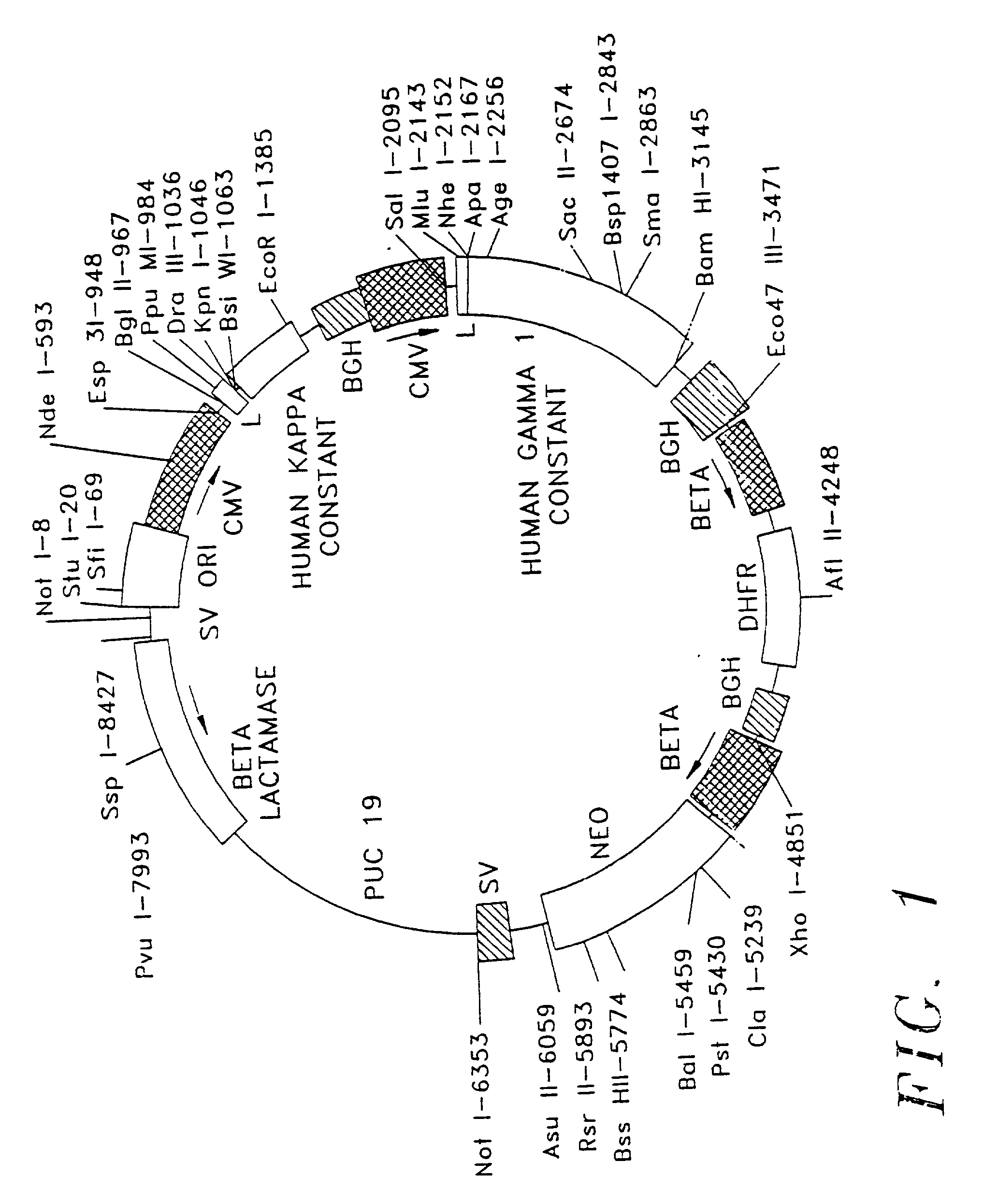
Therapeutic application of chimeric and radiolabelled antibodies to human B lymphocyte restricted differentiation antigen for treatment of B cell lymphoma
a technology of restricted differentiation and immunoglobulin, applied in the field of b cell lymphoma treatment, can solve the problems of induction of immune responses, non-human monoclonal antibodies (eg, murine monoclonal antibodies) typically lack human effector function,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0028] Generally, antibodies are composed of two light chains and two heavy chain molecules; these chains form a general "Y" shape, with both light and heavy chains forming the arms of the Y and the heavy chains forming the base of the Y. Light and heavy chains are divided into domains of structural and functional homology. The variable domains of both the light ("V.sub.L") and the heavy ("V.sub.H") chains determine recognition and specificity. The constant region domains of light ("C.sub.L") and heavy ("C.sub.H") chains confer important biological properties, eg antibody chain association, secretion, transplacental mobility, Fc receptor binding complement binding, etc. The series of events leading to immunoglobulin gene expression in the antibody producing cells are complex. The variable domain region gene sequences are located in separate germ line gene segments referred to as "V.sub.H," "D," and "J.sub.H," or "V.sub.L" and "J.sub.L." These gene segments are joined by DNA rearrang...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com