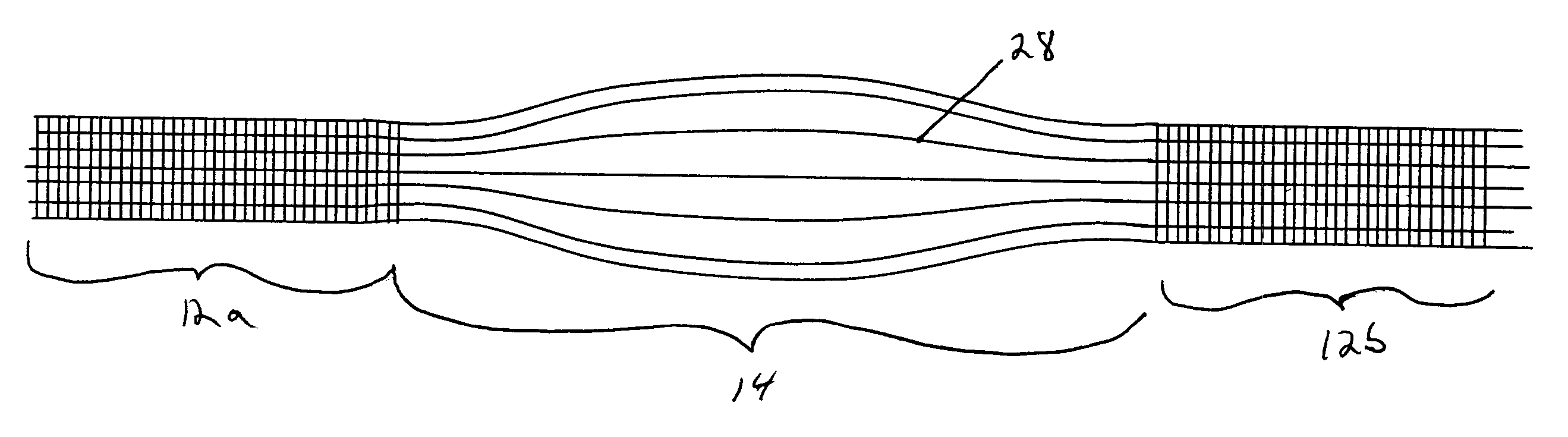
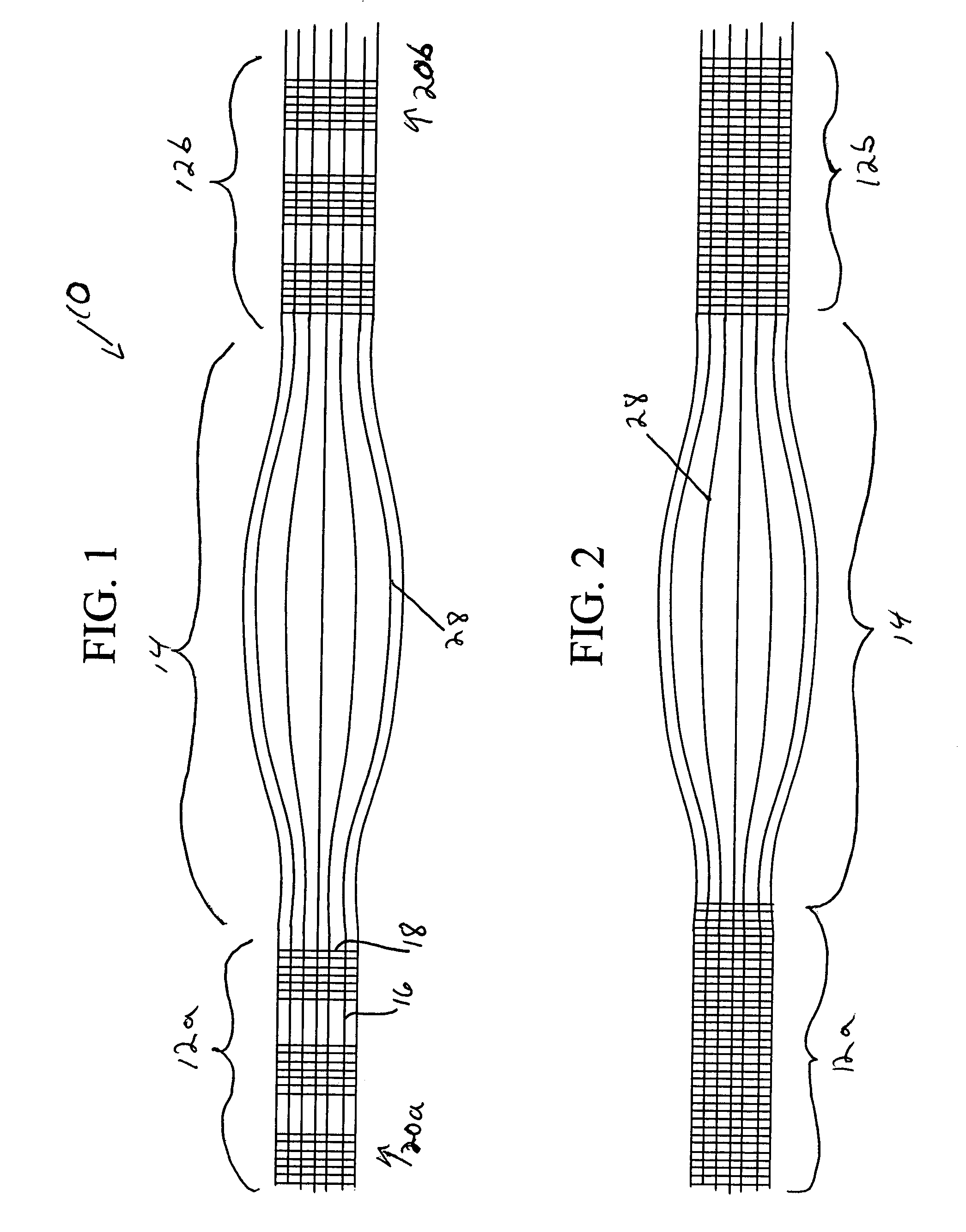
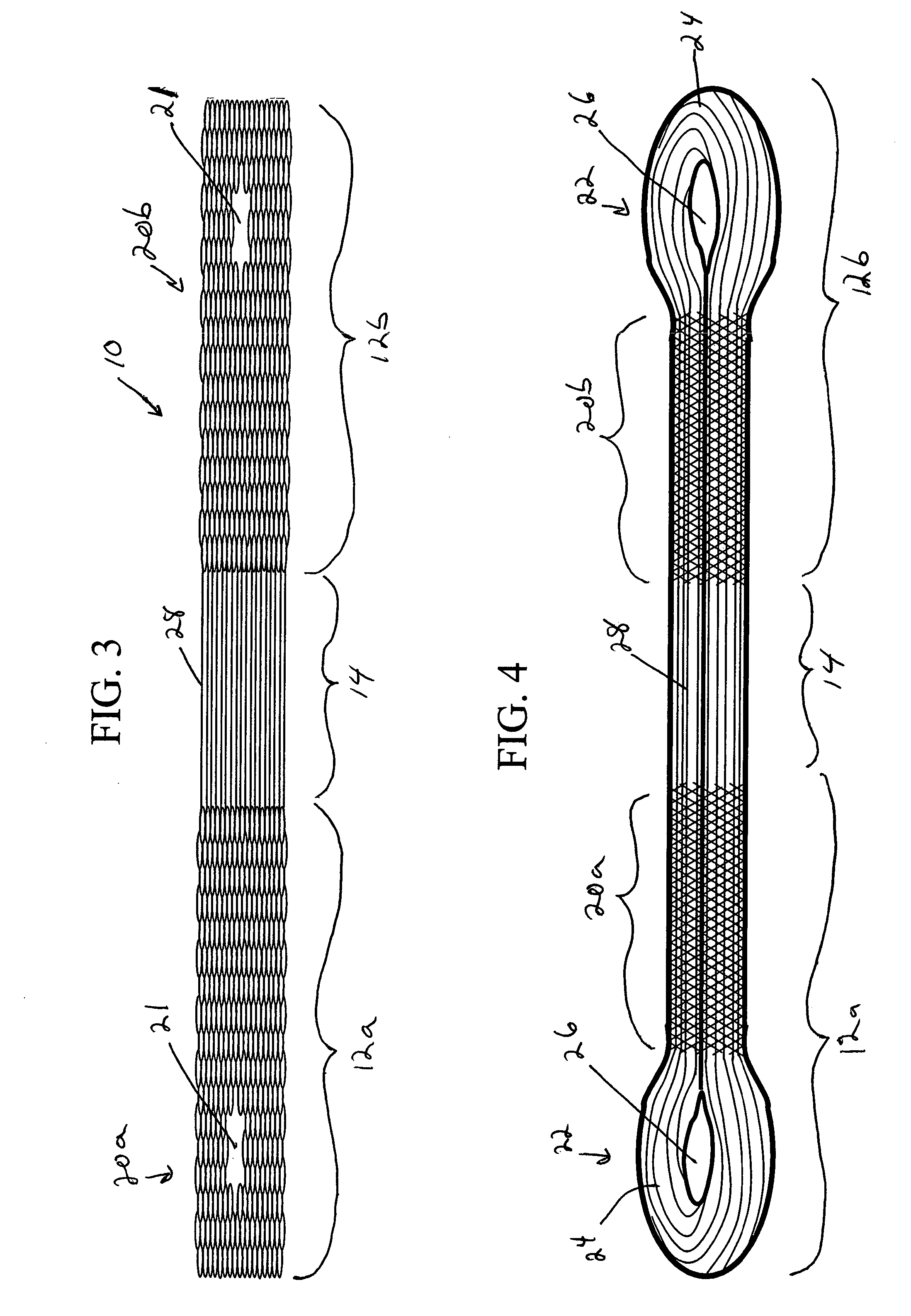
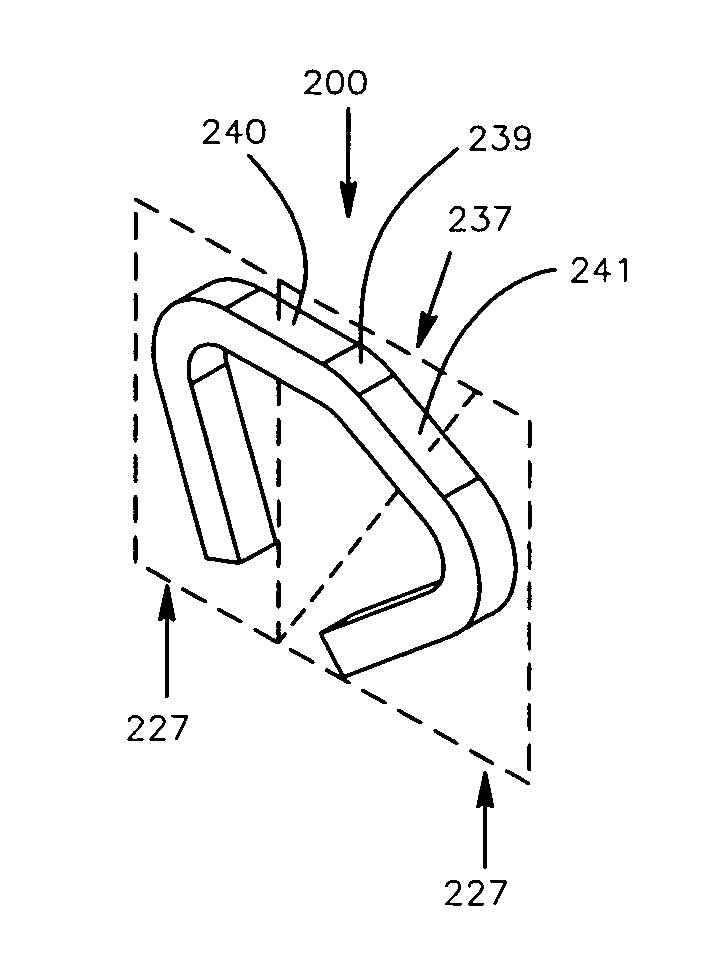
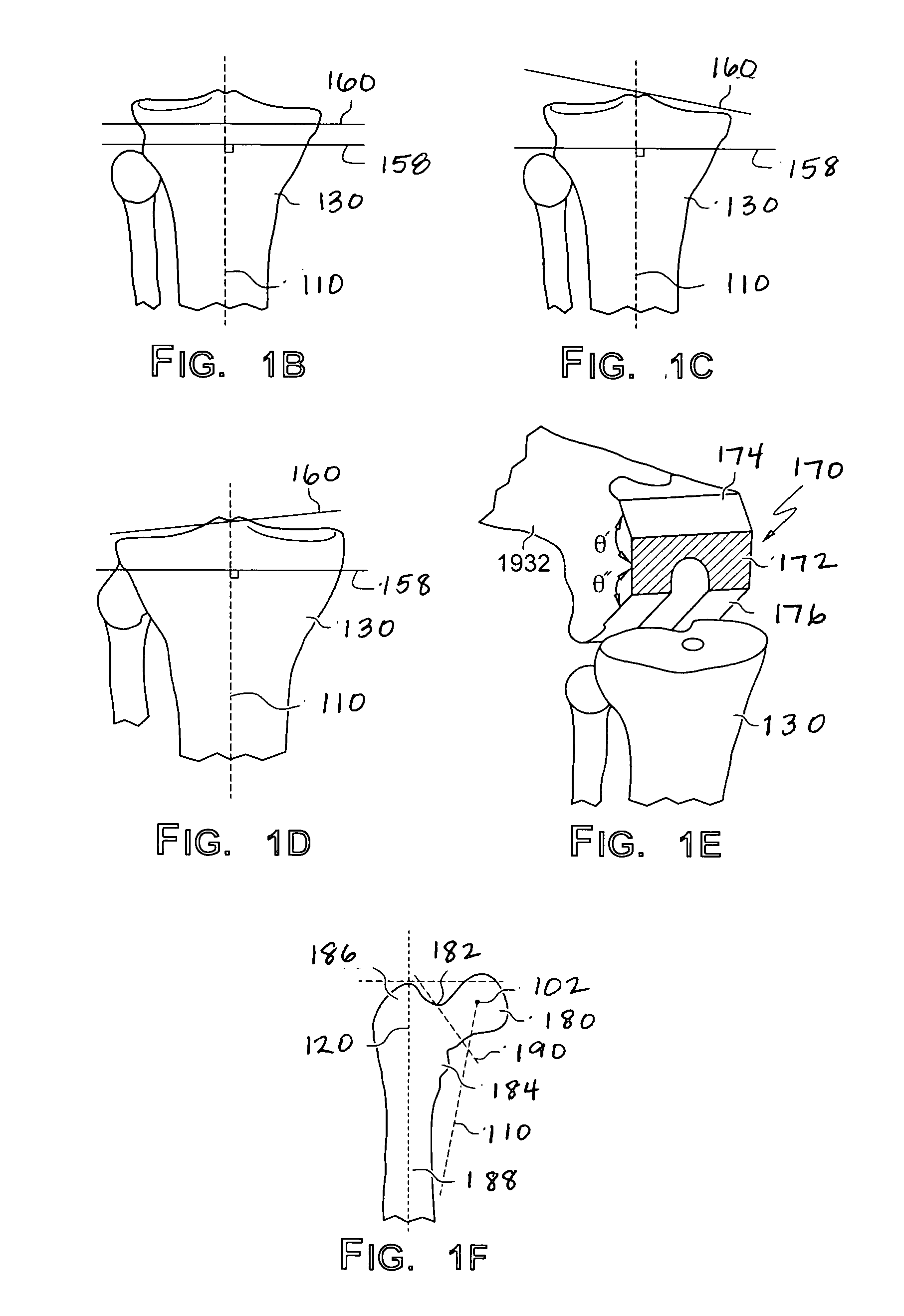Patents
Literature
4758results about "Ligaments" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Medical devices and applications of polyhydroxyalkanoate polymers
InactiveUS6838493B2High porosityReduce probabilitySuture equipmentsOrganic active ingredientsTissue repairBiocompatibility Testing
Devices formed of or including biocompatible polyhydroxyalkanoates are provided with controlled degradation rates, preferably less than one year under physiological conditions. Preferred devices include sutures, suture fasteners, meniscus repair devices, rivets, tacks, staples, screws (including interference screws), bone plates and bone plating systems, surgical mesh, repair patches, slings, cardiovascular patches, orthopedic pins (including bone filling augmentation material), adhesion barriers, stents, guided tissue repair / regeneration devices, articular cartilage repair devices, nerve guides, tendon repair devices, atrial septal defect repair devices, pericardial patches, bulking and filling agents, vein valves, bone marrow scaffolds, meniscus regeneration devices, ligament and tendon grafts, ocular cell implants, spinal fusion cages, skin substitutes, dural substitutes, bone graft substitutes, bone dowels, wound dressings, and hemostats. The polyhydroxyalkanoates can contain additives, be formed of mixtures of monomers or include pendant groups or modifications in their backbones, or can be chemically modified, all to alter the degradation rates. The polyhydroxyalkanoate compositions also provide favorable mechanical properties, biocompatibility, and degradation times within desirable time frames under physiological conditions.
Owner:TEPHA INC
Method and apparatus for bone fracture fixation
InactiveUS7052499B2Easy to separateEasy to useSuture equipmentsLigamentsBone fixationBiomedical engineering
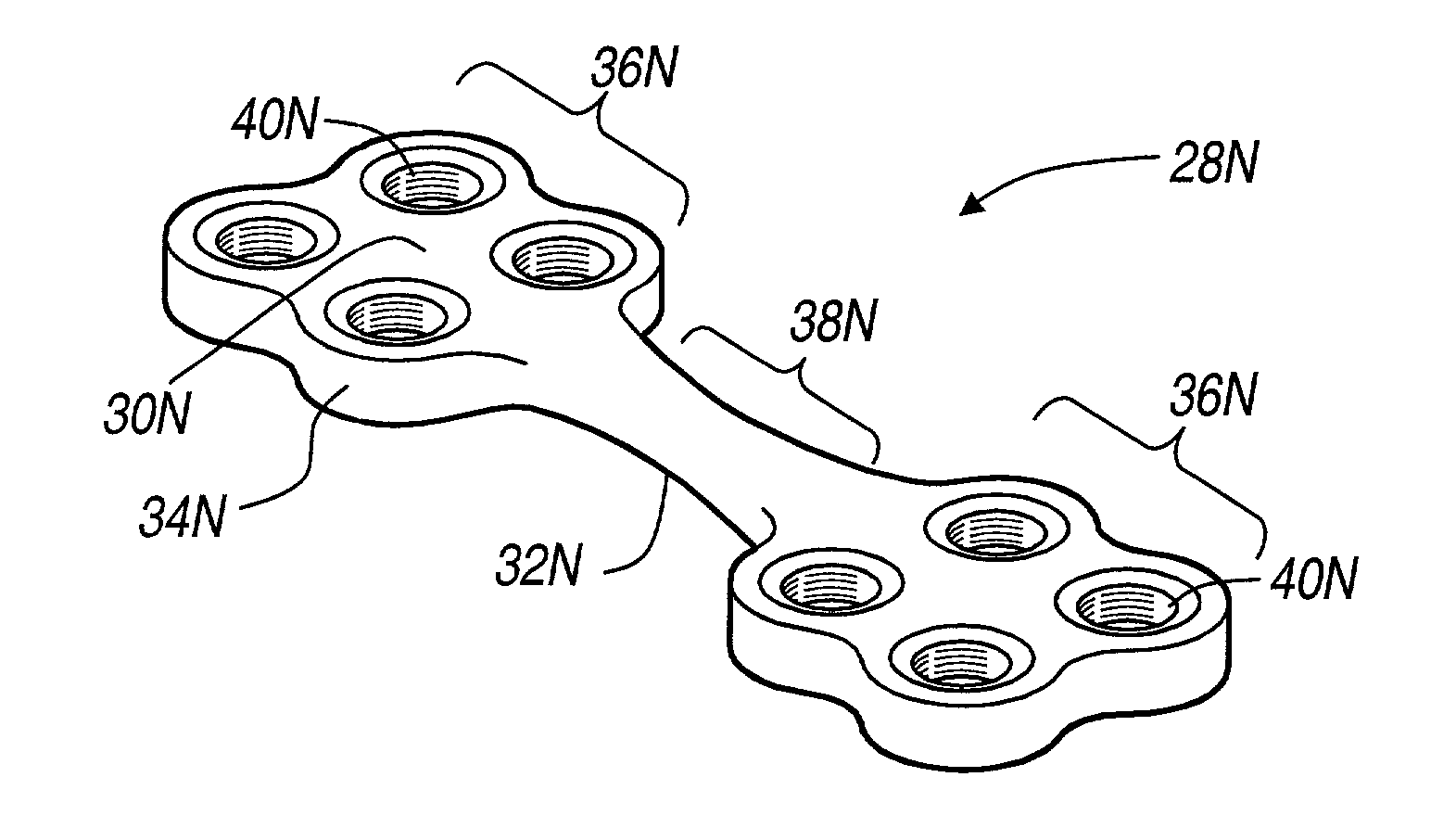
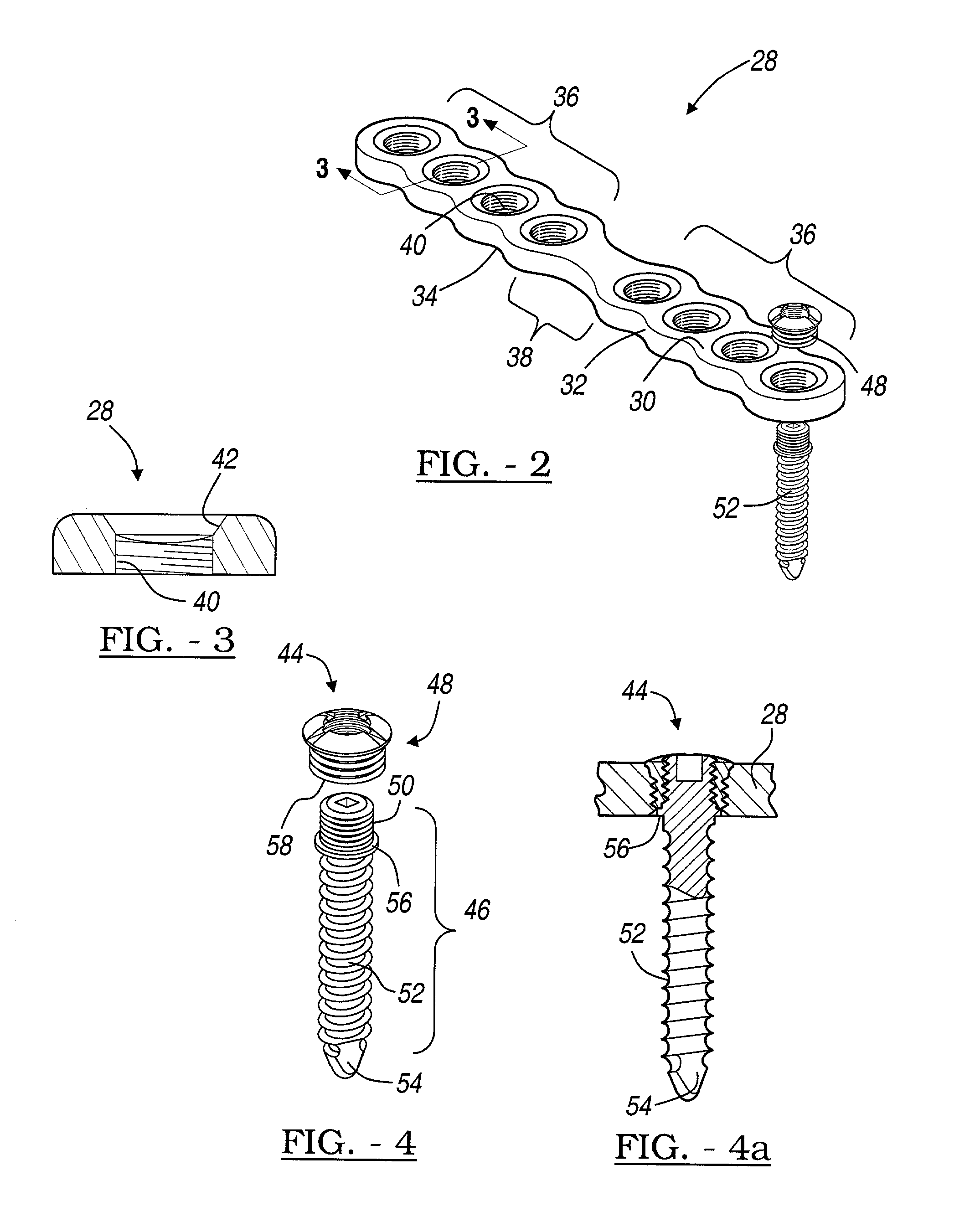
An elongated plate for coupling severed bone regions comprising at least one bridge region, the at least one bridge region terminating in at least two bone fixation regions. The at least two bone fixation regions each contain at least one aperture for receiving a suitable fastening device for securing the elongated plate to the bone regions to be coupled. The bridge region may be configured so as to be easily severed by a suitable severing device such as surgical scissors. The elongated plate and fastening device may be formed from a bio-compatible or bio-resorbable material.
Owner:ZIMMER BIOMET CMF & THORACIC
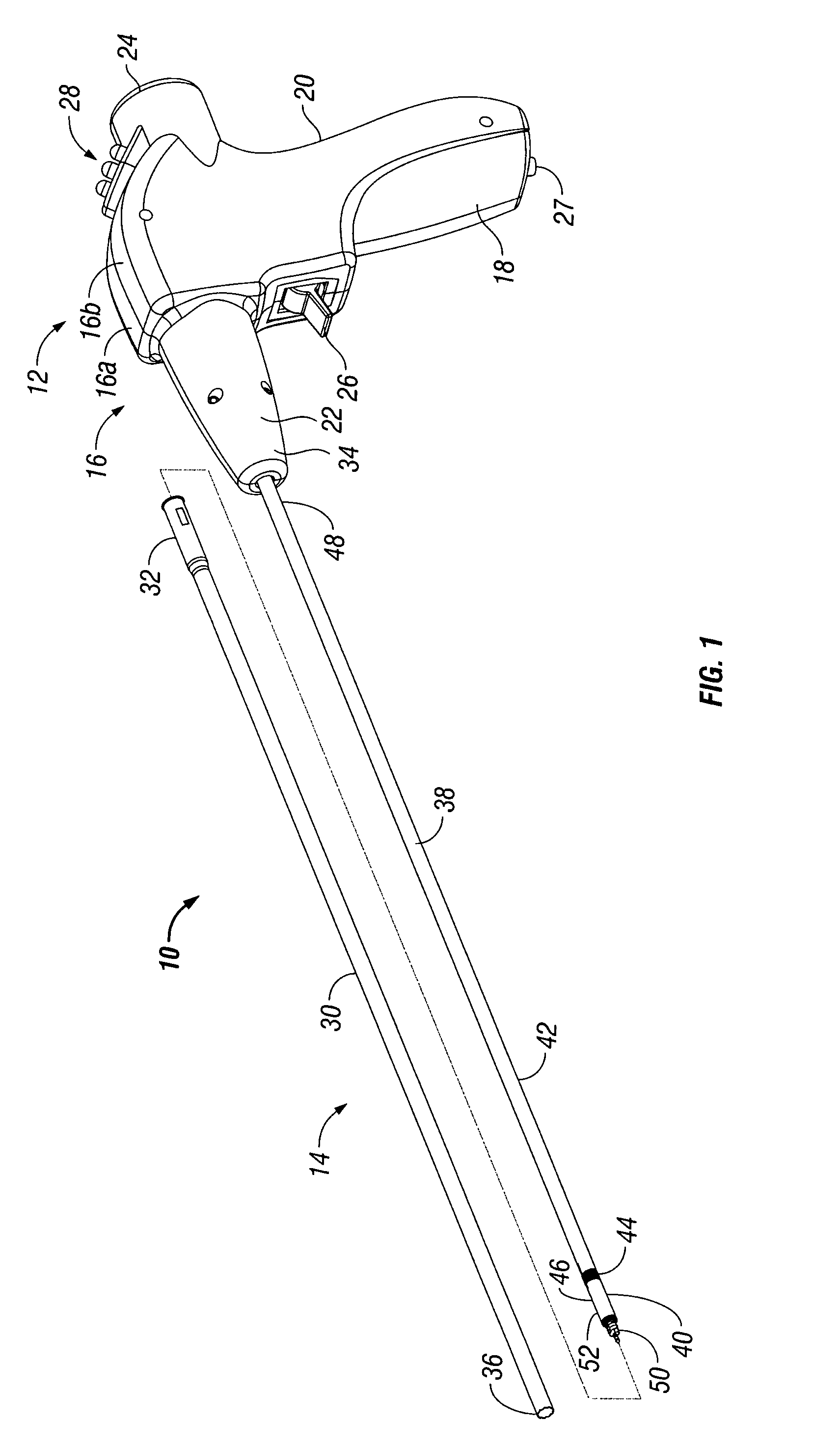
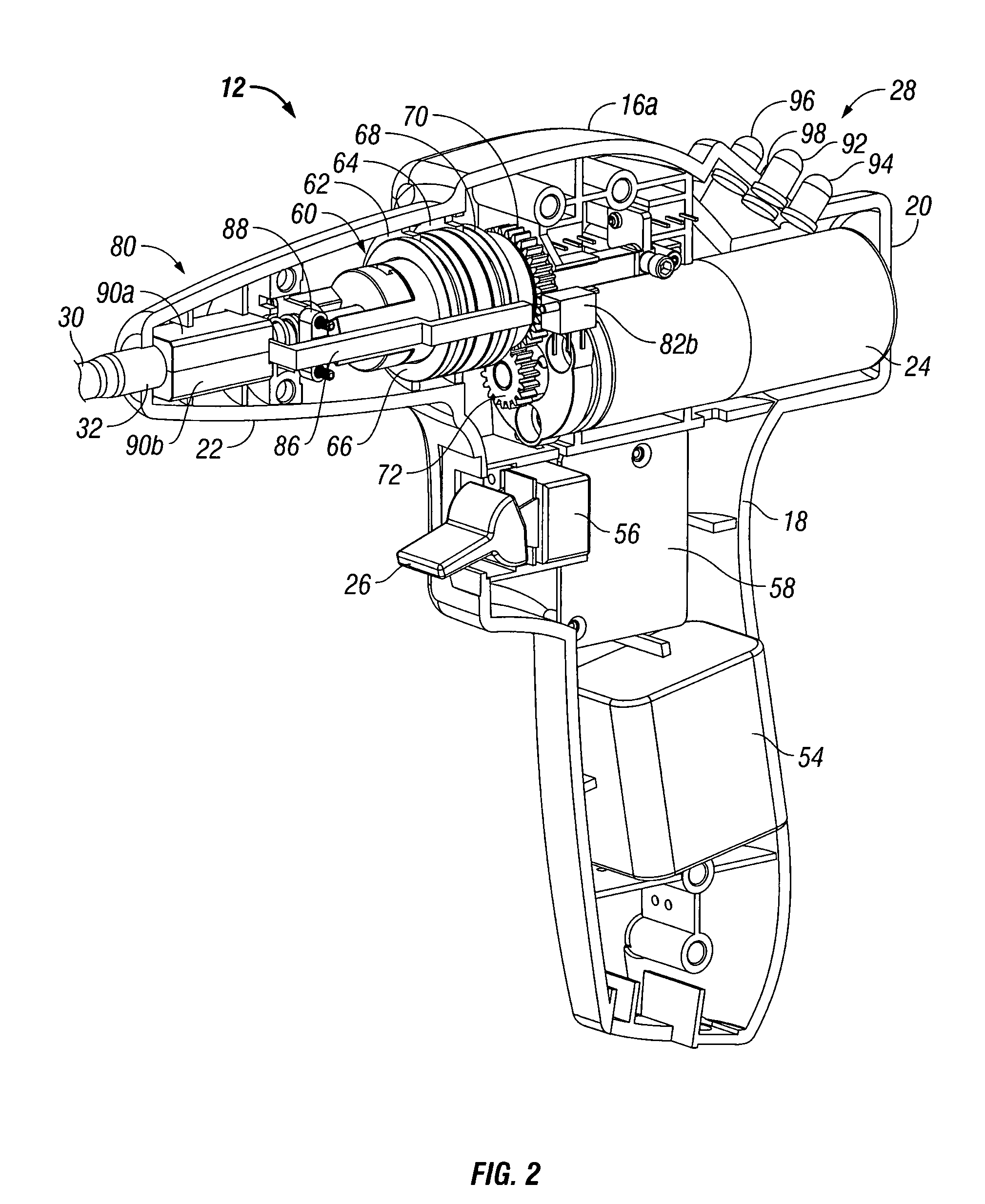
Powered tacker instrument
ActiveUS7931660B2Prevent rotationActuation can be preventedLigamentsMusclesBiomedical engineeringSurgical Fasteners
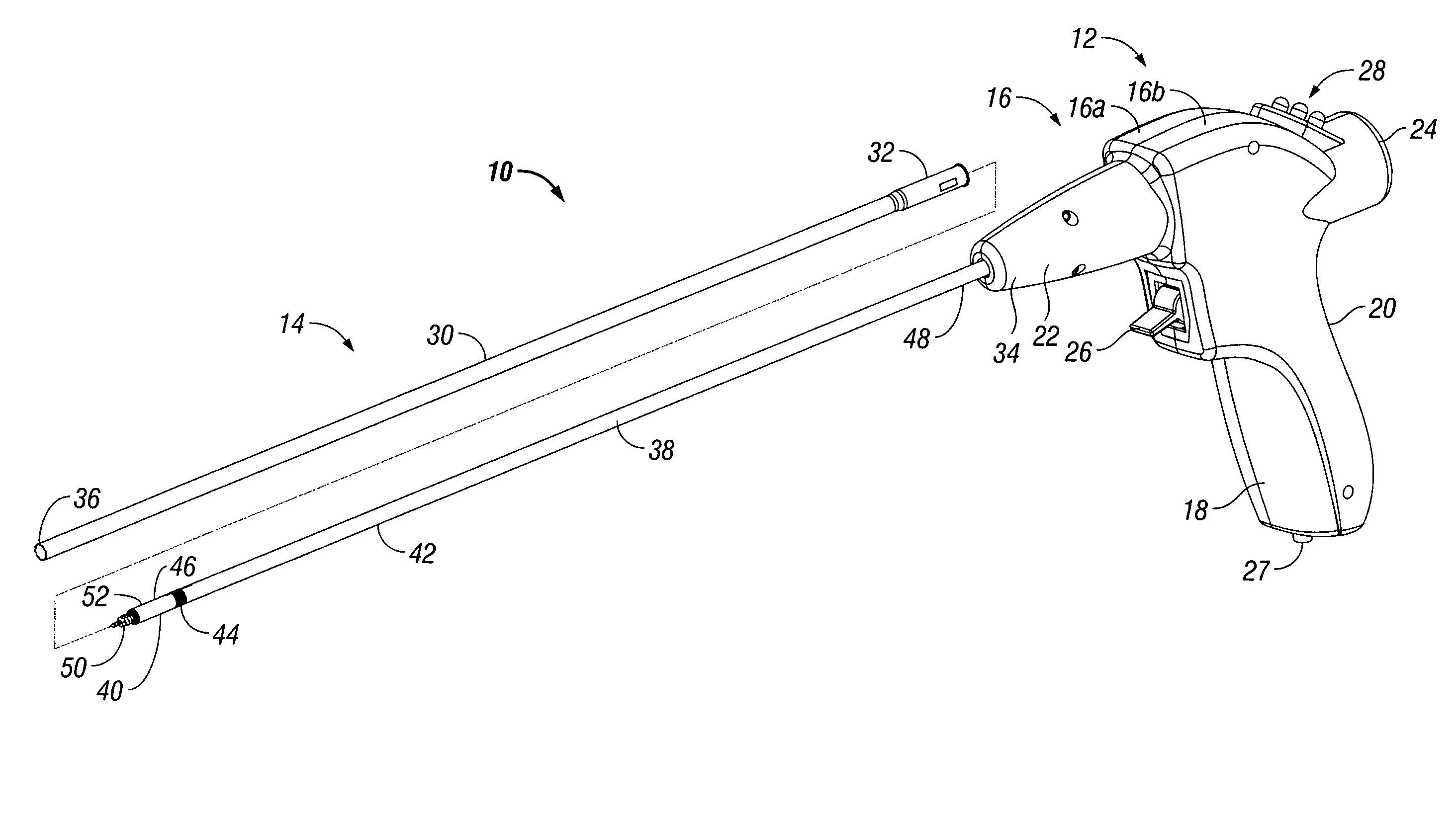
There is provided a powered tacker device for use in installing multiple surgical fasteners through a prosthetic mesh into tissue. The powered tacker device generally includes a handle assembly and a tacker assembly extending distally from the handle assembly. The handle assembly includes a motor and self-contained power assembly to rotate the surgical fasteners into tissue. The handle assembly is provided with a drive assembly which allows for rotation, as well as distal longitudinal movement, of a surgical fastener relative to the powered tacker device. The tacker assembly includes an inner tube for containing the plurality of surgical fasteners and a driver which is movable out of alignment with the inner tube so as to install a single fastener at a time into tissue.
Owner:TYCO HEALTHCARE GRP LP
Systems for securing sutures, grafts and soft tissue to bone and periosteum
InactiveUS7326213B2Great degree of tensionSecure attachmentSuture equipmentsStaplesSoft tissue neckSynthetic materials
Devices for affixing sutures, grafts and tissues to bone, and soft tissue such as periosteum. Such devices are designed to be deployed and selectively positioned at a target site and remain seated thereat. The devices are further provided with attachment structures for securing sutures, grafts, synthetic materials or tissues thereto which facilitates the ability of such devices to remain more firmly in position.
Owner:SPRINGBOARD MEDICAL VENTURES
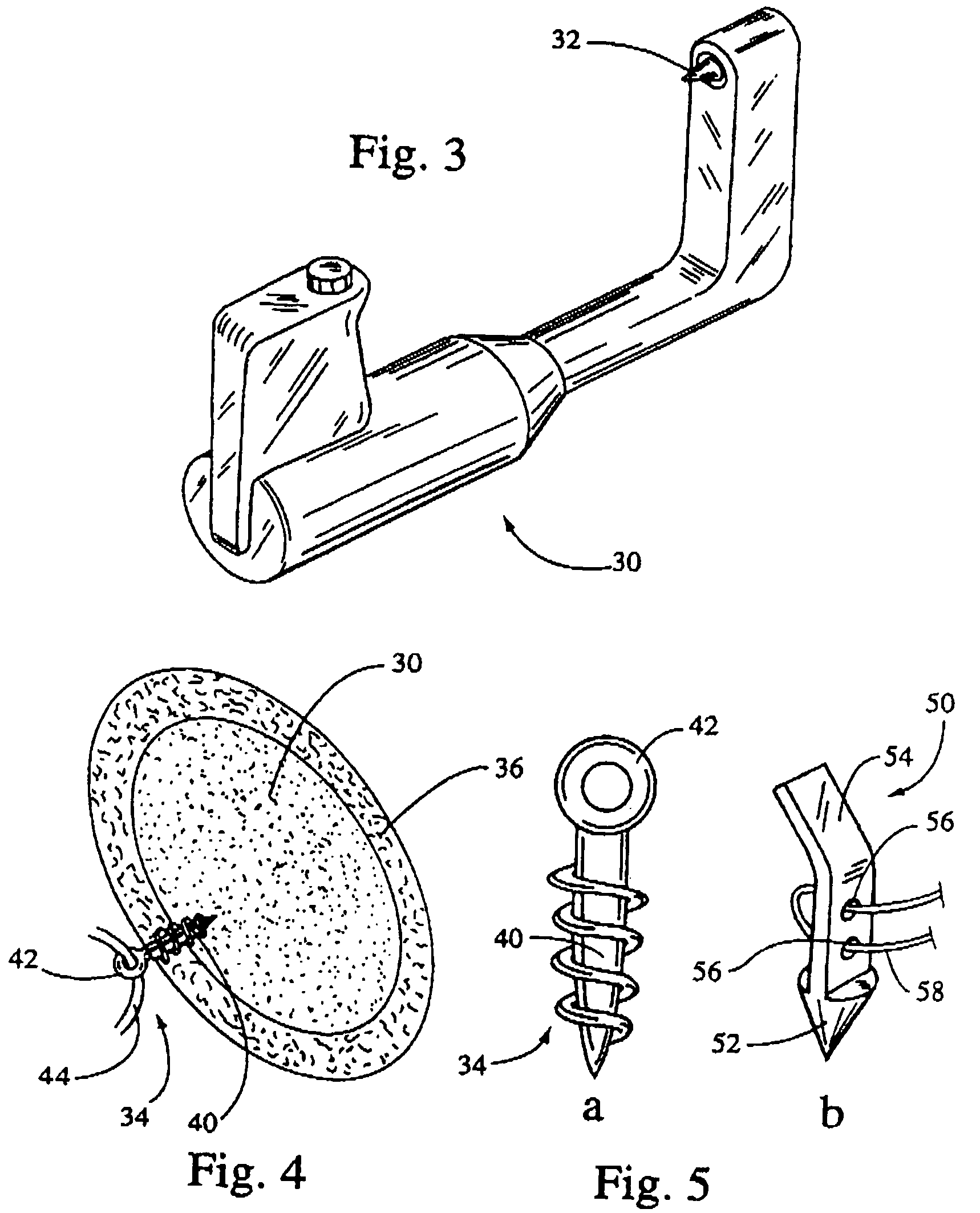
Multi-point tissue tension distribution device and method, a chin lift variation
InactiveUS7510566B2Increase healing responseRelieve painSuture equipmentsDiagnosticsChinWound healing
A tissue approximation device and processes for using the device are provided. The device is an implantable, biodegradable construct that has attachment points emanating from a supportive backing. The device improves the mechanical phase of wound healing and optimally distributes tension over the contact area between the device and tissue. Processes for using the device include soft tissue attachment and soft tissue to bone attachment. Several variations are particularly applicable to facilitating tissue approximation in surgical cosmetic applications, particularly chin lifts. Generally, tissue to be lifted may be set on a chin lift device via attachment points before or after the device is secured to a patient's bone. Variations of the device are described along with a method of installing the chin lift device. Also described is a tool particularly useful for installing a chin lift device.
Owner:MICROAIRE SURGICAL INSTR +2
Scaffold for connective tissue repair
A connective tissue scaffold including opposed first and second anchoring segments formed from a plurality of bioresorbable polymeric fibers oriented in a direction substantially parallel to a longitudinal axis of the scaffold and a plurality of bioresorbable polymeric fibers oriented in a direction substantially transverse to a longitudinal axis of the scaffold. A central segment joins the first and second anchoring segments and includes a plurality of bioresorbable polymeric fibers oriented in a direction substantially parallel to the longitudinal axis of the scaffold. The scaffold can also a tissue particle and / or biological component.
Owner:DEPUY SYNTHES PROD INC
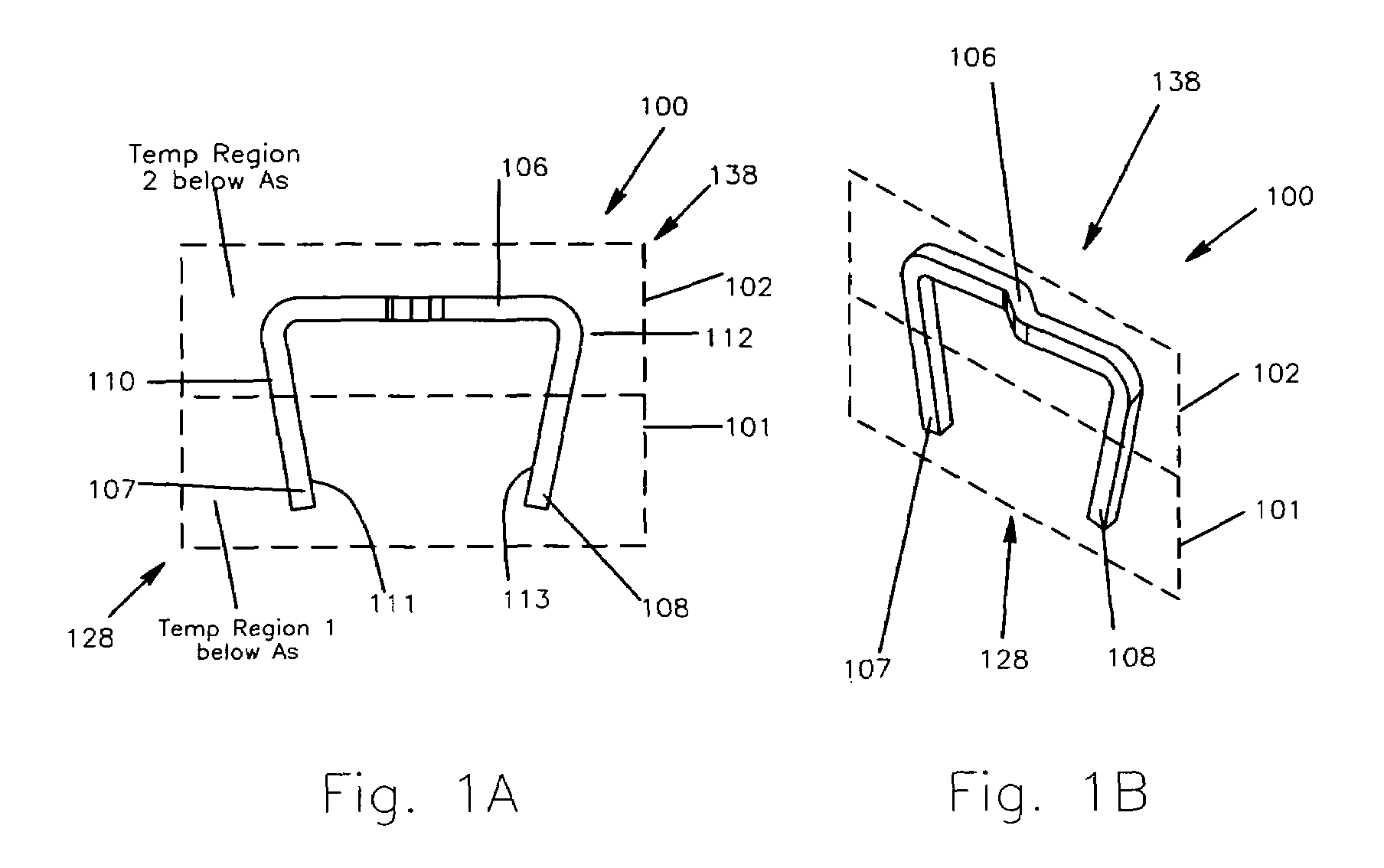
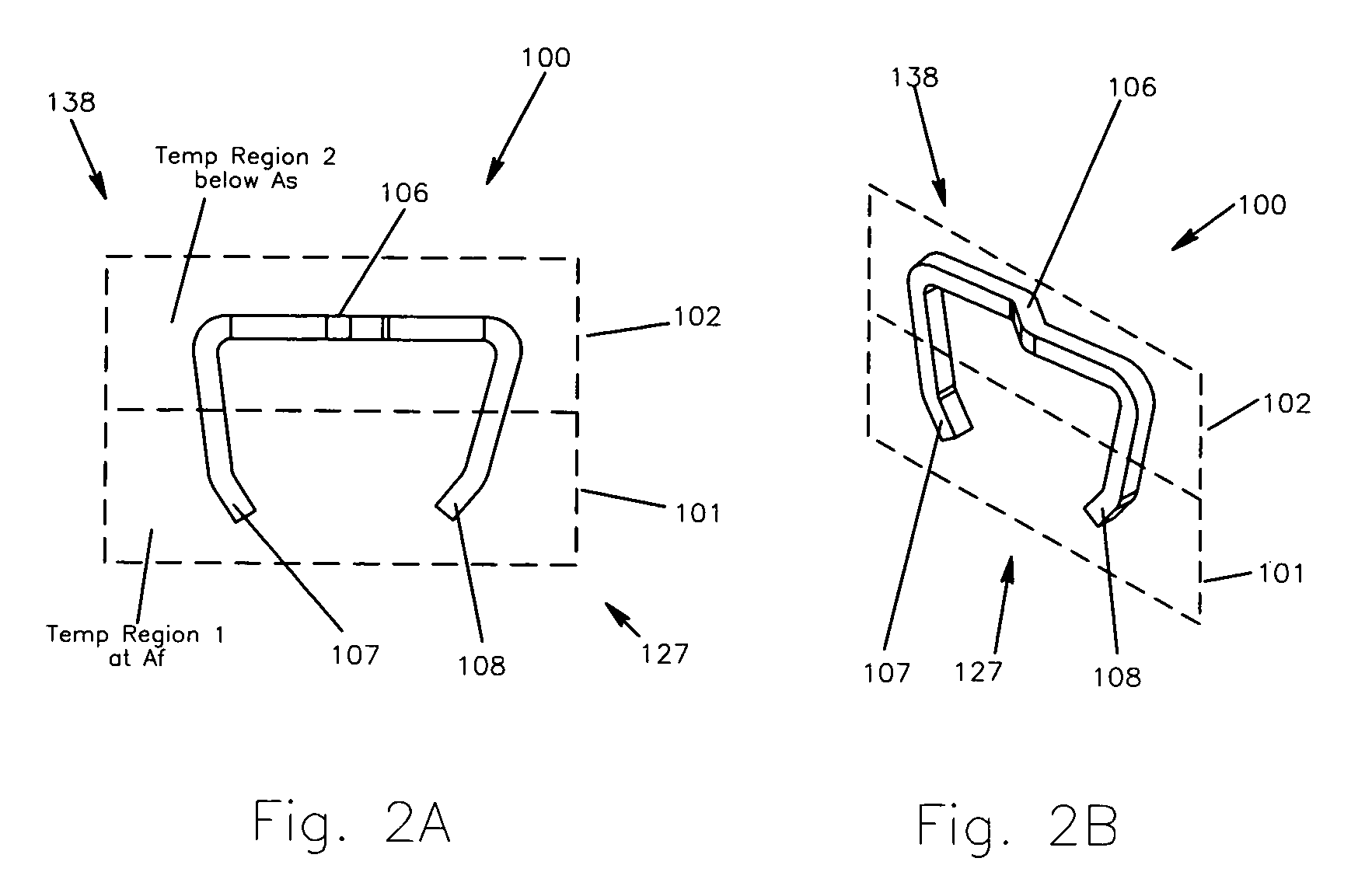
Method and apparatus for a multiple transition temperature implant
A shape-memory device manufactured from shape memory material includes multiple activation temperatures. The multiple activation temperatures arise from either the heat treatment of the device during manufacturing, or by combining different elements with different activation temperatures. To manufacture a shape-memory device with multiple activation temperatures, it is formed into a first shape. A first portion of the shape-memory device is heated to a first temperature, and a second portion of the shape-memory device is heated to a second temperature. The shape-memory device is then worked into a second shape. Accordingly, the first portion has a first transition temperature, and the second portion has a second transition temperature. In use, the shape-memory device is placed into a desired position. Energy is applied such that the first portion, second portion, or both portions are transformed.
Owner:FOX WILLIAM CASEY
Non-metallic implant devices and intra-operative methods for assembly and fixation
This invention relates to orthopedic implants and to methods of treating bone defects. More specifically, but not exclusively, the present invention is directed to non-metallic implants and to methods for intra-operative assembly and fixation of orthopedic implants to facilitate medical treatment. The non-metallic implant assembly can be secured to underlying tissue by a fastener, such as a bone screw, that is capable of swelling on contact with fluid in the underlying tissue. Alternatively, the non-metallic implant assembly can be assembled intra-operatively using a fastener that is adhesively bonded to a bone plate or the bone plate can be deformed using heat, force, or solvents to inhibit withdrawal of the fastener. In preferred embodiments, both the fastener and the bone plate are formed of biodegradable material.
Owner:WARSAW ORTHOPEDIC INC
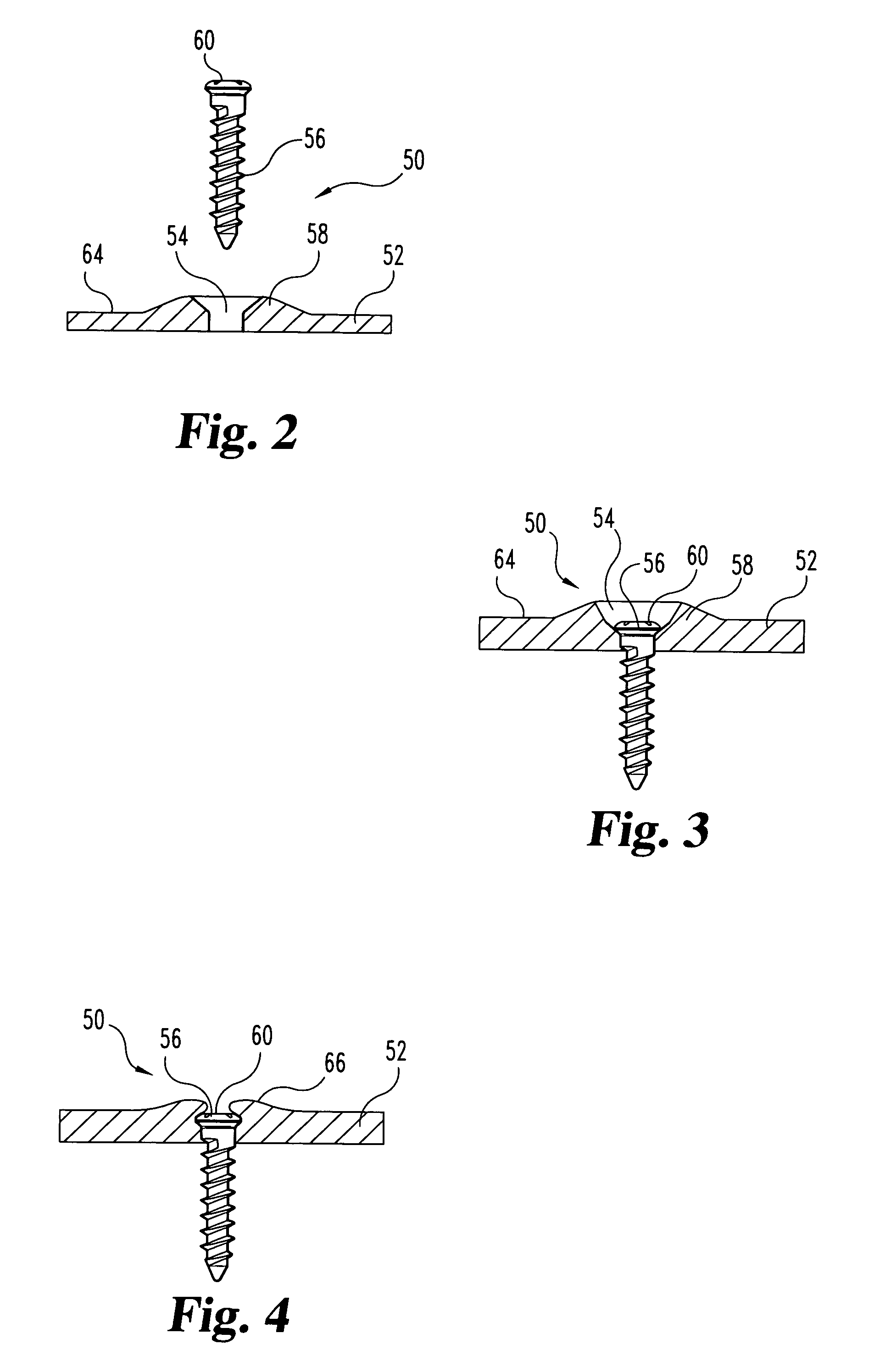
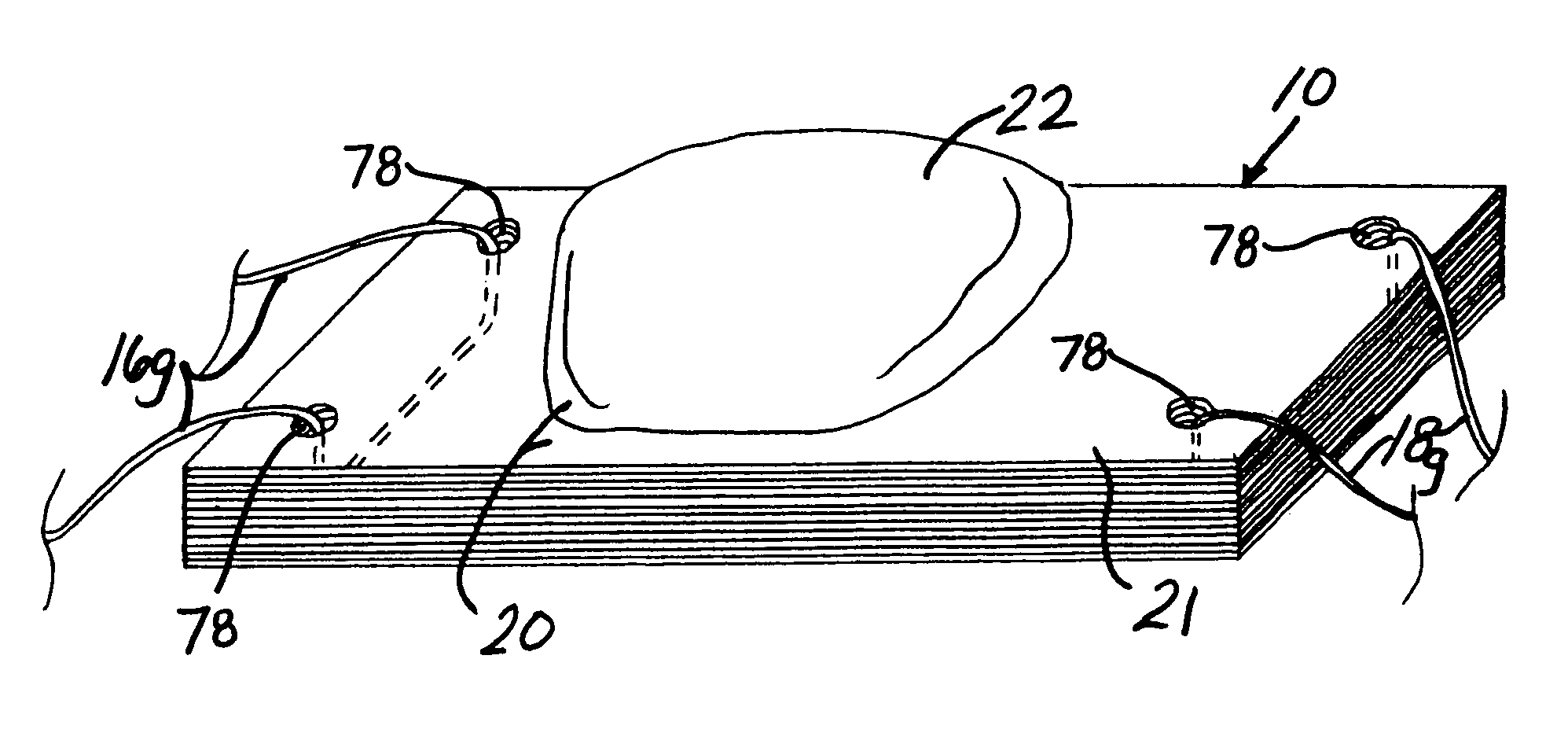
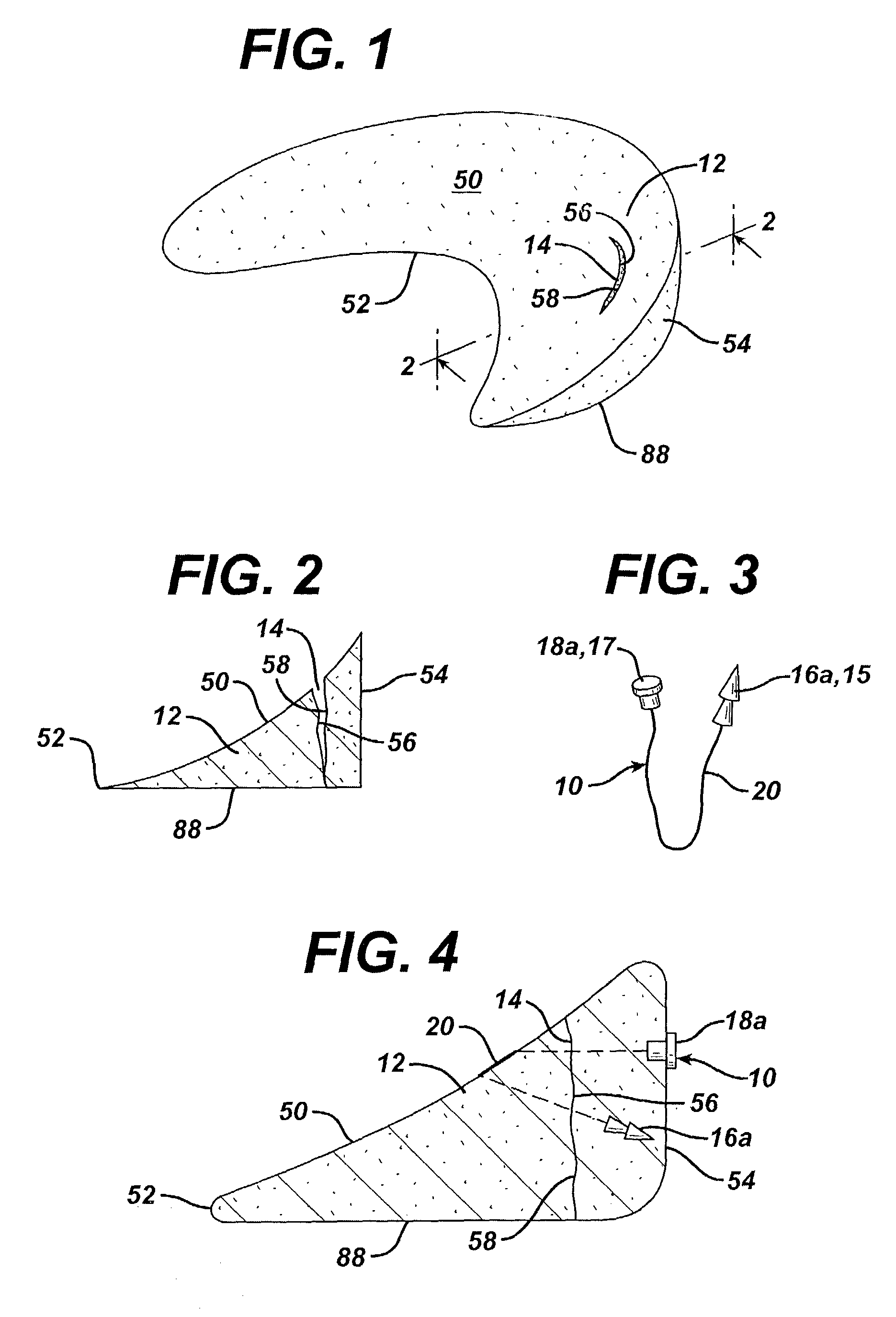
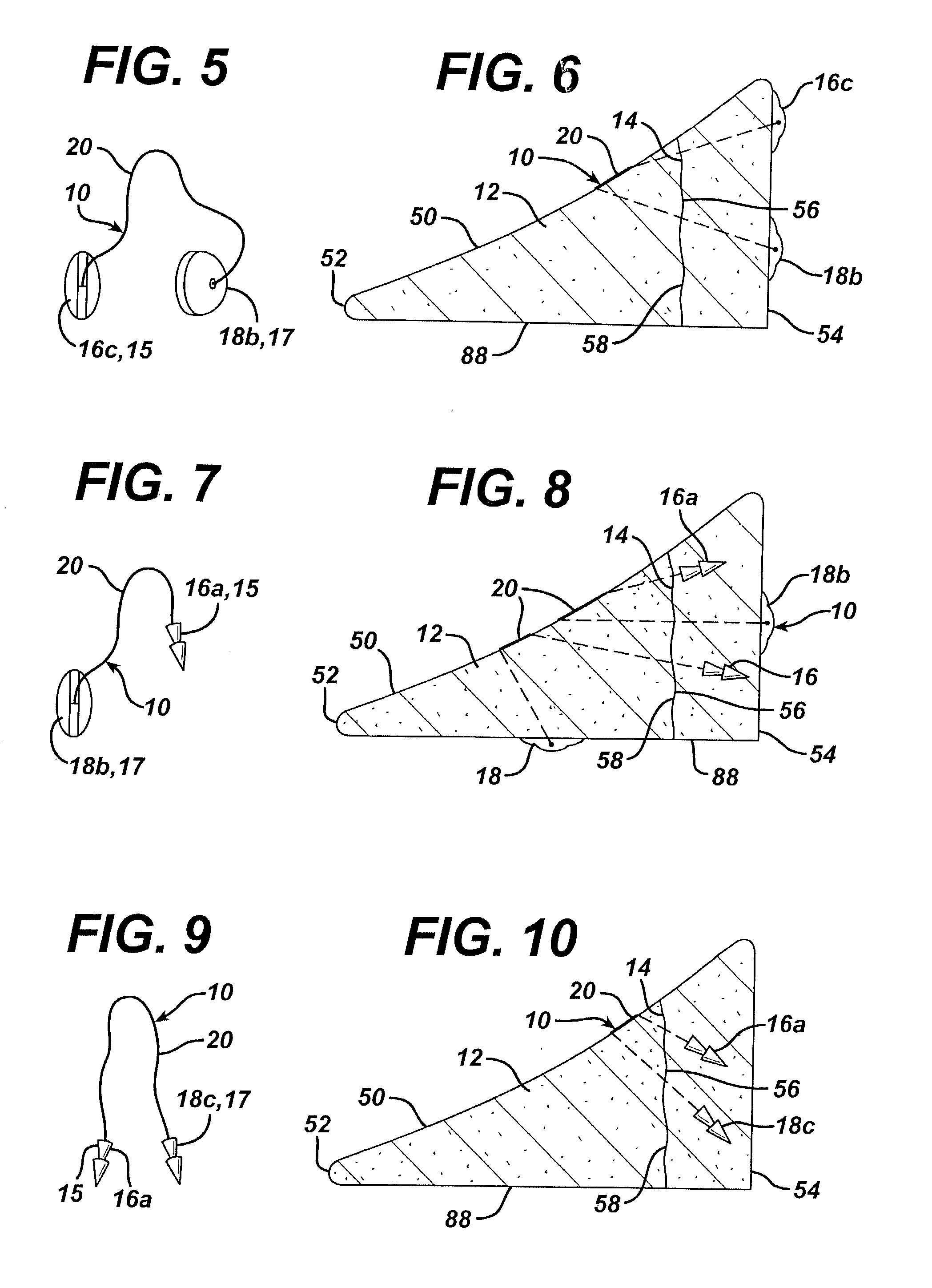
Unitary surgical device and method
Unitary surgical devices (10) are disclosed. One group of the illustrated devices has a pair of biocompatible, bioresorbable anchors (16,18) connected to fixed lengths suture. The anchors (16,18) and fixed length of suture are connected to each other prior to surgery. Another group of unitary surgical devices has a pair of fixating mechanisms (15,17) connected to a base (21) prior to surgery. The second group of illustrated devices generally includes extracellular matrix material either as part of the base (21) or supported on the base (21). The extracellular matrix material serves as tissue regenerating material. In the second group of unitary surgical devices, the fixating mechanisms illustrated generally comprise suture, anchors or pre-formed holes in the base. All of the illustrated unitary surgical devices are useful in repairing a damaged meniscus. The first group of unitary surgical devices can be used to approximate inner surfaces of a tear in the meniscus. The second group of devices can be used either as an insert to be placed between and approximated to the inner surfaces of the tear or as an insert to replace a void in the meniscus left after a meniscectomy.
Owner:DEPUY SYNTHES PROD INC
Prosthesis for replacement of cartilage
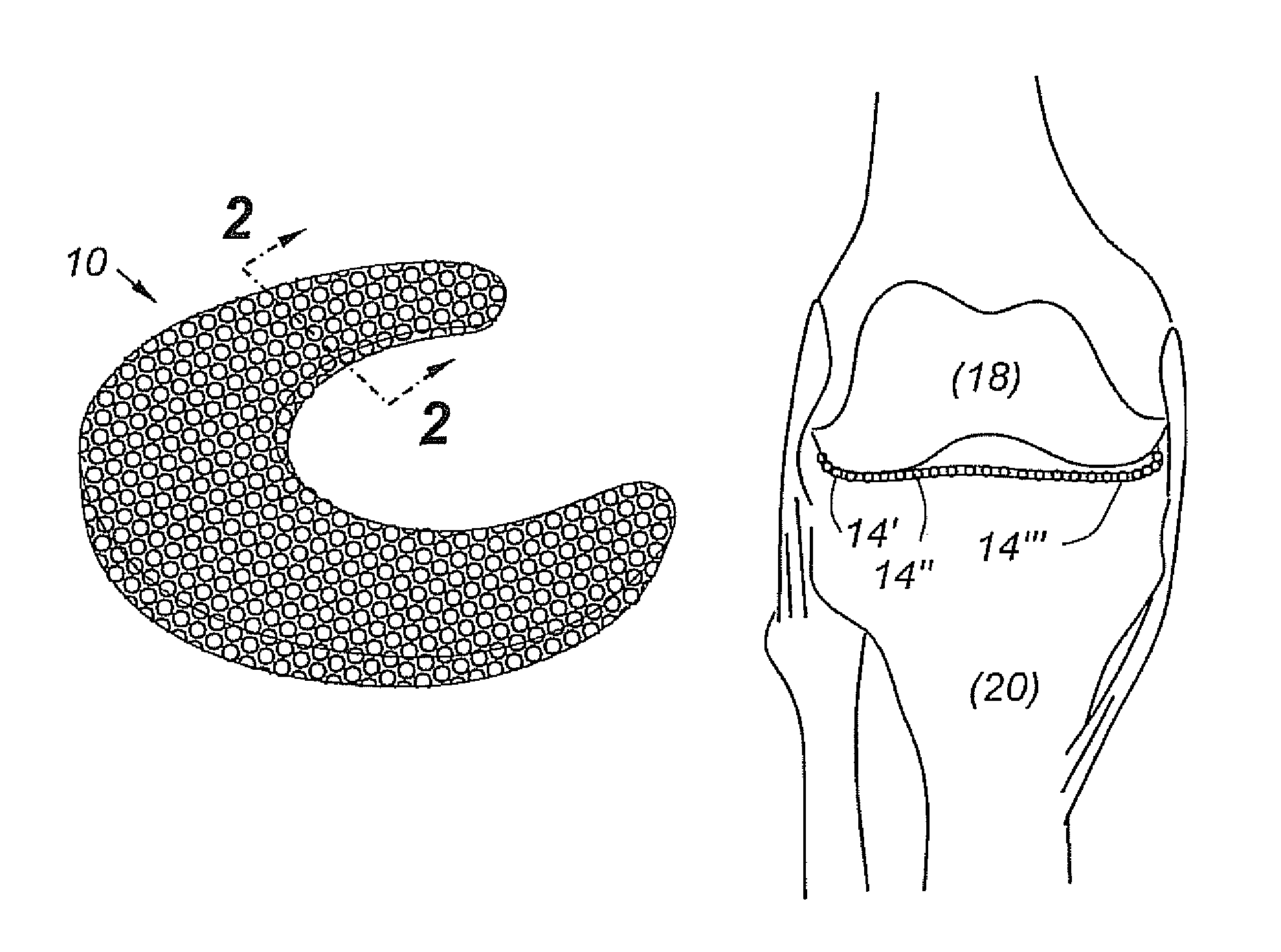
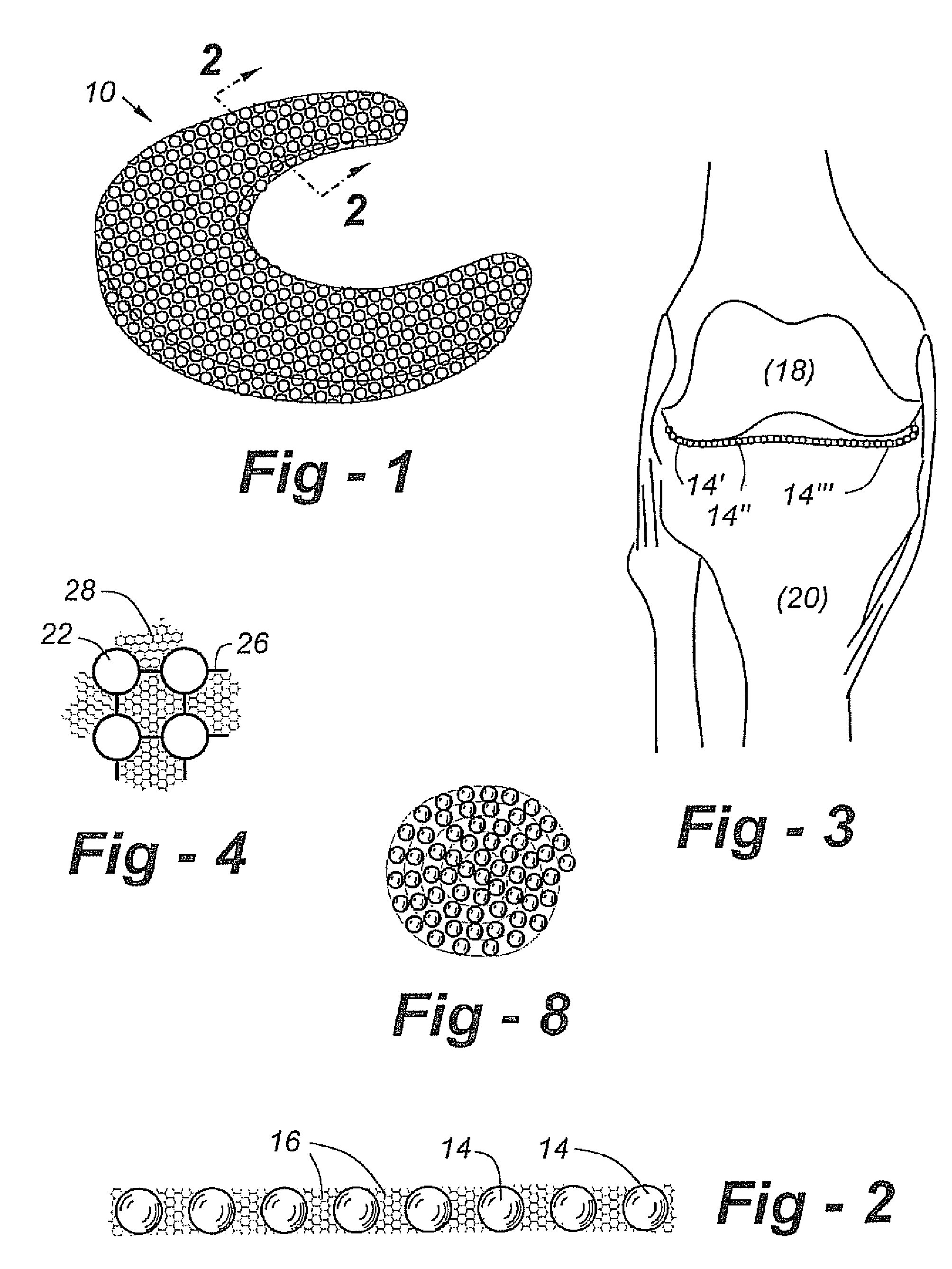
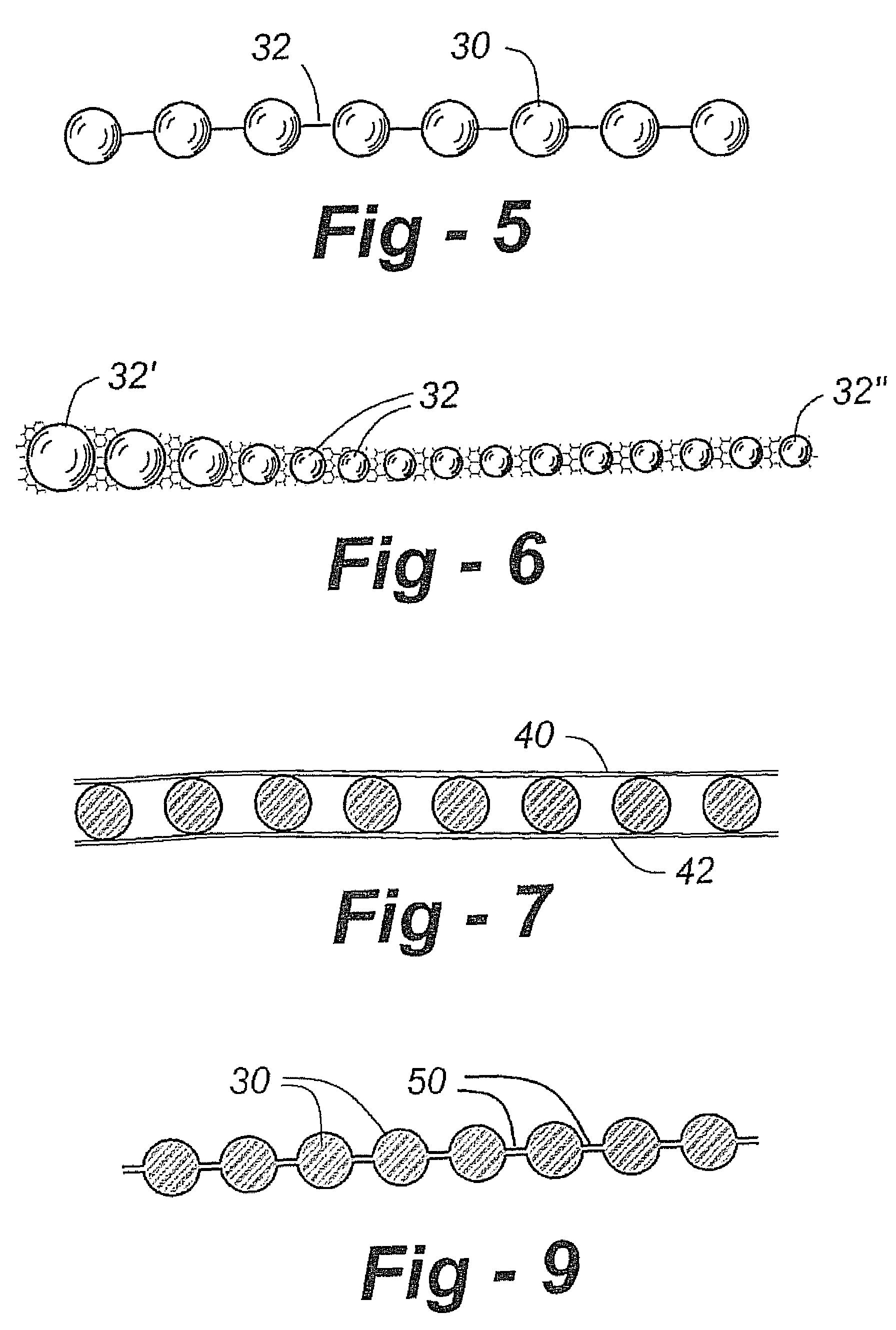
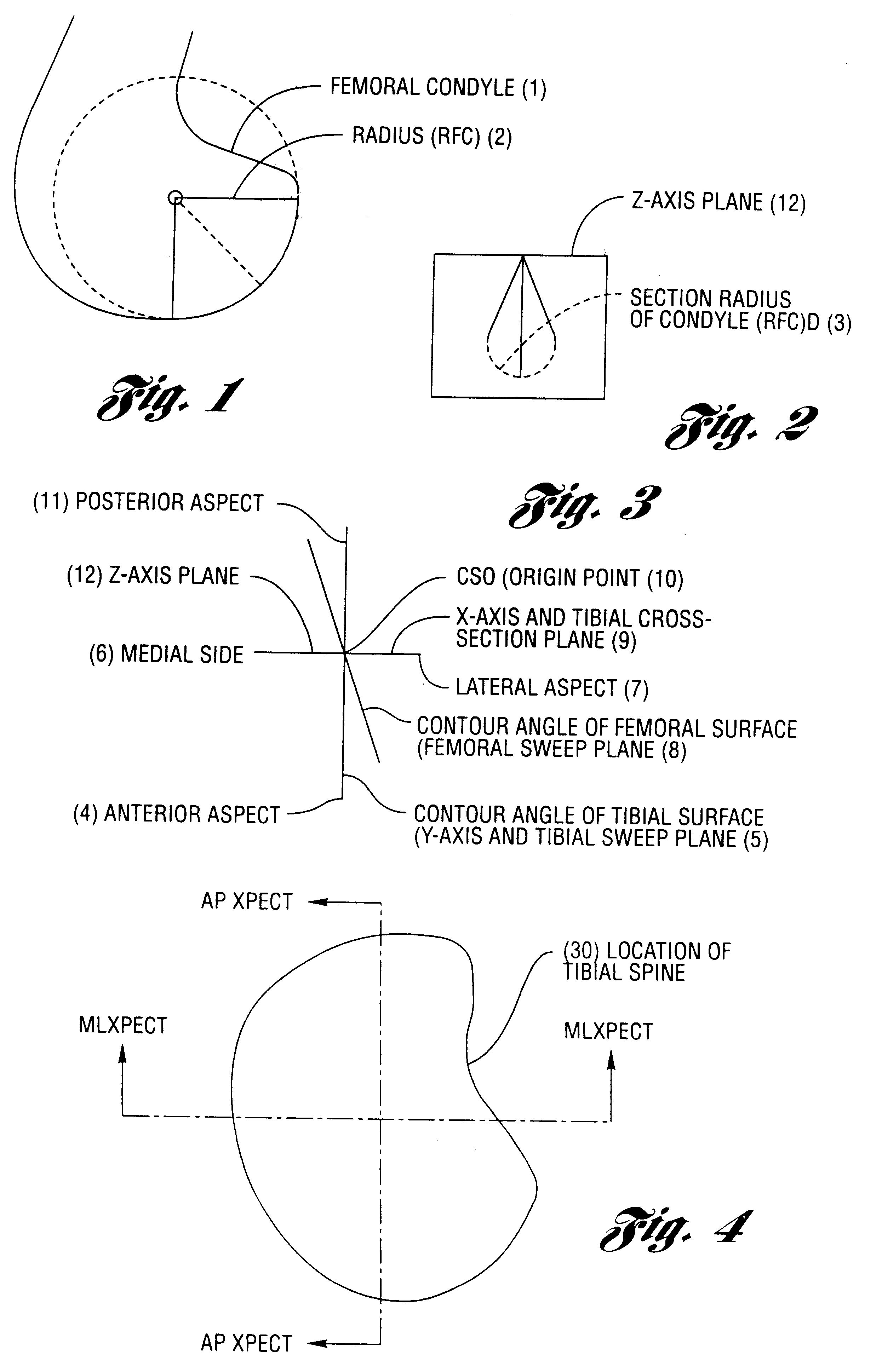
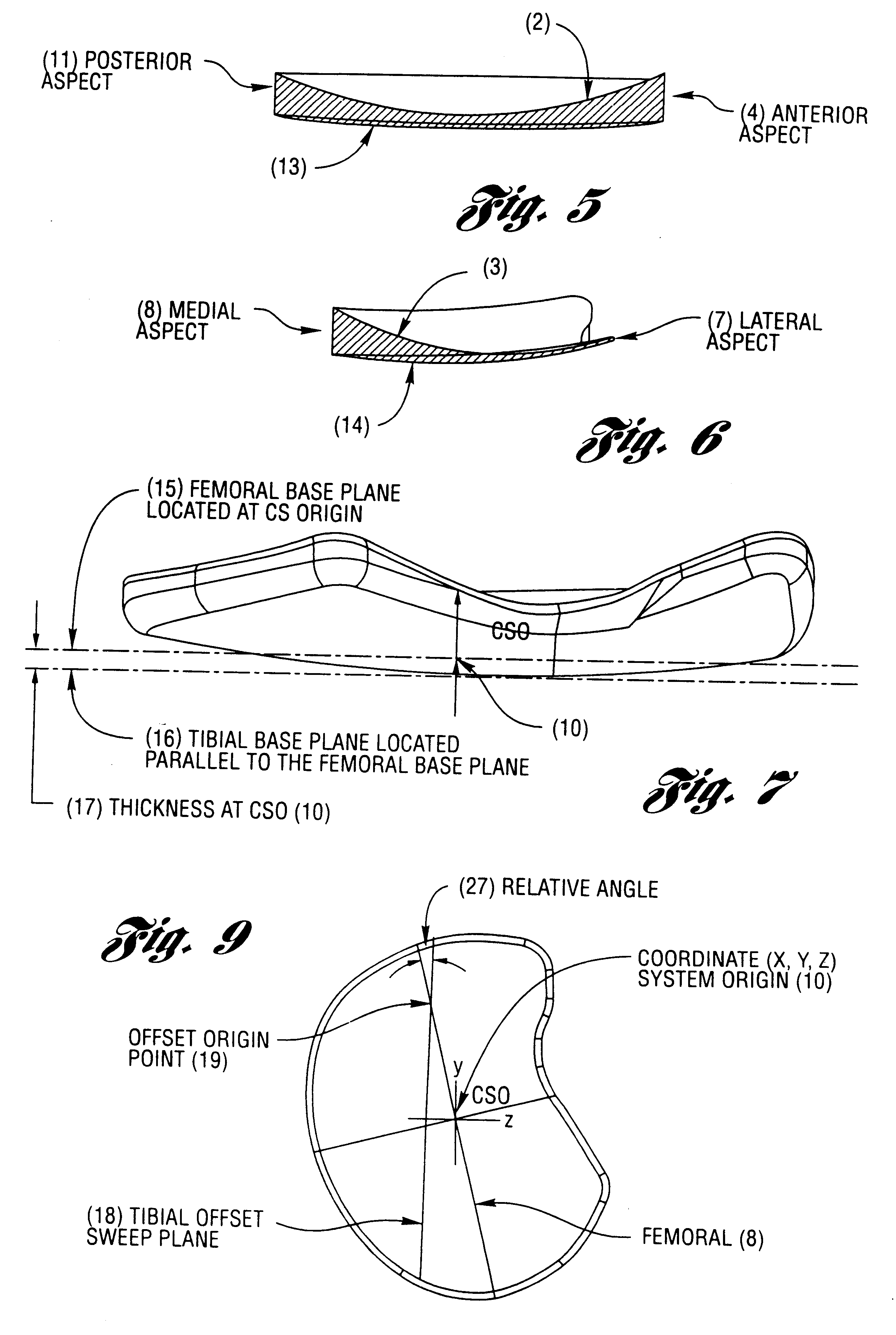
A cartilage replacement or repair prosthesis comprises a layer of streamlined elastomer elements, preferably in the form of spheres, supported in a matrix material so that the radially opposed surfaces of the spheres are positioned on opposite surfaces of the layer and make contact with the opposed surfaces of the femur and tibia and the forces exerted between these bones extend through the streamlined elements. The matrix material has a substantially lower resistance to deformation than the spheres to control the position of the spheres relative to one another without significantly restraining their load-responsive deformation under forces exerted between the femur and tibia. The layer, with its elastomeric inserts, is sufficiently thin and flexible to allow it to be rolled for arthroscopic insertion into a knee joint.
Owner:SUCCESSOR TRUSTEE OF THE EUGENE RIVIN LIVING TRUST +2
Tissue-derived mesh for orthopedic regeneration
An implant including a substantially cohesive aggregate comprising bone-derived particles. Cohesiveness is maintained by a member of mechanical interlocking, engagement of adjacent bone-derived particles with one another through engagement with a binding agent, thermal bonding, chemical bonding, or a matrix material in which the bone-derived particles are retained. The aggregate is shaped as a one-dimensional or two-dimensional body.
Owner:WARSAW ORTHOPEDIC INC
Hybrid biologic-synthetic bioabsorbable scaffolds
ActiveUS8366787B2Increase surface areaGood mechanical integritySuture equipmentsBone implantBioabsorbable scaffoldCell-Extracellular Matrix
A bioprosthetic device is provided for soft tissue attachment, reinforcement, and or reconstruction. The device comprises a naturally occurring extracellular matrix portion and a three-dimensional synthetic portion. In illustrated embodiments, the naturally occurring extracellular matrix portion comprises layers of small intestine submucosa, and the three-dimensional synthetic portion comprises a foam or a three-dimensional mesh, textile, or felt.
Owner:DEPUY SYNTHES PROD INC
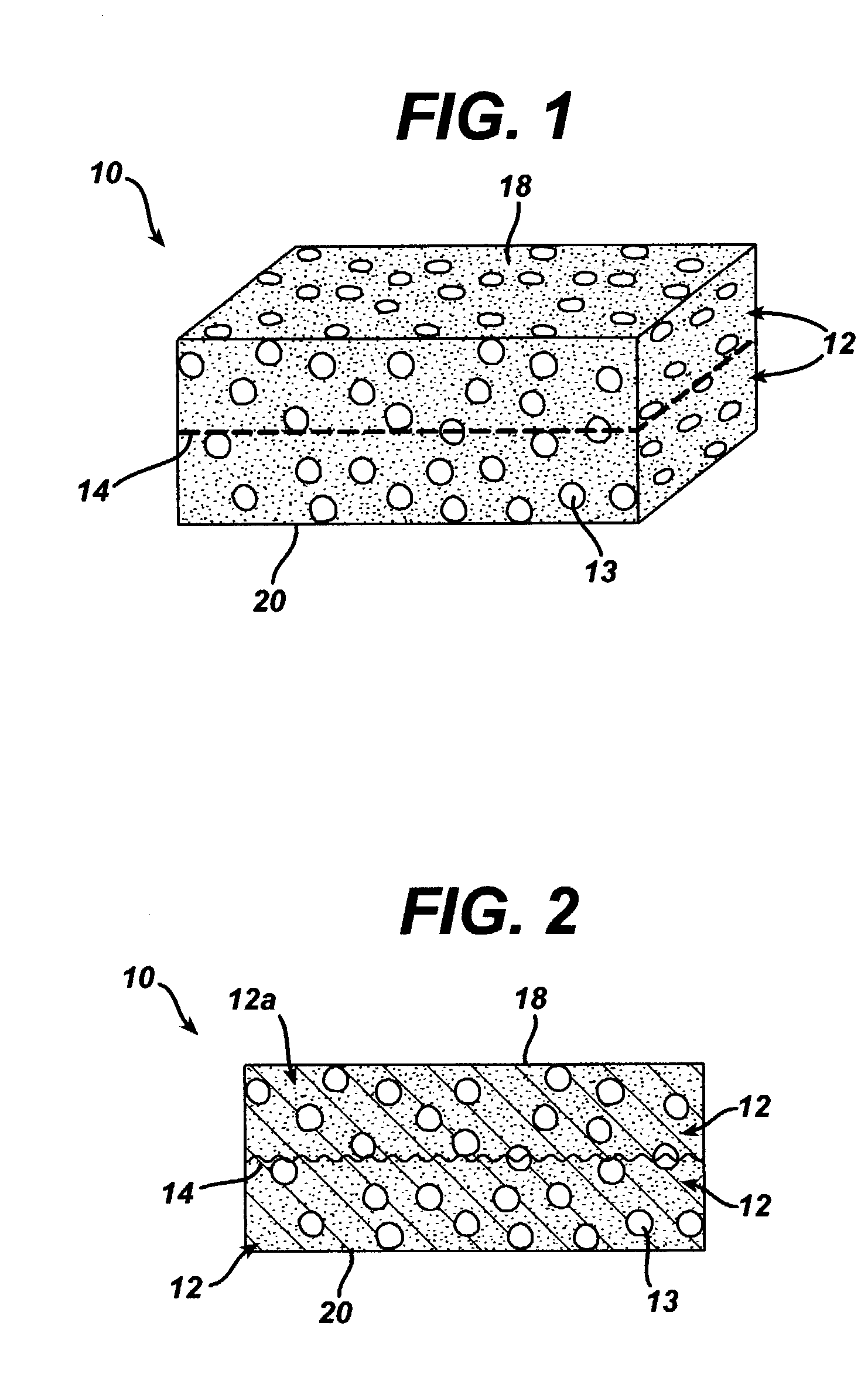
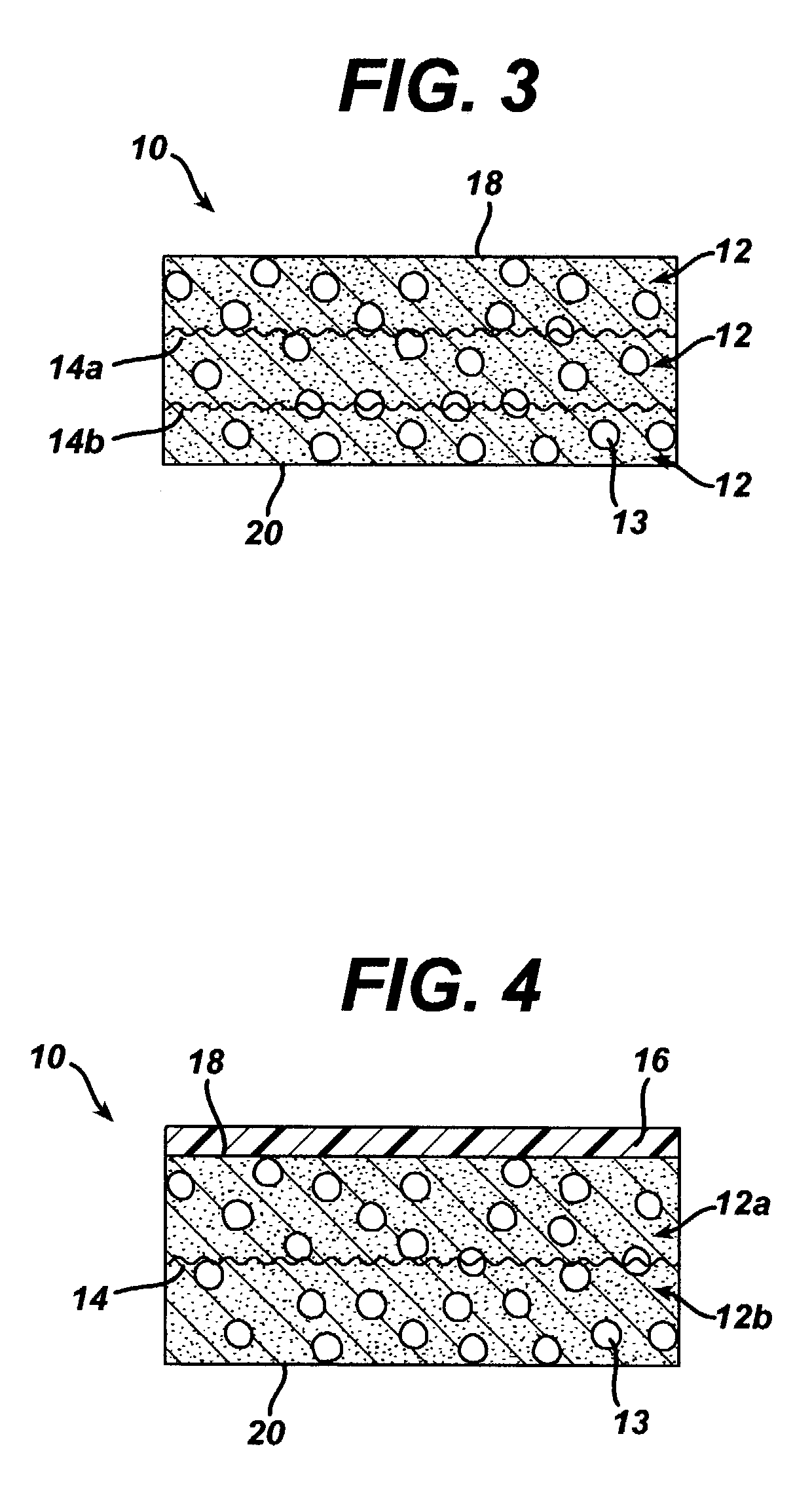
Method of preparation of bioabsorbable porous reinforced tissue implants and implants thereof
A biocompatible tissue implant. The tissue implant may be bioabsorbable, consists of a biocompatible polymeric foam. The tissue implant also includes a biocompatible reinforcement member. The polymeric foam and the reinforcement member are soluble in a lyophilizing solvent. The reinforcement may be annealed and / or coated.
Owner:DEPUY MITEK INC
Method of preparation of bioabsorbable porous reinforced tissue implants and implants thereof
Owner:DEPUY SYNTHES PROD INC
Unitary surgical device and method
Owner:DEPUY SYNTHES PROD INC
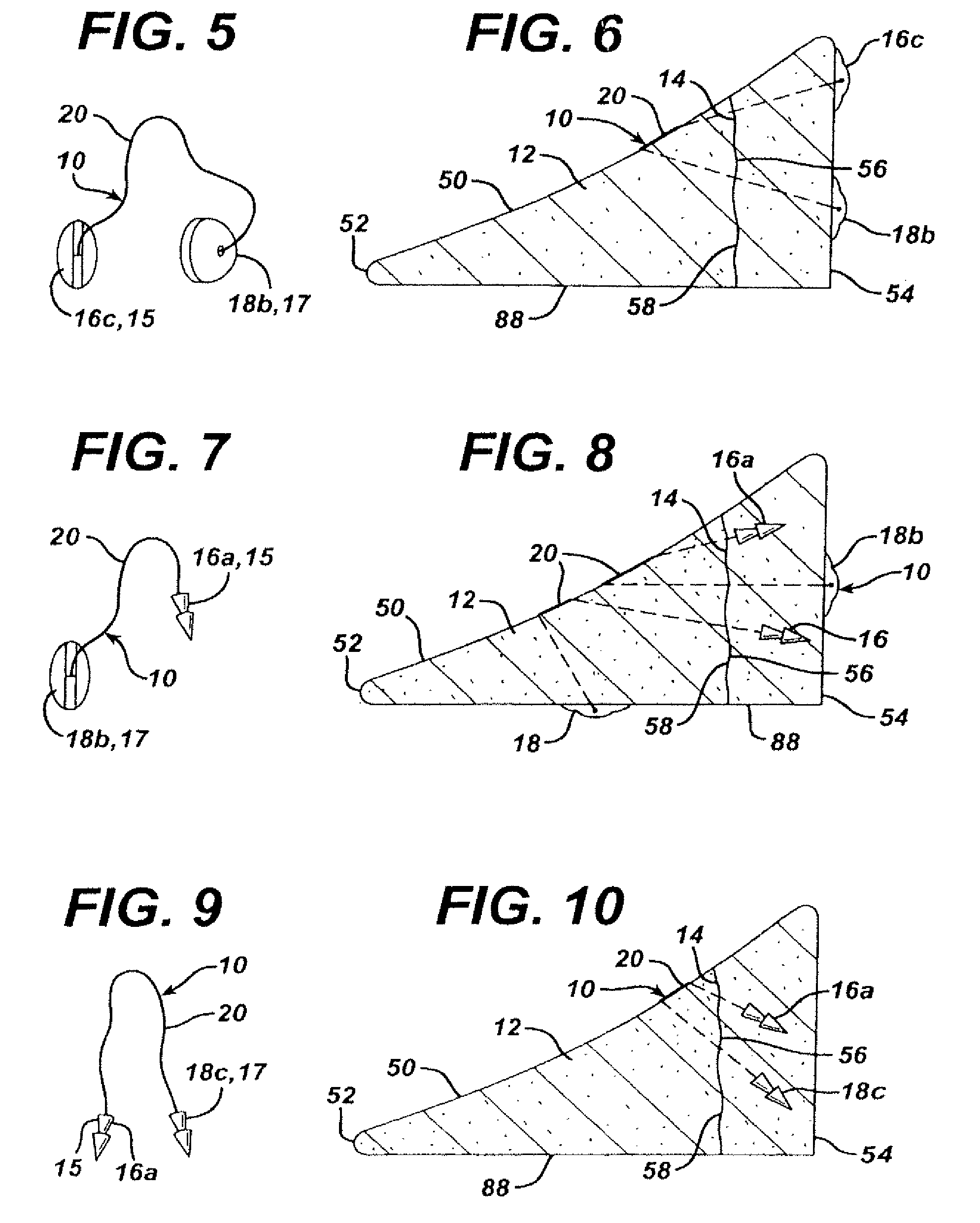
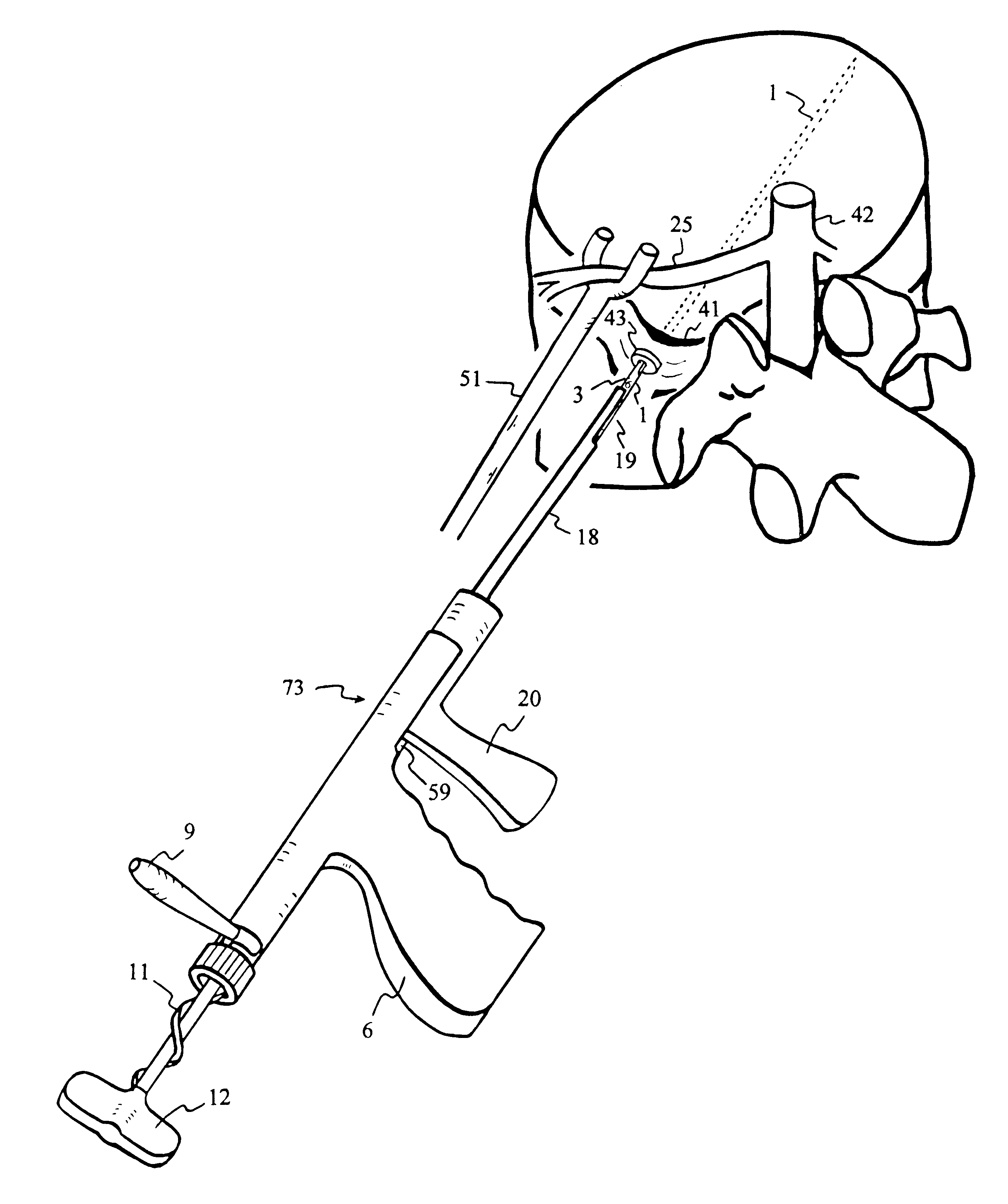
Methods and devices for fastening bulging or herniated intervertebral discs
InactiveUS6530933B1Increase speedShorten post-surgical hospital staysSuture equipmentsDiagnosticsEngineeringIliac screw
Methods and apparatuses for compressing and holding a bulging or herniated intervertebral disc. A number of fastener embodiments are disclosed, including a resiliently curved fastener, suture, screw, staple, tack and others. The curved fastener is resiliently straightened and loaded into a needle for delivery and deployment. In the intervertebral disc, the deployed fastener resumes the curvature to grip and hold the bulge. Alternatively, the suture is anchored and used to tie down the bulging tissue. The screw, staple or tack can also be used to securely fasten, compress and hold the bulge from impinging upon adjacent nerves or tissues.
Owner:KENSEY NASH CORP
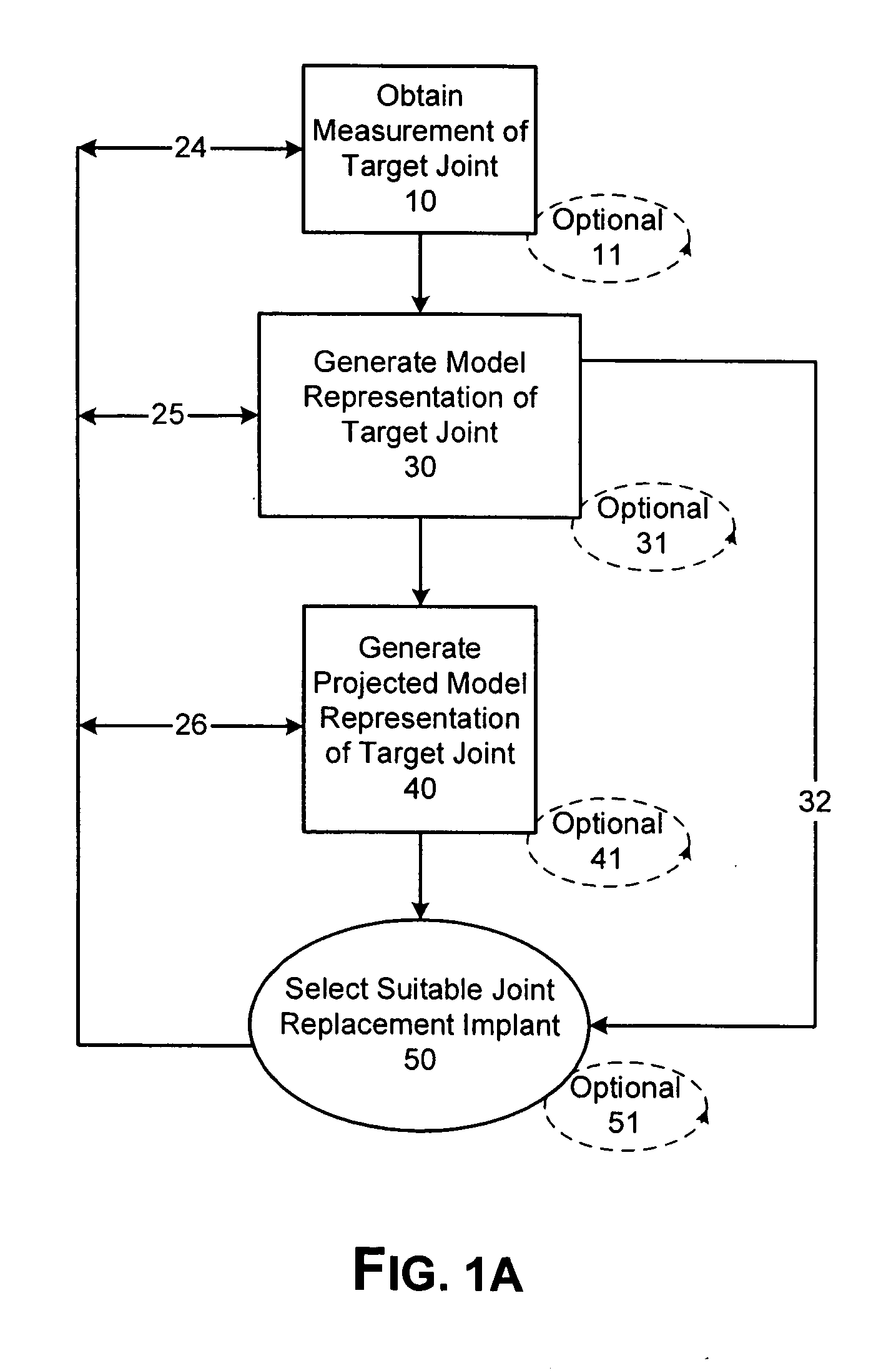
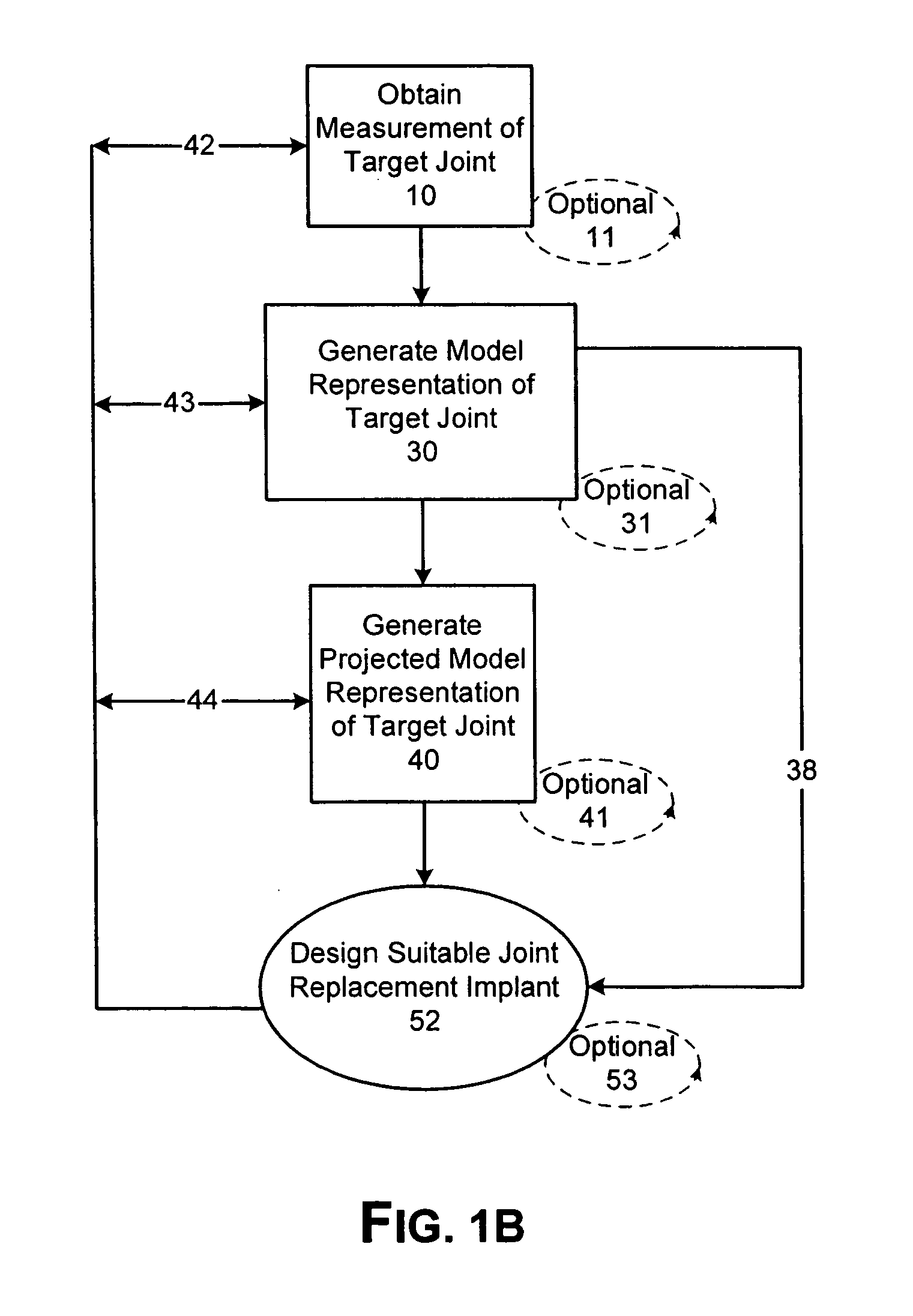
Patient Selectable Joint Arthroplasty Devices and Surgical Tools
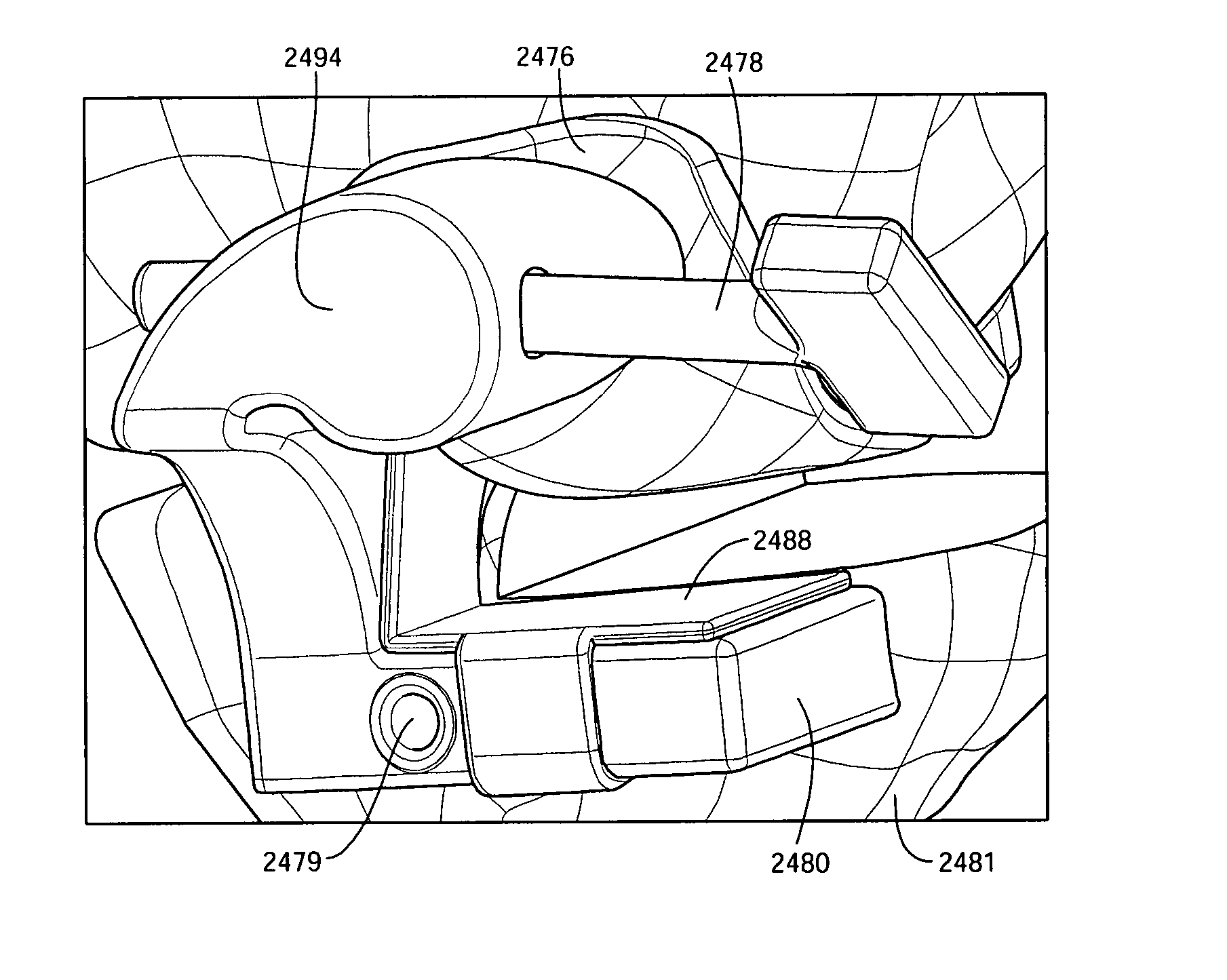
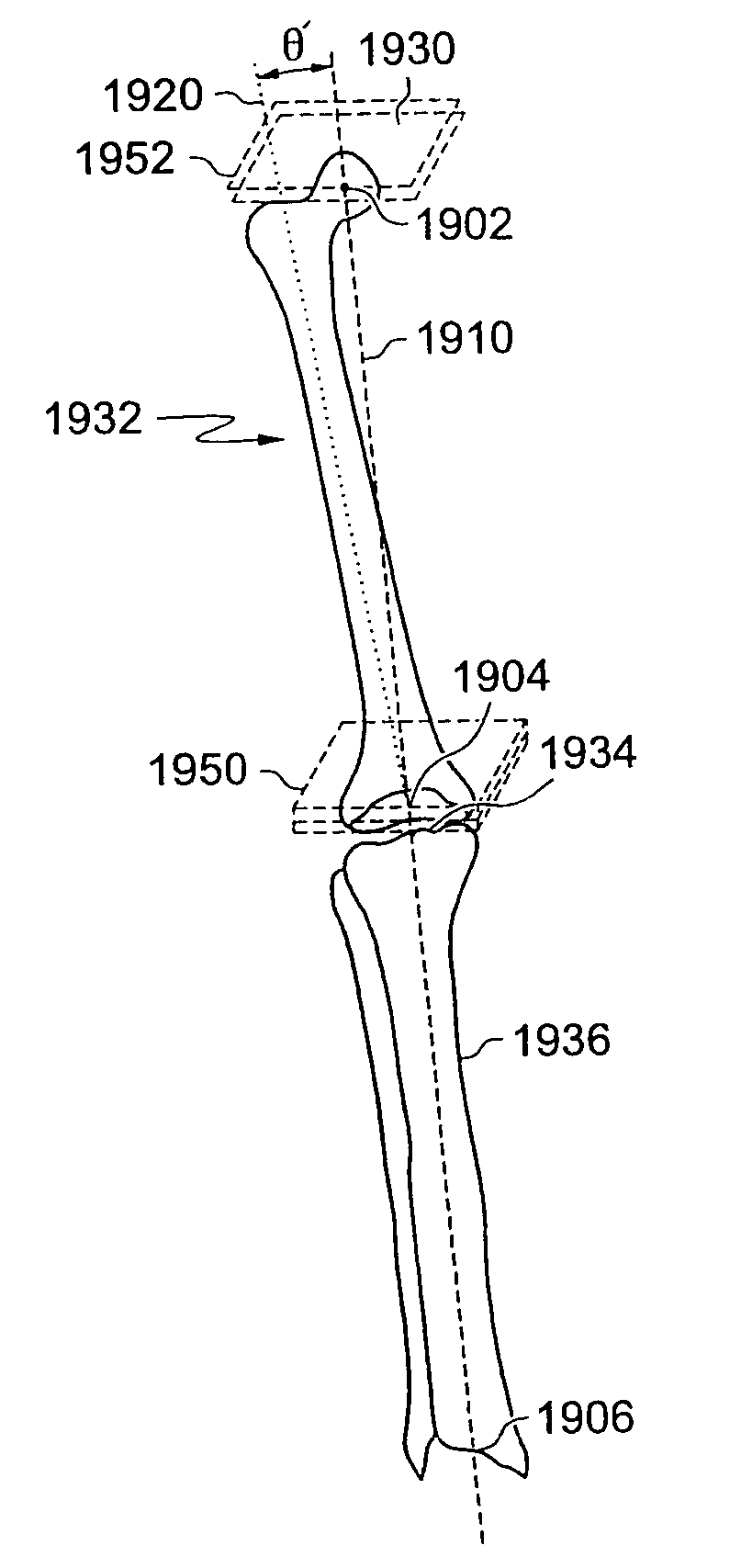
ActiveUS20070198022A1Accurate placementGeometric CADPerson identificationArticular surfacesArticular surface
Disclosed herein are methods, compositions and tools for repairing articular surfaces repair materials and for repairing an articular surface. The articular surface repairs are customizable or highly selectable by patient and geared toward providing optimal fit and function. The surgical tools are designed to be customizable or highly selectable by patient to increase the speed, accuracy and simplicity of performing total or partial arthroplasty.
Owner:CONFORMIS
Surgically implantable knee prothesis
A self-centering meniscal prosthesis device suitable for minimally invasive, surgical implantation into the cavity between a femoral condyle and the corresponding tibial plateau is composed of a hard, high modulus material shaped such that the contour of the device and the natural articulation of the knee exerts a restoring force on the free-floating device.
Owner:CENTPULSE ORTHOPEDICS
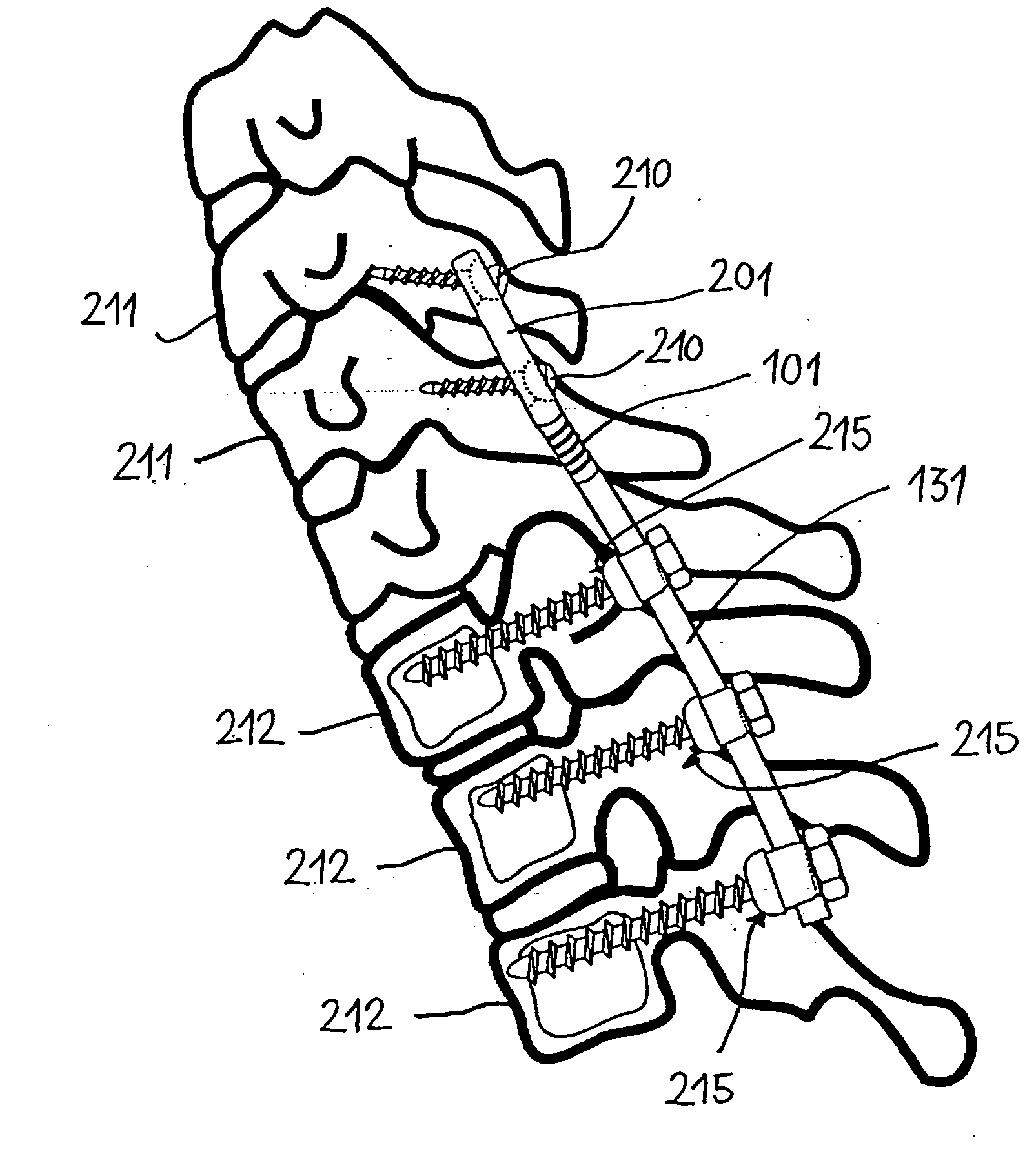
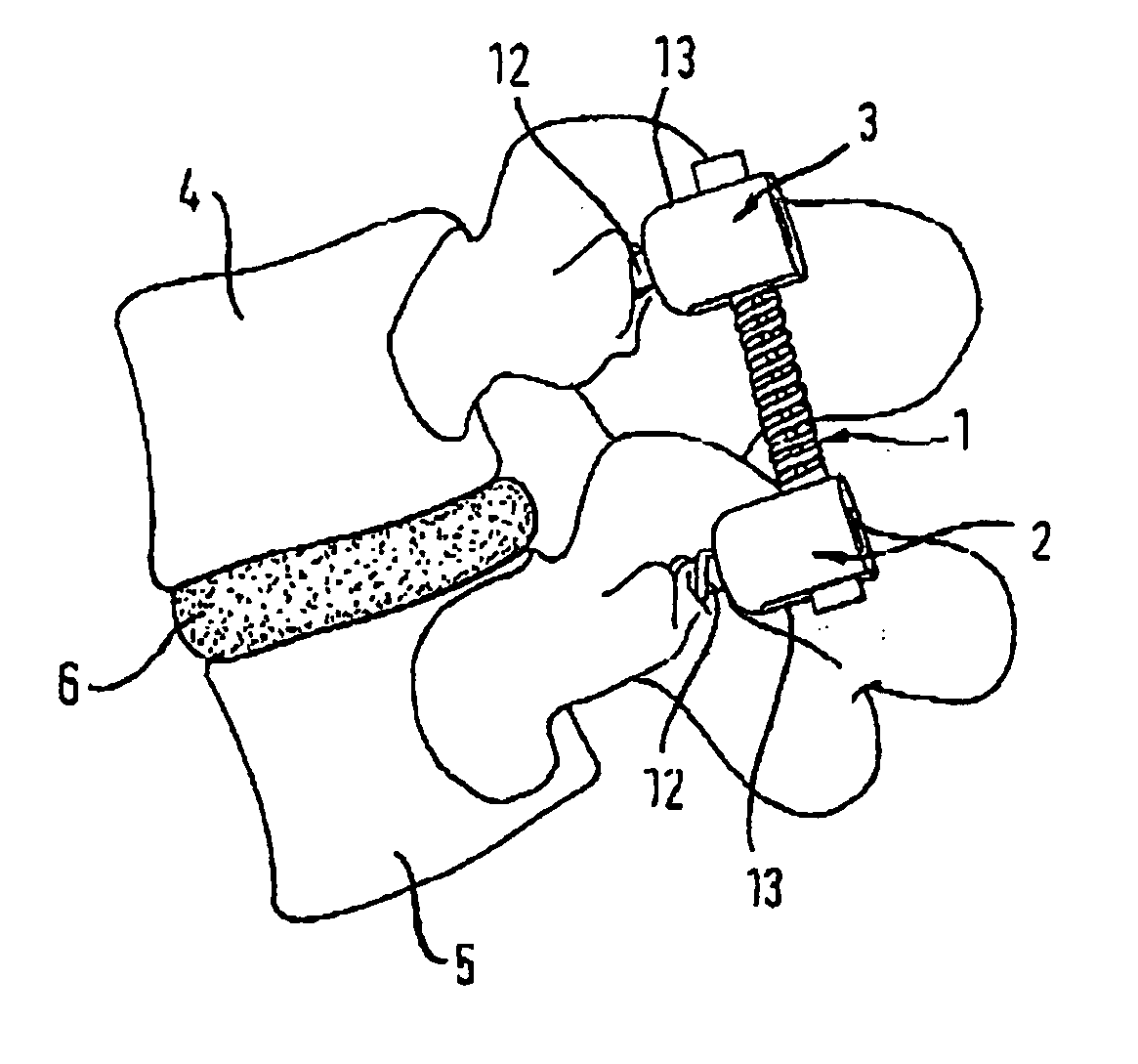
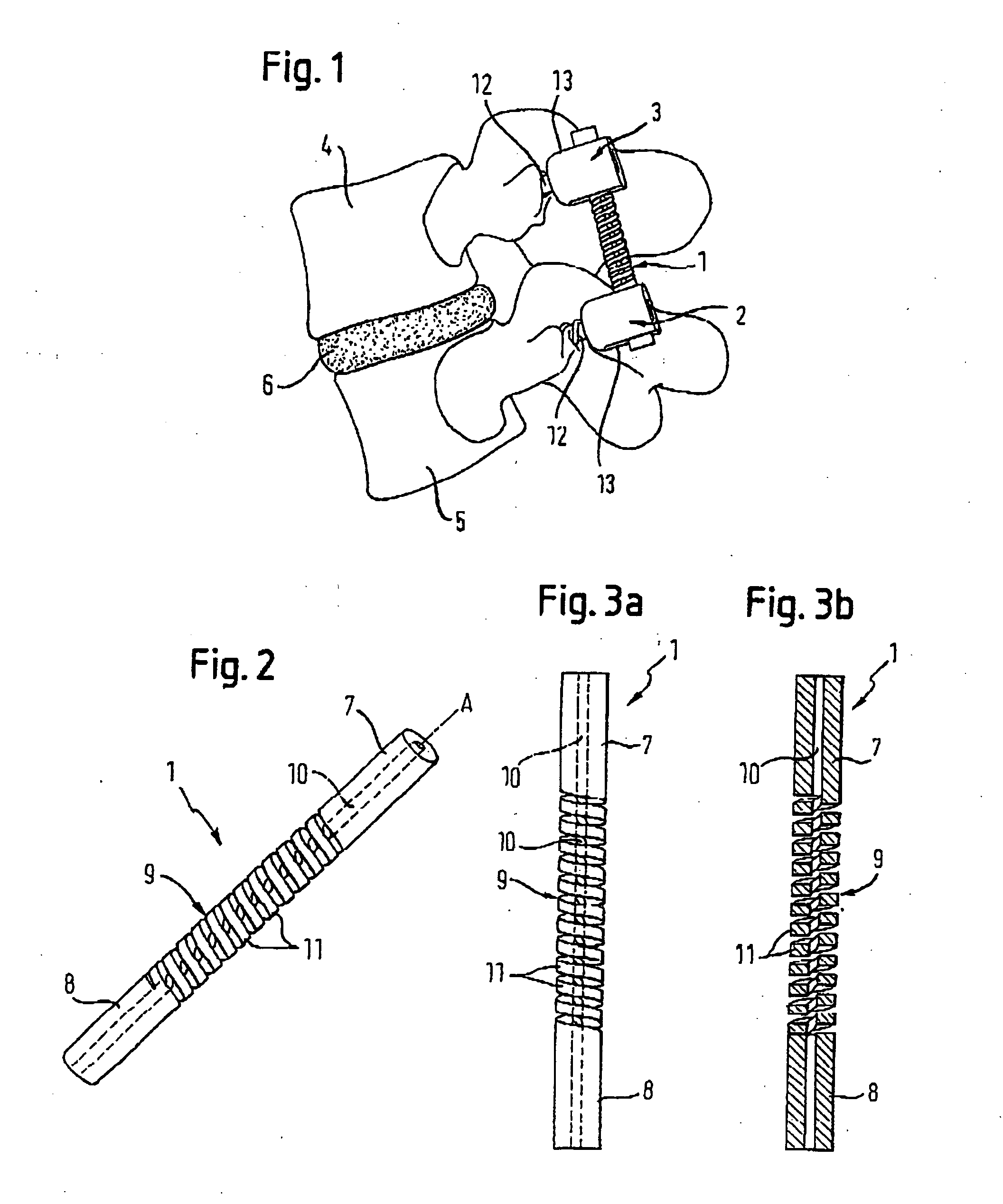
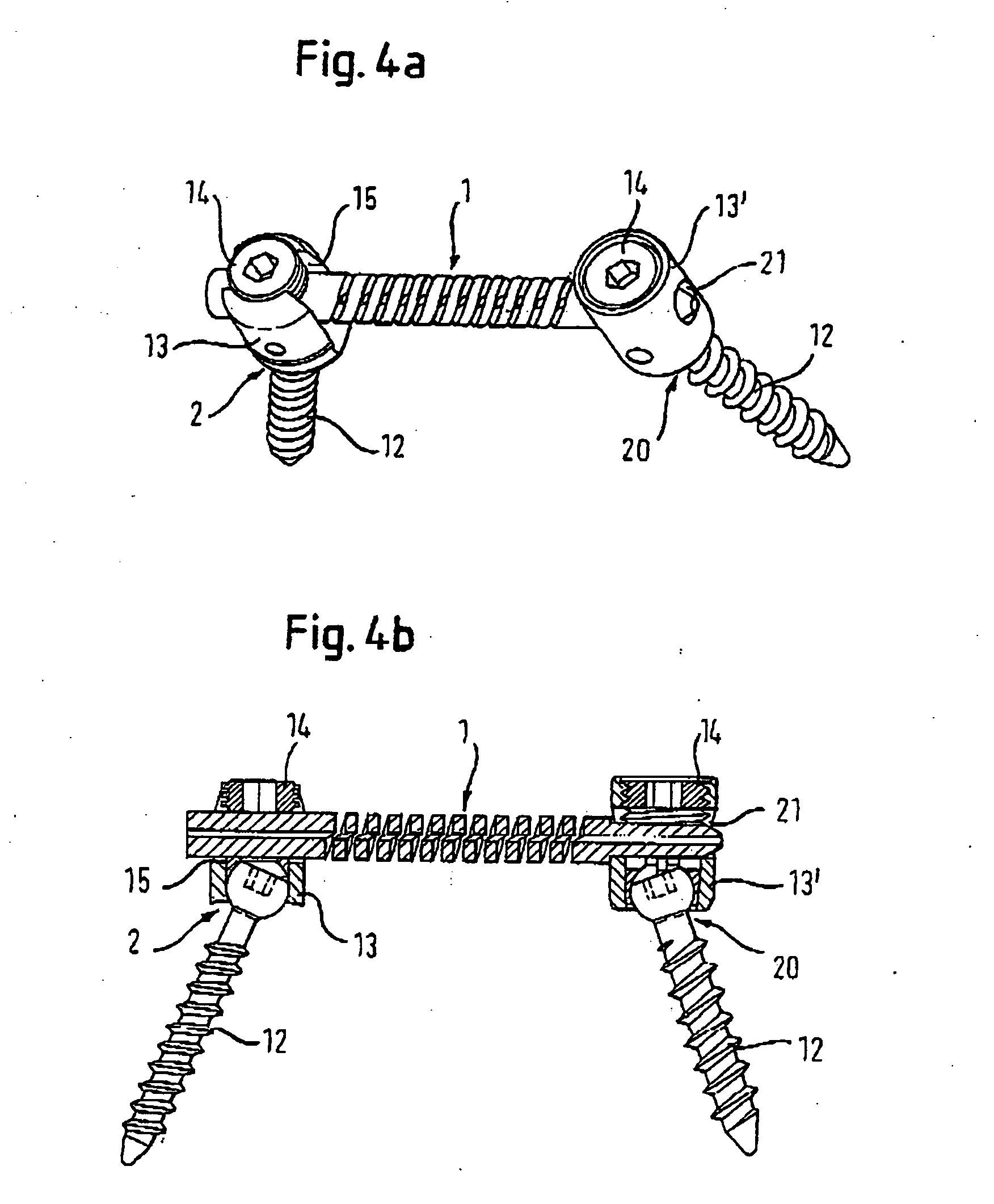
Spinal stabilization systems and methods
InactiveUS7250052B2Minimize damageProvide stabilitySuture equipmentsInternal osteosythesisMinimally invasive proceduresSkin incision
A spinal stabilization system may be formed in a patient. In some embodiments, a minimally invasive procedure may be used to form a spinal stabilization system in a patient. Bone fastener assemblies may be coupled to vertebrae. Each bone fastener assembly may include a bone fastener and a collar. The collar may be rotated and / or angulated relative to the bone fastener. Detachable members may be coupled to the collar to allow for formation of the spinal stabilization system through a small skin incision. The detachable members may allow for alignment of the collars to facilitate insertion of an elongated member in the collars. An elongated member may be positioned in the collars and a closure member may be used to secure the elongated member to the collars.
Owner:HIGHRIDGE MEDICAL LLC
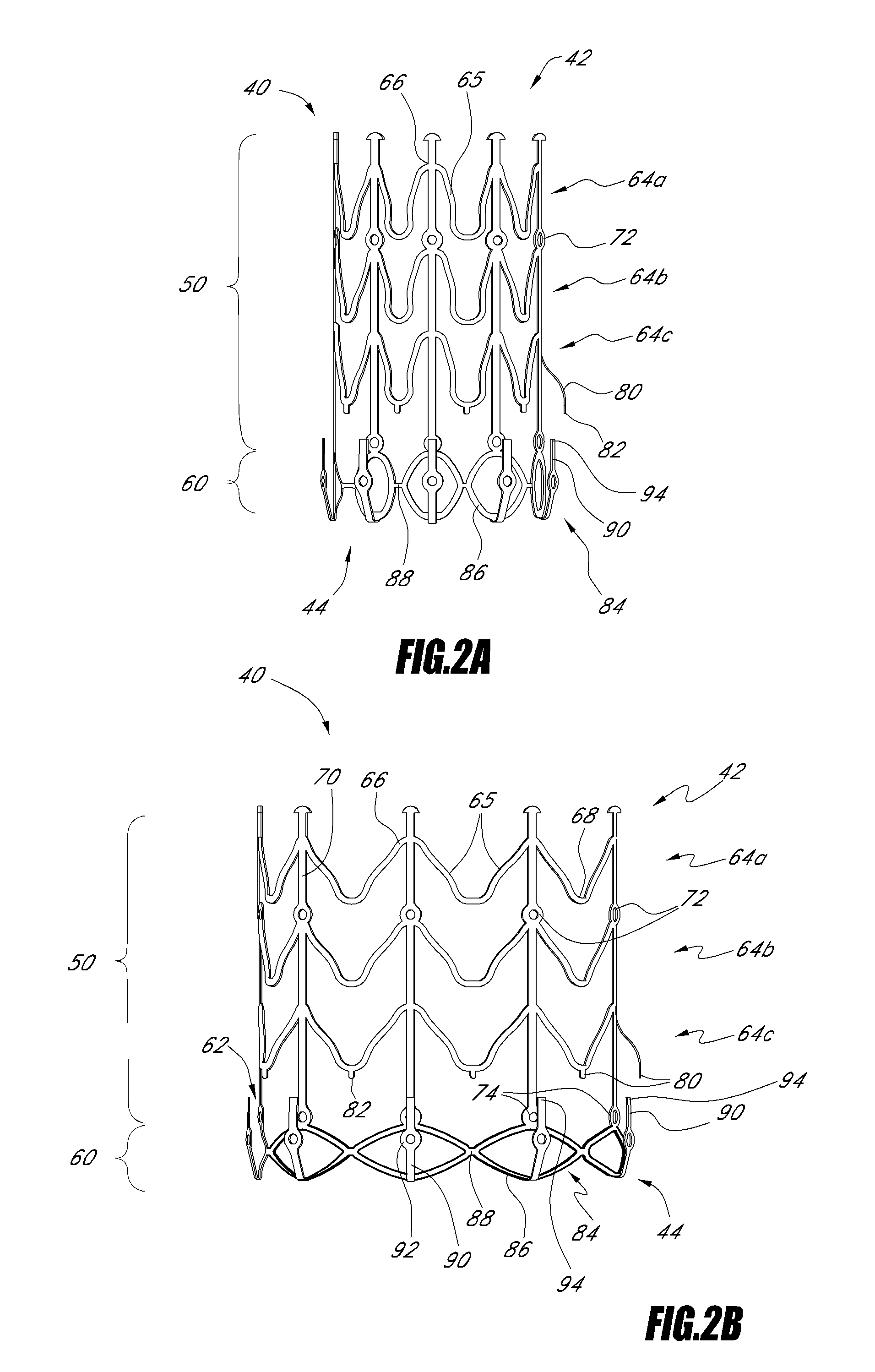
Vascular implant and delivery system
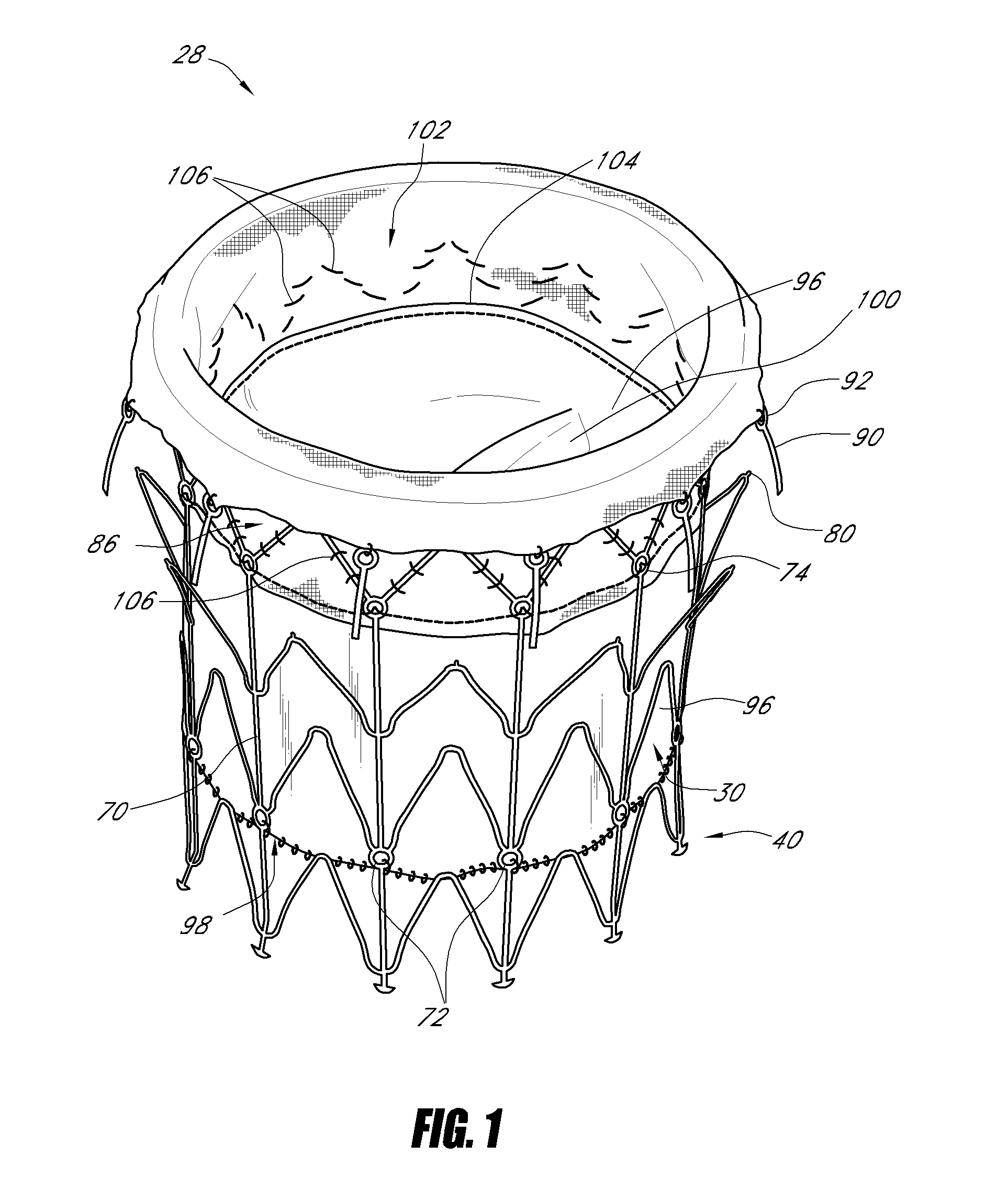
ActiveUS20100298931A1Reliable and controlled mannerStentsHeart valvesVascular implantInsertion stent
A vascular implant for replacing a native heart valve comprises a self expanding stent supporting a valve body having leaflets. The stent preferably comprises an anchoring structure configured to prevent the implant from passing through the valve annulus. For delivery, the implant is compacted within a delivery device and secured at one end. During delivery the implant is partially released from the delivery device, and positioning of the implant is verified prior to full release. The implant can be at least partially resheathed and repositioned if desired.
Owner:EDWARDS LIFESCI CARDIAQ
Porous Substrates for Implantation
InactiveUS20100137990A1Easy to integrateGood biocompatibilitySuture equipmentsDental implantsPorous substrateAnimal body
A porous substrate or implant for implantation into a human or animal body constructed from a structural material and having one or more regions which when implanted are subjected to a relatively lower mechanical loading. The region(s) are constructed with lesser mechanical strength by having a lesser amount of structural material in said region(s) relative to other regions. This is achieved by controlling pore volume fraction in the regions. A spacer is adapted to define an open-cell pore network by taking a model of the required porous structure, and creating the spacer to represent the required porous structure using three-dimensional modelling. Material to form the substrate about the spacer in infiltrated the scaffold structure formed.
Owner:NATIONAL UNIVERSITY OF IRELAND
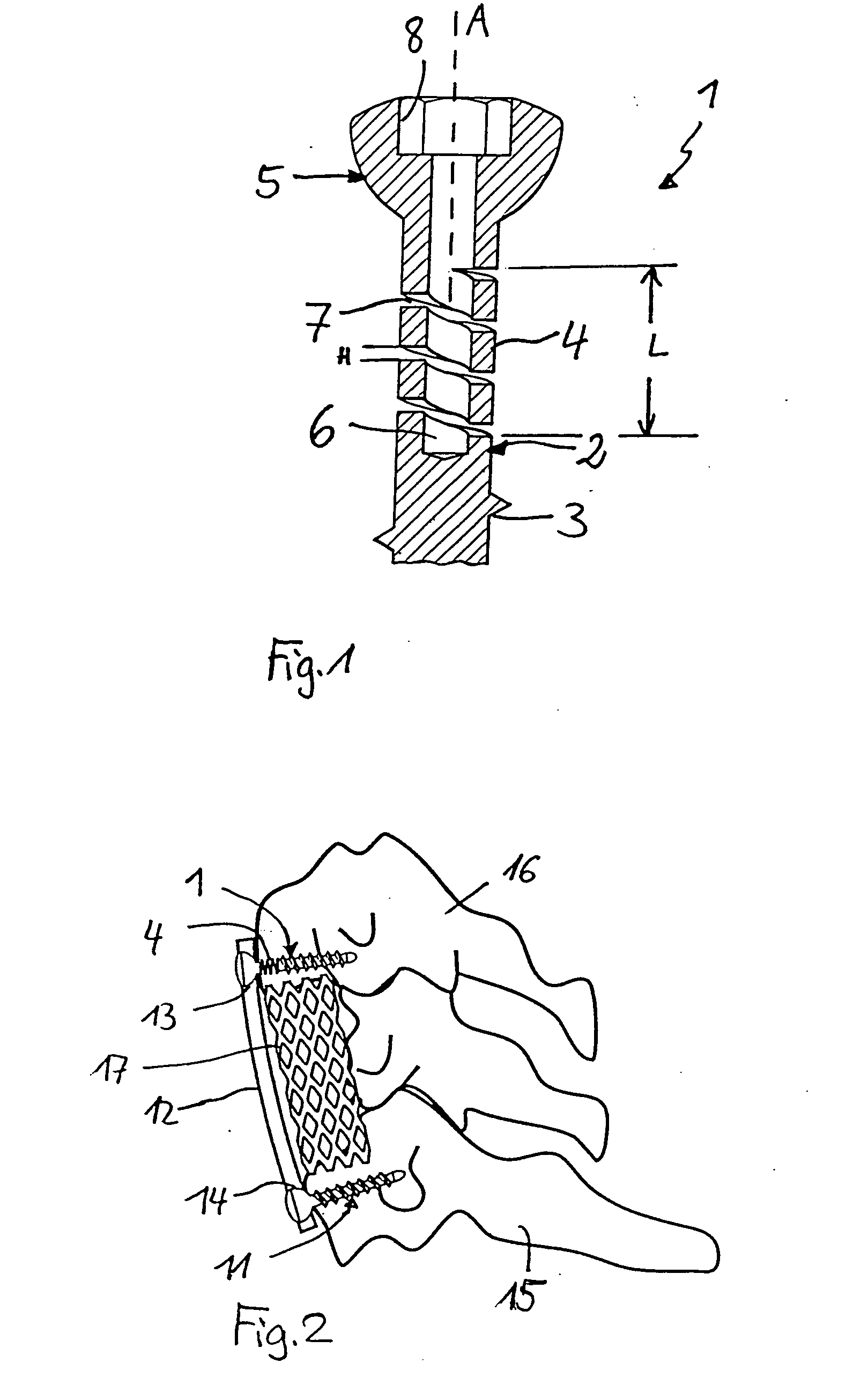
Stabilization device for bones comprising a spring element and manufacturing method for said spring element
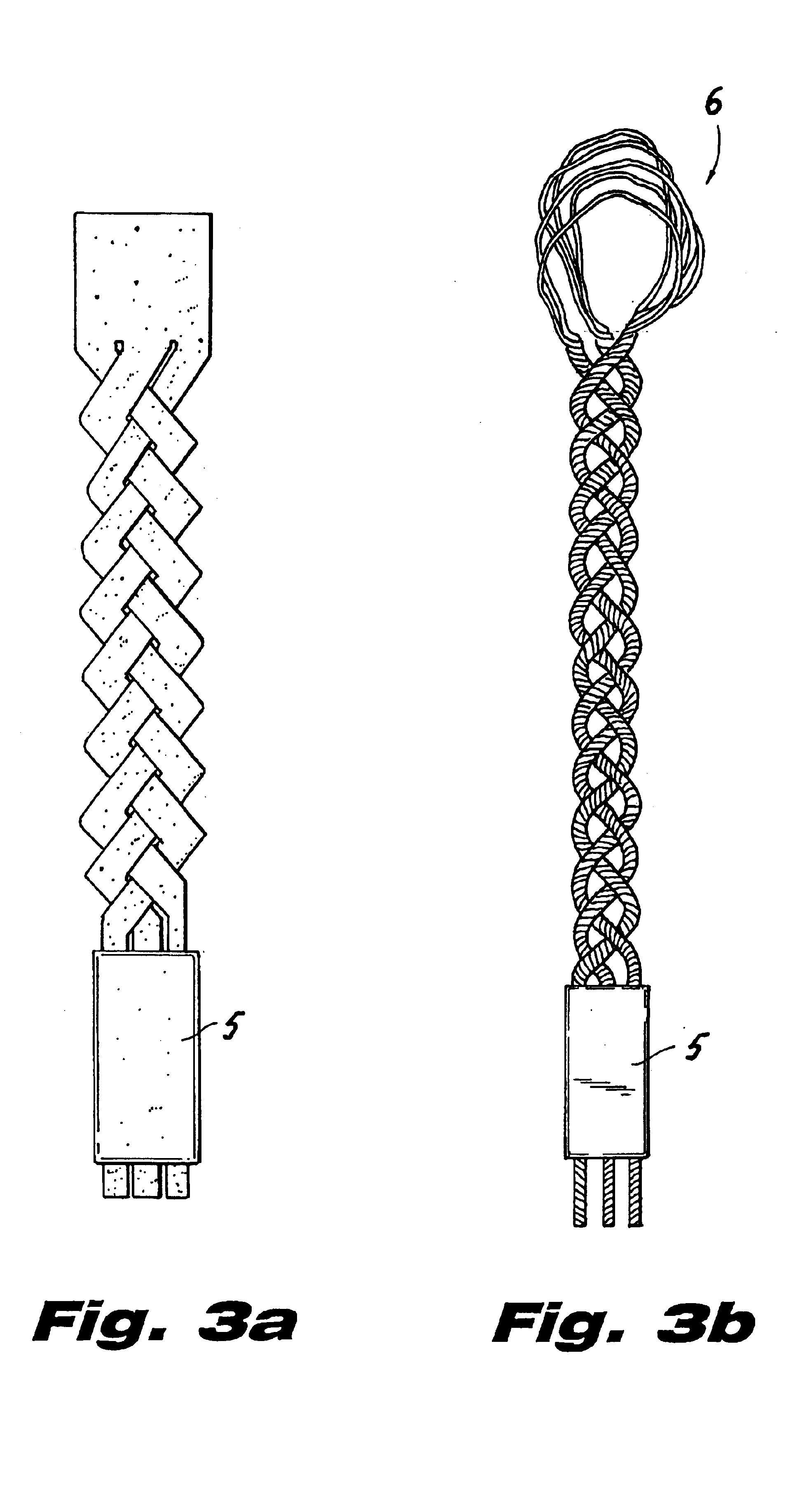
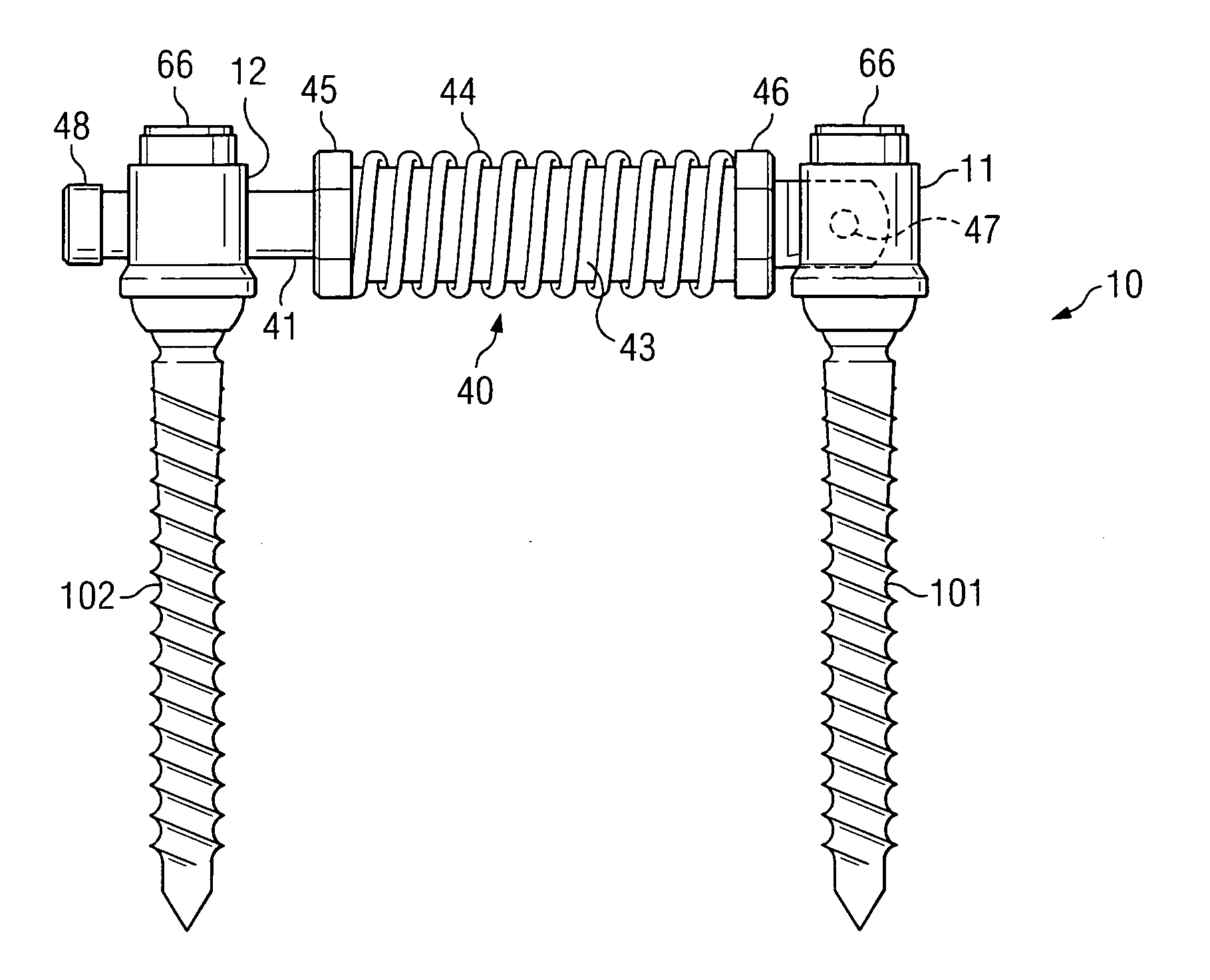
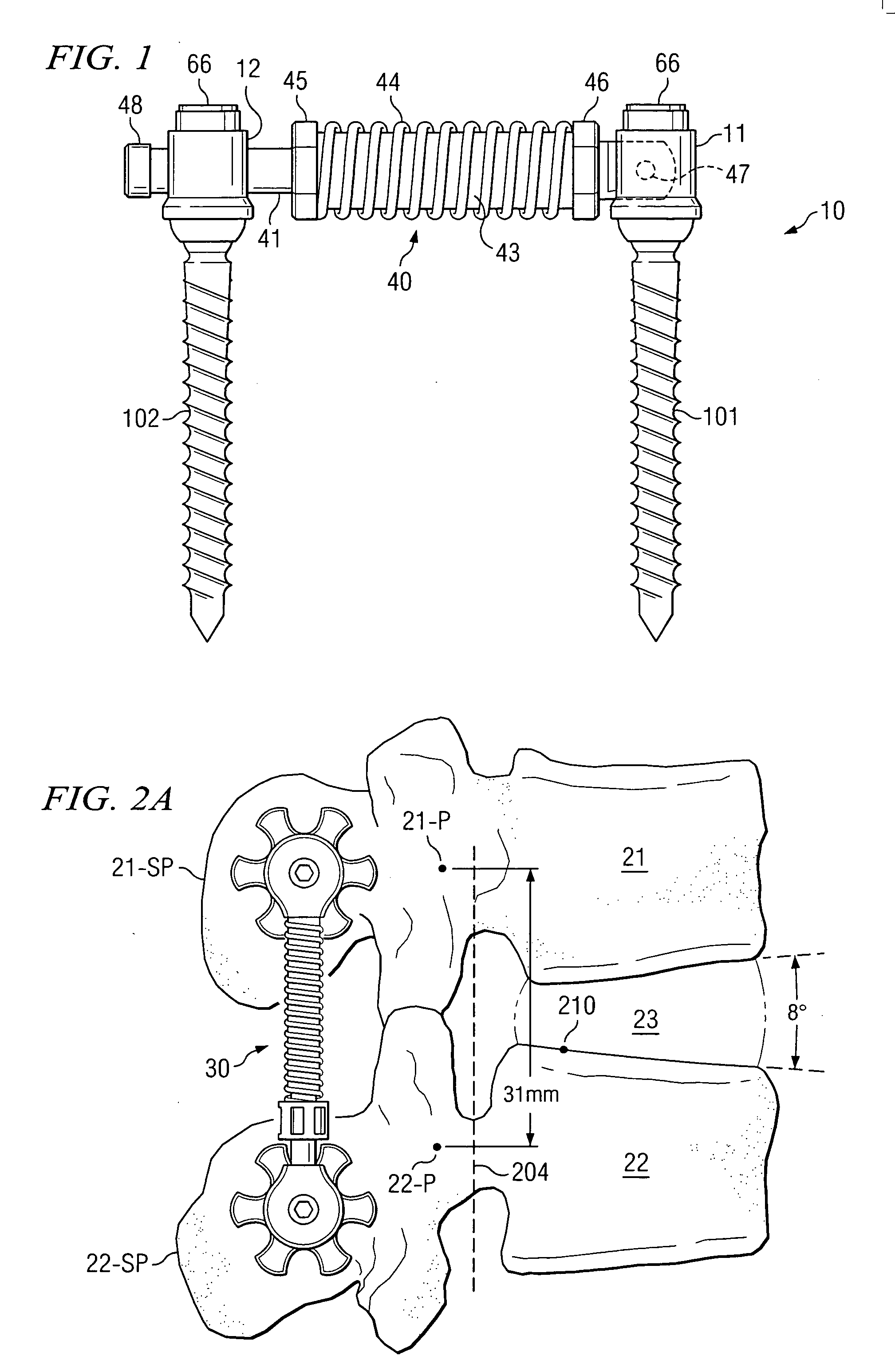
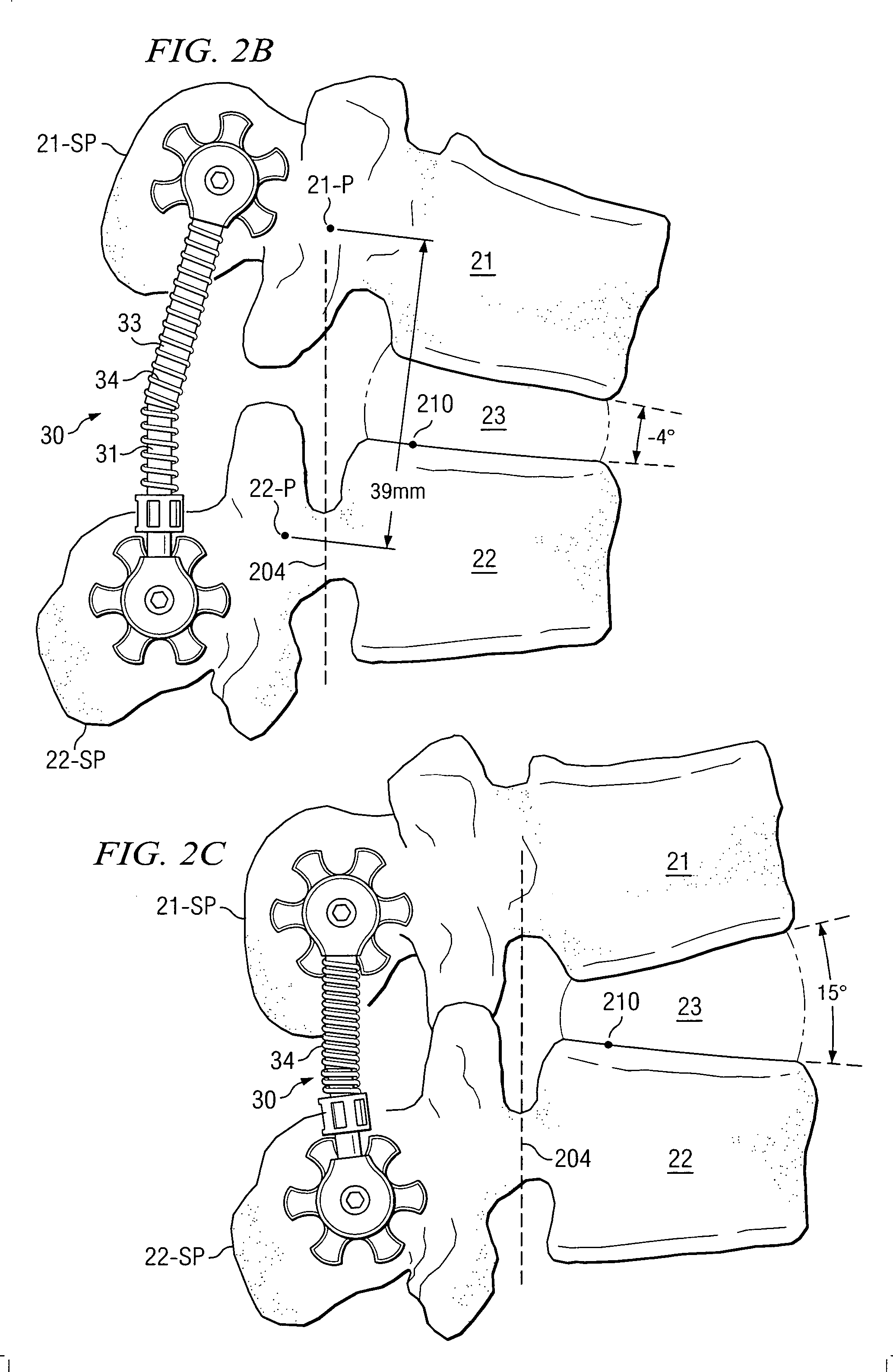
ActiveUS20050154390A1Easy to implementElastic fitSuture equipmentsInternal osteosythesisCoil springBiomedical engineering
An elastic or flexible element for use in a stabilization device for bones or vertebrae is provided. The elastic or flexible element is provided in the form of an essentially cylindrical body with a first end and a second end opposite thereto, wherein at least one of the opposite ends of the cylindrical body comprises a coaxial bore hole with an internal thread for connecting to a shaft and / or a head of a bone screw or for connecting to a rod section. The present invention further provides a bone anchoring element, e.g. a bone screw, with a shaft for the anchoring in a bone, whereby the shaft comprises an elastic or flexible section which is formed integrally with the shaft or as a separate elastic or flexible element. It is preferable for the elastic section to be implemented in the form of a helical spring. Moreover, the present invention provides a stabilization device for bones, for instance for vertebrae, said device comprising at least one bone anchoring element according to the invention, a second bone anchoring element and a rod or plate connecting the bone anchoring elements.
Owner:BIEDERMANN TECH GMBH & CO KG
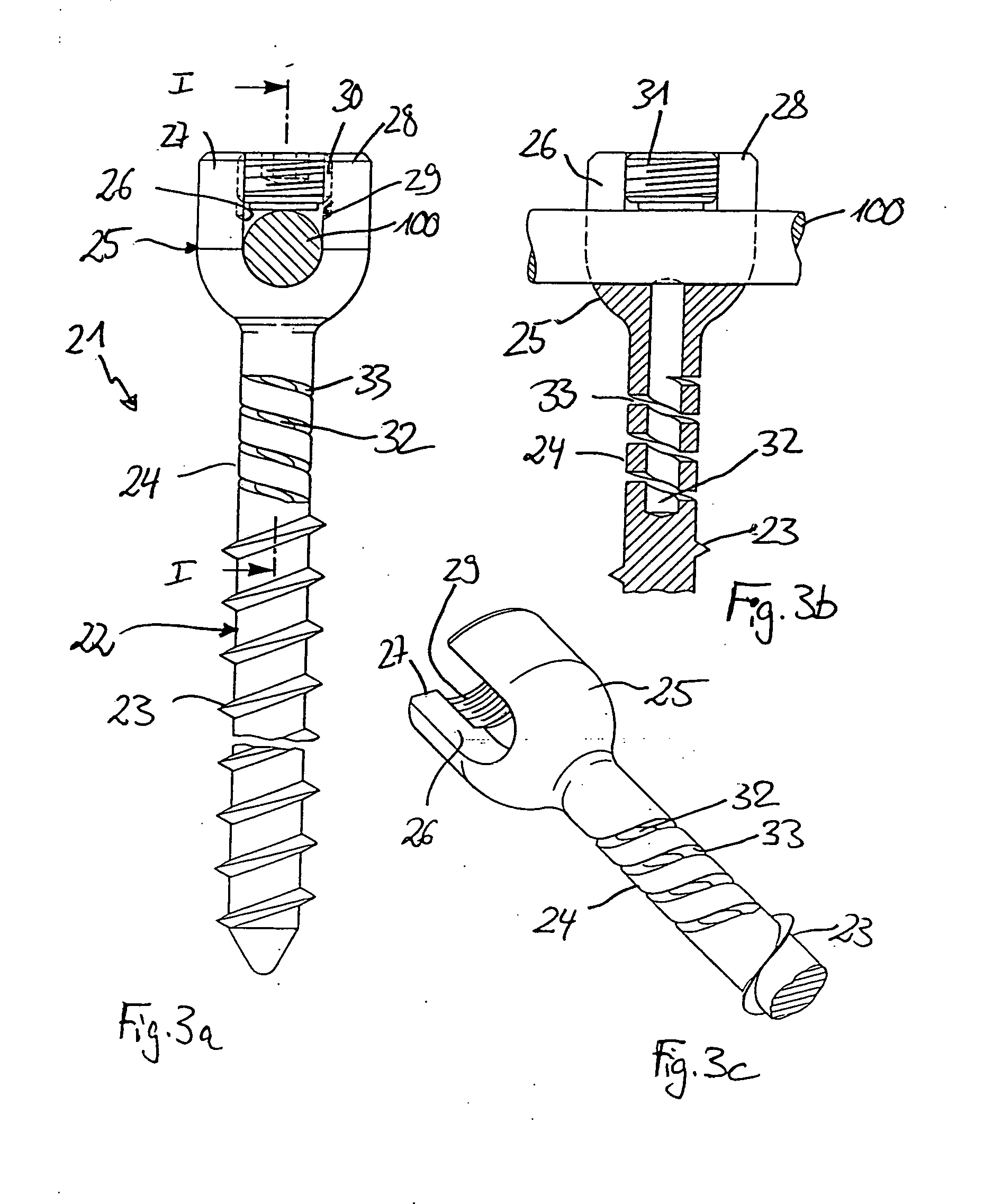
Rod-shaped implant element for application in spine surgery or trauma surgery, stabilization apparatus comprising said rod-shaped implant element, and production method for the rod-shaped implant element
ActiveUS20050085815A1Reliable fixed positioningEasily realizedSuture equipmentsInternal osteosythesisTrauma surgeryBone anchor
A rod-shaped implant element (1, 100, 101, 102, 300) is disclosed for the connection of bone anchoring elements, Each bone anchoring element has an anchoring section (12) to be anchored in the bone and a receiver member (13) to be connected to the rod-shaped implant element The rod-shaped implant element has at least one rigid section (7, 8) that is configured to be placed in the receiver member (13). It also has a flexible section (9, 90, 900, 902) that is adjacent to the rigid section. The flexible section and the rigid section are formed in one piece. Also disclosed is a stabilization apparatus using a rod-shaped implant element and at least two of the bone anchoring elements. The stabilization apparatus can limit the movement of two vertebrae or parts of a bone in relation to each other in a defined manner.
Owner:BIEDERMANN TECH GMBH & CO KG
Surgical tools facilitating increased accuracy, speed and simplicity in performing joint arthroplasty
Disclosed herein are tools for repairing articular surfaces repair materials and for repairing an articular surface. The surgical tools are designed to be customizable or highly selectable by patient to increase the speed, accuracy and simplicity of performing total or partial arthroplasty.
Owner:CONFORMIS
Patient selectable joint arthroplasty devices and surgical tools facilitating increased accuracy, speed and simplicity in performing total and partial joint arthroplasty
Disclosed herein are methods, compositions and tools for repairing articular surfaces repair materials and for repairing an articular surface. The articular surface repairs are customizable or highly selectable by patient and geared toward providing optimal fit and function. The surgical tools are designed to be customizable or highly selectable by patient to increase the speed, accuracy and simplicity of performing total or partial arthroplasty.
Owner:CONFORMIS
Dynamic stabilization assemblies, tool set and method
A hinged bone screw and tool set is used for implanting such bone screws in a human spine, followed by the implantation of a longitudinal connecting member into the bone screws. The hinged bone screw includes a shank with an upper portion and a receiver with integral arms forming a U-shaped channel. A lower curved seat partially defining the U-shaped channel cooperates with an upper portion of the bone screw shank for hinged movement of the shank with respect to the receiver. The tool set includes an insertion tool, a bone screw driver, a reduction tool and a closure starter. The insertion tool includes a bone screw attachment structure and a laterally opening channel. The insertion tool further includes a threaded portion for cooperation with the reduction tool to provide synchronized placement of a closure structure in the bone screw receiver while reducing and capturing a longitudinal connecting member within the receiver. Further alternative bone screws are hinged, polyaxial or fixed and include lordosing or kyphosing lateral surfaces.
Owner:NUVASIVE
Patient selectable knee joint arthroplasty devices
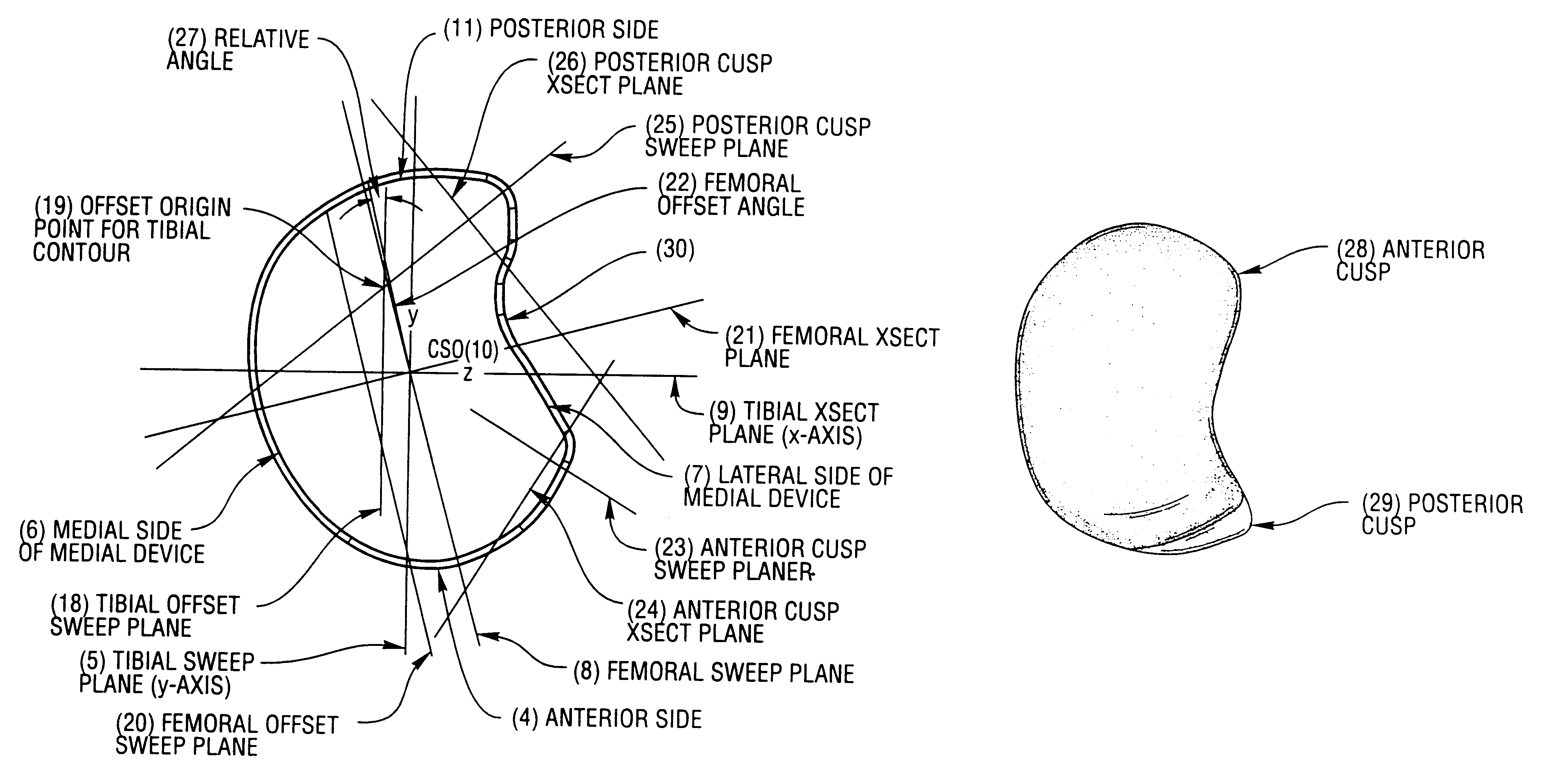
ActiveUS20050267584A1Improve anatomic functionalityPromote resultsJoint implantsLigamentsArticular surfacesArticular surface
Disclosed herein are methods and devices for repairing articular surfaces in a knee joint. The articular surface repairs are customizable or highly selectable for each patient and geared toward providing optimal fit and function. Kits are also provided to enable customized repairs to be performed.
Owner:CONFORMIS
System and method for dynamic skeletal stabilization
InactiveUS20060036240A1Large range of motionSuture equipmentsInternal osteosythesisDistractionKinematics
There is disclosed a system and method for dynamic stabilization which provides for distraction of the inter-vertebral space while still allowing a patient a substantial range of motion. In one embodiment, an inter-vertebral dynamic brace is used to maintain proper distraction. The dynamic brace is designed to allow the vertebrae to which it is attached to move through their natural arc, maintaining the correct instantaneous center of rotation. An adjustable tensioning device is used to maintain the proper distraction and compression forces to restore and maintain proper kinematics, while allowing the dynamic brace to move through an arc centered with respect to the center of rotation of the portion of the spine between the vertebrae. In one embodiment, a method is provided for adjusting the dynamic brace both with respect to the center of rotation of the vertebrae in both the flexion / extension axis and in the superior / inferior axis.
Owner:THEKEN SPINE
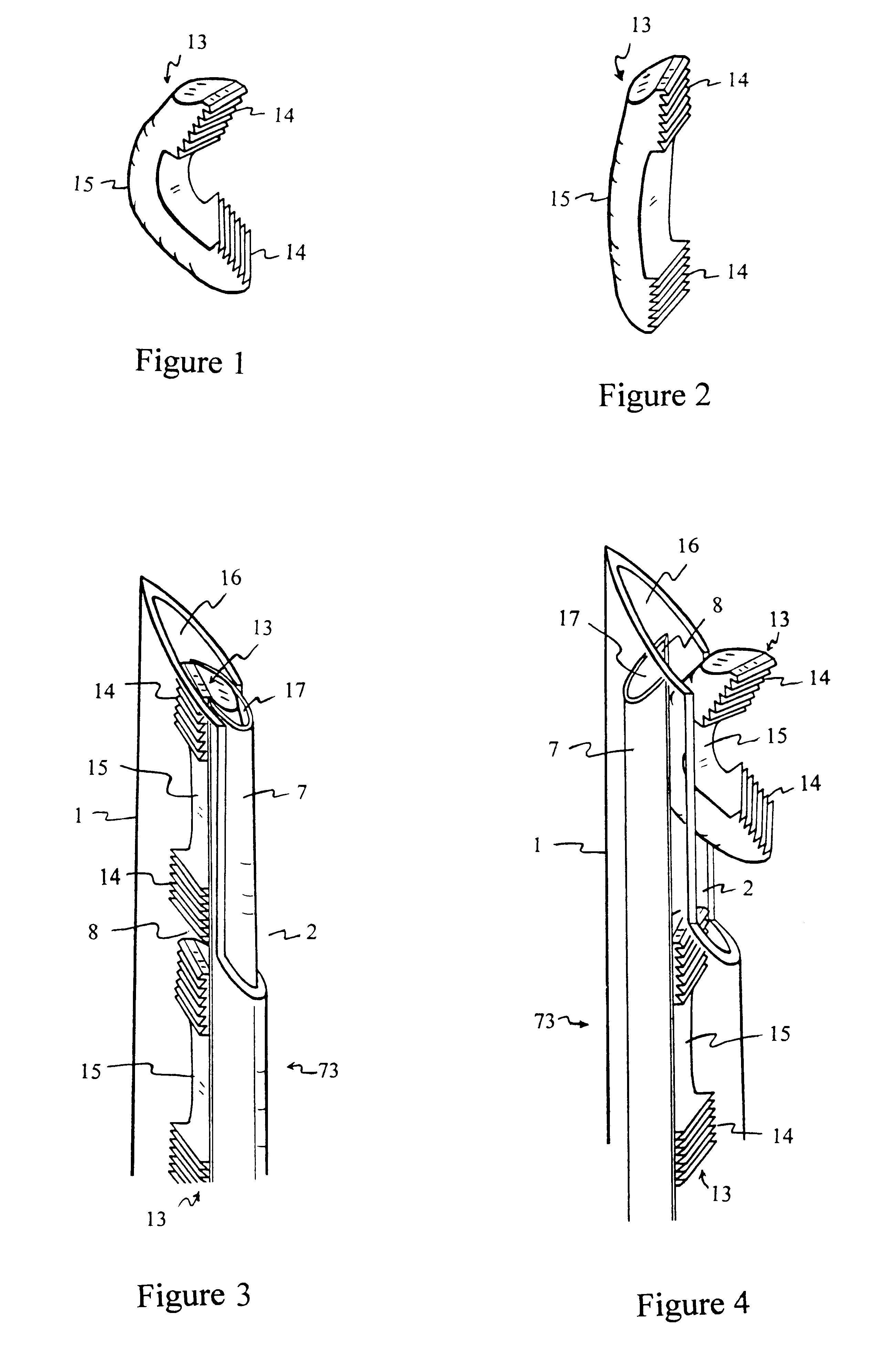
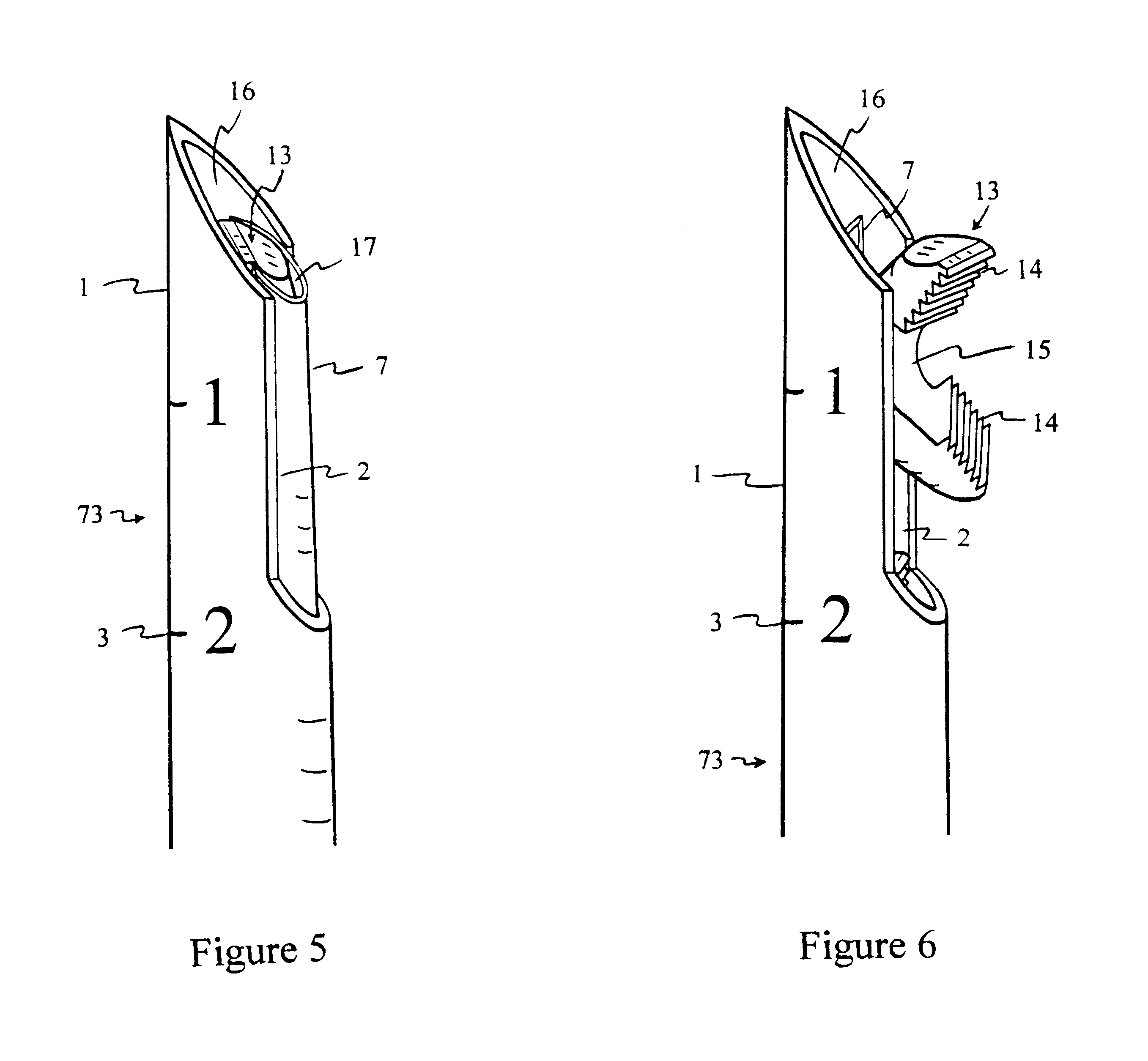
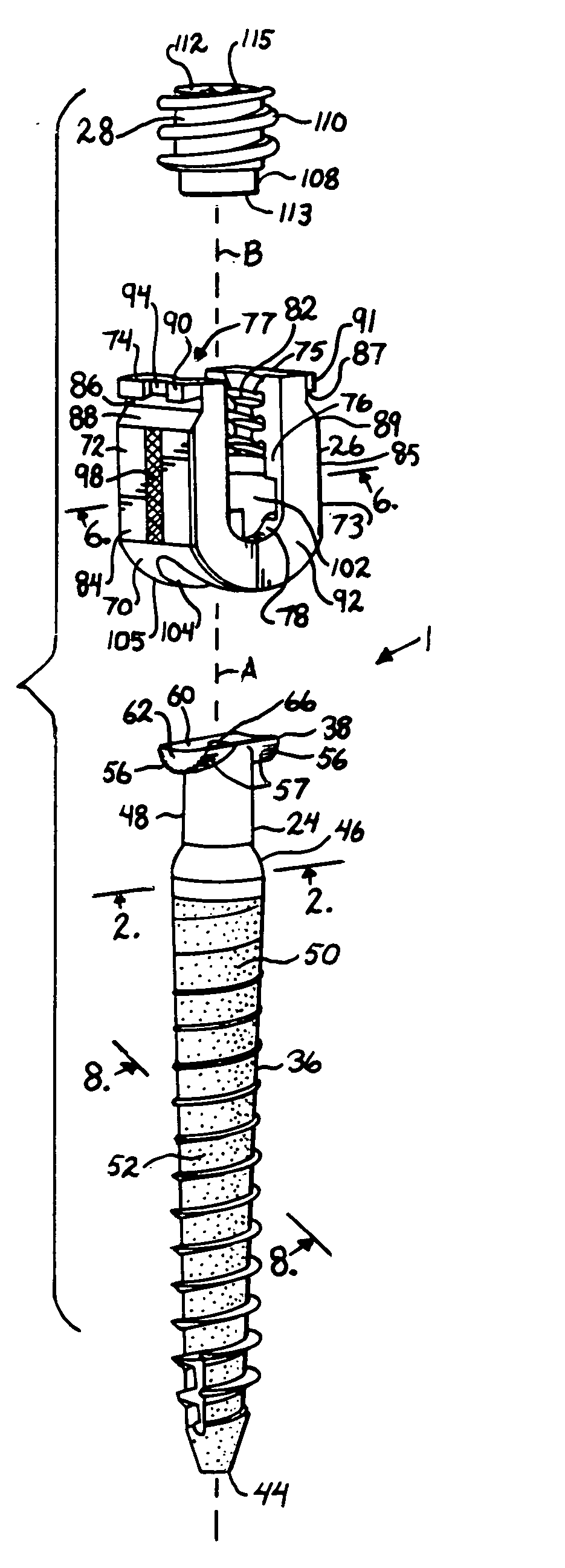
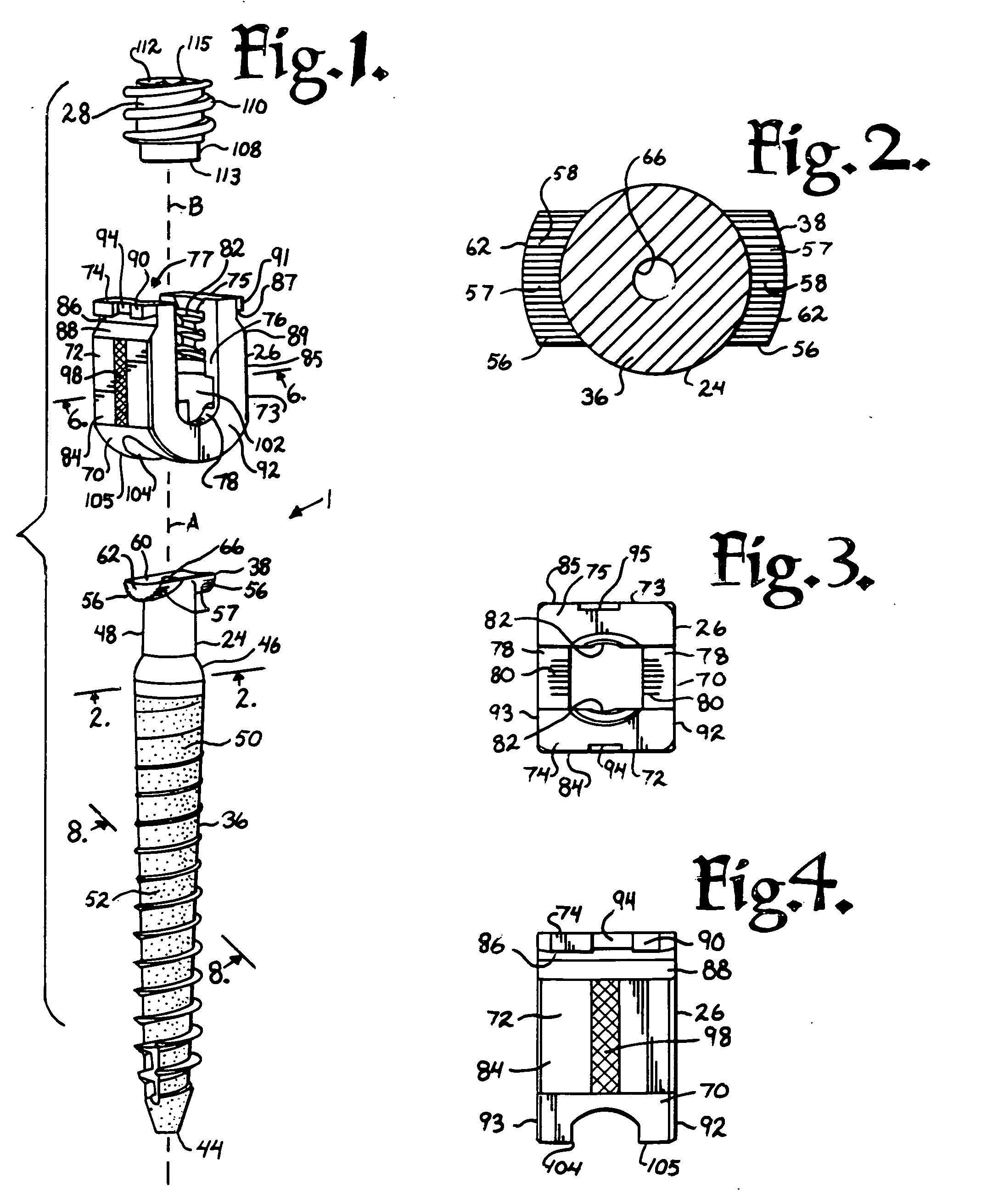
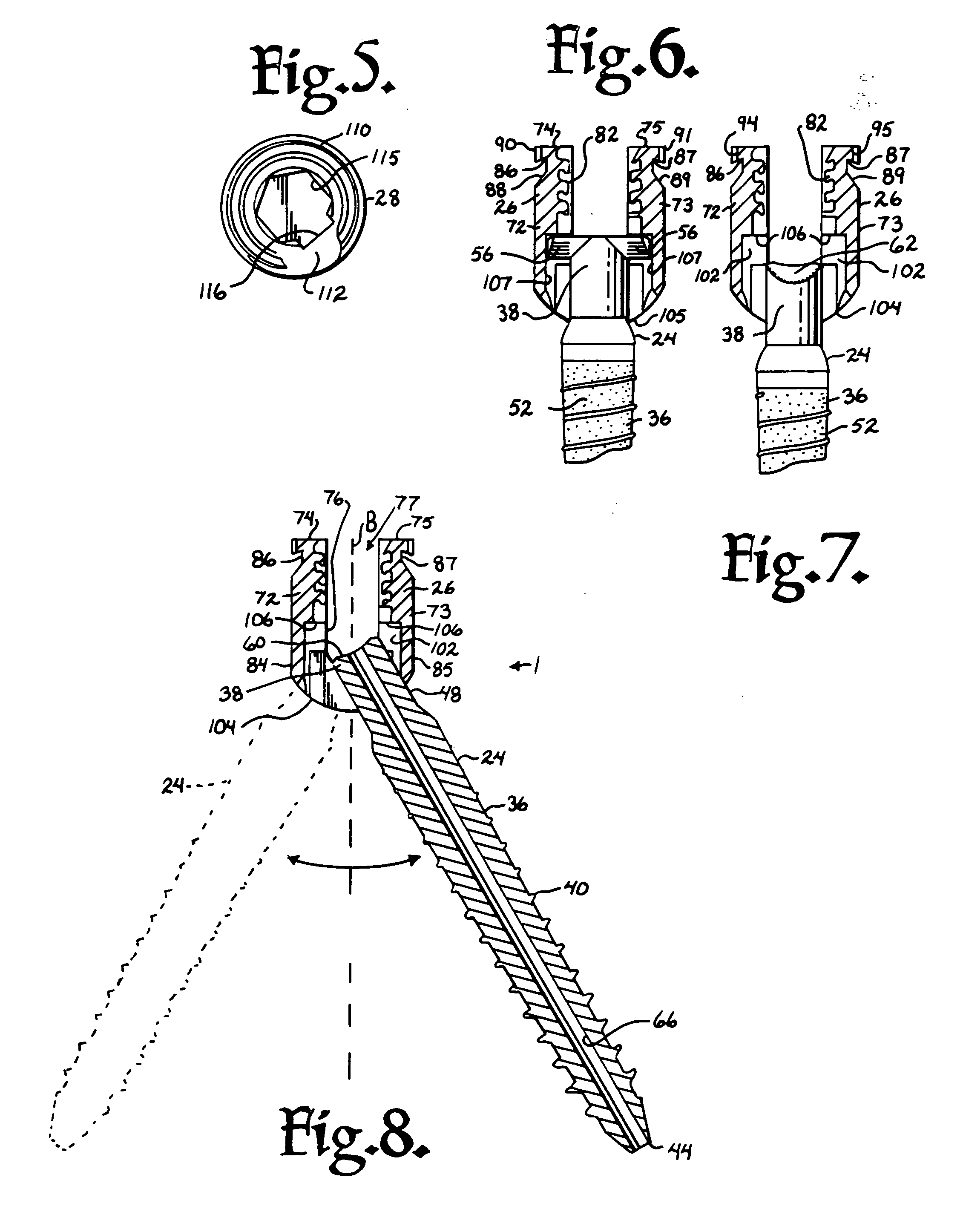
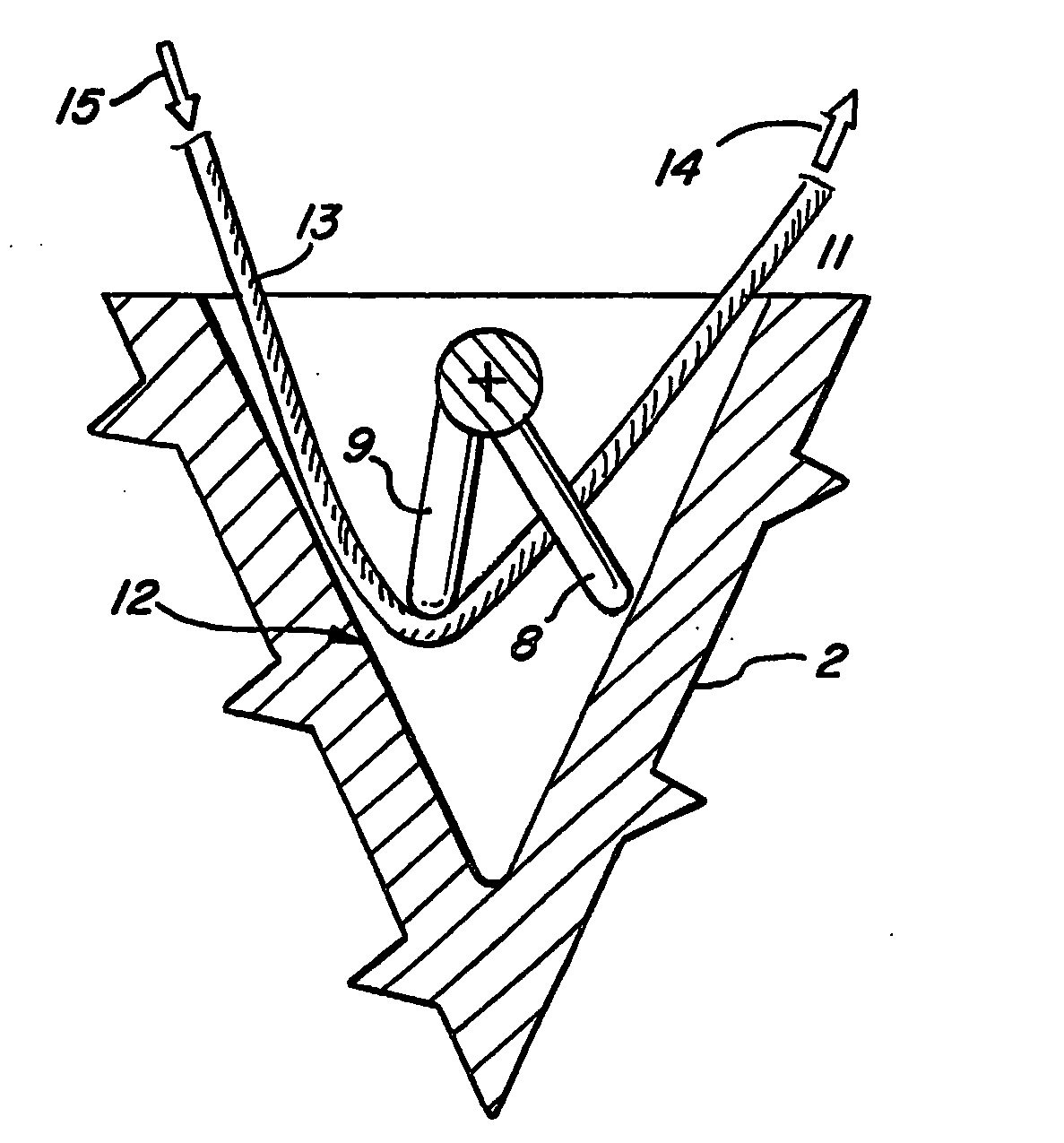
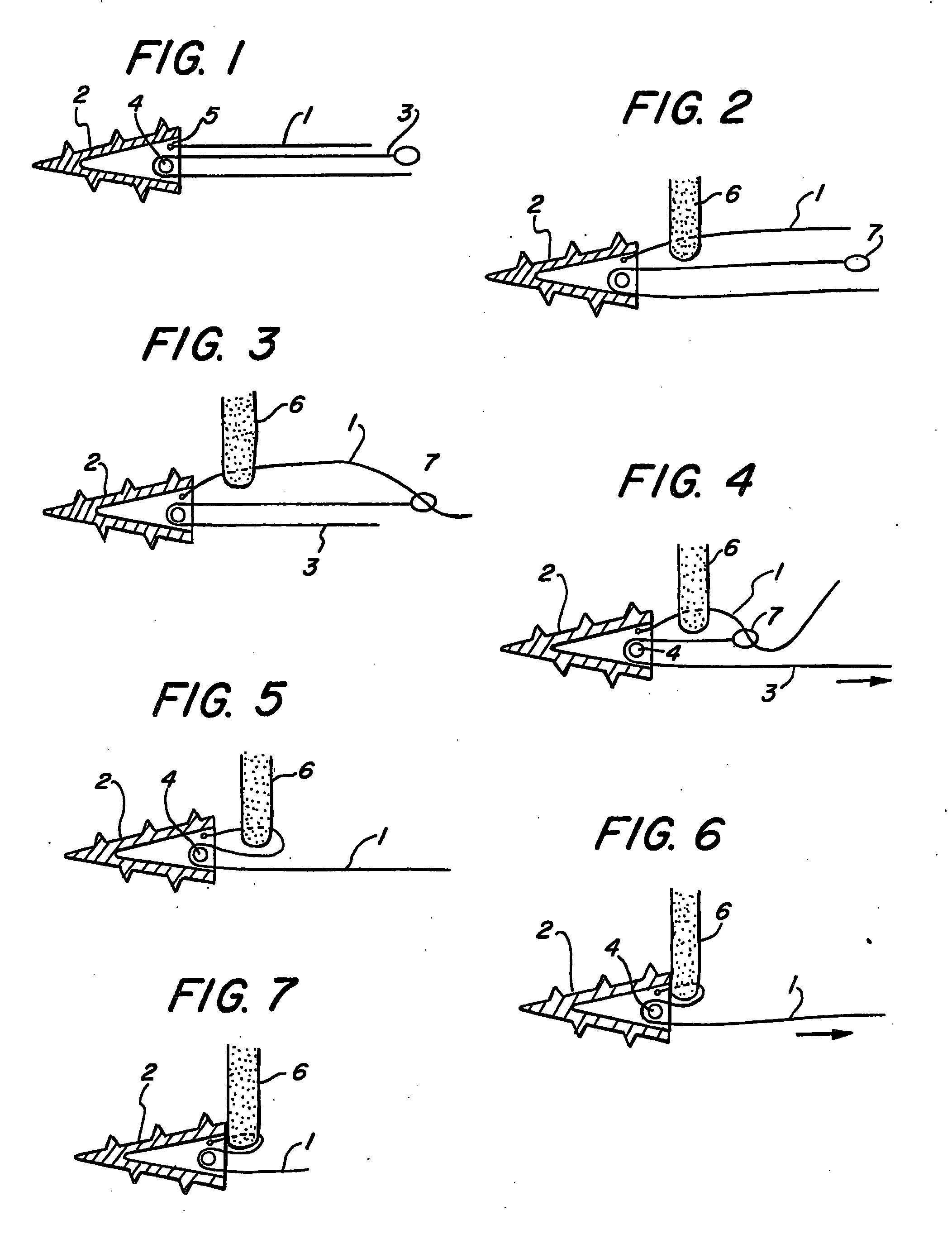
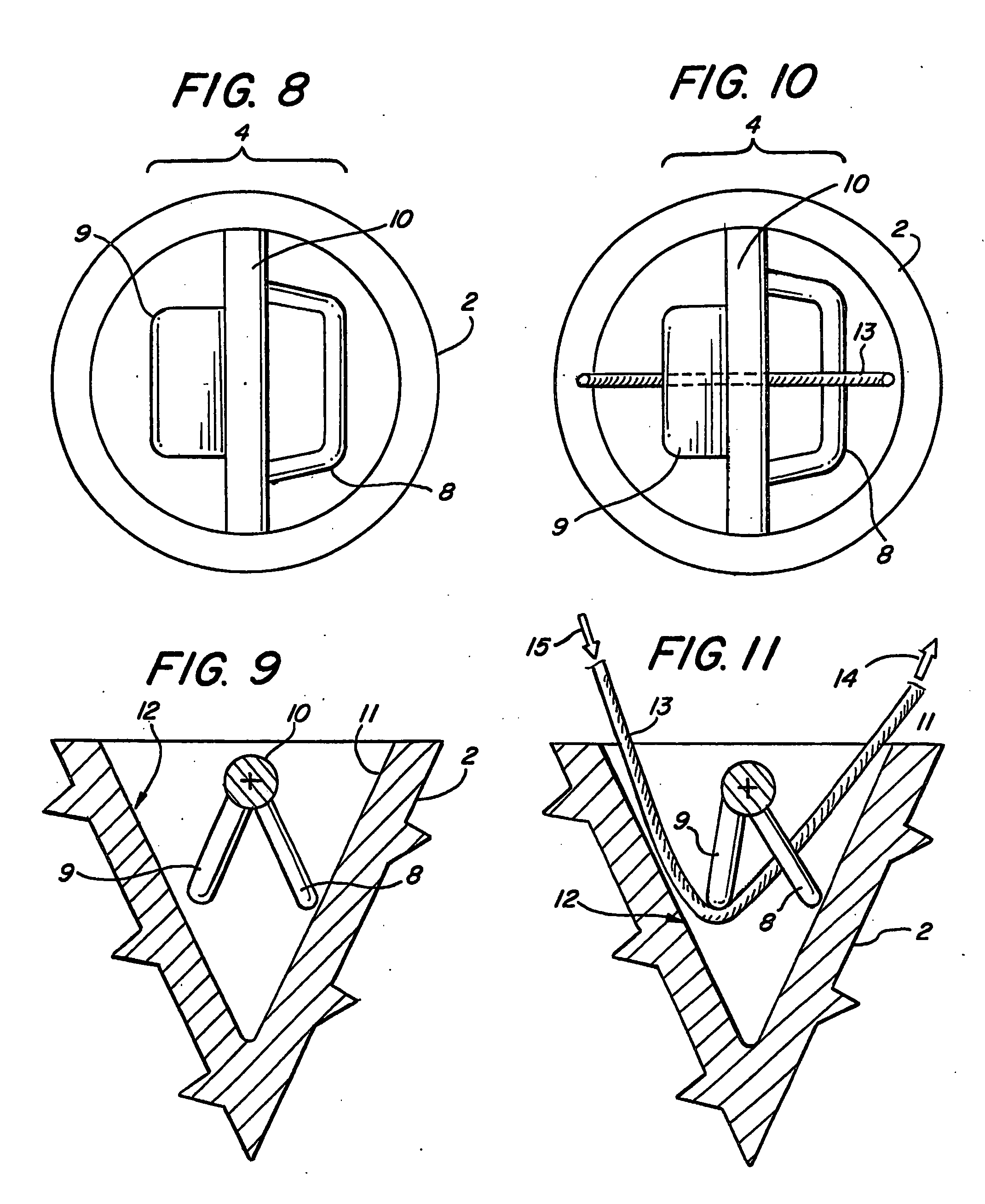
Suture anchor
ActiveUS20060106423A1Easy and intuitivePrevent movementSuture equipmentsLigamentsLocking mechanismSuture anchors
A suture anchor (e.g. 100) for knotlessly securing nearby tissue to bone is formed from an anchor body (e.g. 101 / 102) that includes a mechanism for being securely anchored to the bone, an elongated suture puller (e.g. 122) extending through the anchor body with a proximal end for being pulled in a proximal direction by a surgeon and a distal end (e.g. 125) with a suitable mechanism for engaging suture (e.g. 127), suture (e.g. 132) carried by the engaging mechanism (e.g. 127) at the distal end (e.g. 125) of the elongated suture puller (e.g. 122), and a suture locking mechanism (e.g. 110) that substantially prevents the suture (e.g. 122) from moving in at least a first direction after being pulled into and through the anchor body (e.g. 101 / 102) by the elongated suture puller (e.g. 122).
Owner:BANNERMAN BRETT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com