Patents
Literature
70 results about "Drug test" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A drug test is a technical analysis of a biological specimen, for example urine, hair, blood, breath, sweat, and/or oral fluid/saliva—to determine the presence or absence of specified parent drugs or their metabolites. Major applications of drug testing include detection of the presence of performance enhancing steroids in sport, employers and parole/probation officers screening for drugs prohibited by law (such as cannabis, cocaine, methamphetamine, and heroin) and police officers testing for the presence and concentration of alcohol (ethanol) in the blood commonly referred to as BAC (blood alcohol content). BAC tests are typically administered via a breathalyzer while urinalysis is used for the vast majority of drug testing in sports and the workplace. Numerous other methods with varying degrees of accuracy, sensitivity (detection threshold/cutoff), and detection periods exist.
Testing a patient population having a cardiovascular condition for drug efficacy
Lumenectomy material is tested to determine the efficacy of a test drug in a patient population having a cardiovascular condition. The material is removed from at least a first and a second patient and tested for one or more markers of a cardiovascular condition. The first patient is administered the test drug, and the second patient is administered a placebo. At a later date, more lumenectomy material is removed and tested for the same marker or markers. The presence, absence or amount of the markers is compared in the first patient receiving the drug and the second patient receiving the placebo to determine whether the drug is effective in the patient population. The patient population can comprise as little as two individuals or as many as dozens, hundreds or thousands of patients. The drugs tested include drugs believed to be effective in treating a cardiovascular condition. The markers used can include any marker that can indicate the effectiveness of the drug being tested, including amino acid and nucleic acid markers and markers that indicate a cardiovascular condition.
Owner:TYCO HEALTHCARE GRP LP
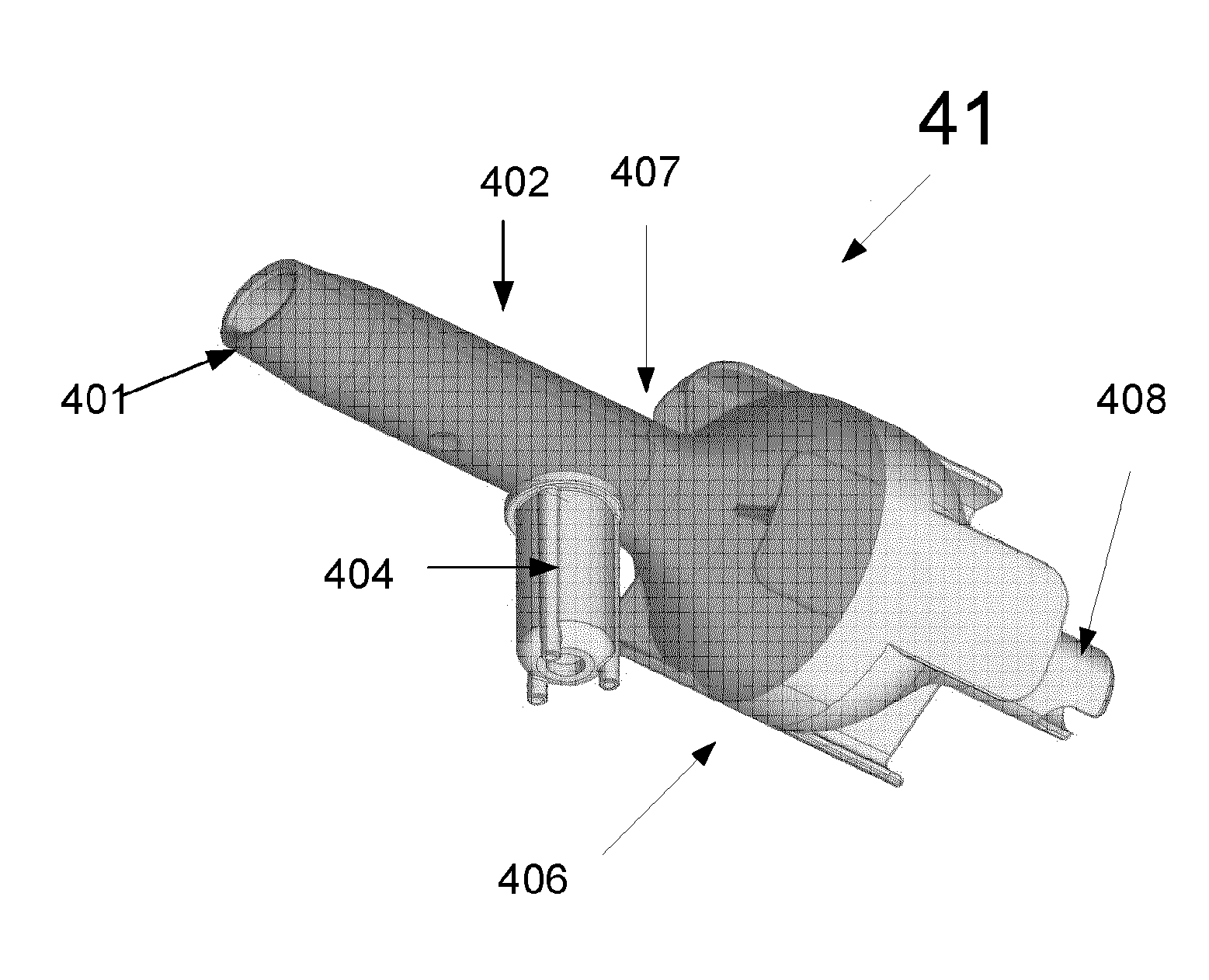
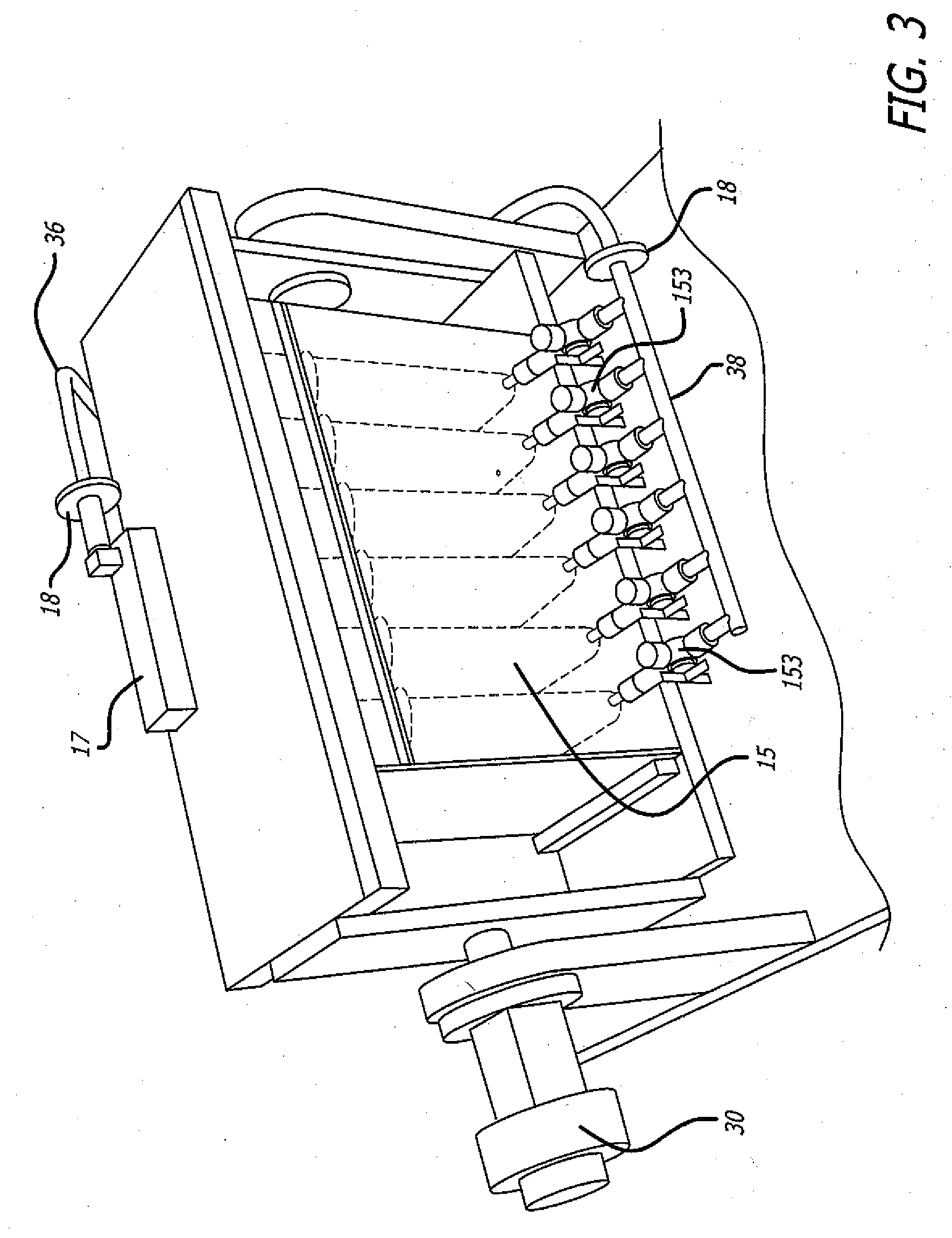
Portable Sampling Device and Method for Sampling Drug Substances From Exhaled Breath
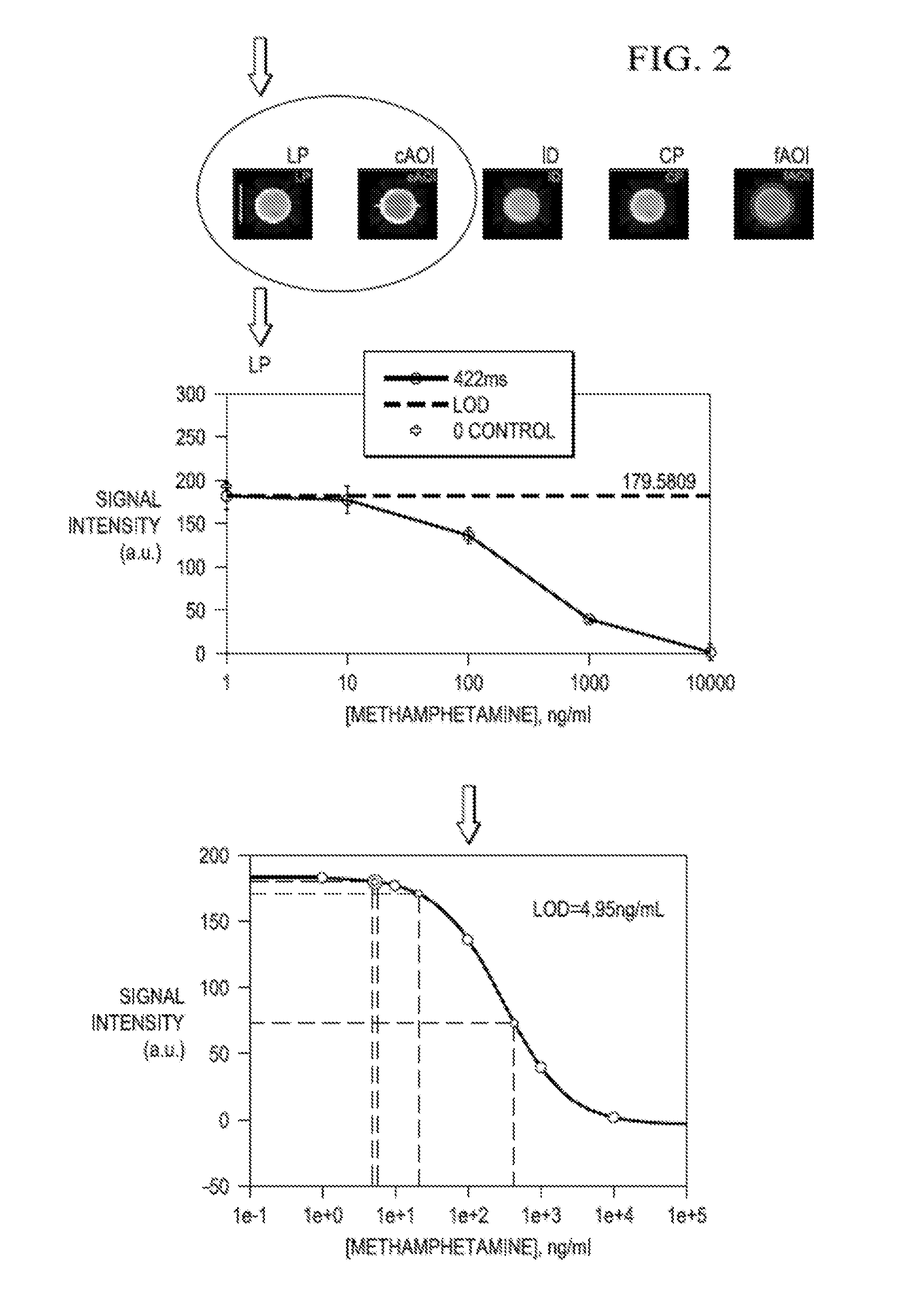
ActiveUS20140366609A1Quality improvementProven robustnessWithdrawing sample devicesLaminationEnvironmental healthDrug test
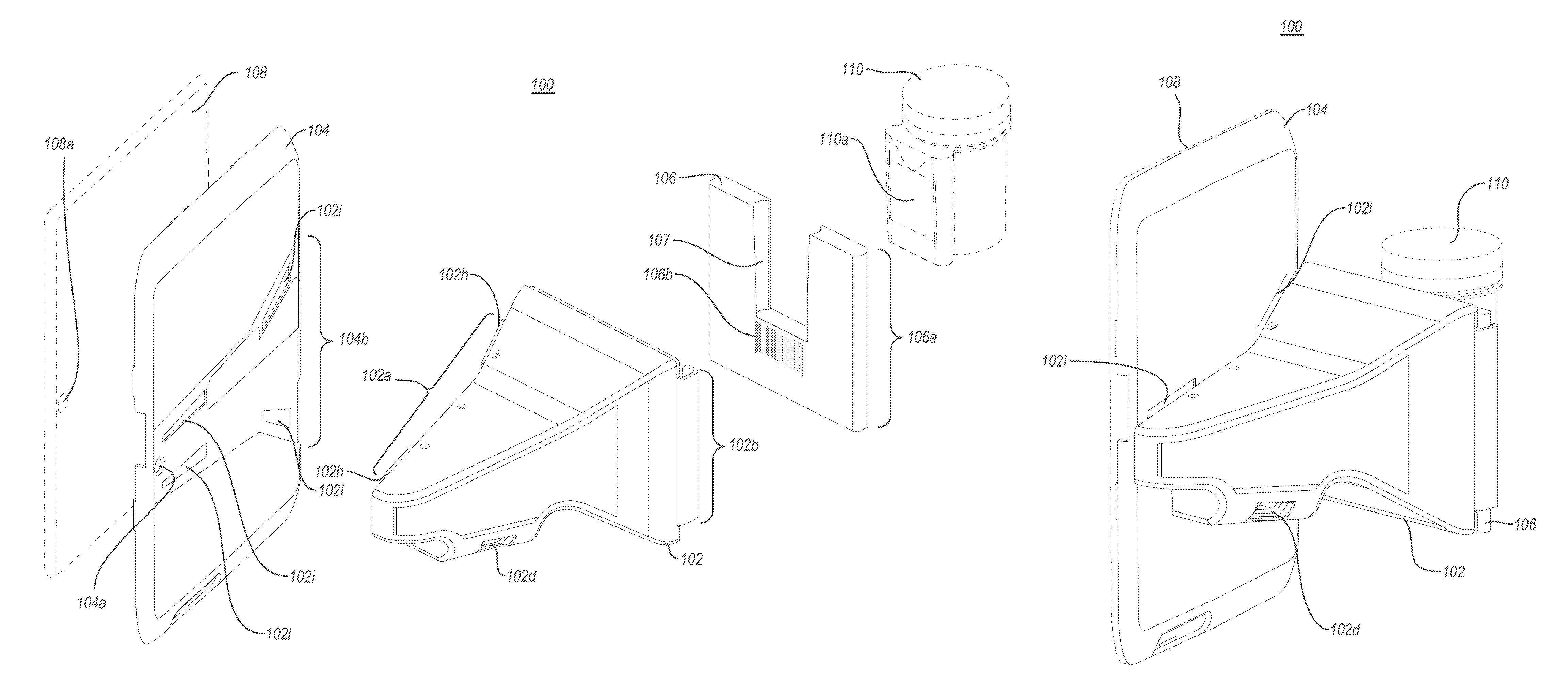
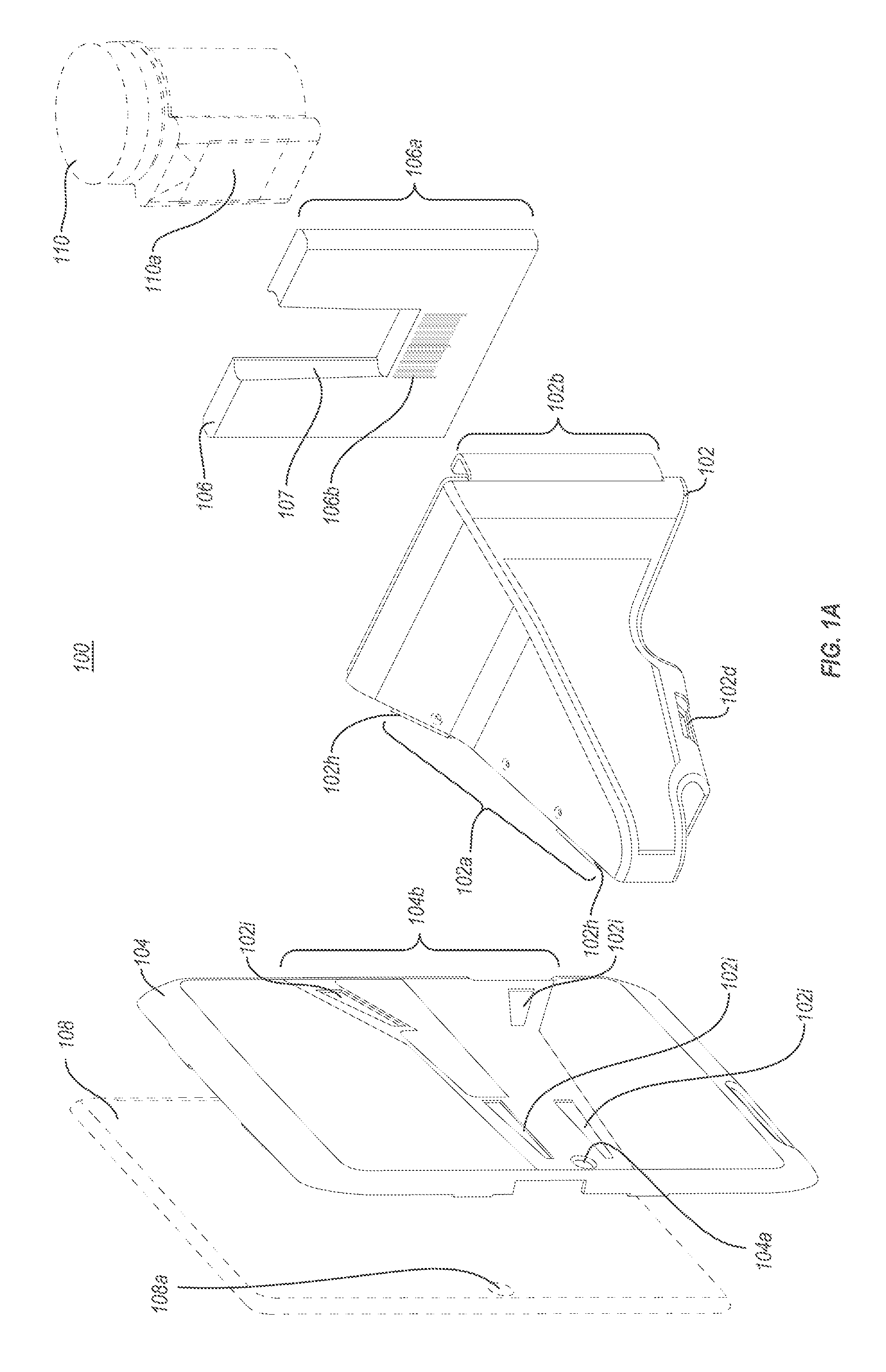
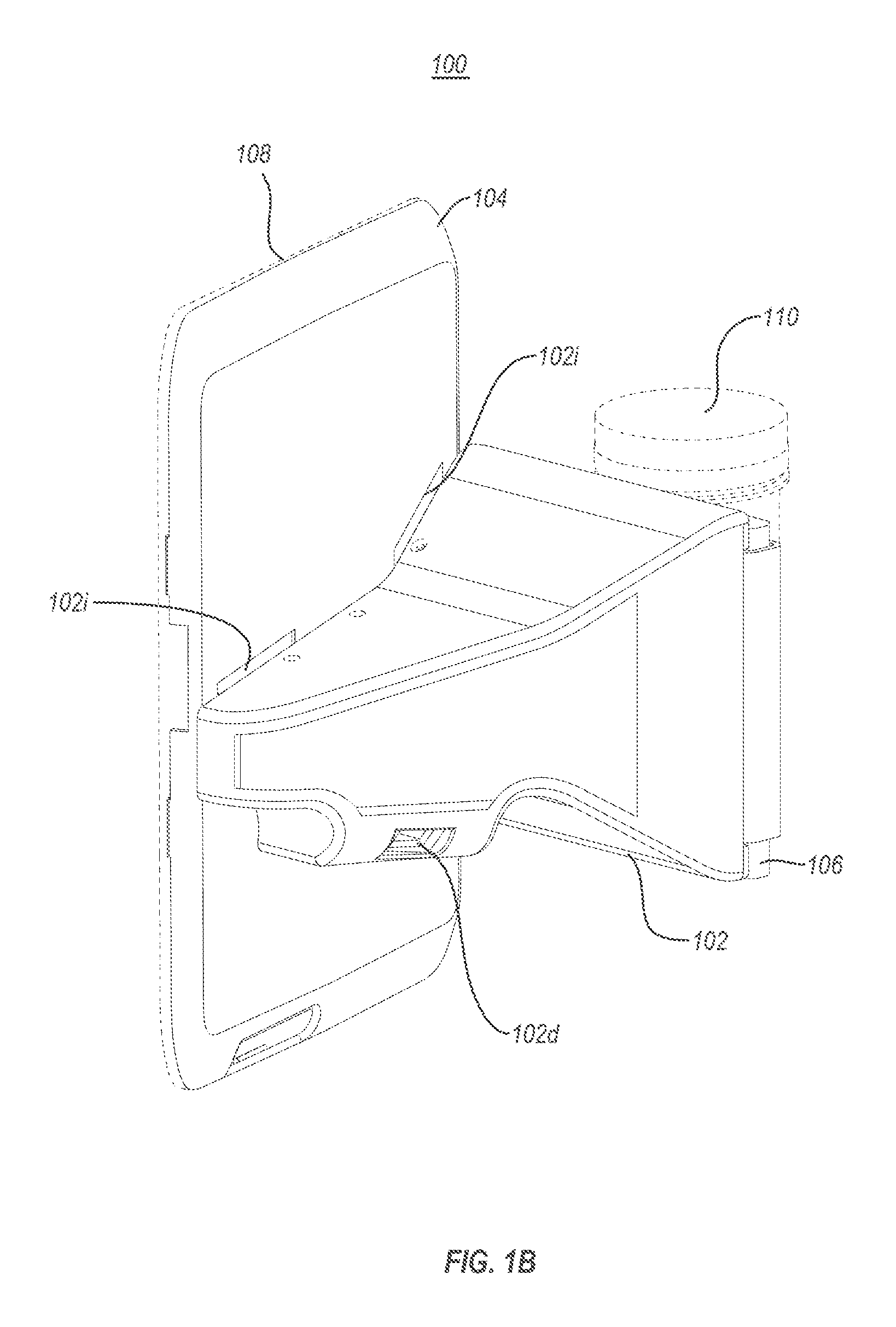
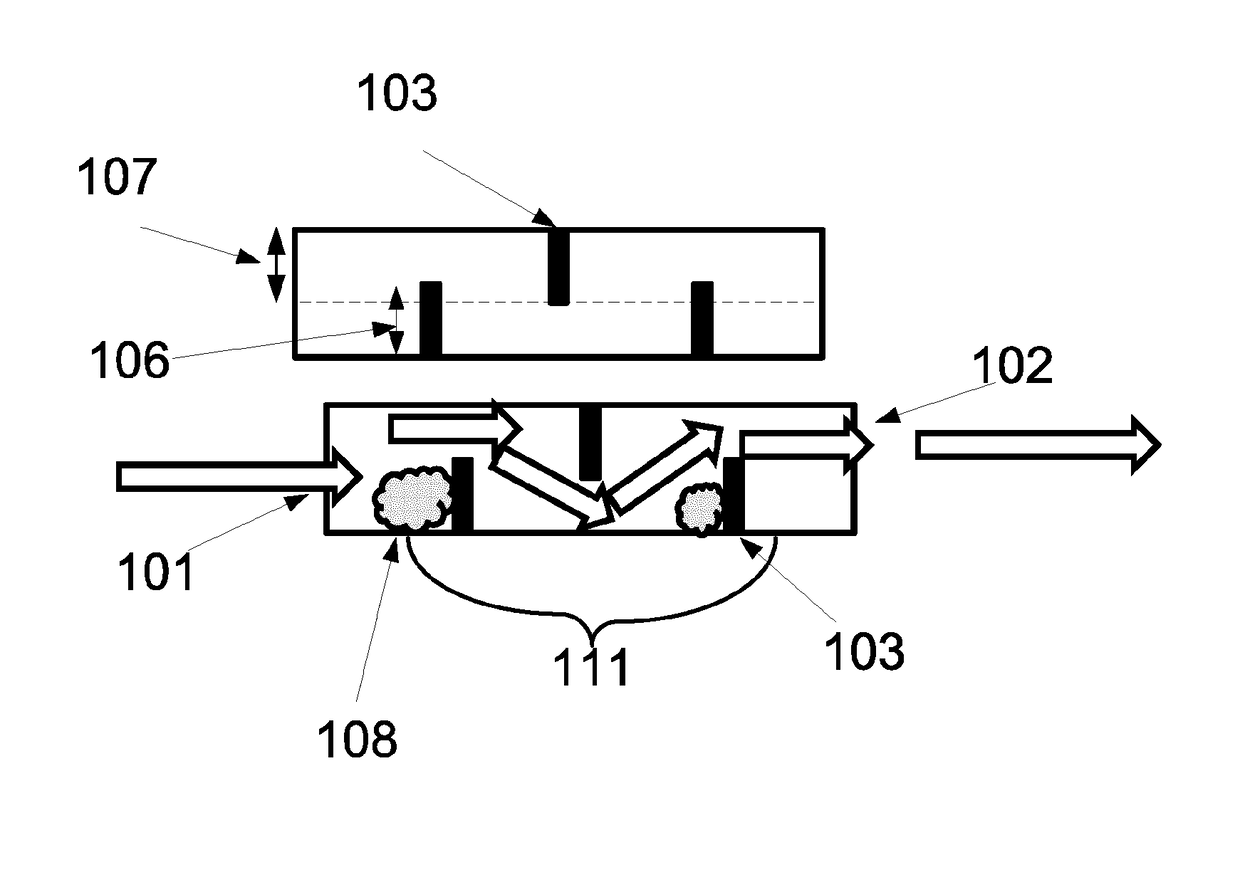
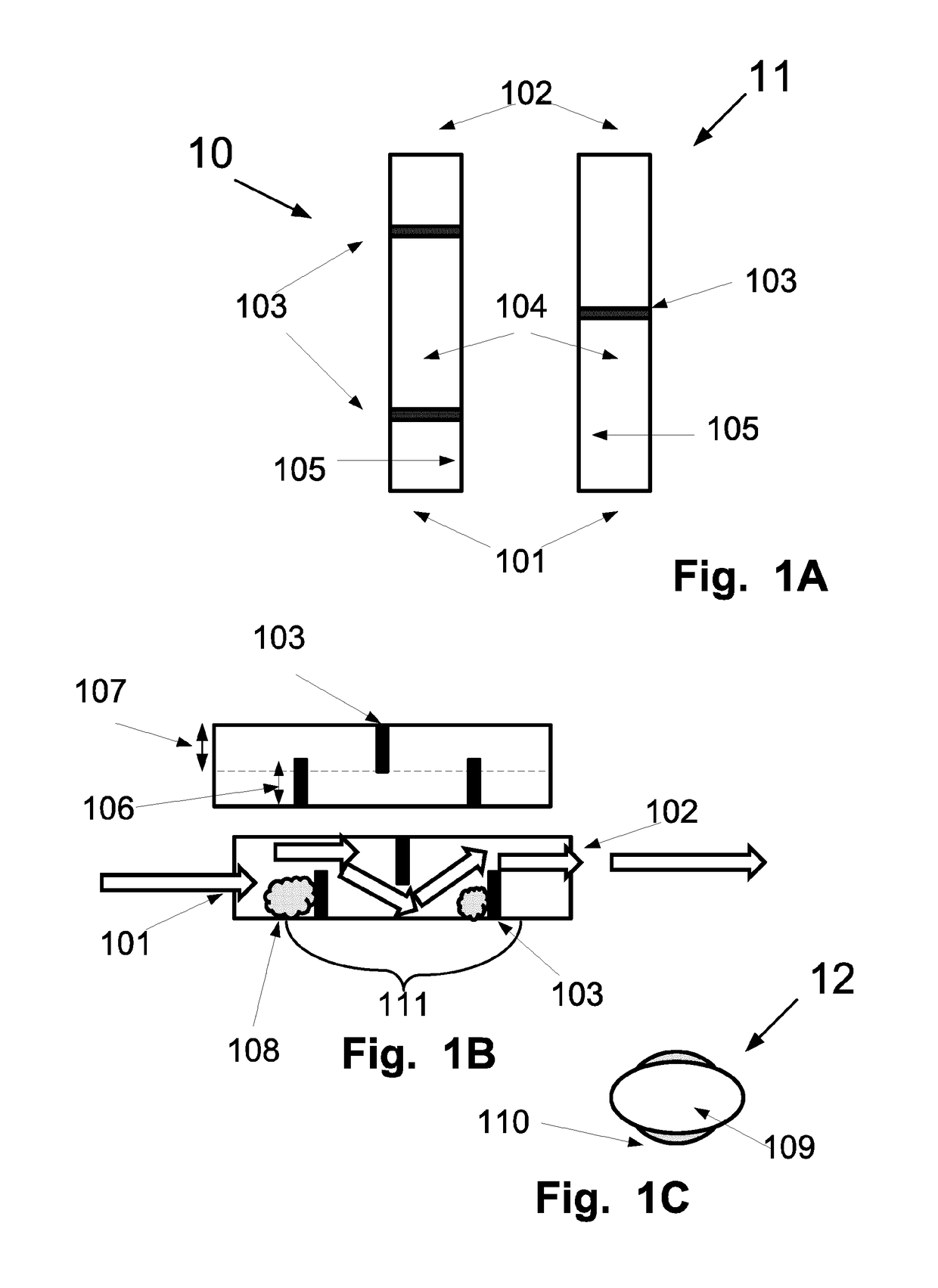
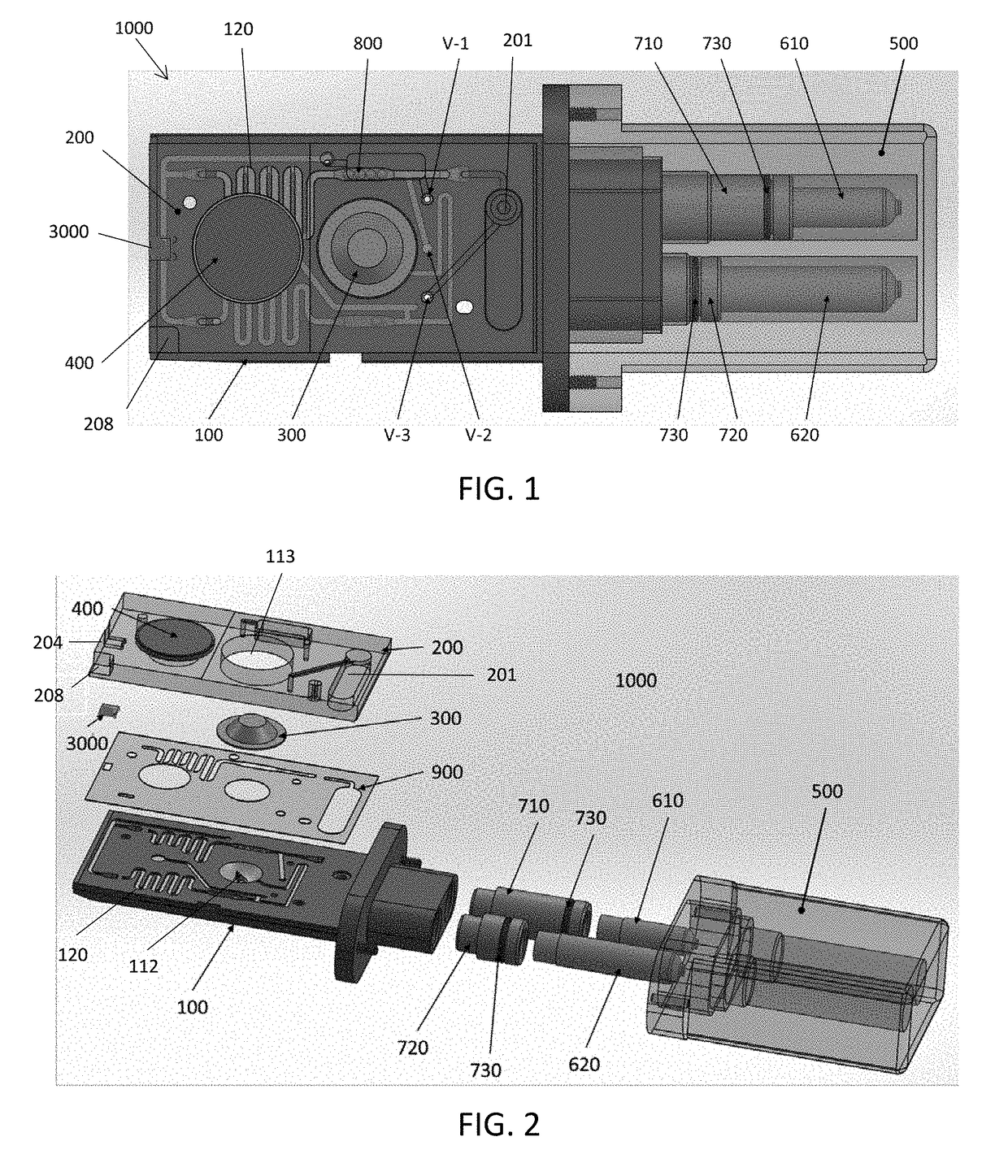
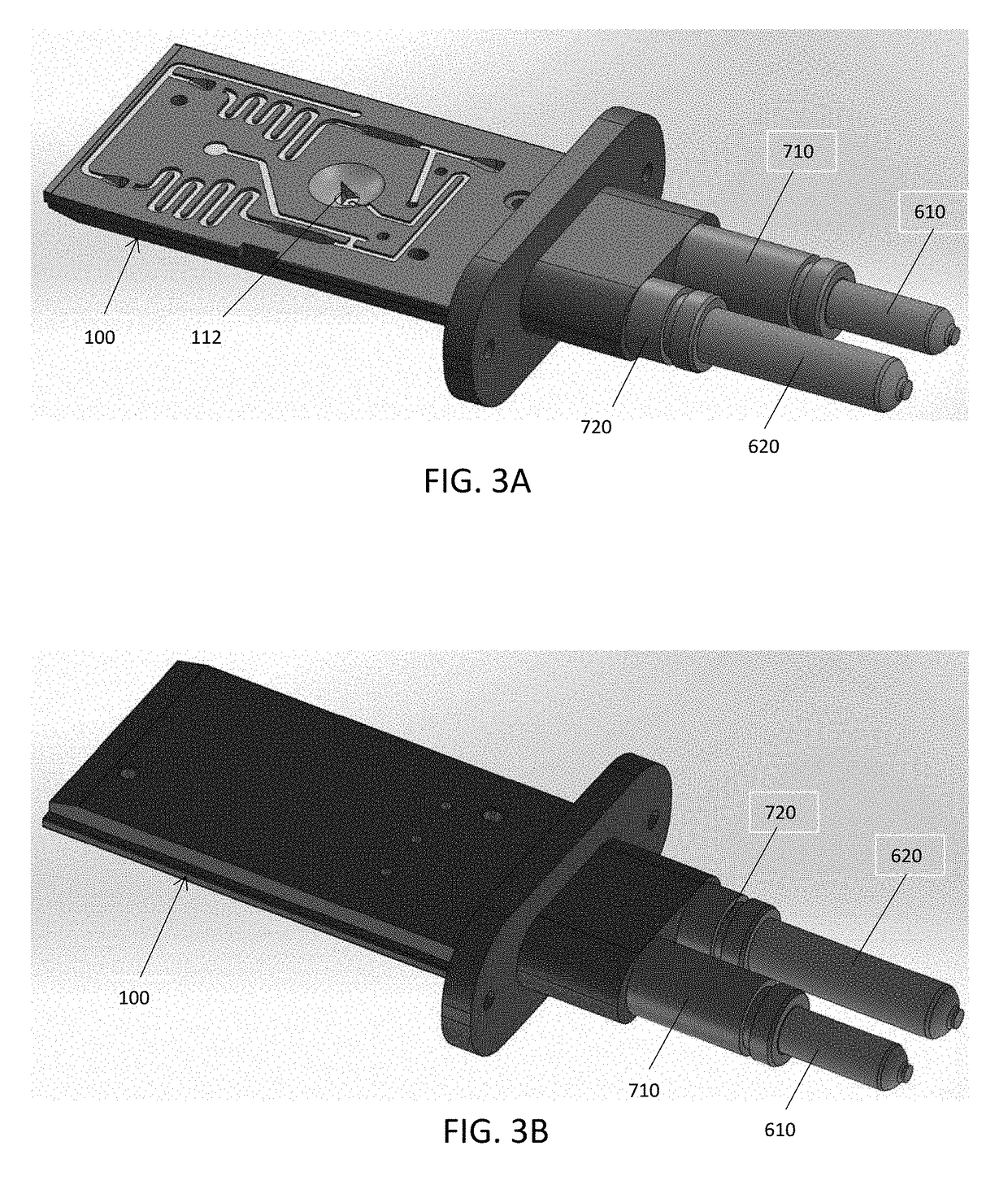
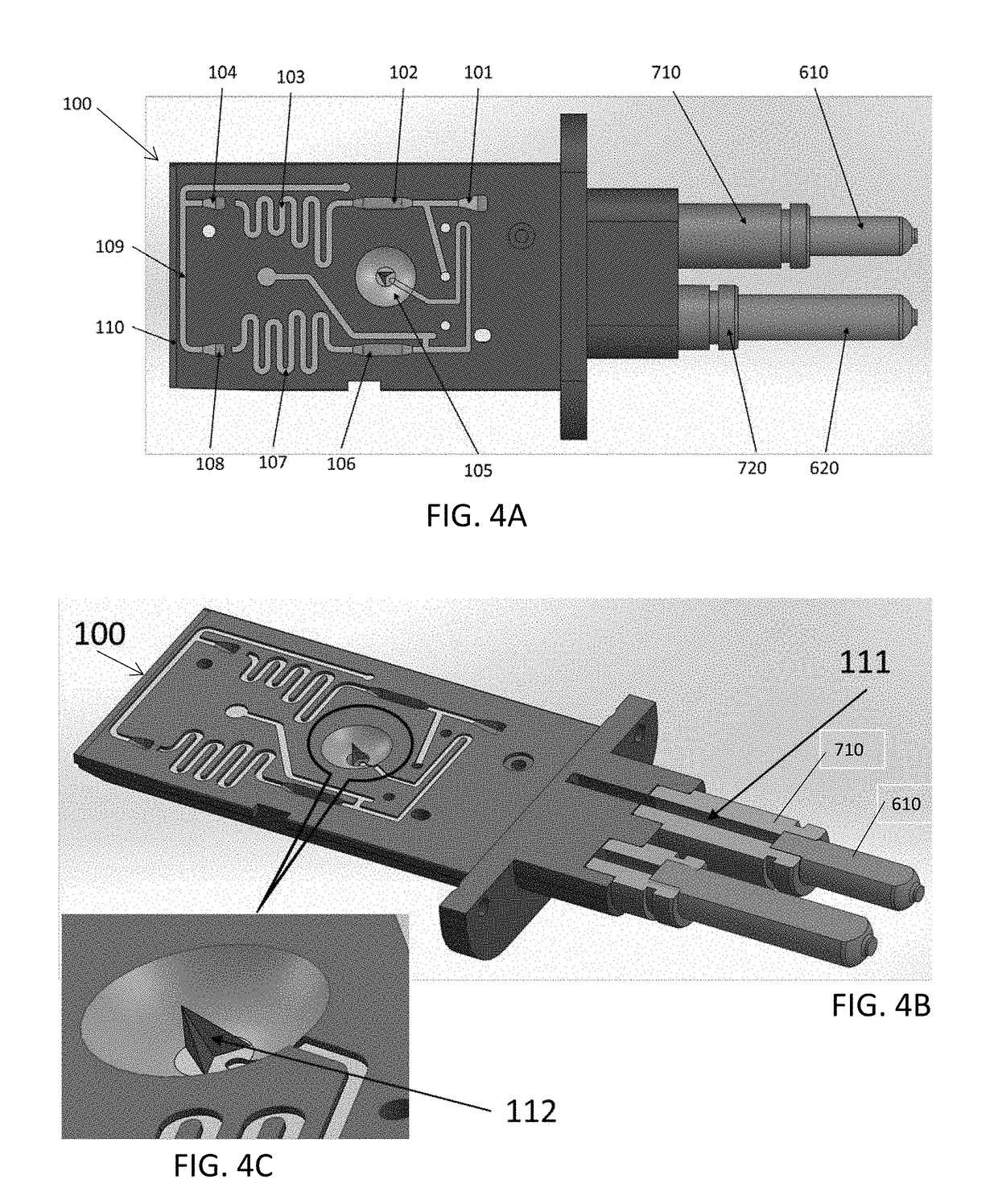
A portable drug sampling device for handheldly collecting a sample from exhaled breath of a subject for further sensor based analysis. The device comprising: a housing (406) comprising at least one inlet (407) and at least one outlet (408) for the exhaled breath to exit through, and a sampling membrane (302) arranged in the housing. A tubular element (40) having a mouthpiece section (401) for the subject to exhale into, and a saliva trap section comprising baffles (103) to create a non-straight gas flow path for letting aerosols pass through the tubular element. The sampling membrane (302) is arranged to collect the aerosols from the exhaled breath. The portable drug testing device further comprises a volume collecting element (208).
Owner:SENSA BUSB
Apparatus and method for passive testing of alcohol and drug abuse
InactiveUS20100204600A1Digital data processing detailsPerson identificationCompound organicAlcohol and drug
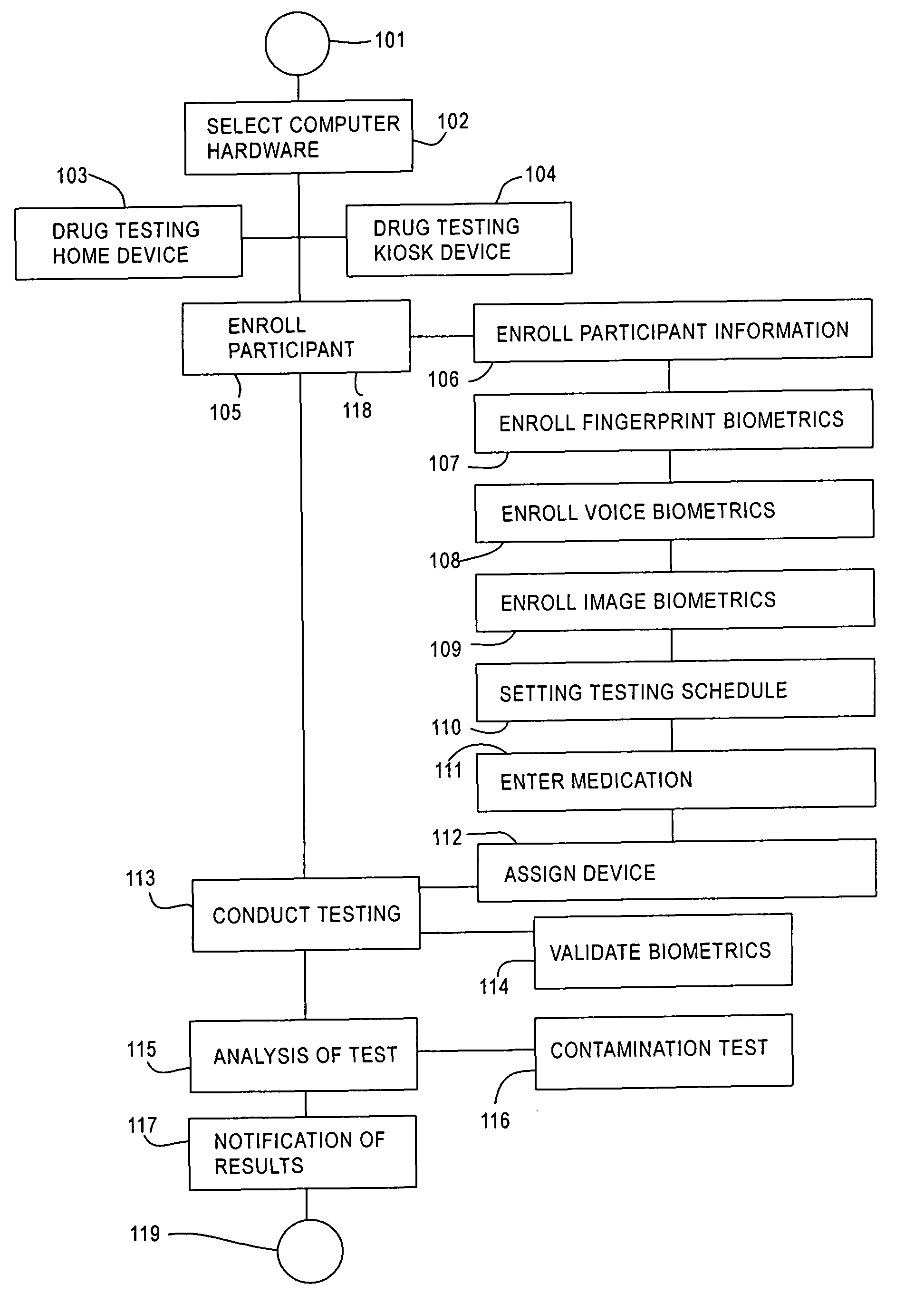
An automated system and method for passive testing of alcohol and drug abuse. The system enters a participant or subject into the system who is to be monitored during a probationary or other program for alcohol or drug abuse offenders. The system provides a drug testing home device or a drug testing kiosk device for use by the participant. The system enrolls the biometrics information of the participant into the computer system (e.g., finger print, voice, image, volatile compound organic gas level, and pH level). When the participant is to be tested in accordance with a testing schedule, the system validates these same biometrics of the participant, conducts the test, and then analyzes the test information for determining if the participant has been using alcohol or other drugs and should be subjected to a confirming urinalysis exam.
Owner:JUSTICE EZ TRAC
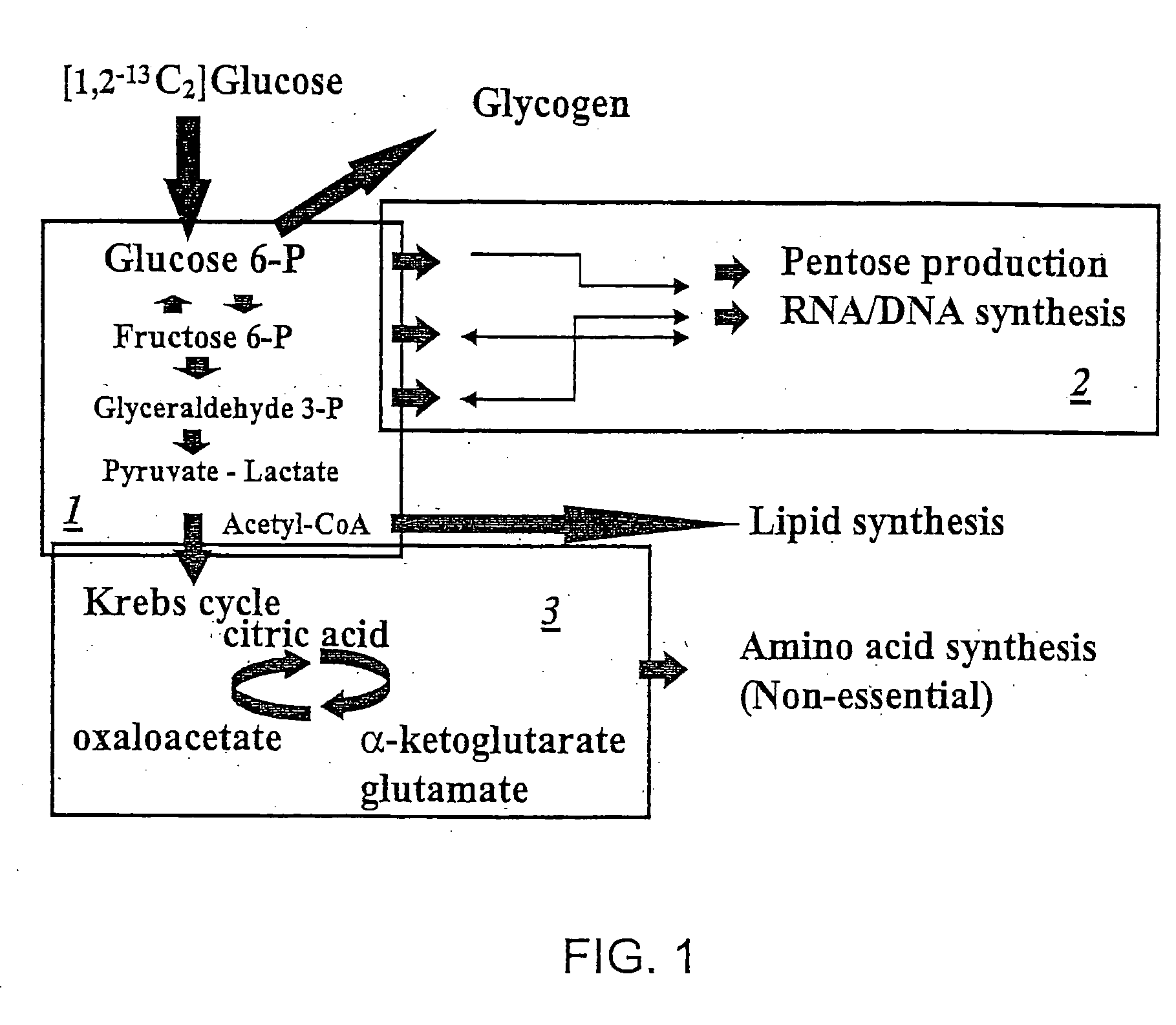
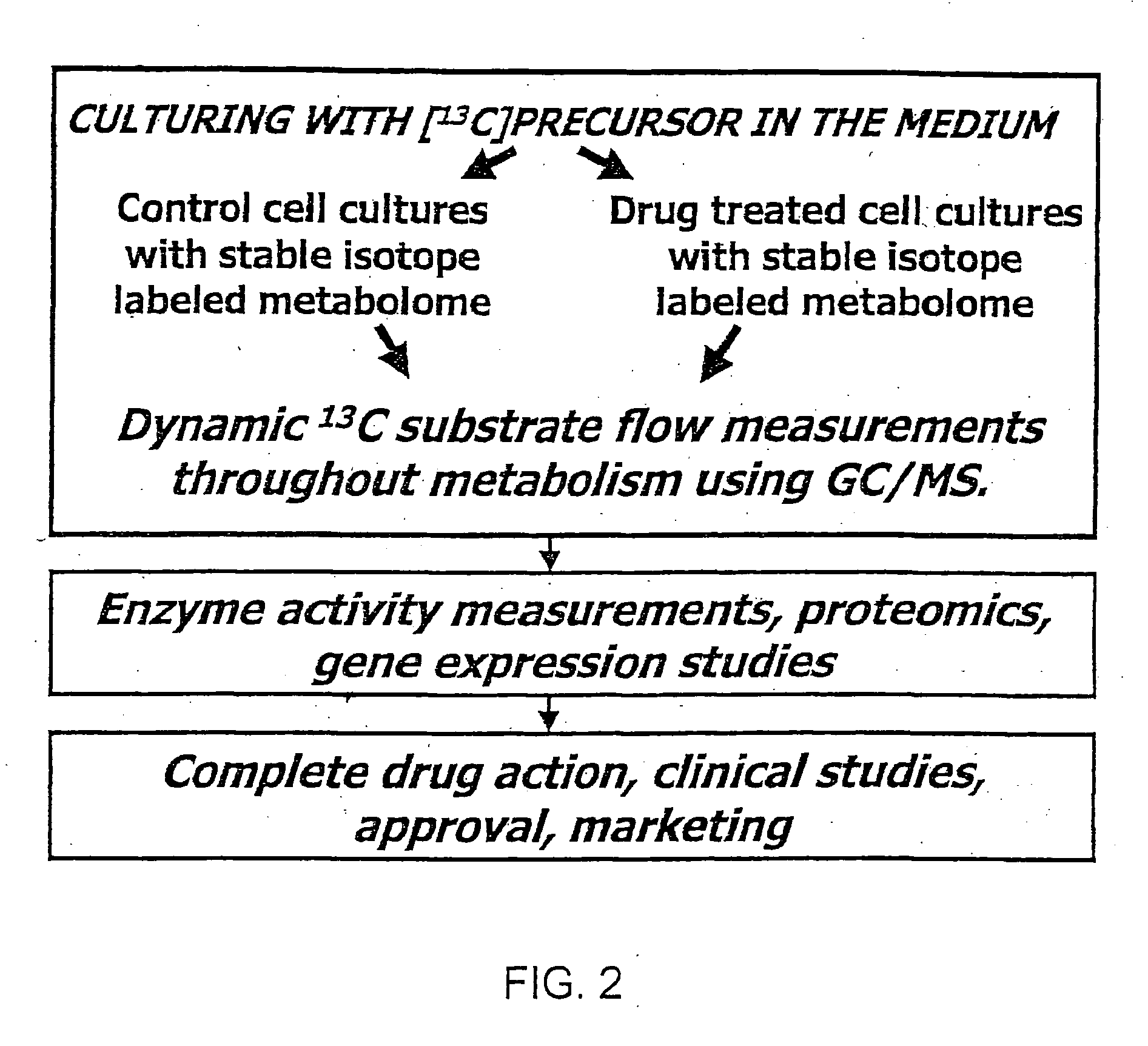
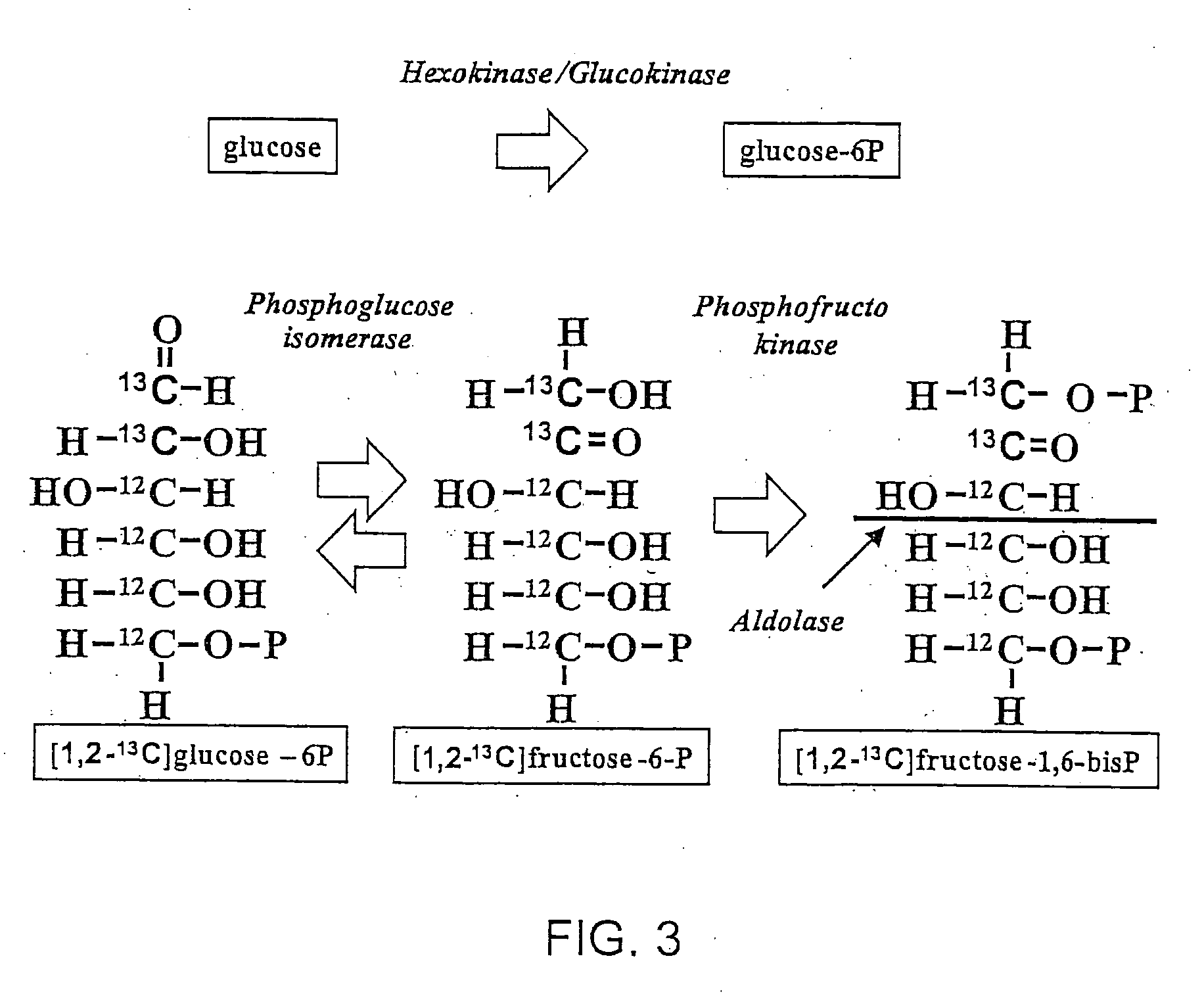
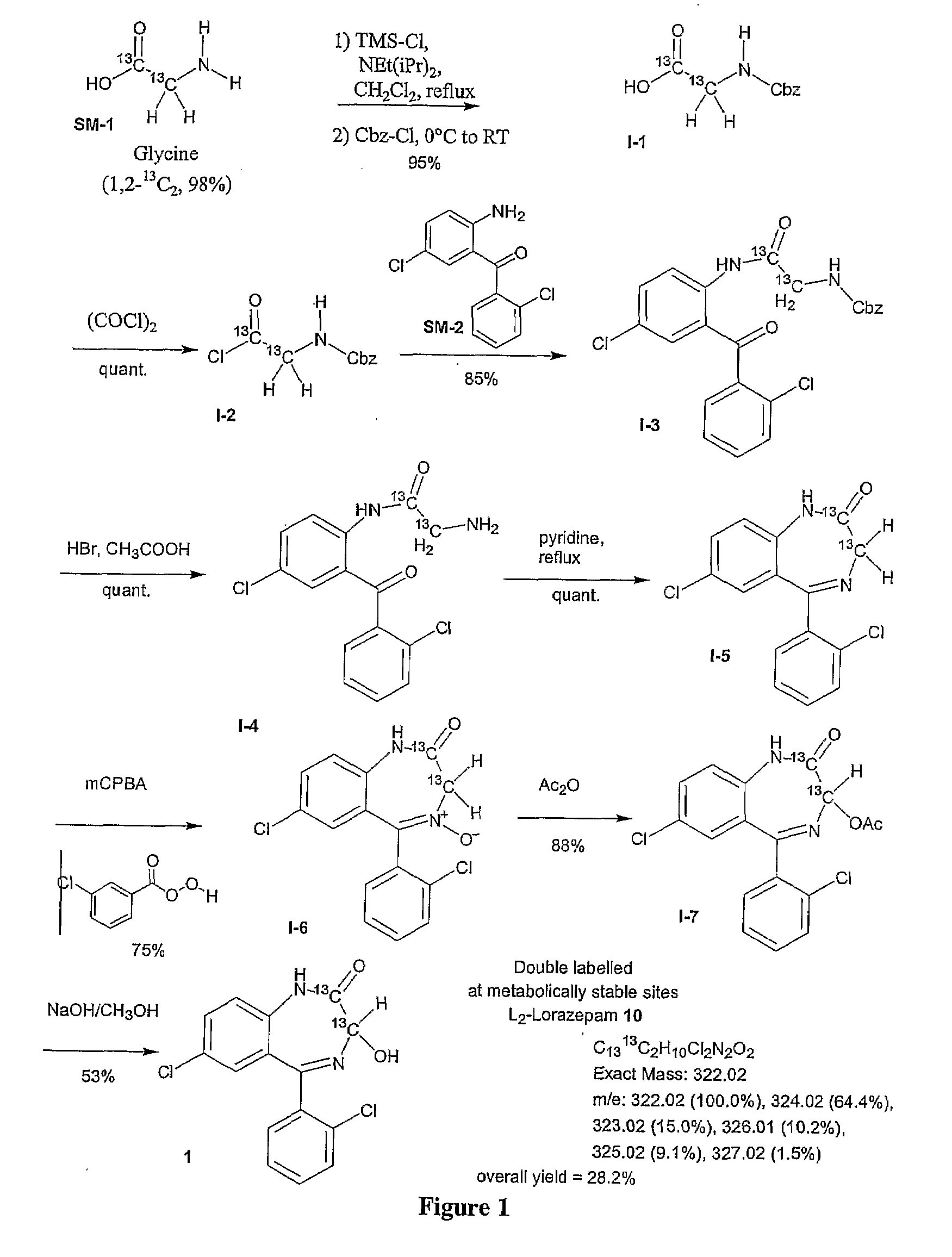
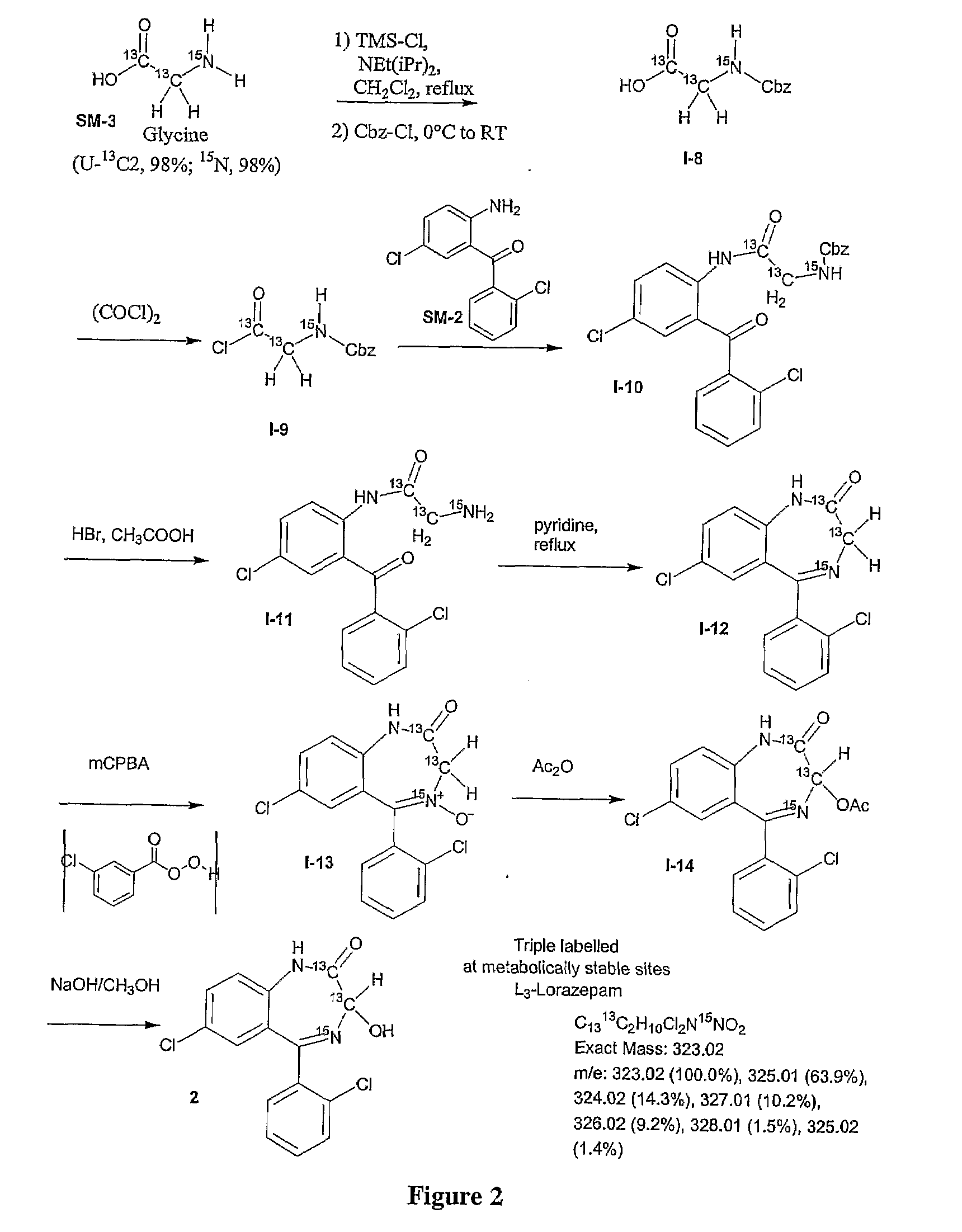
Stable isotope based dynamic metabolic profiling of living organisms for characterization of metabolic diseases, drug testing and drug development
The metabolic processes involved in the formation of any glucose-based metabolite of a metabolic network are determined. A precursor molecule is labeled with a stable carbon (13C) isotope at specific positions. The label is allowed to distribute and rearrange in the system. Metabolites are recovered and analyzed against a control system to determine a set of metabolic pathway substrate fluxes caused by changes to the test system relative to the control system such as the addition of compound being tested as a potential drug.
Owner:LOS ANGELES BIOMEDICAL RES INST AT HARBOR UCLA MEDICAL CENT
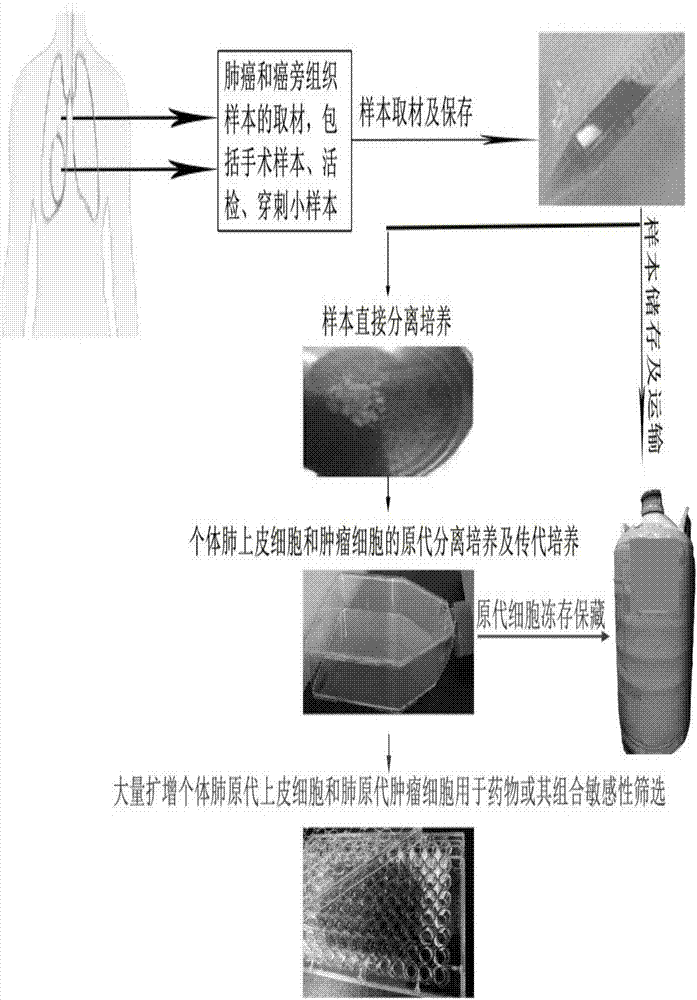
In-vitro individualized medicine test method for lung cancer and culture medium
InactiveCN107151645AProlong survival timeLow toxicityCell dissociation methodsCompound screeningProcess systemsIndividualized treatment
The invention discloses an in-vitro individualized medicine test method for lung cancer and a culture medium, and belongs to the field of cell biology and pharmacology. The in-vitro individualized medicine test method and the culture medium have the advantages that the culture medium M can be applied to normal epithelial cells and primary tumor cells of human or mammals, accordingly, in-vitro culture systems for tumor cells and para-carcinoma cells of lung cancer patients can be established, and primary cells, with continuous passage and quick proliferation functions, of patient individuals can be obtained; the culture systems can be used for detecting the sensitivity and the safety of medicines or combination groups of the medicines, and accordingly stable, accurate and reliable lung cancer individualized treatment-medicine sensitivity test standard detection process systems can be established; individualized sensitive chemotherapy medicines or compositions screened by the aid of the lung cancer individualized treatment-medicine sensitivity test standard detection process systems can be combined with clinical medicine or relevant subjects, accordingly, clinical individualized treatment schemes can be formulated, and the in-vitro individualized medicine test method and the culture medium can ultimately serve for clinical application.
Owner:SHENZHEN RES INST OF WUHAN UNIVERISTY
Capturing and processing instant drug test results using a mobile device
ActiveUS20150031412A1Privacy protectionPreserve subject privacyVaccination/ovulation diagnosticsDiagnostic recording/measuringMobile deviceComputer science
Systems, methods, and apparatus allow for capturing the results of drug tests using mobile devices. One or more implementations include apparatus that interface a mobile computing device with a drug test, while ensuring that an imaging device of the mobile computing device is optically aligned with a test display areas of the drug test. One or more additional implementations include a method for analyzing drug testing results. The method includes receiving and analyzing drug testing data, which includes an identification of a type drug testing apparatus being used at a remote testing device, and an image that visually represents a portion of a drug test. The method also includes analyzing the test results of the drug test based on the identification of the type drug testing apparatus.
Owner:FORMFOX INC
Registry Method and Control System for Dea Schedule II-V Medicines
InactiveUS20090208413A1Contained costFacilitating replacement drug prescriptionIn-vivo radioactive preparationsOrganic compound preparationPatient complianceDrug policies
The present invention provides compositions and methods for synthesizing labeled drugs. The present invention further provides methods for preventing or stopping prescription drug abuse for all agents registered as a Drug Enforcement Agency (DEA) schedule II through schedule V medications. According to the present invention, methods are provided for monitoring patient compliance with prescribed drug treatment. The present invention also provides methods for facilitating a replacement prescription when a patient is left without access to their prescribed drug. Furthermore, the present invention provides a method to improve employee compliance with an employer's drug policies via either a voluntary or compulsory system for enhanced drug testing.
Owner:DR PHARMA NOVA
Bio-nano-chips for on-site drug screening
ActiveUS20140094391A1Reduce signalingMinimizing size and complexityPeptide librariesSurgical furniturePharmaceutical drugNon invasive
A bio-nano-chip (BNC) technology that works in connection with non-invasive samples, such as saliva, cheek swab or urine samples that can be easily performed by non-specialists, such as security personnel and police officers is disclosed. The microfluidic system for drug testing includes an analyzer or reader having a housing containing a slot for receiving a cartridge, a drug testing cartridge, a processor having a user interface, an optical or energy sensing means, and a means for moving fluid.
Owner:RICE UNIV
Method for detecting drugs, and a solvent and a decomposition solution used therefor
InactiveUS20010051379A1Analysis using chemical indicatorsPreparing sample for investigationDrug testing methodsOrganic solvent
The invention relates to a method for the quick and direct on-site detection of drugs and drug substitutes which are in solid form. According to the invention, the drugs or drugs substitutes are transferred to a decomposition vessel or reaction vessel without being pretreated, especially without being reduced in size. Afterwards, they are mixed with a solvent or a solvent mixture containing at least one organic solvent. In addition, the suspension and / or solvent containing the drugs or drugs substitutes is / are directly subjected to a known drug test method, especially using drug test strips. The invention also provides a solvent or solvent mixture and a decomposition solution therefor, and a device for carrying out the method.
Owner:UNLTD PHARMA DEUTLAND
Drug detection kit
InactiveCN107167593ASimple preparation processLow costBiological testingAntibody antigen reactionsControl line
The invention provides a drug detection kit and its preparation process, which combines highly specific antibody antigen reaction with immune colloidal gold chromatography technology, improves on the basis of this prior art, and detects antibody in colloidal gold labeling At the same time, mark the control line antibody, and correspondingly point the second antibody on the membrane at the quality control area (C line) for color reaction with the colloidal gold-labeled control line antibody, so as to ensure that the C line will always develop color, and the color will develop The degree will not change, so it can be judged whether one or more drugs are contained by observing whether the detection area (T line) is colored, and one or more drugs can be semi-quantitatively compared by observing the degree of color development of the T line . The drug detection kit has the characteristics of qualitative and semi-quantitative comparison of drugs, and also has the characteristics of rapidity, sensitivity, easy operation, low cost, and no need for professional detection.
Owner:BOZHOU CITY THE NEW HEALTH TECH CO LTD
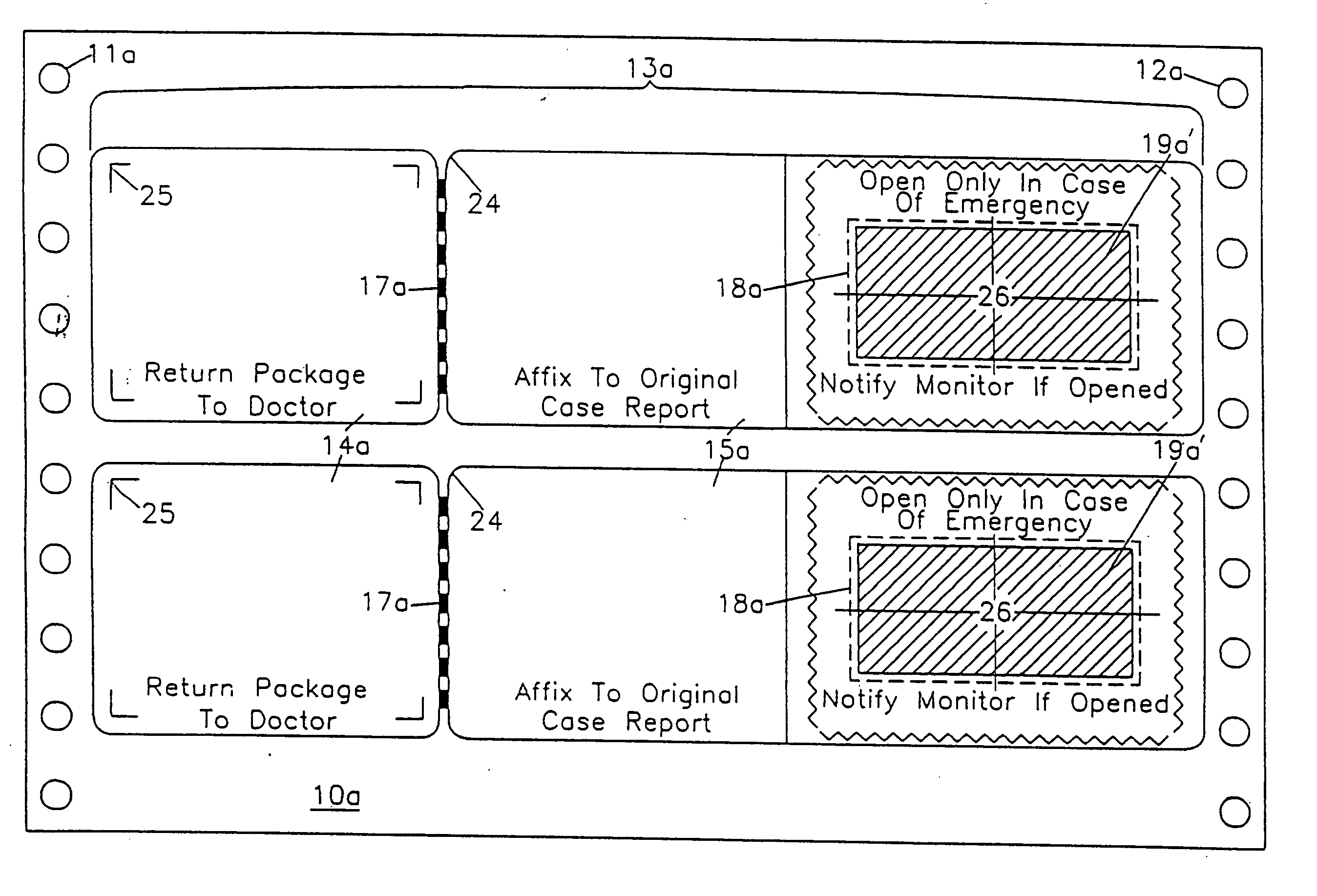
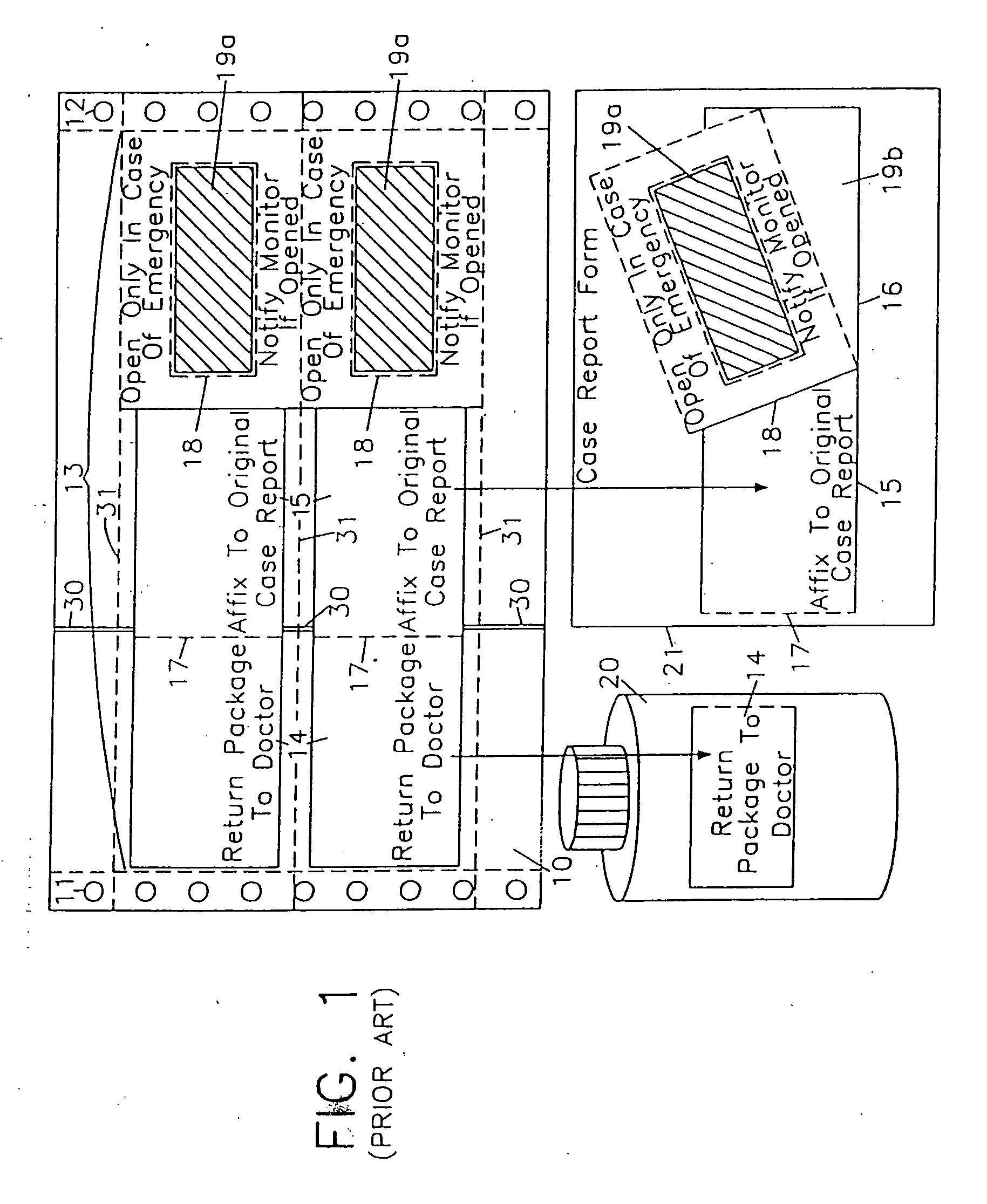
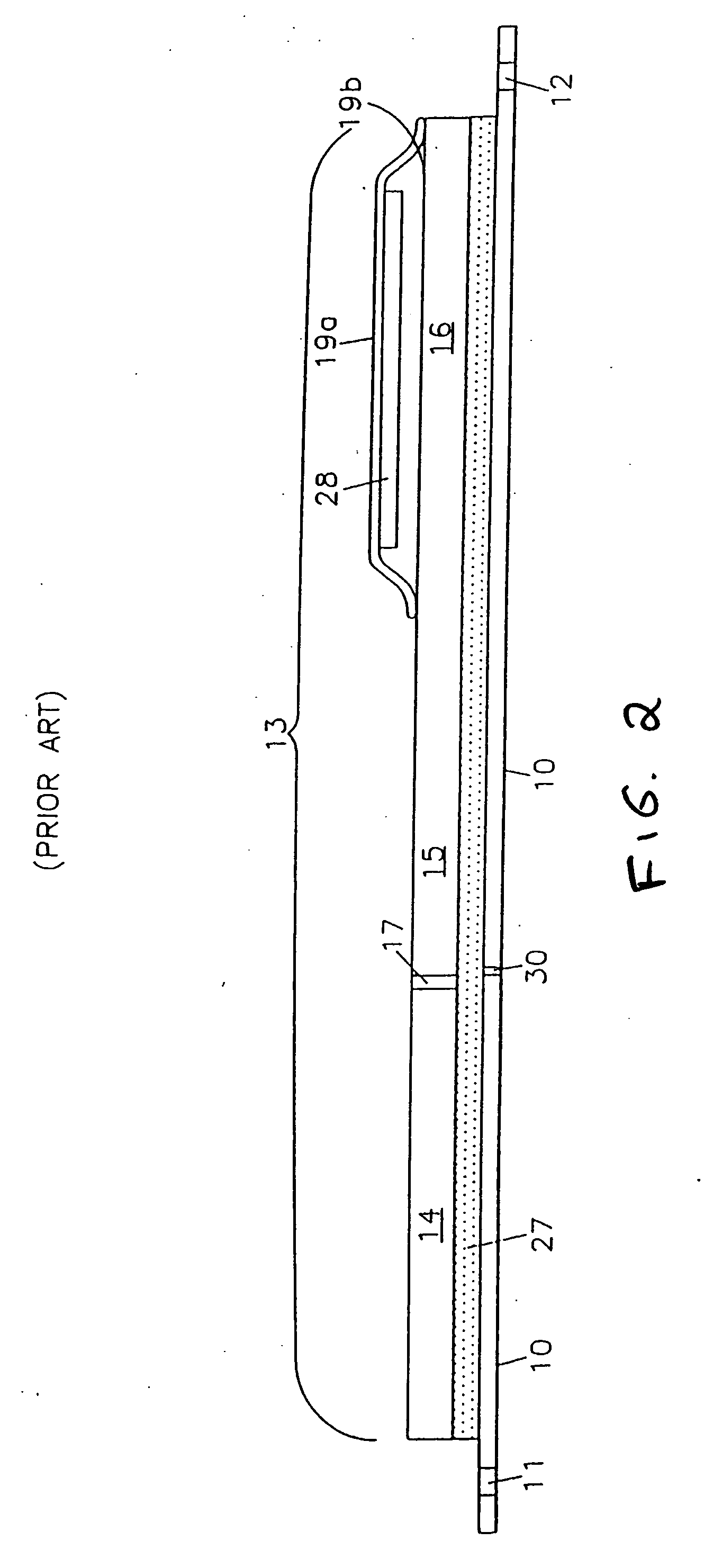
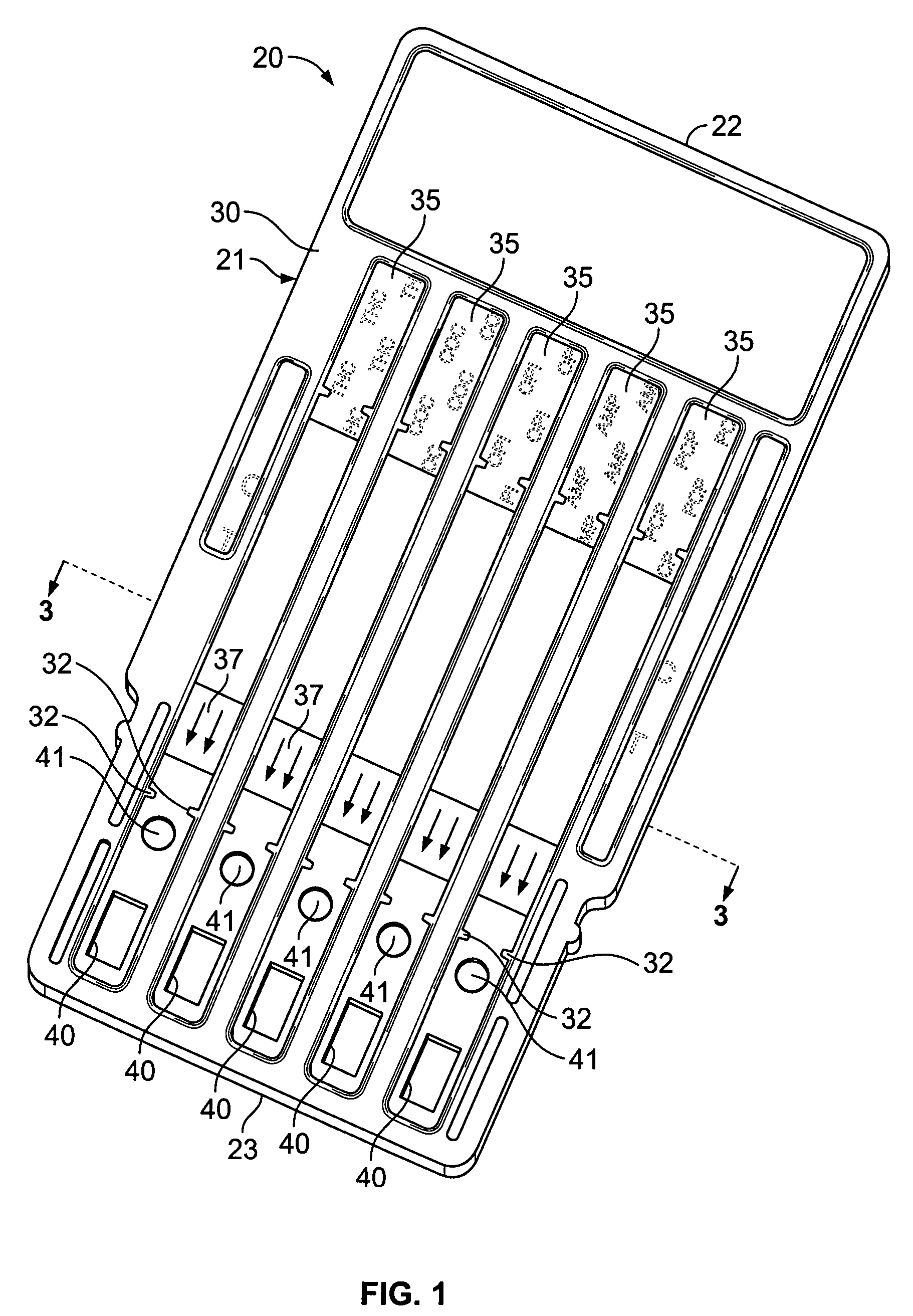
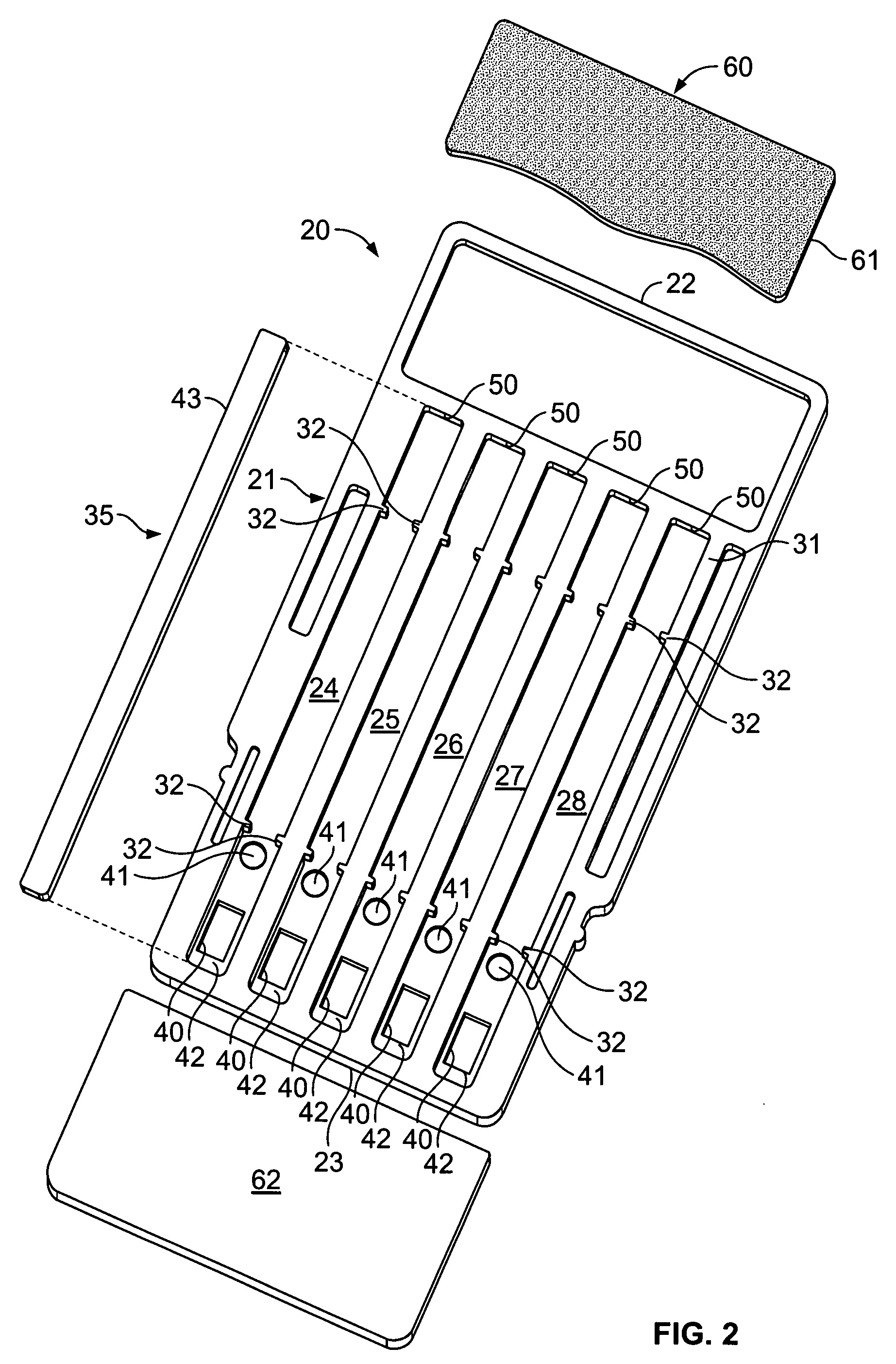
Label form for use in drug testing and method for applying the same
InactiveUS20060029766A1Easy to disassembleEasy to disengageStampsFlexible coversAdhesivePharmaceutical drug
A label form especially for use in testing of drugs. One embodiment has a first label segment with a permanent adhesive backing layer and second label segment with a permanent adhesive backing layer. The first and second label segments may contain information to identify the patient participating in the test, the drug being tested, and other study information and are mounted on mounting sheet having a first mounting sheet segment and a second mounting sheet segment. Each of the mounting sheet segments has a first major surface and a second major surface with the second major surface of the first and the second mounting sheet segments provided with a permanent affixation adhesive layer. The first major surface of the second mounting sheet segment is provided with a nonstick surface for detachably receiving the second major surface of the second label segment. The first major surface of the first mounting sheet segment is attached to the permanent affixation adhesive on the first label segment. The second major surfaces of the first and the second mounting sheet segments are detachably attached to the nonstick surface of the carrying sheet from which the label forms are removed and applied to corresponding containers.
Owner:BOLNICK MARTIN M +1
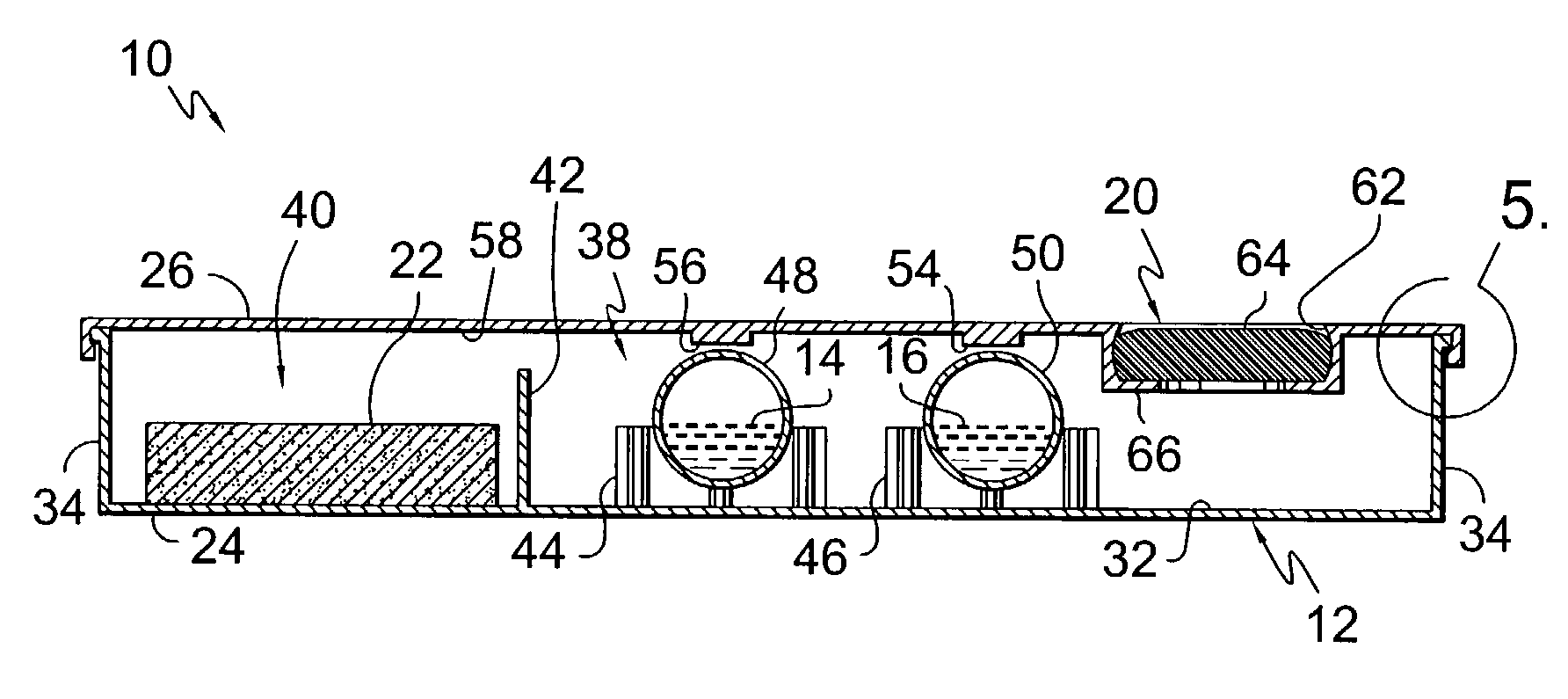
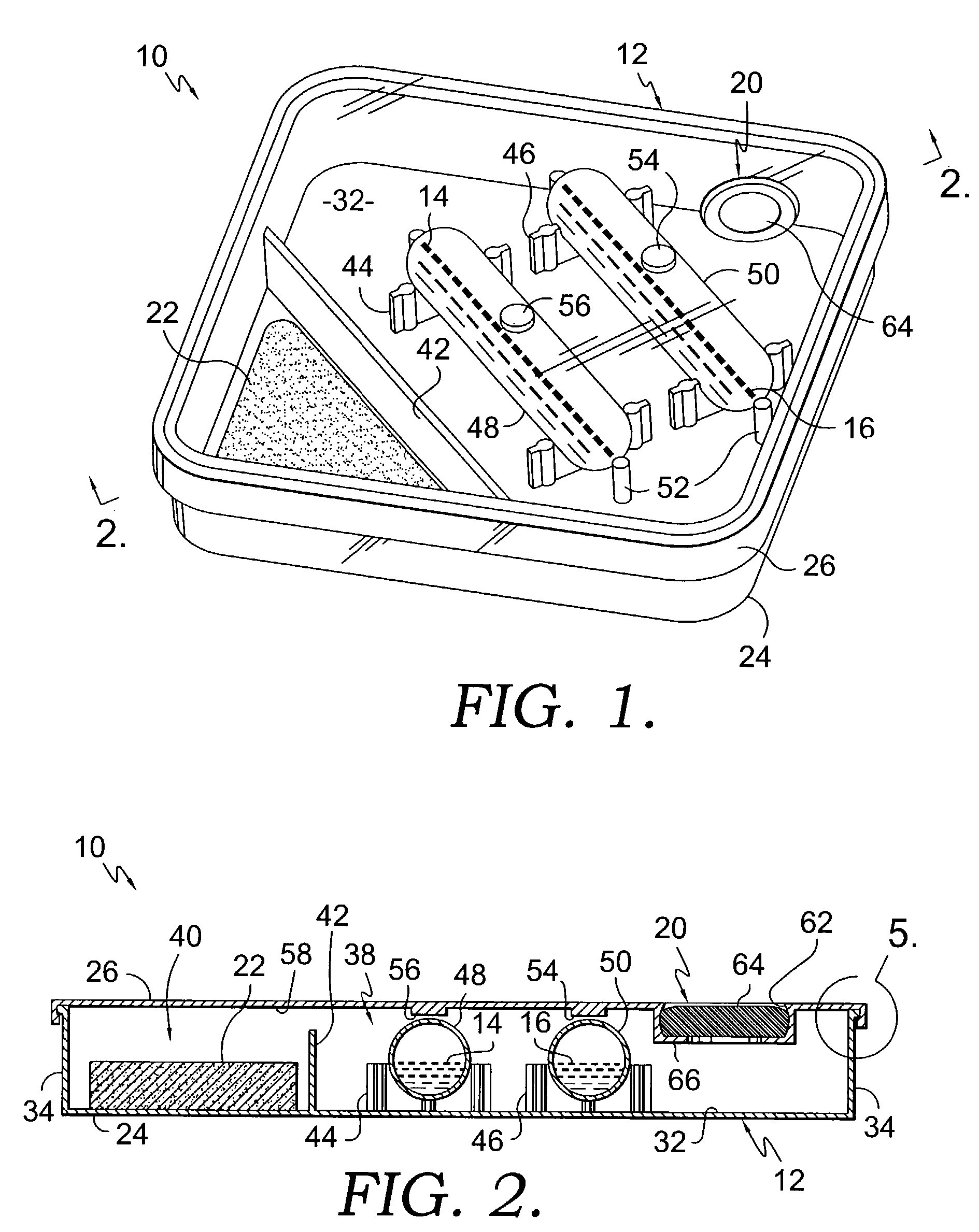
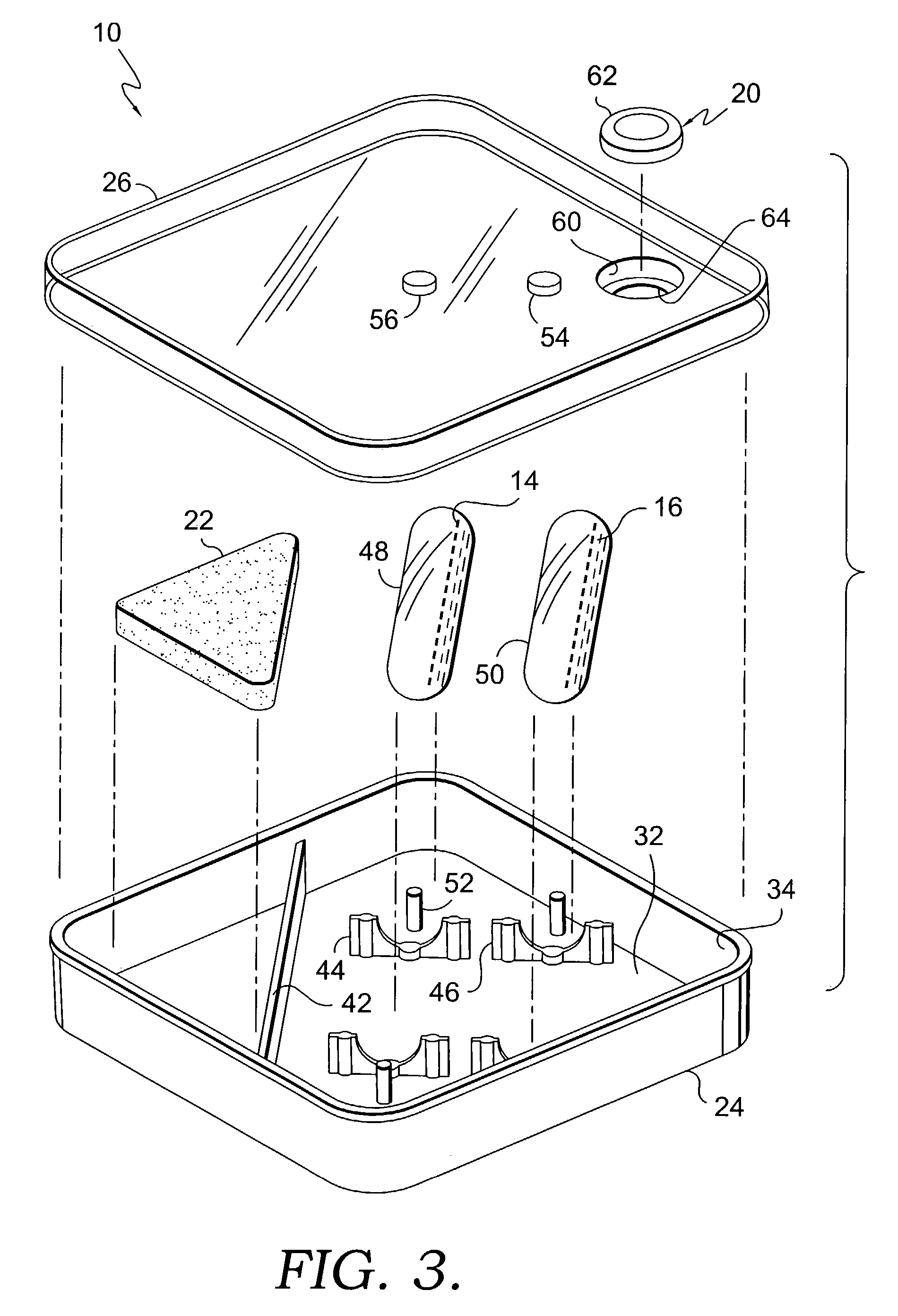
Apparatus for drug testing
InactiveUS7384599B2Analysis using chemical indicatorsLaboratory glasswaresControl substancesControlled substance
An apparatus for testing for the presence of a drug is provided. The apparatus includes a first drug testing reagent, a first ampule, a vessel that contains the first ampule, and a fracturing means. The first drug testing reagent contains a controlled substance detecting chemical reagent. The vessel serves as a mixing chamber for mixing the reagents with the test subject fluid. Further, the vessel may include a transparent wall through which changes in coloration of mixtures of test subject fluids and reagents may be viewed. The vessel may also comprise a test subject fluid injection port to allow for the injection of a test subject fluid into the vessel. The vessel may also contain means for fracturing the ampule without damaging or opening the vessel. A method for conducting a drug test is also provided.
Owner:WITNESS MEDCO
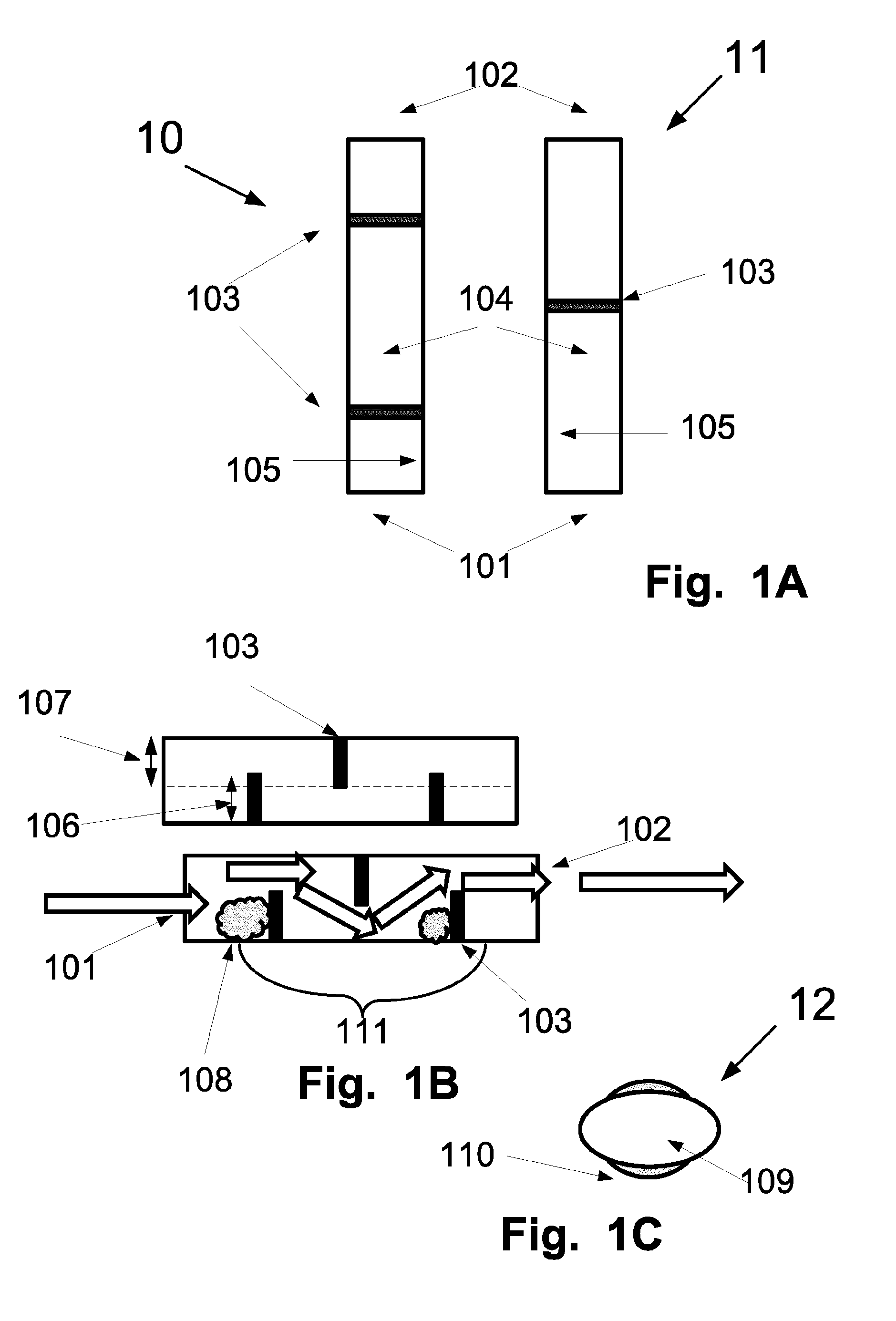
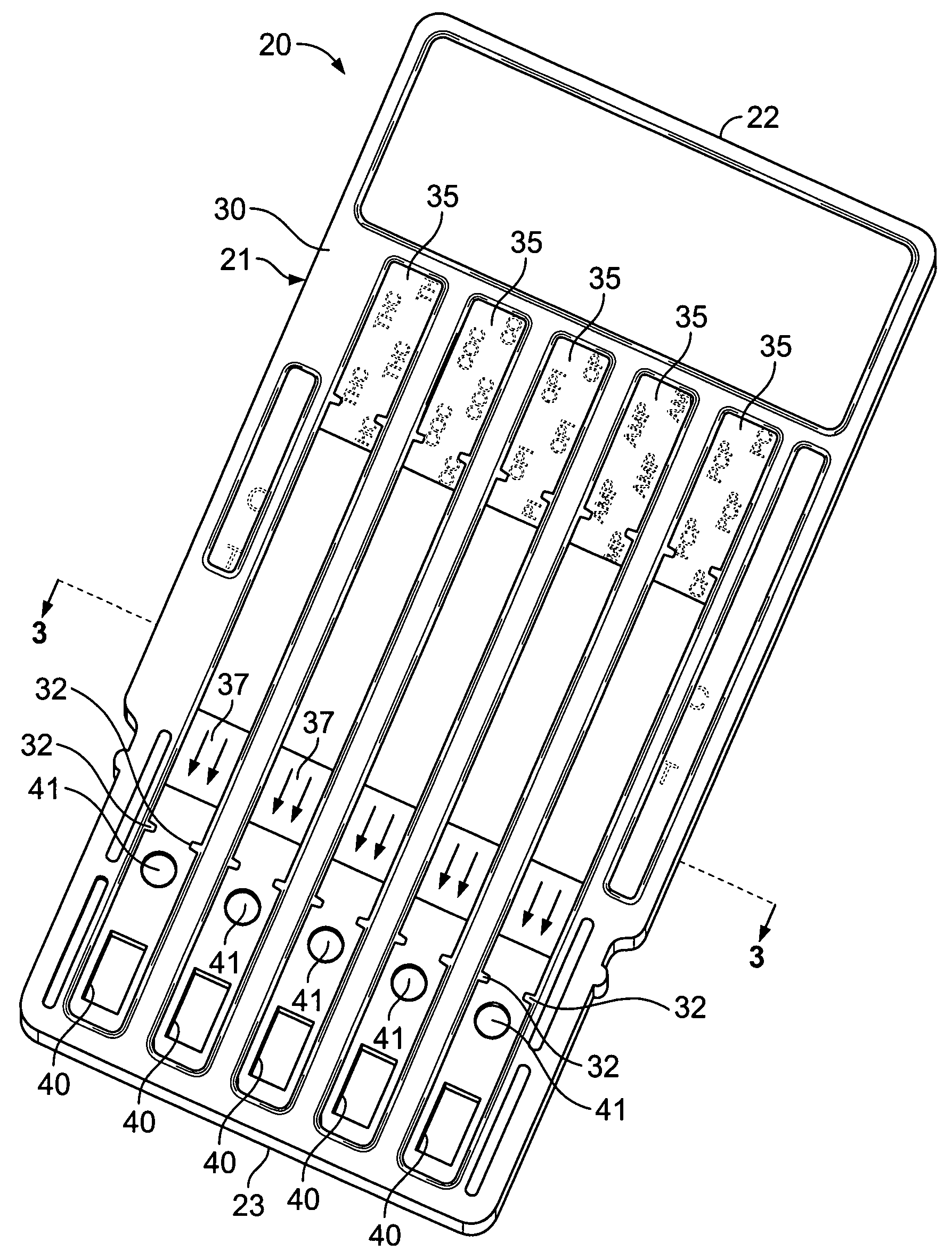
Versatile drug testing device
ActiveUS20090068061A1Not cumbersomeAnalysis using chemical indicatorsMaterial analysis by observing effect on chemical indicatorTest fixtureBody fluid
A versatile drug testing device (a lateral flow diagnostic testing device) includes a flat transparent carrier with a top and a bottom with the carrier having a series of independent parallel grooves formed therein running from adjacent to the top to adjacent to the bottom of the carrier, each groove having a first opening and a second opening above the first opening therein adjacent to the bottom of the carrier, at least one drug test strip installed in one of said grooves with its absorbent pad contiguous to the openings and a cover layer attached to the carrier operable to sealing close each of said grooves whereby the bottom of the device can be immersed in a specimen of “urine”, “body fluid” or “other biological specimen” to wet the pad of the at least one test strip though the ingress of the specimen though the associated openings and the test results on the test strip can be easily viewed through the transparent carrier. Because of the unique construction the device will give accurate reading if temporarily immersed in the specimen or left in the specimen for an extended period of time, making it very user friendly.
Owner:CHEN JIAN FENG
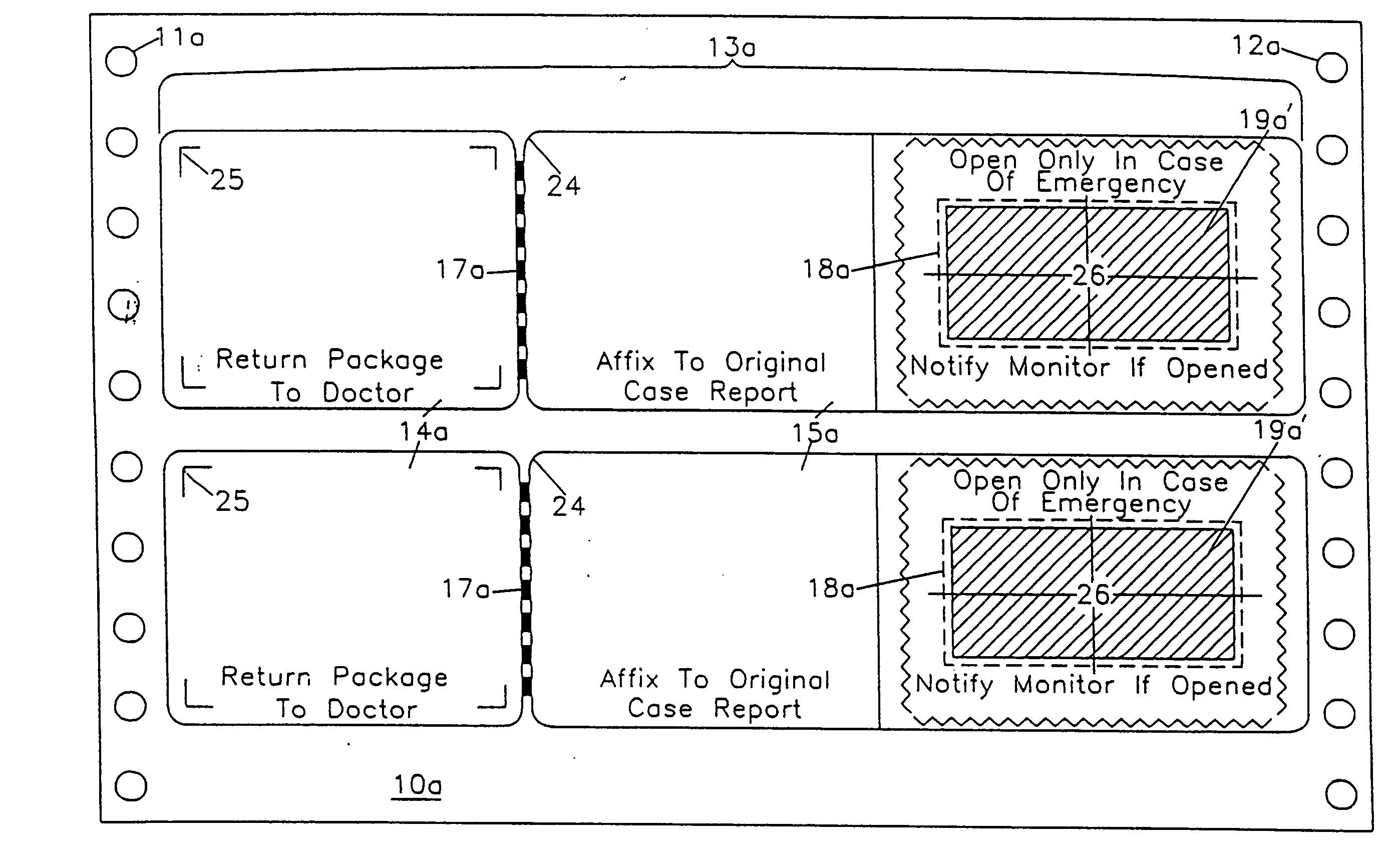
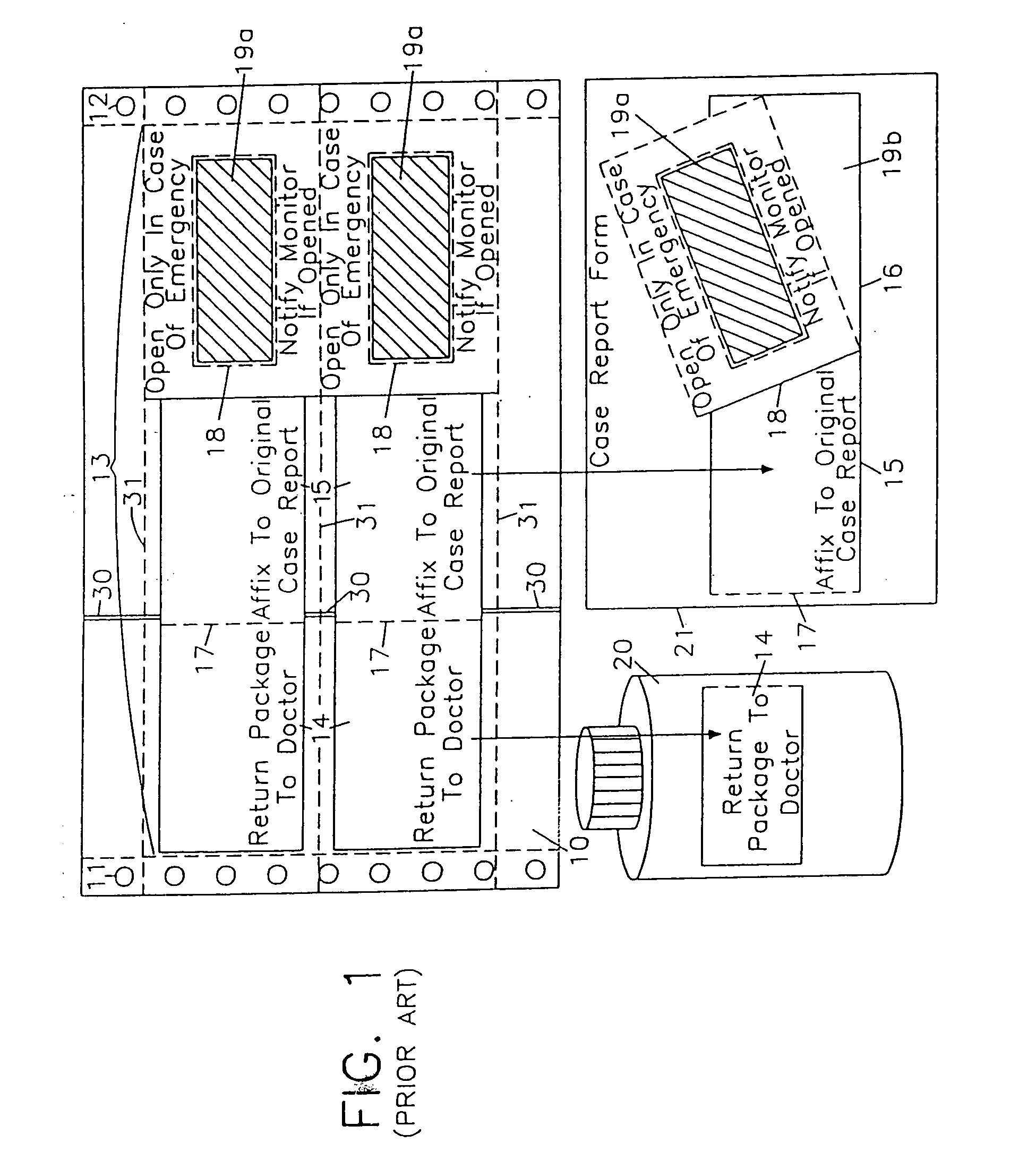
Label form for use in drug testing and method for applying the same
InactiveUS20040009347A1Easy to disassembleEasy to disengageStampsThin material handlingAdhesiveBiomedical engineering
A label form especially for use in testing of drugs. One embodiment has a first label segment with a permanent adhesive backing layer and second label segment with a permanent adhesive backing layer. The first and second label segments may contain information to identify the patient participating in the test, the drug being tested, and other study information and are mounted on mounting sheet having a first mounting sheet segment and a second mounting sheet segment. Each of the mounting sheet segments has a first major surface and a second major surface with the second major surface of the first and the second mounting sheet segments provided with a permanent affixation adhesive layer. The first major surface of the second mounting sheet segment is provided with a nonstick surface for detachably receiving the second major surface of the second label segment. The first major surface of the first mounting sheet segment is attached to the permanent affixation adhesive on the first label segment. The second major surfaces of the first and the second mounting sheet segments are detachably attached to the nonstick surface of the carrying sheet from which the label forms are removed and applied to corresponding containers.
Owner:BOLNICK MARTIN M +1
Micro-fluidic chip-based multiple-organ tumor targeting drug testing platform and application thereof
PendingCN106811409AImprove drug yieldApparatus sterilizationMicrobiological testing/measurementTumor targetTumor targeted
The invention provides a micro-fluidic chip-based multiple-organ tumor targeting drug testing platform and application thereof. A micro-fluidic chip consists of an upper layer chip, a lower layer chip and a porous filter membrane, wherein the upper part of the porous filter membrane is connected with the upper layer chip, and the lower part of the porous filter membrane is connected with the lower layer chip; the structure of the upper layer chip is of a large cell culture chamber, and the structure of the lower layer chip is of independent multiple channels, wherein the number of the channels is 1-9. A construction method of the micro-fluidic chip-based multiple-organ tumor targeting drug testing platform comprises the following steps: (1) manufacturing and modifying the chip; (2) inoculating and culturing cells in the upper layer chip; (3) inoculating and culturing cells in the lower layer chip. The upper layer chip is inoculated with hepatocytes so as to simulate the process of liver metabolism in a body, the lower layer chip is inoculated with different tumor cells and normal tissue cells, so that the effect of drugs, subjected to liver metabolism, on the different cells can be observed, and the effects of drugs, subjected to liver metabolism, on multiple groups of cells can be also observed; therefore, the screening and testing of the tumor targeting drugs can be realized, and the medicine formation rate of the screened drugs is greatly improved.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Capturing and processing instant drug test results using a mobile device
ActiveUS9350956B2Privacy protectionSubject privacyTelevision system detailsCharacter and pattern recognitionTest fixtureMobile device
Systems, methods, and apparatus allow for capturing the results of drug tests using mobile devices. One or more implementations include apparatus that interface a mobile computing device with a drug test, while ensuring that an imaging device of the mobile computing device is optically aligned with a test display areas of the drug test. One or more additional implementations include a method for analyzing drug testing results. The method includes receiving and analyzing drug testing data, which includes an identification of a type drug testing apparatus being used at a remote testing device, and an image that visually represents a portion of a drug test. The method also includes analyzing the test results of the drug test based on the identification of the type drug testing apparatus.
Owner:FORMFOX INC
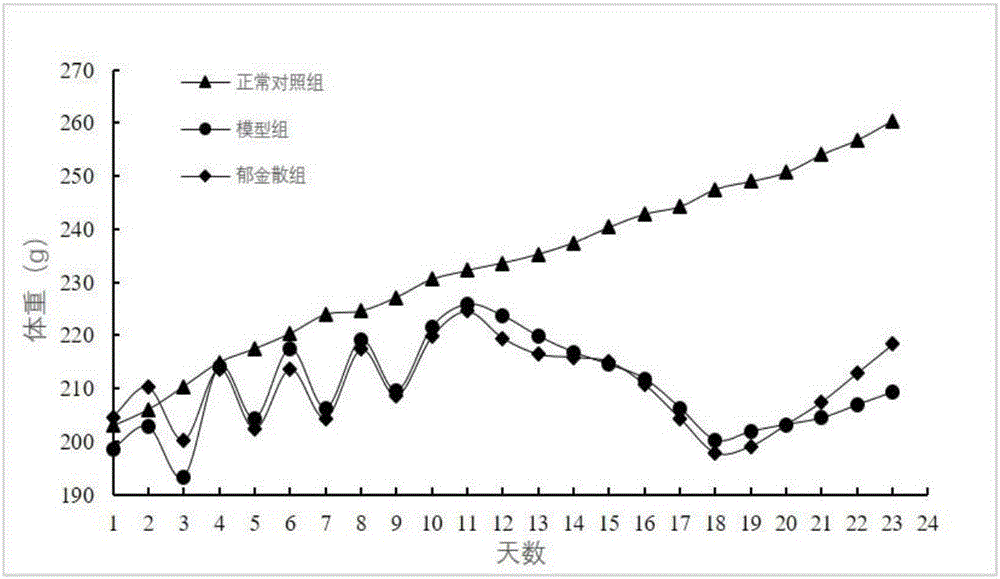
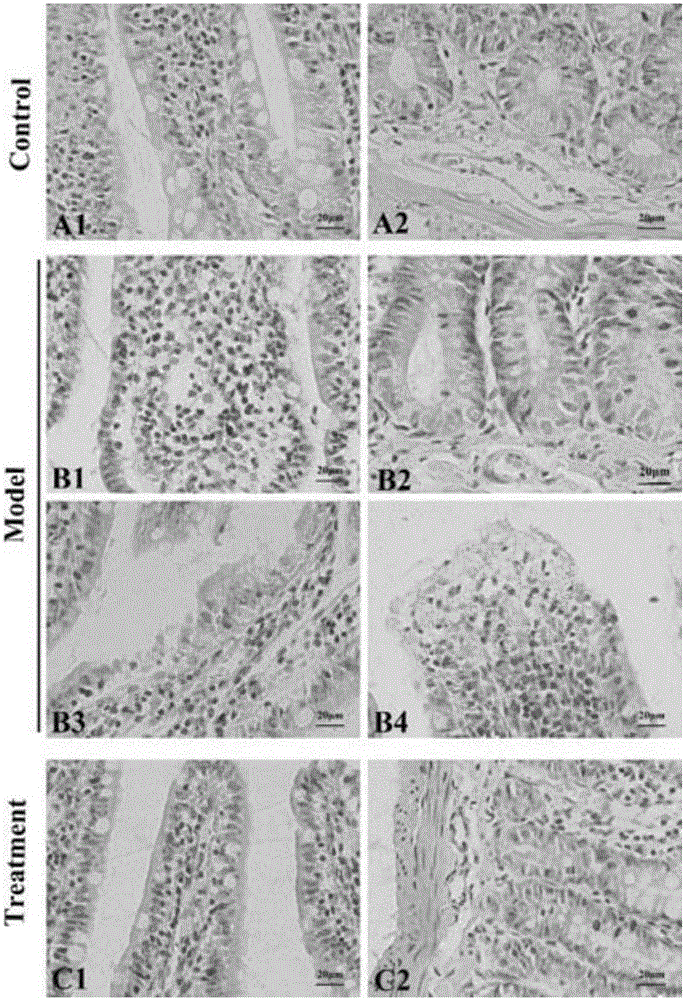
Method for establishing damp-heat diarrhea animal model
The invention relates to a method for establishing a damp-heat diarrhea animal model. The method includes the steps of putting a Wistar rat in a metabolism cage and letting the rat freely drink hydromel; alternatively carrying out fasting, sufficient forage feeding, and lard gavage every other day for 10 days; filling a proper amount of liquor on the 10-15th days, and placing the rat in high temperature and humidity environment for 5 days; and injecting Escherichia coli into the abdominal cavity on the 16th day, and injecting again in 24 hours. By observing the general condition of the rat and detecting the blood, blood viscosity, blood biochemistry and serum cytokines, it is shown that the damp-heat diarrhea animal model is established successfully by the method. The animal model constructed by the invention has good reproducibility and stability, and can withstand the test of drug test, so as to provide an ideal animal model for developing new therapeutic drugs.
Owner:GANSU AGRI UNIV
Culturing patch, culturing method, culture test method, culture test device, drug test method, and drug test device
The present disclosure relates to a culturing patch, culturing method, culture test method, culture test device, drug test method, and drug test device, and the culturing patch according to an aspect of the present disclosure includes component required for growth of an object to be cultured, and a mesh structural body provided in a mesh structure forming micro-cavities in which the component required for growth are contained that is configured to come into contact with a reaction region in which the object to be cultured is placed and provide some of the contained component required for growth to the reaction region.
Owner:NOUL CO LTD
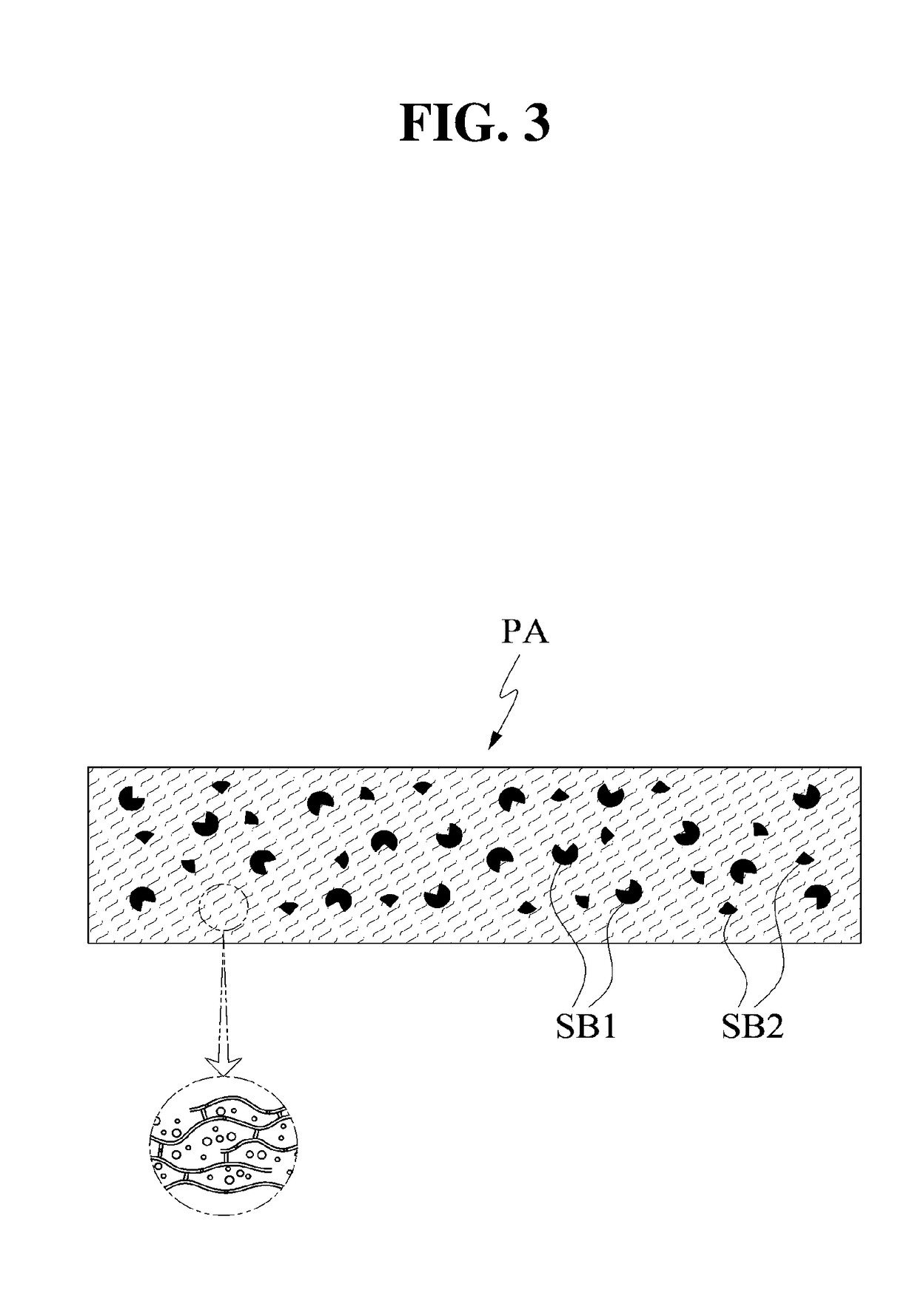
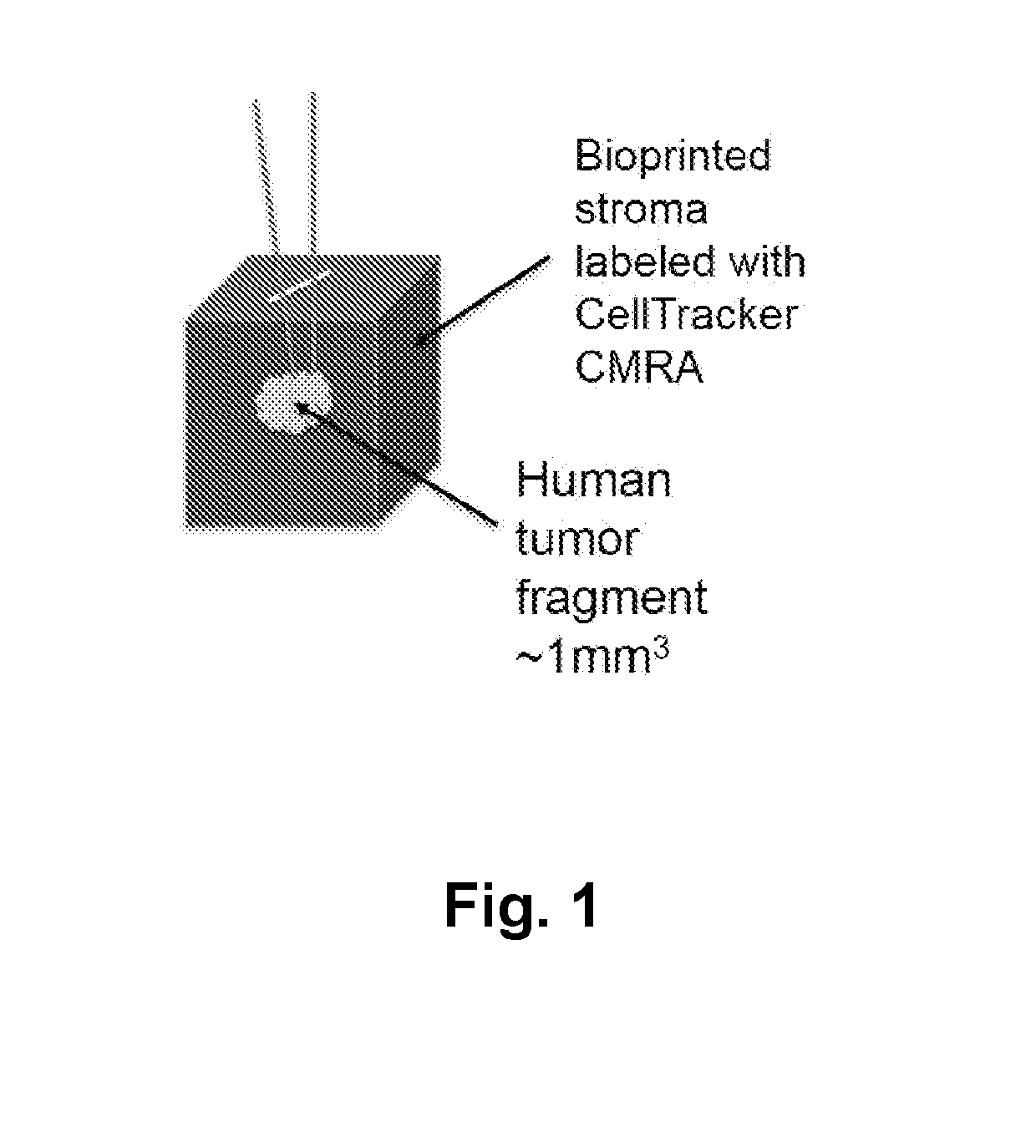
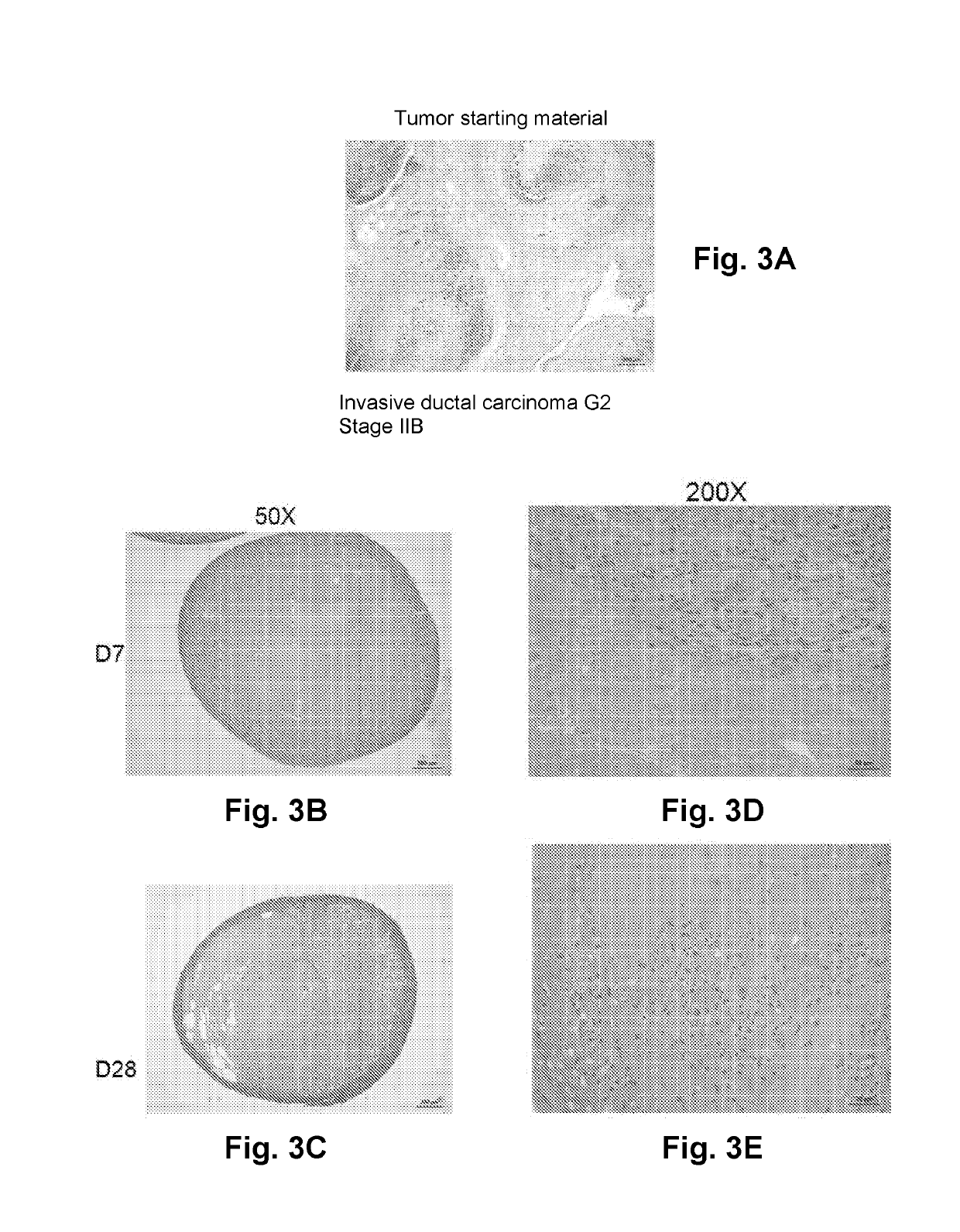
Three Dimensional Bioprinted Tumor Models for Drug Testing
PendingUS20190309264A1Precise screeningUseful measurementCompounds screening/testingAdditive manufacturing apparatusAbnormal tissue growthMedicine
Owner:ORGANOVO +1
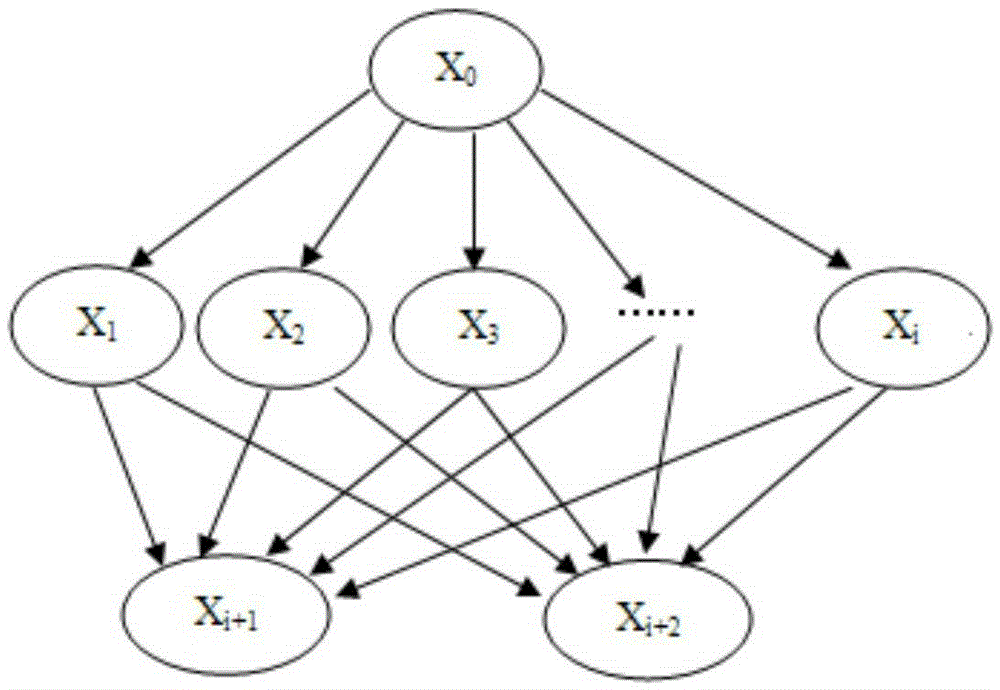
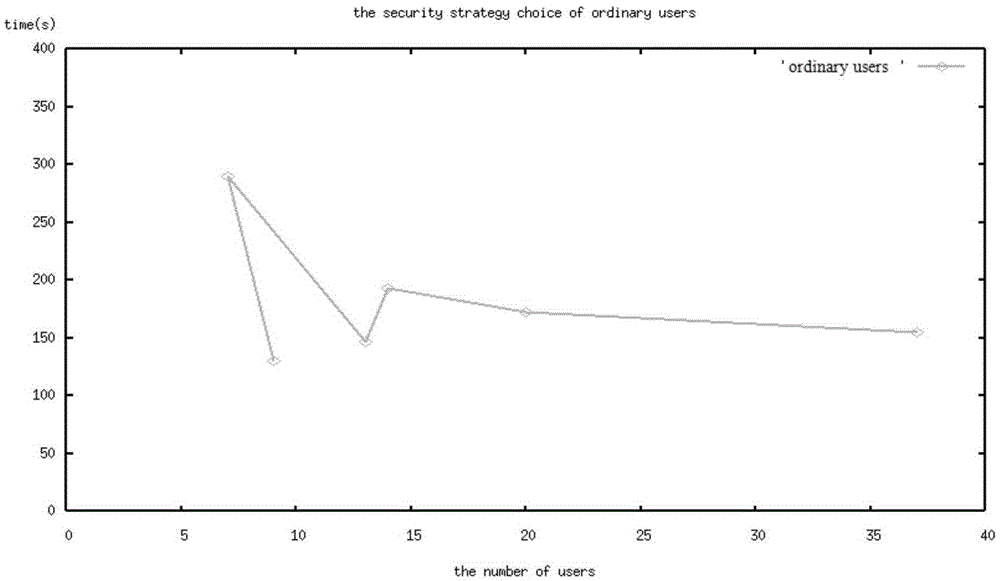
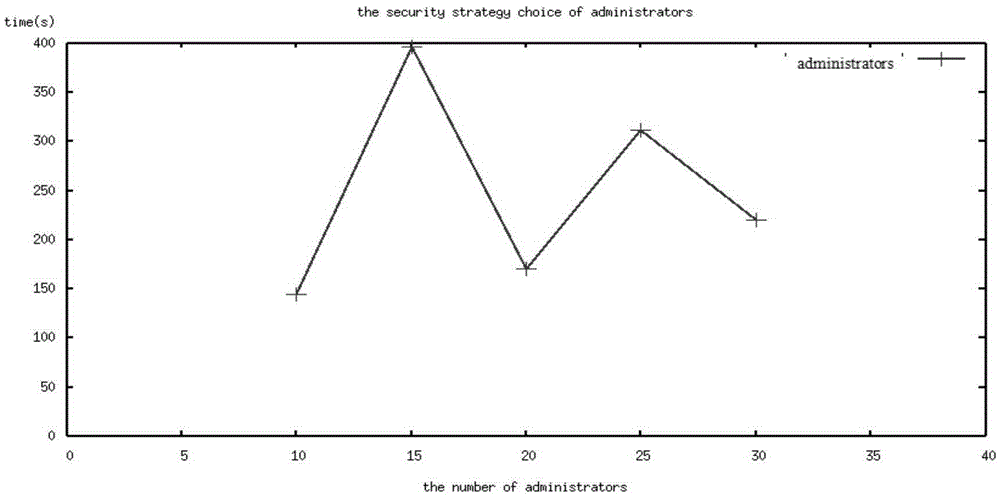
Markov process-based drug test cloud computing security state evaluation method
InactiveCN105306486AReasonable allocation of resourcesSave resourcesTransmissionComputer securityThird party
The invention discloses a Markov process-based drug test cloud computing security state evaluation method, and provides an efficient and safe access state strategy which adopts efficient multi-user access, safe different permission guarantee, high resource utilization rate, reliable third-party storage, extendable new algorithm and the like as key points. According to the strategy, four state security libraries are built for different types of classification of users according to behaviors, a user can select an appropriate security access state strategy according to individual needs and drug test cloud computing security and efficiency quantization as well as evaluation rules, and a result obtained by utilizing a Markov chain state transition matrix and using efficiency security and user evaluation as probability coefficients of the transition matrix through transformation calculation is used for evaluating the benefits of the security strategy selected by the user. Through the adoption of the method, resources are saved during drug test cloud computing, the safety is improved, and an appropriate, reasonable and more user-friendly choice is provided for the user.
Owner:WUHAN UNIV OF TECH
Portable sampling device and method for sampling drug substances from exhaled breath
ActiveUS9977011B2Proven robustnessQuick collectionRespiratory organ evaluationSensorsEnvironmental healthDrug test
A portable drug sampling device for handheldly collecting a sample from exhaled breath of a subject for further sensor based analysis. The device comprising: a housing (406) comprising at least one inlet (407) and at least one outlet (408) for the exhaled breath to exit through, and a sampling membrane (302) arranged in the housing. A tubular element (40) having a mouthpiece section (401) for the subject to exhale into, and a saliva trap section comprising baffles (103) to create a non-straight gas flow path for letting aerosols pass through the tubular element. The sampling membrane (302) is arranged to collect the aerosols from the exhaled breath. The portable drug testing device further comprises a volume collecting element (208).
Owner:SENSA BUSB
Hypromellose-graft-chitosan and methods thereof for sustained drug delivery
ActiveUS20160310440A1Effective drug encapsulationExcessive drug releaseTetracycline active ingredientsSurgeryCelluloseControl release
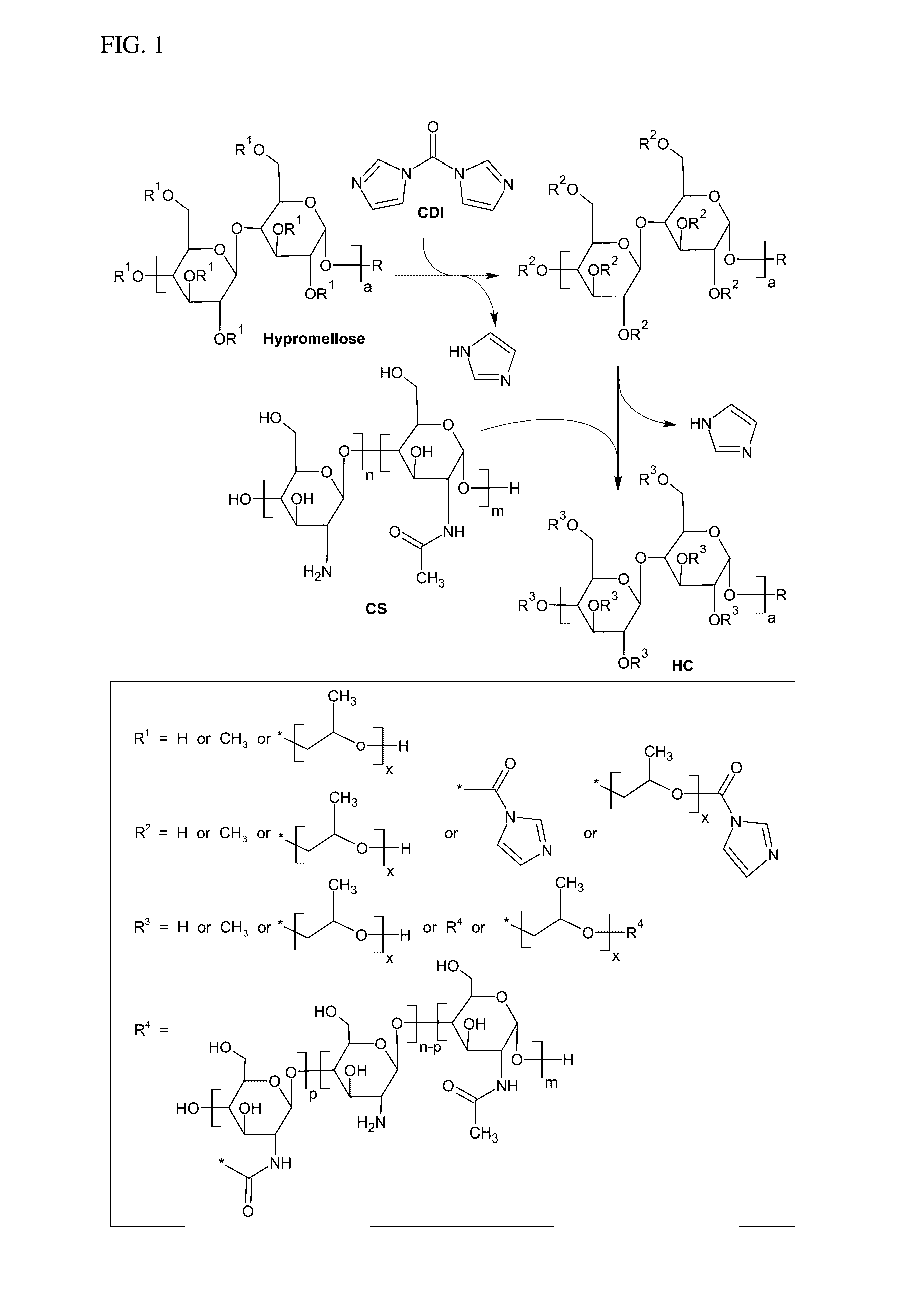
Described herein is a drug delivery system, which is based on a novel polymer namely hypromellose-graft-chitosan (HC), useful to deliver a drug to a patient in sustained and controlled release fashion. HC is highly water soluble across the pH range from 1.2 to 10, and has a high pH buffering capacity to provide a pH-stable environment for drug delivery. In addition, the drug delivery system provided herein exhibited a drug loading efficiency of over 90% in all drugs tested, which is 1-2 fold higher than the efficiency attainable by conventional chitosan, and achieved a 2-3 fold longer duration of sustained drug release.
Owner:THE UNIVERSITY OF HONG KONG
Remote-controlled automated system for drug testing and screening
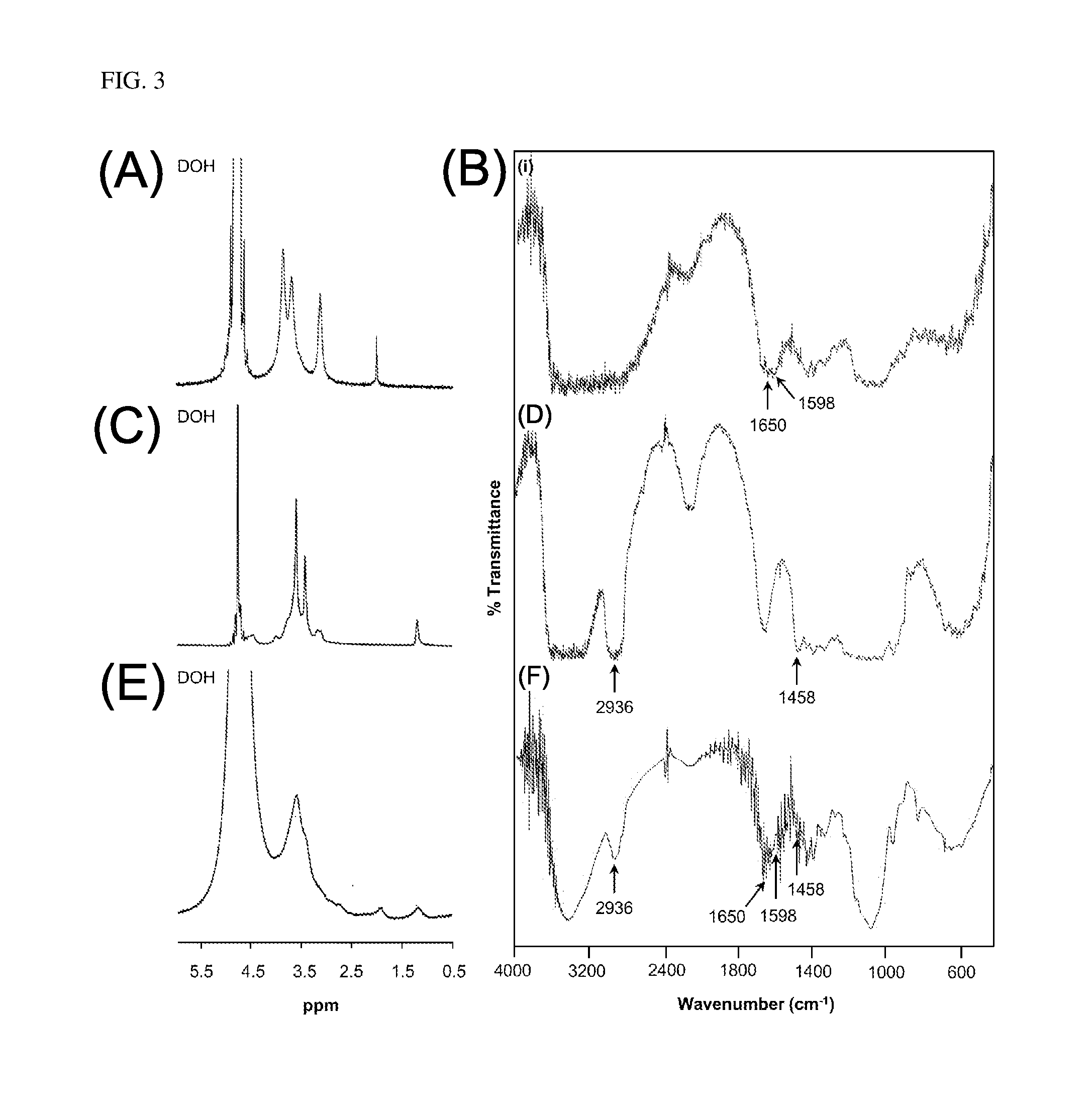
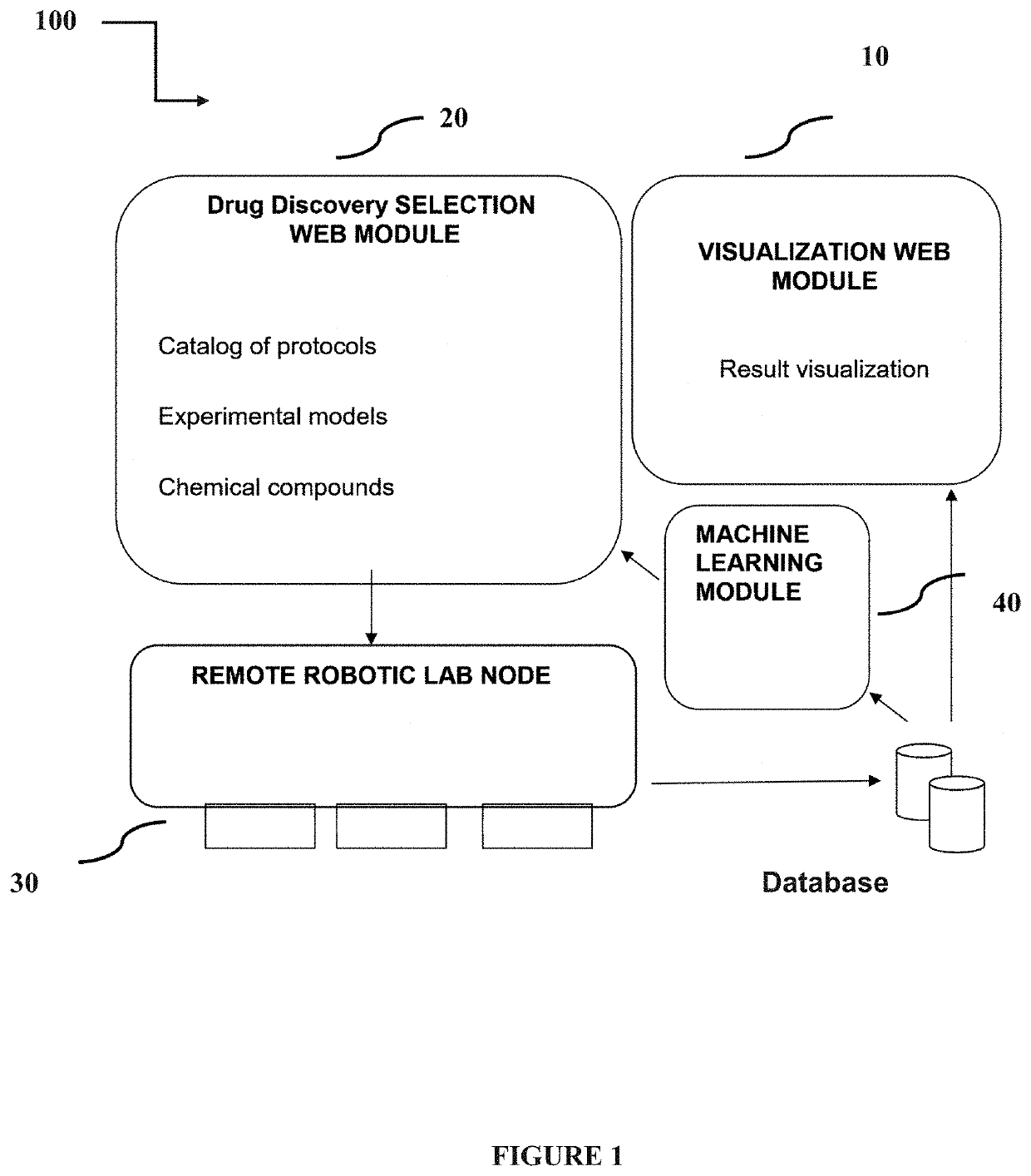
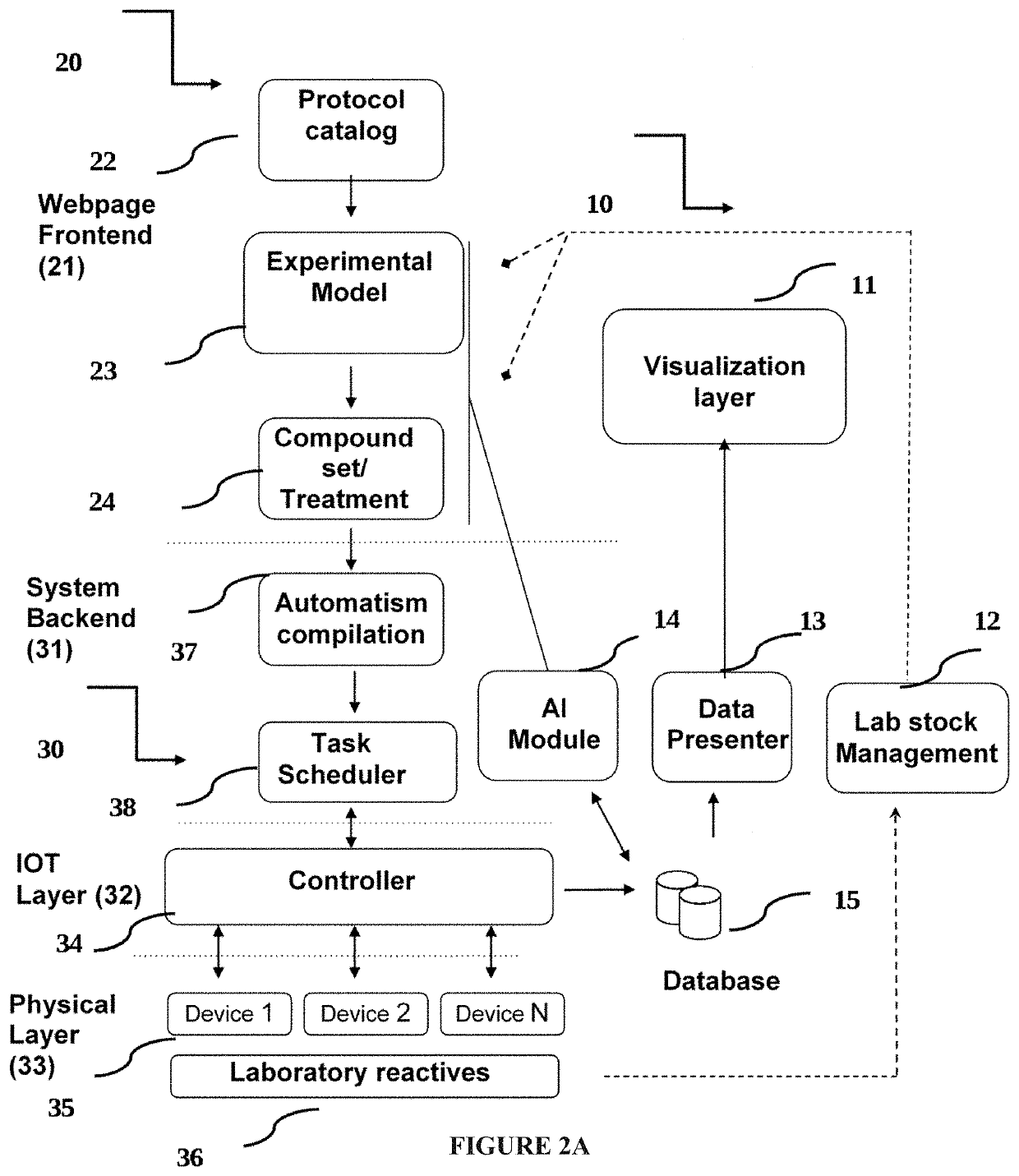
PendingUS20200098449A1Chemical property predictionCheminformatics programming languagesRemote laboratoryEfficacy
A remote-controlled automated system for drug testing and screening. Systems and methods for the discovery of new pharmaceuticals according to their toxicity and / or efficacy, where the discovery process is guided by a computer assisted system and performed into a remote laboratory; additionally, a machine learning algorithm is configured to obtain the results.
Owner:PHYLUMTECH SA
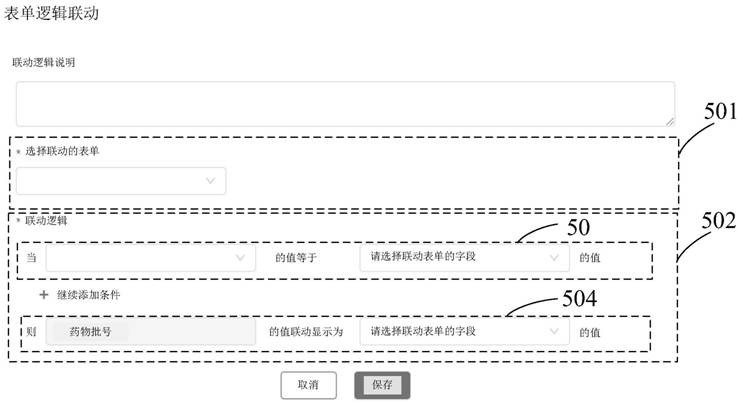
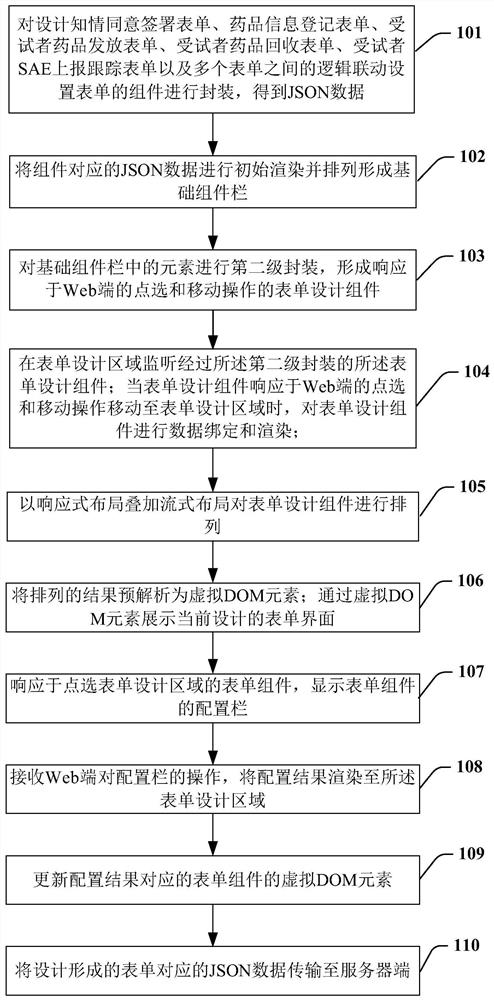
Implementation method and device of field management system for drug tests in clinical research
PendingCN111916163AGuaranteed uptimeQuick ViewNatural language data processingMedical reportsSoftware engineeringPharmaceutical drug
The invention provides an implementation method for a clinical research drug test field management system, and the field management system comprises an informed agreement signing form, a drug information registration form, a subject drug distribution and recovery form, a subject SAE reporting and tracking form, and a logic linkage setting form among a plurality of forms. The step of designing theplurality of forms comprises the following sub-steps: packaging components of the designed forms to obtain JSON data; carrying out initial rendering on the JSON data and arranging the JSON data to form a basic component column; forming a form design component responding to clicking and moving operations of the Web end; monitoring the form design component subjected to the second-level packaging inthe form design area; performing data binding and rendering on the form design component; arranging the form design components; pre-analyzing an arrangement result into a virtual DOM element; displaying a configuration column of the form component; receiving an operation on the configuration bar; and transmitting JSON data corresponding to the designed and formed form to a server side.
Owner:上海太美星云数字科技有限公司
Apparatus and method for passive testing of alcohol and drug abuse
InactiveUS8529462B2Digital data processing detailsPerson identificationCompound organicAlcohol and drug
An automated system and method for passive testing of alcohol and drug abuse. The system enters a participant or subject into the system who is to be monitored during a probationary or other program for alcohol or drug abuse offenders. The system provides a drug testing home device or a drug testing kiosk device for use by the participant. The system enrolls the biometrics information of the participant into the computer system (e.g., finger print, voice, image, volatile compound organic gas level, and pH level). When the participant is to be tested in accordance with a testing schedule, the system validates these same biometrics of the participant, conducts the test, and then analyzes the test information for determining if the participant has been using alcohol or other drugs and should be subjected to a confirming urinalysis exam.
Owner:JUSTICE EZ TRAC
Cartridges for oral fluid analysis and methods of use
ActiveUS20190021704A1Accurate measurementPrevent leakageMaterial analysis by observing effect on chemical indicatorWithdrawing sample devicesAnalyteMagnetic tape
A disposable cartridge can be used for biofluid sample collection, preparation, and mixing with reagents. After sample collection, the cartridge can be inserted into a reader for sample analysis. This system can be used for detecting and measuring analytes, such as drugs, in saliva for example. This is useful for point of test detection of drugs in applications such as workplace drug testing and driving under the influence of drugs testing.
Owner:EVANOSTICS LLC
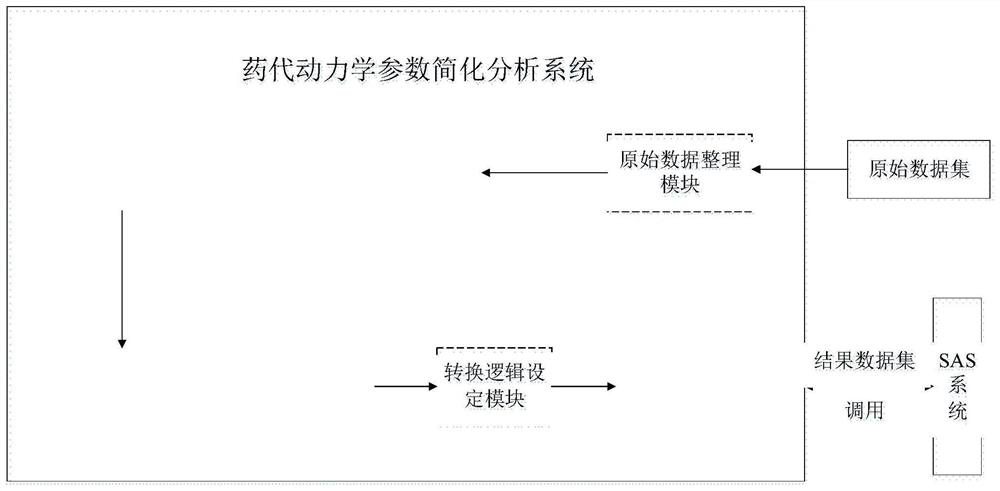
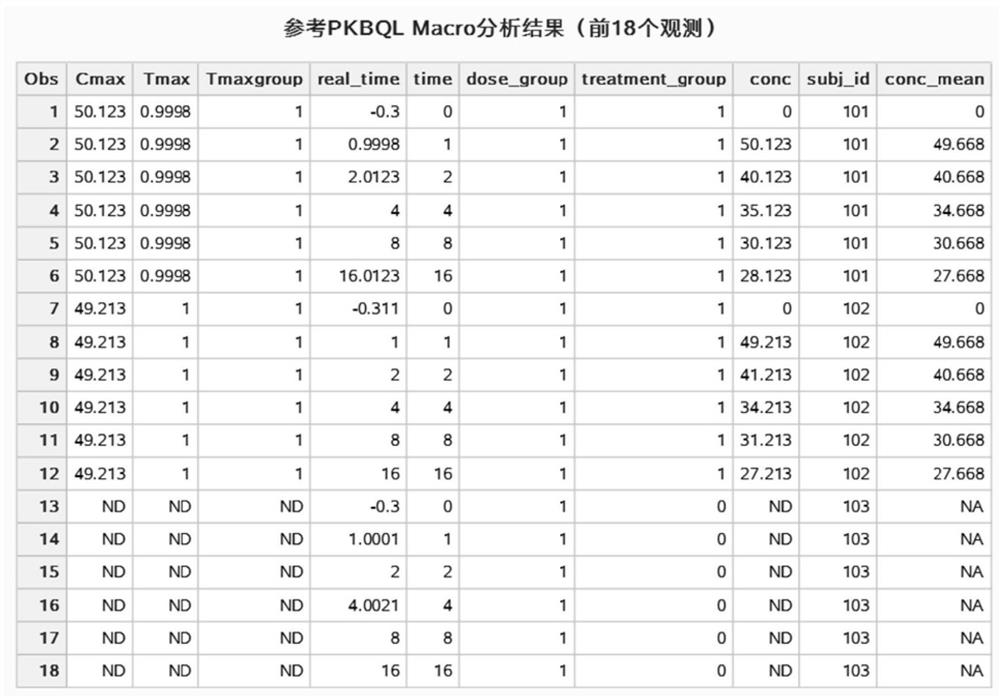
Simplified analysis method and system for pharmacokinetic parameters
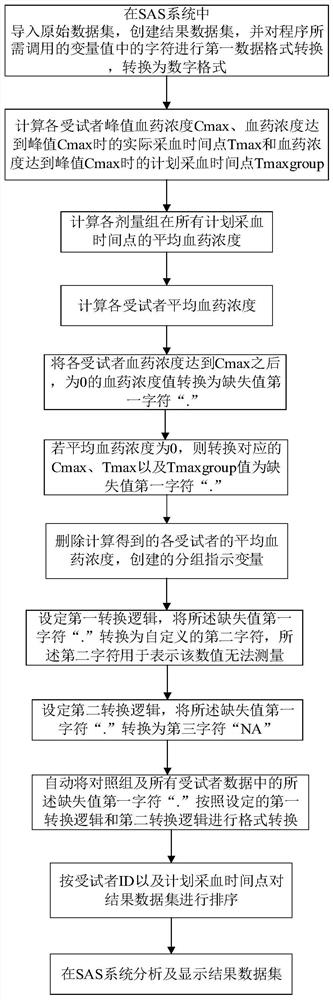
ActiveCN112259251AEasy to handleEasy to operateDrug referencesChemical machine learningData setOriginal data
The invention provides a simplified analysis method and system for pharmacokinetic parameters, and belongs to the technical field of pharmacokinetics. According to the method, when pharmacokinetics ofa single administration test of a clinical drug test is analyzed, values which are in a data set and are lower than the lower limit of a measurement method and cannot be measured are subjected to self-defined conversion, and important parameter results such as Cmax, Tmax, the average blood concentration value of each dose group in a test group at each planned blood sampling time point and the like are obtained; an operation result is merged into the original data set under the condition that the format of the original data set and other variable data in the data set are not influenced and changed, and a new data set is generated or directly covers the original data set. Statists can simply use the method provided by the invention in SAS software to quickly obtain the required operation result, and can directly carry out subsequent analysis and research work without spending a lot of time in importing, merging or sorting data sets among different software.
Owner:昭衍(北京)医药科技有限公司
Novel enhanced processes for drug testing and screening using human tissue
InactiveUS20070048733A1Microbiological testing/measurementTissue/virus culture apparatusHuman bodyTissue sample
A novel method for testing human tissue in a testing system is more effective than conventional cell culture systems and functions by treating the human tissue slice system's samples with at least one compound and observing the effect on the human tissue slices resident therein, or cells, tissue samples or other derivatives from the testing process.
Owner:HEPAHOPE
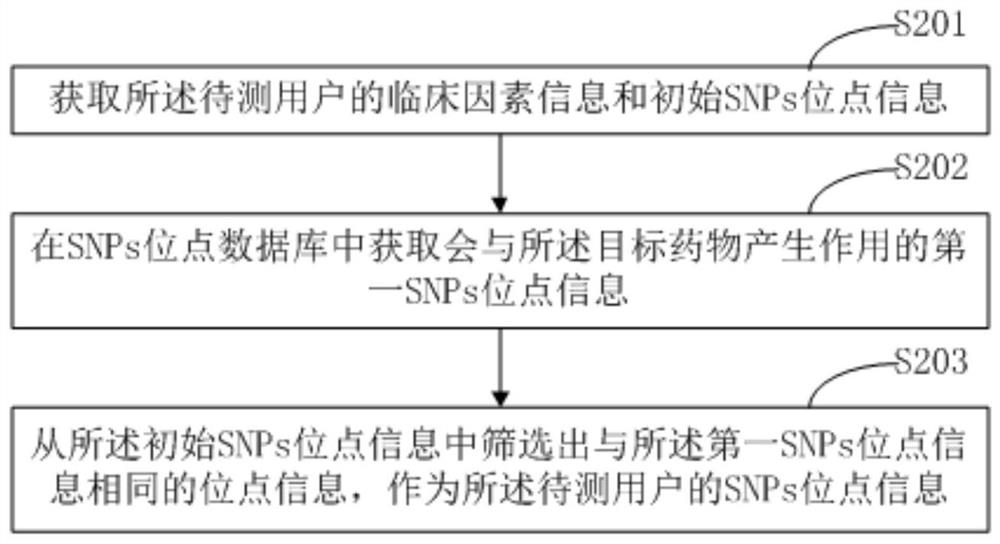
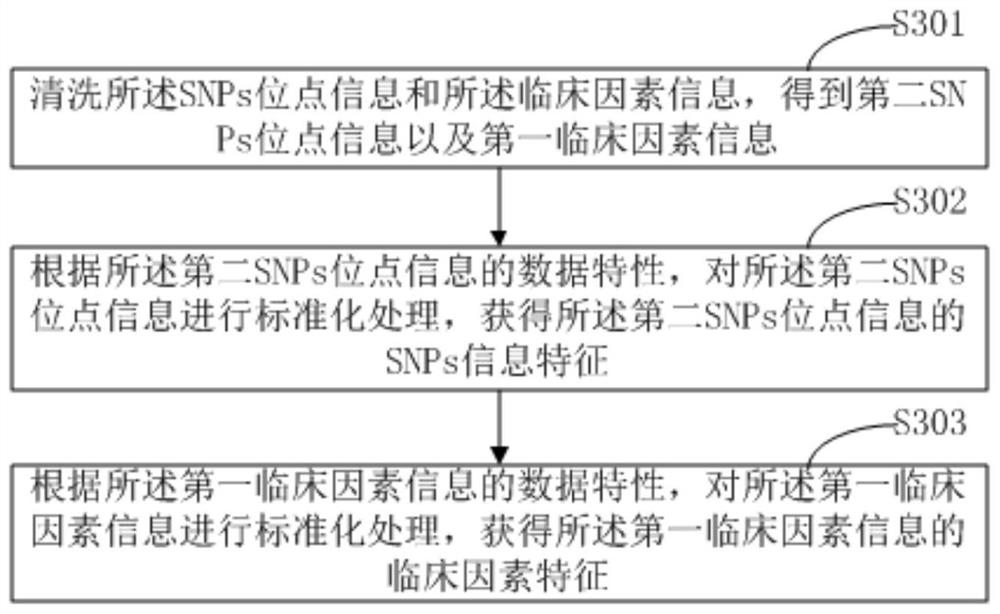
Prediction method and device for sensitivity of target drug, terminal equipment and storage medium
PendingCN111724911AImprove accuracyCharacter and pattern recognitionProteomicsNucleotidePharmaceutical drug
The invention belongs to the technical field of drug testing, and provides a prediction method and device for the sensitivity of a target drug, a terminal device and a storage medium. The method comprises the following steps: acquiring clinical factor information and single nucleotide polymorphism (SNP) site information of a user to be tested, wherein the SNP site information is the information ofa site, that may act with a target drug, in a gene of the user to be tested; extracting SNP information features according to the SNP site information, and extracting clinical factor features according to the clinical factor information; and according to the SNP information features and the clinical factor features, predicting the sensitivity of the to-be-tested user taking the target drug. According to the method, the information of the site, that may act with the target drug, in the gene of the user to be tested and the clinical factor information are taken as prediction information, and corresponding SNP information features and clinical factor features are extracted for prediction, so higher prediction accuracy is obtained in detection of generation of sensitivity when the to-be-tested user takes the target drug.
Owner:深圳哲源生物科技有限责任公司
System and method for automated determination of the relative effectiveness of anti-cancer drug candidates
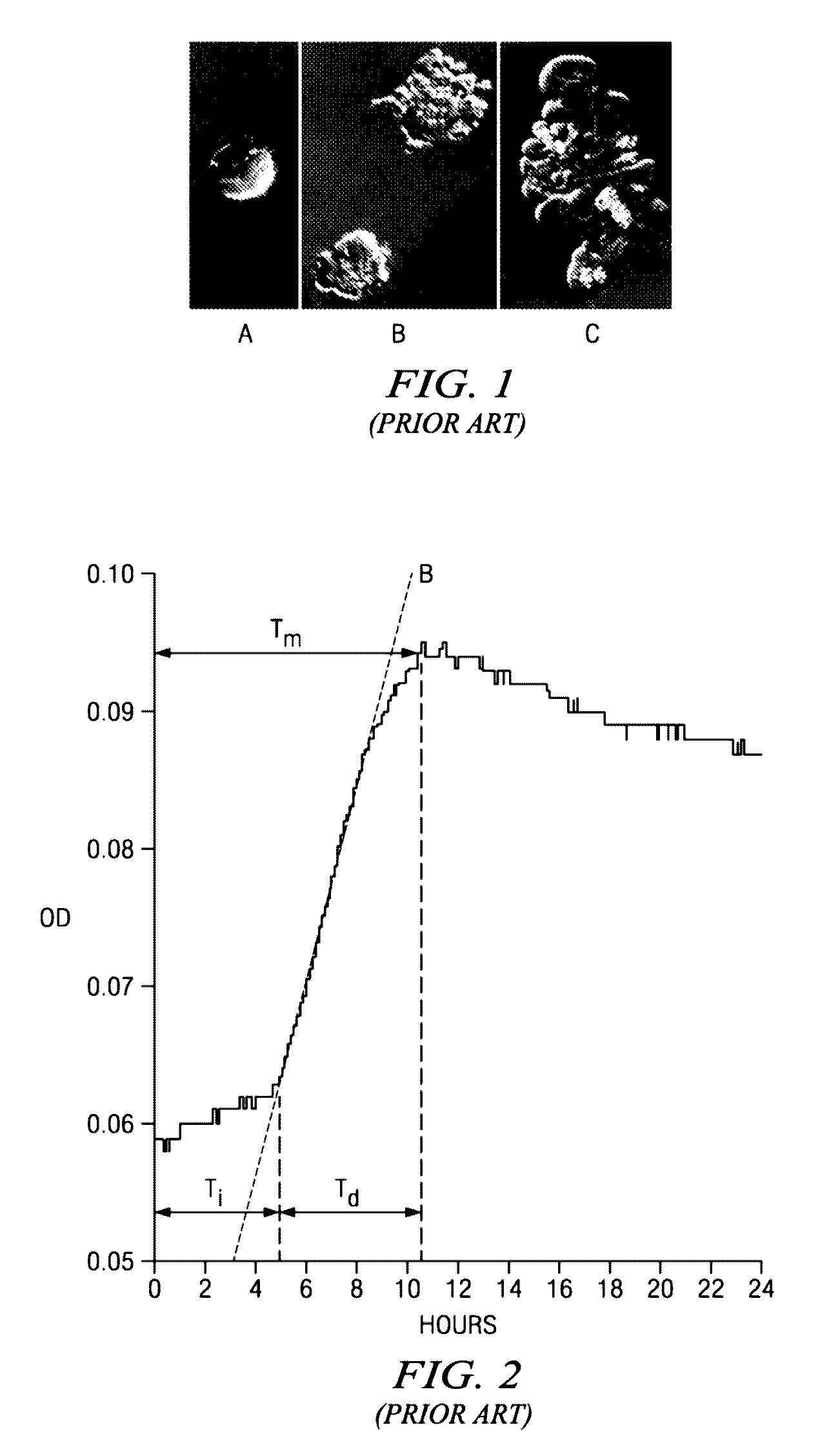
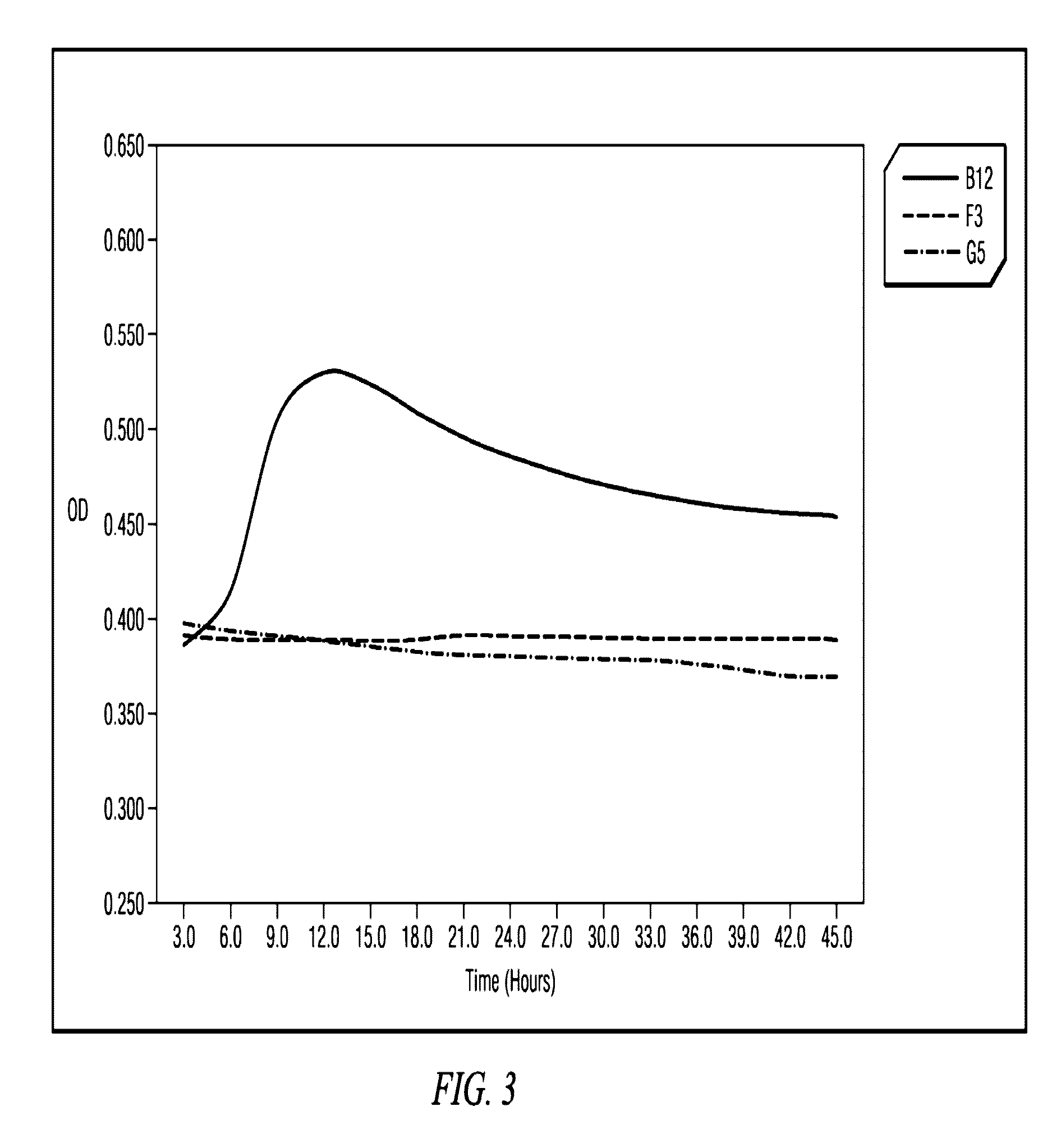
A computer system is provided for determining the relative effectiveness of anti-cancer drugs. The interface has selectable options, including an option to manage drug testing parameters, and enables user selection of desired drug testing parameters in relation to a virtual well plate associated with a physical well plate of a spectrophotometer. The computer system causes the spectrophotometer to start a drug test, wherein the physical well plate includes at least one test well containing viable cancer cells; and at least one drug candidate in a predetermined concentration; and at least one control well containing the viable cancer cells alone. The system records the optical density of the well at a predetermined wavelength at selected time intervals for a selected duration of time, and stores the optical density and time measurements in the database. An activity value is calculated from the optical density and time measurements, and a correlation is displayed between the activity value and the drug candidate's ability to induce apoptosis in the cancer cells.
Owner:PIERIAN BIOSCI LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com