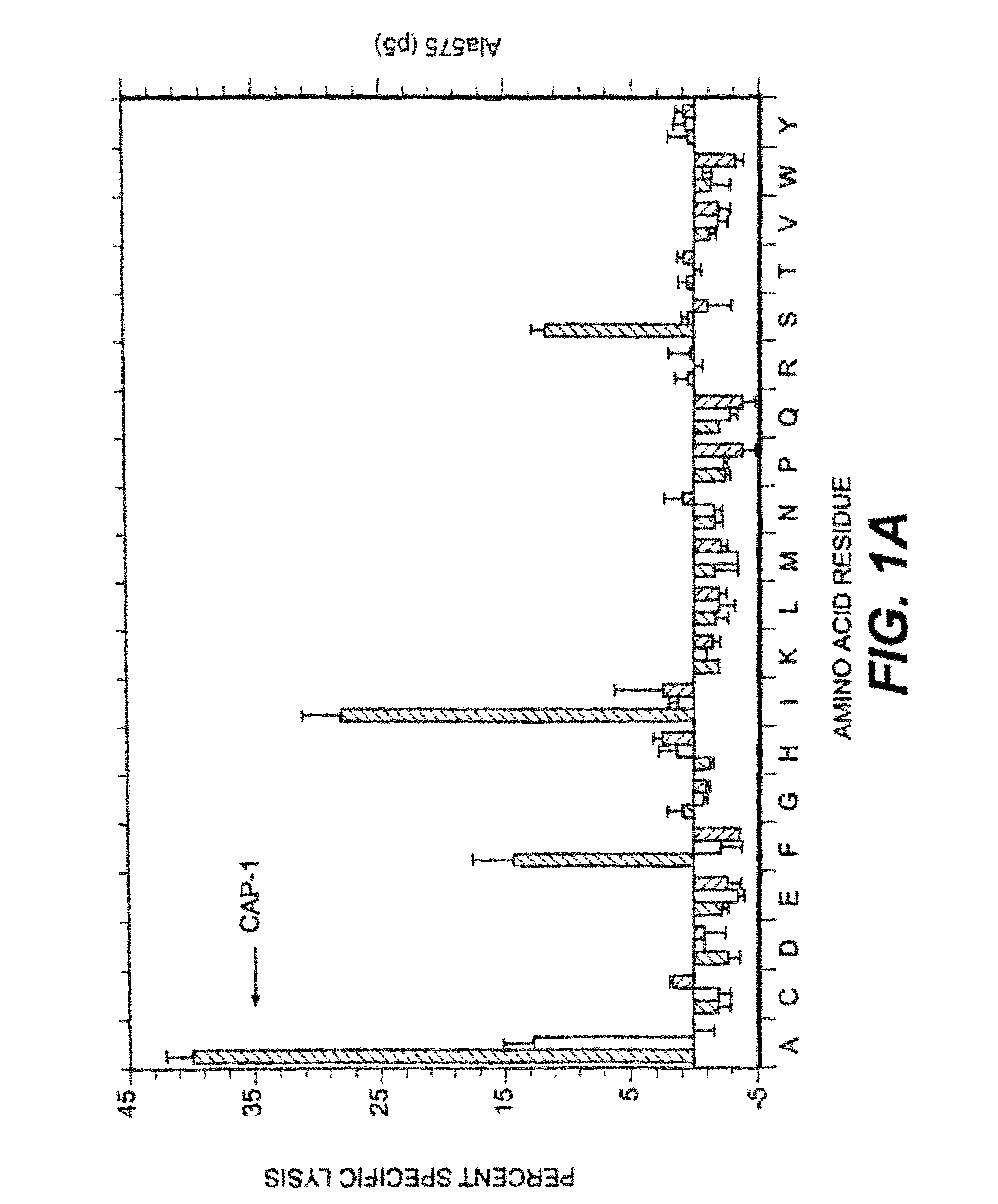
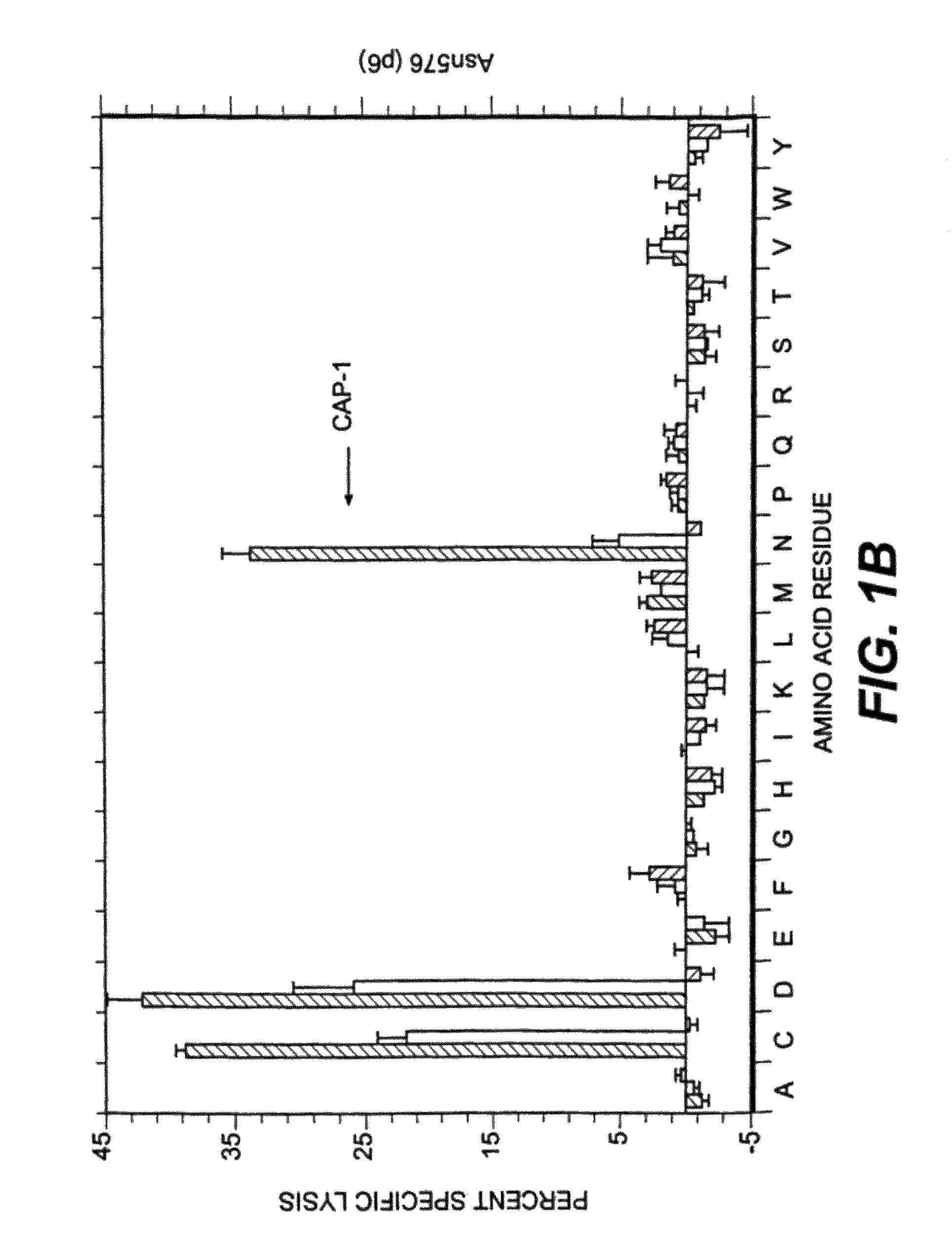
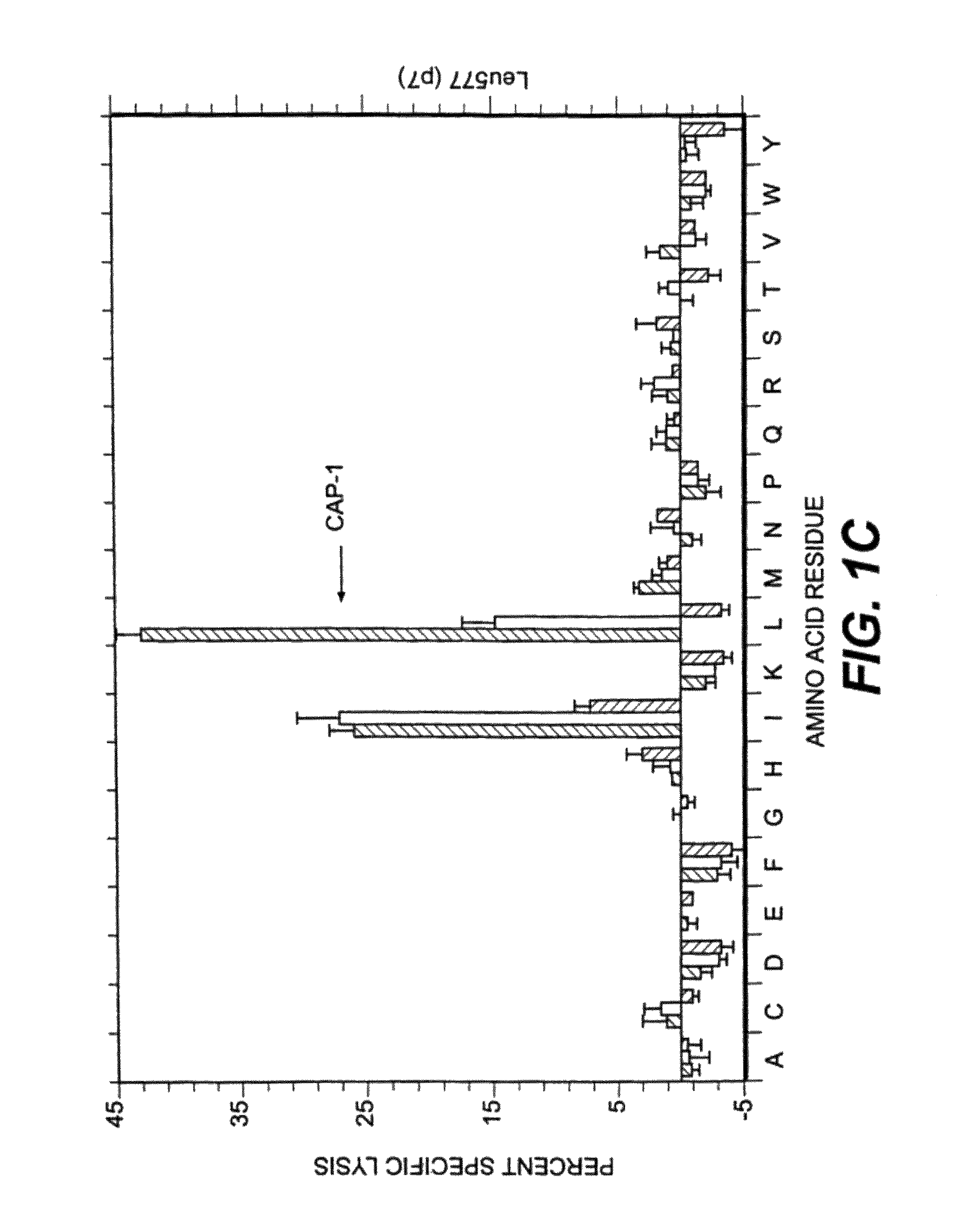
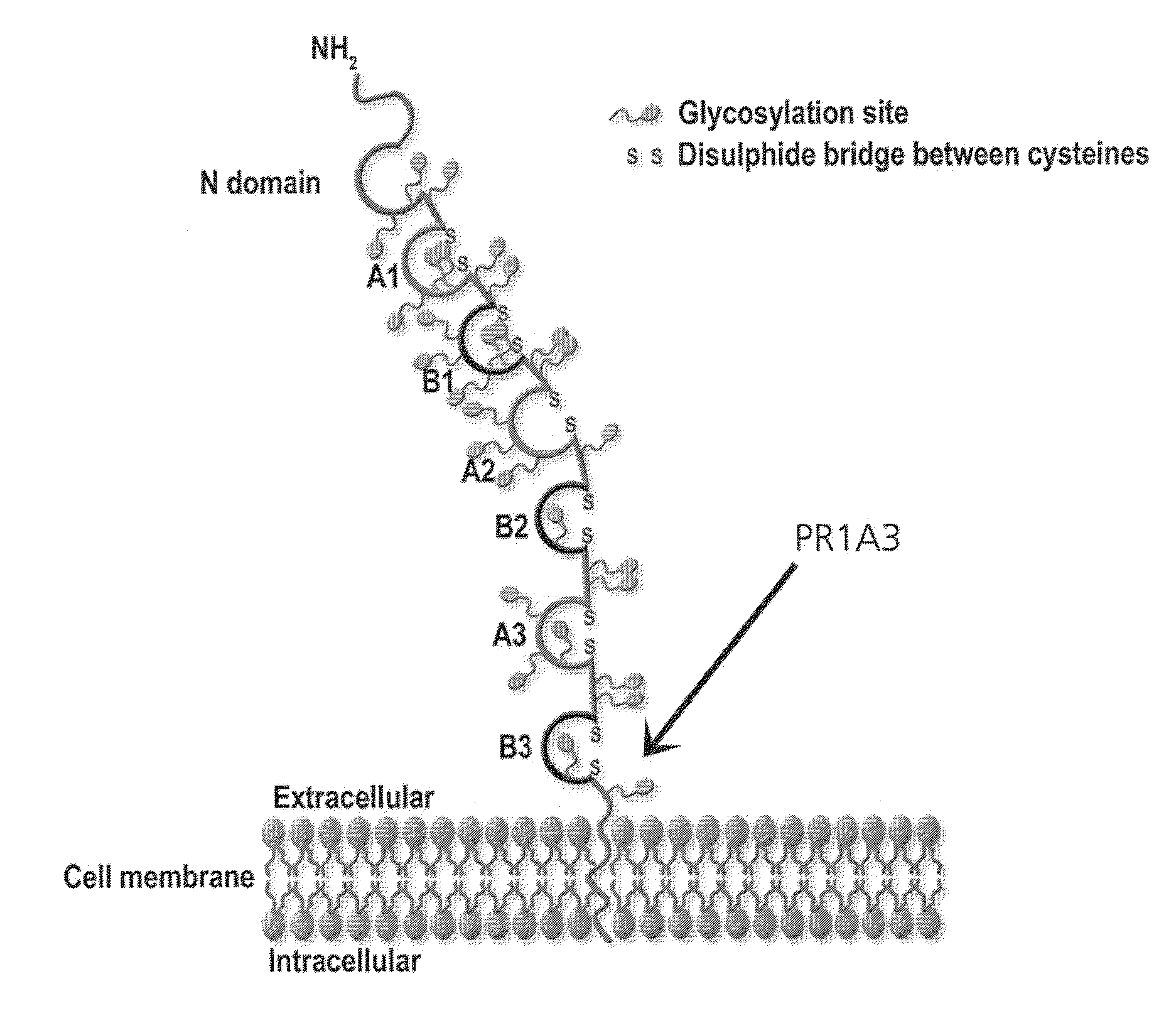
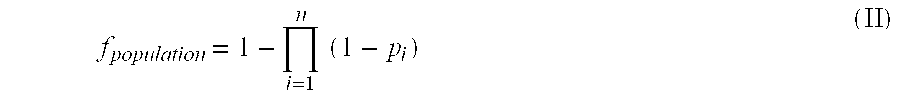Patents
Literature
145 results about "Carcinoembryonic antigen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Carcinoembryonic antigen (CEA) describes a set of highly related glycoproteins involved in cell adhesion. CEA is normally produced in gastrointestinal tissue during fetal development, but the production stops before birth. Consequently, CEA is usually present at very low levels in the blood of healthy adults (about 20 ng/mL). However, the serum levels are raised in some types of cancer, which means that it can be used as a tumor marker in clinical tests. Serum levels can also be elevated in heavy smokers.
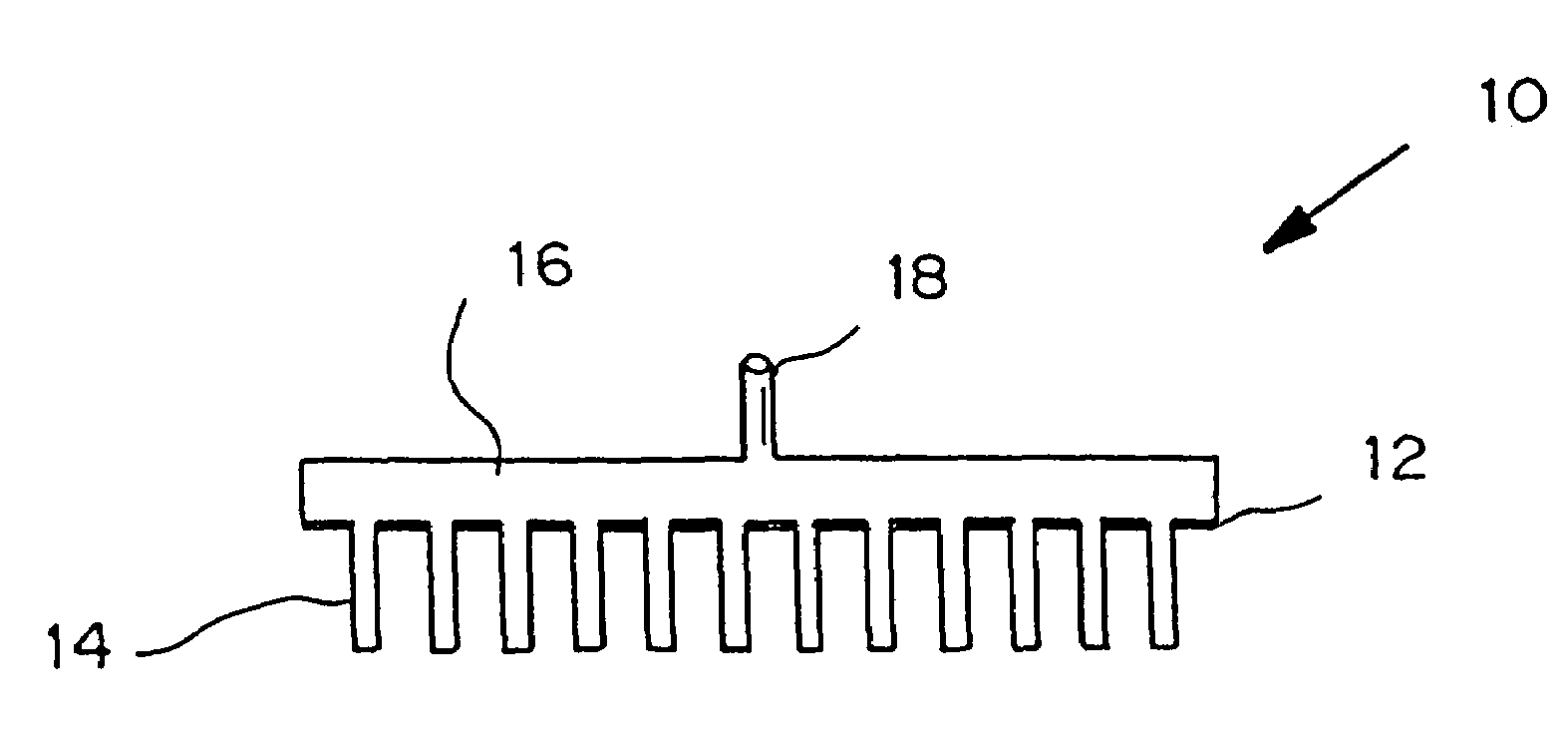
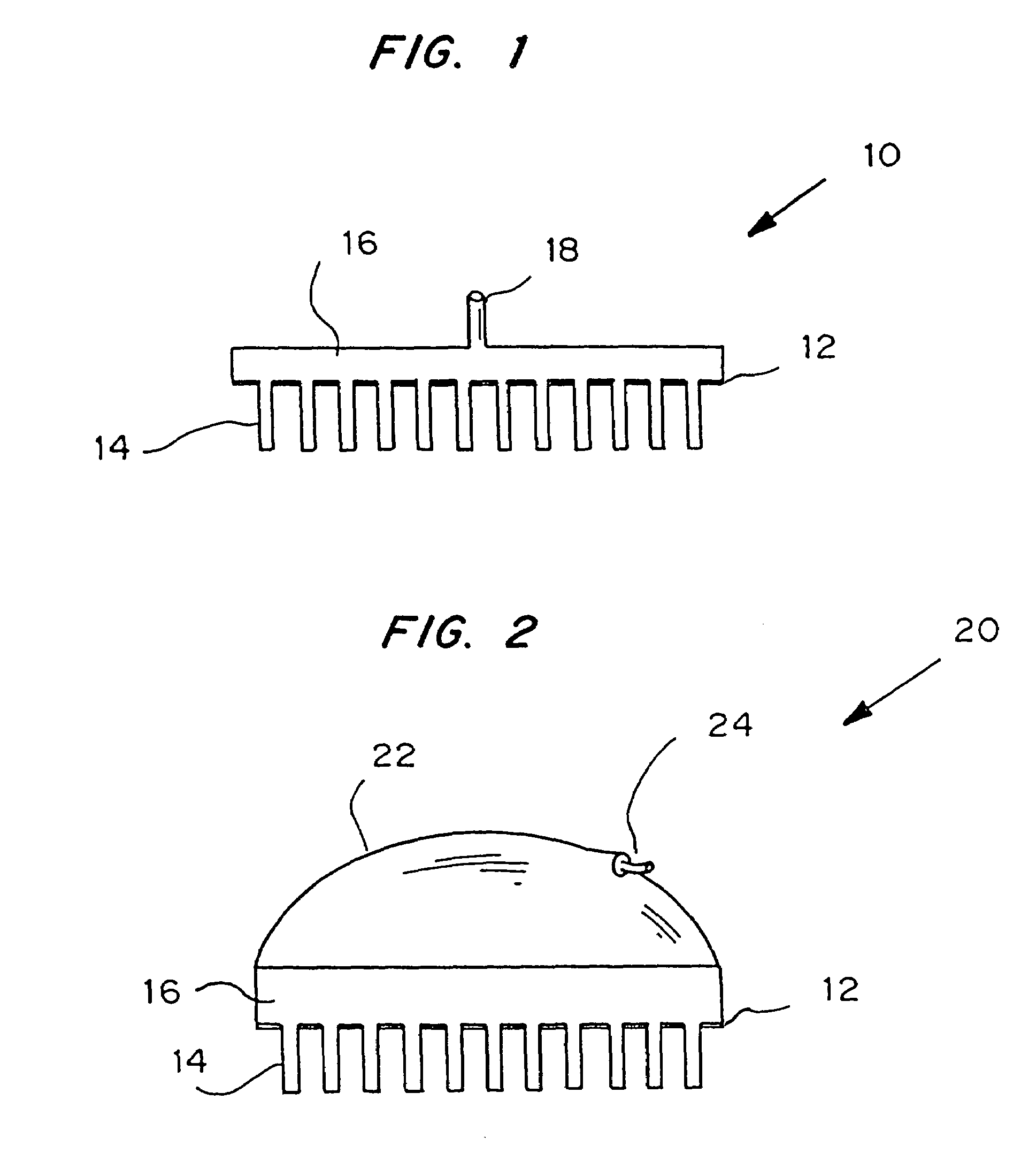
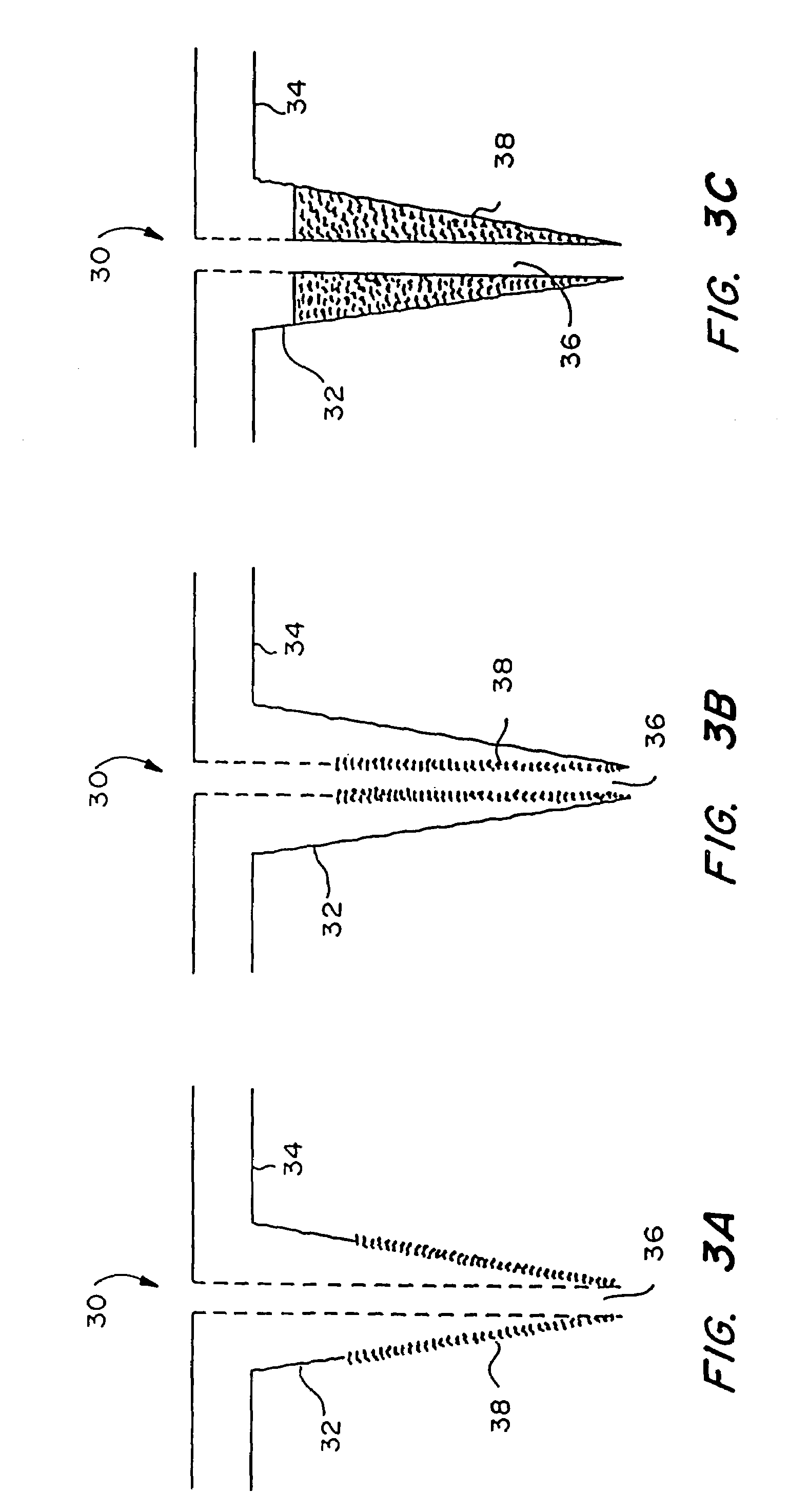
Microneedle device for extraction and sensing of bodily fluids
InactiveUS7344499B1Simple wayMinimal and no damageAdditive manufacturing apparatusMicroneedlesMetaboliteIrritation
Microneedle devices are provided for controlled sampling of biological fluids in a minimally-invasive, painless, and convenient manner. The microneedle devices permit in vivo sensing or withdrawal of biological fluids from the body, particularly from or through the skin or other tissue barriers, with minimal or no damage, pain, or irritation to the tissue. The microneedle device includes one or more microneedles, preferably in a three-dimensional array, a substrate to which the microneedles are connected, and at least one collection chamber and / or sensor in communication with the microneedles. Preferred embodiments further include a means for inducing biological fluid to be drawn through the microneedles and into the collection chamber for analysis. In a preferred embodiment, this induction is accomplished by use of a pressure gradient, which can be created for example by selectively increasing the interior volume of the collection chamber, which includes an elastic or movable portion engaged to a rigid base. Preferred biological fluids for withdrawal and / or sensing include blood, lymph, interstitial fluid, and intracellular fluid. Examples of analytes in the biological fluid to be measured include glucose, cholesterol, bilirubin, creatine, metabolic enzymes, hemoglobin, heparin, clotting factors, uric acid, carcinoembryonic antigen or other tumor antigens, reproductive hormones, oxygen, pH, alcohol, tobacco metabolites, and illegal drugs.
Owner:GEORGIA TECH RES CORP +1
PCR method
InactiveUS7101663B2Permit useReduce errorsMicrobiological testing/measurementRecombinant DNA-technologySentinel lymph nodeCarcinoembryonic antigen
A method for balancing multiplexed PCR methods is provided. In the method, two or more sequential temporal PCR stages are used to effectively separate two or more PCR reactions in a single tube as an alternative to primer limiting to modulate the relative rate of production of a first amplicon by a first primer set and a second amplicon by a second primer set during the first and second amplification stages. Also provided are rapid RT-PCR methods that find particular use in intraoperative diagnoses and prognoses, for instance in diagnosing malignant esophageal adenocarcenoma by determining expression levels of carcinoembryonic antigen (CEA) in sentinel lymph nodes.
Owner:UNIVERSITY OF PITTSBURGH
Noninvasive genetic immunization, expression products therefrom and uses thereof
InactiveUS6348450B1Improve vaccination schemeEfficient methodSsRNA viruses negative-senseBiocideHemagglutininWhole body
Disclosed and claimed are methods of non-invasive genetic immunization in an animal and / or methods of inducing a systemic immune or therapeutic response in an animal, products therefrom and uses for the methods and products therefrom. The methods can include contacting skin of the animal with a vector in an amount effective to induce the systemic immune or therapeutic response in the animal. The vector can include and express an exogenous nucleic acid molecule encoding an epitope or gene product of interest. The systemic immune response can be to or from the epitope or gene product. The nucleic acid molecule can encode an epitope of interest and / or an antigen of interest and / or a nucleic acid molecule that stimulates and / or modulates an immunological response and / or stimulates and / or modulates expression, e.g., transcription and / or translation, such as transcription and / or translation of an endogenous and / or exogenous nucleic acid molecule; e.g., one or more of influenza hemagglutinin, influenza nuclear protein, tetanus toxin C-fragment, anthrax protective antigen, HIV gp 120, human carcinoembryonic antigen, and / or a therapeutic, an immunomodulatory gene, such as co-stimulatory gene and / or a cytokine gene. The immune response can be induced by the vector expressing the nucleic acid molecule in the animal's cells. The immune response can be against a pathogen or a neoplasm. A prophylactic vaccine or a therapeutic vaccine or an immunological composition can include the vector.
Owner:UAB RES FOUND
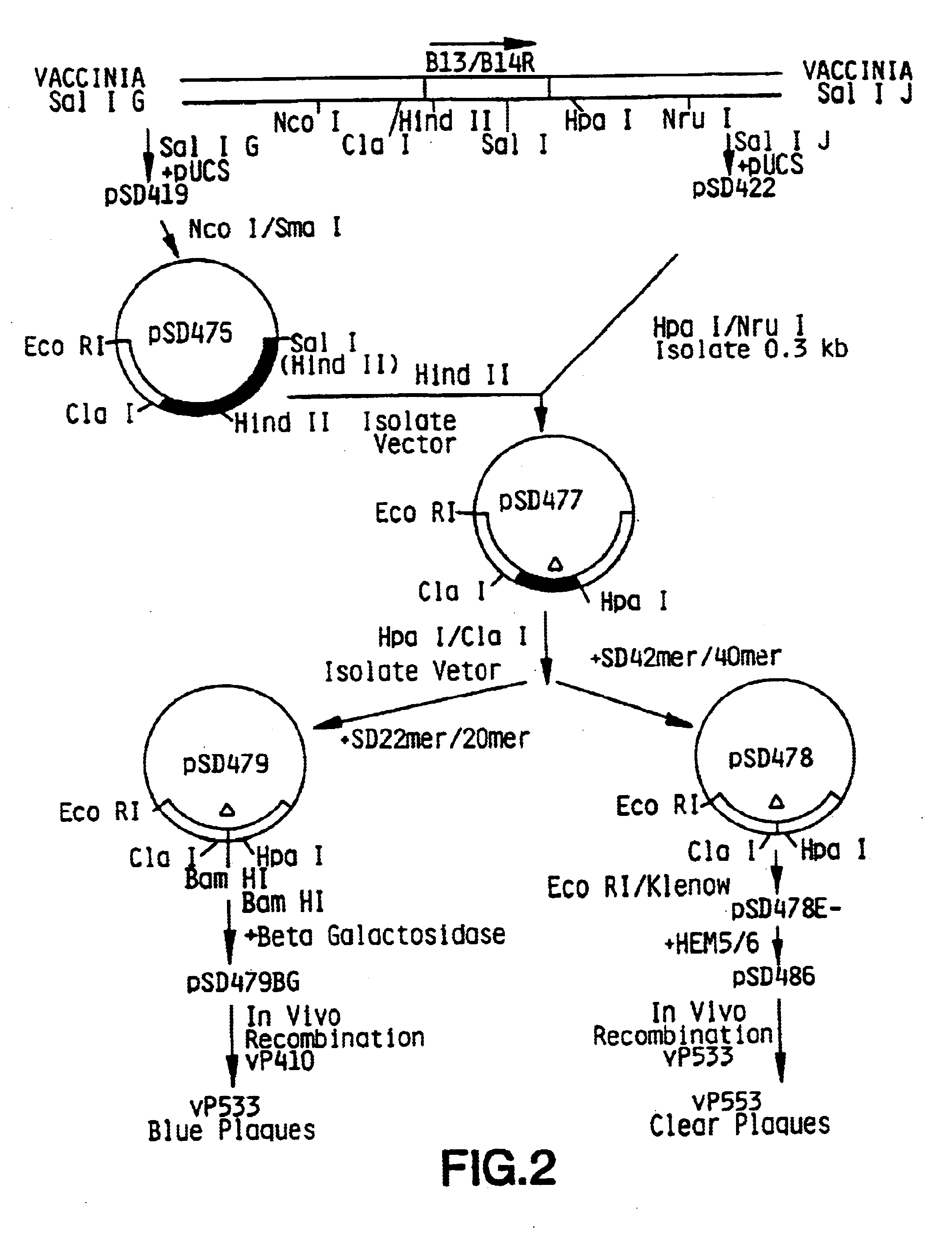
Pox virus containing DNA encoding a cytokine and/or a tumor associated antigen
InactiveUS6265189B1Improve securityImprove security levelVirusesPeptide/protein ingredientsHuman tumorWild type
Attenuated recombinant viruses containing DNA coding for a cytokine and / or a tumor associated antigen, as well as methods and compositions employing the viruses, are disclosed and claimed. The recombinant viruses can be NYVAC or ALVAC recombinant viruses. The DNA can code for at least on of: human tumor necrosis factor; nuclear phosphoprotein p53, wildtype or mutant; human melanoma-associated antigen; IL-2; IFNgamma; IL-4; GNCSF; IL-12; B7; erb-B-2 and carcinoembryonic antigen. The recombinant viruses and gene products therefrom are useful for cancer therapy.
Owner:VIROGENETICS
Noninvasive genetic immunization, expression products therefrom, and uses thereof
InactiveUS6716823B1Improve vaccination schemeEfficient methodSsRNA viruses negative-senseBiocideMalariaNon invasive
Disclosed and claimed are methods of non-invasive genetic immunization in an animal and / or methods of inducing a systemic immune or therapeutic response in an animal, products therefrom and uses for the methods and products therefrom. The methods can include contacting skin of the animal with a vector in an amount effective to induce the systemic immune or therapeutic response in the animal. The vector can include and express an exogenous nucleic acid molecule encoding an epitope or gene product of interest. The systemic immune response can be to or from the epitope or gene product. The nucleic acid molecule can encode an epitope of interest and / or an antigen of interest and / or a nucleic acid molecule that stimulates and / or modulates an immunological response and / or stimulates and / or modulates expression, e.g., transcription and / or translation, such as transcription and / or translation of an endogenous and / or exogenous nucleic acid molecule; e.g., one or more of influenza hemagglutinin, influenza nuclear protein, influenza M2, tetanus toxin C-fragment, anthrax protective antigen, anthrax lethal factor, rabies glycoprotein, HBV surface antigen, HIV gp 120, HIV gp 160, human carcinoembryonic antigen, malaria CSP, malaria SSP, malaria MSP, malaria pfg, and mycobacterium tuberculosis HSP; and / or a therapeutic, an immunomodulatory gene, such as co-stimulatory gene and / or a cytokine gene. The immune response can be induced by the vector expressing the nucleic acid molecule in the animal's cells. The animal's cells can be epidermal cells. The immune response can be against a pathogen or a neoplasm. A prophylactic vaccine or a therapeutic vaccine or an immunological composition can include the vector. The animal can be a vertebrate, e.g., a mammal, such as human, a cow, a horse, a dog, a cat, a goat, a sheep or a pig; or fowl such as turkey, chicken or duck. The vector can be one or more of a viral vector, including viral coat, e.g., with some or all viral genes deleted therefrom, bacterial, protozoan, transposon, retrotransposon, and DNA vector, e.g., a recombinant vector; for instance, an adenovirus, such as an adenovirus defective in its E1 and / or E3 and / or E4 region(s). The method can encompass applying a delivery device including the vector to the skin of the animal, as well as such a method further including disposing the vector in and / or on the delivery device. The vector can have all viral genes deleted therefrom. The vector can induce a therapeutic and / or an anti-tumor effect in the animal, e.g., by expressing an oncogene, a tumor-suppressor gene, or a tumor-associated gene. Immunological products generated by the expression, e.g., antibodies, cells from the methods, and the expression products, are likewise useful in in vitro and ex vivo applications, and such immunological and expression products and cells and applications are disclosed and claimed. Methods for expressing a gene product in vivo and products therefor and therefrom including mucosal and / or intranasal administration of an adenovirus, advantageously an E1 and / or E3 and / or E4 defective or deleted adenovirus, such as a human adenovirus or canine adenovirus, are also disclosed and claimed.
Owner:UAB RES FOUND
Vaccina virus comprising cytokine and/or tumor associated antigen genes
InactiveUS6537594B1Improve securityImprove security levelVirusesPeptide/protein ingredientsHuman tumorWild type
Owner:VIROGENETICS
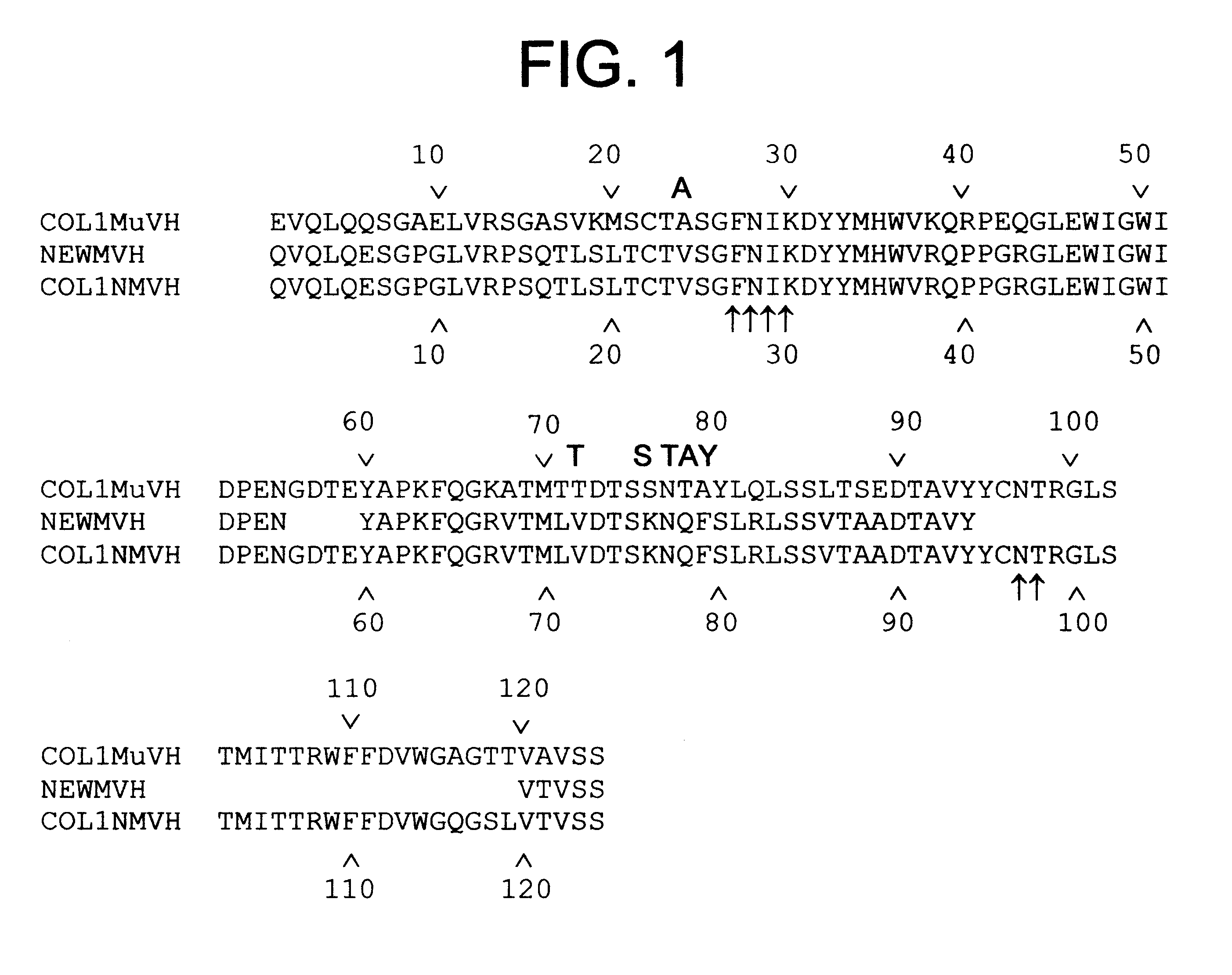
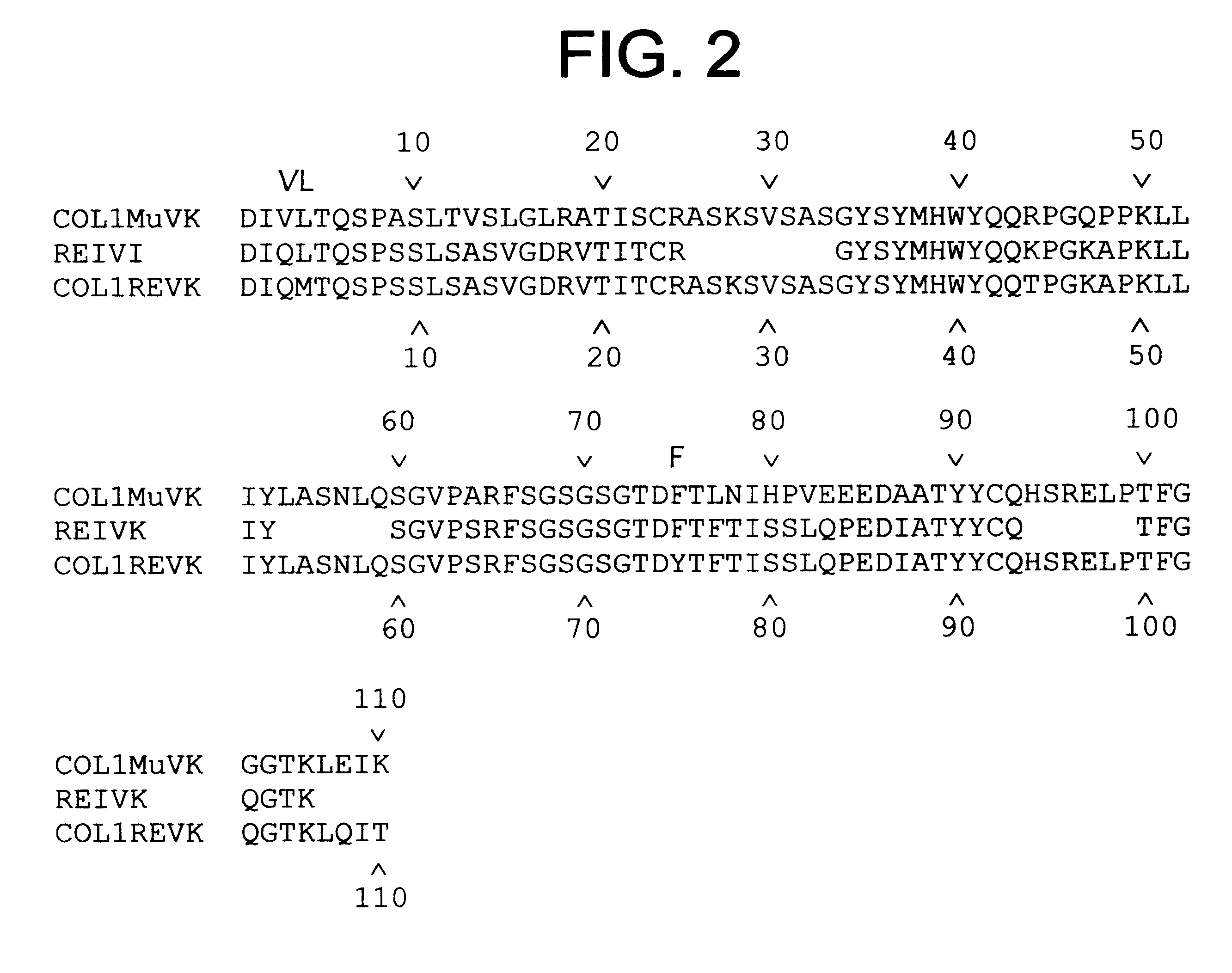
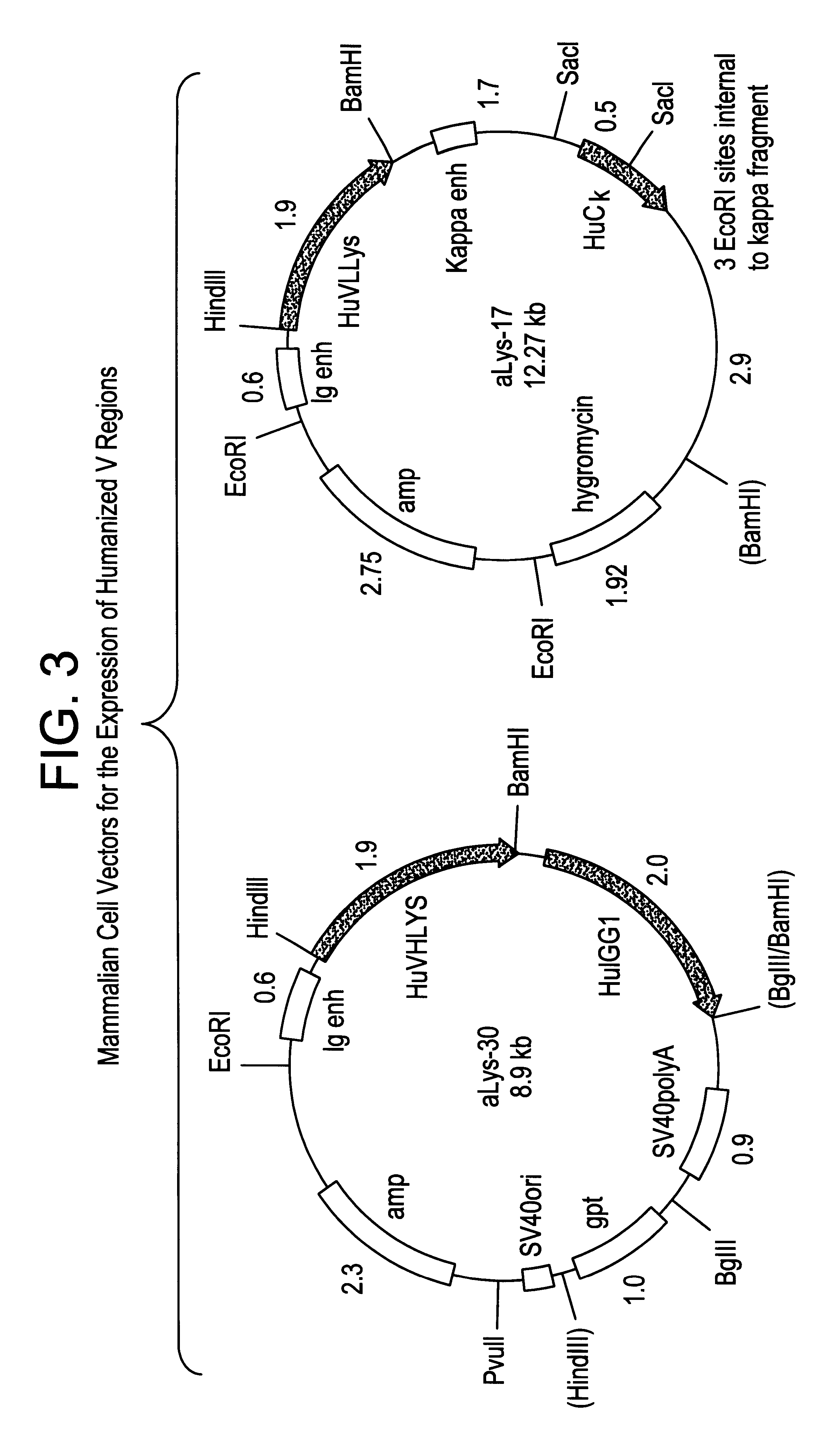
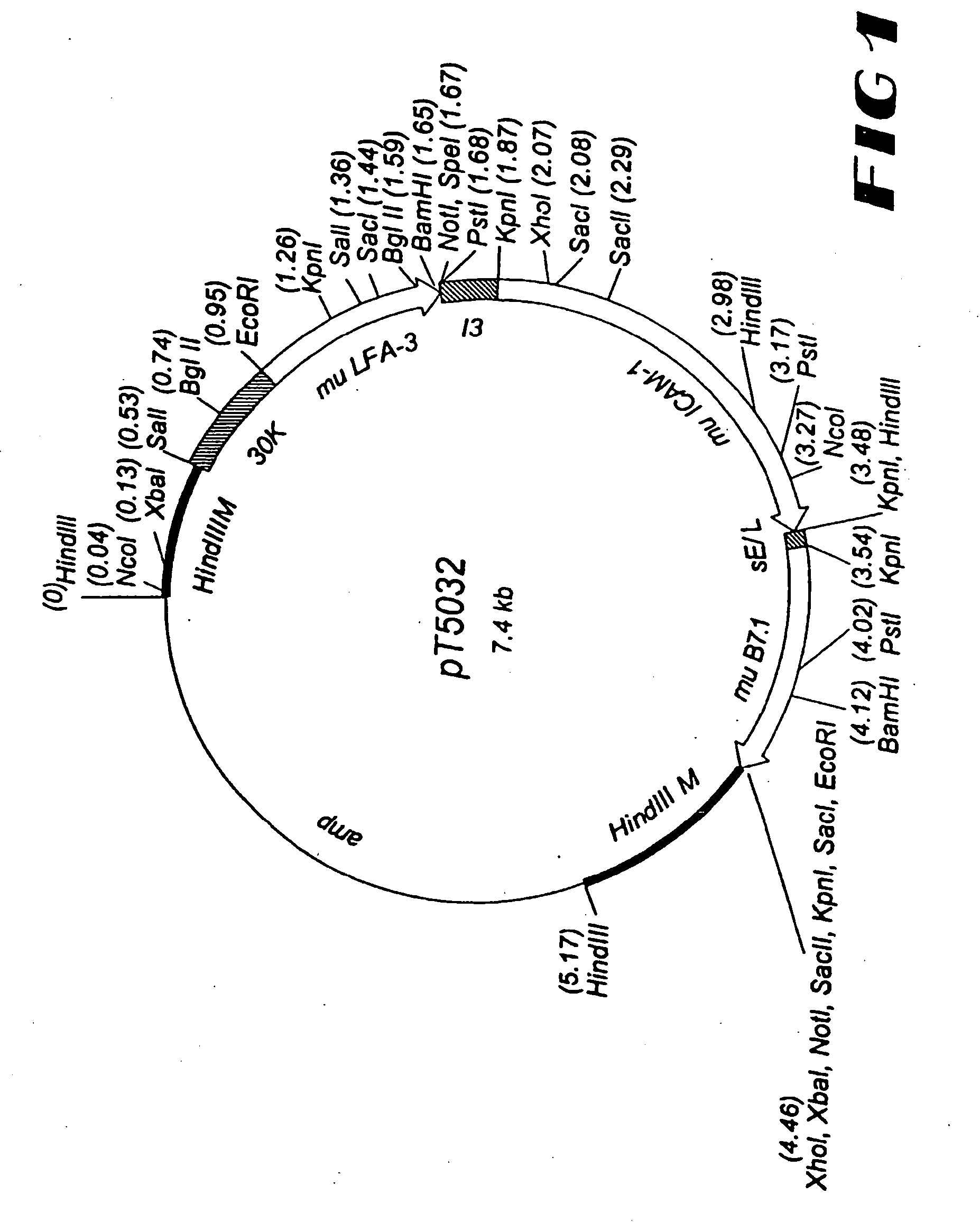
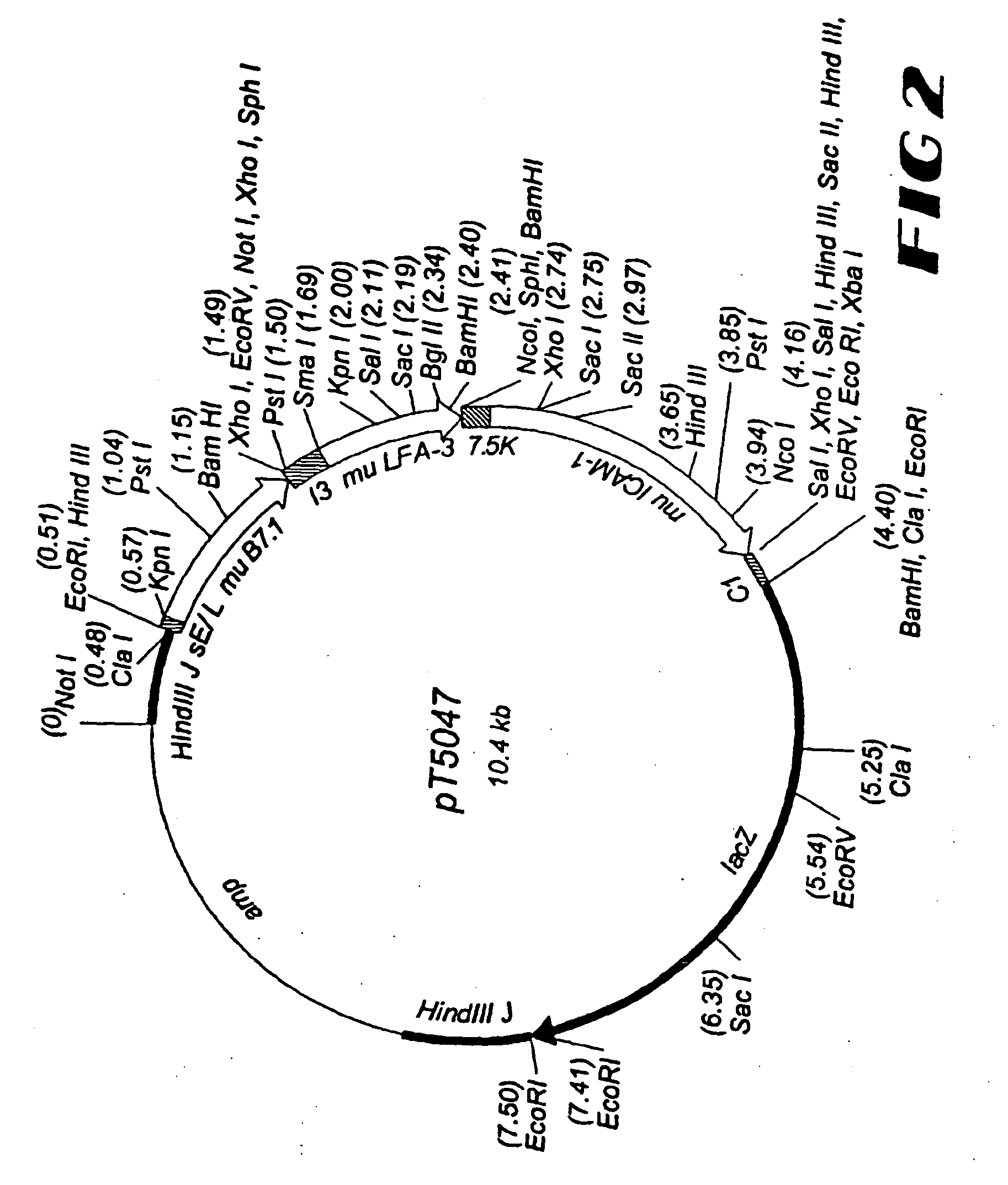
High affinity humanized anti-CEA monoclonal antibodies
InactiveUS6333405B1Reduce complicationsReduce and potentially eliminate elicitingSugar derivativesAntibody mimetics/scaffoldsCancer cellCarcinoembryonic antigen
Novel humanized monoclonal antibodies, fragments or derivatives thereof which specifically bind carcinoembryonic antigen (CEA) are provided as well as methods for their manufacture. These humanized antibodies are useful in the treatment of cancers which express CEA as well as for diagnostic purposes, e.g., for in vivo imaging of tumors or cancer cells which express CEA.
Owner:THE DOW CHEM CO
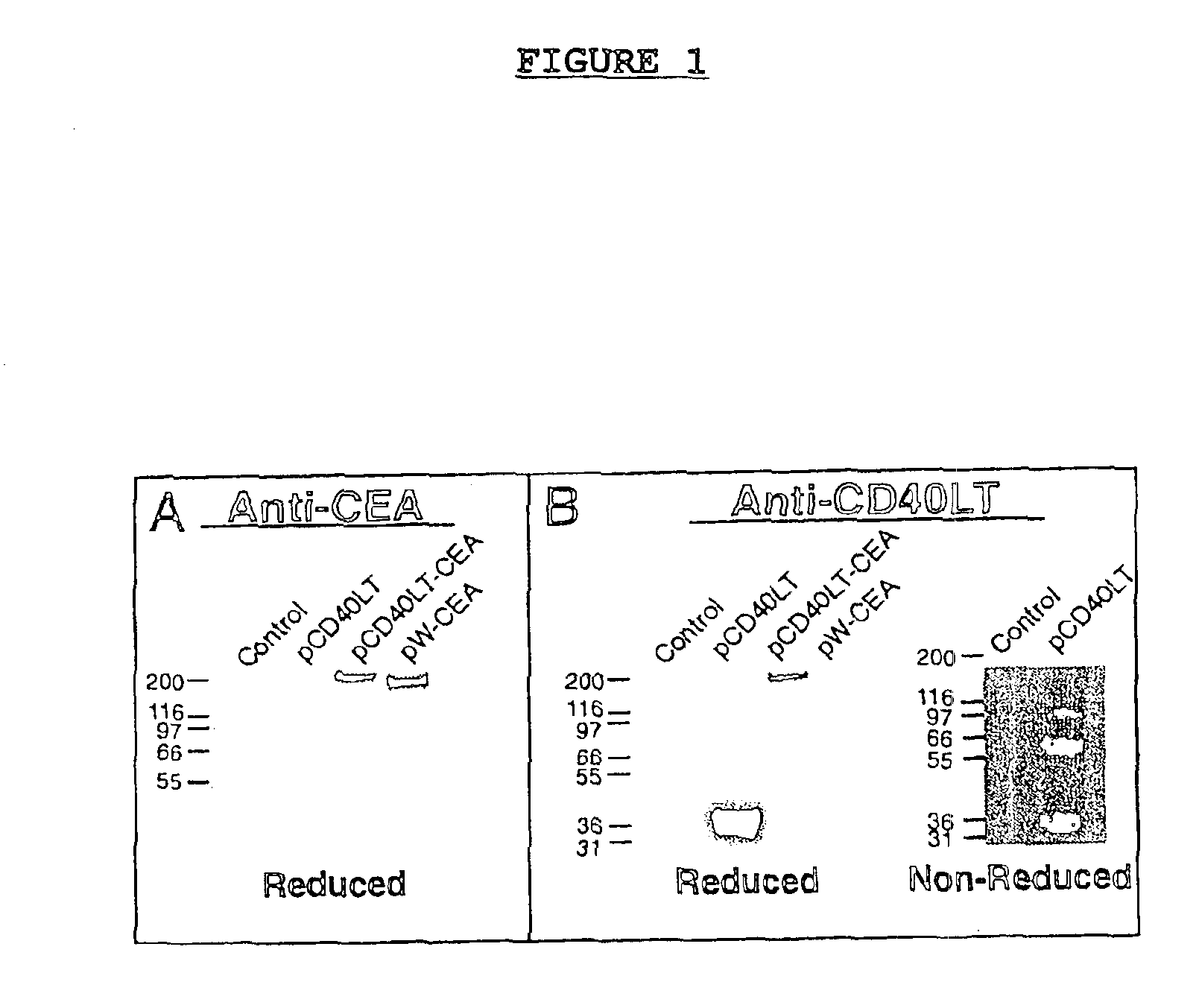
DNA vaccines encoding CEA and a CD40 ligand and methods of use thereof
InactiveUS6923958B2Enhance immune responseEffective immune responseOrganic active ingredientsPeptide/protein ingredientsLipofectamineMammal
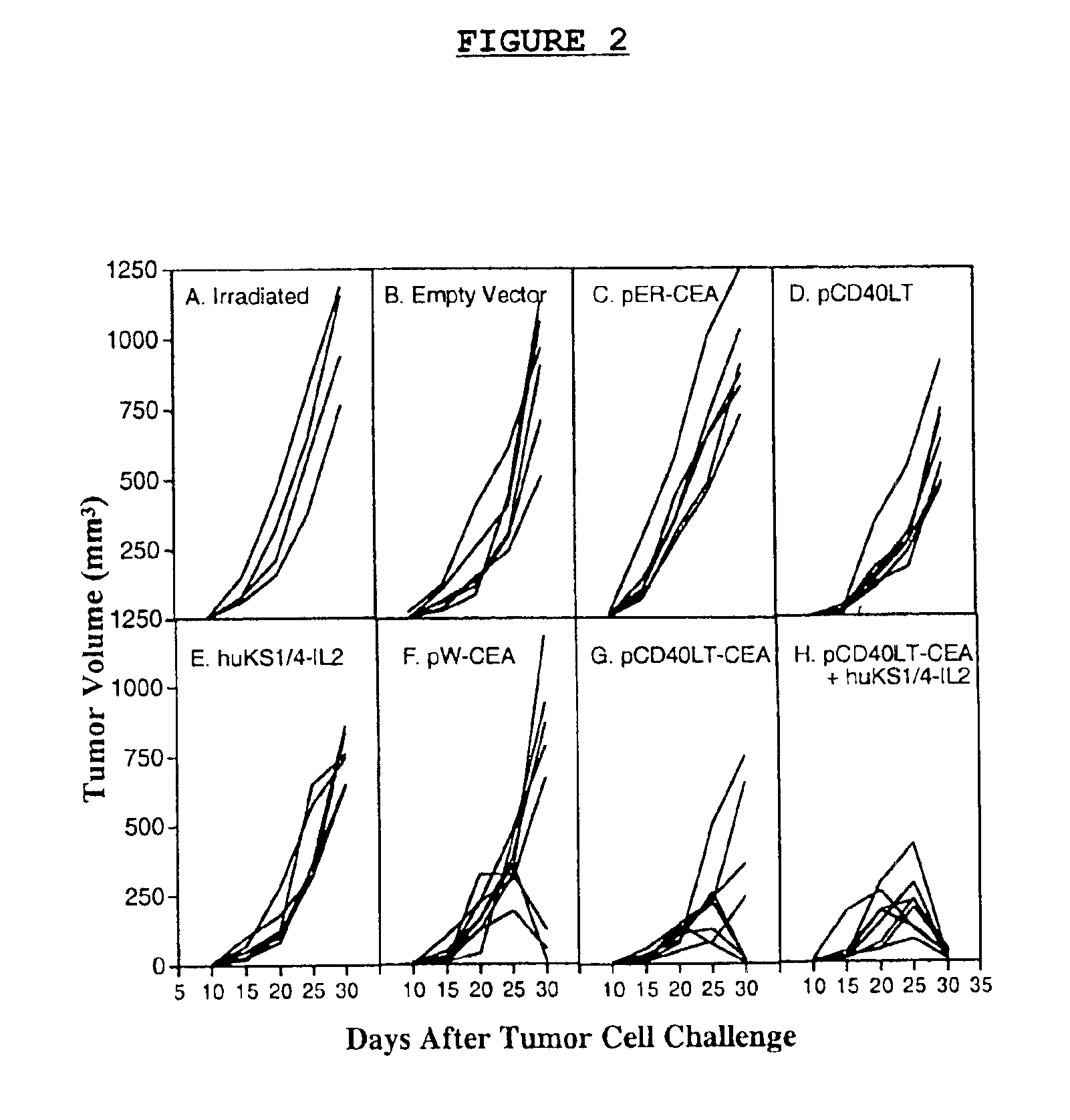
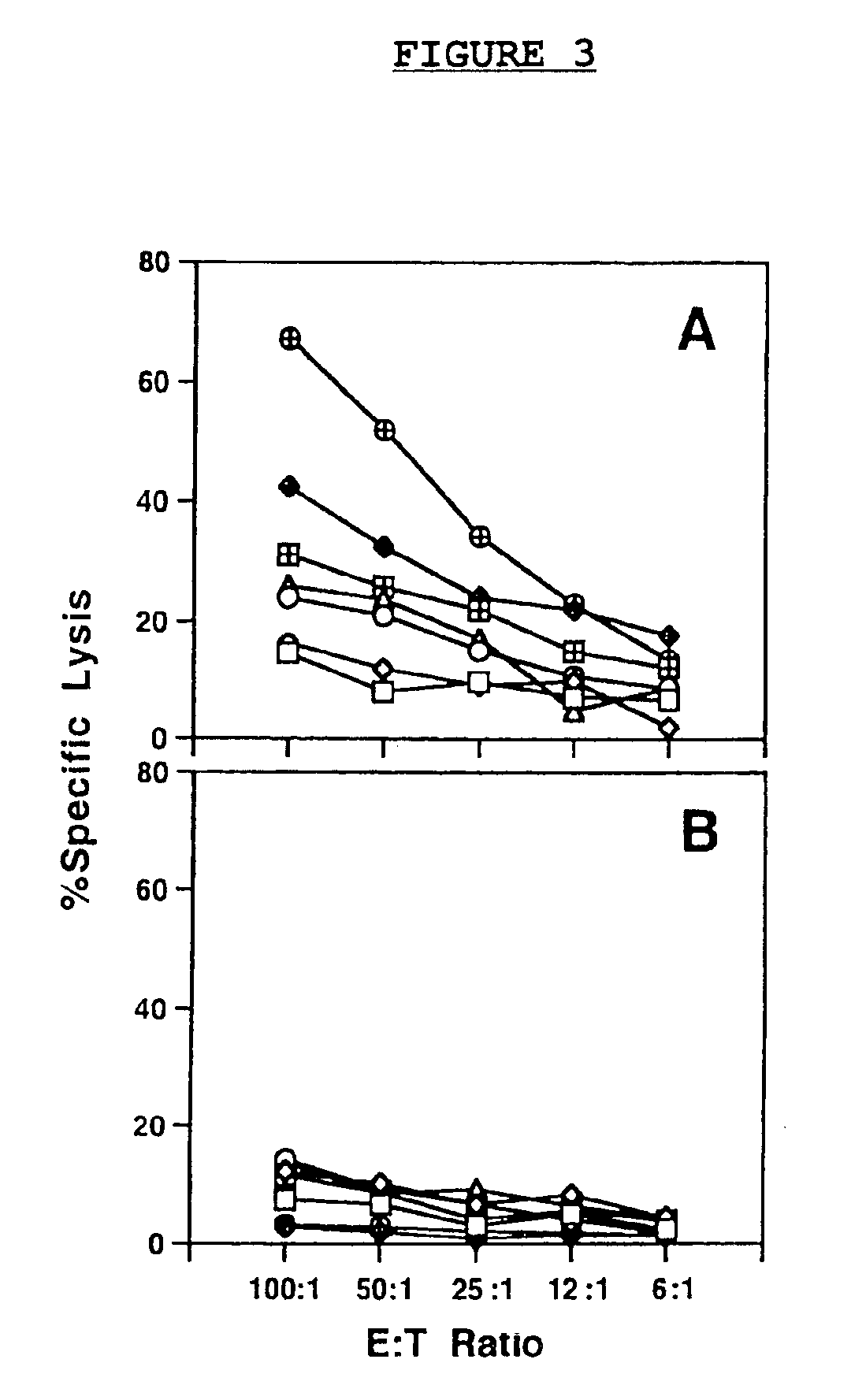
A DNA vaccine effective for eliciting an immune response against cells that present a carcinoembryonic antigen (CEA) comprises a DNA operably encoding a CEA and a DNA operably encoding a CD40 ligand, SEQ ID NO:1 and SEQ ID NO: 2, respectively, or its homotrimer, CD40LT. The DNA vaccine can be incorporated in a delivery vector such as an attenuated live bacterium or virus, or a liposome carrier. In a method embodiment, the DNA vaccine is administered orally to a mammal, such as a human, to elicit an immune response against CEA presenting cells such as colon cancer cells. A preferred method embodiment includes the additional step of treating the mammal with recombinant antibody fusion protein huKS1 / 4-IL2 to enhance the immune response effectiveness of the vaccine.
Owner:THE SCRIPPS RES INST
Pox virus comprising DNA sequences encoding CEA and B7 antigen
Attenutated recombinant viruses containing DNA coding for a cytokine and / or a tumor associated antigen, as well as methods and compositions employing the viruses, are disclosed and claimed. The recombinant viruses can be NYVAC or ALVAC recombinant viruses. The DNA can code for at least one of: human tumor necrosis factor; nuclear phosphoprotein p53, wiltype or mutant; human melanoma-associated antigen; IL-2; IFNgamma; IL-4; GMCSF; IL-12; B7; erb-B-2 and carcinoembryonic antigen. The recombinant viruses and gene products therefrom are useful for cancer therapy.
Owner:AVENTIS PASTEUR LTD
Antibodies against tumor surface antigens
ActiveUS7232888B2Simple methodExtended retention timePeptide/protein ingredientsMammal material medical ingredientsCarcinoembryonic antigenScFv Antibodies
The present invention relates to improved antibodies against tumor surface antigens and their use in the treatment of tumors. Of particular interest are highly stable, humanized, high affinity antibodies against carcinoembryonic antigen (CEA), especially the antibody we have termed sm3E, which is derived from the scFv antibody MFE-23. Such antibodies have the potential for improved therapeutic efficacy.
Owner:MASSACHUSETTS INST OF TECH +1
Antibodies against tumor surface antigens
ActiveUS20050147614A1Good effectExtended retention timePeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAbnormal tissue growthCarcinoembryonic antigen
The present invention relates to improved antibodies against tumor surface antigens and their use in the treatment of tumors. Of particular interest are highly stable, humanized, high affinity antibodies against carcinoembryonic antigen (CEA), especially the antibody we have termed sm3E, which is derived from the scFv antibody MFE-23. Such antibodies have the potential for improved therapeutic efficacy.
Owner:MASSACHUSETTS INST OF TECH +1
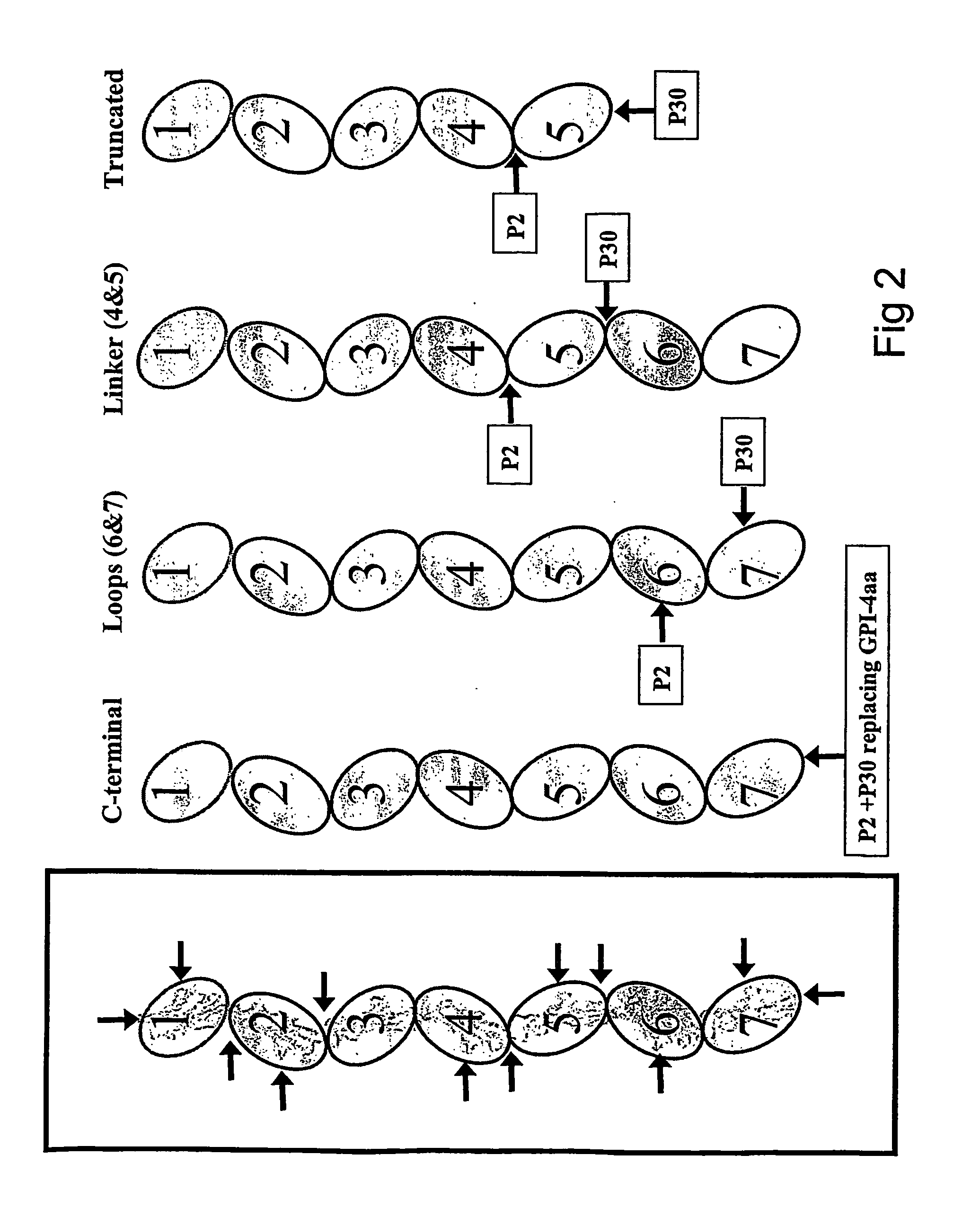
Carcinoembryonic antigen (CEA) peptides
InactiveUS20070048860A1Immunoglobulin superfamilyViral antigen ingredientsEpitopeCarcinoembryonic antigen
The present invention relates to novel modified CEA agonist (or antagonist) peptides, polypeptides and proteins containing a modified epitope therein, nucleic acids coding therefor, vectors comprising said nucleic acids, mixtures and compositions of the aforementioned agents, and their advantageous use in generating CEA-specific immune responses and / or in the treatment of cancers and the present invention further relates to the foregoing combined with one or more costimulatory molecules.
Owner:HEALTH & HUMAN SERVICES US SEC THE DEPT OF THE +1
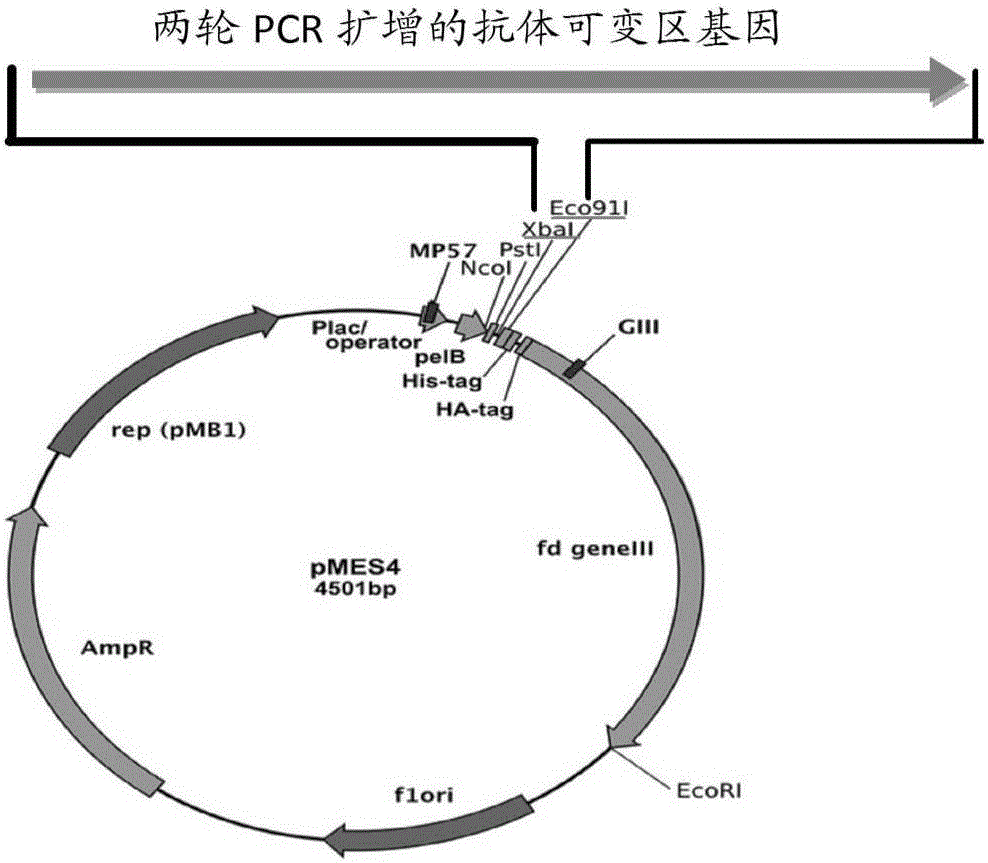
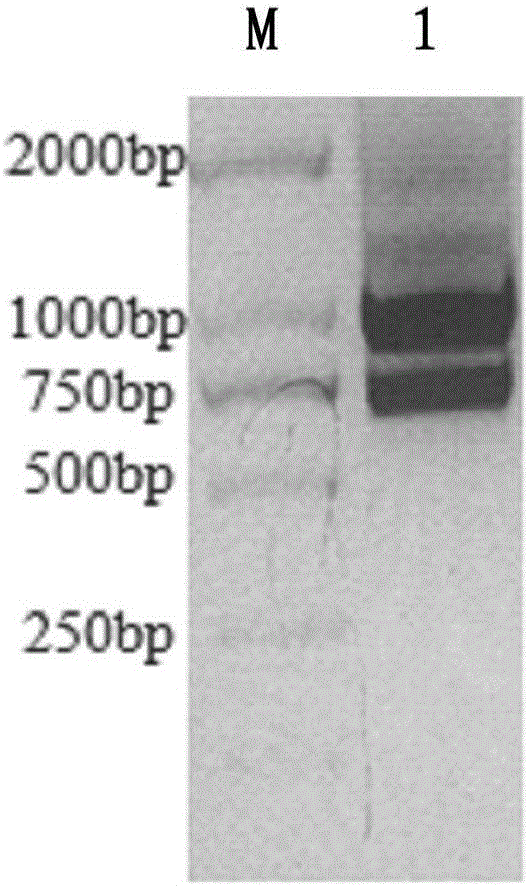
Nanobody resisting CEA (carcinoembryonic antigen) and application of nanobody
ActiveCN106749667ASignificant ADCC effectSpecific recognition abilityIn-vivo radioactive preparationsBacteriaAntigenCancer cell
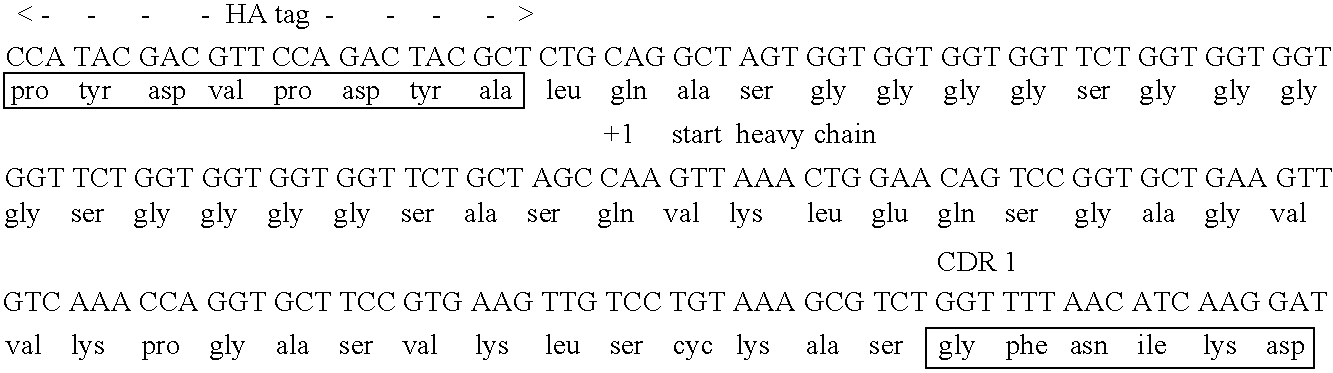
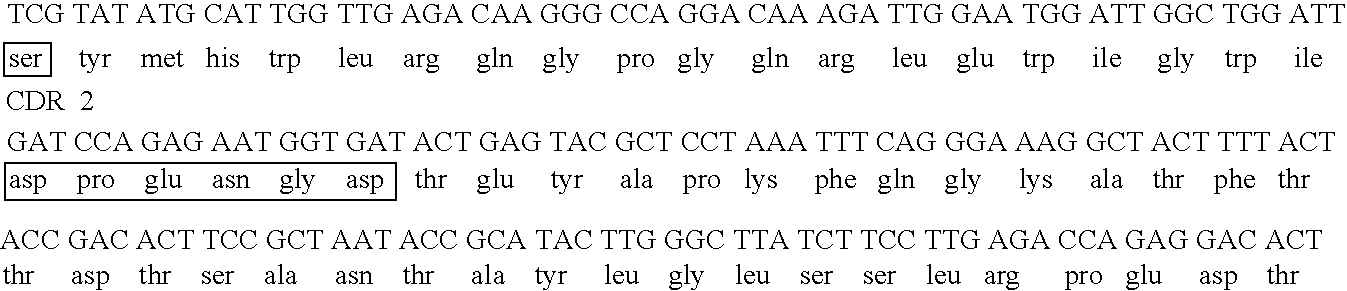
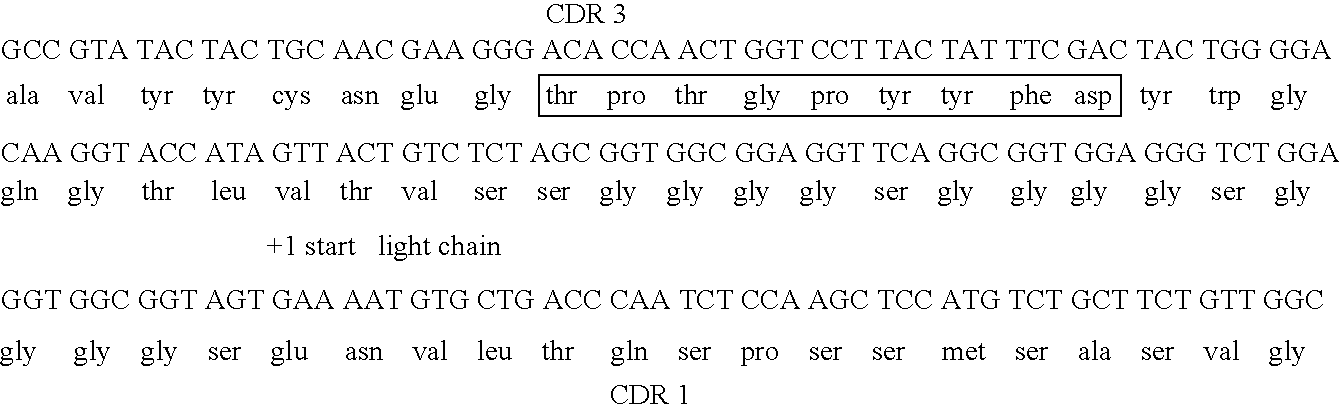
The invention discloses a nanobody resisting CEA (carcinoembryonic antigen). The nanobody has three unique CDRs (complementary determining regions), namely, CDR1, CDR2 and CDR3. The invention further discloses an application of the nanobody in preparation of a cancer therapeutic drug. The nanobody resisting CEA has specific recognition and binding capability for the CEA and a remarkable ADCC effect on cancer cells MKN-45, and can realize accurate imaging of cancer in a mouse body.
Owner:深圳市国创纳米抗体技术有限公司
Preparation and application of photoelectrochemical immunosensor for detection of carcinoembryonic antigen
InactiveCN107064509AImprove conductivityGood biocompatibilityMaterial nanotechnologyMaterial analysis by electric/magnetic meansTin dioxideManganese
The invention discloses a preparation and application study of photoelectrochemical immunosensor for detection of carcinoembryonic antigen. ZnO nanorod-nanosheet with three-dimensional layered structure is grown on transparent conductive tin dioxide glass doped with fluoride, the ZnO nanorod-nanosheet is used to load a large amount of CdS quantum dots doped with manganese, aptamers for identifying carcinoembryonic antigen and DNA probes marked with CdTe / CdSe core shell quantum dots and hybridized with the aptamers to form a multivariate sensitizing structure based on ZnO, so that an initial signal amplification is achieved; in combination with the aptamers for specific recognition of carcinoembryonic antigen, the DNA probes are disintegrated from the hybridization to depart from the weakened sensitizing effect on the electrode surface and the steric hindrance of the conjugate formed by the carcinoembryonic antigen and the aptamers so that a further signal amplification is achieved, and further the sensitive detection of carcinoembryonic antigen is realized.
Owner:UNIV OF JINAN
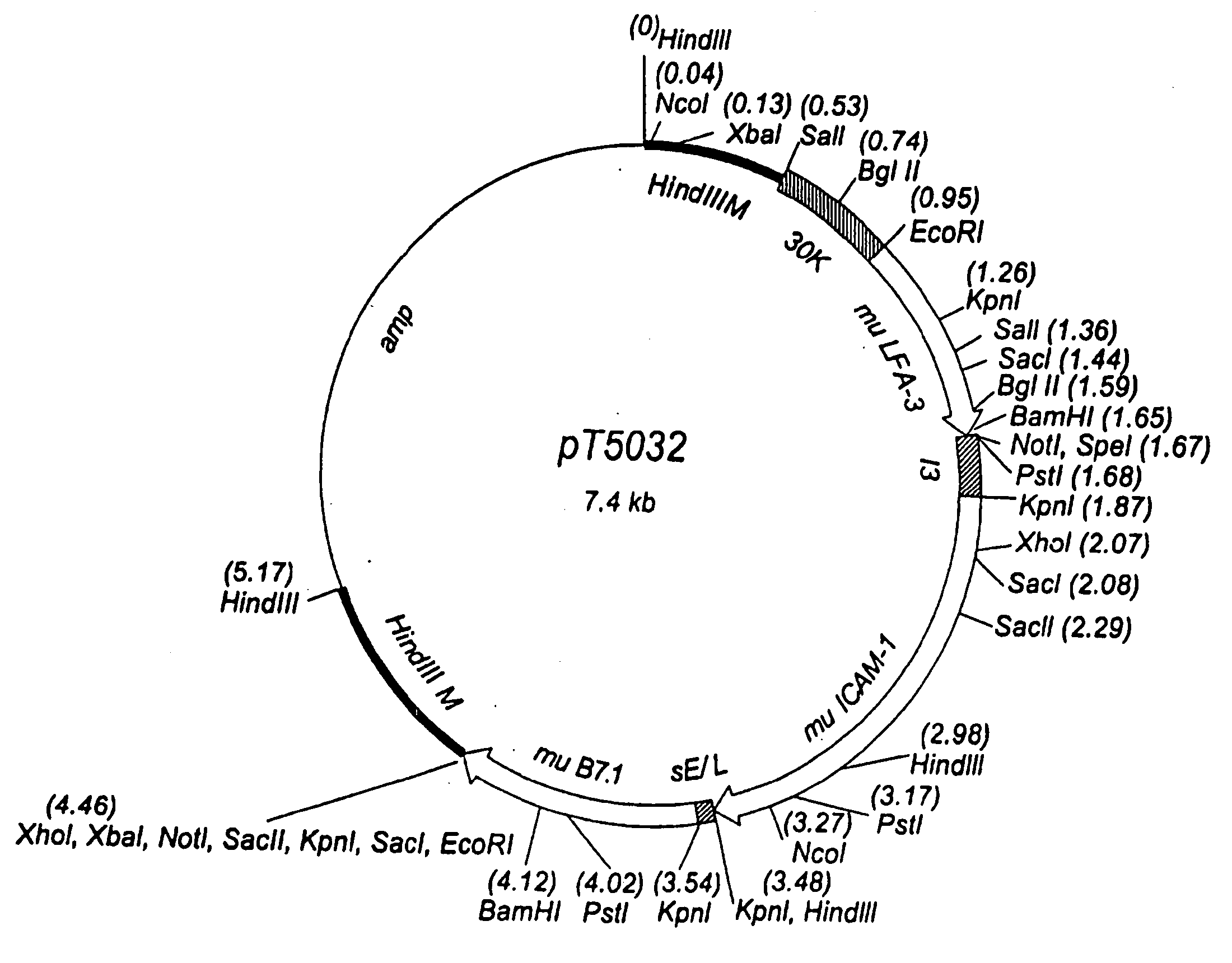
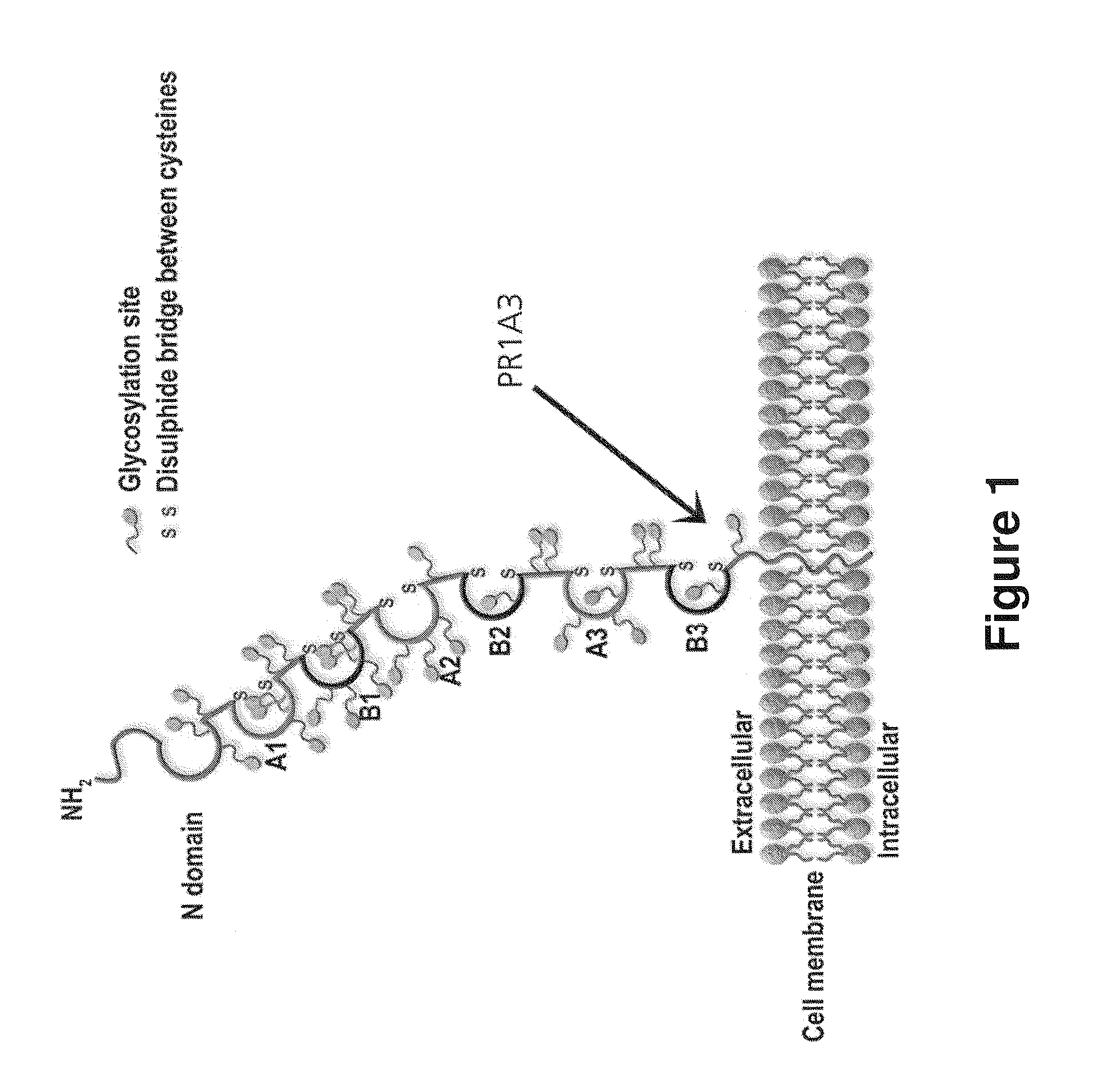
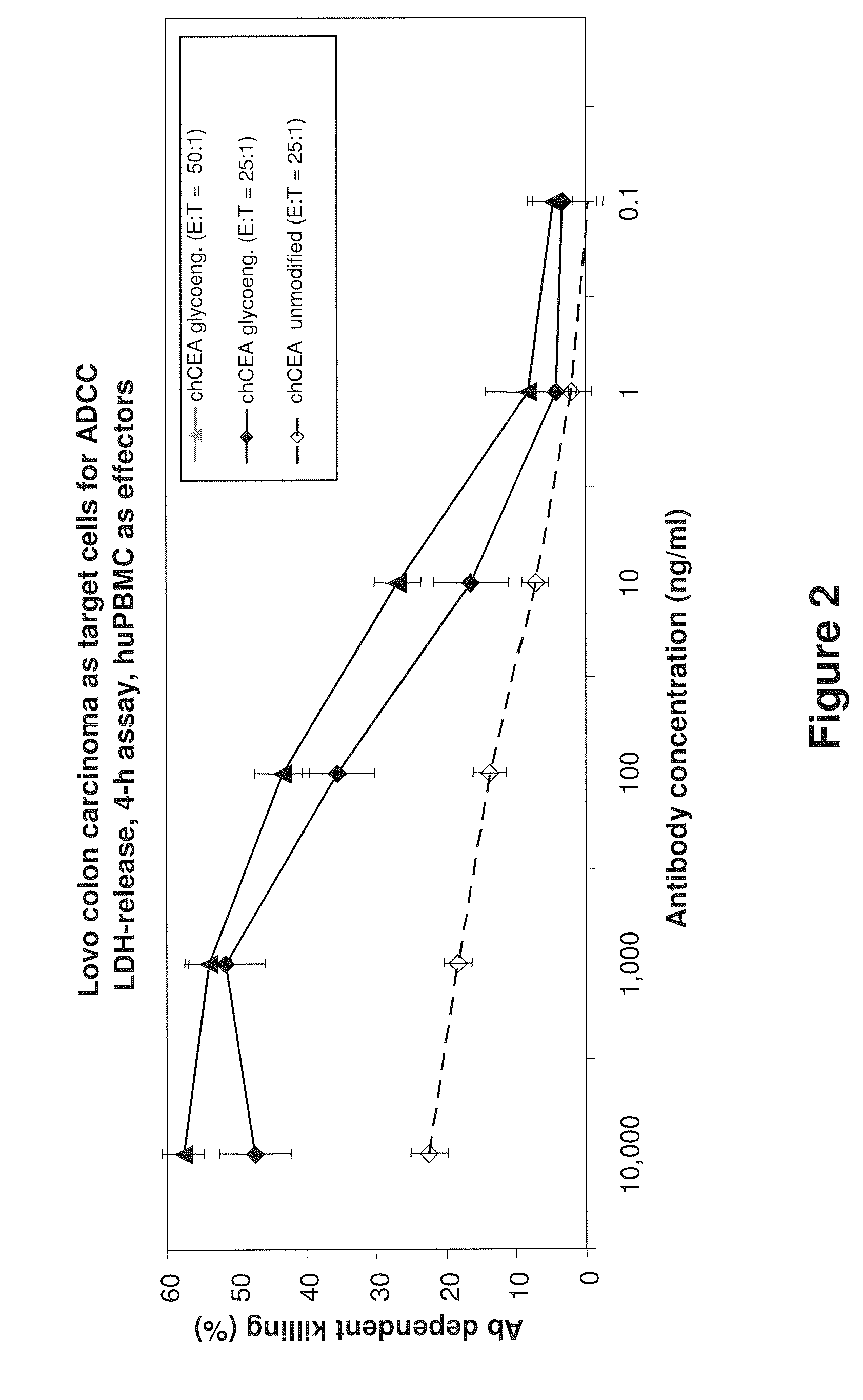
Antibodies to carcinoembryonic antigen (CEA), methods of making same, and uses thereof
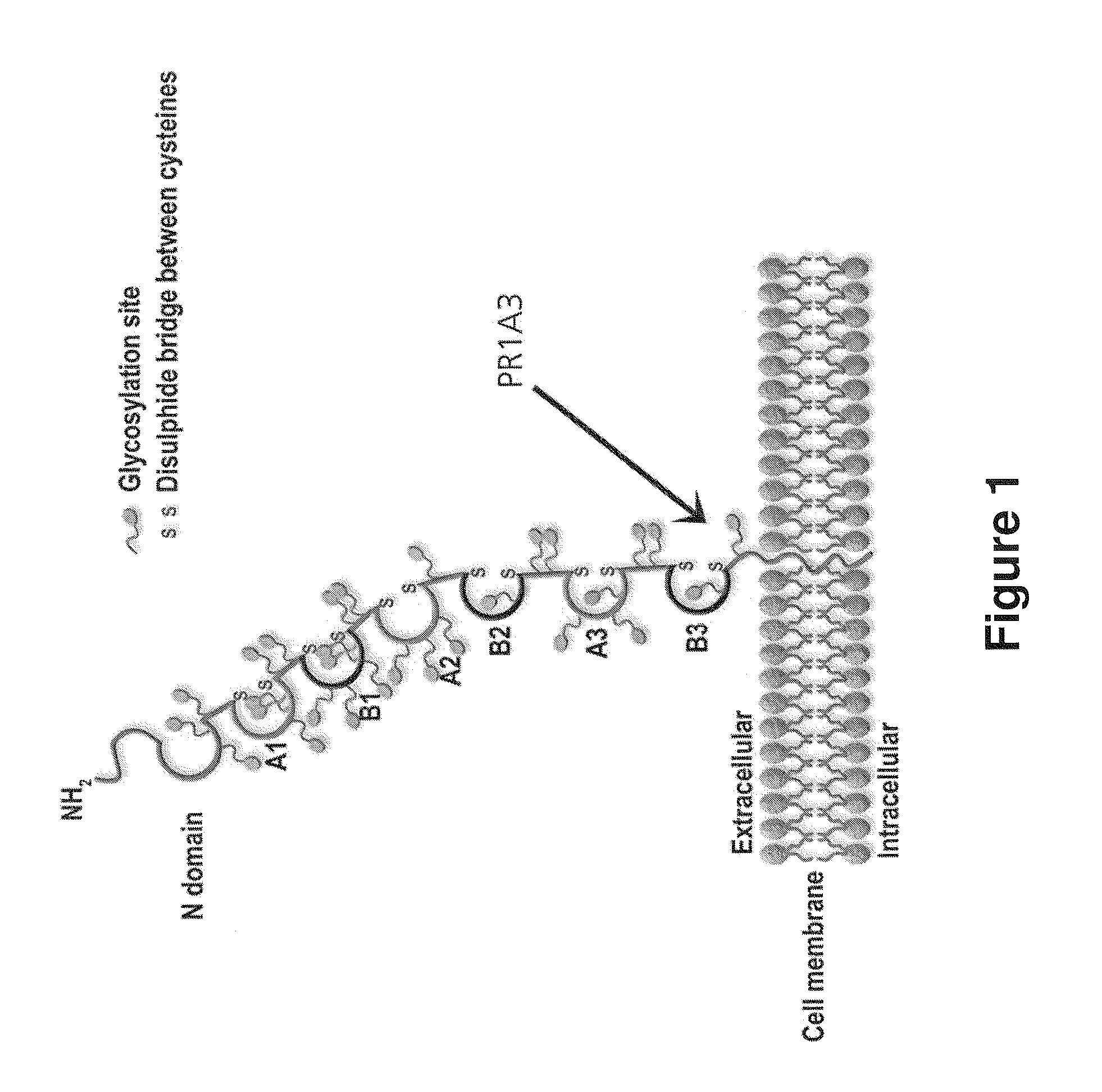
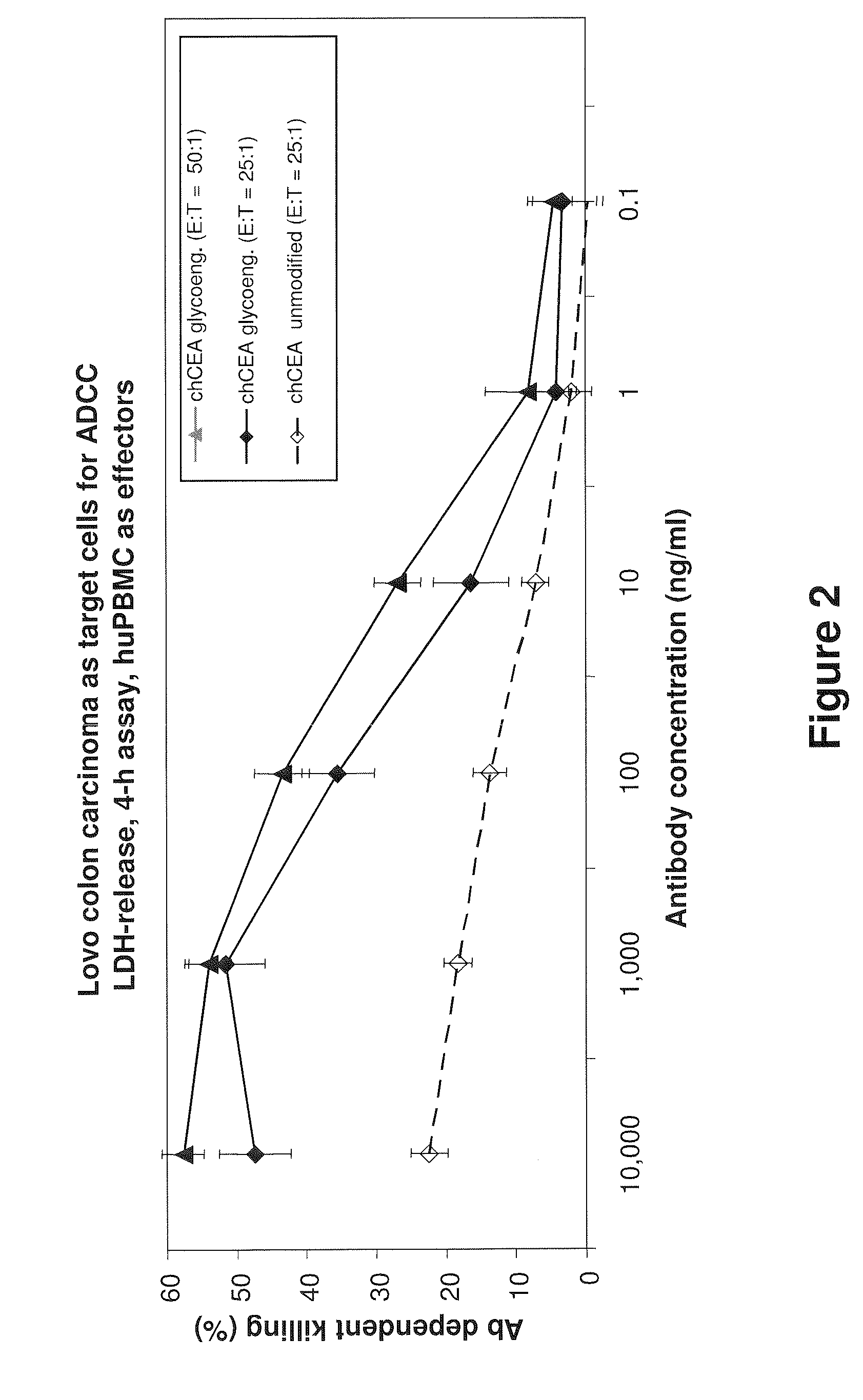
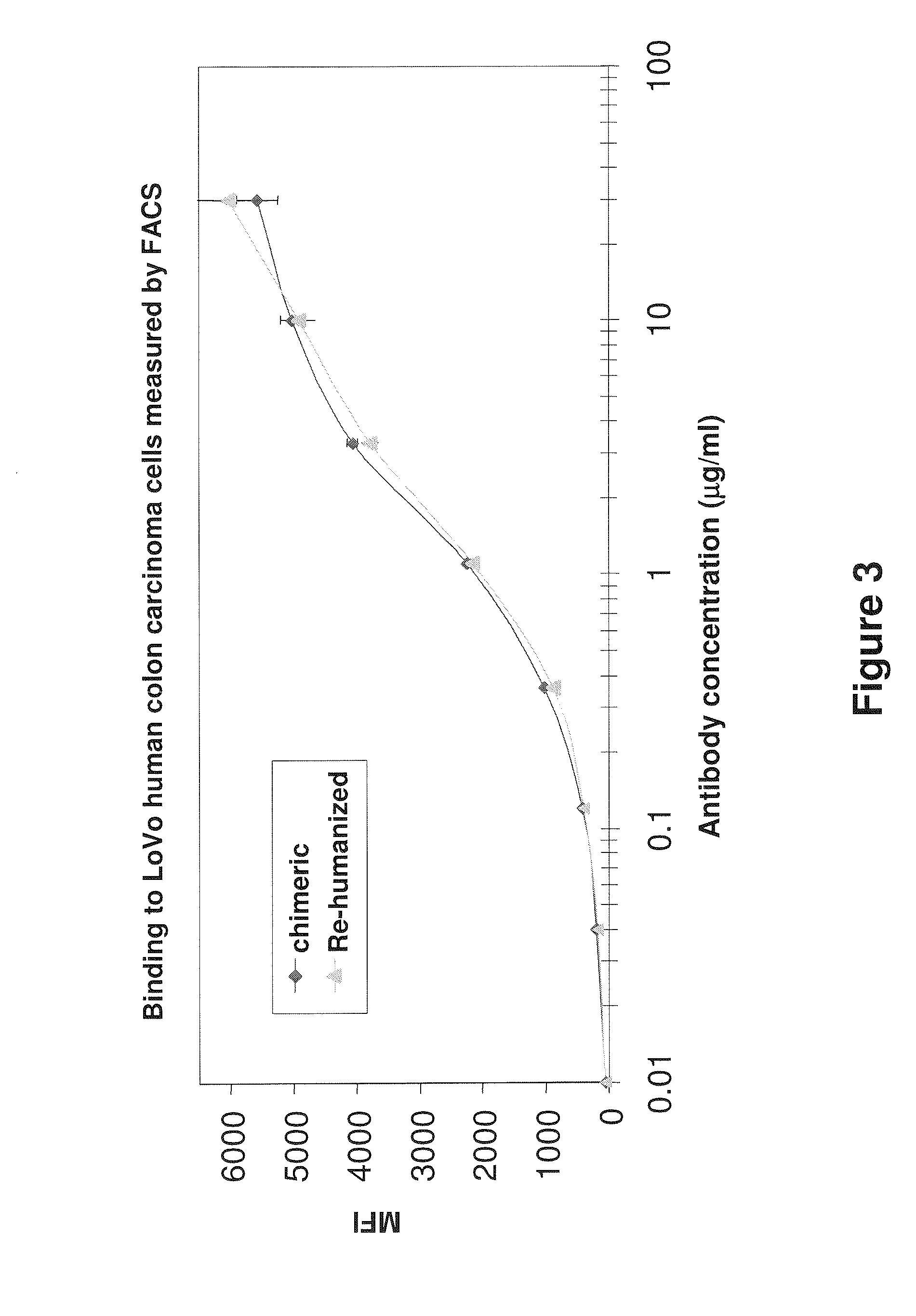
The present invention relates to antigen binding molecules (ABMs). In particular embodiments, the present invention relates to recombinant monoclonal antibodies, including chimeric, primatized or humanized antibodies or variants thereof specific for cell surface or membrane bound human CEA. In addition, the present invention relates to nucleic acid molecules encoding such ABMs, and vectors and host cells comprising such nucleic acid molecules. The invention further relates to methods for producing the ABMs of the invention, and to methods of using these ABMs in treatment of disease. In addition, the present invention relates to ABMs with modified glycosylation having improved therapeutic properties, including antibodies with increased Fc receptor binding and increased effector function.
Owner:ROCHE GLYCART AG
Immunogenic CEA
InactiveUS20050063952A1Effective immune responseBiocideGenetic material ingredientsVaccinationCarcinoembryonic antigen
The present invention provides for methods for immunizing actively against autologous carcinoembryonic antigen (CEA). The method encompasses that the immune system is engaged with variant CEA which is either administered as a protein vaccine, or is effected expressed by nucleic acid vaccination or live-viral vaccination. Preferred embodiments include immunization with variants that include at least one foreign T-helper epitope introduced in the CEA sequence. Also disclosed is variant proteins, DNA, vectors, and host cells useful for practising the method of the invention.
Owner:PHARMEXA
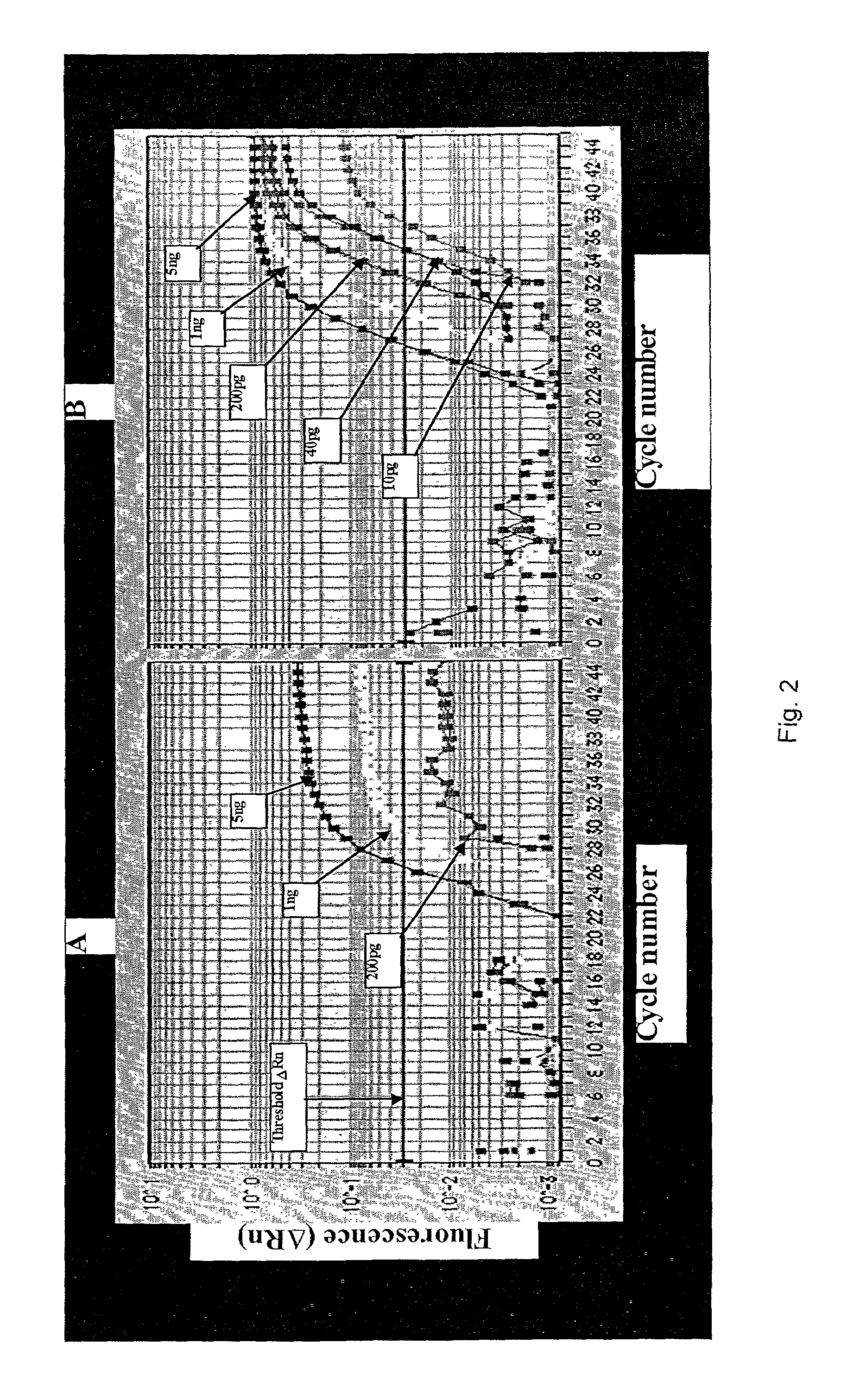
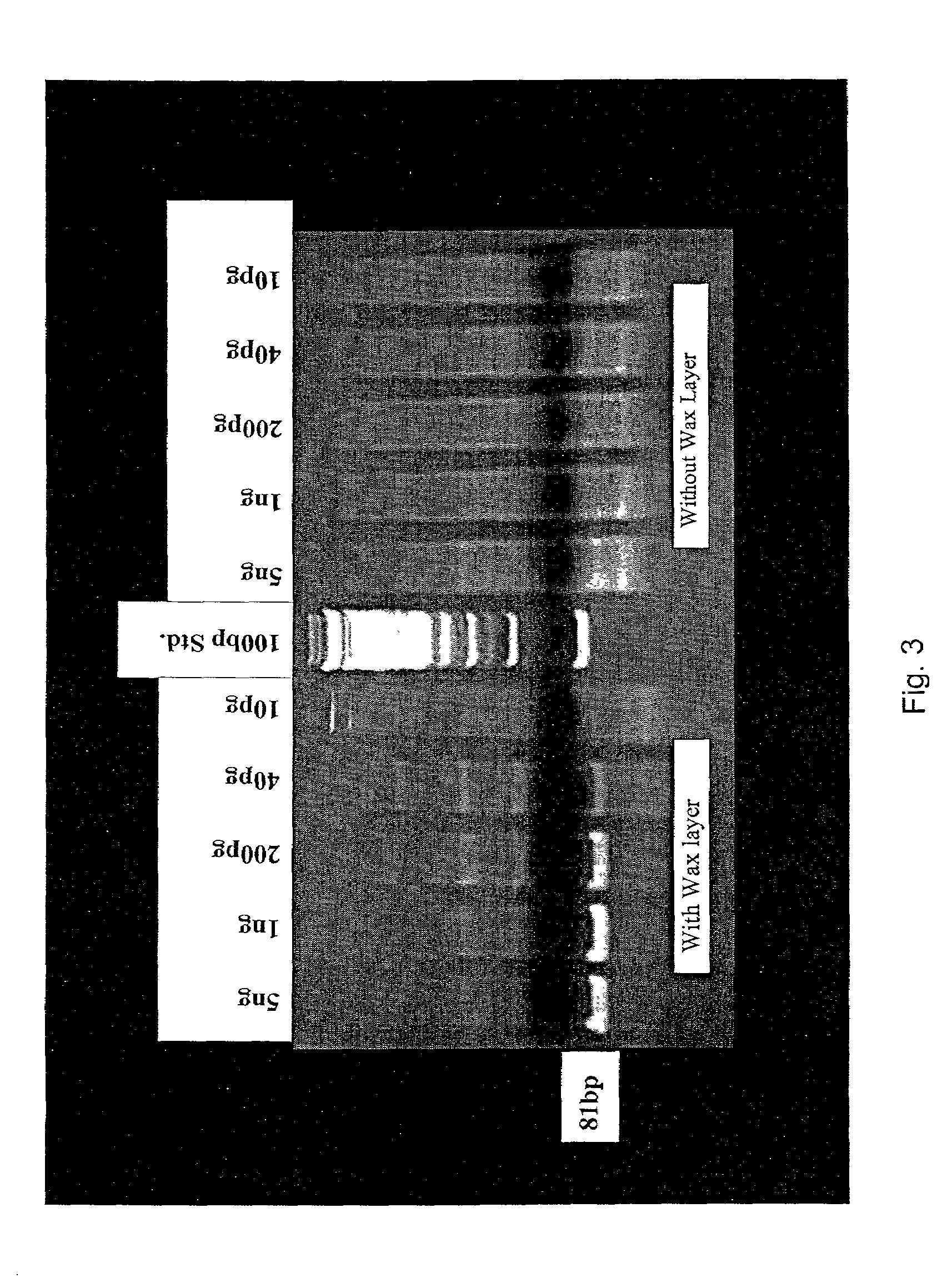
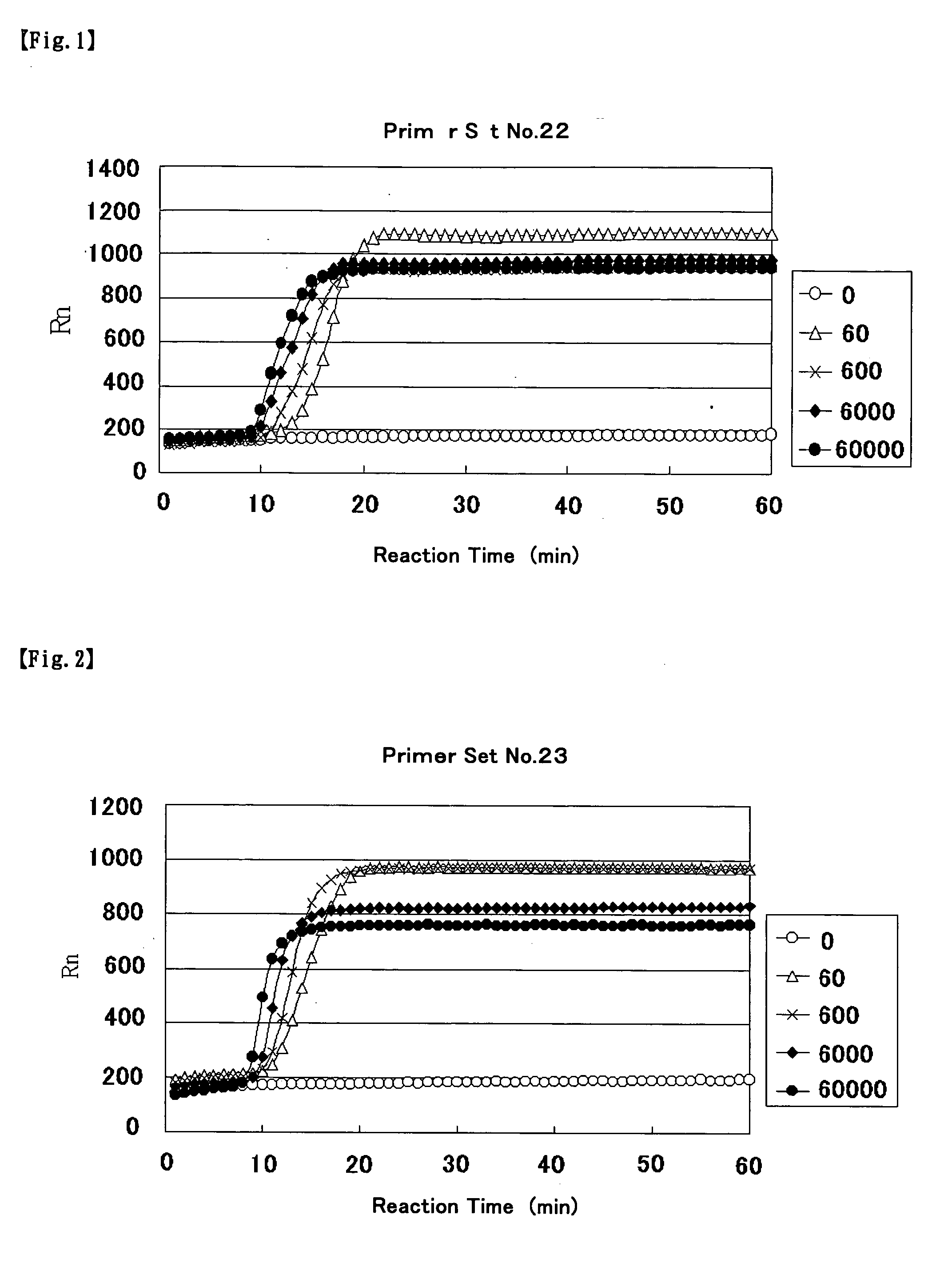
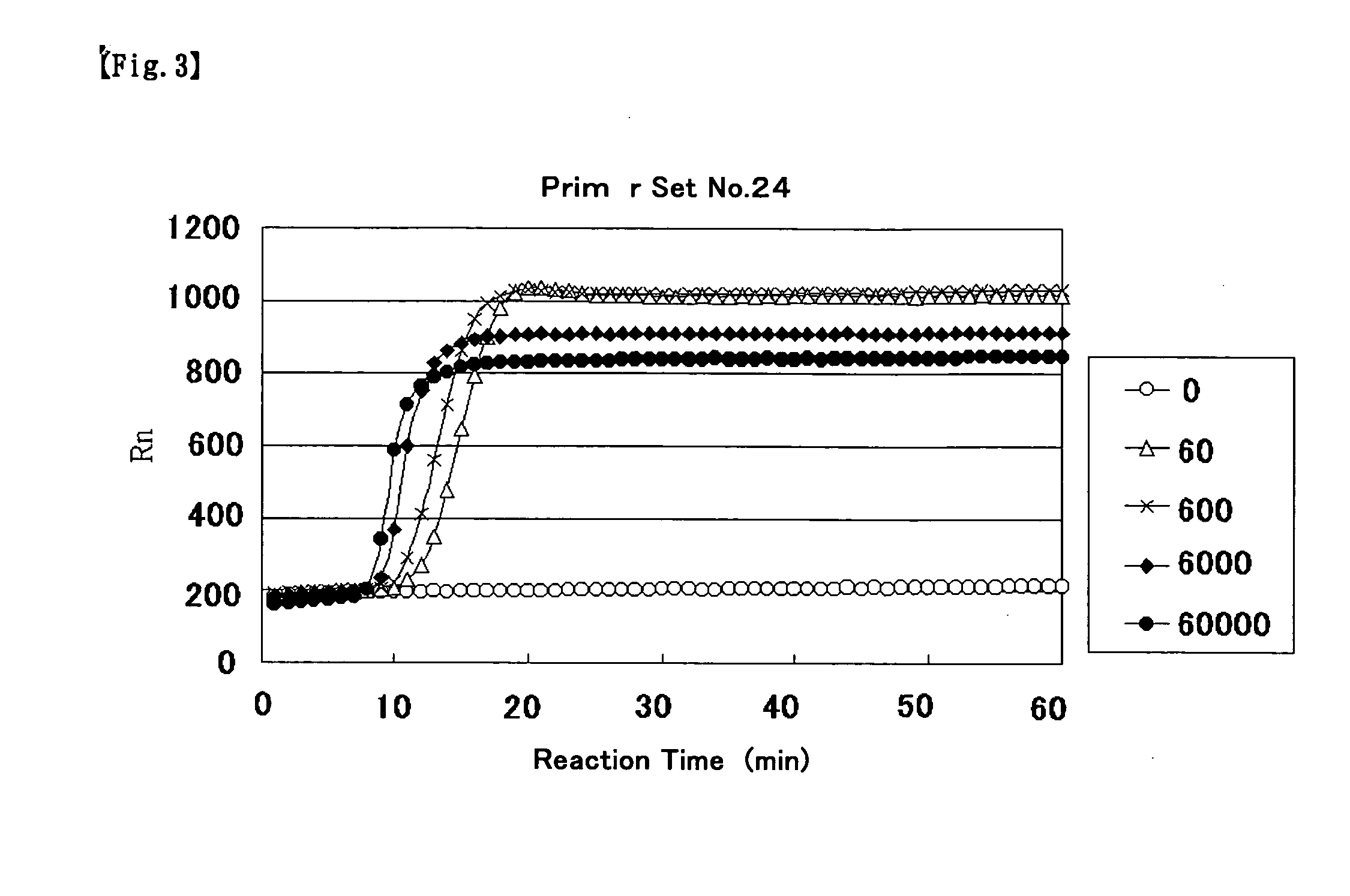
Primer for nucleic acid amplification to detect carcinoembryonic antigen and test method using such primer
InactiveUS20040175729A1Method is applicableSugar derivativesMicrobiological testing/measurementCarcinoembryonic antigenOligonucleotide
An object is to provide a new primer to use in genetic amplification reactions to detect carcinoembryonic antigen (CEA). A primer for nucleic acid amplification is constructed by selecting from the region from No. 1900 to No. 2200 of the base sequence having SEQ ID No. 1 and its complementary strand, and by including oligonucleotides containing at least 5 or more continuous bases of SEQ ID No. 1 and / or its complementary strand, and oligonucleotides comprising a sequence selected from oligonucleotides or the complementary strand thereof comprising a base sequence having SEQ ID Nos. 2 to 24.
Owner:SYSMEX CORP
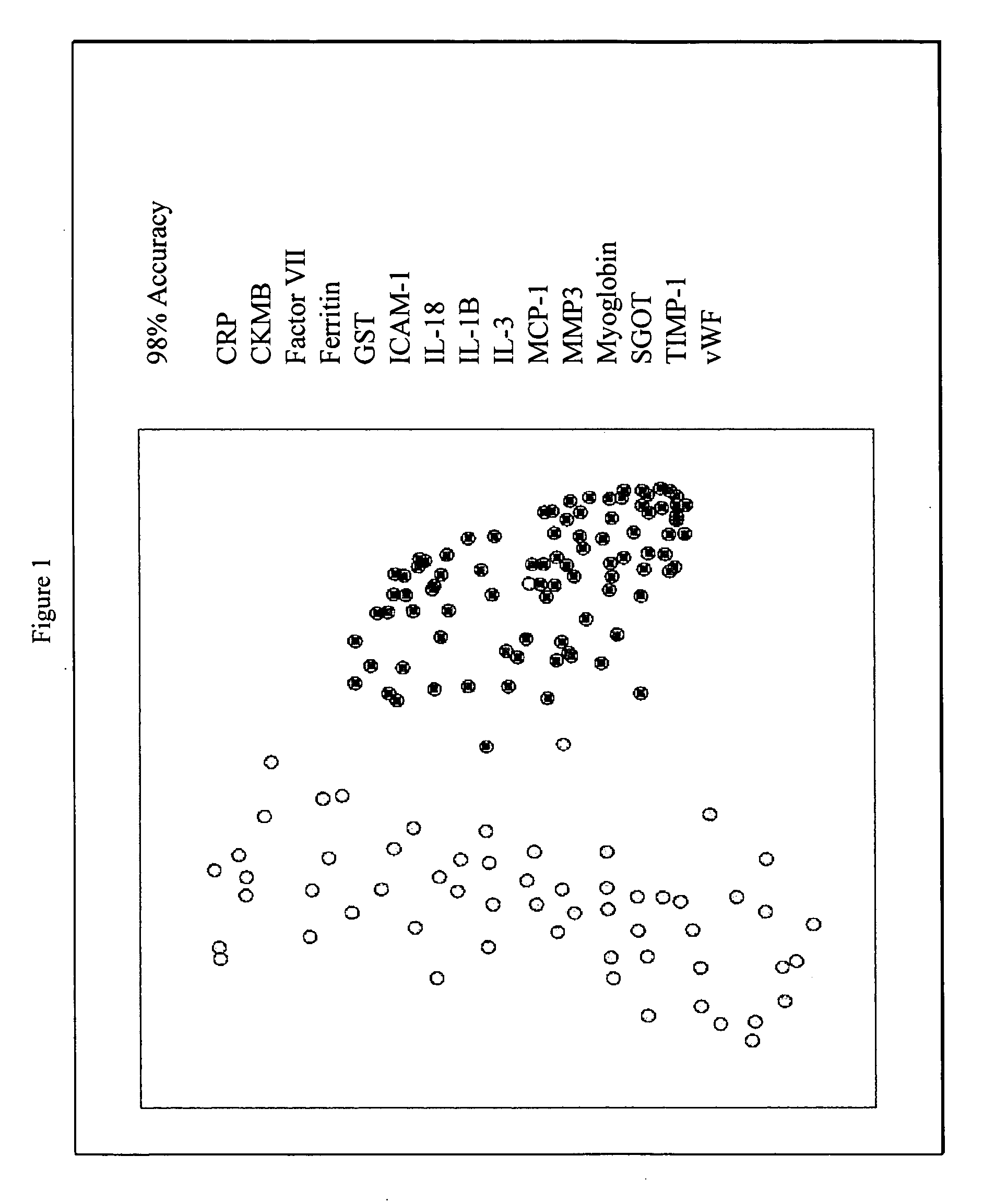
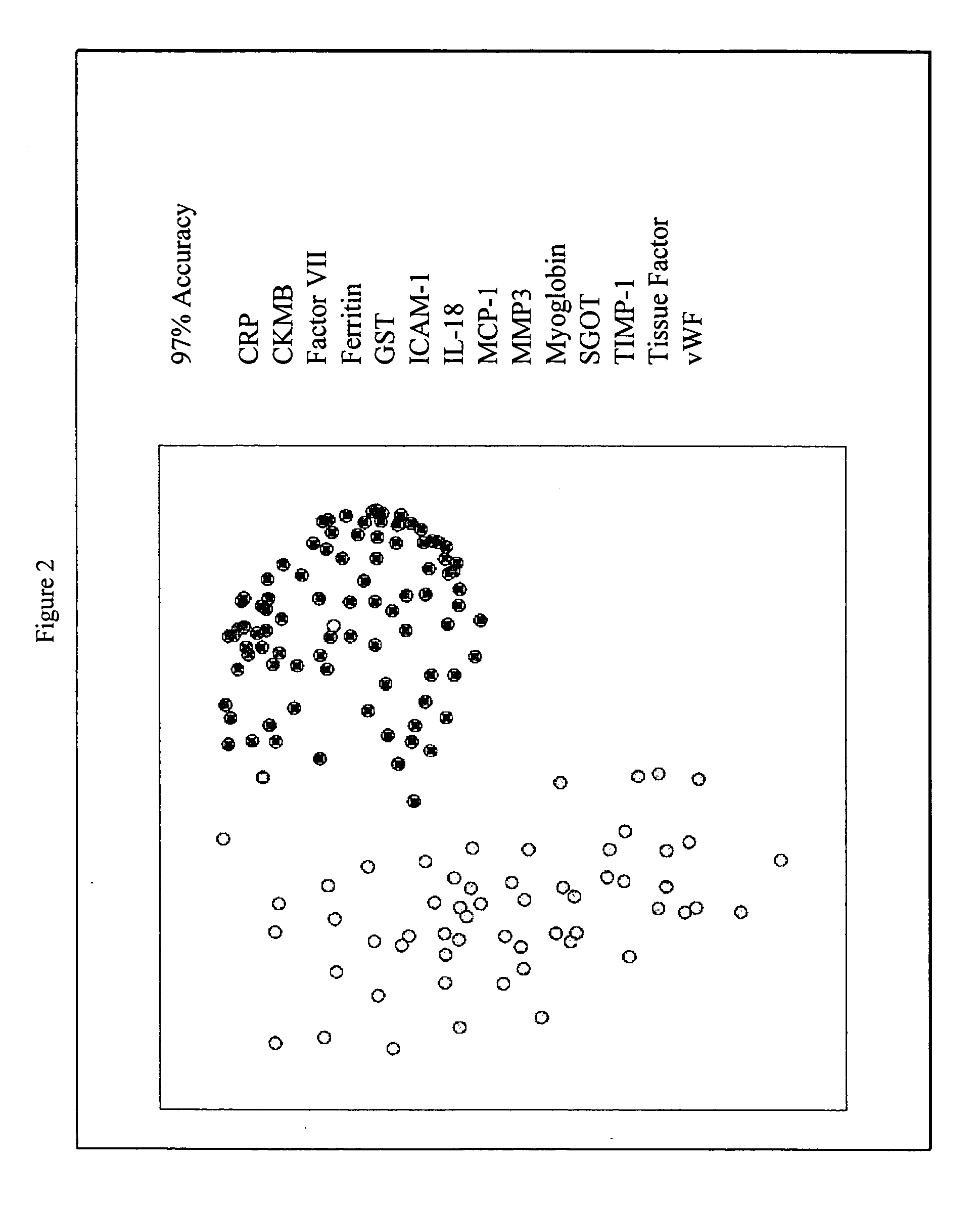
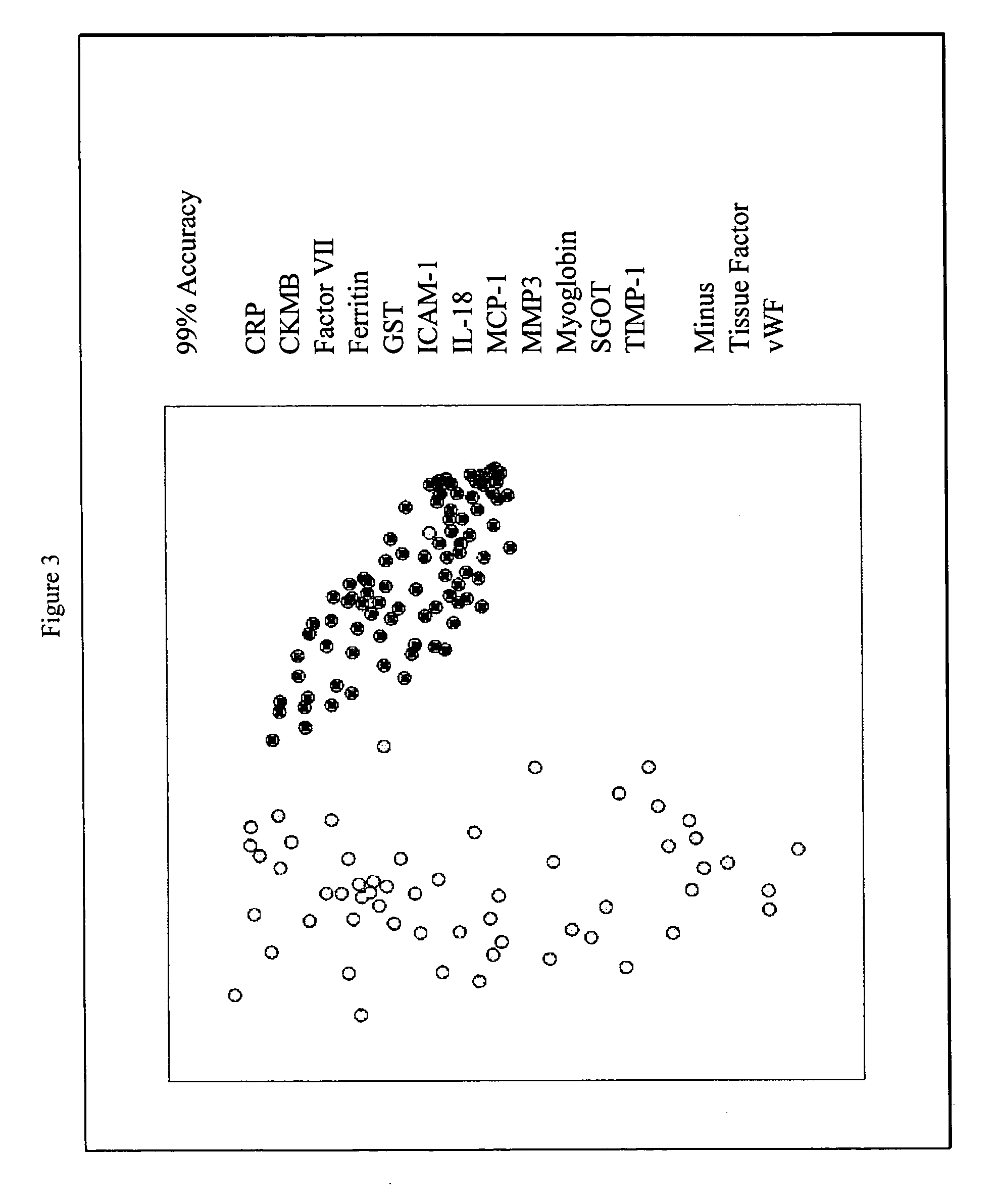
Methods and kits for the diagnosis of acute coronary syndrome
InactiveUS20070003981A1Quick checkAccurate diagnosisMicrobiological testing/measurementDisease diagnosisComplement 3Factor VII
Provided are methods for the detection and diagnosis of acute coronary syndrome or ACS. The methods are based on the discovery that abnormal levels of selected analytes in sample fluid, typically blood samples, of patients who are at risk are supportive of a diagnosis of ACS. At least two new biomarkers for ACS are thus disclosed, MMP-3 and SGOT. Altogether the concentrations of twelve analytes provide a sensitive and selective picture of the patient's condition, namely, whether the patient is suffering a heart attack. Other important biomarkers for ACS are described, including but not limited to IL-18, Factor VII, ICAM-1, Creatine Kinase-MB, MCP-1, Myoglobin, C Reactive Protein, von Willebrand Factor, TIMP-1, Ferritin, Glutathione S-Transferase, Prostate Specific Antigen (free), IL-3, Tissue Factor, alpha-Fetoprotein, Prostatic Acid Phosphatase, Stem Cell Factor, MIP-1-beta, Carcinoembryonic Antigen, IL-13, TNF-alpha, IgE, Fatty Acid Binding Protein, ENA-78, IL-1-beta, Brain-Derived Nerotrophic Factor, Apolipoprotein A1, Serum Amyloid P, Growth Hormone, Beta-2 microglobulin, Lipoprotein (a), MMP-9, Thyroid Stimulating hormone, alpha-2 Macroglobulin, Complement 3, IL-7, Leptin, and IL-6. Kits containing reagents to assist in the analysis of fluid samples are also described.
Owner:RULES BASED MEDICINE
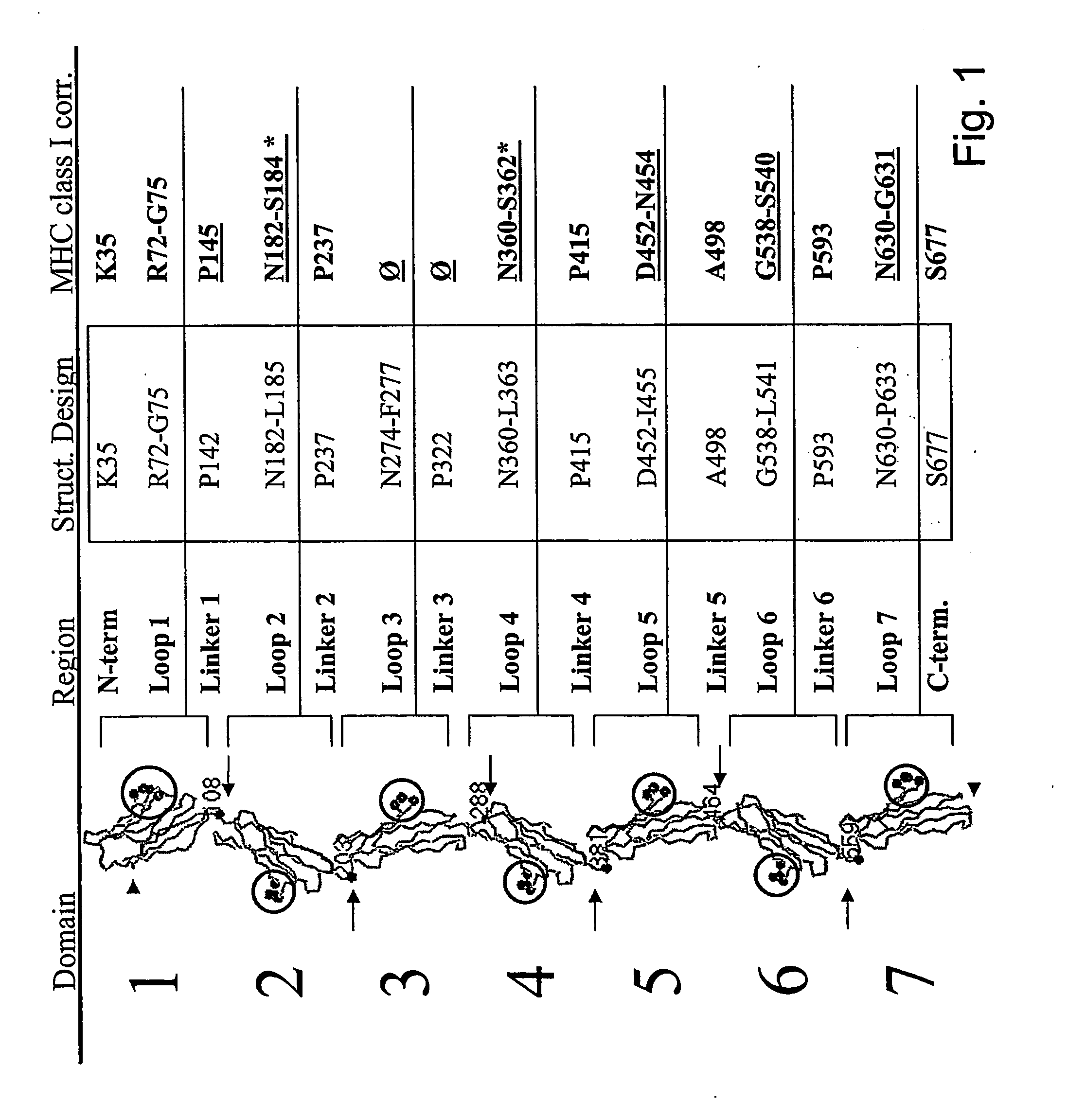
Agonist peptides of carcinoembryonic antigen (CEA) and nucleic acid sequences encoding the agonist peptides
InactiveUS7723096B2Reduce eliminate CEA-specific T-cellReduce or eliminate CEA-specific T-cell activation and killingBacteriaPeptide/protein ingredientsMHC class ICarcinoembryonic antigen
A nucleic acid molecule encoding an amino acid sequence consisting of an agonist of a MHC class I binding native sequence of CEA having an amino acid substitution and enhanced immunogenicity compared to the native sequence is described. A vector comprising the nucleic acid molecule, host cell comprising the vector and a kit comprising the encoded agonist peptide are also described.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
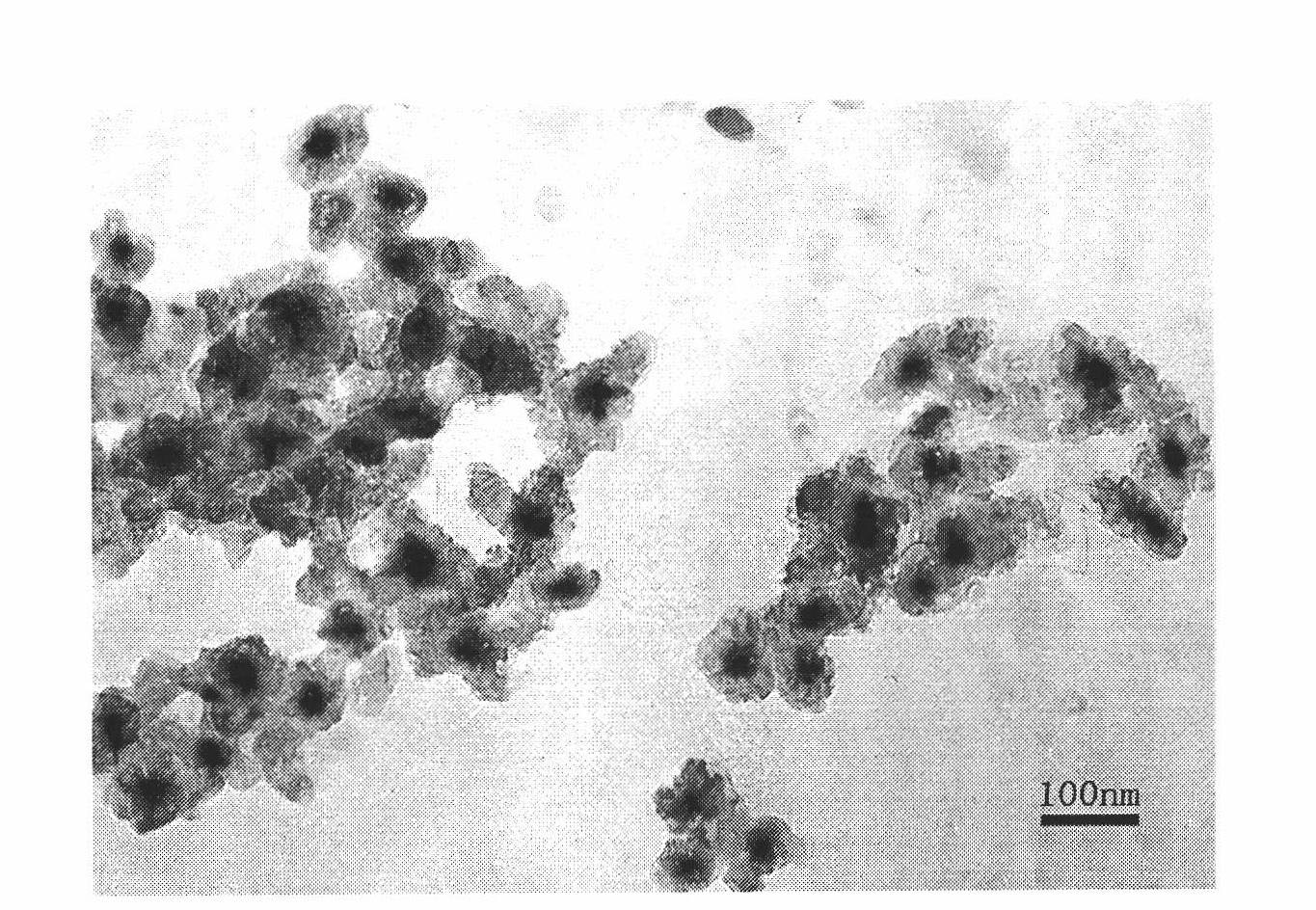
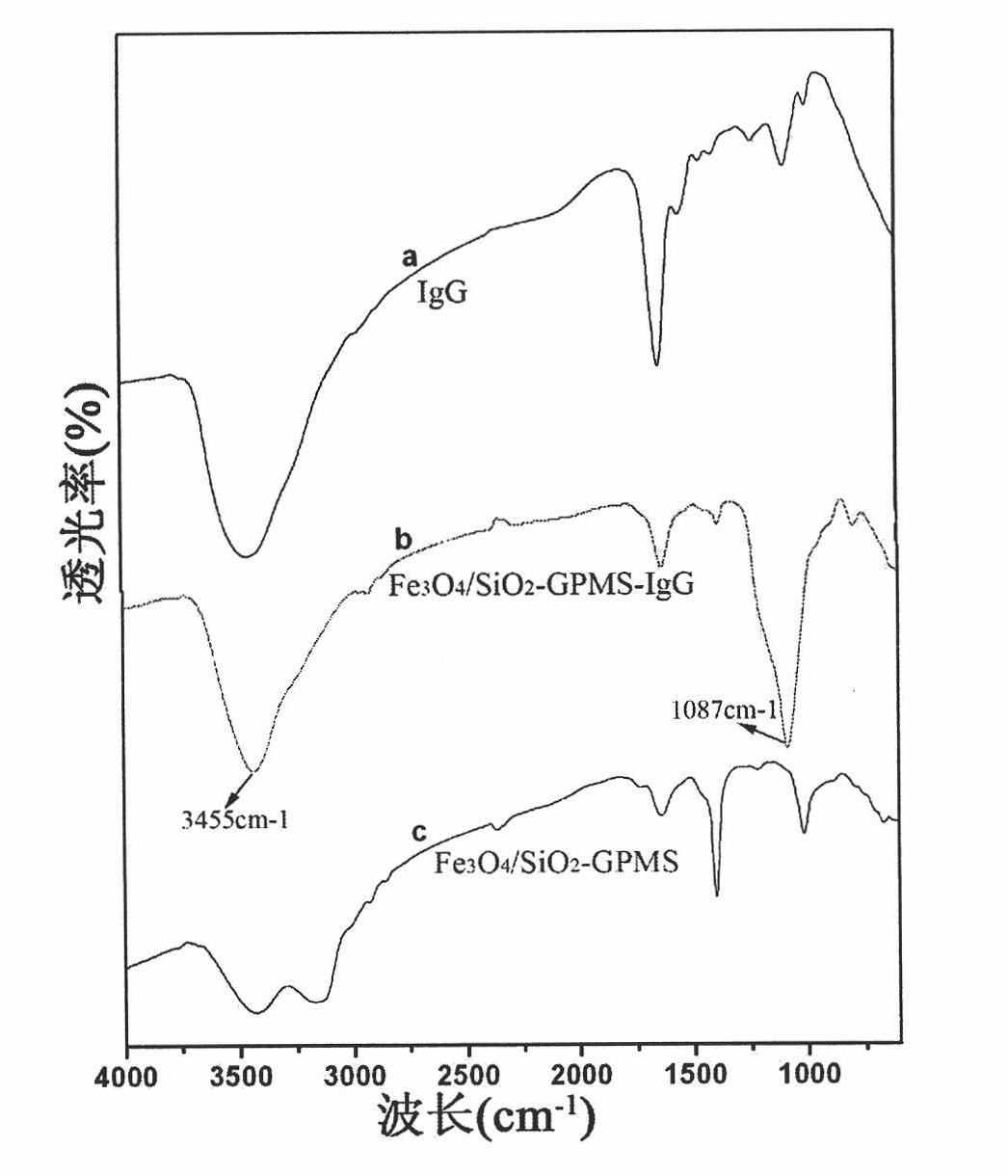
Luminescent and magnetic nano material-based method for detecting carcinoembryonic antigen
InactiveCN101776683APromote enrichmentEasy to detectFluorescence/phosphorescenceCarcinoembryonic antigenMedicine
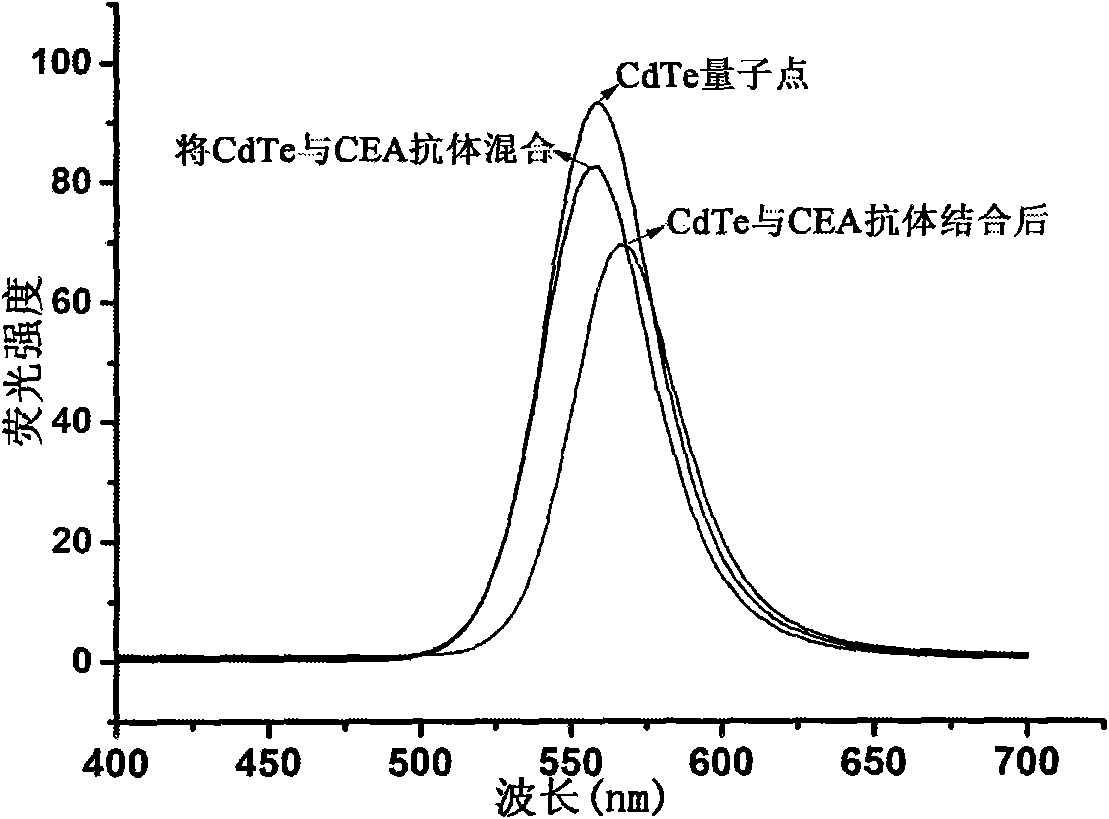
The invention relates to a luminescent and magnetic nano material-based method for detecting carcinoembryonic antigen (CEA), which comprises the following steps: (1) preparing a FE3O4 / SiO2 nuclear shell-first antibody compound; (2) preparing a CdTe-second antibody compound; (3) drawing a 'fluorescence intensity-CEA concentration' standard working curve and calculating a working equation; and (4) detecting a fluorescence intensity value, and obtaining the content of the CEA of the patient through the working equation. The detection method has the advantages of simpleness and high sensitivity, and can easily detect the illness state of patients in the early stage of cancer.
Owner:DONGHUA UNIV
Preparation method of liquid phase protein chip
InactiveCN101144815AEarly detectionEarly treatmentMaterial analysisHigh risk populationsFluorescence
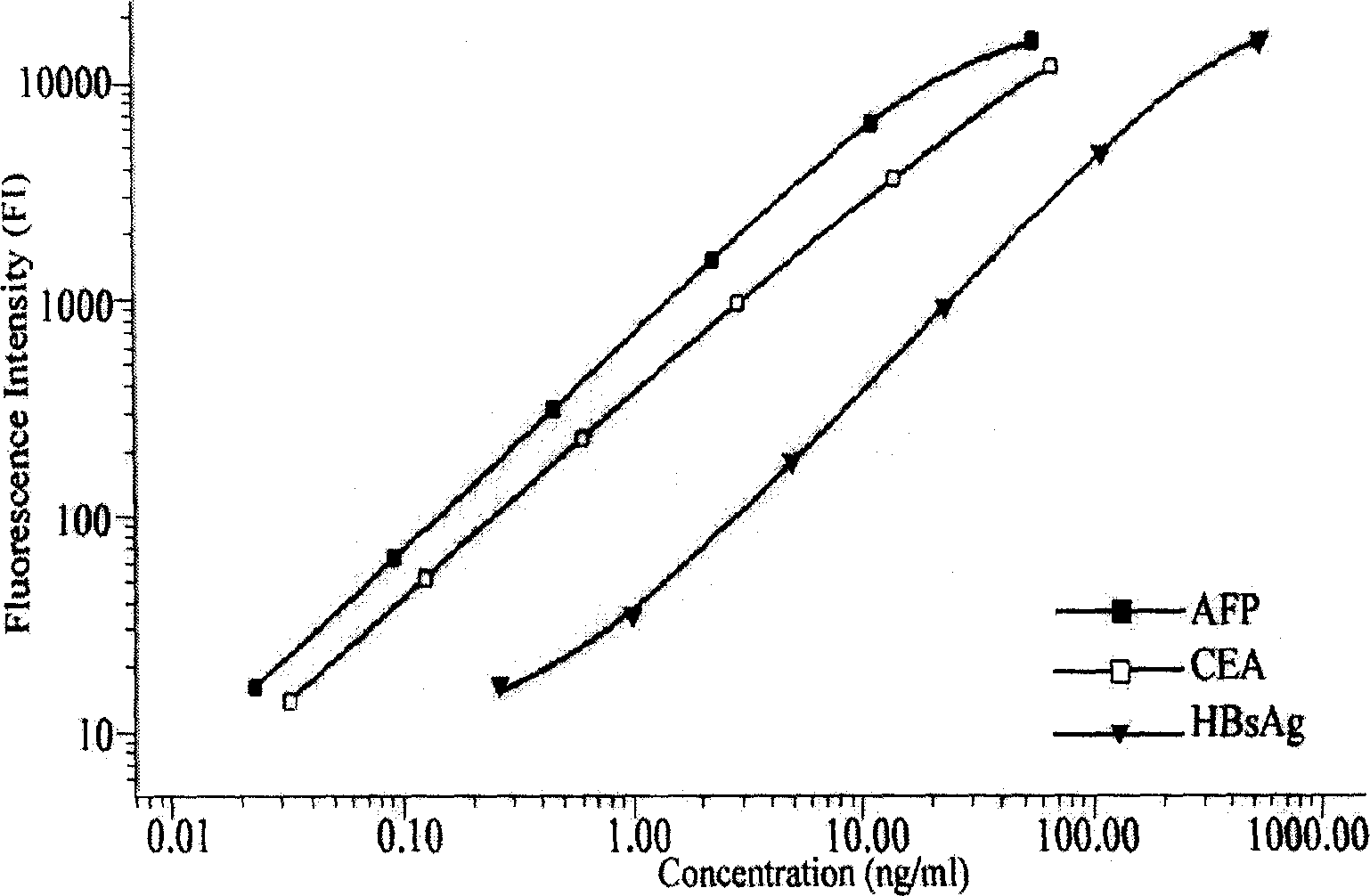
The present invention relates to the biologic technology field, and discloses a liquid phase albumen chip and the preparation and the usage method thereof which can simultaneously test human serum carcinoma embryonic antigen (CEA), Alpha fetoprotein (AFP), and hepatitis B surface antigen (HBsAg). The present invention couples the specificity antibody of CEA, AFP, and HBsAg on different fluorescence micro-spheres, and uses the test antibody marked by biotin or phycoerythrin to determine the nature quickly and quantitatively analyze the above three indexes with the double antibody sandwich method. The present invention uses the filtering membrane board when testing, and washes the board 3 times after each reaction is finished to increase the signal and improve the sensitivity. The present invention has high sensitivity, strong specificity, stable result, excellent repeatability, and simple operation; 1 micro liter serum sample can test three indexed simultaneously. The present invention is applicable to the health test and the general examination as well as the clinic test of the high risk population, and can facilitate the early diagnosis and the early treatment of the knub.
Owner:GUANGZHOU DARUI BIOTECH
Humanized anti-CEA T84.66 antibody and uses thereof
ActiveUS20050244333A1In-vivo radioactive preparationsImmunoglobulins against cell receptors/antigens/surface-determinantsAbnormal tissue growthCarcinoembryonic antigen
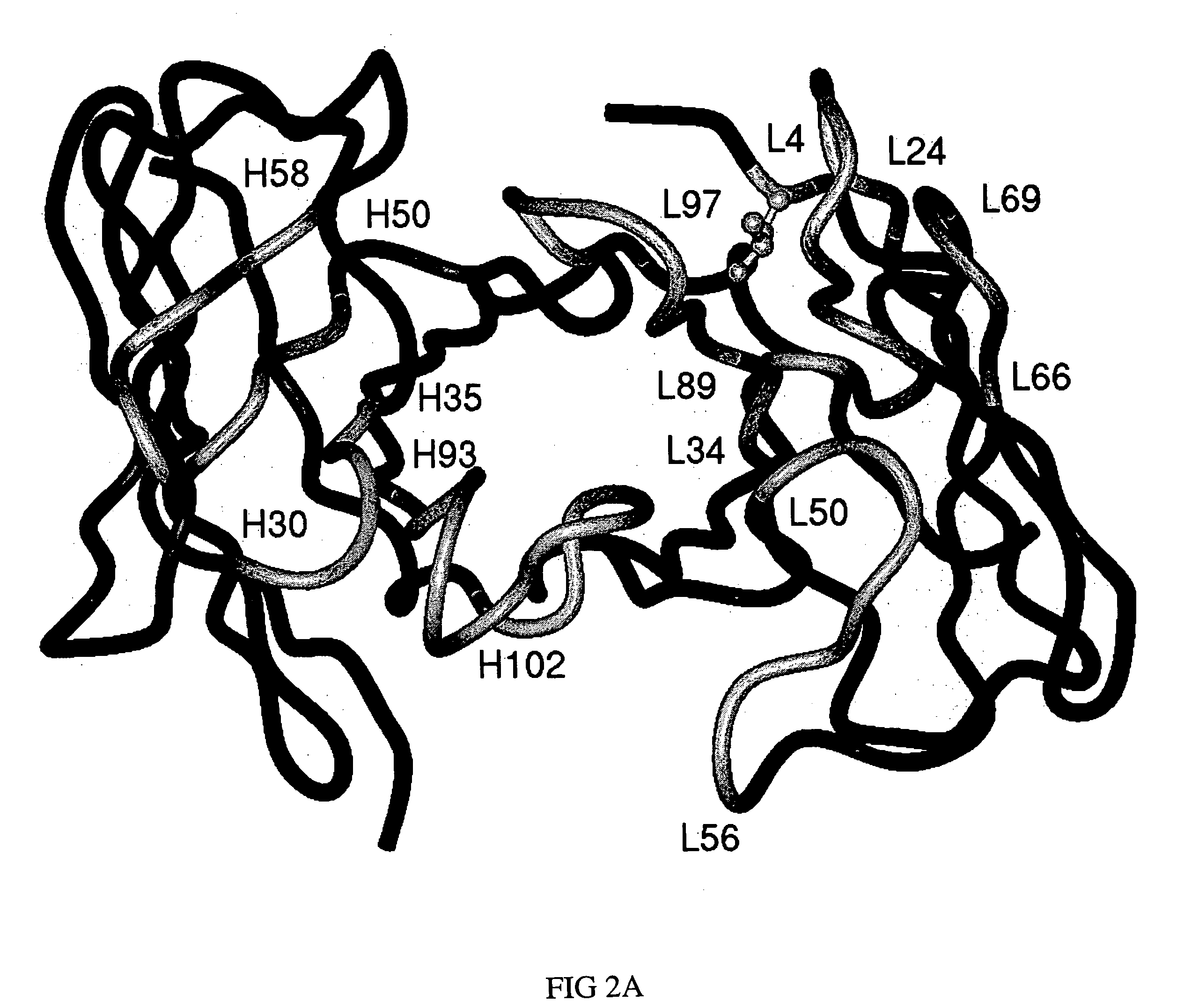
Embodiments of the present invention utilize a more efficient CDR grafting technique to generate humanized versions of the T84.66 antibody. The technique used to generate these antibodies utilizes crystallographic structural data to select an immunoglobulin framework having maximum structural overlap with a non-human donor molecule. This technique was used to develop humanized T84.66 antibodies exhibiting in vitro binding affinity and specificity for carcinoembryonic antigen (CEA) nearly identical to that of T84.66 and the ability to specifically target tumors expressing CEA in vivo.
Owner:CITY OF HOPE
Method for establishing electrochemical immunosensor for detecting carcino-embryonic antigen
InactiveCN104614527AMaterial electrochemical variablesCarcinoembryonic antigenBiocompatibility Testing
The invention relates to a method for establishing an electrochemical immunosensor for detecting carcino-embryonic antigen, in particular to a method for establishing an electrochemical immunosensor for detecting carcino-embryonic antigen based on a graphite / bovine serum albumin stabilized silver nanoparticle (GR / Ag@BSA) composite material. The method is characterized by comprising the following steps: firstly, depositing a layer of gold nanoparticles on the surface of a glassy carbon electrode by using a potential deposition method, sequentially immobilizing primary antibodies and antigen on the surface of the electrode, and finally immobilizing a second antibody solution marked by GR / Ag@BSA through specific reaction of antigen antibodies. The gold nanoparticles are excellent in conductivity, the used GR / Ag@BSA composite material not only integrates excellent conductivity of GR and good biocompatibility of BSA, but also is high in catalytic activity to hydrogen peroxide reduction, and the electrochemical immunosensor which is established based on the material is relatively high in detection sensitivity on carcino-embryonic antigen and relatively in detection limit.
Owner:UNIV OF JINAN
Method and device for detecting carcinoembryonic antigen content
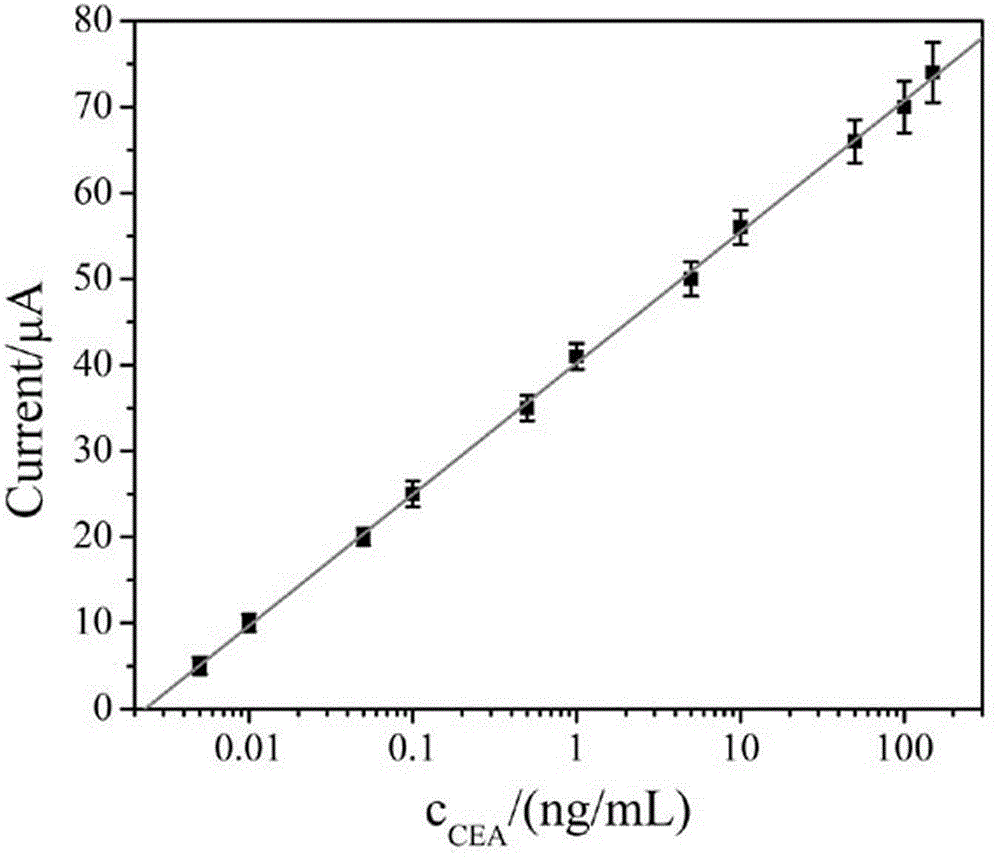
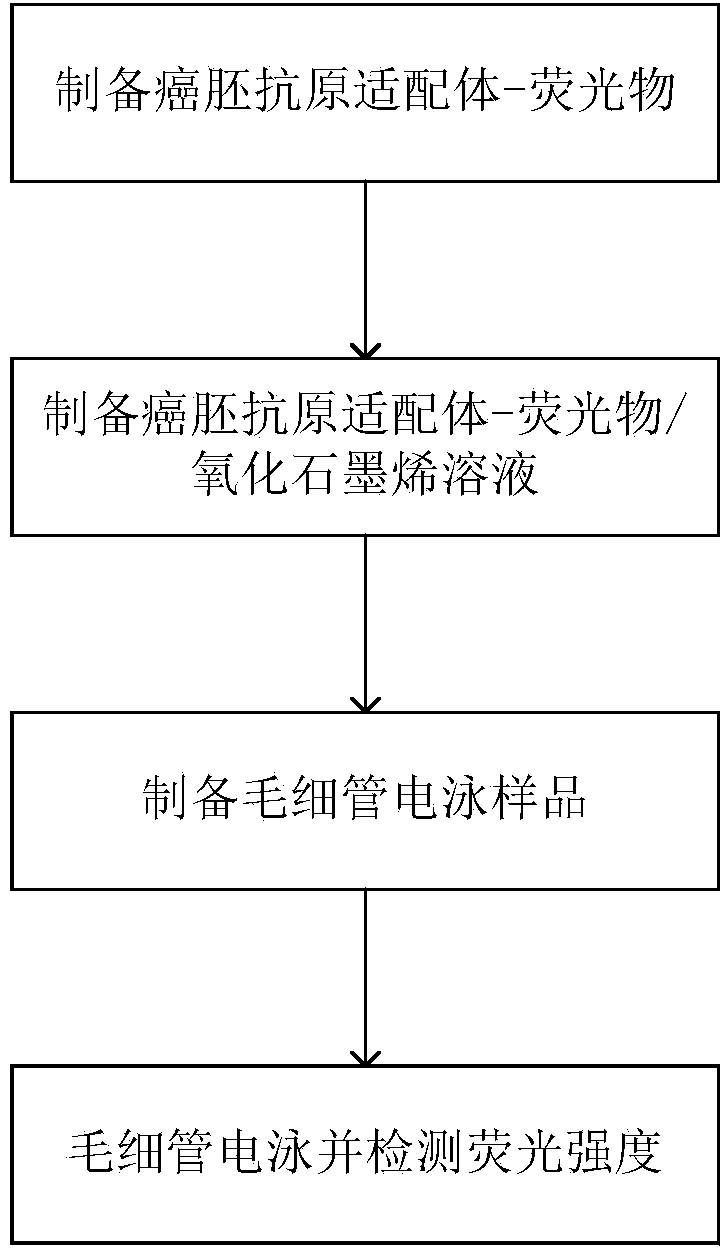
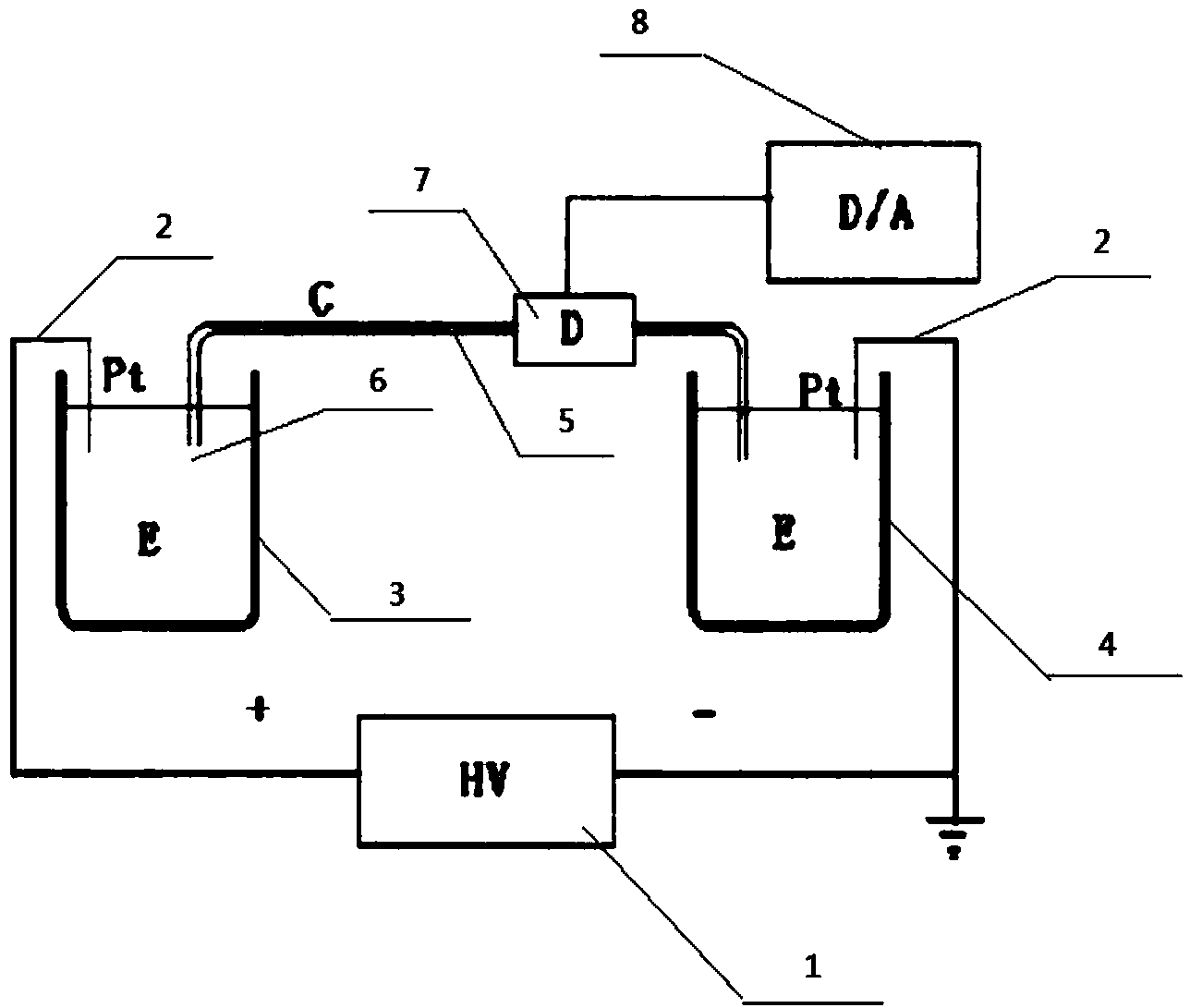
InactiveCN103901197AThe test result is accurateMinimizes the effect of restoring fluorescenceElectrophoretic profilingAptamerCapillary electrophoresis
The invention discloses a method and a device for detecting the carcinoembryonic antigen content. The method comprises the following steps of (1) uniformly mixing a fluorescent substance with a connection agent to obtain a first mixture, and then mixing the first mixture with a carcinoembryonic antigen aptamer solution; (2) uniformly mixing a mixed solution with a graphene oxide solution to cause fluorescence quenching so as to obtain a carcinoembryonic antigen aptamer-phosphor / graphene oxide solution; (3) uniformly mixing a sample to be detected and the carcinoembryonic antigen aptamer-phosphor / graphene oxide solution to cause fluorescence recovery; and (4) performing capillary electrophoresis, performing fluorescent detection at a position meeting the requirement that the distance from the position to a positive electrode is 1 / 2-2 / 3 of the total length of a capillary, and measuring the concentration of a carcinoembryonic antigen. The device comprises a capillary electrophoresis device and a fluorescence inspection device, wherein the total length of the capillary of the capillary electrophoresis device is 50-100cm, and the inner diameter is 50-100 microns; a detection window is arranged in a position meeting the requirement that the distance from the position to a positive electrode buffering solution tank is 1 / 2-2 / 3 of the total length of the capillary; the fluorescence inspection device is arranged at the capillary detection window. The method and the device are high in sensitivity, low in detection limit and high in detection efficiency and can be applied to early screening of cancers.
Owner:HUAZHONG UNIV OF SCI & TECH
Synthetic gene encoding human carcinoembryonic antigen and uses thereof
InactiveUS20070104685A1Easy to insertEasy to removeVirusesGenetic material ingredientsNucleotideCarcinoembryonic antigen
Synthetic polynucleotides encoding human carcinoembryonic antigen (CEA) are provided, the synthetic polynucleotides being codon-optimized for expression in a human cellular environment. The gene encoding CEA is commonly associated with the development of human carcinomas. The present invention provides compositions and methods to elicit or enhance immunity to the protein product expressed by the CEA tumor-associated antigen, wherein aberrant CEA expression is associated with a carcinoma or its development. This invention specifically provides adenoviral vector and plasmid constructs carrying codon-optimed human CEA and discloses their use in vaccines and pharmaceutical compositions for preventing and treating cancer.
Owner:LA MONICA NICOLA +3
Agonist and antagonist peptides of carcinoembryonic antigen (CEA)
InactiveUS8609395B2High activityBacteriaPeptide/protein ingredientsMHC class ICarcinoembryonic antigen
The invention provides a polypeptide comprising an agonist of a MHC Class I binding native sequence having amino acid substitution(s) and enhanced immunogenicity compared to the native sequence. The invention provides DNA encoding the polypeptide, as well as vectors and cells comprising the DNA and methods comprising the administration of the polypeptide.
Owner:UNITED STATES OF AMERICA
Antibodies to Carcinoembryonic Antigen (CEA), Methods of Making Same, and Uses Thereof
ActiveUS20110104148A1Increase heightGood curative effectBacteriaPeptide/protein ingredientsDiseaseCarcinoembryonic antigen
The present invention relates to antigen binding molecules (ABMs). In particular embodiments, the present invention relates to recombinant monoclonal antibodies, including chimeric, primatized or humanized antibodies or variants thereof specific for cell surface or membrane bound human CEA. In addition, the present invention relates to nucleic acid molecules encoding such ABMs, and vectors and host cells comprising such nucleic acid molecules. The invention further relates to methods for producing the ABMs of the invention, and to methods of using these ABMs in treatment of disease. In addition, the present invention relates to ABMs with modified glycosylation having improved therapeutic properties, including antibodies with increased Fc receptor binding and increased effector function.
Owner:ROCHE GLYCART AG
Method for employing double indicatrix immunochromatography by semi-quantity to diagnose carcinoembryonic antigen
InactiveCN102221612ADetection object is singleStrong targetingMaterial analysis by observing effect on chemical indicatorBiological testingPregnancySorbent
The invention discloses a detection method in the technical field of biological engineering and relates to a method for employing double indicatrix immunochromatography by semi-quantity to diagnose carcinoembryonic antigen in particular. The carcinoembryonic antigen is a broad-spectrum tumor marker, can reflect the existences of multi-tumors to people and is a better tumor marker for curative effect judgment, disease development, monitoring and preoperative prognosis on colorectal cancer, breast cancer and lung cancer. Clinic detection on CEA (carcinoembryonic antigen) is usually served as a detection criterion for the early aided diagnosis and cancer postoperation recrudescence of colon cancer, gastric cancer, esophagus cancer, rectal cancer, etc. At present, common CEA detection methods on clinic detection comprise ELISA (enzyme-linked immuno sorbent assay), CLIA and so on, waste time and labor, is not beneficial to popularization and has high cost. The technology of immune collaurum develops rapidly and is applied widely to the detection on infectious diseases, early pregnancy, cancer and the like. The method adopts the collaurum immune chromatography to build a method for quickly detecting the CEA, and can detect the range of a CEA gray area from 5ng / mL-20ng / mL with semi quantity. The CEA can be taken as the basis for differential diagnosis of benign and malignant tumors.
Owner:正元盛邦(天津)生物科技有限公司
Preparation method of paper-based electrochemical luminescence converter and applications of paper-based electrochemical luminescence converter in carcino-embryonic antigen detection
InactiveCN106248658ALow costImprove accuracyChemiluminescene/bioluminescenceProtein insertionEngineering
The invention discloses a preparation method of a paper-based electrochemical luminescence converter and applications of the electrochemical luminescence converter in carcino-embryonic antigen detection. Through wax printing technology, screen printing technology and the functionalization of paper chips, an input signal of protein is converted into an output signal of a specific aptamer chain to further cause the change of luminol electrochemical luminescence intensity, so that the high-sensitive and portable detection of low-concentration tumor markers can be realized.
Owner:UNIV OF JINAN
Electrochemiluminescence multicomponent immunodetection method based on spectral resolution principle
ActiveCN106124487AGood radiation monochromaticityReduce consumptionChemiluminescene/bioluminescenceBiological testingAntigenCarcinoembryonic antigen
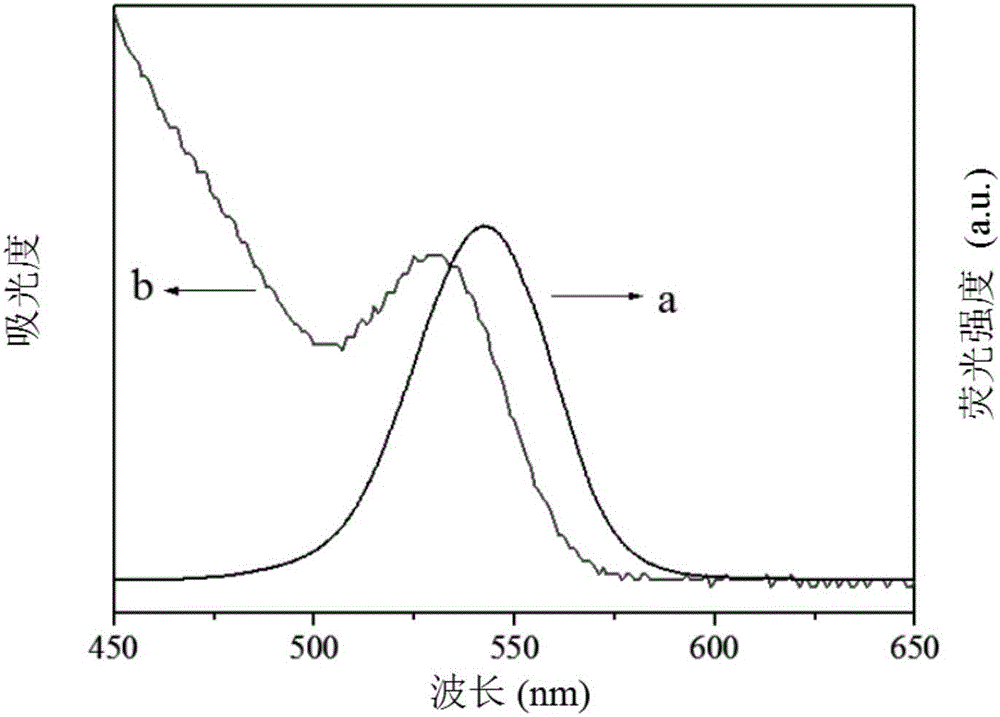
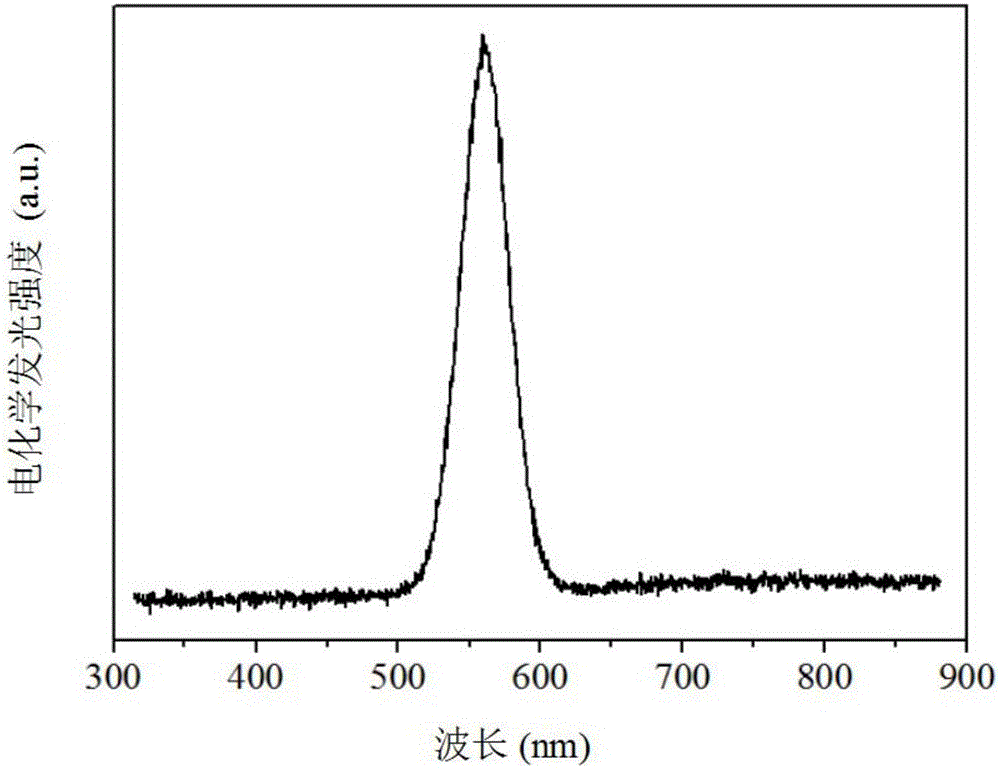
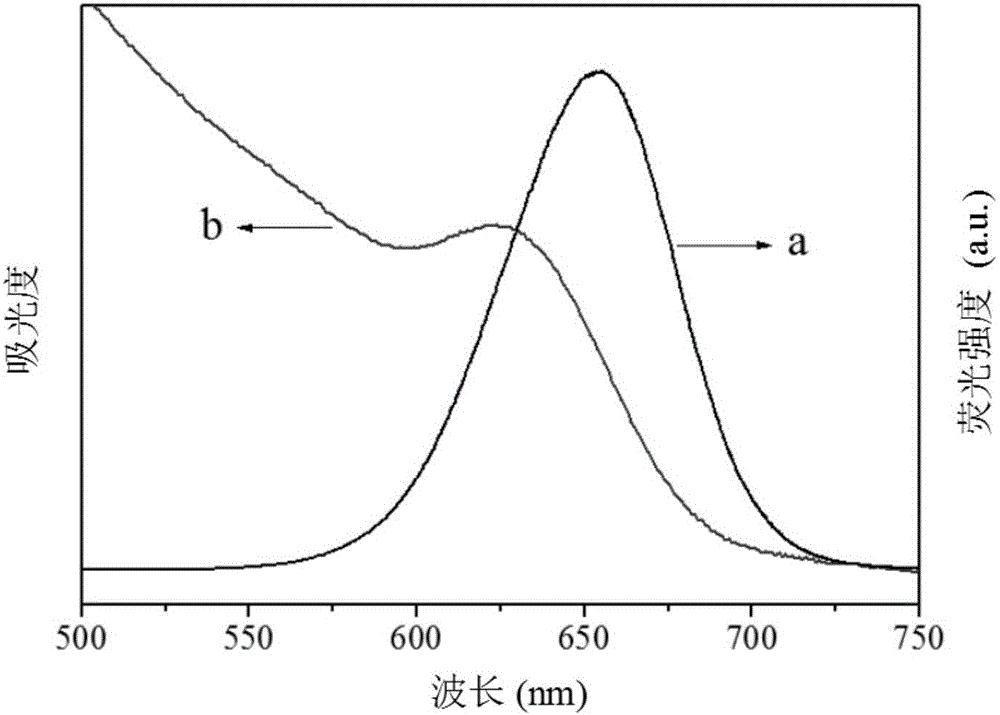
The present invention relates to an electrochemiluminescence multicomponent immunodetection method based on a spectral resolution principle, wherein CdSe quantum dots and CdTe quantum dots are adopted as markers, and the ECL signals of different markers are identified based on a spectral resolution principle so as to provide the ECL multicomponent detection method. The method mainly comprises: (1) preparing three quantum dot labeled corresponding secondary antibody (QDs-Ab2); (2) preparing a multicomponent ECL immunosensor using the three quantum dots as markers; and (3) carrying out multicomponent ECL immunodetection. According to the present invention, the detection sensitivity of the method is high, the detection limit of the carcinoembryonic antigen achieves 1.0 pg / mL, the detection limit of the prostate specific antigen achieves 10.0 pg / mL, the detection limit of the alpha fetoprotein antigen achieves 10.0 fg / mL, the concurrent detection of a variety of antigens can be achieved, and the selectivity is high.
Owner:SHANDONG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com