Patents
Literature
10246results about "Non-surgical orthopedic devices" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
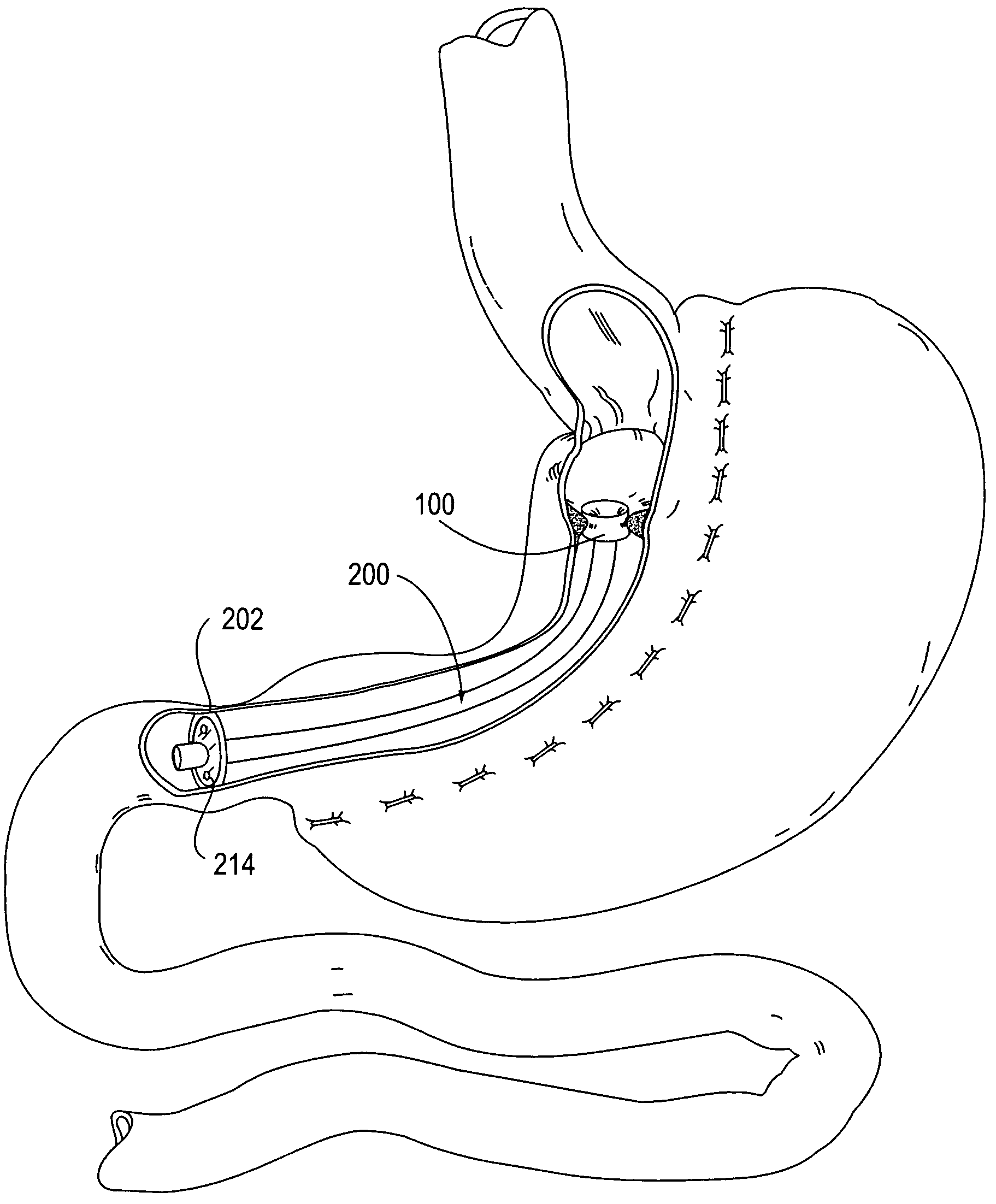
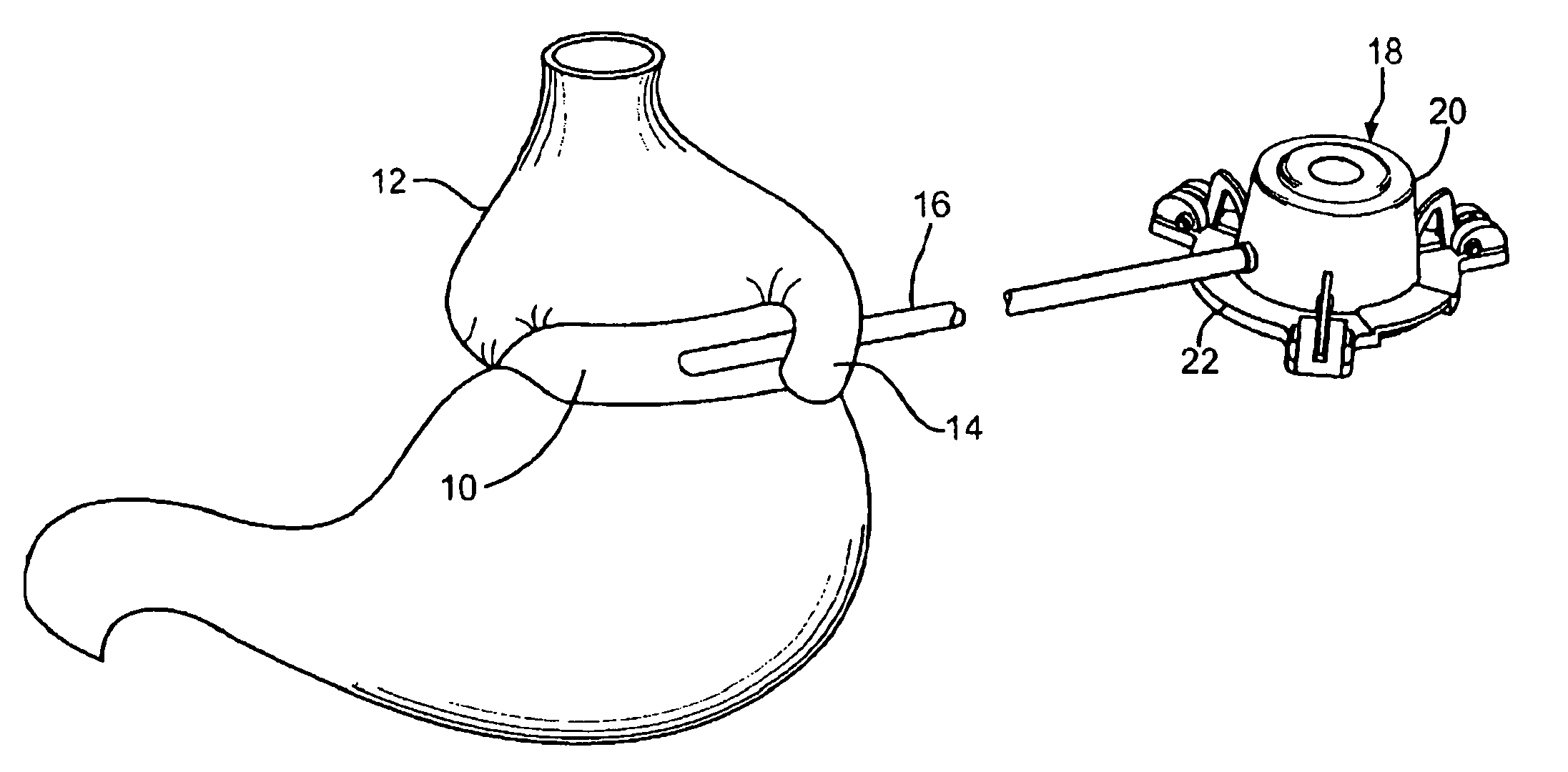
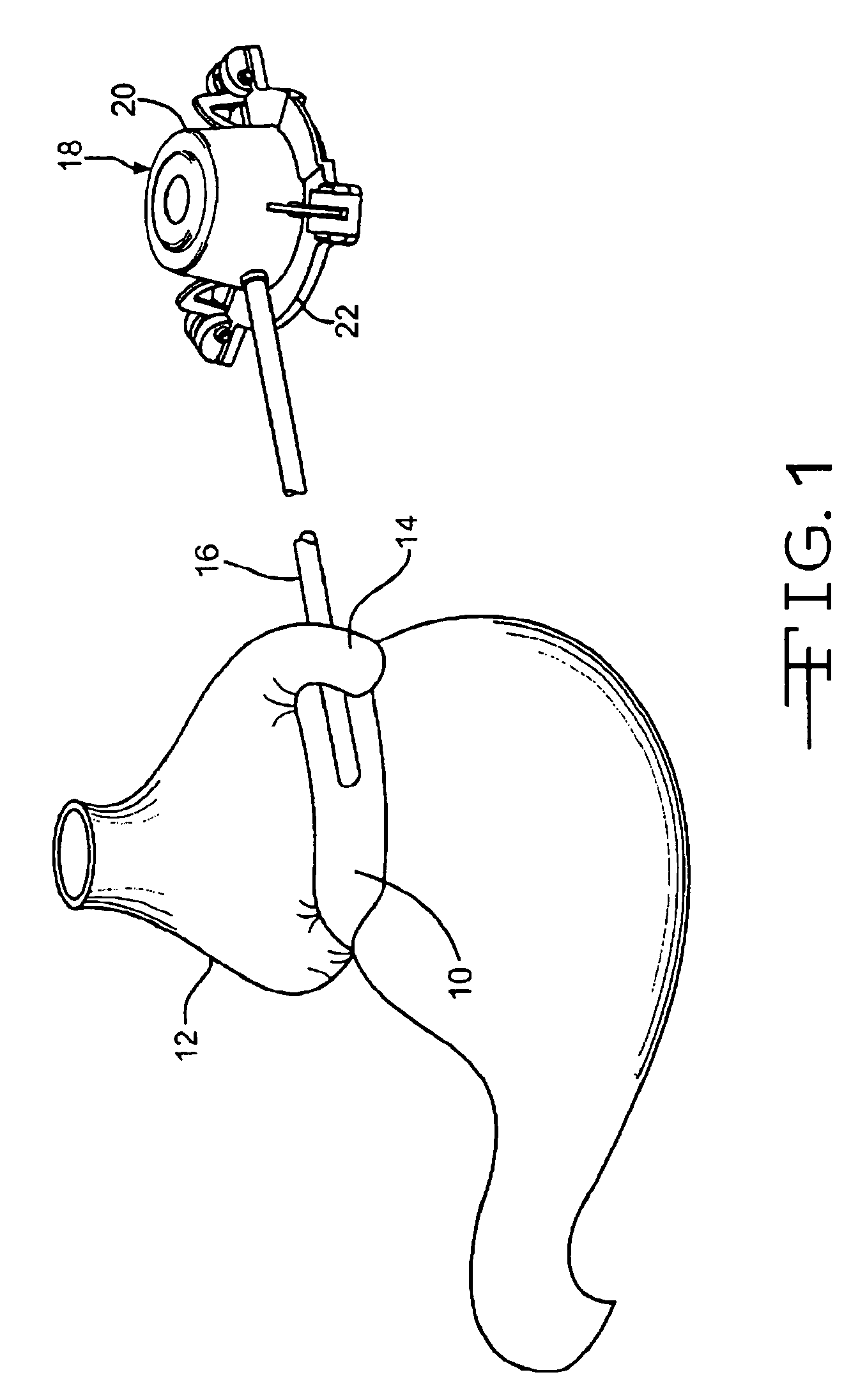
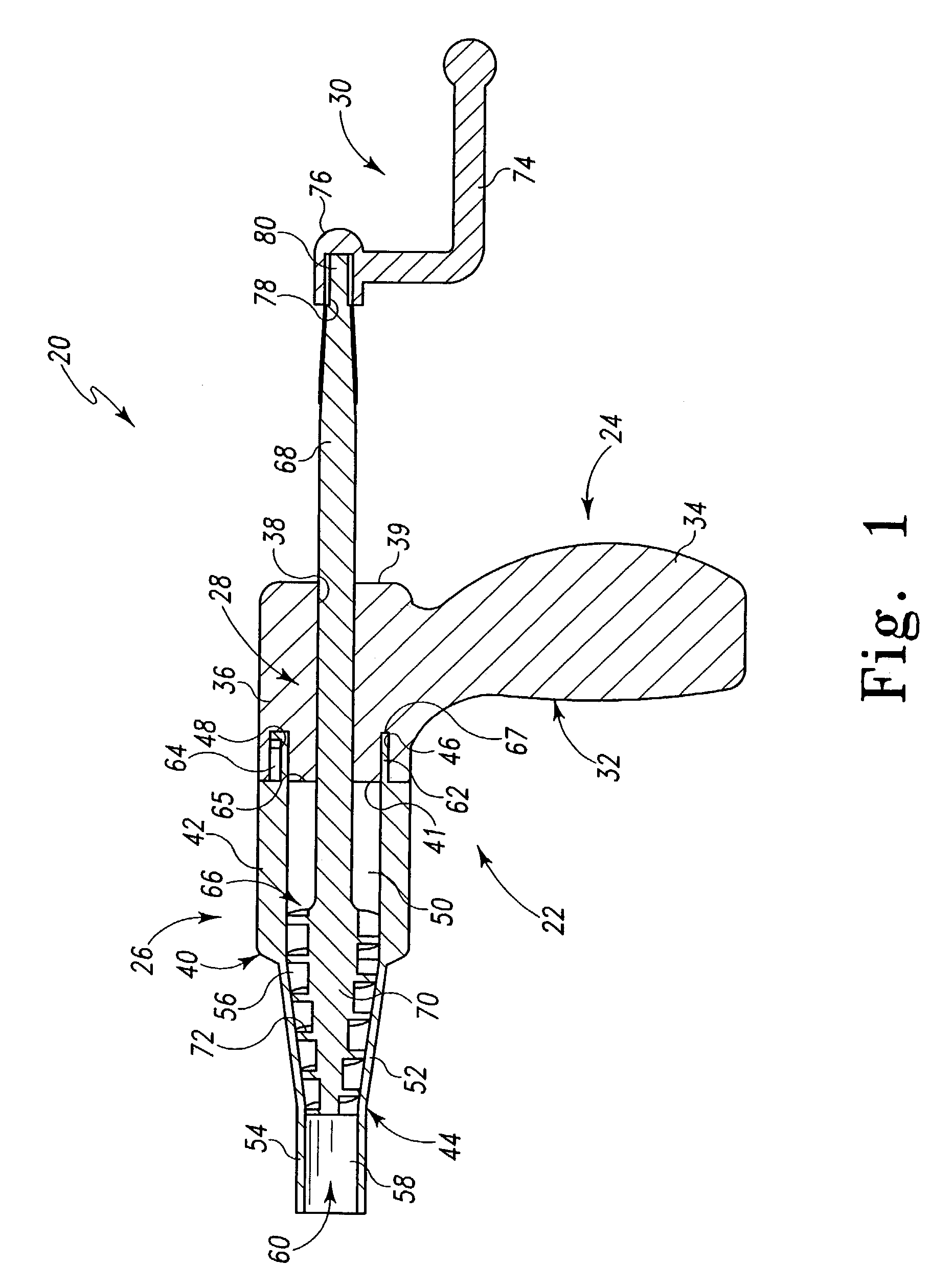
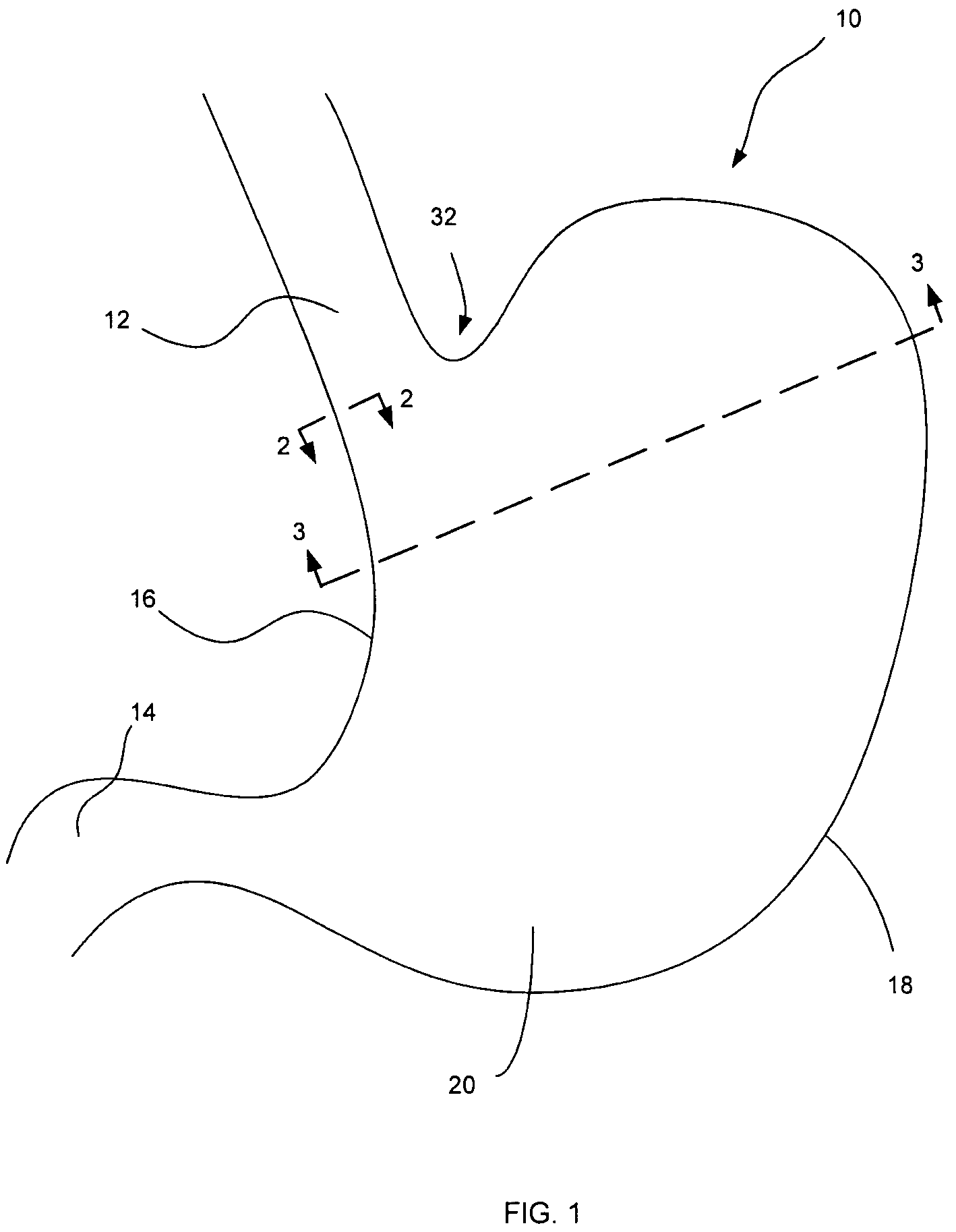
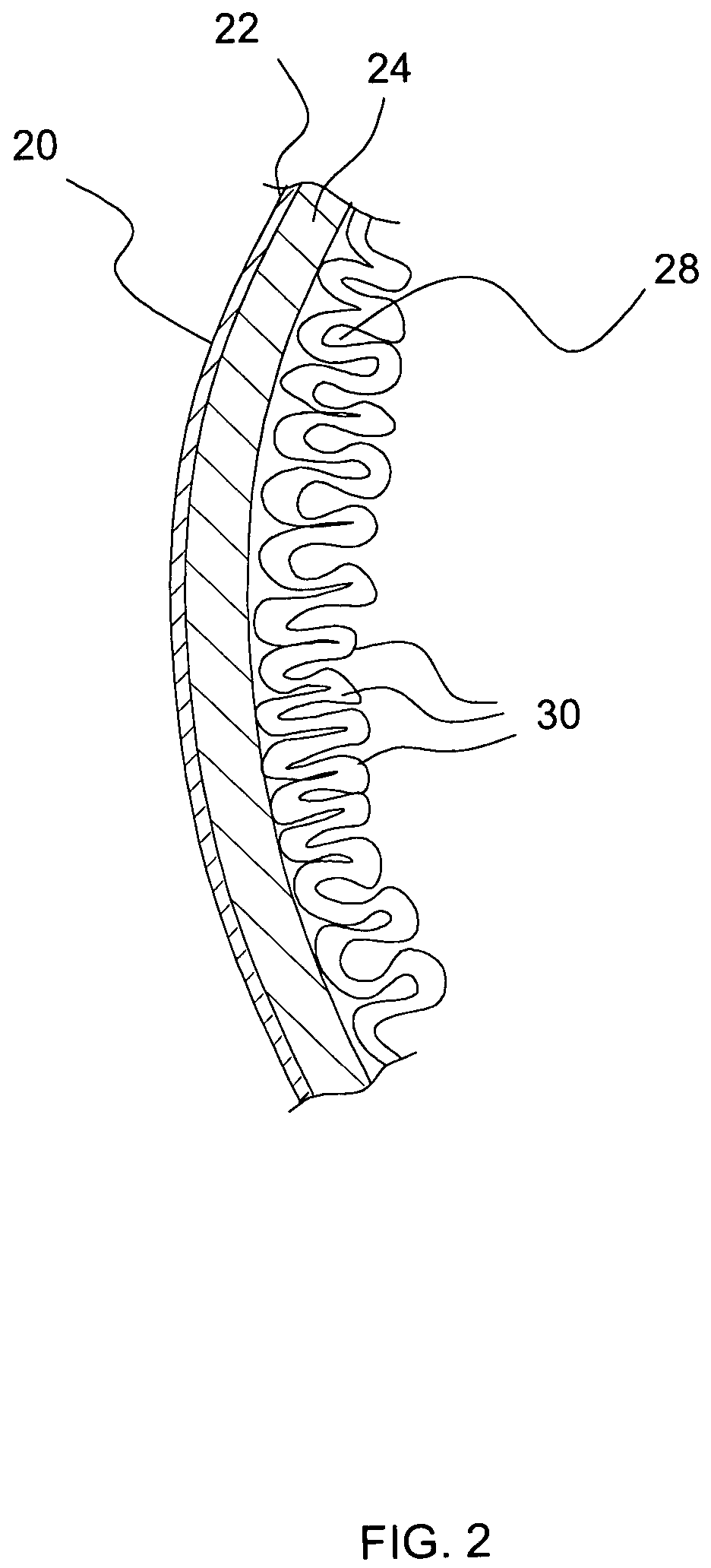
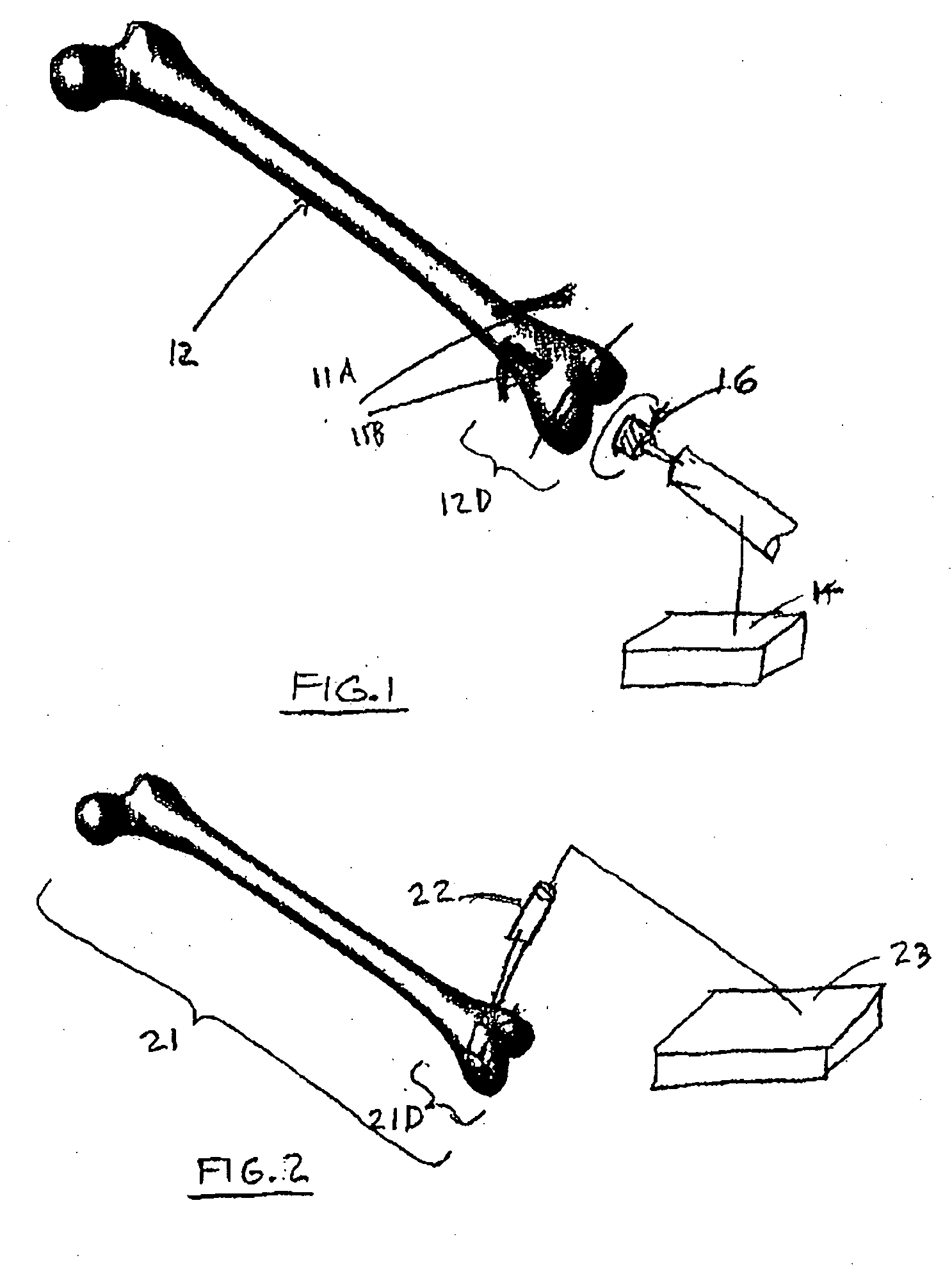
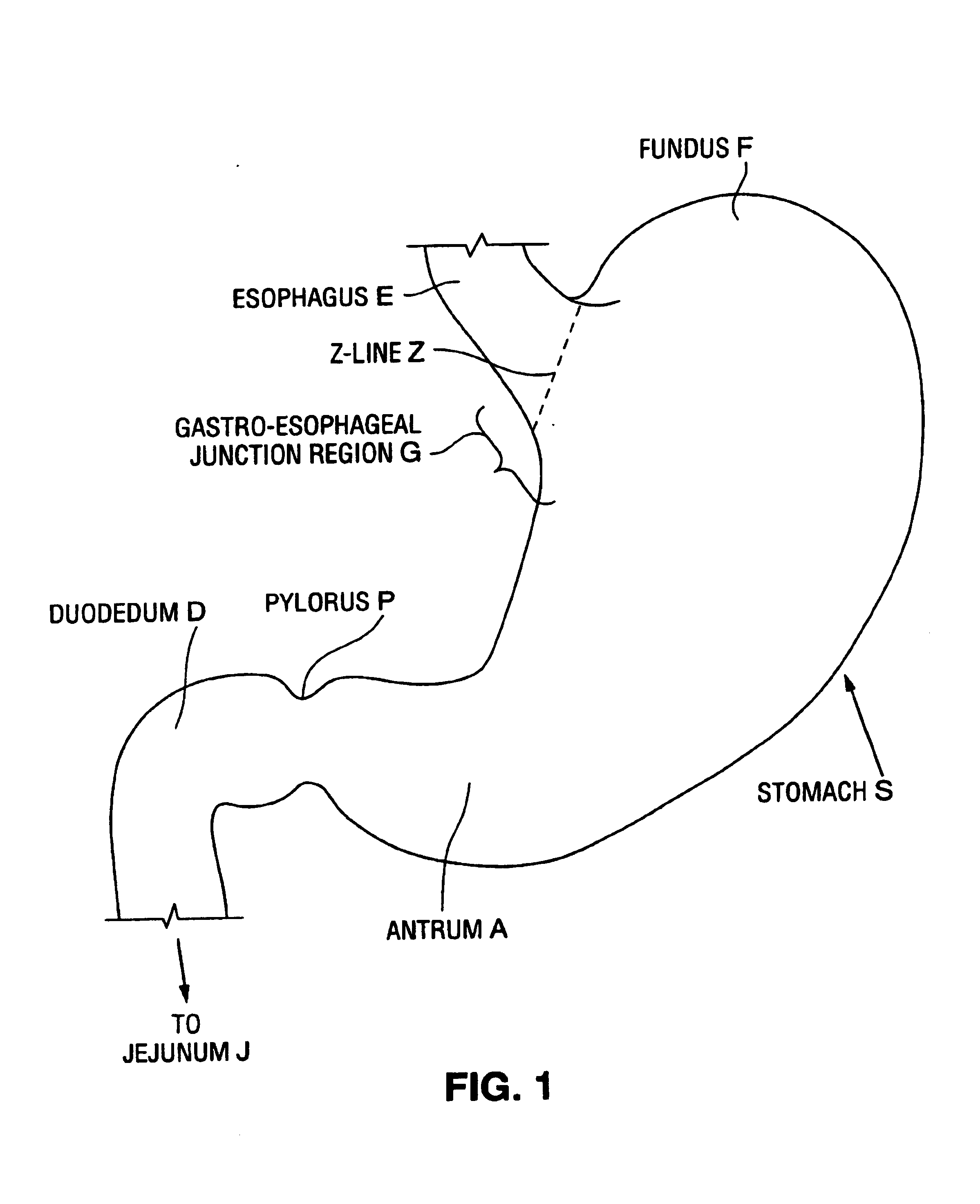
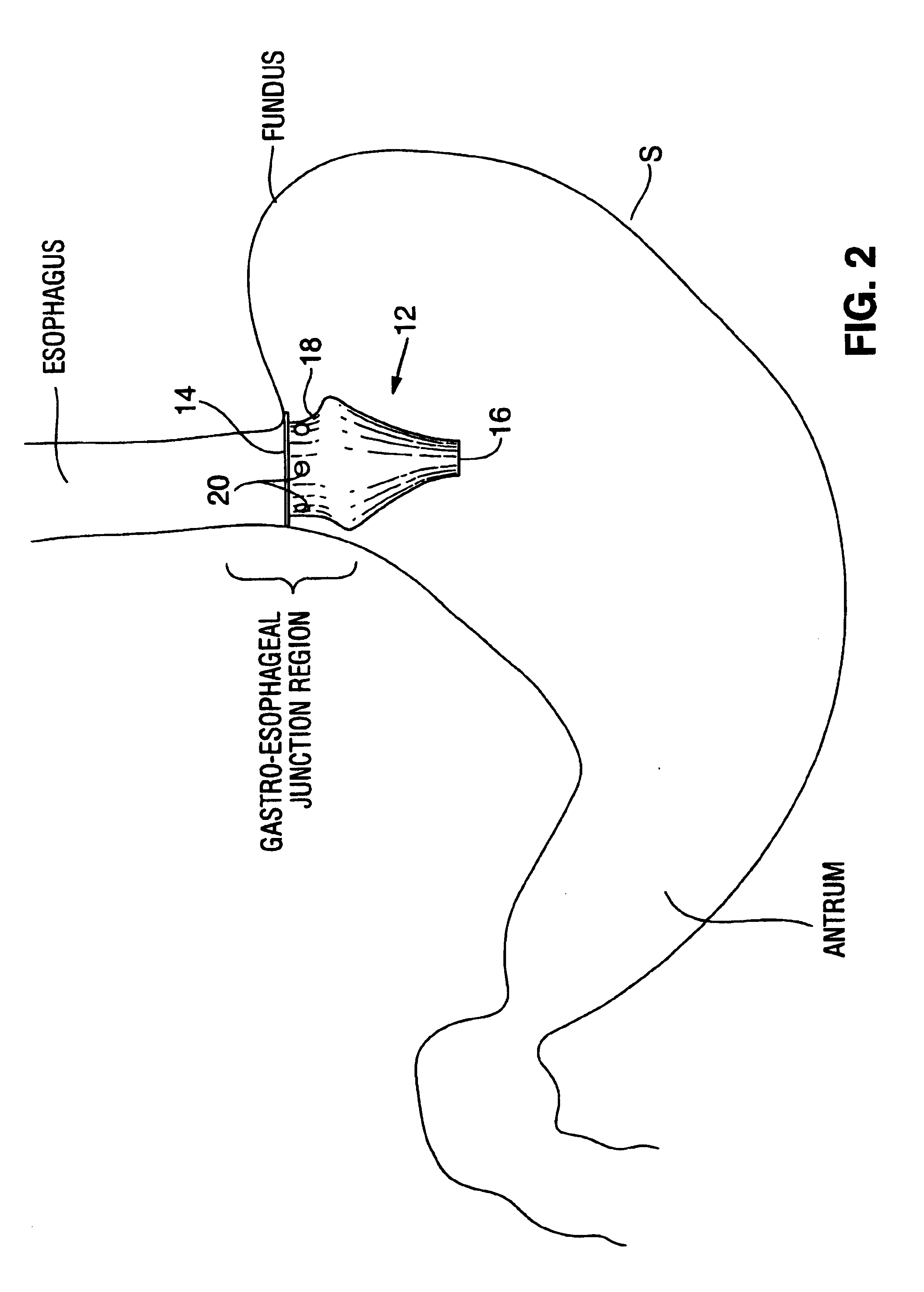
Apparatus and methods for treatment of morbid obesity
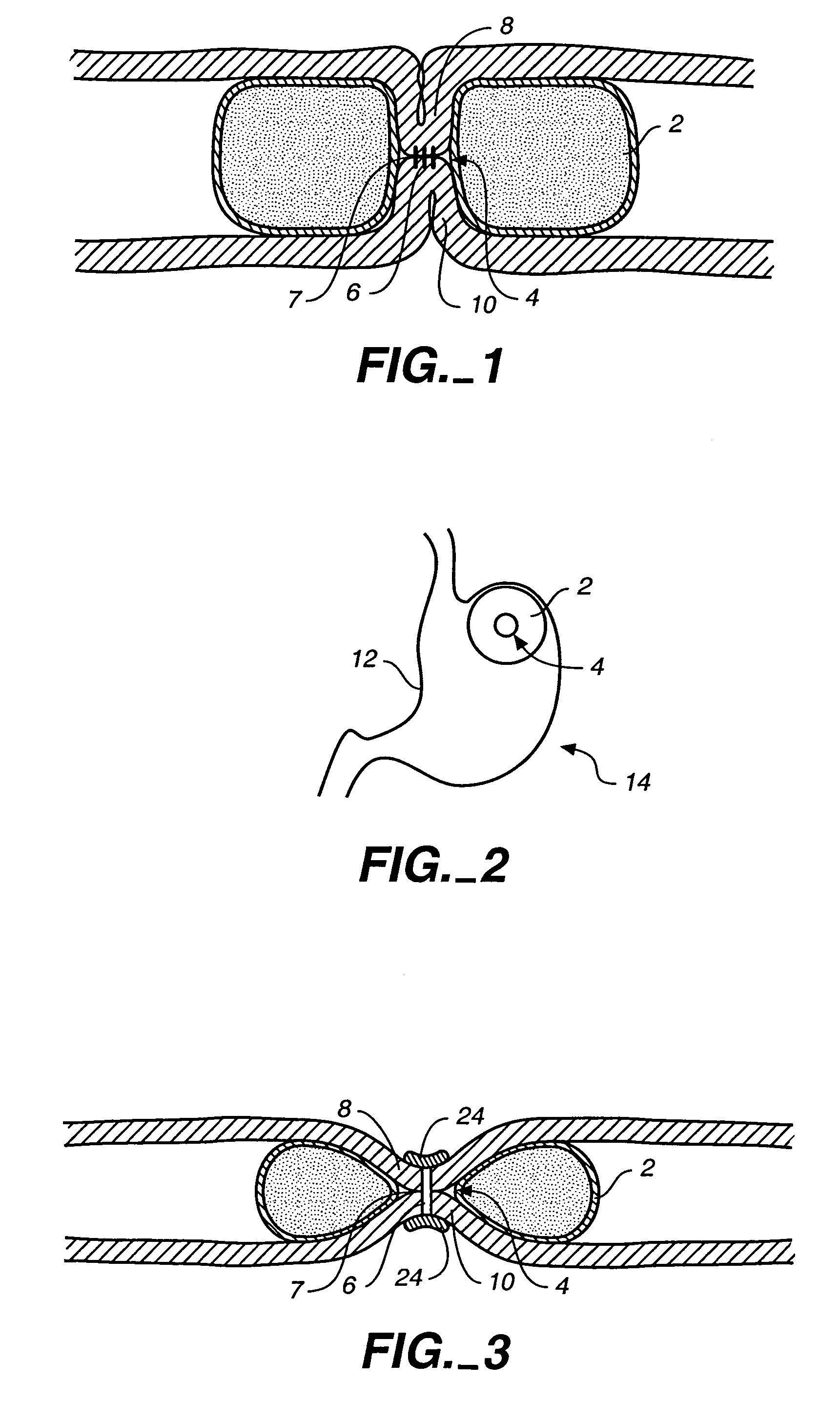
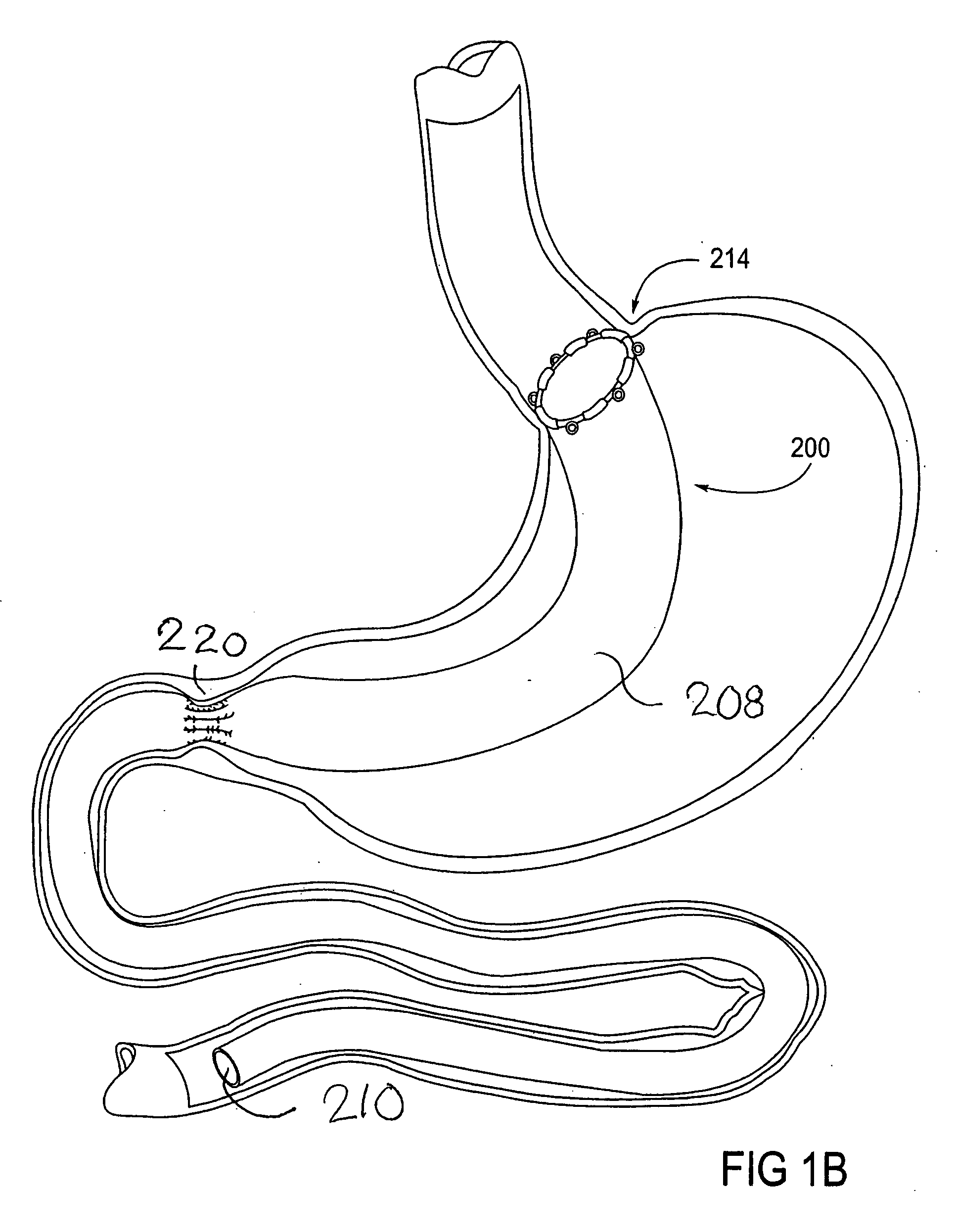
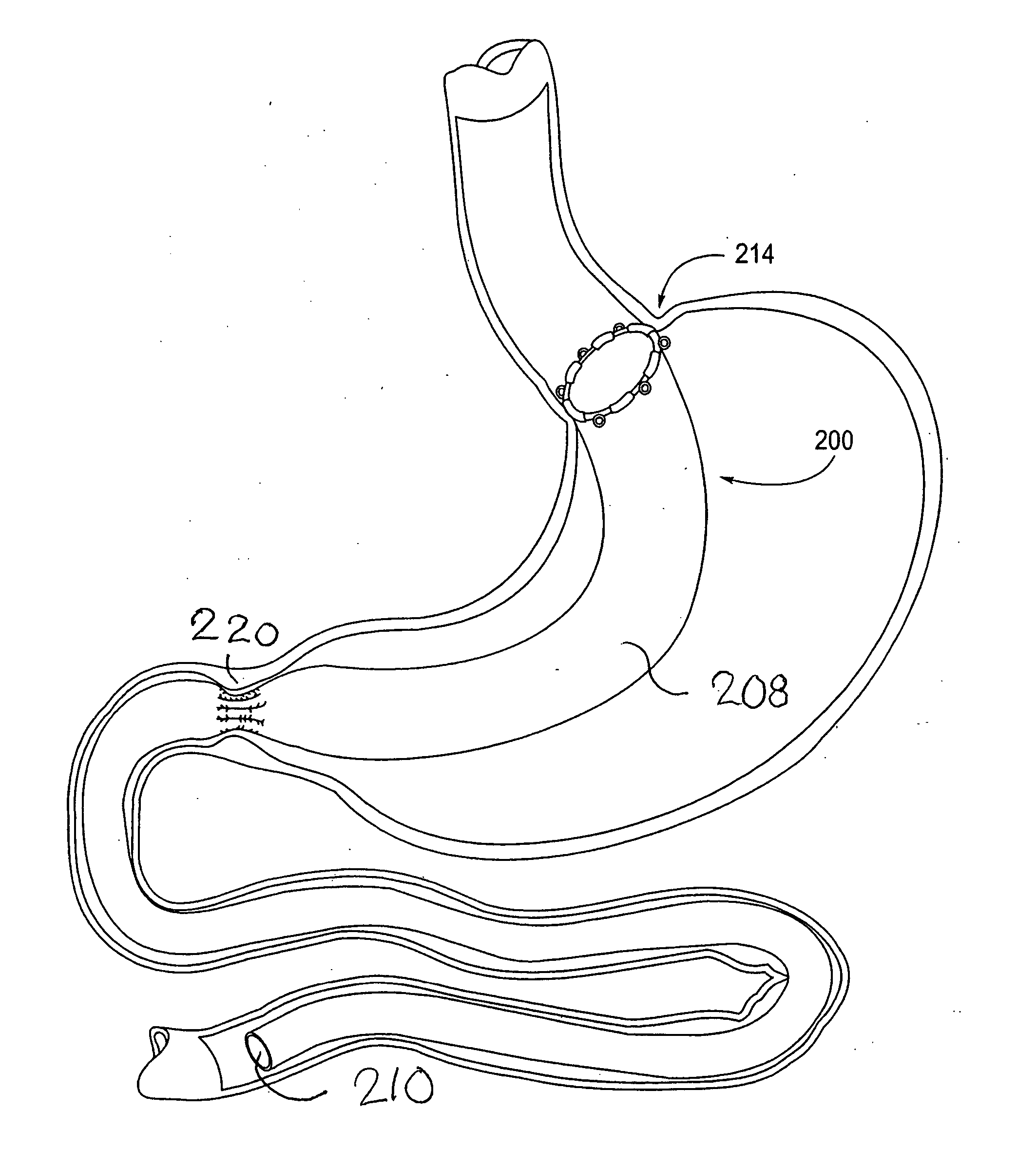
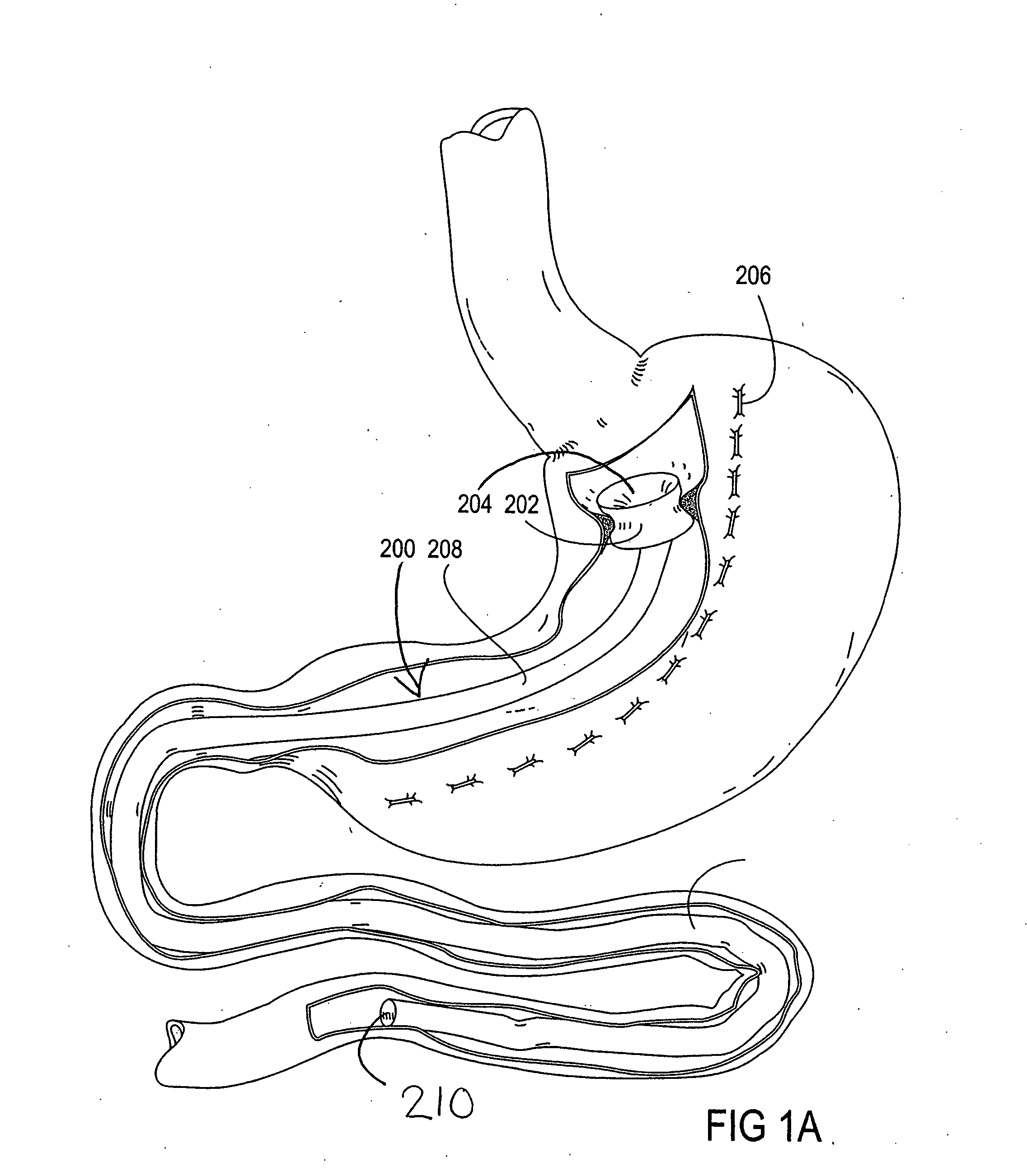
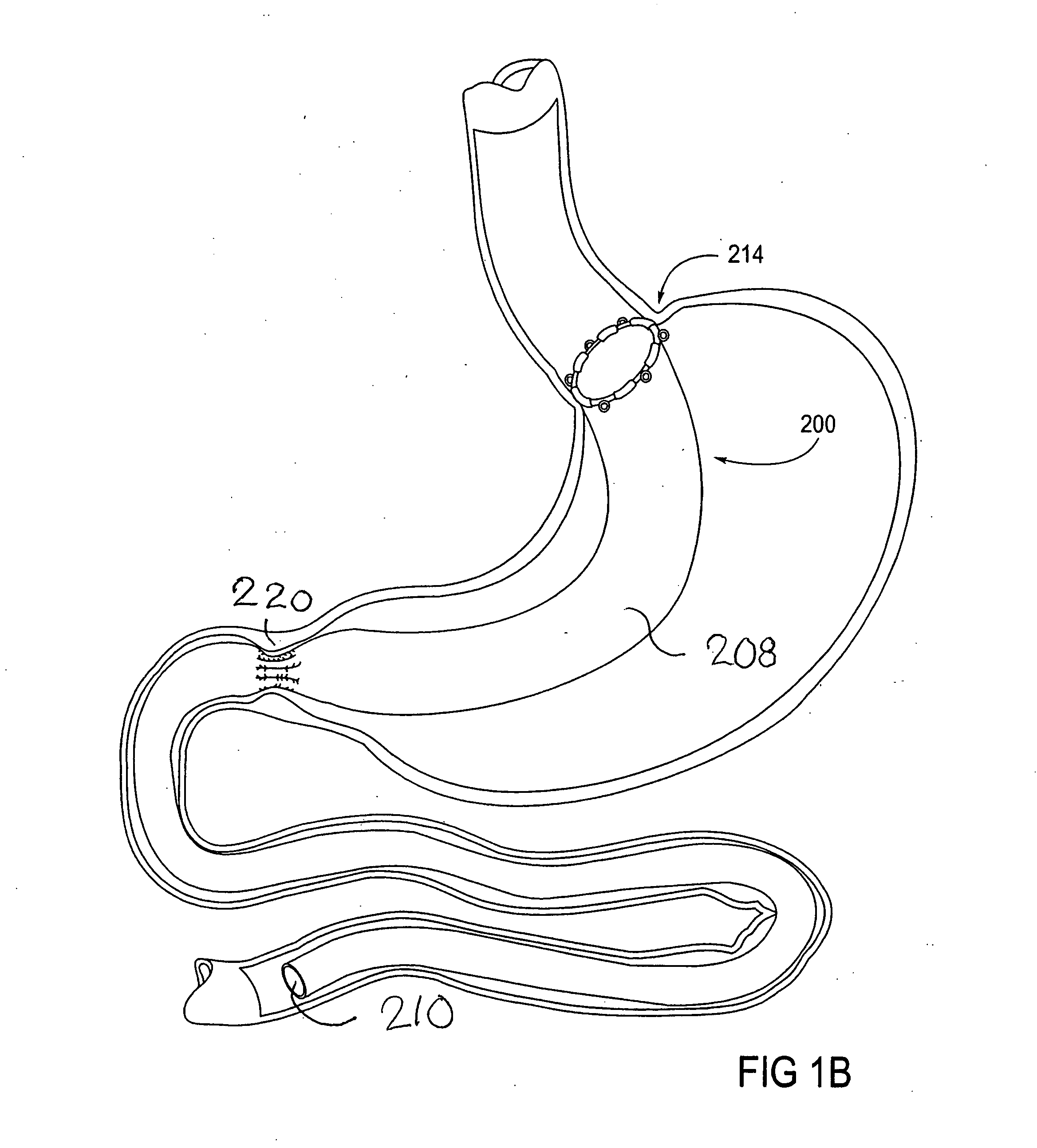
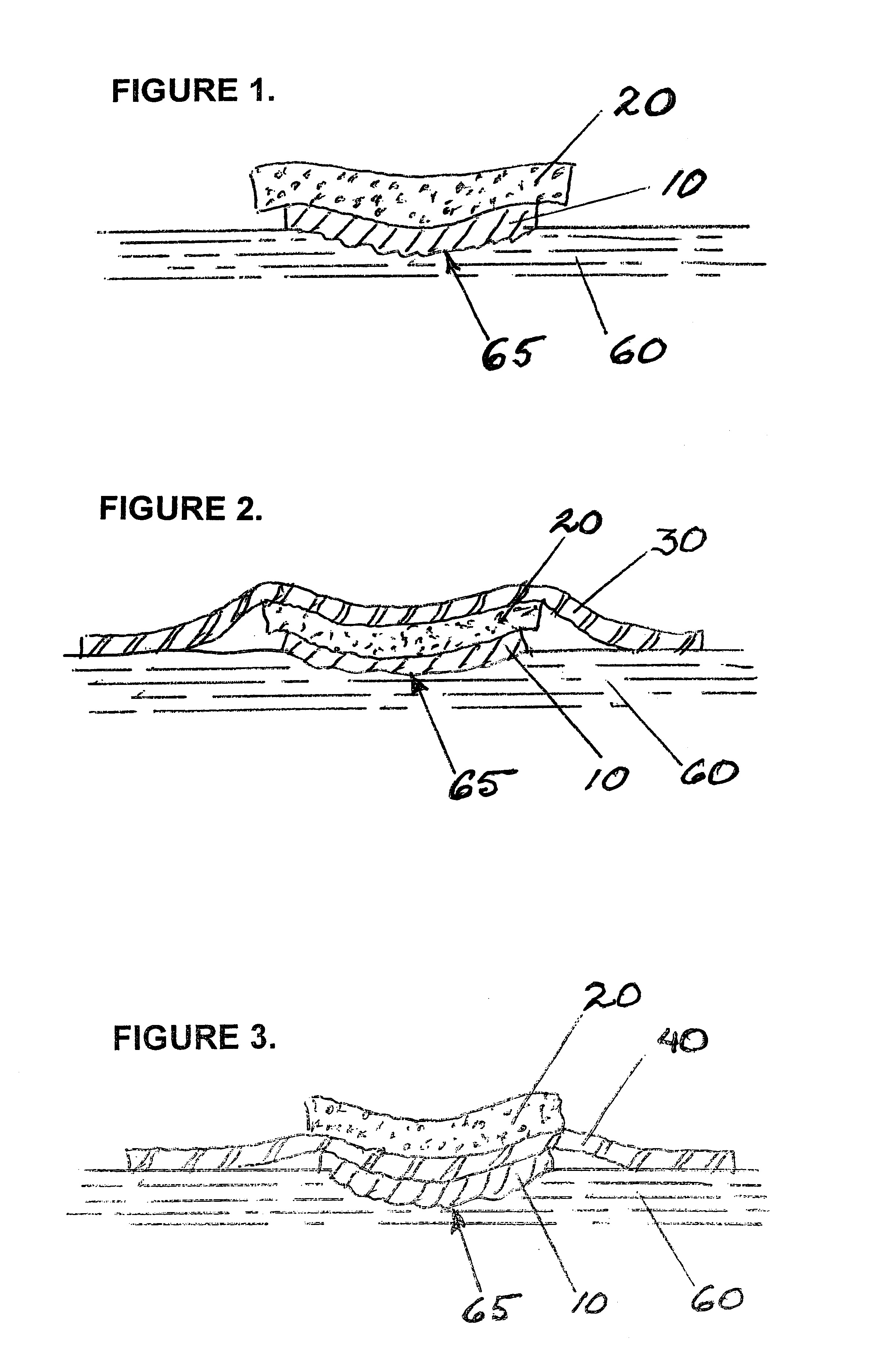
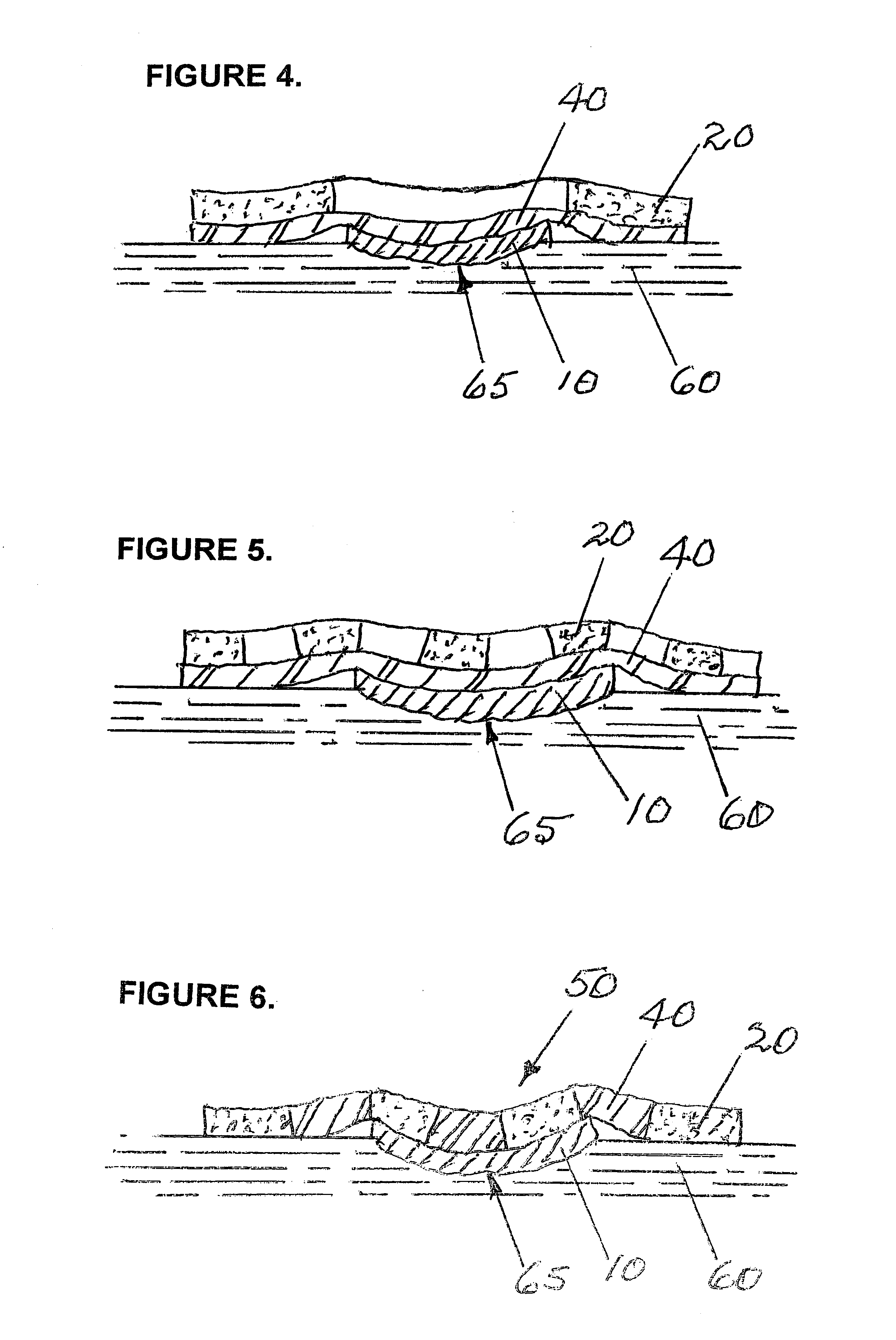
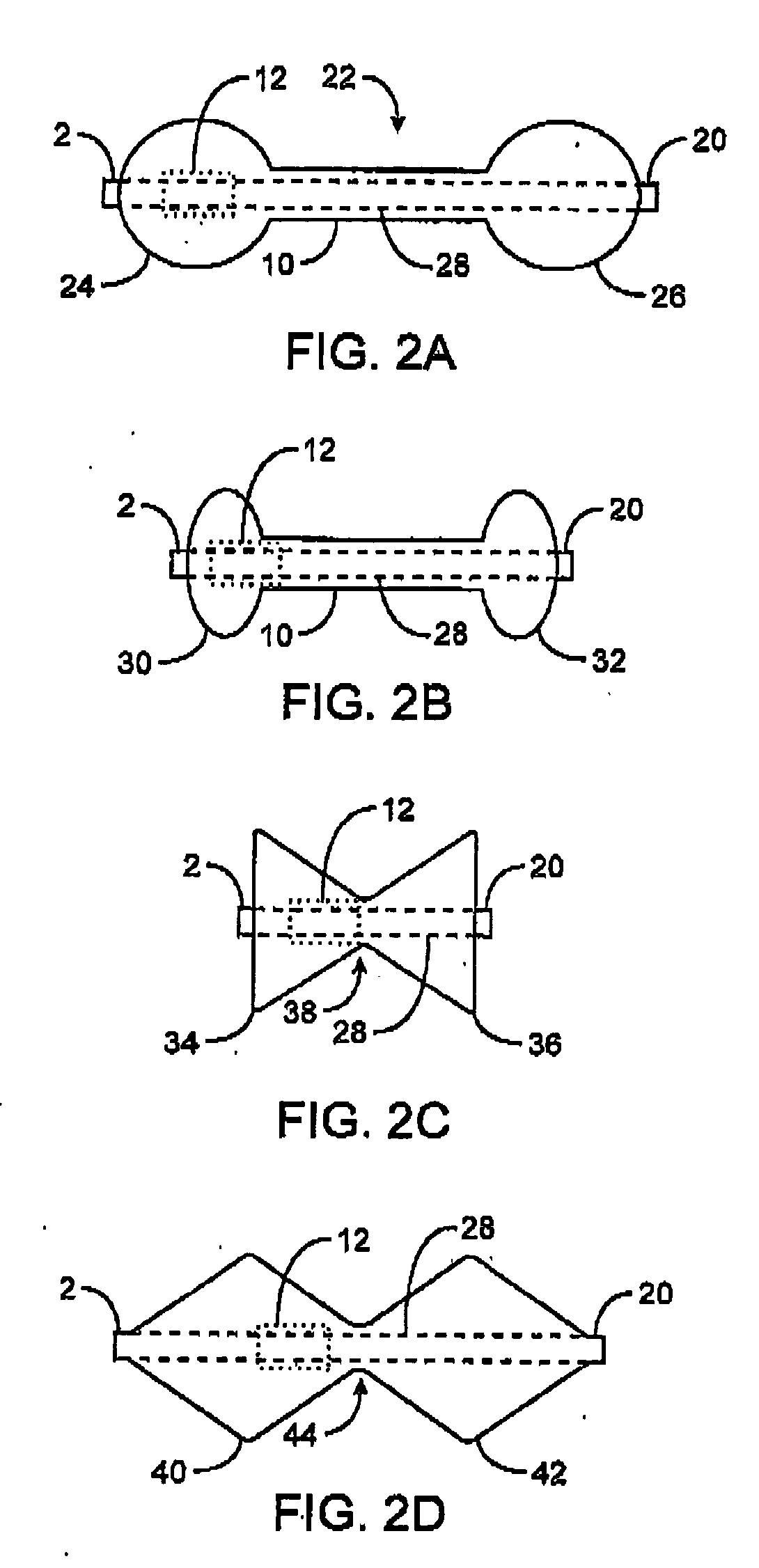
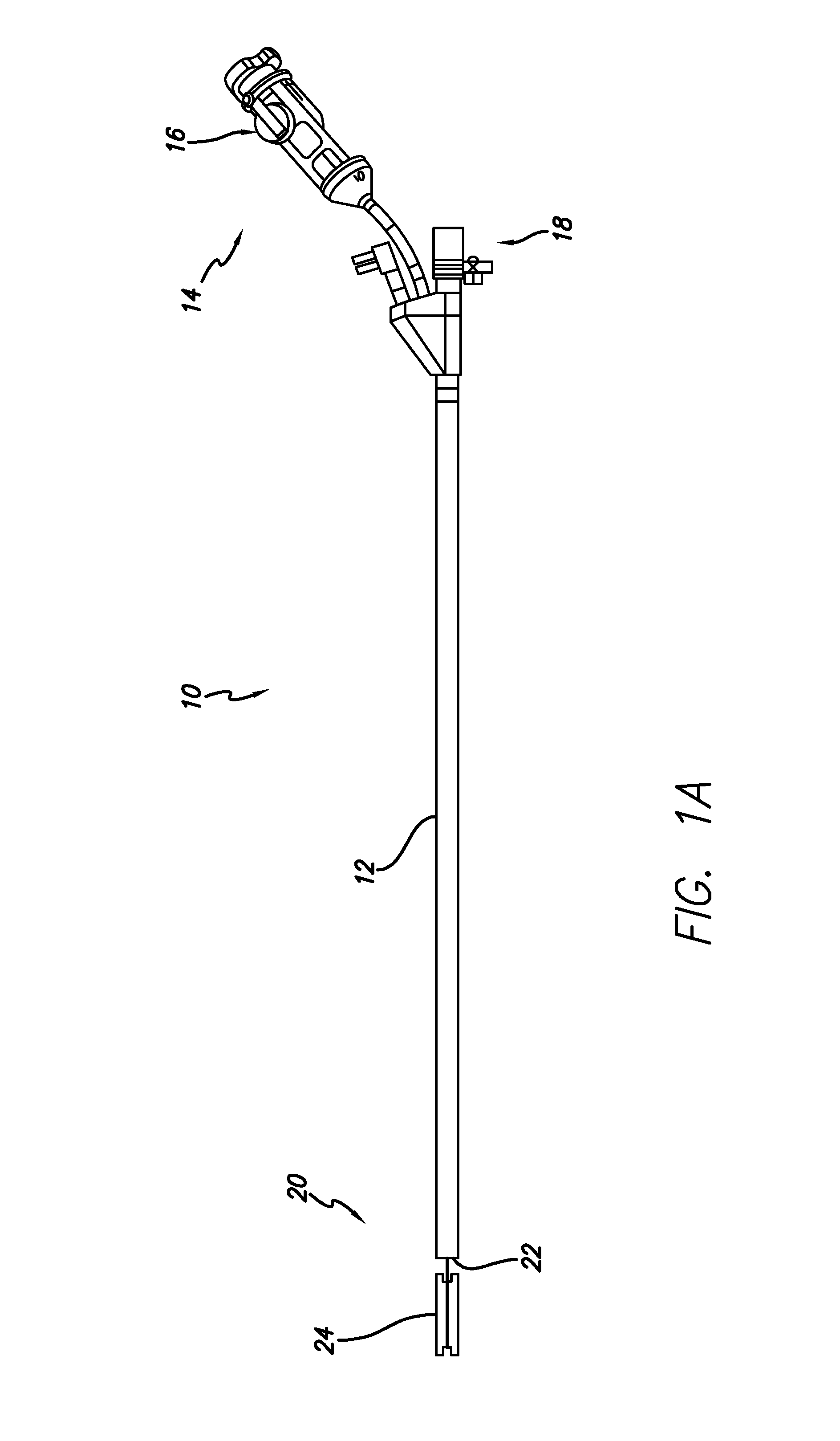
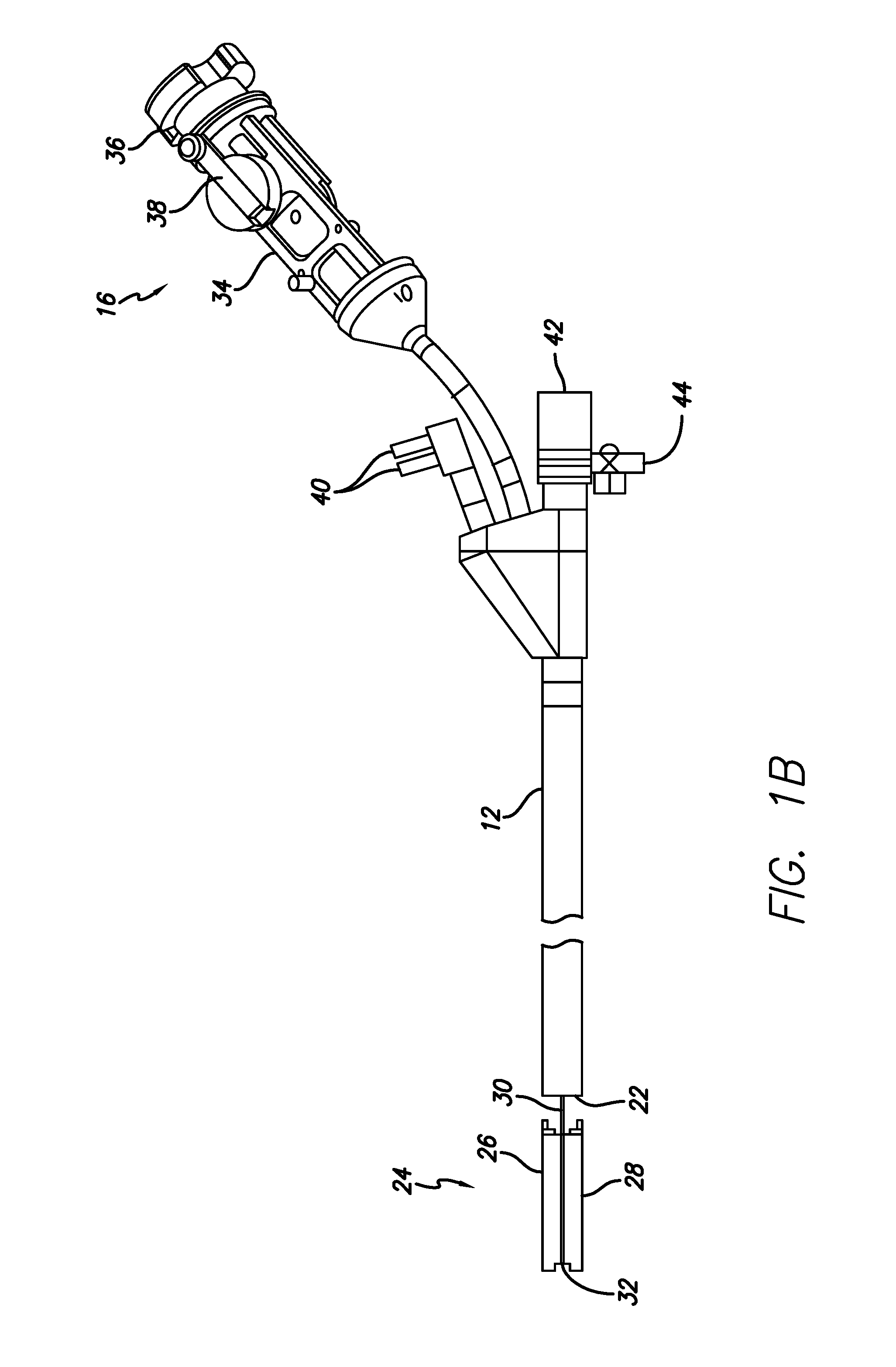
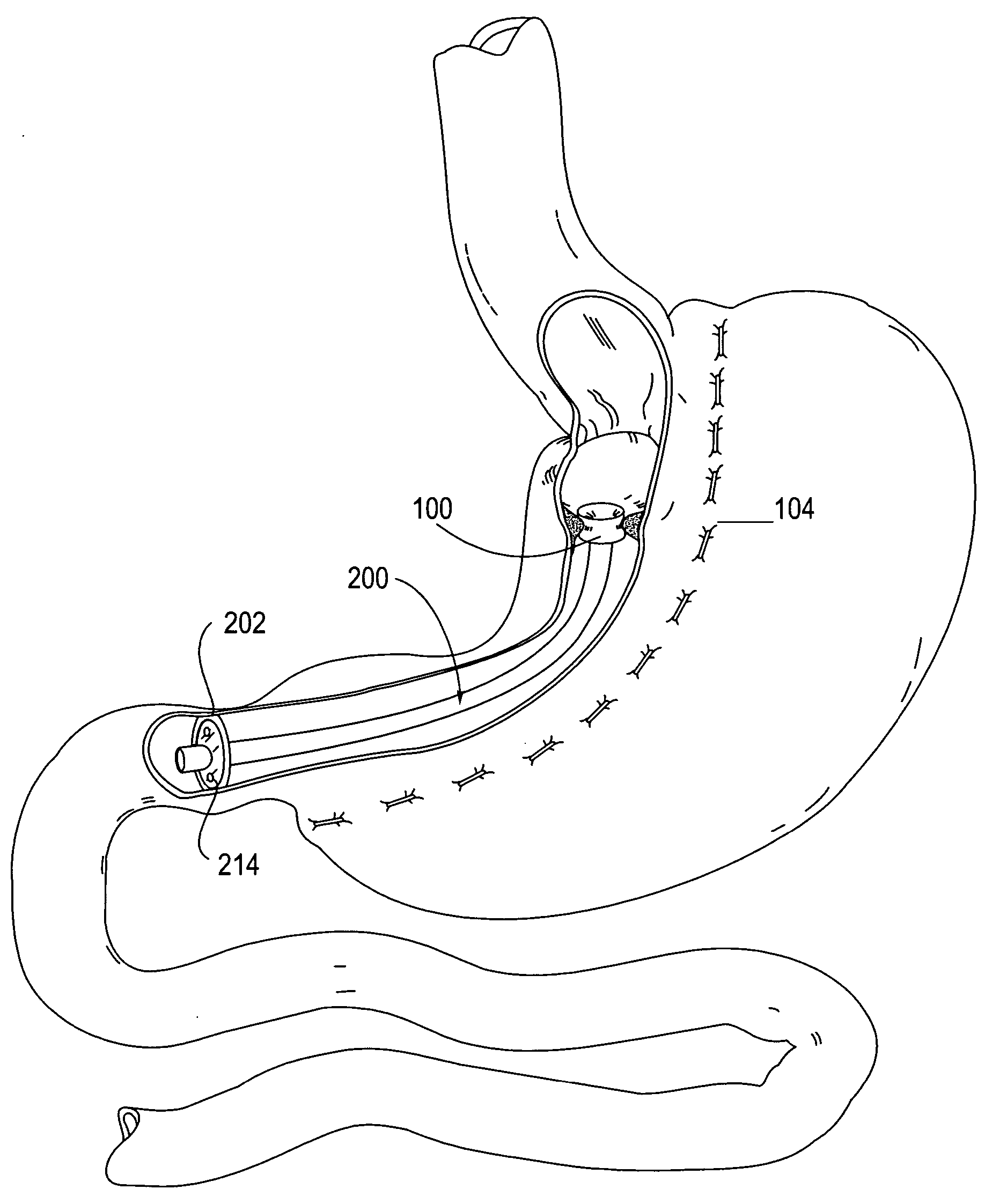
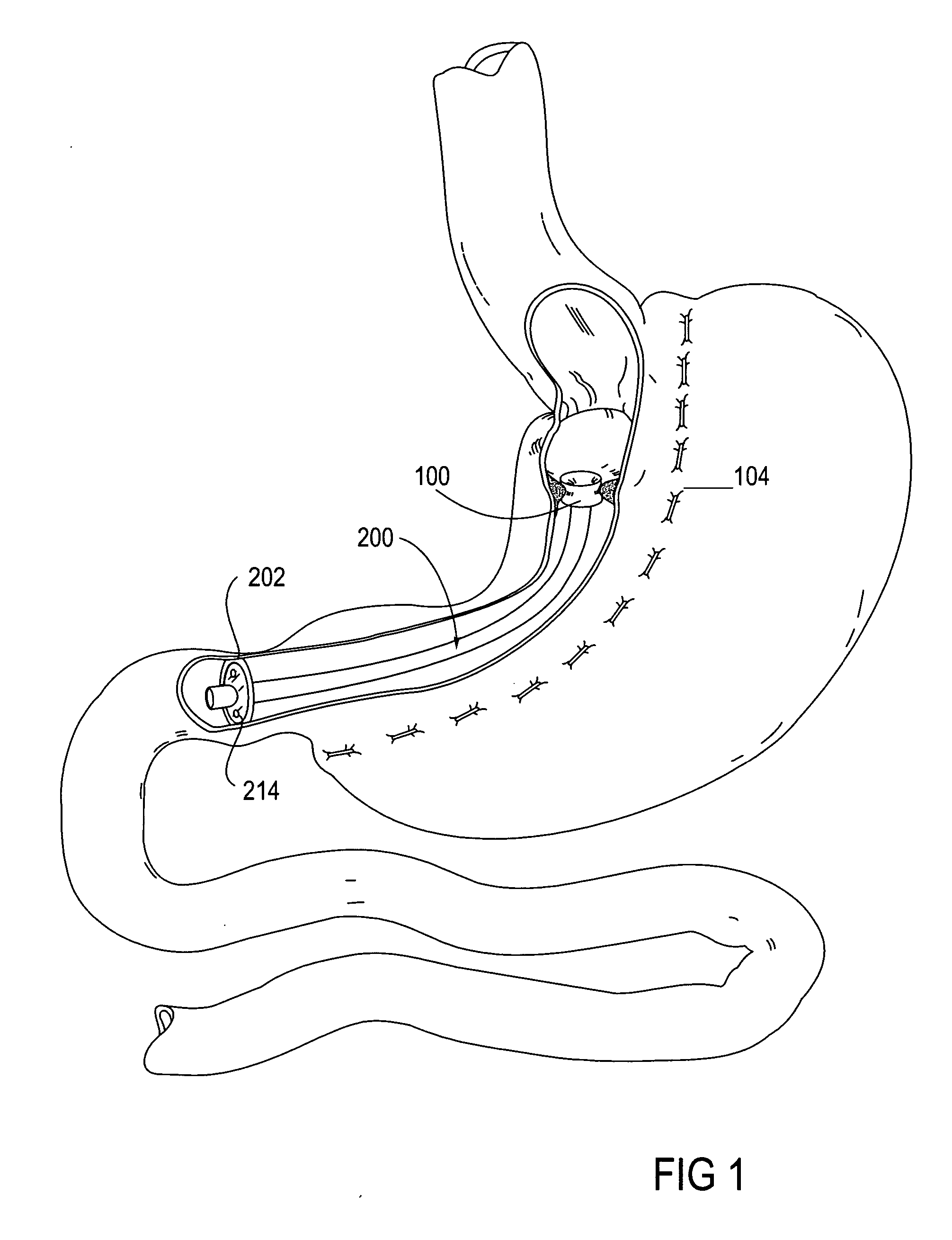
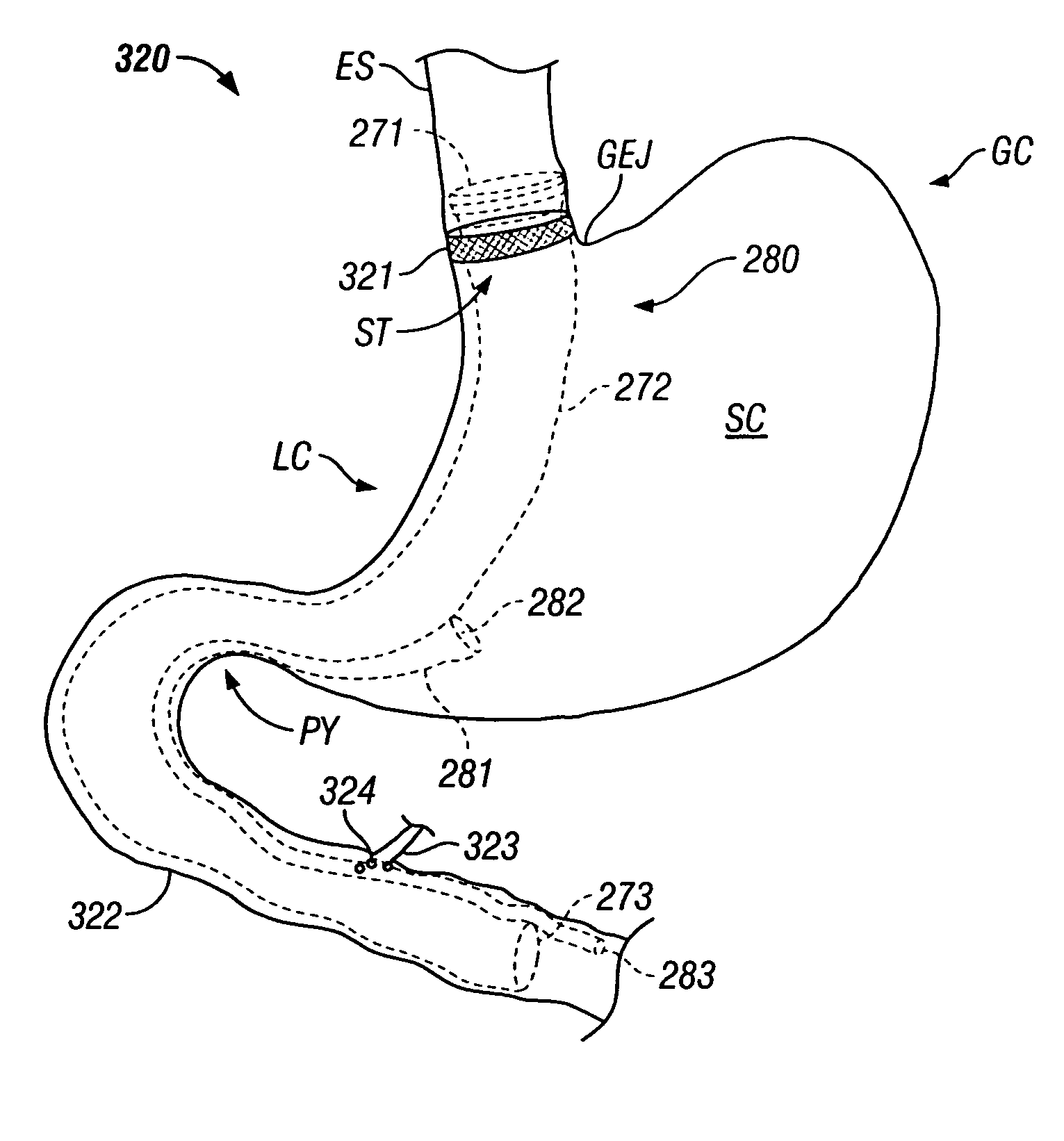
Apparatus and methods are described for treatment of morbid obesity using minimally invasive techniques. The apparatus includes a system of components that may be used separately or in combination for effectively reducing stomach volume, bypassing a portion of the stomach and / or small intestines, reducing nutrient absorption in the stomach and / or small intestines and / or depositing minimally or undigested food farther than normal into the intestines, thereby stimulating intestinal responses. The components described include an artificial stoma device, a gastric sleeve device, an intestinal sleeve device and a combined gastrointestinal sleeve device.
Owner:VALENTX
Methods and devices for maintaining a space occupying device in a relatively fixed location within a stomach
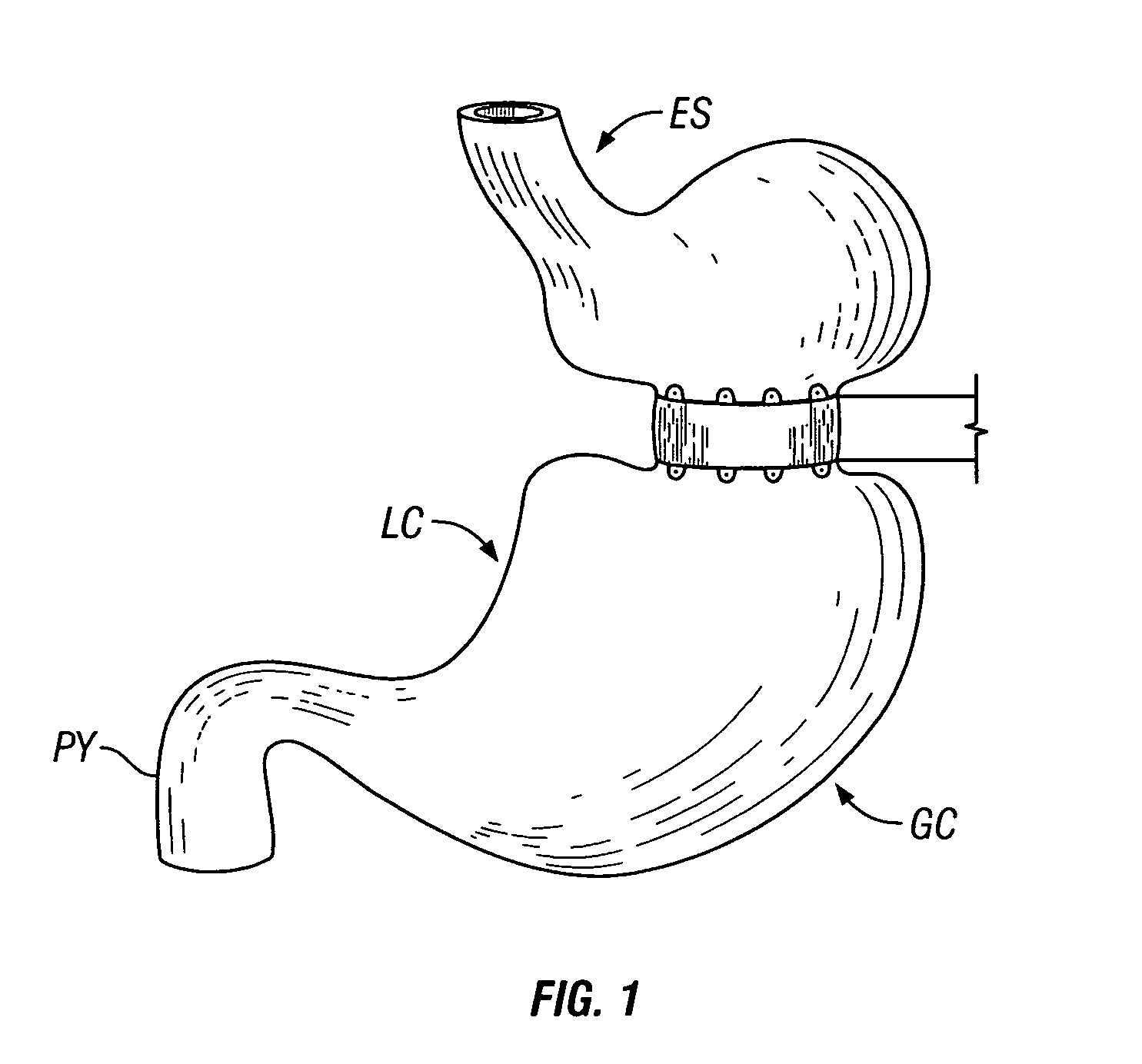
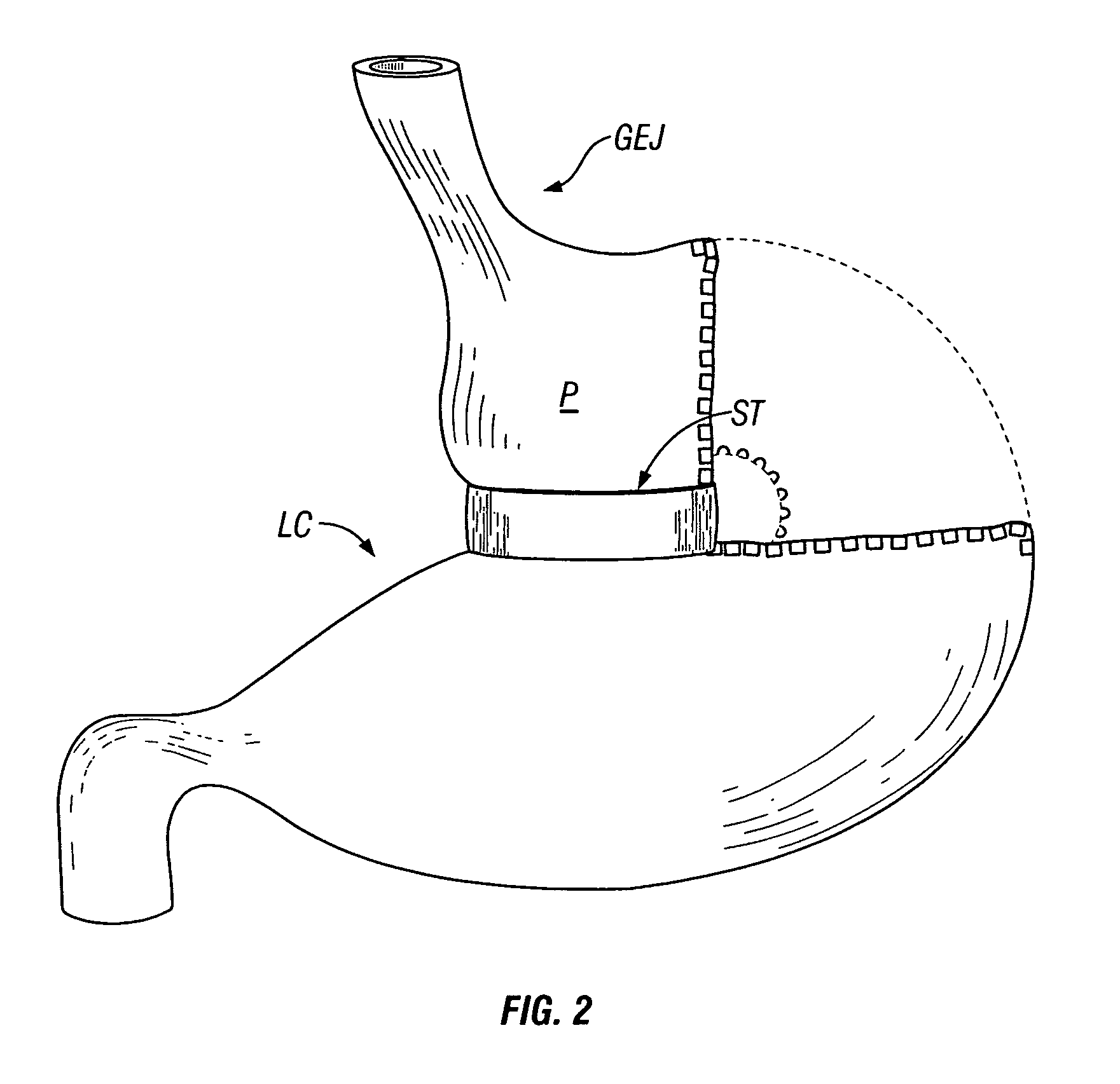
Methods and devices for maintaining a space-occupying device in a fixed relationship relative to a patient's stomach by manipulation of the stomach. In one variation, two or more regions of the stomach wall are brought into appromixation with one another and secured together in a manner that secures a space-occupying device within the stomach of the patient. In another variation, two or more regions of the stomach wall are wrapped around a space-occupying device to maintain the position of the space-occupying device relative to the stomach wall. In another variation, a system having a space-occupying member and a locking member capable holding the space-occupying member against the inner wall of the stomach are provided. In a further variation, a pouch is created within the stomach that receives and retains a space-occupying device.
Owner:ETHICON ENDO SURGERY INC
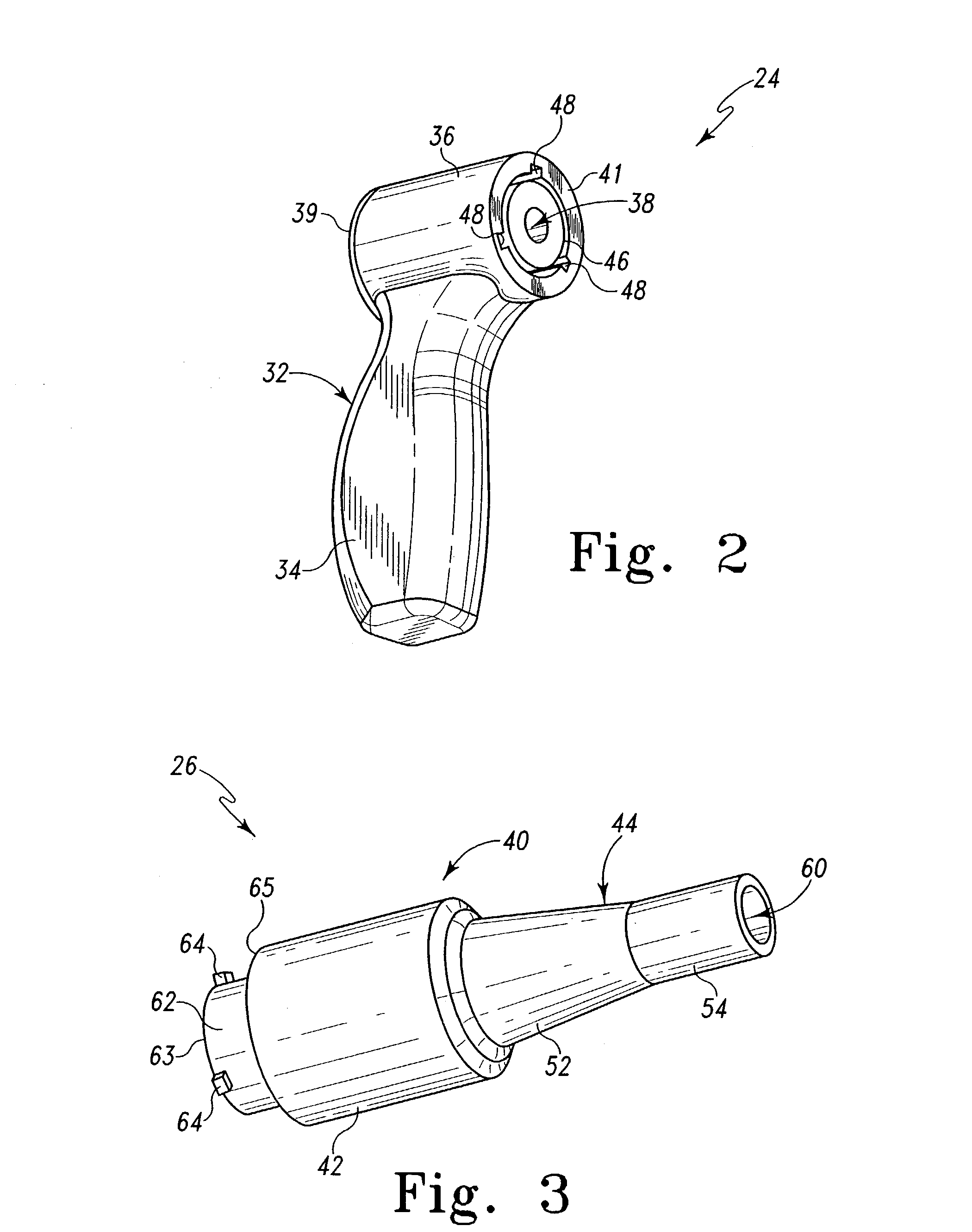
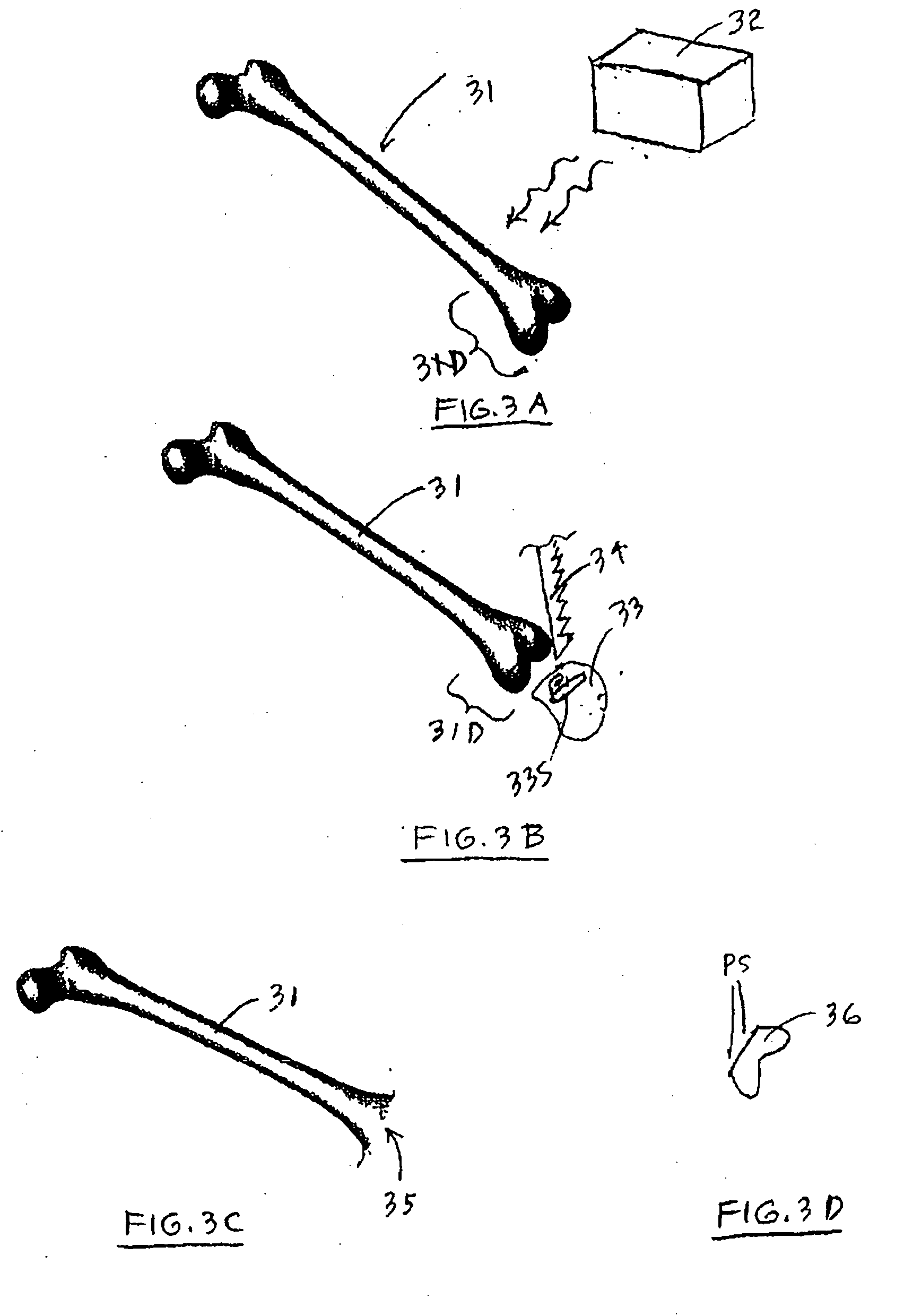
Devices and methods for treating morbid obesity
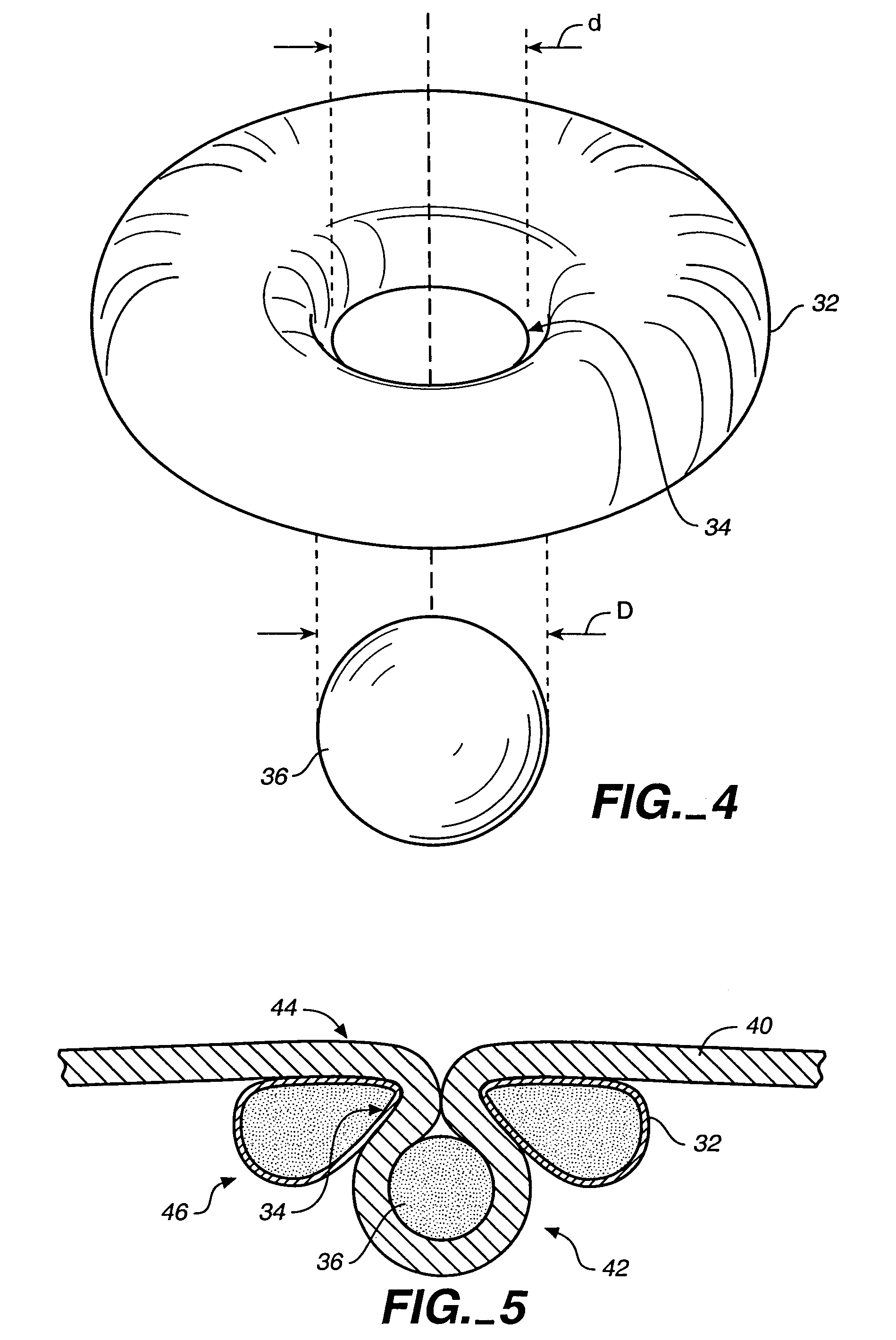
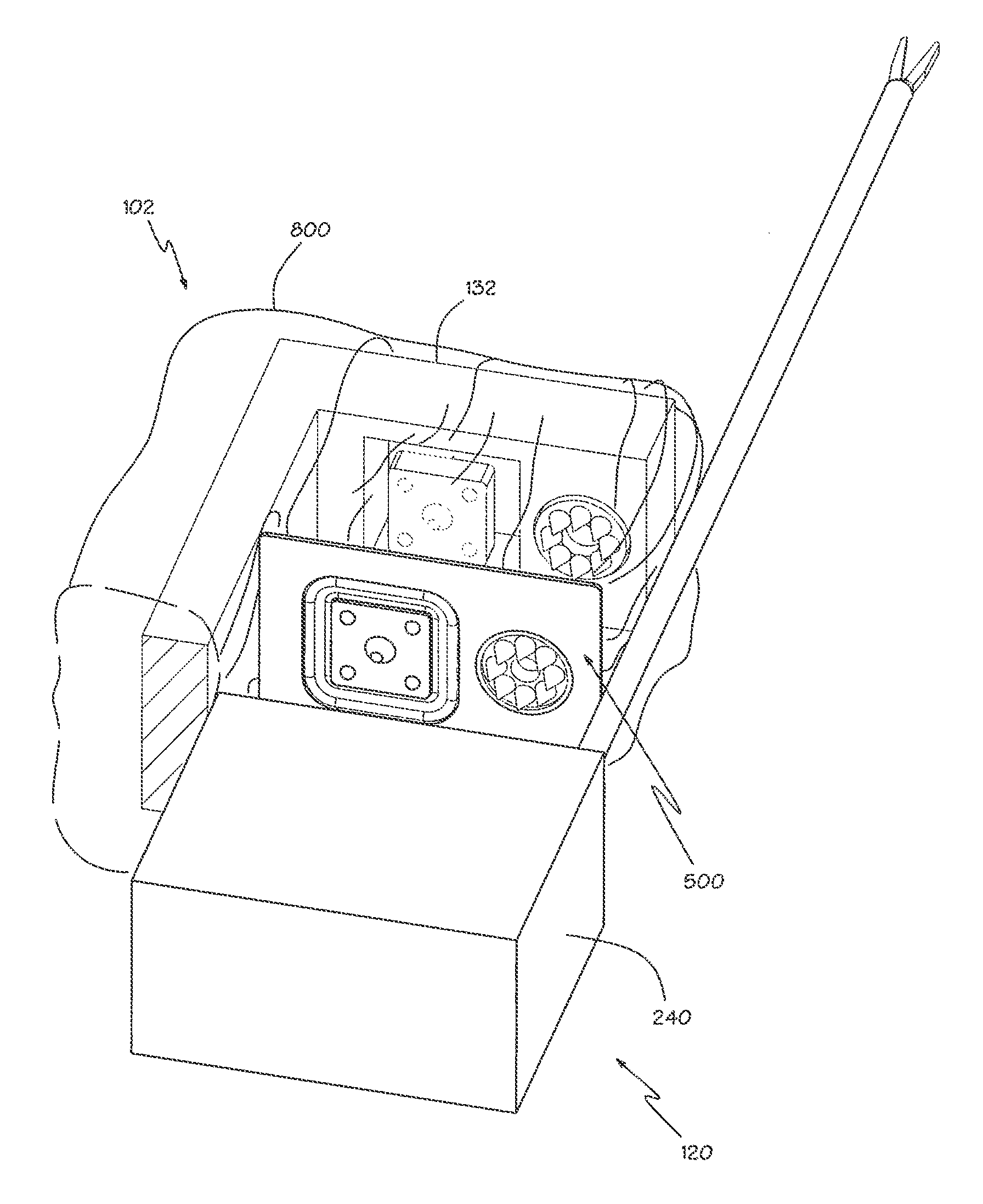
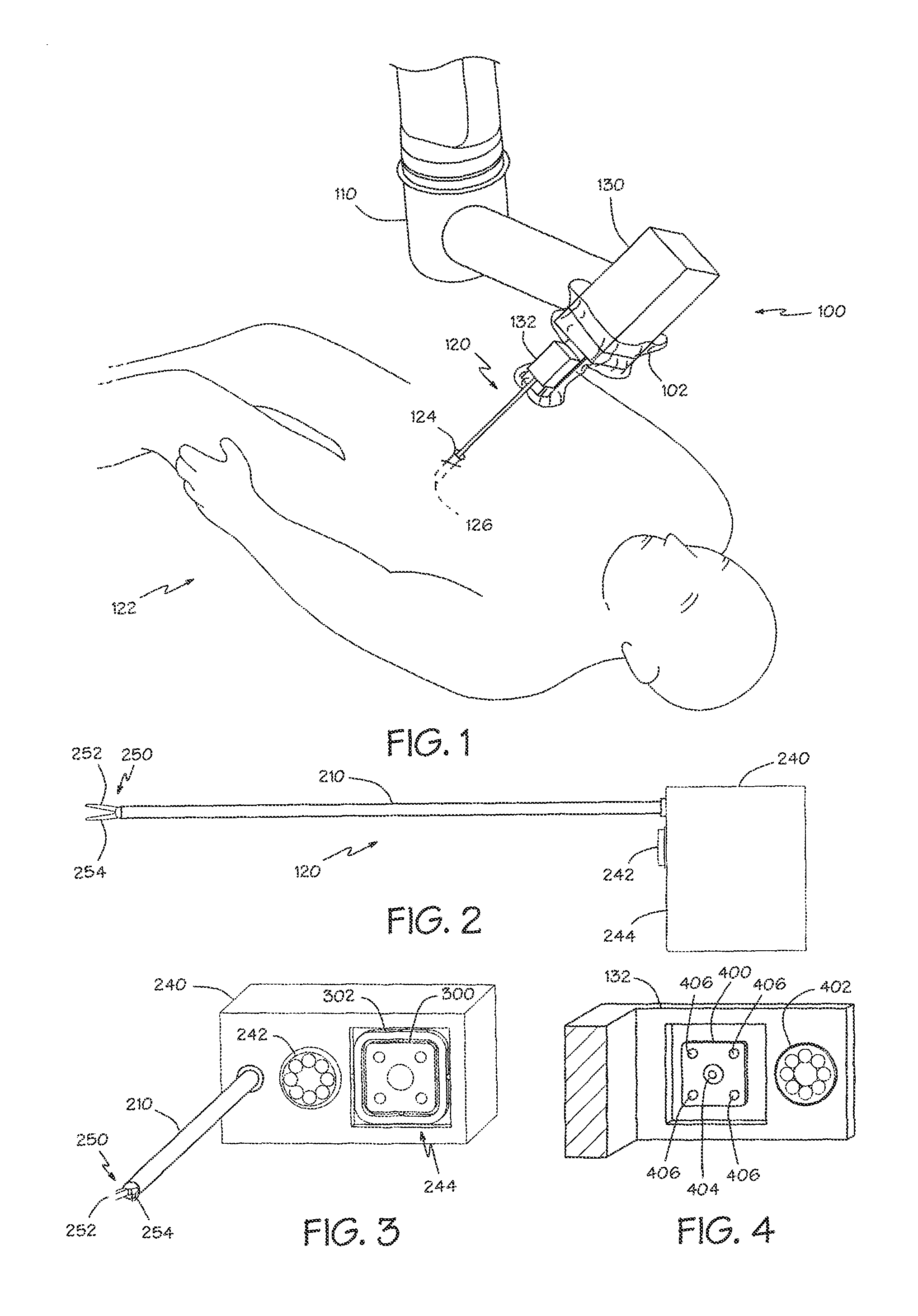
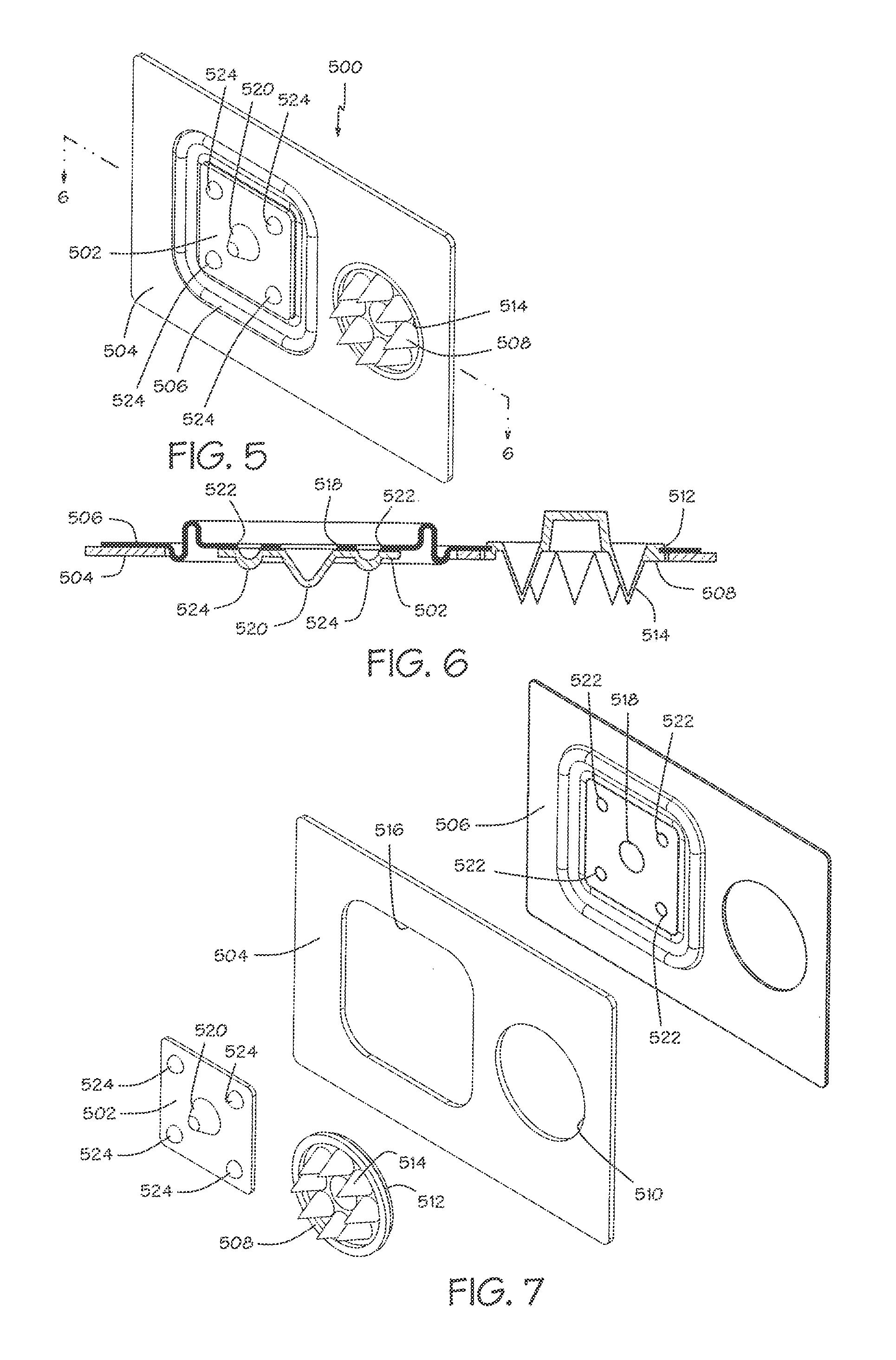
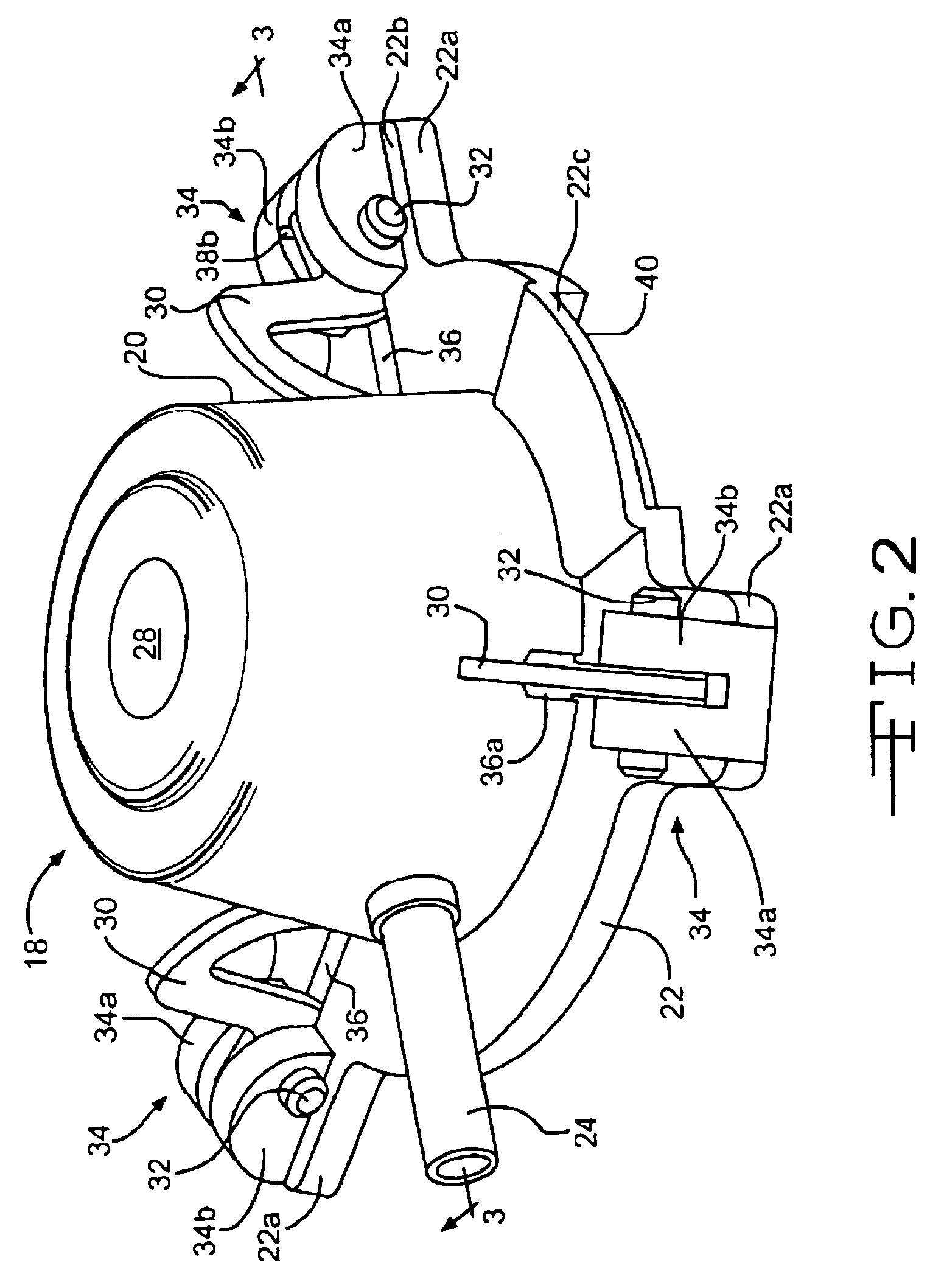
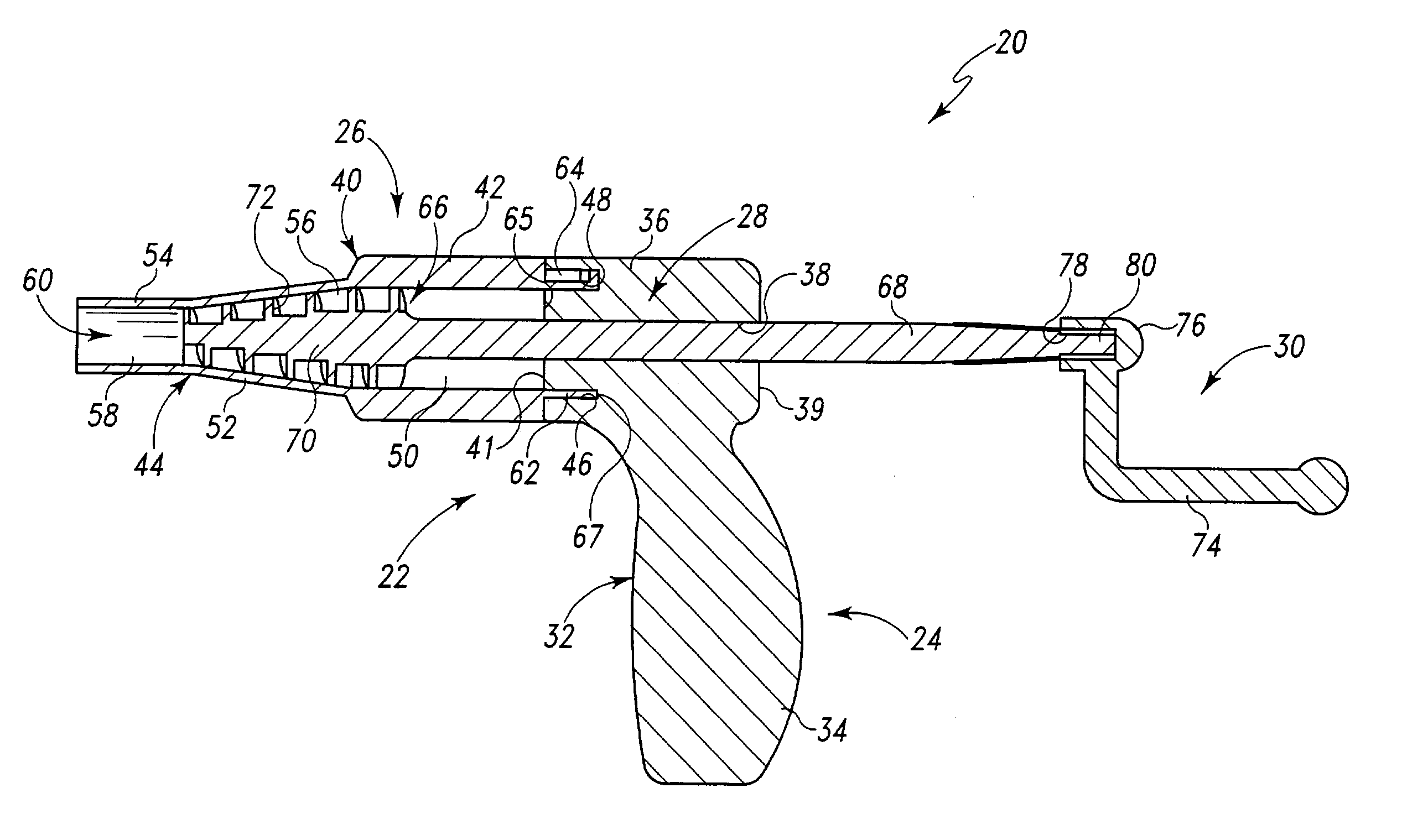
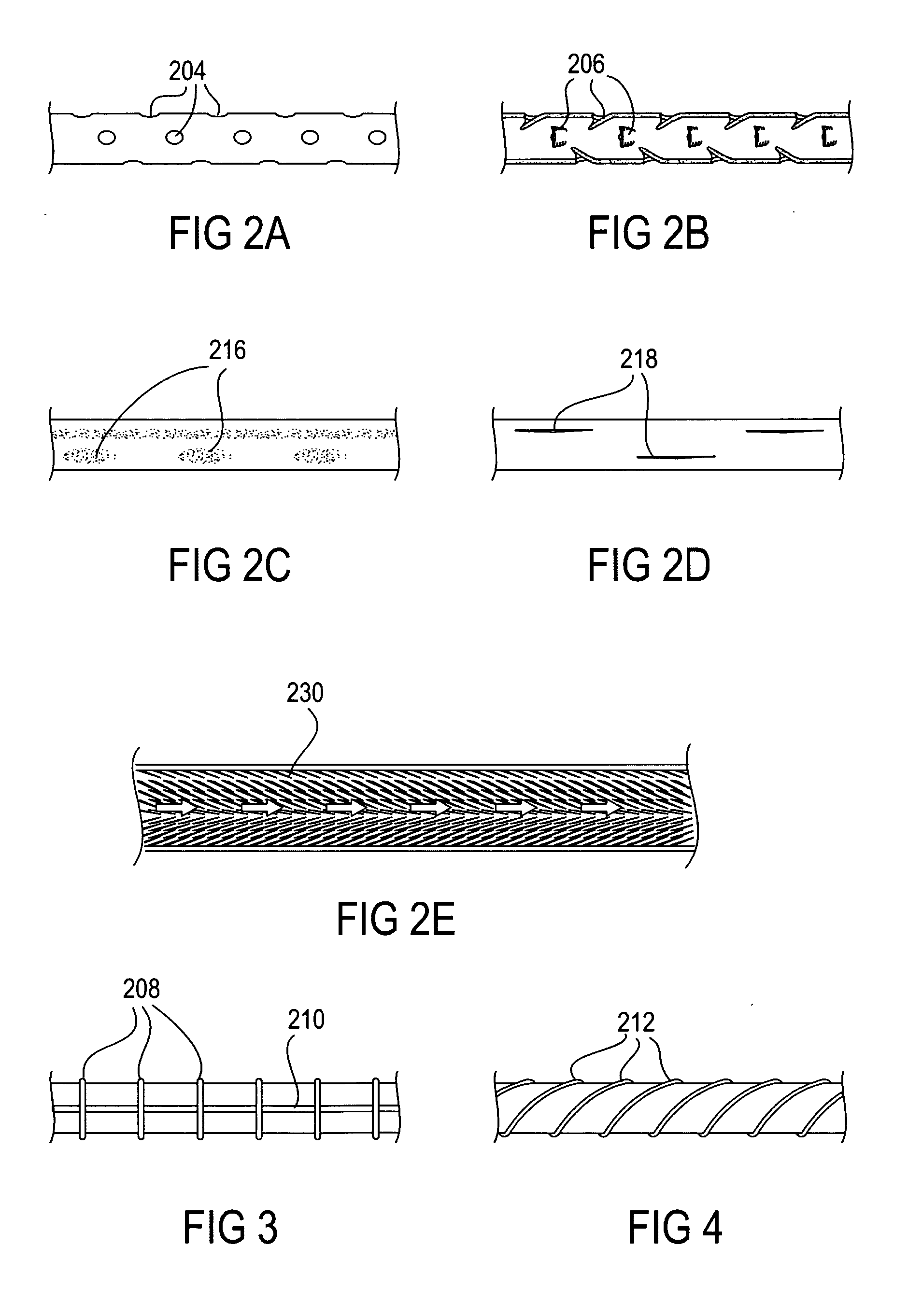
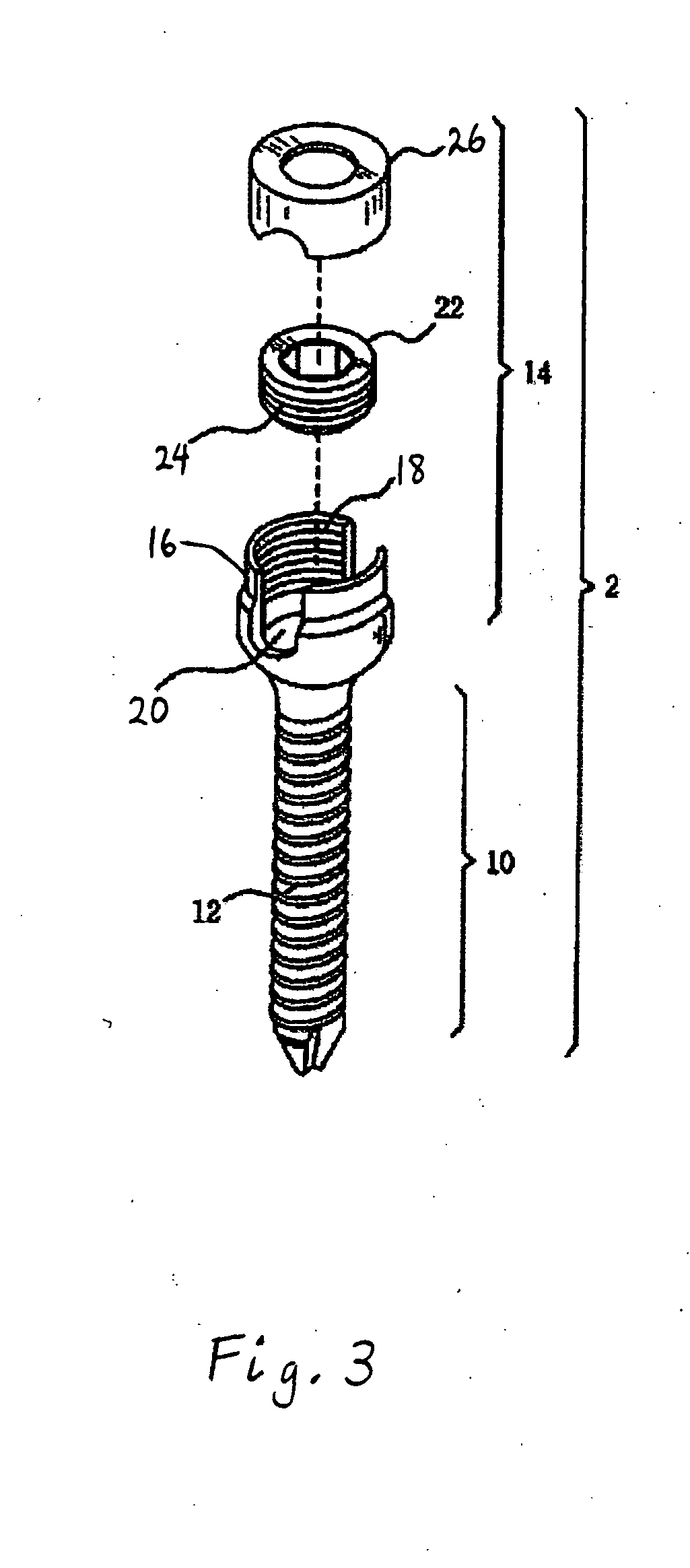
The present invention provides devices and methods for attachment of an implanted device, such as an artificial stoma device, a gastrointestinal sleeve device or an attachment cuff, within a patient's digestive tract for treatment of obesity. Special surgical fasteners provide a lasting and durable attachment to the gastrointestinal tissue without causing excessive pressure that could result in tissue erosion and detachment of the implanted device. Fastener delivery devices that facilitate peroral placement and deployment of fasteners and secondary devices are also provided. Also described are implantable devices and attachment means that avoid causing excessive pressure within the tissue by having compliance that is compatible with the gastrointestinal tissues where it is attached.
Owner:VALENTX
Medical devices and applications of polyhydroxyalkanoate polymers
InactiveUS6838493B2High porosityReduce probabilitySuture equipmentsOrganic active ingredientsTissue repairBiocompatibility Testing
Devices formed of or including biocompatible polyhydroxyalkanoates are provided with controlled degradation rates, preferably less than one year under physiological conditions. Preferred devices include sutures, suture fasteners, meniscus repair devices, rivets, tacks, staples, screws (including interference screws), bone plates and bone plating systems, surgical mesh, repair patches, slings, cardiovascular patches, orthopedic pins (including bone filling augmentation material), adhesion barriers, stents, guided tissue repair / regeneration devices, articular cartilage repair devices, nerve guides, tendon repair devices, atrial septal defect repair devices, pericardial patches, bulking and filling agents, vein valves, bone marrow scaffolds, meniscus regeneration devices, ligament and tendon grafts, ocular cell implants, spinal fusion cages, skin substitutes, dural substitutes, bone graft substitutes, bone dowels, wound dressings, and hemostats. The polyhydroxyalkanoates can contain additives, be formed of mixtures of monomers or include pendant groups or modifications in their backbones, or can be chemically modified, all to alter the degradation rates. The polyhydroxyalkanoate compositions also provide favorable mechanical properties, biocompatibility, and degradation times within desirable time frames under physiological conditions.
Owner:TEPHA INC
Devices and methods for treating morbid obesity
The present invention provides devices and methods for attachment of an implanted device, such as an artificial stoma device, a gastrointestinal sleeve device or an attachment cuff, within a patient's digestive tract for treatment of obesity. Special surgical fasteners provide a lasting and durable attachment to the gastrointestinal tissue without causing excessive pressure that could result in tissue erosion and detachment of the implanted device. Fastener delivery devices that facilitate peroral placement and deployment of fasteners and secondary devices are also provided. Also described are implantable devices and attachment means that avoid causing excessive pressure within the tissue by having compliance that is compatible with the gastrointestinal tissues where it is attached.
Owner:VALENTX
Sterile drape interface for robotic surgical instrument
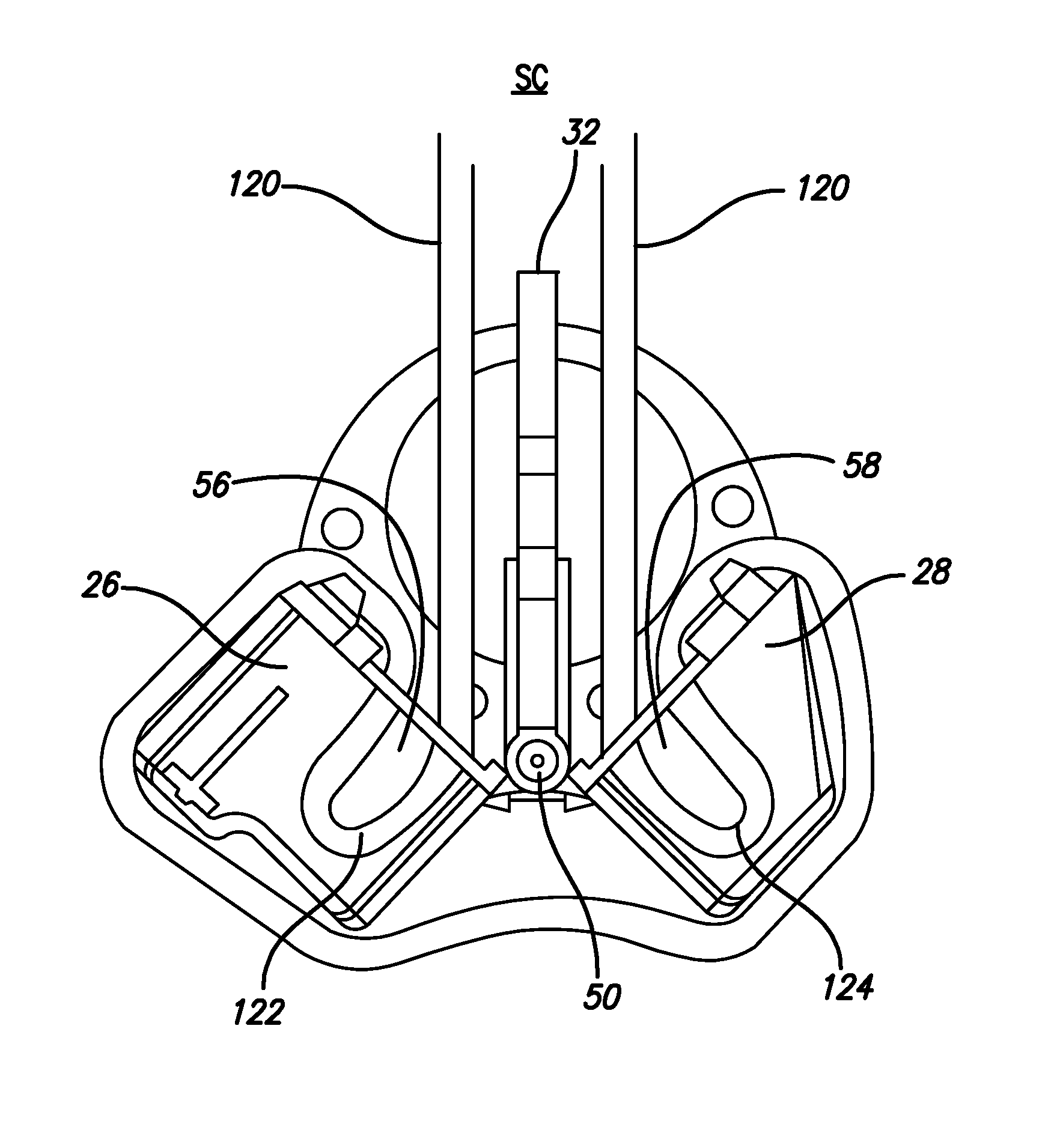
A robotic surgical system includes a sterile surgical instrument, a robotic surgical manipulator, and a sterile drape covering at least a portion of the robotic surgical manipulator. The surgical instrument has a proximal interface and a distal end effector. The proximal interface includes a gimbal assembly with two intersecting rotational axes coupled to the distal end effector. The robotic surgical manipulator has a drive plate that bears against the gimbal assembly. The drive plate has two degrees of rotational freedom about a center of motion that is coincident with an intersection of the axes of the gimbal assembly. The sterile drape includes a sterile sheet covers at least a portion of the robotic surgical manipulator, a frame bonded to the sterile sheet, an instrument interface that covers the drive plate of the robotic surgical manipulator, and a diaphragm that connects the instrument interface to the frame.
Owner:INTUITIVE SURGICAL OPERATIONS INC
Bioabsorbable wound dressing
ActiveUS7041868B2Promote wound healingEasy to useAdhesive dressingsNon-surgical orthopedic devicesCell adhesionAdhesion process
A wound dressing includes a first layer located adjacent the wound and which comprises a material that is bioabsorbable, porous and adapted for serving as a scaffold for cell attachment and proliferation; and a second layer which is in contact with the first layer and which comprises an absorbent, gel forming material adapted for serving as a barrier to cell adhesion and penetration. A method of treating a wound with the dressing is also disclosed.
Owner:AVENT INC
Device for controlling an electric appliance used in the sterile area during medical operations
InactiveUS6755195B1Easy to operateInserting operationManual control with multiple controlled membersDiagnosticsControl signalEngineering
The invention is a device for application in the sterile area for controlling an electric instrument provided in the non-sterile area, with the sterile area being separated from the non-sterile area substantially by a covering. The invention comprises at least one sensor responsive to the magnetic field which is provided in the non-sterile area and a unit generating a magnetic field is provided in the sterile area. The at least one sensor responsive to the magnetic field and the unit generating a magnetic field are separated from each other at least by the covering. The unit generating the magnetic field is disposed relative to the at least one sensor responsive to a magnetic field so that a variation of the magnetic field in space and / or time, which is induced by the unit generating the magnetic field, is detectable by the at least one sensor responsive to the magnetic field and a sensor signal can be generated. A controller unit is provided that generates a control signal for controlling the electric instrument in response to the sensor signal.
Owner:NORBERT LEMKE +1
Subcutaneous self attaching injection port with integral moveable retention members
InactiveUS7862546B2Improve overall utilizationMedical devicesNon-surgical orthopedic devicesInjection portEngineering
A self attaching injection port has integral moveable fasteners which are moveable from a undeployed state to a deployed state engaging tissue. The fasteners may be disposed radially or tangentially, and rotated to pierce the fascia. The fasteners may be rigid or elastically deformable.
Owner:ETHICON ENDO SURGERY INC
Bone graft delivery device and method of use
InactiveUS7014640B2Easy to cleanEasy to disassembleBone implantJoint implantsParticulatesSubject matter
A dispensing device for granule bone graft of varying and / or irregular shape is characterized by a body defining a handle / hopper portion, a dispensing portion, and a feed system. The subject device permits reloading or refilling of bone graft at the time of use of the device. The bone graft dispensing device also accepts vials of bone graft. The vials are loaded onto and releasably retained by the dispensing device. In both forms, the feed system allows a controlled and / or variable rate of flow of bone graft during dispensing. The subject device may be made disposable as well as re-usable. The subject device is also modular in design allowing easy assembly / disassembly. The is subject bone graft dispenser is particularly suited for the dispensing of dry, particulate and / or granule bone graft. Particularly, the bone graft dispensing device is especially suited for the dispensing of particulate or granule bone graft having particulates or granules of various and / or irregular size, shape and combinations thereof.
Owner:DEPUY PROD INC
Indwelling fecal diverting device
ActiveUS8398669B2Improve securityEasy to installBalloon catheterAnti-incontinence devicesFecesSurgery
Disclosed is an indwelling fecal diverting device. The device comprises an elongate tube formed, at an upper end thereof, with a tubular body part; a pair of fixing balloons attached up and down to an outer surface of the tubular body part such that a clamping portion is defined between the fixing balloons; and a tube opening and closing balloon attached to an inner surface of the tubular body part. An injection passage is defined in the tube so that a remedial liquid can be injected through the injection passage to the outside of the tube to medically treat an anastomosed portion of an intestinal tract of a patient. The indwelling fecal diverting device is fitted into the intestinal tract of the patient, air is supplied into the fixing balloons to inflate them, and the intestinal tract is clamped around the clamping portion using a clamping band.
Owner:YUSHIN MEDICAL +1
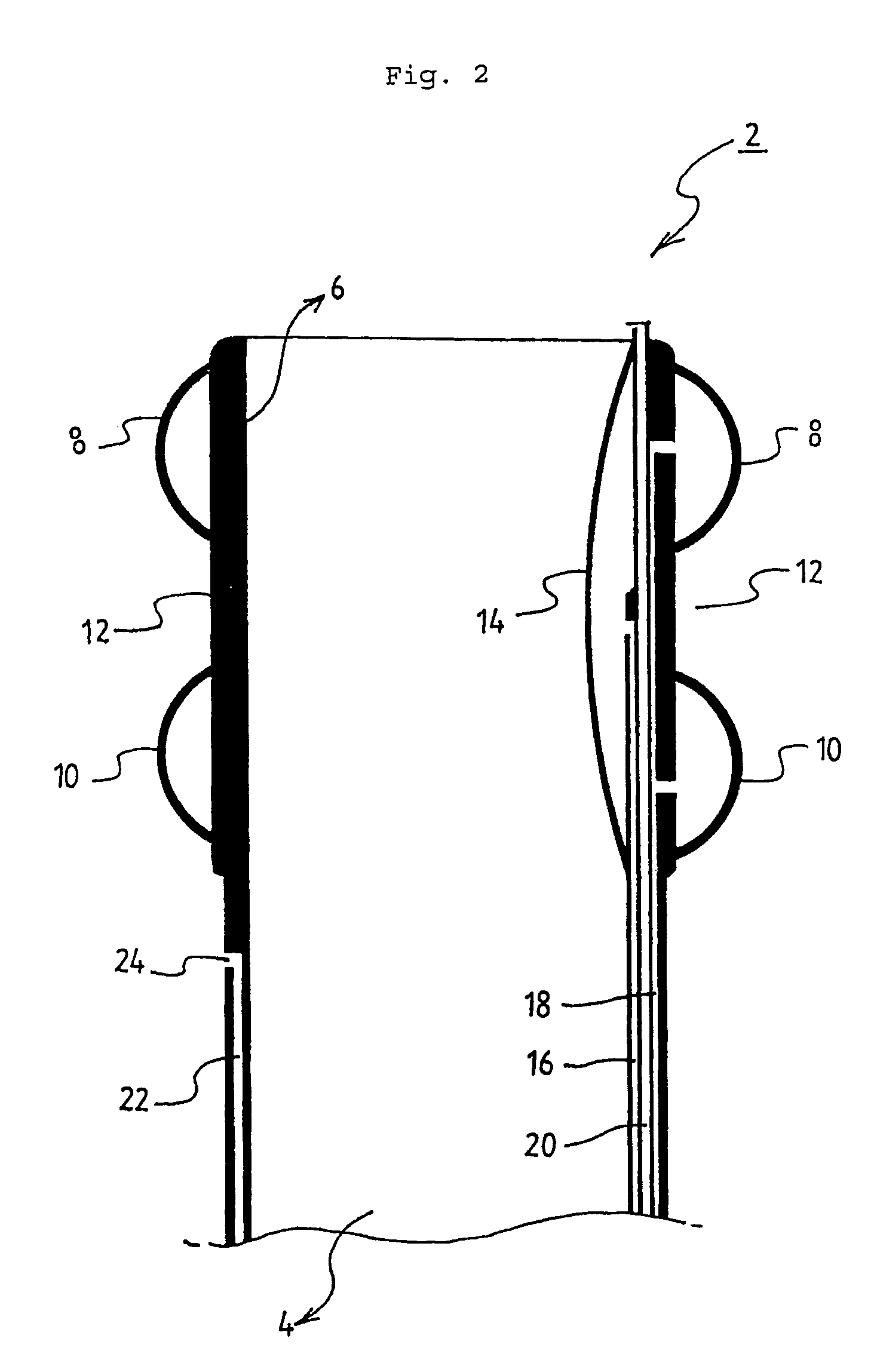
Implant systems and methods for treating obstructive sleep apnea
ActiveUS8561617B2Easy to anchorLarge cross-sectional widthDiagnosticsTracheaeButtressBiomedical engineering
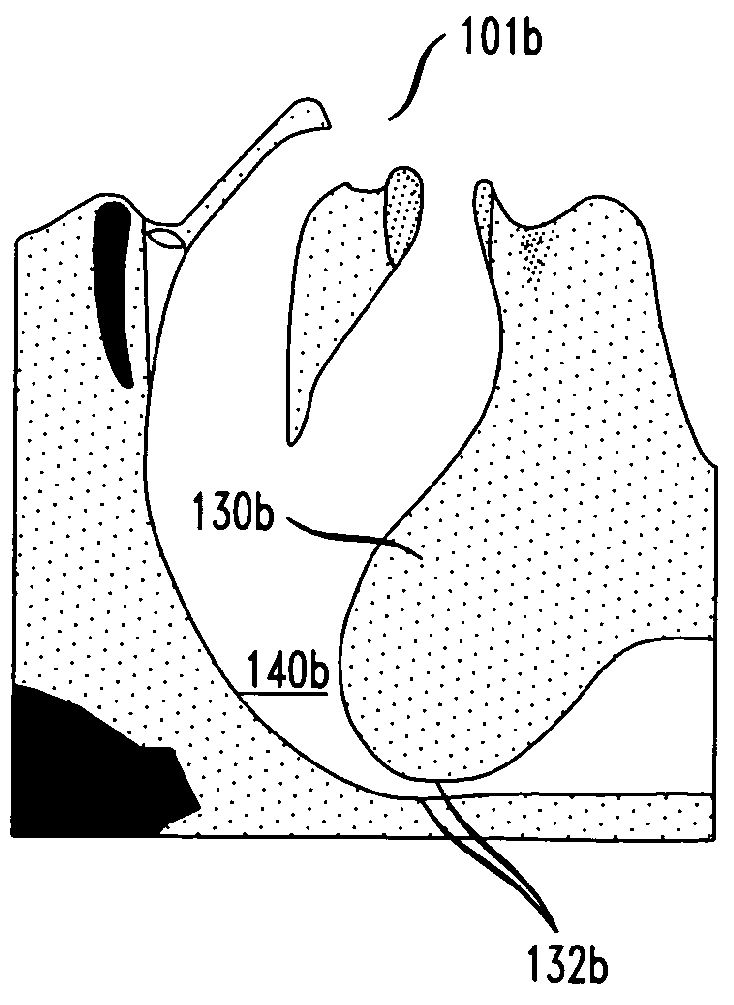
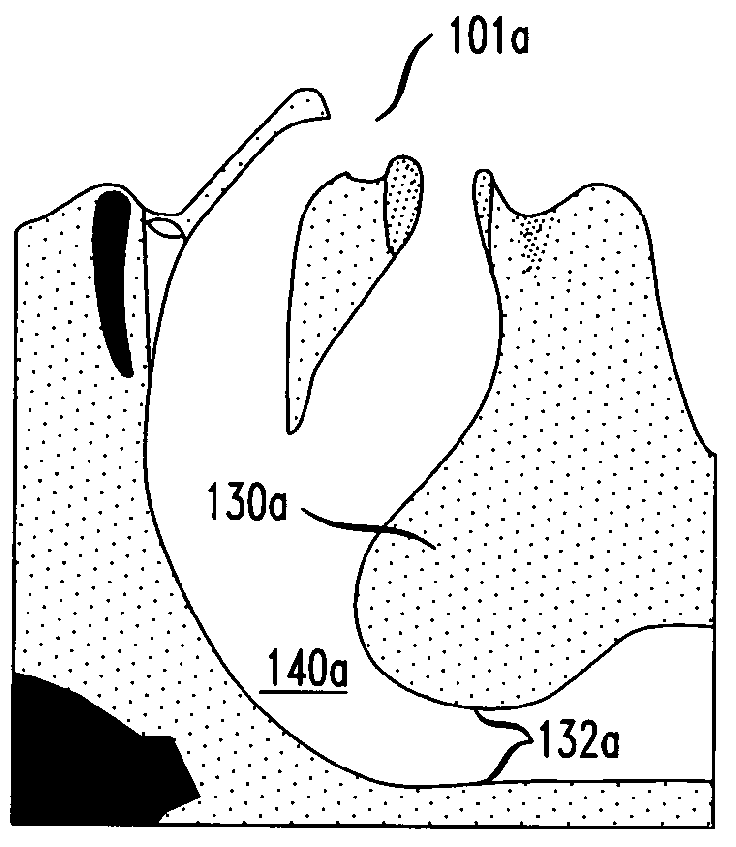
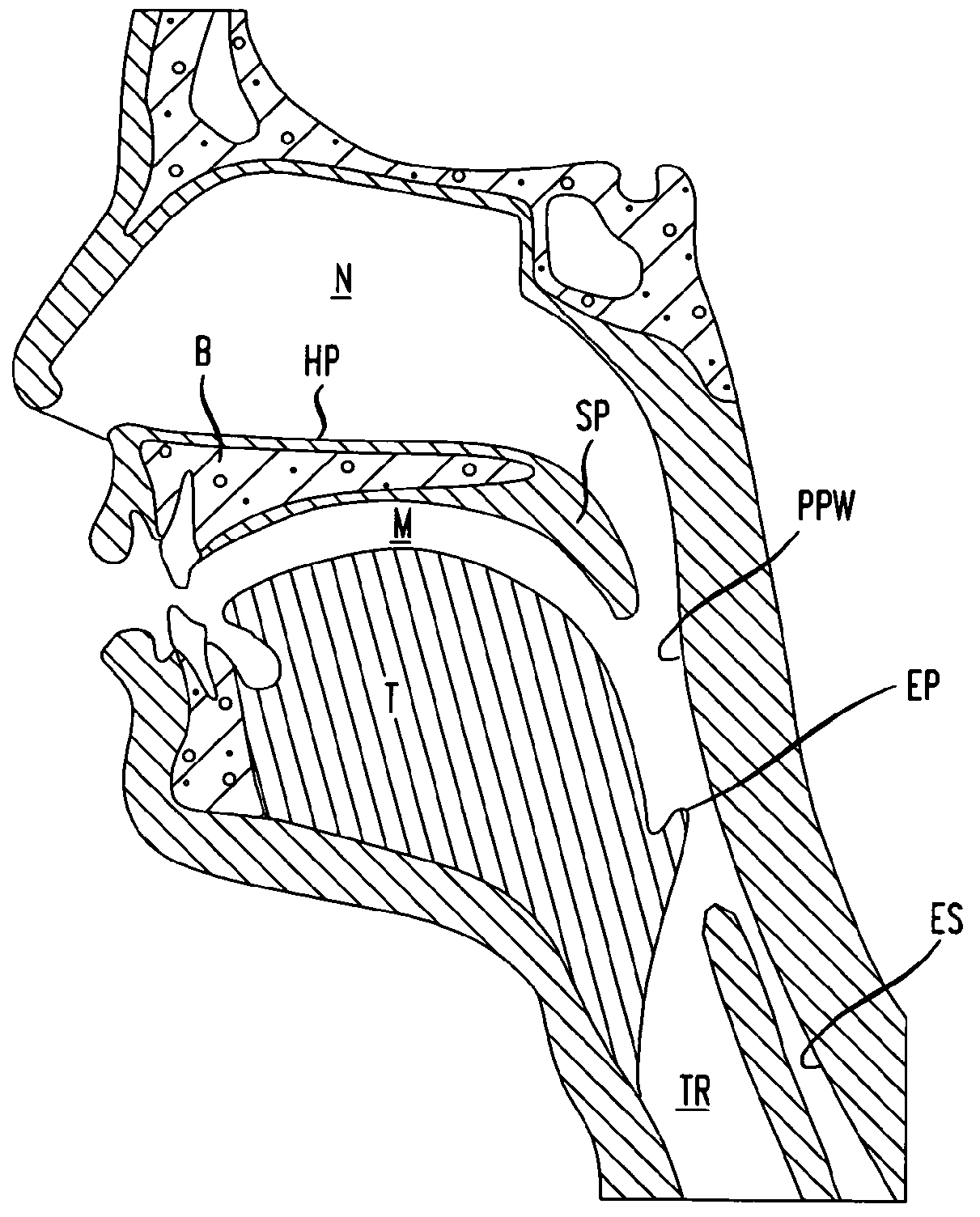
A method of treating obstructive sleep apnea includes providing an elongated element having a central buttress area and first and second arms extending from opposite ends of the central buttress area. The method includes implanting the central buttress area in a tongue so that a longitudinal axis of the central buttress area intersects an anterior-posterior axis of the tongue. The first and second arms are advanced through the tongue until the first and second arms engage inframandibular musculature. Tension is applied to the first and second arms for pulling the central buttress area toward the inframandibular musculature for moving a posterior surface of the tongue away from an opposing surface of a pharyngeal wall. The first and second arms are anchored to the inframandibular musculature for maintaining a space between the posterior surface of the tongue and the opposing surface of the pharyngeal wall.
Owner:ETHICON INC
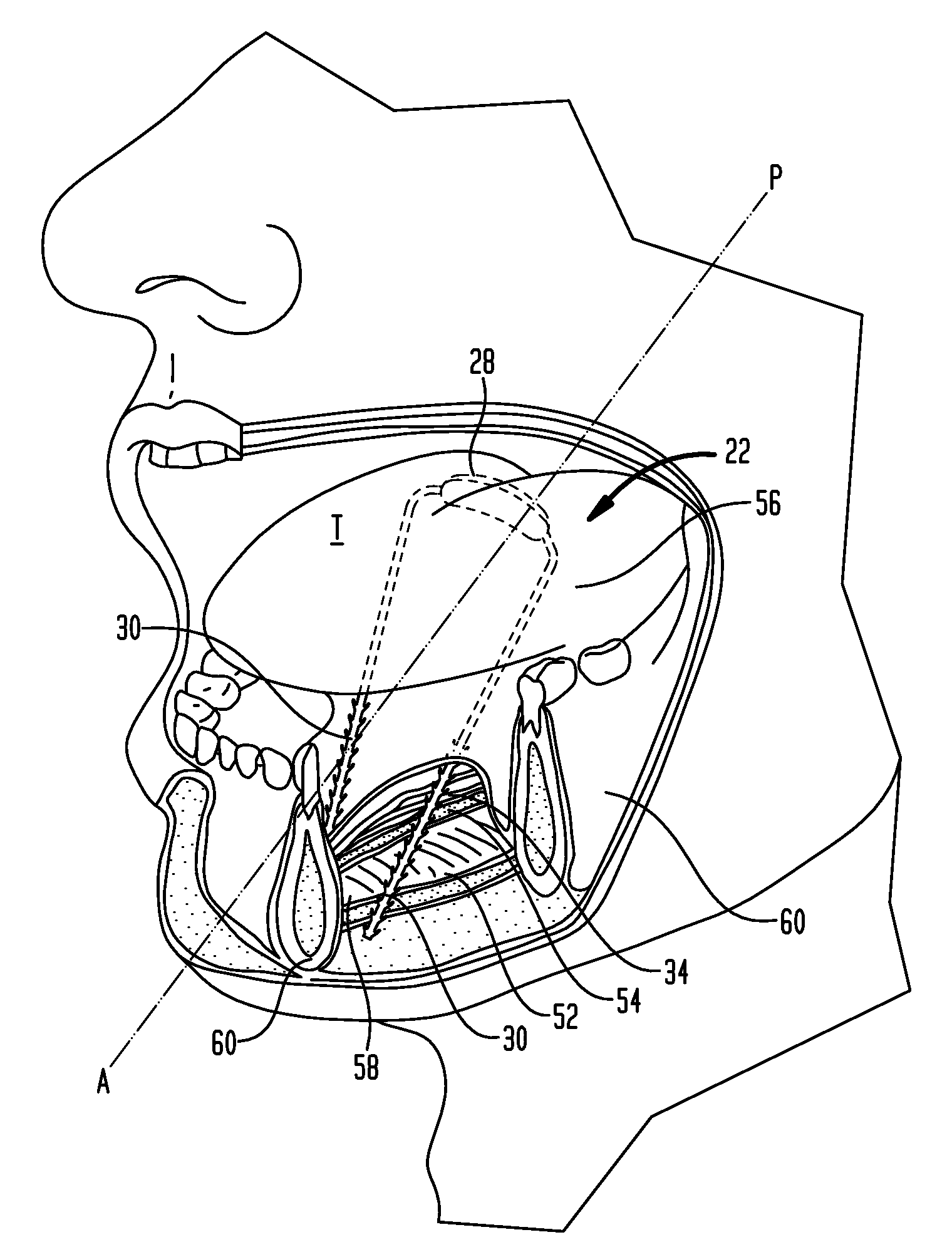
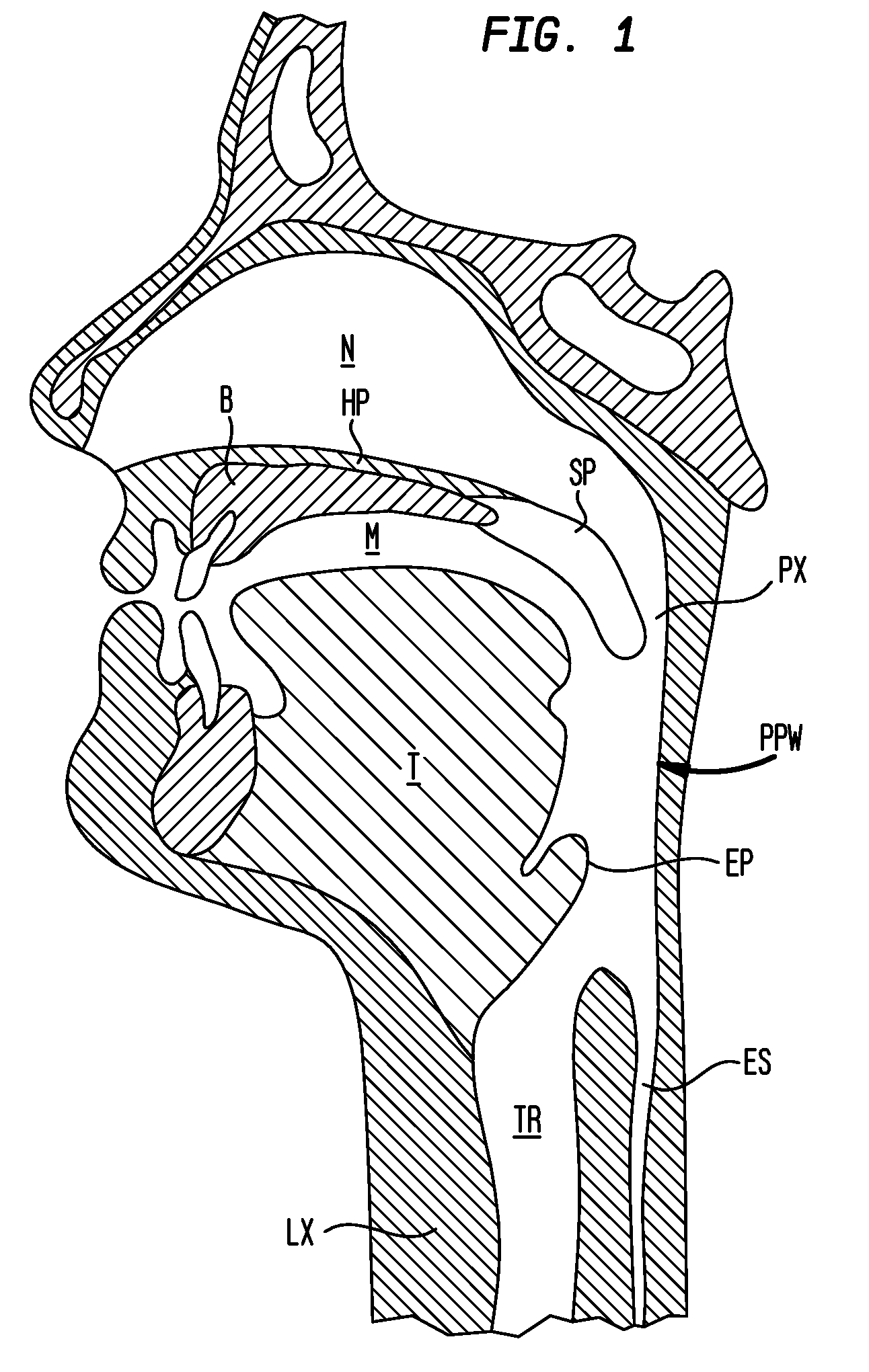
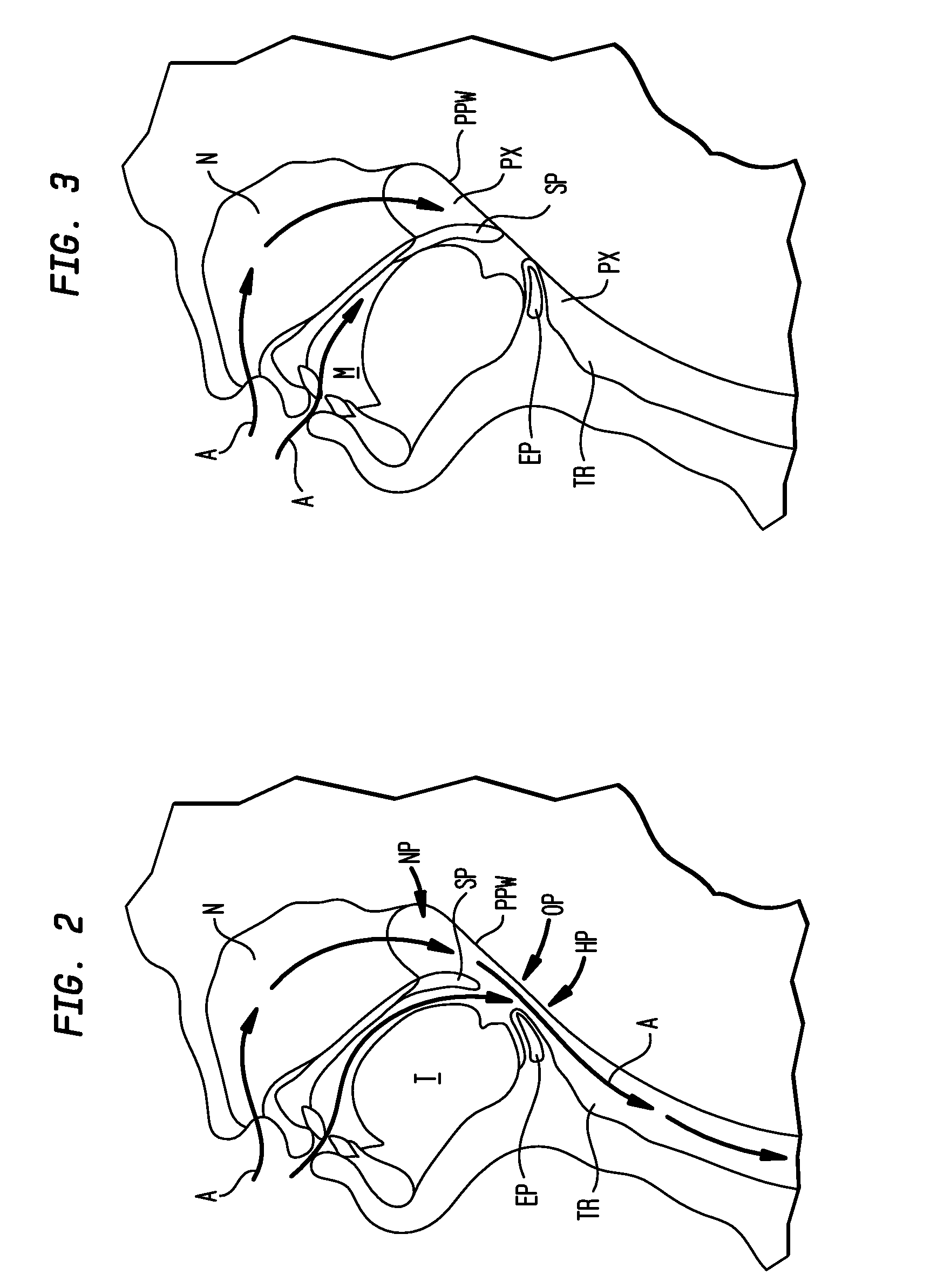
Methods and devices for treatment of obstructive sleep apnea
ActiveUS8413661B2Avoid blockingAlters the geometry of the airwayElectrotherapyDiagnosticsHand heldNeck of pancreas
Owner:ETHICON INC
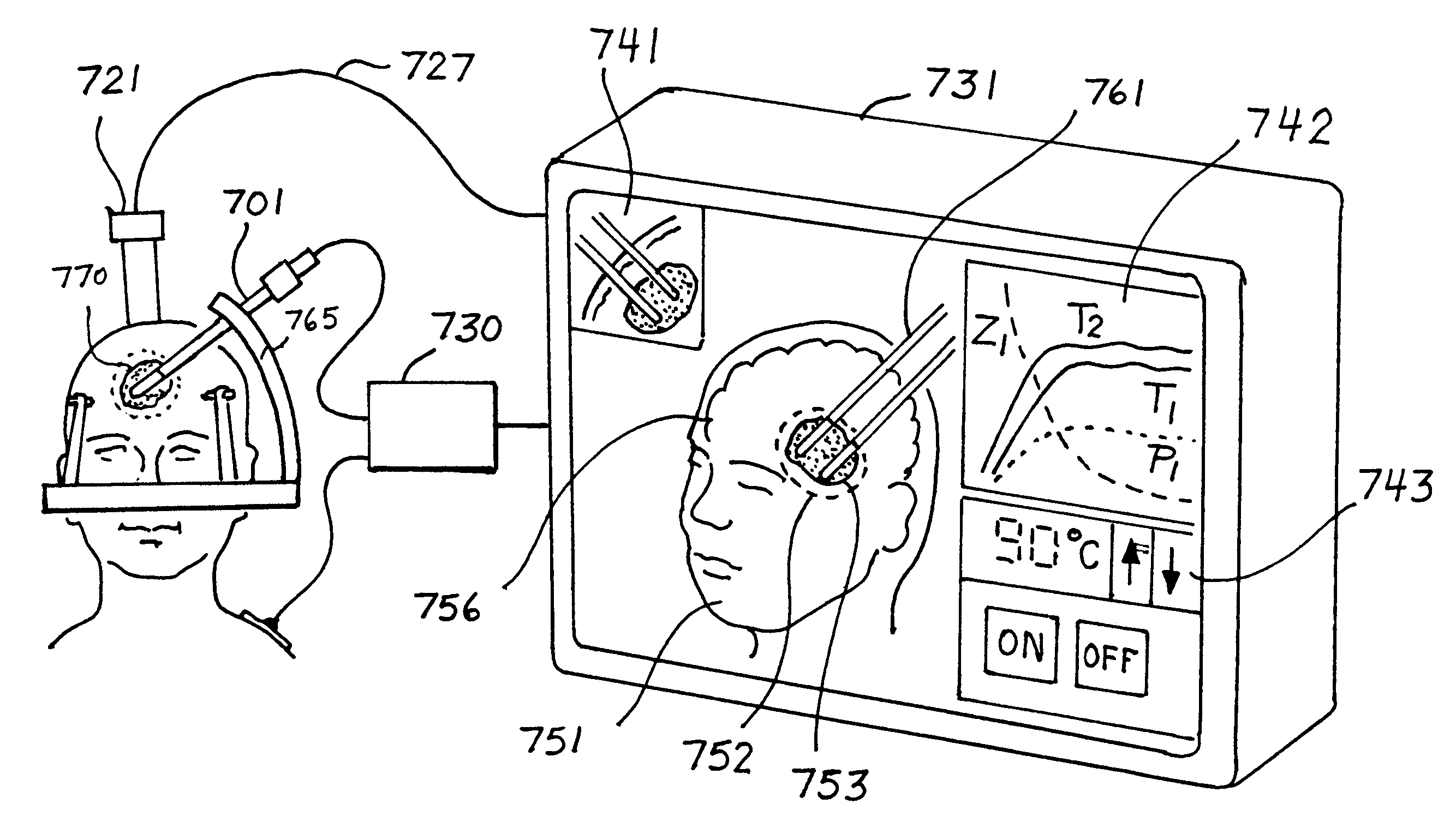
High frequency thermal ablation of cancerous tumors and functional targets with image data assistance
InactiveUS6241725B1Ultrasonic/sonic/infrasonic diagnosticsSurgical needlesAbnormal tissue growthTumour volume
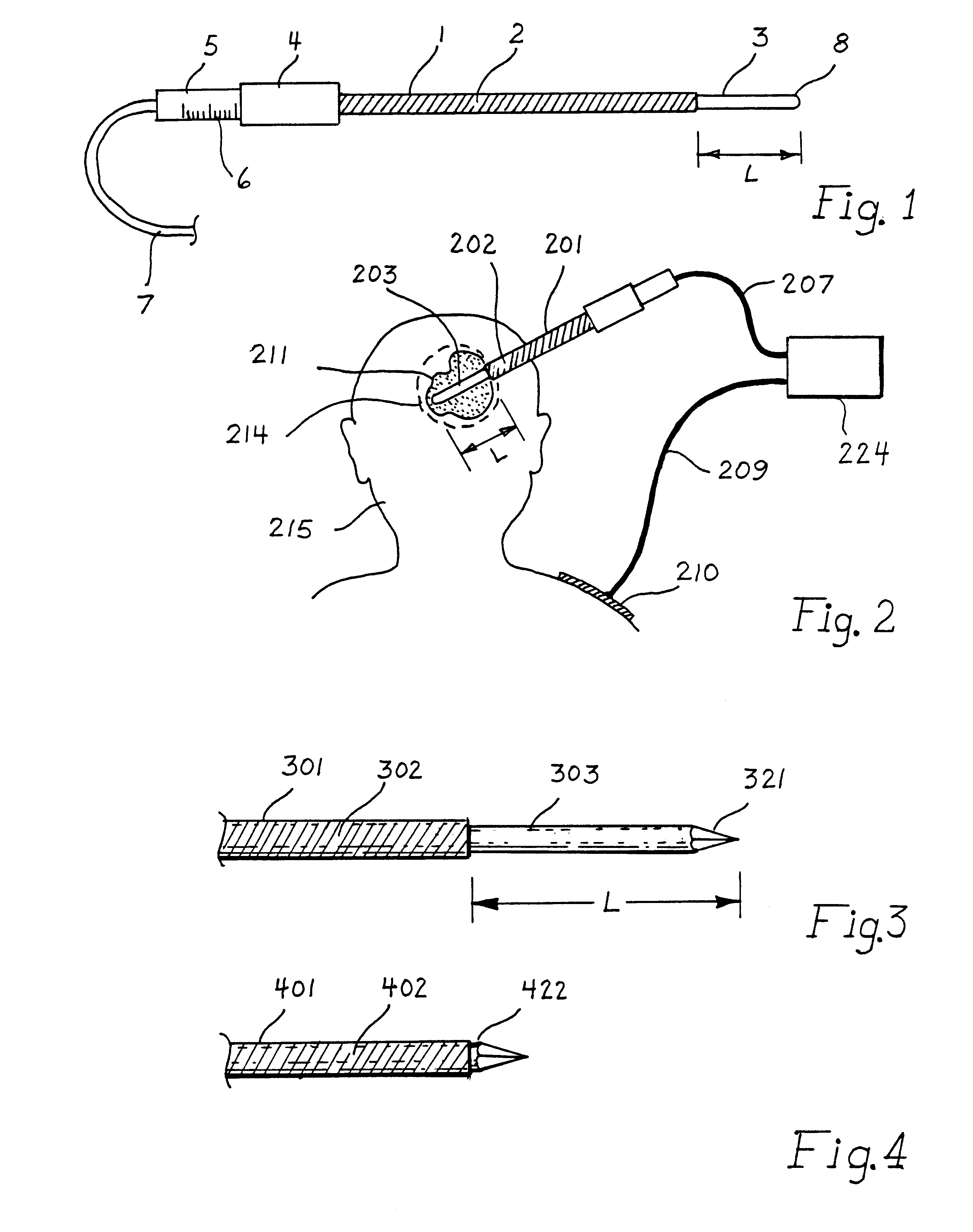
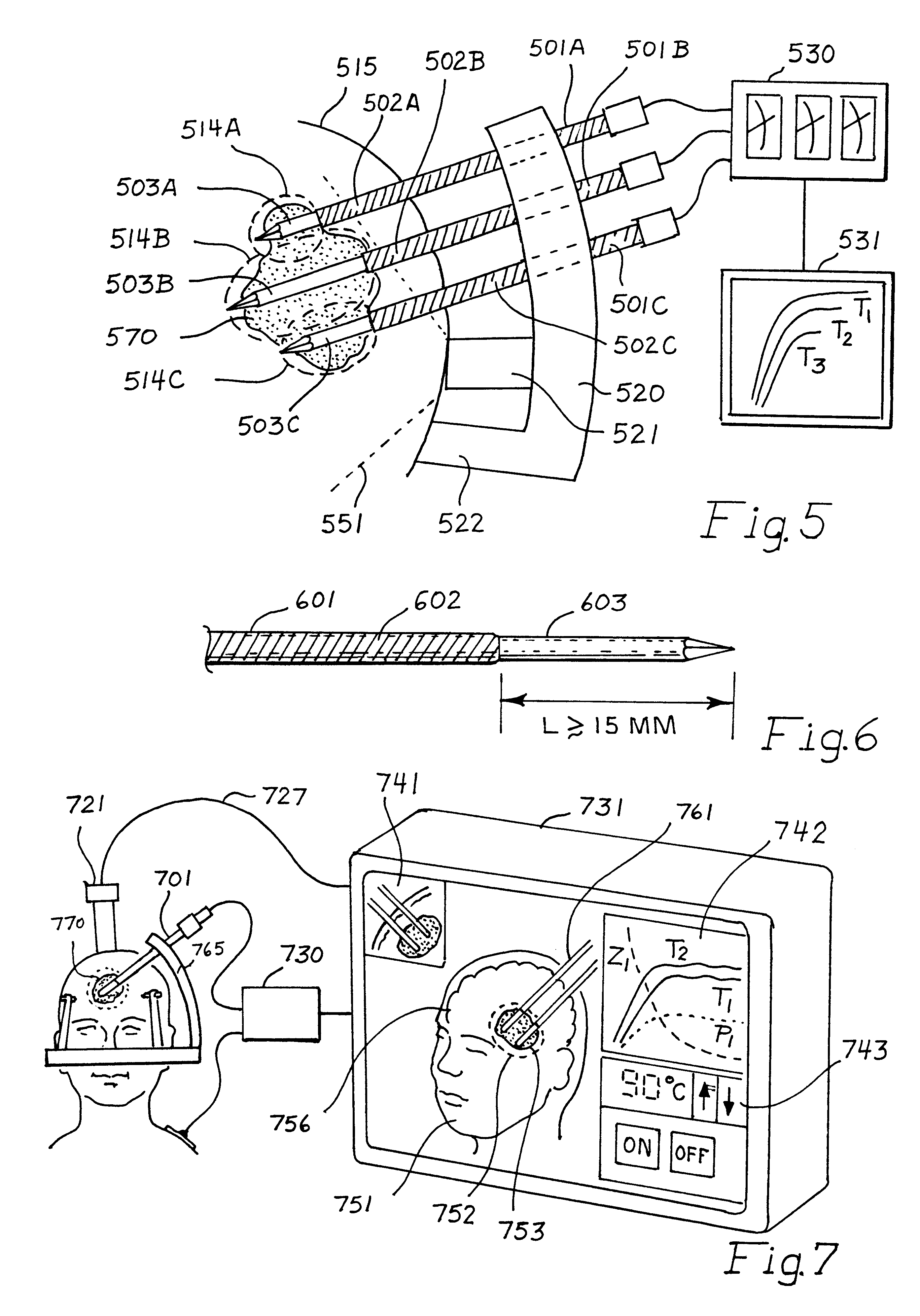
This invention relates to the destruction of pathological volumes or target structures such as cancerous tumors or aberrant functional target tissue volumes by direct thermal destruction. In the case of a tumor, the destruction is implemented in one embodiment of the invention by percutaneous insertion of one or more radiofrequency probes into the tumor and raising the temperature of the tumor volume by connection of these probes to a radiofrequency generator outside of the body so that the isotherm of tissue destruction enshrouds the tumor. The ablation isotherm may be predetermined and graded by proper choice of electrode geometry and radiofrequency (rf) power applied to the electrode with or without temperature monitoring of the ablation process. Preplanning of the rf electrode insertion can be done by imaging of the tumor by various imaging modalities and selecting the appropriate electrode tip size and temperature to satisfactorily destroy the tumor volume. Computation of the correct three-dimensional position of the electrode may be done as part of the method, and the planning and control of the process may be done using graphic displays of the imaging data and the rf ablation parameters. Specific electrode geometries with adjustable tip lengths are included in the invention to optimize the electrodes to the predetermined image tumor size.
Owner:COVIDIEN AG
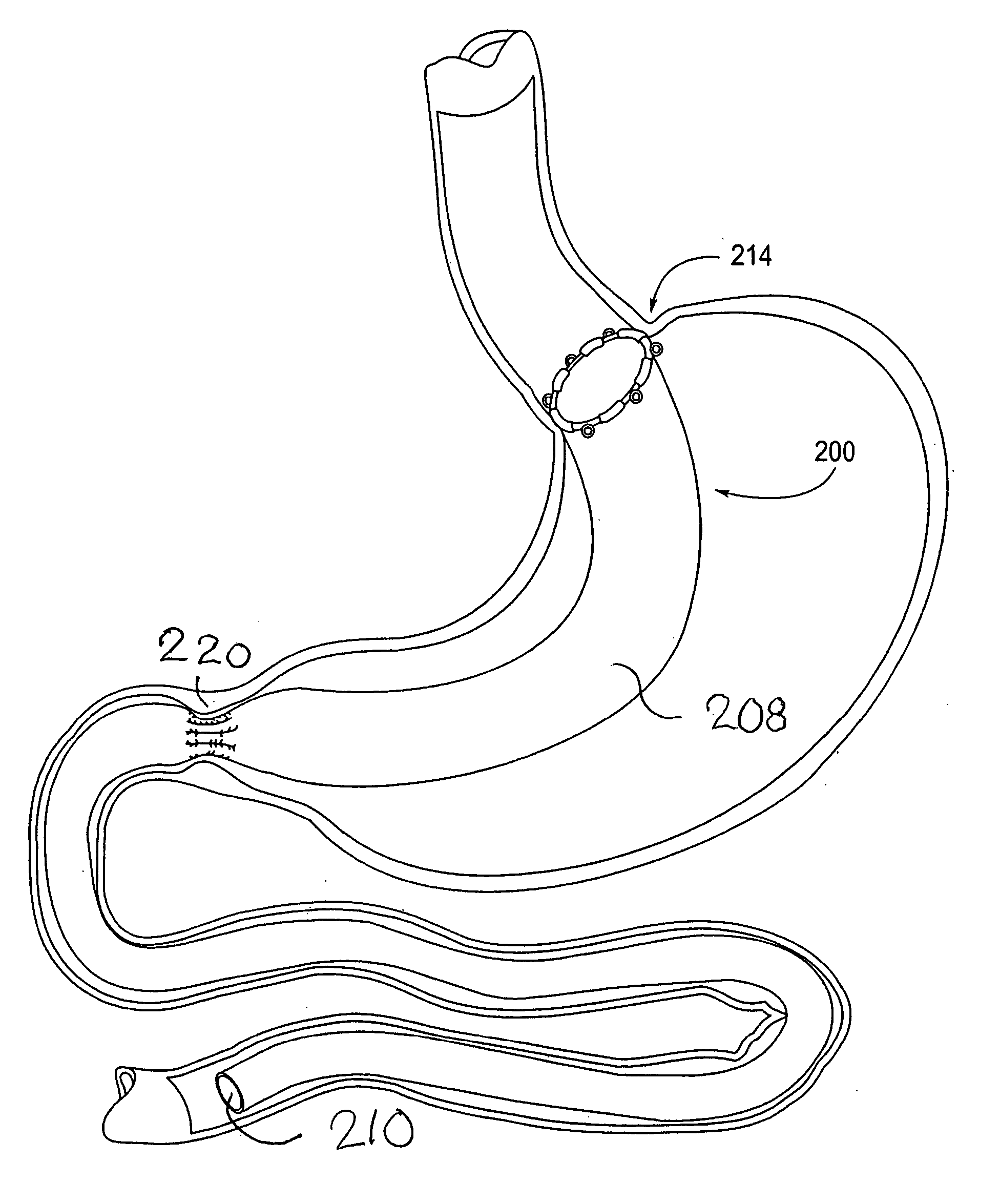
Methods and devices for measuring impedance in a gastric restriction system
Methods and devices are provided for gathering impedance data related to implantable restriction devices. In general, the methods and devices can enable patients, health care providers, and others to use gathered data as a feedback mechanism to non-invasively monitor efficacy of an implantable restriction device in a patient and to identify, modify, and / or prescribe a treatment plan for the patient considering the gathered data. Impedance data can be gathered and analyzed for tissue proximate to the restriction device, e.g., a fat pad between a gastric band and the patient's stomach. Electrodes in contact with the tissue can measure an impedance of the tissue, with the impedance between the electrodes changing as the tissue reduces in size (e.g., as fat cells shrink) and / or changes configuration.
Owner:ETHICON ENDO SURGERY INC
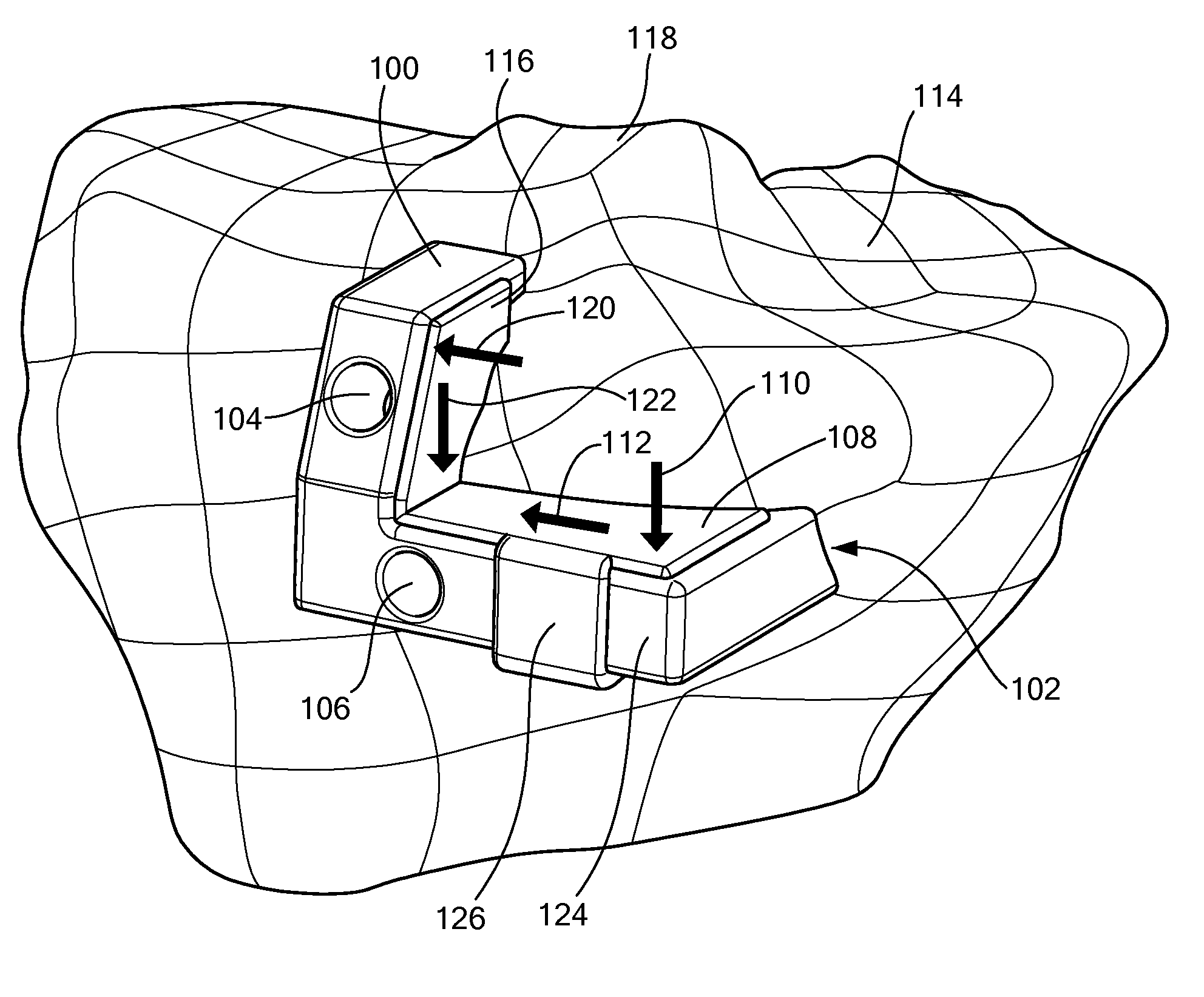
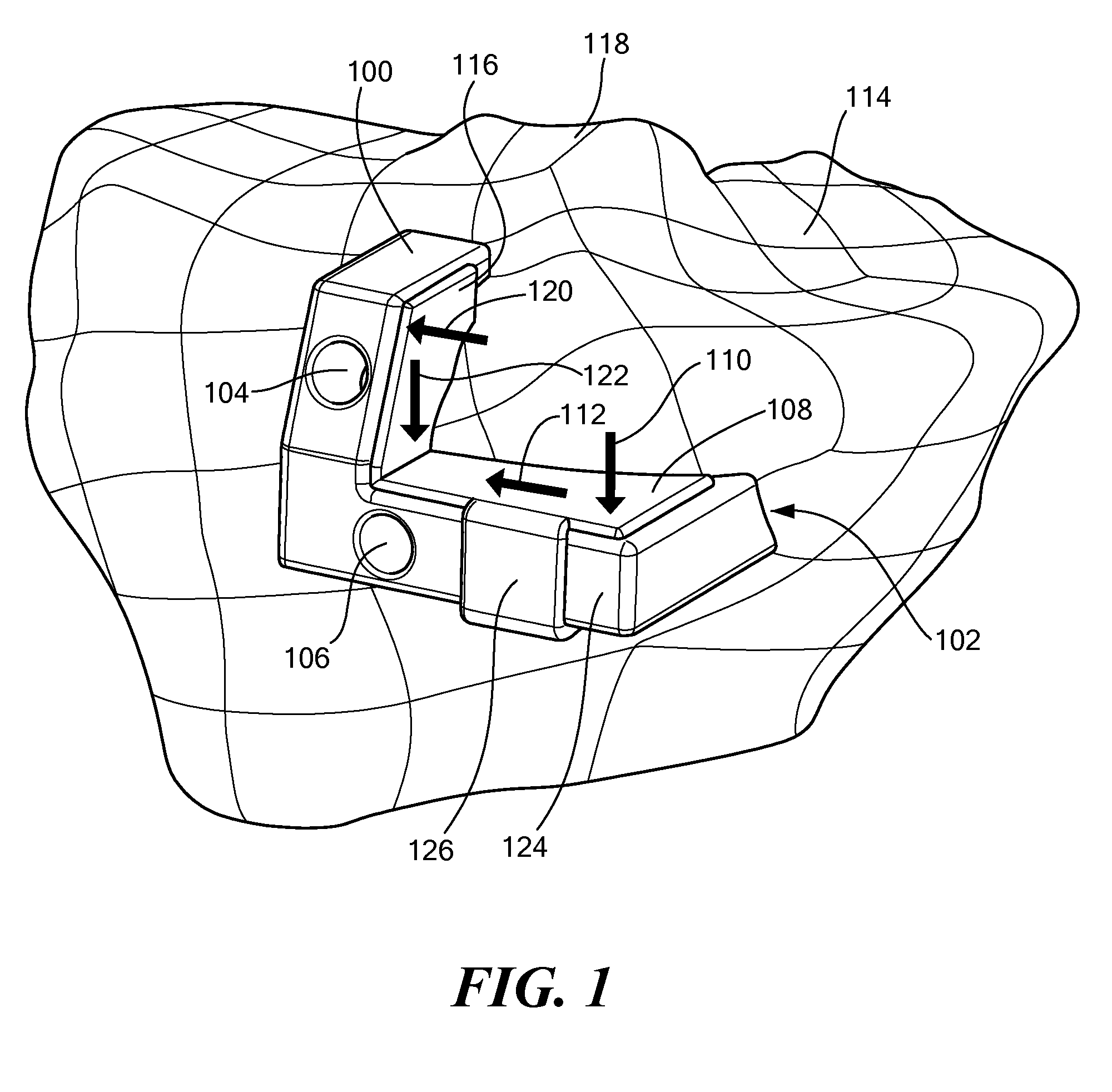
Surgical Tools for Arthroplasty
ActiveUS20080243127A1Accurate placementAdditive manufacturing apparatusSurgical navigation systemsTarsal JointKnee surface
A patellar 3-D guidance tool includes a template. The template includes at least one contact surface for engaging a surface of the patella. The at least one contact surface substantially conforms with the surface associated with the patellar. At least one guide aperture directs movement of a surgical instrument, wherein the shape and / or position of the guide aperture is based, at least in part, on three or more anatomic reference points associated with the patellar.
Owner:CONFORMIS
Patient selectable joint arthroplasty devices and surgical tools facilitating increased accuracy, speed and simplicity in performing total and partial joint arthroplasty
Disclosed herein are methods, compositions and tools for repairing articular surfaces repair materials and for repairing an articular surface. The articular surface repairs are customizable or highly selectable by patient and geared toward providing optimal fit and function. The surgical tools are designed to be customizable or highly selectable by patient to increase the speed, accuracy and simplicity of performing total or partial arthroplasty.
Owner:CONFORMIS
Device and method for endoluminal therapy
InactiveUS7666195B2Minimizing dilationMinimize timeSuture equipmentsSurgical needlesOrgan wallOrgan of Corti
A device and method for selectively engaging or penetrating a layer of a luminal organ wall where the luminal organ wall has a plurality of layers including an outermost layer and an innermost layer adjacent to the lumen of the organ. The device and method select one of the plurality of layers of the organ wall other than the innermost layer and deploy from within the lumen of the organ a tissue device through the innermost layer to a specific depth to engage or penetrate the selected one of the plurality of layers. The device and method may be employed to create luminal pouches or restrictive outlets. In a stomach organ, the device and methods may be employed to treat obesity by forming a gastric pouch with or without a restrictive outlet.
Owner:KELLEHER BRIAN +1
Devices and methods for pyloric anchoring
ActiveUS20050055039A1Avoiding erosion and ulcerationSuture equipmentsElectrotherapyPylorusPatient characteristics
A device for performing one or more functions in a gastrointestinal tract of a patient includes an anchoring member and at least one actuator, sensor, or combination of both coupled with the anchoring device. The anchoring device is adapted to maintain at least part of the device within a pyloric portion of the patient's stomach and to intermittently engage, without directly attaching to, stomach tissue. Actuators perform any suitable function, such as transmitting energy to tissue, acting as a sleeve to reduce nutrient absorption, occupying space in the stomach, eluting a drug and / or the like. Sensors may be adapted to sense any suitable patient characteristic within the patient's gastrointestinal tract, such as pH, temperature, bile content, nutrient content, fats, sugars, alcohol, opiates, drugs, analytes, electrolytes and / or hemoglobin.
Owner:BARONOVA
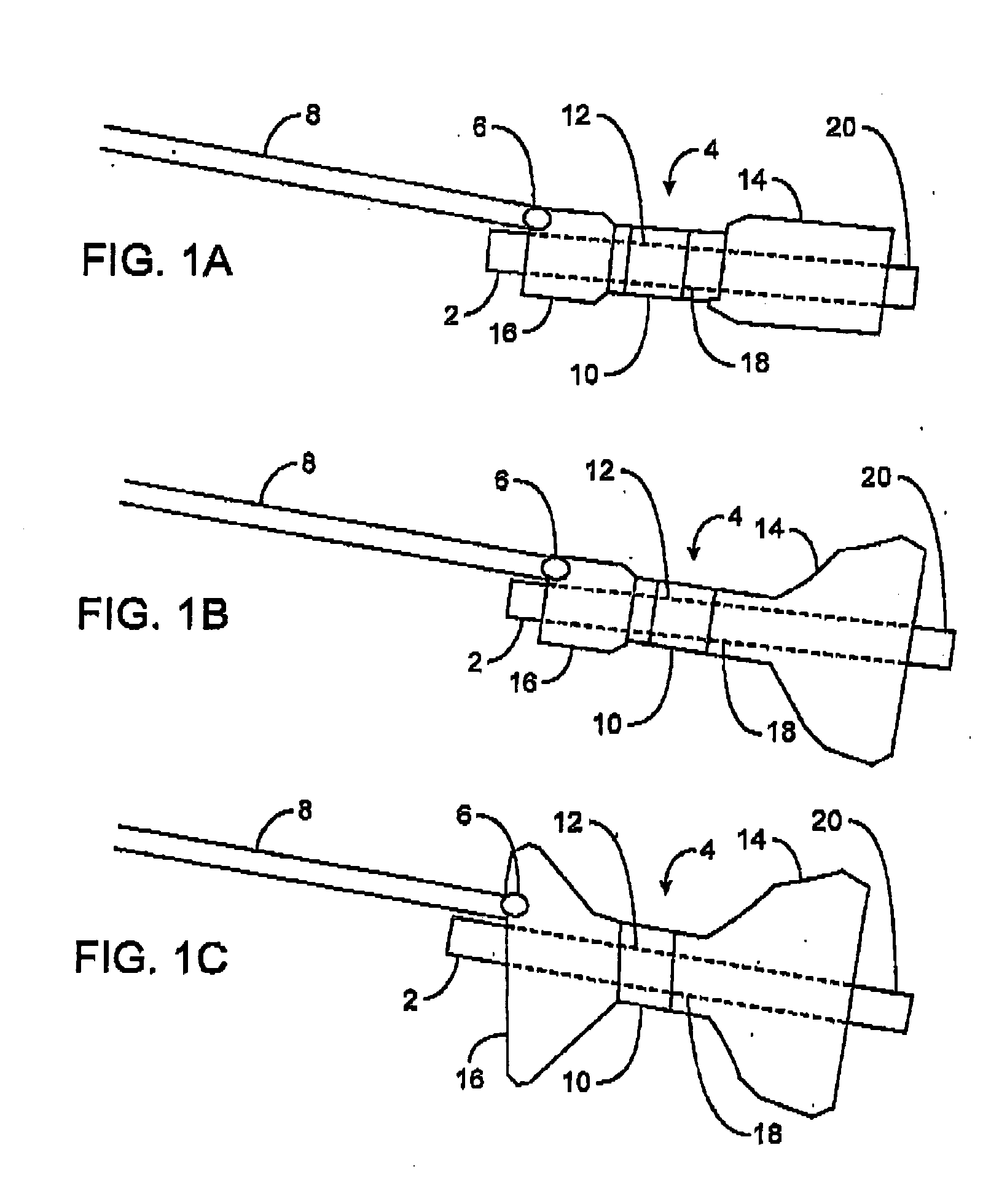
Devices and methods for placement of partitions within a hollow body organ
ActiveUS9028511B2Minimize and eliminate cross acquisitionFacilitate acquisitionNon-surgical orthopedic devicesObesity treatmentBody organsGastric bypass
Devices and methods for tissue acquisition and fixation, or gastroplasty, are described. Generally, the devices of the system may be advanced in a minimally invasive manner within a patient's body, e.g., transorally, endoscopically, percutaneously, etc., to create one or several divisions or plications within the hollow body organ. Such divisions or plications can form restrictive barriers within a organ, or can be placed to form a pouch, or gastric lumen, smaller than the remaining stomach volume to essentially act as the active stomach such as the pouch resulting from a surgical Roux-En-Y gastric bypass procedure. Moreover, the system is configured such that once acquisition of the tissue by the gastroplasty device is accomplished, any manipulation of the acquired tissue is unnecessary as the device is able to automatically configure the acquired tissue into a desired configuration.
Owner:ETHICON ENDO SURGERY INC
Gastrointestinal sleeve device and methods for treatment of morbid obesity
InactiveUS20050049718A1Effectively reducing stomach volumeStimulating intestinal responseMedical devicesTubular organ implantsIntestinal structureMorbid obesity
Apparatus and methods are described for treatment of morbid obesity using minimally invasive techniques. The apparatus includes a system of components that may be used separately or in combination for effectively reducing stomach volume, bypassing a portion of the stomach and / or small intestines, reducing nutrient absorption in the stomach and / or small intestines and / or depositing minimally or undigested food farther than normal into the intestines, thereby stimulating intestinal responses. The components described include a gastric sleeve device, an intestinal sleeve device, and a combined gastrointestinal sleeve device.
Owner:VALENTX
Total joint arthroplasty system
ActiveUS20090131941A1Precise alignmentImprove visualizationMedical simulationProgramme controlJoint arthroplastyTotal hip arthroplasty
A method and system for performing a total joint arthroplasty procedure on a patient's damaged bone region. A CT image or other suitable image is formed of the damaged bone surfaces, and location coordinate values (xn,yn,zn) are determined for a selected sequence of bone surface locations using the CT image data. A mathematical model z=f(x,y) of a surface that accurately matches the bone surface coordinates at the selected bone spice locations, or matches surface normal vector components at selected bone surface locations, is determined. The model provides a production file from which a cutting jig and an implant device (optional), each patient-specific and having controllable alignment, are fabricated for the damaged bone by automated processing. At this point, the patient is cut open (once), the cutting jig and a cutting instrument are used to remove a selected portion of the bone and to provide an exposed planar surface, the implant device is optionally secured to and aligned with the remainder of the bone, and the patient's incision is promptly repaired.
Owner:HOWMEDICA OSTEONICS CORP
Surgical Cutting Guide
ActiveUS20080275452A1Prevent deviationComputer-aided planning/modellingNon-surgical orthopedic devicesEngineeringSurgical department
The present invention is directed to a surgical cutting guide for guiding a surgical instrument along a cutting path located on a biological tissue. The surgical guide includes a contact surface that conforms to a surface associated with the tissue and at least one guide for restricting movement of a surgical instrument in a first direction and for allowing the movement of the surgical instrument in a second direction along a cutting path across the surface of the tissue. The guide further contains a stop for restricting movement of the surgical instrument in the second direction along the cutting path. The stop is based at least, in part, on patient specific information.
Owner:CONFORMIS
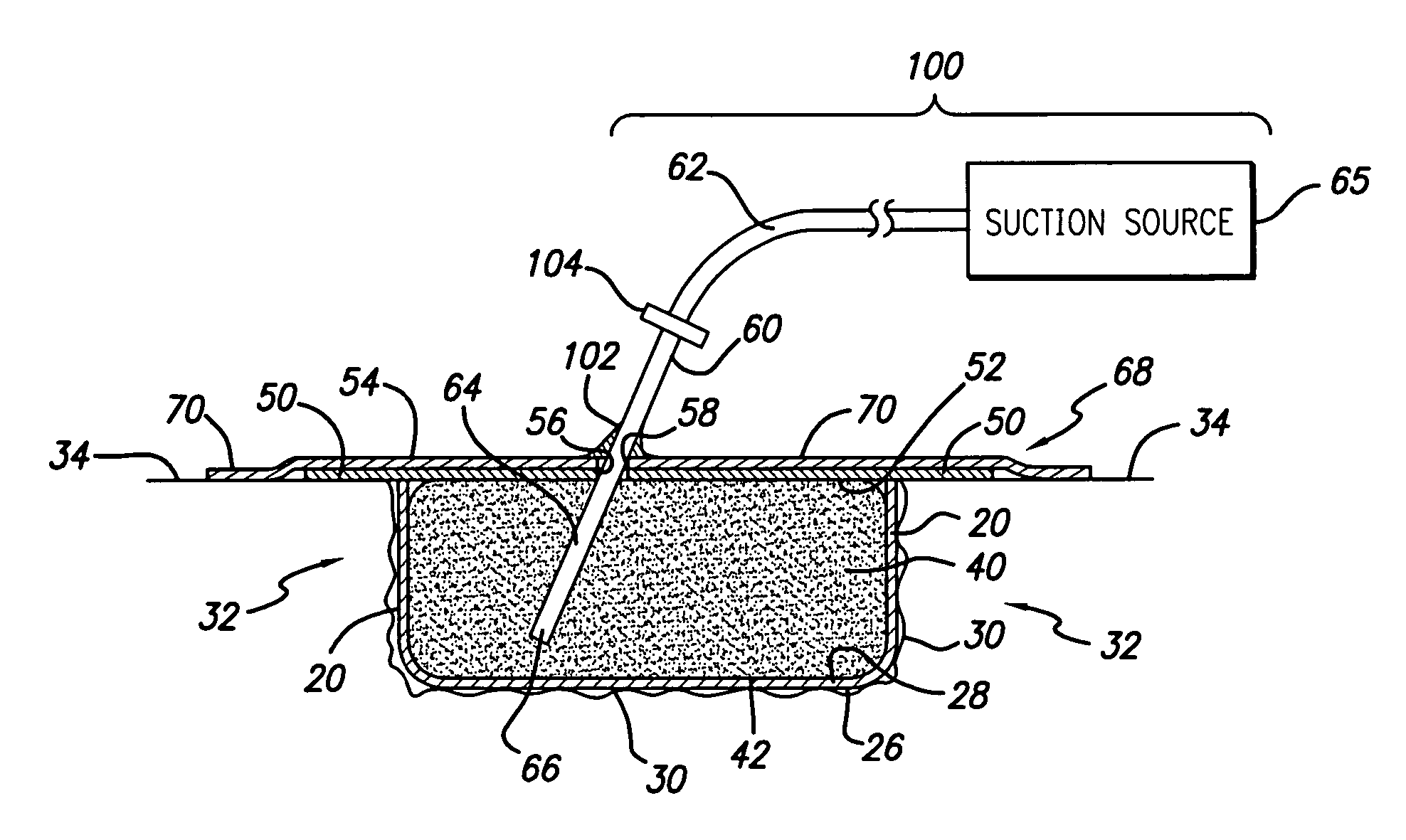
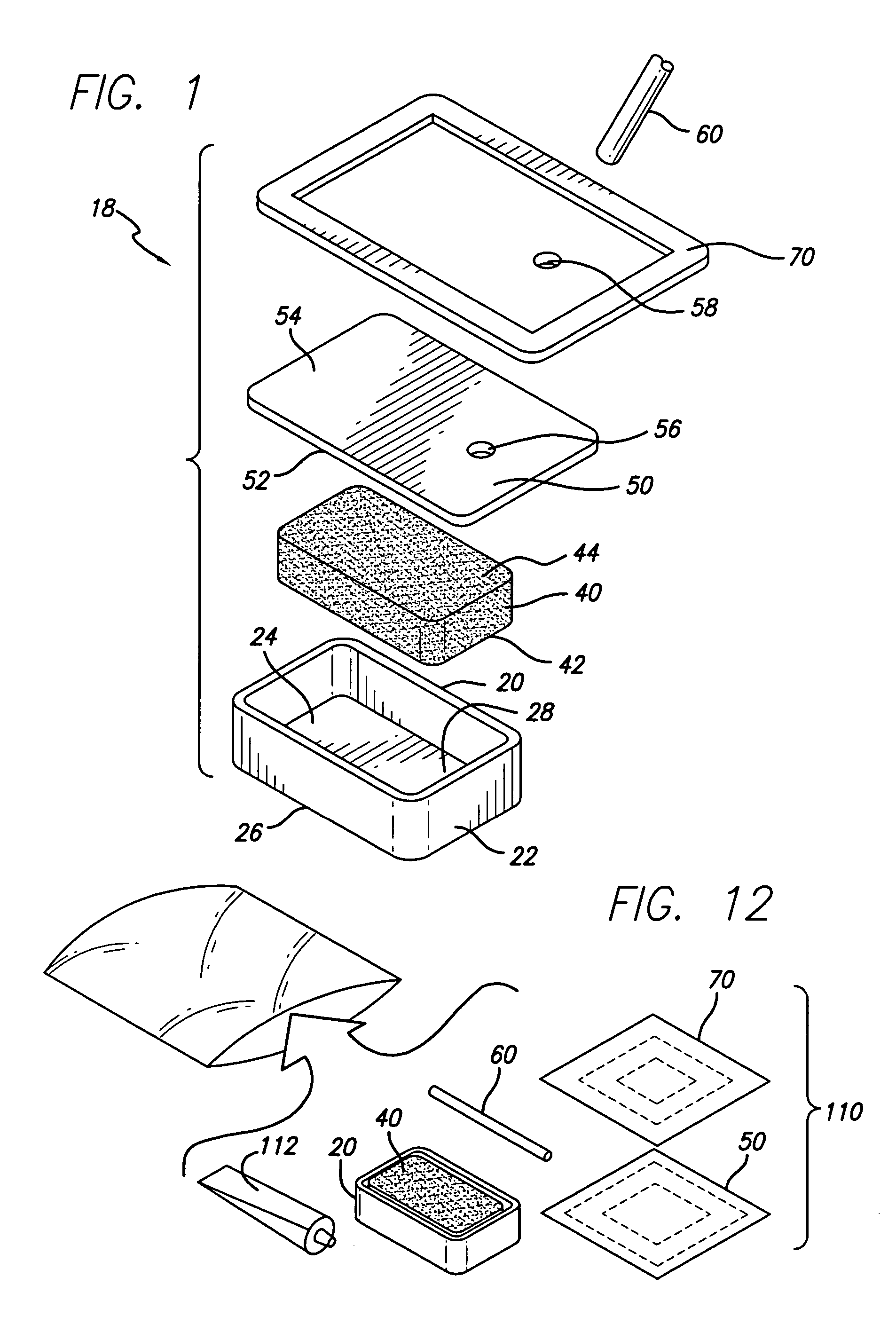
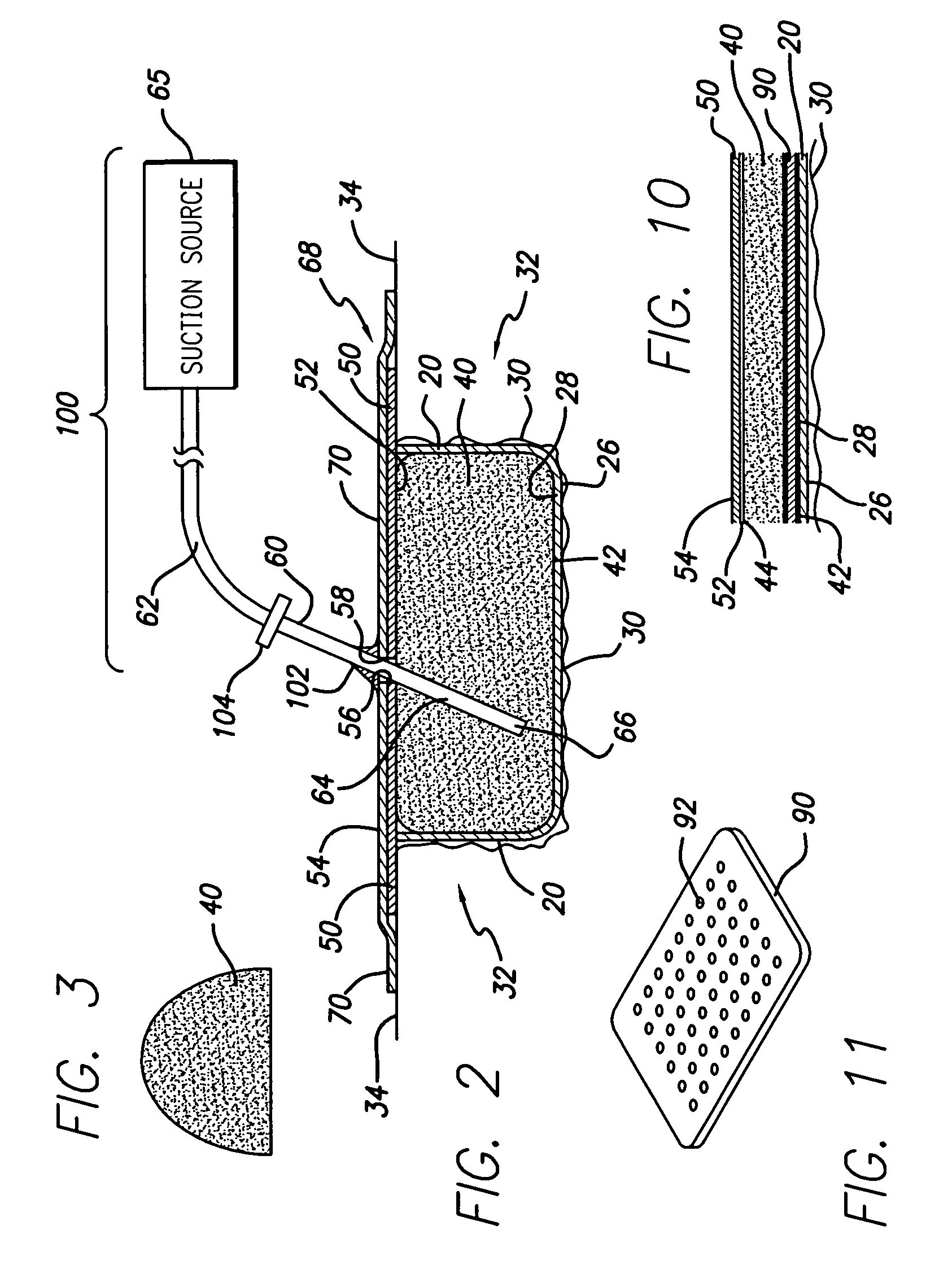
Laminar construction negative pressure wound dressing including bioabsorbable material
InactiveUS20070027414A1Safer and efficient and less painfulWound drainsAdhesive dressingsWound dressingDressing change
A laminated negative pressure wound dressing system and method is described. The wound dressing is disposed in the wound in layers including at least one bioabsorbable layer that contacts the wound bed, a bioabsorbable fluid communicating layer, an atmospheric barrier layer, and a tube for applying a negative pressure to the wound bed. Ingrowth of granulation tissue into the bioabsorbable wound bed layer does not need to be inhibited as the bioabsorbable material need not be removed during dressing changes. A kit containing the components of the wound dressing system is also disclosed as well as a method for applying the dressing.
Owner:INTEGRA LIFESCI
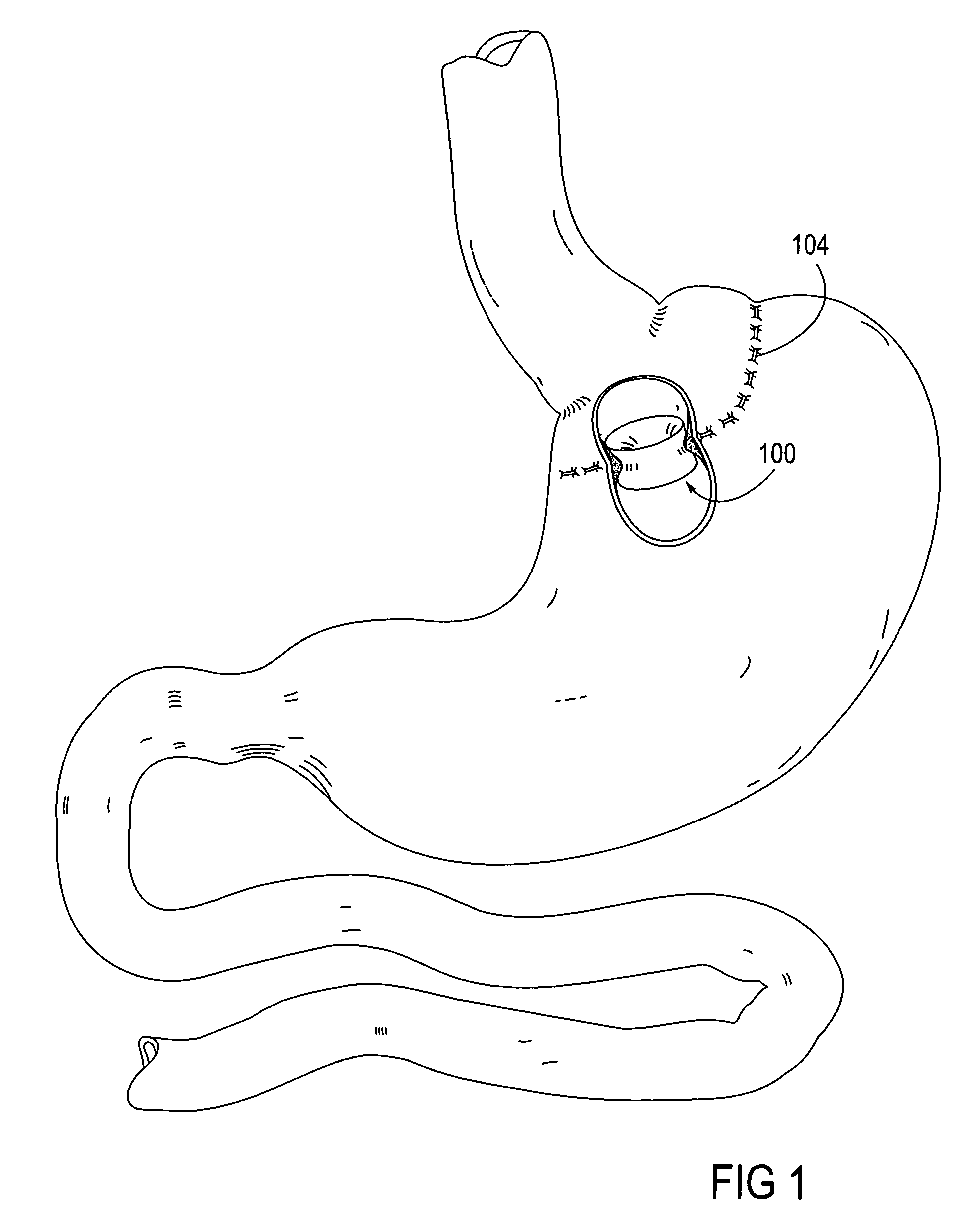
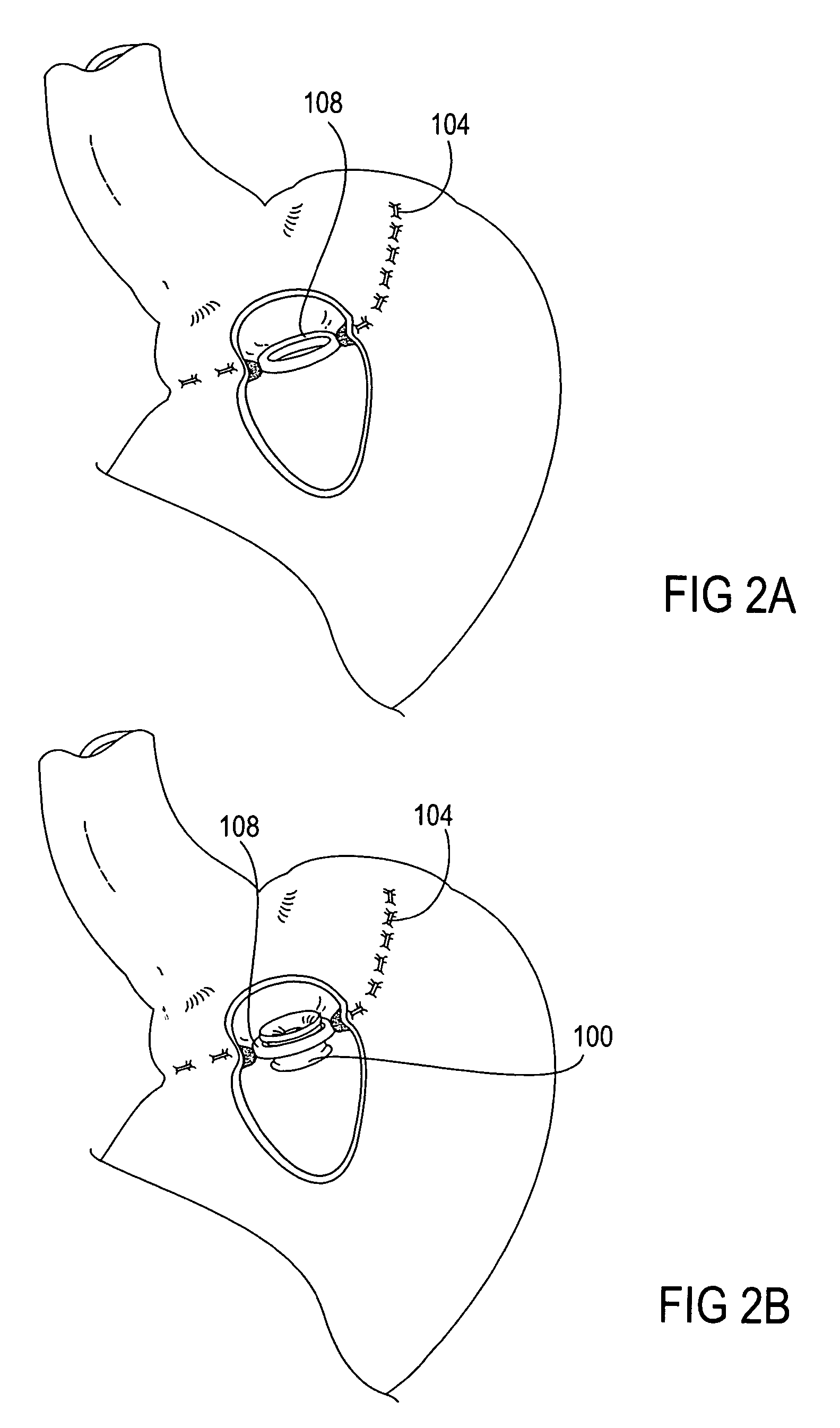
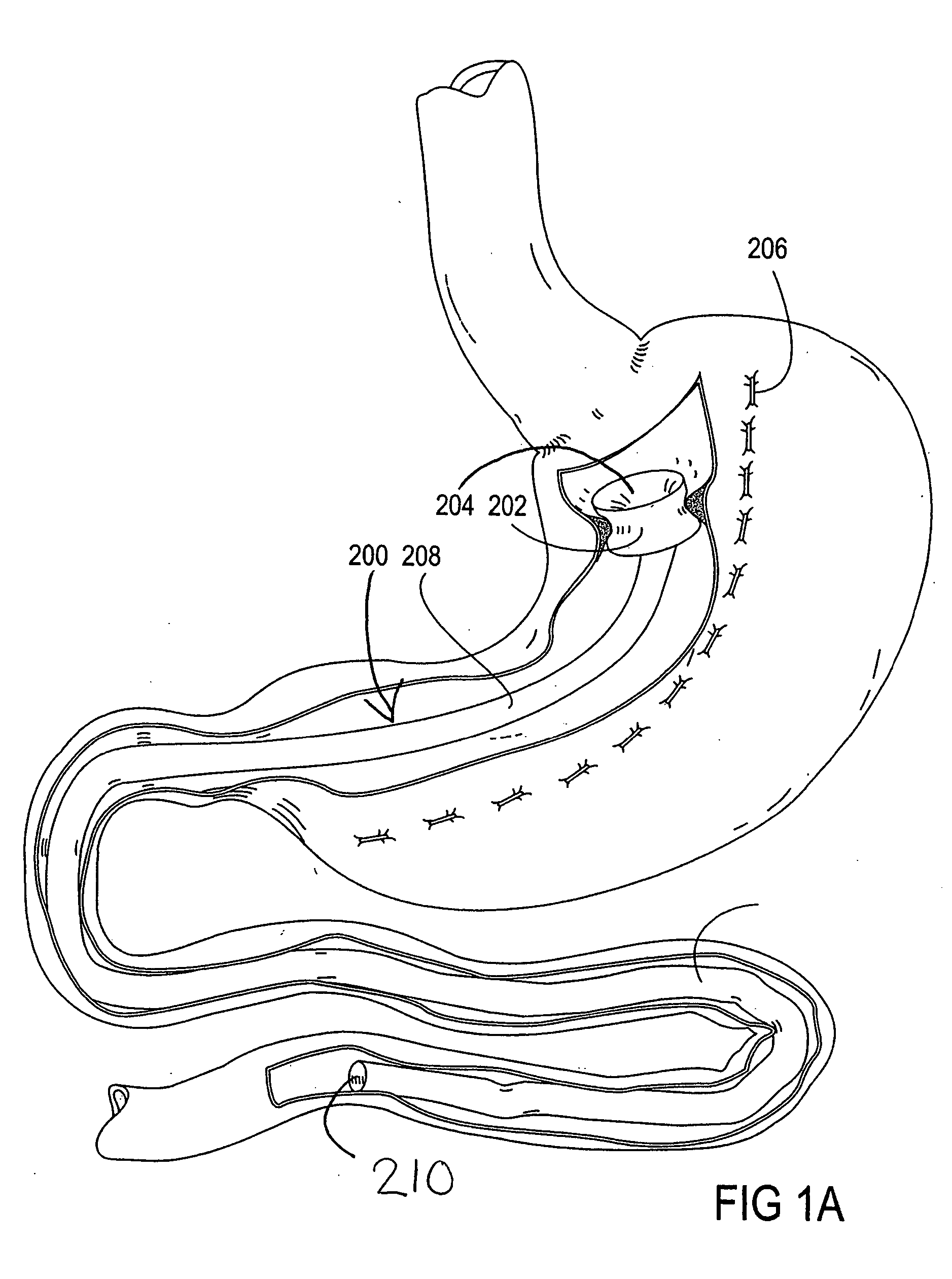
Method and device for use in endoscopic organ procedures
InactiveUS7220237B2Maintain alimentary flowImprove adhesionDiagnosticsSurgical instrument detailsStomaBody organs
Methods and devices for use in tissue approximation and fixation are described herein. The present invention provides, in part, methods and devices for acquiring tissue folds in a circumferential configuration within a hollow body organ, e.g., a stomach, positioning the tissue folds for affixing within a fixation zone of the stomach, preferably to create a pouch or partition below the esophagus, and fastening the tissue folds such that a tissue ring, or stomas, forms excluding the pouch from the greater stomach cavity. The present invention further provides for a liner or bypass conduit which is affixed at a proximal end either to the tissue ring or through some other fastening mechanism. The distal end of the conduit is left either unanchored or anchored within the intestinal tract. This bypass conduit also includes a fluid bypass conduit which allows the stomach and a portion of the intestinal tract to communicate.
Owner:ETHICON ENDO SURGERY INC
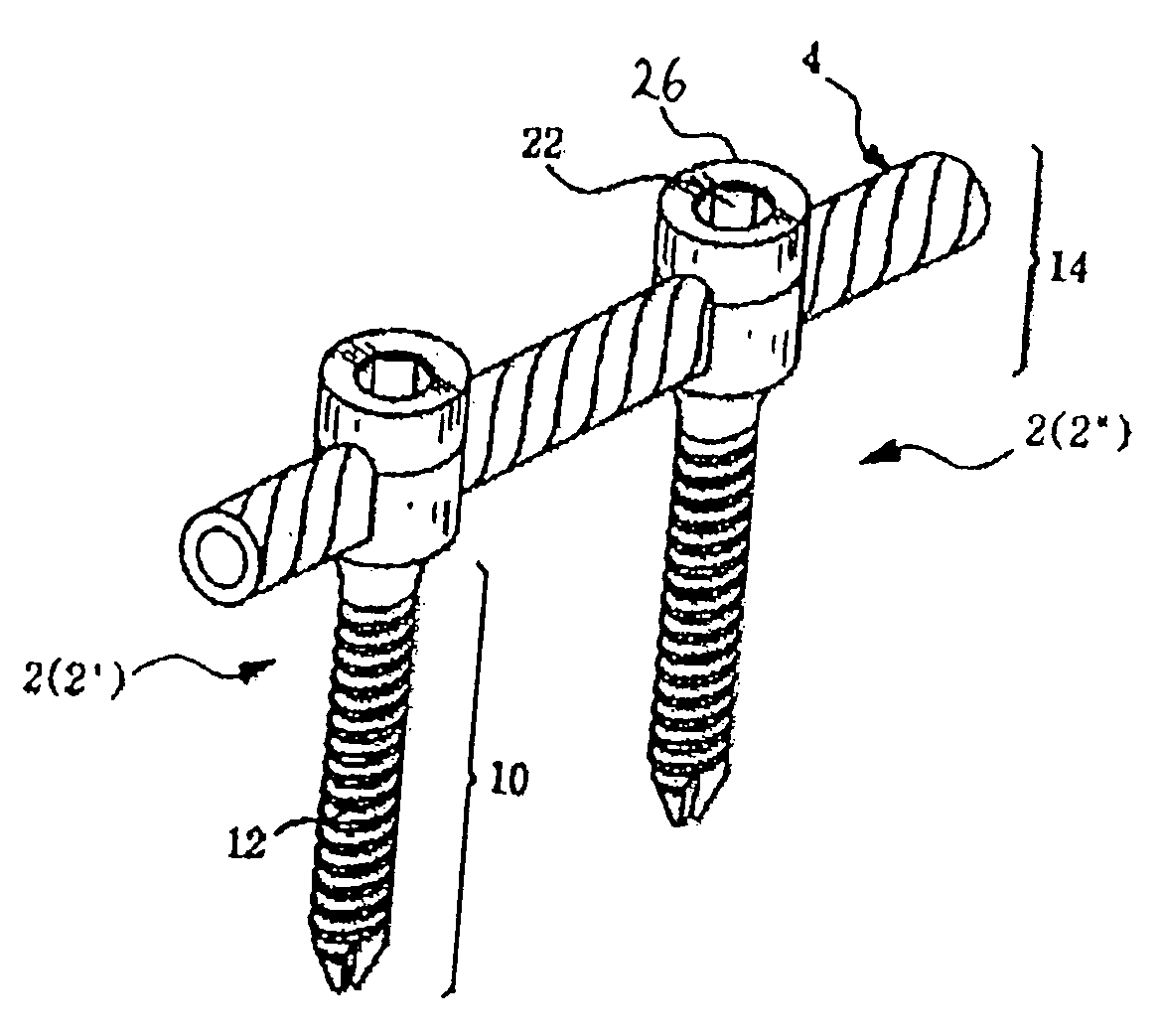
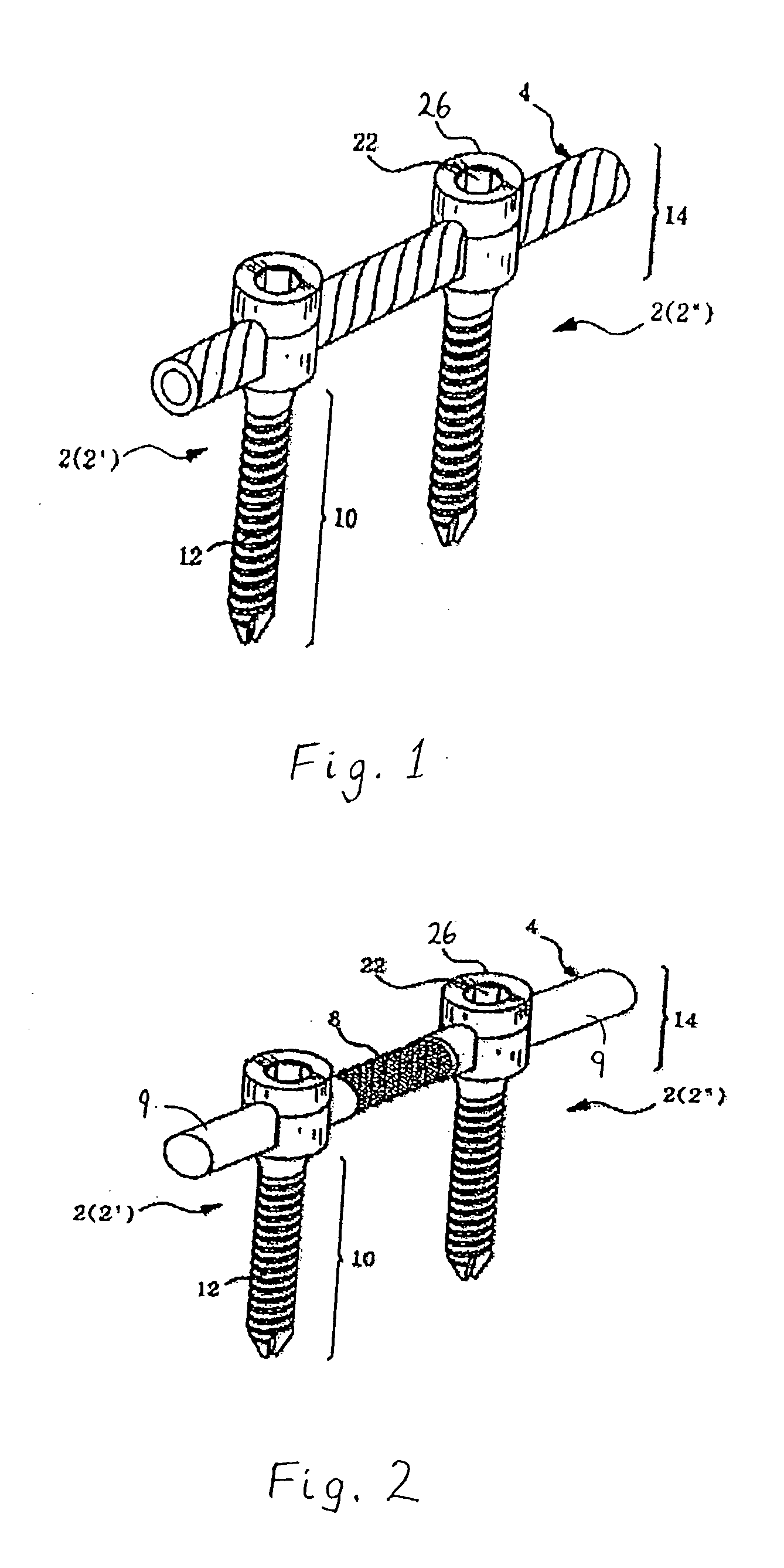
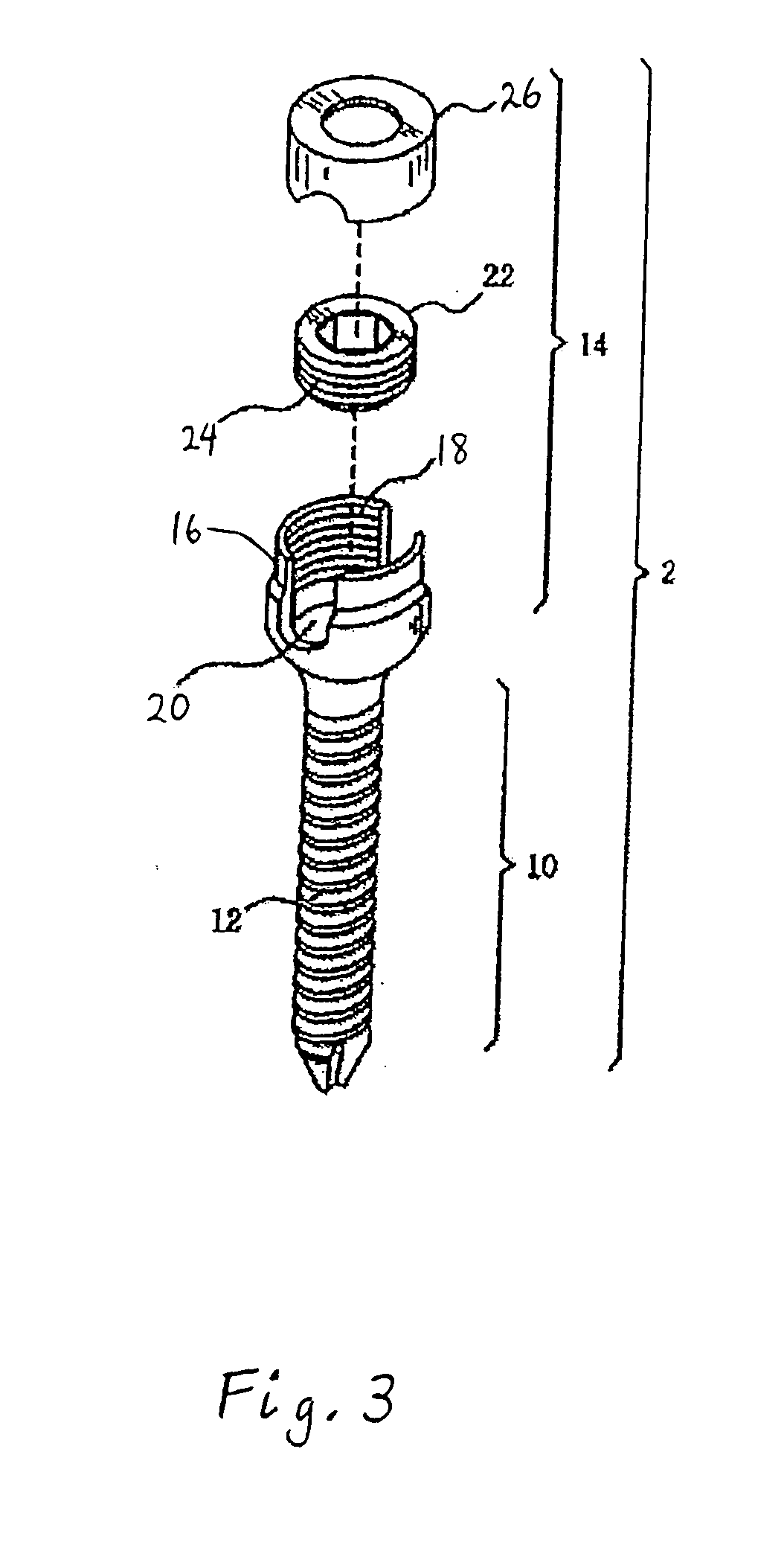
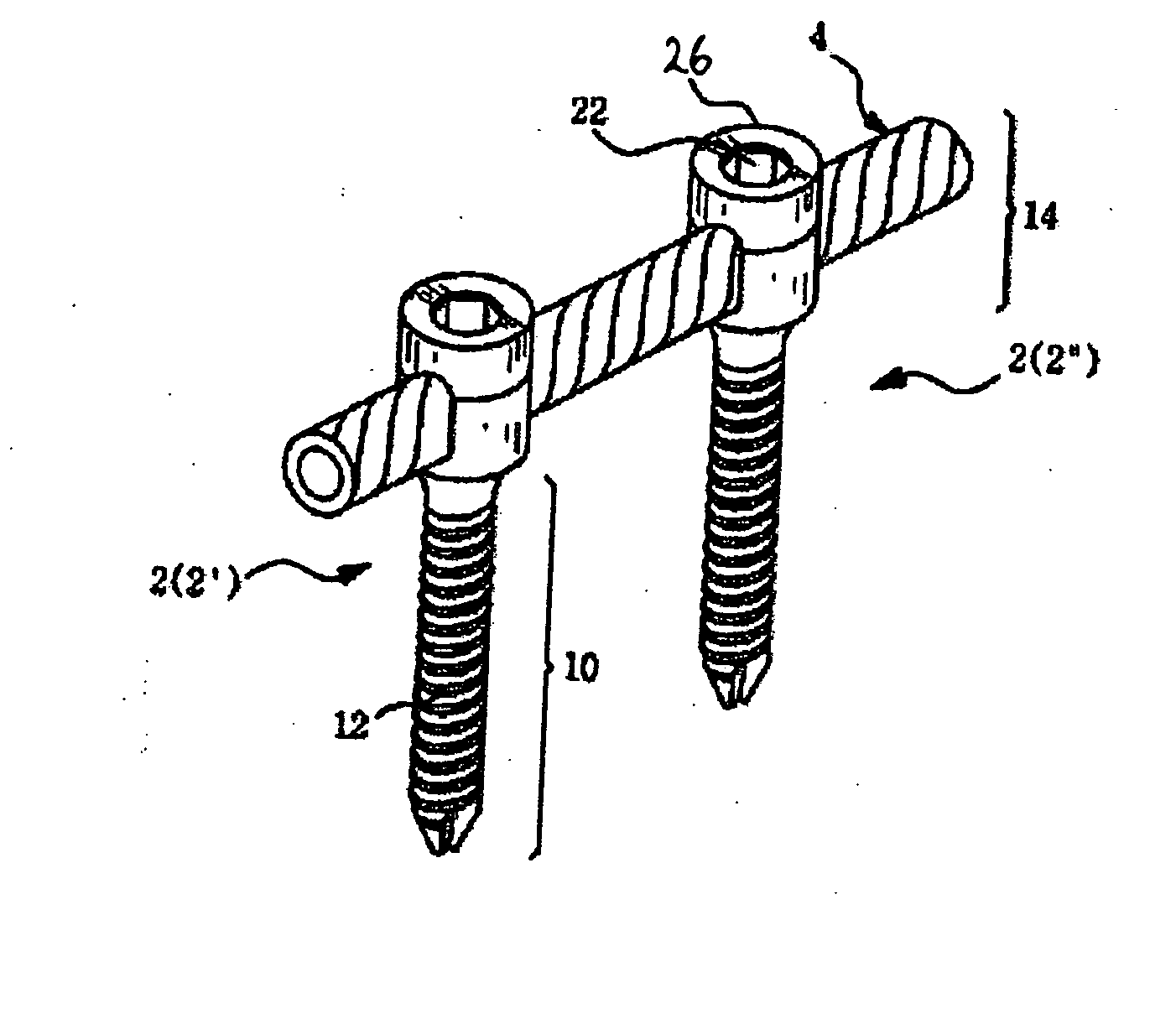
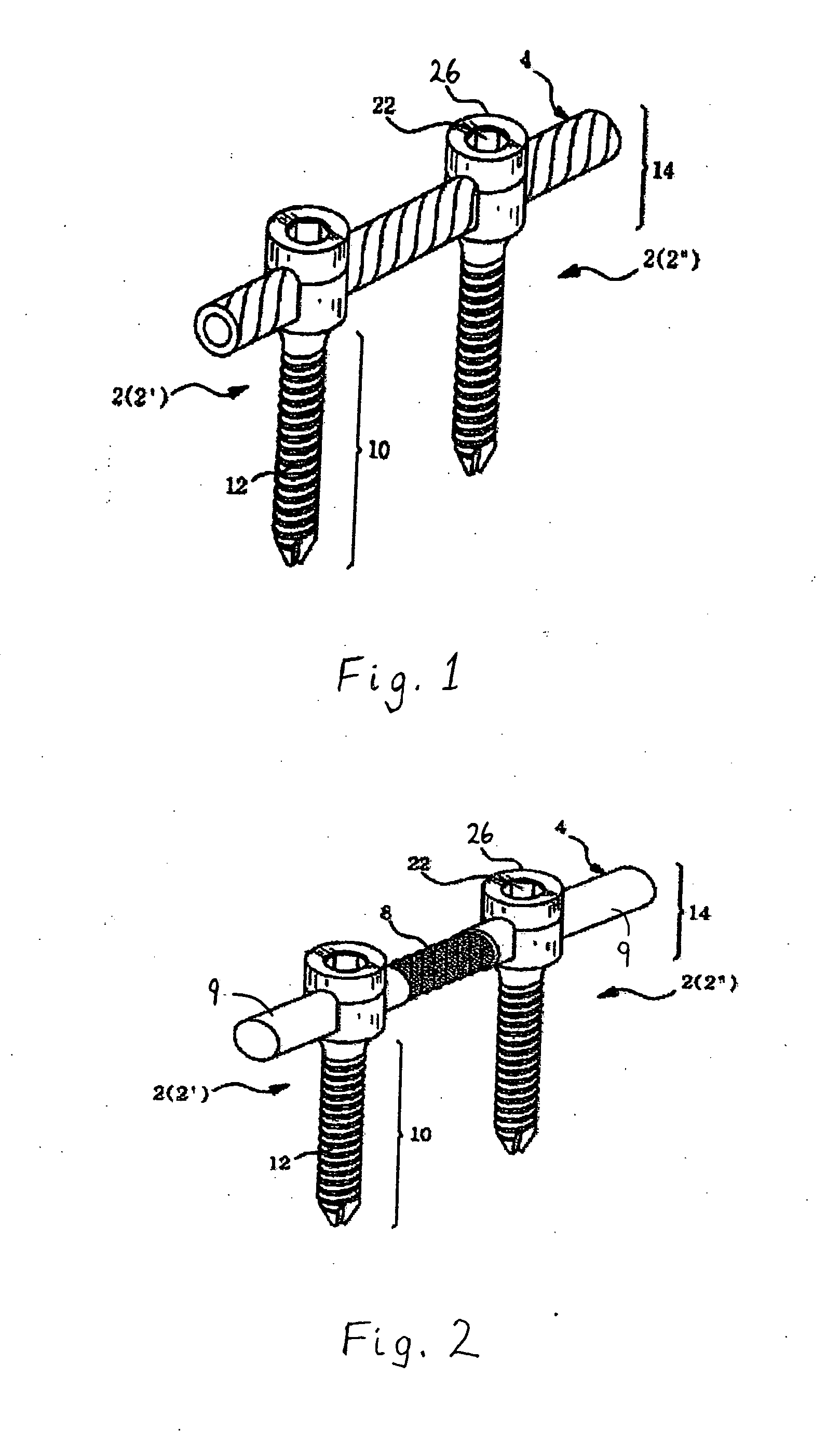
Spinal stabilization device
ActiveUS20050203517A1Easy constructionSimple designInternal osteosythesisEar treatmentEngineeringMechanical engineering
Owner:DEPUY SYNTHES PROD INC
Method and apparatus for flexible fixation of a spine
InactiveUS20050065516A1Easy constructionSimple designInternal osteosythesisEar treatmentSpinal columnCoupling
A flexible spinal fixation device having a flexible metallic connection unit for non-rigid stabilization of the spinal column. In one embodiment, the fixation device includes at least two securing members configured to be inserted into respective adjacent spinal pedicles, each securing member each including a coupling assembly. The fixation device further includes a flexible metal connection unit configured to be received and secured within the coupling assemblies of each securing member so as to flexibly stabilize the affected area of the spine.
Owner:DEPUY SYNTHES PROD INC
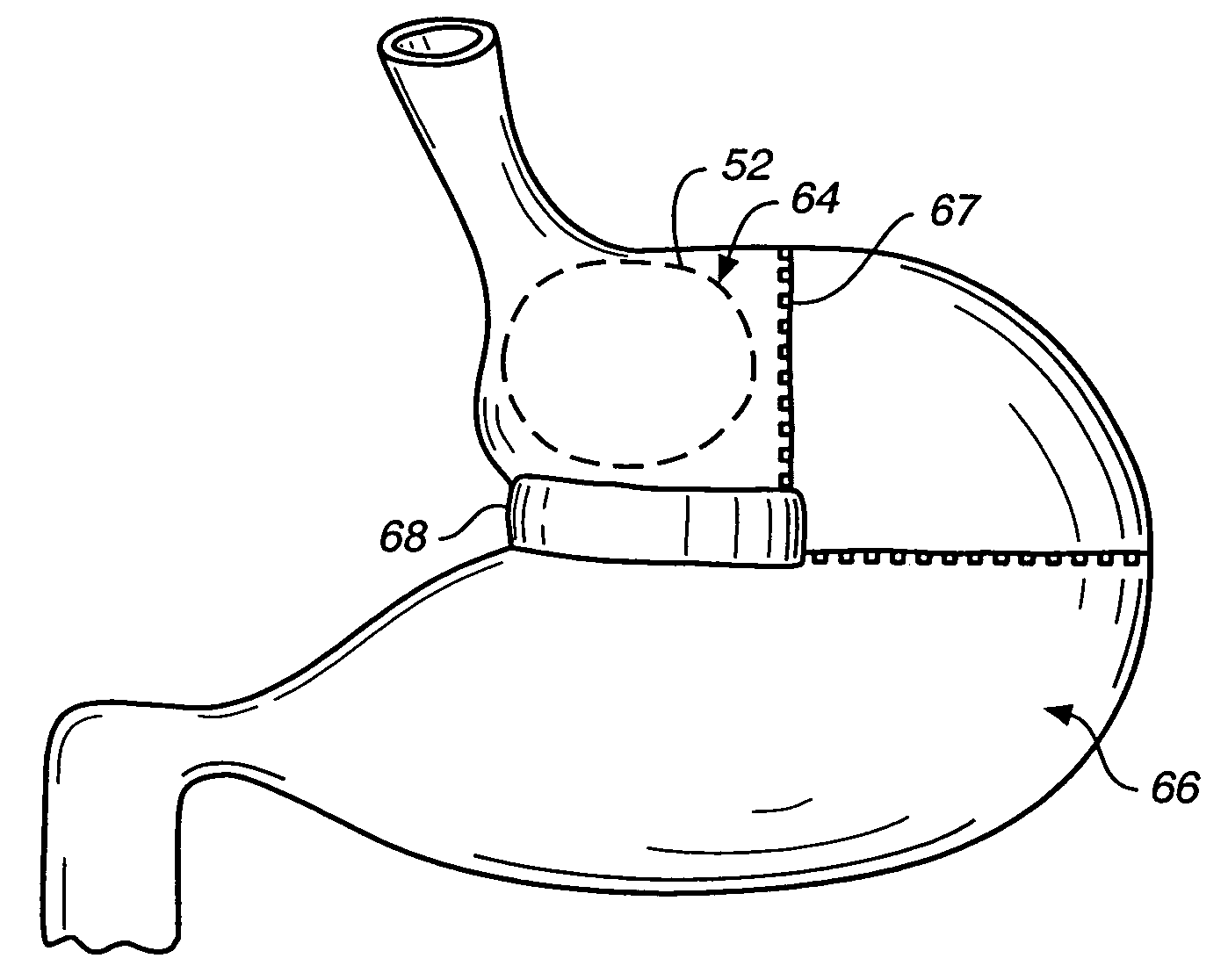
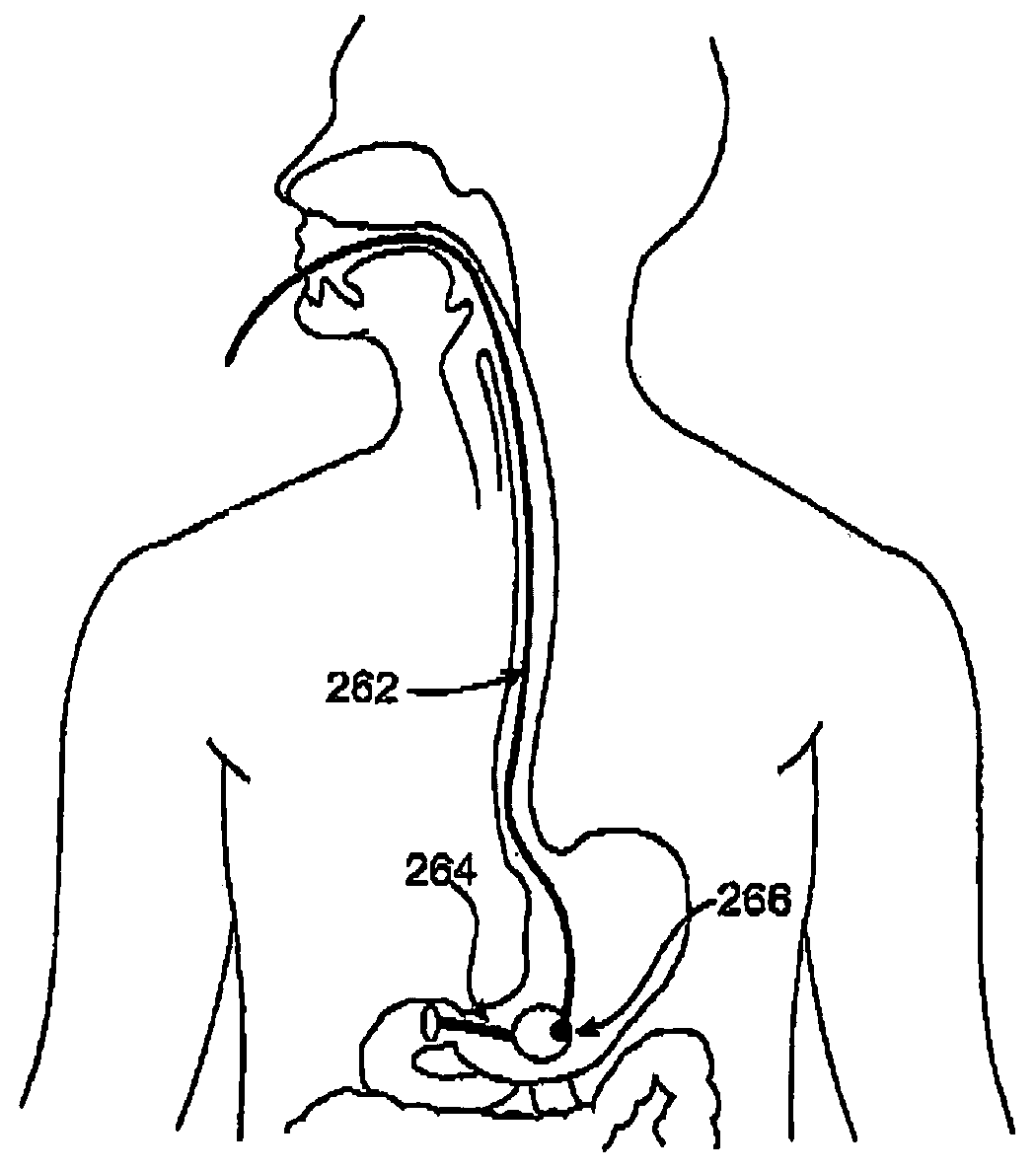
Satiation devices and methods
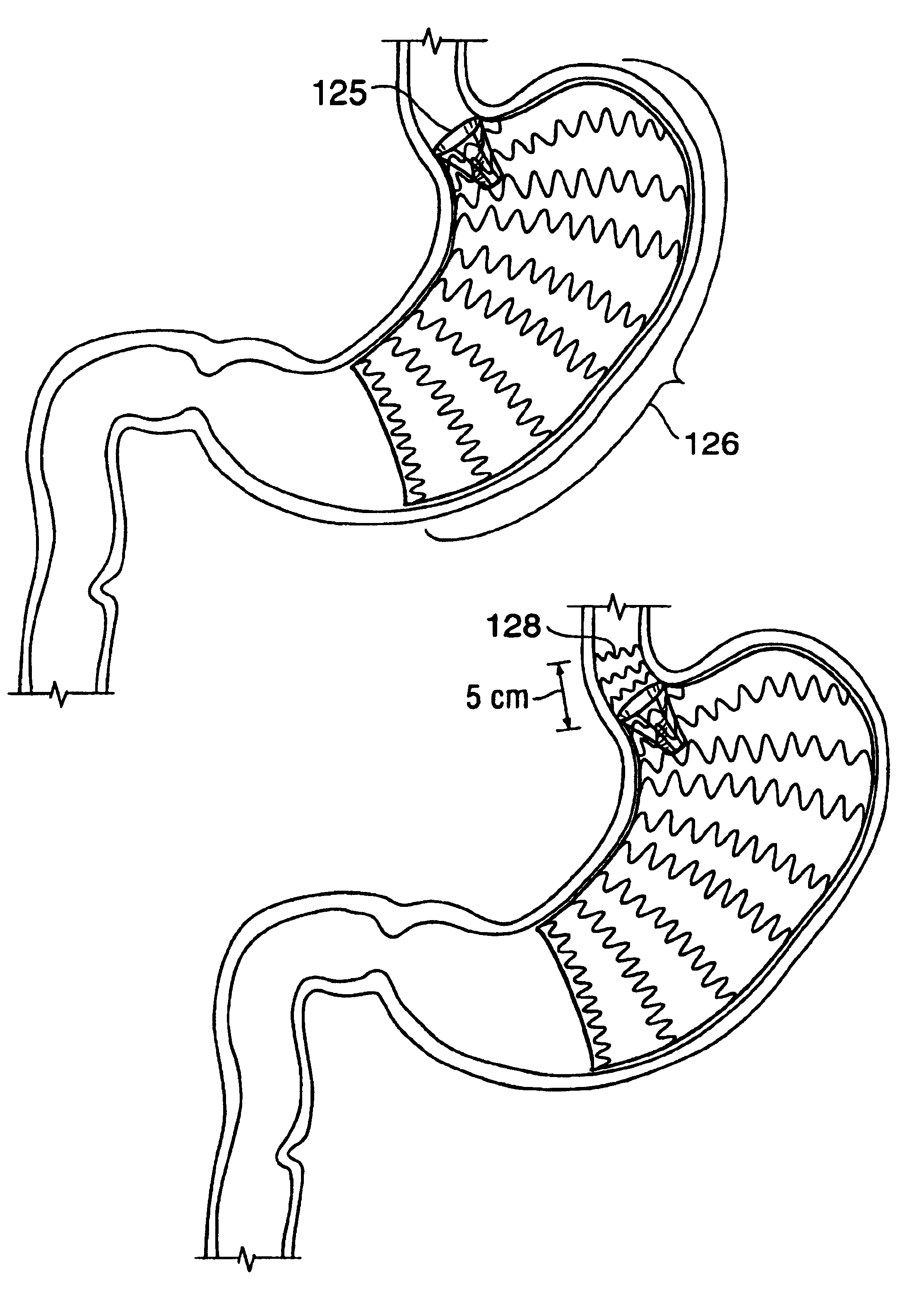
A device for inducing weight loss in a patient includes a tubular prosthesis positionable at the gastro-esophageal junction region, preferably below the z-line. In a method for inducing weight loss, the prosthesis is placed such that an opening at its proximal end receives masticated food from the esophagus, and such that the masticated food passes through the pouch and into the stomach via an opening in its distal end.
Owner:BOSTON SCI SCIMED INC
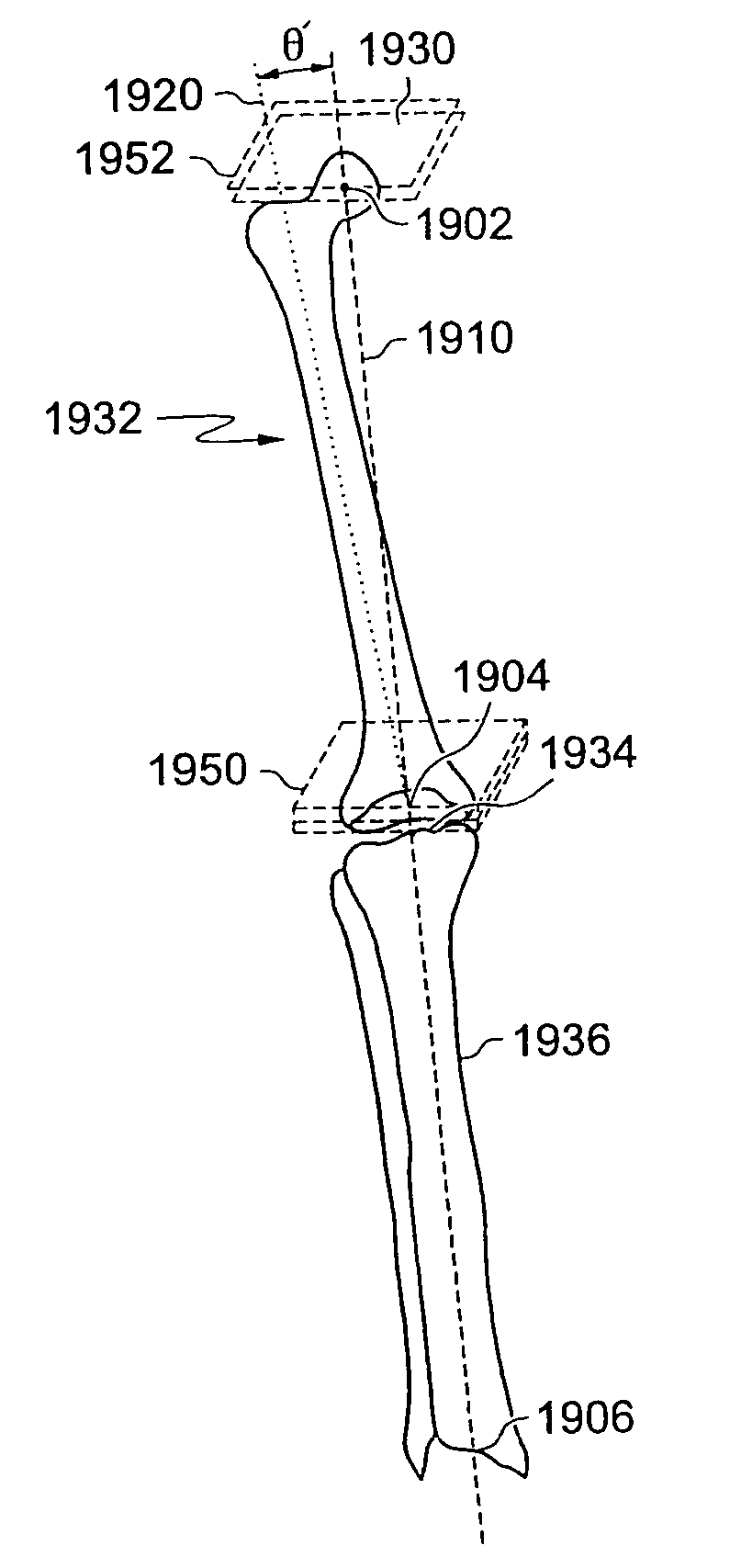
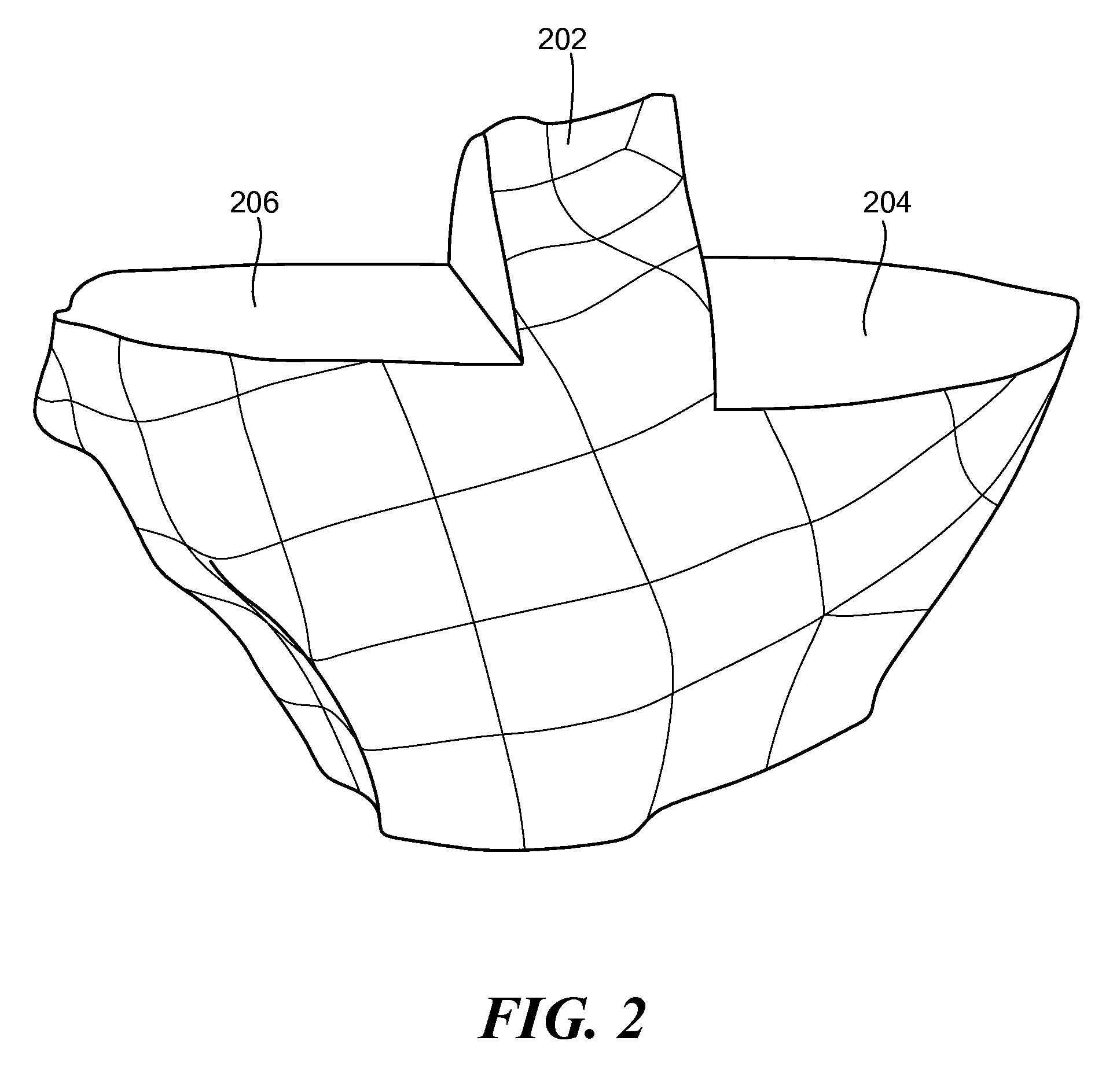
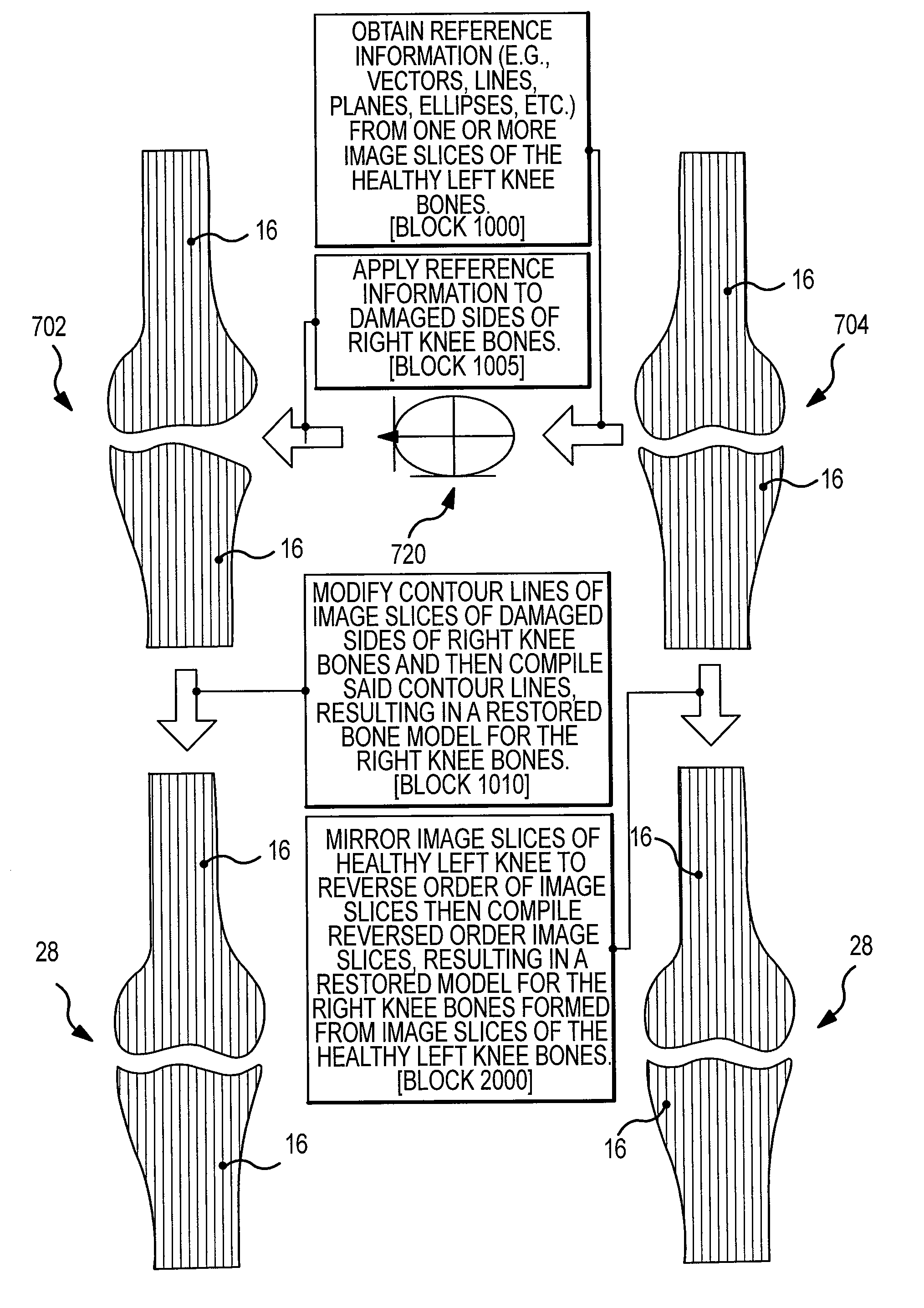
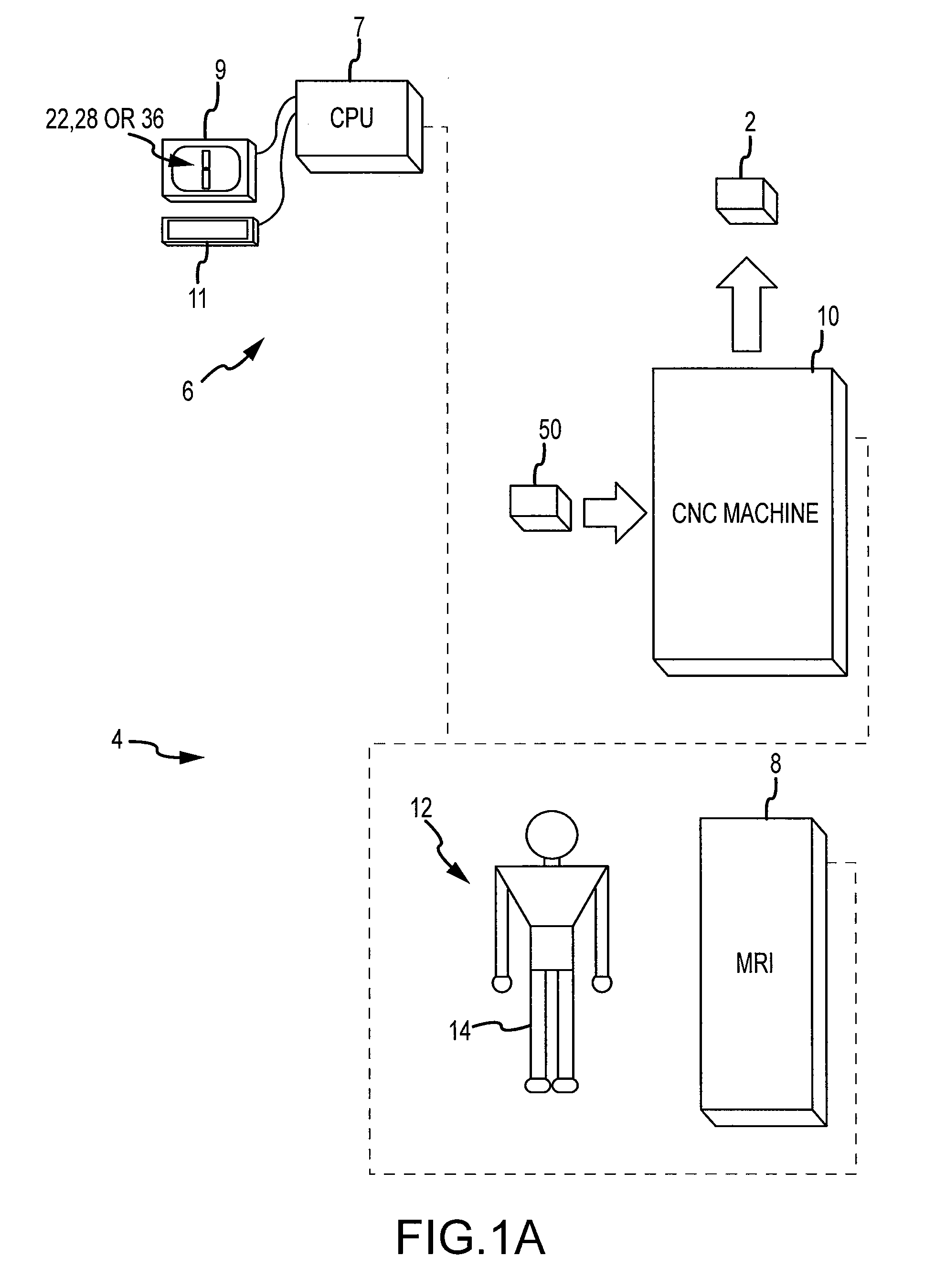
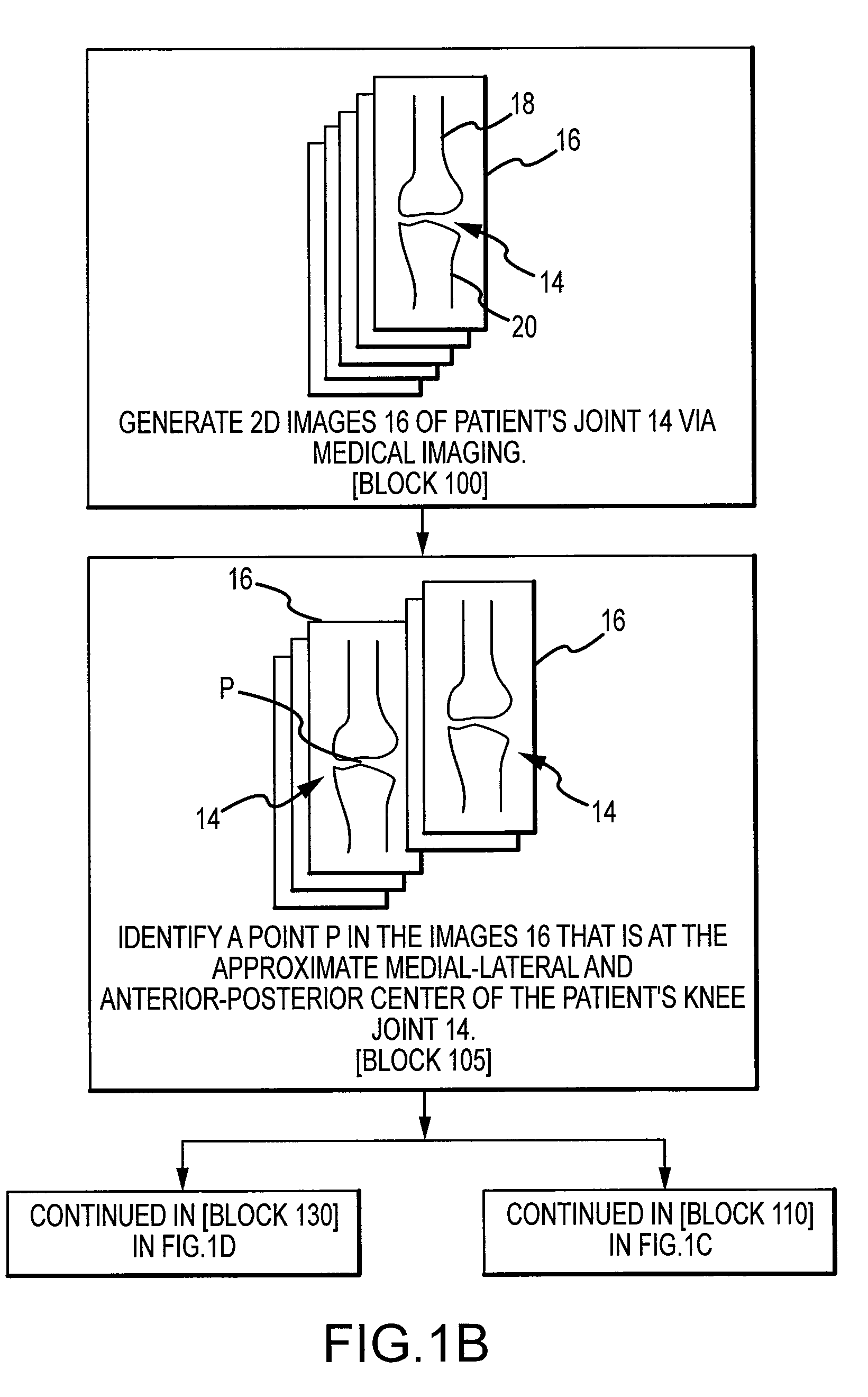
Generation of a computerized bone model representative of a pre-degenerated state and useable in the design and manufacture of arthroplasty devices
ActiveUS20090270868A1Reduce the possibilityIncrease success rateDiagnosticsAnalogue computers for chemical processesKnee JointProsthesis
Disclosed herein is a method of generating a computerized bone model representative of at least a portion of a patient bone in a pre-degenerated state. The method includes: generating at least one image of the patient bone in a degenerated state; identifying a reference portion associated with a generally non-degenerated portion of the patient bone; identifying a degenerated portion associated with a generally degenerated portion of the patient bone; and using information from at least one image associated with the reference portion to modify at least one aspect associated with at least one image associated the generally degenerated portion. The method may further include employing the computerized bone model representative of the at least a portion of the patient bone in the pre-degenerated state in defining manufacturing instructions for the manufacture of a customized arthroplasty jig. Also disclosed herein is a customized arthroplasty jig manufactured according to the above-described method. The customized arthroplasty jig is configured to facilitate a prosthetic implant restoring a patient joint to a natural alignment. The prosthetic implant may be for a total joint replacement or partial joint replacement. The patient joint may be a variety of joints, including, but not limited to, a knee joint.
Owner:HOWMEDICA OSTEONICS CORP
System and method for manufacturing arthroplasty jigs having improved mating accuracy
ActiveUS20100023015A1Facilitate arthroplasty implantsDiagnosticsComputer-aided planning/modellingSacroiliac jointOrthodontics
Disclosed herein is a method of defining a mating surface in a first side of an arthroplasty jig. The mating surface is configured to matingly receive and contact a corresponding patient surface including at least one of a bone surface and a cartilage surface. The first side is oriented towards the patient surface when the mating surface matingly receives and contacts the patient surface. The method may include: a) identifying a contour line associated with the patient surface as represented in a medical image; b) evaluating via an algorithm the adequacy of the contour line for defining a portion of the mating surface associated with the contour line; c) modifying the contour line if the contour line is deemed inadequate; and d) employing the modified contour line to define the portion of the mating surface associated with the contour line.
Owner:HOWMEDICA OSTEONICS CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com