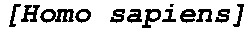Patents
Literature
115 results about "Invasive Procedure" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A medical procedure that invades (enters) the body, usually by cutting or puncturing the skin or by inserting instruments into the body.
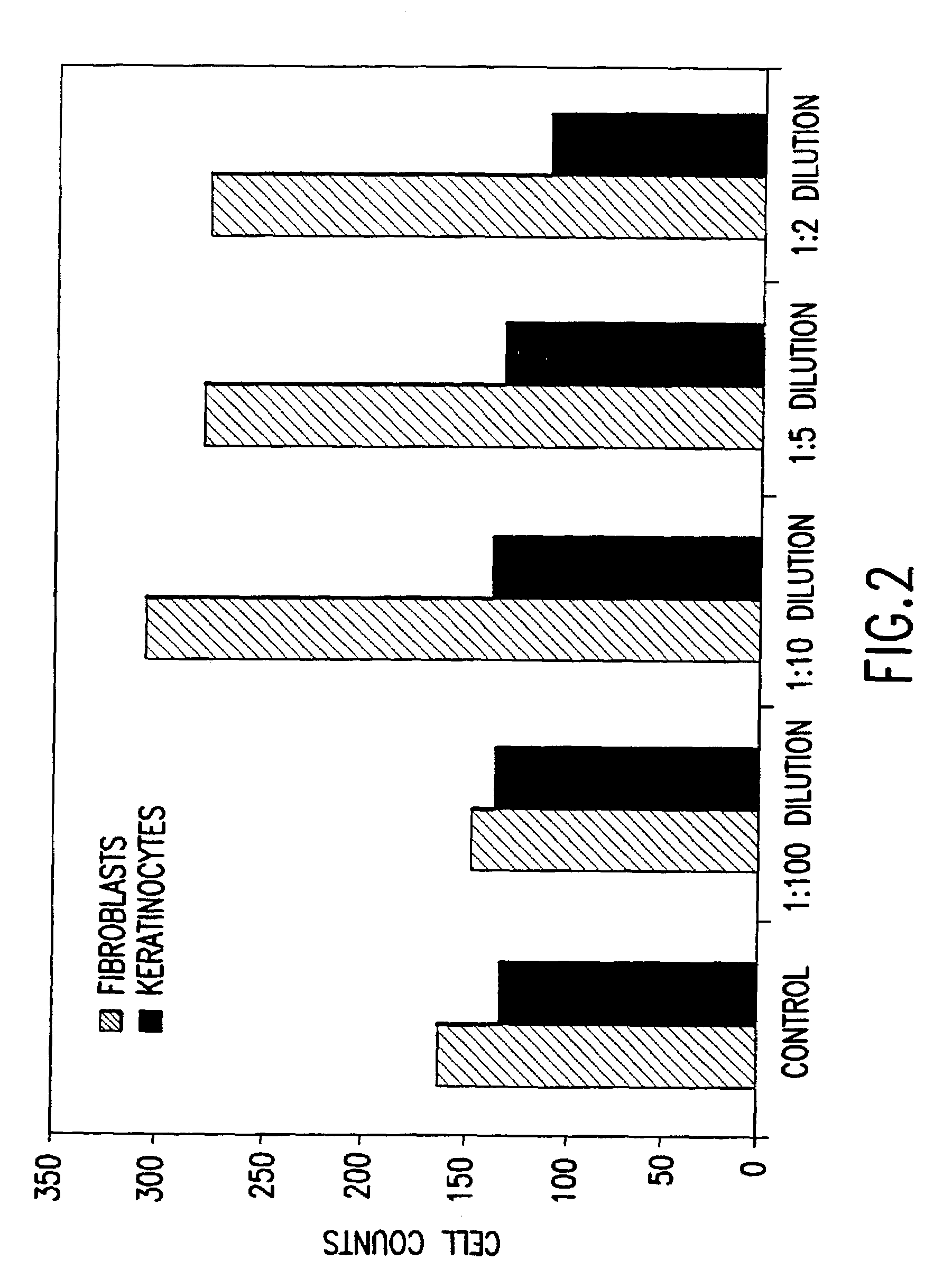
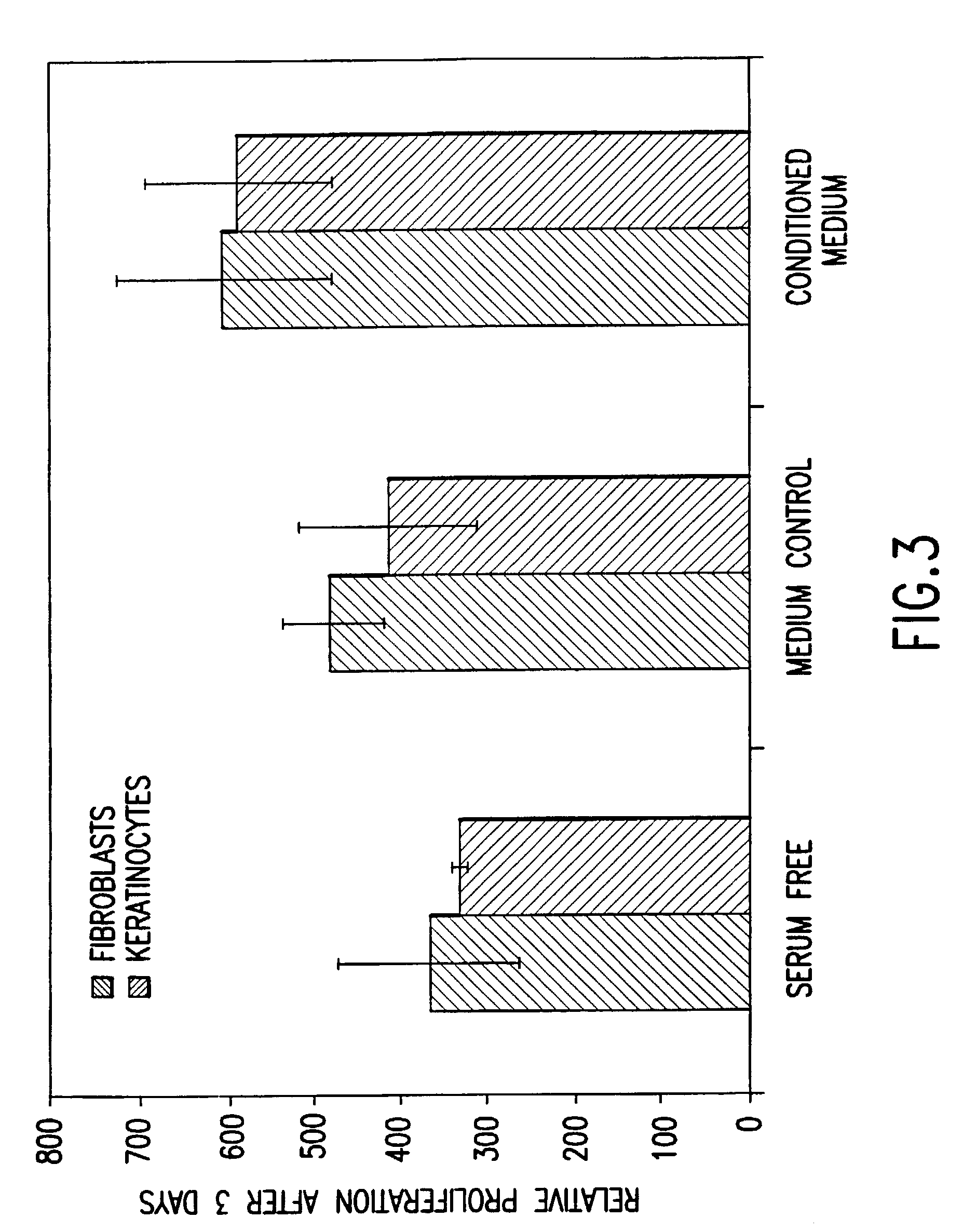
Methods of making conditioned cell culture medium compositions
InactiveUS6372494B1Eliminate wrinklesEliminate frown lineCosmetic preparationsPeptide/protein ingredientsReserve CellCell culture media
Novel products comprising conditioned cell culture medium compositions and methods of use are described. The conditioned cell medium compositions of the invention may be comprised of any known defined or undefined medium and may be conditioned using any eukaryotic cell type. The medium may be conditioned by stromal cells, parenchymal cells, mesenchymal stem cells, liver reserve cells, neural stem cells, pancreatic stem cells and / or embryonic stem cells. Additionally, the cells may be genetically modified. A three-dimensional tissue construct is preferred. Once the cell medium of the invention is conditioned, it may be used in any state. Physical embodiments of the conditioned medium include, but are not limited to, liquid or solid, frozen, lyophilized or dried into a powder. Additionally, the medium is formulated with a pharmaceutically acceptable carrier as a vehicle for internal administration, applied directly to a food item or product, formulated with a salve or ointment for topical applications, or, for example, made into or added to surgical glue to accelerate healing of sutures following invasive procedures. Also, the medium may be further processed to concentrate or reduce one or more factors or components contained within the medium.
Owner:ALLERGAN INC
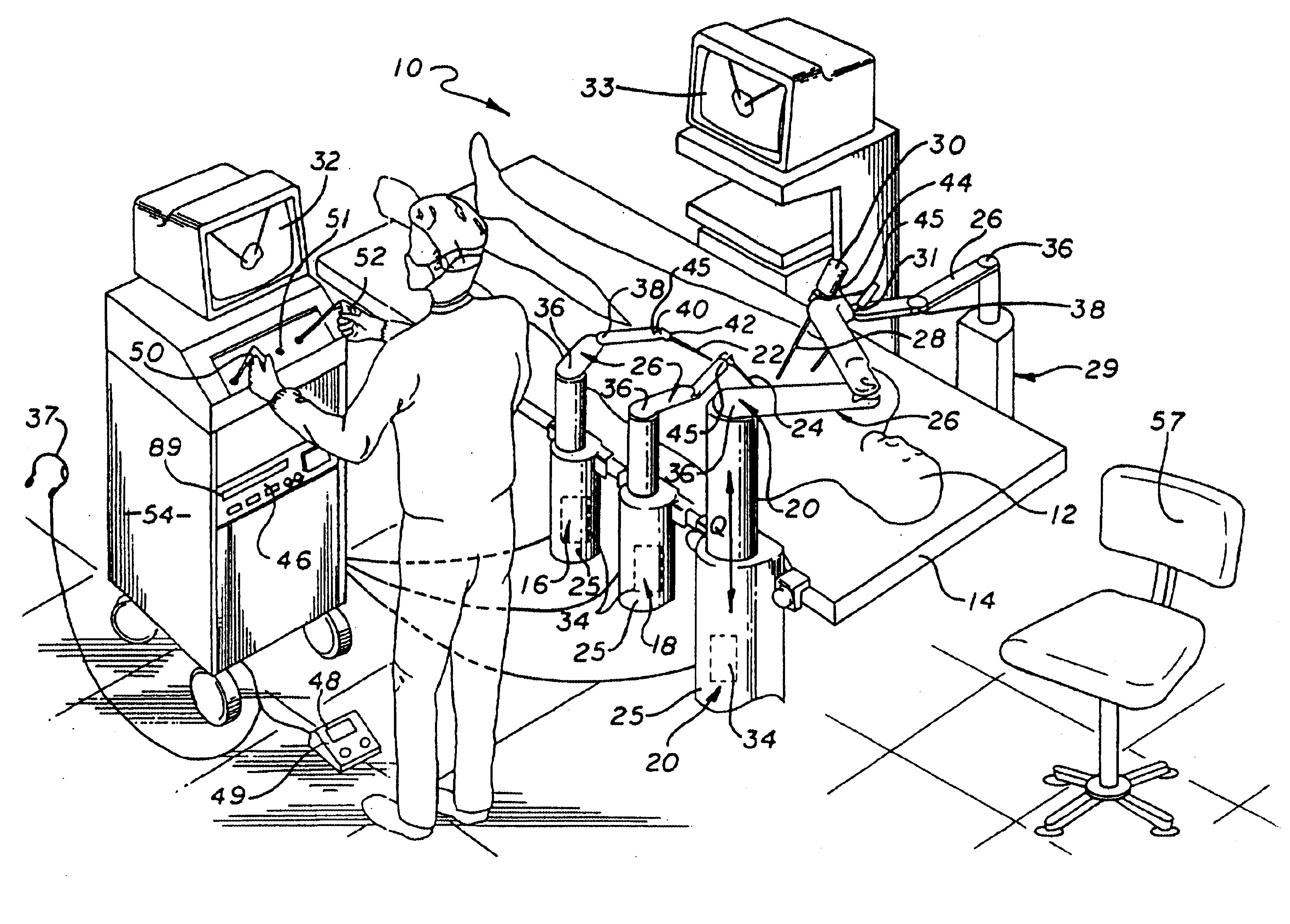
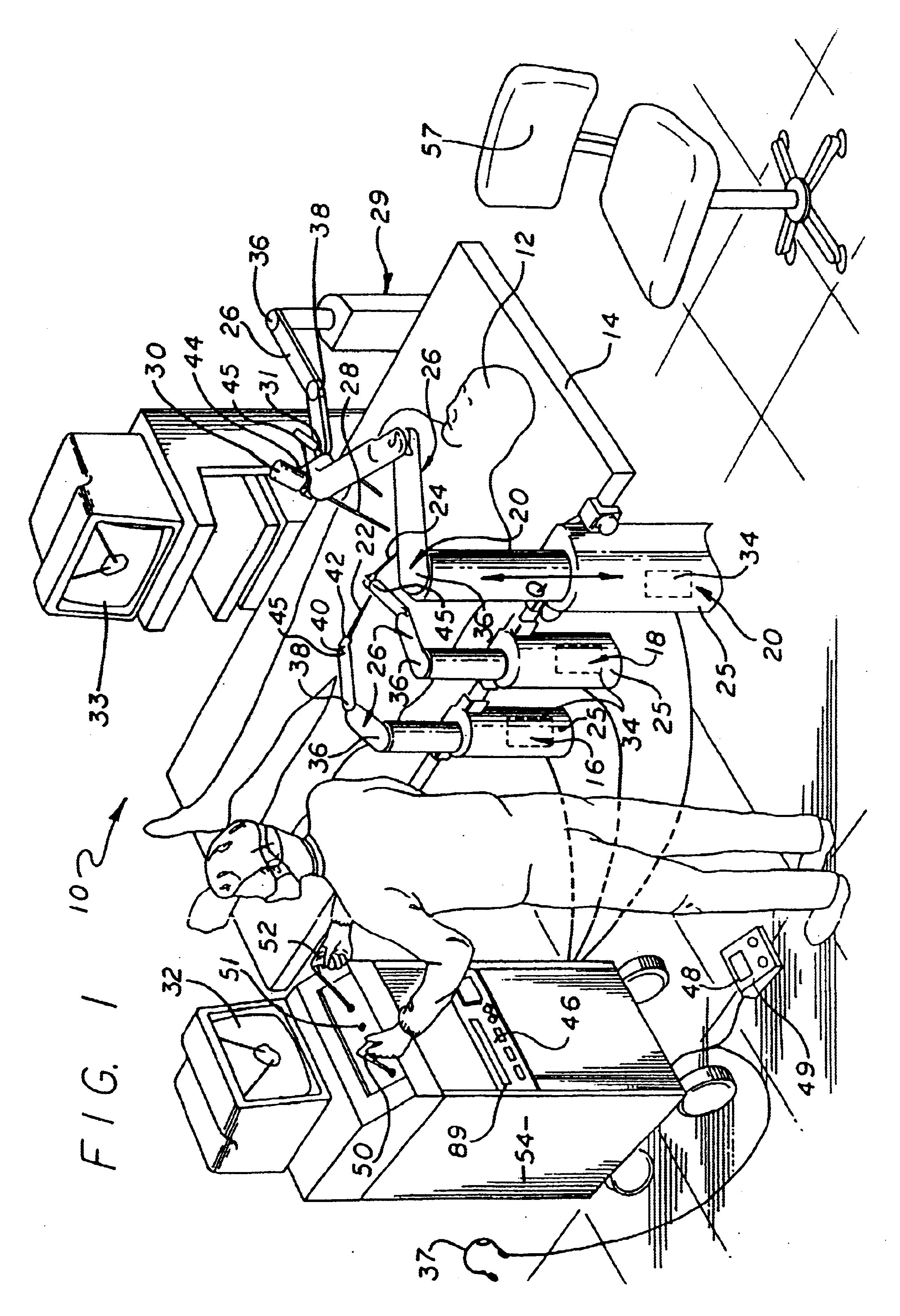
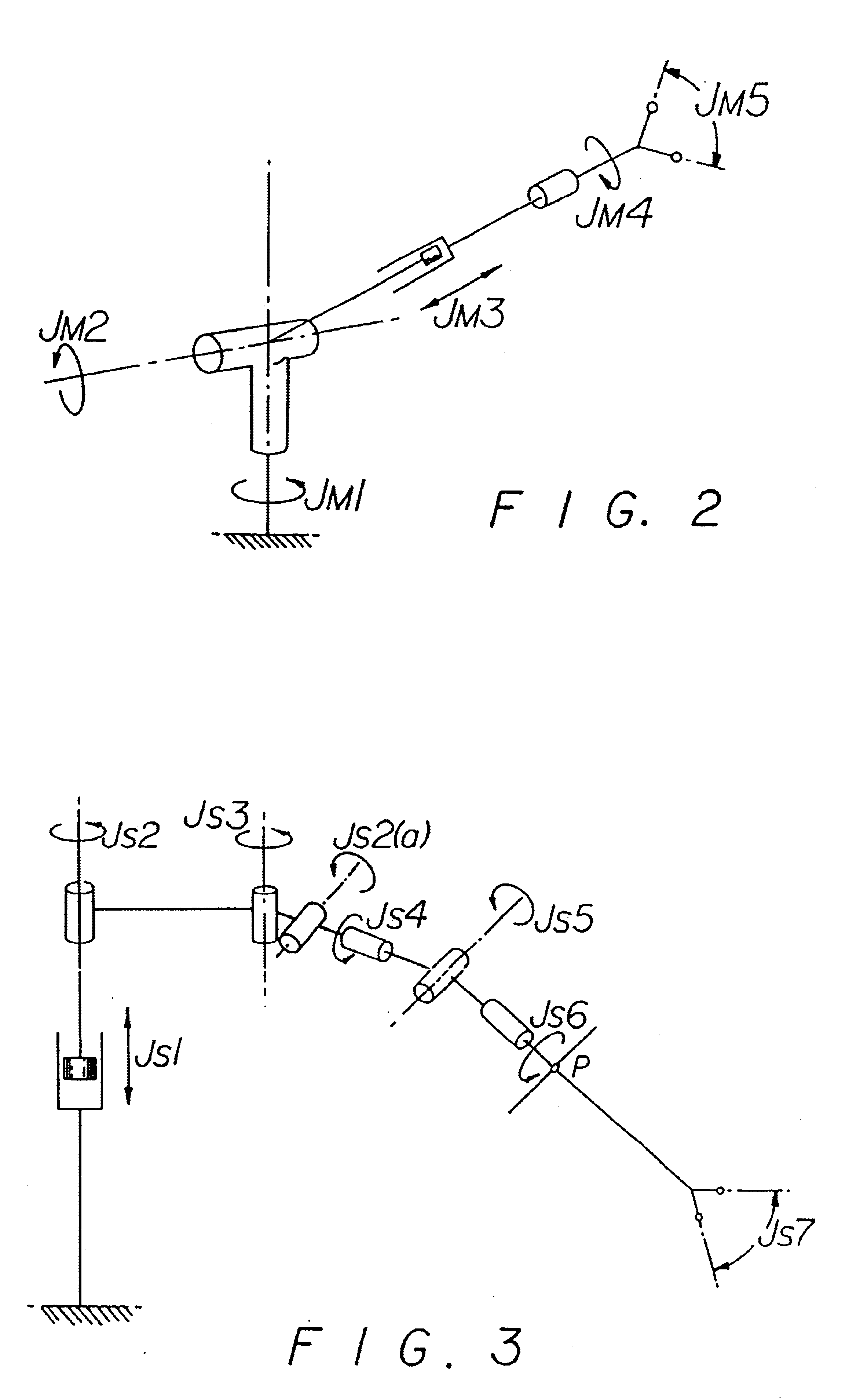
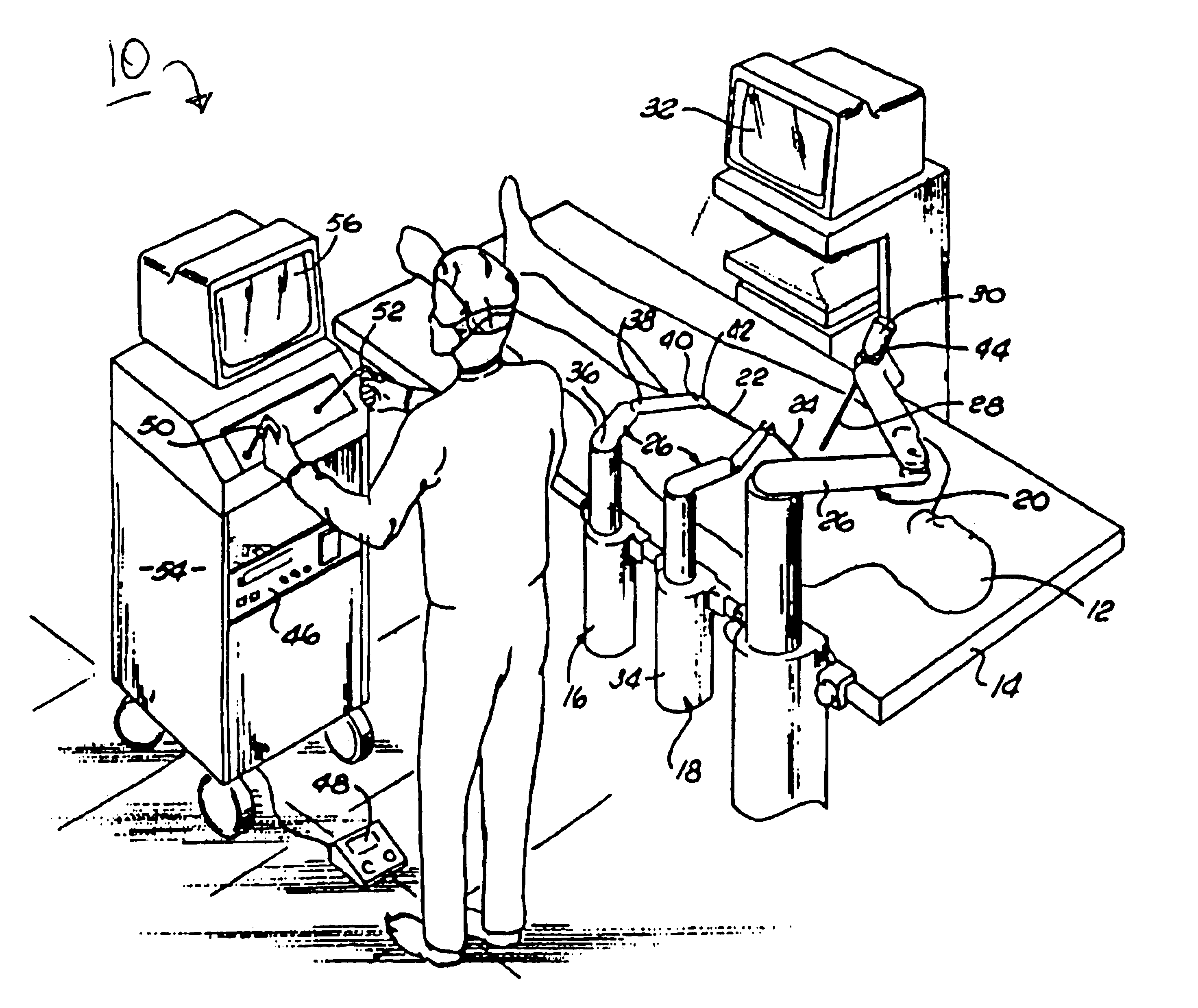
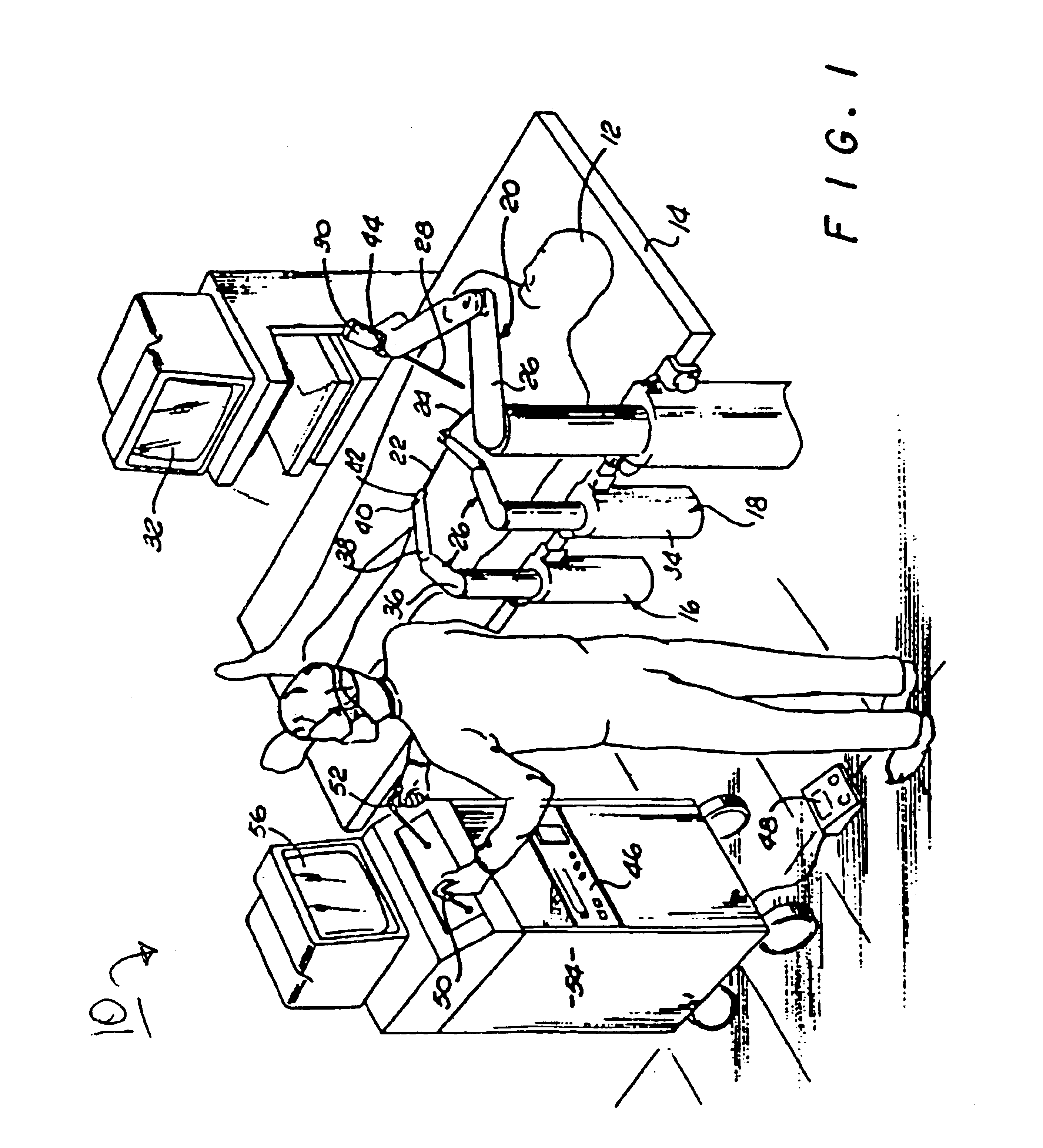
Method and apparatus for performing minimally invasive surgical procedures
InactiveUS6905460B2Reserved functionHigh precisionProgramme controlSuture equipmentsRobotic armSurgical site
A system for performing minimally invasive cardiac procedures. The system includes a pair of surgical instruments that are coupled to a pair of robotic arms. The instruments have end effectors that can be manipulated to hold and suture tissue. The robotic arms are coupled to a pair of master handles by a controller. The handles can be moved by the surgeon to produce a corresponding movement of the end effectors. The movement of the handles is scaled so that the end effectors have a corresponding movement that is different, typically smaller, than the movement performed by the hands of the surgeon. The scale factor is adjustable so that the surgeon can control the resolution of the end effector movement. The movement of the end effector can be controlled by an input button, so that the end effector only moves when the button is depressed by the surgeon. The input button allows the surgeon to adjust the position of the handles without moving the end effector, so that the handles can be moved to a more comfortable position. The system may also have a robotically controlled endoscope which allows the surgeon to remotely view the surgical site. A cardiac procedure can be performed by making small incisions in the patient's skin and inserting the instruments and endoscope into the patient. The surgeon manipulates the handles and moves the end effectors to perform a cardiac procedure such as a coronary artery bypass graft.
Owner:INTUITIVE SURGICAL OPERATIONS INC
Method and apparatus for performing minimally invasive cardiac procedures
InactiveUS7074179B2Minimally invasiveComfortable postureSuture equipmentsGripping headsRobotic systemsRobotic arm
A robotic system that moves a surgical instrument in response to the actuation of a foot pedal that can be operated by the foot of a surgeon. The robotic system has an end effector that is adapted to hold a surgical instrument such as an endoscope. The end effector is coupled to a robotic arm assembly which can move the endoscope relative to the patient. The system includes a computer which controls the movement of the robotic arm in response to input signals received from the foot pedal.
Owner:INTUITIVE SURGICAL OPERATIONS INC
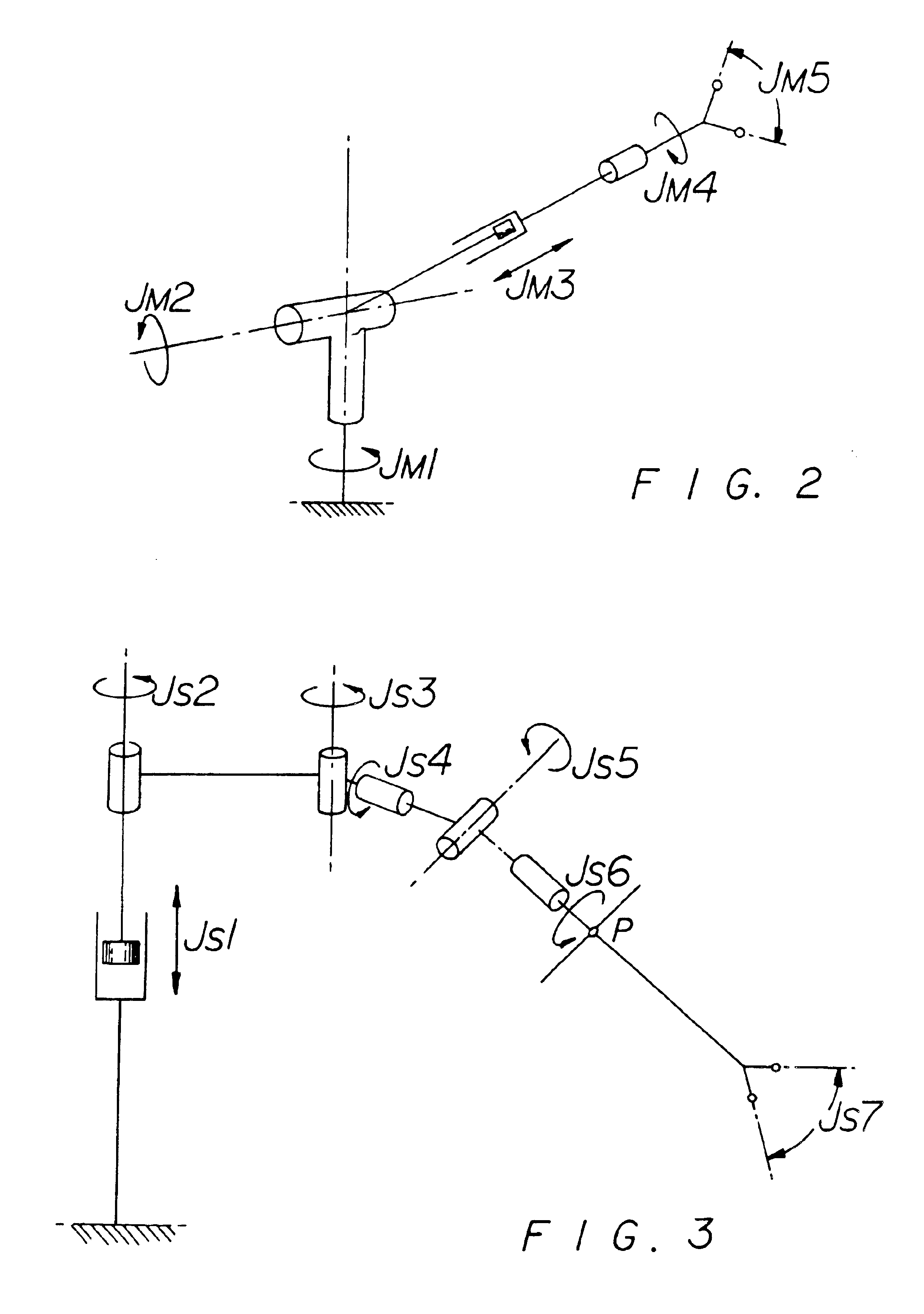
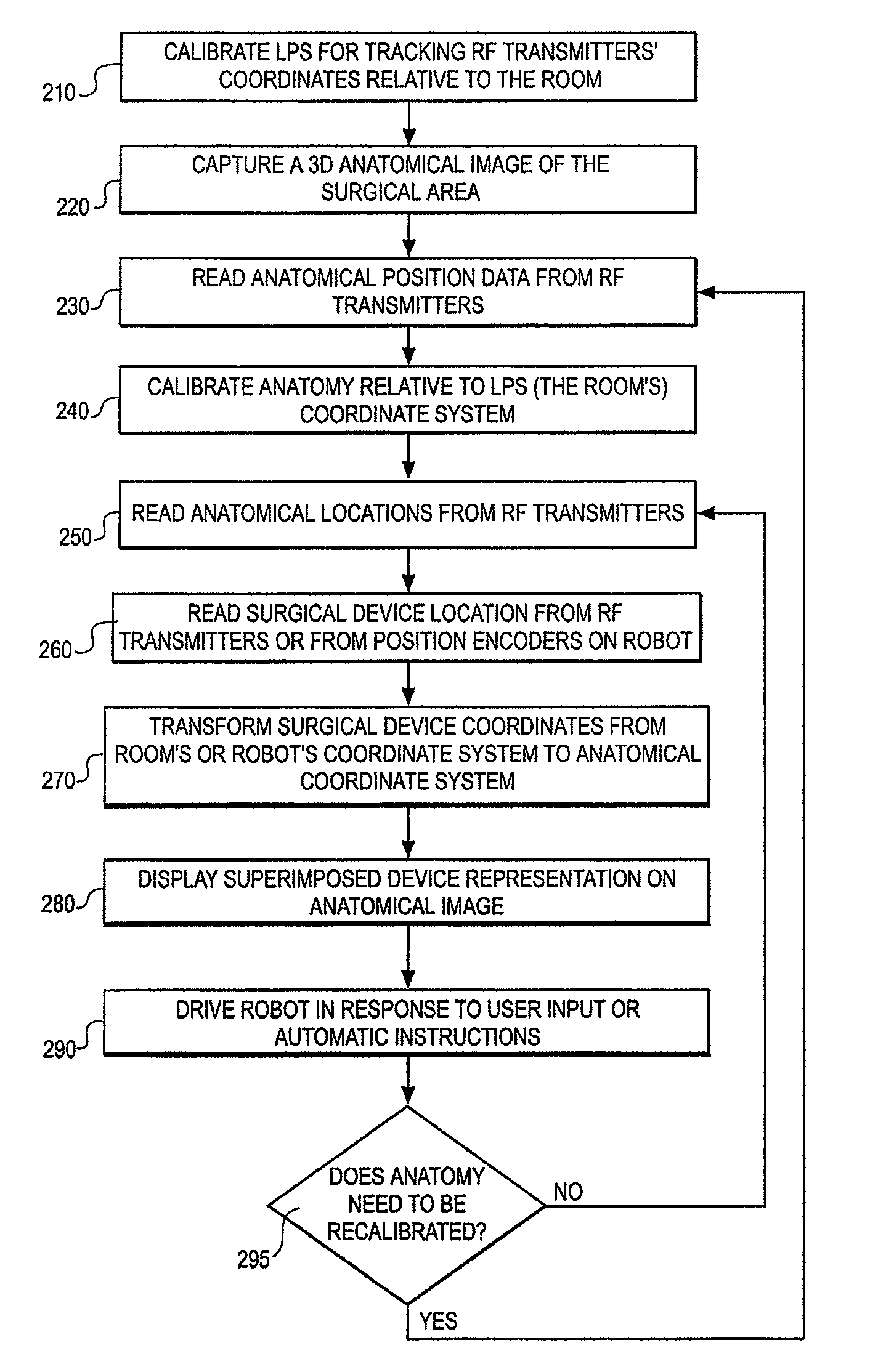
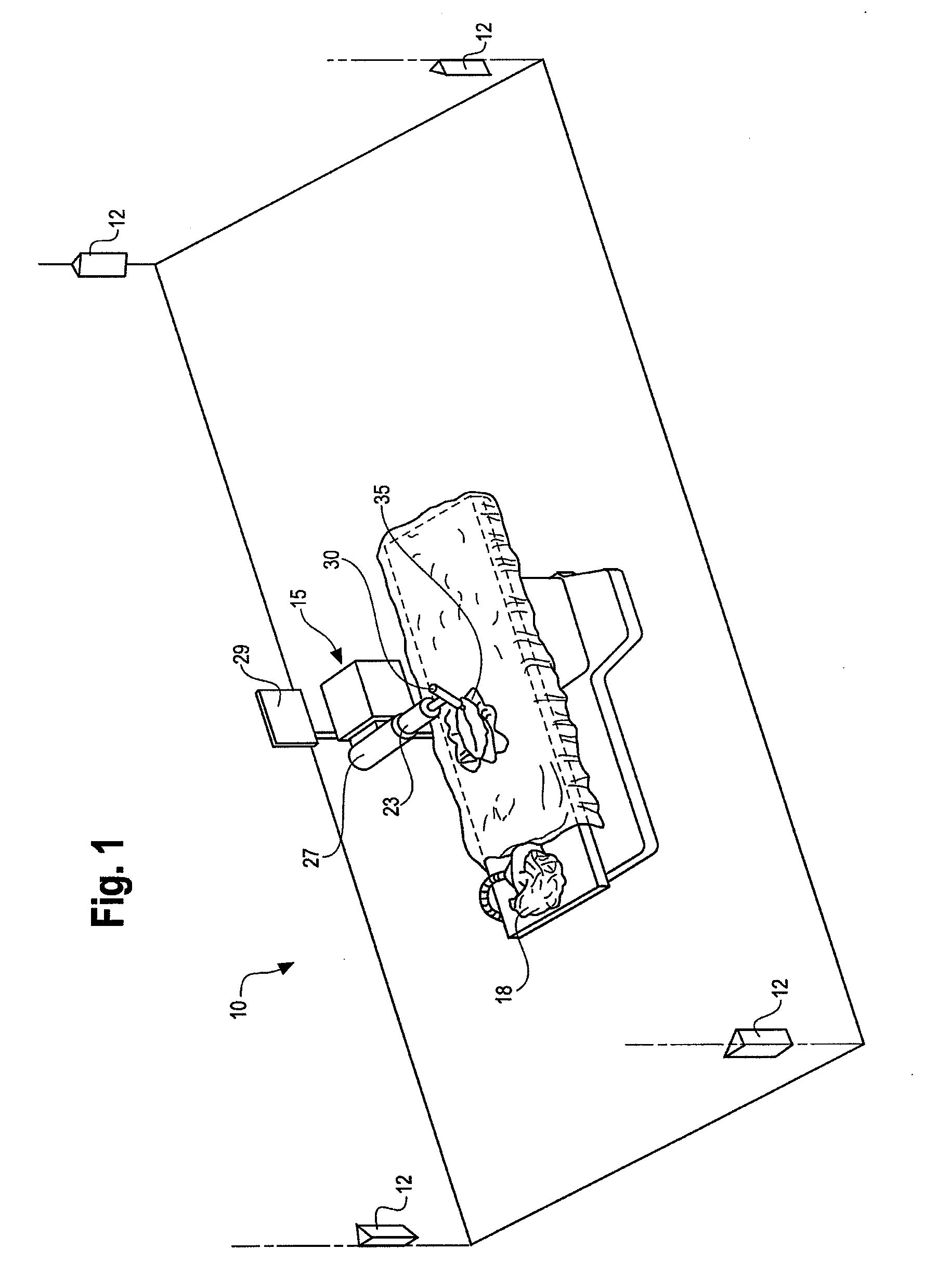
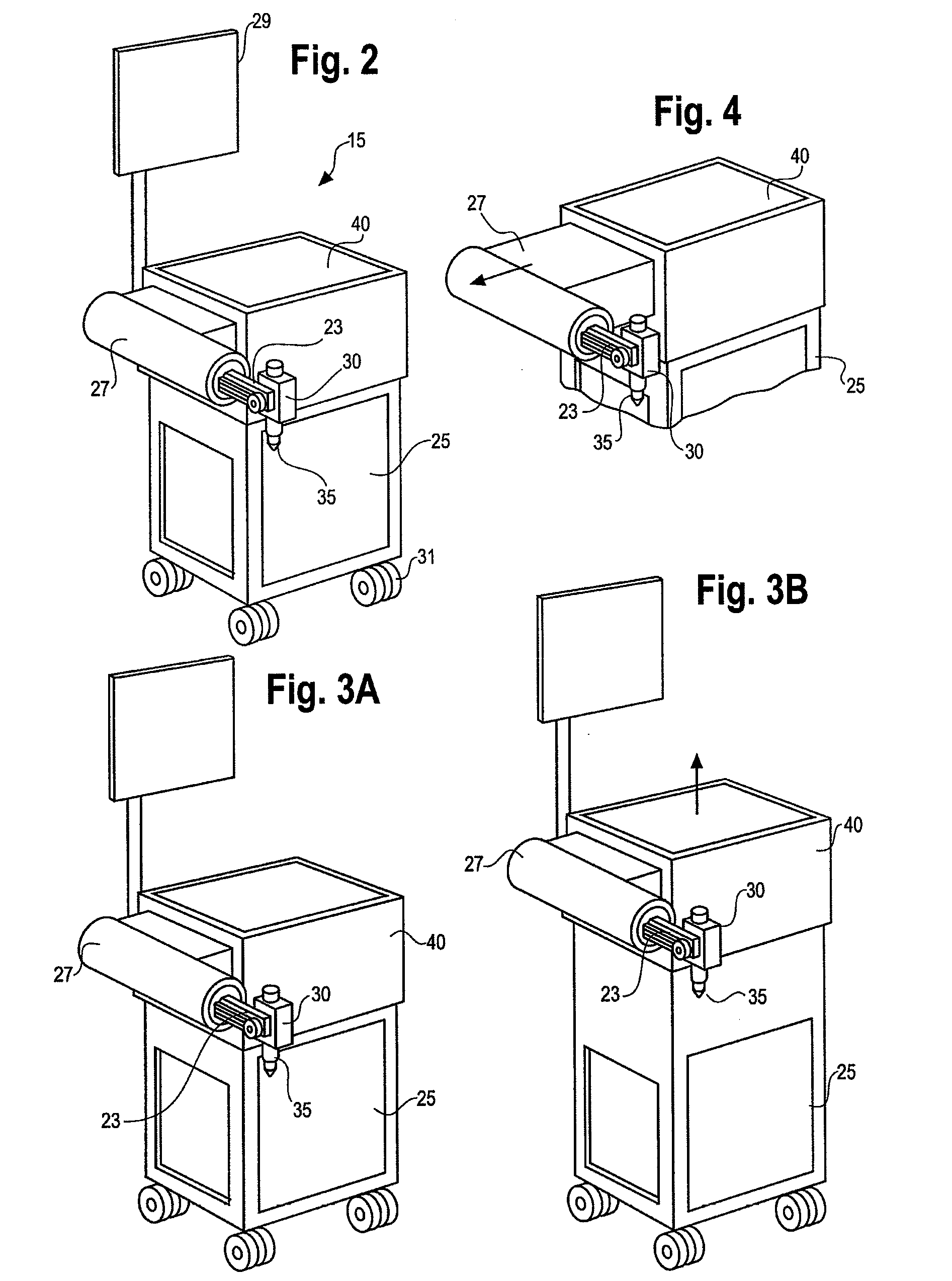
Method and system for performing invasive medical procedures using a surgical robot
ActiveUS8219178B2Degree of precisionInternal osteosythesisSurgical needlesGuidance systemSurgical robot
A method and system for performing invasive procedures includes a surgical robot which is controlled by a guidance system that uses time of flight calculations from RF transmitters embedded in the robot, surgical instrument, and patient anatomy. Sensors around the room detect RF transmissions emitted by the RF transmitters and drive the robot according to a preprogrammed trajectory entered into the guidance system.
Owner:CATHOLIC HEALTHCARE WEST ST JOSEPHS HOSPITAL +1
Electromagnetic photonic catheter for reducing restenosis
InactiveUS6962584B1Reduce spreadImprove scalabilitySurgical instrument detailsCatheterPhotonicsPercent Diameter Stenosis
The method of vascular treatment for restenosis or vulnerable plaque after an invasive procedure, such as for example angioplasty, stenting with or without drug coating, or drug delivery, comprises: inserting a catheter or hollow guide wire to the treatment location; delivering light through the catheter in the wavelength range of about 700–2500 nm; and moving the light to treat the affected region.
Owner:STONE GREGG W +5
Method and apparatus for performing intra-operative angiography
InactiveUS6915154B1Accurately determine extentSensorsBlood flow measurementAfter treatmentAv fistulas
Method for assessing the patency of a patient's blood vessel, advantageously during or after treatment of that vessel by an invasive procedure, comprising administering a fluorescent dye to the patient; obtaining at least one angiographic image of the vessel portion; and evaluating the at least one angiographic image to assess the patency of the vessel portion. Other related methods are contemplated, including methods for assessing perfusion in selected body tissue, methods for evaluating the potential of vessels for use in creation of AV fistulas, methods for determining the diameter of a vessel, and methods for locating a vessel located below the surface of a tissue.
Owner:STRYKER EUROPEAN OPERATIONS LIMITED
Method and system for performing invasive medical procedures using a surgical robot
ActiveUS8219177B2Degree of precisionSurgical needlesSurgical navigation systemsGuidance systemSurgical robot
A method and system for performing invasive procedures includes a surgical robot which is controlled by a guidance system that uses time of flight calculations from RF transmitters embedded in the robot, surgical instrument, and patient anatomy. Sensors around the room detect RF transmissions emitted by the RF transmitters and drive the robot according to a preprogrammed trajectory entered into the guidance system.
Owner:CATHOLIC HEALTHCARE WEST ST JOSEPHS HOSPITAL +1
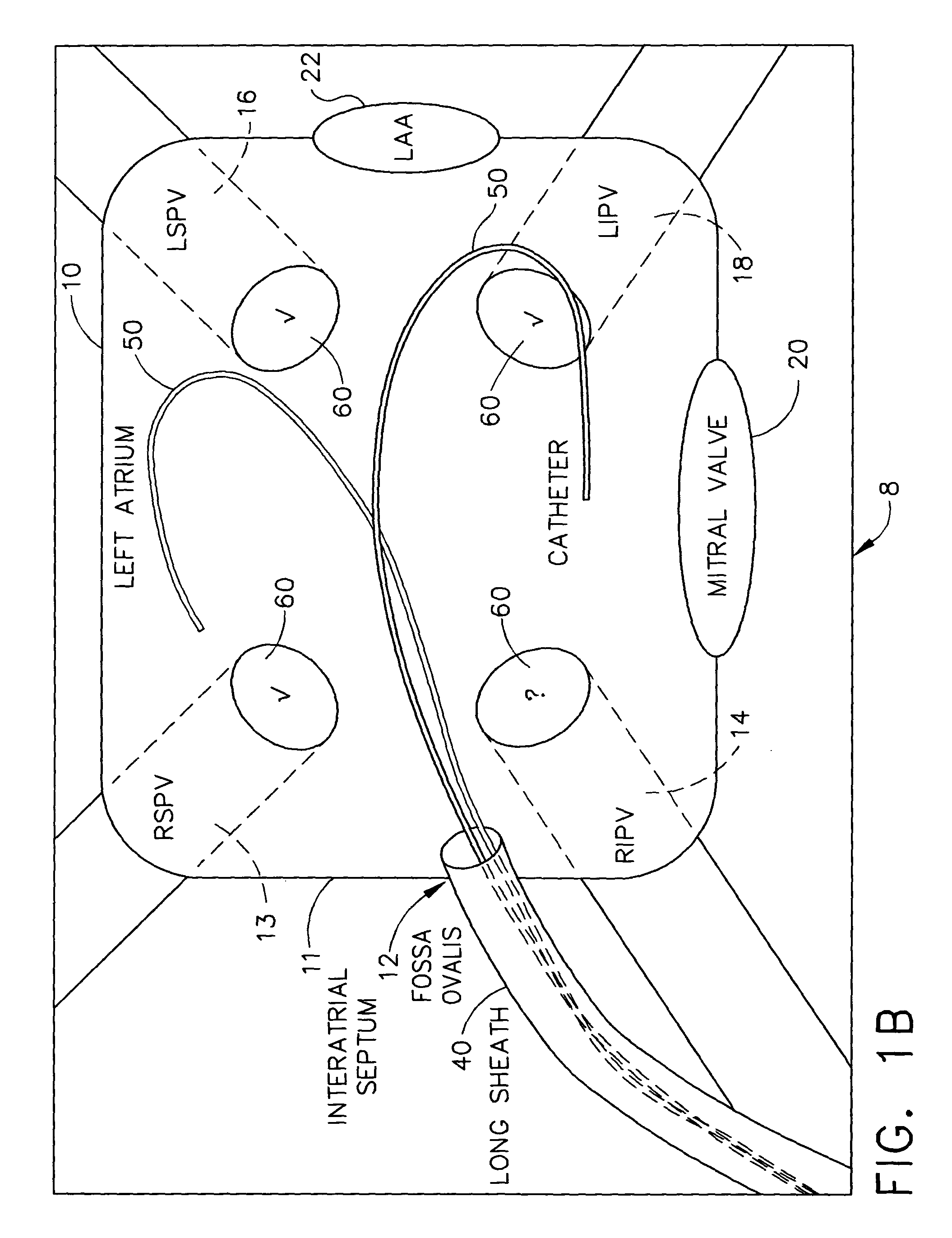
Externally applied RF for pulmonary vein isolation
Owner:BIOSENSE WEBSTER INC
Method and system for performing invasive medical procedures using a surgical robot
ActiveUS20080154389A1Degree of precisionSurgical needlesSurgical navigation systemsGuidance systemEngineering
A method and system for performing invasive procedures includes a surgical robot which is controlled by a guidance system that uses time of flight calculations from RF transmitters embedded in the robot, surgical instrument, and patient anatomy. Sensors around the room detect RF transmissions emitted by the RF transmitters and drive the robot according to a preprogrammed trajectory entered into the guidance system.
Owner:CATHOLIC HEALTHCARE WEST ST JOSEPHS HOSPITAL +1
Conditioned cell culture medium compositions and methods of use
InactiveUS7118746B1Eliminate wrinkles, frown lines, scarringCondition the skinOrganic active ingredientsCosmetic preparationsReserve CellCell culture media
Novel products comprising conditioned cell culture medium compositions and methods of use are described. The conditioned cell medium compositions of the invention may be comprised of any known defined or undefined medium and may be conditioned using any eukaryotic cell type. The medium may be conditioned by stromal cells, parenchymal cells, mesenchymal stem cells, liver reserve cells, neural stem cells, pancreatic stem cells and / or embryonic stem cells. Additionally, the cells may be genetically modified. A three-dimensional tissue construct is preferred. Once the cell medium of the invention is conditioned, it may be used in any state. Physical embodiments of the conditioned medium include, but are not limited to, liquid or solid, frozen, lyophilized or dried into a powder. Additionally, the medium is formulated with a pharmaceutically acceptable carrier as a vehicle for internal administration, applied directly to a food item or product, formulated with a salve or ointment for topical applications, or, for example, made into or added to surgical glue to accelerate healing of sutures following invasive procedures. Also, the medium may be further processed to concentrate or reduce one or more factors or components contained within the medium.
Owner:ALLERGAN INC
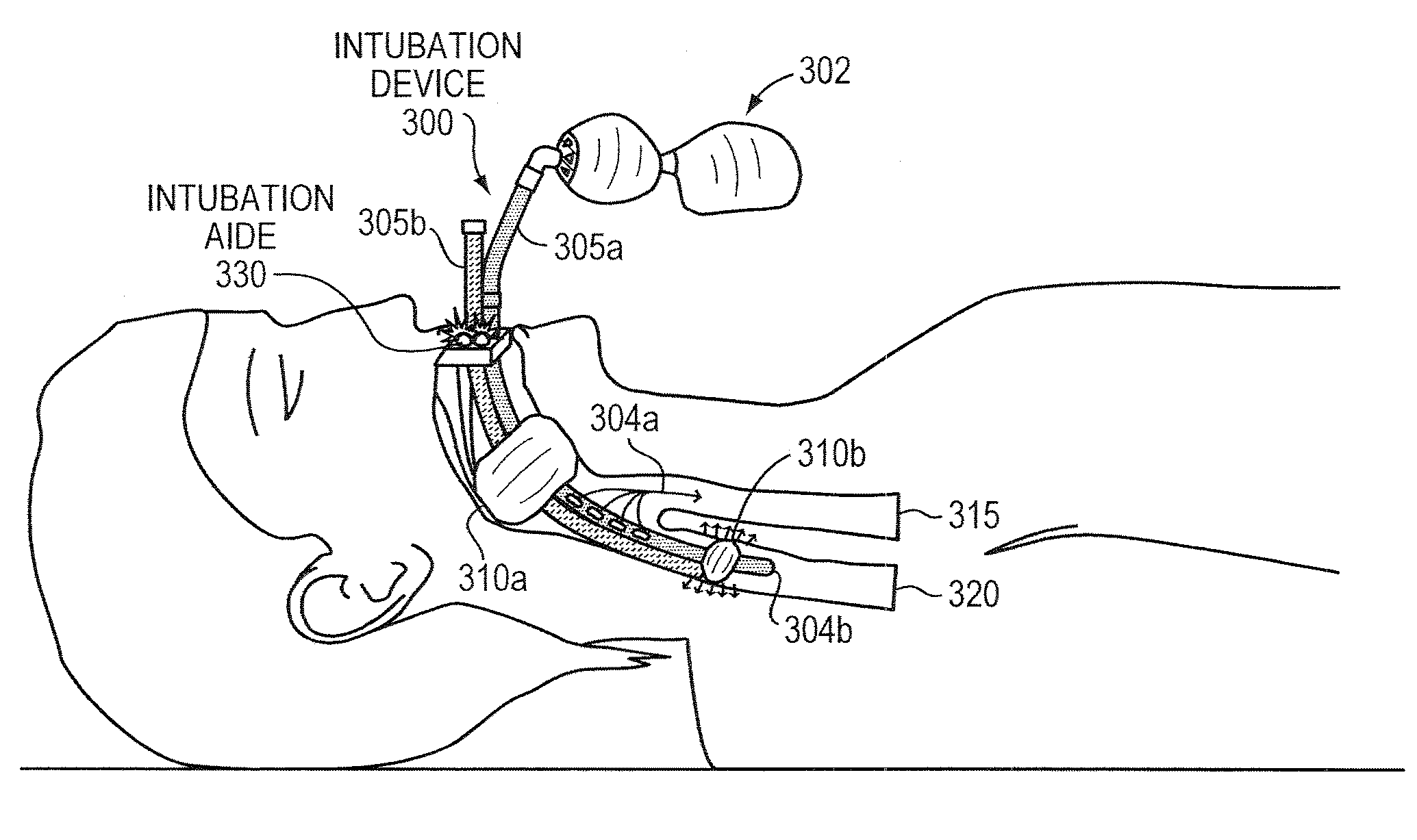
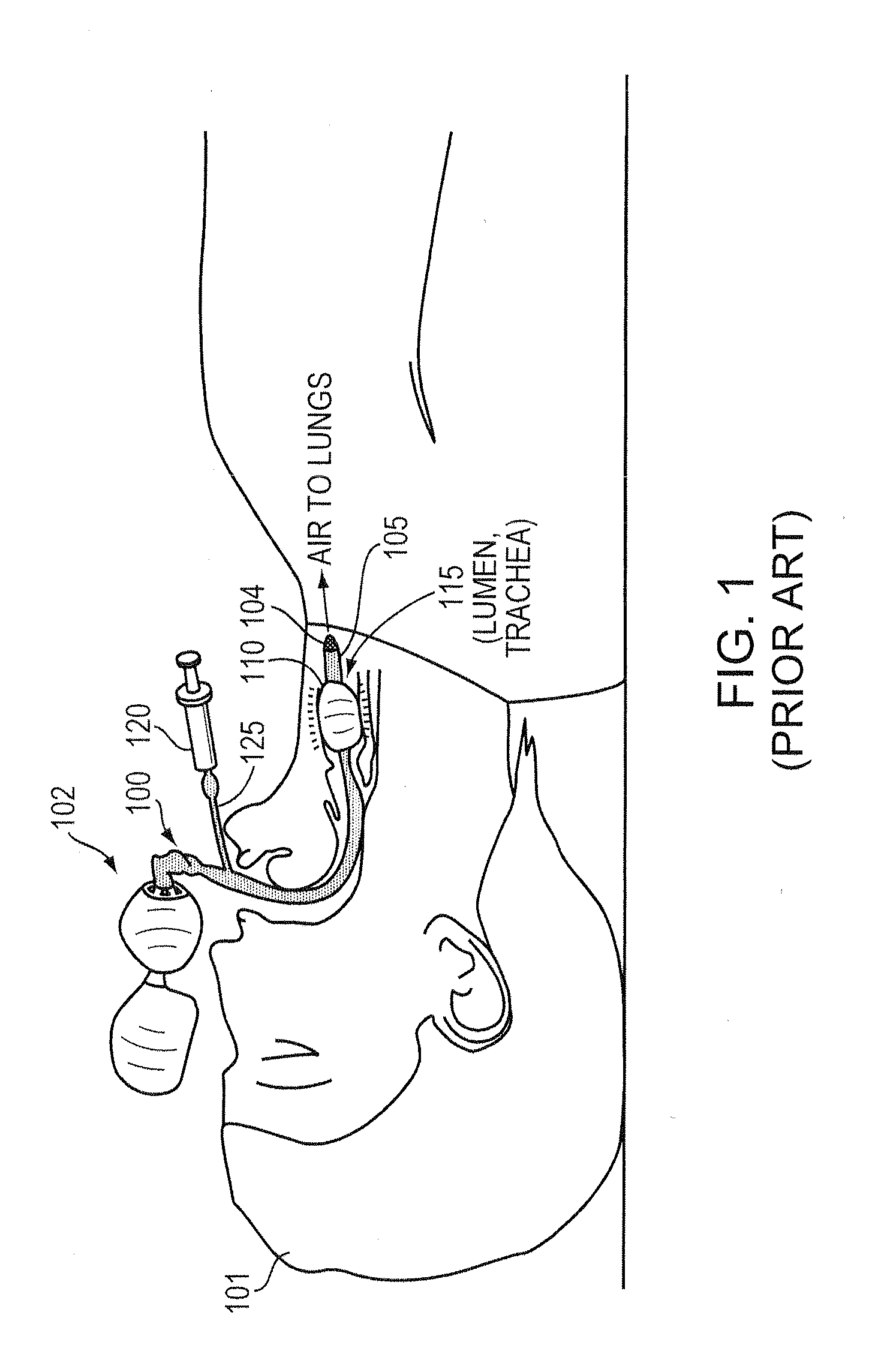
Methods and apparatus for safe application of an intubation device
InactiveUS20100163023A1Accurately maintains pressureDirect contact guaranteeRespiratory device testingMedical devicesInternal bleedingCuff
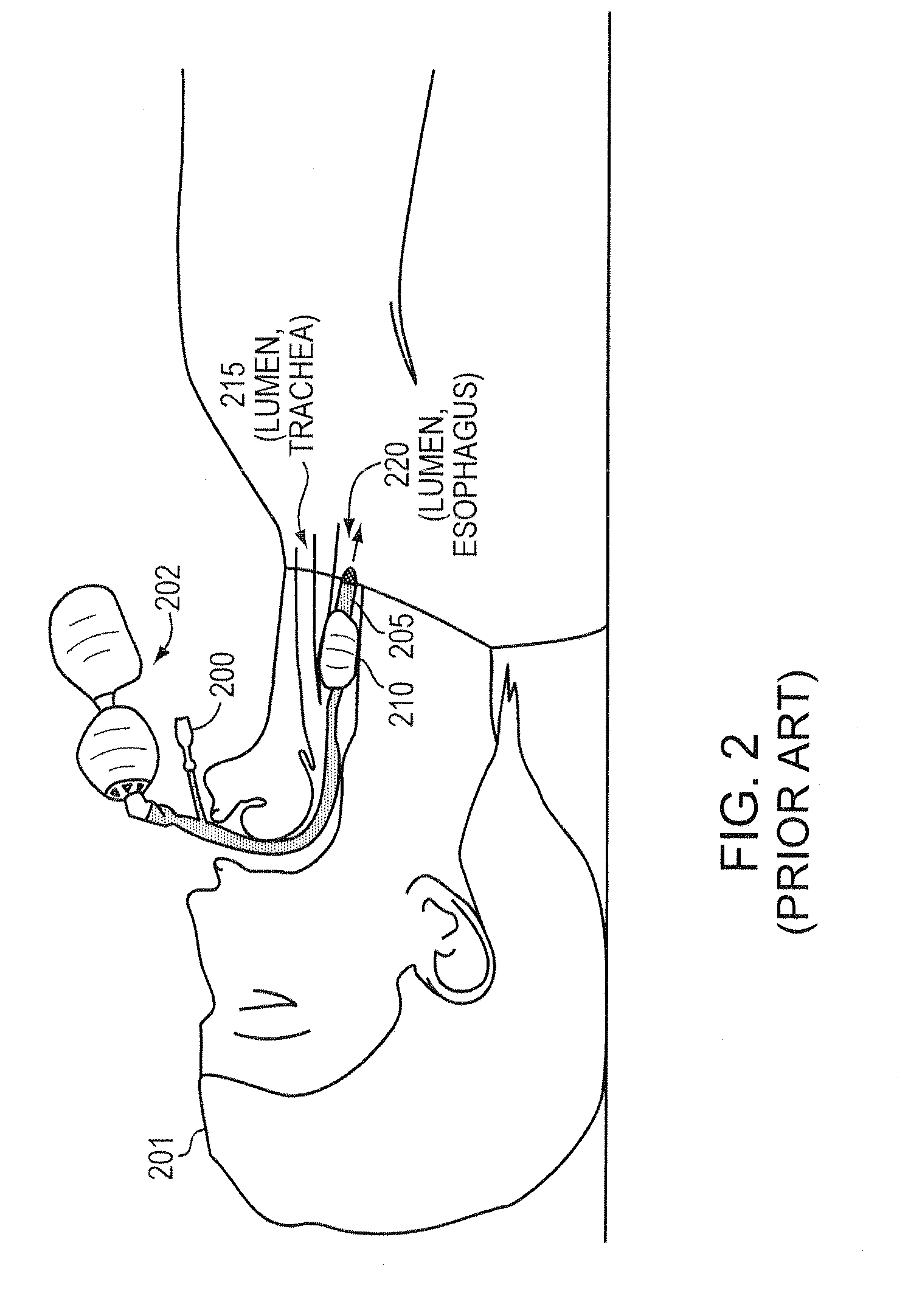
Intubation is a potentially dangerous invasive procedure with many plausible errors, such as over-inflation of a cuff and insertion of an intubation tube in the wrong lumen, potentially resulting in a patient's internal bleeding, suffocation, or even death. An intubation aide according to example embodiments of the present invention allows intubation of a patient, while eliminating potential injury to the patient, increasing accuracy and reliability of the placement of the intubation tube, and drastically decreasing procedural time. Within moments of insertion of the device into a patient, the medical caregiver knows, with complete certainty, the location of the intubation device without applying traditional time-consuming tasks. Embodiments also provide patient safety, if intubated for a prolonged periods, by regulating an inflation pressure of the cuff. The intubation aide can also be used for training purposes and is ideal for intubation in hospital and field settings.
Owner:SINGH MANU B
Medical instruments for performing minimally-invasive procedures
Owner:LUMENDI LTD
Externally applied RF for pulmonary vein isolation
InactiveUS20050101946A1Eliminate needInternal electrodesDiagnostic recording/measuringVeinInsertion stent
A resonant circuit is incorporated in a stent, which implantable in a pulmonary vein using known cardiac catheterization techniques. When an external RF field is generated at the resonant frequency of the stent, RF energy is re-radiated by the stent toward electroconductive tissue in the wall of the pulmonary vein, and produces a circumferential conduction block. The stent can be made of biodegradable materials, so that it eventually is resorbed. Following an ablation procedure, the stent may be left in situ. Repeated ablation can be performed using the inserted stent until it has been determined that the desired lesions have been formed. Furthermore, the same stent can potentially be used even years after being inserted should the treated arrhythmia reoccur or a new arrhythmia develop, thereby possibly obviating the need for an invasive procedure at that future time.
Owner:BIOSENSE WEBSTER INC
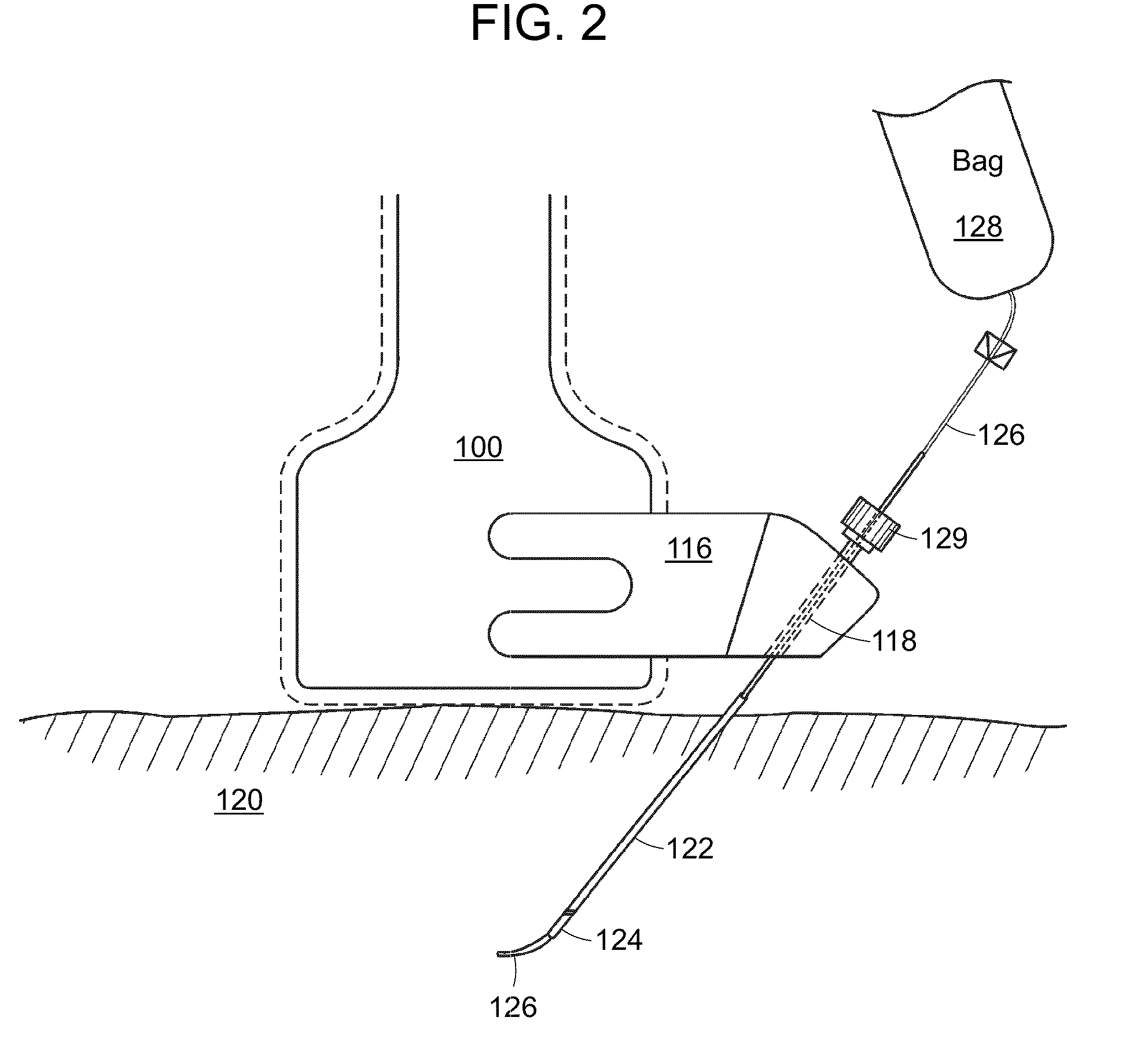
Sequential implant delivery system
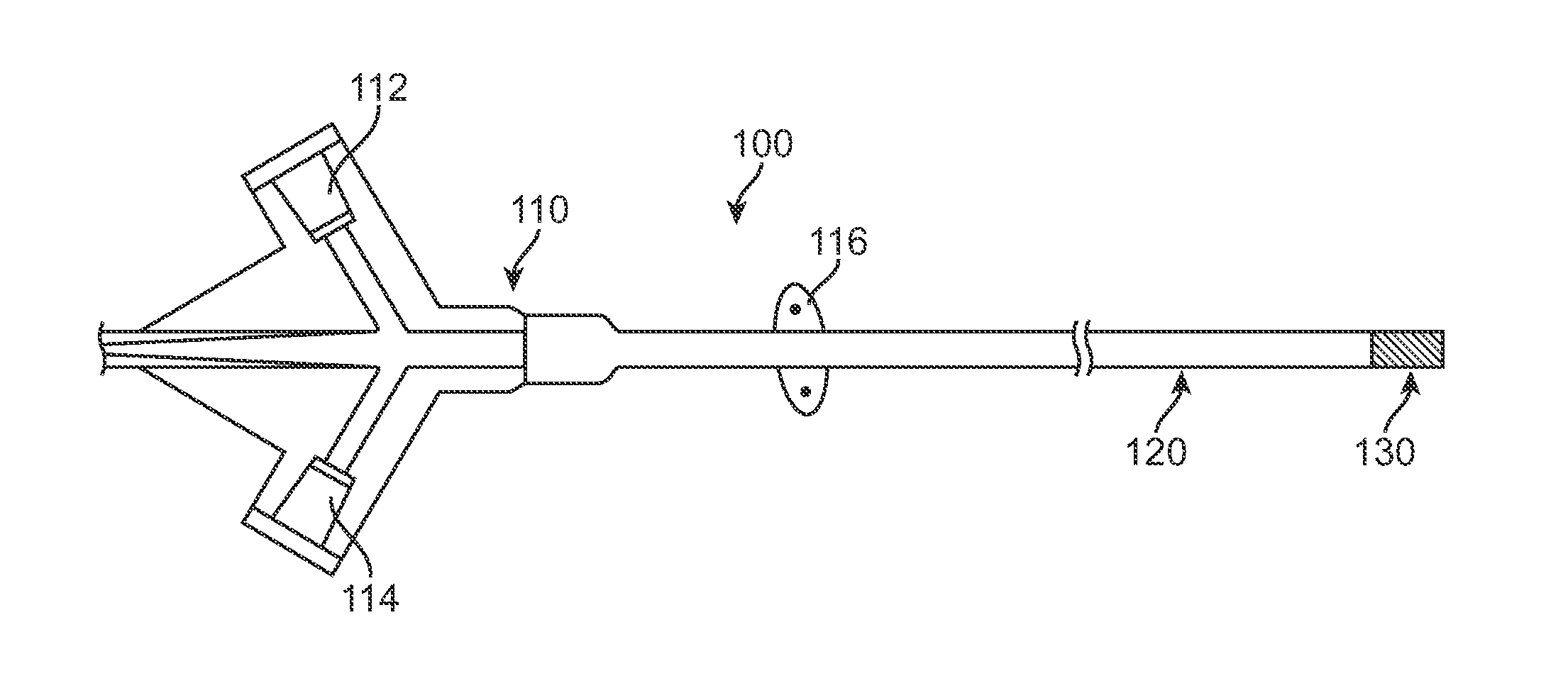
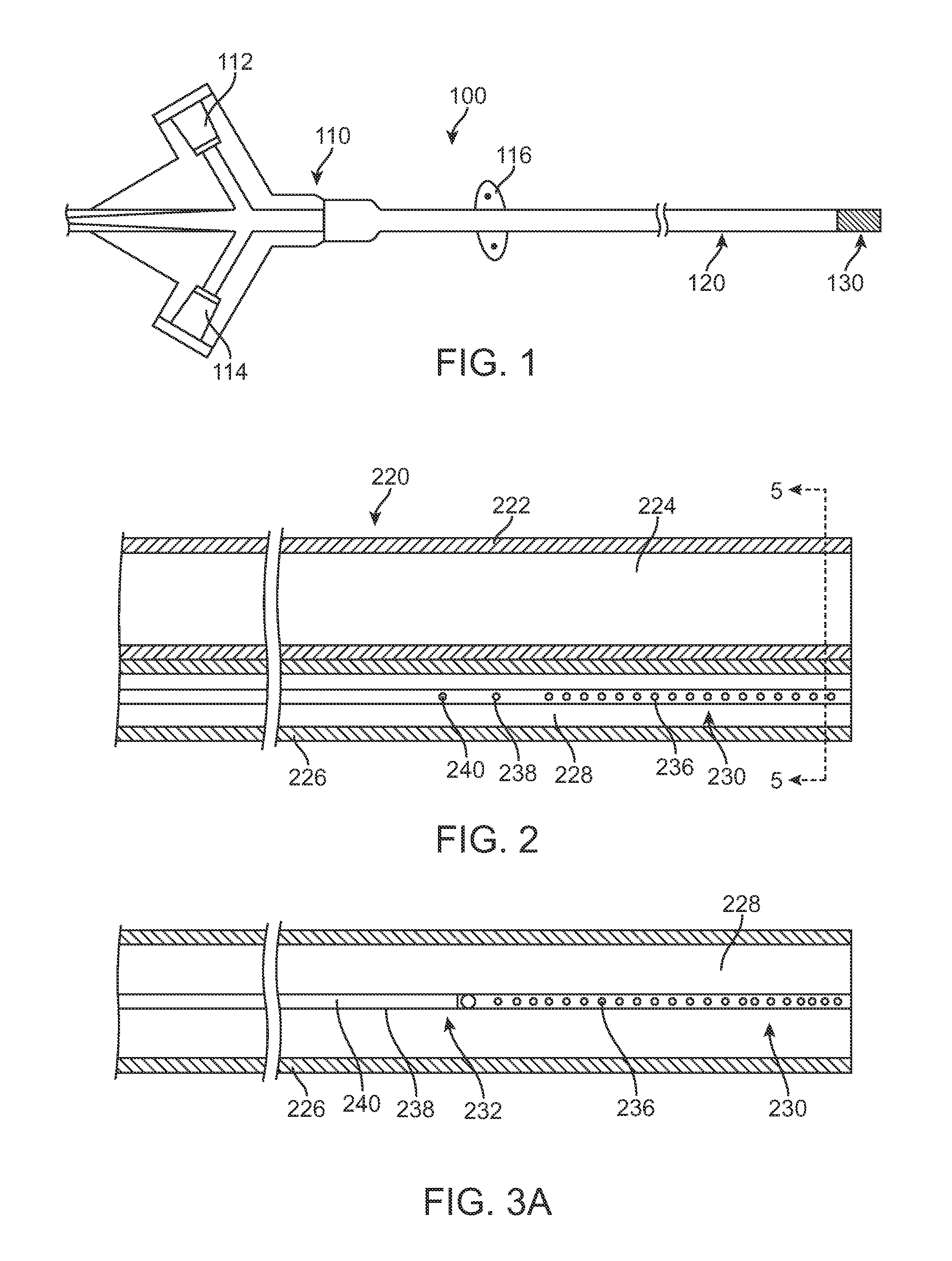
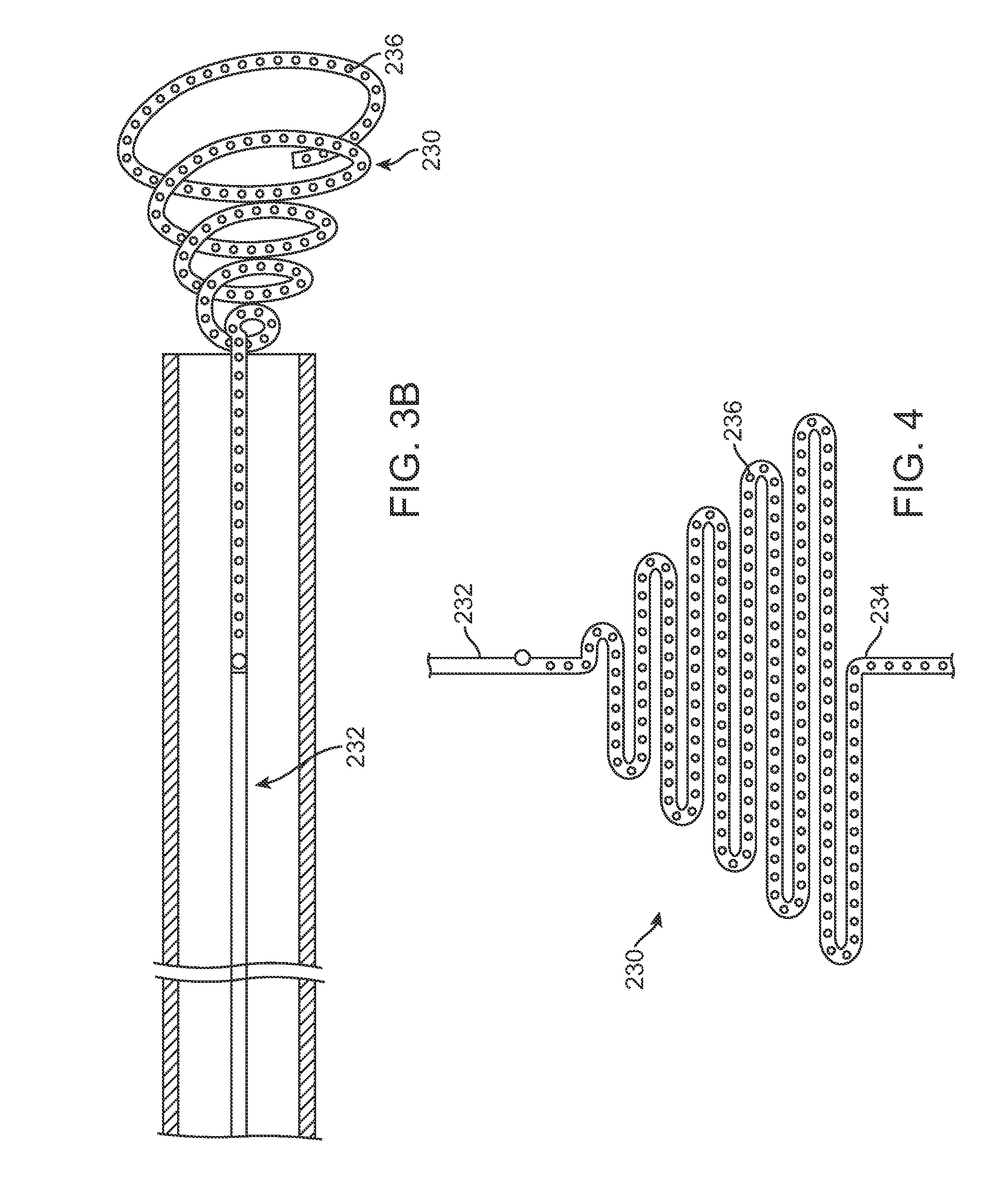
A method and device includes advancing a first stent and a second stent into a stenosed region of a blood vessel to protect or shield the vessel from possible blockage. The delivery device may include placement rings, selectively engagable by positioning members disposed on the outer wall of in inner catheter. The positioning members and the placement rings may be utilized to accurately place multiple stents within an afflicted vessel, in a single invasive procedure.
Owner:COOK MEDICAL TECH LLC
Needle Guides for Catheter Delivery
InactiveUS20100041990A1Improve visualizationOvercome limitationsUltrasonic/sonic/infrasonic diagnosticsCannulasVeinInvasive Procedure
Provided are needle locks and methods of using the same for ultrasound guided catheter insertion. The needle locks hereof substantially immobilizes a needle relative to an ultrasound probe to permit manipulation of a catheter or other device through the needle. The needle locks of the present invention may be used in nerve block, venous catheter insertion procedures or other invasive procedures.
Owner:ANES VENTURES
Method and apparatus for performing intra-operative angiography
Method for assessing the patency of a patient's blood vessel, advantageously during or after treatment of that vessel by an invasive procedure, comprising administering a fluorescent dye to the patient; obtaining at least one angiographic image of the vessel portion; and evaluating the at least one angiographic image to assess the patency of the vessel portion. Other related methods are contemplated, including methods for assessing perfusion in selected body tissue, methods for evaluating the potential of vessels for use in creation of AV fistulas, methods for determining the diameter of a vessel, and methods for locating a vessel located below the surface of a tissue.
Owner:NOVADAG TECH INC
Apparatus and methods for performing minimally-invasive surgical procedures
InactiveUS20150142041A1Prevent axial movementPrevent rotationCannulasSurgical needlesSurgical departmentInvasive Procedure
Devices, tools and methods for performing minimally invasive surgical procedures. Methods of performing minimally invasive ablation procedures. Methods of performing rapid exchange of tools in a device while the device remains in a reduced-access surgical space.
Owner:MAQUET CARDIOVASCULAR LLC
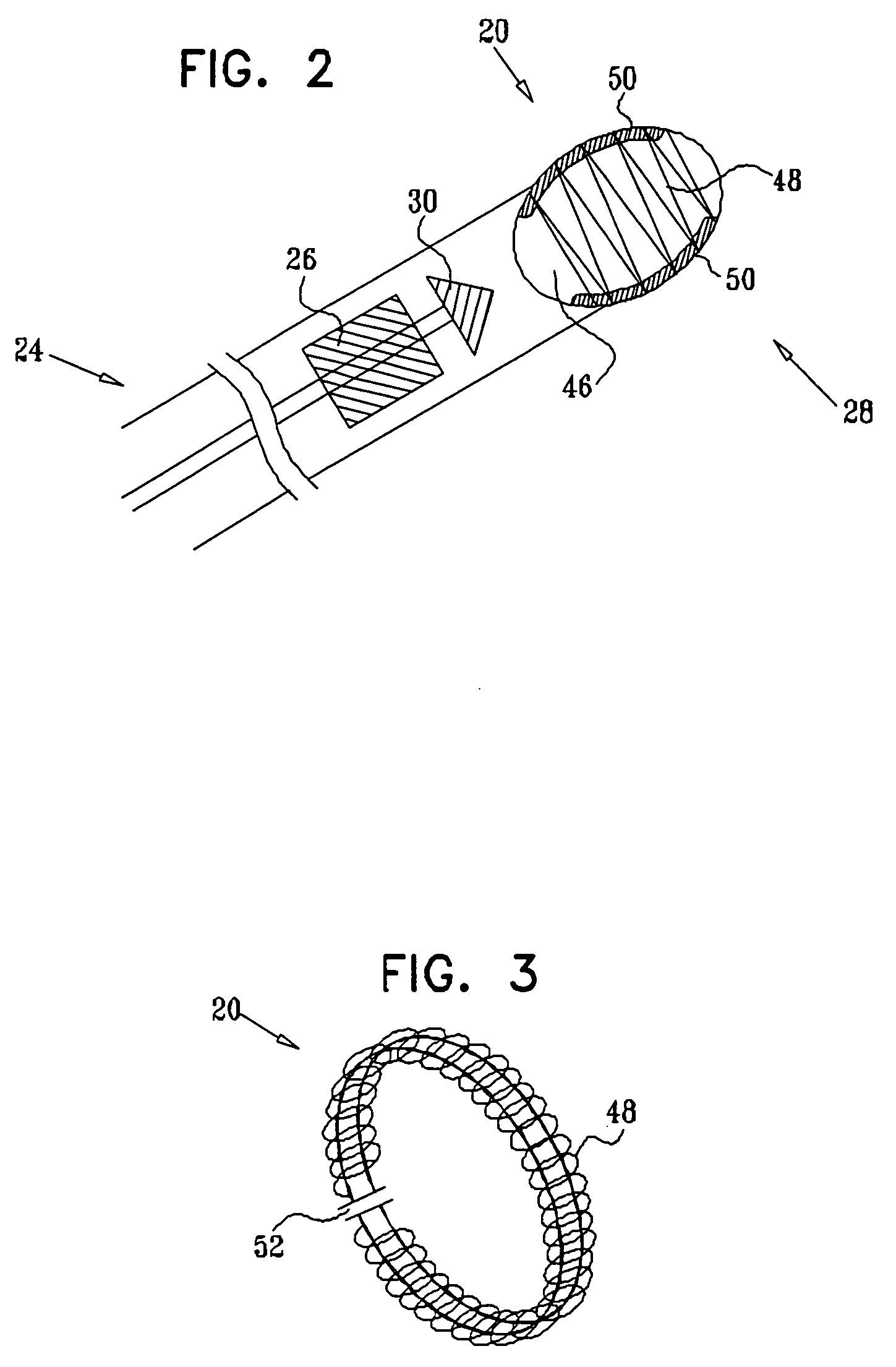
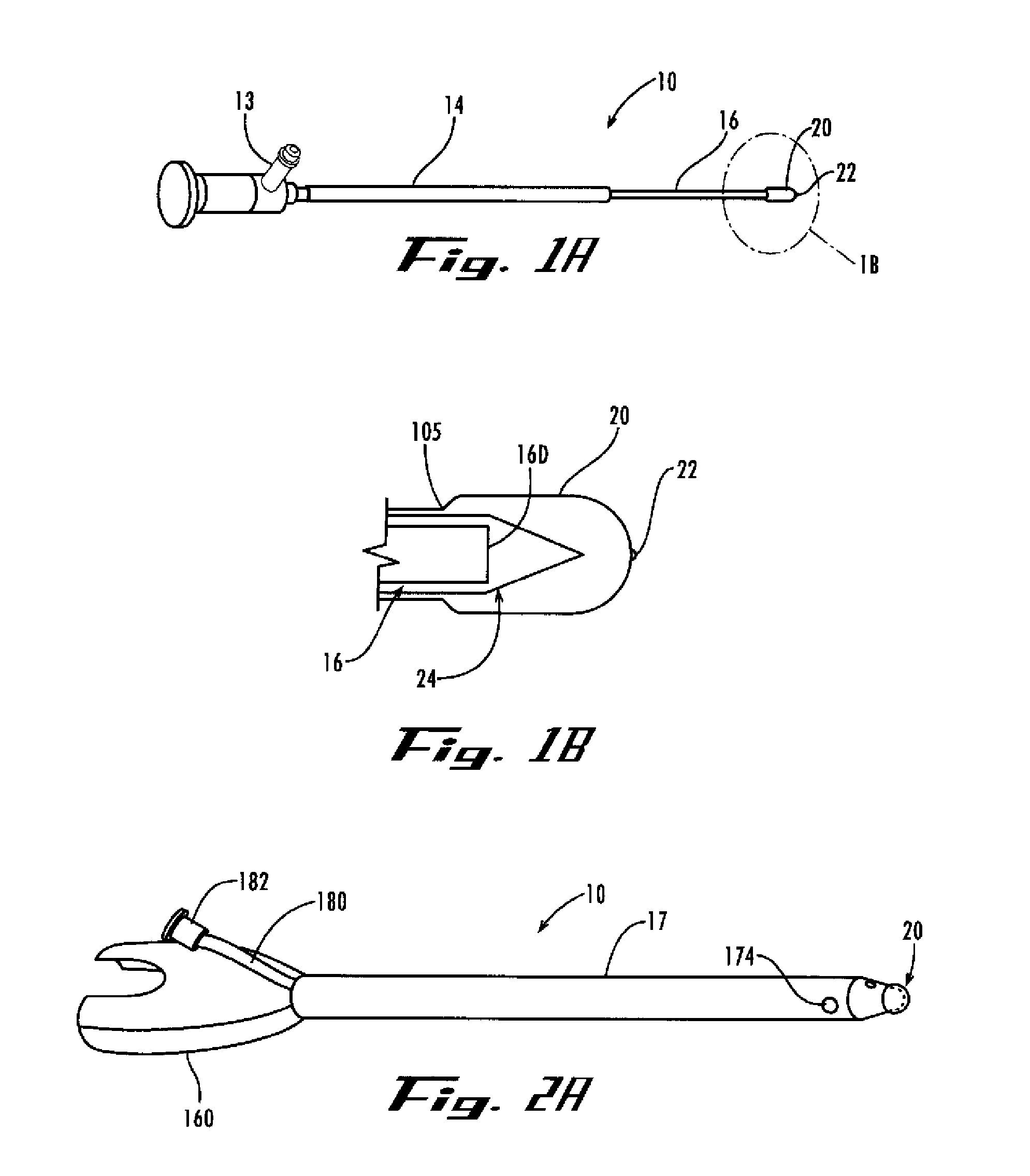
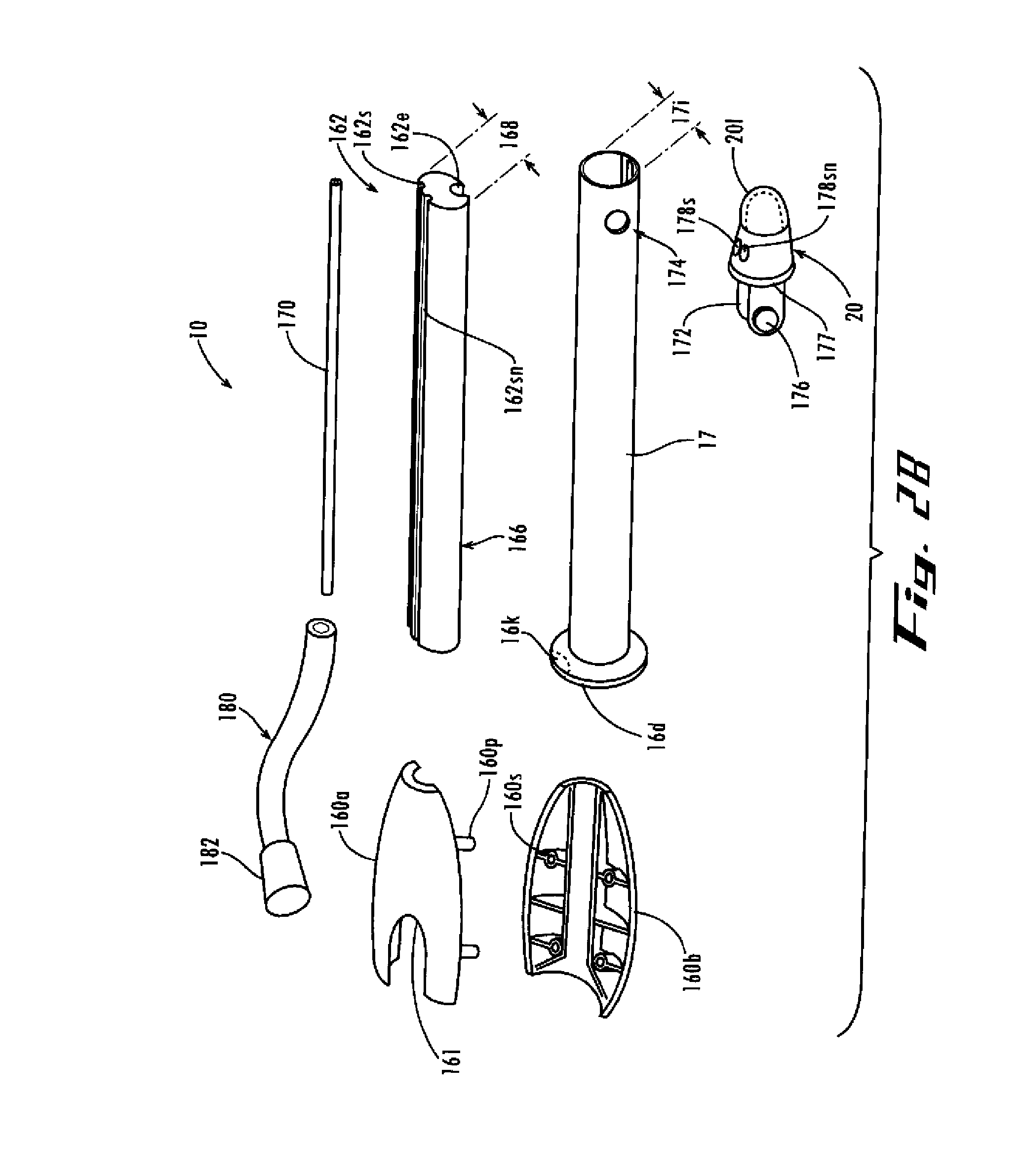
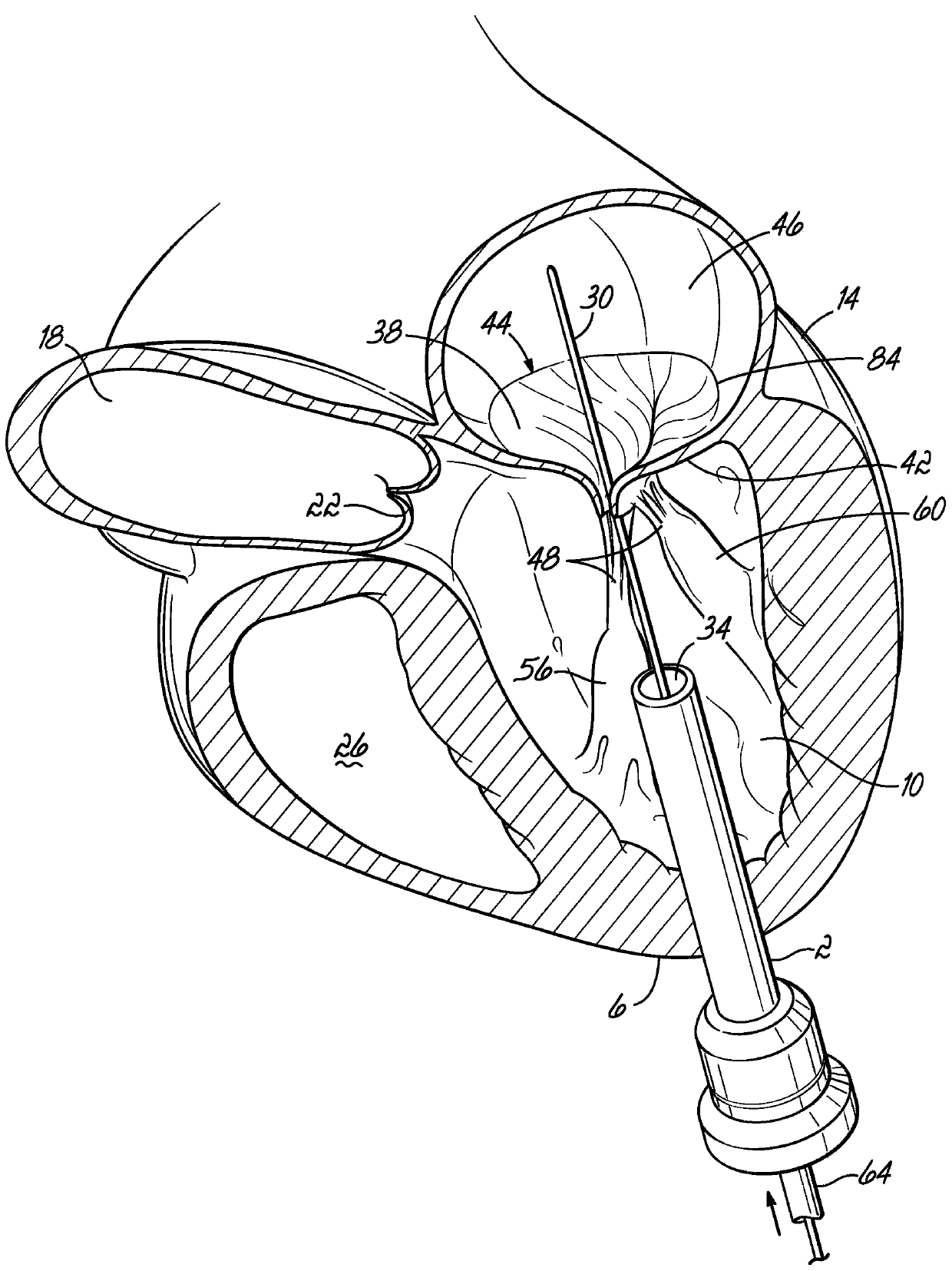
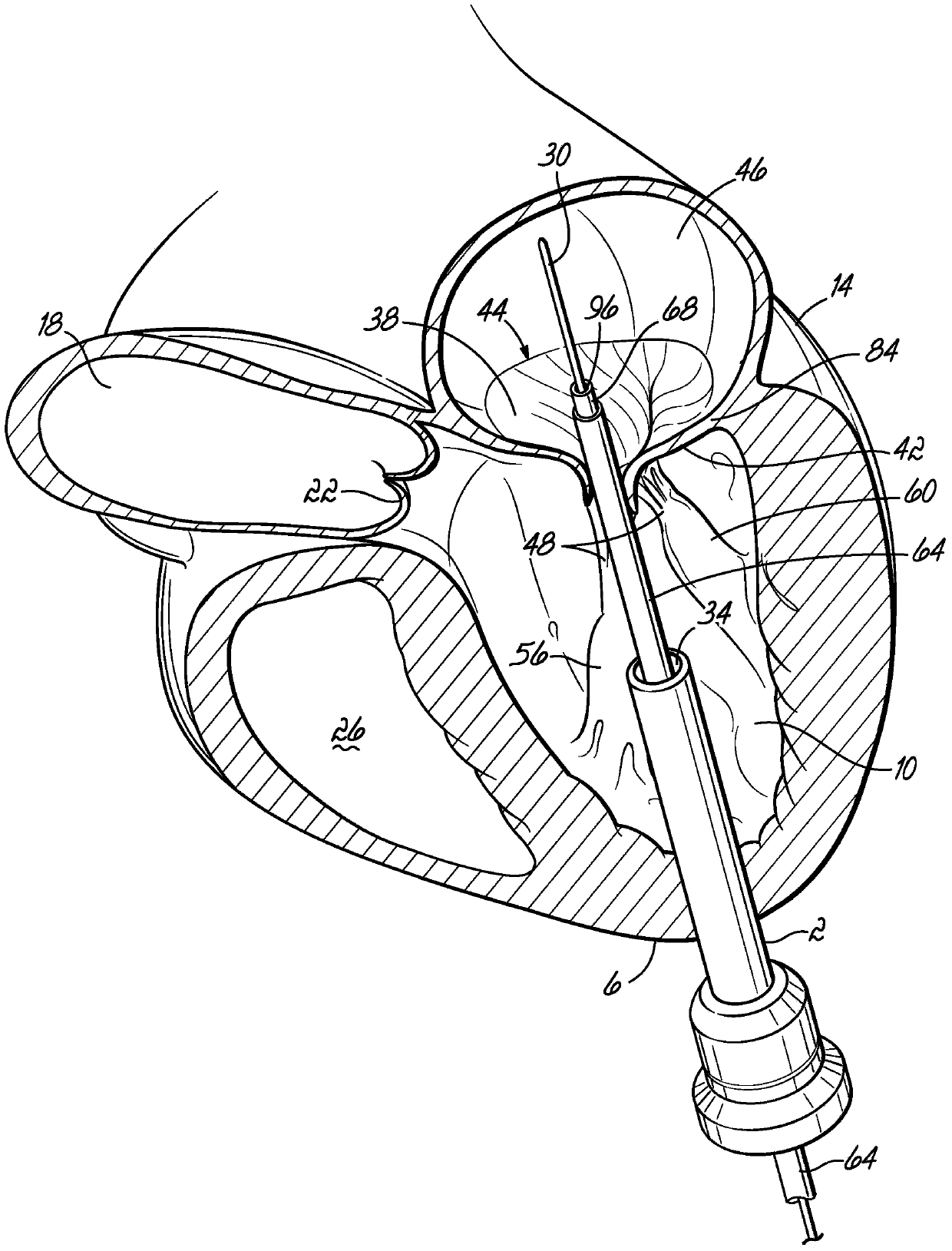
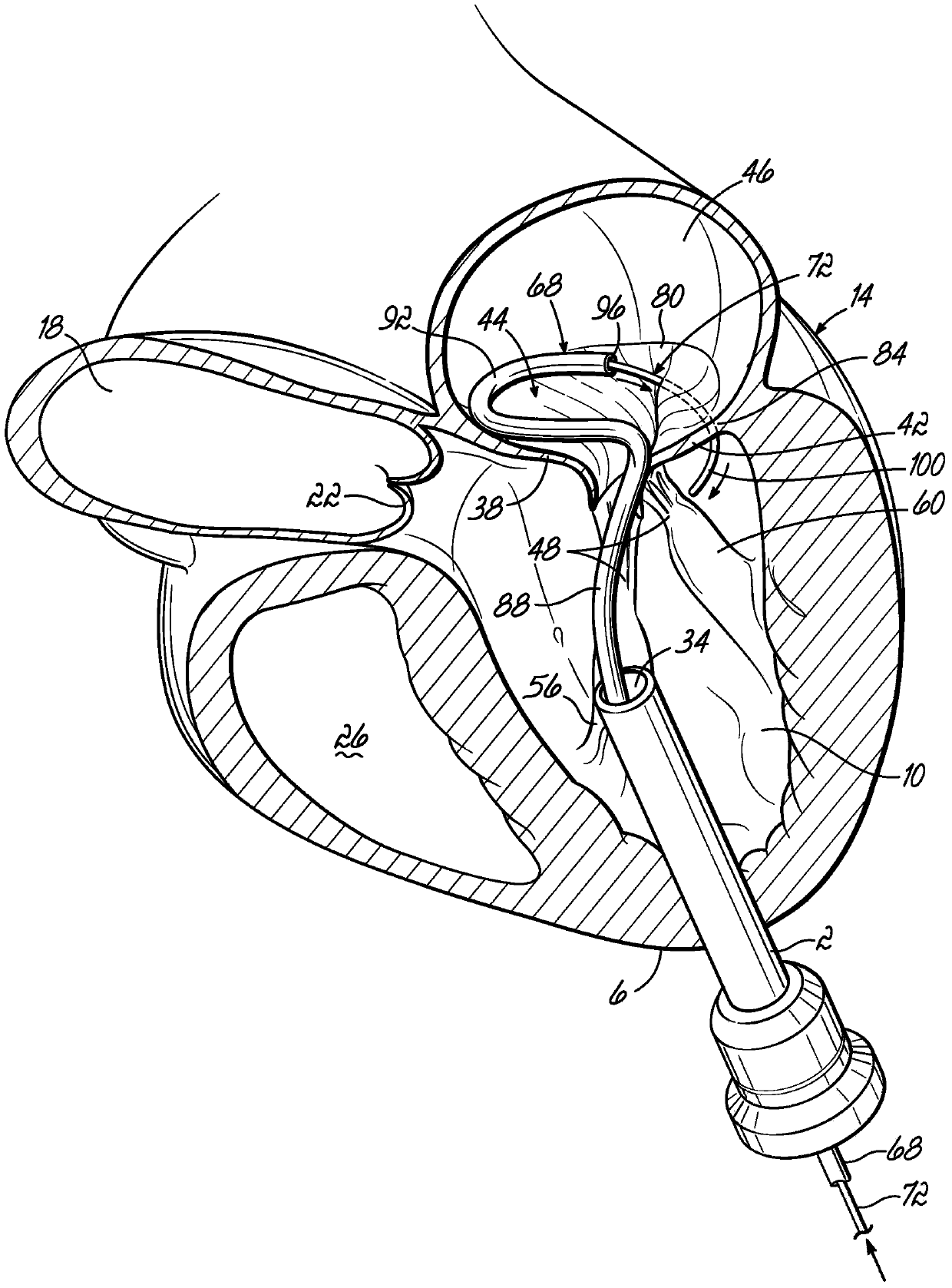
Mitral valve docking devices, systems and methods
Various systems, devices and methods associated with the placement of a dock or anchor (72) for a prosthetic mitral valve (120). The anchor (72) may take the form of a helical anchor having multiple coils (104, 108) and / or a stent-like structure. Various methods include different levels of minimal invasive procedures for delivering the prosthetic valve anchor (72) and prosthetic valve (120), as well as tissue anchors for plication or other purposes to the mitral valve position in the heart (14).
Owner:MITRAL VALVE TECHNOLOGIES SARL
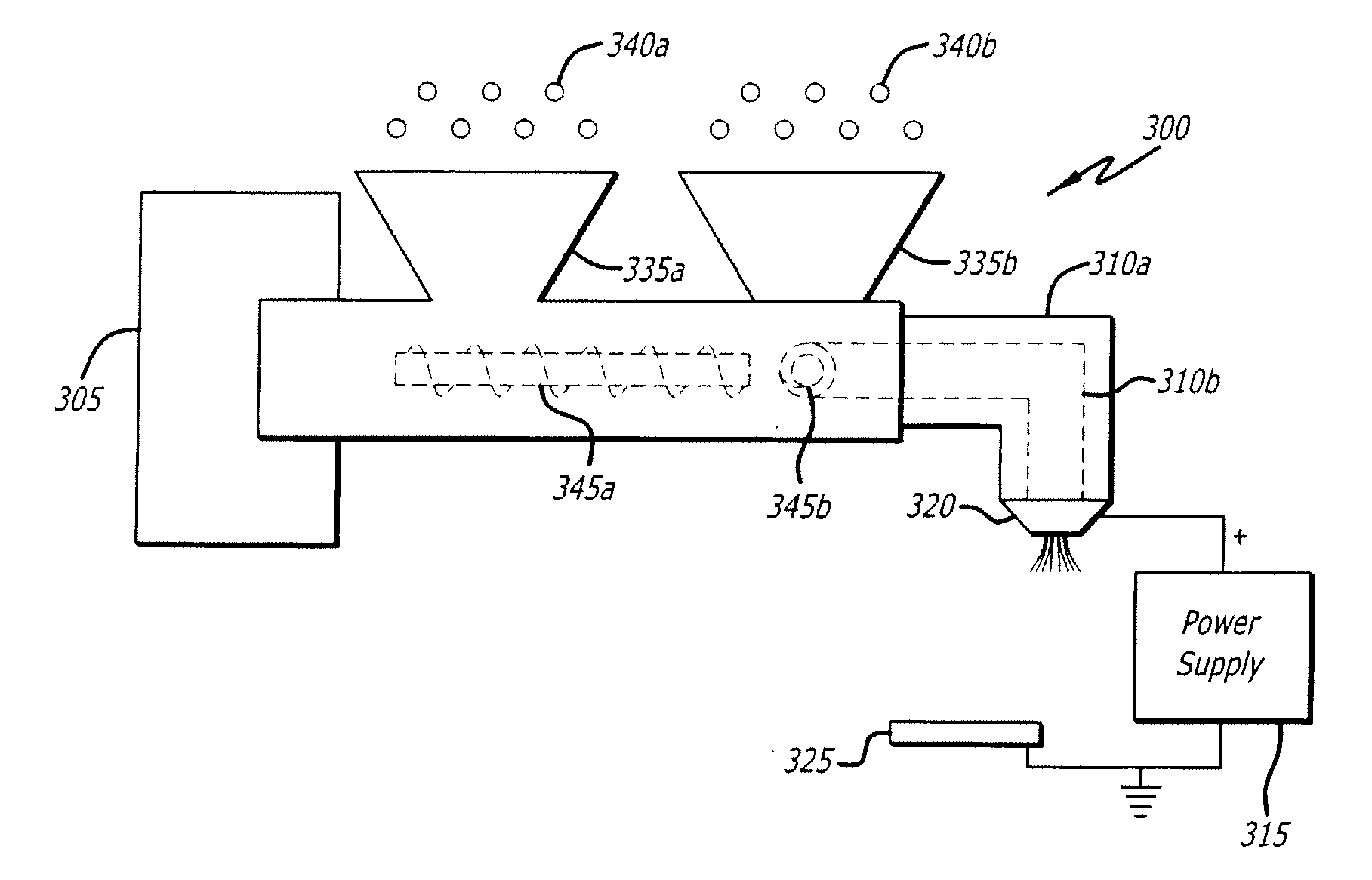
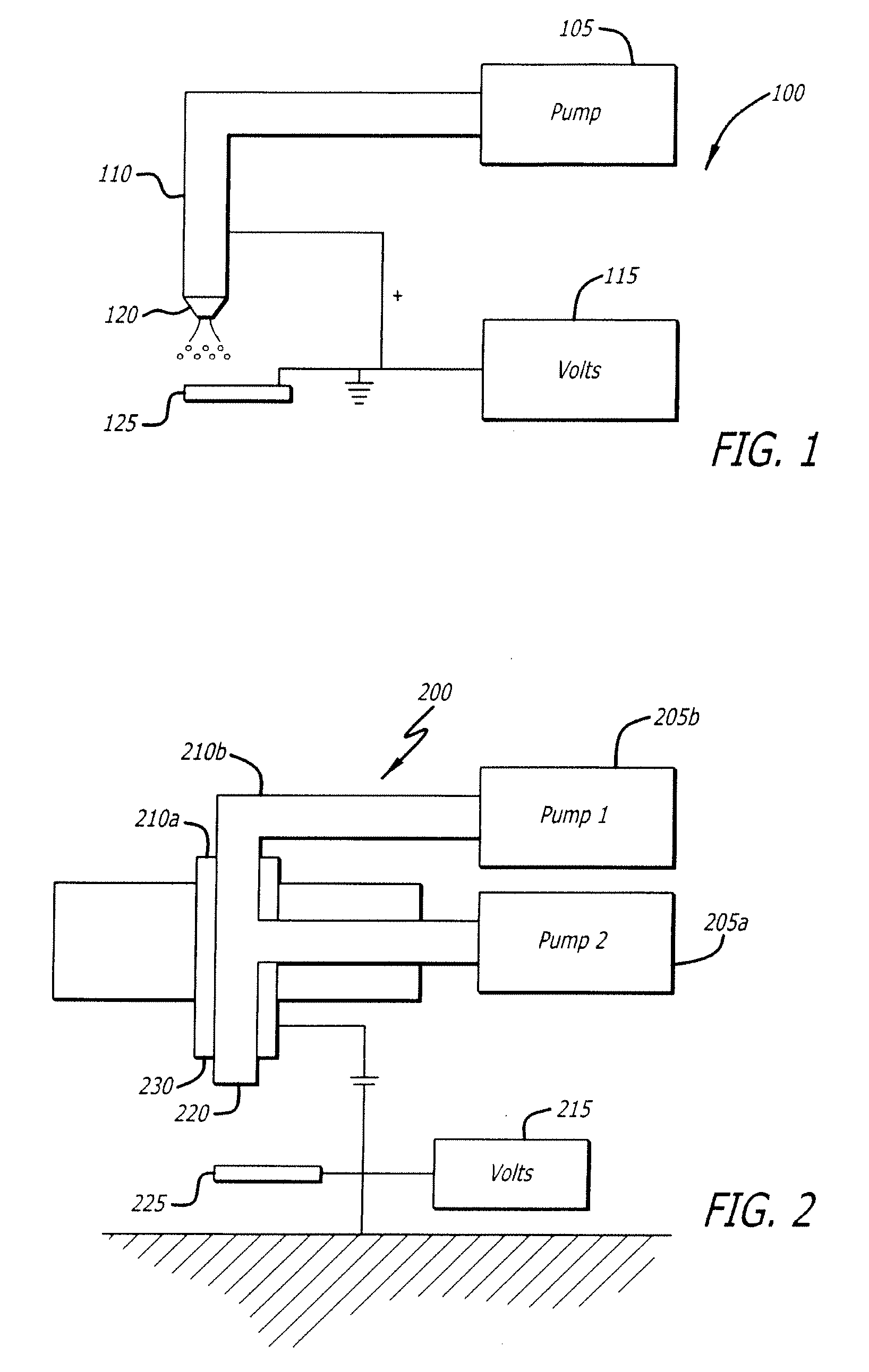
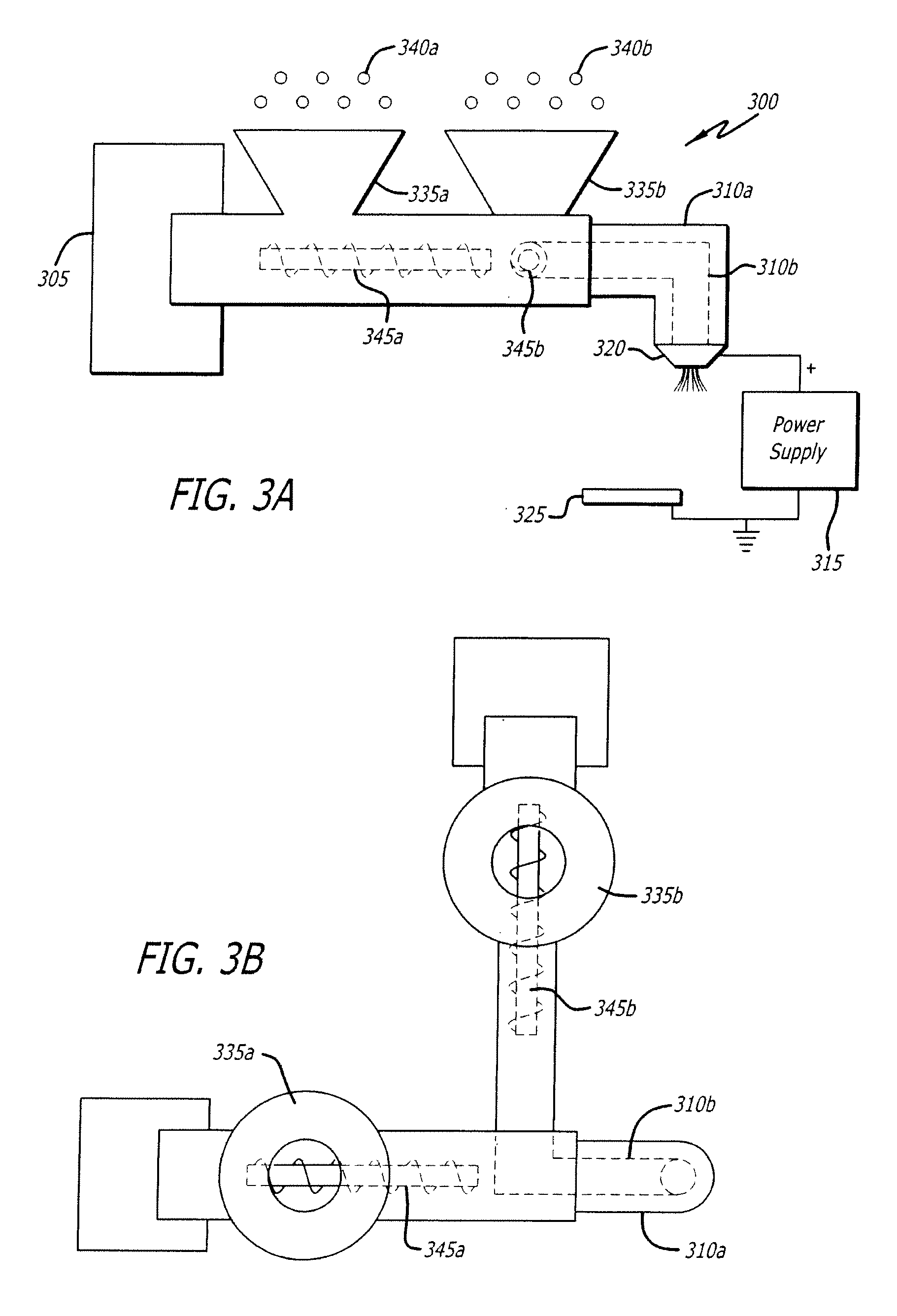
Electrospraying method for fabrication of particles and coatings and treatment methods thereof
Electrospray systems and modified electrospray systems for the fabrication of core-shell particles for controlled-release and / or sustained-release treatment and delivery are herein disclosed. The electrospray system may include between one and a plurality of co-axially situated tubes. Each tube may be electrically connected to a power supply wherein a voltage may be applied thereto. Core-shell particles may be collected on a collection target, which may be a wet or dry collector, and electrically connected to the power supply. Core-shell particles and methods of manufacture are also disclosed. The precursors of the core-shell particles may be polymer- or biomacromolecule-based solutions and may include at least one treatment agent incorporated therein. The number of “core” particle(s) within the “shell” may vary and may provide different treatment agent release profiles depending on the material and / or chemical characteristics of the polymer and / or biomacromolecule used. Methods of treating a condition are also disclosed. A treatment may include delivery of a plurality of core-shell particles which include a treatment agent to a treatment site. Delivery may be performed by a surgical procedure or by a non-invasive procedure such as catheter delivery.
Owner:ABBOTT CARDIOVASCULAR
Transillumination of body members for protection during body invasive procedures
InactiveUS6597941B2Discriminated with easeProtect body partsDiagnostics using lightSurgeryLight energyLight guide
An apparatus and method for identifying a body member during a body intrusive procedure includes infrared light energy which is applied to the body member. Substantially visible infrared free light is introduced into the region. The body member is located by detecting the infrared light energy applied thereto. The infrared light energy may be applied to the body member via a light guide which is inserted into or placed in contact with the body member. In addition, a body member to be located may be illuminated from one side and the location of the illuminator detected by a probe on the other side of the body member.
Owner:STRYKER CORP
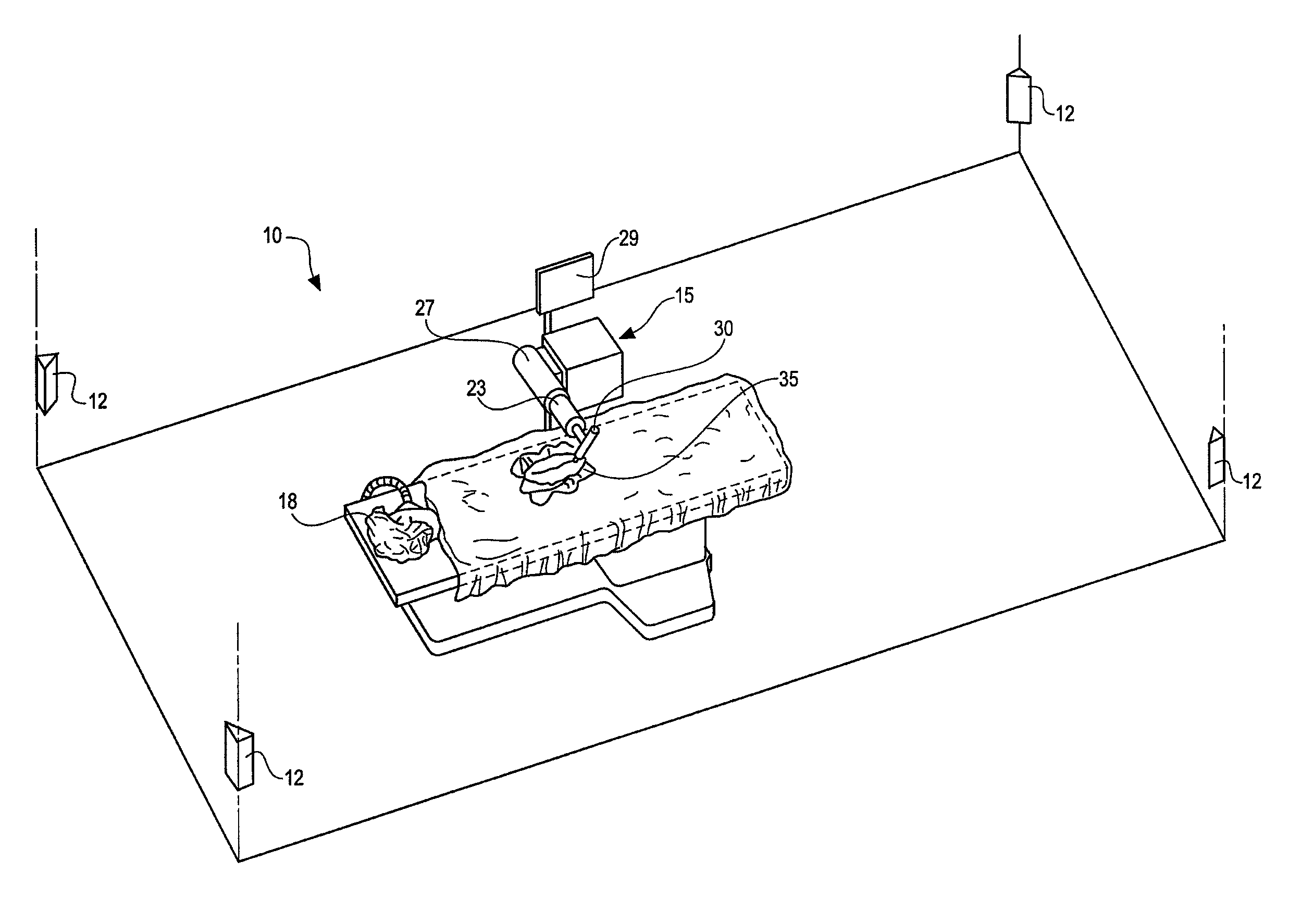
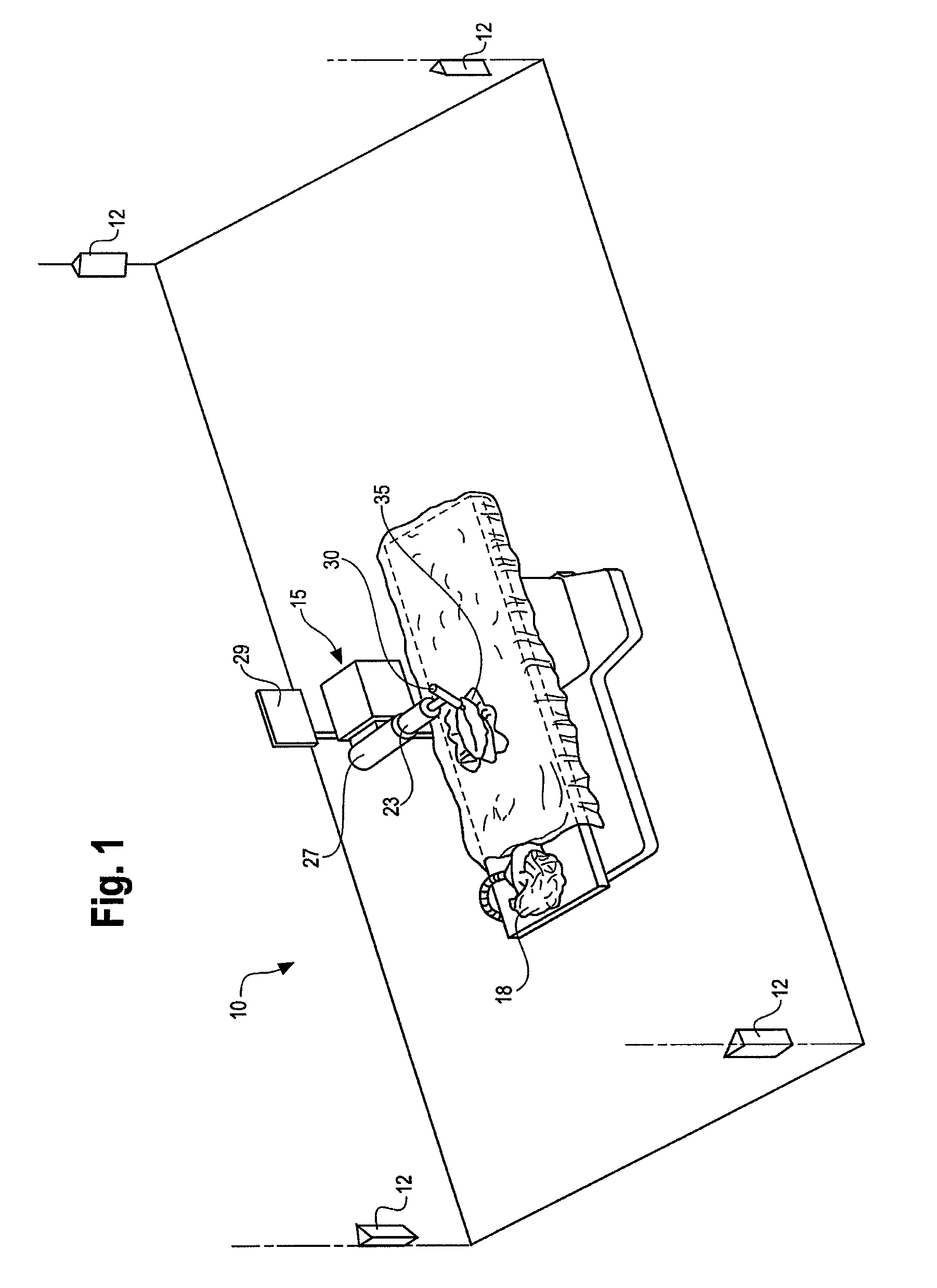
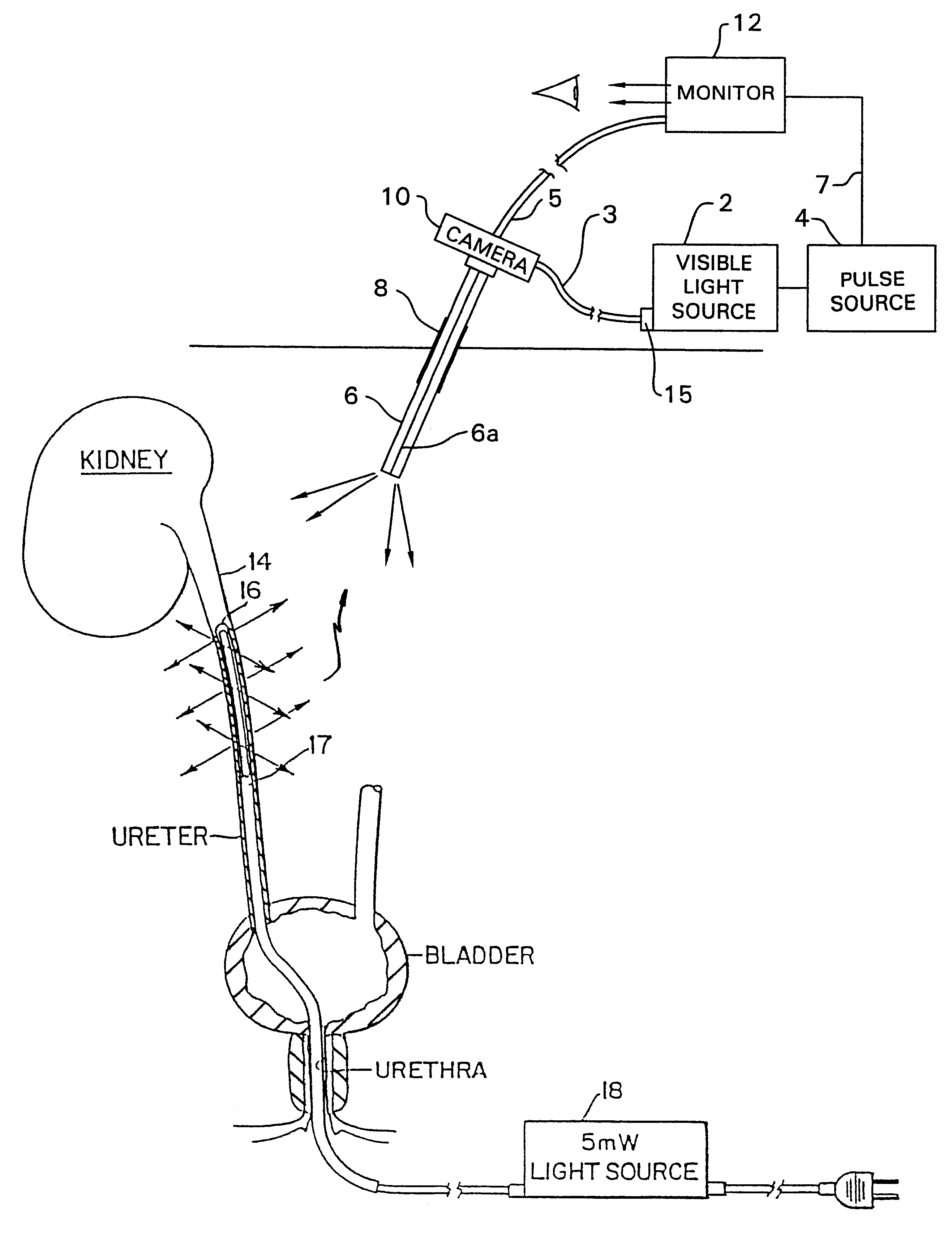
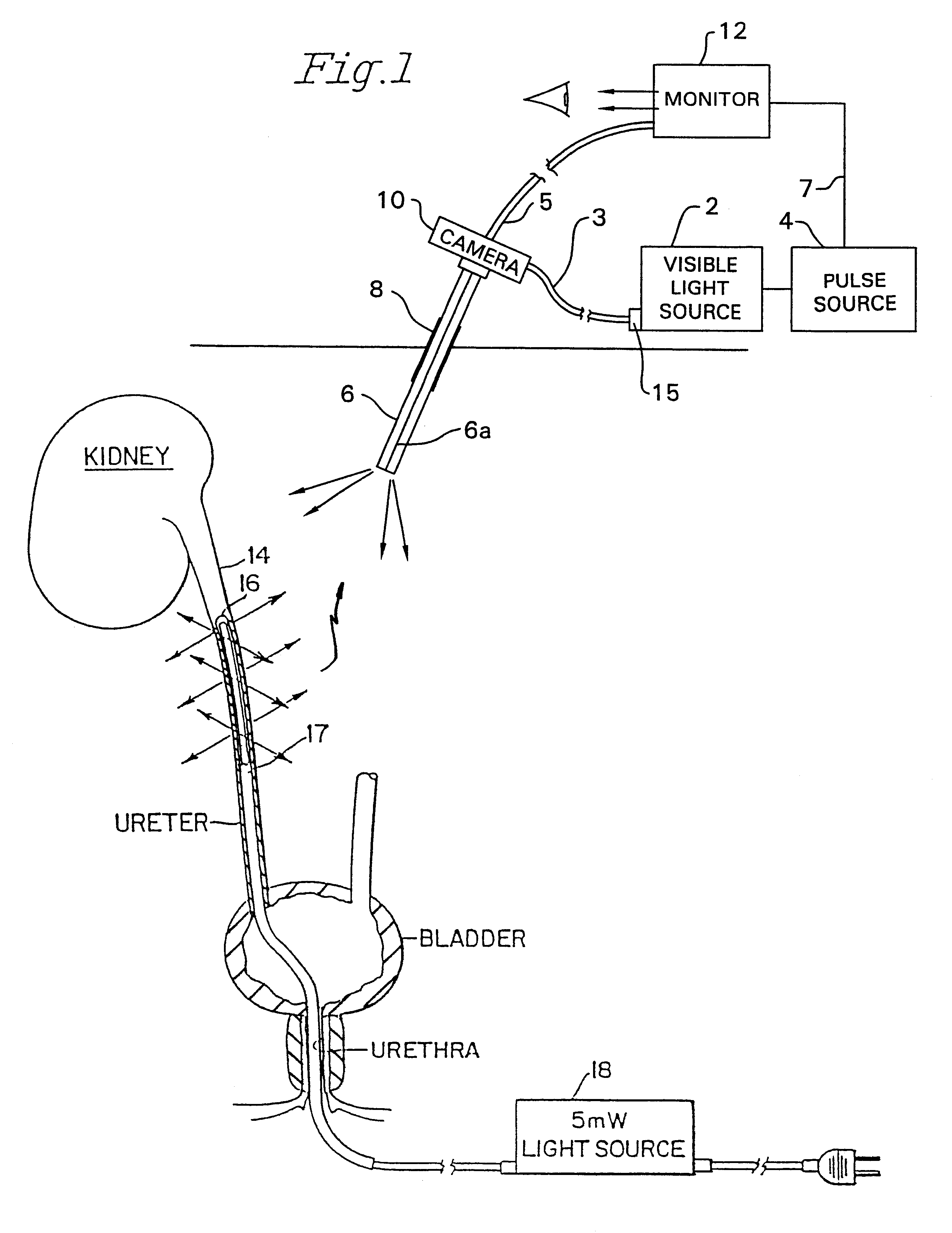
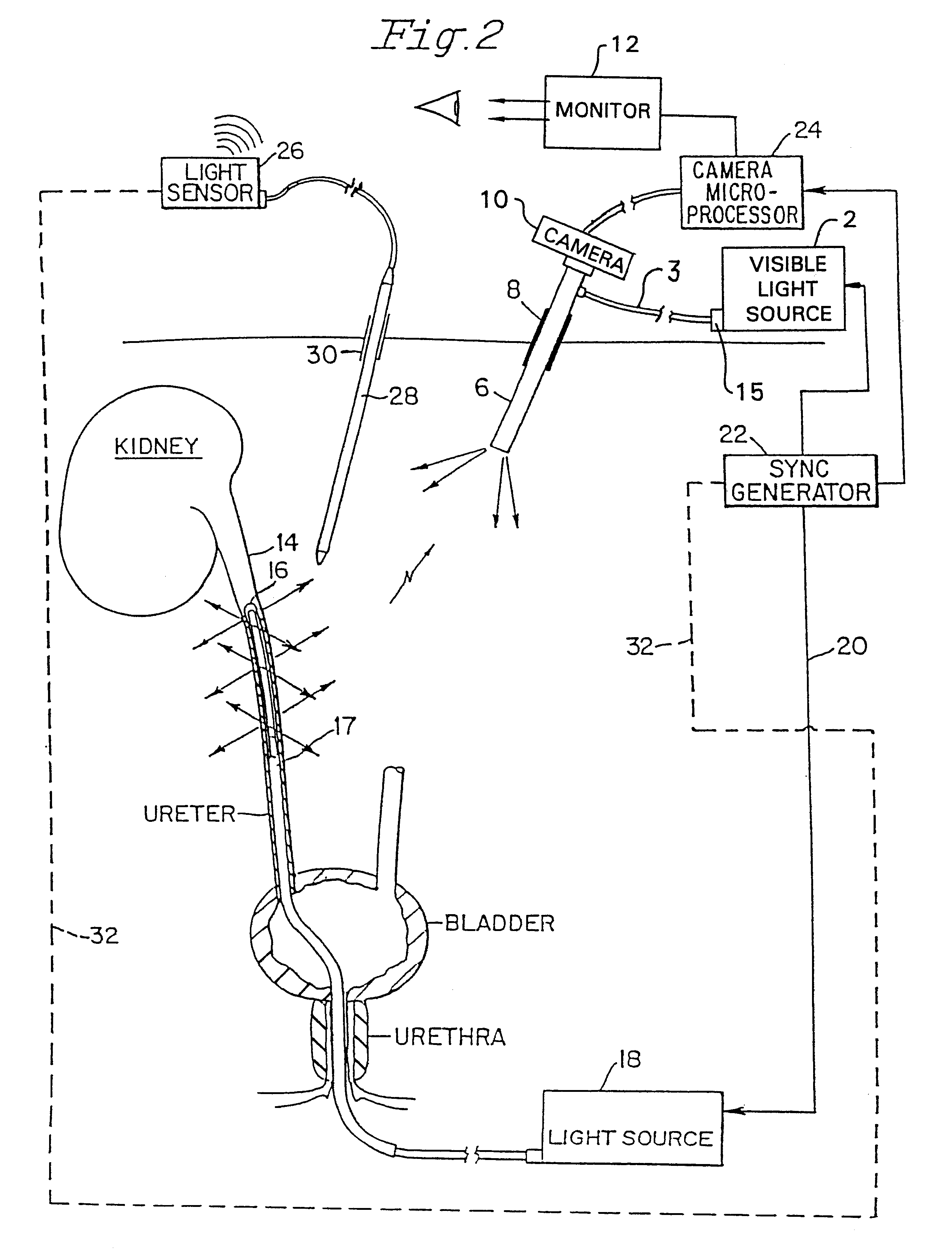
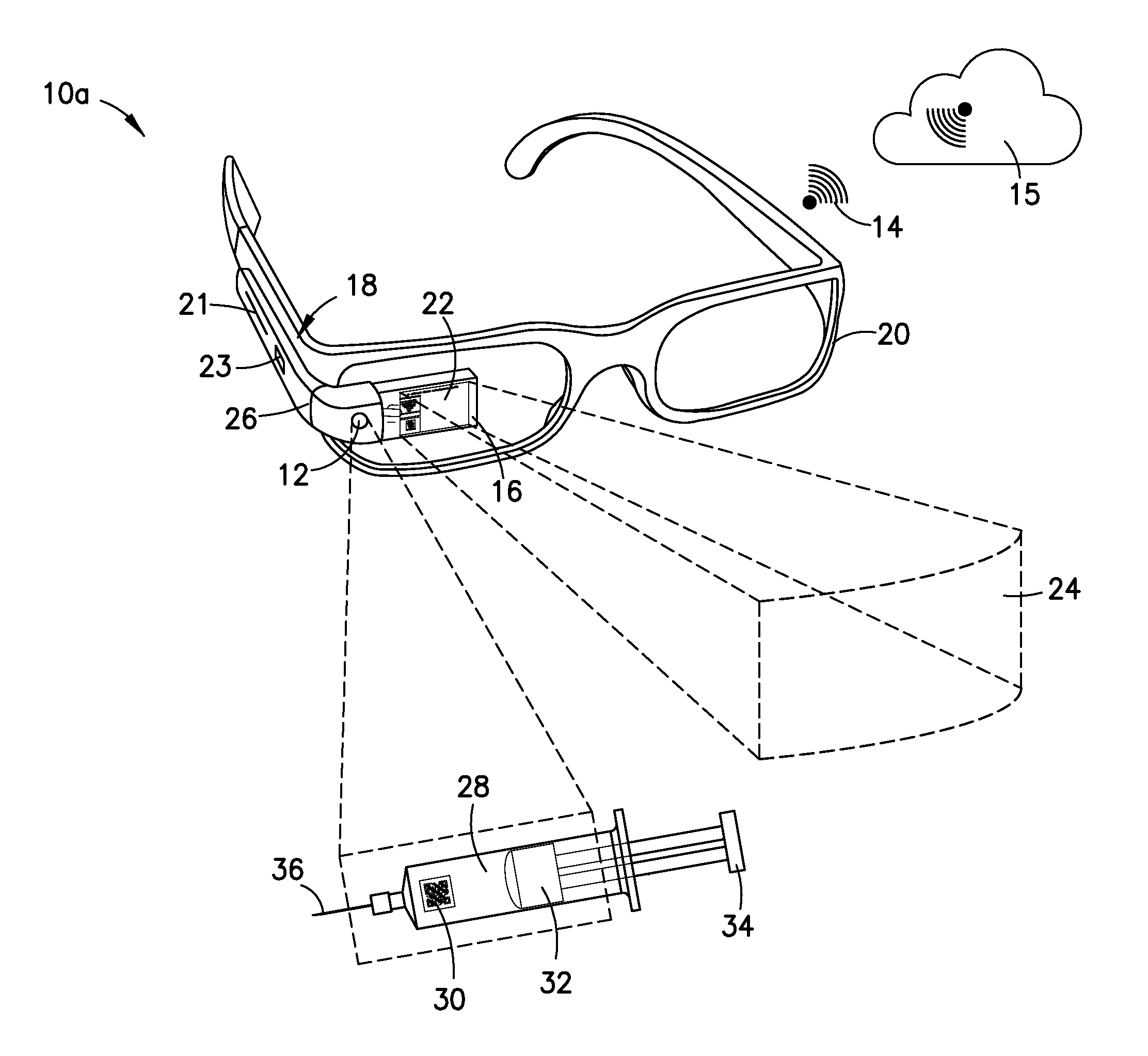
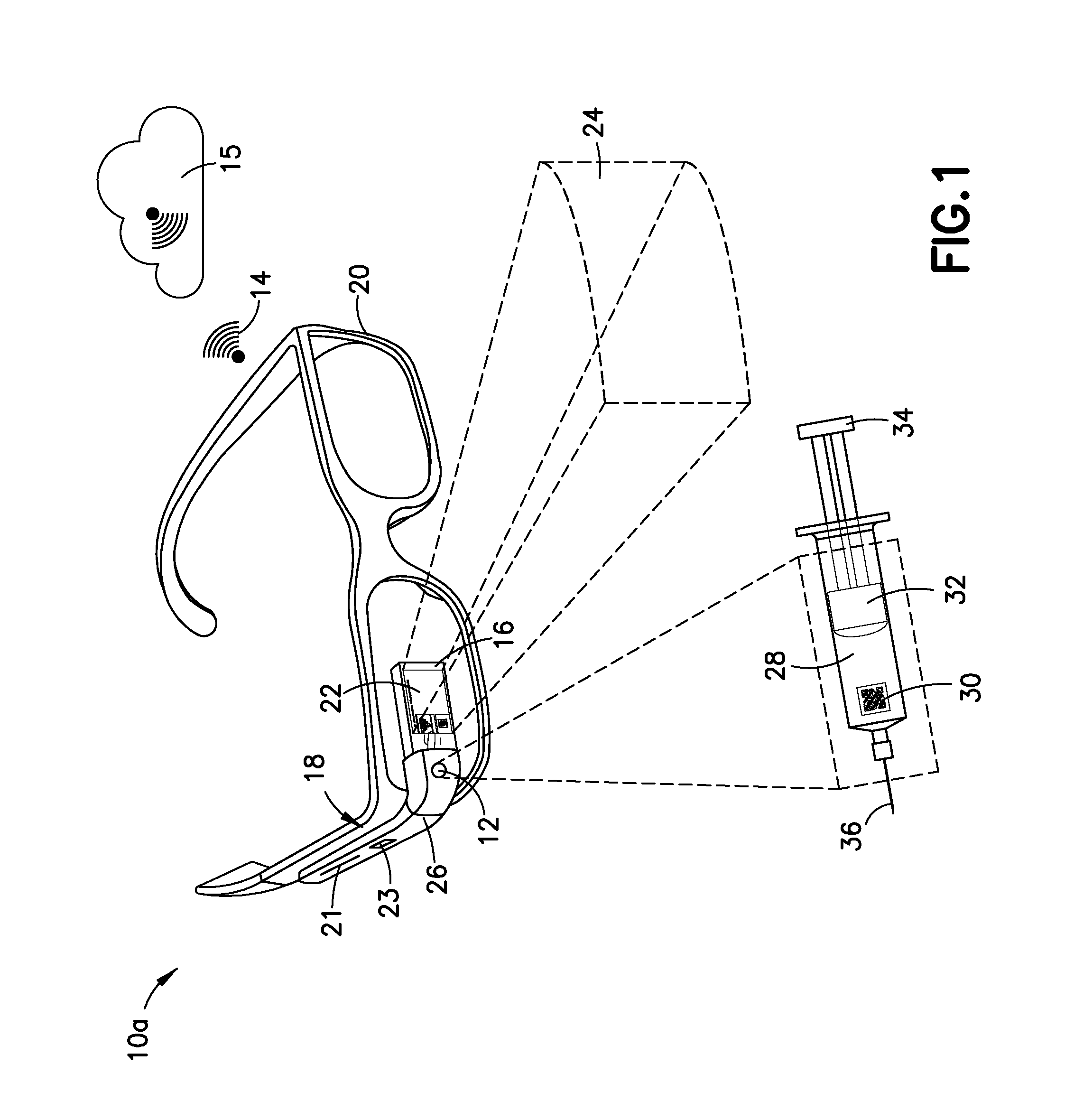
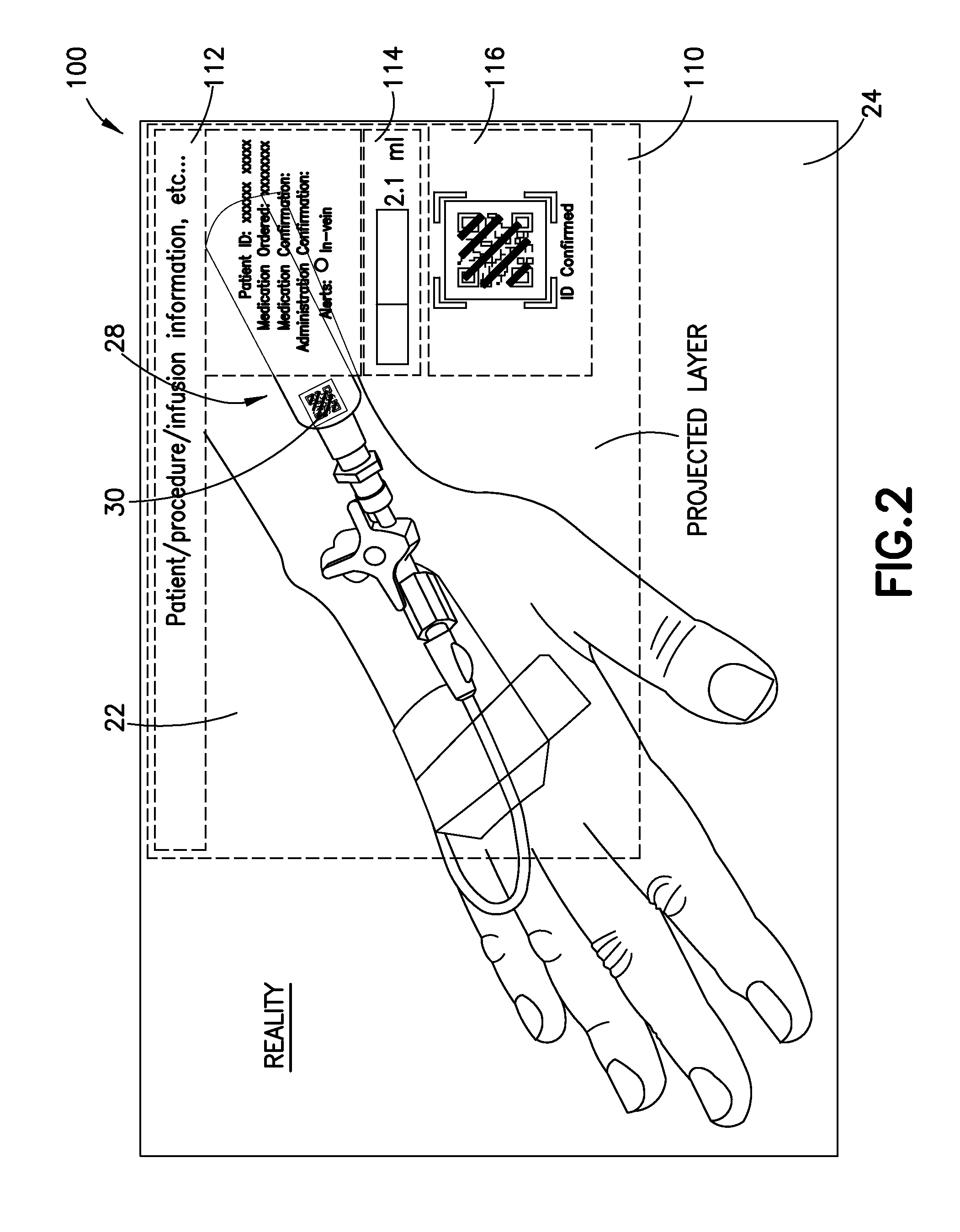
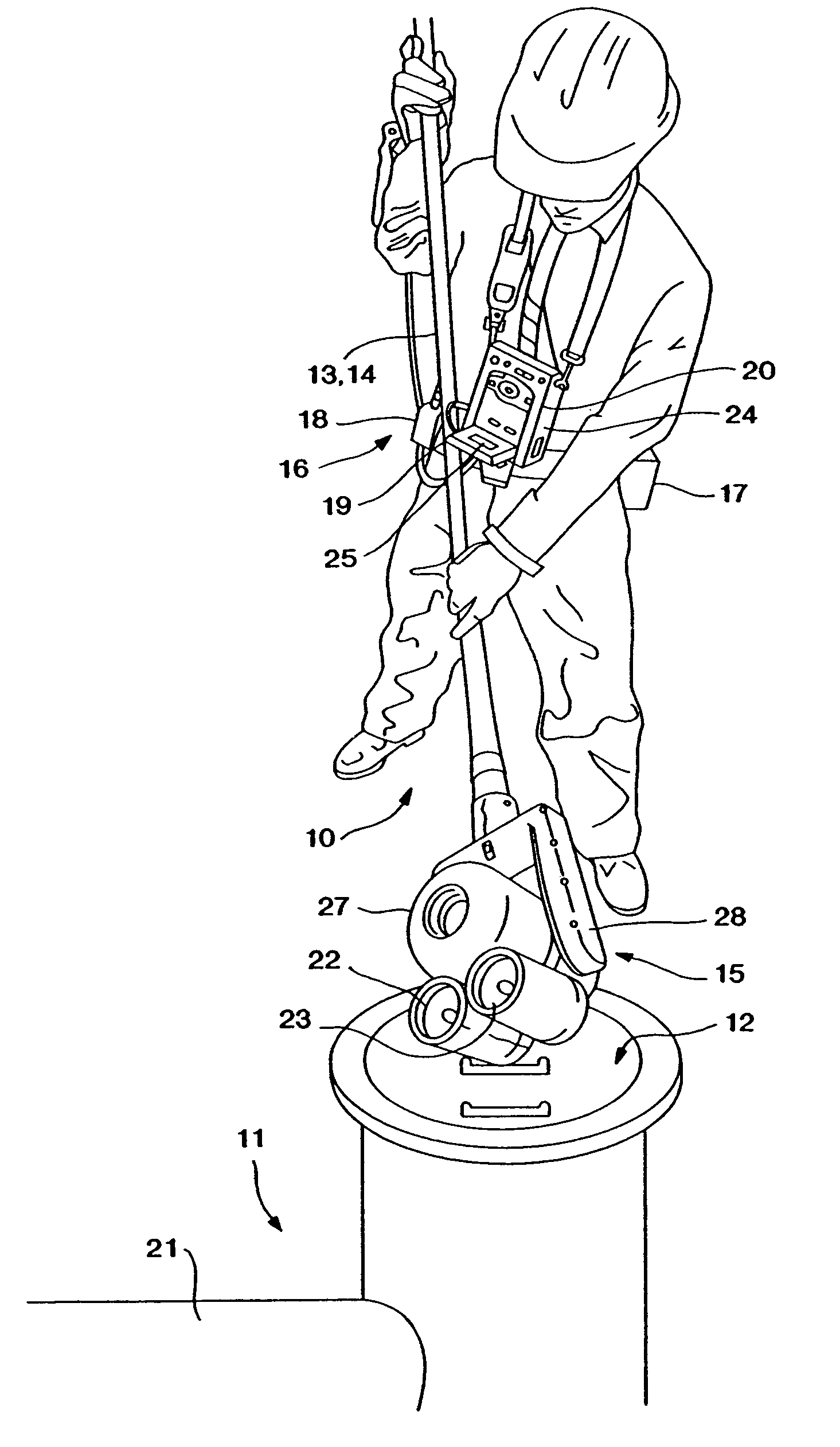
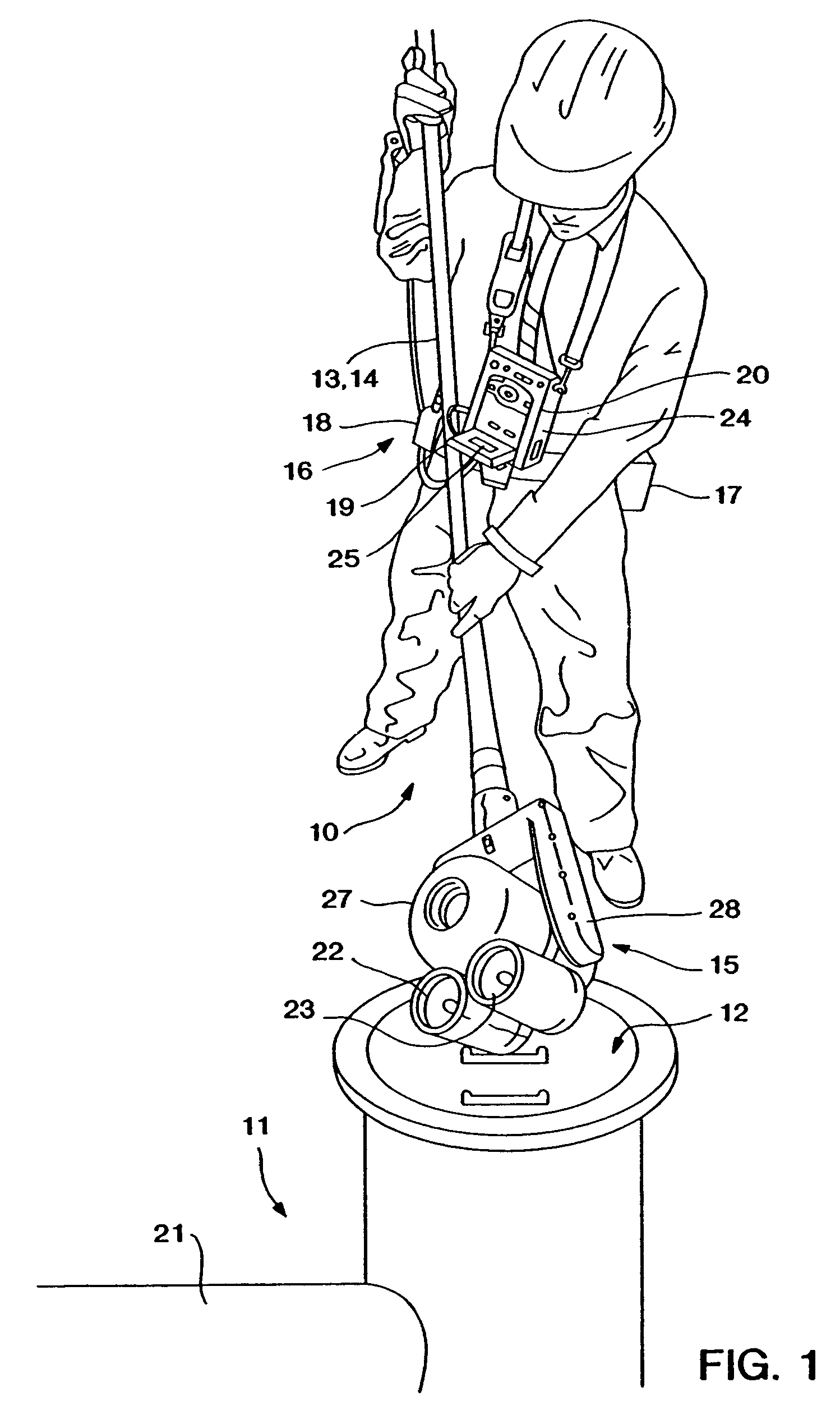
Wearable Electronic Device for Enhancing Visualization During Insertion of an Invasive Device
ActiveUS20150209113A1Reduce riskImproves clinical workflowMechanical/radiation/invasive therapiesOrgan movement/changes detectionAnatomical structuresDisplay device
A wearable electronic device configured to be worn by a user while performing an invasive procedure for enhancing visualization of desired anatomical structures is provided. The wearable electronic device includes: a housing; at least one imaging sensor associated with the housing; and a visual display integrally formed with or associated with the housing. The device is configured to acquire an image of an invasive access site of a patient with the at least one imaging sensor, process the image to determine a location of a desired anatomical structure, and display a virtual trace of the location to the user via the visual display.
Owner:BECTON DICKINSON & CO
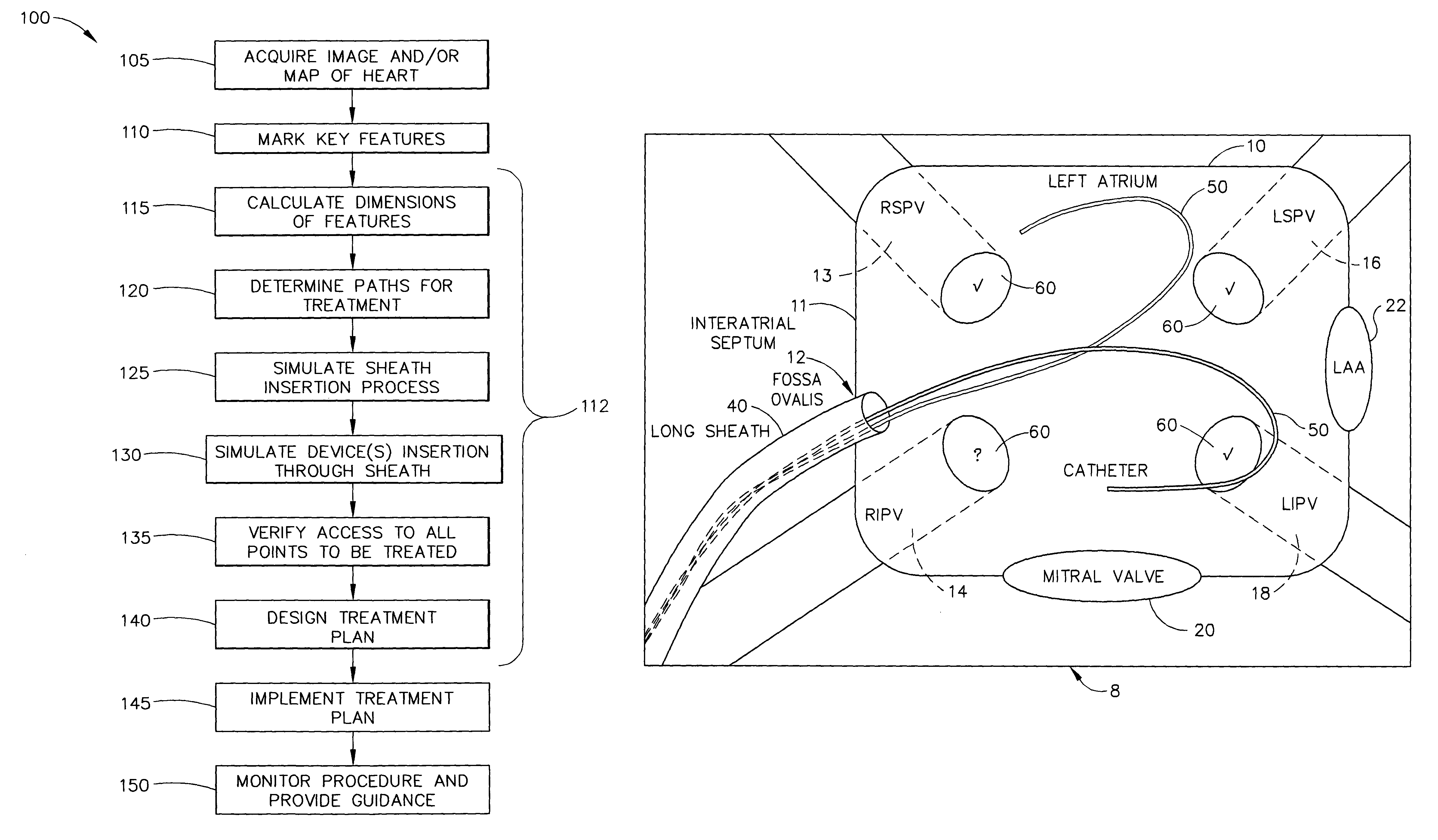
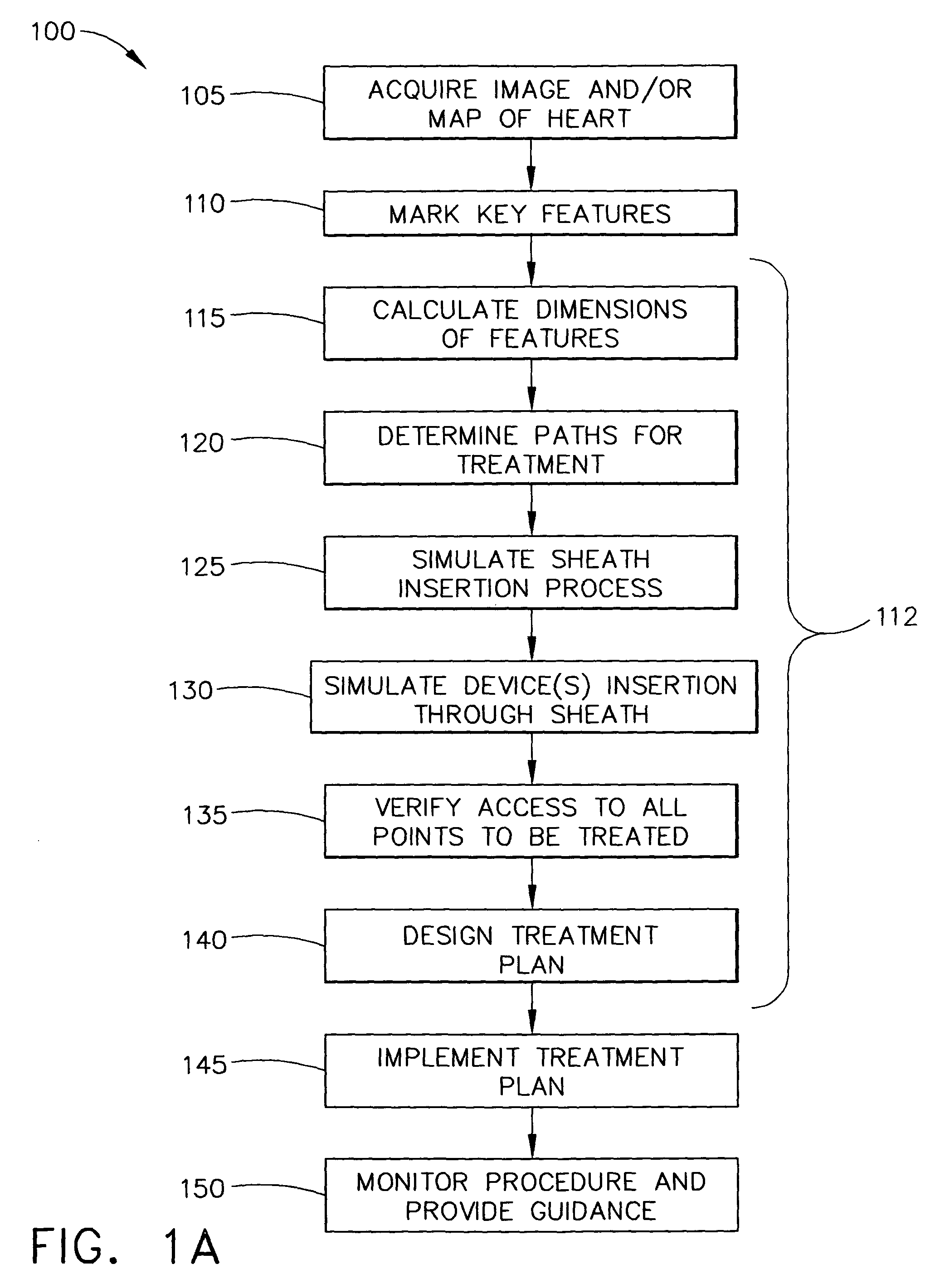
Simulation of invasive procedures
ActiveUS7877128B2Ultrasonic/sonic/infrasonic diagnosticsMedical simulationInvasive ProcedureCardiac procedures
Owner:BIOSENSE WEBSTER INC
Sequential implant delivery system
A method and device includes advancing a first stent and a second stent into a stenosed region of a blood vessel to protect or shield the vessel from possible blockage. The delivery device may include placement rings, selectively engagable by positioning members disposed on the outer wall of in inner catheter. The positioning members and the placement rings may be utilized to accurately place multiple stents within an afflicted vessel, in a single invasive procedure.
Owner:COOK MEDICAL TECH LLC
Inspection system and method
InactiveUS7009698B2Quick and convenient non-invasive approachPrecise positioningProjectorsMaterial analysis by optical meansSupporting systemMagnification
A process for maintaining an area using a non-invasive inspection device, the inspection device comprising at least: an imaging system having an adjustable field of view and an imaging device for transmitting an electrical signal corresponding to an area within the field of view; a portable support system operatively connected to the imaging system and adapted to provide functional support to the imaging system; and a positioning system connected to the imaging system and adapted for moving the imaging system independently of the portable support system; the process comprising: (a) positioning the imaging system independently of the support system such that a target area is in the field of view while at a first magnification level; (b) imaging a the target area at a second magnification level greater than the first magnification level; (c) outputting an image of the target area; (d) evaluating the image to determine whether the target area is acceptable or whether an invasive procedure needs to be performed; and (e) performing the invasive procedure on the target area if needed.
Owner:ENVIROSIGHT
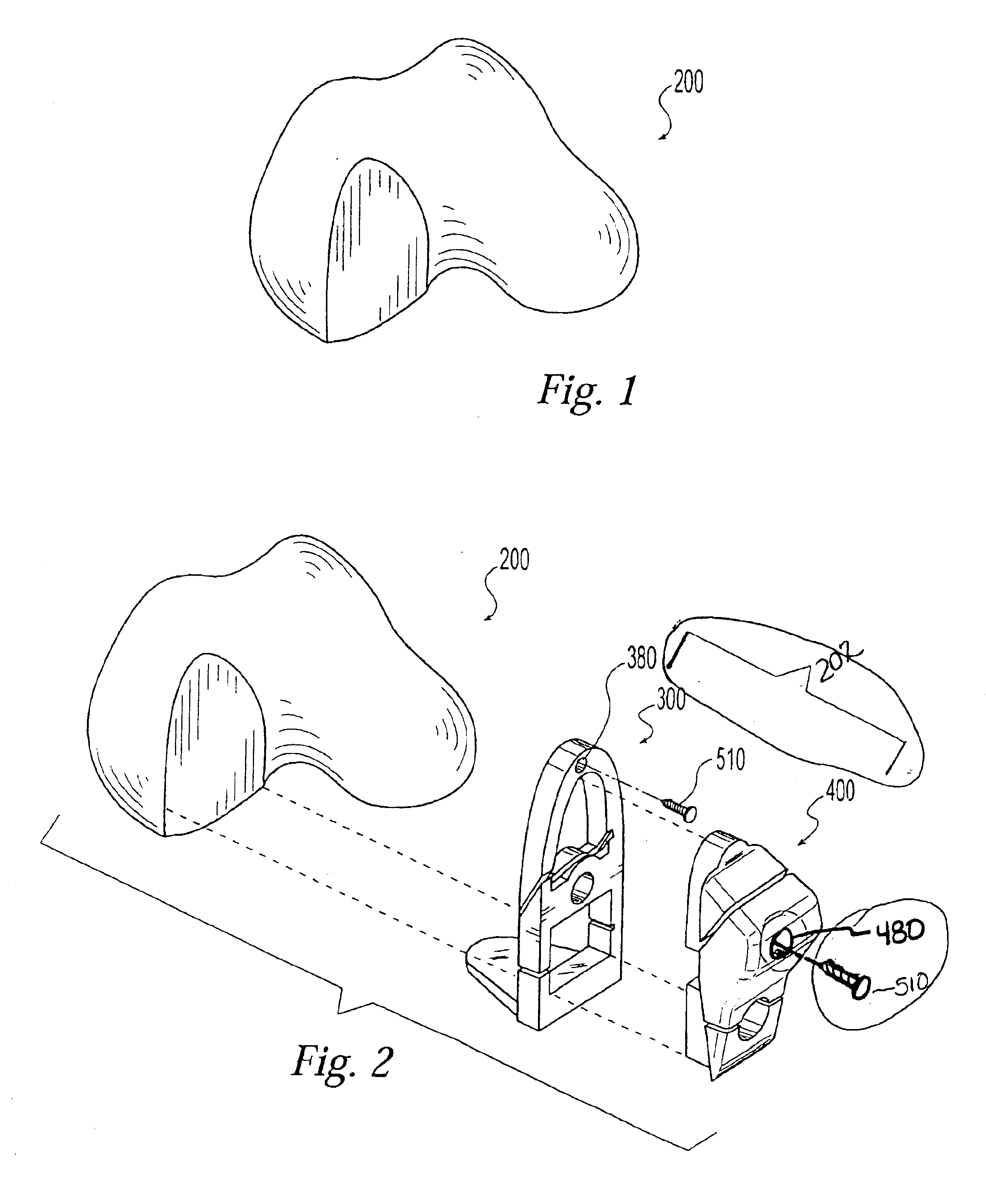
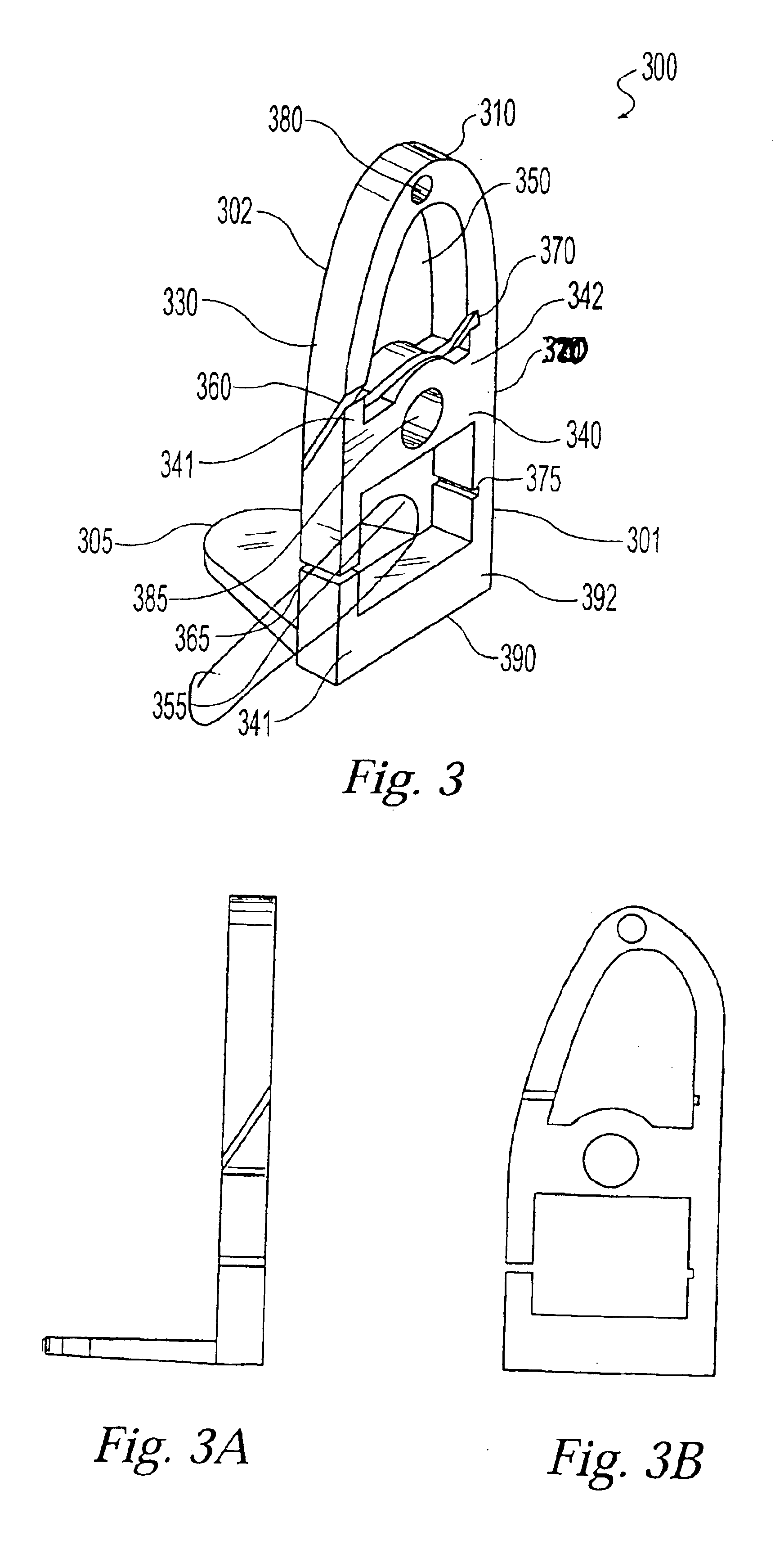
Two-piece cut block for minimally invasive surgical procedure
InactiveUS6962593B2Less initial traumaFast recovery timeJoint implantsNon-surgical orthopedic devicesTotal hip arthroplastyLess invasive surgery
A two-piece cut block for performing a minimally invasive partial or total knee arthroplasty. The present invention comprises a cut block that can be inserted into an incision in two parts then assembled in vivo. The two-piece design allows the relatively large surgical instrument to fit into a small, minimally invasive, surgical incision
Owner:ZIMMER INC
Biomarkers for neurological conditions
InactiveUS20100159486A1Microbiological testing/measurementImmunoglobulins against animals/humansNervous systemCognitive diseases
Low molecular weight (LMW) peptides have been discovered that are indicative of neurological conditions, such as Alzheimer's Disease (AD), cognitive impairment and brain microhemmorhages. Evaluating patient samples for the presence of such LMW peptides is an effective means of detecting neurological conditions and monitoring the progression of the disease. The LMW peptides are particularly useful in detecting neurological conditions during the early stages without invasive procedures.
Owner:GEORGE MASON INTPROP INC +1
Compositions and methods for surface treatment in medical and surgical procedures
The present invention comprises compositions and methods for contemporaneously anesthetizing and antiseptically treating or pretreating anatomic surfaces for invasive surgical or treatment procedures. In particular, compositions and methods according to the present invention are used to treat ocular surfaces for intravitreal injections and other ophthalmic procedures. Compositions of the present invention comprise antiseptic agents and anesthetic agents in an aqueous gel or semi-gel formulation base to provide for enhanced methods of treating anatomic surfaces such as the eye prior to surgical or other invasive procedures.
Owner:LADD BYRON S
RF and/or microwave energy conveying structure, and an invasive electrosurgical scoping device incorporating the same
ActiveUS20170215955A1Secure locationReduces and eliminates power reflectionTwo pole connectionsEndoscopesMicrowavePERITONEOSCOPE
Embodiments of the invention provide an energy conveying structure for delivering RF and / or microwave energy to an electrosurgical instrument, where the energy conveying structure is incorporated into an insertion tube of a surgical scoping device (e.g. endoscope, laparoscope or the like). The insertion tube is a flexible conduit that is introduced into a patient's body during an invasive procedure, and can include an instrument channel and an optical channel. The energy conveying structure may be a layered coaxial structure that formed a liner that fits within the scoping device, e.g. within an instrument channel. Alternatively, the energy conveying structure may be a coaxial structure integrally formed as part of the flexible conduit.
Owner:CREO MEDICAL LTD
Indwelling Temporary IVC Filter System With Drug Delivery and Aspiration
InactiveUS20110106135A1Inhibition of dissolutionInhibition formationSurgeryDilatorsVeinInferior vena cava filter
Indwelling temporary inferior vena cava filter systems are disclosed. Such filter systems provide for easy removal of the filter without the need for additional invasive procedures and provide for dissolution and aspiration of captured emboli. Methods of using such systems for the dissolution, capture and removal of emboli are described.
Owner:MEDTRONIC VASCULAR INC
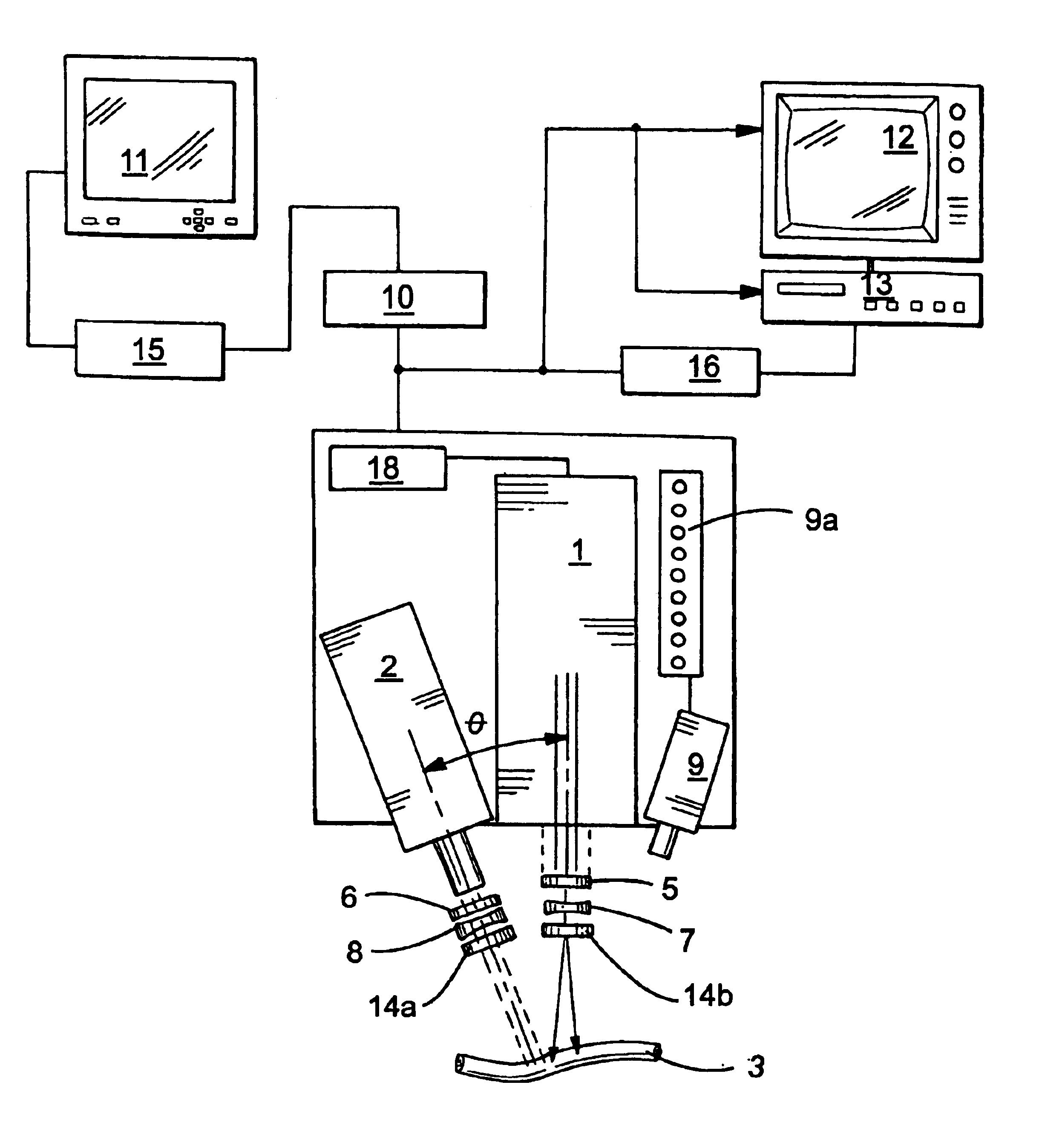
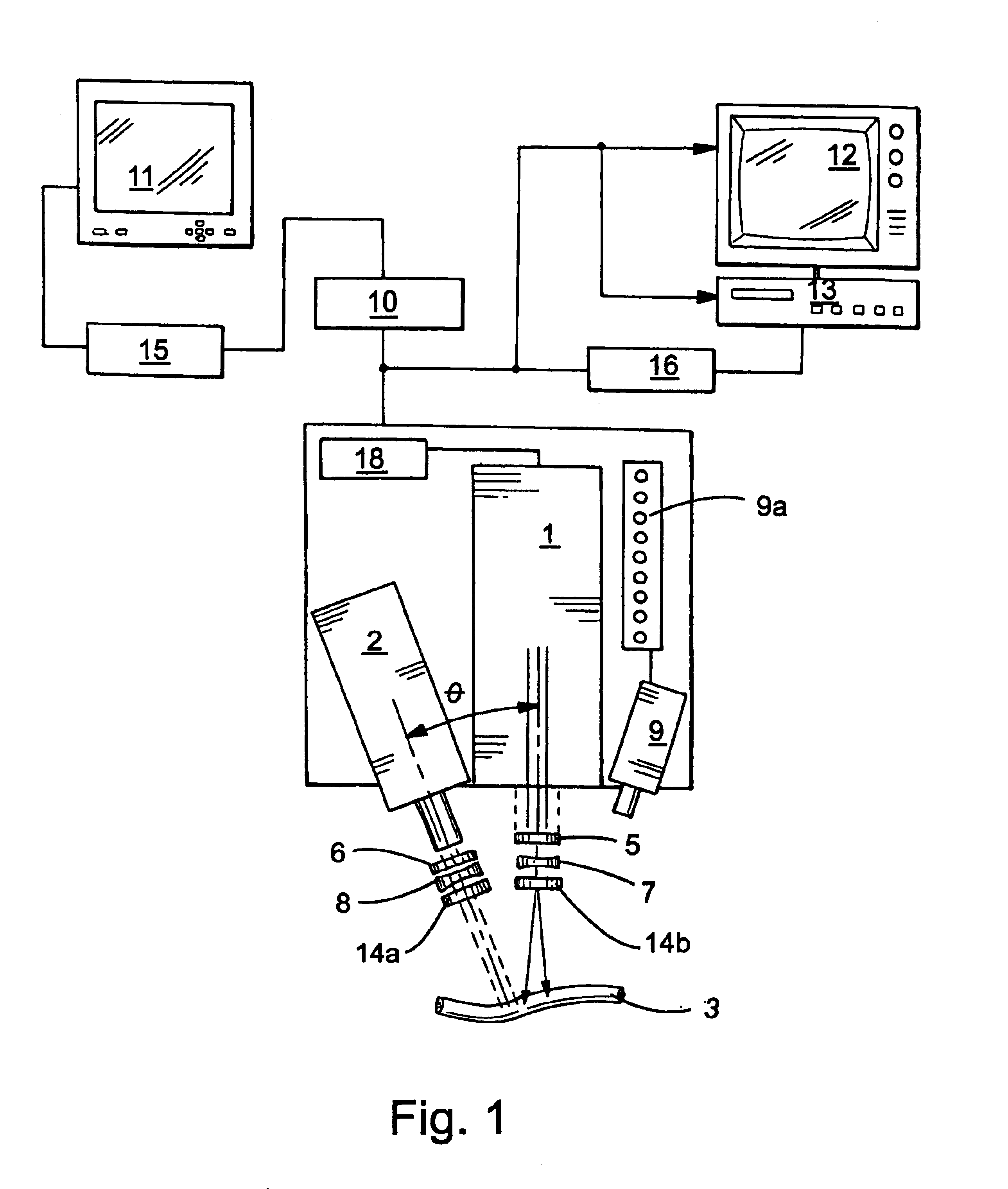
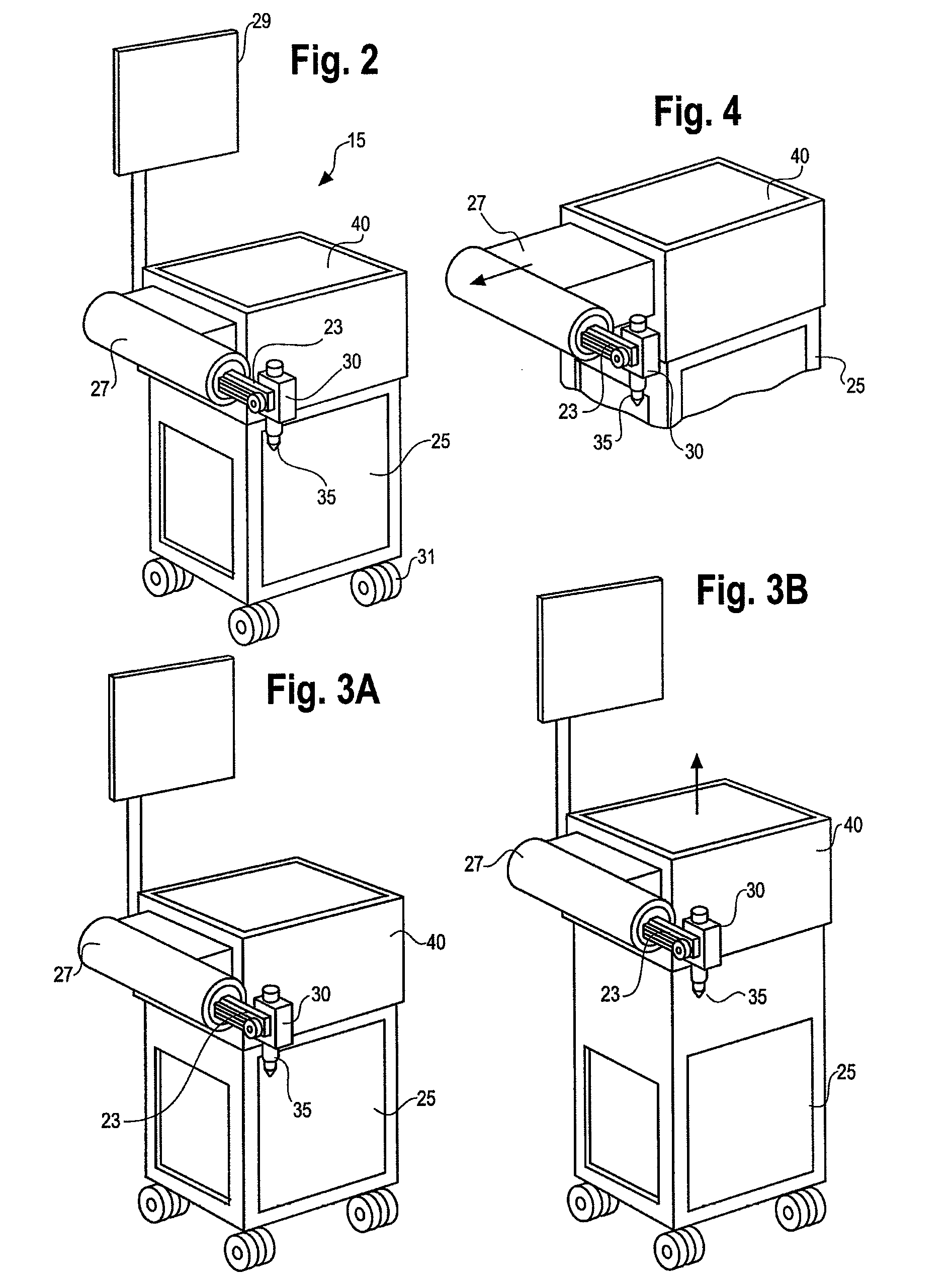
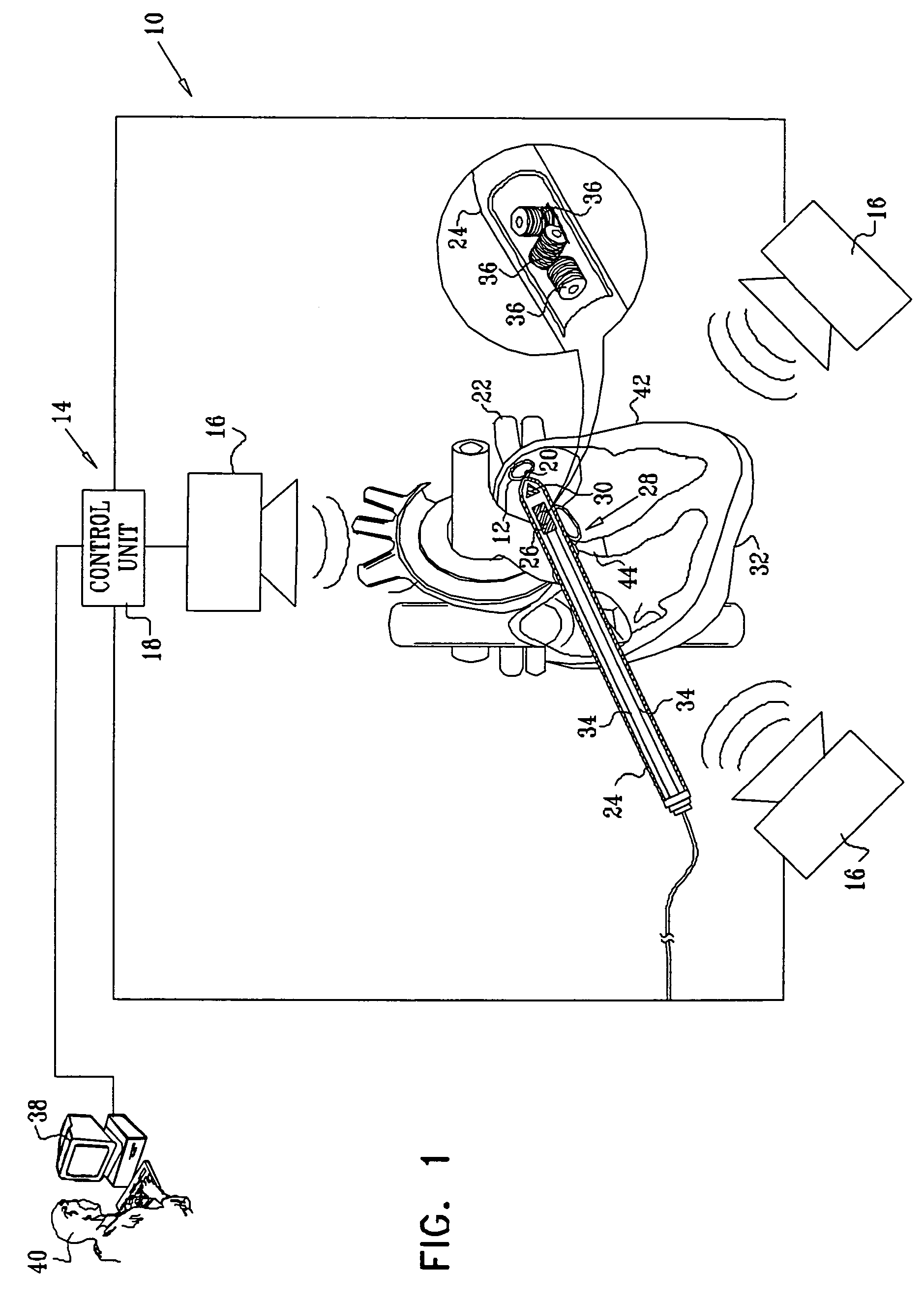
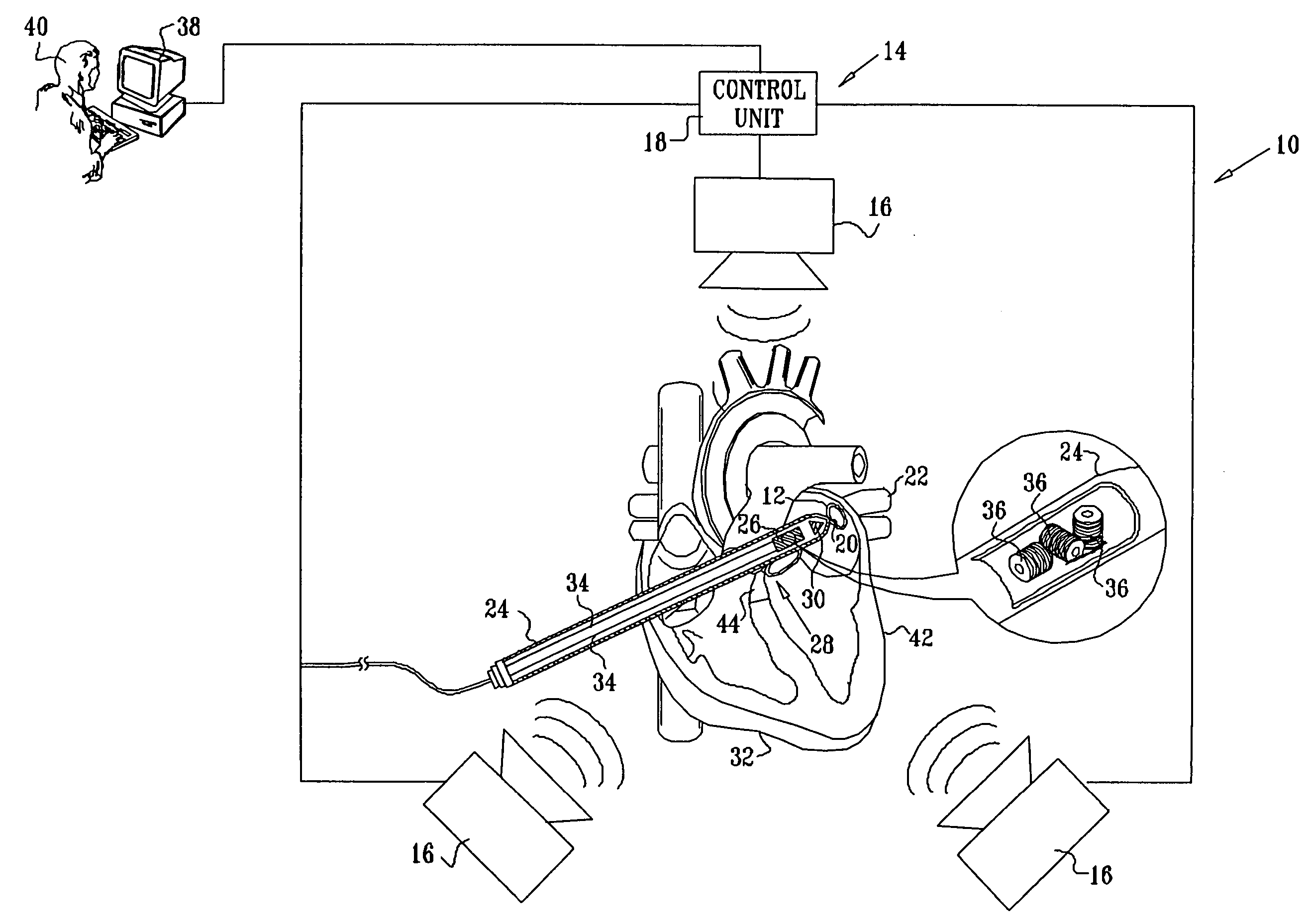
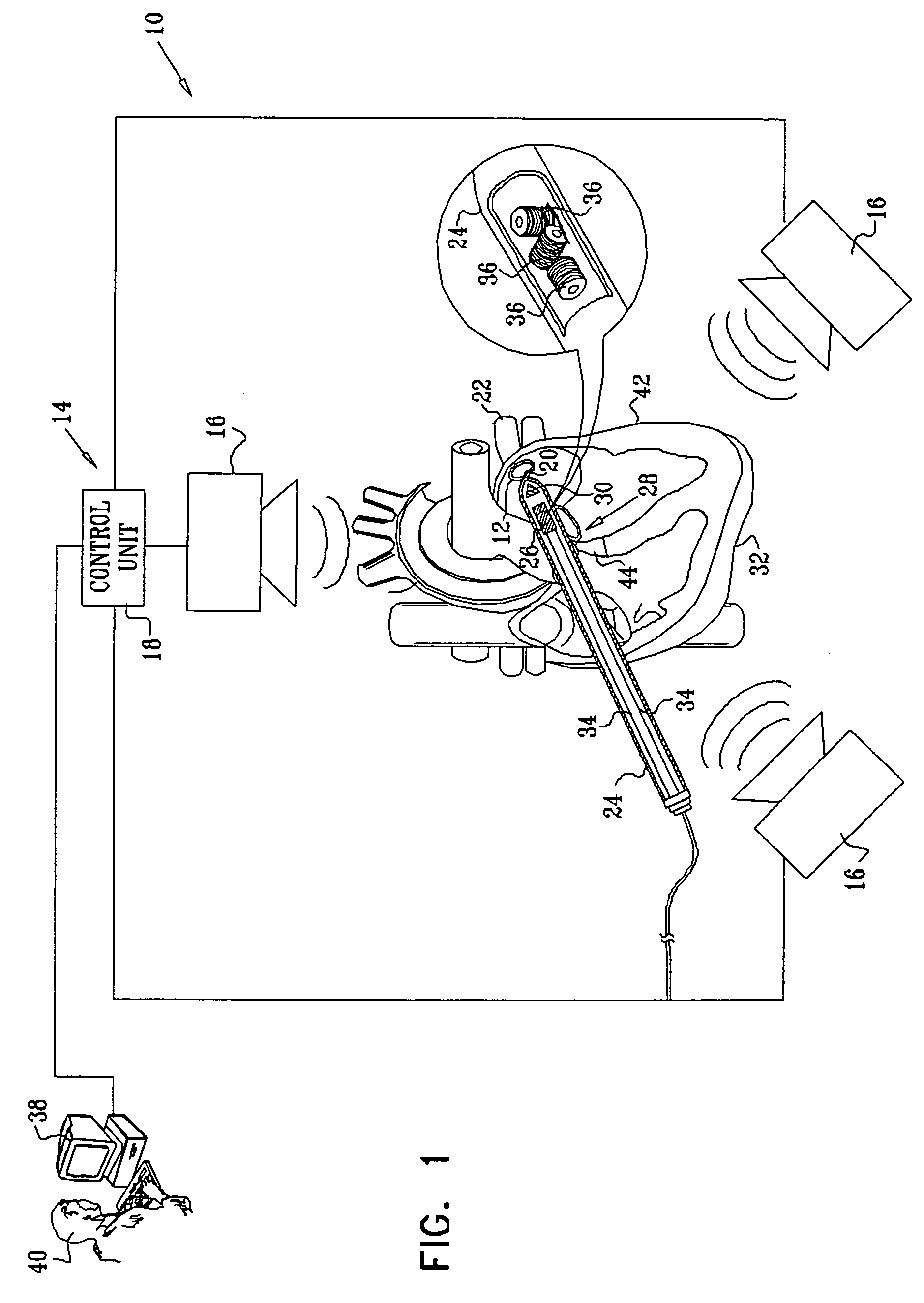
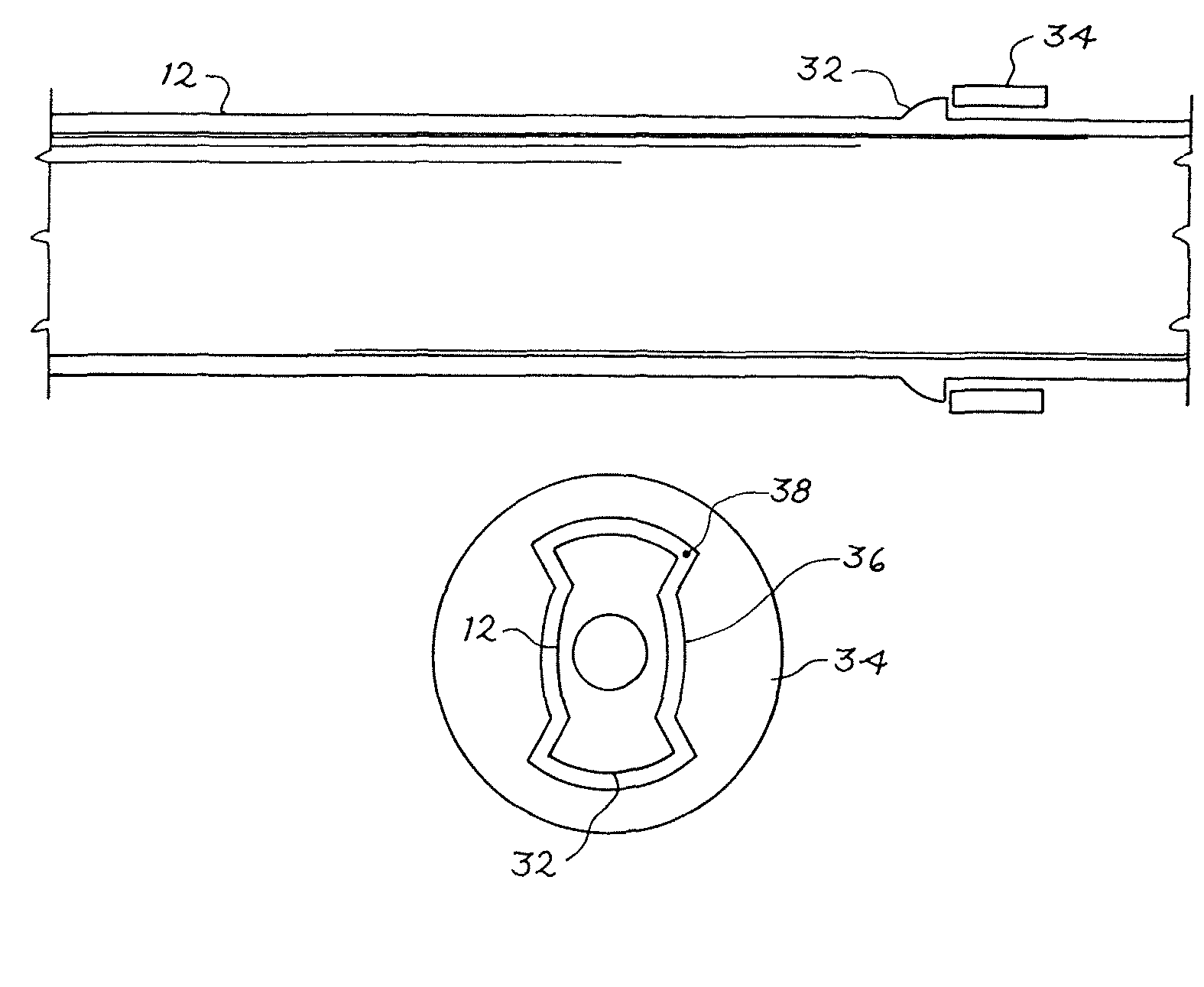
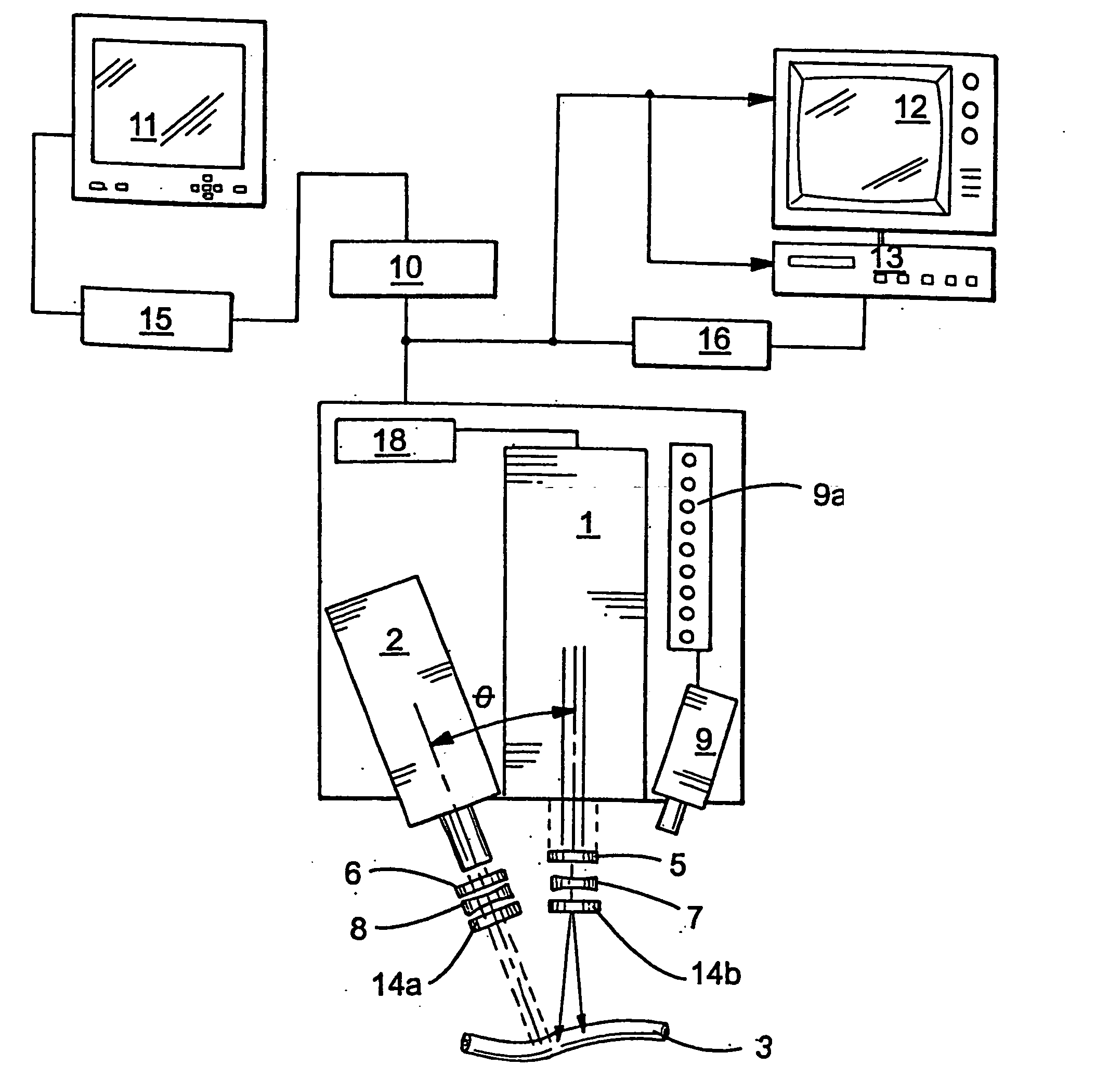
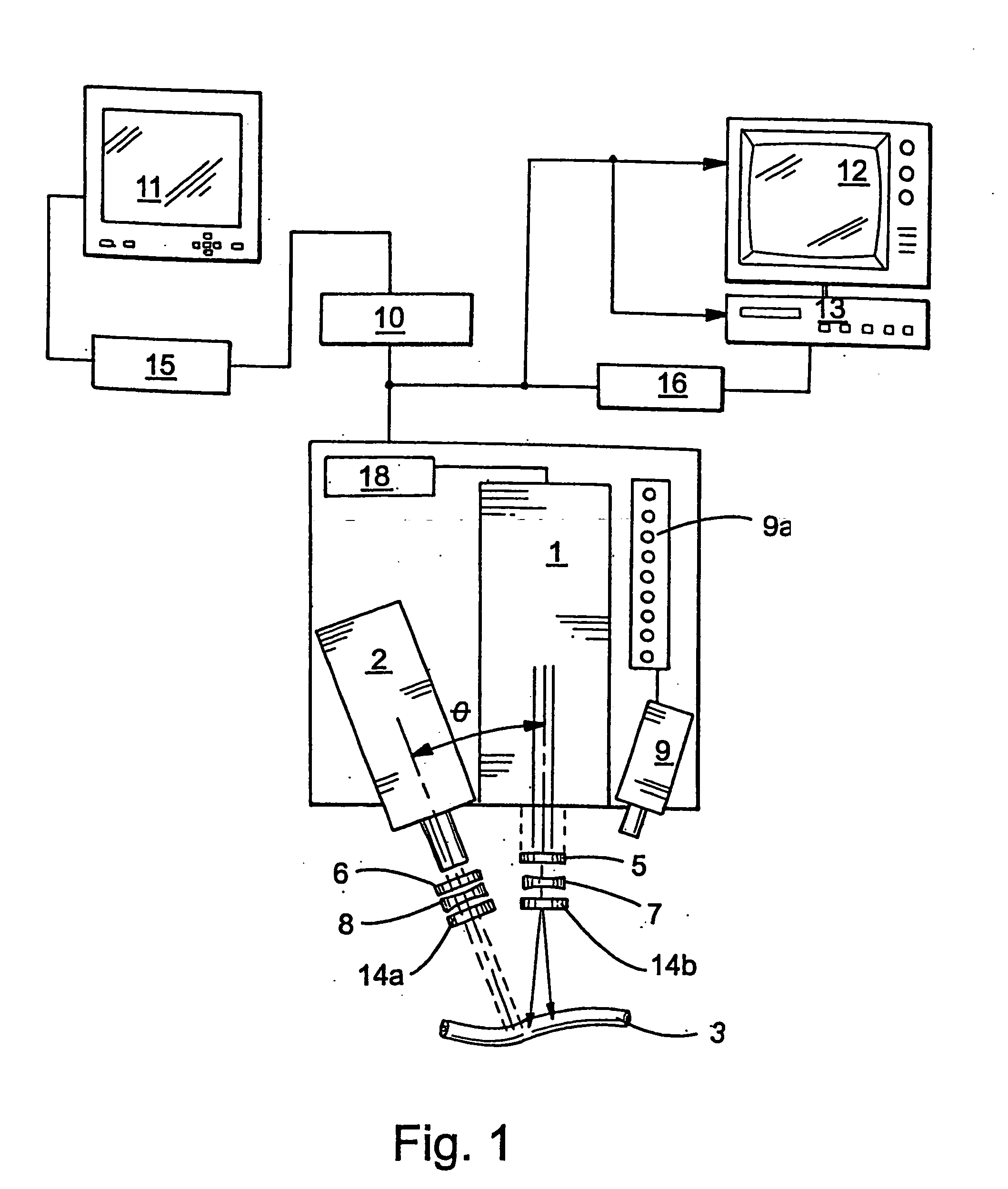
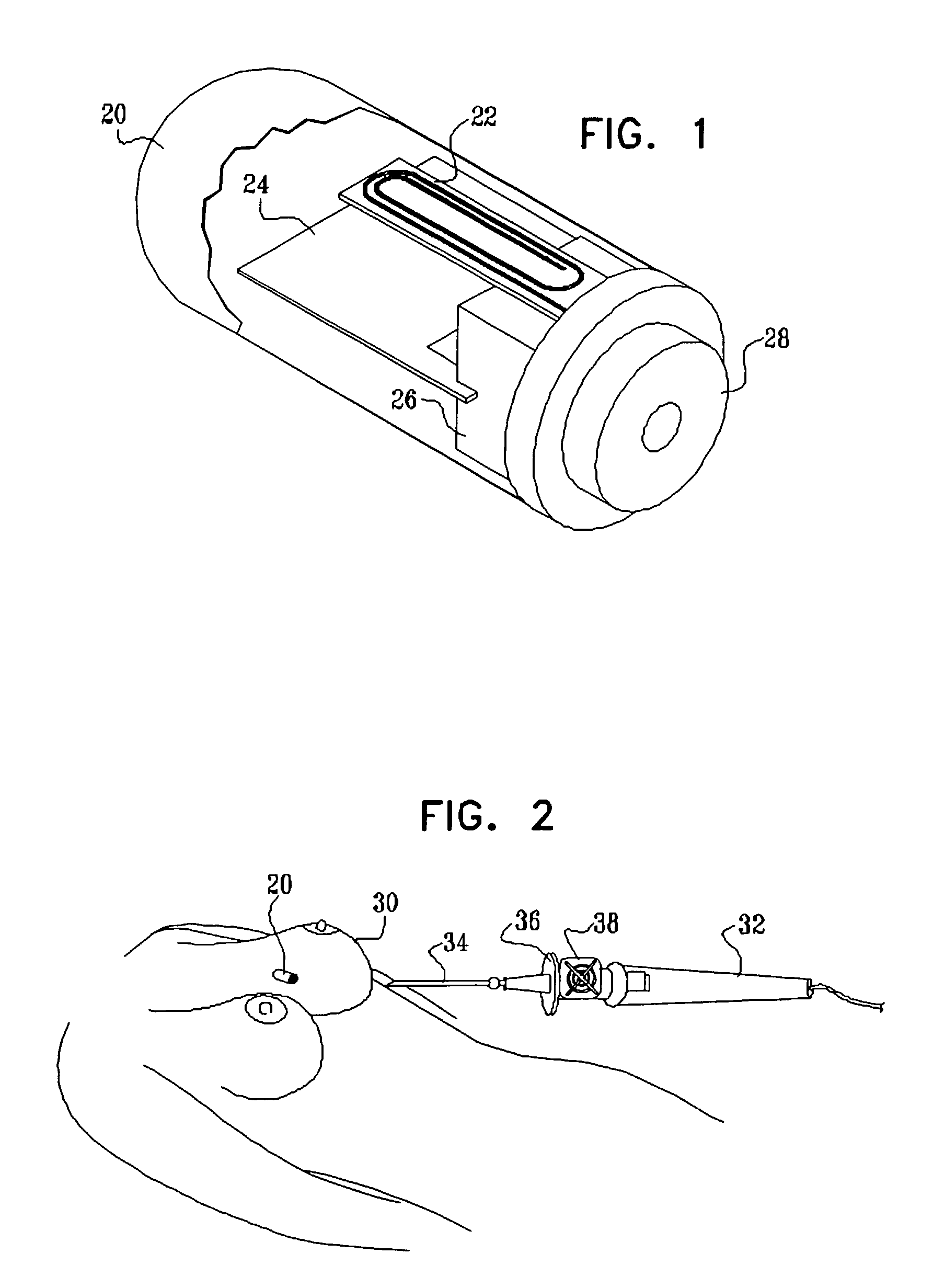
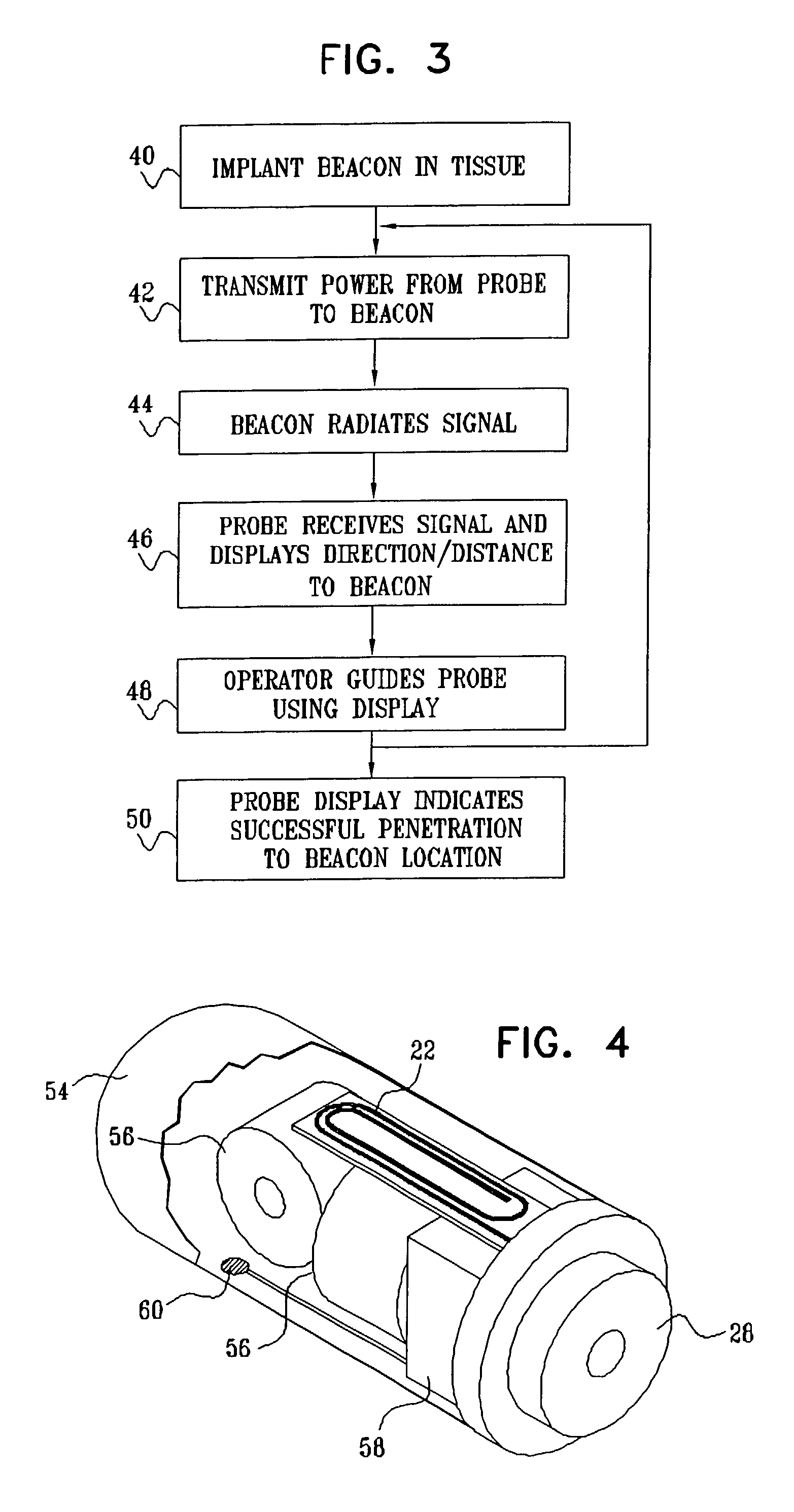
Guidance of invasive medical procedures using implantable tags
InactiveUS7558616B2Small and simple and inexpensiveThe location information is accurateUltrasonic/sonic/infrasonic diagnosticsSurgical needlesTransducerAcoustic wave
Apparatus for locating a tissue within a body of a subject includes an acoustic tag configured to be fixed to the tissue and adapted, responsive to acoustic waves incident thereon, to return acoustic echoes. Acoustic transducers are placed at respective positions so as to direct the acoustic waves into the body toward the tissue and to receive the acoustic echoes returned from the tag responsive to the acoustic waves, generating first signals responsive to the received echoes. Transducer position sensors are coupled respectively to the acoustic transducers so as to generate second signals indicative of the respective positions of the acoustic transducers in an external frame of reference. A processing unit processes the first signals and the second signals so as to determine coordinates of the acoustic tag in the external frame of reference.
Owner:BIOSENSE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com