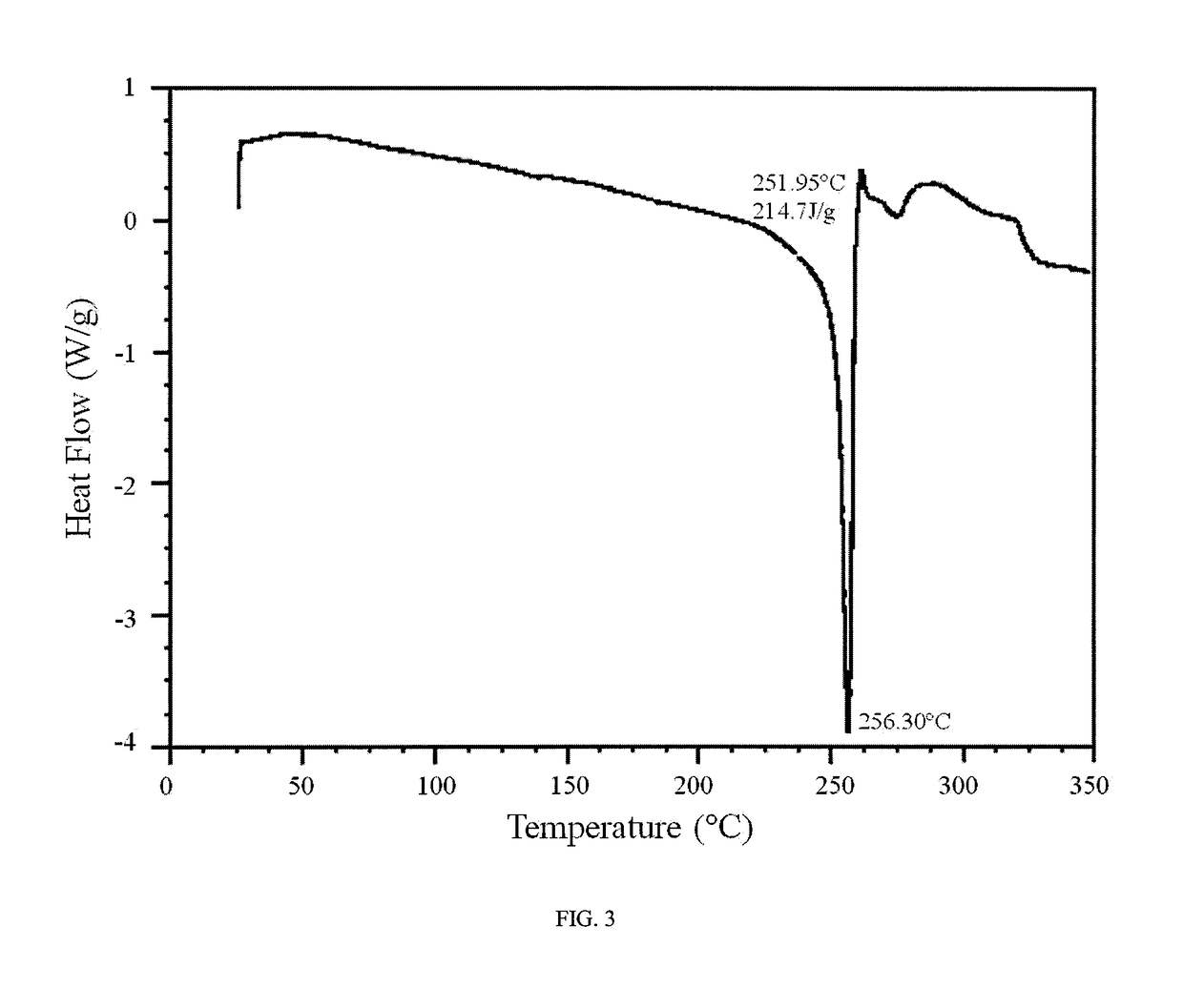
An inflatable nasopharyngeal stent is disclosed along with a method of using same. The stent comprises a central tube having a lumen defining a central inner chamber. A plurality of inflatable spokes are disposed along the central tube. The spokes are in fluid communication with the inner chamber of the central tube and are preferably aligned in groupings along the central tube. The outer ends of the spokes connect to a rib. The un-inflated stent is inserted into the nasal passageway through a naris and positioned such that so that a portion of the device is proximal to an anatomic structure exhibiting undesirable inflammation, configuration, growth or motility. Once positioned, the stent is inflated. Upon inflation, the ribs and adjoining web members move outwardly from the central tube and press upon the tissues of the nasopharyngeal cavity. Spaces between the inflatable spokes permit the passage of air along the stent and maintain airway patency.
Owner:OBERLE FAMILY TRUST
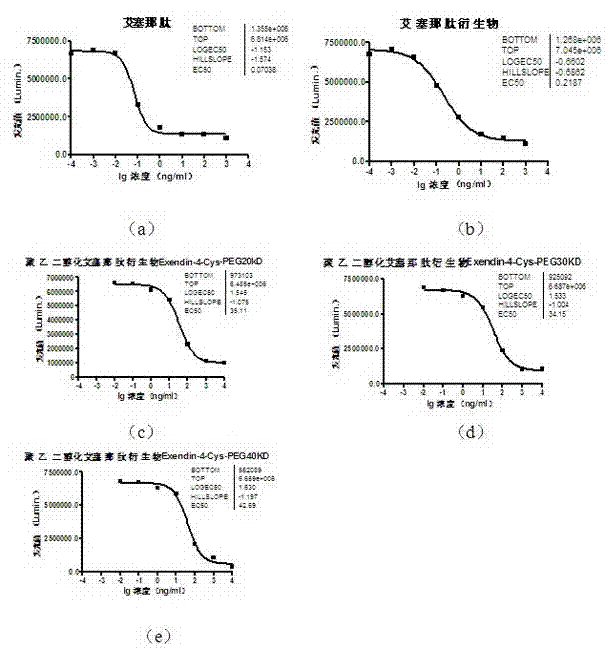
Pegylated exenatide ramification and use thereof
InactiveCN102827270AReduce clearanceExtended half-lifeHormone peptidesPeptide/protein ingredientsClearance rateMotility
The invention provides a pegylated exenatide ramification (Exendin-4-Cys-PEG (polyethylene glycol)) and the use of the pegylated exenatide ramification, belonging to the technical field of the preparation and the application of a polypeptide drug. A preparation method of the pegylated exenatide ramification provided by the invention comprises the following steps of: introducing a cysteine at the C-end of exenatide, and coupling maleic acylamino polyethylene glycol, so that the pegylated exenatide ramification can be formed. The pegylated exenatide ramification provided by the invention has slow clearance rate in the blood circulation and prolonged half-life period, and can be used for preparing the related illness treatment drugs for reducing the blood sugar, reducing the ingestion, reducing the gastrointestinal emptying, increasing the beta cell quantity or reducing the gastrointestinal motility.
Owner:无锡和邦生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com