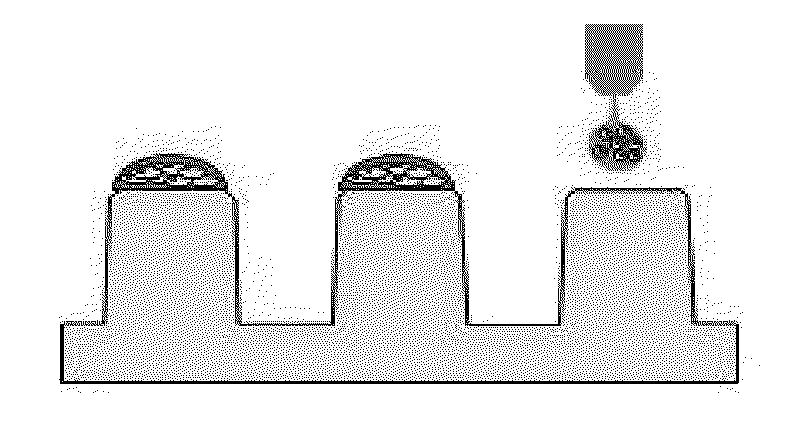
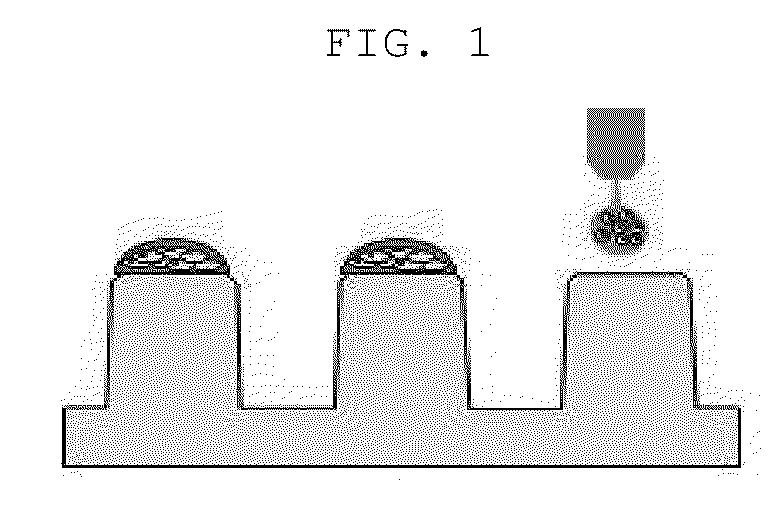
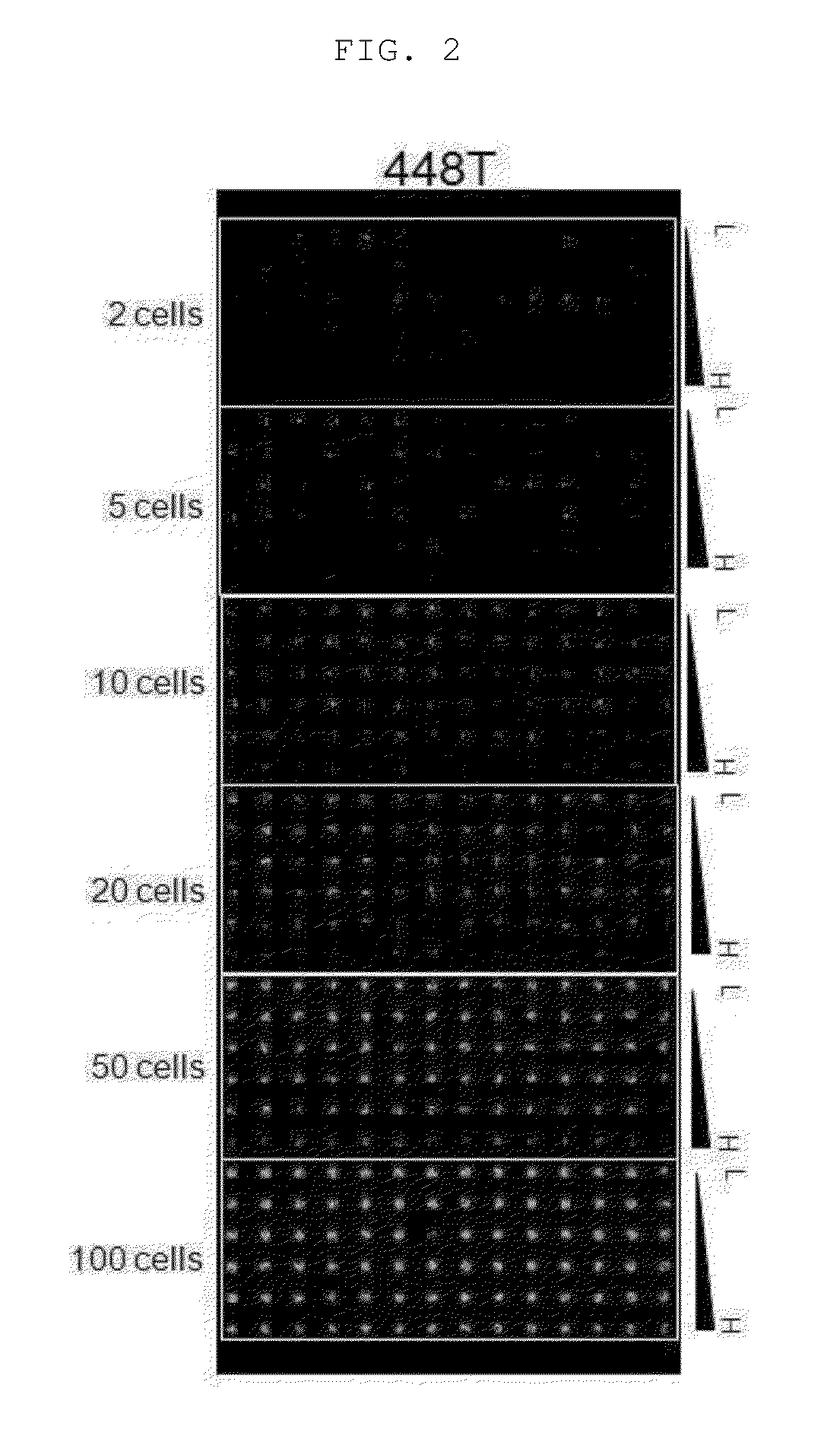
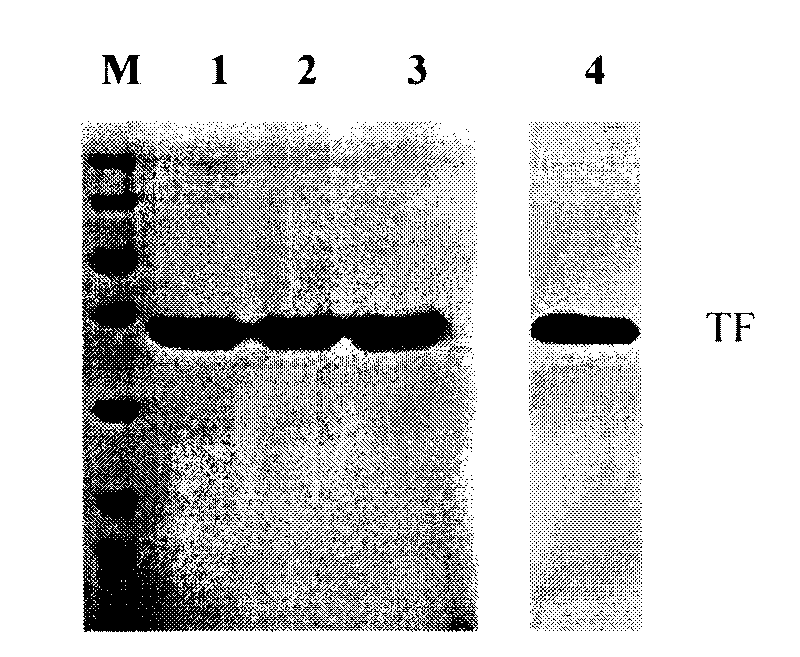
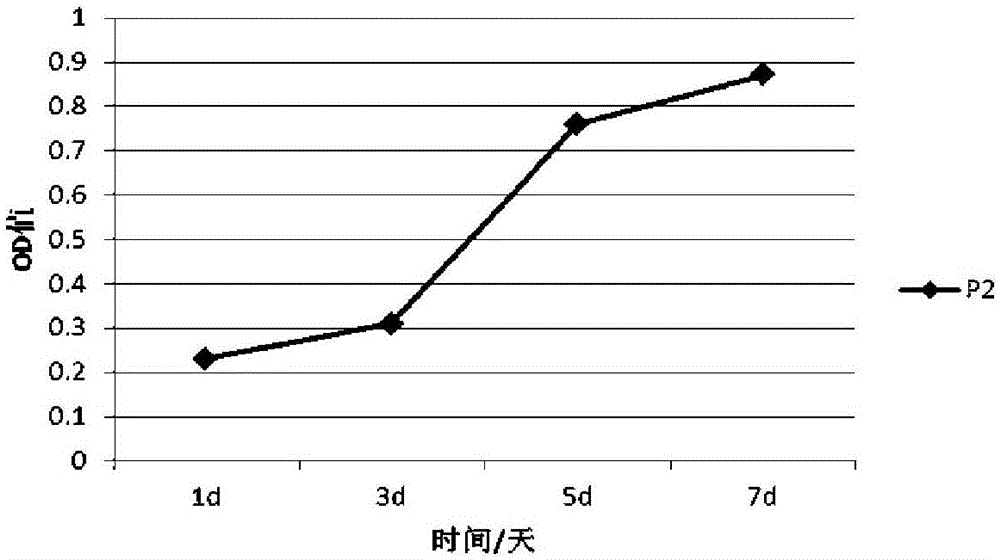
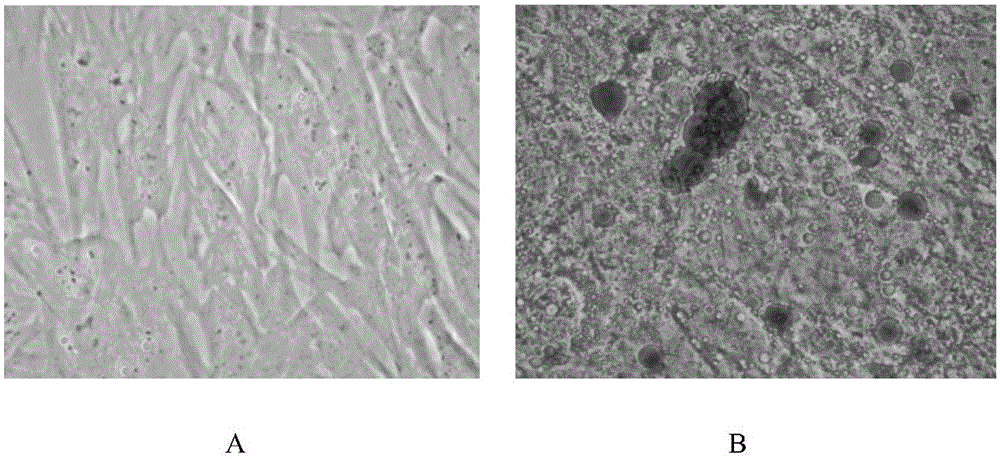
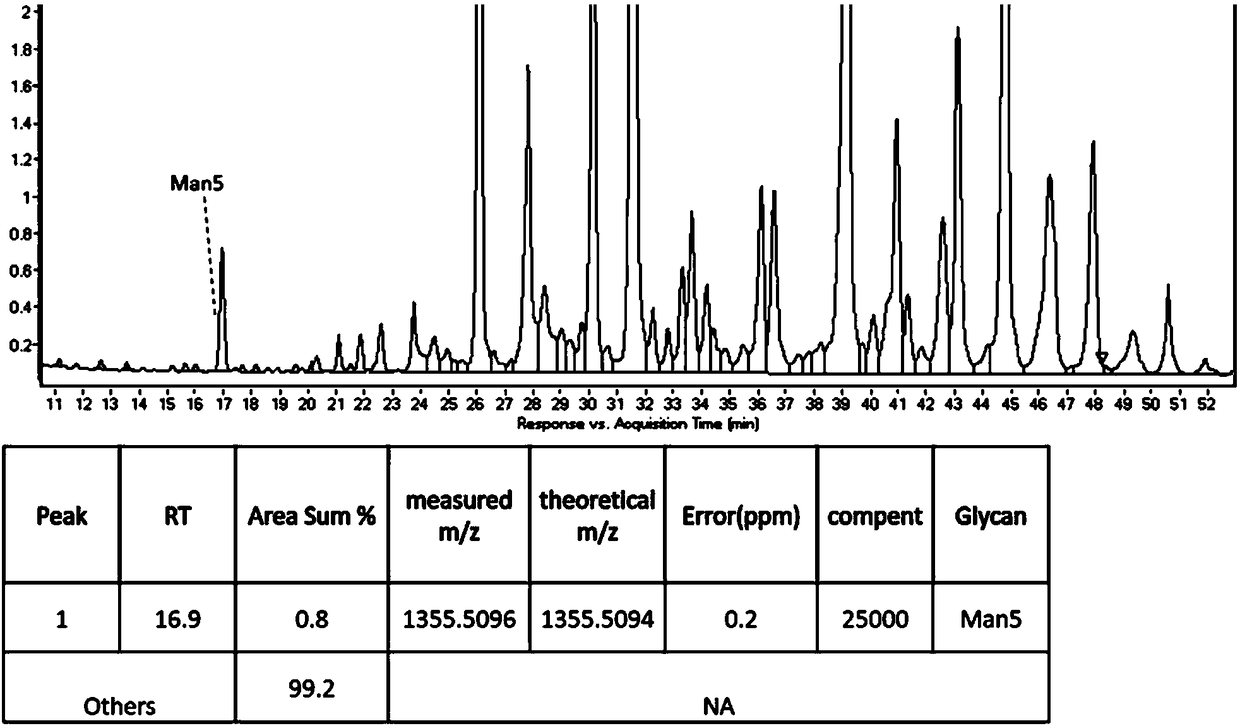
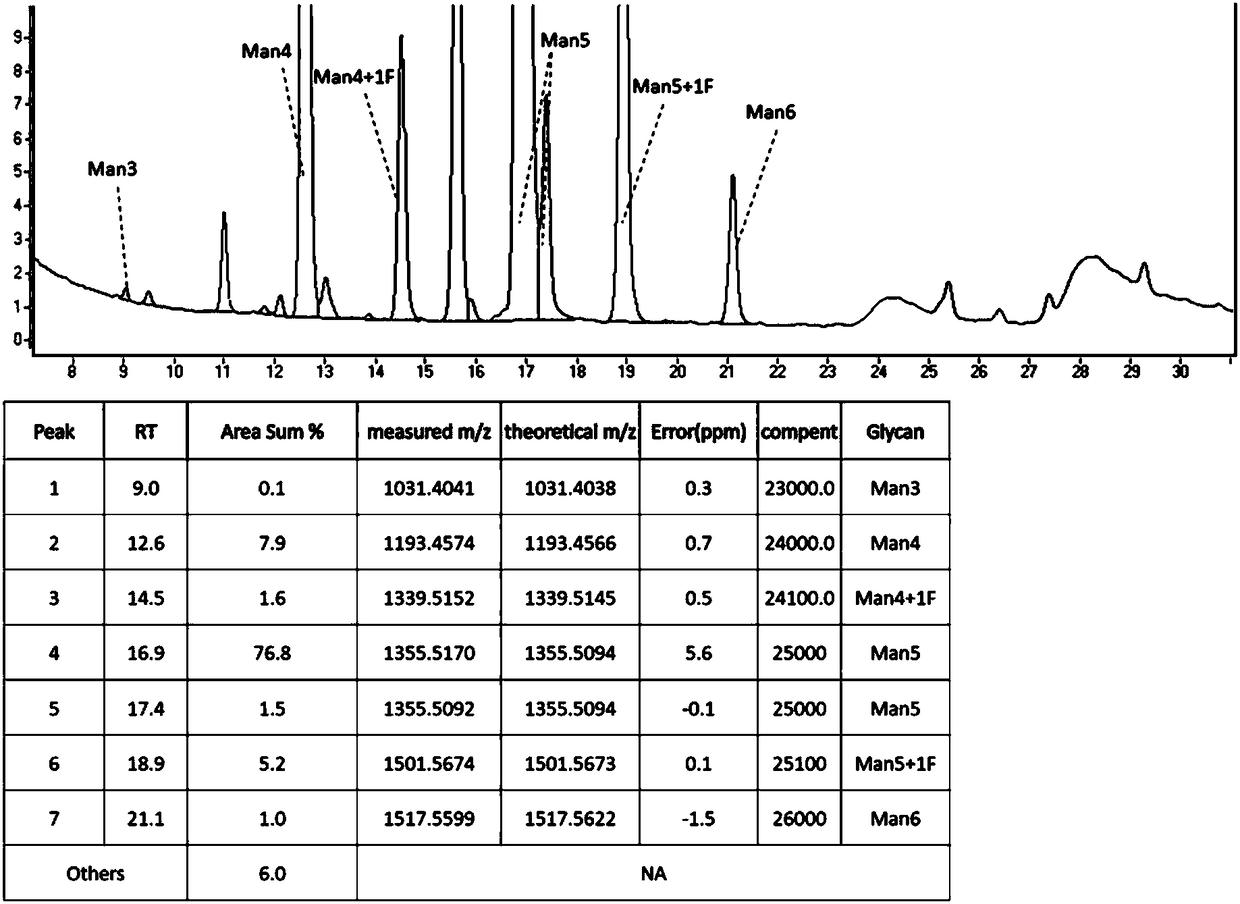
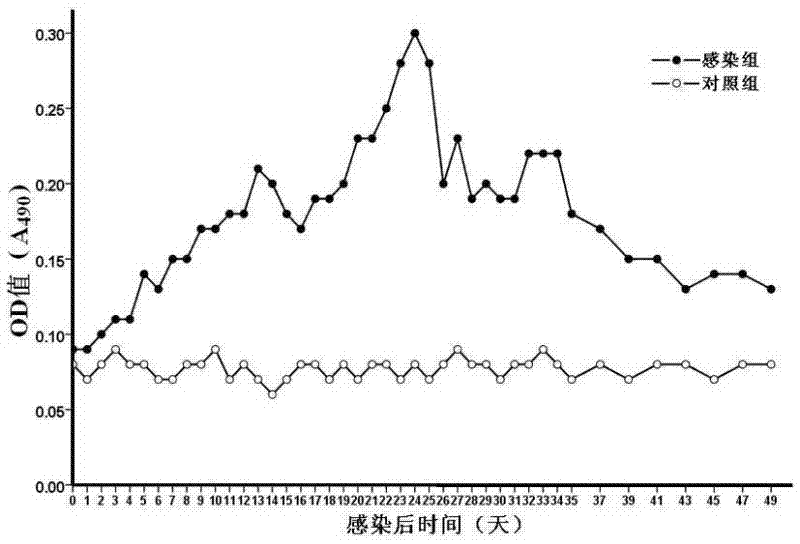
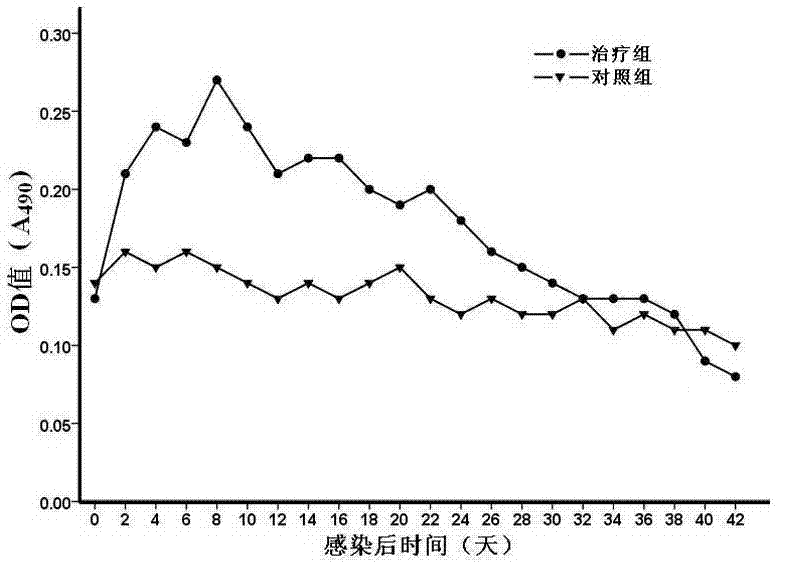
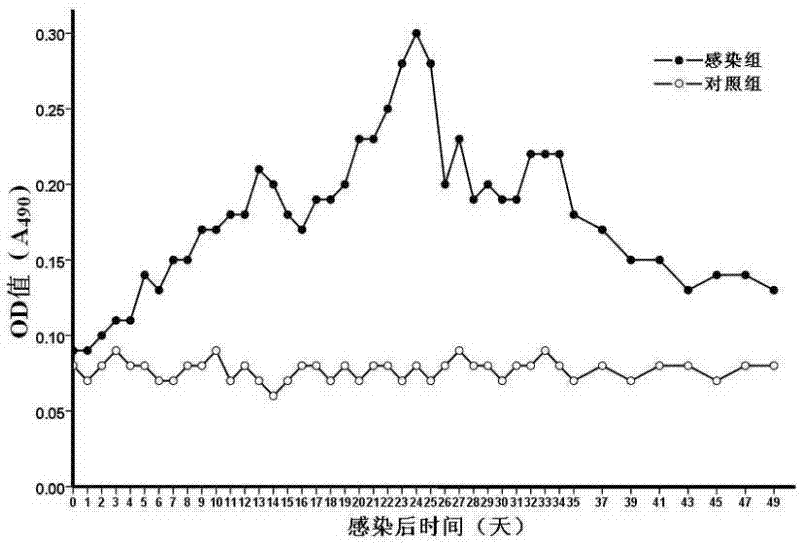
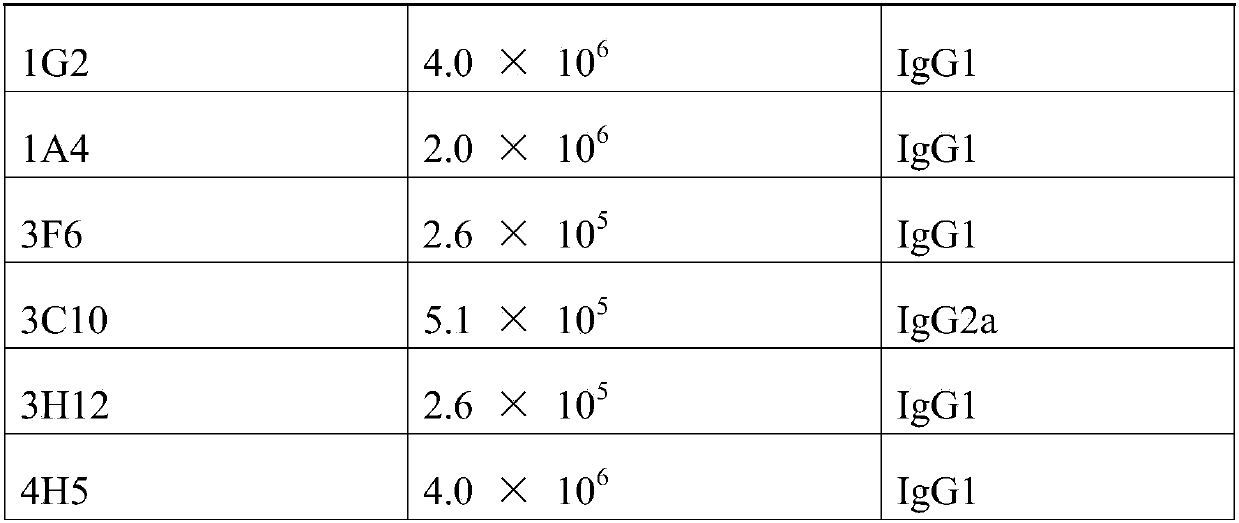
Patents
Literature
73 results about "Limiting dilution" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Limiting Dilution Analysis So limiting dilution analysis is used to measure the abundance of cells able to perform a particular function. ... Limiting dilution analysis. A semilogarithmic plot is made of the fraction of negative cultures as a function of the dose of cells placed in each culture.
Spodoptera frugiperda single cell suspension cell line in serum-free media, methods of producing and using
InactiveUS6103526AAvoid infectionHigh densityConnective tissue peptidesInvertebrate cellsSerum free mediaAdjuvant
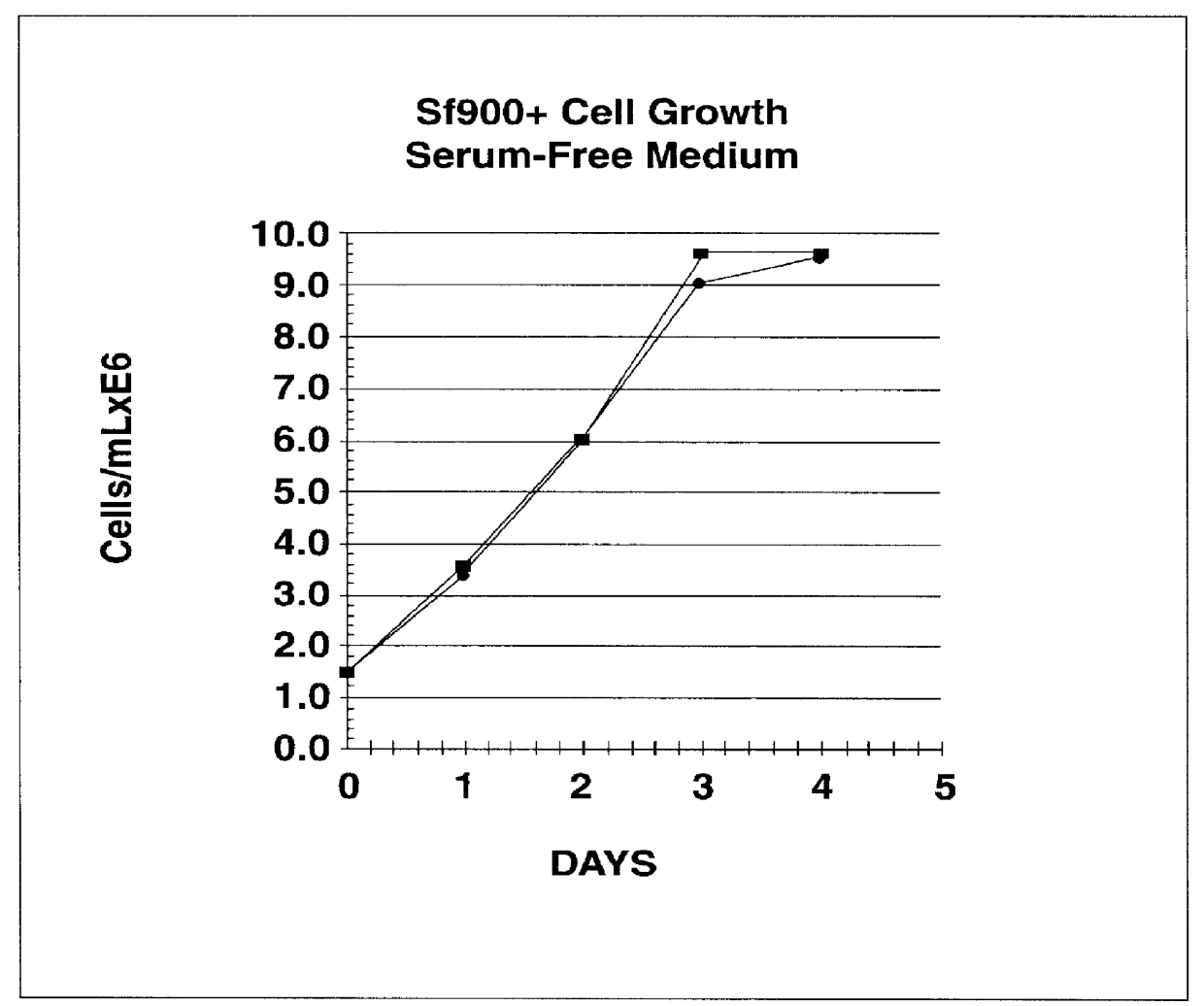
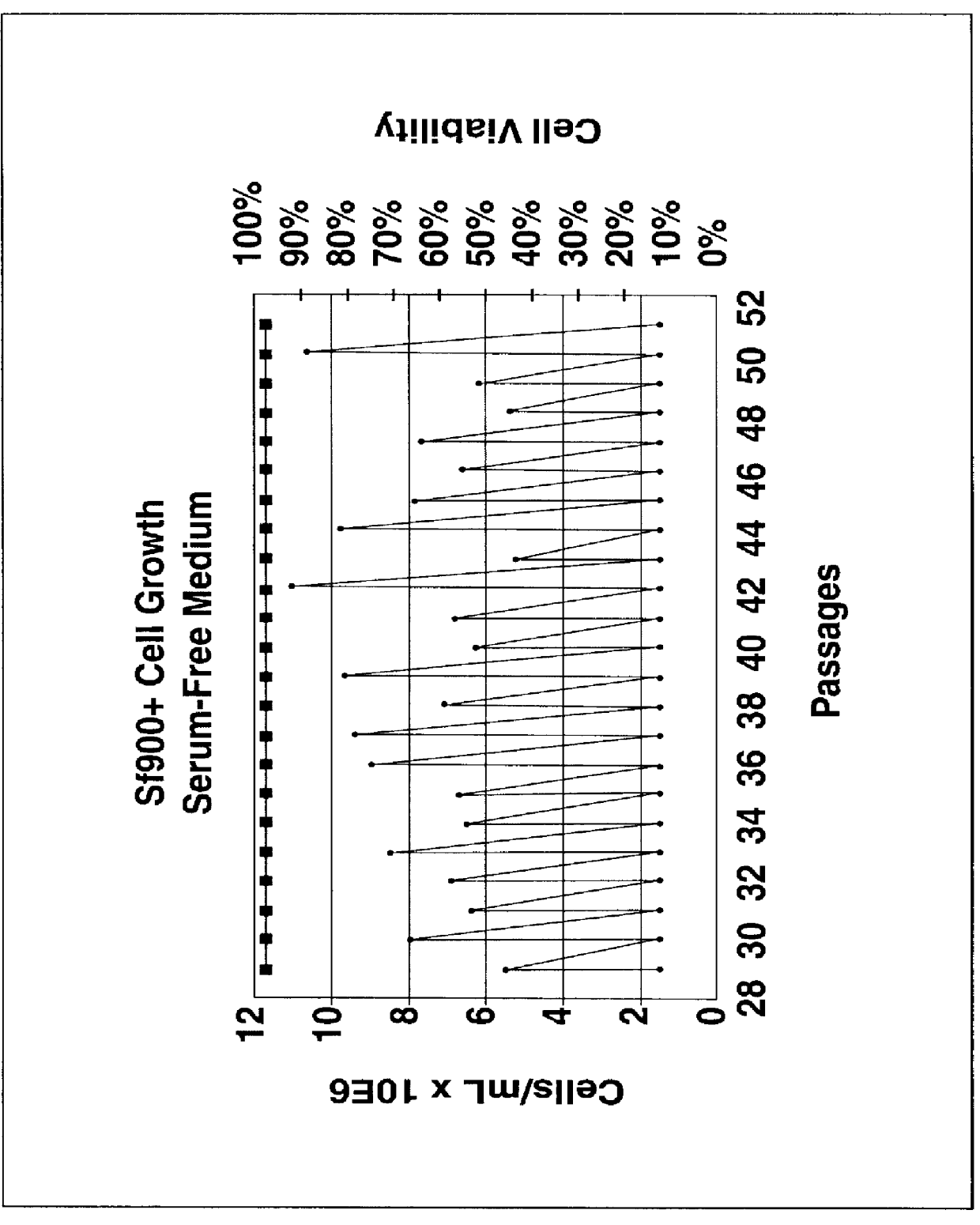
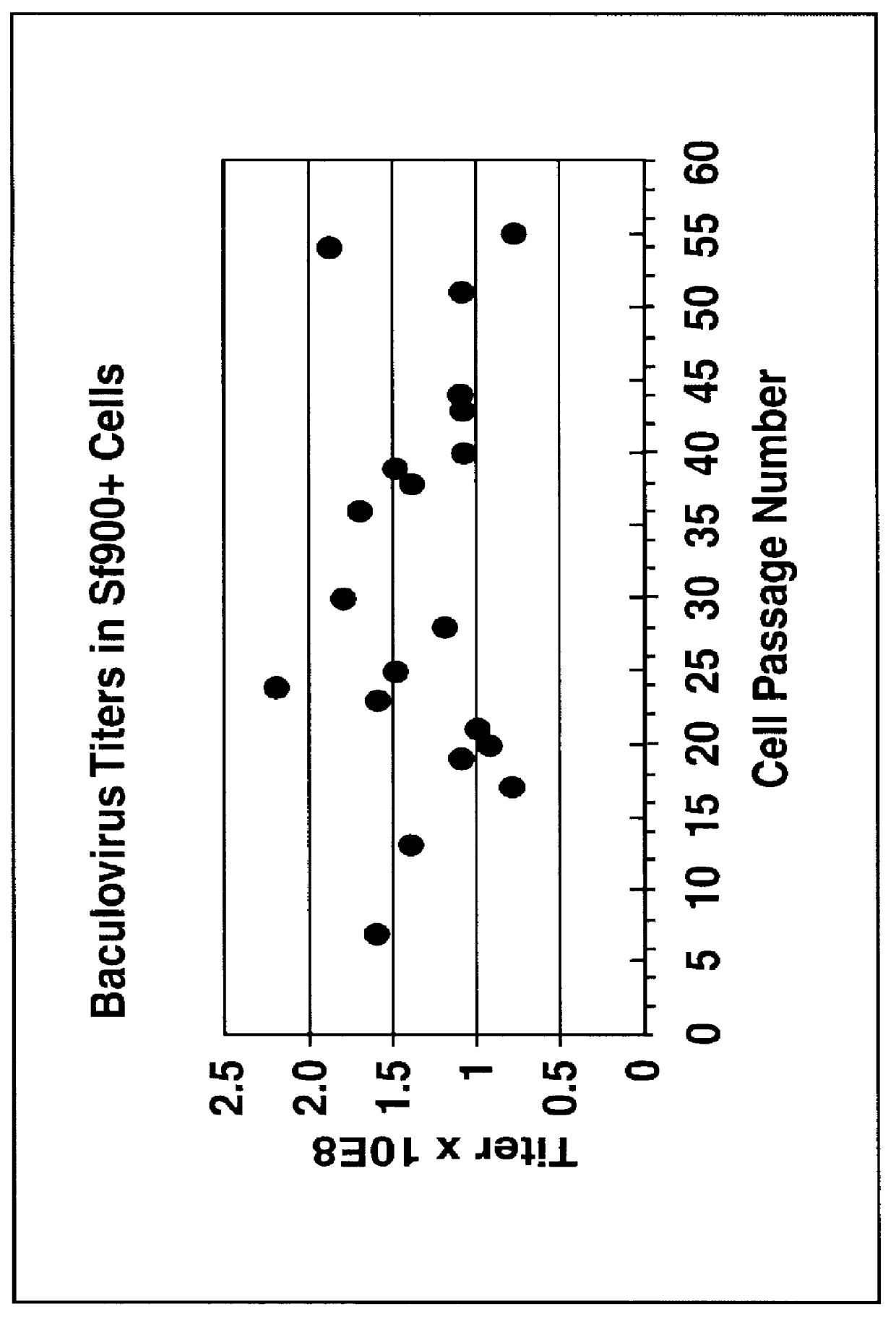
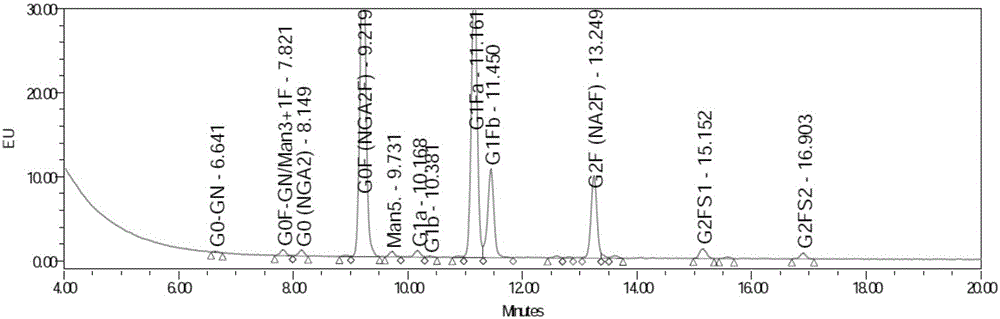
Disclosed and claimed is a new insect cell line, Sf900+, ATCC CRL-12579. The insect cell line was established from Lepidoptera, Noctuidae, Spodoptera frugiperda Sf-9 (ATCC CRL-1711) through multiple rounds of limiting dilution and selection in a serum-free insect medium supplemented with added human insulin. The insect cell line is useful in BEVS or as an adjuvant and has many characteristics and advantages. Also disclosed and claimed are recombinant proteins from recombinant baculovirus expression in insect cells such as Sf900+ cells, for instance, HA, NA, EPO, CD4, CEA, and thrombospondin.
Owner:PROTEIN SCI
FUT8 gene knockout method based on CRISPR technology
InactiveCN106399360AAchieve the purpose of inactivationImprove ADCC activityVector-based foreign material introductionHeterologousDouble stranded
The invention discloses a FUT8 gene knockout method based on a CRISPR technology. The FUT8 gene knockout method comprises: 1) designing a sgRNA sequence; 2) recombining the sgRNA sequence into a first vector to obtain a second vector; 3) transfecting the second vector into cells, and extracting DNA; 4) carrying out PCR amplification; 5) carrying out denaturation and annealing on the PCR product to form a heterologous hybrid double-stranded DNA; 6) cutting the heterologous hybrid double-stranded DNA, and analyzing the product to obtain the NHEJ occurring proportion in each cell population; and 7) selecting the cell population having the high NHEJ occurring proportion, carrying out limiting dilution method cloning, screening the monoclonal cell, and carrying out expanded culture to obtain the FUT8 gene knockout antibody. The invention further discloses the sgRNA sequence for the method. According to the present invention, the sgRNA capable of recognizing the FUT8 gene specific sequence is encoded, and the sgRNA and the sequence encoding the endonuclease are transfected into the cells, such that the FUT8 gene inactivation purpose is achieved, and the activity of the antibody ADCC is improved.
Owner:SHANGHAI WUXI BIOLOGIC TECH CO LTD +1
GPC3 (glypican-3) monoclonal antibody hybridoma cell strain 7D11 and preparation method and application thereof
ActiveCN102634486AReduce manufacturing costConducive to clinical target validationImmunoglobulins against animals/humansMicroorganism based processesMonoclonalSpleen cell
The invention discloses a GPC3 (glypican-3) monoclonal antibody hybridoma cell strain 7D11 and a preparation method and application thereof, which belong to the technical field of bioengineering. The GPC3 monoclonal antibody hybridoma cell strain 7D11 has a preservation code of CGMCC No.5426. The preparation method of the hybridoma cell strain 7D11 includes: using GPC3 protein as an immunogen to immunize a mouse, subjecting spleen cells of the mouse with serum titer more than 1:104 and myeloma cells SP2 / 0 to fusion, using a HATRPMI-1640 medium to screen fused cells, screening by ELISA (enzyme-linked immuno sorbent assay) and multiple limiting dilution process, and finally obtaining the hybridoma cell strain 7D11. The GPC3 monoclonal antibody hybridoma cell strain 7D11 is high in yield of secreted antibodies and easy to survive, the secreted monoclonal antibodies are high in titer, flexible in reaction, easy to detect, low in production cost and widely applicable to detection of GPC3 protein expression.
Owner:GUANGZHOU DARUI BIOTECH
Method for constructing hybridoma cell line of heterogeneity of seereting human monoclonal antibody for anti hepatitis B
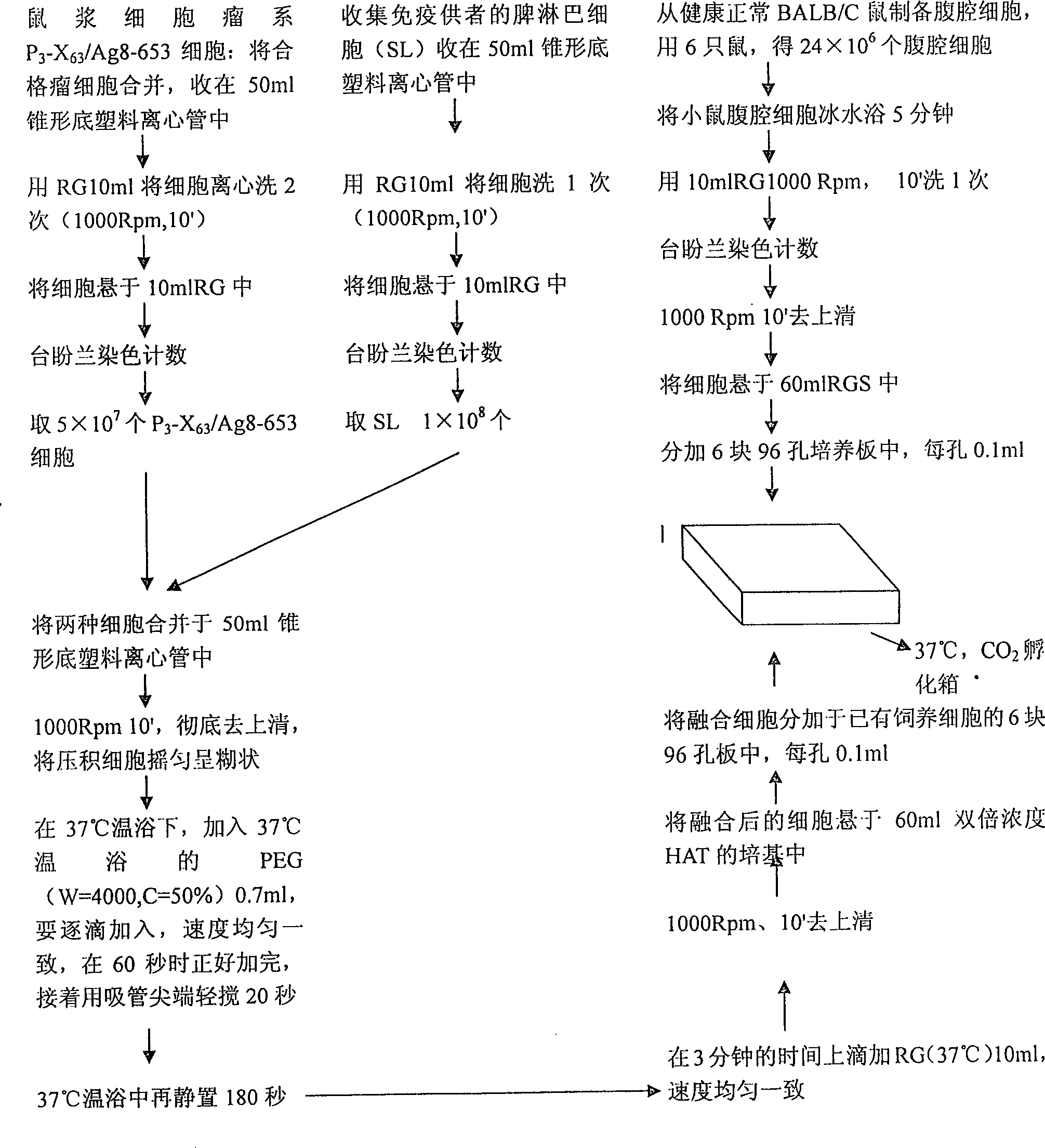
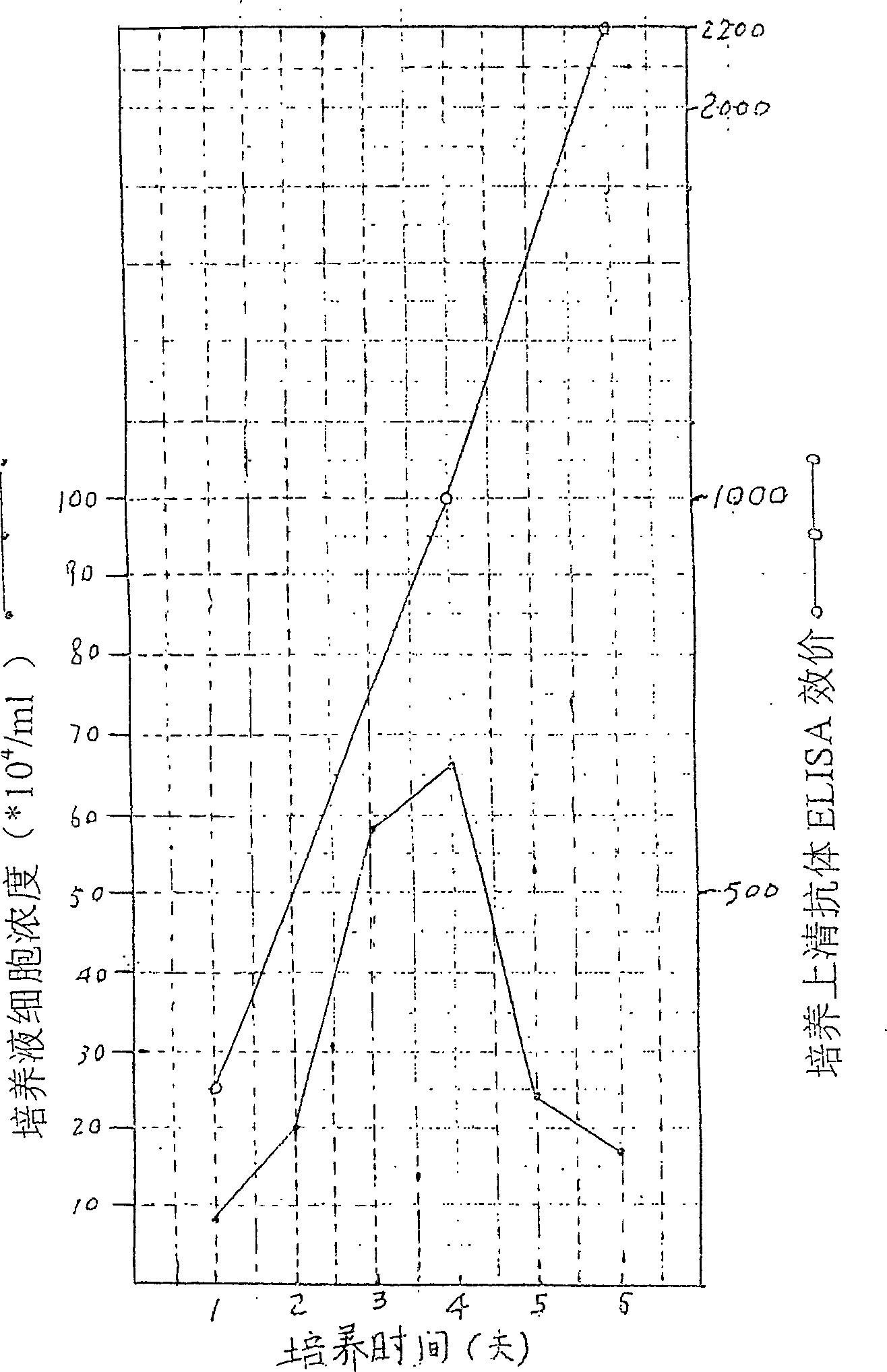
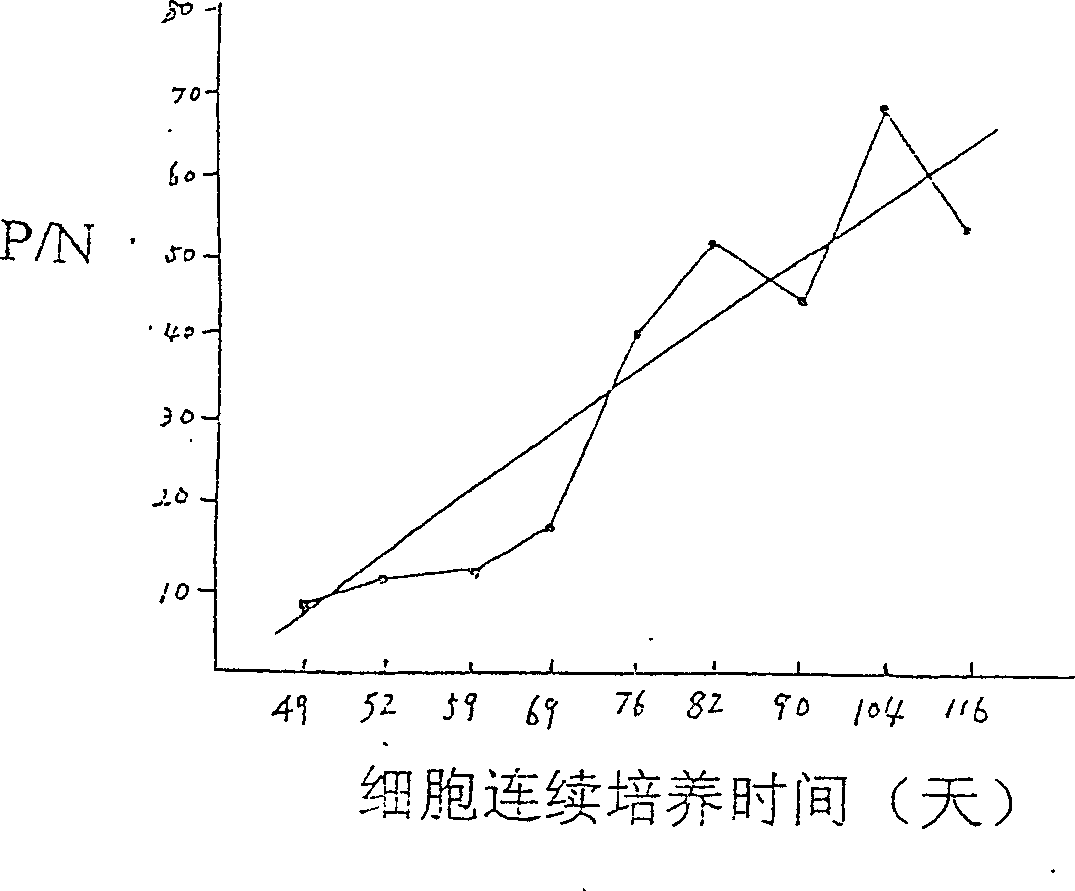
This invention provides a method for establishing hetero-hybrid tumor engineering cell sequence for producing human monoclonal antibody for anti-hepatitis B virus. The anti-HBs positive human splenic lymph cell (SL) is fused with mouse plasmacytoma sequence P3-X63 / Ag8-653 cell. Limiting dilution method is used for cloning the positive antibody poro-hybrid-tumor cell to obtain hetero hybrid tumor engineering cell sequence which produces human monoclonal antibody for anti-hepatitis B virus, with stable and high product, rate. Said monoclonal antibody is proceeded with purification and cross-linkage with interferon to produce target prepn. of anti-hepatitis B virus human monoclonal antibody crosslinking with itnerferon, and passive immune prepn. for hepatitis B, and testing agent for hepatitis B.
Owner:SHAANXI JIUZHOU BIOTECH
Method for preparing tussah silk fibroin protein monoclonal antibody
ActiveCN107266569AStrong specificityStrong immune responseImmunoglobulins against animals/humansBiological testingAntibody secretionSuccinic acid
The present invention relates to THE archaeological detection field, and discloses a method for preparing a tussah silk fibroin protein monoclonal antibody, a FeCl2 and tetrasodium iminodisuccinate mixed solution system is used for extraction of tussah silk fibroin, the tussah silk fibroin as a complete antigen is injected into the body of rabbits, a rabbit with high immunizing potency is selected, splenic lymphocytes and myeloma cells of the rabbit with the high immunizing potency are fused, after obtained hybridoma is cultured, a bacterial strain with positive secreted antibodies, strong antibody secreting ability and good cell growth conditions can be selected by indirect ELISA method, the bacterial strain is cloned by limiting dilution method until the secreting positive rate of the antibody with grown hybridoma is 100%, the selected cells are used for large-scale production, and then a chromatographic column is used for purification of the monoclonal antibody. The antibody prepared by the method has strong specificity, and in the application process, the operation is simple and quick, and the detection accuracy is high.
Owner:ZHEJIANG SCI-TECH UNIV
Method for screening patient-specific Anti-cancer agent using limiting dilution assay
ActiveUS20150101070A1Useful for developmentEfficient screeningCompound screeningApoptosis detectionPersonalizationAnticarcinogen
A screening method is described for selecting patient-specific anti-cancer agents reflecting individual genetic properties, in a precise and rapid manner, using an extremely small amount of cancer cells. Such screening method is useful for development of novel anti-cancer agents and the personalized medical field.
Owner:SAMSUNG LIFE PUBLIC WELFARE FOUND
Method for preparing antihuman recombinant tissue factor monoclonal antibody
ActiveCN101717447AStrong specificityEasy to prepareImmunoglobulins against cell receptors/antigens/surface-determinantsFreund adjuvantElisa method
The invention discloses a method for preparing an antihuman recombinant tissue factor monoclonal antibody. The method comprises the following steps: combining a truncated human recombinant tissue factor obtained by purification after prokaryotic expression with a freund adjuvant immunized mouse; fusing mouse spleen cells with myeloma cells; selectively cultivating and screening an HAT selective medium; after the subcloning cultivation is subjected to limiting dilution, selecting target hybridoma cells by an ELISA method, performing massive proliferation, and injecting into an abdominal cavity of a syngeneic mouse to prepare ascites; and obtaining the antihuman recombinant tissue factor monoclonal antibody which has excellent potency and higher yield. The antihuman recombinant tissue factor monoclonal antibody prepared by the method of the invention has the advantages of high sensitivity and strong specificity, can be used for immunoaffinity purification of the human recombinant tissue factor, and also can be used for research and development of anticoagulant medicaments and medicaments for treating tumors.
Owner:山西省生物研究院有限公司 +1
Vomitoxin hybridoma, monoclonal antibody, and preparation method and application of monoclonal antibody
InactiveCN102952782AHigh sensitivityQuick checkMicroorganism based processesTissue cultureBALB/cElisa kit
The invention discloses vomitoxin hybridoma, a monoclonal antibody, and a preparation method and application of the monoclonal antibody. The preparation method comprises the steps of: immunizing a Balb / c mouse with a vomitoxin and bovine serum albumin conjugate as an immunizing antigen; preparing a hybridoma by the spleen cells and the myeloma cell SP2 / 0 of the mouse; obtaining a monoclonal antibody hybridoma strain D-2-1CGMCC No. 5161 through an indirect ELISA (Enzyme-Linked Immunosorbent Assay) screening and a limiting dilution method; and injecting the hybridoma strain into the abdominal cavity of the mouse to produce a monoclonal antibody, wherein the monoclonal antibody is used for indirect competitive ELISA testing of the vomitoxin. The monoclonal antibody is capable of specifically detecting the vomitoxin, and high in sensitivity; and when an ELISA kit prepared by the monoclonal antibody is used for detecting the vomitoxin, the cost is low, large equipment is not needed, the operation is simple and convenient, and the preparation method is convenient for popularization and utilization.
Owner:BEIJING KWINBON BIOTECH
GPC3 (glypican-3) monoclonal antibody hybridoma strain 8G6, and preparation method and application thereof
ActiveCN102634487AReduce manufacturing costFunction increaseImmunoglobulins against animals/humansTissue cultureMicrobiological cultureMonoclonal
The invention discloses a GPC3 (glypican-3) monoclonal antibody hybridoma strain 8G6, and a preparation method and application thereof, which belong to the technical field of cell engineering. The preservation code of the GPC3 monoclonal antibody hybridoma strain 8G6 is CGMCC (china general microbiological culture collection center) No.5427. The preparation method of the GPC3 monoclonal antibody hybridoma strain 8G6 includes: using GPC3 protein as immunogen to immunize a mouse; and fusing mouse splenocytes having serum titer more than 1:104 with SP2 / 0 myeloma cells, using an HATRPMI-1640 medium to screen fusion cells, and finally obtaining the hybridoma strain 8G6 by ELISA (enzyme-linked immunosorbent assay) and repeated limiting dilution. The GPC3 monoclonal antibody hybridoma strain 8G6 is high in yield of secretory antibodies, and is easy to survive, and the secreted monoclonal antibodies are high in titer, sensitive in reaction, easy to detect, low in production cost and widely applicable to detection of GPC3 protein expression.
Owner:GUANGZHOU DARUI BIOTECH
Monoclonal antibody of bluetongue virus (BTV) and preparation method and application thereof
InactiveCN101597334ASame structureUniform compositionImmunoglobulins against virusesFermentationBALB/cHamster
The invention relates to the biotechnology field. A monoclonal antibody against a BTV VP7 protein of a bluetongue virus (BTV) of the invention is prepared by the following steps: adopting the hybridoma cell technology, taking splenocytes of BALB / c mice immunized with purified BTV for fusion with a mouse myeloma cell (SP2 / 0), after culturing the cells with an HAT selective medium, carrying out screening with indirect ELISA coated by a purified BTV antigen, a BTV VP7 protein antigen expressed by a gene engineering and a control antigen of a normal hamster kidney continuous cell (BHK21), carrying out screening and cloning by limiting dilution to obtain a hybridoma cell line which has the capacities of stable continuous culture and secretion of the monoclonal antibody (McAb) against the specific BTV VP7 protein and preparing McAb mouse ascites of the BTV VP7 protein. The monoclonal antibody against the BTV VP7 protein features strong specificity, high ascites titer, high affinity and simple preparation method, can be used in the detection method of the BTV antibody and antigen and provides an important technical means for prevention and control of bluetongue in China.
Owner:花群义
Japanese encephalitis virus like particles as well as preparation method and application thereof
InactiveCN102329784AReserve spaceReserved epitopeViral antigen ingredientsInactivation/attenuationJapanese B Encephalitis VirusReverse transcriptase
The invention discloses Japanese encephalitis virus like particles as well as a preparation method and an application thereof. The Japanese encephalitis virus like particles are prepared by assembling a PrM / E gene of a Japanese Encephalitis virus (JEV) and a JEVRNA replicor with a nucleotide sequence as shown in SEQ ID NO: 1. The preparation method of the Japanese encephalitis virus like particles comprises the steps of: connecting the JEV PrM / E gene amplified by an RT-PCR (Reverse Transcriptase Polymerase Chain Reaction) to a plasmid pTRE-Tight of a Tet-on advance expression system to form a recombination vector, transfecting a Hela Tet-on advanced cell by using an Xfect method, after screening and identifying through HygB, obtaining a Hela cell line for controllably and stably expressing the PrM / E gene through a limiting dilution process, inducing for expressing a PrM / E protein, and packaging to obtain the conventional virus like particles; and transfecting the Hela cell line by using a JEV replicor RNA vector and packaging to obtain the virus like particles with a capacity of transient infection. The Japanese encephalitis virus like particles can be used for preparing a vaccine or used as a detection agent, has better immunogenicity and reactivity, and is safe and reliable.
Owner:SOUTH CHINA AGRI UNIV
T cell receptor and uses thereof
ActiveUS20140212888A1Efficient detectionDistinguish clearlyTumor rejection antigen precursorsPeptide/protein ingredientsWild typeCellular receptor
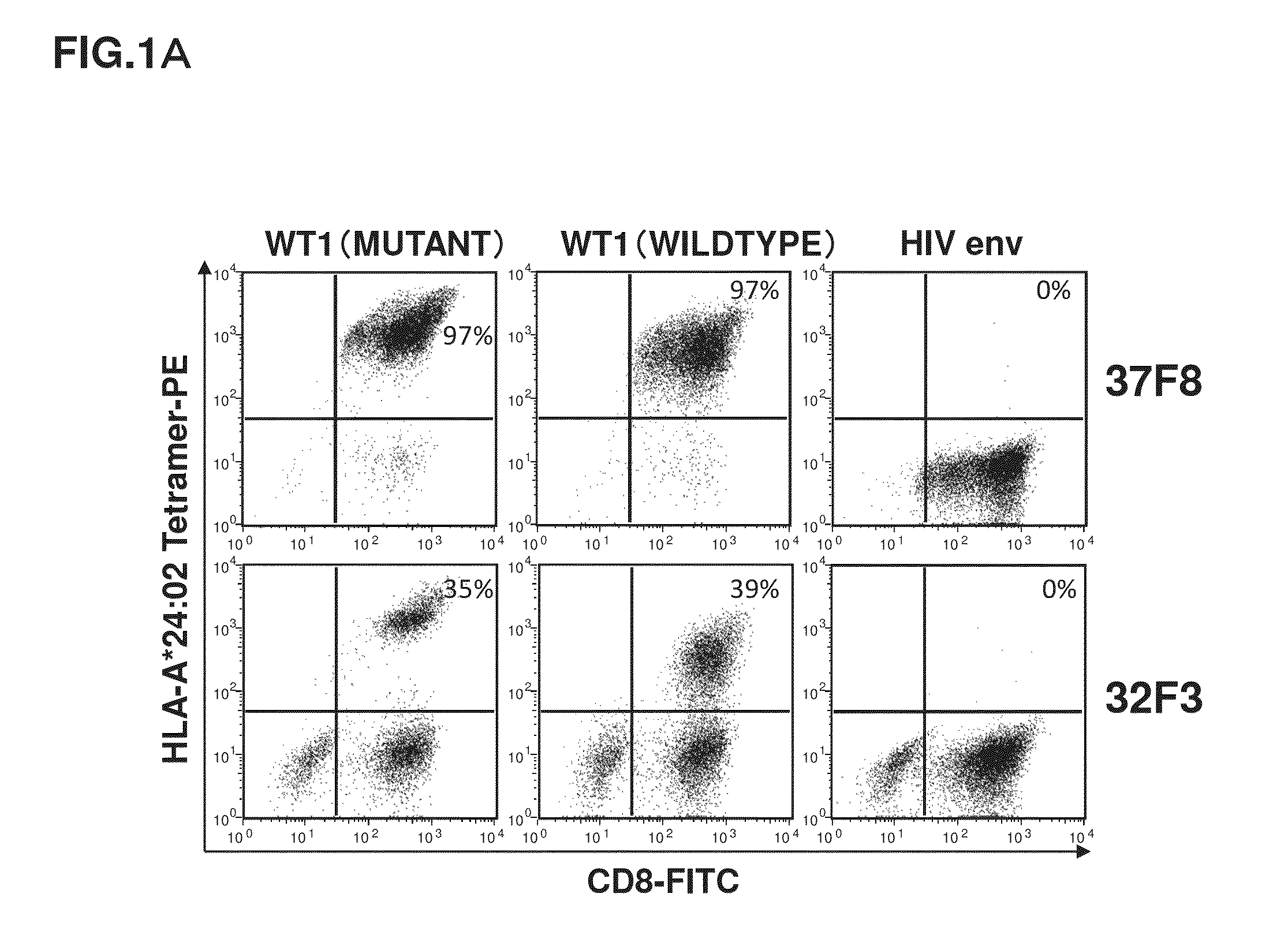
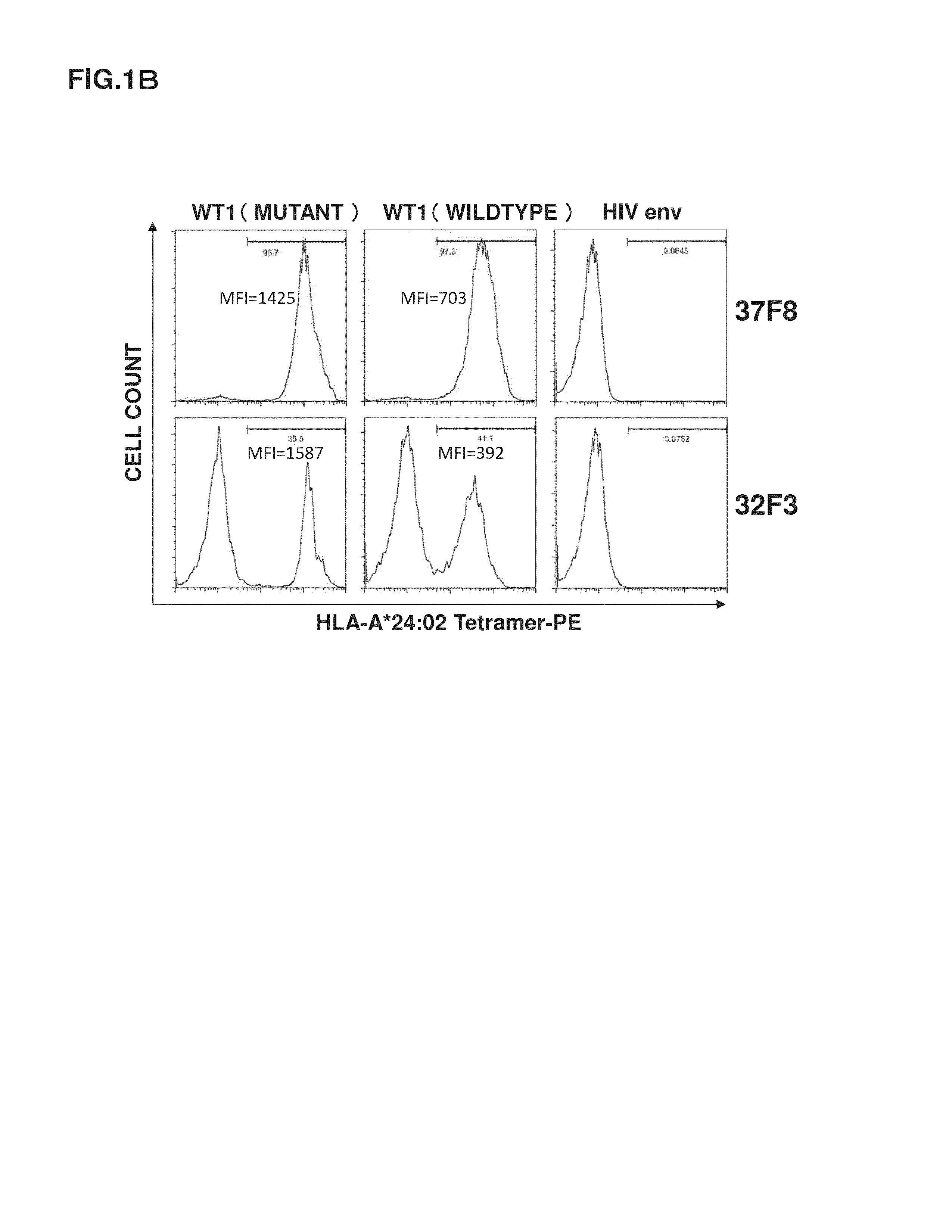
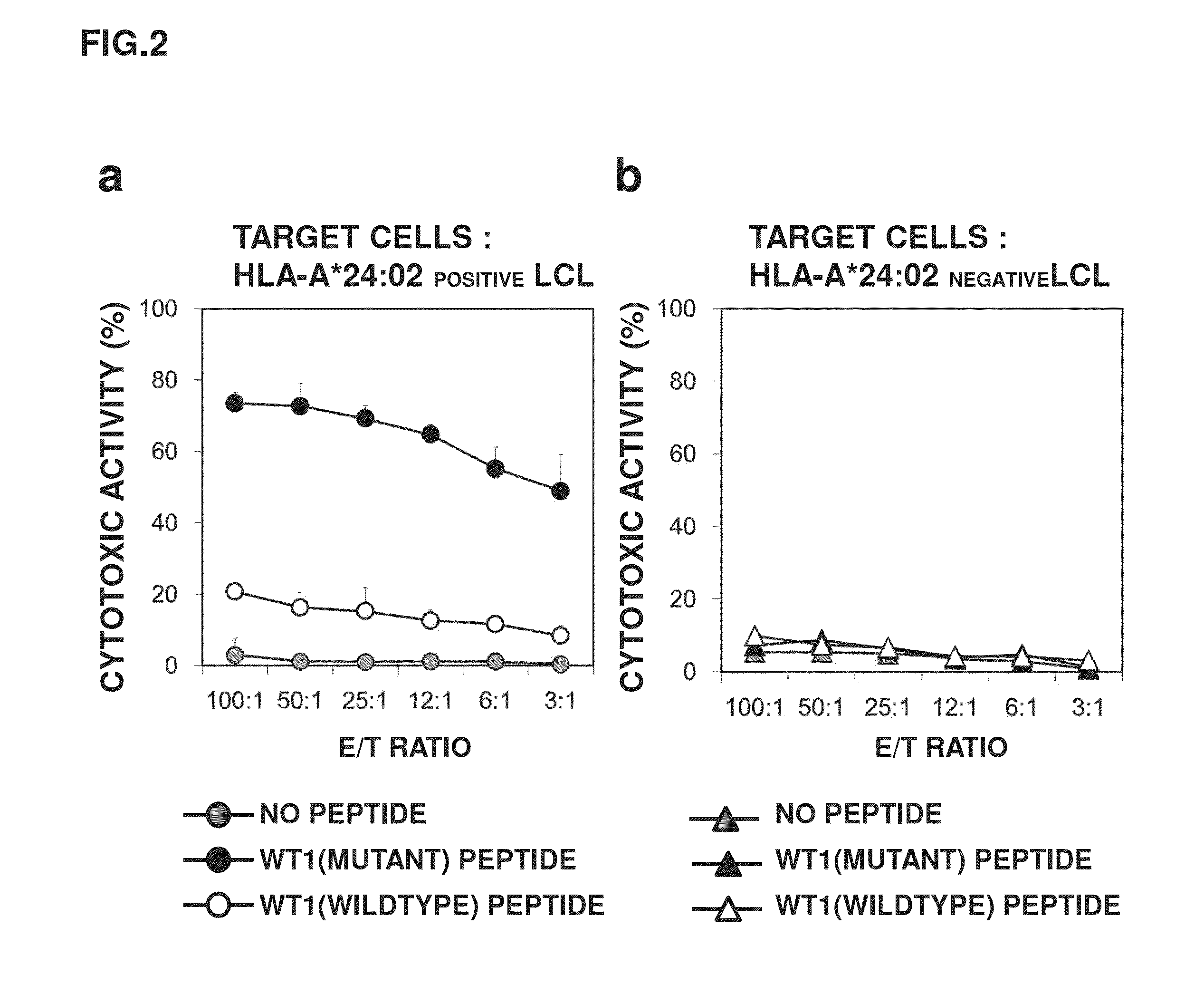
A method has been developed to efficiently proliferate and culture a CTL specific to WT1 peptides under limiting dilution conditions. Utilizing this method, CTLs capable of recognizing both a state where a wildtype WT1 specific peptide is presented by HLA-A*24:02 and a state where a mutant WT1 specific peptide is presented by HLA-A*24:02 have been successfully obtained.
Owner:MEDICAL & BIOLOGICAL LAB CO LTD
Immune detection method for residual aryl-N-methyl carbamate pesticide
InactiveCN101314618AImprove practicalityLarge analysis capacityImmunoglobulinsMaterial analysisBALB/cMethyl carbamate
The invention relates to a method for detecting the residual immunity of an aryl-N-methylcarbamates pesticide, and belongs to the biotechnology field. The method includes the steps as follows: a carrier protein is coupled with a general hapten (HA) of the aryl-N-methylcarbamates pesticide to obtain a complete antigen; by applying the hybridoma technique, the antigen immunized Balb / C mice are screened by adopting the ELISA method and subcloned by adopting the limiting dilution method to obtain the hybridoma cell strains capable of stably secreting the aryl-N-methylcarbamates pesticide; and monoclonal antibodies are prepared. Based on the monoclonal antibodies, four ELISA detection methods for detecting the residual immunity of the aryl-N-methylcarbamates pesticide are established. The method has high specificity, sensitivity and stability, and provides a quick, sensitive and specific technology for trace-detecting the aryl-N-methylcarbamates pesticides.
Owner:NANJING AGRICULTURAL UNIVERSITY
Preparation, identification and application of HA tag monoclonal antibody
InactiveCN101532009AHigh titer of ascitesNo cross reactionFermentationGenetic engineeringBALB/cIndirect elisa
The invention relates to preparation, identification and application of HA tag monoclonal antibody, which belong to the technical field of biological medicines. A carbodiimide method is used for synthesizing an HA-tag complete antigen to immunize a BALB / c mouse, a hybrid tumor method is adopted to perform cell fusion, and positive hybrid tumor cell strains are cloned and screened by a limiting dilution method and an indirect ELISA method. Ascites is prepared conventionally and is purified by an octylic acid-ammonium sulphate method, and specificity identification is performed on a purified mAb. The result of the identification shows that the HA-tag complete antigen is coupled successfully, and a hybrid tumor cell strain excreting an HA tag mAB is obtained successfully through cell fusion, screening and cloning. In the preparation of the ascites, the ascites titer is measured to be more than 1:10<6>, and the strain of the mAb does not have cross reactions with other fusion protein tags; and the monoclonal antibody applied to a Western-blotting and an immunohistochemical detection of fusion proteins so that a method which can be applied to the purification and the detection of HA fusion proteins is established.
Owner:朱晓林
Natural anti-oxidation low-density lipoprotein antibody for suppressing atherosclerosis
InactiveCN101654481AReduce areaInhibition formationImmunoglobulins against animals/humansAntibody ingredientsAntigenOxidized low density lipoprotein
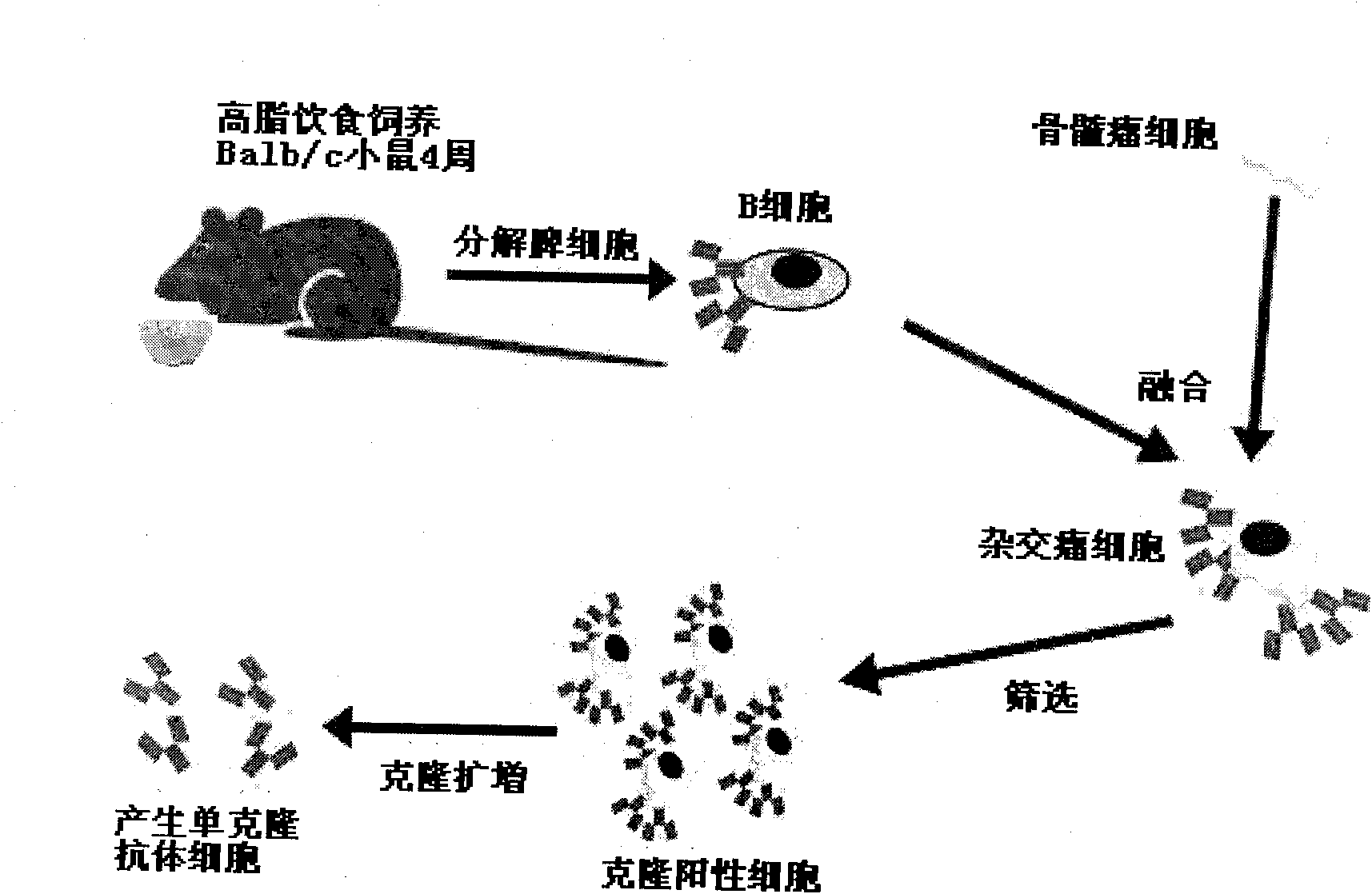
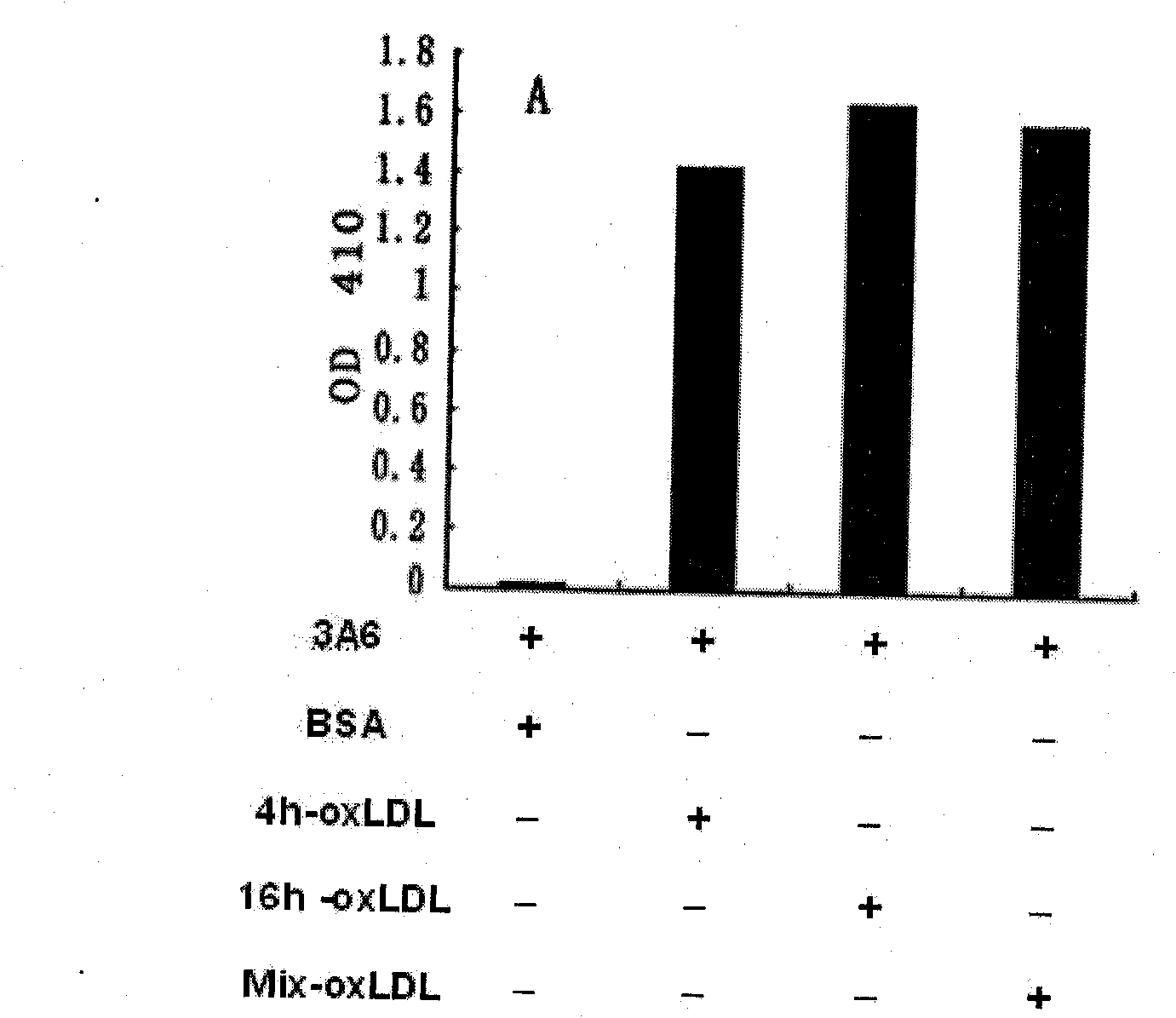
The invention belongs to the technical field of biomedicine, in particular to a natural anti-oxidation low-density lipoprotein (oxLDL) IgM subclass antibody for suppressing atherosclerosis. The natural anti-oxidation low-density lipoprotein (oxLDL) IgM subclass antibody is characterized in that a Babl / c rat is bred with a high-cholesterol diet for 4 weeks under a condition of no special pathogen;splenic cells (B cells primarily) are separated, the B cells and myeloma cells are combined by a chemical method, and hybridoma cells are obtained; and oxidized low-density lipoprotein (oxLDL) is usedas an antigen, an indirect enzyme-linked immunosorbent assay (ELISA) experiment is carried out on the growth holes of the positive hybridoma cells, positive cloning holes are determined, and the required cloning positive cells are screened out by a limiting dilution method. The obtained cloning positive cells are cloned and multiplied, cells generating a monoclonal antibody are obtained, and thenatural oxLDL-resisting immunoglobulin M (IgM) subclass antibody 3A6 is obtained. The antibody can be used for lowering the formation of the atherosclerosis of the rat and provides a novel idea and anovel method for researching the generation, the development and the treatment of the atherosclerosis.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Infectivity resistant bursal disease virus VP4 protein monoclonal antibody
InactiveCN101121748AEasy to prepareHigh purityImmunoglobulins against virusesEscherichia coliInclusion bodies
The anti-infectious bursal disease virus (IBDV) VP4 protein monoclonal antibody of the invention belongs to the field of biotechnology and relates to genetic engineering. The IBDV VP4 gene was cloned into the prokaryotic expression vector pET-28a, induced and expressed in Escherichia coli BL21(DE3); the inclusion body was isolated, dissolved with 8M urea, and purified by nickel chelate affinity chromatography to obtain the VP4 recombinant protein. BALB / c mice were immunized with VP4 recombinant protein, and the splenocytes were taken for cell fusion with myeloma cell SP2 / 0. After selective culture in HAT medium, cells infected with VP4 recombinant protein and IBDV were screened by double ELISA and limited dilution The hybridoma cell lines secreting anti-IBDV VP4 protein monoclonal antibody were obtained by cloning by the method. The ELISA titers of 4C3, 6A4 and 6H8 ascites were 6.4×105, 2.6×106 and 5.2×106, respectively, all of which could specifically recognize IBDV The viral protein that infects cells has an Ig subtype of IgG1 for the heavy chain and κ for the light chain.
Owner:ZHEJIANG UNIV
Hybridomas cell strain of anti-human macroglobulin monoclonal antibody
The invention relates to a hybridomas cell strain secreting anti-human macroglobulin monoclonal antibody and a preparation method thereof. The preparation method comprises using a conjugate obtaining by performing coupling on bovine serum albumin (BSA) and human macroglobulin as an antigen for immunizing Balb / c mouse, getting spleen cell of the mouse and performing cell fusion with SP2 / 0 myeloma cell, performing selective culture by using HAT medium, performing multiple cloning by using a limiting dilution process, and finally screening and obtaining the hybridomas cell strain stably secreting anti-human macroglobulin monoclonal antibody. The prepared hybridomas cell strain is preserved in China Center for Type Culture Collection with the preservation number of CCTCC C2015190.
Owner:TSINGHUA UNIV
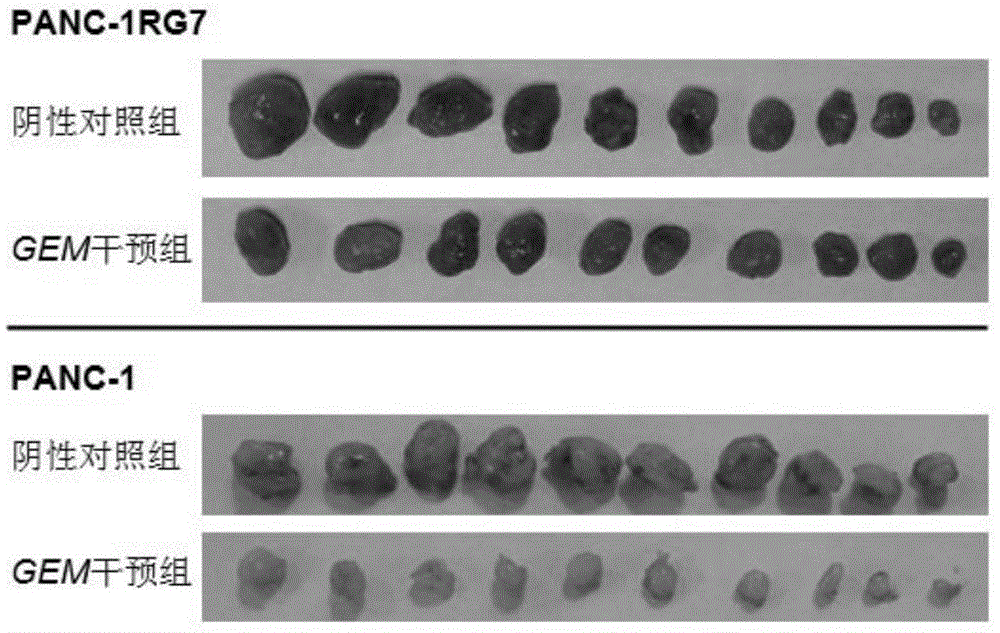
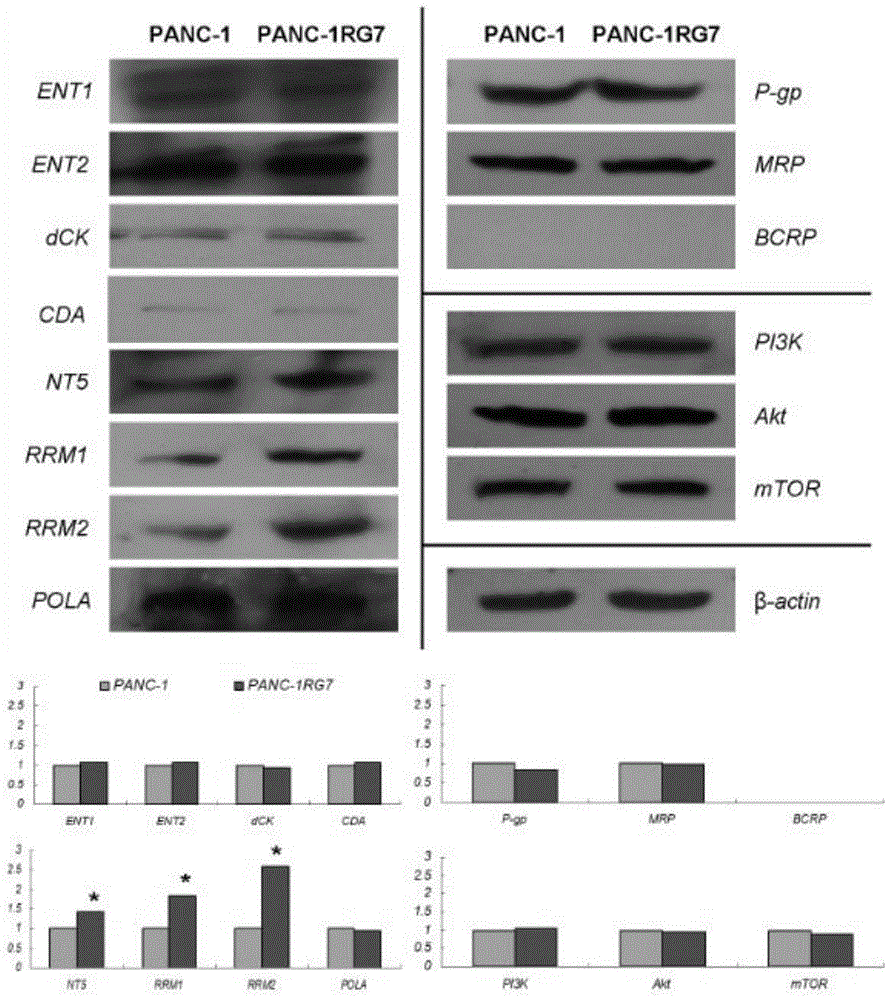
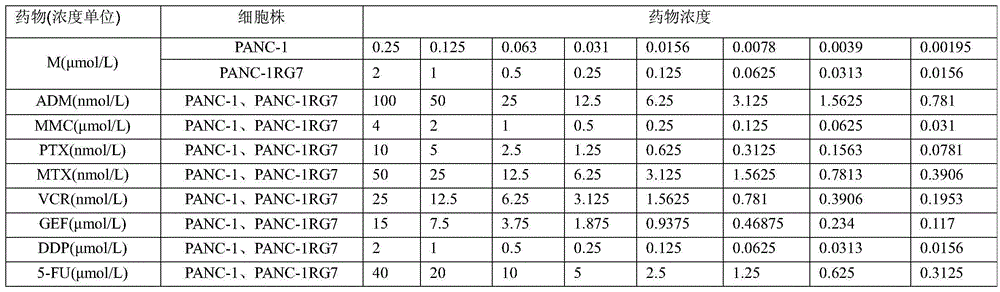
Establishing method of human pancreatic cancer gemcitabine-resistant cell line, and application of cell line
PendingCN104946594AHigh drug resistance indexImprove stabilityMicrobiological testing/measurementMicroorganism based processesCell growthCell state
The purpose of the invention is providing an establishing method of a human pancreatic cancer gemcitabine-resistant cell line, and an application of the cell line. The method comprises the following steps: putting a PANC-1 cell line in an exponential growth stage in a culture box, and culturing; observing the state of a cell until the growth of the cell goes back to normal; increasing the final concentration of GEM by 25-50nmol / L; gradually increasing the concentration until the final induction concentration is 1.9-2.1[mu]mol / L; and carrying out cloning culture on obtained drug-resistant cells through a limiting dilution process, selecting monoclonal cell growth holes with good cell state, and carrying out cloning culture. In the invention, the establishing method of the human pancreatic cancer gemcitabine-resistant cell line has the advantages of high efficiency and easy implementation; and the human pancreatic cancer gemcitabine-resistant cell line established in the invention has the advantages of high drug resistance index, good stability, and certain resistance to gemcitabine.
Owner:FUJIAN MEDICAL UNIV
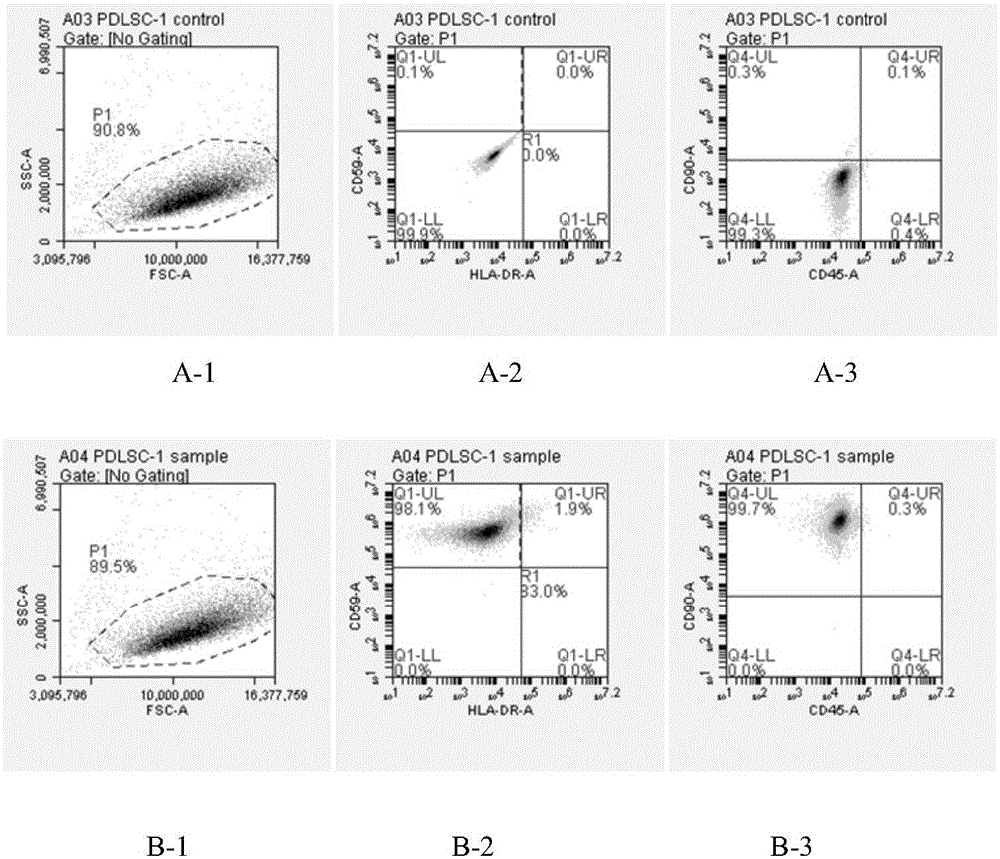
Primary separation and culture method for periodontal ligament stem cells
ActiveCN105062960AHigh affinityLow costArtificially induced pluripotent cellsNon-embryonic pluripotent stem cellsPeriodontiumPeriodontal ligament stem cells
The invention discloses a primary separation and culture method for periodontal ligament stem cells, which comprises the following steps: separating periodontal ligaments, carrying out enzymolysis on periodontal ligament tissues by a mixed enzyme of collagenase V of which the mass-volume concentration is 0.05-0.25% and pancreatin of which the mass-volume concentration is 0.05-0.25%, carrying out primary culture on periodontal ligament stem cells, and separating the periodontal ligament stem cells by adopting a limiting dilution method. The primary separation and culture method disclosed by the invention has the following advantages: (1) the cost is reduced because the cost of the pancreatin is lower than that of dispase; (2) the digestion time is shortened, the periodontal ligaments are dense connective tissues, the affinity of the collagenase V to the connective tissues is superior to that of collagenase I, and the collagenase V and the pancreatin are combined, so that the enzymolysis effect is obviously improved.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
Cell strain capable of expressing glucocerebrosidase with high mannose content as well as preparation method and applications of cell strain
PendingCN108588127AIncrease enzyme activityHigh mannose glycoform contentPeptide/protein ingredientsMetabolism disorderHigh mannoseEukaryotic plasmids
The invention discloses a preparation method of a cell strain capable of expressing glucocerebrosidase with high mannose content. The preparation method comprises the following steps: (1) carrying outlipofection transfection, namely, aiming at the sequence of a GNT1 gene, designing a sgRNA sequence guiding cutting of endonuclease, recombining the sgRNA sequence into a knock-out vector, thus obtaining synthetic plasmids with coexpression of sgRNA and endonuclease, and transfecting a cell pool stably expressing glucocerebrosidase by adopting the synthetic plasmids through a liposome transfection method; (2) carrying out cloning by adopting a limiting dilution method, namely, screening out monoclonal cells, and carrying out enlarged culturing; (3) carrying out mutation cloning screening on the target gene, namely, acquiring monoclone with the GNT1 gene knocked out through cell PCR sequencing, and thus the cell strain capable of expressing glucocerebrosidase with high mannose content is obtained. The cell strain can stably express glucocerebrosidase, meanwhile, the content of the mannose is obviously improved, the binding capacity with mannose receptor is effectively improved, the enzyme activity capacity is improved, and the purpose of increasing the efficacies is achieved.
Owner:WUXI BIOLOGICS IRELAND LTD
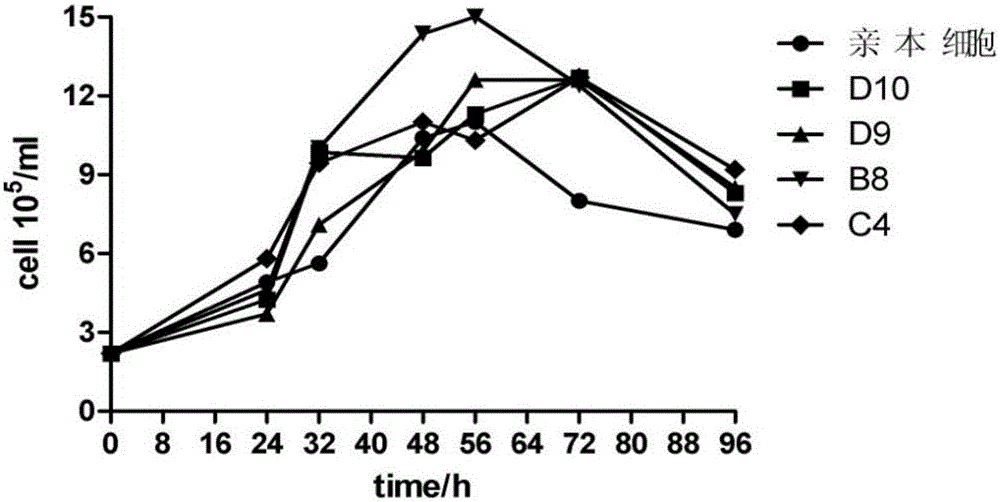
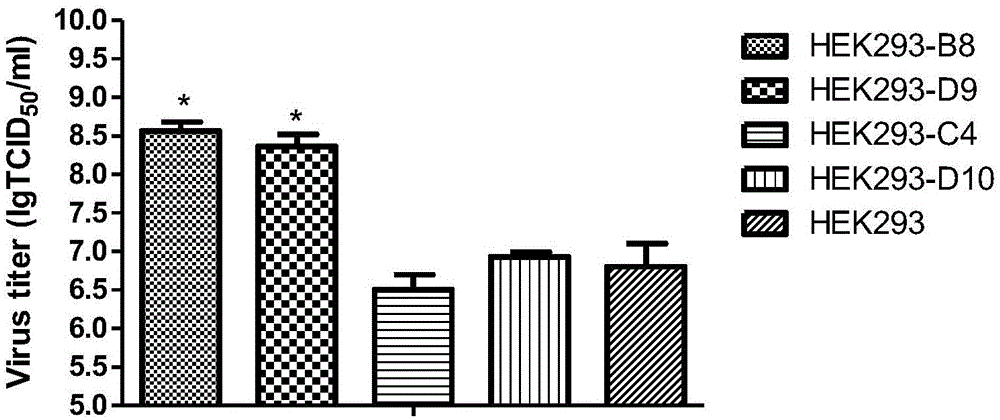
High-adaptability HEK293 clonal cell strains for recombinant adenovirus vaccine strains and application of high-adaptability HEK293clonal cell strains
ActiveCN105154390AEfficient ProliferationHigh titerMicroorganism based processesViruses/bacteriophagesMicroorganismMicrobiology
The invention discloses high-adaptability HEK293 clonal cell strains for recombinant adenovirus vaccine strains and application of the high-adaptability HEK293 clonal cell strains, which belongs to the field of HEK293 clonal cell strains. The high-adaptability HEK293 clonal cell strains and the application thereof are characterized in that a limiting dilution method is adopted for cloning HEK293 single cells; and two monoclonal cell strains with excellent performance are finally screened by subcloning the cloned HEK293 single cells, with microbial preservation numbers of CGMCC (China General Microbiological Culture Collection Center) No. 11096 and CGMCC No. 11097. Compared with a female cell, the two HEK293 monoclonal cell strains provided by the invention have the advantages that the growth speed is remarkably improved, recombinant adenovirus rAdV-SFV-E2 strains can be better replicated, the replication level of viruses is obviously improved, and the better stability is obtained; the two HEK293 monoclonal cell strains are used for replicating the recombinant adenovirus rAdV-SFV-E2 strains, so that virus bulk with higher titer can be obtained, and the virus titer is significantly improved.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI +1
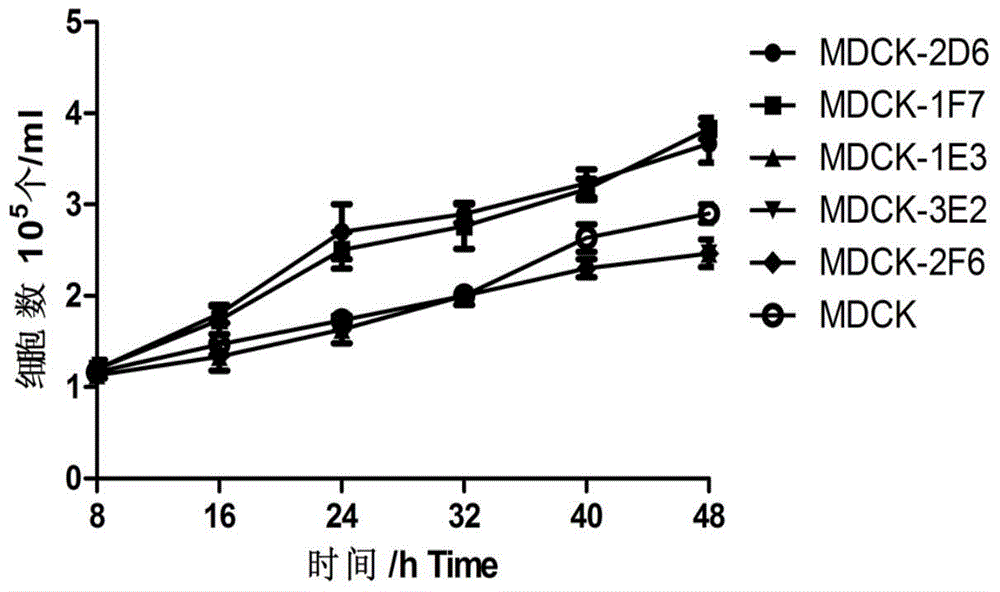
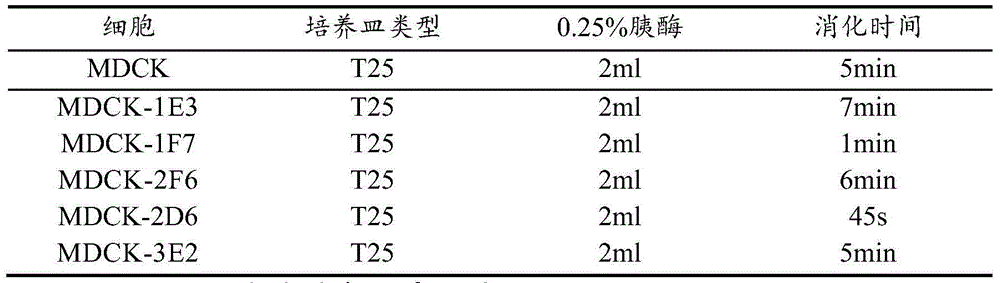
MDCK (Madin-Darby Canine Kidney) clone cell strains and application thereof
ActiveCN104694458AHigh potencyImprove performanceMicroorganism based processesVertebrate cellsVaccine ProductionTGE VACCINE
The invention discloses MDCK (Madin-Darby Canine Kidney) clone cell strains and application thereof, belonging to the field of MDCK clone cell strains. A limiting dilution process is adopted to perform MDCK unicell cloning, and subcloning is further performed to finally obtain 2 monoclonal cell strains with excellent performance. The two monoclonal cell strains have higher growth rate than the female parent, can well reproduce avian influenza virus, obviously enhances the duplication level of the virus in cells, and obtains the virus solution with higher potency. Compared with the virus by female parent cell culture, the HA potency titer is obviously enhanced. The microbe collection numbers of the two cell strains are respectively CGMCC No.10008 and CGMCC No.10009. The MDCK clone cell strains disclosed by the invention can efficiently culture viruses, obviously enhances the virus titer, and has high application value in influenza virus vaccine production.
Owner:哈药集团生物疫苗有限公司
MDS (myelodysplastic syndrome) transfection leukocyte line with capacity of stable expression of GFP (green fluorescent protein)
ActiveCN105670999ATo prevent lossUniform fluorescenceMicroorganism based processesPeptidesBiologyIndividual animal
The invention belongs to the field of microbial and animal cell lines and provides an MDS (myelodysplastic syndrome) transfection leukocyte line with capacity of stable expression of GFP (green fluorescent protein) as well as establishment method and an application of the MDS transfection leukocyte line. A human MDS transfection leukocyte line SKM-1 is taken as maternal cells and is transfected through lentiviruses carrying GFP genes, GFP-positive single cells are obtained with a limiting dilution method and cloned, cells after amplified culture are screened in a mouse body in a subcutaneous injection manner, tumor masses are separated after tumorigenesis and cultured continuously, and the MDS transfection leukocyte line SKM-1 / GFP with stable expression of GFP is obtained. The morphology, growth characteristics and the growth curve of the cell line have no difference with those of the maternal cells, and the cell line can express GFP stably, has tumorigenicity, can be further applied to establishment of a murine animal model and provides a platform for MDS, minimal residual disease and other preclinical study.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
Method for detecting trichinella circulating antigen by utilizing IgY-McAb sandwich ELISA (enzyme-linked immuno sorbent assay)
InactiveCN102331501AEnable early detectionIncreased sensitivityMaterial analysisTrichinella speciesZoology
The invention discloses a method for detecting a trichinella circulating antigen by utilizing IgY-McAb sandwich ELISA (enzyme-linked immnuo sorbent assay). With an anti-trichinella muscle larval ES antigen egg yolk antibody IgY as a capture antibody and an anti-trichinella ES antigen McAb as a detection antibody, the preparation method of the IgY comprises the following steps of: adding the trichinella muscle larval into a culture medium to carry out sterile culture, purifying and dialyzing supernate and then concentrating and drying to obtain the ES antiagen, determining protein concentration and then immunizing roman legehenne by utilizing the ES antigen, collecting the IgY from the produced egg yolk by adopting a saturate ammonium sulphate precipitation method, purifying and determining concentration, and sub-packaging for later use; and the preparation method of the McAb comprises the following steps of: establishing a hybridoma cell strain secreting the anti-trichinella muscle larval ES antigen, cloning by adopting a limiting dilution method, purifying McAb by applying an octanoic acid-ammonia sulphate method, determining the concentration and sub-packaging for later use. The invention has the advantage that a new method which has high sensitivity and specificity and has wide application prospect is provided for early detection of trichinella CAg in blood serum of an infected animal.
Owner:ZHENGZHOU UNIV
Flp-In-293 cell line for expressing bovine gamma interferon and establishment method thereof
InactiveCN102321589AVector-based foreign material introductionForeign genetic material cellsHygromycin BLipofectamine
The invention relates to an Flp-In-293 cell line for expressing a bovine gamma interferon. The cell line is named B1 and contains a gene for encoding the bovine gamma interferon. A method for constructing the cell line B1 comprises the following steps of: constructing preBoIFN-gamma fragments which are correctly sequenced into a recombinant plasmid pcDNA5 / FRT-preBoIFN-gamma-FLAG, cotransfecting Flp-In-293 cells by using the recombinant plasmid pcDNA5 / FRT-preBoIFN-gamma-FLAG and a recombinase expression vector pOG44 by a liposomal transfection method, screening positive clones by a dulbecco's modified eagle medium (DMEM) containing 120mu g / mL Hygromycin B, performing amplification culture, subcloning through limiting dilution analysis, and identifying to obtain a recombinant cell line. The bovine gamma interferon secreted by the cell line B1 has extremely high specific activity and stability.
Owner:YANGZHOU UNIV
Preparation method of beta-casein monoclonal antibody, beta-casein colloidal gold test strip and preparation method thereof
InactiveCN103864929AEasy to operateHigh resolutionImmunoglobulins against animals/humansBiological testingBALB/cAntigen
The invention relates to the technical field of biology and provides a preparation method of a beta-casein monoclonal antibody, a beta-casein colloidal gold test strip and a preparation method thereof. The technical scheme is as follows: immunizing a BALB / c mouse by taking beta-casein as an antigen, taking mouse splenocytes with best immune effects to perform fusion with myeloma cells SP2 / 0, taking beta-casein, alpha s1-casein and K-casein as detection antigens, detecting supernatant of hybridoma cells by enzyme-linked immunosorbent assay, screening, retaining positive holes, further cloning by using a limiting dilution method to obtain a hybridoma cell strain which can stably secrete the anti-beta-casein monoclonal antibody, and then performing large-scale preparation and purification of the obtained monoclonal antibody. After the beta-casein monoclonal antibody obtained by the method is labeled by colloidal gold, the beta-casein monoclonal antibody can be adsorbed on a gold standard conjugate pad to prepare the colloidal gold test strip. The beta-casein colloidal gold test strip disclosed by the invention can realize fast specific detection and high resolution and shorten detection time when used for judging whether the casein content in raw milk achieves a standard or not.
Owner:长沙安迪生物科技有限公司
Single cell clone culture method
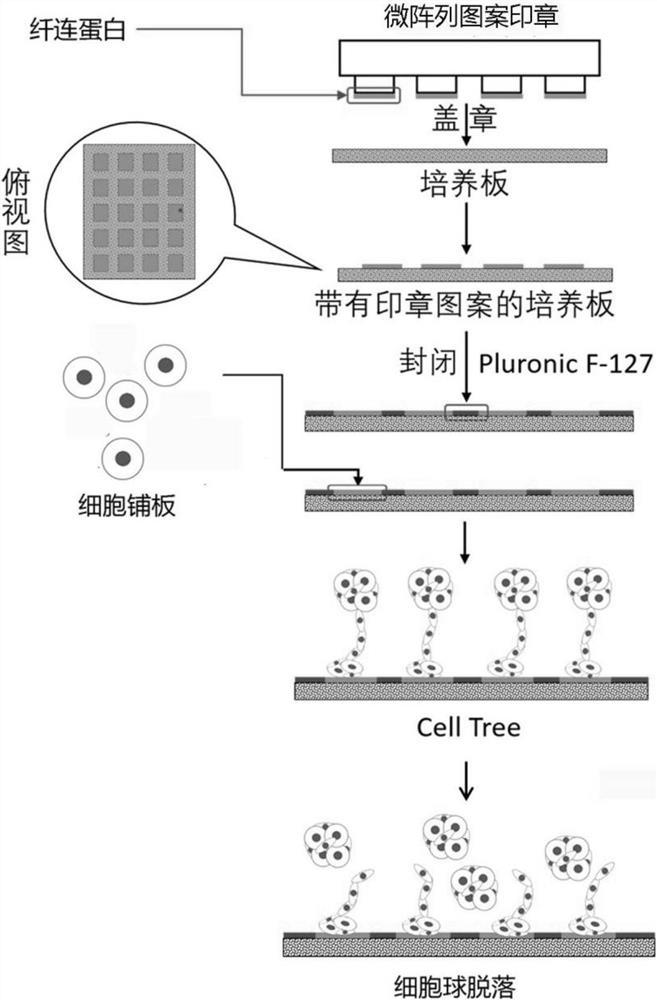
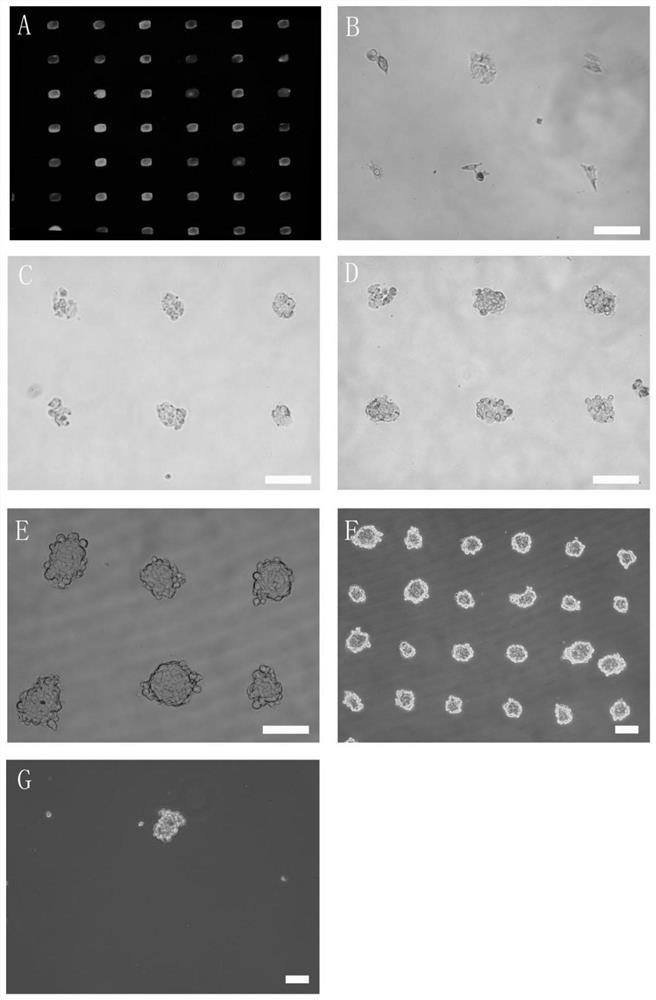
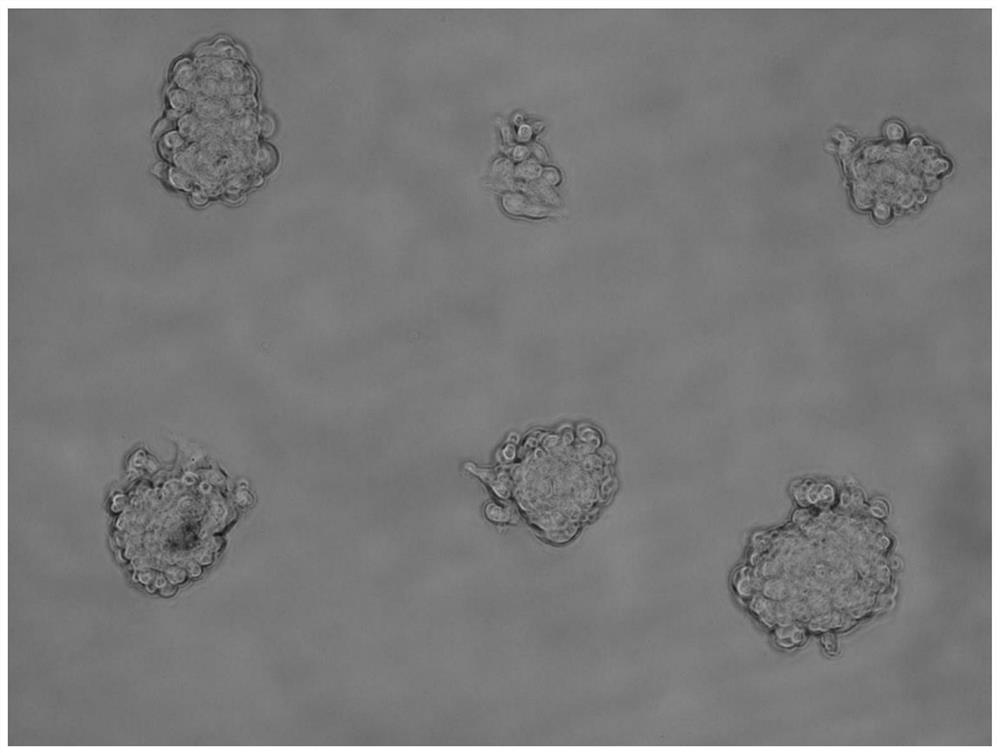
ActiveCN111826285AImprove efficiencyImprove throughputGenetically modified cellsStable introduction of DNAFibronectinsSingle cell sequencing
The invention discloses a single cell clone culture container. The single cell clone culture container is a cell culture container with a fibronectin imprinting block array on the bottom surface, an area, that is not covered with fibronectin, on the bottom surface is sealed by a sealing agent, and an area of a single imprinting block is 225-625 [mu]m<2>. The invention also discloses a single cellclone culture method, and the method is to inoculate cells into the aforementioned culture container for culture. The single cell clone culture method of the invention has high throughput, can culturethousands of single cell clone groups at a time, and can also overcome the problem that the single cell clone is impure and cannot grow normally caused by the traditional limiting dilution method. Inaddition, the method can be applied to many fields such as gene editing, tumor clone culture, and single cell sequencing sample preparation, and has broad application prospects.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV +1
Method for preparing cassava calmodulin monoclonal antibody, and antibody prepared with method
The invention provides a method for preparing a manihot esculanta crantz calmodulin (CaM) monoclonal antibody, and the antibody prepared with the method. The method comprises the following steps: immunizing mice by purified fusion protein ACP-CaM and adjuvants; intraperitoneally impacting the mice with the fusion protein ACP-CaM diluted by normal saline; fusing spleen cells of the mice and myeloma cells; after fusion, performing cultivation by adopting HAT culture liquid and performing indirect ELISA detection screening to obtain positive hybridoma cells; cloning the positive hybridoma cells with a limiting dilution method until the positive monocloning rate is 100 percent; excluding the positive hybridoma cells, of which the antibodies crossly react with tag protein ACP; preparing a calmodulin monoclonal antibody with a mouse in vivo ascites induction method; and purifying the calmodulin monoclonal antibody. The calmodulin monoclonal antibody prepared with the method provided by the invention has high valence, high stability and high specificity.
Owner:TROPICAL CORP STRAIN RESOURCE INST CHINESE ACAD OF TROPICAL AGRI SCI
Beta-glucosidase monoclonal antibody and preparing method thereof
The invention provides a preparing method for a beta-glucosidase monoclonal antibody. The method comprises the steps of 1, injecting antigen beta-glucosidase into immunized mice; 2, measuring the serum titer of each immunized mouse with the indirect ELISA method; 3, selecting the mice with serum titer higher than 20000, and fusing splenocytes with myeloma cells; 4, conducting culture with HAT culture solution and conducting indirect ELISA test screening to obtain positive hybridoma cells; 5, cloning the positive hybridoma cells with the limiting dilution method till positive monoclonal rate is 100%; 6, preparing the beta-glucosidase monoclonal antibody with the mouse in-vivo ascites induction method; 7, purifying the beta-glucosidase monoclonal antibody. The beta-glucosidase monoclonal antibody prepared with the method has high titer, stability and specificity.
Owner:TROPICAL CORP STRAIN RESOURCE INST CHINESE ACAD OF TROPICAL AGRI SCI
Cell model and screening method for screening calcium-activated chloride ion channel inhibitor
InactiveCN103898059AQuick filterSensitive and Specific ScreeningMicrobiological testing/measurementVector-based foreign material introductionHigh concentrationTwo-vector
The invention provides a cell model and a screening method for screening a calcium-activated chloride ion channel inhibitor. The screening method sequentially comprises the steps of (1) constructing eukaryotic expression vectors of Anoctamin1 or Anocatmin2 and YFP-H148Q / I152L; (2) stably transfecting the two vectors into FRT (Fisher rat thyroid) cells respectively to ensure that the RFT cells co-express two proteins of YFP-H148Q / I152l and Anoctamin1 or Anocatmin2, and performing multiple times of limiting dilution to obtain an FRT cell model; (3) transferring the FRT cell model to a black-wall microporous plate with a black wall and a transparent bottom; (4) adding a small molecular compound to be detected into the black-wall microporous plate, incubating, then adding a regent for raising the concentration of calcium ions in cells and high-concentration iodide ions, and screening the calcium-activated chloride channel inhibitor by detecting the change of relative fluorescence intensity.
Owner:JINLIN MEDICAL COLLEGE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com