Liquid ready-to-use prothrombin time detection reagent
A technology for prothrombin time and detection reagents, which is applied in the field of biomedical diagnosis, can solve the problems of large batch-to-batch variation and freeze-dried powder preparation bottle-to-bottle variation, and achieves good stability, easy quality control, and high sensitivity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] The preparation of embodiment 1 prothrombin time detection reagent
[0019] (1) Lipidation of tissue factor
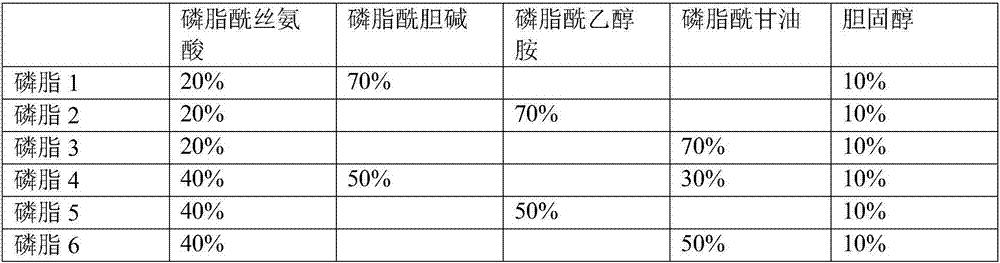
[0020] Take an appropriate amount of synthetic phospholipids by weighing respectively: phosphatidylserine, phosphatidylcholine, phosphatidylglycerol, cholesterol, and dissolve in the surfactant OGP solution in different proportions (accounting for the mass percentage of synthetic phospholipids). See Table 1 for details. The final concentration is 25mg / ml, mix until the synthetic phospholipids are completely dissolved, add 100μg / ml recombinant rabbit tissue factor, stir and incubate at room temperature for 2 hours, transfer the solution to a dialysis bag, change the dialysate every 4 hours, dialyze Overnight, remove the surfactant OGP. The obtained lipidated recombinant rabbit tissue factor solution was diluted with Hepes buffer to prepare a PT reagent containing 250 μg / ml synthetic phospholipids and 1 μg / ml recombinant rabbit tissue factor.
[0021] Table 1 Di...
Embodiment 2
[0035] The mensuration of embodiment 2 stability
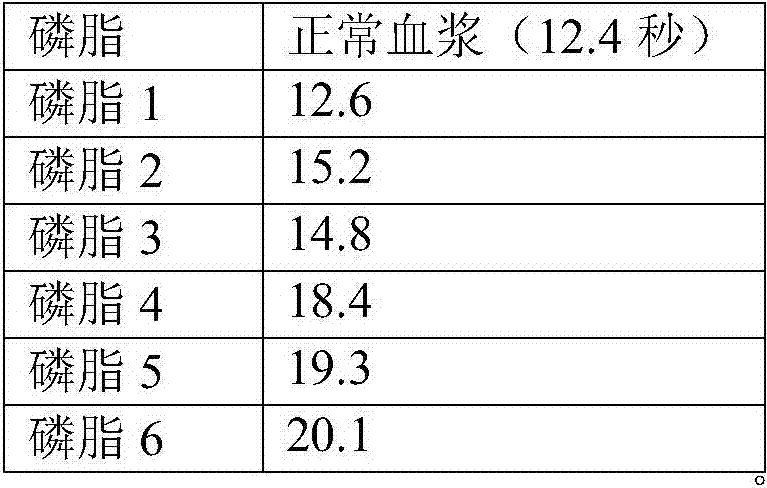
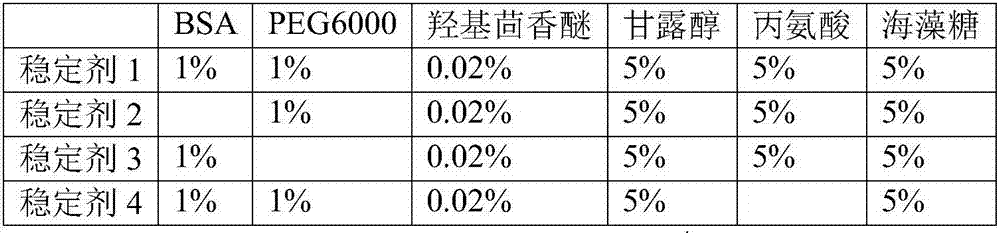
[0036] According to the method of Example 1, the PT detection reagent was prepared using phospholipid 1 and stabilizer 5. PT detection reagent contains 20mM, HEPES buffer, 200mM sodium chloride, 5% alanine, 5% trehalose, 1% BSA, 1% PEG6000, 0.02% hydroxyanisole, 0.05% sodium azide, 250μg / ml synthetic Phospholipids, 1 μg / ml recombinant rabbit tissue factor. The PT detection reagents were placed at 4°C and incubated at 37°C, and samples were taken every 7 days. The PT test was performed on the normal quality control plasma and the pathological quality control plasma respectively. The coagulation instrument CA1500 was used. The results are shown in Table 5. The results show that the results of PT testing at 37°C for 28 days, whether it is normal blood samples or pathological blood samples, have little change with the results at 0 days, and the test results at 4°C for 28 days do not change much, indicating that the stability of ...
Embodiment 3
[0039] The mensuration of difference between batches of embodiment 3
[0040] Three batches of prothrombin time detection reagent samples were continuously prepared, and then the quality control was tested simultaneously. The results showed that the difference between batches was very small (CV<5%) (as shown in Table 6).
[0041] Table 6 Difference between batches
[0042] batch
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com