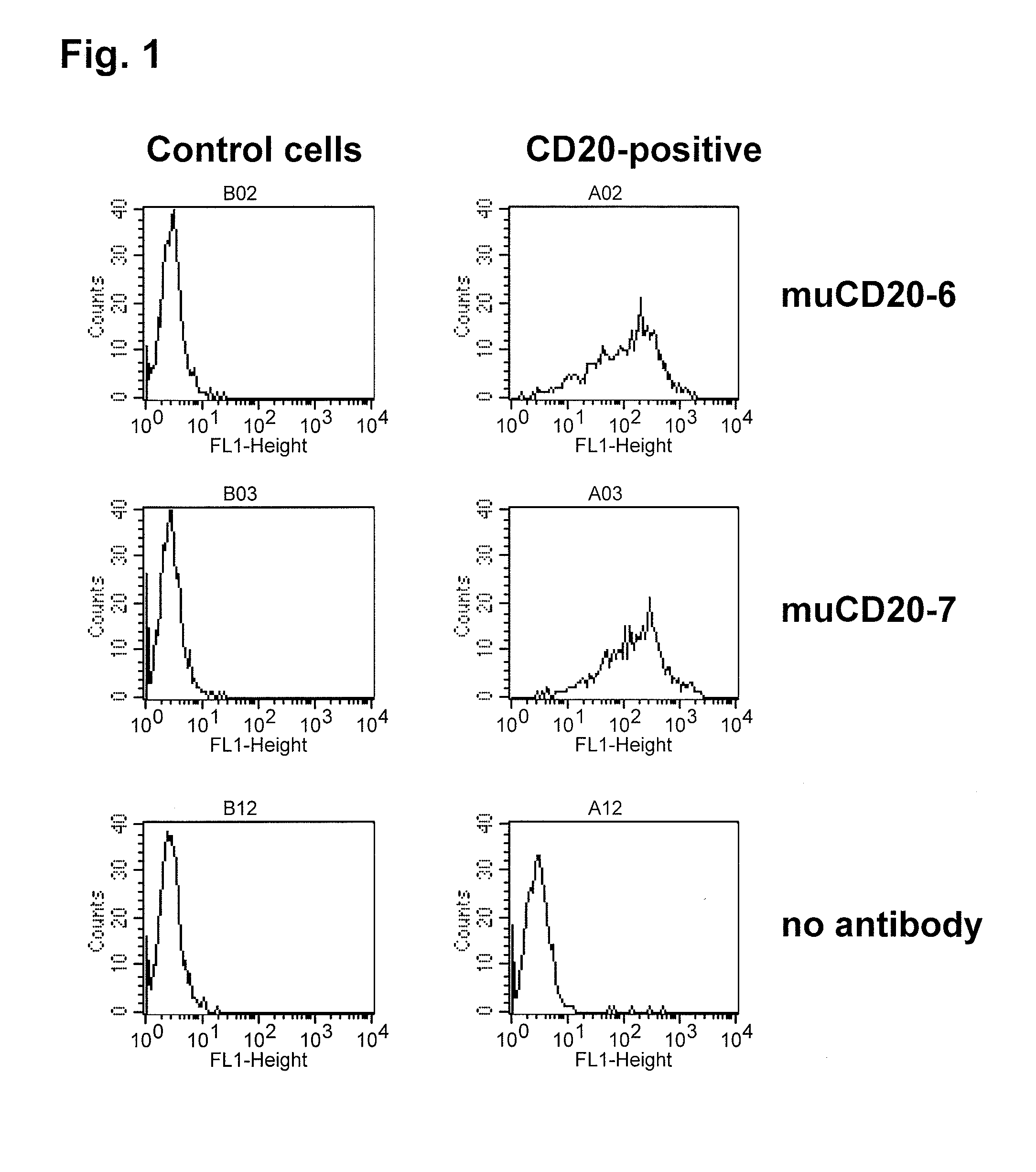
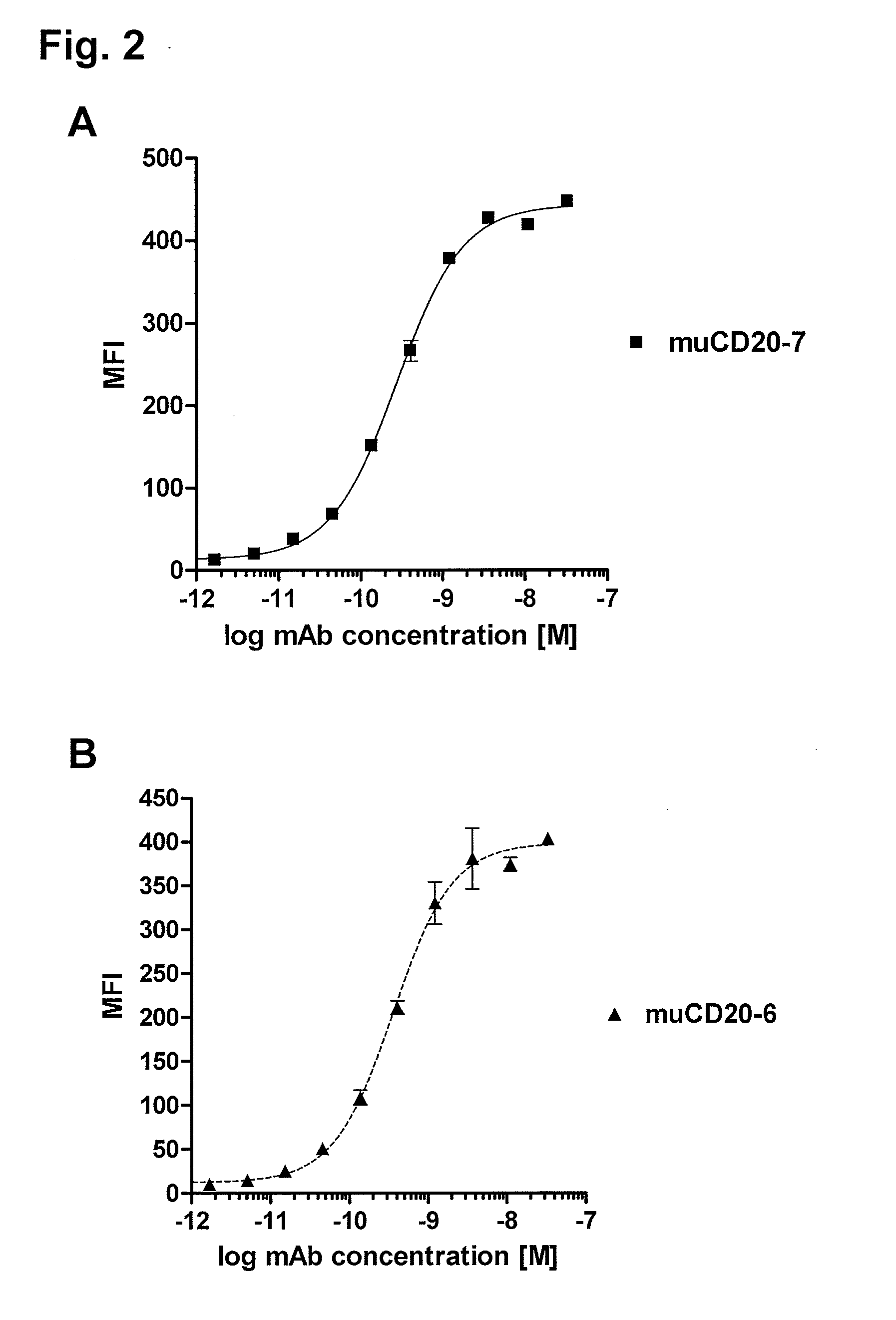
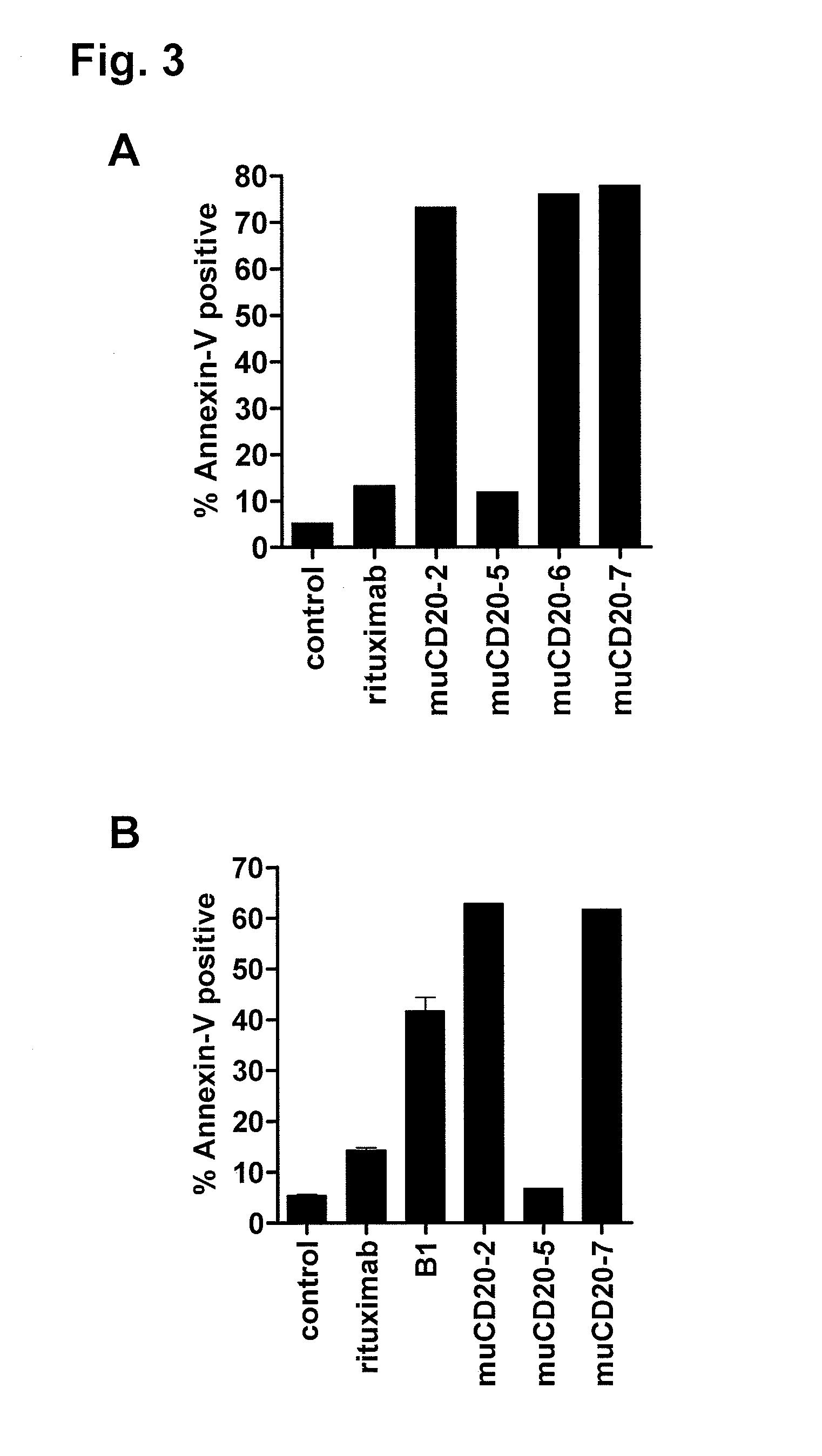
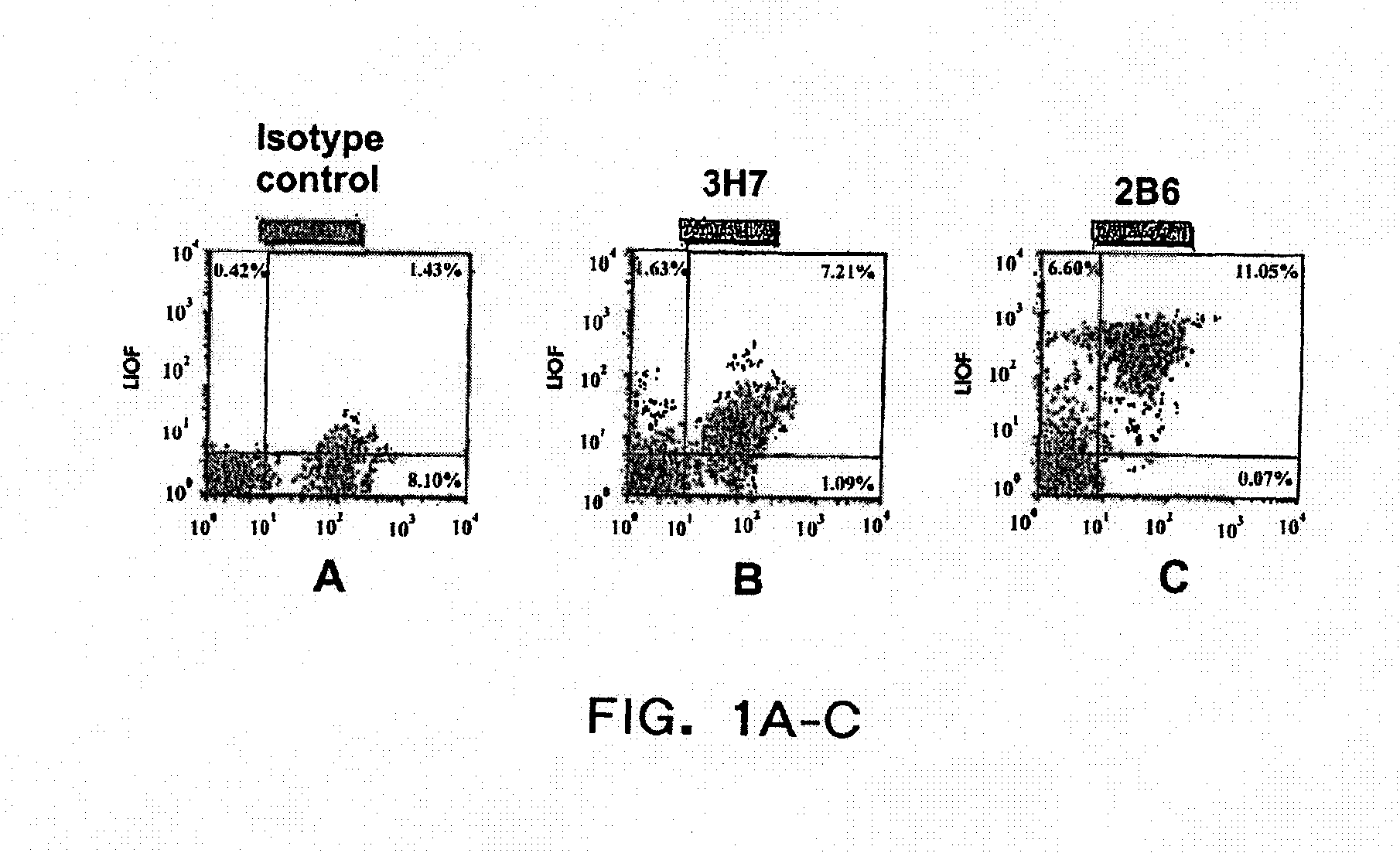
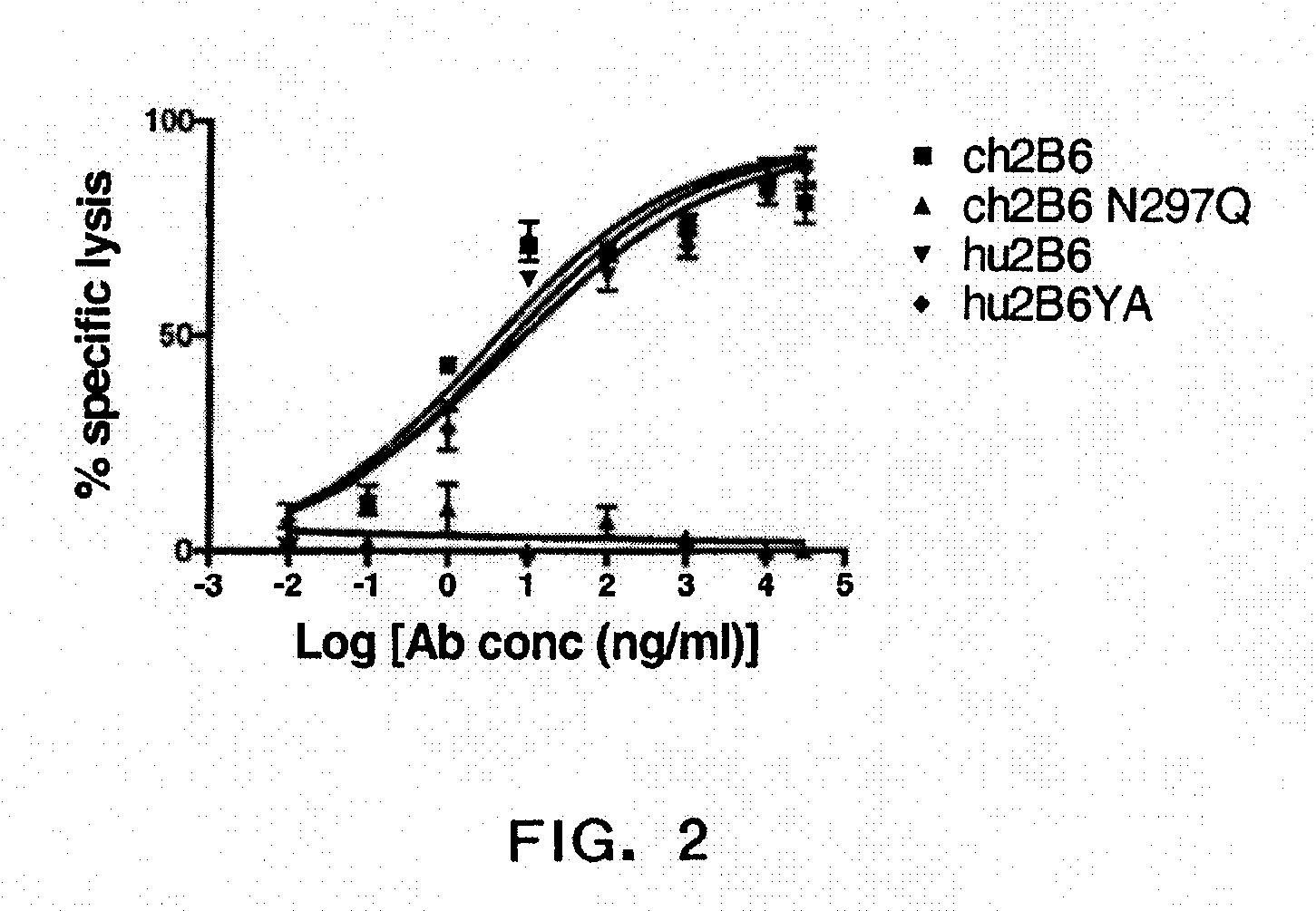
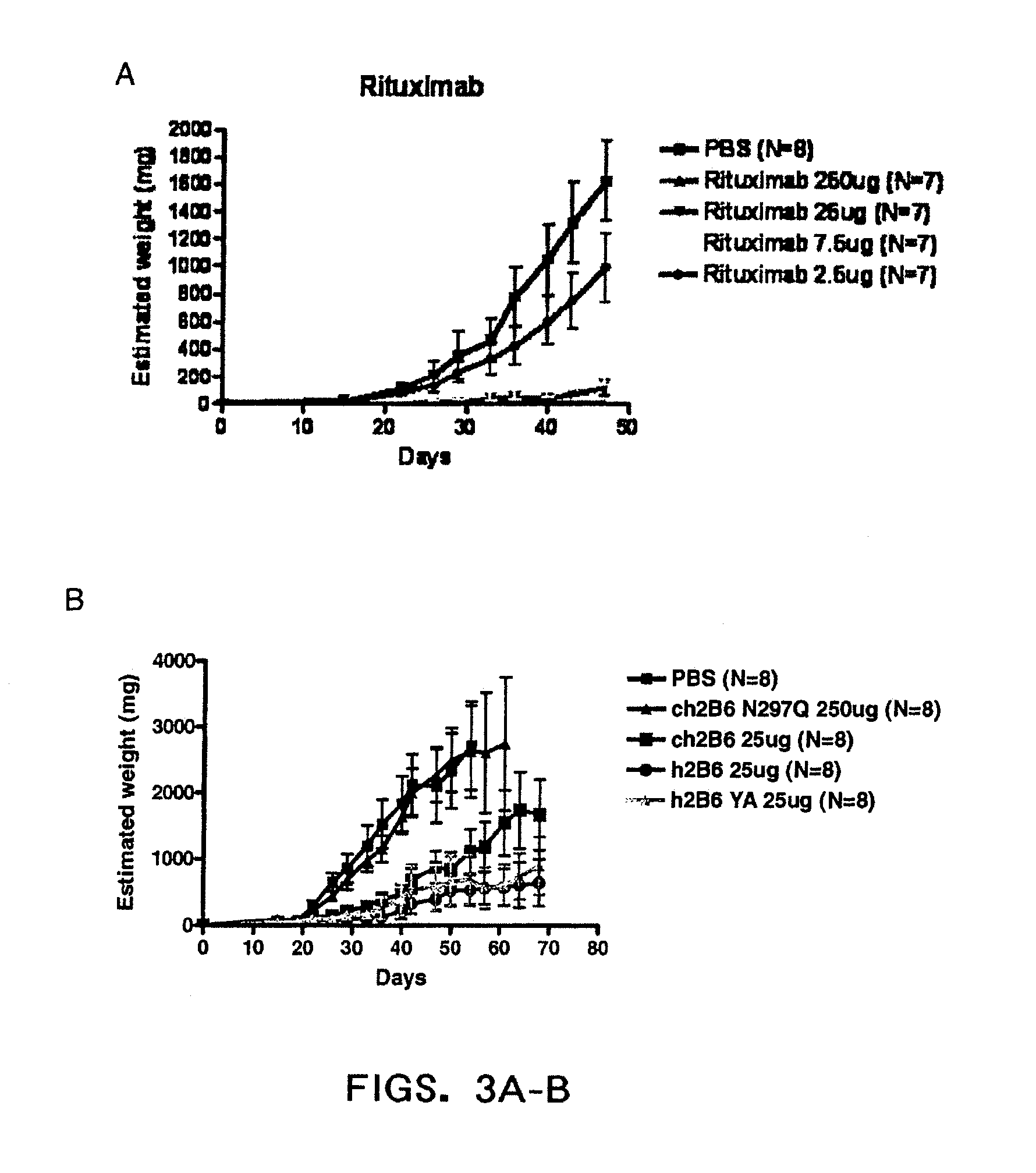
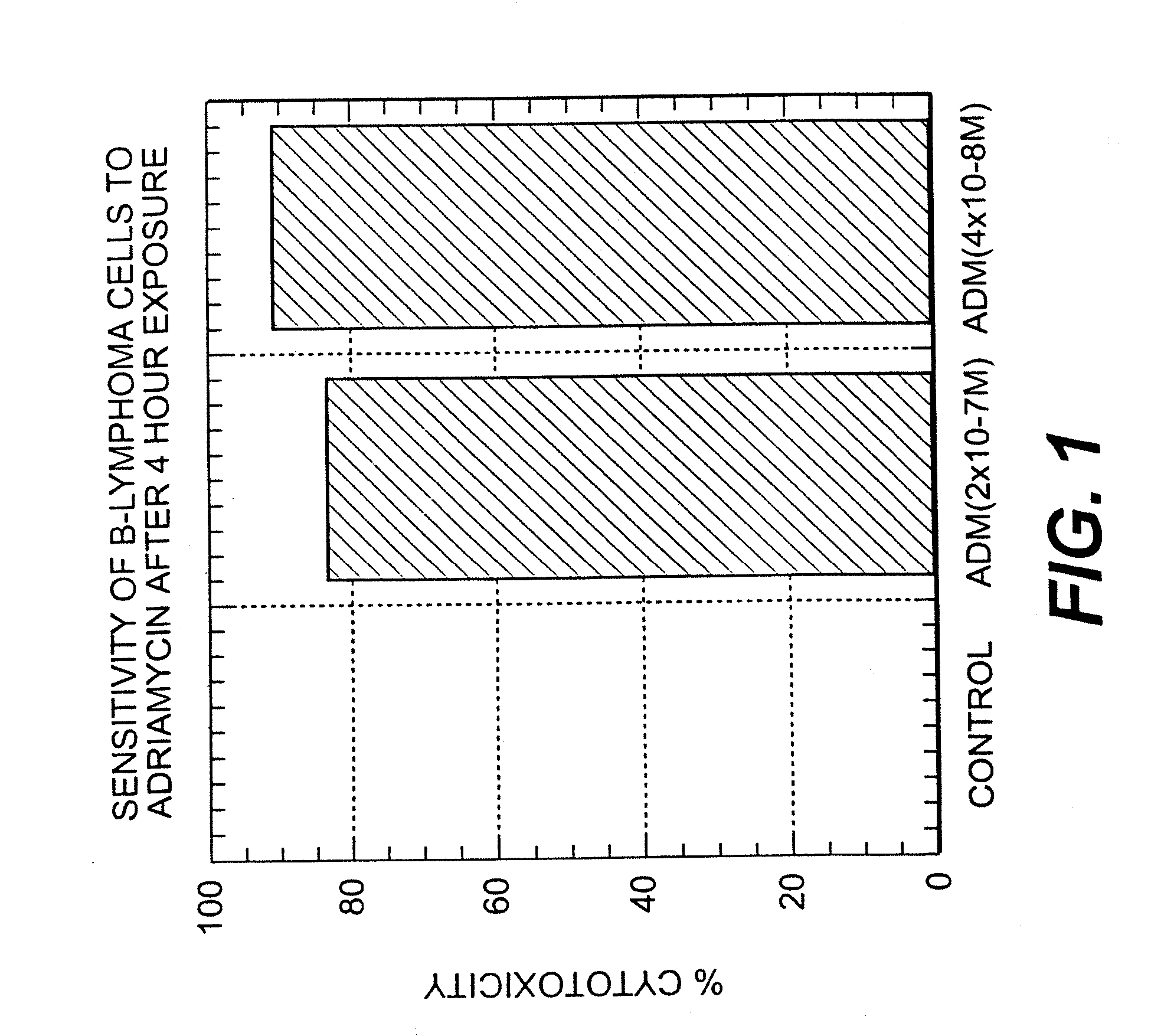
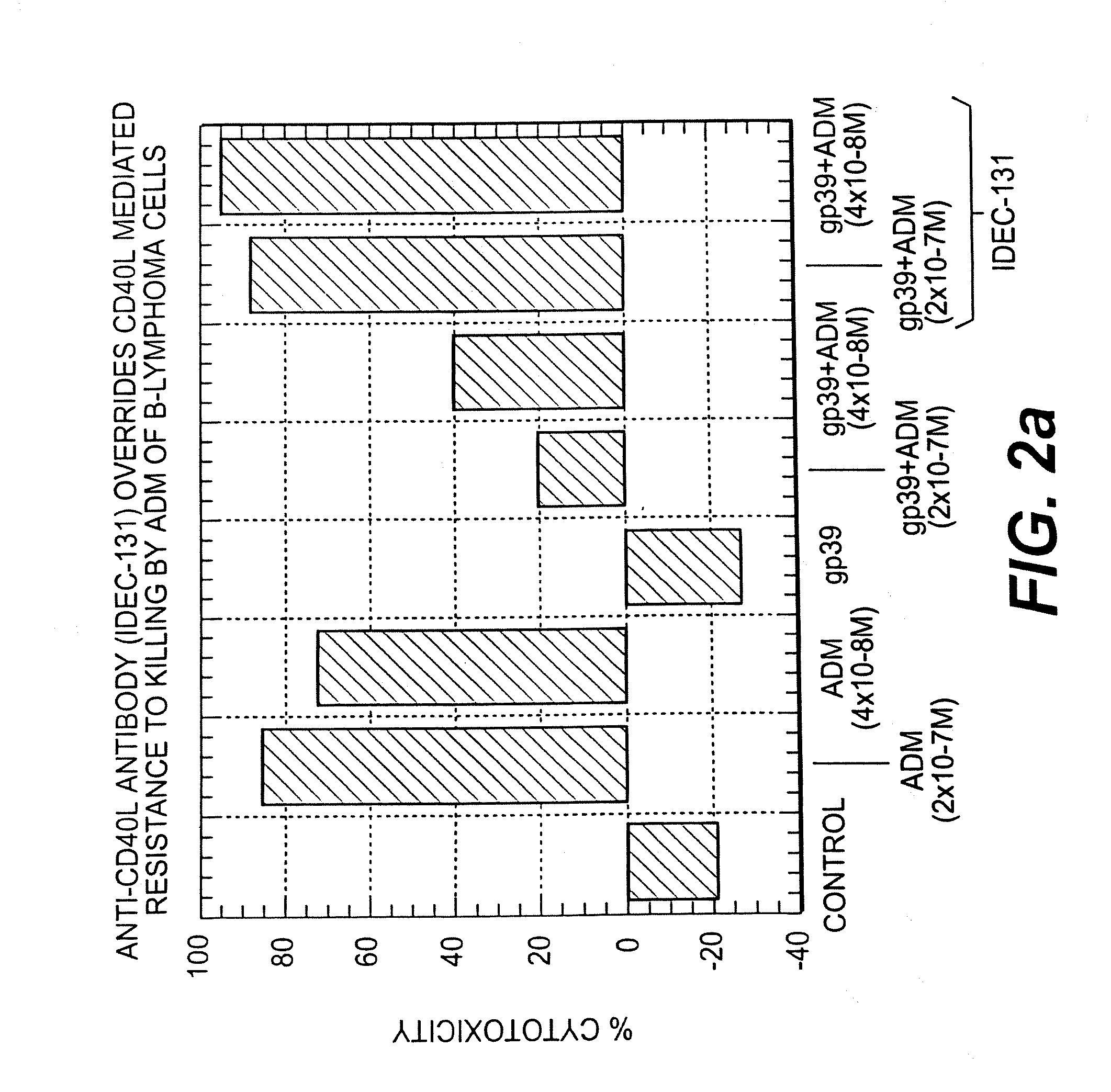
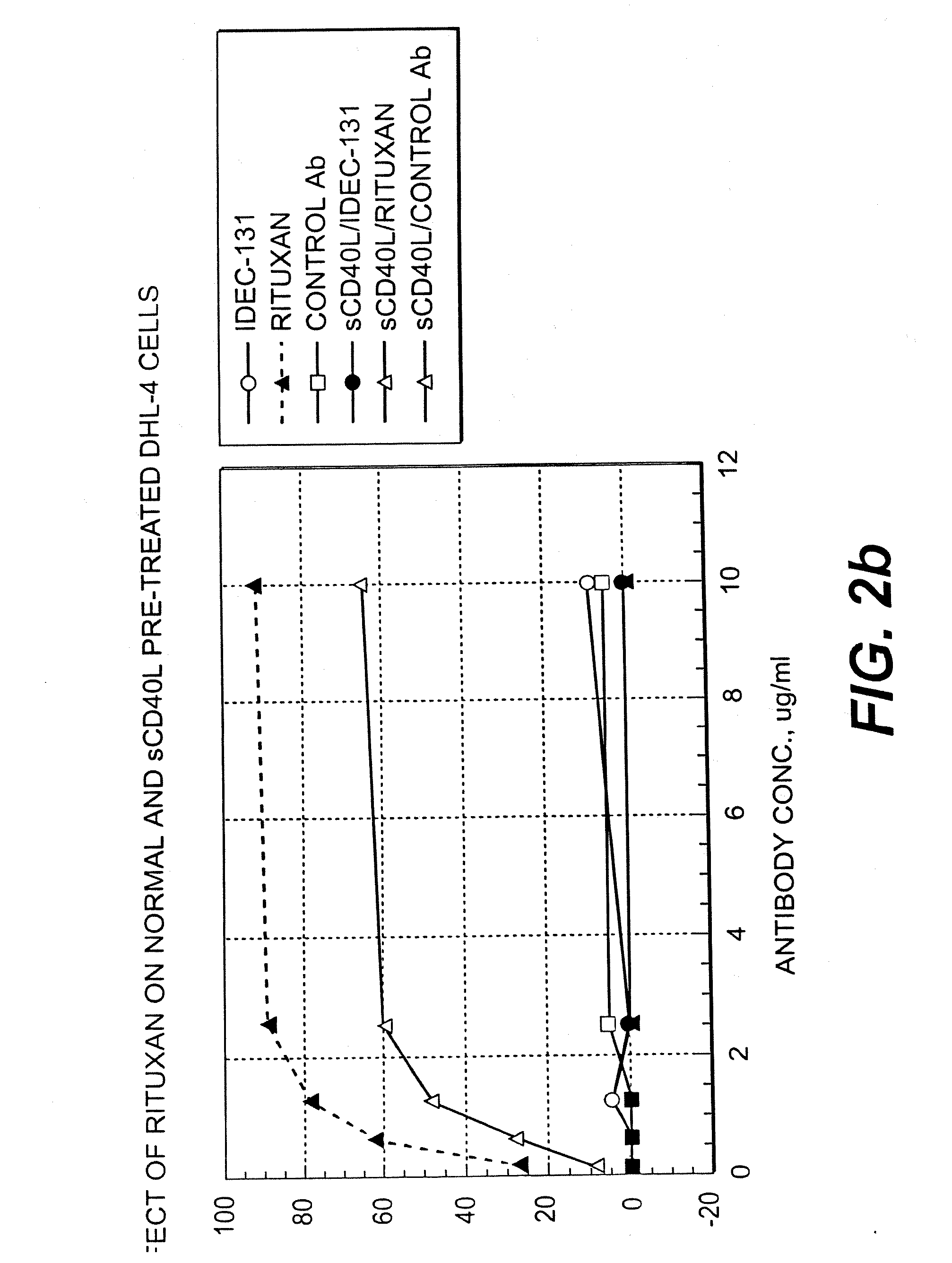
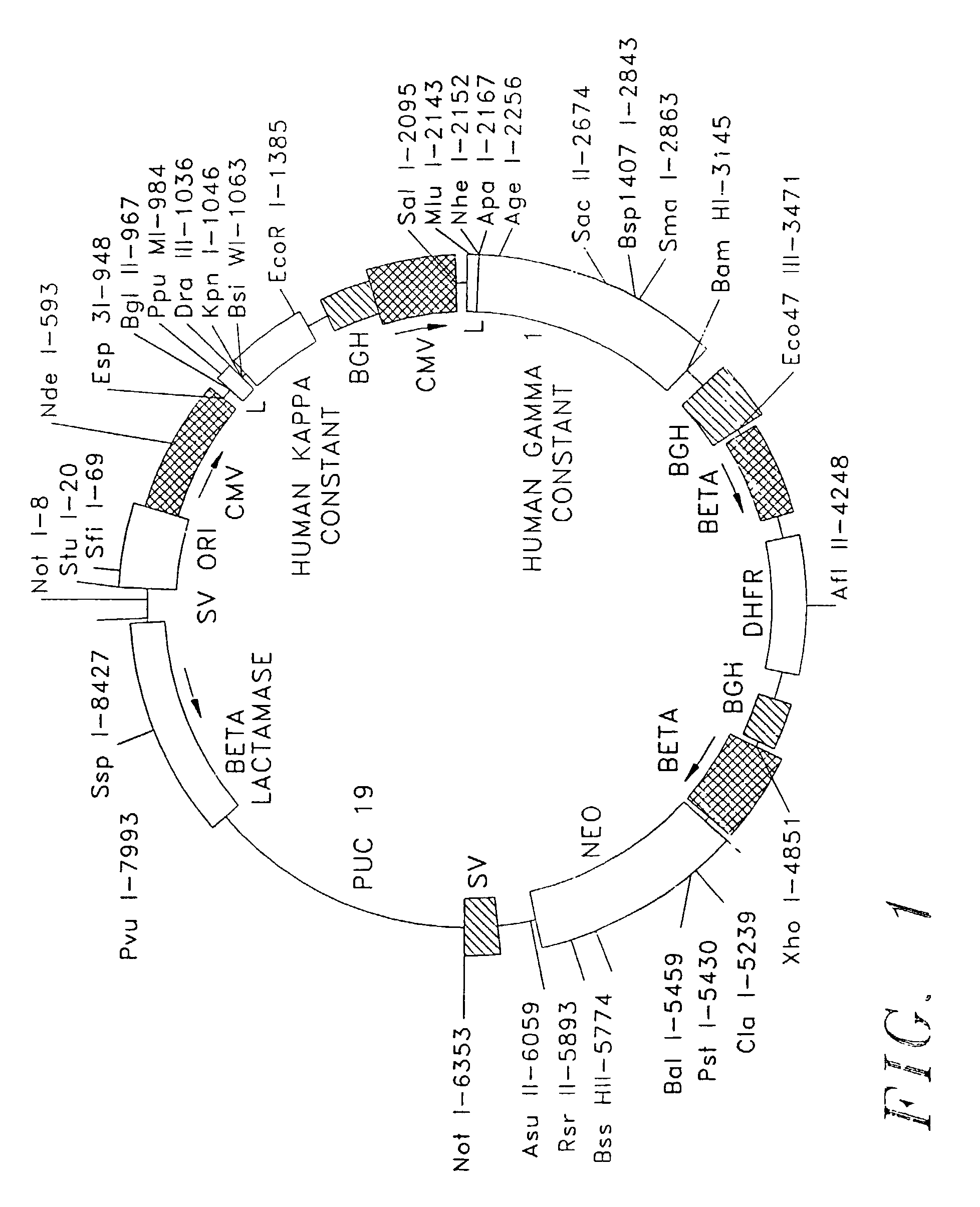
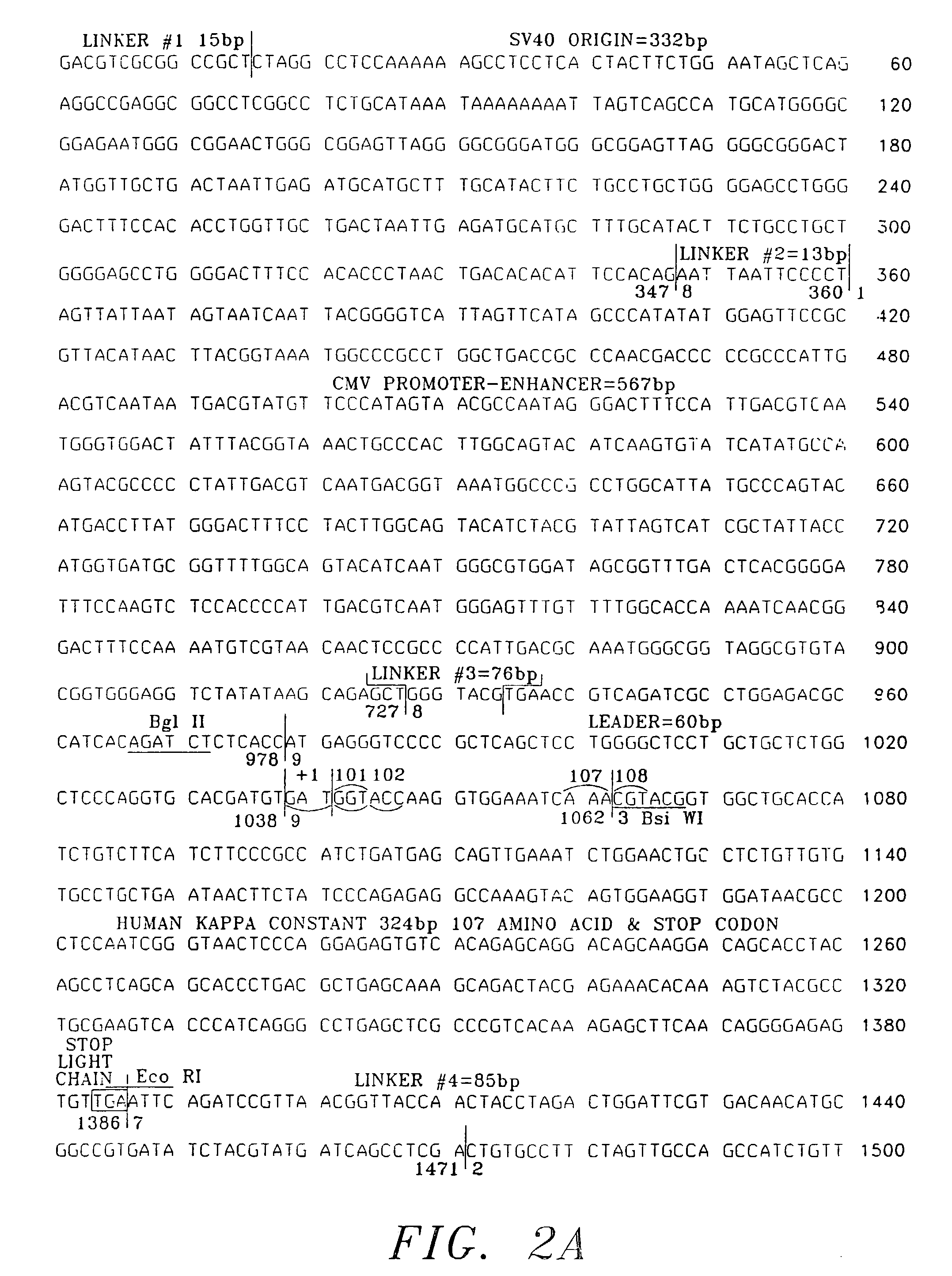
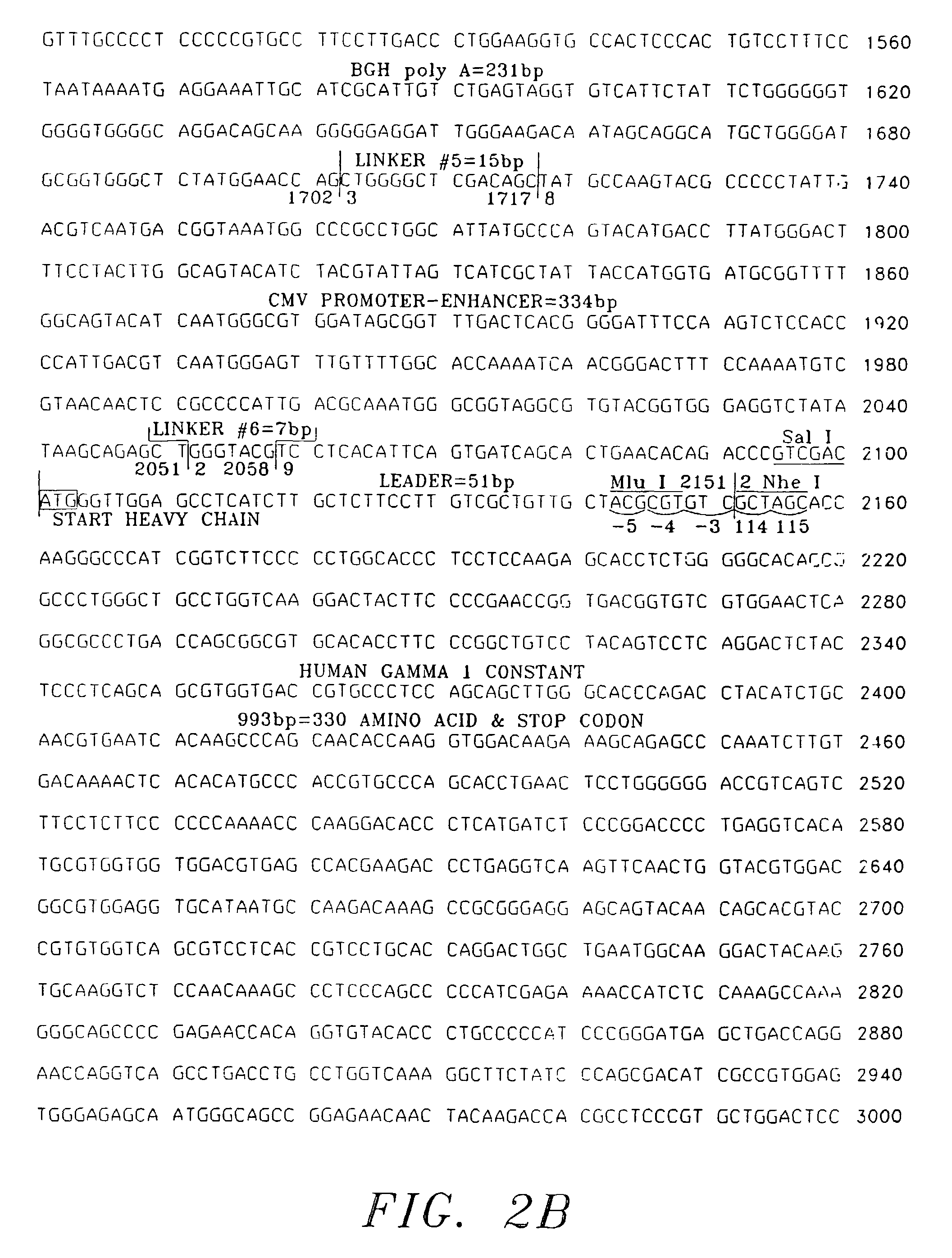
Patents
Literature
133 results about "Anti cd20 antibody" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Rituxan (rituximab) is an anti-CD20 monoclonal antibody indicated for the treatment of Non-Hodgkin’s Lymphoma (NHL), CLL, rheumatoid arthritis, and gramulomatosis.
Radioimmunotherapy of lymphoma using anti-CD20 antibodies
Owner:GLAXO SMITHKLINE LLC
Intrathecal administration of rituximab for treatment of central nervous system lymphomas
InactiveUS20020009444A1Prevent intermolecular disulfide formationPromote recoveryBiocidePeptide/protein ingredientsMeningesImmunocompromised patient
This invention describes methods of using anti-B cell antibodies, preferably anti-CD20 antibodies, and most preferably Rituximab, to treat B cell lymphomas of the brain, especially primary central nervous system lymphomas (PCNSLs), and to prevent meningeal relapse. The antibodies can be administered intrathecally alone, or in combination with other chemotherapeutics, such as methotrexate, or other anti-B cell antibodies to treat PCNSL in both immunocompromised and non-immunocompromised patients. These antibodies can also be used to diagnose patients with CNS lymphoma, especially in immunocompromised patients.
Owner:BIOGEN INC
Radioimmunotherapy of lymphoma using anti-CD20 antibodies
Methods for the treatment of lymphoma by administration of a B cell-specific antibody are described. The invention encompasses providing to a patient both unlabeled antibodies and antibodies labeled with a radioisotope. A principal advantage of the method is that tumor responses can be obtained in a radiometric dose range that does not require hematopoietic stem cell replacement as an adjunct therapy also described is a composition useful in the treatment of lymphoma.
Owner:RGT UNIV OF MICHIGAN +2
Therapeutic application of chimeric and radiolabelled antibodies to human B lymphocyte restricted differentiation antigen for treatment of B cell lymphoma
Disclosed herein are therapeutic treatment protocols designed for the treatment of B cell lymphoma. These protocols are based upon therapeutic strategies which include the use of administration of immunologically active mouse / human chimeric anti-CD20 antibodies, radiolabeled anti-CD20 antibodies, and cooperative strategies comprising the use of chimeric anti-CD20 antibodies and radiolabeled anti-CD20 antibodies.
Owner:IDEC PHARM CORP
Therapeutic application of chimeric and radiolabelled antibodies to human B lymphocyte restricted differentiation antigen for treatment of B cell lymphoma
Disclosed herein are therapeutic treatment protocols designed for the treatment of B cell lymphoma. These protocols are based upon therapeutic strategies which include the use of administration of immunologically active mouse / human chimeric anti-CD20 antibodies, radiolabeled anti-CD20 antibodies, and cooperative strategies comprising the use of chimeric anti-CD20 antibodies and radiolabeled anti-CD20 antibodies.
Owner:BIOGEN INC
Immunoregulatory antibodies and uses thereof
InactiveUS20030103971A1Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsCD37CD23
A combination antibody therapy for treating B cell malignancies using an immunoregulatory antibody, especially an anti-B7, anti-CD23, or anti-CD40L antibody and a B cell depleting antibody, especially anti-CD19, anti-CD20, anti-CD22 or anti-CD37 antibody is provided. Preferably, the combination therapy will comprise anti-B7 and anti-CD20 antibody administration.
Owner:BIOGEN INC
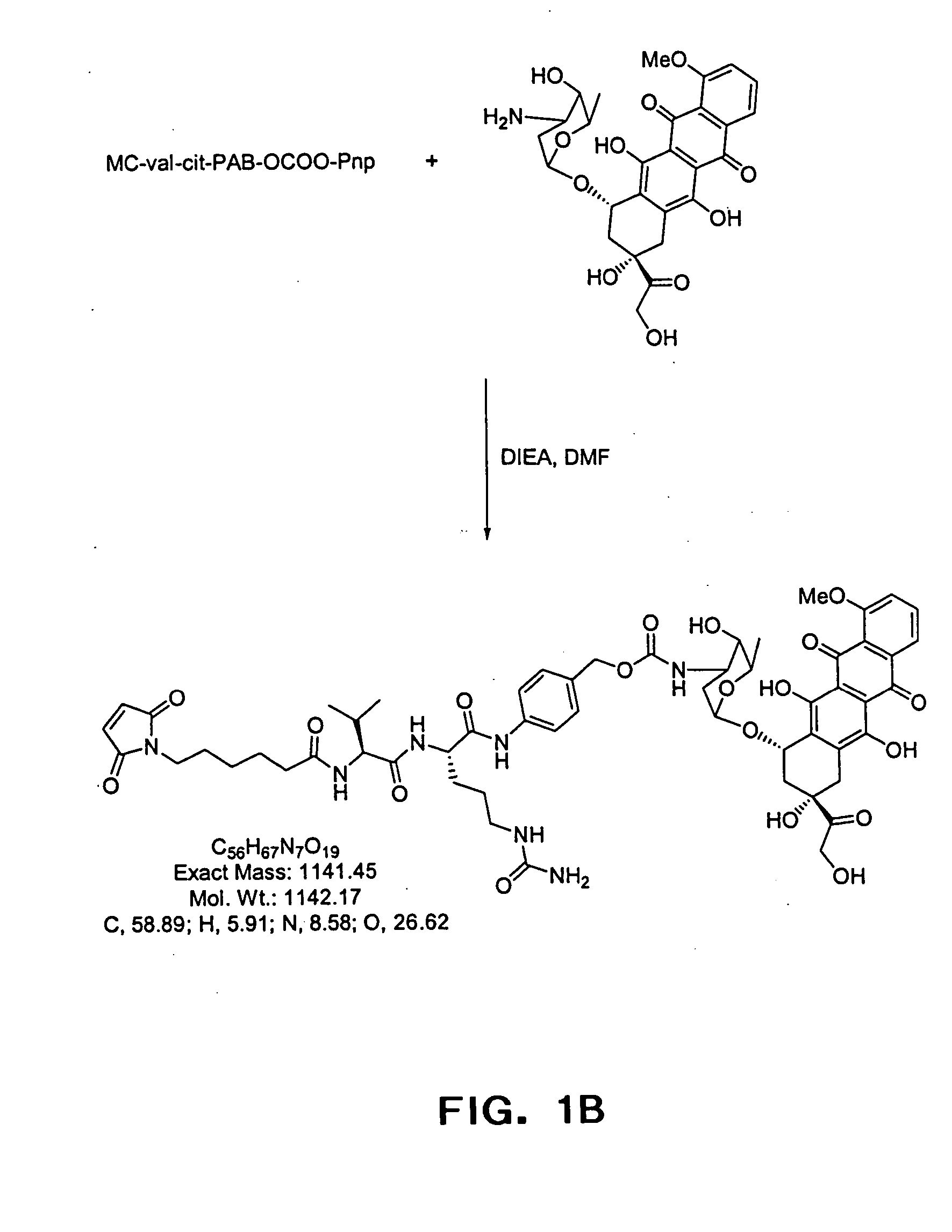
Anti-CD20 antibody-drug conjugates for the treatment of cancer and immune disorders
The present invention relates to methods and compositions for the treatment of CD20-expressing cancers and immune disorders involving CD20-expressing cells. The present methods comprise administering to a subject an anti CD20 antibody-drug conjugate that has a high potency and / or is capable of internalizing into CD20-expressing cells. The present invention further provides pharmaceutical compositions and kits comprising such conjugates. The present invention yet further provides methods of and compositions relating to combination therapy of cancer and immune disorders involving CD20-expressing cells using the anti-CD20 antibody-drug conjugates of the invention.
Owner:SEATTLE GENETICS INC
Treatment of B-cell associated diseases
InactiveUS6846476B2Enhanced killing and depletionReduce the possibilityRadioactive preparation carriersAntibody ingredientsDiseaseAutoimmune responses
Treatment of B-cell associated diseases including autoimmune and B-cell malignancies such as leukemias, lymphomas, using the combination of an anti-CD20 antibody, preferably RITUXAN® and a radiolabeled anti-CD22 antibody, preferably an 90Y labeled humanized anti-CD22 antibody, is described. These therapeutic regimens provide for enhanced depletion of B cells, and therefore reduce the risk in B cell malignancy treatment of relapse associated with RITUXAN® and, moreover, provide for prolonged immunosuppression of B-cell immune responses, especially in the context of autoimmune diseases and transplant.
Owner:BIOGEN INC
Anti-cd20 antibodies and fusion proteins thereof and methods of use
InactiveUS20070020259A1Useful in diagnosisUseful in treatmentAntipyreticAnalgesicsAutoimmune conditionAutoimmune disease
The present invention provides humanized, chimeric and human anti-CD20 antibodies and CD 20 antibody fusion proteins that bind to a human B cell marker, referred to as CD20, which is useful for the treatment and diagnosis of B-cell disorders, such as B-cell malignancies and autoimmune diseases, and methods of treatment and diagnosis.
Owner:IMMUNOMEDICS INC
Therapy of rituximab-refractory rheumatoid arthritis patients
A method is disclosed of treating a rituximab-refractory rheumatoid arthritis (RA) patient comprising administering an anti-CD20 antibody other than rituximab to the patient in an amount effective to treat the RA.
Owner:GENENTECH INC
Treatment of B-cell associated diseases such as malignancies and autoimmune diseases using a cold anti-CD20 antibody/radiolabeled anti-CD22 antibody combination
InactiveUS20050112060A1Enhanced killing and depletionPrevent and inhibit relapseRadioactive preparation carriersAntibody ingredientsAutoimmune conditionRegimen
Owner:BIOGEN INC
Treatment of B cell malignancies using combination of B cell depleting antibody and immune modulating antibody related applications
InactiveUS20050123540A1Radioactive preparation carriersImmunoglobulins against cell receptors/antigens/surface-determinantsCD37Combination therapy
A combination antibody therapy for treating B cell malignancies using an immunoregulatory antibody, especially an anti-B7, anti-CD23, or anti-CD40L antibody and a B cell depleting antibody, especially anti-CD19, anti-CD20, anti-CD22 or anti-CD37 antibody is provided. Preferably, the combination therapy will comprise anti-B7 and anti-CD20 antibody administration.
Owner:IDEC PHARM CORP +1
Use of chimeric Anti-cd20 antibody as in vitro or in vivo purging agent in patients receiving bmt or pbsc transplant
InactiveUS20110165159A1Reduce morbidityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsIn vivoDISEASE RELAPSE
Owner:BIOGEN INC
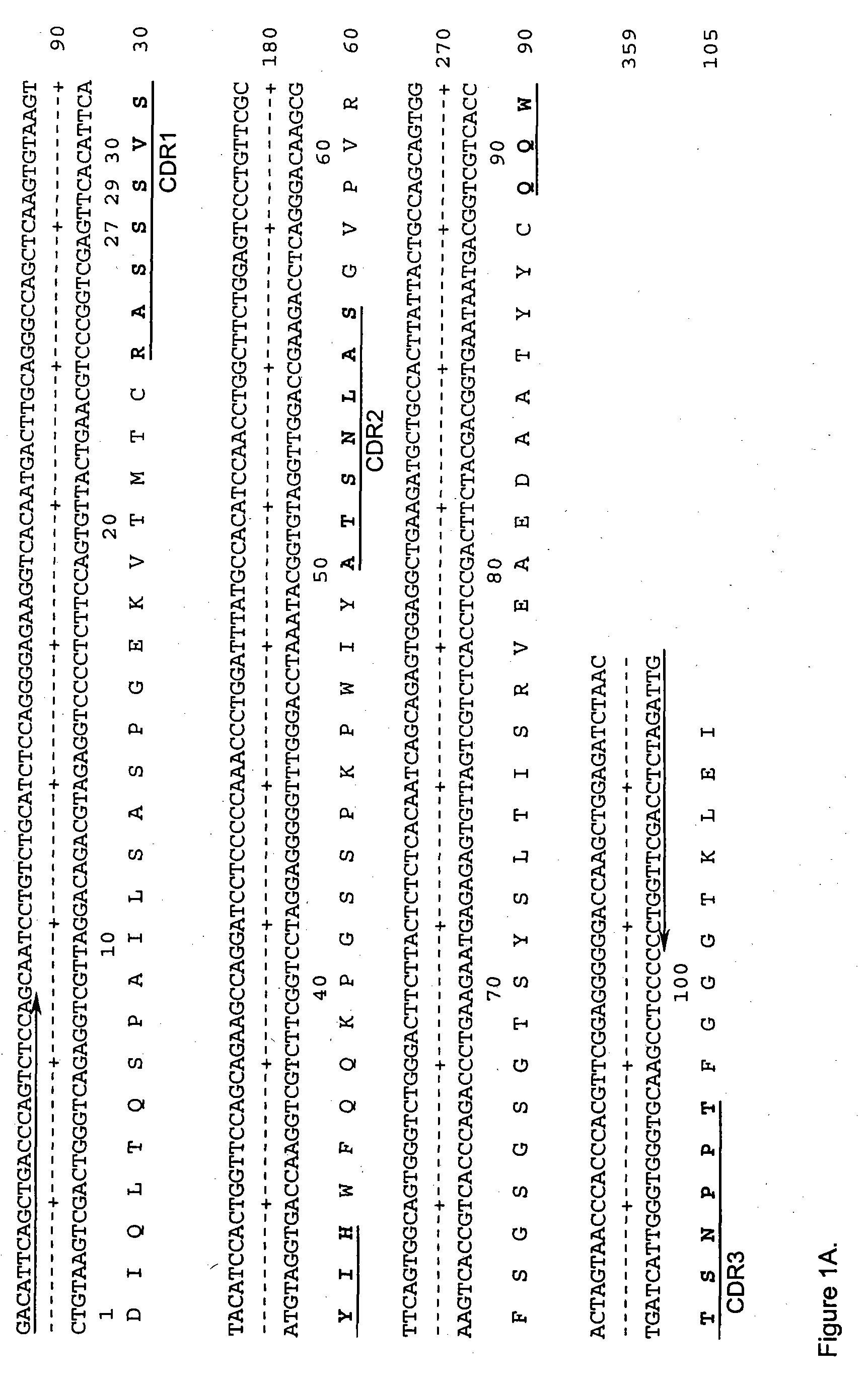
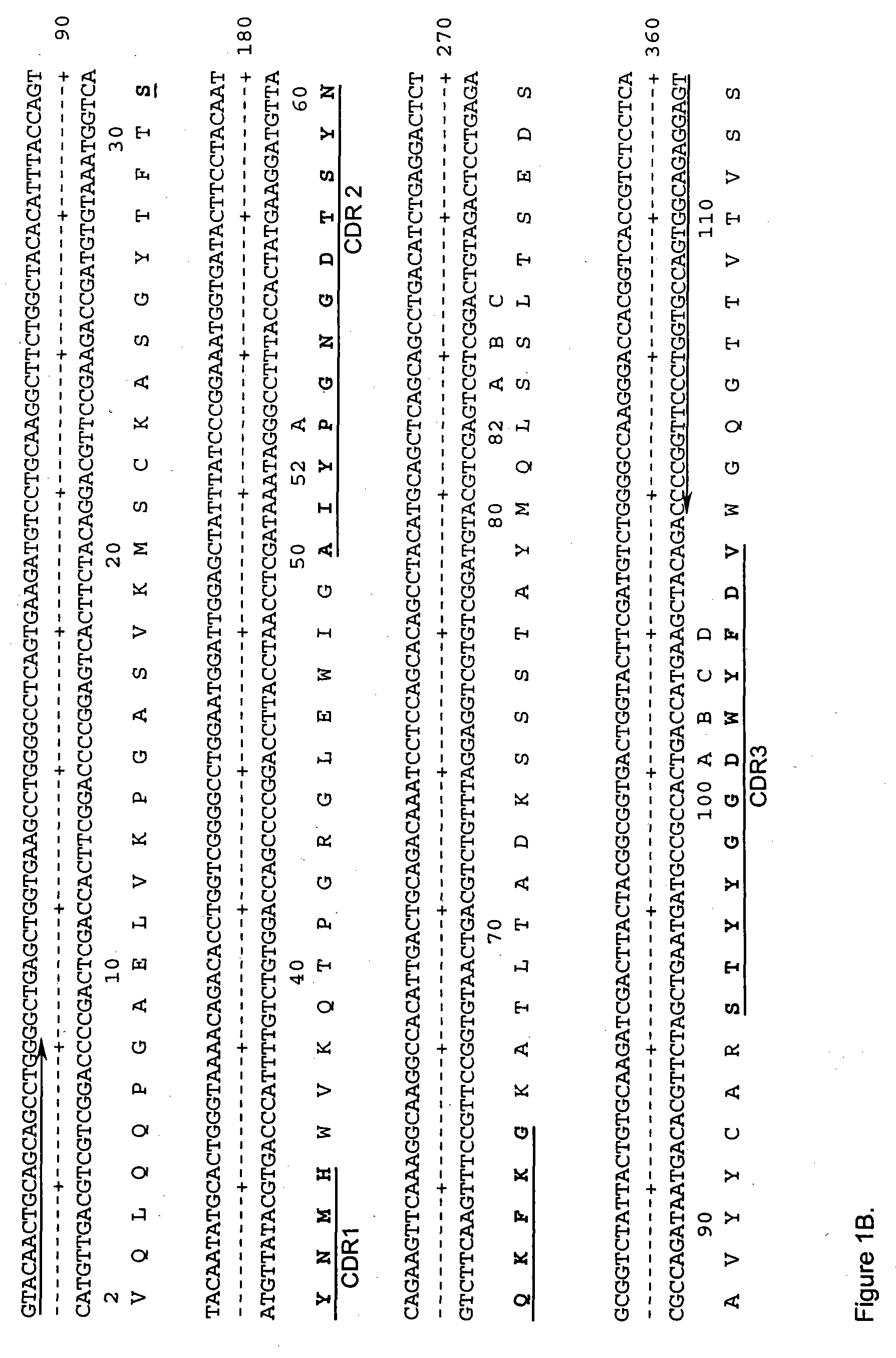
Cd20 antibodies and uses thereof
InactiveUS20110195021A1Improve stabilityImproved ADCC functionSenses disorderAntipyreticDiseaseMalignancy
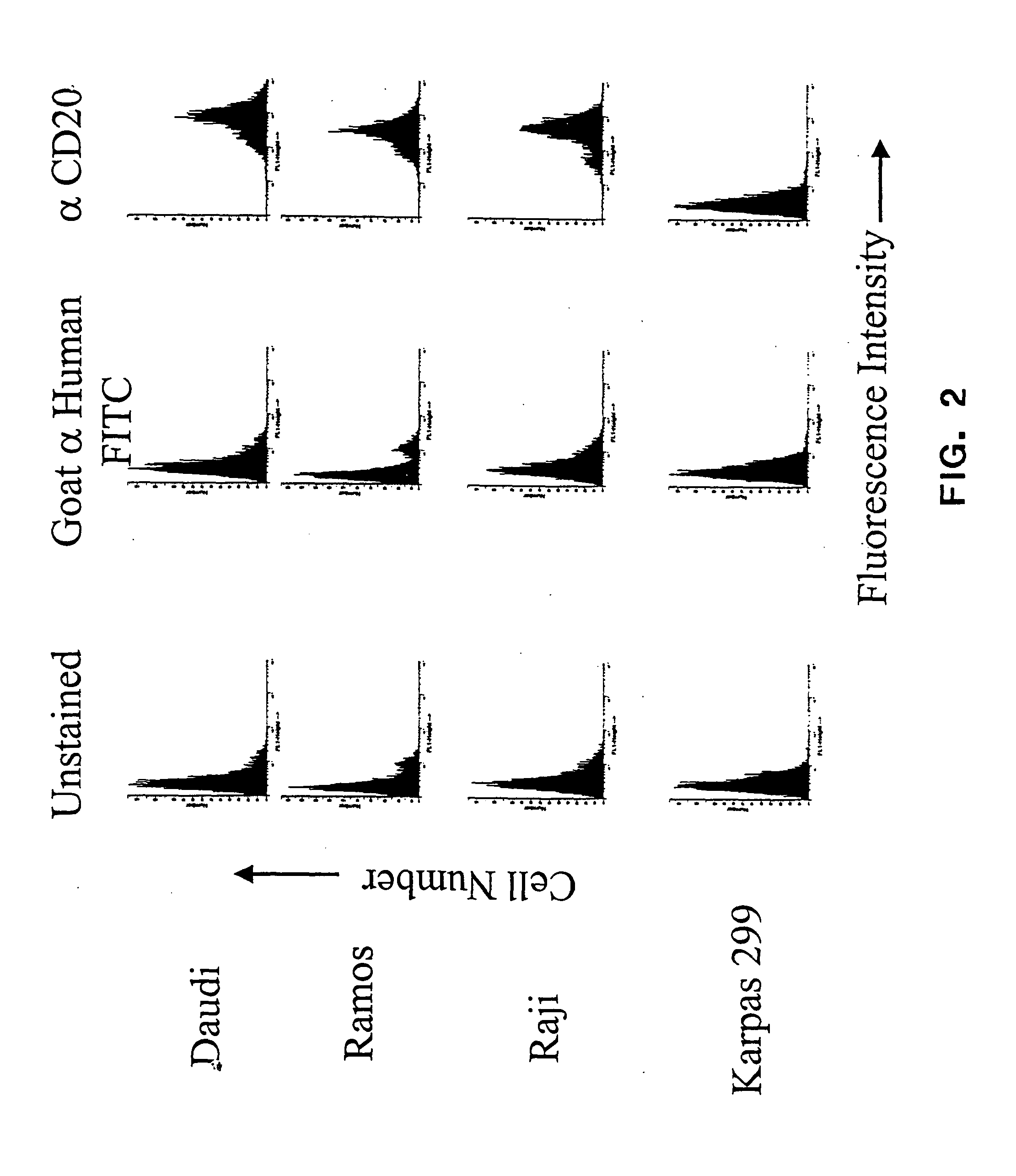
CD20 is a transmembrane protein of the tetra-spanin family expressed on the surface of B-cells and has been found on B-cells from peripheral blood as well as lymphoid tissues. CD20 expression persists from the early pre-B cell stage until the plasma cell differentiation stage. Conversely, it is not found on hematopoietic stem cells, pro-B cells, differentiated plasma cells or non-lymphoid tissues. In addition to expression in normal B-cells, CD20 is expressed in B-cell derived malignancies such as non-Hodgkin's lymphoma (NHL) and B-cell chronic lymphocytic leukemia (CLL). CD20 expressing cells are known to play a role in other diseases and disorders, including inflammation. The present invention includes anti-CD20 antibodies, forms and fragments, having superior physical and functional properties; immunoconjugates, compositions, diagnostic reagents, methods for inhibiting growth, therapeutic methods, improved antibodies and cell lines; and polynucleotides, vectors and genetic constructs encoding same.
Owner:IMMUNOGEN INC
Combination of FcgammaRIIB-Specific Antibodies and CD20-Specific Antibodies and Methods of Use Thereof
InactiveUS20090191195A1Good curative effectAvoid managementAntipyreticAnalgesicsB cellSpecific antibody
The present invention relates to methods of treatment, prevention, management or amelioration of one or more symptoms of diseases or disorders associated with CD20 expression that encompass administration of a combination of: (A) one or more antibodies that specifically bind FcγRIIB, particularly human FcγRIIB, with greater affinity than said antibodies bind FcγRIIA, and (B) one or more antibodies that specifically bind to CD20. Such methods include methods of treating, preventing, managing or ameliorating one or more symptoms of a B cell related disease or disorder or an inflammatory disorder. The invention also provides pharmaceutical compositions comprising an anti-FcγRIIB antibody and an anti-CD20 antibody.
Owner:MACROGENICS INC
Immunoregulatory Antibodies and Uses Thereof
InactiveUS20070009519A1Convenient treatmentIn-vivo radioactive preparationsPharmaceutical containersCD37Combination therapy
A combination antibody therapy for treating B cell malignancies using an immunoregulatory antibody, especially an anti-B7, anti-CD23, or anti-CD40L antibody and a B cell depleting antibody, especially anti-CD19, anti-CD20, anti-CD22 or anti-CD37 antibody is provided. Preferably, the combination therapy will comprise anti-B7 and anti-CD20 antibody administration.
Owner:BIOGEN INC
Expression and use of anti-CD20 Antibodies
Disclosed herein are methods of depleting peripheral blood B cells in a human host comprising administering to the host an immunologically active anti-CD20 antibody in an amount effective to deplete peripheral blood B cells in the host.
Owner:BIOGEN INC
Treatment of aggressive non-Hodgkins lymphoma with anti-CD20 antibody
InactiveUS8557244B1Organic active ingredientsIn-vivo radioactive preparationsDiseaseHodgkins lymphomas
This invention concerns methods for the treatment of intermediate and high-grade non-Hodgkins lymphomas, comprising the administration of anti-CD20 monoclonal antibodies and fragments thereof. Particular embodiments include the administration of chimeric anti-CD20 antibody, either alone or in combination with radiolabeled antibodies or chemotherapy. The invention is particularly useful for lymphomas characterized by a high number of circulating B cells, possibly bone marrow involvement, bulky disease, or the involvement of extralymphatic organs or sites.
Owner:BIOGEN INC
Novel uses
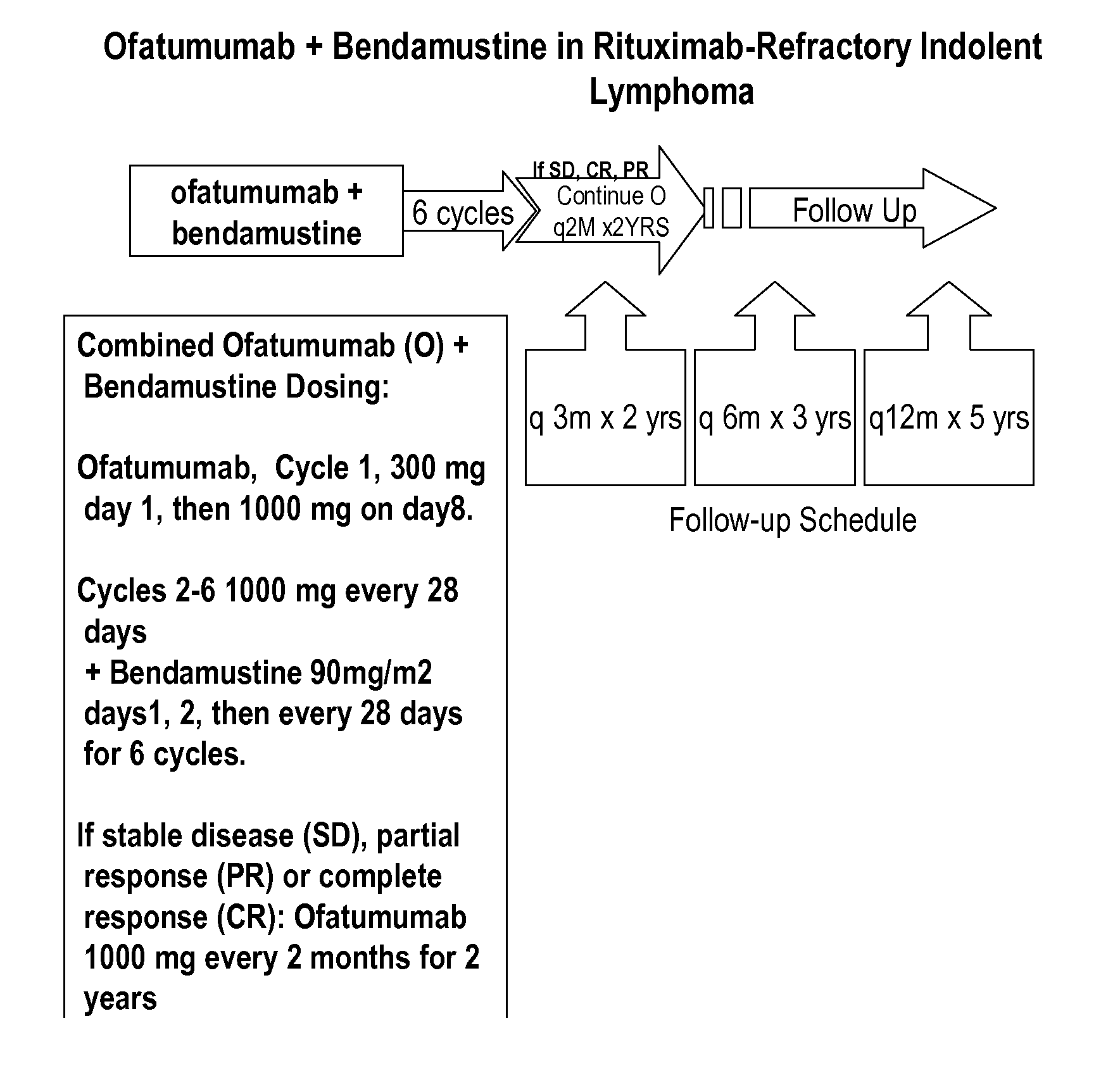
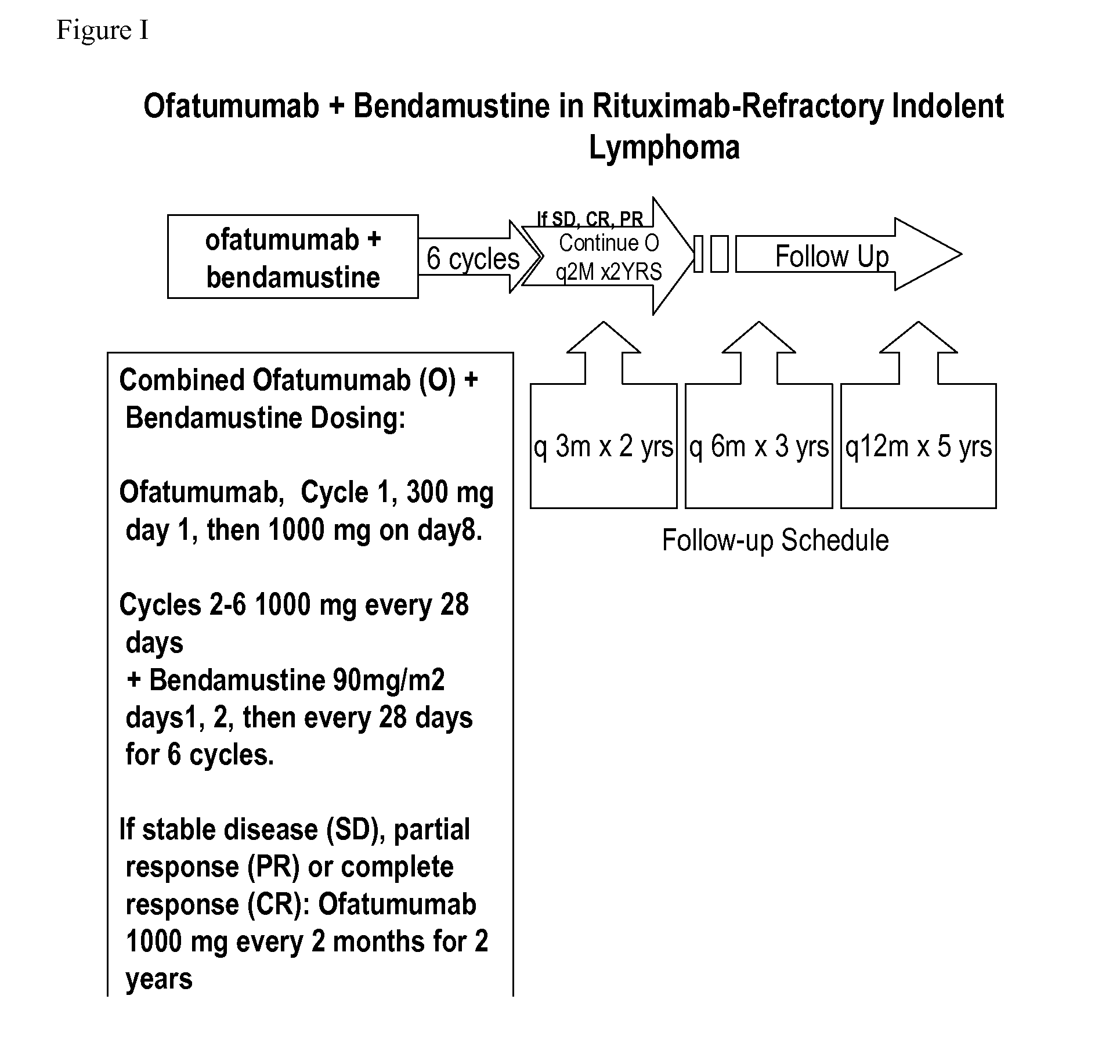
The present invention relates generally to the use of bendamustine in combination with an anti-CD20 antibody to treat cancer.
Owner:GLAXO SMITHKLINE LLC
Glycan-optimized Anti-cd20 antibodies
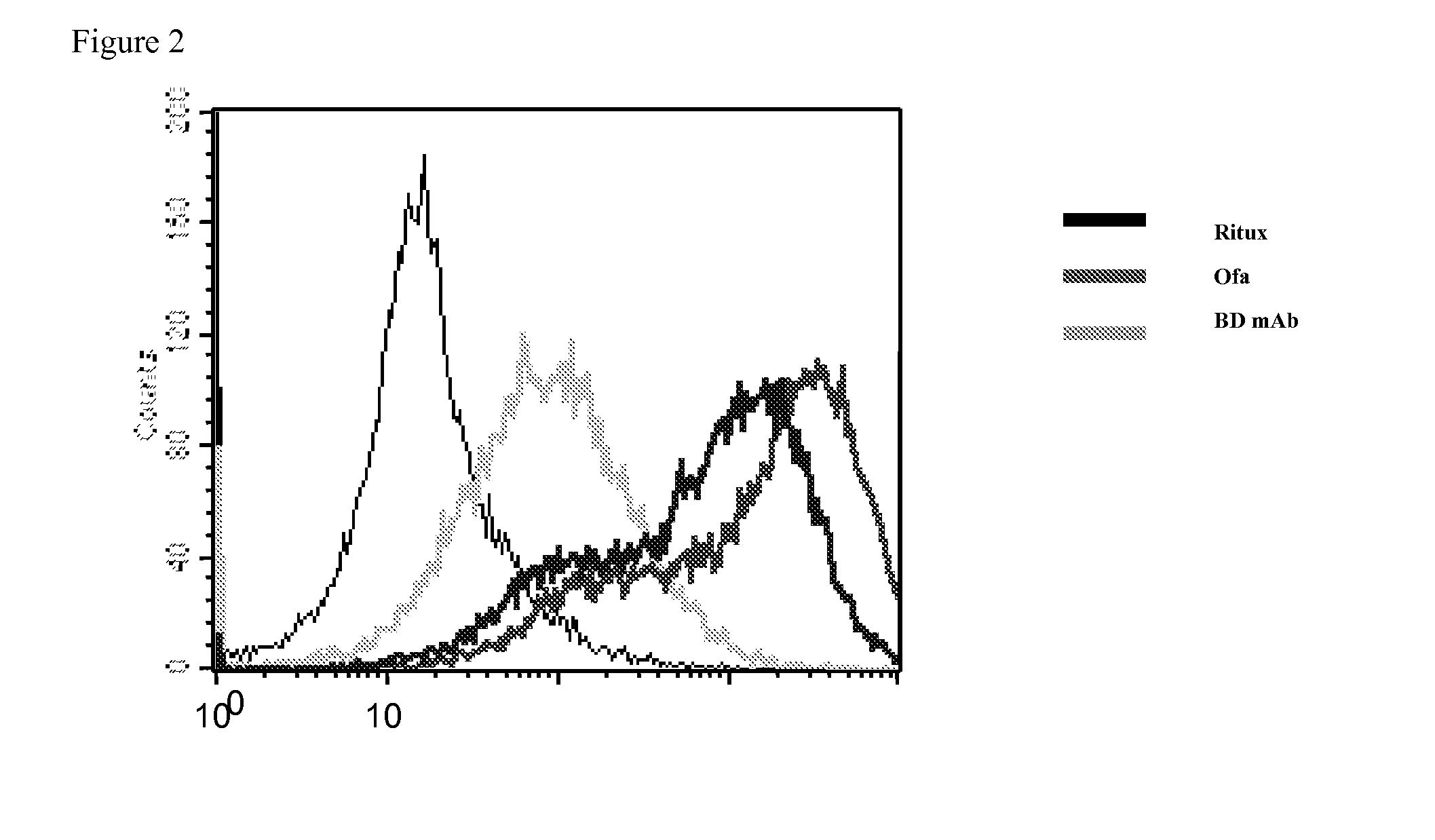
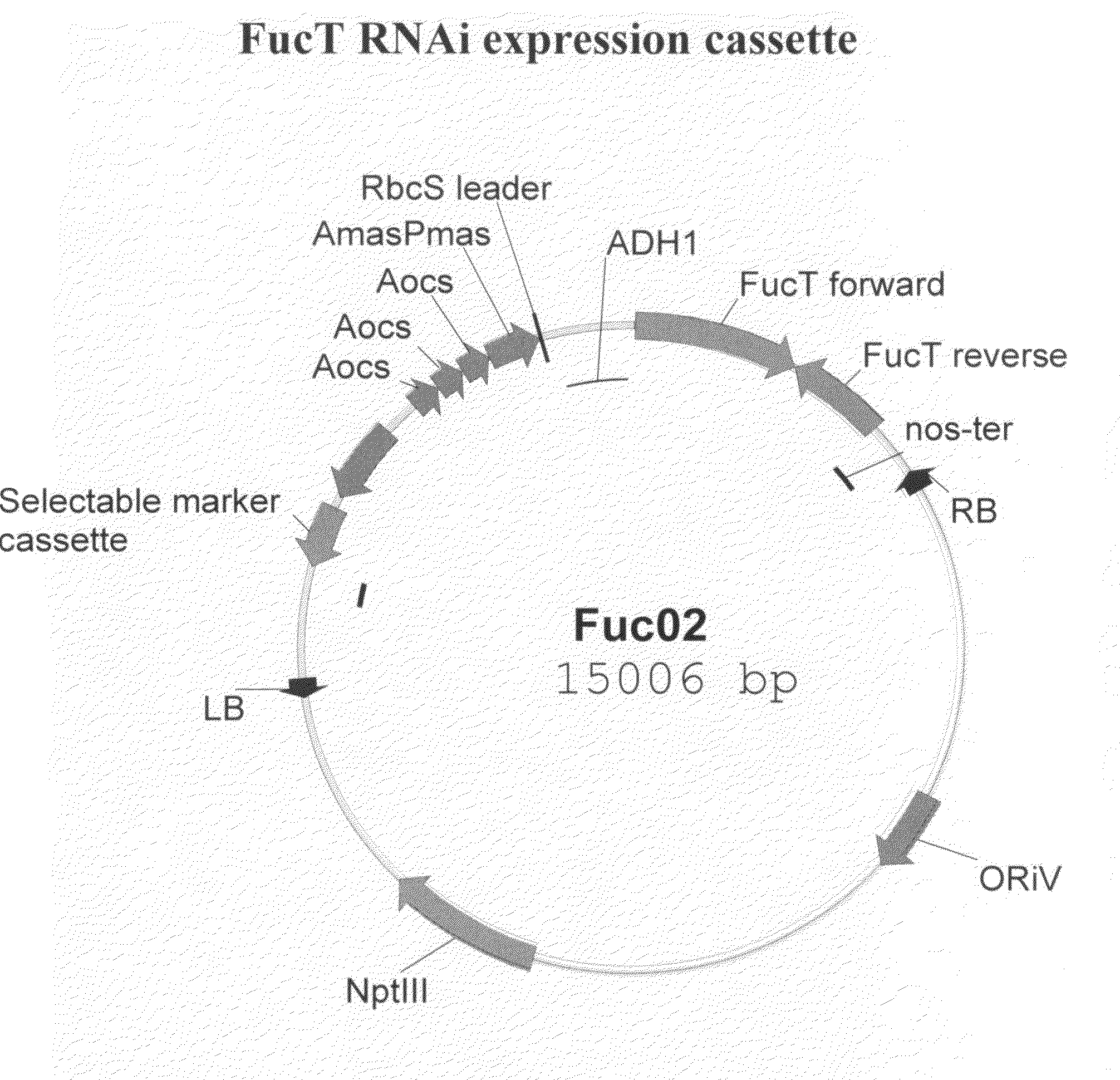
InactiveUS20090060921A1High antibody activityEnhanced effector functionImmunoglobulins against animals/humansVaccinesAntigenFucosylation
Glycan-optimized monoclonal antibodies that specifically bind CD20 antigen and which have improved effector function are provided. The anti-CD20 antibodies of the invention have a glycosylation pattern that results in an antibody composition having predominately the G0 glycoform, and thus comprise N-glycans that lack fucose (i.e., afucosylated) and galactose residues attached thereto. In some embodiments, these anti-CD20 antibodies comprise the light chain and heavy chain sequences of the rituximab anti-CD20 antibody, and thus represent afucosylated rituximab. Methods for producing these glycan-optimized anti-CD20 antibodies are also provided.
Owner:SYNTHON BIOPHARMACEUTICALS BV
Anti-tumor agent
InactiveUS20080118978A1Improve expression levelHigh expressionTissue cultureAntineoplastic agentsBULK ACTIVE INGREDIENTActive ingredient
The present invention provides an agent which is effective to a patient who is administered by an agent comprising anti-CD20 antibody as an active ingredient.
Owner:KYOWA HAKKO KIRIN CO LTD
Method for the Production of a Monoclonal Antibody to CD20 for the Treatment of B-Cell Lymphoma
InactiveUS20090285795A1Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsDNA constructMammalian expression
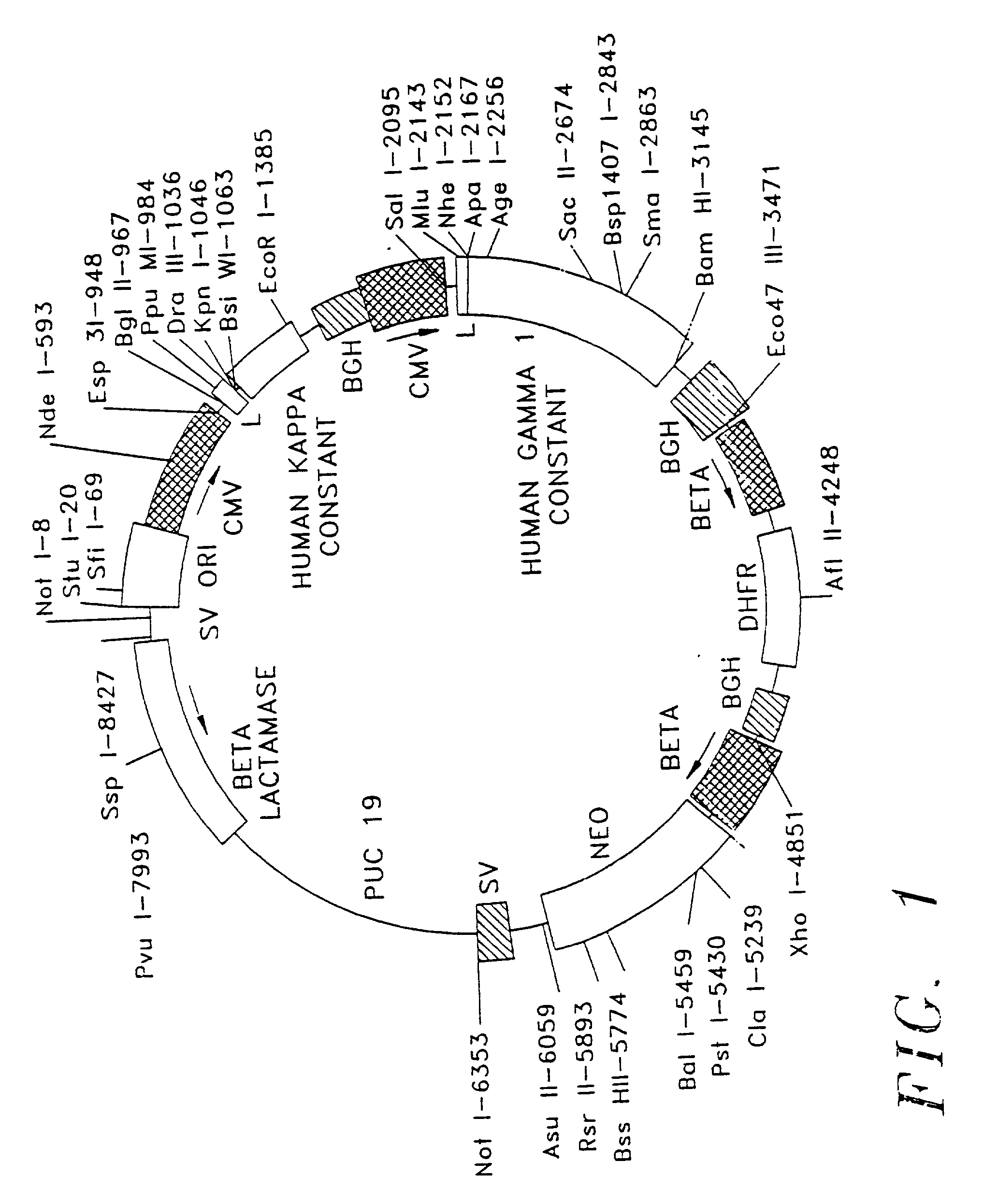
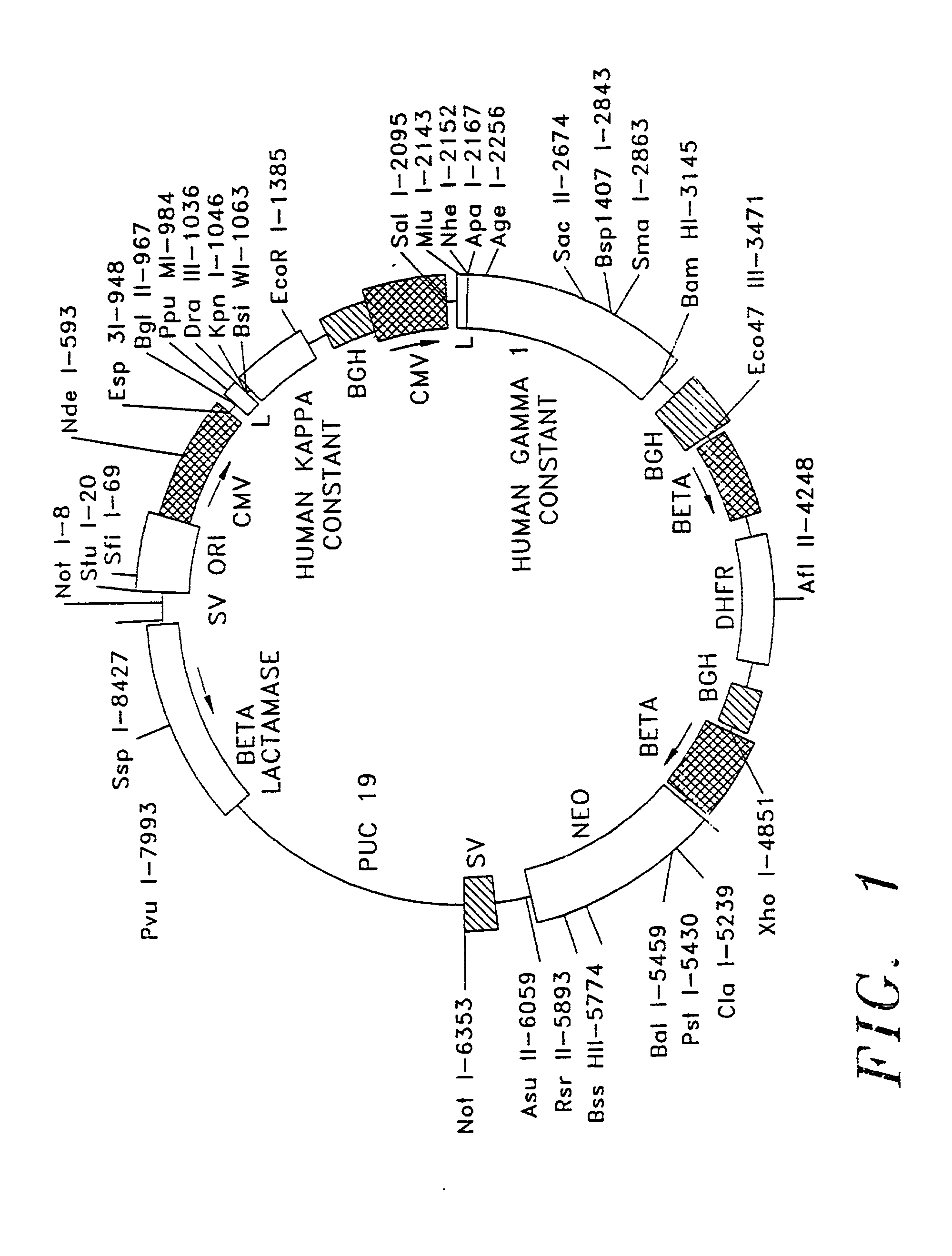
The present invention relates to the recombinant method used for the production of soluble form of an antibody that binds to CD20 for treatment of patients with relapsed or refractory, low-grade or follicular, CD20-positive, B-cell non-Hodgkin's lymphoma (NHL). The treatment will comprise the use of immunologically active anti-CD20 antibodies; or radiolabeled anti-CD20 antibodies and or cooperative strategies where both labeled and non-labeled antibodies will be used for treatment of NHL. The procedure describes the de novo synthesis of the nucleic acid sequence encoding anti-CD20, transformation of the constructed nucleic acid sequences into competent bacteria and the sub-cloning of the same into mammalian expression vectors for expression of the desired protein. DNA constructs comprising the control elements associated with the gene of interest has been disclosed. The nucleic acid sequence of interest has been codon optimized to permit expression in the suitable mammalian host cells.
Owner:AVESTHAGEN
Use of antibodies against CD20 for the treatment of the graft versus host disease
It is described the use of antibodies against exogenous surface antigens not present on normal human T lymphocytes for the preparation of compositions for the treatment of the graft versus host disease in patients who have received T lymphocytes transduced with such exogenous surface antigens.
Owner:CONSIGLIO NAT DELLE RICERCHE
Combination therapy of an anti cd20 antibody with a btk inhibitor
InactiveUS20150125446A1Enhanced antiproliferative effectGood effectAntibody ingredientsImmunoglobulinsFucosylationOncology
The present invention is directed to the combination therapy of an anti-CD20 antibody with a BTK inhibitor for the treatment of cancer, especially to the combination therapy of CD20 expressing cancers with a type I anti-CD20 antibody or an afucosylated humanized B-Ly1 antibody and a BTK inhibitor.
Owner:ONO PHARMA CO LTD +1
Combination therapies for b-cell lymphomas comprising administration of Anti-cd20 antibody
InactiveUS20080038261A1Good synergyHigh response rateOrganic active ingredientsPeptide/protein ingredientsRegimenHodgkins lymphomas
New combined therapeutic regimens for treatment of B-cell lymphomas are disclosed which comprise in particular administration of anti-CD20 antibodies to patients having low-, intermediate- or high-grade non-Hodgkins lymphomas.
Owner:BIOGEN INC
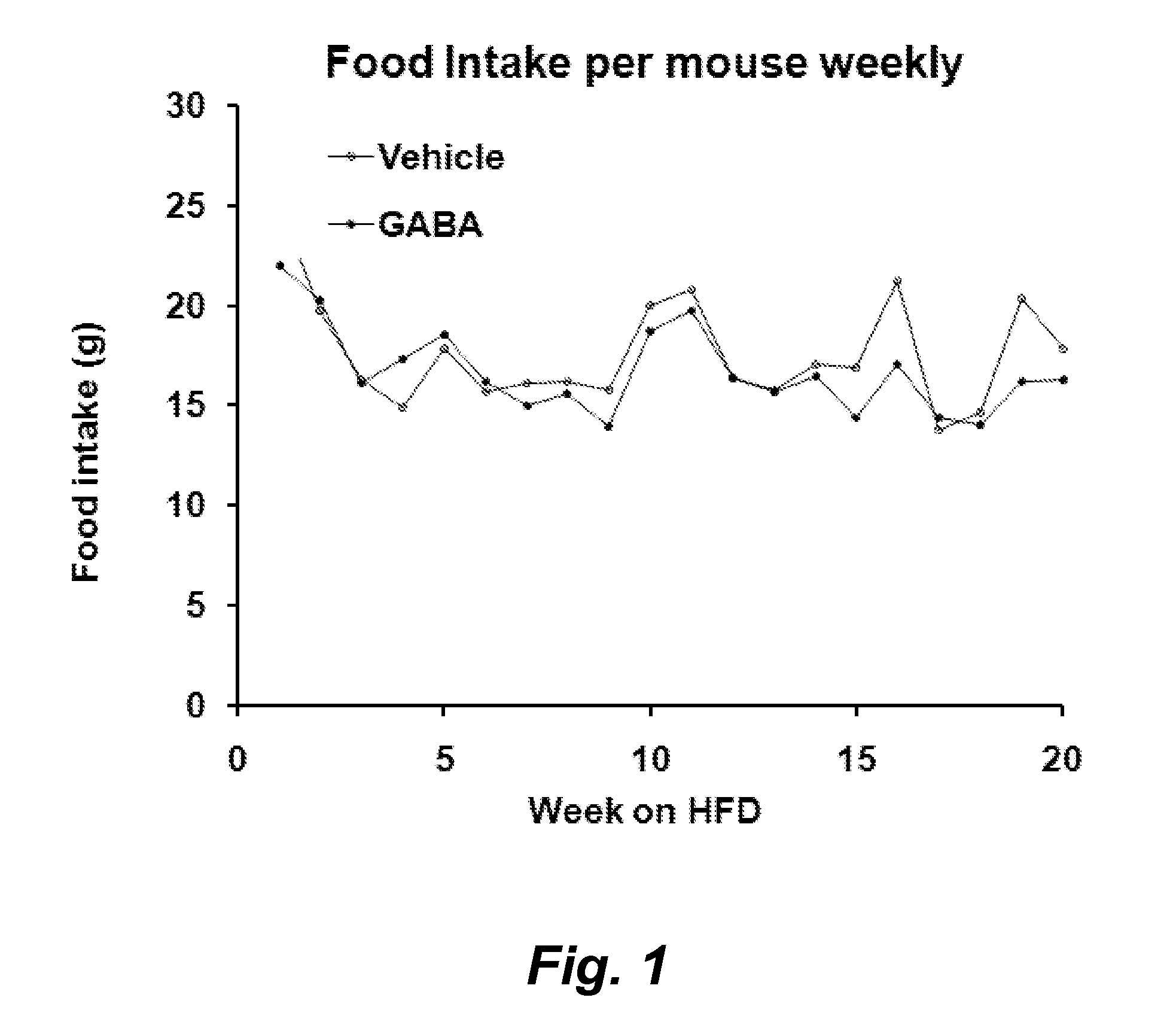
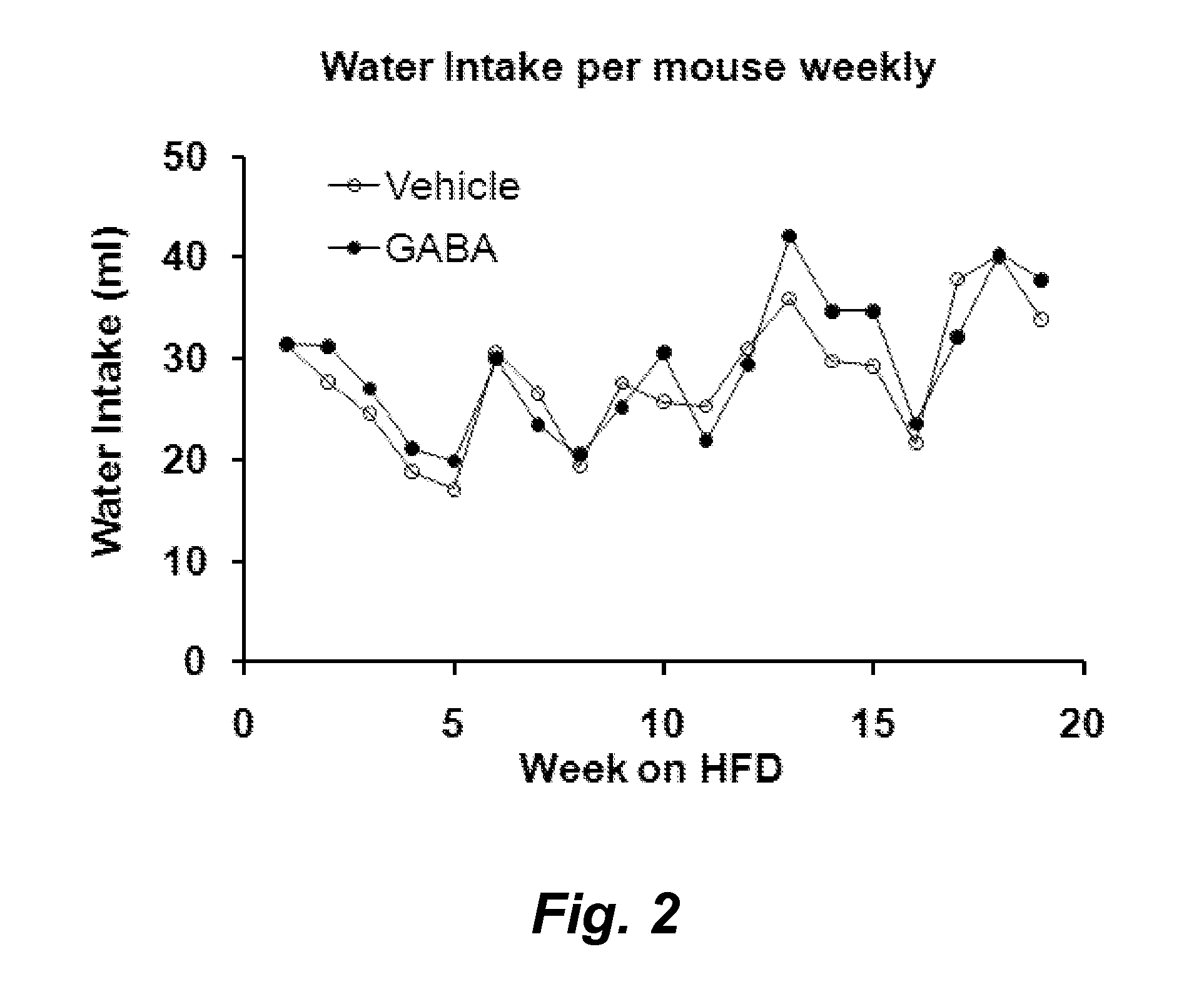
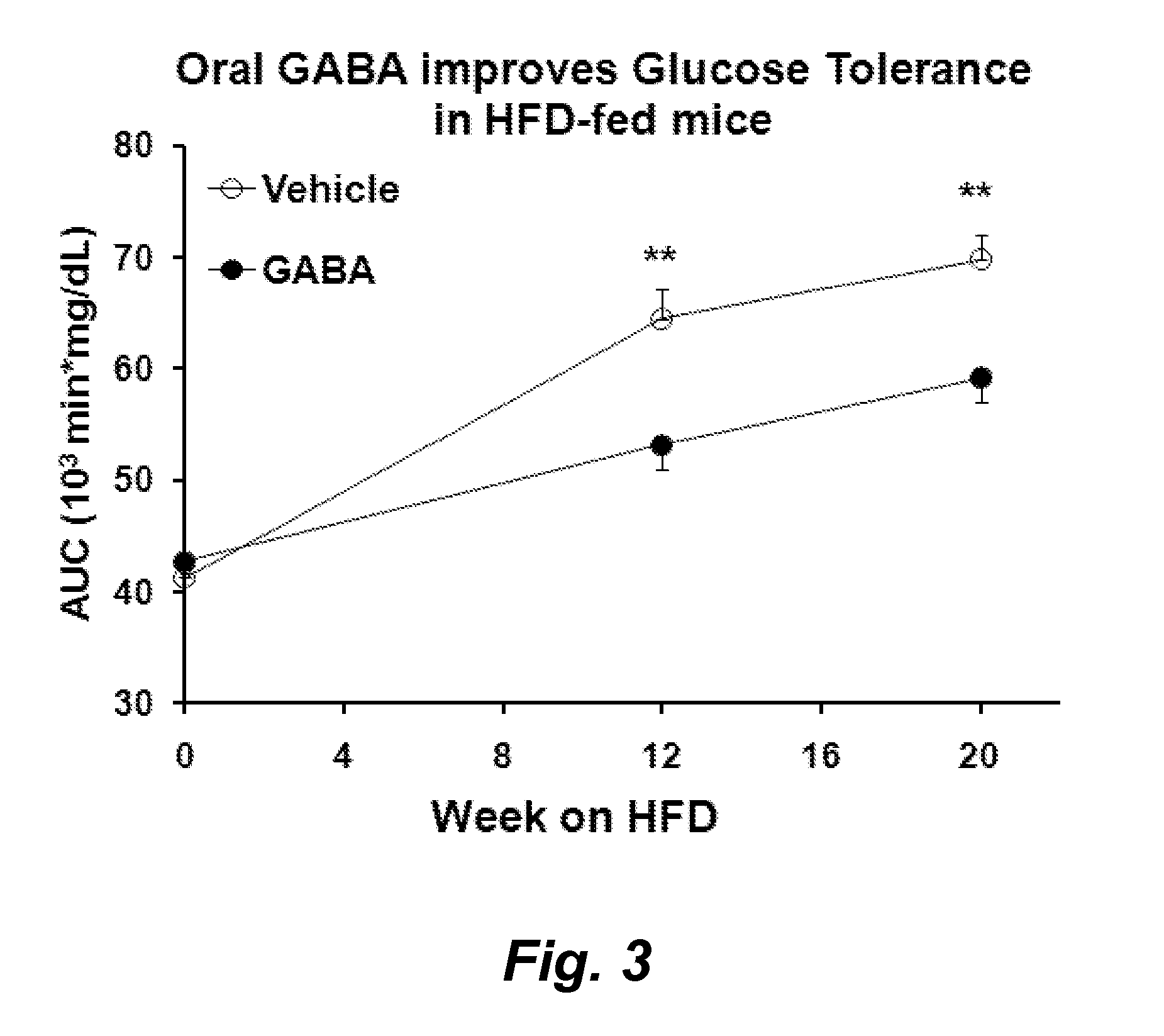
Gaba agonists in the treatment of disorders associated with metabolic syndrome and gaba combinations in treatment or prophylaxis of type i diabetes
In certain embodiments methods are provided for the therapeutic or prophylactic amelioration of one or more symptoms or disorders associated with metabolic syndrome. In various embodiments the methods involve administering to a subject in need thereof, a GABA receptor agonist, in an amount sufficient to ameliorate said one or more symptoms. In certain embodiments methods are provided for the prophylaxis or treatment of type I diabetes and related pathologies that involve the use of GABA or GABA agonists in combination with certain other compounds (e.g., one more antigens (e.g., GAD) that have a therapeutic effect in type I diabetes and / or an anti-CD3 antibody, an anti-CD20 antibody, exendin-4, and / or or a pro-insulin therapeutic).
Owner:RGT UNIV OF CALIFORNIA
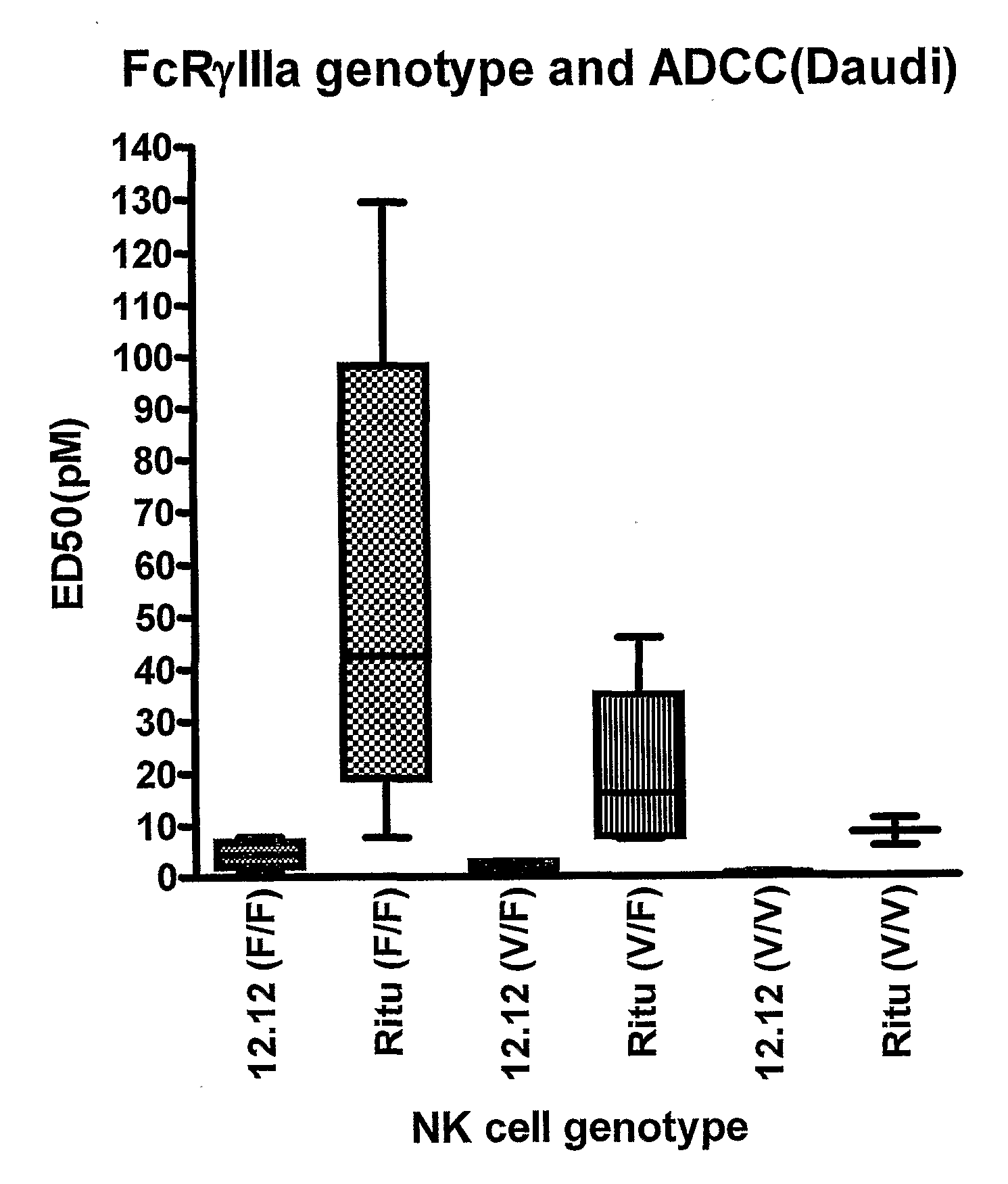
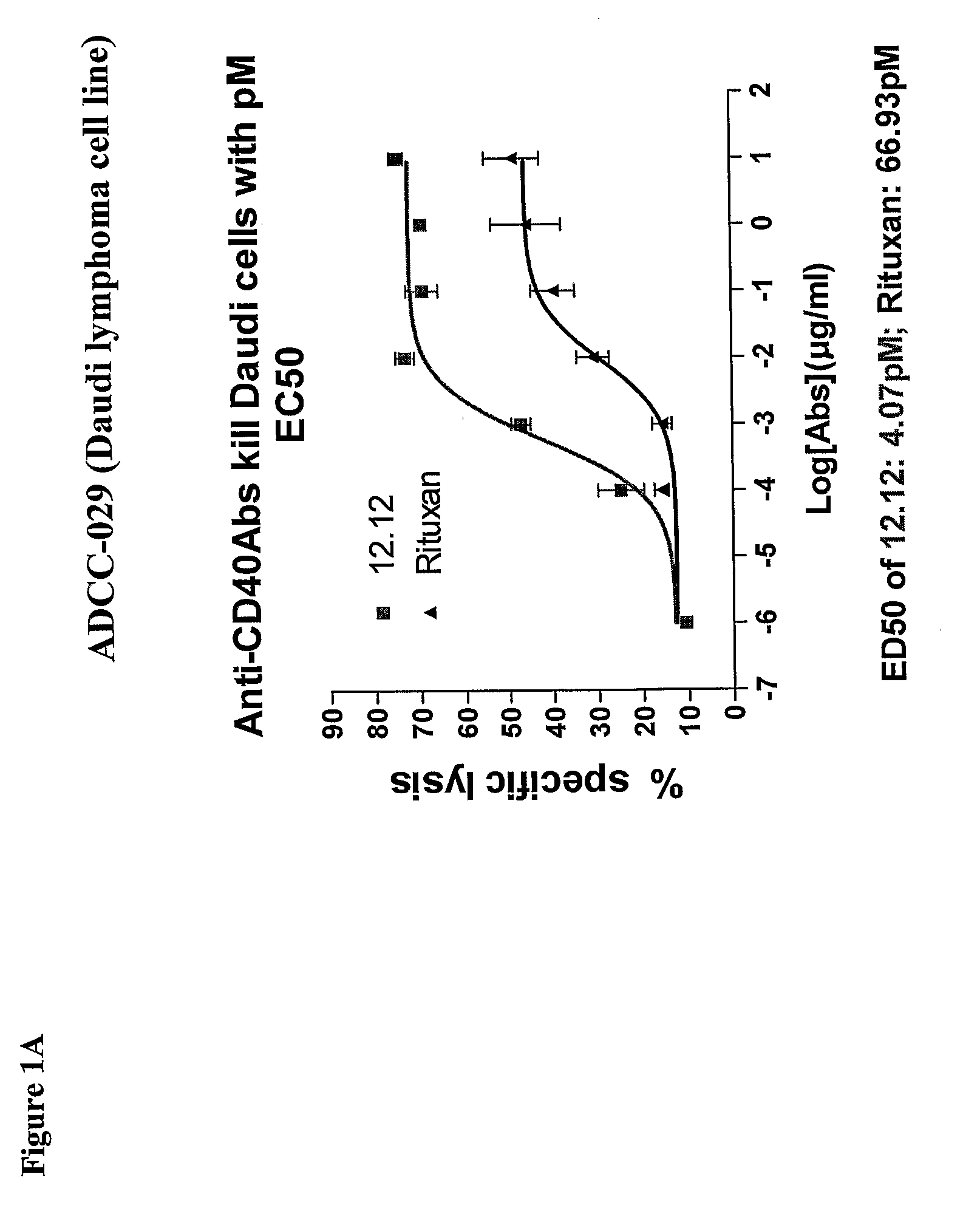
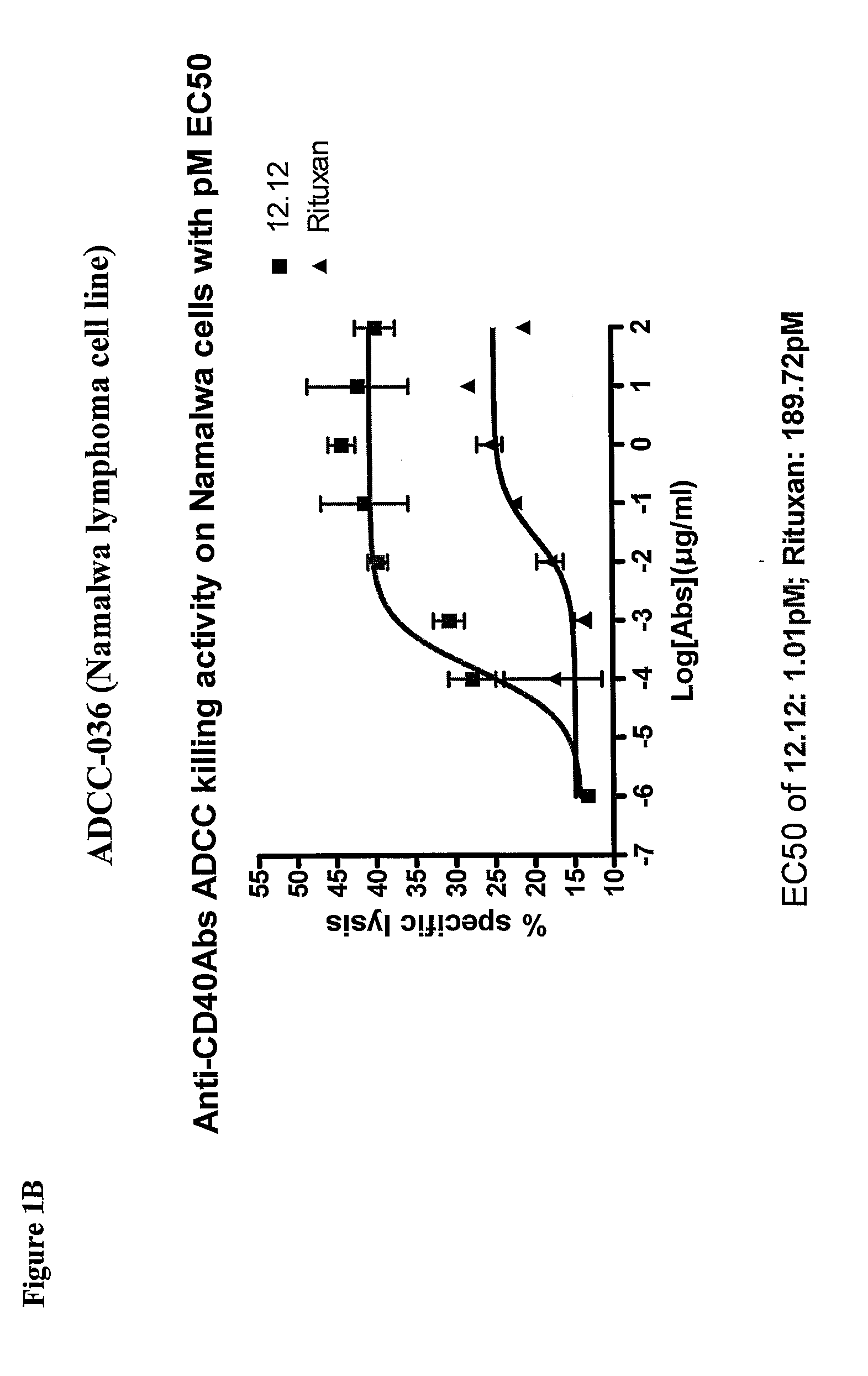
Uses of Anti-cd40 antibodies
Methods for treating a human patient for a cancer or pre-malignant condition that is associated with CD40-expressing cells are provided, where the human patient is heterozygous or homozygous for FcγRIIIa-158F (genotype V / F or F / F). Also provided are methods of inhibiting antibody production by B cells in a human patient who is heterozygous or homozygous for FcγRIIIa-158F (genotype V / F or F / F). The methods comprise administering to the human patient a therapeutically or prophylactically effective amount of an anti-CD40 antibody. Methods and kits for identifying a human patient with a cancer or pre-malignant condition that is treatable with an anti-CD40 antibody and which is refractory to treatment with rituximab (Rituxan®), as well as methods and kits for selecting an antibody therapy for treatment of a human patient having a cancer or pre-malignant condition that is refractory to treatment with rituximab (Rituxan®), are also provided. The methods of the present invention find use in treatment of cancers and pre-malignant conditions that are associated with CD40-expressing cells. These methods are particularly advantageous with respect to cancers and pre-malignant conditions that are associated with cells expressing both CD40 and CD20, as the methods enable the treatment of patients having a cancer or pre-malignant condition that is refractory to therapy with other oncotherapeutic agents such as anti-CD20 antibodies.
Owner:XOMA TECH LTD
Treatment of B-cell lymphoma
InactiveUS20060029543A1Increase ratingsConducive to survivalBiocideIn-vivo radioactive preparationsRegimenChemotherapy regimen
A method of treating B-cell lymphoma comprises administering to a patient a chemotherapeutic regimen, followed by treatment with a radiolabeled anti-CD20 antibody, wherein at the time of said treatment with said radiolabeled antibody said patient is not refractory to said chemotherapeutic regimen and has not relapsed.
Owner:BAYER PHARMA AG
Combination therapies for b-cell lymphomas comprising administration of Anti-cd20 antibody
InactiveUS20120258101A1Good synergyHigh response rateOrganic active ingredientsPeptide/protein ingredientsCombination therapyB cell
New combined therapeutic regimens for treatment of B-cell lymphomas are disclosed which comprise, in particular, administration of anti-CD20 antibodies to patients having low-, intermediate- or high-grade non-Hodgkin's lymphomas.
Owner:GRILLO LOPEZ ANTONIO J
Combination therapies for b-cell lymphomas comprising administration of Anti-cd20 antibody
InactiveUS20120251534A1Good synergyHigh response rateOrganic active ingredientsPeptide/protein ingredientsCombined Modality TherapyCombination therapy
New combined therapeutic regimens for treatment of B-cell lymphomas are disclosed which comprise, in particular, administration of anti-CD20 antibodies to patients having low-, intermediate- or high-grade non-Hodgkin's lymphomas.
Owner:GRILLO LOPEZ ANTONIO J
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com