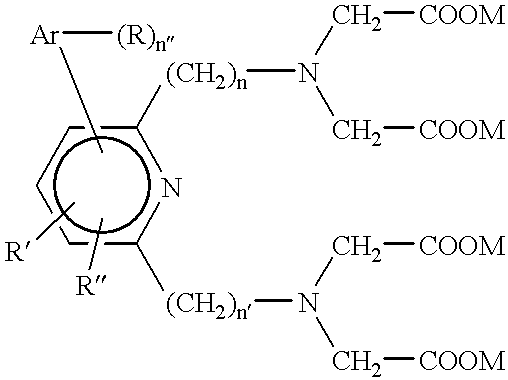
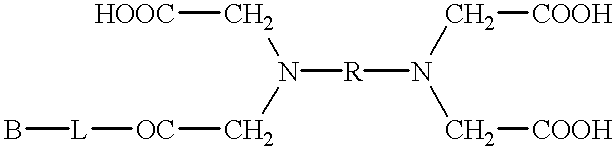
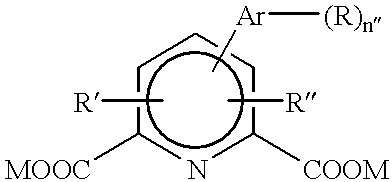
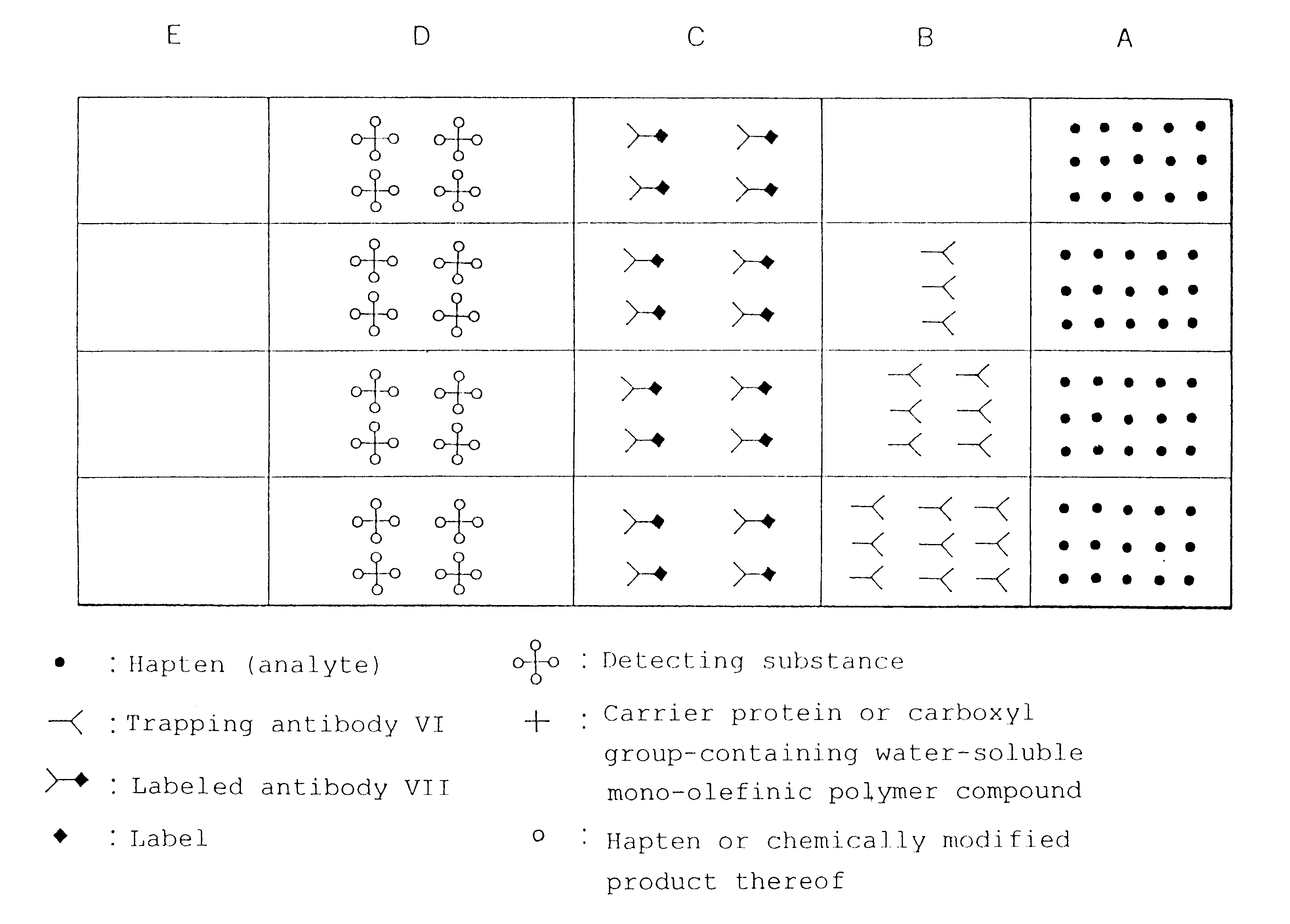
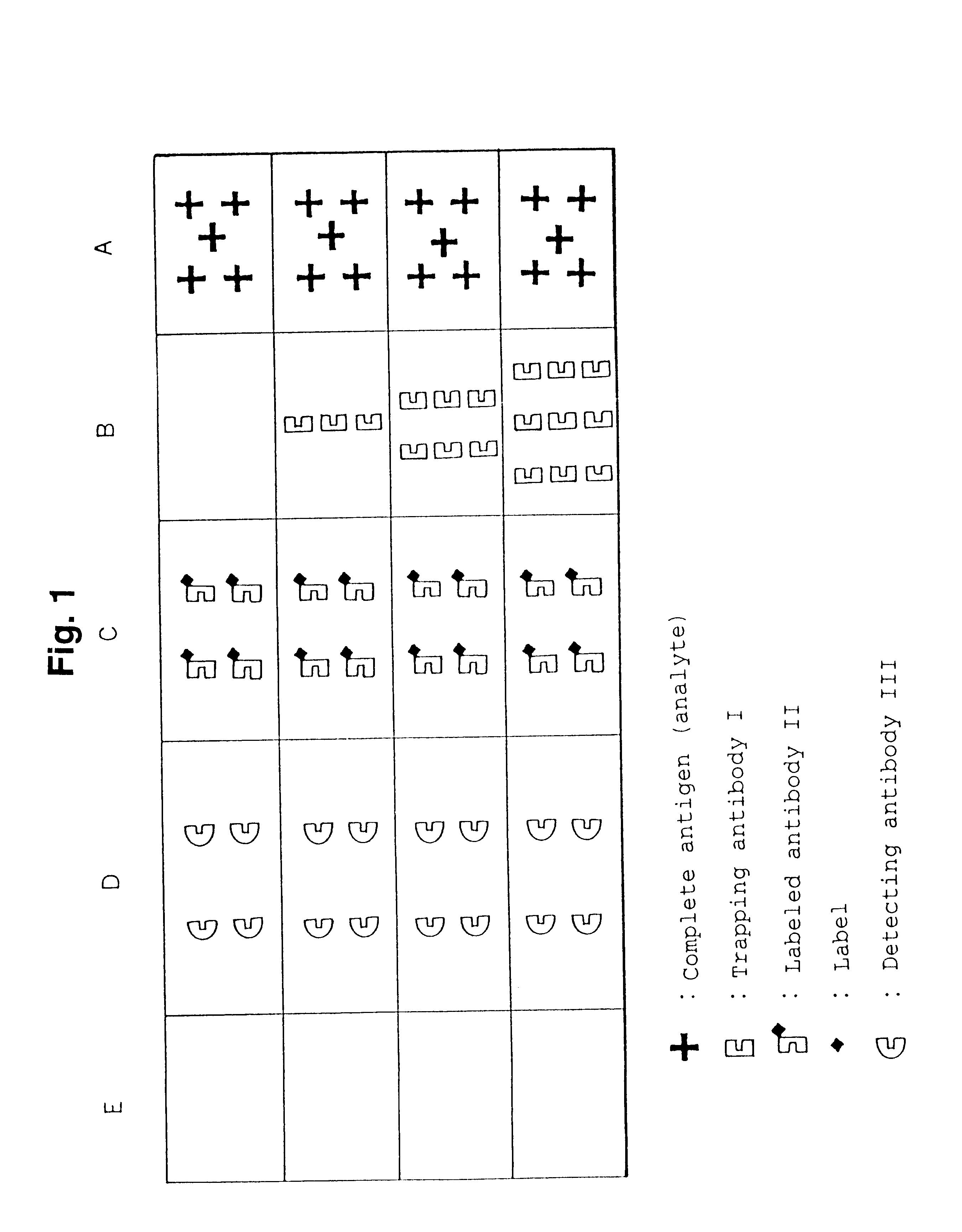
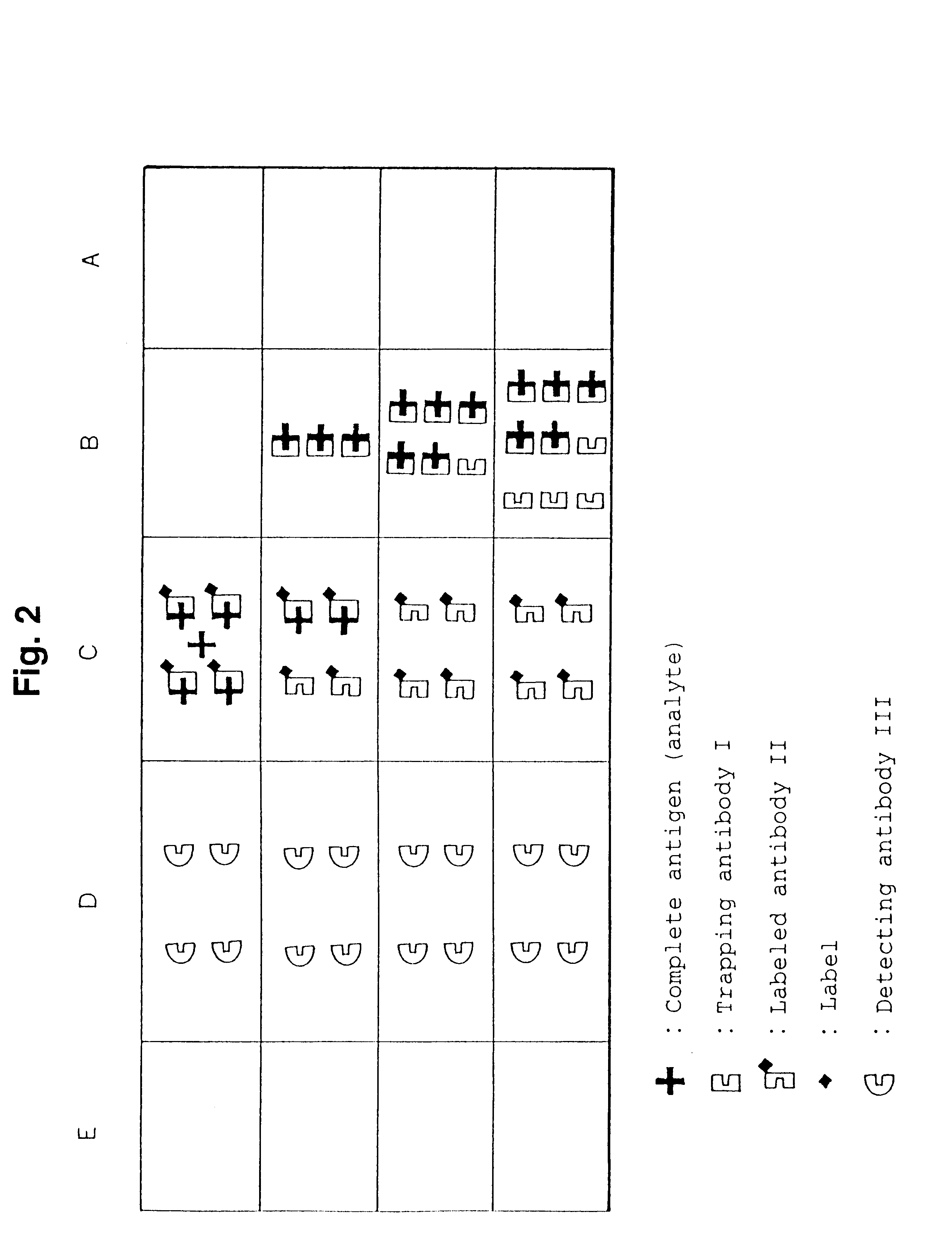
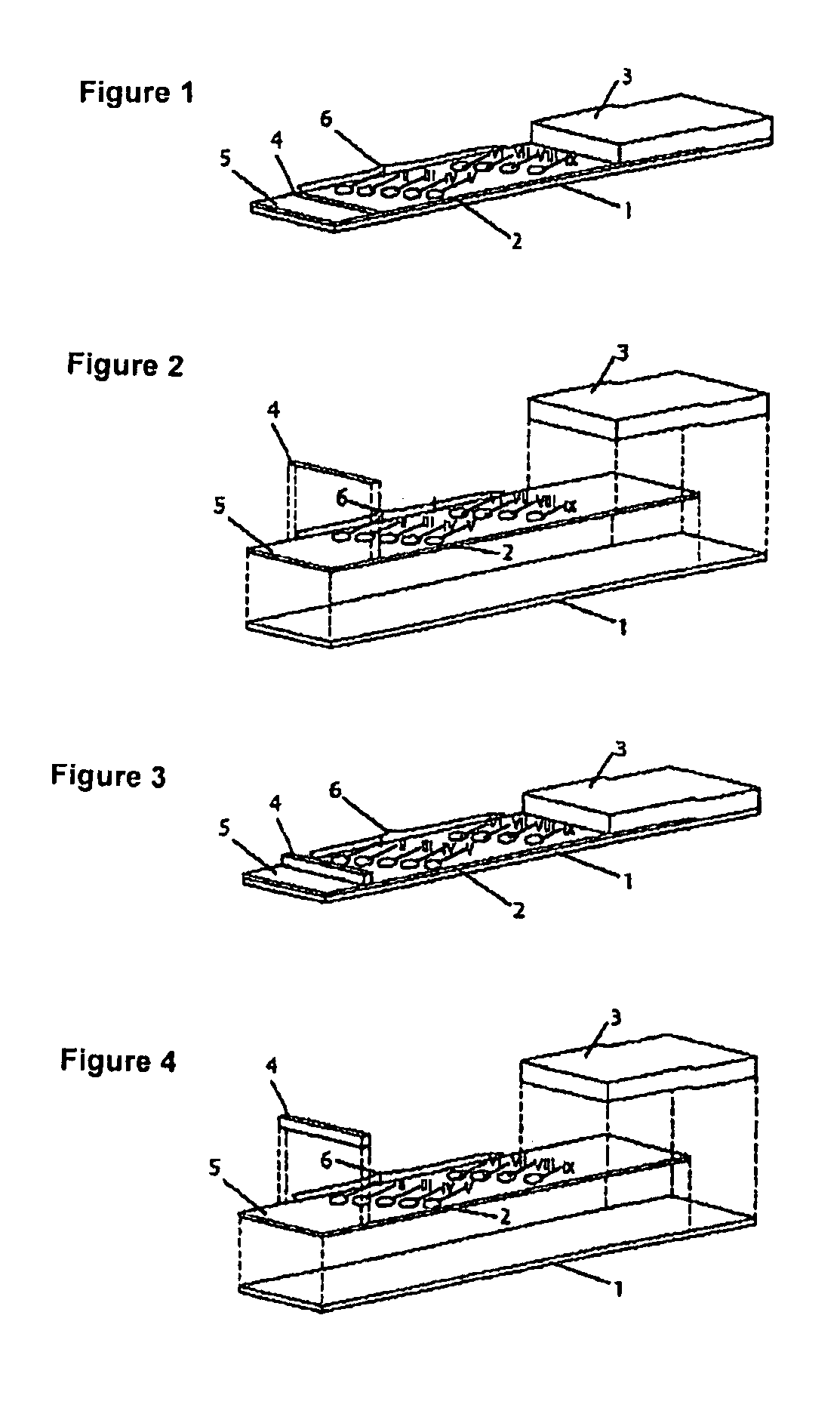
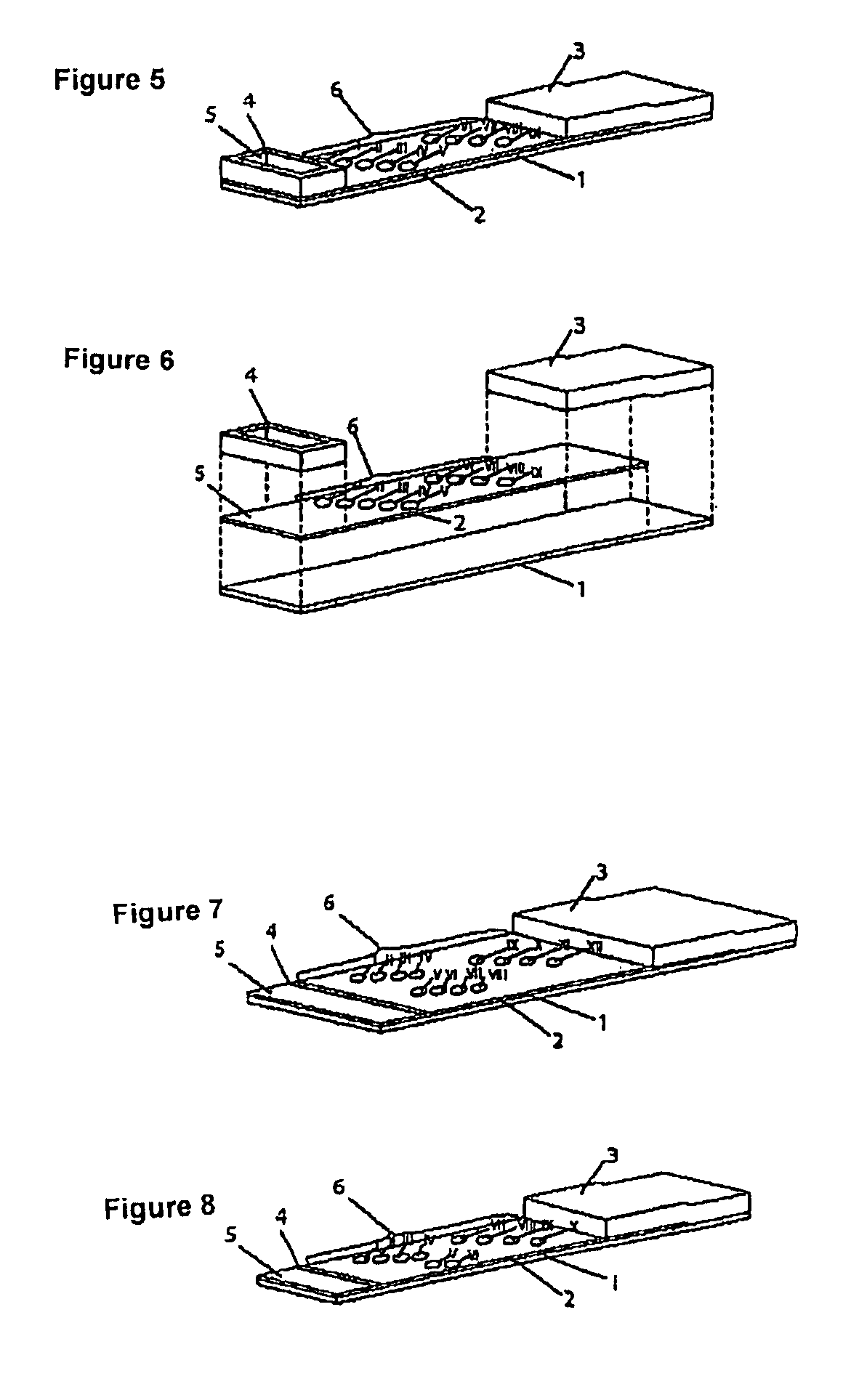
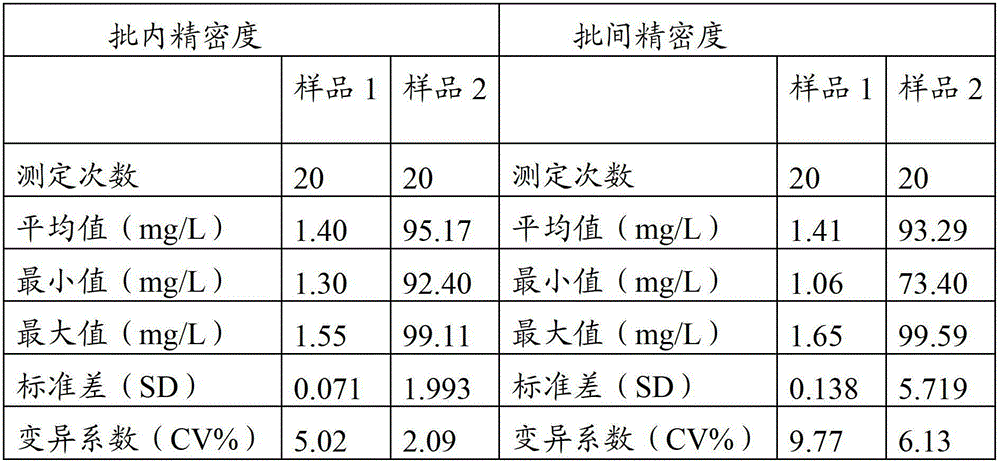
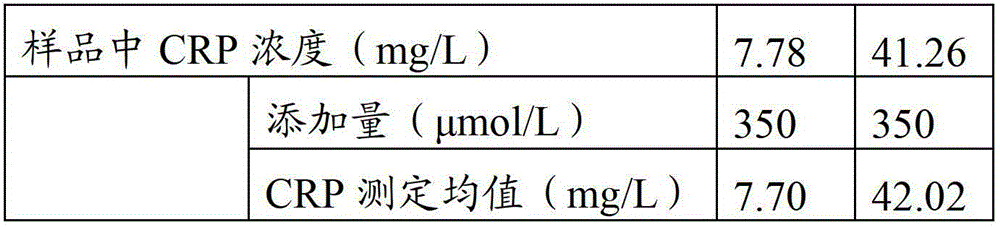
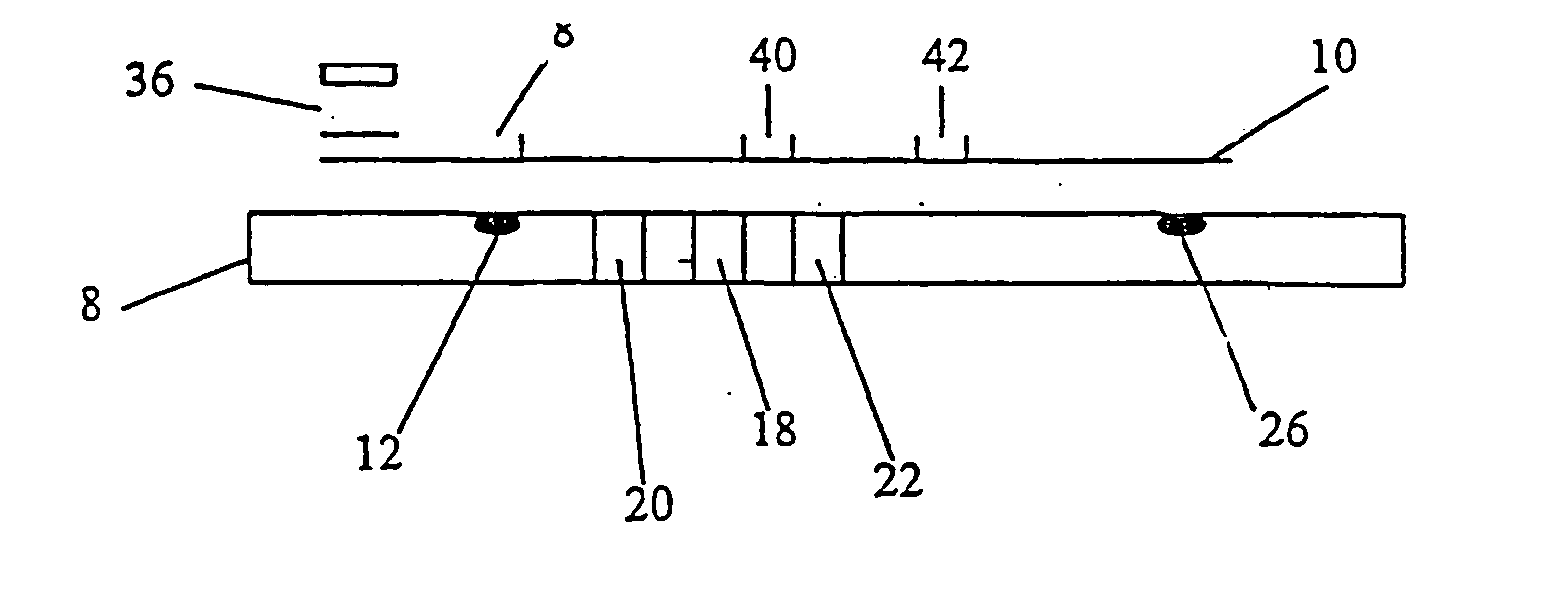
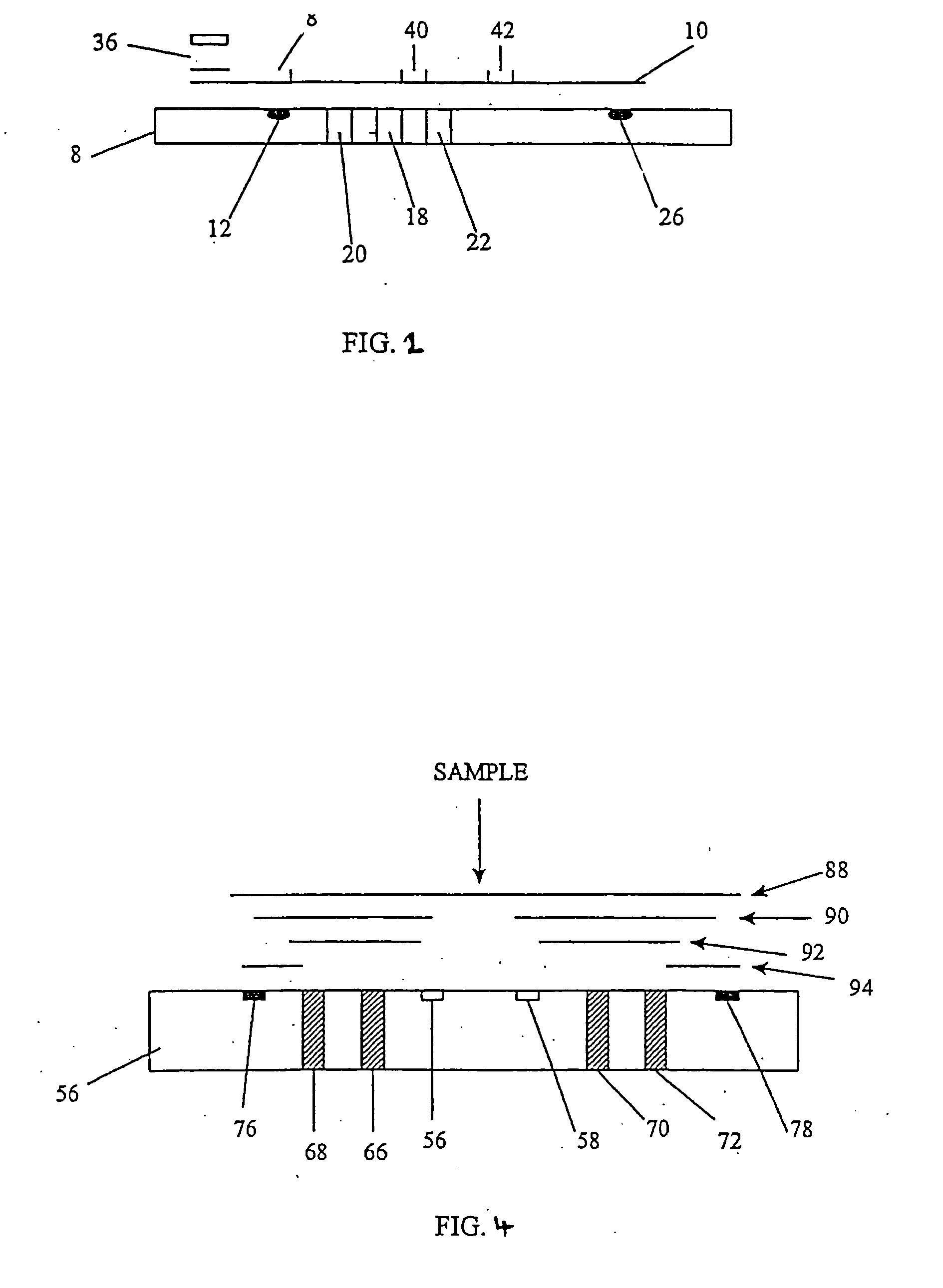
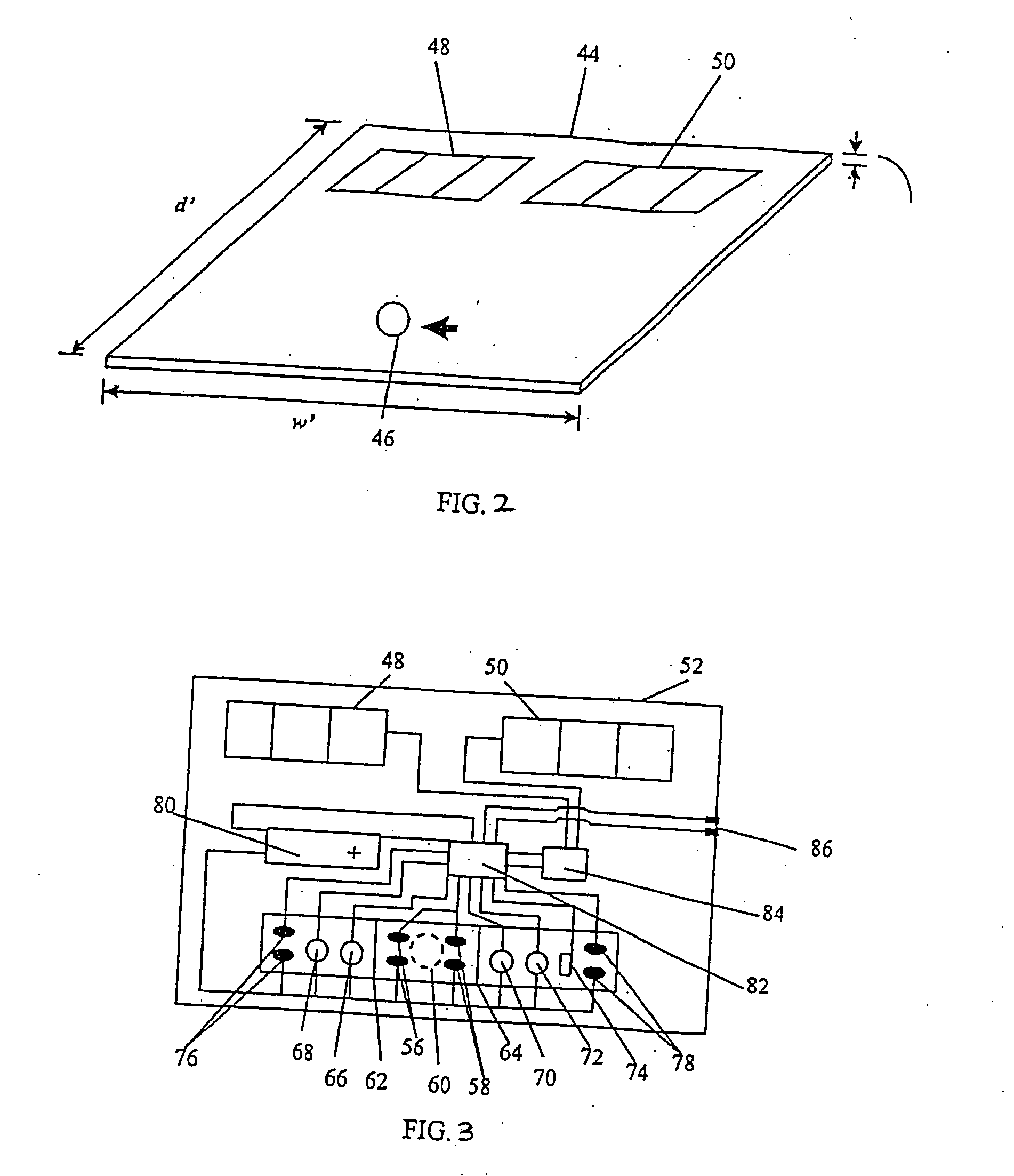
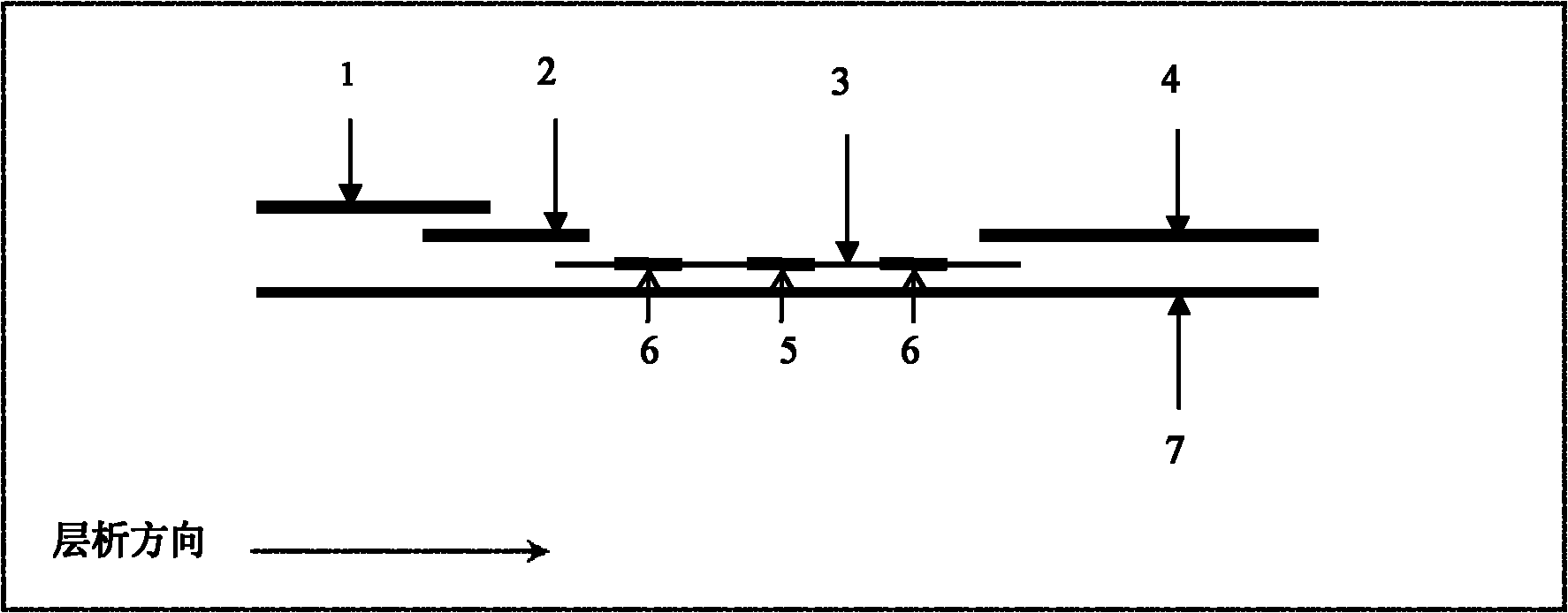
Patents
Literature
3145 results about "Quantitative assay" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Assay - a quantitative or qualitative test of a substance (especially an ore or a drug) to determine its components; frequently used to test for the presence or concentration of infectious agents or antibodies etc.
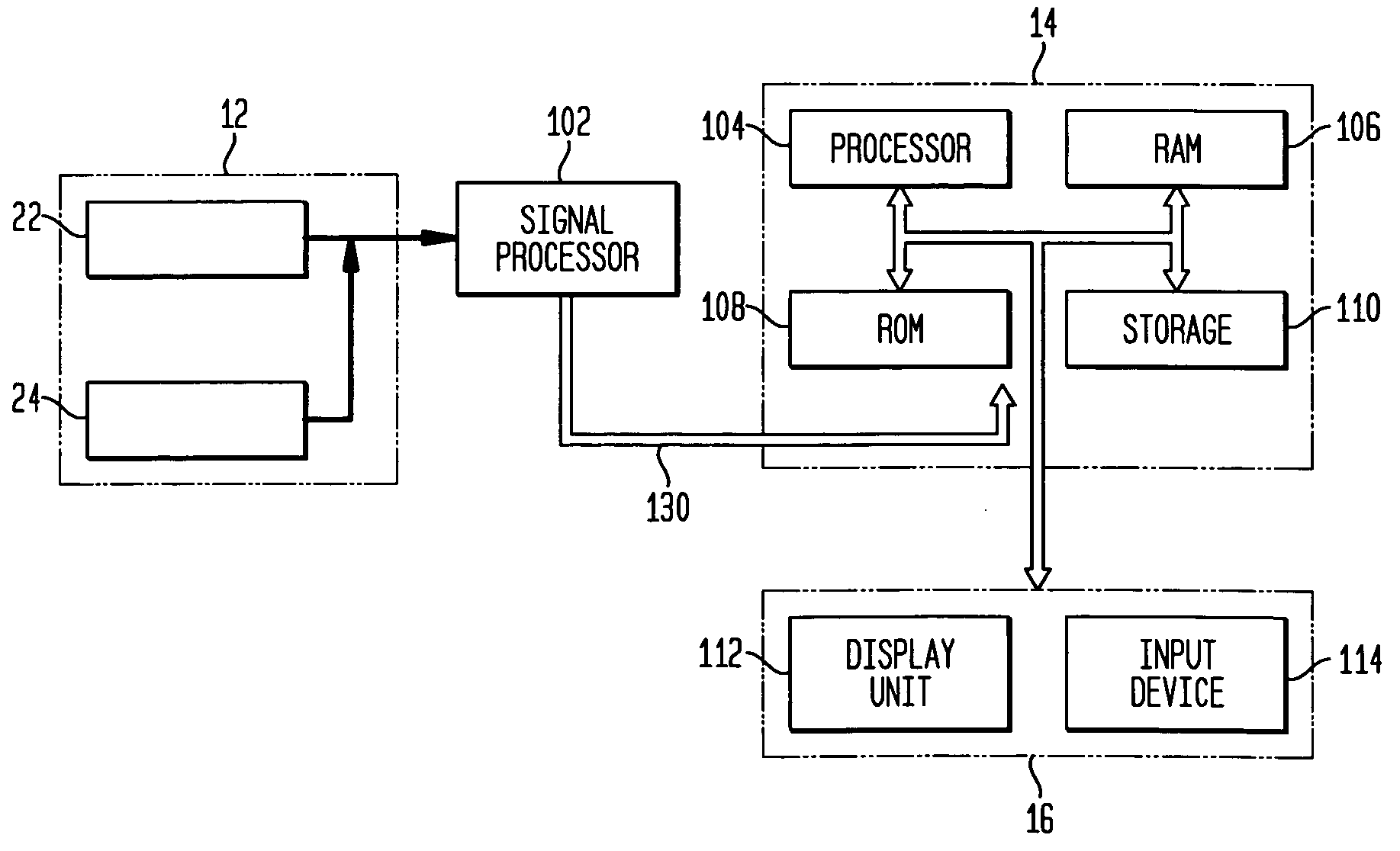
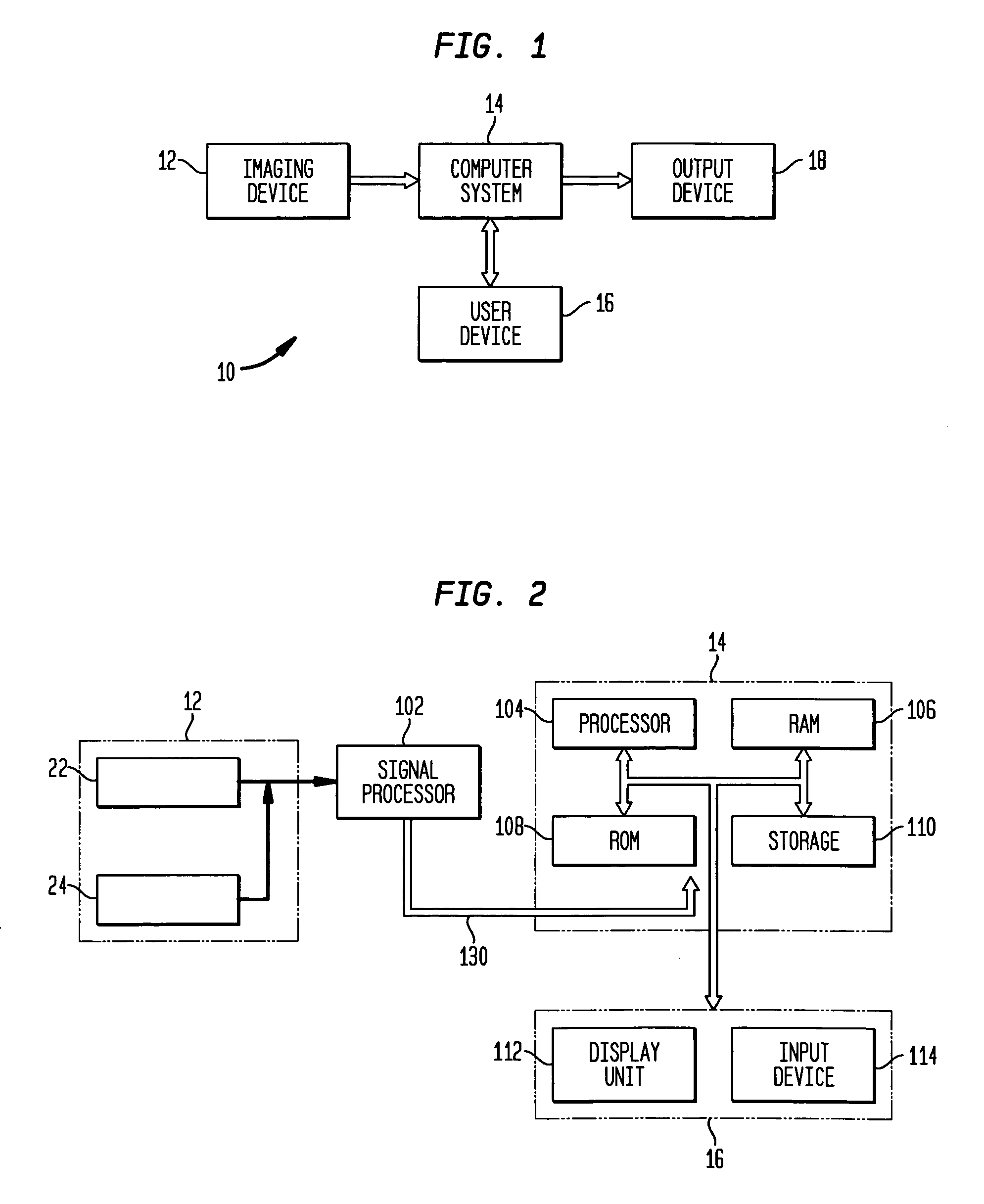
Diagnostic radio frequency identification sensors and applications thereof
ActiveUS20060290496A1Low costDevices with bluetooth interfacesBurglar alarm mechanical actuationPower sensorPoint of care
An integrated passive wireless chip diagnostic sensor system is described that can be interrogated remotely with a wireless device such as a modified cell phone incorporating multi-protocol RFID reader capabilities (such as the emerging Gen-2 standard) or Bluetooth, providing universal easy to use, low cost and immediate quantitative analyses, geolocation and sensor networking capabilities to users of the technology. The present invention can be integrated into various diagnostic platforms and is applicable for use with low power sensors such as thin films, MEMS, electrochemical, thermal, resistive, nano or microfluidic sensor technologies. Applications of the present invention include on-the-spot medical and self-diagnostics on smart skin patches, Point of Care (POC) analyses, food diagnostics, pathogen detection, disease-specific wireless biomarker detection, remote structural stresses detection and sensor networks for industrial or Homeland Security using low cost wireless devices such as modified cell phones.
Owner:ALTIVERA
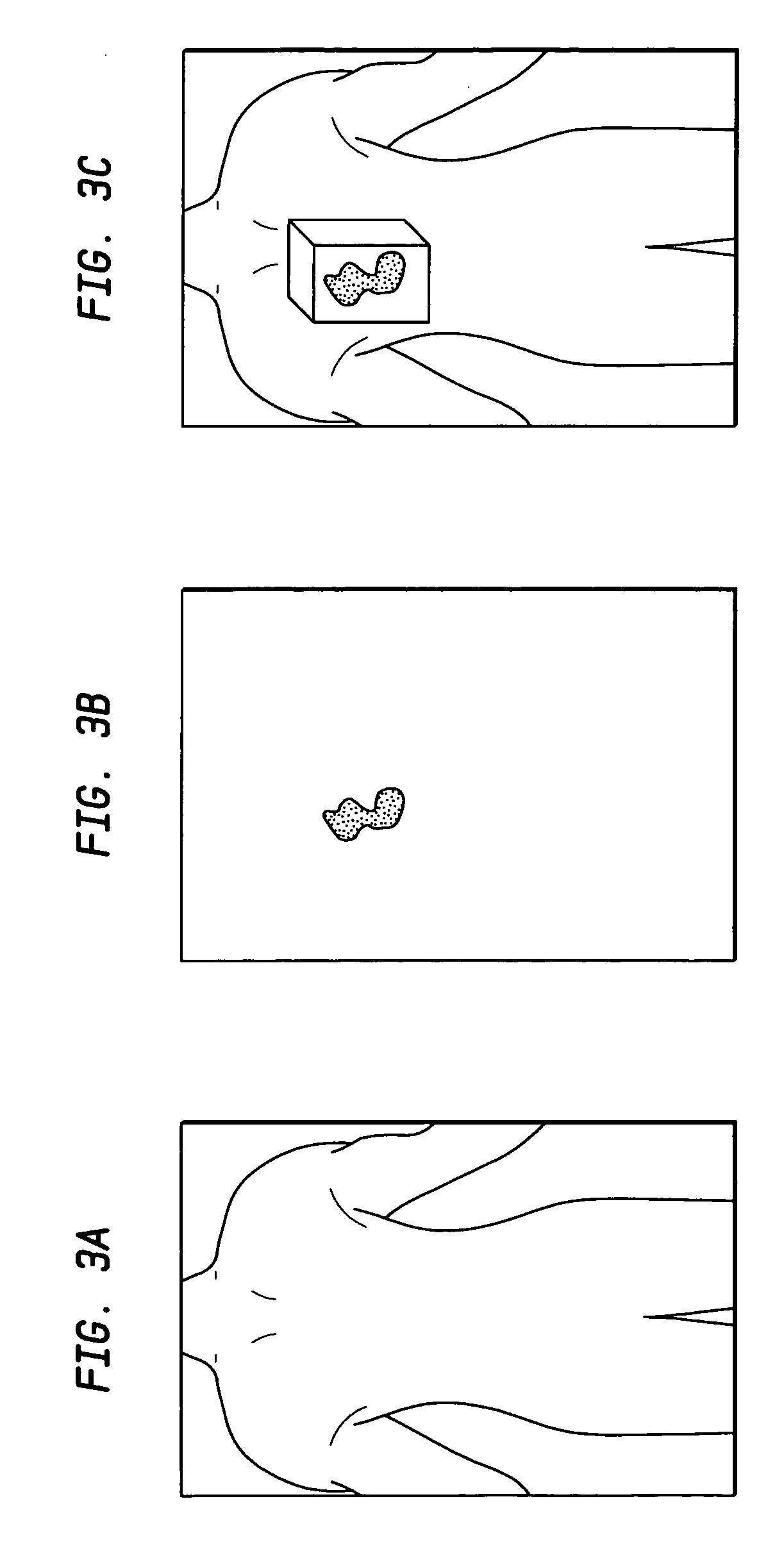
System and method of measuring disease severity of a patient before, during and after treatment
ActiveUS20050065421A1Material analysis using wave/particle radiationImage analysisData setImaging data
A system and method of obtaining serial biochemical, anatomical or physiological in vivo measurements of disease from one or more medical images of a patient before, during and after treatment, and measuring extent and severity of the disease is provided. First anatomical and functional image data sets are acquired, and form a first co-registered composite image data set. At least a volume of interest (ROI) within the first co-registered composite image data set is identified. The first co-registered composite image data set including the ROI is qualitatively and quantitatively analyzed to determine extent and severity of the disease. Second anatomical and functional image data sets are acquired, and form a second co-registered composite image data set. A global, rigid registration is performed on the first and second anatomical image data sets, such that the first and second functional image data sets are also globally registered. At least a ROI within the globally registered image data set using the identified ROI within the first co-registered composite image data set is identified. A local, non-rigid registration is performed on the ROI within the first co-registered composite image data set and the ROI within the globally registered image data set, thereby producing a first co-registered serial image data set. The first co-registered serial image data set including the ROIs is qualitatively and quantitatively analyzed to determine severity of the disease and / or response to treatment of the patient.
Owner:SIEMENS MEDICAL SOLUTIONS USA INC
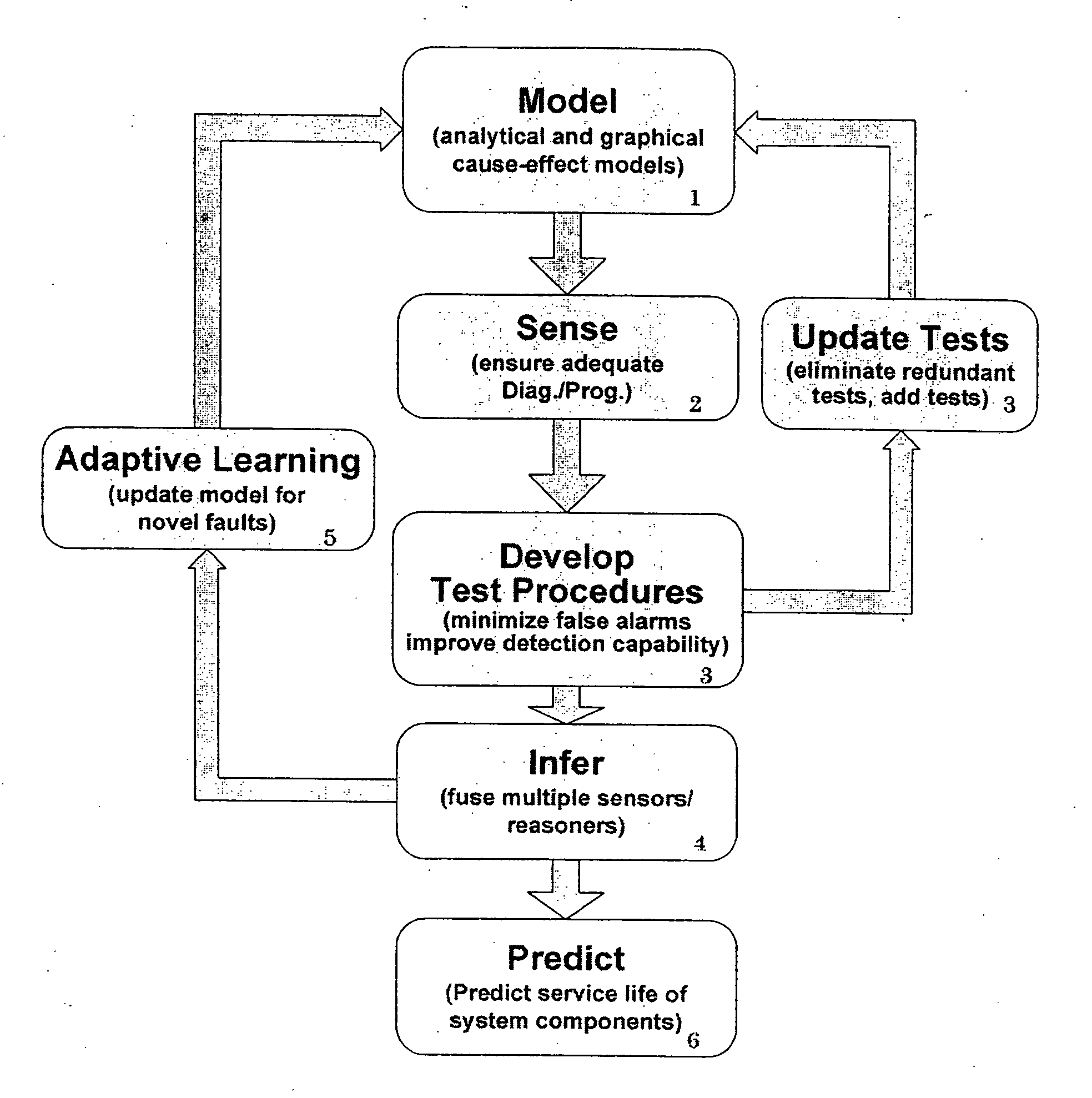
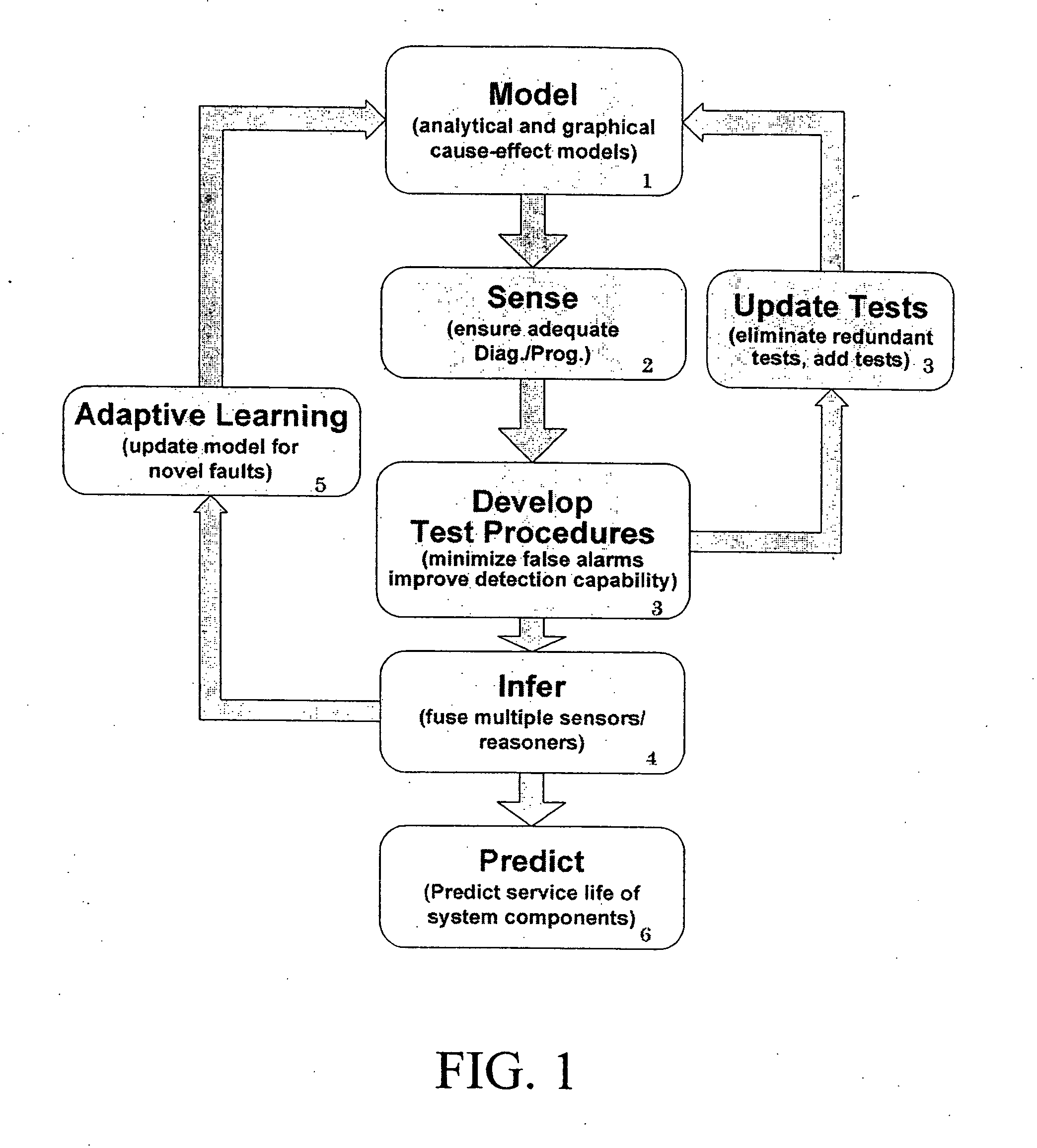
Intelligent model-based diagnostics for system monitoring, diagnosis and maintenance
InactiveUS20060064291A1Improve accuracyImprove consistencyAmplifier modifications to reduce noise influenceTesting/monitoring control systemsElectric power systemCompound (substance)
Systems and methods are provided for monitoring, diagnosis and condition-based maintenance of mechanical systems. The disclosed systems and methods employ intelligent model-based diagnostic methodologies to effectuate such monitoring, diagnosis and maintenance. According to exemplary embodiments of the present disclosure, the intelligent model-based diagnostic methodologies combine or integrate quantitative (analytical) models and graph-based dependency models to enhance diagnostic performance. The disclosed systems and methods may be employed a wide variety of applications, including automotive, aircraft, power systems, manufacturing systems, chemical processes and systems, transportation systems, and industrial machines / equipment.
Owner:TOYOTA TECHN CENT USA +1
Pyrophosphorolysis and incorporation of nucleotide method for nucleic acid detection
InactiveUS7090975B2Useful in detectionSugar derivativesMicrobiological testing/measurementDepolymerizationNucleic acid detection
Processes are disclosed using the depolymerization of a nucleic acid hybrid and incorporation of a suitable nucleotide to qualitatively and quantitatively analyze for the presence of predetermined nucleic acid target sequences. Applications of those processes include the detection of single nucleotide polymorphisms, identification of single base changes, genotyping, medical marker diagnostics, mirosequencing, and.
Owner:PROMEGA
Method for quantitative analysis of a nucleic acid amplification reaction
InactiveUS6911327B2Reduce decreaseReduce in quantityBioreactor/fermenter combinationsHeating or cooling apparatusTest sampleNucleic acid sequencing
A method for determining an unknown starting quantity of a target nucleic acid sequence in a test sample comprises amplifying the unknown starting quantity of the target nucleic acid sequence in the test sample and known starting quantities of a calibration nucleic acid sequence in respective calibration samples; and determining a respective threshold value for each of the nucleic acid sequences using a derivative of a growth curve derived for the sequence. The starting quantity of the target nucleic acid sequence in the test sample is determined using the threshold value determined for the target sequence and a calibration curve derived from the threshold values determined for the known starting quantities of the calibration nucleic acid sequences.
Owner:CEPHEID INC
Homogeneous fluorassay methods employing fluorescent background rejection and water-soluble rare earth metal chelates
InactiveUS6242268B1High binding constantNon-metal conductorsGlass making apparatusHalf-lifeMetal chelate
Homogeneous assays for determining quantitatively the extent of a specific binding reaction can be carried out effectively on very dilute solutions using measurements of fluorescence if a fluorescence measurement scheme that is capable of rejecting short-lived background fluorescence is employed and if the fluorescent group being measured has the following properties: a. the group being measured must be a rare earth metal chelate complex combination; b. the chelate must be water-soluble; c. the complex combination must also be stable in extremely dilute aqueous solutions, that is, the measured chelate must have at least one ligand having a metal-to-ligand binding constant of at least about 1013M-1 or greater and it must have a fluorescent emission that is long-lived compared to the longest decay lifetime of ambient substances and have a half life of from 0.01 to 50 msec.
Owner:EG&G WALLAC
Chemical analysis apparatus and method
InactiveUS6020147ABioreactor/fermenter combinationsAnalysis using chemical indicatorsAnalyteQuantitative determination
A method and apparatus for the quantitative determination of an analyte in a liquid employs a liquid-permeable solid medium defining a liquid flow path. The medium includes a number of reactant-containing reaction zones spaced apart along the flow path and in which reaction occurs with the analyte or an analyte derivative (e.g., a labeled analyte) to result in the formation of a predetermined product. Detector means are employed to detect analyte, analyte derivative, reactant or predetermined product in the reaction zones, the number of such zones in which such detection occurs indicating the amount of analyte in the liquid.
Owner:SURMODICS INC
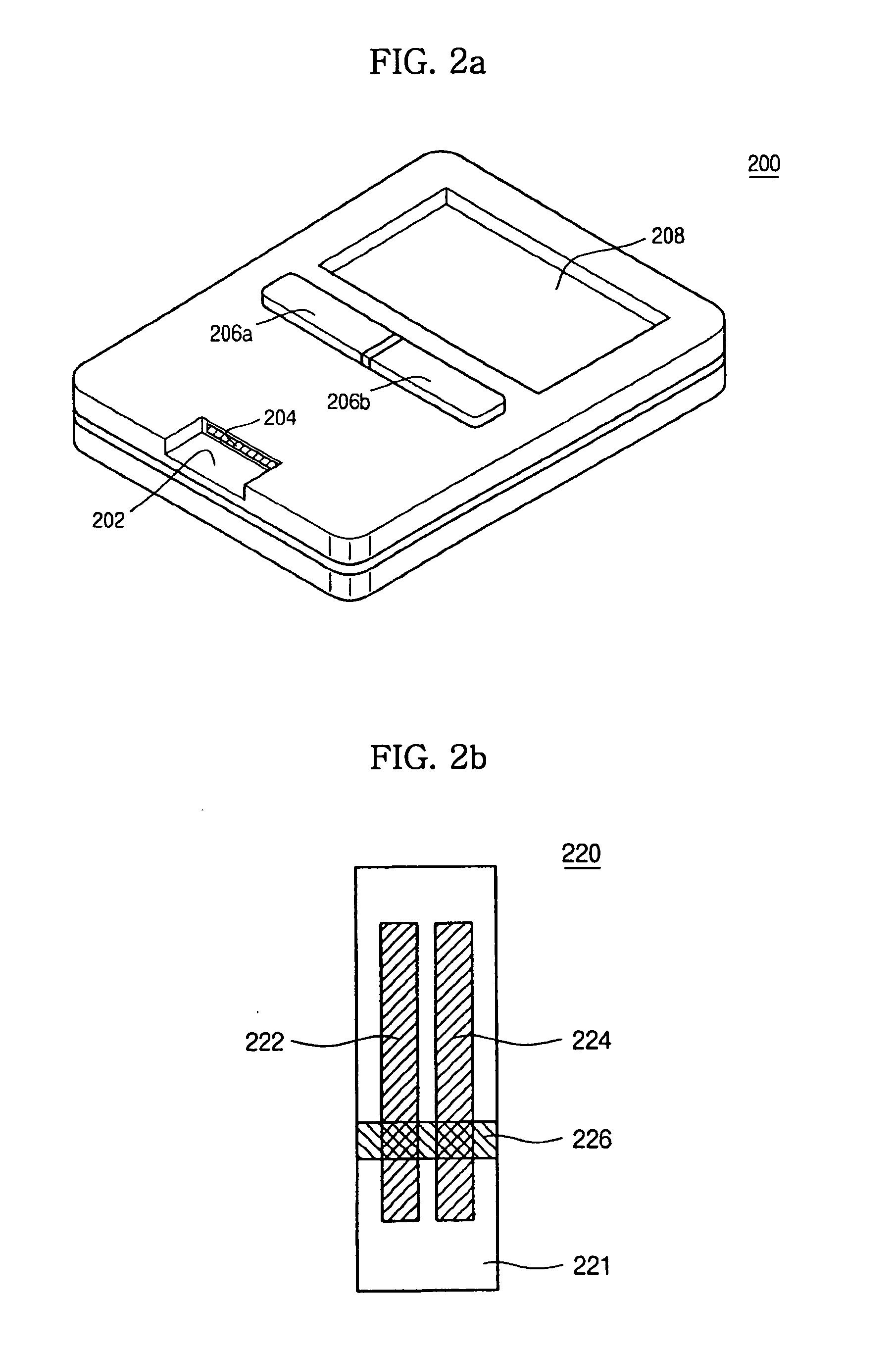
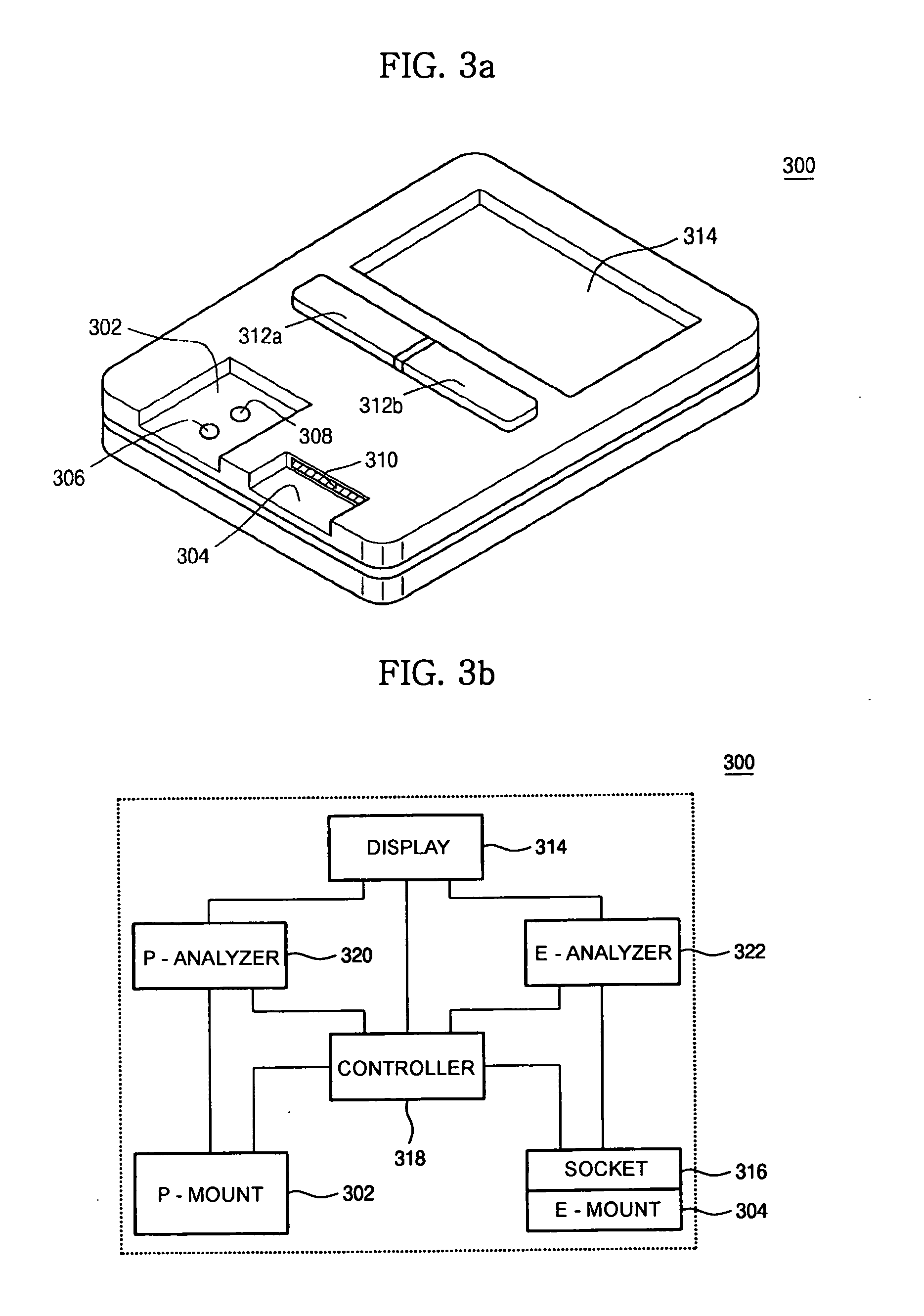
Device for quantitative analysis of biological materials
InactiveUS20060099703A1Easy to useHigh precision analysisBioreactor/fermenter combinationsBiological substance pretreatmentsEngineeringBiological materials
Disclosed is a device for quantitatively analyzing material of a living creature. The device includes an analyzing unit using photometric method, and an analyzing unit using electrochemical method. The device has a socket for mounting a photometric test strip and a socket for mounting an electrochemical test strip, separately or in a body. Also, the test strip may have a recognition electrode formed on a specific position of the test strip. The position of the recognition electrode is determined by analyzing method and target material. When used together with a test strip having the recognition electrode, the device has a terminal for identifying the position of the recognition electrode.
Owner:ALL MEDICUS CO LTD
Method and apparatus for quantitative microimaging
InactiveUS20120224053A1Easy to operateSimple designMaterial analysis by optical meansLaboratory glasswaresQuantitative determinationAnalytical chemistry
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST +1
Measurement of protein expression using reagents with barcoded oligonucleotide sequences
ActiveUS20180267036A1Quality improvementFast and efficient and highly scalablePolypeptide with localisation/targeting motifMicrobiological testing/measurementProtein targetOrganic chemistry
Some embodiments disclosed herein provide a plurality of compositions each comprising a protein binding reagent conjugated with an oligonucleotide. The oligonucleotide comprises a unique identifier for the protein binding reagent it is conjugated with, and the protein binding reagent is capable of specifically binding to a protein target. Further disclosed are methods and kits for quantitative analysis of a plurality of protein targets in a sample and for simultaneous quantitative analysis of protein and nucleic acid targets in a sample. Also disclosed herein are systems and methods for preparing a labeled biomolecule reagent, including a labeled biomolecule agent comprising a protein binding reagent conjugated with an oligonucleotide.
Owner:BECTON DICKINSON & CO
Qualitative and quantitative analytical modeling of sales performance and sales goals
InactiveUS20140324521A1Facilitate business risk analysisFinanceForecastingRisk profilingDecision taking
The present invention is applicable in the field of sales performance management coupled with corporate finance, corporate capital investments, economics, math, business risk analysis, simulation, decision analysis, qualitative risk analysis, risk management, quantitative risk analysis, and business statistics, and relates to the modeling and valuation of investment decisions and sales performance management and analysis under uncertainty and risk within all companies, allowing these firms to properly identify, assess, quantify, value, diversify, and hedge their corporate capital investment and sales management decisions and their associated risks. Specifically, the present invention looks at starting from comprehensive qualitative sales performance management and moving the analysis into the realms of quantitative risk-based sales performance modeling, simulation, and optimization.
Owner:MUN JOHNATHAN
Affinity-shifted probes for quantifying analyte polynucleotides
InactiveUS20080131892A1Good quantitative resultNegative effectMaterial analysis by observing effect on chemical indicatorMicrobiological testing/measurementAmpliconHybridization probe
Compositions, methods and devices for detecting and quantifying levels of an analyte polynucleotide in homogeneous assays using collections of soluble or immobilized hybridization probes. In certain preferred embodiments, the probes are immobilized in an array format. Polynucleotides may be quantified directly, or amplified in an in vitro nucleic acid amplification reaction prior to detection and quantitation. Amplification reactions may be performed in contact with the invented probes, and analyte amplicons quantified in real-time or end-point assays.
Owner:GEN PROBE INC
Lateral flow quantitative assay method and strip and laser-induced fluoerescence detection device therefor
ActiveUS20050214951A1Bioreactor/fermenter combinationsBiological substance pretreatmentsAnalyteDetection limit
Disclosed is a lateral flow quantitative assay method which can measure one or more analyte species at the same time, with high sensitivity. Also, the present invention relates to a strip which can measure one or more analyte species at the same time, with high sensitivity and a package in which the strip is integrated with a laser-induced surface fluorescence detector. The present invention can quantify multiple analytes with a minimum detection limit of pg / ml. Therefore, the present invention provides an advantage capable of quantifying a plurality of analytes at the same time using a simple lateral flow assay strip.
Owner:BODITECHMED
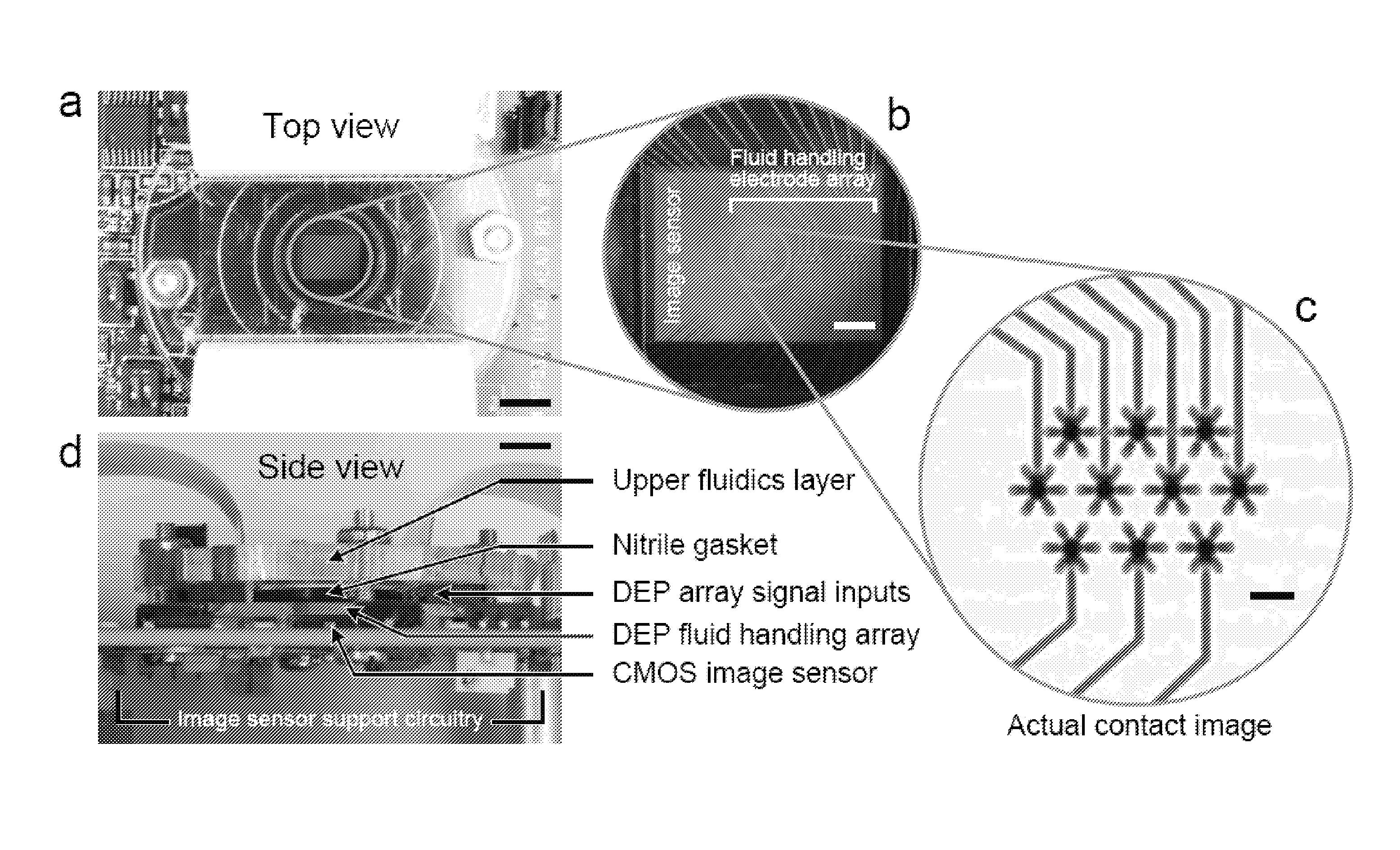
Methods and systems for analyzing images of specimens processed by a programmable quantitative assay
ActiveUS20120163681A1Material analysis by optical meansCharacter and pattern recognitionTissue sampleBioinformatics
Disclosed are methods and systems for analyzing images of specimens processed by a programmable quantitative assay or more specifically a robust programmable quantitative dot assay, PDQA, that enable specimens to be imaged and assessed across a wide variety of conditions and applications. Specific embodiments directed to immunohistochemical applications provide more quantitative methods of imaging and assessing biological samples including tissue samples.
Owner:AGILENT TECH INC
Simple immunochemical semi-quantitative assay method and apparatus
InactiveUS6177281B1Easy to adjustHigh sensitivityBioreactor/fermenter combinationsAnalysis using chemical indicatorsAnalyteQuantitative determination
The present invention relates to a simple immunochemical semi-quantitative assay method according to chromatography, which comprises trapping a certain amount of an analyte in a sample with a predetermined amount of a fixed antibody for the analyte before qualitative analysis of the analyte, the certain amount corresponding to the amount of the fixed antibody, and thereby decreasing a concentration of the analyte to be subjected to subsequent immunochemical qualitative determination, and an apparatus therefor.
Owner:TEIKOKU HORMONE MFG
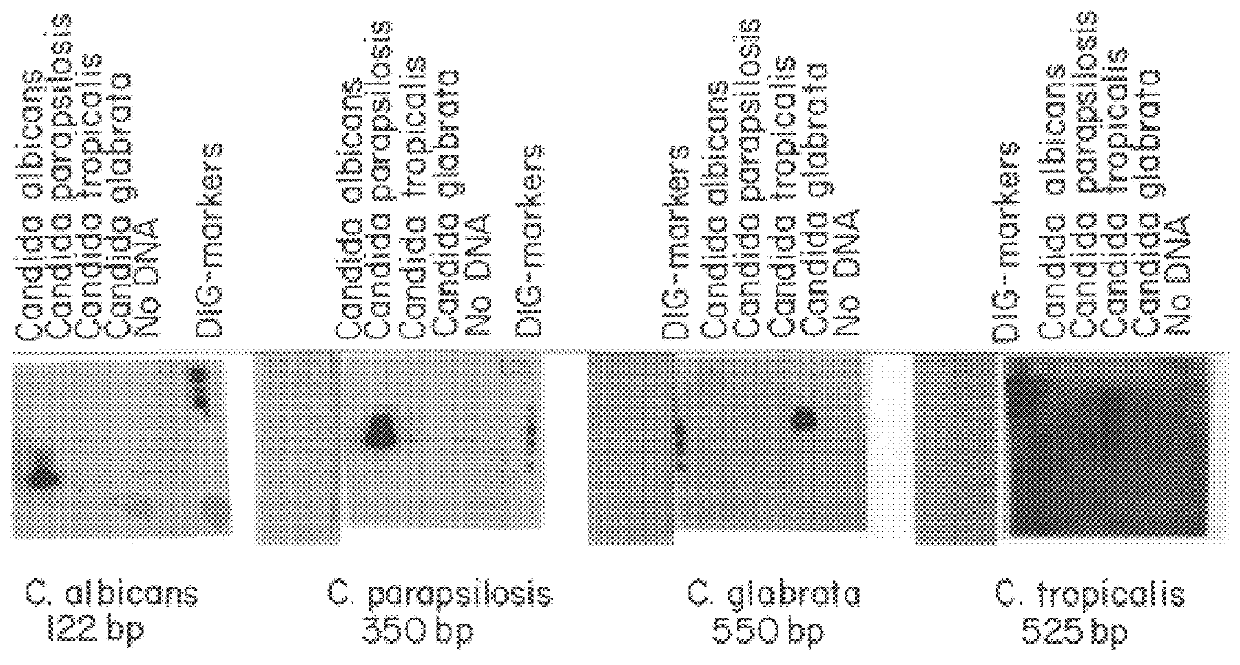
PCR identification and quantification of important Candida species
InactiveUS6017699AConvenient amountHigh sensitivitySugar derivativesMicrobiological testing/measurementQuantitative determinationCombined use
The subject invention relates to a set of DNA primers which, when utilized in conjunction with the polymerase chain reaction (PCR) assay, can amplify and speciate DNA from five medically important Candida species. Furthermore, the PCR amplified products, generated by the primers, can also be used to create species specific probes which can also detect and confirm the five species of Candida. Thus, the present invention allows for early diagnosis and treatment of an infection. The assay is useful in the context of monitoring antifungal treatment regimens, screening potential antifungal agents, and similar applications requiring quantitative determinations.
Owner:UNIV OF PITTSBURGH THE
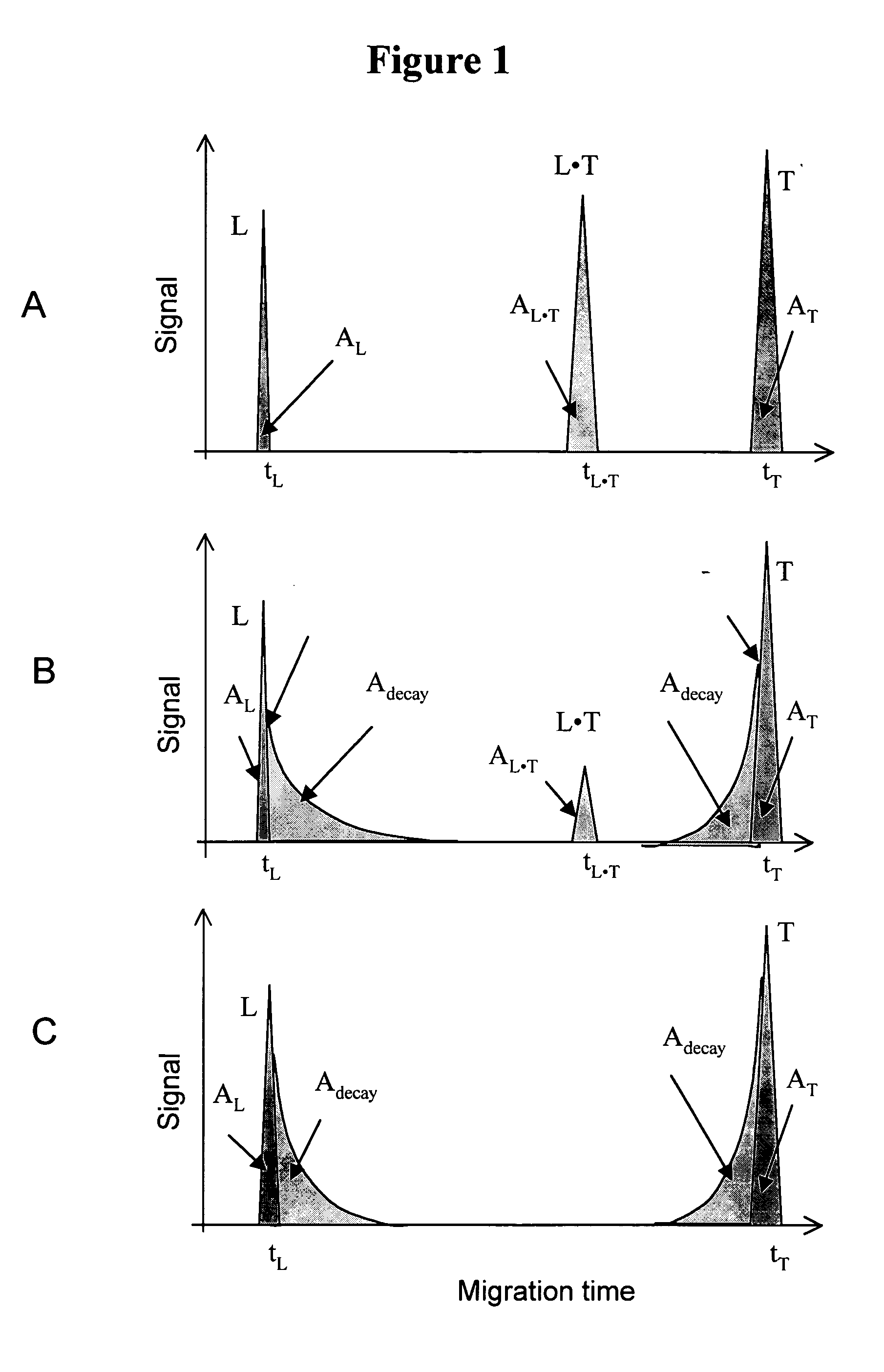
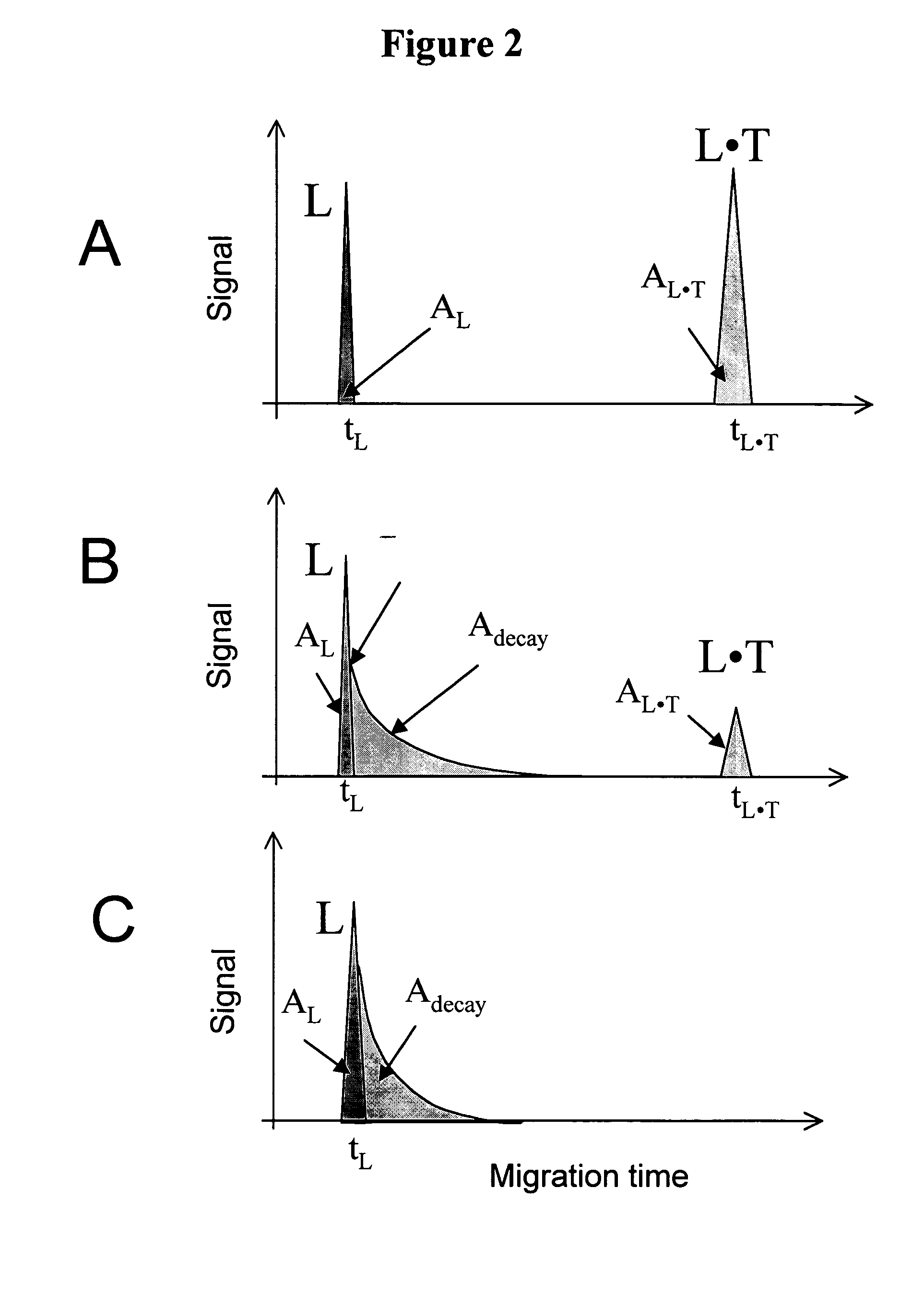
Non-equilibrium capillary electrophoresis of equilibrium mixtures (NECEEM) - based methods for drug and diagnostic development
ActiveUS20050003362A1Enhances electrophoretic separationEasy to separateCompound screeningApoptosis detectionSolid substrateNucleotide Aptamers
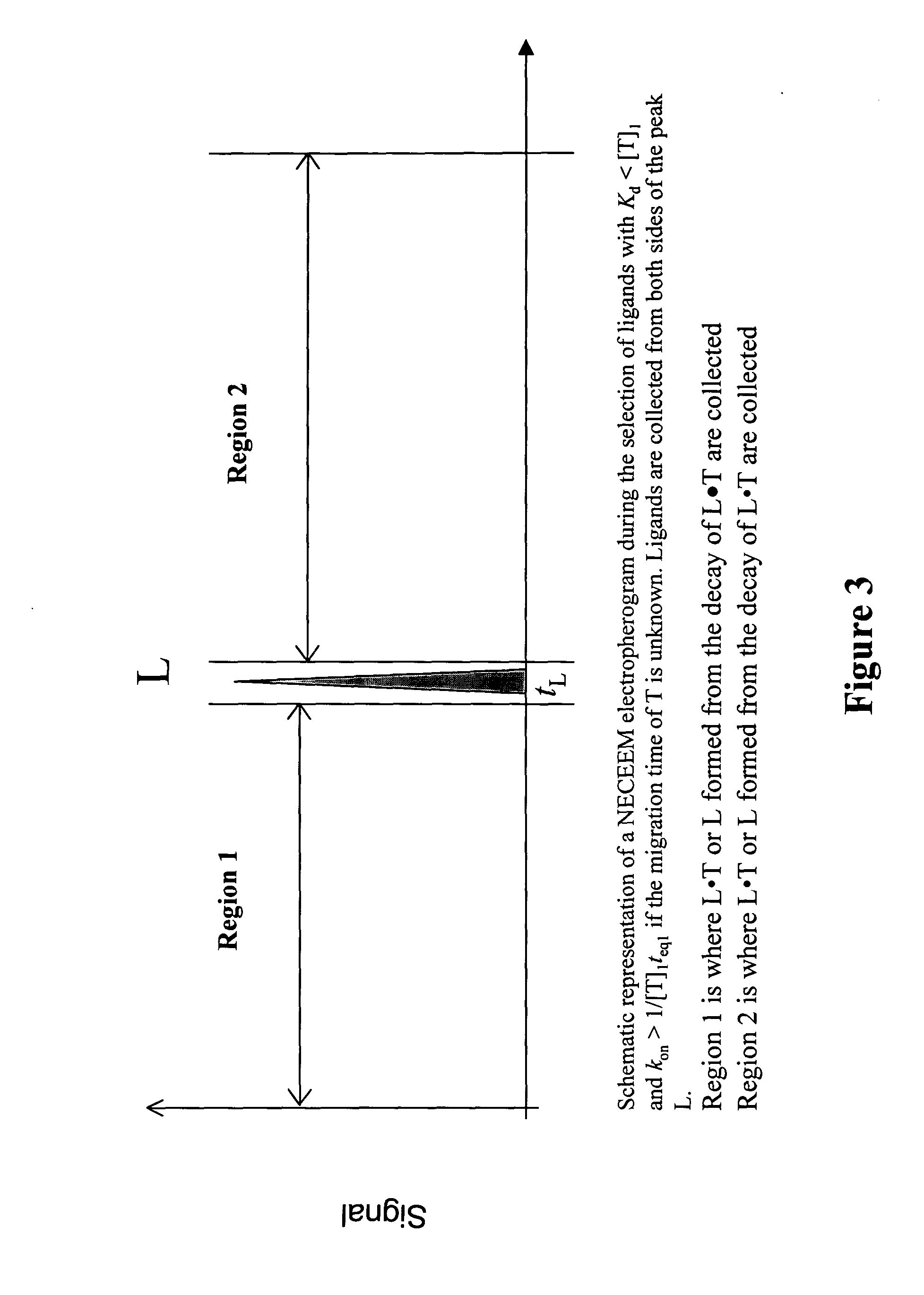
The invention discloses a Non-Equilibrium Capillary Electrophoresis of Equilibrium Mixtures (NECEEM) method and NECEEM-based practical applications. The NECEEM method is a homogeneous technique, which, in contrast to heterogeneous methods, does not require affixing molecules to a solid substrate. The method of the invention facilitates 3 practical applications. In the first application, the method allows the finding of kinetic and thermodynamic parameters of complex formation. It advantageously allows for revealing two parameters, the equilibrium dissociation constant, Kd, and the monomolecular rate constant of complex decay, koff, in a single experiment. In the second practical application, the method of this invention provides an approach for quantitative affinity analysis of target molecules. It advantageously allows for the use of affinity probes with relatively high values of koff. In the third practical application, the method of this invention presents a new and powerful approach to select target-binding molecules (ligands) from complex mixtures. Unique capabilities of the method in its third application include but not limited to: (a) the selection of ligands with pre-determined ranges of kinetic and thermodynamic parameters of target-ligand interactions, (b) the selection of ligands present in minute amounts in complex mixtures of biological or synthetic compounds such as combinatorial libraries of oligonucleotides, and (c) the selection of ligands for targets available in very low amounts. In particular, the method of this invention provides a novel approach for the selection of oligonucleotide aptamers. The NECEEM-based method can be used for discovery and characterization of drug candidates and the development of new diagnostic methods.
Owner:KRYLOV SERGEY
Full-automatic fluorescent quantitative immunity analyzer and detection method
ActiveCN105572407AProcessing supportRealize fully automated detection and analysisMaterial analysisFluorescenceData acquisition
The invention relates to a full-automatic fluorescent quantitative immunity analyzer and a method, and belongs to the technical field of detection. The full-automatic fluorescent quantitative immunity analyzer comprises a sample feeding device, a hatching device, a sampling device, a data acquisition device, a reagent card device and a system control module; the purpose of automatic quantitative immunity analysis is achieved through organic combination of all the devices and modules and mutual cooperation of all elements. According to the fluorescent quantitative immunity detection method, the full-automatic fluorescent quantitative immunity analyzer is adopted, therefore, full-automatic instrumental analysis is achieved, the detection efficiency is improved, and the professional requirement on an operator is lowered.
Owner:GUANGZHOU WONDFO BIOTECH
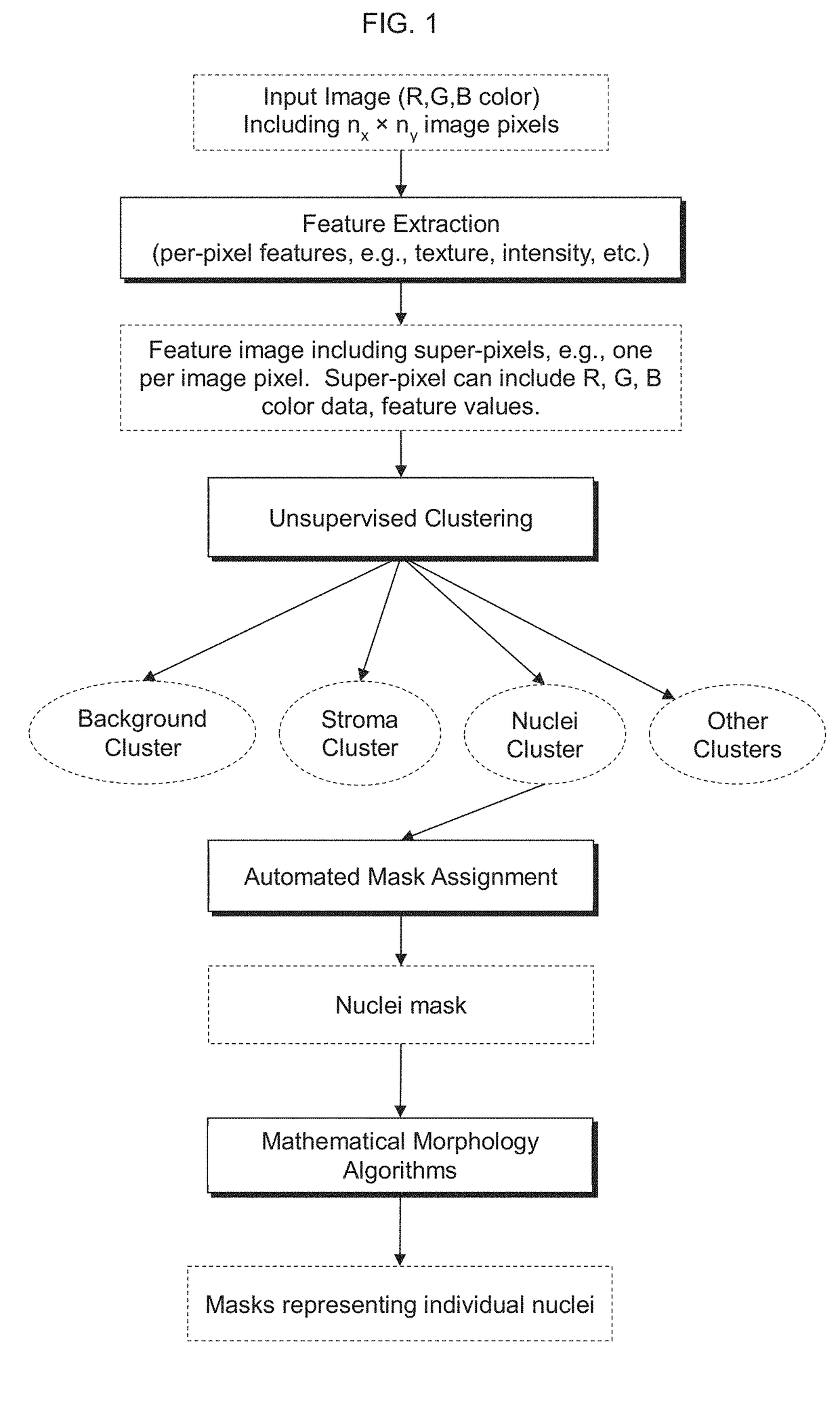
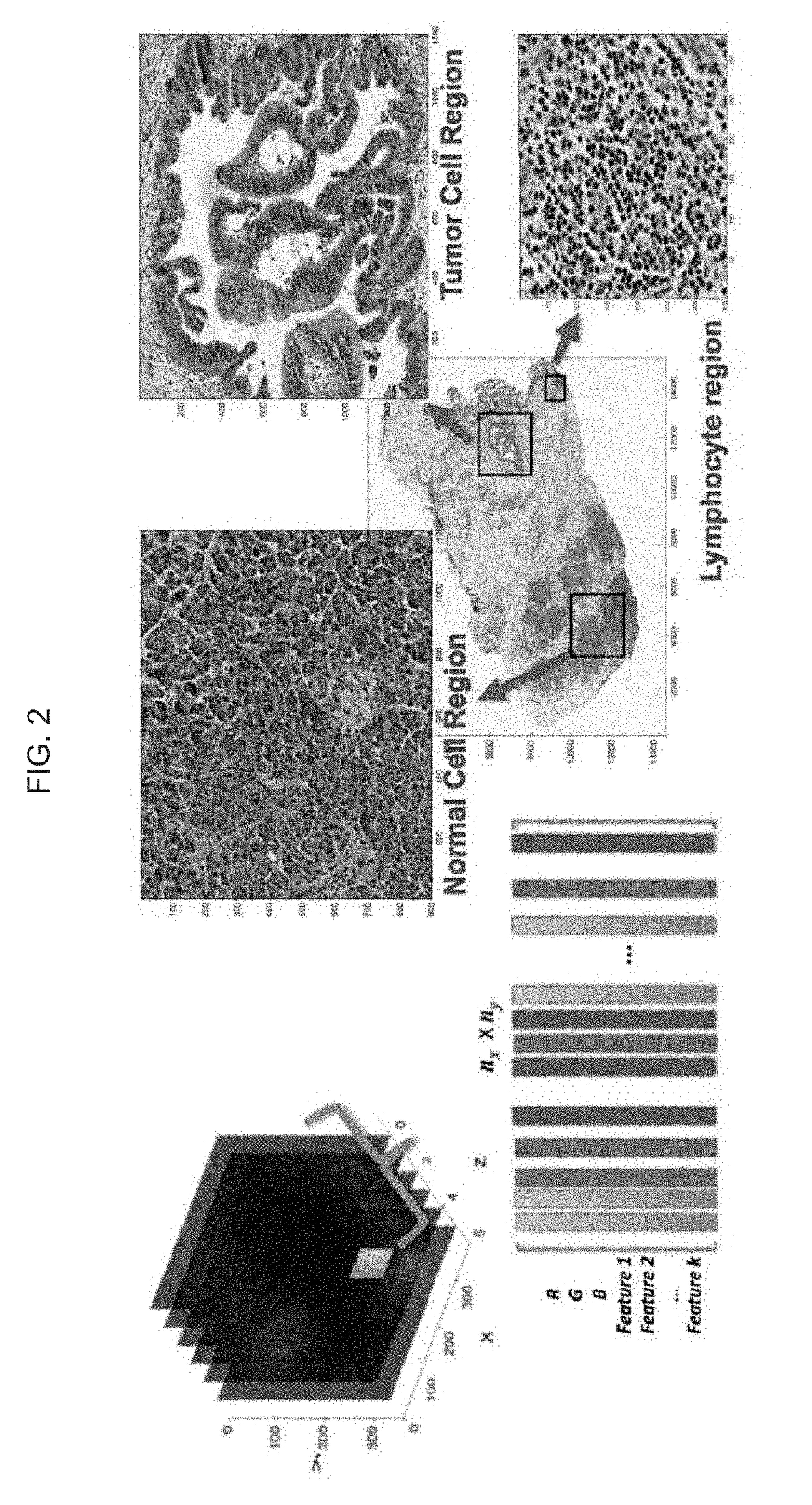
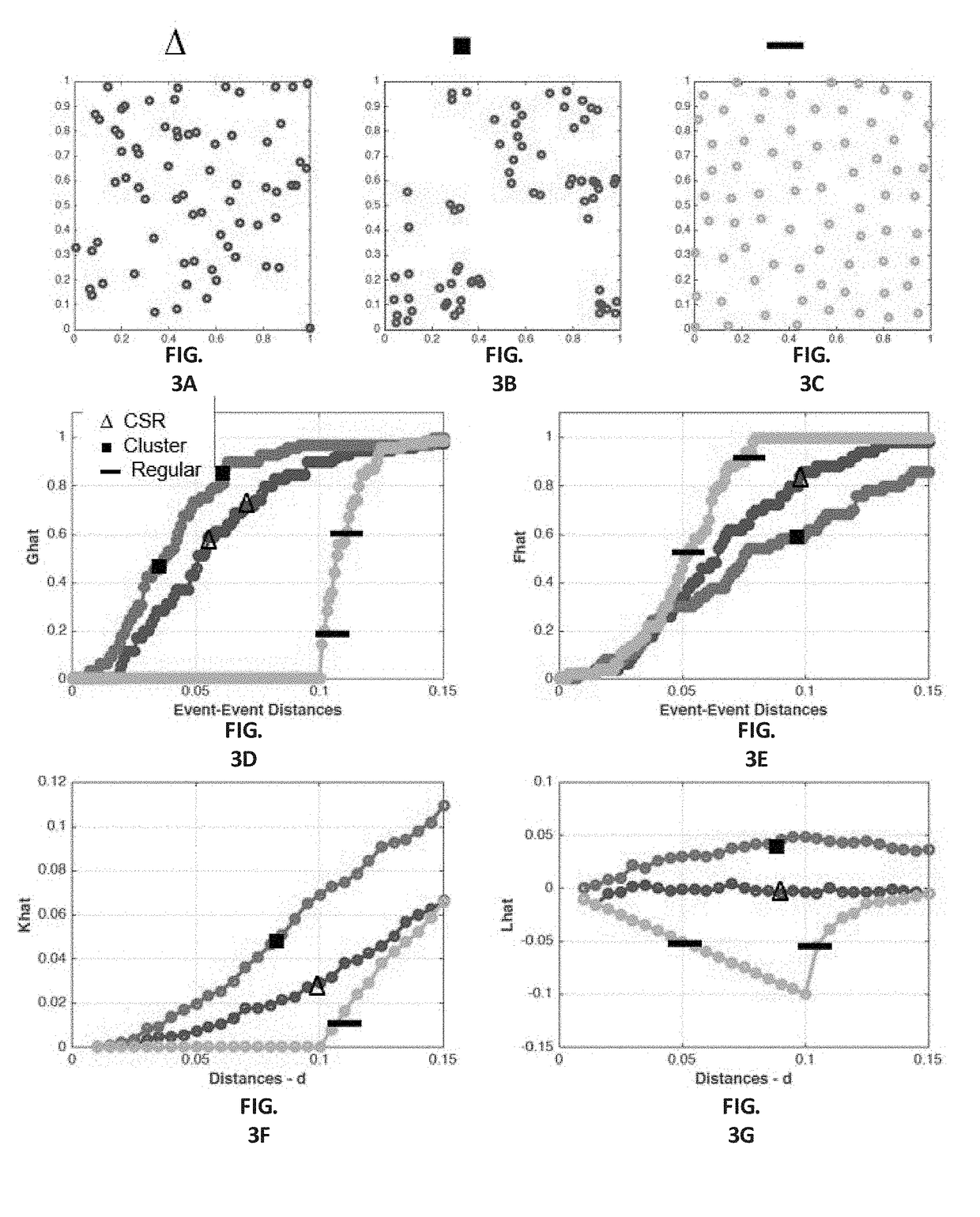
Automatic nuclei segmentation in histopathology images
Provided herein are systems and computer-implemented methods for quantitative analyses of tissue sections (including, histopathology samples, such as immunohistochemically labeled or H&E stained tissue sections), involving automatic unsupervised segmentation of image(s) of the tissue section(s), measurement of multiple features for individual nuclei within the image(s), clustering of nuclei based on extracted features, and / or analysis of the spatial arrangement and organization of features in the image based on spatial statistics. Also provided are computer-readable media containing instructions to perform operations to carry out such methods. A quantitative image analysis pipeline for tumor purity estimation is also described
Owner:OREGON HEALTH & SCI UNIV
Device for simultaneously carrying out blood group determination, serum cross-check and antibody detection test
ActiveUS7745228B2Practical and easily mannerFunction increaseBioreactor/fermenter combinationsBiological substance pretreatmentsAnalyteQuantitative determination
This invention relates to a device for the simultaneous qualitative or quantitative determination of several analytes in a liquid sample. The device comprises a membrane with a charging zone, for the application of the liquid sample, at least two indicator zones which can interact with the analyte(s) and at least one absorption region, which accepts the fluid after passing through the indicator zones, whereby the indicator zones lie between the charging zone and an absorption region, characterized in that the flow directions (flow tracks) are essentially parallel from the application zone through each indicator zone to an absorption region and at least two different flow tracks are present. The invention further relates to a method for the determination of several analytes or derivatives thereof in a liquid sample, comprising: application of the sample to the charging zone of a membrane of the device, whereby said sample is present in sufficient amounts to permit the sample fluid to flow in the direction of the absorption region through the indicator zones and to permit the analytes or derivatives thereof in the liquid sample to form a complex in the indicator zone.
Owner:GRIFOLS DIAGNOSTIC SOLUTIONS INC
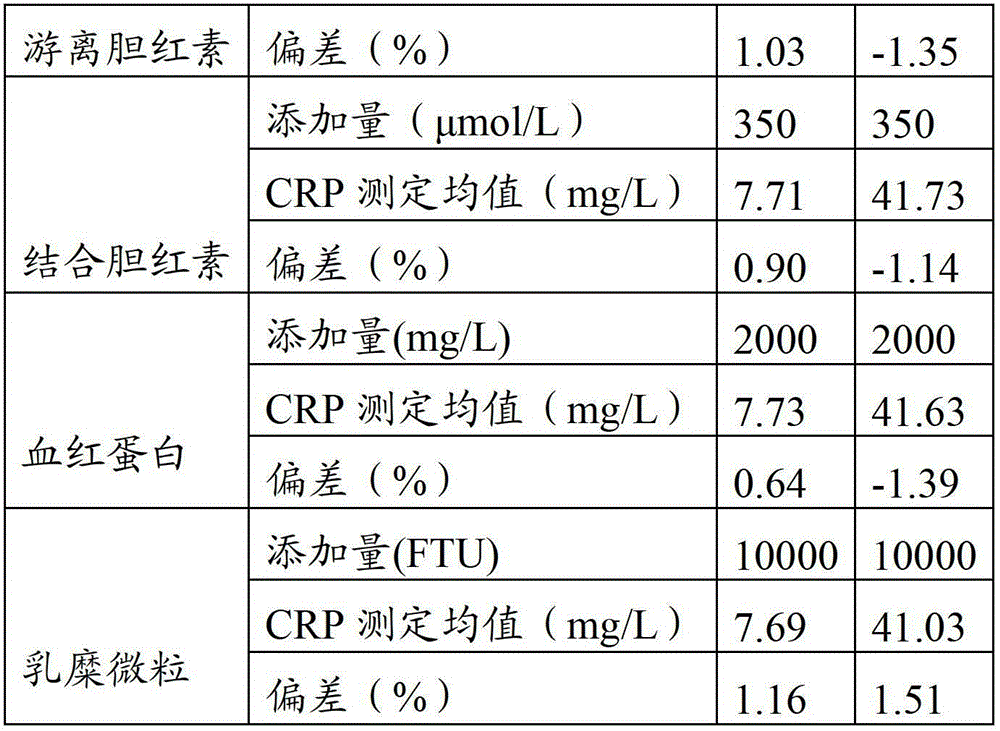
Full-scale C-reactive protein (CRP) colloidal gold immunoturbidimetric assay kit
The invention provides a full-scale CRP colloidal gold immunoturbidimetric assay kit. A quantitative assay purpose is reached through enhancing the turbidity by the colloidal gold. The method used by the kit provided by the invention solves a problem that the generation of a precipitate after a latex reinforced immunoturbidimetric reaction goes against biochemical instrument cleaning. The kit provided in the invention comprises a reagent R1 and a reagent R2, wherein the reagent R2 is a proper buffer solution; and the reagent R2 is a buffer solution of the colloidal gold combined with an antihuman CRP antibody. The kit has the characteristics of high sensitivity, strong specificity and good stability, can be used for assaying the content of the CRP in serum or blood plasma, and is suitable for clinical fully-automatic biochemical analyzers. The CRP assay sensitivity of the kit can reach 0.01mg / L, and the upper CRP assay limitation of the kit is 500mg / L.
Owner:重庆沃康生物科技有限公司
Quantitative assay for low abundance molecules
InactiveUS7074586B1Bioreactor/fermenter combinationsBiological substance pretreatmentsQuantitative determinationBioinformatics
A method for quantitatively assaying one or more target molecules in a sample uses a nucleic acid aptamer that is specific for each target molecule. A quantitative replicative procedure is used to determine a quantity of aptamer specific for each molecule.
Owner:SOURCE PRECISION MEDICINE INC
Quantitative assay with extended dynamic range
InactiveUS20060019404A1Material analysis by observing effect on chemical indicatorBiological testingAnalyteChemical composition
An efficient design for an expanded dynamic range in a lateral flow one step assay for the detection of an analyte in a biological sample is disclosed. The device comprises a multiple strip design, each constructed of four zones; a sample receiving zone, a sample treatment zone, a labeling zone, and a capture zone. The sample containing analyte is accepted in the sample receiving zone in the form of blood, serum, plasma, or urine. It is then carried into the sample treatment zone where it is rendered compatible with the chemistries of the assay strip. The treated sample then flows into the labeling zone where it interacts with visible particles that are coupled to analyte specific binding proteins. The flow continues, carrying the labeled analyte into the capture zone where it is immobilized in specific regions with analyte specific binding proteins. Excess flow is absorbed in an absorbent zone that is in contact with the capture zone. A positive result is interpreted by detection of the visible particles in the specified regions of the capture zone.
Owner:BAYER HEALTHCARE LLC
Immunochromatographic test strip for full quantitative detection of C-reactive protein and preparation method thereof
The invention discloses an immunochromatographic test strip for full quantitative detection of C-reactive protein and a preparation method thereof. The test strip is composed of a sample pad, a mark pad, a coating film and absorbent paper which are successively overlapped and adhered on a baseplate, wherein the mark pad is coated with C-reactive protein (CRP) monoclonal antibody labelled by fluorescent latex particles and rabbit IgG labelled by fluorescent latex particles; and the coating film is composed of a detection region and a quality control region, and the detection region is coated with another CRP monoclonal antibody which is at different epitope with the CRP monoclonal antibody labelled by fluorescent latex particles. The C-reactive protein immunochromatographic test strip can perform sensitive quantitative detection on the full CRP in 10 seconds, can diagnose diseases and identify infection more rapidly and accurately, and can detect infectious illness state and determine the curative effect of antibiotics; and the full CRP has two detection results, namely hs-CRP and conventional CRP, comprehensive linear range and good detection sensitivity and less required sample amount, and is very convenient to operate.
Owner:GUANGZHOU WONDFO BIOTECH
In-situ composite system based on carbon quantum dot/manganese dioxide nanometer sheet layer and using method for detecting content of glutathione
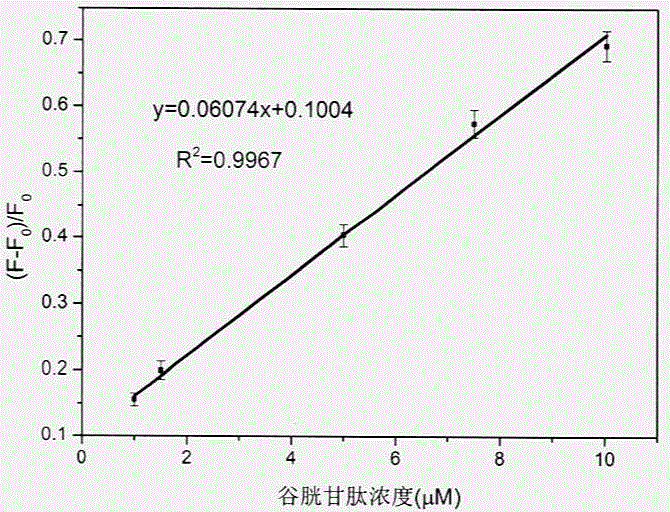
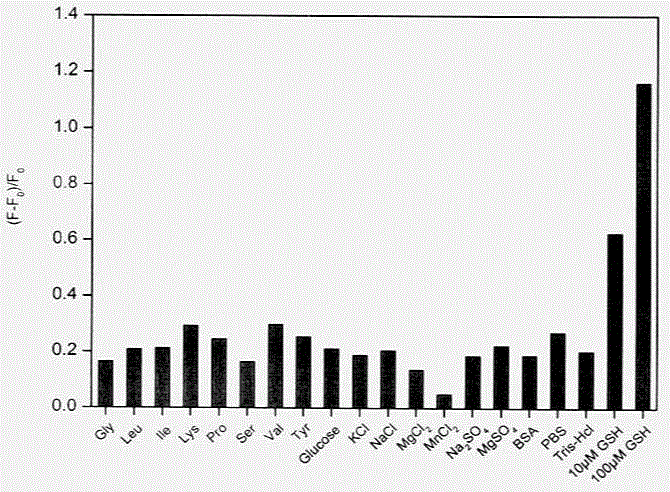
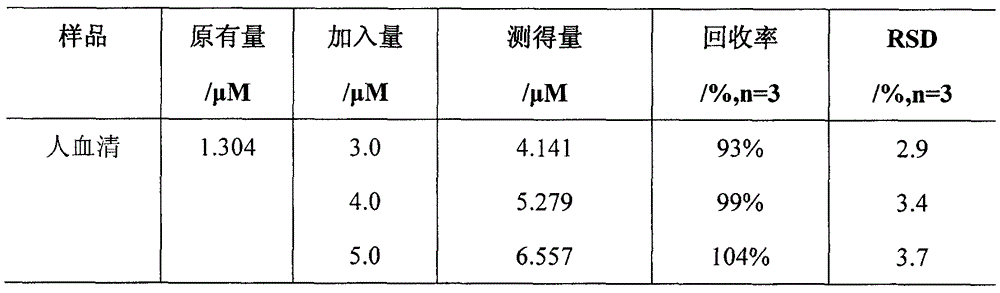
ActiveCN104597019ANo pollution in the processHigh precisionFluorescence/phosphorescenceTest sampleRedox
The invention discloses an in-situ composite system based on a carbon quantum dot / manganese dioxide nanometer sheet layer and a using method of the in-situ composite system. The using method comprises the following steps: firstly, completely quenching fluorescence by using a manner that carbon quantum dots are adsorbed on a manganese dioxide nanometer sheet layer prepared by reducing potassium permanganate in situ, and then quantitatively analyzing and determining the content of the GSH in a GSH to-be-tested sample based on a principle of causing digestion of the manganese dioxide nanometer sheet layer, releasing the carbon quantum dots and recovering the fluorescence thereof by virtue of a redox reaction between glutathione (GSH) and manganese dioxide. According to the in-situ composite system, the detection method is high in precision, the repeatability is good, a used carbon material is low in price, easily available, and free from polluting the environment, and has relatively good biological safety; meanwhile, the in-situ composite system is simple and convenient to operate, relatively strong in practicability, high in sensitivity, and excellent in selectivity of the GSH.
Owner:ZHENGZHOU UNIV
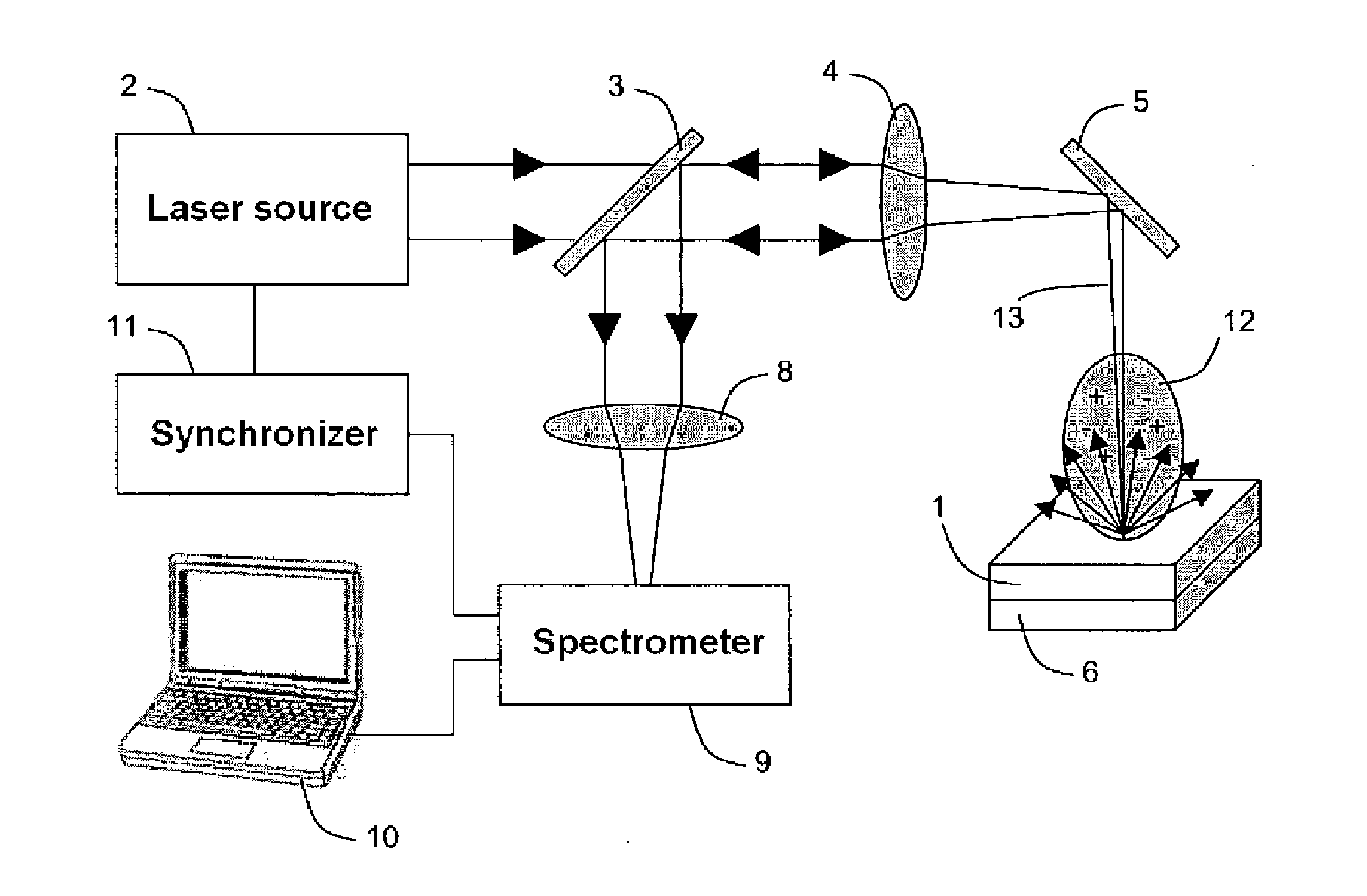
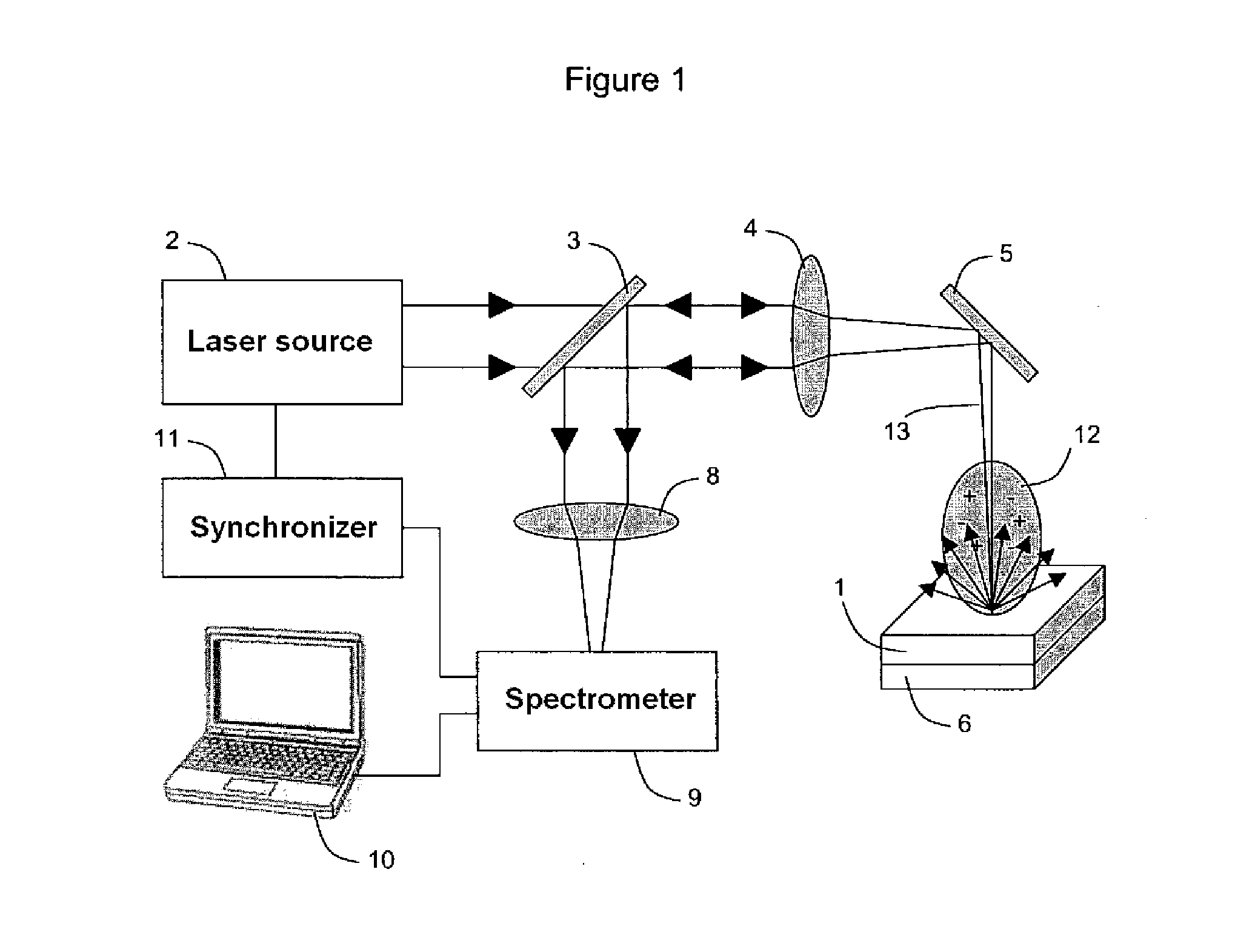
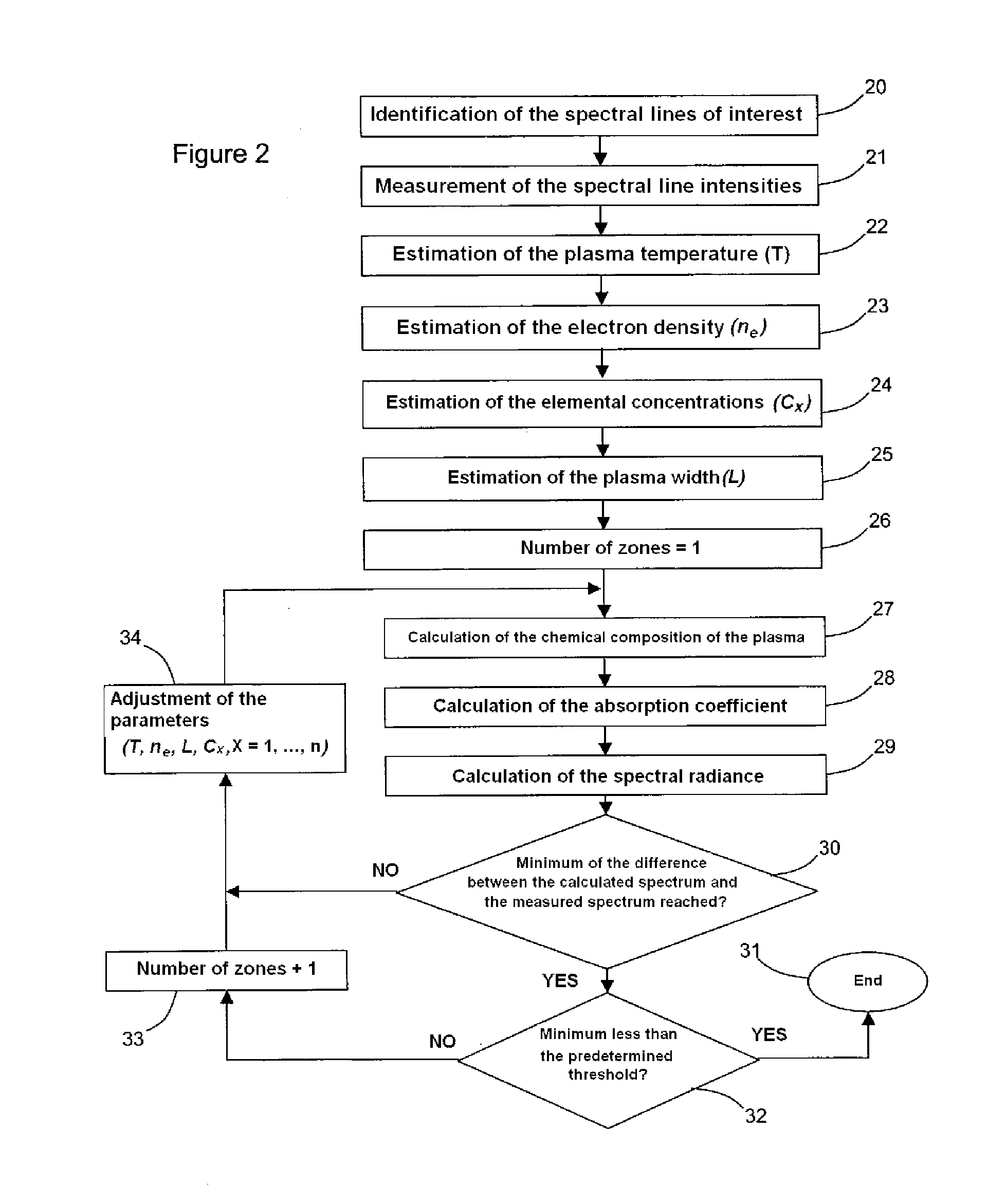
System and method for quantitative analysis of the elemental composition of a material by laser-induced breakdown spectroscopy (LIBS)
ActiveUS20120029836A1Quick calculationShort calculation timeEmission spectroscopyAnalysis by thermal excitationElemental compositionChemical composition
A system and method for measuring elemental concentrations of a material from a sample containing several elements by LIBS analysis. The material is heated to generate plasma and its chemical composition is determined from spectral analysis of its radiation. The spectral lines of interest are identified among those emitted by the constituents of each element composing sample. The intensities of the spectral lined identified are measured. From an estimate of temperature, electron density and relative concentration values, the chemical composition of the plasma is calculated. The absorption coefficient according to wavelength is calculated for the spectral zones of the lines of interest. From an estimate of the plasma width, the spectral radiance of the plasma is calculated for the same spectral zones and then a comparison of the intensity and shape of the spectrum thus calculated with those of the spectrum measured is performed. These calculations and this comparison are repeated iteratively in order to adjust the temperature, electron density, relative values of the elemental concentrations and width of the plasma.
Owner:CENT NAT DE LA RECHERCHE SCI
Emissive compositions with internal standard and related techniques
InactiveUS20080085566A1Chemiluminescene/bioluminescenceSynthetic resin layered productsAnalyteQuantitative determination
The present invention provides materials, devices, and methods related to determination of an analyte. In some embodiments, an analyte may be determined by monitoring, for example, a change in an optical signal of a luminescent material (e.g., particle) upon exposure to an analyte. The present invention may be particularly advantageous in that some embodiments may comprise an emissive species useful as an internal reference standard. Methods of the invention may also be useful in the quantitative determination of an analyte. In some cases, the present invention may allow for selective determination of an analyte.
Owner:MASSACHUSETTS INST OF TECH
Quantitative analysis of protein isoforms using matrix-assisted laser desorption/ionization time of flight mass spectrometry
InactiveUS20040119010A1Particle separator tubesMicrobiological testing/measurementMass Spectrometry-Mass SpectrometryMatrix assisted laser desorption ionization time of flight
The present invention provides for methods of quantitating the amounts of proteins or peptides, including those that are closely related isoforms, using matrix-assisted laser desorption / ionization time of flight mass spectrometry (MALDI-TOF-MS). Measurement of protein concentrations in vivo has been extremely difficult and problematic, and protein concentrations have not been shown to correlate well with mRNA levels, the standard used in the past. The present invention overcomes the deficiencies of prior methodologies by taking advantage of MALDI-TOF-MS technology and applying it to proteins and peptides in a way that allows for accurate, quantitative measurement in vivo of protein or peptide concentrations.
Owner:UNIV OF COLORADO THE REGENTS OF
Hybrid phase lateral flow assay
ActiveUS20050227371A1Increased binding surface areaHigher irreversible immobilizationCompound screeningApoptosis detectionAnalyteReagent
The invention relates to devices for performing single step assays for the determination of the presence or absence of an analyte in a liquid sample, and methods of determining the presence or absence of such analytes using such devices. Devices disclosed comprise a labeled analyte-binding reagent reversibly-immobilized on a non-porous solid material, which solid material is in physical contact with a dry porous carrier bearing an immobilized analyte-binding reagent. Also provided are quantitative assay devices.
Owner:QUIDEL
Device and method for qualitative and quantitative determination of intravenous fluid components
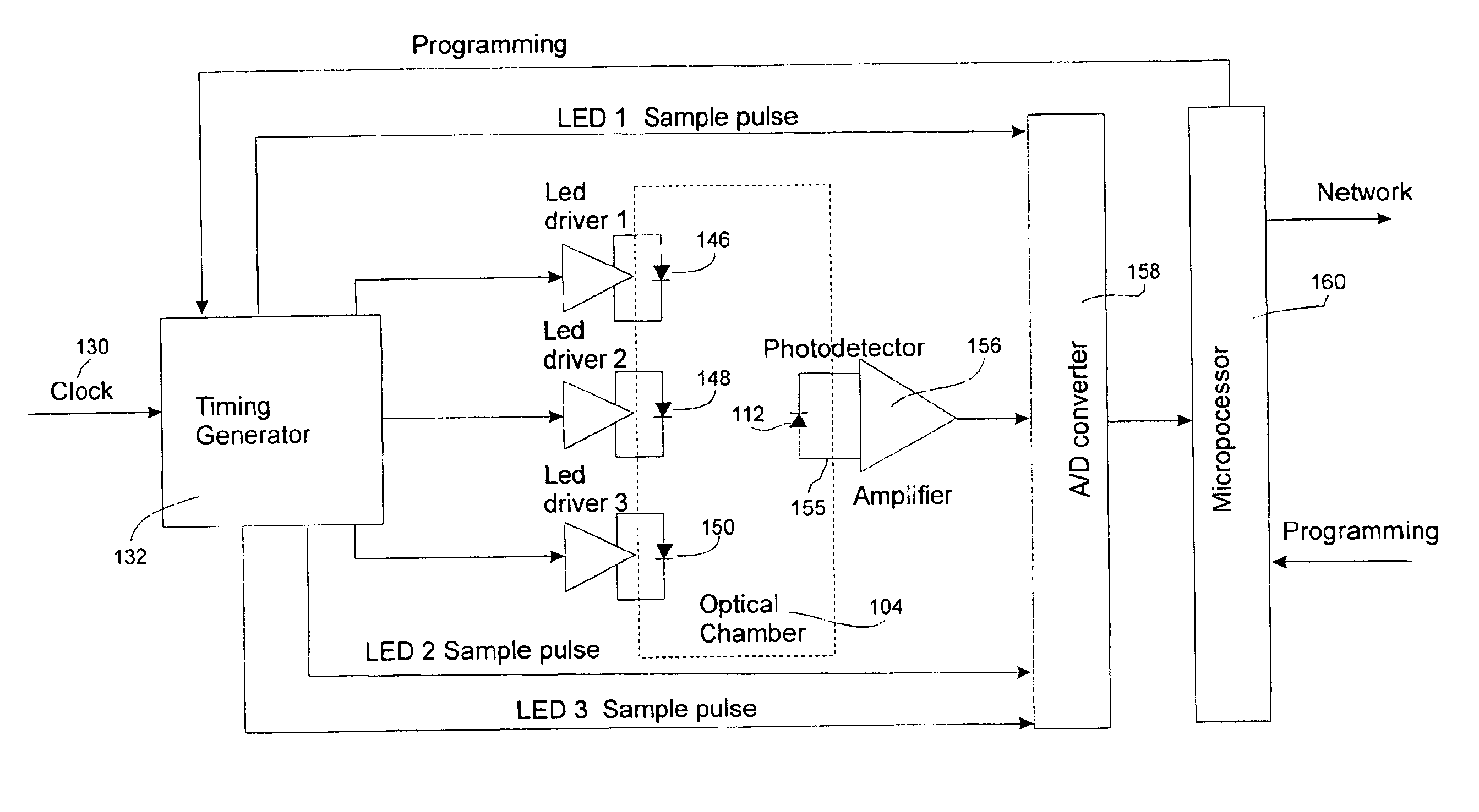
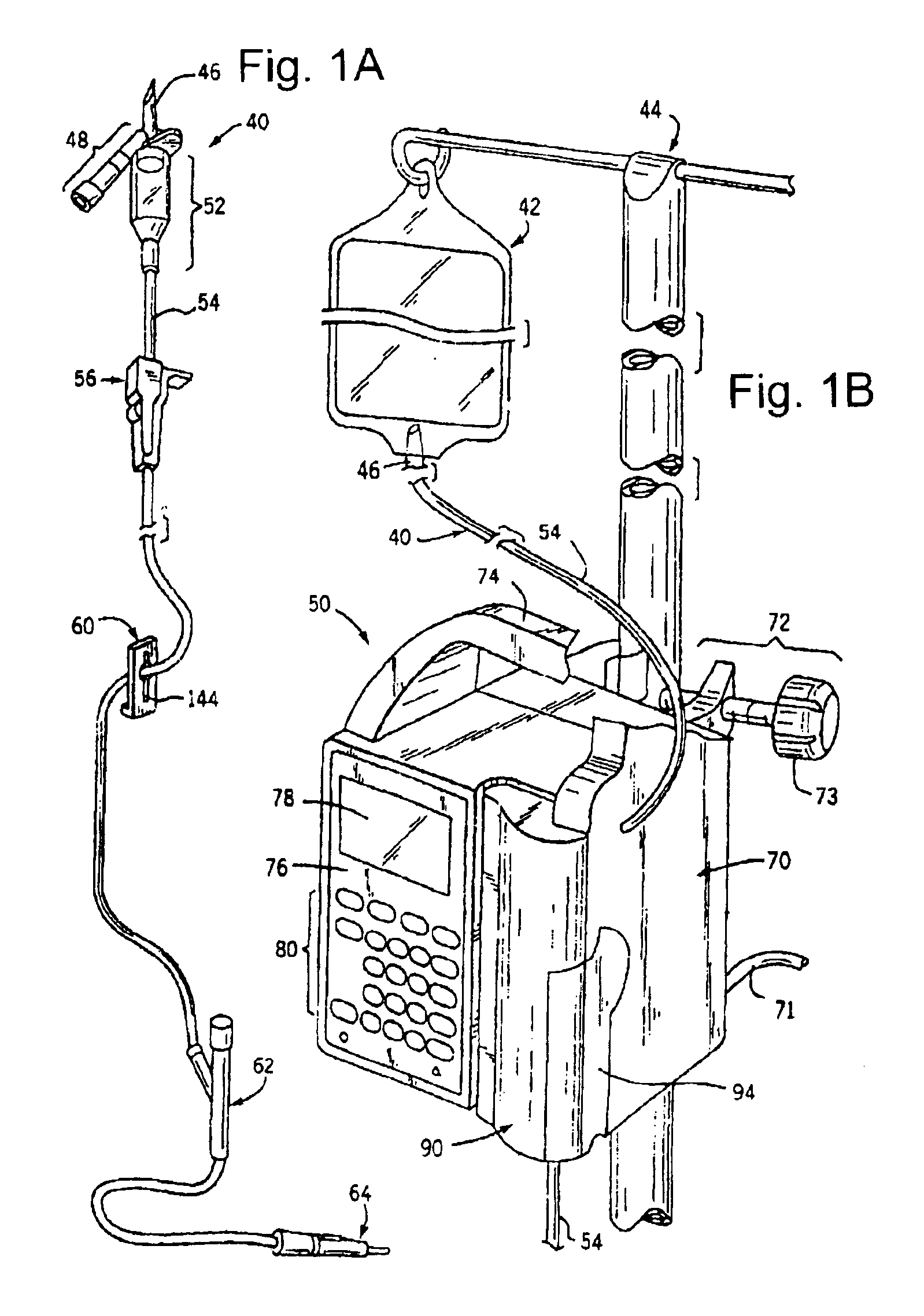
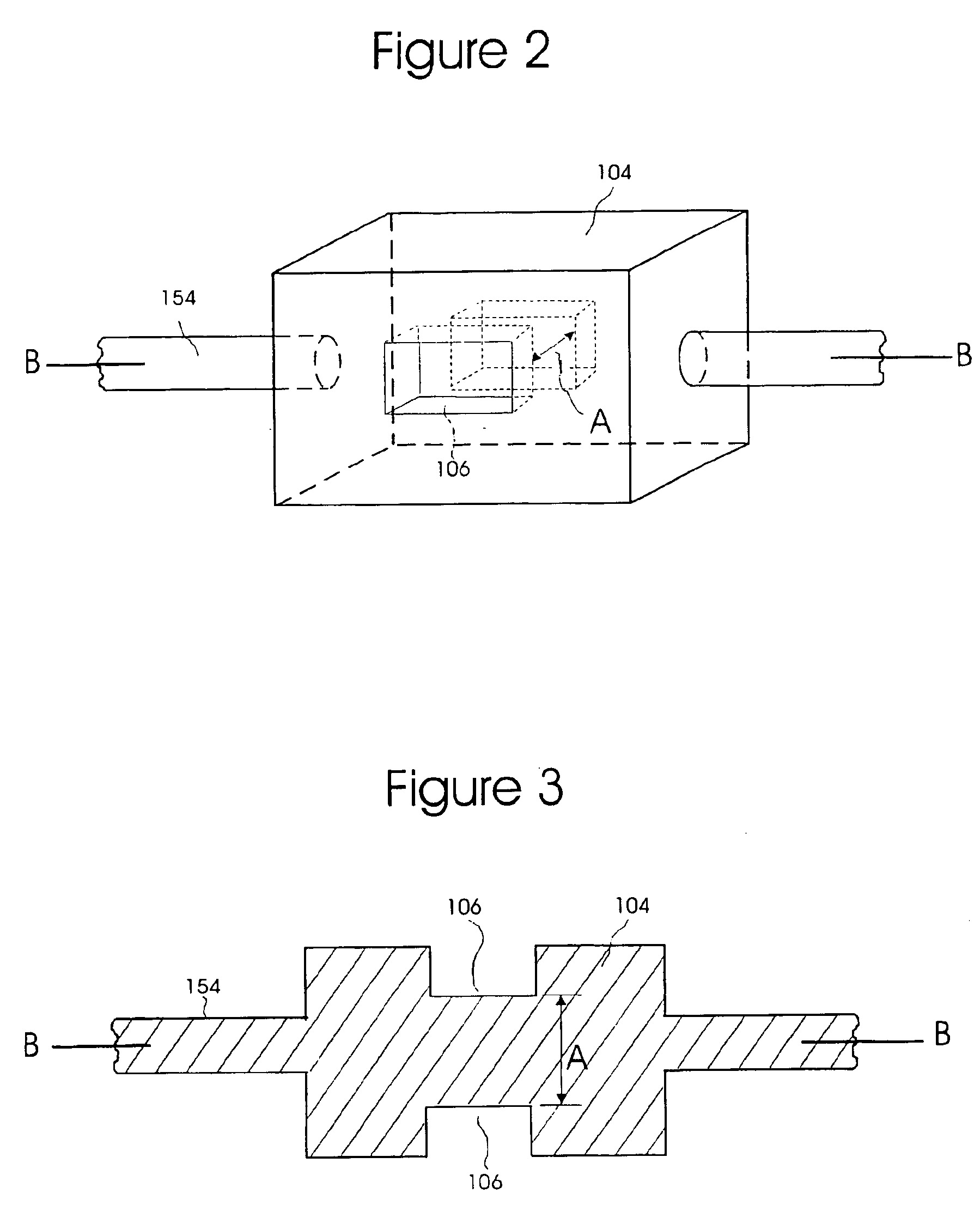
InactiveUS6847899B2Minimize and eliminate errorConvenient careOptical radiation measurementDrug and medicationsMedicineQuantitative determination
A device and process for preventing medical errors due to the improper administration of an intravenously delivered medication includes the spectroscopic analysis of intravenous fluid components. An emission source and detector are placed adjacent to the intravenous tubing of an administration set to generate signals for spectroscopic analysis. The signals are processed to identify the medication and, in certain embodiments of the invention, can determine the medication's concentration. In a preferred embodiment, the emission source, detector, and hardware and software for the spectroscopic analysis are placed in an infusion pump.
Owner:DEAN ALLGEYER M D
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com