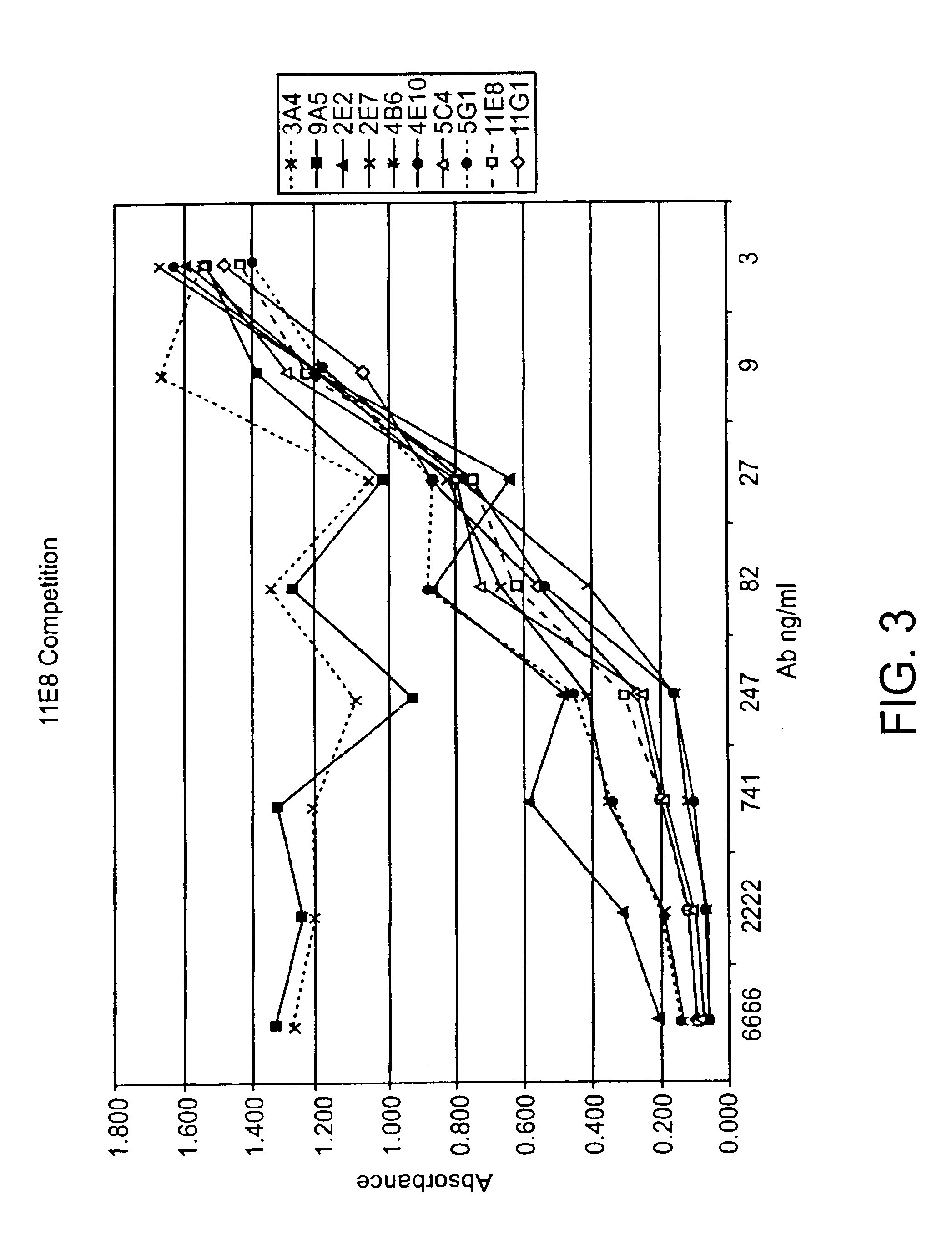
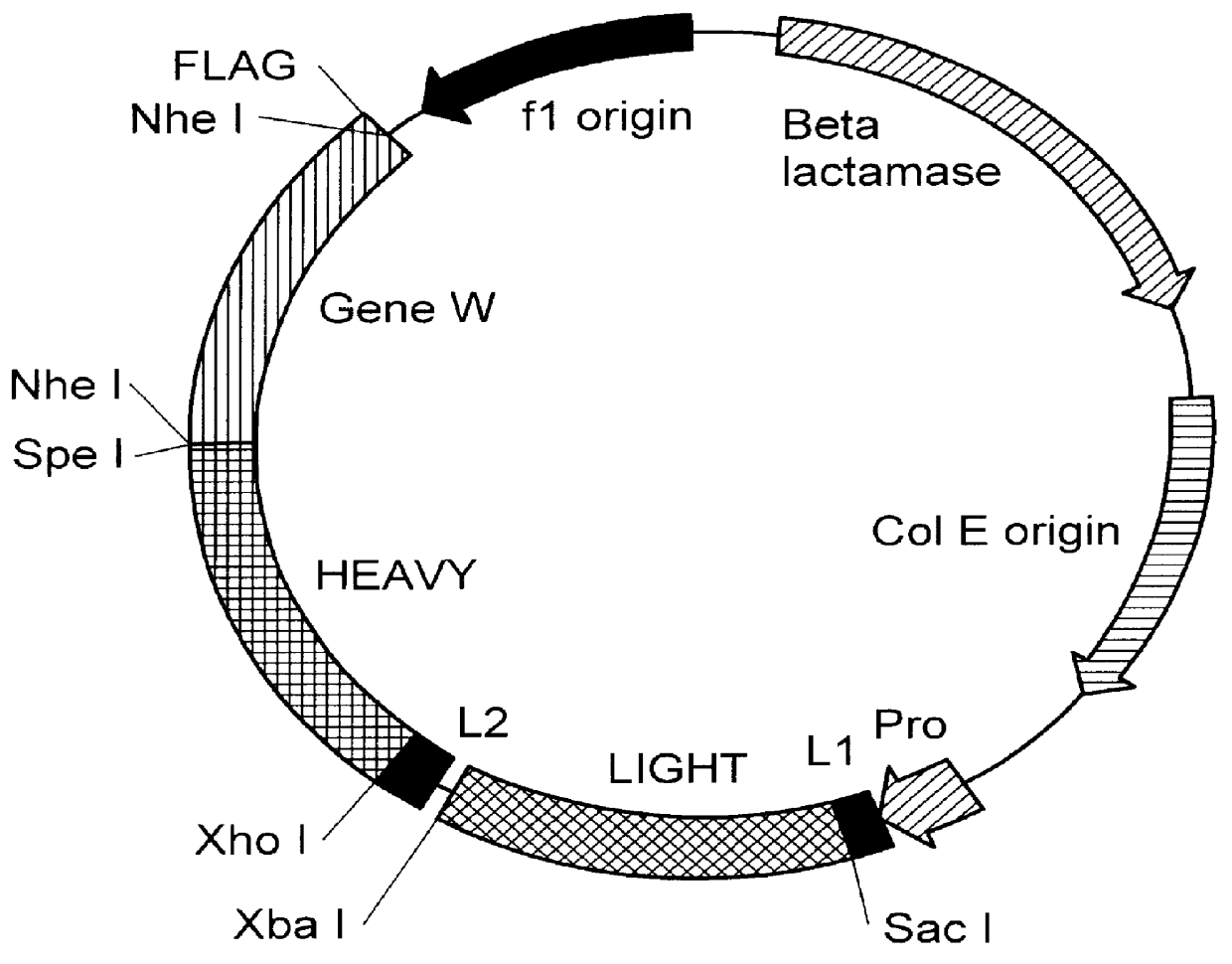
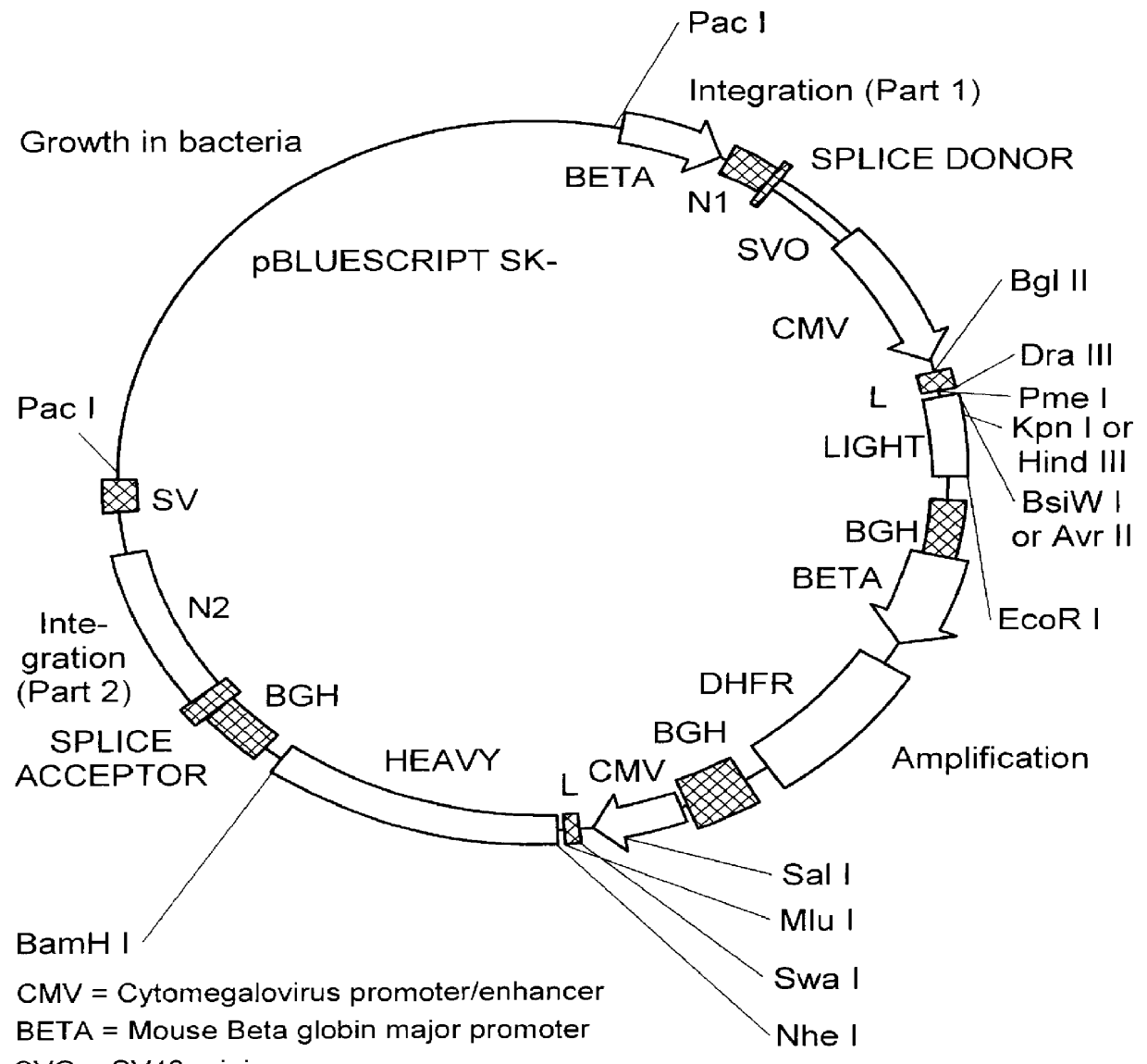
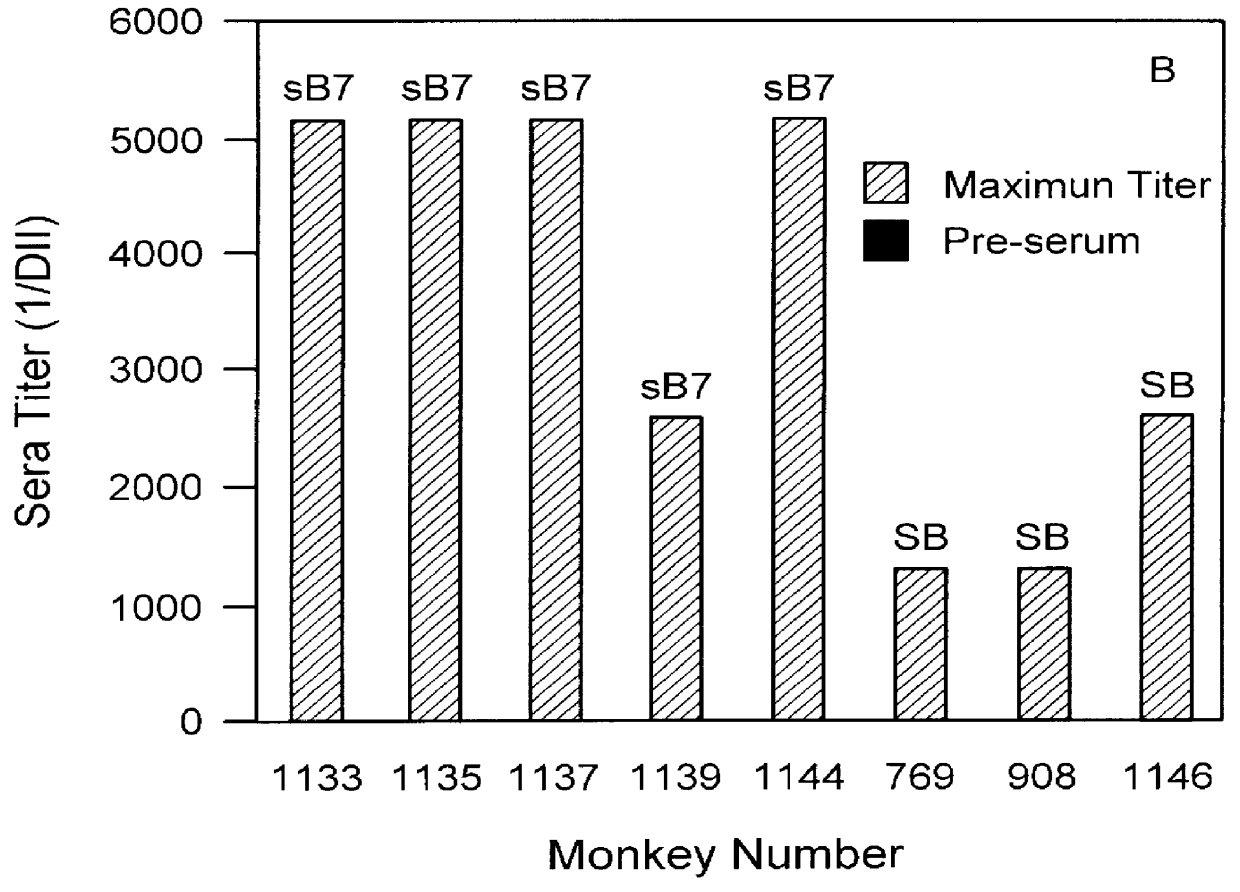
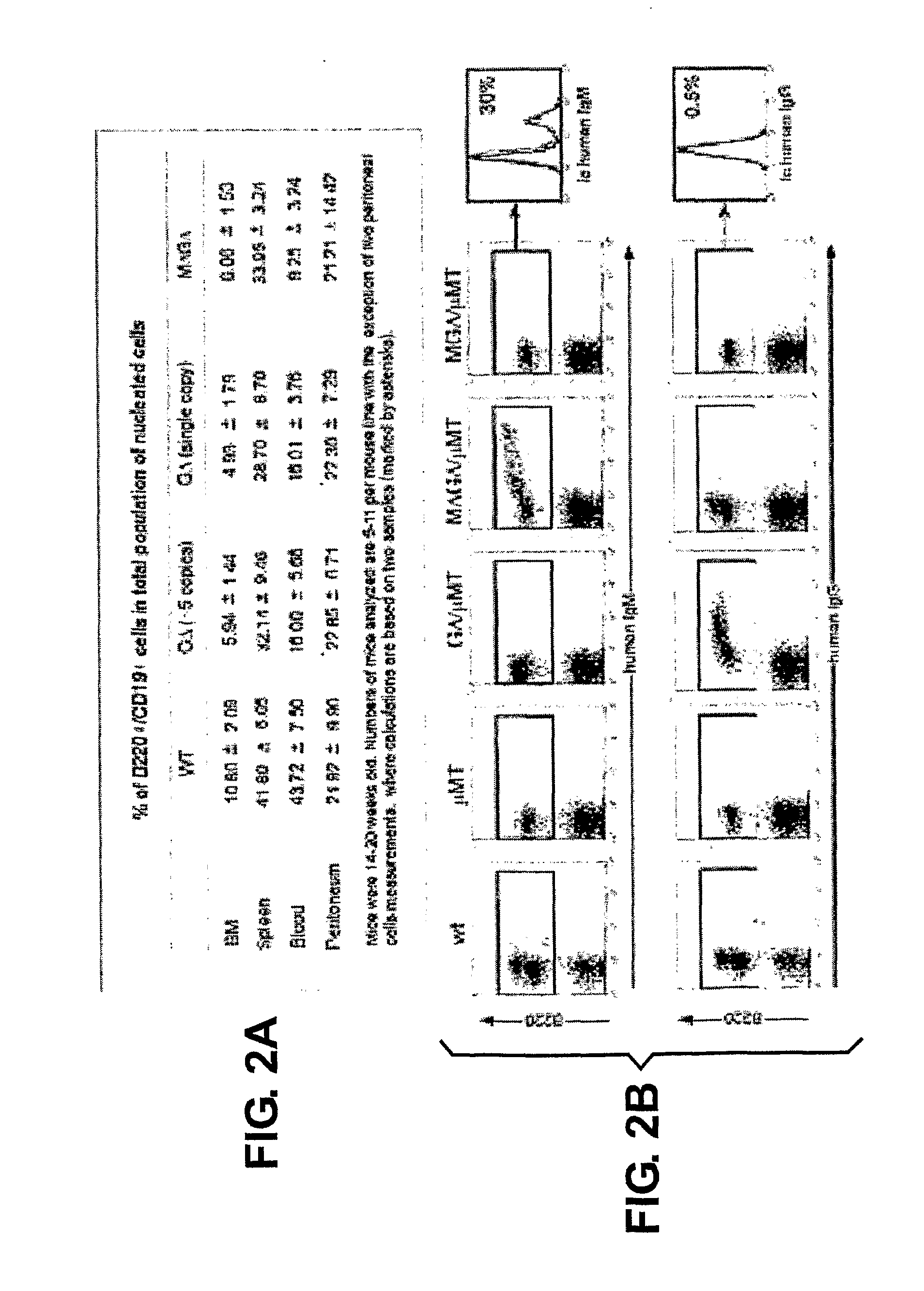
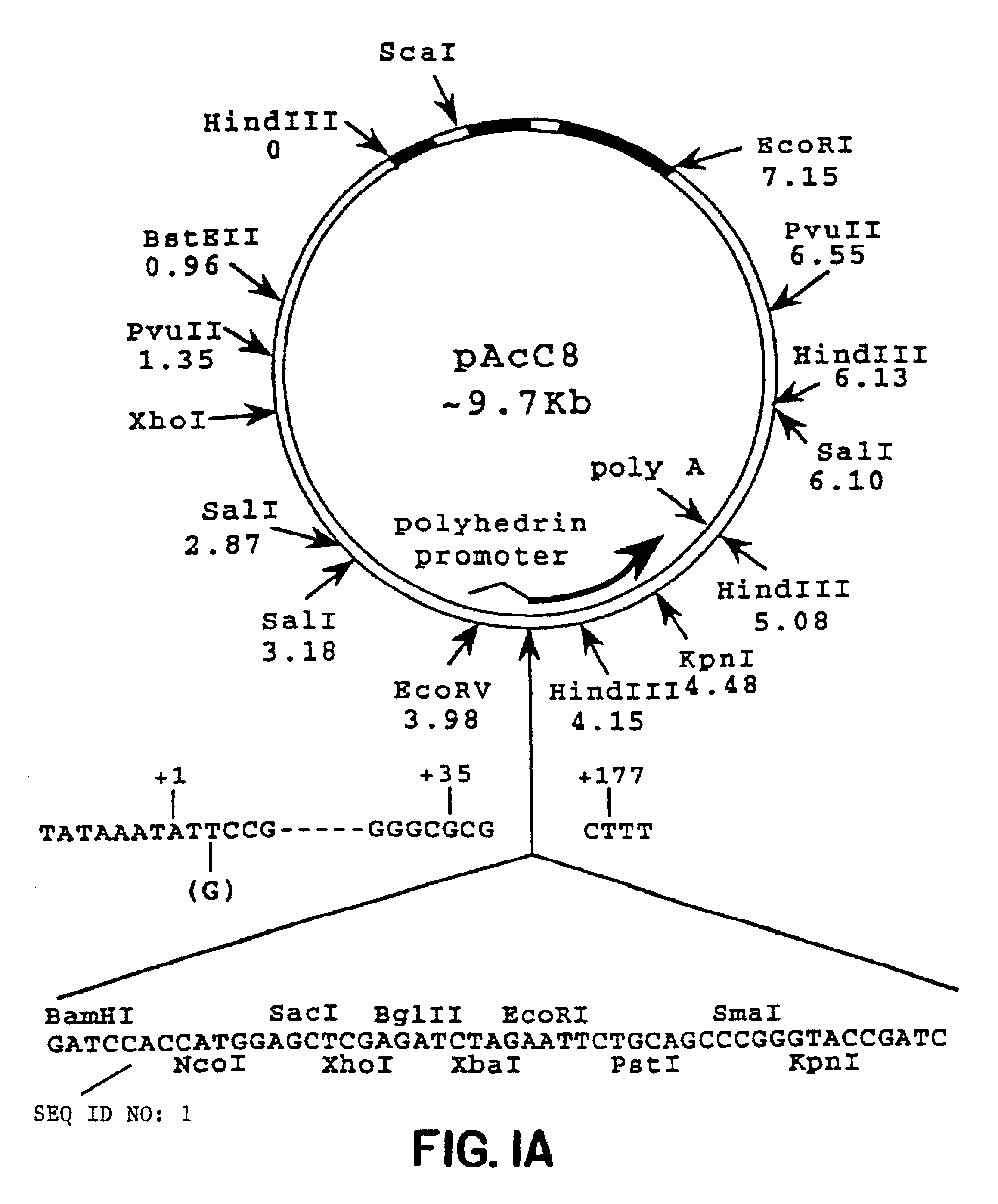
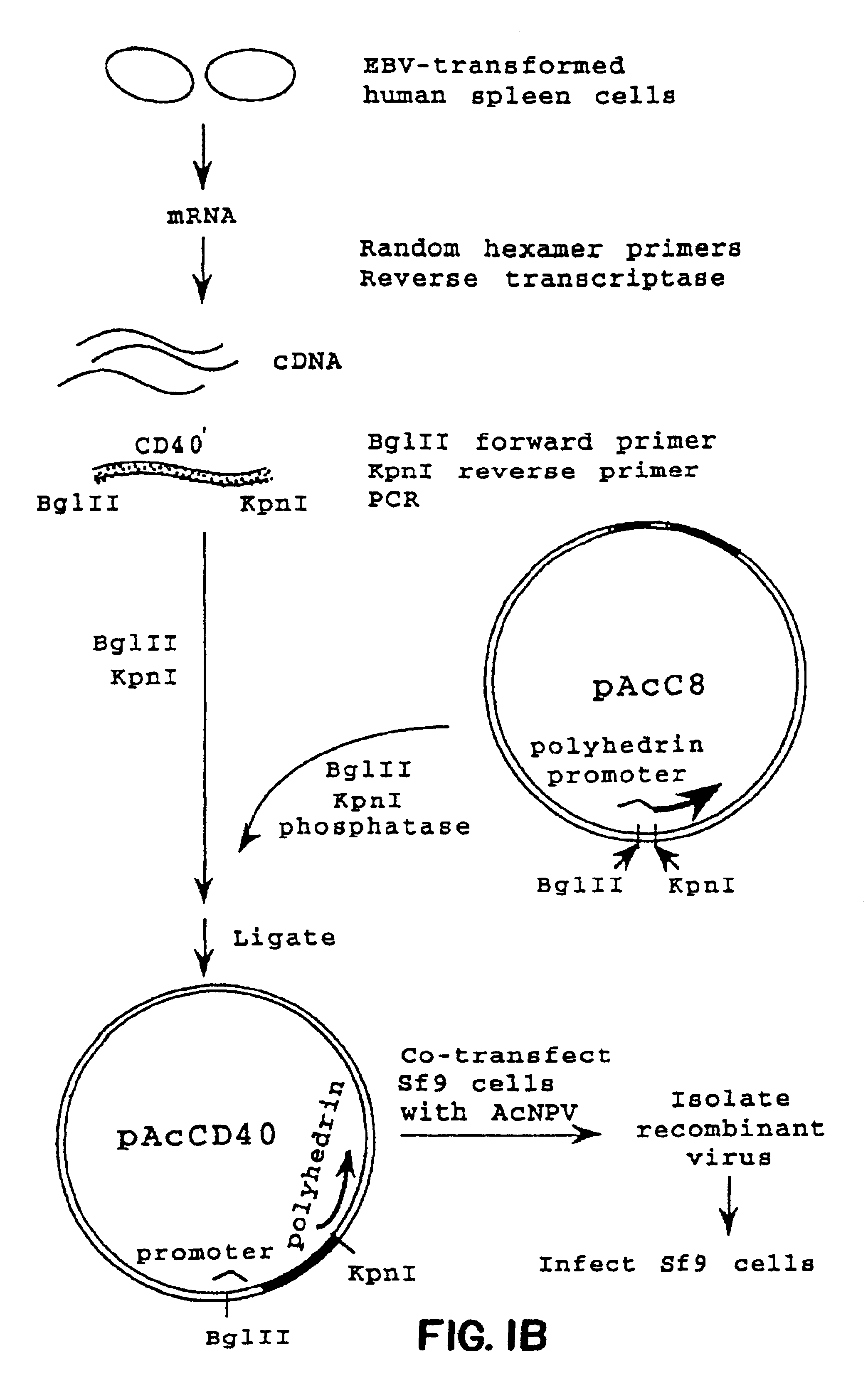
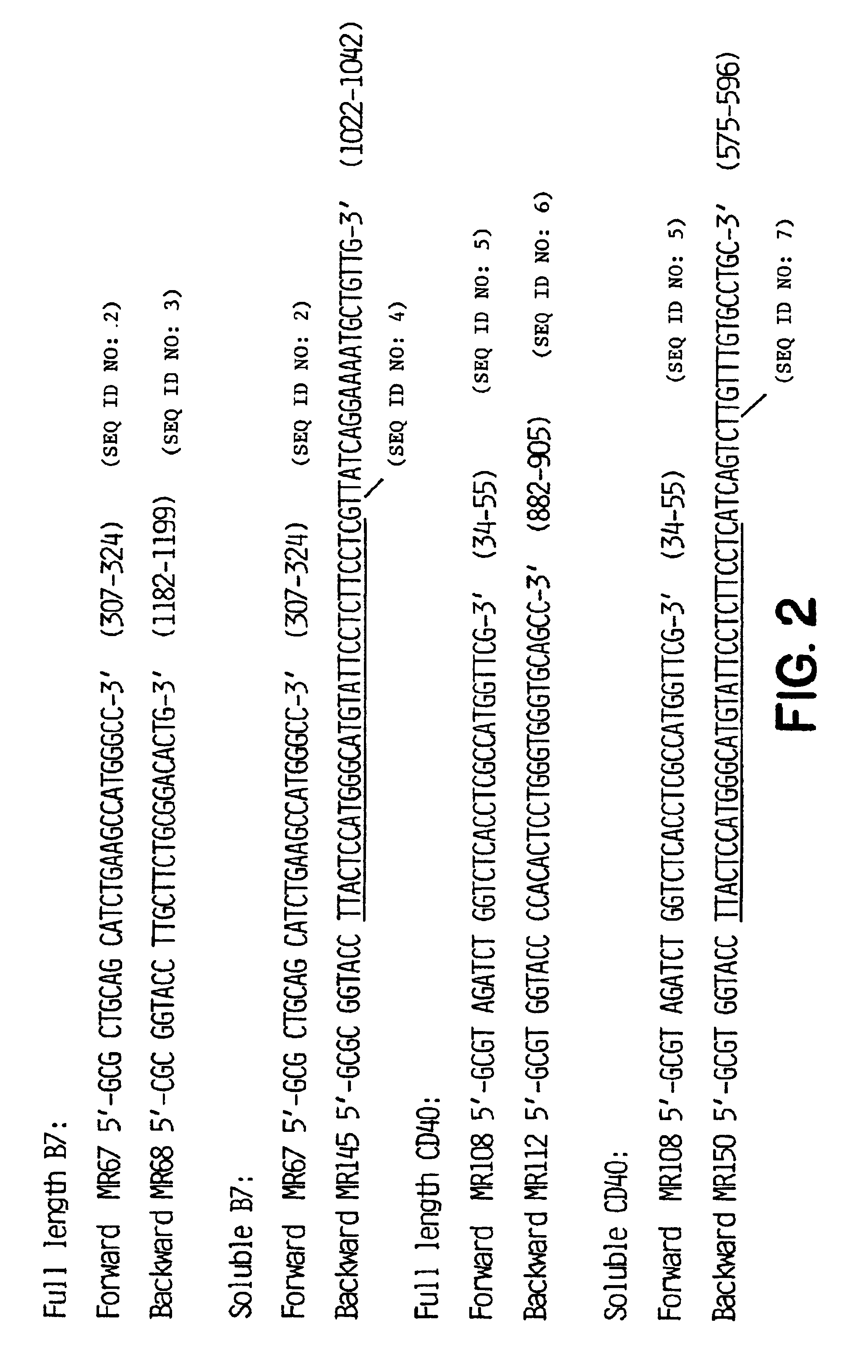
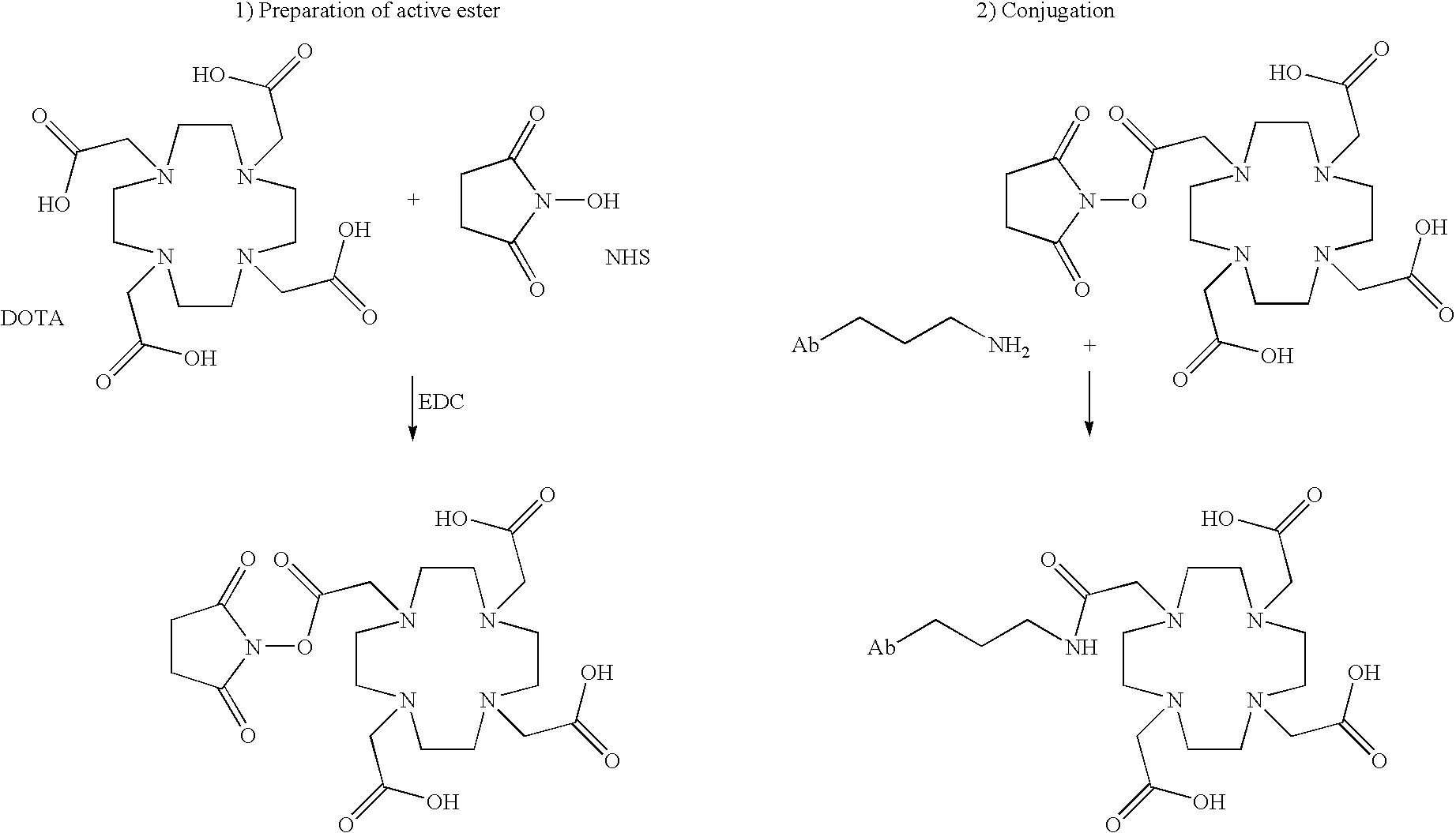
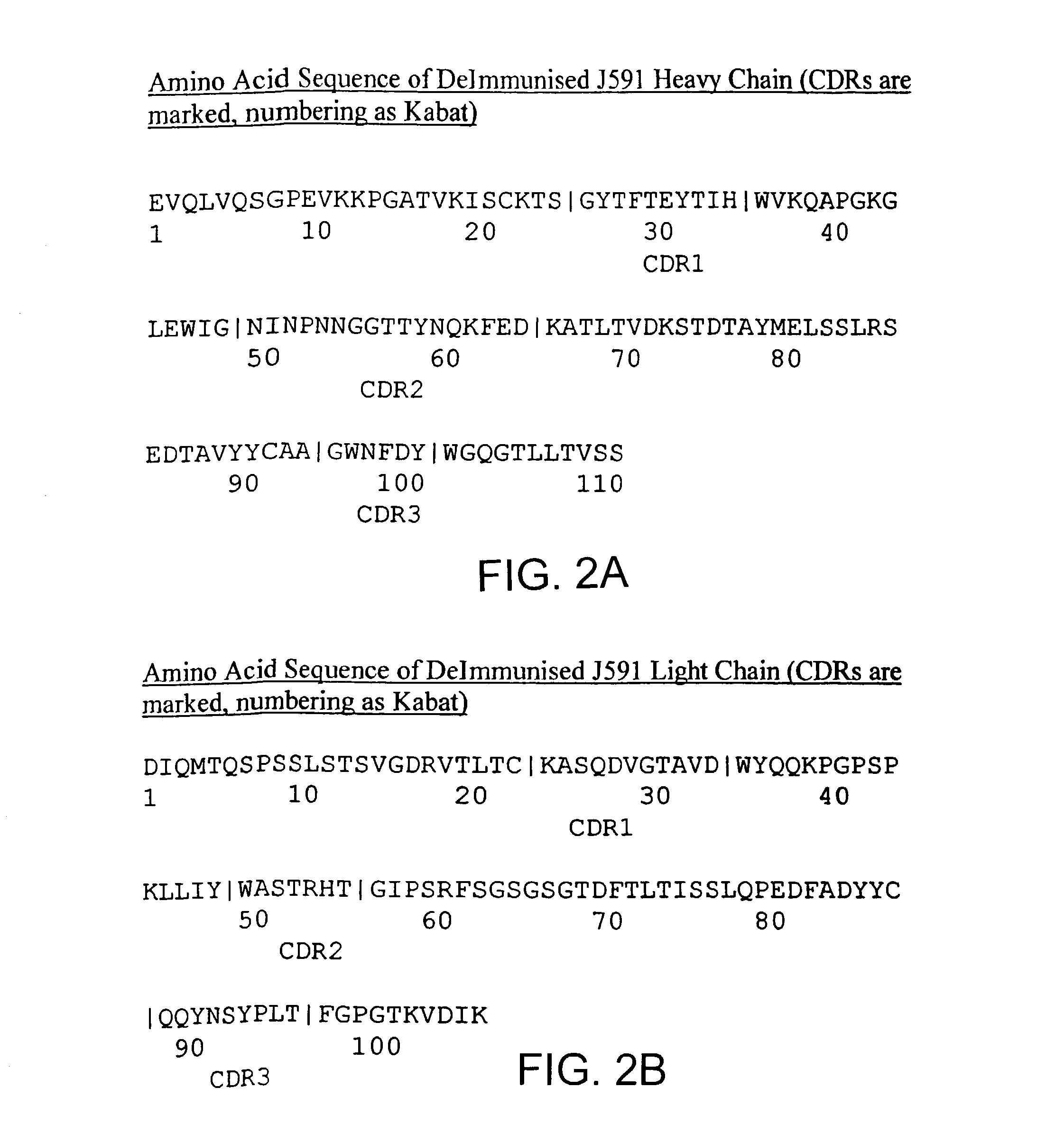
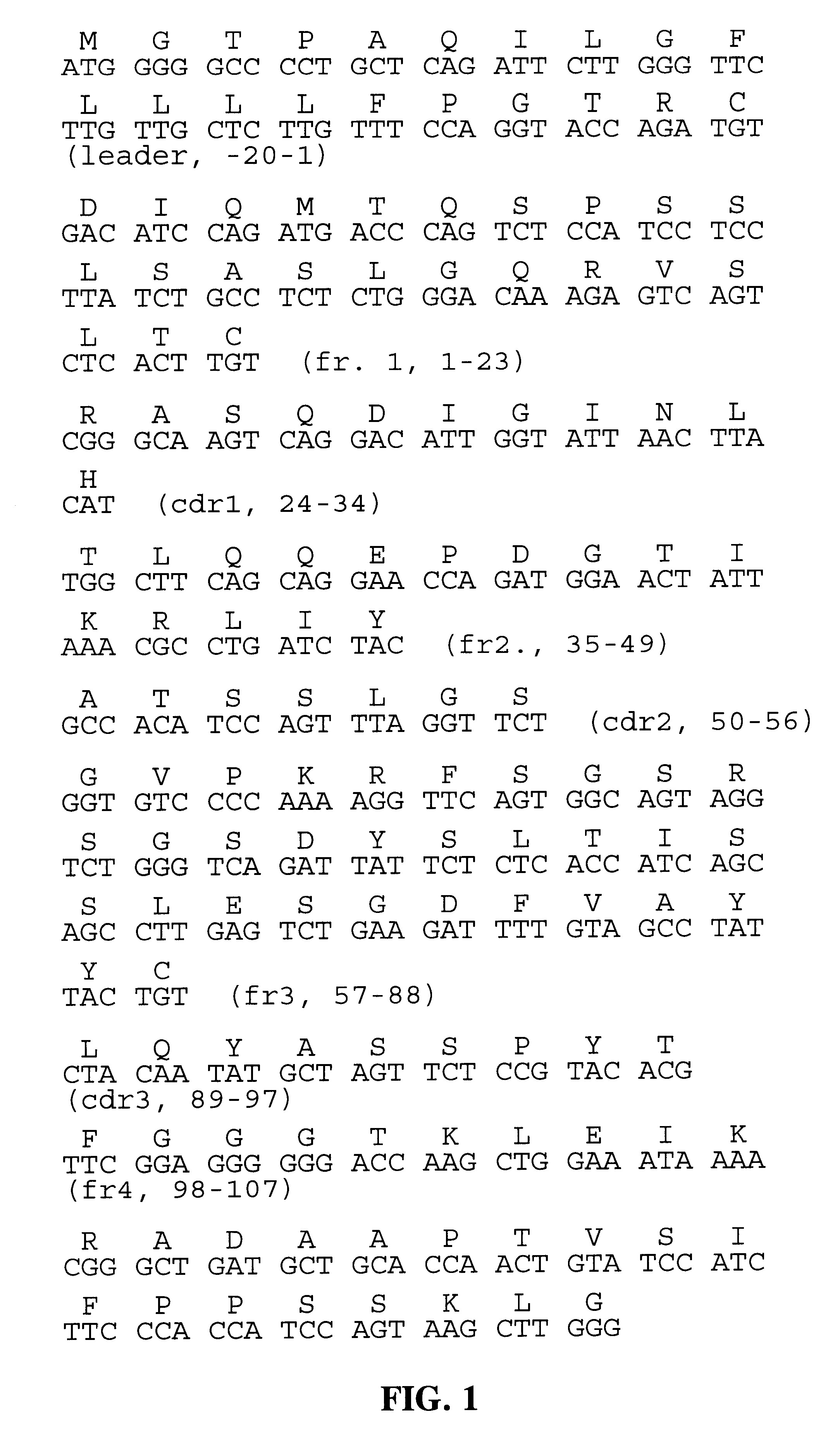Patents
Literature
1097results about "Hybrid cell preparation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
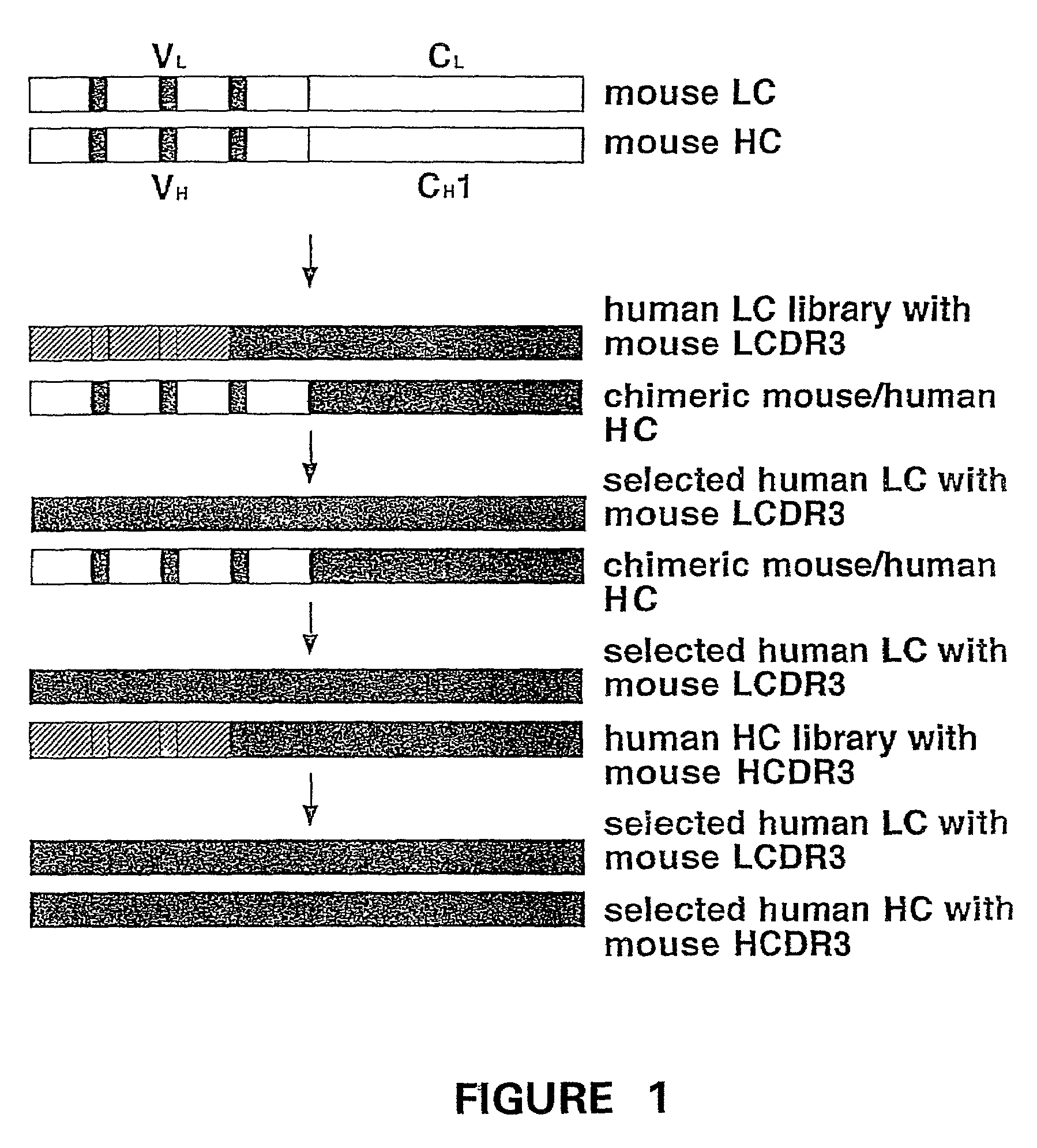
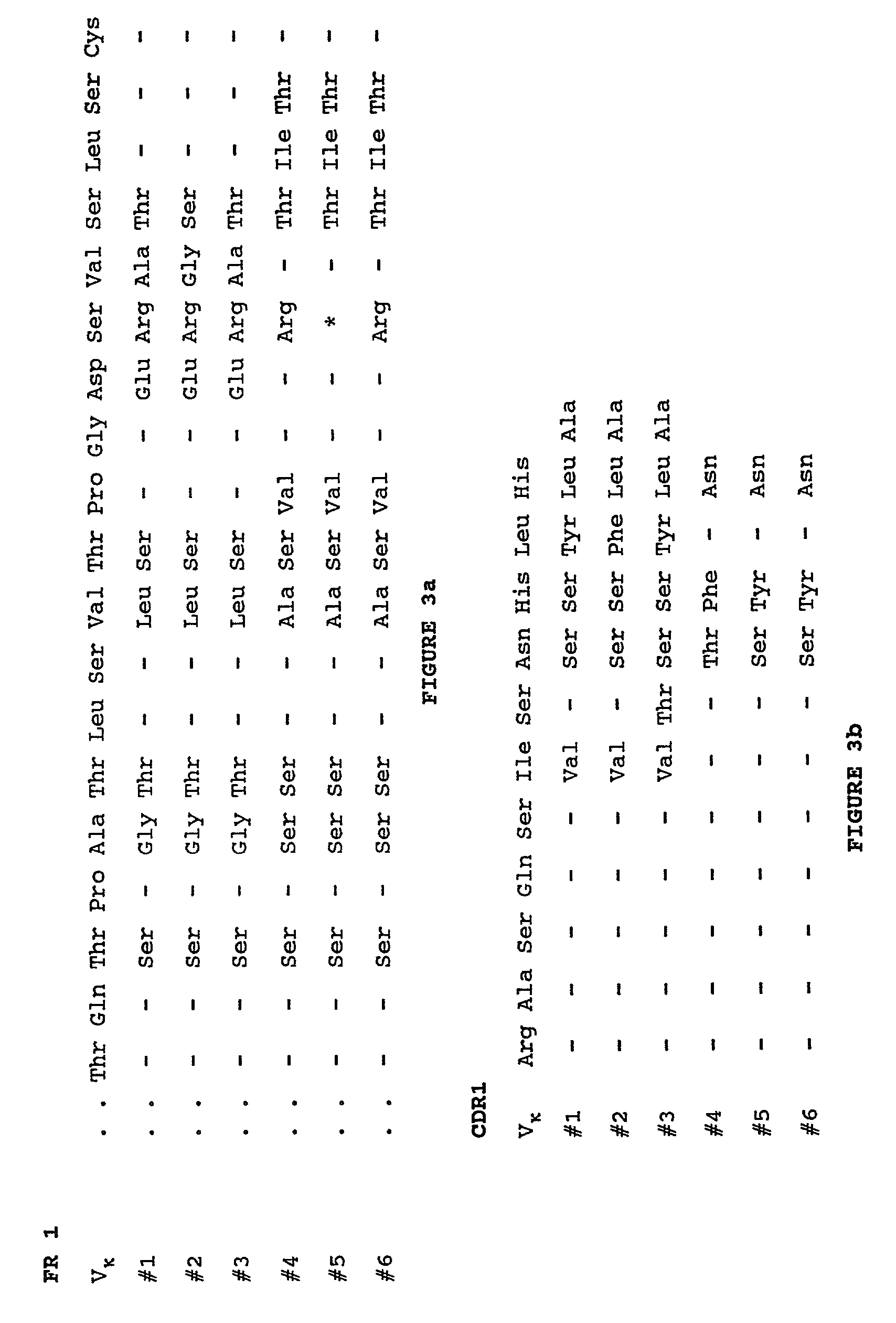
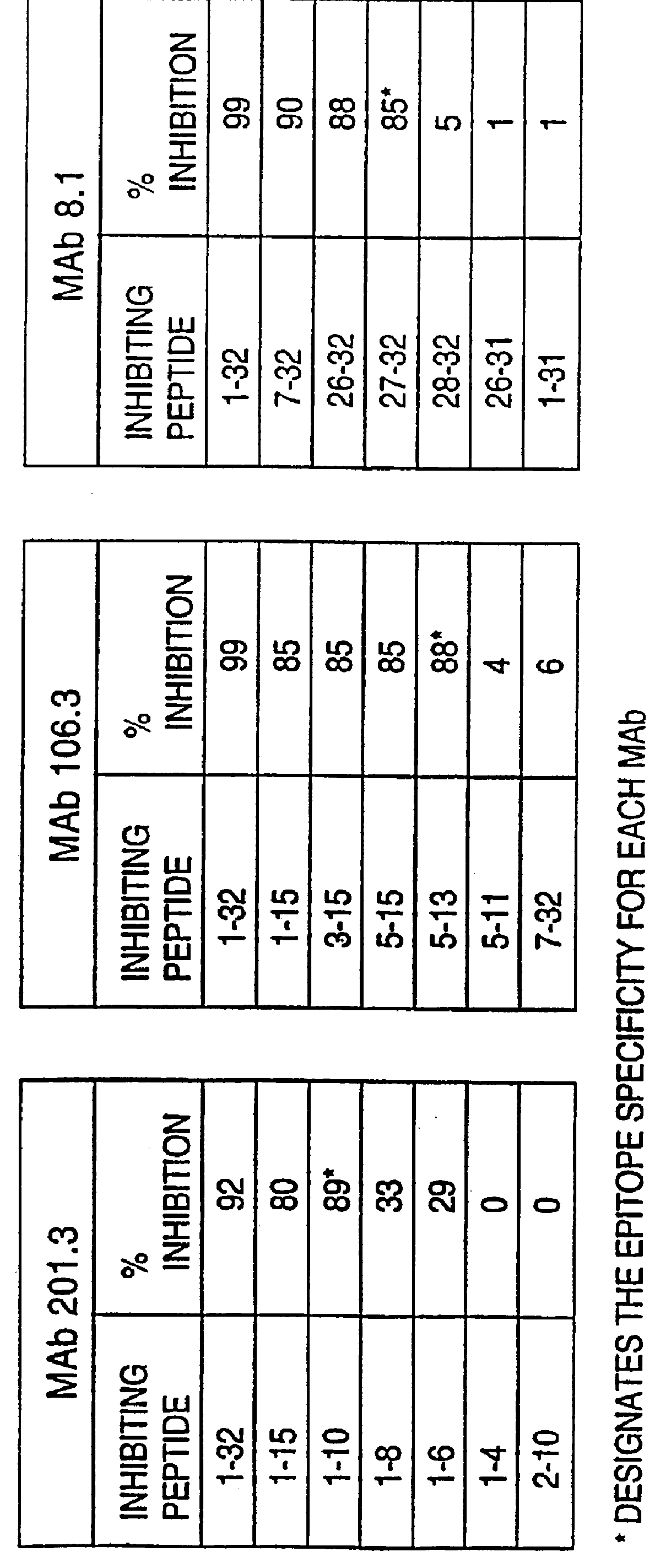
Humanization of murine antibody
InactiveUS7087409B2Hybrid immunoglobulinsSugar derivativesComplementarity determining regionHeavy chain
A humanized murine antibody is provided. The amino acid sequences of a light chain complementarity determining region from a mouse antibody are grafted onto a human light chain, and a heavy chain complementarity determining region from a mouse antibody are grafted onto a human antibody heavy chain to produce libraries from which a humanized murine antibody having the desired specificity is selected.
Owner:THE SCRIPPS RES INST
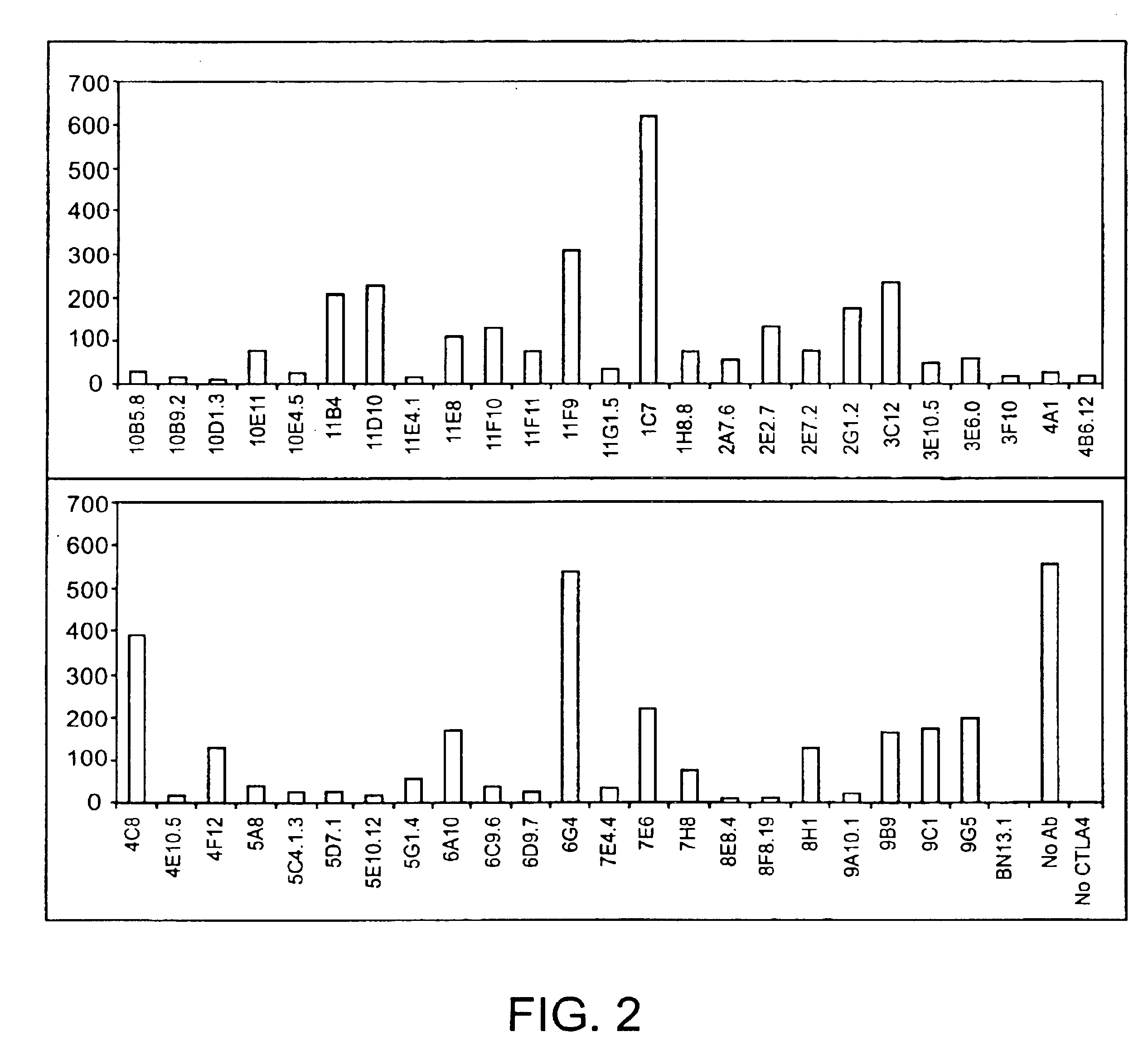
Human CTLA-4 antibodies
The present invention provides human sequence antibodies against CTLA-4 and methods of treating human diseases, infections and other conditions using these antibodies.
Owner:ER SQUIBB & SONS INC
Human B7.1-specific primatized antibodies and transfectomas expressing said antibodies
InactiveUS6113898AShrink tumorInhibit tumor growthPeptide/protein ingredientsAntipyreticDiseaseOrgan transplant rejection
The present invention relates to the identification of macaque antibodies to human B7.1 and B7.2 by screening of phage display libraries or monkey heterohybridomas obtained using B lymphocytes from B7.1 and / or B7.2 immunized monkeys. More specifically, the invention provides four monkey monoclonal antibodies 7B6, 16C10, 7C10 and 20C9 which inhibit the B7:CD28 pathway and thereby function as effective immunosuppressants. The invention further provides the complete DNA and amino acid sequences of the light and heavy chain of three primatized antibodies derived from those monkey monoclonal antibodies which bind B7.1 and possibly B7.2, primatized 7C10, primatized 7B6 and primatized 16C10. These primatized and monkey antibodies may be used as specific immunosuppressants, e.g., for the treatment of autoimmune diseases and to prevent organ transplant rejection.
Owner:BIOGEN INC
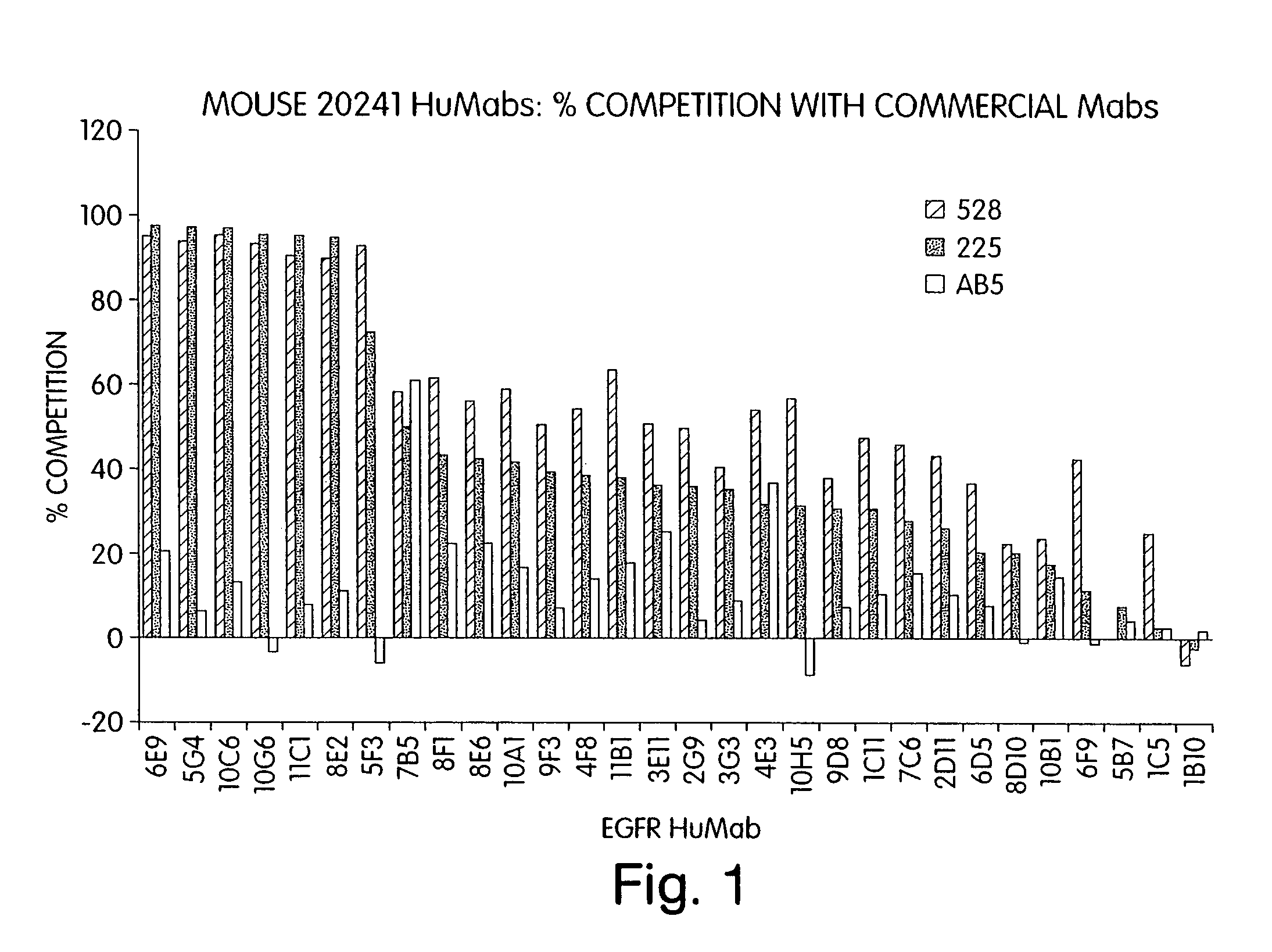
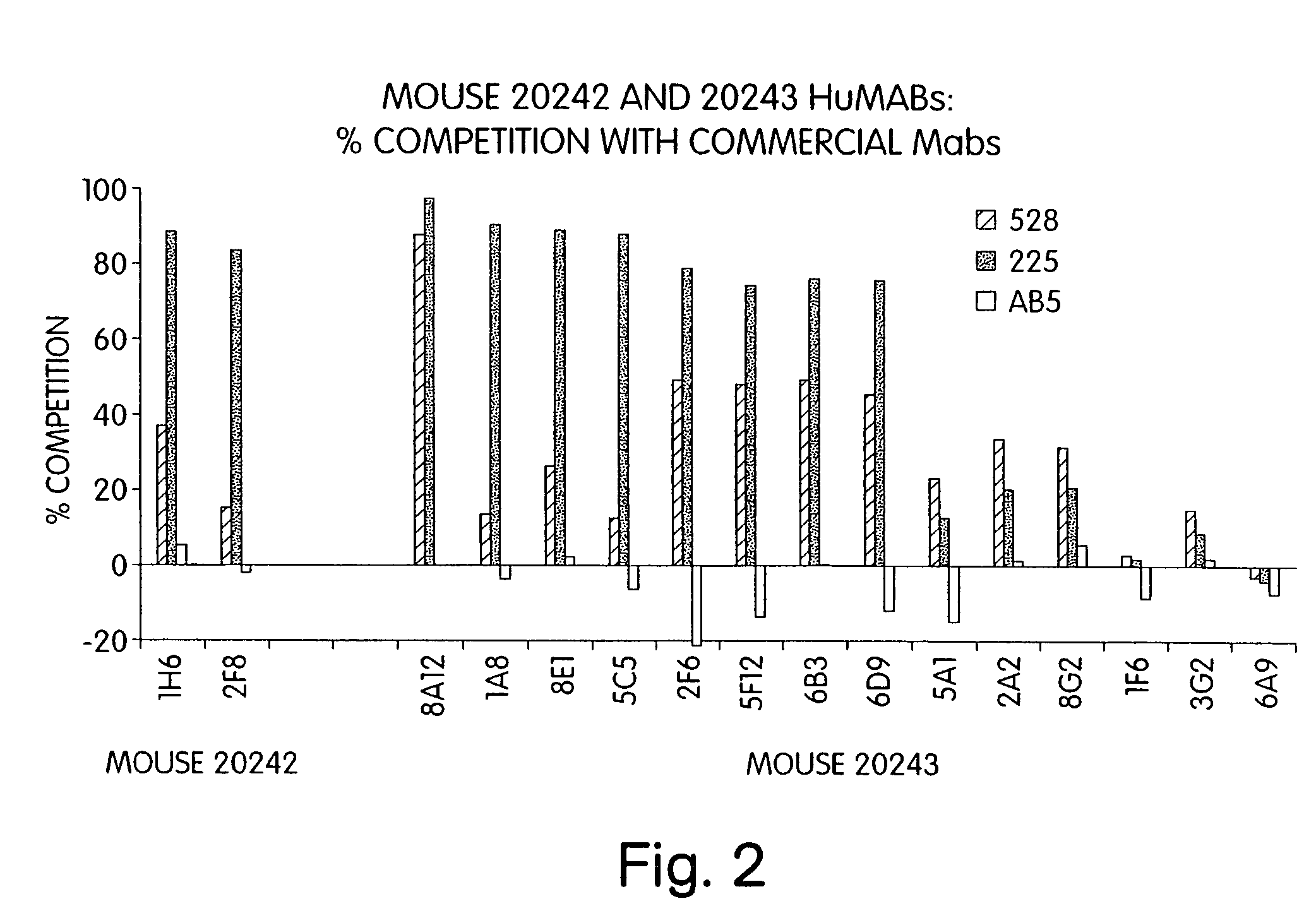
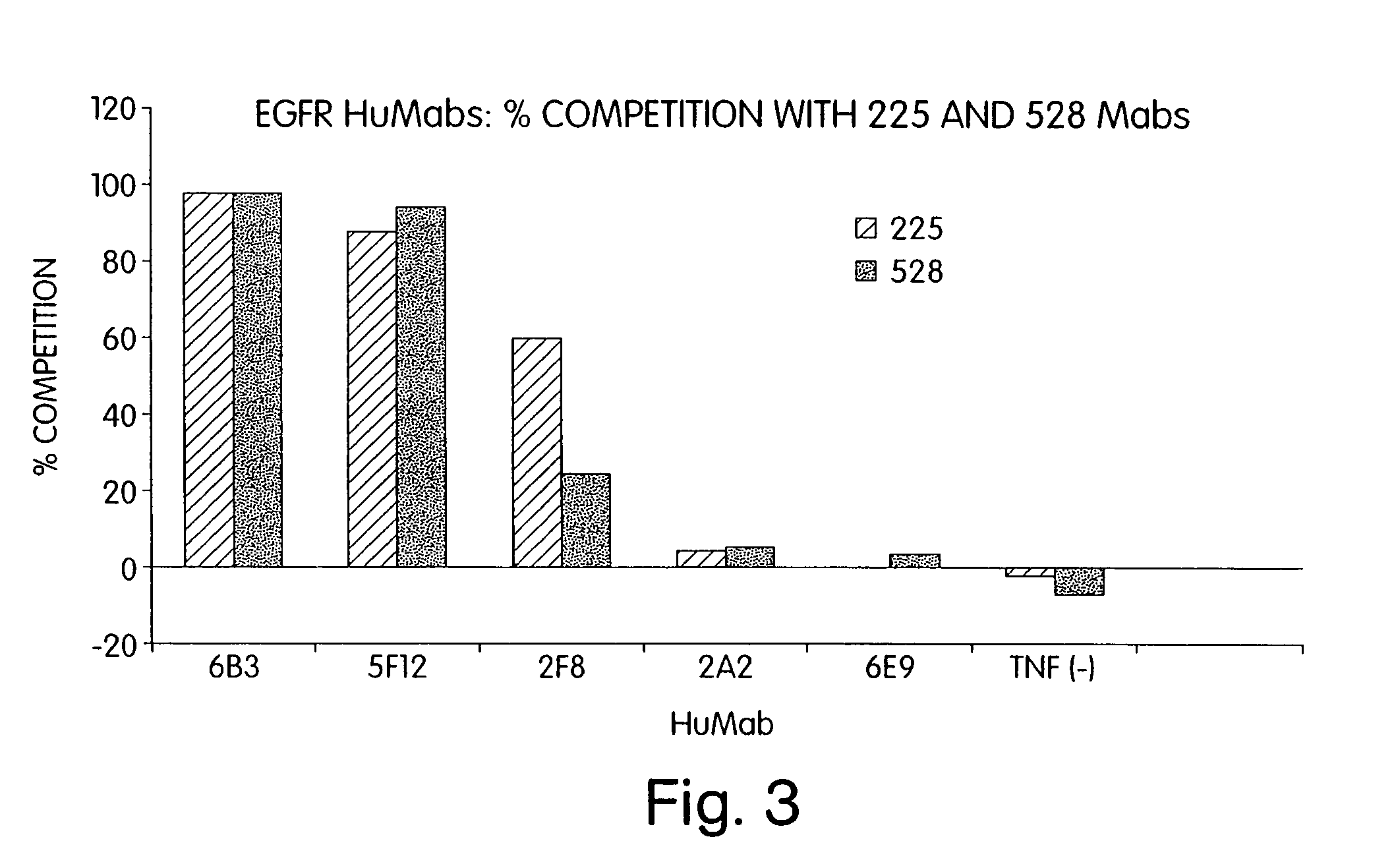
Human monoclonal antibodies to epidermal growth factor receptor (EGFR)
InactiveUS7247301B2Less immunogenicReduce adverse side effectsInorganic active ingredientsImmunoglobulins against cytokines/lymphokines/interferonsV(D)J recombinationHuman epidermal growth factor receptor
Isolated human monoclonal antibodies which specifically bind to human EGFR, and related antibody-based compositions and molecules, are disclosed. The human antibodies can be produced by a transfectoma or in a non-human transgenic animal, e.g., a transgenic mouse, capable of producing multiple isotypes of human monoclonal antibodies by undergoing V-D-J recombination and isotype switching. Also disclosed are pharmaceutical compositions comprising the human antibodies, non-human transgenic animals and hybridomas which produce the human antibodies, and therapeutic and diagnostic methods for using the human antibodies.
Owner:GENMAB INC
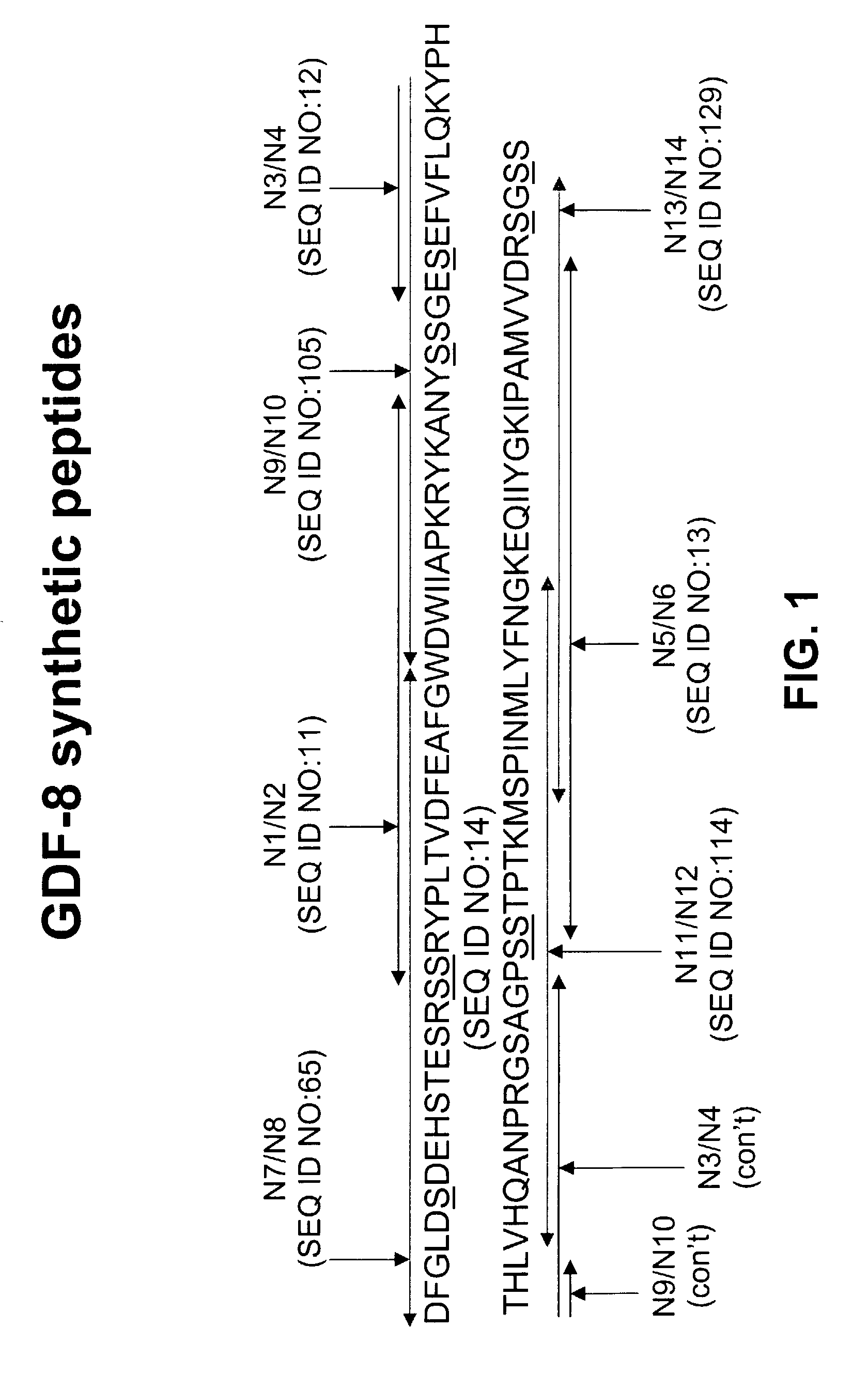
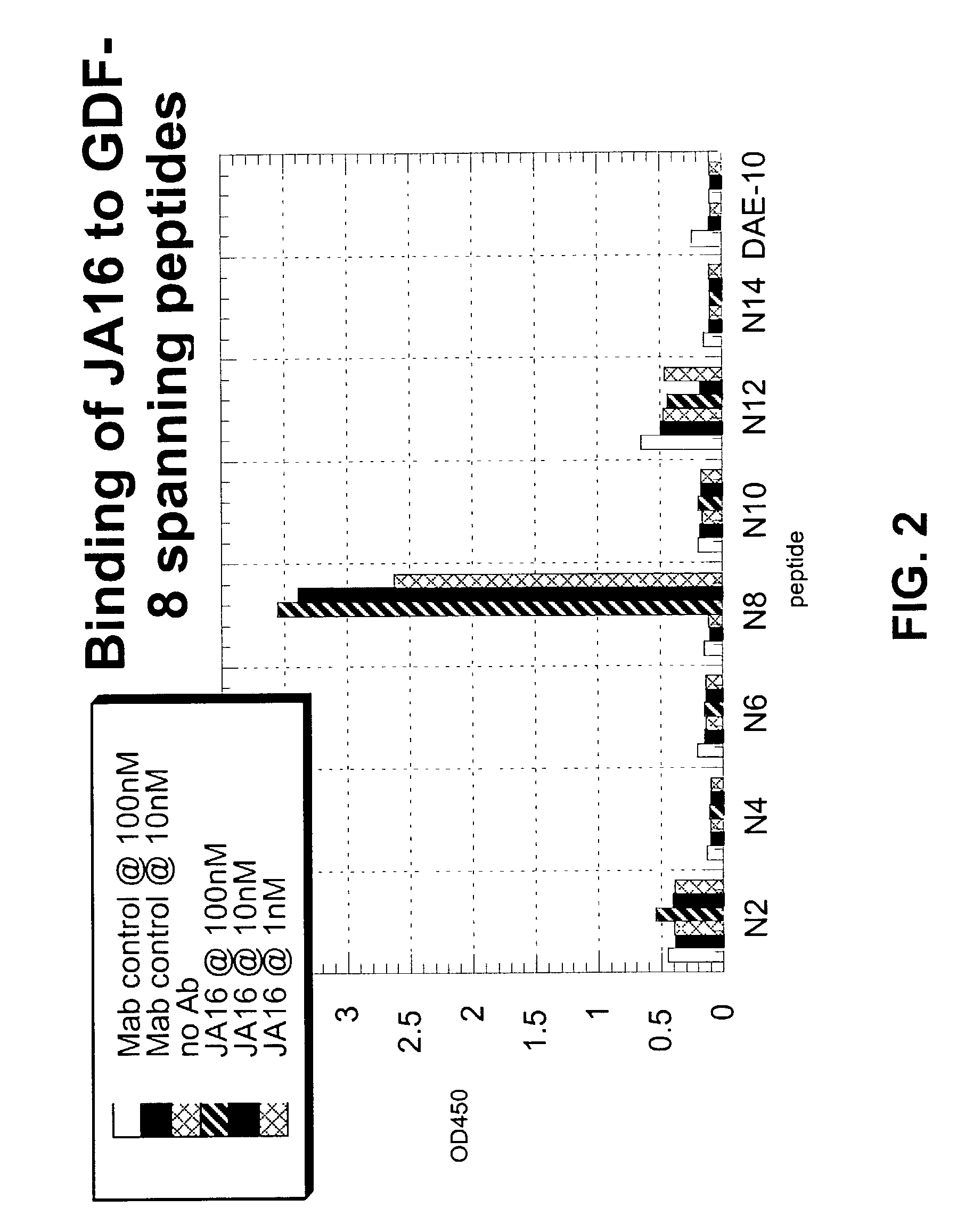
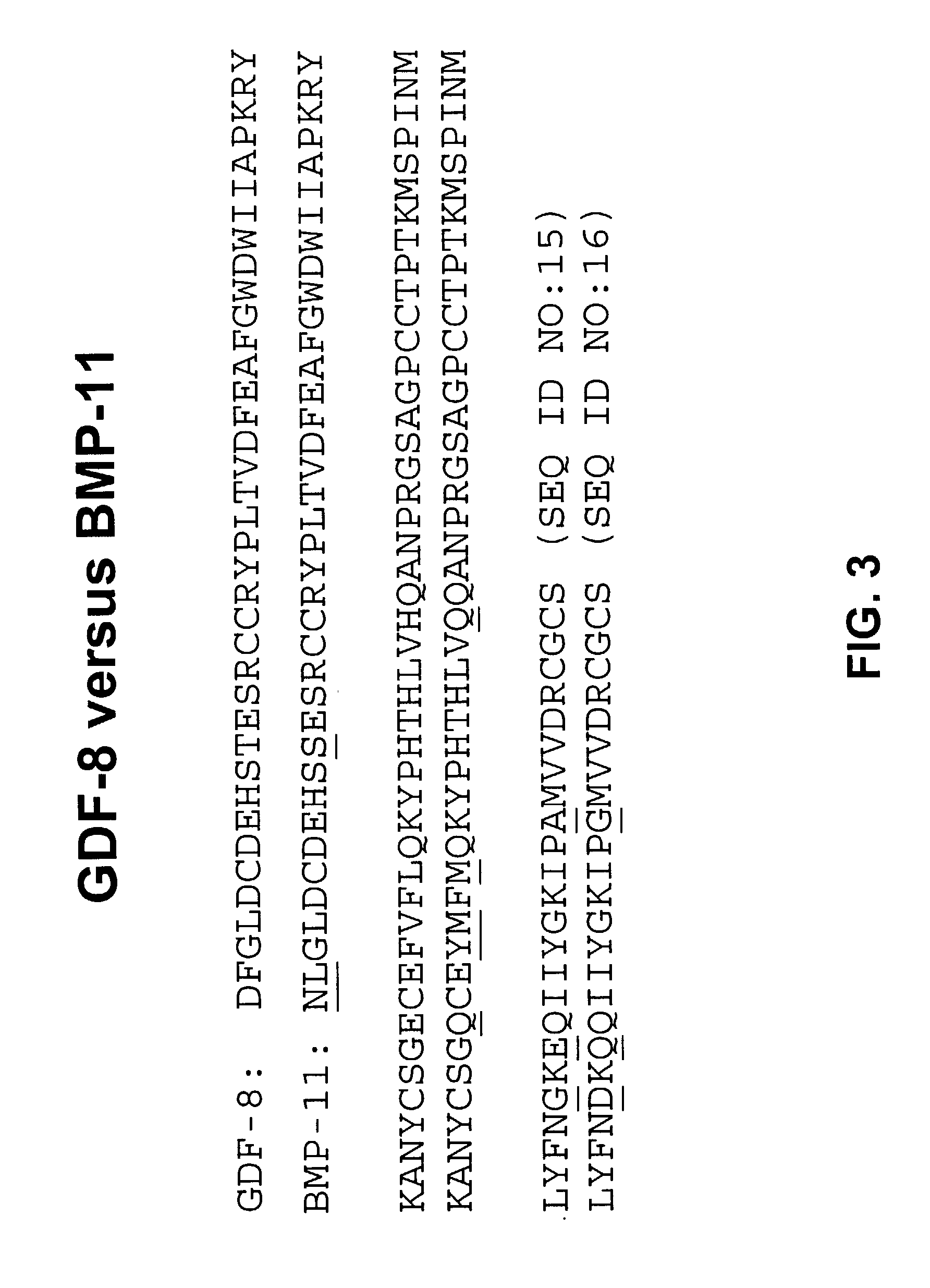
Antibody inhibitors of GDF-8 and uses thereof
ActiveUS7320789B2Reduction in one or more of the biological activitiesReduced activityNervous disorderMuscular disorderAntibody inhibitorAntibody fragments
The disclosure provides novel antibodies against growth and differentiation factor-8 (GDF-8), including antibody fragments, which inhibit GDF-8 activity in vitro and in vivo. The disclosure also provides methods for diagnosing, preventing, or treating degenerative disorders of muscle, bone, or insulin metabolism.
Owner:WYETH LLC
Monoclonal antibodies specific for the extracellular domain of prostate-specific membrane antigen
The present invention relates to monoclonal antibodies that bind to the extracellular domain of prostate-specific membrane antigen (PSMA), hybridoma cell lines producing the antibodies, and methods of using such antibodies for diagnosis and treatment of cancer. In particular, thirty-five monoclonal antibodies reactive with PSMA expressed on the cell surface are exemplified. Additionally, the present invention relates to a novel protein variant (PSM') of PSMA detected by a number of the antibodies of the invention. The hydrolase activity of PSMA and PSM' allows the use of an immunoenzymatic assay for their detection.
Owner:ER SQUIBB & SONS INC
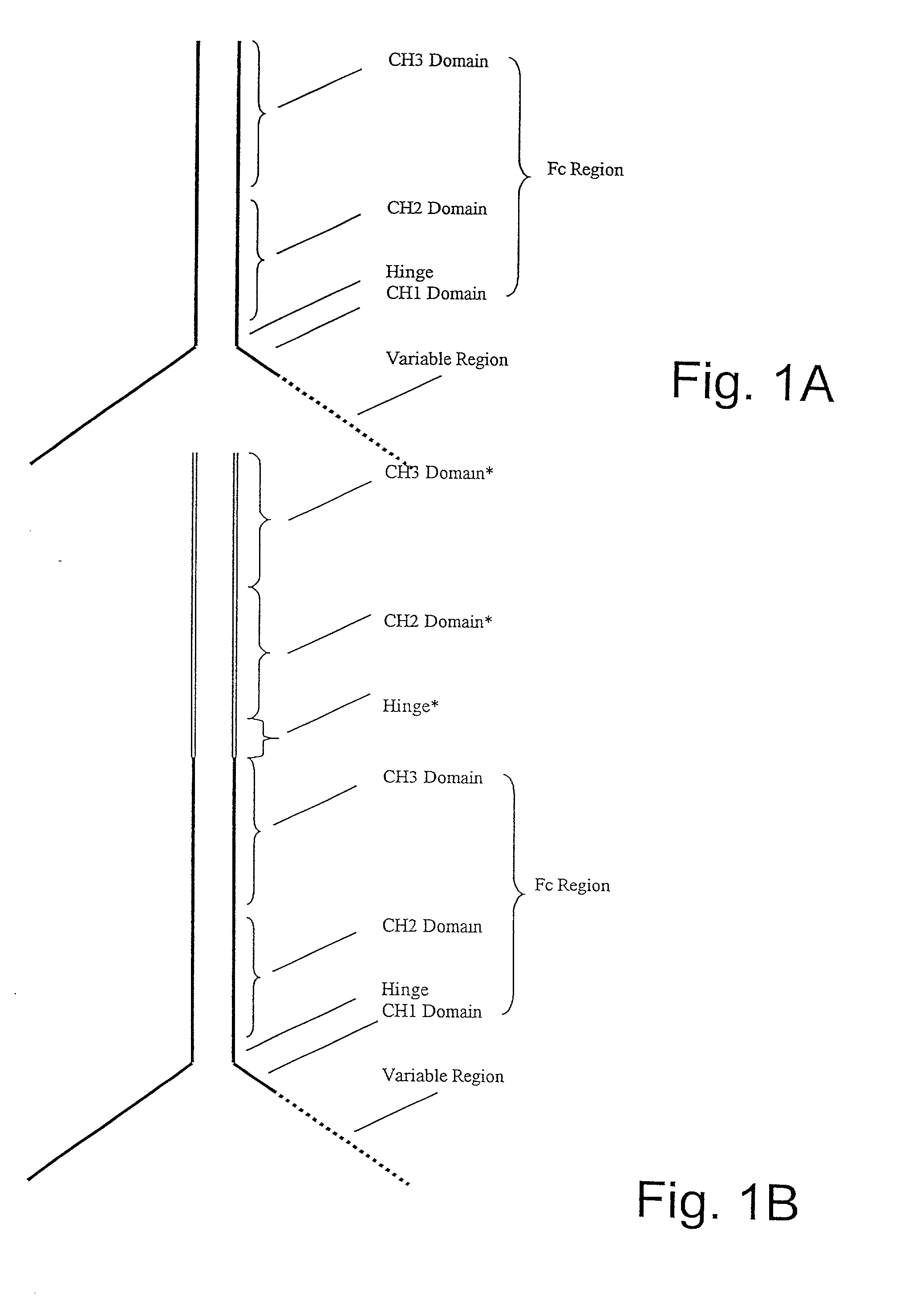
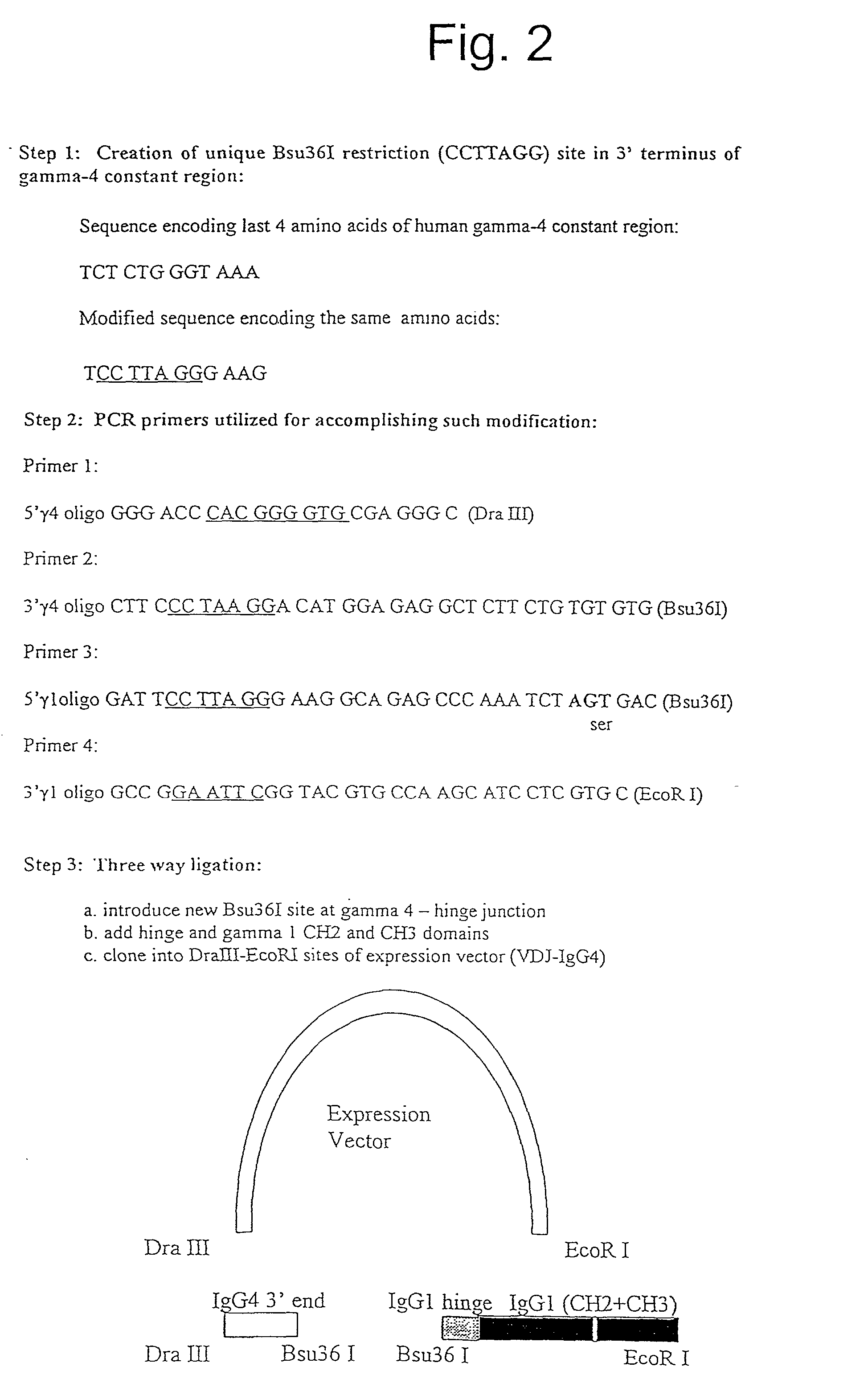
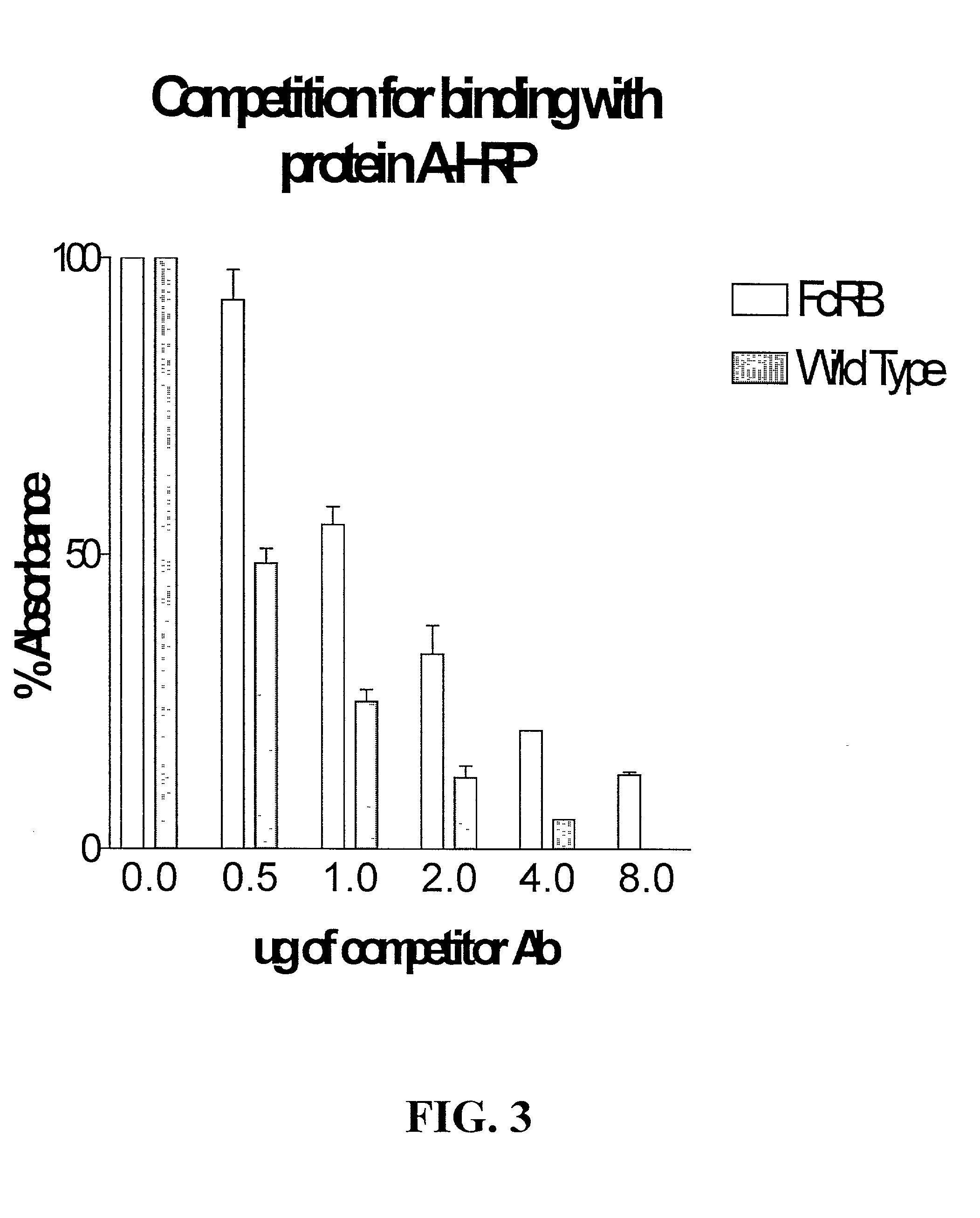
Generation of modified molecules with increased serum half-lives
InactiveUS20020142374A1Antibody mimetics/scaffoldsImmunoglobulins against cytokines/lymphokines/interferonsSerum igeHalf-life
In accordance with the present invention, there are provided methods for the extension of serum half-lives of proteinaceous molecules, particularly antibody molecules, and compositions of molecules modified in accordance with the methods of the invention. In accordance with a first aspect of the present invention, there is provided a method of modifying the half-life of an antibody through providing an antibody containing an FcRn binding domain or the genes encoding such antibody and physically linking the antibody or the antibody as encoded to a second FcRn binding domain. In accordance with a second aspect of the present invention, there is provided a molecule that contains at least two distinct FcRn binding moieties.
Owner:ABQENIX INC
Compositions and methods for silencing apolipoprotein B
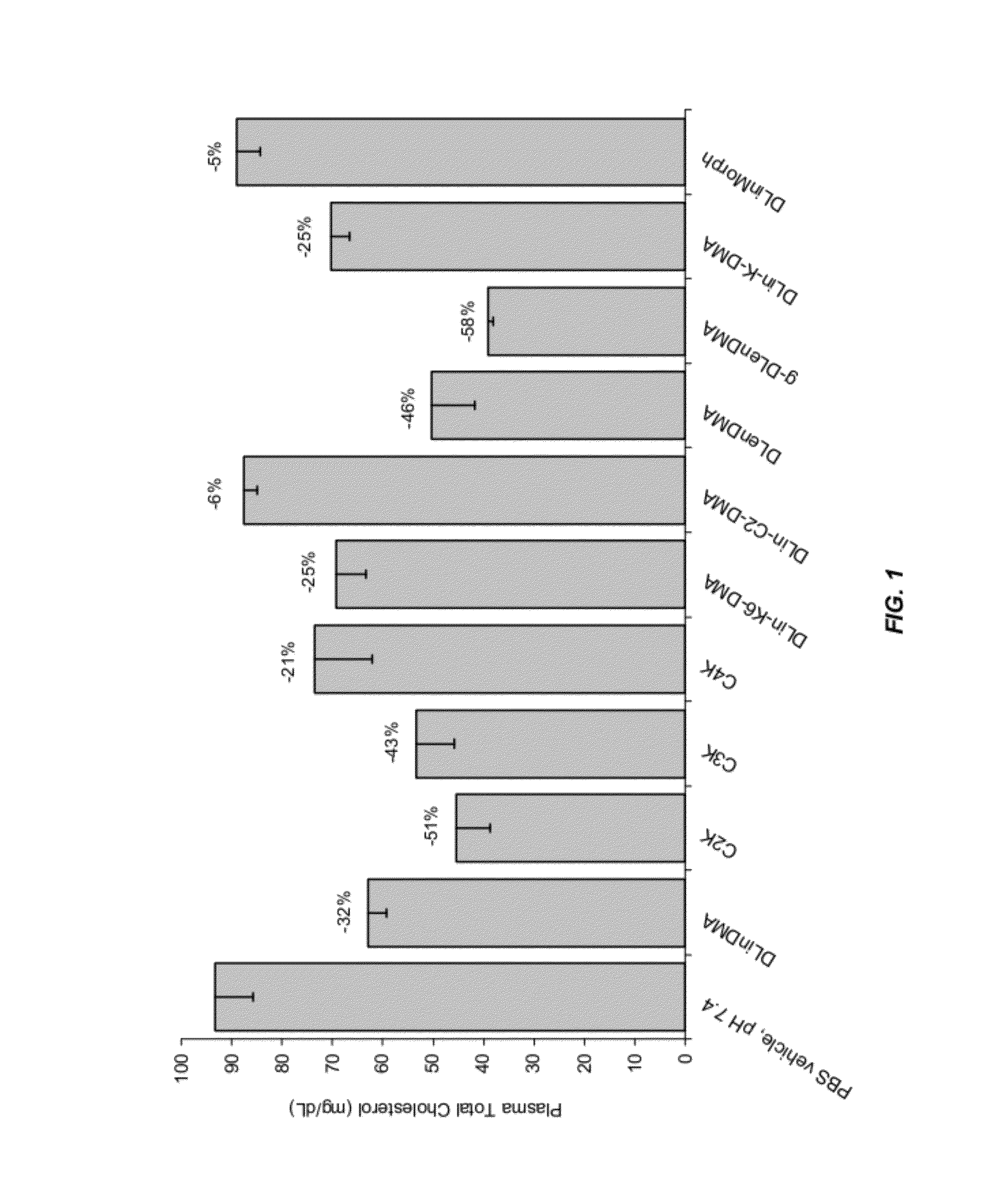
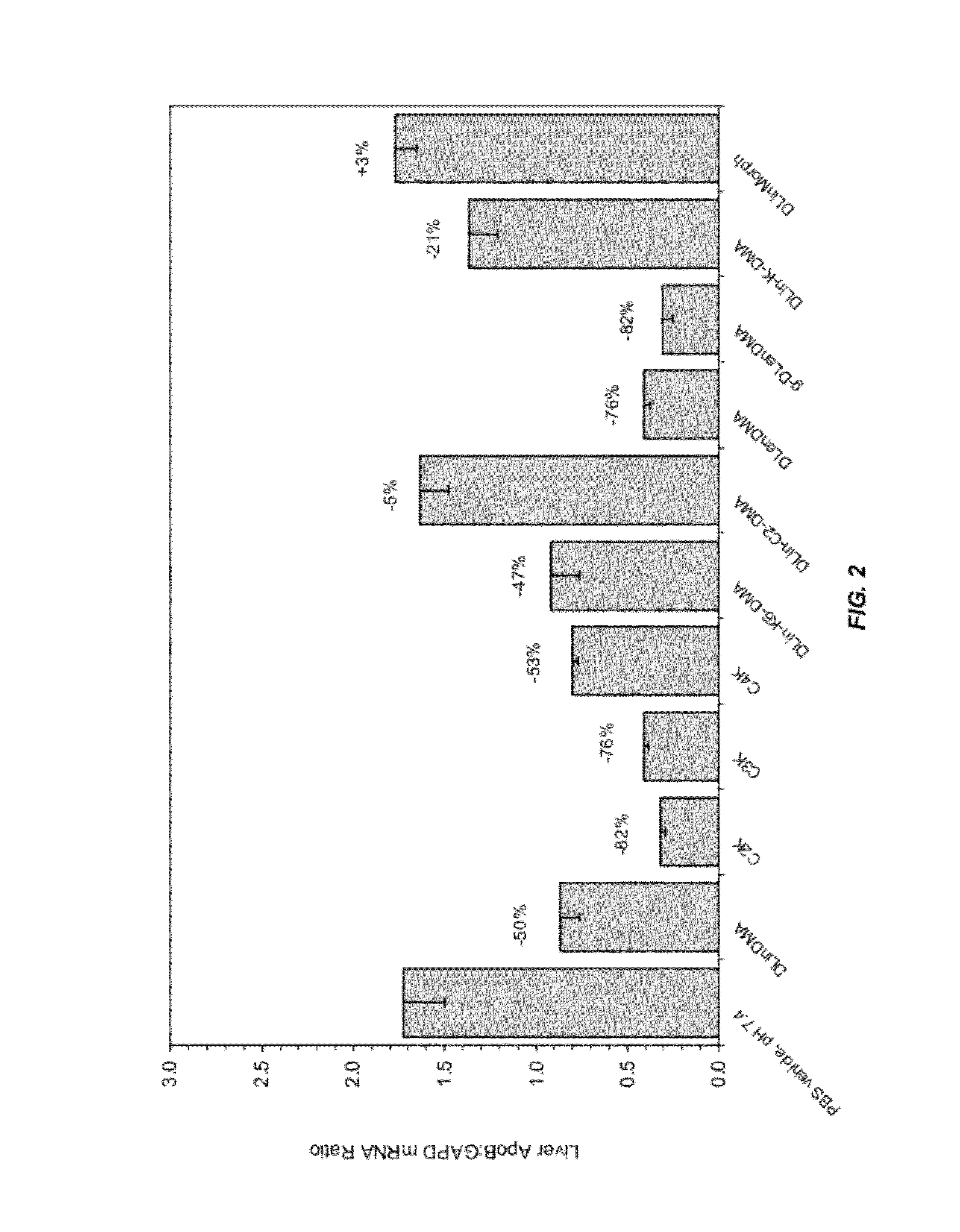
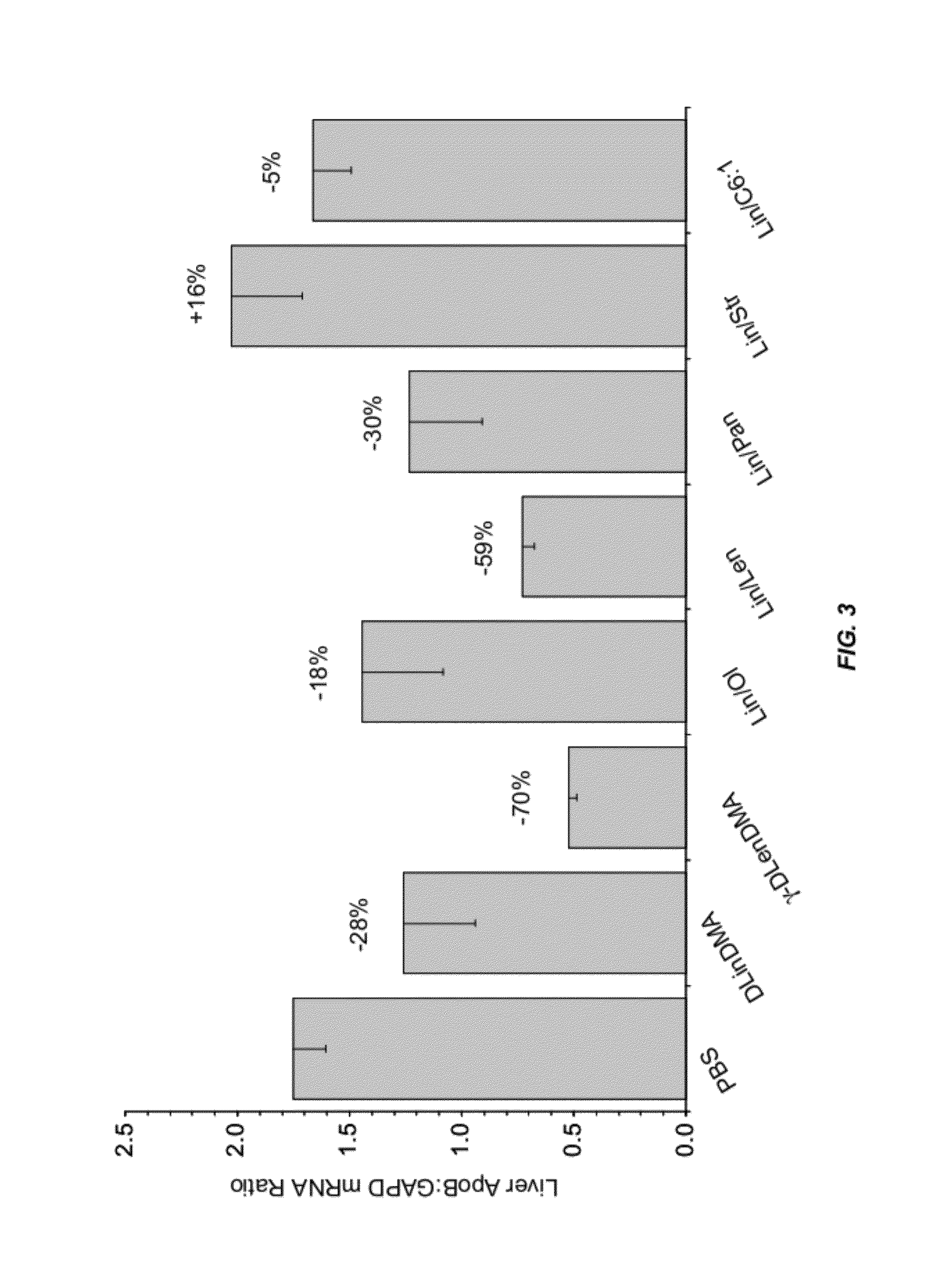
InactiveUS8236943B2Improve effectivenessHigh activityOrganic active ingredientsNanotechLipid particleApolipoproteins b
The present invention provides compositions and methods for the delivery of interfering RNAs that silence APOB expression to liver cells. In particular, the nucleic acid-lipid particles provide efficient encapsulation of nucleic acids and efficient delivery of the encapsulated nucleic acid to cells in vivo. The compositions of the present invention are highly potent, thereby allowing effective knock-down of APOB at relatively low doses. In addition, the compositions and methods of the present invention are less toxic and provide a greater therapeutic index compared to compositions and methods previously known in the art.
Owner:ARBUTUS BIOPHARMA CORPORAT ION
Therapeutic application of chimeric and radiolabelled antibodies to human B lymphocyte restricted differentiation antigen for treatment of B cell lymphoma
Disclosed herein are therapeutic treatment protocols designed for the treatment of B cell lymphoma. These protocols are based upon therapeutic strategies which include the use of administration of immunologically active mouse / human chimeric anti-CD20 antibodies, radiolabeled anti-CD20 antibodies, and cooperative strategies comprising the use of chimeric anti-CD20 antibodies and radiolabeled anti-CD20 antibodies.
Owner:IDEC PHARM CORP
Therapeutic application of chimeric and radiolabelled antibodies to human B lymphocyte restricted differentiation antigen for treatment of B cell lymphoma
Disclosed herein are therapeutic treatment protocols designed for the treatment of B cell lymphoma. These protocols are based upon therapeutic strategies which include the use of administration of immunologically active mouse / human chimeric anti-CD20 antibodies, radiolabeled anti-CD20 antibodies, and cooperative strategies comprising the use of chimeric anti-CD20 antibodies and radiolabeled anti-CD20 antibodies.
Owner:BIOGEN INC
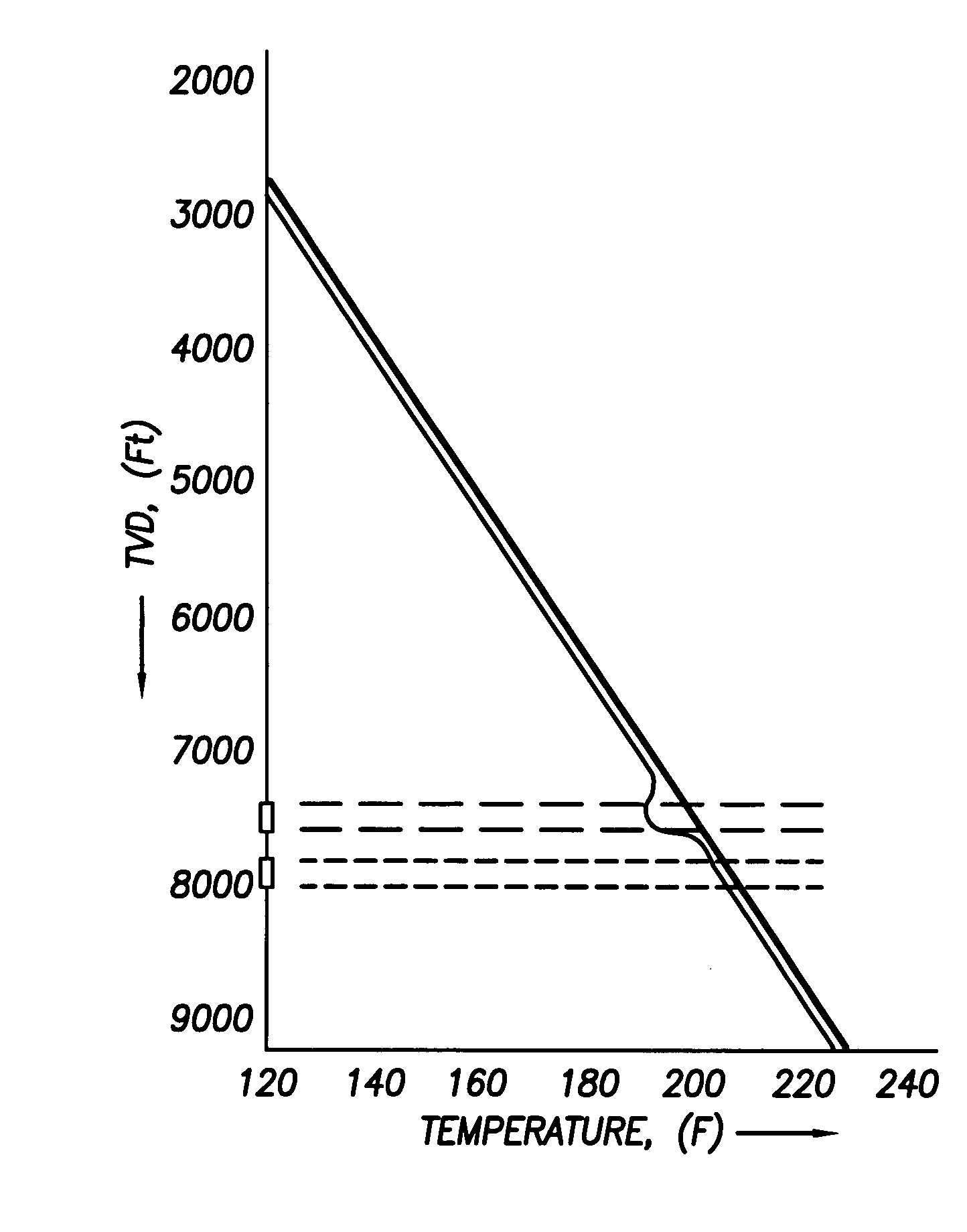
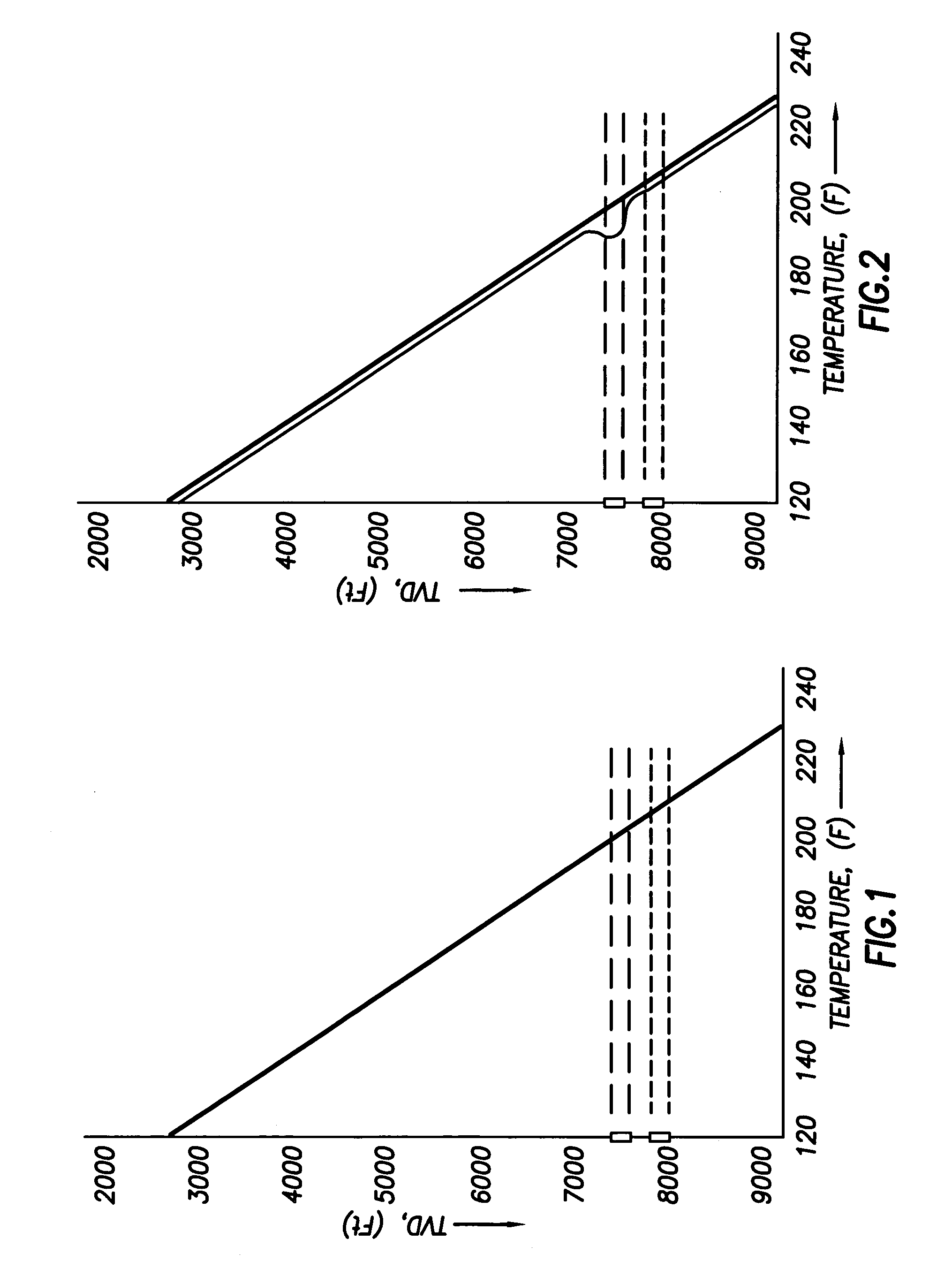
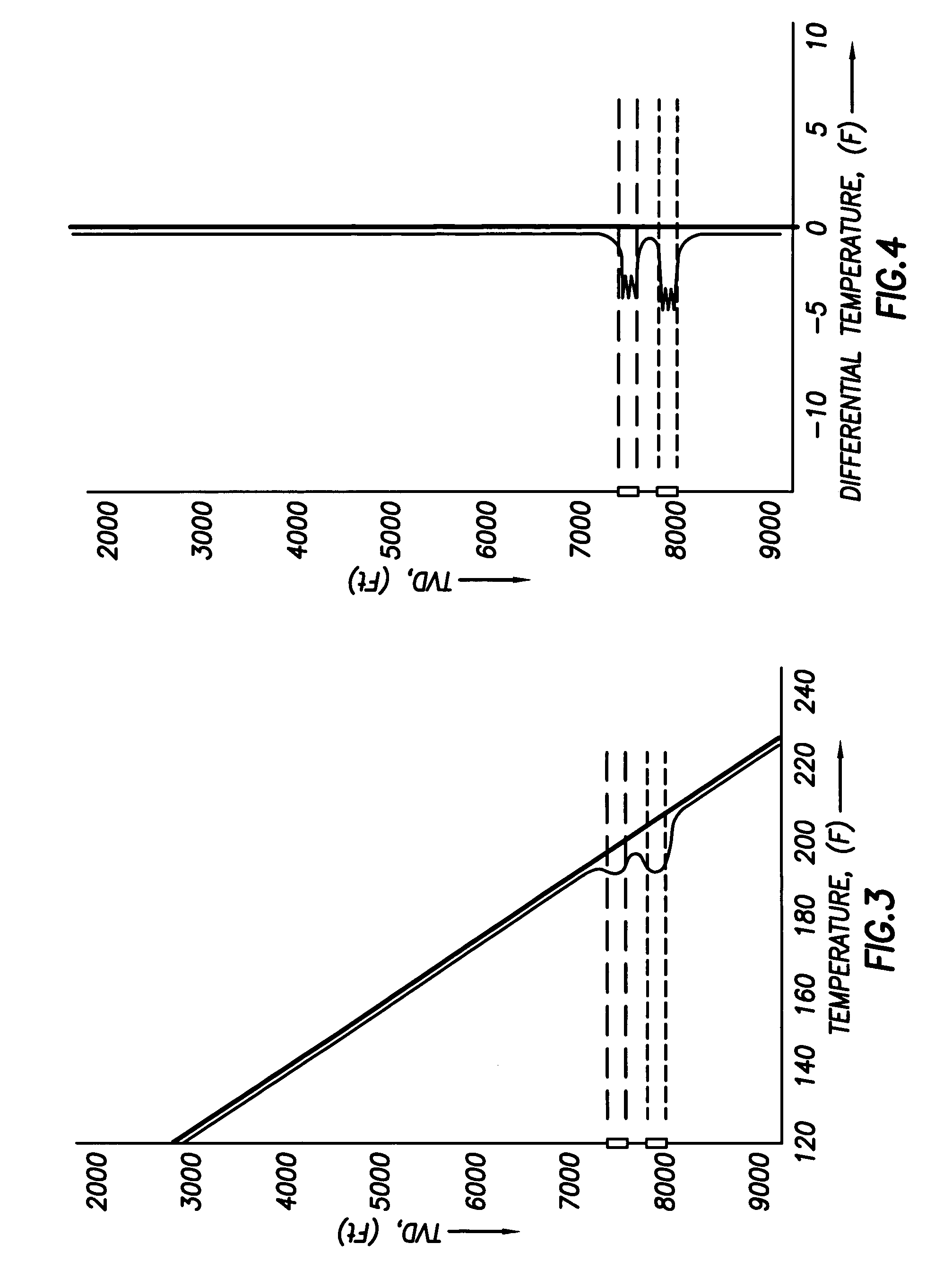
Use of distributed temperature sensors during wellbore treatments
The invention relates to a method for treating subterranean formation comprising providing distributed temperature sensors, injecting a treatment fluid and monitoring the temperature across the treatment interval during the injection process.
Owner:SCHLUMBERGER TECH CORP
Anti-mesothelin antibodies having high binding affinity
InactiveUS7081518B1Peptide/protein ingredientsHybrid cell preparationAnti-Mesothelin AntibodyAntiendomysial antibodies
Mesothelin is a differentiation antigen present on the surface of ovarian cancers, mesotheliomas and several other types of human cancers. Because among normal tissues, mesothelin is only present on mesothelial cells, it represents a good target for antibody mediated delivery of cytotoxic agents. The present invention is directed to anti-mesothelin antibodies, including Fv molecules with particularly high affinity for mesothelin, and immunoconjugates employing them. Also described are diagnostic and therapeutic methods using the antibodies. The anti-mesothelin antibodies are well-suited for the diagnosis and treatment of cancers of the ovary, stomach, squamous cells, mesotheliomas and other malignant cells expressing mesothelin.
Owner:UNITED STATES OF AMERICA
Monoclonal antibodies with enhanced ADCC function
InactiveUS20050249722A1Preventing alloimmunizationAntibacterial agentsImmunoglobulins against blood group antigensMonoclonal antibodyAnti-d antibody
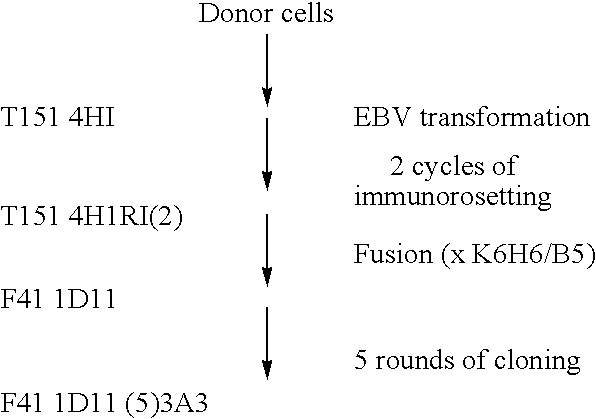
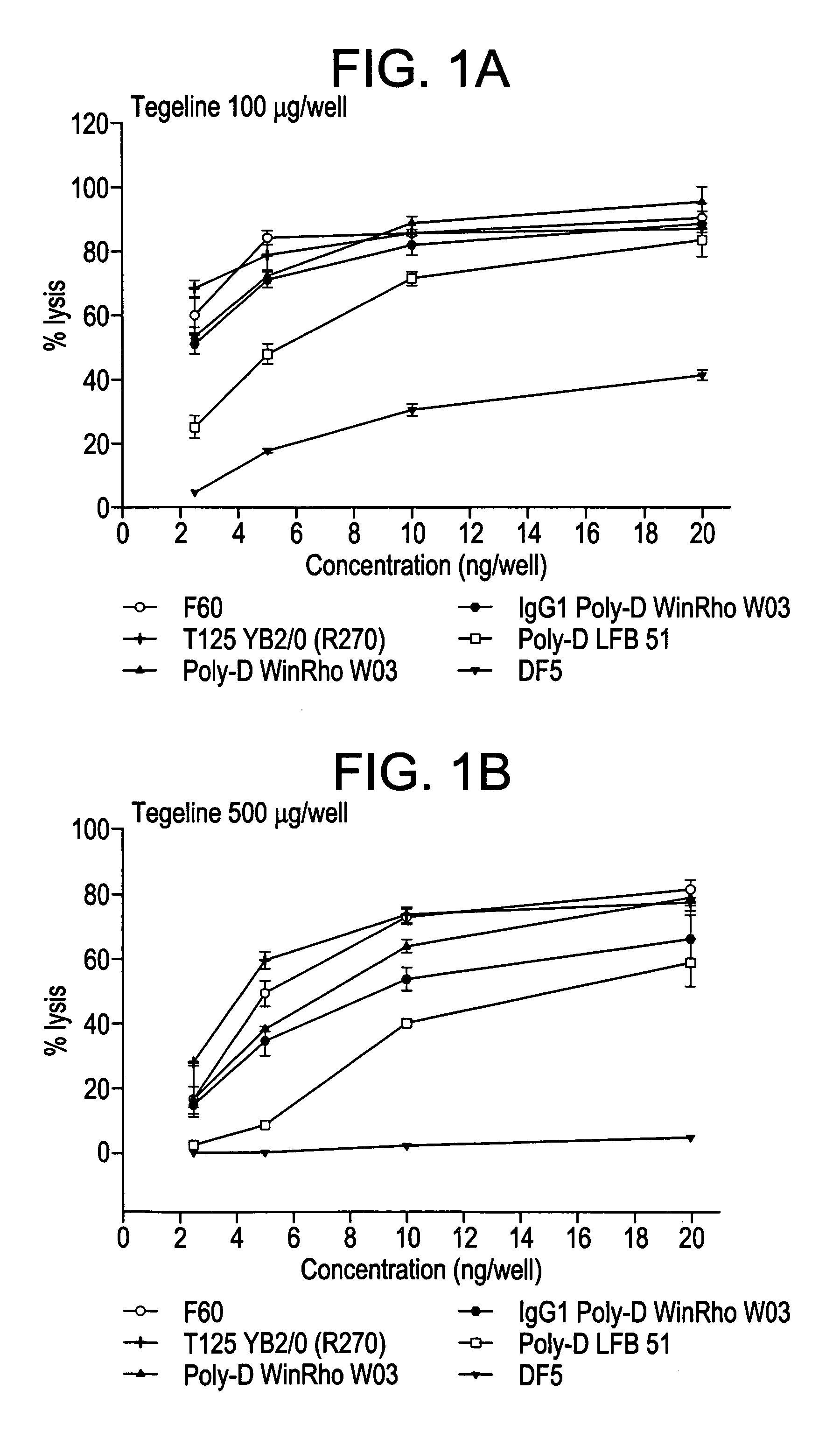
The invention concerns a method for obtaining and selecting monoclonal antibodies by an ADDC-type test, said antibodies capable of activating type III Fcy receptors and having a particular glycan structure. The inventive anti-D antibodies can be used for preventing Rhesus isoimmunisation in Rh negative persons, in particular for haemolytic disease in a new-born baby of for uses such as idiopathic thrombocytopenic pupura 9ITP)
Owner:LABE FR DU FRACTIONNEMENT & DES BIOTECH SA
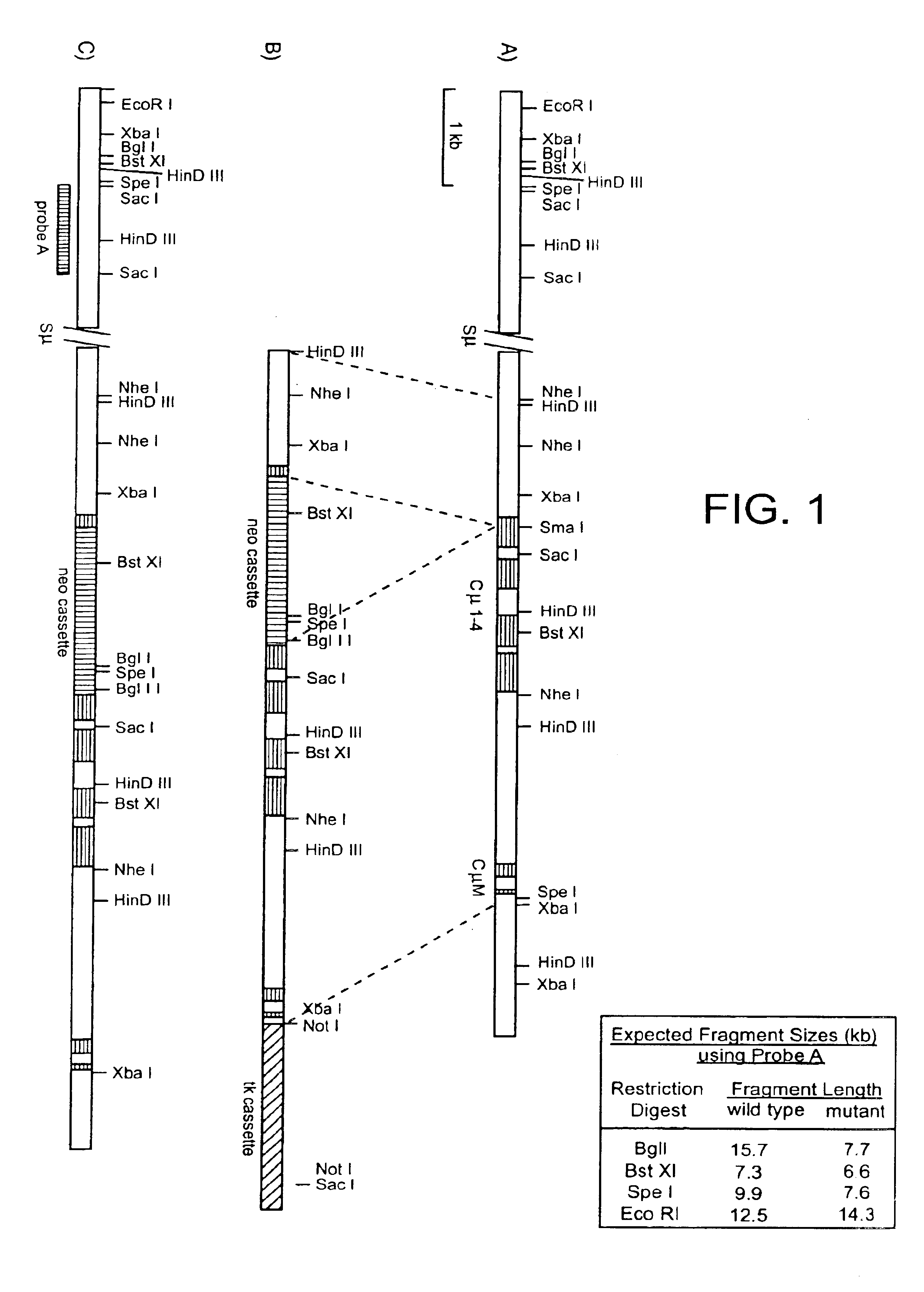
Generation of heavy-chain only antibodies in transgenic animals
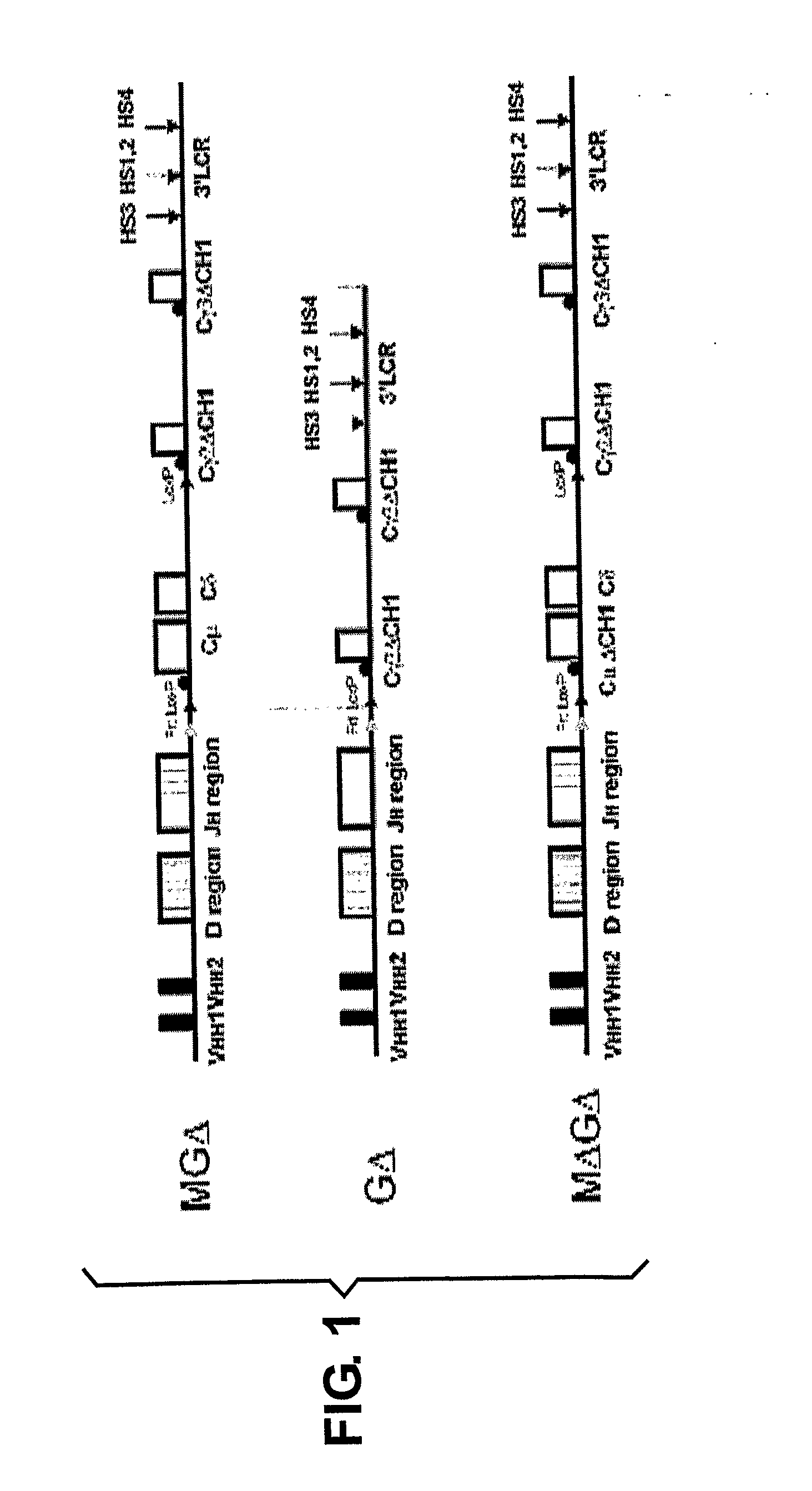
InactiveUS20090307787A1Maximizing numberIncrease diversityHybrid cell preparationImmunoglobulinsHeavy chainMammal
The present invention relates to a method for the generation of VH heavy chain-only antibodies in a transgenic non-human mammal. In particular, the present invention relates to a method for the production of a VH heavy chain-only antibody in a transgenic non-human mammal comprising the step of expressing more than one heterologous VH heavy chain locus in that mammal.
Owner:ERASMUS UNIV MEDICAL CENT ROTTERDAM ERASMUS MC
Method for treating an IgE-mediated disease in a patient using anti-CD40 monoclonal antibodies
InactiveUS6899879B2Inhibition of differentiationInhibit growthOrganic active ingredientsVirusesDiseaseEpitope
Methods for preventing or treating an IgE-mediated allergic disease in a patient are presented, the methods comprising administration of a monoclonal antibody capable of binding to a human CD40 antigen located on the surface of a human B cell, wherein binding of the antibody to the CD40 antigen prevents the growth or differentiation of the B cell. Monoclonal antibodies useful in these methods, and epitopes immunoreactive with such monoclonal antibodies are also presented.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
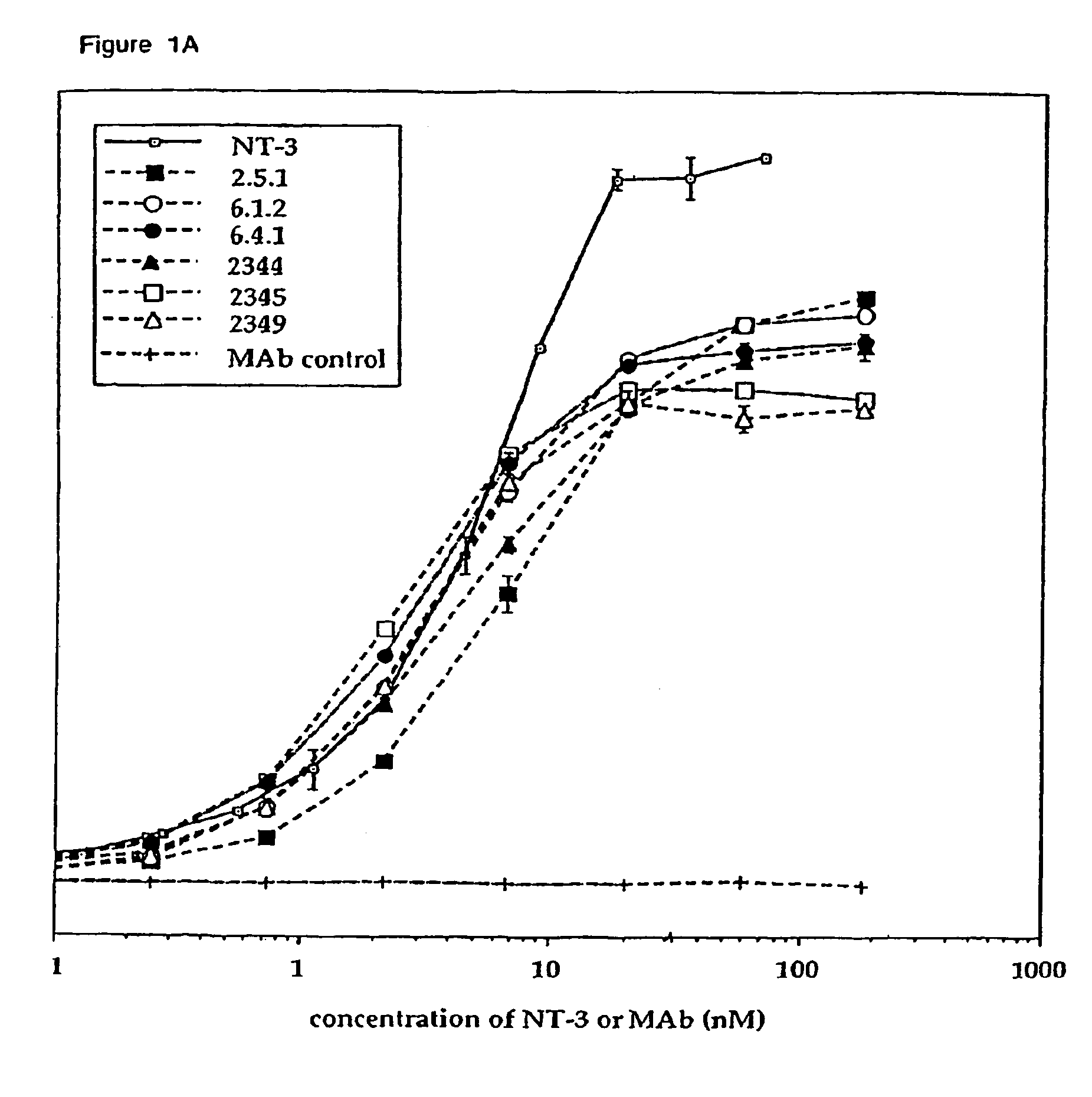
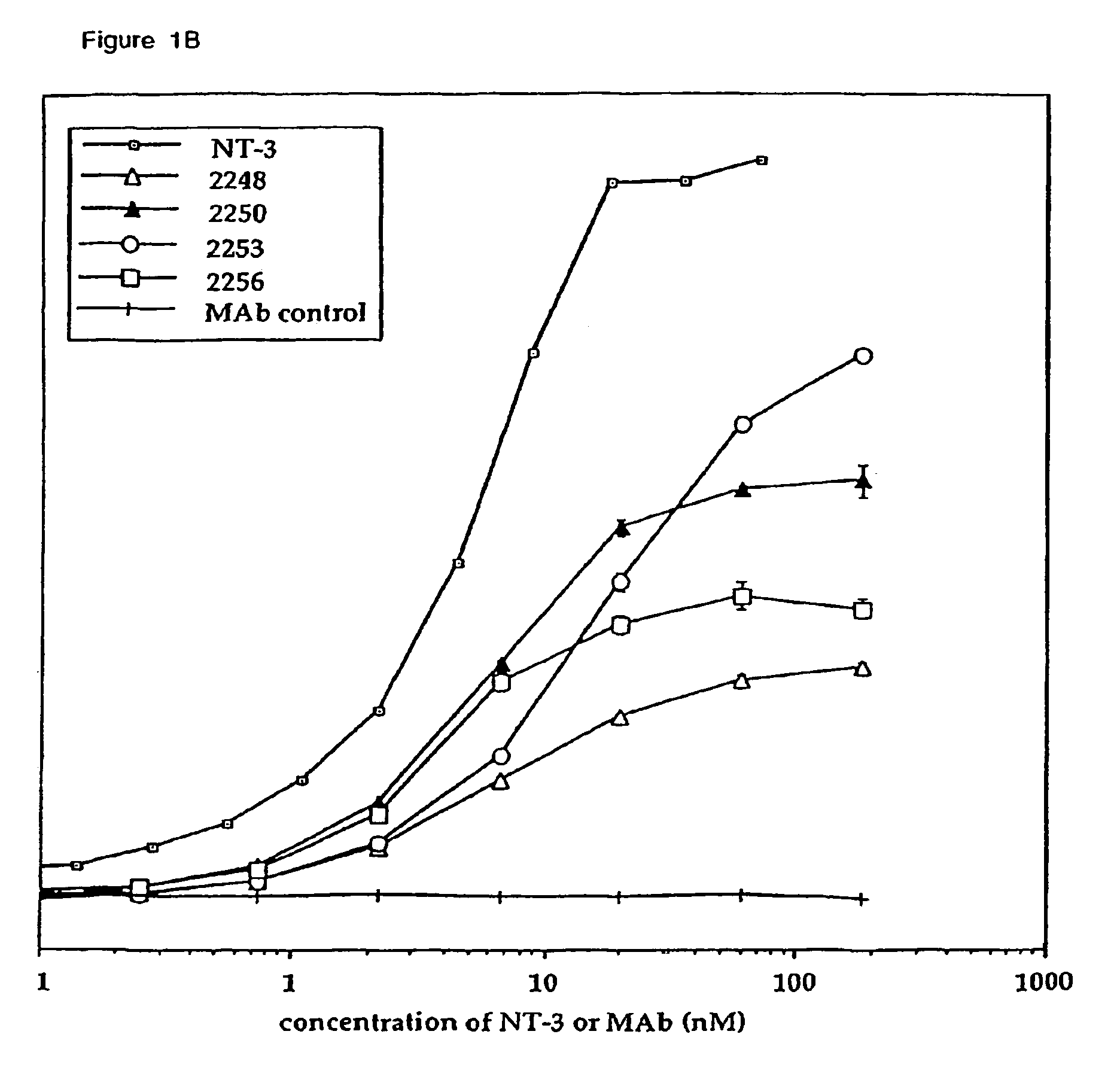
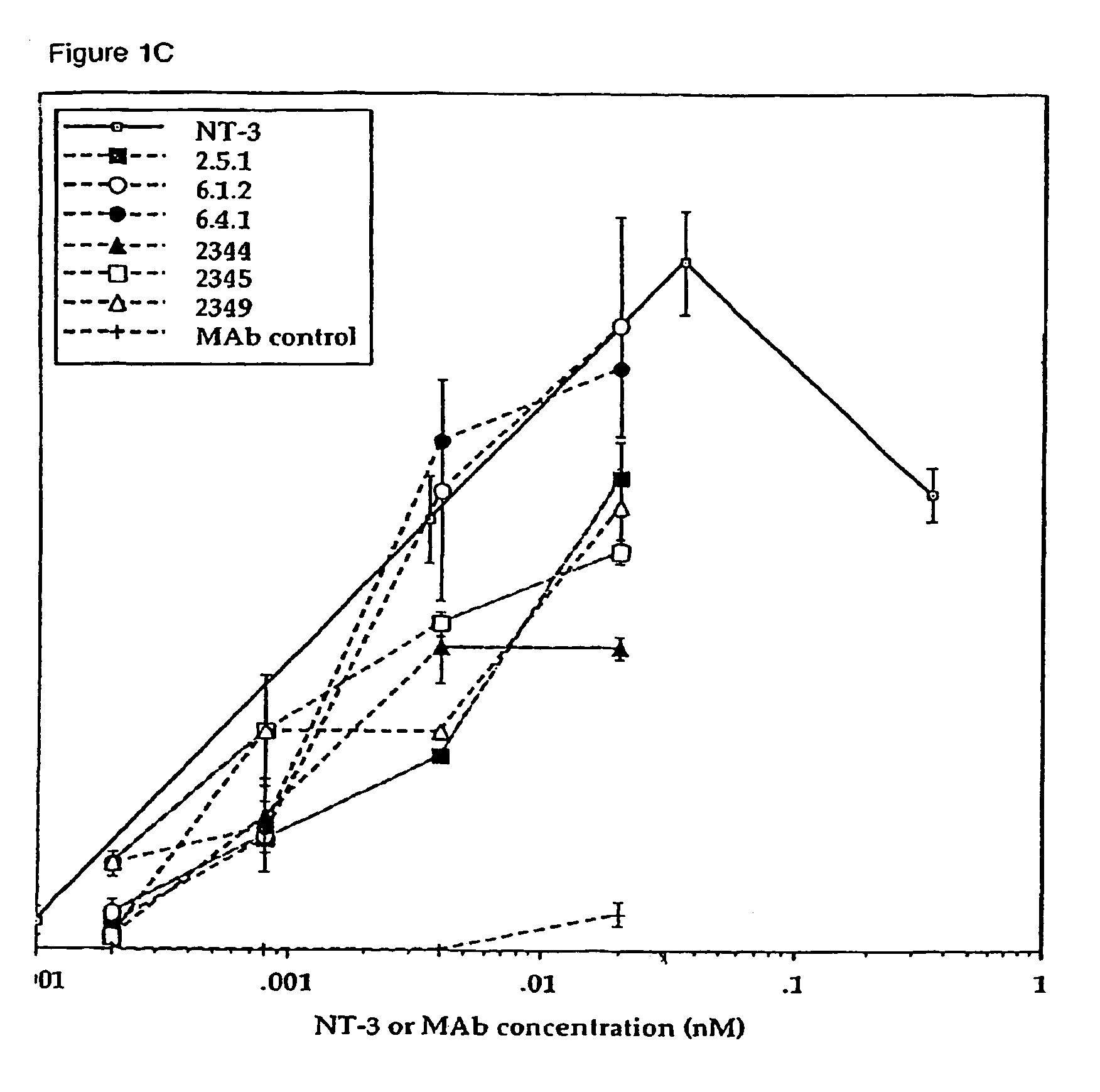
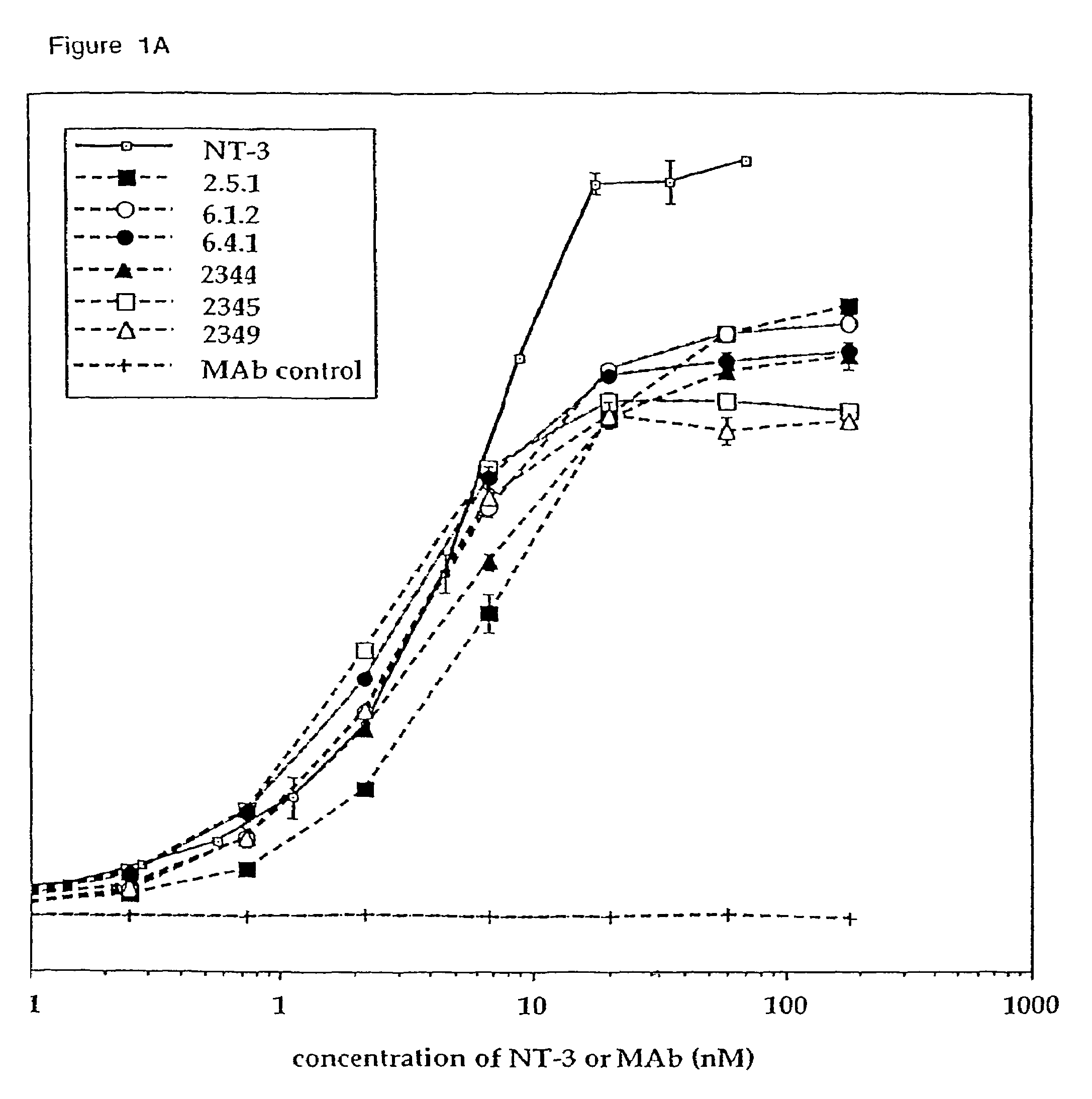
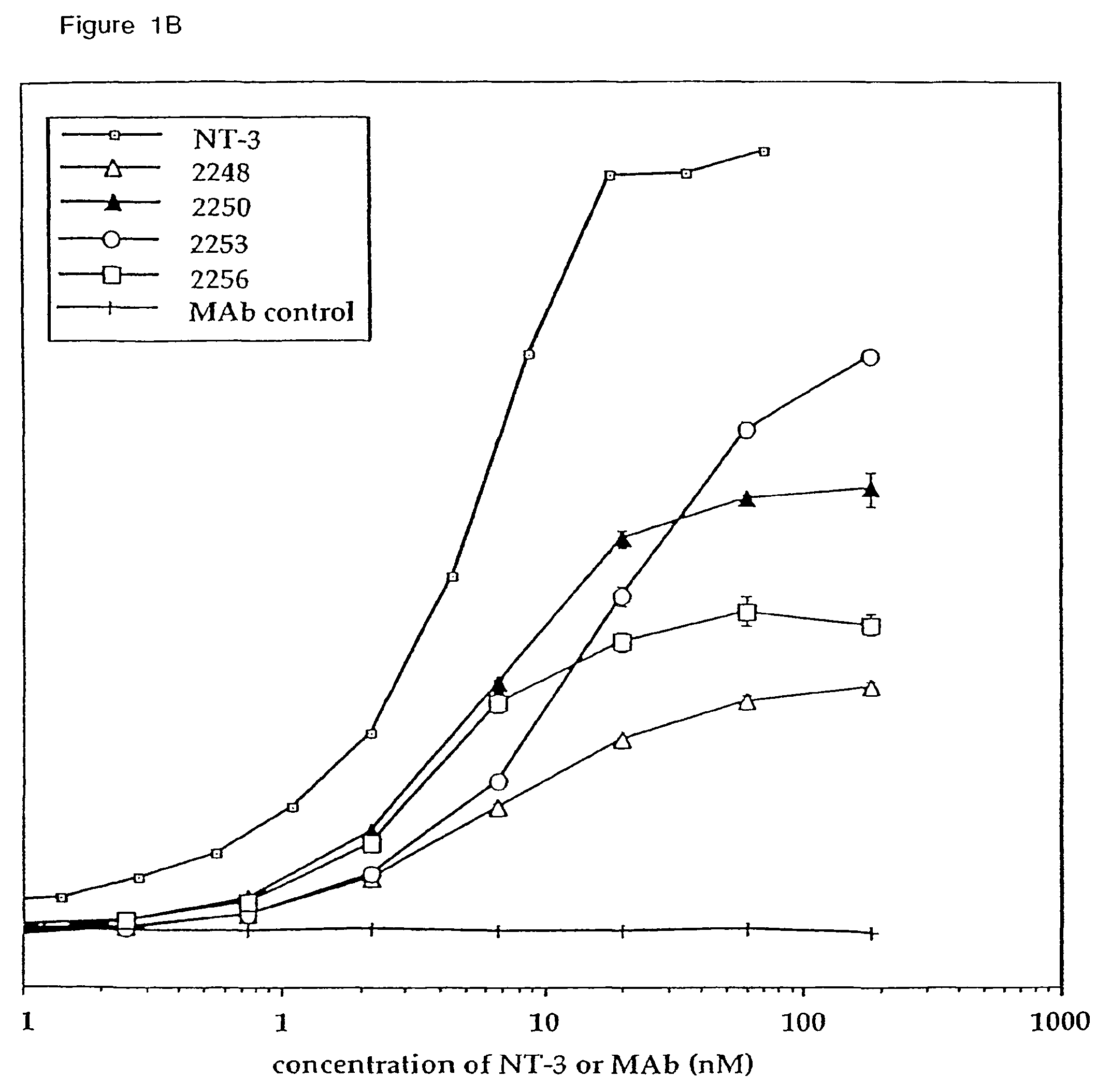
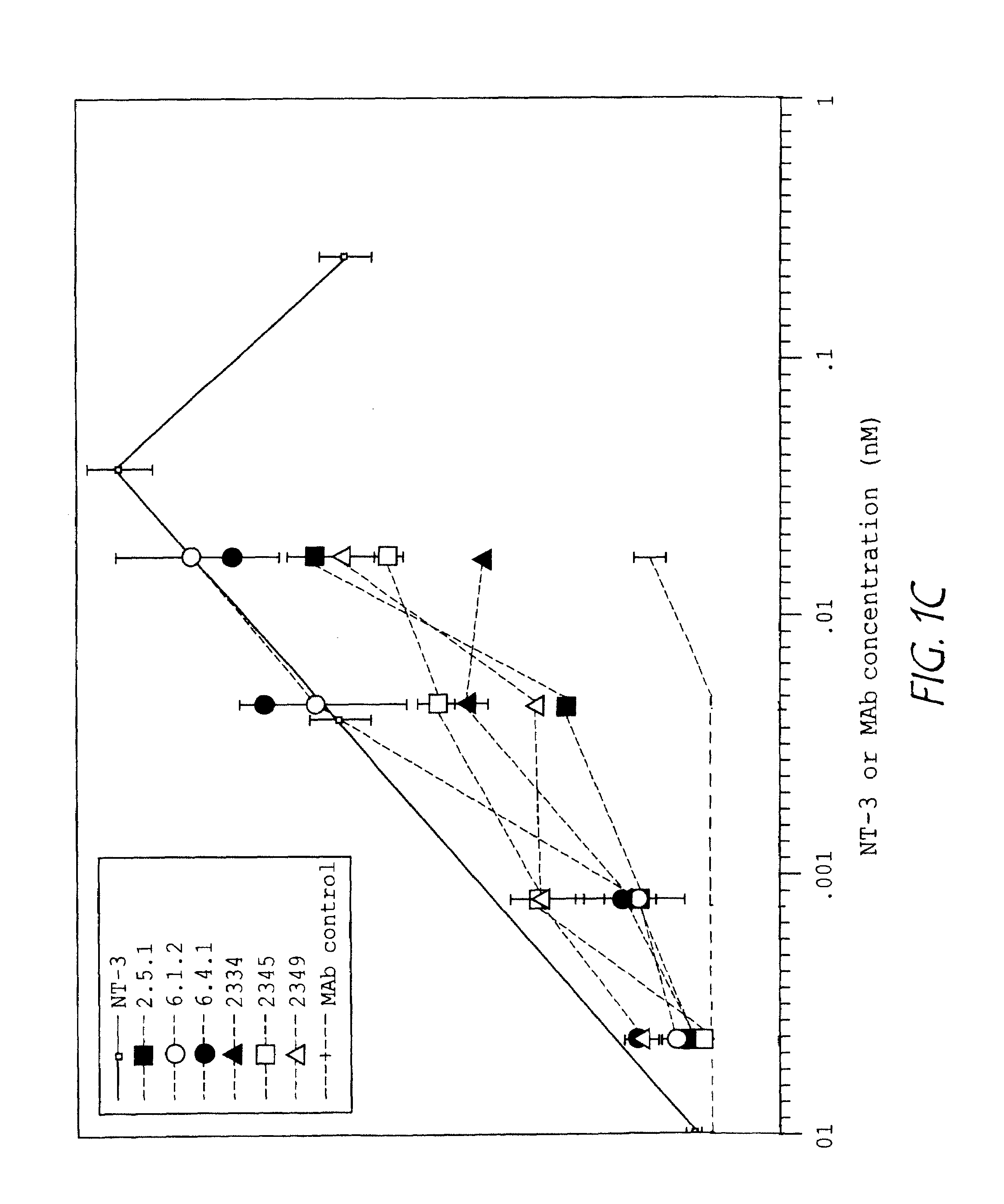
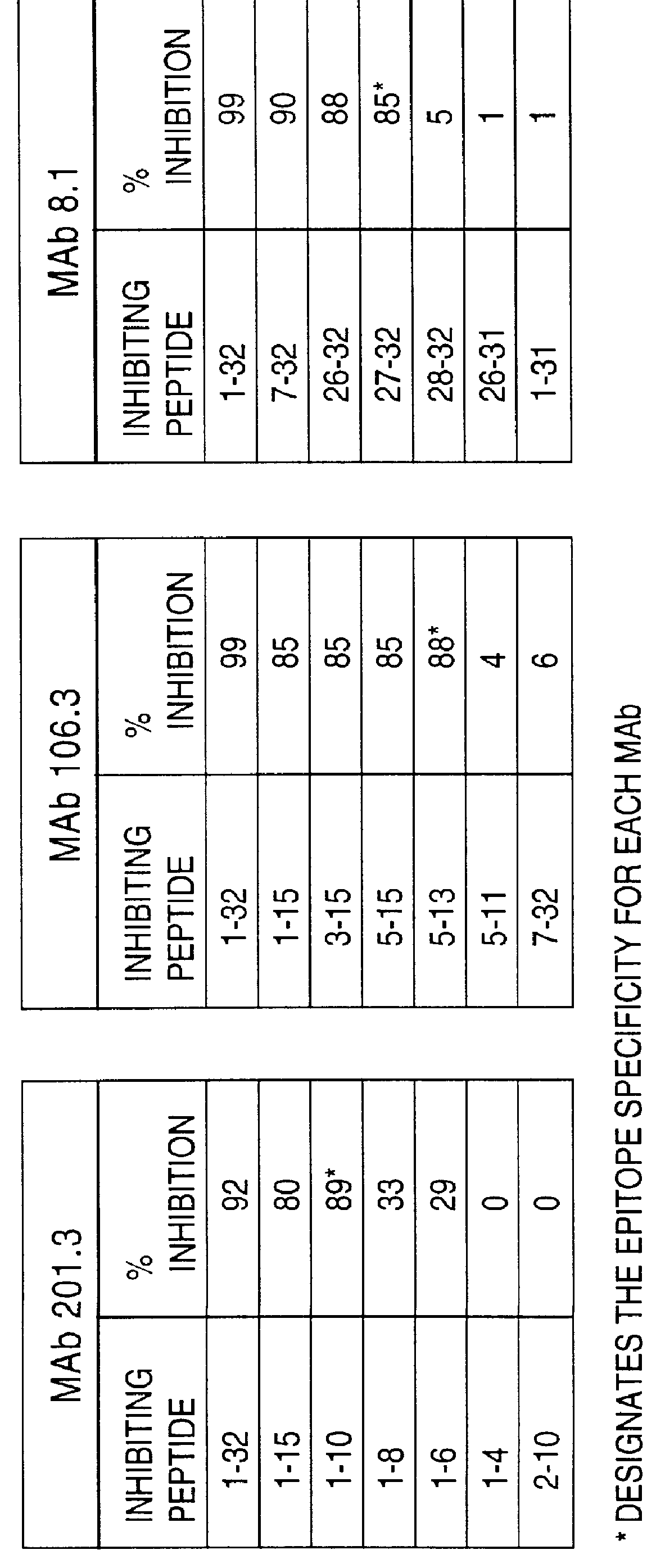
Methods for treating neuropathy by agonist anti-trk-C monoclonal antibodies
InactiveUS7615383B2Effective prevention and treatmentMaintain good propertiesNervous disorderGenetic material ingredientsBiologic DMARDCell system
The invention concerns agonist anti-trkC monoclonal antibodies which mimic certain biological activities of NT-3, the native ligand of trkC. The invention further concerns the use of such antibodies in the prevention and / or treatment of cellular degeneration, including nerve cell damage associated with acute nervous cell system injury and chronic neurodegenerative diseases, including peripheral neuropathy.
Owner:GENENTECH INC
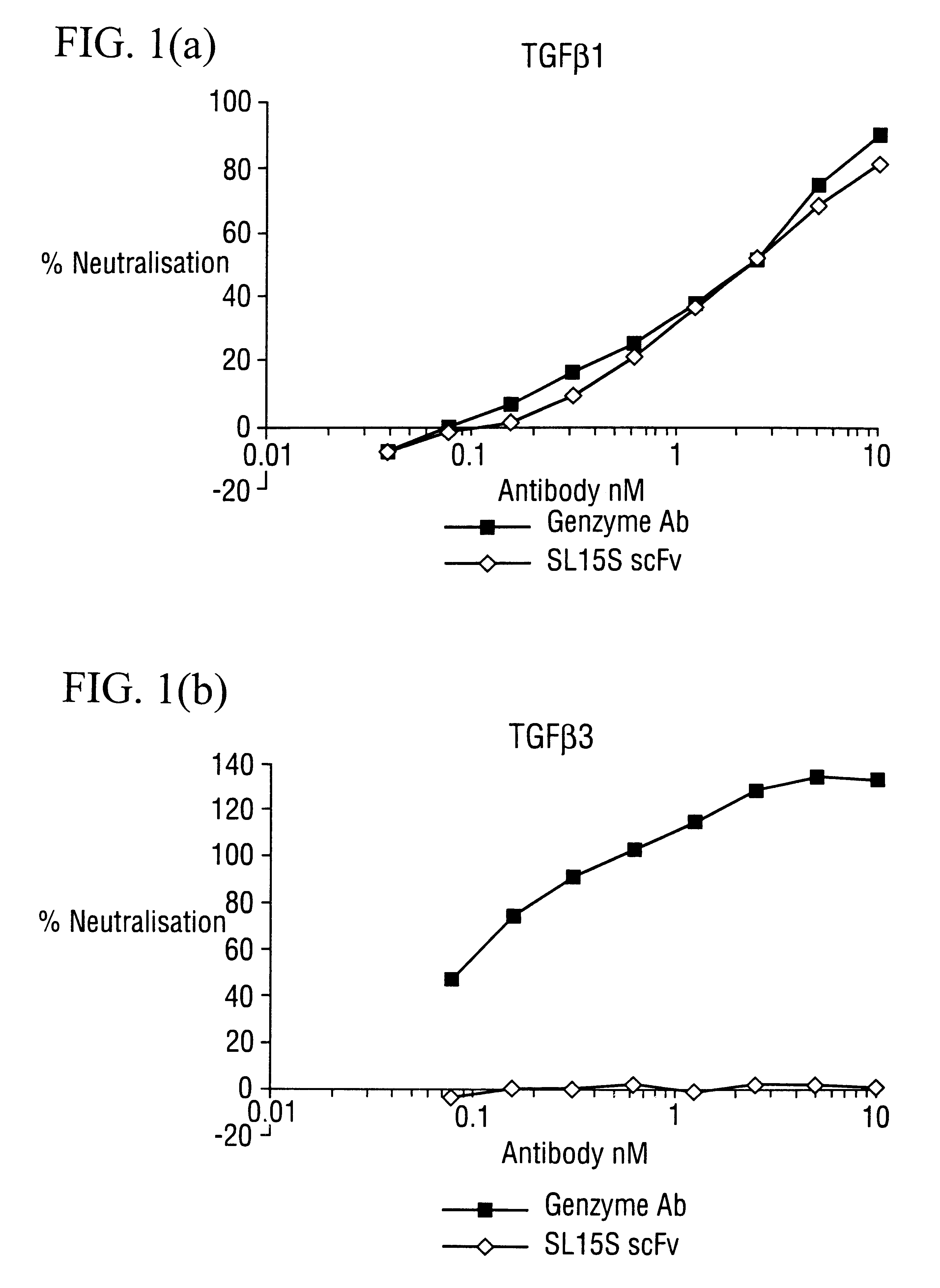
Specific binding members for TGFbeta1
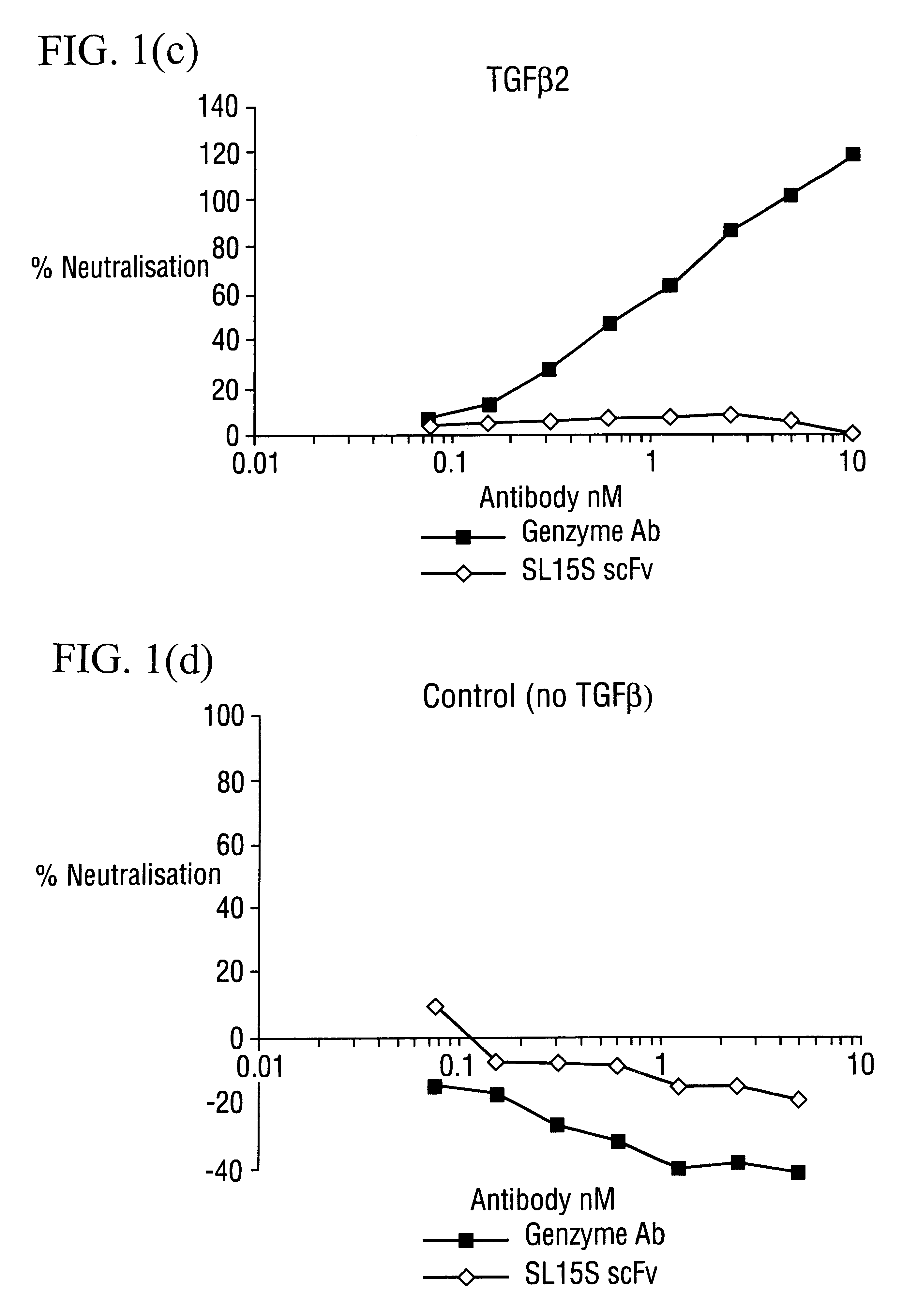
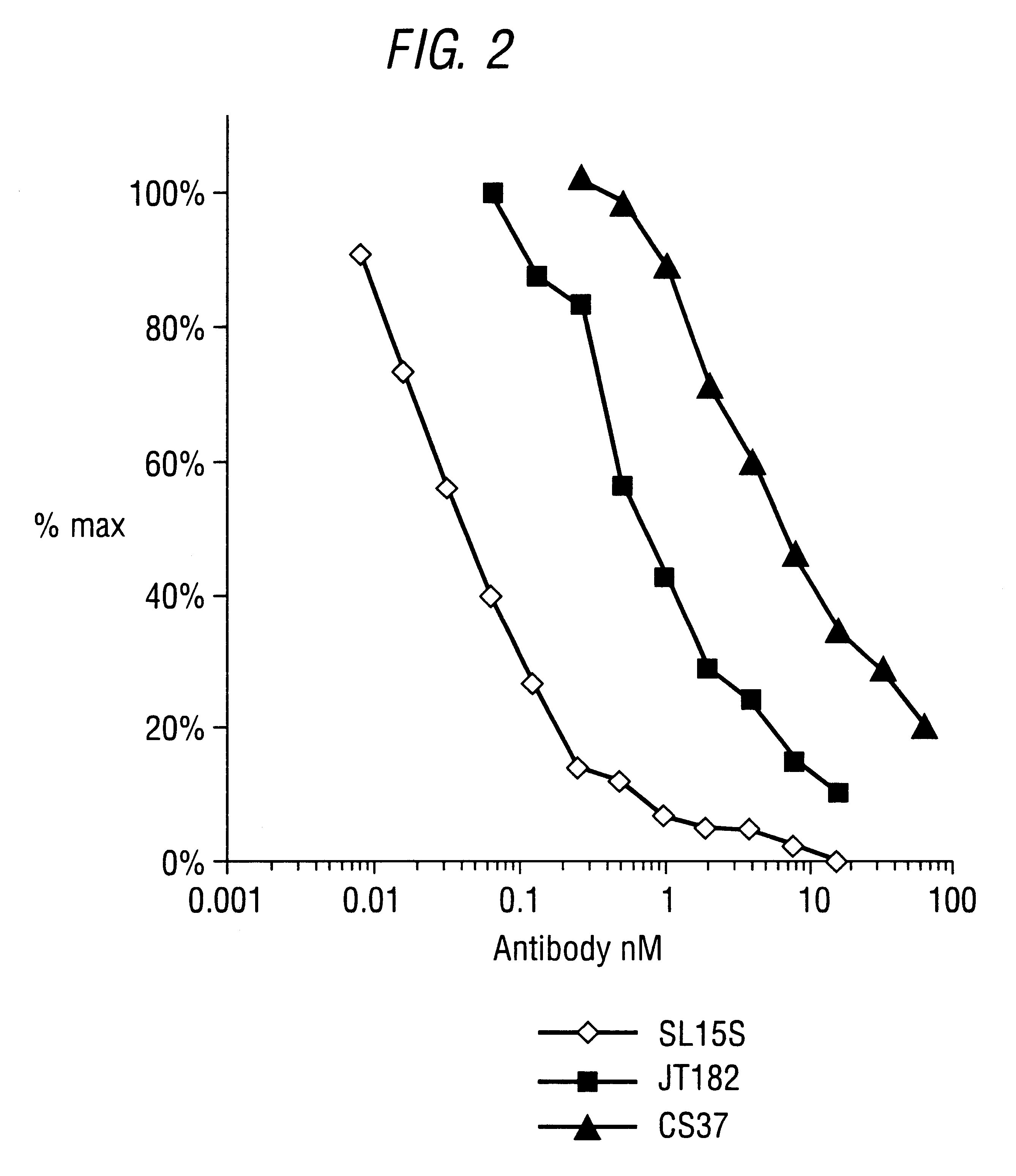
The invention provides specific binding members, for example in the form of antibody variable domains, based on the CDR3 sequences of the antibody VH regions of SL15 (SEQ ID NO:4) and JT182 (SEQ ID NO:10). The antibodies have strong neutralizing activity for TGFbeta1 and are useful in treating conditions associated with excess TGFbeta1 activity, such as fibrosis, immune responses and tumor progression.
Owner:MEDIMMUNE LTD
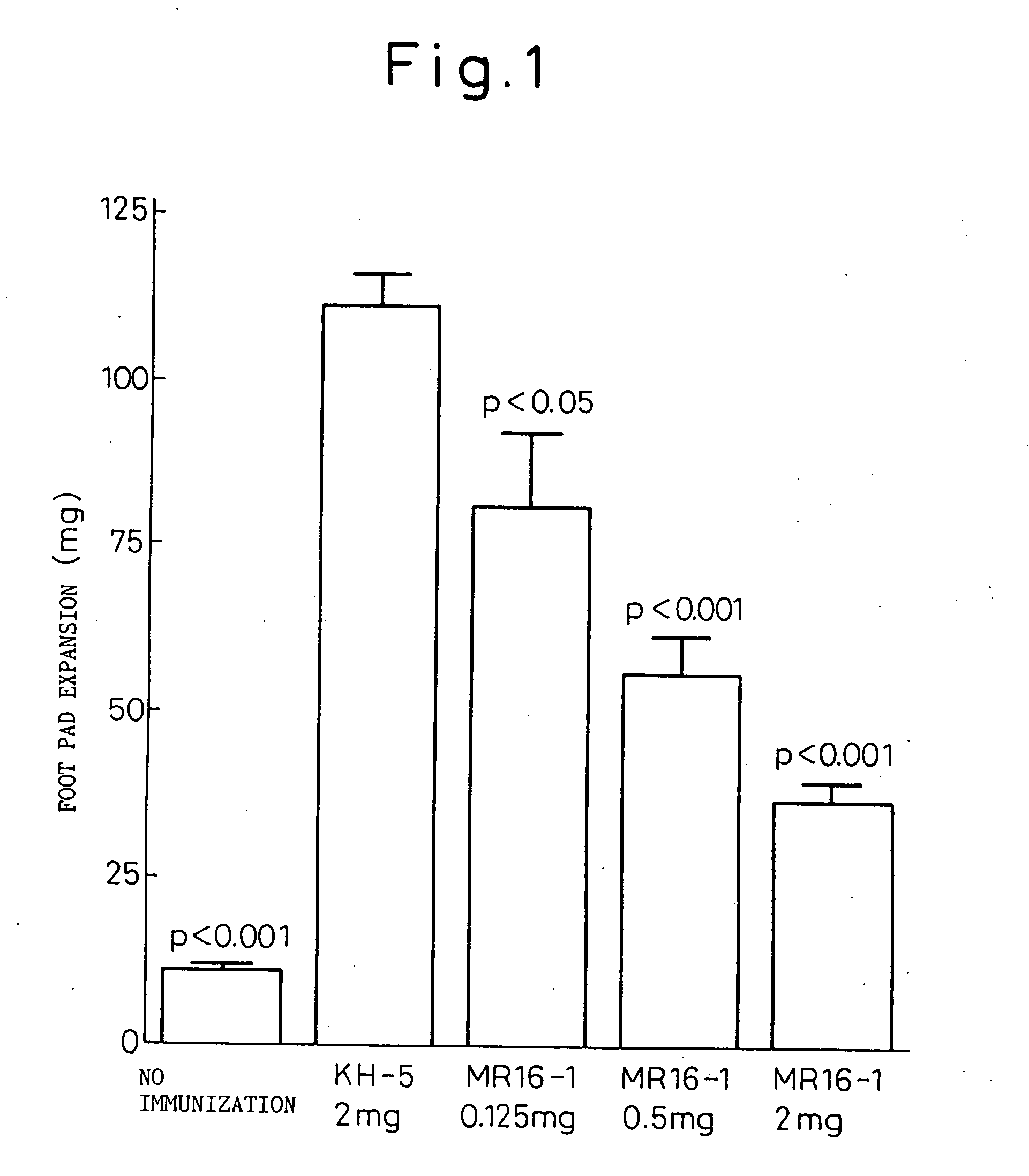
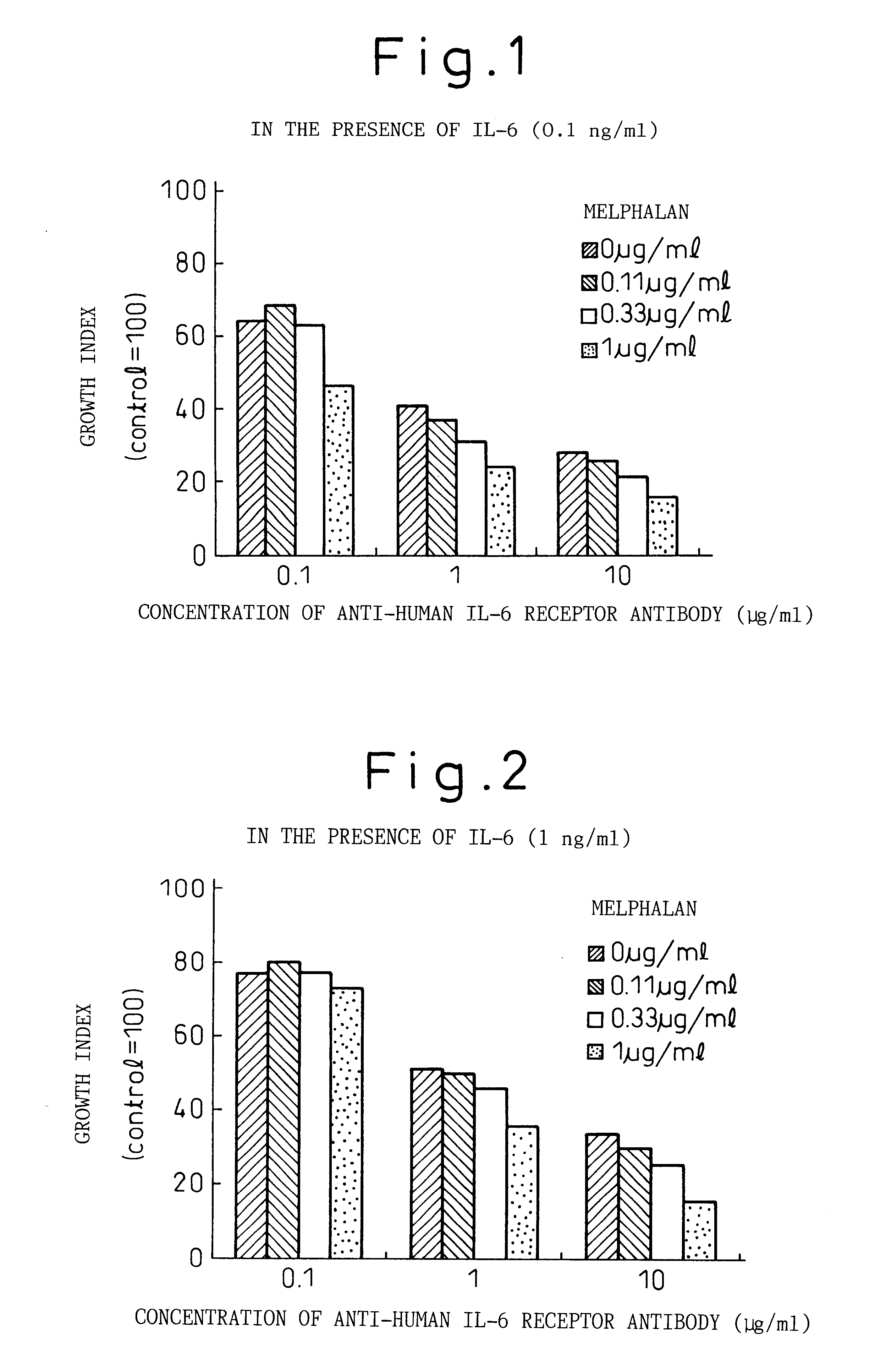
Preventive or therapeutic agent for sensitized T cell-mediated diseases comprising IL-6 antagonist as an active ingredient
InactiveUS20060134113A1Avoid delayPeptide/protein ingredientsImmunoglobulins against cytokines/lymphokines/interferonsInterleukin 6T cell
A preventive or therapeutic agent for sensitized T cell-mediated diseases comprising an interleukin-6 (IL-6) antagonist, for example an antibody directed against IL-6 receptor, an antibody directed against IL-6, an antibody directed against gp130, and the like.
Owner:CHUGAI PHARMA CO LTD
Murine monoclonal anti-idiotype antibody 11D10 and methods of use thereof
Owner:UNIVERSITY OF KENTUCKY
Agonist anti-trk-C monoclonal antibodies
InactiveUS7384632B2Effective prevention and treatmentMaintain good propertiesNervous disorderGenetic material ingredientsMonoclonal antibodyAgonist
Owner:GENENTECH INC
Herbicide resistant rice
InactiveUS6274796B1Growth inhibitionGood flexibilityBiocideMutant preparationRice plantsAcetohydroxy Acid Synthetase
Rice plants are disclosed with two separate, but synergistic mechanisms for resistance to herbicides that normally inhibit a plant's acetohydroxyacid synthase (AHAS) enzyme. The herbicide resistance of plants with both resistance mechanisms is substantially greater than one would expect from a simple combination of the two types of resistance. The first of the two resistance mechanisms is a metabolic pathway that is not fully understood, but that does not itself involve a mutant AHAS enzyme. The second resistance mechanism is a mutant AHAS enzyme, an enzyme that shows direct resistance to levels of herbicide that normally inhibit the enzyme, in both in vivo and in vitro assays. Besides controlling red rice, many AHAS-inhibiting herbicides also effectively control other weeds that are common in rice fields. Several of these herbicides have residual activity, so that a treatment controls both existing weeds as well as weeds that sprout later. No herbicide currently available for use on rice has residual activity against a broad spectrum of weeds including red rice. With effective residual activity against red rice and other weeds, rice producers now have a weed control system superior to those currently used.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE
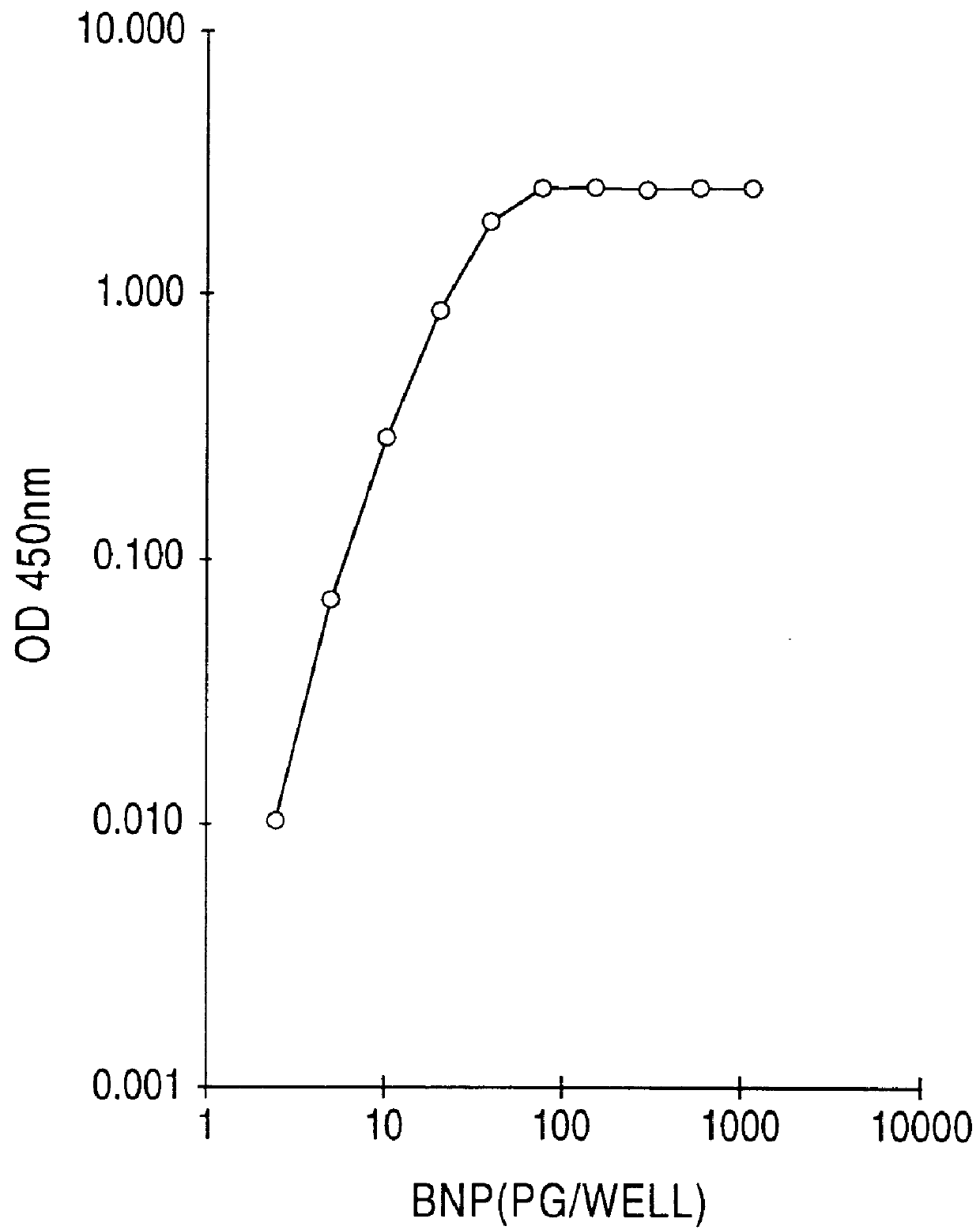
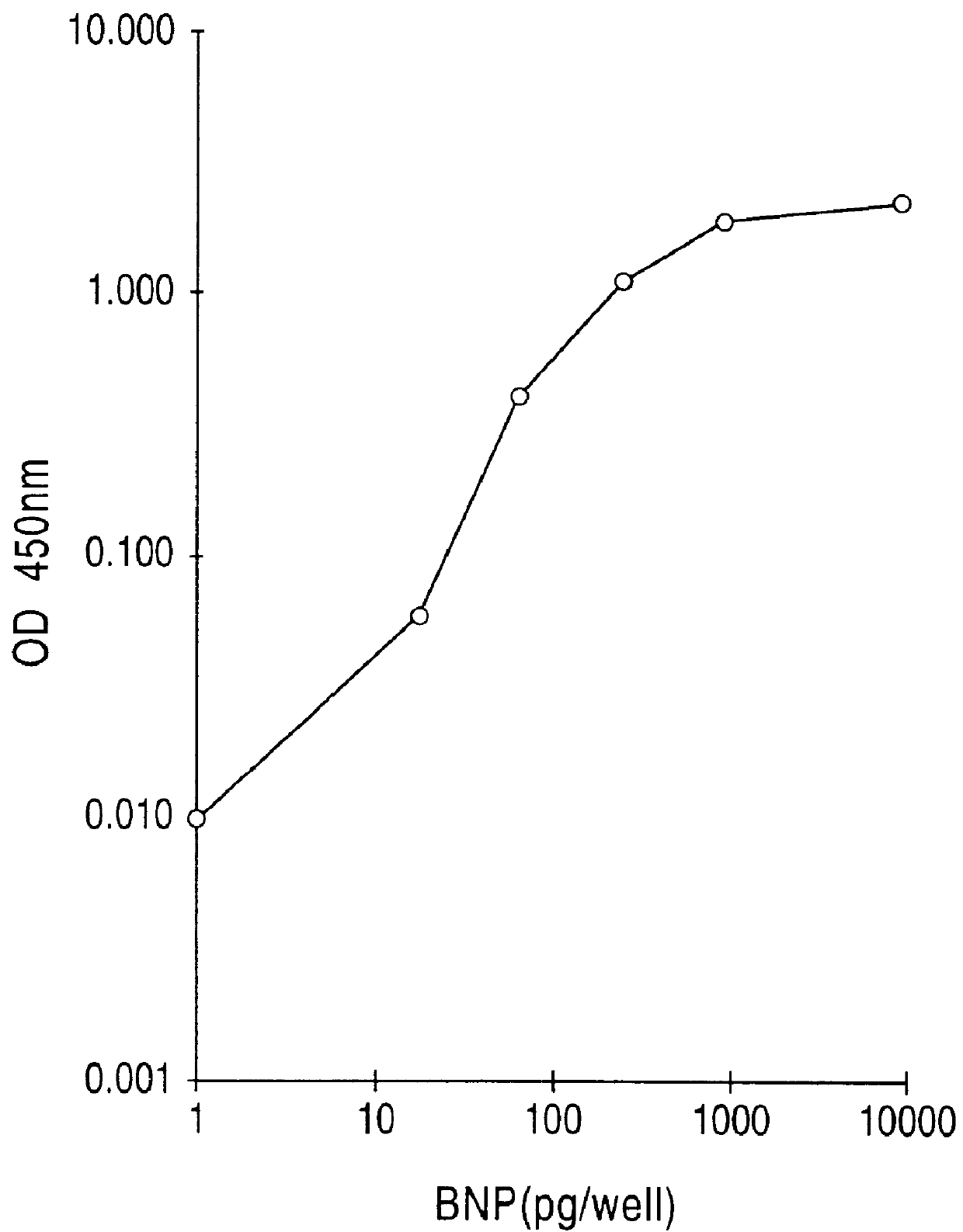
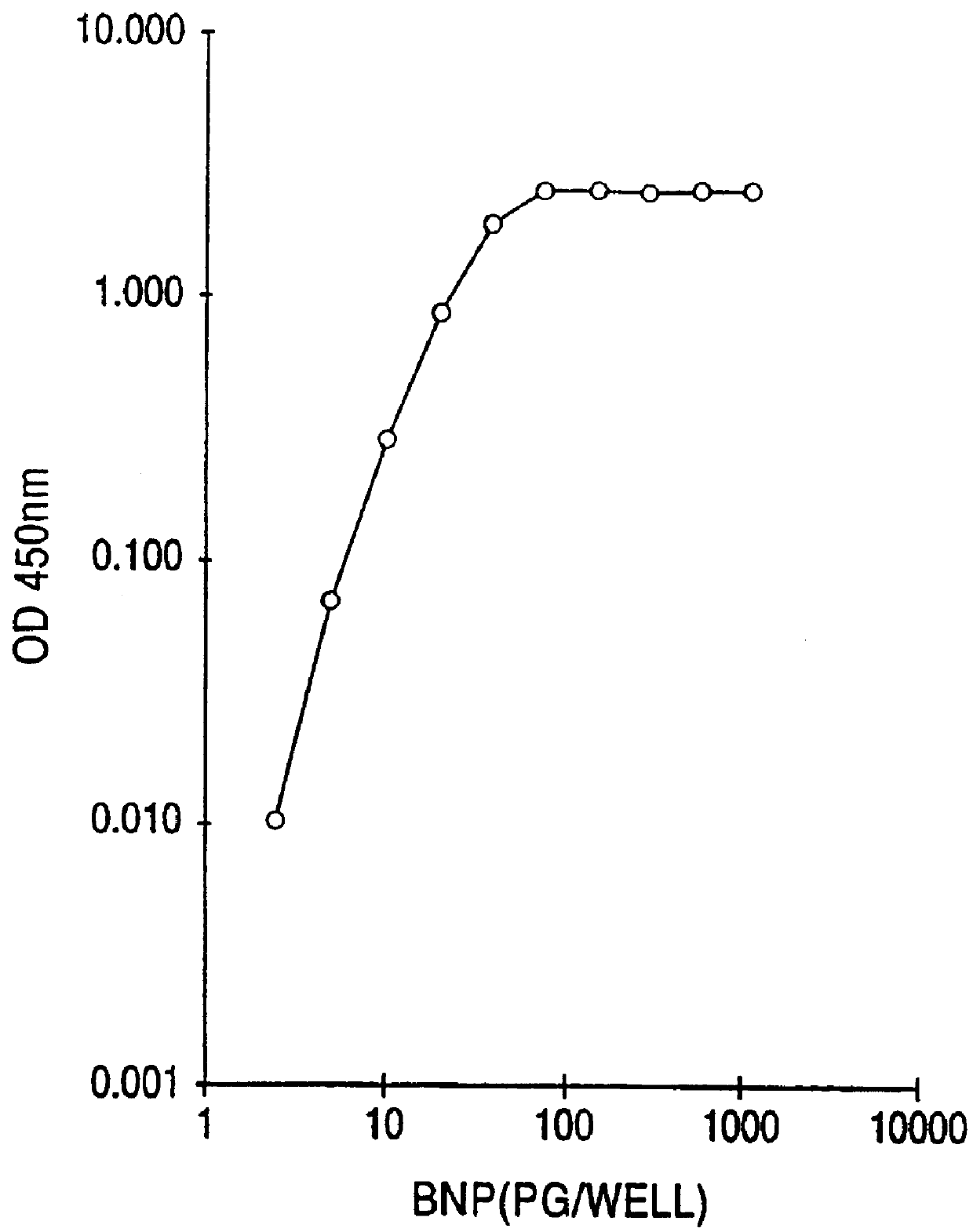
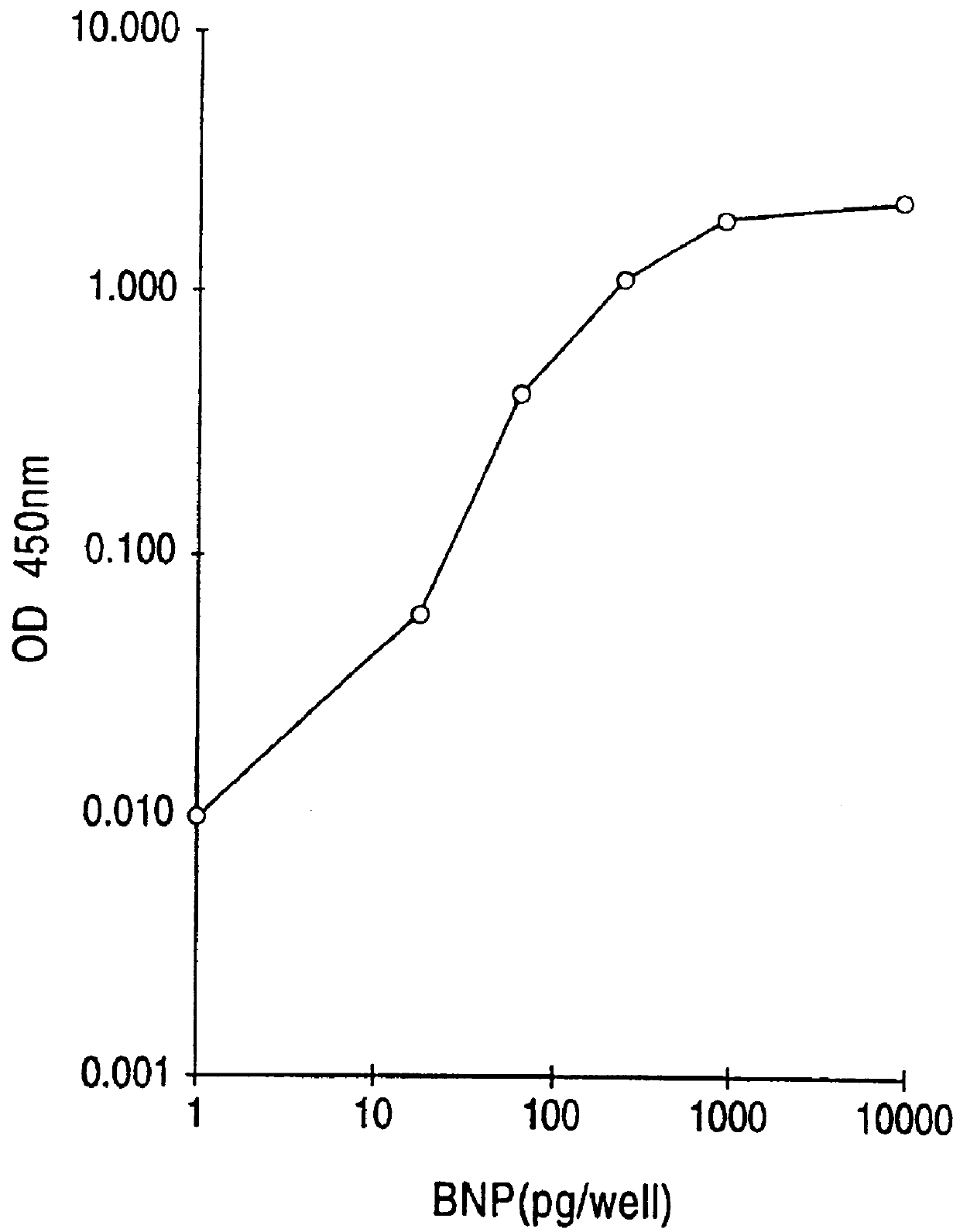
Human BNP-specific antibodies
Owner:SCIOS
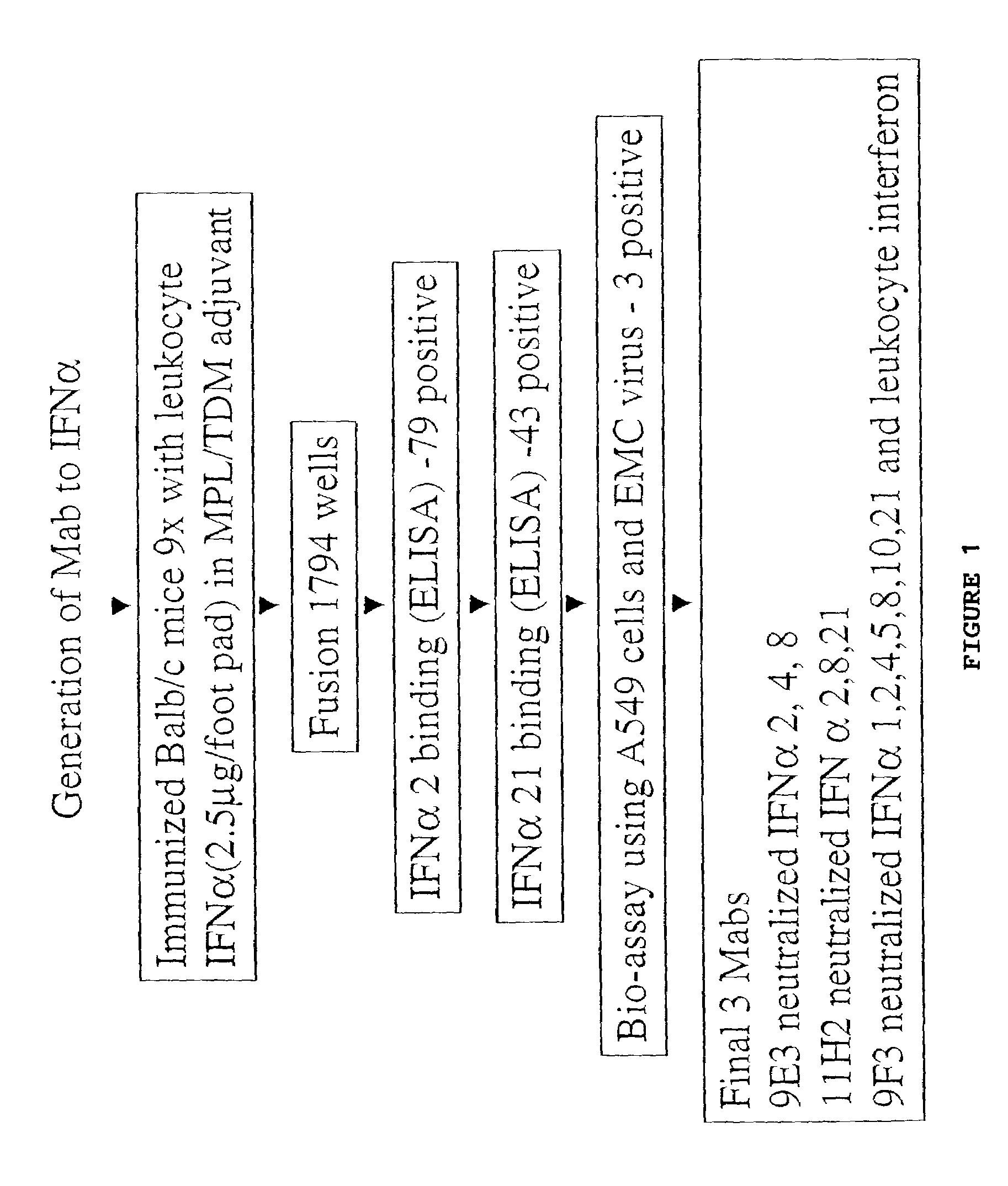
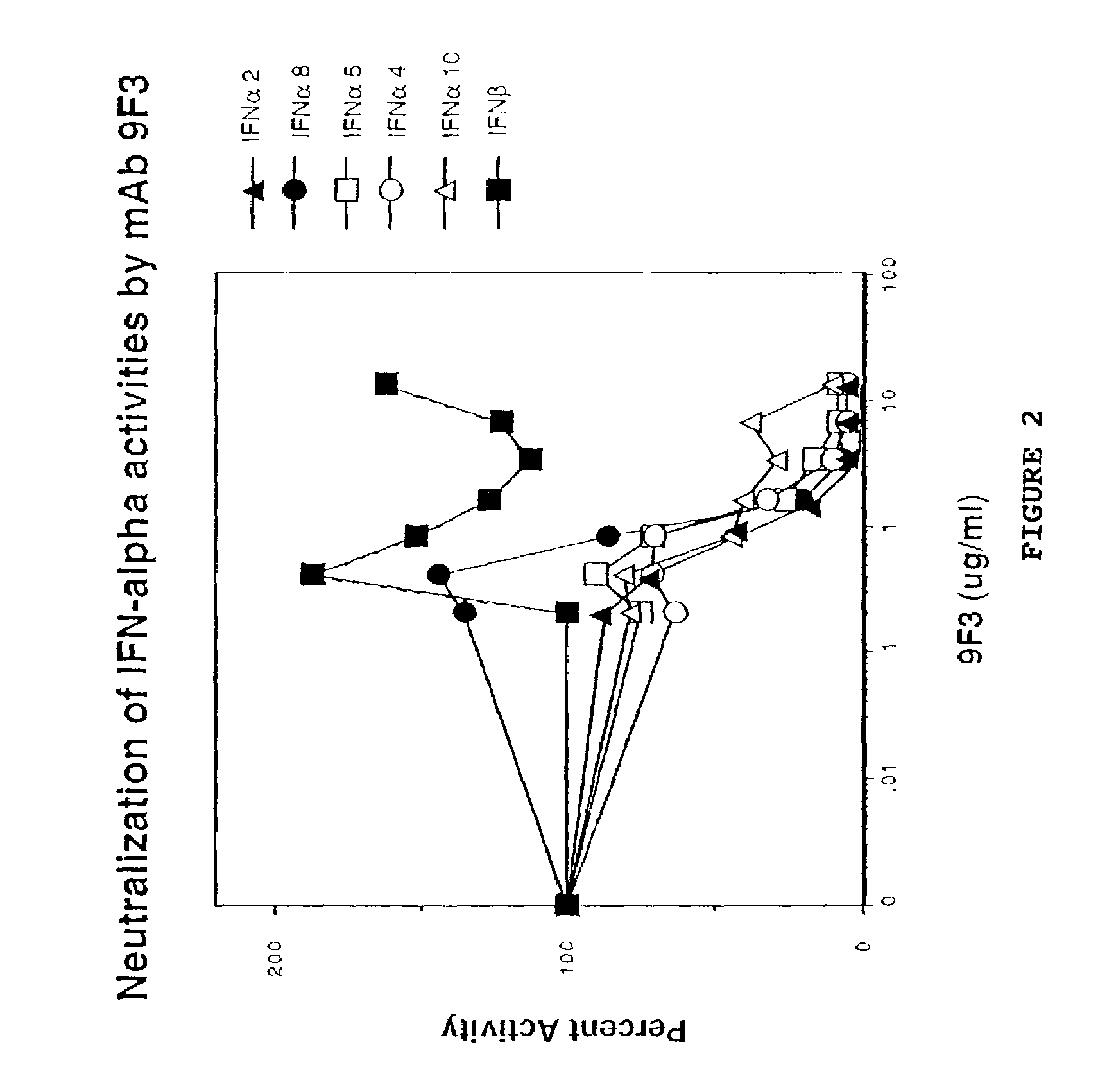
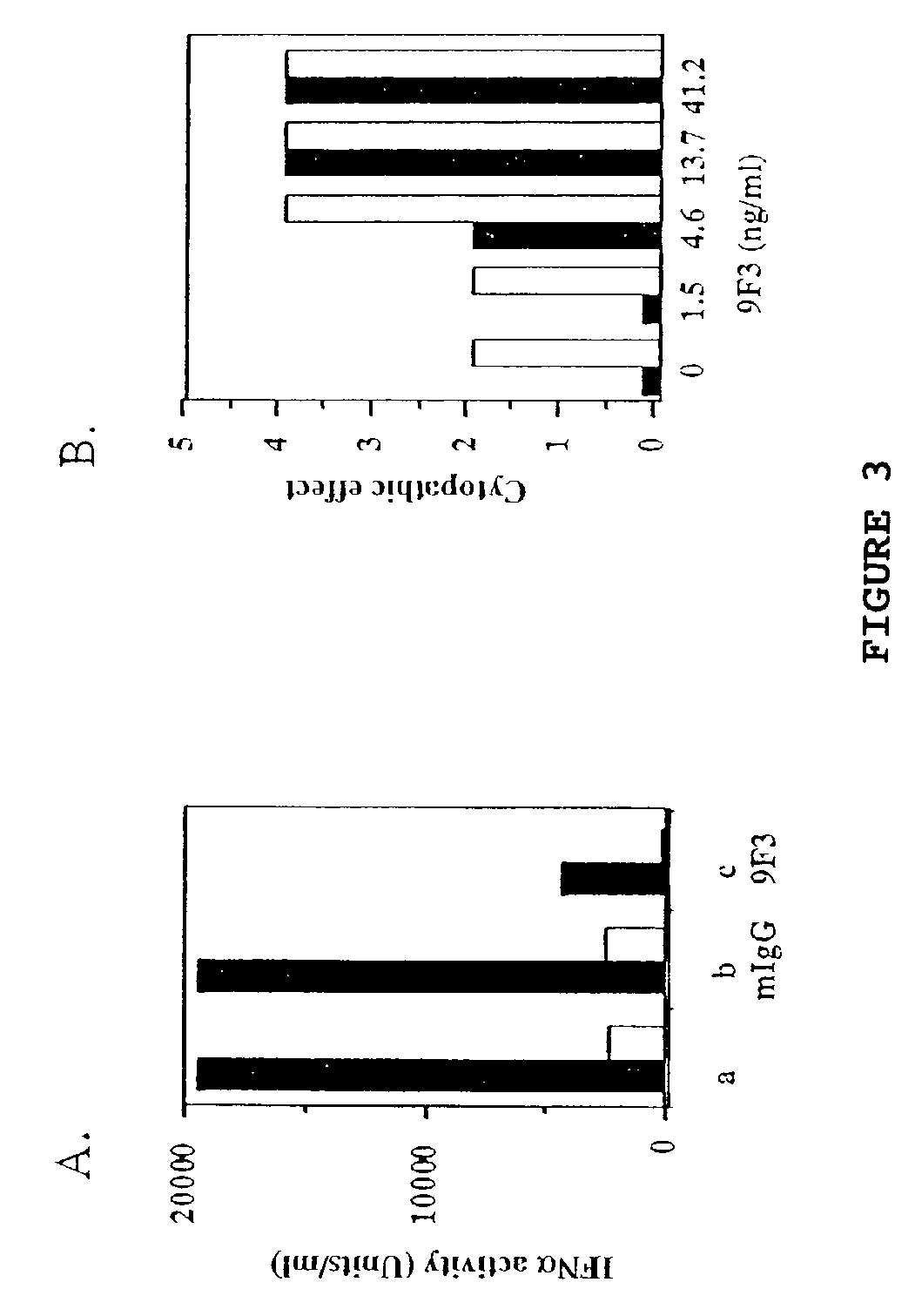
Anti-interferon-α antibodies
InactiveUS7087726B2Reduce and eliminate biological activityFungiBacteriaDiseaseAntiendomysial antibodies
The present invention relates generally to the generation and characterization of neutralizing anti-IFN-α monoclonal antibodies with broad reactivity against various IFN-α subtypes. The invention further relates to the use of such anti-IFN-α antibodies in the diagnosis and treatment of disorders associated with increased expression of IFN-α, in particular, autoimmune disorders such as insulin-dependent diabetes mellitus (IDDM) and systemic lupus erythematosus (SLE).
Owner:GENENTECH INC
Human brain natriuretic peptides
The present invention provides reagents and assays for the quantification of hBNP in biological fluid samples such as plasma or serum. Antibodies are provided which are monospecific to epitopes comprising the amino acid sequences 5-13, 1-10 and 15-25 of hBNP. These antibodies, and peptide fragments containing these sequences, can be employed in the assays of the invention, which may be carried out in a sandwich format or a competition format.
Owner:SCIOS
Modified antibodies to prostate-specific membrane antigen and uses thereof
InactiveUS7045605B2Less immunogenicHigh affinityNervous disorderHybrid cell preparationAntigen Binding FragmentAntigen binding
Modified antibodies, or antigen-binding fragments thereof, to the extracellular domain of human prostate specific membrane antigen (PSMA) are provided. The modified anti-PSMA antibodies, or antigen-binding fragments thereof, have been rendered less immunogenic compared to their unmodified counterparts to a given species, e.g., a human. Pharmaceutical compositions including the aforesaid antibodies, nucleic acids, recombinant expression vectors and host cells for making such antibodies and fragments are also disclosed. Methods of using the antibodies of the invention to detect human PSMA, or to ablate or kill a PSMA-expressing cell, e.g., a PSMA-expressing cancer or prostatic cell, either in vitro or in vivo, are also provided.
Owner:CORNELL RES FOUNDATION INC
Methods of promoting immunopotentiation and preparing antibodies with anti-CD3 antibodies
Disclosed are immunopotentiating agents, and vaccines thereof, which enhance and / or otherwise modify immune responses, and method for their preparation and use in vivo. Immunopotentiating agents can be single agents that act directly, adjuvants added concurrently with the agents, or heteroconjugates wherein the immunopotentiating agent is chemically coupled to the compound against which an immune response is desired. Examples of immunopotentiating agents include monoclonal antibodies, such as anti-CD3, anti-CD2) and anti-CD5 antibodies, and proteins derived from microorganisms (e.g., enterotoxins) which activate T cells. The compounds against which an immune response can be generated, which may be the second component in a heteroconjugate, include compound from abnormal or diseased tissues such as tumors, or infectious agents, such as viruses, bacteria, fungi, protozoal or metozoal parasites, and can be obtained by natural or recombinant means. Methods of using the invention to prepare monoclonal antibodies are particularly disclosed.
Owner:MACROGENICS INC
Fluidic device
InactiveUS20070105206A1Great electric field intensityBioreactor/fermenter combinationsBiological substance pretreatmentsLysisElectroporation
A fluidic device for cell electroporation, cell lysis, and cell electrofusion based on constant DC voltage and geometric variation is provided. The fluidic device can be used with prokaryotic or eukaryotic cells. In addition, the device can be used for electroporative delivery of compounds, drugs, and genes into prokaryotic and eukaryotic cells on a microfluidic platform.
Owner:PURDUE RES FOUND INC
Method and apparatus for manipulation of cells and cell-like structures focused electric fields in microfludic systems and use thereof
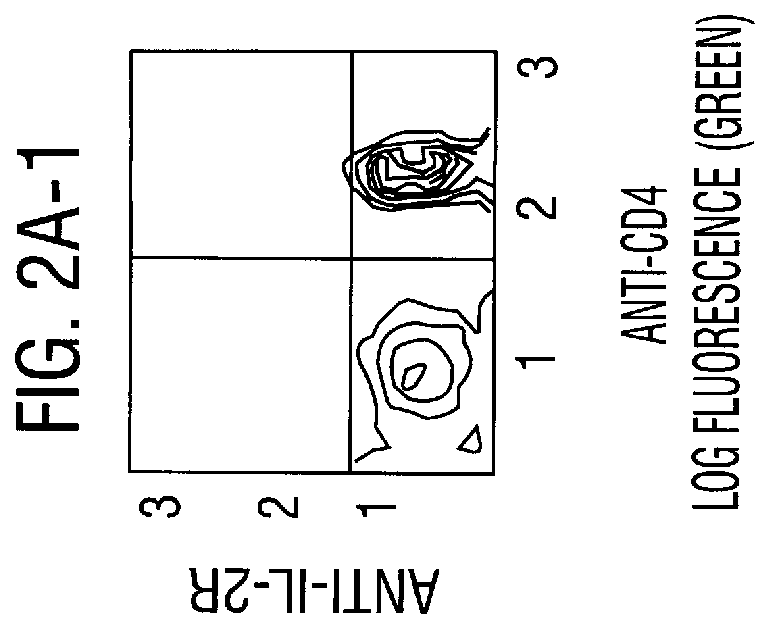
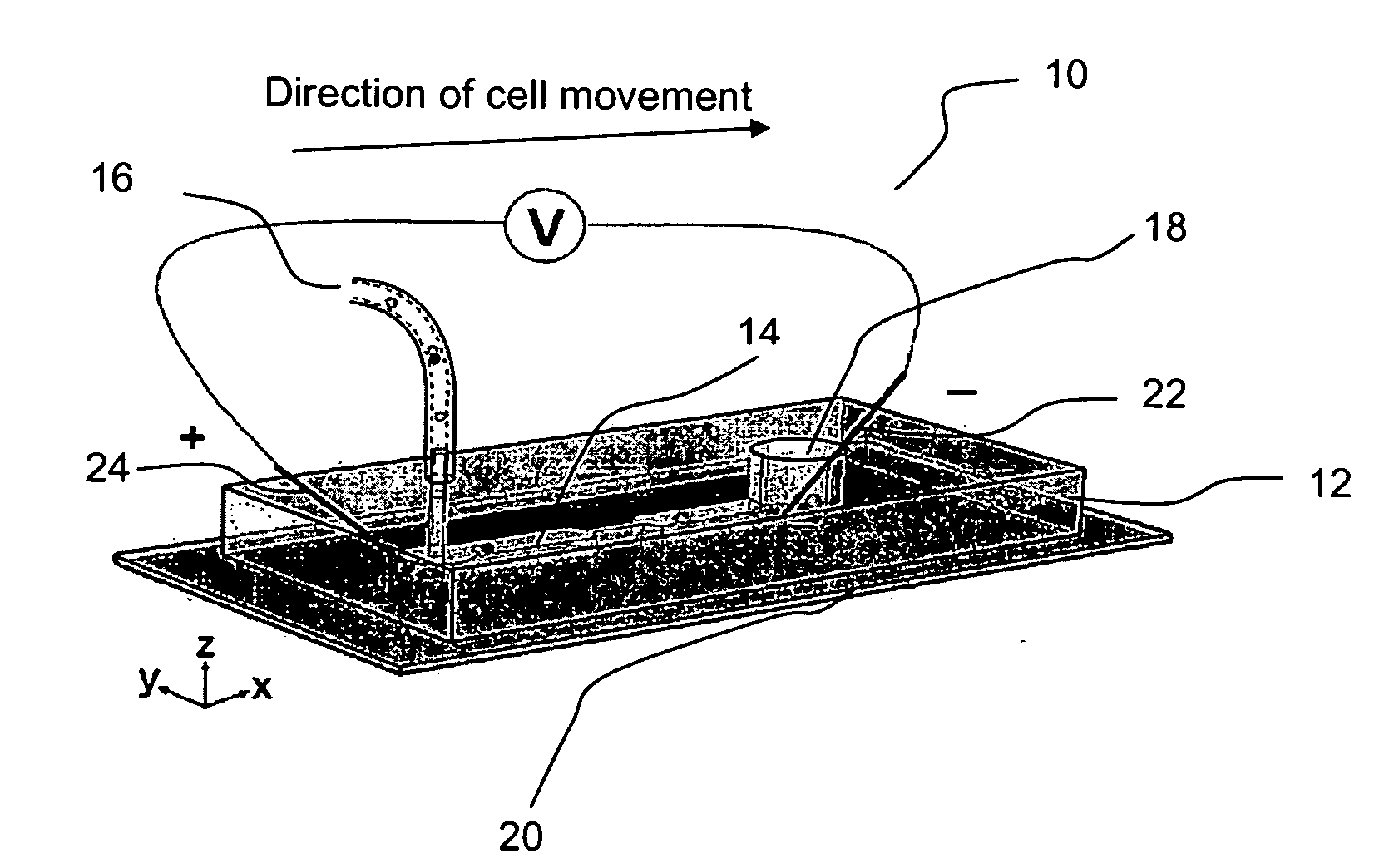
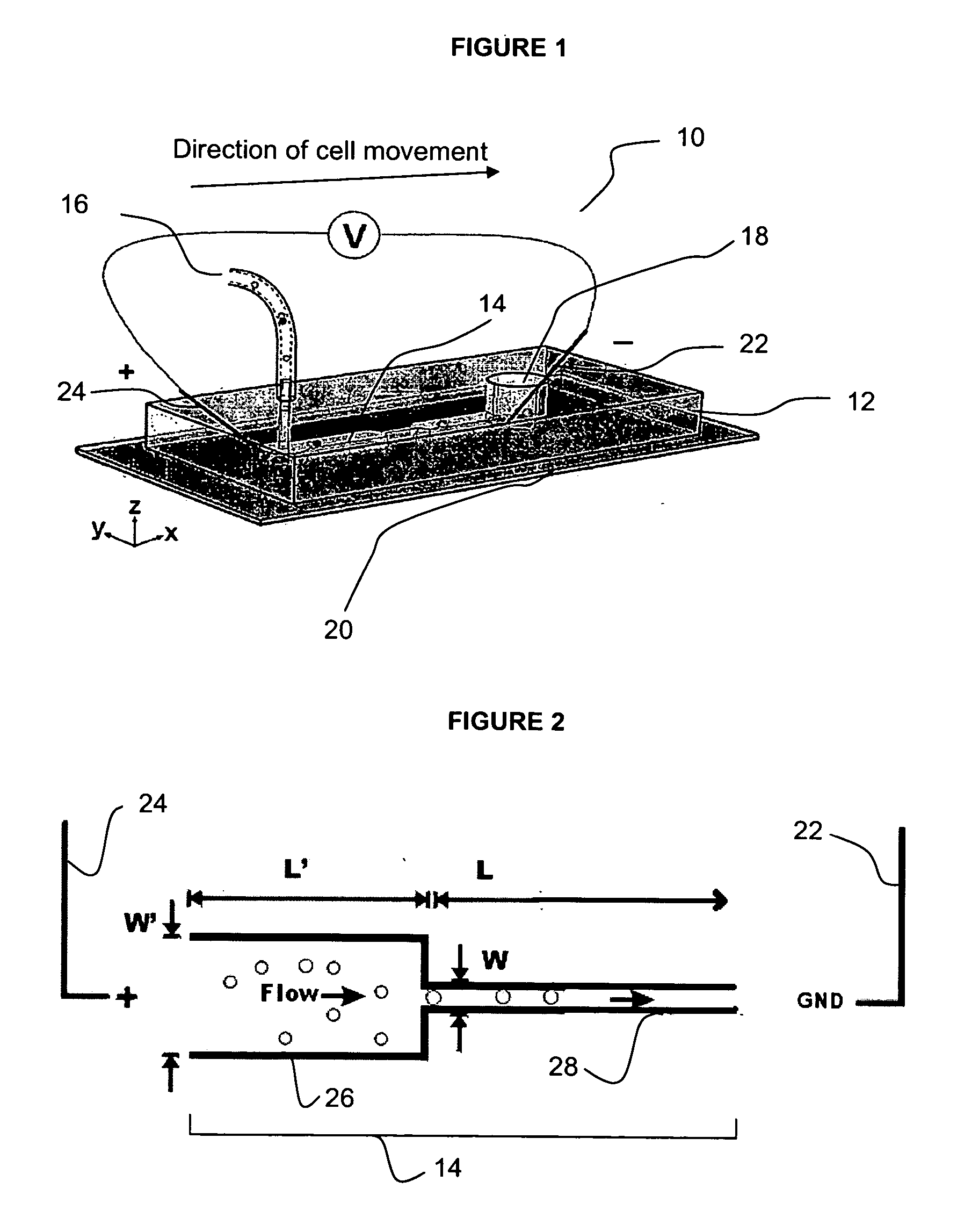
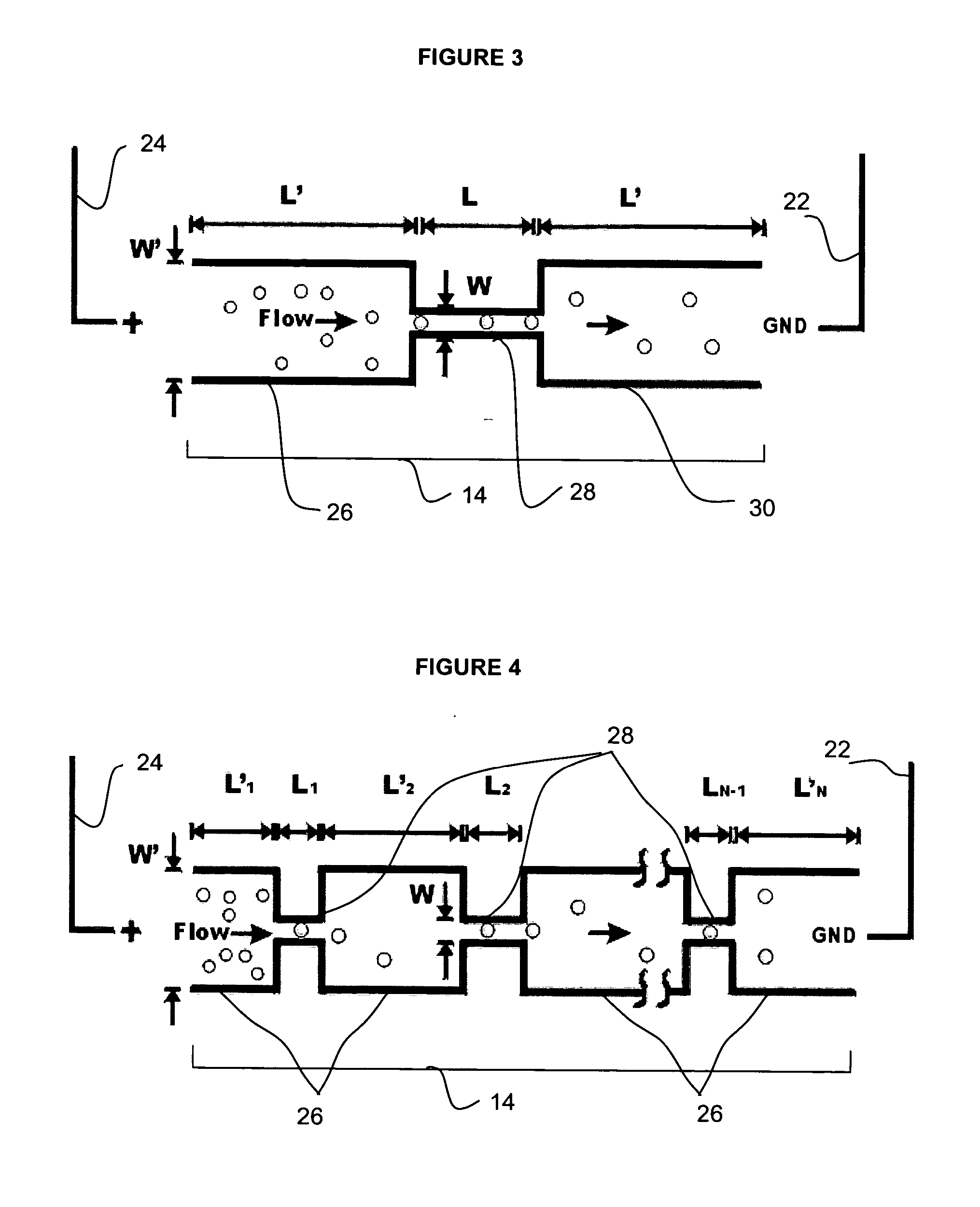
InactiveUS7018819B2Understand natureOvercomes shortcomingHybrid cell preparationOther foreign material introduction processesVoltage generatorEngineering
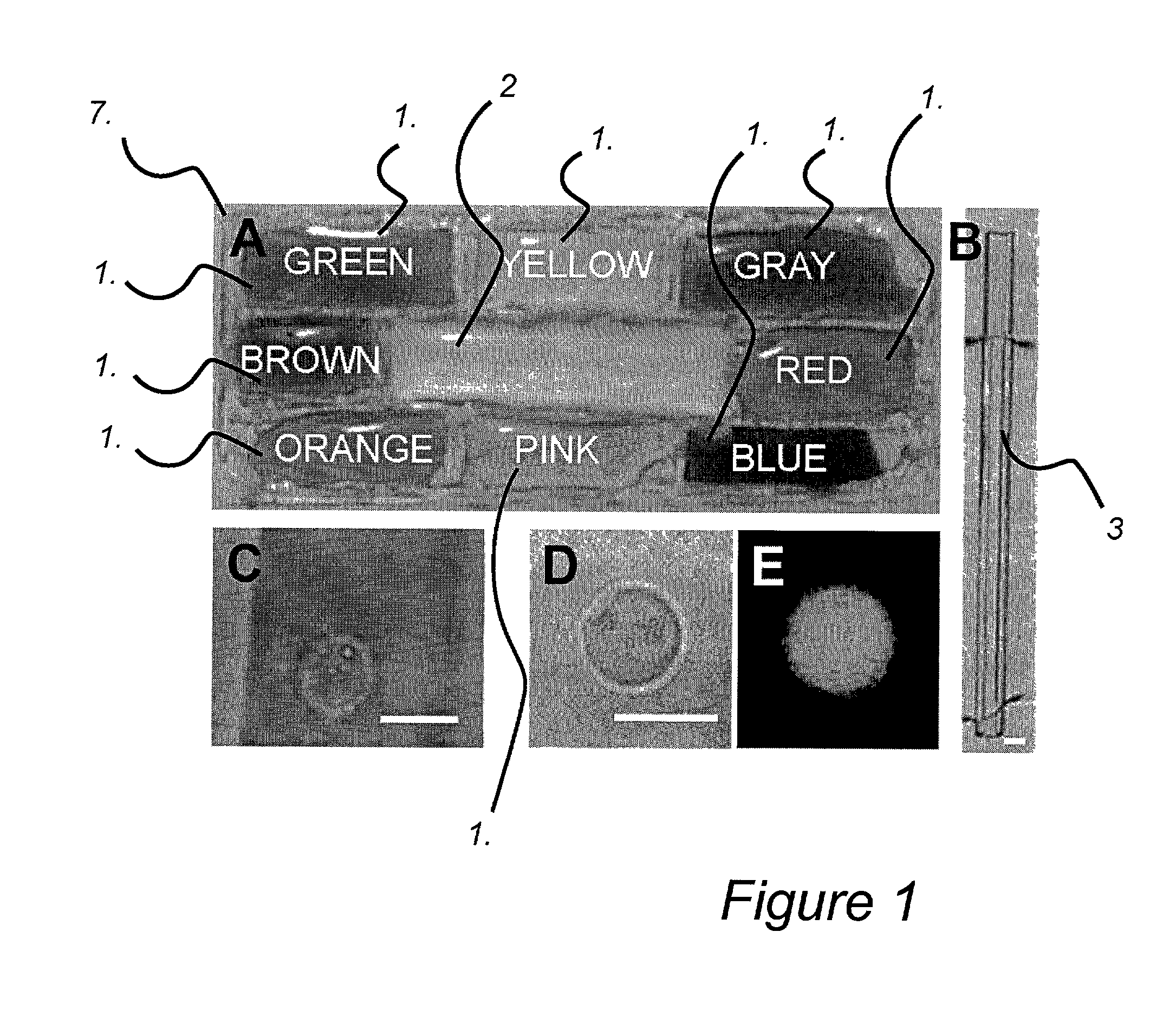
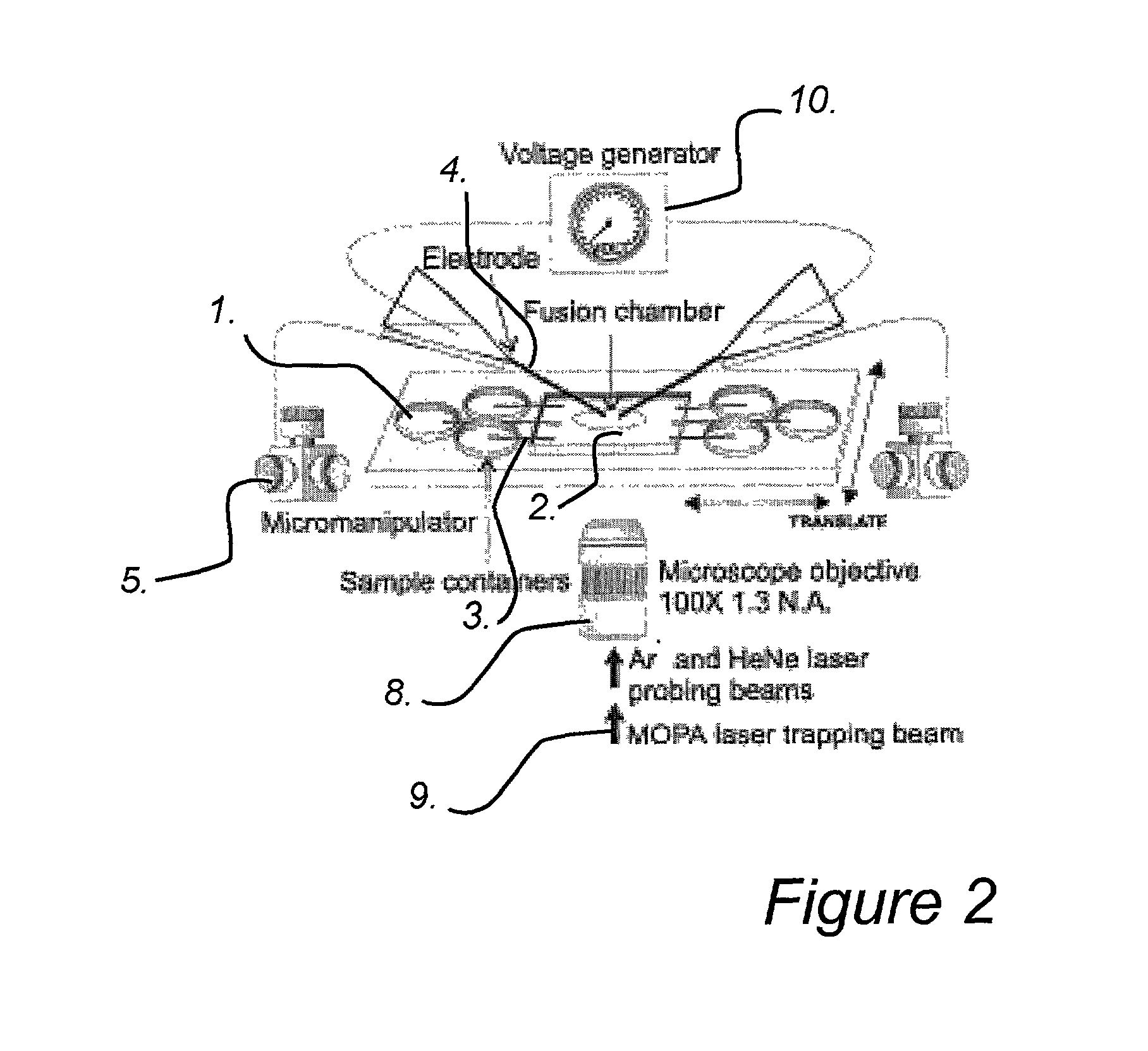
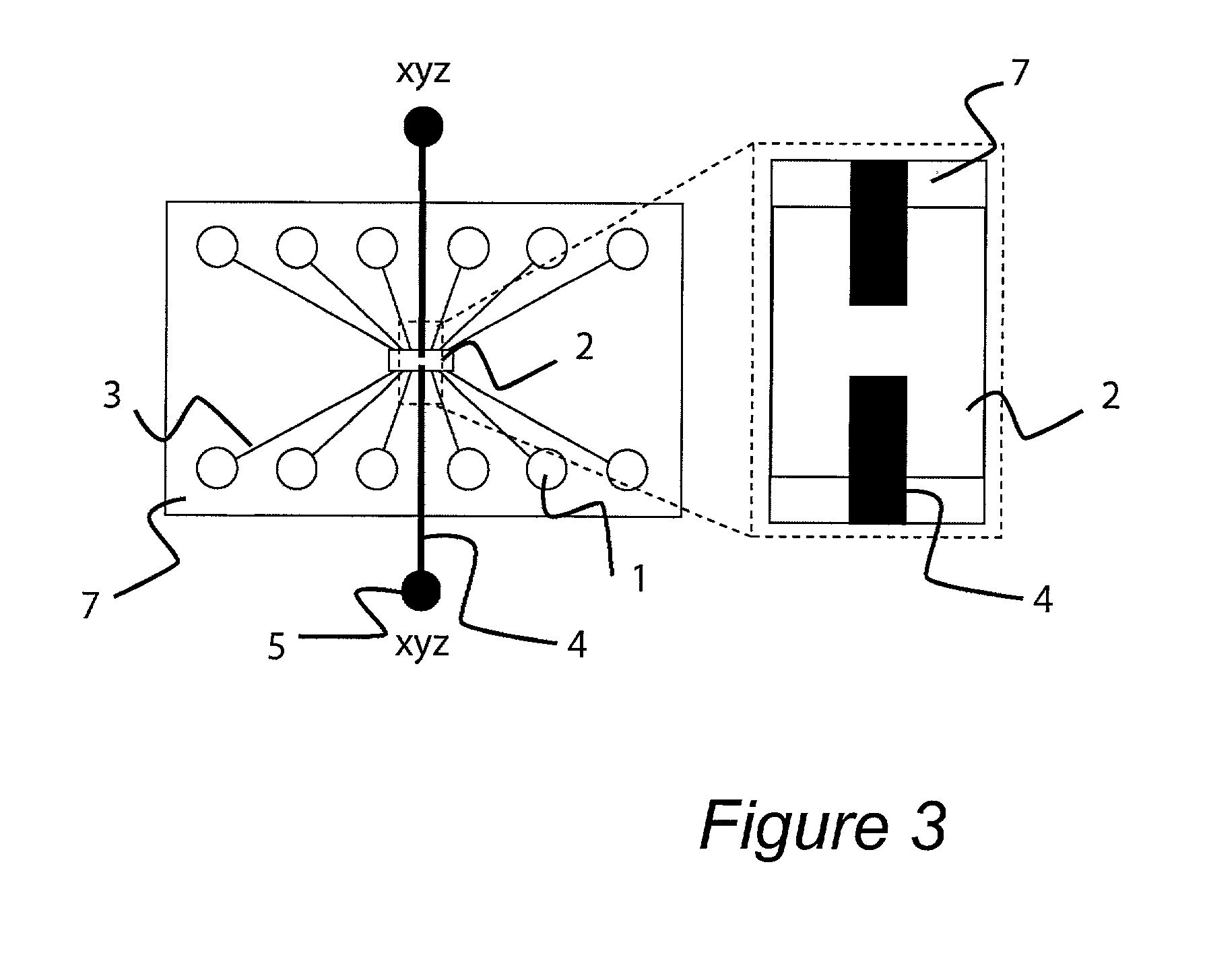
An apparatus and method are disclosed for electromanipulation of at least one cell or cell-like structure having cell-like membranes, the method comprising the steps: (a) at least one cell or cell-like structure is transported from one or more sample containers located on a chip through microchannel(s) located on said chip into a chamber located on said chip, wherein said chamber contains electrode(s) connected to a voltage generator, wherein said microchannel provides a fluid contact between the sample containers, (b) said cell or cell-like structure(s) is placed close to said at least one electrode, and (c) an electrical field is applied and focused on said cell or cell-like structure(s), said electrical field being of a strength sufficient to obtain pore-formation or fusion of said at least one cell or cell-like structure with another cell or cell-like structure(s) present in said chamber.
Owner:CELLECTRICON
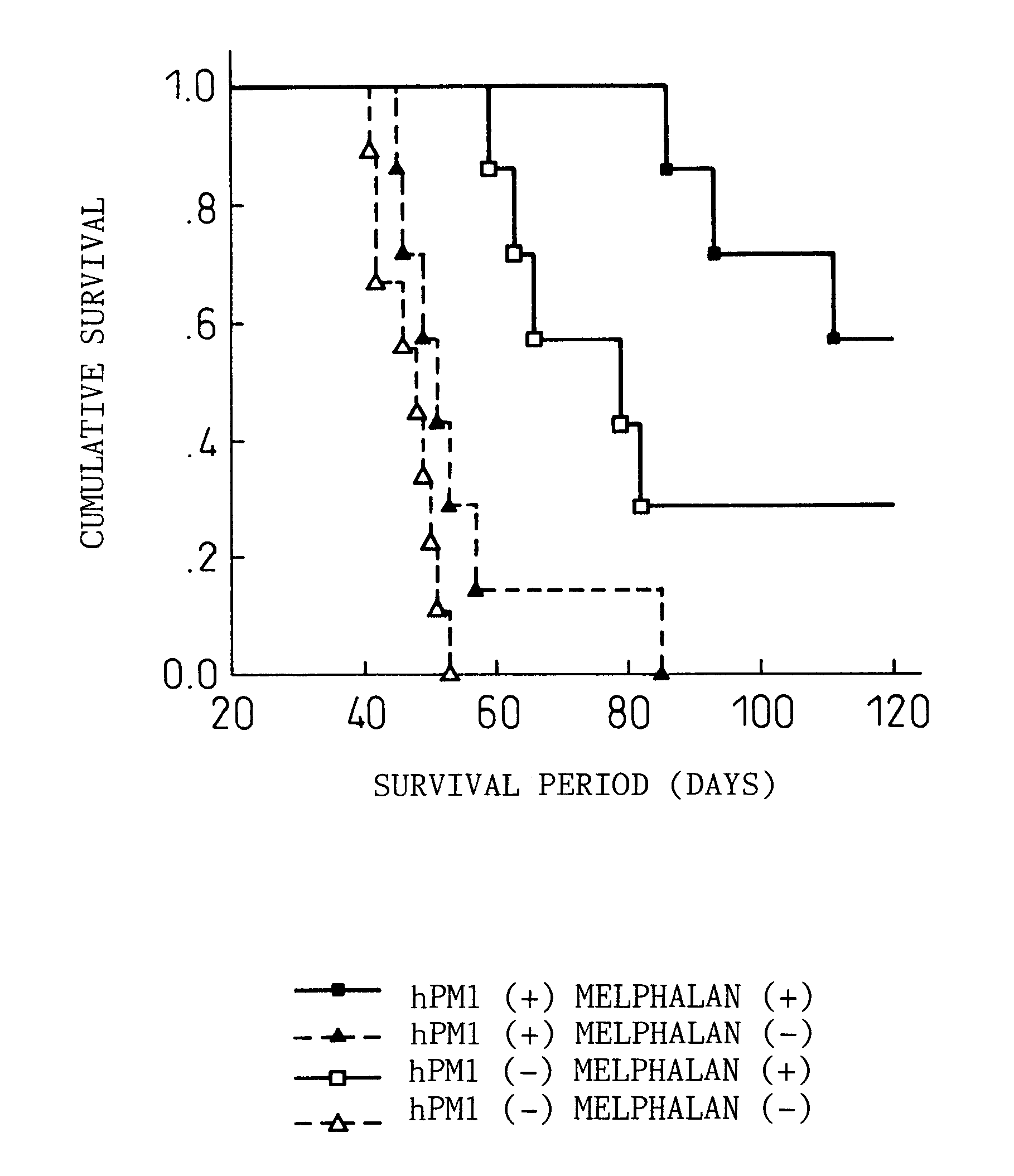
Remedies for myeloma to be used together with nitrogen mustard antitumor agents
A therapeutic agent for myeloma comprising a combined use of a nitrogen mustard anticancer agent and anti-IL-6 receptor antibody. Thus, a therapeutic agent for myeloma comprising anti-IL-6 receptor antibody for use in combination with a nitrogen mustard anticancer agent; a therapeutic agent for myeloma comprising a nitrogen mustard anticancer agent for use in combination with anti-IL-6 receptor antibody; and a therapeutic agent for myeloma comprising a nitrogen mustard anticancer agent and anti-IL-6 receptor antibody.
Owner:CHUGAI PHARMA CO LTD
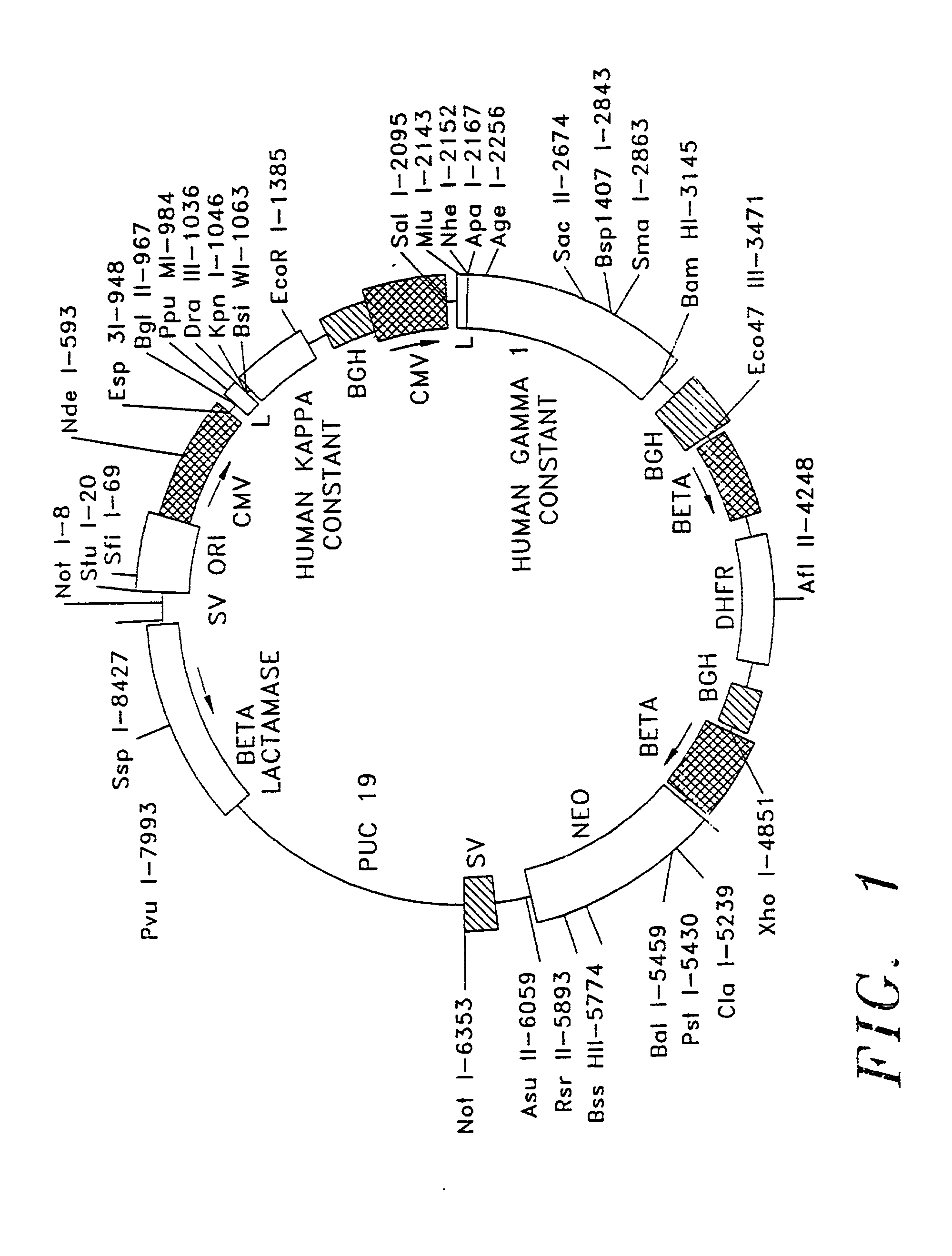
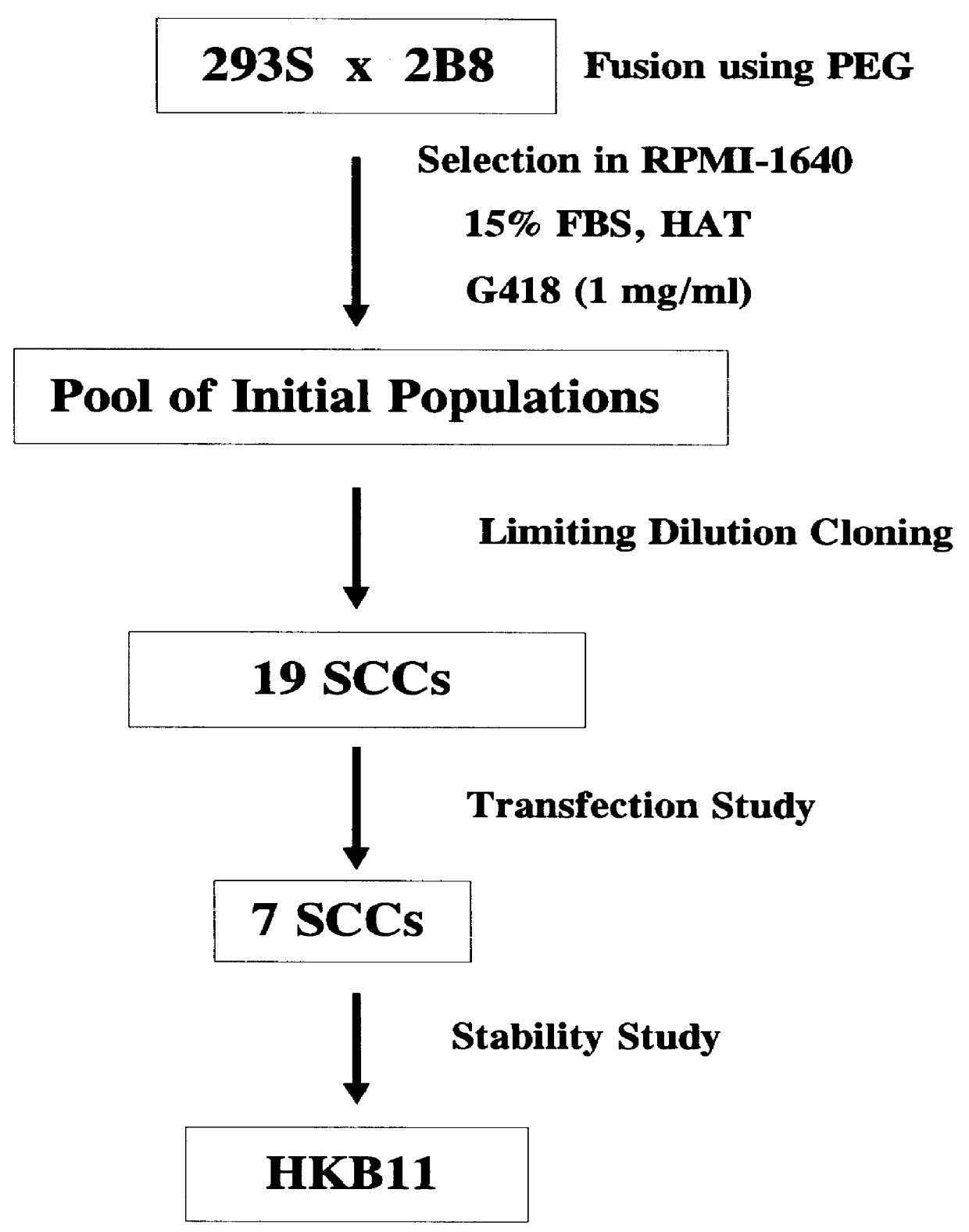
Human hybrid host cell for mammalian gene expression
InactiveUS6136599AEasily transfectedEasy to adaptGenetically modified cellsMutant preparationHeterologousMammal
Human / human hybrid cells were made via fusion of human embryonic kidney cells (293S) and modified Burkitt's lymphoma cells (2B8). The fusion cells are useful as host cells for the recombinant expression of mammalian genes. The advantages of using these hybrid clones of human kidney- and B-cells, called HKBs, for mammalian gene expression, include (i) the cells are negative for immunoglobulin expression, (ii) the cells grow easily in plasma protein-free medium (with or without the addition of recombinant insulin) as suspension cultures in a shake flask or in a fermenter (iii) the cells are very susceptible for transfection of DNA, and (iv) the cells secrete high levels of heterologous recombinant proteins, such as recombinant monoclonal antibodies, soluble ICAM-1, rIL-4, and rFVIII.
Owner:BAYER HEALTHCARE LLC +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com