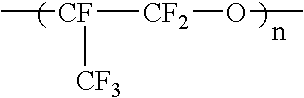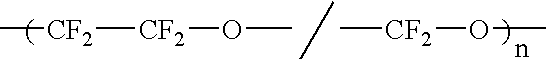Patents
Literature
98results about How to "Promote tissue growth" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
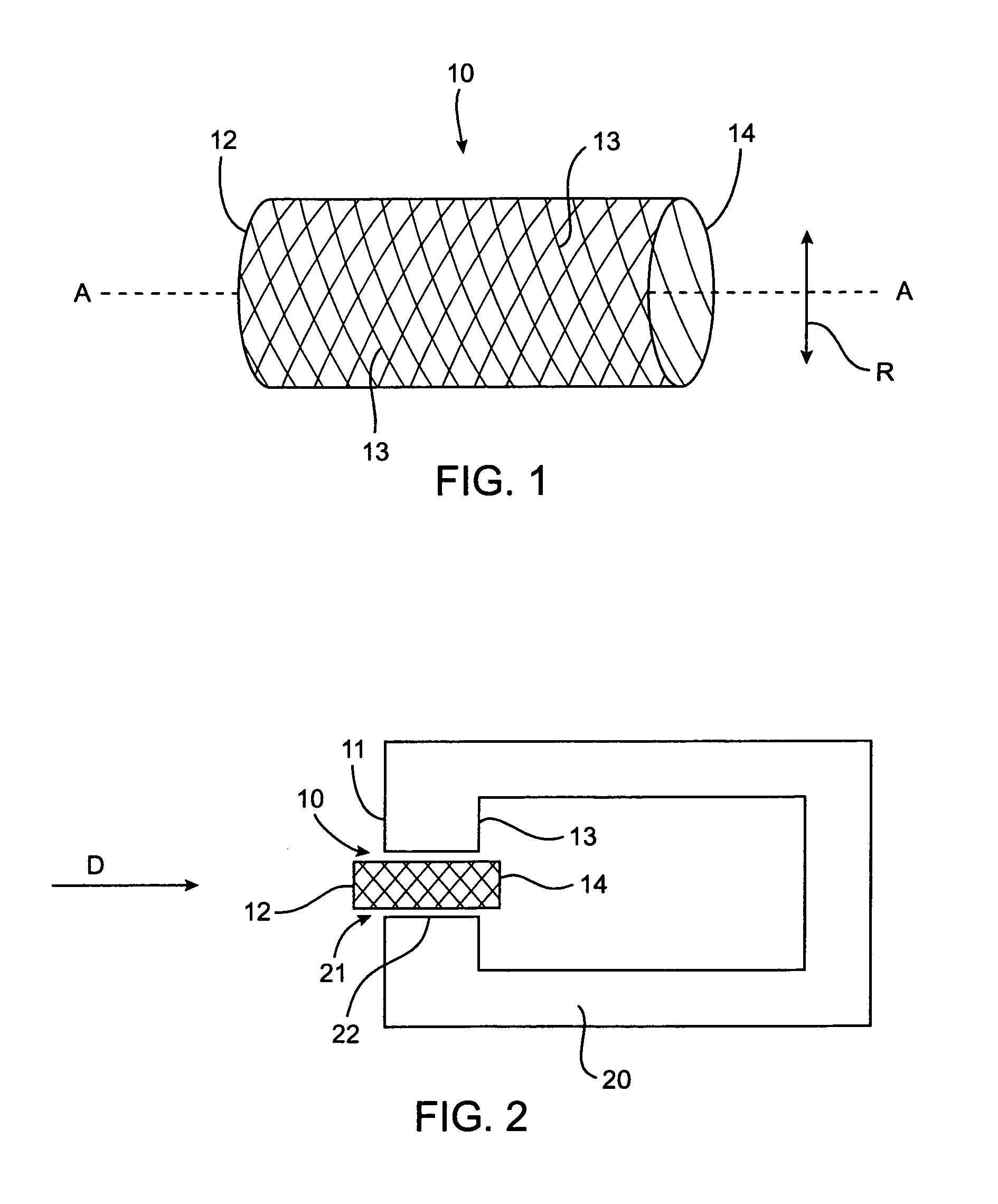
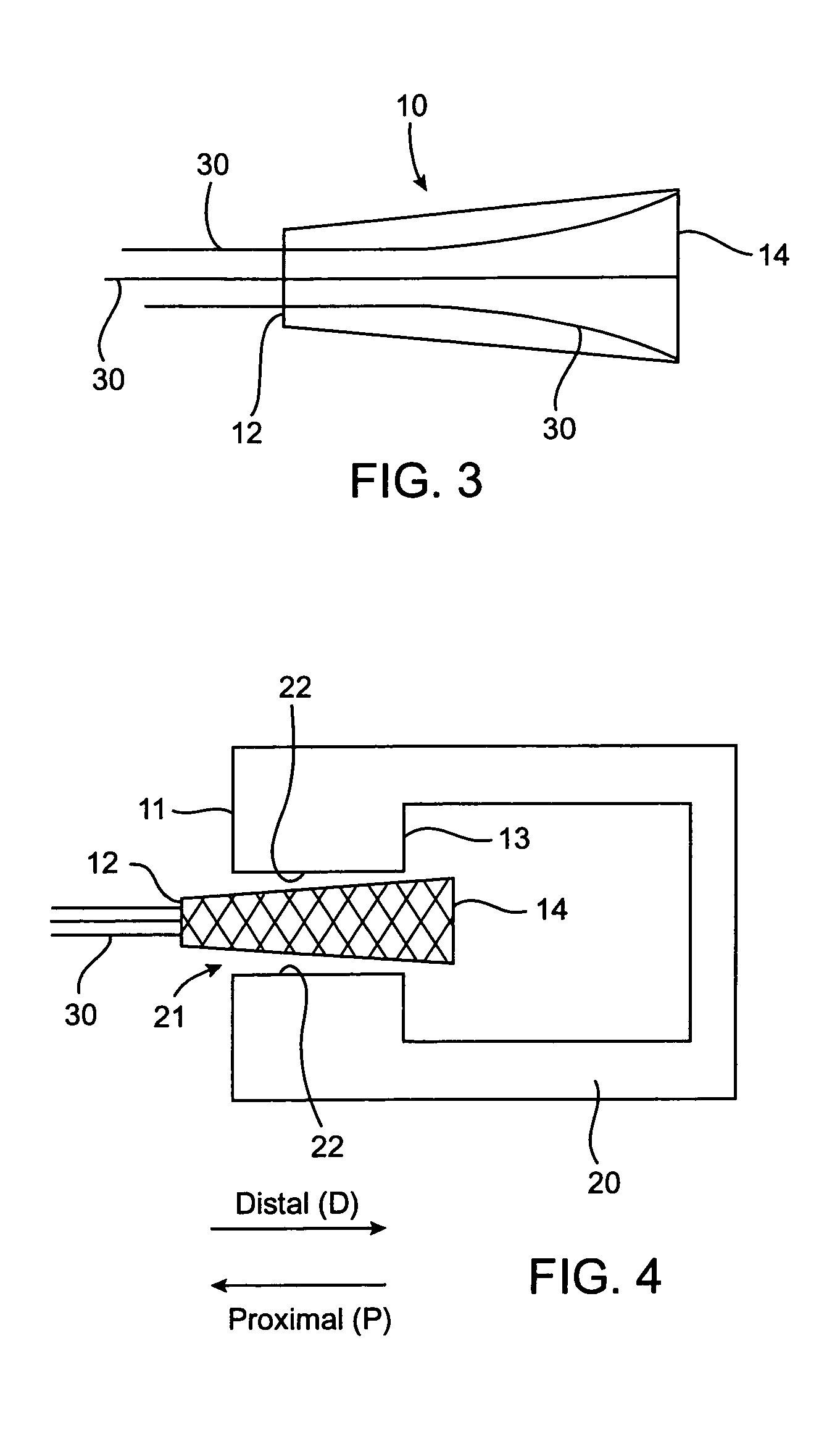
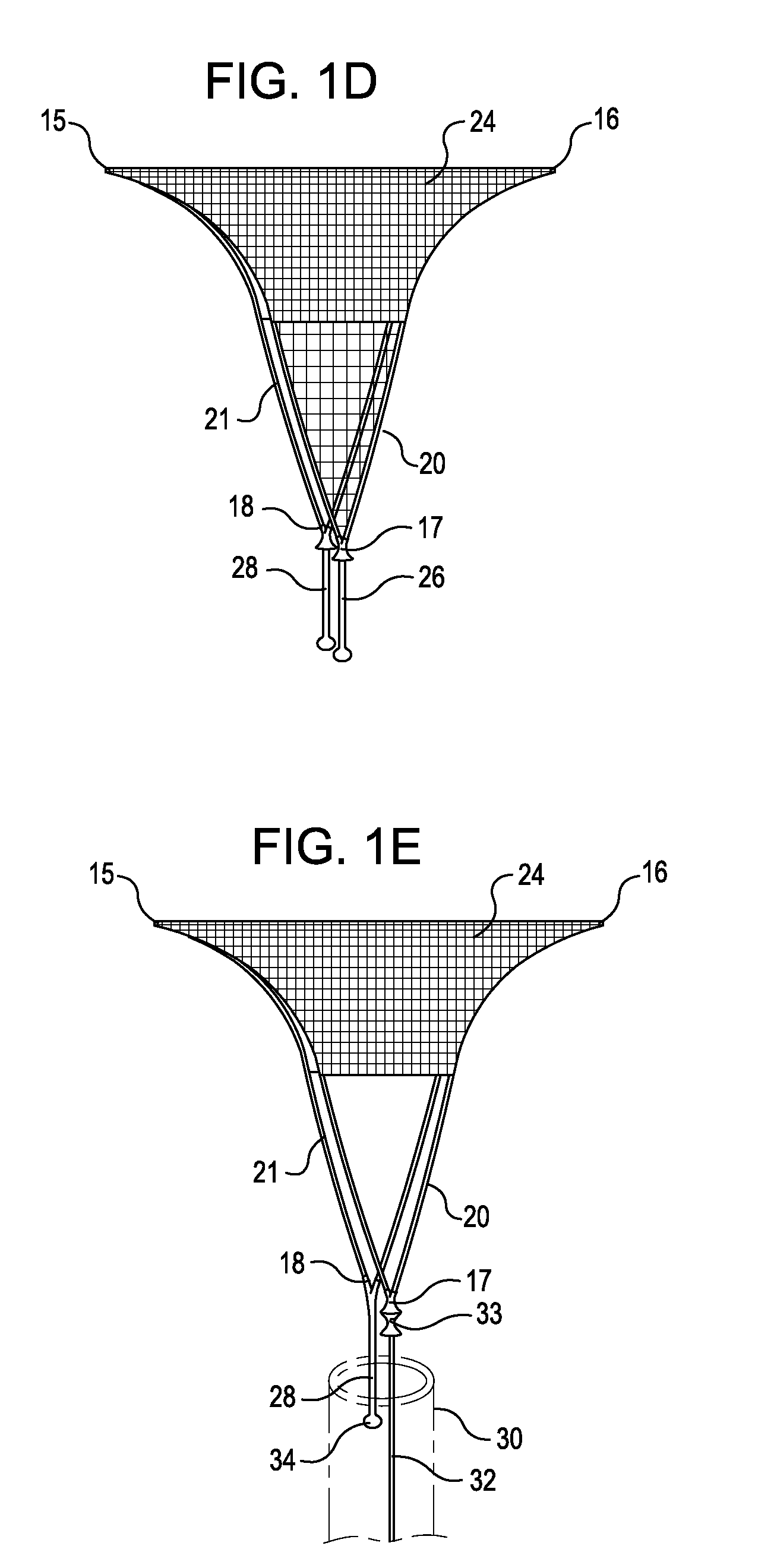
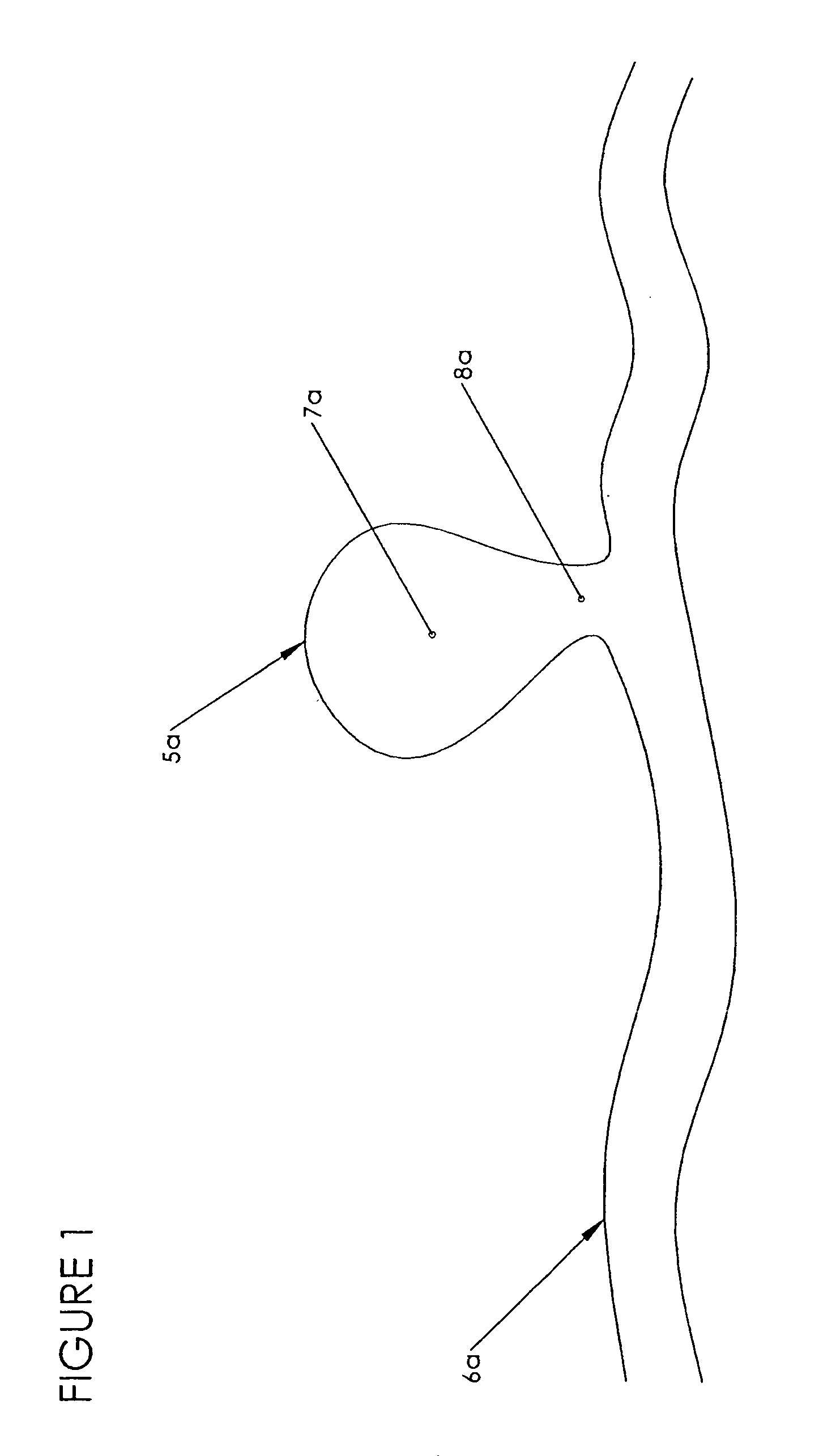
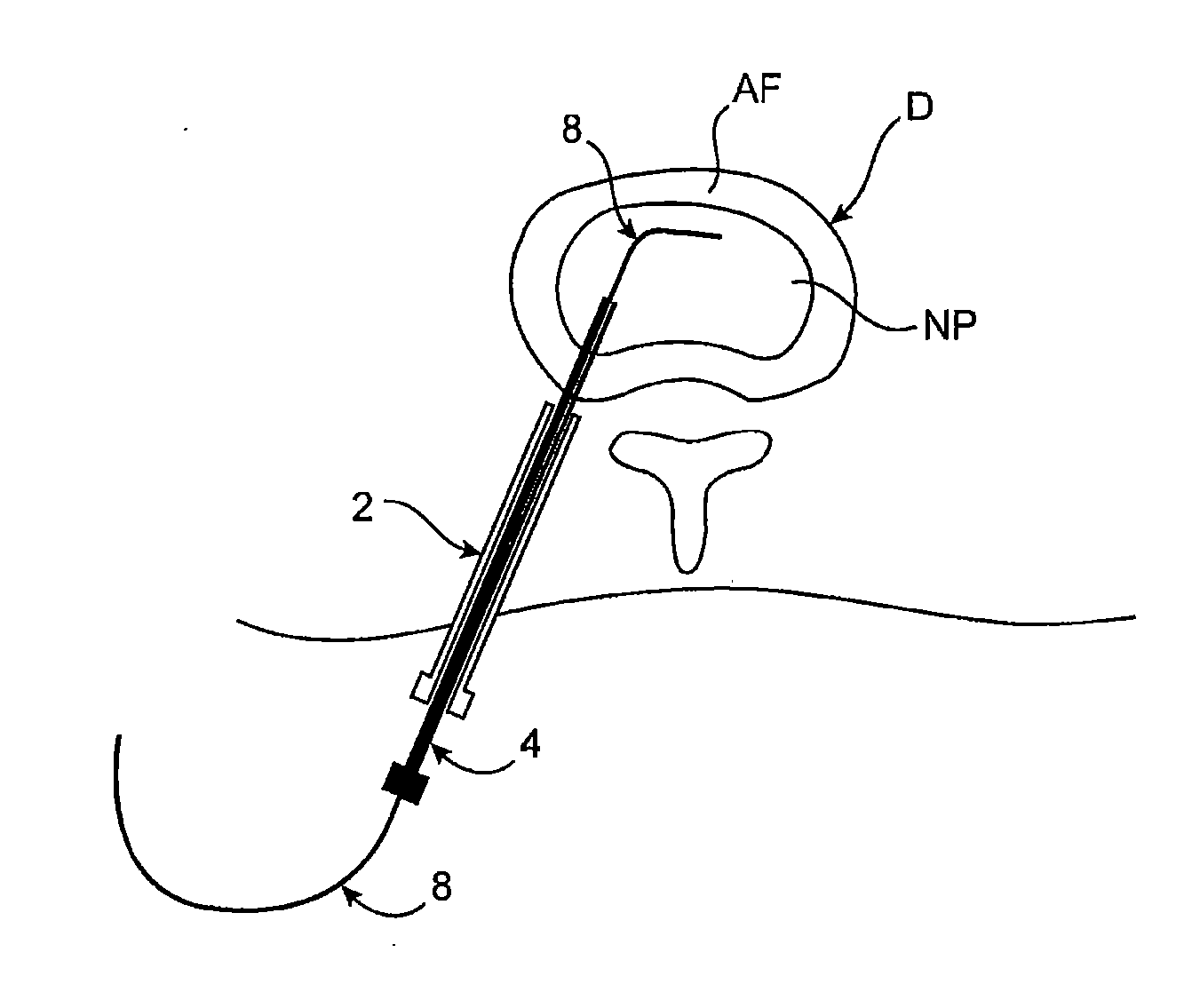
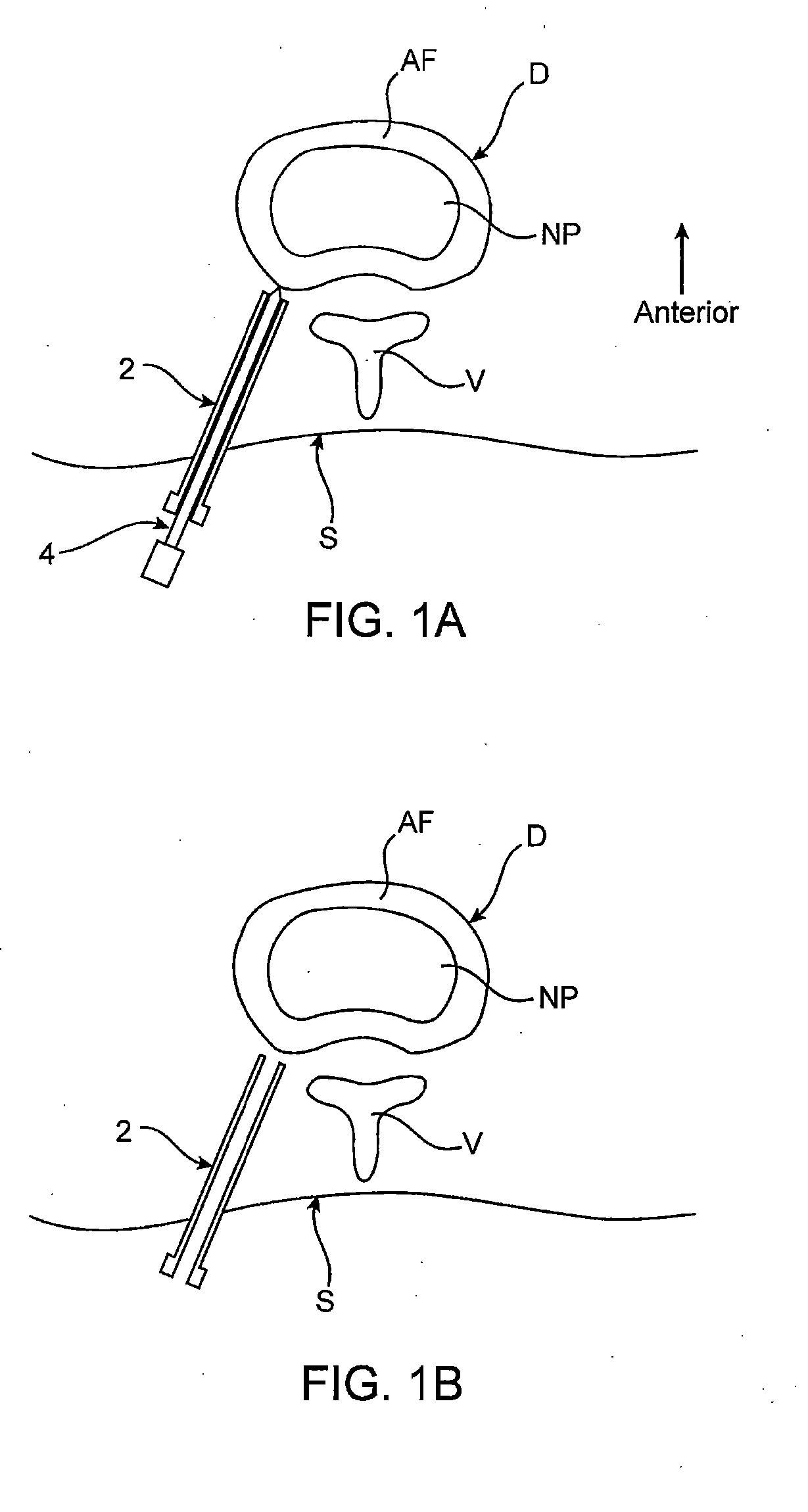
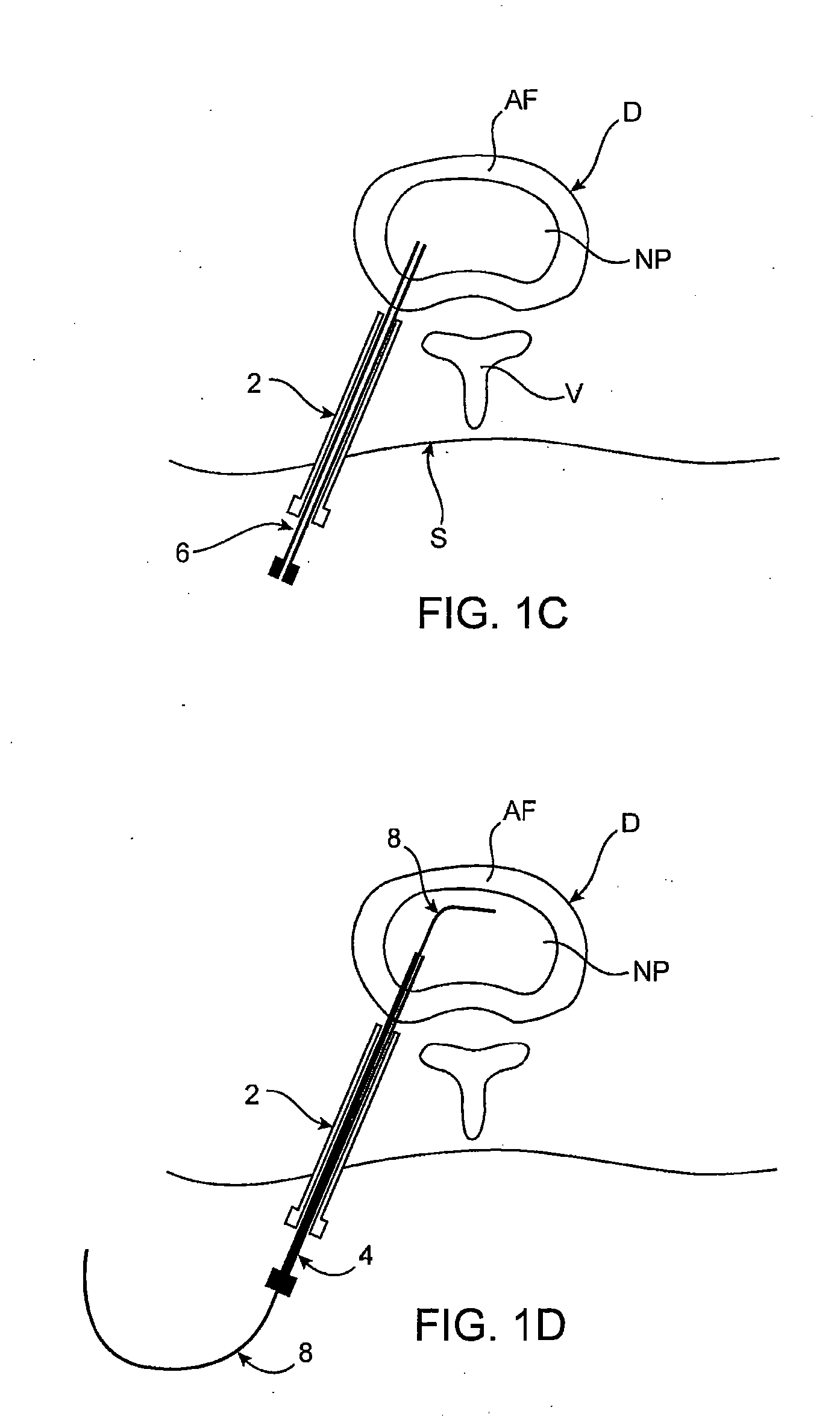
Intervertebral disc treatment devices and methods
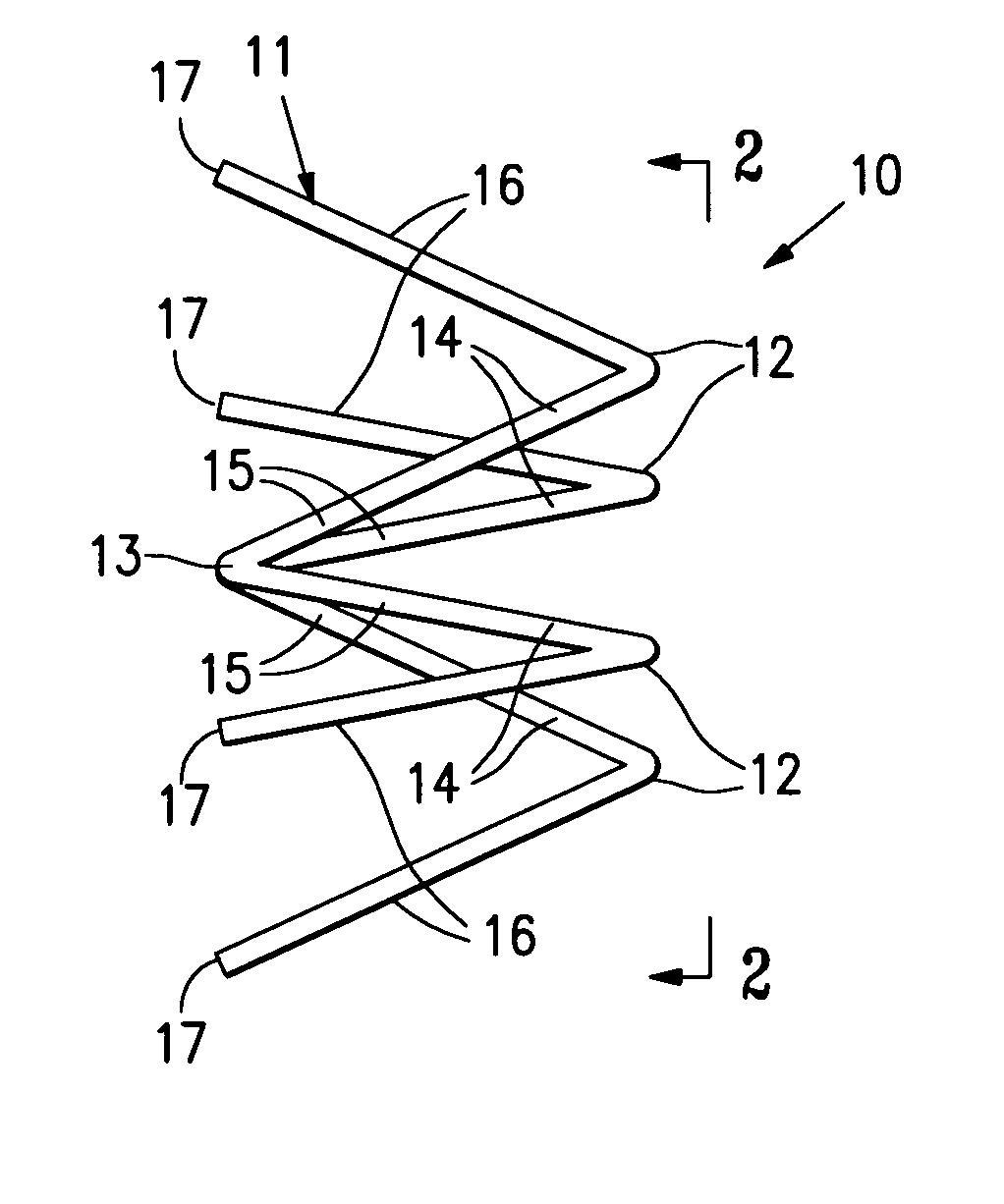
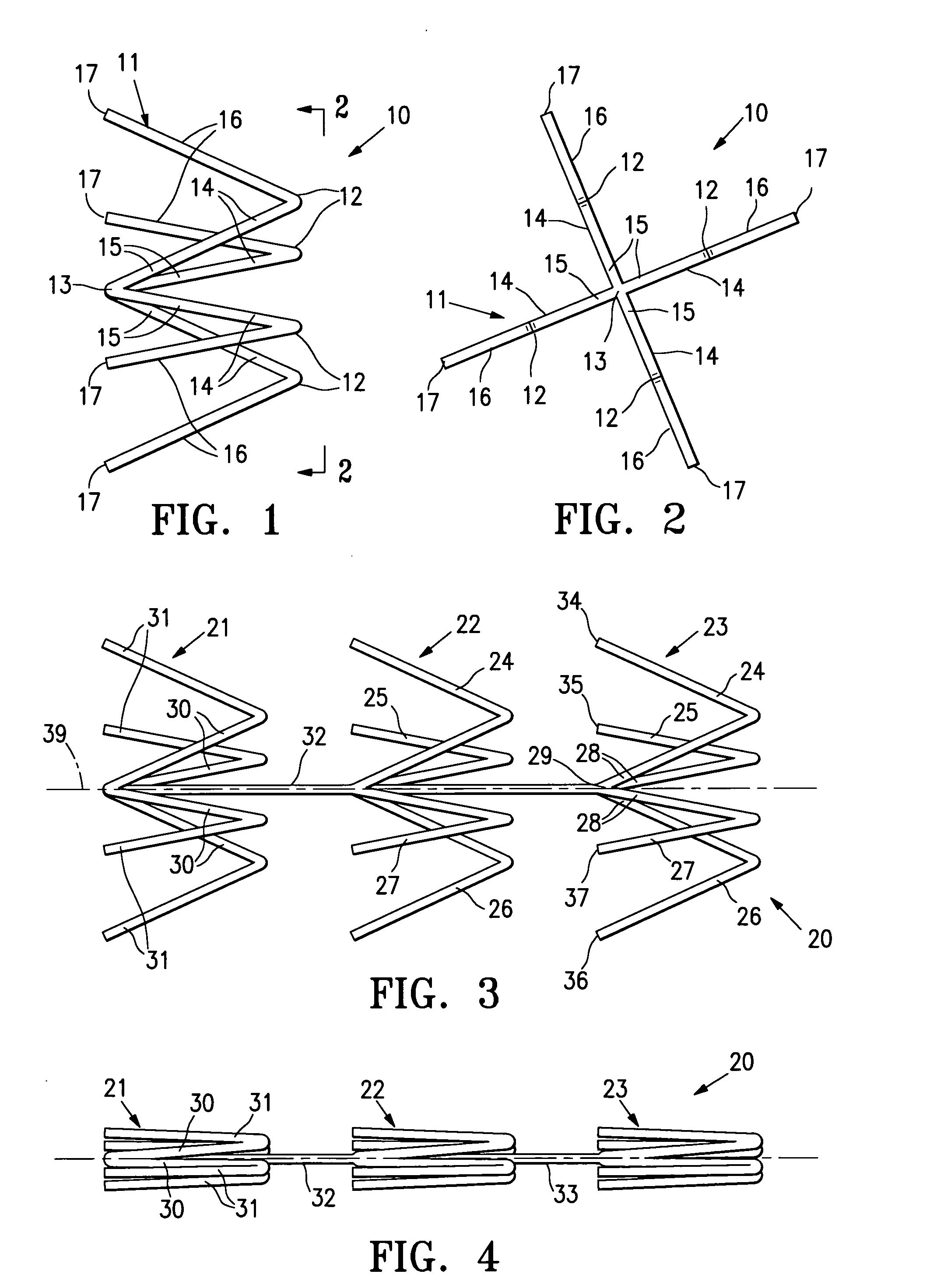
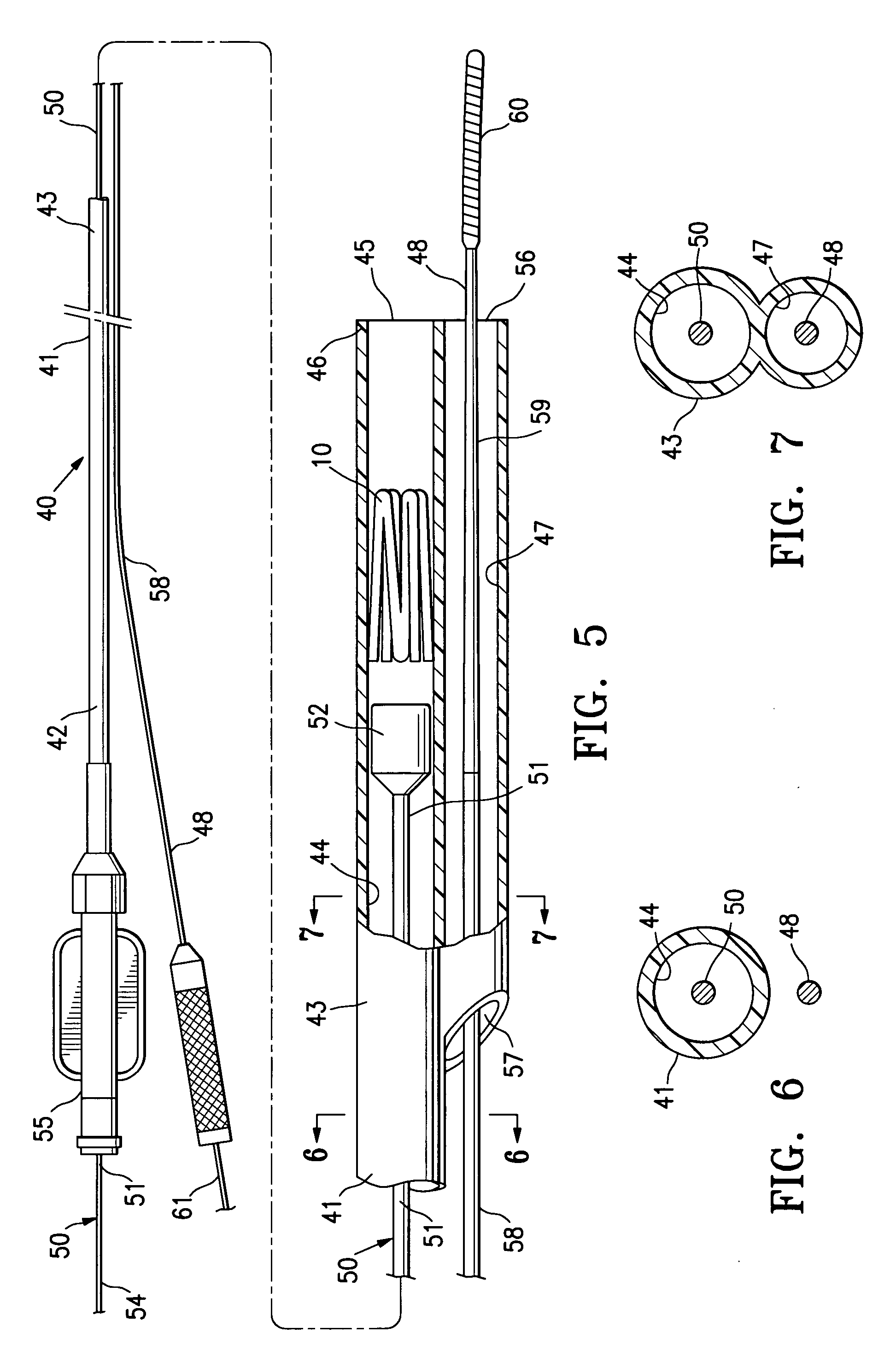
InactiveUS7318840B2Promote tissue growthFunction increasePeptide/protein ingredientsBone implantFibrous bodyIntervertebral disk
Intervertebral disc treatment devices and methods are provided. An intervertebral disc treatment device includes a fibrous body sized for introduction into a disc cavity of a damaged disc wherein the body incorporates an effective amount of a tissue growth factor. Intervertebral disc treatment apparatuses are also described that include such a disc treatment device in combination with a delivery apparatus for retaining and selectively releasing the device into the disc cavity. Methods for treatment include providing a disc treatment device as described above and inserting the device into an opening in an annulus fibrous and into the disc cavity. The methods further include stimulating tissue growth within the disc cavity of the intervertebral disc.
Owner:SDGI HLDG
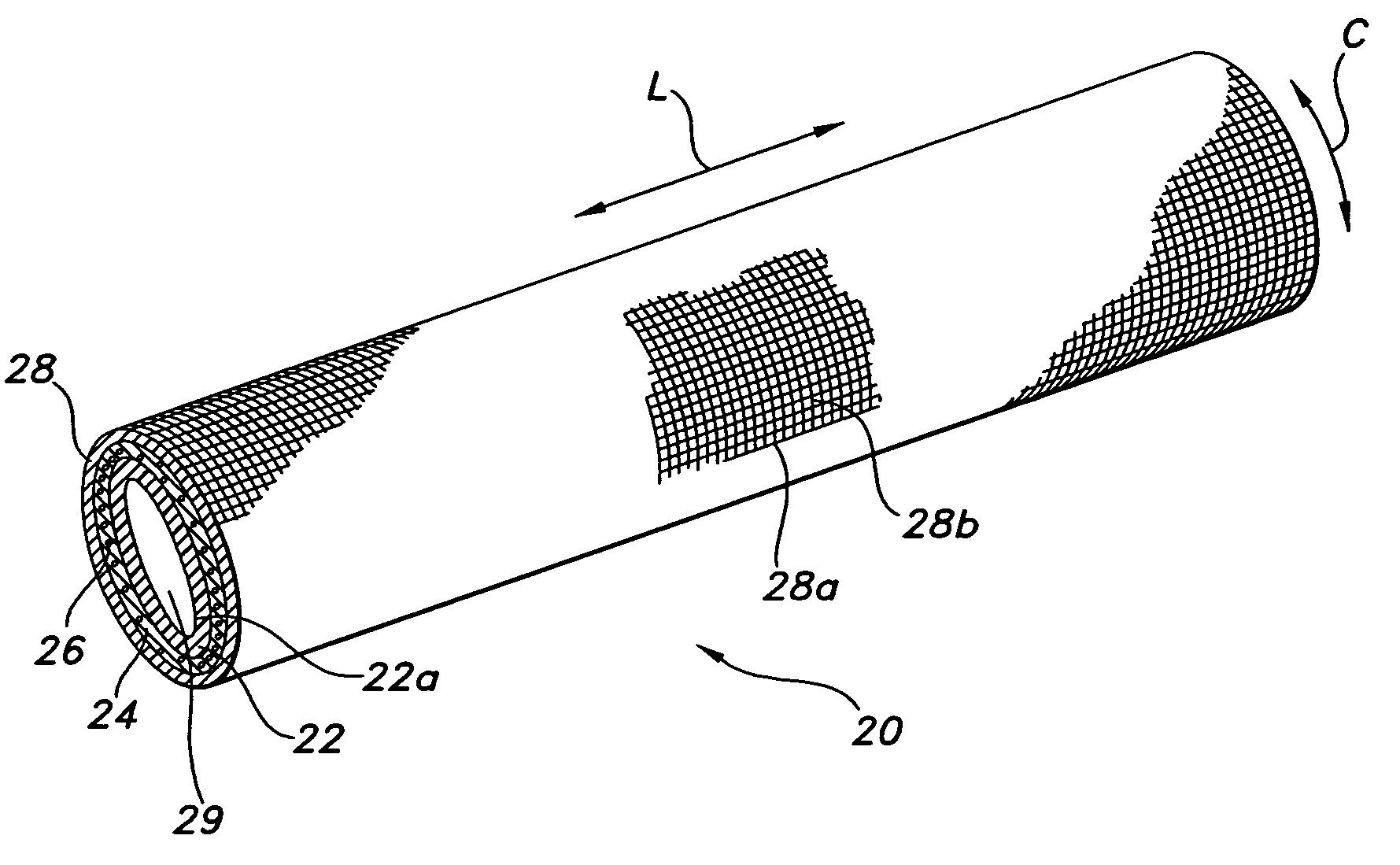
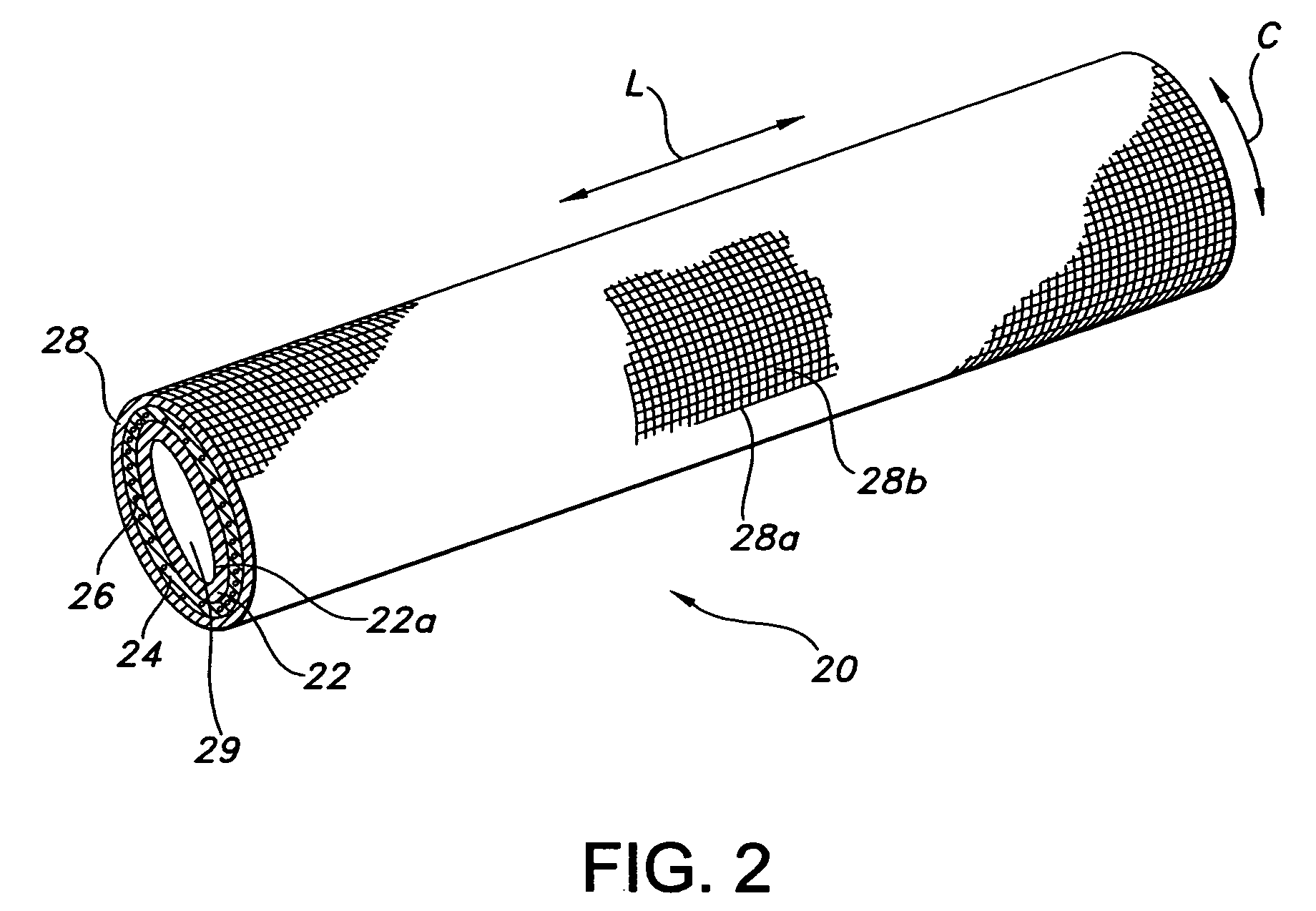
Annulotomy closure device
A system for sealing a hole in a body, comprising a generally cylindrical mesh formed from a plurality of helical strands which is inserted into the hole, with at least one end of the cylindrical mesh being moved least partially through an interior portion of the cylindrical shaped mesh such that the mesh expands radially outwards against sides of the hole.
Owner:NUVASIVE
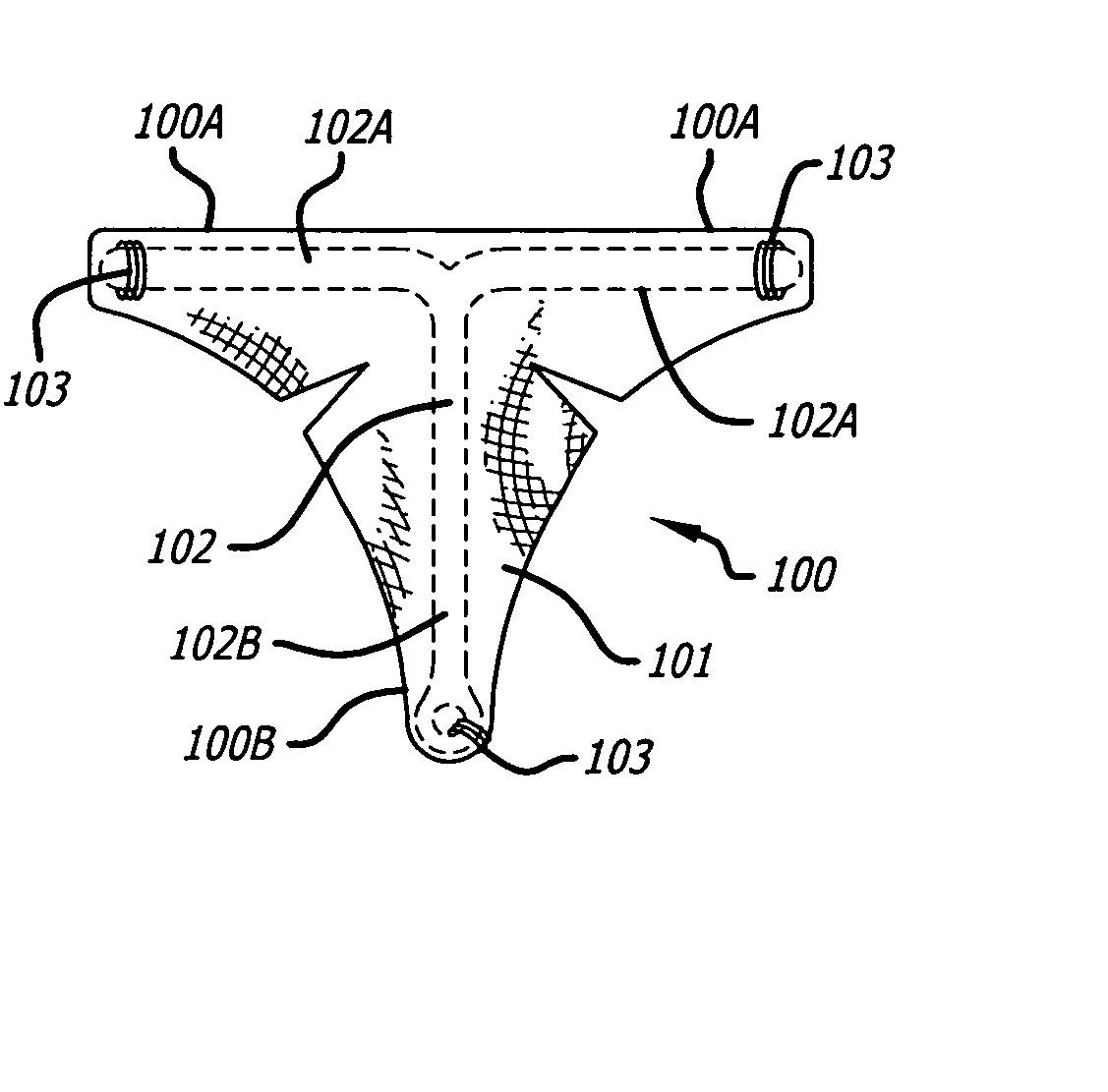
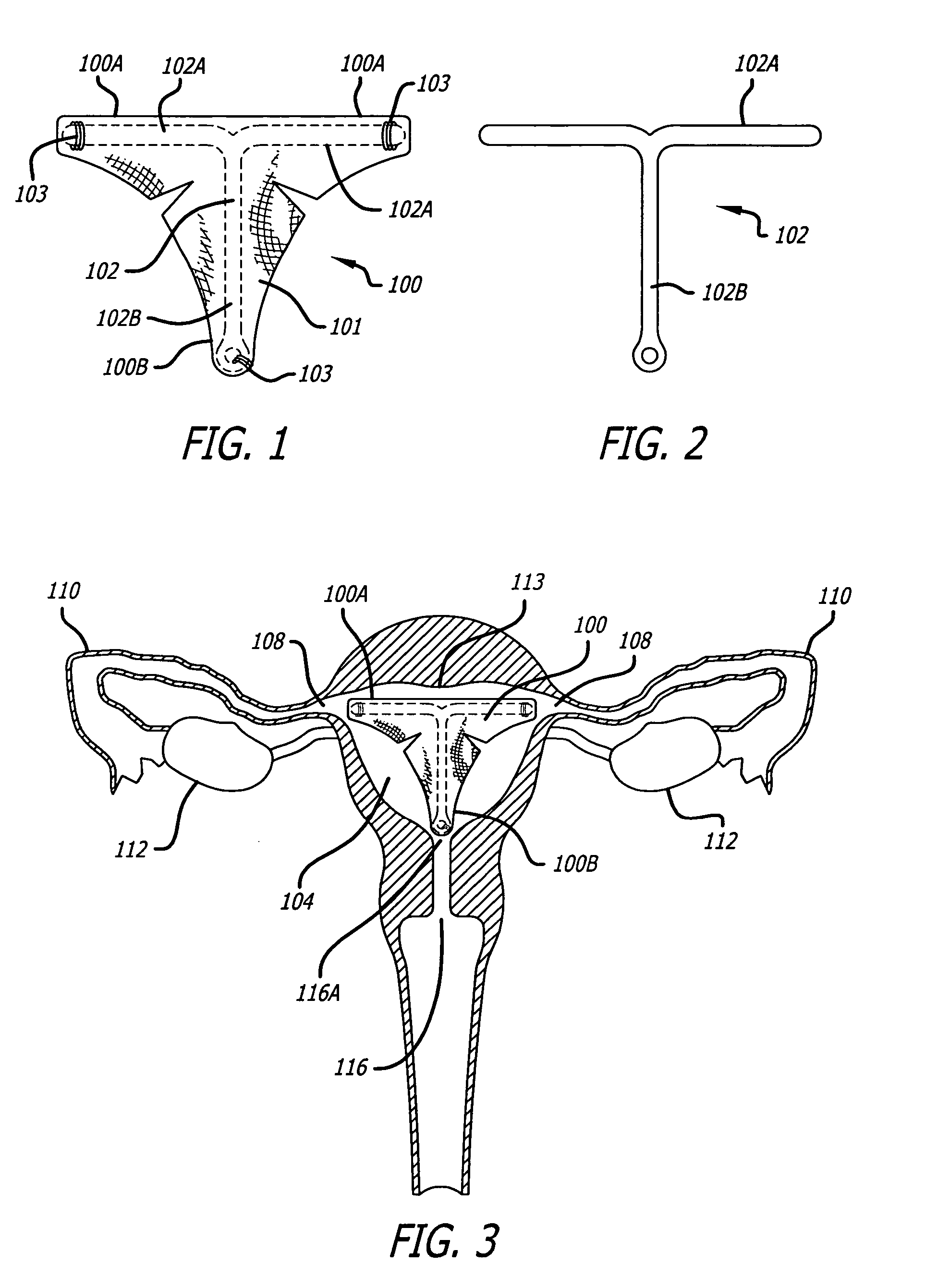
Method and apparatus for creating intrauterine adhesions
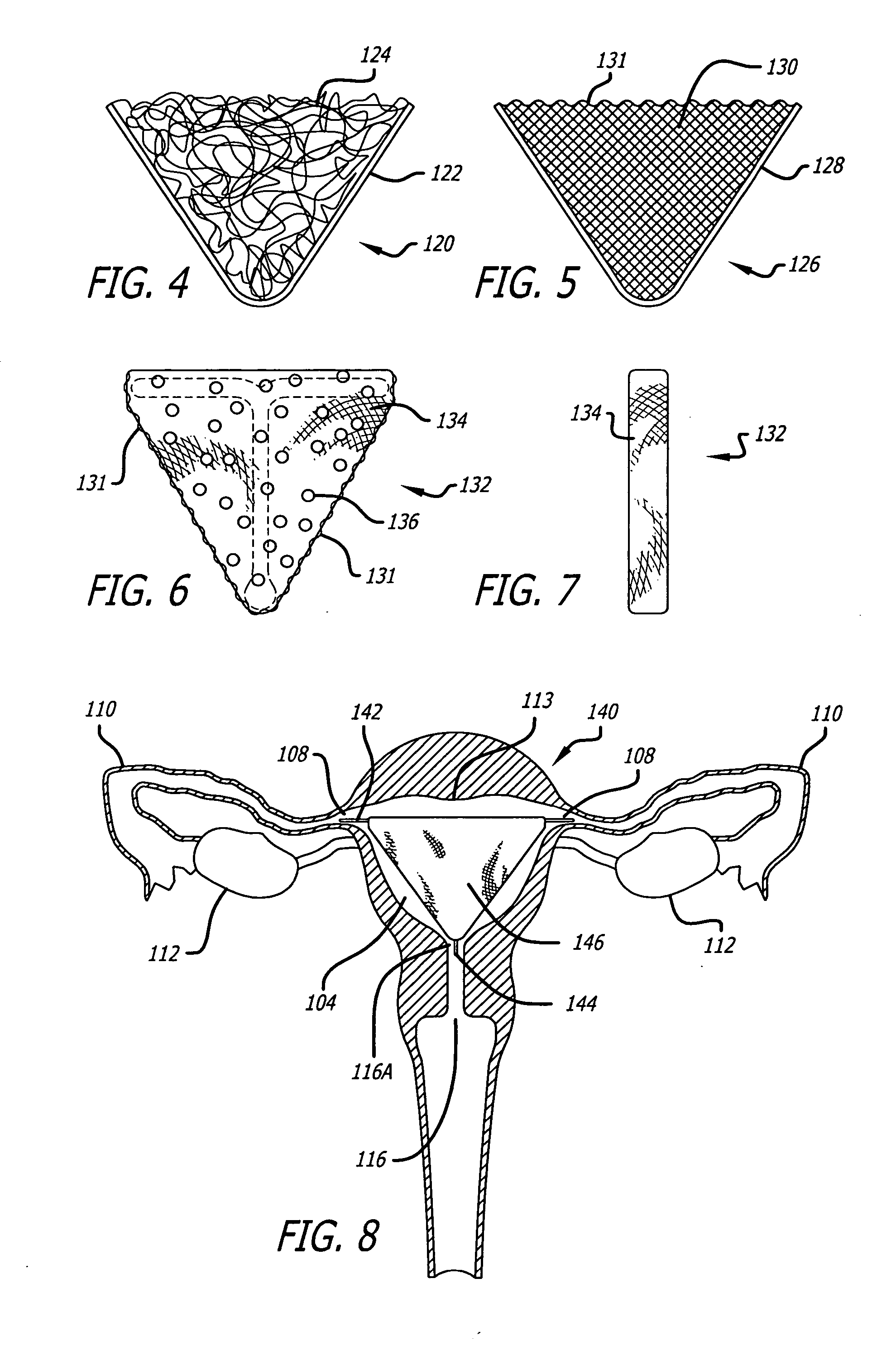
InactiveUS20050171569A1Better treat excessive bleedingPromote tissue growthUltrasonic/sonic/infrasonic diagnosticsSuture equipmentsExcessive BleedingGynecology
In general, the present invention contemplates an implantable device for treating excessive bleeding in a body cavity. The device comprises a biocompatible material, for example polyethylene teraphathalate (PET), which is deliverable into the body cavity. The biocompatible material contains an attribute(s) that promotes tissue reaction or growth that results in a tissue response and / or adhesion formation within the body cavity to reduce or stop the excessive bleeding.
Owner:AUB HLDG
Apparatus and method for wound treatment employing periodic sub-atmospheric pressure
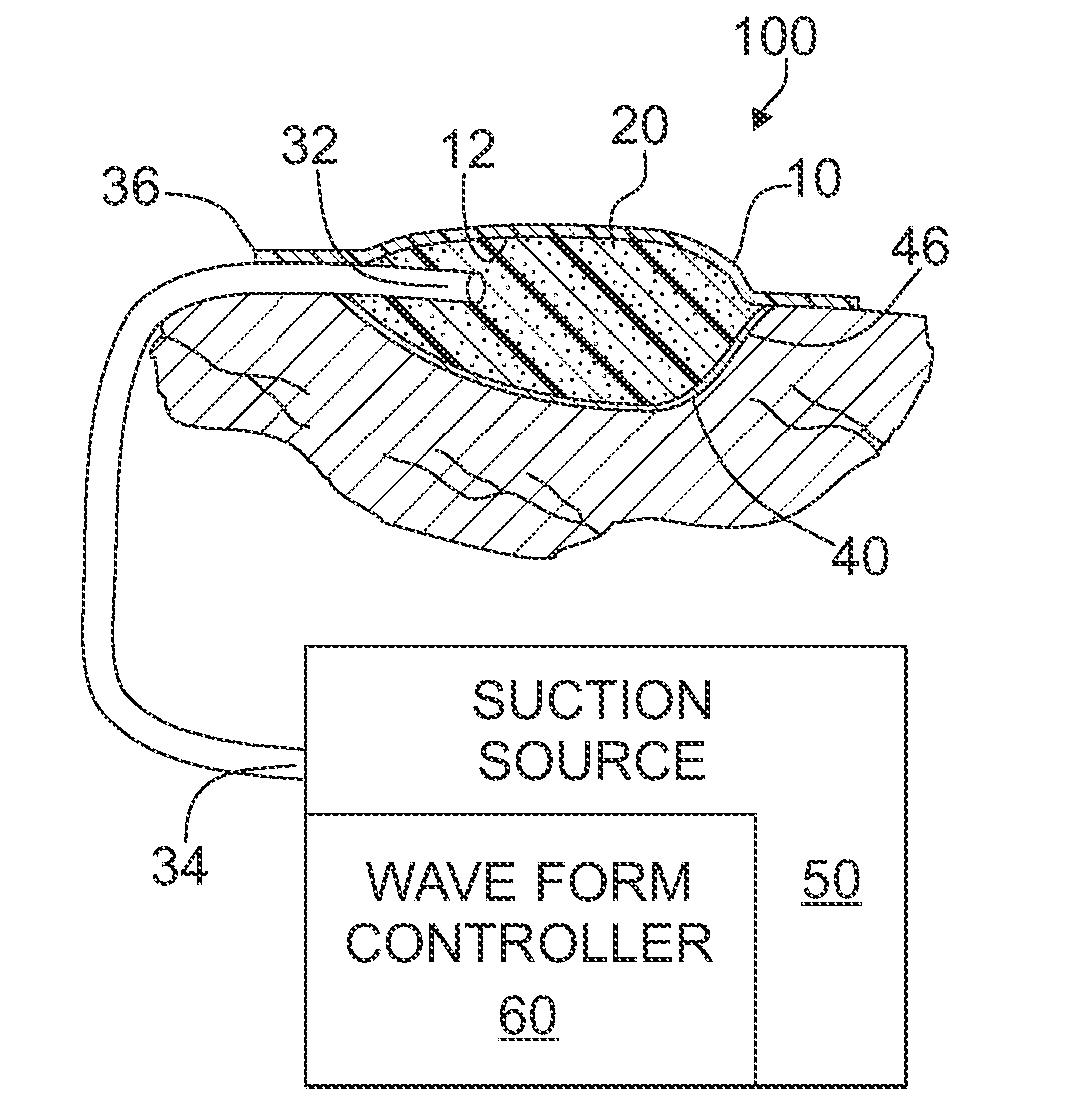
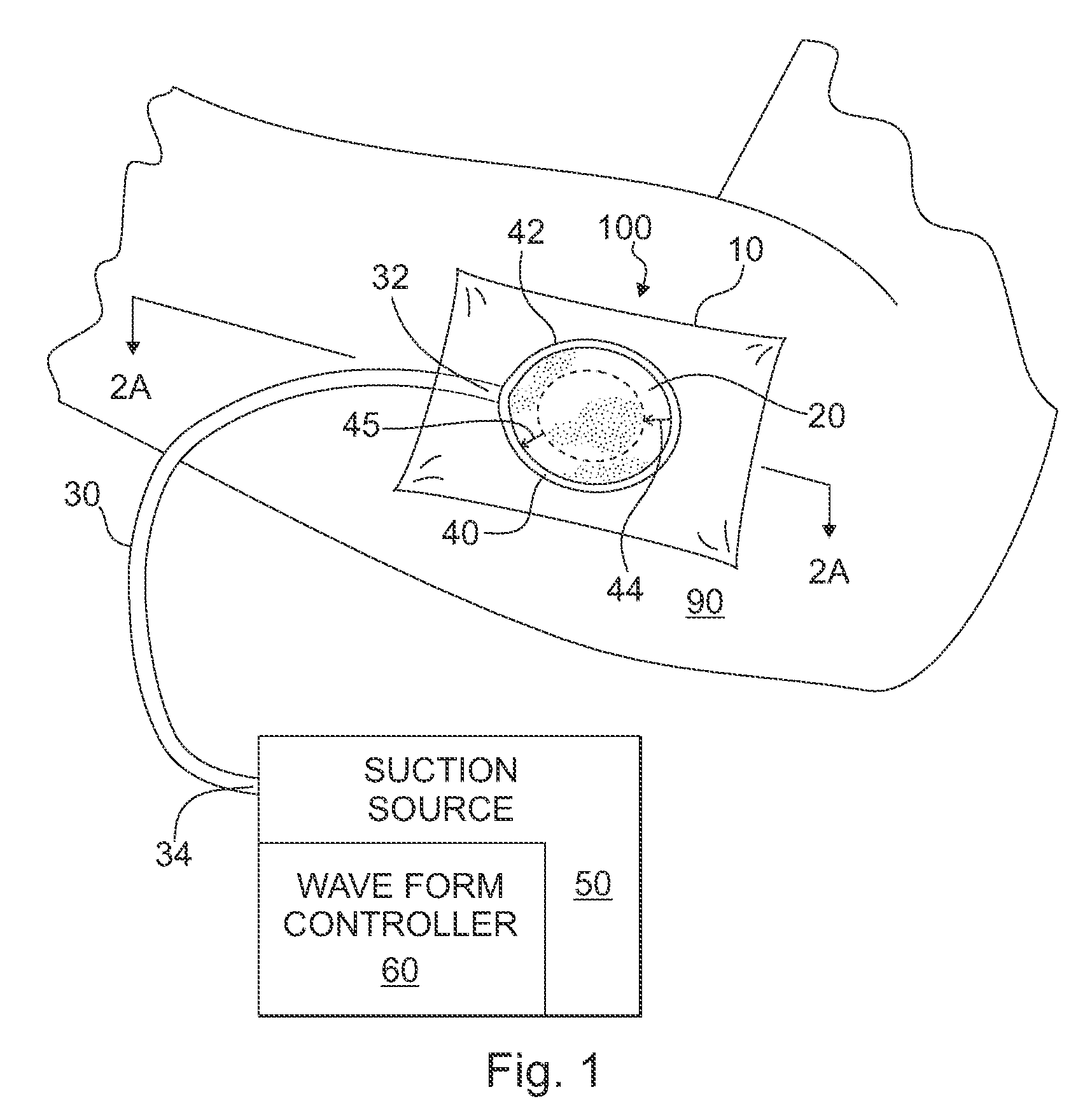
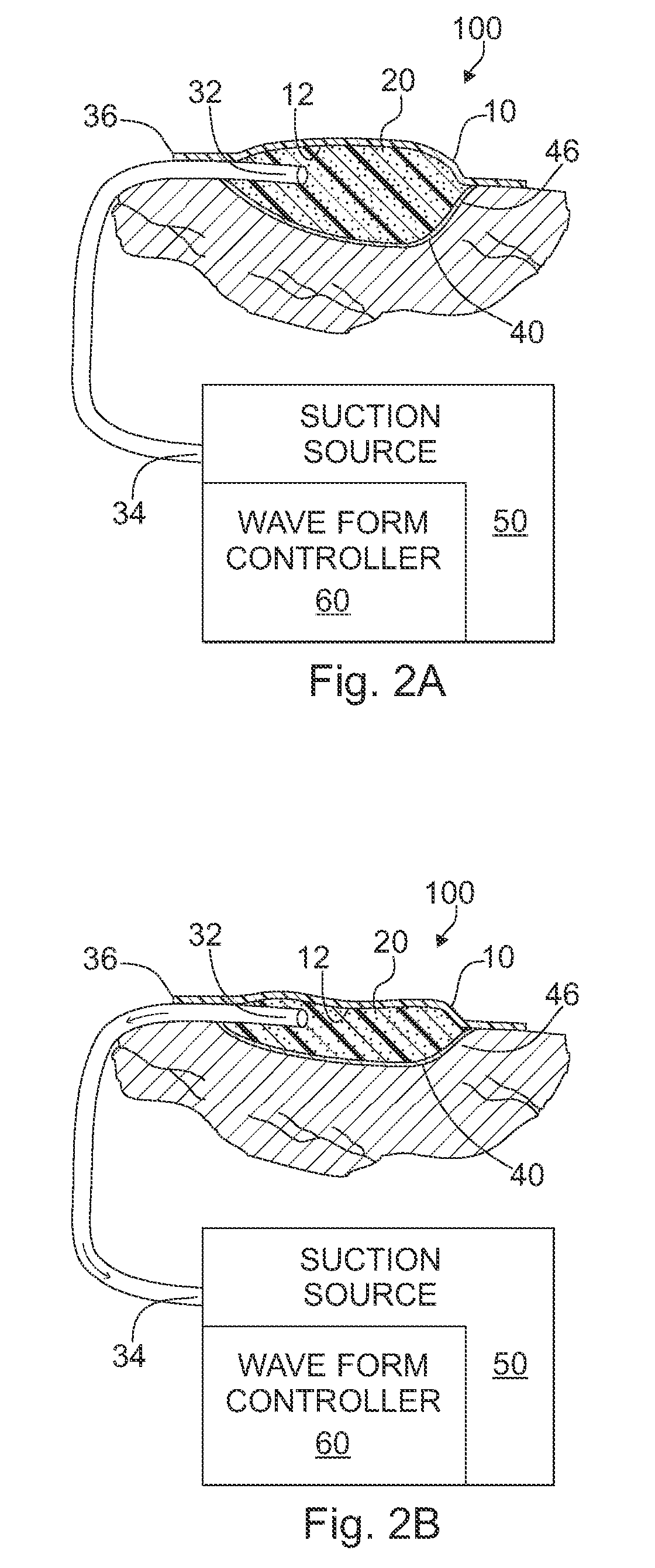
ActiveUS20080208147A1Promote tissue growthWound drainsAdhesive dressingsAtmospheric pressurePneumatic pressure
A tissue treatment apparatus and method are provided for treating tissue by the application of the time-varying sub-atmospheric pressure. The apparatus includes a cover adapted to cover a wound and adapted to maintain sub-atmospheric pressure the site of the wound. The apparatus further includes a source of suction configured to generate a time-varying sub-atmospheric pressure having a periodic waveform comprising a gradual change in pressure. The suction source cooperates with the cover to supply the time-varying sub-atmospheric pressure under the cover to the wound. The time-varying sub-atmospheric pressure may vary between a first pressure value below the inherent tissue tension of the wound tissue and a second pressure value above the inherent tissue tension of the wound tissue.
Owner:WAKE FOREST UNIV HEALTH SCI INC
Implantable prosthesis
InactiveUS7404819B1Limit incidencePromote tissue growthLigamentsMusclesPostoperative adhesionImplantable prosthesis
An implantable prosthesis and a method of repairing an anatomical defect, such as a tissue or muscle wall defect, by promoting tissue growth thereto, while limiting the incidence of postoperative adhesions between a portion of the prosthesis and tissue or organs. The prosthesis is formed of a biologically compatible, flexible layer of repair fabric suitable for reinforcing tissue or muscle and closing anatomical defects, and a barrier layer for physically isolating at least a portion of one side of the fabric from areas likely to form adhesions. A peripheral barrier extends about at least a portion of the outer peripheral edge of the repair fabric to inhibit adhesions between the outer peripheral edge and adjacent tissue and organs. The repair fabric may include an outer margin that has been melted and resolidified to render the outer peripheral edge substantially impervious to tissue ingrowth. The barrier layer may be joined to the repair fabric with connecting stitches formed from PTFE to inhibit the formation of adhesions thereto.
Owner:CR BARD INC
Methods and devices for intervertebral augmentation
InactiveUS20070150064A1Promote tissue growthHigh modulusBone implantLigamentsFilling materialsSmall intestine
Devices and methods for treating diseased or damaged portions of an intervertebral region are provided. In particular, intervertebral implants that can include use of a tissue regeneration structure having small intestine submucosa are described. The intervertebral implants can be utilized with any combination of load bearing structures for supporting loading on the implant, shaping structures for biasing the configuration of the implant, collapsible support structures for shaping the implant, and other features. Implants can also be formed with an enclosure to contain a filling material, such as an injectable small intestine submucosa formulation. Methods of delivering and utilizing the various implants are also discussed.
Owner:DEPUY SPINE INC (US) +1
Systems and methods for supporting or occluding a physiological opening or cavity
ActiveUS20100094335A1Promote re-endothelializationPromote tissue growthStentsDilatorsImplanted deviceEngineering
Implantable devices for placement at a cavity or opening such as an aneurysm are disclosed. The implantable devices, in a deployed condition, have a generally inverted U-shaped profile with a curved or angled framework support structure sized and configured for placement in proximity to tissue surrounding the opening and anchoring legs extending proximally from the framework structure sized and configured to contact the wall of a neighboring lumen at opposed locations. Occlusive and semi-occlusive membranes may be associated with the framework support structure and deployed over the opening to provide exclusion of the opening and flow diversion. Proximal anchoring segments providing additional lumen wall surface area contact for the implantable device following deployment may be incorporated.
Owner:PULSAR VASCULAR
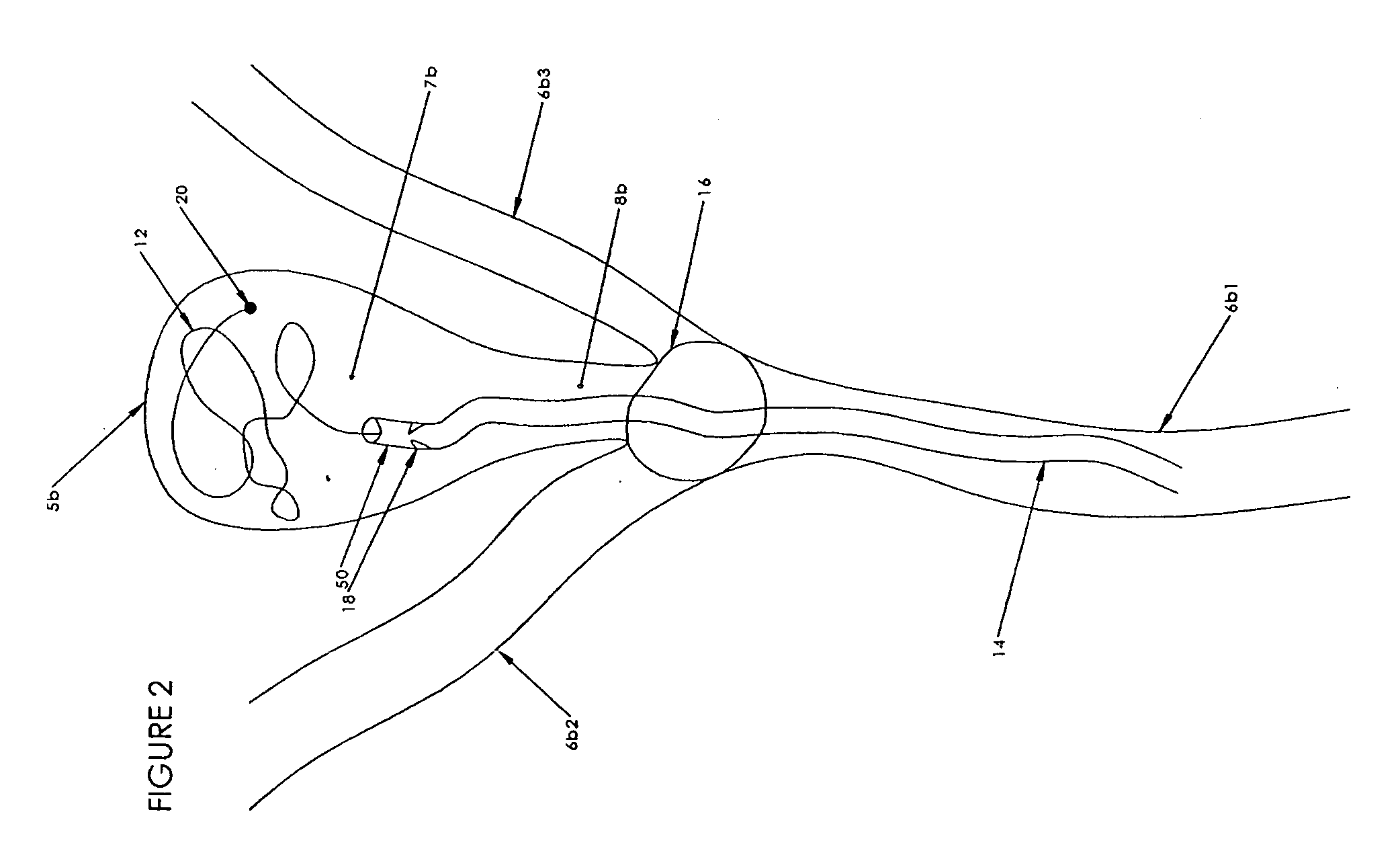
Intervertebral disc treatment devices and methods
InactiveUS20060004456A1Prevent outflowPromote tissue growthPeptide/protein ingredientsDiagnosticsFibrous bodyIntervertebral disk
Intervertebral disc treatment devices and methods are provided. An intervertebral disc treatment device includes a fibrous body sized for introduction into a disc cavity of a damaged disc wherein the body incorporates an effective amount of a tissue growth factor. Intervertebral disc treatment apparatuses are also described that include such a disc treatment device in combination with a delivery apparatus for retaining and selectively releasing the device into the disc cavity. Methods for treatment include providing a disc treatment device as described above and inserting the device into an opening in an annulus fibrous and into the disc cavity. The methods further include stimulating tissue growth within the disc cavity of the intervertebral disc.
Owner:SDGI HLDG
Implantable medical devices with antimicrobial and biodegradable matrices
InactiveUS20050283224A1Inhibition formationPromote tissue growthStentsBlood vesselsVenous graftActive agent
A composite vascular graft is provided, which incorporates bioactive agents that can be controllably delivered to the implantation site to deliver therapeutic materials and / or to reduce infection of the implant. The vascular graft of the present invention includes a luminal layer of ePTFE; and a biodegradable polymer layer including a bioactive agent, such as an antimicrobial agent. The biodegradable polymer layer is posited on the external surface of the luminal ePTFE layer. The graft also includes a fabric layer, which is posited on the external surface of the biodegradable layer. The graft is particularly useful as an arterial-venous graft for hemodialysis procedures.
Owner:MAQUET CARDIOVASCULAR LLC +1
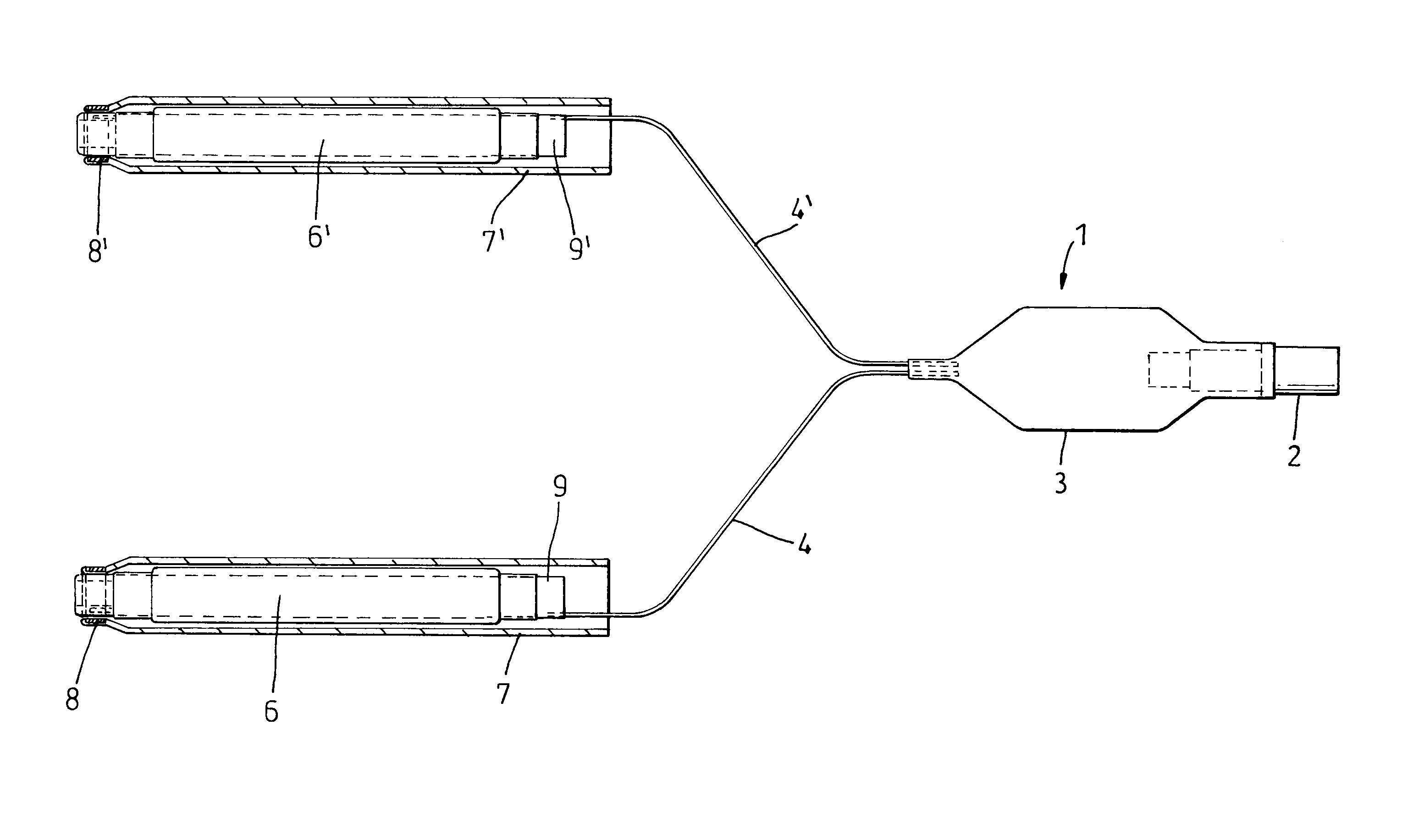
Nasal packing device
A kit for packing and supporting the nasal cavities after surgical procedures performed on the nose comprising two inflatable non-elastomeric balloons (6′, 6″) and inflation means (27) arranged so that, in use, each balloon (6′, 6″) can be located in a nasal cavity and inflated.
Owner:ARTHROCARE
Devices for intervertebral augmentation and methods of controlling their delivery
InactiveUS20070150063A1Promote tissue growthHigher compressive modulusBone implantLigamentsFilling materialsSmall intestine
Devices and methods for treating diseased or damaged portions of an intervertebral region are provided. In particular, intervertebral implants that can include use of a tissue regeneration structure having small intestine submucosa are described. The intervertebral implants can be utilized with any combination of load bearing structures for supporting loading on the implant, shaping structures for biasing the configuration of the implant, collapsible support structures for shaping the implant, and other features. Implants can also be formed with an enclosure to contain a filling material, such as an injectable small intestine submucosa formulation. Methods of delivering and utilizing the various implants are also discussed.
Owner:DEPUY SYNTHES PROD INC +1
Enhancing tissue ingrowth for contraception
InactiveUS20050209633A1Facilitate tissue ingrowthAvoid easy placementFallopian occludersFemale contraceptivesMetallic materialsElectric energy
This invention is directed to occluding devices and methods of using such devices for occluding a patient's body lumen, such as a reproductive lumen for contraceptive purposes. The occluding device generally has an occluding component, a first metallic element associated with the occluding component, a second metallic element associated with the occluding component. The first and second metallic elements are configured to generate electrical activity which enhances tissue growth into and / or onto the occluding component to aid in lumen occlusion. In one embodiment the first and second metallic elements are formed of different metallic materials and generate galvanic activity. In a second embodiment electrical power is applies to the first and second metallic elements to generate electrical activity that enhances tissue growth.
Owner:BAYER HEALTHCARE LLC
Agents for controlling biological fluids and methods of use thereof
InactiveUS20070059350A1Good hemostasisHigh viscosityOrganic active ingredientsBiocideMedicineIncision Site
Therapeutic formulations adapted for positive-pressure application for controlling biological fluid at a desired site in a subject, absorbent articles comprising therapeutic formulations, and anti-infective devices coated with therapeutic formulations, said formulations comprising about 25% to about 99% by weight liquid-crystal forming compound and 0% to about 75% by weight solvent. In addition, methods of using said formulations including methods for controlling biological fluid at a desired site in a subject, methods for controlling blood loss, and methods for facilitating effective closure of a vascular wound or incision site at a desired site in a subject are disclosed, the methods comprising administering particular formulations comprising liquid-crystal forming compounds and solvents that are described herein.
Owner:SOUTHEASTERN MEDICAL TECH
Biodegradable scaffolds and uses thereof
InactiveUS20060002978A1Strengthen cellsEnhanced tissue growthPowder deliveryPeptide/protein ingredientsBiodegradable scaffoldPolymer chemistry
The invention is directed to scaffolds containing porous polymer material prepared by a process of gas foaming / particulate leaching and a wet granulation step prior to gas foaming and particulate leaching, particularly having a characteristic interconnected pore structure, as well as sustained release of protein, DNA or cells, and to methods for using such porous polymer material for preparation of scaffolds, particularly for tissue engineering.
Owner:NORTHWESTERN UNIV
Implantable Tendon Protection Systems and Related Kits and Methods
InactiveUS20100191332A1Promote tissue growthEasy to packLigamentsMusclesEnthesisBiomedical engineering
An implantable tendon protection system includes a body adapted to be implanted within a bursa overlying a tendon of a patient to protect the tendon. The body may be fixed to the tendon with adhesive, sutures, staples, and / or anchors. A surgical kit is provided with such a tendon protection system and an insertion cannula. Methods of protecting a tendon of a patient are also disclosed.
Owner:ROTATION MEDICAL
Wound irrigation containment arrangement
InactiveUS20060069357A1Safely and effectively treatAvoid problemsInfusion syringesMilking pumpMedicineWound irrigation
A containment arrangement for safely and effectively treating a wound on a patient without contaminating attending personnel. The arrangement comprises a patient receiving first enclosure having a patient contacting periphery, a pressurizable source in communication with the patient through a wall of the enclosure for enlarging the enclosure, and a sealing means arranged in the patient contacting periphery. A hand manipulable fluid discharge nozzle is extendably arranged through the enclosure for providing controllable treatment fluid to the wound on the patient within the enclosure.
Owner:PULSECARE MEDICAL
Flattened tubular mesh sling and related methods
Owner:BOSTON SCI SCIMED INC
Multi-Functional Wound Dressing Matrices and Related Methods
InactiveUS20100047324A1Promote tissue growthPromote optimal healingAdhesive dressingsAbsorbent padsParticulatesPolyphosphazene
Various embodiments are directed to multi-functional wound-care dressing matrices that can protect and promote new tissue growth at a wound site. The multi-functional wound care matrix can incorporate polyphosphazenes of formula I, as a component that can be configured into various forms, including as fibrous mats, porous membranes, nonporous films, particulate formulations, and equivalents. The multi-functional wound-care dressing matrix of the present disclosure exhibit high-performance properties conferred by polyphosphazenes of formula I. Exceptional biocompatible properties of polyphosphazenes of formula I provide an ideal tissue-contacting surface for the multi-functional wound-care dressing matrix of interest.
Owner:CELONOVA BIOSCIENCES INC
Implantable tendon protection systems and related kits and methods
ActiveUS20130158661A1Promote tissue growthEasy to packLigamentsMusclesSurgical procedure kitAdhesive
An implantable tendon protection system includes a body adapted to be implanted within a bursa overlying a tendon of a patient to protect the tendon. The body may be fixed to the tendon with adhesive, sutures, staples, and / or anchors. A surgical kit is provided with such a tendon protection system and an insertion cannula. Methods of protecting a tendon of a patient are also disclosed.
Owner:ROTATION MEDICAL
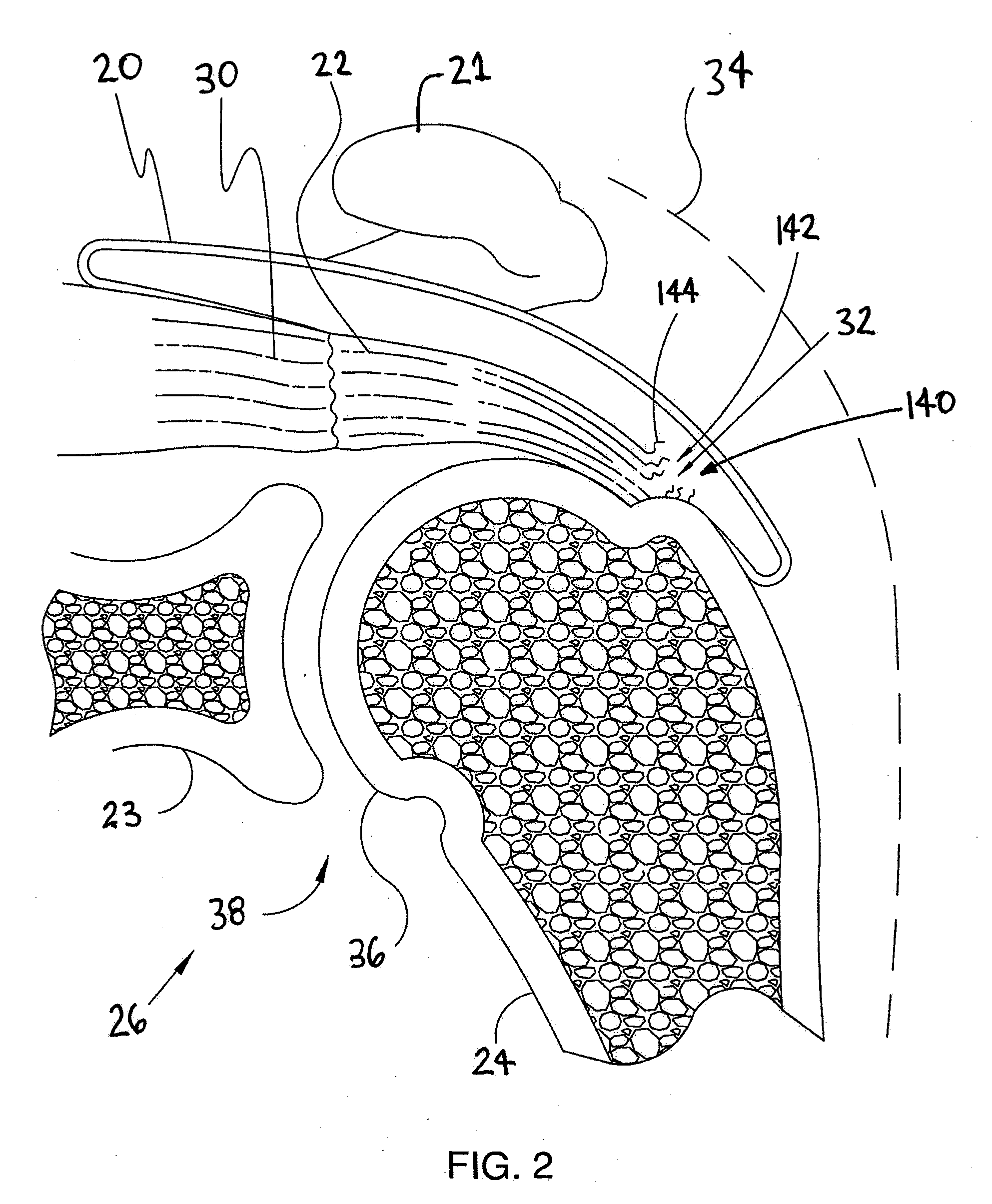
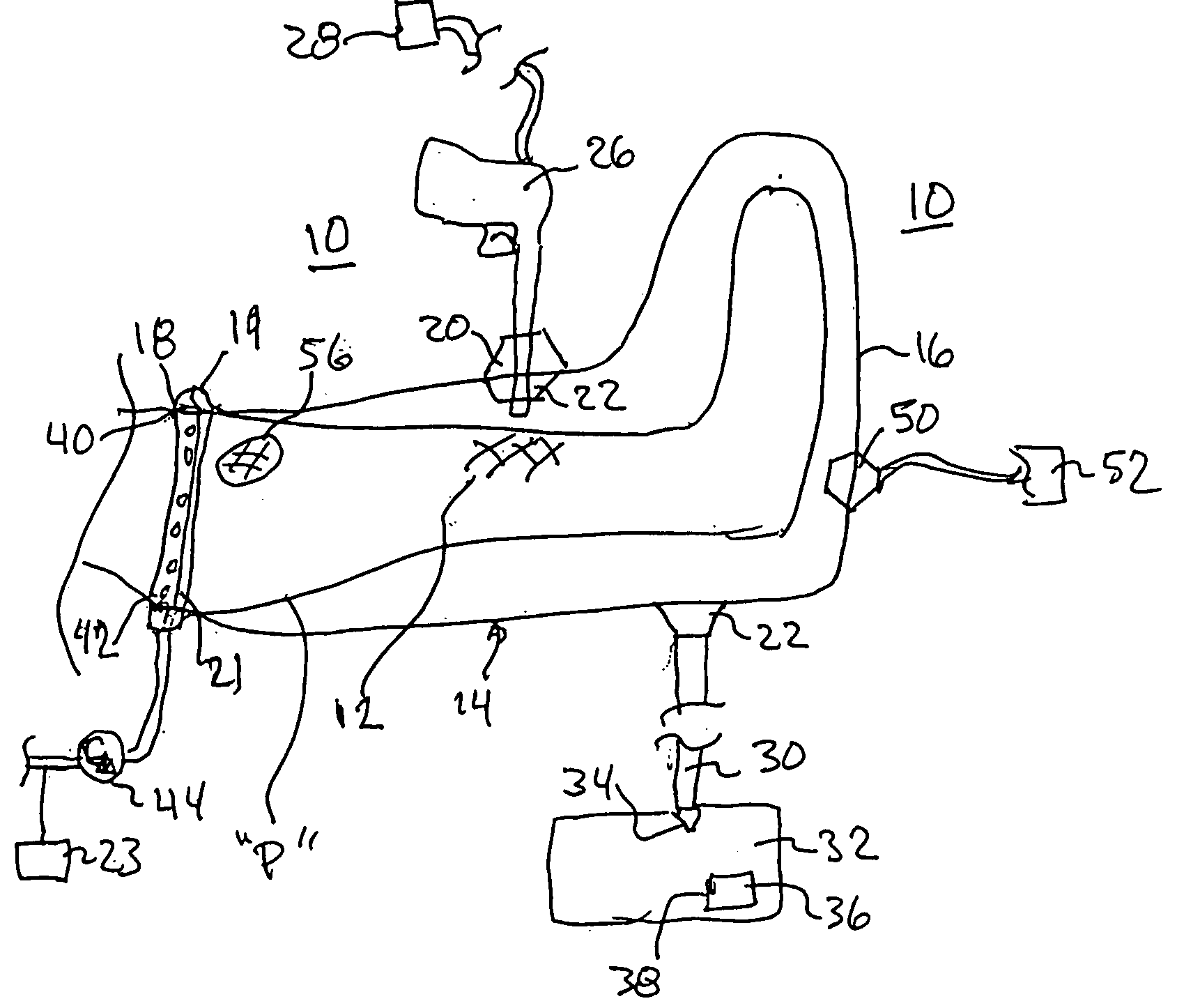
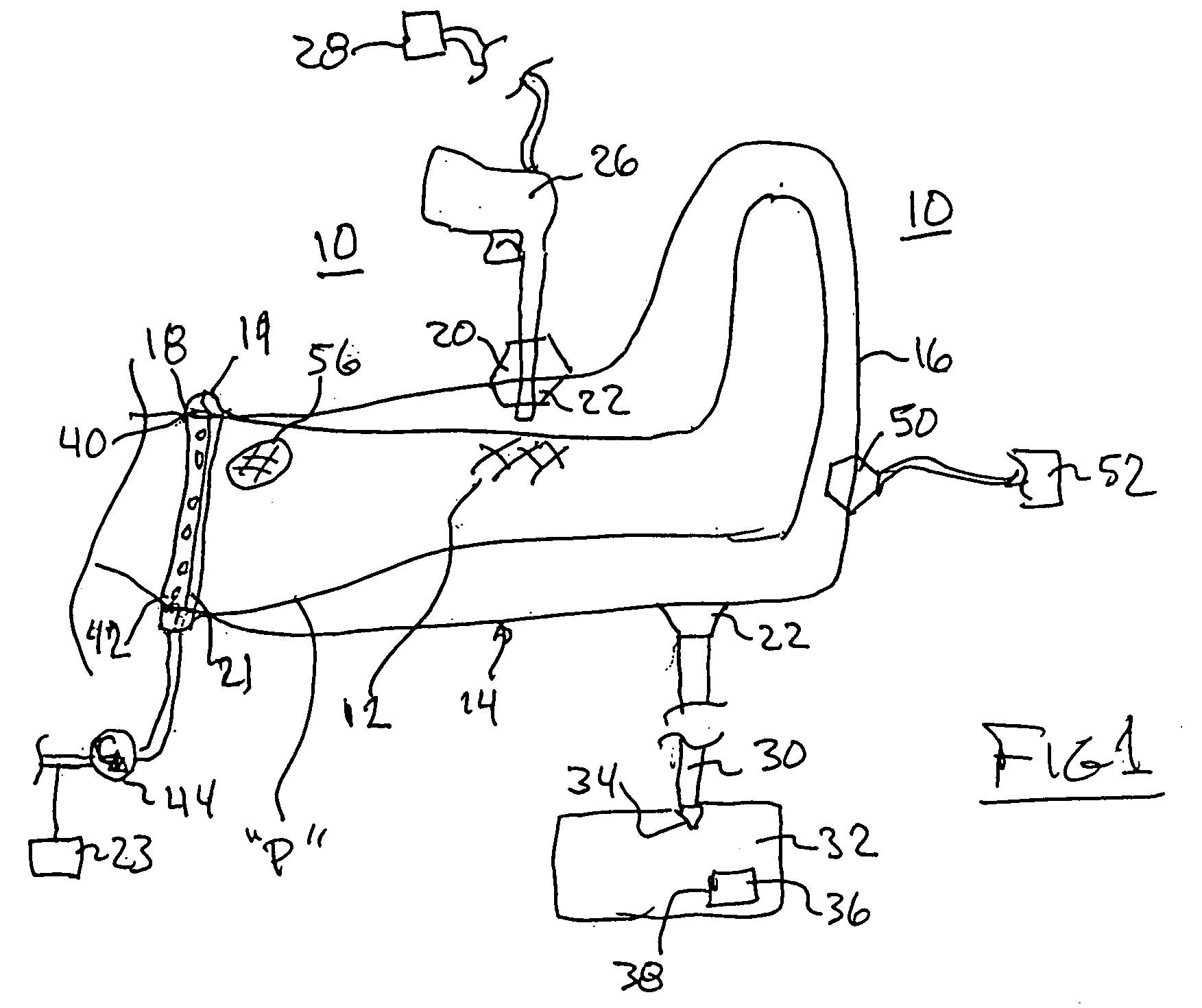
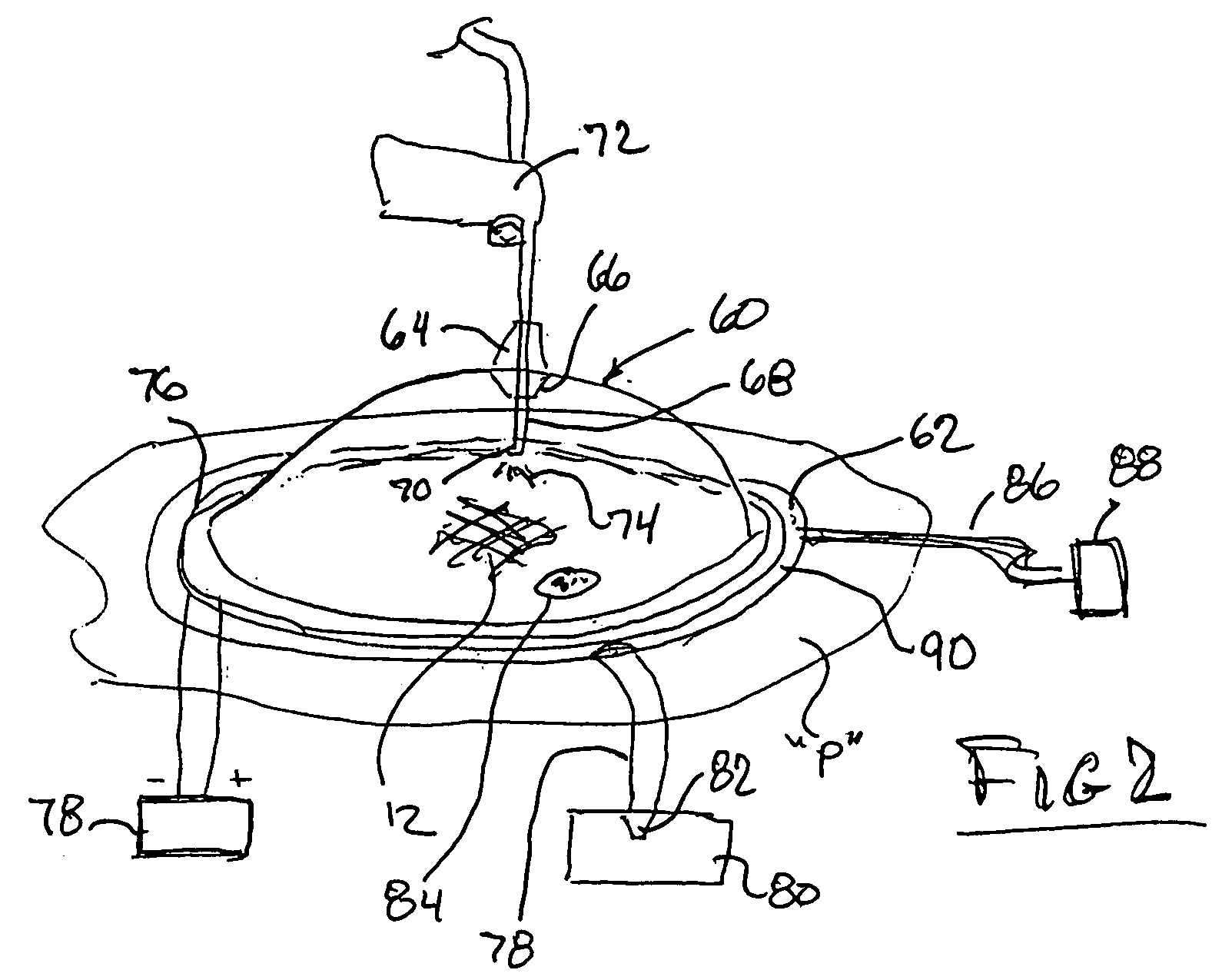
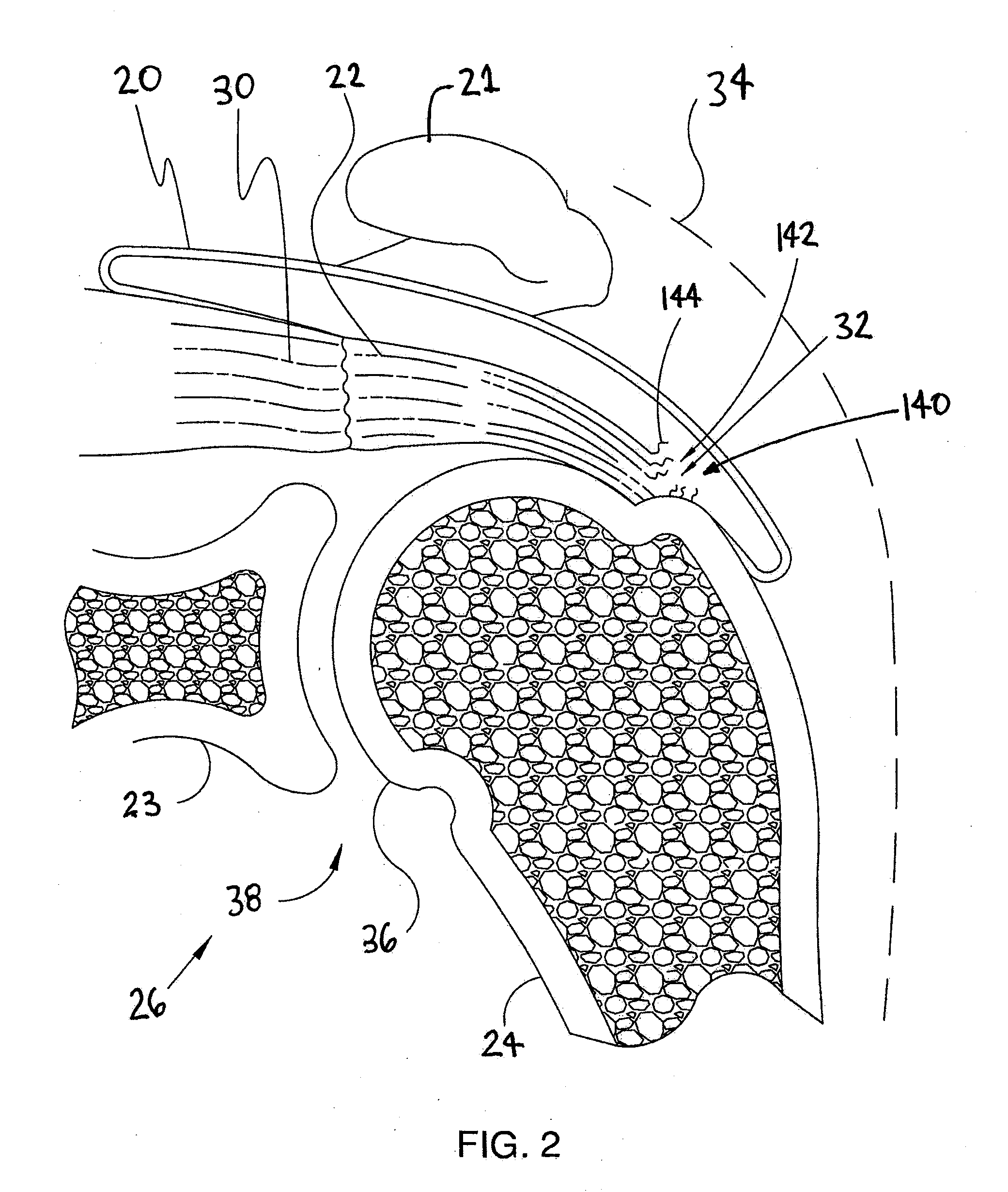
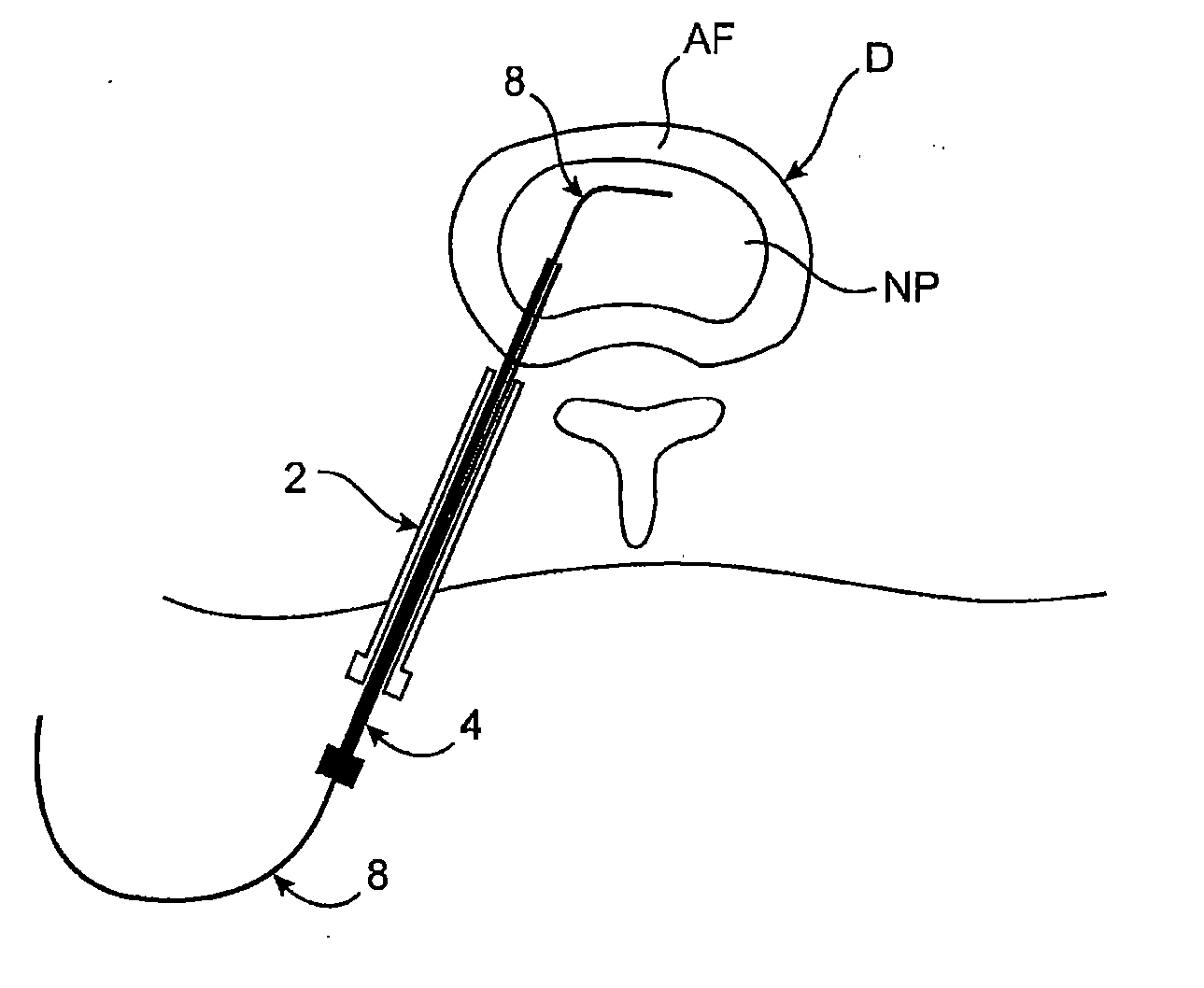
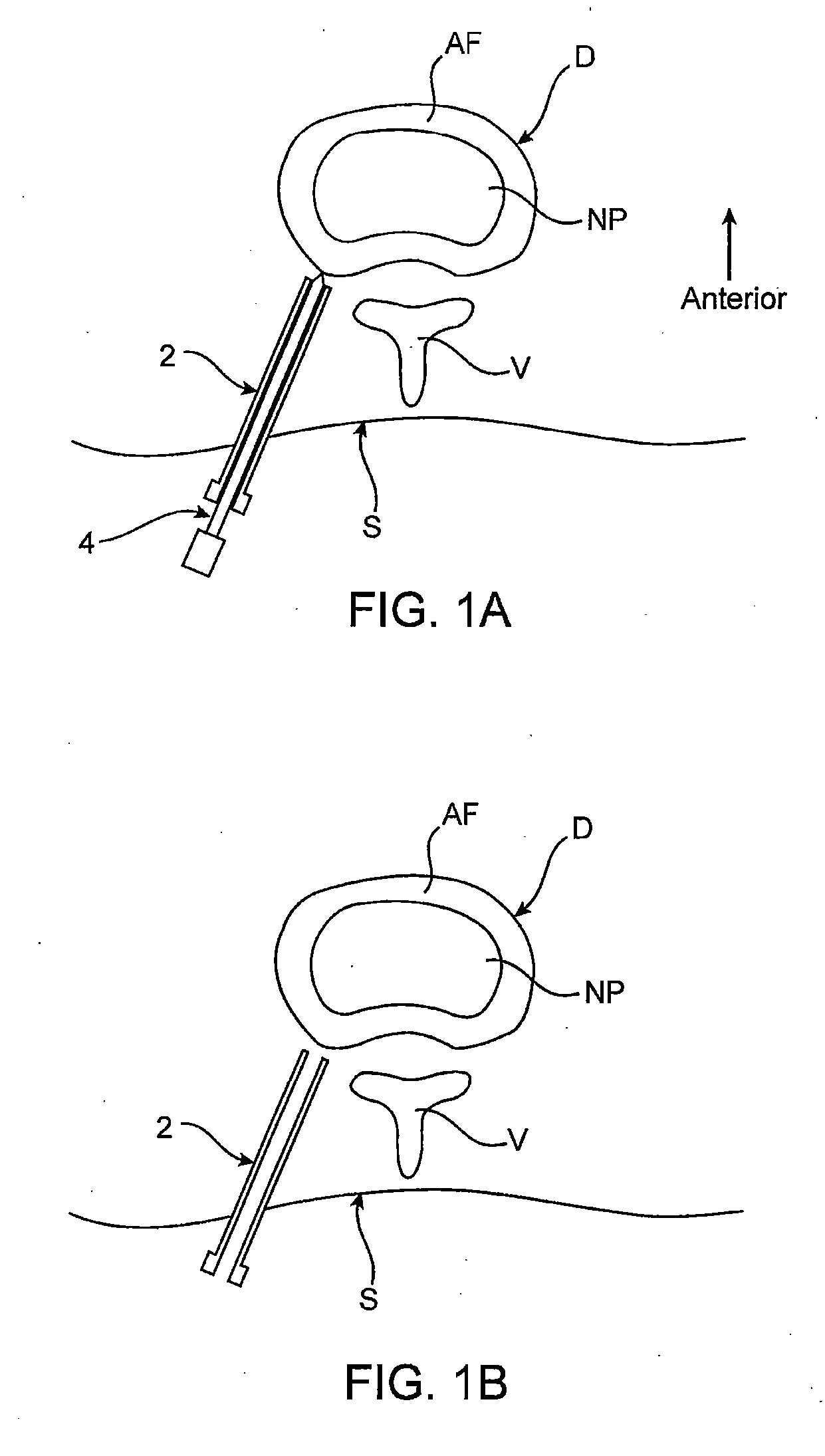
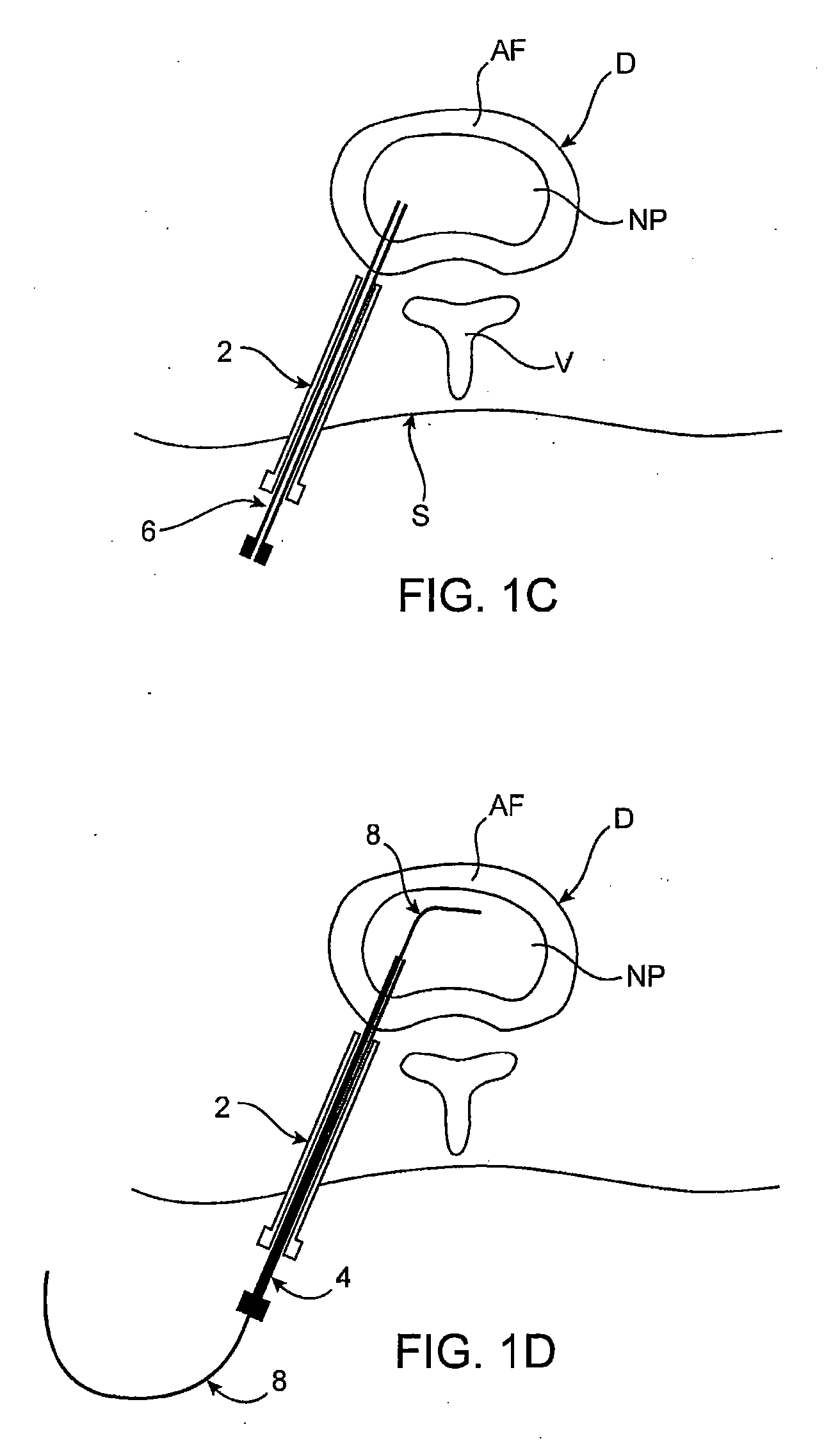
Spinal diagnostic methods and apparatus
ActiveUS20080009826A1Easy diagnosisRelieve painCannulasSurgical needlesSpinal columnAnesthetic Agent
Methods, devices and systems facilitate diagnosis, and in some cases treatment, of back pain originating in intervertebral discs. Methods generally involve introducing one or more substances into one or more discs using a catheter device. In one embodiment, a patient assumes a position that causes back pain, and a substance such as an anesthetic or analgesic is introduced into the disc to determine whether the substance relieves the pain. Injections into multiple discs may optionally be performed, to help pinpoint a disc as a source of the patient's pain. In some embodiments, the catheter device is left in place, and possibly coupled with another implantable device, to provide treatment of one or more discs. A catheter device includes at least one anchoring member for maintaining a distal portion of the catheter within a disc.
Owner:GLOBUS MEDICAL INC
Contraceptive device and delivery system
InactiveUS20050085844A1Promote tissue growthOcclusion effectFallopian occludersDilatorsFiber bundleEngineering
The invention described is directed to an intracorporeal occluding device having at least one spider segment with at least two, preferably dog-leg shaped expansive elements which are secured to a central location. The occluding device preferably has a plurality of spider segments which are axially aligned and preferably secured together by connecting elements such as beams, S-shaped or Z-shaped connecting elements. The spider segments or connecting elements may be provided with fibrous strands or other elements which facilitate tissue growth.
Owner:OVION +1
Embolic prosthesis for treatment of vascular aneurysm
InactiveUS20070237720A1Promote tissue growthConstant cross-sectionSuture equipmentsSurgical adhesivesMedicineProsthesis
The invention relates to an implantable embolic medical device comprising a non-erodible, erodible or biodegradable material. The device preferably comprises one or more longitudinal filament members of varying cross sectional shapes which may or may not be coiled to suit a particular clinical need. The embolic device is placed through lumens and cavities to reach areas in the body which require embolism to achieve a particular clinical objective.
Owner:REVA MEDICAL
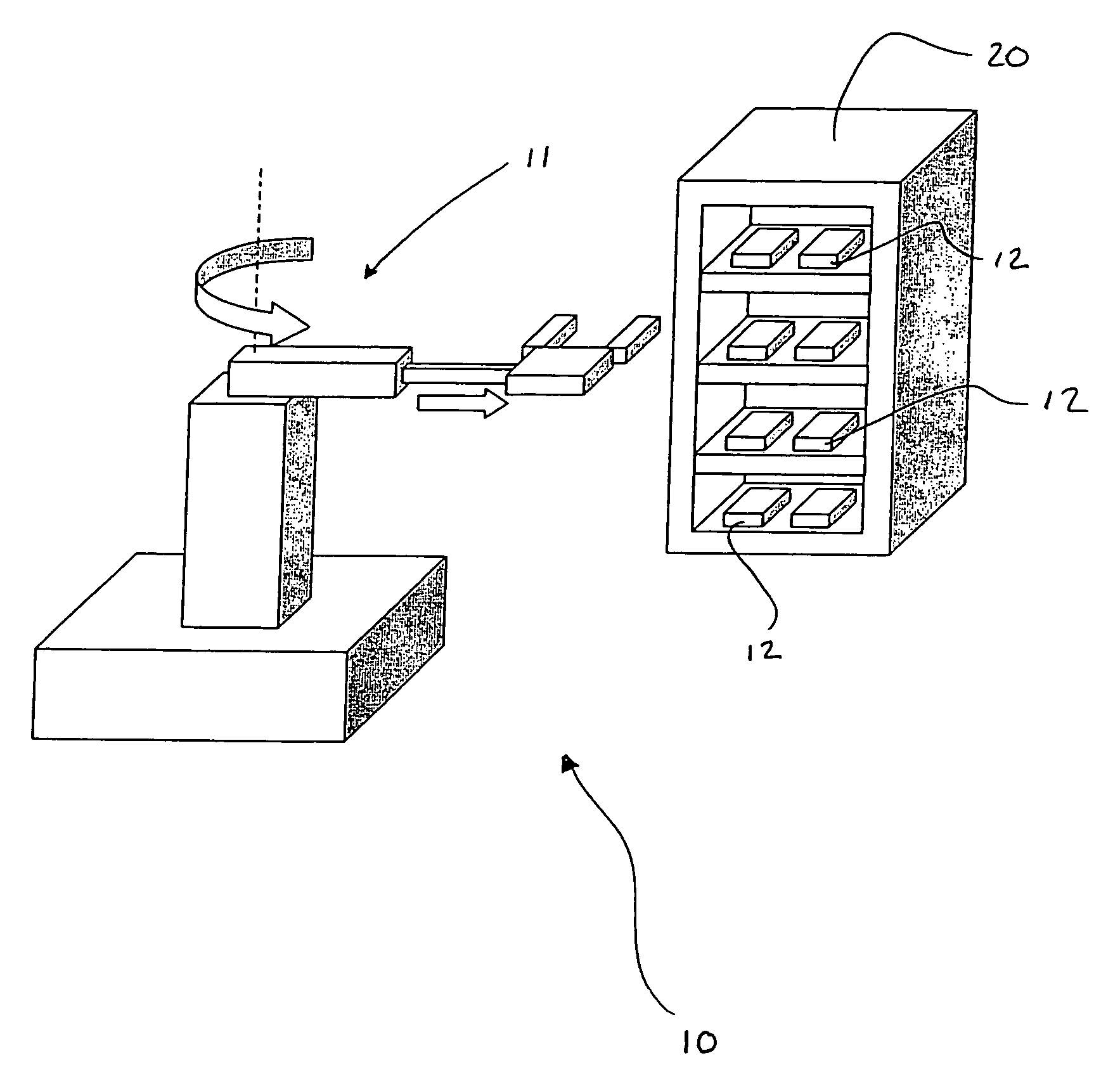
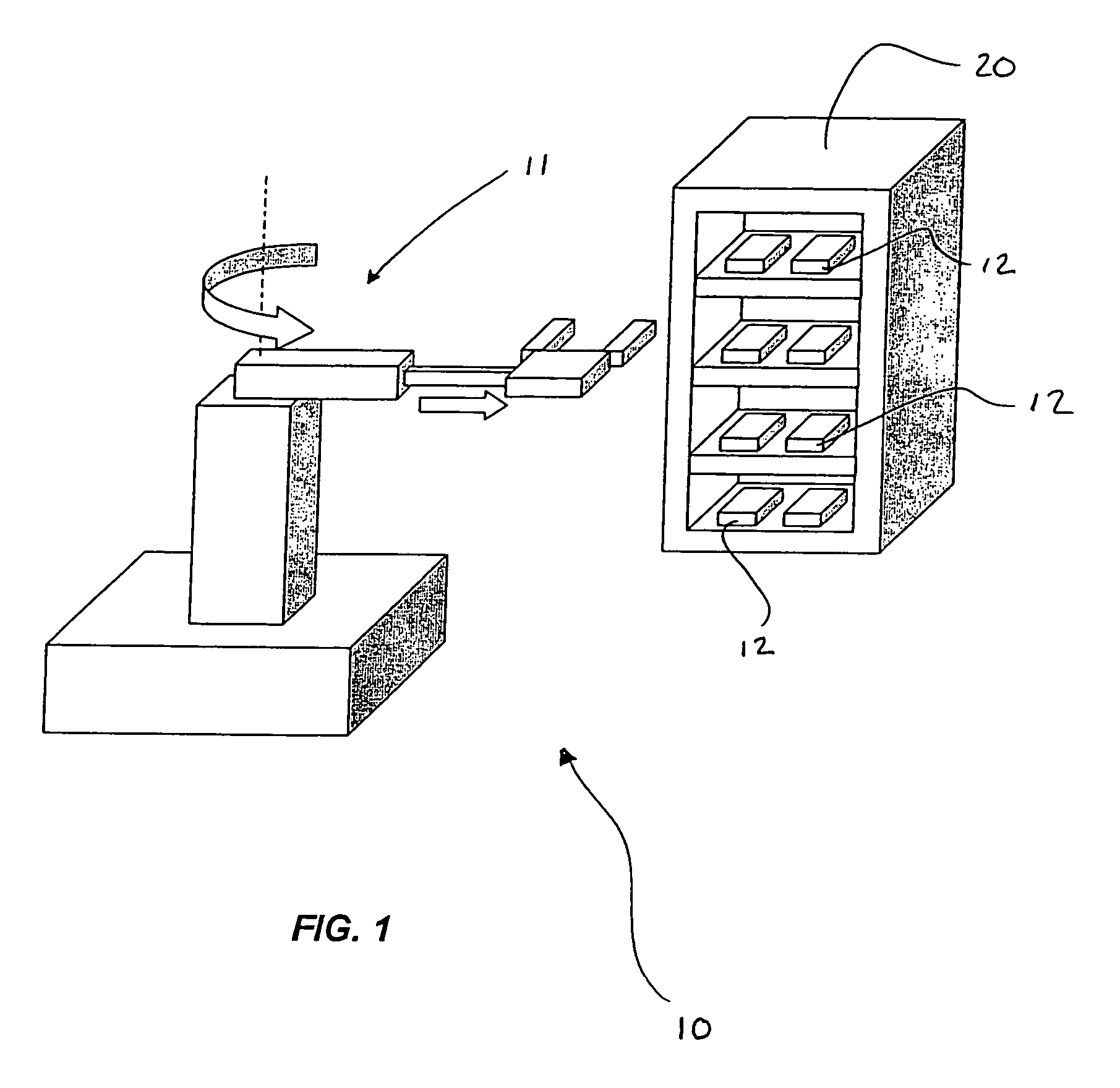
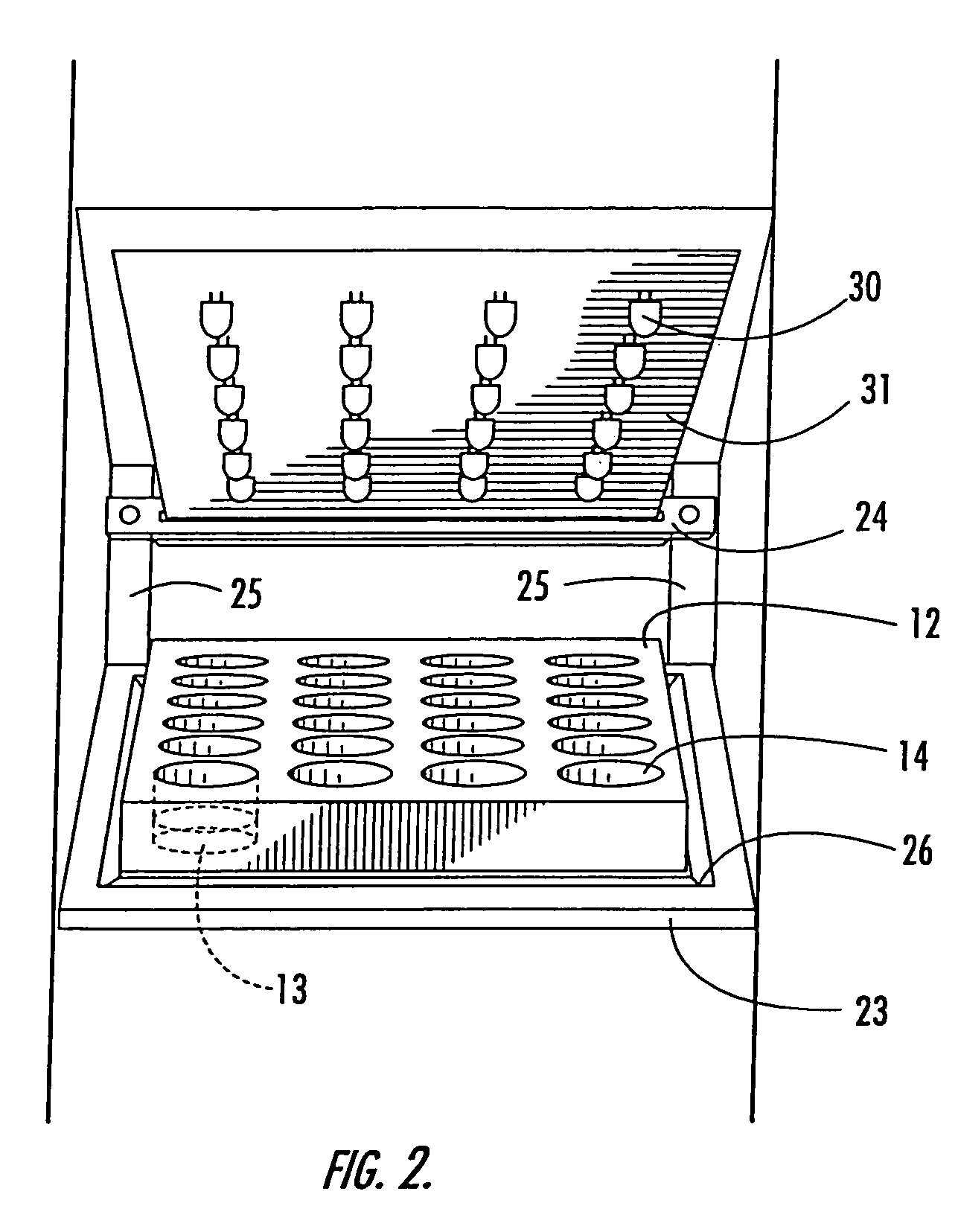
LED array for illuminating cell well plates and automated rack system for handling the same
InactiveUS7160717B2Improve storage efficiencyReduce amountBioreactor/fermenter combinationsBiological substance pretreatmentsCells/wellPlant tissue
An assembly for promoting the growth of plant tissues that includes a plurality of plates each defining an array of wells wherein each of the wells contains a tissue sample. Support for the plates is provided by a rack having a plurality of vertically stacked shelves that may include one or more register depressions that urge the plates into predetermined positions. Light for the tissue samples is provided by a plurality of light-emitting diode arrays each mounted on a circuit board. Each circuit board is supported by a respective card edge connector of the rack so that the light-emitting diodes are in proximity to the plates supported on one of the shelves therebelow. Preferably, the light-emitting diode array corresponds to the well array supported in the registered position on the shelf therebelow so that each light-emitting diode is centered above a respective one of the wells.
Owner:BIOLEX THERAPEUTICS INC
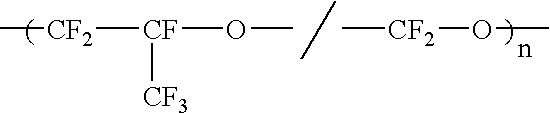
Liquid perfluoropolymers and medical and cosmetic applications incorporating same
InactiveUS20050271794A1Reduce removalTissue in-growth can be minimizedSuture equipmentsElectrotherapyPerfluoropolyetherOxygen
Liquid curable perfluoropolyether (PFPE) materials are provided for use as coatings, sealants, flexible fillers, and structural parts for a wide variety of medical and cosmetic applications, particularly where silicone has been utilized conventionally. The PFPE material is oxygen permeable and bacterial impermeable and may contain one or more pharmacological agents elutably trapped therewithin for delivery within the body of a subject.
Owner:LIQUIDIA TECH
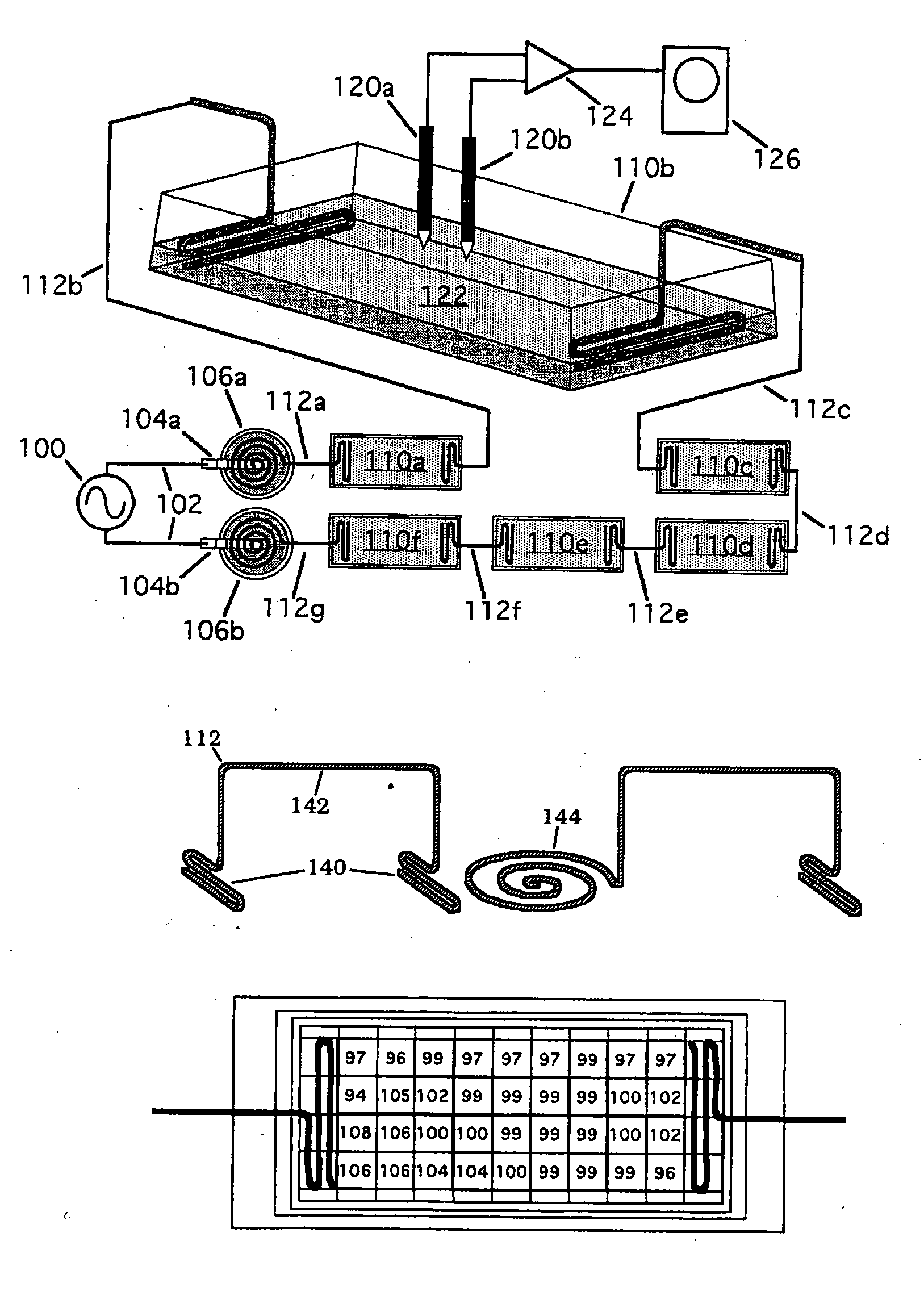
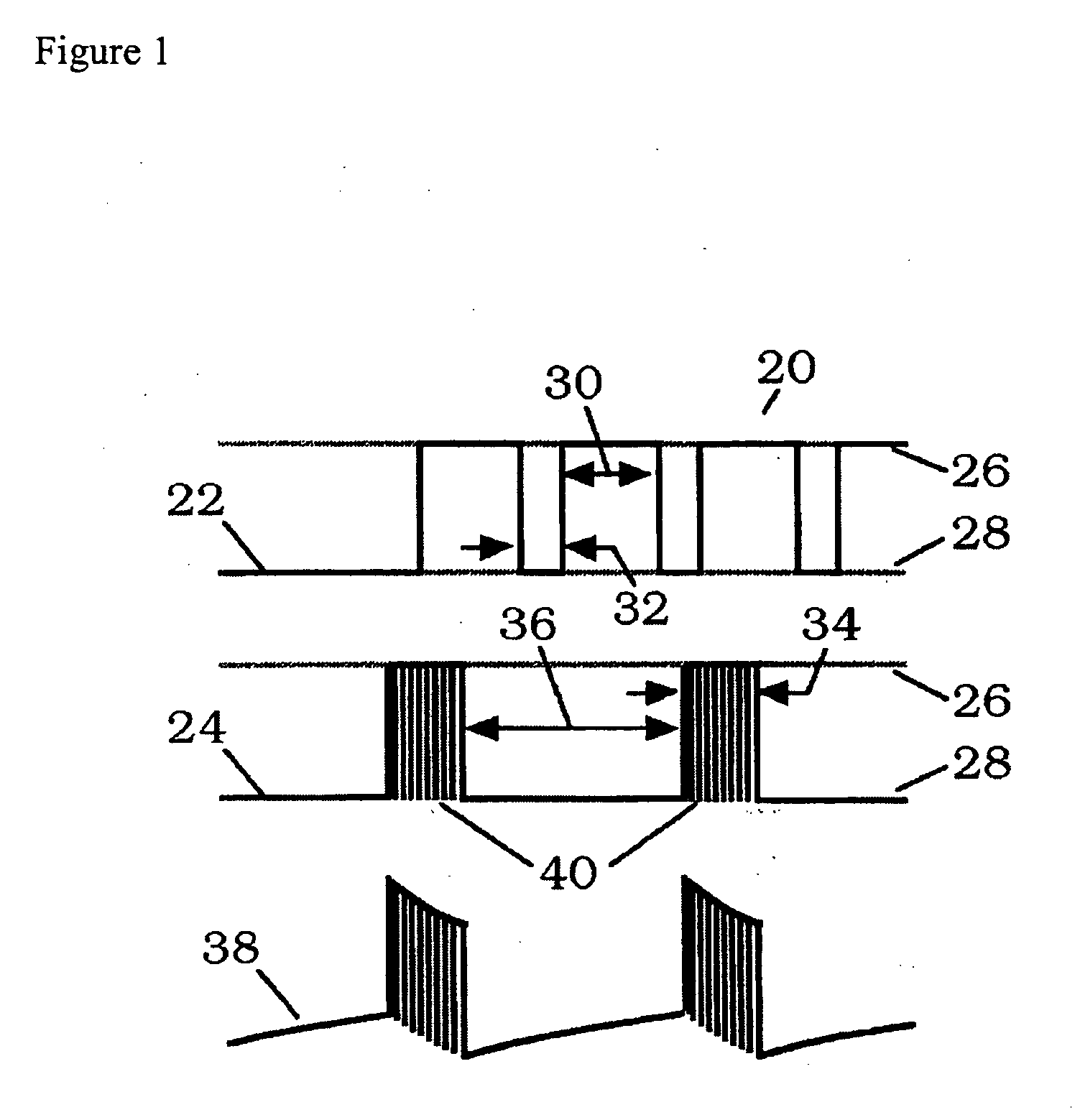
Methods for modulating osteochondral development using bioelectrical stimulation
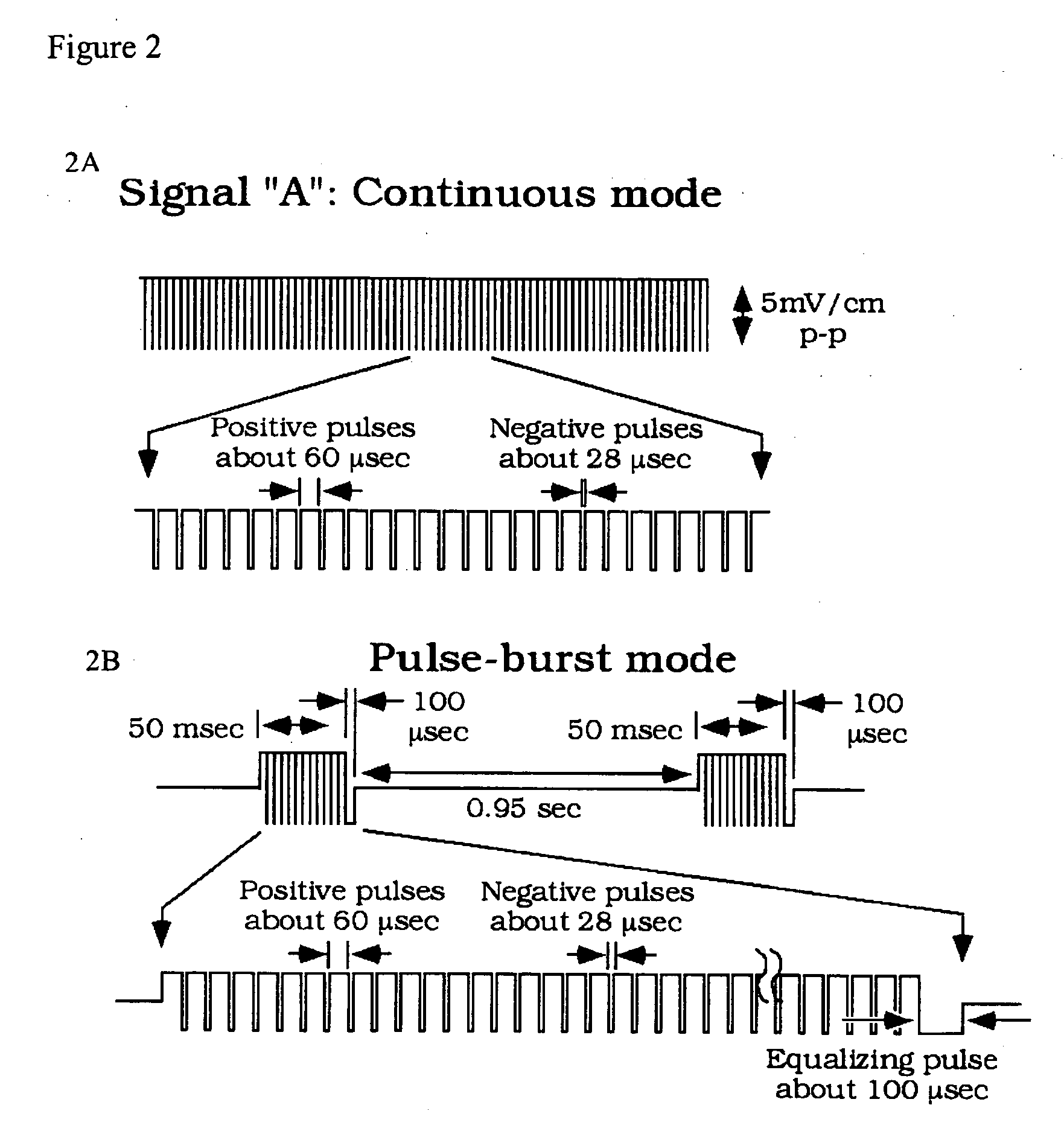
ActiveUS20060293724A1Maximize utilizationMaximize applicationElectrotherapyStress based microorganism growth stimulationCo administrationOsteoblast
Compositions and methods are provided for modulating the growth, development and repair of bone, cartilage or other connective tissue. Devices and stimulus waveforms are provided to differentially modulate the behavior of osteoblasts, chondrocytes and other connective tissue cells to promote proliferation, differentiation, matrix formation or mineralization for in vitro or in vivo applications. Continuous-mode and pulse-burst-mode stimulation of cells with charge-balanced signals may be used. Bone, cartilage and other connective tissue growth is stimulated in part by nitric oxide release through electrical stimulation and may be modulated through co-administration of NO donors and NO synthase inhibitors. Bone, cartilage and other connective tissue growth is stimulated in part by release of BMP-2 and BMP-7 in response to electrical stimulation to promote differentiation of cells. The methods and devices described are useful in promoting repair of bone fractures, cartilage and connective tissue repair as well as for engineering tissue for transplantation.
Owner:MEDRELIEF
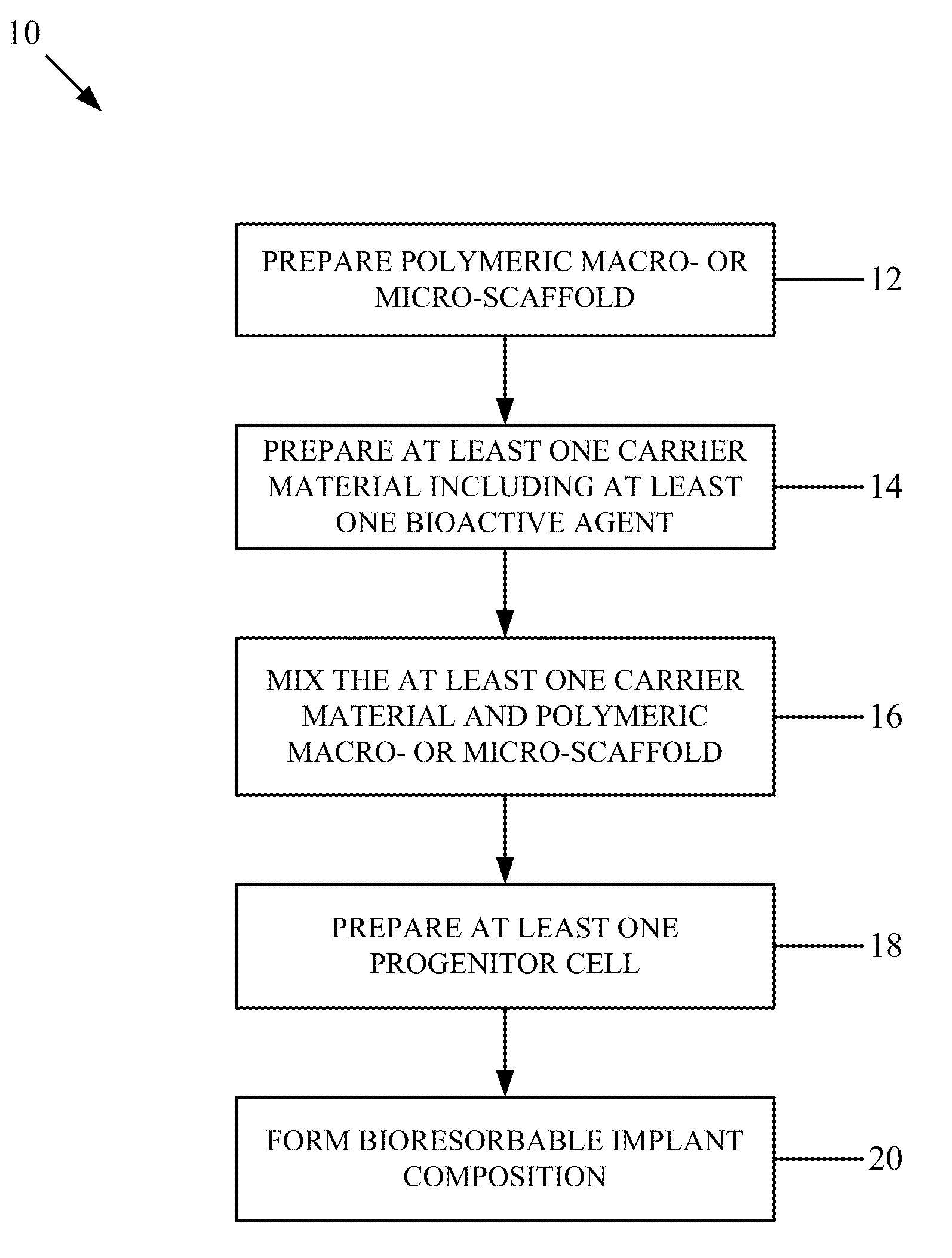
Bioresorbable implant composition
PendingUS20090081276A1Promote tissue growthOrganic active ingredientsBiocideActive agentBiomedical engineering
A bioresorbable implant composition includes a polymeric macro- or micro-scaffold and first and second bioactive agents respectively incorporated on or within the polymeric macro- or micro-scaffold. The first and second bioactive agents modulate a different function and / or characteristic of a cell.
Owner:CASE WESTERN RESERVE UNIV
Wound treatment-dressing and method of manufacture
InactiveUS20070161936A1Cleanse woundPromote resultsNon-adhesive dressingsPlastersWound dressingActive agent
A wound treatment-dressing is shown which includes a dressing body formed from a medically inert, moisture permeable, urethane open-cell foam which is hydrophilic in nature. The foam body has a foam matrix of interconnected foam cells with cell walls which have incorporated therein a combination of inorganic antimicrobials as active agents, the active agents being incorporated into the foam matrix both topically on a foam cell surface and integrally within the foam cell wall. The antimicrobials are manufactured in a selected particle size range which improves predictability and performance of the wound dressing.
Owner:SVETLIK HARVEY E
Agents for controlling biological fluids and methods of use thereof
ActiveUS20070053957A1Good hemostasisHigh viscosityBiocidePeptide/protein ingredientsMedicinePositive pressure
Therapeutic formulations adapted for positive-pressure application for controlling biological fluid at a desired site in a subject, absorbent articles comprising therapeutic formulations, and anti-infective devices coated with therapeutic formulations, said formulations comprising about 25% to about 99% by weight liquid-crystal forming compound and 0% to about 75% by weight solvent. In addition, methods of using said formulations including methods for controlling biological fluid at a desired site in a subject, methods for controlling blood loss, and methods for facilitating effective closure of a vascular wound or incision site at a desired site in a subject are disclosed, the methods comprising administering particular formulations comprising liquid-crystal forming compounds and solvents that are described herein.
Owner:SOUTHEASTERN MEDICAL TECH
Surgical slings
InactiveUS20060205998A1Promotes collagenous tissue growthMaintaining continenceAnti-incontinence devicesSurgical implantSurgical department
The invention relates generally to surgical implants, and in various embodiments to surgical implants configured for promoting growth of collagenous tissue at an anatomical site.
Owner:BOSTON SCI SCIMED INC
Spinal diagnostic methods and apparatus
ActiveUS20080009828A1Easy diagnosisRelieve painCannulasSurgical needlesAnesthetic AgentSpinal column
Methods, devices and systems facilitate diagnosis, and in some cases treatment, of back pain originating in intervertebral discs. Methods generally involve introducing one or more substances into one or more discs using a catheter device. In one embodiment, a patient assumes a position that causes back pain, and a substance such as an anesthetic or analgesic is introduced into the disc to determine whether the substance relieves the pain. Injections into multiple discs may optionally be performed, to help pinpoint a disc as a source of the patient's pain. In some embodiments, the catheter device is left in place, and possibly coupled with another implantable device, to provide treatment of one or more discs. A catheter device includes at least one anchoring member for maintaining a distal portion of the catheter within a disc.
Owner:GLOBUS MEDICAL INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com