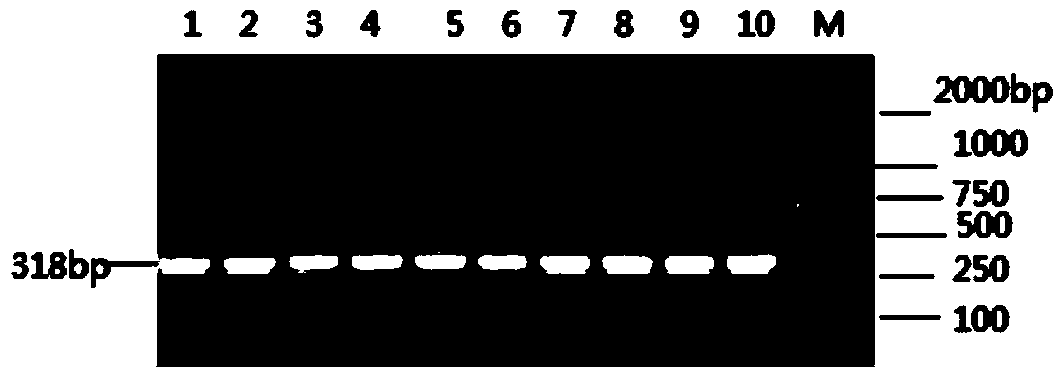
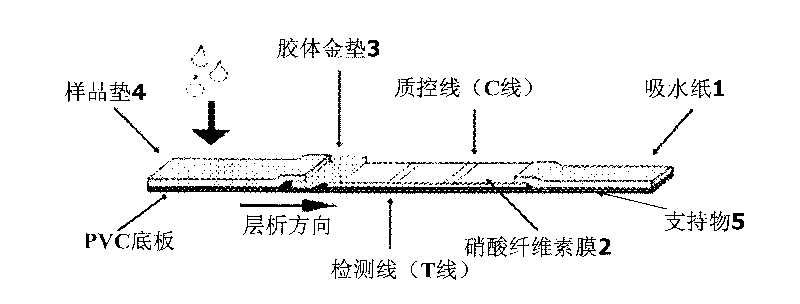
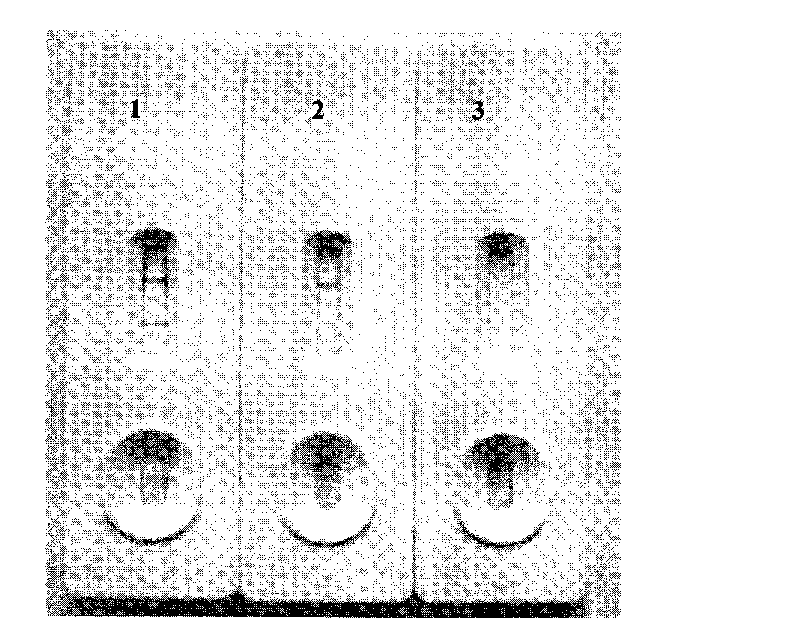
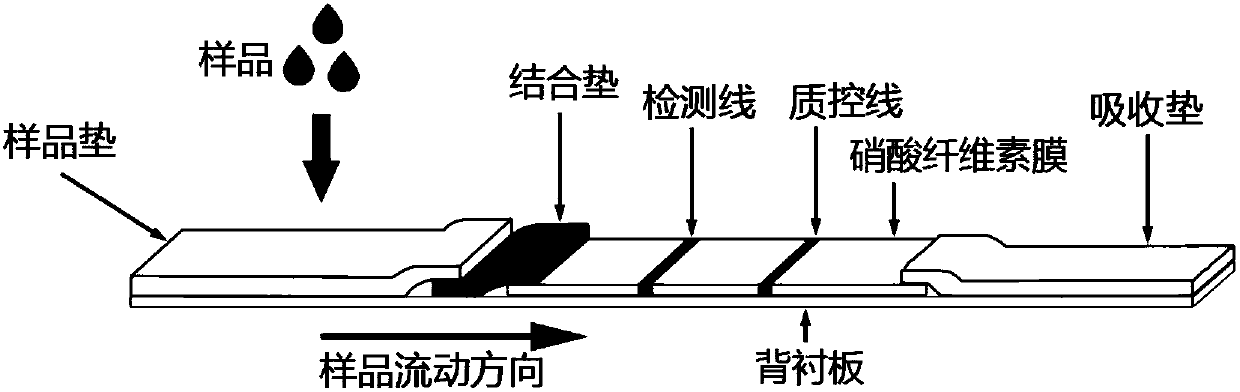
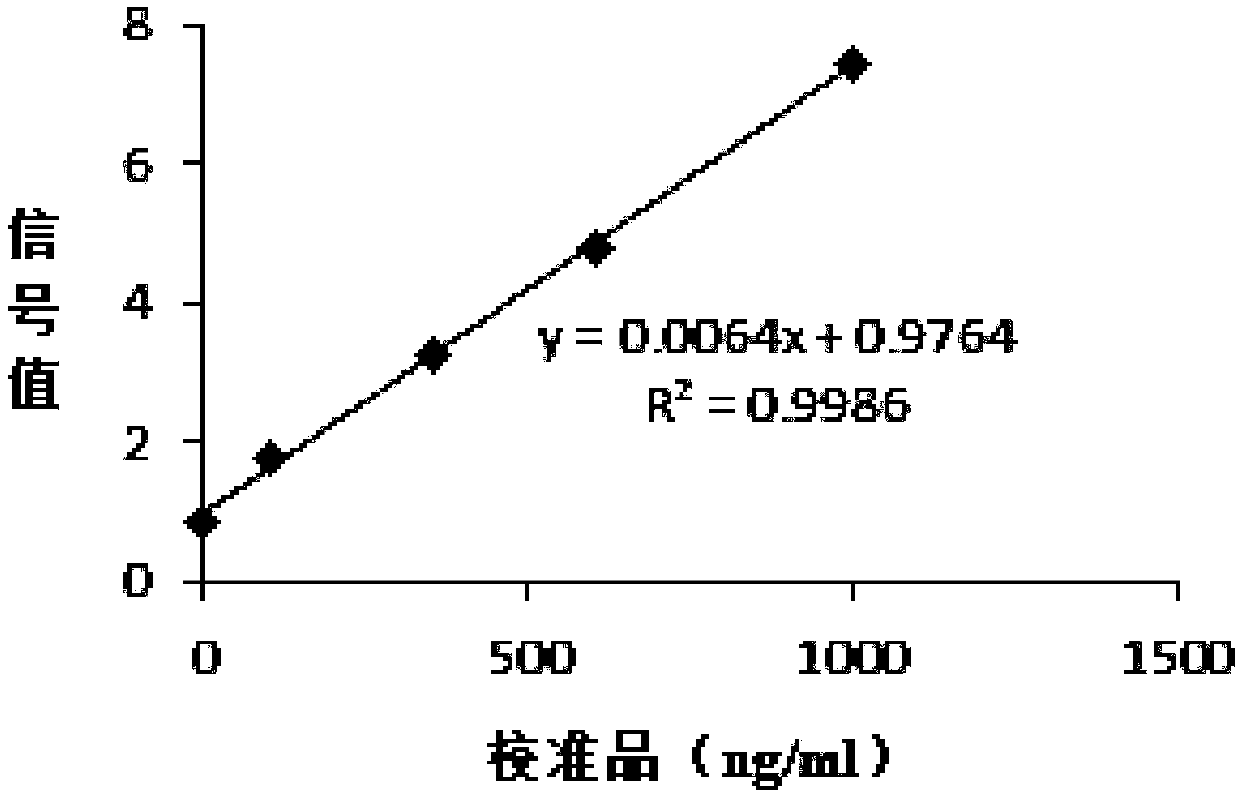
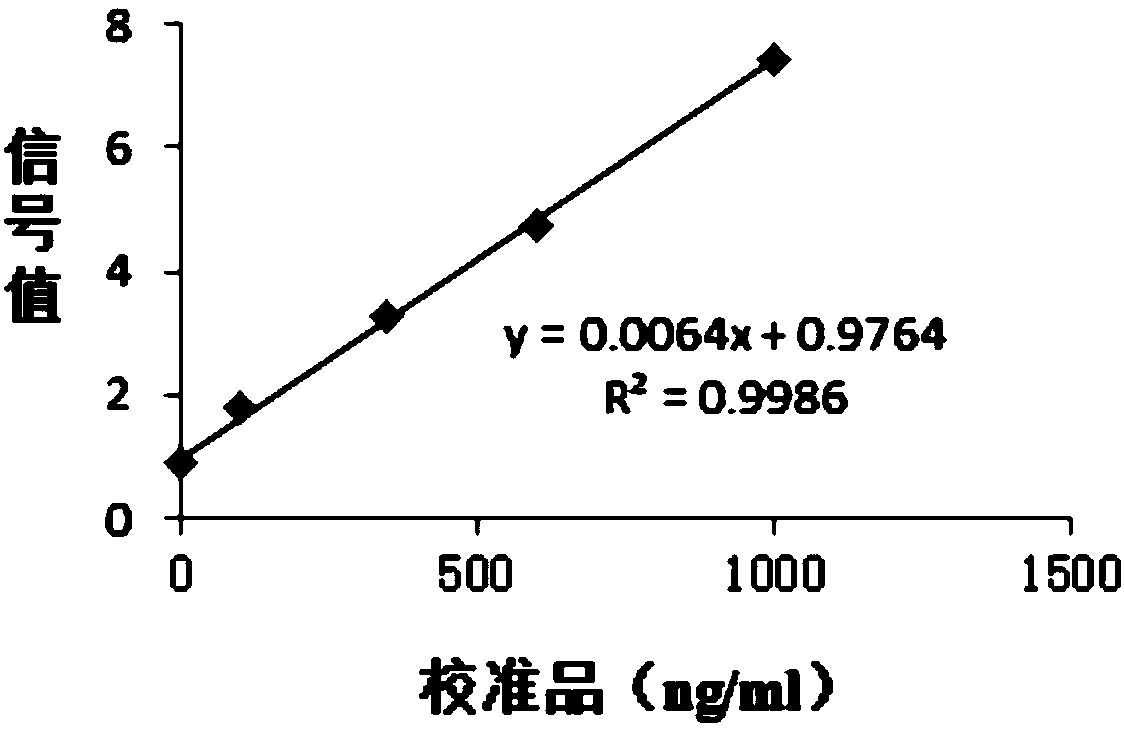
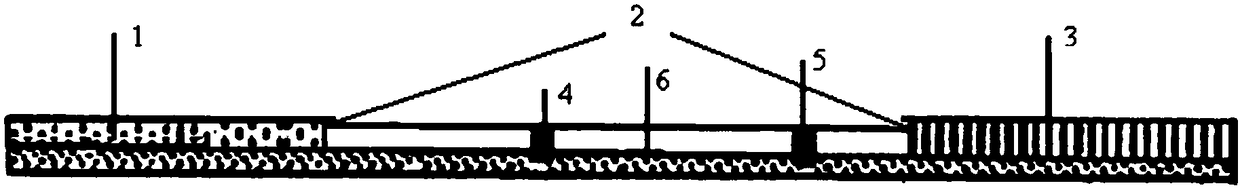
Patents
Literature
717 results about "Immunochromatographic test" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
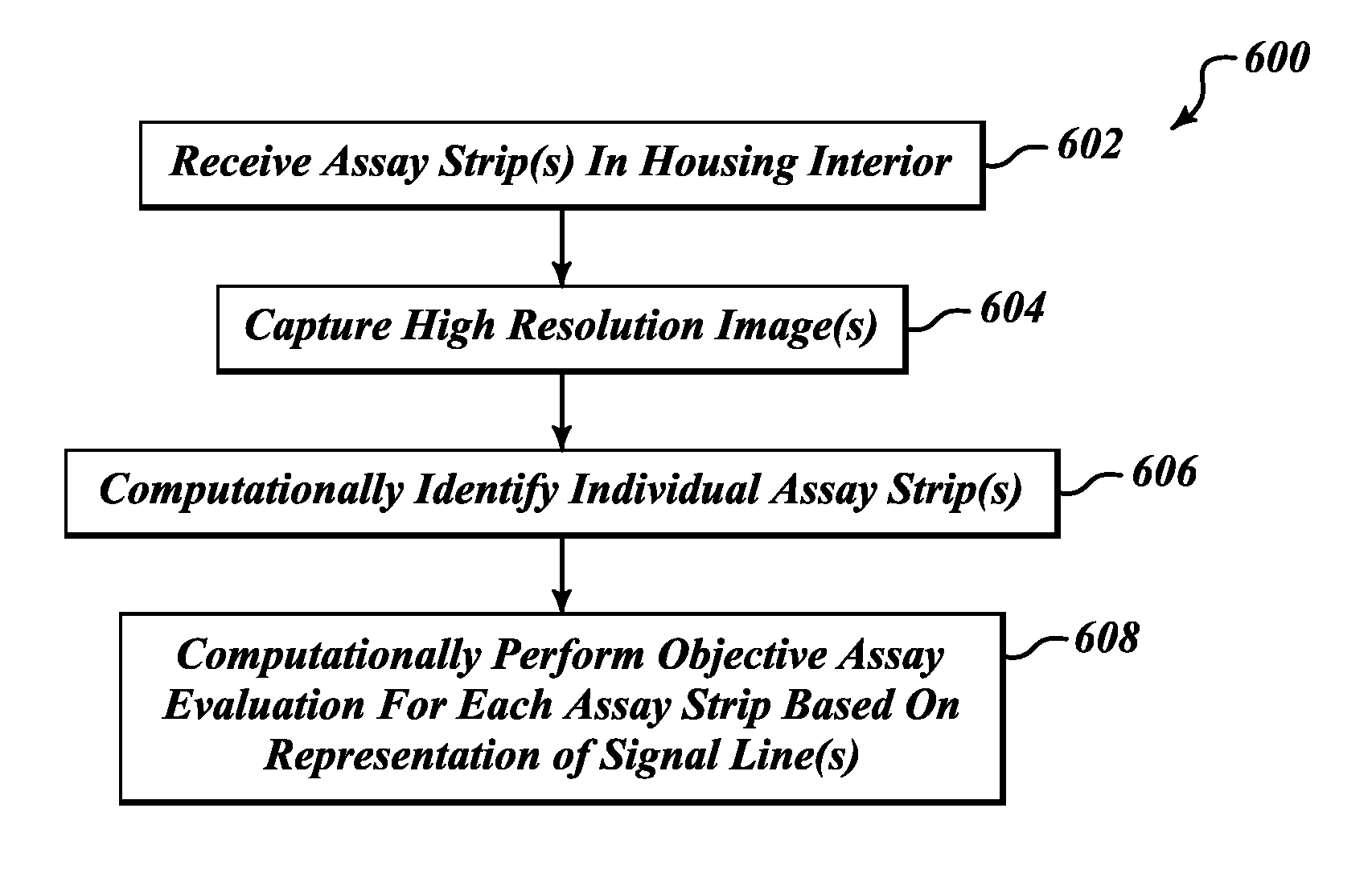
Apparatus, method and article to perform assays using assay strips
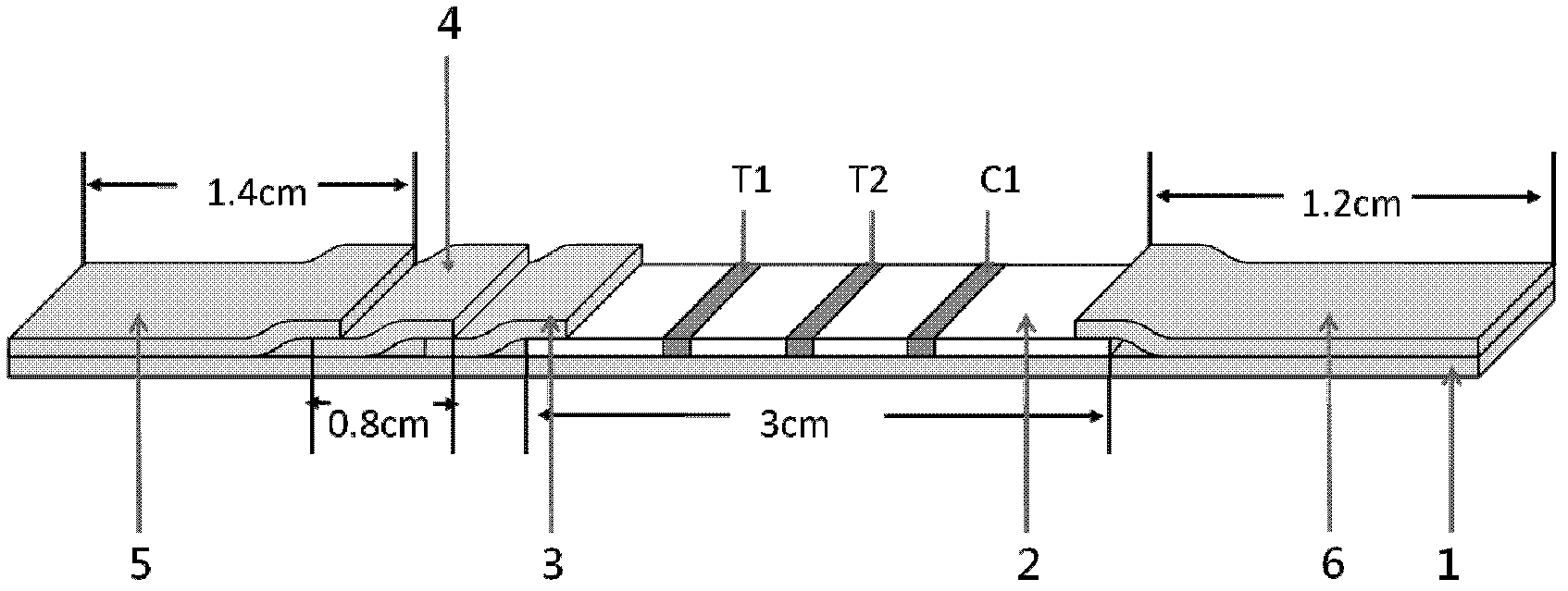
ActiveUS20100045789A1Material analysis by observing effect on chemical indicatorLocal control/monitoringControl signalControl line
An assay system includes an optical imager to acquire high resolution images of assay strips (e.g., lateral flow immunochromatographic test strips) and performs image processing to identify individual assay strips and determine results for each assay strip, by quantifies the presence or absence of test signal line(s) and control signal line(s). Assay strips may be in a holder or carrier contained in a specimen container also holding a specimen. The assay system automatically logs all results and data to a database that stores a high resolution image of the original immunochromatographic assay, the values of test line(s) and control line(s), and the test result. A user interface directs an end user through operation.
Owner:GENPRIME
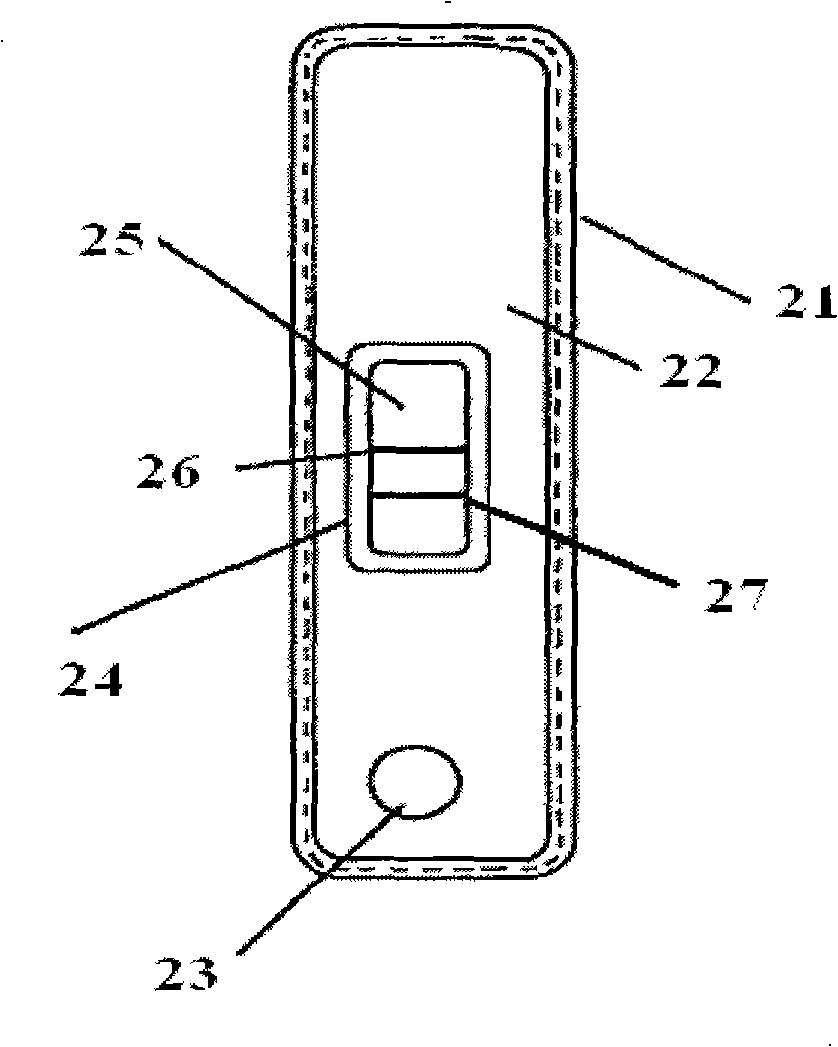
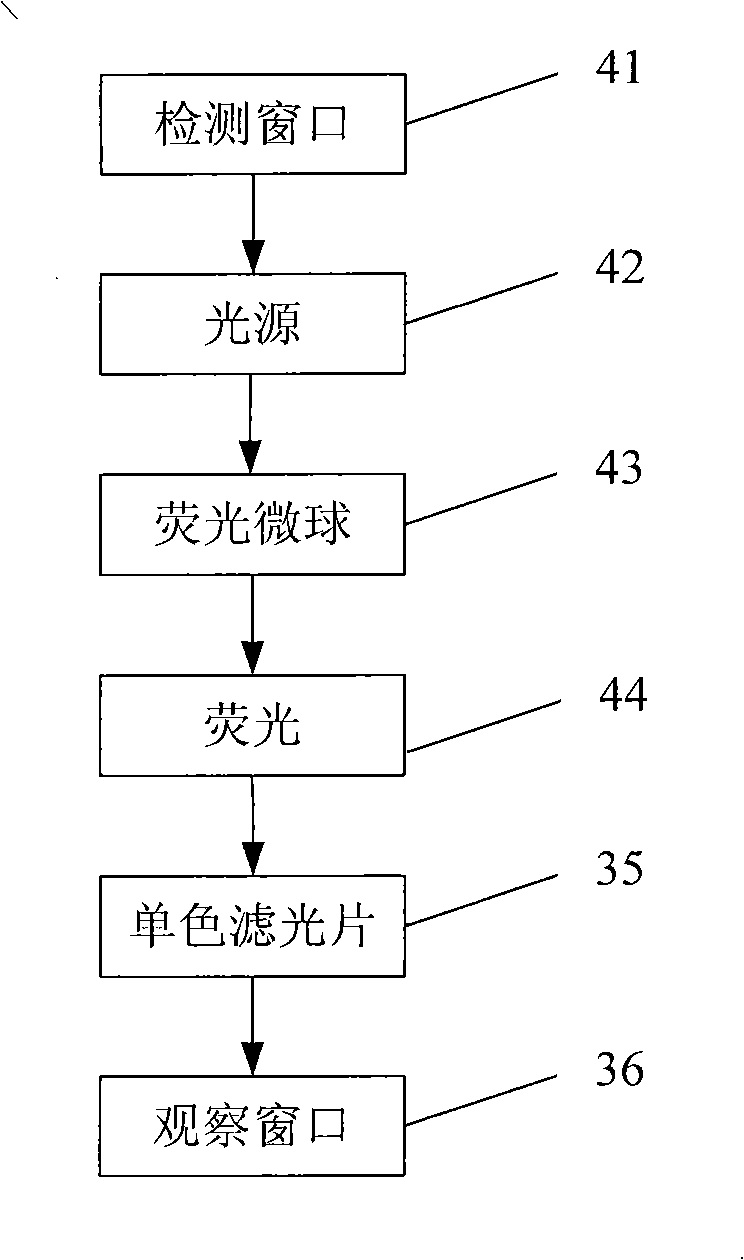
Method for producing fluorescent microballoons immune chromatography test paper stripe and quantitative determination method
ActiveCN101493460ASimple and fast operationHigh sensitivityFluorescence/phosphorescenceFiberCompound organic
The invention discloses a method for preparing fluorescent microspheres immunochromatographic test paper strip and quantitative detection method. The invention takes the luminous nano-particles of dual-structure silicon dioxide compound organic dye as a marker, uses the immunochromatographic technology for preparing fluorescent microspheres immunochromatographic test paper strip, and then prepares a detection card which consists of a sample pad, a glass fiber membrane, a nitrocellulose membrane and absorbent paper, wherein the nitrocellulose membrane is fixedly provided with a detection line and a quality control line. In the detection process, the best excitation light souce of fluorescent microspheres is used for excitation; after the emitted fluorescence passes through a filter, a CCD scanning technology or fiber-optic technology is used for collecting, accumulating or multiplicating the emitted spectra which is then converted into a numerical signal; then the measured fluorescence intensity of the detection line is multiplied by a correction coefficient, and later the corrected fluorescence intensity is substituted in a standard curve which is preset in a fluorescence analyzer; and finally, the concentration of an object to be measured in the sample can be automatically calculated and obtained by the fluorescence analyzer. The invention has high sensitivity, accurate quantization and easy operation.
Owner:江西中德生物工程股份有限公司
Method for detecting by using quantum dot fluorescence immunochromatographic test strips
ActiveCN103048460AHigh fluorescence intensityOperational securityMaterial analysisFluorescenceQuantum dot
The invention provides a method for detecting by using quantum dot fluorescence immunochromatographic test strips. The method comprises the following specific steps: (1) preparing a quantum dot labeled protein liquid; (2) preparing a quantum dot labeled protein detecting liquid; (3) preparing a reaction film coated with a detection index; (4) bonding a sample pad, the reaction film prepared in the step (3) and a water absorbing pad on a PVC backing in sequence so as to obtain a test paper board; cutting the test paper board into test strips; and loading the test strips into a test paper clip; and (5) qualitatively or quantitatively detecting. The method for detecting by using the quantum dot fluorescence immunochromatographic test strips, provided by the invention, is a rapid detecting method using the quantum dot fluorescence immunochromatographic test strips, which is featured by high sensitivity, synchronicity in multi-index detection, simplicity, convenience, intuitive nature, low price and wide application.
Owner:WUHAN JIAYUAN BIOMEDICAL ENG
Immune chromatograph testing strip and production thereof
An immune chromatographic test bar consists of supporter, drinking fibrous membrance, nitrate fibrous membrance, sample membrance, waterproof membrance and colloidal gold labeled membrance, Its preparing method utilizes colloidal gold labeling technique and quantitative immune competition method to produce test bar for being used in quick detection.
Owner:李人
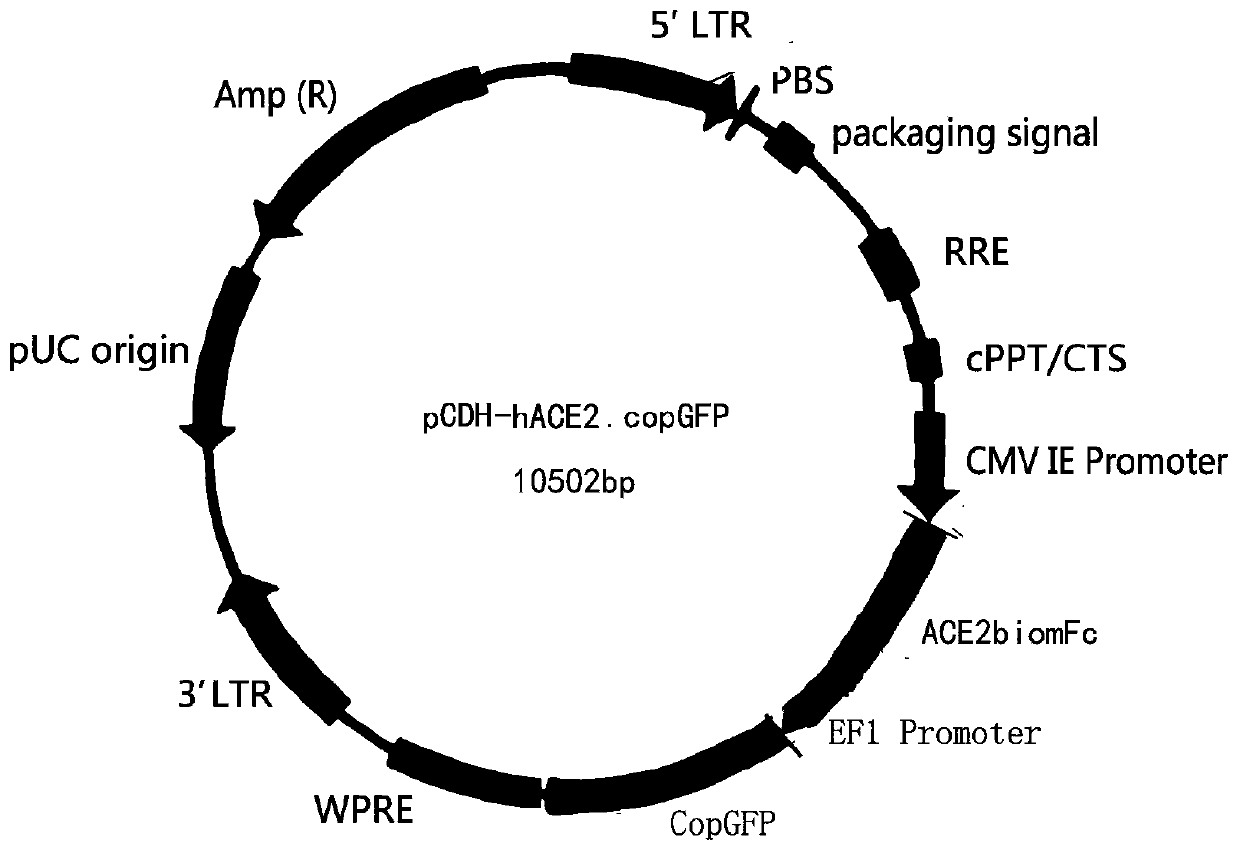
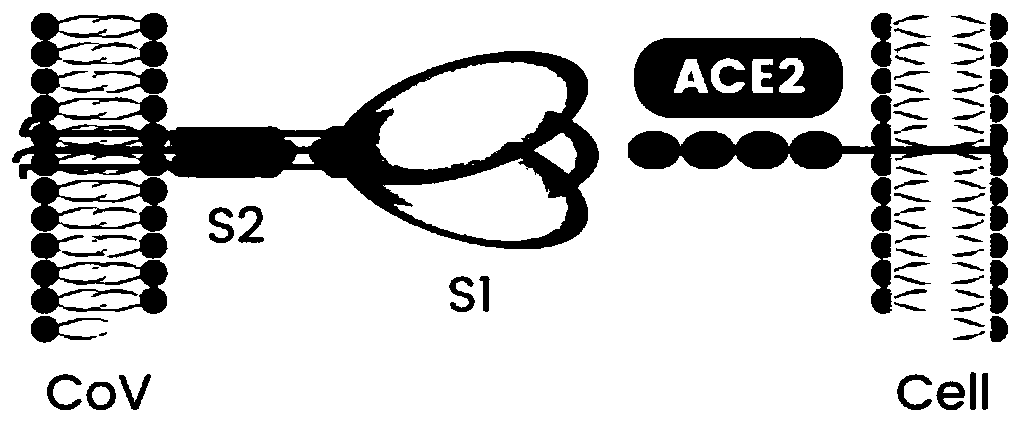
Coronavirus rapid detection kit based on S protein ligand and ACE2 receptor competitive chromatography
ActiveCN111273016AImmunochromatographic fastEasy immunochromatographyCell receptors/surface-antigens/surface-determinantsAntibody mimetics/scaffoldsReceptorBlood plasma
Owner:浙江诺迦生物科技有限公司 +1
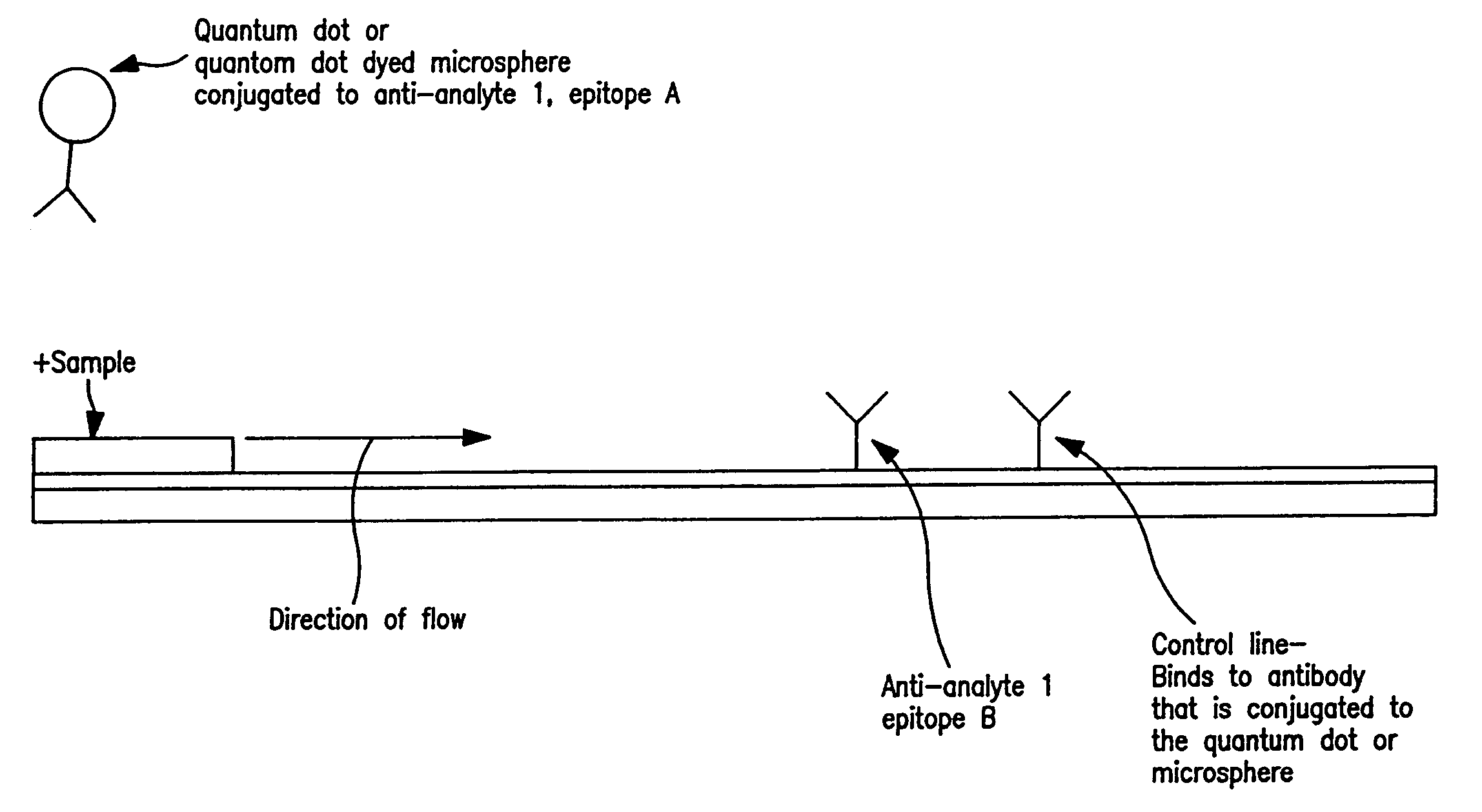
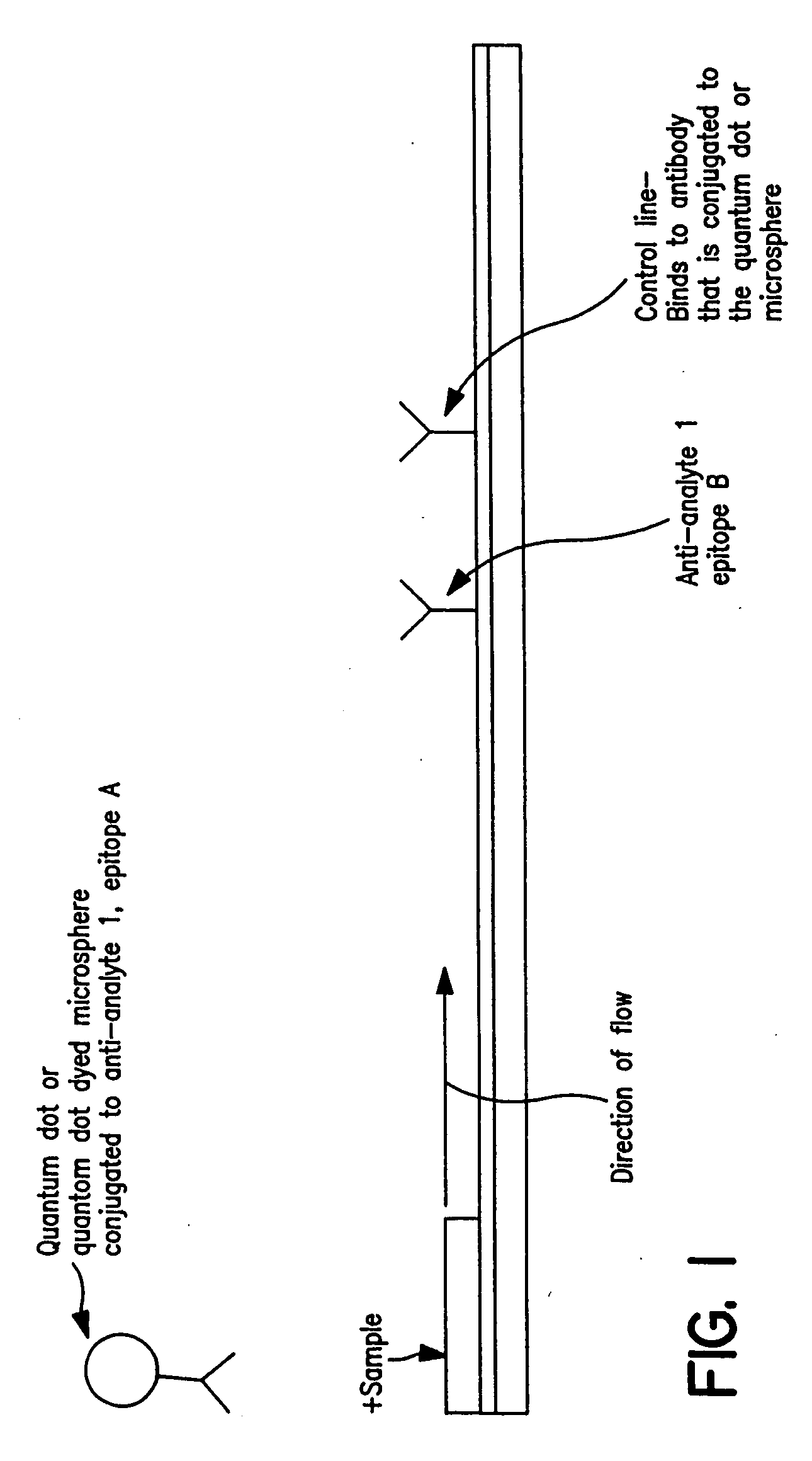
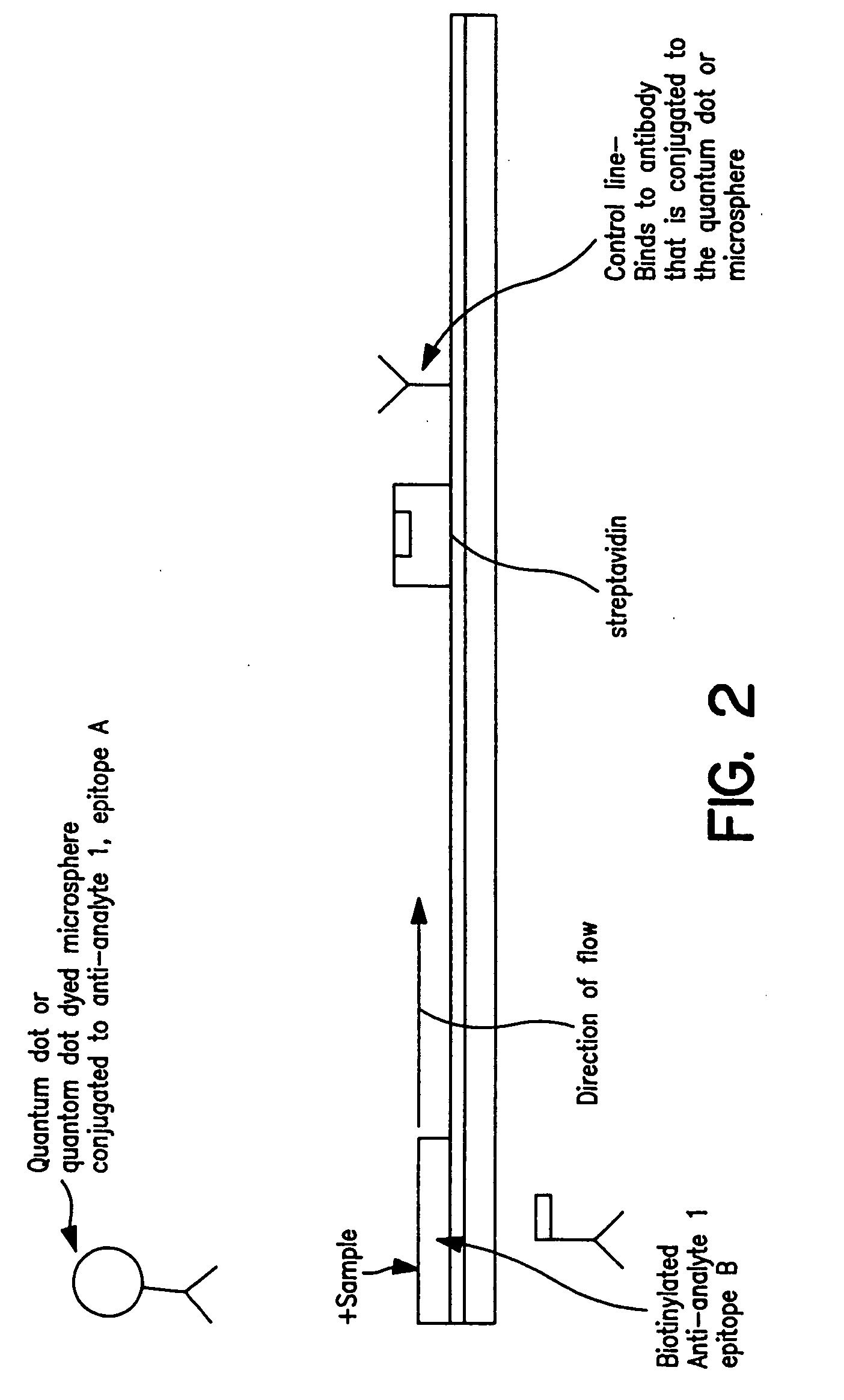
Immunochromatographic methods for detecting an analyte in a sample which employ semiconductor nanocrystals as detectable labels
InactiveUS20060008921A1High resolutionHigh energyComponent separationSolid sorbent liquid separationAssayAnalyte
Immunochromatographic test strip assays which employ semiconductor nanocrystals as detectable labels are disclosed, as are methods for detecting and quantifying one or more analytes of interest in a test sample using those assays. The test strips of the present invention permit detection and quantitation of one or more analytes of interest present in a test sample suspected of containing them, by using more than one semiconductor nanocrystal as a detectable label, each of which emits exhibits a unique emission peak.
Owner:INVITROGEN
Immunochromatographic test strip for full quantitative detection of C-reactive protein and preparation method thereof
The invention discloses an immunochromatographic test strip for full quantitative detection of C-reactive protein and a preparation method thereof. The test strip is composed of a sample pad, a mark pad, a coating film and absorbent paper which are successively overlapped and adhered on a baseplate, wherein the mark pad is coated with C-reactive protein (CRP) monoclonal antibody labelled by fluorescent latex particles and rabbit IgG labelled by fluorescent latex particles; and the coating film is composed of a detection region and a quality control region, and the detection region is coated with another CRP monoclonal antibody which is at different epitope with the CRP monoclonal antibody labelled by fluorescent latex particles. The C-reactive protein immunochromatographic test strip can perform sensitive quantitative detection on the full CRP in 10 seconds, can diagnose diseases and identify infection more rapidly and accurately, and can detect infectious illness state and determine the curative effect of antibiotics; and the full CRP has two detection results, namely hs-CRP and conventional CRP, comprehensive linear range and good detection sensitivity and less required sample amount, and is very convenient to operate.
Owner:GUANGZHOU WONDFO BIOTECH
Digital immunochromatographic test strip for semi-quantitative detection of aflatoxin B1 and preparation method thereof
InactiveUS20120034711A1Quick checkSimple procedureAnalysis using chemical indicatorsLamination ancillary operationsPaperboardBovine serum albumin
The present invention belongs to the field of biological detection. Multi-line immunochromatographic test strip for semi-quantitative detection of aflatoxin B1 comprises a paperboard, wherein a water-absorbing pad, a detection pad, a gold-labeled pad and a sample pad are adhered sequentially on one surface of the paperboard from top to bottom, wherein each adjacent pads is overlapped and connected, the detection pad uses a nitrocellulose film as a backing pad, the nitrocellulose film is provided with a transverse control line, a test line I, a test line II and a test line III, wherein the control line is coated with a rabbit anti-mouse polyclonal antibody, and the test line I, test line II and test line III are coated with aflatoxin B1-bovine serum albumin conjugate (AFB1-BSA), respectively: and the gold-labeled pad is transversely coated with a nanogold-labeled anti-aflatoxin B1 monoclonal antibody. Said test strip is used for semi-quantitative detection of aflatoxin B1, and is characterized by quick detection, simple procedure and high sensitivity.
Owner:INST OF OIL CROPS RES CHINESE ACAD OF AGRI SCI
Closed nucleic acid chromatographic test paper detection kit preserved at normal temperature and detection method
InactiveCN103243087AChemically stableHigh recovery rateMicrobiological testing/measurementDNA preparationNucleic acid detectionImmunochromatographic test
The invention discloses a method for preserving nucleic acid amplification reagents at normal temperature. Partial nucleic acid amplification reagents or all reagents are converted to solid state through a solidifying process and preserved stably at normal temperature. The invention further discloses a closed nucleic acid chromatographic test paper detection kit preserved at normal temperature. The kit comprises a nucleic acid extraction reagent of a sampler, the nucleic acid amplification reagent preserved at normal temperature, a closed chromatographic test paper detection device and an innocent treatment method of nucleic acid amplification products. The closed chromatographic test paper detection device comprises immunochromatographic test paper and a closed detection device, wherein the immunochromatographic test paper is fixedly arranged in the closed detection device, and sample liquor detected cannot be contacted with external environment. In addition, the invention further discloses a method for nucleic acid detection by using the kit. According to the invention, the nucleic acid reagents can be conveyed and preserved at normal temperature and extracted simply and quickly, and the nucleic acid amplification products are hermetically, quickly and sensitively detected, so that cross infection is avoided.
Owner:QUICKING BIOTECH
Multi-test-line immunochromatographic test strip for semi-quantitatively detecting aflatoxin B1 and preparation method thereof
InactiveCN101900728AHigh practical application valueEasy to handleBiological material analysisAflatoxin BMonoclonal antibody
The invention belongs to the field of bioinstrumentation and relates to a multi-test-line immunochromatographic test strip for semi-quantitatively detecting aflatoxin B1, comprising a paperboard, wherein a water absorbent pad, a detection pad, a gold-labeled pad and a sample pad are adhered on one surface of the paperboard from top to bottom in sequence, wherein adjacent pads are overlapped and connected at the connection part, the detection pad takes a nitrocellulose film as a base pad, the nitrocellulose film is provided with a transverse quality control line, a detection line I, a detection line II and a detection line III from top to bottom, wherein the quality control line is wrapped with a rabbit anti-mouse polyclonal antibody, and the detection line I, the detection line II and the detection line III are respectively wrapped with an aflatoxin B1-bovine serum albumin (AFB1-BSA) conjugate; and the gold-labeled pad is transversely sprayed with a nanogold-labeled aflatoxin B1 monoclonal antibody. The immunochromatographic test strip is used for semi-quantitatively detecting the aflatoxin B1 and has the characteristics of fast detection, simple operation and high sensitivity.
Owner:INST OF OIL CROPS RES CHINESE ACAD OF AGRI SCI
One-step nucleic acid test method based on CRISPR/Cas and isothermal amplification and kit
InactiveCN110184329AQuick checkEasy to detectMicrobiological testing/measurementVisual testFreeze-drying
The invention discloses a one-step nucleic acid test method based on CRISPR / Cas (clustered regularly interspaced short palindromicrepeats / CRISPR-associated protein) and isothermal amplification and akit. The method is a quick field test method used for testing nucleic acid and displaying visual test results through fluorescent light or lateral side immunochromatographic test paper. The test accuracy is effectively ensured through specificity of crRNA (RISPR-derived ribonucleic acid); at a room temperature, whether a test sample contains test nucleic acid can be determined through the test paper or the fluorescent light within 1-2h; and compared with the traditional test method, the precision, simplicity and convenience in operation of the test method are improved to some extent. The kit with purified freeze-drying LbaCas12a, crRNA and RPA (recombinase polymerase amplification) can be formed; a nucleic acid segment of a specific sequence can be conveniently and quickly tested on site;a complicated temperature control instrument such as a PCR (polymerase chain reaction) instrument and other electrophoresis and centrifugation equipment are not required; and nucleic acid of the specific sequence can be tested quickly and sensitively only by a thermostatic device and fluorescence detection equipment and even visual observation.
Owner:SOUTH CHINA UNIV OF TECH
Immunochromatographic quantitative test reagent based on near-infrared fluorescent marker
ActiveCN102680692AHigh detection sensitivityHigh sensitivityMaterial analysisMicroorganismFood safety
The invention relates to an immunochromatographic quantitative test reagent based on a near-infrared fluorescent marker. A near-infrared fluorescent molecule is adopted as a marker, and the immunochromatographic technology is adopted to prepare a near-infrared fluorescent immunochromatographic test strip. In the test process, a conventional near-infrared test instrument is used for respectively scanning a quality control line and a sample line through near-infrared rays, and after being corrected by utilizing the fluorescent strength of the quality control line, the fluorescent strength of a detection line is substituted in a standard curved line in a fluorescent analysis instrument, so that the concentration of an object to be tested in a test specimen can be analyzed. The reagent can be applied to the detection of microorganism, the food safety detection, the drug detection and the quick detection of dangerous chemicals. The immunochromatographic quantitative test reagent has characteristics of high sensitivity, accuracy in quantization and convenience in operation.
Owner:BEIJING RUNBO FUDE BIOLOGICAL TECH DEV
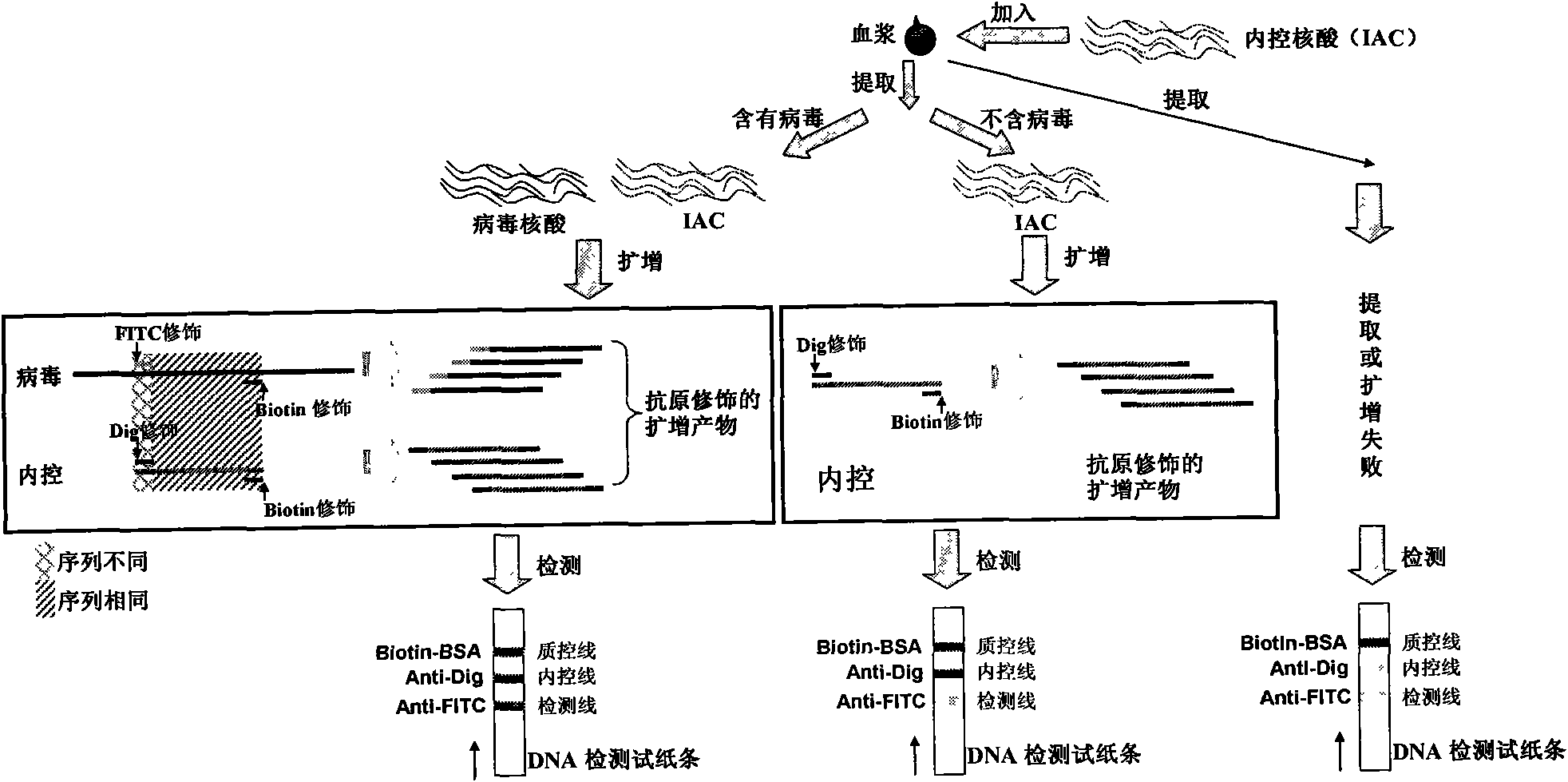
Method for semi-quantitatively detecting pathogenic nucleic acid by adding internal control nucleic acid
InactiveCN101957373AAvoid diagnostic problems that are prone to false negativesAvoid problems prone to false negativesMicrobiological testing/measurementMaterial analysisTest sampleQuality control
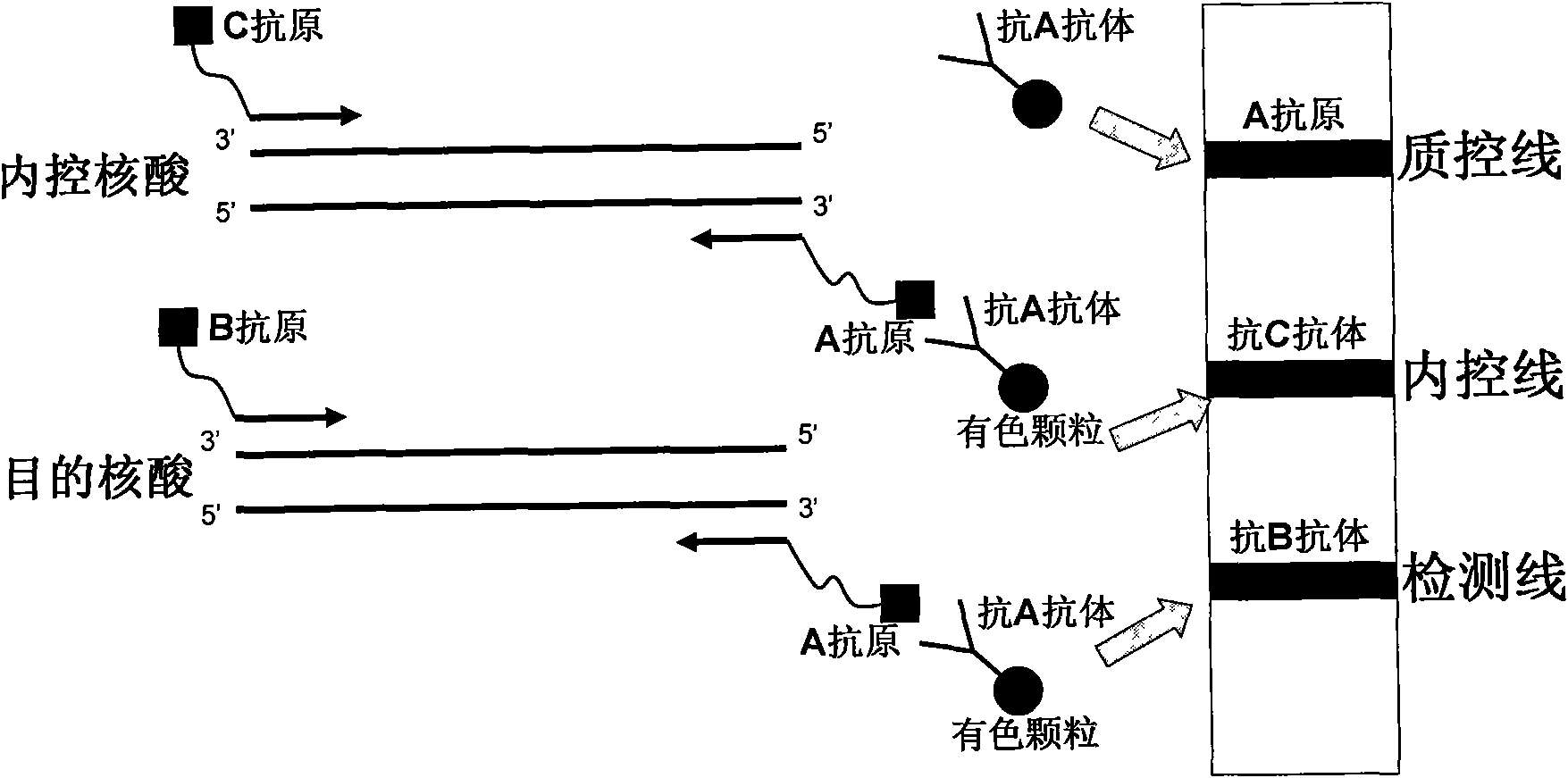
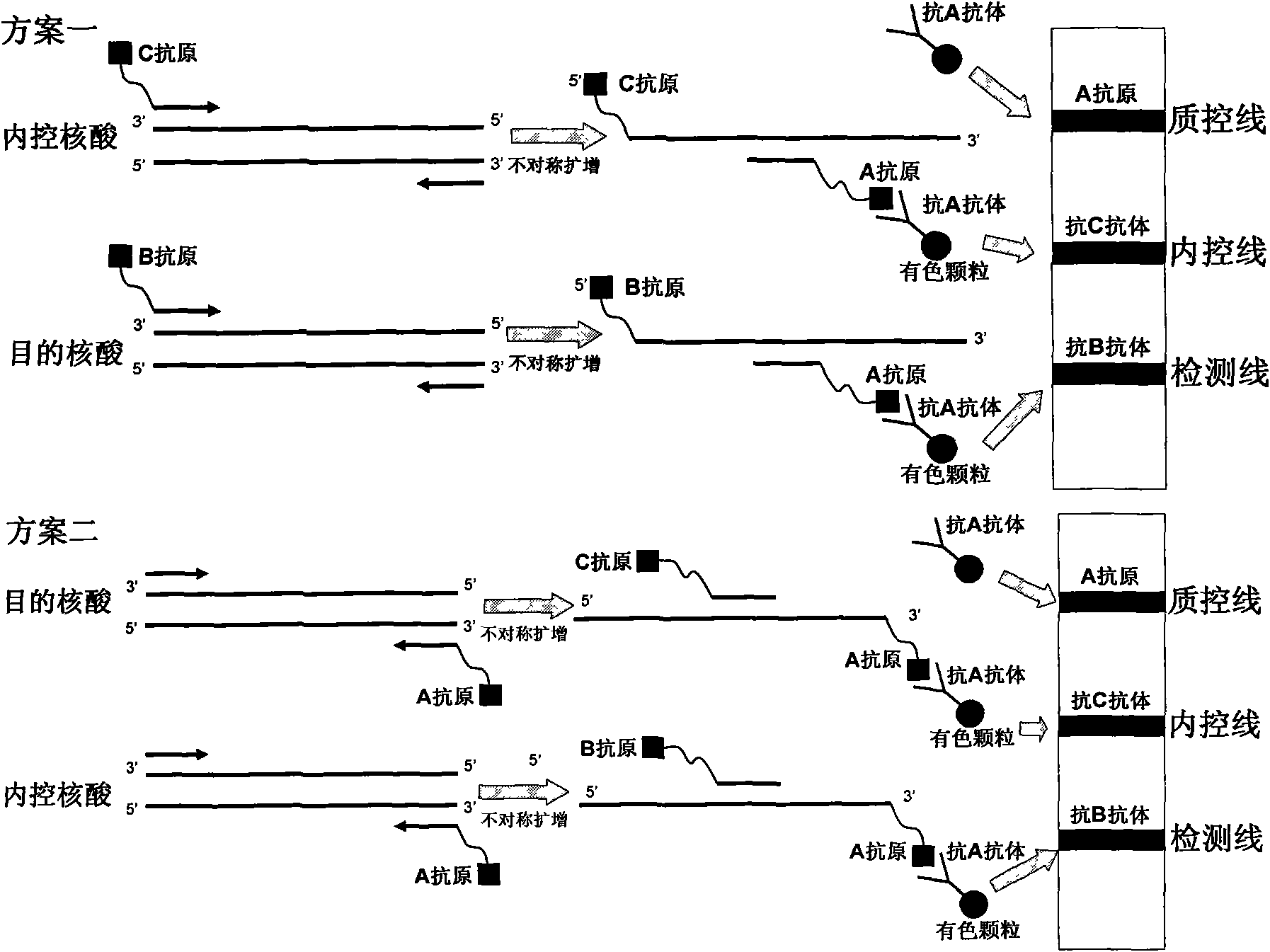
The invention belongs to the field of nucleic acid detection and discloses a method for semi-quantitatively detecting pathogenic nucleic acid by adding internal control nucleic acid. Corresponding internal control is added in the whole process of extracting and amplifying target nucleic acid and testing by using a test paper, so that the internal control and a target segment are parallelly operated, and the semi-quantitative detection is performed finally through color development and intensity contrast of three strips, namely a detection line, an internal control line and a quality control line on the test paper. In the method, in the whole process of processing the target nucleic acid, the corresponding internal control is taken as a positive contrast, and false negative results due to links such as extraction, amplification or sample application errors are avoided in the processing of detecting by using the test paper. Meanwhile, by comparing color development intensity of the internal control line and a sample line and introducing the semi-quantitative function on the basis of the qualitative function of the immunochromatographic test paper to estimate the copy number of tested samples, the detection results are more detailed, accurate and reliable. The method has the advantages of convenient and quick operation and capacity of meeting the actual clinical requirement.
Owner:HUADONG RES INST FOR MEDICINE & BIOTECHNICS
Reagent for detecting acute myocardial infarction by immunological method and test strip
ActiveCN101806804AHigh sensitivityImprove featuresImmunoglobulins against animals/humansTissue cultureSpecific antibodyHuman heart
The invention relates to a medical diagnostic reagent, in particular to a quick detection reagent for the early diagnosis of acute myocardial infarction and a test strip. The invention provides a double-index united detection reagent containing a specific antibody resisting a human heart-type fatty acid binding protein H-FABP and a human cardiac troponin cTnI. The invention also provides a colloidal gold labeling immunological chromatographic test strip containing the specific antibody resisting the H-FABP and the cTnI, which is used for quickly detecting the acute myocardial infarction. The double-index united detection reagent can carry out early diagnosis on a patient having the acute myocardial infarction, can also prevent the missed diagnosis of a patient having long-time chronic myocardial infarction with mild symptoms and better solves the influence caused by a difference existing in the detection time. The colloidal gold labeling immunological chromatographic test strip provides a quick, convenient, cheap and practical detection tool for the early diagnosis of the acute myocardial infarction, is hopefully used for hospitals at all levels and can also be used for the self monitoring of the patient.
Owner:LANZHOU INST OF BIOLOGICAL PROD
Performance correction method for gold mark immunochromatographic test strip detection system
ActiveCN102707051AReduce measurement errorGuaranteed accurate quantitative measurementsMaterial analysisObservational errorPhotovoltaic detectors
The invention relates to a performance correction method for a gold mark immunochromatographic test strip detection system. The method comprises the steps of: first, recording data information corresponding to a gold mark strip colorimetric card by a photoelectric detector of the gold mark immunochromatographic test strip detection system; then determining and storing the optimal response curve of the data of the gold mark strip colorimetric card and the concentration of the object detected according to the corresponding relation between the color of the gold mark strip colorimetric card and the concentration of the detected substance; then, using the gold mark strip colorimetric card to test current state of the gold mark test strip system when performance of the gold mark test strip system is needed to be corrected, determining a current response curve of the gold mark test strip system, then comparing the optimal response curve with linear sections of the current response curve one by one and section by section; determining and storing the mapping functions corresponding to the linear sections of the current response curve; and finishing correction. According to the performance correction method for the gold mark immunochromatographic test strip detection system provided by the invention, the truth-value of the concentration of the object detected can be obtained by calculating the data collected by the gold mark test strip system, thereby reducing the measurement error caused by problems of system aging and the like, and ensuring long time accurate and quantitative measurement of the gold mark test strip system.
Owner:SHANGHAI INST OF OPTICS & FINE MECHANICS CHINESE ACAD OF SCI
Immunochromatographic test strip for semi-quantitatively and simultaneously detecting cTnI and Myo and preparation method thereof
ActiveCN102323422AHigh sensitivitySolve and make up for the defect of narrow linear rangeBiological material analysisNanosensorsNitrocellulosePhysical chemistry
The invention relates to an immunochromatographic test strip for semi-quantitatively and simultaneously detecting cTnI and Myo and a preparation method thereof. The test strip comprises a base plate, a nitrocellulose membrane, a first bonding pad, a second bonding pad, a sample pad and a water absorbing pad. According to the preparation method, the pretreated nitrocellulose membrane, first bonding pad, second bonding pad, sample pad and water absorbing pad are sequentially and mutually staggered and attached to the base plate so as to obtain the immunochromatographic test strip for simultaneously detecting the cTnI and the Myo. The immunochromatographic test strip disclosed by the invention can be used for simultaneously detecting two proteins with greater abundance difference, providing great convenience for quick diagnosis of clinical myocardial infarction and overcoming and making up the defects of low sensitiveness and narrow linear range during detection of polyprotein in a traditional immunochromatographic test strip technology. The preparation method has the advantages of simple process, low cost, simpleness and convenience in operation and favorable application prospect.
Owner:SHANGHAI INST OF MICROSYSTEM & INFORMATION TECH CHINESE ACAD OF SCI
High-sensitivity immunochromatographic test strip for detecting total aflatoxin content quickly and preparation method thereof
The invention belongs to the field of biological detection and provides a high-sensitivity immunochromatographic test strip for detecting total aflatoxin content quickly, which is characterized by comprising a test strip, wherein a water-absorbing pad, a detection pad, a gold label pad and a sample pad are adhered on one side of the test strip from top down; the pads are overlapped at the connected parts; the detection pad uses a nitrocellulose film as a substrate pad; transverse quality control lines and detection lines are arranged on the nitrocellulose film from top down; the detection lines are coated with aflatoxin B1-bovine serum albumin (AFB1-BSA) coupling and the quality control lines are coated with rabbit anti-mouse polyclonal antibody; and a nano gold-labeled anti-aflatoxin common monoclonal antibody, which is generated by hybrid tumor cell strain 1C11 having a collection number of CCTCC No.C201013, is sprayed on the gold label pad transversely. The test strip is used for detecting total aflatoxin content and has the characteristics of quick detection, simple operation and high flexibility.
Owner:INST OF OIL CROPS RES CHINESE ACAD OF AGRI SCI
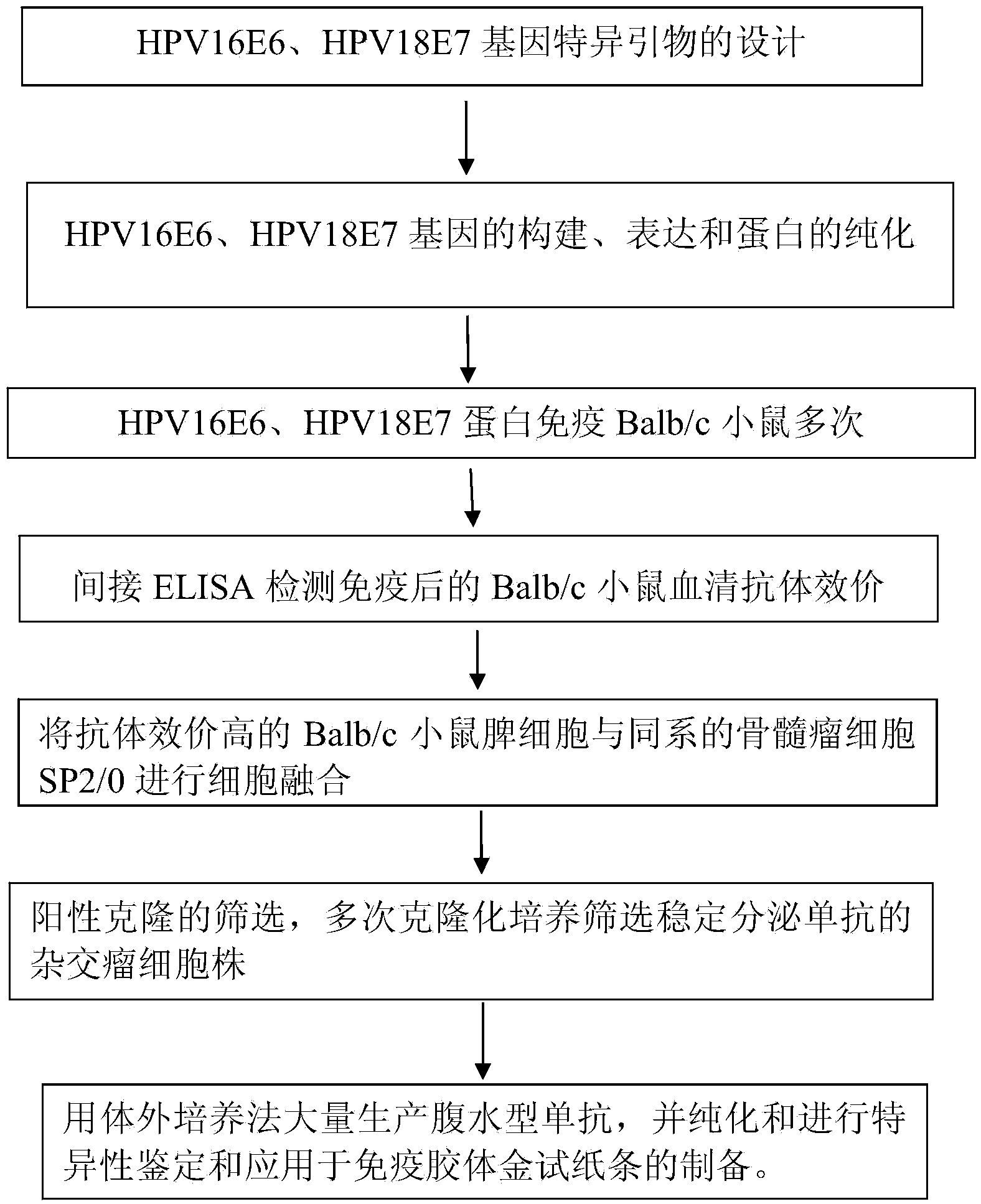
Monoclonal antibodies for resisting high-risk human papillomavirus proteins and application of monoclonal antibodies
InactiveCN103865883AStrong specificityImprove bindingImmunoglobulins against virusesMicroorganism based processesHuman papillomavirusLatex particle
The invention relates to monoclonal antibodies for resisting high-risk human papillomavirus proteins HPV16E6 and HPV18E7, a hybridoma cell strain secreting the monoclonal antibodies and the application of the monoclonal antibodies. The monoclonal antibodies can be used for specifically detecting the proteins HPV16E6 and HPV18E7. The two antibodies can be prepared into immunochromatographic test strips for rapidly detecting the proteins HPV16E6 and HPV18E7 by virtue of labeled colloidal gold or color latex particles. The monoclonal antibodies for detecting the proteins HPV16E6 and HPV18E7 have the characteristics of high speed (results can be obtained within 10 minutes), simplicity, specificity, sensitivity, low cost and easiness in popularization.
Owner:CHONGQING UNIV OF TECH
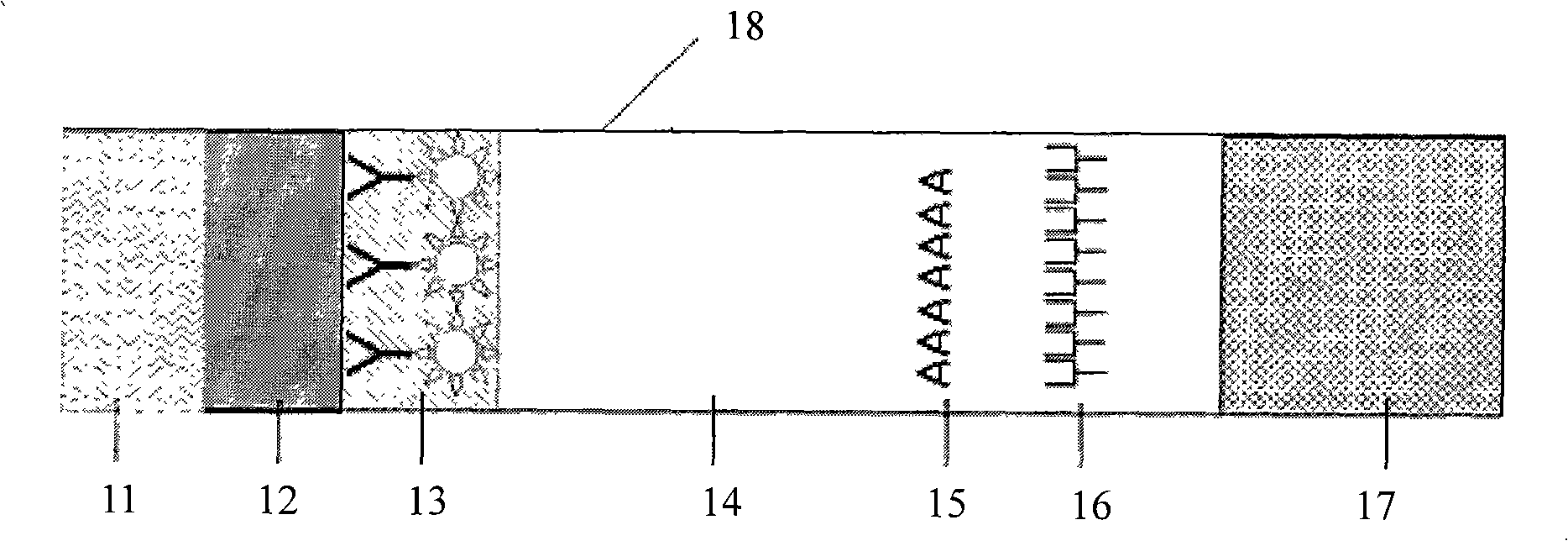
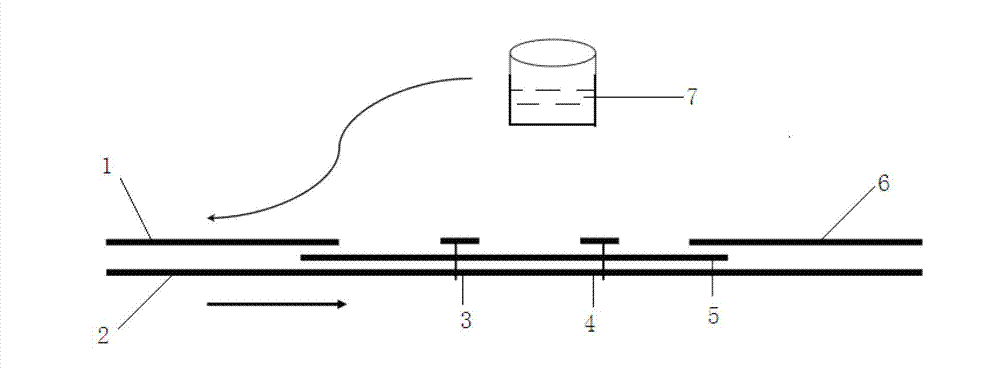
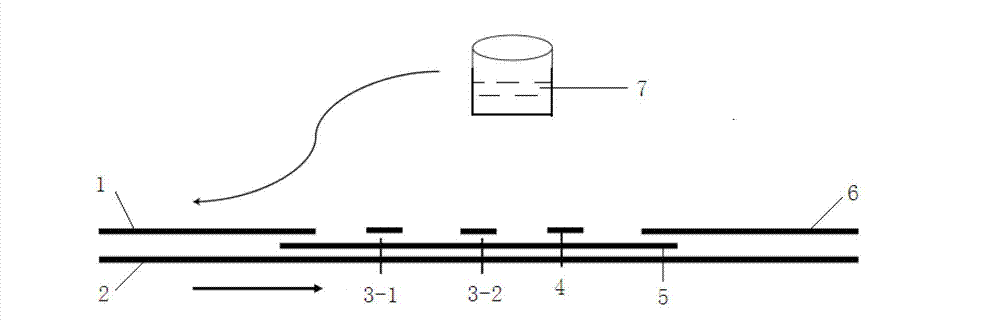
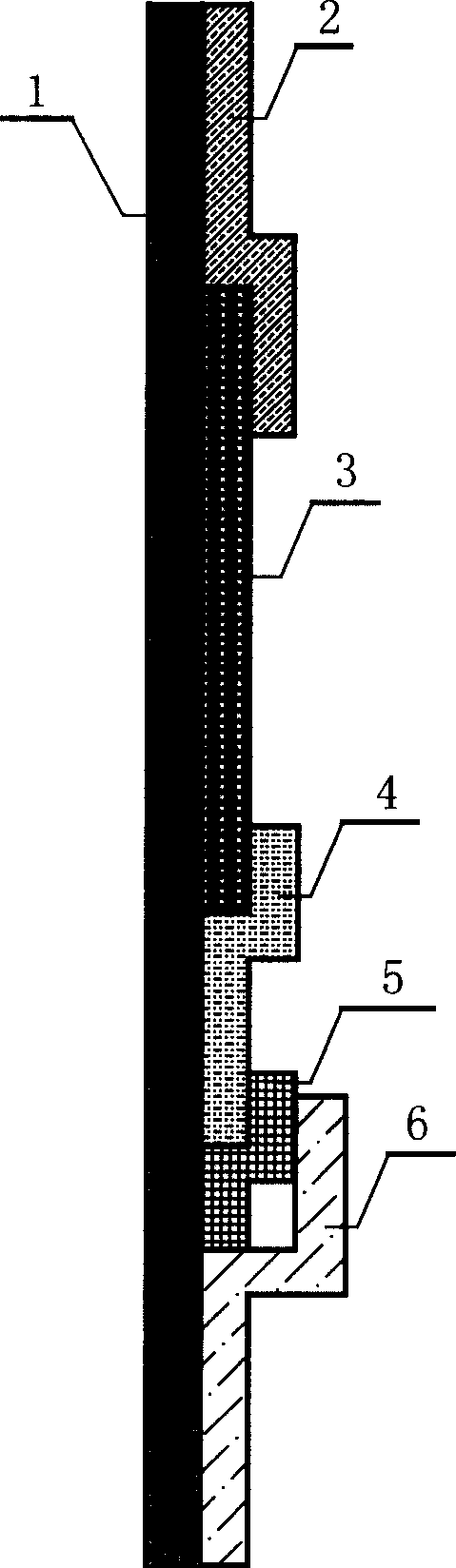
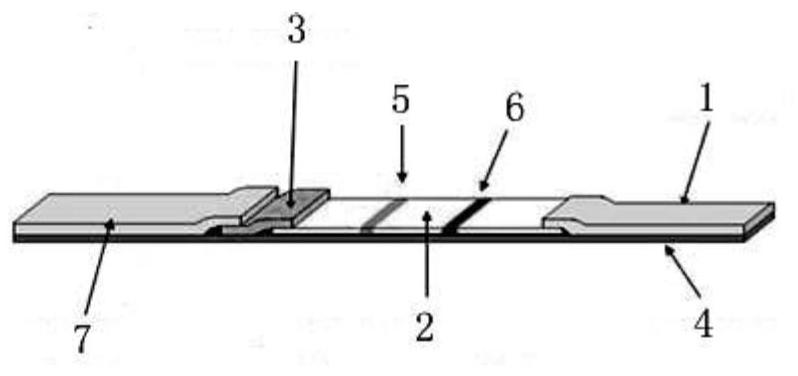
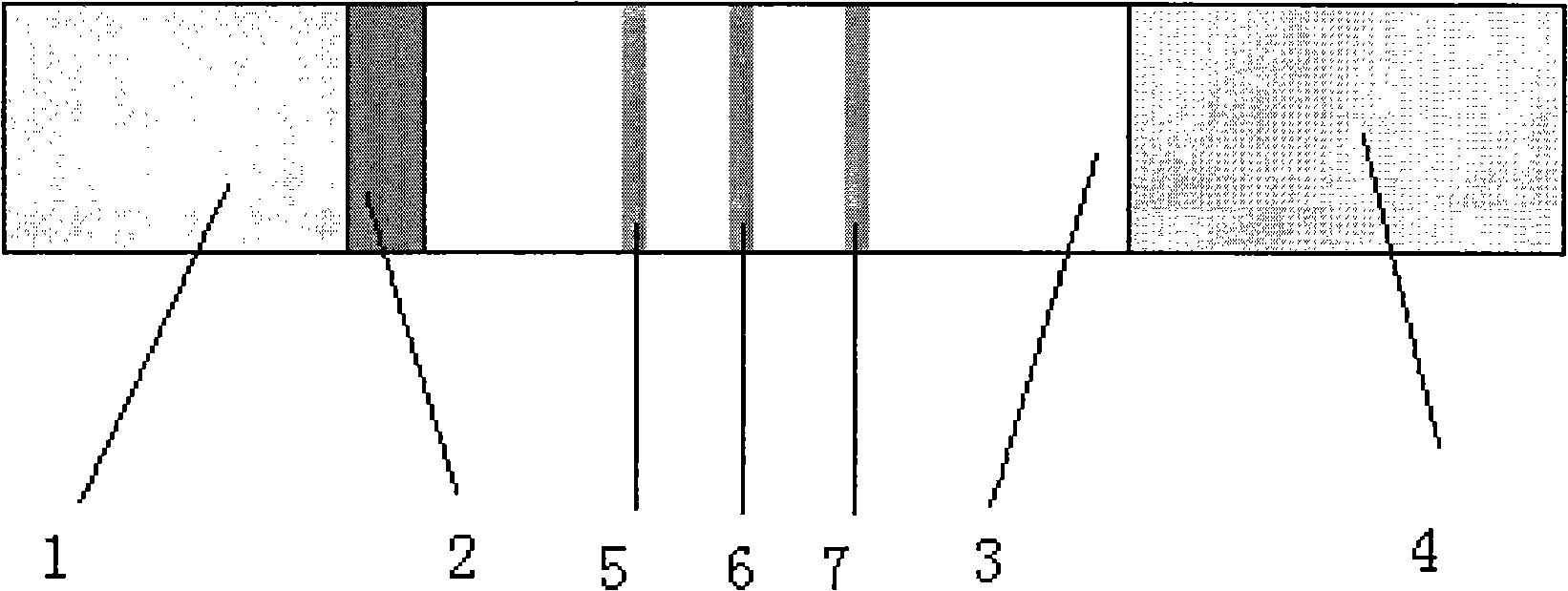
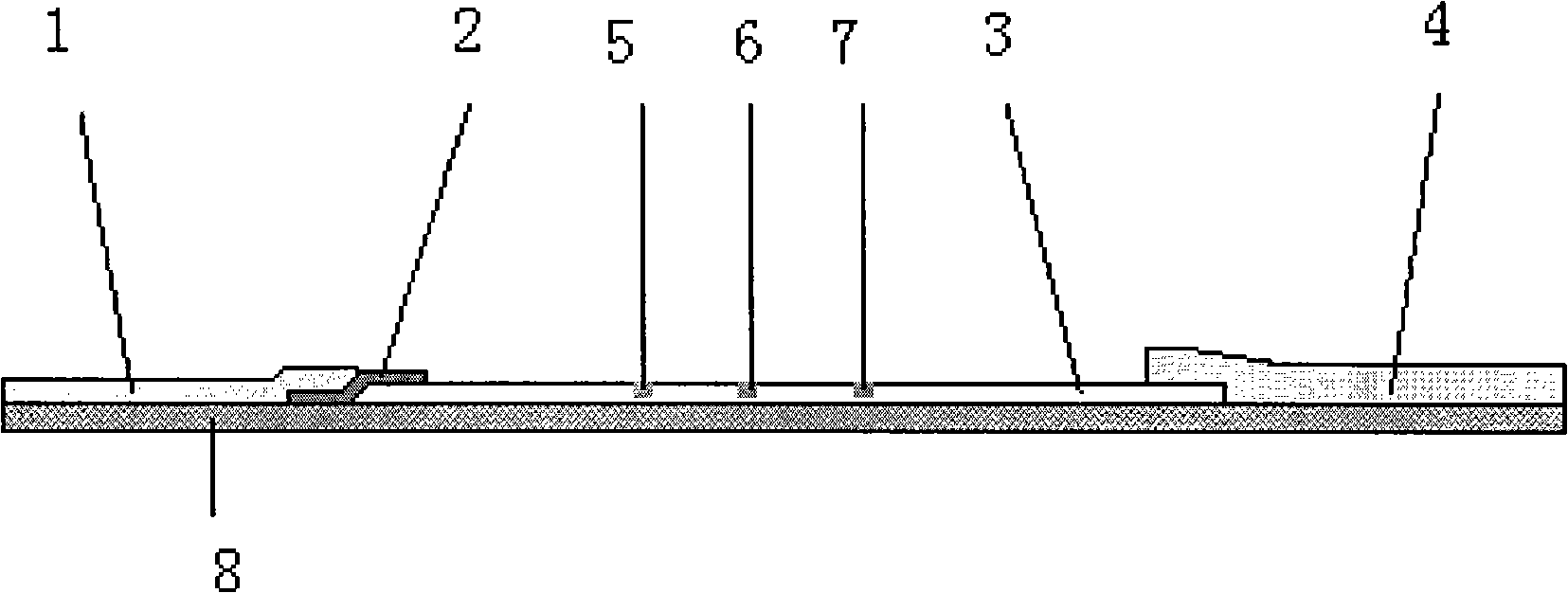
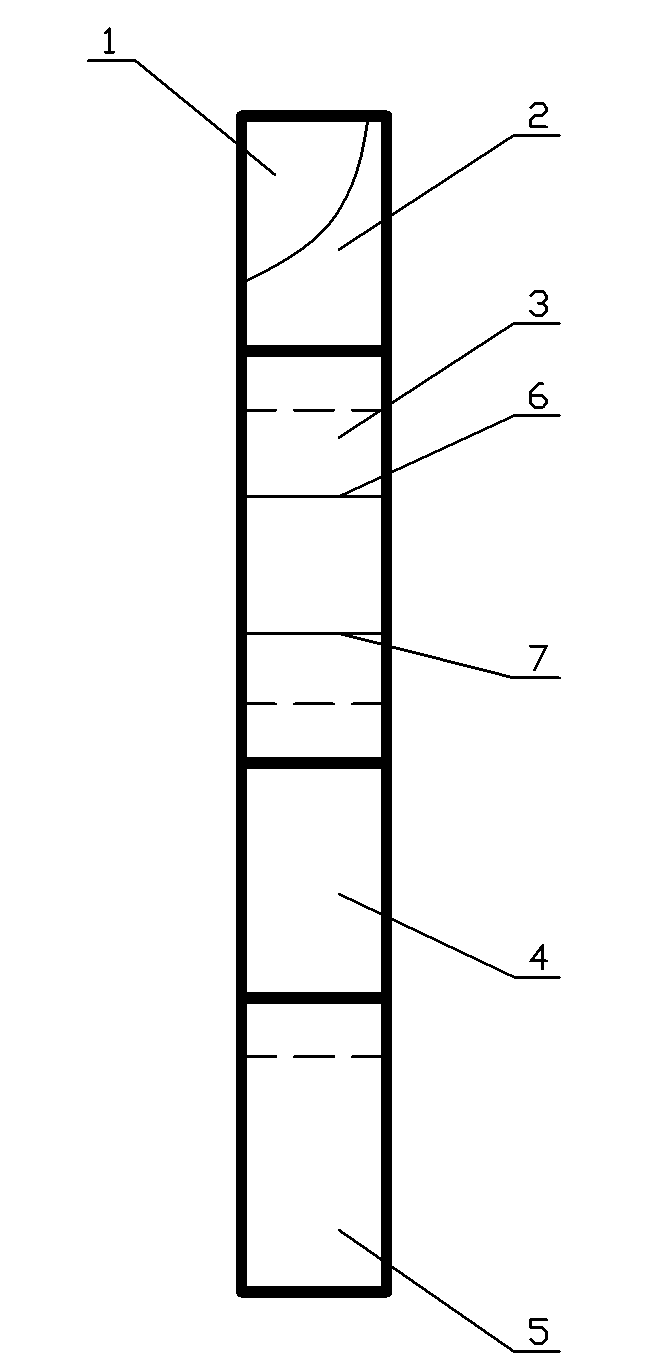
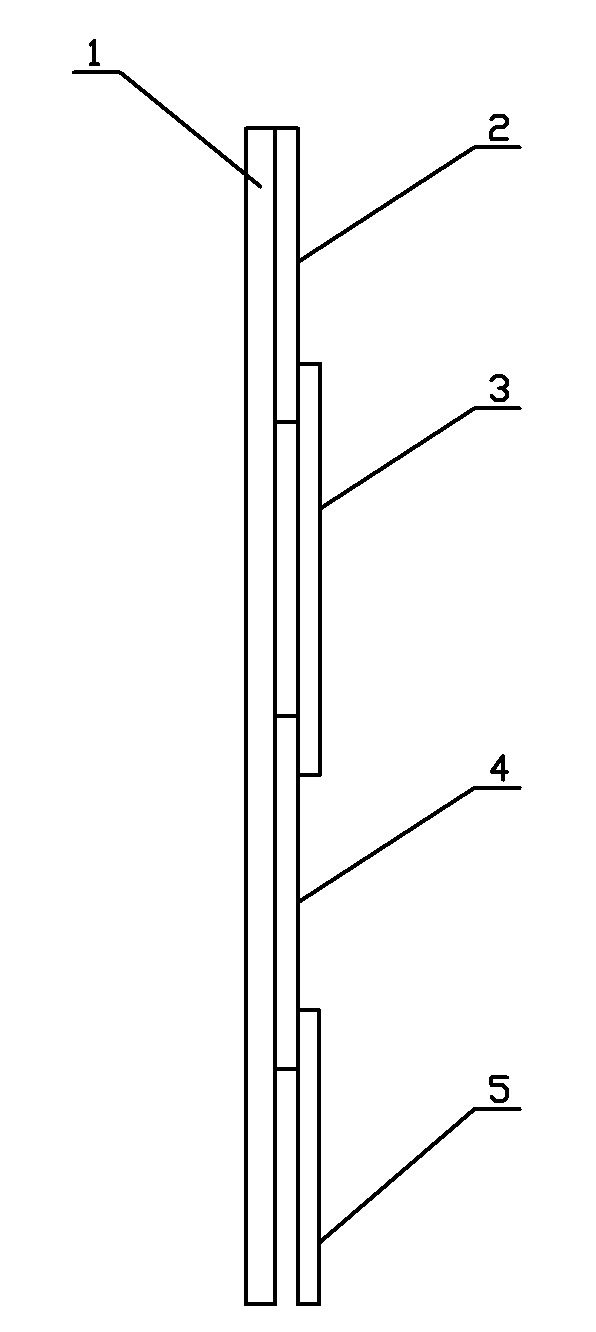
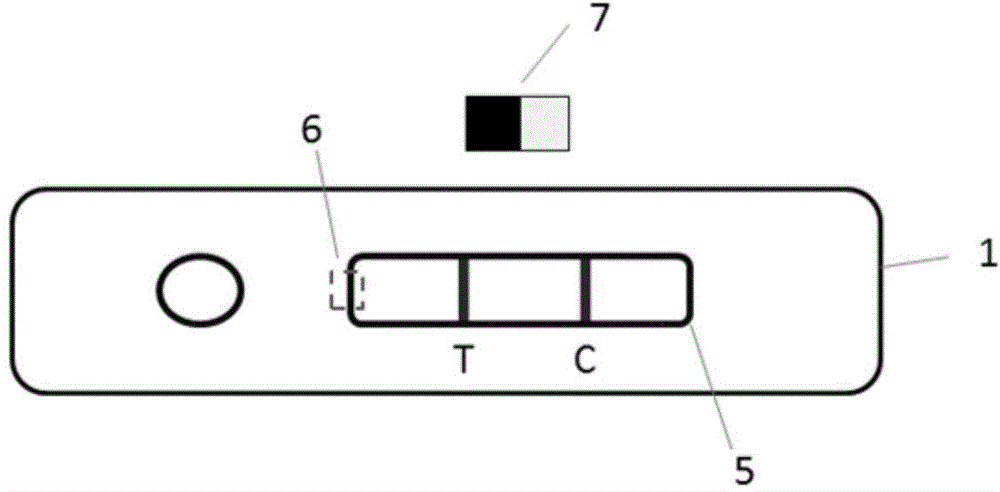
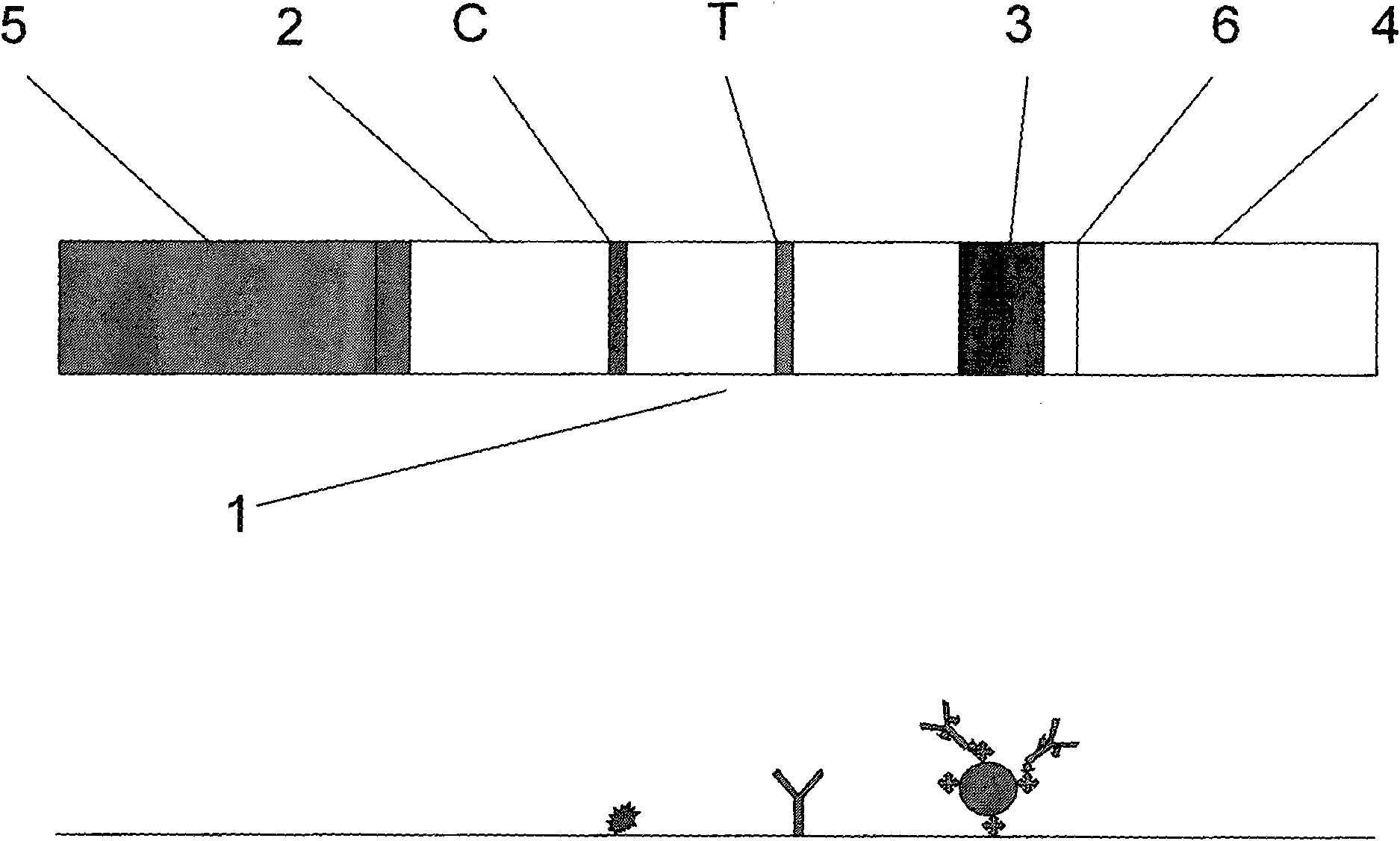
Colloidal gold immunochromatographic test strip for detecting wild-type classical swine fever virus
The invention discloses a colloidal gold immunochromatographic test strip for detecting wild-type classical swine fever virus, which consists of water absorbent paper (1), a cellulose nitrate membrane (2), a colloidal gold pad (3), a sample pad (4) and a support (5), wherein the cellulose nitrate membrane contains a detection line which is formed by coating monoclonal antibody HQ06 of anti-classical swine fever virus E2 protein and a quality control line which is formed by coating rabbit anti-mouse IgG antibody; and the colloidal gold pad is combined with colloidal gold-labeled monoclonal antibody 6E10 of the anti-classical swine fever virus E2 protein. The test strip does not react with C-strain of classical swine fever virus, bovine viral diarrhea virus, porcine reproductive and respiratory syndrome virus, transmissible gastroenteritis virus, porcine epidemic diarrhea virus, porcine rotavirus, pseudorabies virus, porcine parvovirus and porcine circovirus type 2, and can accurately and sensitively identify the wild-type classical swine fever virus, thereby having good specificity, sensitivity and repeatability.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Lung cancer marker detection immunochromatographic test paper and application
InactiveCN101609096AAchieve early diagnosisIncreased sensitivityMaterial analysisAntigenWilms' tumor
The invention belongs to the cancer detection technology, relating to a lung cancer marker detection immunochromatographic test paper and an application. The detection of a single tumour marker in the prior art can not meet the requirements of clinical early diagnosis and differential diagnosis. The invention realizes the early diagnosis of lung cancer by jointly detecting NSE and CEA in serum with a double antibody sandwich immunochromatography. The invention comprises the following preparation steps: preparing aurosol-neure NSE and aurosol-carcinoembryonic antigen CEA antibody compounds; preparing double antibody sandwich NSE and CEA detection test strips. The invention comprises the following steps in lung cancer detection: configurating series concentration NSE and CEA standard substances; preparing NSE and CEA mixed antigen solution with serum of normal people; inserting the sample pad ends of the test strips into the above concentration series standard substances respectively to observe the changes in colours of T and C lines on a NC film. The invention has the advantages of convenient detection operation, high sensitivity and precise result, and can determine lung cancer recurrence 4-12 weeks ahead.
Owner:SHANGHAI NORMAL UNIVERSITY
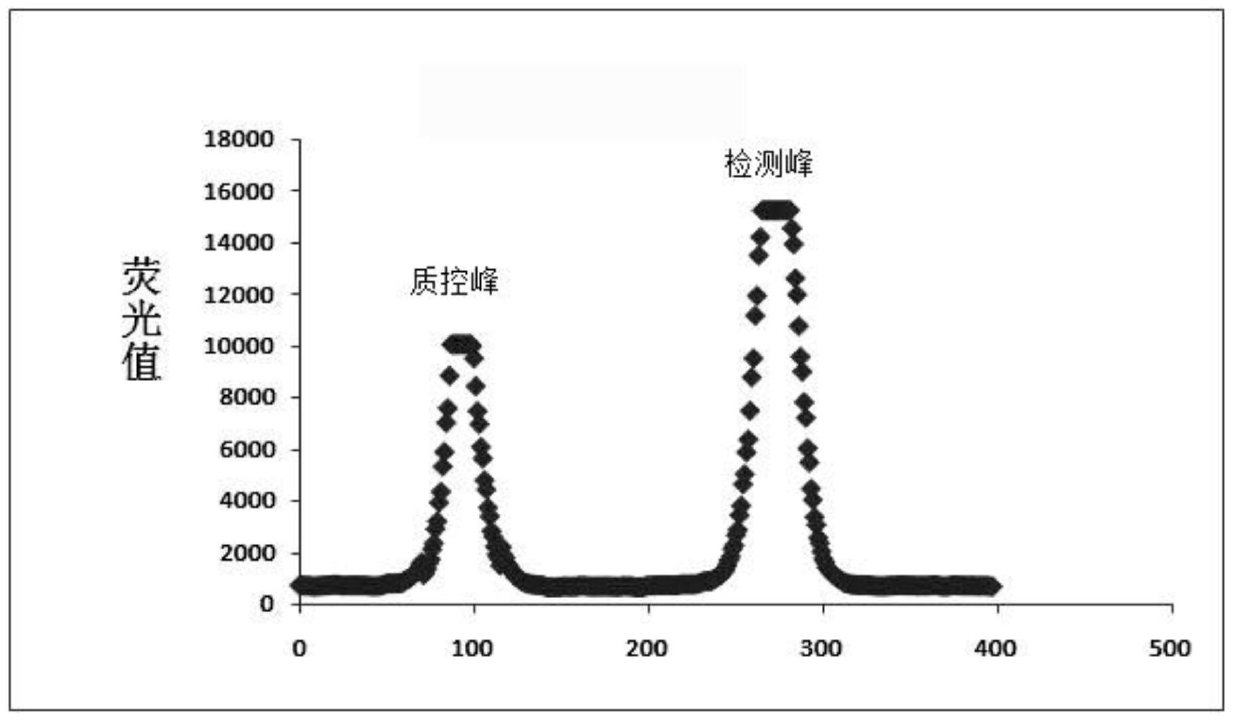
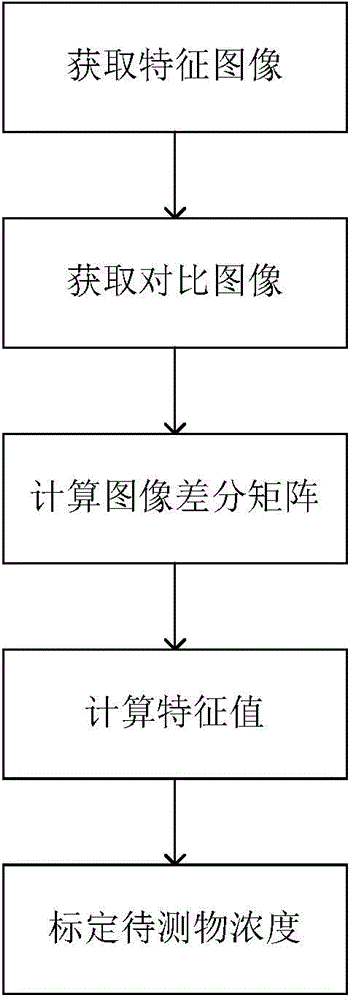
Method for quantitative detection of immunochromatographic test card
ActiveCN104101704AImprove accuracyImprove signal-to-noise ratioColor/spectral properties measurementsSignal-to-noise ratio (imaging)Immunochromatographic test
The invention discloses a method for quantitative detection of an immunochromatographic test card. The method comprises the following steps: (1) placing an immunochromatographic test card to be tested on a detection light path of a first light source to acquire a first image data matrix Pic 1; (2) placing the immunochromatographic test card to be tested on a detection light path of a second light source to acquire a second image data matrix Pic 2; (3) selecting a reference zone to calculate the image difference matrix a Pic; (4) adopting the chromatographic peak detection algorithm to calculate the characteristic value; (5) calibrating the concentration of an object to be tested. The method for the quantitative detection of the immunochromatographic test card can eliminate the problem of baseline deflection caused by nonuniform lighting of the light paths, dead angles of a lens of a detector and the like, and meanwhile, can effectively inhibit spurious signal peaks brought by shadows, impurity particle interferents and the like, thereby improving the accuracy in distinguishing chromatographic peaks, enhancing the signal-to-noise ratio of the chromatographic peak of an immune marker and improving the accuracy and the sensitivity.
Owner:广纳达康(广州)生物科技有限公司
Ultrasensitive and quantitative immunochromatographic device and detection method using same
InactiveCN102305854ASuitable for qualitative detectionHigh detection sensitivityMaterial analysisNanoparticleFluorescence
The invention belongs to the technical field of biomedicine and discloses an immunochromatographic device and a detection method using the same. The immunochromatographic device is composed of an immunochromatographic test strip and a reaction tank. In the immunochromatographic device, quick, ultrasensitive and quantitative detection of a target substance can be achieved by virtue of time distinguishing detection equipment by taking a rare-earth fluorescence nanoparticle as a marker. Compared with the traditional immunochromatographic test strip, the immunochromatographic device has high sensitivity. The device can be widely applied to quick, qualitative and quantitative detection items in the fields of biology, medicine, food safety and the like.
Owner:汤凌霄 +1
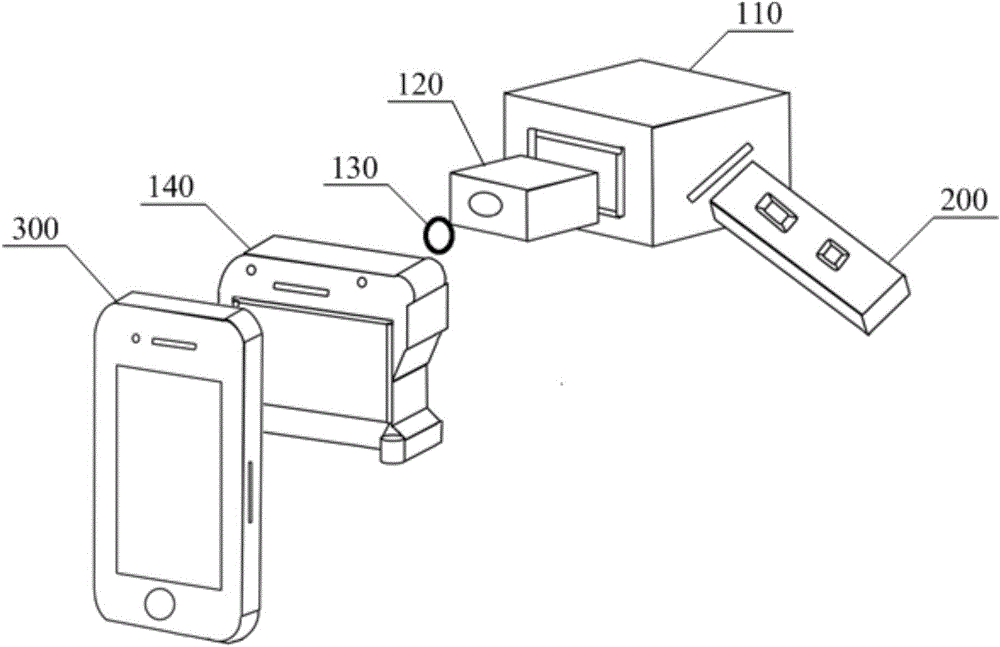
Intelligent mobile phone type rapid detection device matched with immunochromatographic test strip
The invention discloses an intelligent mobile phone type rapid detection device matched with an immunochromatographic test strip. The intelligent mobile phone type rapid detection device comprises a collimating lens, a light-guide fiber and a test strip insertion hole unit, wherein the test strip insertion hole unit is provided with an insertion hole for containing the immunochromatographic test strip, a light source for illuminating the test strip is arranged in the test strip insertion hole unit, one end of the light-guide fiber is connected with the test strip insertion hole unit and is used for leading reflecting light of an image of the test strip out of the test strip insertion hole unit, and the collimating lens enables emergent light of the light-guide fiber to be irradiated on a lens of an intelligent mobile phone in a collimated manner, so that the intelligent mobile phone can acquire the image of the test strip, the image of the test strip can be processed by processing software arranged the intelligent mobile phone and a detection result is output. According to the intelligent mobile phone type detection device, the intelligent mobile phone is used as an image and data processing core carrier and the test strip can be detected. The intelligent mobile phone type rapid detection device has good universality, can be carried out conveniently and is suitable for detection of the test strip in various environments.
Owner:SHANGHAI JIAO TONG UNIV
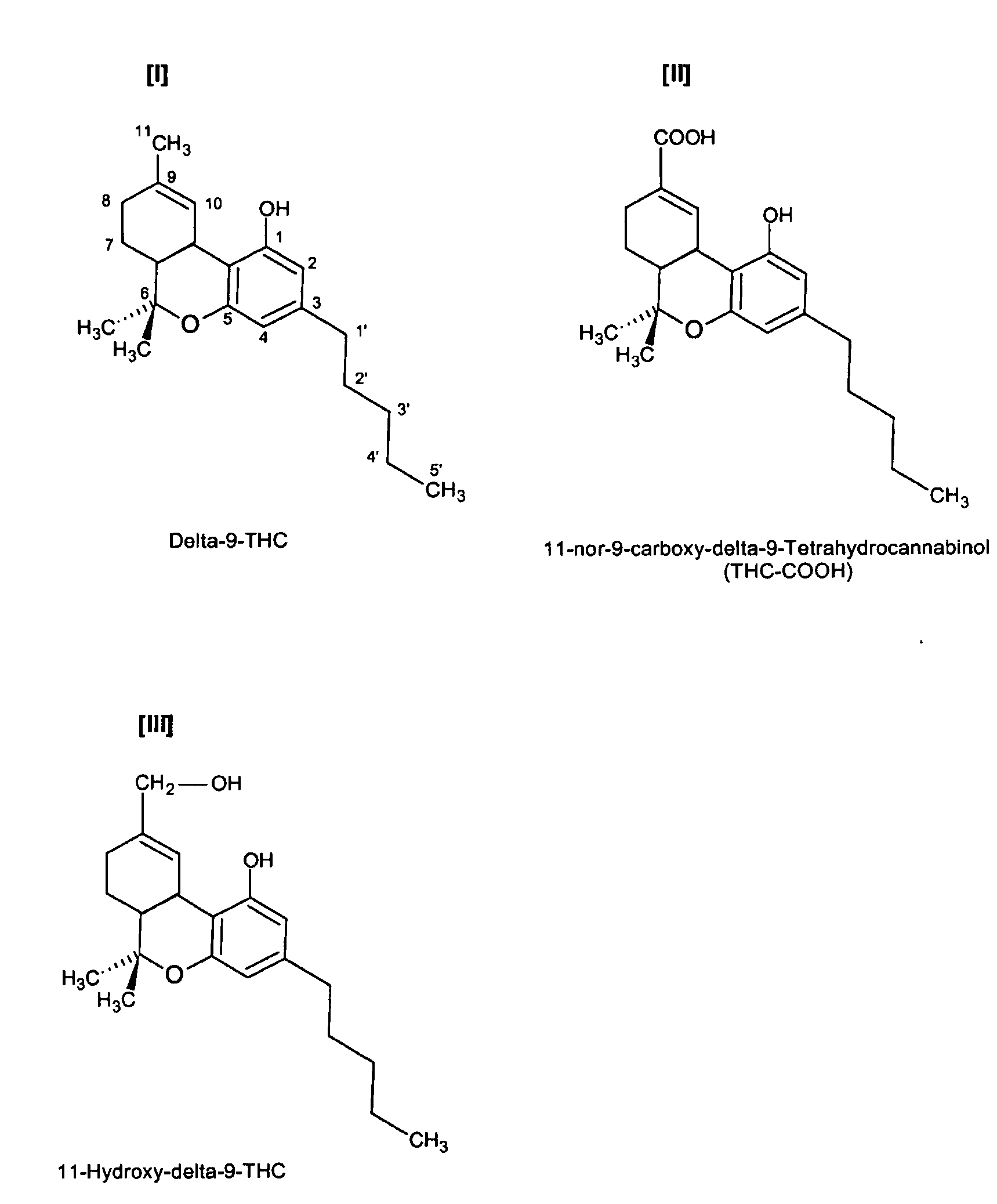
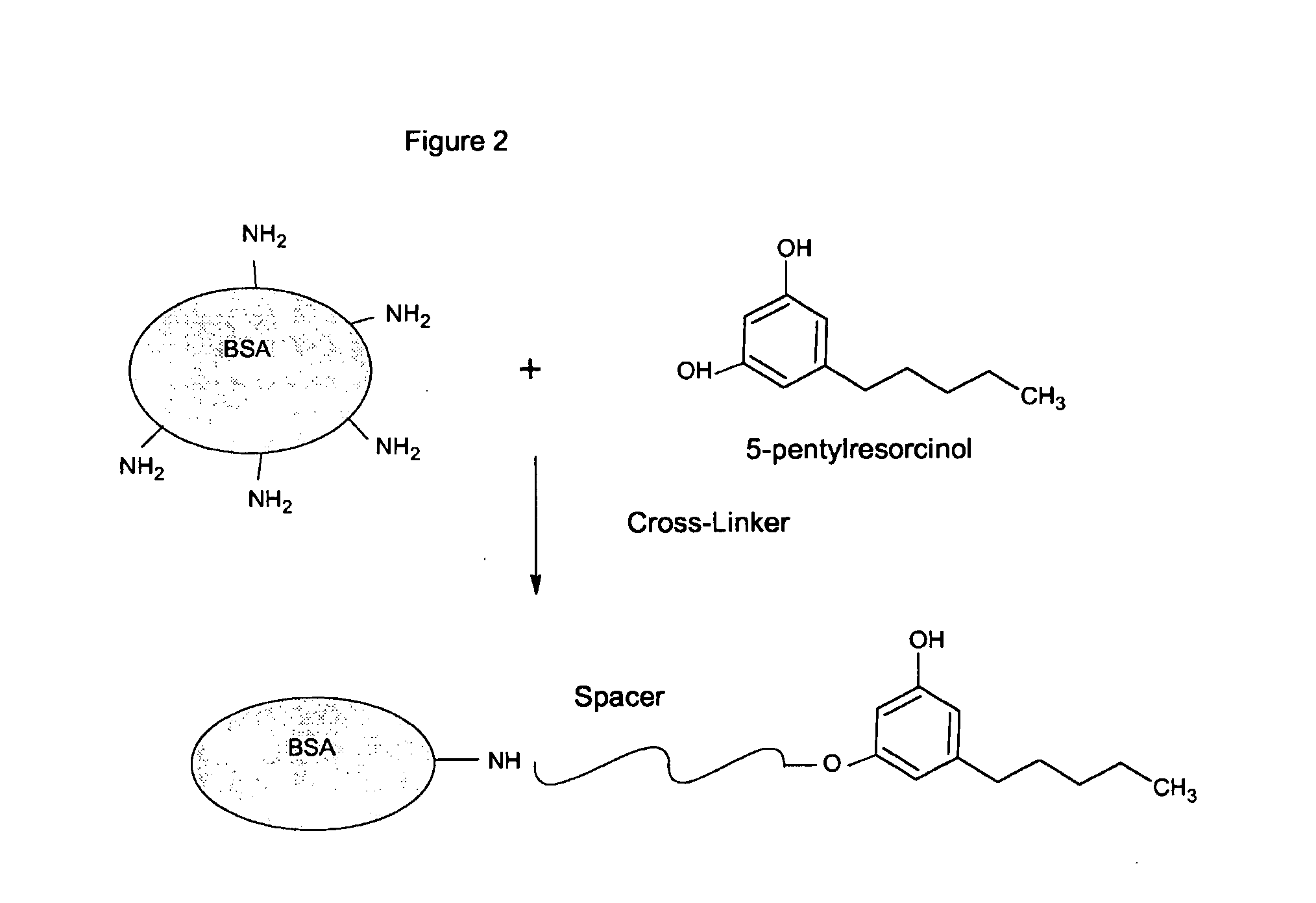
Delta-9-tetrahydrocannabinol detection method
InactiveUS20090017555A1High sensitivityAnalysis using chemical indicatorsAnalysis by subjecting material to chemical reactionDelta-9-tetrahydrocannabinolImmunochromatographic test
The invention provides competitive immunoassay techniques for high sensitivity detection of delta-9-tetrahydro-cannabinol (cannabis; THC) employing a carrier conjugate of an intermediate in the biosynthesis of cannabis, more particularly 5-pentylresorcinol conjugated to a macromolecular carrier via its hydroxyl groups. By employing such a conjugate with anti-THC antibody in a lateral flow immunochromatography test device convenient on-site testing for low levels of cannabis in liquid samples may be achieved. Such testing is particularly favoured for roadside testing for cannabis in oral fluid samples.
Owner:CONCATENO UK
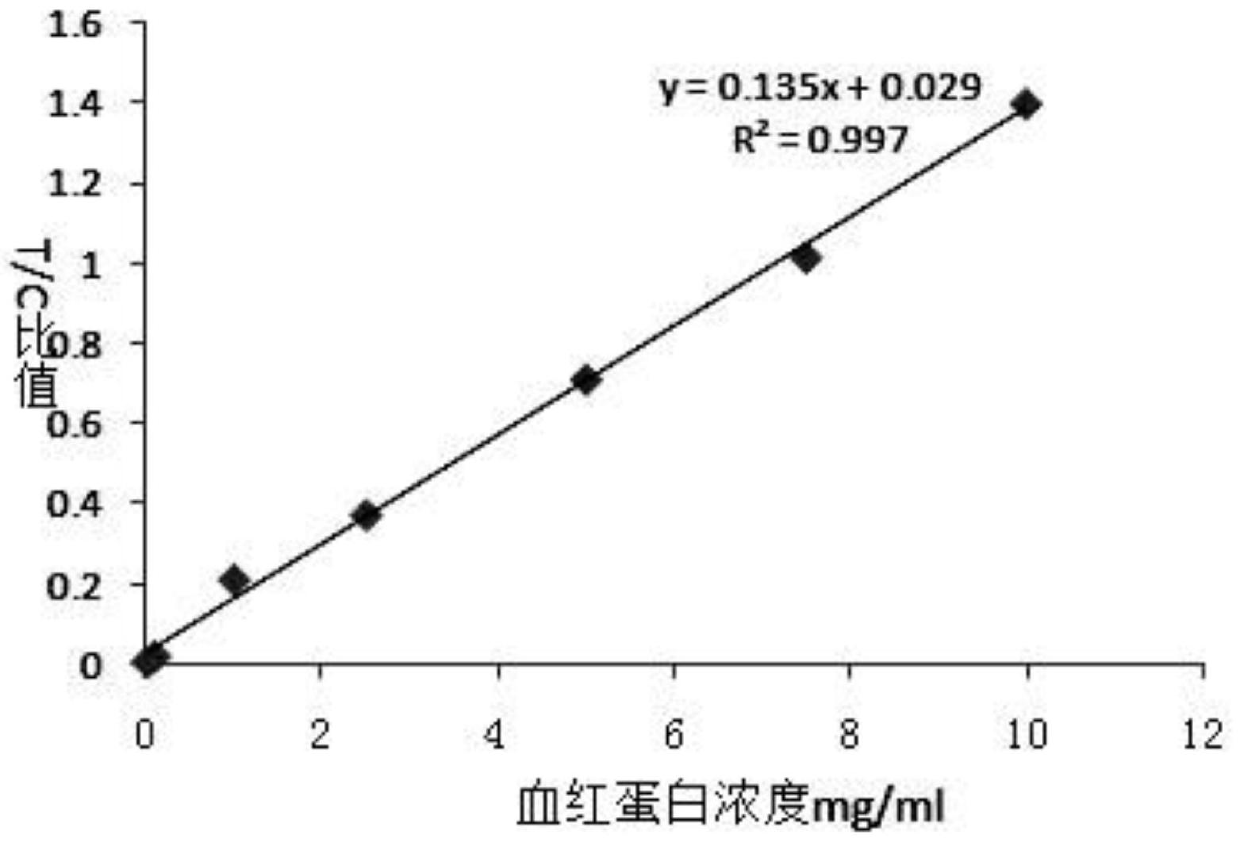
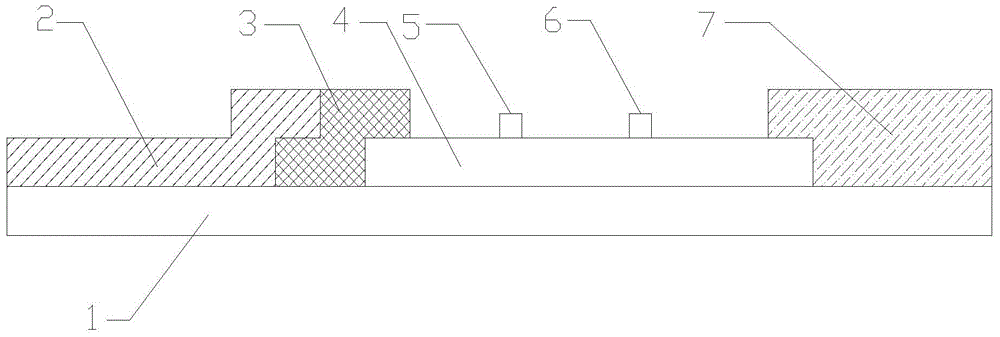
Magnetic immunochromatographic test strip for quantitatively detecting alpha-fetoprotein in blood and preparation method thereof
ActiveCN101566636ARealize single-serving wide-range quantitative detectionClinically convenientBiological testingMedicineQuantitative Result
The invention relates to a magnetic immunochromatographic test strip for quantitatively detecting alpha-fetoprotein in blood and a preparation method thereof. The test strip is assembled by pasting a coated film, magnetic particles combined with an AFP antibody, a sample pad and a water-absorbing pad which are mutually staggered by 2 millimeters in turn on a bottom plate and then covering an upper layer with a transparent plastic sealing film, wherein the coated film is precoated with an AFP-antibody detection line and a quality control line. As the test strip introduces a magnetic immunochromatography technique and a biotin-avidin system into the quantitative detection of AFP in blood, the test strip has the advantages of greatly increasing detection sensitivity, providing accurate quantitative results, reducing the labor intensity of operators and ensuring that clinicians can quickly diagnose patients.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
Fluorescence immunochromatographic assay method of serum amyloid protein A and kit
InactiveCN105675879AHigh sensitivity in vitroImprove accuracyBiological testingFluorescenceAmyloid A Protein
The invention relates to a fluorescence immunochromatographic method of serum amyloid protein A and a detection kit thereof. The method includes the steps of preparing a probe fixing pad, preparing an immunochromatographic test strip, preparing a sample diluting liquid and detecting a sample. The probe fixing pad is prepared by mixing serum amyloid protein A monoclonal antibody fluorescent latex particles and goat-anti-rabbit IgG fluorescent latex particles, and diluting the mixture with a gold diluent and spraying the liquid onto glass fibers. A detection line is coated with serum amyloid protein A monoclonal antibody and a control line is coated with goat-anti-rabbit IgG to prepare the immune-chromatographic test strip. The detection kit includes an immune-chromatographic detection card including a PVC lining plate, a sample pad, the probe fixing pad, a nitrocellulose membrane and a water absorbing paper. The probe fixing pad is prepared by mixing and drying the serum amyloid protein A monoclonal antibody fluorescent latex particles and the goat-anti-rabbit IgG fluorescent latex particles, so that the detection kit is high in detection sensitivity and accuracy, is simple in operation and is low in cost.
Owner:SUZHOU BIONANOTECH CO LTD
A fluorescence immunochromatographic detecting method for anti-mullerian hormone and a kit
InactiveCN105527448AHigh sensitivity in vitroImprove accuracyBiological material analysisBiological testingGlass fiberFluorescence
The invention relates to a fluorescence immunochromatographic detecting method for anti-mullerian hormone and a kit thereof. The method includes steps of preparing a probe fixing pad, preparing an immunochromatographic test paper strip, preparing a sample diluting liquid and detecting a sample, wherein the probe fixing pad is prepared by mixing anti-mullerian hormone monoclonal antibody fluorescence latex microparticles and goat anti-rabbit IgG fluorescence latex microparticles, diluting with a gold diluting liquid and spraying glass fibers with the diluted solution. A detection line is covered with an anti-mullerian hormone monoclonal antibody, and a control line is covered with goat anti-rabbit IgG so as to prepare the immunochromatographic test paper strip. The kit comprises an immunochromatographic detecting card. The immunochromatographic detecting card comprises a PVC liner plate, a sample pad, the probe fixing pad, a nitrocellulose membrane and water absorbing paper. The probe fixing pad is prepared by mixing the anti-mullerian hormone monoclonal antibody fluorescence latex microparticles and the goat anti-rabbit IgG fluorescence latex microparticles and drying. The method and the kit are advantaged by high detection sensitivity, high accuracy, simple operation and a low cost.
Owner:SUZHOU BIONANOTECH CO LTD
Colloidal gold immunochromatographic test strip for detecting in-vivo neutralizing antibody after injection of novel coronavirus vaccine and preparation method of colloidal gold immunochromatographic test strip
PendingCN112485436ARapid Qualitative DetectionHigh sensitivityBiological testingImmunoassaysNeutralising antibodyColloidal au
The invention discloses a colloidal gold immunochromatographic test strip for detecting a neutralizing antibody secreted in vivo after injection of a novel coronavirus vaccine and a preparation methodof the colloidal gold immunochromatographic test strip. The test strip comprises a PVC bottom plate, and a sample pad, a colloidal gold pad, a nitrocellulose membrane and water absorption filter paper are sequentially lapped on the PVC bottom plate from left to right; the colloidal gold pad is coated with a recombinant novel coronavirus RBD protein marked by colloidal gold; and the nitrocellulosemembrane is coated with an angiotensin converting enzyme 2 (ACE2) detection line and coated with a mouse anti-chicken IgG antibody as a quality control line. According to the colloidal gold immunochromatography test strip, a competitive method is adopted to detect the neutralizing antibody in a human body, the sensitivity, specificity, repeatability and stability are high, the recovery rate of atarget compound is high, and the detection result is accurate and reliable. The test strip realizes rapid qualitative detection of the neutralizing antibody in a human body after injection of a novelcoronavirus vaccine, has high sensitivity and small intra-batch and inter-batch difference, and provides great convenience for clinical use.
Owner:GUILIN UNIV OF ELECTRONIC TECH
Immunochromatographic test strip, and production method and application thereof
InactiveCN107907679AGuaranteed specificityIndicating validityMaterial analysisQuality control systemMonoclonal antibody
The invention discloses an immunochromatographic test strip, and a production method and an application thereof. The test strip is characterized in that a labeling compound carries an inert protein connected with a special label and used for quality control, and a quality control line contains a monoclonal antibody specifically binding to the special label carried by the inert protein. The test strip has an independent quality control system, so the obtained test result has a high specificity and an anti-interference property, non-specific reactions brought by the self-bearing antibodies in human blood samples are effectively avoided, and the effectiveness of the test strip is effectively indicated.
Owner:NANJING VAZYME MEDICAL TECH CO LTD +1
Time-resolved fluorescent immunochromatographic test strip for detecting tetracyclines and preparation method and application thereof
InactiveCN108508215ALarge capacity adsorptionRelease fullyBiological testingFreeze-dryingCarrier protein
The invention discloses a time-resolved fluorescent immunochromatographic test strip for detecting tetracyclines and a preparation method and application thereof. The test strip comprises test paper and a microporous reagent, the test paper comprises a bottom plate and a sample absorbing pad, a nitrocellulose membrane and a water absorbing pad which are sequentially overlapped and bonded to the bottom plate, a detecting area and a quality control area are arranged on the nitrocellulose membrane, hapten-carrier protein conjugate of the tetracyclines is sprayed on the detecting area, goat anti-mouse antiantibody is sprayed on the quality control area, and the monoclonal antibody of the tetracyclines marked by fluorescent microspheres is frozen dry in the microporous reagent. The invention further provides the preparation method of the test strip and a method for detecting the tetracyclines in samples by using the test strip. The test strip and the detecting method have the advantages ofbeing simple in operation, high in sensitivity, fast in detecting speed and low in cost, and the rapid detecting and on-spot monitoring of the tetracyclines in samples in large batches can be achieved.
Owner:BEIJING KWINBON BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com