Use of methylnaltrexone and related compounds
a technology of methylnaltrexone and related compounds, which is applied in the direction of heterocyclic compound active ingredients, biocide, drug compositions, etc., can solve the problems of limited efficacy of common treatments of bulking agents and laxatives, and associated with undesirable side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
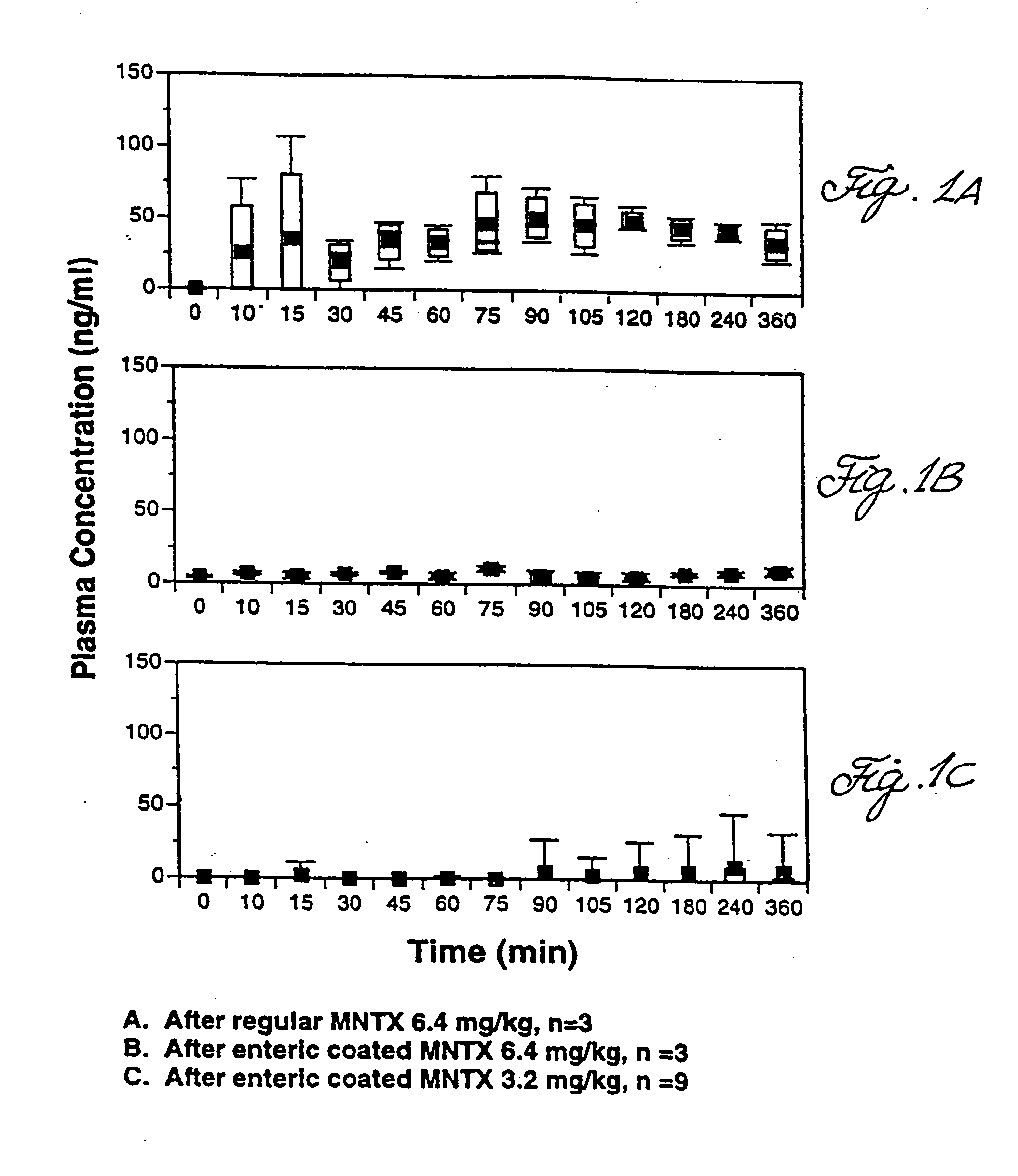
Effects Of Enterically Coated MNTX On Oral-Cecal Transit Time and Plasma Levels of MNTX
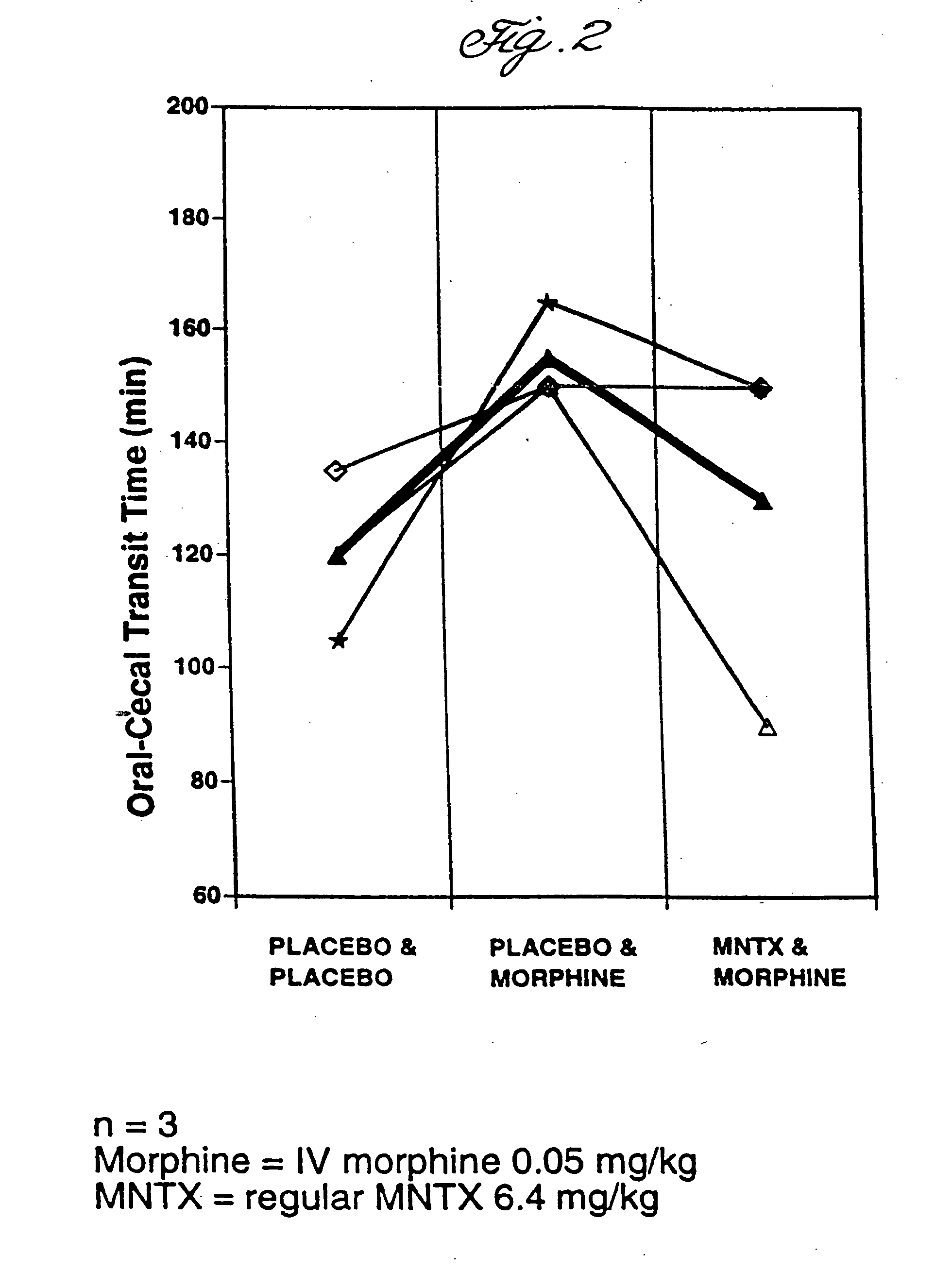
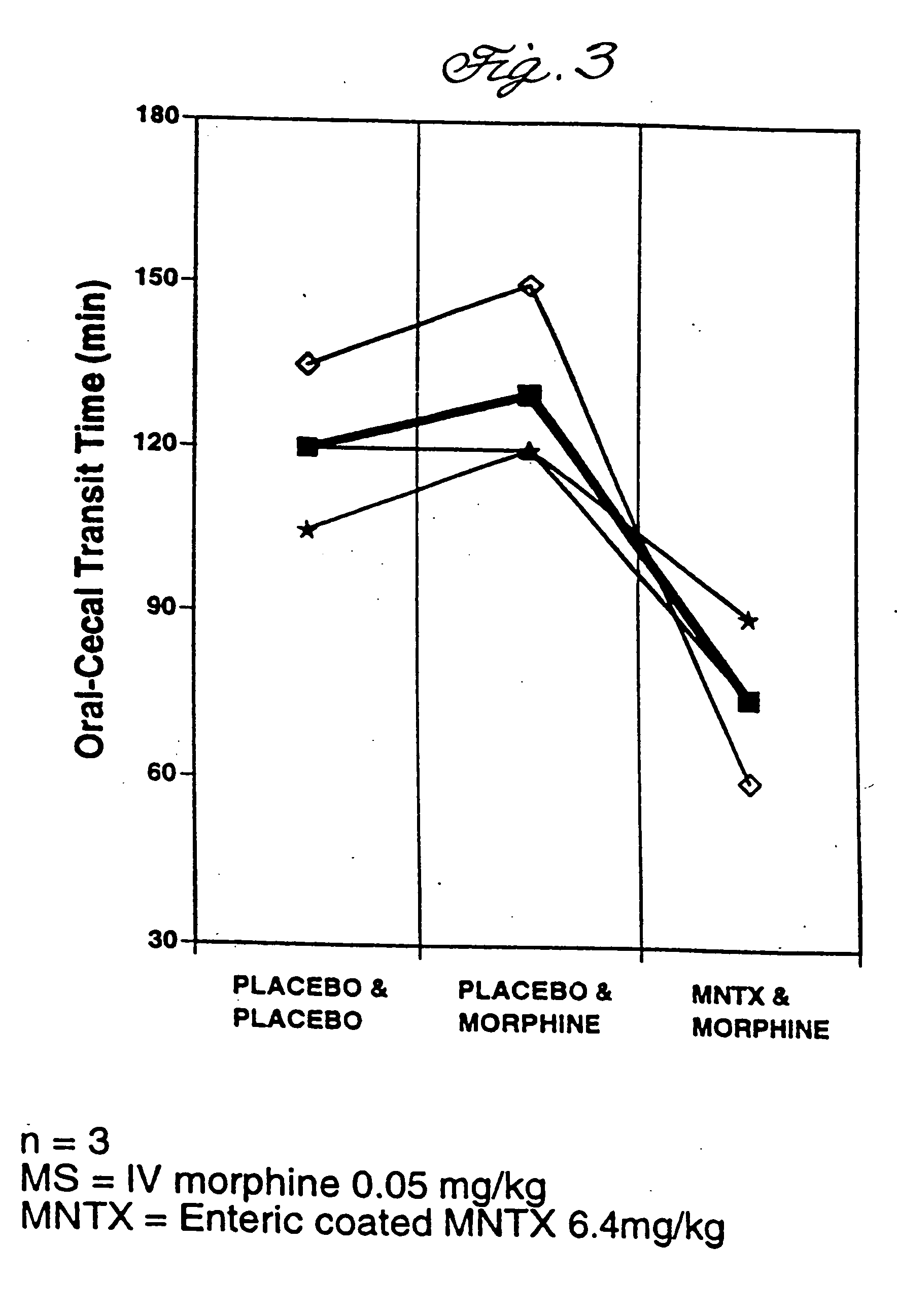
Oral methylnaltrexone, whether enterically coated or uncoated, was shown to reverse the inhibitory effects of opioid administration on gastrointestinal motility as measured by oral-cecal transit time. As compared to non-enterically coated MNTX, however, treatment with enterically coated MNTX enhanced the efficacy of the drug at a lower dose while producing lower plasma levels of MNTX.
Subjects were divided into five treatment groups A-E. With the exception of subjects in Group A, who were given a placebo in place of morphine, all were given an intravenous dose of morphine at 0.05 mg / kg. Prior to morphine administration, subjects were given either a placebo or MNTX in various doses and formulations (see Table 1). The subjects in Group A and B were given a placebo in place of MNTX. Group C received uncoated MNTX at 6.4 mg / kg, Group D received enterically coated MNTX at 6.4 mg / kg active drug, and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| composition | aaaaa | aaaaa |
| lipid soluble | aaaaa | aaaaa |
| polarity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com