Patents
Literature
113 results about "Placebo" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A placebo (/pləˈsiːboʊ/ plə-SEE-boh) is an inert substance or treatment which is designed to have no therapeutic value. Common placebos include inert tablets (like sugar pills), inert injections (like saline), sham surgery, and other procedures.
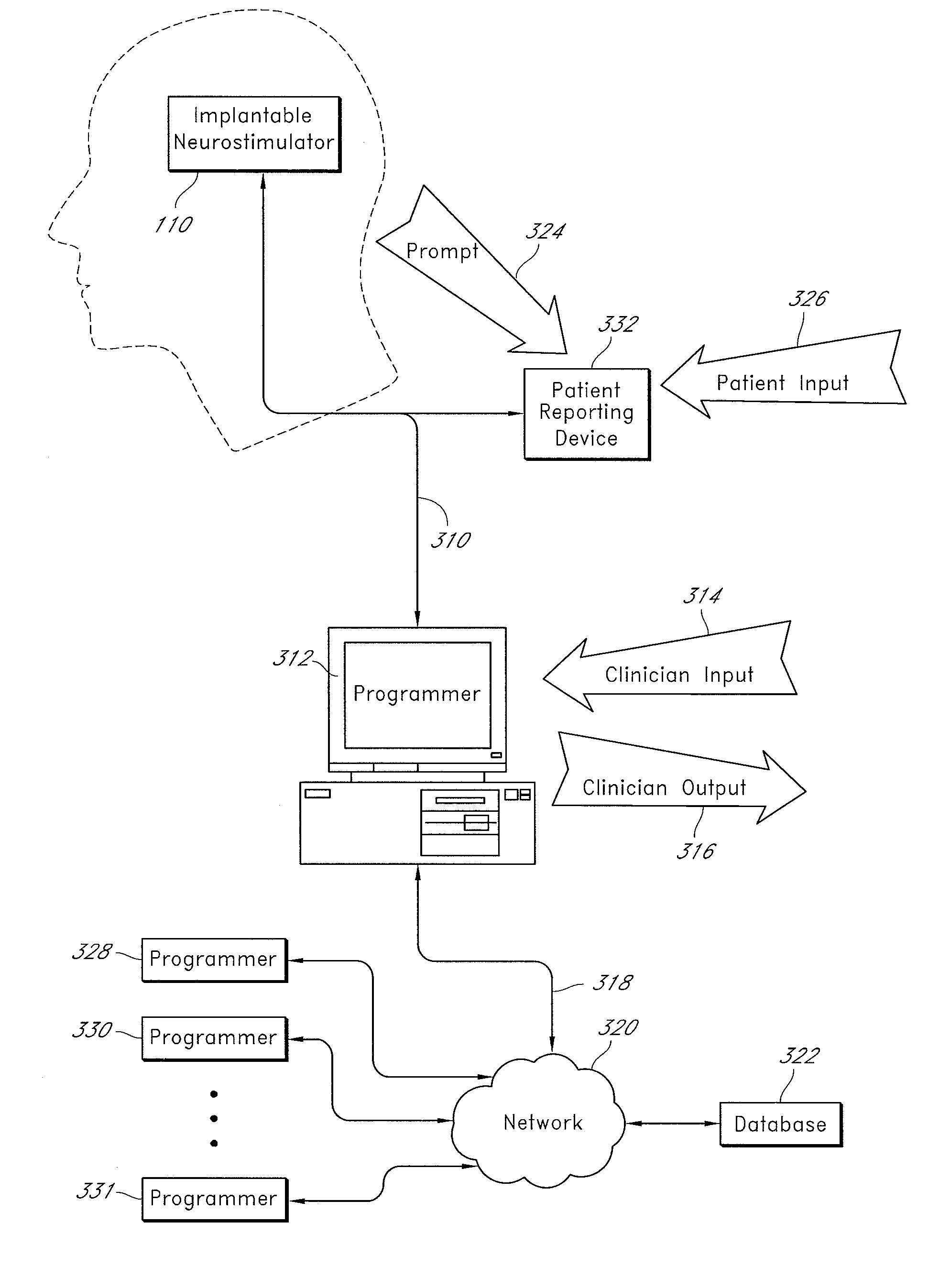
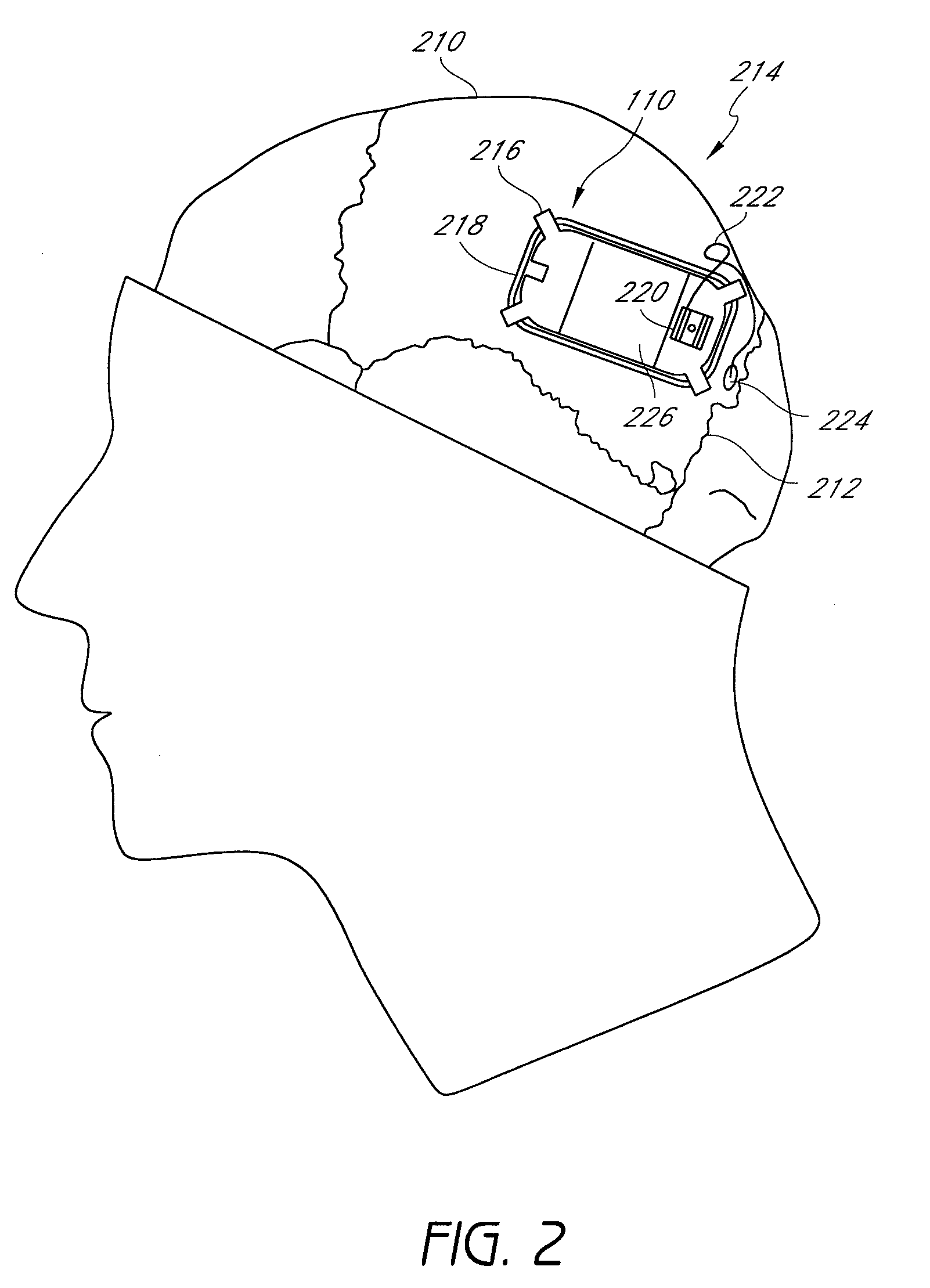
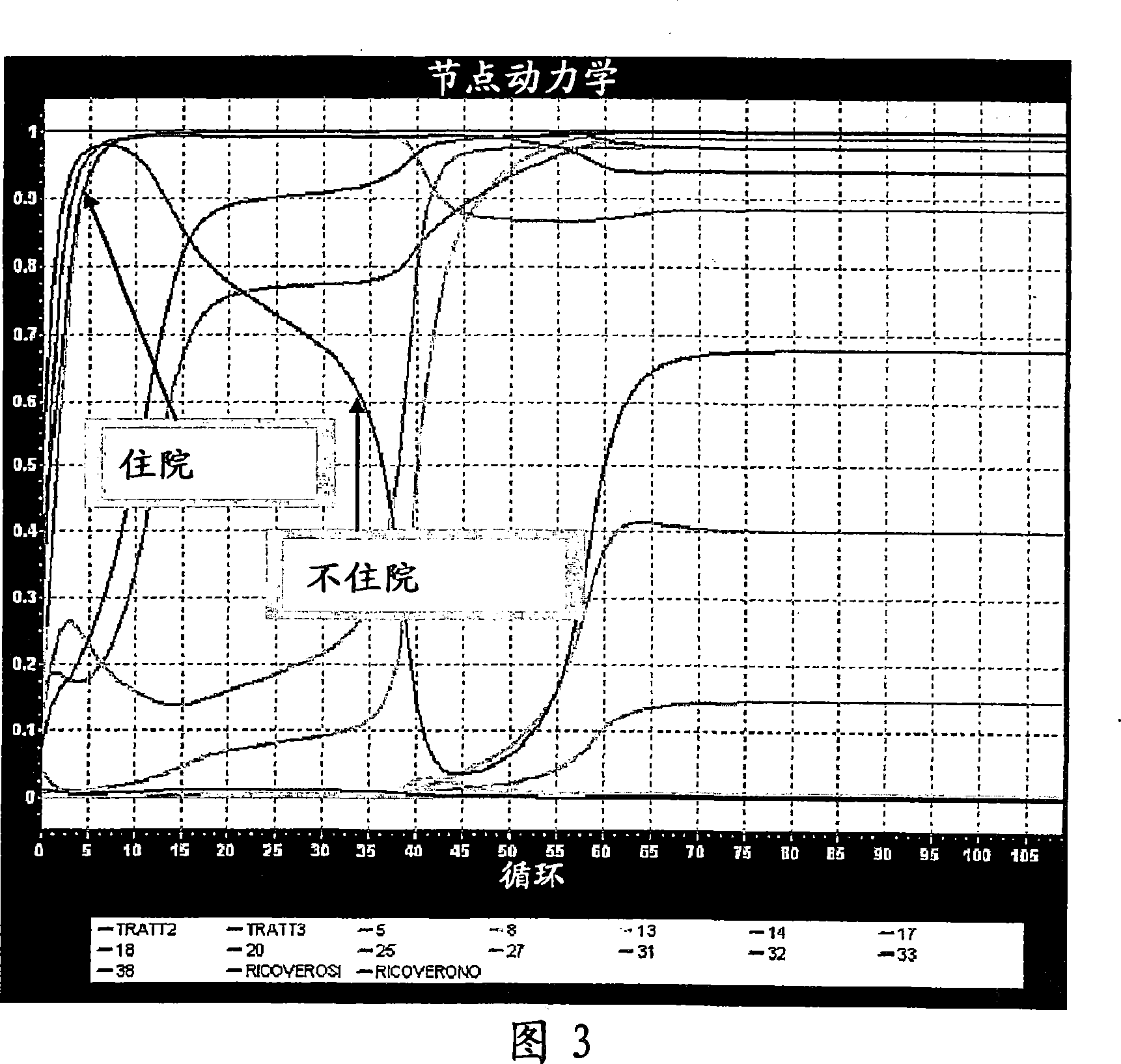
Auto adjusting system for brain tissue stimulator
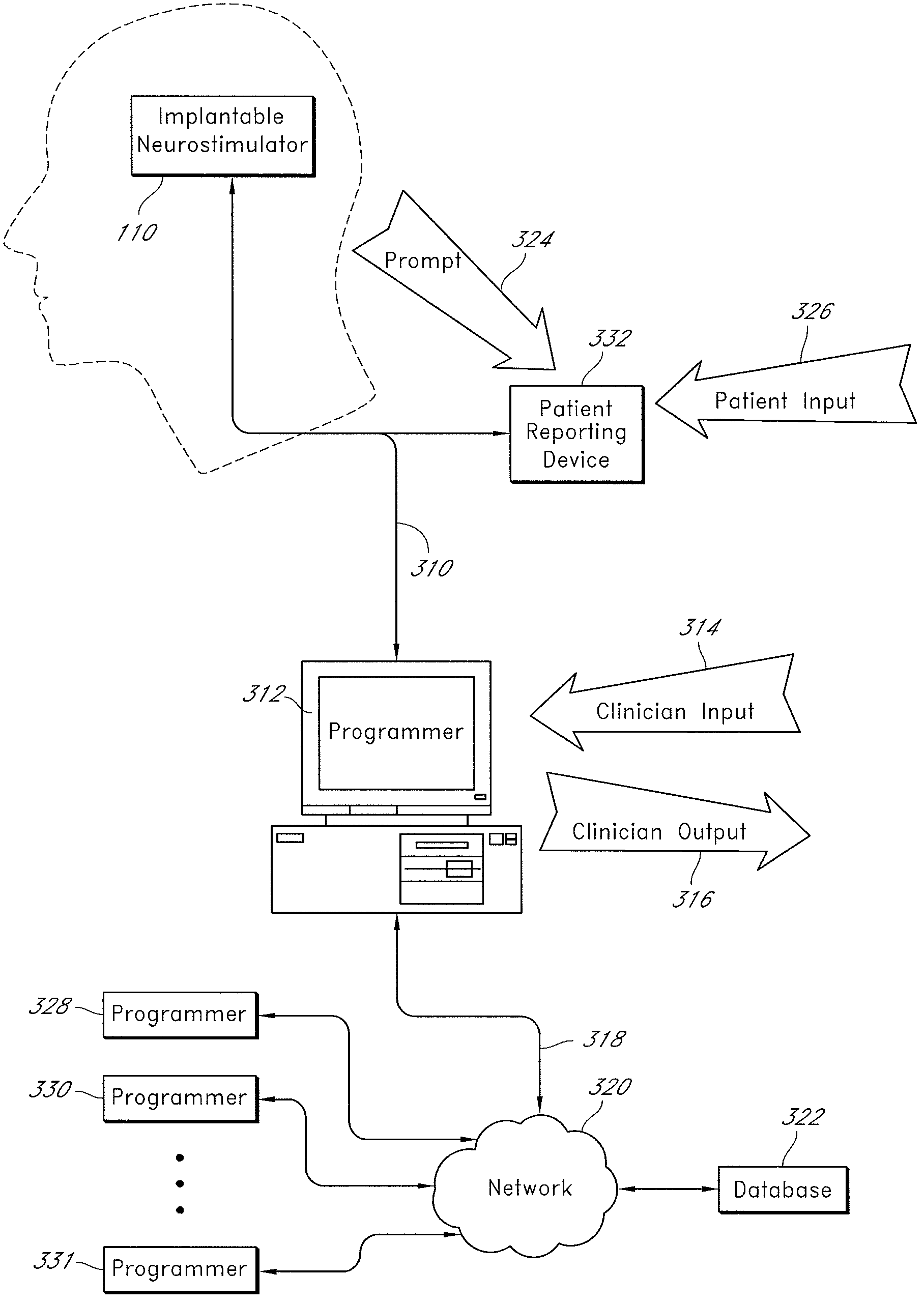
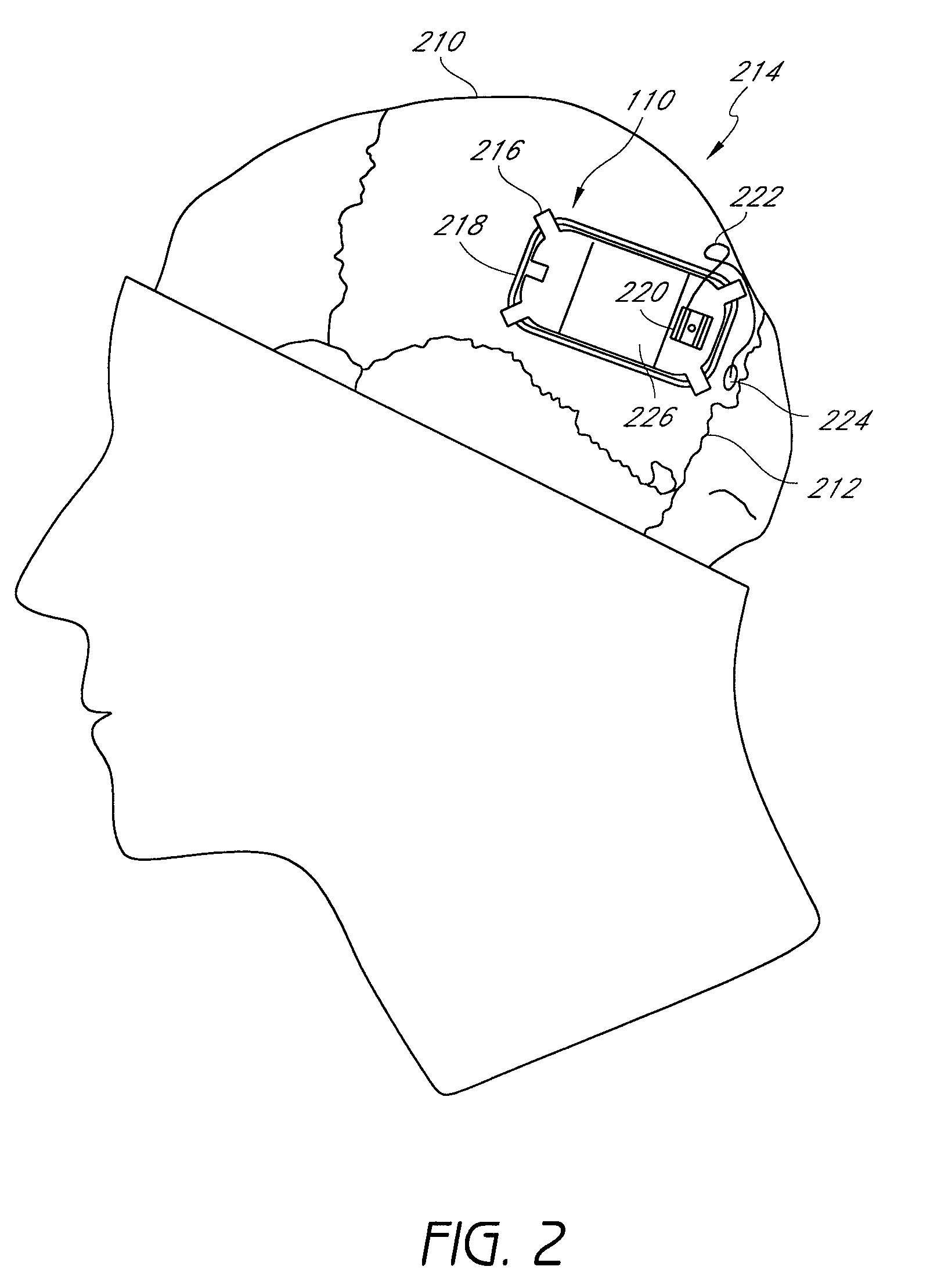
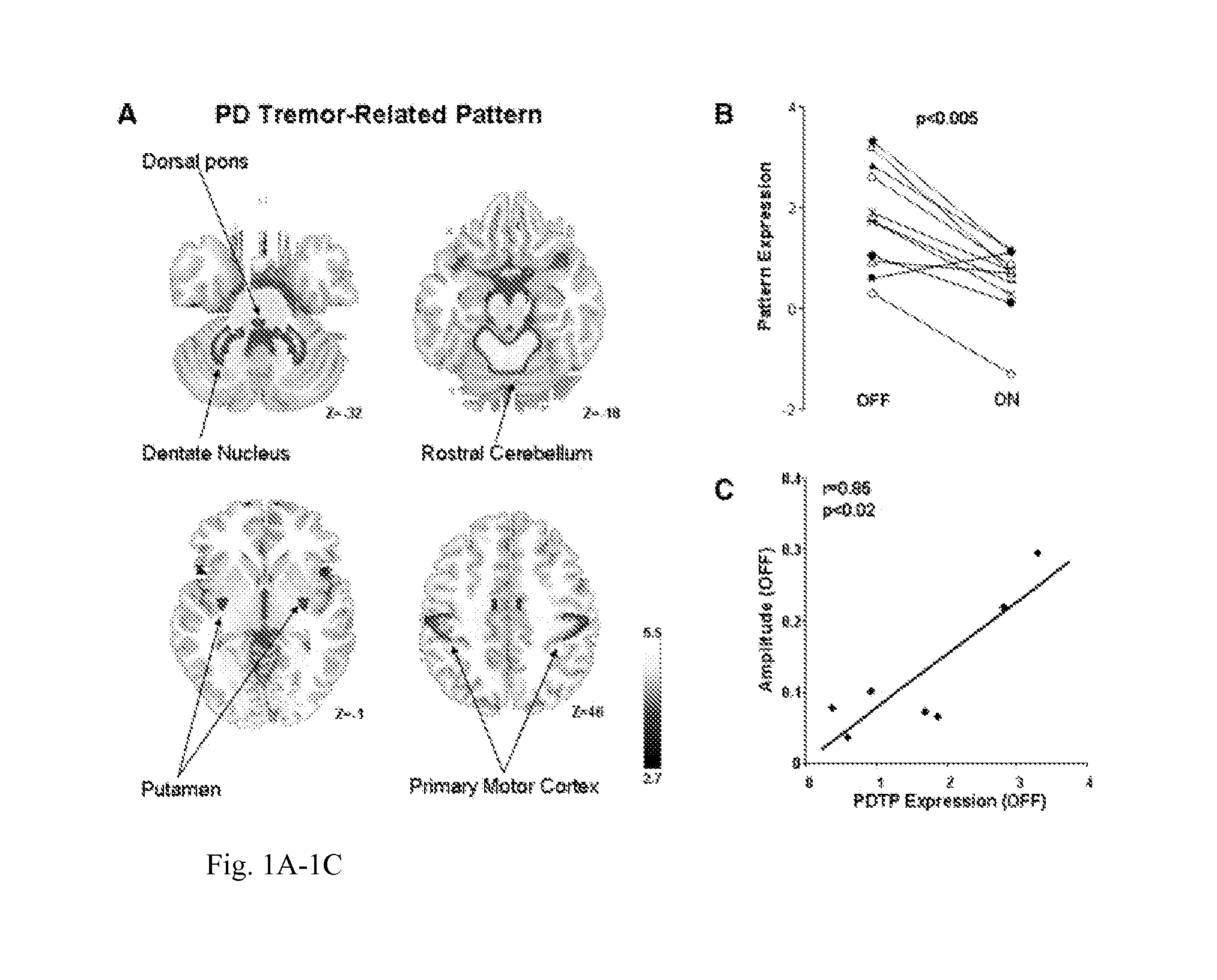
An implantable neurostimulator for treating disorders such as epilepsy, pain, movement disorders and depression includes a detection subsystem capable of detecting a physiological condition and a therapy subsystem capable of providing a course of therapy in response to the condition. The therapy subsystem includes an auto-adjust module for automatically adjusting one or more parameters of the therapy so that the therapy subsystem can provide an adjusted parameter to the patient and solicit the patient's feedback concerning the adjustment without requiring the presence of, or immediate involvement with, a clinician or physician. The patient feedback can be analyzed by computer, clinician or a combination of both to determine an optimal range of parameters for subsequent courses of therapy. In this manner, information useful in tuning the neurostimulator therapy parameters to optimize them for individual patient can be acquired automatically outside of the traditional clinical setting, saving time and minimizing patient fatigue that otherwise would be experience in marathon, in-clinic tuning sessions. The auto-adjust module also can be configured to prompt the patient to provide feedback even when parameters are not being adjusted, so as to acquire information for a baseline or about any placebo effect when the patient is otherwise expecting changes to the therapy to be made.
Owner:NEUROPACE
Testing a patient population having a cardiovascular condition for drug efficacy
Lumenectomy material is tested to determine the efficacy of a test drug in a patient population having a cardiovascular condition. The material is removed from at least a first and a second patient and tested for one or more markers of a cardiovascular condition. The first patient is administered the test drug, and the second patient is administered a placebo. At a later date, more lumenectomy material is removed and tested for the same marker or markers. The presence, absence or amount of the markers is compared in the first patient receiving the drug and the second patient receiving the placebo to determine whether the drug is effective in the patient population. The patient population can comprise as little as two individuals or as many as dozens, hundreds or thousands of patients. The drugs tested include drugs believed to be effective in treating a cardiovascular condition. The markers used can include any marker that can indicate the effectiveness of the drug being tested, including amino acid and nucleic acid markers and markers that indicate a cardiovascular condition.
Owner:TYCO HEALTHCARE GRP LP
N-acetylcysteine compositions and methods for the treatment and prevention of cysteine/glutathione deficiency in diseases and conditions
InactiveUS20050070607A1Low toxicityAllow administrationBiocideOrganic active ingredientsCysteine thiolateClinical settings
Life-threatening hepatotoxicity in the setting of acetaminophen overdose is due to depletion of glutathione (GSH), a vital cysteine-containing tripeptide that protects cells and organs against oxidant injury. Rapid administration of N-acetylcysteine (NAC), which provides the cysteine necessary to replenish the depleted GSH, is the standard of care for preventing injury in acetaminophen overdose. Beneficial effects of NAC treatment have also been demonstrated in respiratory, cardiovascular, endocrine and infectious and other diseases. In fact, over fifty randomized placebo-controlled trials conducted in diverse clinical settings document positive responses to NAC treatment. The present invention relates to cysteine / glutathione (GSH) deficiency as a previously unrecognized clinical entity that can complicate the course of commonly encountered diseases and methods of treatment of this generalized deficiency involving administering N-acetylcysteine (NAC) or a pharmaceutically acceptable salt or derivative to a subject in need thereof and monitoring the subjects appropriate glutathione blood levels as needed.
Owner:ANDRUS JAMES +4
Extended cycle multiphasic oral contraceptive method
ActiveUS8063030B2Reduce in quantityQuantity minimizationBiocideOrganic active ingredientsGynecologyObstetrics
A multiphasic method of contraception comprising the steps of sequentially administering to a female of child bearing age a Phase I composition containing a progestogen in an amount equivalent to about 0.3 to about 1.5 mg norethindrone acetate and an estrogen in an amount equivalent to about 5 to about 15 mcg of ethinyl estradiol for about 7 to about 14 days; a Phase II composition containing a progestogen in an amount equivalent to about 0.3 to about 1.5 mg of norethindrone acetate and an estrogen in an amount equivalent to about 10 to about 25 mcg of ethinyl estradiol for about 14 to about 22 days; a Phase III composition containing a progestogen in an amount equivalent to about 0.3 to about 1.5 mg of norethindrone acetate and an estrogen in an amount equivalent to about 15 to about 35 mcg of ethinyl estradiol for about 20 to about 31 days; and an optional Phase IV composition containing (i) an estrogen in an amount equivalent to about 5 to about 20 mcg of ethinyl estradiol, or (ii) a placebo or a non-steroidal component, or (iii) a combination of (i) and (ii), for about 2 to about 8 days. The ethinyl estradiol equivalent amount of estrogen in each of the successive Phases II and III is at least 5 mcg greater than the ethinyl estradiol equivalent amount of estrogen in the immediately-preceding phase.
Owner:APTALIS PHARMA
Method and apparatus for performing microcurrent stimulation (MSC) therapy
A method and apparatus for providing microcurrent stimulation (MSC) therapy. In accordance with the present invention, it has been determined that the application of microcurrent signals at particular frequencies to the eye for particular periods of time stabilizes and even improves conditions of macular degeneration and other ocular diseases. Experimental data from clinical trials shows that results of persons who underwent therapy are at least better than placebo (i.e., efficacious), and that the therapy is safe. In fact, experimental data from clinical trials showed that approximately 98% of the patients who underwent the MCS therapy of the invention experienced either stabilization or improvement of macular degeneration within one year of starting therapy. Of this percentage, approximately 65% of the patients subjected to the MCS therapy experienced improved vision, while approximately 32% experienced stabilization of macular degeneration (i.e., no further loss of vision).
Owner:ATLANTIC MEDICAL
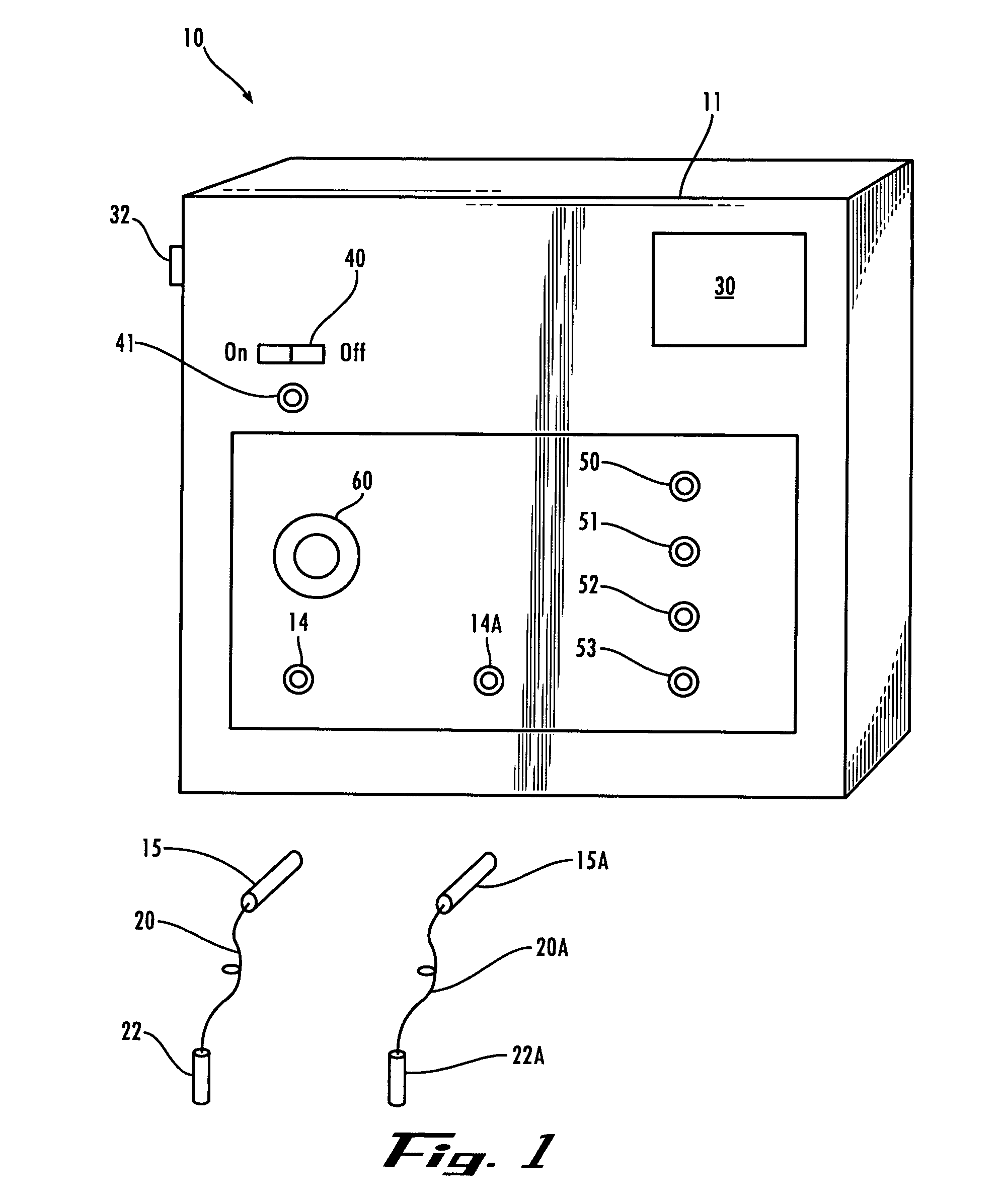
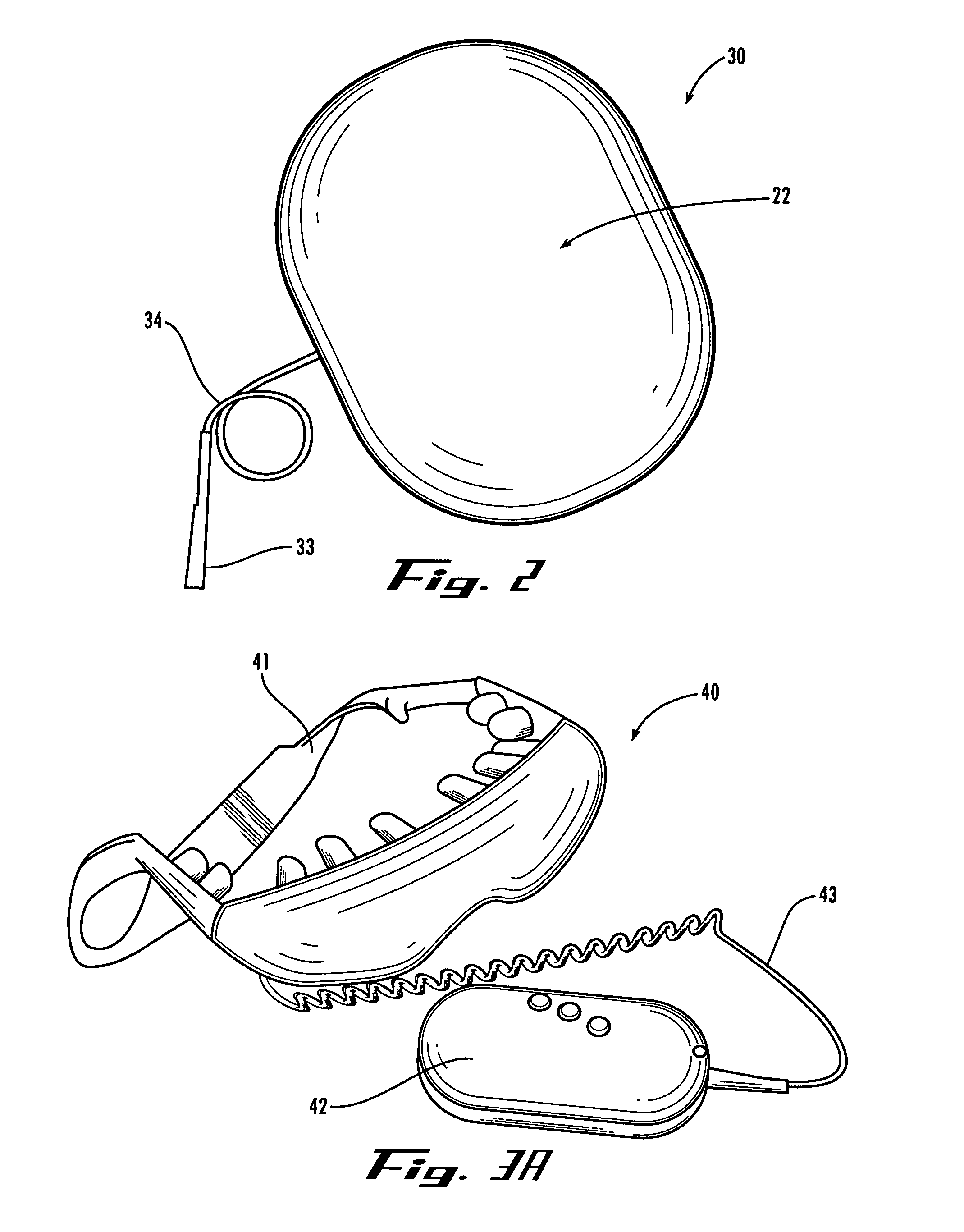
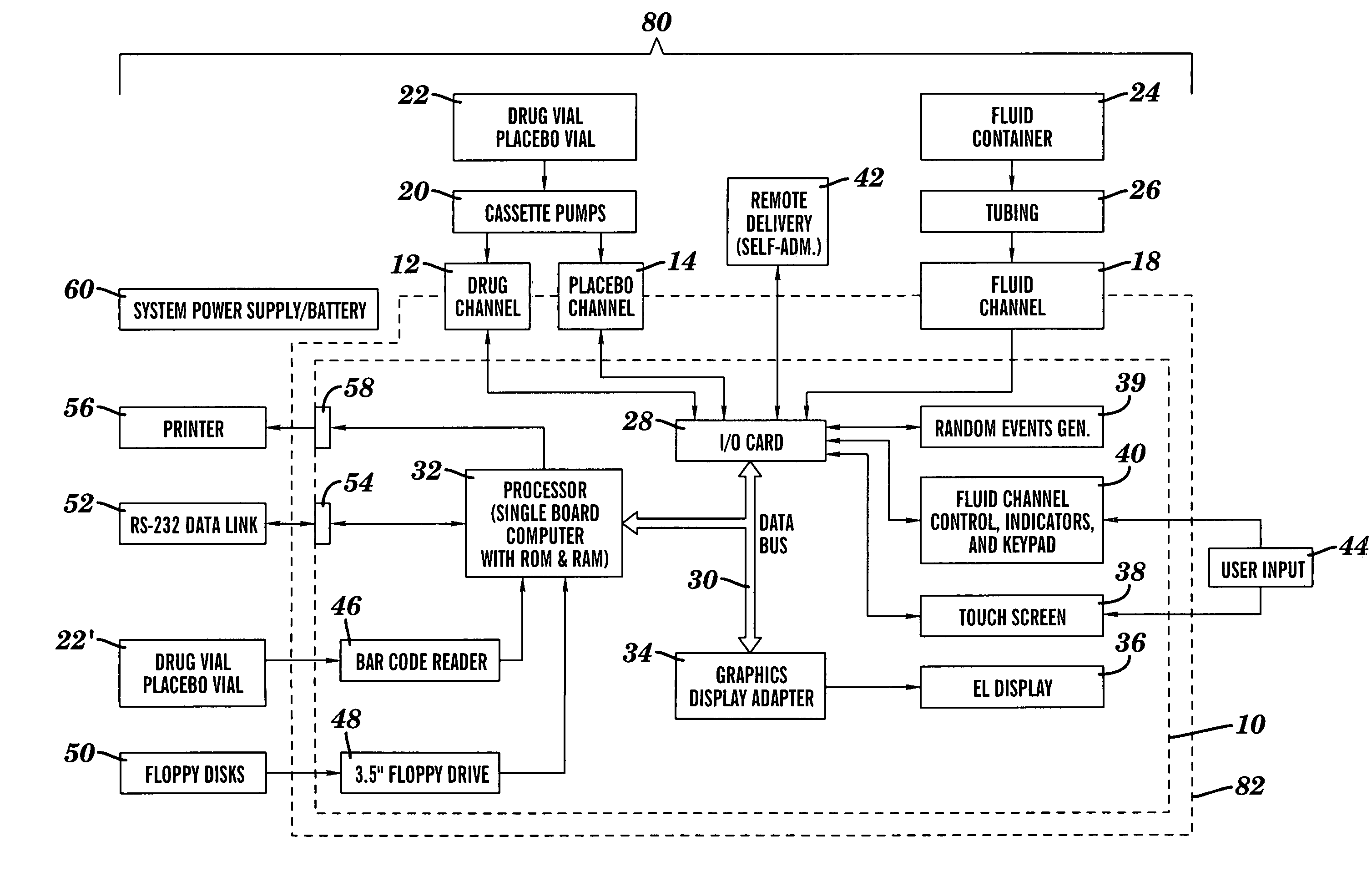
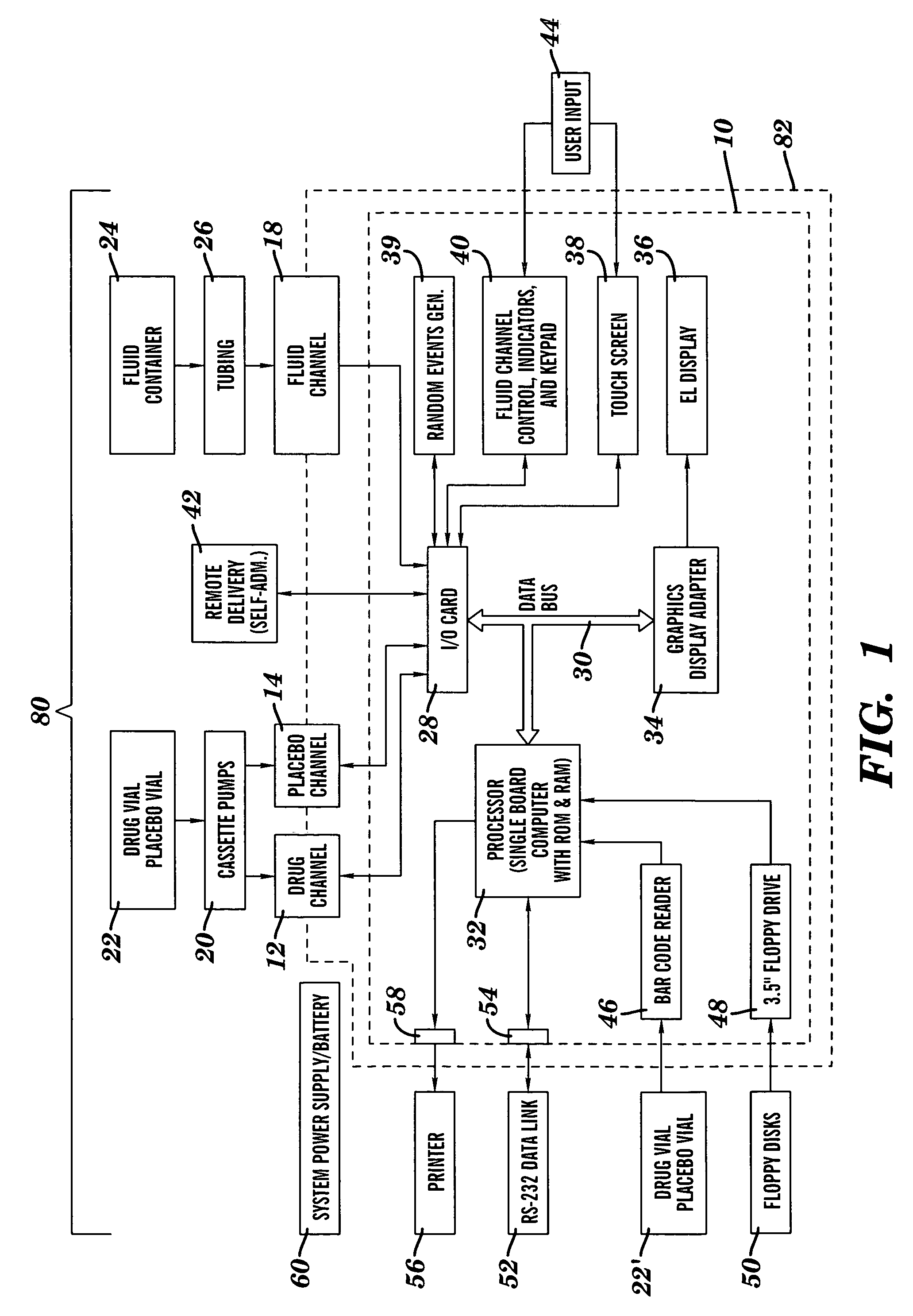
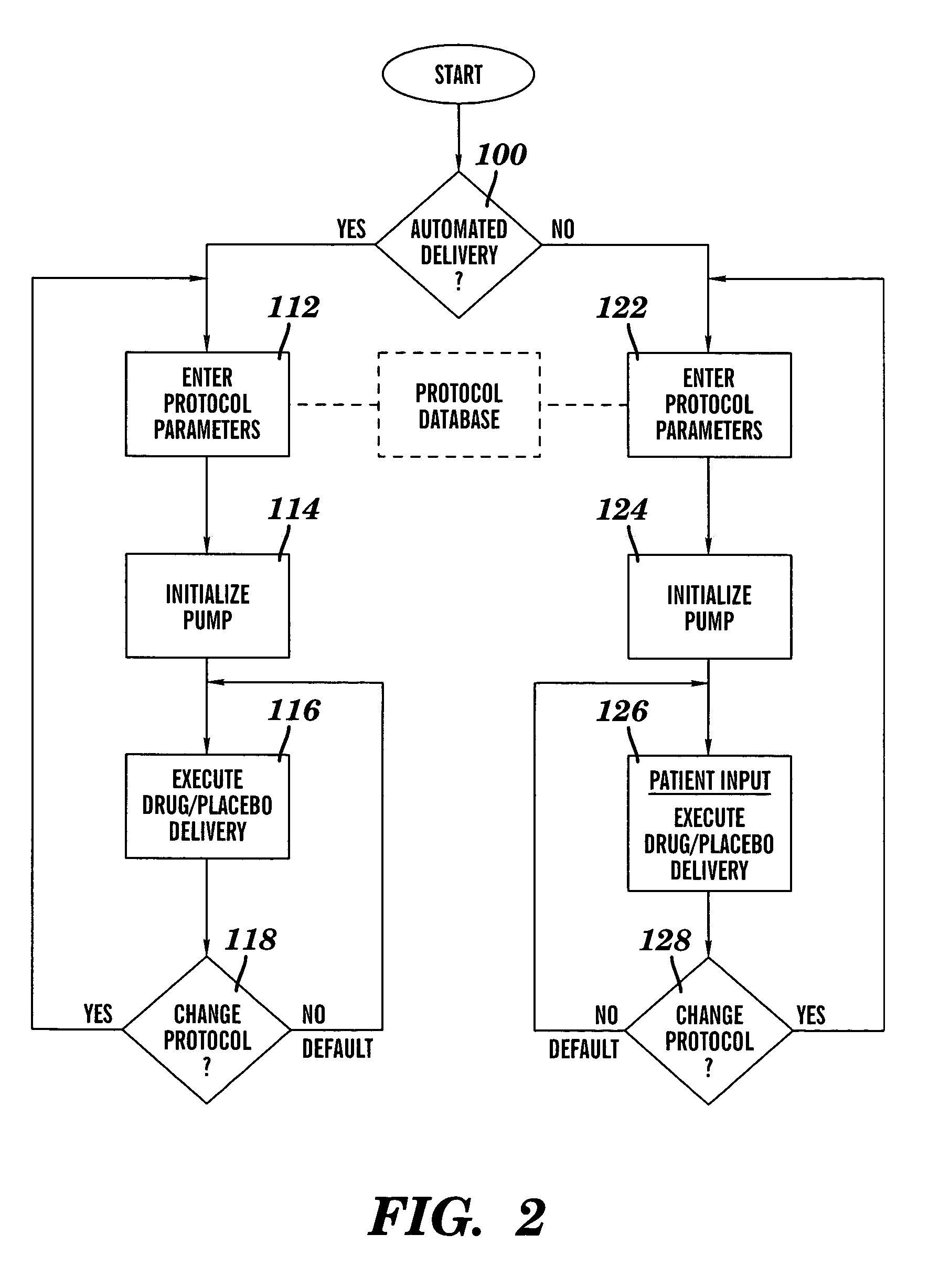
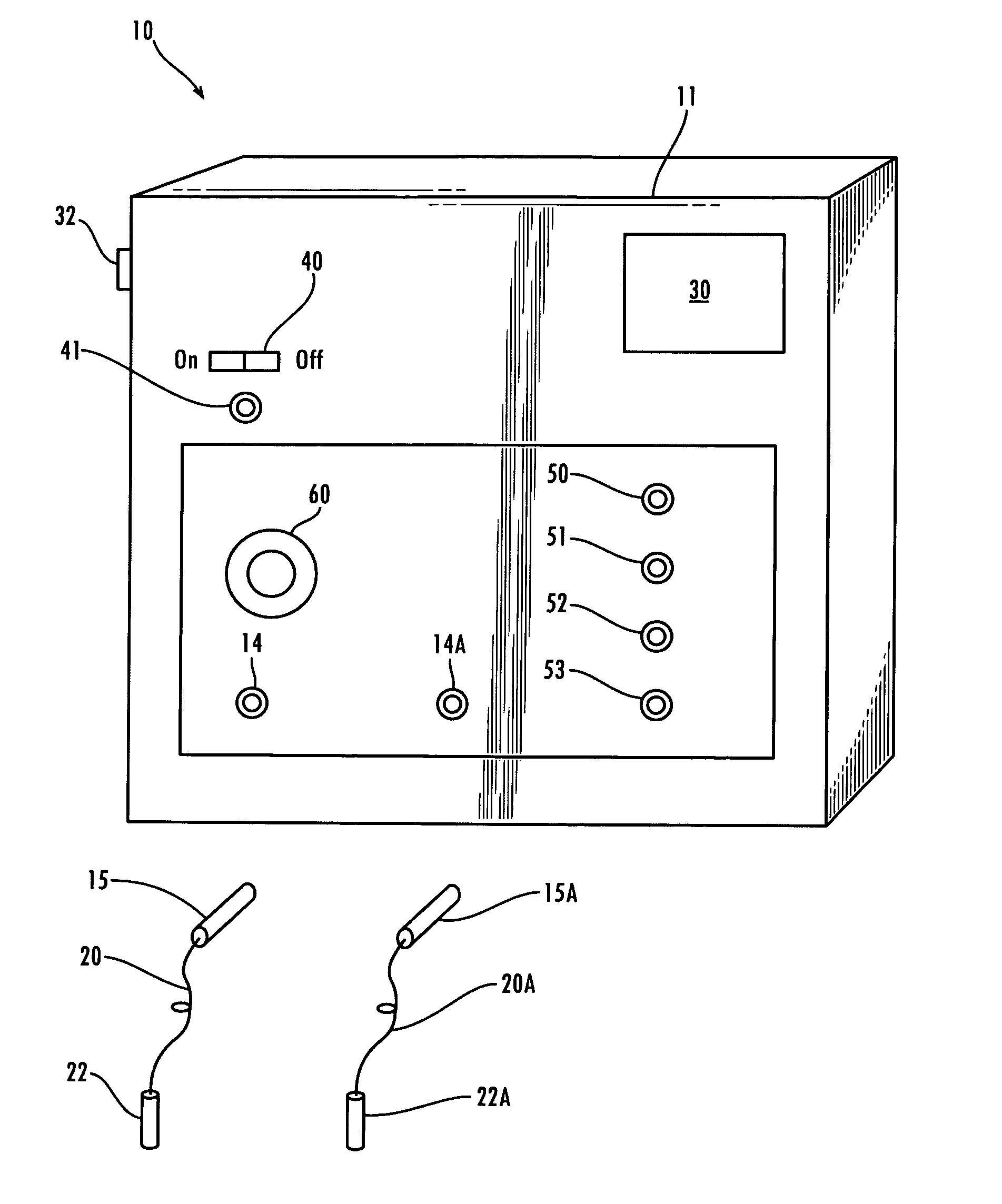
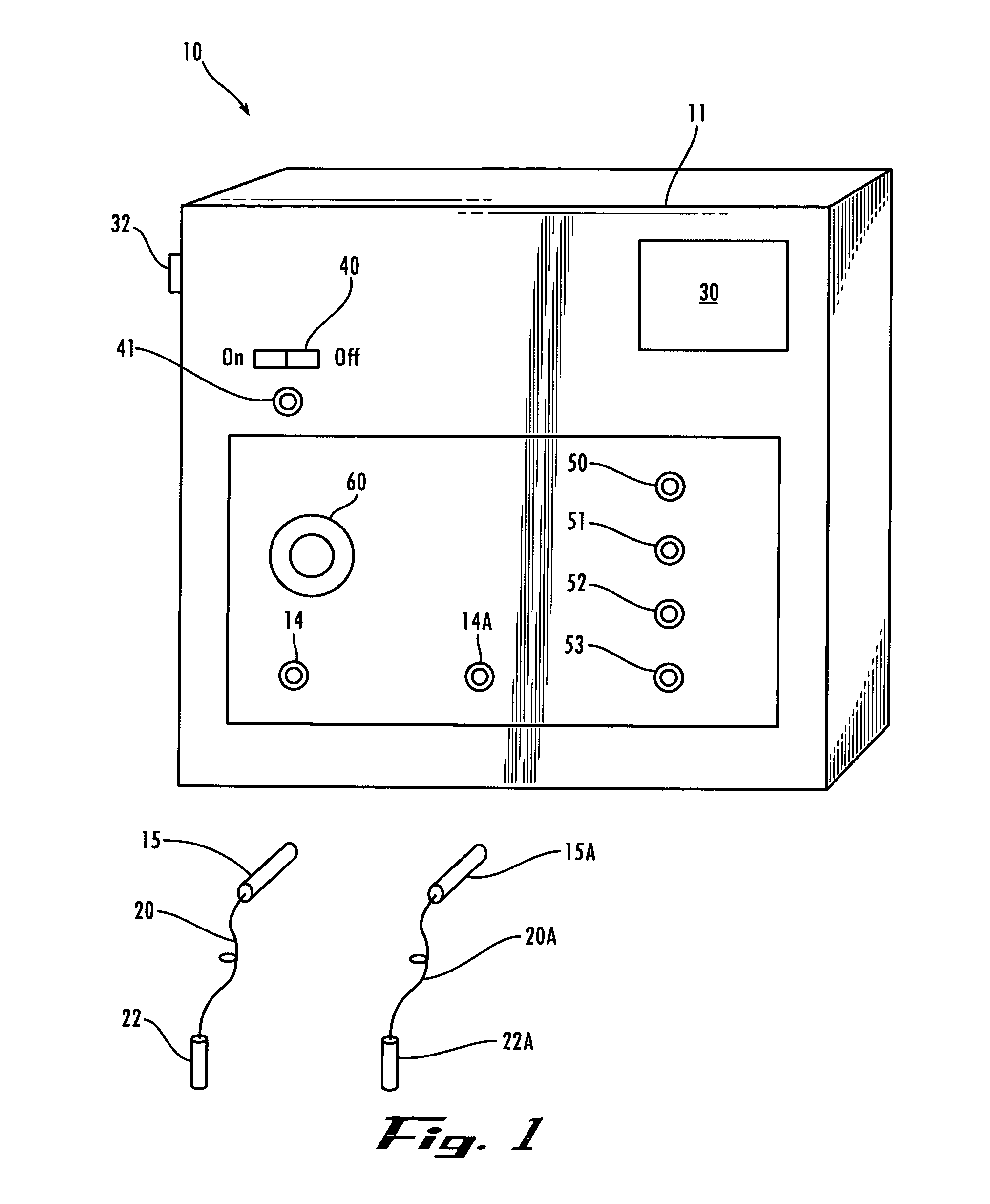
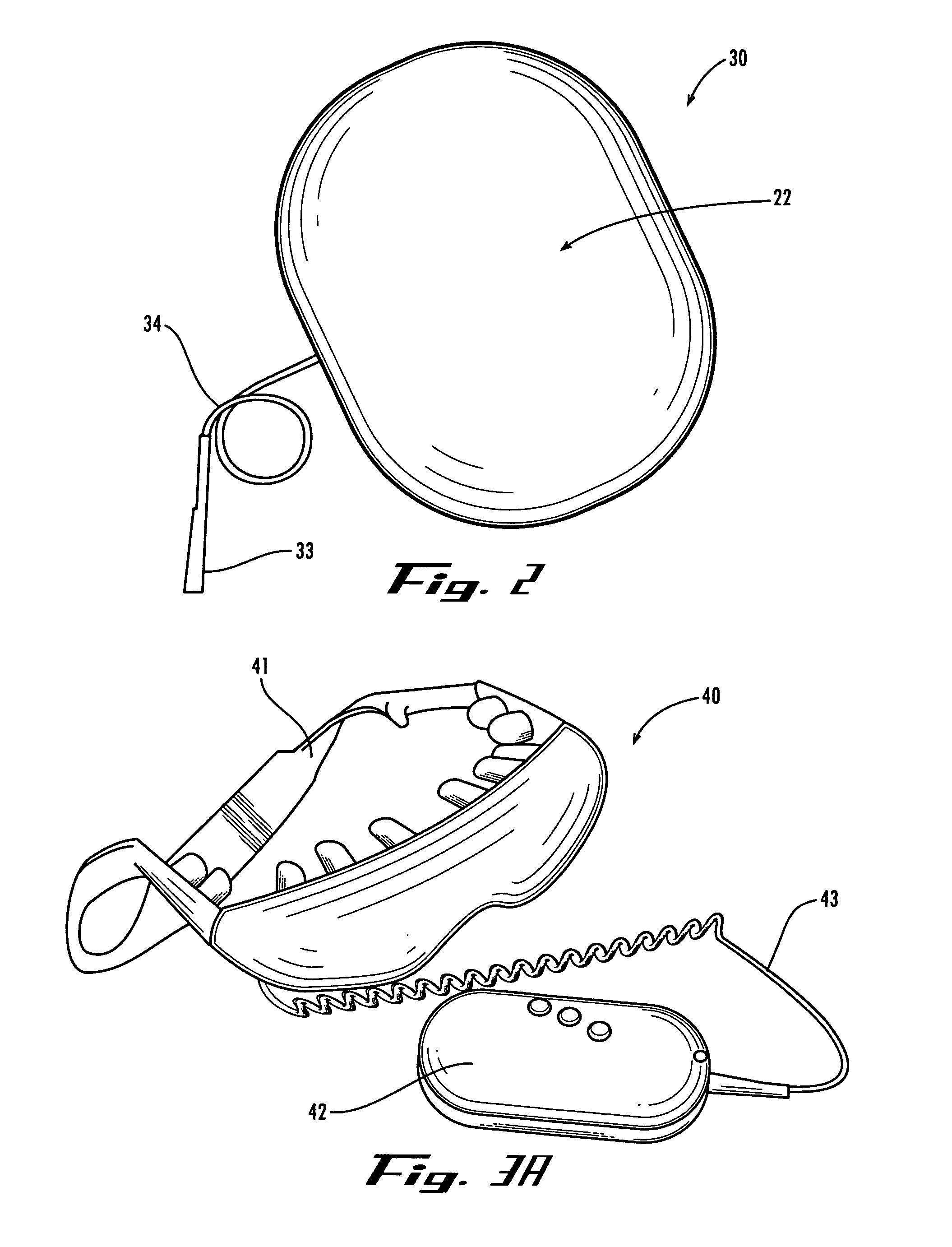
Method and device for administering medication and/or placebo
InactiveUS6973374B2Weakening rangeGood treatment effectLevel controlDrug and medicationsMedicinePlacebo
A control device and a drug delivery system containing such a control device are disclosed for delivery to a patient of a drug or a placebo (or ineffective dose of the drug) according to a partial reinforcement schedule, whereby the placebo (or ineffective dose of the drug) is delivered in whole or in part during one or more delivery events. Methods of modulating the drug / placebo delivery to a patient are also provided, whereby the delivery event is initiated either according to a patient-initiated signaling event (i.e., a self-medication mode) or an automated signaling event (i.e., automated mode).
Owner:UNIVERSITY OF ROCHESTER
Method and apparatus for performing microcurrent stimulation (MSC) therapy
A method and apparatus for providing microcurrent stimulation (MSC) therapy. In accordance with the present invention, it has been determined that the application of microcurrent signals at particular frequencies to the eye for particular periods of time stabilizes and even improves conditions of macular degeneration and other ocular diseases. Experimental data from clinical trials shows that results of persons who underwent therapy are at least better than placebo, and that the therapy is safe and efficacious. In fact, experimental data from clinical trials showed that approximately 98% of the patients who underwent the MCS therapy of the invention experienced either stabilization or improvement of macular degeneration within one year of starting therapy. Of this percentage, approximately 65% of the patients subjected to the MCS therapy experienced improved vision, while approximately 32% experienced stabilization of macular degeneration (i.e., no further loss of vision).
Owner:ATLANTIC MEDICAL
Auto adjusting system for brain tissue stimulator
An implantable neurostimulator for treating disorders such as epilepsy, pain, movement disorders and depression includes a detection subsystem capable of detecting a physiological condition and a therapy subsystem capable of providing a course of therapy in response to the condition. The therapy subsystem includes an auto-adjust module for automatically adjusting one or more parameters of the therapy so that the therapy subsystem can provide an adjusted parameter to the patient and solicit the patient's feedback concerning the adjustment without requiring the presence of, or immediate involvement with, a clinician or physician. The patient feedback can be analyzed by computer, clinician or a combination of both to determine an optimal range of parameters for subsequent courses of therapy. In this manner, information useful in tuning the neurostimulator therapy parameters to optimize them for individual patient can be acquired automatically outside of the traditional clinical setting, saving time and minimizing patient fatigue that otherwise would be experience in marathon, in-clinic tuning sessions. The auto-adjust module also can be configured to prompt the patient to provide feedback even when parameters are not being adjusted, so as to acquire information for a baseline or about any placebo effect when the patient is otherwise expecting changes to the therapy to be made.
Owner:NEUROPACE
Method and apparatus for simulating application workloads on an e-business application server
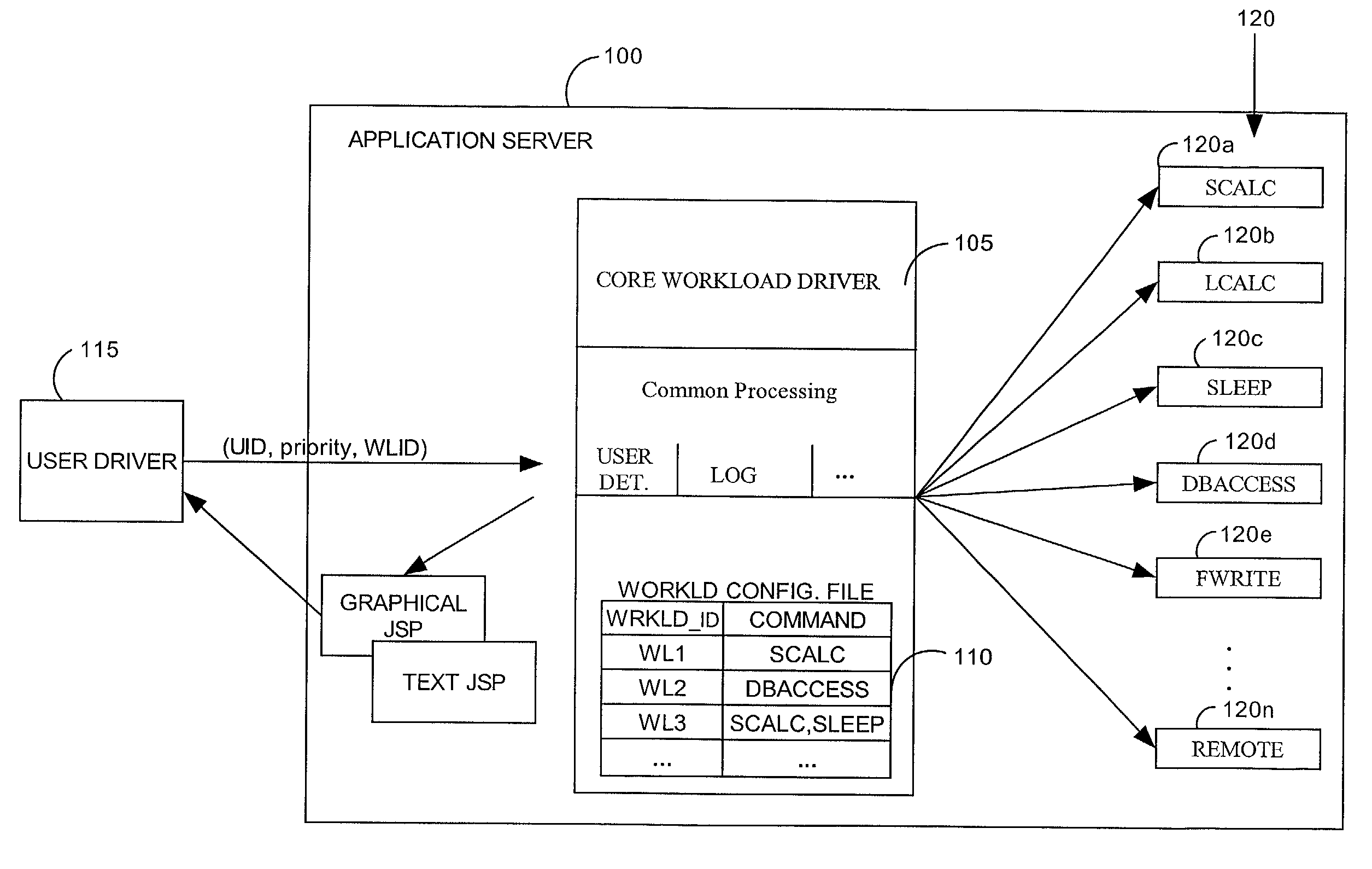
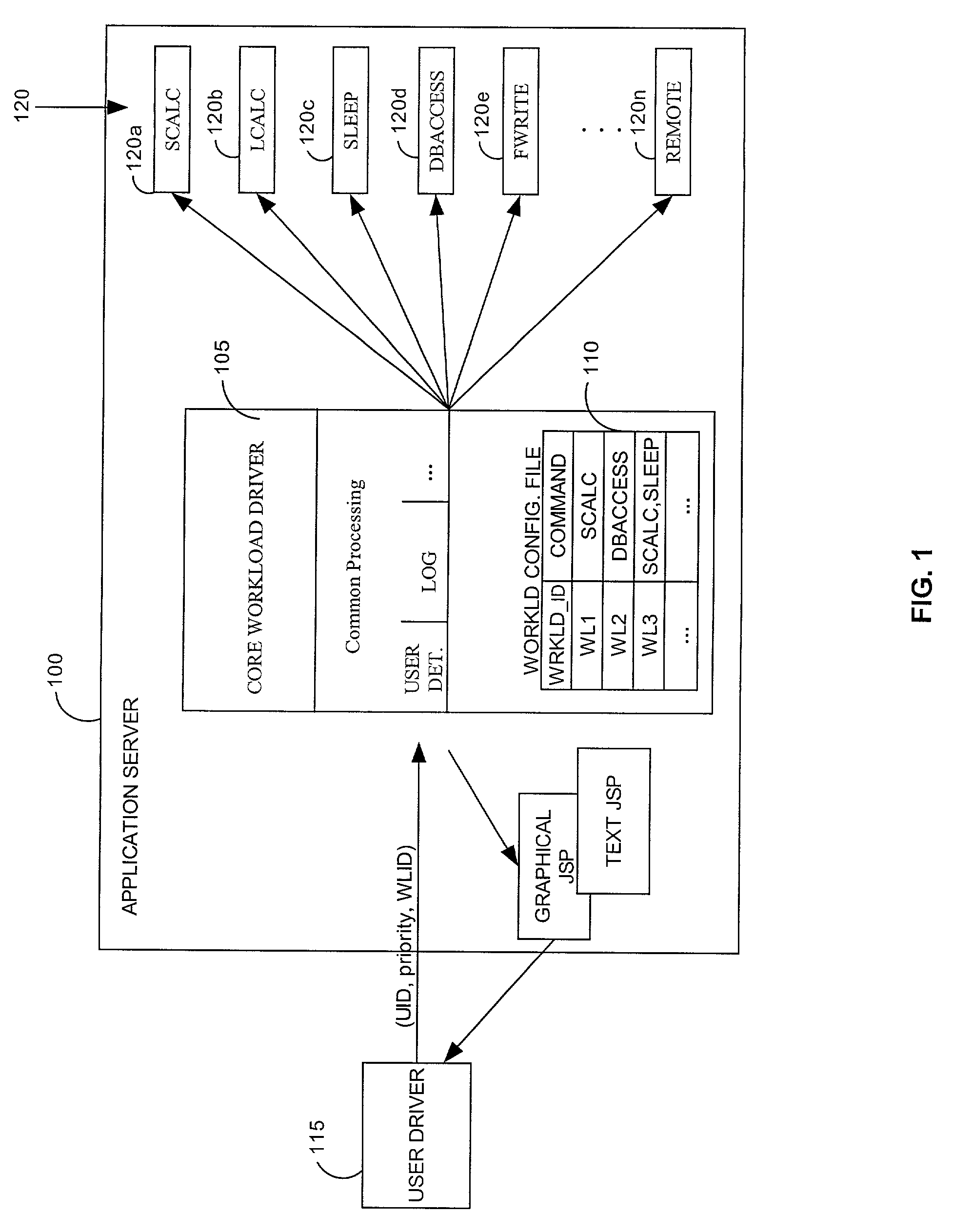
A method and system for simulating application workloads on an e-business application sewer hosting e-business application programs. A test driver can simulate different workloads and enable monitoring of e business applications. A work request for a placebo transaction can be forwarded to the application server for processing. The placebo transaction can be a workload used to test the application server by placing a load on the application server. The placebo transaction can emulate realtime tasks and activities within the application server. The workloads for the placebo transactions can require varying levels of processing resources. The placebo transaction can include hypertext transfer protocol (HTTP) traffic. A work request can include HTTP requests for accomplishing e-business related calculations, and retrieving and / or storing information in a database.
Owner:IBM CORP
Dispenser for progestin used for acute and maintenance treatment of DUB
InactiveUS20050269238A1Easy to useMaximum effectivenessHeavy metal active ingredientsOrganic active ingredientsPlaceboDysfunctional uterine bleeding
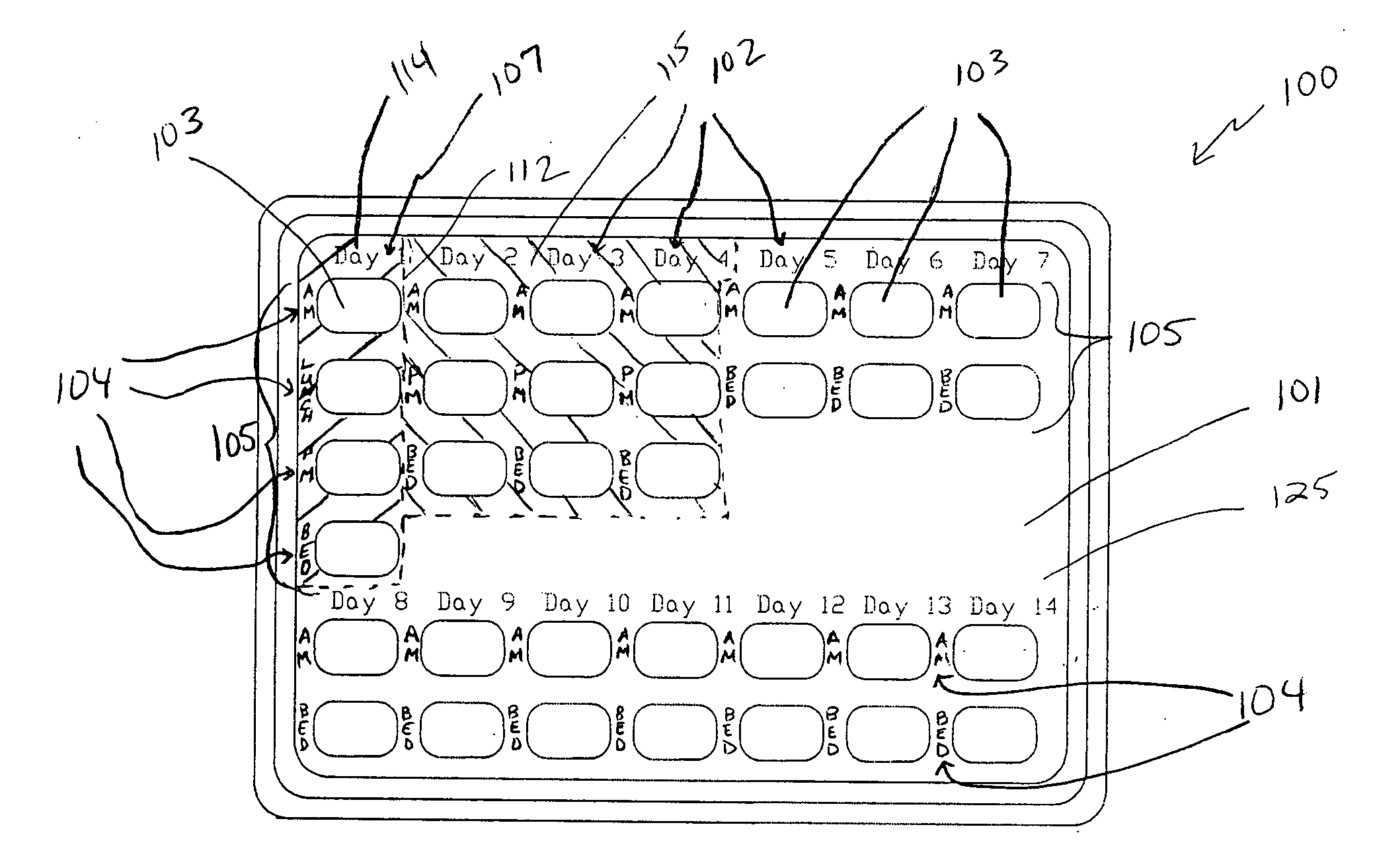
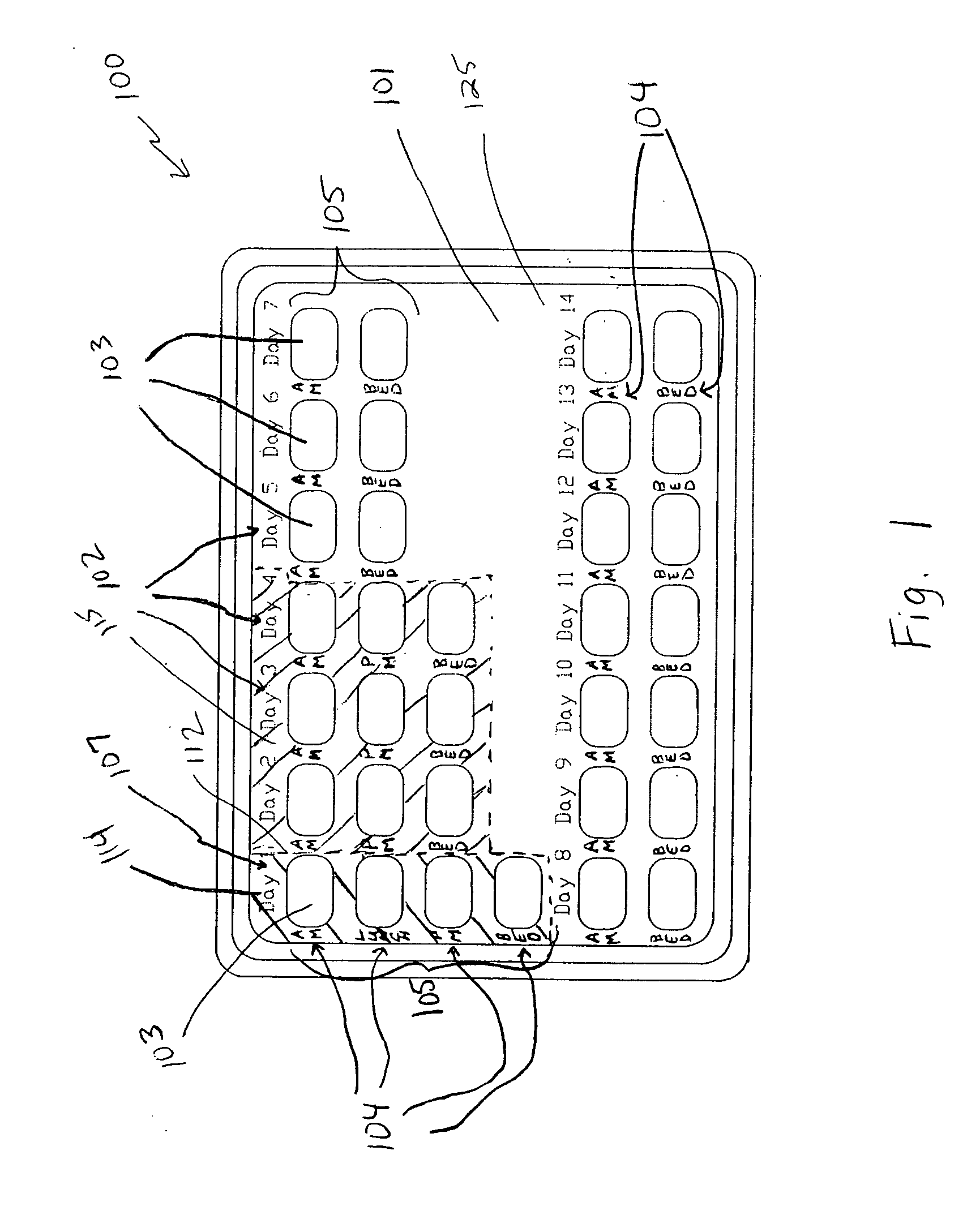
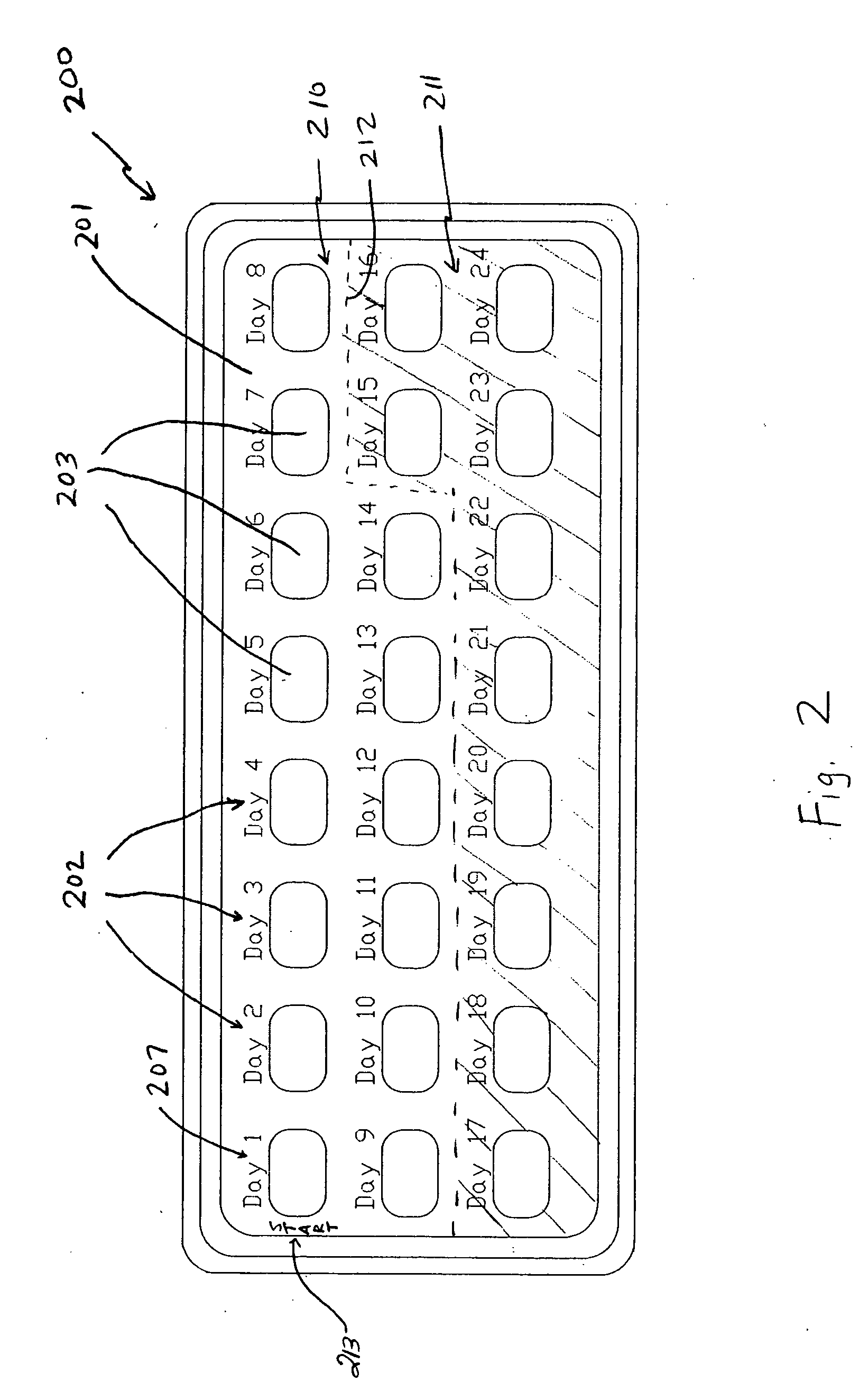
A dispenser including a first dispensing portion including one group of storage units for each of ten to twenty-eight days, and a second dispensing portion, including at least one storage unit for each of seventeen to thirty days. In the first dispensing portion, a first set includes a group of four storage units for each day, a second set includes a group of three storage units for each day, and a third set includes a group of two storage units for each day. The first dispensing portion contain a progestin. In the second dispensing portion, the first fourteen storage units contain a placebo and the remaining storage units contain a progestin. First and second dispensing portions may be provided as separate dispensing packs, or as a multi-pack of second dispensing portions. The dispenser is useful for treatment of acute episodes of dysfunctional uterine bleeding (DUB) and maintenance treatment for preventing future episodes of DUB.
Owner:TEVA WOMENS HEALTH
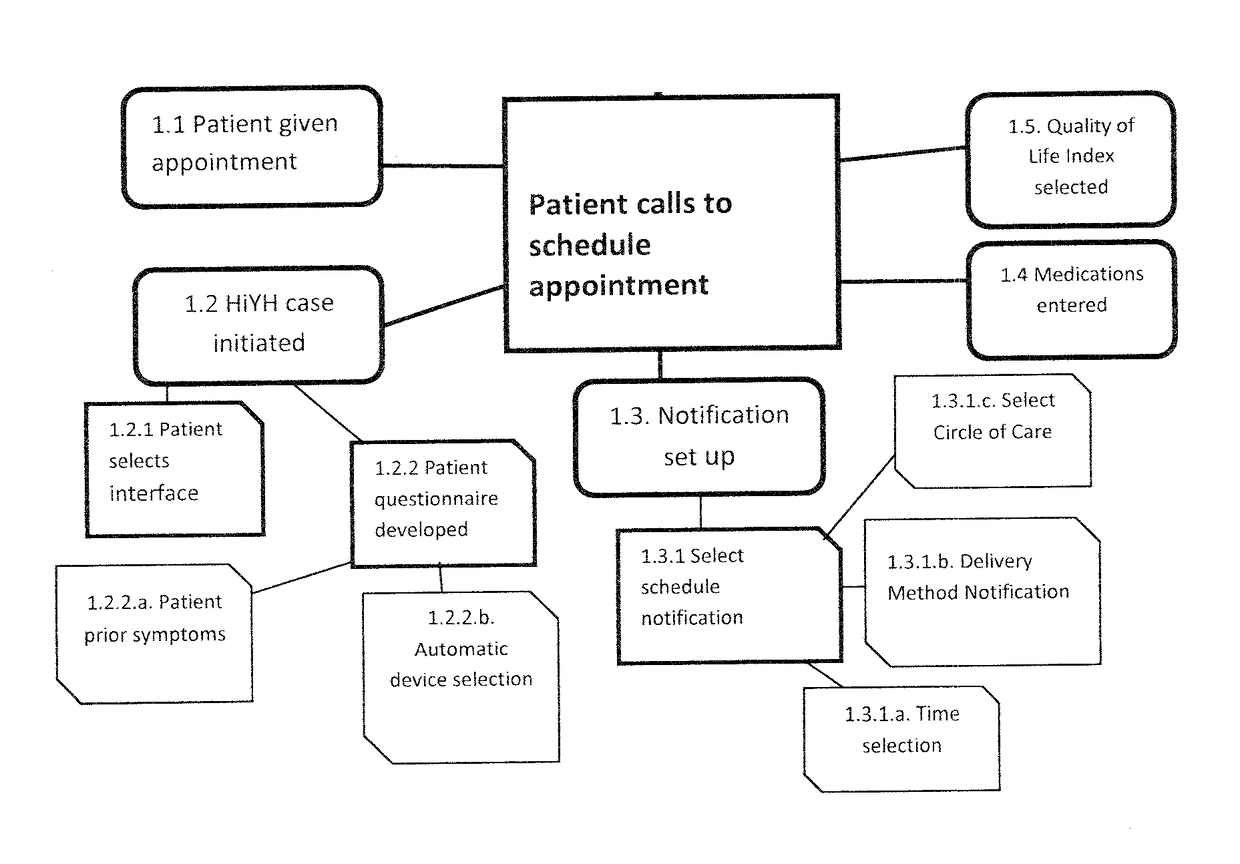
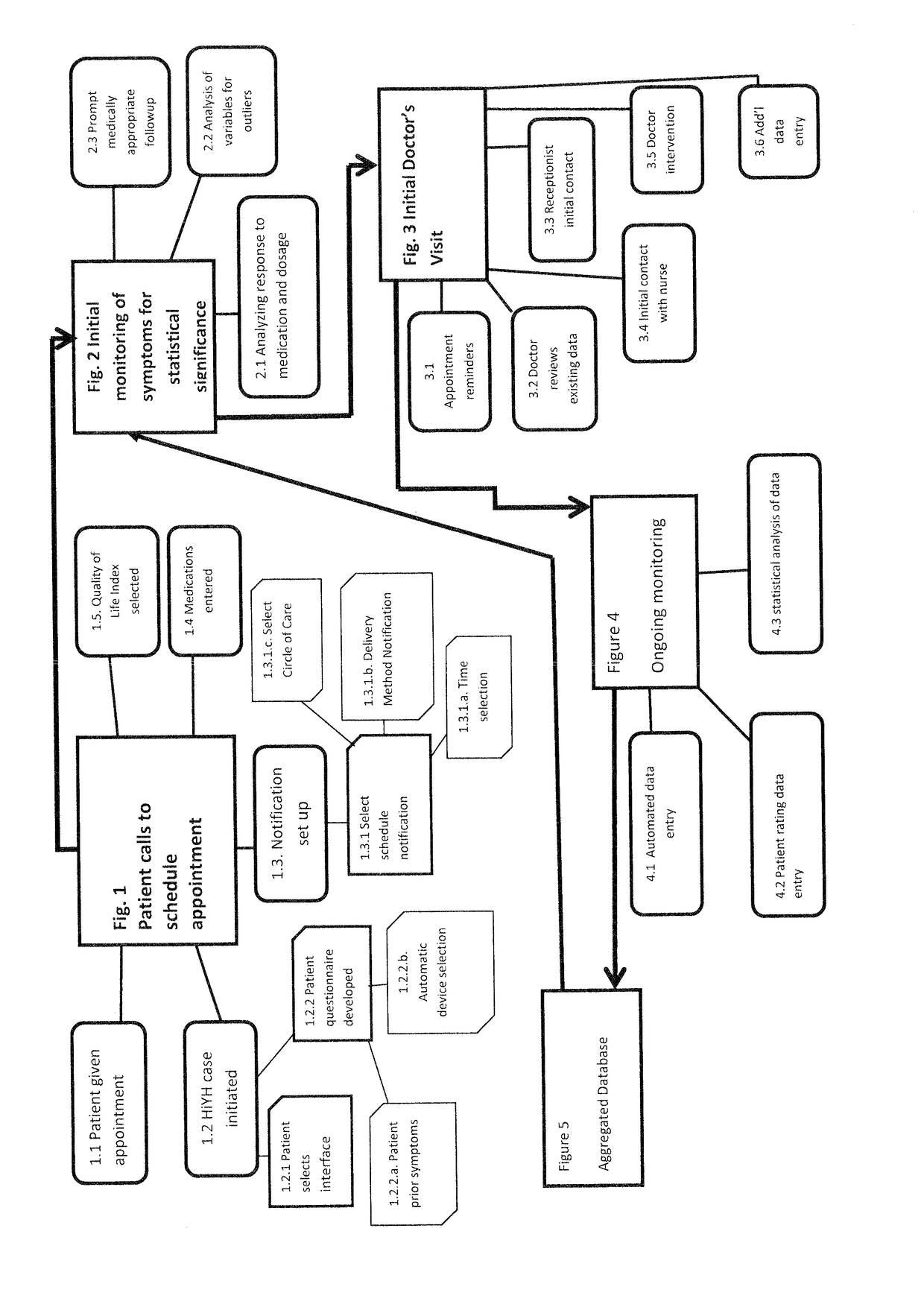
Health In Your Hands
InactiveUS20170300647A1Relieve symptomsEasy to monitorDrug and medicationsComputer-assisted medical data acquisitionPlaceboSystems design
The invention in this application is a decision-support system that utilizes single system design (SSD) and a proprietary algorithm to determine real change is for each individual patient. It pinpoints right medication and dosage for an individual patient, targets standards for functionality, called Activities of Daily Living (ADLs), and Quality of Life Indicators (QoLIs) to maximize personal health and satisfaction. Further, the system has as hallmarks a specific communication system, provision of coordination of services and a continuum of care for seamless delivery of treatment targeted to the needs of an individual patient and his / her care network. It incorporates data collected from genomic testing, patient entry, and automated devices. It is a safety-enhancing, cost-saving, time-saving system that will address placebo effect. It will utilize a variety of technologies to gather data with flexibility for patient needs. It will utilize machine learning for added safety enhancement.
Owner:GOLDBERG MARC DANIEL +1
Method for improving endothelial function and decreasing cardiovascular morbidity using shilajit
InactiveUS20140079729A1Function increaseGood for healthOrganic active ingredientsBiocideEndothelial dysfunctionPlacebo
Shilajit in a standardized composition produces a significant improvement in several cardiovascular parameters including RI, AIx and SEVR. Further, significant reductions in malondialdehyde and increases in nitric oxide levels are provided suggesting improvement in endothelial function. Shilajit may be used to reduce inflammatory biomarker HsCRP levels significantly compared to baseline and placebo. Additionally, Shilajit can provide significant improvement in lipid parameters including total cholesterol, LDL-C, and HbA1c (%) Inhibition of platelet aggregation using Shilajit performed using ADP as aggregant also provides highly significant inhibition of platelet aggregation compared to baseline and with placebo. Thus, Shilajit may be used for improvement of endothelial function and to help reduce cardiovascular morbidity, particularly for the diabetic individual.
Owner:NATREON INC
Extended cycle multiphasic oral contraceptive method
ActiveUS20070207945A1Reduce in quantityQuantity minimizationBiocideOrganic active ingredientsGynecologyObstetrics
A multiphasic method of contraception comprising the steps of sequentially administering to a female of child bearing age a Phase I composition containing a progestogen in an amount equivalent to about 0.3 to about 1.5 mg norethindrone acetate and an estrogen in an amount equivalent to about 5 to about 15 mcg of ethinyl estradiol for about 7 to about 14 days; a Phase II composition containing a progestogen in an amount equivalent to about 0.3 to about 1.5 mg of norethindrone acetate and an estrogen in an amount equivalent to about 10 to about 25 mcg of ethinyl estradiol for about 14 to about 22 days; a Phase III composition containing a progestogen in an amount equivalent to about 0.3 to about 1.5 mg of norethindrone acetate and an estrogen in an amount equivalent to about 15 to about 35 mcg of ethinyl estradiol for about 20 to about 31 days; and an optional Phase IV composition containing (i) an estrogen in an amount equivalent to about 5 to about 20 mcg of ethinyl estradiol, or (ii) a placebo or a non-steroidal component, or (iii) a combination of (i) and (ii), for about 2 to about 8 days. The ethinyl estradiol equivalent amount of estrogen in each of the successive Phases II and III is at least 5 mcg greater than the ethinyl estradiol equivalent amount of estrogen in the immediately-preceding phase.
Owner:APTALIS PHARMA
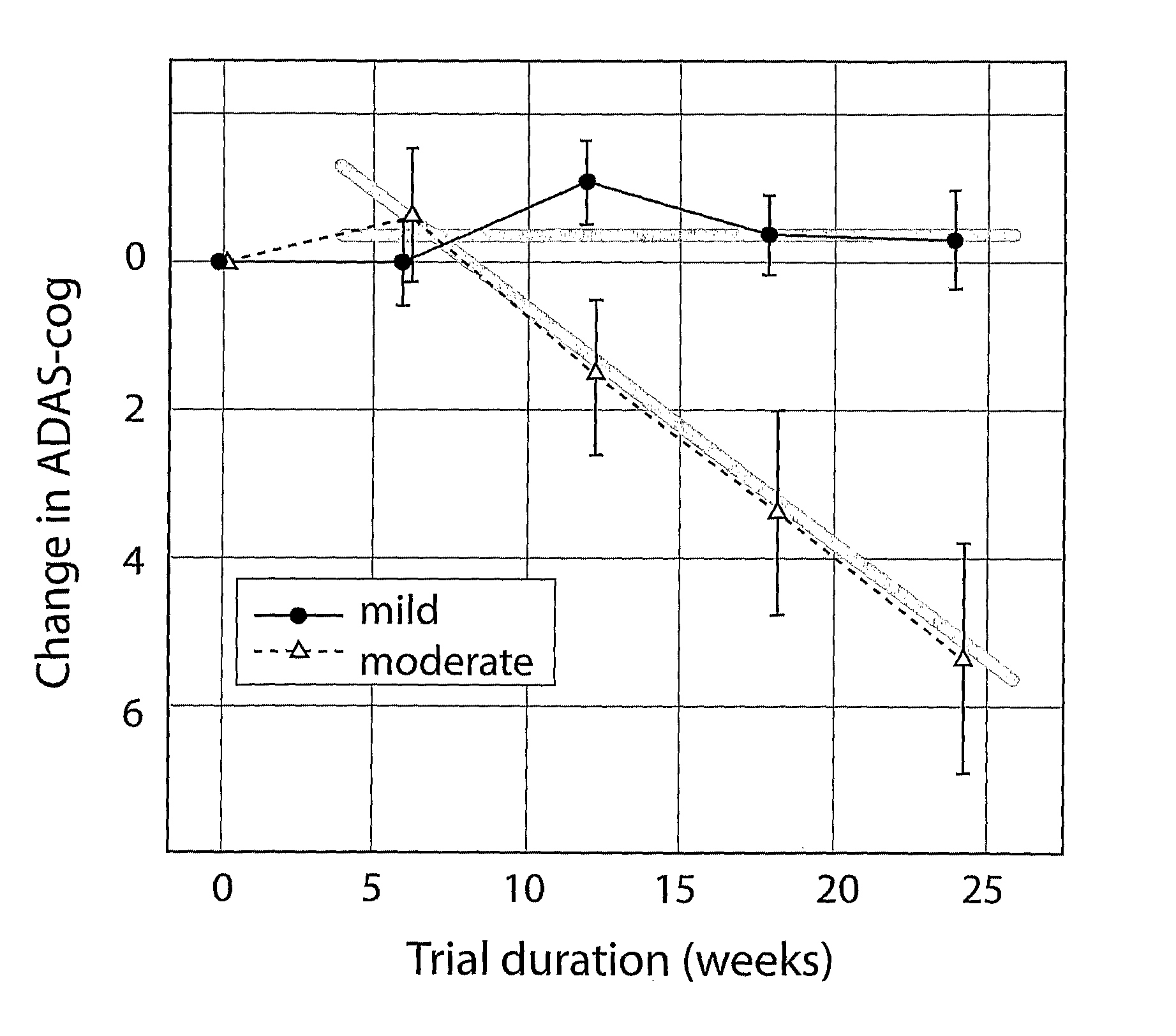
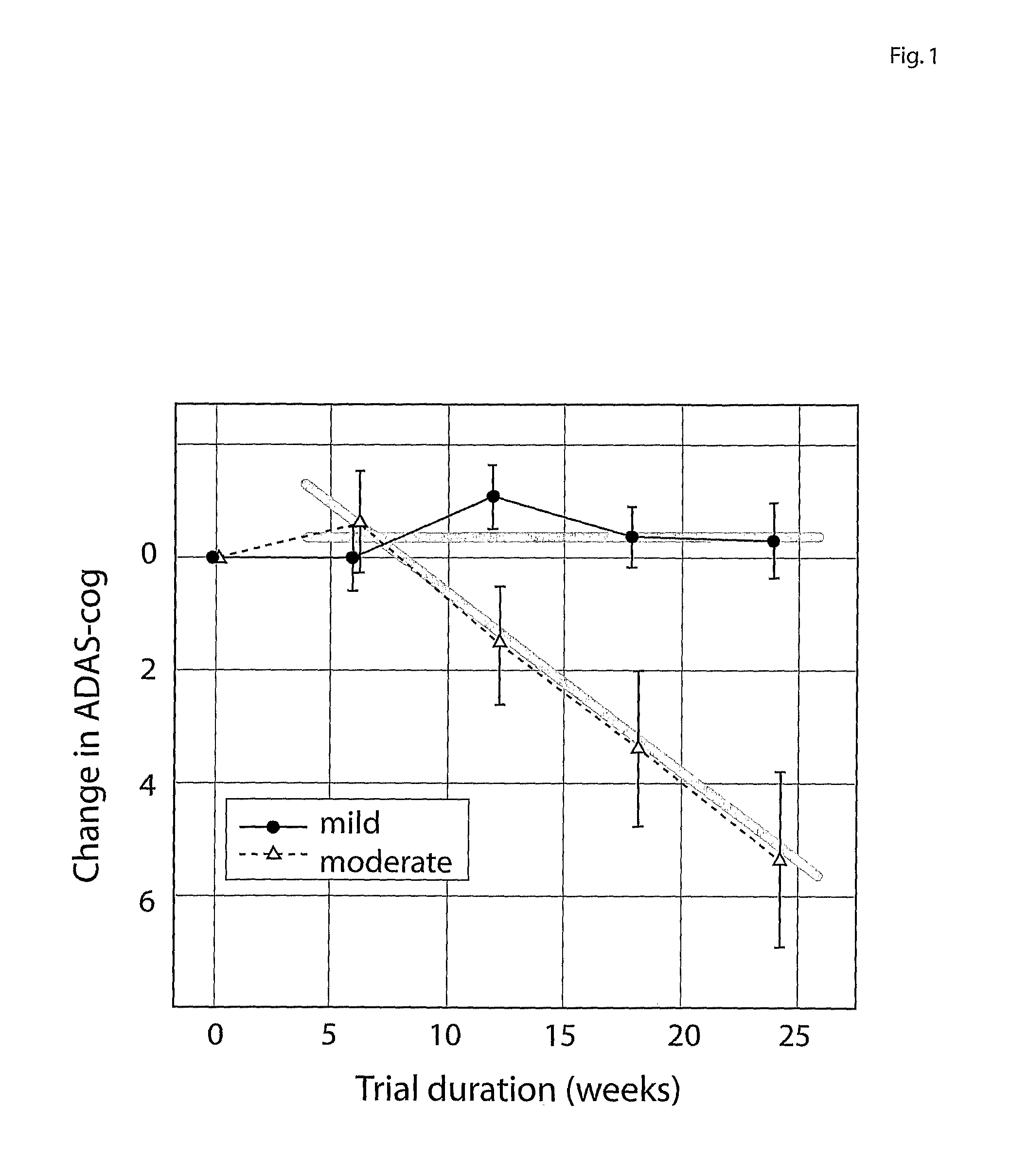
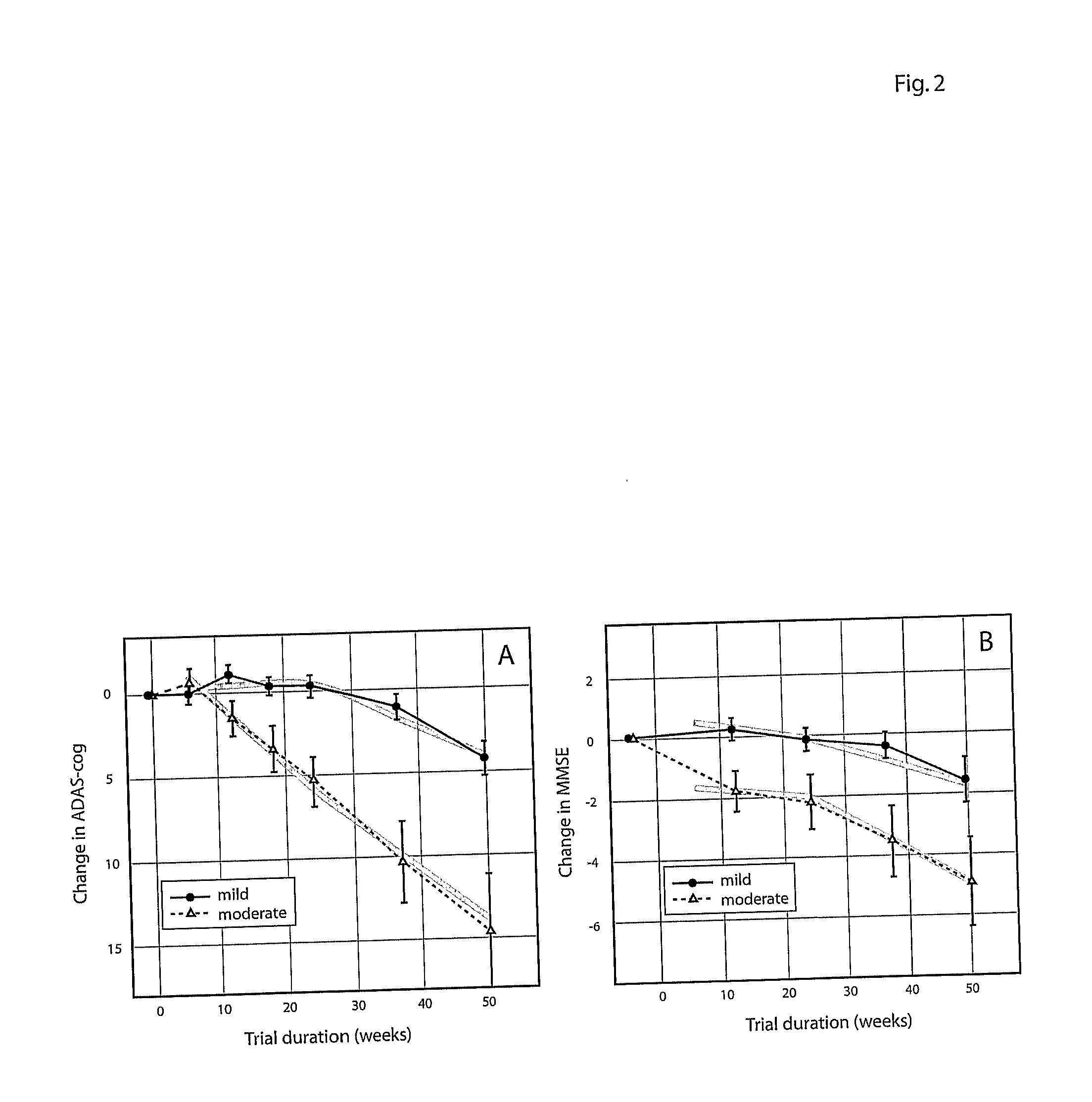
Systems for clinical trials
InactiveUS20100280975A1Medical simulationData processing applicationsBaseline IndicatorTreatment Arm
The invention provides methods and systems for assessing the efficacy of a pharmaceutical which is putatively disease modifying of a cognitive disorder, for use in the treatment or prophylaxis of that cognitive disorder, the method comprising the steps of: (1) stratifying a subject group into at least 2 sub-groups according to a baseline indicator of likely disease progression, (2) treating members of each subject group with the pharmaceutical for a treatment time frame, (3) deriving psychometric and optionally physiological outcome measures for each treated patient group, (4) comparing the outcomes at (3) with a comparator arm of said sub-groups which is optionally a placebo or minimal efficacy comparator arm, (5) using the comparison in (4) to derive an efficacy measure for the pharmaceutical. The methods and systems of the invention address problems such as low rate of decline over the treatment time-frame of patients who have mild-disease severity at baseline and biased withdrawal, particularly in the placebo / comparator treatment arm.
Owner:WISTA LAB LTD
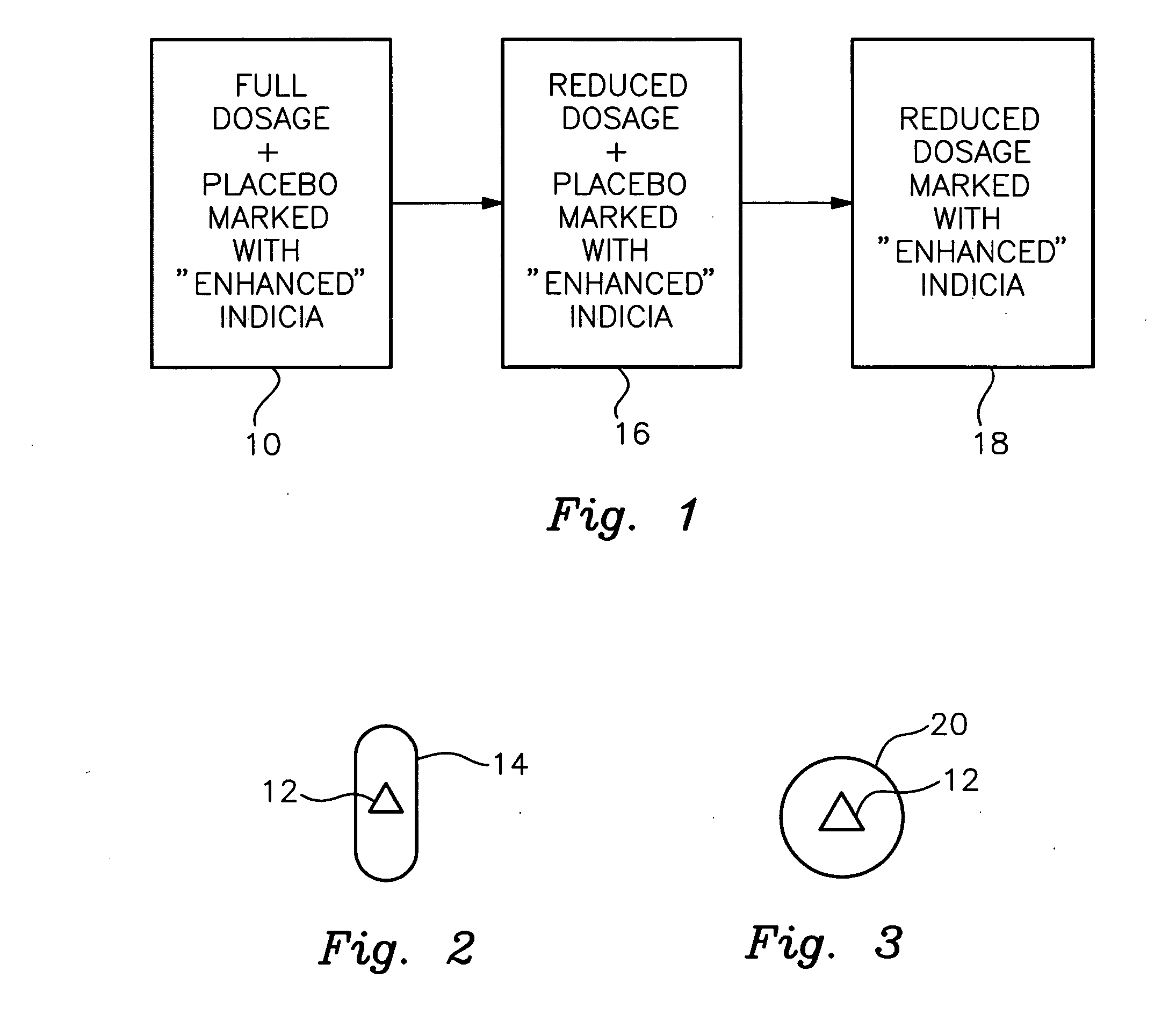
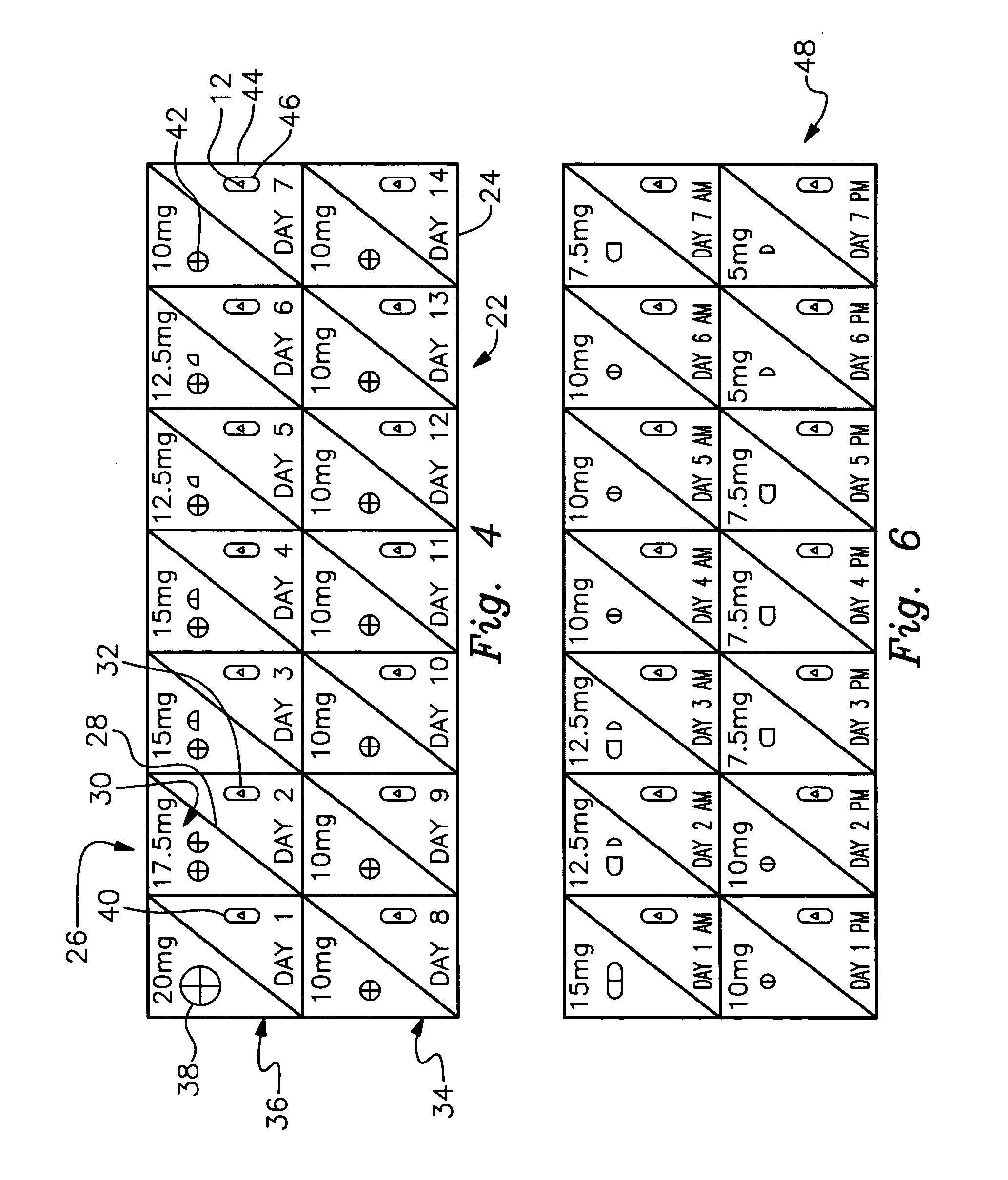
Therapeutic placebo enhancement of commonly used medications
InactiveUS20050136106A1Less pharmaceuticalReduced effectivenessPill deliveryHeterocyclic compound active ingredientsUse medicationDisease
There is provided a method and associated kit for reducing the normal dosage of a pharmaceutical given to a patient for the treatment of a disorder without substantially reducing its effectiveness. During a first predetermined time period, a substantially full dosage of the pharmaceutical is administered to the patient, preferably with a placebo. During a second predetermined time period, a reduced dosage of the pharmaceutical is administered to the patient, also with a placebo. The second predetermined time period is subsequent to the first predetermined time period. Preferably, the placebo has a distinctive indicia. The placebo, in association with the decreased pharmaceutical, augments the effectiveness of the pharmaceutical by heightening the patient's conditioned response and expectation of effectiveness.
Owner:SANDLER ADRIAN
Combination preparation for contraception based on natural estrogens
InactiveUS6884793B2Improves cycle bleeding behaviorMinimizes and prevents side effectOrganic active ingredientsBiocidePhysiologyPlacebo
The combination preparation for contraception includes from 2 to 4 first stage daily dosage portions each including an effective amount of at least one natural estrogen as sole active ingredient, from 16 to 22 second stage daily dosage portions each including an effective amount of a combination of at least one natural estrogen and at least one natural or synthetic gestogen as active ingredient; from 2 to 4 third stage daily dosage portions each including an effective amount of at least one natural estrogen as sole active ingredient; and from 2 to 4 final stage daily dosage portions containing a pharmaceutically acceptable placebo. The estrogen may be estradiol, an estradiol compound that is metabolized to estradiol when taken into the body, a conjugated equine estrogen or a phytoestrogen. The natural or synthetic gestogen can be natural progesterone or a synthetic gestogens, such as medroxyprogesterone acetate.
Owner:BAYER INTELLECTUAL PROPERTY GMBH
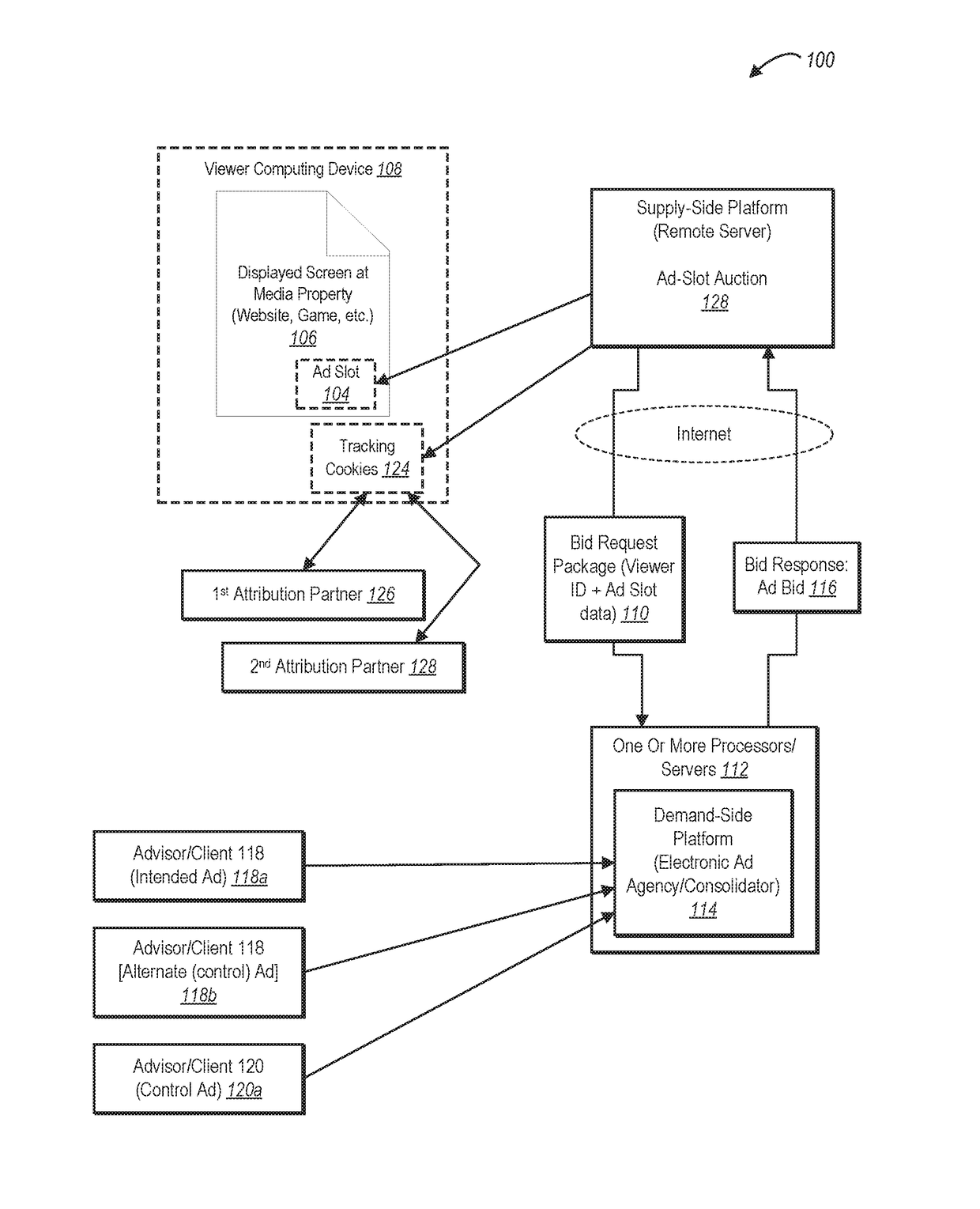
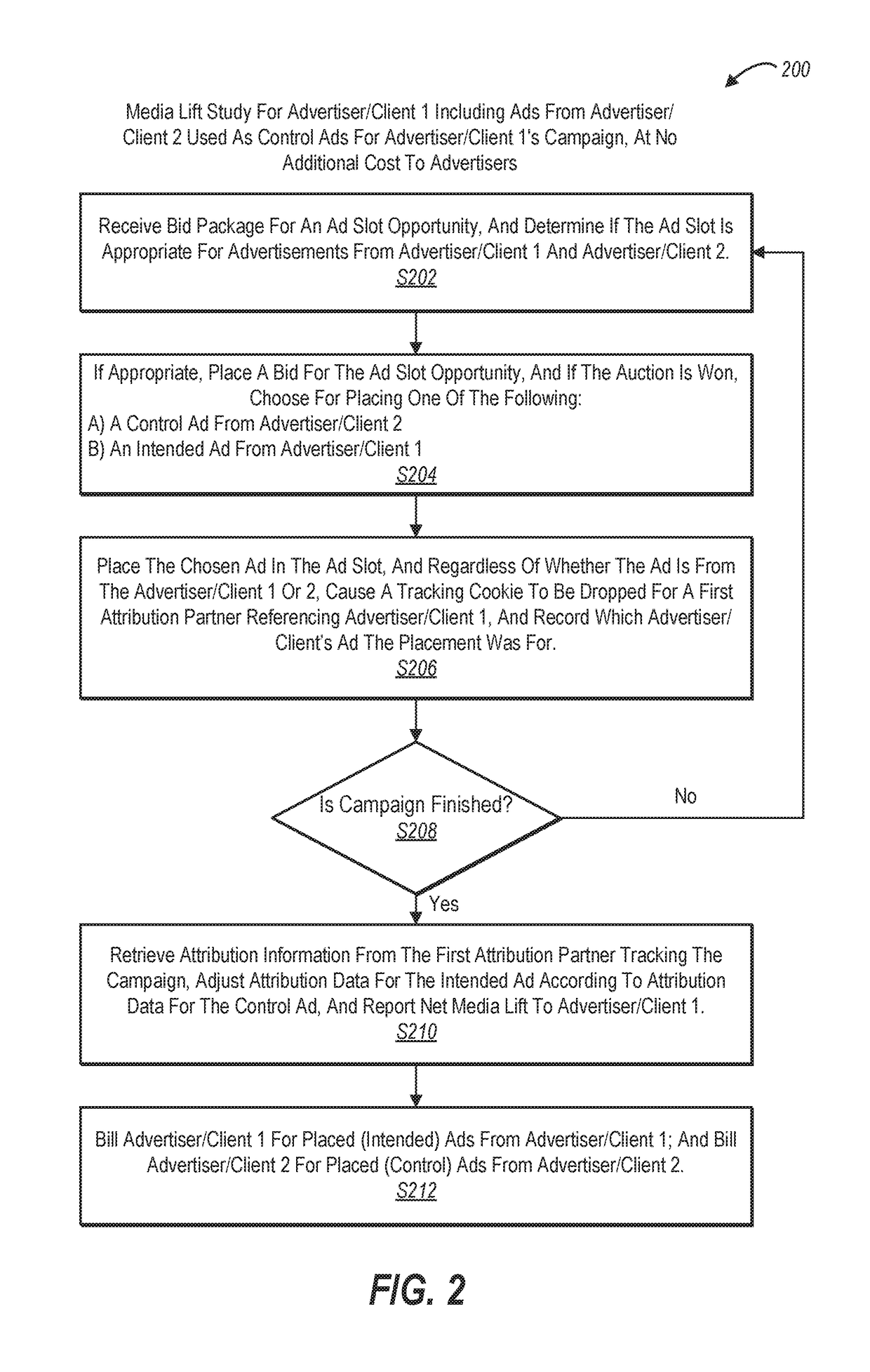
Real-time bidding through placebo-based experimentation
Systems and methods for operating placebo-based experiments are described for online advertisements. One or more embodiments of the disclosed systems and methods utilizes an ad swapping approach to offer placebo media exposures, at no additional cost to an advertiser. One or more embodiments further provide a native experimentation platform that allows users to run tests of ad placements to measure the effectiveness of ads and view results displayed on a user interface. The disclosed systems and methods can assign viewers into a test group if shown the test ad or a control group if shown a control ad. The control ad can be provided at no cost to the advertiser for embodiments where the placebo ad belongs to an alternative advertiser. Effectiveness of third party attribution can also be evaluated. The disclosed systems and methods can define experiment parameters, including control frequency, test viewer groups, and control viewer groups.
Owner:ADOBE INC
Guidance/self-learning process for using inhalers
InactiveUS20150339953A1Readily availableEasy to learnDrug and medicationsMedical devicesPlaceboAnimation
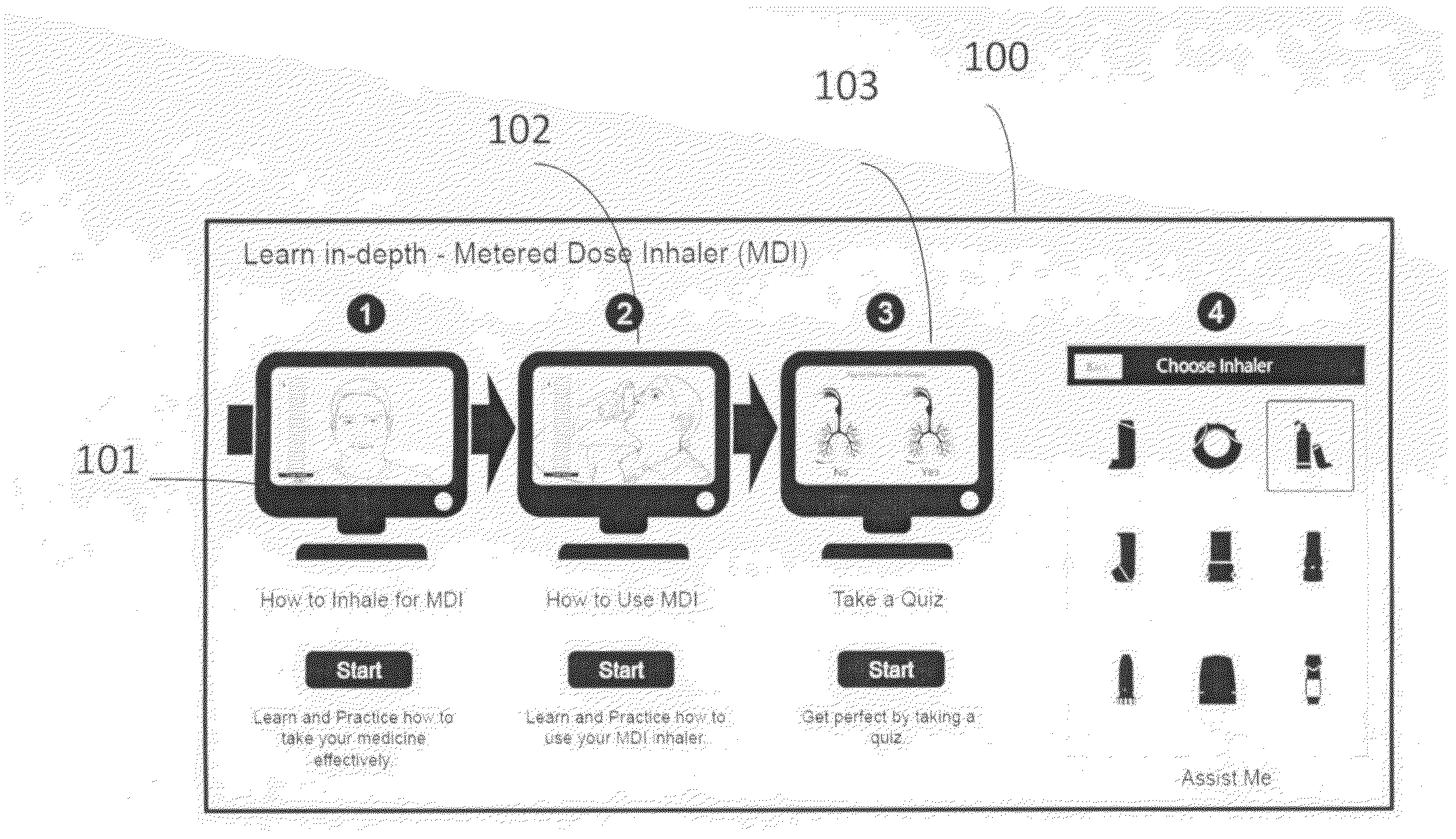
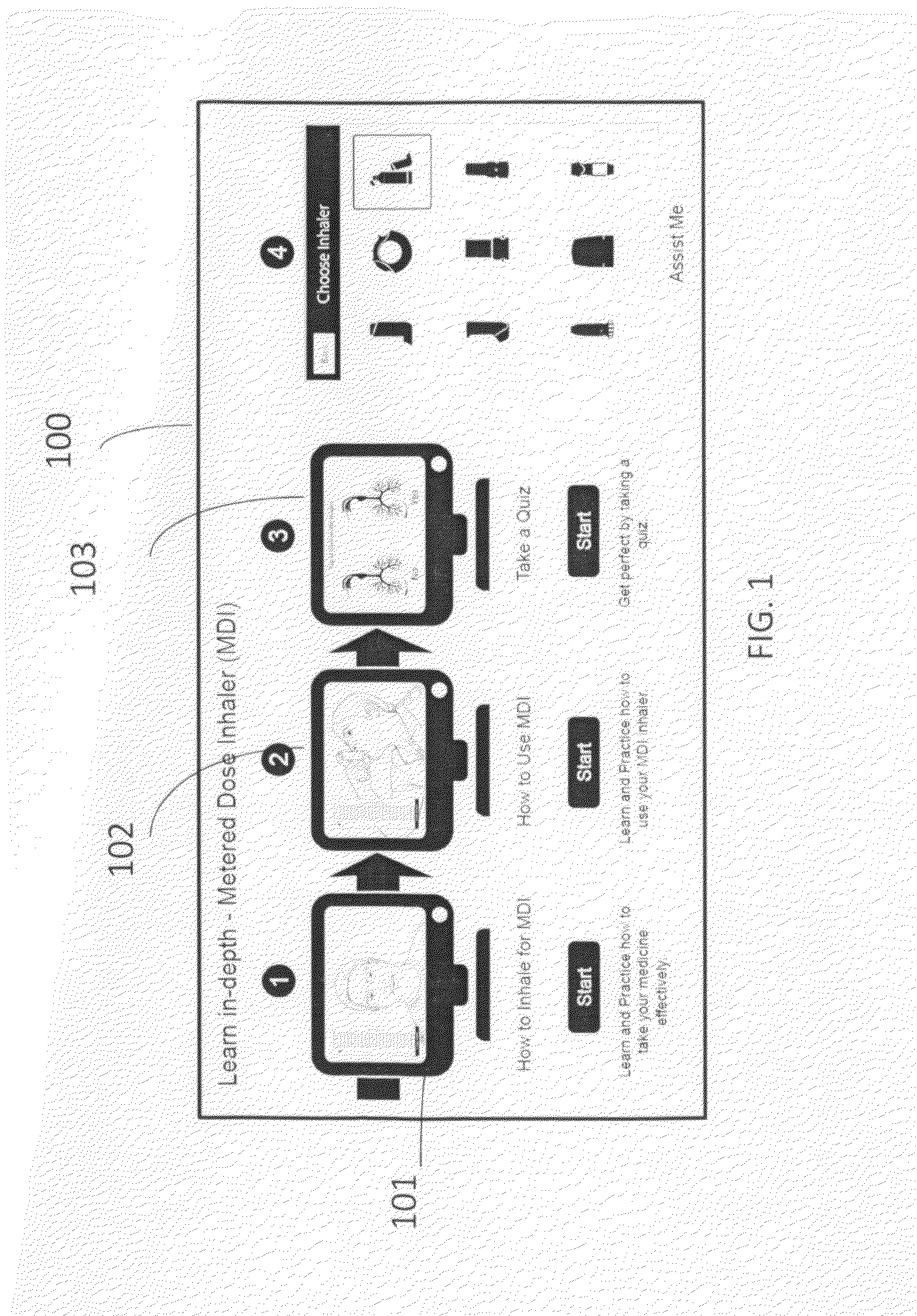
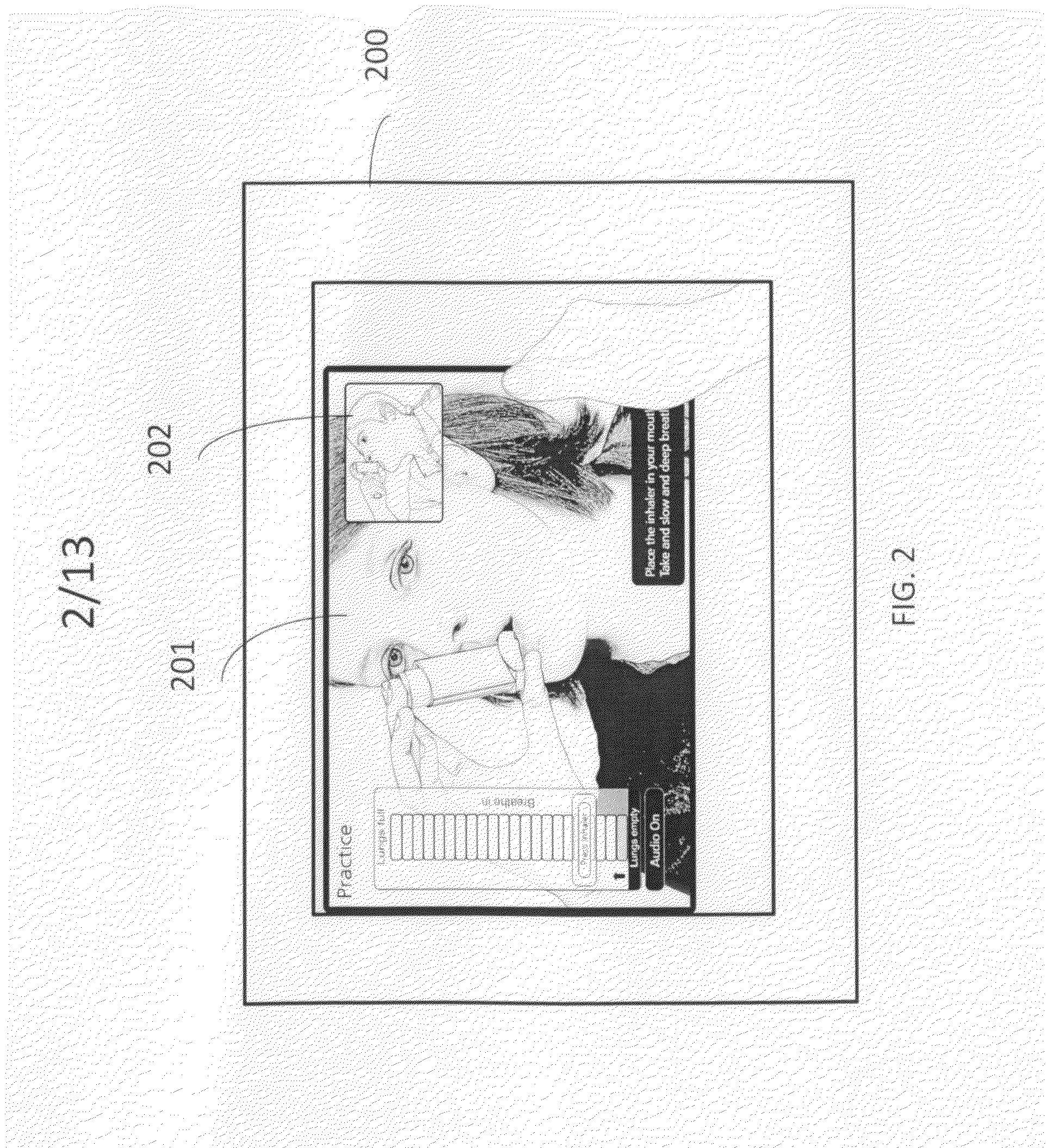
A self-learning system of providing guidance on using inhaler devices, provided using a portable or mobile computing device. Guidance comprises of multiple stages(modes), and provides a unique feature of real-time learning using a camera device. It provides simultaneous viewing of the instructional video and the patient's real-time video in real time. The said guidance is provided in step-by-step instructions in an incremental manner of increasing complexity. The steps of the guidance are linked together by an audio-visual indicator. The self-learning system of using inhaler devices has various features including action-focusing videos, a technique to superimpose an animation on the learner's real-time video and virtual placebo inhaler. The said system of using inhalers also provides guidance on learning, practicing / reinforcing the correct method of using inhalers and assists the patient in using the inhaler on a regular basis by logging, analyzing and alerting about the time and dosage of the inhaler.
Owner:SHAH FENIL
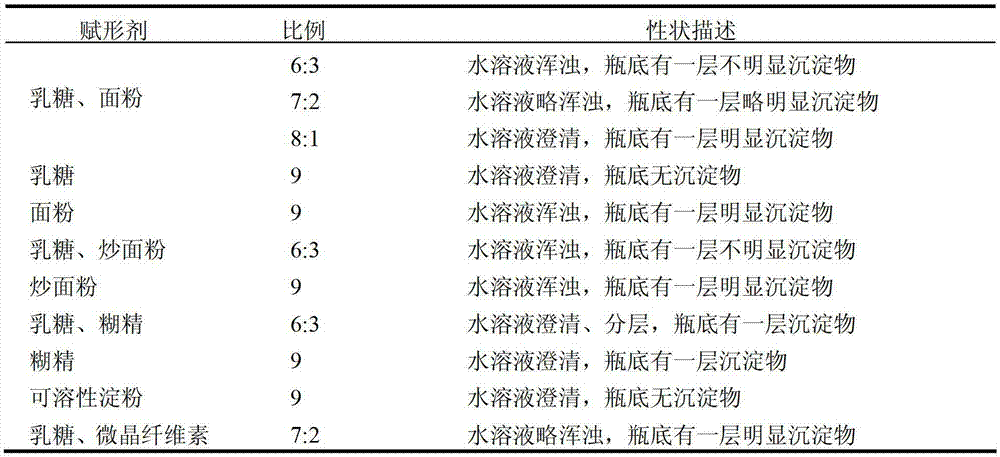
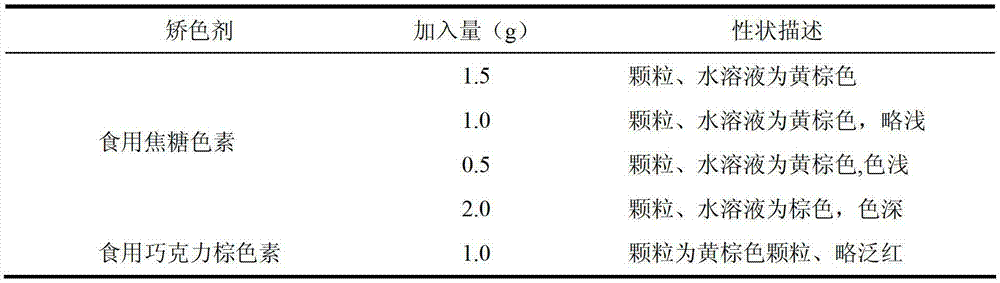
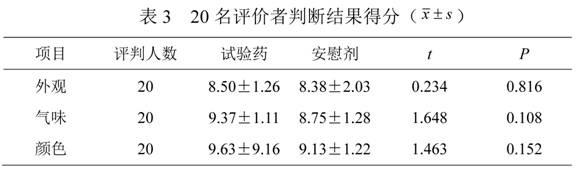
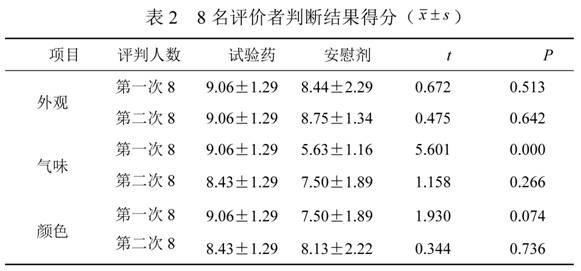
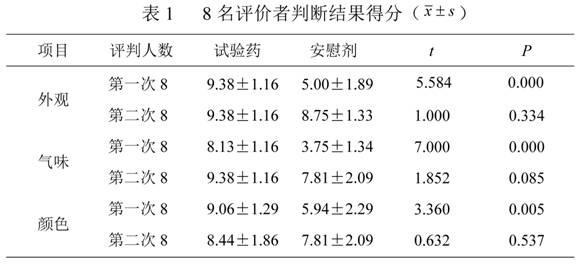
Traditional Chinese medicine placebo, as well as preparation process and evaluation method thereof
InactiveCN103110962AAchieve objective quantificationHigh similarityIn-vivo testing preparationsTesting medicinal preparationsHuman bodyExperimental research
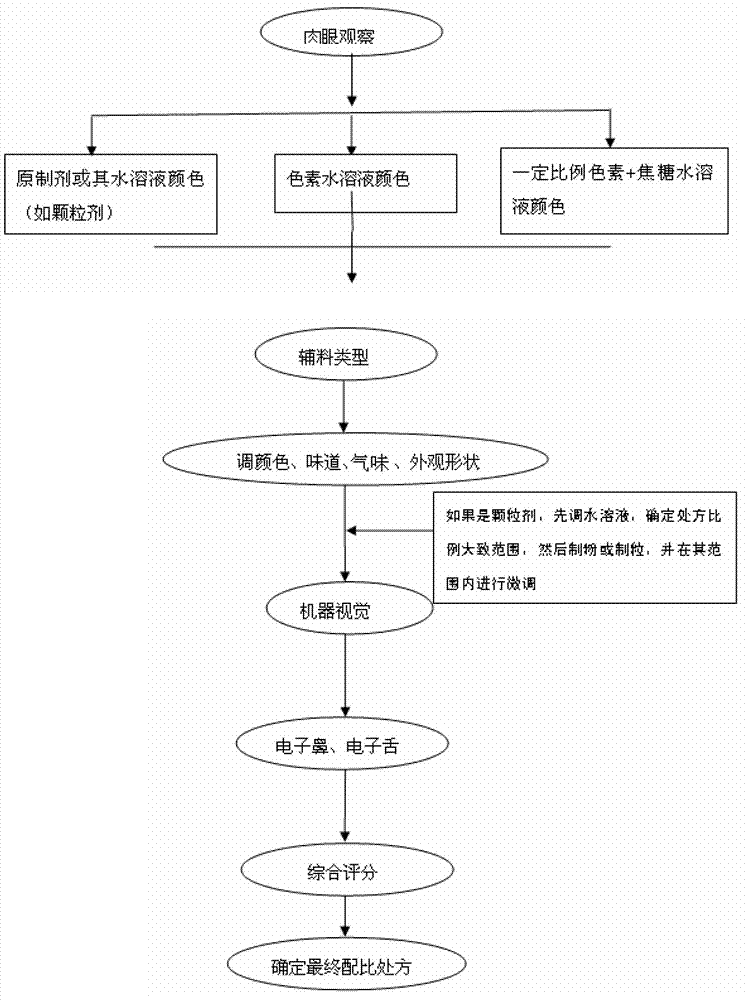
The invention provides a traditional Chinese medicine placebo preparation process. The invention also provides a traditional Chinese medicine placebo and an evaluation method of the placebo. According to the invention, a small amount of an original preparation (granule, mixture, tablet, capsule, pill, external preparation, and the like) is taken and prepared into a traditional Chinese medicine placebo, the similarity of properties of the placebo and the original preparation is high, and the placebo has no physiological activity, therefore, the placebo is more suitable for clinical and experimental research; meanwhile, the invention also provides a multi-sensing information fusion based intelligent sensory evaluation method for the traditional Chinese medicine placebo, which is implemented through simulating three sensory organs, namely, eye, nose and tongue of the human body respectively by using a machine vision (visual sensor), an electronic nose (smell sensor) and an electronic tongue (taste sensor), so that the objective quantification of properties evaluation of the traditional Chinese medicine placebo is realized, and the subjective deviation caused by the experiential sensory evaluation of people is avoided.
Owner:CHENGDU UNIV OF TRADITIONAL CHINESE MEDICINE
Oral food challenge meal formulations
ActiveUS20180177895A1Reduce the amount of solutionMaintaining blindingCompounds screening/testingFood ingredient as antigenicityMedicinePlacebo
Owner:REACTA BIOTECH
Extended cycle multiphasic oral contraceptive method
A multiphasic method of contraception that provides for sequentially administering to a female of child bearing age: (a) a Phase I composition containing a progestogen in an amount equivalent to about 0.5 to about 1.5 mg norethindrone acetate and an estrogen in an amount equivalent to about 5 to about 30 mcg of ethinyl estradiol for about 4 to about 7 days; (b) a Phase II composition containing a progestogen in an amount equivalent to about 0.5 to about 1.5 mg of norethindrone acetate and an estrogen in an amount equivalent to about 10 to about 40 mcg of ethinyl estradiol for about 8 to about 16 days; (c) a Phase III composition containing a progestogen in an amount equivalent to about 0.5 to about 1.5 mg of norethindrone acetate and an estrogen in an amount equivalent to about 5 to about 30 mcg of ethinyl estradiol for about 4 to about 7 days; and (d) optionally, a Phase IV composition which is a placebo or a non-steroidal component, such as for example, ferrous fumarate, for about 2 to about 9 days, wherein the ethinyl estradiol equivalent amount of estrogen in the Phase II composition is at least 5 mcg greater than the ethinyl estradiol equivalent amount of estrogen in each of the Phase I and III compositions. Preferably the sequential administration of the Phase I, II, and II compositions is repeated the day following the completion of the administration of the Phase III compositions to provide an extended cycle multiphasic oral contraceptive method.
Owner:APTALIS PHARMA
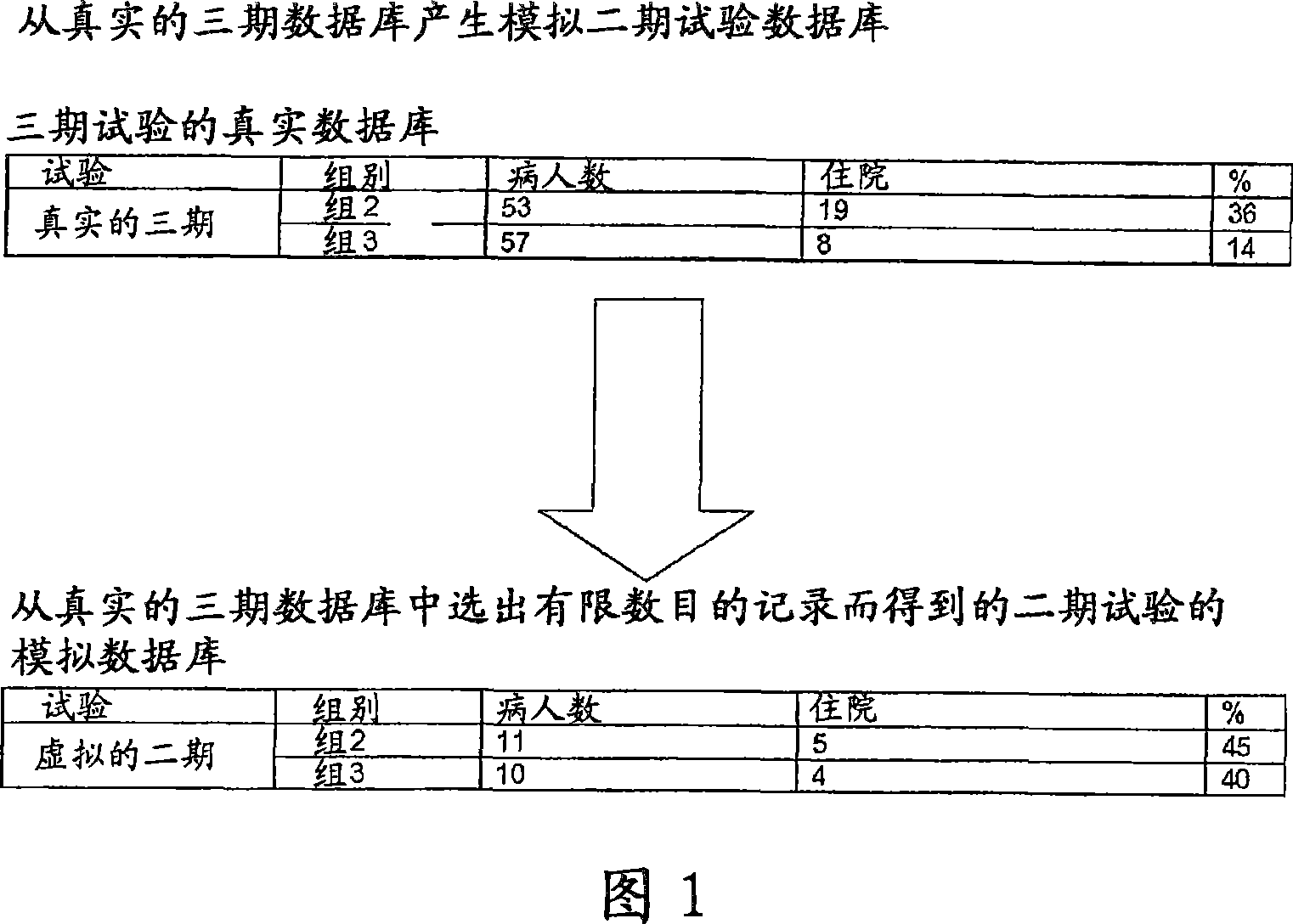
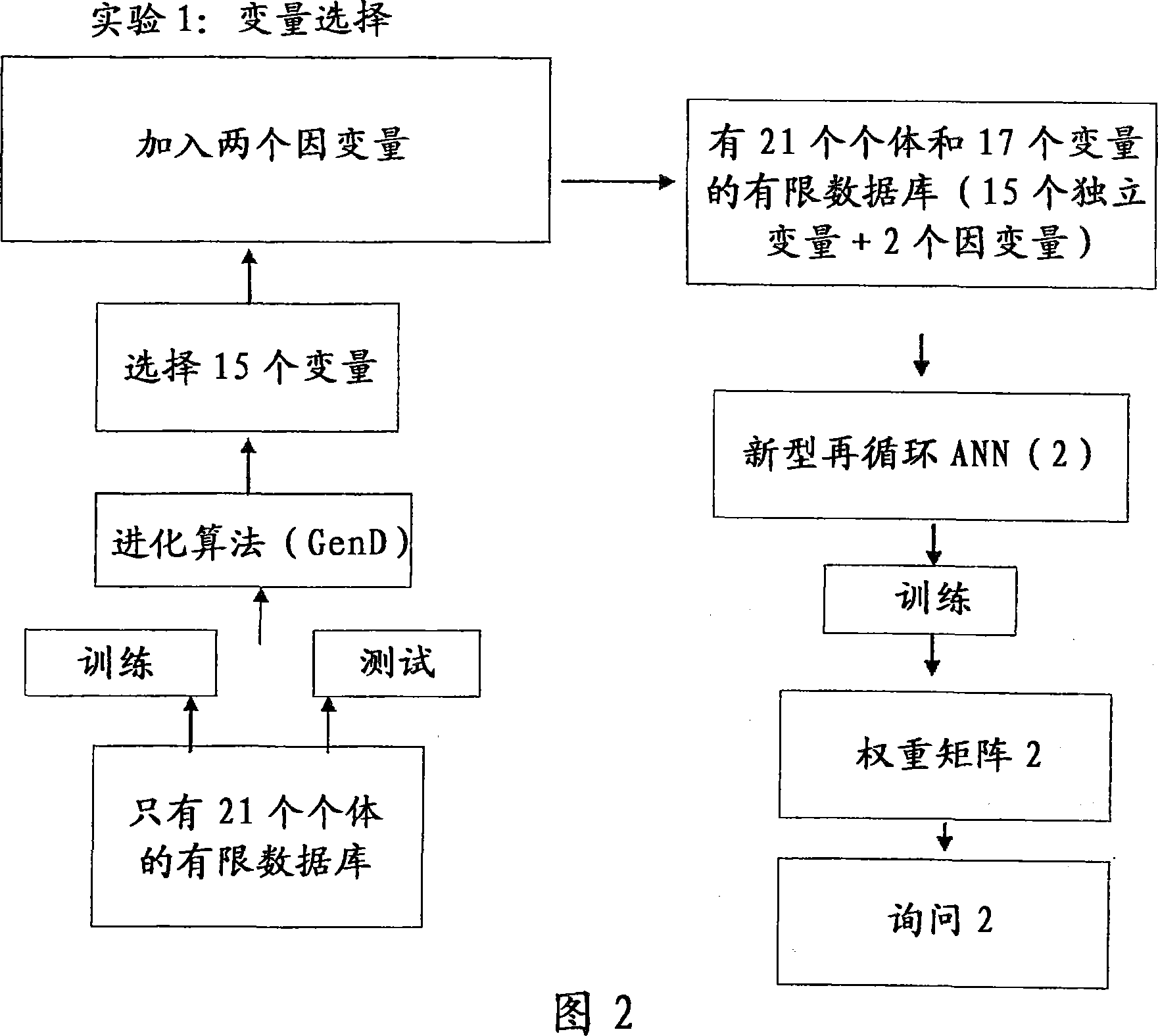
Clinical trial phase simulation method and clinical trial phase simulator for drug trials
InactiveCN1977270AMedical simulationComputer-assisted medicine prescription/deliveryNerve networkMedicine
A clinical trial phase simulation method for drug trials, which method allows to predict the trend of the results of a clinical trial phase of a drug with the steps of providing a database comprising for each of a certain number of individuals a predefined number of independent variables each of which corresponds to a certain clinical parameter relevant or characteristic for a disease condition against which the drug to be tested is oriented and at least a further independent variable describing the specific treatment to which the individual has been subjected between at least two different treatments one with the said drug and the second with a placebo or with another known drug, the database comprising also for each individuals one or more dependent variables describing the effects of the said treatments; carrying out an input variable selection; adding to the independent variables selected as input variables the dependent variables describing the effects of the treatments; training and validating an artificial neural network with the selected variables as input variables and with the dependent variables; interrogating the said neural network by inputting the values of the variable describing one of the treatments and obtaining as an output the variable values of the effectiveness of the treatment to which the inputted values of the variable of the treatment correspond according to the trained artificial neural network.
Owner:BRACCO IMAGINIG SPA
Bubble pack toy containing comestibles
InactiveUS6283762B1Simple and economical to manufactureDollsFruit and vegetables preservationPlaceboThree dimensional shape
A toy peripherally formed of flexible bubble packing material in a shape simulating an object defines an internal cavity that is stuffed with a plurality of relatively smaller pieces of comestible material such as candy to provide a three dimensional shape. The toy is destructible so that a user may gain pleasure not only by reason of ownership and manual manipulation as with any three dimensional toy, but also from manually popping the bubbles of the peripheral covering to create sound and provide an additional manipulative function and from opening the cavity of the toy to gain access to the contained and consuming the comestible. The toy is useful as a placebo in juvenile counseling, psychology, psychiatry and education to alleviate tension and introversion and to gain attention and establish a responsive communicative relationship with the toy user.
Owner:WIGGINS WARREN MORRIS
Placebo for coronary heart disease
InactiveCN102133408ANot easy to break the blindIn-vivo testing preparationsSucrose octa acetateCoronary heart disease
The invention discloses placebo for coronary heart disease and a preparation method thereof. The placebo contains malto dextrin, caramel pigment, sunset yellow pigment, tartrazine, sucrose octaacetate, tangerine essence, agastache rugosus essence, Beta-cyclodextrin and non-decoction coronary heart disease particles. The preparation method comprises the following steps: taking the Beta-cyclodextrin and adding water for dissolving, then adding the tangerine essence and the agastache rugosus essence and mixing uniformly, arranging the mixture into a colloid mill for milling ten minutes so as to obtain essence compound; taking the malto dextrin, the caramel pigment, the sunset yellow pigment, the tartrazine, and the sucrose octaacetate and adding water for dissolving, then adding the essence compound in the mixture and mixing uniformly, spraying and drying the mixture liquid so as to obtain spray drying powder; and sieving the spray drying powder by a sieve with 100 meshes, and mixing the spray drying powder with the non-decoction coronary heart particles uniformly, mixing the obtained drying powder is mixed, granulating by a drying method, packaging and then obtaining the placebo. The placebo for the coronary heart disease is more similar to the non-decoction coronary heart particles in smell, taste and color, is not easy to be taken as a placebo by a tester, so that blindness breaking of a double-blind experiment can be guaranteed to be difficult.
Owner:GUANGDONG HOSPITAL OF TRADITIONAL CHINESE MEDICINE
Preparation of microparticles having improved flowability
InactiveUS7247319B2Preserve sterilityReadily availablePowder deliveryOrganic active ingredientsActive agentPlacebo
Methods for preparing microparticles having improved flowability to facilitate processing in automated equipment. Microparticles are conditioned so that a flowability index of the microparticles is greater than about 60. The conditioning preferably includes maintaining the microparticles at a conditioning temperature for a period of time. The conditioning can be used with microparticles containing an active agent, and with placebo microparticles, and it is reversible.
Owner:ALKERMES INC
Oral contraceptive containing a gestagen and an estrogen combined with pharmaceutically acceptable auxiliary agents and/or excipients, but not containing lactose, and method of making same
InactiveUS20090117183A1Easy to solveLow costBiocideOrganic active ingredientsPhysiologyAdditive ingredient
The method produces a lactose-free oral contraceptive composition containing a combination of a gestagen and an estrogen together with one or more pharmaceutically acceptable auxiliary agents and / or excipients. The contraceptive composition is a tablet, powder, or capsule that contains the gestagen and estrogen, filler material such as microcrystalline cellulose and a binder such as hydroxypropylcellulose, but no lactose. Preferably the gestagen is dienogest, chlormadinone acetate, or levonorgestrel and the estrogen is ethinylestradiol, 17β-estradiol, or estradiol valerate. A method is provided for improving the prophylaxis of lactose intolerance in women taking oral contraceptives. The oral contraceptive preparations for a standard 28-day cycle or for long-term use contain at least 21 daily dose units of the gestagen and the estrogen in a low-dosage but without lactose and at most 7 daily dose units containing no active ingredient or a placebo.
Owner:BAYER SCHERING PHARMA AG
Encapsulation machine with valved injection wedge
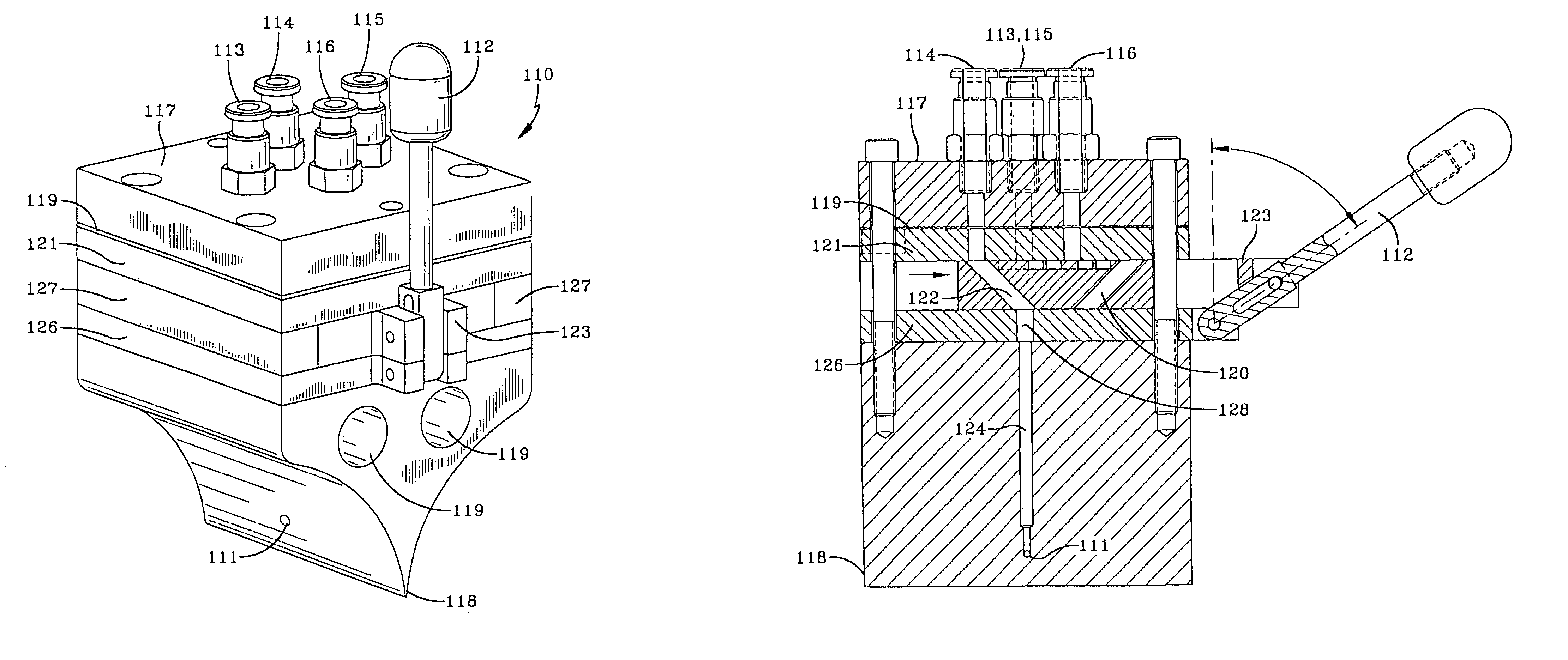
InactiveUS6990791B2Facilitate evenFacilitate consistent extrusionConfectionerySweetmeatsPlaceboEngineering
This invention relates to a method and apparatus for forming soft capsules and provides novel processing flexibility. The apparatus comprises a three-way valve injector wedge. This injector wedge allows for set-up of the encapsulation machine with a placebo fill and quick change over to active fill. This conserves use of the active ingredient.
Owner:CATALENT USA WOODSTOCK INC +3
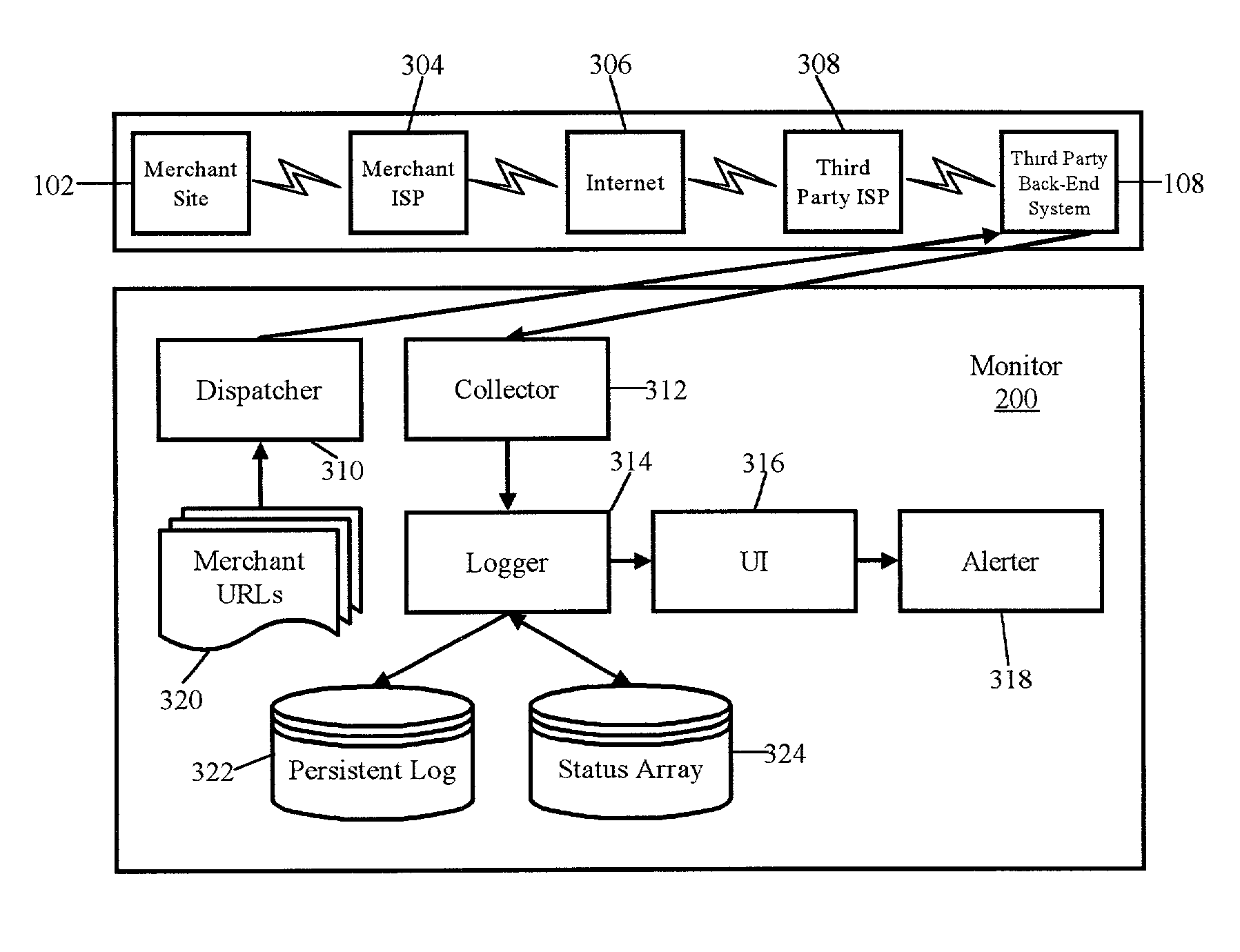
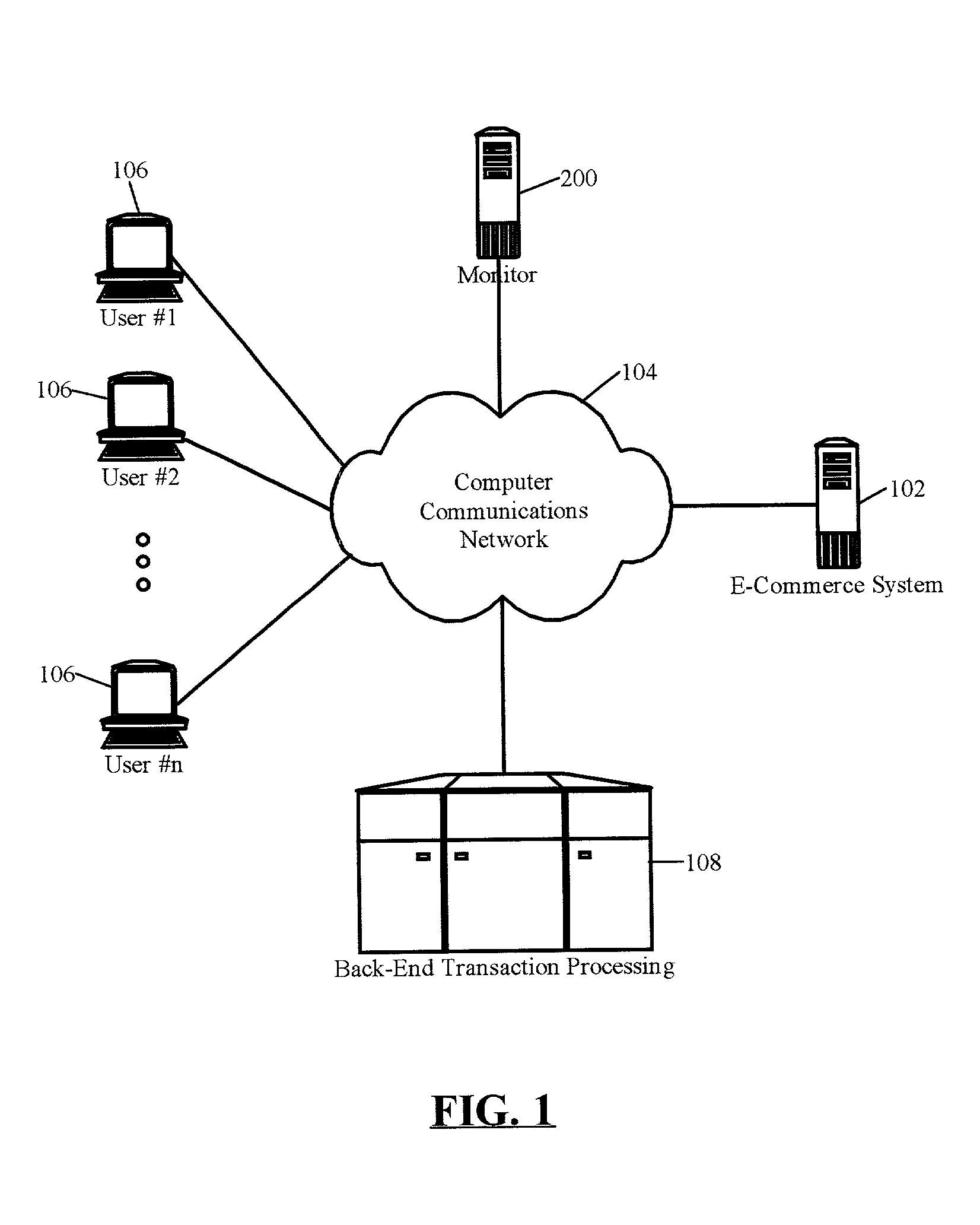
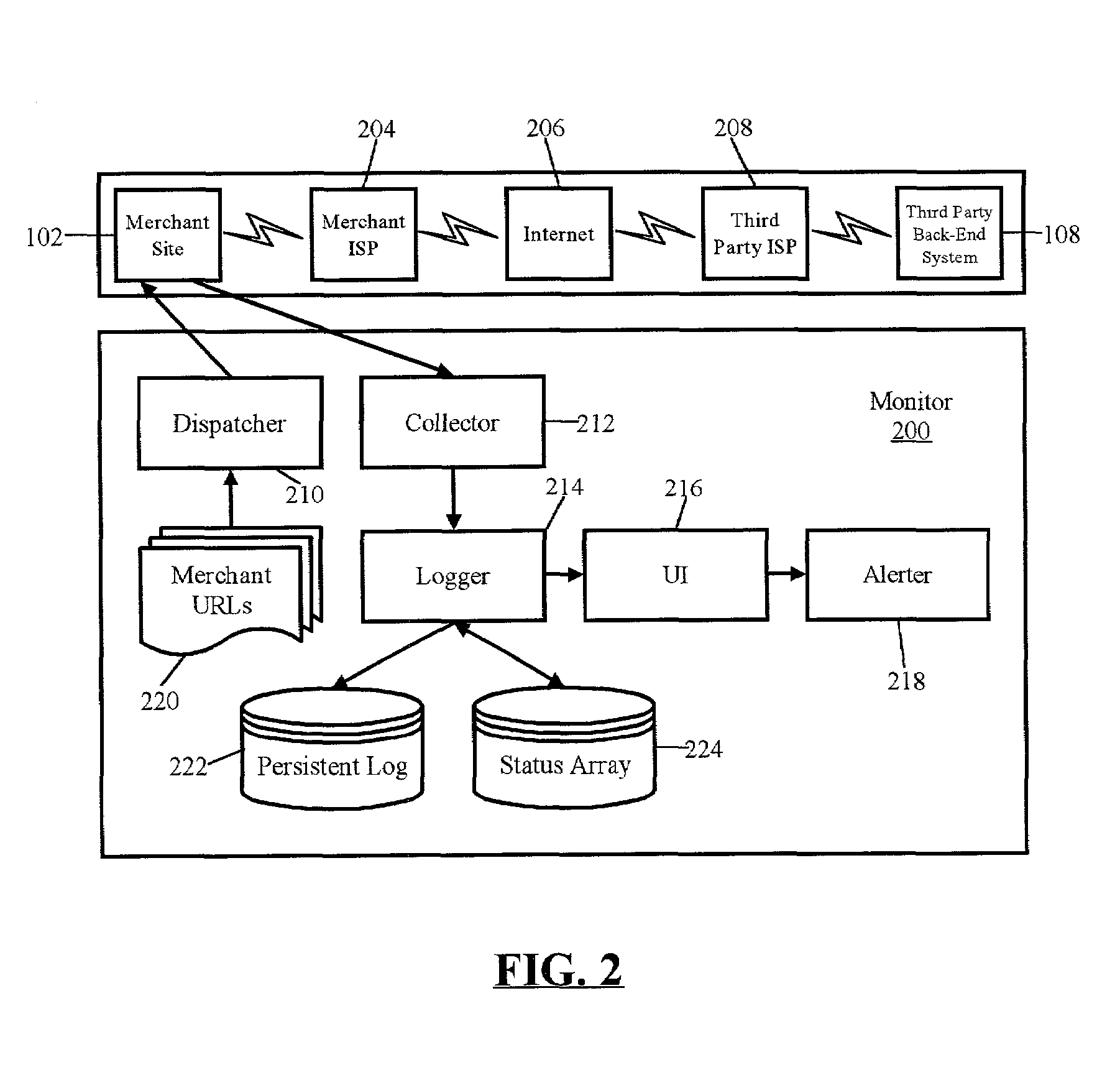
Monitoring tool
A method for detecting unreliable response conditions in a plurality of back-end transaction processing systems also can include the steps of: reading a list of references to a plurality of subscribing e-commerce systems; generating and dispatching placebo transactions to each e-commerce system in the list; receiving responses to the dispatched placebo transactions; computing transaction latency data based upon when each placebo transaction is dispatched to a subscribing e-commerce system, and when a corresponding response is received; and, notifying individual subscribing e-commerce systems when computed transaction latency data for the individual subscribing e-commerce systems indicates an unreliable response condition in an associated back-end transaction processing system.
Owner:IBM CORP
Menstrual cycle control and improvement of conception rates in females
InactiveUS20050171071A1Raise the possibilitySuppressing ovulationOrganic active ingredientsBiocideOvulation timesPlacebo
The present invention relates to methods of increasing likelihood of conception, treatment of low fecundity and / or providing menstrual cycle control without suppressing ovulation in a female comprising administering an effective dose of at least one estrogen and at least one progestin within a treatment period of at least 21 days to 35 days or multiple periods thereof. Furthermore, the invention relates to the combination of at least one estrogen and at least one progestin for the preparation of a medicament to be used in the abovementioned indications. A pharmaceutical kit, according to the invention, comprises 21 to 35 dosage units or a multiple of 21 to 35 dosage units, wherein a first section comprises at most 21 dosage units of at least one estrogen; and a second section comprises from 2 to 34 dosage units of at least one estrogen and from 2 to 34 dosage units of at least one progestin or comprises from 2 to 34 dosage units of at least one estrogen and at least one progestin provided that in each dosage unit containing a progestin or a progestin and an estrogen the progestin is in a non-ovulation inhibiting amount; and and optionally a third section comprises at most 10 dosages of a pharmaceutically acceptable placebo or a blank.
Owner:BAYER SCHERING PHARMA AG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com