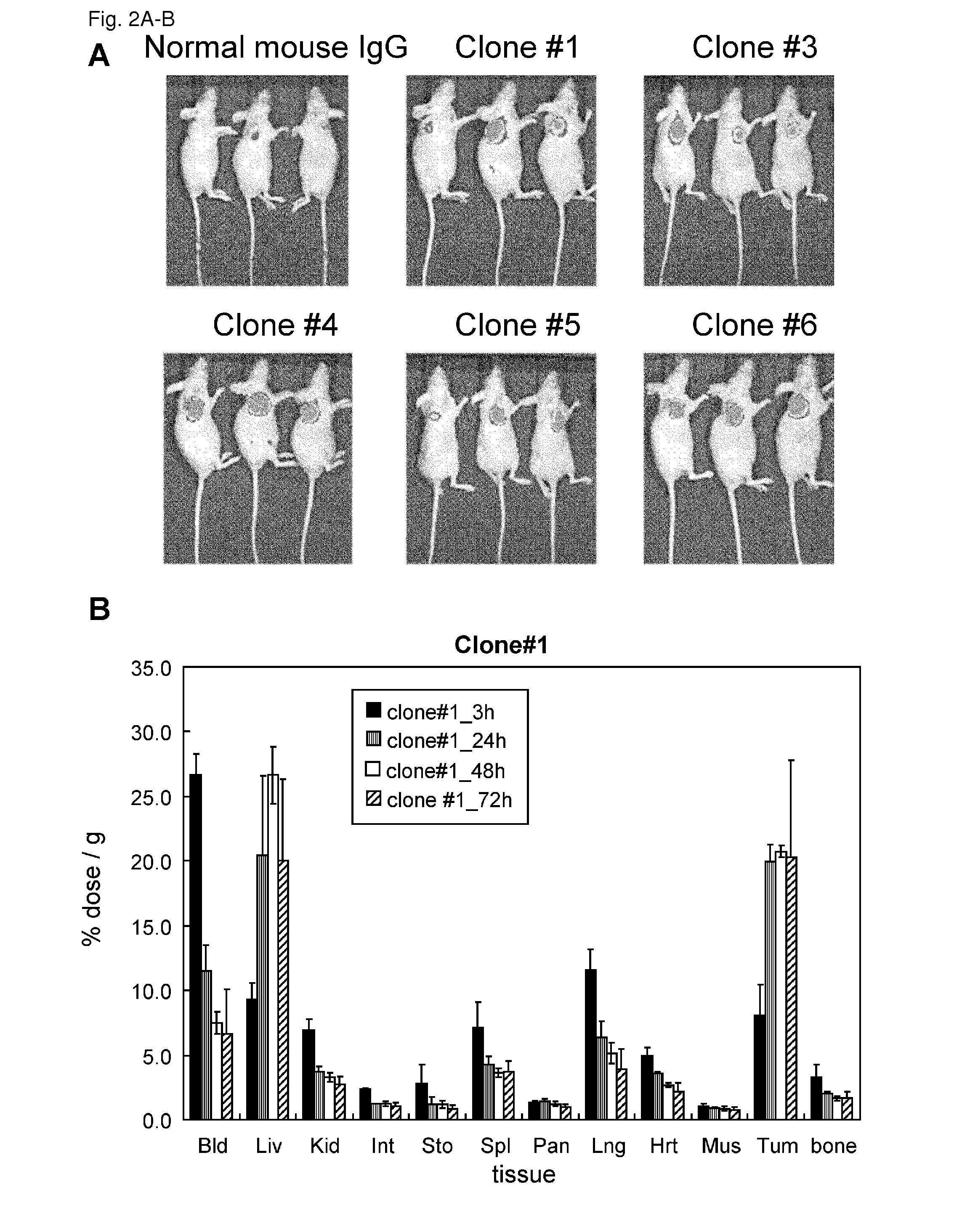
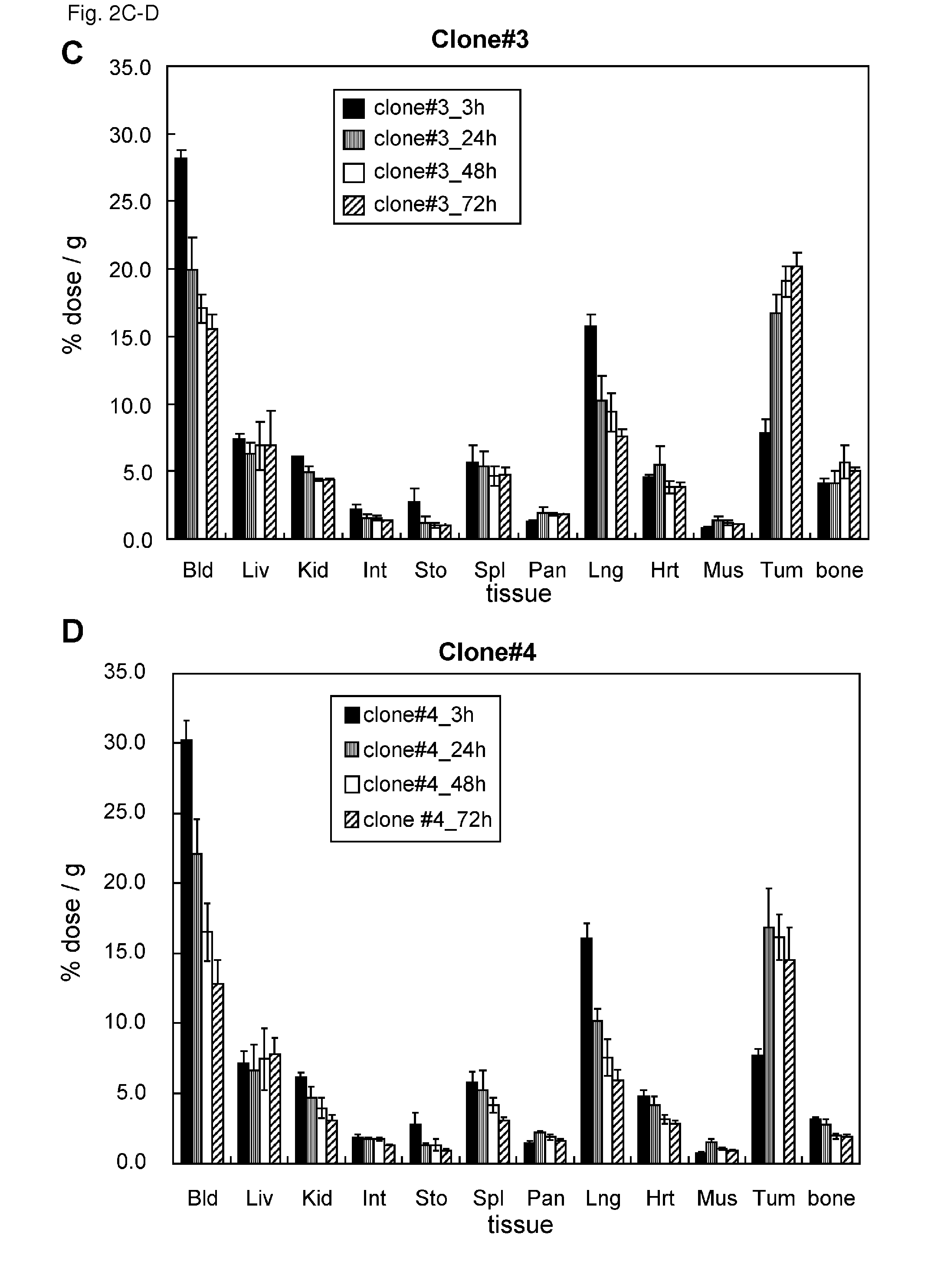
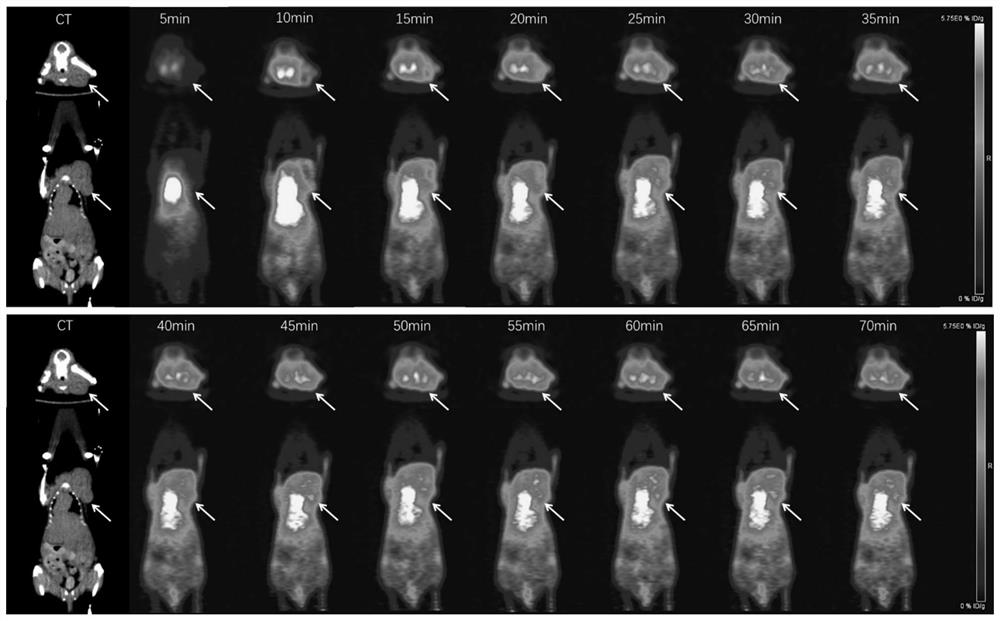
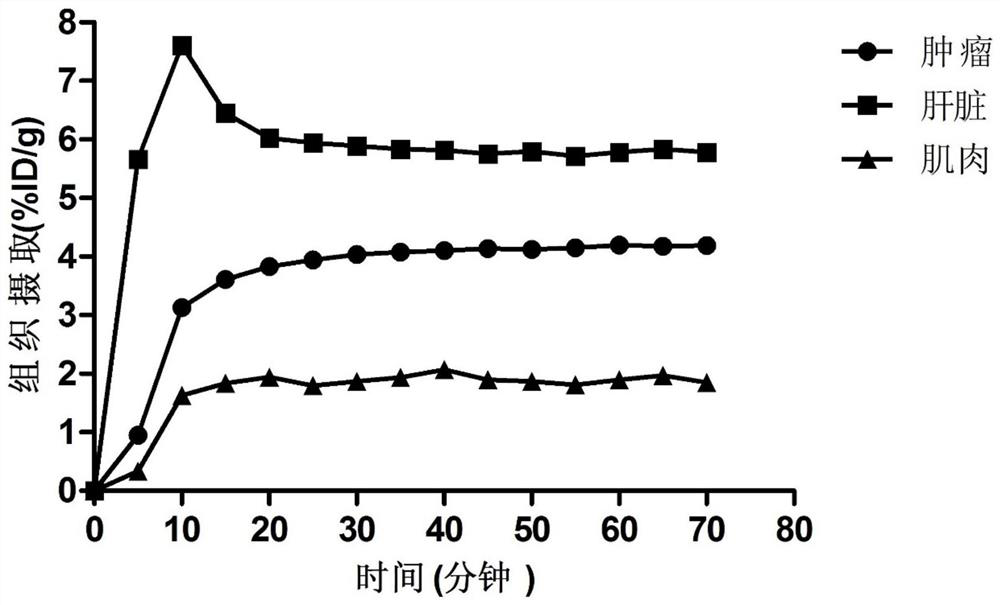
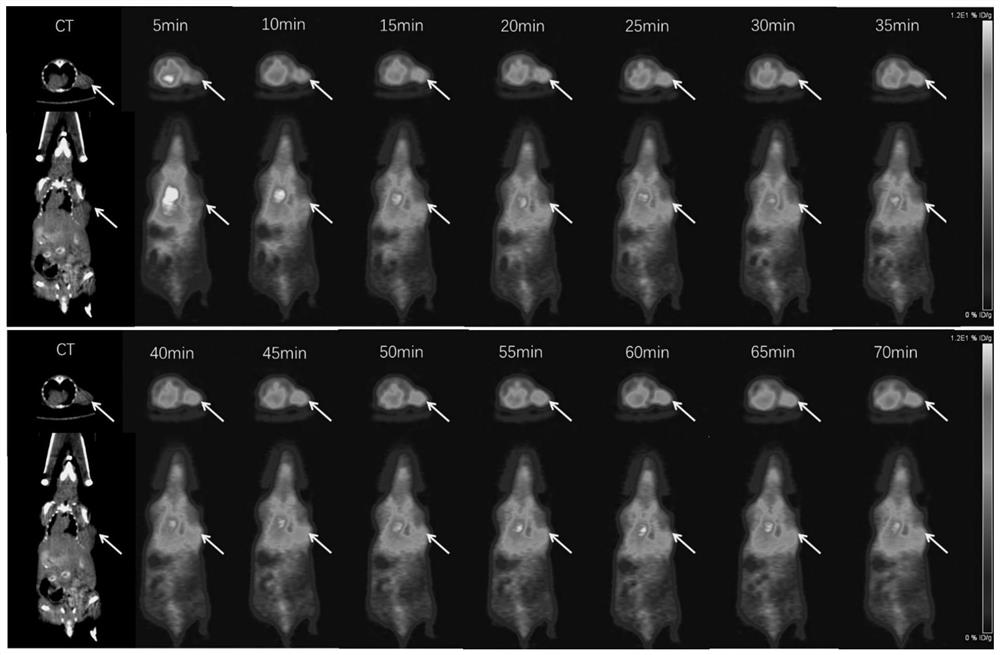
Patents
Literature
129 results about "Radioisotopic Labeling" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A laboratory procedure that results in the incorporation of a radioactive isotope into a molecule of interest.
Radioimmunotherapy of lymphoma using anti-CD20 antibodies
Owner:GLAXO SMITHKLINE LLC
Radioimmunotherapy of lymphoma using anti-CD20 antibodies
Methods for the treatment of lymphoma by administration of a B cell-specific antibody are described. The invention encompasses providing to a patient both unlabeled antibodies and antibodies labeled with a radioisotope. A principal advantage of the method is that tumor responses can be obtained in a radiometric dose range that does not require hematopoietic stem cell replacement as an adjunct therapy also described is a composition useful in the treatment of lymphoma.
Owner:RGT UNIV OF MICHIGAN +2
Methods for measuring cellular proliferation and destruction rates in vitro and in vivo
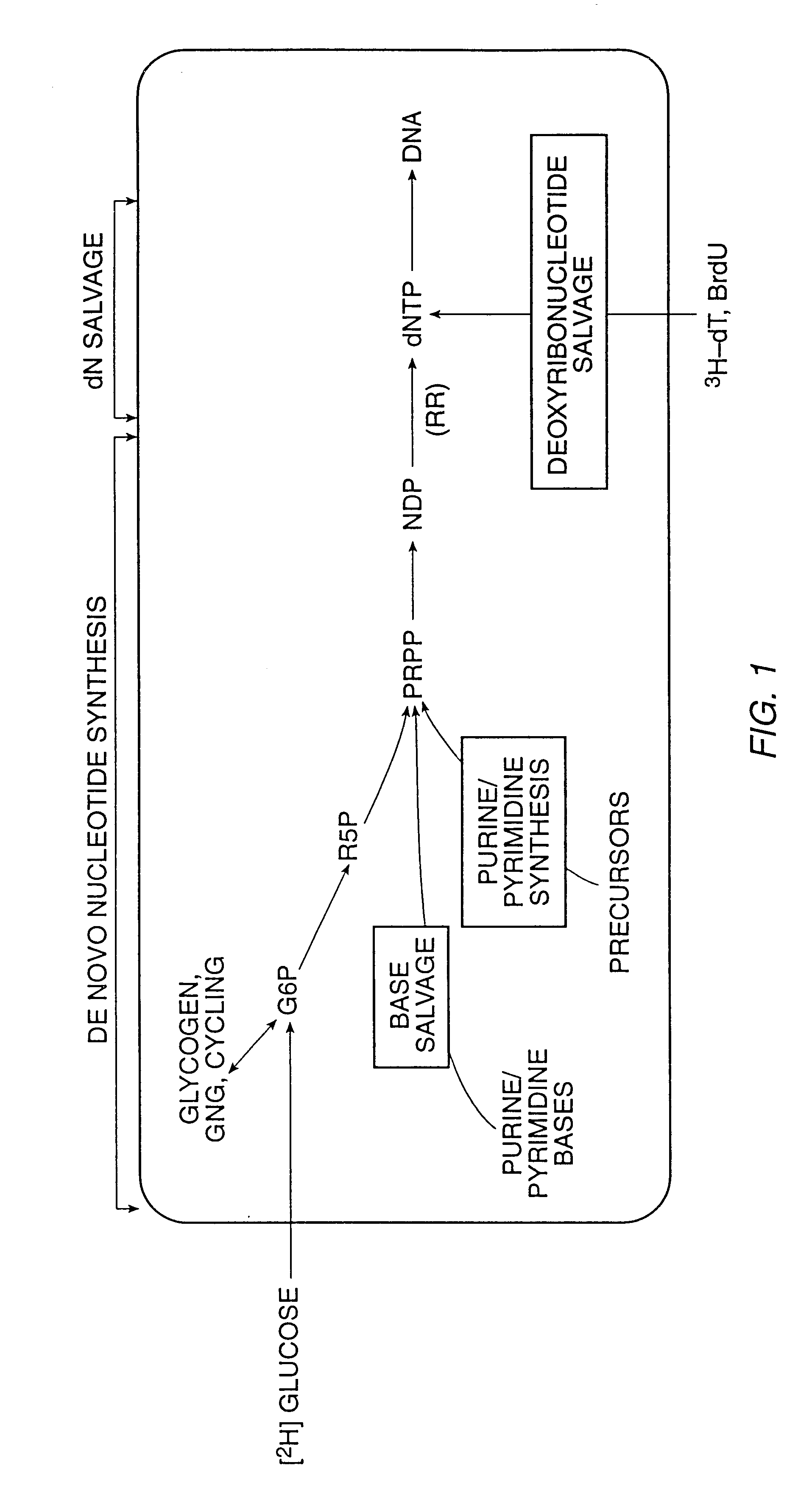
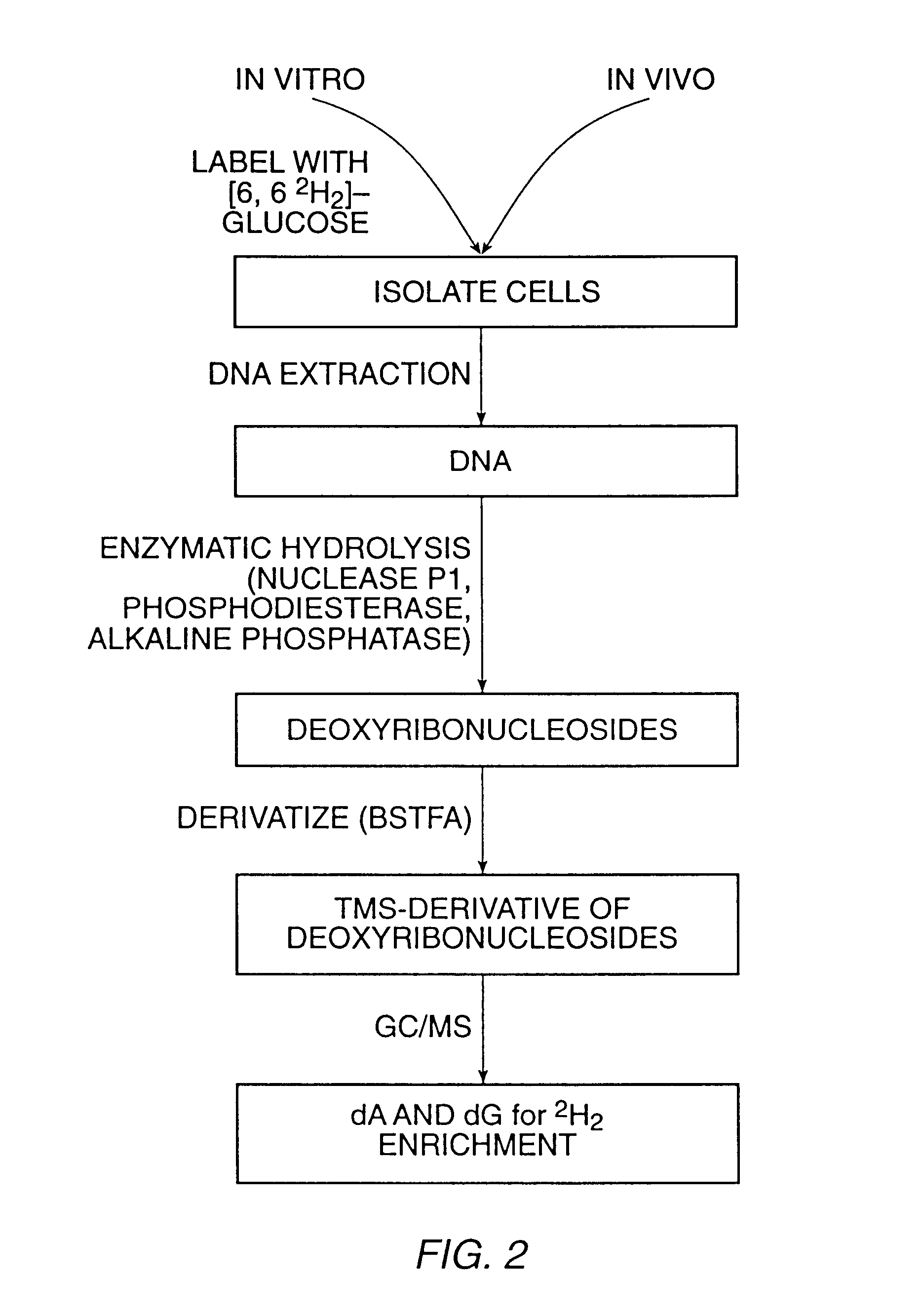
InactiveUS6461806B1Organic active ingredientsIn-vivo radioactive preparationsStable Isotope LabelingDisease
The present invention relates to methods for measuring the proliferation and destruction rates of cells by measuring deoxyribonucleic acid (DNA) synthesis and / or destruction. In particular, the methods utilize non-radioactive stable isotope labels to endogenously label DNA synthesized through the de novo nucleotide synthesis pathway in a cell. The amount of label incorporated in the DNA is measured as an indication of cellular proliferation. The decay of labeled DNA over time is measured as an indication of cellular destruction. Such methods do not involve radioactivity or potentially toxic metabolites, and are suitable for use both in vitro and in vivo. Therefore, the invention is useful for measuring cellular proliferation or cellular destruction rates in humans for the diagnosis, prevention, or management of a variety of disease conditions in which cellular proliferation or cellular destruction is involved. The invention also provides methods for measuring proliferation or destruction of T cells in a subject infected with human immunodeficiency virus (HIV) and methods of screening an agent for a capacity to induce or inhibit cellular proliferation or destruction. In addition, the invention provides methods for measuring cellular proliferation in a proliferating population which utilize both radioactive isotope labels and stable isotopes to endogenously label DNA through the de novo nucleotide synthesis pathway.
Owner:RGT UNIV OF CALIFORNIA
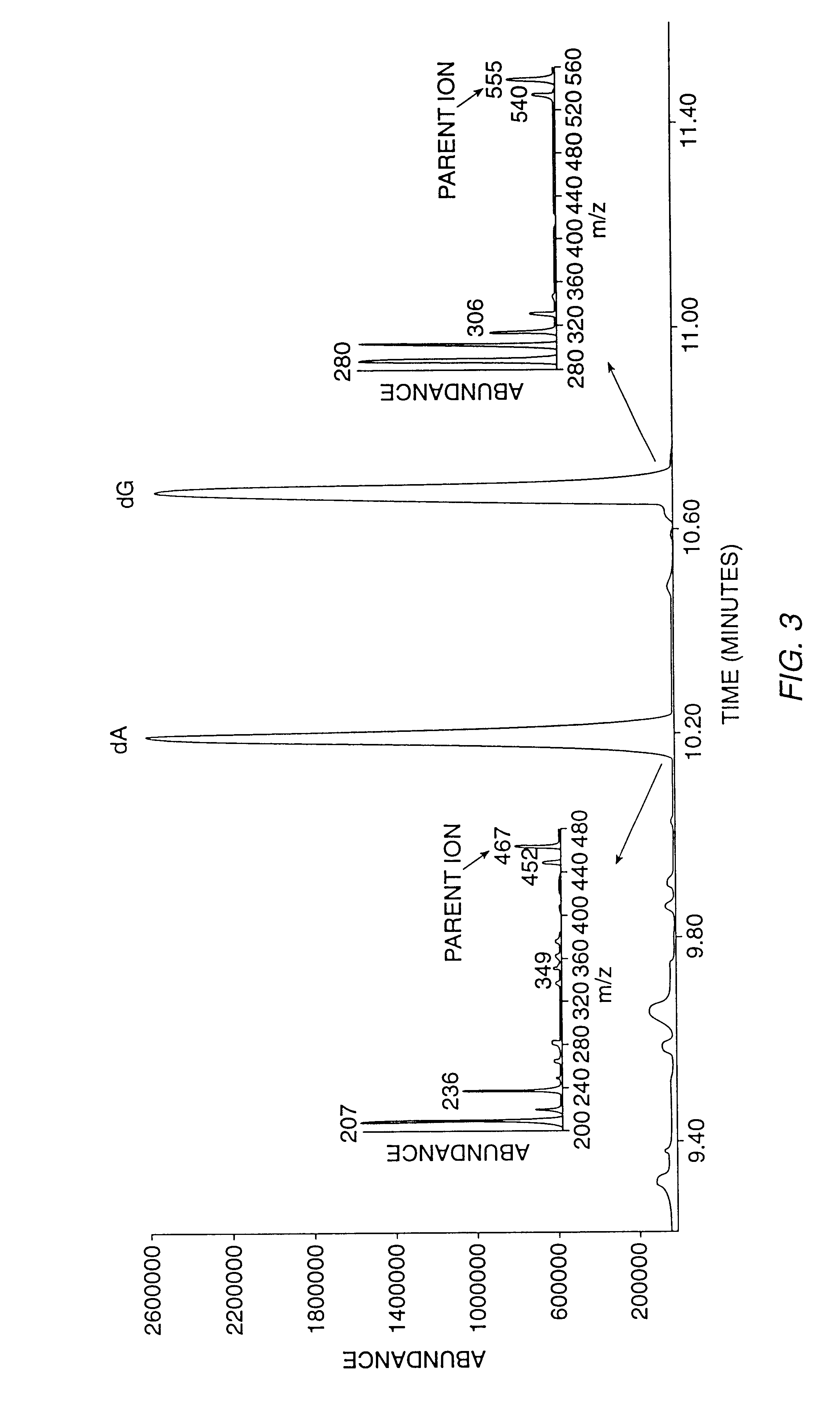
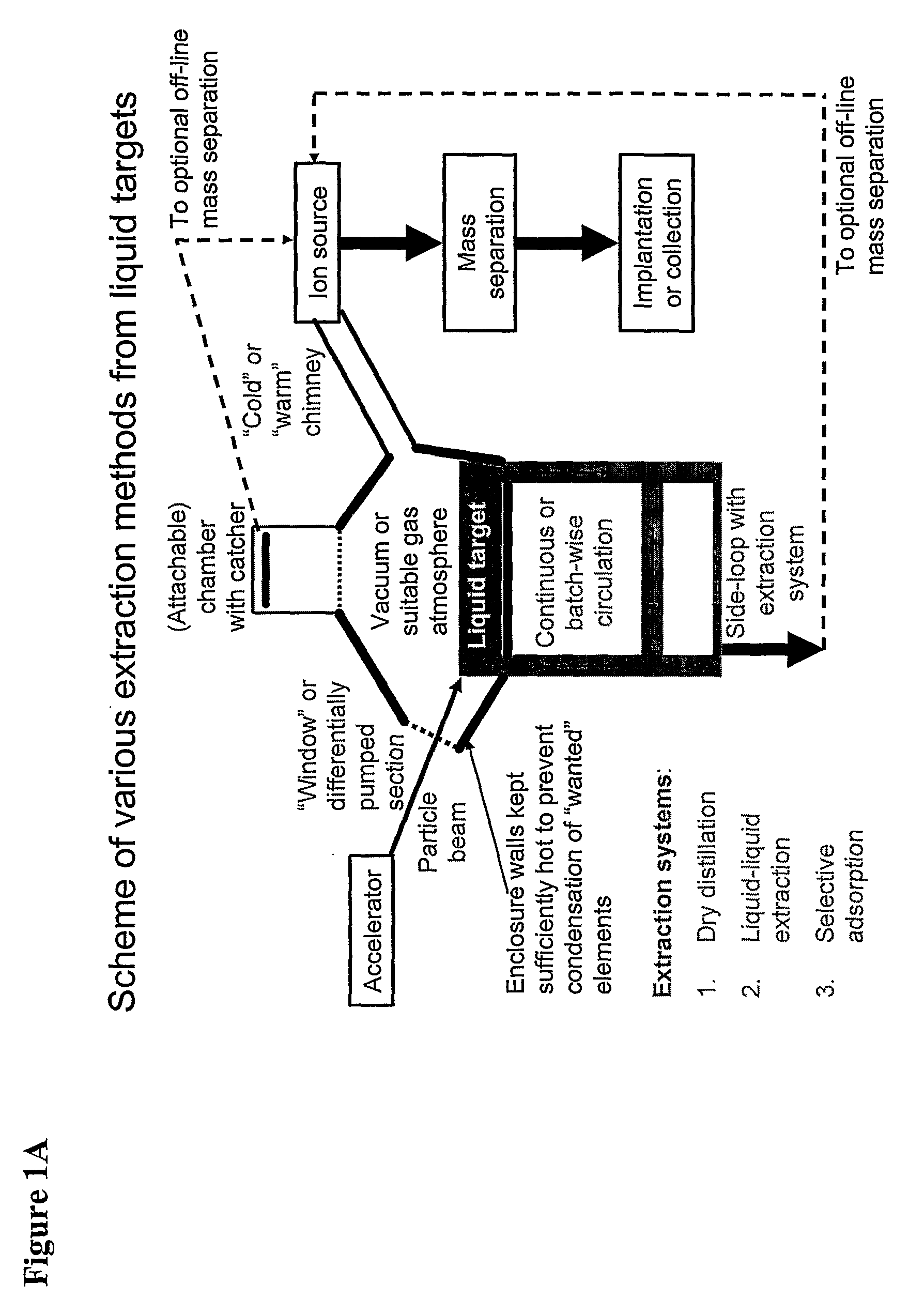
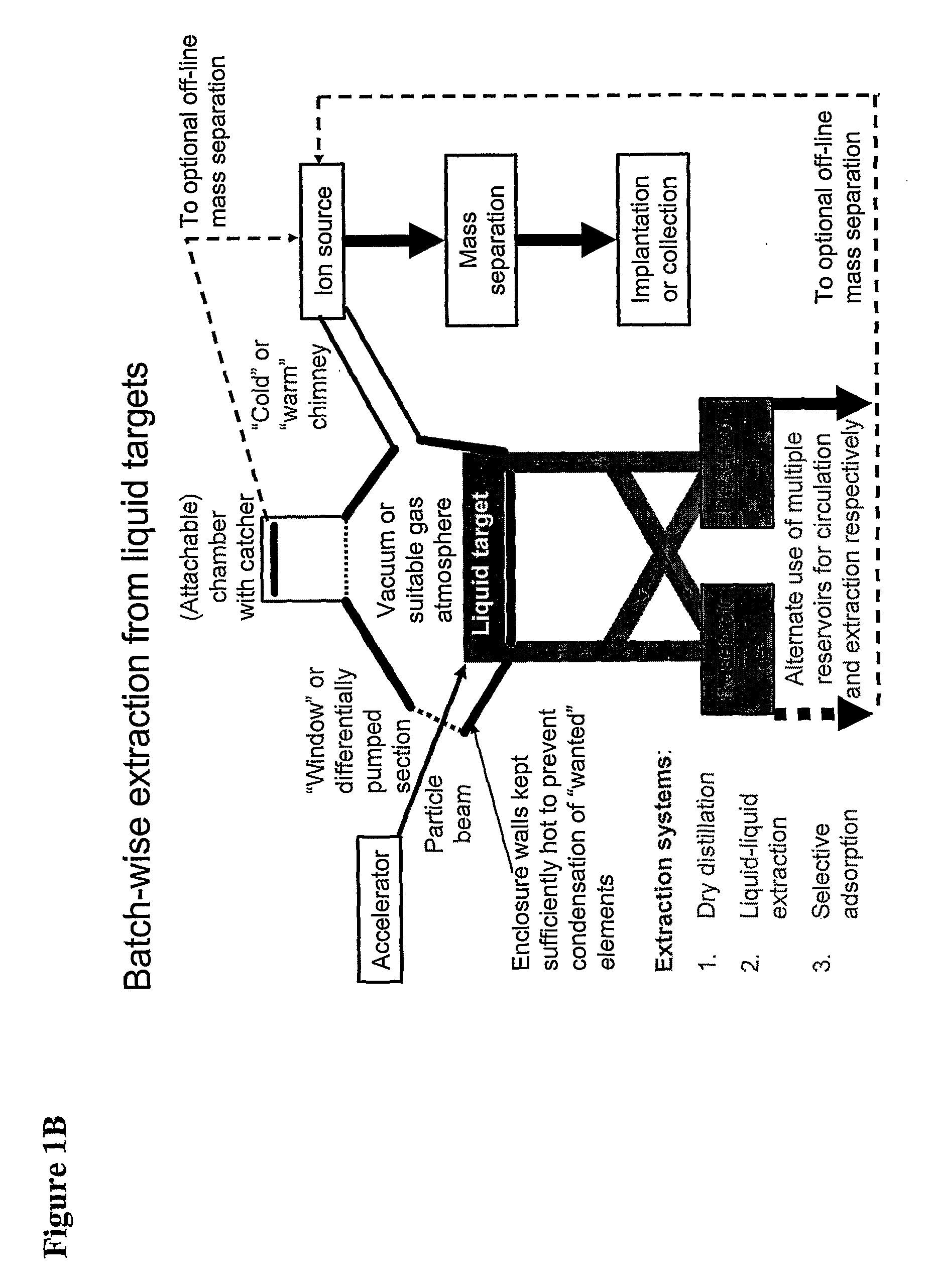
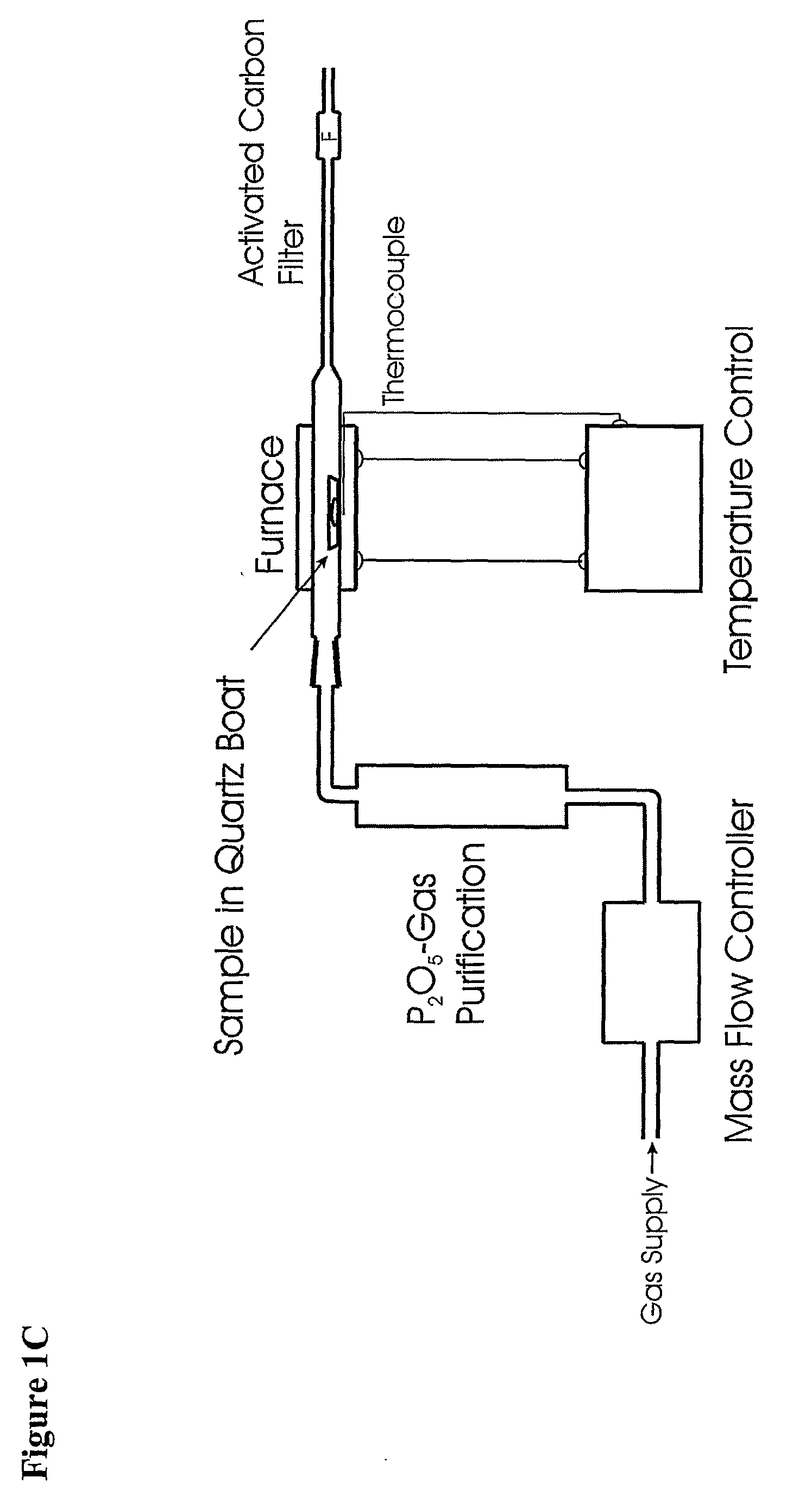
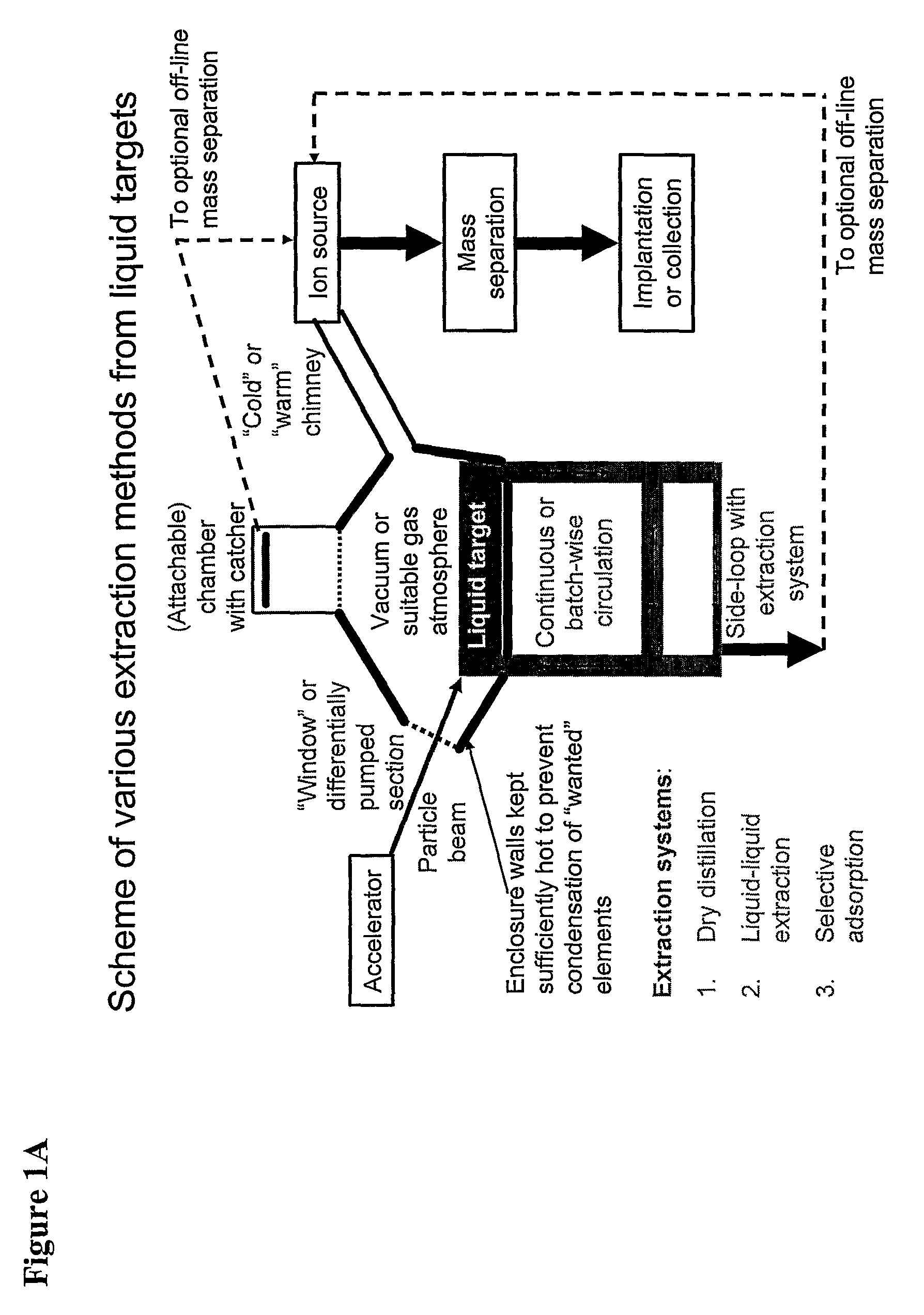
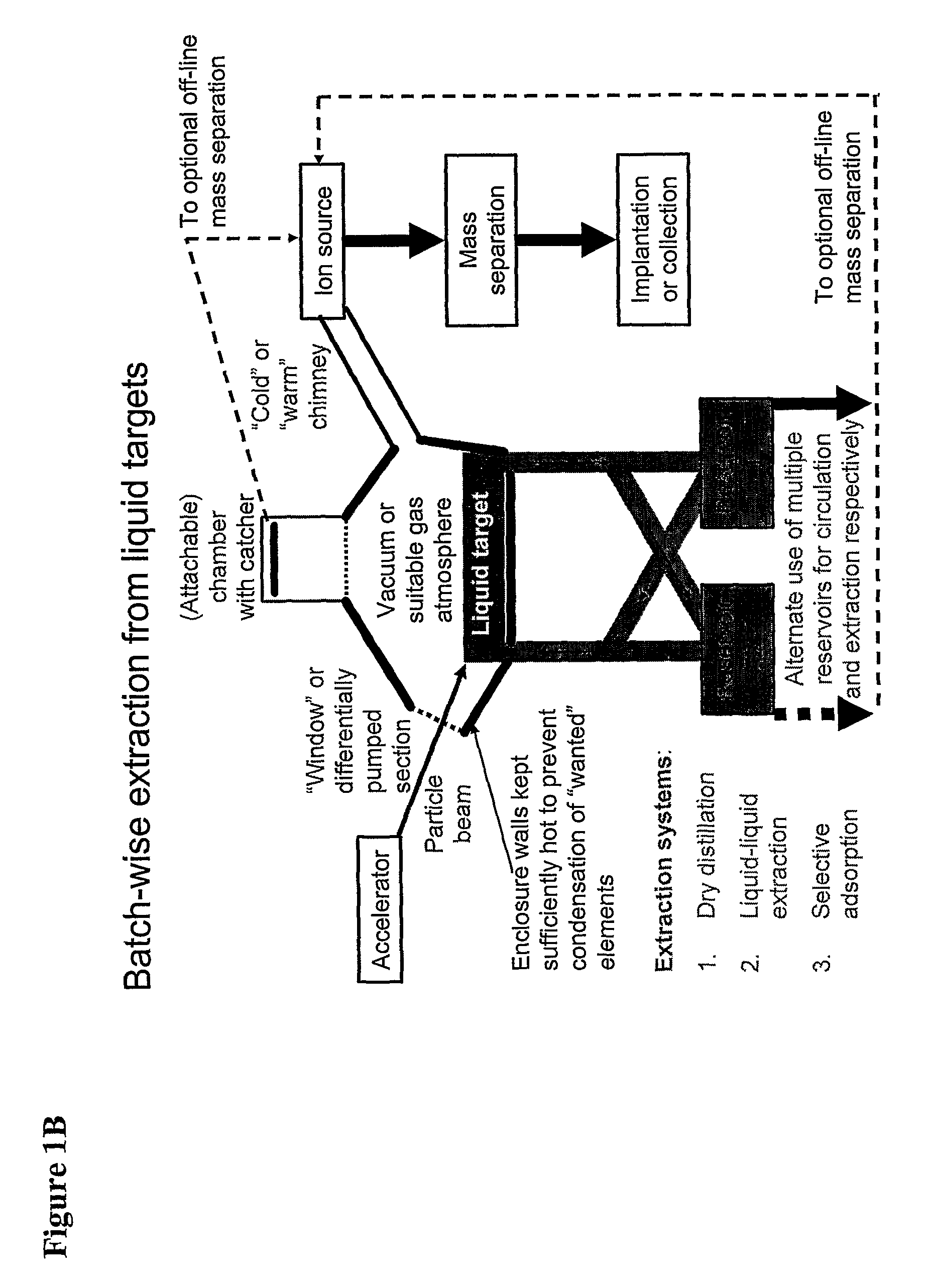
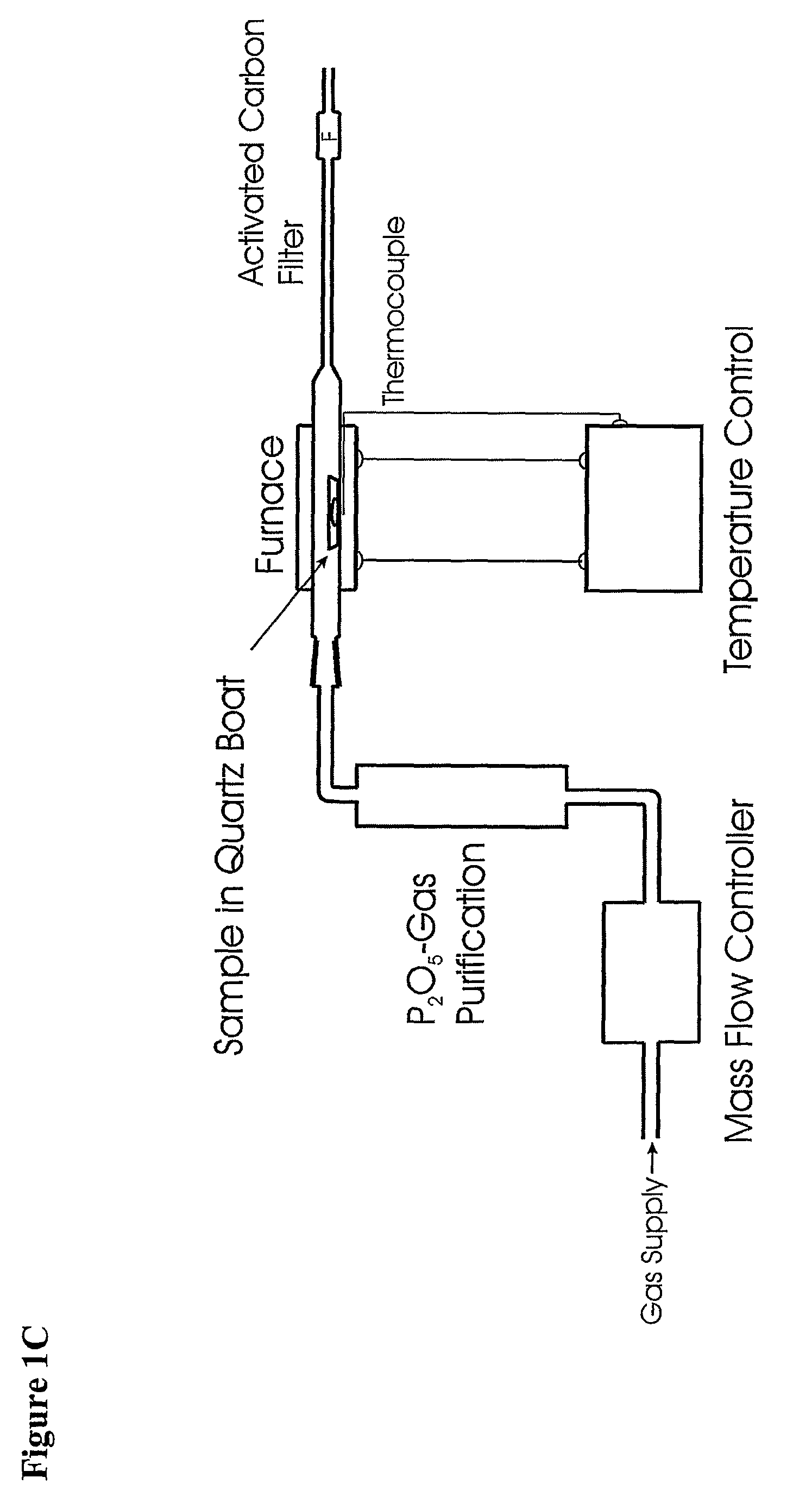
Method for Production of Radioisotope Preparations and Their Use in Life Science, Research, Medical Application and Industry
The present invention relates to an universal method for the large scale production of high-purity carrier free or non carrier added radioisotopes by applying a number of “unit operations” which are derived from physics and material science and hitherto not used for isotope production. A required number of said unit operations is combined, selected and optimised individually for each radioisotope production scheme. The use of said unit operations allows a batch wise operation or a fully automated continuous production scheme. The radioisotopes produced by the inventive method are especially suitable for producing radioisotope-labelled bioconjugates as well as particles, in particular nanoparticles and microparticles.
Owner:EUROPEAN ORGANIZATION FOR NUCLEAR RESEARCH +1
Silica nanoparticles postloaded with photosensitizers for drug delivery in photodynamic therapy
InactiveUS20110288234A1Promote resultsOvercomes drawbackPowder deliveryEnergy modified materialsSilica nanoparticlesTumor target
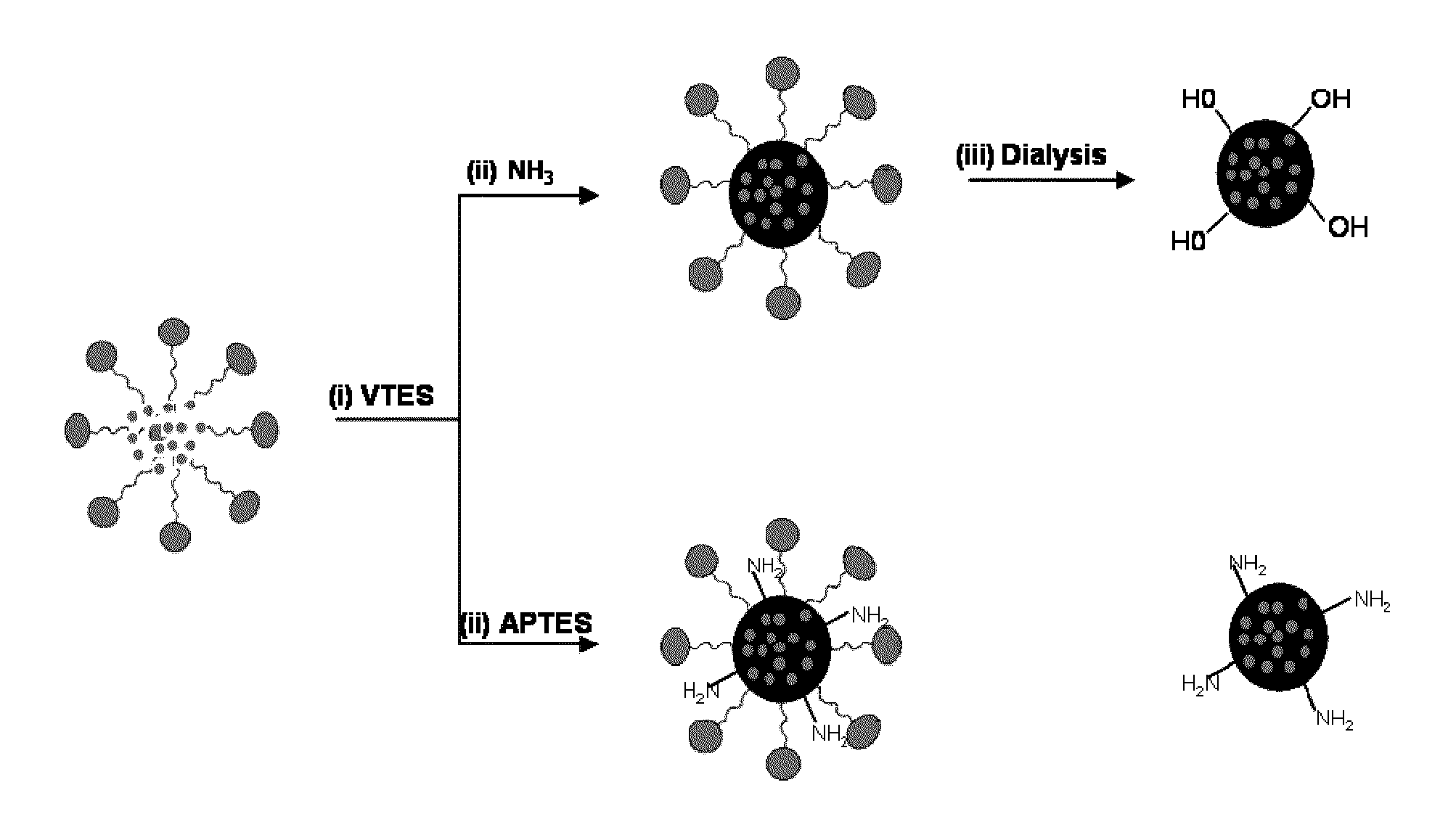
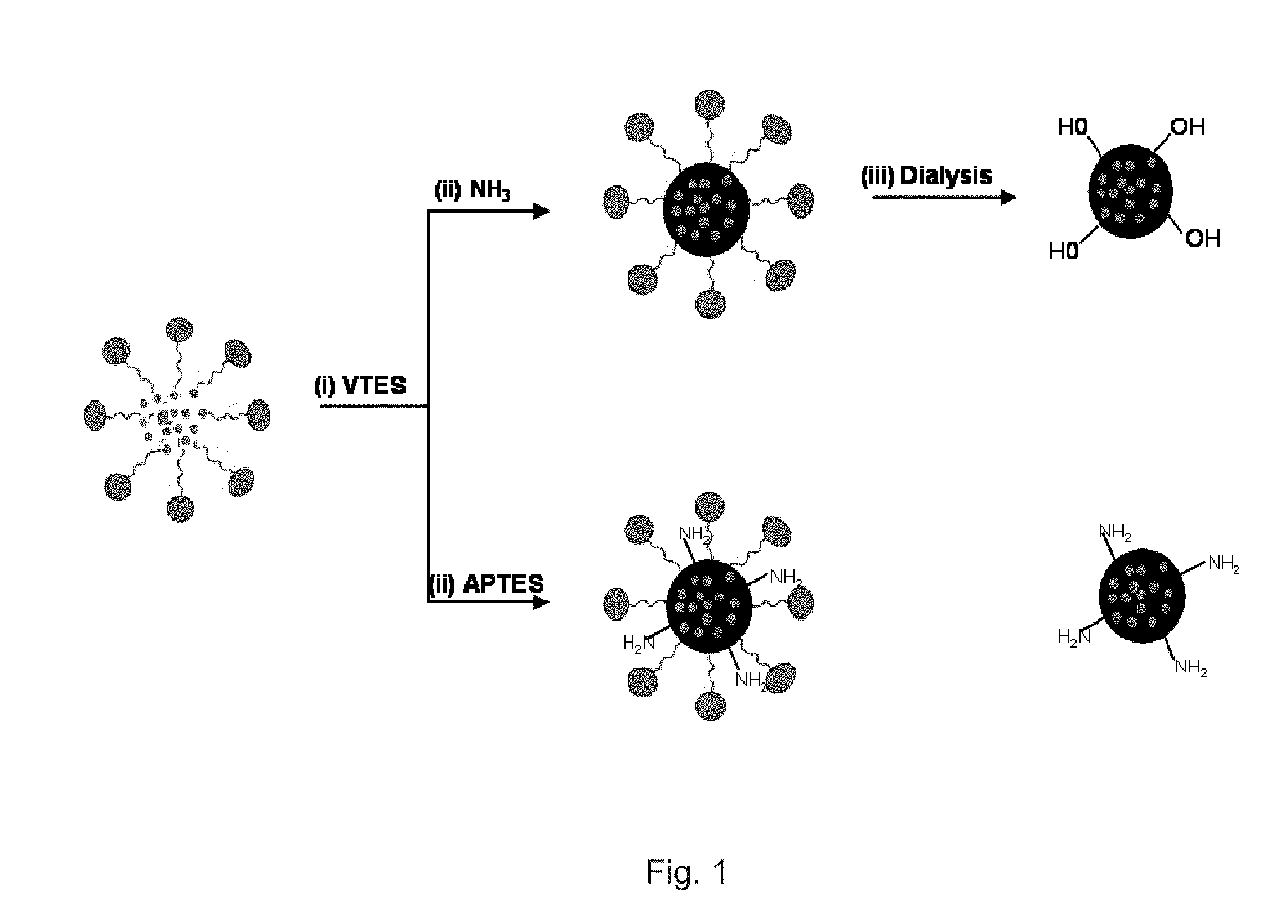
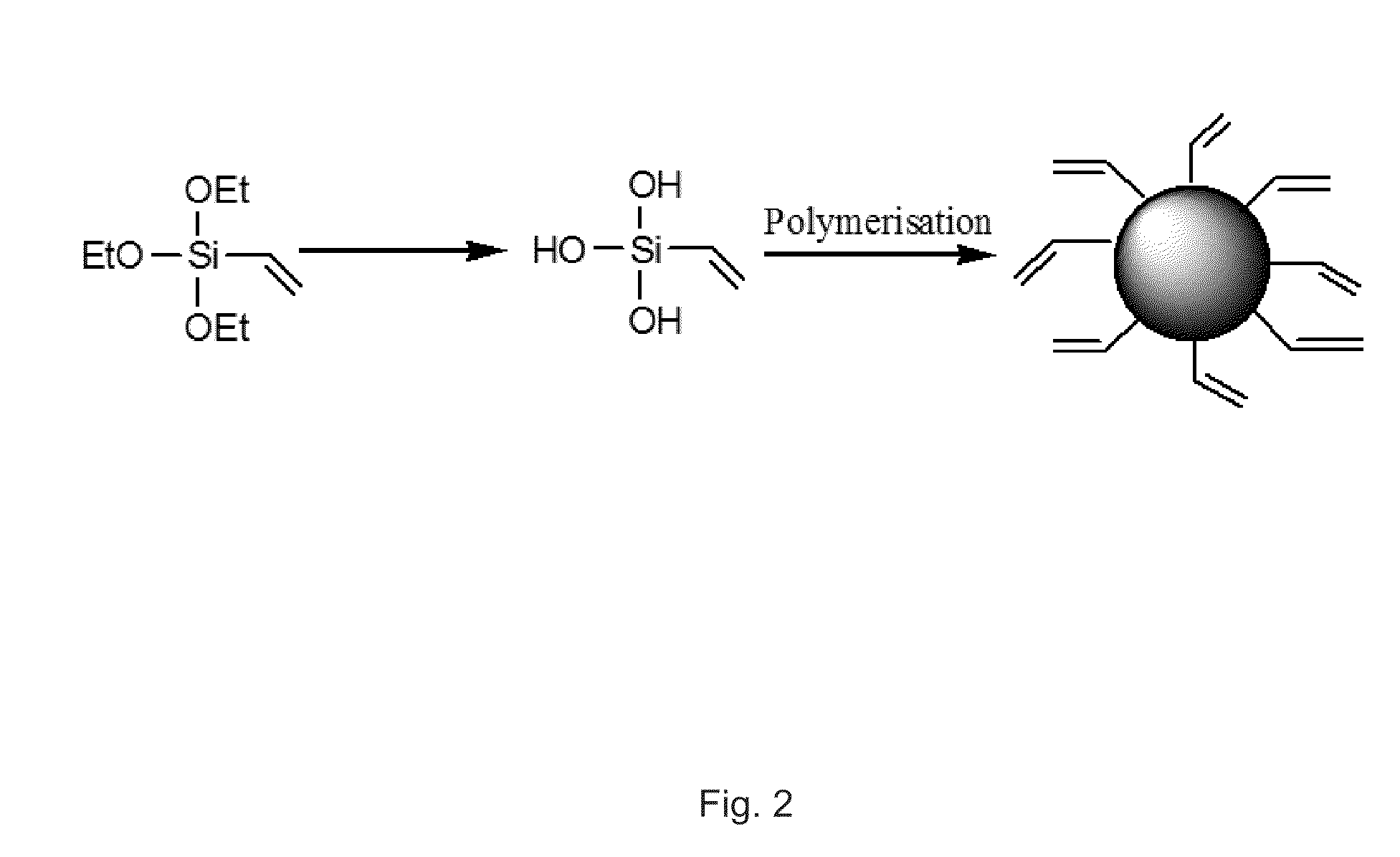
A nanoparticle including a polysiloxane base having an exterior surface and having a photosensitizer at least partly exposed at its exterior surface, said photosensitizer being secured to the exterior surface by loading the photosensitizer onto the surface after formation of the polysiloxane base of the nanoparticle. The nanoparticle may have tumor targeting moieties and may be post loaded with cyanine dye. The nanoparticle preferably includes post loaded moieties providing at least two of tumor specificity, photodynamic properties and imaging capabilities and the photosensitizer is tagged with a radioisotope. A method for preparation of the nanoparticle is also provided.
Owner:THE RES FOUND ON STATE UNIV OF NY +1
Sorter and sorting method for separating cells and particles
ActiveCN102019277ARich feature informationNon-invasiveNanoparticle analysisPreparing sample for investigationParticle sortingFluorescence
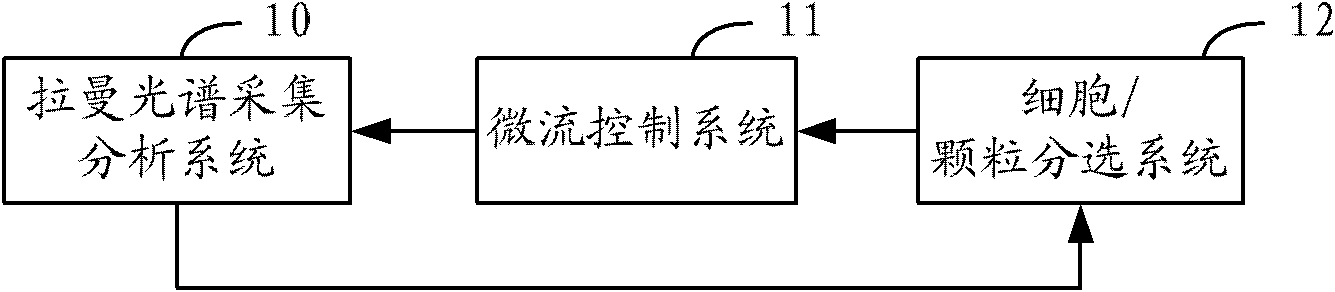
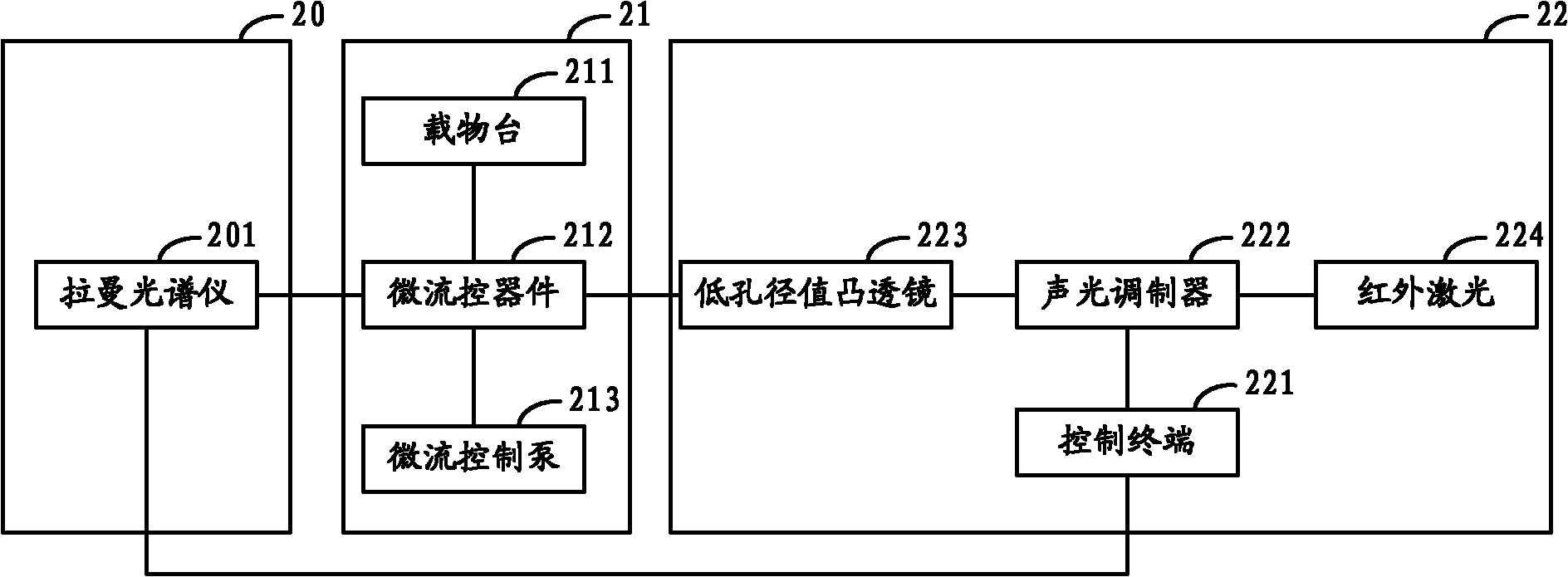
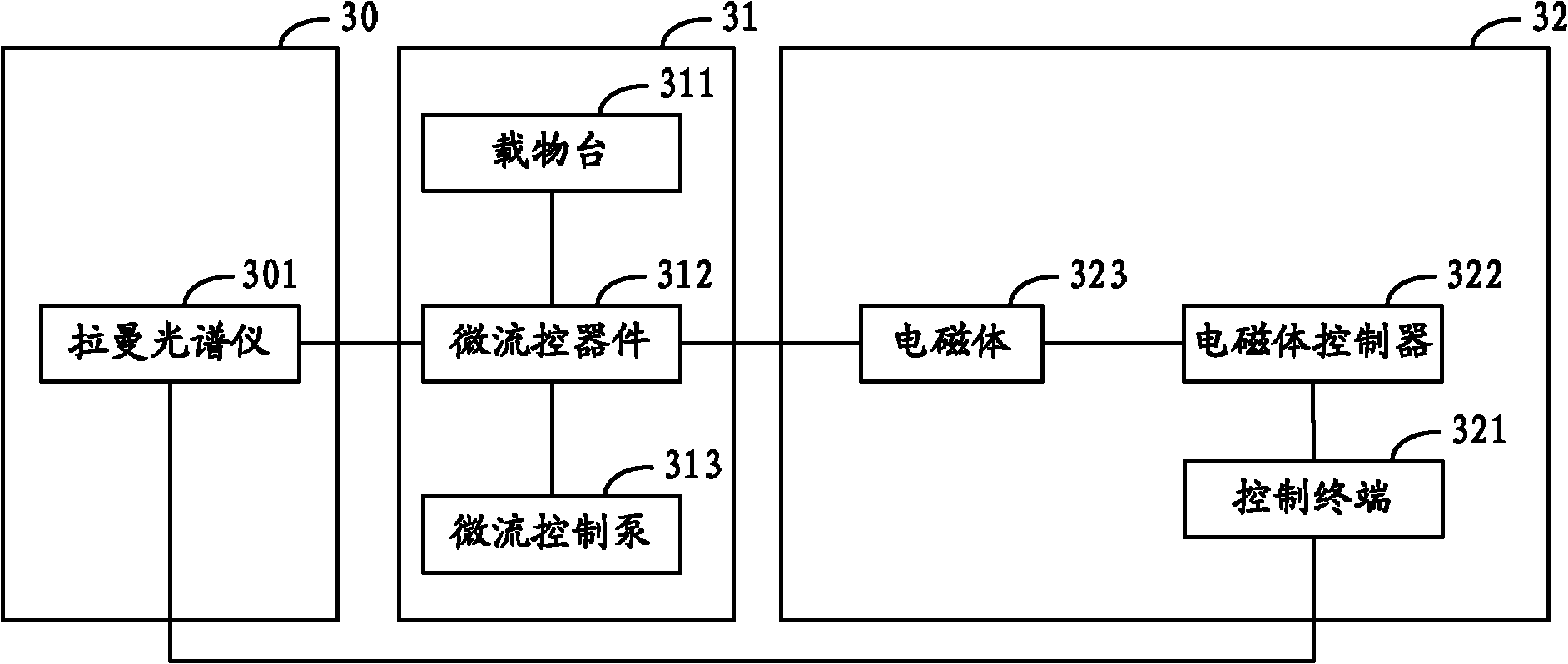
The invention relates to a sorter and a sorting method for separating cells and particles. The sorter comprises a Raman spectrum acquisition analysis system, a micro-flow control system and a cell / particle sorting system, wherein the micro-flow control system is connected with the Raman spectrum acquisition analysis system; the Raman spectrum acquisition analysis system is connected with the cell / particle sorting system; and the cell / particle sorting system is connected with the micro-flow control system. The invention also provides a sorting method for separating the cells and the particles.The sorter can effectively identify and sort nanometer-micron sized cells, nano materials, particles and cell contents, and because the Raman spectrum has the characteristics of high speed, non-invasive property, high information content and the like, the sorting method provides richer characteristic information of the cells or the particles in the same time period compared with methods of fluorescence or radio isotope labeling and the like.
Owner:长春长光辰英生物科学仪器有限公司
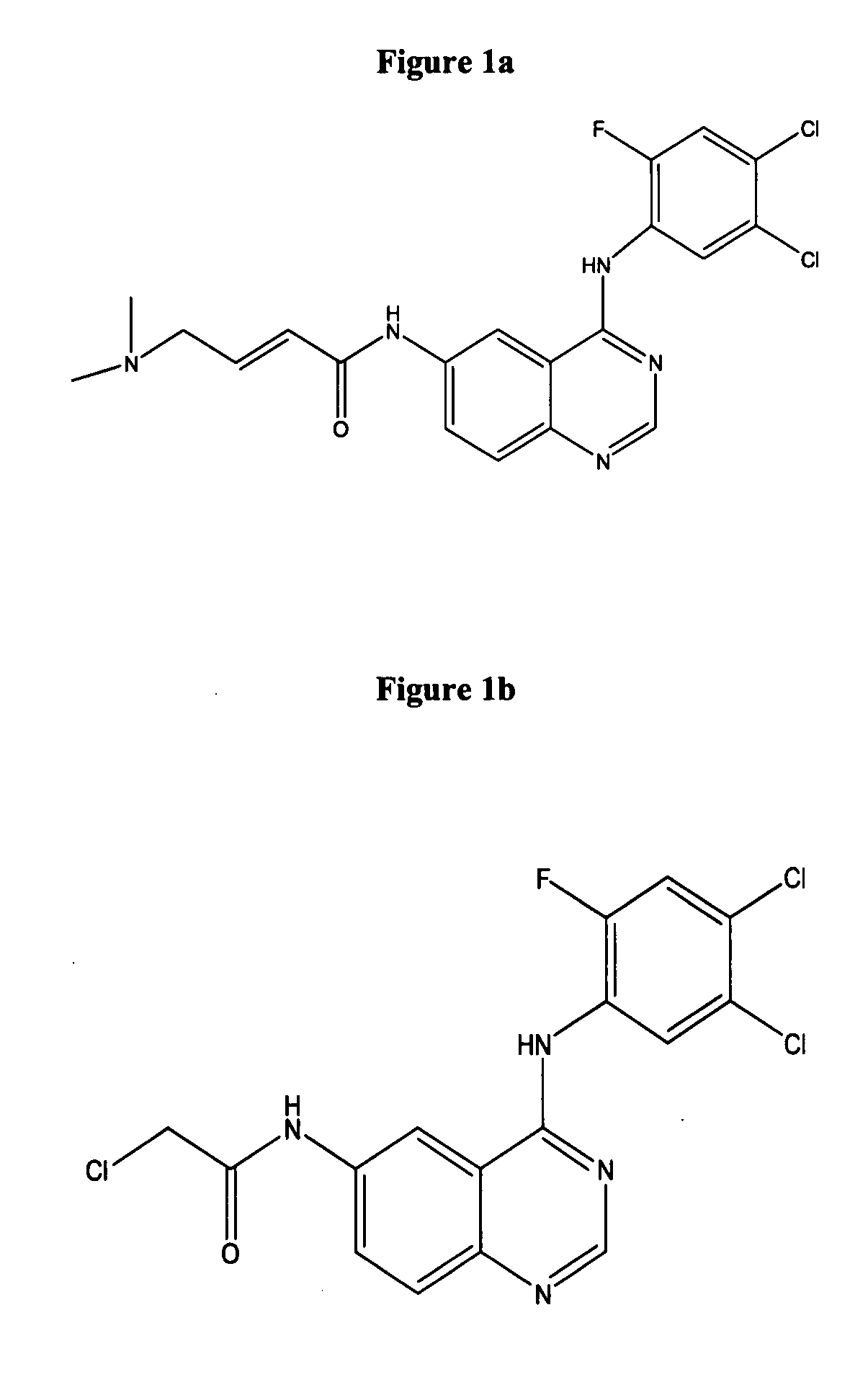
2-arylbenzothiophene derivatives or pharceutically acceptable salts thereof, preparation method thereof, and pharceutical composition for the diagnosis or treatment of degenerative brain disease containing the same as active ingredient
InactiveUS20100261727A1Suppress generationHigh binding affinityBiocideNervous disorderThiophene derivativesBULK ACTIVE INGREDIENT
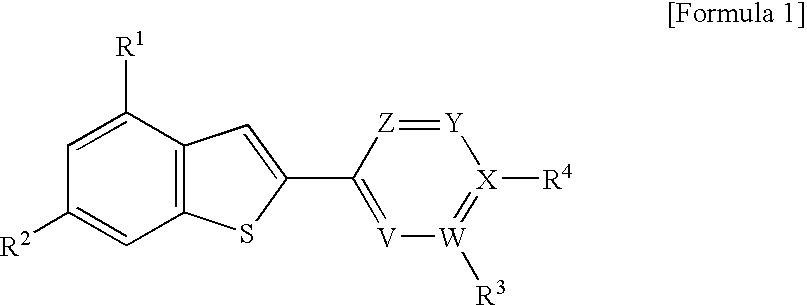
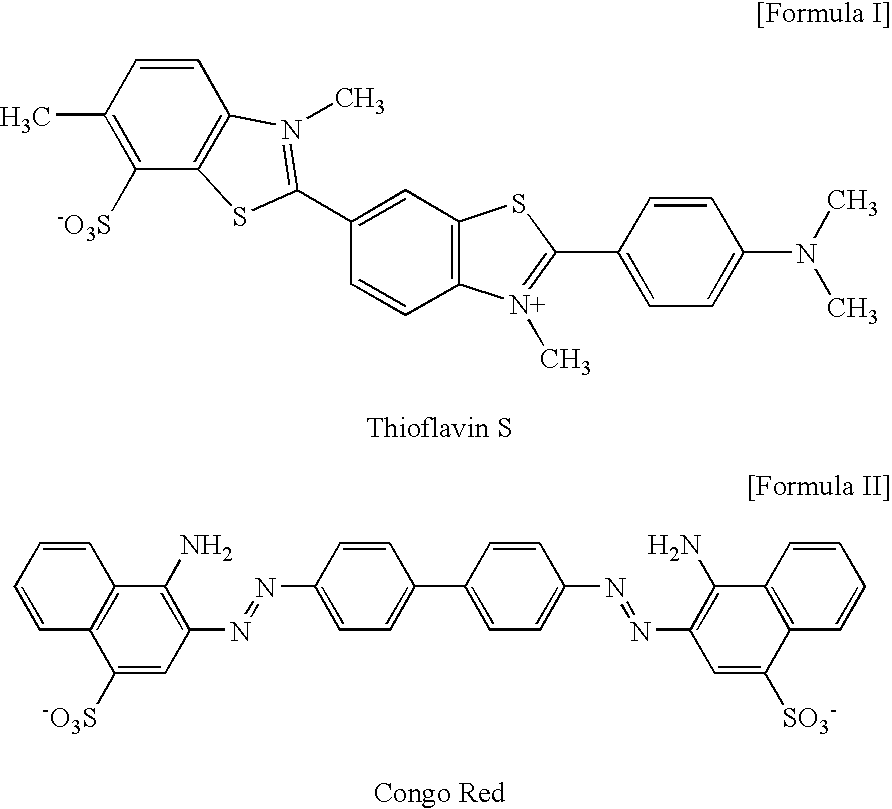
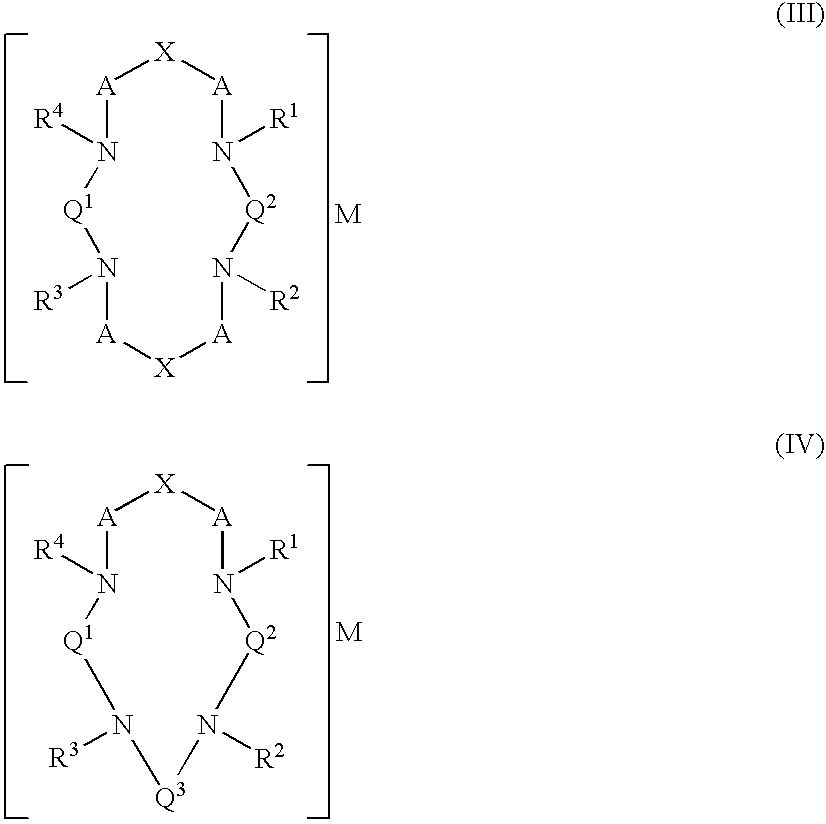
2-arylbenzothiophene derivatives or pharmaceutically acceptable salts thereof, a preparation method thereof, and a pharmaceutical composition for the diagnosis or treatment of degenerative brain disease containing the same as an active ingredient. Since the 2-arylbenzothiophene derivatives of Formula 1 have a relatively high binding affinity for β-amyloid, they can be used as diagnostic reagents for diagnosing Alzheimer's disease at an early stage by non-invasive techniques when they are labeled with radioisotopes:wherein R1-R4, V, W, X, Y and Z are as defined in the Detailed Descript of the specification. Further, when the pharmaceutical composition containing the 2-arylbenzothiophene derivative binds with a low-molecular weight β-amyloid peptide binding compound, generation of malignant high-molecular weight β-amyloid deposits is minimized. Accordingly, the pharmaceutical composition can be used as a therapeutic agent of degenerative brain disease such as Alzheimer's disease.
Owner:IND UNIV COOP FOUND SOGANG UNIV
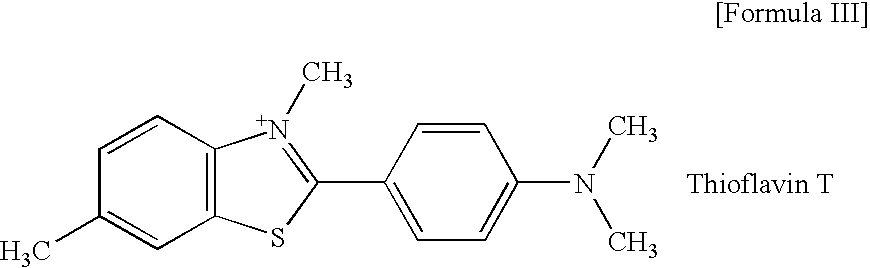
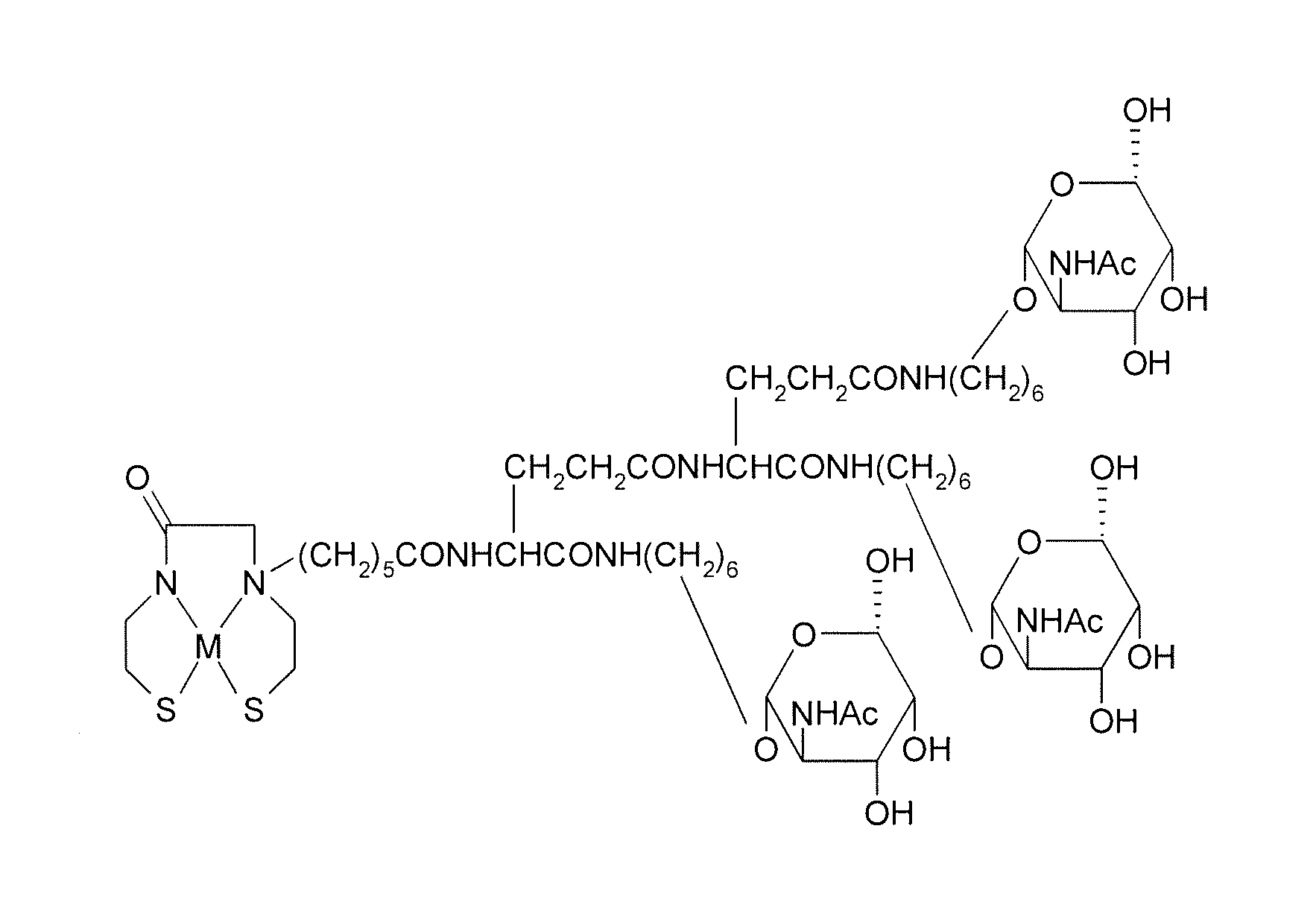
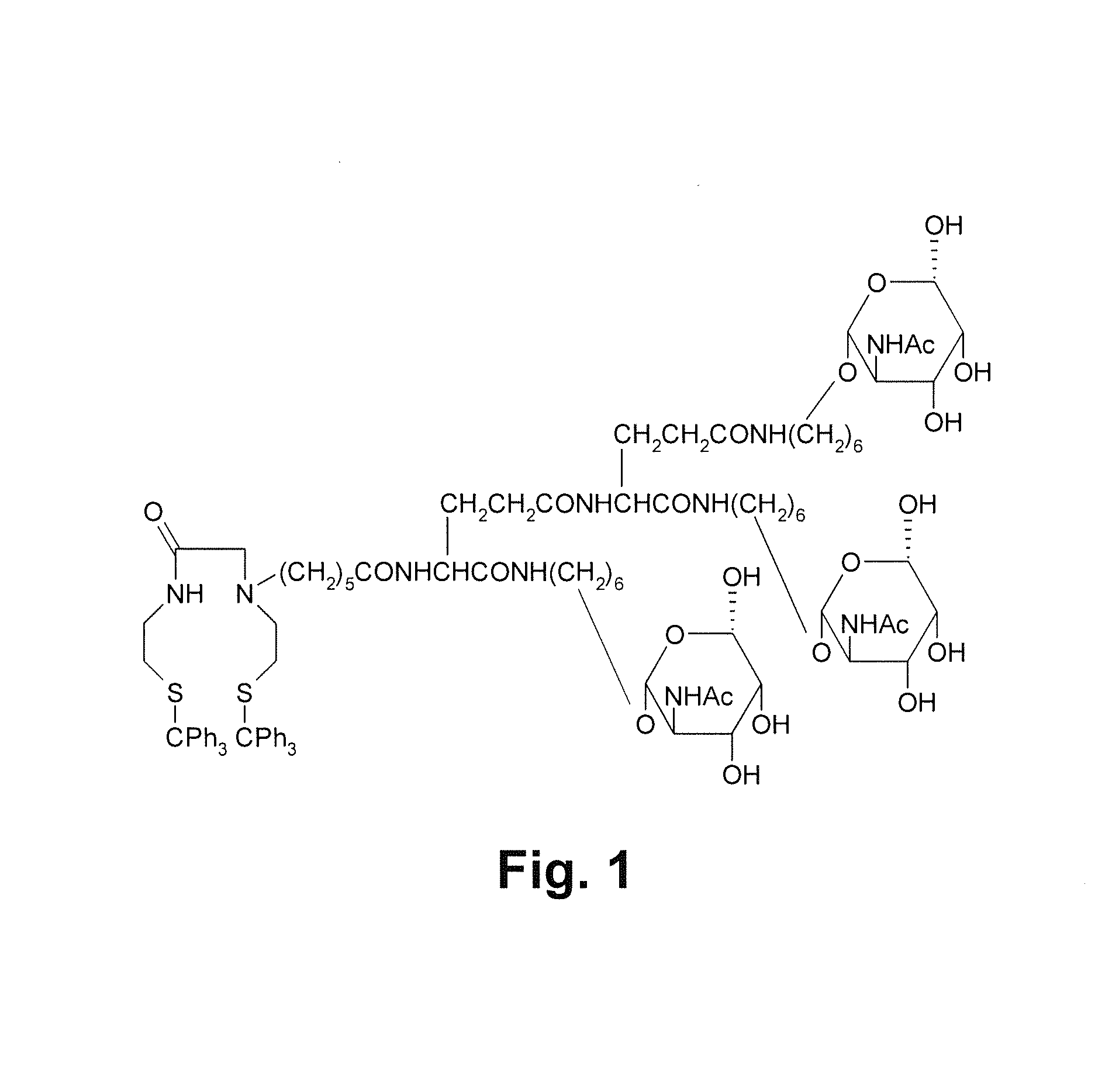
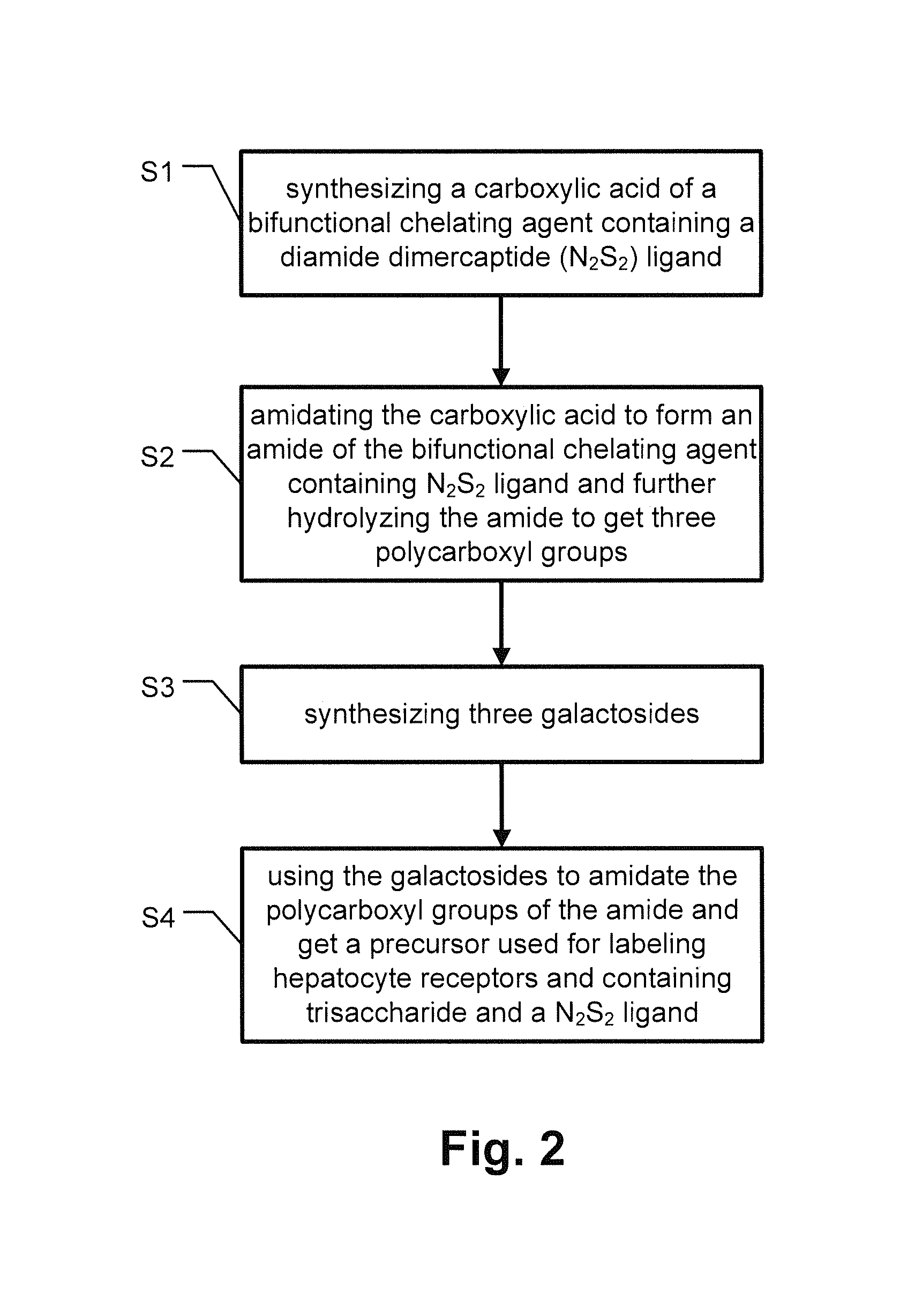
Precursor used for labeling hepatorcyte receptor and containing trisaccharide and diamide demercaptide ligand, method for preparing the same, radiotracer and pharmaceutical composition of the same
InactiveUS20140031533A1Easy to useFacilitated releaseSugar derivativesRadioactive preparation carriersRadioactive tracerThiol
A precursor used for labeling hepatocyte receptors and applied to radiotracers for imaging or pharmaceutical compositions for liver cancers is revealed. The precursor is a bifunctional compound. The bifunctional group includes a trisaccharide structure and a diamide dimercaptide (N2S2) ligand. The trisaccharide has high affinity to asialoglycoprotein receptors (ASGPR) on surfaces of hepatocytes while N2S2 ligand reacts with radioisotopes to form neutral complexes. Thus the precursor stays on surfaces of hepatocytes to provide radioisotope labeling or treatment effect of liver cancers.
Owner:INST NUCLEAR ENERGY RES ROCAEC
Radiolabelling Method Using Polymers
InactiveUS20080305042A1Efficient responseDelayed recoveryRadioactive preparation carriersRadiation therapyImaging agentCombinatorial chemistry
The present invention provides a method for the preparation of radioisotopically-labelled imaging agent compositions. The method uses precursors which are bound to soluble polymers, so that the radiolabelling reaction is carried out in solution. Also described are radiopharmaceutical compositions, automated versions of the radiolabelling method and disposable cassettes for use in the automated method.
Owner:GACEK MICHEL +2
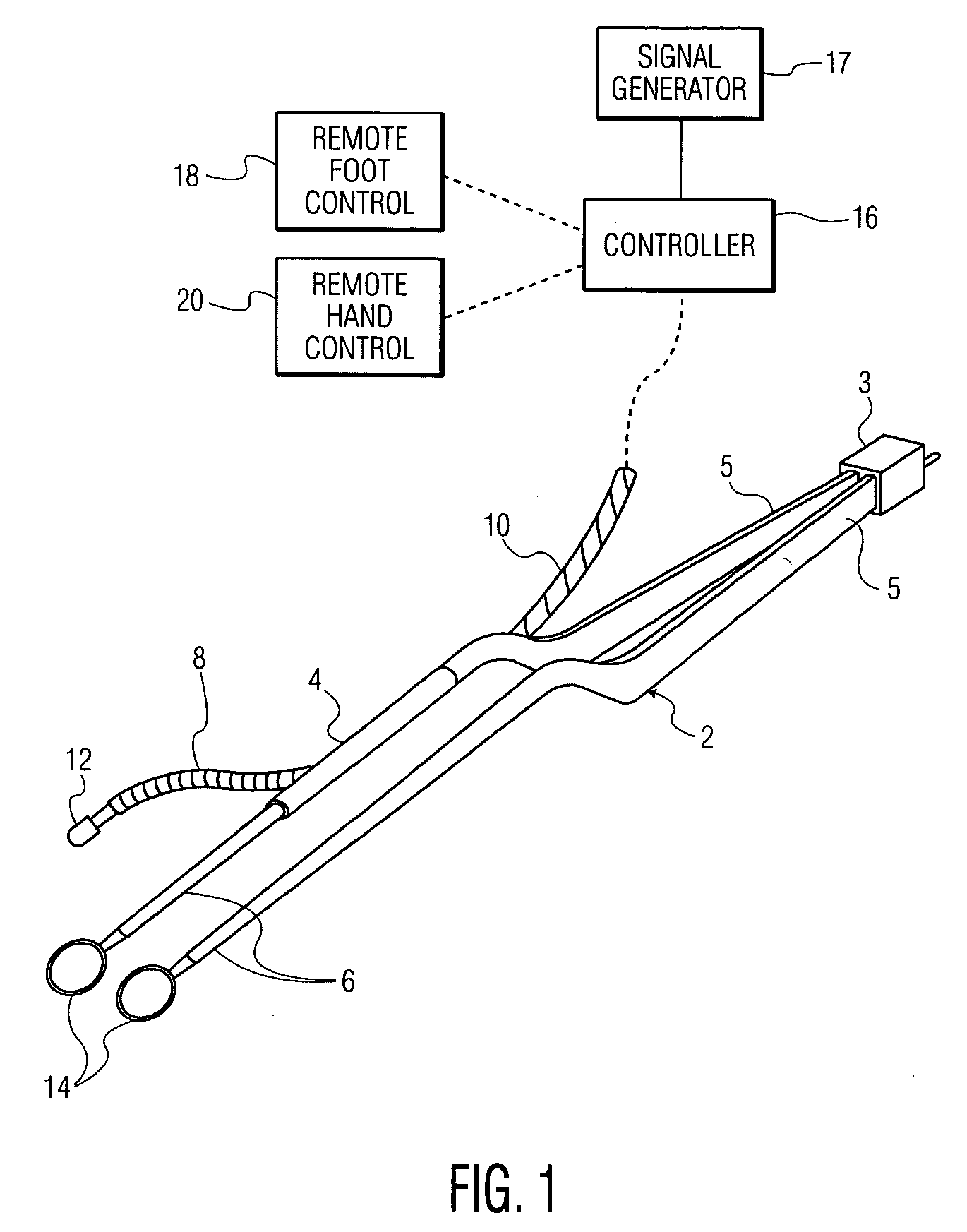
Neurosurgical instrument with gamma ray detector
InactiveUS20050203331A1Rapid positioningIn-vivo radioactive preparationsSurgical instruments for heatingBrain tumorEngineering
A neurosurgical instrument includes a radiation detecting sensor adjustably positioned proximate a distal end of an arm of the instrument. A controller is programmed to receive and process electrical signals from the sensor. The controller operates to control an audio tone generator to emit a tone of higher intensity as the surgeon moves the sensor and its associated coagulator closer to radioactively tagged malignant tissue or a brain tumor of a patient, and of lower intensity as the coagulator moves away from the tissue or tumor, thereby permitting the surgeon to accurately locate and remove the malignant tissue or brain tumor. Also, in place of or in combination with the audio tone generator, a light emitter provides for emitting light of intensity directly related to the distance the sensor is from the malignant tissue or brain tumor tagged with a radioactive isotope.
Owner:NEUROGENESIS
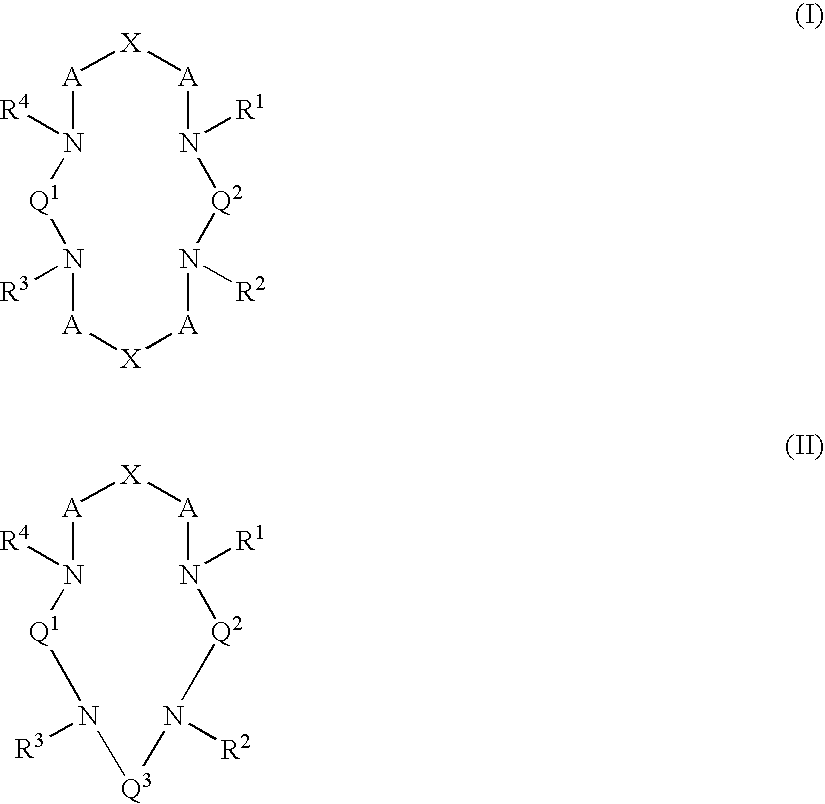
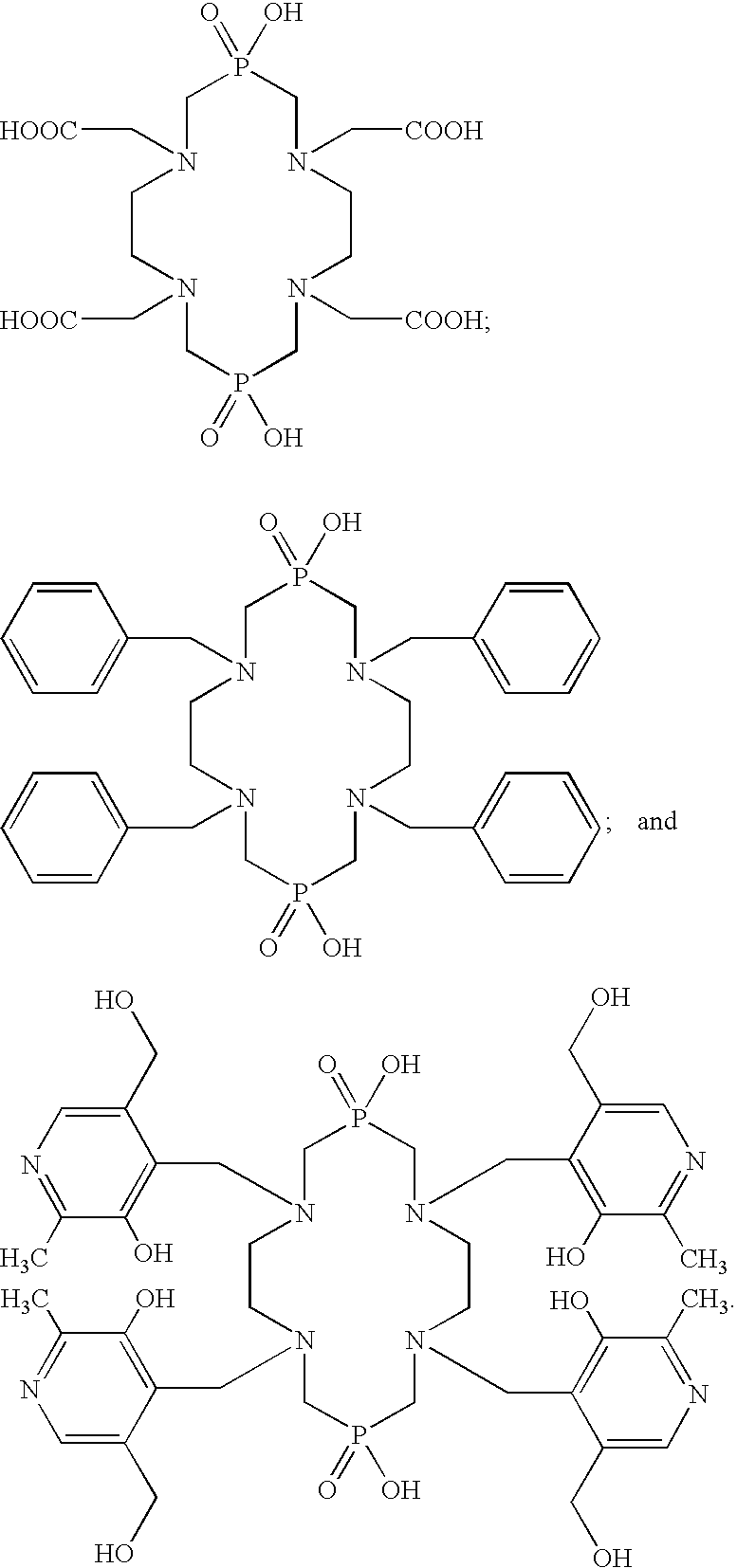
Macrocyclic chelants for metallopharmaceuticals
InactiveUS6916460B2High binding affinityGroup 5/15 element organic compoundsRadioactive preparation carriersMetal chelateHeteroatom
This invention relates to macrocyclic chelants comprised of one or two heteroatom-containing bridges, compositions containing them and their use in medicine, particularly in diagnostic imaging and radiotherapy. This invention relates especially to the use of metal chelates of the macrocyclic chelants as metallopharmaceuticals in Magnetic Resonance Imaging (MRI) and radiopharmaceuticals. This invention also relates to macrocyclic chelants as bifunctional chelating agents (BFC's) for the labeling of biologically active targeting molecules such as proteins, peptides, peptidomimetics, and non-peptide receptor ligands, with metal ions and radioisotopes.
Owner:BRISTOL MYERS SQUIBB PHARMA CO
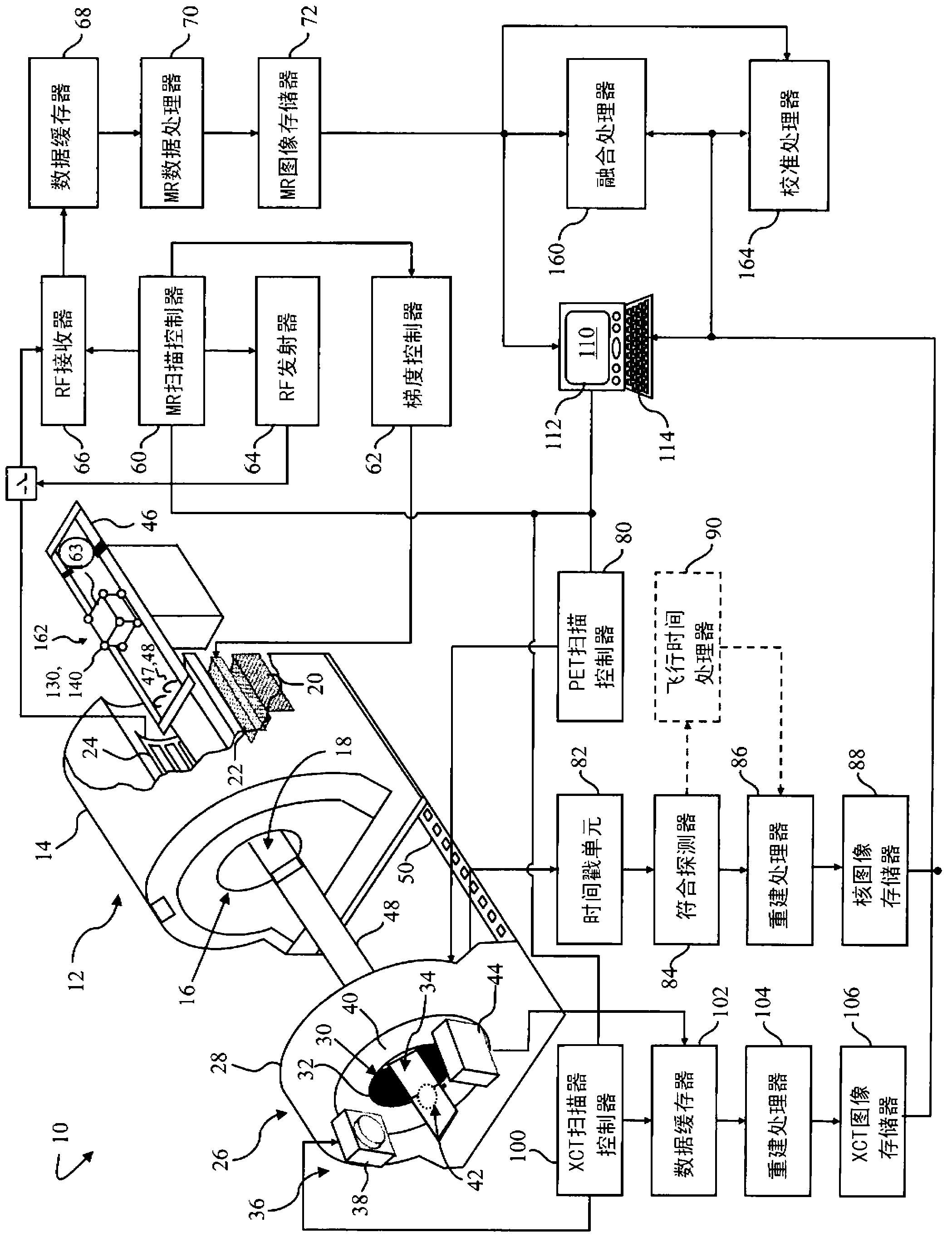
Apparatus for CT-RI and nuclear hybrid imaging, cross calibration, and performance assessment
InactiveCN103260522AReduce registration errorOptimize workflowSurgeryDiagnostic markersCt scannersCt imaging
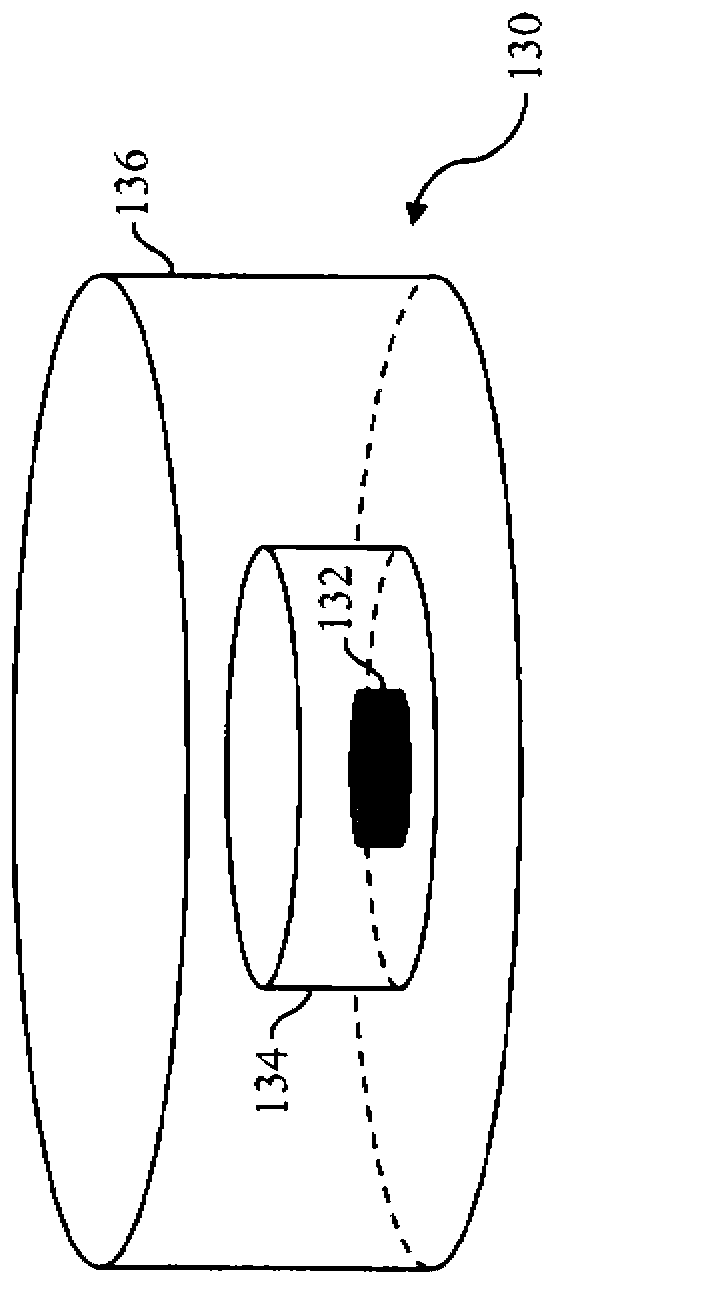
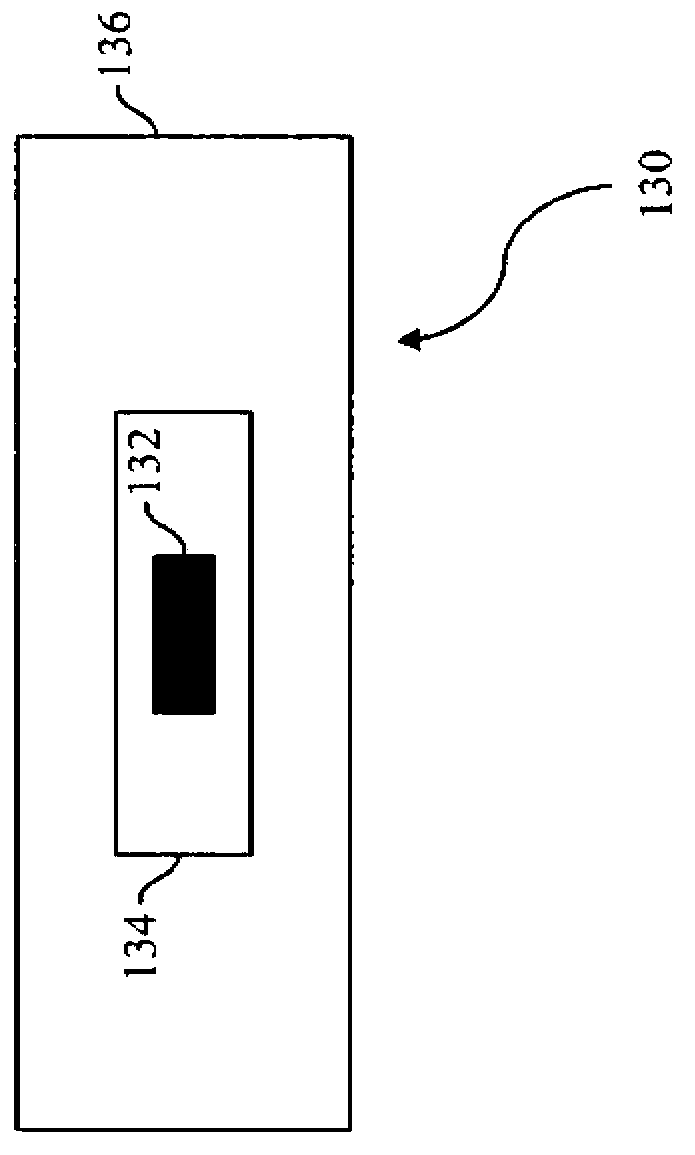
A multiple modality imaging system (10) includes a MR scanner (12) which defines an MR imaging region (18), a nuclear imaging scanner (26) which defines a nuclear imaging region (34), an CT scanner (36) which defines an CT imaging region (42). Each scanner (12, 26, 36) having a longitudinal axis along which a common patient support (46) moves linearly through the MR, nuclear, and CT imaging regions (18, 34, 42). A marker (130, 140, 150), for use with the system (10), includes a radio-isotope marker (132) which is imageable by the nuclear imaging scanner (26) and the CT scanner (36) surrounded by a flexible silicone MR marker (134) which is imageable by the MR scanner (12) and the CT scanner (36). A calibration phantom(162), for use with the image scanner (10), includes a plurality of the markers (130, 140, 150) supported by a common frame having a known and predictable geometry.
Owner:KONINKLIJKE PHILIPS ELECTRONICS NV
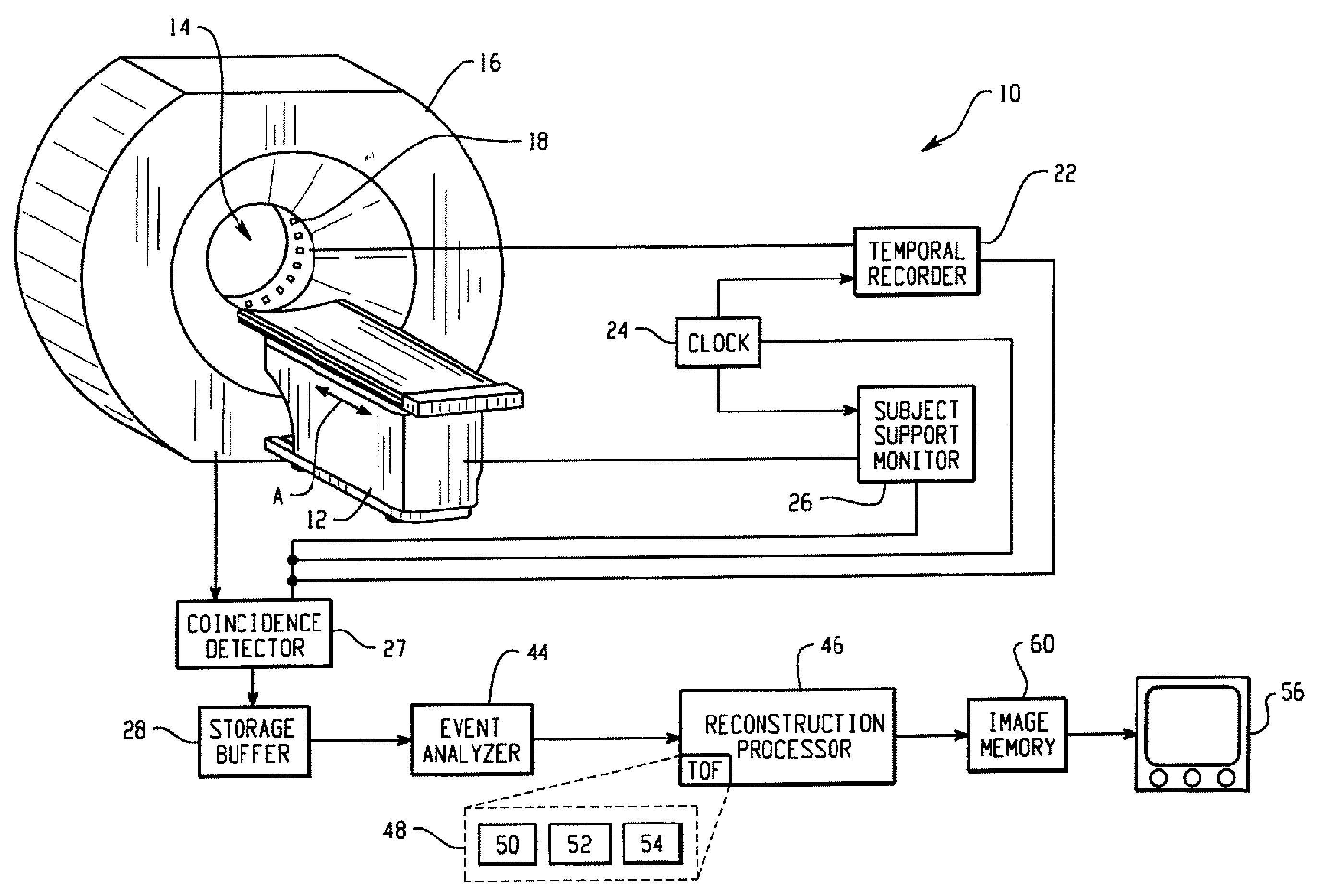
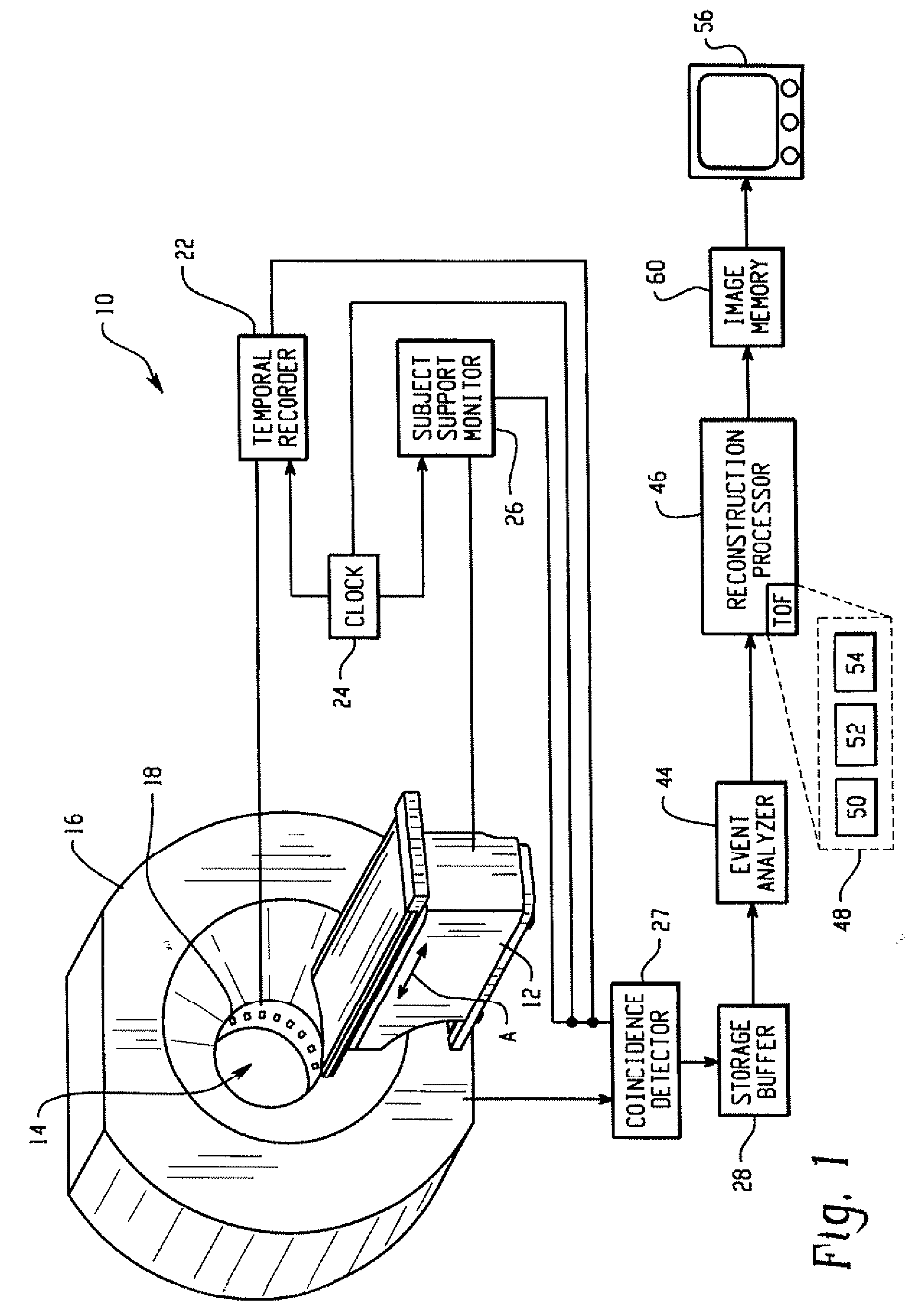
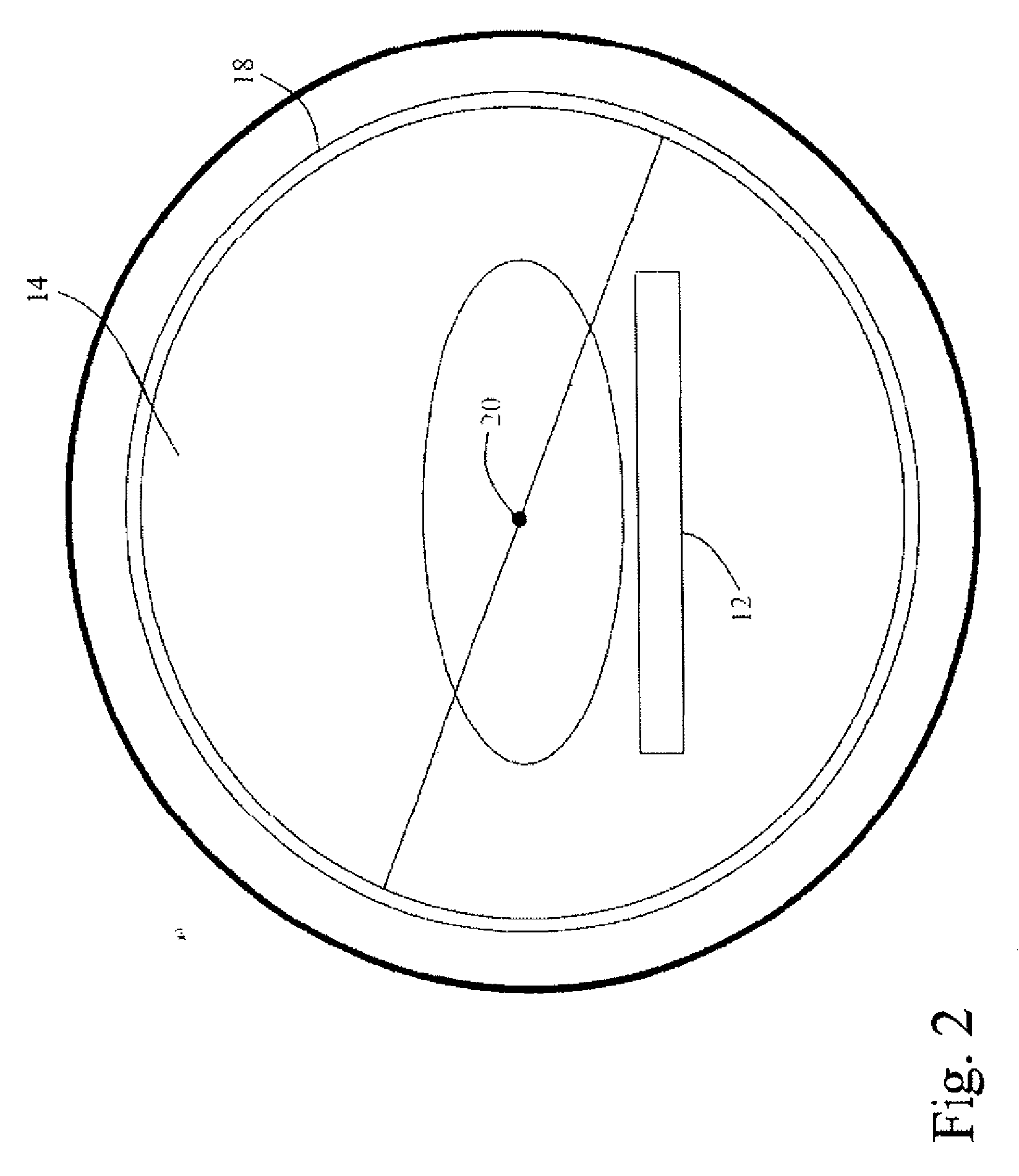
Real-time list mode reconstruction
In positron emission tomography, a nuclear medicine scanner is utilized to detect γ-ray events resulting from positron annihilation events. Molecules with known behaviors are tagged with radioactive isotopes which decay into γ-ray pairs which are detected coincidentally, i.e. in a near-simultaneous fashion, by radiation detectors. A temporal recorder and a subject support monitor indicate the time and position of the subject when the coincident γ-rays were detected. A storage buffer collects γ-ray detection times and locations along with support positions. Every 1 / 100th- 1 / 10th second, a batch of data collected in the buffer is reconstructed into overlapping portions of an image memory as the support moves continuously through the scanner.
Owner:KONINKLIJKE PHILIPS ELECTRONICS NV
Polyalkylene glycol derivatives of inhibitors of epidermal growth factor receptor tyrosine kinase
InactiveUS20080056990A1Improve solubilityImprove bioavailabilityUltrasonic/sonic/infrasonic diagnosticsBiocideDiseasePositron emission tomography
Novel epidermal growth factor receptor tyrosine kinase (EGFR-TK) inhibitors, pharmaceutical compositions including same and their use in the treatment of EGFR-TK related diseases or disorders are disclosed. Novel radiolabeled EGFR-TK inhibitors as their use as biomarkers for medicinal radioimaging such as Positron Emission Tomography (PET) and Single Photon Emission Computed Tomography (SPECT) and as radiopharmaceuticals for radiotherapy are further disclosed. The disclosed EGFR-TK inhibitors comprise a polyalkylene glycol moiety and / or a hydroxy-containing moiety and are characterized by improved solubility, biostability and bioavailability. Processes of preparing the disclosed EGFR-TK inhibitors and of radiolabeling same, via, for example, one-step radiosyntheses, are also disclosed.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD +1
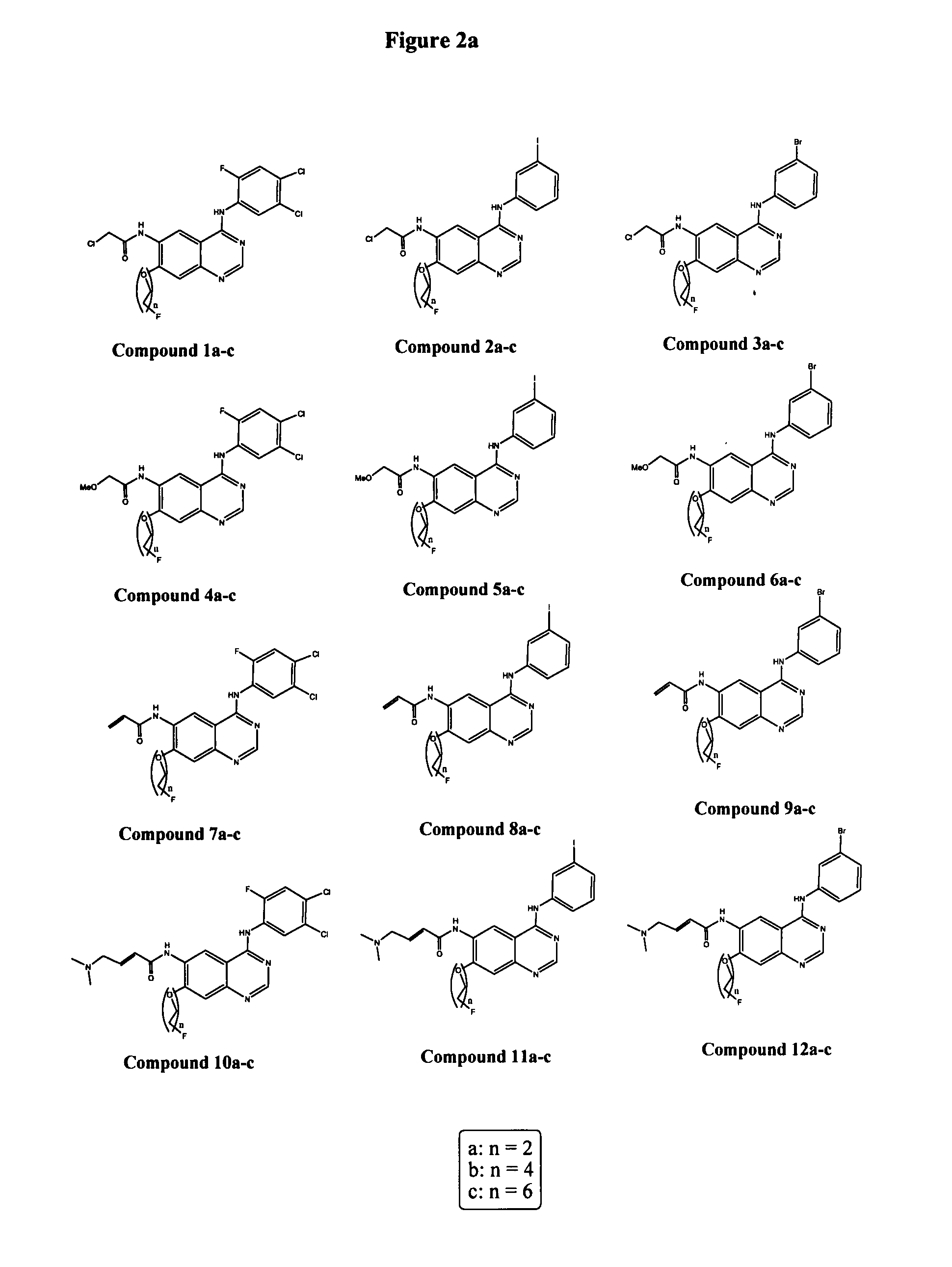
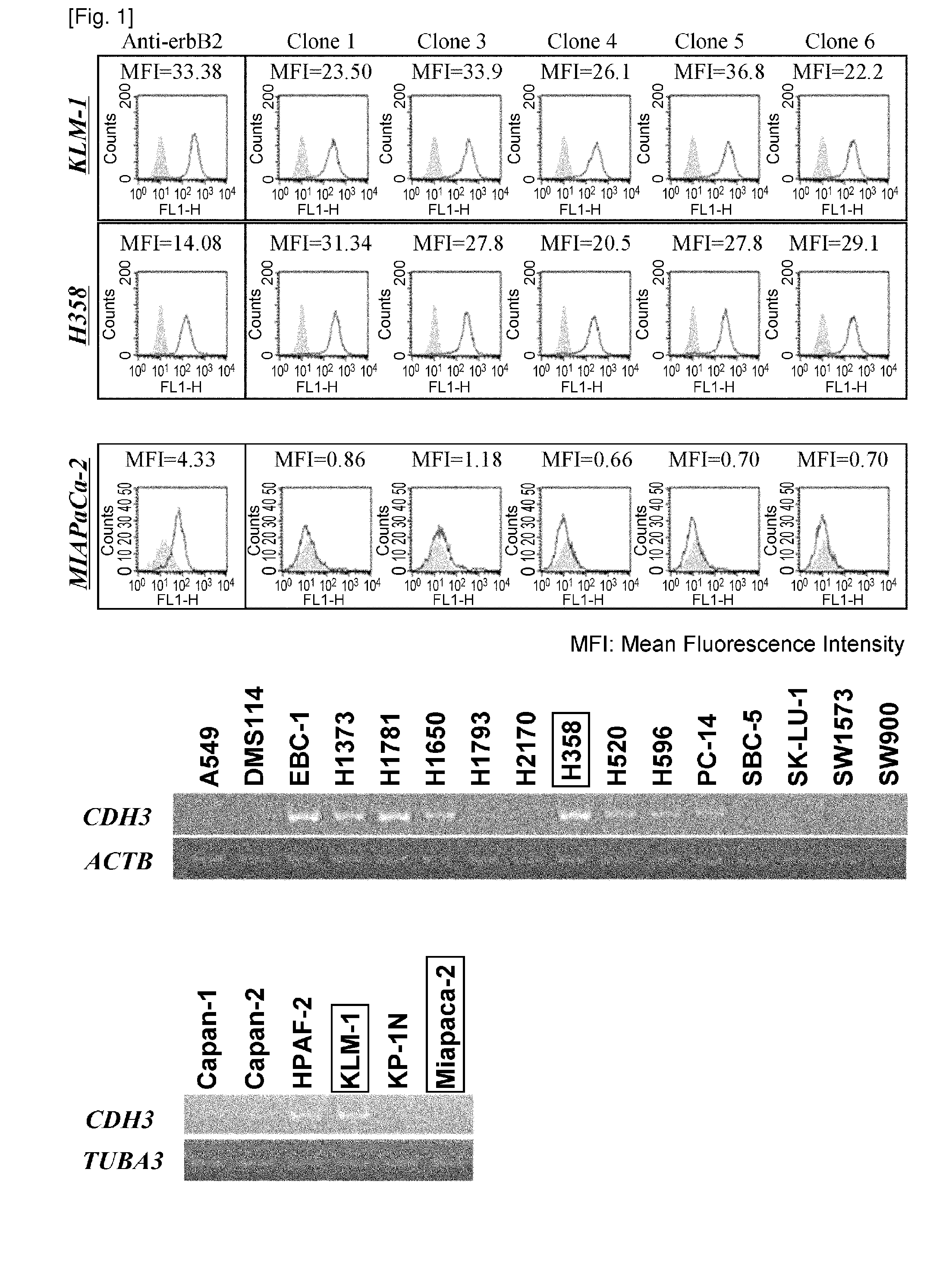
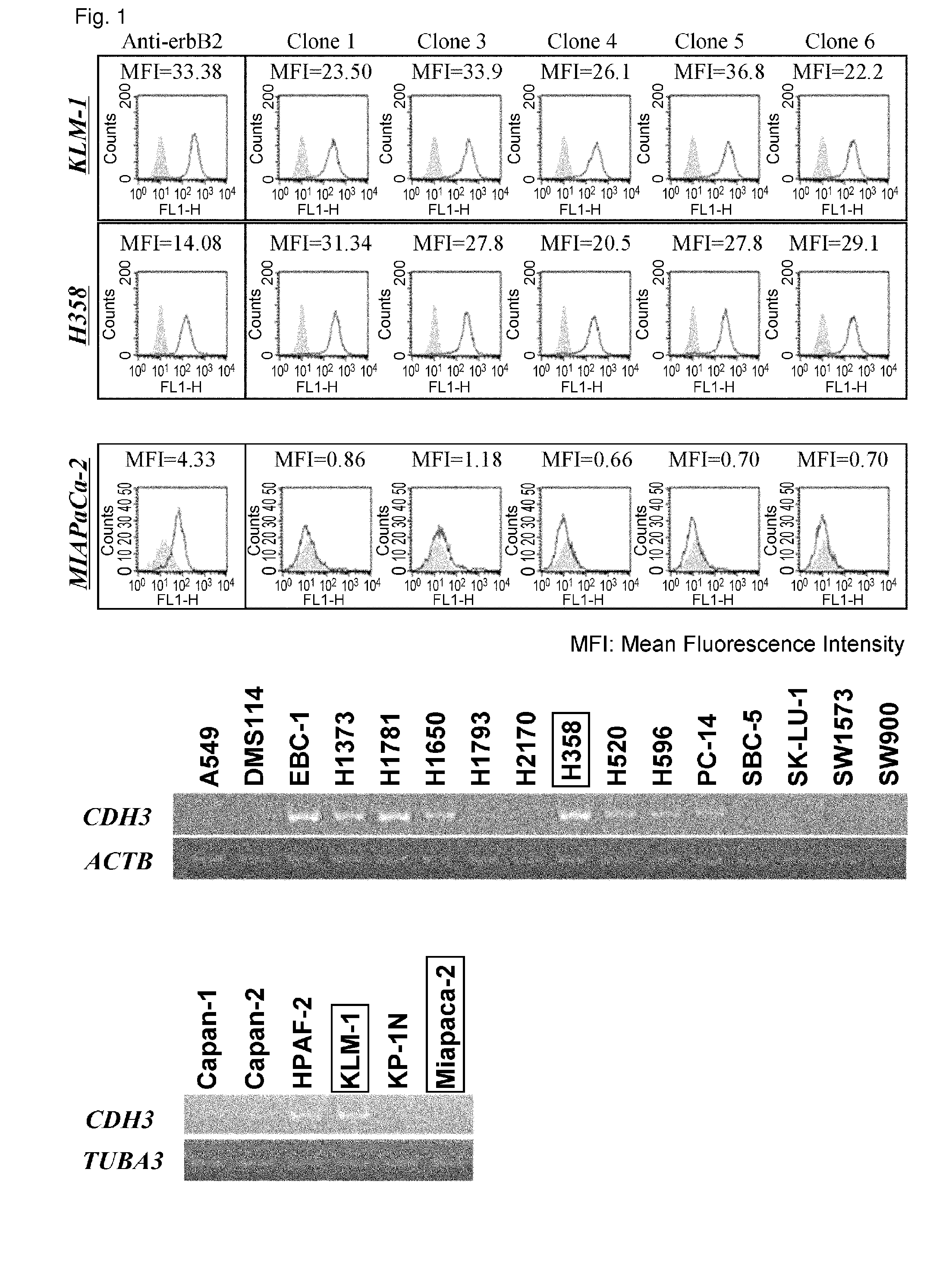
Anti-cdh3 antibodies labeled with radioisotope label and uses thereof
ActiveUS20120128584A1Remarkable effectBiological material analysisRadioactive preparation carriersProstate cancerActive ingredient
The present invention relates to anti-CDH3 antibodies, which can be labeled with a radioisotope. Moreover, the present invention provides methods and pharmaceutical compositions that comprise an anti-CDH3 antibody as an active ingredient. Since CDH3 is strongly expressed in pancreatic, lung, colon, prostate, breast, gastric or liver cancer cells, the present invention is useful in pancreatic, lung, colon, prostate, breast, gastric or liver cancer therapies.
Owner:ONCOTHERAPY SCI INC
Method for labeling a prostate-specific membrane antigen ligand with a radioactive isotope
InactiveUS20160256579A1Fast and simple and safe mannerFast and simple and safeRadioactive preparation carriersGroup 3/13 element organic compoundsAntigenProstate specific membrane
A method for labeling a prostate-specific membrane antigen (PSMA) ligand with a radioactive isotope, such as 68Ga, 177Lu, or 90Y, includes providing a reaction vial containing a sodium-based buffering agent and the PSMA ligand, both in dried form; adding a solution of the radioactive isotope in HCl to the reaction vial, thus obtaining a solution of the PSMA ligand and the radioactive isotope in the HCl; and mixing the solution and then incubating it for a sufficient period of time, thus reacting the PSMA ligand with the radioactive isotope to thereby obtain the PSMA ligand labeled with the radioactive isotope, At least 90% of the radioactive isotope in the solution is bound to the PSMA ligand. A kit can be used for carrying out the method. Radiolabeled PSMA ligands prepared by this method can be used for both imaging and therapy.
Owner:ISOTOPIA MOLECULAR IMAGING
Heterocyclic indene derivatives and their radioisotope labeled compounds for imaging beta-amyloid deposition
InactiveUS20080299041A1Easy to radiolabelHigh binding affinityNervous disorderOrganic chemistryCombinatorial chemistryIsotope
The invention is directed to heterocyclic indene derivatives useful for β-amyloid plaque imaging, their radiolabeled compounds and their preparation methods. The compounds of the invention are easily labeled with radioisotopes and have high affinities to β-amyloid depositions, thus they facilitate diagnosis of Alzheimer's disease by imaging the distribution of β-amyloid depositions.
Owner:SEOUL NAT UNIV R&DB FOUND
Fluorescent silica nanoparticle with radioactive tag and the detecting method of pet and fluorescent dual imaging using thereof
InactiveUS20110262351A1Powder deliveryGroup 4/14 element organic compoundsDual imagingRadioactive Tag
The present invention relates to nuclear medicine using fluorescent silica nanoparticle and detecting method of optical dual imaging, and more particularly to radioisotope labeled fluorescent silica nanoparticles which are used for PET (positron emission tomography) and fluorescence detecting, and detecting method of PET and fluorescent dual imaging using thereof. Functionalized silica nanoparticles of this invention have promising potential as a role for organic lymphatic tracer in biomedical imaging such as pre- and intra-operative surgical guidance.
Owner:SEOUL NAT UNIV R&DB FOUND +1
Method for highly sensitive hybridization of nucleic acids, and method for gene analysis using the same
A method for hybridizing nucleic acids, which includes an annealing step of preparing a first single stranded nucleic acid fragment immobilized on a surface of an immobilizing material and a second single stranded nucleic acid fragment labeled with fluorescence or radioisotope and forming a complementary double strand from the first single stranded nucleic acid fragment and the second single stranded nucleic acid fragment, and an enzyme treatment step. In the annealing step, the complementary double strand is formed by performing a temperature gradient processing performed from a high temperature area to a low temperature area, and in the enzyme treatment step, a noncomplementary nucleic acid portion contained in the complementary double stand is recognized and cleaved by adding endonuclease. This method has high measurement sensitivity and the operation is simple.
Owner:NGK INSULATORS LTD
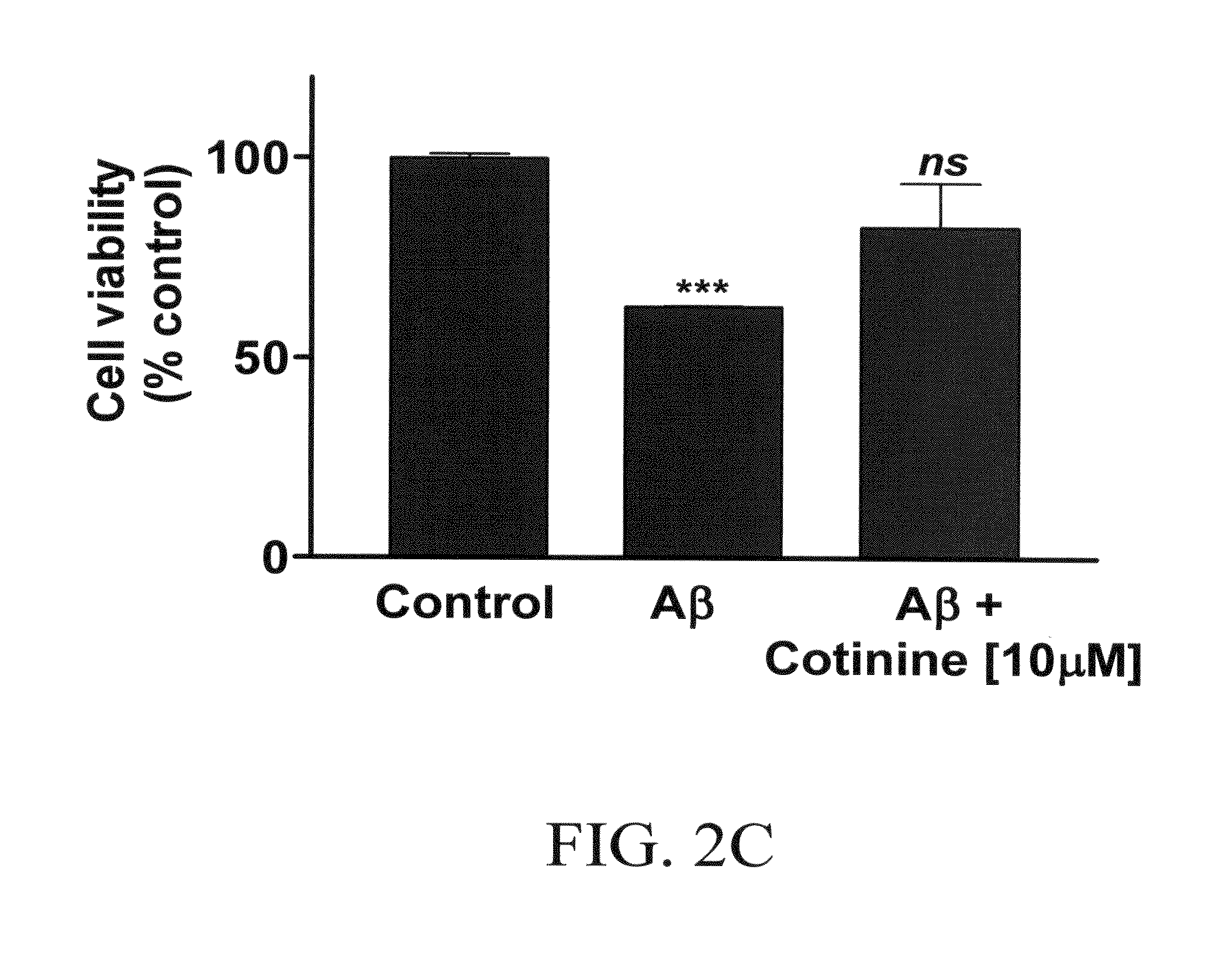
Materials and methods for diagnosis, prevention and/or treatment of stress disorders and conditions associated with abeta peptide aggregation
The subject invention concerns materials and methods for treating and / or preventing diseases associated with accumulation of Aβ peptide in neural tissue. The subject invention also concerns materials and methods for treating and / or preventing stress disorders, such as post-traumatic stress disorder (PTSD). In one embodiment, a method of the invention comprises administering a therapeutically effective amount of cotinine, or a pharmaceutically acceptable salt thereof, to a person or animal in need of treatment. The methods of the invention can be used to prevent and / or treat Alzheimer's disease, Parkinson's disease, and / or Down's syndrome. The subject invention also concerns compositions that comprise cotinine, or a pharmaceutically acceptable salt thereof, and a pharmaceutically acceptable carrier, diluent or adjuvant.The subject invention concerns materials and methods for detecting and diagnosing conditions associated with accumulation of Aβ peptide in neural tissue, such as Alzheimer's disease and Parkinson's disease, using the chemical cotinine. In one embodiment, the method comprises administering cotinine labeled with a detectable label to a person or animal. The presence of labeled cotinine in neural tissue is then determined. The level and / or location of cotinine can be analyzed and a diagnosis made. The subject invention also concerns cotinine labeled with a detectable label. In one embodiment, the cotinine is labeled with a radioisotope that can be detected by Positron Emission Tomography (PET) or single photon emission computed tomography (SPECT).
Owner:UNIV OF SOUTH FLORIDA +1
Method for production of radioisotope preparations and their use in life science, research, medical application and industry
The present invention relates to an universal method for the large scale production of high-purity carrier free or non carrier added radioisotopes by applying a number of “unit operations” which are derived from physics and material science and hitherto not used for isotope production. A required number of said unit operations is combined, selected and optimized individually for each radioisotope production scheme. The use of said unit operations allows a batch wise operation or a fully automated continuous production scheme. The radioisotopes produced by the inventive method are especially suitable for producing radioisotope-labelled bioconjugates as well as particles, in particular nanoparticles and microparticles.
Owner:EUROPEAN ORGANIZATION FOR NUCLEAR RESEARCH +1
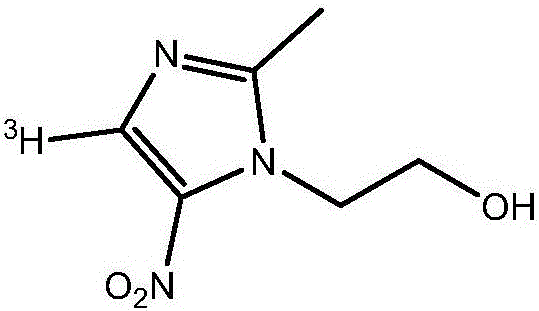
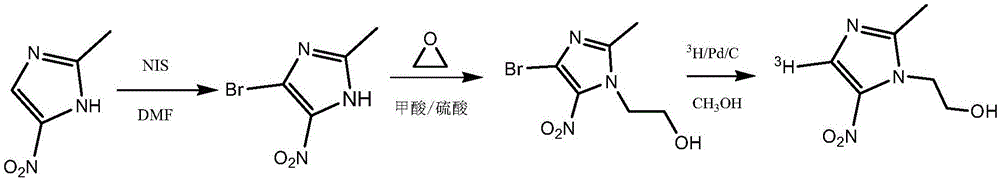
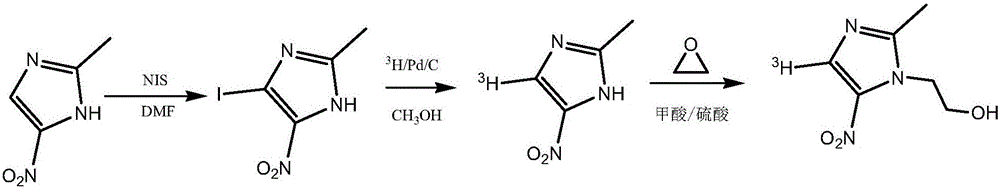
Tritiated metronidazole and preparation method thereof
InactiveCN106674123ARaw materials are easy to getReduce pollutionOrganic chemistry methodsNitroimidazoleRadioactive tracer
The invention belongs to the field of radioactive isotope labeling preparation, and particularly relates to tritiated metronidazole and a preparation method thereof. The preparation method includes: using 2-methyl-5-nitroimidazole as a raw material to react with N-iodosuccinimide to obtain 4-iodine-2-methyl-5nitroimidazole; under catalysis of palladium carbon, enabling 4-iodine-2-methyl-5nitroimidazole and tritium gas to be in tritium-halogen exchange to generate 4-3H-2-methyl-5-nitroimidazole; enabling 4-3H-2-methyl-5-nitroimidazole to react with ethylene oxide to obtain 4-3H-metronidazole. A synthetic product is purified through a prepared liquid phase to obtain4-3H-metronidazole with high specific activity (22.08Ci / g), high radiochemical purity (greater than or equal to 98%) and high chemical purity (greater than or equal to 98%). The tritiated metronidazole can be used as a radioactive tracer in studying absorption, distribution, metabolism and residue elimination of metronidazole in animal bodies.
Owner:HUAZHONG AGRI UNIV
Anti-CDH3 antibodies labeled with radioisotope label and uses thereof
ActiveUS8435749B2In-vivo radioactive preparationsBiological material analysisProstate cancerBULK ACTIVE INGREDIENT
The present invention relates to anti-CDH3 antibodies, which can be labeled with a radioisotope. Moreover, the present invention provides methods and pharmaceutical compositions that comprise an anti-CDH3 antibody as an active ingredient. Since CDH3 is strongly expressed in pancreatic, lung, colon, prostate, breast, gastric or liver cancer cells, the present invention is useful in pancreatic, lung, colon, prostate, breast, gastric or liver cancer therapies.
Owner:ONCOTHERAPY SCI INC
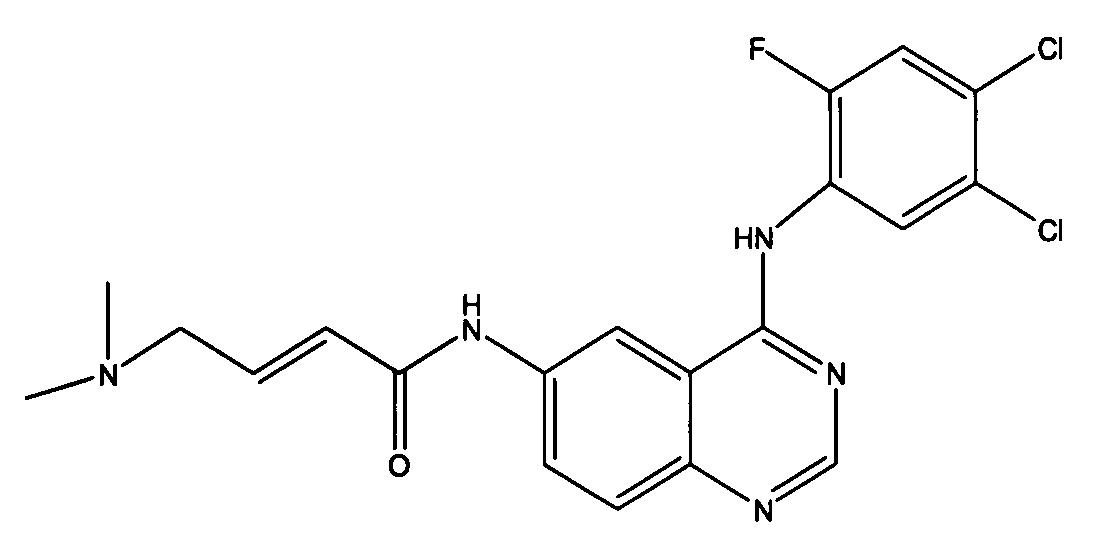
Application of radiolabelled monoanthracene nucleus anthraquinone compound in preparation of medicine used for detecting myocardial viability
The invention relates to the field of medicines, in particular relates to the application field of monoanthracene nucleus anthraquinone compounds and in particular relates to application of a radiolabelled monoanthracene nucleus anthraquinone compound in preparation of a medicine used for detecting myocardial viability. The radiolabelled monoanthracene nucleus anthraquinone compound has a specific binding force on myocardial necrotic cells, and a radiation source produced by a radioactive isotope is combined with a detector, thus the aim of effectively detecting the myocardial necrotic cells is achieved.
Owner:JIANGSU PROVINCE INST OF TRADITIONAL CHINESE MEDICINE
Radioisotope labeled polypeptide developer of targeted transferrin receptor and application of radioisotope labeled polypeptide developer
PendingCN112043839AAdaptabilityAdapt to needsPeptidesRadioactive preparation carriersImaging agentTumor specific
The invention discloses a radioisotope labeled polypeptide developer of a targeted transferrin receptor and application of the radioisotope labeled polypeptide developer. The invention particularly discloses a 68Ga chelation reaction labeling method of two polypeptide precursors and application of the two polypeptide precursors as an imaging agent in positron emission tomography (PET) of a transferrin receptor overexpressed tumor. The radioisotope labeled product has radiochemical purity of more than 95% and good in-vivo stability, can be rapidly aggregated within 5 minutes at a tumor site, has obvious tumor muscle contrast, can realize clear imaging of tumor tissues and margins, and has a certain difference between pharmacokinetics and biodistribution due to structural modification of different linking groups, so that the compound can be used as a tumor-specific imaging agent of a targeted transferrin receptor.
Owner:SUN YAT SEN UNIV CANCER CENT
Radioisotope labeled meso-porous bioglass porous scaffold and making method thereof
InactiveCN104117090AMarked validDetection of in vivo degradationProsthesisIn vivo absorptionIn vivo degradation
The invention provides a radioisotope labeled meso-porous bioglass porous scaffold. A meso-porous bioglass porous scaffold is labeled with radioisotope, and the radioisotope can be one or more of <31>Si, <32>P and <45>Ca. The radioisotope labeled meso-porous bioglass porous scaffold can effectively label the meso-porous bioglass porous scaffold, can quantitatively and intuitively detect the in vivo degradation situation of the meso-porous bioglass porous scaffold, and can be used to research the in vivo absorption and the organism distribution of the bioglass scaffold.
Owner:SHANGHAI NINTH PEOPLES HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
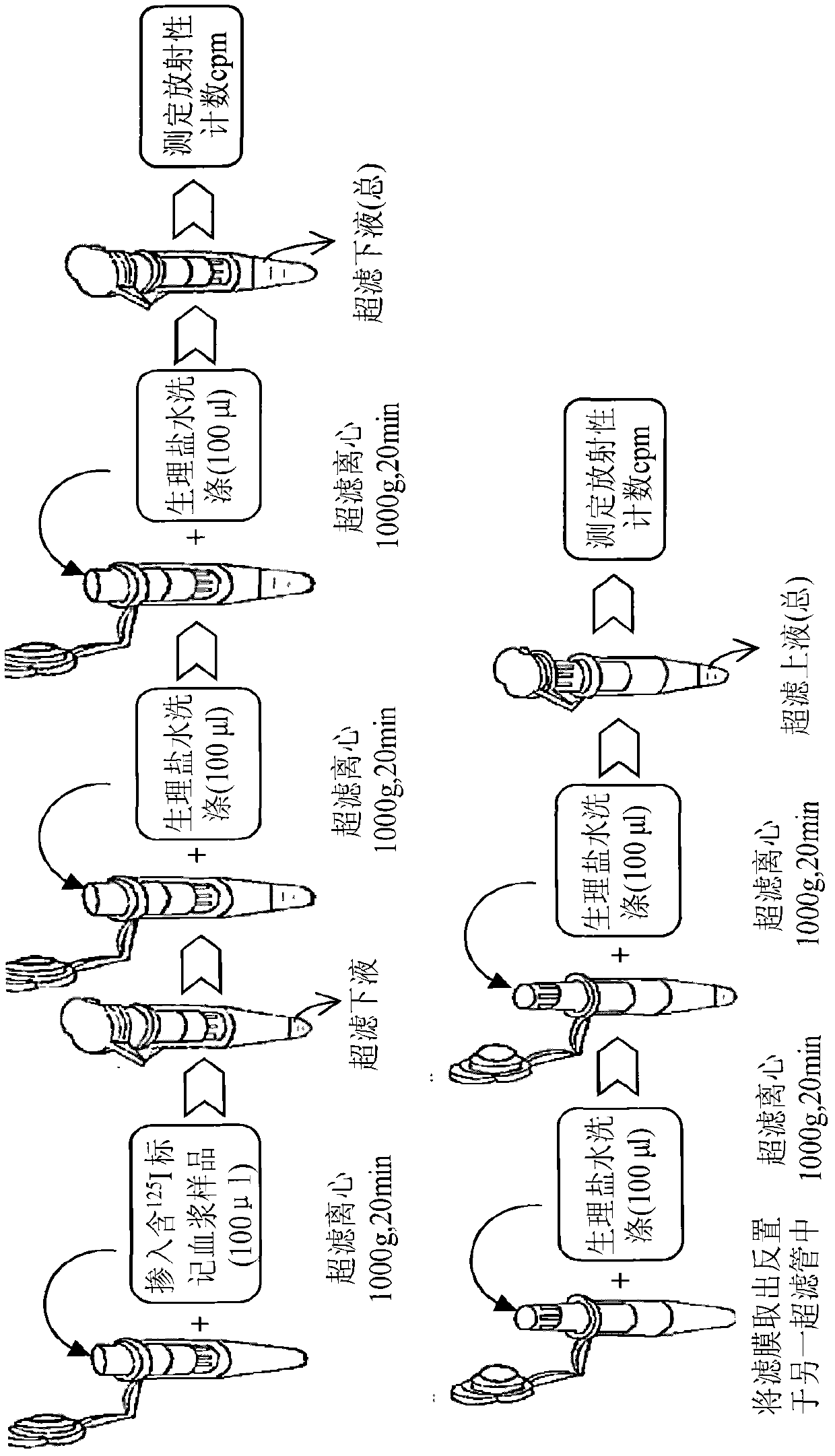
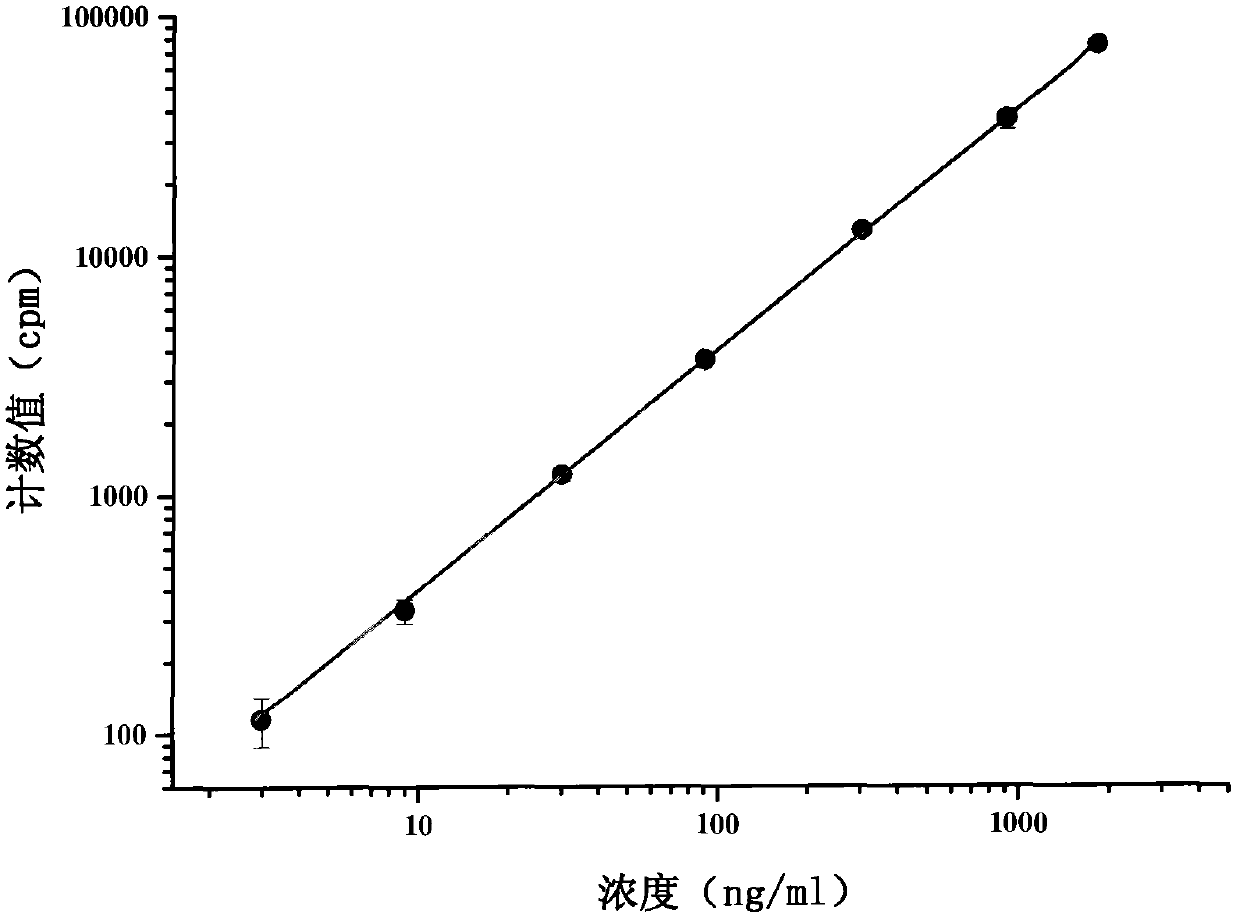
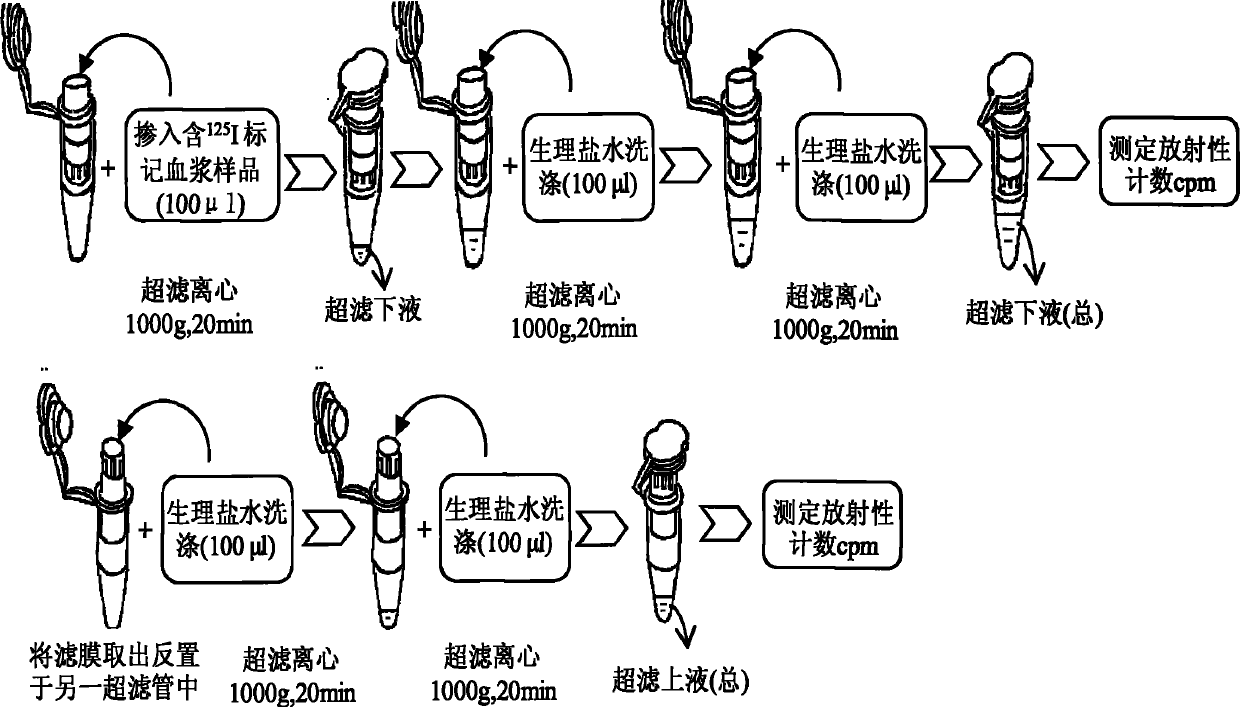
Method for detecting combination of polypeptide medicine and plasma proteins
ActiveCN102565414AImprove adsorption capacityReduce adsorptionBiological testingUltrafiltrationHigh doses
The invention provides a method for detecting the combination of polypeptide medicine and plasma proteins. The method comprises the following steps: marking part of the polypeptide medicine through radioactive isotopes; preparing part of the marked polypeptide medicine into a series of polypeptide medicine plasma solutions with known concentrations, quantificationally detecting the radioactivity of the polypeptide medicine plasma solutions with the known concentrations respectively, and drawing standard curves corresponding to the concentrations of the polypeptide medicine plasma solutions respectively; and preparing part of the marked polypeptide medicine into to-be-detected polypeptide medicine plasma solutions with known concentrations, performing centrifugal ultrafiltration on the to-be-detected polypeptide medicine plasma solutions, and quantificationally detecting the radioactivity of filtered solutions and unfiltered solutions, wherein the concentrations of the to-be-detected polypeptide medicine plasma solutions can be set according to plasma concentration peak values after the administration of a low dose group, a medium dose group and a high dose group in a polypeptide medicine pharmacokinetics research, so as to set the three concentrations which are approximately consistent with peak concentrations or are different from the peak concentrations with the variation amplitudes less than 10 percent.
Owner:天津天诚新药评价有限公司 +1
Support and method for cell based assays
InactiveUS7101719B2Layered productsMicrobiological testing/measurementMeasurement testCellular process
Disclosed is a method for the measurement of a cellular process, or for the measurement of the effect of a test compound on a cellular process, in one or more different populations of cells. The method comprises providing separate samples of one or more different populations of cells adhering to support particles, the support particles comprising a scintillant substance and being adapted for cell growth. In one embodiment, different samples of cells are introduced into separate reaction vessels in a fluid medium, together with a reagent labelled with a radioisotope, in the presence or the absence of the test compound, under conditions so as to cause a portion of said radiolabelled reagent to become associated with the cells. In another embodiment, multiparameter analysis may be performed to determine the effect of a test compound on a cellular process using two or more different cell populations present in the same well. Measurement of the cellular process, or the effect of a test compound on a cellular process may be made by detecting light emission from the scintillant particles caused by radioactive decay of the radioisotope.
Owner:GE HEALTHCARE LTD
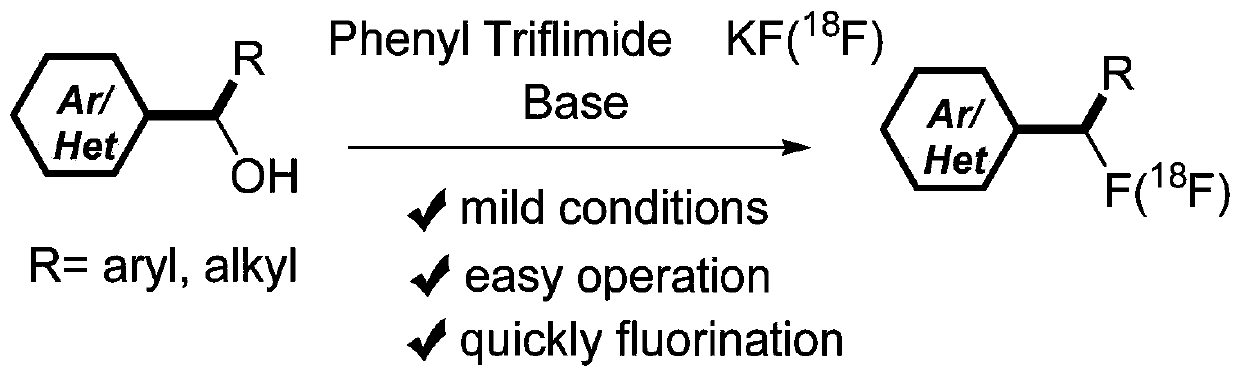
Alcohol compound-based in-situ deoxygenation fluorination synthesis method and alcohol compound-based <18>F radiolabeling method
PendingCN110724026ASimple and fast operationSimple processSugar derivativesCarboxylic acid nitrile preparationOrganic basePotassium fluoride
The invention discloses an alcohol compound-based in-situ deoxidation fluorination synthesis method and an alcohol compound-based <18>F radiolabeling method. Alkyl alcohols such as 4-phenyl-1-butanol,which are used as raw materials, react with trifluoromethylsulfonyl fluoride gas, generated by N-phenylbis(trifluoromethanesulphonimide) and potassium fluoride in a short time, under the catalysis ofan organic alkali to realize the deoxygenation fluorination reaction of alcoholic hydroxyl groups, and monofluoro-substituted compounds are obtained after separation and purification. The synthesis method has the characteristics of high atom economy, simplicity in preparation, greenness, high efficiency, high selectivity and few elimination byproducts, is suitable for large-scale production, andcan be applied to the radioactive isotope <18>F labeling process to label drug molecules containing alcoholic hydroxyl groups.
Owner:HEFEI UNIV OF TECH
Nano-sensor for detecting O-acetylglucosamine transferase and detection method of nano-sensor
InactiveCN107723339AHigh sensitivityGood choiceMicrobiological testing/measurementBiological material analysisEnzyme kineticsDonor specific antibodies
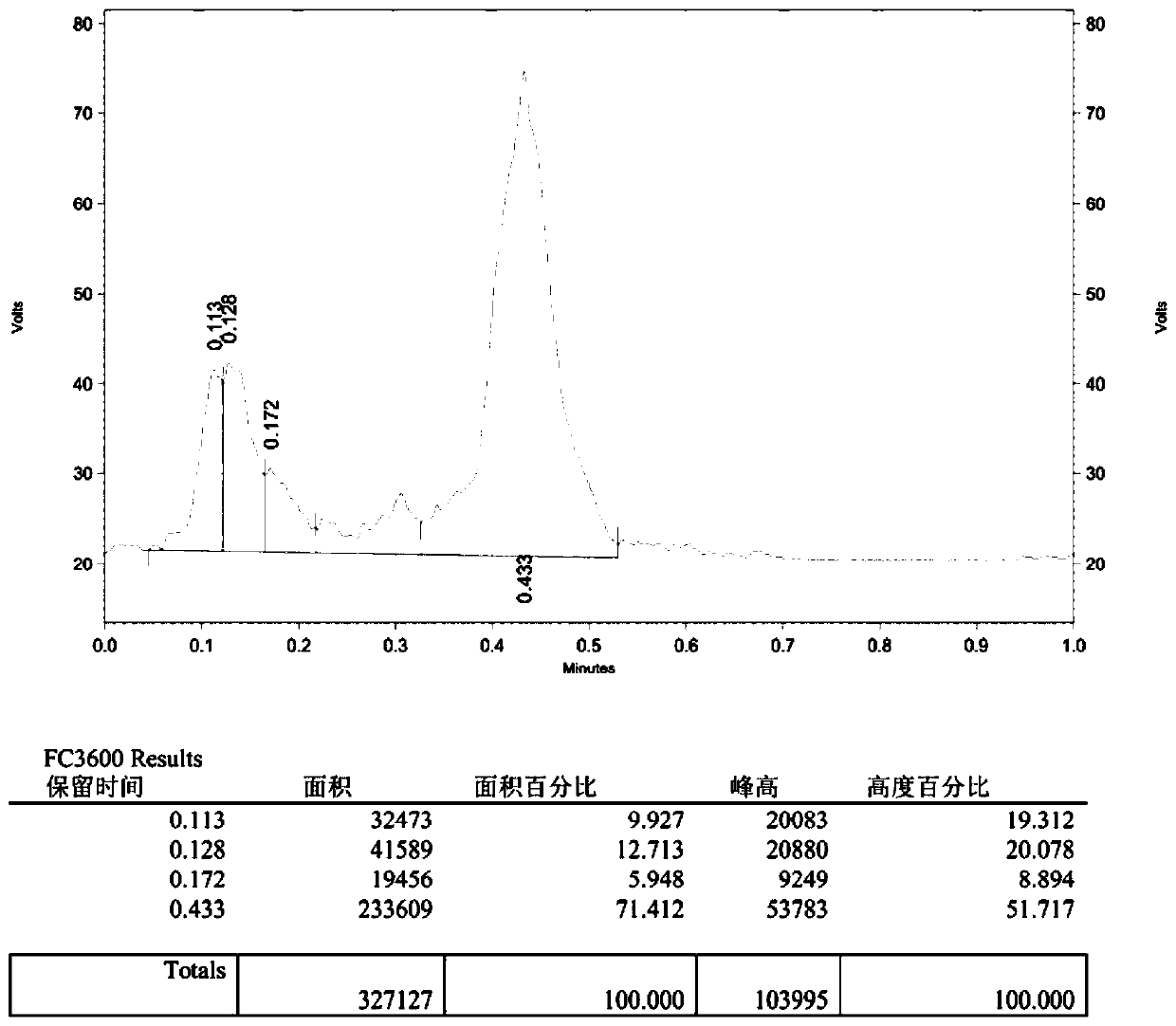
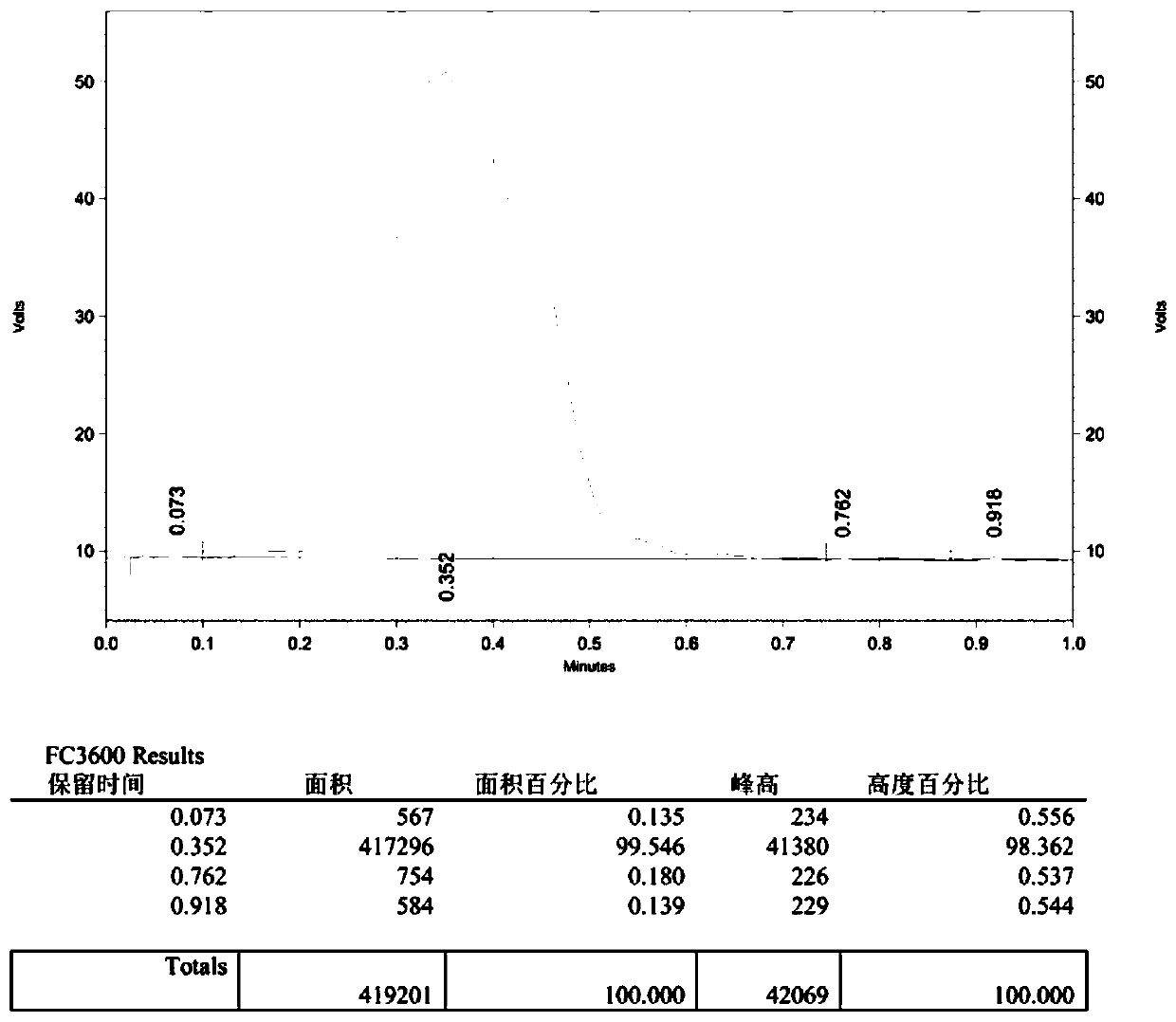
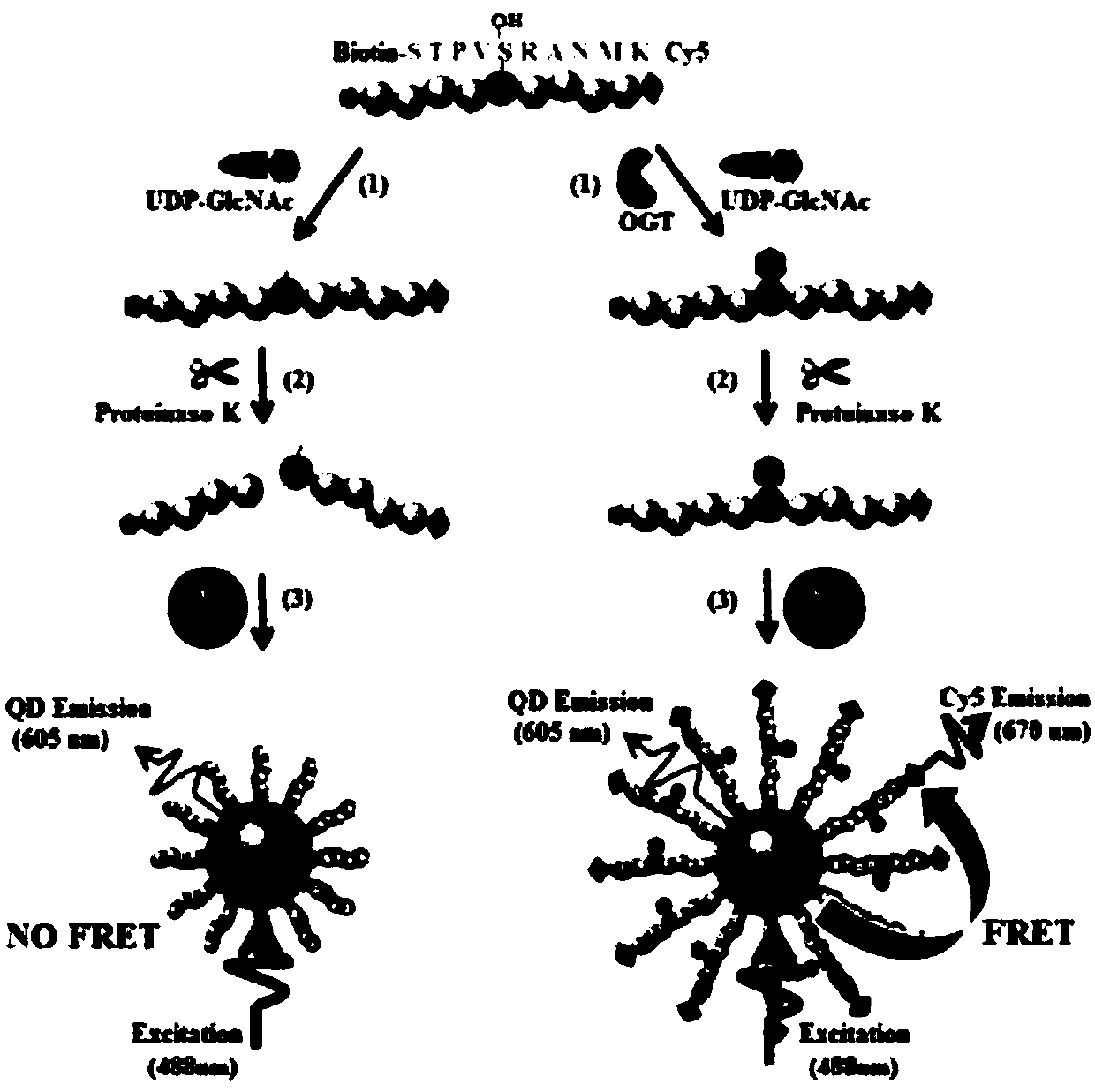
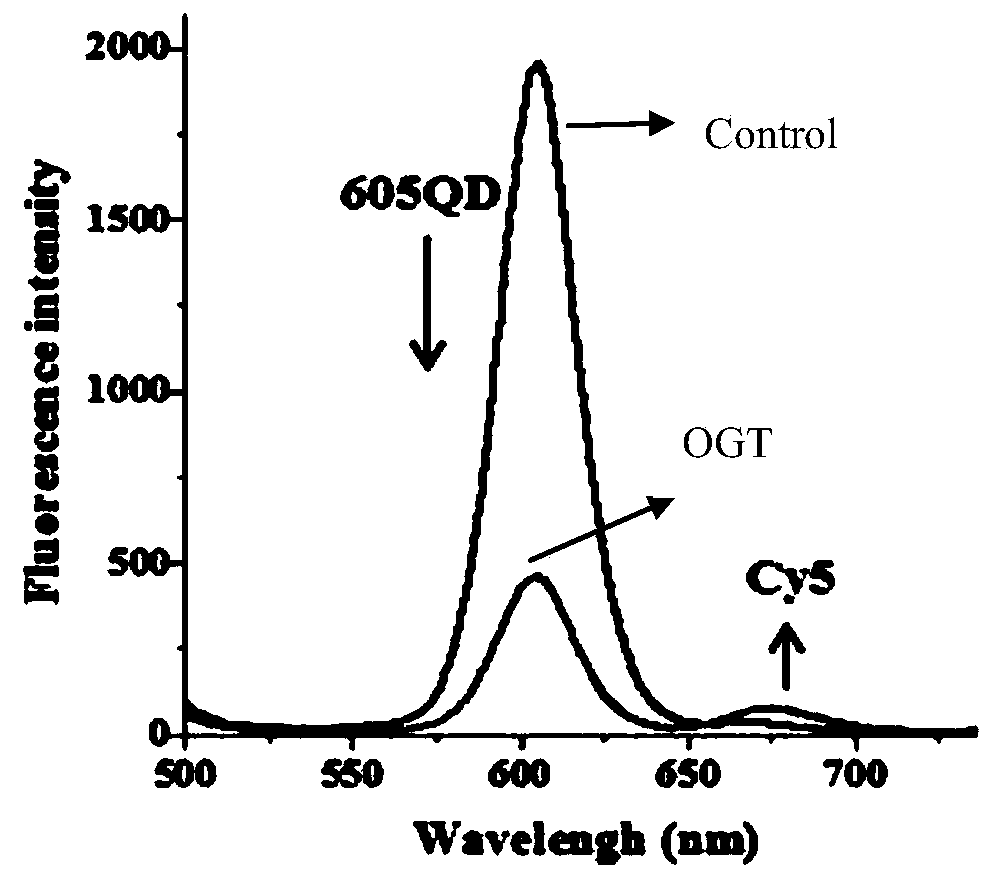
The invention belongs to the technical fields of biological analysis and detection and discloses a nano-sensor for detecting O-acetylglucosamine transferase based on a single quantum dot and a detection method of the nano-sensor. The nano-sensor comprises a cyanine 5 / biotin modified peptide chain, non-radioactive uridine-acetylglucosamine and the quantum dot coated with streptavidin, wherein a cyanine 5 / biotin modified peptide chain is Biotin-Ser-Thr-Pro-Val-Ser-Arg-Ala-Asn-Met-Lys-Cy5. The detection method of the nano-sensor is very simple, enzyme purification is not required, a radioactive isotope labeled sugar donor, a specific antibody and a fluorescence UDP-GlcNAc analogue do not need to be synthesized, the detection limit can be decreased to 3.47*10<-13> mol / L, and the sensitivity isvery high. The nano-sensor can be further applied to the measurement of enzyme kinetics parameters, the screening of an OGT inhibitor and the quantitative determination of OGT activity of cells.
Owner:SHANDONG NORMAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com