Patents
Literature
36 results about "Morbid obesity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
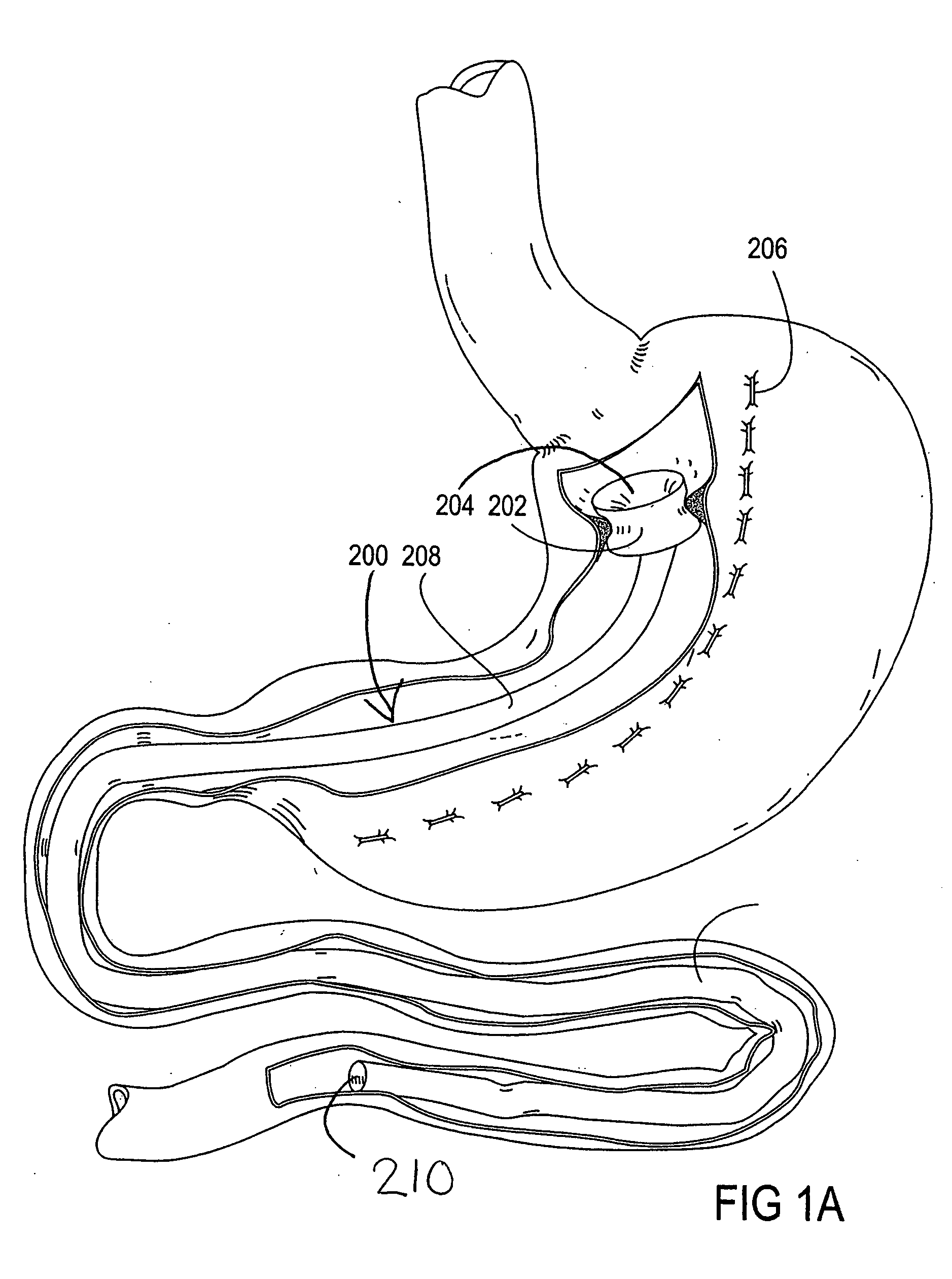
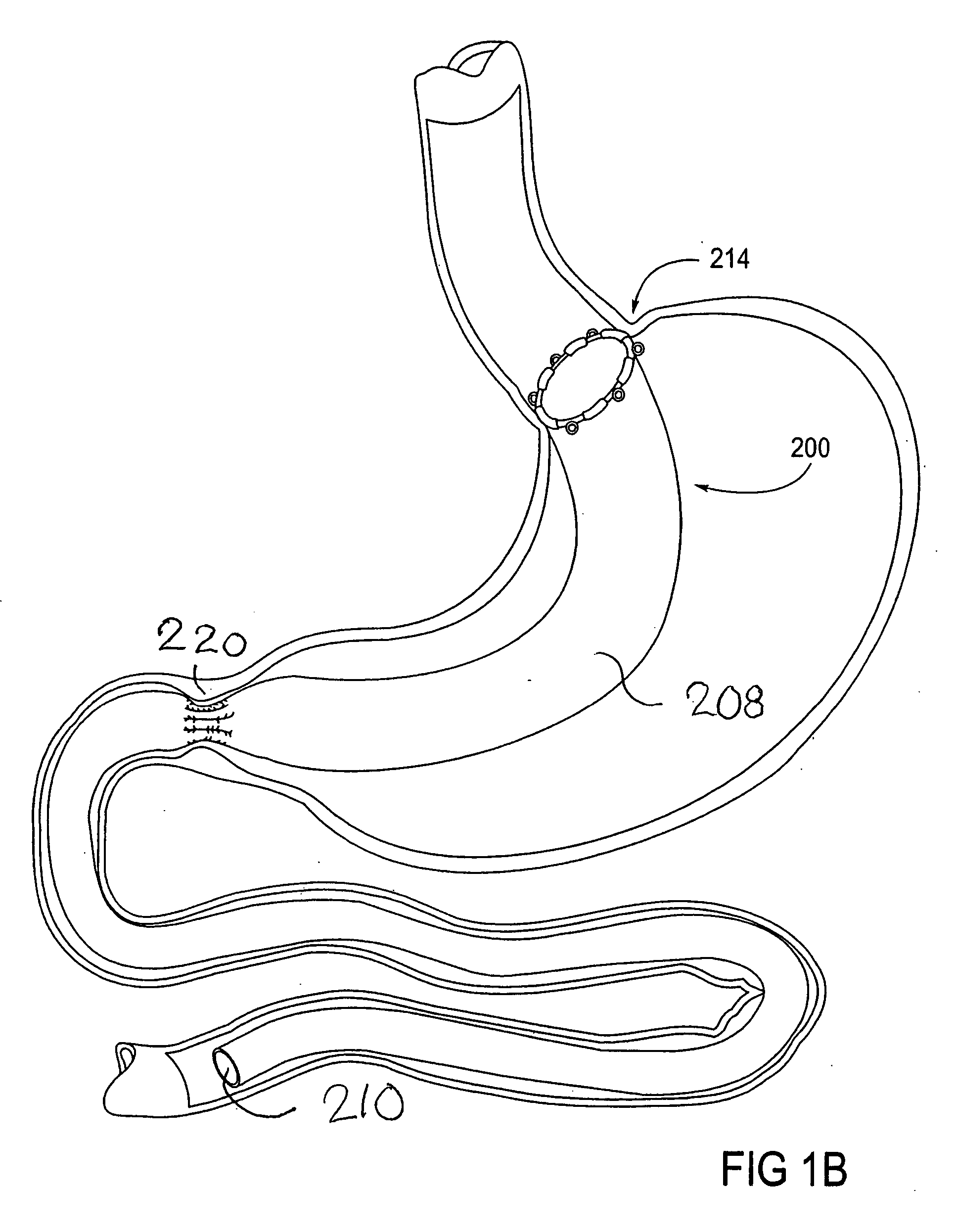
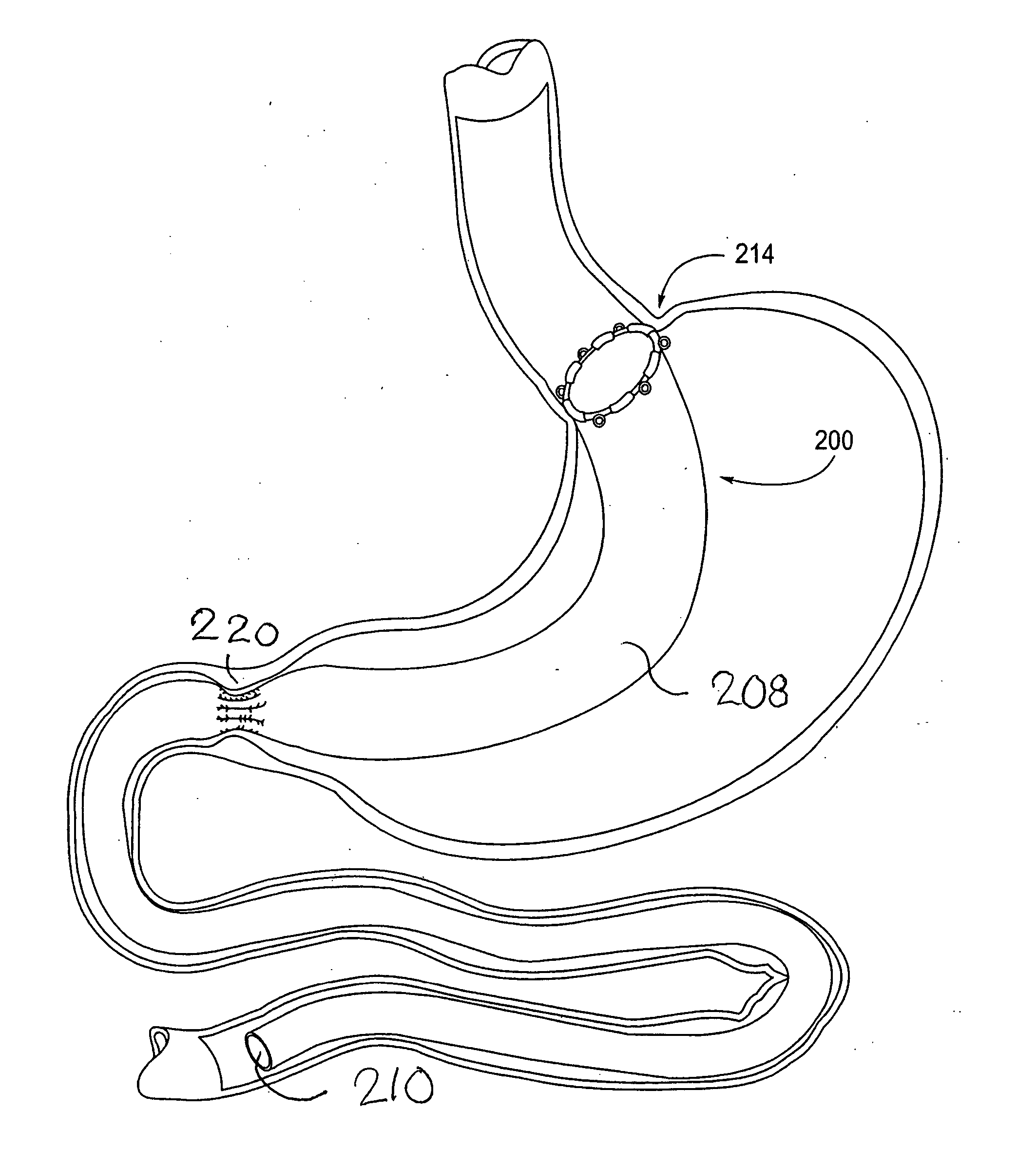
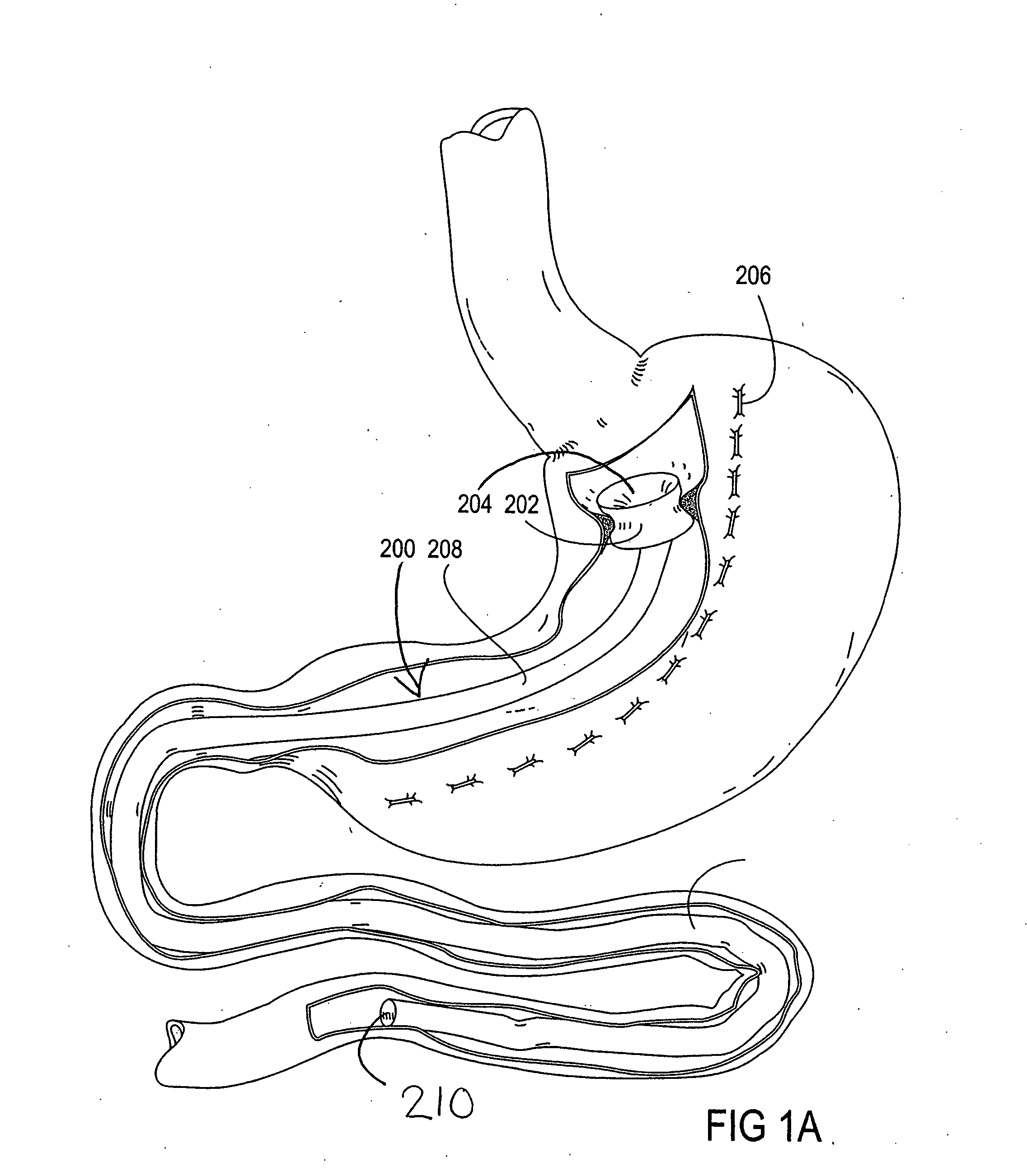
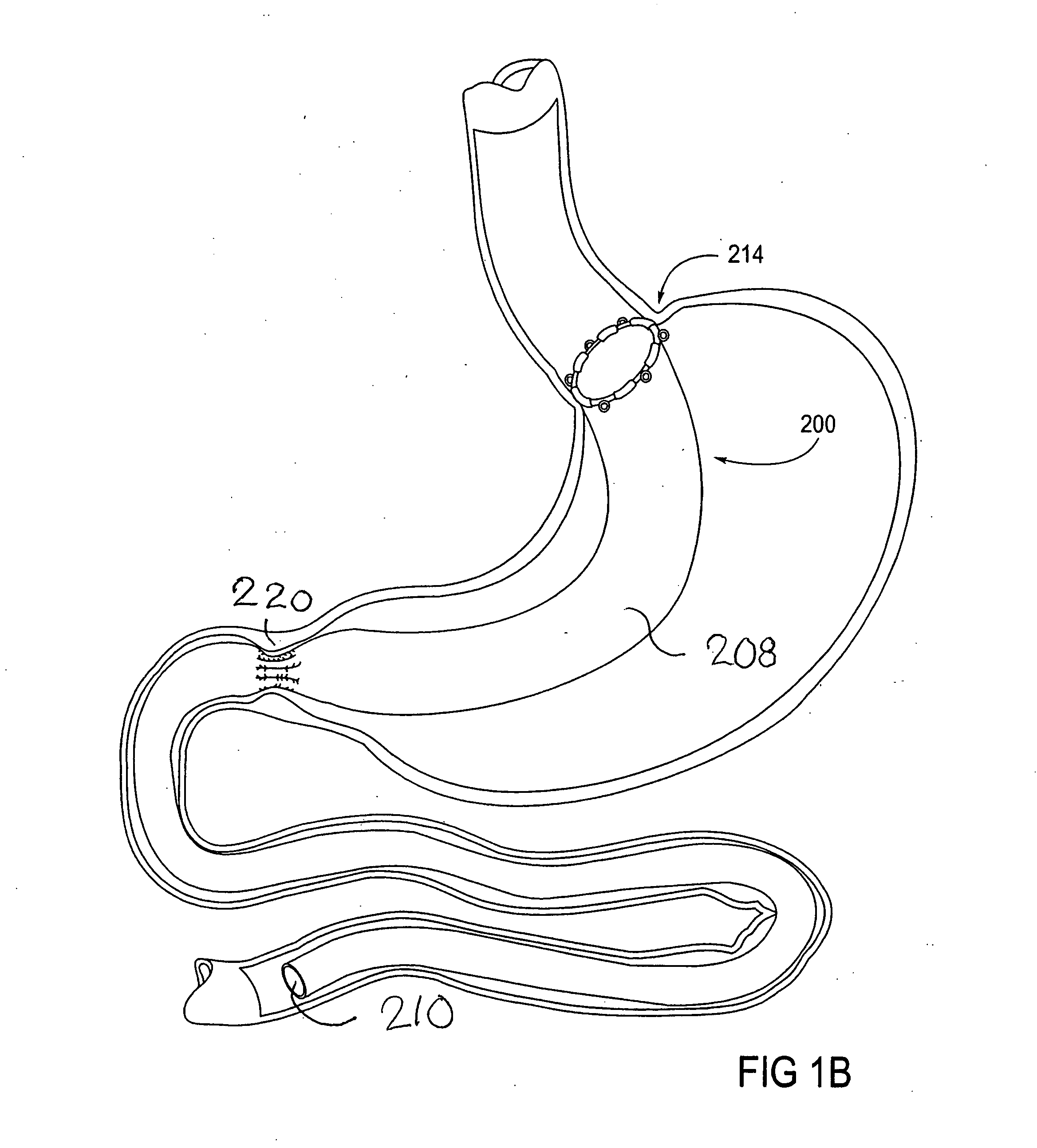
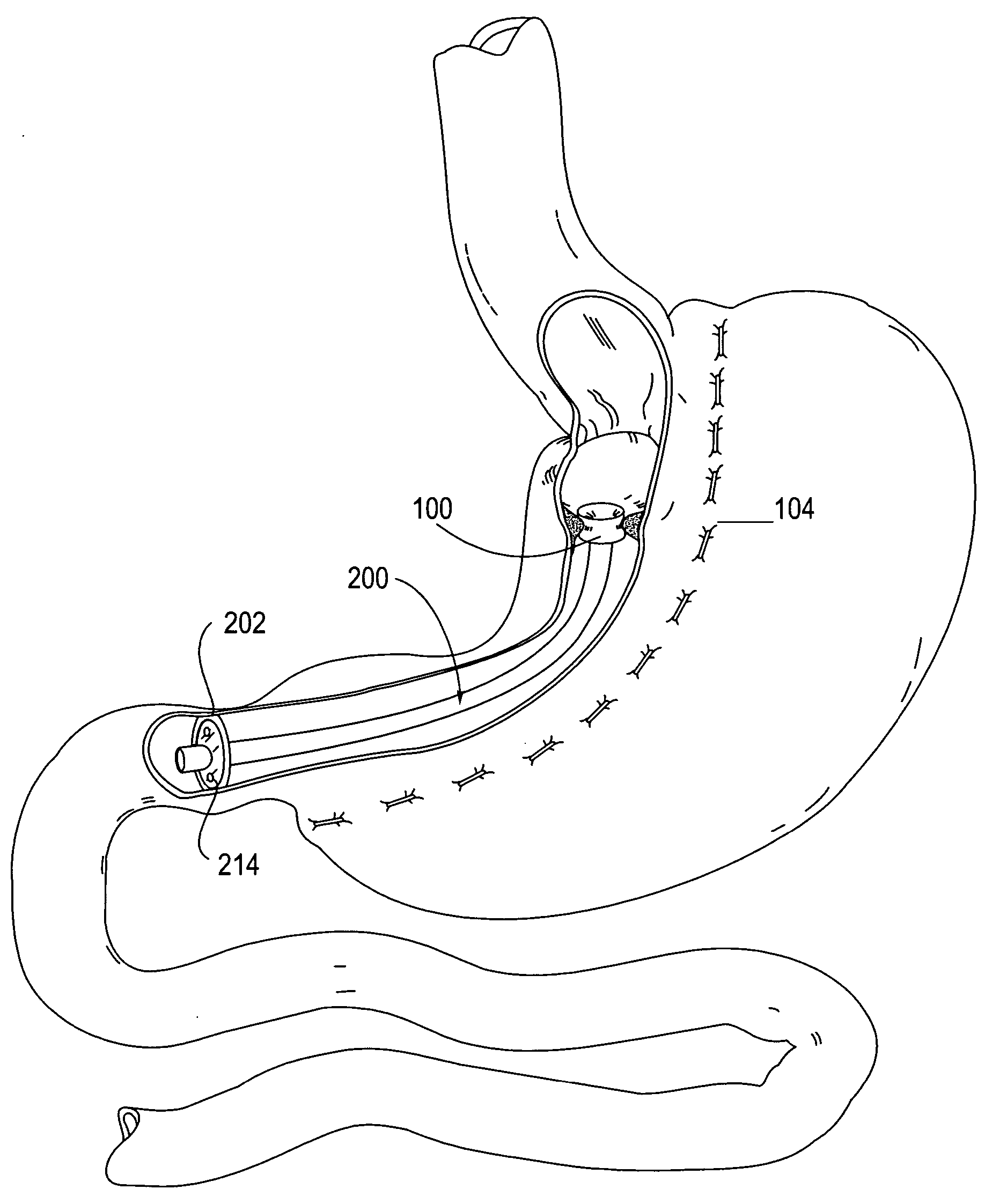
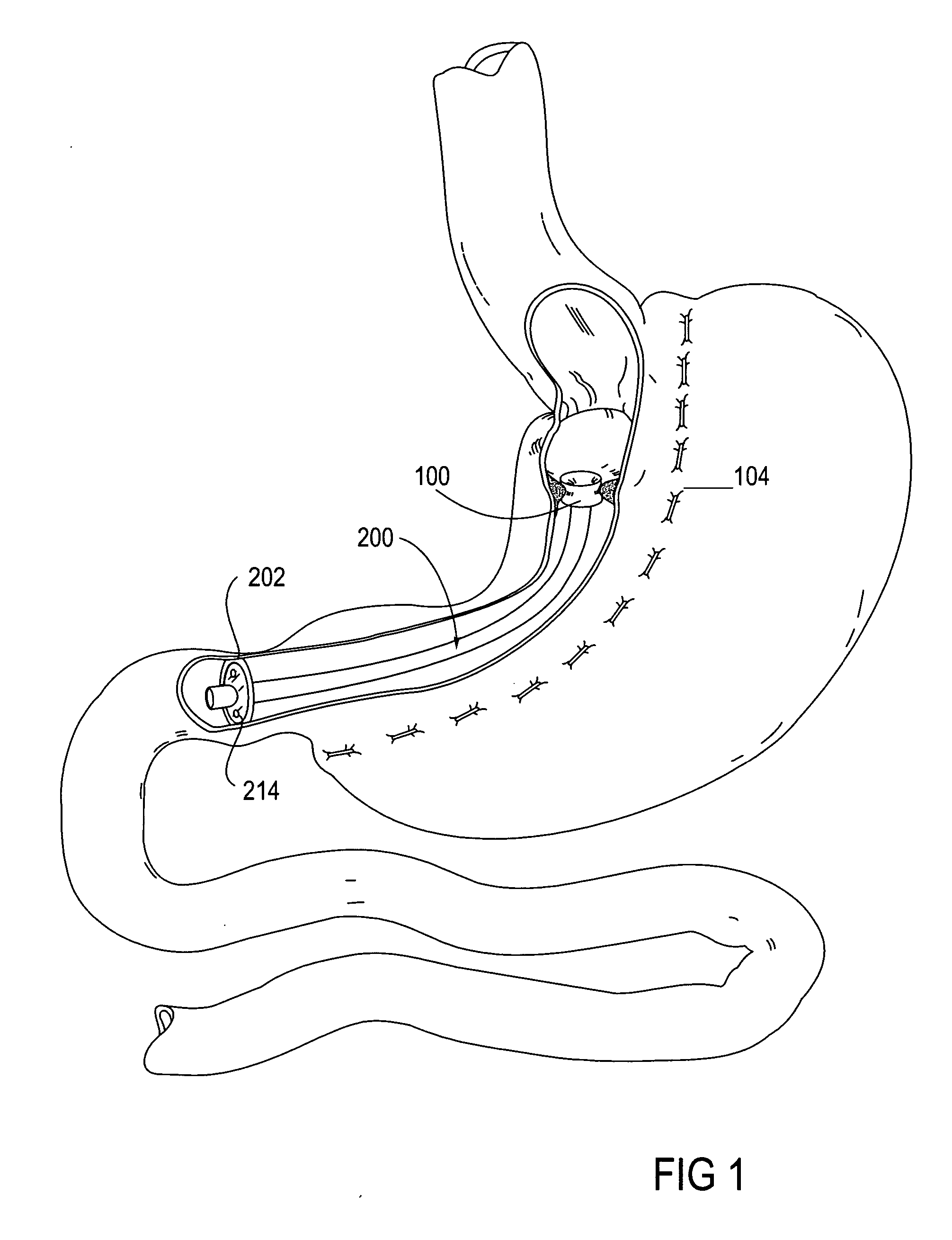
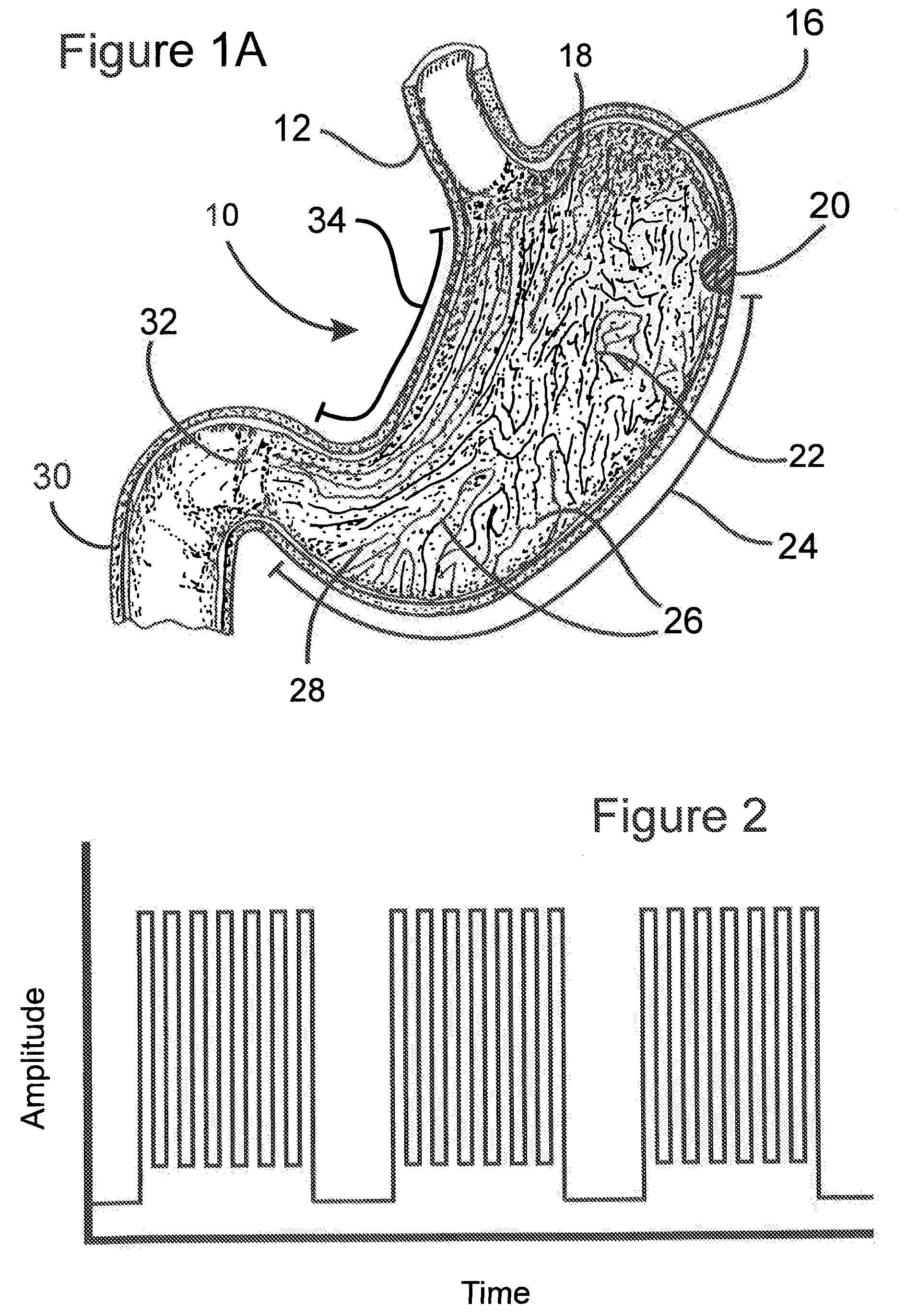
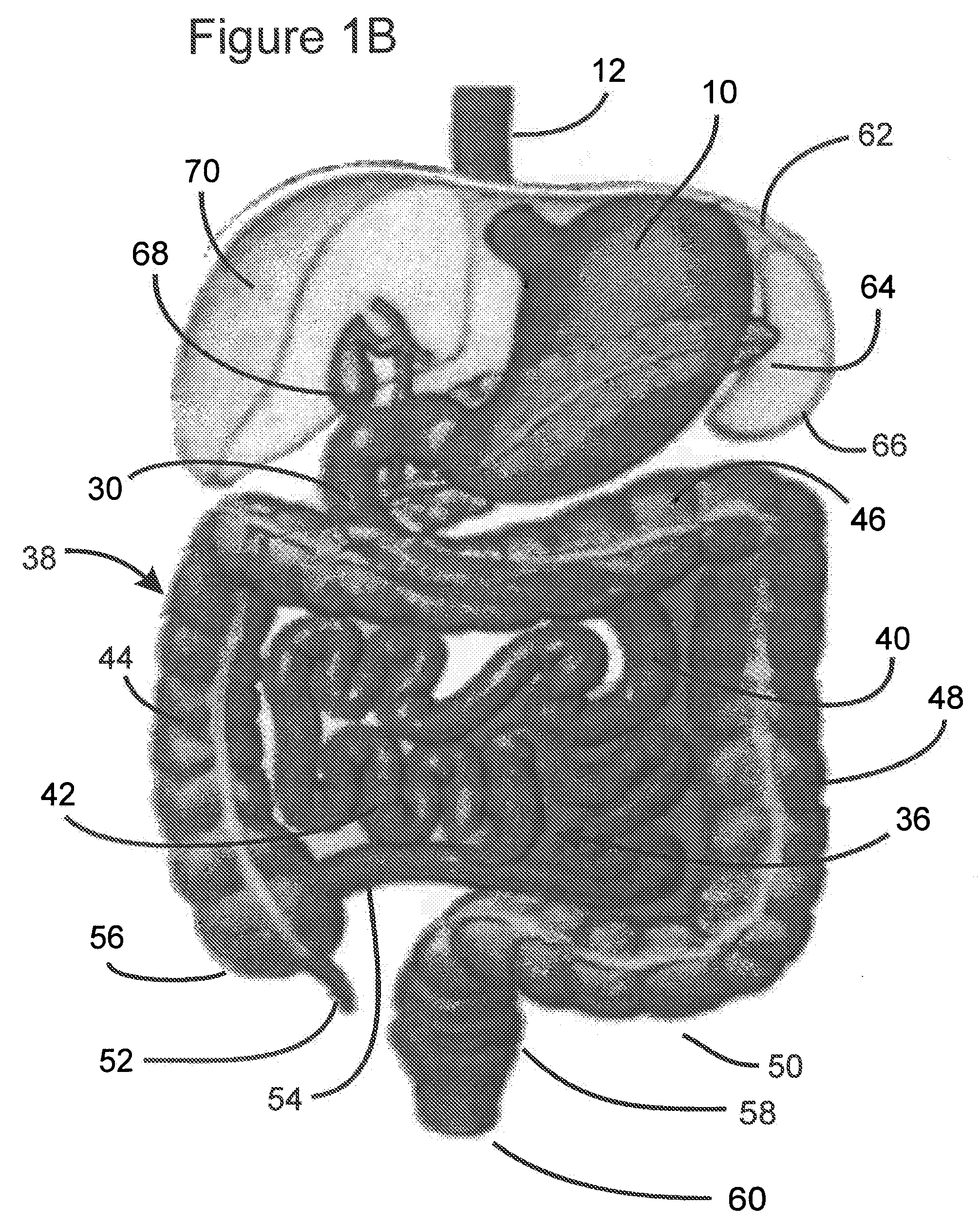
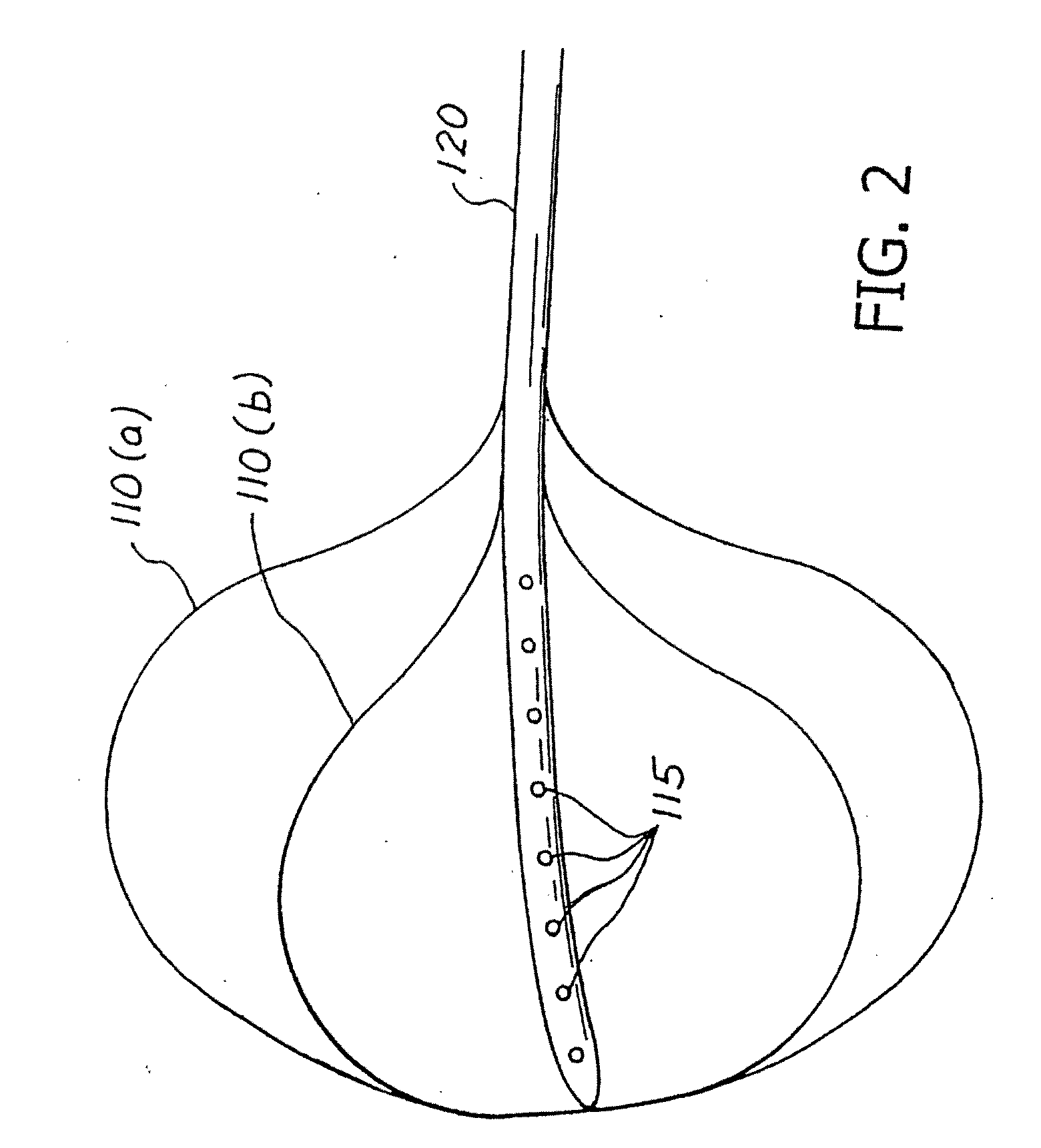
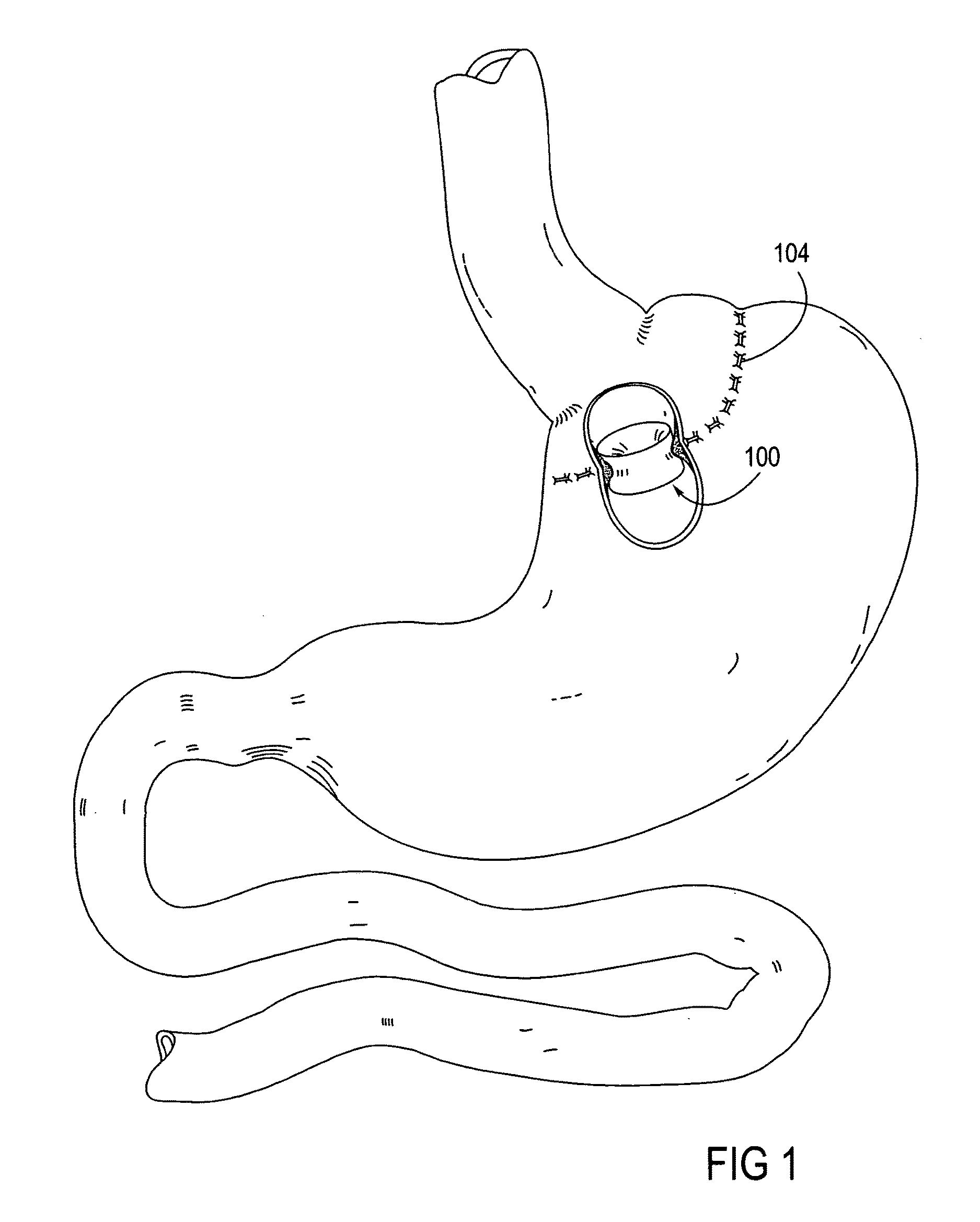
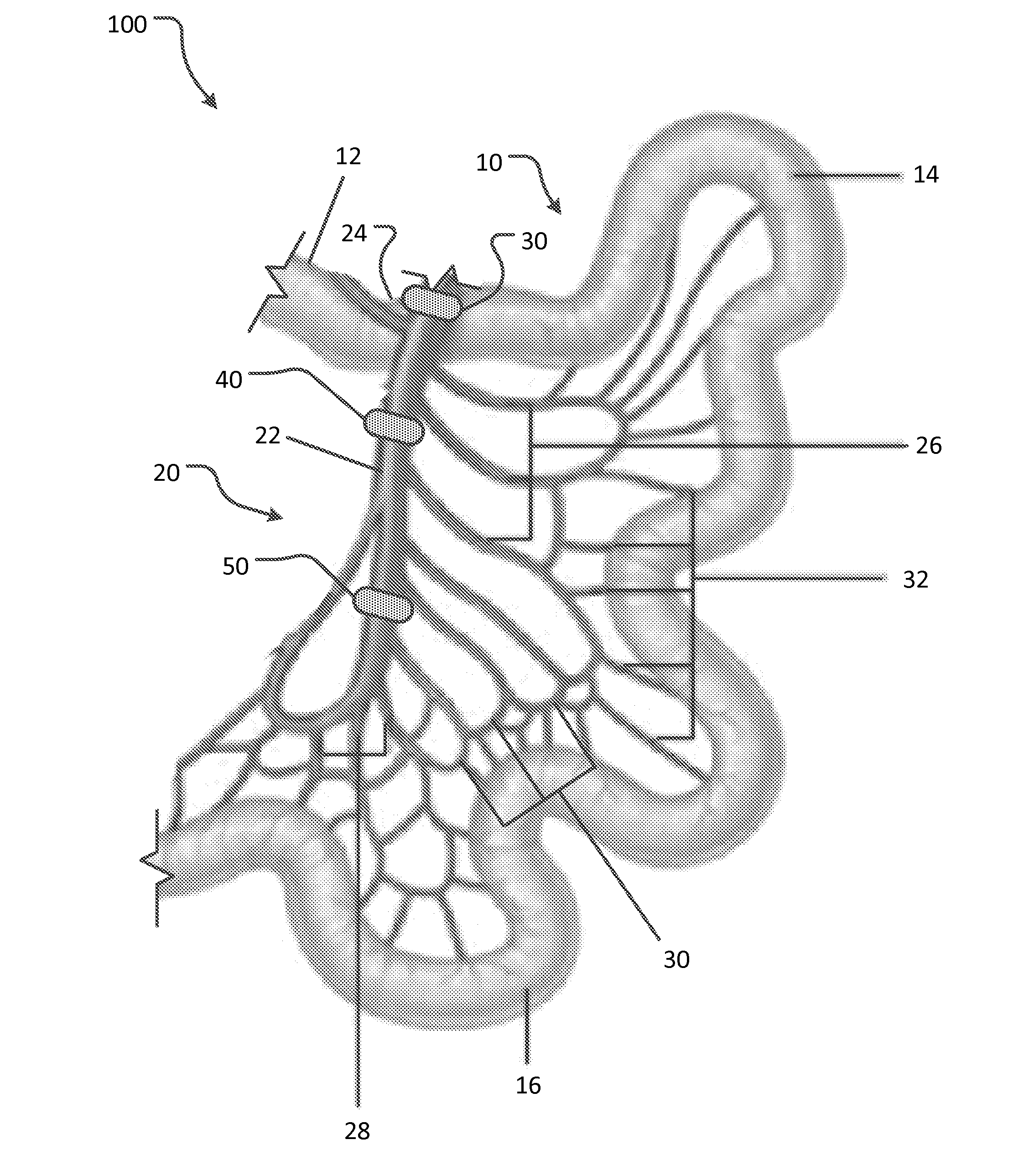
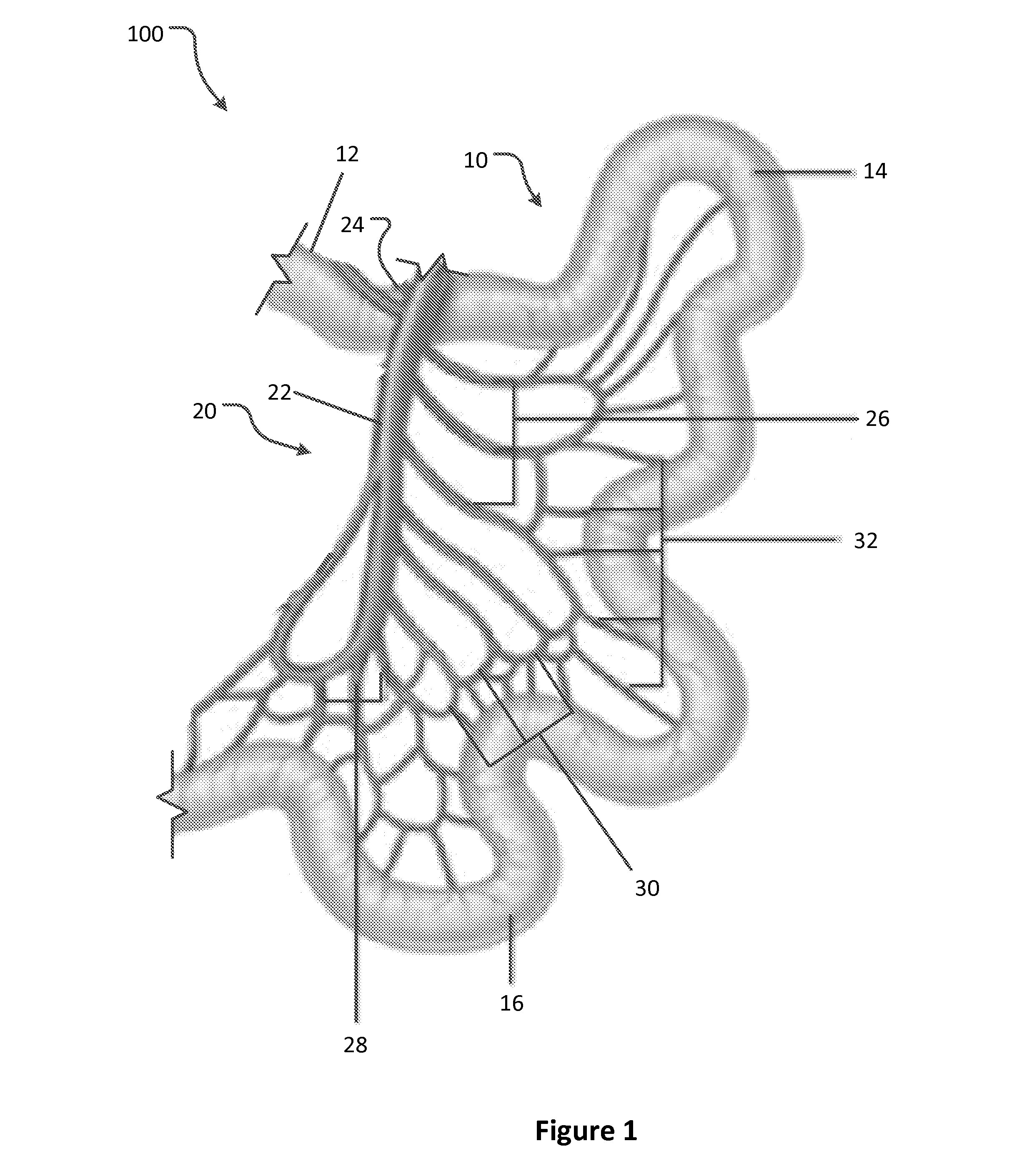
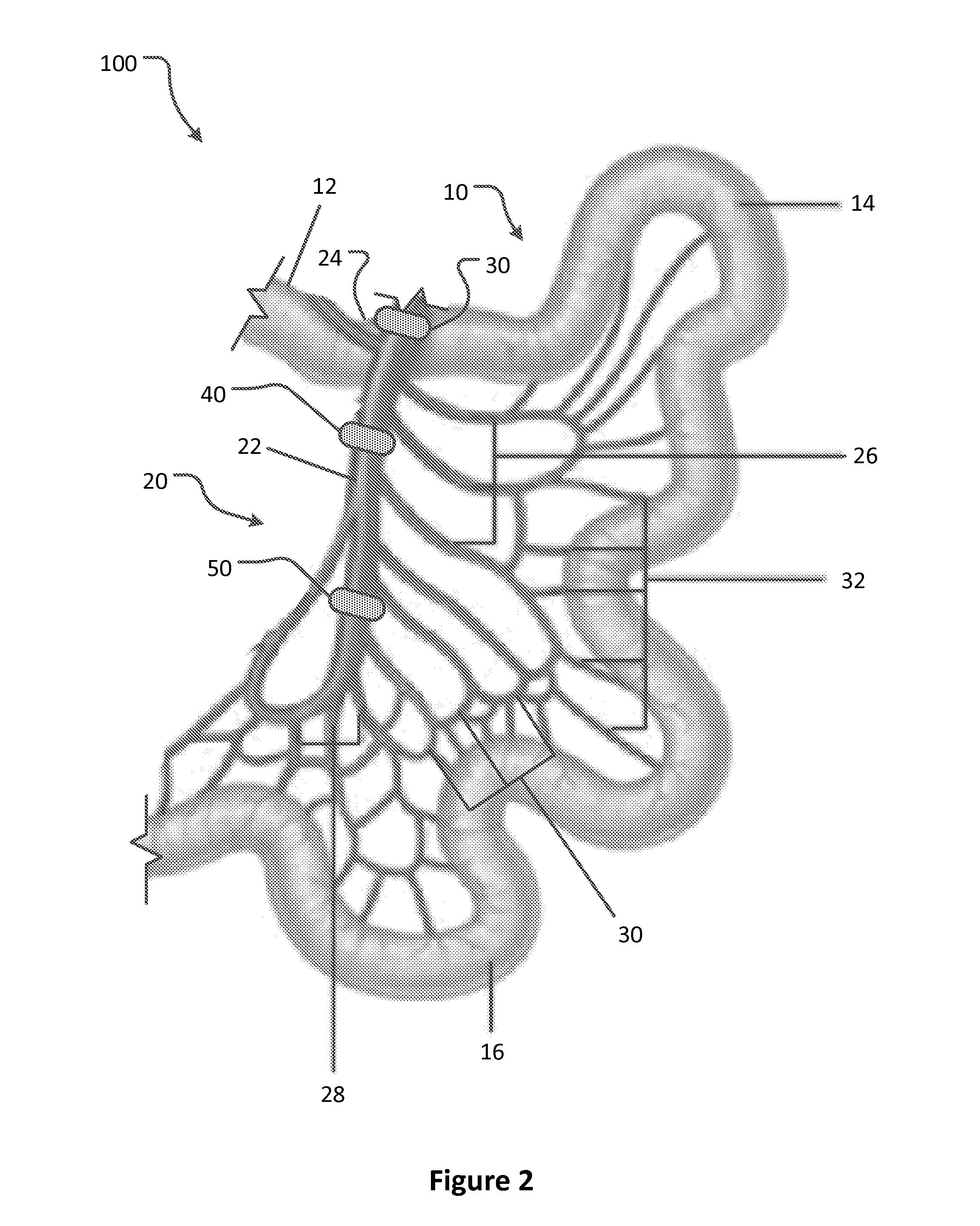
Apparatus and methods for treatment of morbid obesity
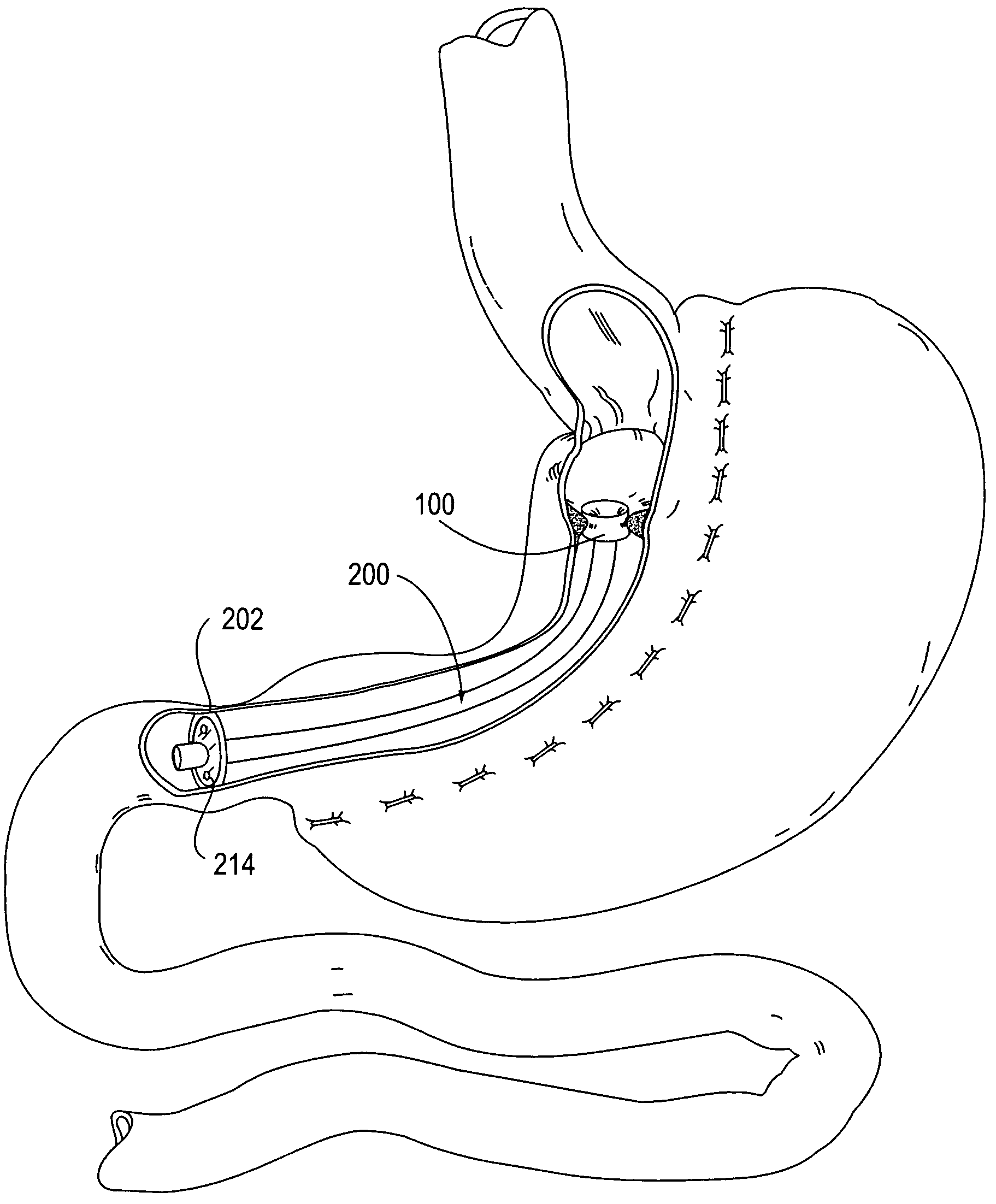
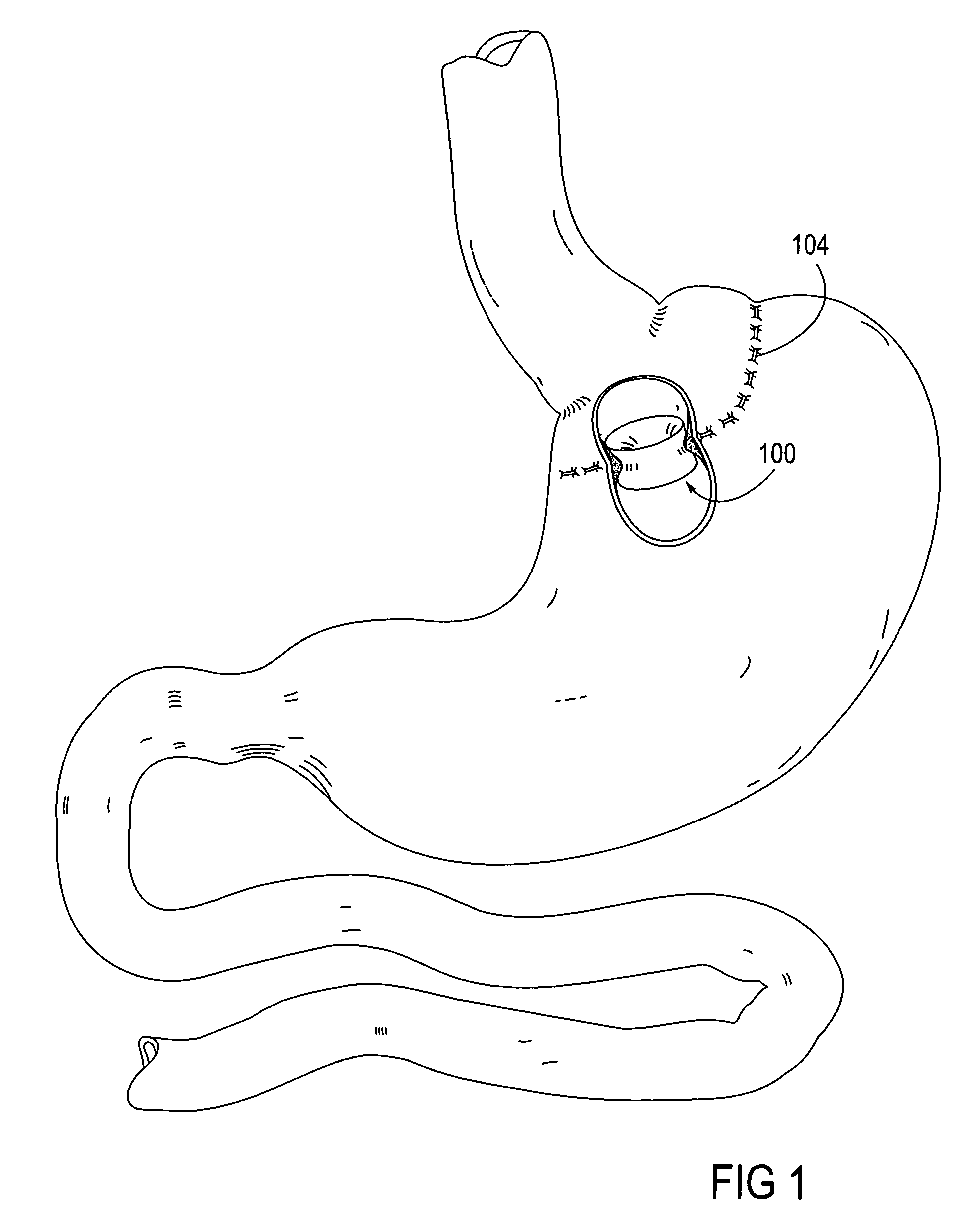
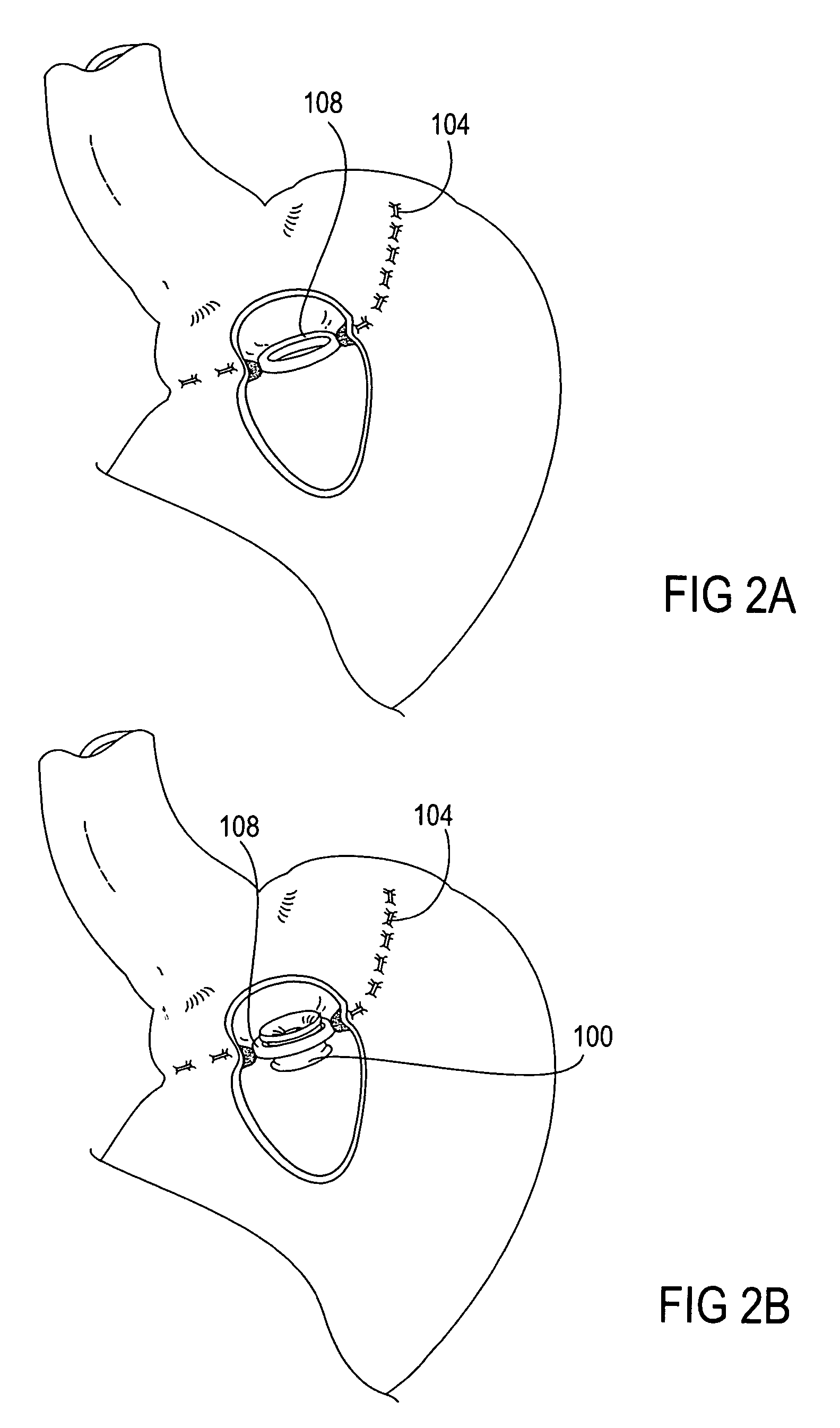
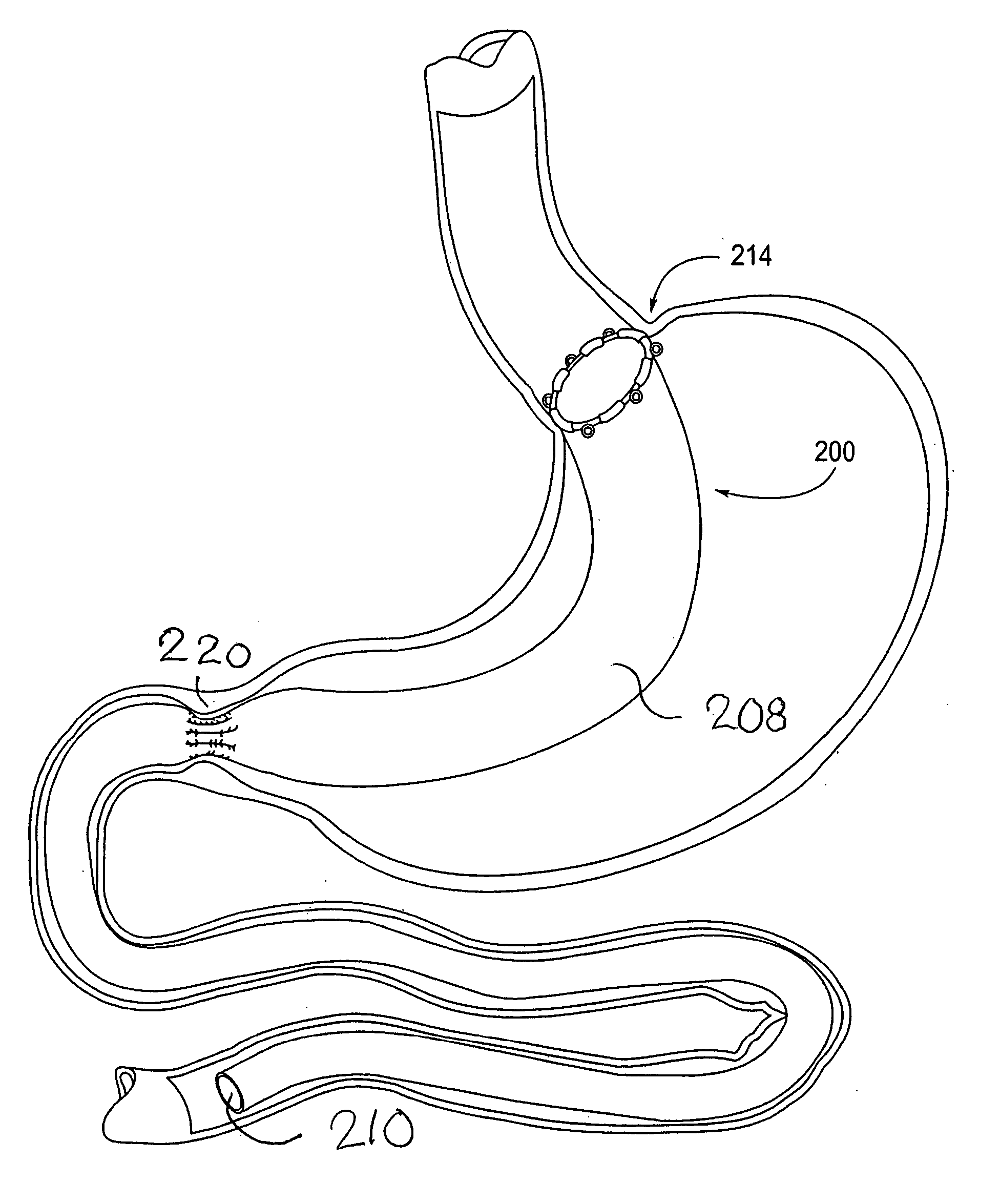
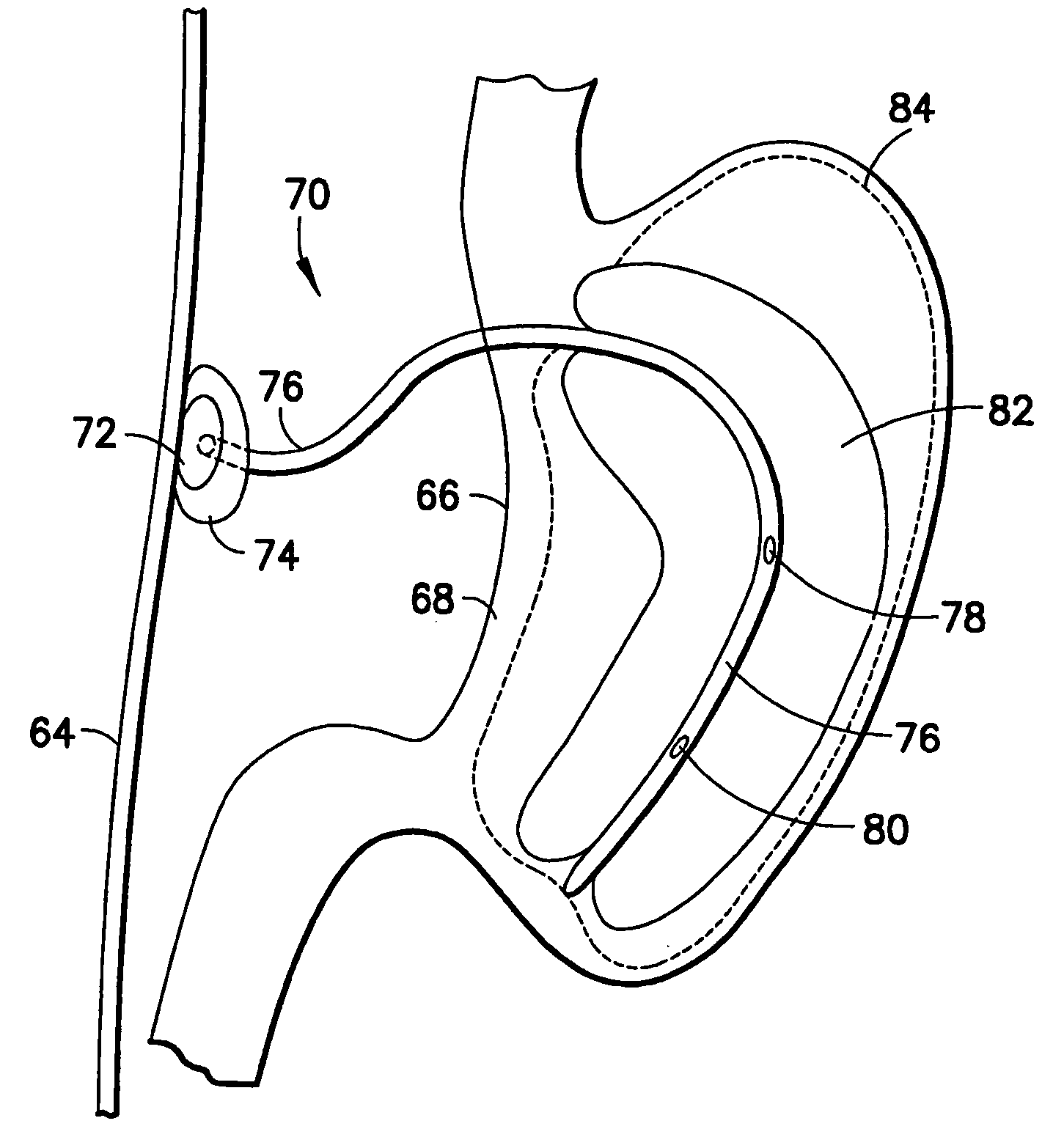
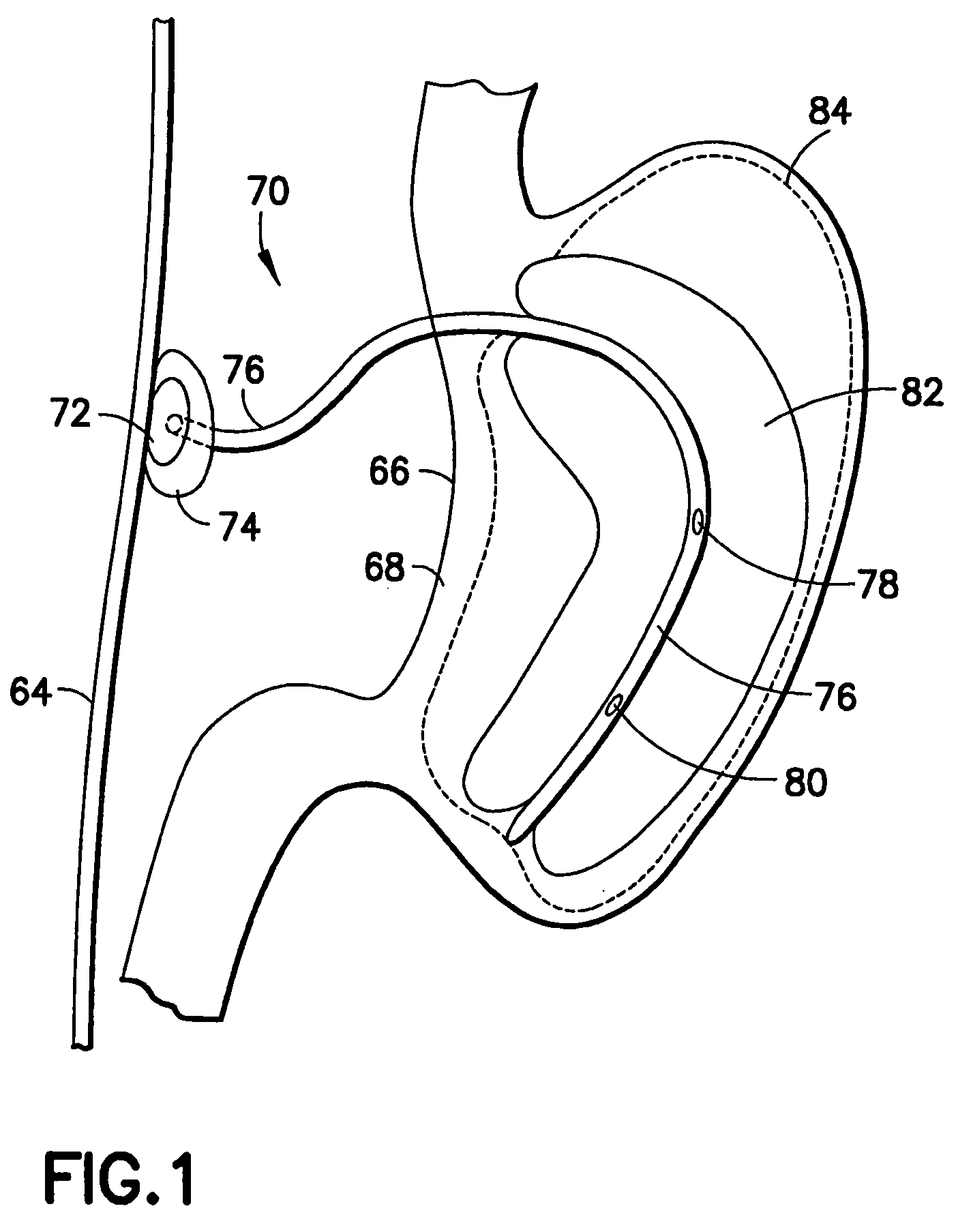
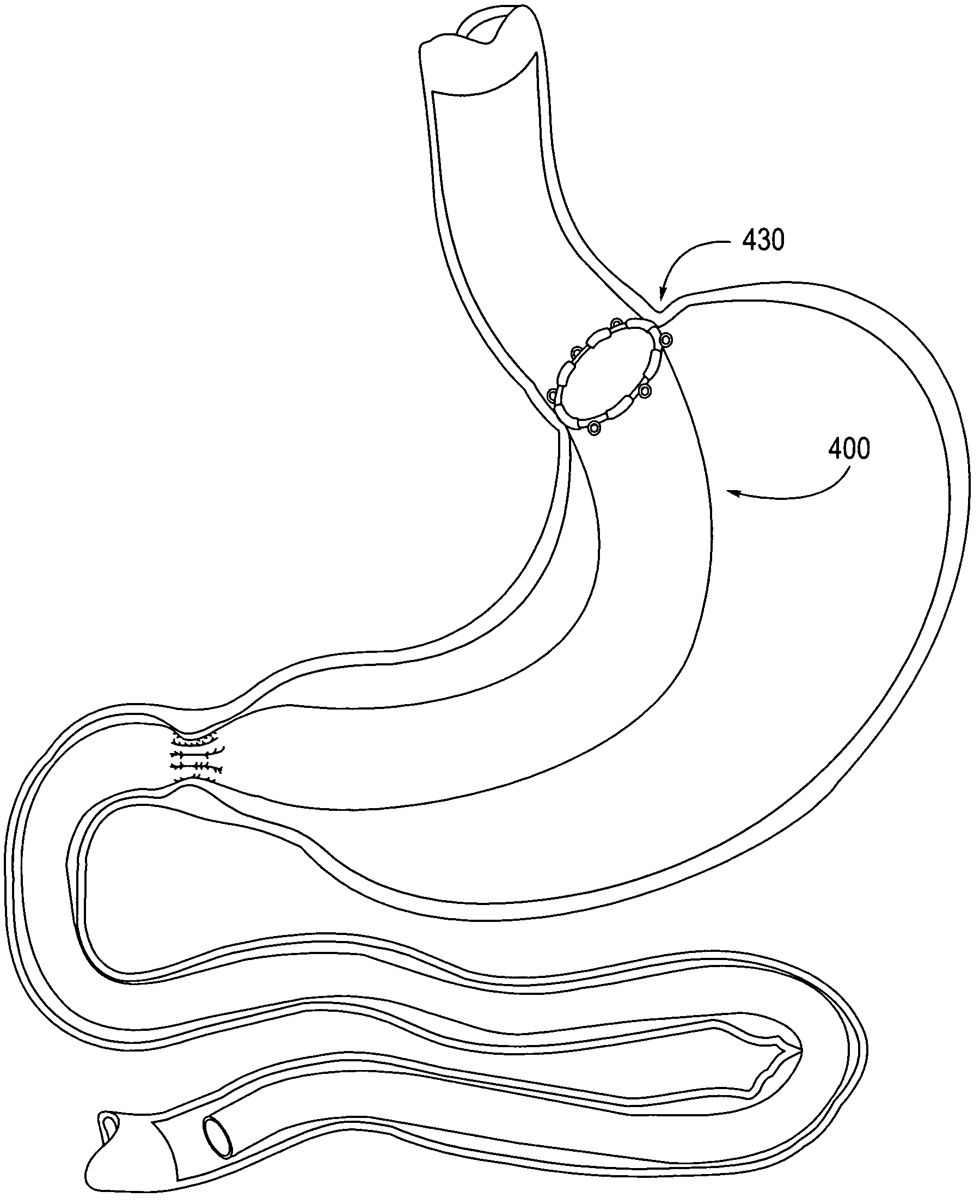
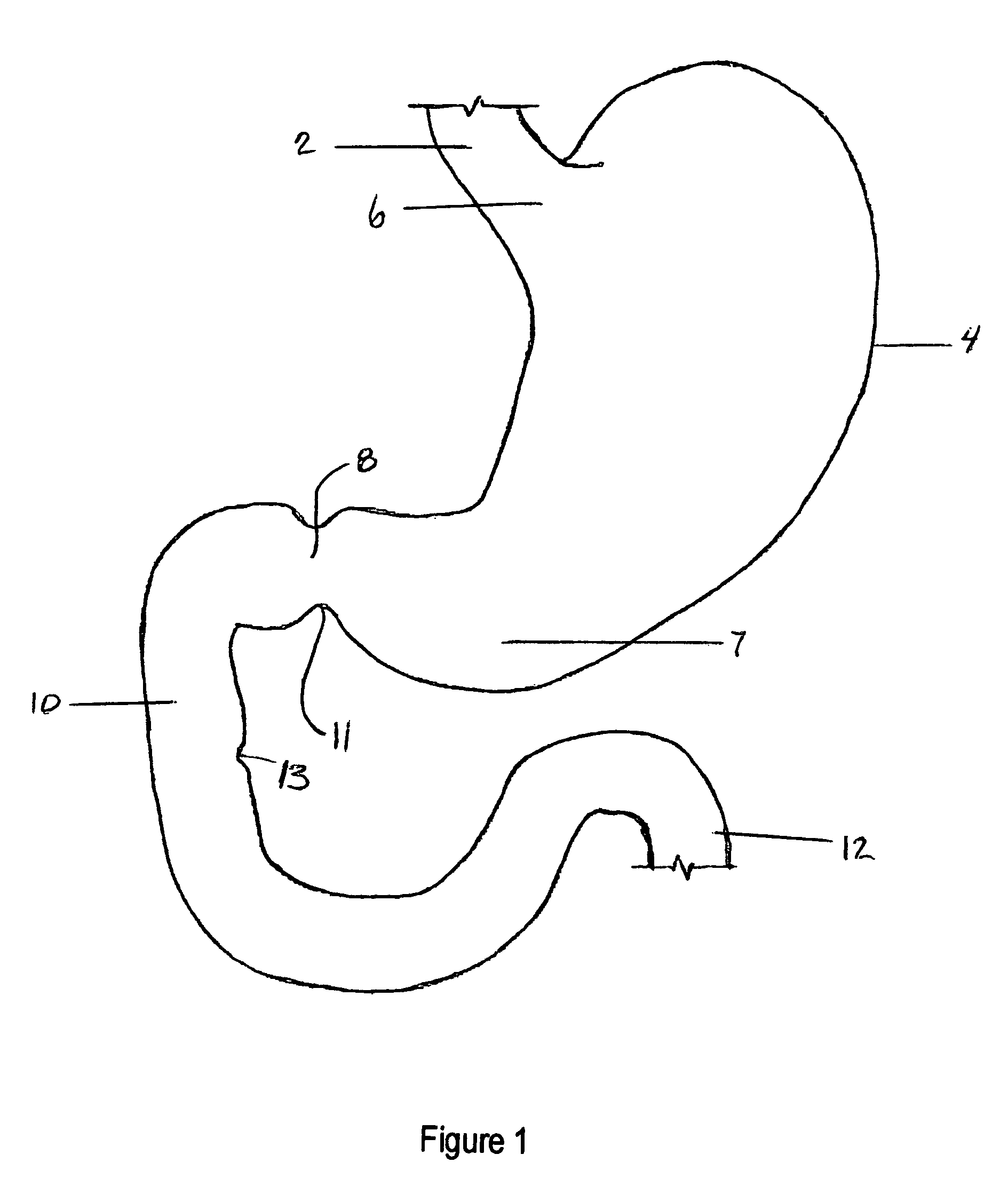
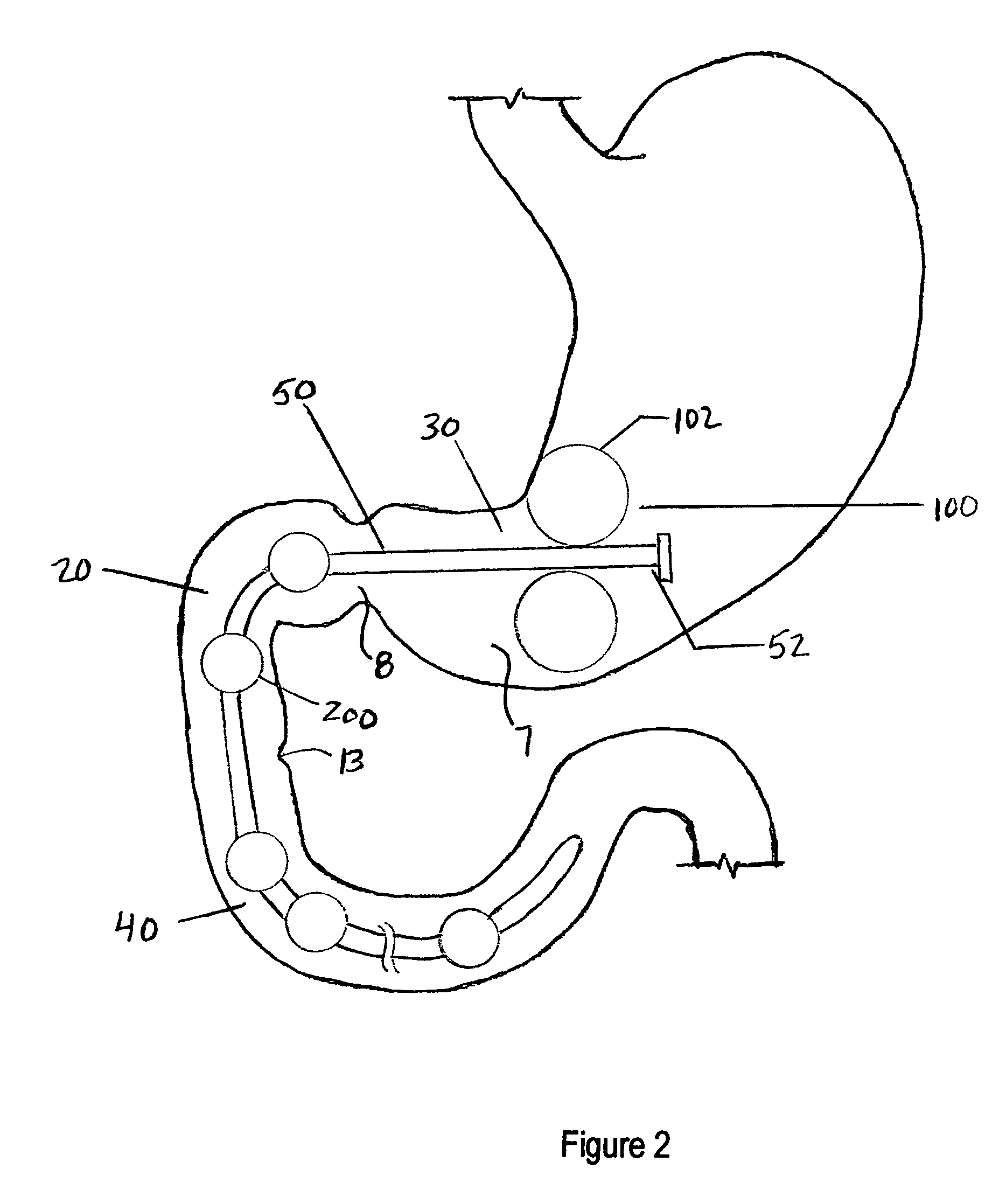
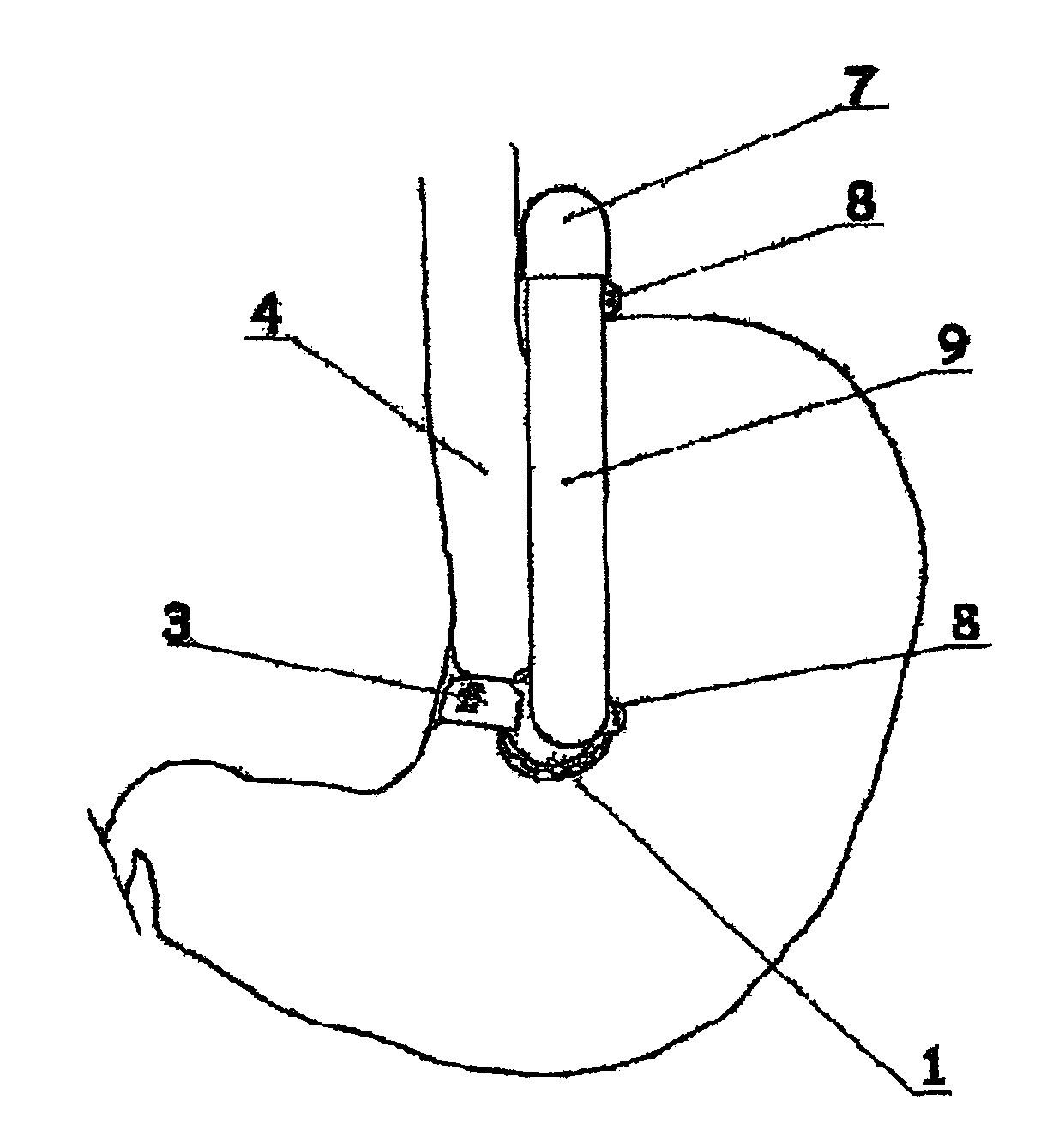
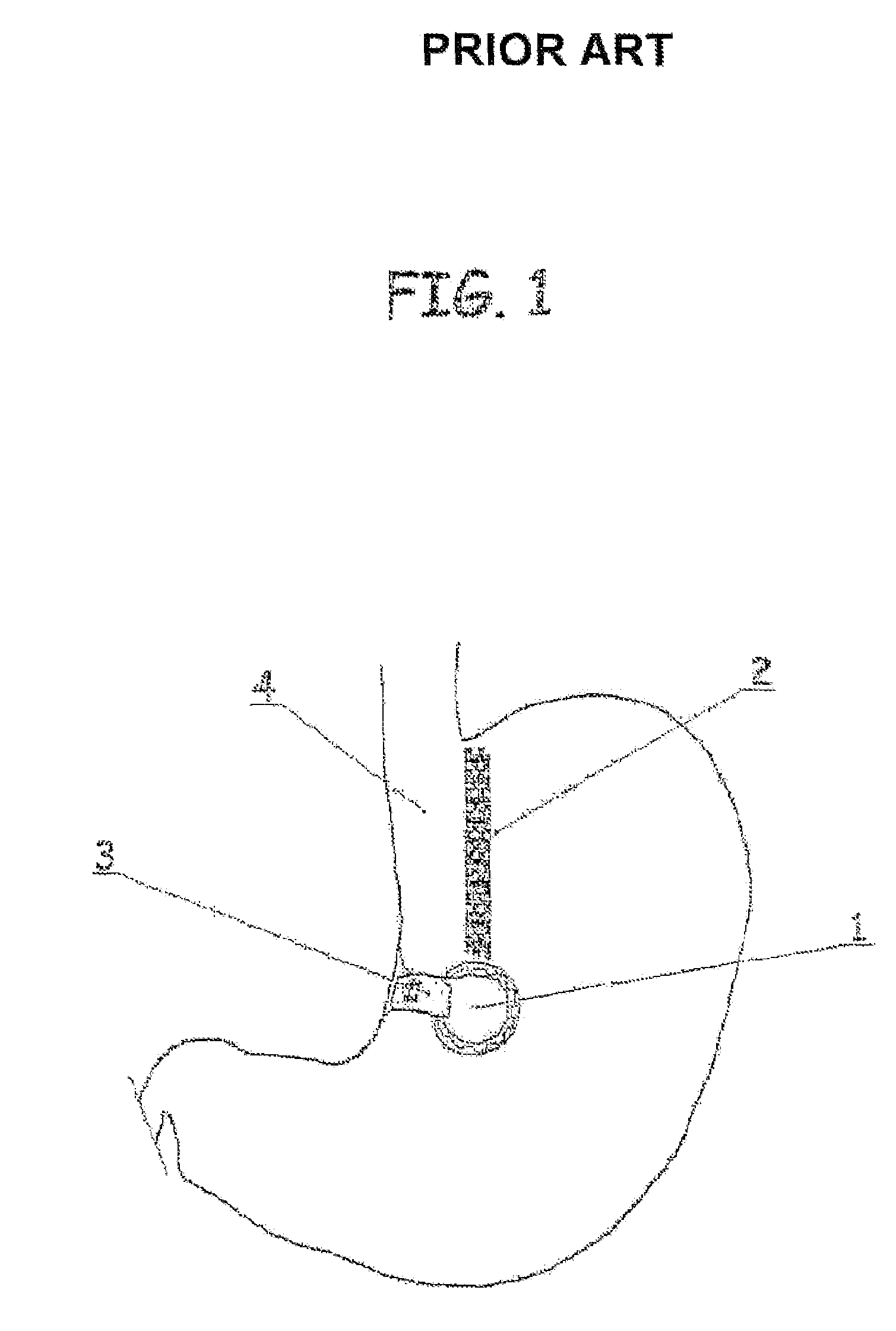
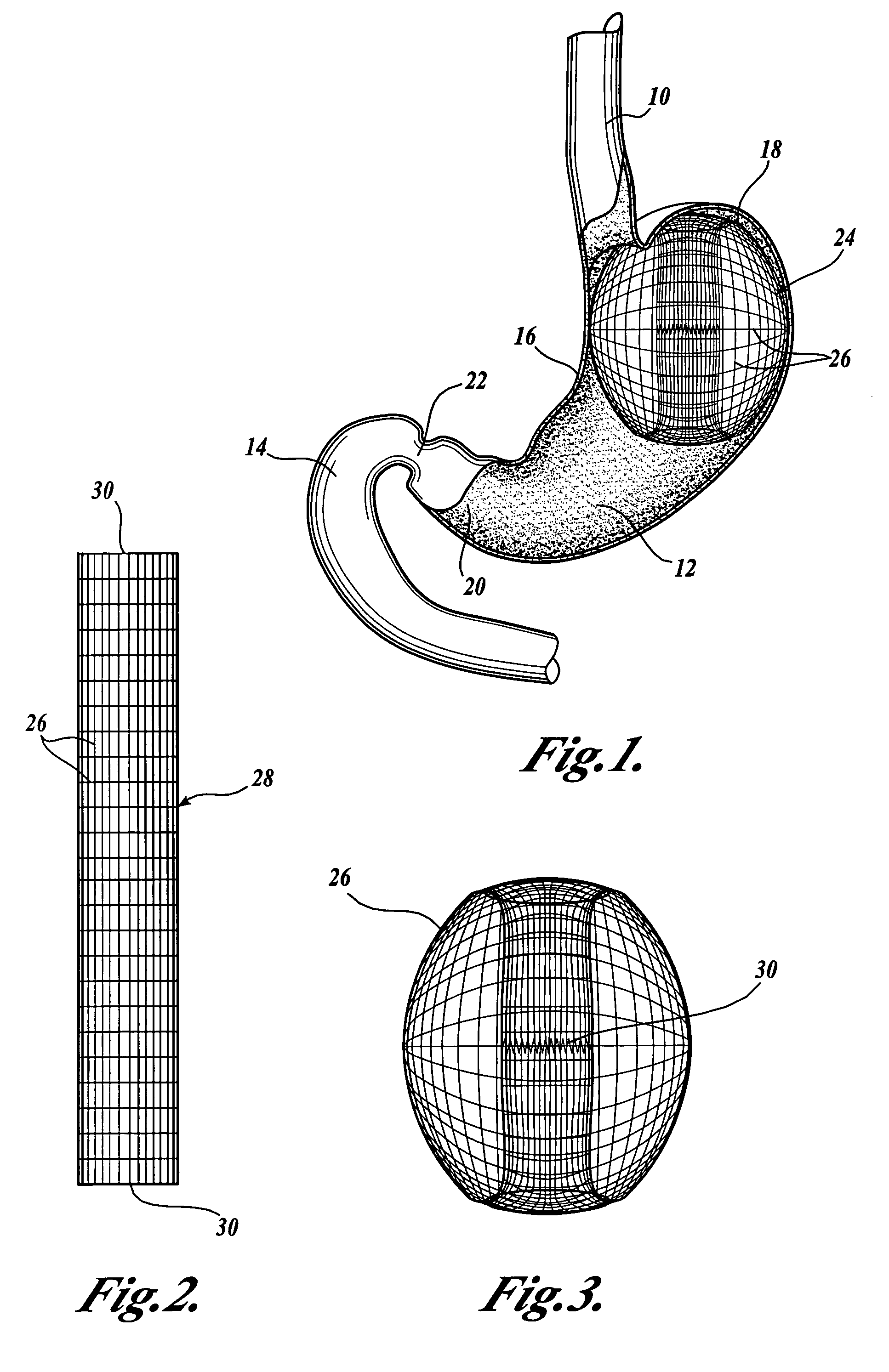
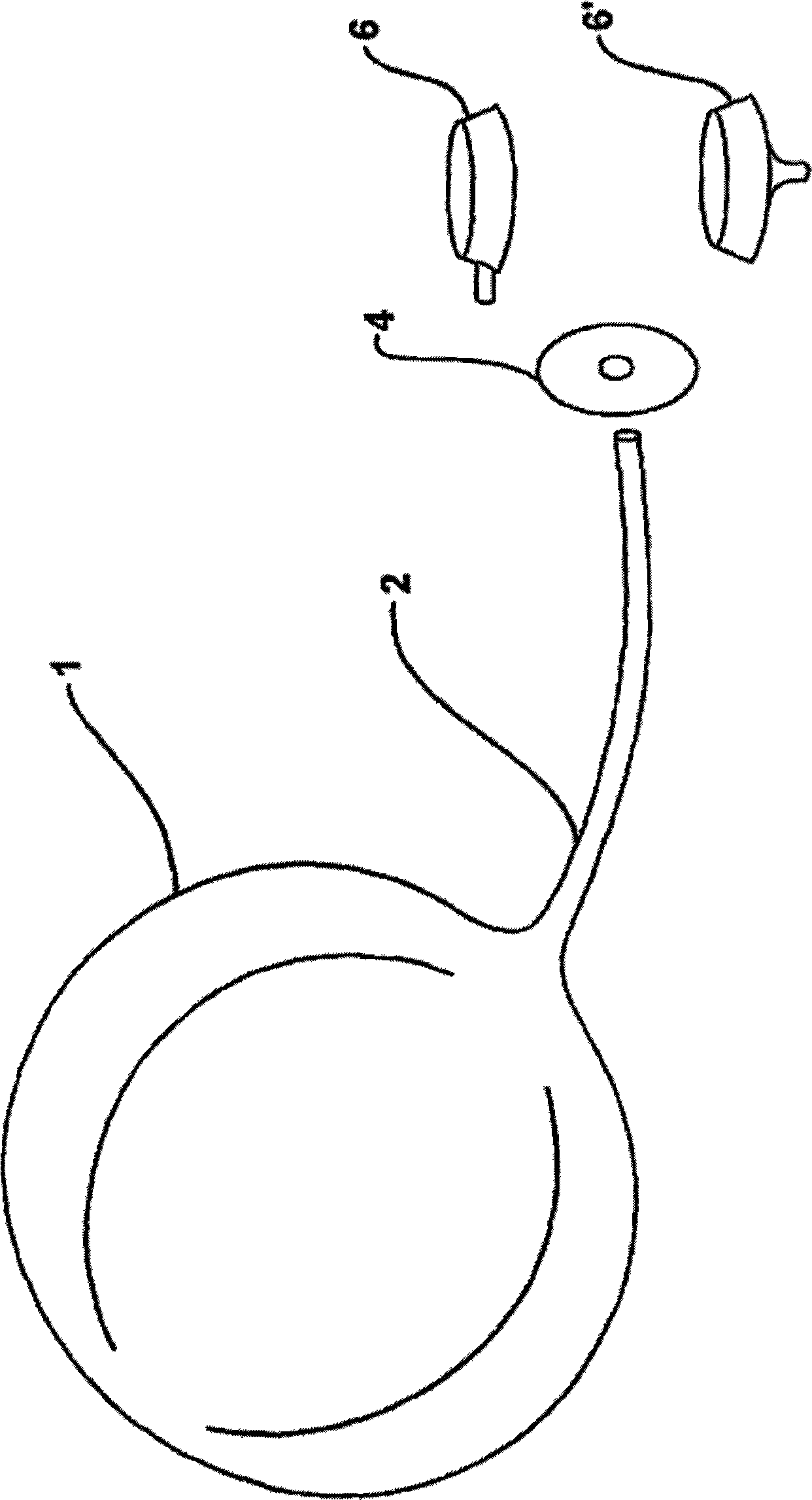
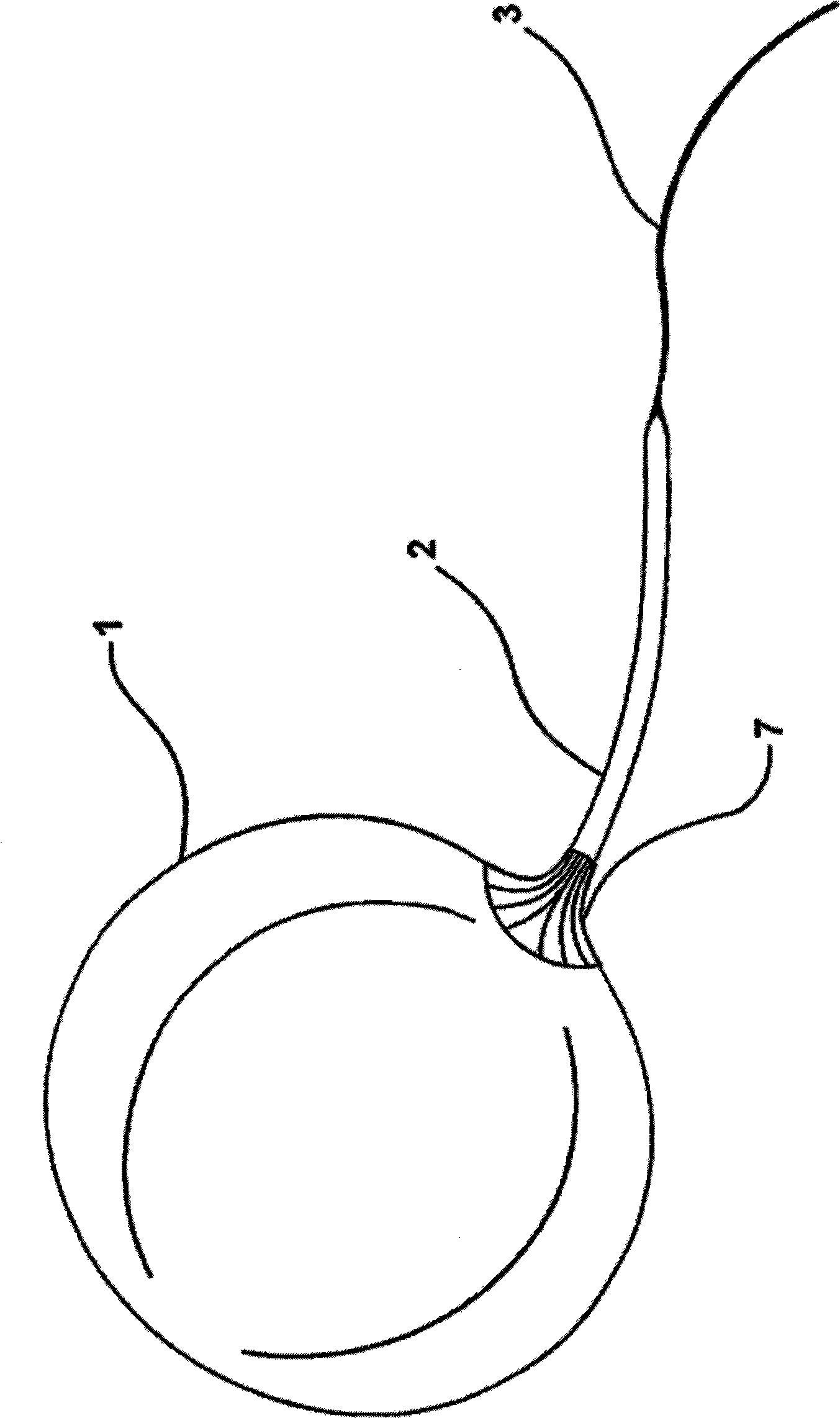
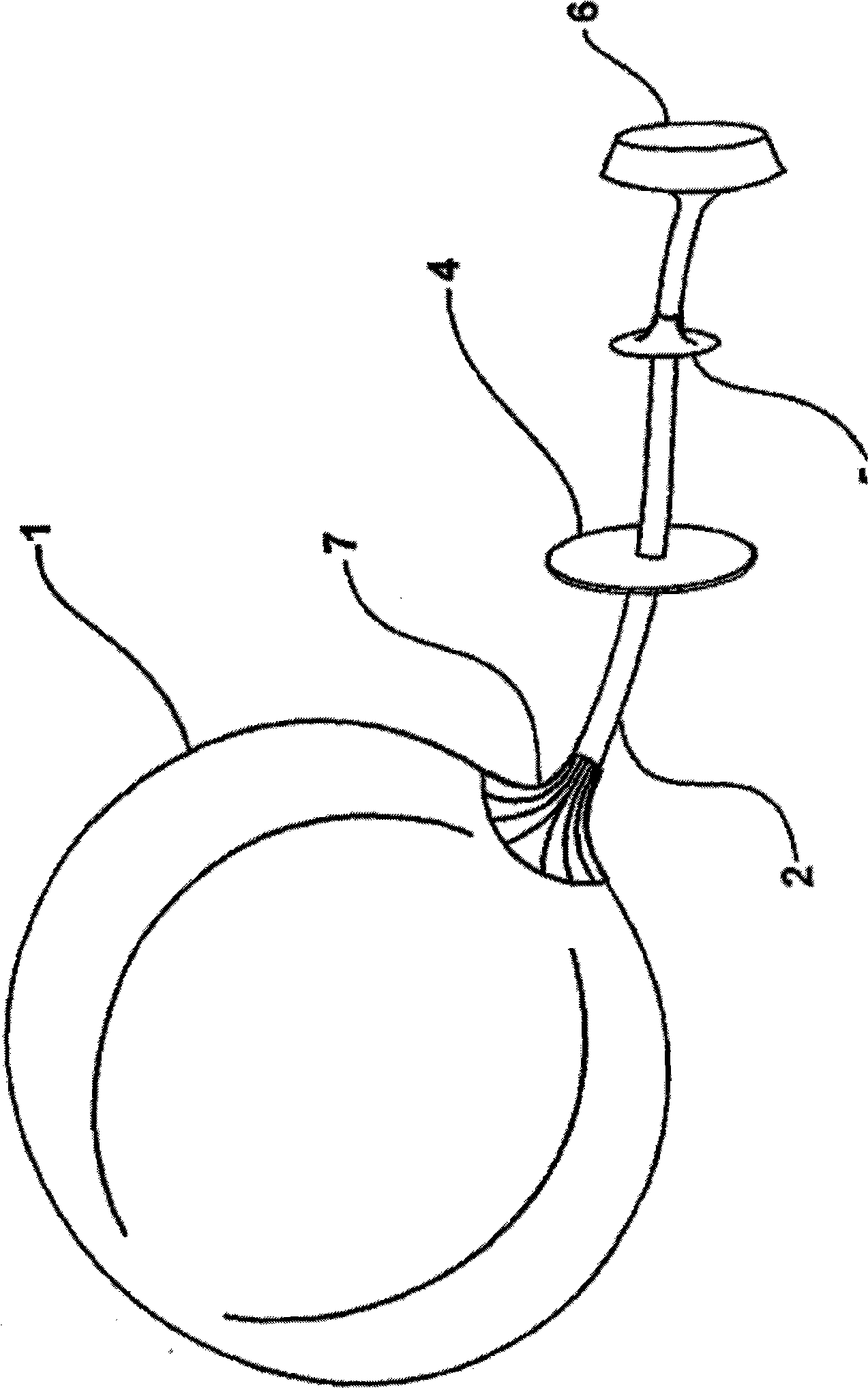
Apparatus and methods are described for treatment of morbid obesity using minimally invasive techniques. The apparatus includes a system of components that may be used separately or in combination for effectively reducing stomach volume, bypassing a portion of the stomach and / or small intestines, reducing nutrient absorption in the stomach and / or small intestines and / or depositing minimally or undigested food farther than normal into the intestines, thereby stimulating intestinal responses. The components described include an artificial stoma device, a gastric sleeve device, an intestinal sleeve device and a combined gastrointestinal sleeve device.
Owner:VALENTX
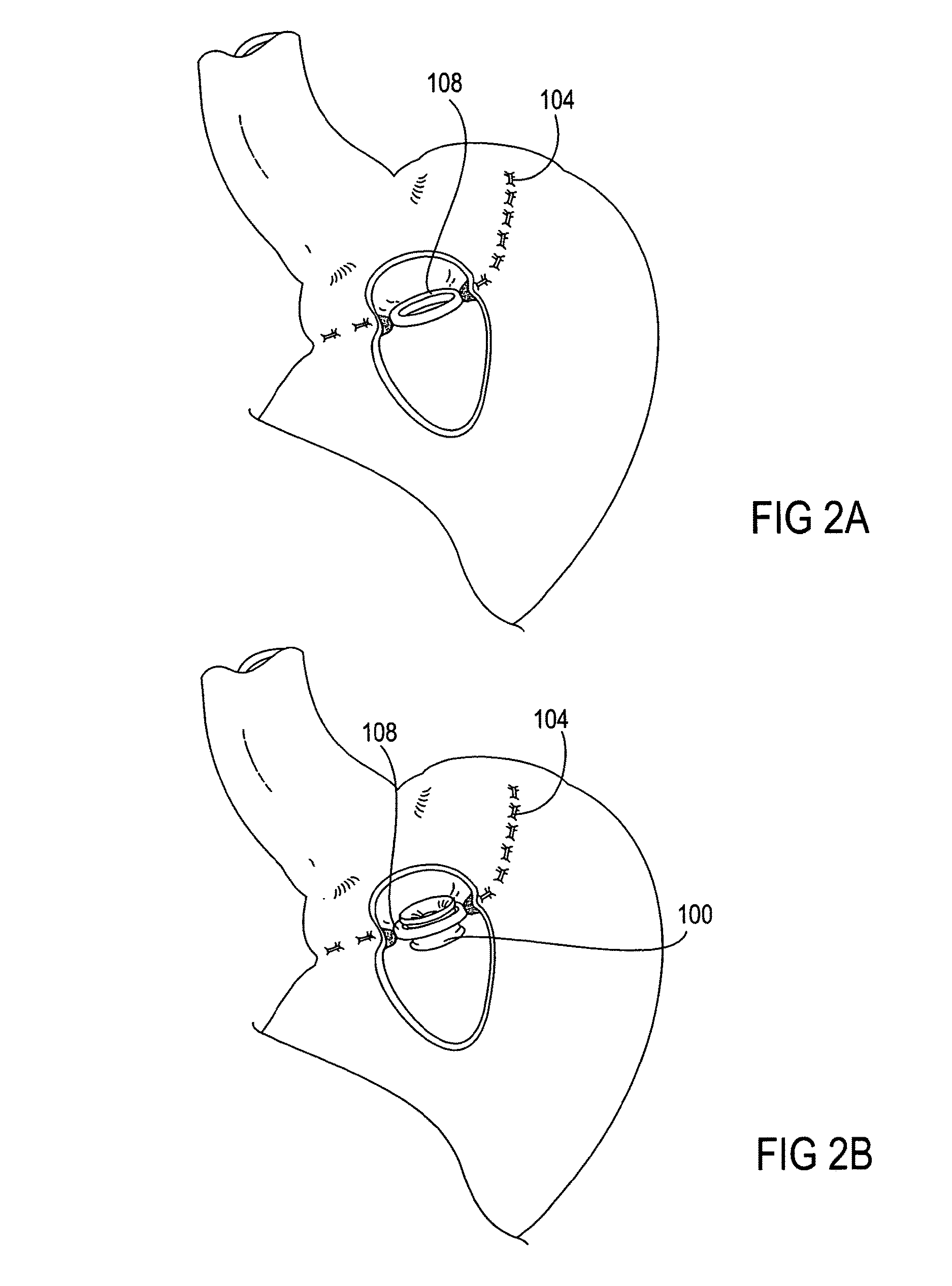
Devices and methods for treating morbid obesity
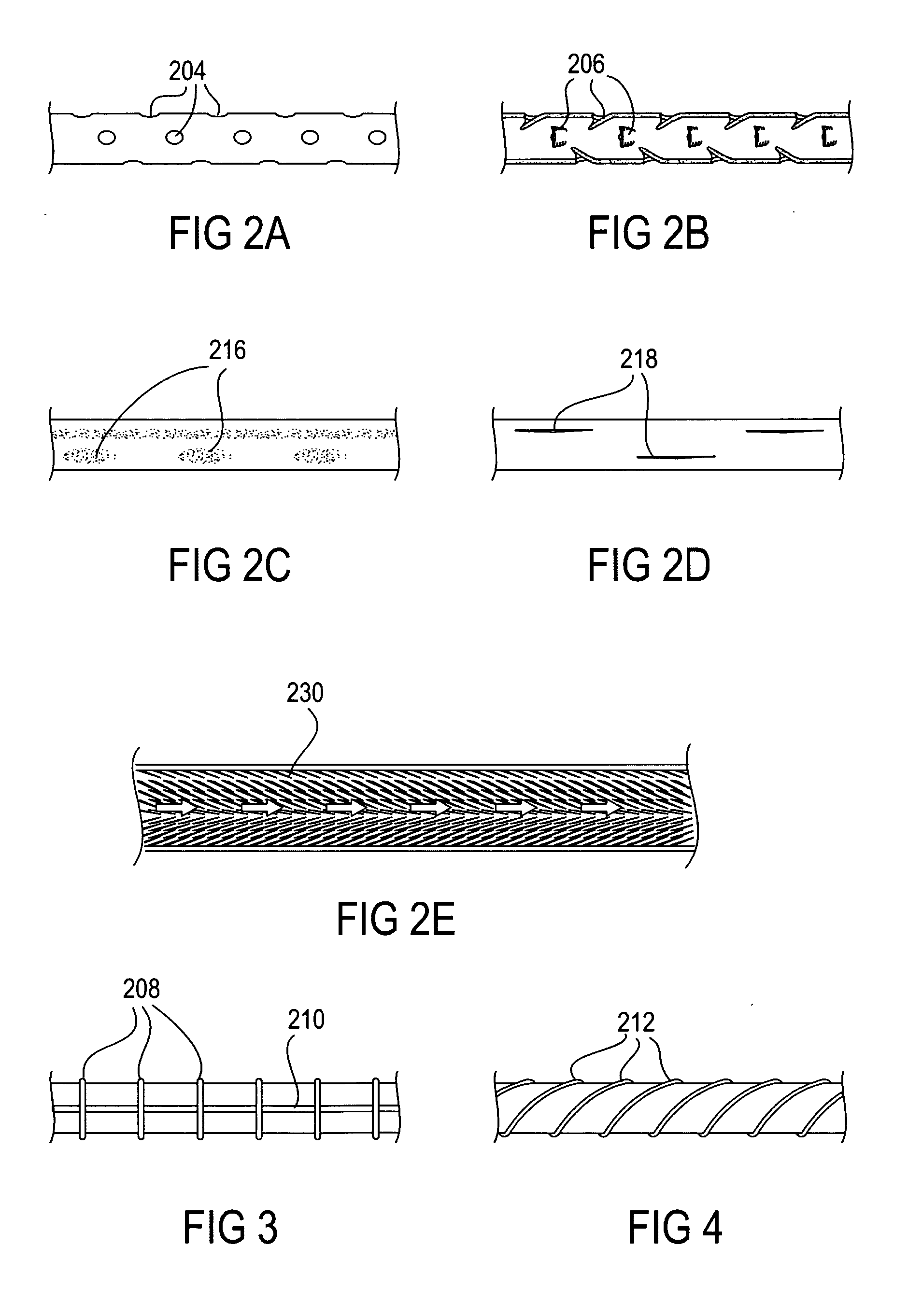
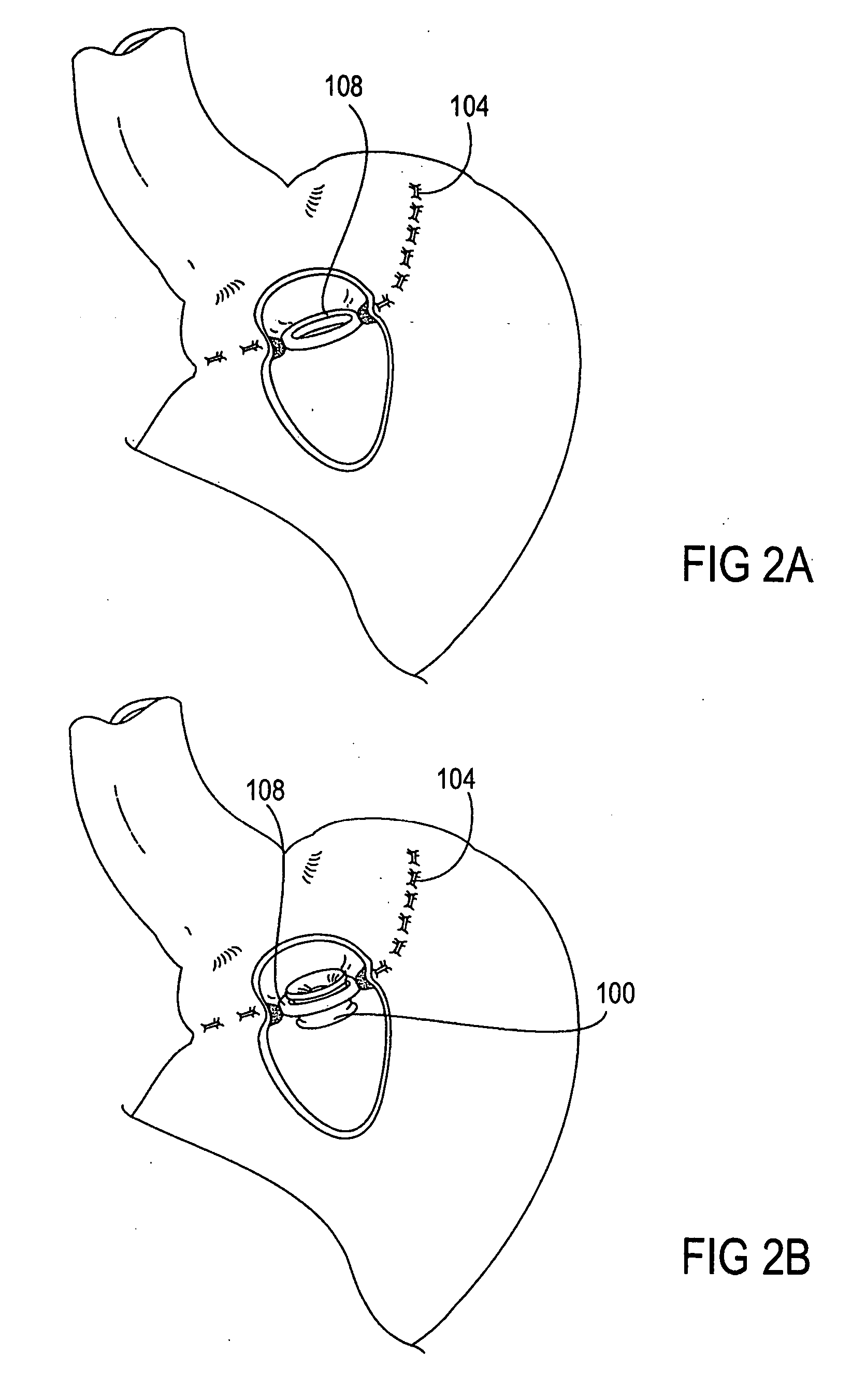
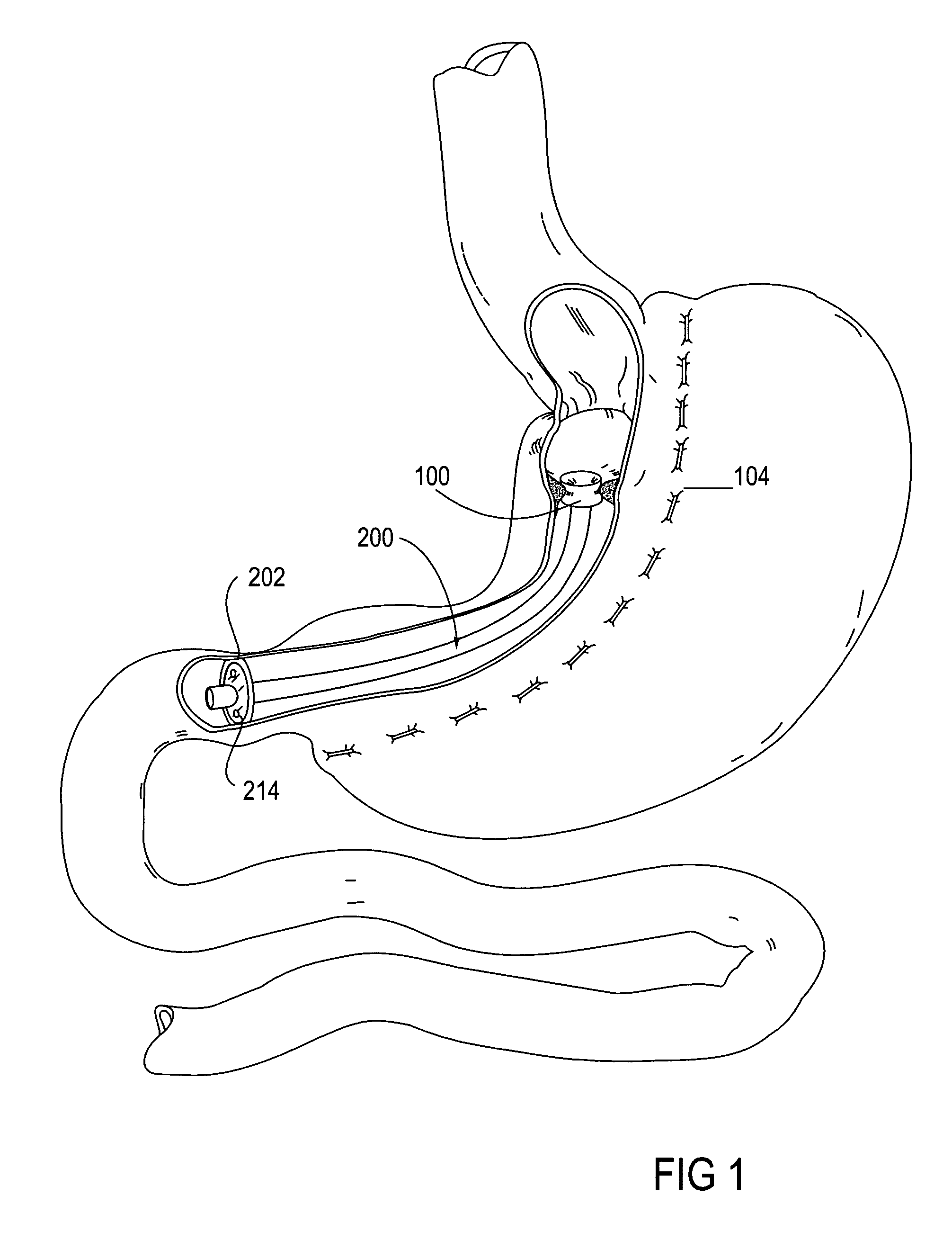
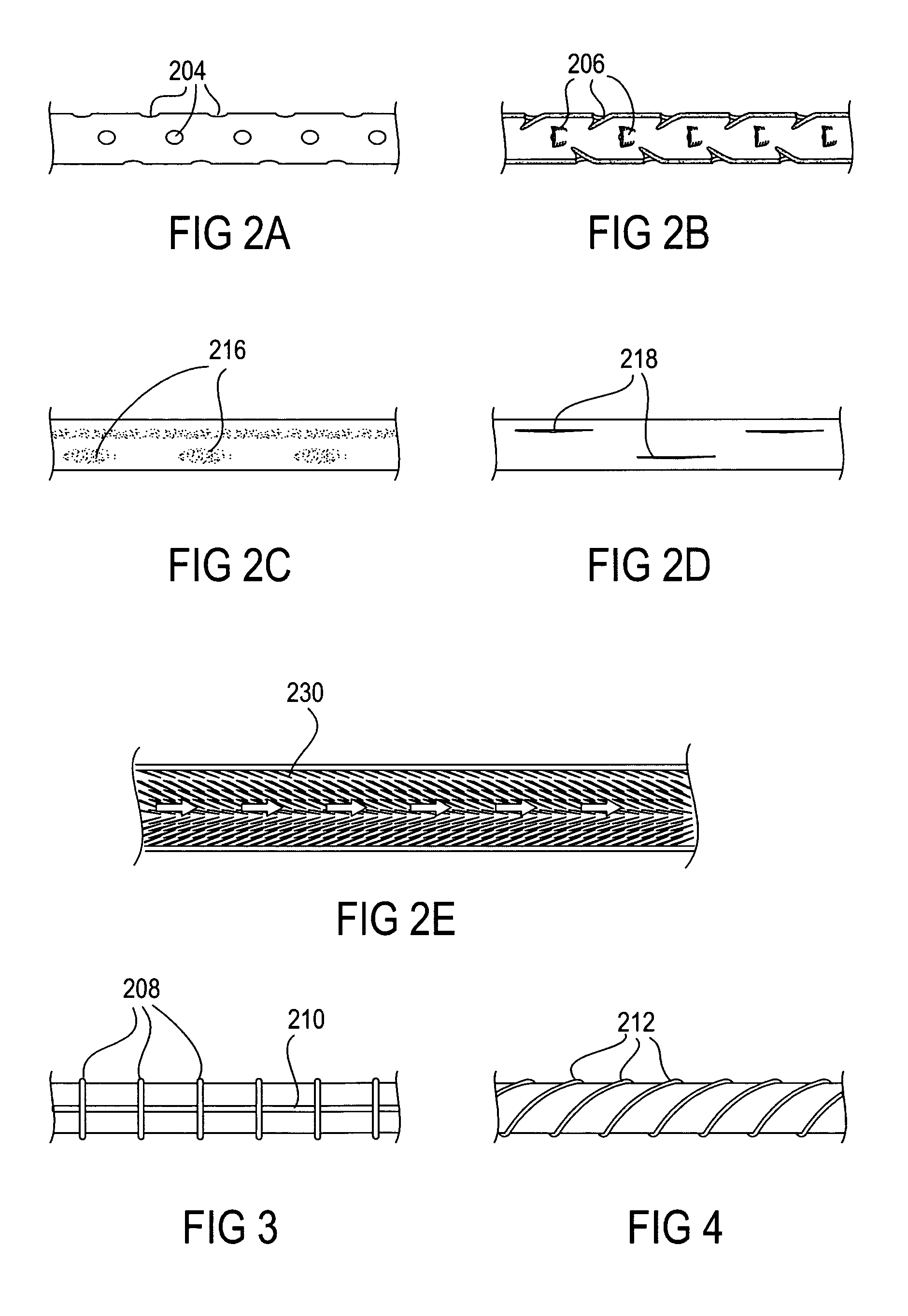
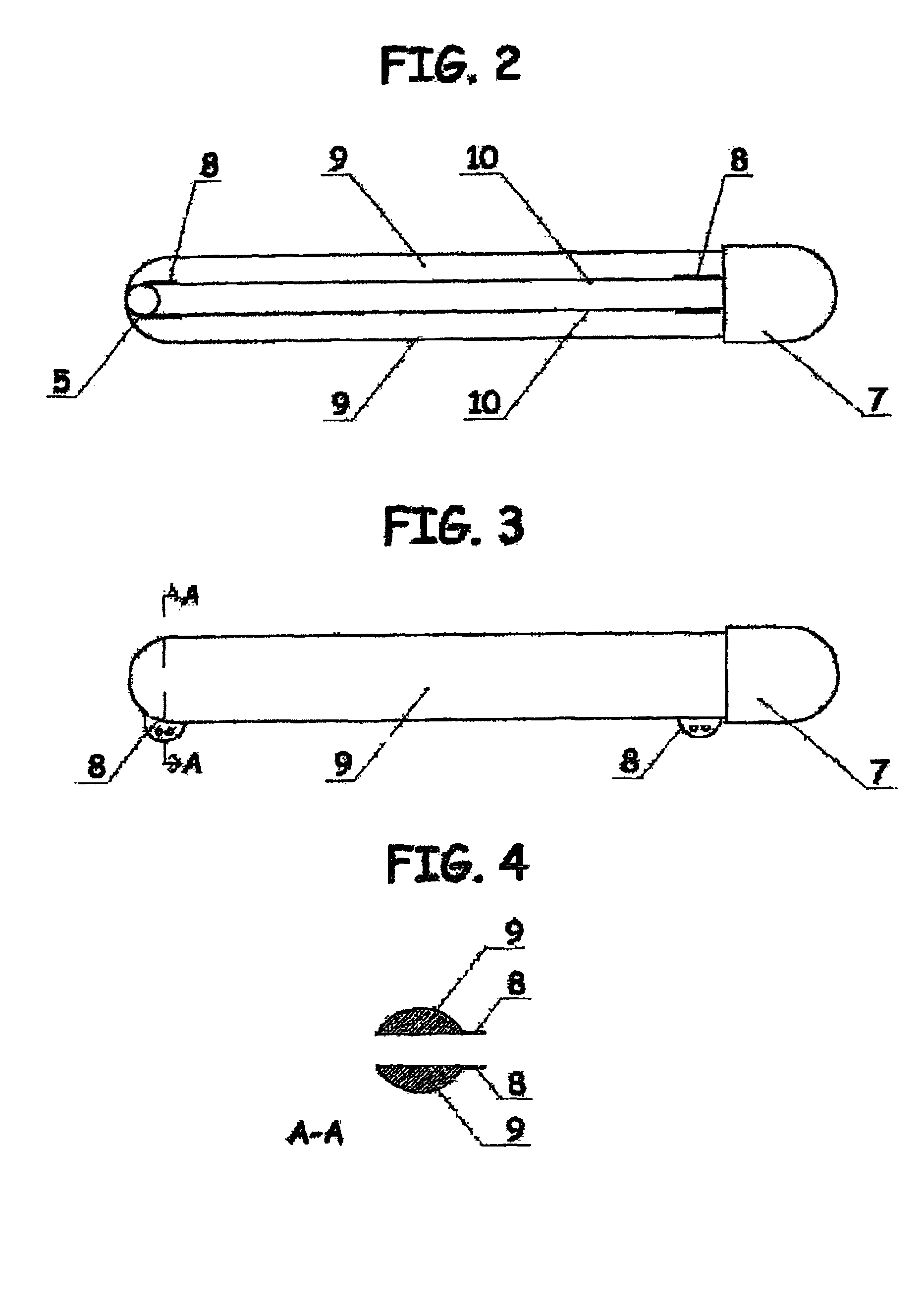
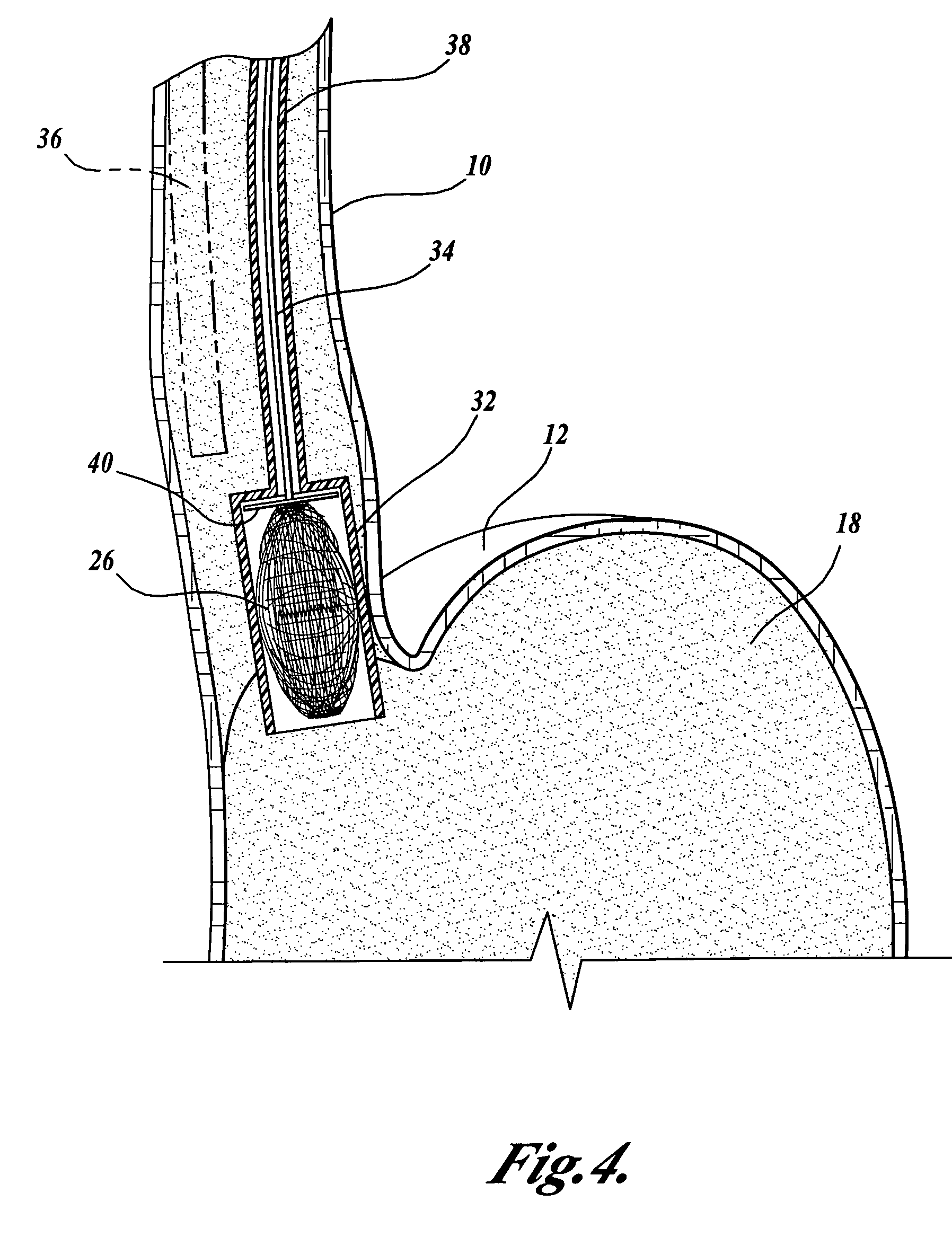
The present invention provides devices and methods for attachment of an implanted device, such as an artificial stoma device, a gastrointestinal sleeve device or an attachment cuff, within a patient's digestive tract for treatment of obesity. Special surgical fasteners provide a lasting and durable attachment to the gastrointestinal tissue without causing excessive pressure that could result in tissue erosion and detachment of the implanted device. Fastener delivery devices that facilitate peroral placement and deployment of fasteners and secondary devices are also provided. Also described are implantable devices and attachment means that avoid causing excessive pressure within the tissue by having compliance that is compatible with the gastrointestinal tissues where it is attached.
Owner:VALENTX
Devices and methods for treating morbid obesity
The present invention provides devices and methods for attachment of an implanted device, such as an artificial stoma device, a gastrointestinal sleeve device or an attachment cuff, within a patient's digestive tract for treatment of obesity. Special surgical fasteners provide a lasting and durable attachment to the gastrointestinal tissue without causing excessive pressure that could result in tissue erosion and detachment of the implanted device. Fastener delivery devices that facilitate peroral placement and deployment of fasteners and secondary devices are also provided. Also described are implantable devices and attachment means that avoid causing excessive pressure within the tissue by having compliance that is compatible with the gastrointestinal tissues where it is attached.
Owner:VALENTX
Gastrointestinal sleeve device and methods for treatment of morbid obesity
InactiveUS20050049718A1Effectively reducing stomach volumeStimulating intestinal responseMedical devicesTubular organ implantsIntestinal structureMorbid obesity
Apparatus and methods are described for treatment of morbid obesity using minimally invasive techniques. The apparatus includes a system of components that may be used separately or in combination for effectively reducing stomach volume, bypassing a portion of the stomach and / or small intestines, reducing nutrient absorption in the stomach and / or small intestines and / or depositing minimally or undigested food farther than normal into the intestines, thereby stimulating intestinal responses. The components described include a gastric sleeve device, an intestinal sleeve device, and a combined gastrointestinal sleeve device.
Owner:VALENTX
Sensor based gastrointestinal electrical stimulation for the treatment of obesity or motility disorders
InactiveUS20050222638A1Reduce riskAvoid stimulationElectrotherapyArtificial respirationGastroparesisMotility
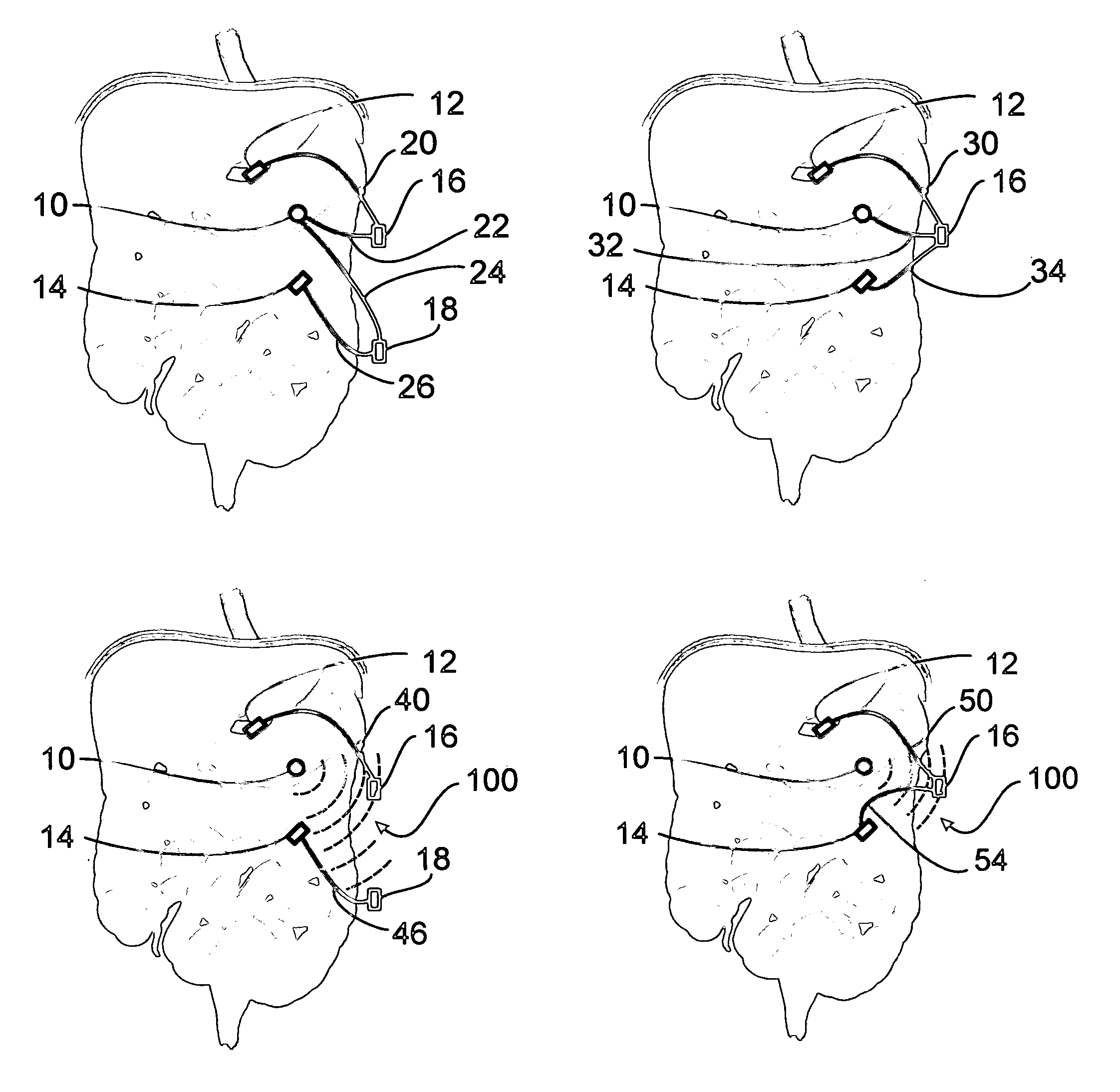
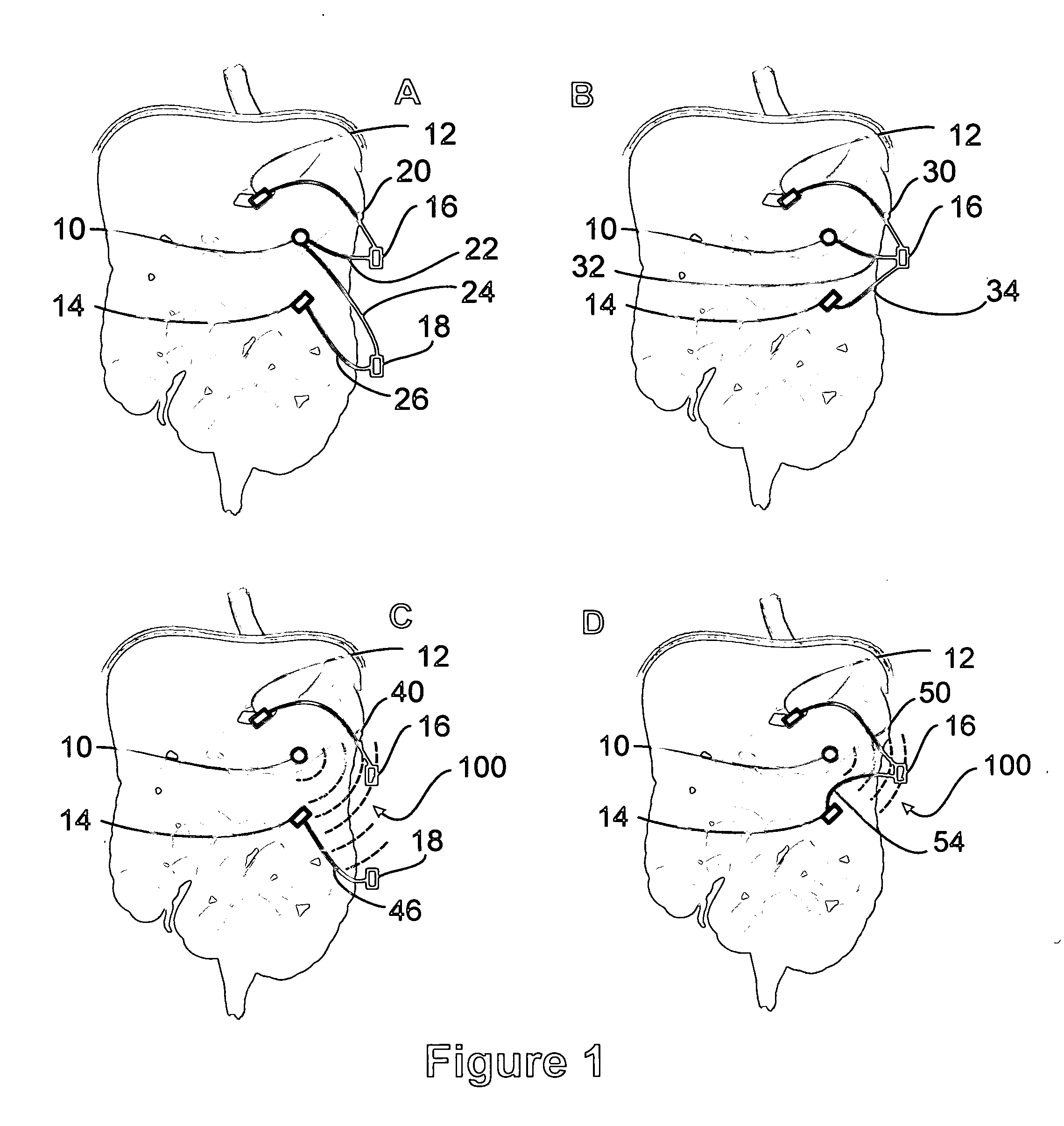
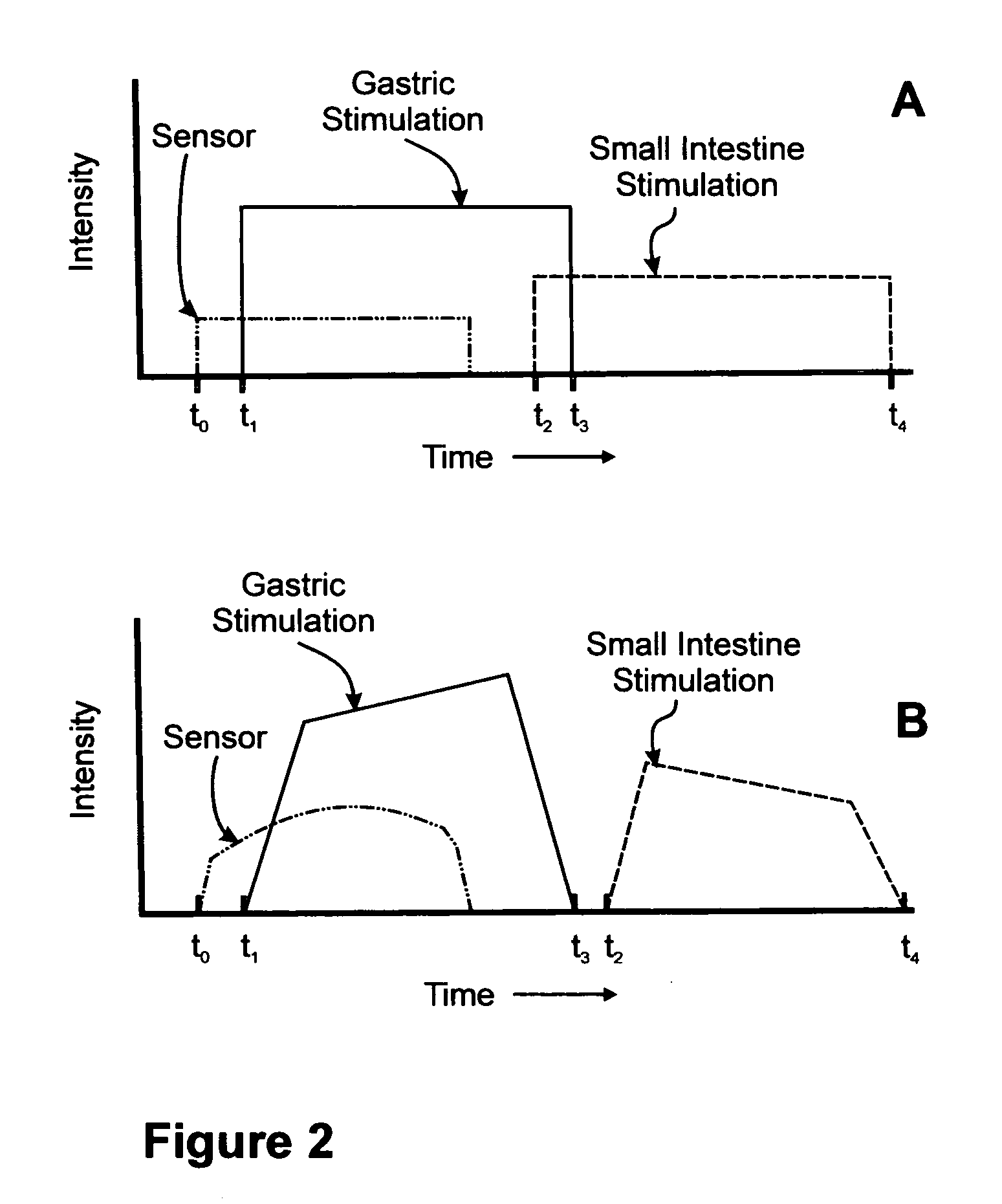
A method for treatment of obesity, especially morbid obesity, gastroparesis and other syndromes related to motor disorders of the stomach. The method of this invention utilizes a sensor to detect food entering the patient's stomach, thereby the sensor communicates with and activates at least one electrical stimulation device attached to either the stomach or the small intestine.
Owner:MEDTRONIC TRANSNEURONIX
Gastro-occlusive device
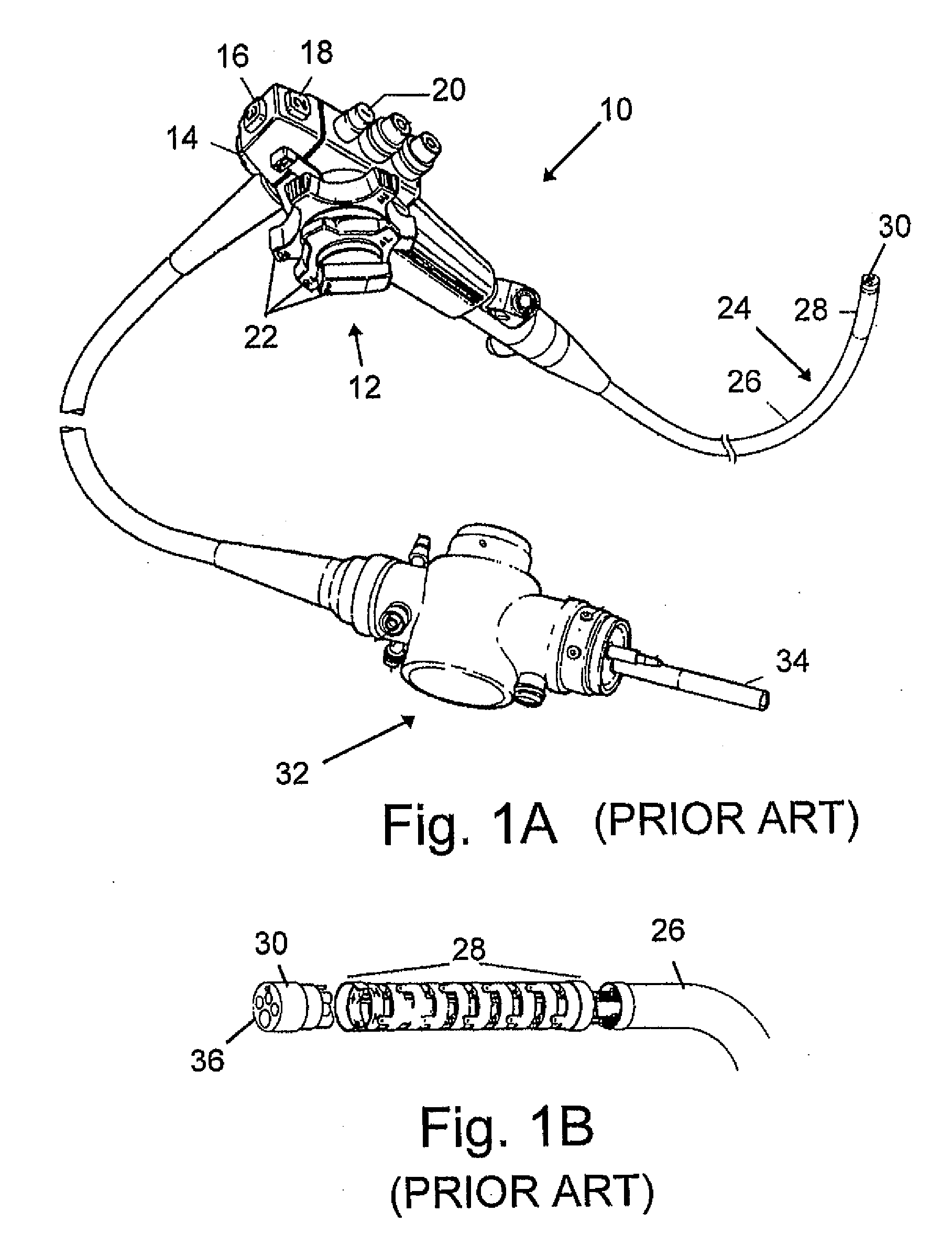
A gastro-occlusive device, comprising a balloon disposable in a stomach cavity of a patient, and inflatable therein to occlude a portion of the volume of the stomach cavity, a gas flow tube coupled at a distal end thereof with the balloon and extending outwardly through a stomach wall for coupling with a gas source for selectively inflating the balloon, to occlude at least a portion of the volume of the stomach cavity. The gastro-occlusive device may be employed in combination with a feeding tube unit, a drain unit or other ancillary apparatus, and is useful for treatment of morbid obesity and various eating disorders.
Owner:POLYZEN INC
Gastrointestinal sleeve device and methods for treatment of morbid obesity
InactiveUS20050240279A1Effectively reducing stomach volumeStimulating intestinal responseTubular organ implantsNon-surgical orthopedic devicesIntestinal structureStoma
Apparatus and methods are described for treatment of morbid obesity using minimally invasive techniques. The apparatus includes a system of components that may be used separately or in combination for effectively reducing stomach volume, bypassing a portion of the stomach and / or small intestines, reducing nutrient absorption in the stomach and / or small intestines and / or depositing minimally or undigested food farther than normal into the intestines, thereby stimulating intestinal responses. The components described include an artificial stoma device, a gastric sleeve device, an intestinal sleeve device and a combined gastrointestinal sleeve device.
Owner:VALENTX
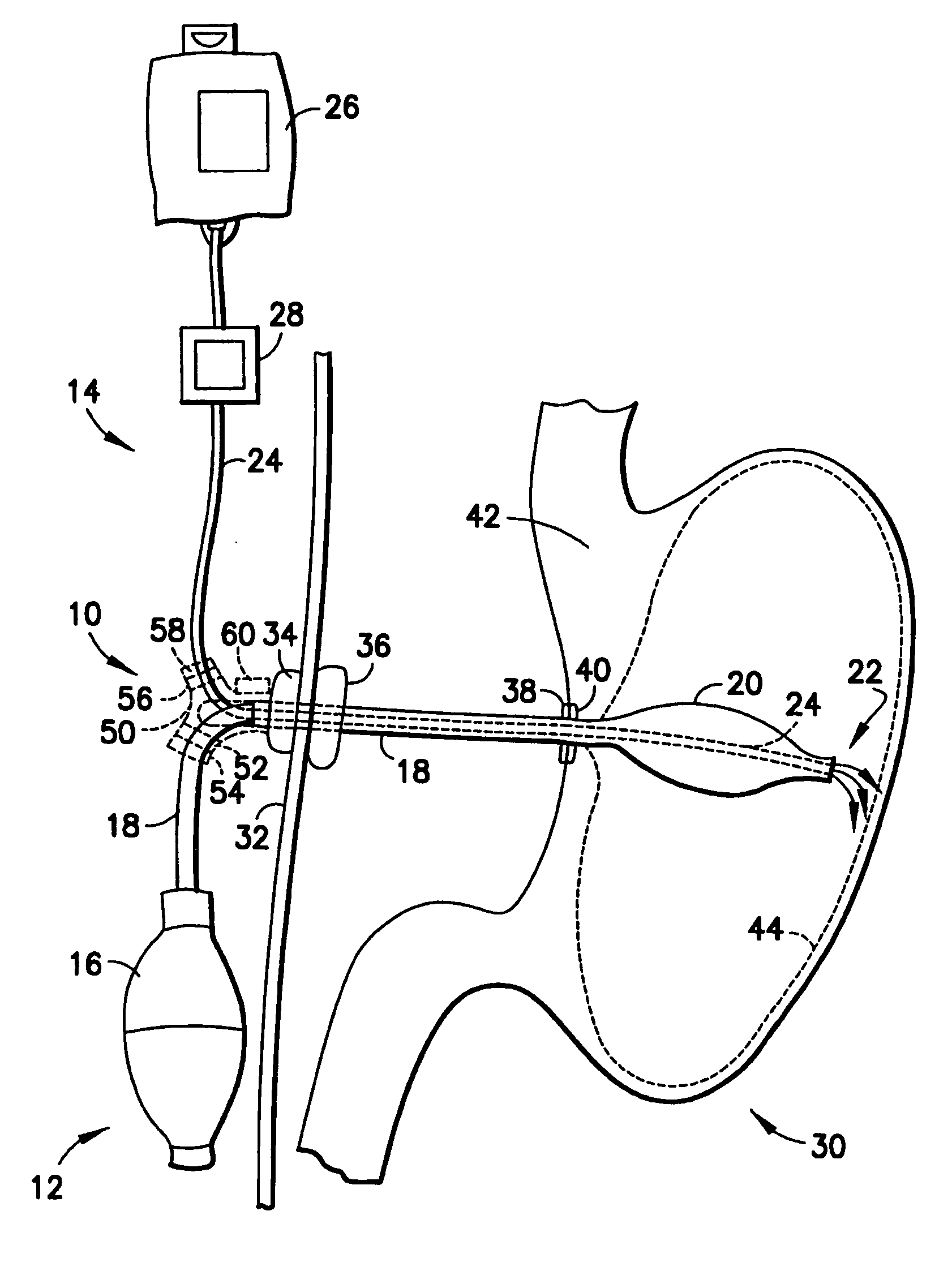
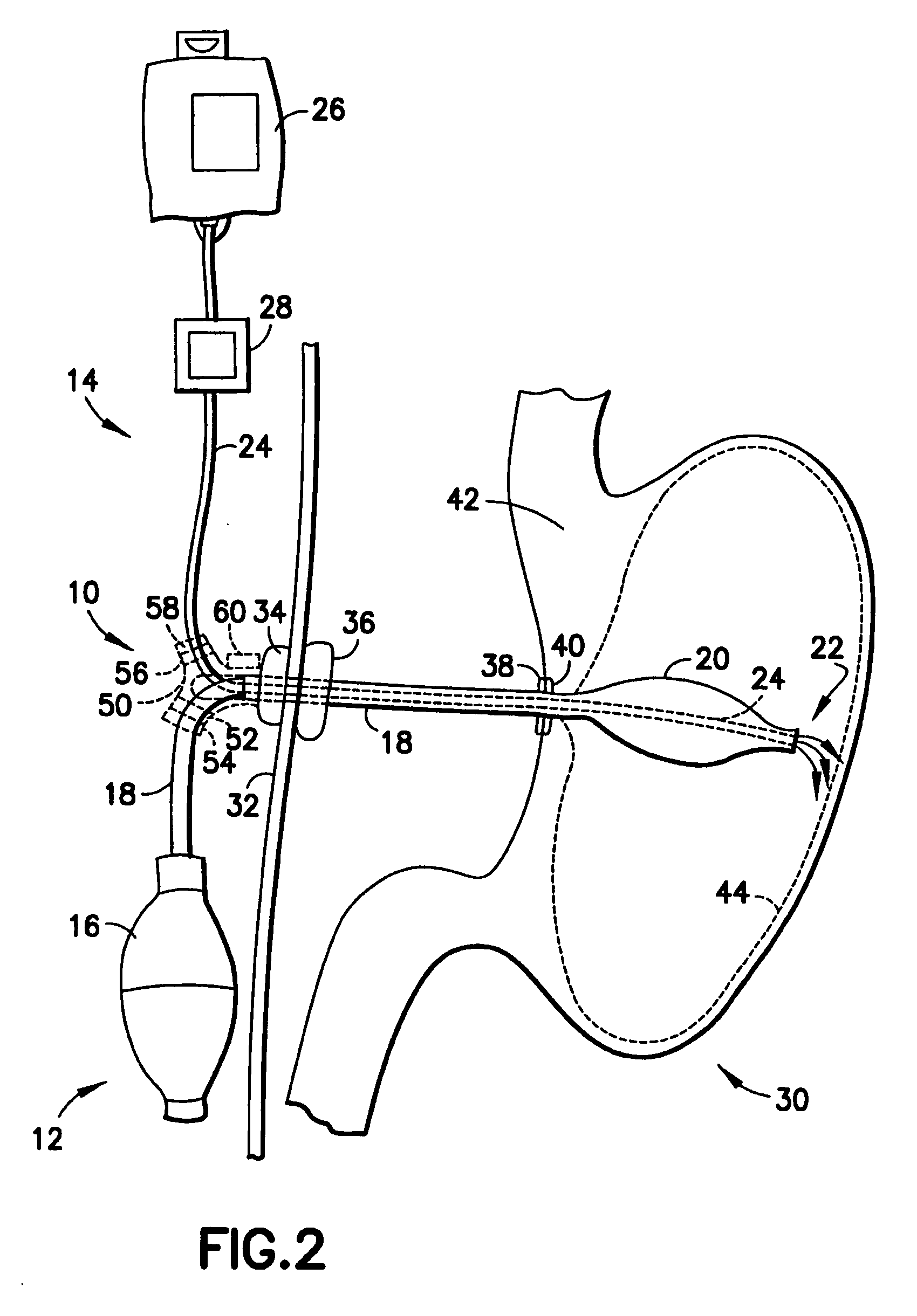
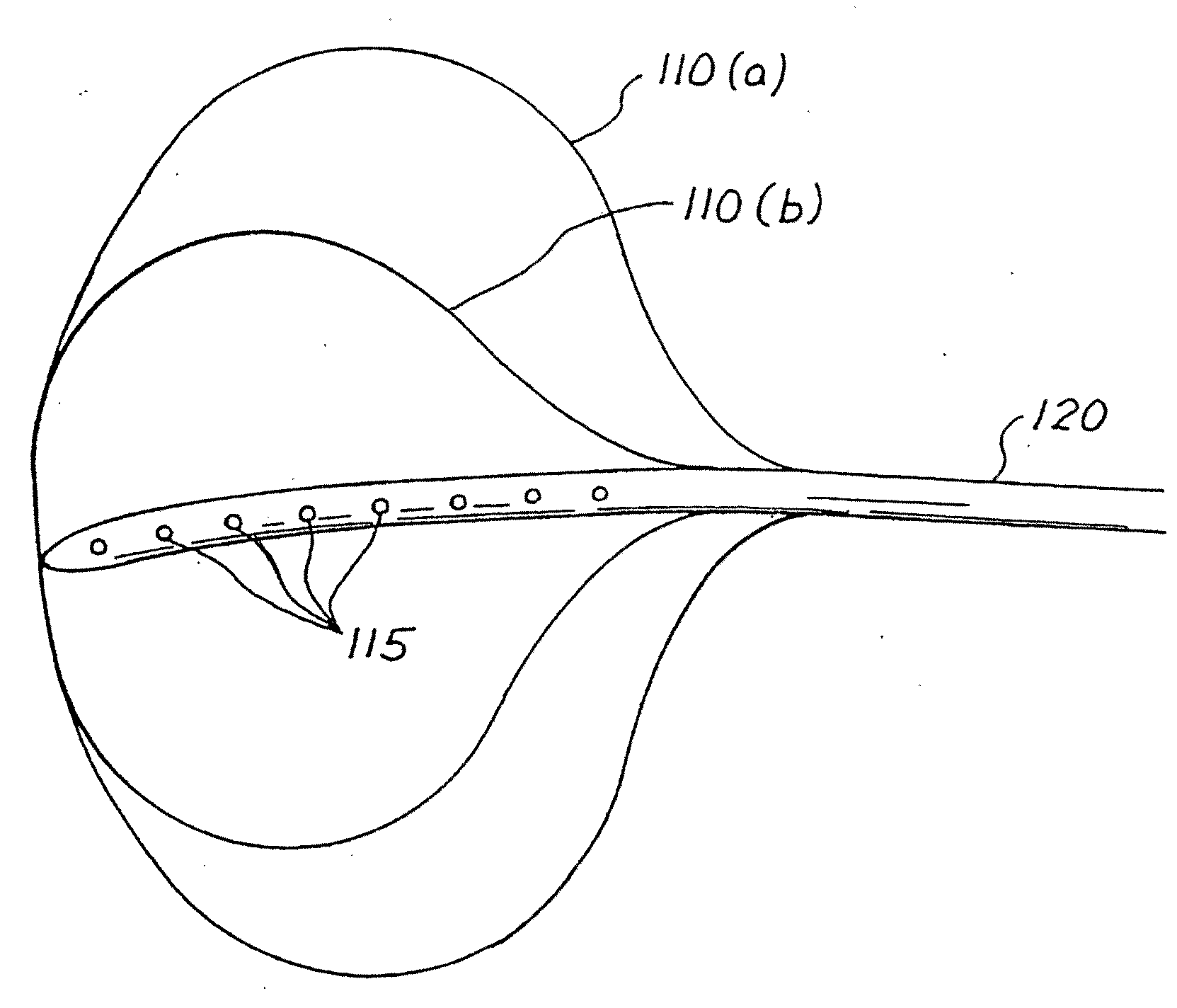
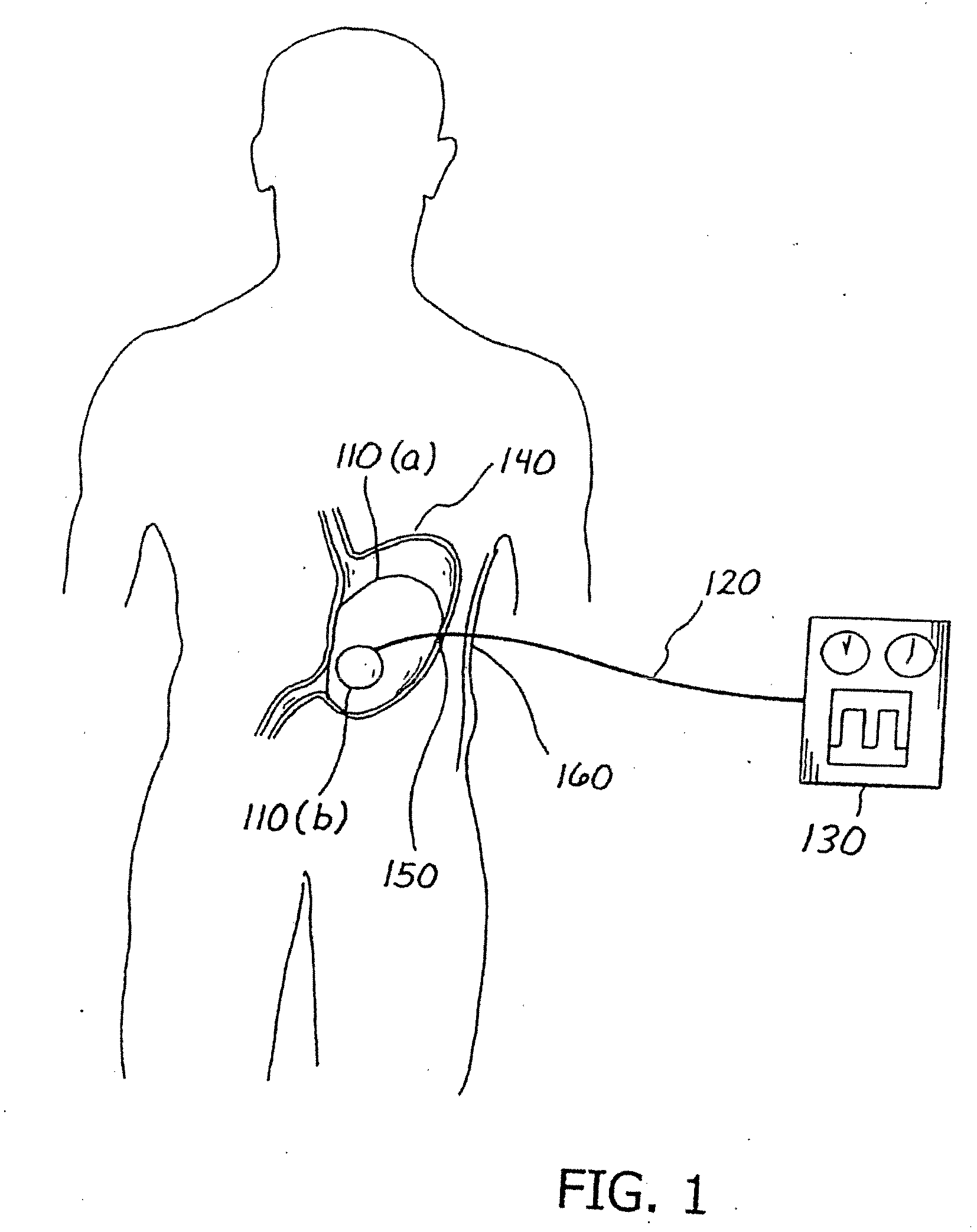
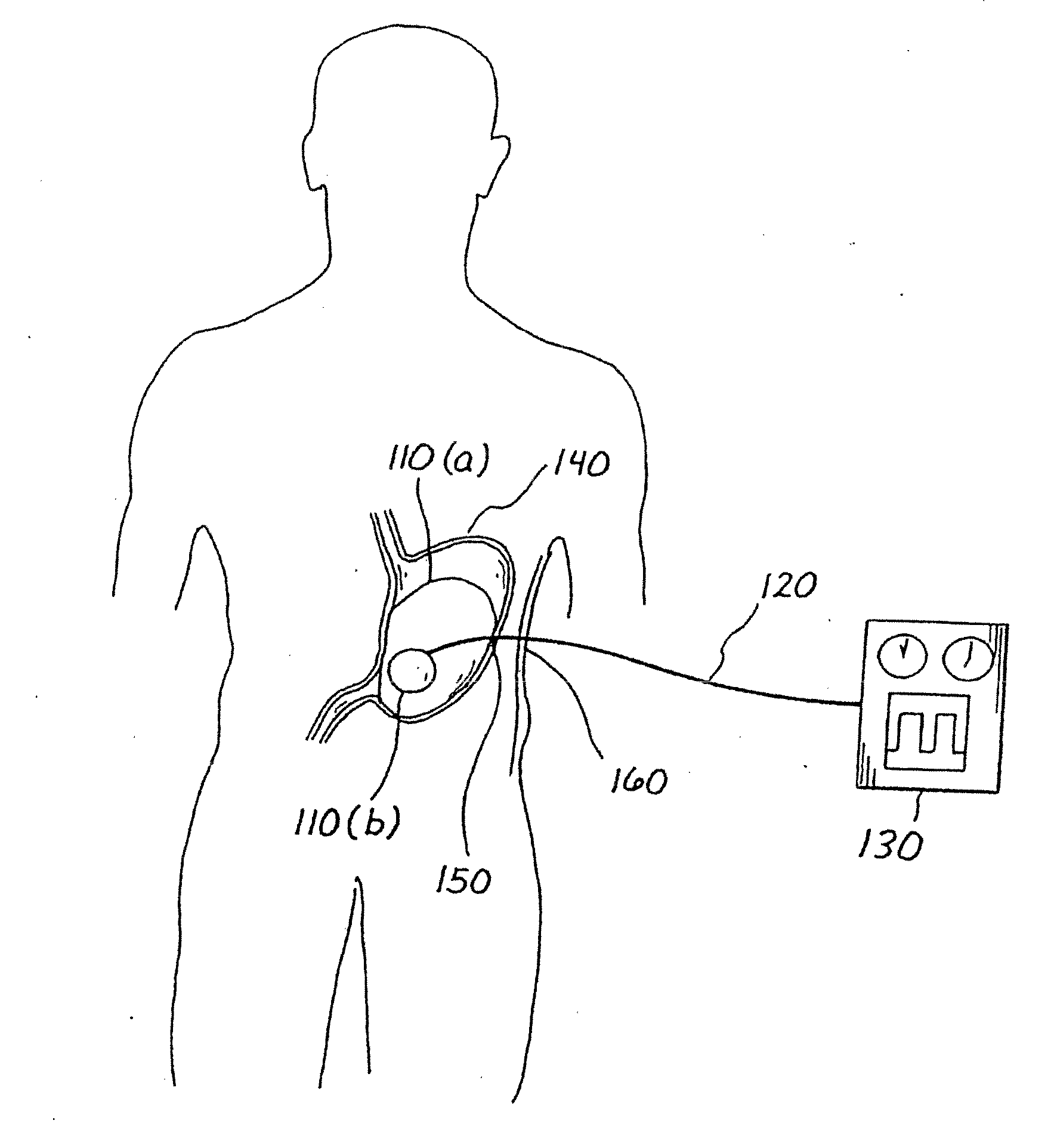
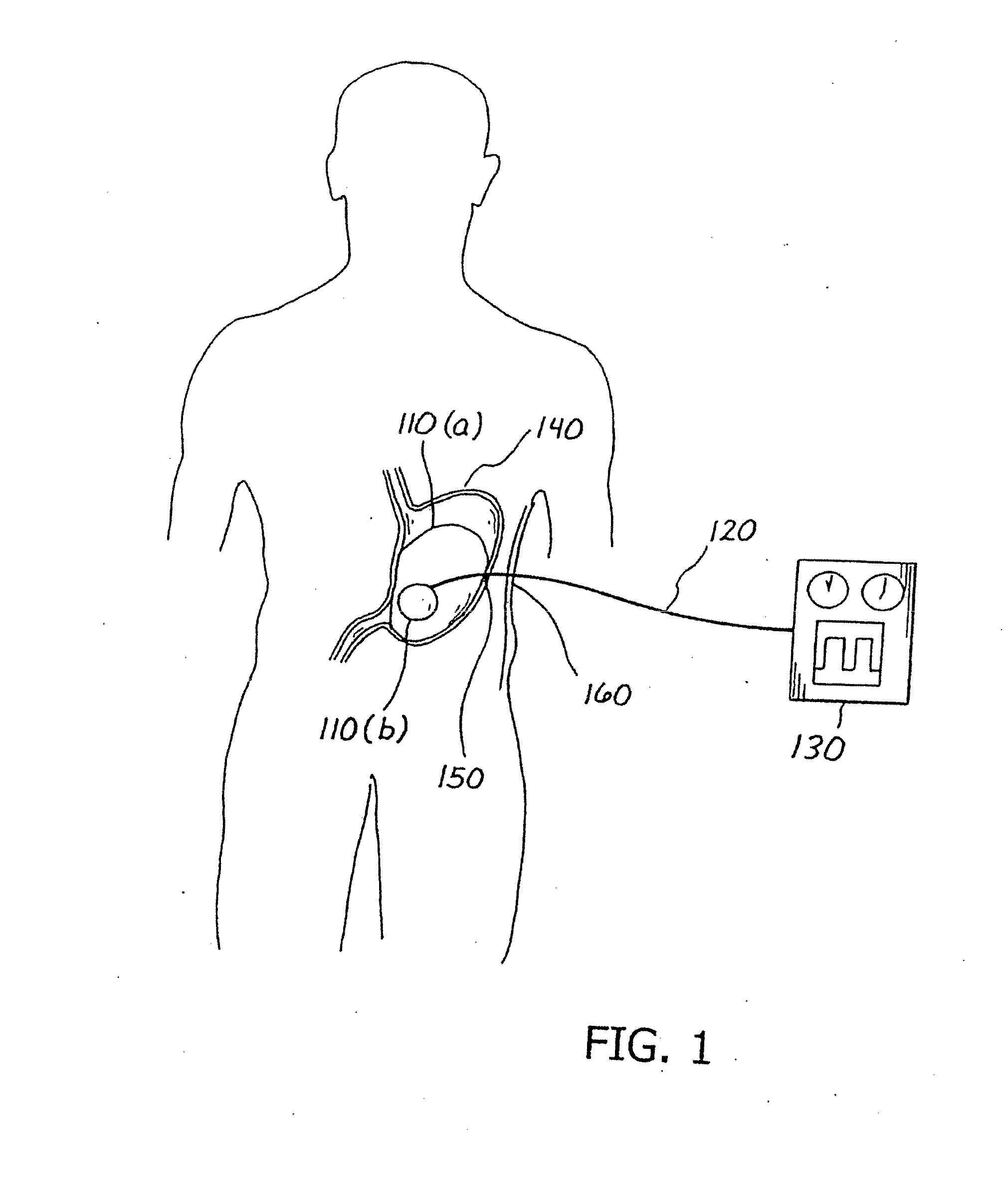
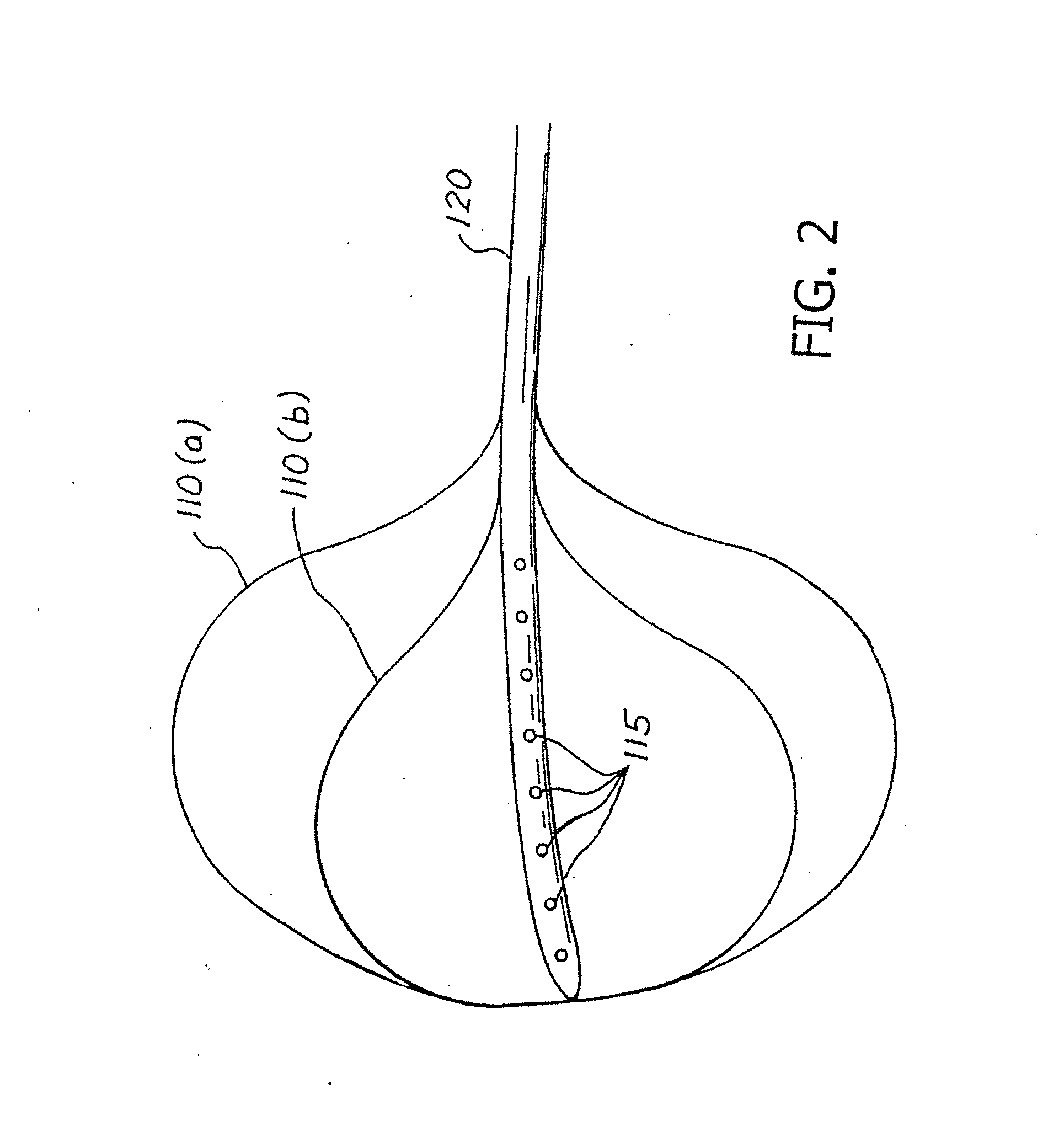
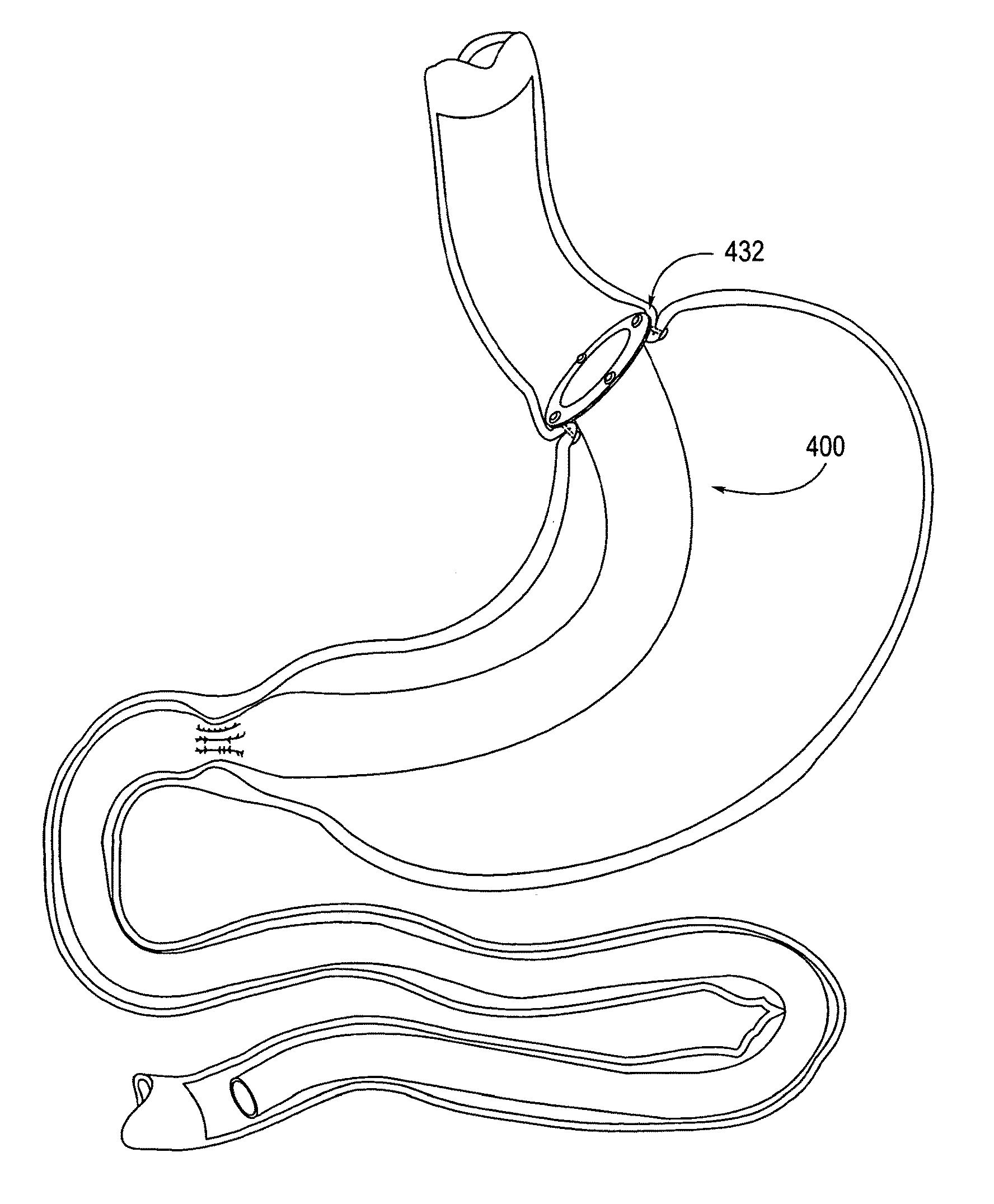
Balloon system and methods for treating obesity
InactiveUS20050159769A1Cause a feeling of satiety with less foodLess invasiveStentsSurgeryGastric cavityCritical time
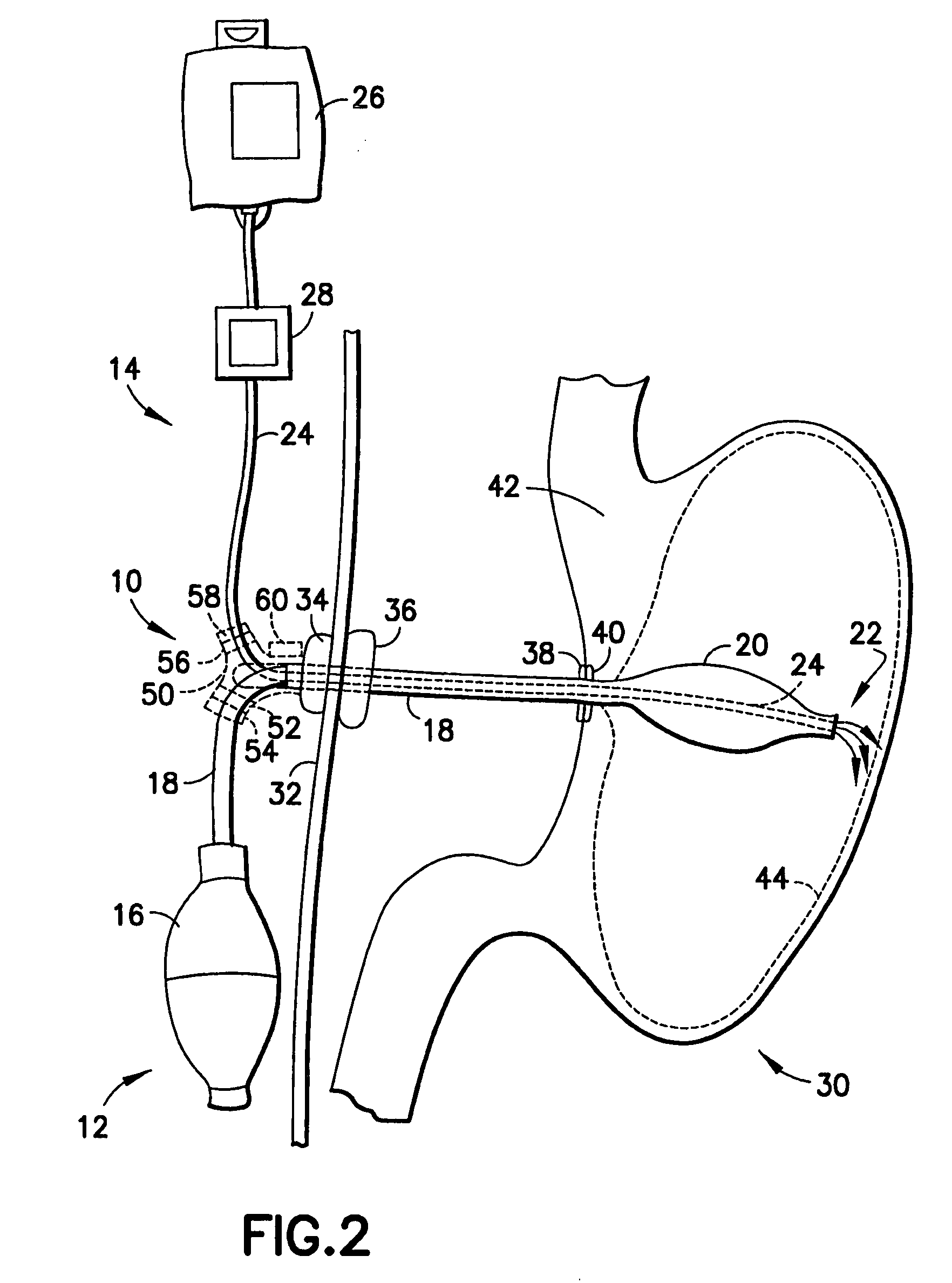
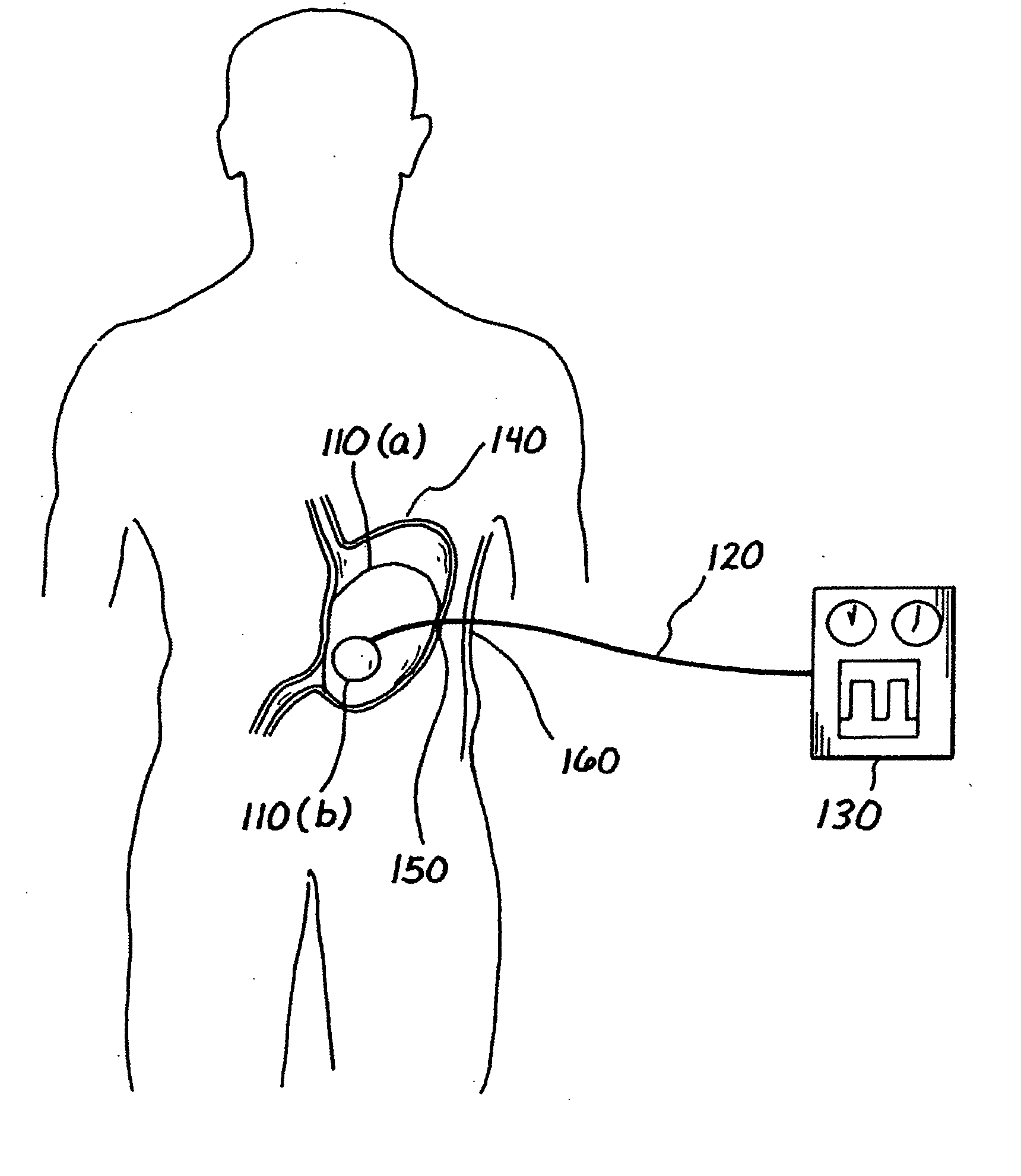
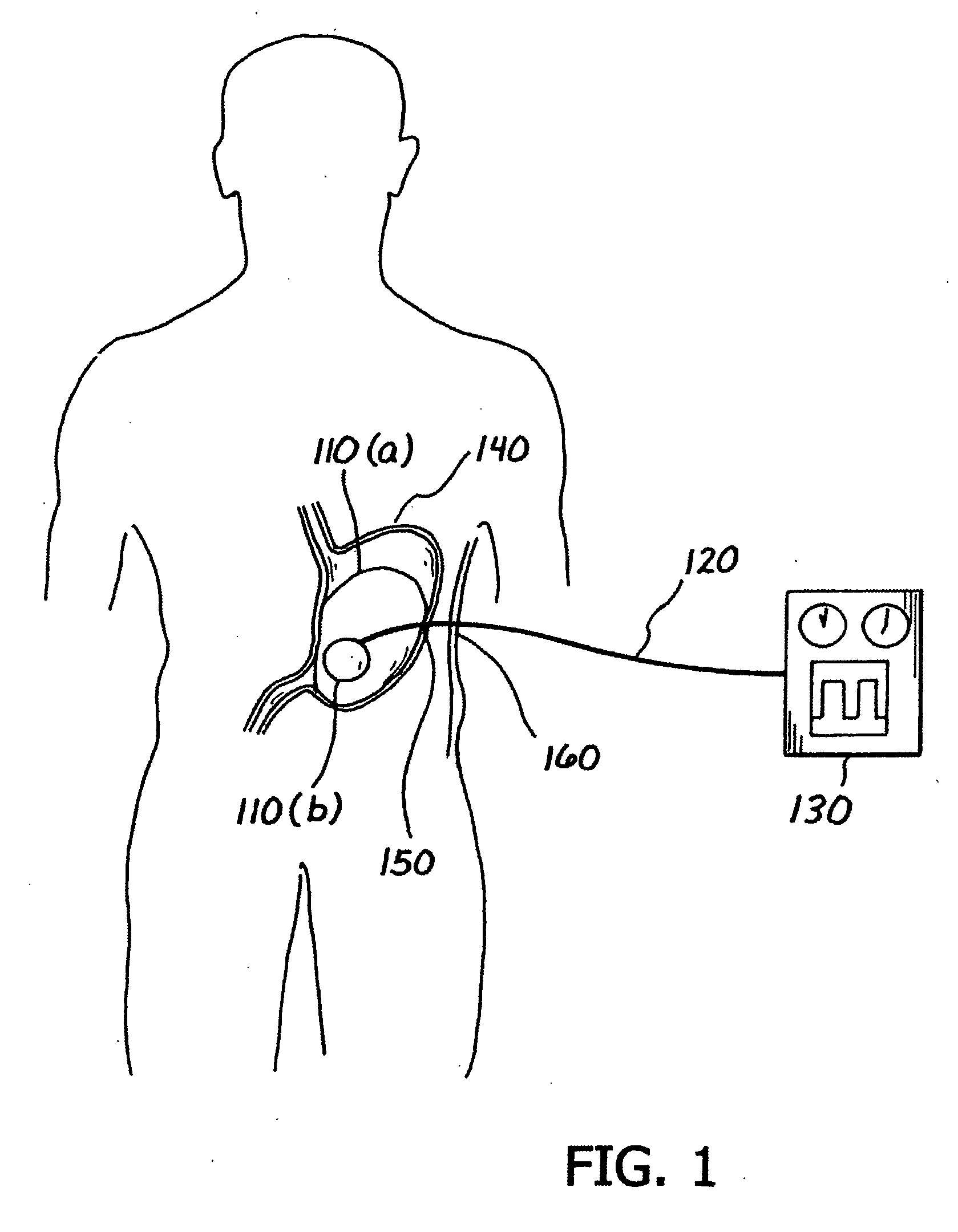
A medical system (100) for the treatment of morbid obesity comprising an inflatable balloon (110) implanted in a gastric cavity, a percutaneous fillant delivery tube (120) and a control module (130) connected to the tube for regulating the inflation and deflation of the balloon. The balloon may be individually contoured and inflated to occupy a large volume of the gastric cavity to provide a feeling of satiety. The balloon may also be deflated to give the gastric cavity lining a rest during less critical time.
Owner:RESHAPE MEDICAL LLC
Gastrointestinal sleeve device and methods for treatment of morbid obesity
InactiveUS7794447B2Reduce volumeReduce absorptionMedical devicesTubular organ implantsIntestinal structureSmall intestine
Owner:VALENTX
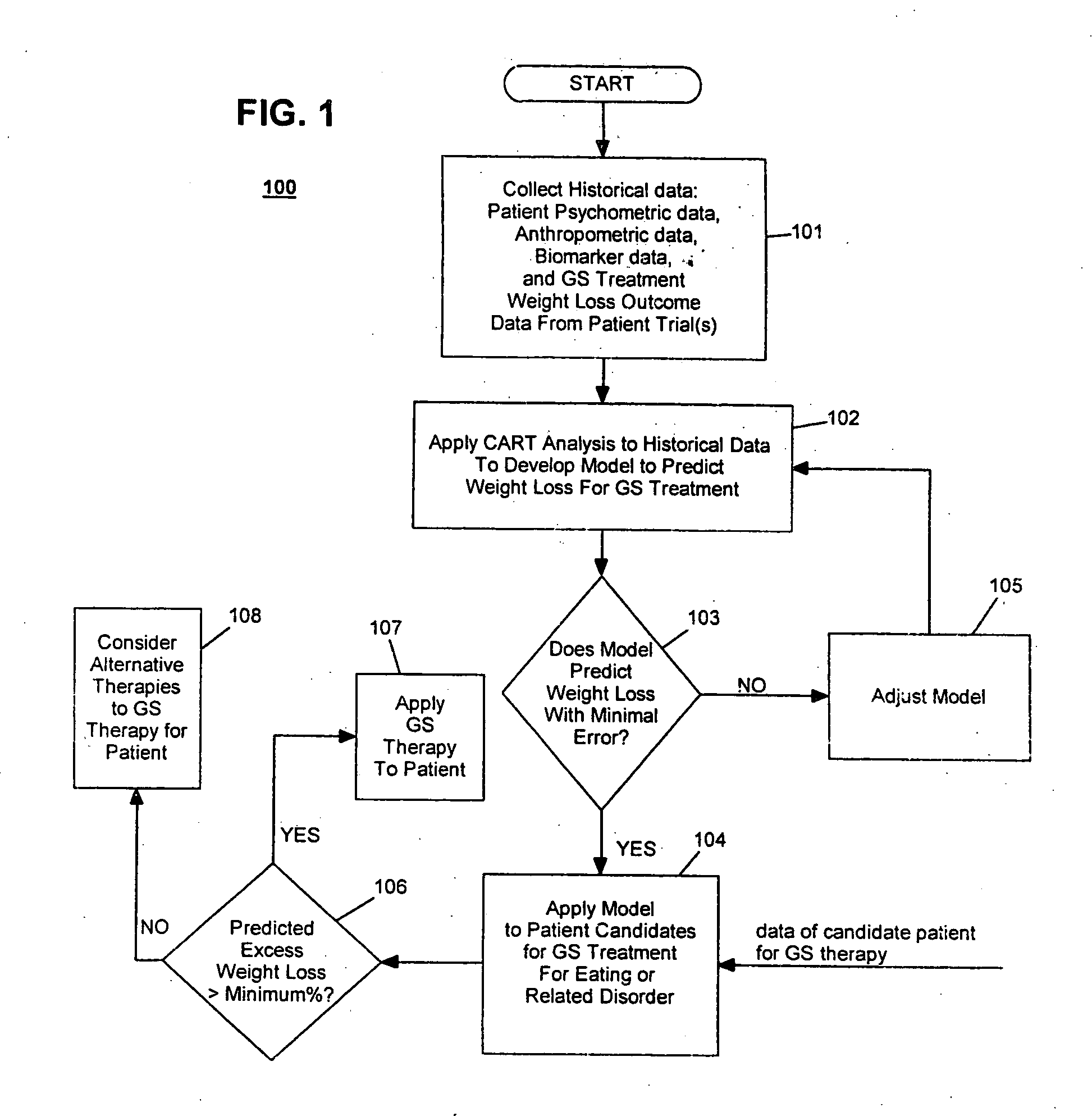
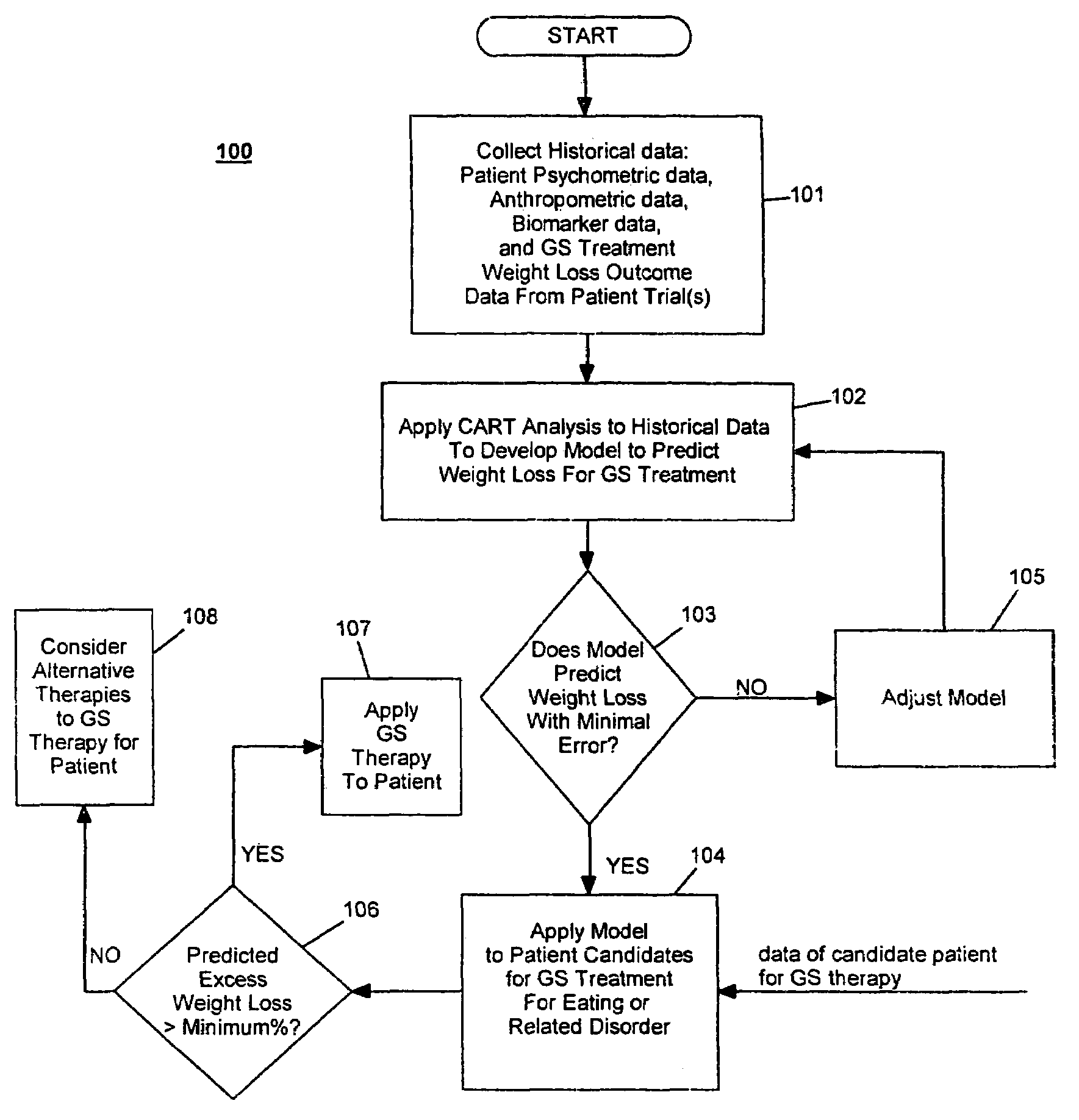
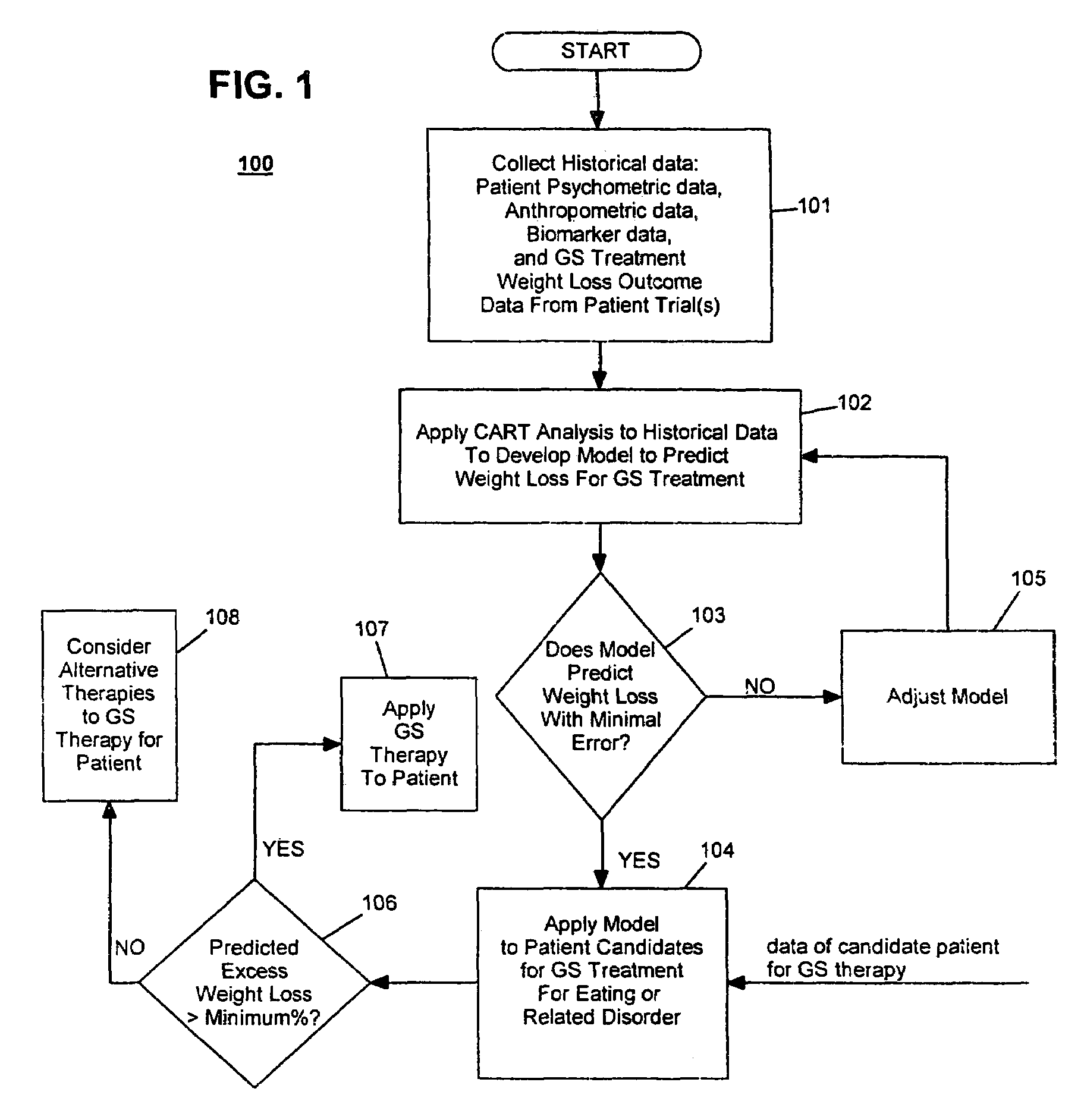
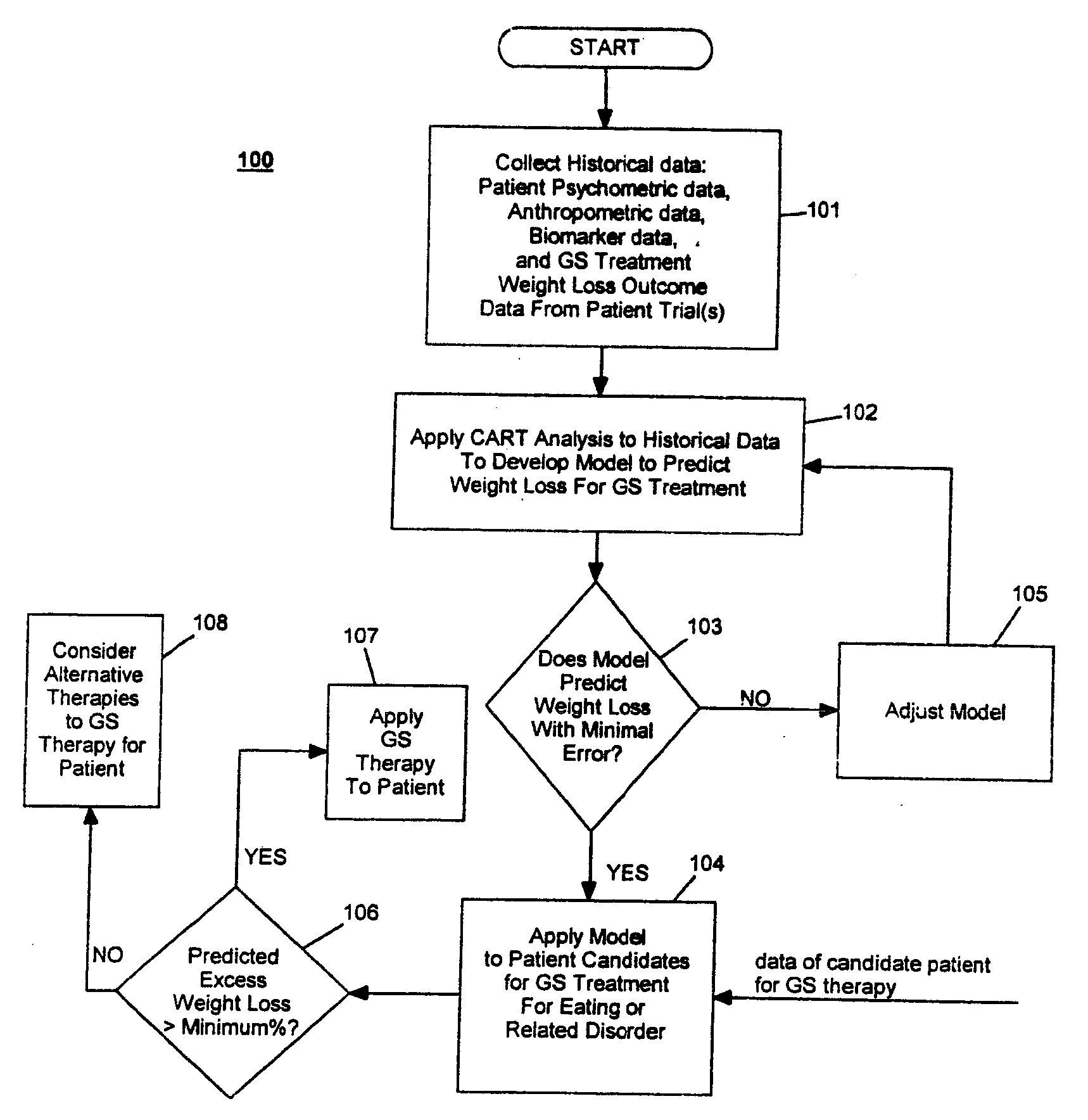
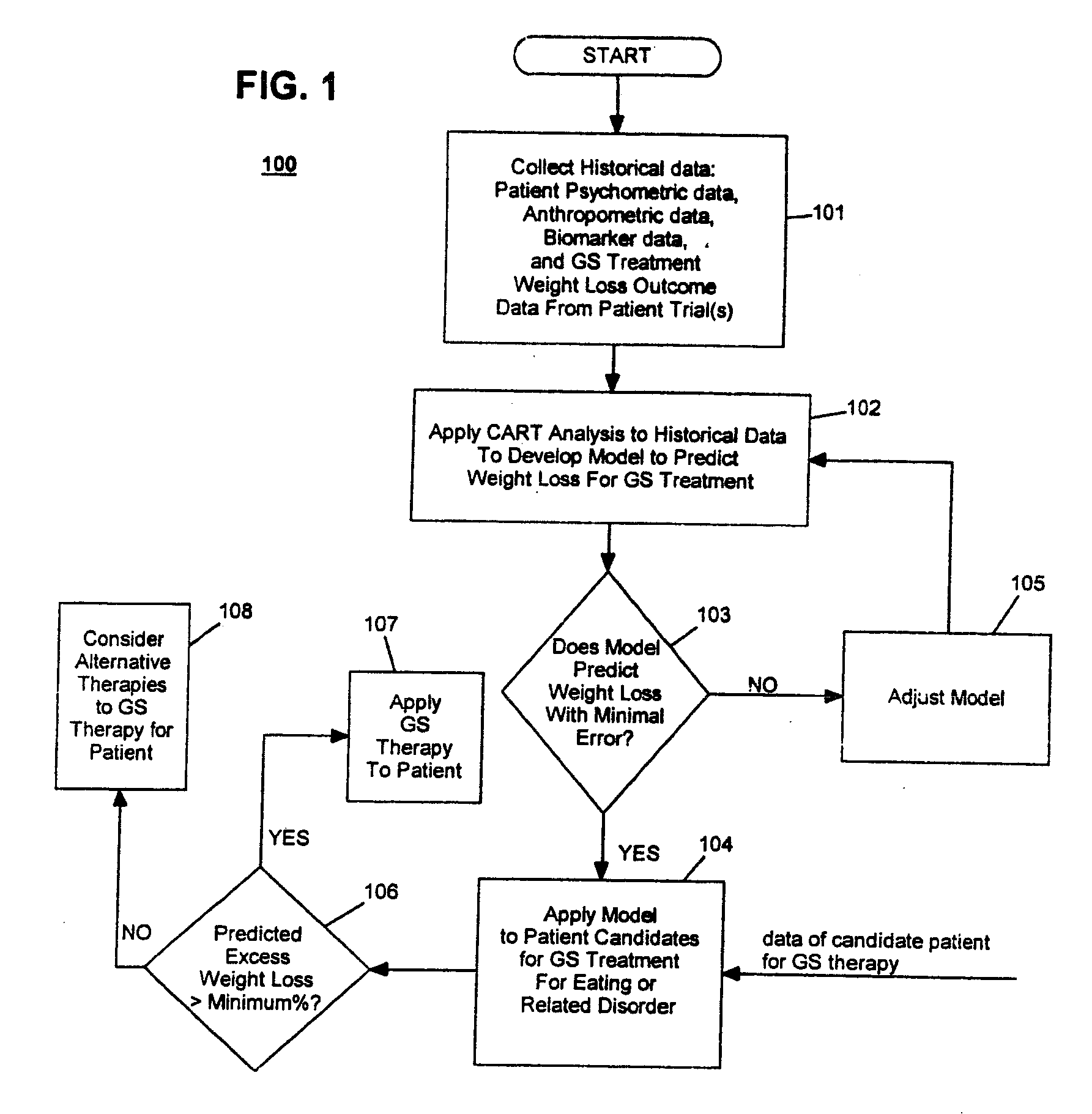
Method for screening and treating patients at risk of medical disorders
InactiveUS20050080462A1Reduced health costAccurate predictionMedical simulationElectrotherapyMedical disorderMorbid obesity
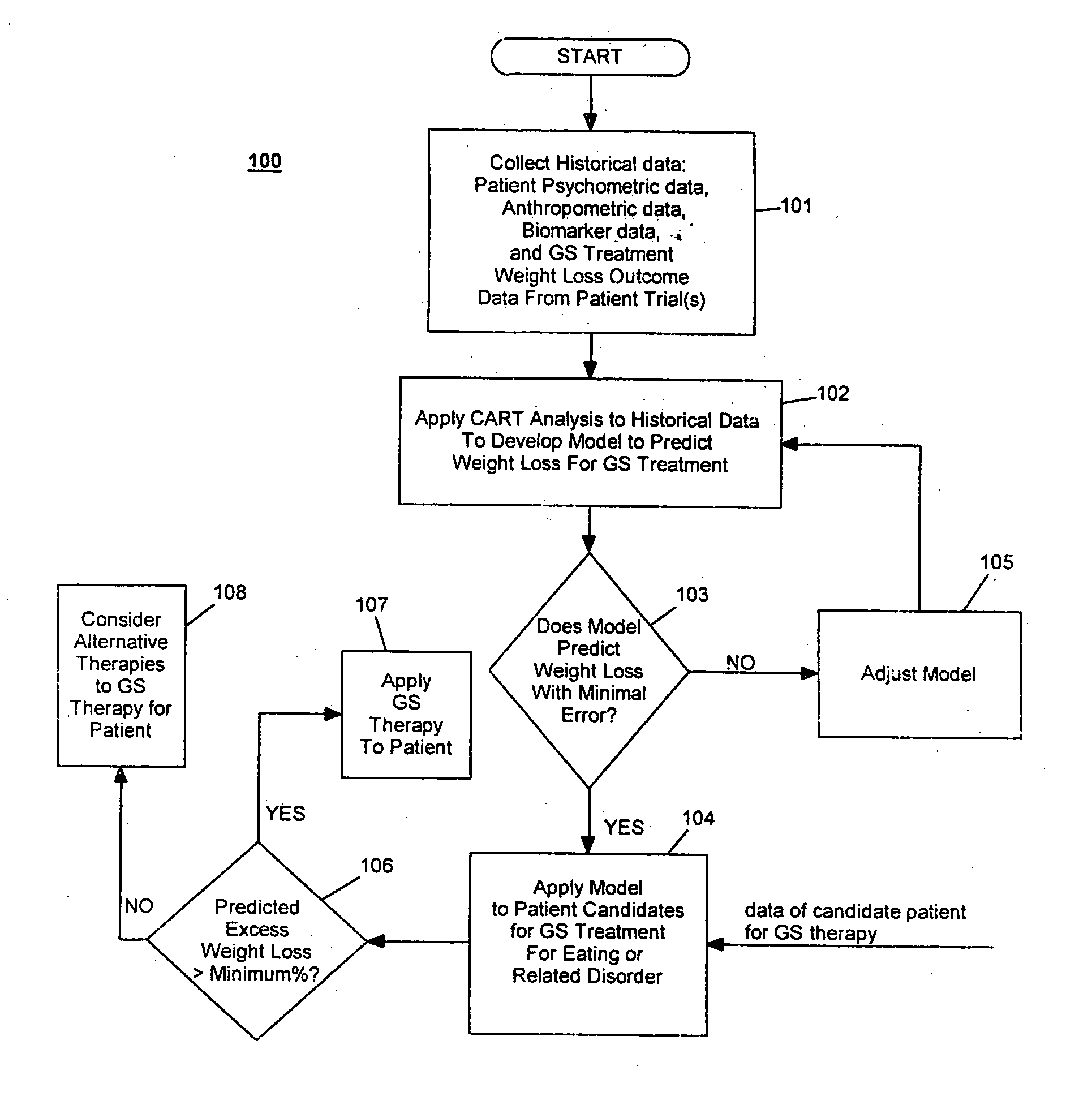
Method for screening patients to predict which patients at risk of a medical disorder, such as morbid obesity, gastrointestinal problems, or gastroesophageal problems, will be responders, and conversely, which patients will not, to achieve a favorable outcome from therapy for that disorder. This method supports an intervention strategy for patients having weight or gastrointestinal problems that will cut health costs. It enables patients and care-givers alike to more efficiently use their time, efforts and resources by enabling an early selection of an appropriate treatment modality for a given patient. Its application also extends to other implantable medical devices and therapies using them.
Owner:TRANSNEURONIX INC
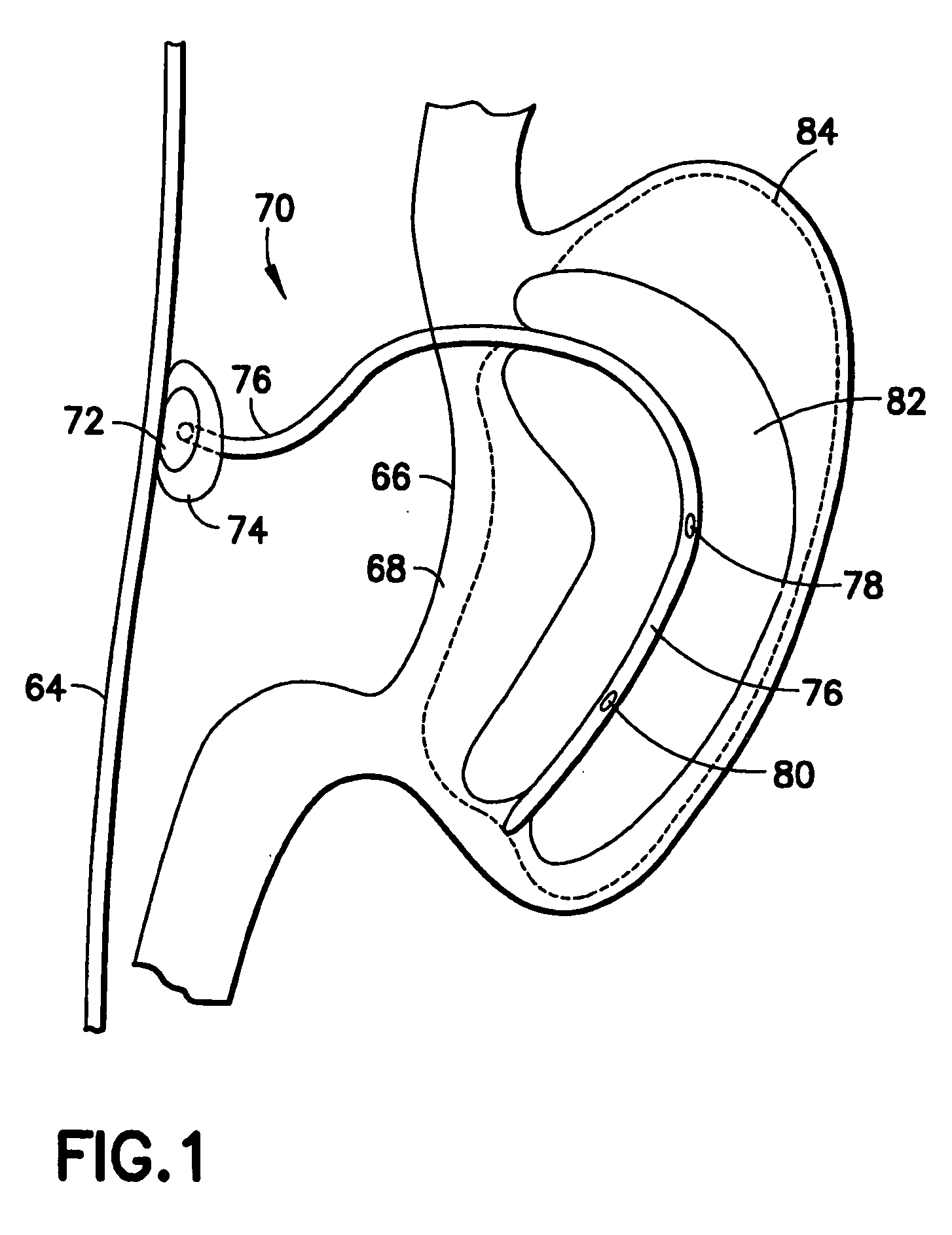
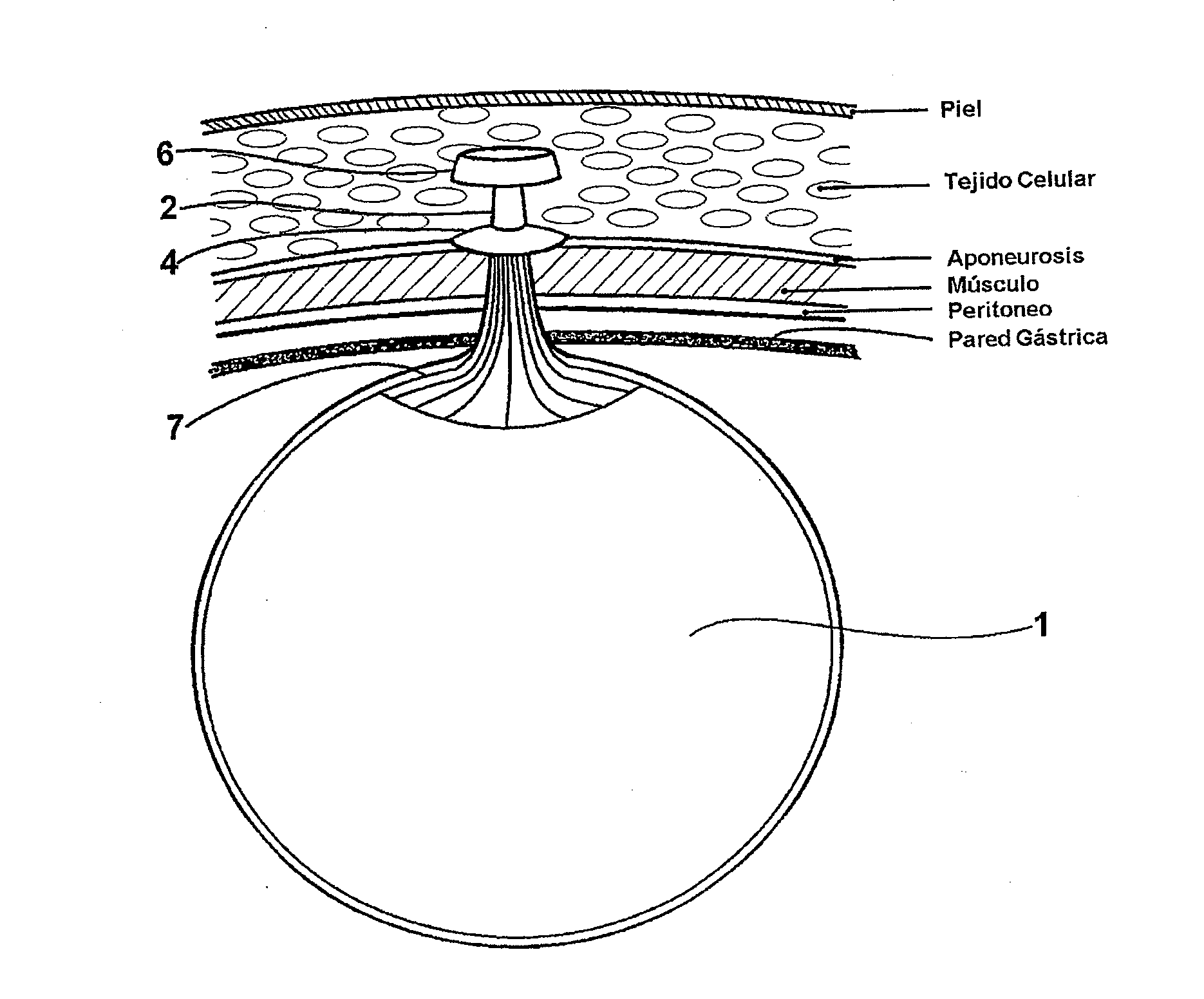
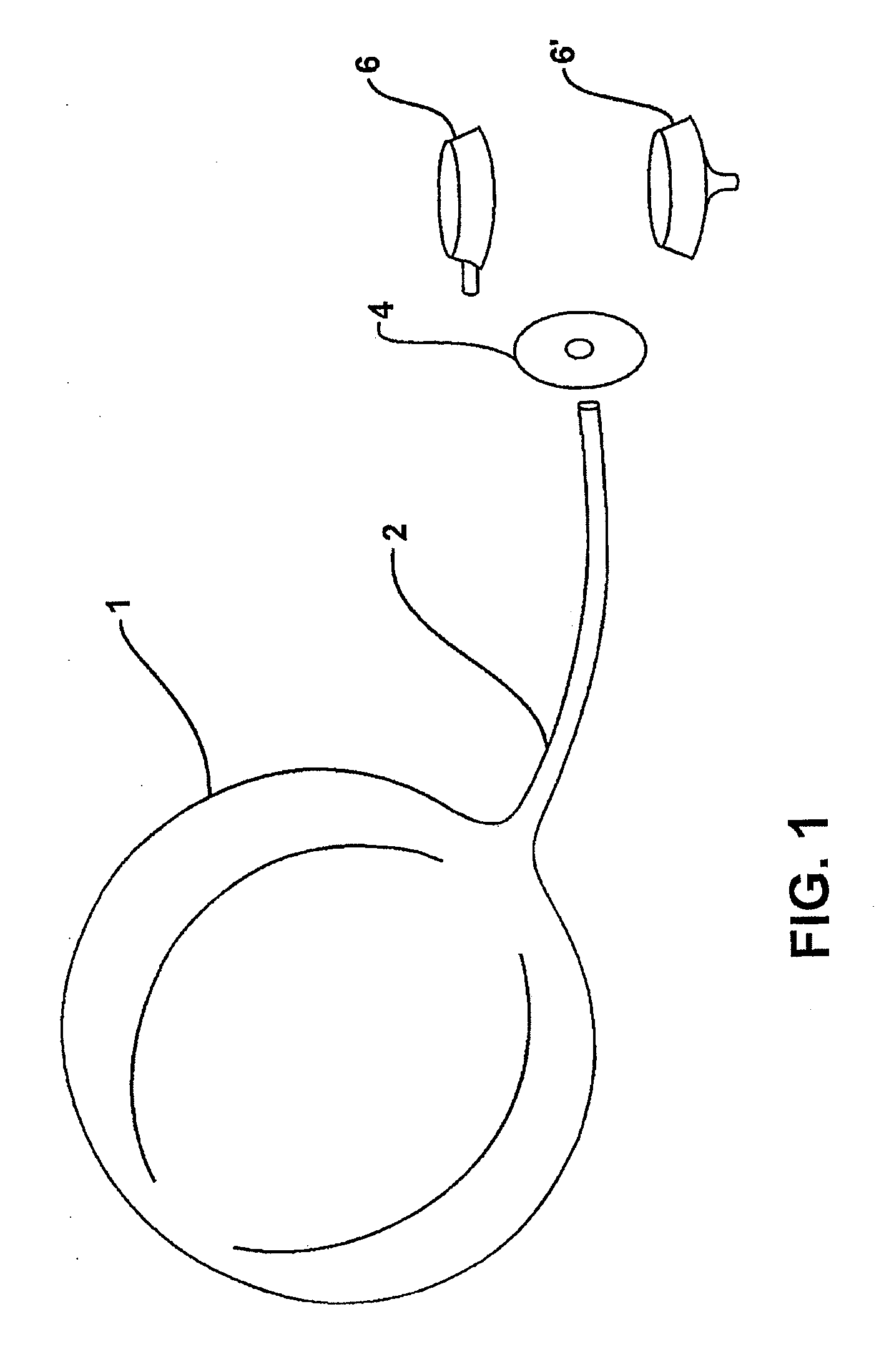
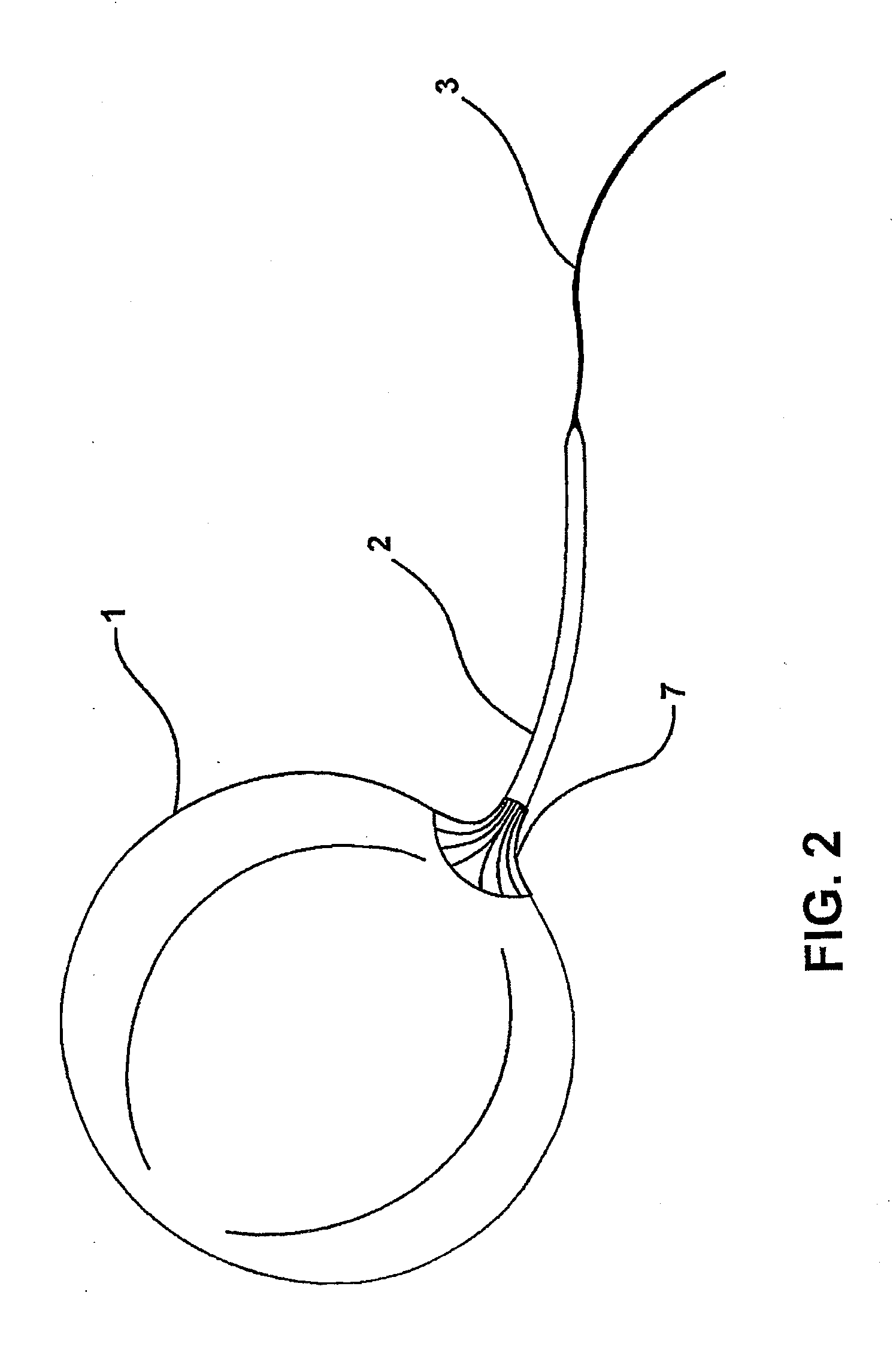
Intragastric device for treating morbid obesity
An intragastric device inserted by endoscopic path into a patient's stomach. The device includes a balloon or envelope having a specific nominal volume. The balloon is sealingly connected to connecting elements consisting of a disc forming a support base for the balloon against an inner wall of the stomach. The device also includes a flexible tube or catheter for connecting the balloon to a filling device and catching element integral with the tube or catheter. The connection elements enable a doctor to set and / or remove the balloon and to fix, either inside the patient's body, or subcutaneously the filling device and to be able to bring the balloon or envelope to its predetermined nominal volume.
Owner:DISTRICLASS MEDICAL
Devices and methods for treating morbid obesity
A surgical method of treating morbid obesity via bariatric procedures, carried out endoluminally and transluminally using endoscopic devices that are introduced through natural body openings without the necessity of creating any incisions in the abdominal wall.
Owner:MEDIGUS LTD
Method and apparatus for reducing obesity
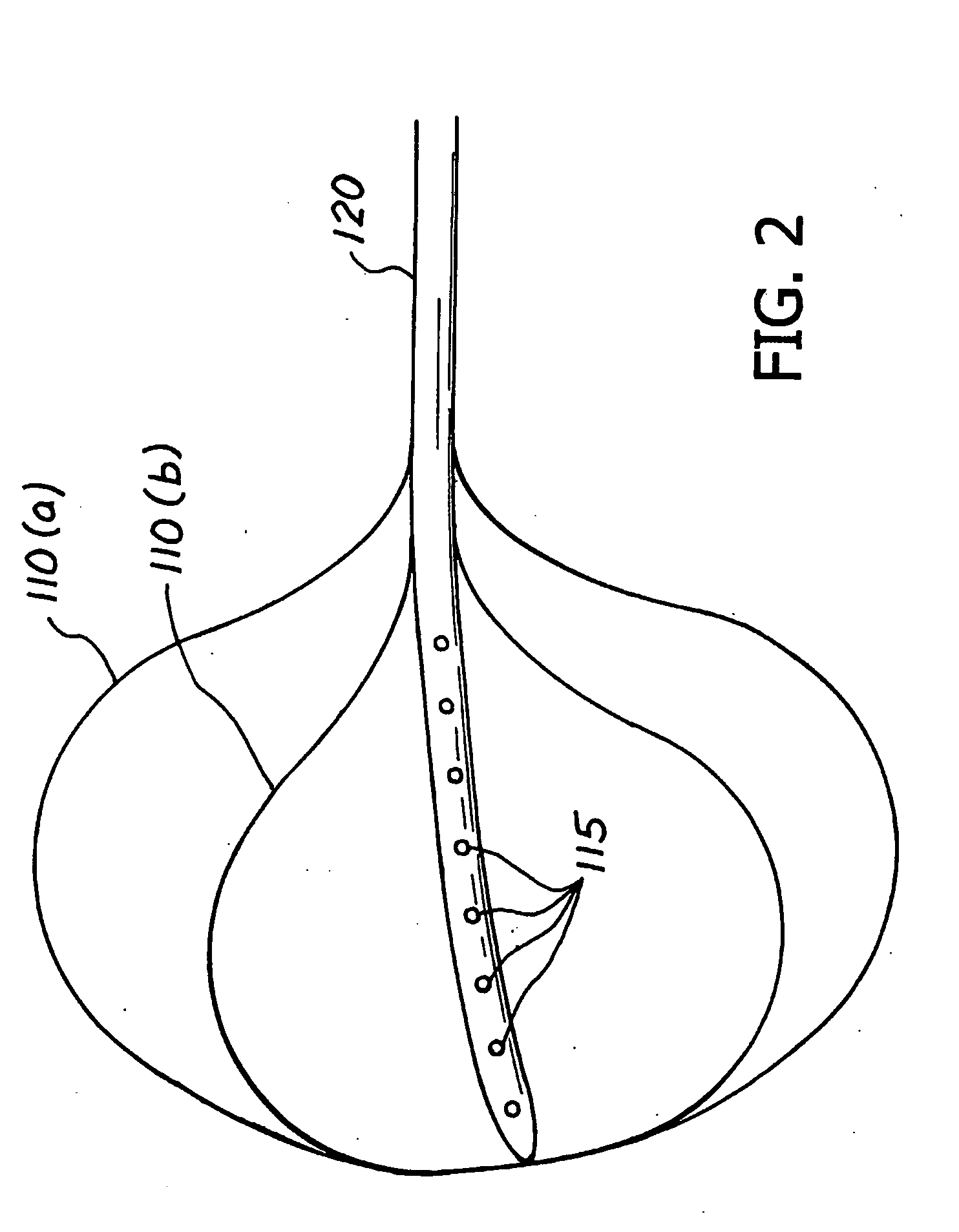
Method and apparatus for treatment of morbid obesity by placement of a series of flow reduction elements in the small intestine to induce satiety are disclosed. The flow reduction elements restrict the movement of partially digested food and reduce the flow rate through the small intestine which causes the emptying of the stomach and the duodenum to occur slower. The flow reduction elements are attached to an elongated tube and are constructed from various shapes and configurations. The flow reduction elements may be inflated with fluid or may be constructed from self-expandable materials. The device is anchored in the antrum of the stomach with an anchoring member. The transoral gastric device can be inserted with a delivery catheter through the working lumen of an endoscope or alongside an endoscope and may be removed with the aid of an endoscope if desired.
Owner:ENDOSPHERE
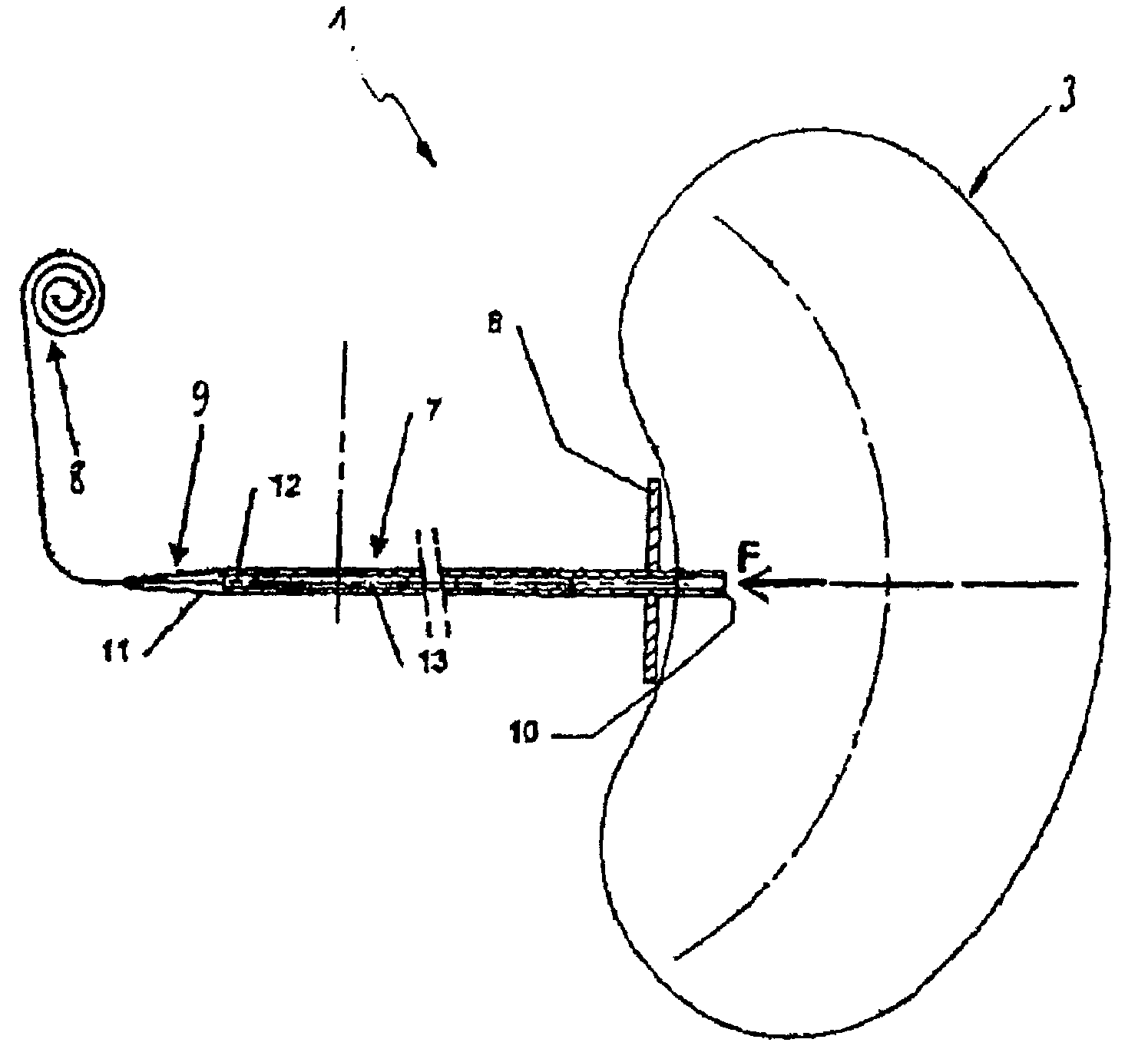
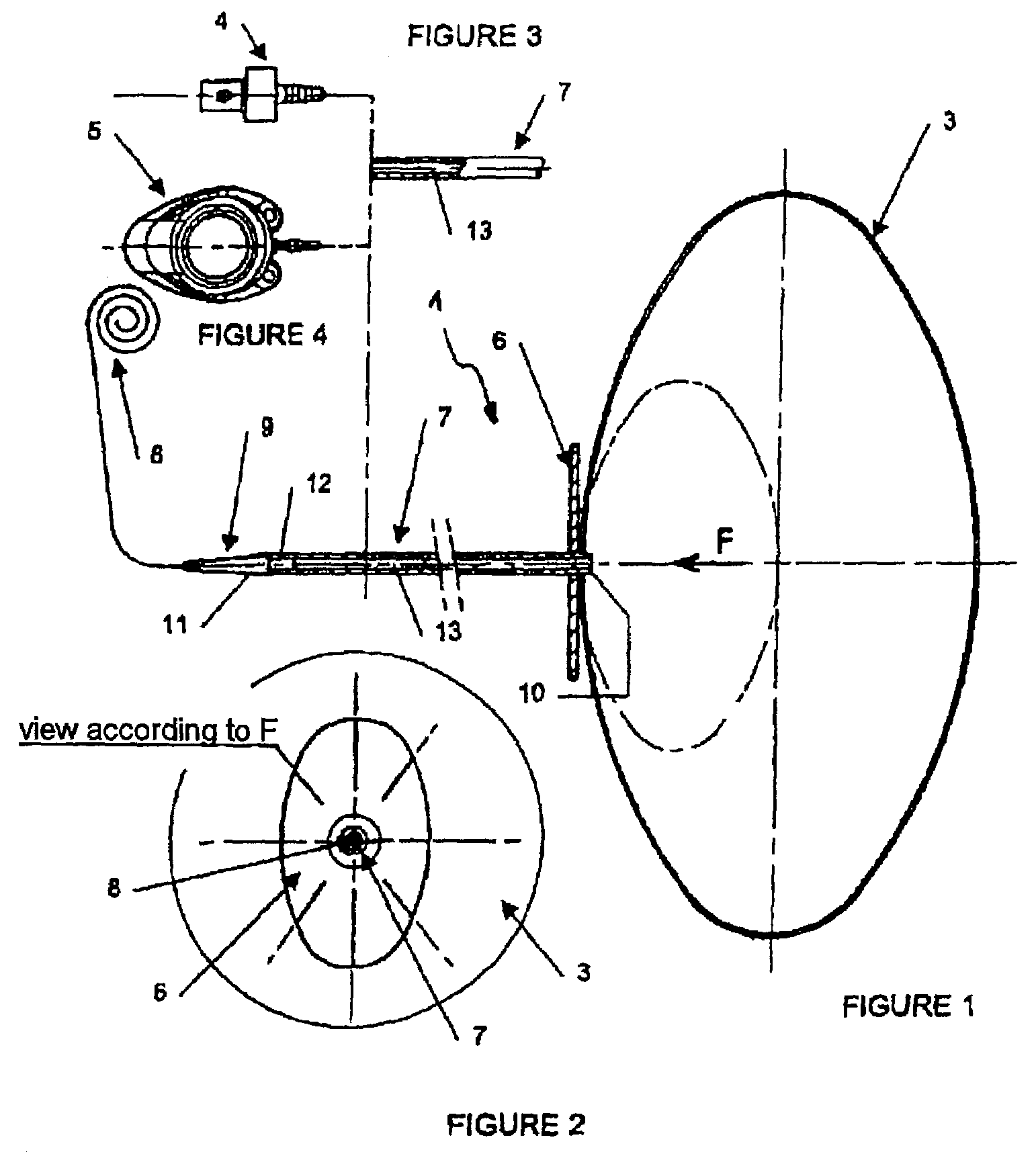
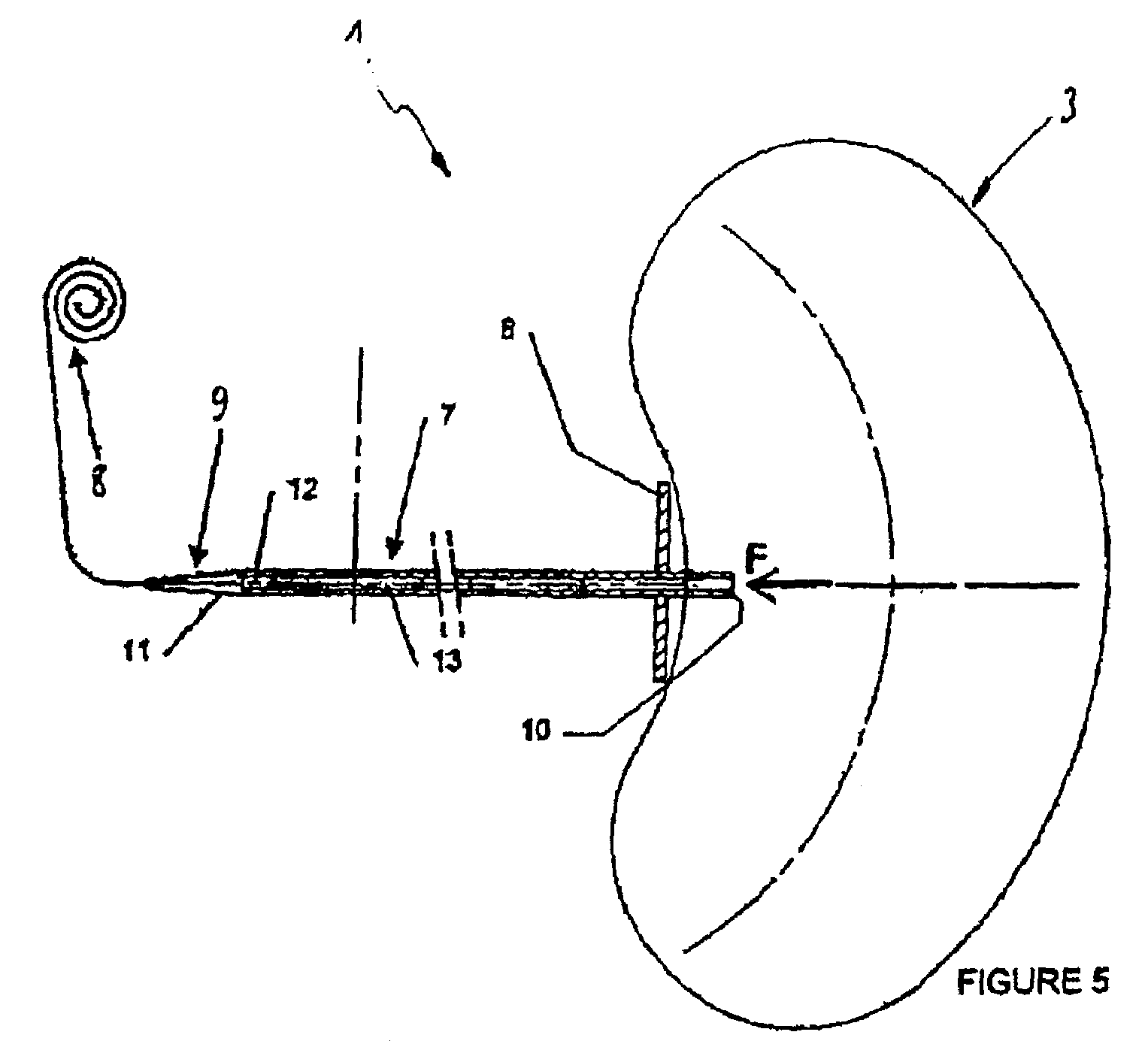
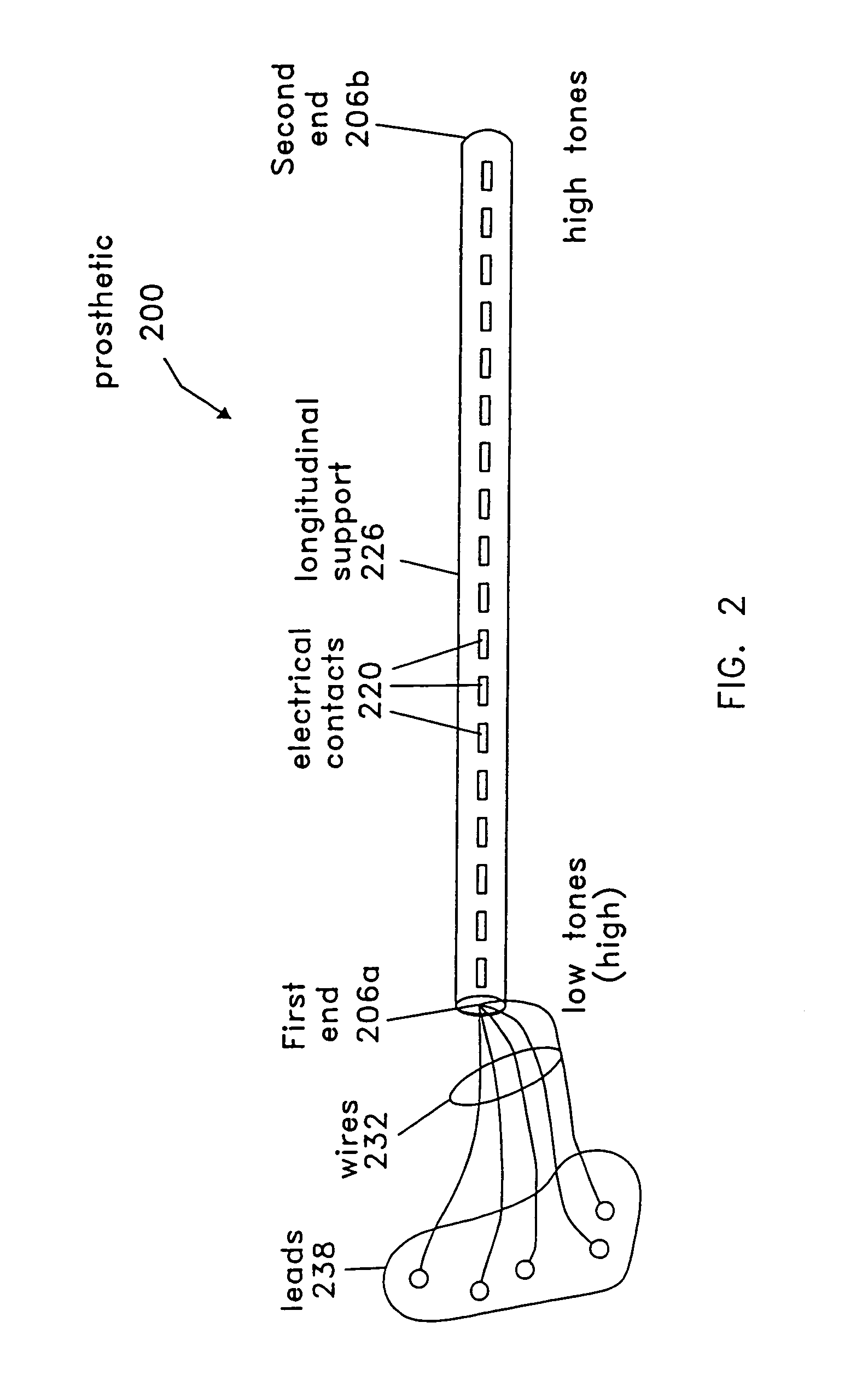
Stereotactic hypothalamic obesity probe
InactiveUS7077822B1High resolution stimulationReadily currentHead electrodesInfusion syringesMedicineMorbid obesity
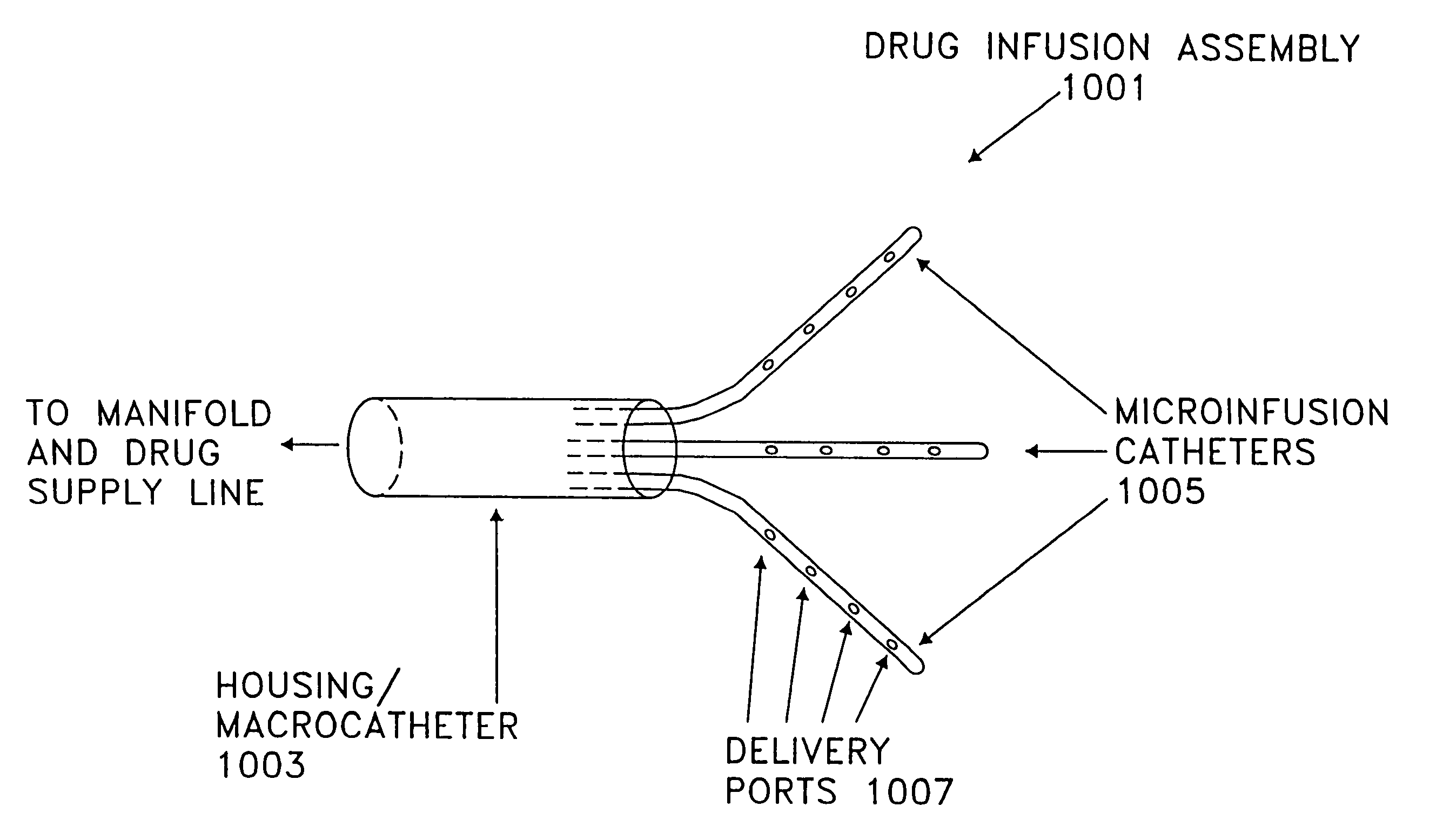
Apparatus and methods for regulating the appetite of an individual suffering from morbid obesity, the apparatus including a plurality of stimulation electrodes arranged longitudinally on at least one electrode support shaft for insertion within the hypothalamus for outputting electrical discharges to specific sites within the hypothalamus. Each of the plurality of stimulation electrodes may be independently controlled. Electrical discharge of various frequencies transmitted from one or more of the plurality of stimulation electrodes, and delivered to a region of the hypothalamus that is involved with either stimulating or inhibiting appetite, may be used to regulate appetite in the individual. Alternatively, an individual's appetite may be regulated by the microinfusion from at least one microinfusion catheter of an appropriate quantity of a suitable drug to a distinct site or region within the hypothalamus.
Owner:UNIV OF IOWA RES FOUND
Gastro-occlusive device
A gastro-occlusive device, comprising a balloon disposable in a stomach cavity of a patient, and inflatable therein to occlude a portion of the volume of the stomach cavity, a gas flow tube coupled at a distal end thereof with the balloon and extending outwardly through a stomach wall for coupling with a gas source for selectively inflating the balloon, to occlude at least a portion of the volume of the stomach cavity. The gastro-occlusive device may be employed in combination with a feeding tube unit, a drain unit or other ancillary apparatus, and is useful for treatment of morbid obesity and various eating disorders.
Owner:POLYZEN INC
Method for screening and treating patients at risk of medical disorders
InactiveUS7194301B2Timely useAccurate predictionMedical simulationMedical data miningMedical disorderTreatment modality
Owner:TRANSNEURONIX INC
Method for Screening and Treating Patients at Risk of Medical Disorders
InactiveUS20070244375A1Reduced health costAccurate predictionElectrotherapySurgeryMorbidly obeseProper treatment
Method for screening patients to predict which patients at risk of a medical disorder, such as morbid obesity, gastrointestinal problems, or gastroesophageal problems, will be responders, and conversely, which patients will not, to achieve a favorable outcome from therapy for that disorder. This method supports an intervention strategy for patients having weight or gastrointestinal problems that will cut health costs. It enables patients and care-givers alike to more efficiently use their time, efforts and resources by enabling an early selection of an appropriate treatment modality for a given patient. Its application also extends to other implantable medical devices and therapies using them.
Owner:MEDTRONIC TRANSNEURONIX
Gastric clamp for performing vertical band gastroplasty and a gastric bypass with lesser curvature
InactiveUS7288100B2Efficient compressionNon-surgical orthopedic devicesObesity treatmentSurgical treatmentStomach walls
The invention relates to the production of a vertical gastric reservoir for the surgical treatment of morbid obesity. The reservoir consists of a clamp having two plates articulated at the lower end thereof and an automatic closing system in the upper part thereof, reinforced with a sealing system. Both plates are provided with two extensions (one at each end of the instrument) on the right-hand edge thereof. Both extensions are provided with two openings that are used to fix the instrument to the stomach wall. Application of the instrument and the technical solution provided are as follows: the substitution of metal staples which are subsequently eliminated in between 15% and 20% of patients and which result in failure of the vertical band gastroplasty operation. The instrument could also be used for producing small gastric reservoirs used for gastric bypass with minor curvature instead of metal staples.
Owner:SURGICAL IOC
Process for electrostimulation treatment of morbid obesity
InactiveUS20080183238A1Prevent and slow down stomach emptyingTransit delayInternal electrodesExternal electrodesMorbid obesityPulse rate
An improved process using electrostimulation for treating obesity, especially morbid obesity, is provided. The improved method of this invention provides electrostimulation on or along the small intestines, preferably on or along the duodenum and / or jejunum, which provides improved control of obesity. In one embodiment, the process employs stimulation of the lesser curvature at a rate of about 2 to about 30 pulses / minute with each pulse lasting about 0.1 to about 4 seconds such that there is a pause of about 3 to about 30 seconds between the pulses. More preferably, the pulse rate is about 12 to about 14 pulses / minute with each pulse lasting about 0.1 to about 0.5 seconds with a pause of about 4.5 to about 5 seconds between pulses. Preferably, the pulse amplitude is about 0.5 to about 15 milliamps. More preferable, each pulse consists of a train of micro-bursts with a frequency of about 5 to about 100 Hz.
Owner:MEDTRONIC INC
Balloon System and Methods for Treating Obesity
ActiveUS20100130998A1Cause a feeling of satiety with less foodLess invasiveSurgeryDilatorsGastric cavityMorbid obesity
A medical system for the treatment of morbid obesity comprising an inflatable balloon implanted in a gastric cavity, a percutaneous fillant delivery tube and a control module connected to the tube for regulating the inflation and deflation of the balloon. The balloon may be individually contoured and inflated to occupy a large volume of the gastric cavity to provide a feeling of satiety. The balloon may also be deflated to give the gastric cavity lining a rest during less critical time.
Owner:APOLLO ENDOSURGERY INC +1
Intragastric balloon assembly
InactiveUS20100191270A1Convenient design and constructionEasy to manufactureSurgeryDilatorsPull forceStomach walls
The invention relates to an intragastric balloon for the treatment of persons having severe or morbid obesity problems, wherein the number of elements in the already known systems is reduced, and provides a material savings in the manufacture thereof. The intragastric balloon assembly comprises a balloon connected to a catheter to inflate the same, and includes a means for fixing the same to a patient body, consisting in a plain fixing plate with a sole orifice through which said inflation catheter is passed, to be fixed subcutaneously in the abdominal region of said patient; and in a preferred embodiment, an additional fastening element is provided allowing firmly subject said inflation catheter to said fixing plate, and consisting in a semi-rigid washer or ring made of silicon, which is fastened to the catheter and fixing plate by means of a suitable adhesive; thus maintaining said catheter with a pulling suitable for the balloon to be pasted perfectly but without pressure at all, on the stomach wall. Said assembly also comprises a multi-puncture valve to which said inflation catheter is connected to inflate said balloon, which in turn can be arranged on the fixing plate, whereby an alternative element to fasten the catheter and, therefore, the balloon assembly is obtained.
Owner:GARZA ALVAREZ JOS RAFAEL
Balloon system and methods for treating obesity
InactiveUS20140257358A1Cause a feeling of satiety with less foodLess invasiveSurgeryDilatorsGastric cavityMorbid obesity
A medical system for the treatment of morbid obesity comprising an inflatable balloon implanted in a gastric cavity, a percutaneous fillant delivery tube and a control module connected to the tube for regulating the inflation and deflation of the balloon. The balloon may be individually contoured and inflated to occupy a large volume of the gastric cavity to provide a feeling of satiety. The balloon may also be deflated to give the gastric cavity lining a rest during less critical time.
Owner:RESHAPE MEDICAL LLC +1
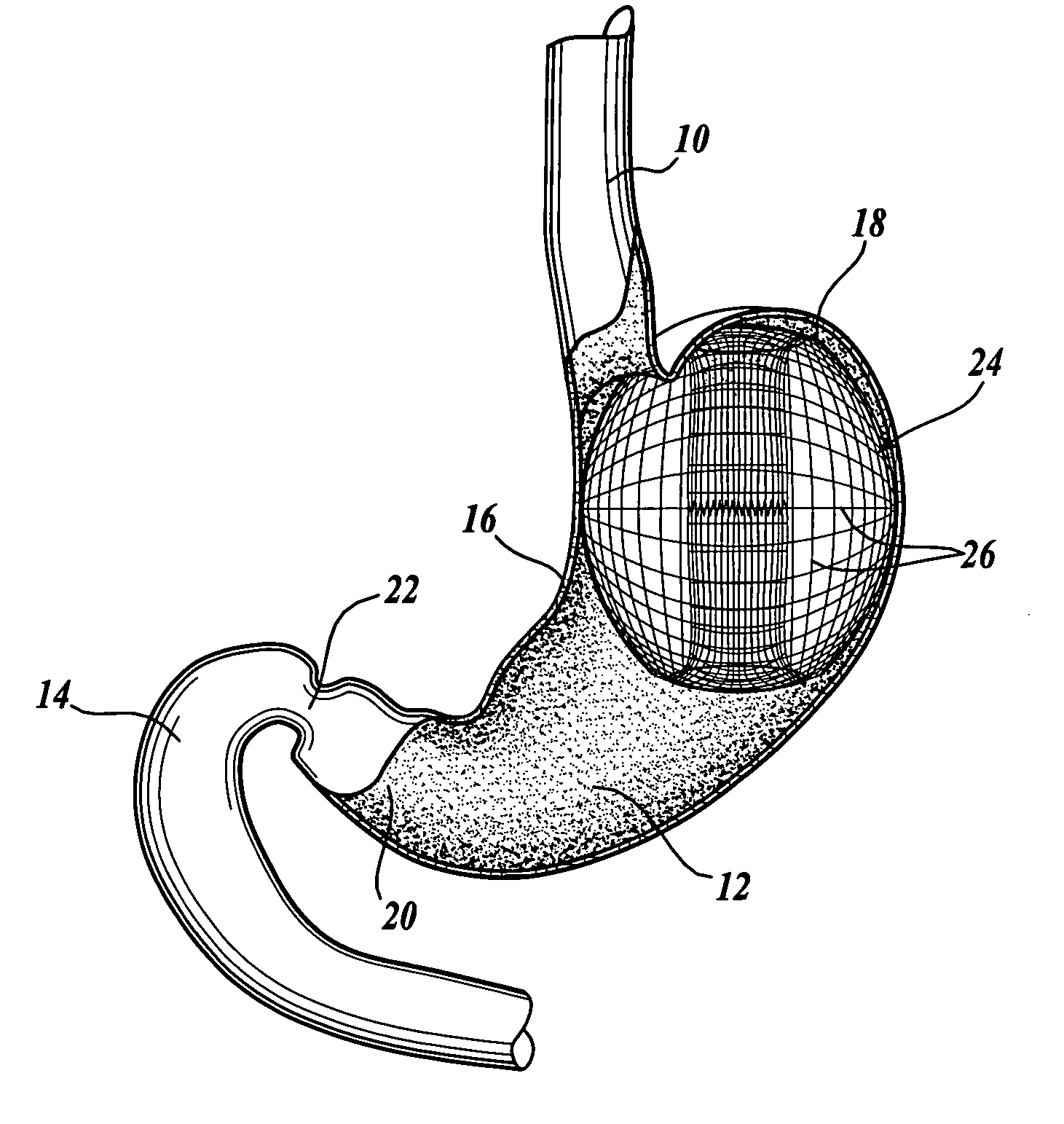
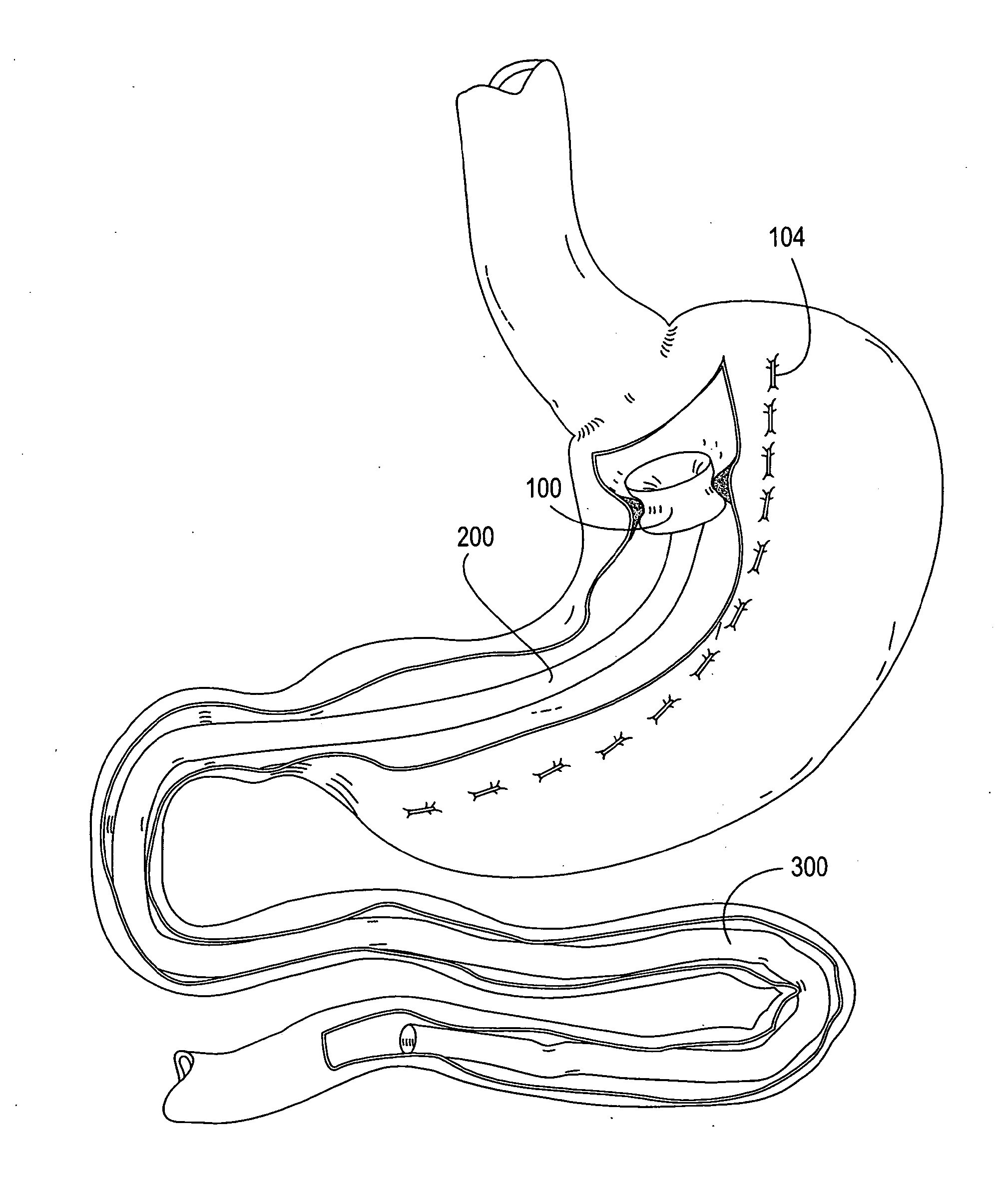
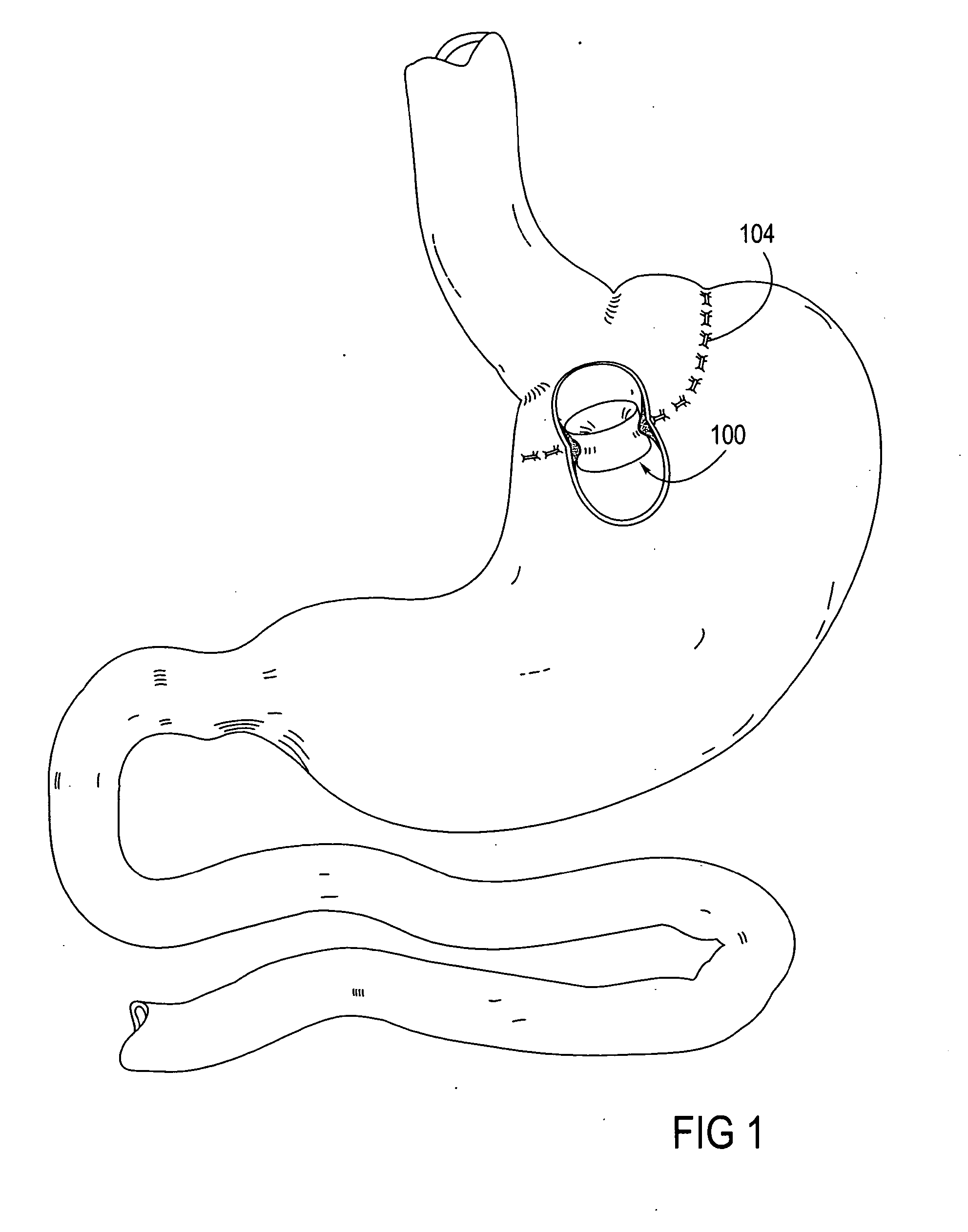
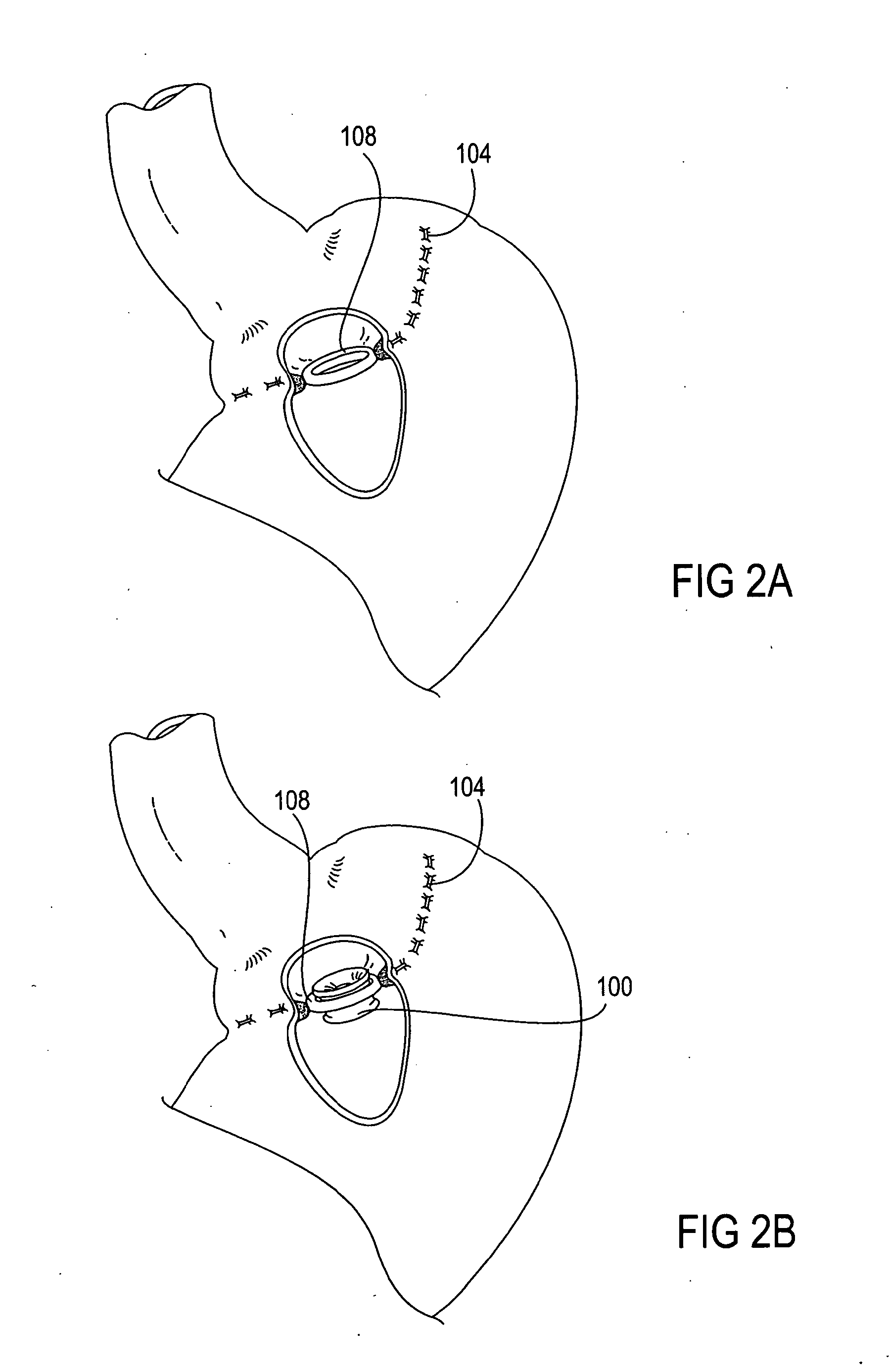
Intragastric prosthesis for the treatment of morbid obesity
A porous weave of bioabsorbable filaments having an open mesh configuration is formed into an oblate shape having dimensions greater than the esophageal opening and gastric outlet of a stomach. The resulting prosthesis is deployed in the stomach and is of a size to be retained in the proximate portion thereof for exerting pressure on the upper fundus. The prosthesis limits the amount of food that may be held within the stomach, and exerts pressure on the fundus to create a sensation of being full, resulting in weight loss.
Owner:BOSTON SCI SCIMED INC
Devices and Methods for Treating Morbid Obesity
A surgical method of treating morbid obesity via bariatric procedures, carried out endoluminally and transluminally using endoscopic devices that are introduced through natural body openings without the necessity of creating any incisions in the abdominal wall.
Owner:MEDIGUS LTD
Apparatus and methods for treatment of morbid obesity
InactiveUS20150366693A1Effectively reducing stomach volumeStimulating intestinal responseSuture equipmentsIntravenous devicesStomaIntestinal structure
Apparatus and methods are described for treatment of morbid obesity using minimally invasive techniques. The apparatus includes a system of components that may be used separately or in combination for effectively reducing stomach volume, bypassing a portion of the stomach and / or small intestines, reducing nutrient absorption in the stomach and / or small intestines and / or depositing minimally or undigested food farther than normal into the intestines, thereby stimulating intestinal responses. The components described include an artificial stoma device, a gastric sleeve device, an intestinal sleeve device, a combined gastrointestinal sleeve device and permanent and detachable attachment systems. Also described are devices for delivering and deploying the components of the system.
Owner:VALENTX
Intragastric balloon assembly
InactiveCN101500517AFew partsLow costNon-surgical orthopedic devicesObesity treatmentStomach wallsMorbid obesity
The invention relates to an intragastric balloon for the treatment of persons having severe or morbid obesity problems, which reduces the number of elements in known systems and makes possible a saving in material in its manufacture. The intragastric balloon assembly comprises a balloon connected to a catheter in order to inflate it and includes a means for attaching it to the patient's body comprising a flat fixing plate with a single orifice through which the inflation catheter is passed, which plate is fixed subcutaneously in the patient's abdominal region and in a preferred embodiment an additional supporting member is provided through which the inflation catheter is firmly supported on the fixing plate and comprises a semi-rigid silicone washer or ring which is secured to the catheter and the supporting plate with appropriate adhesive, holding the catheter in place with adequate tension for the balloon to lie against the stomach wall perfectly but without any pressure. In addition to this the assembly comprises a multiple-puncture valve to which the inflation catheter is connected to inflate the balloon, which may in turn be located on the fixing plate, thus providing an alternate element for supporting the catheter and therefore the balloon assembly.
Owner:乔斯·拉斐尔·加尔扎·艾尔瓦热兹
Apparatus and methods for treatment of morbid obesity
InactiveUS9561127B2Reduce volumeReduce absorptionSuture equipmentsIntravenous devicesIntestinal structureStoma
Apparatus and methods are described for treatment of morbid obesity using minimally invasive techniques. The apparatus includes a system of components that may be used separately or in combination for effectively reducing stomach volume, bypassing a portion of the stomach and / or small intestines, reducing nutrient absorption in the stomach and / or small intestines and / or depositing minimally or undigested food farther than normal into the intestines, thereby stimulating intestinal responses. The components described include an artificial stoma device, a gastric sleeve device, an intestinal sleeve device, a combined gastrointestinal sleeve device and permanent and detachable attachment systems. Also described are devices for delivering and deploying the components of the system.
Owner:VALENTX
Duodenum casing pipe and manufacturing method thereof
InactiveCN102697590AThe process of processing tiny structures is simpleEasy to operateNon-surgical orthopedic devicesGas phaseBiocompatibility Testing
The invention discloses a duodenum casing pipe and a manufacturing method of the duodenum casing pipe. The duodenum casing pipe comprises an anchoring part made of nitinol and a casing pipe with a micro-nanometer type anti-sticking structure. The manufacturing method of the duodenum casing pipe comprises the following steps: step one, manufacturing a casing pipe mold with the micro-nanometer type anti-sticking structure; step two, depositing a layer of thin polymer film sleeve on the surface of the mold in the chemical vapor mode; and step three, taking the thin polymer film sleeve from the mould. According to the duodenum casing pipe and the manufacturing method of the duodenum casing pipe, the existing casing pipe can be better applied in treating morbid obesity. The manufacturing method of the duodenum casing pipe is simple in the small structure processing process and easy in operation. The duodenum casing pipe takes biocompatible polymer as the small structure material, so that the biocompatibility is favorable. Meanwhile, the duodenum casing pipe has sufficient physical and chemical stability, and is hard to oxidize or corrode, so that the duodenum casing pipe can be directly applied in the biomedicine field.
Owner:SHANGHAI JIAO TONG UNIV
Arterial constrictor for weight loss treatment
InactiveUS20160022459A1Reduce absorptionControl digestive efficiencyGuide wiresSurgical navigation systemsMorbid obesityArterial blood supply
This document provides methods and devices involved in medical treatment of morbid obesity. For example, this document provides methods and devices for reducing the digestive efficiency of the intestines by decreasing the arterial blood supply to the intestines.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES +1
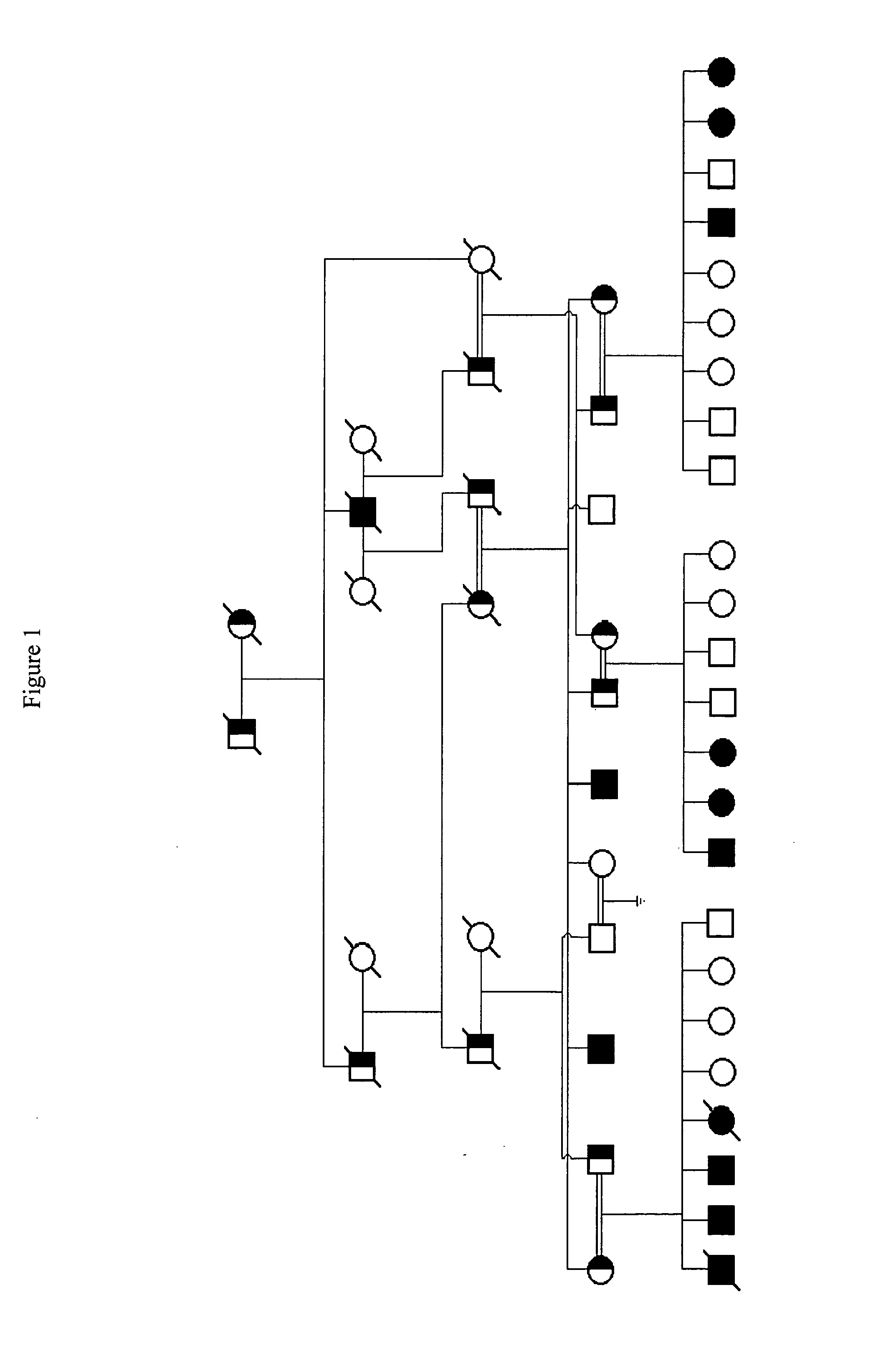
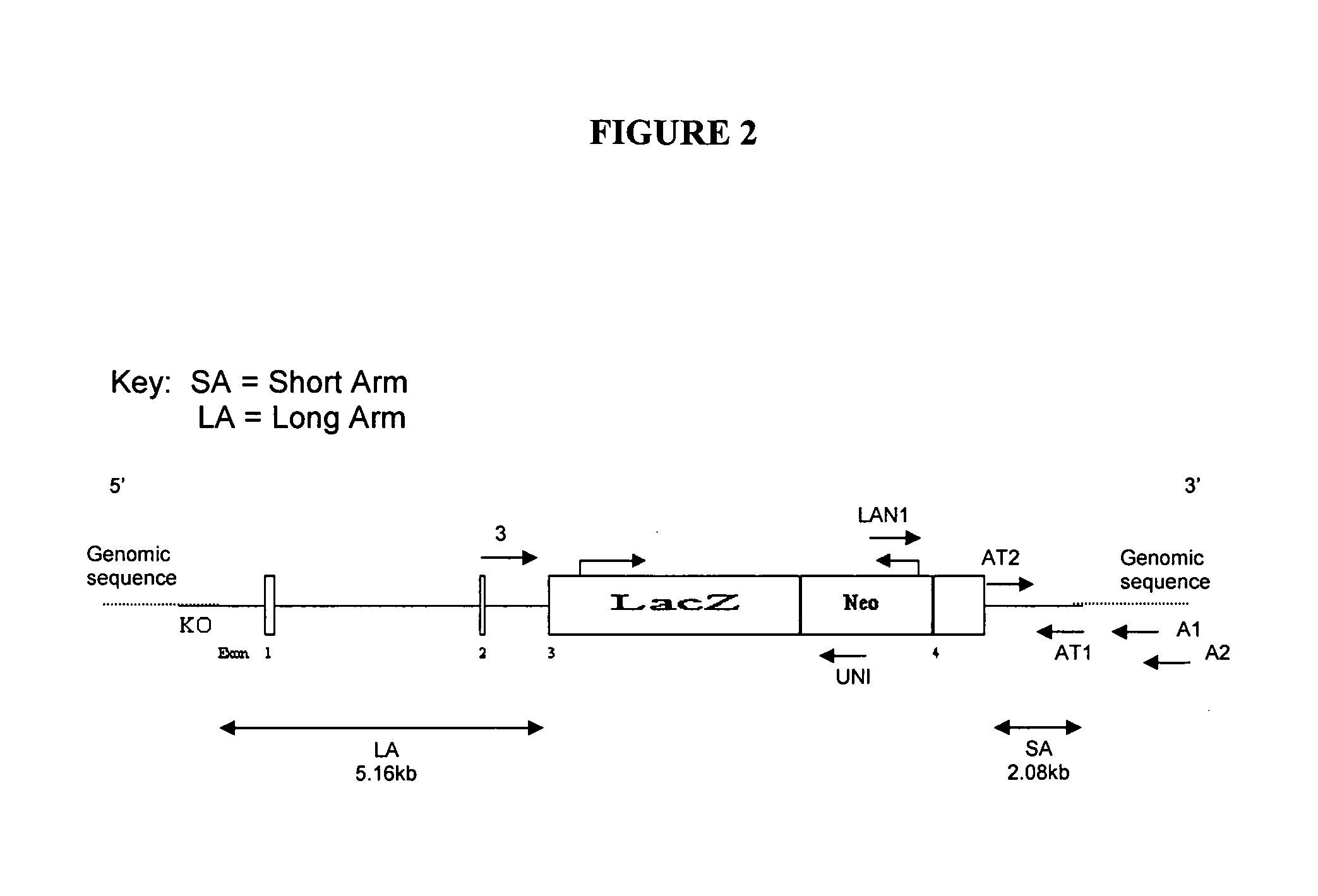
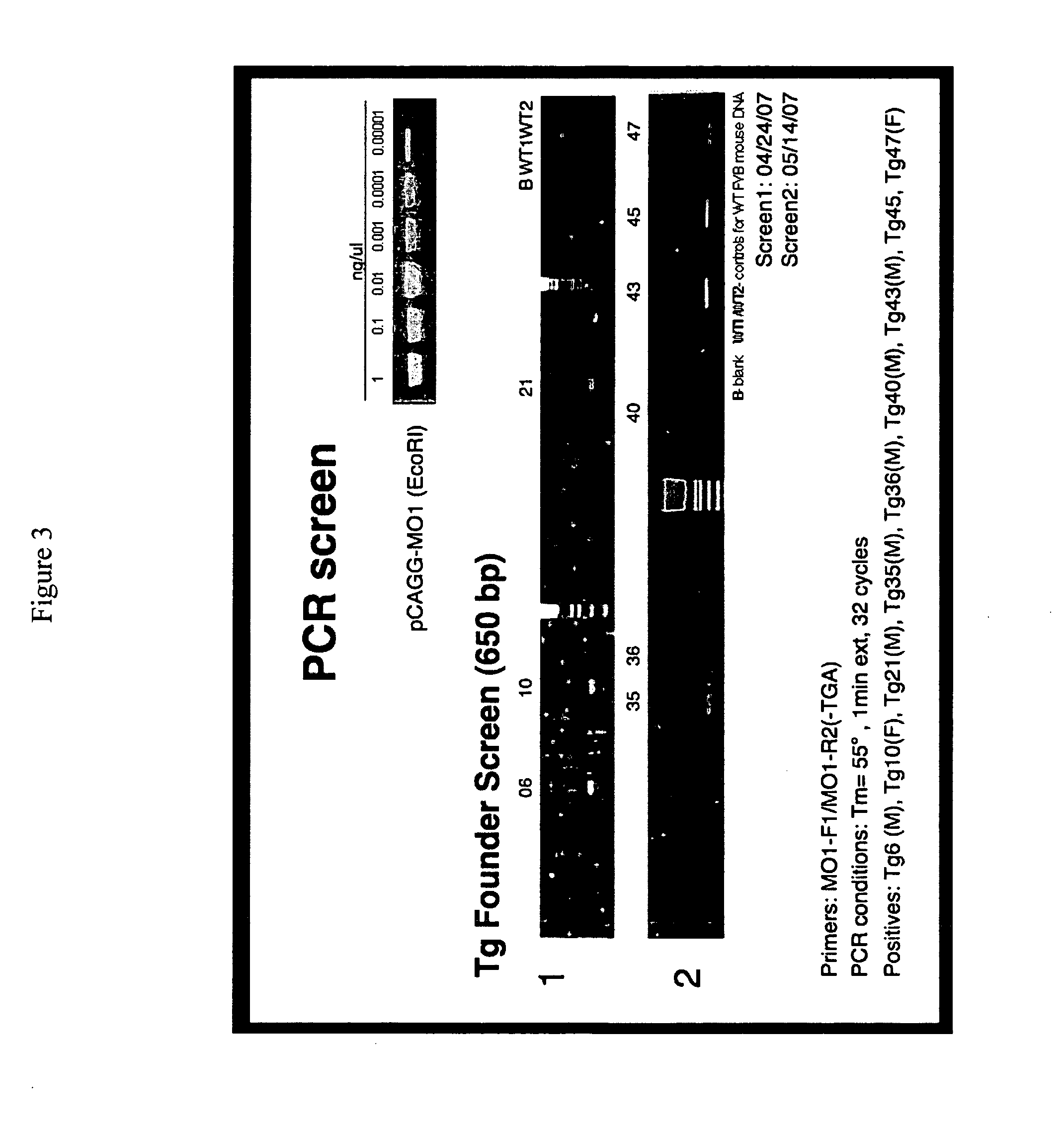
MO-1, A Gene Associated With Morbid Obesity
InactiveUS20100077496A1High activityReduced activityAntibacterial agentsOrganic active ingredientsHuman animalMorbid obesity
MO-1 is a newly identified gene and gene product associated with morbid obesity. Isolated MO-1 nucleic acids, MO-1 polypeptides, oligonucleotides that hybridize to MO-1 nucleic adds, and vectors, including expression vectors, comprising MO-1 nucleic acids are disclosed, as are isolated host cells, antibodies, transgenic non-human animals, compositions, and kits relating to MO-1. Methods of detecting the presence of MO-1 nucleic acid, screening for agents which affect MO-1 activity, and screening for MO-1 variants are also disclosed.
Owner:MT SINAI SCHOOL OF MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com