Patents
Literature
2236 results about "Colloidal gold" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
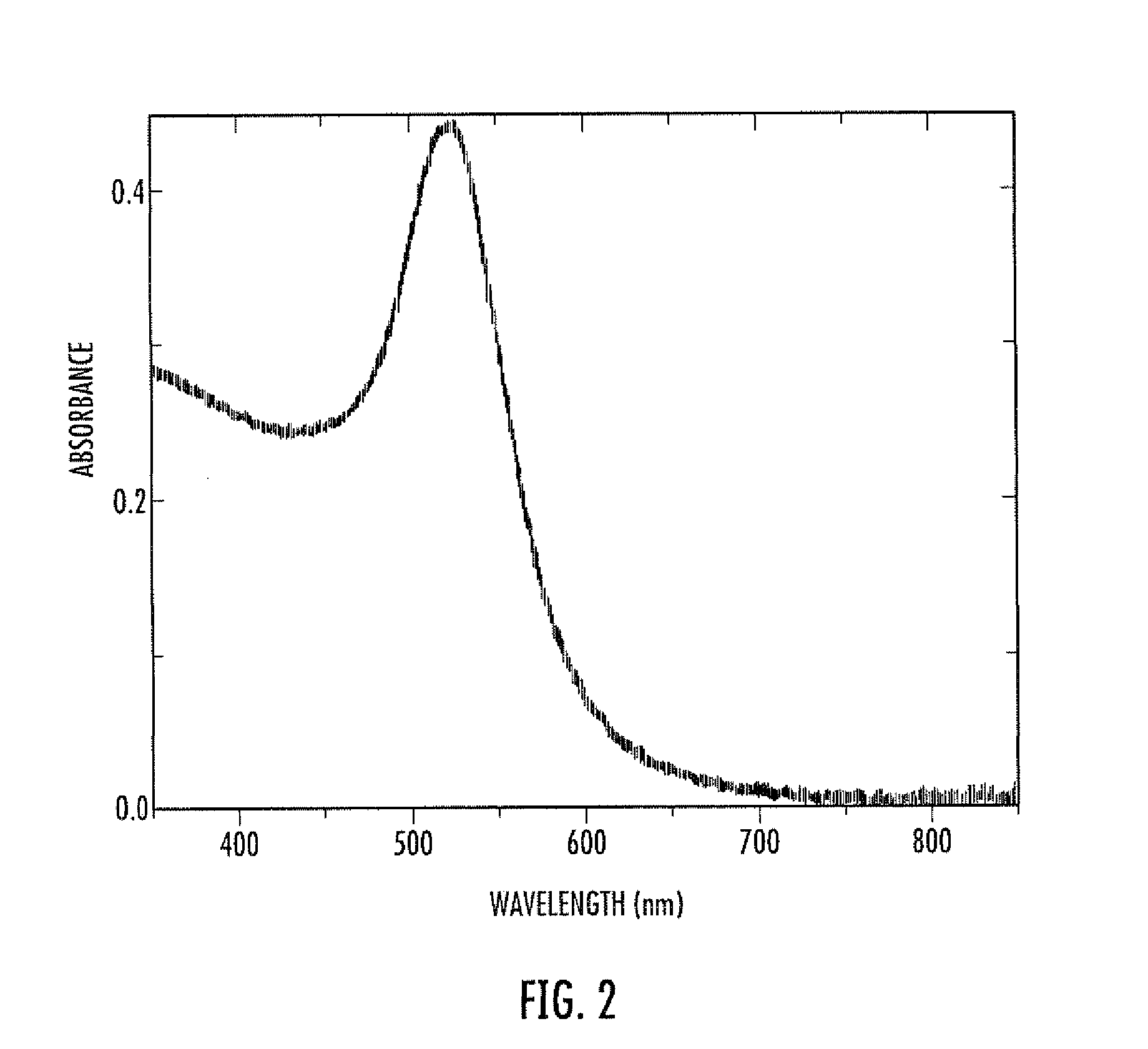
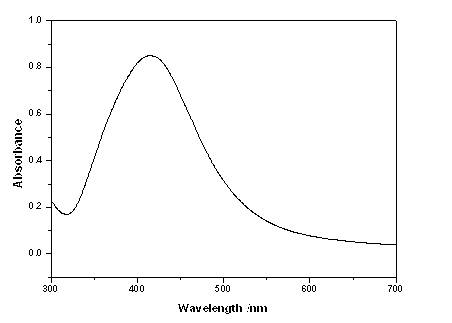
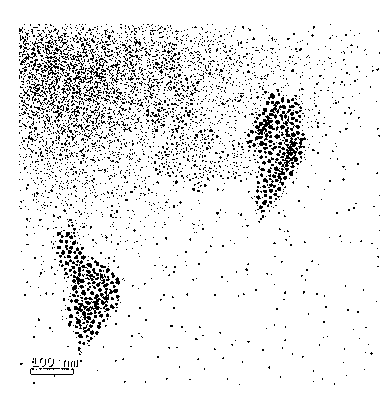
Colloidal gold is a sol or colloidal suspension of nanoparticles of gold in a fluid, usually water. The colloid is usually either an intense red colour (for spherical particles less than 100 nm) or blue/purple (for larger spherical particles or nanorods). Due to their optical, electronic, and molecular-recognition properties, gold nanoparticles are the subject of substantial research, with many potential or promised applications in a wide variety of areas, including electron microscopy, electronics, nanotechnology, materials science, and biomedicine.
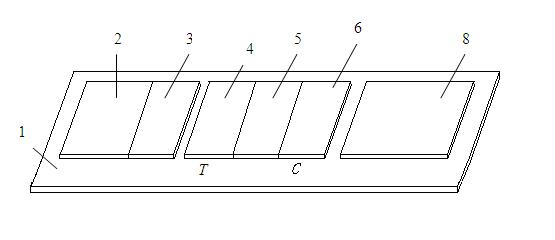
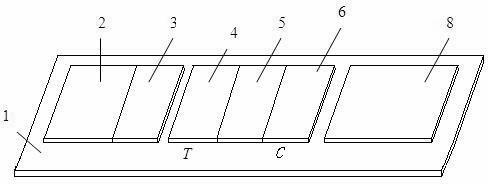
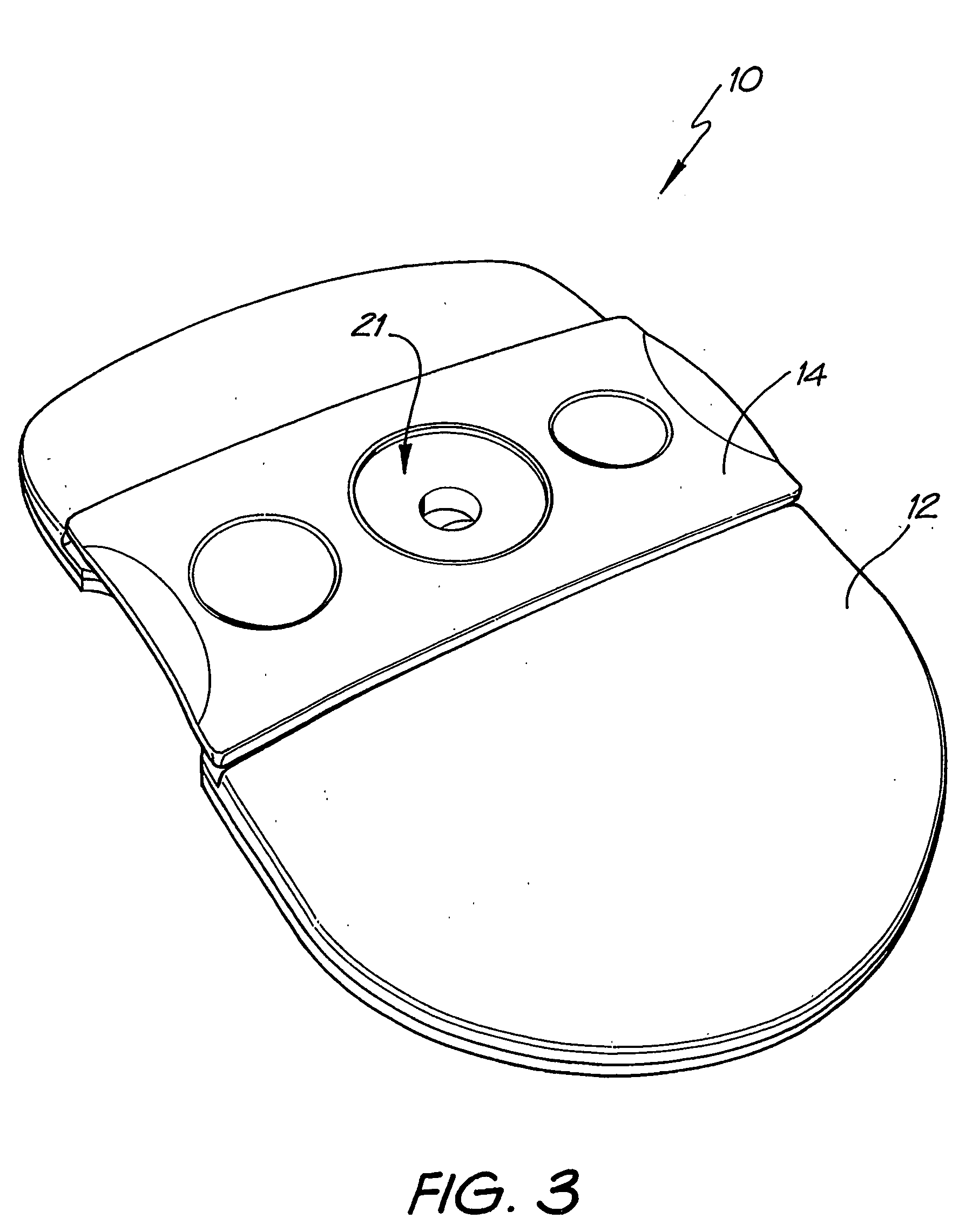
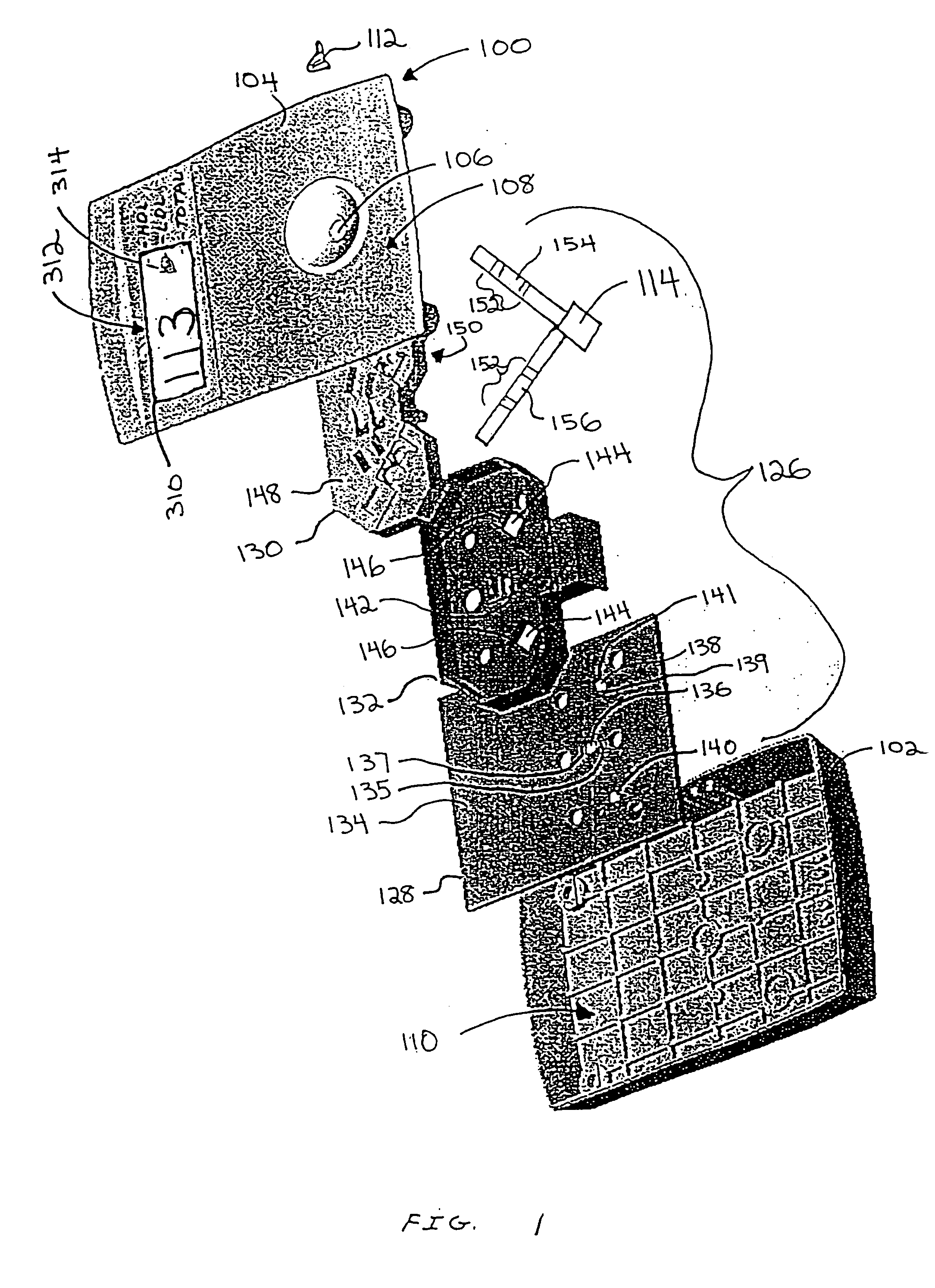
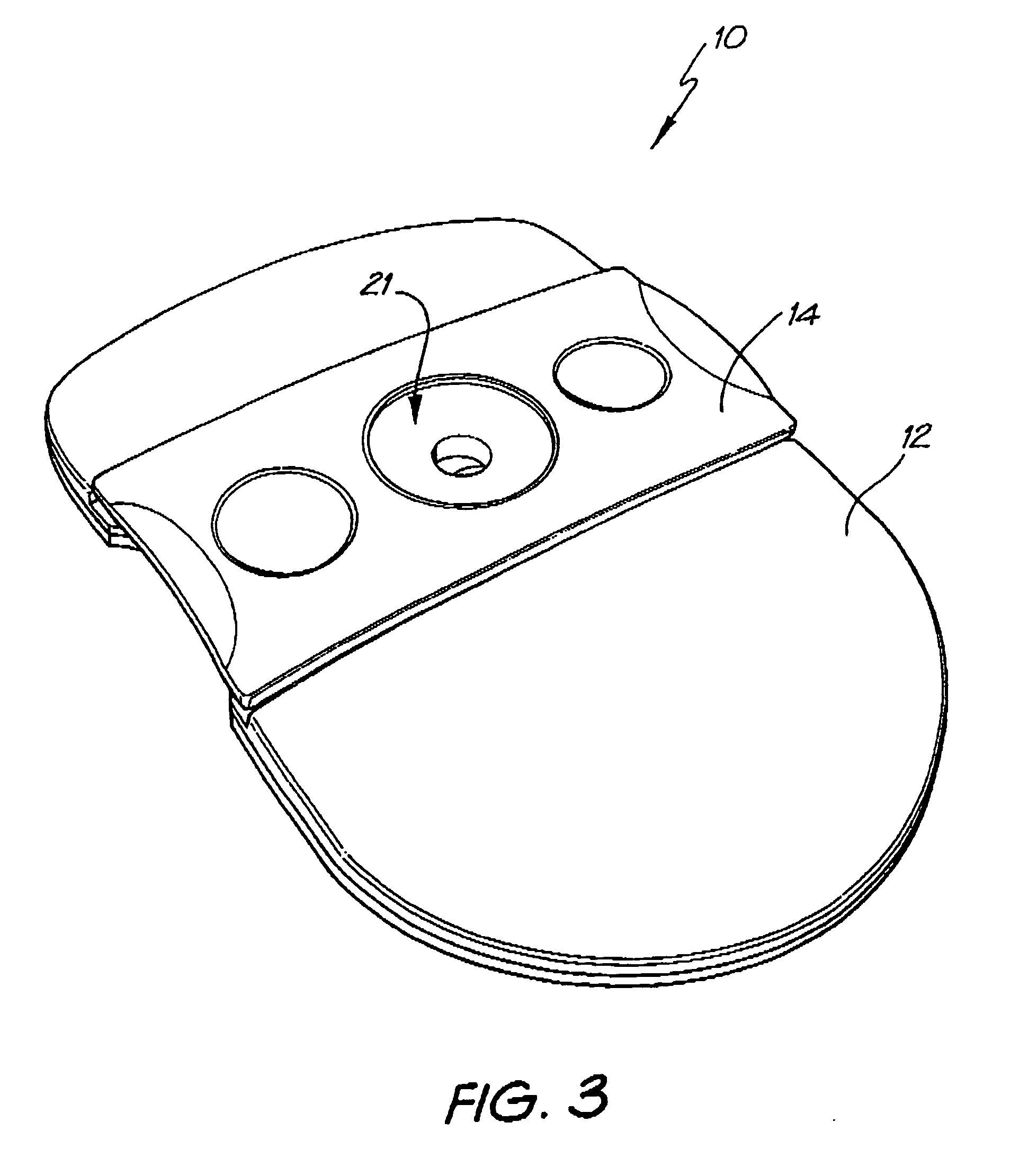
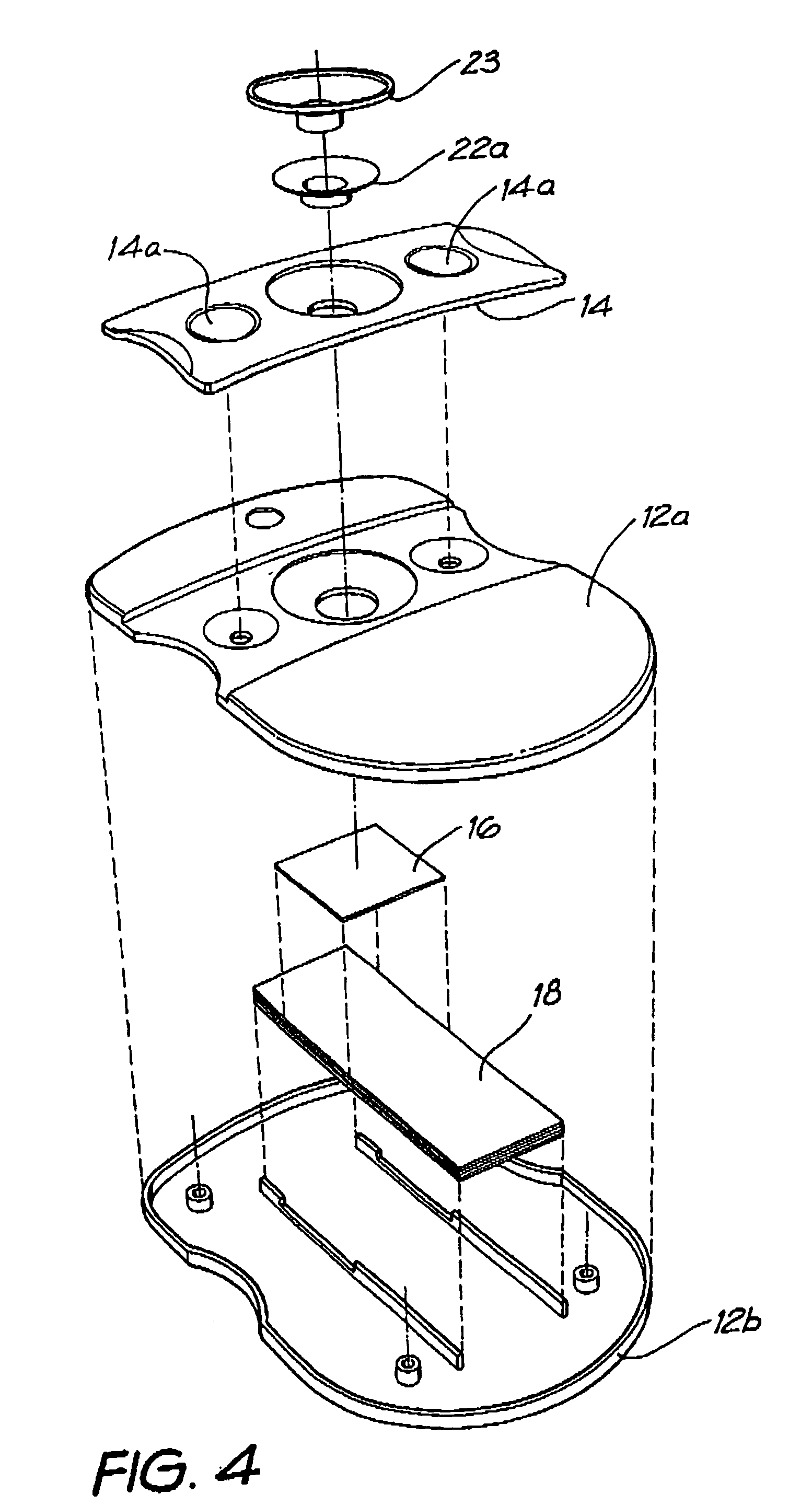
Diagnostic testing process and apparatus
InactiveUS7205159B2High detection sensitivityReduce volumeBioreactor/fermenter combinationsBiological substance pretreatmentsAnalyteAntibody
A method and apparatus for use in a flow through assay process is disclosed. The method is characterised by a “pre-incubation step” in which the sample which is to be analysed, (typically for the presence of a particular protein), and a detection analyte (typically an antibody bound to colloidal gold or a fluorescent tag) which is known to bind to the particular protein may bind together for a desired period of time. This pre incubation step occurs before the mixture of sample and detection analyte come into contact with a capture analyte bound to a membrane. The provision of the pre-incubation step has the effect of both improving the sensitivity of the assay and reducing the volume of sample required for an assay. An apparatus for carrying out the method is disclosed defining a pre-incubation chamber for receiving the sample and detection analyte having a base defined by a membrane and a second membrane to which a capture analyte is bound. In one version the pre-incubation chamber is supported above the second membrane in one position but can be pushed into contact with the membrane carrying the capture analyte thus permitting fluid transfer from the incubation chamber through the capture membrane. In another version the membrane at the base of the incubation chamber is hydrophobic and its underside contacts the capture membrane and when a wetting agent is applied to the contents of the pre-incubation chamber fluid transfer occurs.
Owner:PROTEOME SYST LTD
Nanoparticles for therapeutic and diagnostic applications
InactiveUS20060222595A1Easy to useFacilitate multiple modeUltrasonic/sonic/infrasonic diagnosticsMaterial nanotechnologyDiagnostic agentProviding material
This document provides materials and methods related to nanoparticles. For example, nanoparticle compositions, methods for making nanoparticle compositions, and methods for using nanoparticle compositions are provided. In some cases, the nanoparticles are gold (e.g., colloidal gold) nanoparticles. A nanoparticle can include one or more agents linked to its surface, such as therapeutic and / or diagnostic agents, and can be from about 1 nm to about 10 nm in size.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
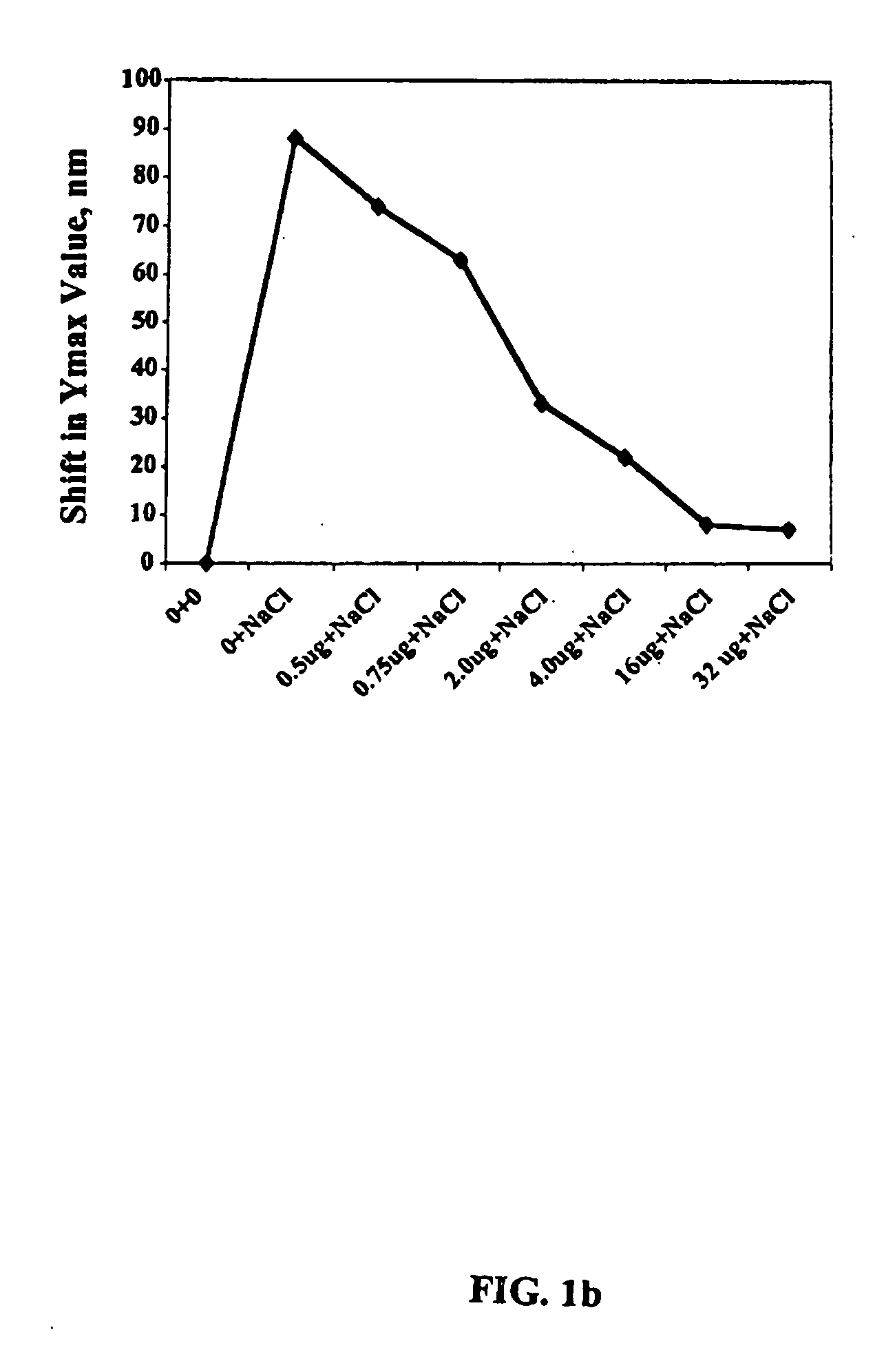
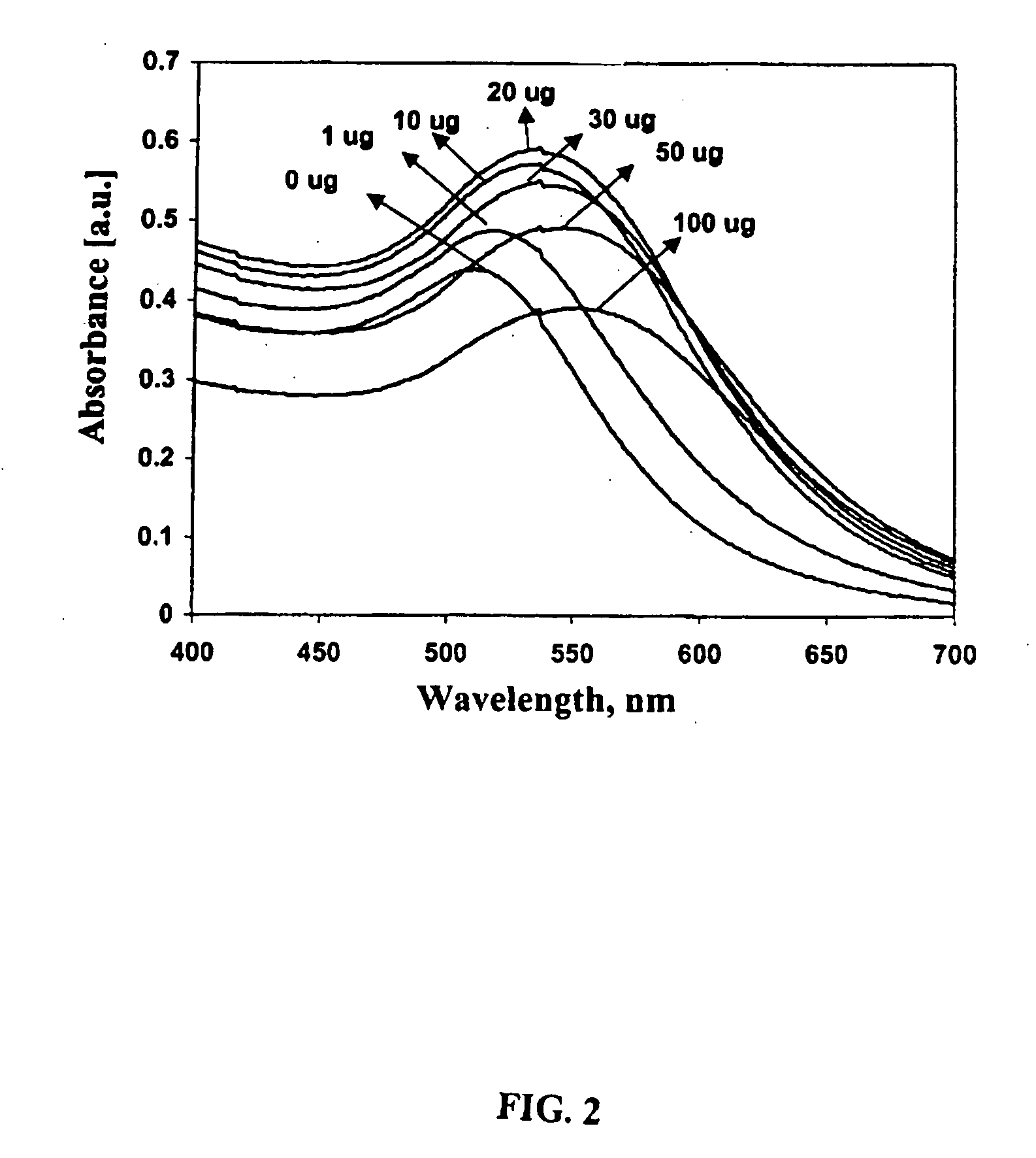
Detection kit of IgM/IgG antibodies of novel coronavirus (SARS-CoV-2)
ActiveCN111187354AImprove response accuracyOvercoming cumbersome detection operationsSsRNA viruses positive-senseAntibody mimetics/scaffoldsEpitopeCoronavirus antibody
The invention provides a detection kit of IgM / IgG antibodies of novel coronavirus (SARS-CoV-2), and relates to the technical field of biology. According to the detection kit of IgM / IgG antibodies of novel coronavirus (SARS-CoV-2), the IgM / IgG antibodies of novel coronavirus (SARS-CoV-2) are detected through adoption of a colloidal gold capture method, a colloidal gold indirect method and a colloidal gold double antigen sandwich method, wherein adopted antigens are highly active recombinant antigens which are obtained through fusion expression of N protein fragments and S protein dominant epitope fragments, and colloidal gold is labeled indirectly, so that steric hindrance is reduced, and the activity, reaction consistency and accuracy of the antigens are increased. Since a sample pad is pretreated, the interference of complex components in a blood sample to a reaction can be reduced greatly, the detection kit has no requirements for instruments during detection, simple and flexible operation, direct and manual interpretation of results and a short test time, the results can be interpreted in only 15 minutes, and the test results are accurate.
Owner:北京新创生物工程有限公司
Human mycoplasma pneumoniae gold-marked silver-stained immunochromatographic assay kit and preparation method and application thereof
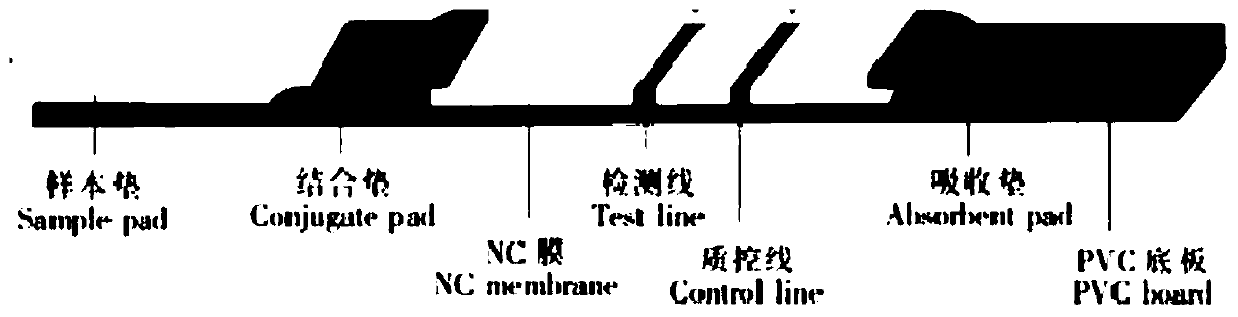
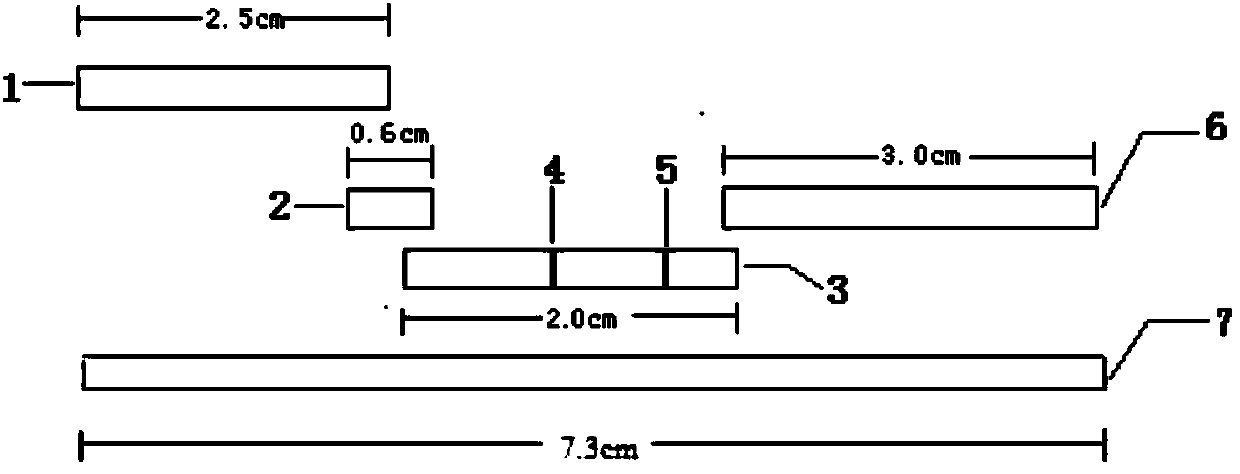
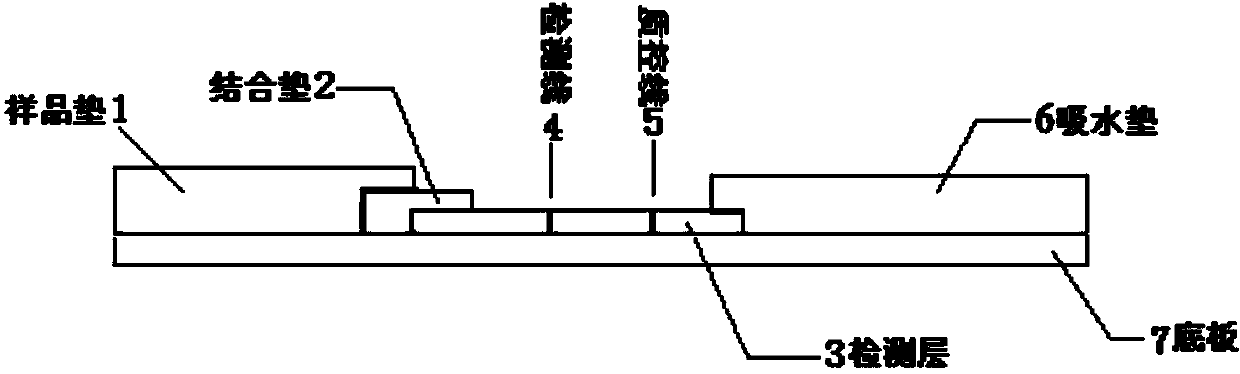
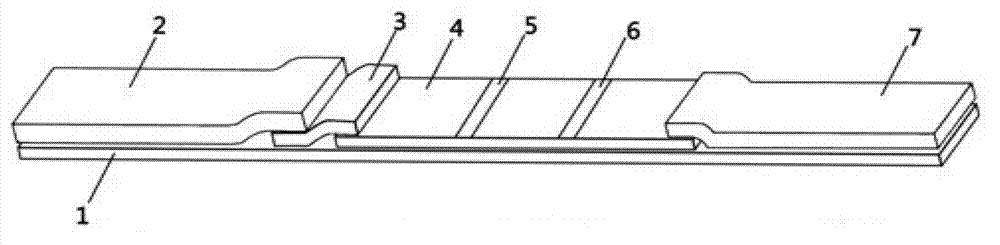
The invention provides a human mycoplasma pneumoniae gold-marked silver-stained immunochromatographic assay kit and a preparation method and application thereof. The assay kit comprises a detection card and a silver-stained sensitivity-enhanced pad, wherein the detection card is composed of a bottom plate, a sample pad, an absorbent pad, a conjugate pad and a detection layer; the conjugate pad is coated with a colloidal gold-marked polyclonal antibody mixture of colloidal gold marked rabbit anti-human mycoplasma pneumoniae P1 protein and P30 protein; the detection layer is composed of a solid phase nitrocellulose membrane with a detection line and a quality control line; the detection layer is bonded on the bottom plate, the conjugate pad and the absorbent pad are partially overlapped with the detection layer respectively and are bonded with the detection layer and the bottom plate respectively; the sample pad and the conjugate pad are partially overlapped to be bonded with the conjugate pad and the bottom plate respectively; and the silver-stained sensitivity-enhanced pad consists of a AgNO3 pad and a restoring pad. The human mycoplasma pneumoniae gold-marked silver-stained immunochromatographic assay kit can effectively improve the detection sensitivity of the human mycoplasma pneumoniae, has the strong specificity and has the high application value in the aspects of clinical diagnosis of human mycoplasma pneumoniae, etiology identification, epidemiological investigation and the like.
Owner:HUBEI UNIV OF TECH +1
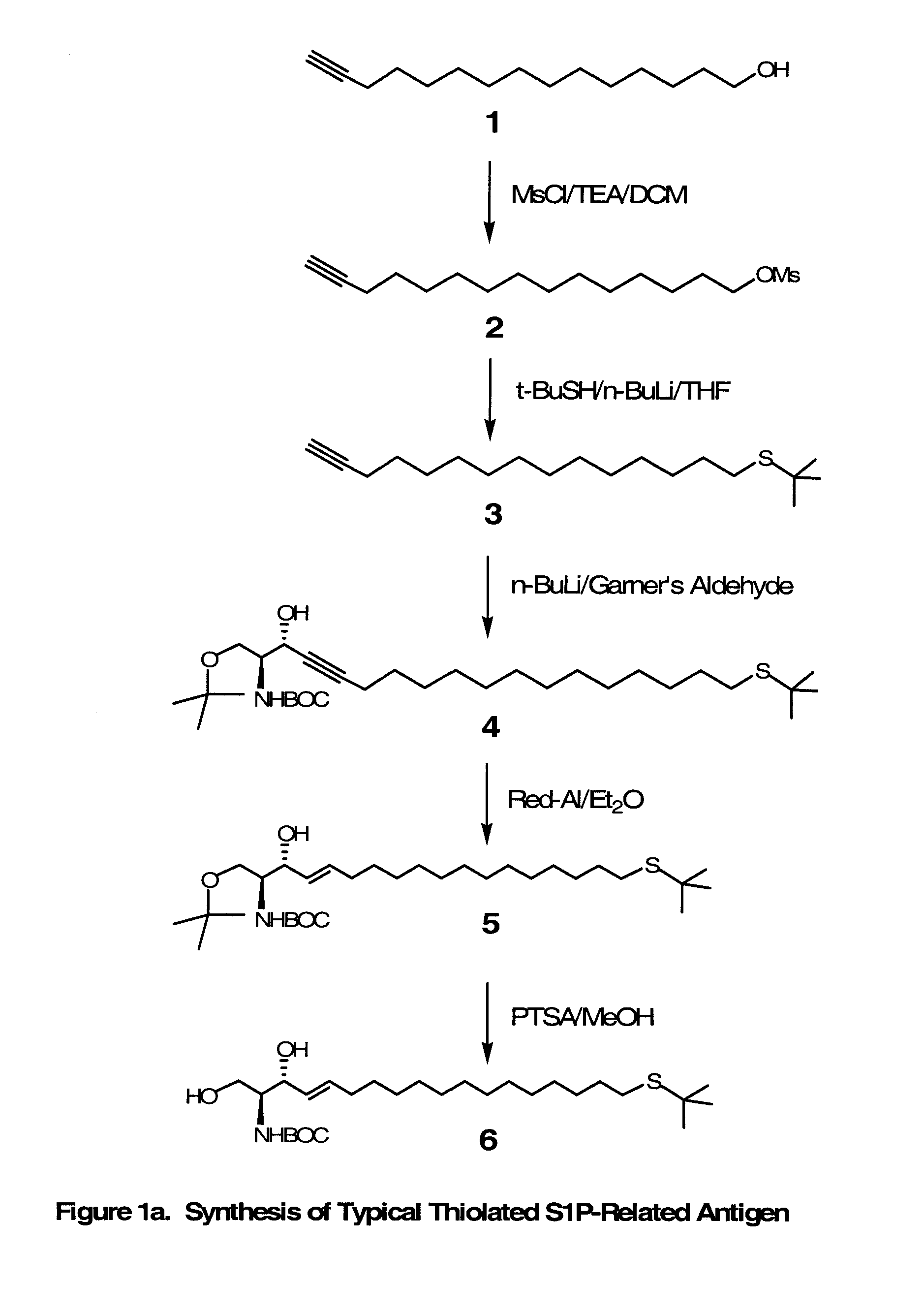
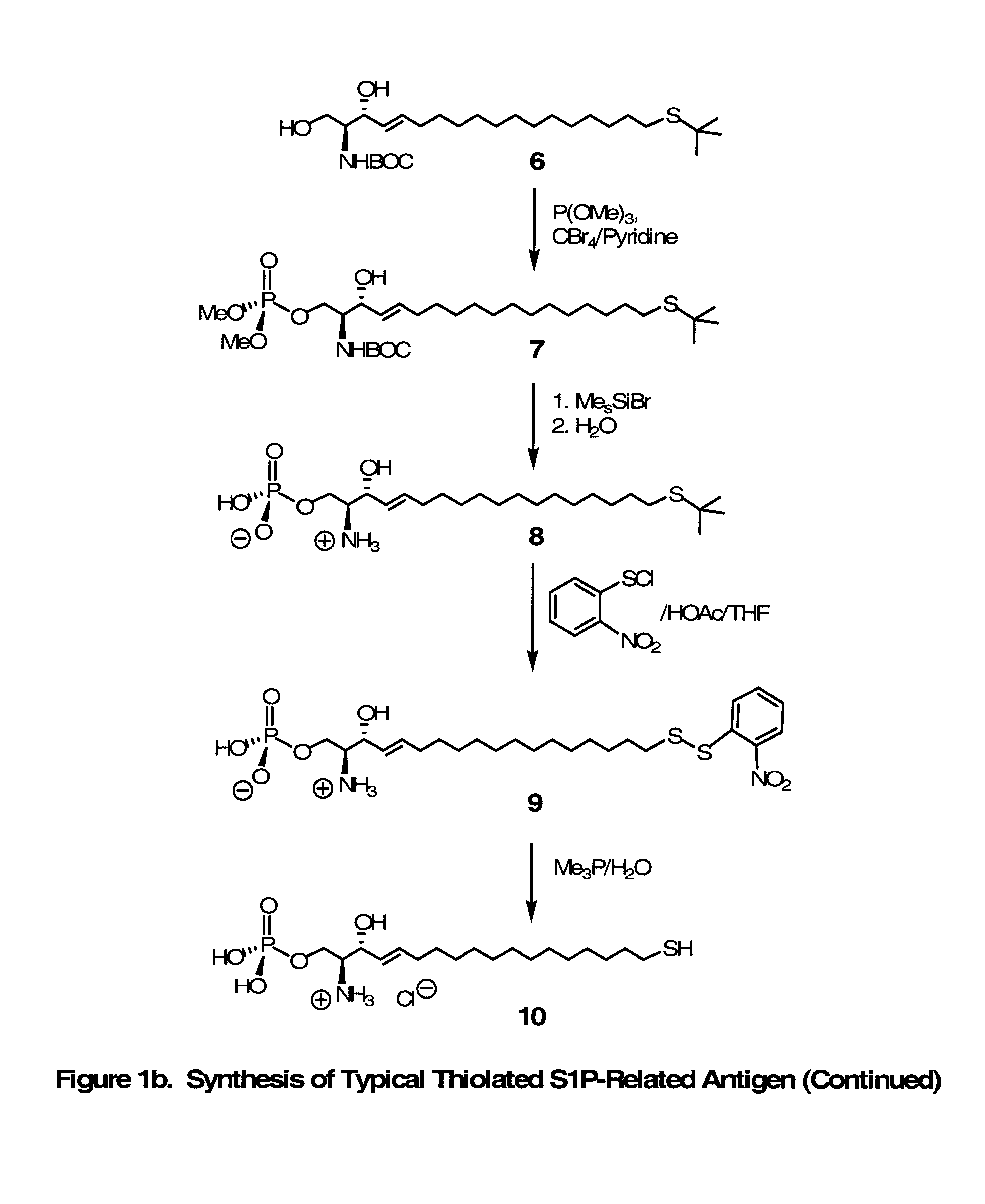
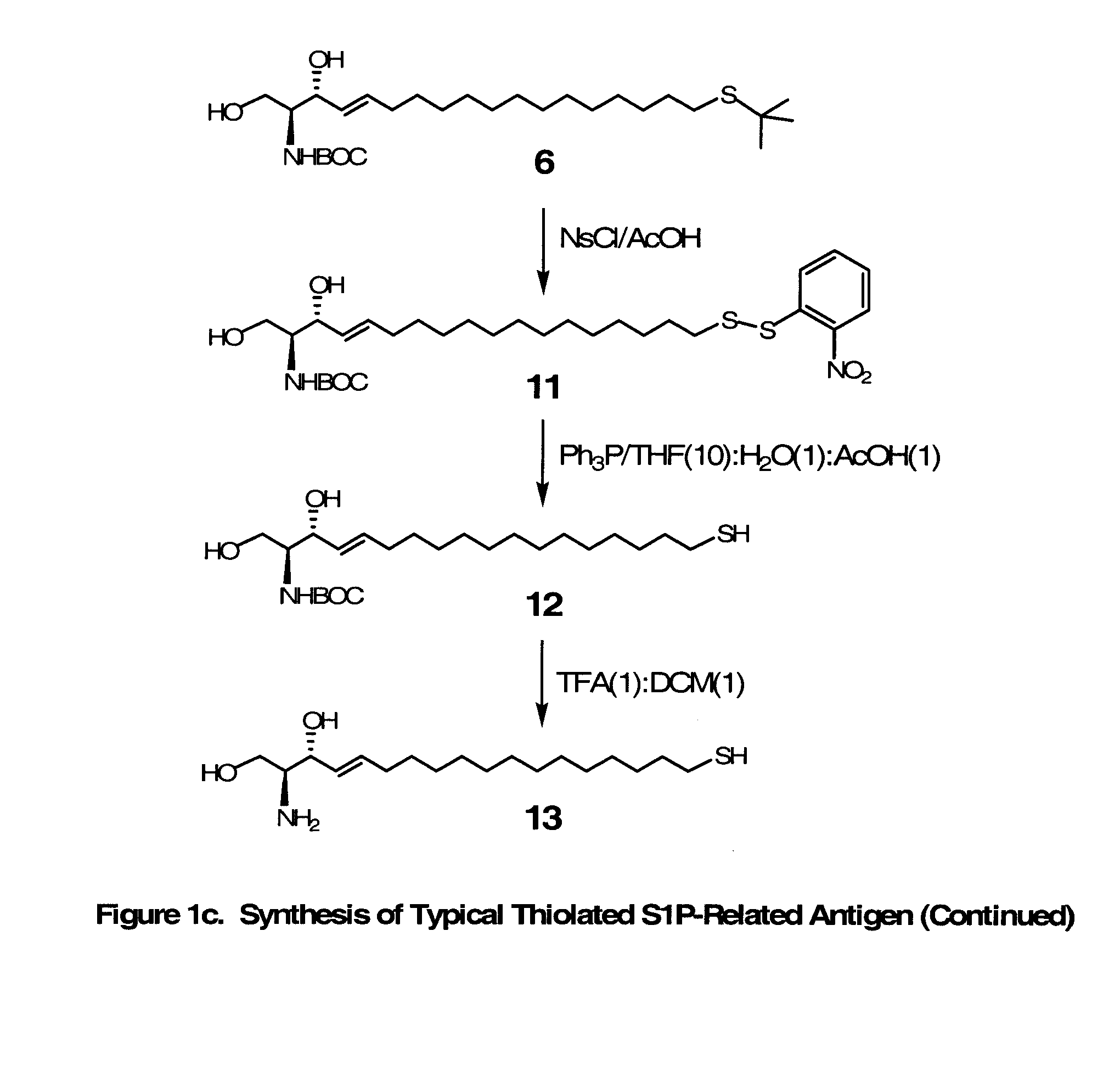
Novel Bioactive Lipid Derivatives, and Methods of Making and Using Same
InactiveUS20070281320A1Minimize the possibilityImmunoglobulins against animals/humansCulture processPolyethylene glycolCarrier protein
Compositions and methods for producing monoclonal antibodies and their derivatives reactive against bioactive lipid targets are described. These compositions include derivatized lipids, each of which comprises a bioactive lipid that having a polar head group and at least one hydrocarbon chain (e.g., a lysolipid such as lysophosphatidic acid or sphingosine-1-phosphate) in which a carbon atom has been derivatized with a pendant reactive group; immunogens made by linking a derivatized lipid to a carrier moiety (e.g., a carrier protein, polyethylene glycol, colloidal gold, alginate, or a silicone bead); monoclonal antibodies and derivatives produced by immunizing an animal with such an immunogen; and therapeutic and diagnostic compositions containing such antibodies and antibody derivatives. Methods for making such derivatized lipids, immunogens, and monoclonal antibodies and derivatives, methods for detecting such antibodies once generated, and therapeutic and diagnostic methods for using such antibodies and derivatives, are also described.
Owner:APOLLO ENDOSURGERY INC
Method for highly sensitive quantitative detection of quantum dot fluorescence immunochromatographic assay
ActiveCN102520165ASensitive quantitative detection fastRealize detectionMaterial analysisCritical illnessLinear range
The invention discloses a method for highly sensitive quantitative detection of quantum dot fluorescence immunochromatographic assay. The method includes: building a fluorescence immunochromatographic assay test strip on the basis of optimizing the structure of the test strip and components by the aid of excellent fluorescent characteristics of quantum dots and by means of combining quantum dot fluorescence labeling technology and immunochromatographic assay; detecting fluorescence signal strength of a quantitative belt and a quality control belt by the aid of a fluorescence quantometer and correcting the fluorescence strength of the quantitative belt by the aid of the quality control belt after immunochromatographic assay of the test strip; and further quantitatively detecting analyte according to a standard curve obtained by the fluorescence quantometer. The method is simple, rapid, accurate, low in cost and quite high in sensitivity. Compared with a conventional colloidal gold immunochromatographic assay method, the method has the advantages of fine labeling stability, low non-specificity, high sensitivity, wide linear range and accuracy in quantization. The method is applicable to samples such as blood samples, urine samples, spittle, excrement and the like, and can be applied to detection of critical illness, poison, food safety and the like.
Owner:BEIJING KANGMEI TIANHONG BIOTECH
Test paper reading method and test paper reading device
InactiveCN104198482ASuitable for popular promotion and useEasy to operateMaterial analysis by observing effect on chemical indicatorPesticide residueTest card
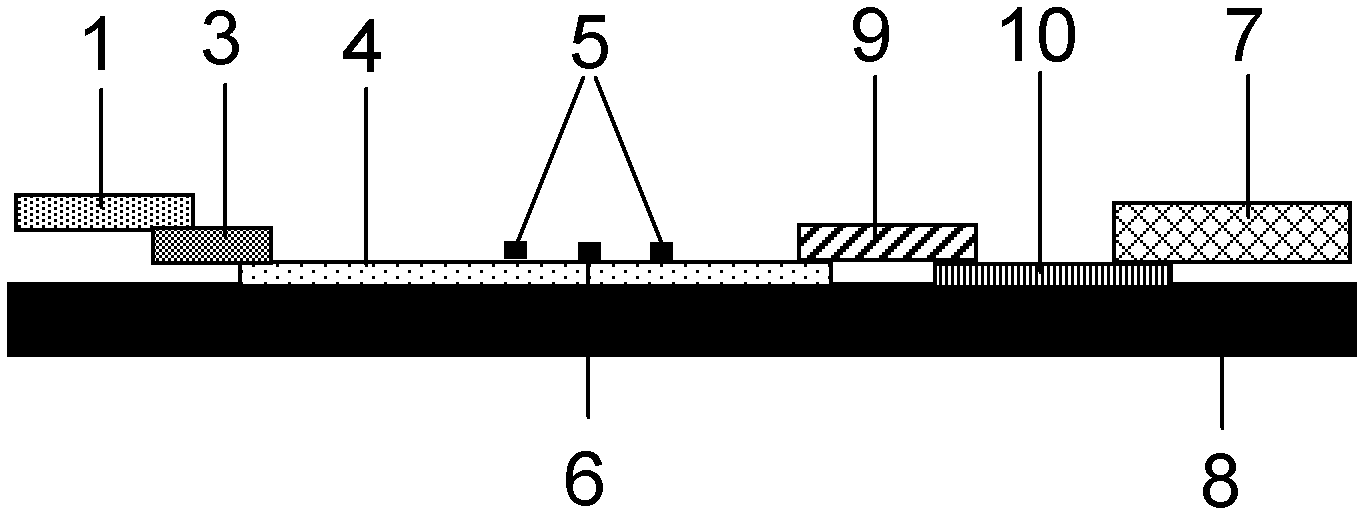
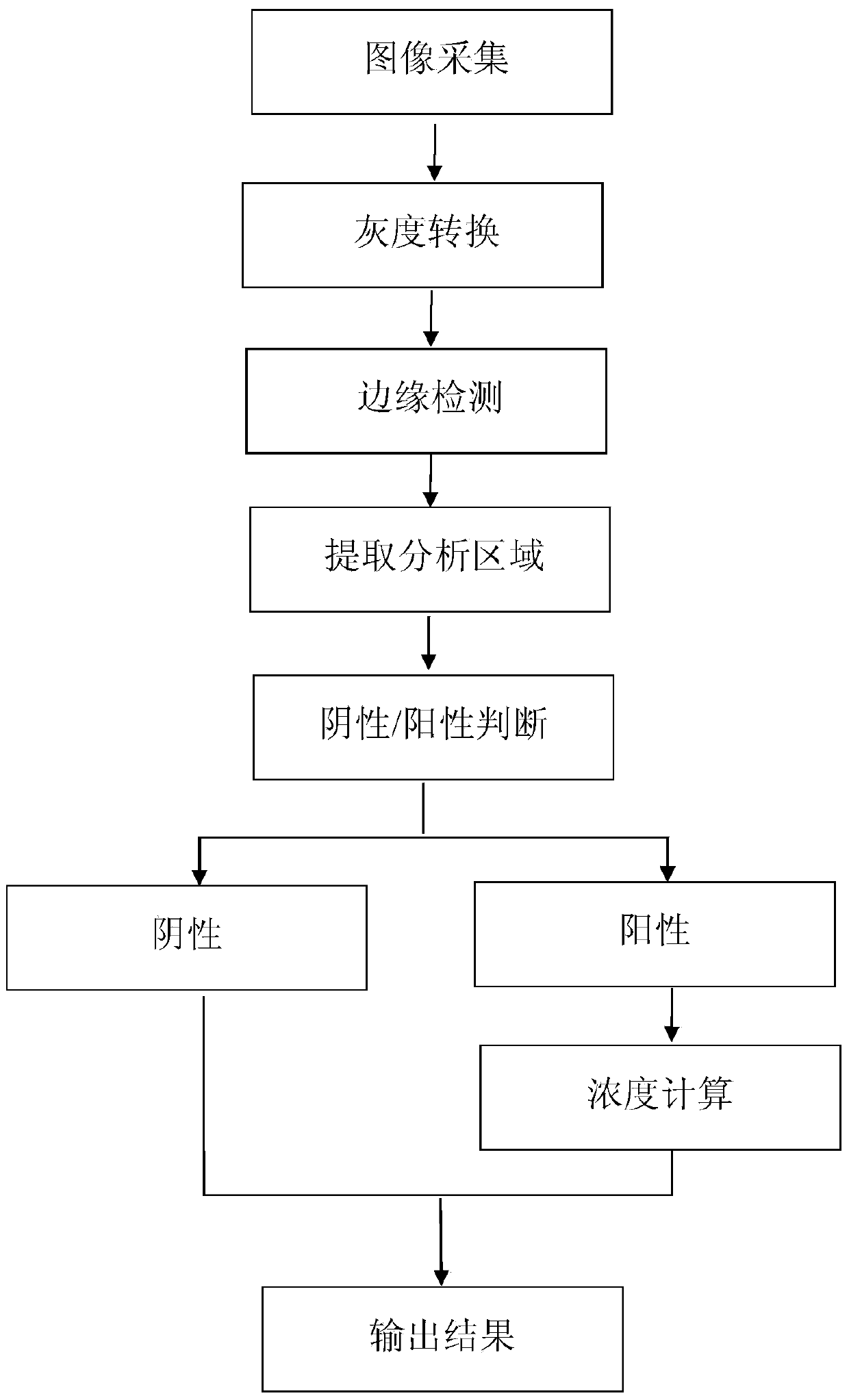
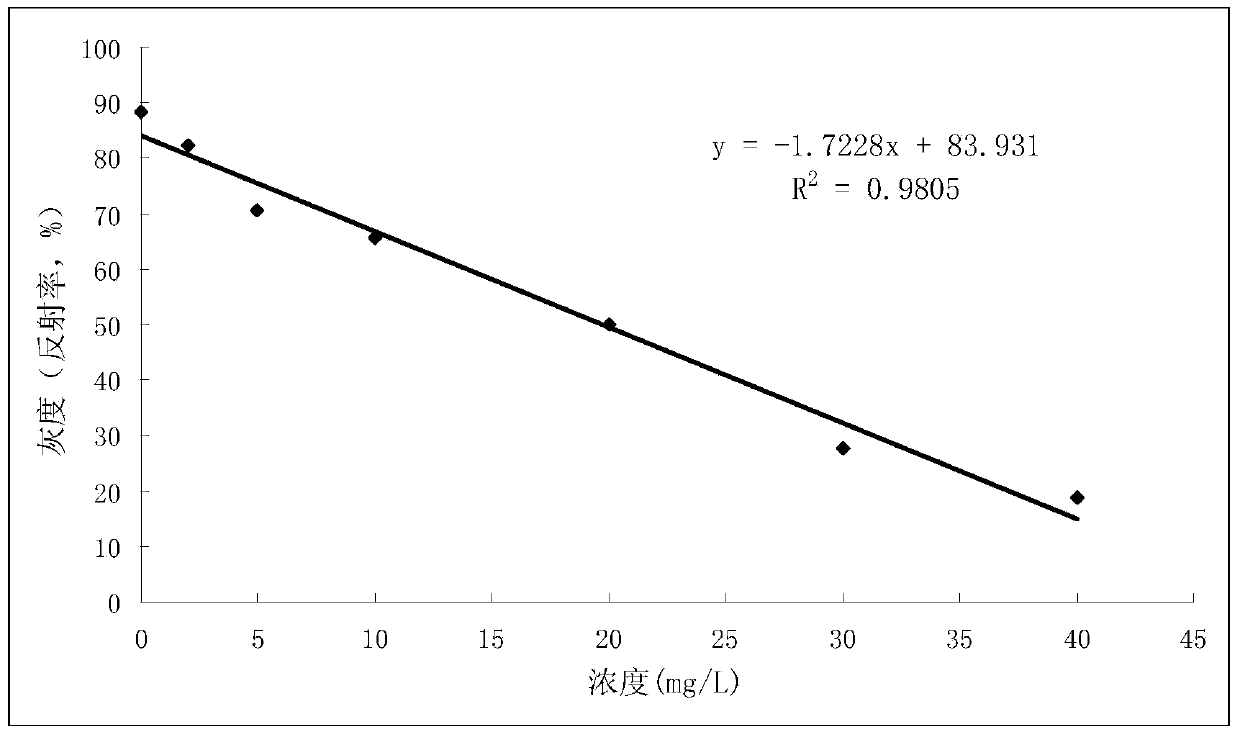
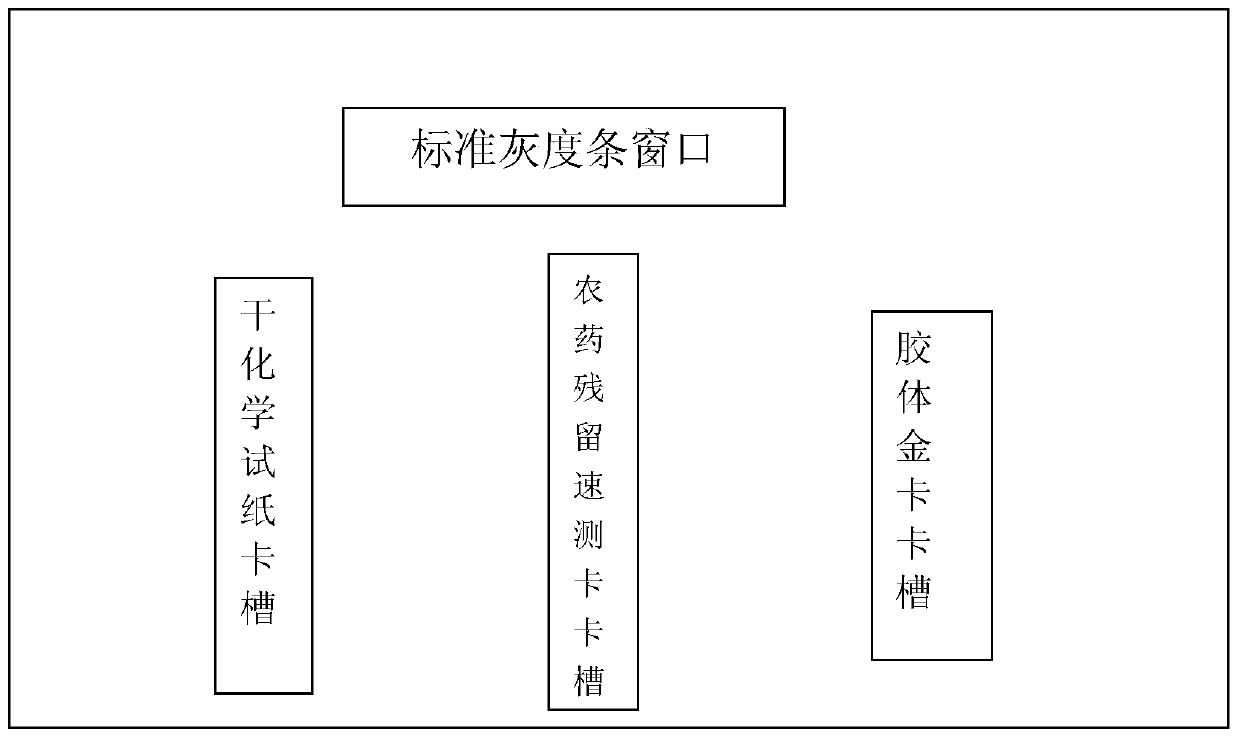
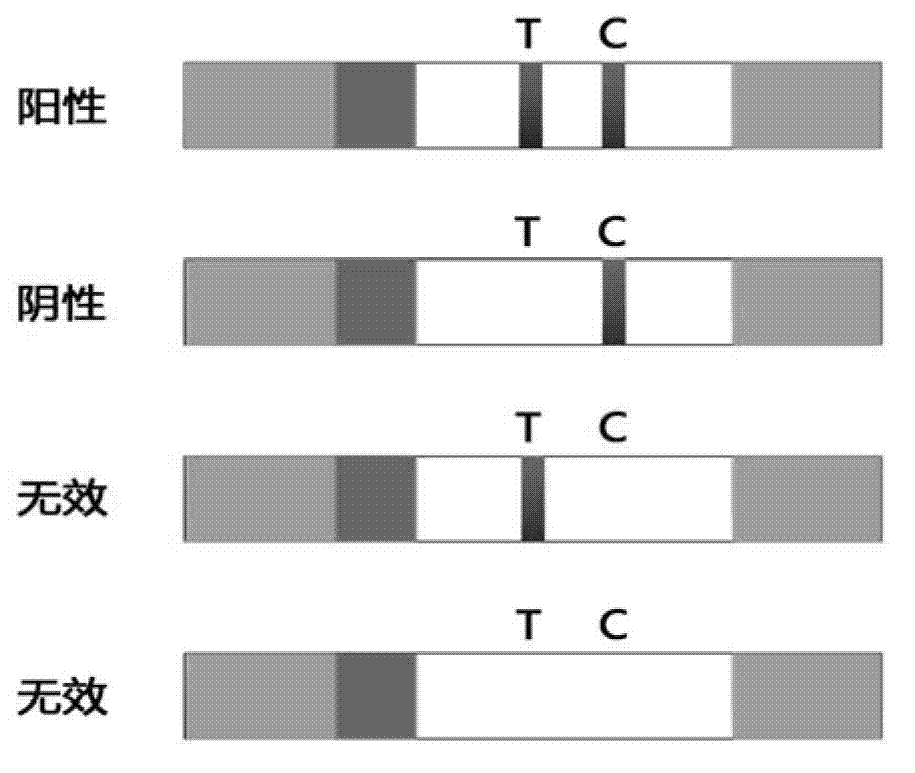
The invention provides a test paper reading method and a test paper reading device, which can read dry chemical test paper strips, colloidal gold immunochromatograohic assay test paper strips / cards and rapid pesticide residue test cards, are easy to operate, need no extra detection instrument and equipment, can automatically judge and read the detection result by only acquiring corresponding developing pictures through electronic equipment such as computers or mobile phones, have reliable testing accuracy and are particularly suitable for personal and household wide application. The method comprises the following steps: A. acquiring images; B. processing the acquired images; C. performing negative and positive discrimination; and D. performing concentration conversion on a sample which is discriminated to be positive in the step C through a concentration-gray scale relation to obtain a concentration value of the to-be-tested paper card / strip. The device comprises a shell and a built-in standard gray scale strip, wherein the shell is provided with a standard gray scale strip display window and is also provided with a first card slot, a second card slot and a third card slot for arranging the colloidal gold card, the rapid pesticide residue test card and the dry chemical test paper respectively.
Owner:BEIJING ZHIYUNDA TECH CO LTD
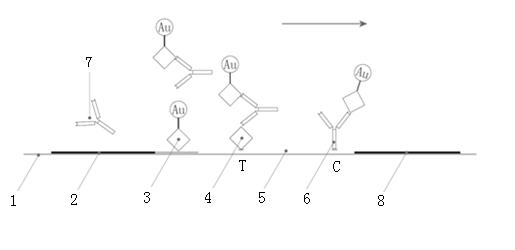
Immune chromatograph testing strip and production thereof
An immune chromatographic test bar consists of supporter, drinking fibrous membrance, nitrate fibrous membrance, sample membrance, waterproof membrance and colloidal gold labeled membrance, Its preparing method utilizes colloidal gold labeling technique and quantitative immune competition method to produce test bar for being used in quick detection.
Owner:李人
Fluorescence proximity assay
InactiveUS20030059850A1Easy to operateEasy to useMicrobiological testing/measurementBiological testingColloidFluorophore
The present invention provides binding assays, referred to here as fluorescence proximity assays or FPA. The inventions detect binding of target molecules in a sample to a molecular probe or probes that specifically bind or hybridize to those molecules. In particular, the molecular probes are immobilized to a bead or particle, such as colloidal gold, the reflects fluorescent energy from a fluorophore. The derivatized beads are contacted to a sample of fluorescently labeled target molecules, and binding of the target is indicated by an increase in the fluorescent signal. Kits are also provided that contain materials and reagents to performing a fluorescence proximity assay.
Owner:STANLEY MEDICAL RES HLDG
Milk allergen test plate and preparation method thereof
ActiveCN102636650AReduce medical costsSave medical resourcesBiological testingBeta lactoglobulin AbImmune complex deposition
The invention discloses a milk allergen test plate, which belongs to the gold immunochromatography detection. According to the milk allergen test plate disclosed by the invention, two ends of a PVC (polrvinyl chloride) base plate are respectively provided with a to-be-tested sample zone and an absorption zone. Colloidal gold mark antigens prepared by respectively marking colloidal gold into casein, beta lactoglobulin and alpha lactalbumin and mixing are orderly arranged between the sample zone to be tested and the absorption zone, and a nitrocellulose membrane is respectively provided with a detection zone coated with mixed milk allergen and a quality control zone coated with anti-beta lactoglobulin antibodies. In detection, color lines are formed in the detection zone and the quality contol zone when an immune complex is formed by specific milk antibodies contained in samples. If the samples do not contain specific milk antibodies, the detection zone does not display color, and only one color line is formed in the quality control zone. The milk allergen test plate disclosed by the invention with the design has the advantages of strong pertinence to the allergen detection, simplicity in operation, low cost and high sensitivity and the like, and can prevent the phenomenon of missed diagnosis in the single antibody detection. The milk allergen test plate is applied for rapidly screening patient allergic to milk, and is especially suitable for being used by primary medical treatment units.
Owner:江苏迈源生物科技有限公司
Full-scale C-reactive protein (CRP) colloidal gold immunoturbidimetric assay kit
The invention provides a full-scale CRP colloidal gold immunoturbidimetric assay kit. A quantitative assay purpose is reached through enhancing the turbidity by the colloidal gold. The method used by the kit provided by the invention solves a problem that the generation of a precipitate after a latex reinforced immunoturbidimetric reaction goes against biochemical instrument cleaning. The kit provided in the invention comprises a reagent R1 and a reagent R2, wherein the reagent R2 is a proper buffer solution; and the reagent R2 is a buffer solution of the colloidal gold combined with an antihuman CRP antibody. The kit has the characteristics of high sensitivity, strong specificity and good stability, can be used for assaying the content of the CRP in serum or blood plasma, and is suitable for clinical fully-automatic biochemical analyzers. The CRP assay sensitivity of the kit can reach 0.01mg / L, and the upper CRP assay limitation of the kit is 500mg / L.
Owner:重庆沃康生物科技有限公司
Diagnostic testing process and apparatus
InactiveUS20050124077A1High detection sensitivityReduce sample volumeBioreactor/fermenter combinationsBiological substance pretreatmentsAnalyteMembrane configuration
A method and apparatus for use in a flow through assay process is disclosed. The method is characterised by a “pre-incubation step” in which the sample which is to be analysed, (typically for the presence of a particular protein), and a detection analyte (typically an antibody bound to colloidal gold or a fluorescent tag) which is known to bind to the particular protein may bind together for a desired period of time. This pre incubation step occurs before the mixture of sample and detection analyte come into contact with a capture analyte bound to a membrane. The provision of the pre-incubation step has the effect of both improving the sensitivity of the assay and reducing the volume of sample required for an assay. An apparatus for carrying out the method is disclosed defining a pre-incubation chamber for receiving the sample and detection analyte having a base defined by a membrane and a second membrane to which a capture analyte is bound. In one version the pre-incubation chamber is supported above the second membrane in one position but can be pushed into contact with the membrane carrying the capture analyte thus permitting fluid transfer from the incubation chamber through the capture membrane. In another version the membrane at the base of the incubation chamber is hydrophobic and its underside contacts the capture membrane and when a wetting agent is applied to the contents of the pre-incubation chamber fluid transfer occurs.
Owner:PROTEOME SYST LTD
Chromatographic detection kit based on aptamer, as well as preparation method and detection method thereof
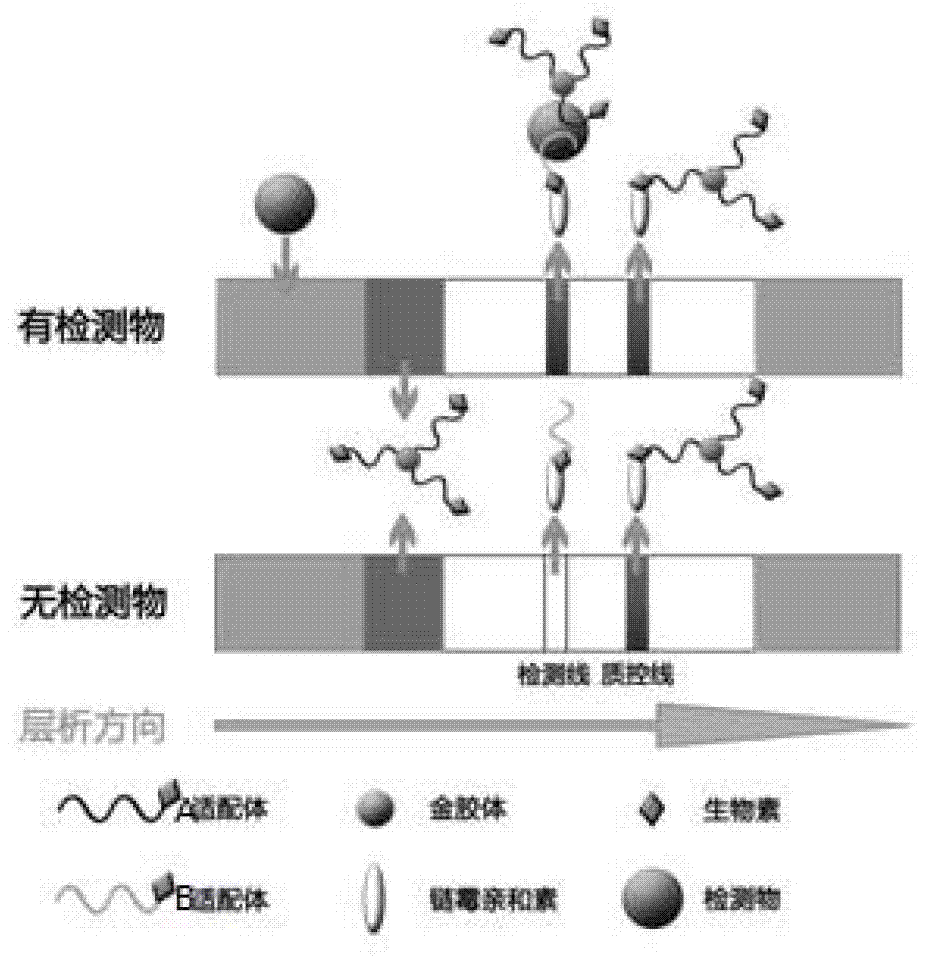
The invention discloses a chromatographic detection kit based on an aptamer, as well as a preparation method and detection method of the kit. The test paper of the kit includes a bottom board, and a sample pad, a bonding pad, a cellulose nitrate film and a water absorption pad, which are adhered on the bottom board and overlap one another; a detection line is arranged on the side of the cellulose nitrate film close to the bonding pad, and a quality control line is arranged on the side of the cellulose nitrate film close to the water absorption pad; an A aptamer labeled by colloidal gold is applied on the bonding pad; a composite containing a B aptamer and streptavidin is applied on the detection line; streptavidin is applied on the quality control line; and both of the A aptamer and the B aptamer are aptamers labeled by biotin and capable of specifically identifying a same test object. The chromatographic detection kit can display the result in five minutes only by directly dropping a diluted sample to be tested into a sample hole, is simple, quick, sensitive, easy to read result, and convenient and simple to prepare, and so on, and has the advantages of simplicity in operation and high specificity without using antibodies and instruments.
Owner:谭蔚泓
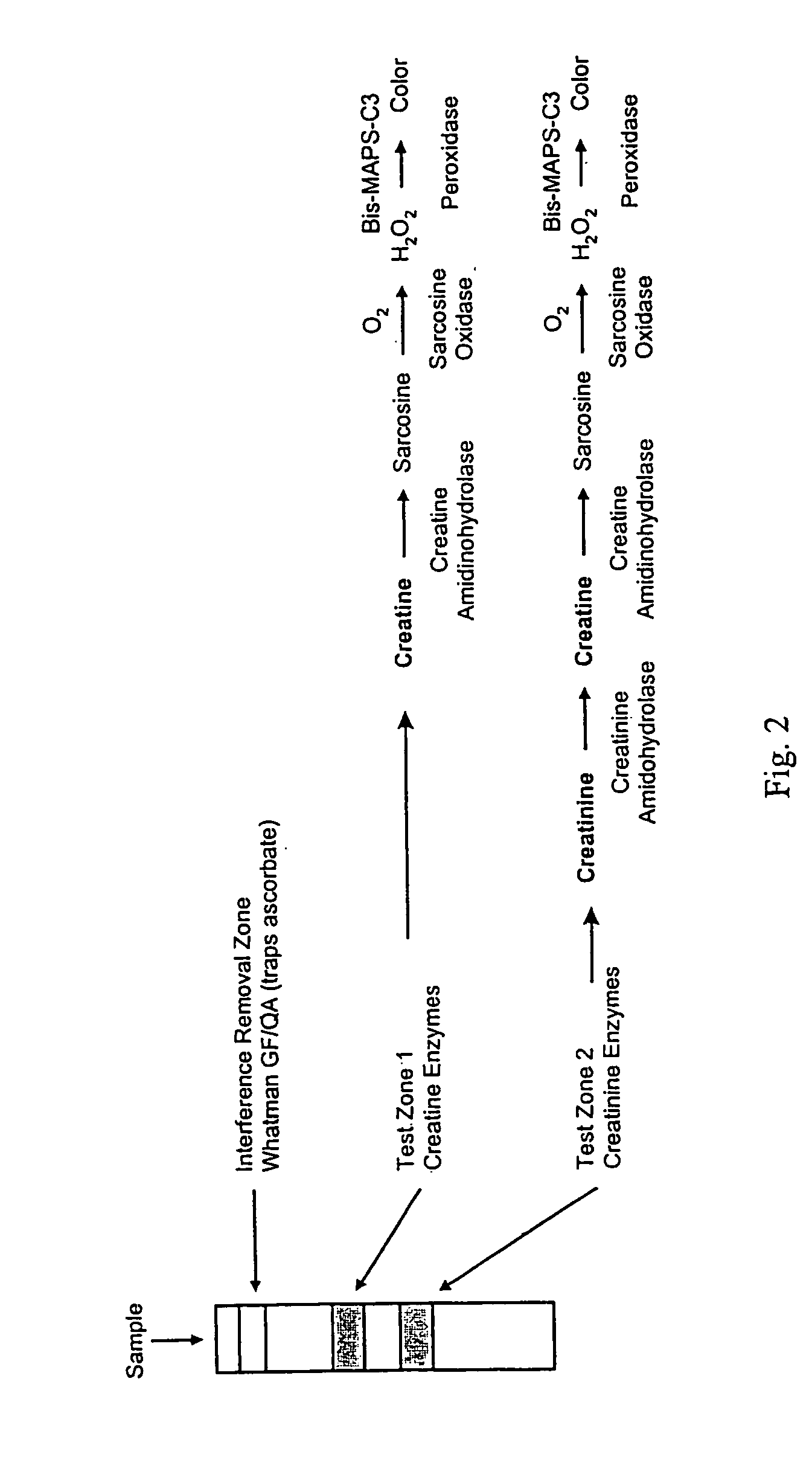
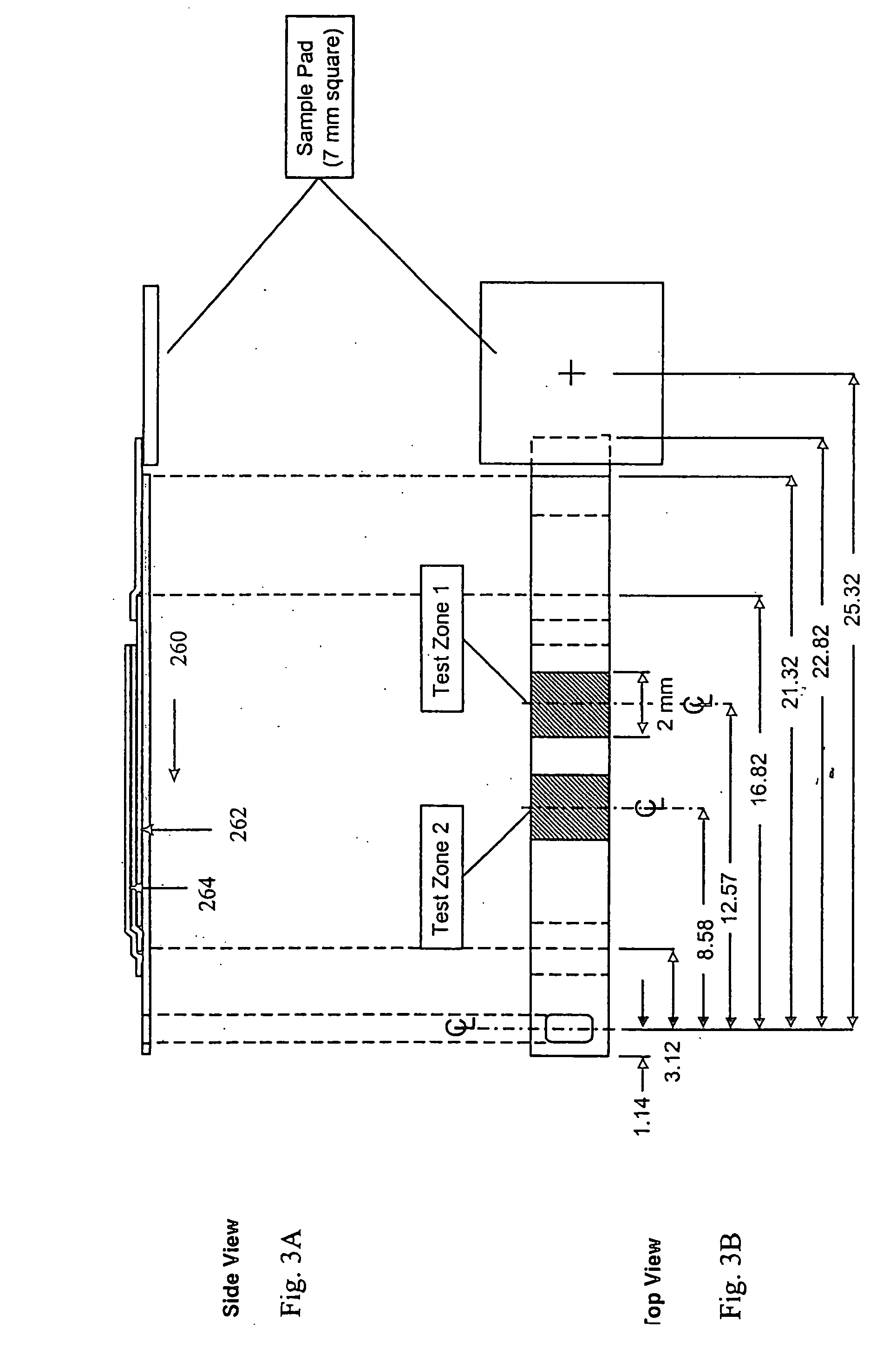
Dry reagent strip configuration, composition and method for multiple analyte determination
InactiveUS20050130293A1Quantitative results are accurateBioreactor/fermenter combinationsBiological substance pretreatmentsReagent stripTransverse axis
An assay device, analytical instrument and assay method for determining the presence or amount of an analyte in a fluid is disclosed. The device is a one-step lateral flow dry reagent immunoassay with one, two or more zones along a transverse axis of the device, each zone can contain or be preceded by diffusively or non-diffusively bound reagents. The invention measures an indicator in one or two test zones for each analyte to determine its presence or concentration. The signal reagent (indicator) may be a particle such as a colored latex or colloidal gold. The assay quantitation may be read by an instrument.
Owner:POLYMER TECH SYST
Diagnostic testing process
InactiveUS20050164404A1High detection sensitivityReduce sample volumeBioreactor/fermenter combinationsBiological substance pretreatmentsAnalyteMembrane configuration
A method and apparatus for use in a flow through assay process is disclosed. The method is characterised by a “pre-incubation step” in which the sample which is to be analysed (typically for the presence of a particular protein), and a detection analyte (typically one or more antibodies bound to colloidal gold or a fluorescent tag) which is known to bind to the particular protein may bind together for a desired period of time. This pre-incubation step occurs before the mixture of sample and detection analyte come into contact with a capture analyte bound to a membrane. The provision of the pre-incubation step has the effect of both improving the sensitivity of the assay and reducing the volume of sample required for an assay. An apparatus for carrying out the method is disclosed defining a pre-incubation chamber for receiving the sample and detection analyte having a base defined by a membrane and a second membrane to which a capture analyte is bound. In one version the pre-incubation chamber is supported above the second membrane in one position but can be pushed into contact with the membrane carrying the capture analyte thus permitting fluid transfer from the incubation chamber through the capture membrane. In another version the membrane at the base of the incubation chamber is hydrophobic and its underside contacts the capture membrane and when a wetting agent is applied to the contents of the pre-incubation chamber fluid transfer occurs.
Owner:PROTEOME SYST LTD
Anti-wrinkle cosmetic composition
ActiveUS20090136595A1Improving dermal fibroblast regulationEasy to superviseHeavy metal active ingredientsCosmetic preparationsWrinkle skinAdditive ingredient
The present invention relates to a composition for treating skin comprising an acylated short chain bioactive peptide and fulvic acid, and optionally colloidal gold. The invention further relates to a method for topically administering the composition in an amount therapeutically effective to reduce wrinkles by building the dermal fibroblast matrix. The invention further relates to a method of treating wrinkled skin by topically administering the composition to an individual in need of such treatment.
Owner:GRANT INDS
Device and method for integrated diagnostics with multiple independent flow paths
InactiveUS20030040021A1Avoid flowHigh sensitivityBioreactor/fermenter combinationsBiological substance pretreatmentsWater insolubleHydrophobic polymer
Devices and methods for performing assays to determine the presence or quantity of a specific analyte of interest in a fluid sample. In devices according to this invention two separate flow paths are established sequentially in the device with a single user activation step. The first flow path delivers the analyte of interest (if present in the sample) and conjugate soluble binding reagents to the solid phase. If analyte is present, an analyte:conjugate complex is formed and immobilized. The volume of sample delivered by this first path is determined by the absorbent capacity of the solid phase, and not by the amount of sample added to the device, relieving the user from the necessity of measuring the sample. The sample / conjugate mixture is prevented from entering the second flow path because the capillarity and the surface energy of the second flow path prevent it from being wetted by this mixture. The second flow path allows a wash reagent to remove unbound conjugate and sample from the solid phase to the absorbant, and optionally to deliver detection reagents. The invention may be adapted to many assay formats including, sandwich immunoassays, colloidal gold, or sol particle assays, heterogeneous generic capture assays and competitive assays. In one embodiment, sandwich assays can be performed by immobilizing an analyte binding reagent on the solid phase, and drying a labeled analyte binding reagent in the first flow path. In a competitive assay embodiment, the first flow path would contain labeled analyte that is dissolved by the sample, and the analyte binding reagent is immobilized on the solid phase. In each of these embodiments, the assay can be further modified to run in a "generic capture" format, where the solid phase binding reagent is instead conjugated to a generic ligand such as biotin, and dried in the first flow path (either together or separately from the other assay reagents), and a generic ligand binding reagent (such as avidin) is immobilized on the solid phase. Another aspect of the present invention includes a subassembly for the immunoassay device that is comprised of a plastic housing and a means for delivering fluid and / or wash solution. This subassembly comprises a structure formed from a hydrophobic polymer selectively treated with a water insoluble surface active agent that has been applied as a solution in an organic solvent rendering portions of the surface hydrophilic. When the surface is contacted with an aqueous liquid, it flows only along the treated areas, creating a defined fluid flow path, thereby delivering sample / conjugate solutions to said solid phase.
Owner:CLARK SCOTT M +4
Stable colloidal gold nanoparticles with controllable surface modification and functionalization
ActiveUS20120225021A1Ultrasonic/sonic/infrasonic diagnosticsMaterial nanotechnologySource materialColloid
In the present invention, a method of producing stable bare colloidal gold nanoparticles is disclosed. The nanoparticles can subsequently be subjected to partial or full surface modification. The method comprises preparation of colloidal gold nanoparticles in a liquid by employing a top-down nanofabrication method using bulk gold as a source material. The surface modification of these nanoparticles is carried out by adding one or multiple types of ligands each containing functional groups which exhibit affinity for gold nanoparticle surfaces to produce the conjugates. Because of the high efficiency and excellent stability of the nanoparticles produced by this method, the fabricated gold nanoparticle conjugates can have surface coverage with functional ligands which can be tuned to be any percent value between 0 and 100%.
Owner:IMRA AMERICA
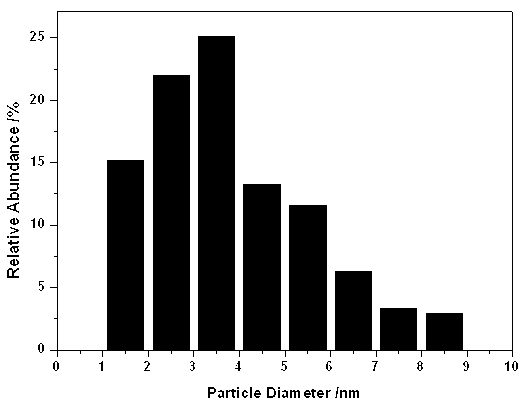
Stable nanometer silver colloidal sol and preparation method thereof
The invention relates to a stable nanometer silver colloidal sol and a preparation method thereof. The stable nanometer silver colloidal sol adopts a silver containing compound, a protective agent and a solvent, water is used as the solvent, and the silver containing compound, the protective agent and the water are mixed so as to prepare the colloidal sol; and the particle diameters of nanometer silver grains are 1nm-10nm, and the nanometer silver grains are uniformly distributed in the colloidal sol, and can be preserved for 6 months to 24 months without changing at room temperature. The preparation method provided by the invention comprises the following steps of: adopting the silver containing compound and the protective agent as raw materials, adopting the water as the solvent, mixing the silver containing compound, the protective agent and the water so as to form a mixed solution which contains 200-10000ppm of silver, 0.02%-1% of protective agent, and the balance of water according to weight percent; and dissolving and mixing the components when the stable nanometer silver colloidal sol is prepared, then uniformly stirring, conducting ultrasonic processing, using a light source containing ultraviolet rays to light 30 minutes to 24 hours to obtain the nanometer silver colloidal sol containing the nanometer silver grains. The stable nanometer silver colloidal sol and the preparation method thereof provided by the invention have the advantages that the cost is low, the process is simple, and the stable nanometer silver colloidal sol is suitable for large-scale preparation.
Owner:WUHAN KANGYIN GAOKE
Colloidal gold immune chromatography test paper for detecting biotoxin and detecting method thereof
ActiveCN101281195AStrong specificityMeet the demand for rapid detection of biotoxin residuesMaterial analysisAntigenBiology
The invention pertains to the biotoxin testing technique field, especially to a colloidal gold immunity chromatography reagent paper for testing biotoxin and testing method thereof. The colloidal gold immunity chromatography reagent paper for testing biotoxin includes a colloidal gold marking mat and testing wires, wherein, the substance covered by the colloidal gold marking mat may be a mixer ofcolloidal gold marking articles of a biotoxin antibody or a plurality of biotoxin antibodies, the number of the testing wires is corresponding to the number of the biotoxin antibodies covered by the colloidal gold marking articles, and each testing wire is cover with a single biotoxin testing antigen corresponding to the colloidal gold marking antibody. The inventive colloidal gold immunity chromatography reagent paper has strong specificity; has convenient and simple operation, which can direct detect milk and animal urine, can test samples such as feed, wheat, corn, barley and the like after simple processing, and can observe result 3-5min later. The inventive method has wide application, can satisfy requirements of food product safety, feed safety as well as fast detecting of biotoxin residual by governmental detection mechanism.
Owner:CHINA JILIANG UNIV +1
Colloidal metal labeled microparticles and methods for producing and using the same
InactiveUS20050158393A1Easy to detectReadily visibleBiocideHeavy metal active ingredientsMicrosphereMicroparticle
The present invention relates to polymeric materials that are labeled with colloidal metals, preferably colloidal gold, to processes for producing the labeled polymeric material, and to methods of using the materials in prophylactic, therapeutic and cosmetic applications. Specifically, the invention relates to porous injectable and implantable microparticles, preferably microspheres, that are associated with colloidal metals such that the microparticles are visible or detectable under regular light, by radiological and / or magnetic resonance imaging techniques, or both. The microparticles having colloidal metals are particularly useful for embolization, dermal augmentation and tissue bulking, drug delivery, gene therapy, and other prophylactic, therapeutic or cosmetic medical applications.
Owner:BIOSPHERE MEDICAL INC
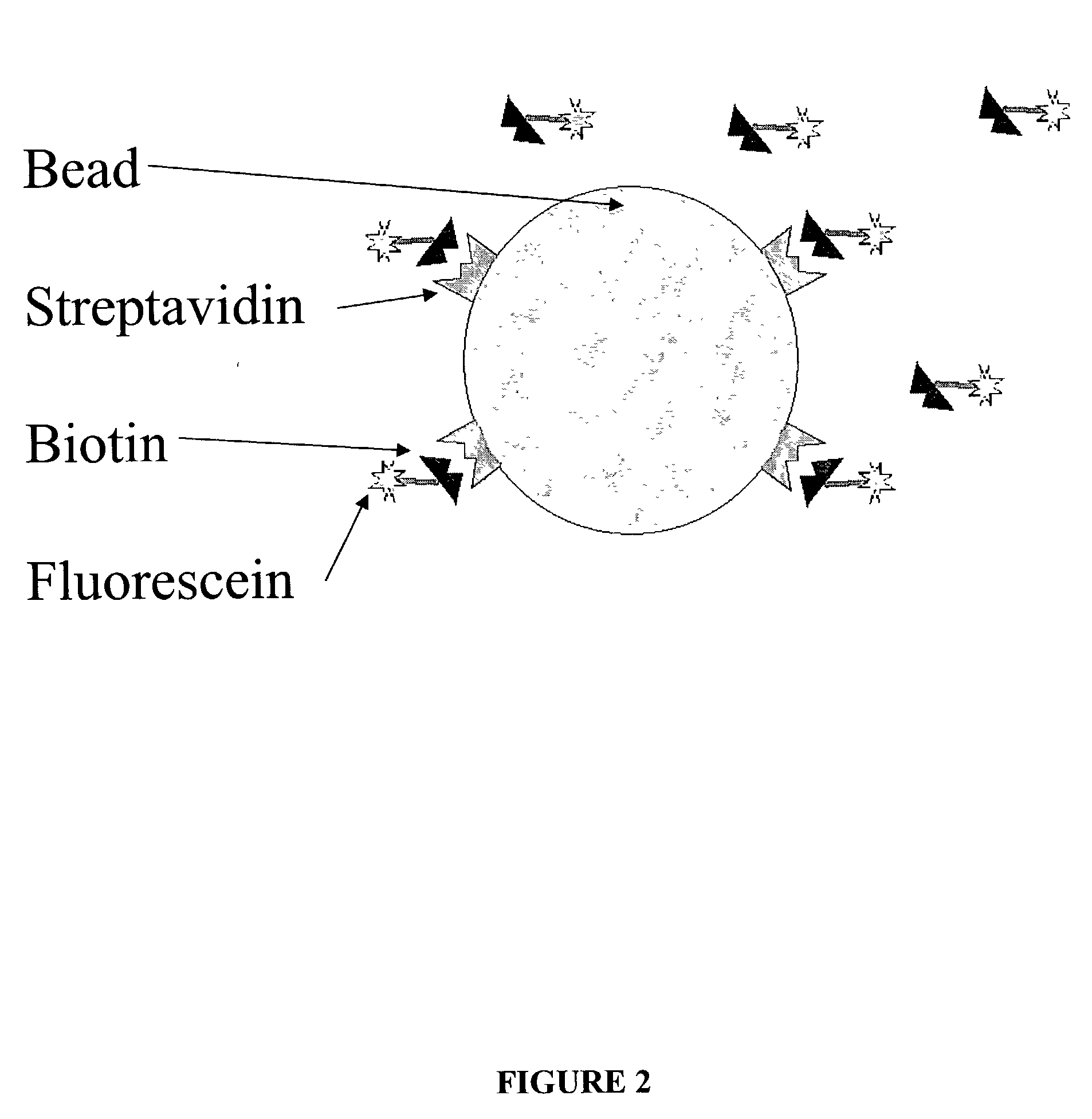
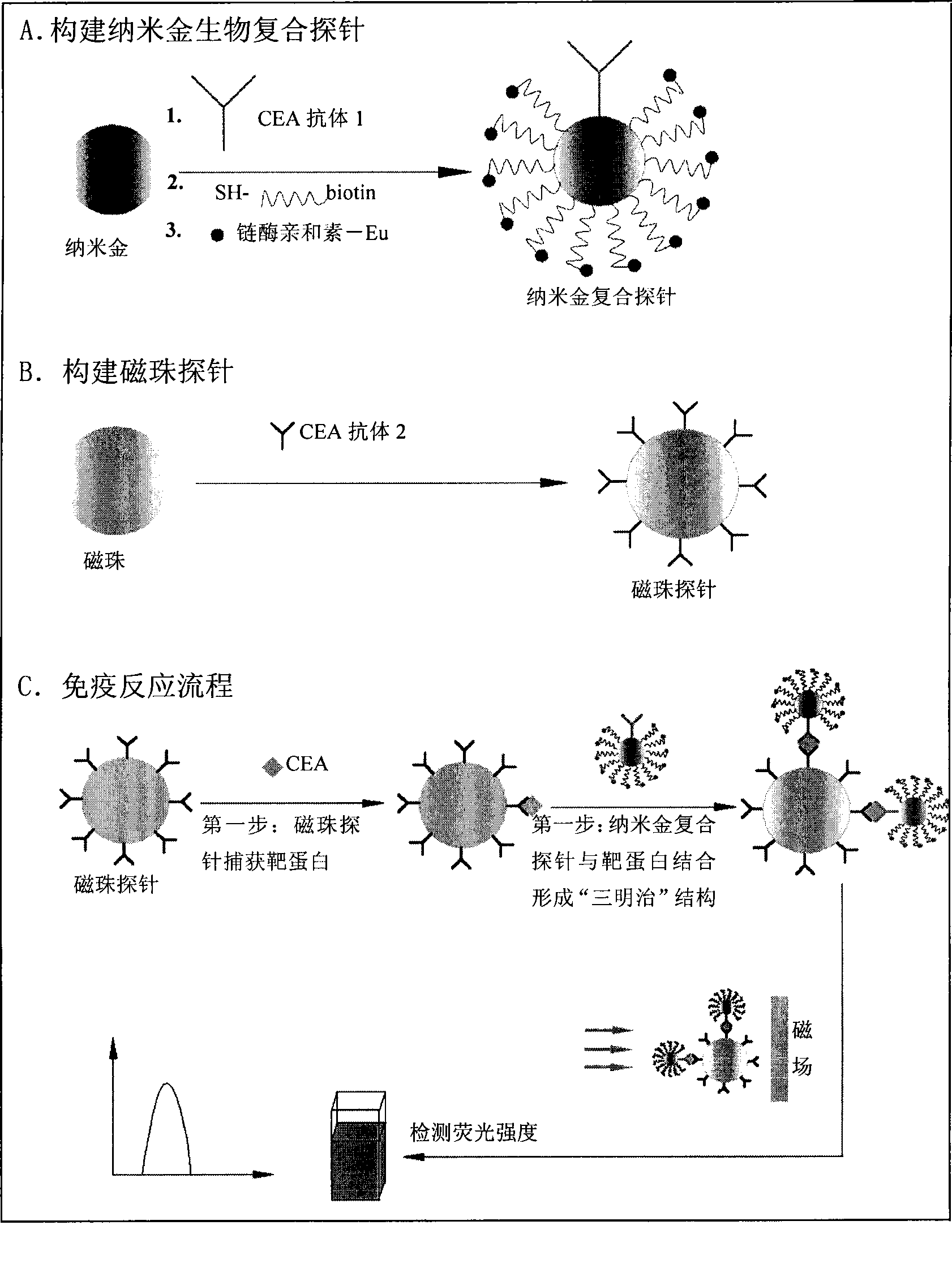
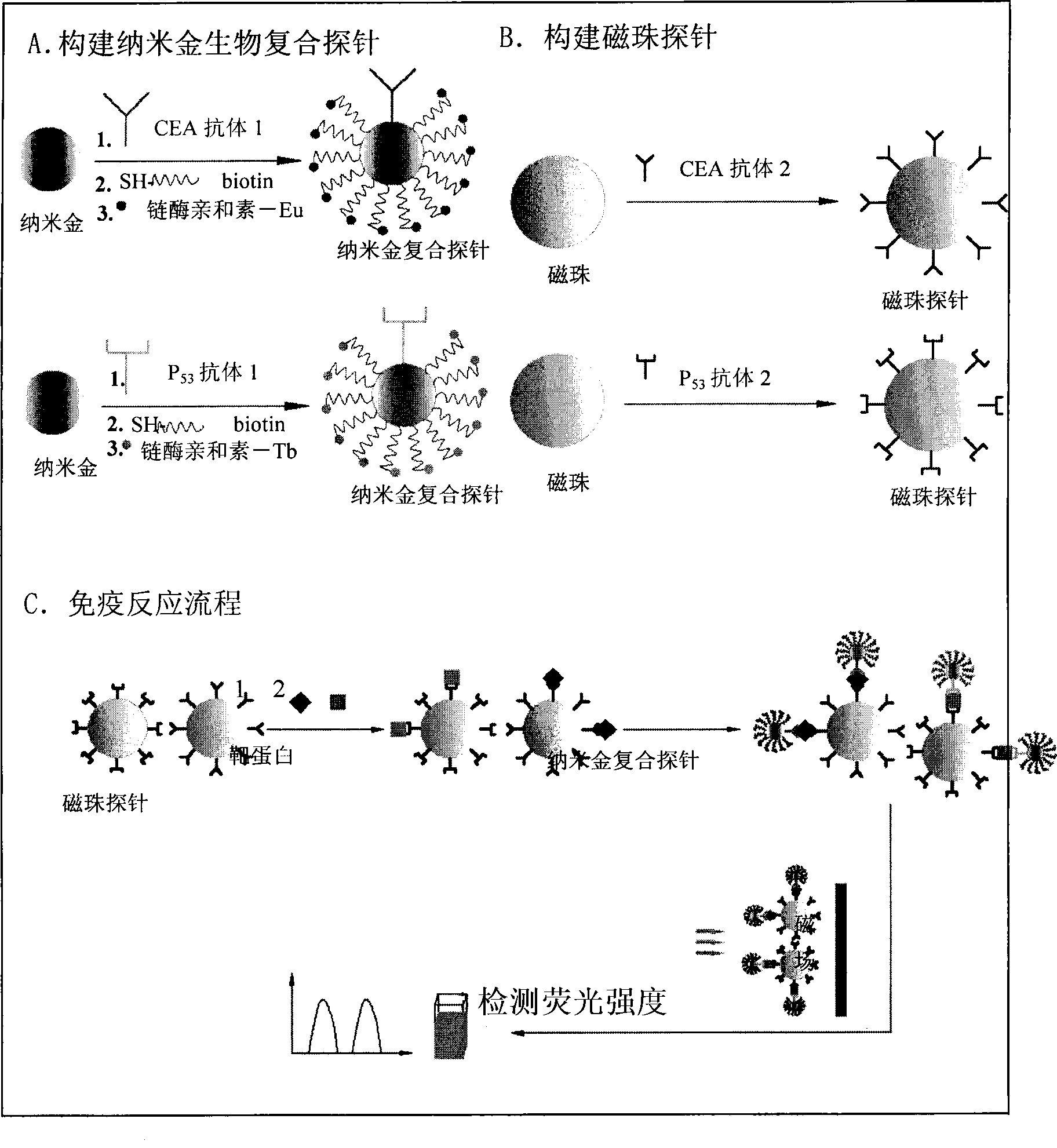
Nano gold biological composite probe, detection method and application thereof
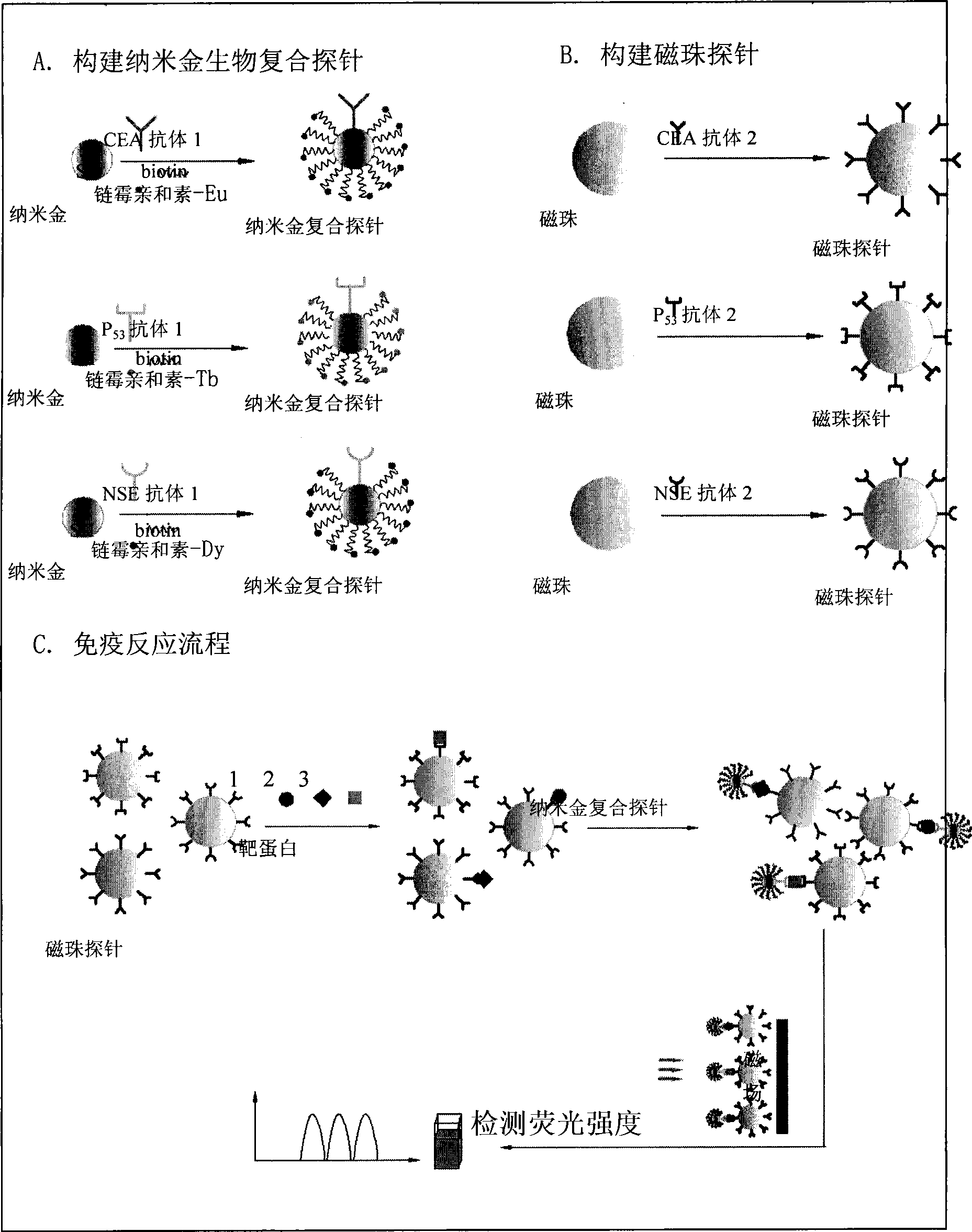
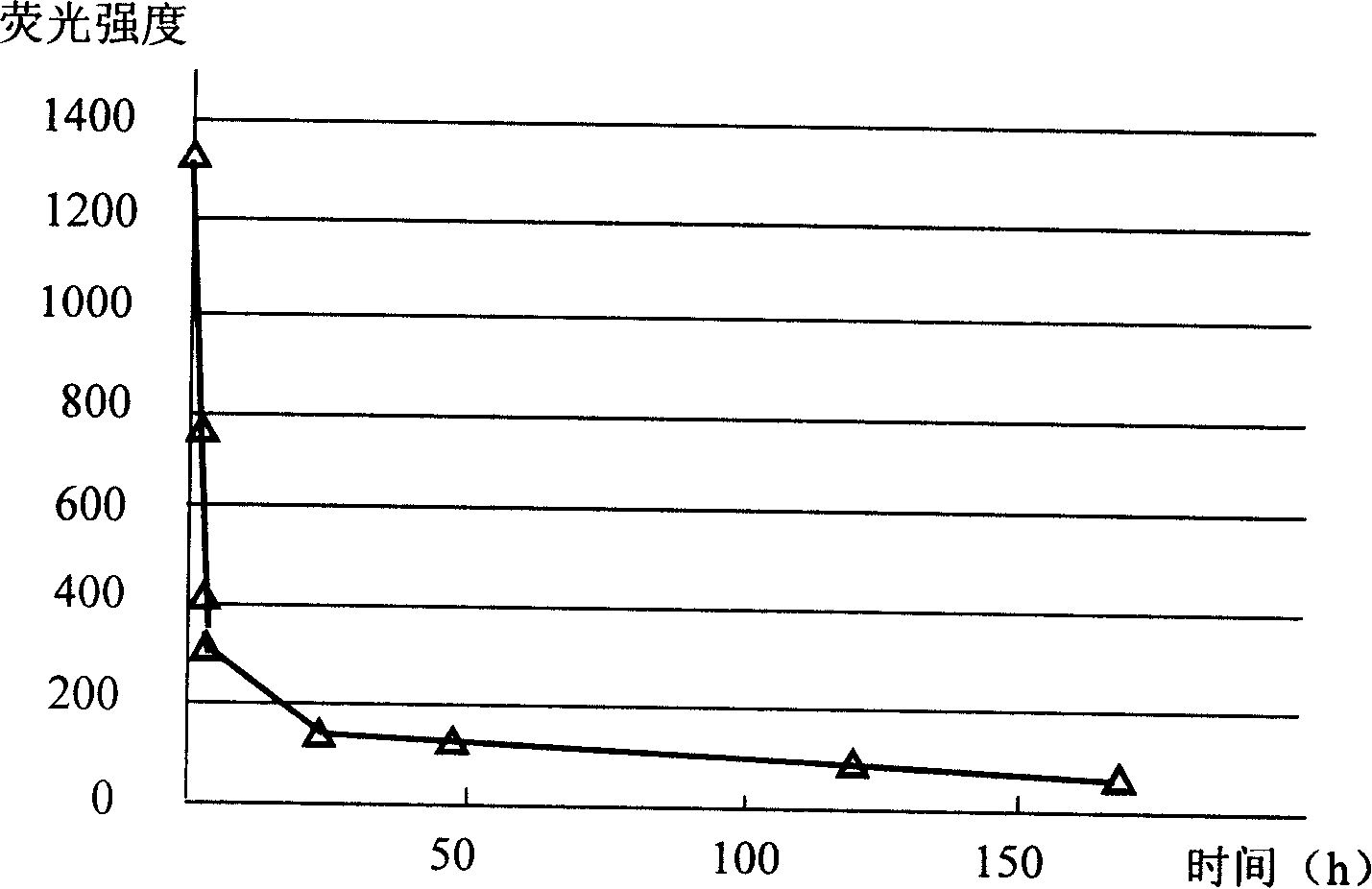
InactiveCN101545007AHigh sensitivityEasy to detectMicrobiological testing/measurementFluorescence/phosphorescenceBiotin-streptavidin complexMagnetic bead
The invention relates to a nano gold biological composite probe, a detection method and application thereof. The detection method is characterized in that: the method comprises the following steps: firstly marking a bead through a monoclonal antibody of a protein to be tested; marking the polyclonal antibody of the protein to be tested on nano gold while and simultaneously marking a DNA probe with a biotin label; carrying out a biotin-streptavidin reaction on the DNA probe on the nano gold to make a lanthanide bonded with colloidal gold to construct the nano gold biological composite probe; mixing the bead of marked monoclonal antibody of the protein to be tested, the nano gold biological composite probe and a protein sample to be tested, carrying out the incubation on the mixture for a certain time at 37 DEG C; cleaning away the nano gold probe which does not react; adding a reinforcing liquid; and determining the fluorescence intensity so as to realize the aim of carrying out the quantitative determination on the protein to be tested. The method obviously improves the detection sensitivity of biomolecules, and is used for detecting a plurality of kinds of biomolecules synchronously. The method is widely applied in the fields of clinical diagnosis, antigen, antibody and nucleic acid detection, health quarantine, environmental tests, and the like.
Owner:SHANGHAI INST OF MICROSYSTEM & INFORMATION TECH CHINESE ACAD OF SCI
Nano compound oxide preparation method
ActiveCN1865151ALarge particle sizeHigh yieldCatalyst carriersCatalyst activation/preparationMolten saltNanometre
This invention discloses a method for producing nanometer composite oxides. The procedures comprise: solubilise the molten substance in the mixture of hydrocarbon component and surfactantto form the molten salt-in-oil super solubilising colloidal group, which limits the hydroxide generated by the reaction of molten substance and precipitant in the colloidal group, so as to avoid the increase of particle size, and then obtain the nanometer composite oxides by burning.This invention is characterized of low cost of surfactant and hydrocarbon component, single distributed colloidal groups, narrow particle size distribution range and high yield. Besides, the process is simple and is adapted for industrial mass production. The nanometer composite oxide produced by this invention has large specific surface area and pore size, which makes it suitable for being used as catalyst carrier.
Owner:CHINA PETROLEUM & CHEM CORP +1
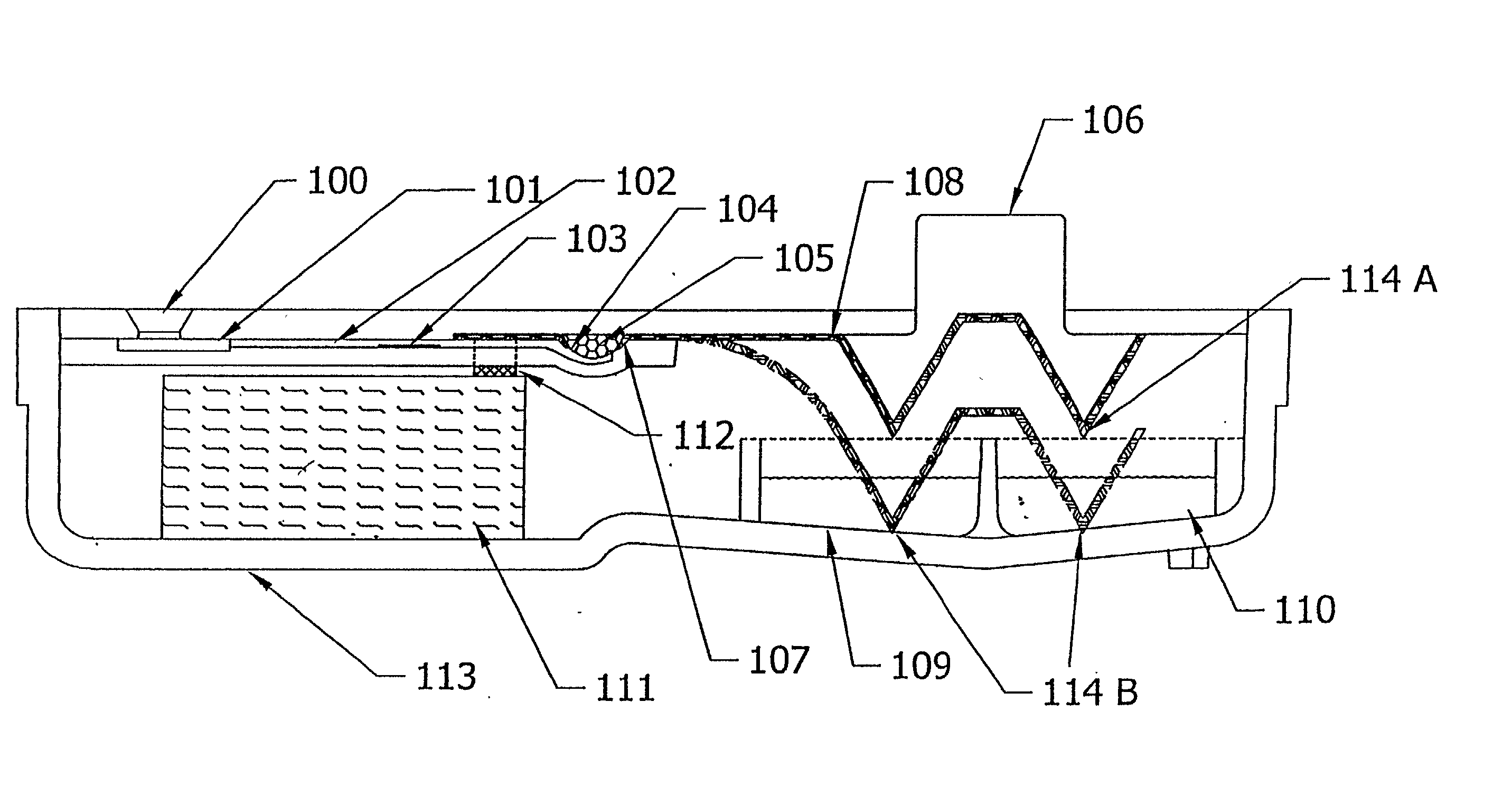
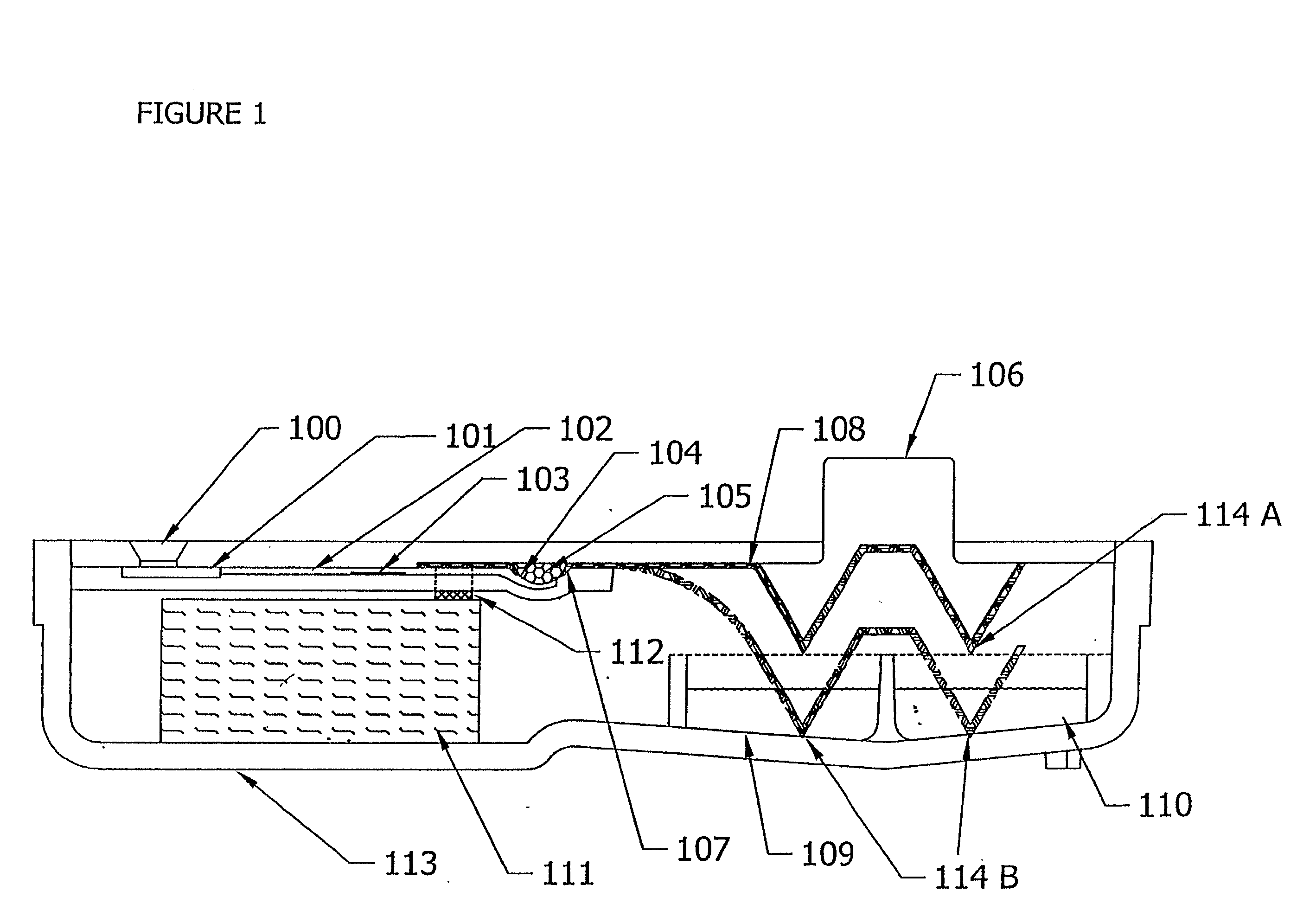
Diagnostic Testing Process and Apparatus Incorporating Controlled Sample Flow
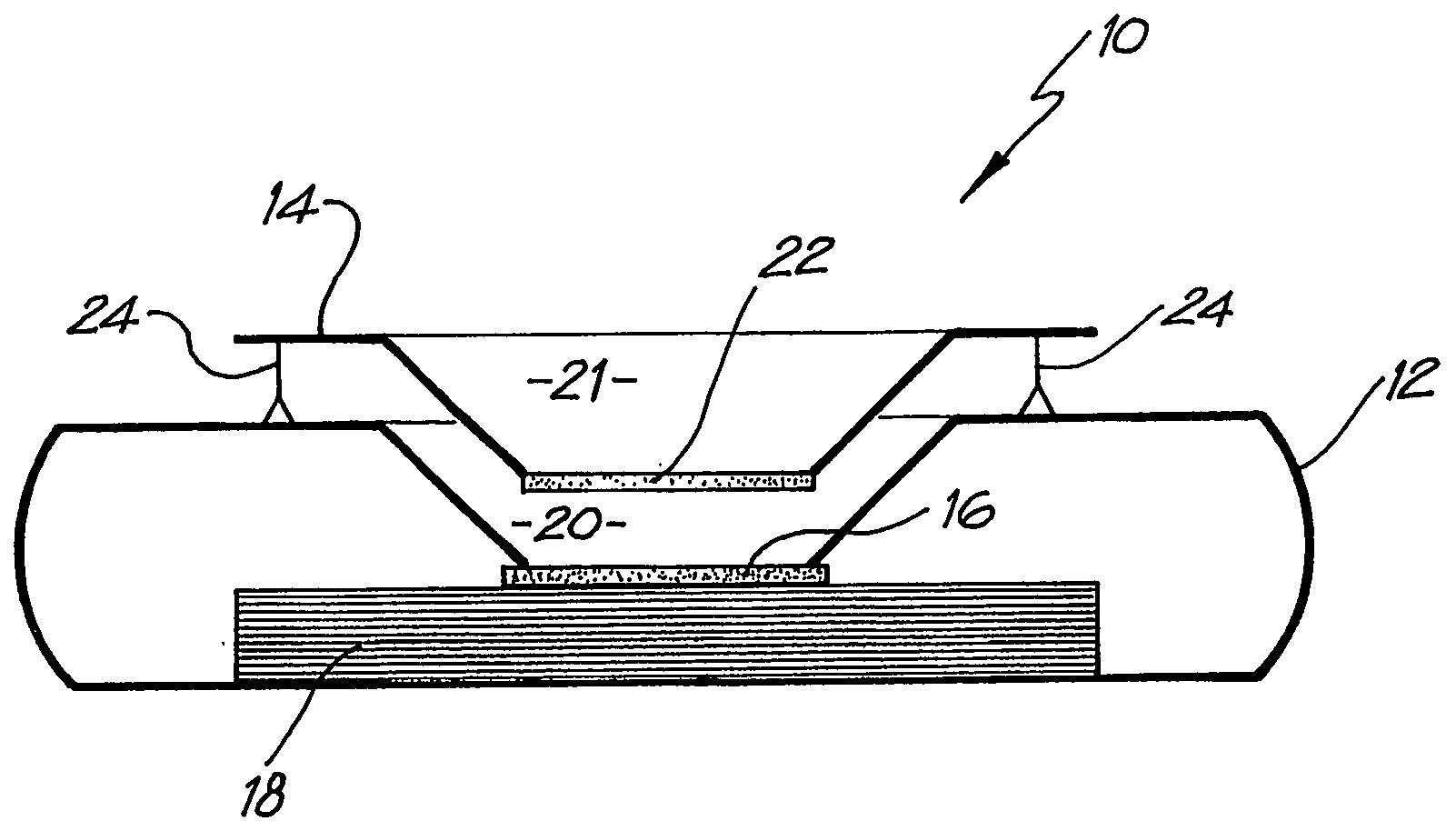
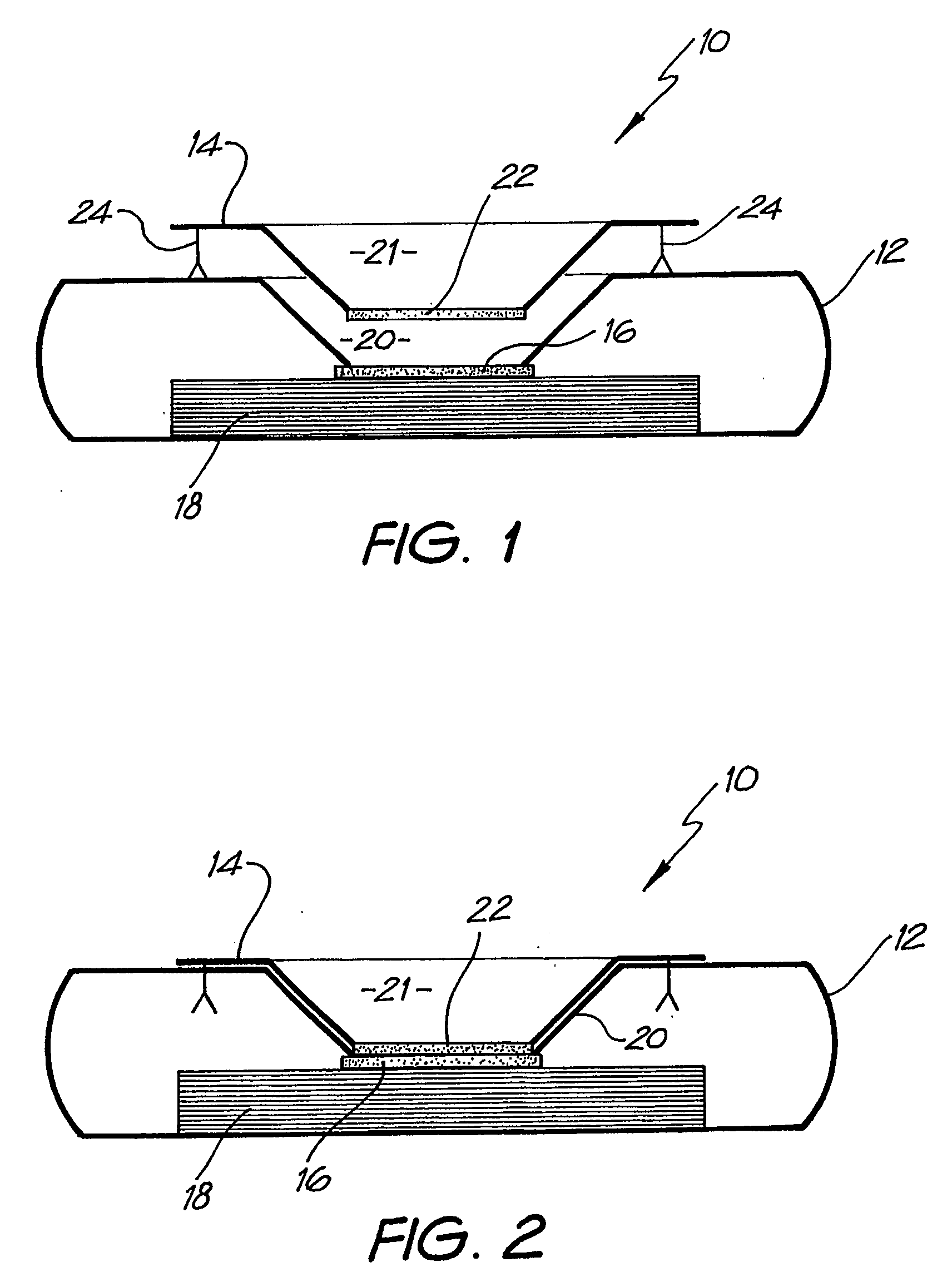
InactiveUS20080318342A1Avoid accumulationPrevents wickingLaboratory glasswaresBiological testingActuatorPiston
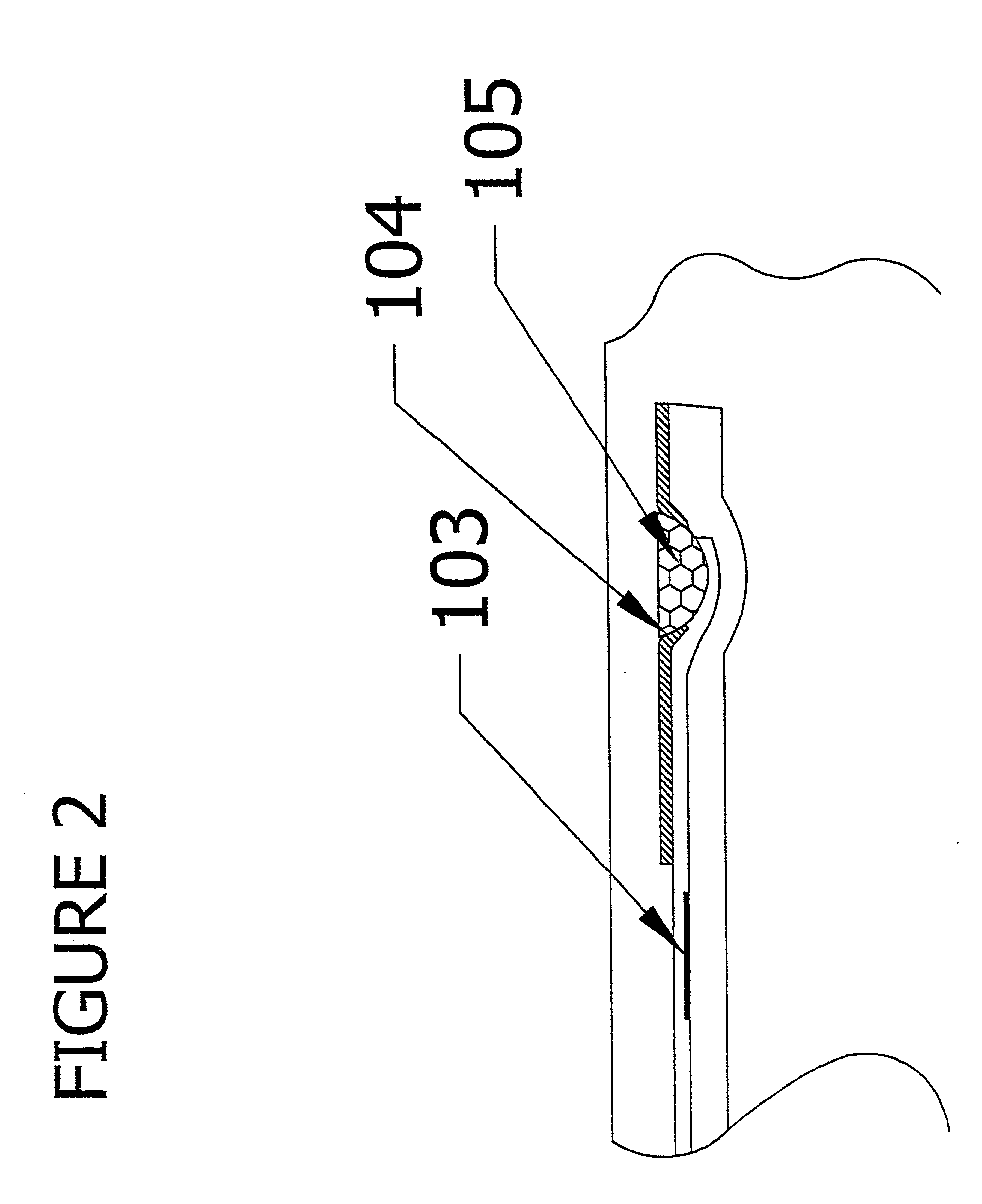
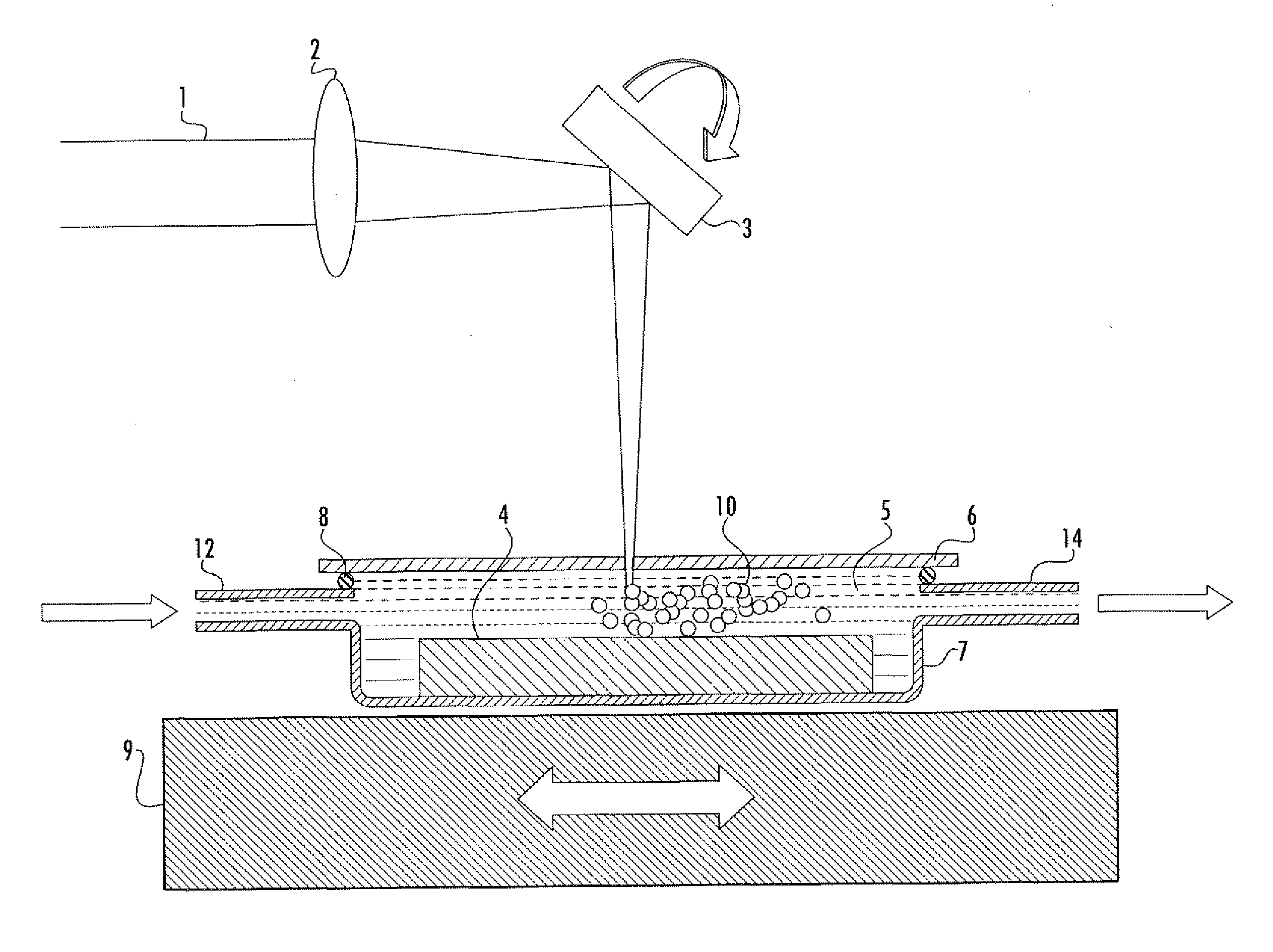
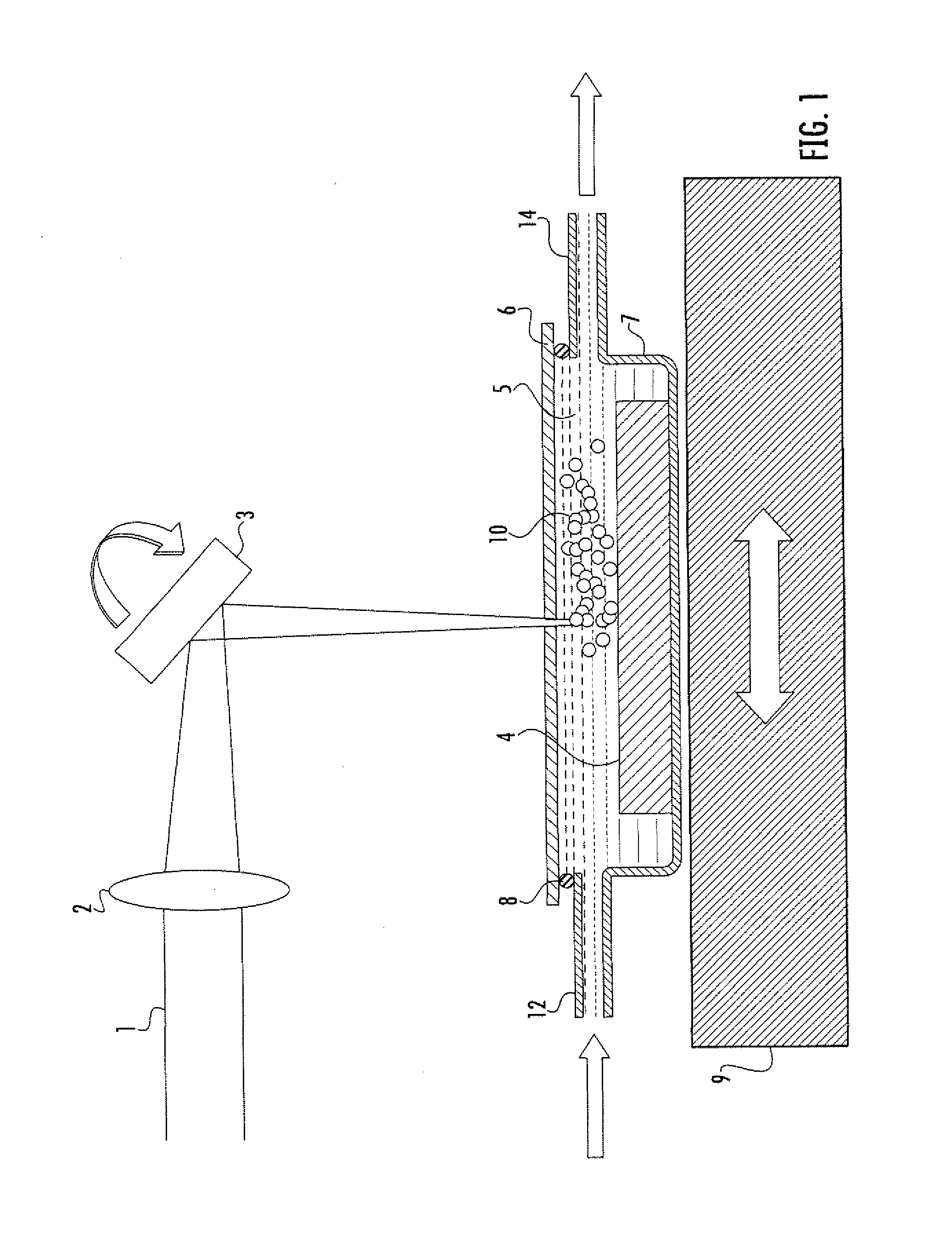
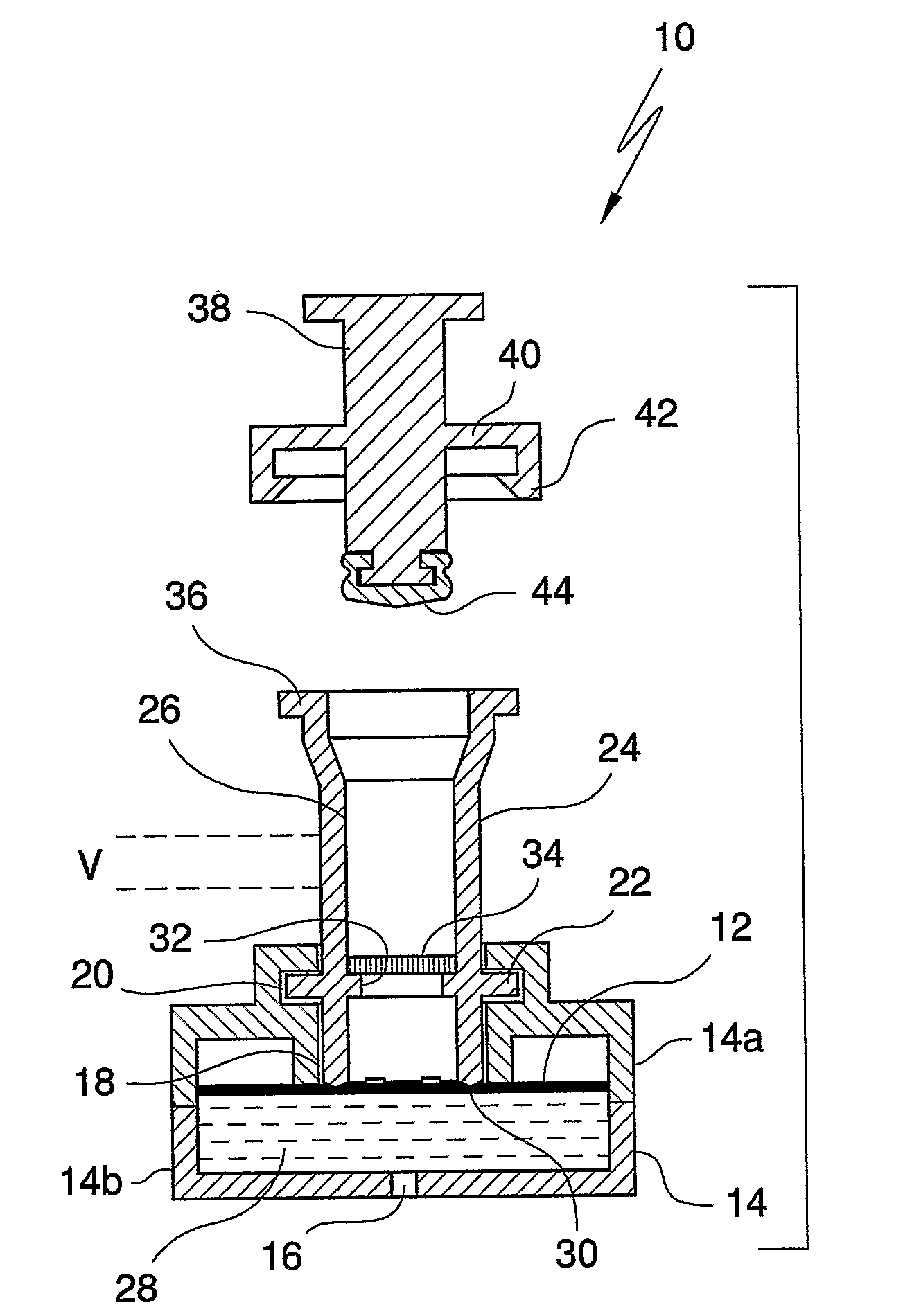
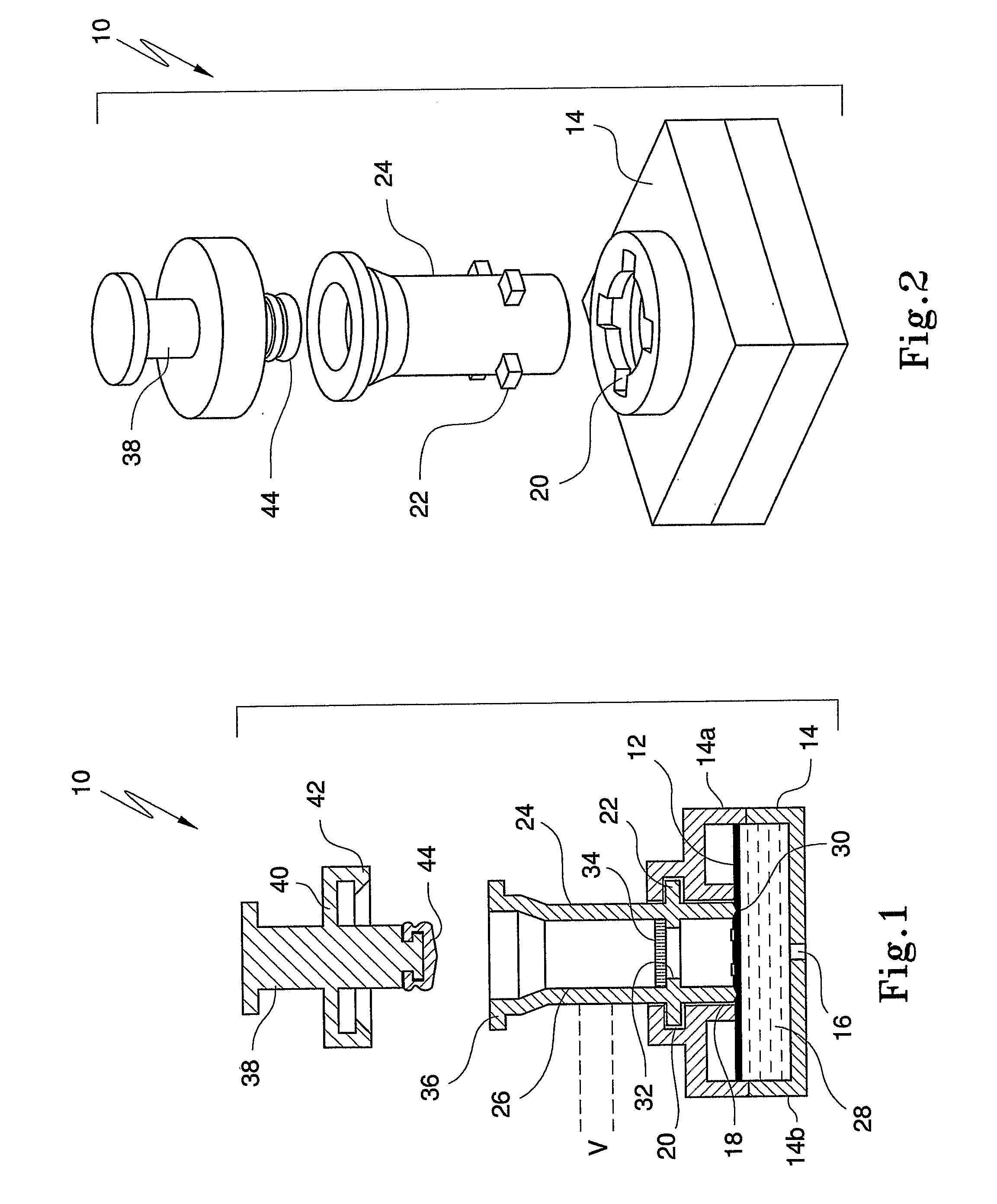
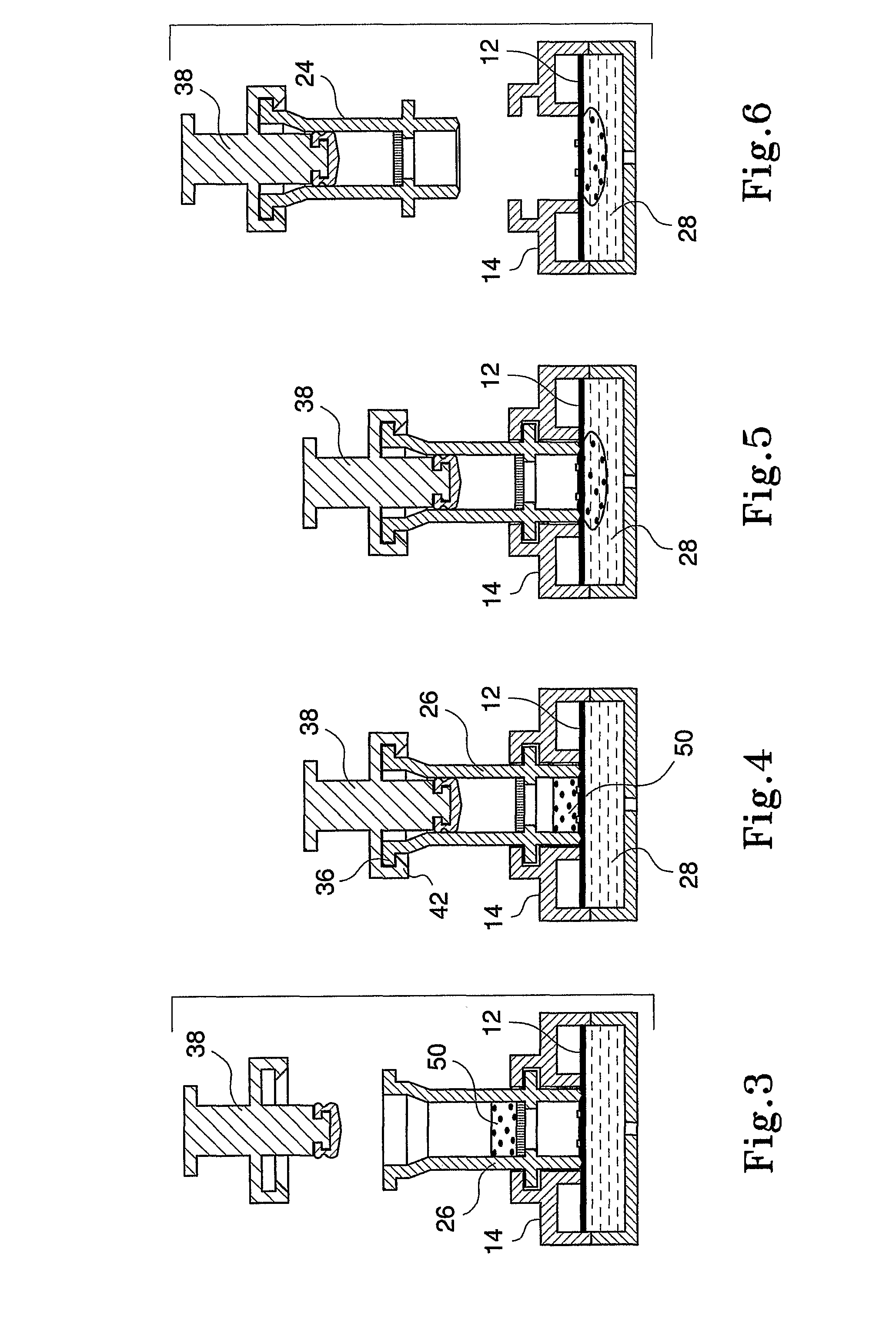
An apparatus (10) and method for use in a vertical flow-through assay process is characterised by applying pressure to a sample to force the sample through a reaction / capture membrane (12), to which one or more ligands are bound, at a controlled rate. Typically, the method includes a pre-incubation step in which the sample and a detection analyte typically an antibody bound to colloidal gold or a fluorescent tag bind together The pre-incubation step typically takes place in a chamber (26) spaced above the capture membrane (12). The base of the chamber is defined by a porous hydrophobic frit (34) typically formed from polyethylene. It is preferred that the chamber (26) is defined by the upper part of a cylinder (24) extending from a seal (30) compressing the reaction membrane (12) against an absorbent pad (28). The seal (30) has the effect of compressing tie reaction membrane and preventing wicking of the sample in the lateral direction outside of the circular seal. A piston (28) compresses air located in the chamber above atmospheric pressure to farce the sample to pass through the hydrophobic frit (34). Alternatively, a hydraulic actuator (60) may directly act on the sample to force the sample through the frit and reaction membrane at a predetermined rate.
Owner:PROTEOME SYST LTD
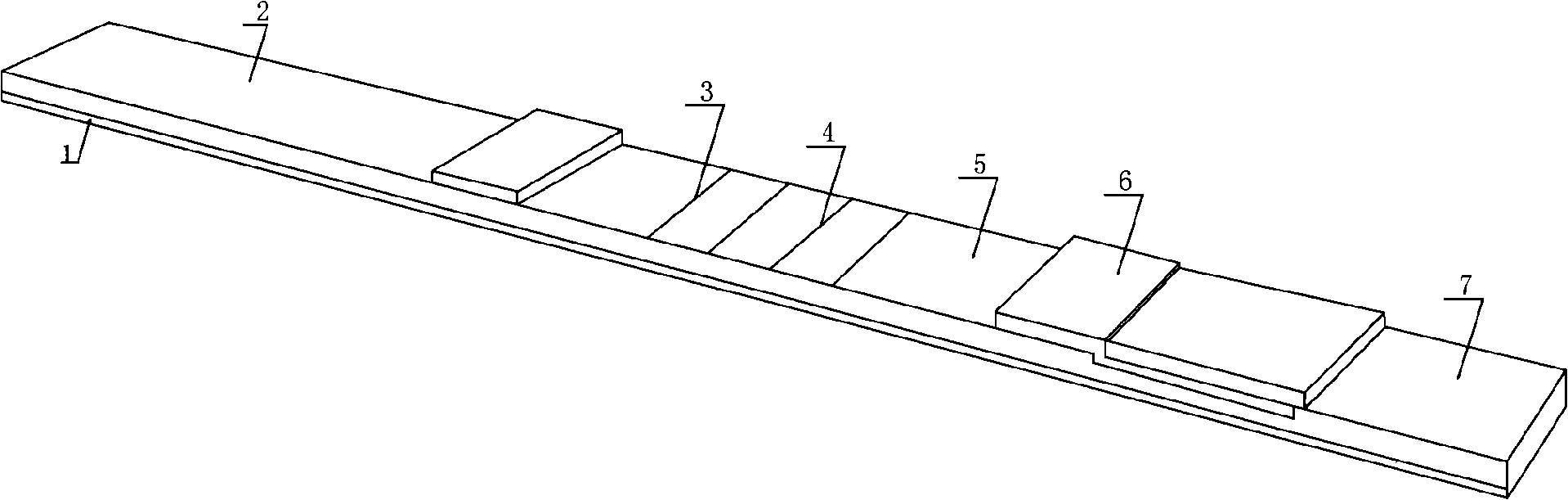
Multi-linked immunity chromatography test paper for detecting saliva abusing drug, system and its preparation method
InactiveCN101241136ANo pollution in the processOperational securityMaterial analysisAntigenAbuse drugs
The present invention discloses a multi-linked chromatographic test paper bar for detecting drug abuse in saliva and preparation thereof. The testing paper bar is a reaction film bar made of sample mats lap jointed mutually in turn pasted on the base, glass fiber film coated with colorful latex, colloidal gold or colloid selenium particles labeled protein, custodite film and absorbent paper. Control region coated anti-rat antibody, at least two detecting region of which coated with corresponding drug antigen are set on custodite film. The present invention has merits of safety and simple operation, suitable for detecting by single person / share and high detecting speed, and multi-detection of drug antigen in saliva at the same time.
Owner:GUANGZHOU WONDFO BIOTECH
Reagent for detecting acute myocardial infarction by immunological method and test strip
ActiveCN101806804AHigh sensitivityImprove featuresImmunoglobulins against animals/humansTissue cultureSpecific antibodyHuman heart
The invention relates to a medical diagnostic reagent, in particular to a quick detection reagent for the early diagnosis of acute myocardial infarction and a test strip. The invention provides a double-index united detection reagent containing a specific antibody resisting a human heart-type fatty acid binding protein H-FABP and a human cardiac troponin cTnI. The invention also provides a colloidal gold labeling immunological chromatographic test strip containing the specific antibody resisting the H-FABP and the cTnI, which is used for quickly detecting the acute myocardial infarction. The double-index united detection reagent can carry out early diagnosis on a patient having the acute myocardial infarction, can also prevent the missed diagnosis of a patient having long-time chronic myocardial infarction with mild symptoms and better solves the influence caused by a difference existing in the detection time. The colloidal gold labeling immunological chromatographic test strip provides a quick, convenient, cheap and practical detection tool for the early diagnosis of the acute myocardial infarction, is hopefully used for hospitals at all levels and can also be used for the self monitoring of the patient.
Owner:LANZHOU INST OF BIOLOGICAL PROD
Super-paramagnetic composite particle drug-loaded body and preparation method thereof
ActiveCN101164621ALarge specific surface areaImprove adsorption capacityOrganic active ingredientsGenetic material ingredientsCross-linkSuperparamagnetism
The present invention relates to a superparamagnetic composite microparticle medicine-carrying body which can be mainly used for making magnetic target therapy and its preparation method. The invented magnetic composite microparticle medicine-carrying body is composed of magnetic composite microparticle and medicine-carrying layer which can be used for covering exterior of said magnetic composite microparticle. Said invention is characterized by that it utilizes the property of high surface activity of gold shell layer or colloidal gold of superparamagnetic composite microparticle to directly cover medicine or firstly utilizes the property of high surface activity of the gold shell layer or colloidal gold of superparamagnetic composite microparticle to make its surface be covered with high-molecular material, then utilizes the affinity adsorption or covalent linkage of high-molecular material to medicine to cover the medicine, also can make the surface of superparamagnetic composite microparticle be covered with the high-molecular material and medicine cross-linked or target preparation and medicine cross-linked or high-molecular material, target preparation and medicine cross-linked composite body layer.
Owner:XIAN GOLDMAG NANOBIOTECH
Mesoporous nanocrystaline zeolite composition and preparation from amorphous colloidal metalosilicates
Owner:ECOLAB USA INC
Isothermal amplification kit for detecting SARS-COV-2 and primer probe set
PendingCN111074007AStrong specificityHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationViral nucleic acidBiotin
The invention discloses an isothermal amplification kit for detecting SARS-COV-2 and a primer probe set. The kit comprises (1) inactivated lysate; and (2) an isothermal amplification system: a reaction buffer solution Buffer A, magnesium acetate Buffer B, negative control, nuclease-free water, a primer probe set and RAA enzyme. The lysate is used for rapid inactivation and release of viral nucleicacid, an isothermal amplification technology is utilized for rapidly enriching and amplifying a target area, whether a specific amplified product exists is rapidly determined through combination of aprobe with modification groups and a product with biotin, by a lateral chromatography technology and by use of colloidal gold developing. The kit is simple to operate, professional extraction reagents and detection instruments are not required limitation of detection laboratories and professionals is freed, the kit can perform rapid detection in an instrument-free state outdoors, the whole process only needs 30 min, and reading is very convenient.
Owner:上海世和医学检验实验室有限公司
Test paper for rapidly detecting immunochromatography of cadmium ion colloidal gold and preparation method and application thereof
The invention discloses test paper for rapidly detecting the immunochromatography of cadmium ion colloidal gold and a preparation method and application thereof. The test paper consists of a sample pad, a colloidal gold combination pad, a nitrocellulose membrane, absorbent paper and a plastic base plate; The sample pad, the colloidal gold combination pad, the nitrocellulose membrane and the absorbent paper are sequentially stuck on the plastic base plate; an anti-cadmium ion monoclonal colloidal gold label is coated on the colloidal gold combination pad; a goat anti-rat IgG antibody and a complete antigen Cd-iEDTA-BSA of heavy metal cadmium are sequentially coated on the nitrocellulose membrane; the goat anti-rat IgG antibody is used as a quality control line; and the complete antigen Cd-iEDTA-BSA of the heavy metal cadmium is used as a test line. The test paper detects residual metal cadmium in ambient soil, a water body and an aquatic product based on the immunology theory of antigen antibody; and compared with the prior detection system, the test paper has the advantages of rapidness, cheapness, convenience, sensitivity and speciality, can give a result within five minutes and is convenient to carry along to carry out field monitoring.
Owner:JINAN UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com