Method for preparing antihuman recombinant tissue factor monoclonal antibody
A technology of monoclonal antibody and tissue factor, which is applied in the biological field to achieve the effects of stable quality, increased yield and high specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
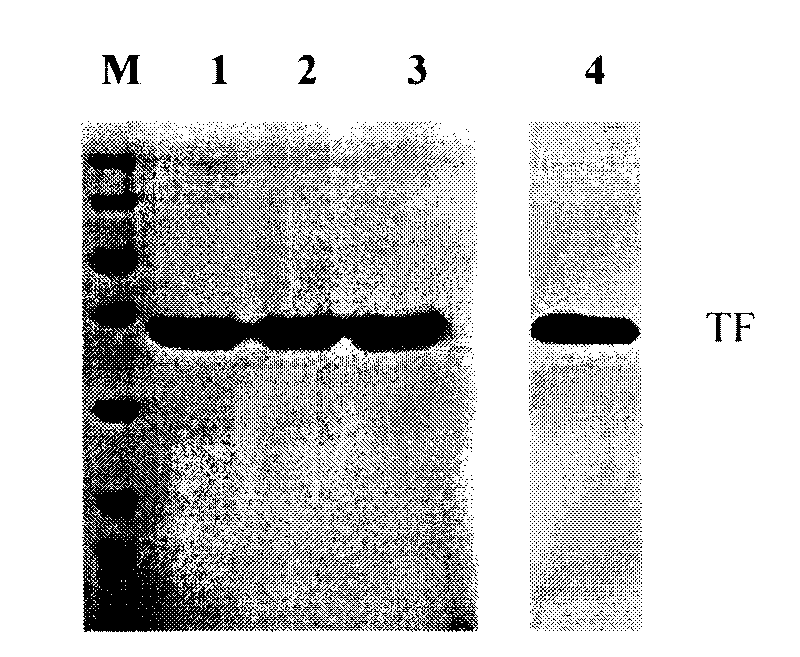
Image
Examples
Embodiment 1
[0034] Preparation of anti-human recombinant tissue factor monoclonal antibody
[0035] 1. Immunogen
[0036] Select 200510012414.7 patent to construct and express human recombinant tissue factor (rhTF 243 ) as an immunogen.
[0037] 2. Immunization of mice
[0038] Basic immunity: take rhTF stored in Tris-HCl buffer 243 (1mg / ml) was diluted to 120μg / ml, fully emulsified and mixed with Freund's complete adjuvant or Freund's incomplete adjuvant according to the volume ratio of 1:1, disinfected the abdomen of BALB / c mice with alcohol cotton balls, and selected 5 The mice were immunized by subcutaneous injection, and the injection volume was 0.5ml / only. Immunization volume 0.5ml, 30μg rhTF 243 / only, after the injection, disinfect the injection site with alcohol cotton balls to prevent infection.
[0039] Booster immunization: two weeks later, take rhTF stored in buffer 243 Dilute to 60 μg / ml, fully emulsify and mix with Freund’s complete adjuvant or Freund’s incomplete a...
Embodiment 2
[0056] Preparation of Anti-human Recombinant Tissue Factor Monoclonal Antibody Ascites with Different Inoculation Density
[0057] 1. Preparation of hybridoma cells
[0058] The anti-human recombinant tissue monoclonal antibody hybridomas obtained in Example 1 were selected and conventionally expanded and cultivated in DMEM high-glucose cell culture medium before use.
[0059] 2. Selection and pretreatment of experimental animals
[0060] Select 14-week-old, female, multiparous BALB / c mice, use liquid paraffin as a sensitizer, and sensitize one week before inoculating hybridoma cell lines, 0.5ml / mouse.
[0061] 3. Inoculation of hybridoma cell lines
[0062] The hybridoma cell line was subcultured through tissue culture flask cells, and the cells were collected after the cells grew to the logarithmic phase (1000rpm, 6min, centrifuged to remove the supernatant), and the adjusted cells were resuspended in serum-free basal medium (avoiding the introduction of bovine antibody) ...
Embodiment 3
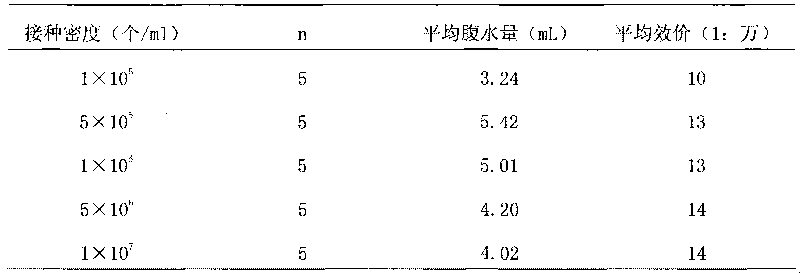
[0075] Preparation of anti-human recombinant tissue factor monoclonal antibody ascites in different treatment groups
[0076] The anti-human recombinant tissue monoclonal antibody hybridomas obtained in Example 1 were selected and conventionally expanded and cultivated for use.
[0077] Select 21 14-week-old BALB / c mice purchased from the same batch, including 7 female, male and female multiparous mice, and divide them into 3 groups. Sensitize with liquid paraffin one week before hybridoma cell line inoculation, 0.5ml / only. One week later, the same density (5×10 5 cells / ml) of the hybridoma cell suspension, and the ascites was collected when the mice were dying.
[0078] The results of ascites production are shown in Table 2.
[0079] Table 2 Ascites production and antibody titer of mice in different treatment groups
[0080]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com