Application of lipid-modified substance of chlorogenic acid and derivative thereof
A technology of lipid modification and derivatives, which is applied in the field of medicine, can solve the problems of low bioavailability and poor membrane permeability, and achieve the effects of enhancing membrane permeability, improving lipophilicity, and facilitating processing and molding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
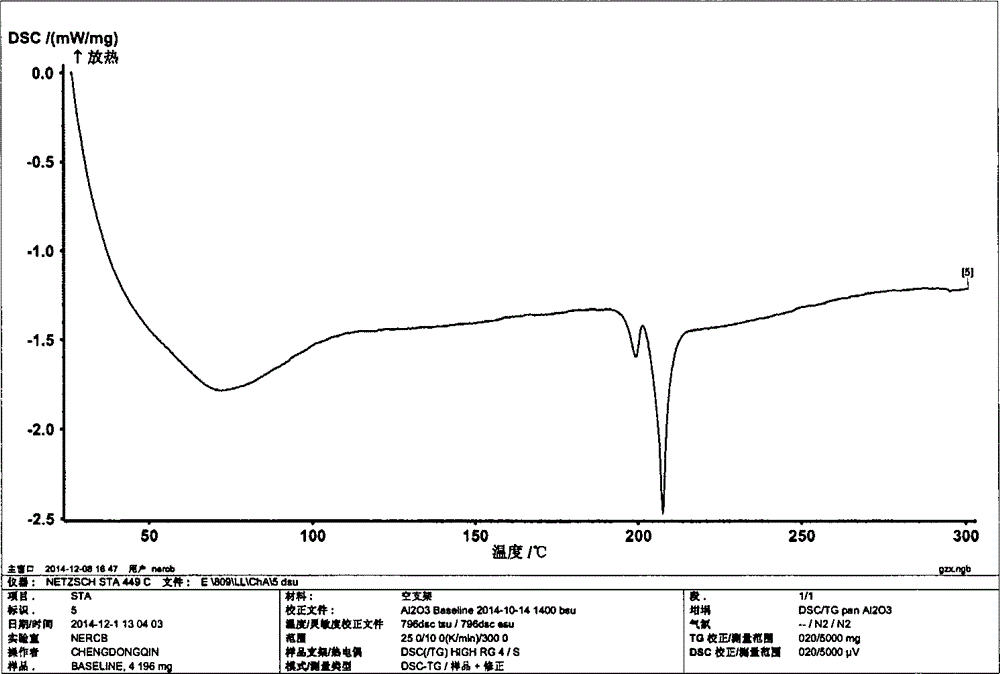
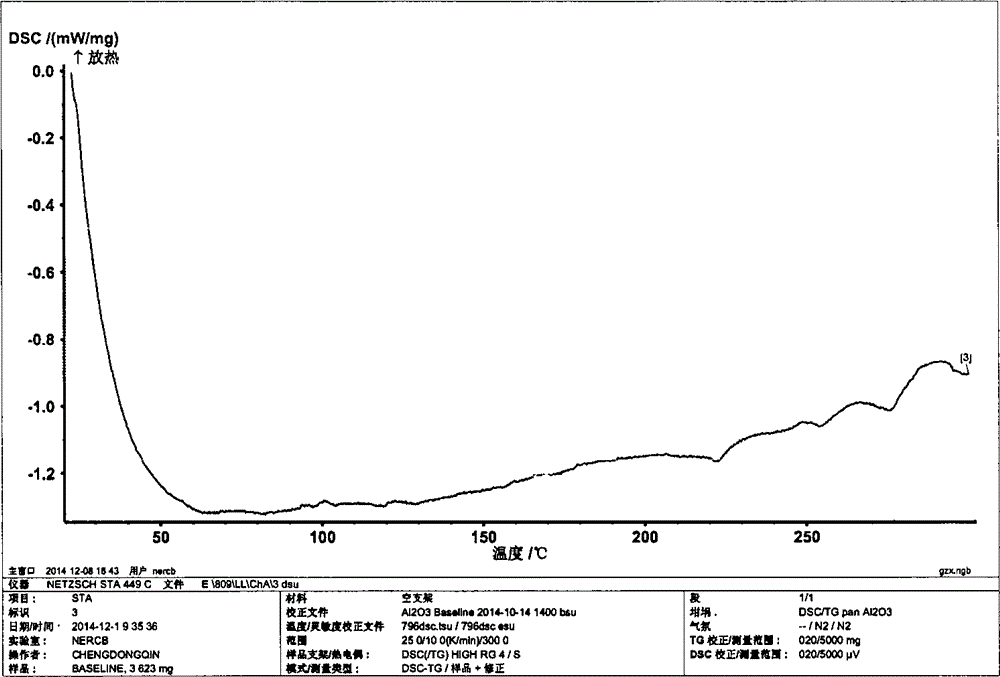
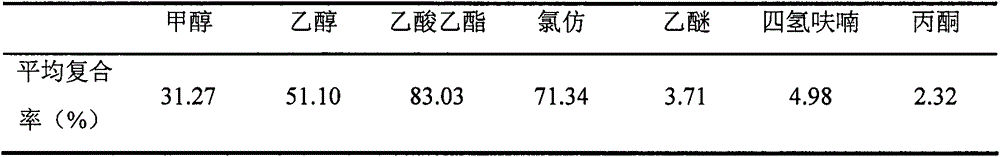
Image
Examples
Embodiment 1
[0037] Get chlorogenic acid and derivatives thereof and lipid equivalent to 0.5 times the weight of chlorogenic acid and derivatives thereof; add an appropriate amount of ethyl acetate, the amount of ethyl acetate used is to make the reaction concentration of chlorogenic acid and derivatives thereof 6mg / ml; reflux at 55°C with constant magnetic stirring for 0.5h to obtain the reaction solution; freeze-dry the reaction solution or dry it under reduced pressure to obtain the crude lipid film of chlorogenic acid and its derivative lipid modification; add diethyl ether to the crude product, Make it fully dispersed to obtain a dispersion liquid; filter the above dispersion liquid, take the filtrate and freeze-dry it again or dry it under reduced pressure to obtain the refined lipid-modified product of chlorogenic acid and its derivatives.
Embodiment 2
[0039] Get chlorogenic acid and derivatives thereof and lipid equivalent to 25 times the weight of chlorogenic acid and derivatives thereof; add appropriate amount of chloroform, the amount of chloroform used is to make the reaction concentration of chlorogenic acid and derivatives thereof be 48mg / ml; Under the condition of 15°C, constant temperature magnetic stirring and reflux for 3.5 hours to obtain the reaction solution; freeze-dry the reaction solution or dry it under reduced pressure to obtain the crude product of chlorogenic acid and its derivative lipid modified lipid film; add ethyl acetate to the crude product to make Fully disperse to obtain a dispersion liquid; filter the above dispersion liquid, and take the filtrate to freeze-dry or dry under reduced pressure again to obtain chlorogenic acid and its derivative lipid modified products.
Embodiment 3
[0041] Take chlorogenic acid and its derivatives and lipid equivalent to 1 times the weight of chlorogenic acid and its derivatives; add an appropriate amount of ethanol, the amount of ethanol used is such that the reaction concentration of chlorogenic acid and its derivatives is 20 mg / ml; Under the condition of 40°C, constant temperature magnetic stirring and reflux for 2.5h to obtain the reaction solution; freeze-dry the reaction solution or dry it under reduced pressure to obtain the crude product of chlorogenic acid and its derivative lipid modified lipid film; add dichloromethane to the crude product to make it Fully disperse to obtain a dispersion liquid; filter the above dispersion liquid, and take the filtrate to freeze-dry or dry under reduced pressure again to obtain chlorogenic acid and its derivative lipid modified products.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com