Implantable Tendon Protection Systems and Related Kits and Methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0028]The following detailed description should be read with reference to the drawings in which similar elements in different drawings are numbered the same. The drawings, which are not necessarily to scale, depict illustrative embodiments and are not intended to limit the scope of the invention.
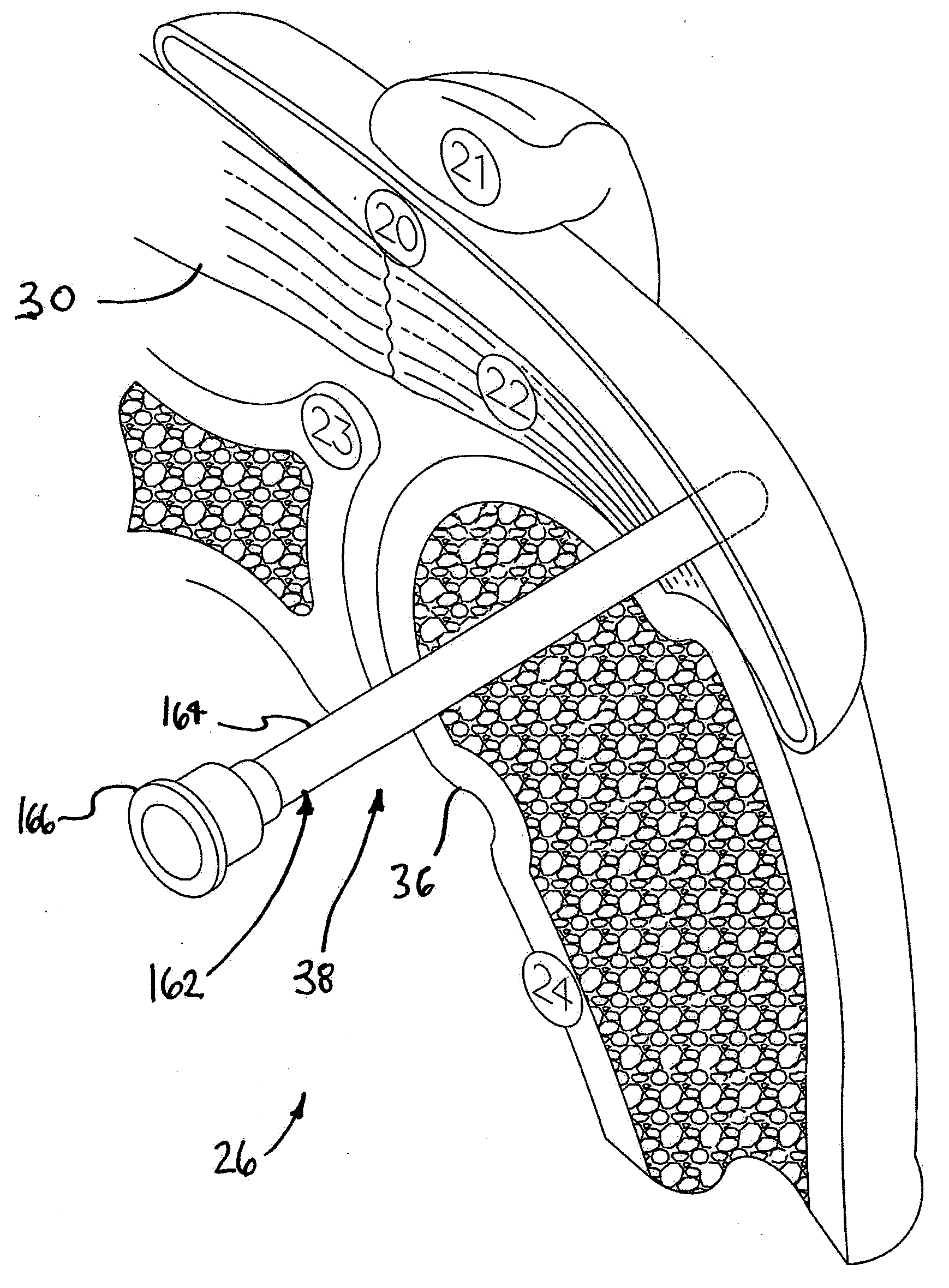
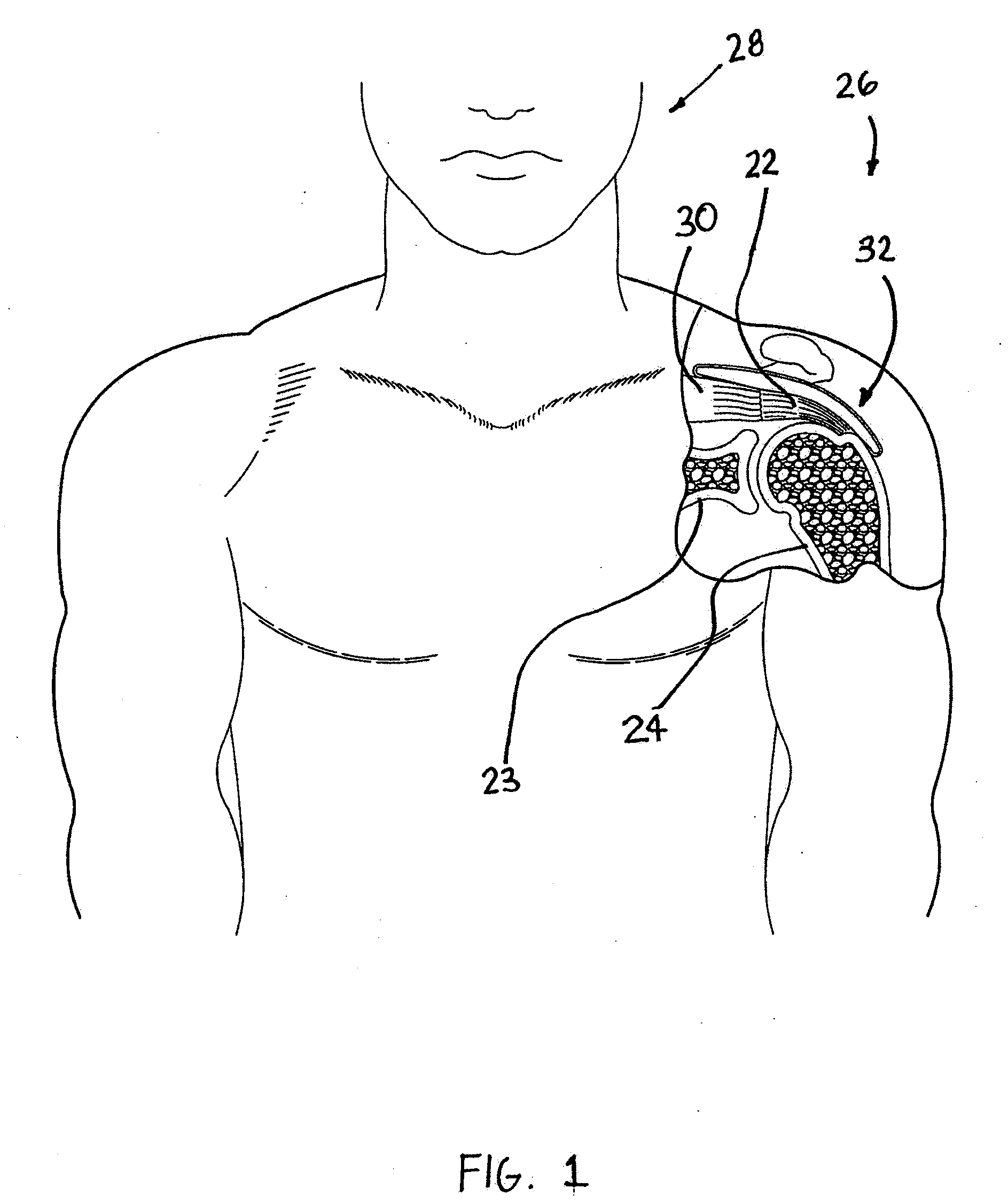
[0029]FIG. 1 is a stylized anterior view of a patient 28. For purposes of illustration, a shoulder 26 of patient 28 is shown in cross-section in FIG. 1. Shoulder 26 includes a humerus 24 and a scapula 23. The movement of humerus 24 relative to scapula 23 is controlled by a number of muscles including: the deltoid, the supraspinatus, the infraspinatus, the subscapularis, and the teres minor. For purposes of illustration, only the supraspinatus 30 is shown in FIG. 1. With reference to FIG. 1, it will be appreciated that a distal tendon 22 of the supraspinatus 30 meets humerus 24 at an insertion point 32.
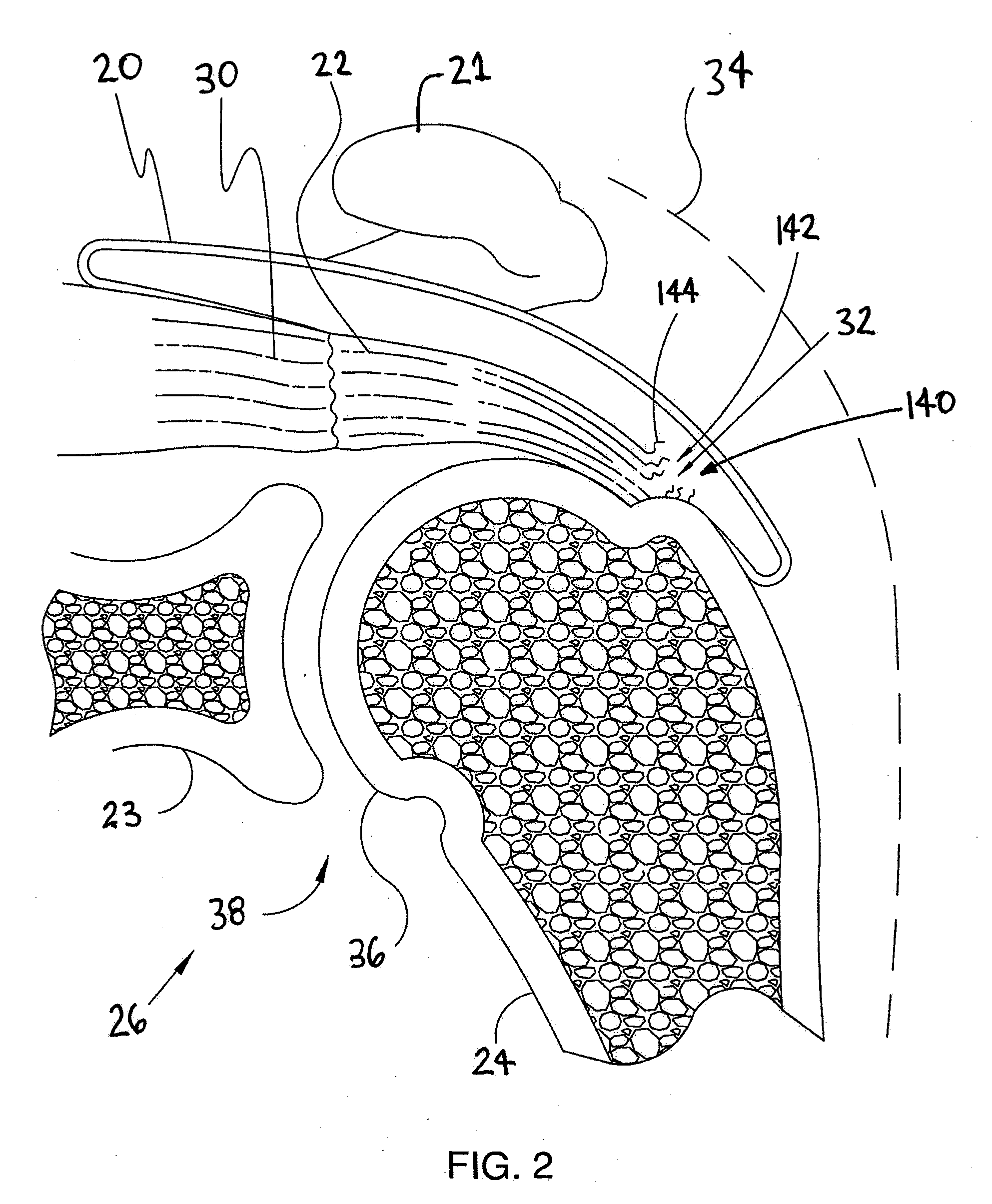
[0030]FIG. 2 is an enlarged cross sectional view of shoulder 26 shown in the previous figur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com