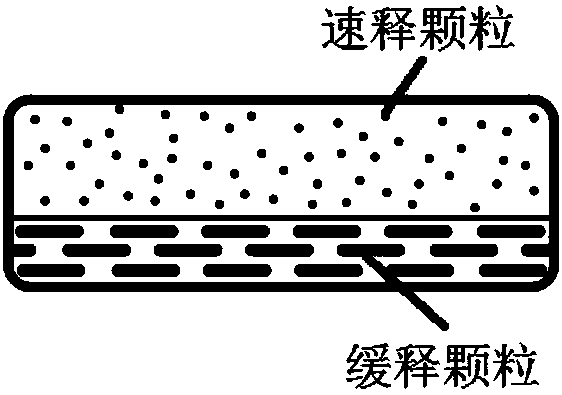
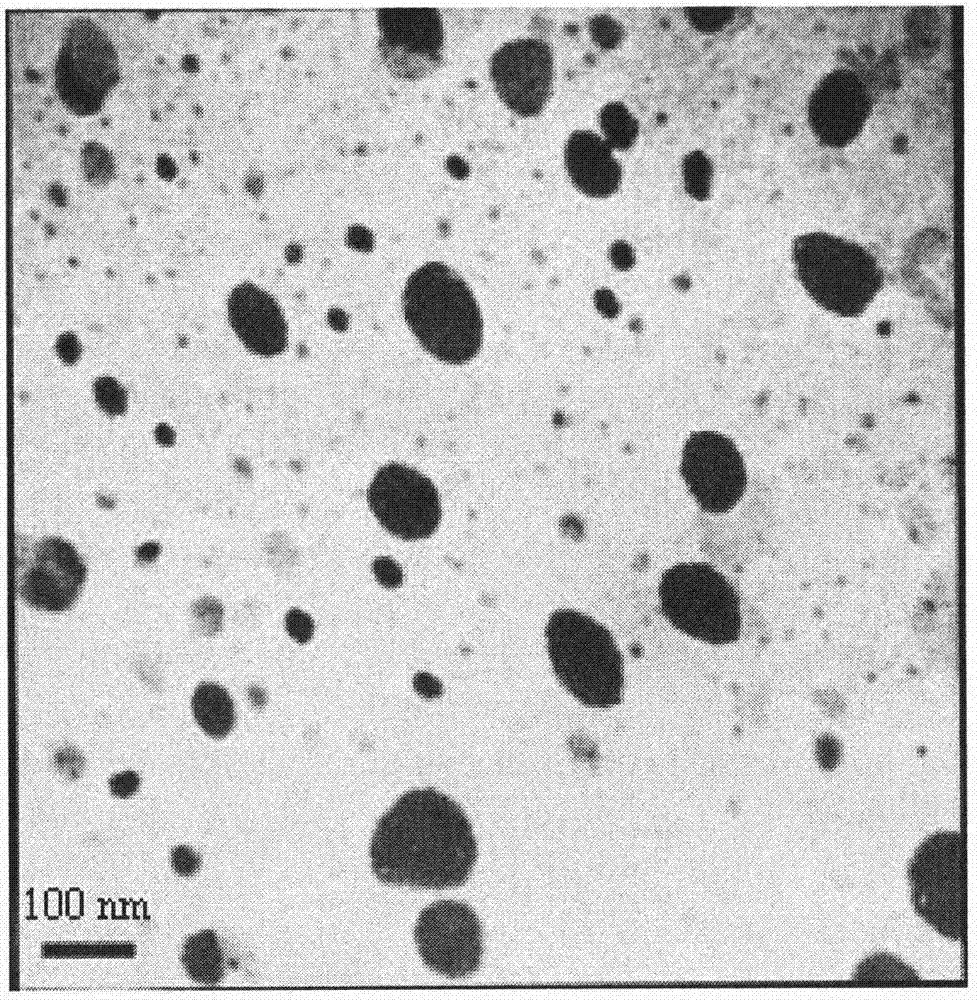
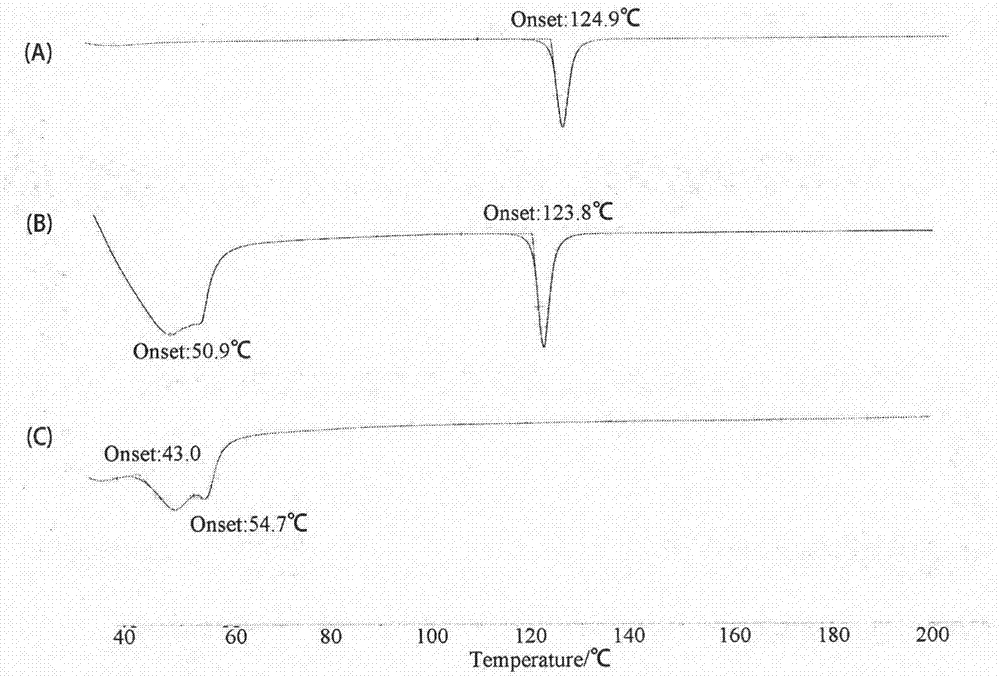
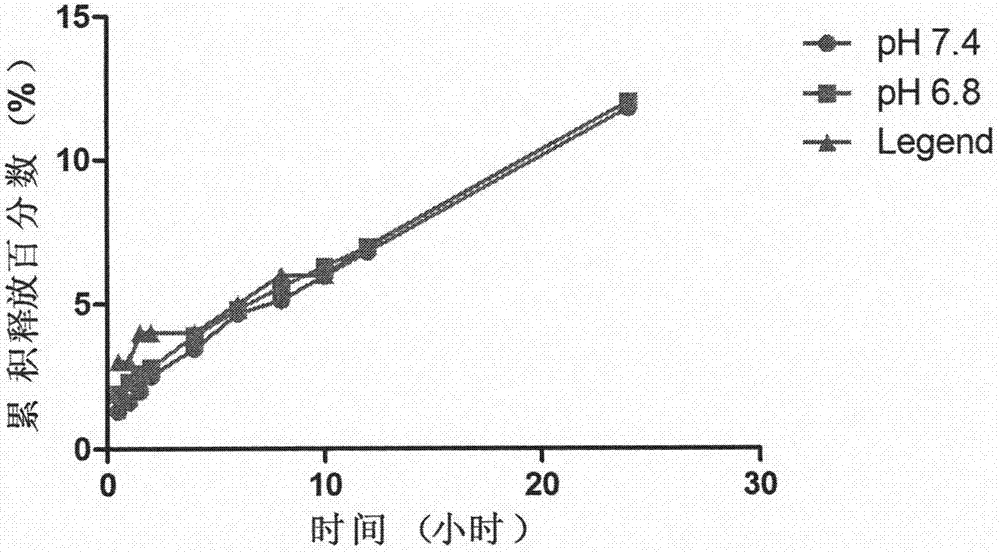
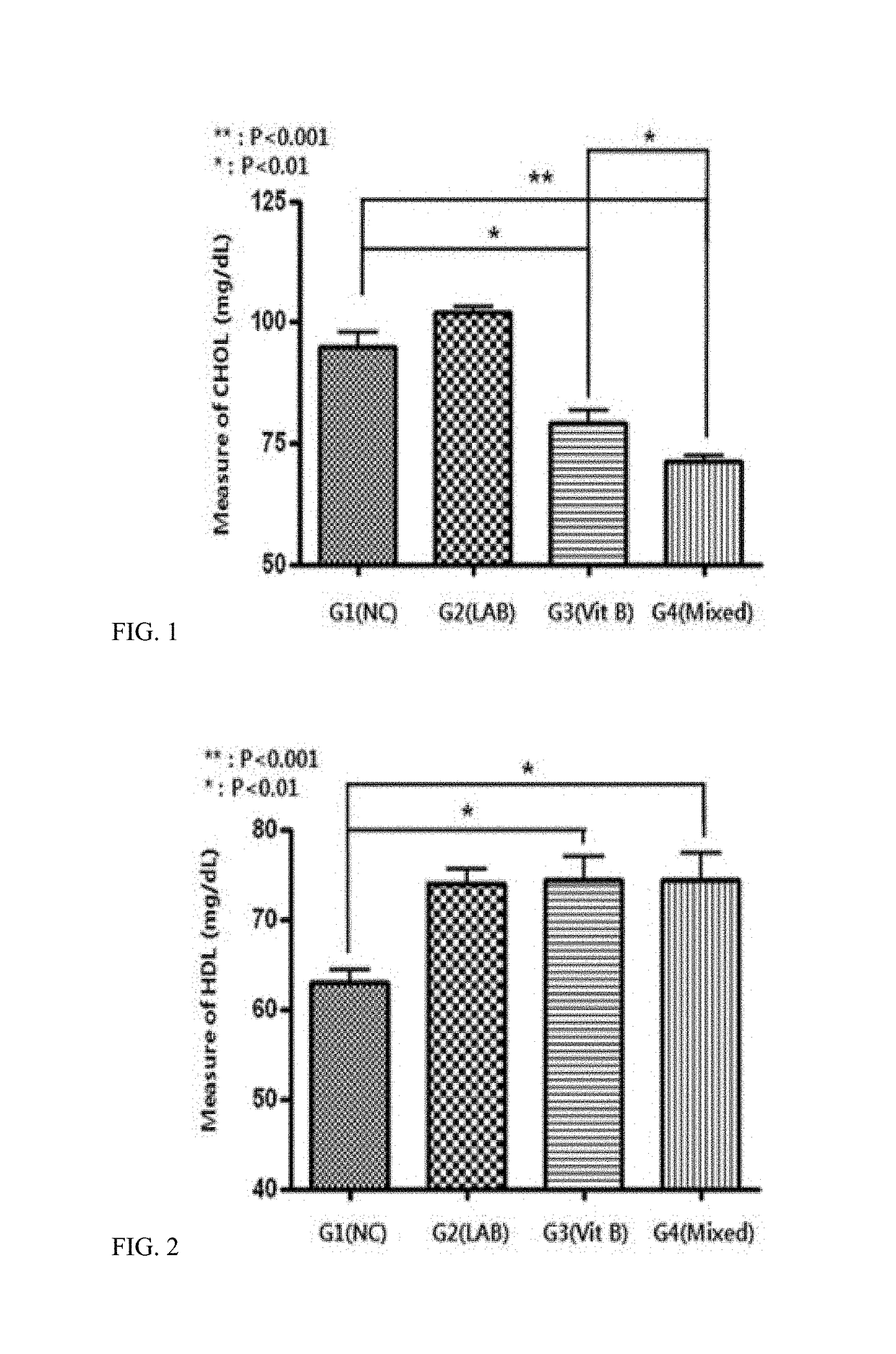
Patents
Literature
180 results about "In vivo absorption" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Liquid cannabinoid formulations
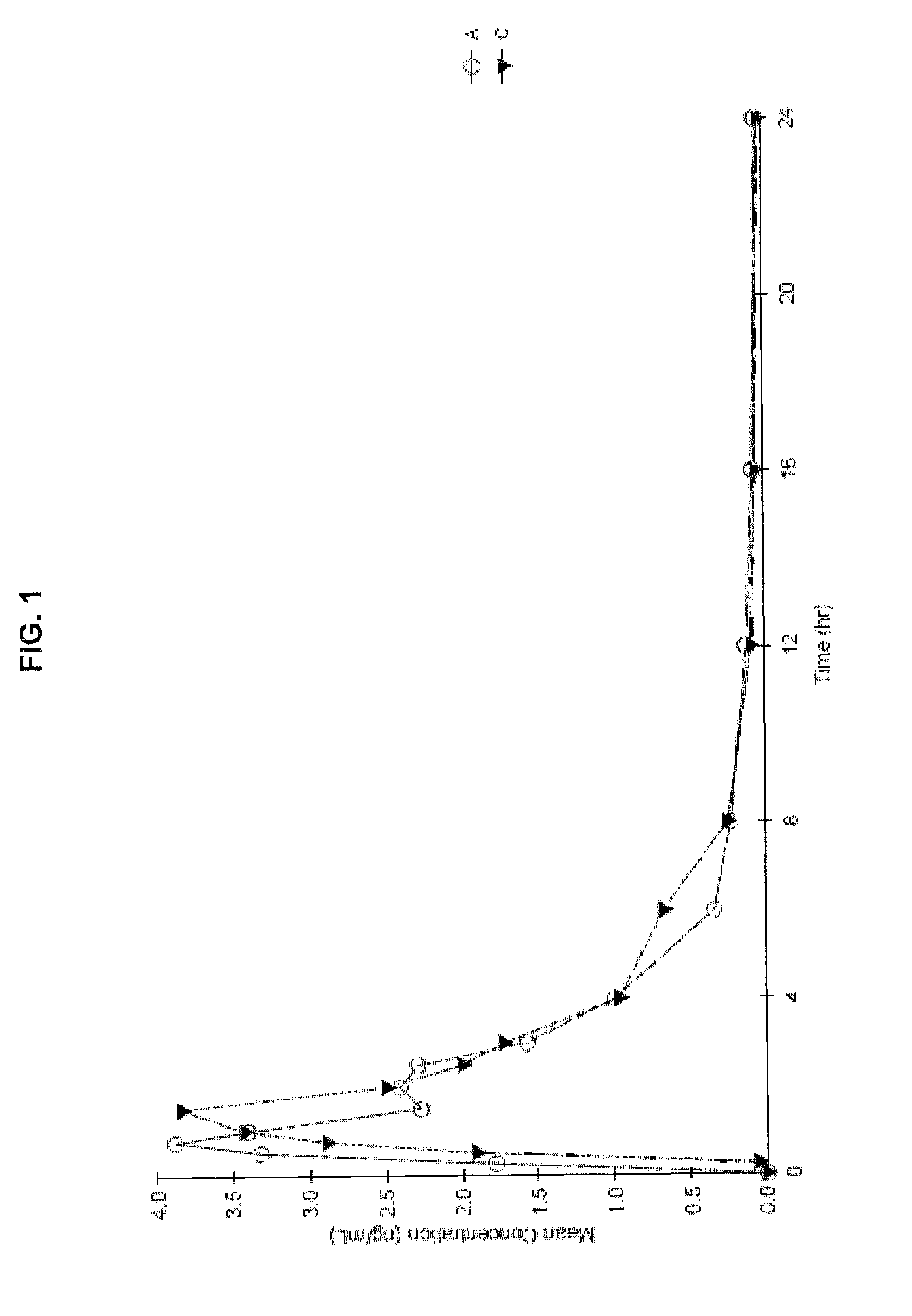
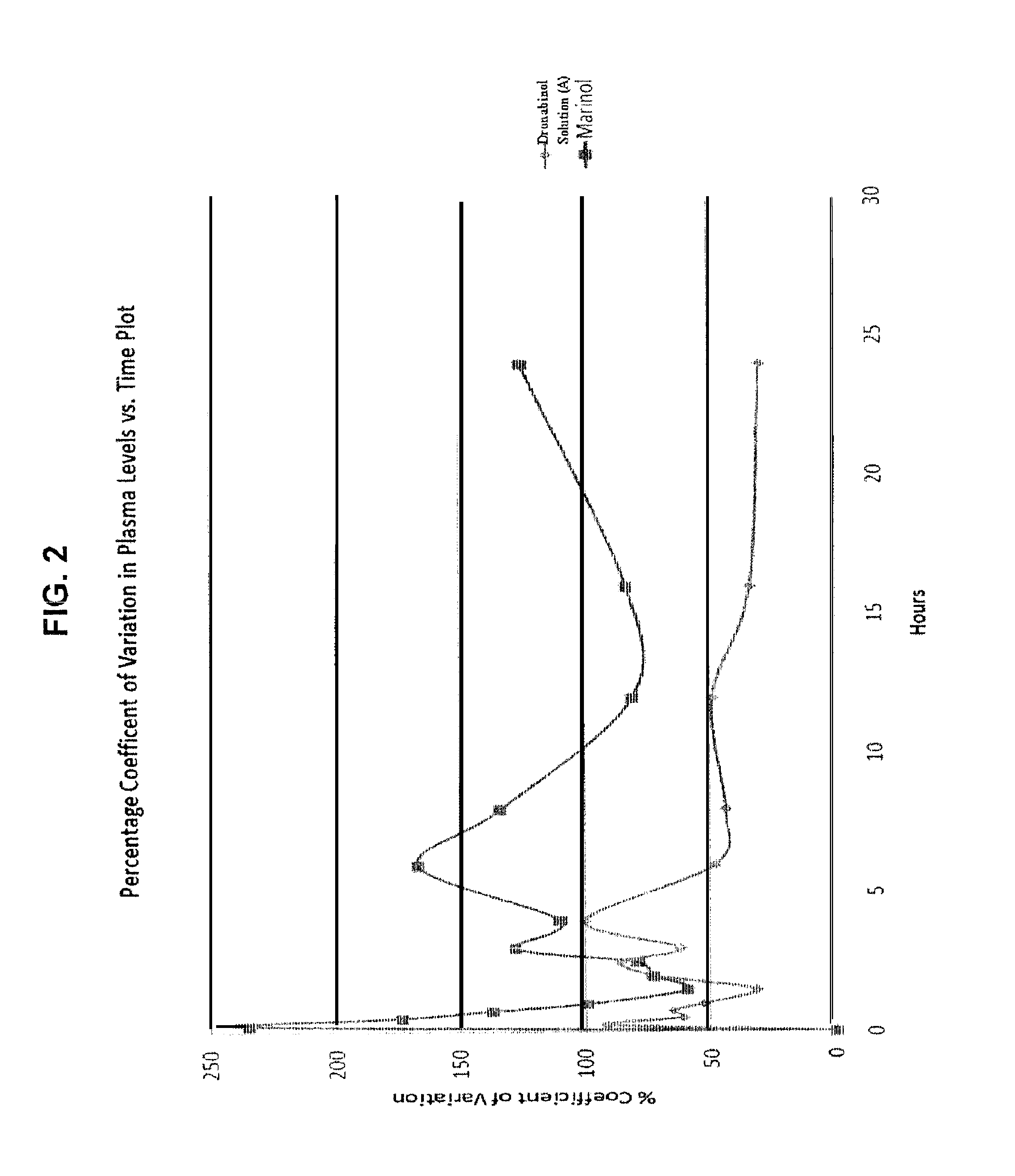
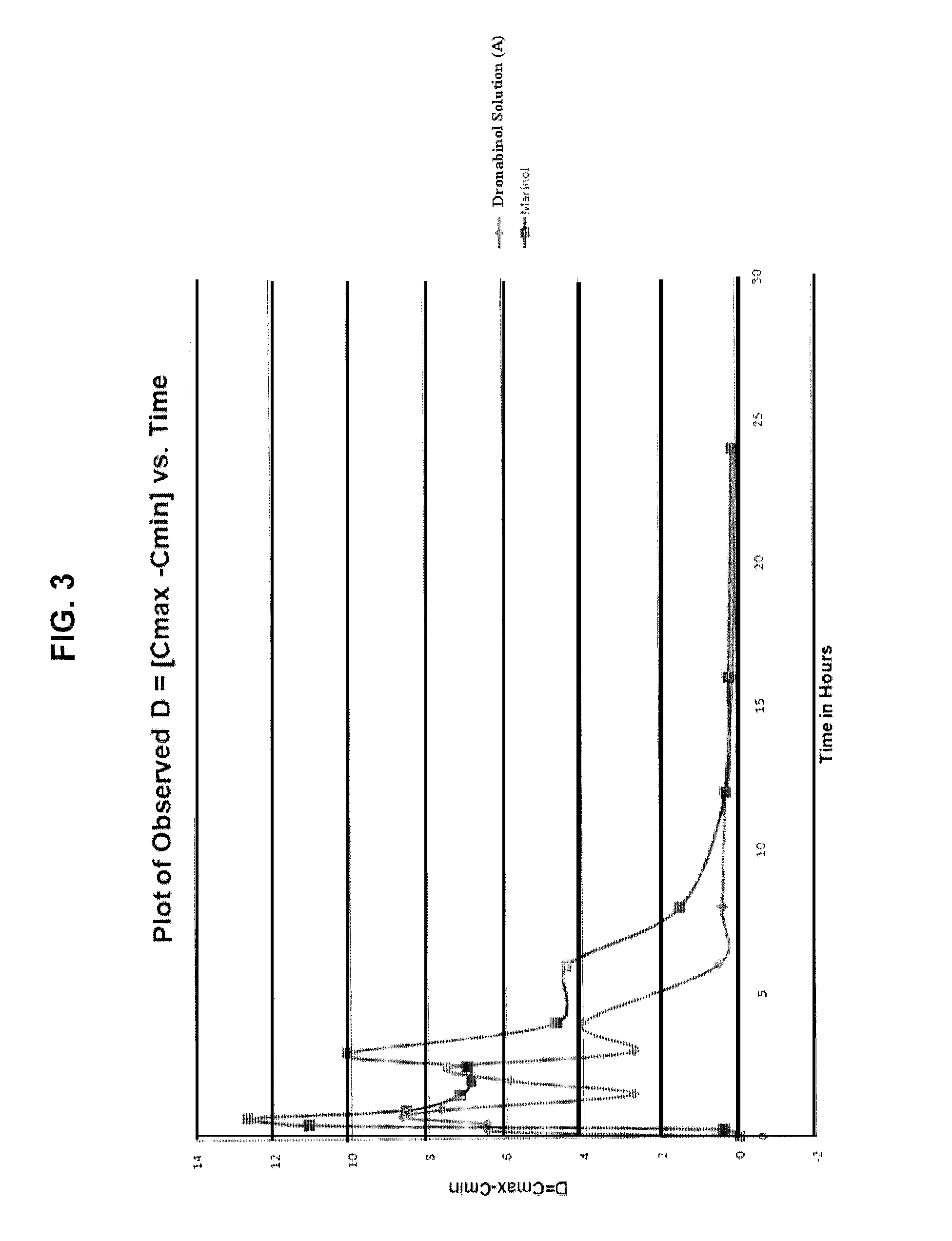
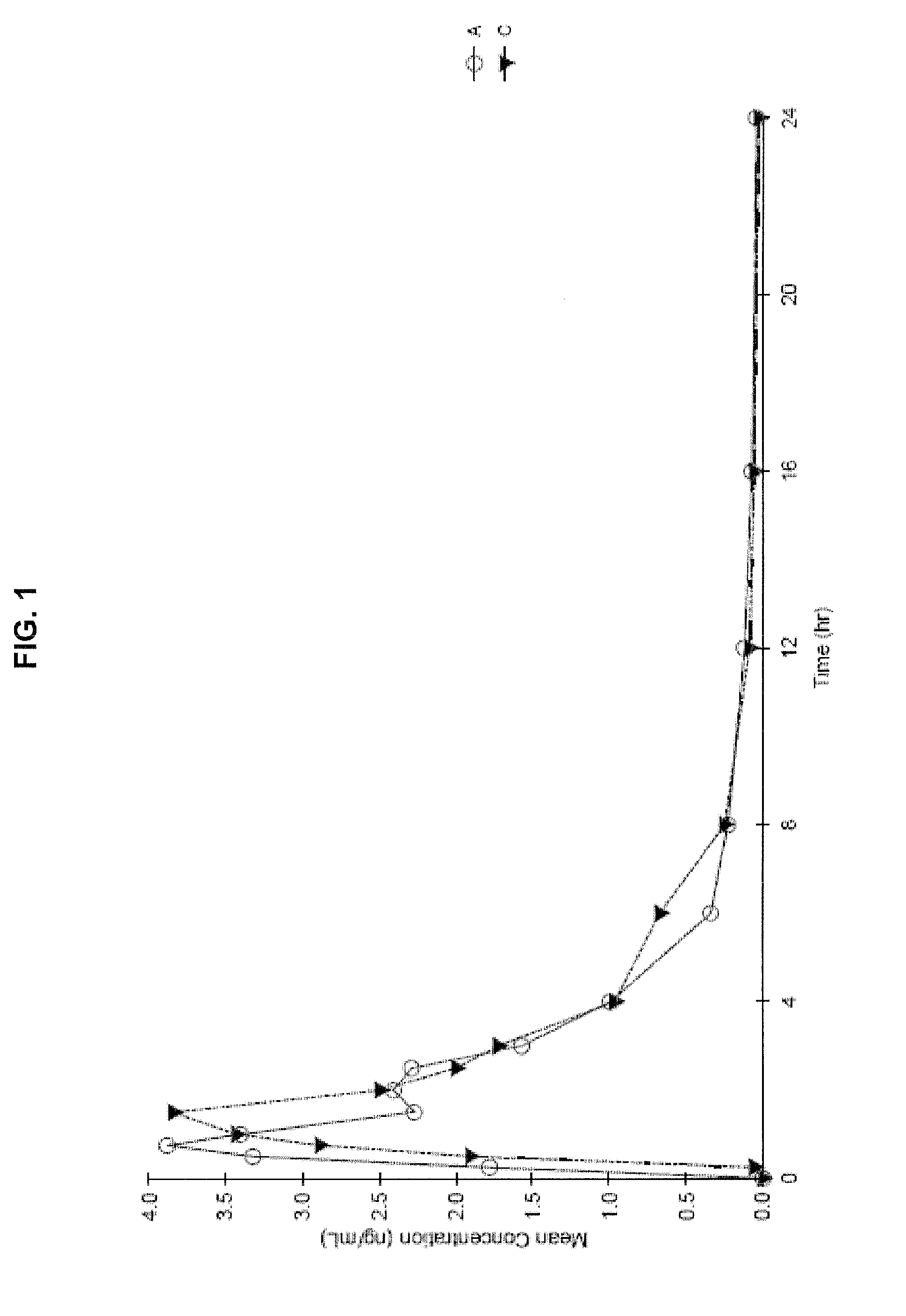
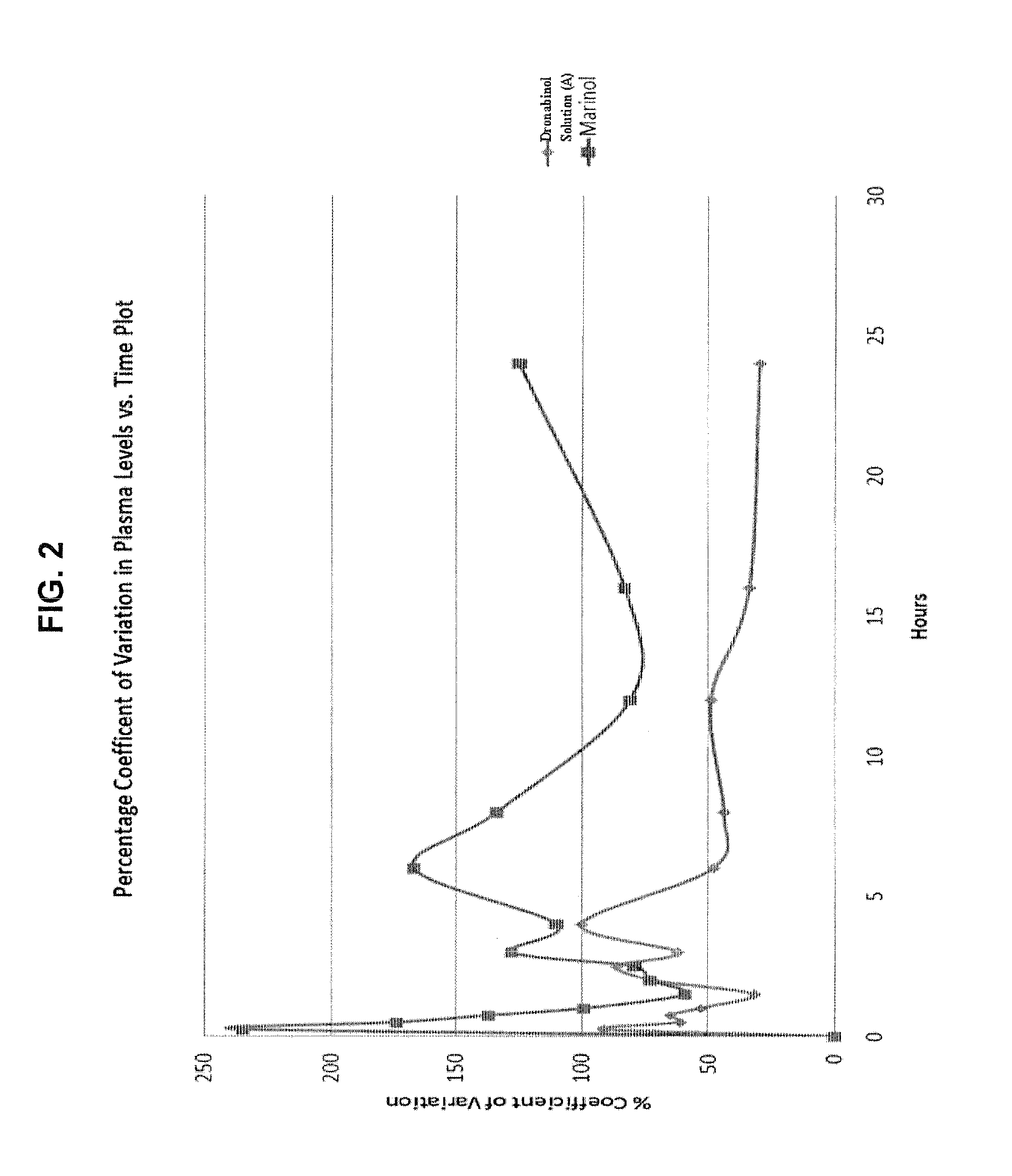
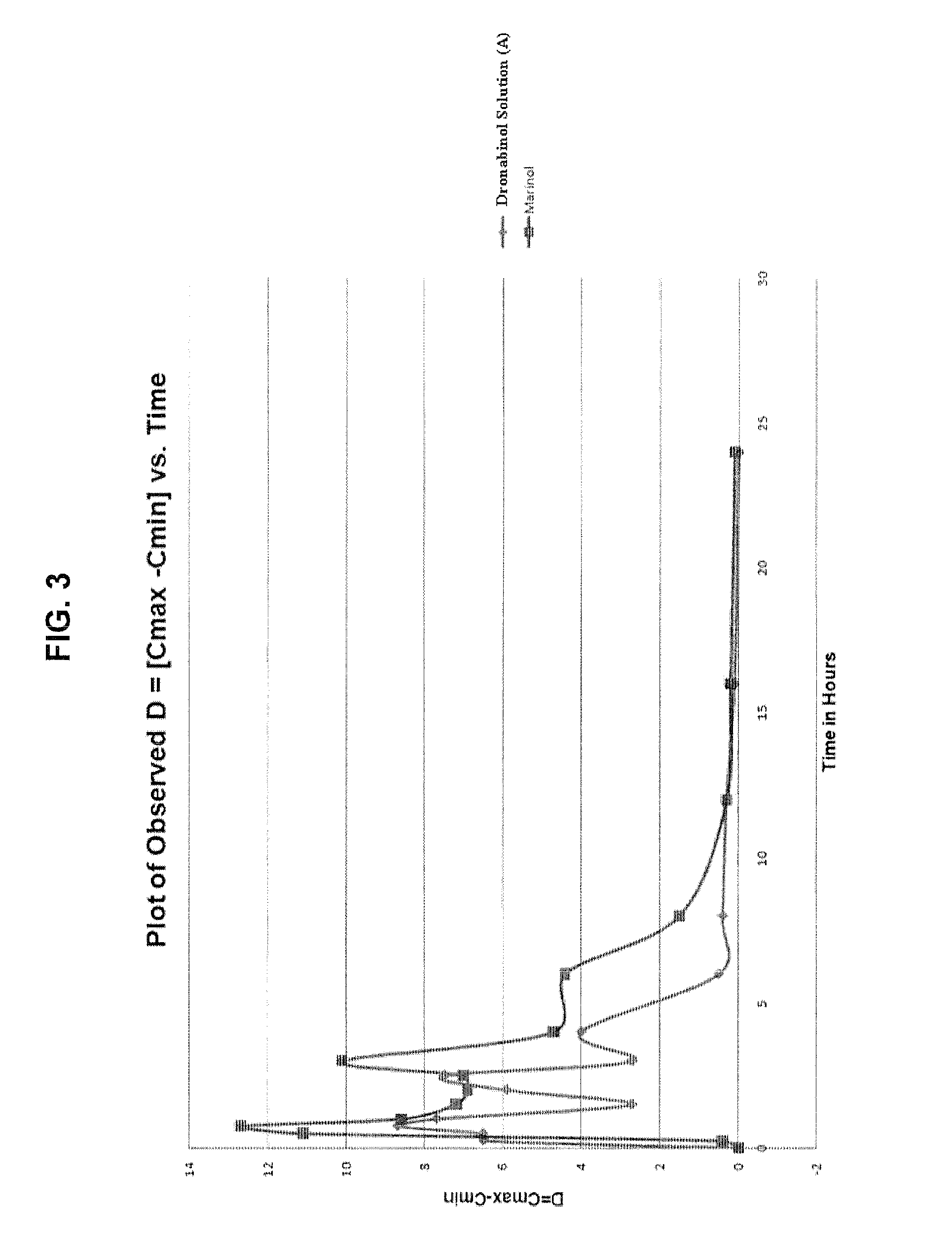
Oral cannabinoid formulations, including an aqueous-based oral dronabinol solution, that are stable at room or refrigerated temperatures and may possess improved in vivo absorption profiles with faster onset and lower inter-subject variability.
Owner:BENUVIA OPERATIONS LLC
Oral cannabinoid formulations
ActiveUS20140100269A1Improve stabilityEffective amountBiocideDispersion deliveryIn vivo absorptionSubject variability
Oral cannabinoid formulations, including an aqueous-based oral dronabinol solution, that are stable at room or refrigerated temperatures and may possess improved in vivo absorption profiles with faster onset and lower inter-subject variability.
Owner:BENUVIA OPERATIONS LLC
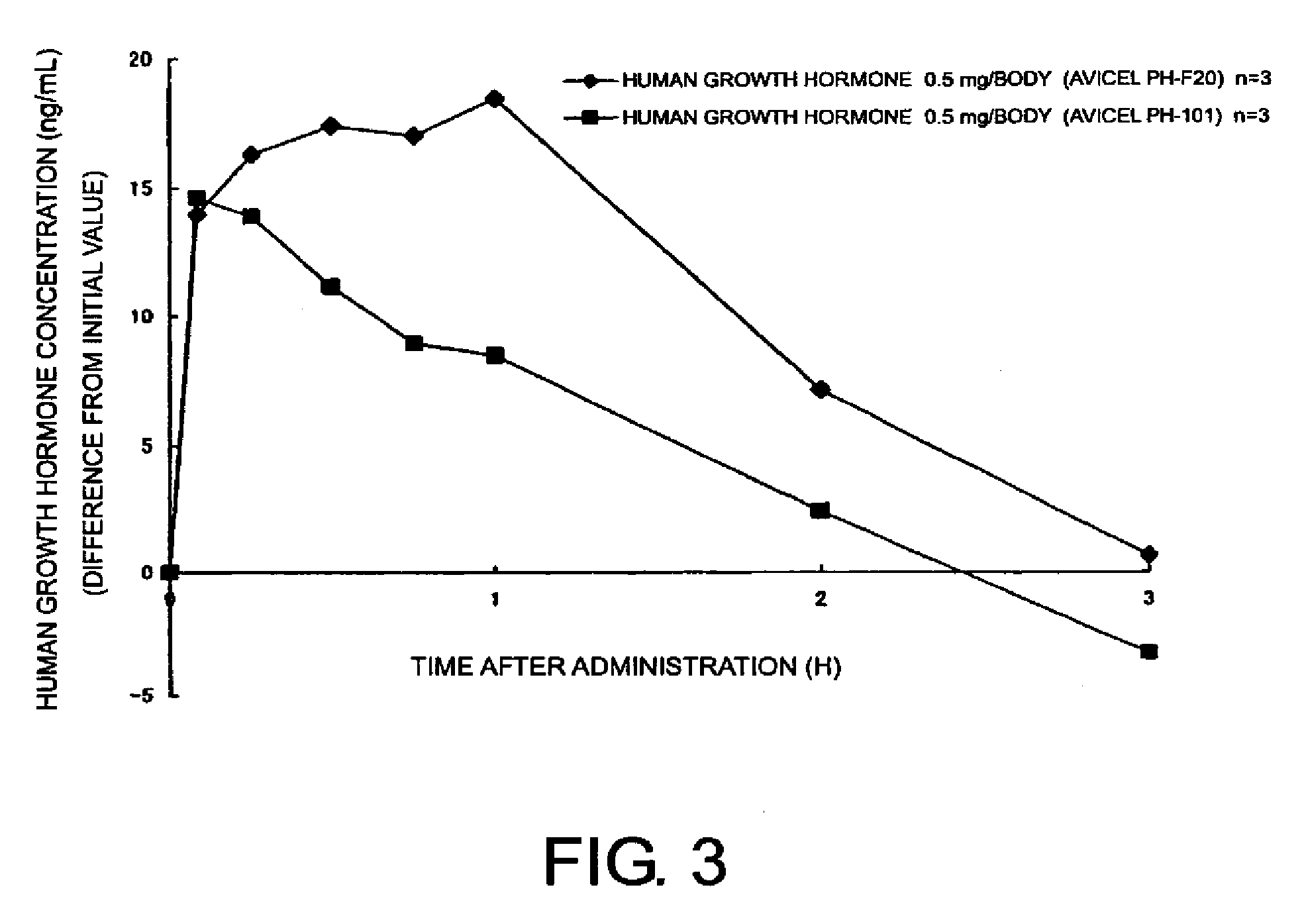
Compositions for nasal administration of pharmaceuticals
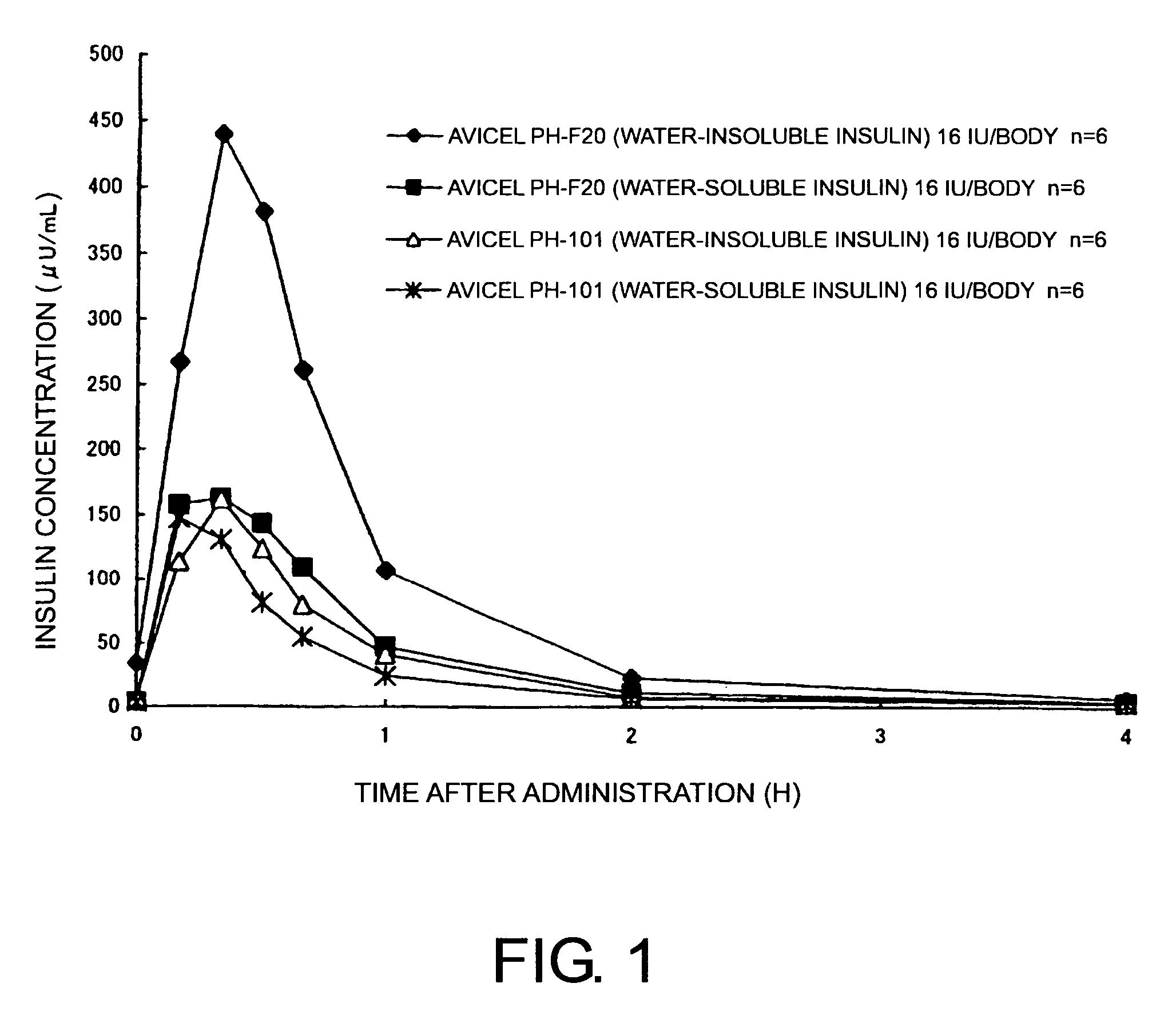
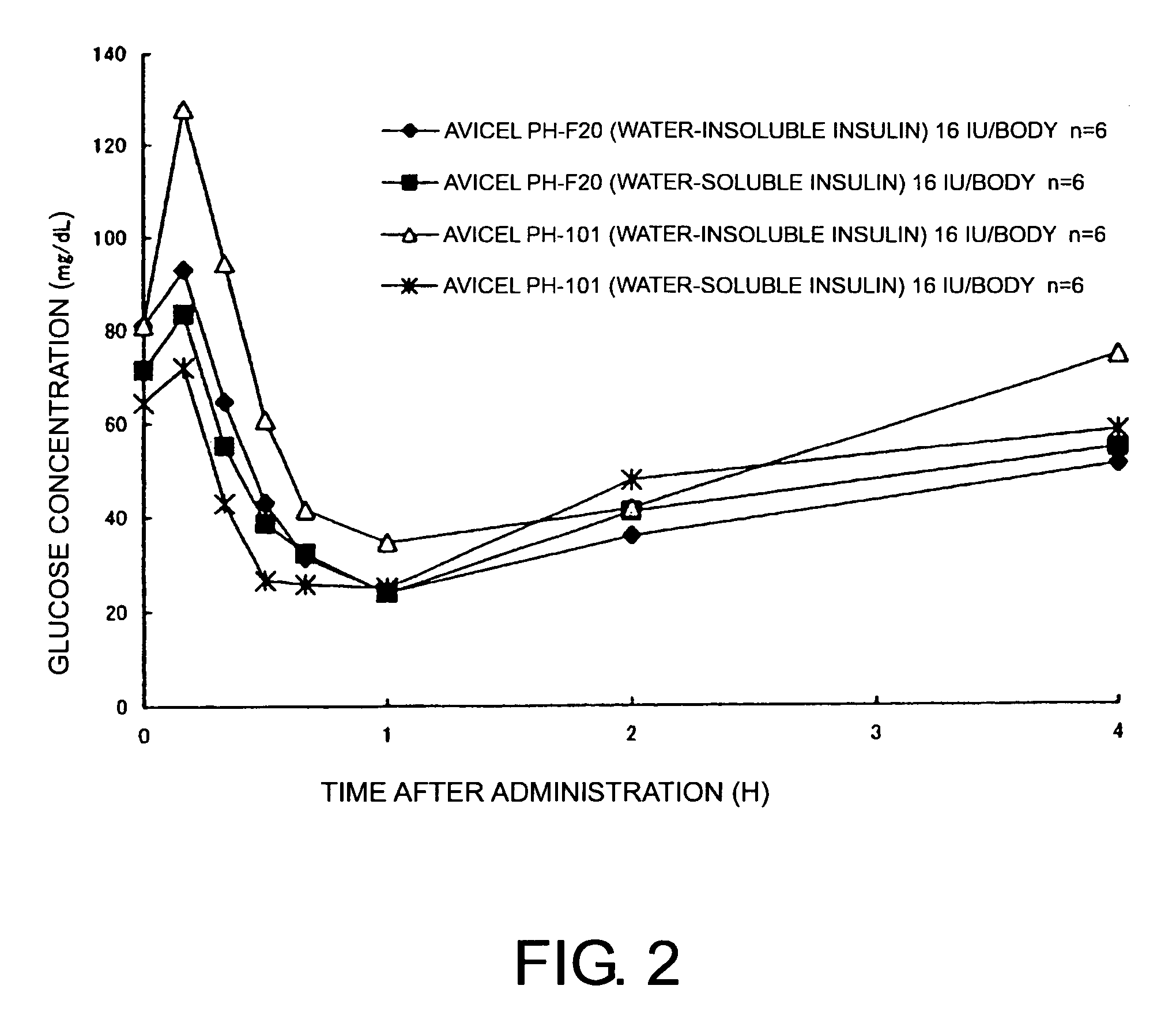
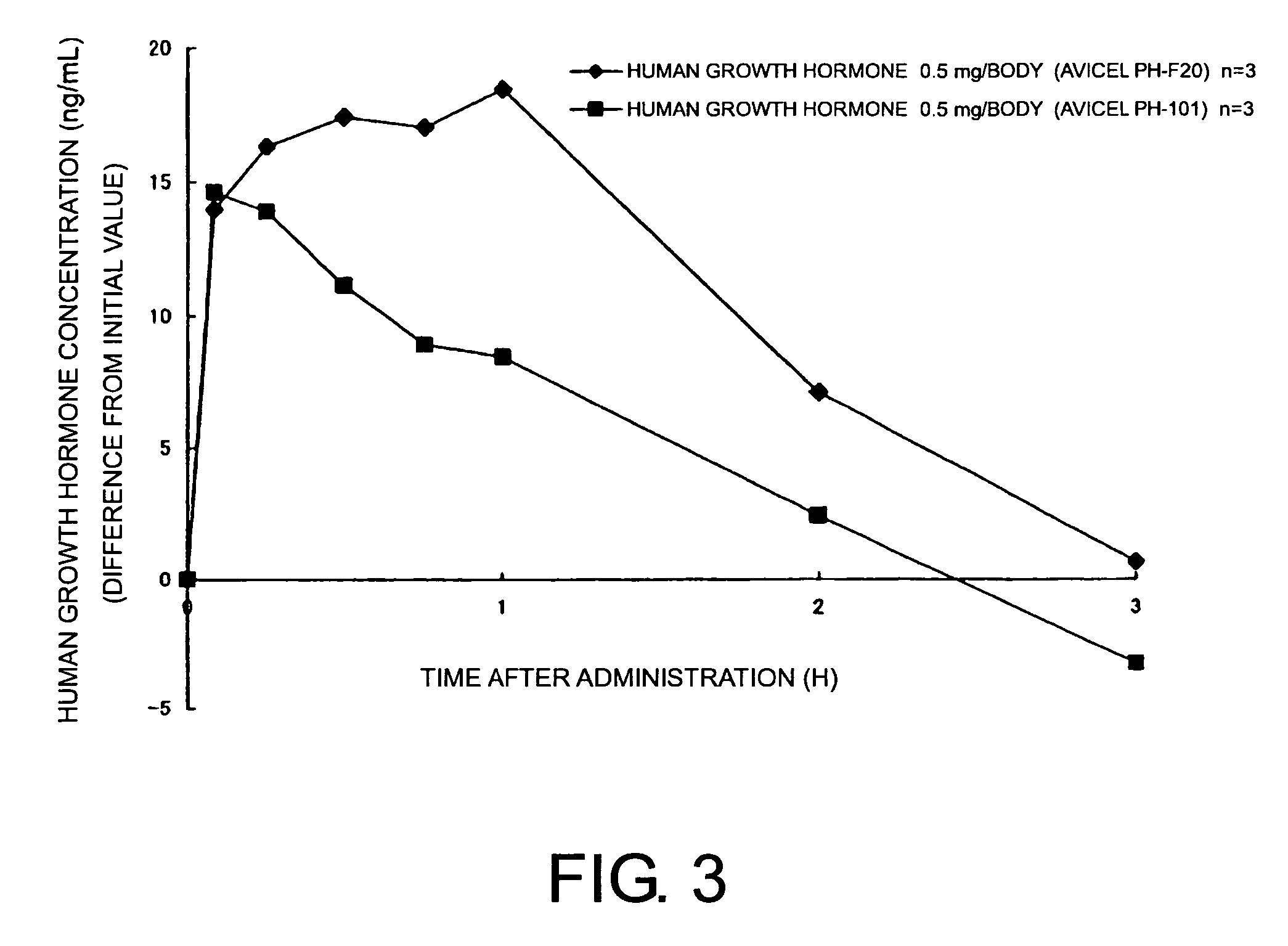
Compositions for nasal administration, which comprise a pharmaceutical, a physiologically active peptide, or a peptide-related compound, and as the carrier thereof, crystalline cellulose with a specific particle diameter and / or partially pregelatinized starch are provided. Such compositions improve the in vivo absorption efficiency of pharmaceuticals.
Owner:SHIN NIPPON BIOMEDICAL LAB
(3S)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid derivatives, and synthesis method and application thereof
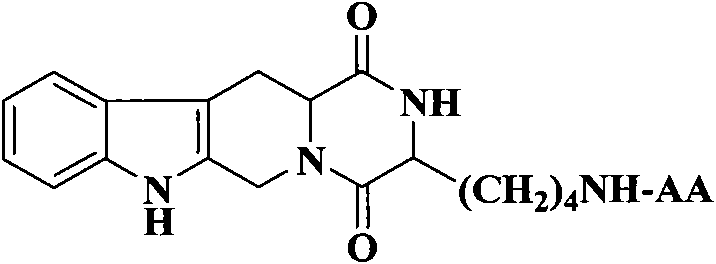
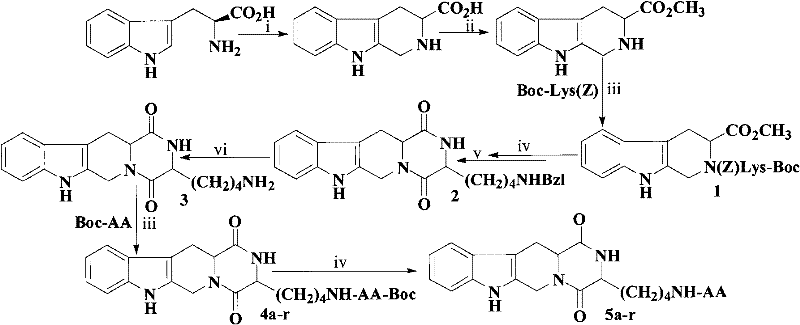
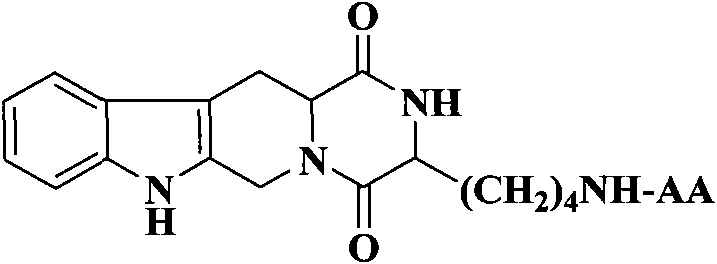
The invention discloses (3S)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid derivatives, and a synthesis method and application thereof. The structural formula of the compounds is shown as a general formula I. Hexa hydro-pyrazino-pyridino-indoldione compounds are structurally modified by natural amino acid, the water solubility and in-vivo absorption are improved and the anti-thrombotic activity is improved. In-vitro and in-vivo anti-thrombotic activity experiments indicate that the compounds in the general formula I have excellent anti-thrombotic activity, and can be prepared into anti-thrombotic medicines.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
Medicinal preparation of palbociclib and preparing method thereof
ActiveCN105816437AImprove in vitro dissolutionReduce drug particle sizePowder deliveryOrganic active ingredientsPharmacyAdjuvant
The invention belongs to the field of pharmacy and particularly relates to a medicinal preparation of palbociclib and a preparing method thereof. The medicinal preparation comprises palbociclib, an acidic adjuvant and an optional hydrophily high-molecular material. Compared with a conventional preparation, the medicinal preparation has more excellent solubility and in-vitro dissolution property, and can be used for enhancing in-vivo adsorption and bioavailability of palbociclib.
Owner:SHENZHEN PHARMACIN CO LTD
Liquid cannabinoid formulations
ActiveUS20110306660A1Improve stabilityEffective amountBiocideDispersion deliveryIn vivo absorptionSubject variability
Oral cannabinoid formulations, including an aqueous-based oral dronabinol solution, that are stable at room or refrigerated temperatures and may possess improved in vivo absorption profiles with faster onset and lower inter-subject variability.
Owner:BENUVIA OPERATIONS LLC
Oral cannabinoid formulations
Oral cannabinoid formulations, including an aqueous-based oral dronabinol solution, that are stable at room or refrigerated temperatures and may possess improved in vivo absorption profiles with faster onset and lower inter-subject variability.
Owner:BENUVIA OPERATIONS LLC
Pralmorelin-containing nasal drop preparations
InactiveUS7008927B2Promote absorptionImprove stabilitySenses disorderPeptide/protein ingredientsPralmorelinIn vivo absorption
The preparation for intranasal administration comprising D-alanyl-3-(naphthalen-2-yl)-D-alanyl-L-alanyl-L-tryptophyl-D-phenylalanyl-L-lysinamide (pralmorelin) and / or an acid addition salt thereof as an active ingredient and water permits a marked increase in the in vivo absorption of pralmorelin and hence provides adequate efficacy even if it is administered in a small dose at a time. The preparation also allows pralmorelin to be dissolved in an increased amount, so it can be formulated pharmaceutically with great ease. It also high stability over time.
Owner:KAKEN PHARMA CO LTD
Drug-containing nanoparticle, process for producing the same and parenterally administered preparation from the nanoparticle
InactiveCN1917859AGood slow releaseStrong targetingPowder deliveryHydroxy compound active ingredientsNanoparticleIn vivo absorption
An external preparation or injectable solution that exerts the effect of enabling percutaneous or transmucous in vivo absorption of fat-soluble drugs and water-soluble drugs not having been satisfactorily attained hitherto and that contains a highly absorbable fat-soluble / water-soluble drug. The injectable solution especially aims at sustained release and target effects. In particular, drug-containing nanoparticles (secondary nanoparticles) are provided by causing primary nanoparticles containing a fat-soluble drug or fat-solubilized water-soluble drug to act with a divalent or trivalent metal salt. Further, drug-containing nanoparticles (tertiary nanoparticles) are provided by first causing primary nanoparticles containing a fat-soluble drug or fat-solubilized water-soluble drug to act with a divalent or trivalent metal salt to thereby obtain secondary nanoparticles and thereafter causing a monovalent to trivalent basic salt to act on the secondary nanoparticles. Still further, there are provided a process for producing these nanoparticles, and a percutaneous or transmucous external preparation or injectable solution in which these nanoparticles are contained.
Owner:LTT BIO PHARMA
Compositons for nasal administration of pharmaceuticals
Compositions for nasal administration, which comprise a pharmaceutical, a physiologically active peptide, or a peptide-related compound, and as the carrier thereof, crystalline cellulose with a specific particle diameter and / or partially pregelatinized starch are provided. Such compositions improve the in vivo absorption efficiency of pharmaceuticals.
Owner:SHIN NIPPON BIOMEDICAL LAB
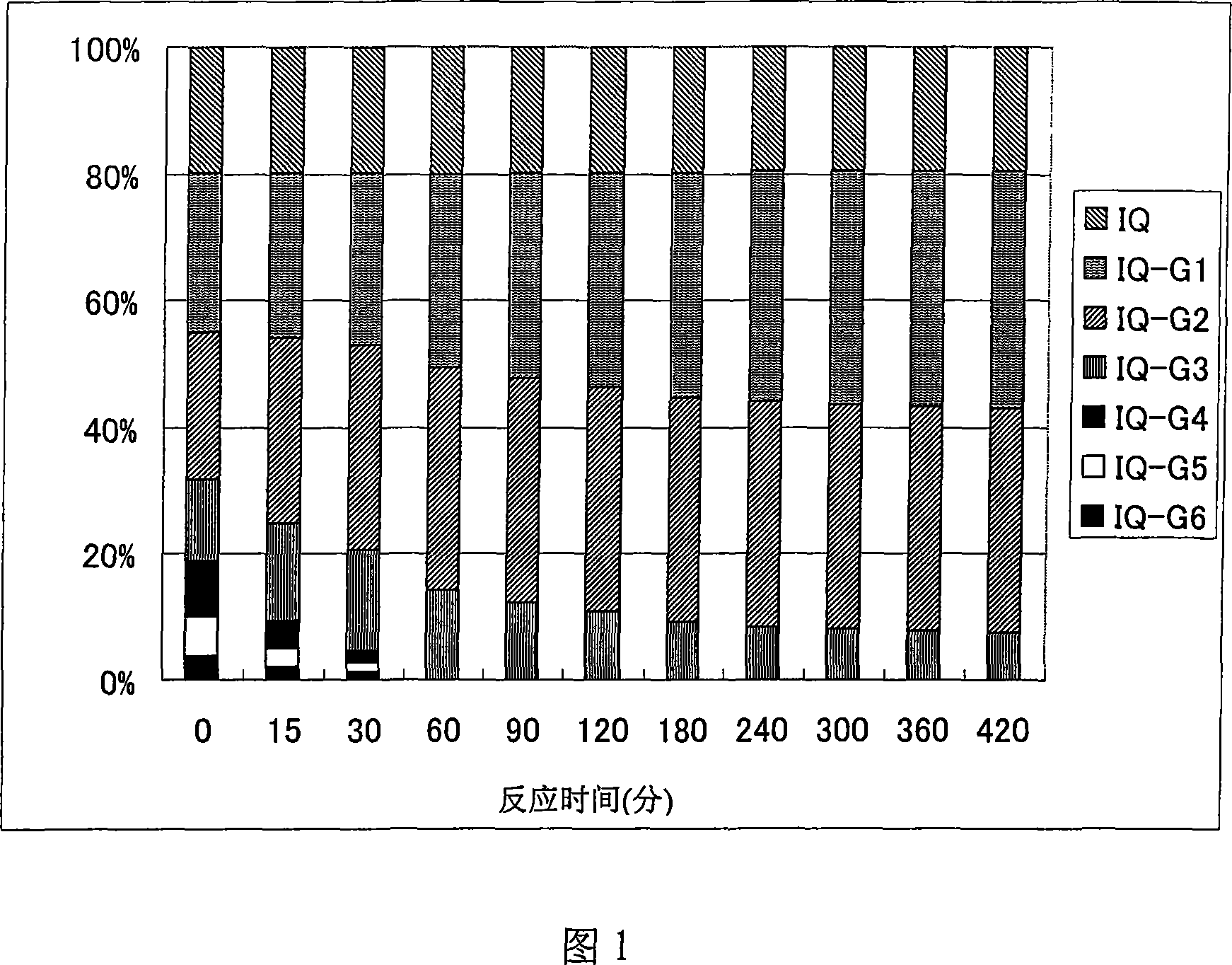
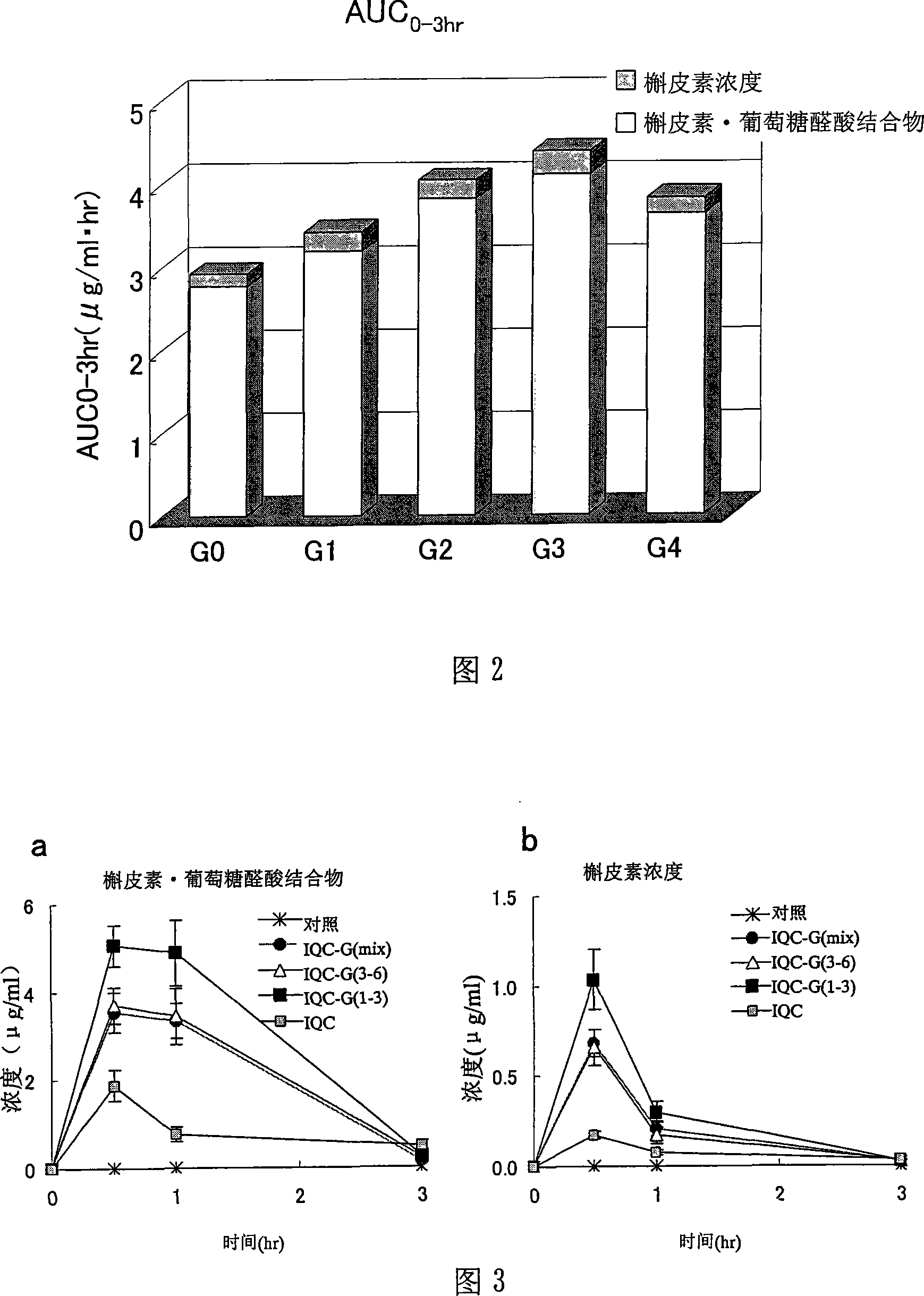
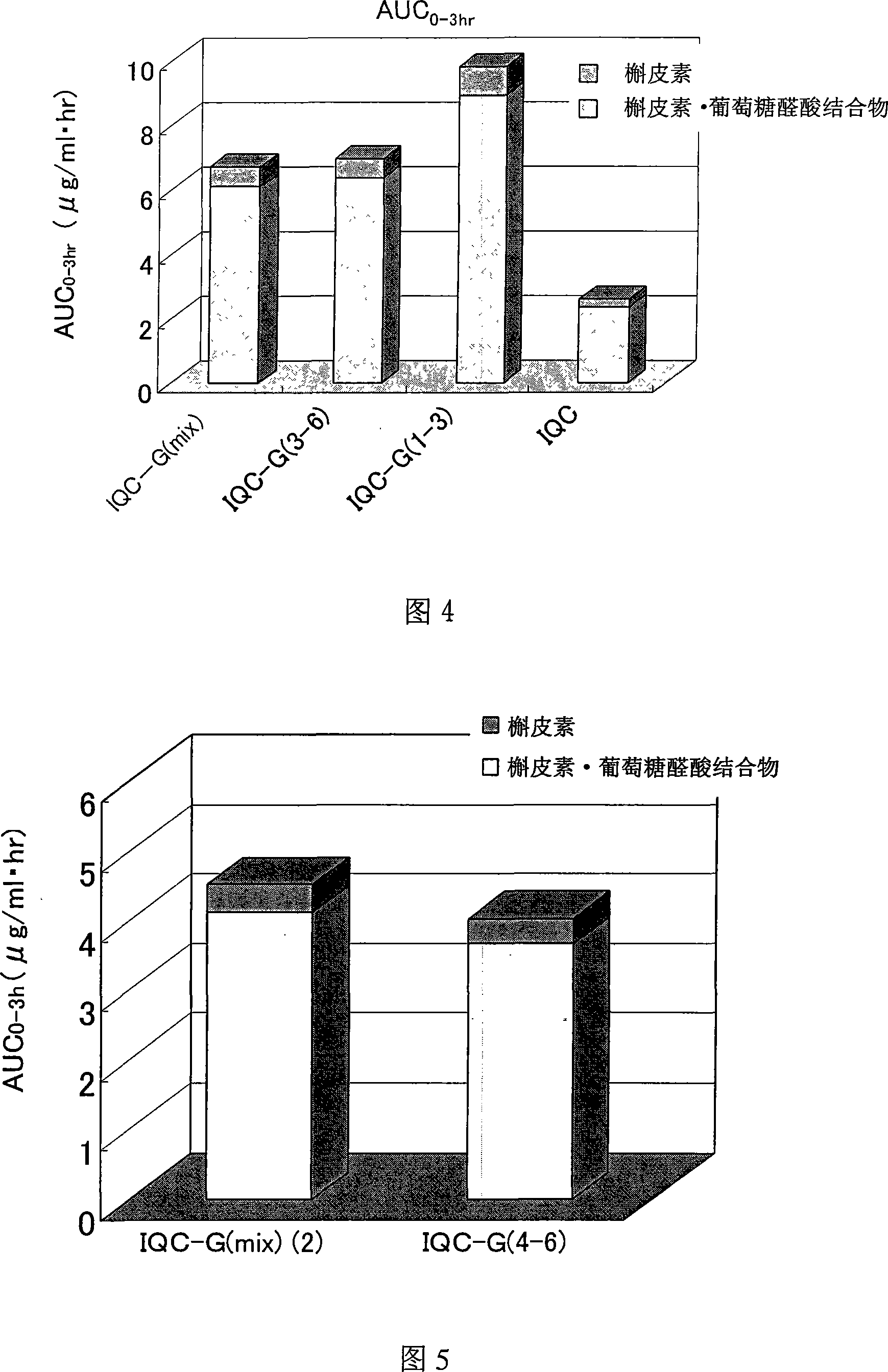
Quercetin glycoside composition and method of preparing the same
The present invention provides an +--glycosyl isoquercitrin-containing novel composition which has a high in vivo absorbability, and hence exhibits a significant in vivo antioxidative activity. The present invention further provides preparation methods for such a composition. The composition contains a mixture of quercetin glycosides represented by the following formula: wherein Glc represents a glucose residue, and n is 0 or a positive integer of 1 or more, includes at least a quercetin glycoside wherein n is 3, and satisfies the following requirement (a): (a) the total proportion of quercetin glycosides in which n is 3, and in which other n values may be 1 or 2, or 1 and 2, is 50 mol% or more, and the total proportion of quercetin glycosides wherein n is 4 or more is 15 mol% or less, in the composition. The composition can be prepared by treating an enzymatically modified isoquercitrin with -amylase.
Owner:SUNTORY HLDG LTD +1
Degradable stephanoporate magnesium basis complex tissue project bracket stuff within biosome
ActiveCN101156960AGood biocompatibilityImprove mechanical propertiesProsthesisMagnesium matrix compositeChemical reaction
The invention relates to porous magnesium matrix composite tissue engineering scaffold biomaterial which can degrade in the bio-body. The components and the volume percent contents are 70 percent to 20 percent of high purity magnesium and magnesium alloy powder, 10 percent to 20 percent of HA powder and 20 percent to 70 percent of pore forming agent, the high purity magnesium and the magnesium alloy powder are HA alloys, high purity magnesium alloys, high purity Mg-Zn alloys, high purity Mg-Zn-Ca alloys, high purity Mg-Zn-Ca-Fe alloys, high purity Mg-Mn alloys and high purity Mg-Zn-Mn alloys, and the purity rate of the alloy is higher than or equal to 99.99 percent. The bone tissue engineering scaffold biomaterial prepared by the invention which adopts the powder metallurgy technology utilizes the chemical reaction of the magnesium in aqueous medium to be converted into magnesium ions, and the magnesium ions adjust the balance through the in vivo absorption and the kidney metabolism, thereby leading the scaffold to be gradually degraded and absorbed in vivo.
Owner:CHANGSHU MICROTUBE TECH
Berberine hydrochloride medicinal composition and preparation method thereof
InactiveCN102370643ASolve the problem of insoluble and unstable absorptionDisintegration and dissolution fastAntibacterial agentsOrganic active ingredientsSolubilityIn vivo absorption
The invention discloses a berberine hydrochloride medicinal composition and a preparation method thereof, which are used for increasing the dissolution rate of berberine hydrochloride in water and enhance the in-vivo absorption of berberine hydrochloride. According to the specific technical scheme, berberine hydrochloride serves as a medicinal active ingredient of the composition; and a filling agent, a disintegrating agent, a flavoring agent and a lubricating agent in the weight ratio (1-50):(25-60):(3-15):(1.5-3):(0.4-1.2) serve as auxiliary materials. The method for preparing the berberine hydrochloride medicinal composition comprises the following steps of: (1) dissolving; (2) drying; (3) screening; and (4) tableting. The berberine hydrochloride medicinal composition prepared with the method has high disintegrating and dissolving speeds and a good medicament effect, and the problems of low solubility and unstable absorption of berberine hydrochloride are solved. The berberine hydrochloride medicinal composition is effective to enteral infection caused by dysentery bacillus and colon bacillus, and is used for treating enteral infection and diarrhea.
Owner:JILIN AODONG GROUP DALIAN PHARMACEUTICAL CO LTD
Puerarin self-microemulsion composition based on mixed oil and preparation method thereof
InactiveCN101810576AReduce the amount requiredReduce dosageOrganic active ingredientsPill deliverySolubilitySide effect
The invention discloses a puerarin self-microemulsion composition, which consists of puerarin, mixed oil, an emulsifier and an auxiliary emulsifier by weight percent, wherein the puerarin accounts for 0.5-5 percent; the mixed oil accounts for 36-61 percent; the emulsifier accounts for 10-50 percent; and the auxiliary emulsifier accounts for 5-30 percent. The puerarin self-microemulsion composition prepared in the invention not only solves the problems of the traditional emulsion such as large particle size, and poor stability, large storage volume and the like due to existence of the aqueous phase, but also improves the solubility and dispersion of puerarin, increases the in vivo absorption of drugs, and enhances the bioavailability. The invention has the advantages of low viscosity, stable quality and the like, and can improve the drug absorption, enhance the bioavailability and reduce the toxic and side effects.
Owner:GUANGDONG HUANAN PHARMACEUTICAL GROUP CO LTD
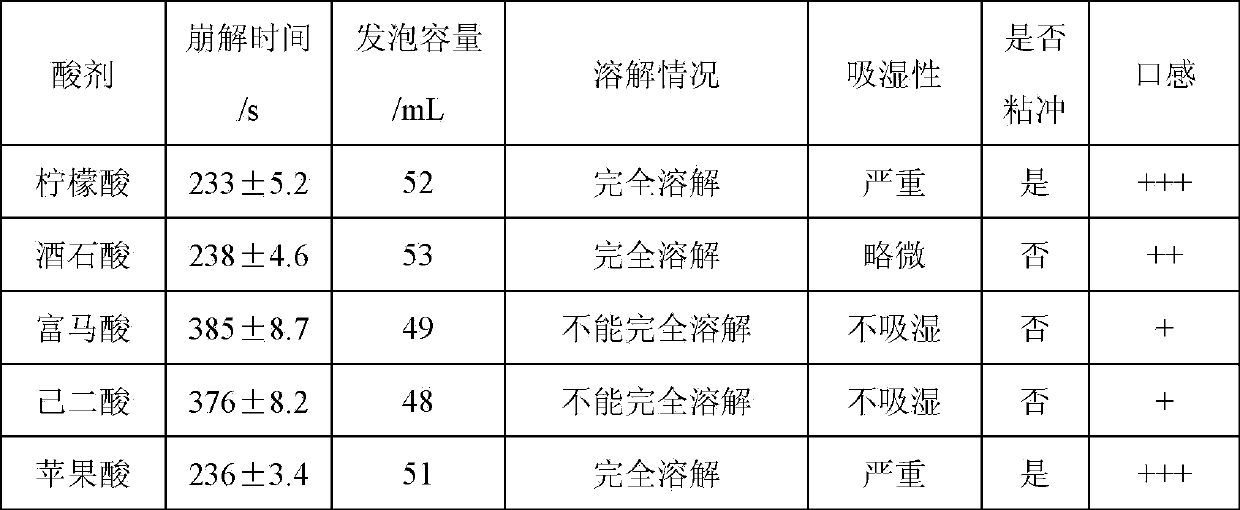
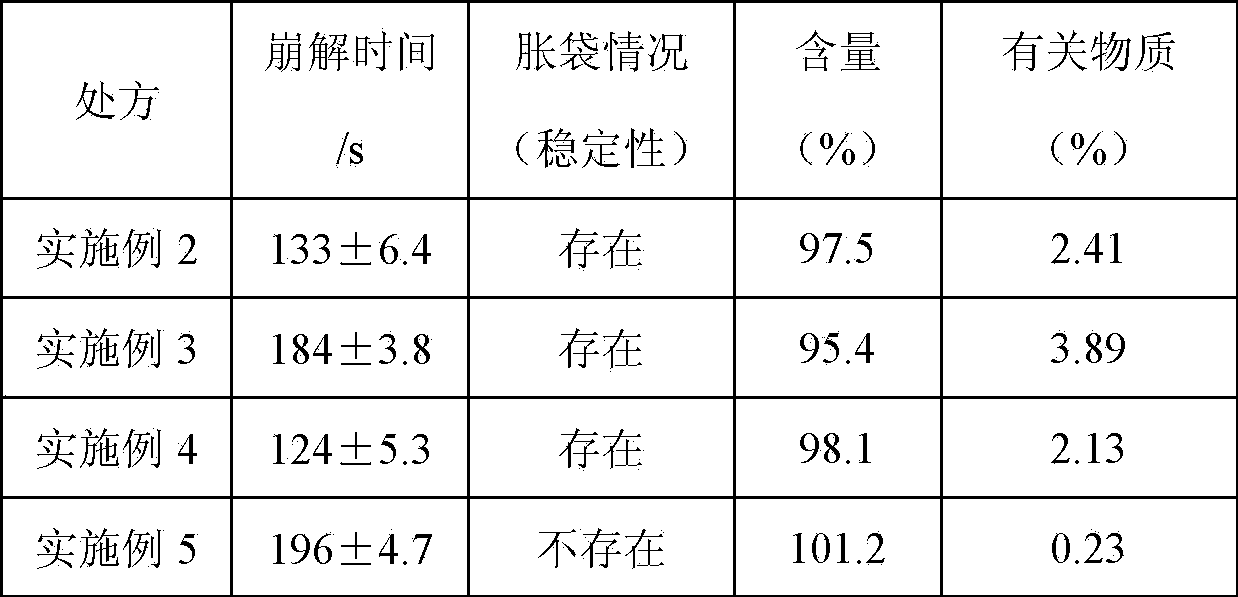
Ornithine aspartate effervescent tablets and preparing process thereof
InactiveCN103860517AAvoid degradationGood disintegrationOrganic active ingredientsDigestive systemEffervescent tabletOrnithine aspartate
The invention provides ornithine aspartate effervescent tablets and a preparing process thereof. The ornithine aspartate effervescent tablets comprise the components by mass percentage: 20%-65% of ornithine aspartate, 0%-5% of a binder, 15%-25% of a disintegrating acid agent, 10%-15% of a disintegrating alkali agent, and 0%-10% of a lubricant. The preparation process comprises a polyethylene glycol wrapping-wet granulation process comprising the steps of wrapping the disintegrating alkali agent with polyethylene glycol, then carrying out wet granulation of ornithine aspartate and the disintegrating acid agent, finally adding additional auxiliary materials, totally mixing, and tabletting. The prepared ornithine aspartate effervescent tablets have the following advantages that the disintegration speed is fast, the effervescent tablets can be disintegrated in 2-3 min, the dissolving speed is fast, the in-vivo absorption speed is fast, the acting is rapid, and the bioavailability is high.
Owner:PEKING UNIV FOUNDER GRP CO LTD +2
Preparation method and application of bioactive nanometer organic zinc and selenium animal and plant nutrient solution
InactiveCN107021847APromote absorptionPromote enrichmentNitrogenous fertilisersOrganic fertiliser preparationBetaineNutrient solution
The invention discloses a preparation method and application of a bioactive nanometer organic zinc and selenium animal and plant nutrient solution. The method comprises the following steps: weighing raw materials in the following mass percent, and adding the following raw materials in every 5000kg of water: 8 to 12kg of maltose, 15 to 25kg of sodium cyclamate, 15 to 25g of gelatin, 15 to 25g of chitosan, 25 to 35g of zinc oxide, 450 to 550g of lysine, 35 to 45g of sodium tetraborate, 95 to 105g of glycine betaine, 95 to 105g of nonprotein nitrogen, 95 to 105g of beta-glucolase, 450 to 550g of saccharomycetes, 450 to 550g of selenium philic bacillus laterosporus, and 25 to 35g of selenium dioxide; weighing and uniformly mixing and stirring the raw materials, and performing fermentation on the raw materials for 15 to 20 days at 25 to 35 DEG C, so as to obtain the nutrient solution. The nutrient solution can not only ensure that plants accelerate absorb and enrich organic zinc and selenium, but also can promote the plants to perform in vivo absorption on various nutrients beneficial to growth and improvement of the mouthfeel, enables the egg dead rate to be greater than 95 percent, is odorless, environmental-friendly, convenient to apply, uniform, does not destroy roots and seedlings, improves the plant quality and yield, and can further improve the antiviral and anti-infective ability of animals.
Owner:赵福振
Celecoxib solid dispersion and preparation method and application thereof
InactiveCN103655478AGood dissolution effectGood disintegrationOrganic active ingredientsPowder deliverySolubilityDispersity
The invention specifically relates to a celecoxib solid dispersion and a preparation method and application thereof. The celecoxib solid dispersion is prepared from celecoxib and poloxamer 188 at a mass ratio of 1:(1-20) by a melting process and a solvent process. The dispersion provided by the invention has good dispersity and high stability; the solubility and dissolution rate of the medicine are increased; the solubility of the medicine in water is 5-200 times higher than that of the raw medicines; with good dissolution rate in water, the in-vivo absorption of the medicine can be enhanced so as to improve the bioavailability. The solid dispersion also can be made into multiple clinically acceptable dosage forms which are used as new dosage forms of celecoxib.
Owner:BEIJING SUNHO PHARMA
Composition containing dronedarone
ActiveCN102349889APromote dissolutionImprove absorption rateOrganic active ingredientsPharmaceutical non-active ingredientsDronedaroneBlood concentration
The invention relates to a pharmaceutical composition which comprises dronedarone or pharmaceutically acceptable salt thereof used as a preparation active ingredient, a phosphatide, an acidity regulator and one or more crystallization inhibitors. The composition is suitable for any oral drug forms, such as tablets, capsules or granules. The composition has the advantages that: the drug dissolution can be effectively increased, thereby increasing the in-vivo absorption rate and degree of the active ingredients; and more importantly, the areas below the in-vivo blood concentration-time curves of the Beagle dog under drug feeding / fasting conditions have no difference almost.
Owner:JILIN BODA PHARMA
Olaparib oral controlled-release and sustained-release pharmaceutical composition and uses thereof
InactiveCN108201536AAchieve absorptionAccurate blood drug concentration in vivoOrganic active ingredientsPill deliveryControl releaseEnzyme inhibition
The present invention relates to an olaparib oral controlled-release and sustained-release pharmaceutical composition, which contains dissolution form improved olaparib and a matrix polymer for release rate adjustment. According to the present invention, the in vivo absorption behavior, the blood drug level and the PARP enzyme inhibition level of the pharmaceutical composition can be controlled, the pharmaceutical composition has the improved olaparib loading and / or oral absorption and / or bioavailability and / or blood drug concentration control and / or enzyme inhibition level control, and can beused as the sole preparation or can be combined with other treatment methods in the treatment of cancer.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Suspension containing andrographolide solid lipid nanoparticles as well as preparation method and application of suspension
ActiveCN102716080AGrowth inhibitory effectImprove tumor inhibition rateOrganic active ingredientsSolution deliveryEmulsionIn vivo absorption
The invention relates to suspension containing andrographolide solid lipid nanoparticles as well as a preparation method and an application of the suspension, wherein the andrographolide solid lipid nanoparticles are dispersed in emulsion formed by purified water and emulsifying agents and consists of andrographolide and lipid materials, the weight ratio of the andrographolide to the lipid materials to the emulsifying agents is (1-15): (35-70):(20-50), and the consumption of the purified water is metered according to the principle that 1ml of purified water is added into 5 to 20mg of emulsifying agents. The suspension has the advantages that the andrographolide is covered and sealed in lipid inner cores of the solid lipid nanoparticles, the in-vivo absorption of the medicine is improved, the bioavailability is improved, meanwhile, and the slow release performance and the targeting performance are realized, so the toxic or side effect is reduced, and the anti-tumor curative effect is improved.
Owner:HENAN UNIVERSITY
Determination method for absorption and transportation amount of six components in rhizoma bletillae in Caco-2 cell model
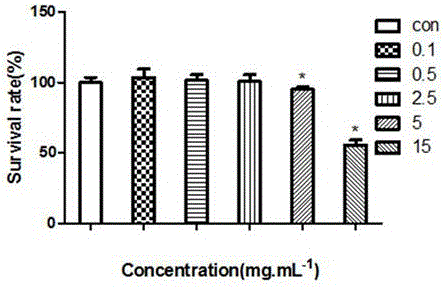
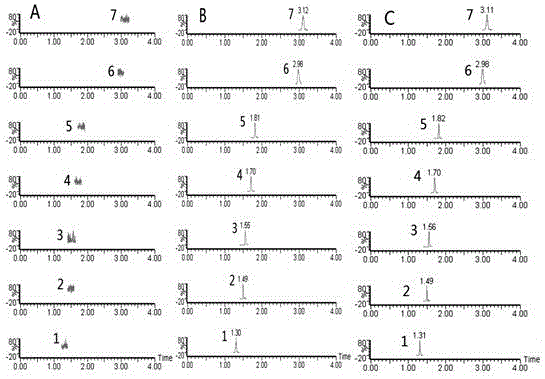
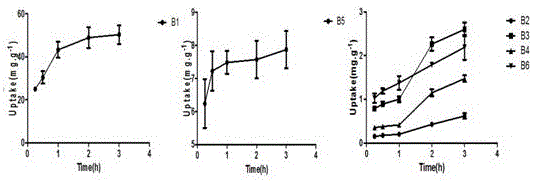
ActiveCN105954411AEvaluation of in vivo absorption propertiesComponent separationInternal standardIn vivo absorption
The invention discloses a determination method for the absorption and transportation amount of six components in rhizoma bletillae in a Caco-2 cell model. The method comprises the steps of: preparing a rhizoma bletillae extract solution, a standard solution serving as a reference substance and an internal standard solution; establishing a human-derived colon adenocarcinoma cell line Caco-2 cell model; preparing a cell suspension through a Caco-2 cell model; determining the content of the six components by UPLC-MS / MS; determining the total protein content according to a Coomassie brilliant blue dye liquor protein determination kit method, and calculating the cell uptake X=the total protein of a to-be-determined substance. The invention adopts UPLC-MS / MS to establish the analysis method for the 6 components in a rhizoma bletillae extract, determines the influence of the rhizoma bletillae extract to absorption and uptake of Caco-2 cells under the conditions of time, concentration, temperature, pH and P-gh inhibitors, preliminarily evaluates the in vivo absorption characteristics of the rhizoma bletillae extract, and provides scientific basis for the research and development of the oral preparation of the rhizoma bletillae extract.
Owner:GUIZHOU MEDICAL UNIV
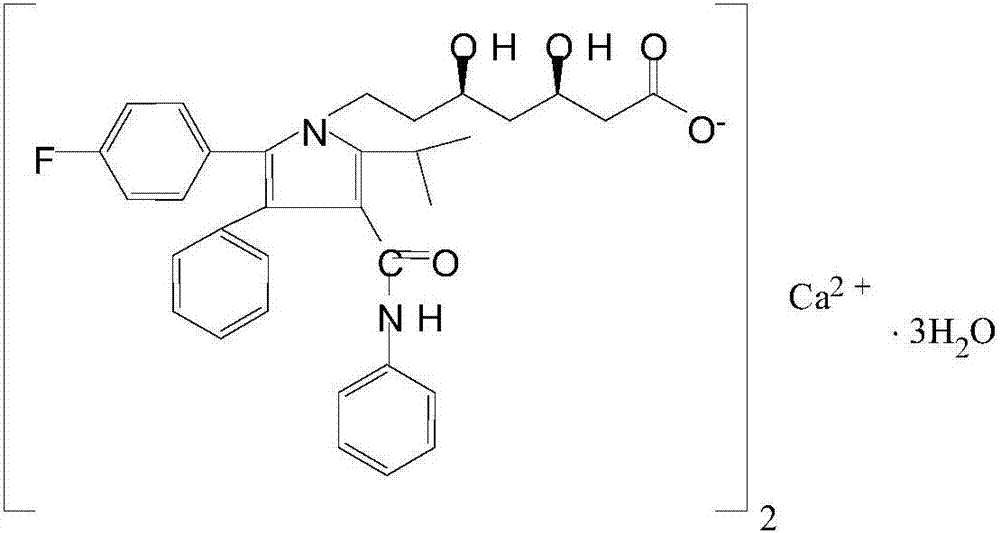
Tablet comprising atorvastatin calcium alkaline solid dispersoid and preparation method of tablet
InactiveCN107998085AHigh dissolution rateImprove stabilityMetabolism disorderPharmaceutical non-active ingredientsHydrogenIn vivo absorption
The invention provides a tablet comprising an atorvastatin calcium alkaline solid dispersoid and a preparation method of the tablet. The solid dispersoid comprises atorvastatin calcium, a water-soluble copolymer and a pH (potential of hydrogen) regulator. The tablet is prepared by adding a filler, a disintegrant and a lubricant into the solid dispersoid and has the characteristics of good dissolubility, high stability, quick in vivo absorption, small individual variation and uniform absorption during administration for many times. At the same time, the method is simple in preparation technology and suitable for large-scale production.
Owner:乐普制药科技有限公司
4-Amino-2-trifluoromethylphenylretinoate self-microemulsion and preparation method thereof
InactiveCN103356504AImprove solubilityImprove stabilityOrganic active ingredientsCapsule deliverySolubilityCell membrane
The invention relates to a technology of improving the in-vitro dissolution of an indissolvable medicament, discloses a self-microemulsion, and especially discloses an ATPR (4-Amino-2-Trifluoromethyl Phenyl Retinoate) self-microemulsion and a preparation method thereof. The ATPR self-microemulsion is characterized by being prepared from the following components by weight percent: 0.1-20% of ATPR, 1-30% of oil phase, 40-85% of surfactant and 1-40% of cosurfactant. The self-microemulsion and the preparation method provided by the invention have the following beneficial effects: the solubility of the medicament is improved by the ATPR self-microemulsion drug release system, the medicament is dissolved in oil drops and thus protected and the stability of the medicament in vivo is improved, the surfactant is capable of changing the liquidity of the cell membrane and improving the membrane permeability of the medicament, so that the in-vivo absorption of the medicament is increased and the relative bioavailability of the medicament is improved. Besides, the solid self-microemulsion is capable of improving the phenomena of softening and oil leakage of the traditional soft capsules and enhancing the stability of the preparation.
Owner:ANHUI MEDICAL UNIV
Radioisotope labeled meso-porous bioglass porous scaffold and making method thereof
InactiveCN104117090AMarked validDetection of in vivo degradationProsthesisIn vivo absorptionIn vivo degradation
The invention provides a radioisotope labeled meso-porous bioglass porous scaffold. A meso-porous bioglass porous scaffold is labeled with radioisotope, and the radioisotope can be one or more of <31>Si, <32>P and <45>Ca. The radioisotope labeled meso-porous bioglass porous scaffold can effectively label the meso-porous bioglass porous scaffold, can quantitatively and intuitively detect the in vivo degradation situation of the meso-porous bioglass porous scaffold, and can be used to research the in vivo absorption and the organism distribution of the bioglass scaffold.
Owner:SHANGHAI NINTH PEOPLES HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Application of lipid-modified substance of chlorogenic acid and derivative thereof
InactiveCN104826118AImprove lipophilicityImprove bioavailabilityAntibacterial agentsOrganic active ingredientsLipid formationPurification methods
The invention provides a lipid-modified substance of chlorogenic acid and a derivative thereof, and a preparation and purification method of the lipid-modified substance, wherein the lipid-modified substance includes the chlorogenic acid or the derivative thereof and phosphoglyceride. A preparation process includes steps of performing constant-temperature magnetic stirring reflux for a certain time to the chlorogenic acid or the derivative thereof and the phosphoglyceride with an aprotic solvent and drying an obtained reaction liquid to obtain a crude product. A purification process includes the steps of re-dissolving the crude product with filtration to obtain a filtrate and then drying the filtrate. The lipid-modified substance of the chlorogenic acid and the derivative thereof is high in composition rate and can significantly improve the fat-solubility of the chlorogenic acid and the derivative thereof, so that a subsequent preparation is convenient to process and shape. The lipid-modified substance can be used as an intermediate for preparing a lipidosome, nano particles, polymer micelles or a dendritic polymer and the like nano drug-delivery systems and micro emulsion, self-micro emulsion and the like micron drug-delivery systems, thereby enhancing in-vivo absorption of the chlorogenic acid and the derivative thereof and further increasing the bioavailability of the medicines in a preparation.
Owner:任静 +1
Novel absorbable hemostatic material
ActiveCN103446619AEasy to prepareReduce manufacturing costAbsorbent padsBandagesHEMOSTATIC POWDERPolyvinyl alcohol
The invention relates to a novel absorbable hemostatic material which is easy in preparation method, low in cost and good in hemostatic effect. At present most medical hemostatic materials in China are water-soluble hemostatic gauze, and the gauze does not have anti-inflammatory and anti-bacterial effects when being used for hemostasis, and has the defects that the hemostatic speed is low, the in vivo absorption time is long, an inflammatory reaction is easy to cause and the like, and therefore people turns to hemostatic powder, however, as existing hemostatic powder is complex to prepare, high in price, not ideal in hemostatic effect and the like, the hemostatic powder is not applied as a mainstream hemostatic product. The novel absorbable hemostatic material is a guluronic acid polymer generated by an complexation reaction or crosslinking reaction of a guluronic acid monomer, polyvinyl alcohol, lavender essential oil and clove essential oil. The novel absorbable hemostatic material is good in hemostatic effect, and has the advantages that the hemostatic speed is high, the in vivo absorption time is short, broad-spectrum sterilization and anti-inflammation effects are achieved, wound healing is promoted and the like, and in addition, the novel absorbable hemostatic material is not affected by the size and position of a wound surface, and widely applicable to the fast hemostasis for war wound, trauma and other conditions.
Owner:QINGDAO ZHONGTENG BIOTECH
Ezetimibe tablet and preparation method thereof
InactiveCN105213340AReduce fluctuations in dissolutionImprove securityOrganic active ingredientsMetabolism disorderIn vivo absorptionDissolution
The invention provides an ezetimibe tablet and a preparation method thereof. The ezetimibe tablet is prepared from the following components in parts by weight: 10 parts of ezetimibe, 10 parts of a disintegrating agent, 2 parts of a solubilizing agent, 55 to 70 parts of a filling agent, 5 to 15 parts of microcrystalline cellulose, 5 parts of an adhesive and 0.5 parts of a lubricant. According to the ezetimibe tablet disclosed by the invention, the fluctuation of the dissolution of the ezetimibe tablet can be effectively reduced, the dissolution difference is reduced, the safety of the ezetimibe tablet in a medicine using process is improved, and the fluctuation of in-vivo absorption of the ezetimibe tablet is reduced; meanwhile, according to the preparation method of the ezetimibe tablet, a production process is simplified, and the method can adapt to large-scale industrial production.
Owner:WUXI FORTUNE PHARMA
Nimodipine lipid nanoparticle and preparation technology thereof
ActiveCN107019682ASafe and reliableImprove uniformityOrganic active ingredientsSenses disorderDispersityOrganic solvent
The invention belongs to the field of pharmaceutical preparations and discloses a nimodipine lipid nanoparticle and a preparation technology thereof. The lipid nanoparticle is prepared by an emulsification-evaporation technique combined with a high pressure homogenization method, and a drug is encapsulated in a lipid carrier to form a stable and homogeneous colloidal solution. The prepared lipid nanoparticle is small in nanoparticle size, high in dispersity, good in stability, and capable of releasing the drug slowly and permanently and improving the in vivo absorption of the drug. The preparation technology is simple, low in cost, free from residual organic solvent, low in biological toxicity, and easy to realize industrial production.
Owner:CHINA PHARM UNIV
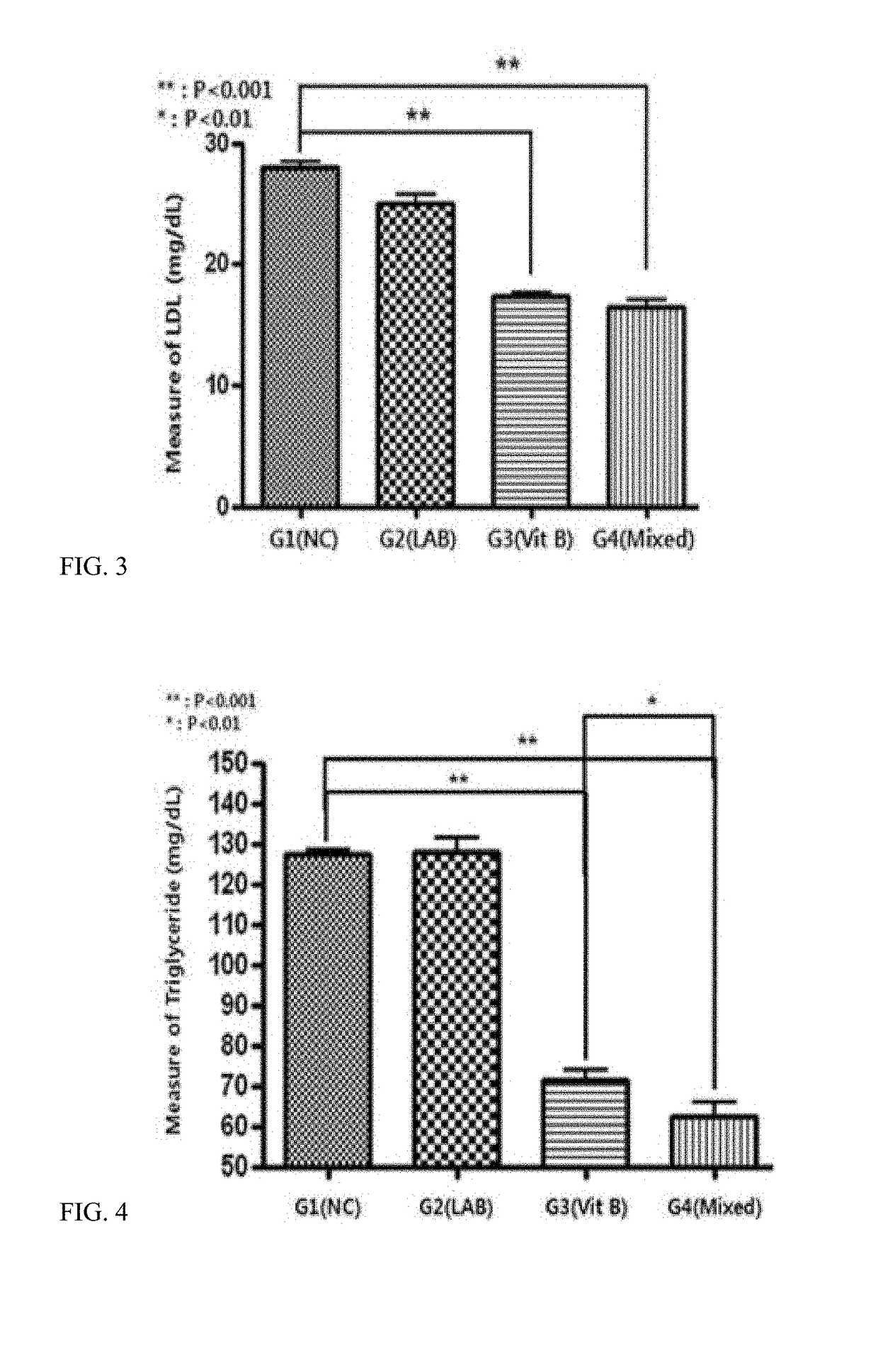
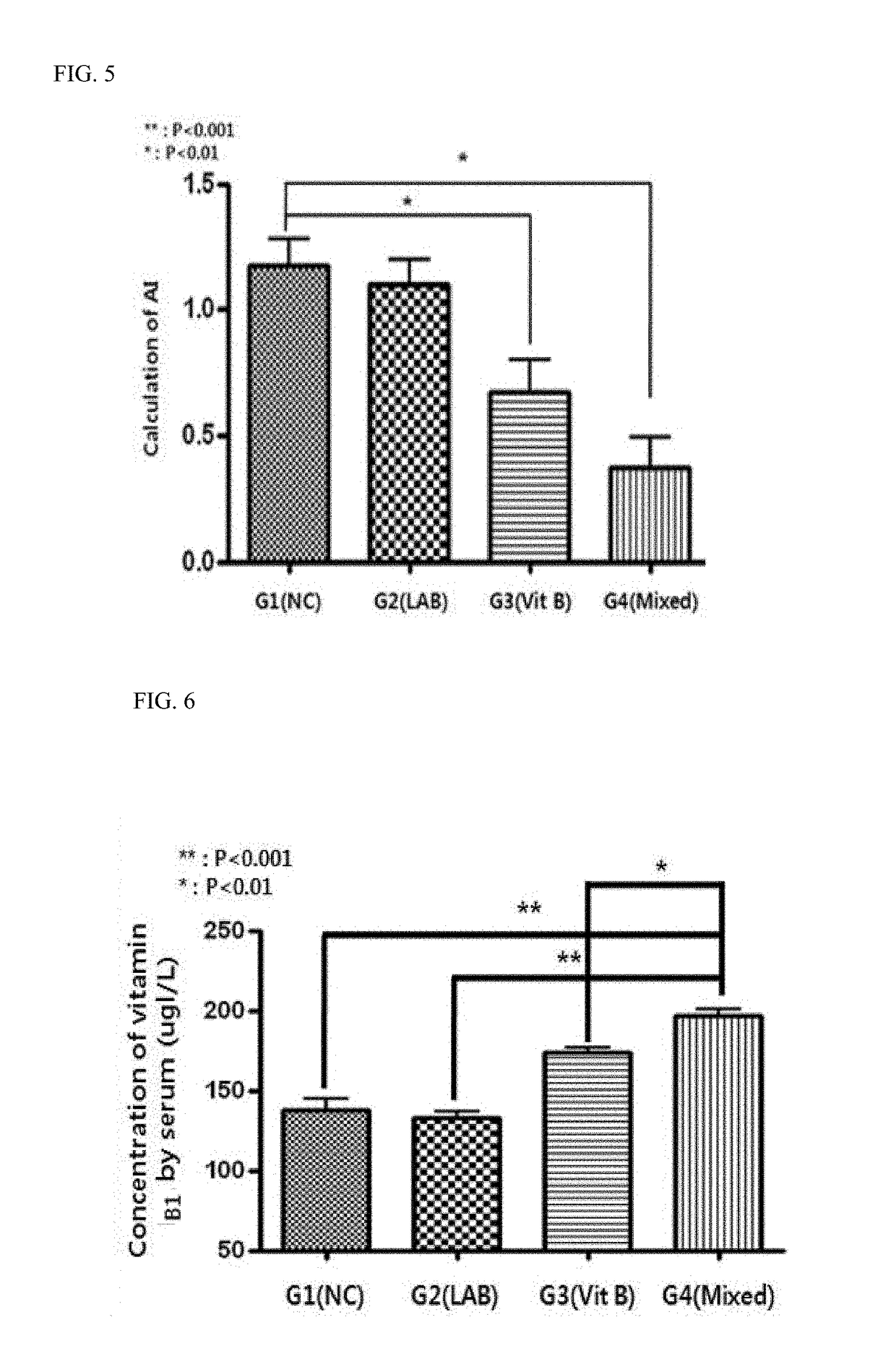
Composition for preventing or treating obesity or lipid-related metabolic disease
ActiveUS20180117102A1Metabolism disorderBacteria material medical ingredientsIn vivo absorptionBlood lipids
The present invention provides a pharmaceutical composition and a food composition for preventing or treating lipid-related metabolic disease, which contain, as active ingredients, probiotics and a vitamin B complex. When the composition comprising the probiotics and the vitamin B complex is used, it may further promote in vivo absorption of the vitamin B complex, and may induce a synergistic effect on a reduction in blood lipid levels, thereby exhibiting an effect on the alleviation, prevention or treatment of obesity and lipid-related metabolic disease.
Owner:CELL BIOTECH
Multi-variant and multi-phase salvia miltiorrhiza solid self-microemulsion, application thereof in preparation of drug and preparation method thereof
InactiveCN103446234AImprove stabilityReduce dosageEmulsion deliveryBlood disorderAdditive ingredientIn vivo absorption
The invention discloses a multi-variant and multi-phase salvia miltiorrhiza solid self-microemulsion, application thereof in preparation of a drug and a preparation method thereof. A solid self-microemulsion composition is prepared from the following components by weight: 0.1-10g of salvia miltiorrhiza multi-component extract, 10-30g of emulsifier, 5-20g of co-emulsifier, 10-30g of oil phase and 20-50g of solid adsorbent. Two components (total tanshinones and salvianolic acids) in the salvia miltiorrhiza are simultaneously loaded to one preparation; in vivo absorption of a plurality of ingredients in the salvia miltiorrhiza is improved. By adopting the preparation method, the stability of the self-microemulsion preparation can be increased; the dosage of a surfactant is greatly reduced; meanwhile, the bioavailability of a multi-component extract of the salvia miltiorrhiza is improved.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com