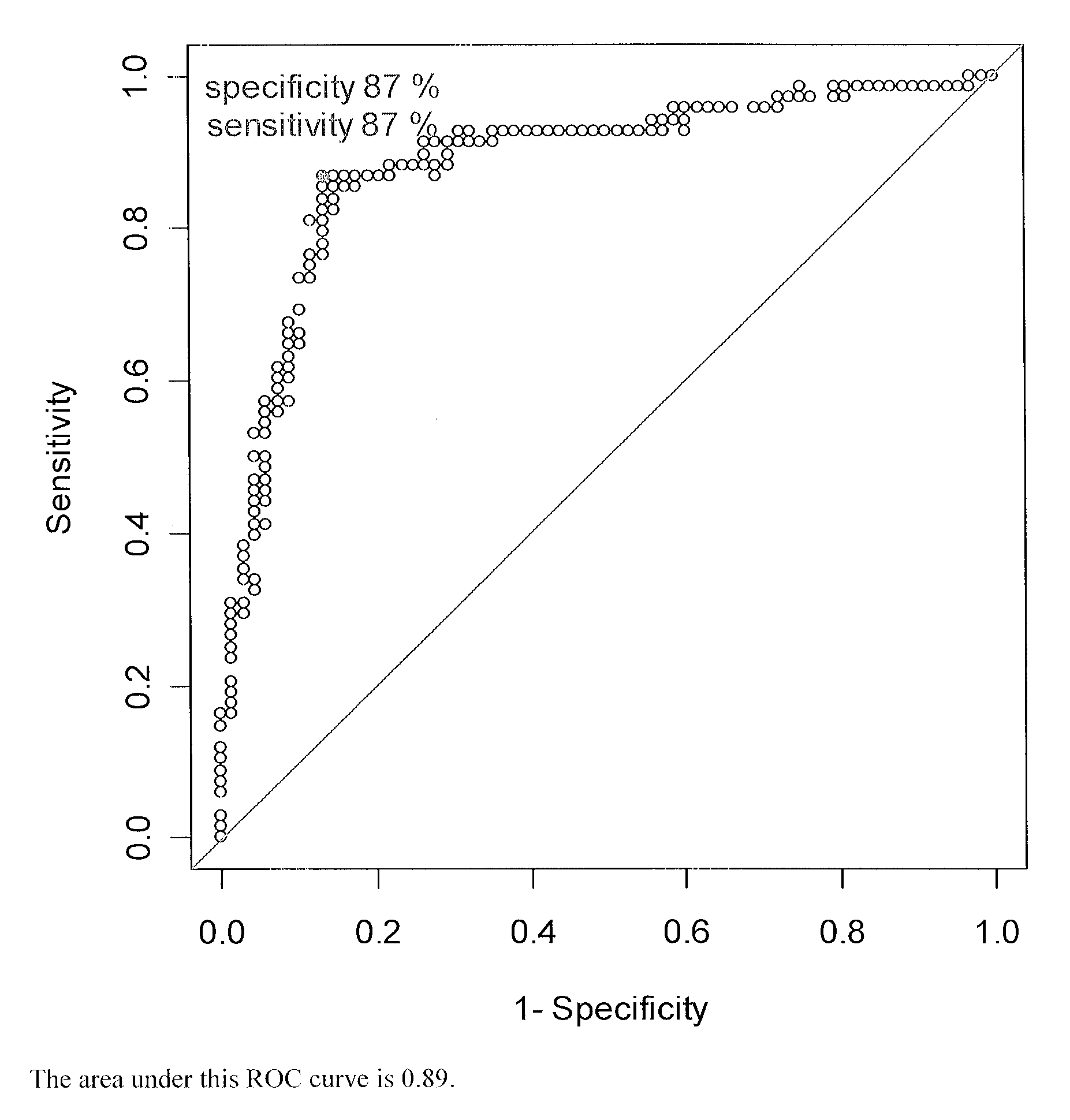
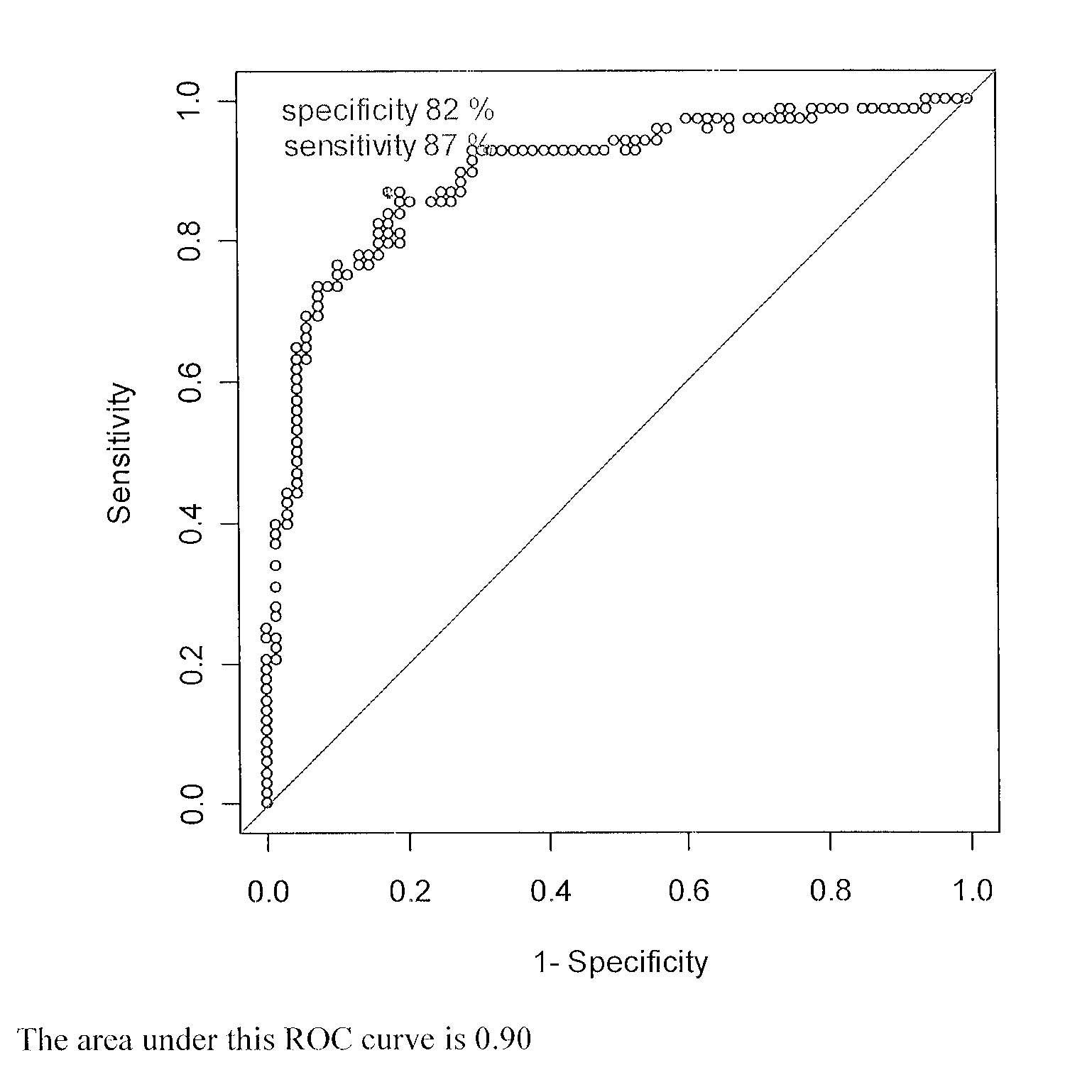
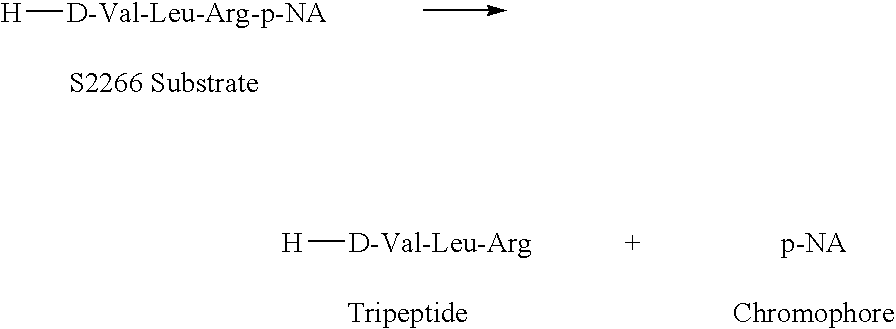
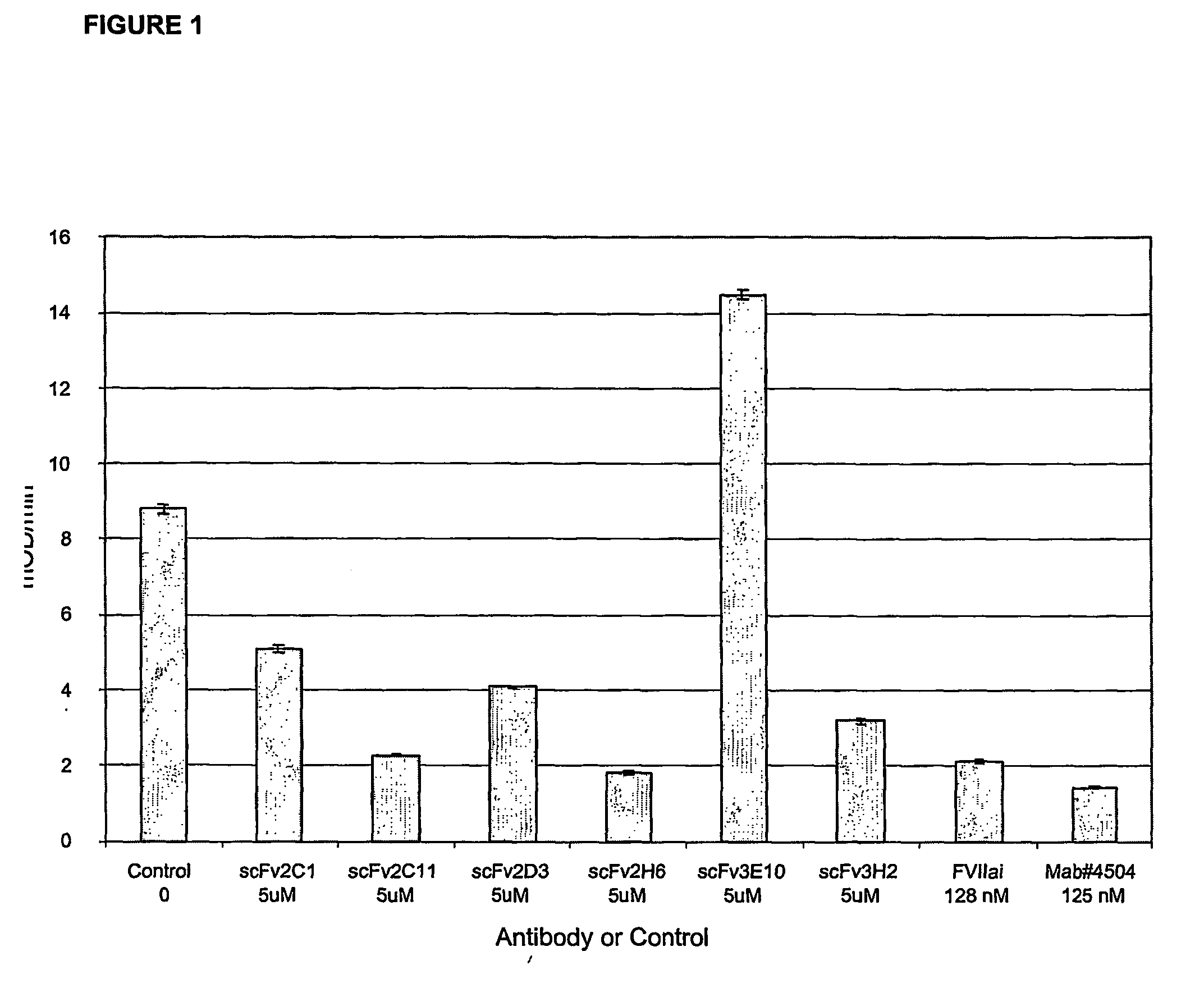
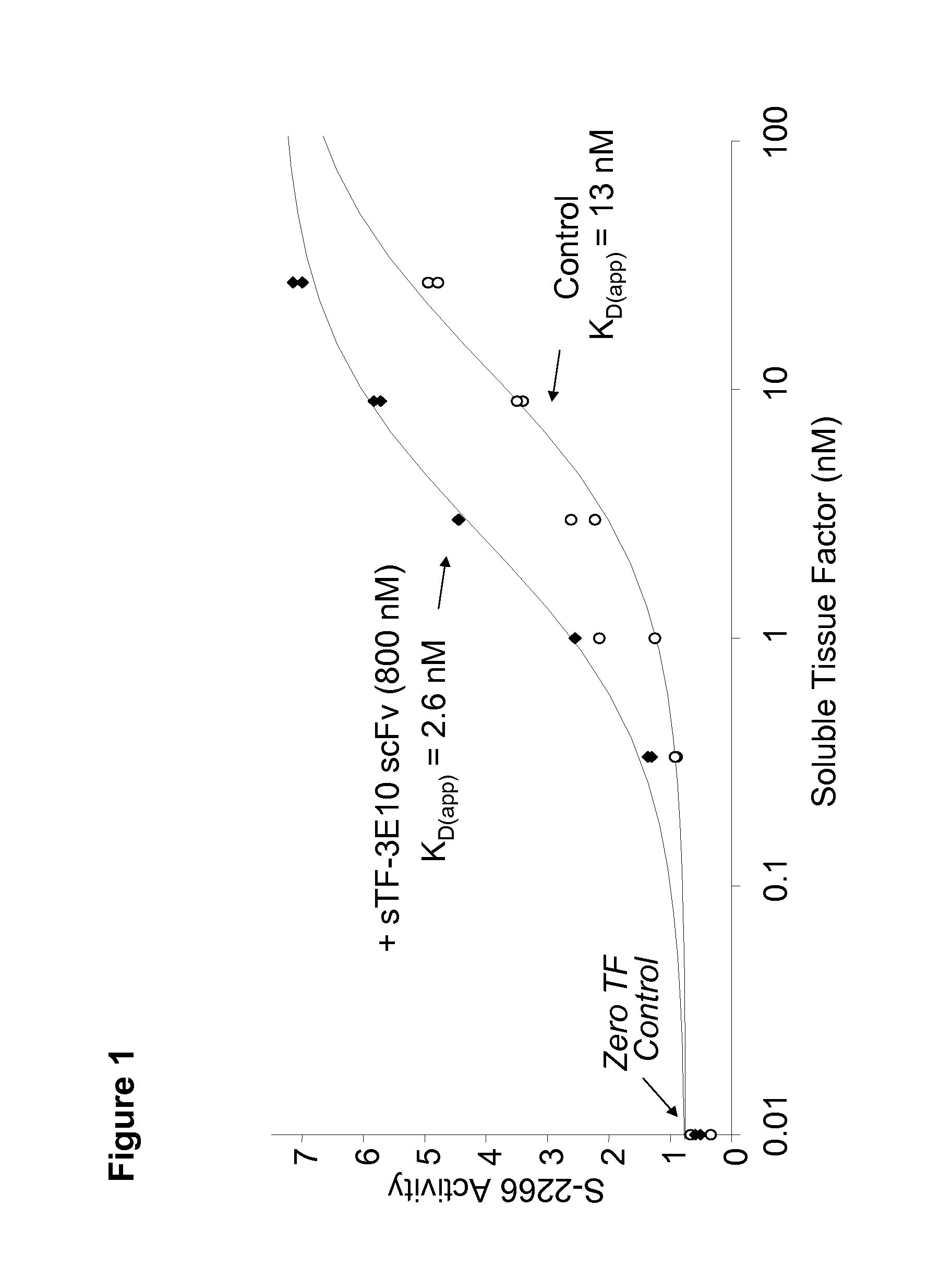
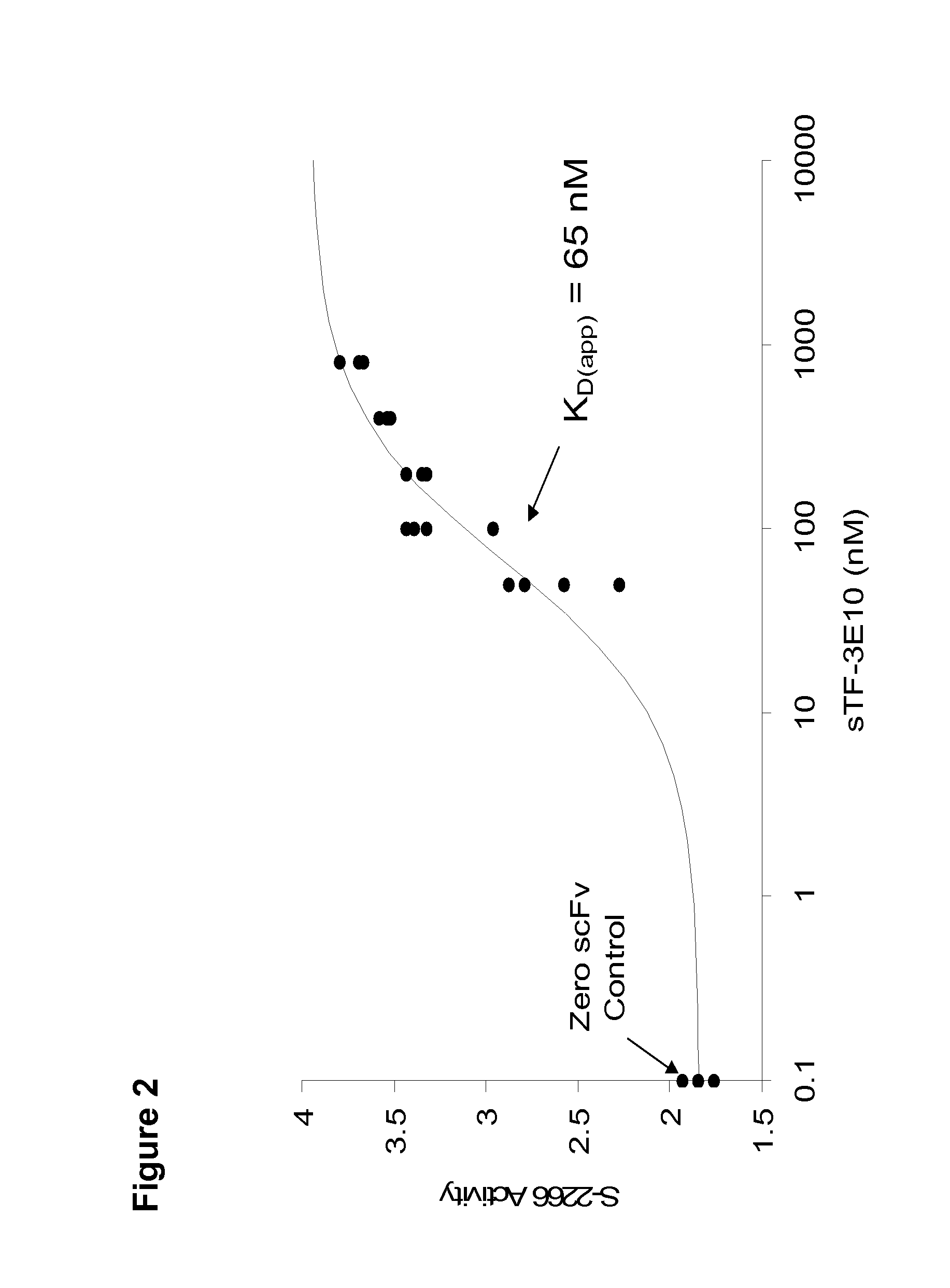
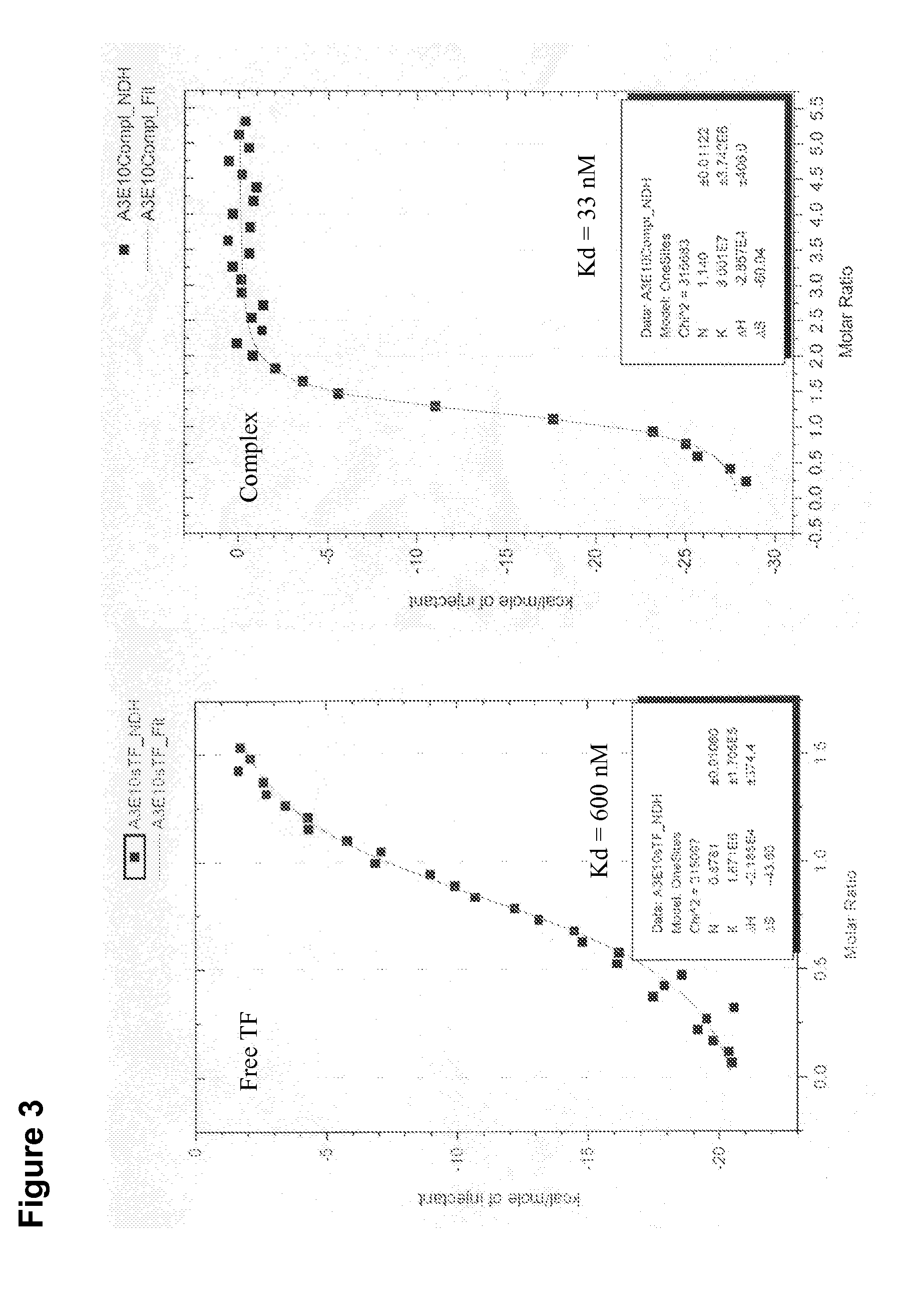
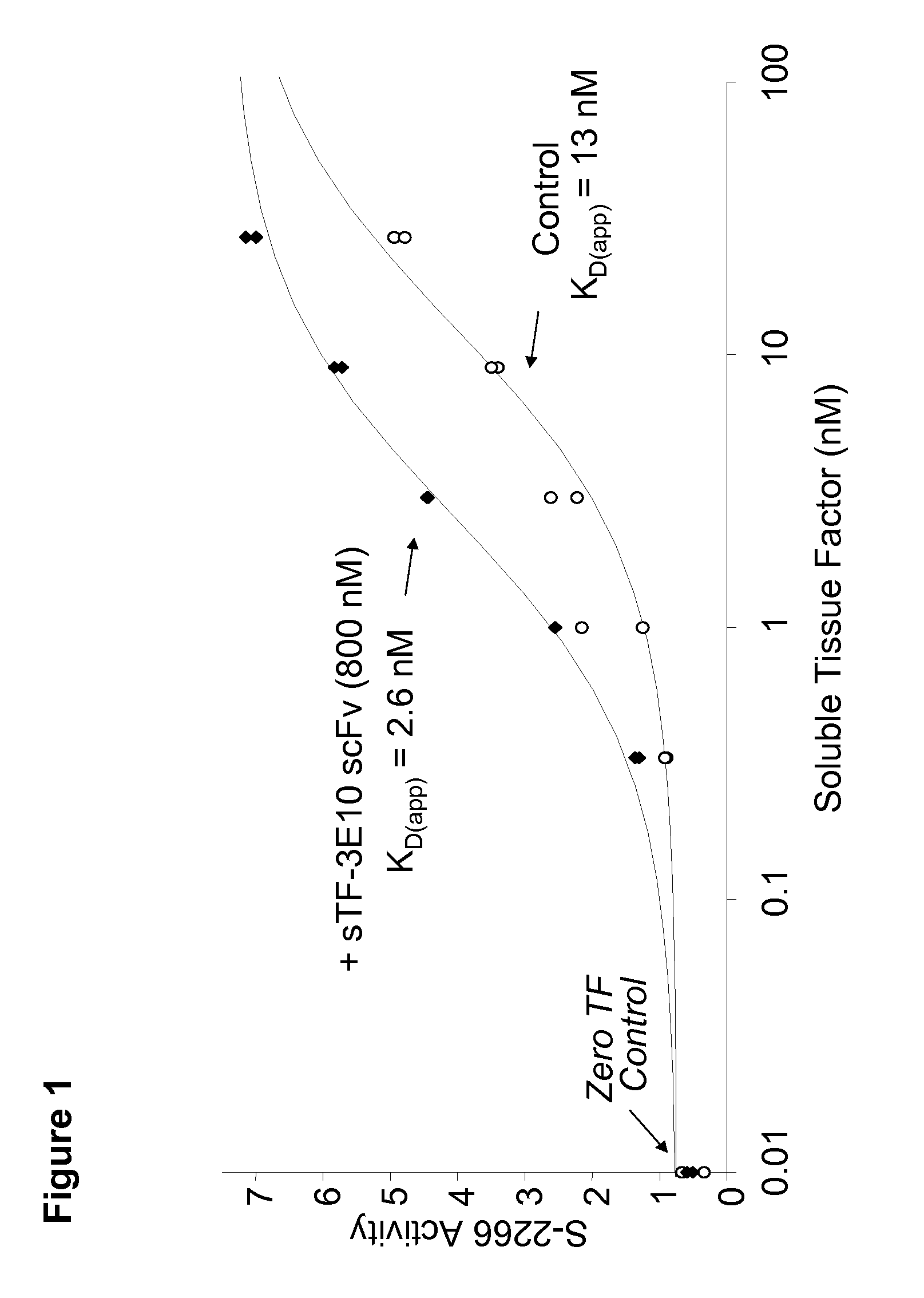
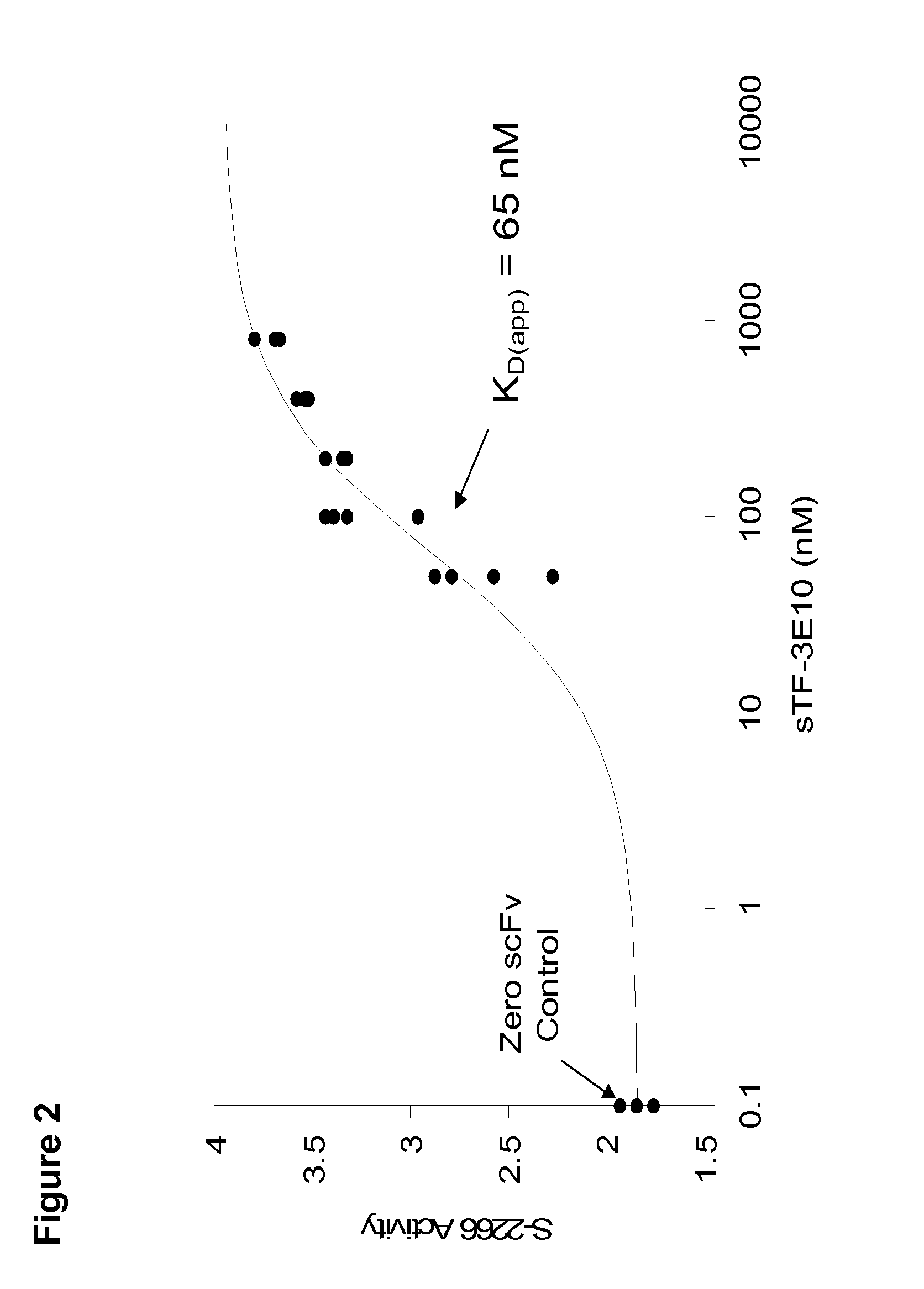
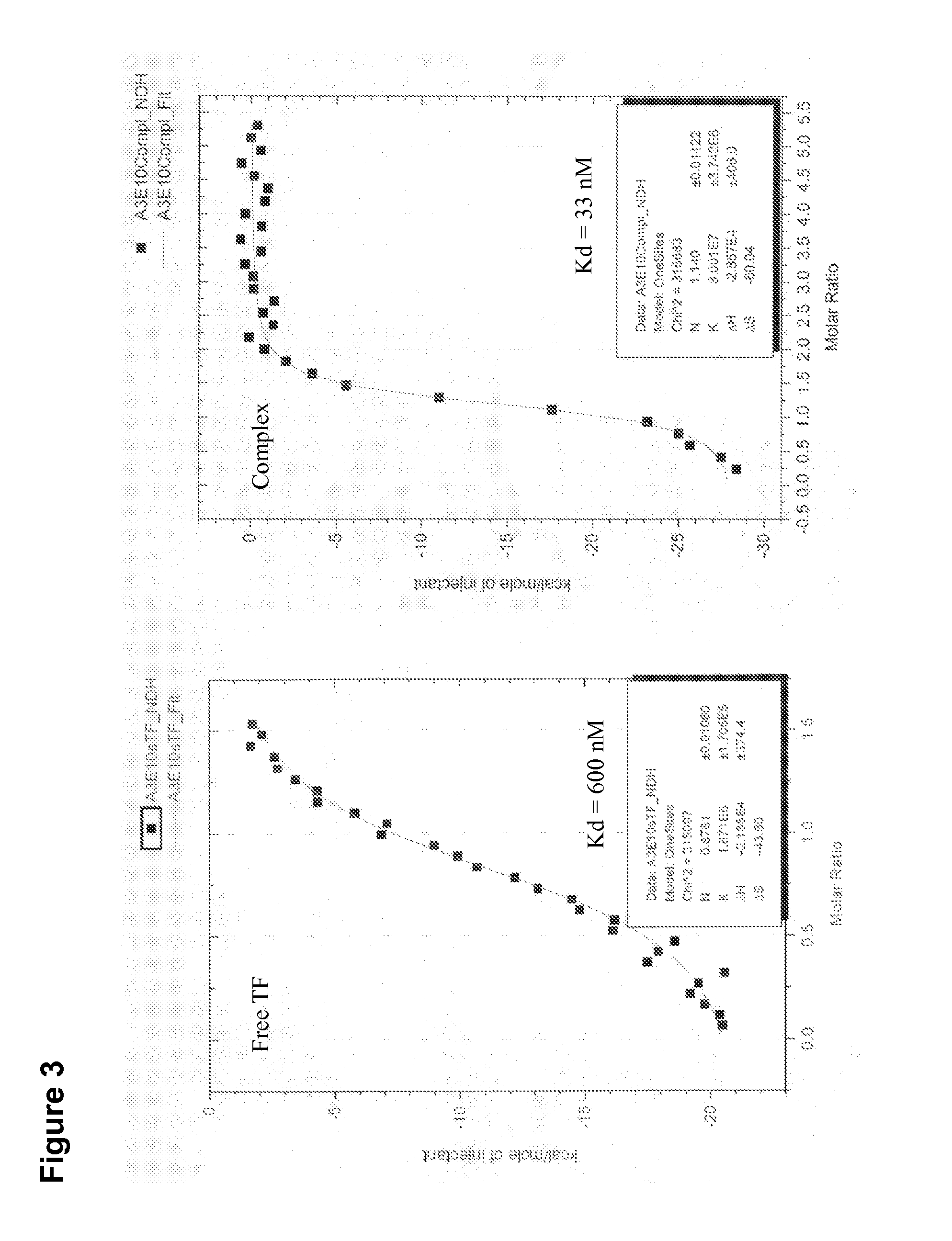
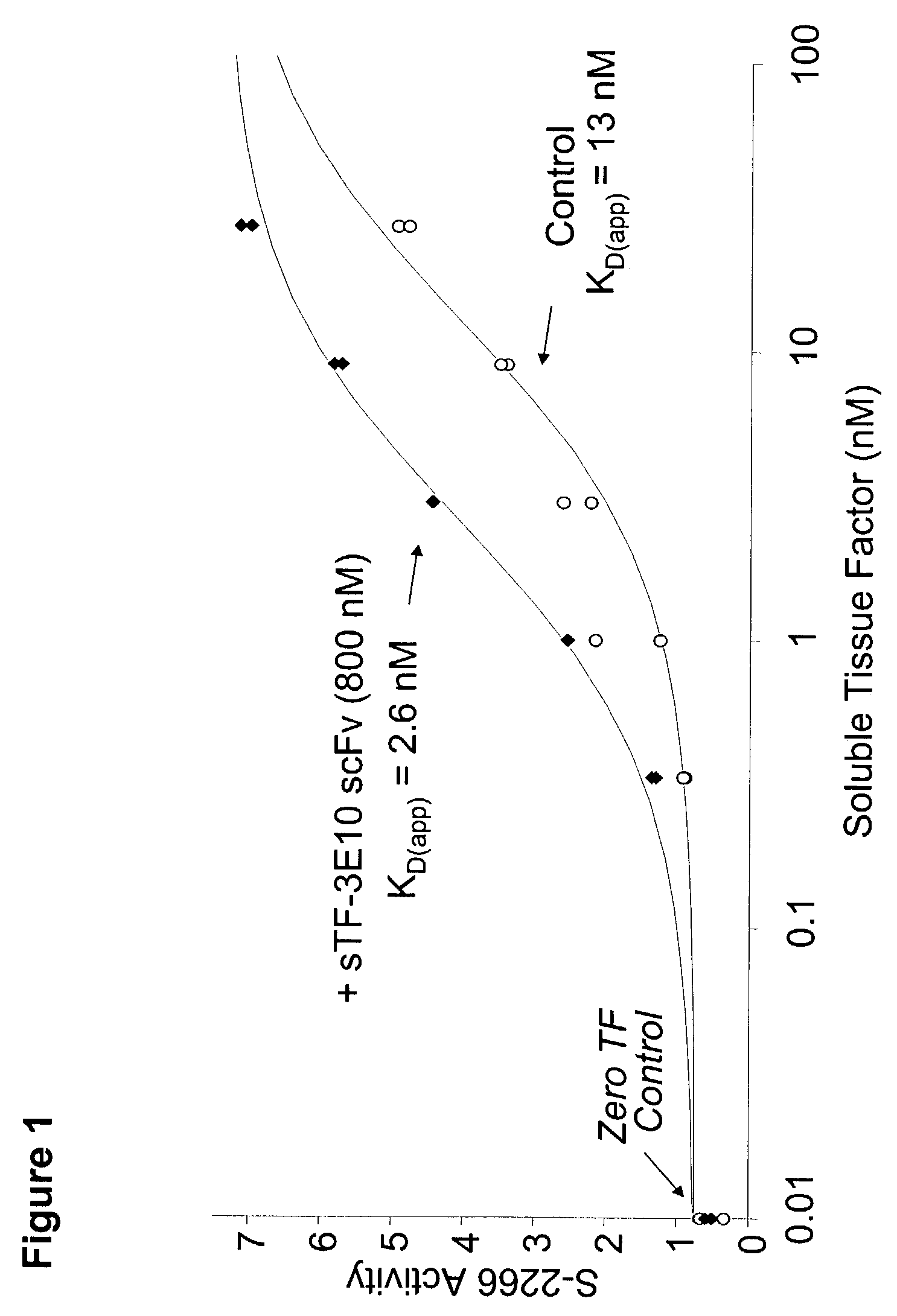
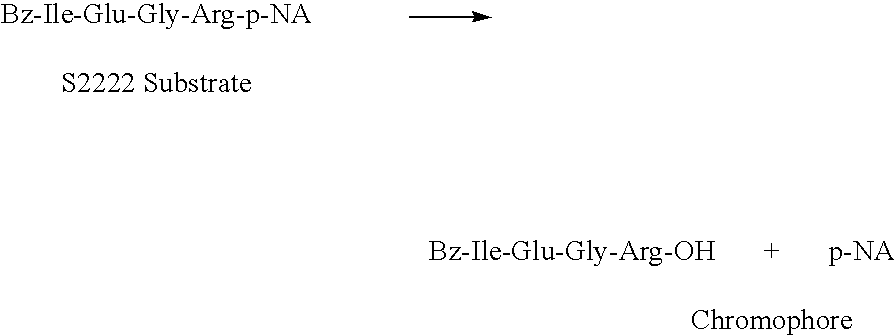Patents
Literature
185 results about "Tissue factor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
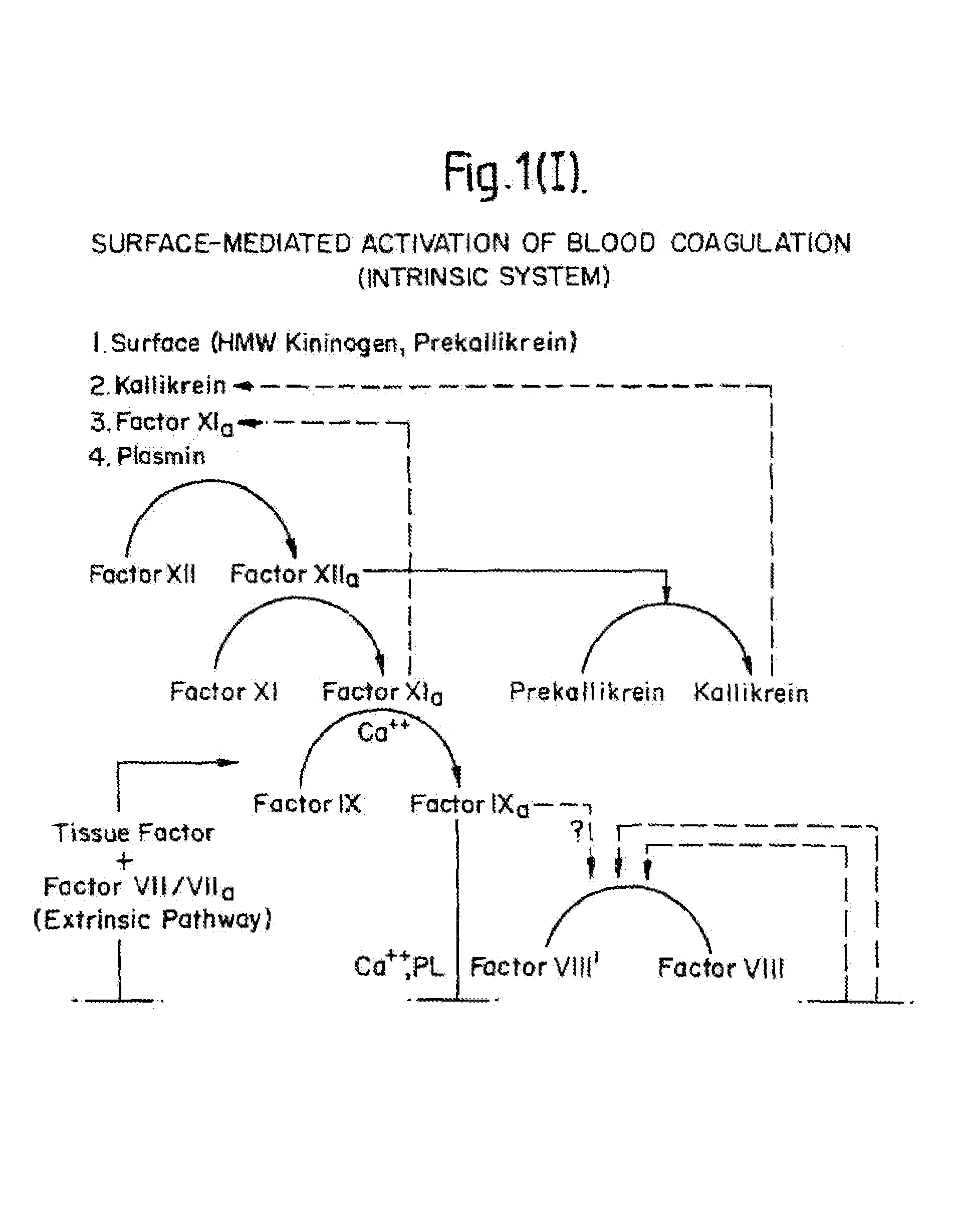
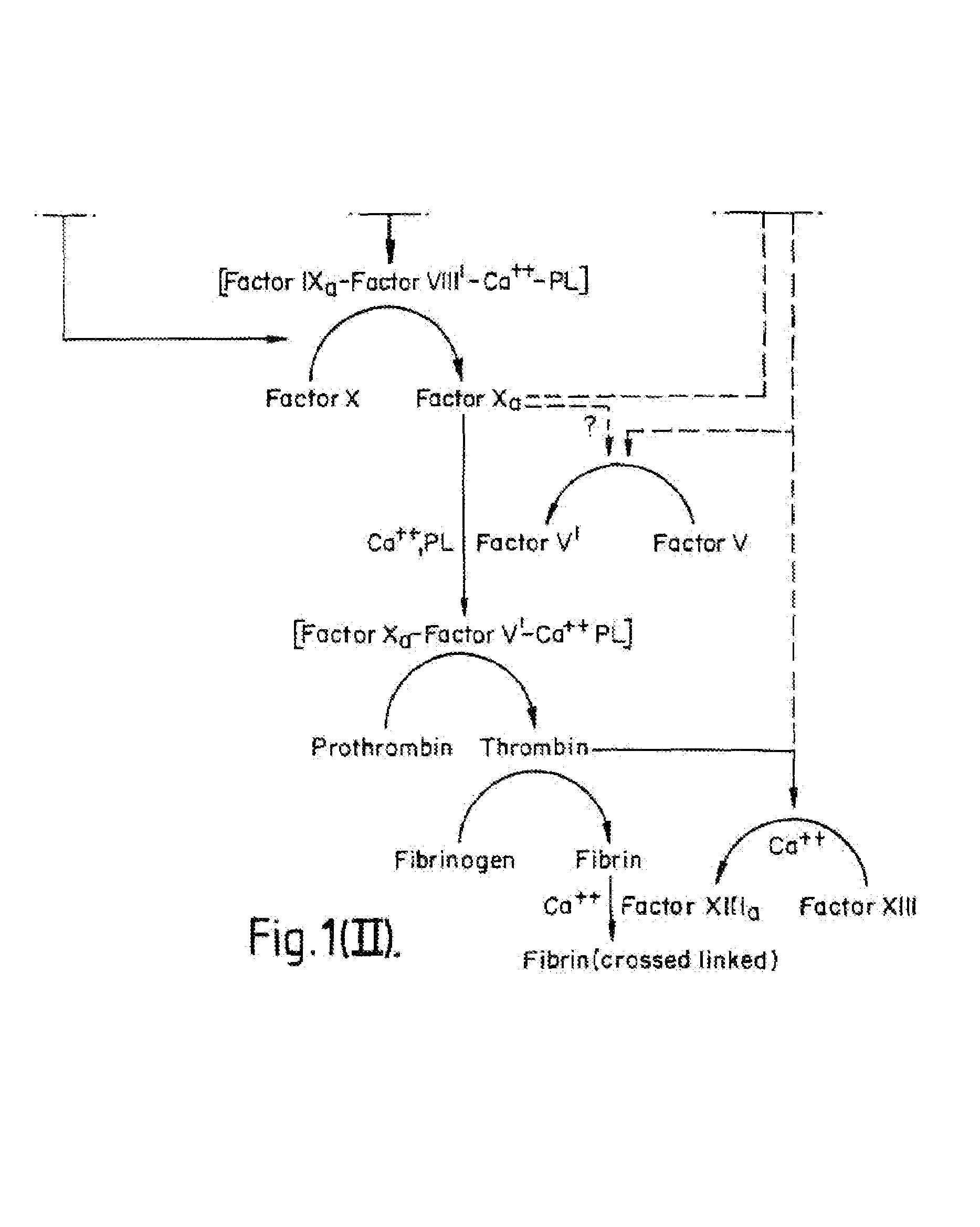
Tissue factor, also called platelet tissue factor, factor III, or CD142, is a protein encoded by the F3 gene, present in subendothelial tissue and leukocytes. Its role in the clotting process is the initiation of thrombin formation from the zymogen prothrombin. Thromboplastin defines the cascade that leads to the activation of factor X—the tissue factor pathway. In doing so, it has replaced the previously named extrinsic pathway in order to eliminate ambiguity.
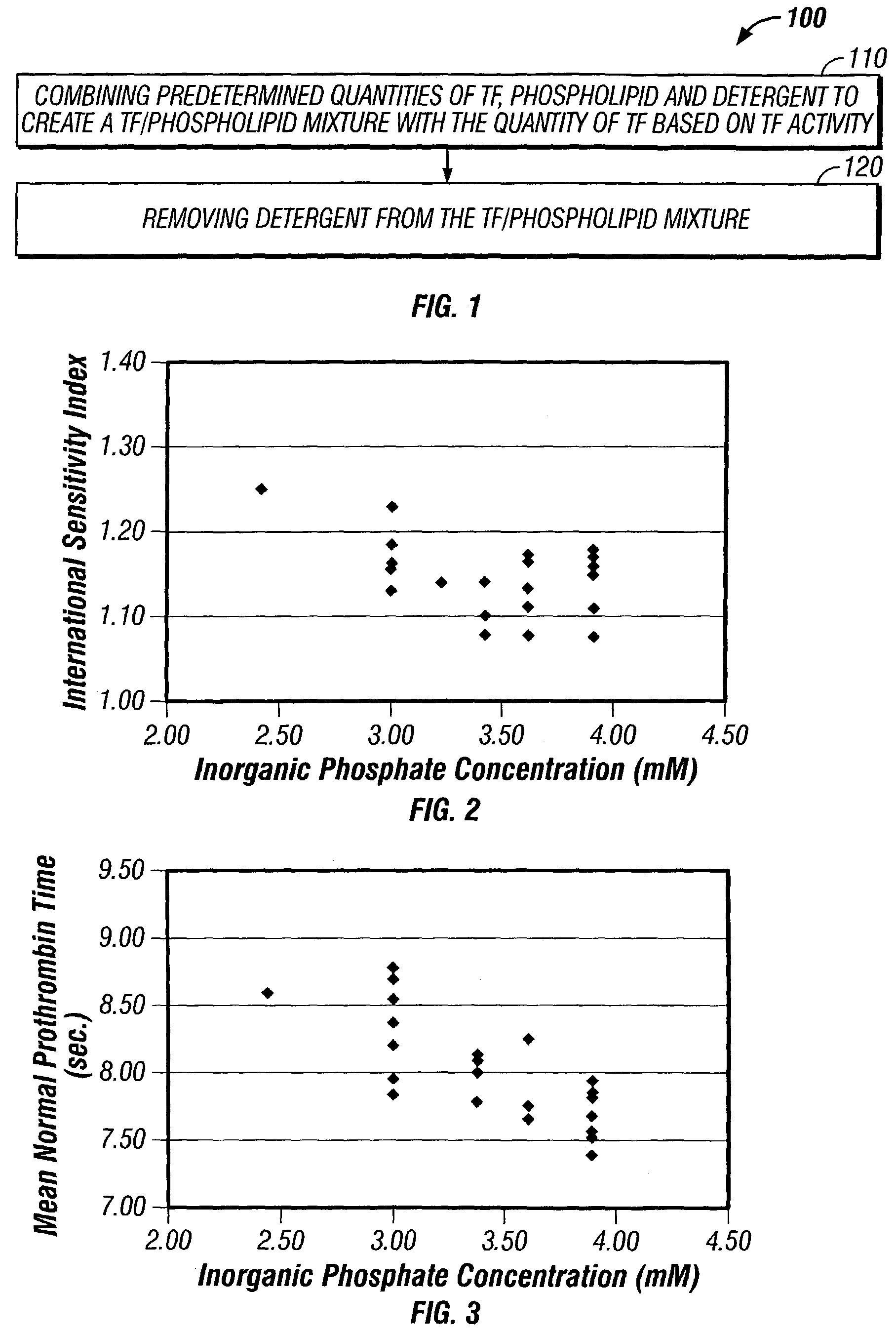
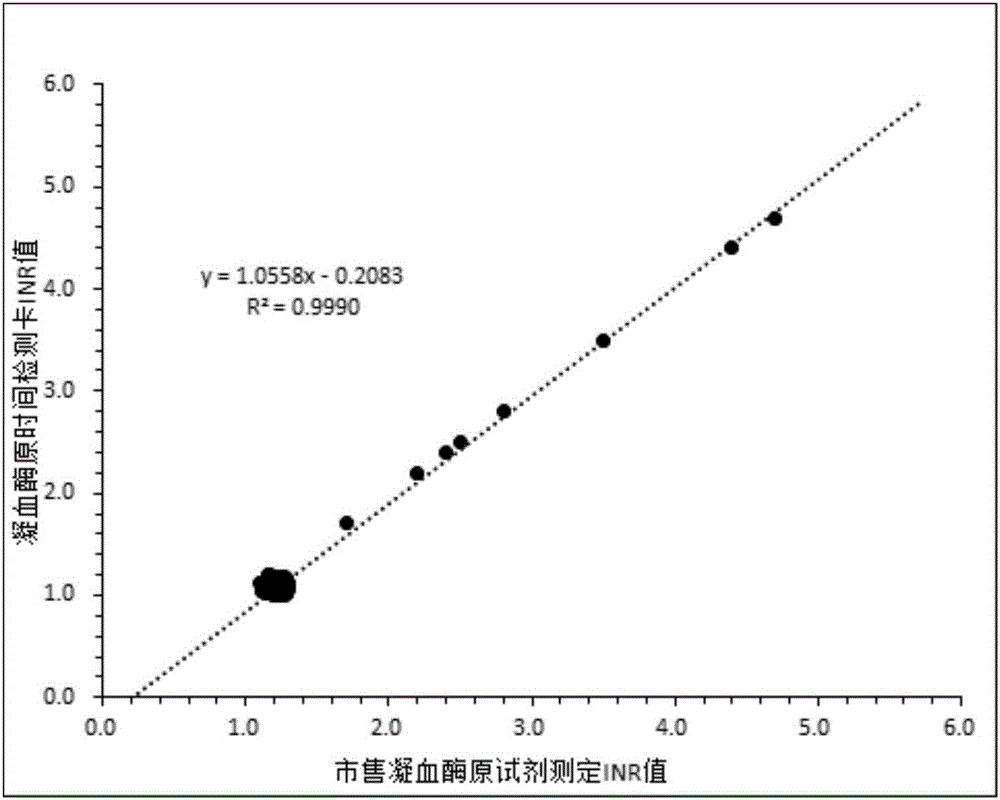
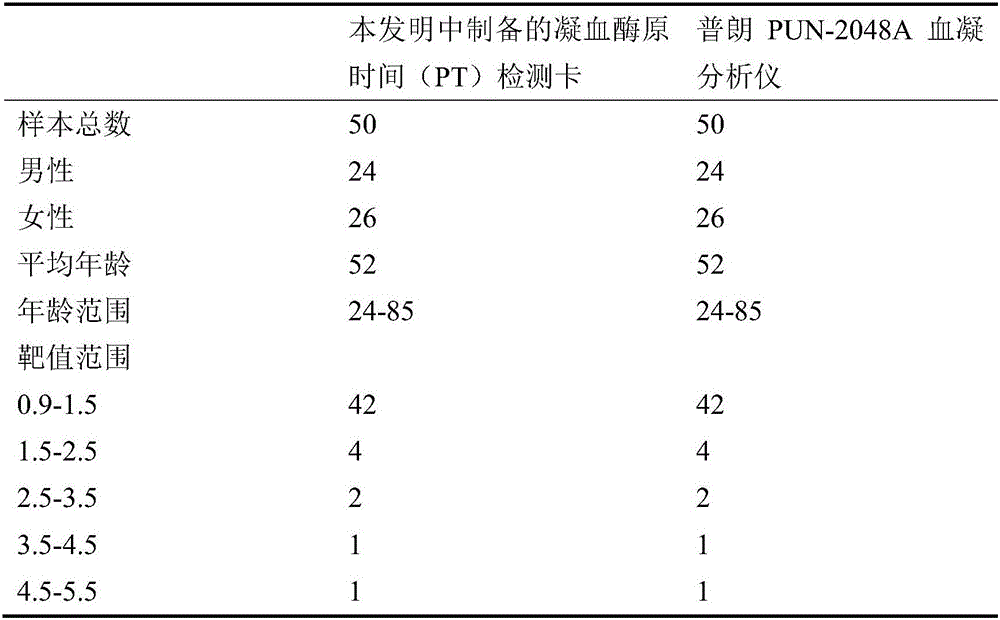
Method for manufacturing a tissue factor-based prothrombin time reagent
ActiveUS7049087B2Microbiological testing/measurementBiological material analysisTissue factorPhospholipid
Owner:LIFESCAN IP HLDG LLC
Compositions and methods for treating coagulation related disorders
InactiveUS20060159675A1Initiate and prolong such disorderRelieve symptomsImmunoglobulins against blood coagulation factorsAntibacterial agentsDiseaseTissue factor
Disclosed are methods for preventing or treating sepsis, a sepsis-related condition or an inflammatory disease in a mammal. In one embodiment, the method includes administering to the mammal a therapeutically effective amount of at least one humanized antibody, chimeric antibody, or fragment thereof that binds specifically to tissue factor (TF) to form a complex in which factor X or factor IX binding to the complex is inhibited and the administration is sufficient to prevent or treat the sepsis in the mammal. The invention has a wide spectrum of useful applications including treating sepsis, disorders related to sepsis, and inflammatory diseases such as arthritis.
Owner:GENENTECH INC
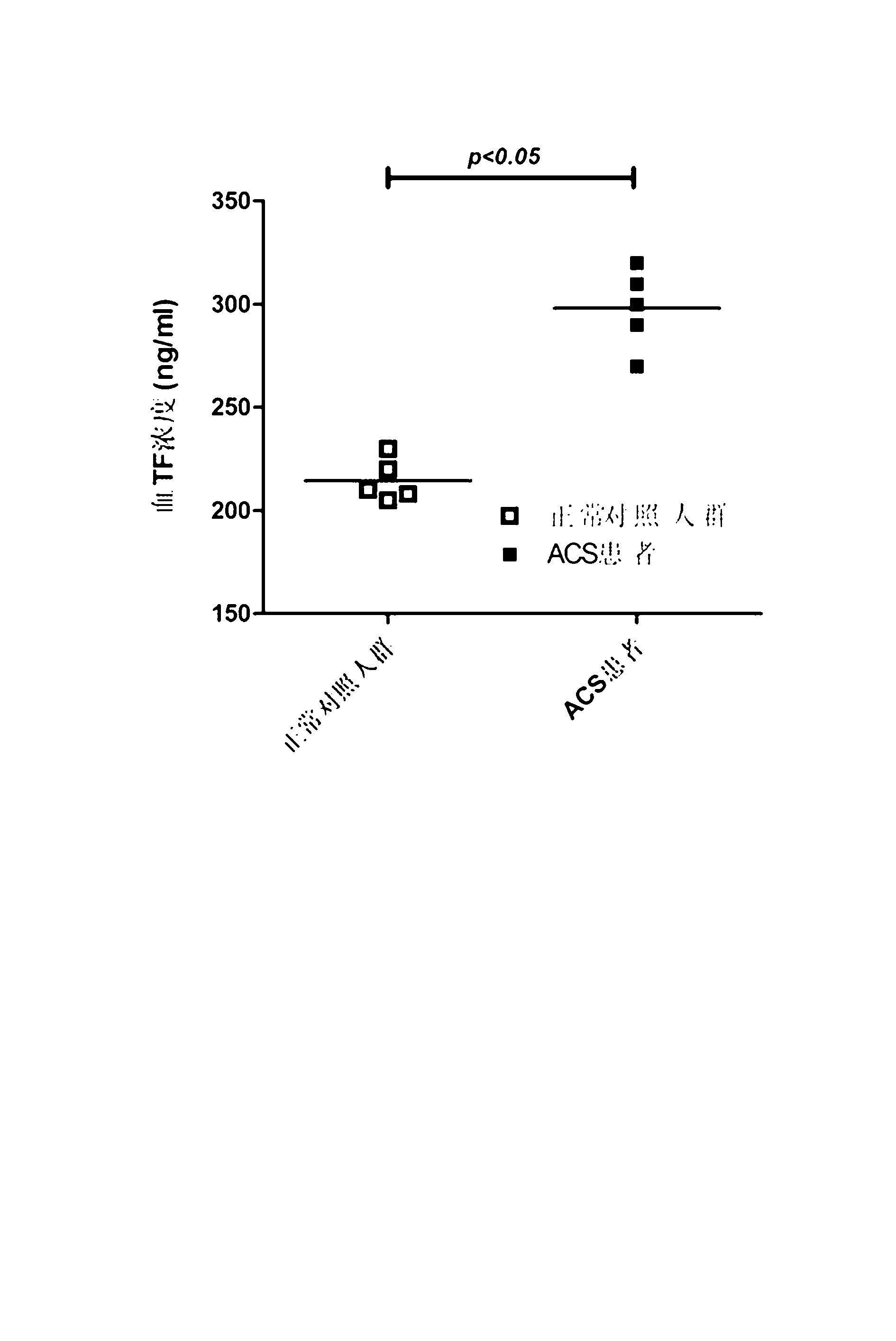
Methods for detecting major adverse cardiovascular and cerebrovascular events
InactiveUS20110045514A1Raise the possibilityMicrobiological testing/measurementDisease diagnosisTissue factorAnalyte
The present teachings relate to a method of assessing the probability of a major adverse cardiovascular or cerebrovascular event in a human. The method can include measuring a concentration, in a blood-based sample of a human, of a set of analytes, for example, alpha-fetoprotein, cancer antigen 125, glutathione S-transferase, and tissue factor. The method also can include determining a MACCE index for the set of analytes and identifying the human as having an increased likelihood of a major adverse cardiovascular or cerebrovascular event if the MACCE index is greater than zero, or a decreased likelihood of a major adverse cardiovascular or cerebrovascular event if the MACCE index is less than or equal to zero.
Owner:BG MEDICINE
Novel tissue factor targeted antibodies as anticoagulants
InactiveUS20060166284A1Inhibiting generation of thrombinPrevent thrombosisImmunoglobulins against blood coagulation factorsAntibody ingredientsTissue factorDisseminated coagulopathy
This invention relates to novel antibodies that bind with greater affinity to the factor VIIa / tissue factor (FVIIa / TF) complex than to tissue factor (TF) alone, do not compete for binding to TF with FVII and FX, an inhibit FX activation. The antibodies bind at the site of injury and prevent the initiation of thrombosis. The antibodies can be used to treat a variety of thrombotic conditions including but not limited to deep vein thrombosis, disseminated intravascular coagulation, and acute coronary syndrome.
Owner:BAYER INTELLECTUAL PROPERTY GMBH
Novel tissue factor targeted thrombomodulin fusion proteins as anticoagulants
InactiveUS20080019985A1Prevent thrombosisMore effectiveAntibacterial agentsPeptide/protein ingredientsProtein targetThrombus
This invention relates to novel fusion proteins which are comprised of a targeting protein that binds tissue factor (TF), which is operably linked to the thrombomodulin (TM) EGF456 domain alone or in combination with at least one other TM domain selected from the group consisting of the N-terminal hydrophobic region domain, the EGF123 domain, the interdomain loop between EGF3 and EGF4, and the O-glycosylated Ser / Thr-rich domain, or analogs, fragments, derivatives or variants thereof. The fusion protein binds at the site of injury and prevents the initiation of thrombosis. The fusion protein can be used to treat a variety of thrombotic conditions including but not limited to deep vein thrombosis, disseminated intravascular coagulation, and acute coronary syndrome.
Owner:BAYER INTELLECTUAL PROPERTY GMBH
Novel tissue factor targeted thrombomodulin fusion proteins as anticoagulants
InactiveUS20080020965A1Prevent thrombosisMore effectiveAntibacterial agentsOrganic active ingredientsProtein targetDisseminated coagulopathy
This invention relates to novel fusion proteins which are comprised of a targeting protein that binds tissue factor (TF), which is operably linked to the thrombomodulin (TM) EGF456 domain alone or in combination with at least one other TM domain selected from the group consisting of the N-terminal hydrophobic region domain, the EGF123 domain, the interdomain loop between EGF3 and EGF4, and the O-glycosylated Ser / Thr-rich domain, or analogs, fragments, derivatives or variants thereof. The fusion protein binds at the site of injury and prevents the initiation of thrombosis. The fusion protein can be used to treat a variety of thrombotic conditions including but not limited to deep vein thrombosis, disseminated intravascular coagulation, and acute coronary syndrome.
Owner:BAYER INTELLECTUAL PROPERTY GMBH
Tissue factor targeted thrombomodulin fusion proteins as anticoagulants
InactiveUS7250168B2Inhibiting generation of thrombinPrevent thrombosisAntibacterial agentsPeptide/protein ingredientsProtein targetDisseminated coagulopathy
This invention relates to novel fusion proteins which are comprised of a targeting protein that binds tissue factor (TF), which is operably linked to the thrombomodulin (TM) EGF456 domain alone or in combination with at least one other TM domain selected from the group consisting of the N-terminal hydrophobic region domain, the EGF123 domain, the interdomain loop between EGF3 and EGF4, and the O-glycosylated Ser / Thr-rich domain, or analogs, fragments, derivatives or variants thereof. The fusion protein binds at the site of injury and prevents the initiation of thrombosis. The fusion protein can be used to treat a variety of thrombotic conditions including but not limited to deep vein thrombosis, disseminated intravascular coagulation, and acute coronary syndrome.
Owner:BAYER SCHERING PHARMA AG
Antibody fragment-polymer conjugates and uses of same
Described are conjugates formed by an antibody fragment covalently attached to a non-proteinaceous polymer, wherein the apparent size of the conjugate is at least about 500 kD. The conjugates exhibit substantially improved half-life, mean residence time, and / or clearance rate in circulation as compared to the underivatized parental antibody fragment. Also described are conjugates directed against human vascular endothelial growth factor (VEGF), human p185 receptor-like tyrosine kinase (HER2), human CD20, human CD18, human CD11a, human IgE, human apoptosis receptor-2 (Apo-2), human tumor necrosis factor-α (TNF-α), human tissue factor (TF), human α4β7 integrin, human GPIIb-IIIa integrin, human epidermal growth factor receptor (EGFR), human CD3, and human interleukin-2 receptor α-chain (TAC) for diagnostic and therapeutic applications.
Owner:GENENTECH INC
Long-acting reconbinant tissue factor channel inhibitor and preparing method thereof
InactiveCN1528894AExtended half-lifeHighly effective anticoagulantFermentationVector-based foreign material introductionBiotechnologyInclusion bodies
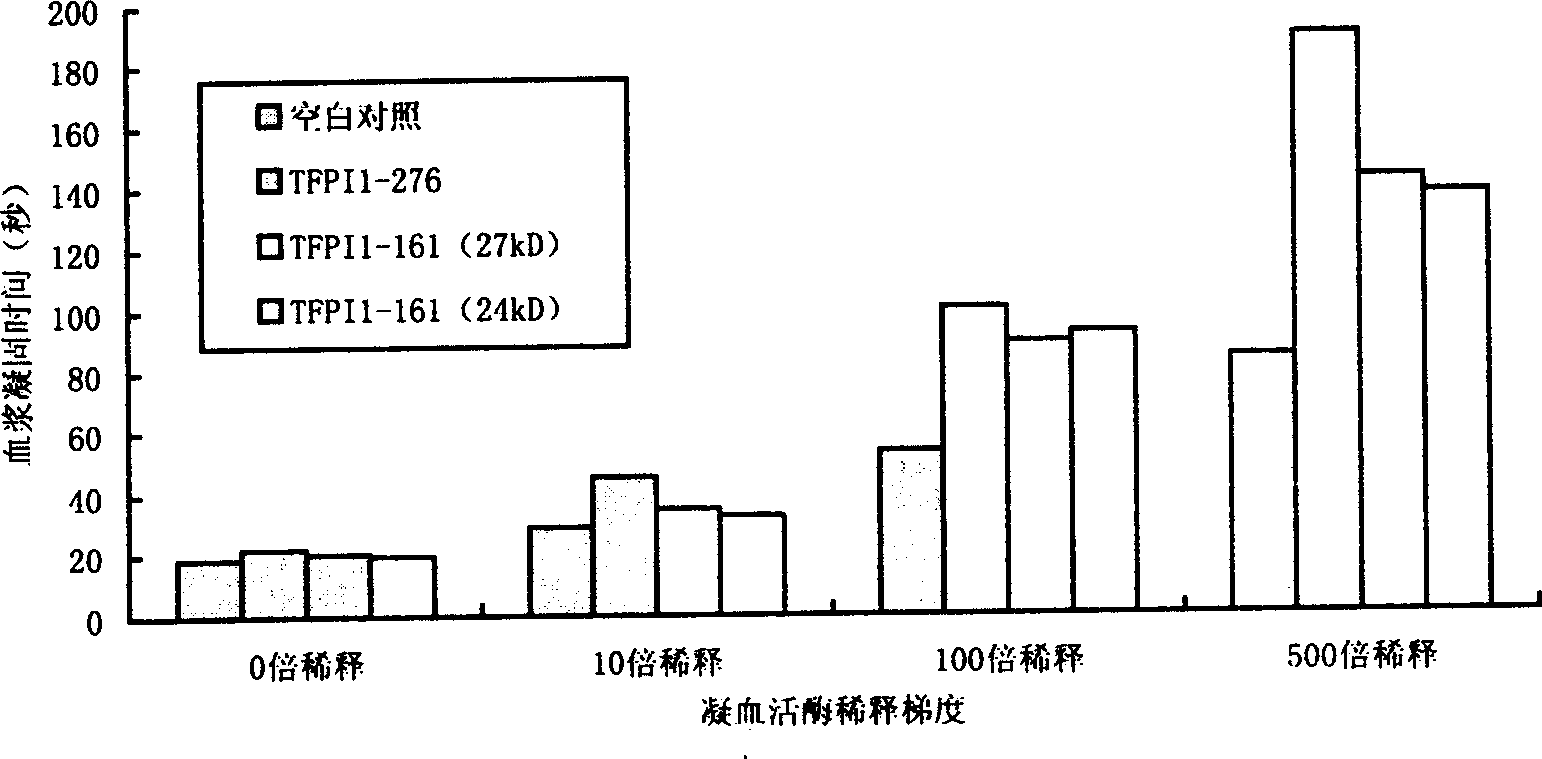
The invention belongs to biology technology field, which concretely refers to controlled release tissue factor path inhibitor (LTFPI) and the manufacturing method, and the application. The invention analyzes and experiments the biology information science and structure molecular biology science of tissue factor path inhibitor (TFPI) and its acceptor low density lipoprotein acceptor correspondent protein (LRP), it ascertains the part where the TFPI carboxy end combines with LRP and is eliminated. It designs TFPI carboxy end mutant, which is recombined with primary nucleus or eukarya expressing carrier after constructing LTFPI gene through PCR location, it converts bacillus coli or bici yeast, sifts the high expression project fungus. The primary project fungus are yeasted and expanded, crushed, then they are centrifugated and collects the inclusion body, purifies the LTFPI through molecular sift and ion interchanging two-step method; the eukarya project fungus are carried on with two-step purifying directly. The half-life are prolonged, it has good pour-depressant function.
Owner:海菲尔(辽宁)生物科技有限公司
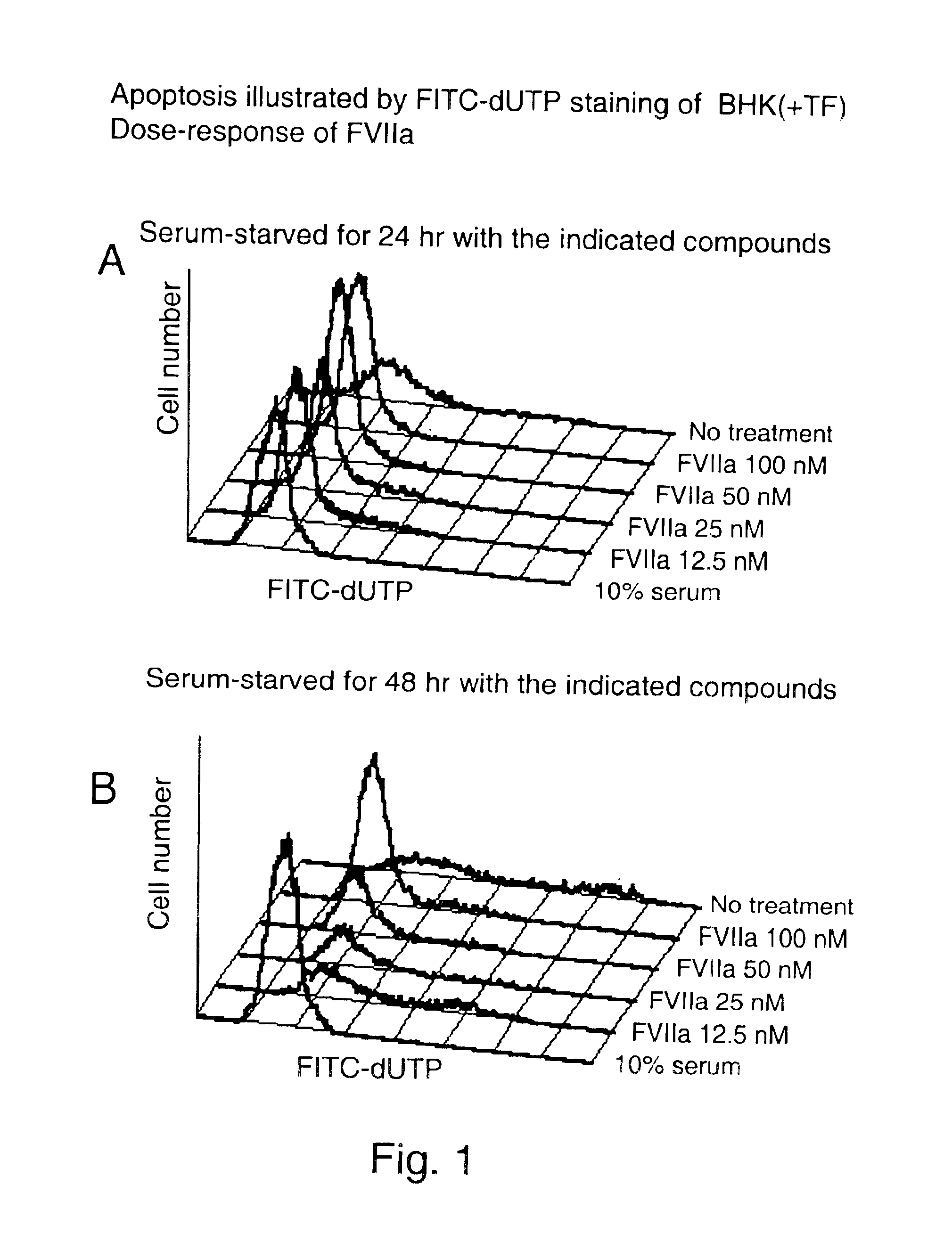
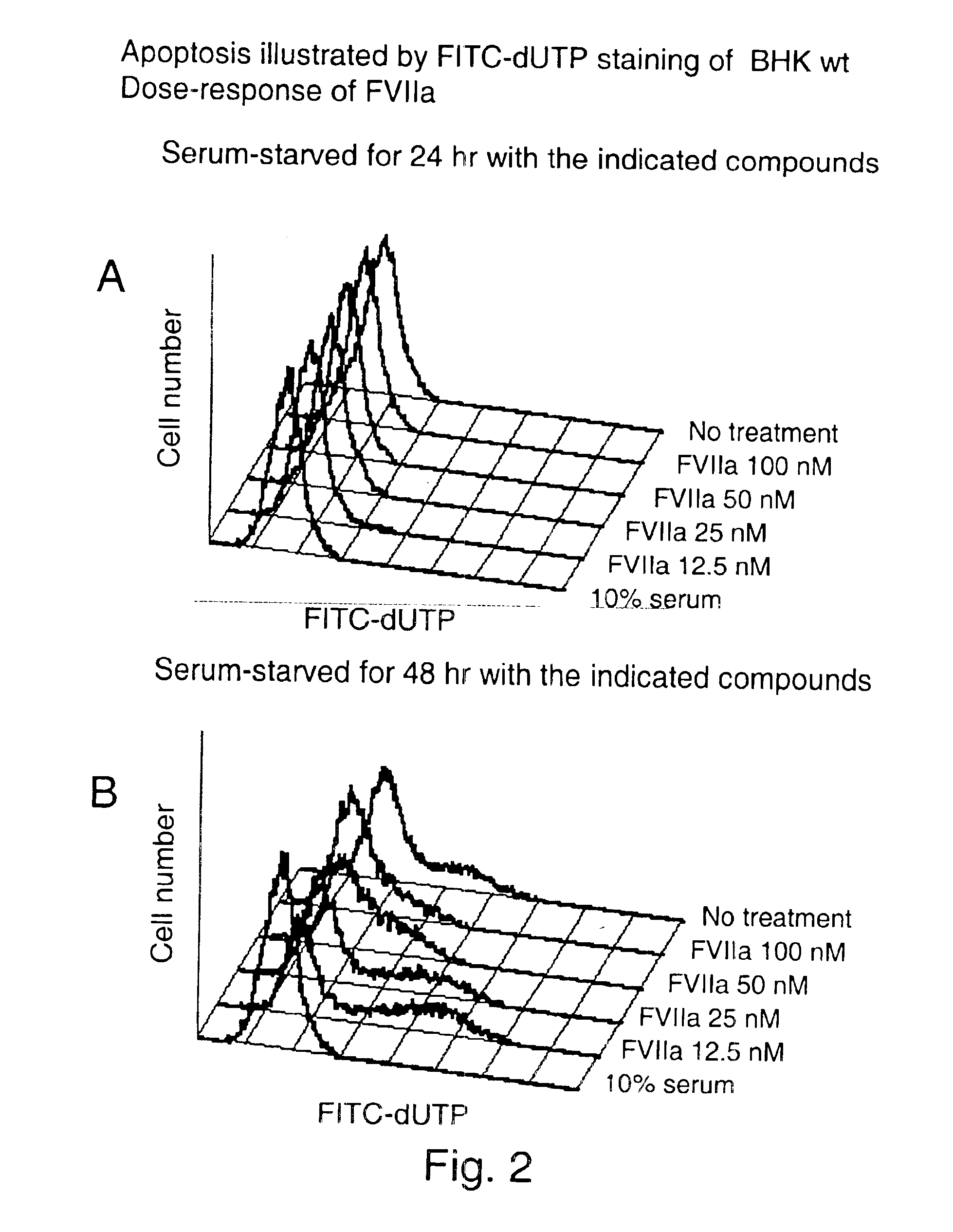
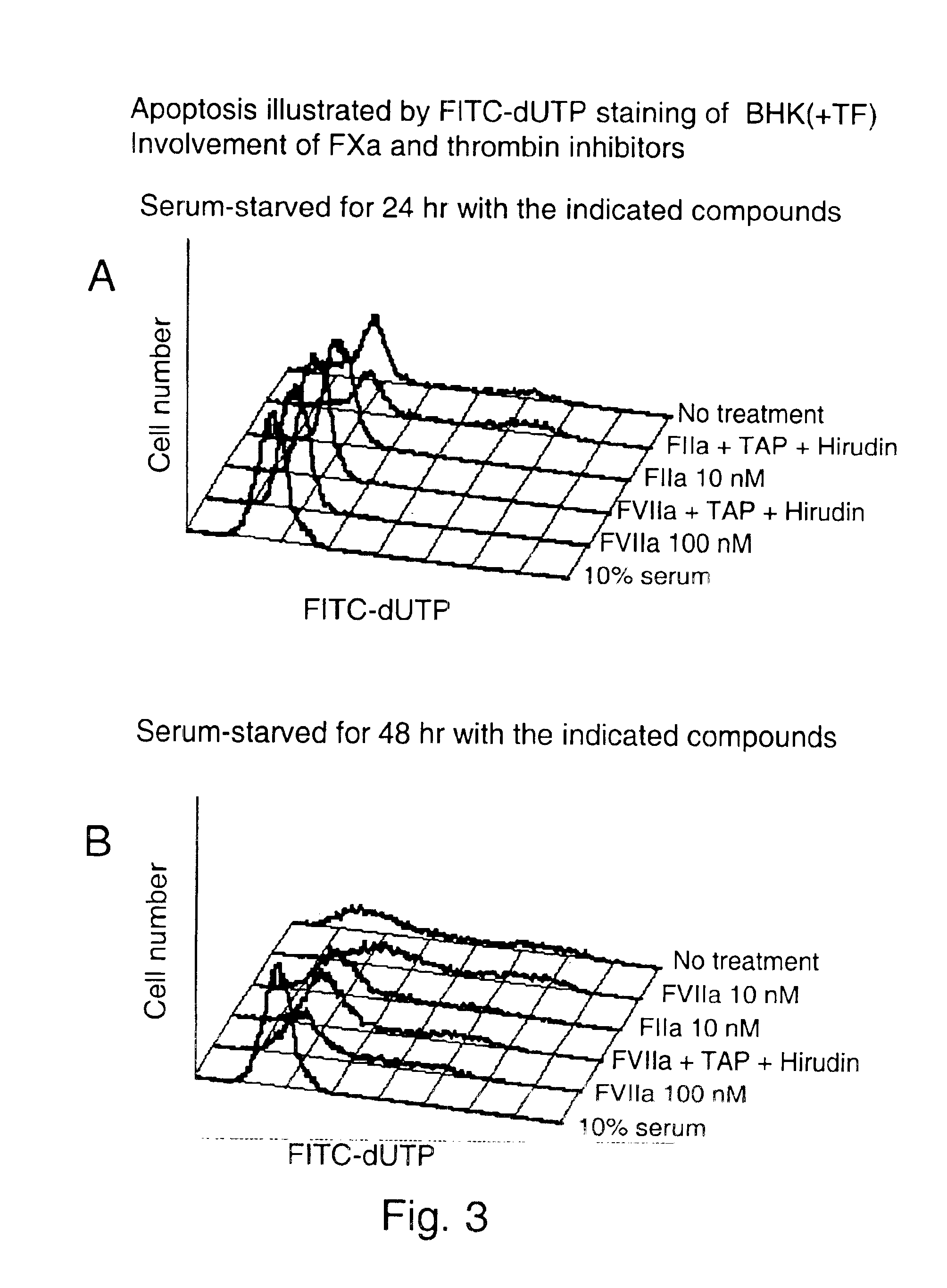
Use of tissue factor agonist or tissue factor antagonist for treatment of conditions related to apoptosis
The present invention relates to use of FVII and / or FVIIa and / or another TF agonist and / or FVIIai and / or another TF antagonist in therapeutic treatment of pathological conditions increased or decreased cell apoptosis is required.
Owner:NOVA NORDISK HEALTH CARE AG
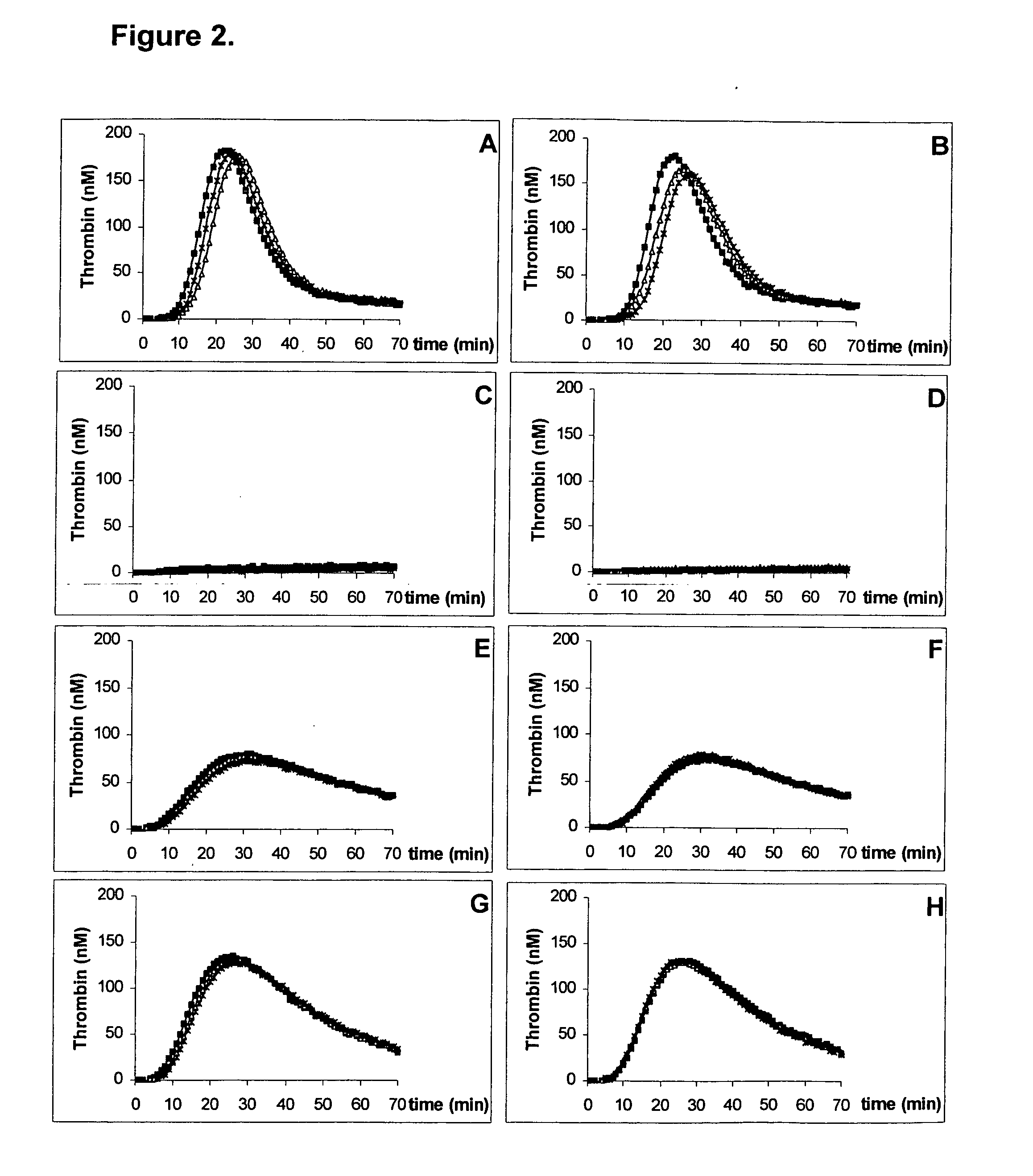
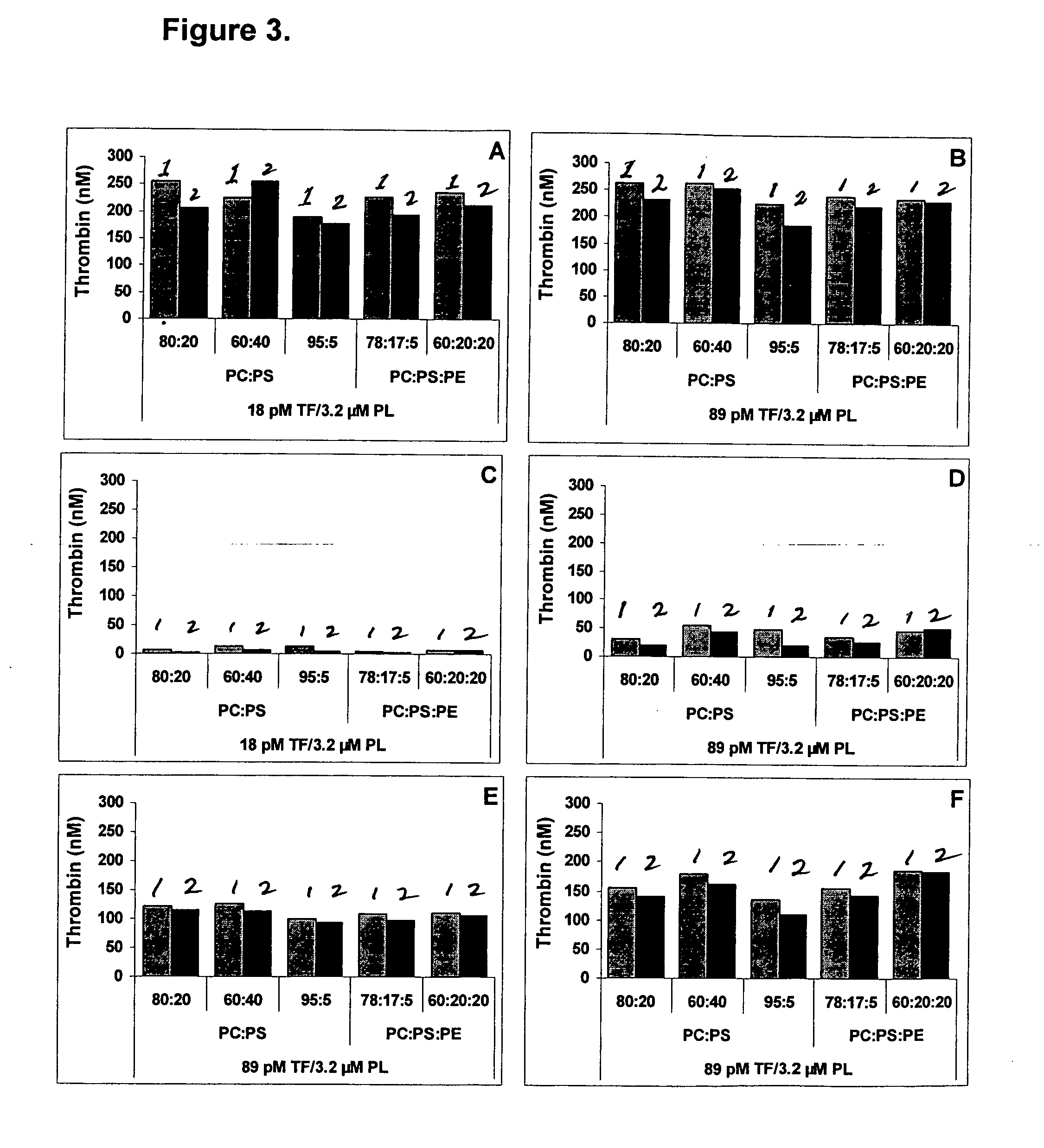
Kit for measuring the thrombin generation in a sample of a patient's blood or plasma
InactiveUS20050221414A1Simple and efficient and fast and reproducible assayConvenient typeMicrobiological testing/measurementBiological material analysisTissue factorThrombin activity
The invention provides a kit for measuring the thrombin generation in a sample of a patient's blood or plasma, or in a sample of clotting factors. The kit contains lyophilized tissue factor / phospholipid-complex and a lyophilized mixture containing a thrombin-substrate and CaCl2. The invention also provides processes for preparing the reagents for the kit. The kit can be used in a method for measuring the thrombin generation in a sample, wherein it is possible to detect changes in thrombin generation kinetics, for example after administration of inhibitor bypassing agents to a patient who has developed inhibitors to an exogenous clotting factor such as Factor VIII.
Owner:BAXTER INT INC +1
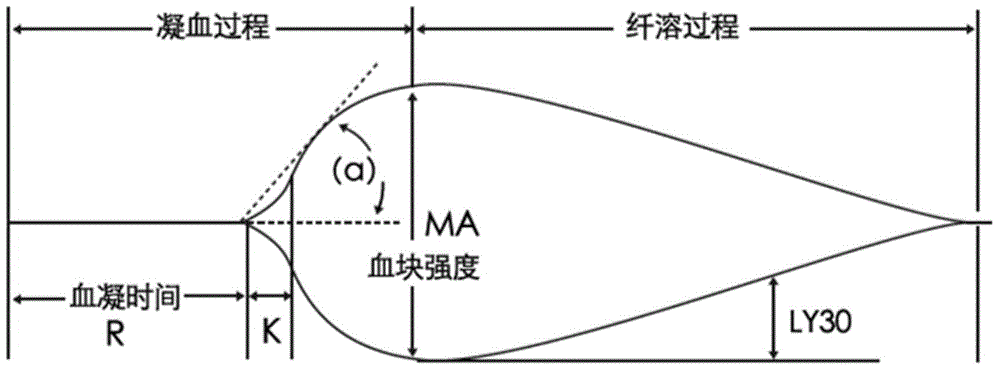
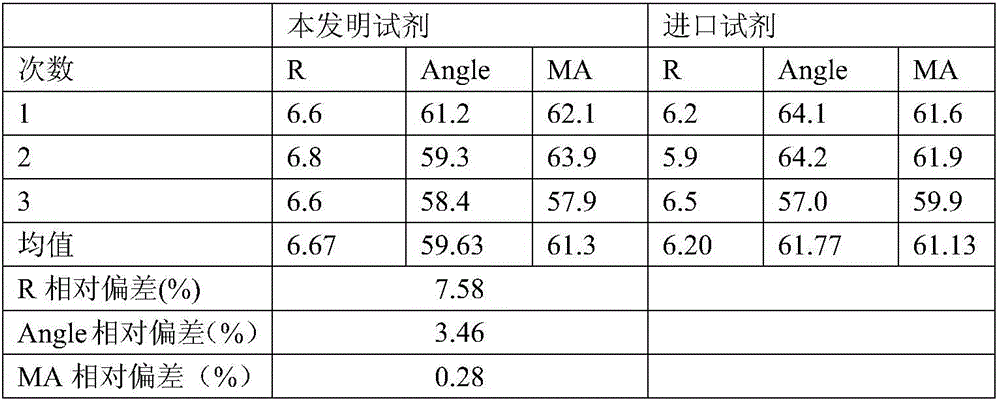
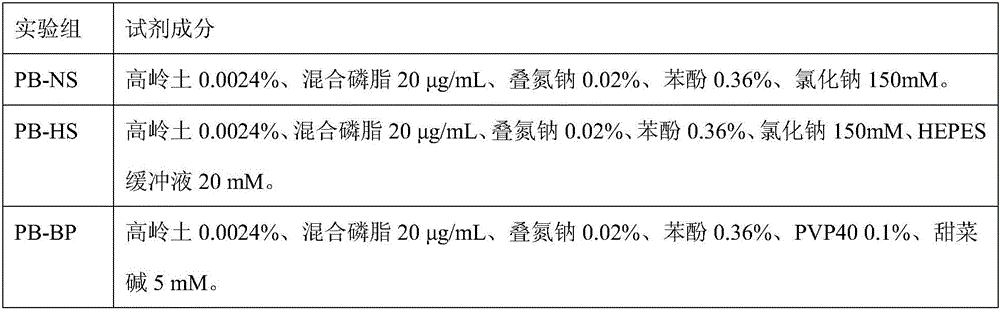
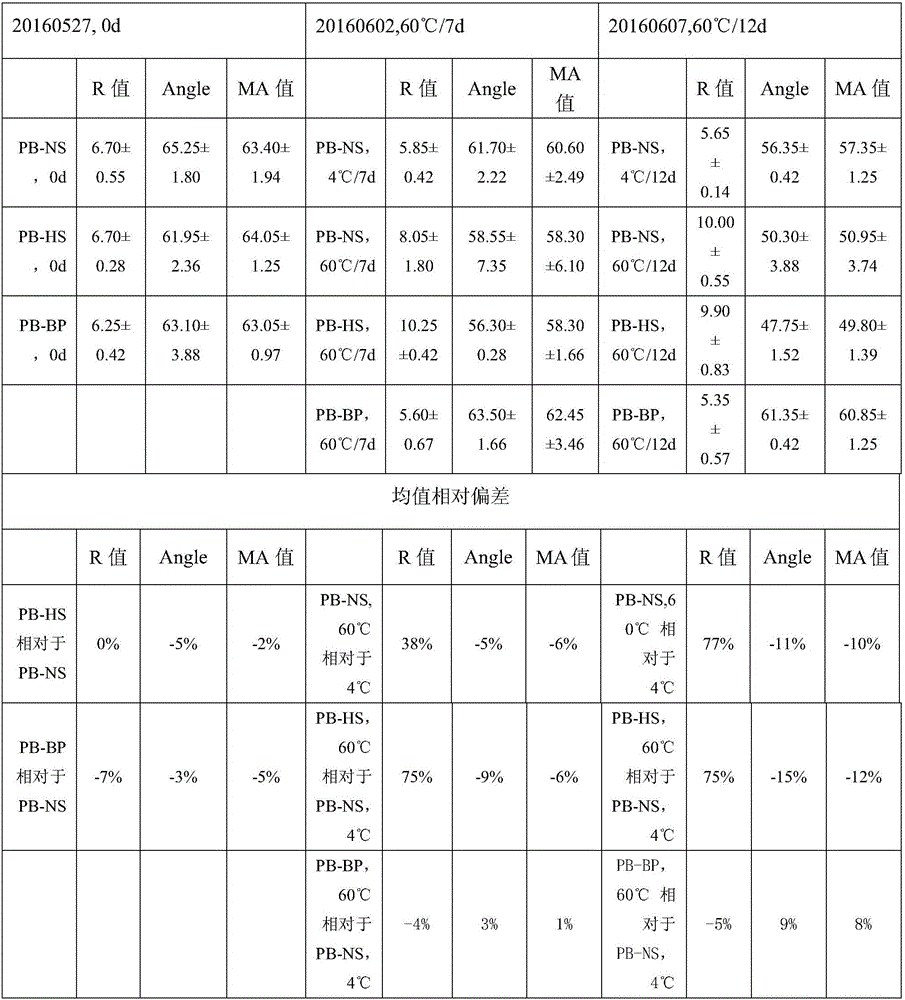
3-level thrombelastogram quality control product and application thereof
The invention relates to the field of quality control of blood coagulation testing items and provides a 3-level thrombelastogram quality control product. The quality control product is prepared by the following steps: respectively mixing pig blood with 3 different concentrations of sodium citrate, centrifuging, taking blood plasma, respectively adding a coagulation activator and a tissue factor and lyophilizing to prepare a powdery quality control product I, adding a coagulation activator and lyophilizing to prepare a powdery quality control product II, and adding a coagulation activator and human lyophilized platelets and lyophilizing to prepare a powdery quality control product III. The 3-level thrombelastogram quality control product provided by the invention is shaped as a lyophilized powder, can be used to monitor R and MA values simultaneously, is better used for quality control of an thromboelastography instrument and a thrombelastogram detection kit, is fast to detect, has accurate results and is simple to operate.
Owner:北京乐普诊断科技股份有限公司
Human antibodies against tissue factor and methods of use thereof
ActiveUS9150658B2Immunoglobulins against blood coagulation factorsAntipyreticTissue factorMonoclonal antibody
Isolated human monoclonal antibodies which bind to human TF and related antibody-based compositions and molecules, are disclosed. Also disclosed are pharmaceutical compositions comprising the antibodies, and therapeutic and diagnostic methods for using the antibodies.
Owner:GENMAB AS
Prothrombin time test kit and preparation method thereof
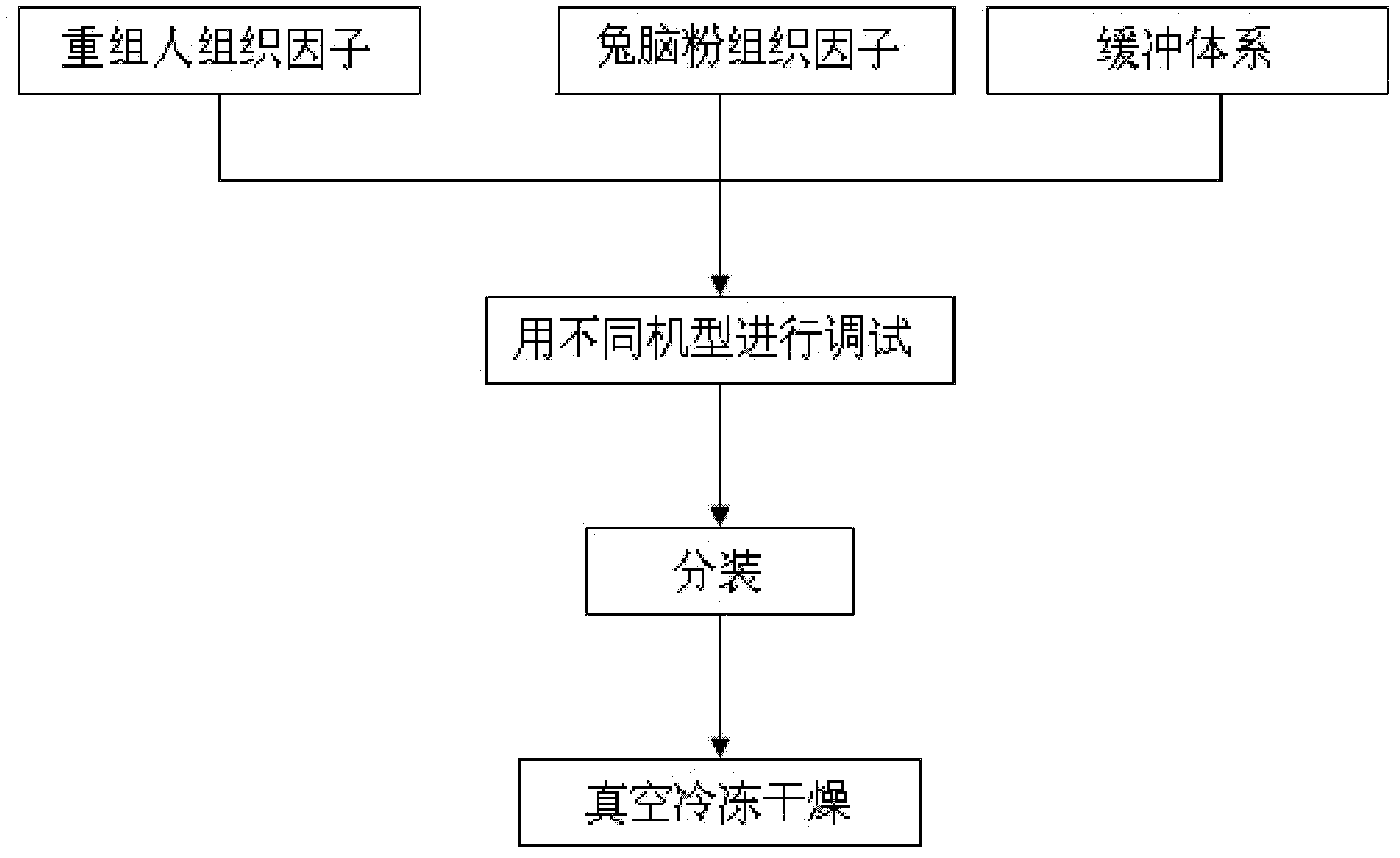
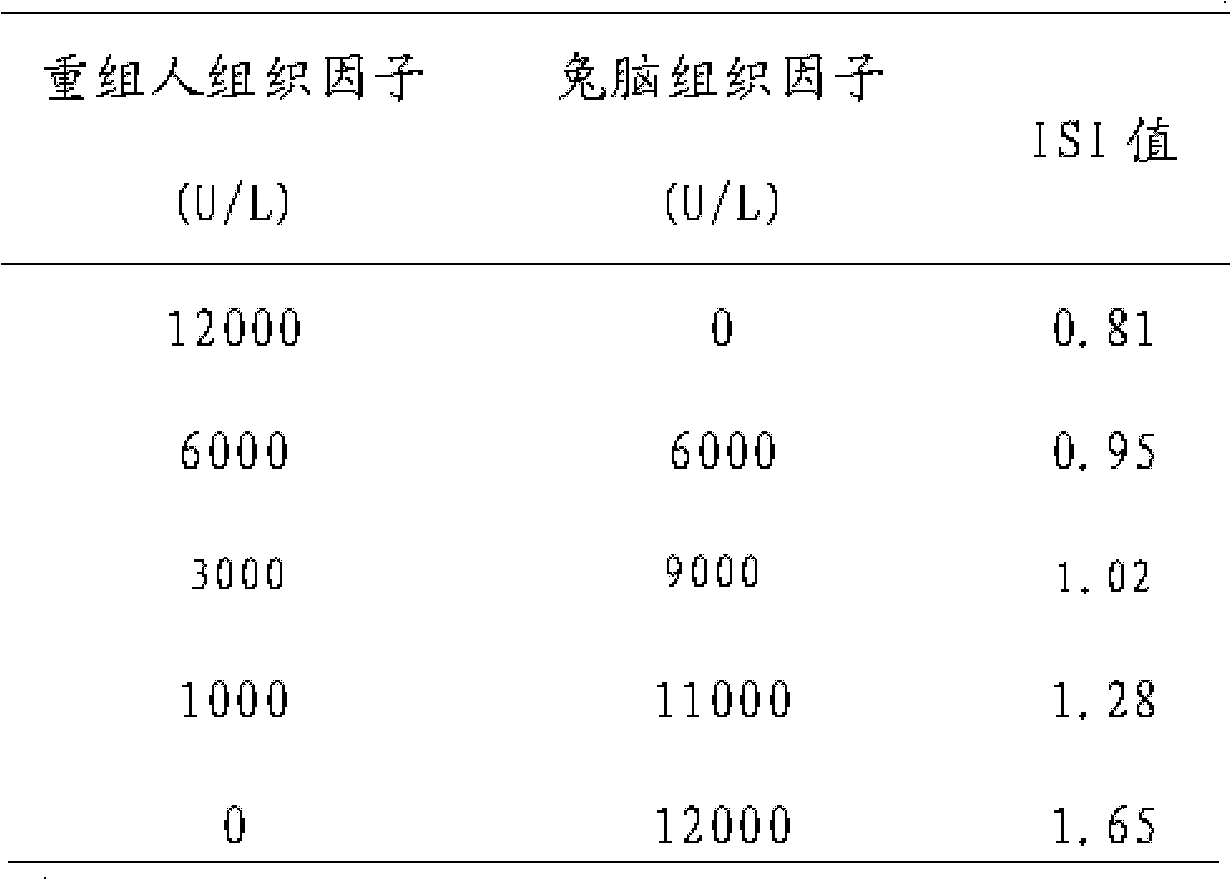
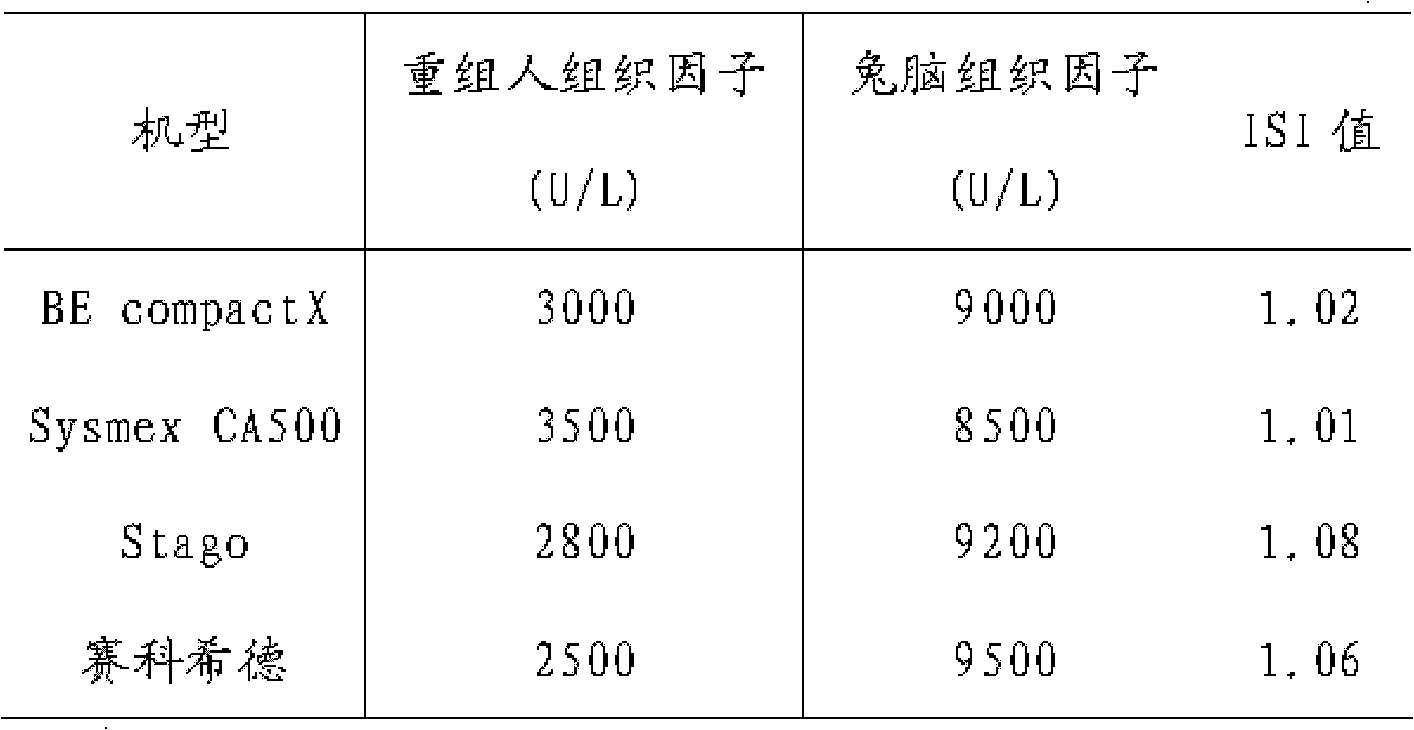
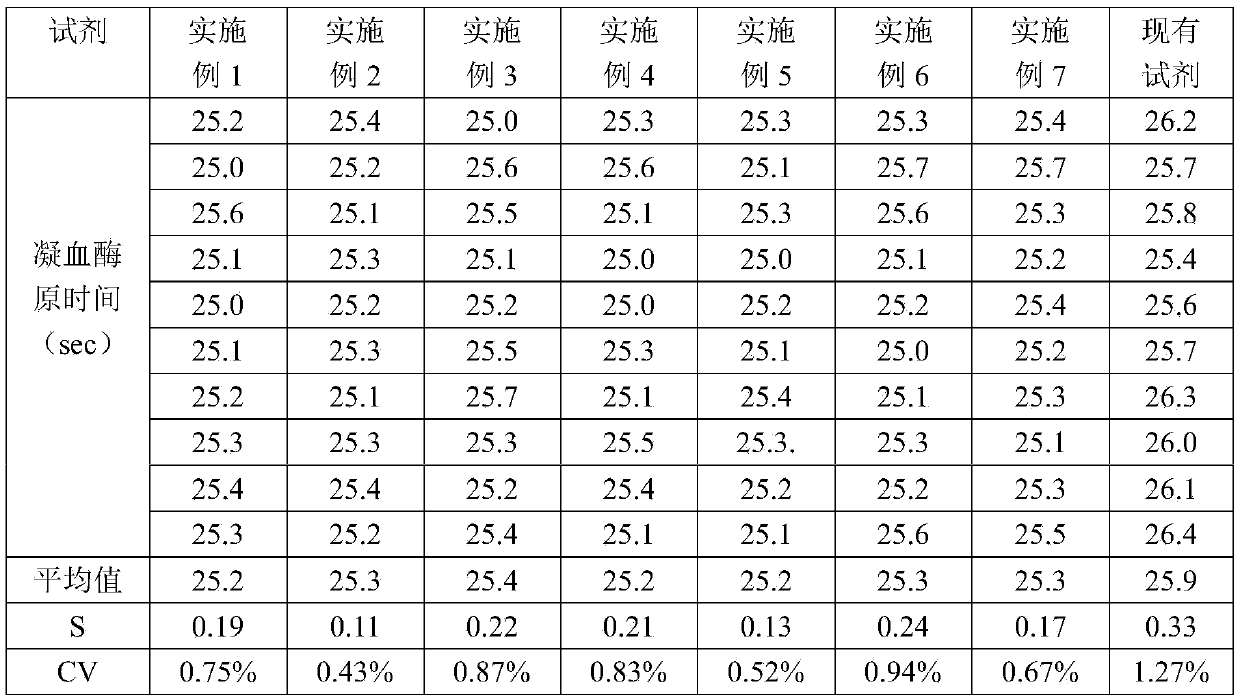
A prothrombin time test kit consists of thromboplastin and a buffer liquid system, wherein the thromboplastin is rabbit brain tissue factors and recombinant tissue factors. The buffer liquid system comprises 30-50mM Tris-HCL, 3-10% of glycine, 1-5% of bovine serum albumin, 0.6-1.3% of sodium chloride, 6.8-8.4mM of calcium chloride and 30.5% of NaN, and the pH value of the buffer liquid system is 6.0-8.0. The thromboplastin also comprises rabbit cephalin. According to the invention, the defects that the sensitiveness indexes of various instruments are different due to the differences of the methods of the different instruments can be overcome, the high-sensitivity human recombinant thromboplastin and low-sensitivity rabbit brain powder extracting thromboplastin are combined, the PT reagent capable of meeting different methods of coagulometer can be prepared according to certain proportion, and the ISI (insulin sensitivity index) value is close to 1.0. The invention also discloses a preparation method of the prothrombin time test kit simultaneously.
Owner:武汉塞力斯生物技术有限公司
Hepaplastin test determination kit and preparation method thereof
InactiveCN105372424AImprove stabilityImprove accuracyMaterial analysisTissue factorBlood Coagulation Factor VII
The invention discloses a hepaplastin test determination kit and a preparation method thereof. Through combination of a buffer solution system a containing multiple effective components, plasma without blood coagulation factors VII, II, X and IX, a buffer system b, rabbit brain powder and tissue factor ester, the hepaplastin test determination kit improves reagent stability. A hepaplastin test determination reagent with good accuracy, good repeatability and good effects is further obtained.
Owner:WUHAN KING DIAGNOSTIC TECH CO LTD
Activated coagulation detection reagent and application thereof
The invention discloses an activated coagulation detection reagent and an application thereof. The reagent is prepared from the following components: an activating agent, mixed phospholipid and composite stabilizer, wherein the activating agent comprises one or more of kaolin, porcellanite, diatomite, ellagic acid, silicon dioxide and a tissue factor, and the composite stabilizer comprises a polymer, an anticorrosive agent and a water soluble antioxidant. The detection reagent does not use a buffer solution adopted in the prior art and does not use normal saline. By combining the activating agent, the mixed phospholipid and the composite stabilizer, the composite stabilizer is used as a solution environment, so that the stability of the detection reagent can be guaranteed. The detection reagent can effectively avoid the adverse influence of the buffer solution used in the prior art for promoting the hydrolysis of the phospholipid; and moreover, the activated coagulation detection reagent is low in cost, good in effect and stable in properties.
Owner:重庆鼎润医疗器械有限责任公司
Microvesicles derived from recombinant yeast having haemostatic activities and uses thereof
Tissue factor-bearing yeast derived microvesicles comprising a yeast membrane and a tissue factor protein, or a fragment thereof, or a tissue factor protein or a fragment thereof fused to another peptide as a fusion protein having pro-coagulant activity are disclosed. Said products can be used as pro-coagulant agents in the treatment of hemorrhages in a subject.
Owner:THROMBOTARGETS EURO SL
Liquid-phase chip kit for acute coronary syndrome and preparation method for same
InactiveCN103163295AComprehensive and comprehensive assessment of prognosisImprove detection efficiencyMaterial analysisA lipoproteinMicrosphere
The invention relates to a liquid-phase chip kit for acute coronary syndrome and a preparation method for the same. A liquid-phase chip mainly comprises microspheres coated with oxidized low density lipoprotein (ox-LDL), soluble CD40 ligand (sCD40L), matrix metallopeptidase 9 (MMP-9), tissue factor (TF), omentin and endogenous secretory receptor of advanced glycation end products (esRAGE) capture antibody respectively, detection antibodies labelled by biotin, and streptavidin phycoerythrin. The liquid-phase chip provided by the invention has the advantages of being high in detection efficiency, low in the quantity of the needed samples, strong in specificity, high in sensitivity, rapid and accurate in detection, and the like. Simultaneously, the chip reflects a protective factor level and an injury factor level in an organism, so that the risk stratification and prognosis conditions of a patient can be much comprehensively assessed. Simultaneously, the preparation method disclosed by the invention is simple and practicable, and good in stability, various process parameters such as the quantities of the microspheres and the antibodies, and the reaction process in the technical scheme of the preparation method are obtained on the basis of lots of experiments, and are the optimal parameter values of the preparation process.
Owner:吴宗贵 +2
Targeting Tissue Factor To Activated Platelets
ActiveUS20160272710A1Peptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsTissue factorPlatelet
The current invention relates to procoagulant fusion proteins, polynucleotides that encode said fusion proteins and cells that expresses said fusion proteins. Furthermore, the current invention relates to fusion proteins for use as a medicament. Individuals that have a coagulopathy, such as haemophilia A and B with or without inhibitors, may be treated with fusions proteins of the current invention.
Owner:NOVO NORDISK AS
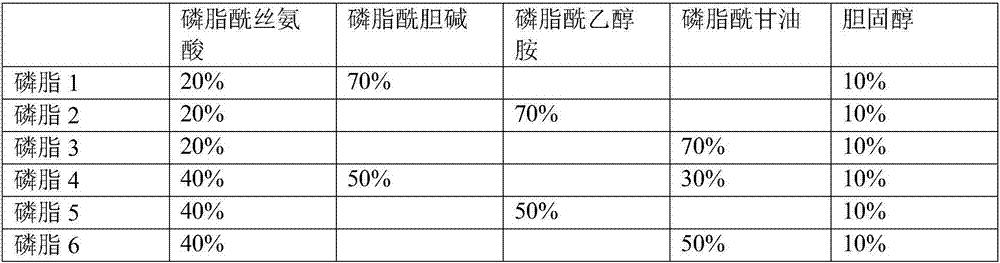
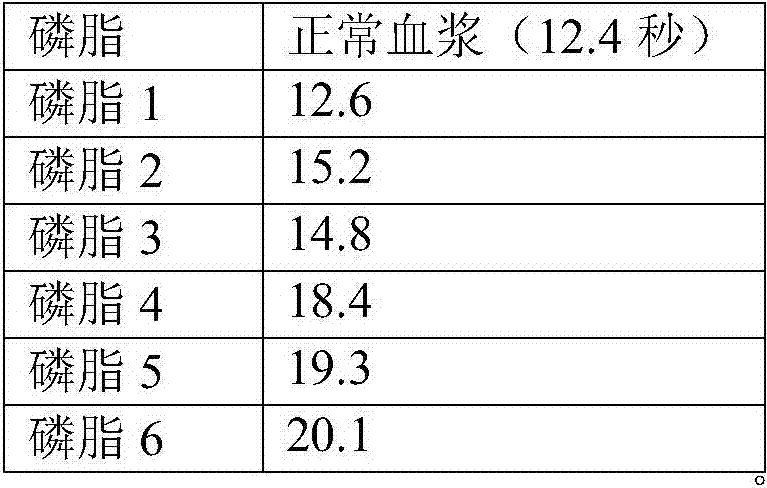
Liquid ready-to-use prothrombin time detection reagent
InactiveCN107356768AOvercome the difference between bottlesOvercome the defect of large batch differenceBiological testingTissue factorCholesterol
The invention discloses a liquid ready-to-use prothrombin time detection reagent, which includes a buffer, a synthetic phospholipid, a recombinant rabbit tissue factor, a surfactant and a stabilizer. The synthetic phospholipid is composed of phosphatidylserine, phosphatidylcholine and Cholesterol composition. The present invention uses rabbit recombinant factors and synthetic phospholipids to prepare prothrombin time detection reagents by selecting synthetic phospholipid components and optimizing stabilizers. It does not need to be reconstituted during use and can be used immediately after opening the bottle. The reagent overcomes the problem of difficult-to-control batch-to-batch variation of existing prothrombin time detection reagents and has high sensitivity, good stability, small batch-to-batch variation, easy quality control, and easy production.
Owner:NINGBO ACCUTECH BIOSCI LTD
Neovascular-targeted immunoconjugates
Immunoconjugates for treating diseases associated with neovascularization such as cancer, rheumatoid arthritis, the exudative form of macular degeneration, and atherosclerosis are described. The immunoconjugates typically consist of the Fc region of a human IgG1 immunoglobulin including the hinge, or other effector domain or domains that can elicit, when administered to a patient, a cytolytic immune response or cytotoxic effect against a targeted cell. The effector domain is conjugated to a targeting domain which comprises a factor VII mutant that binds with high affinity and specificity to tissue factor but does not initiate blood clotting such as factor VII having a substitution of alanine for lysine-341 or of alanine for serine-344.
Owner:YALE UNIV
Global test of the hemostatic system
ActiveUS7235377B2Large spreadIndividual variance is lowMicrobiological testing/measurementDisease diagnosisTissue factorMedicine
Owner:UNIVERSITY OF VERMONT
Fusion protein of tumor blood vessel targeted polypeptide and tissue factor and preparation method thereof
InactiveCN102153653AImprove anti-tumor effectGrowth inhibitionPeptide/protein ingredientsMacromolecular non-active ingredientsFermentationBlood vessel
The invention provides the expression and the purification of a recombinant fusion protein, particularly a fusion protein of the tumor blood vessel targeted polypeptide and tissue factor and a preparation method and application thereof. The preparation method of the fusion protein of the tumor blood vessel targeted polypeptide and tissue factor comprises the following steps: designing primers, carrying out PCR (polymerase chain reaction) to amplify an recombinant gene of the fusion protein EG3287-tTF, guiding the recombinant gene of the fusion protein EG3287-tTF into a carrier, guiding a recombinant carrier into a host cell, constructing a recombinant engineering bacteria for expressing the fusion protein EG3287-tTF, fermenting to culture, and separating and purifying fermentation liquor to obtain the fusion protein of the tumor blood vessel targeted polypeptide and tissue factor. The targeted antitumor fusion protein EG3287-tTF is constructed, a novel tTF derivative with the antitumor advantage of the tTF and the targeting effect is obtained, the antitumor effect of the tTF is improved, and a foundation is laid to the development of a novel medicament for the targeted therapy of the tumor blood vessel.
Owner:XIAMEN UNIV
Chimeric proteins with phosphatidylserine binding domains
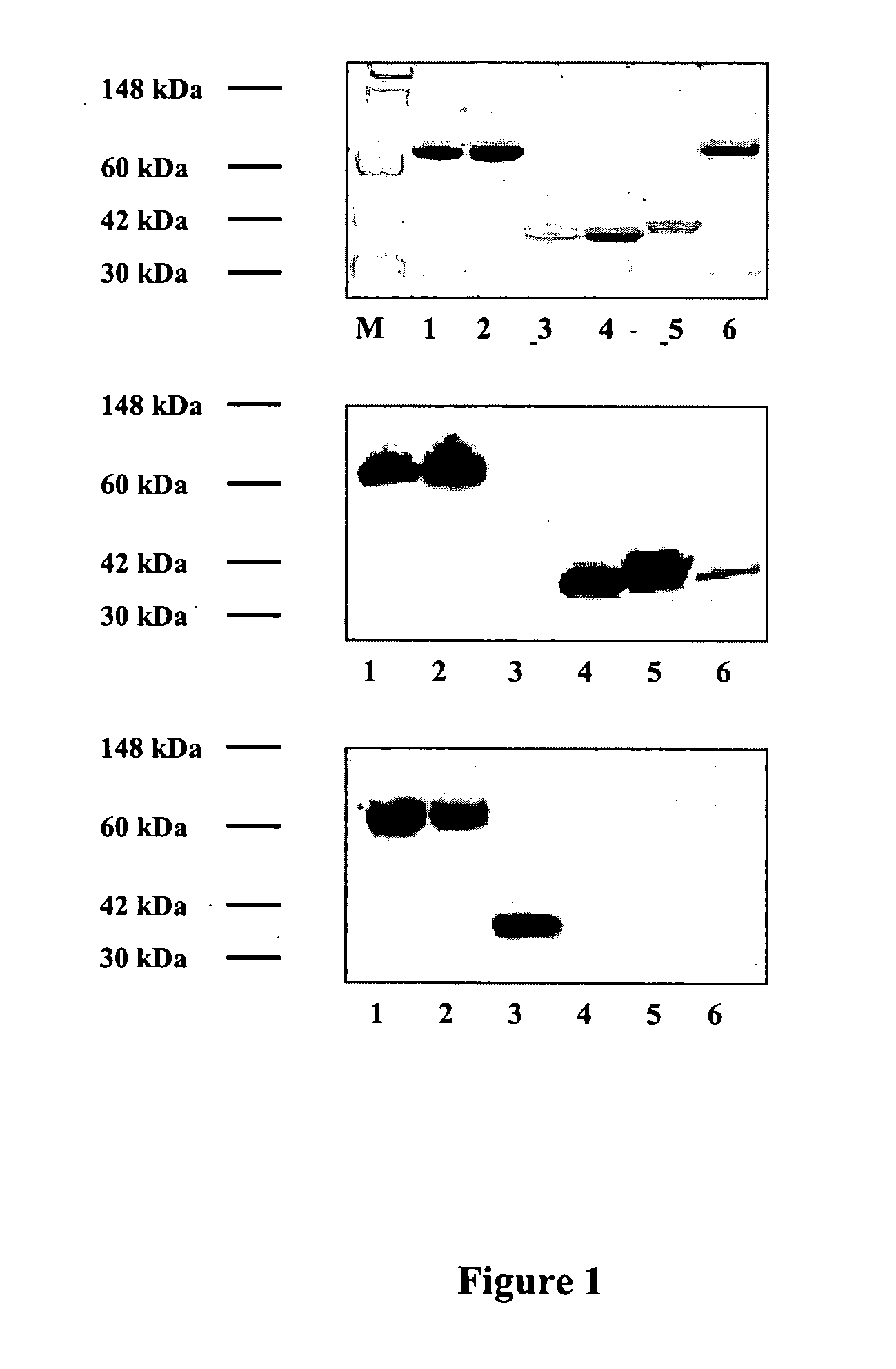
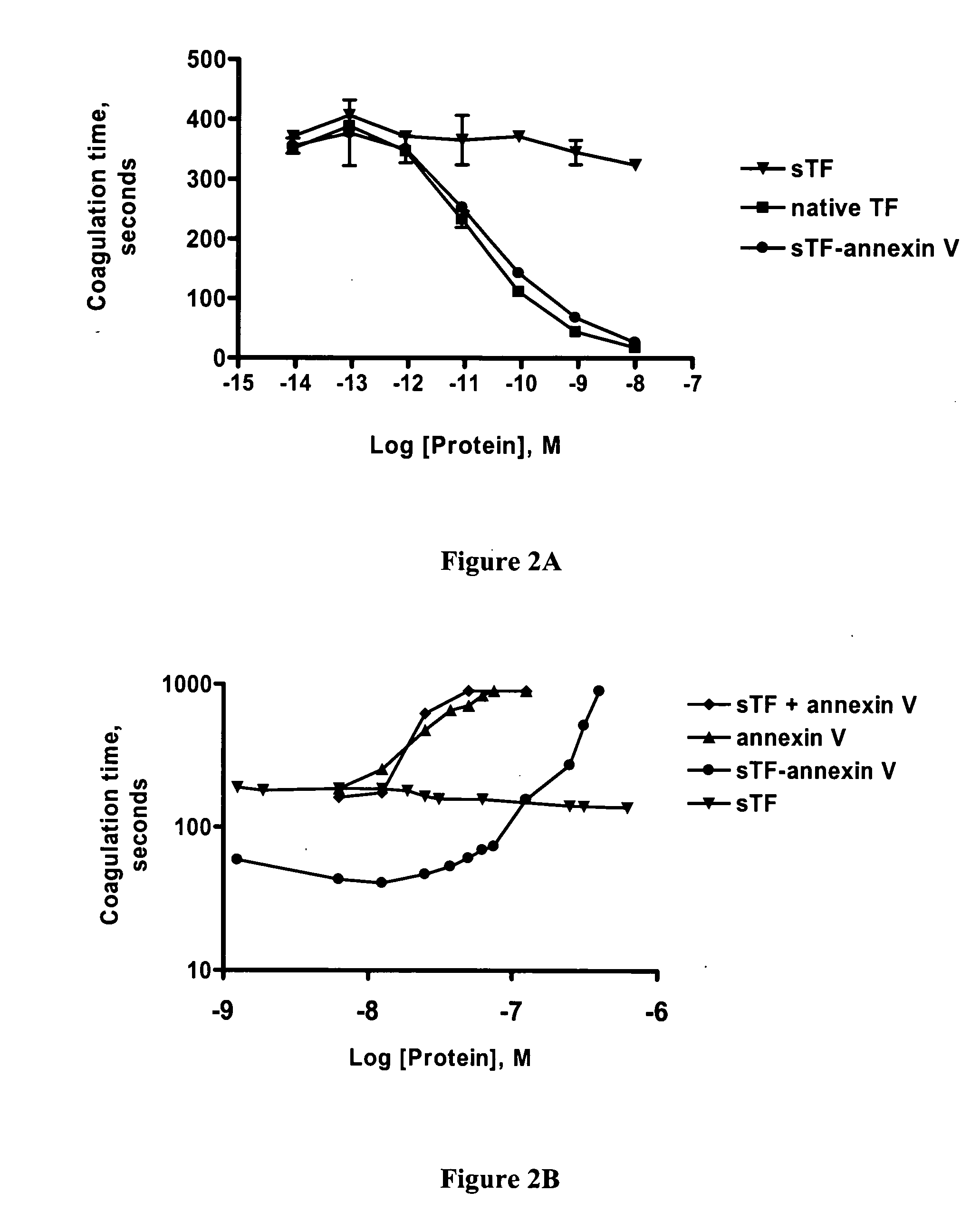
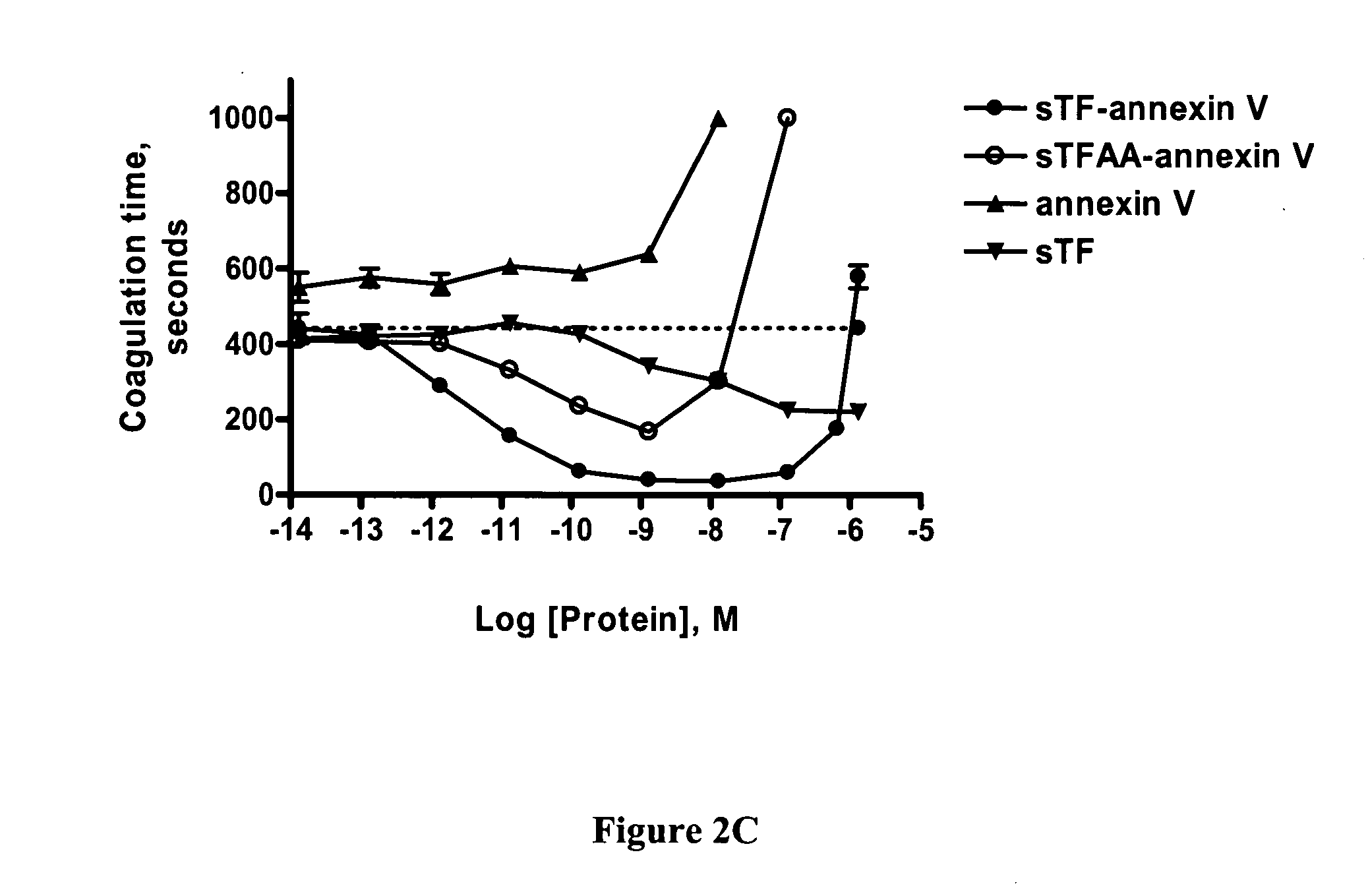
Chimeric proteins comprising soluble Tissue Factor (sTF) and another subunit (e.g., annexin V) are described. The proteins promote blood clotting and / or inhibit cancer by targeting sTF to specific receptors such as phosphatidyl serine (PS) on activated cells. These chimeric proteins are useful in treating patients with excessive bleeding due to inborn problems, drug therapy, trauma or surgery and / or as an anti-cancer therapy, for example by causing blood vessels feeding cancers to become clotted, thereby preventing adequate flow of blood to a tumor, which in turn will lead to tumor inhibition and death or may be used in a therapy to cause clotting within blood vessels that pose a threat in the subject in non-cancerous conditions.
Owner:THE BOARD OF RGT UNIV OF OKLAHOMA
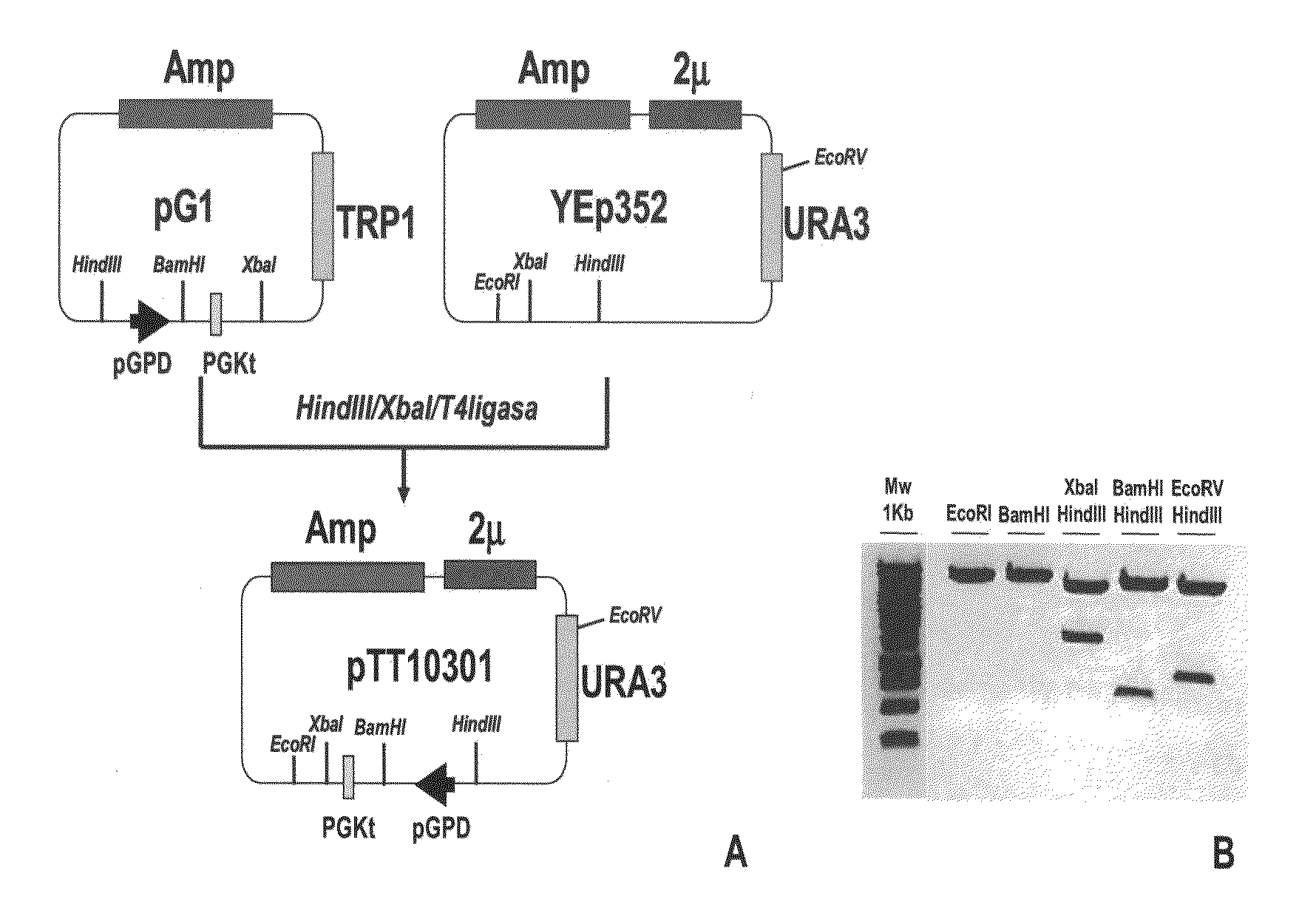
Method for constructing, expressing and purifying human recombination factor and application
InactiveCN1687125AImprove expression rateHigh purityMicrobiological testing/measurementDepsipeptidesEscherichia coliPurification methods
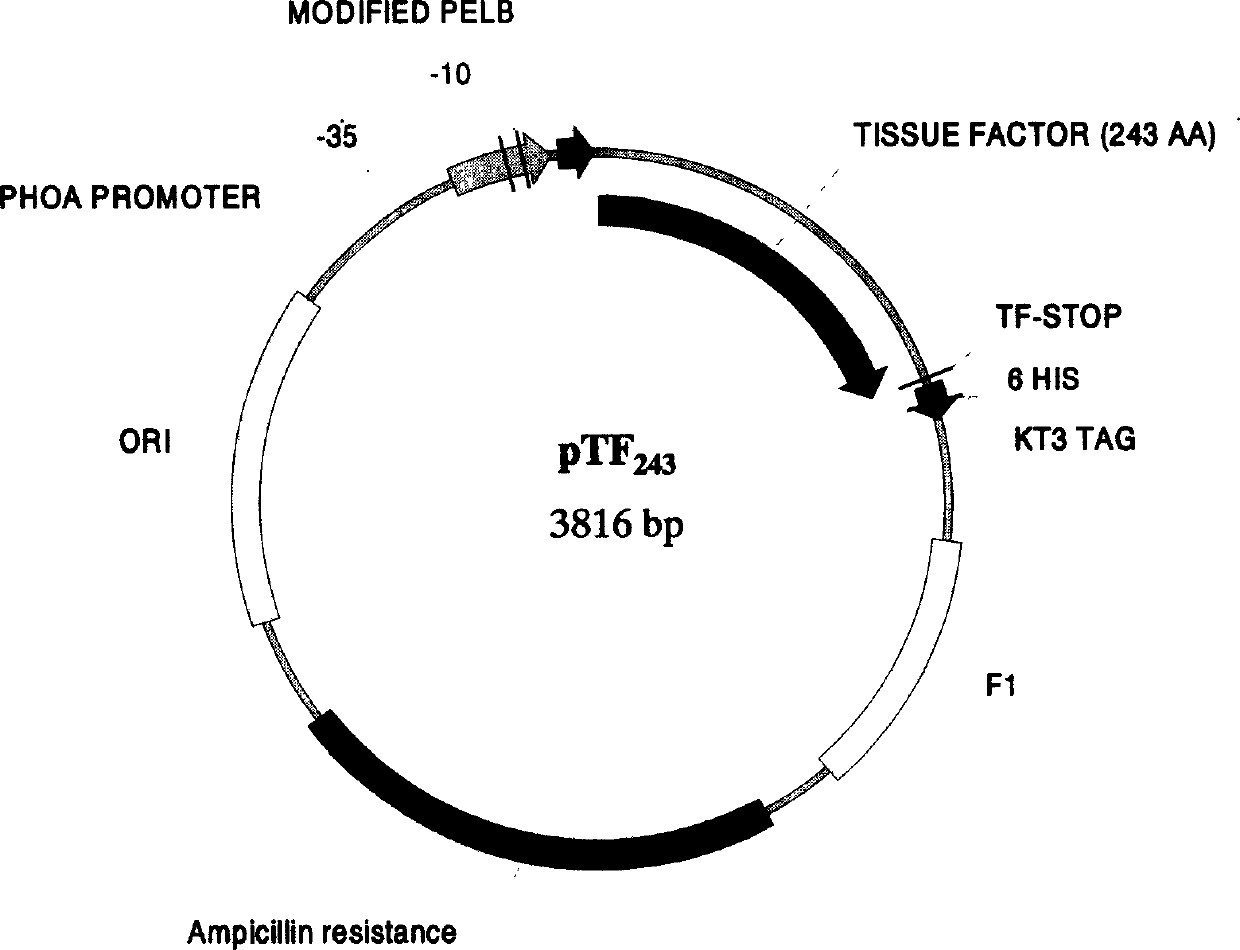
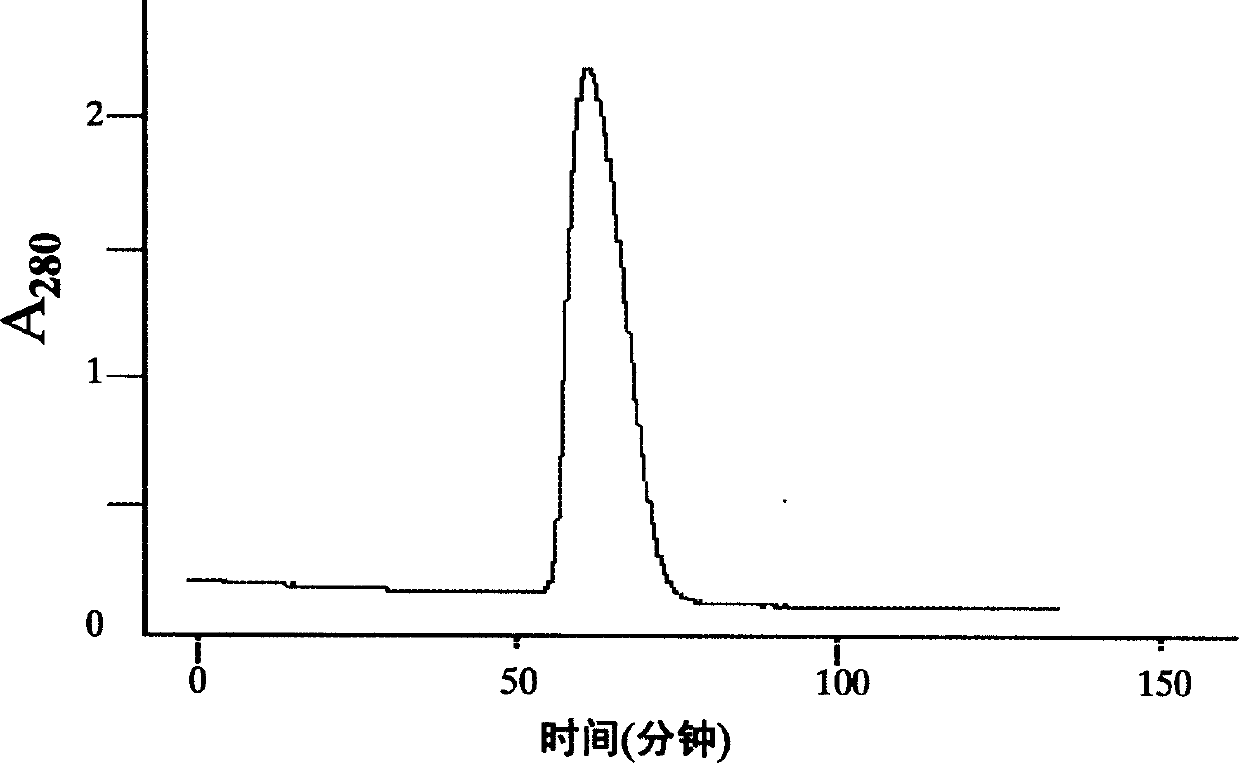
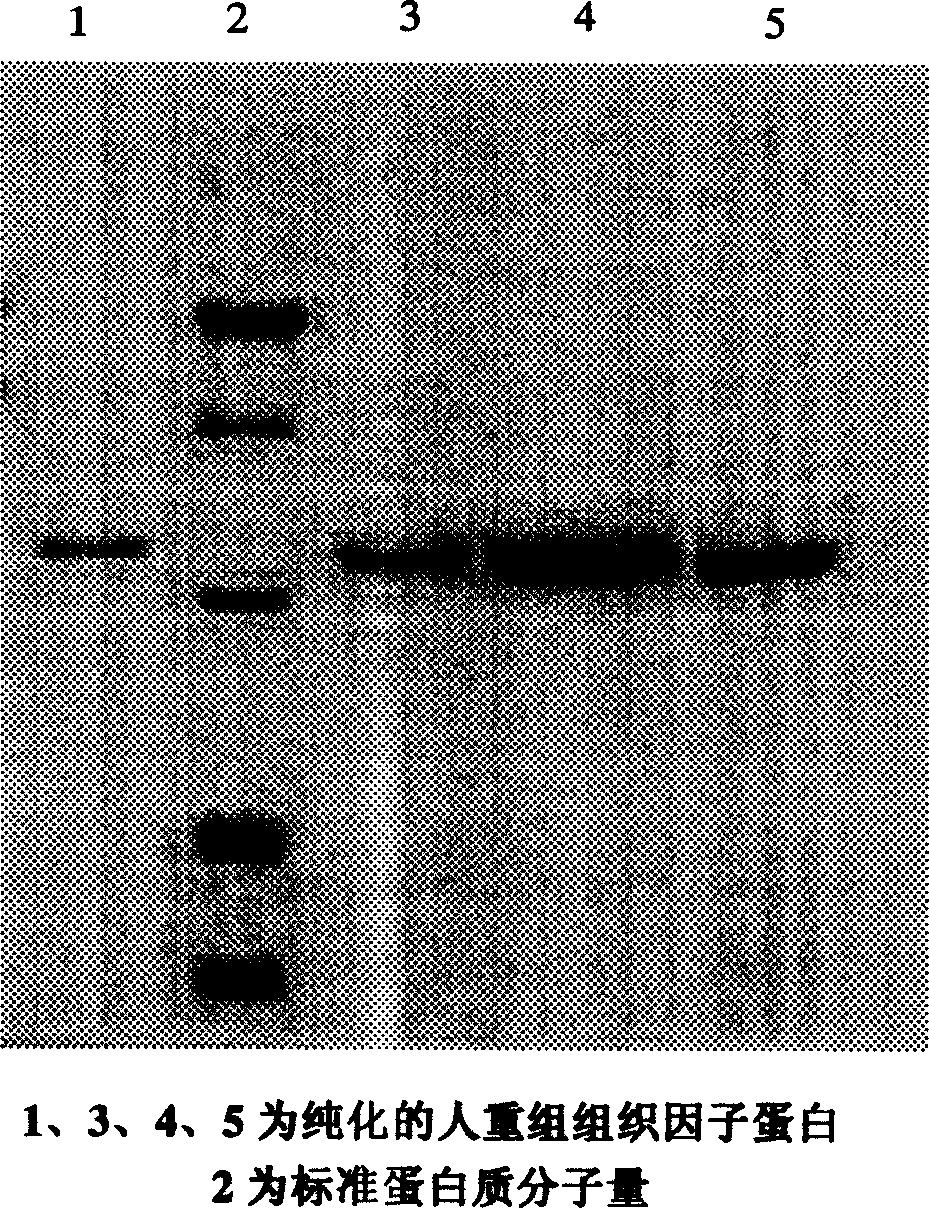
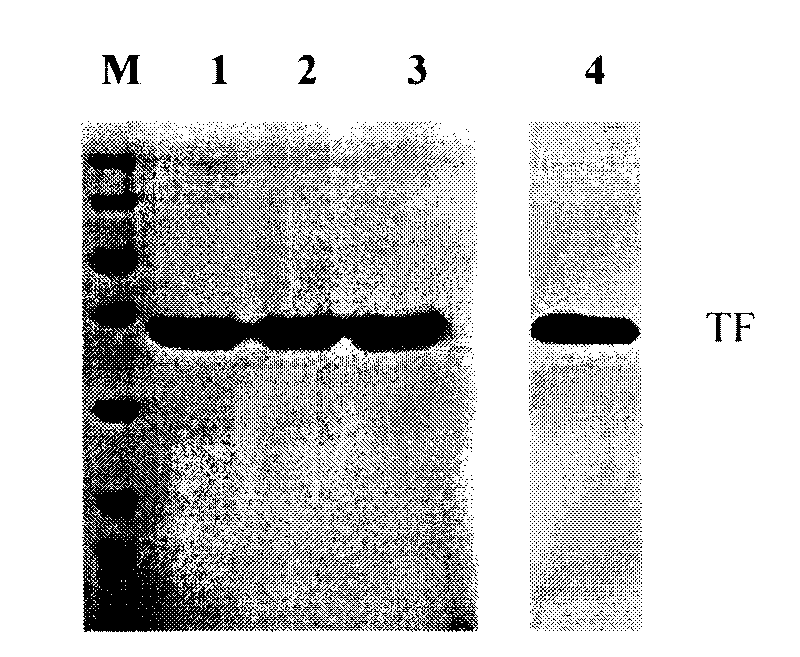
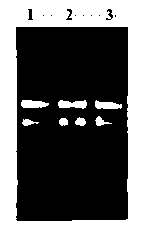
The present invention relates to a human recombinant tissue factor, its construction, expression and purification method and application. Said method includes the following steps: recombining TF gene fragment obtained from human placenta with phoA plasmid, constructing plasmid vector pTF243, transforming it into colibacillus MM294, screening and obtaining engineering strain, inoculating said strain into culture medium to directly make fermentation and expression to obtain bacterial liquor, then using Q-Sepharose Fast Flow matrix to make the bacterial liquor under the process of ion exchange chromatography, then pass through TF monoclonal antibody immunoaffinity chromatographic column to make adsorption and elution so as to obtain the rhTF243 protein.
Owner:山西省博奥特医学检验有限公司
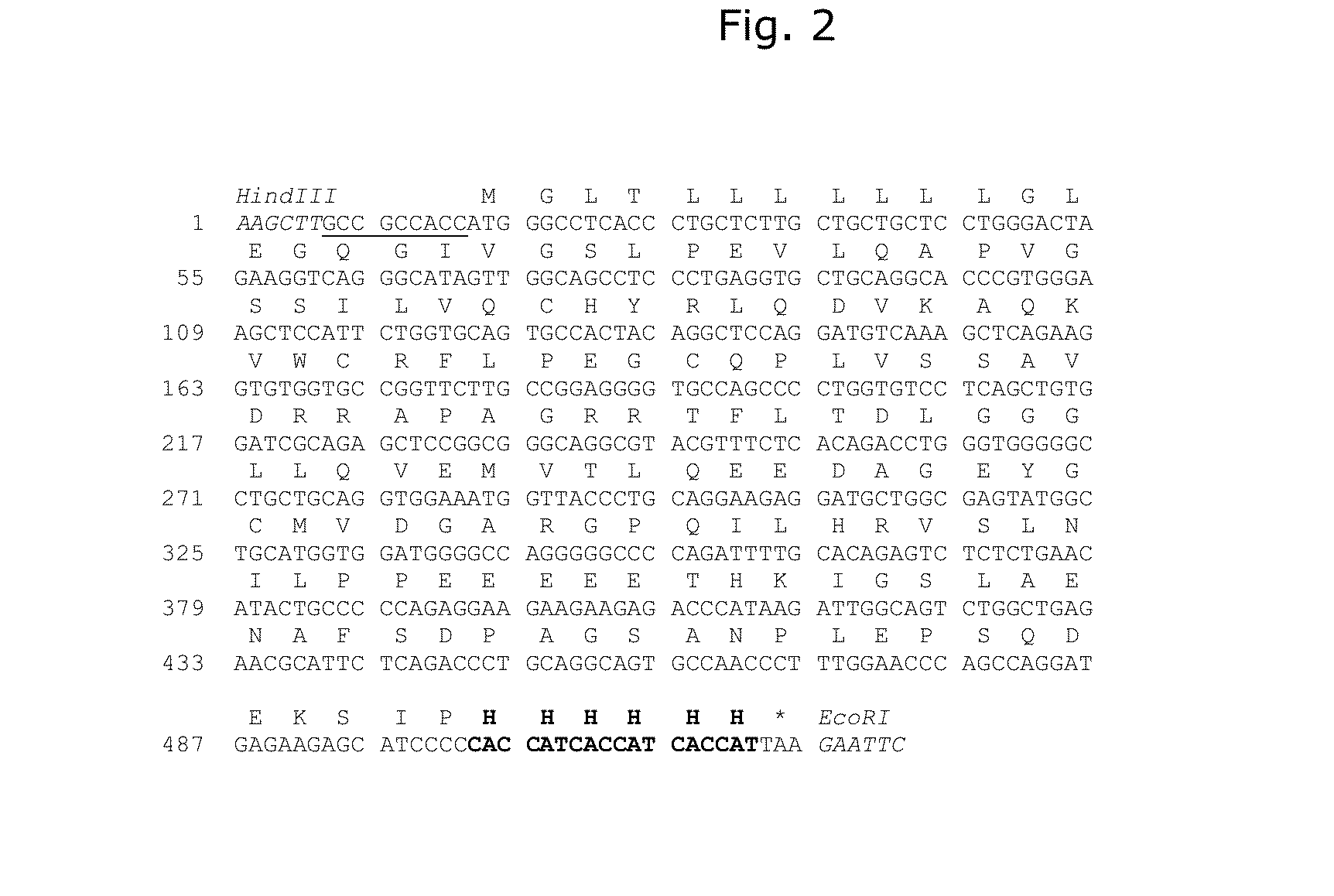
Methods and Deoxyribonucleic acid for the preparation of tissue factor protein
InactiveUS7084251B1Difficult to identifyIsolation of difficultDepsipeptidesPeptide preparation methodsTissue factorCoagulation Disorder
DNA isolates coding for tissue factor protein and methods of obtaining such DNA and producing tissue factor protein using recombinant expression systems for use in therapeutic composition for the treatment of coagulation disorders.
Owner:GENENTECH INC
Electrochemical method-based PT (Prothrombin Time) test card and preparation method thereof
InactiveCN106680339ALow skill level requiredEasy to prepareMaterial electrochemical variablesTissue factorOperability
The invention discloses an electrochemical method-based PT (Prothrombin Time) test card and a preparation method thereof. The electrochemical method-based PT test card is formed by an electric conduction substrate, a chemical reagent and the like, wherein the chemical reagent is specifically prepared from the main components of tissue factors, phospholipid and the like. The electrochemical method-based PT test card prepared by the invention is capable of realizing PT detection on fingertip blood or a blood sample which is not subjected to blood coagulation treatment, and has the advantages of high accuracy, good repeatability and the like; the preparation method is simple, has low technical requirements on equipment and operators, is strong in operability and has a wide application prospect in the fields of clinical examination and the like.
Owner:北京乐普诊断科技股份有限公司
Kit for measuring the thrombin generation in a sample of a sample of a patient's blood or plasma
ActiveUS20130052672A1Rapid diagnosisSimple and efficient and fast and reproducible assayMicrobiological testing/measurementBiological material analysisTissue factorPlasma samples
The invention provides a kit for measuring the thrombin generation in a sample of a patient's blood or plasma, or in a sample of clotting factors. The kit contains lyophilized tissue factor / phospholipid-complex and a lyophilized mixture containing a thrombin-substrate and CaCl2. The invention also provides processes for preparing the reagents for the kit. The kit can be used in a method for measuring the thrombin generation in a sample, wherein it is possible to detect changes in thrombin generation kinetics, for example after administration of inhibitor bypassing agents to a patient who has developed inhibitors to an exogenous clotting factor such as Factor VIII.
Owner:TAKEDA PHARMA CO LTD
Method for preparing antihuman recombinant tissue factor monoclonal antibody
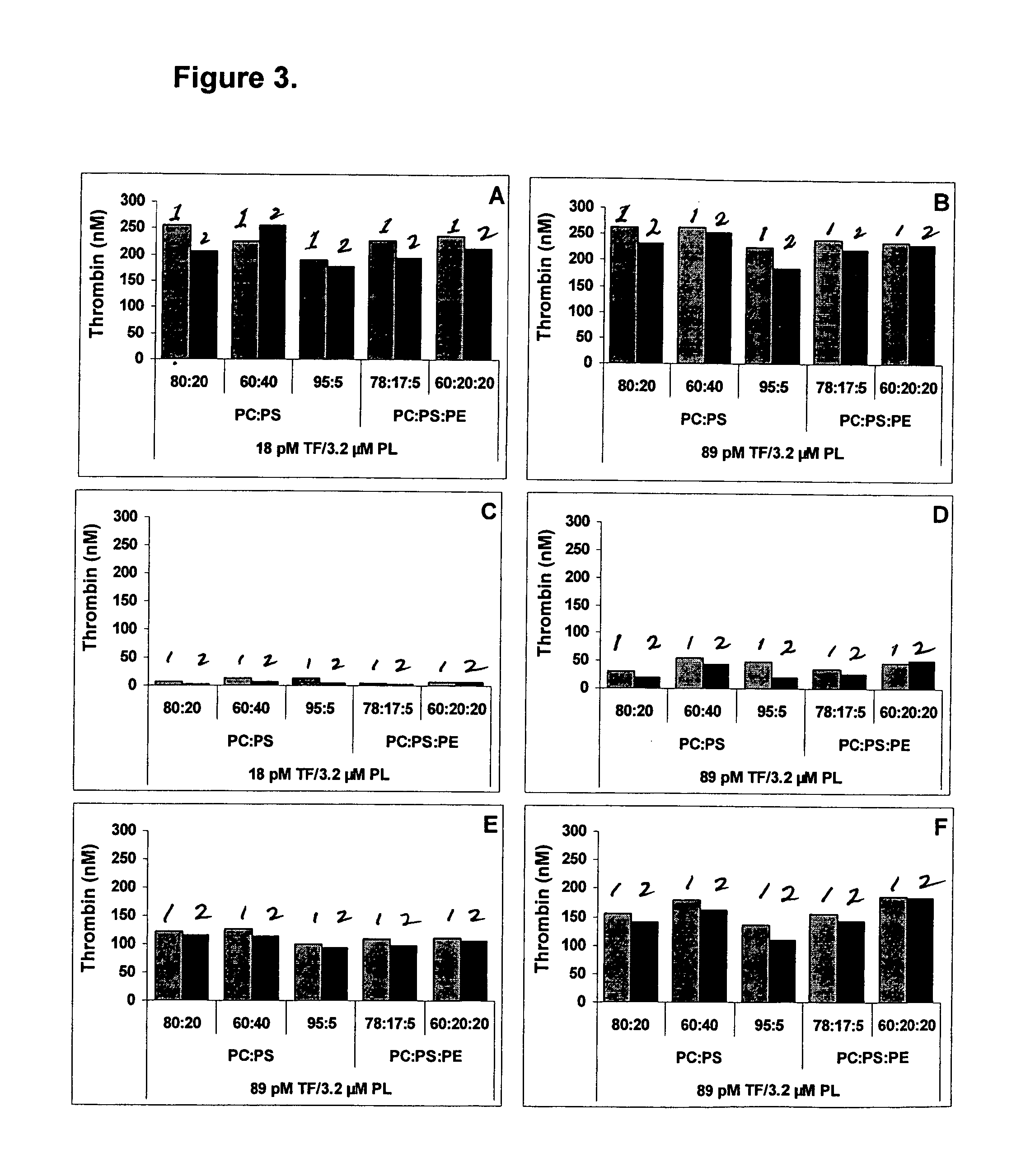
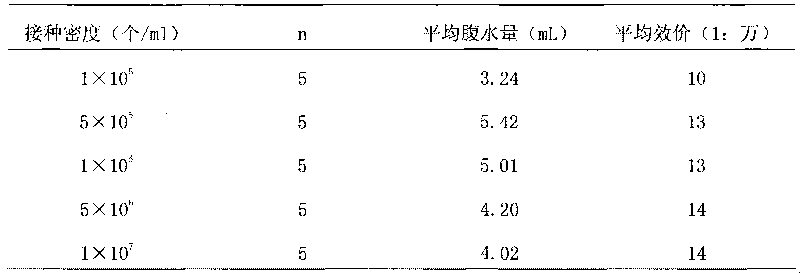
ActiveCN101717447AStrong specificityEasy to prepareImmunoglobulins against cell receptors/antigens/surface-determinantsFreund adjuvantElisa method
The invention discloses a method for preparing an antihuman recombinant tissue factor monoclonal antibody. The method comprises the following steps: combining a truncated human recombinant tissue factor obtained by purification after prokaryotic expression with a freund adjuvant immunized mouse; fusing mouse spleen cells with myeloma cells; selectively cultivating and screening an HAT selective medium; after the subcloning cultivation is subjected to limiting dilution, selecting target hybridoma cells by an ELISA method, performing massive proliferation, and injecting into an abdominal cavity of a syngeneic mouse to prepare ascites; and obtaining the antihuman recombinant tissue factor monoclonal antibody which has excellent potency and higher yield. The antihuman recombinant tissue factor monoclonal antibody prepared by the method of the invention has the advantages of high sensitivity and strong specificity, can be used for immunoaffinity purification of the human recombinant tissue factor, and also can be used for research and development of anticoagulant medicaments and medicaments for treating tumors.
Owner:山西省生物研究院有限公司 +1
Anti-human tissue factor single-chain antibody and preparation method thereof
ActiveCN103342752AAchieve soluble expressionStrong penetrating powerImmunoglobulins against cell receptors/antigens/surface-determinantsFermentationSingle-Chain AntibodiesNucleotide
The invention provides an anti-human tissue factor single-chain antibody and a preparation method thereof. The antibody has characteristics of small molecular weight, strong penetrating force for tumor tissues and low immunogenicity. The single-chain antibody is prepared by using a reverse transcription polymerase chain reaction technology, comprising starting from anti-tissue factor hybridoma cell lines prepared in a Chinese patent CN200910227865.0, amplifying to obtain a light chain variable range gene VL and a heavy chain variable range gene VH of the antibody, and using a linker sequence to link, thereby obtaining the single-chain antibody. A nucleotide sequence of the single-chain antibody is as shown in a SEQIDNO: 1. The preparation method of the single-chain antibody comprises: (1) gene clone of the anti-human tissue factor single-chain antibody; (2) construction of a soluble prokaryotic expression vector for the anti-human tissue factor single-chain antibody; (3) expression and purification of the anti-human tissue factor single-chain antibody in MM294 bacteria; and (4) active identification of the anti-human tissue factor single-chain antibody.
Owner:NANOLATTIX BIOTECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com