Patents
Literature
17973results about "Microcapsules" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Drug depot implant designs
ActiveUS7727954B2Uniform drug distributionMinimal disruptionPowder deliveryPeptide/protein ingredientsSkeletal injuryChronic pain
Owner:WARSAW ORTHOPEDIC INC
Cyclodextrin polymers for carrying and releasing drugs
This invention discloses methods for preparing compositions of cyclodextrin polymers for carrying drugs and other active agents. Methods are also disclosed for preparing cyclodextrin polymer carriers that release drugs under controlled conditions. The invention also discloses methods for preparing compositions of cyclodextrin polymer carriers that are coupled to biorecognition molecules for targeting the delivery of drugs to their site of action. The advantages of the water soluble (or colloidal) cyclodextrin polymer carrier are: (1) Drugs can be used that are designed for efficacy without conjugation requirements. (2) It will allow the use of drugs designed solely for efficacy without regard for solubility. (3) Unmodified drugs can be delivered as macromolecules and released within the cell. (4) Drugs can be targeted by coupling the carrier to biorecognition molecules. (5) Synthesis methods are independent of the drug to facilitate multiple drug therapies.
Owner:KOSAK KENNETH M
Method of producing sustained-release preparation
InactiveUS6267981B1Maintain good propertiesEnhancement of entrapmentPowder deliveryPeptide/protein ingredientsEntrapmentBiodegradable polymer
This invention provides a sustained-release preparation comprising a biodegradable polymer metal salt and broactive polypeptide, with enhanced entrapment of the bioactive polypeptides, a suppression of initial burst, and a constant long-term release of the bioactive polypeptides.
Owner:TAKEDA PHARMA CO LTD
Method of producing a sustained-release preparation
InactiveUS6197350B1Reduce the number of stepsSuitable for industrializationPowder deliveryPeptide/protein ingredientsBlood concentrationOrganic solvent
A method of producing sustained-release microcapsules which comprises dispersing a physiologically active polypeptide into a solution of a biodegradable polymer and zinc oxide in an organic solvent, followed by removing the organic solvent; which provides a sustained-release preparation showing a high entrapment ratio of the physiologically active polypeptide and its constant high blood concentration levels over a long period of time.
Owner:TAKEDA PHARMA CO LTD
Controlled drug delivery system using the conjugation of drug to biodegradable polyester
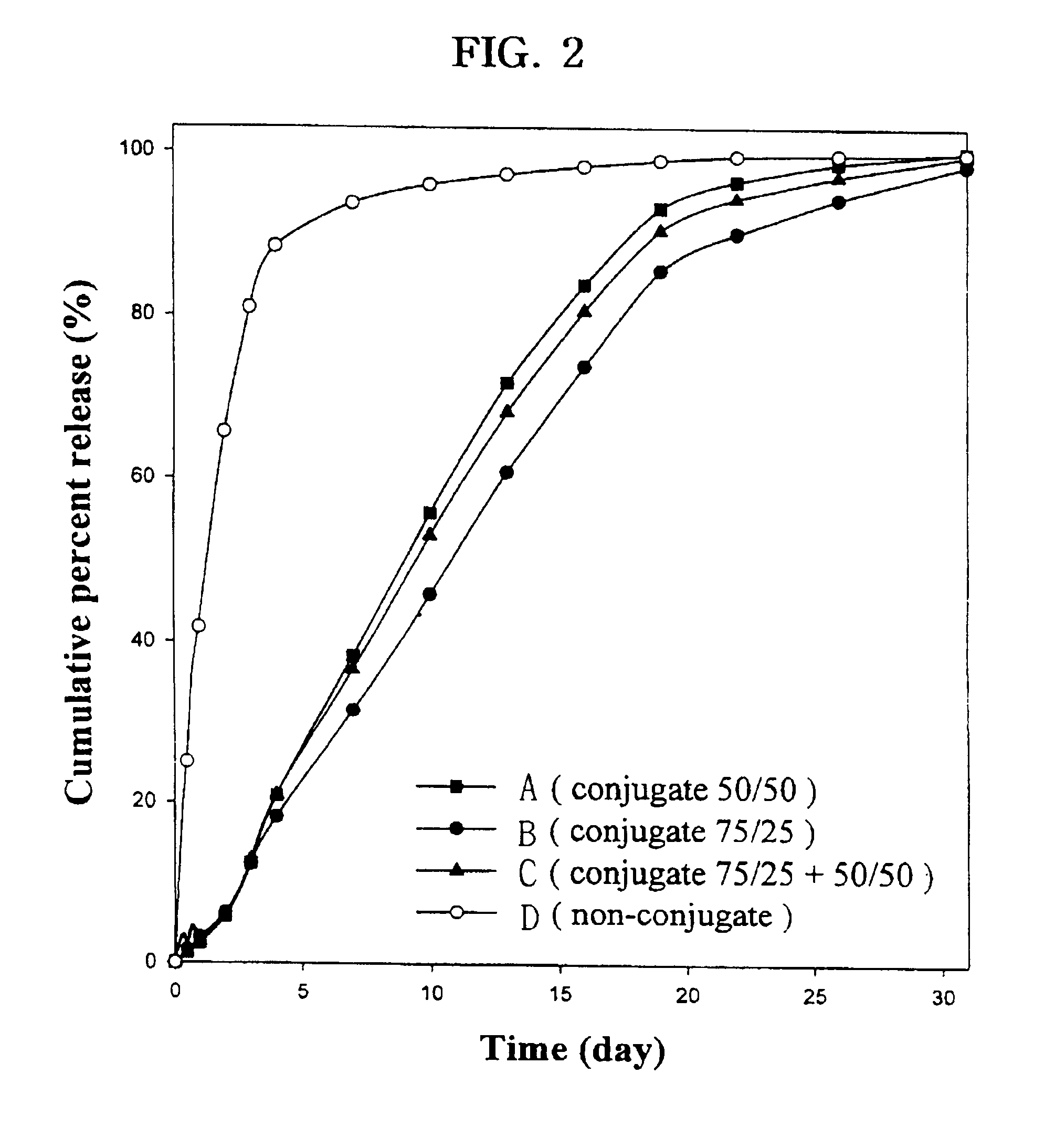
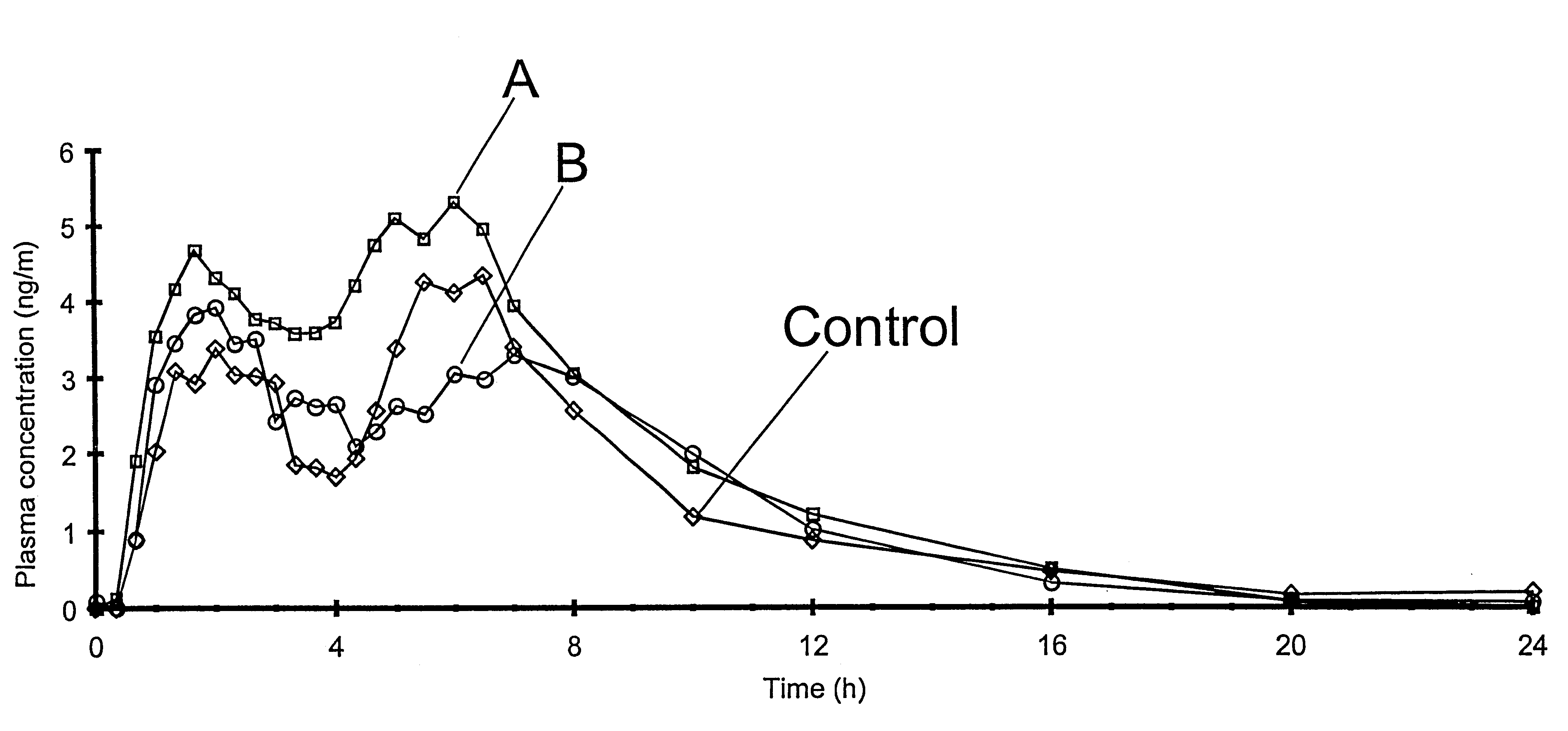
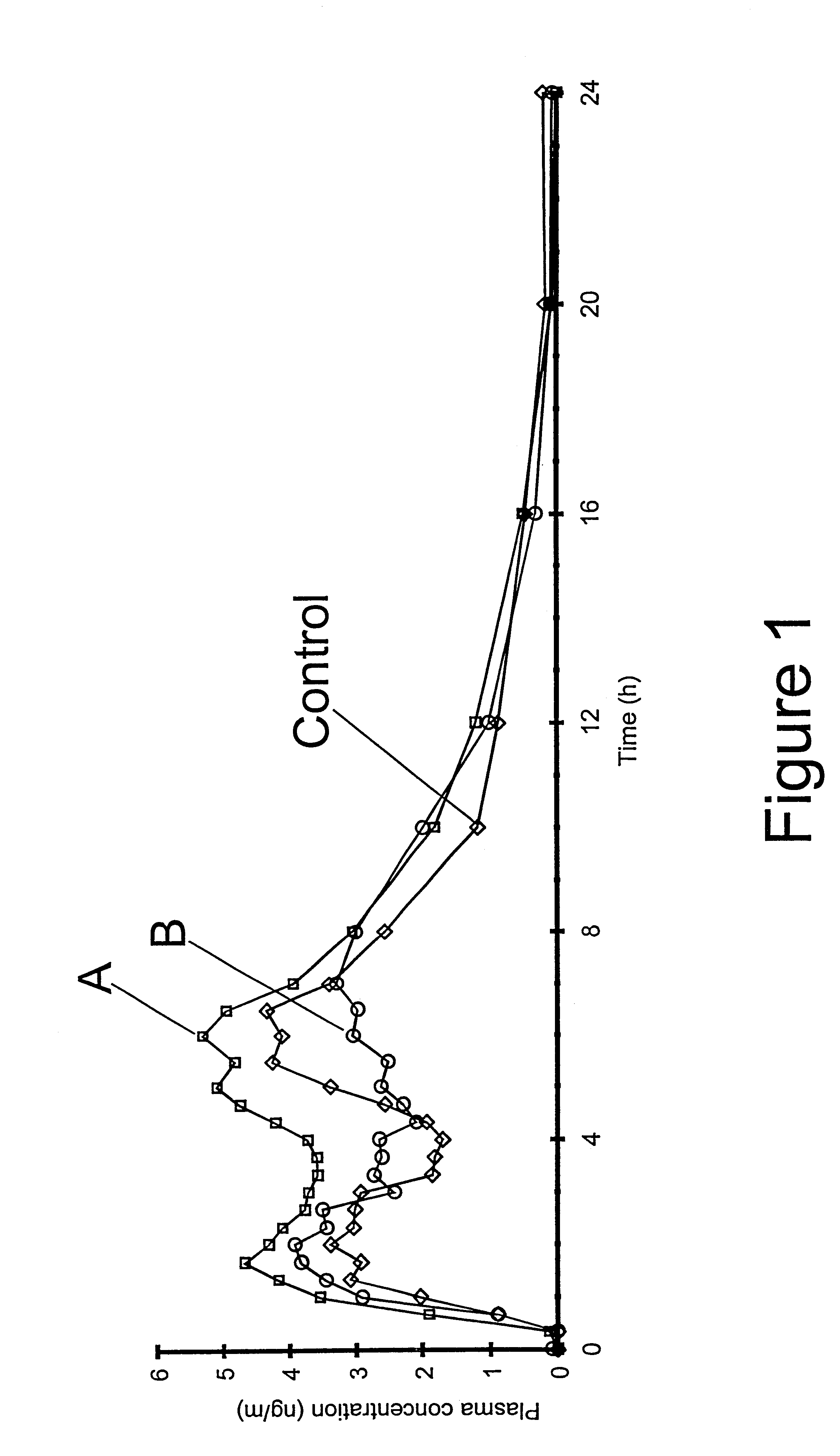
The present invention relates to a molecular sustained controlled release system constructed by the conjugation of molecules to be released with biodegradable polyester polymer via covalent bond and method for preparation thereof. In accordance with the present invention, the system may be formulated into microspheres, nanoparticles, or films. The molecular release rate from the above system can be regulated to be proportional to the chemical degradation rate of the biodegradable polyester polymers, resulting in near zero order kinetics profile of release without showing a burst effect, Moreover, a high loading efficiency of hydrophilic drugs can be achieved.
Owner:MOGAM BIOTECH RES INST +1
Multiparticulate modified release composition
The invention relates to a multiparticulate modified release composition that in operation delivers an active ingredient in a pulsed or bimodal manner. The multiparticulate modified release composition comprises an immediate release component and a modified release component; the immediate release component comprising a first population of active ingredient containing particles and the modified release component compnsimg a second population of active ingredient containing particles coated with a controlled release coating; wherein the combination of the immediate release and modified release components in operation deliver the active ingredient in a pulsed or a bimodal manner. The invention also relates to a solid oral dosage form containing such a multiparticulate modified release composition. The plasma profile achieved by the multiparticulate modified release composition is advantageous in reducing patient tolerance to the active ingredient and in increasing patient compliance by reducing dosage frequency.
Owner:ALKERMES PHARMA IRELAND LTD +1
Sustained release drug delivery devices, methods of use, and methods of manufacturing thereof
InactiveUS6375972B1Without of effectWithout riskOrganic active ingredientsSenses disorderBiological bodySustained release drug
A method and device for treating a mammalian organism to obtain a desired local or systemic physiological or pharmacological effect is provided. The method includes administering a sustained release drug delivery system to a mammalian organism in need of such treatment at an area wherein release of an effective agent is desired and allowing the effective agent to pass through the device in a controlled manner. The device includes an inner core or reservoir including the effective agent, an impermeable tube which encloses portions of the reservoir, and a permeable member at an end of the tube.
Owner:EYEPOINT PHARMA INC
Emulsion compositions
Owner:UK RES & INNOVATION LTD
Methods and compositions useful for administration of chemotherapeutic agents
InactiveUS6096331AReduce morbidityLow toxicityPowder deliveryEchographic/ultrasound-imaging preparationsActive agentIn vivo
In accordance with the present invention, there are provided compositions and methods useful for the in vivo delivery of a pharmaceutically active agent, wherein the agent is associated with a polymeric biocompatible material.
Owner:ABRAXIS BIOSCI LLC
Therapeutic treatment and prevention of infections with a bioactive materials encapsulated within a biodegradable-biocompatible polymeric matrix
InactiveUS6309669B1Sustained release of active agent over timeEfficient and effective usePowder deliveryPeptide/protein ingredientsAdjuvantEnd-group
Novel burst-free, sustained release biocompatible and biodegrable microcapsules which can be programmed to release their active core for variable durations ranging from 1-100 days in an aqueous physiological environment. The microcapsules are comprised of a core of polypeptide or other biologically active agent encapsulated in a matrix of poly(lactide / glycolide) copolymer, which may contain a pharmaceutically-acceptable adjuvant, as a blend of upcapped free carboxyl end group and end-capped forms ranging in ratios from 100 / 0 to 1 / 99.
Owner:ARMY GOVERNMENT OF THE UNITED STATES AS REPRESENTED BY THE SEC OF THE
Compositions and methods for coating medical devices
InactiveUS6143037AStimulates and promotesImproved wound healing characteristicSuture equipmentsInternal osteosythesisDiseaseMedical device
Owner:MICHIGAN REGENTS THE UNIV OF
Protein stabilized pharmacologically active agents, methods for the preparation thereof and methods for the use thereof
InactiveUS6749868B1Low toxicityLong half-lifePowder deliveryEchographic/ultrasound-imaging preparationsSuspended particlesFree protein
In accordance with the present invention, there are provided compositions and methods useful for the in vivo delivery of substantially water insoluble pharmacologically active agents (such as the anticancer drug paclitaxel) in which the pharmacologically active agent is delivered in the form of suspended particles coated with protein (which acts as a stabilizing agent). In particular, protein and pharmacologically active agent in a biocompatible dispersing medium are subjected to high shear, in the absence of any conventional surfactants, and also in the absence of any polymeric core material for the particles. The procedure yields particles with a diameter of less than about 1 micron. The use of specific composition and preparation conditions (e.g., addition of a polar solvent to the organic phase), and careful election of the proper organic phase and phase fraction, enables the reproducible production of unusually small nanoparticles of less than 200 nm diameter, which can be sterile-filtered. The particulate system produced according to the invention can be converted into a redispersible dry powder comprising nanoparticles of water-insoluble drug coated with a protein, and free protein to which molecules of the pharmacological agent are bound. This results in a unique delivery system, in which part of the pharmacologically active agent is readily bioavailable (in the form of molecules bound to the protein), and part of the agent is present within particles without any polymeric matrix therein.
Owner:ABRAXIS BIOSCI LLC
Bioactive agent delivering system comprised of microparticles within a biodegradable to improve release profiles
InactiveUS6589549B2Improve stabilityPowder deliveryPeptide/protein ingredientsActive agentEngineering
A composition and method for releasing a bio-active agent or a drug within a biological environment in a controlled manner is disclosed. The composition is a dual phase polymeric agent-delivery composition comprising a continuous biocompatible gel phase, a discontinuous particulate phase comprising defined microparticles and an agent to be delivered. A microparticle containing a bio-active agent is releasably entrained within a biocompatible polymeric gel matrix. The bioactive agent release may be contained in the microparticle phase alone or in both the microparticles and the gel matrix. The release of the agent is prolonged over a period of time, and the delivery may be modulated and / or controlled. In addition, a second agent may be loaded in some of the microparticles and / or the gel matrix.
Owner:BTG INT LTD
Tamper-resistant oral opioid agonist formulations
InactiveUS6696088B2Lower potentialReduce releasePowder deliveryNervous disorderOpioid AgonistOpioid antagonist
Disclosed is an oral dosage form comprising (i) an opioid agonist in releasable form and (ii) a sequestered opioid antagonist which is substantially not released when the dosage form is administered intact, such that the ratio of the amount of antagonist released from said dosage form after tampering to the amount of said antagonist released from said intact dosage form is about 4:1 or greater, based on the in-vitro dissolution at 1 hour of said dosage form in 900 ml of Simulated Gastric Fluid using a USP Type II (paddle) apparatus at 75 rpm at 37 degrees C. wherein said agonist and antagonist are interdispersed and are not isolated from each other in two distinct layers.
Owner:PURDUE PHARMA LP
Targeted and high density drug loaded polymeric materials
ActiveUS20060002852A1Increase molecular densityHigh densityPowder deliveryBiocideAntigenWound dressing
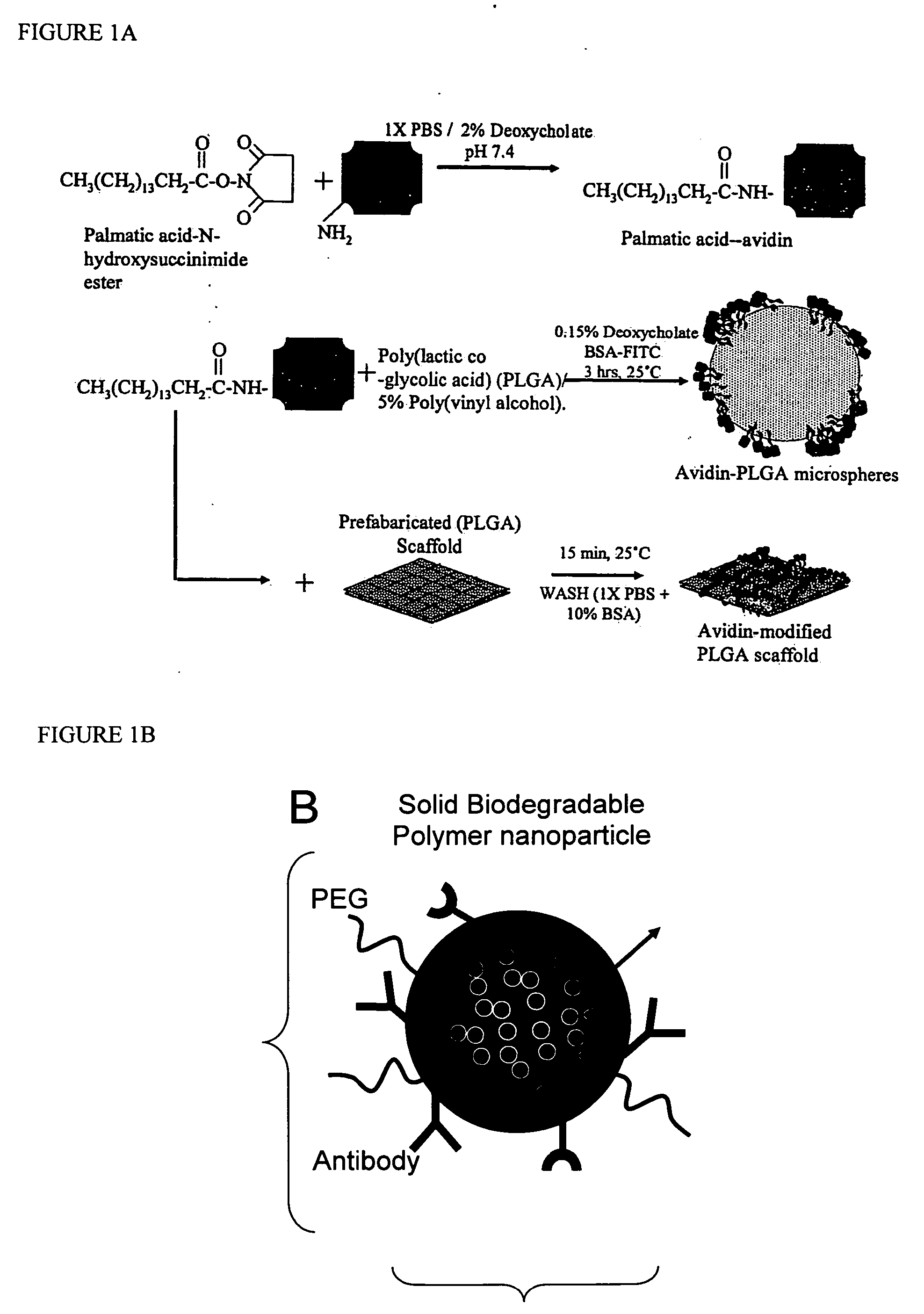
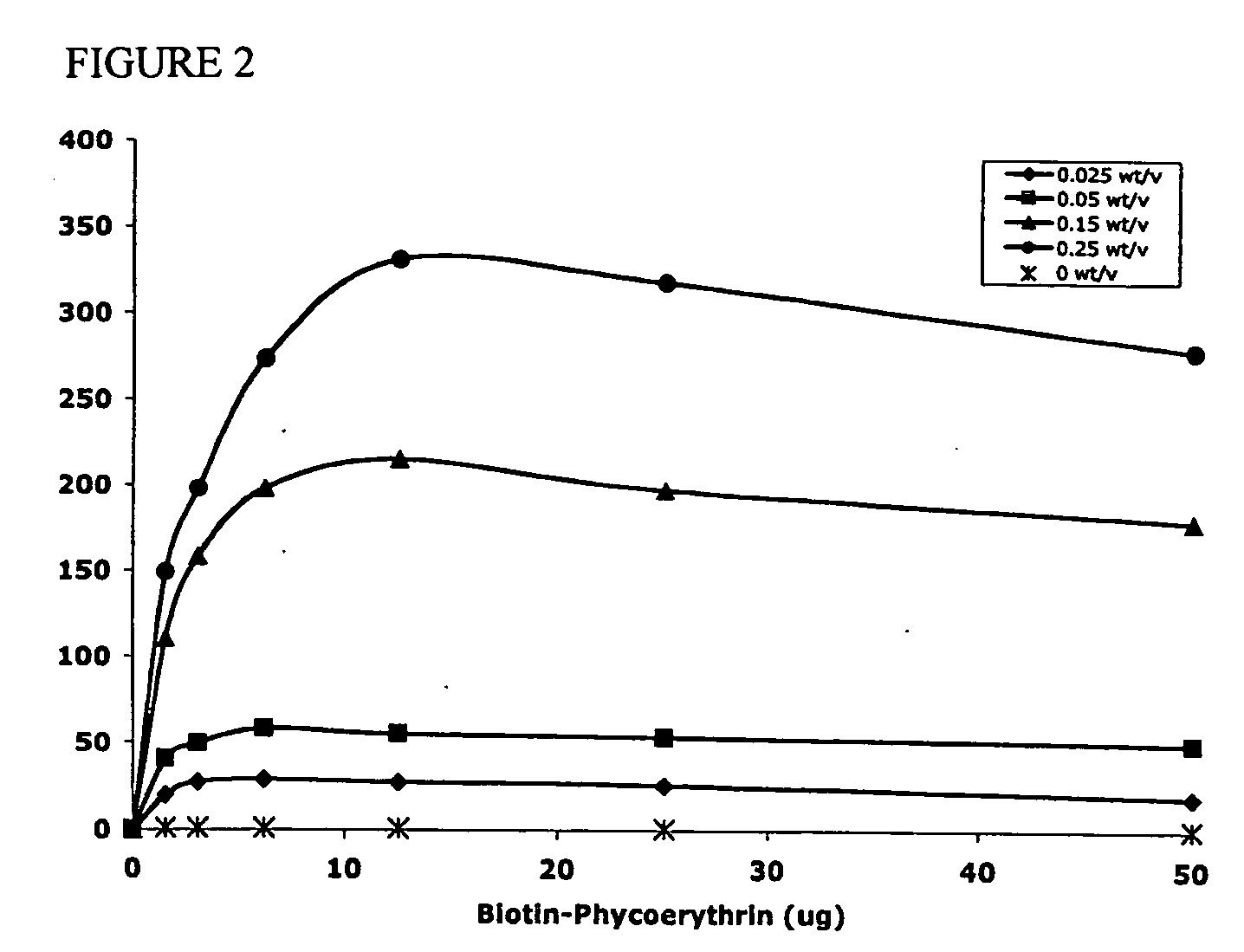
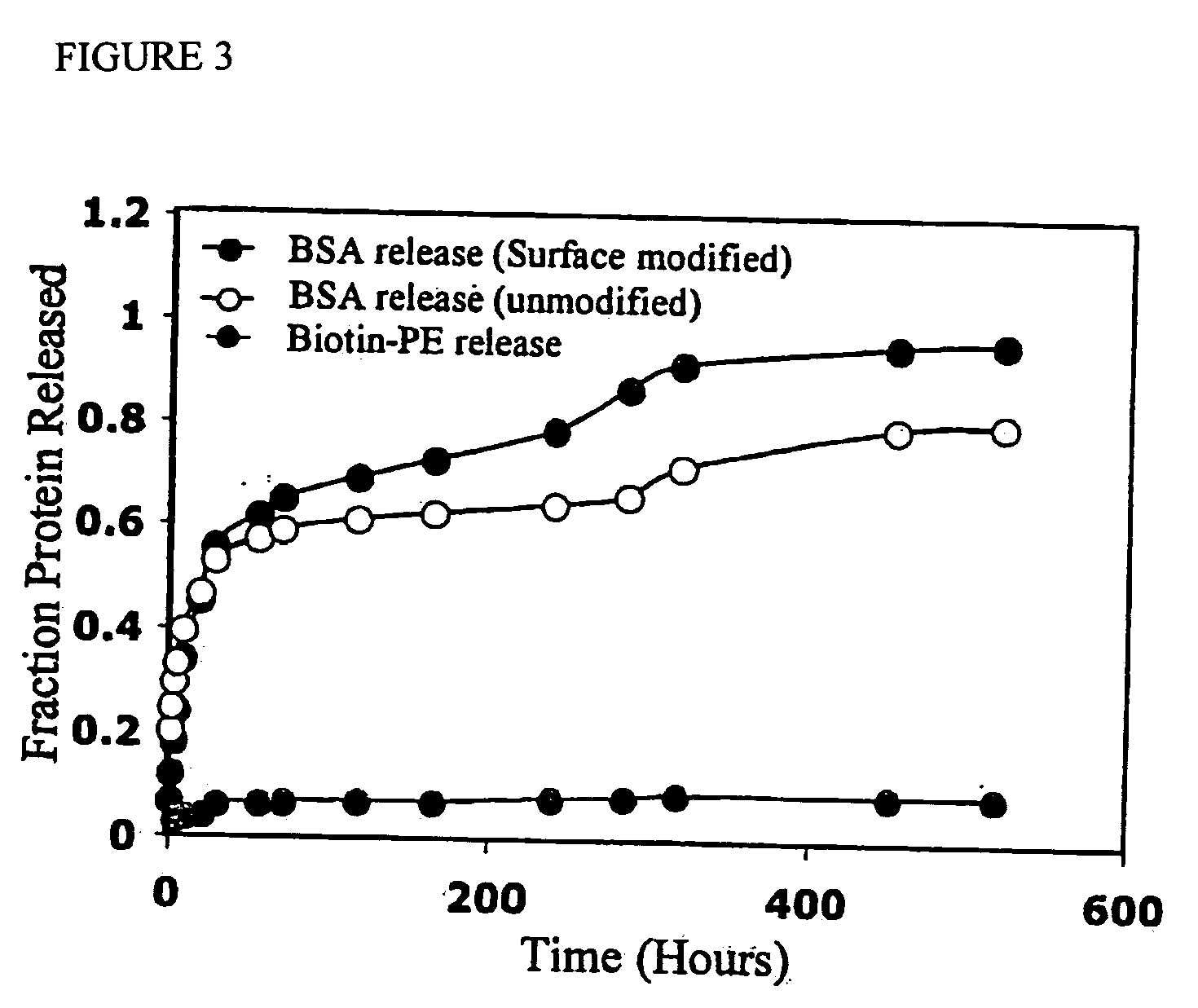
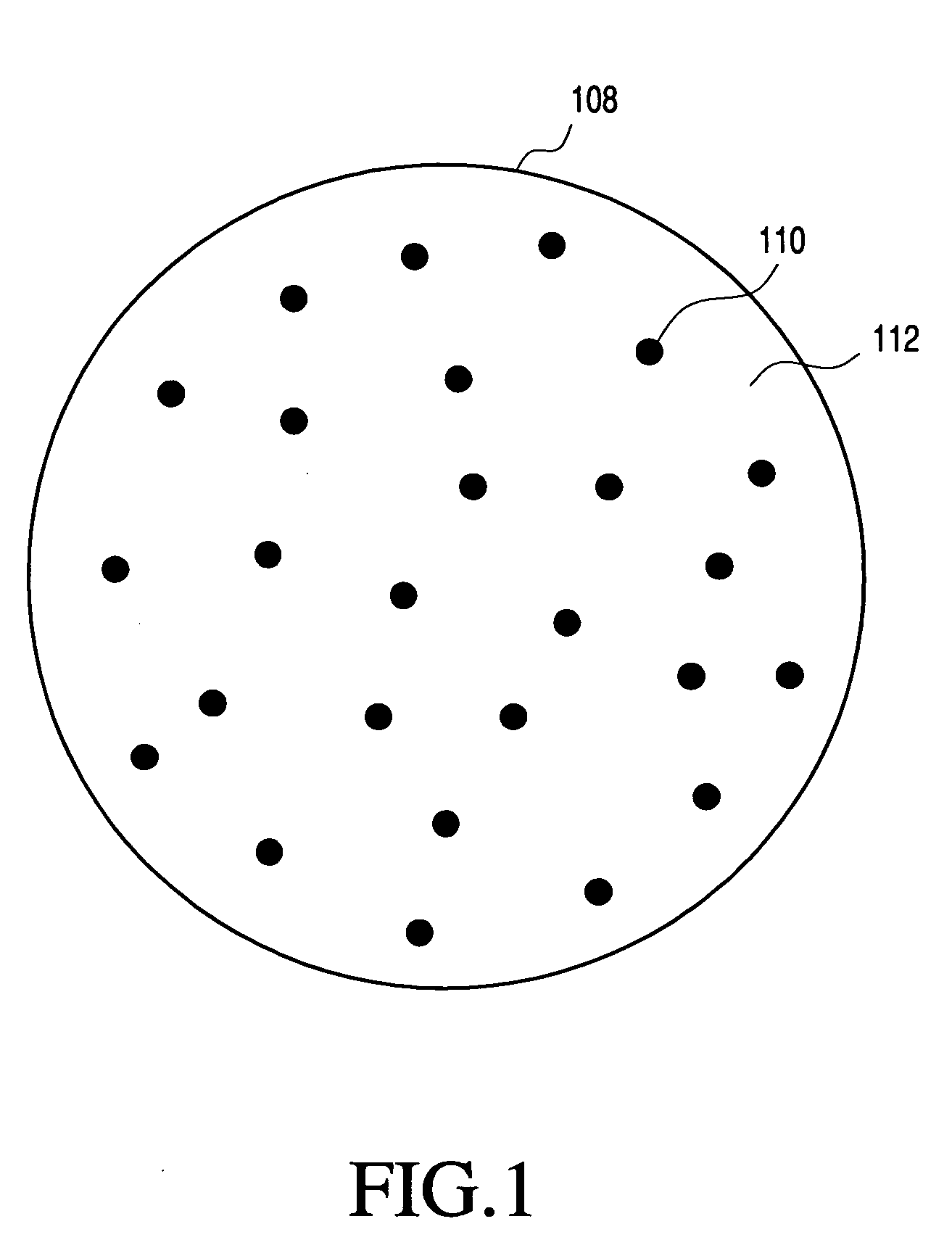
Polymeric delivery devices have been developed which combine high loading / high density of molecules to be delivered with the option of targeting. As used herein, “high density” refers to microparticles having a high density of ligands or coupling agents, which is in the range of 1000-10,000,000, more preferably between 10,000 and 1,000,000 ligands per square micron of microparticle surface area. A general method for incorporating molecules into the surface of biocompatible polymers using materials with an HLB of less than 10, more preferably less than 5, such as fatty acids, has been developed. Because of its ease, generality and flexibility, this method has widespread utility in modifying the surface of polymeric materials for applications in drug delivery and tissue engineering, as well other other fields. Targeted polymeric microparticles have also been developed which encapsulate therapeutic compounds such as drugs, cellular materials or components, and antigens, and have targeting ligands directly bound to the microparticle surface. Preferred applications include use in tissue engineering matrices, wound dressings, bone repair or regeneration materials, and other applications where the microparticles are retained at the site of application or implantation. Another preferred application is in the use of microparticles to deliver anti-proliferative agents to the lining of blood vessels following angioplasty, transplantation or bypass surgery to prevent or decrease restenosis, and in cancer therapy. In still another application, the microparticles are used to treat or prevent macular degeneration when administered to the eye, where agents such as complement inhibitors are administered.
Owner:YALE UNIV
Multi-component particles comprising inorganic nanoparticles distributed in an organic matrix and processes for making and using same
Multi-component particles comprising inorganic nanoparticles distributed in an organic matrix and processes for making and using same. A flowing aerosol is generated that includes droplets of a precursor medium dispersed in a gas phase. The precursor medium contains a liquid vehicle and at least one precursor. At least a portion of the liquid vehicle is removed from the droplets of precursor medium under conditions effective to convert the precursor to the nanoparticles or the matrix and form the multi-component particles.
Owner:CABOT CORP
Pharmaceutical formulation containing opioid agonist, opioid antagonist and irritant
ActiveUS20030068392A1Reduce and eliminate effectInhibition effectBiocideNervous disorderOpioid AgonistOpioid antagonist
Disclosed in certain embodiments is an oral dosage form comprising: a therapeutically effective amount of an opioid analgesic; an opioid antagonist; and an irritant in an effective amount to impart an irritating sensation to an abuser upon administration of the dosage form after tampering.
Owner:PURDUE PHARMA LP
Biodegradable low molecular weight triblock poly (lactide-co-glycolide) polyethylene glycol copolymers having reverse thermal gelation properties
InactiveUS6117949AReduce solubilityReduced stabilityPowder deliveryPeptide/protein ingredientsSolubilityPolymer science
A water soluble biodegradable ABA- or BAB-type triblock polymer is disclosed that is made up of a major amount of a hydrophobic polymer made of a poly(lactide-co-glycolide) copolymer or poly(lactide) polymer as the A-blocks and a minor amount of a hydrophilic polyethylene glycol polymer B-block, having an overall weight average molecular weight of between about 2000 and 4990, and that possesses reverse thermal gelation properties. Effective concentrations of the triblock polymer and a drug may be uniformly contained in an aqueous phase to form a drug delivery composition. At temperatures below the gelation temperature of the triblock polymer the composition is a liquid and at temperatures at or above the gelation temperature the composition is a gel or semi-solid. The composition may be administered to a warm-blooded animal as a liquid by parenteral, ocular, topical, inhalation, transdermal, vaginal, transurethral, rectal, nasal, oral, pulmonary or aural delivery means and is a gel at body temperature. The composition may also be administered as a gel. The drug is released at a controlled rate from the gel which biodegrades into non-toxic products. The release rate of the drug may be adjusted by changing various parameters such as hydrophobic / hydrophilic componenet content, polymer concentration, molecular weight and polydispersity of the triblock polymer. Because the triblock polymer is amphiphilic, it functions to increase the solubility and / or stability of drugs in the composition.
Owner:BTG INT LTD +2
Pharmaceutical formulation containing opioid agonist,opioid antagonist and gelling agent
InactiveUS20030068371A1Reduce and eliminate effectInhibition effectBiocideNervous disorderOpioid antagonistOpioid Agonist
Disclosed in certain embodiments is an oral dosage form comprising a therapeutically effective amount of an opioid analgesic, an opioid antagonist and one or more pharmaceutically acceptable excipients; the dosage form further including a gelling agent in an effective amount to impart a viscosity unsuitable for administration selected from the group consisting of parenteral and nasal administration to a solubilized mixture formed when the dosage form is crushed and mixed with from about 0.5 to about 10 ml of an aqueous liquid.
Owner:PURDUE PHARMA LP
Orally administrable opioid formulations having extended duration of effect
InactiveUS6294195B1Effective steady-state blood levelPowder deliveryBiocideBlood levelOral medication
Sustained release oral solid dosage forms of opioid analgesics are provided as multiparticulate systems which are bioavailable and which provide effective blood levels of the opioid analgesic for at least about 24 hours. A unit dose of the opioid analgesic contains a plurality of substrates including the opioid analgesic in sustained release form. The substrates have a diameter from about 0.1 mm to about 3 mm.
Owner:PURDUE PHARMA LP
Solution-based fabrication of photovoltaic cell
InactiveUS20050183767A1Improve overall utilizationLow costMaterial nanotechnologyNanostructure manufactureNanoparticleSolar cell
An ink for forming CIGS photovoltaic cell active layers is disclosed along with methods for making the ink, methods for making the active layers and a solar cell made with the active layer. The ink contains a mixture of nanoparticles of elements of groups IB, IIIA and (optionally) VIA. The particles are in a desired particle size range of between about 1 nm and about 500 nm in diameter, where a majority of the mass of the particles comprises particles ranging in size from no more than about 40% above or below an average particle size or, if the average particle size is less than about 5 nanometers, from no more than about 2 nanometers above or below the average particle size. The use of such ink avoids the need to expose the material to an H2Se gas during the construction of a photovoltaic cell and allows more uniform melting during film annealing, more uniform intermixing of nanoparticles, and allows higher quality absorber films to be formed.
Owner:AERIS CAPITAL SUSTAINABLE IP
Particulate acellular tissue matrix
A method of processing an acellular tissue matrix to give a particulate acellular tissue matrix includes: cutting sheets of dry acellular tissue matrix into strips; cryofracturing the dry acellular tissue matrix strips at cryogenic temperatures; separating the resulting particles by size at cryogenic temperatures; and freeze drying the fraction of particles desired size to remove any moisture that may have been absorbed to give a dry particulate acellular tissue matrix. Rehydration of the dry particulate acellular tissue matrix may take place just prior to use. The particulate acellular tissue may be applied to a recipient site, by way of injection, spraying, layering, packing, in-casing or combinations thereof. The particulate acellular tissue may further include growth and stimulating agents selected from epidermal growth factor, fibroblast growth factor, nerve growth factor, keratinocyte growth factor, platelet derived growth factor, vasoactive intestinal peptide, stem cell factor, bone morphogetic proteins, chondrocyte growth factor and combinations thereof. Other pharmaceutically active compounds may be combined with the rehydrated particulate material including: analgesic drugs; hemostatic drugs; antibiotic drugs; local anesthetics and the like to enhance the acceptance of the implanted particulate material. The particulate material product may also be combined with stem cells selected from mesenchymal stem cells, epidermal stem cells, cartilage stem cells, hematopoietic stem cells and combinations thereof.
Owner:LIFECELL
Methods and compositions for reducing or eliminating post-surgical adhesion formation
The present invention relates to a method for reducing adhesions associated with post-operative surgery. The present method comprises administering or affixing a polymeric composition preferably comprising chain extended, coupled or crosslinked polyester / poly(oxyalkylene) ABA triblocks or AB diblocks having favorable EO / LA ratios to a site in the body which has been subjected to trauma, e.g. by surgery, excision or inflammatory disease. In the present invention, the polymeric material provides a barrier to prevent or reduce the extent of adhesions forming.
Owner:YISSUM RES DEV CO OF THE HEBREW UNIV OF JERUSALEM LTD
Pharmaceutical formulations and systems for improved absorption and multistage release of active agents
InactiveUS7374779B2Improve bioavailabilityLow variabilityPowder deliveryOrganic active ingredientsActive agentFast release
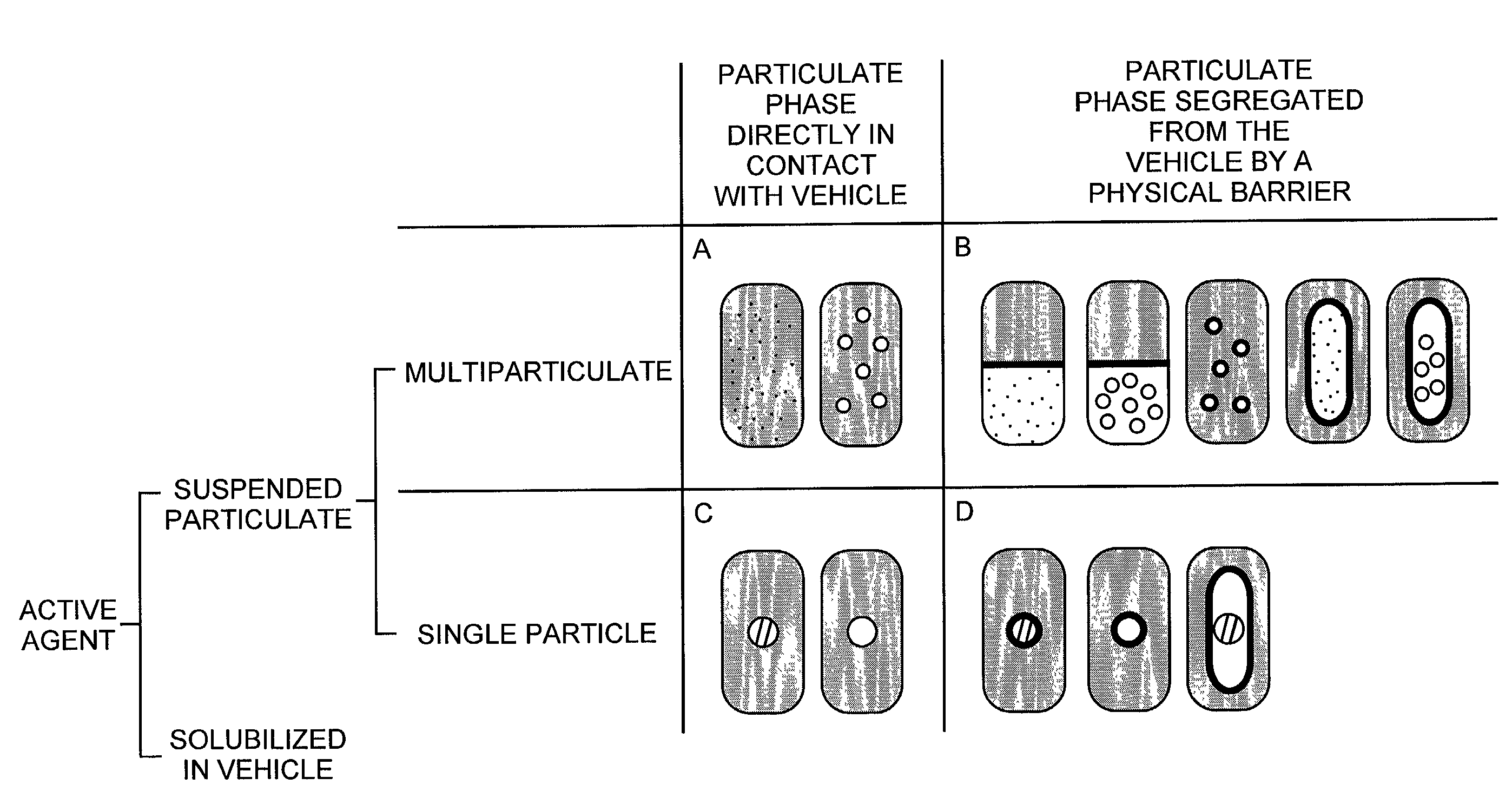
The present invention pertains to pharmaceutical formulations and systems for delivery of active agents, wherein a first fraction of an active agent is suspended in a vehicle and a second fraction of active agent is solubilized in the vehicle, with the suspended fraction representing about 5 wt. % to about 80 wt. % of the active agent and the second fraction representing about 20 wt. % to about 95 wt. % of the active agent. One or more additional active agents, which may be fully solubilized, partially solubilized, or suspended, may also be present. The first and second fractions of the active agent may or may not have different release profiles. Generally, a significant fraction of the solubilized drug will release rapidly, providing for rapid onset, while the suspended drug may be formulated for delayed and / or sustained release.
Owner:LIPOCINE
Solid carriers for improved delivery of active ingredients in pharmaceutical compositions
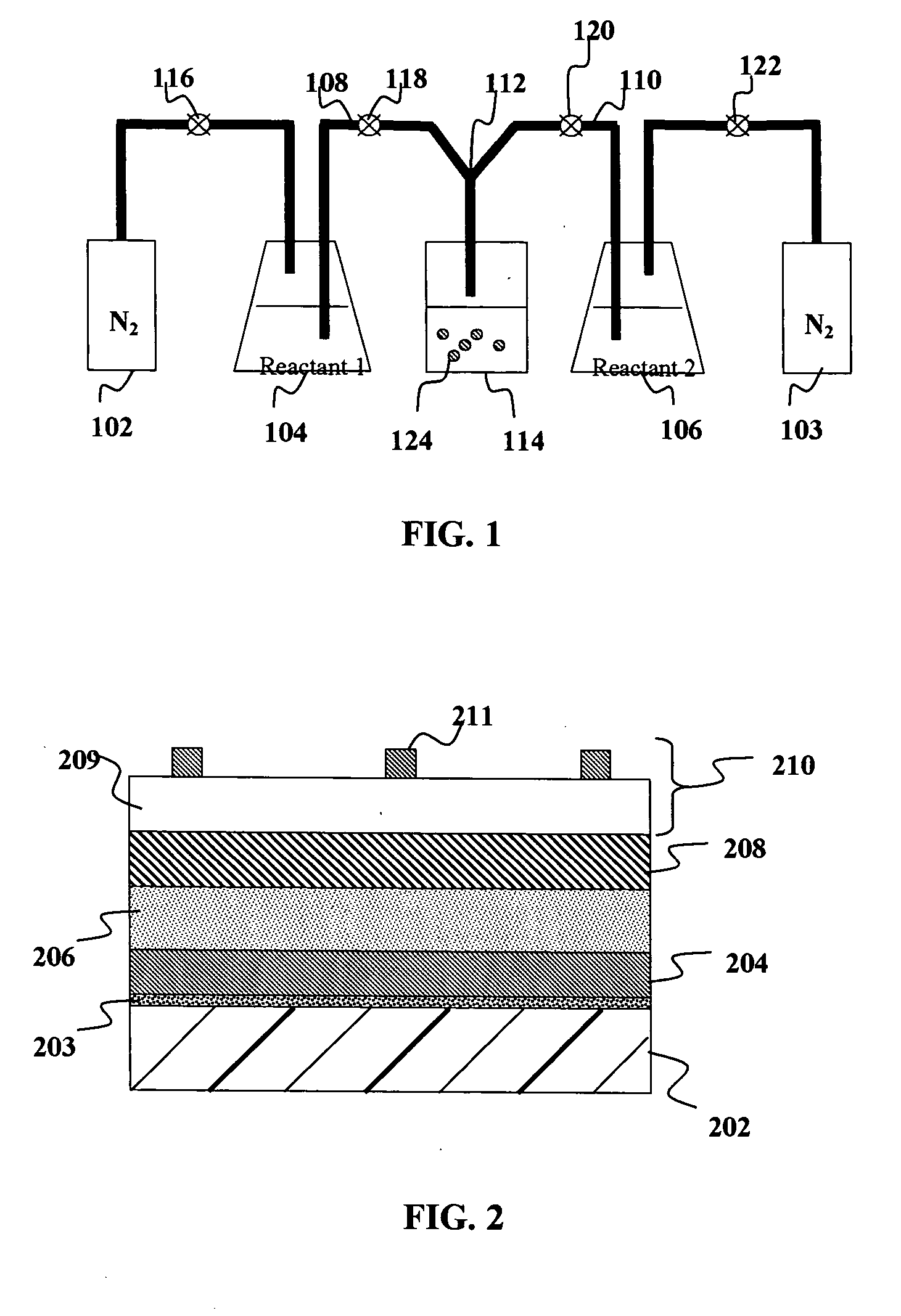
InactiveUS20060034937A1Good chemical stabilityPromote absorptionGranular deliveryMicrocapsulesDiagnostic agentMedicine
The present invention provides solid pharmaceutical compositions for improved delivery of a wide variety of pharmaceutical active ingredients contained therein or separately administered. In one embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier including a substrate and an encapsulation coat on the substrate. The encapsulation coat can include different combinations of pharmaceutical active ingredients, hydrophilic surfactant, lipophilic surfactants and triglycerides. In another embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier being formed of different combinations of pharmaceutical active ingredients, hydrophilic surfactants, lipophilic surfactants and triglycerides. The compositions of the present invention can be used for improved delivery of hydrophilic or hydrophobic pharmaceutical active ingredients, such as drugs, nutritional agents, cosmeceuticals and diagnostic agents.
Owner:LIPOCINE
Tamper resistant dosage forms
ActiveUS20090081290A1Reduces and prevents stickingBiocidePowder deliveryOpioid analgesicsDosage form
The present invention relates to pharmaceutical dosage forms, for example to a tamper resistant dosage form including an opioid analgesic, and processes of manufacture, uses, and methods of treatment thereof.
Owner:PURDUE PHARMA LP
Pharmaceutical compositions for the coordinated delivery of NSAIDs
The present invention is directed to drug dosage forms that release an agent that raises the pH of a patient's gastrointestinal tract, followed by a non-steroidal anti-inflammatory drug. The dosage form is designed so that the NSAID is not released until the intragastric pH has been raised to a safe level. The invention also encompasses methods of treating patients by administering this coordinated release, gastroprotective, antiarthritic / analgesic combination unit dosage form to achieve pain and symptom relief with a reduced risk of developing gastrointestinal damage such as ulcers, erosions and hemorrhages.
Owner:NUVO PHARMA IRELAND DESIGNATED ACTIVITY CO
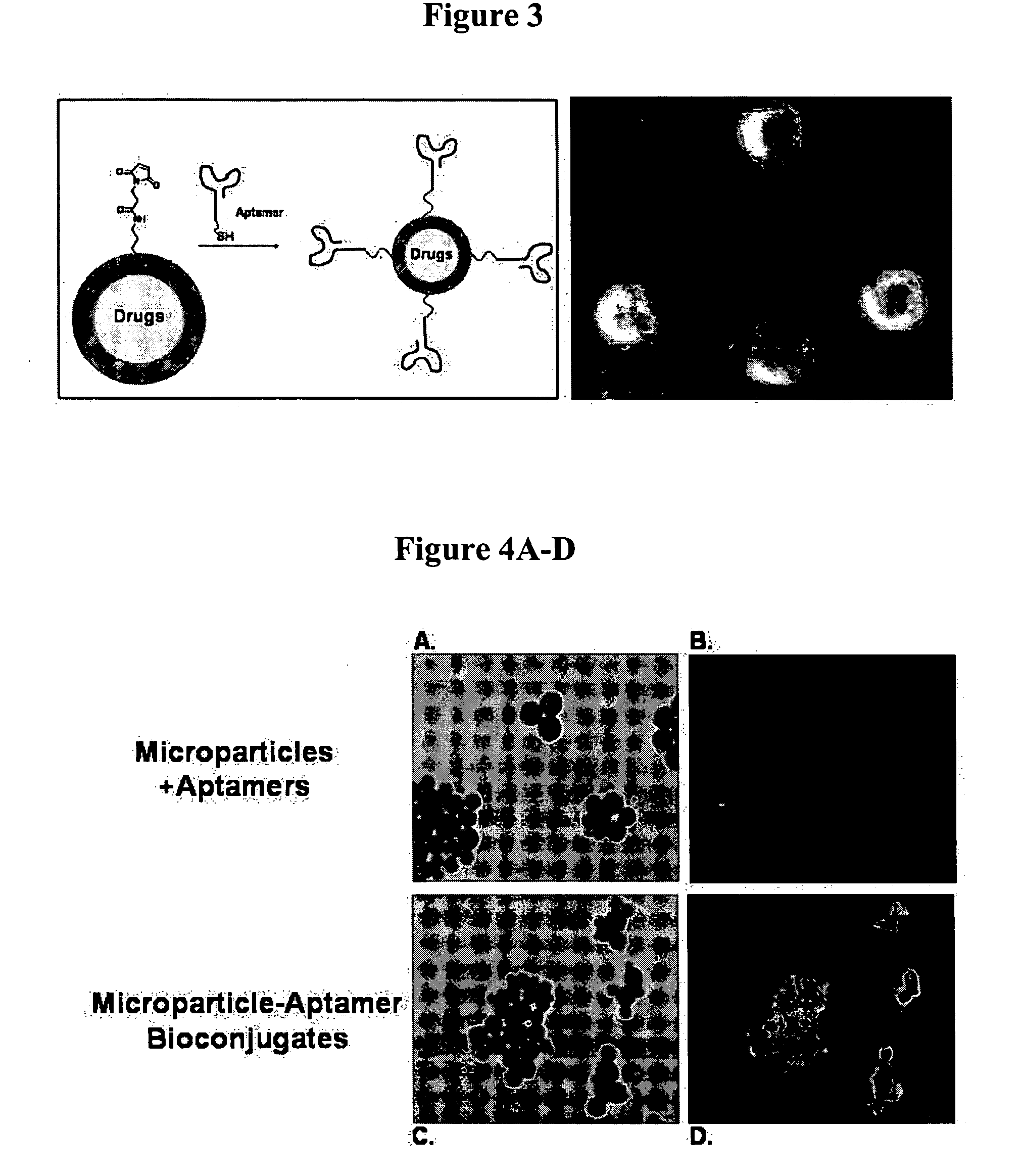
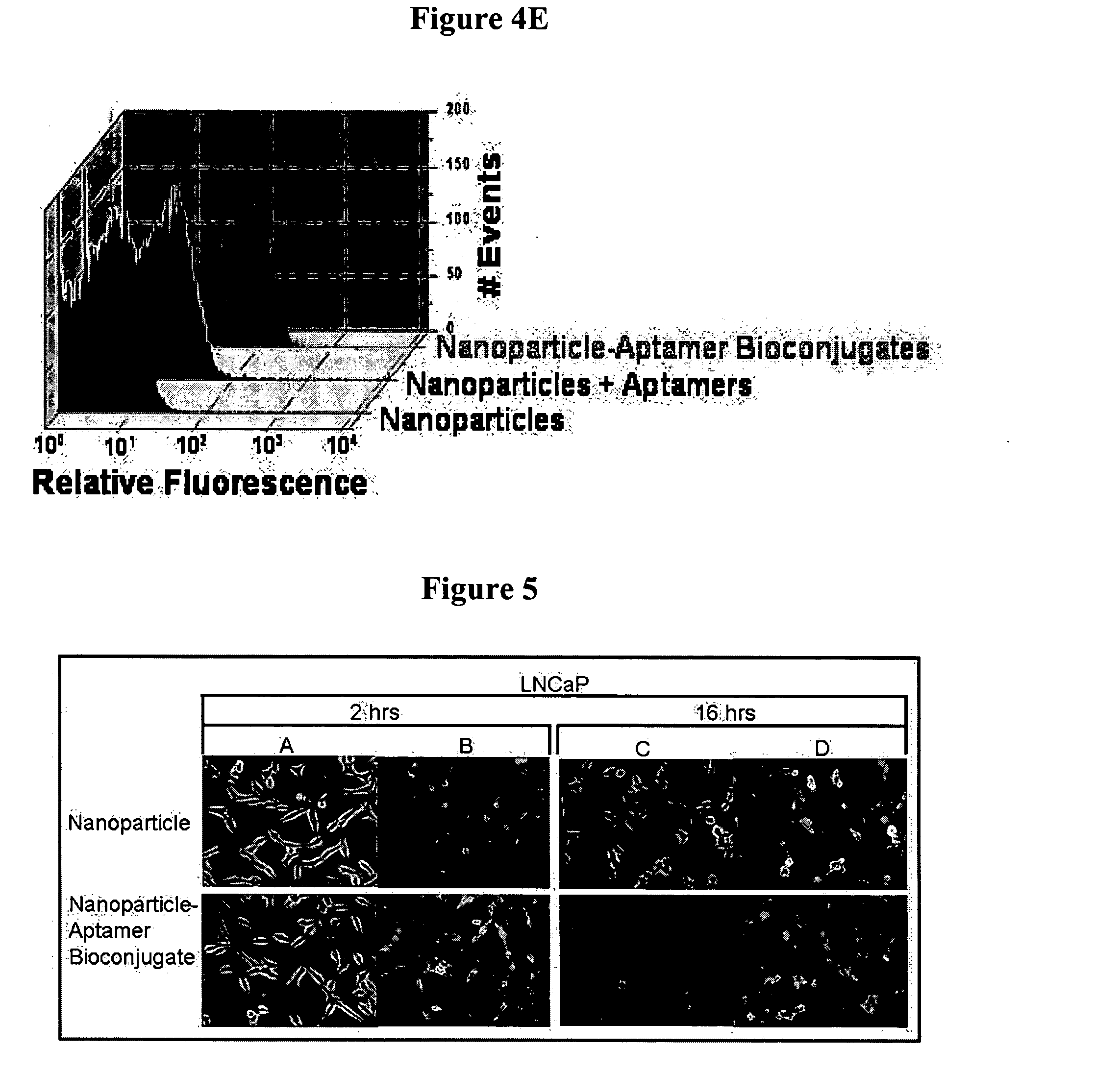
Targeted delivery of controlled release polymer systems
ActiveUS20050037075A1Strong specificityHigh affinityPowder deliveryPeptide/protein ingredientsControlled releaseCell Surface Antigens
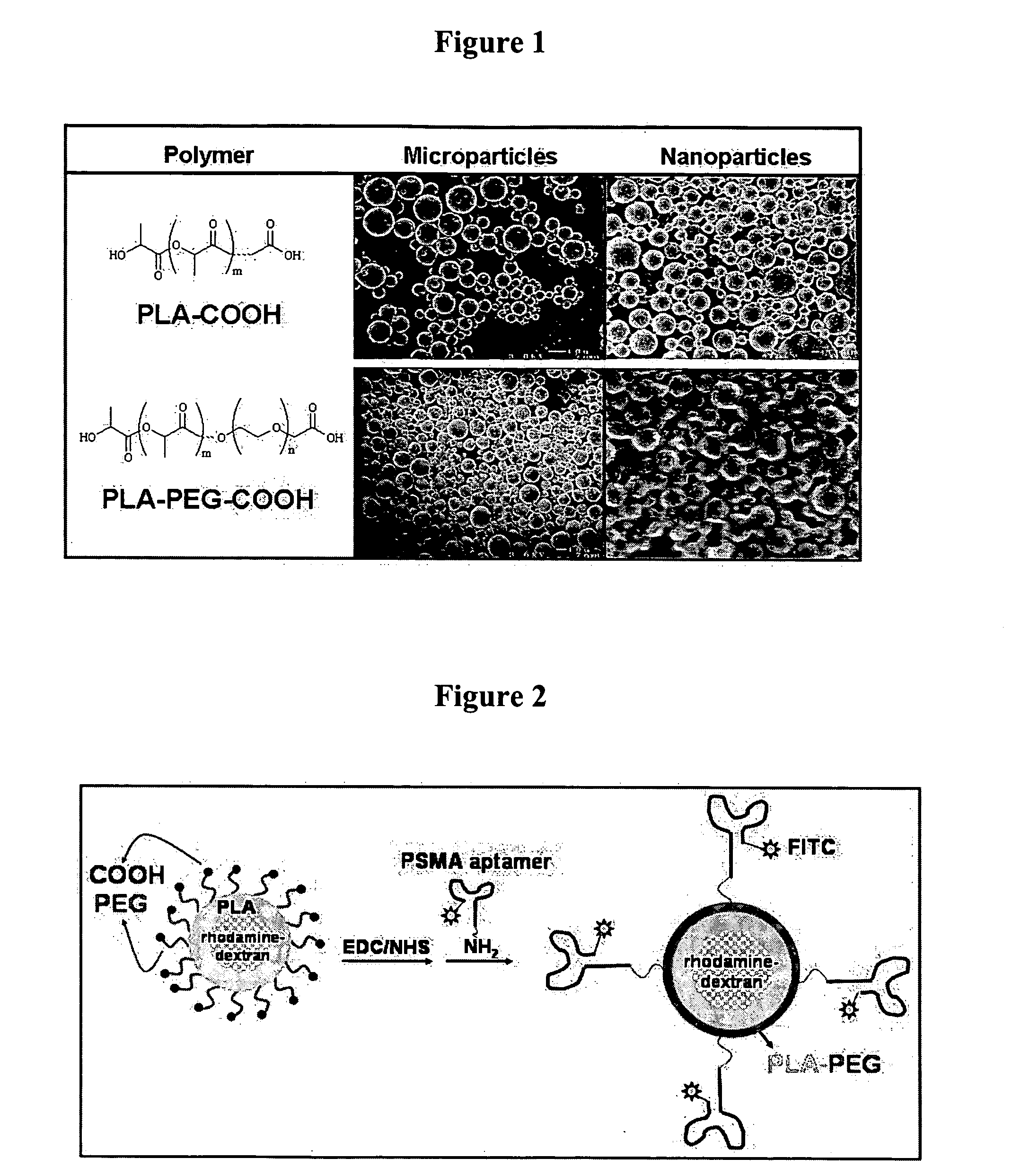
The present invention relates to a conjugate that includes a nucleic acid ligand bound to a controlled release polymer system, a pharmaceutical composition that contains the conjugate, and methods of treatment using the conjugate. The controlled release polymer system includes an agent such as a therapeutic, diagnostic, prognostic, or prophylactic agent. The nucleic acid ligand that is bound to the controlled release polymer system, binds selectively to a target, such as a cell surface antigen, and thereby delivers the controlled release polymer system to the target.
Owner:MASSACHUSETTS INST OF TECH
Microparticle coated medical device
A drug-loaded microparticle is applied to a medical device for subsequent application to biological tissues. A method of formulating a drug-loaded microparticle and applying it to the surface of a medical device, such as a stent, is disclosed. The drug-loaded microparticle is formulated by combining a drug with various chemical solutions. Specified sizes of the microparticles and amounts of drug(s) contained within the microparticles may be varied by altering the proportions of the chemicals / solutions. In addition to various drugs, therapeutic substances and radioactive isotopes may also be loaded into the microparticles. The drug-loaded microparticle are suspended in a polymer solution forming a polymer matrix. The polymer matrix may be applied to the entire surface or only selected portions of the medical device via dipping, spraying or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
Controlled-release compositions containing opioid agonist and antagonist
InactiveUS6716449B2Good curative effectPatient compliance is goodBiocideNervous disorderOpioid antagonistOpioid Agonist
Controlled-release dosage forms containing an opioid agonist; an opioid antagonist; and a controlled release material release during a dosing interval an analgesic or sub-analgesic amount of the opioid agonist along with an amount of the opioid antagonist effective to attenuate a side effect of the opioid agonist. The dosage form provides analgesia for at least about 8 hours when administered to human patients. In other embodiments, the dose of antagonist released during the dosing interval enhances the analgesic potency of the opioid agonist.
Owner:PURDUE PHARMA LP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com