Patents
Literature
1292 results about "Biocompatible material" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In surgery, a biocompatible material is a synthetic or natural material used to replace part of a living system or to function in intimate contact with living tissue. Biocompatible materials are intended to interface with biological systems to evaluate, treat, augment or replace any tissue, organ or function of the body. Biomaterials are usually non-viable, but may also be viable. A biocompatible material is different from a biological material such as bone that is produced by a biological system. Artificial hips, vascular stents, artificial pacemakers, and catheters are all made from different biomaterials and comprise different medical devices. Biomimetic materials are not made by living organisms but have compositions and properties similar to those made by living organisms. The calcium hydroxylapatite coating found on many artificial hips is used as a bone replacement that allows for easier attachment of the implant to the living bone.
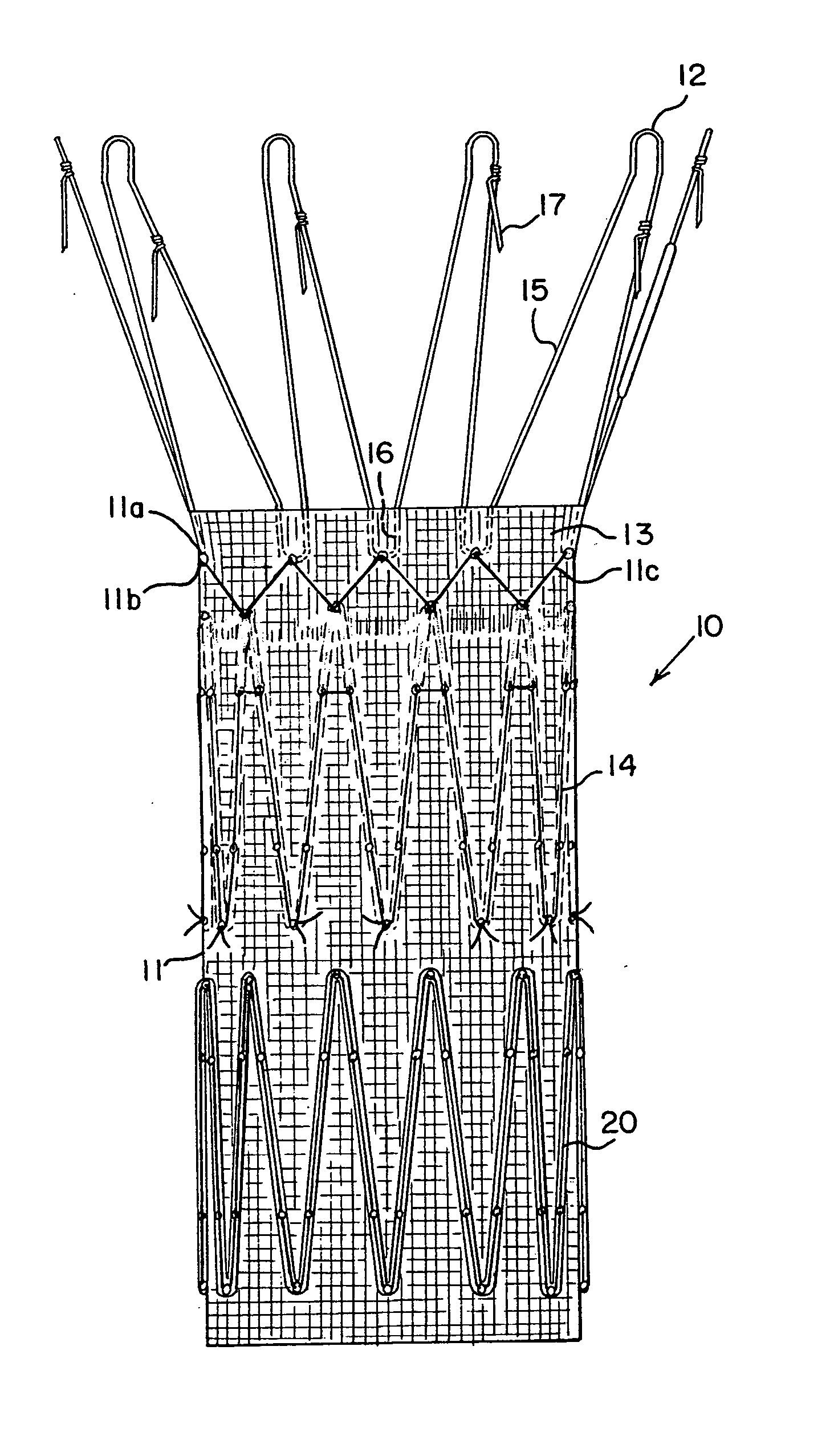
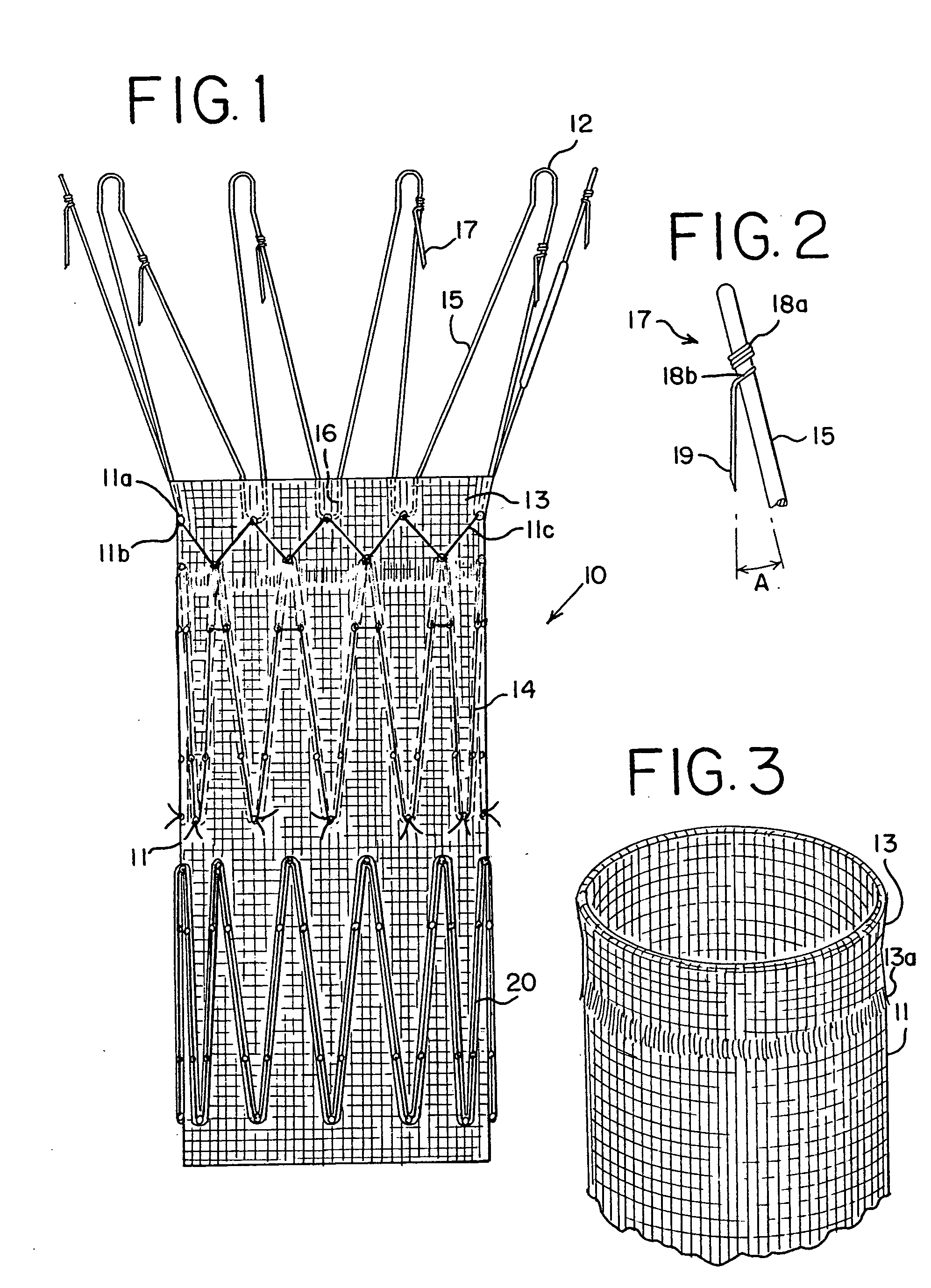
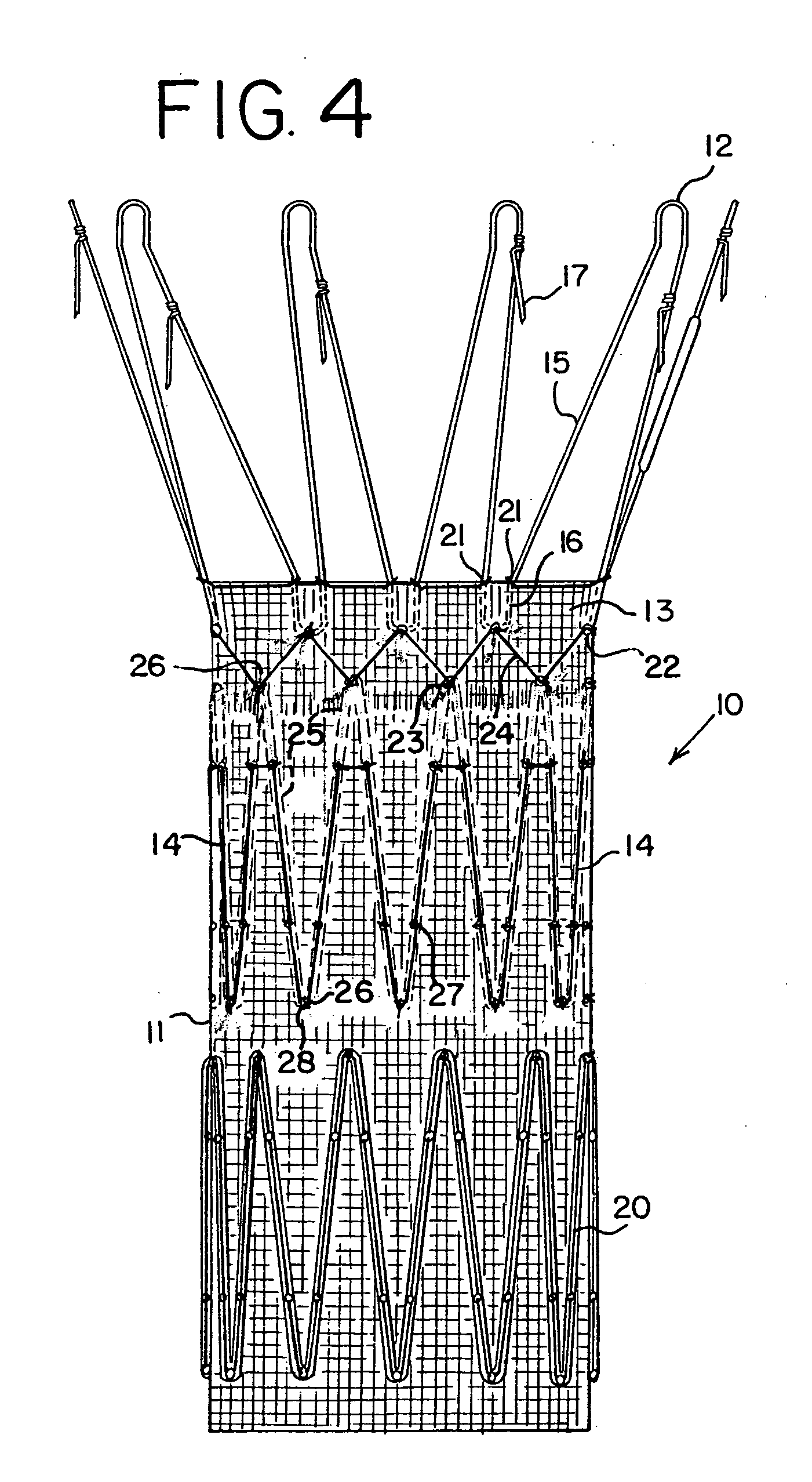
Drug releasing anastomosis devices and methods for treating anastomotic sites
ActiveUS7108701B2Reduce drug toxicityGood curative effectSuture equipmentsSurgical needlesBiological bodyReady to use
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned.
Owner:WYETH
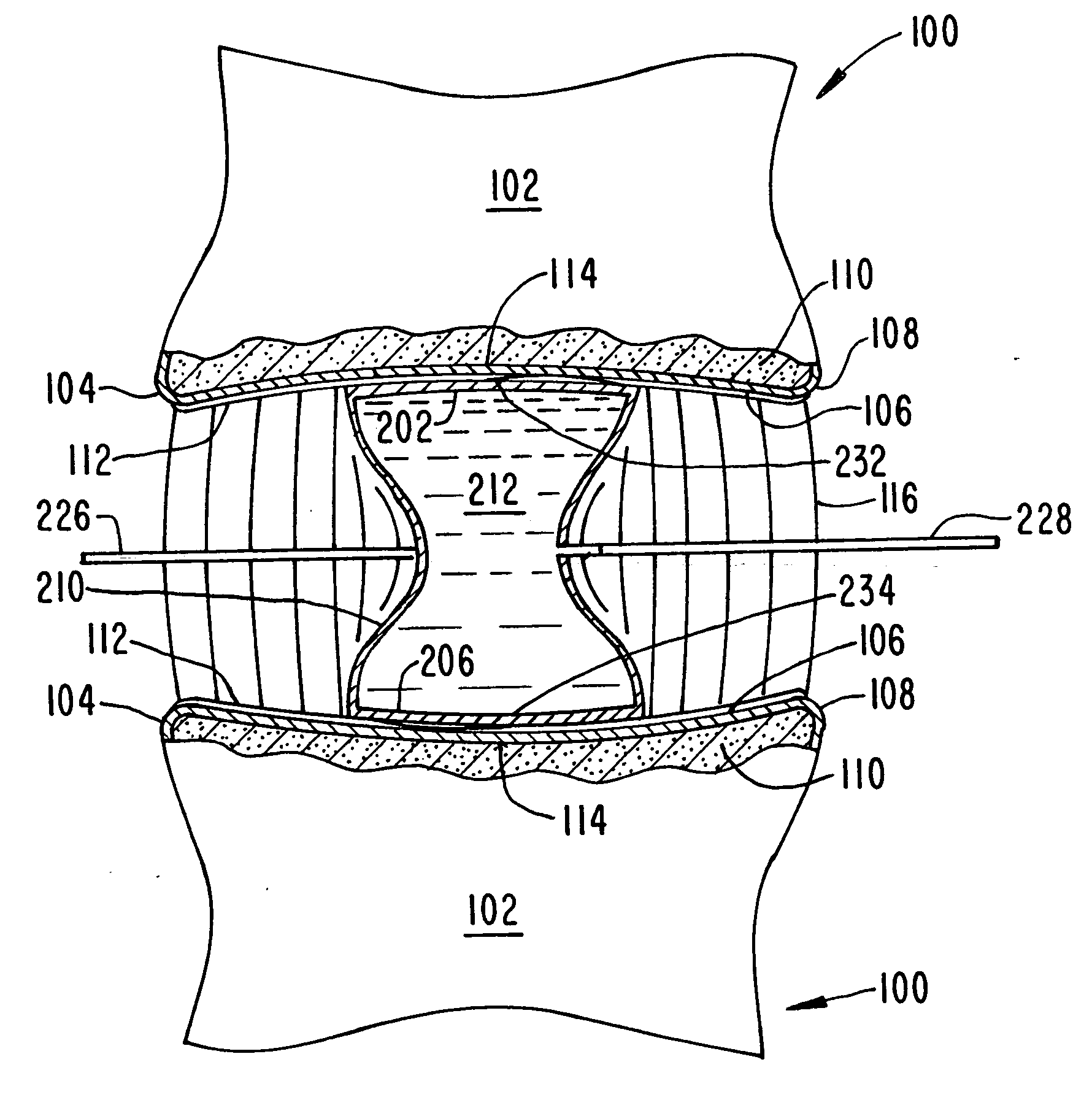
Coated endovascular AAA device
InactiveUS6852122B2Reduce drug toxicityGood curative effectSuture equipmentsStentsBiological bodyReady to use
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Bioabsorbable Polymer, Bioabsorbable Composite Stents
InactiveUS20080249608A1Simple and inexpensive to manufactureVarying of ductilityStentsSurgeryMetallic materialsMedical device
Biocompatible materials may be configured into any number of implantable medical devices including intraluminal stents. The biocompatible material may comprise metallic and non-metallic materials in hybrid structures. In one such structure, a device may be fabricated with one or more elements having an inner metallic core that is biodegradable with an outer shell formed from a polymeric material that is biodegradable. Additionally, therapeutic agents may be incorporated into the microstructure or the bulk material.
Owner:CORDIS CORP
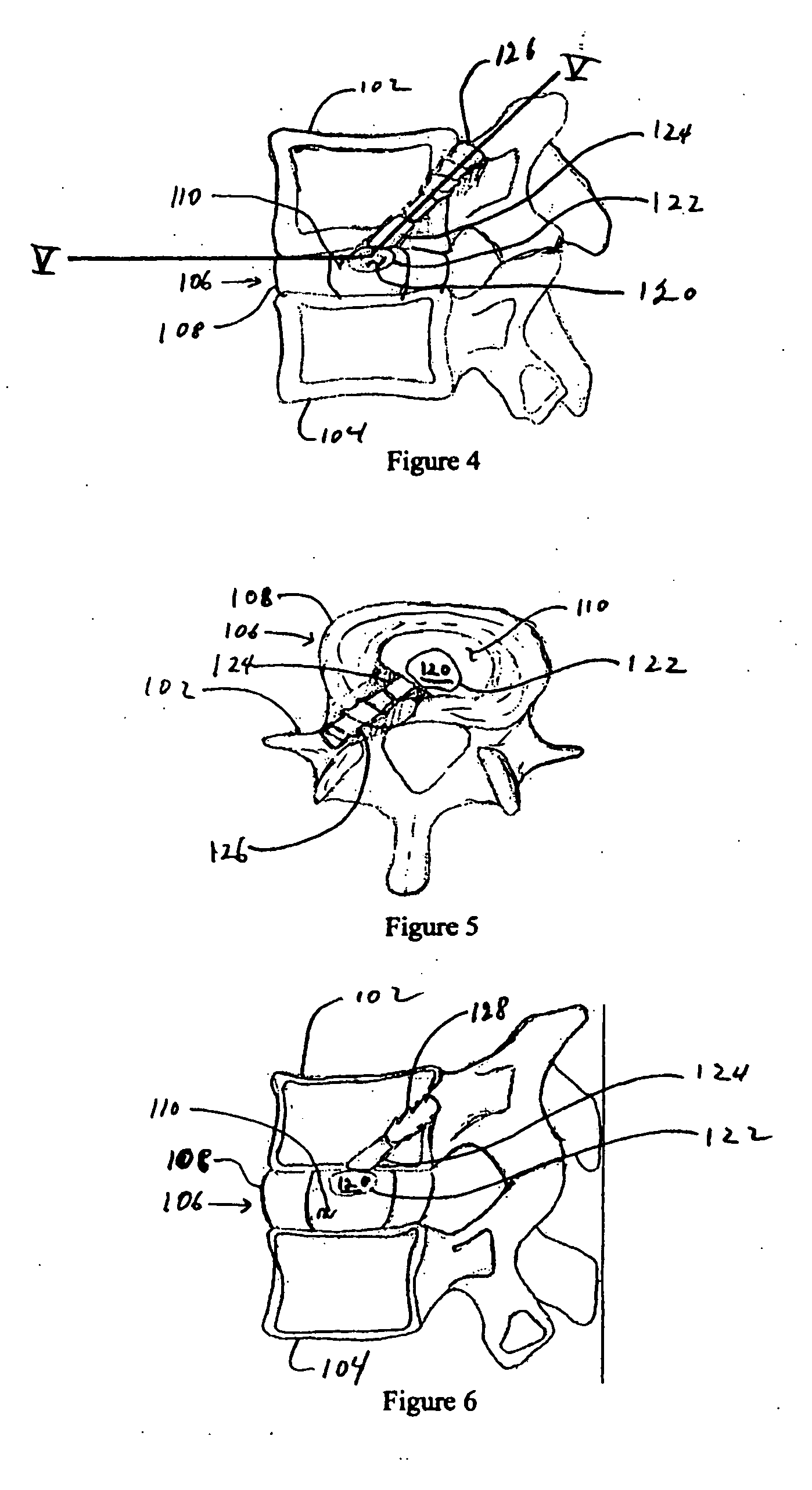
Method of expanding an intradiscal space and providing an osteoconductive path during expansion
Owner:SPINEWAVE
Methods and compositions useful for administration of chemotherapeutic agents
InactiveUS6096331AReduce morbidityLow toxicityPowder deliveryEchographic/ultrasound-imaging preparationsActive agentIn vivo
In accordance with the present invention, there are provided compositions and methods useful for the in vivo delivery of a pharmaceutically active agent, wherein the agent is associated with a polymeric biocompatible material.
Owner:ABRAXIS BIOSCI LLC
In situ formation of intervertebral disc implants
InactiveUS20060089719A1Improve bindingLarge deformationSkeletal disorderSpinal implantsSpinal Disk ImplantShort terms
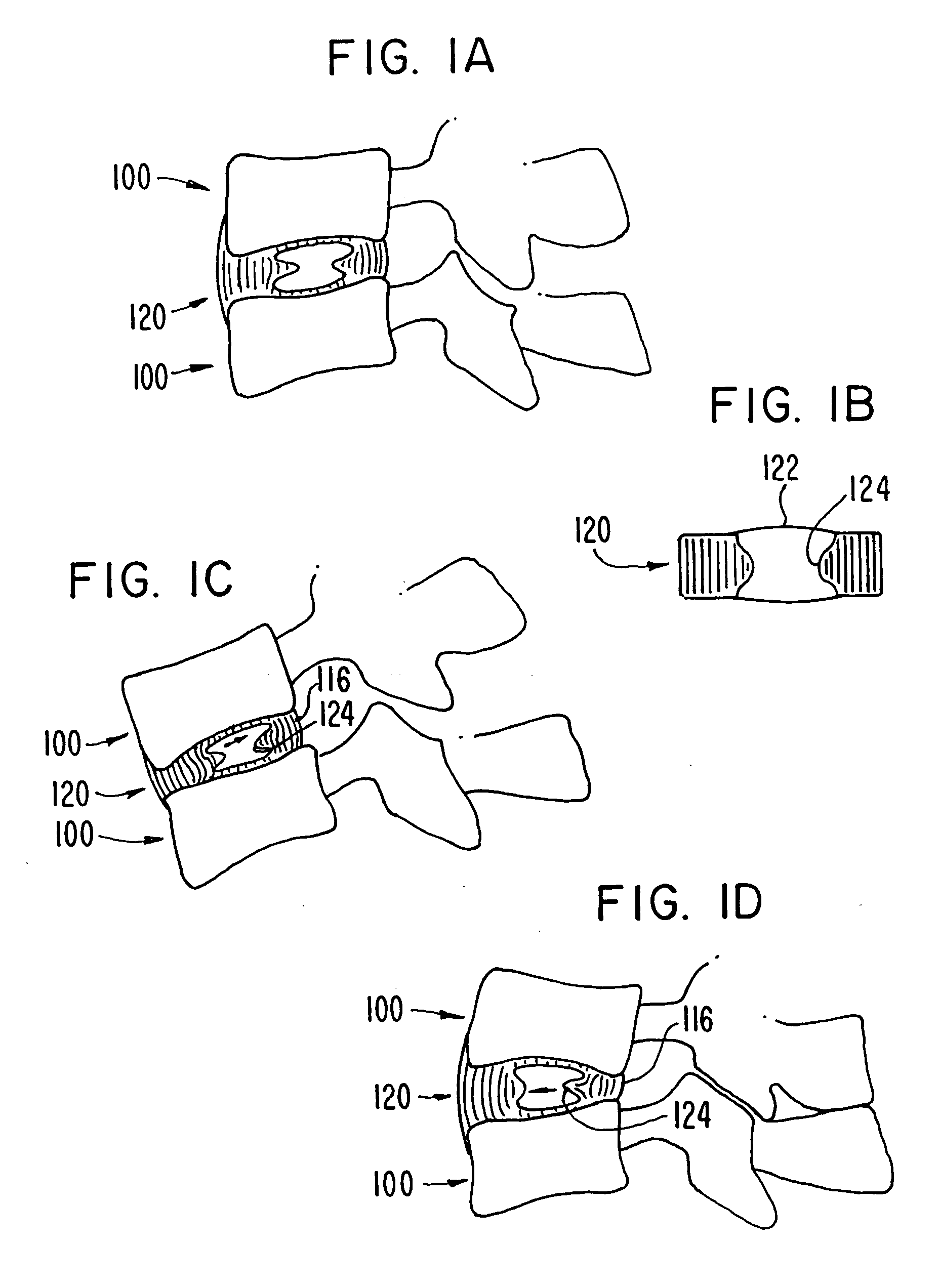
Nucleus pulposus implants that are resistant to migration in and / or expulsion from an intervertebral disc space are provided. In one form of the invention, an implant includes a load bearing elastic body surrounded in the disc space by an anchoring, preferably resorbable, biocompatible material which may be in the form of an outer shell. In certain forms of the invention, the elastic body is surrounded by a supporting member, such as a band or jacket, and the supporting member is surrounded by the outer shell. Kits for forming such implants are also provided. In another form of the invention, an implant is provided that has locking features and optional shape memory characteristics. In yet another aspect of the invention, nucleus pulposus implants are provided that have shape memory characteristics and are configured to allow short-term manual, or other deformation without permanent deformation, cracks, tears, breakage or other damage. Methods of forming and implanting the implants are also described, as are delivery devices and components thereof for delivering the implants.
Owner:SDGI HLDG
Methods, materials and apparatus for treating bone and other tissue
ActiveUS20060079905A1Avoid less flexibilityEasy to useImpression capsSurgical adhesivesBiomedical engineeringVertebra
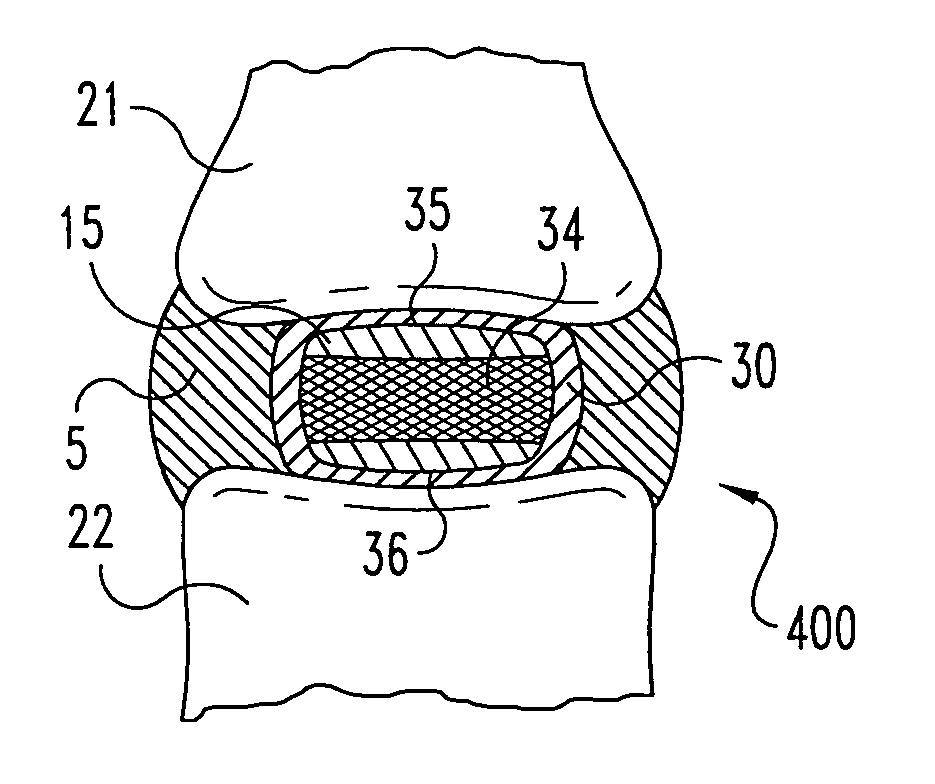
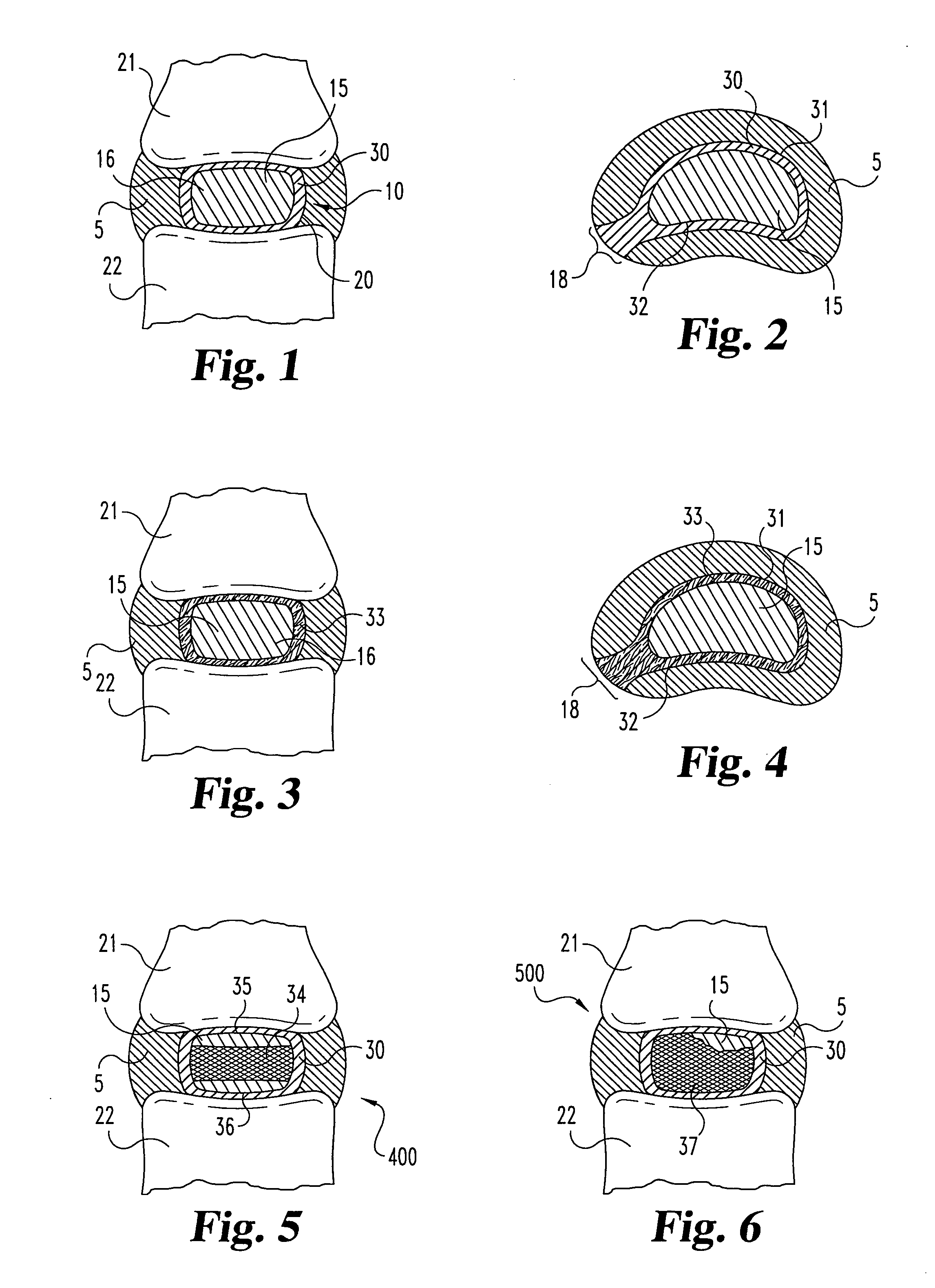
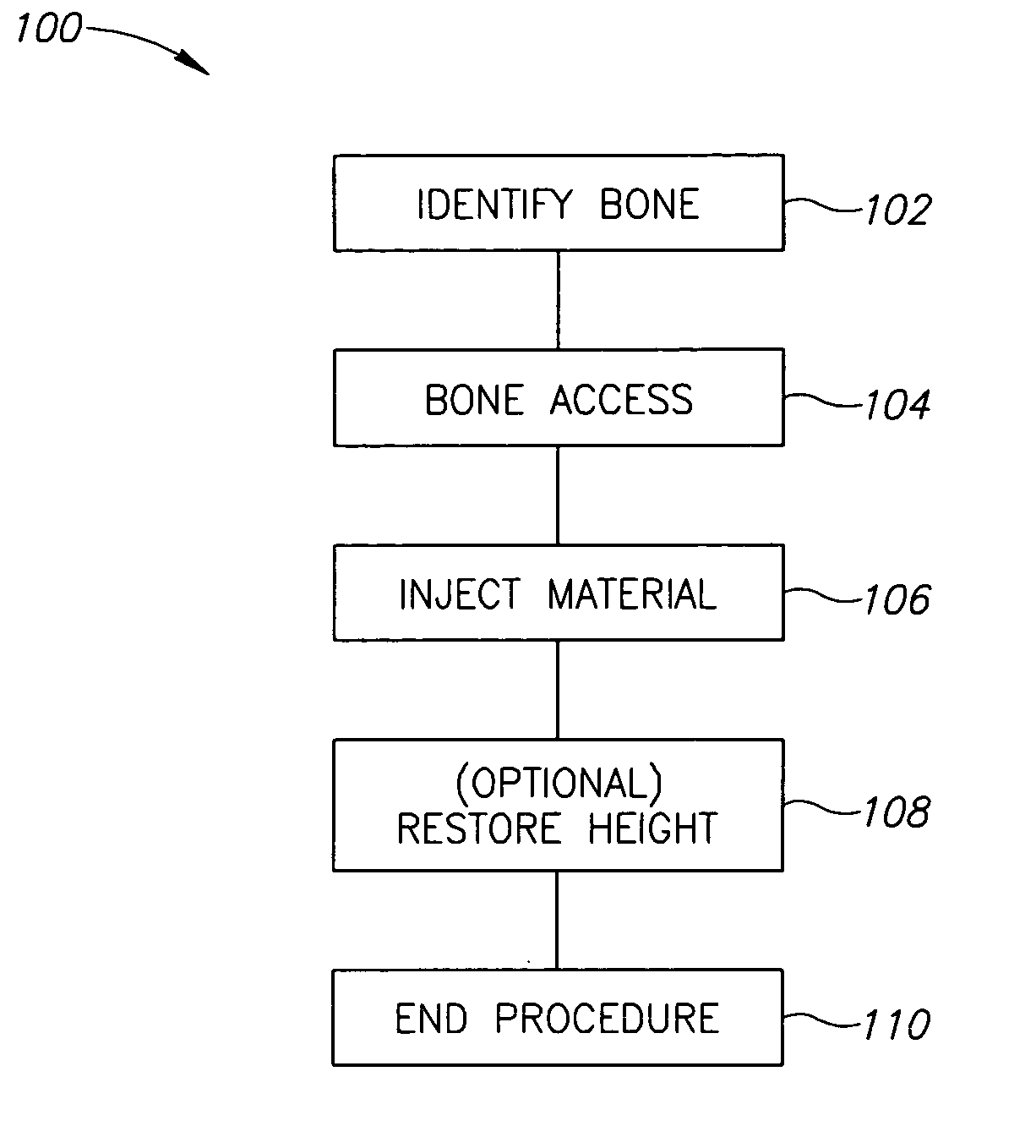
A method of treating a vertebra, comprising: (a) accessing an interior of a vertebra; and (b) introducing a sufficient amount of artificial biocompatible material which does not set to a hardened condition in storage, into said bone, with sufficient force to move apart fractured portions of said bone.
Owner:DEPUY SYNTHES PROD INC
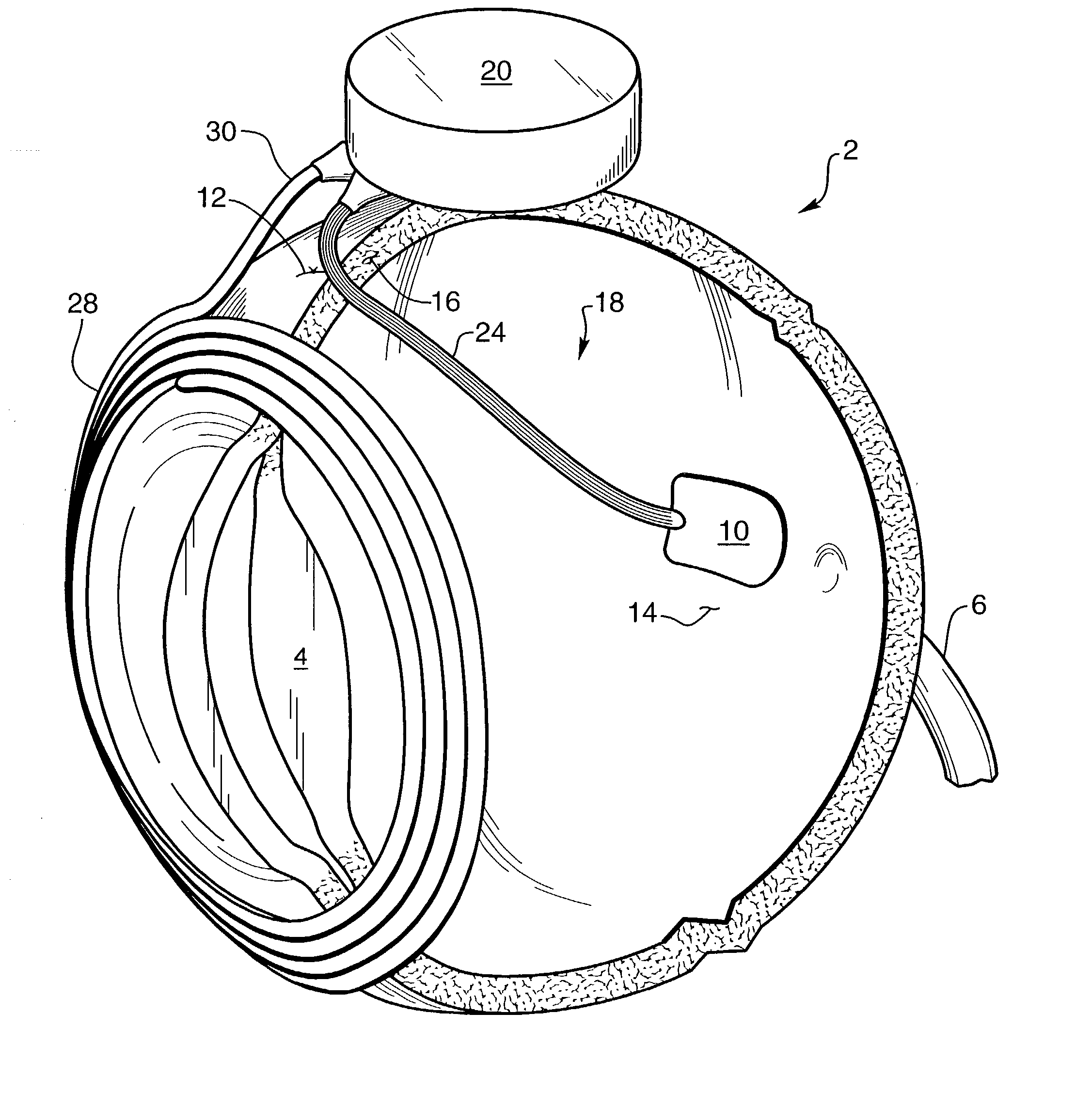
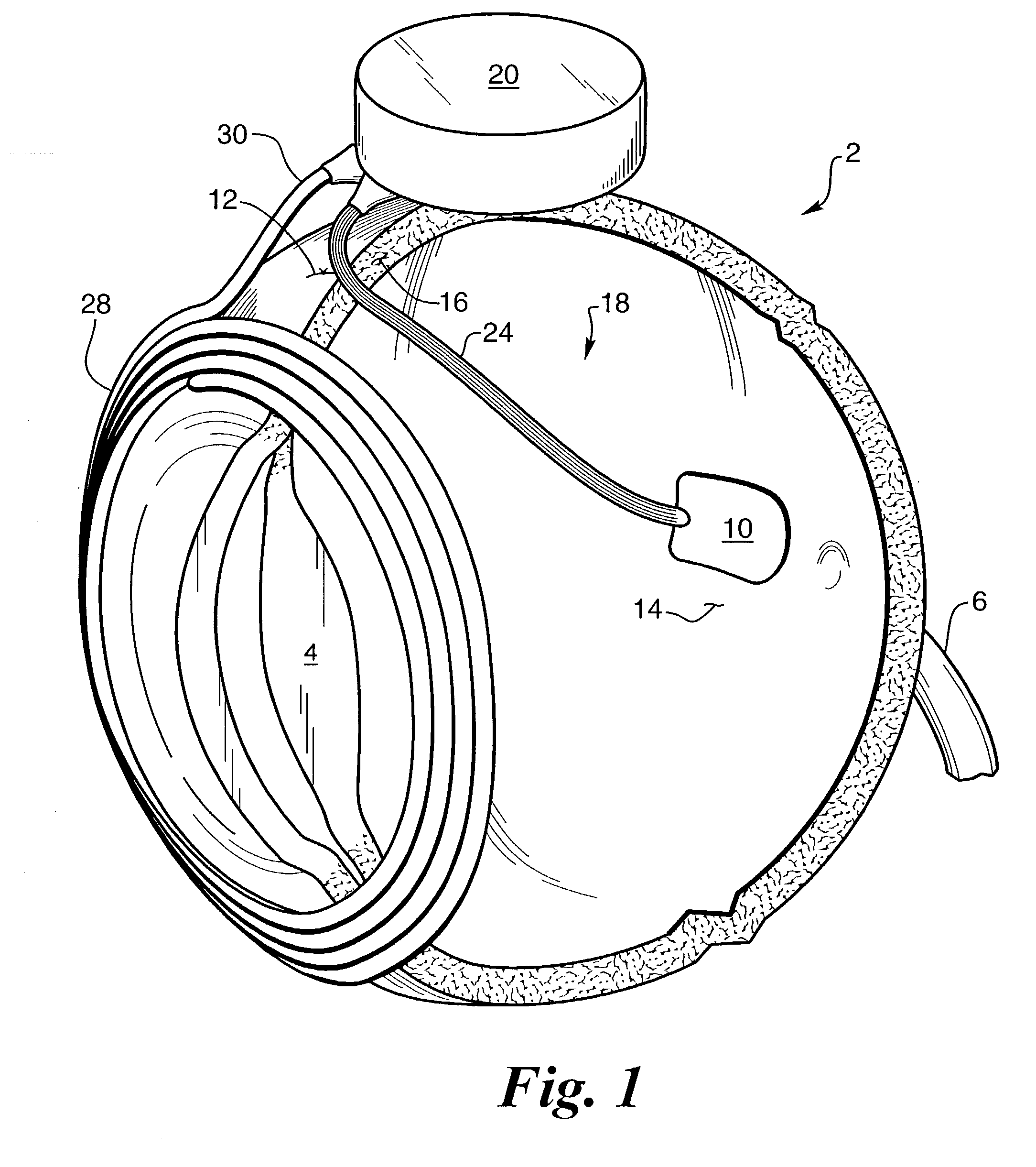
Device for use in the eye
A glaucoma filtration implant is constituted by a generally tubular body section having an oblong external diameter formed of a continuous convex curve. The device preferably has flow resistance structure provided by a portion of the internal lumen having a reduced diameter. The flow resistance structure may have a length of up to 5000 mum and a diameter in the range 15 to 50 mum, the diameter being selected so as to achieve a pressure drop along the flow resistance structure in the range 5 to 15 mm Hg. The internal conduit for liquid flow also may include a removable flow inhibitor, which can be removed after implantation by a laser, such as an ophthalmic YAG laser. The device is made of biocompatible materials.
Owner:UNIV COLLEGE LONDON INST OF OPHTHALMOLOGY +1
Annulus fibrosis augmentation methods and apparatus
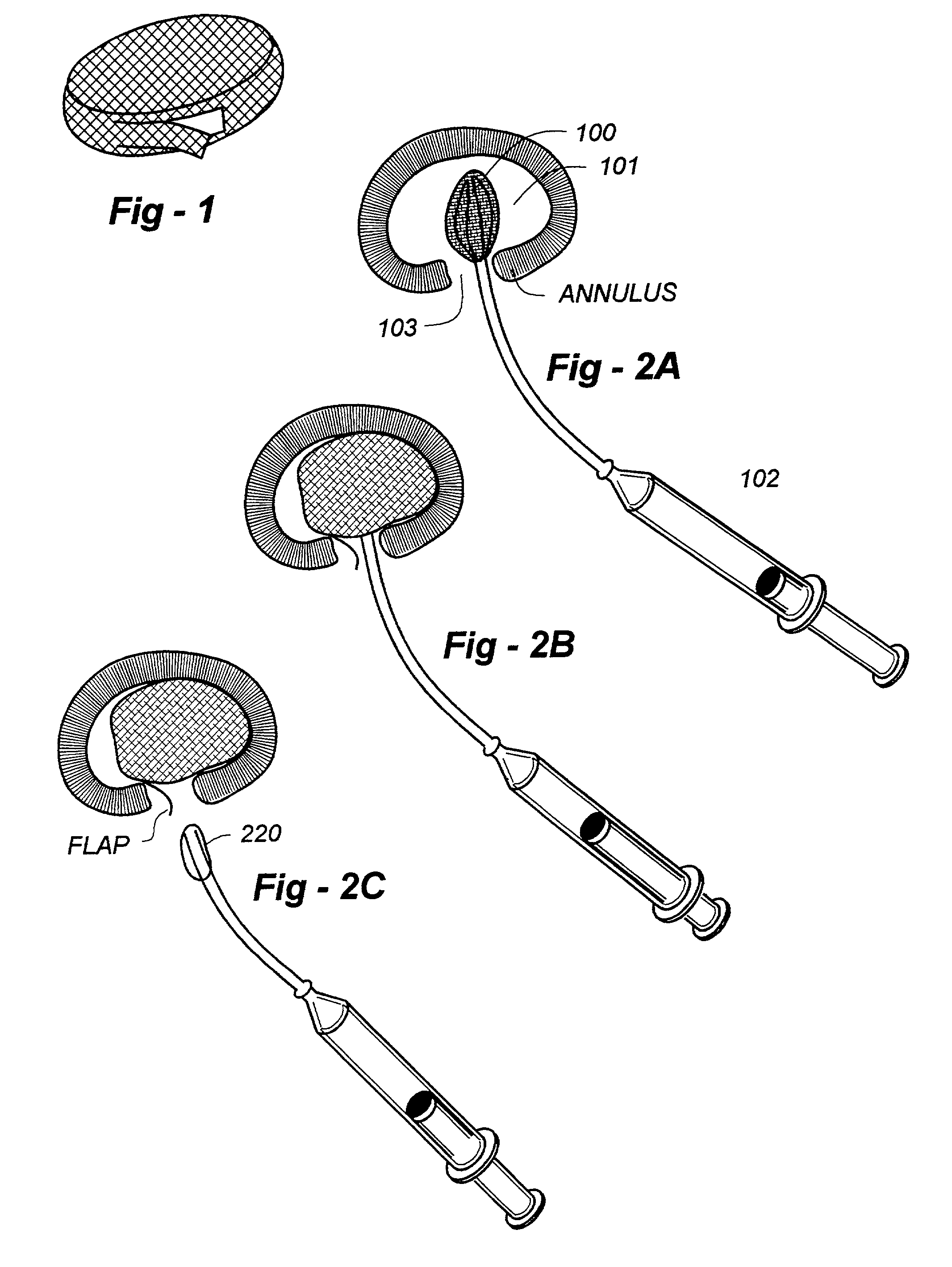
A device and method are used in fortifying an intervertebral disc having an annulus fibrosis with an inner wall. According to the method, a hole is formed through the annulus fibrosis, and a collapsed bag is inserted into the disc through the hole. The bag is inflated, or allowed to expand within the disc space, then filling with one or more biocompatible materials. The hole in the annulus fibrosis is then closed. In one preferred embodiment, the bag includes an inflatable bladder or balloon which is filled with a gas or liquid to expand the bag. In an alternative preferred embodiment, the bag includes a self-expanding frame that assumes a collapsed state for introduction into the disc space and an expanded state once inserted through the hole in the annulus. The self-expanding frame is composed of a shape-memory material, for example. The bag preferably features a wall which is porous to allow for the diffusion of body fluids therethrough, and the bag and / or frame may be fastened to the inner wall of the annulus at one or more points. The biocompatible material may include autograft nucleus pulposis, allograft nucleus pulposis or xenograft nucleus pulposis. In the preferred embodiment, the biocompatible material includes morselized nucleus or annulus from the same disc.
Owner:ANOVA
Biocompatible wires and methods of using same to fill bone void
InactiveUS20050015148A1Reduce compression fractureInternal osteosythesisSpinal implantsWire rodBone structure
Devices, kits, and methods are provided for reducing a bone fracture, e.g., a vertebral compression fracture, is provided. The device comprises a plurality of resilient wires composed of a biocompatible material, such as a biocompatible polymer (e.g., polymethylmethacrylate (PMMA)). The wires can be introduced into the cavity of the bone structure to form a web-like arrangement therein. The web-like arrangement can be stabilized by applying uncured bone cement onto the arrangement to connect the wires at their contacts point. The bone cavity can then be filled with a bone growth enhancing medium.
Owner:BOSTON SCI SCIMED INC
Cardiac valve annulus restraining device
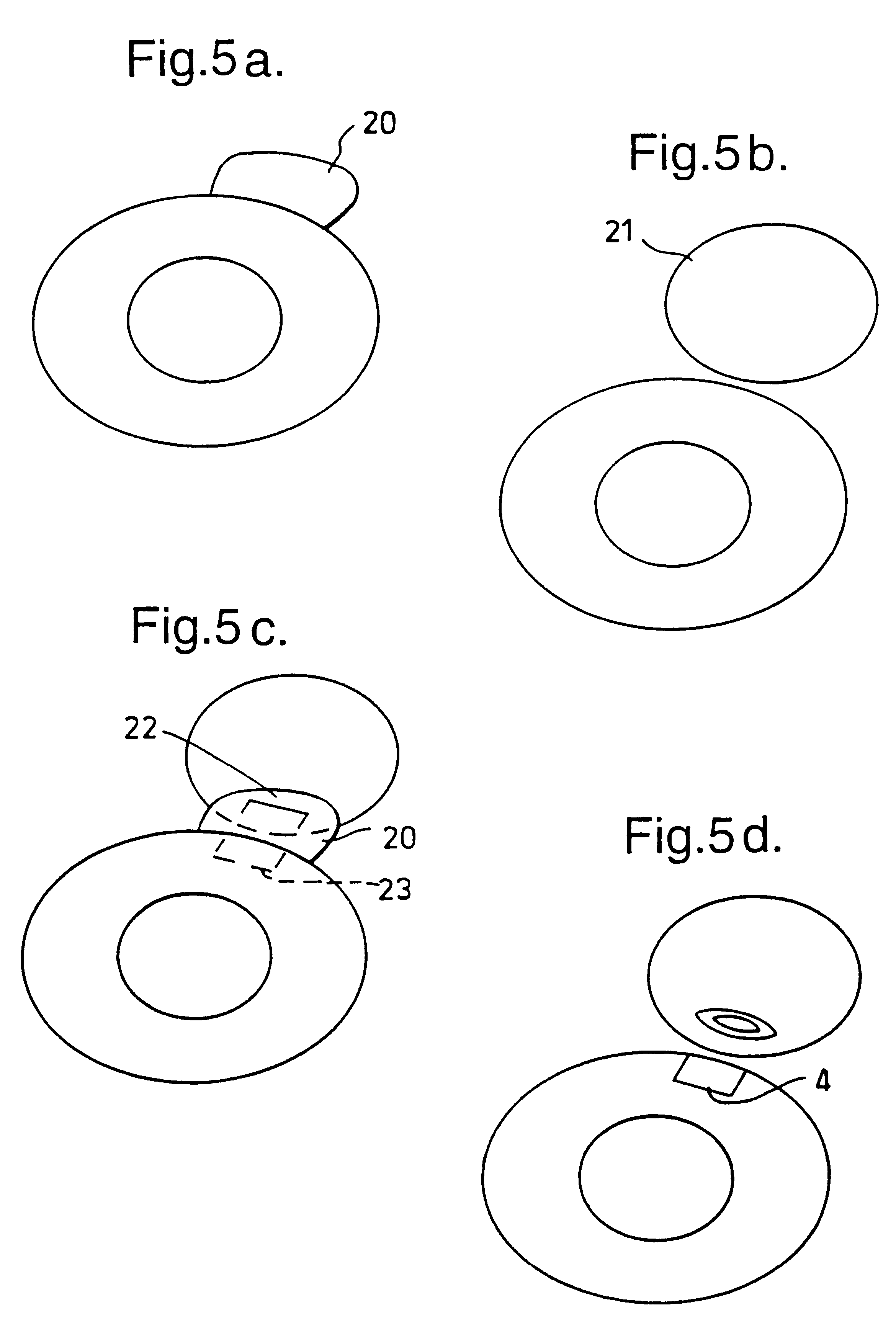
A catheter based system for treating mitral valve regurgitation includes a reshaping device having a body and a plurality of movable anchoring barbs attached to the body of the device. The reshaping device can be made from a biocompatible material having suitable shape memory properties. The devices of the current invention can be self expandable, balloon expandable, or a combination self expandable and balloon expandable. One embodiment of the invention includes a method for attaching a reshaping device to the annulus of a mitral valve, moving the body of the device from a fully expanded configuration to a resting configuration, and thereby reshaping the mitral valve annulus.
Owner:MEDTRONIC VASCULAR INC
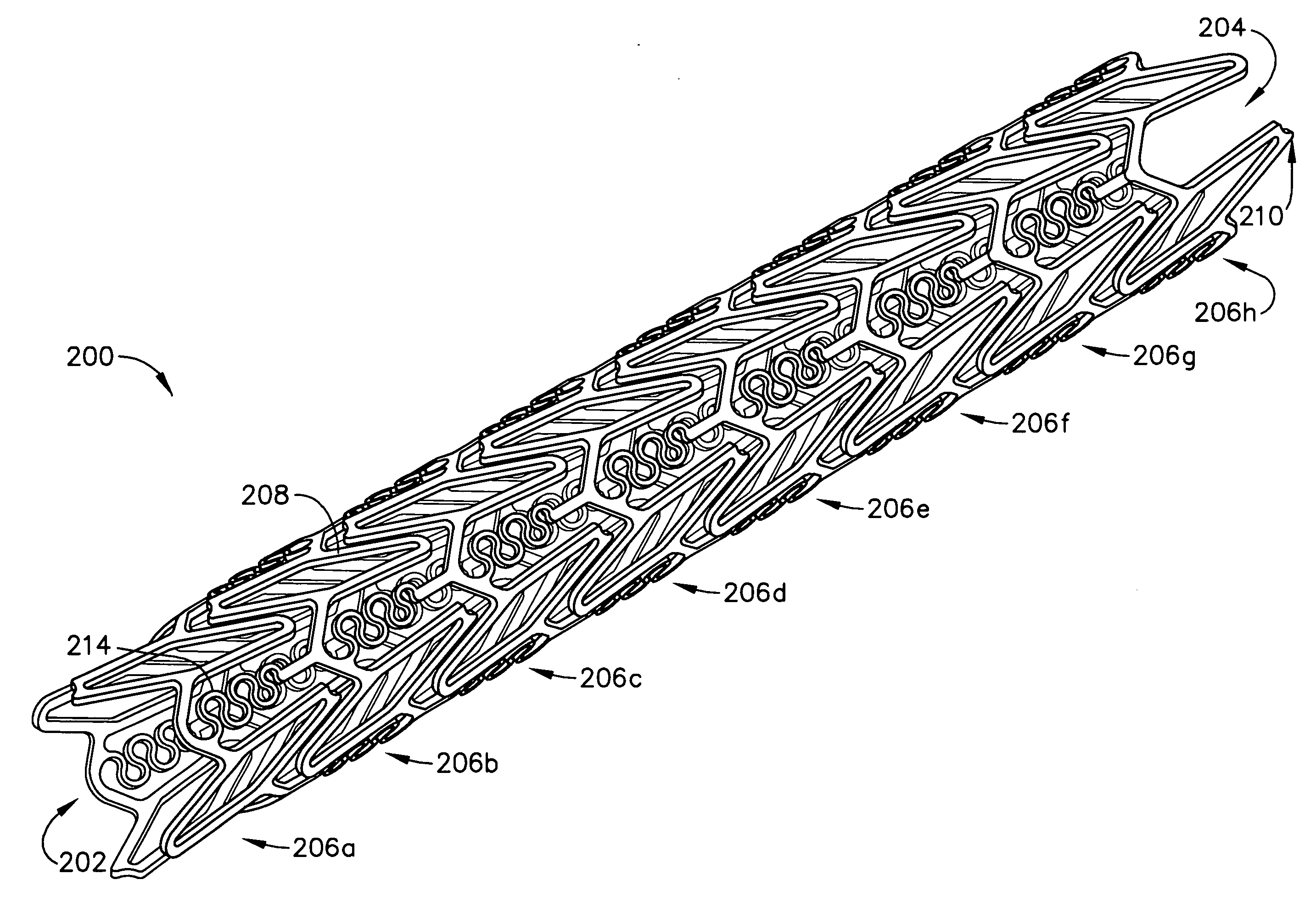
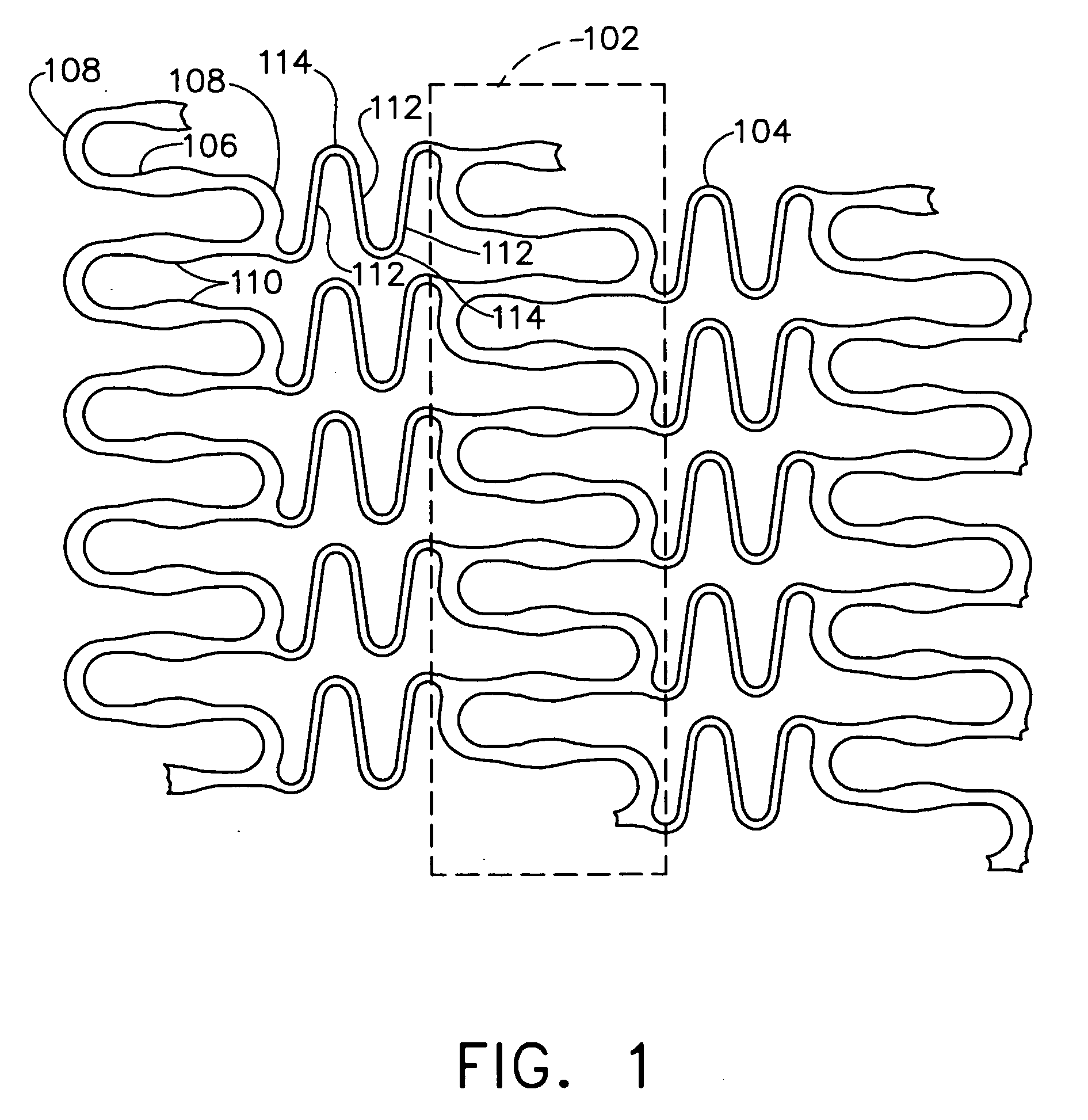
Complaint implantable medical devices and methods of making same
InactiveUS6936066B2Give flexibilityFacilitating transmural endothelializationStentsHeart valvesSurgical GraftMetallic materials
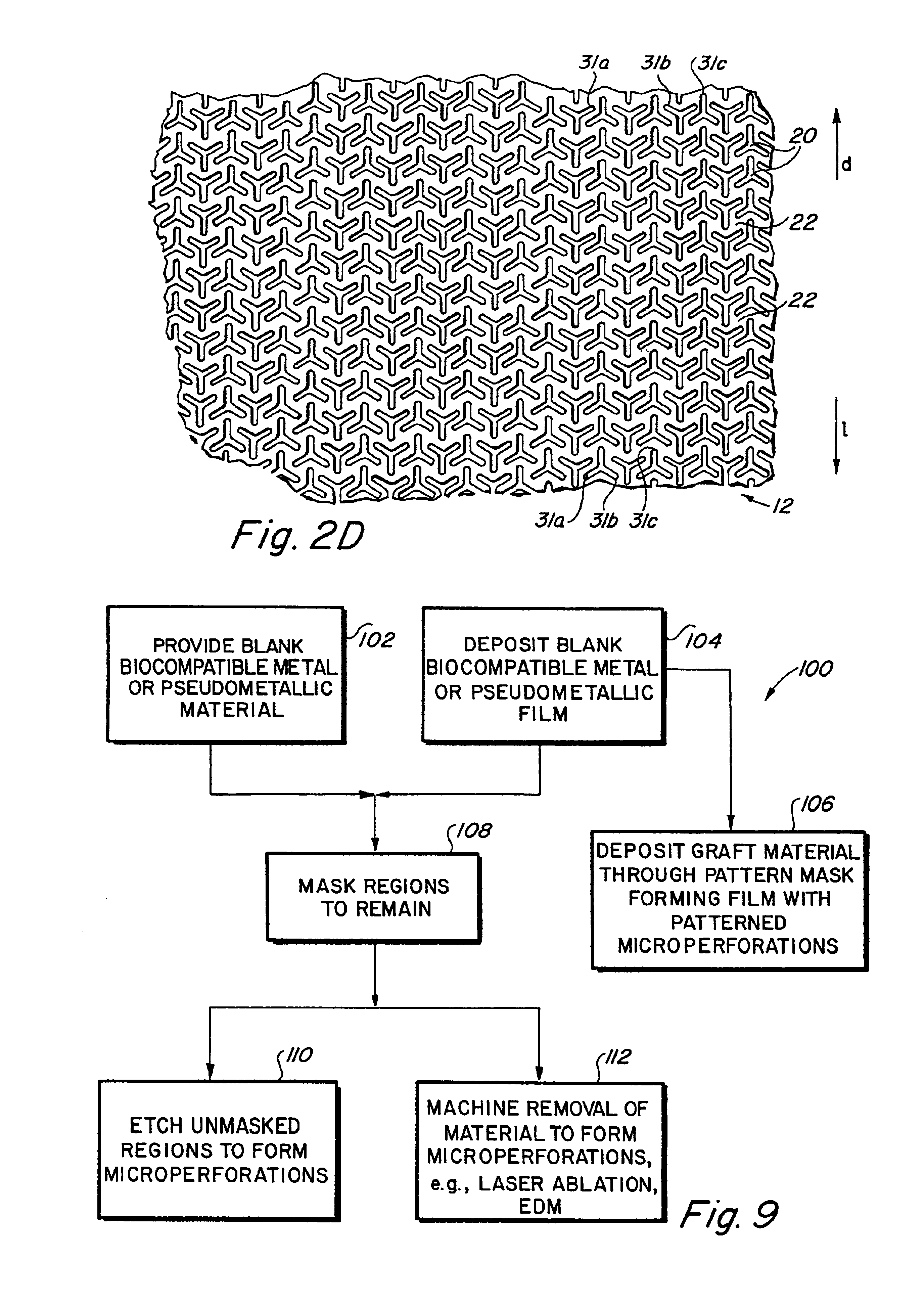
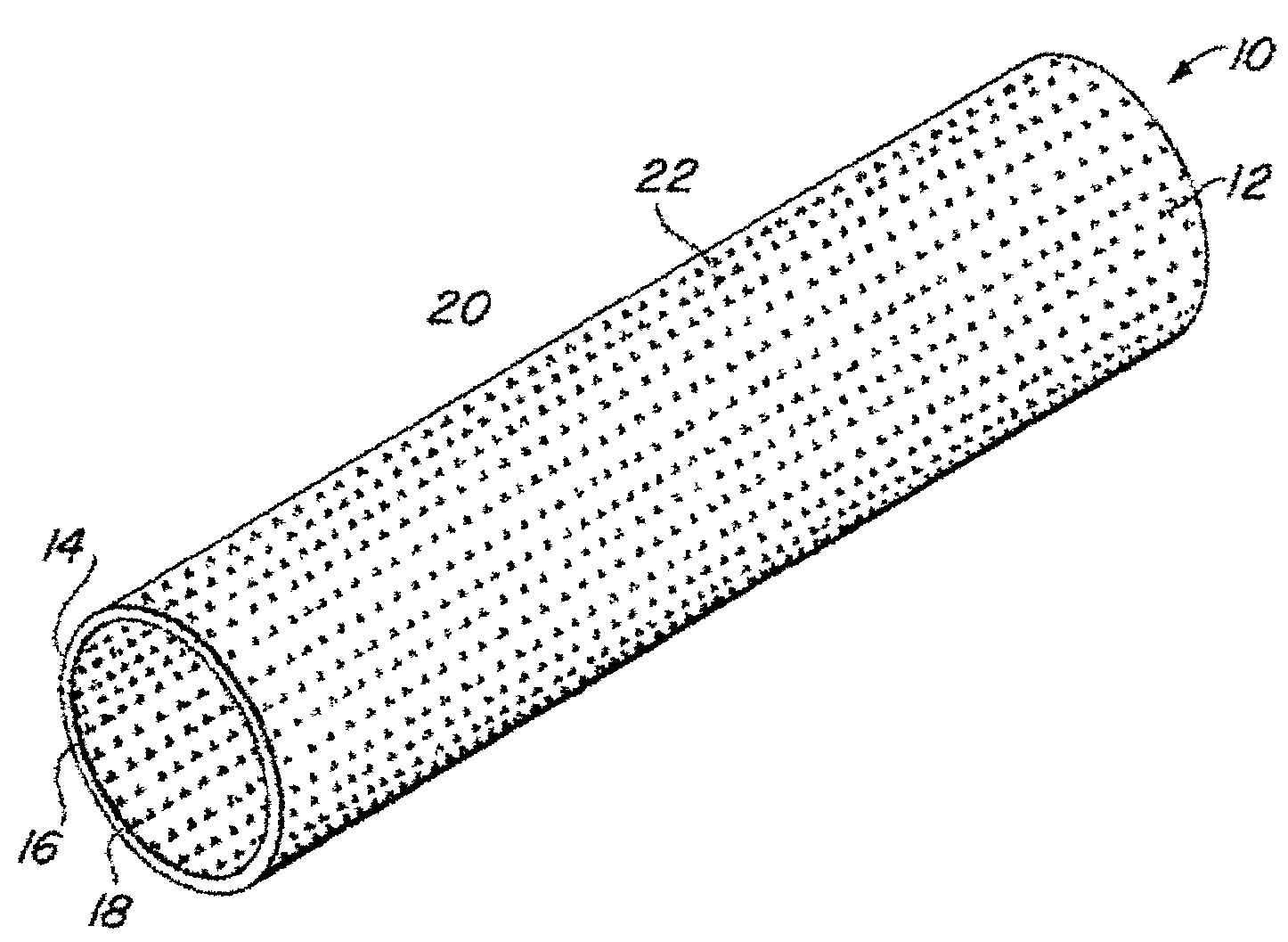
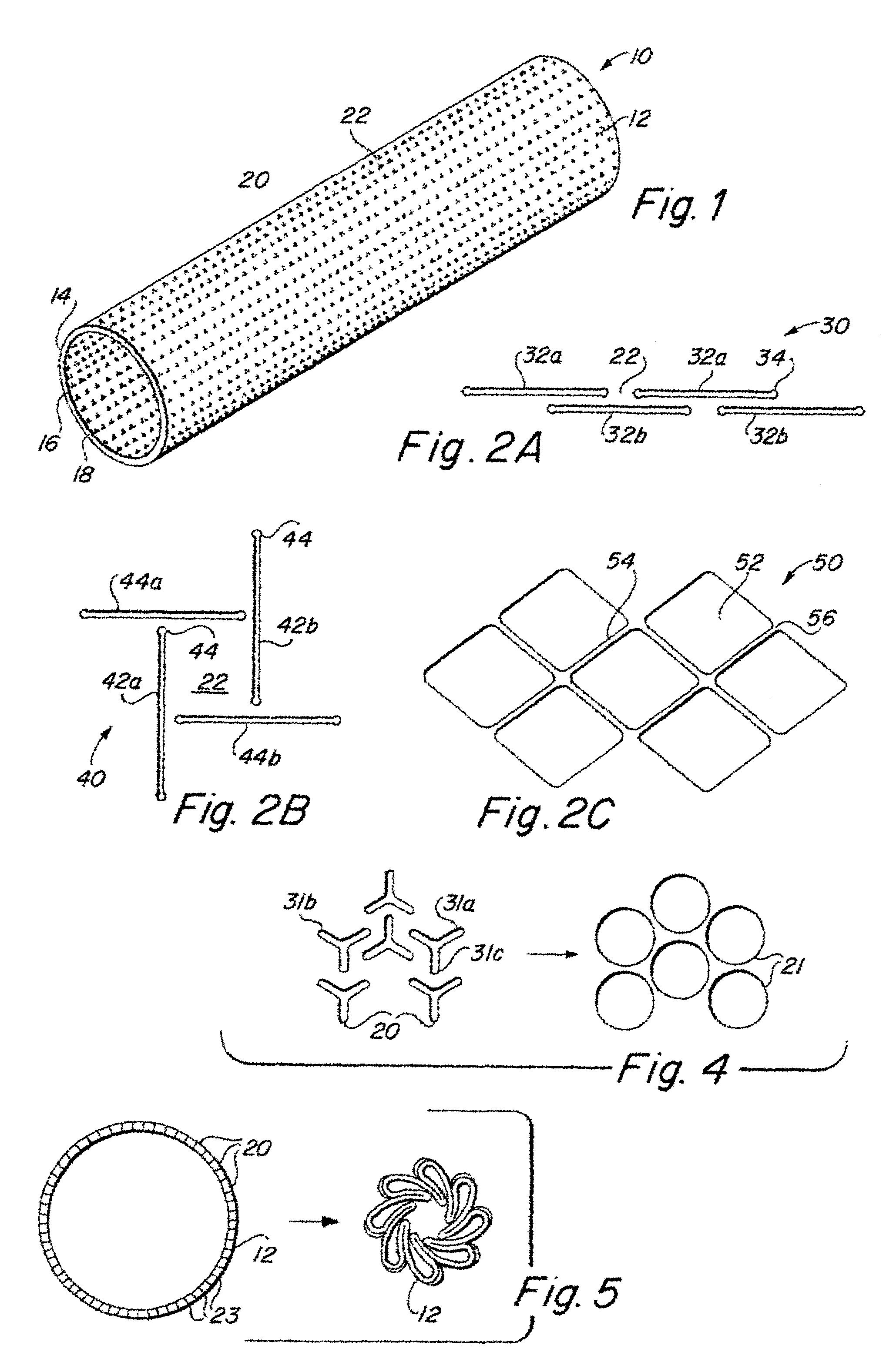
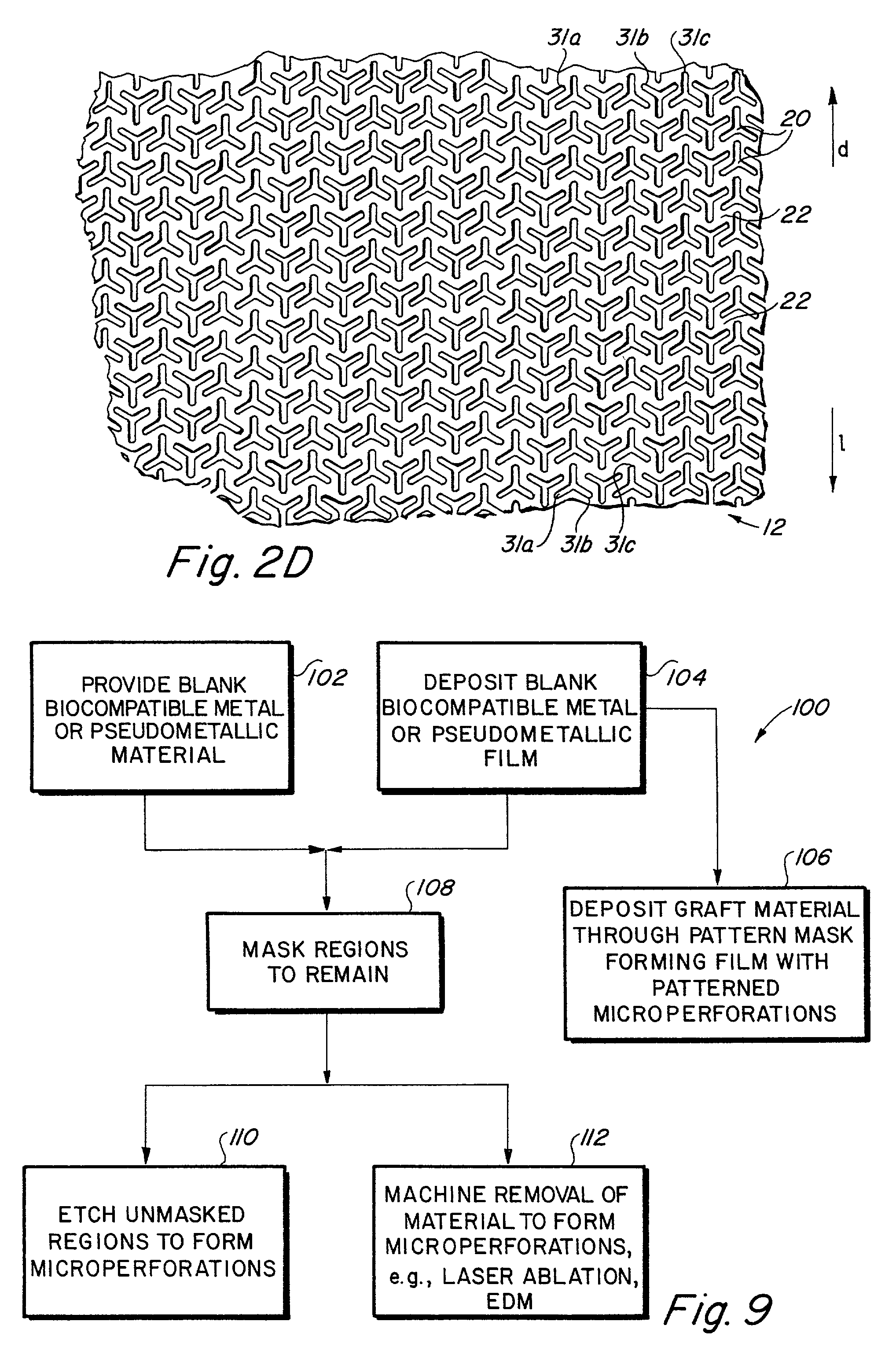
Implantable medical grafts fabricated of metallic or pseudometallic films of biocompatible materials having a plurality of microperforations passing through the film in a pattern that imparts fabric-like qualities to the graft or permits the geometric deformation of the graft. The implantable graft is preferably fabricated by vacuum deposition of metallic and / or pseudometallic materials into either single or multi-layered structures with the plurality of microperforations either being formed during deposition or after deposition by selective removal of sections of the deposited film. The implantable medical grafts are suitable for use as endoluminal or surgical grafts and may be used as vascular grafts, stent-grafts, skin grafts, shunts, bone grafts, surgical patches, non-vascular conduits, valvular leaflets, filters, occlusion membranes, artificial sphincters, tendons and ligaments.
Owner:VACTRONIX SCI LLC
Annulus repair systems and techniques
Systems and methods for repairing annulus defects include at least one blocking member positionable in or adjacent to the annulus defect, at least one attachment portion for securing the blocking member to adjacent tissue, and instruments for placing and engaging the blocking member in and / or adjacent to the annulus defect. The blocking member extends at least partially across the annulus defect for repair of the defect and / or retention of nucleus material, one or more implants, bio-compatible materials or device, and / or other objects positioned in the disc space.
Owner:WARSAW ORTHOPEDIC INC
Device forming an endoluminal intracorporeal endoprosthesis, in particular for the abdominal aorta
The invention relates to a device forming an endoluminal endoprosthesis. The device comprises at least a first segment, and upstream from said segment, at a predetermined distance therefrom, it comprises a "fixing" upstream segment made of a biocompatible material so as to be deployable from a closed or non-deployed position for insertion purposes to a deployed working position, the deployed working position being designed to be located in a healthy zone of blood vessel and being separated from the first segment by a predetermined distance defined by links of predetermined length. The invention makes it easy to recatheterize a blood vessel such as the abdominal aorta suffering from an aneurysm.
Owner:LEGONA ANSTALT
Implantable flexible circuit leads and methods of use
InactiveUS20080140152A1Easy to manufactureStrong specificitySpinal electrodesExternal electrodesDielectricFlexible circuits
Devices, systems and methods are provided for stimulation of tissues and structures within a body of a patient. In particular, implantable leads are provided which are comprised of a flexible circuit. Typically, the flexible circuit includes an array of conductors bonded to a thin dielectric film. Example dielectric films include polyimide, polyvinylidene fluoride (PVDF) or other biocompatible materials to name a few. Such leads are particularly suitable for stimulation of the spinal anatomy, more particularly suitable for stimulation of specific nerve anatomies, such as the dorsal root (optionally including the dorsal root ganglion).
Owner:ST JUDE MEDICAL LUXEMBOURG HLDG SMI S A R L SJM LUX SMI
Supplementation or replacement of a nucleus pulposus of an intervertebral disc
InactiveUS20060122704A1Avoid damageFunction increaseBone implantJoint implantsMedicineIntervertebral disc
A degenerated nucleus pulposus located in a central core region of an intervertebral disc within the annulus fibrosus is supplemented or replaced by a method wherein an amount of a biocompatible material is introduced into the central core region by a process including the steps of 1) forming a channel through a vertebral body adjacent to said intervertebral disc, extending from an exterior surface of the vertebral body to the central core region of the annulus fibrosus; 2) introducing an amount of a biocompatible material through the channel into the central core region of the annulus fibrosus; 3) pressurizing the biocompatible material through the channel to a postsurgical pressure sufficient to alleviate symptoms caused by the degenerated nucleus pulposus; and 4) sealing the channel while maintaining the sufficient postsurgical pressure. After sealing the channel, a vertebroplasty may optionally be performed in the vertebra.
Owner:DEPUY SYNTHES PROD INC
Drug delivery devices and methods
InactiveUS20080051866A1Designing can be facilitatedWide load rangeStentsHeart valvesRadiopaque agentActive agent
A biocompatible material may be configured into any number of implantable medical devices including intraluminal stents. Polymeric materials may be utilized to fabricate any of these devices, including stents. The stents may be balloon expandable or self-expanding. The polymeric materials may include additives such as drugs or other bioactive agents as well as radiopaque agents. By preferential mechanical deformation of the polymer, the polymer chains may be oriented to achieve certain desirable performance characteristics.
Owner:CORDIS CORP
Endoluminal prosthetic device
An endoluminal prosthetic device for placement in a body lumen is formed by stitching stents to a graft. A first or anchoring stent is used for securing a graft made of biocompatible material that forms at least one lumen. There is also a second stent. The first and second stents each include a plurality of struts and apices between the struts. At least two of the apices in each of the first and second stents are secured to the graft by stitches. A running suture links at least one stitch of the first stent and one stitch of the second stent. The running suture linking the first stent and the second stent adds strength to the stitches and better secures the first stent to the device. The endoluminal prosthetic device may be used in an aortic vessel to treat stenoses or aneurysms.
Owner:COOK MEDICAL TECH LLC
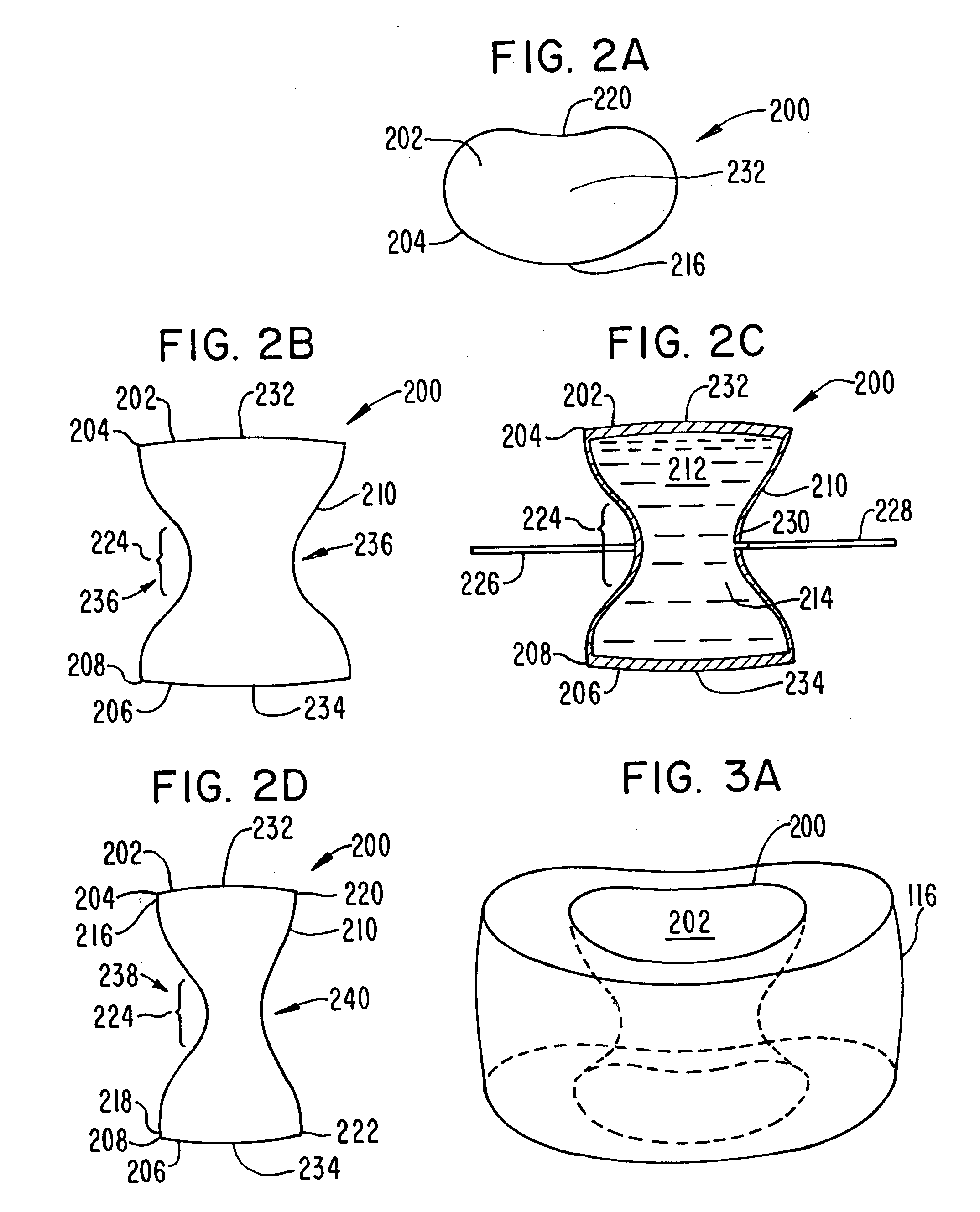
Intervertebral disk and nucleus prosthesis
A prosthetic implant for replacing a nucleus pulposus of an intervertebral disk includes upper and lower endwalls of discoid cross-section, each having an antero-posterior diameter less than its transverse diameter, and an hourglass-shaped sidewall connecting the peripheries of the upper endwall and lower endwall to enclose an interior volume filled with a substantially incompressible liquid or soft plastic material. A total prosthesis for replacing the entire human intervertebral disk has an annular core made of a first biocompatible polymer surrounding a central cavity, transitional plates affixed respectively to the upper and lower surfaces of the annular core, the upper and lower transitional plates being made of a second biocompatible material having an elastic modulus greater than that of the first biocompatible polymer, and upper and lower endplates adapted to contact adjacent vertebrae and affixed respectively to the upper and lower transitional plates.
Owner:K2M
Biocompatible bonding method and electronics package suitable for implantation
ActiveUS20030233134A1Uniform propertySemiconductor/solid-state device detailsSolid-state devicesFlexible circuitsHermetic seal
The invention is directed to a method of bonding a hermetically sealed electronics package to an electrode or a flexible circuit and the resulting electronics package, that is suitable for implantation in living tissue, such as for a retinal or cortical electrode array to enable restoration of sight to certain non-sighted individuals. The hermetically sealed electronics package is directly bonded to the flex circuit or electrode by electroplating a biocompatible material, such as platinum or gold, effectively forming a plated rivet-shaped connection, which bonds the flex circuit to the electronics package. The resulting electronic device is biocompatible and is suitable for long-term implantation in living tissue.
Owner:CORTIGENT INC +1
Implantable intraluminal device and method of using same in treating aneurysms
An intraluminal device implantable in a blood vessel having an aneurysm therein in the vicinity of a perforating vessel and / or of a bifurcation leading to a branch vessel. The intraluminal device includes a mesh-like tube of bio-compatible material having an expanded condition in which the tube diameter is slightly larger than the diameter of the blood vessel in which it is to be implanted, and the tube length is sufficient to straddle the aneurysm and to be anchored to the blood vessel on the opposite sides of the aneurysm. The mesh-like tube also has a contracted condition wherein it is sufficiently flexible so as to be easily manipulatable through the blood vessel to straddle the aneurysm. In its expanded condition, the mesh-like tube has a porosity index of 55%-80% such as to reduce the flow of blood through its wall to the aneurysm sufficiently to decrease the possibility of rupture of the aneurysm but not to unduly reduce the blood flow to a perforating or branch vessel to the degree likely to cause significant damage to tissues supplied with blood by such perforating or branch vessel.
Owner:STRYKER CORP
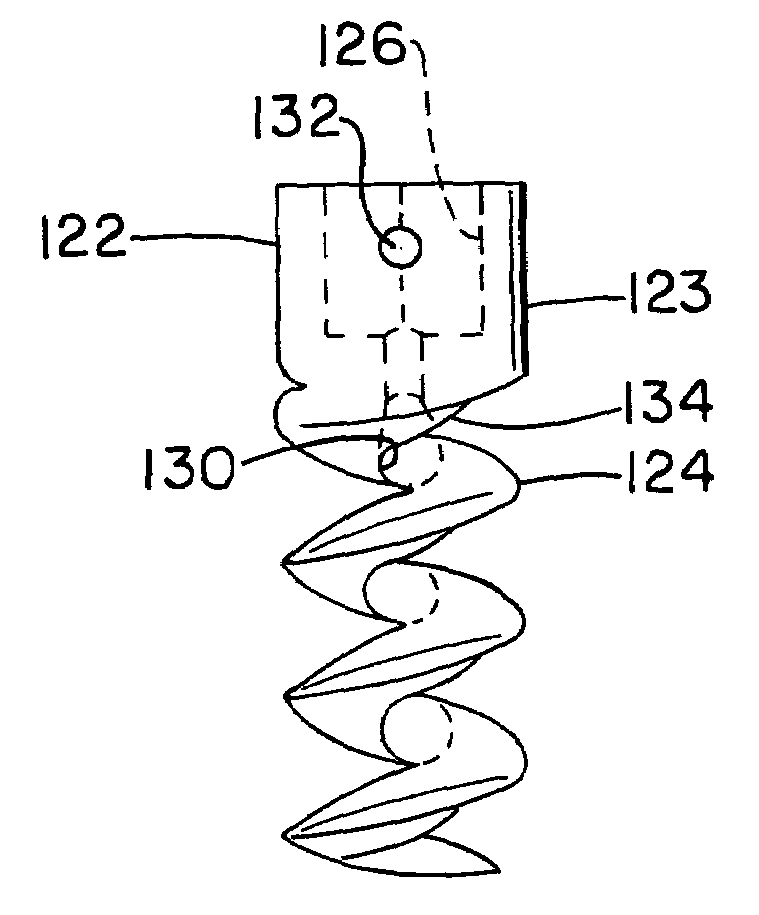
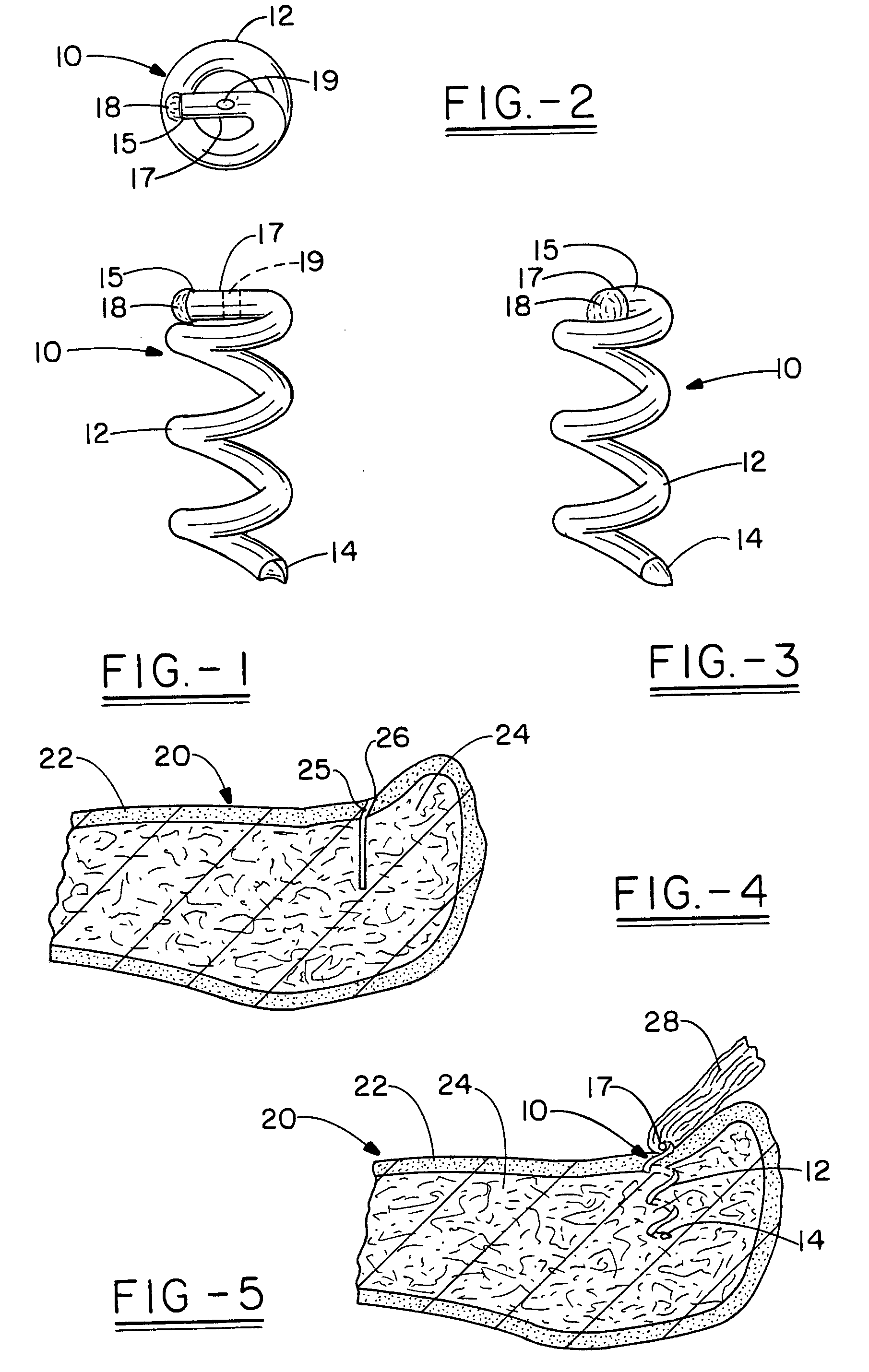
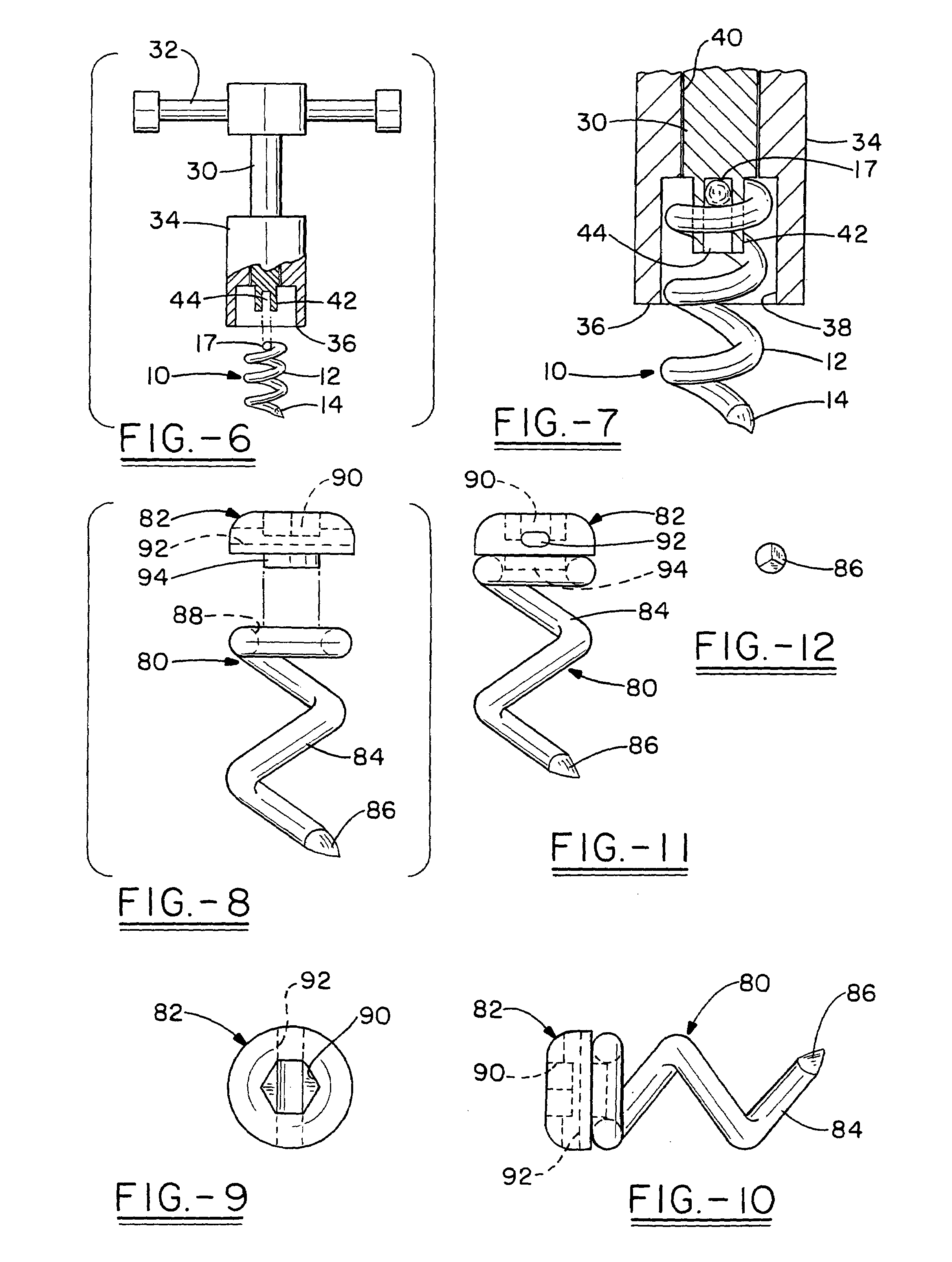
Open helical organic tissue anchor having recessible head and method of making the organic tissue anchor
InactiveUS7189251B2Easy to anchorStrengthens helixSuture equipmentsPinsSelf reinforcedLigament structure
The invention relates to a tissue anchor which is an open helix of biocompatible material having a slope of from 0.5 to 10 turns per centimeter, a length from 3 to 75 millimeters, a diameter of from 1.5 to 11 millimeters, and an aspect ratio of from about 3 to about 5 to 1. The anchor can have a head which is capable of securing or clamping tissue together, such as holding a suture to secure a ligament or tendon to bone. The anchor can also have a head which causes an inward, compressive loading for use in fastening bone to bone, orthopedic plates to bone, or cartilage to bone. The head may be an integral member and may include a self-reinforcing wedge which joins the helix to the head. Further, the elongate member, or filament that forms the helix may have a tapering diameter along its length.
Owner:ORTHOHELIX SURGICAL DESIGNS
Stent for a vascular meniscal repair and regeneration
ActiveUS20070067025A1Promote healingPromote regenerationStentsAdditive manufacturing apparatusMeniscal repairVascular tissue
A surgical stent made of biocompatible material for implantation in human tissue to enable blood and nutrients to flow from an area of vascular tissue to an area of tissue with little or no vasculature.
Owner:HOWMEDICA OSTEONICS CORP
Radiolucent bone graft
An improved bone graft is provided for human implantation, bone graft includes a substrate block of high strength biocompatible material having a selected size and shape to fit the anatomical space, and a controlled porosity analogous to natural bone. The substrate block may be coated with a bio-active surface coating material such as hydroxyapatite or a calcium phosphate to promote bone ingrowth and enhanced bone fusion. Upon implantation, the bone graft provides a spacer element having a desired combination of mechanical strength together with osteoconductivity and osteoinductivity to promote bone ingrowth and fusion, as well as radiolucency for facilitated post-operative monitoring. The bone graft may additionally carry one or more natural or synthetic therapeutic agents for further promoting bone ingrowth and fusion.
Owner:AMEDICA A DELAWARE
Method and apparatus for creating intrauterine adhesions
InactiveUS20050171569A1Better treat excessive bleedingPromote tissue growthUltrasonic/sonic/infrasonic diagnosticsSuture equipmentsExcessive BleedingGynecology
In general, the present invention contemplates an implantable device for treating excessive bleeding in a body cavity. The device comprises a biocompatible material, for example polyethylene teraphathalate (PET), which is deliverable into the body cavity. The biocompatible material contains an attribute(s) that promotes tissue reaction or growth that results in a tissue response and / or adhesion formation within the body cavity to reduce or stop the excessive bleeding.
Owner:AUB HLDG
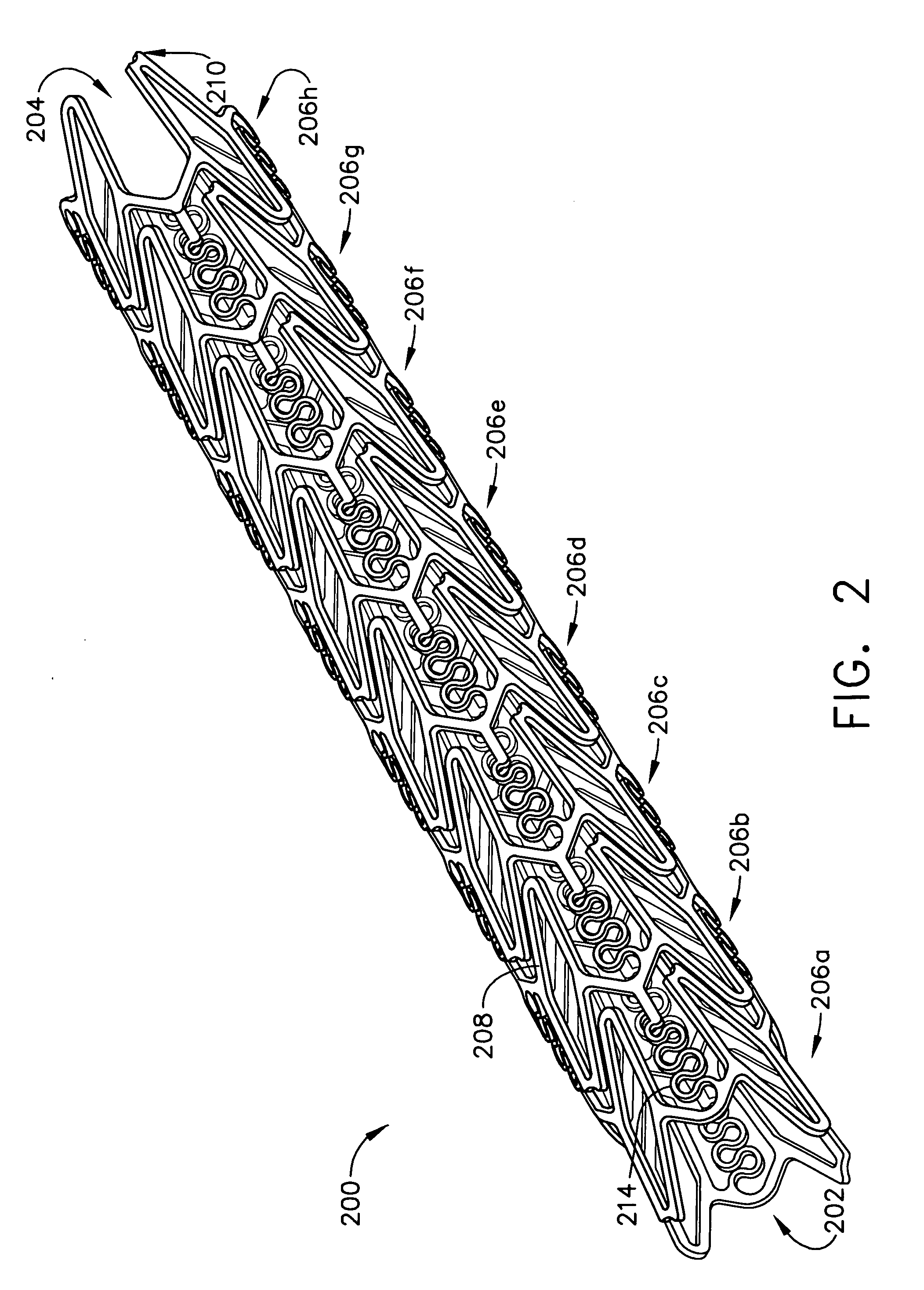
Self-supporting metallic implantable grafts, compliant implantable medical devices and methods of making same
Implantable medical grafts fabricated of metallic or pseudometallic films of biocompatible materials having a plurality of microperforations passing through the film in a pattern that imparts fabric-like qualities to the graft or permits the geometric deformation of the graft. The implantable graft is preferably fabricated by vacuum deposition of metallic and / or pseudometallic materials into either single or multi-layered structures with the plurality of microperforations either being formed during deposition or after deposition by selective removal of sections of the deposited film. The implantable medical grafts are suitable for use as endoluminal or surgical grafts and may be used as vascular grafts, stent-grafts, skin grafts, shunts, bone grafts, surgical patches, non-vascular conduits, valvular leaflets, filters, occlusion membranes, artificial sphincters, tendons and ligaments.
Owner:VACTRONIX SCI LLC
Prophylactic bactericidal implant
InactiveUS20060004431A1Prevent microbial infectionSuture equipmentsElectrotherapyProtozoaSurgical operation
A medical implant system is described for inhibiting infection associated with a joint prosthesis implant. An inventive system includes an implant body made of a biocompatible material which has a metal component disposed on an external surface of the implant body. A current is allowed to flow to the metal component, stimulating release of metal ions toxic to microbes, such as bacteria, protozoa, fungi, and viruses. One detailed system is completely surgically implantable in the patient such that no part of the system is external to the patient while the system is in use. In addition, externally controlled devices are provided which allow for modulation of implanted components.
Owner:AIONX ANTIMICROBIAL TECH INC
Topographic coatings and coating methods for medical devices
InactiveUS20050228477A1Easy to operateImprove adhesionStentsPretreated surfacesInsertion stentElution
Medical devices having topographic coatings are provided. The topographic coatings have regions of high and low elevation and may be composed of polymers, metals, ceramics, proteins and other biocompatible materials. Such topographic coatings facilitate the deposition, elution, and protection of therapeutic agents on the medical device, manipulation of the medical device, and other purposes. In particularly preferred embodiments, the medical device comprises a stent for vascular implantation.
Owner:XTENT INC
Non-destructive tissue repair and regeneration
ActiveUS20070185568A1Facilitate healing and regenerationEasy to fixStentsAdditive manufacturing apparatusNon destructiveTissue repair
A surgical stent made of biocompatible material for implantation in human tissue to enable blood and nutrients to flow from an area of vascular tissue to an area of tissue with little or no vasculature.
Owner:HOWMEDICA OSTEONICS CORP
Articles comprising large-surface-area bio-compatible materials and methods for making and using them
ActiveUS20100303722A1Improve cell adhesionAccelerated cell growth characteristicImmobilised enzymesBioreactor/fermenter combinationsCell culture mediaBone growth
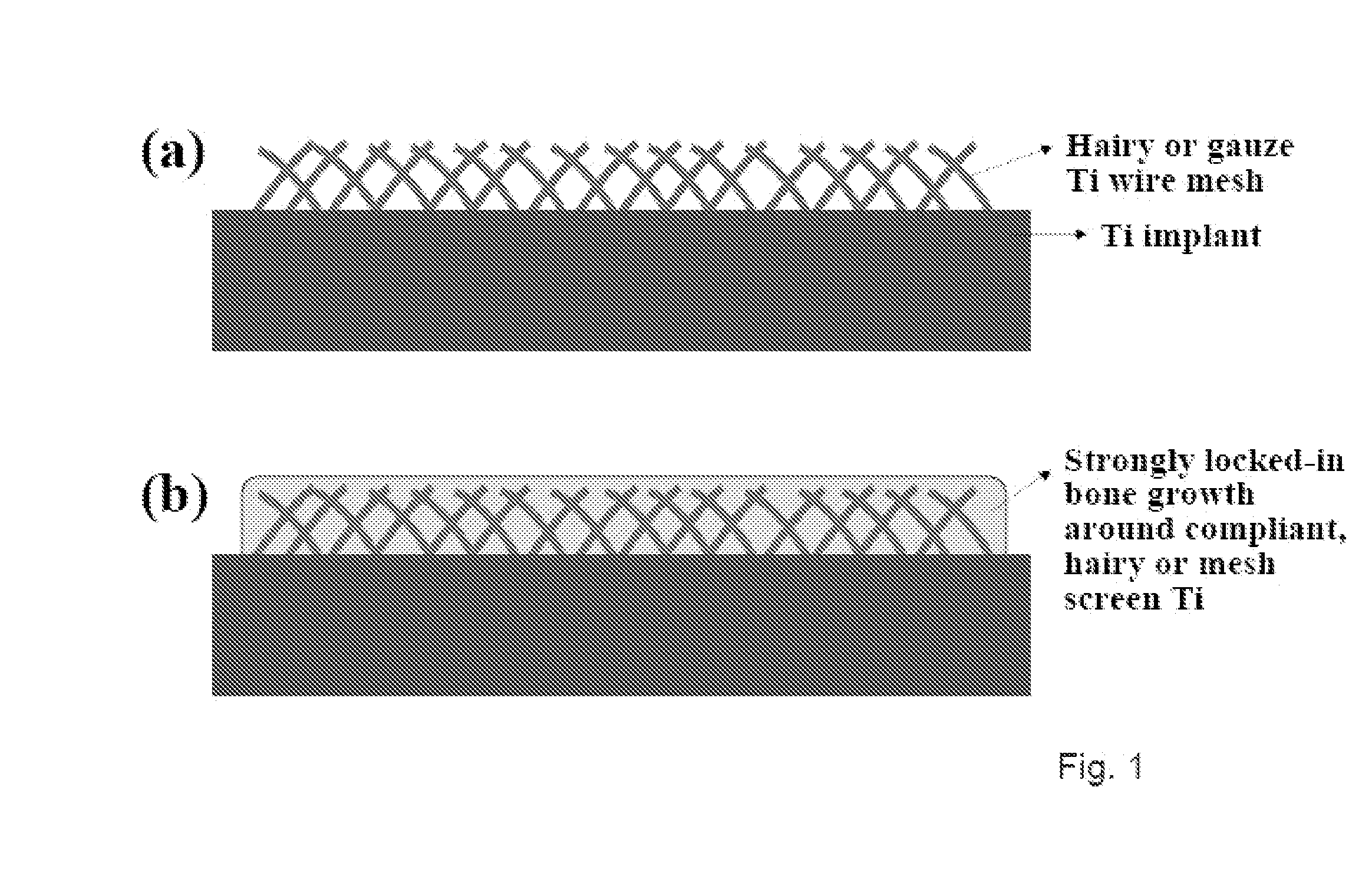
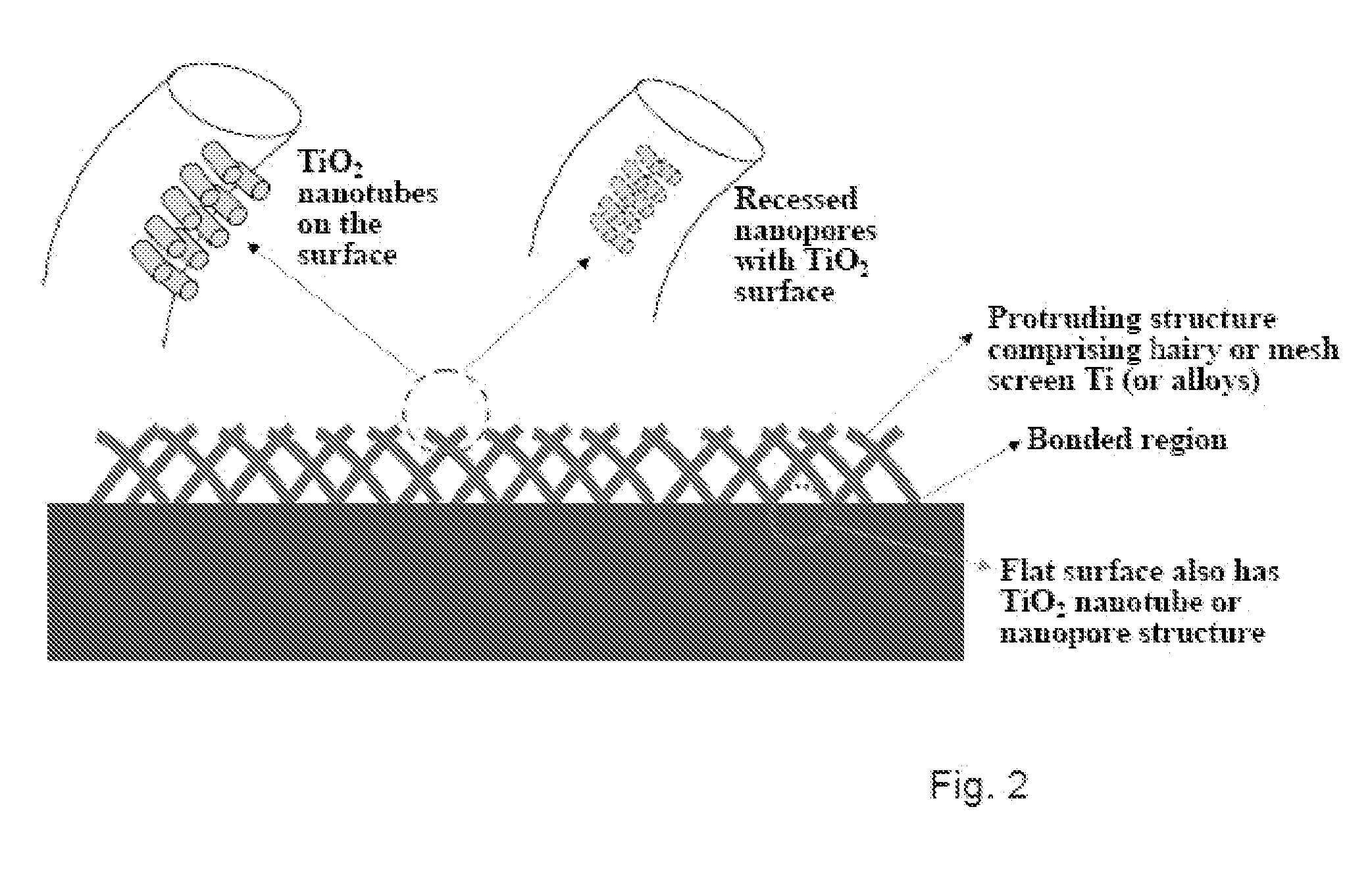
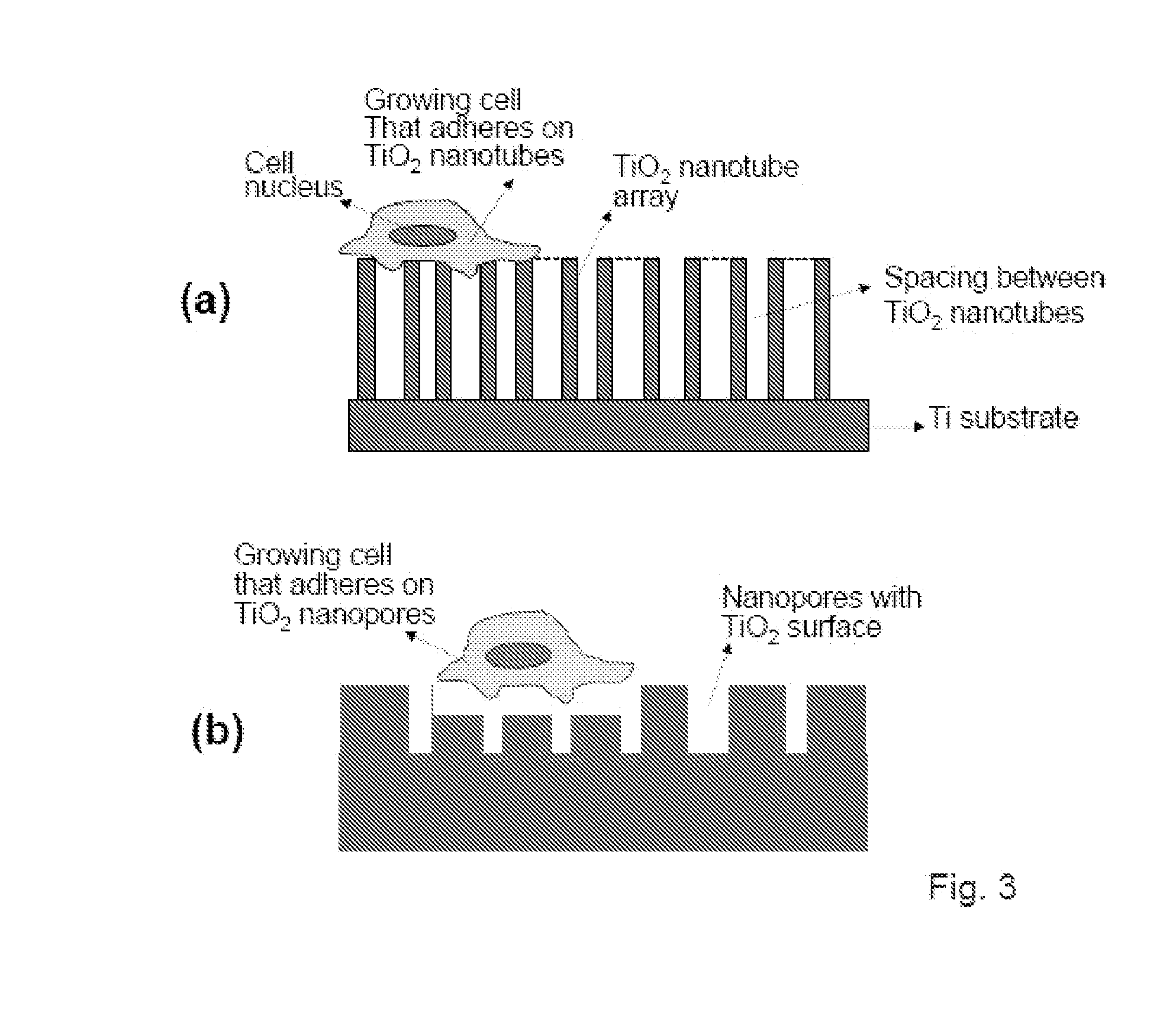
The present invention provides articles of manufacture comprising biocompatible nanostructures comprising significantly increased surface area for, e.g., organ, tissue and / or cell growth, e.g., for bone, tooth, kidney or liver growth, and uses thereof, e.g., for in vitro testing of drugs, chemicals or toxins, or as in vivo implants, including their use in making and using artificial tissues and organs, and related, diagnostic, screening, research and development and therapeutic uses, e.g., as drug delivery devices. The present invention provides biocompatible nanostructures with significantly increased surface area, such as with nanotube and nanopore array on the surface of metallic, ceramic, or polymer materials for enhanced cell and bone growth, for in vitro and in vivo testing, cleansing reaction, implants and therapeutics. The present invention provides optically transparent or translucent cell-culturing substrates. The present invention provides biocompatible and cell-growth-enhancing culture substrates comprising elastically compliant protruding nanostructure substrates coated with Ti, TiO2 or related metal and metal oxide films.
Owner:RGT UNIV OF CALIFORNIA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com