Clinical trial phase simulation method and clinical trial phase simulator for drug trials
A technology for clinical trials, simulation methods, used in electronic clinical trials, computer-aided drug prescription/delivery, medical simulation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
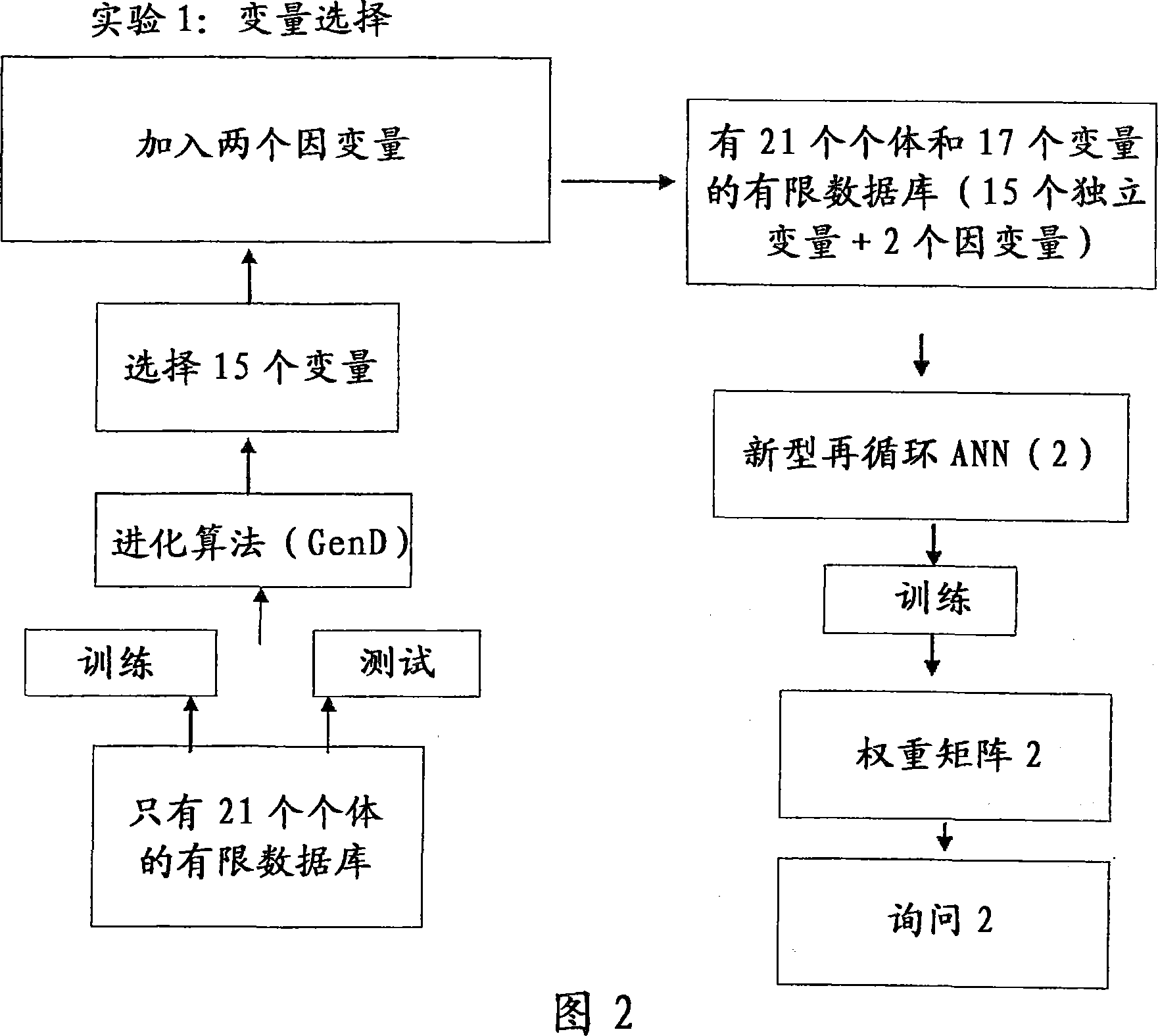
[0071] The method involved in the present invention is explained below through two experiments.
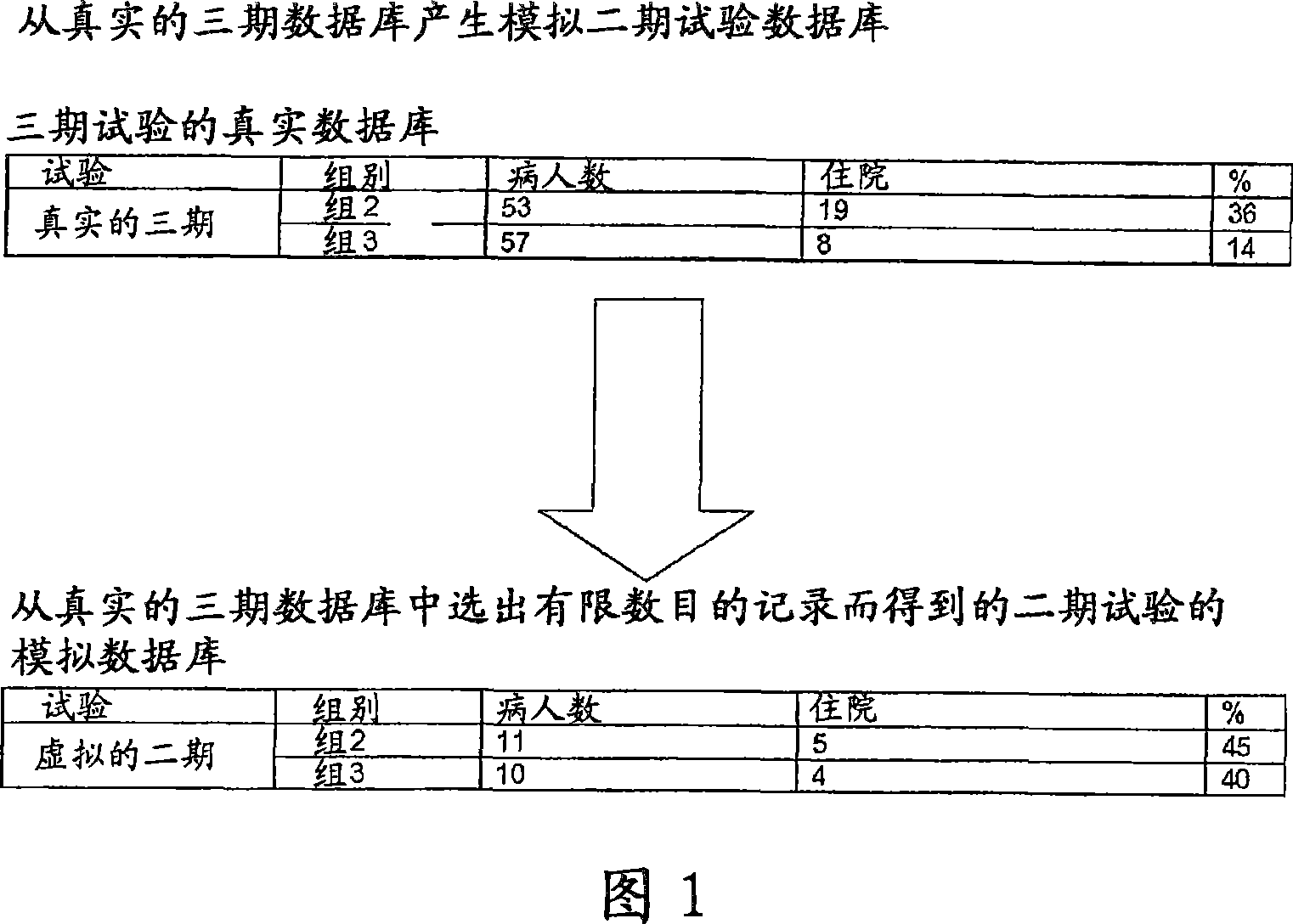
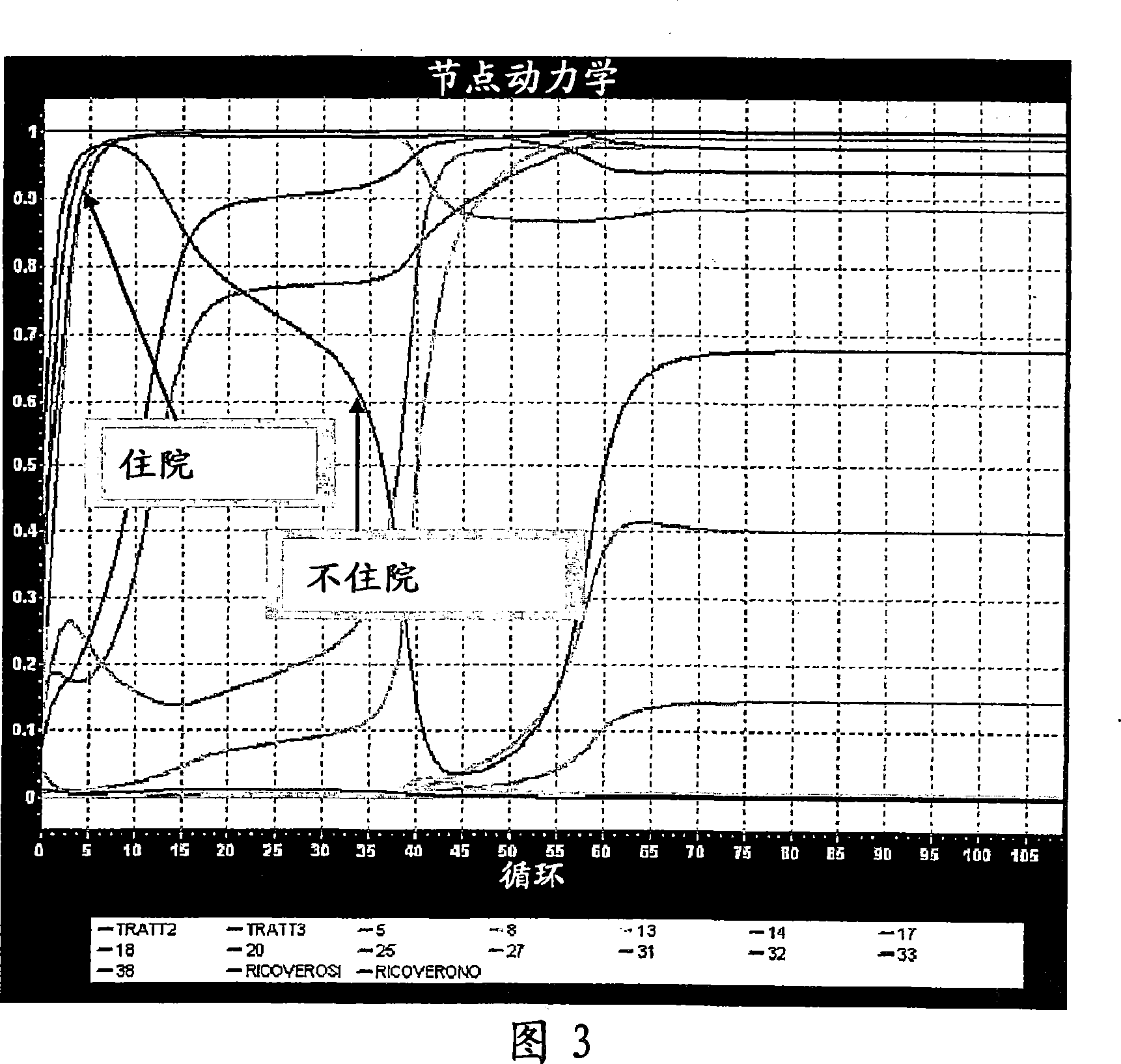
[0072] Both experiments were performed using the same starting database, generated using a database of Phase III trials with known and established outcomes on known drugs. The initial database is generated to simulate Phase II trial database. Also, from each group, select the reduced number of individuals that make up the group. The choice was made to provide results of this trial that did not lead to a clear indication of the efficacy of the drug being tested, contrary to the results of Phase III trials, which had shown that the drug was effective for the The disease has a clear curative effect.
[0073]A typical phase 2 trial scenario is thus constructed whereby the phase 2 trial does not give a clear indication of the efficacy of the drug being tested, when in fact the drug is effective.
[0074] The experimental database used as the basis for constructing the simulated pha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com