Patents
Literature
6677results about How to "Accurate prediction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
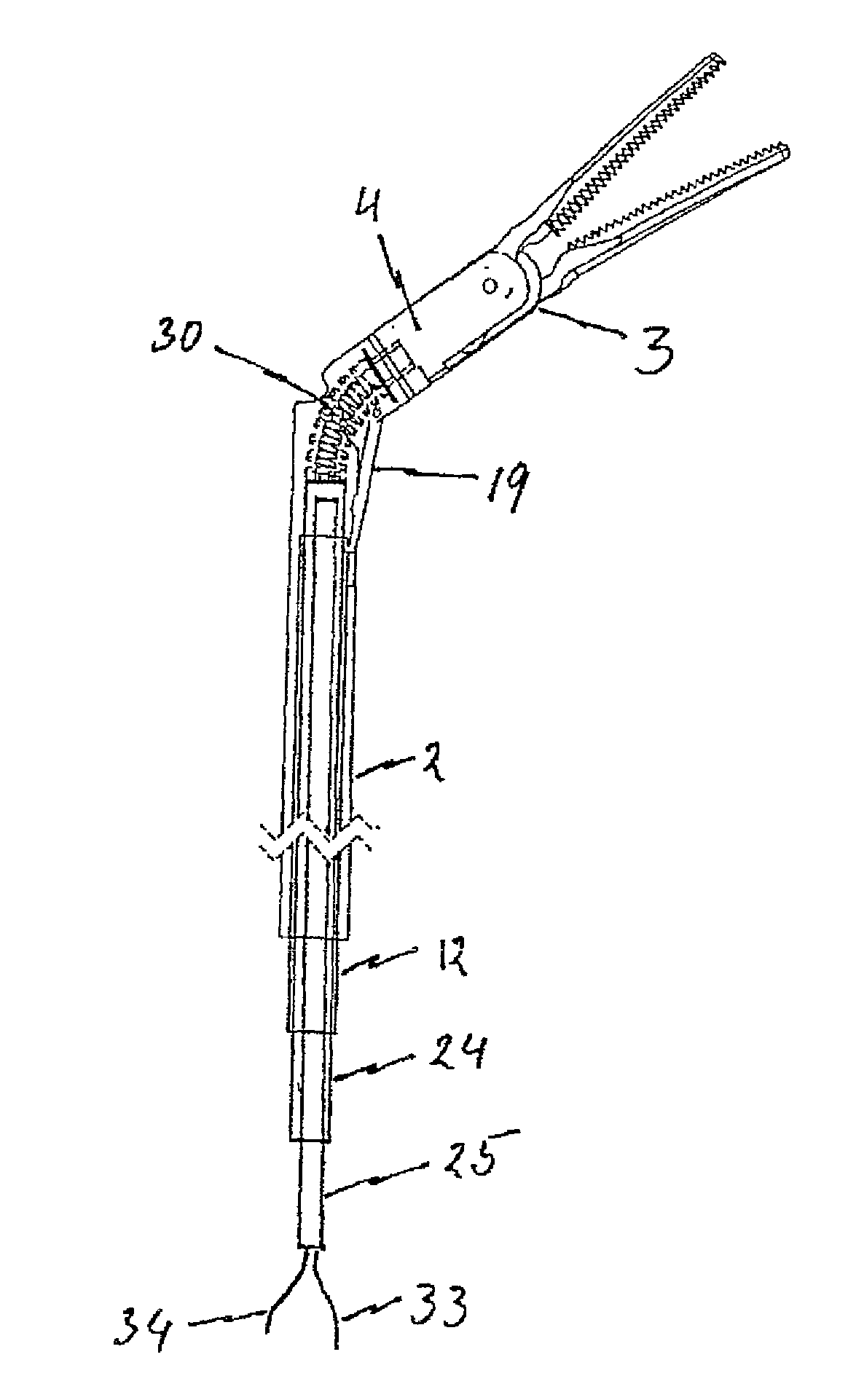
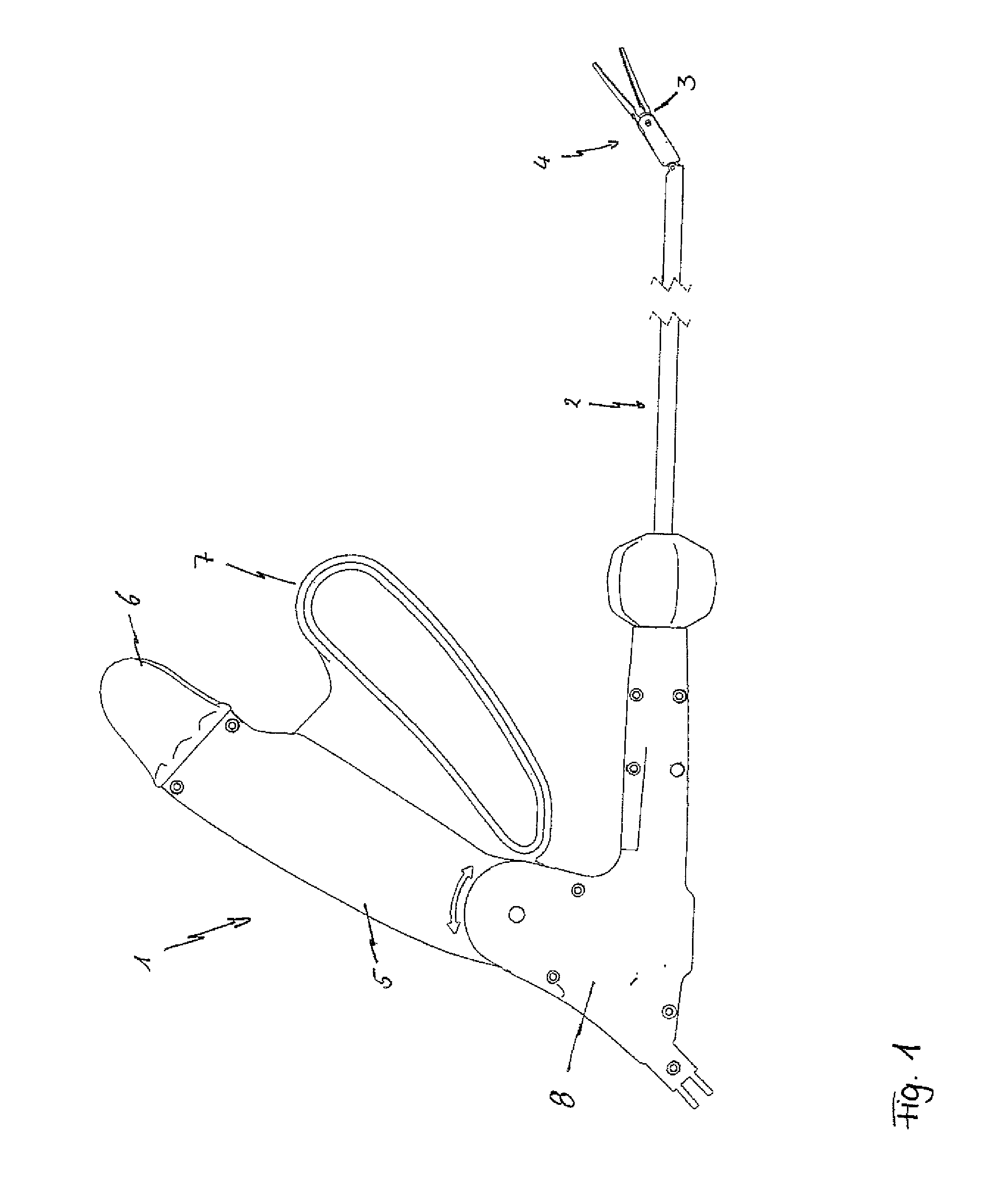
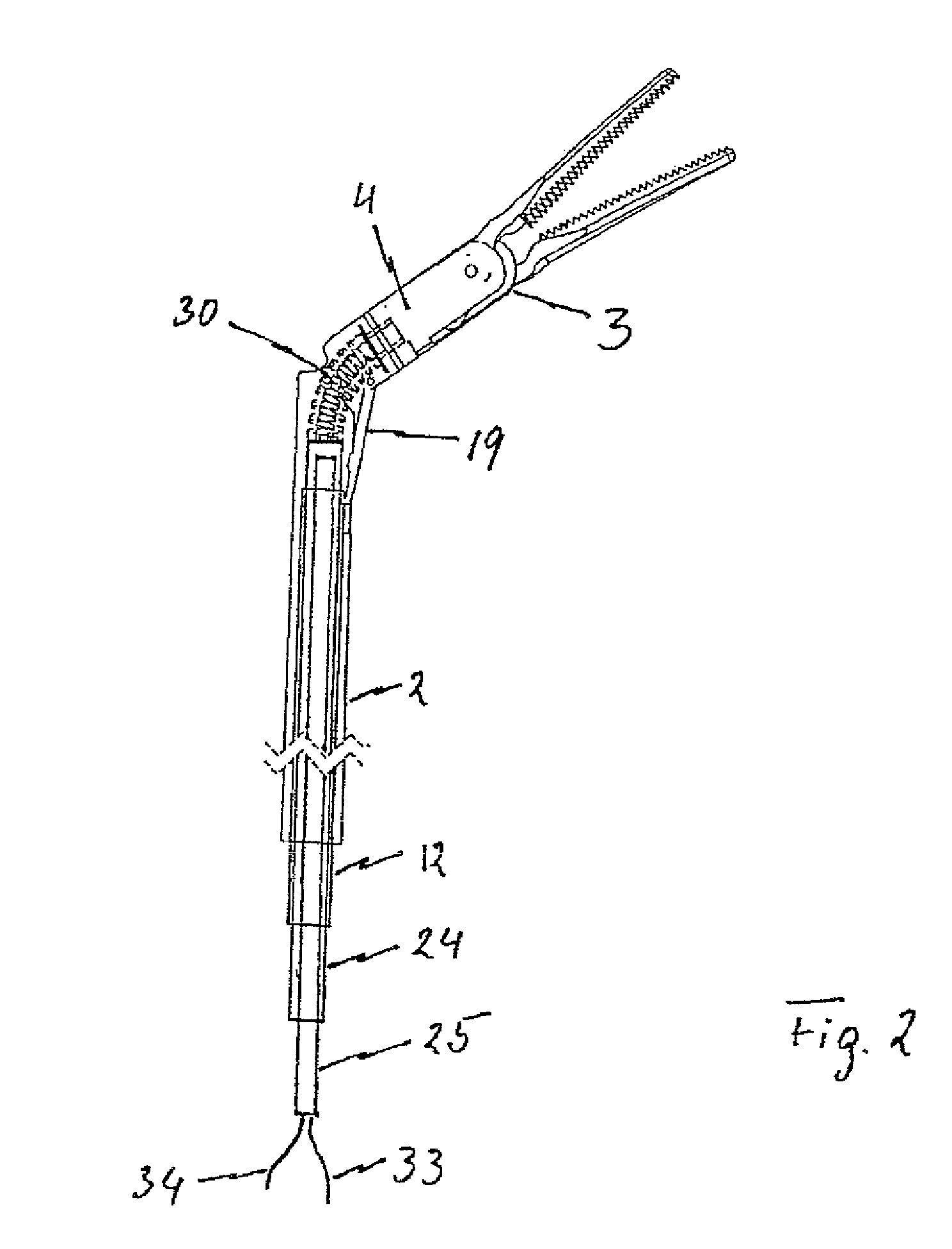
Surgical instrument with elastically movable instrument head
ActiveUS8876858B2More working spaceSufficient torqueSurgical instruments for heatingSurgical forcepsEngineeringSurgical device
Disclosed is a surgical instrument comprising an instrument handle, an instrument shaft shaft having a distal end and a proximal end at which the instrument handle is linked, an instrument head, pivotally linked to the distal end of the instrument shaft via a hinge shaft or pins and comprising an effector rotatably supported in said instrument head around its longitudinal axis as well as a surgical tool held by said effector, and a mechanical transmission system at least partially arranged within said instrument shaft transmitting mechanical operation signals from said instrument handle to said instrument head at least for pivoting and / or rotating motions. A bending flexible as well as rotating rigid, hollow spindle is arranged bypassing said hinge shaft or pins and directly connecting said effector with the mechanical transmission system for transmitting at least rotating signals via said spindle to said effector.
Owner:TUEBINGEN SCI MEDICAL
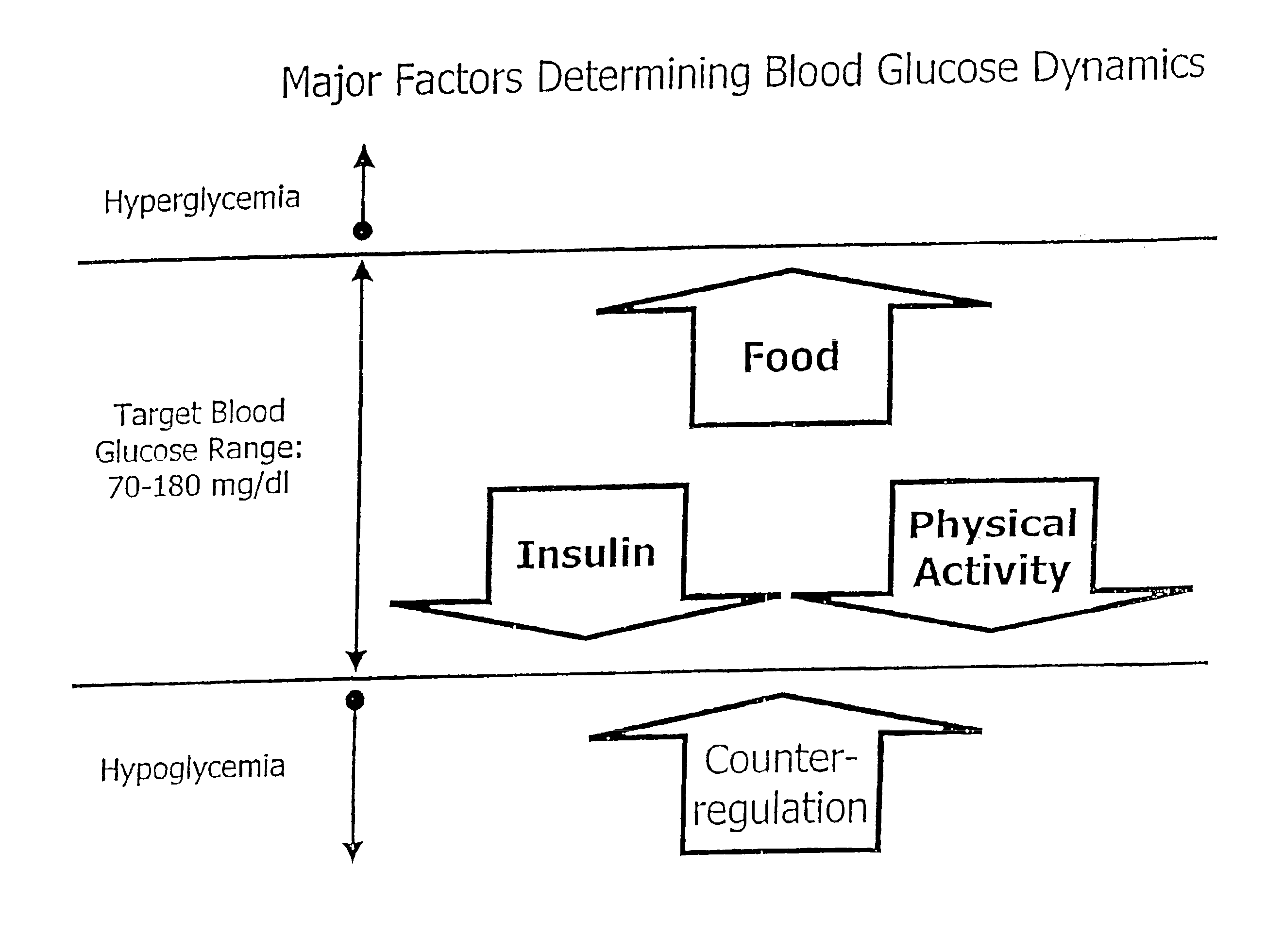
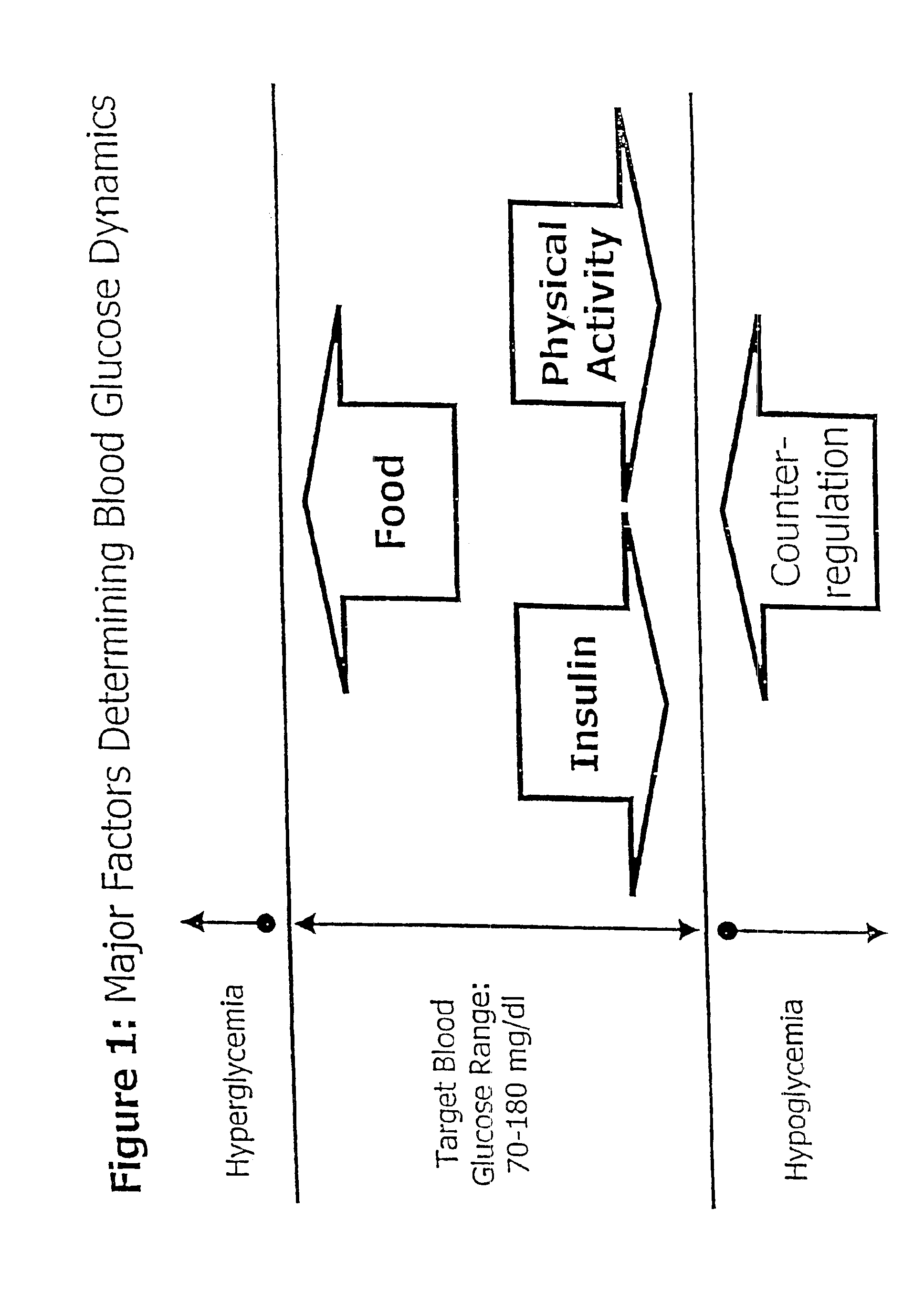
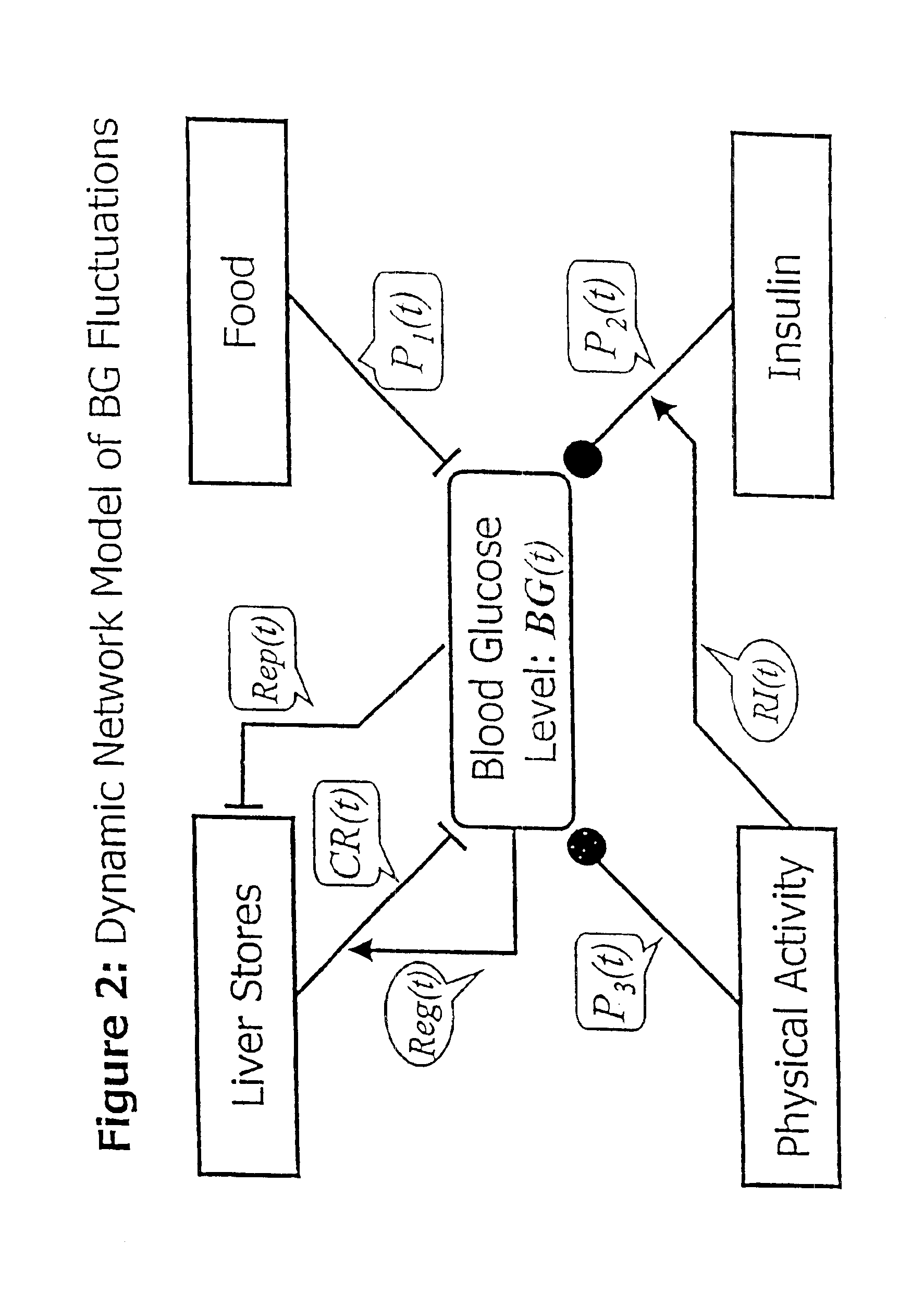
Method and apparatus for predicting the risk of hypoglycemia
InactiveUS6923763B1Reduced responseOptimal glucose controlMedical simulationHealth-index calculationInsulin infusionMedicine
The invention relates to a method which utilizes blood glucose (“BG”) sampling, insulin infusion / injection records, heart rate (“HR”) information and heart rate varability (“HRV”) information to estimate BG in the near future and to estimate of the risk of the onset of hypoglycemia. The invention also relates to an apparatus for predicting BG levels and for assessing the risk of the onset of hypoglycemia in the near future. The invention is based on two predetermined bio-mathematical routines: a network model of BG fluctuations and a BG profile for assessment of the risk of hypoglycemia.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
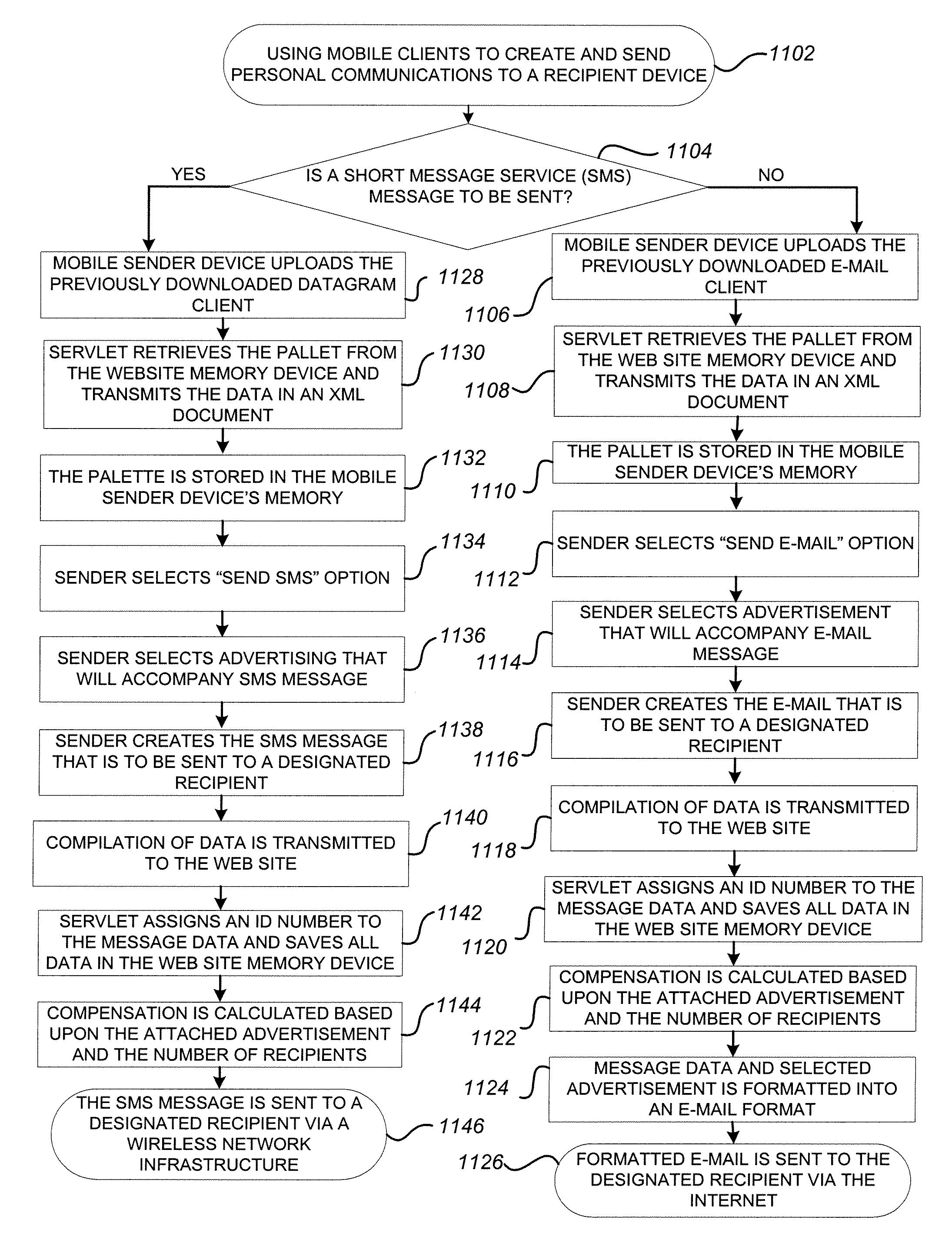
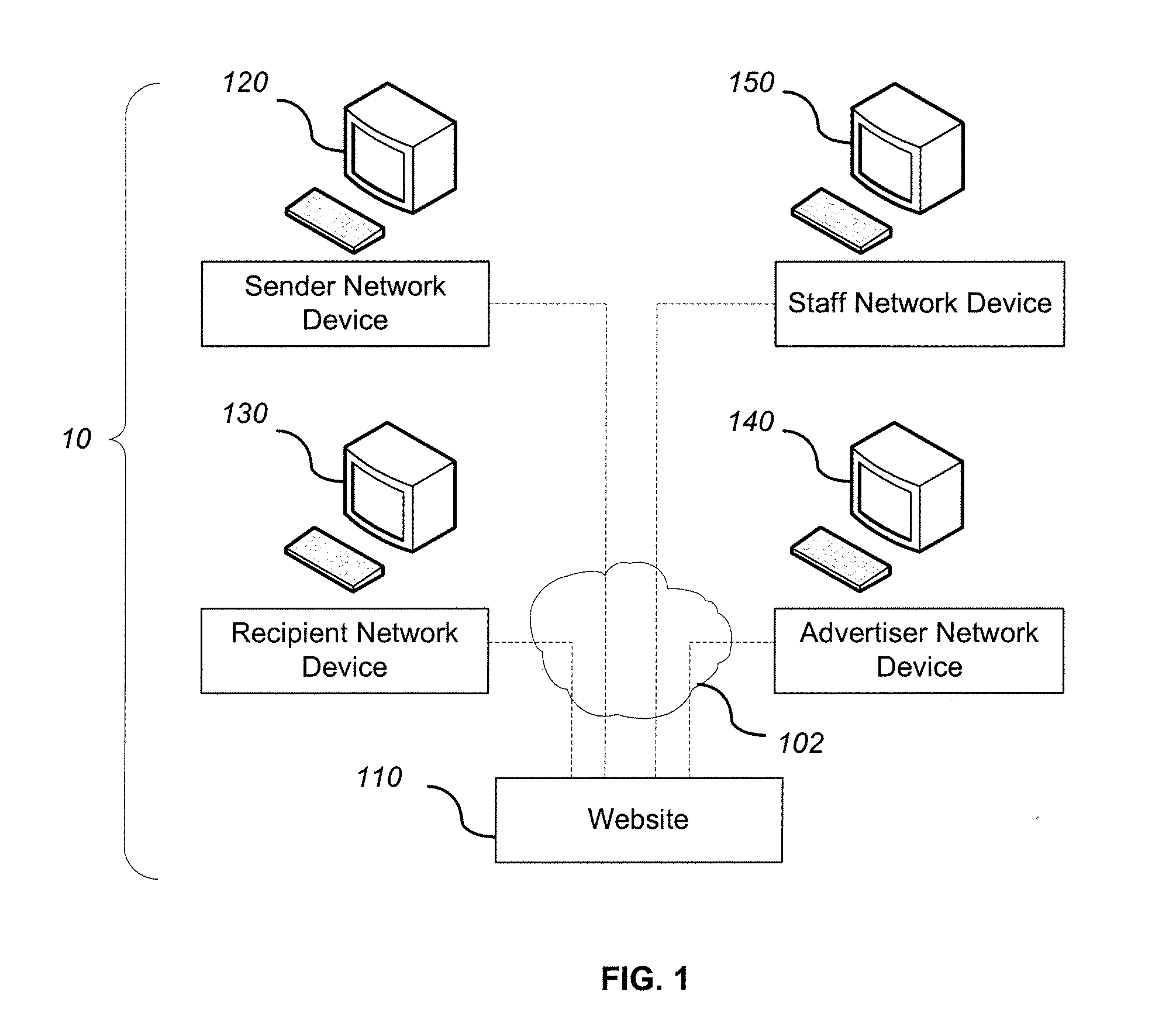
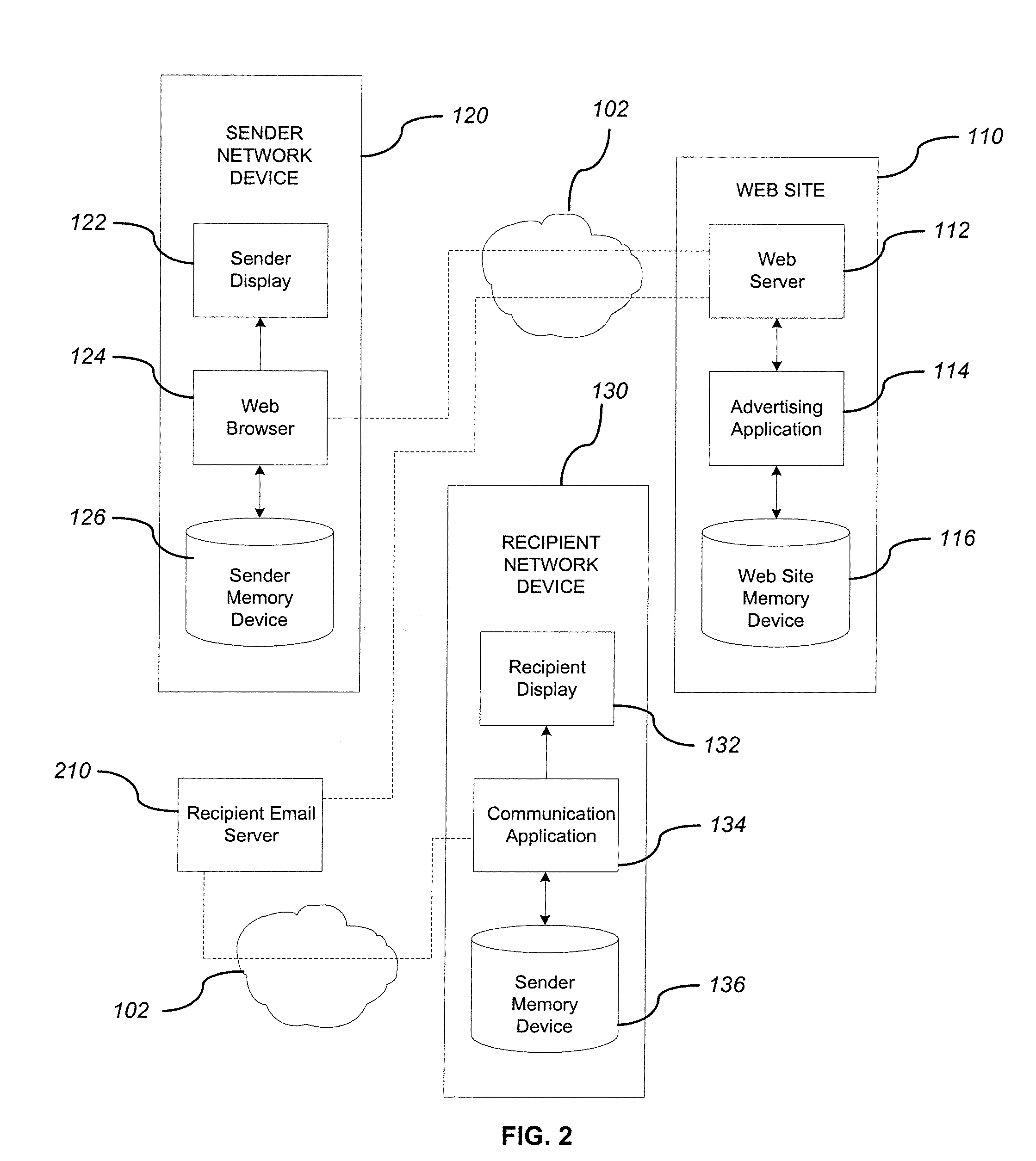
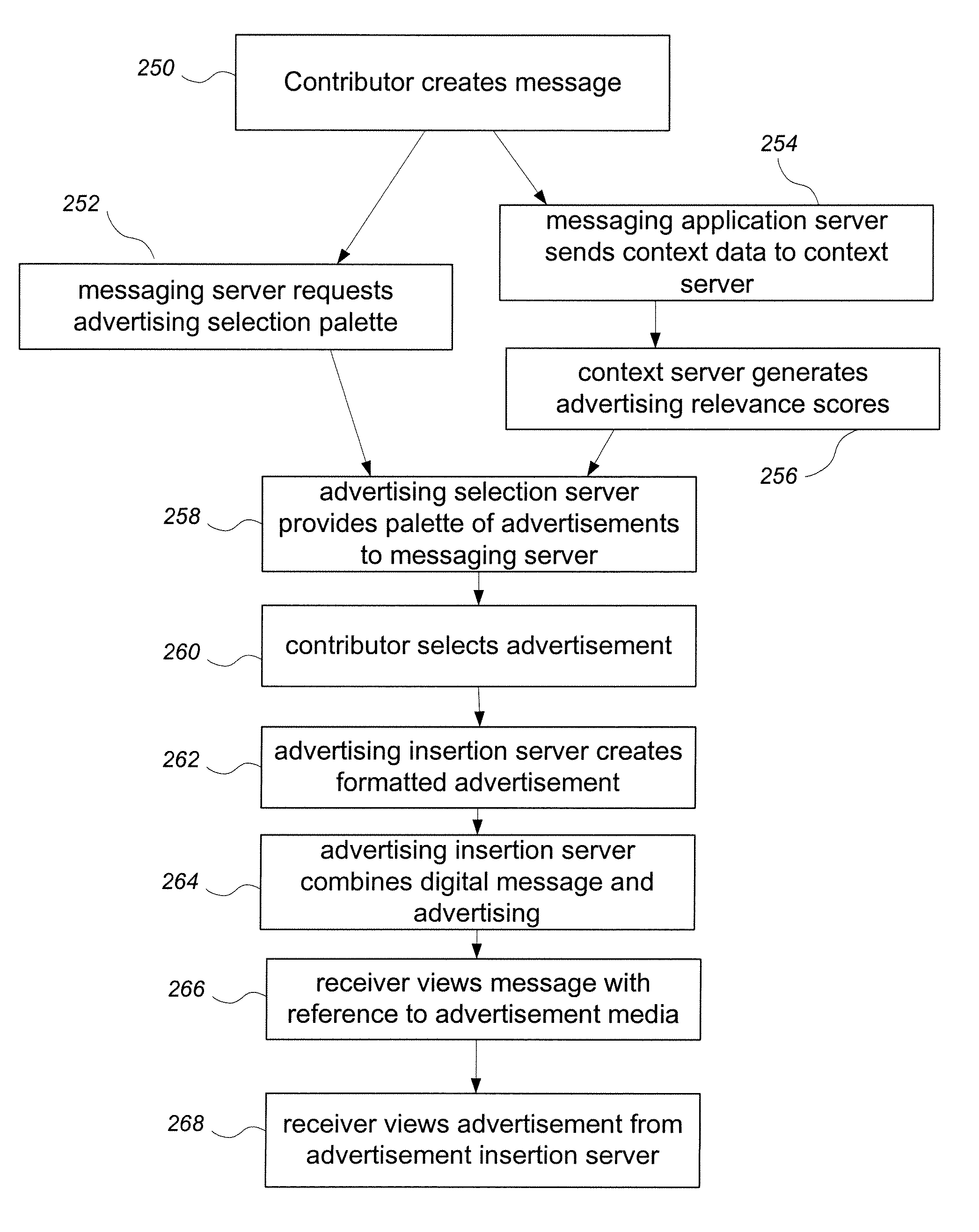
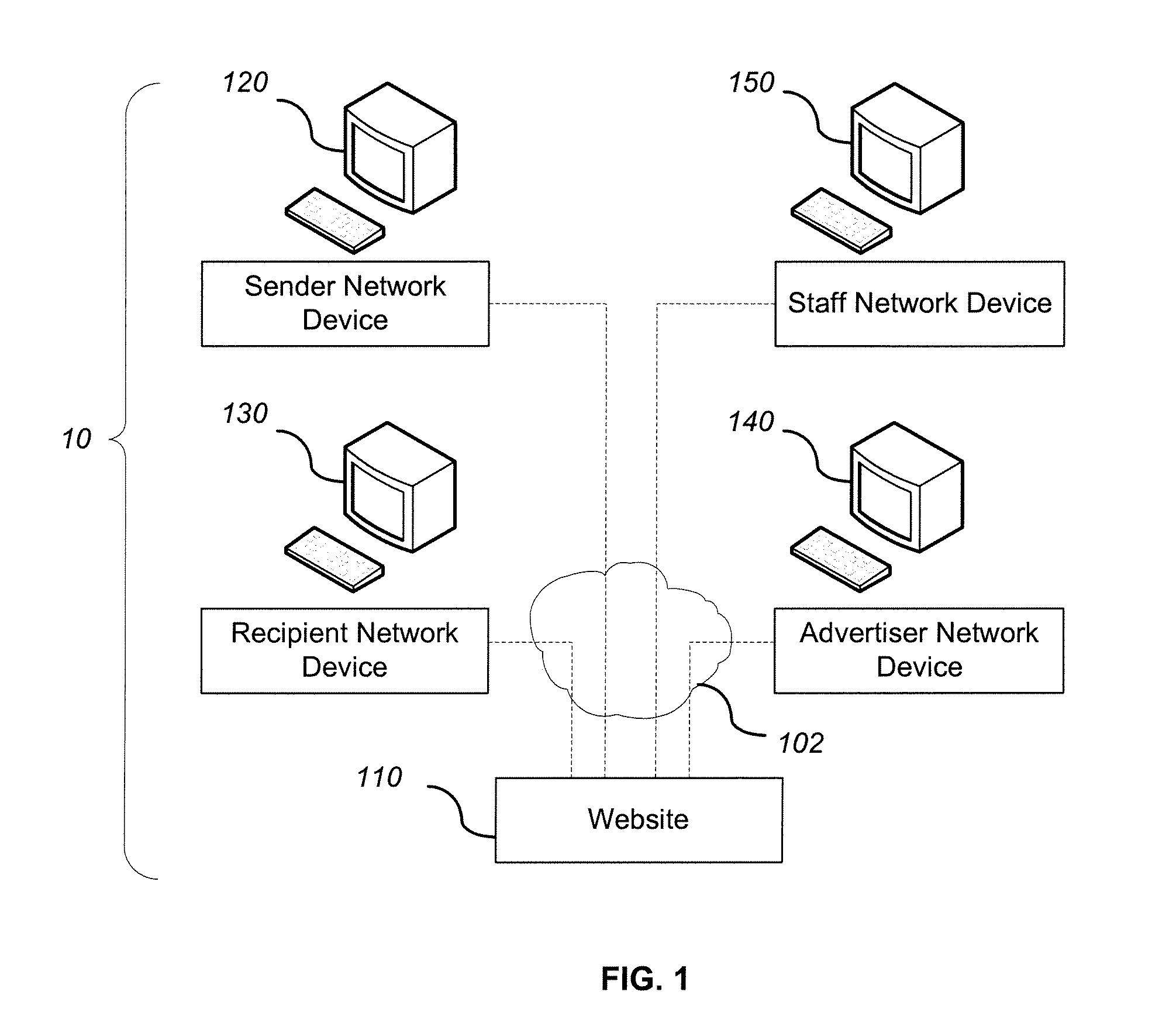
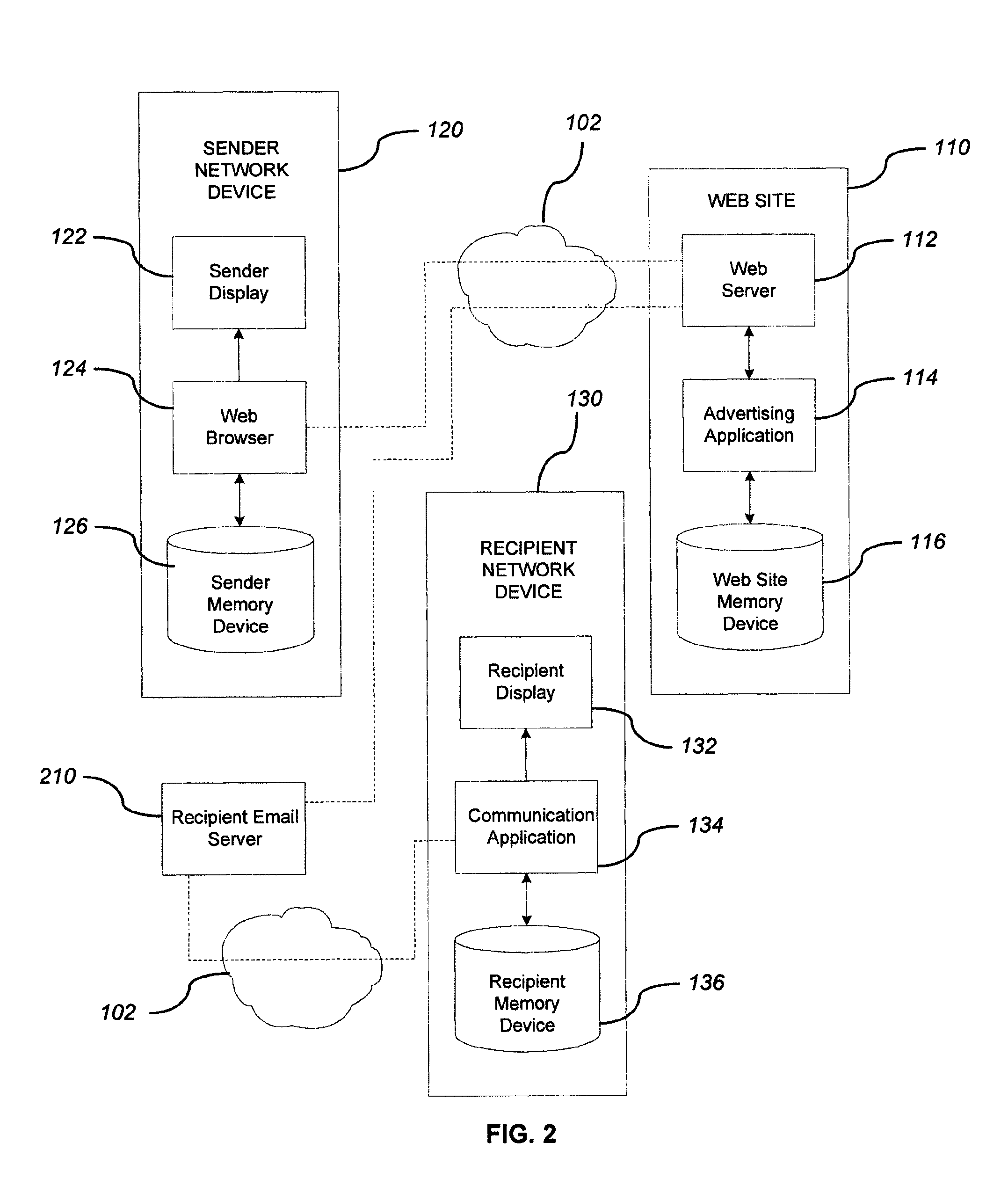
System and method for adding an advertisement to a personal communication
ActiveUS20090030774A1Accurate predictionIncrease probabilityDiscounts/incentivesAdvertisementsWeb siteThird party
A system and method is provided for adding an advertisement to a digital message and providing additional communication data to a recipient that interacts with the advertisement regardless of the network device the recipient is utilizing. An advertisement generator residing on a network host accepts digital messages from contributors and allows the contributors to select an advertisement to be displayed with their contributed messages. These digital messages may be sent to specified recipients or published on a Web site. Using stored personal data associated with the contributor and with the recipient of a digital message, in addition to the content of the message itself, the advertisement generator suggests advertisements to be included with the digital messages based on their contextual relevance. In exchange for including an advertisement with a digital message, a contributor is compensated. If the contributor-selected advertisement is provided by a third party advertiser, the message, contributor, and advertisement data is utilized to compensate the contributor of that message for sending it to at least one recipient or posting it on a Web site. If the advertisement is interactive, and the advertisement is interacted with, the advertisement generator will provide the recipient with additional communication data in a format that can be understood by the recipient network device.
Owner:ROTHSCHILD RICHARD ANTHONY
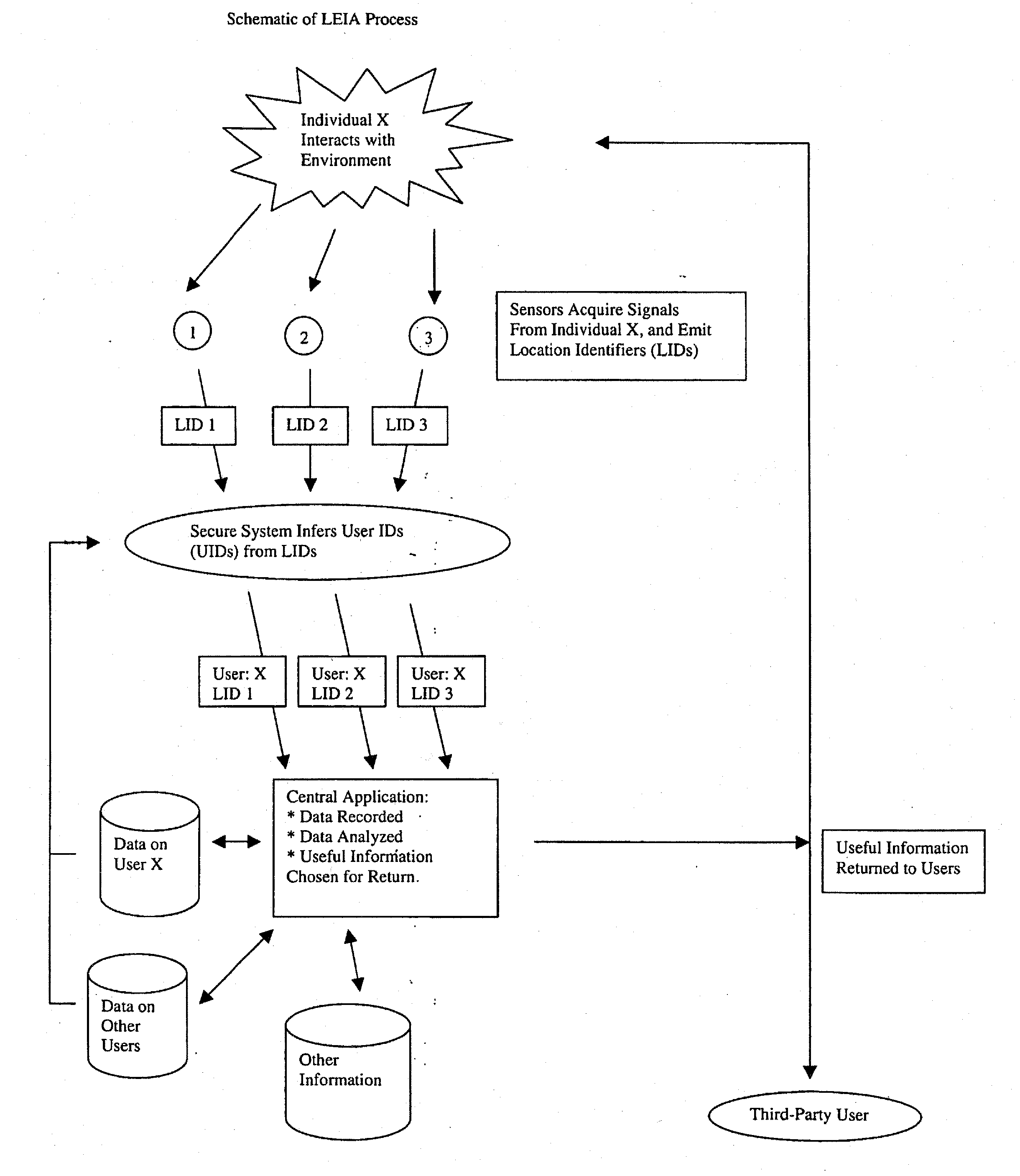
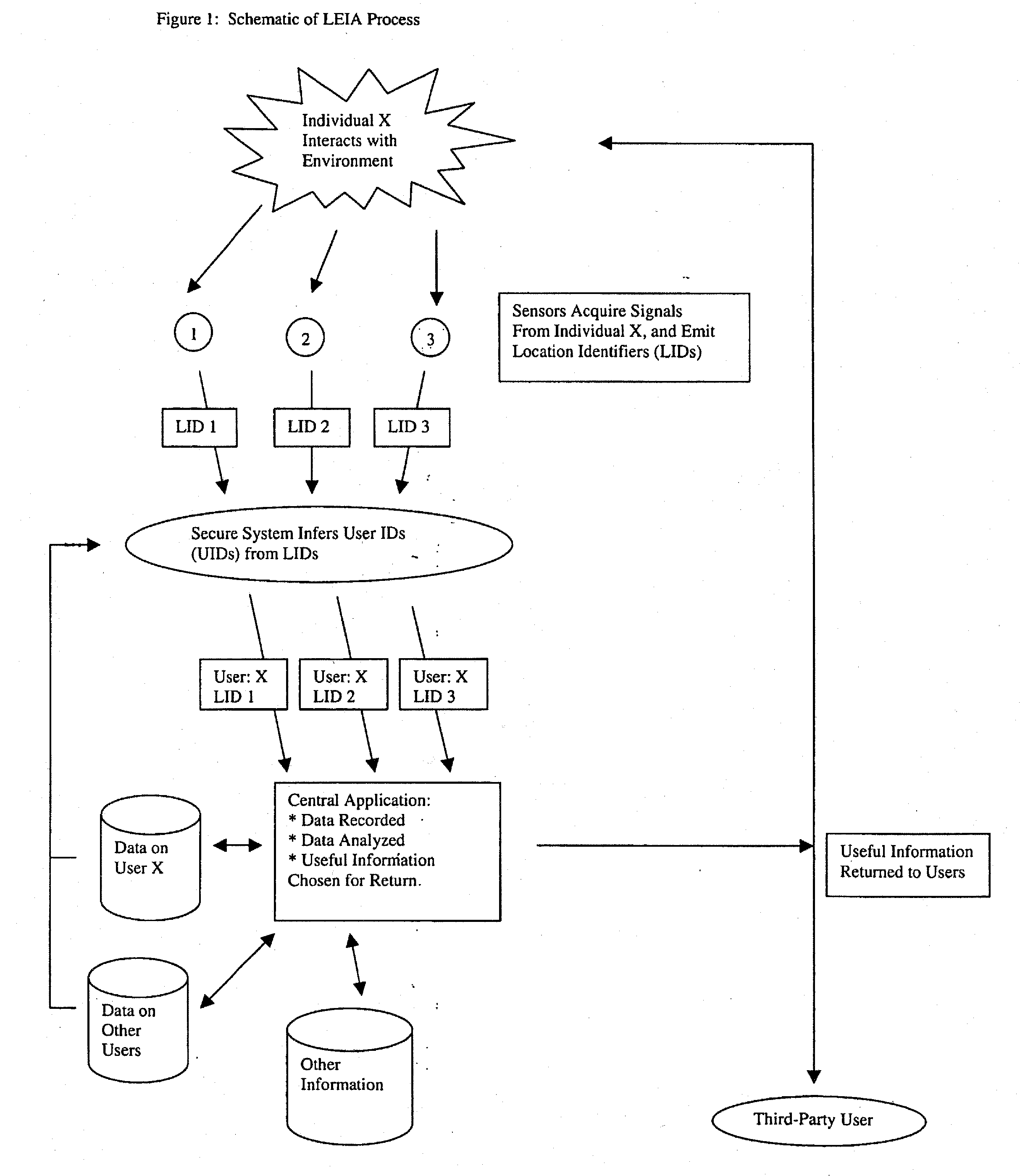
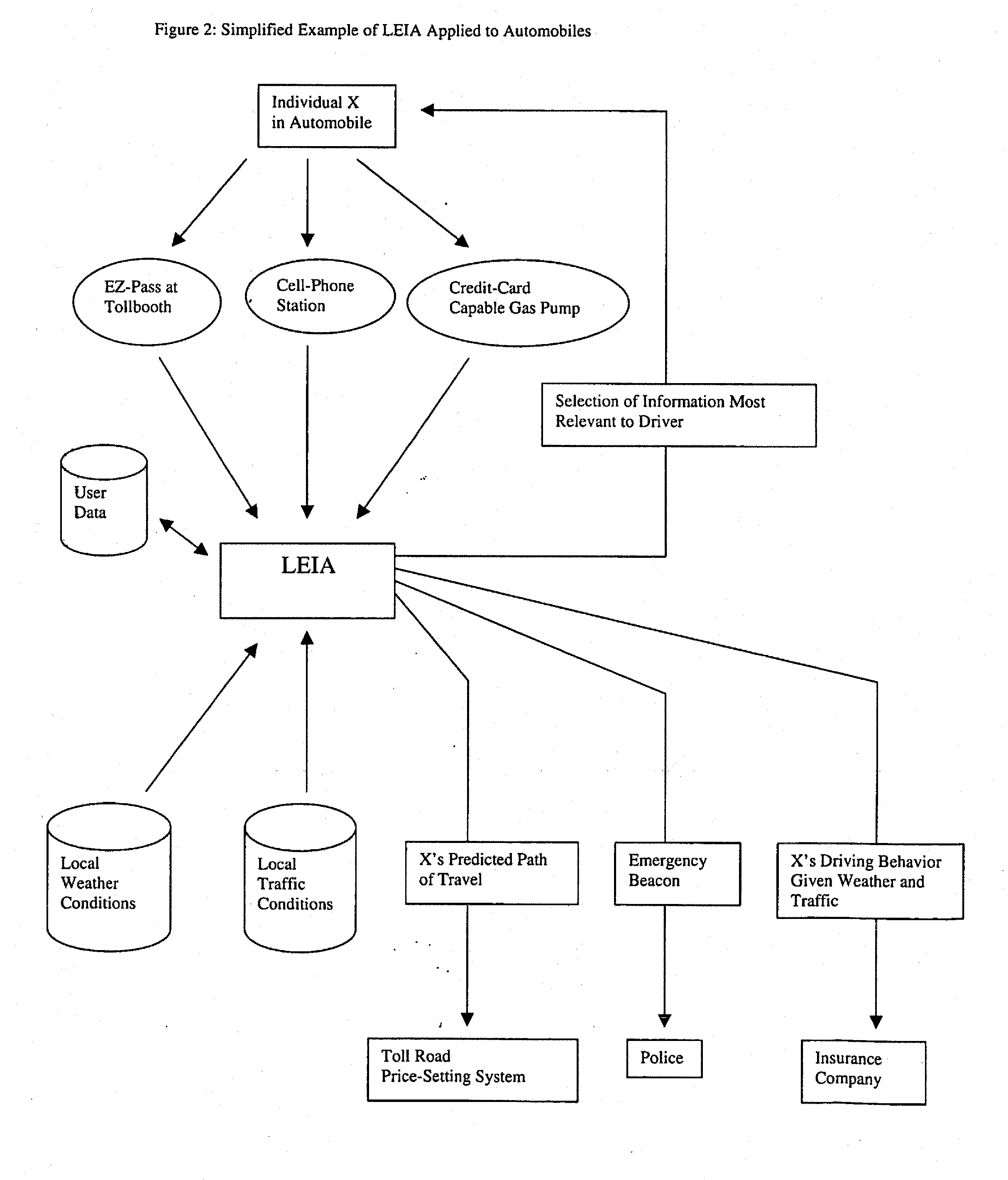
System for collecting, analyzing, and transmitting information relevant to transportation networks
InactiveUS20130059607A1Accurate predictionEmergency connection handlingFinanceThird partyPosition dependent
When individual persons or vehicles move through a transportation network, they are likely to be both actively and passively creating information that reflects their location and current behavior. In this patent, we propose a system that makes complete use of this information. First, through a broad web of sensors, our system collects and stores the full range of information generated by travelers. Next, through the use of previously-stored data and active computational analysis, our system deduces the identity of individual travelers. Finally, using advanced data-mining technology, our system selects useful information and transmits it back to the individual, as well as to third-party users; in short, it forms the backbone for a variety of useful location-related end-user applications.
Owner:APPLE INC
Method and apparatus for providing derived glucose information utilizing physiological and/or contextual parameters
InactiveUS20090177068A1Accurate predictionPredict glucose levelPhysical therapies and activitiesInertial sensorsState parameterD-Glucose
Various methods and apparatuses for measuring a state parameter of an individual using signals based on one or more sensors are disclosed. In one embodiment, a first set of signals is used in a first function to determine how a second set of signals is used in one or more second functions to predict the state parameter. In another embodiment, first and second functions are used where the state parameter or an indicator of the state parameter may be obtained from a relationship between the first function and the second function. The state parameter may, for example, include blood glucose levels, calories consumed or calories burned by the individual. Various methods for making such apparatuses are also disclosed.
Owner:J FITNESS LLC
System and method for integrating and validating genotypic, phenotypic and medical information into a database according to a standardized ontology
InactiveUS20070178501A1Safest and most effective treatmentGood decisionData processing applicationsMicrobiological testing/measurementData validationMedical record
The system described herein enables clinicians and researchers to use aggregated genetic and phenotypic data from clinical trials and medical records to make the safest, most effective treatment decisions for each patient. This involves (i) the creation of a standardized ontology for genetic, phenotypic, clinical, pharmacokinetic, pharmacodynamic and other data sets, (ii) the creation of a translation engine to integrate heterogeneous data sets into a database using the standardized ontology, and (iii) the development of statistical methods to perform data validation and outcome prediction with the integrated data. The system is designed to interface with patient electronic medical records (EMRs) in hospitals and laboratories to extract a particular patient's relevant data. The system may also be used in the context of generating phenotypic predictions and enhanced medical laboratory reports for treating clinicians. The system may also be used in the context of leveraging the huge amount of data created in medical and pharmaceutical clinical trials. The ontology and validation rules are designed to be flexible so as to accommodate a disparate set of clients. The system is also designed to be flexible so that it can change to accommodate scientific progress and remain optimally configured.
Owner:NATERA
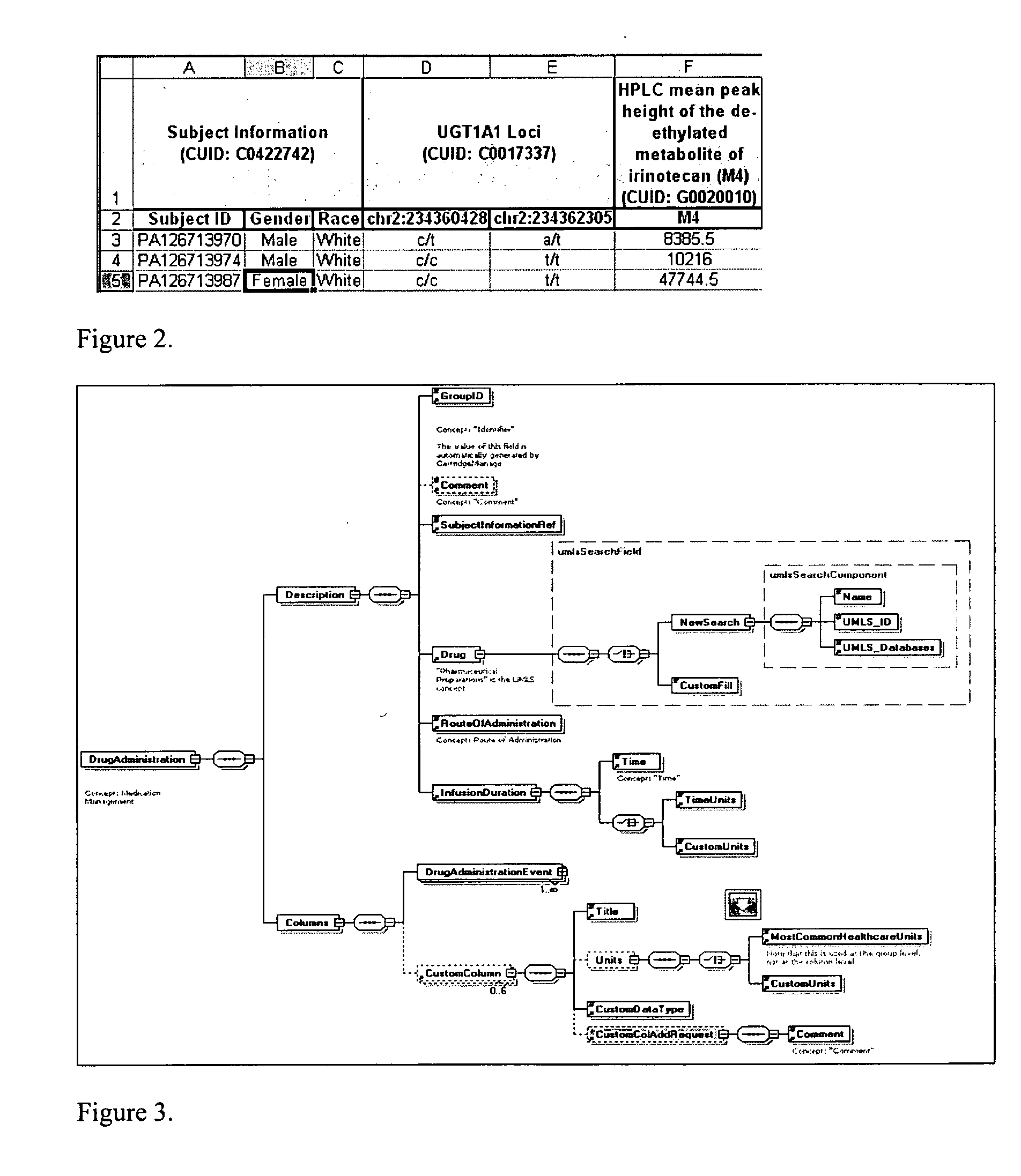
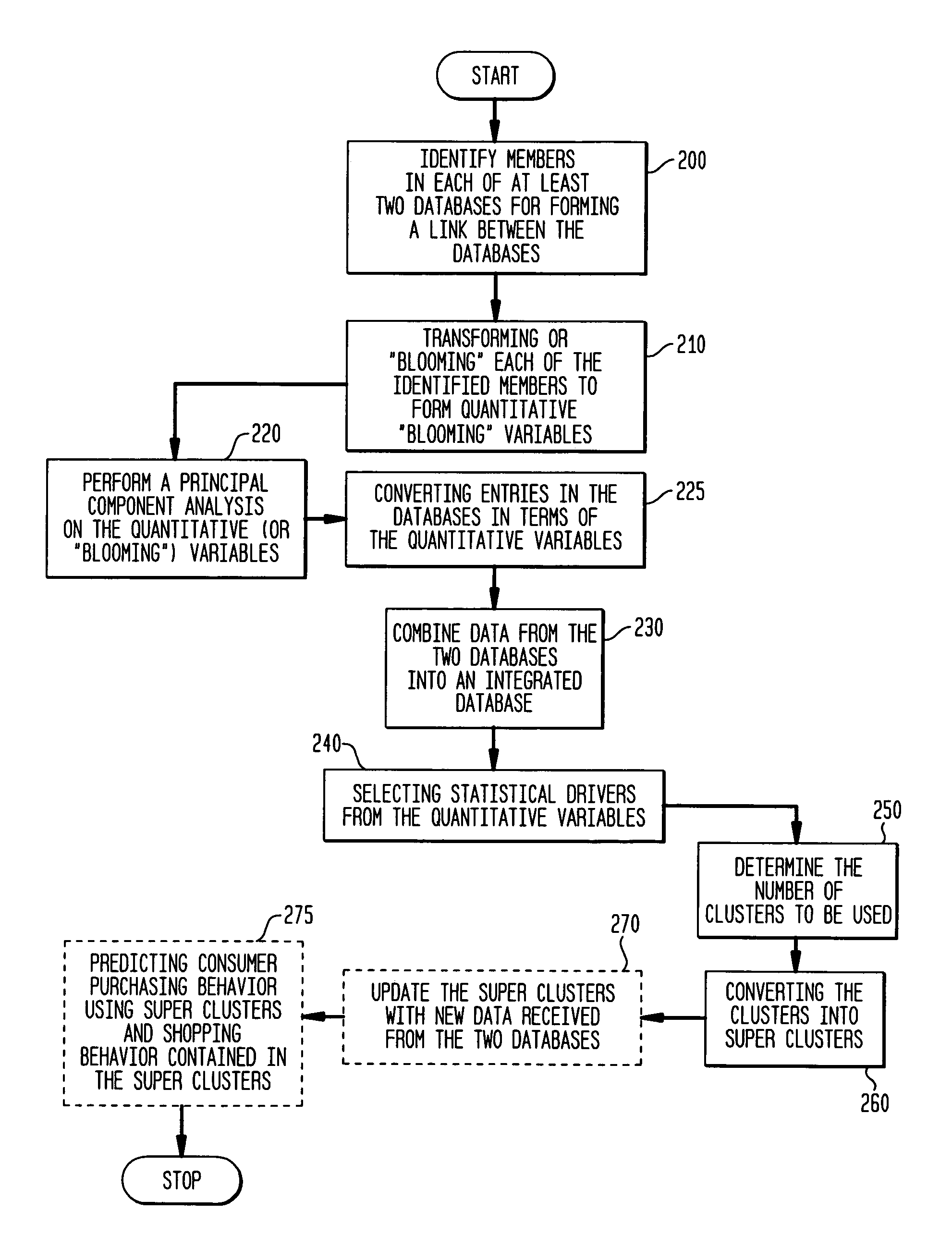
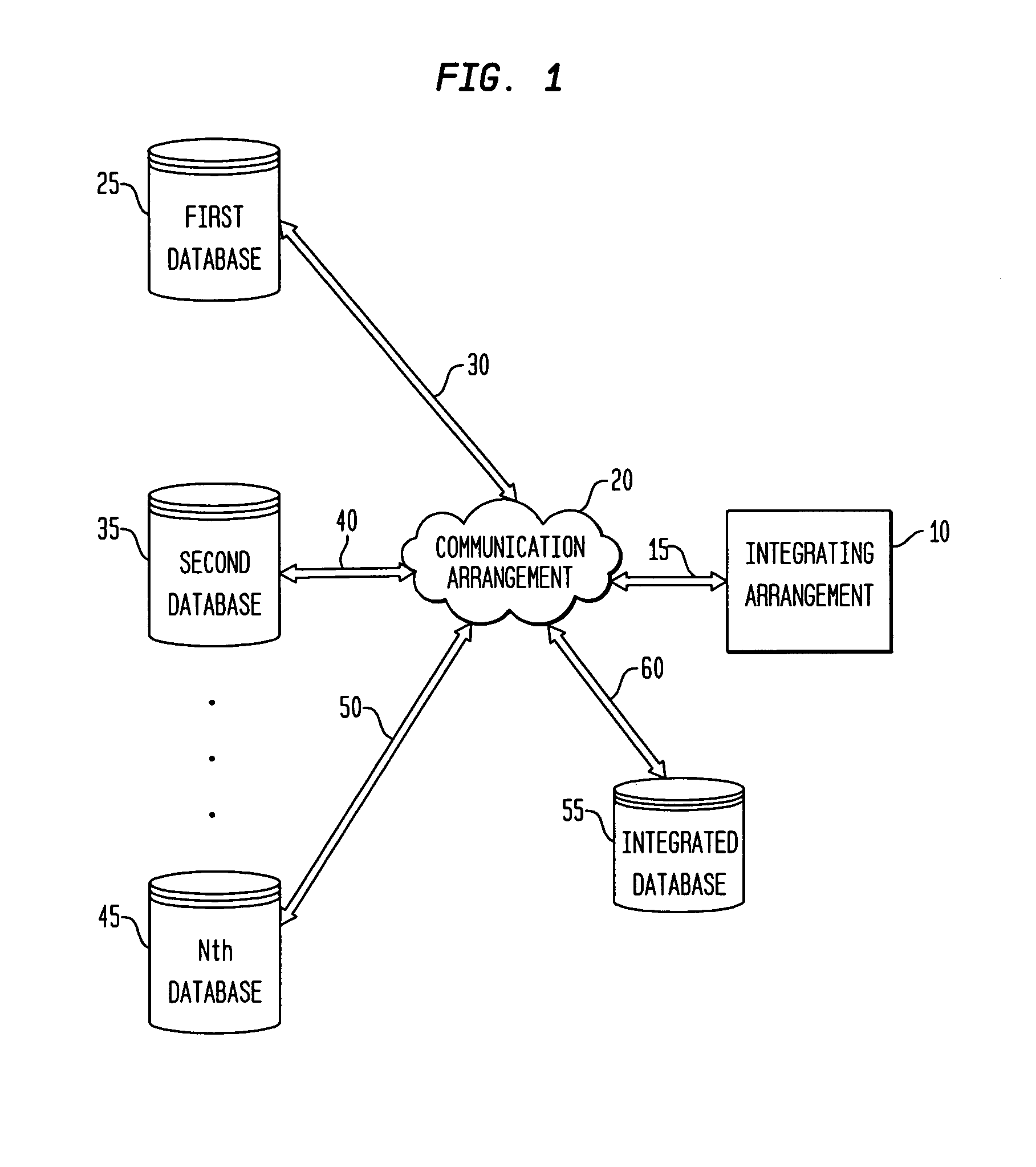
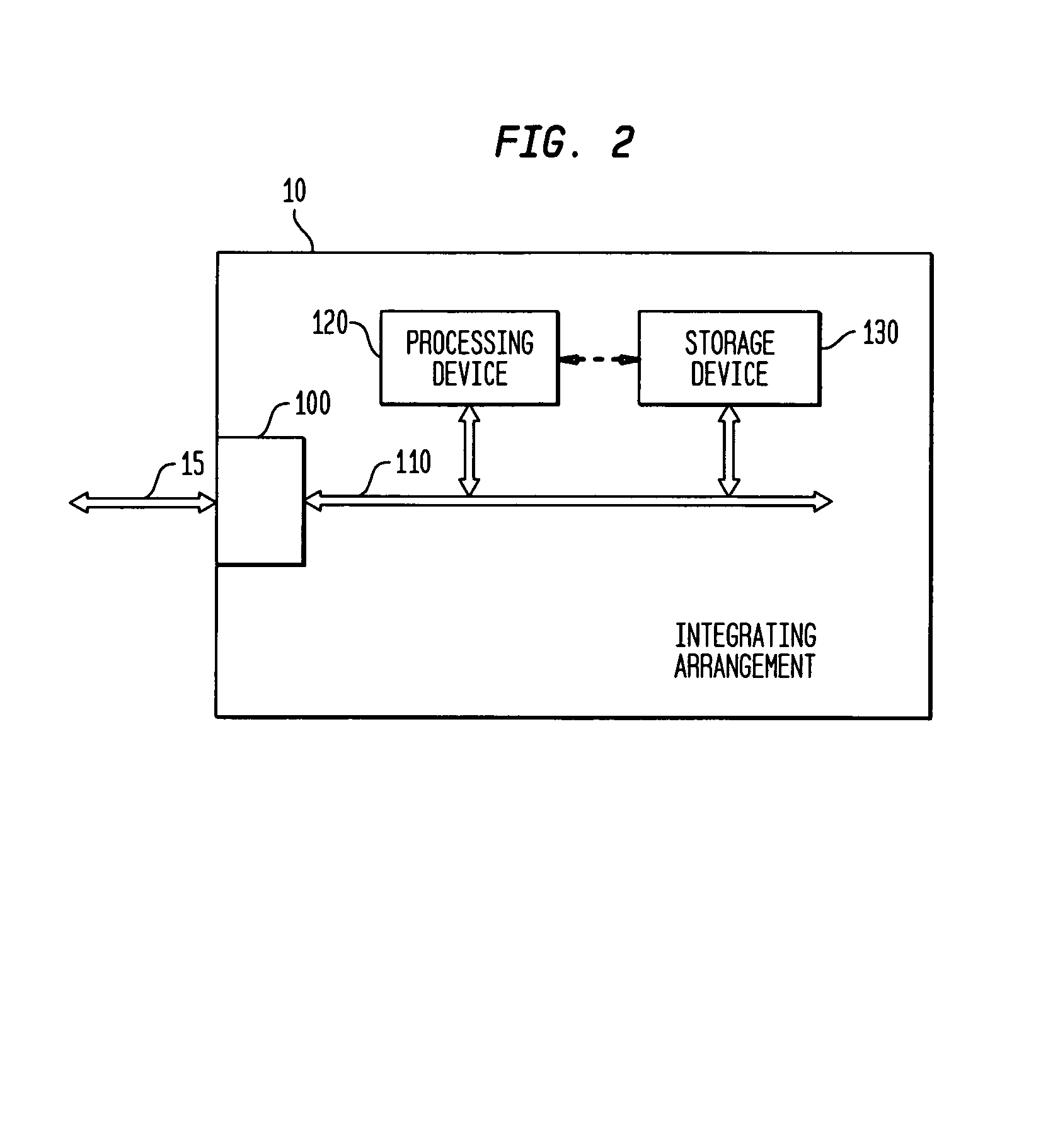
Process and system for integrating information from disparate databases for purposes of predicting consumer behavior
InactiveUS7035855B1Effective predictionEfficient integrationMarket predictionsDigital data processing detailsIntegrated databaseData mining
A process and system for integrating information stored in at least two disparate databases. The stored information includes consumer transactional information. According to the process and system, at least one qualitative variable which is common to each database is identified, and then transformed into one or more quantitative variables. The consumer transactional information in each database is then converted into converted information in terms of the quantitative variables. Thereafter, an integrated database is formed for predicting consumer behavior by combining the converted information from the disparate databases.
Owner:GFK US MRI LLC
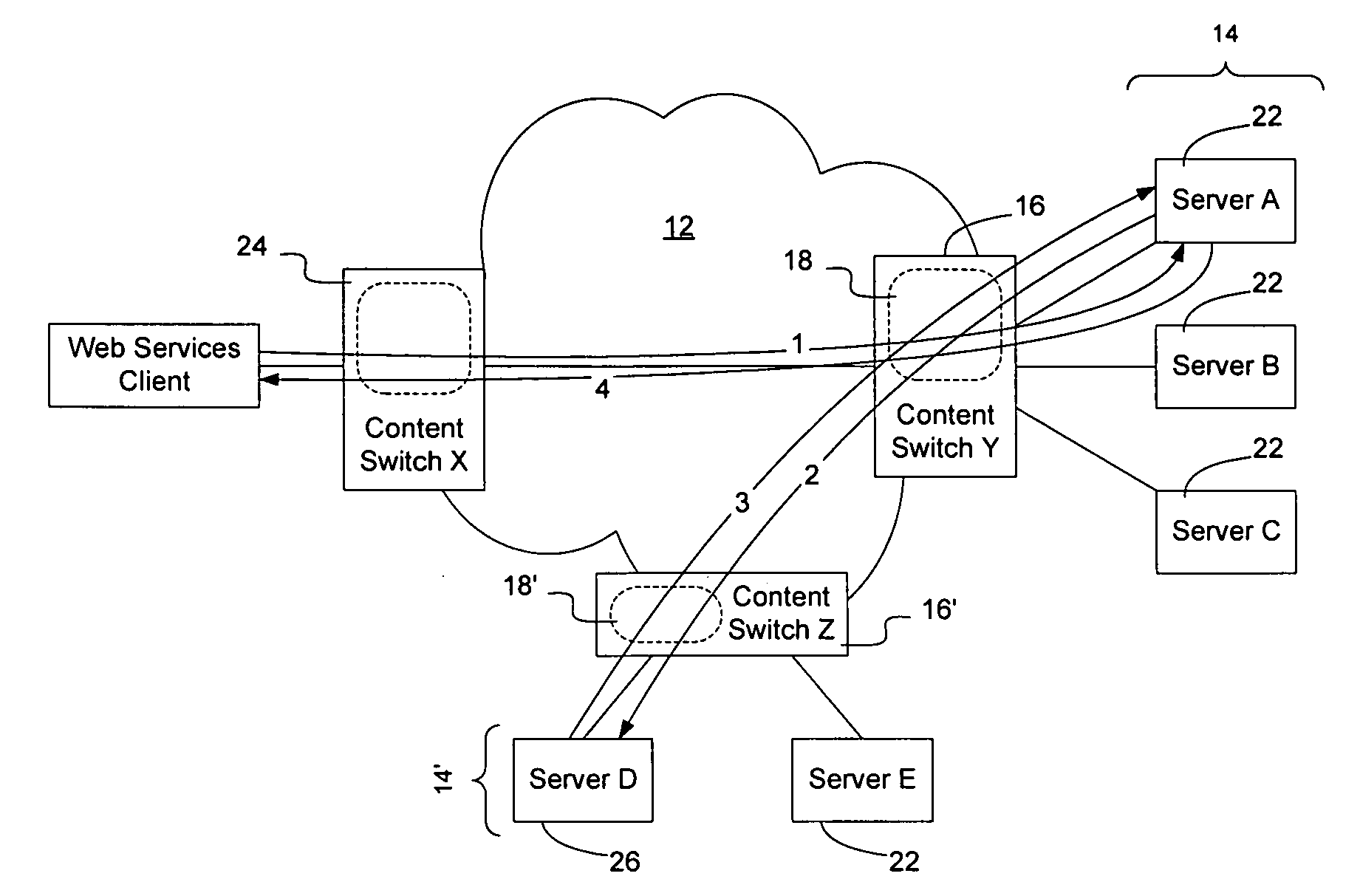
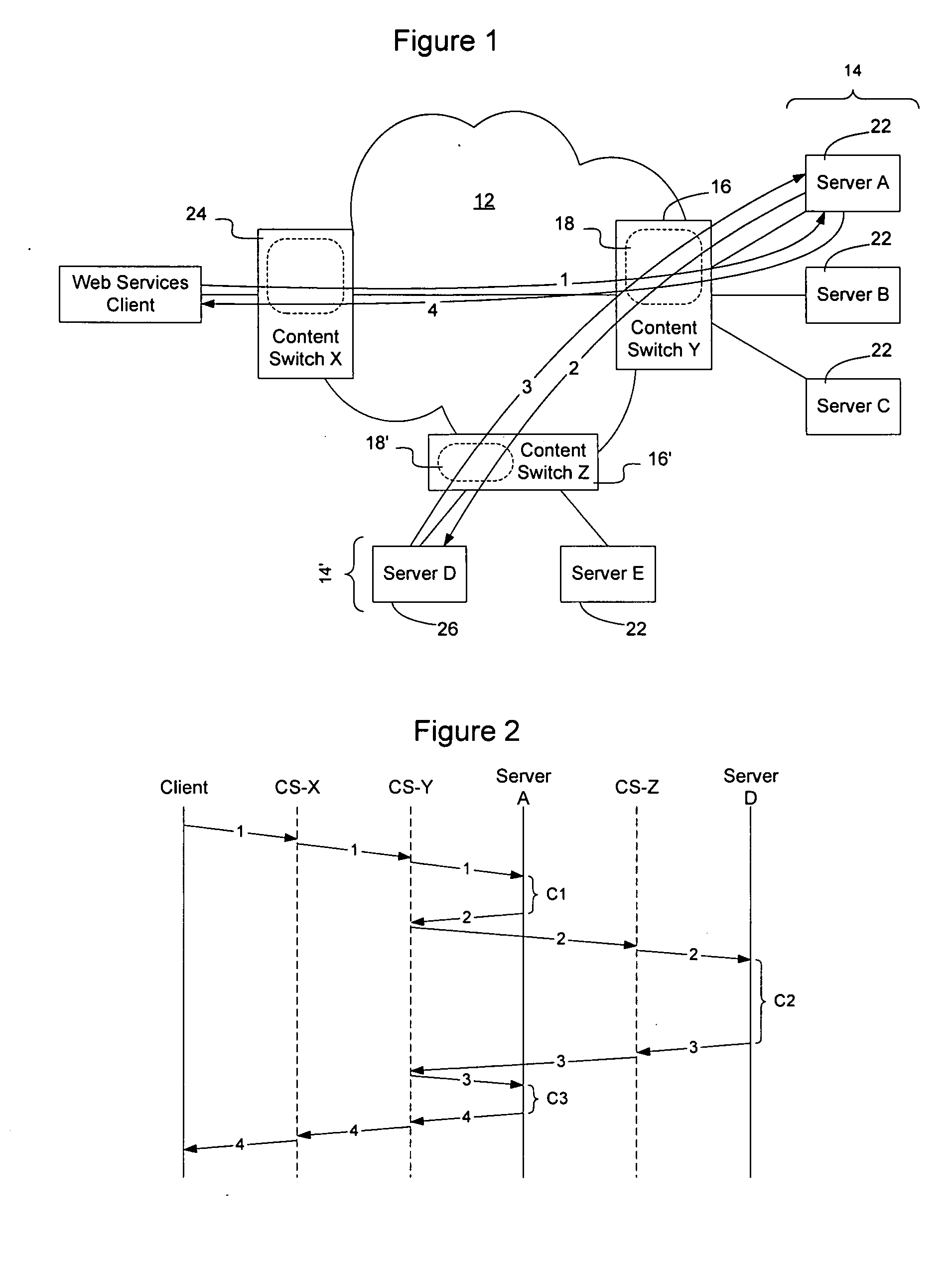
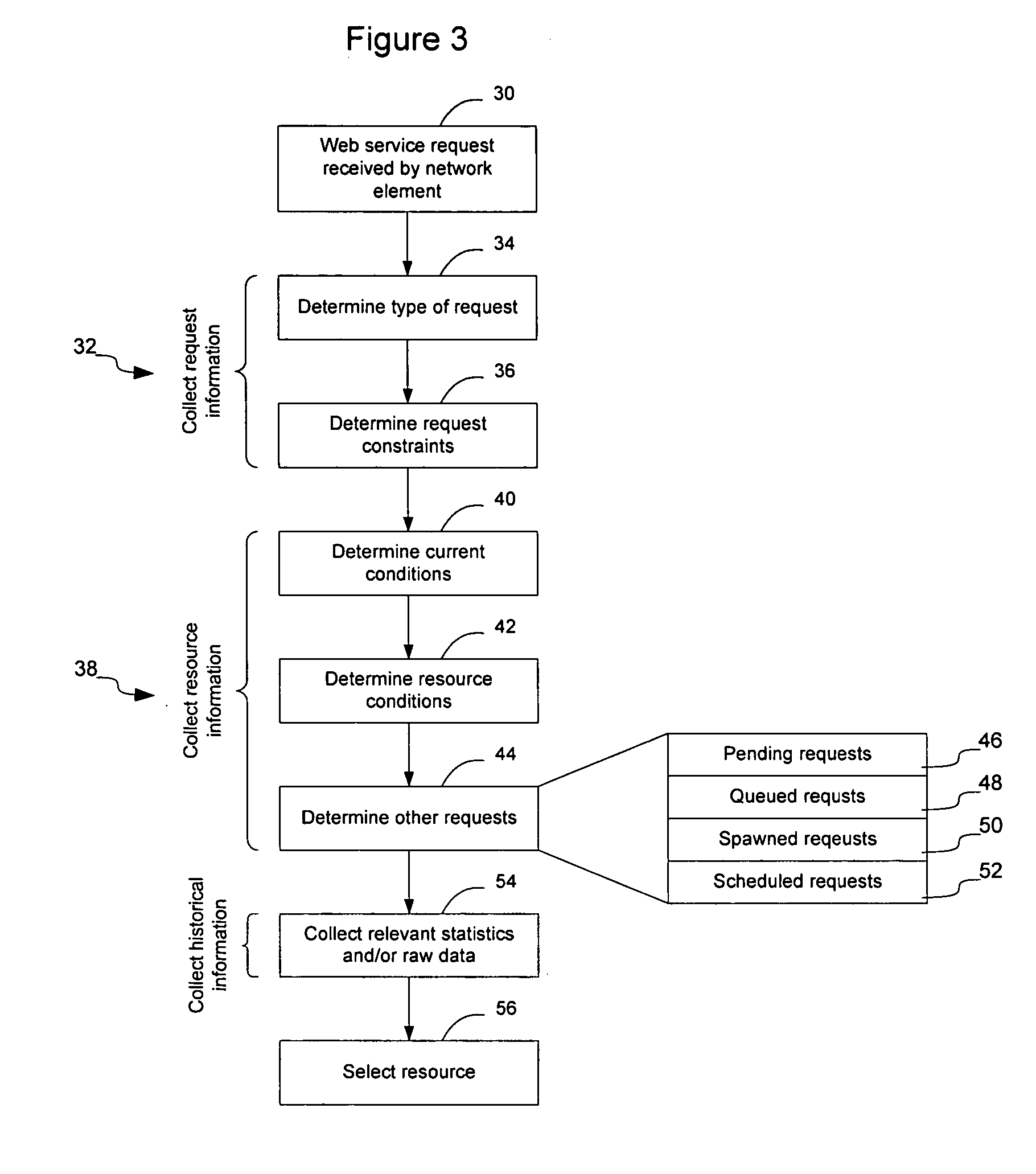
Method and apparatus for facilitating fulfillment of web-service requests on a communication network
InactiveUS20050198200A1Accurate predictionOvercomes drawbackMultiple digital computer combinationsTransmissionDistributed computingWeb service
Fulfillment of web-service requests may be facilitated by intelligently load balancing the web-service requests between servers or server clusters configured to perform the requested web-service. Load balancing may be based on the type of request, target class of server, whether the request is likely to spawn any subsequent requests, relevant historical information, other requests, current and anticipated work load on the servers, the current ability of the servers to handle additional requests, the numbers type and schedule of requests in a queue waiting to be allocated to one or more of the servers, and numerous other factors that may affect the servers' ability to process the request. Requests may be classified to enable historical correlation between how servers have handled previous requests and the present request. Additionally, requests may be scheduled for future execution and monitored during execution.
Owner:RPX CLEARINGHOUSE
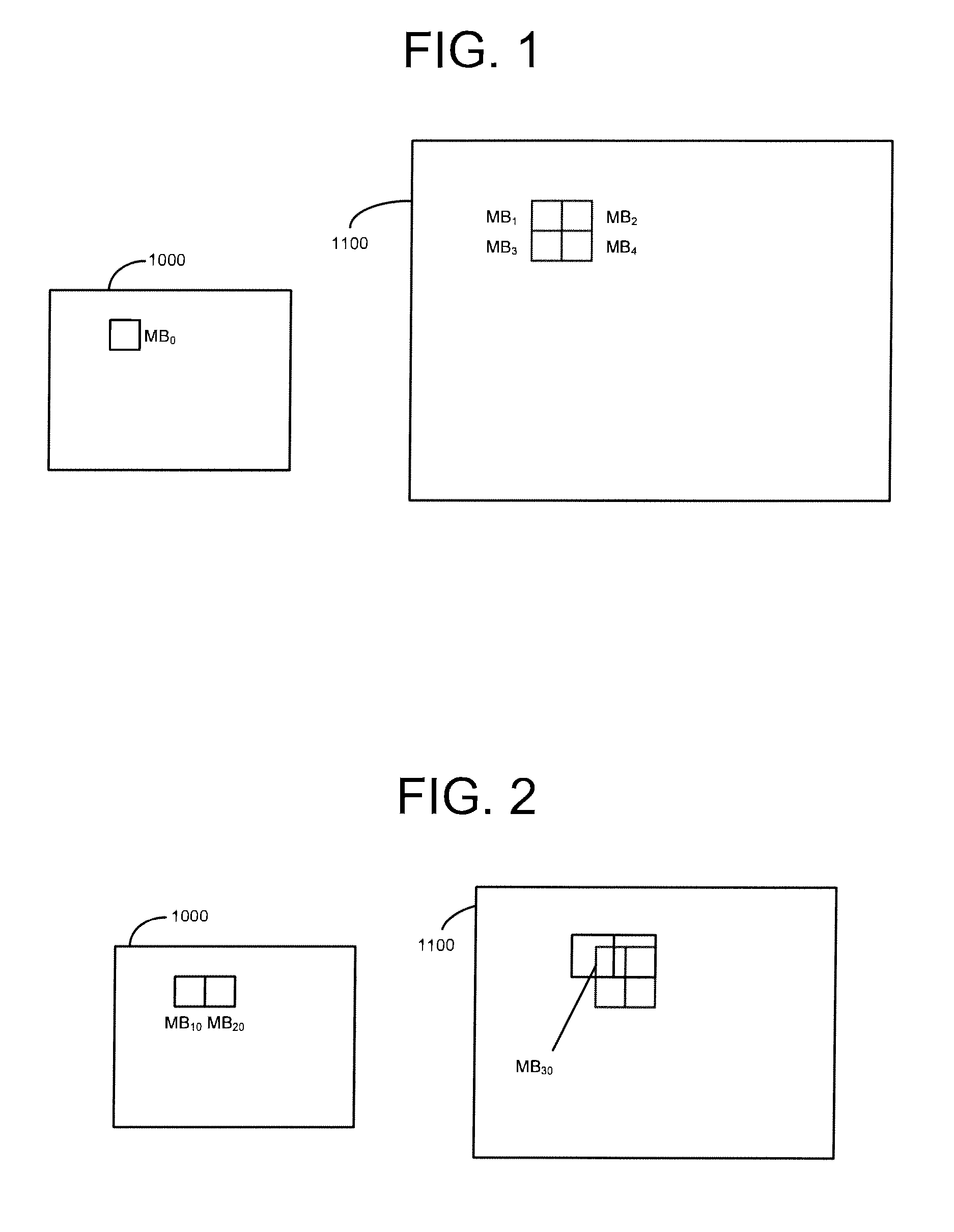
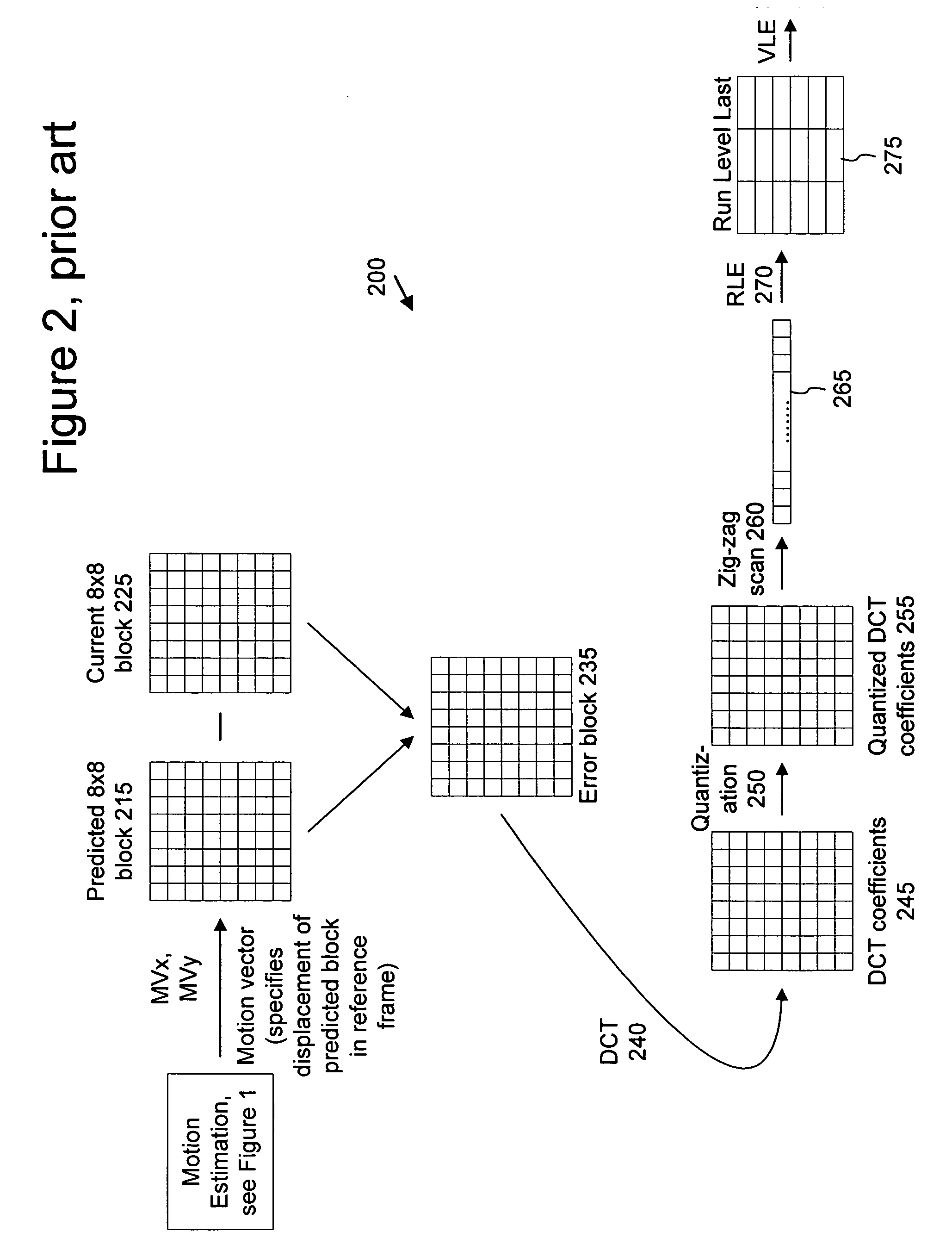
Inter-layer prediction for extended spatial scalability in video coding
ActiveUS20080165855A1Improving inter-layer predictionReduce computational complexityColor television with pulse code modulationColor television with bandwidth reductionInter layerMotion vector
An improved system and method for providing improved inter-layer prediction for extended spatial scalability in video coding, as well as improving inter-layer prediction for motion vectors in the case of extended spatial scalability. In various embodiments, for the prediction of macroblock mode, the actual reference frame index and motion vectors from the base layer are used in determining if two blocks should be merged. Additionally, multiple representative pixels in a 4×4 block can be used to represent each 4×4 block in a virtual base layer macroblock. The partition and motion vector information for the relevant block in the virtual base layer macroblock can be derived from all of the partition information and motion vectors of those 4×4 blocks.
Owner:NOKIA TECHNOLOGLES OY
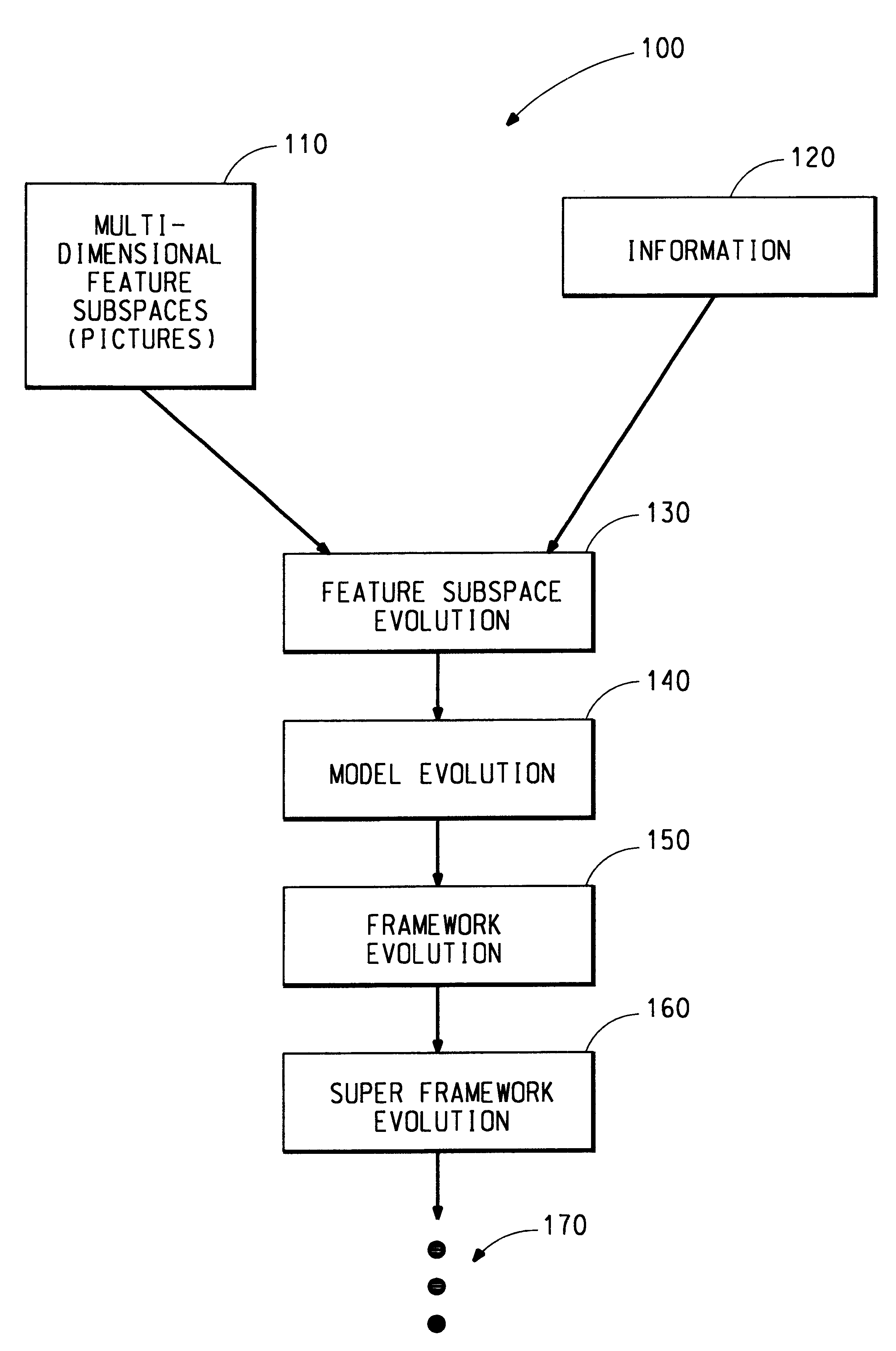
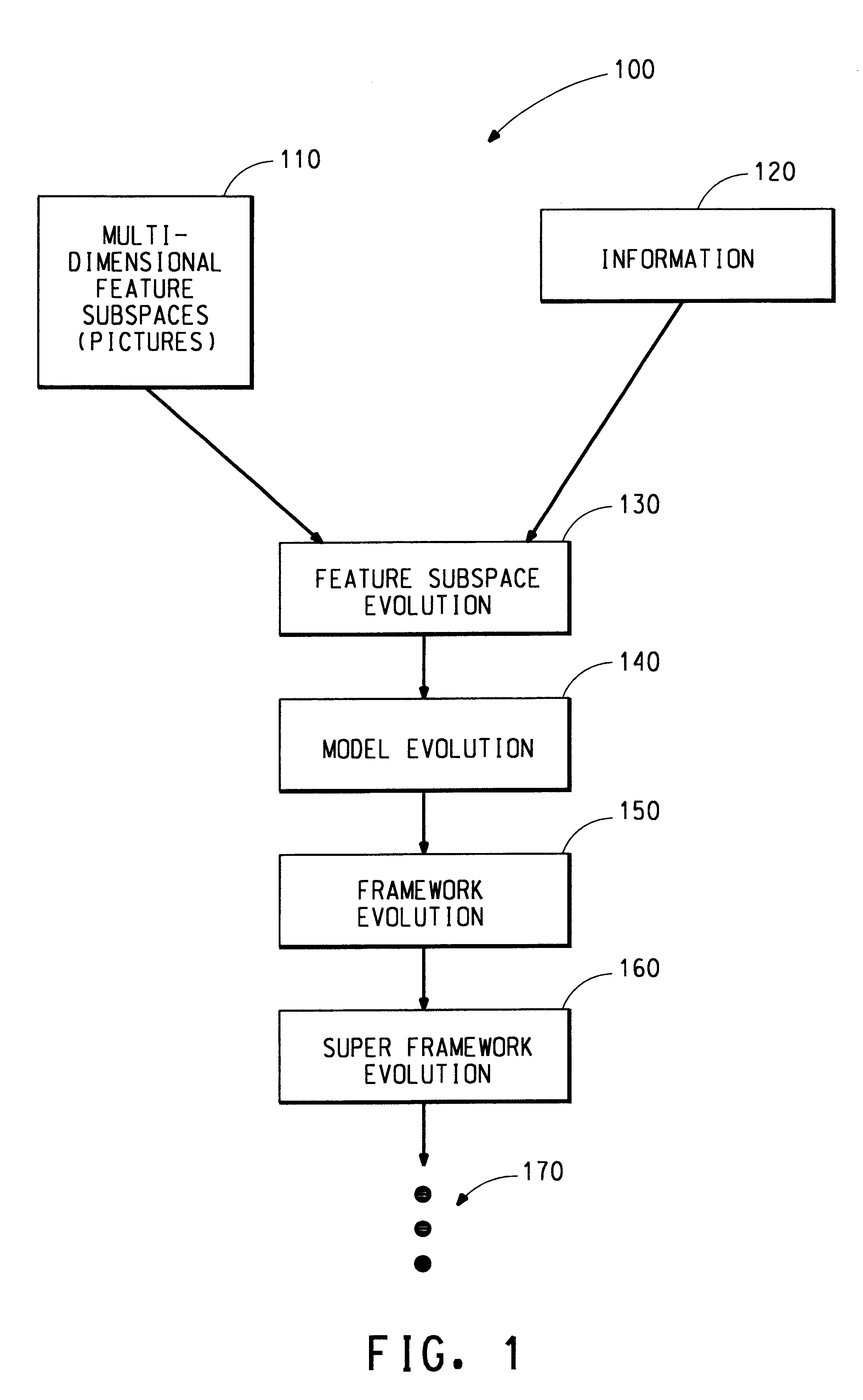
Distributed hierarchical evolutionary modeling and visualization of empirical data
InactiveUS6941287B1Accurate predictionDigital computer detailsCharacter and pattern recognitionEmpirical modellingData set
A distributed hierarchical evolutionary modeling and visualization of empirical data method and machine readable storage medium for creating an empirical modeling system based upon previously acquired data. The data represents inputs to the systems and corresponding outputs from the system. The method and machine readable storage medium utilize an entropy function based upon information theory and the principles of thermodynamics to accurately predict system outputs from subsequently acquired inputs. The method and machine readable storage medium identify the most information-rich (i.e., optimum) representation of a data set in order to reveal the underlying order, or structure, of what appears to be a disordered system. Evolutionary programming is one method utilized for identifying the optimum representation of data.
Owner:EI DU PONT DE NEMOURS & CO
System and methods for automated detection, reasoning and recommendations for resilient cyber systems
ActiveUS20180103052A1Improve applicabilityLarge capacityDigital data information retrievalComputer security arrangementsElastic networkKnowledge extraction
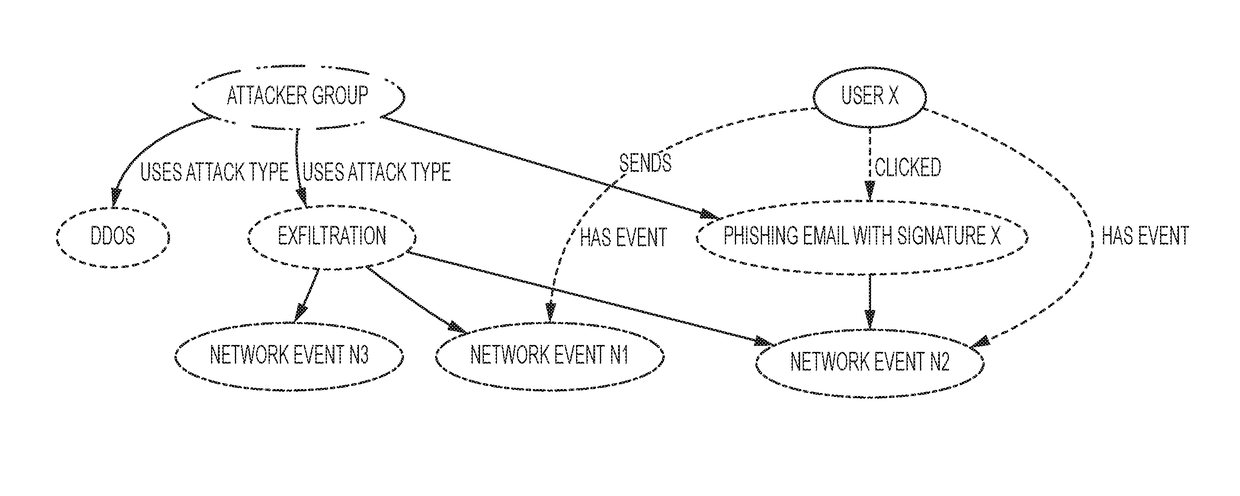
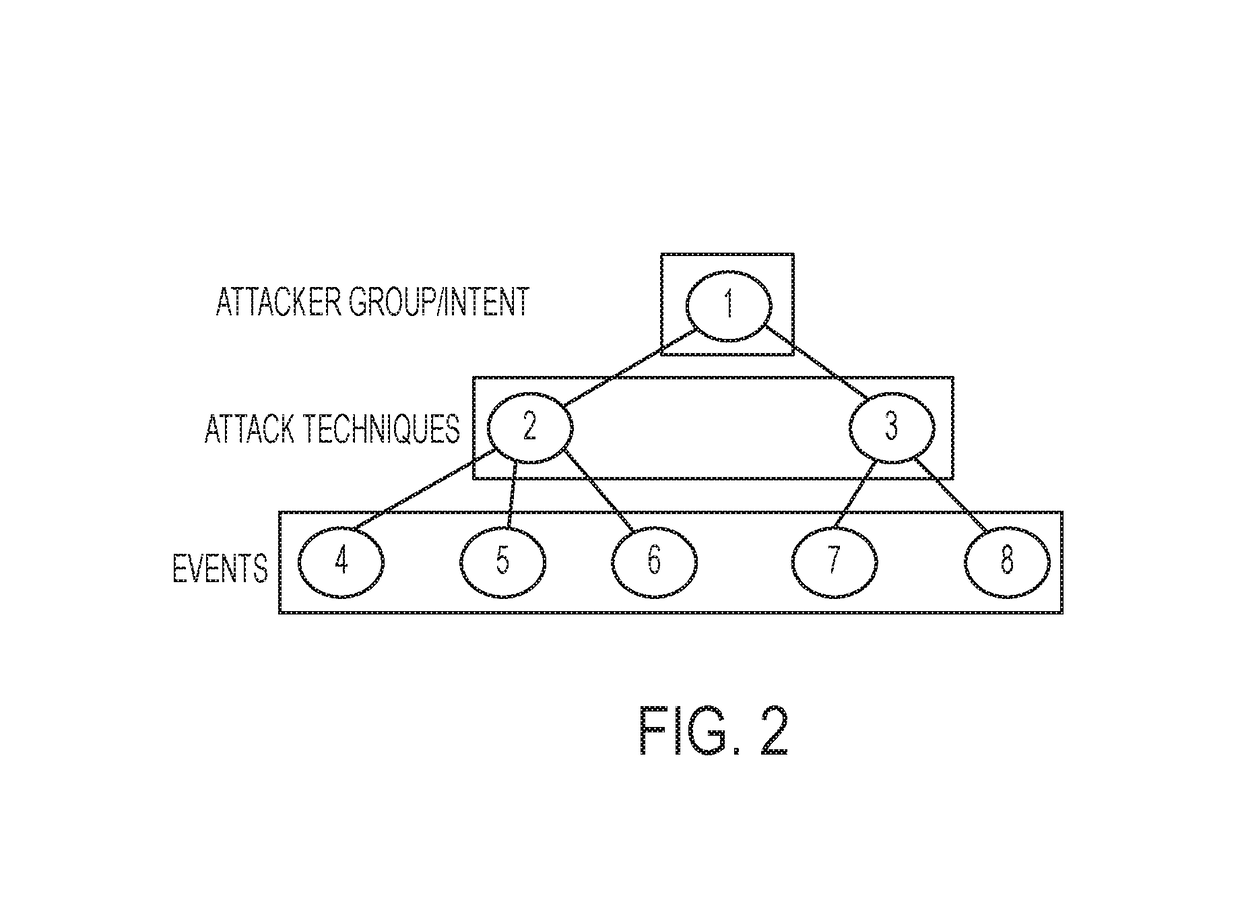
A method for securing an IT (information technology) system using a set of methods for knowledge extraction, event detection, risk estimation and explanation for ranking cyber-alerts which includes a method to explain the relationship (or an attack pathway) from an entity (user or host) and an event context to another entity (a high-value resource) and an event context (attack or service failure).
Owner:BATTELLE MEMORIAL INST
System and method for adding an advertisement to a personal communication
ActiveUS8527345B2Accurate predictionIncrease probabilityDiscounts/incentivesAdvertisementsThird partyWorld Wide Web
A system and method is provided for adding an advertisement to a digital message and providing additional communication data to a recipient that interacts with the advertisement regardless of the network device the recipient is utilizing. An advertisement generator residing on a network host accepts digital messages from contributors and allows the contributors to select an advertisement to be displayed with their contributed messages. These digital messages may be sent to specified recipients or published on a Web site. Using stored personal data associated with the contributor and with the recipient of a digital message, in addition to the content of the message itself, the advertisement generator suggests advertisements to be included with the digital messages based on their contextual relevance. In exchange for including an advertisement with a digital message, a contributor is compensated. If the contributor-selected advertisement is provided by a third party advertiser, the message, contributor, and advertisement data is utilized to compensate the contributor of that message for sending it to at least one recipient or posting it on a Web site. If the advertisement is interactive, and the advertisement is interacted with, the advertisement generator will provide the recipient with additional communication data in a format that can be understood by the recipient network device.
Owner:ROTHSCHILD RICHARD ANTHONY
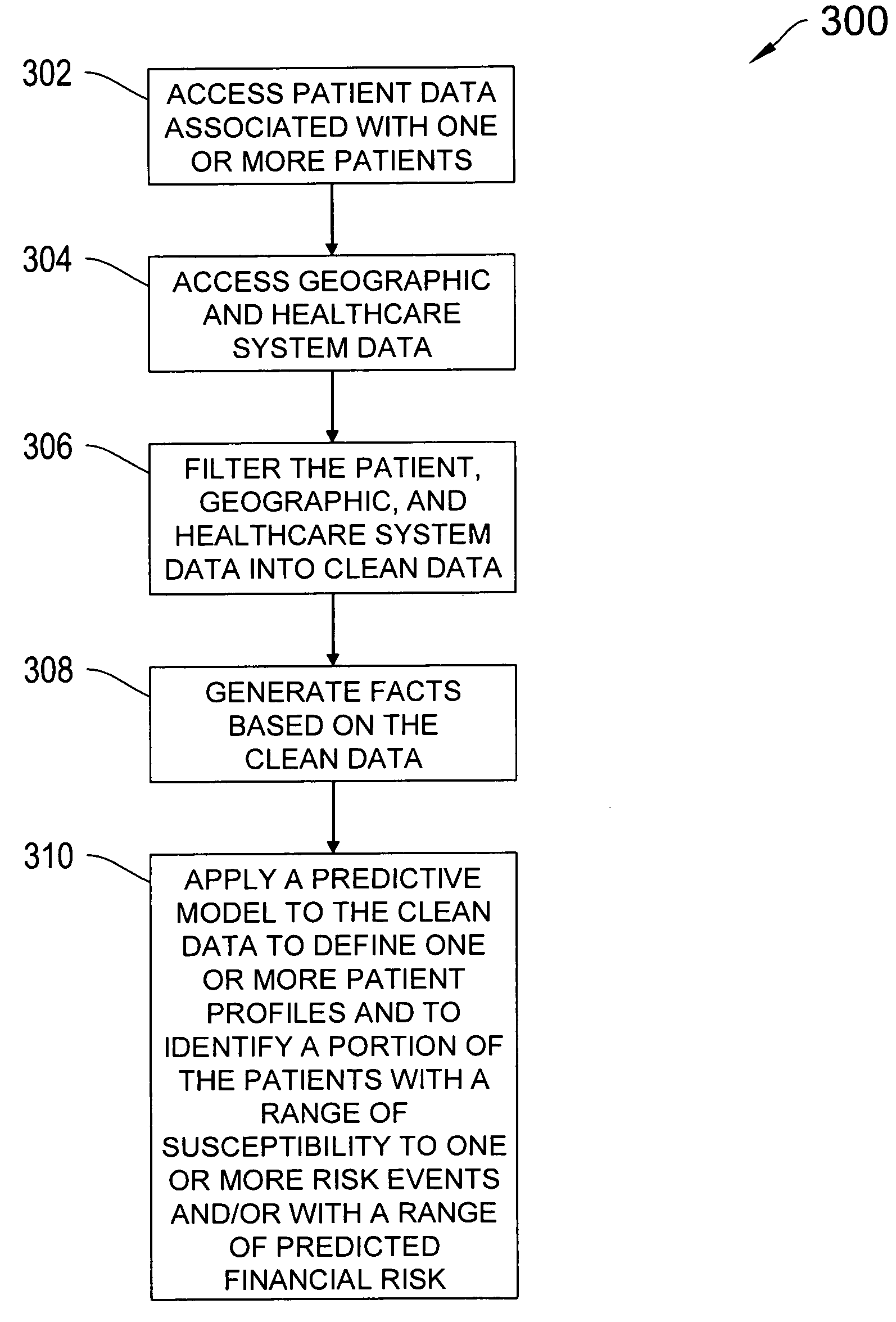
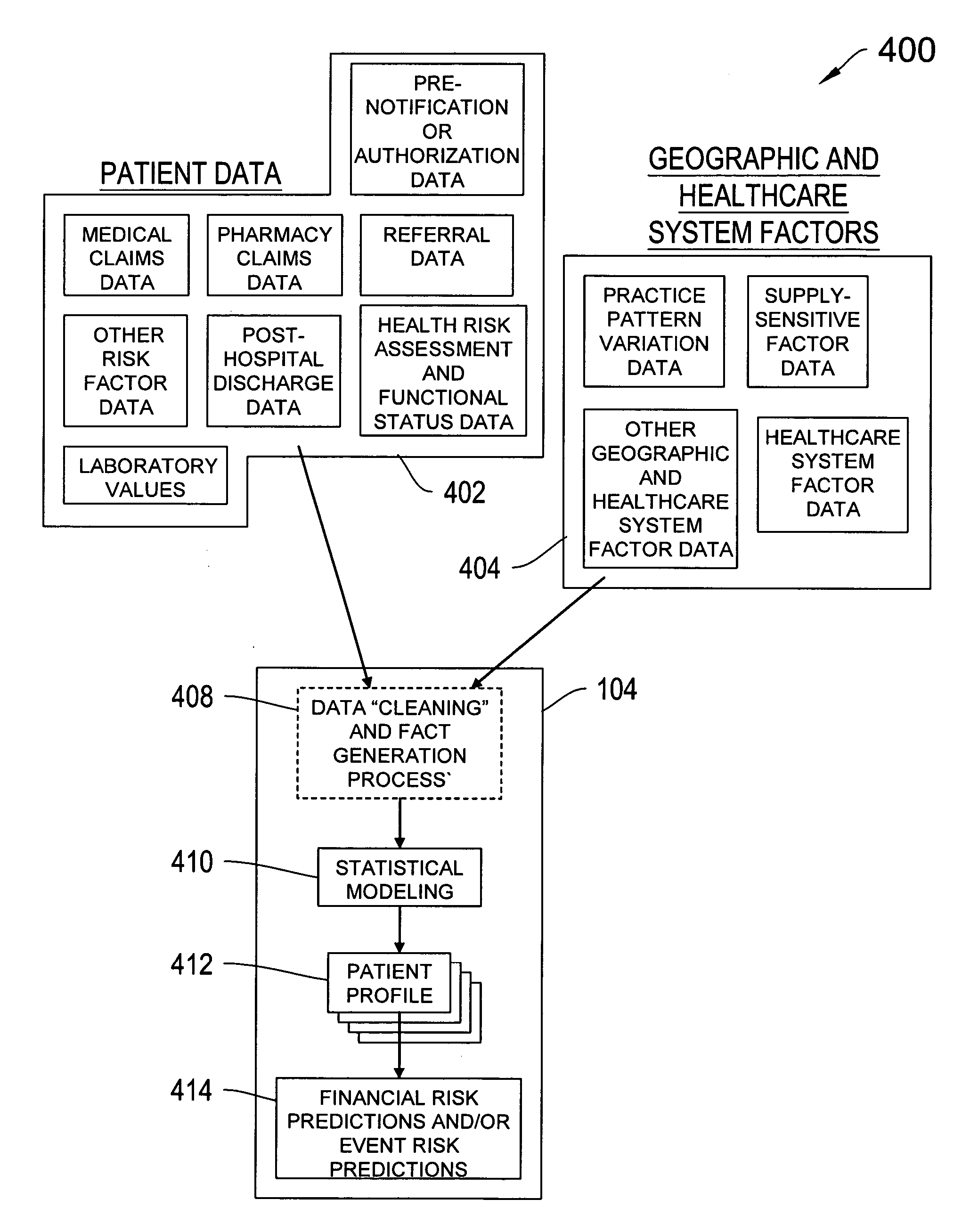
Systems and methods for predicting healthcare related risk events
InactiveUS20060129427A1Reduce healthcare costPrevent and mitigate occurrenceMedical simulationMedical data miningPatient profileRisk model
A system for predicting healthcare risk events including the process of accessing patient data associated with one or more patents, accessing geographic and healthcare system data, filtering the patient data, geographic data, and healthcare system data into clean data, and applying a predictive risk model to the clean data to generate patient profile data and to identify a portion of the patients susceptible to one or more risk events.
Owner:HEALTH DIALOG SERVICES CORP
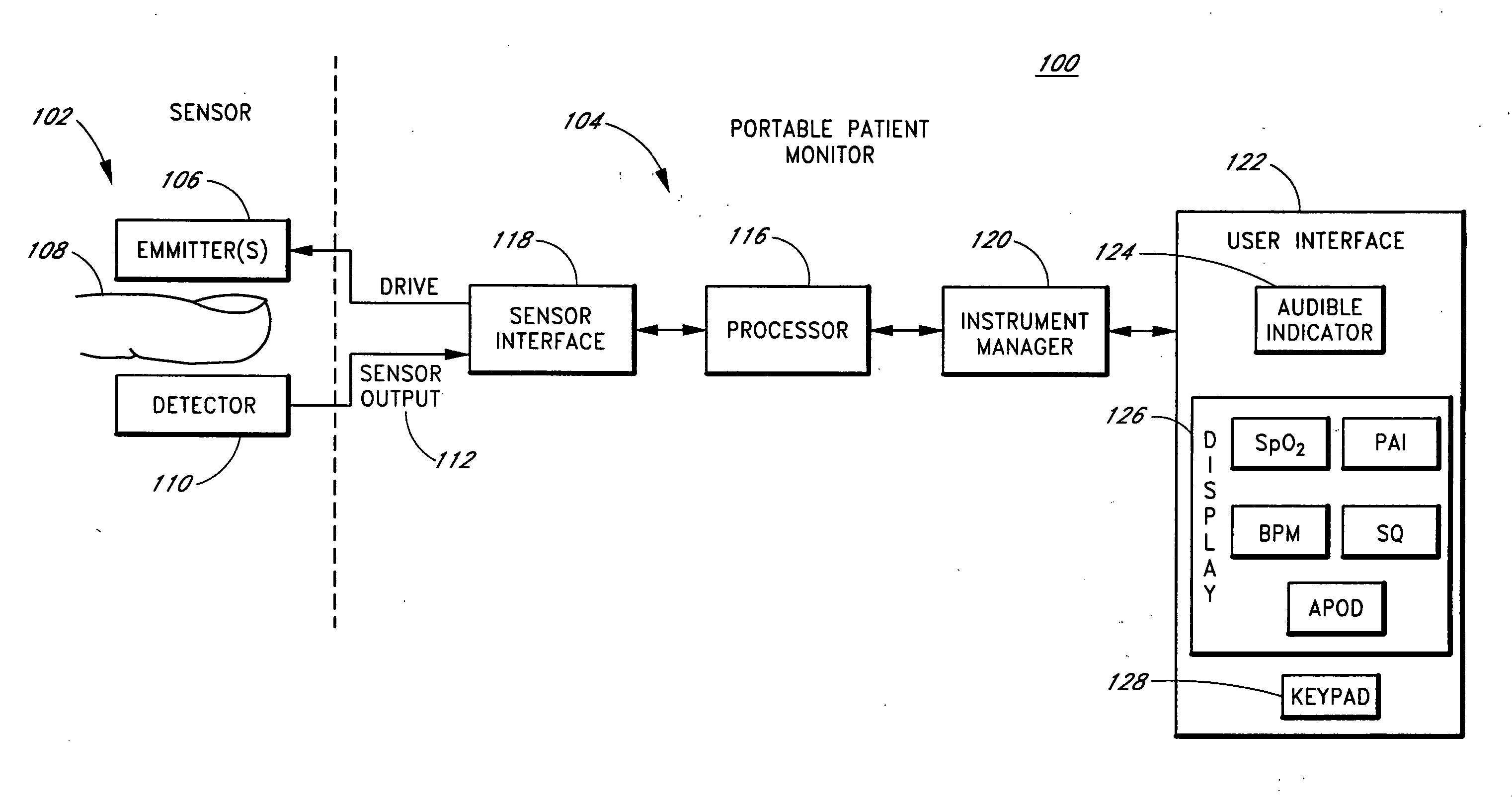
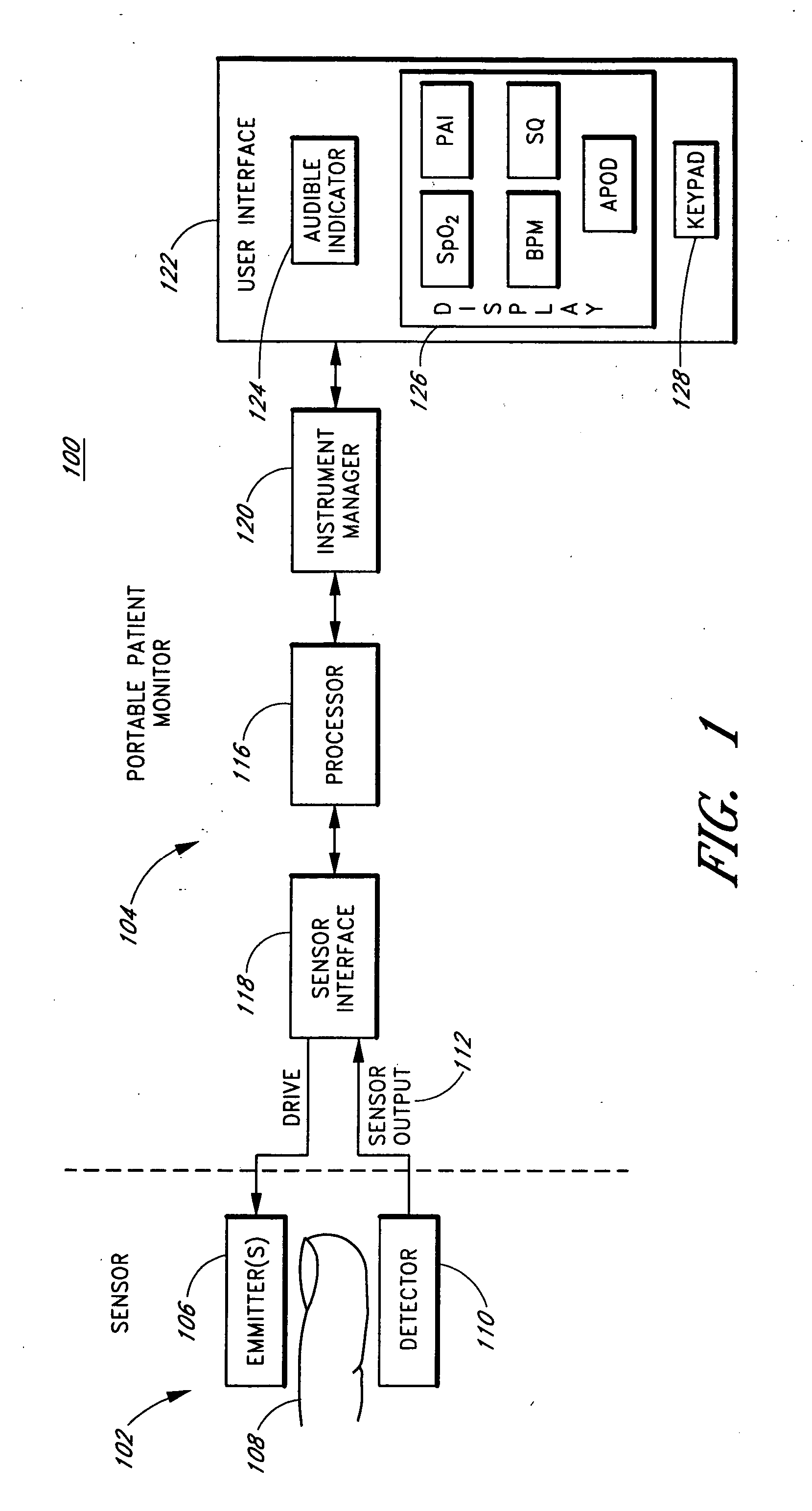
Portable patient monitor
InactiveUS20060189871A1Reduce amountReduce concentrationDiagnostic recording/measuringSensorsEngineeringMarine navigation
Embodiments of the present disclosure includes a portable pulse oximeter, such as a handheld pulse oximeter, that provides a user with intuitive key navigation for device operation, which reduces an amount of visual concentration needed to handle and operate the oximeter. In various embodiments, the portable pulse oximeter includes one or more of user input keys disposed along curve, an alignment edge providing guidance by feel of a user's digits to the input keys, raised convex keys also providing navigation by feel, a protective boot disposed around various portions of the oximeter housing to protect against impacts, a table-top stand, combinations of the same, or the like.
Owner:MASIMO CORP
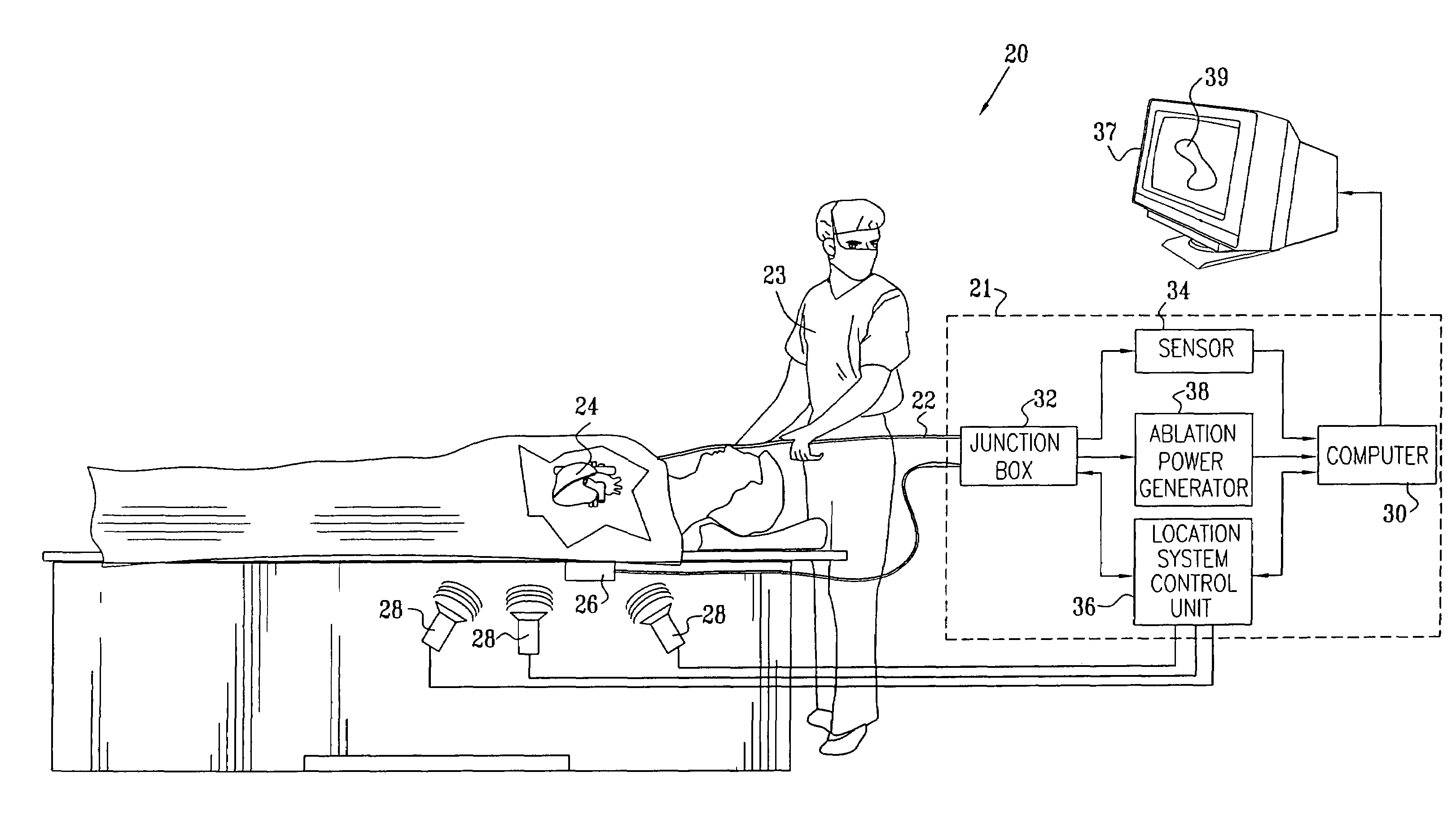
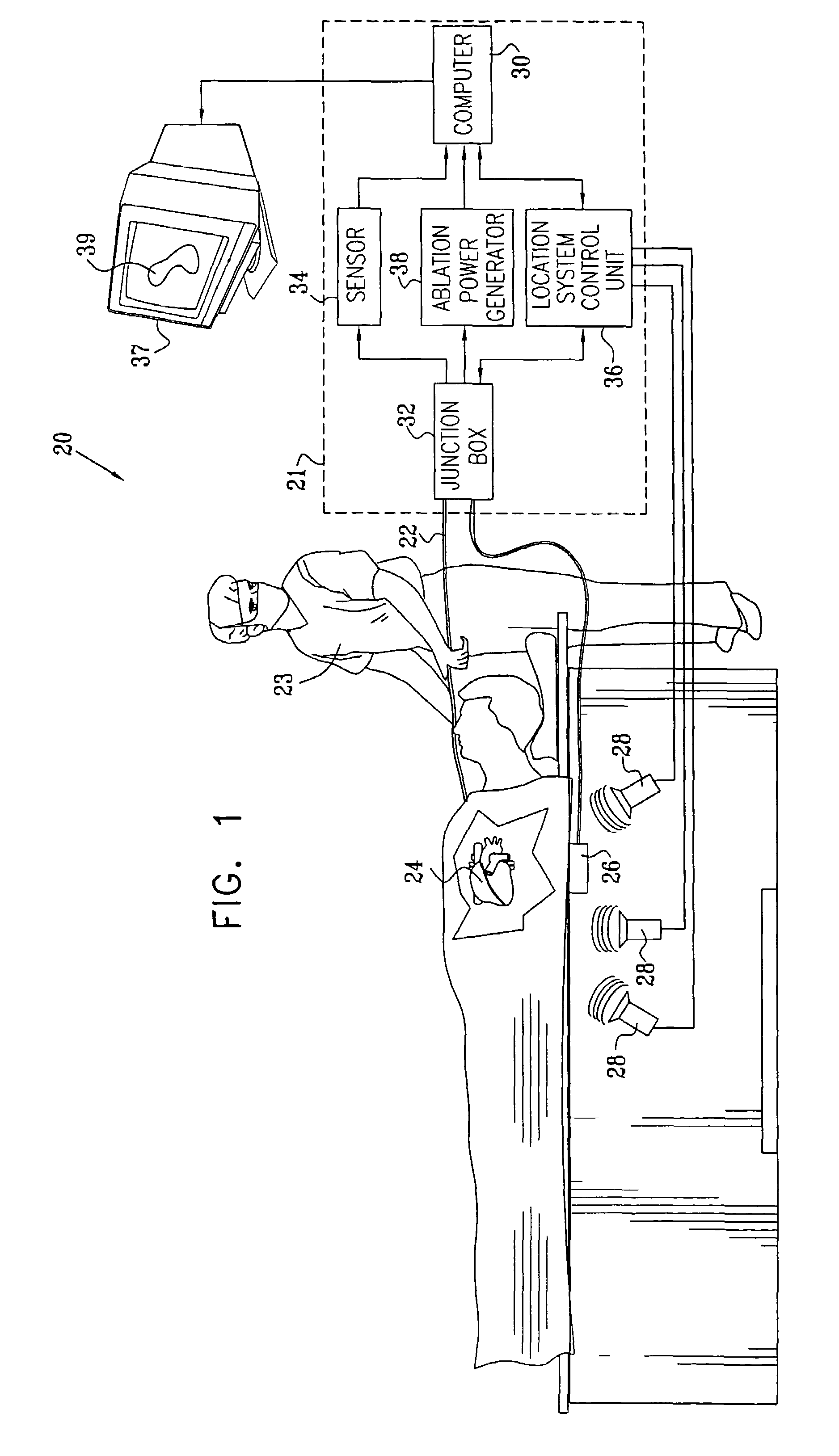
Prediction and assessment of ablation of cardiac tissue
ActiveUS7306593B2Accurately measureAccurate measurementElectrotherapyBlood flow measurement devicesBiomedical engineeringCorneal ablation
A method for ablating tissue in an organ inside a body of a subject includes bringing a probe inside the body into a position in contact with the tissue to be ablated, and measuring one or more local parameters at the position using the probe prior to ablating the tissue. A map of the organ is displayed, showing, based on the one or more local parameters, a predicted extent of ablation of the tissue to be achieved for a given dosage of energy applied at the position using the probe. The given dosage of energy is applied to ablate the tissue using the probe, and an actual extent of the ablation at the position is measured using the probe subsequent to ablating the tissue. The measured actual extent of the ablation is displayed on the map for comparison with the predicted extent.
Owner:BIOSENSE WEBSTER INC
Gas flow control method in a blower based ventilation system
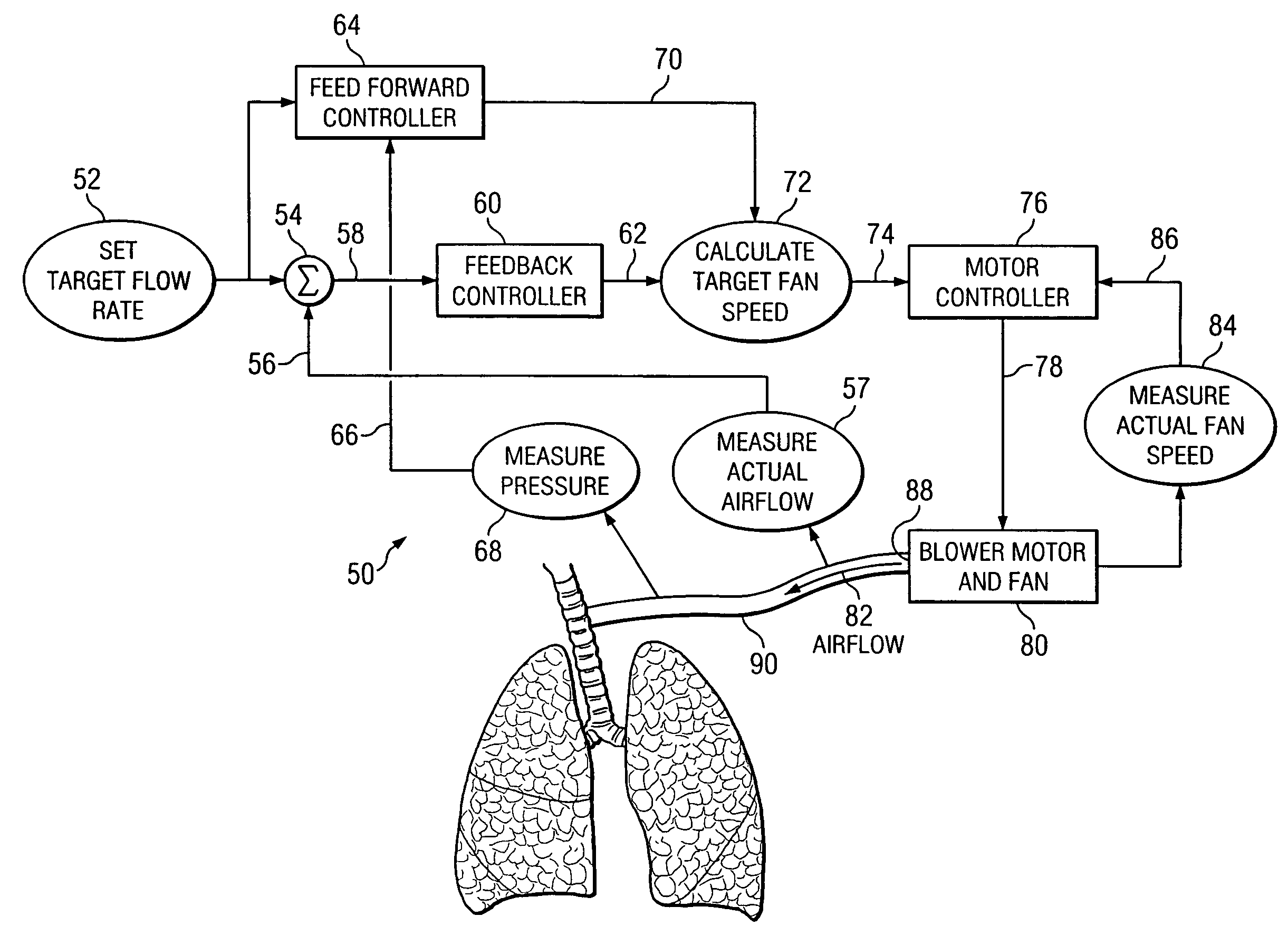
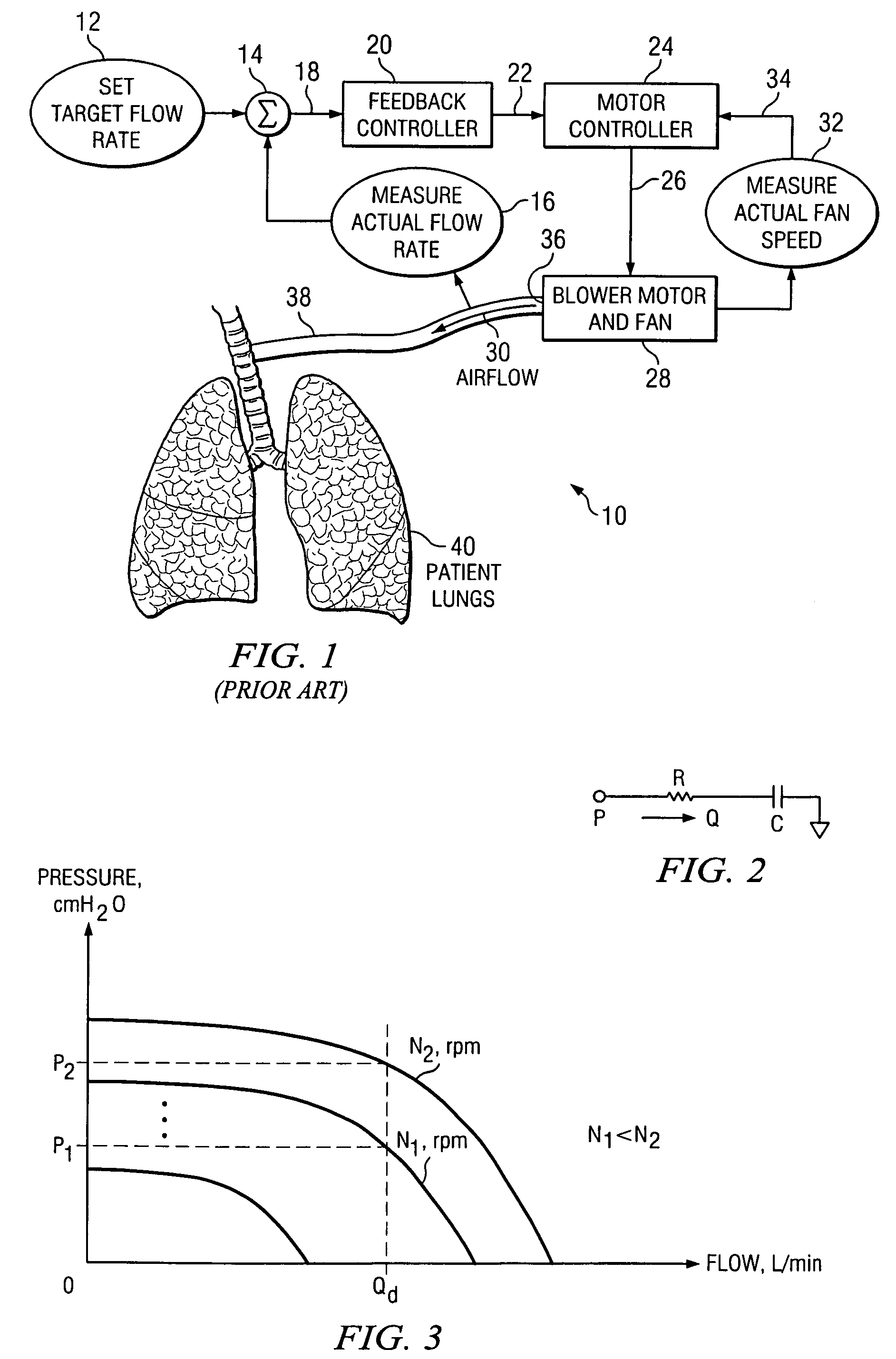
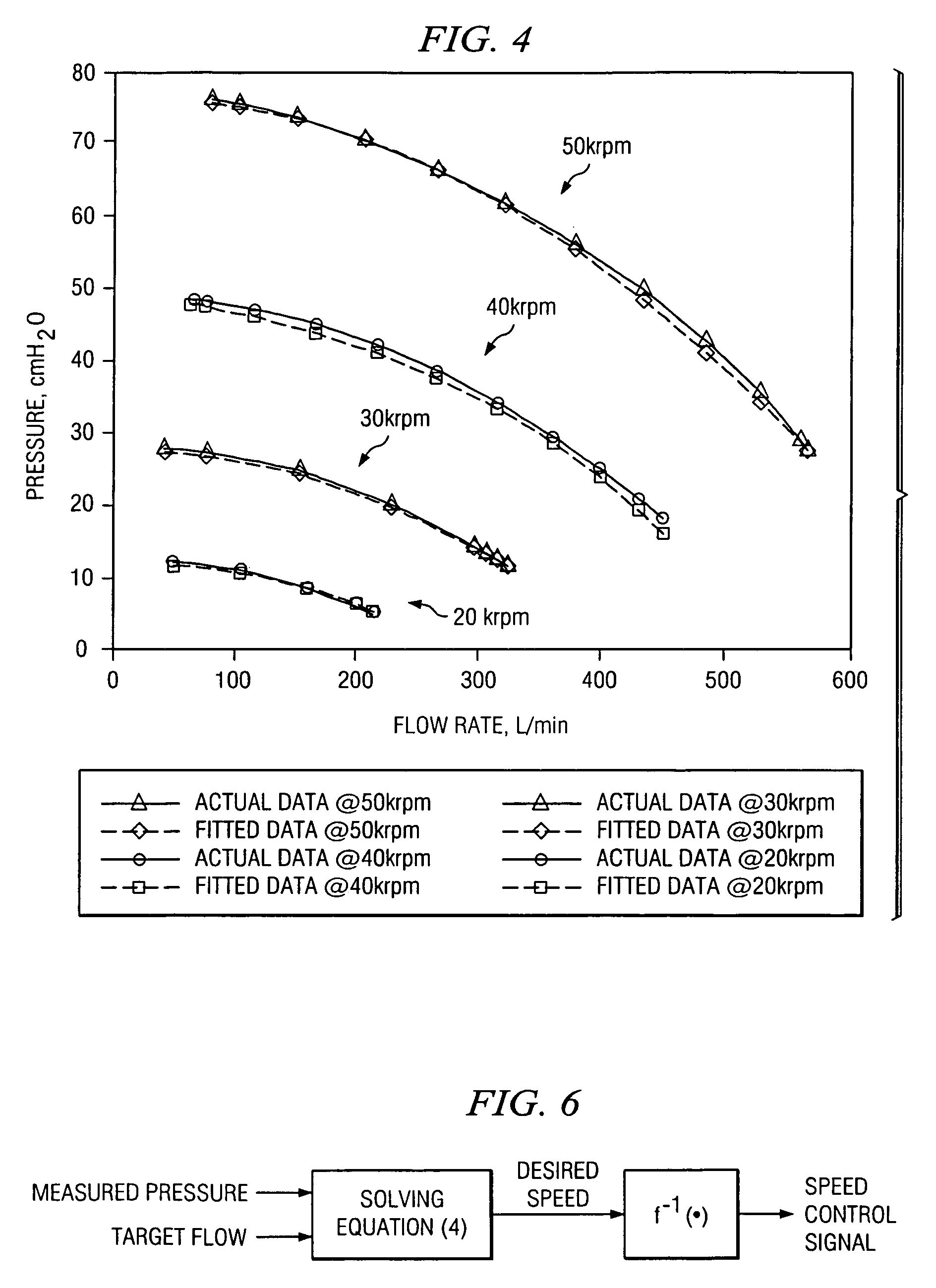
ActiveUS7487773B2Accurate predictionRespiratorsOperating means/releasing devices for valvesControl systemFeedback controller
The invention is directed to a system and method for controlling the flow of gas from a medical ventilator into a patient's lungs. The control system provides for a non-linear feedforward controller to correct for disturbances caused by back pressure at the outlet of the blower of the medical ventilator. For this purpose, a pressure transducer is provided to measure the back pressure. Additionally, the invention allows for a feedback controller to correct for the differences between the rate of the actual gas flow and the targeted gas flow rate. For this purpose a flow rate transducer is provided. The control system may account for each of the gas flow rate error and the back pressure disturbance to provide for a quick and accurate adjustment to achieve the targeted gas flow rate.
Owner:TYCO HEALTHCARE GRP LP
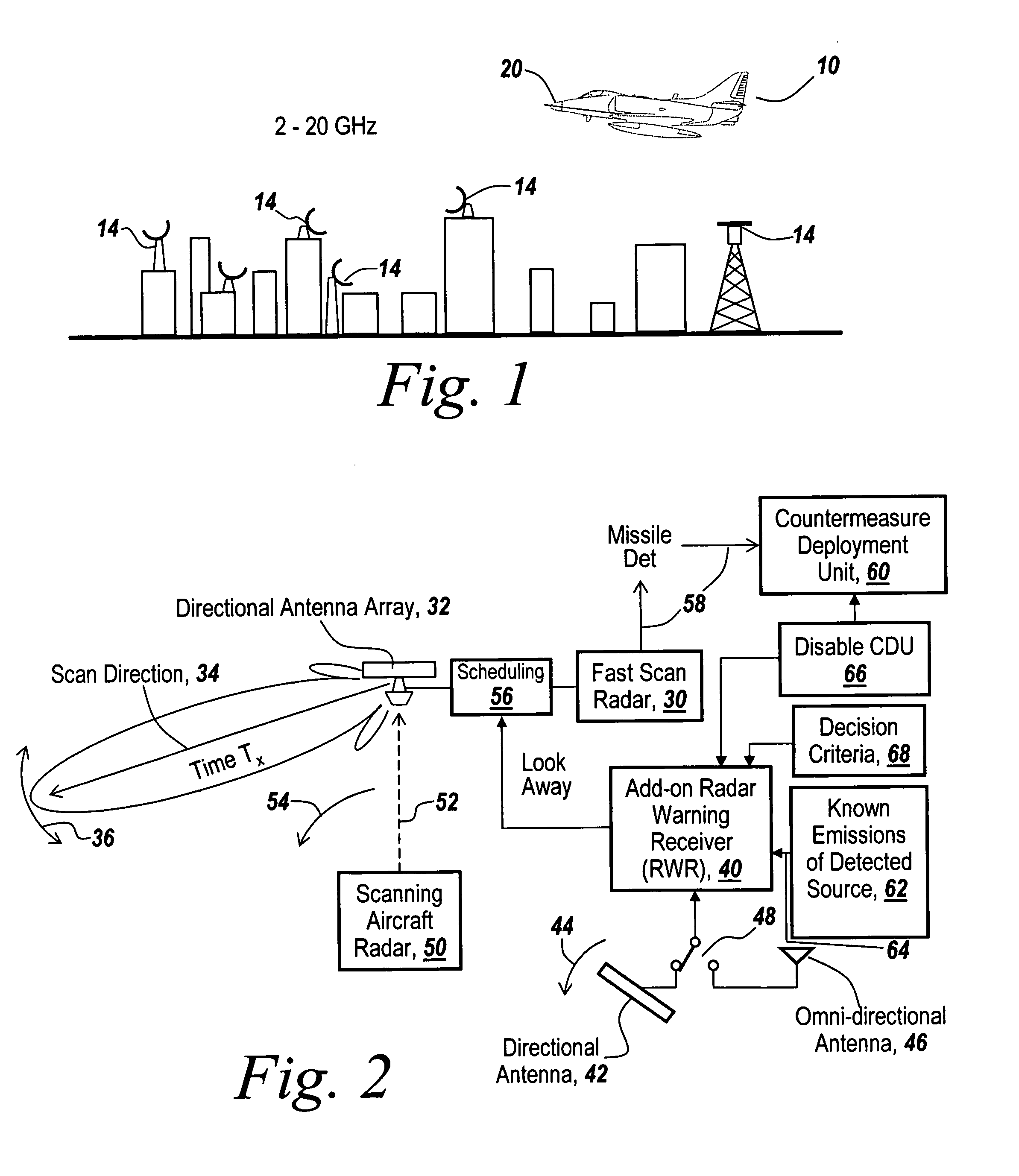
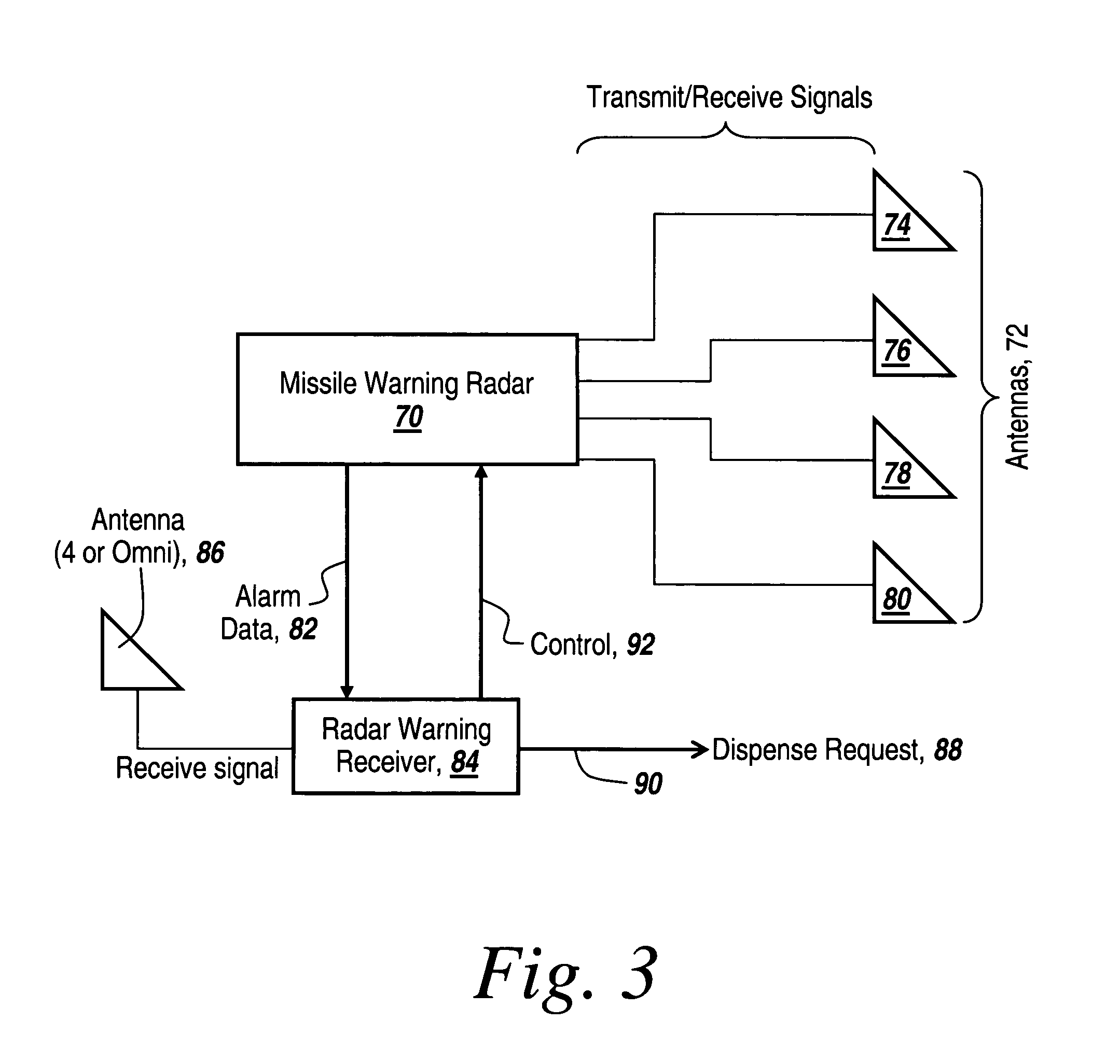
Method and apparatus for monitoring the RF environment to prevent airborne radar false alarms that initiate evasive maneuvers, reactionary displays or actions
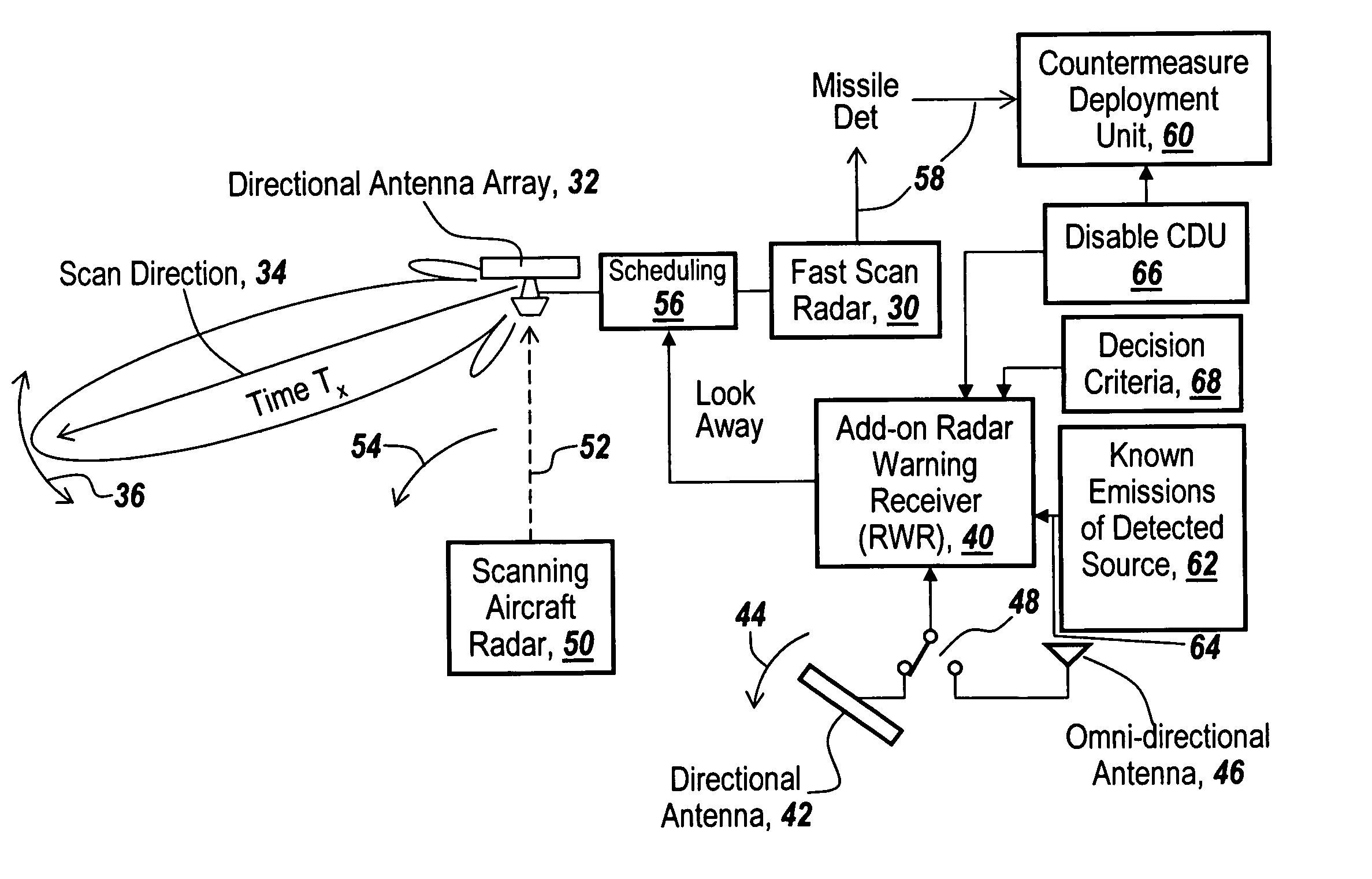
InactiveUS7843375B1Accurate predictionLower false alarms without degrading system performanceCommunication jammingRadio wave reradiation/reflectionRadarFalse alarm
Rather than costly modifications to existing radars, a small, low cost radar warning receiver is used to monitor the RF environment. This add-on receiver can provide situational awareness including RF signal levels and angle of arrival, and recommend or provide antenna scanning synchronization, blanking inputs or gated reactionary outputs to or for the airborne radar. Utilization of this information can be used to reduce false alarms and improve system performance.
Owner:BAE SYST INFORMATION & ELECTRONICS SYST INTERGRATION INC
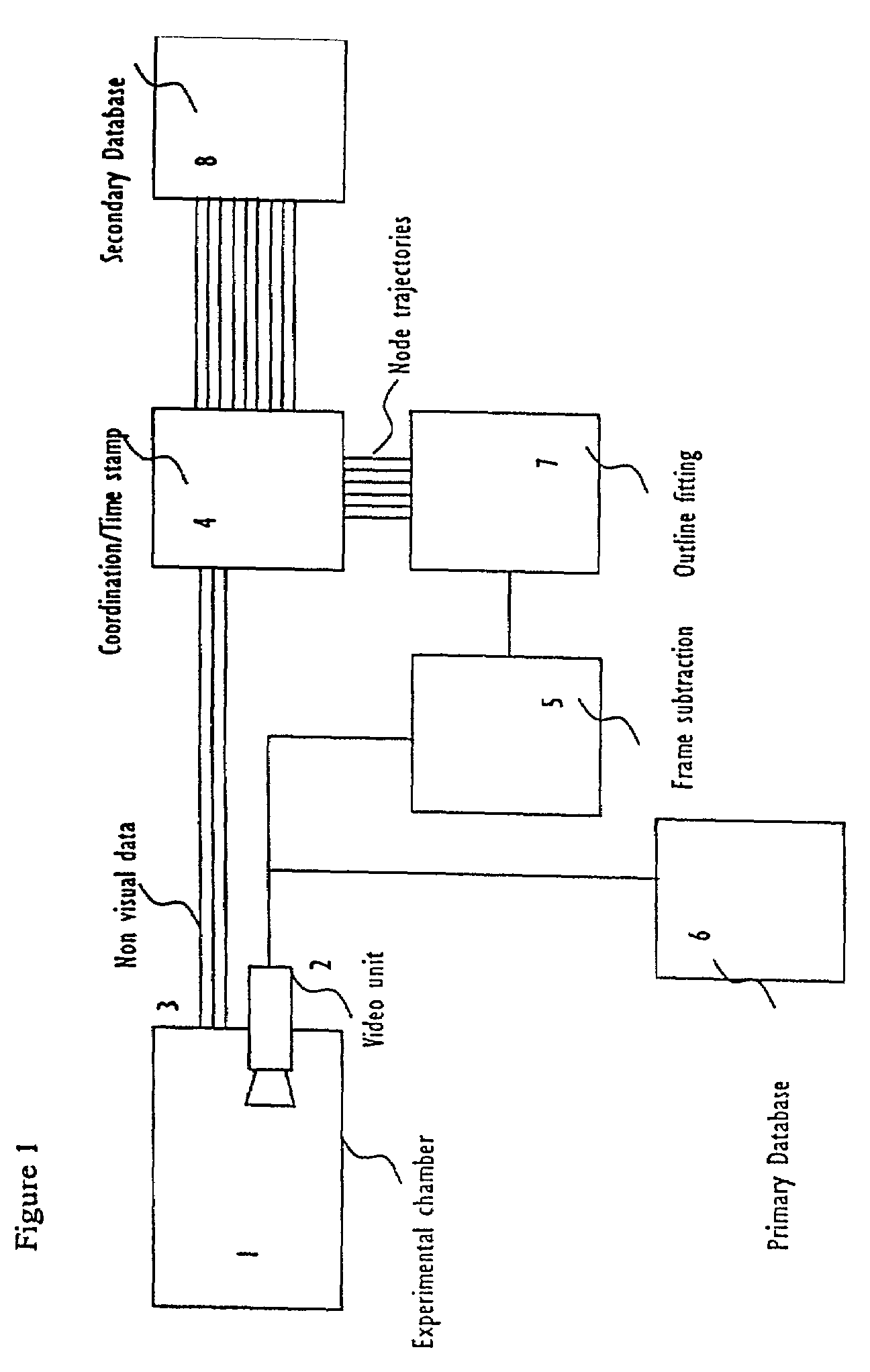
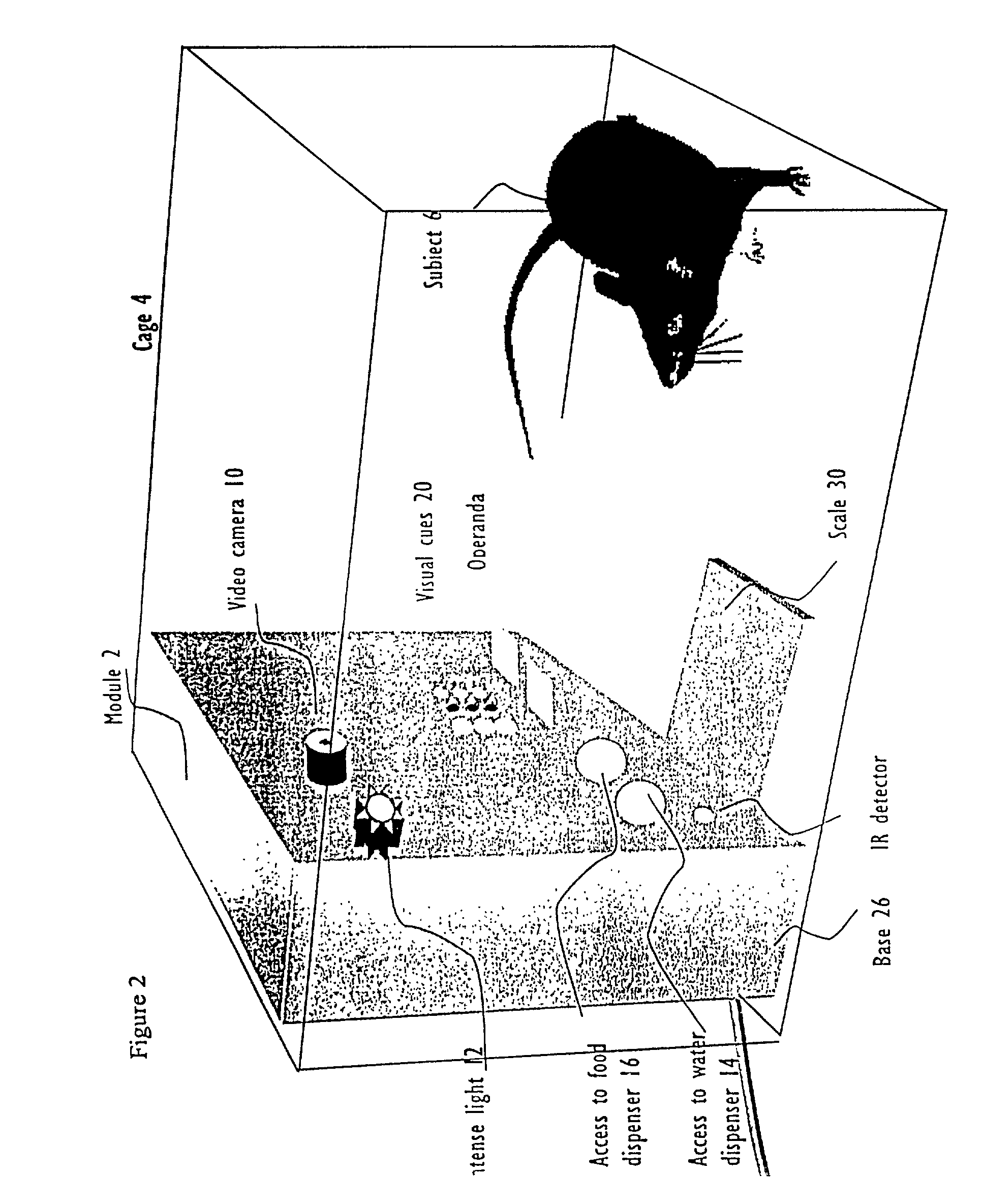
Systems and methods for monitoring behavior informatics
InactiveUS20030100998A2Improve statistics performanceExpand selectionDrug and medicationsBiostatisticsMulti dimensionalOrganism
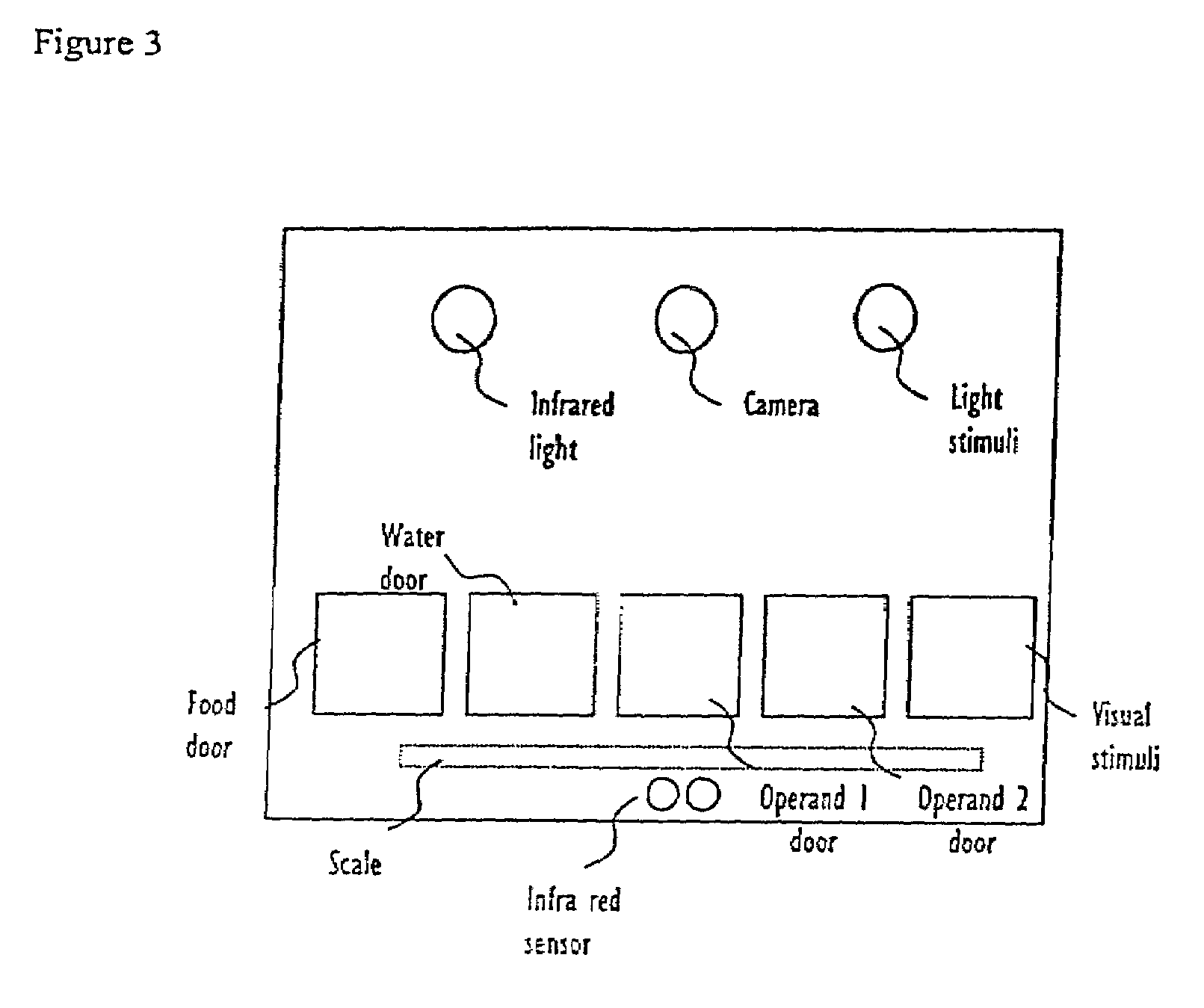
Abstract of Disclosure A system and method used to assess animal behavior includes a module having sensors that collects a variety of physical and biological data from a test subject. Interpretation of the data is provided to assess the test subject's behavior, neurology, biochemistry and physiology. The module is useful in observing the effects of a drug on the test animal and providing information on the drug's signature. Another advantage is module's portability that allows it to be used in standard laboratory cages. This portability allows the animal to be tested in its own habitat, that can reduce any erroneous data due to stressing the animal when removed to a test cage. Additionally, the module's design allows for parallel data collection and interpretation from several laboratory animals undergoing different experiments. Multi-dimensional modeling of the test subject based the system's interpretation of the data allows pattern recognition of the drug signature, and predictive drug analysis.
Owner:CARNEGIE MELLON UNIV +1
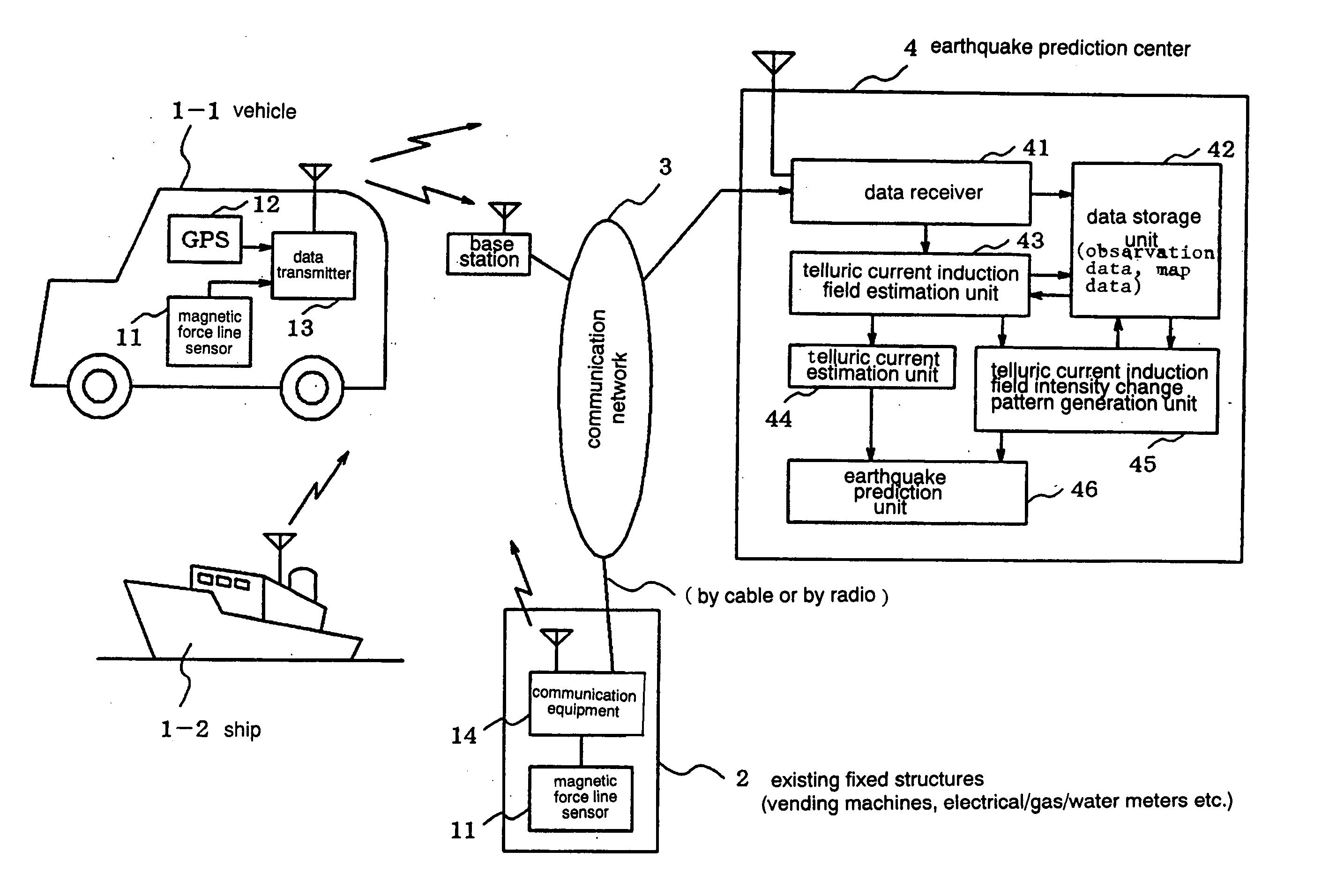
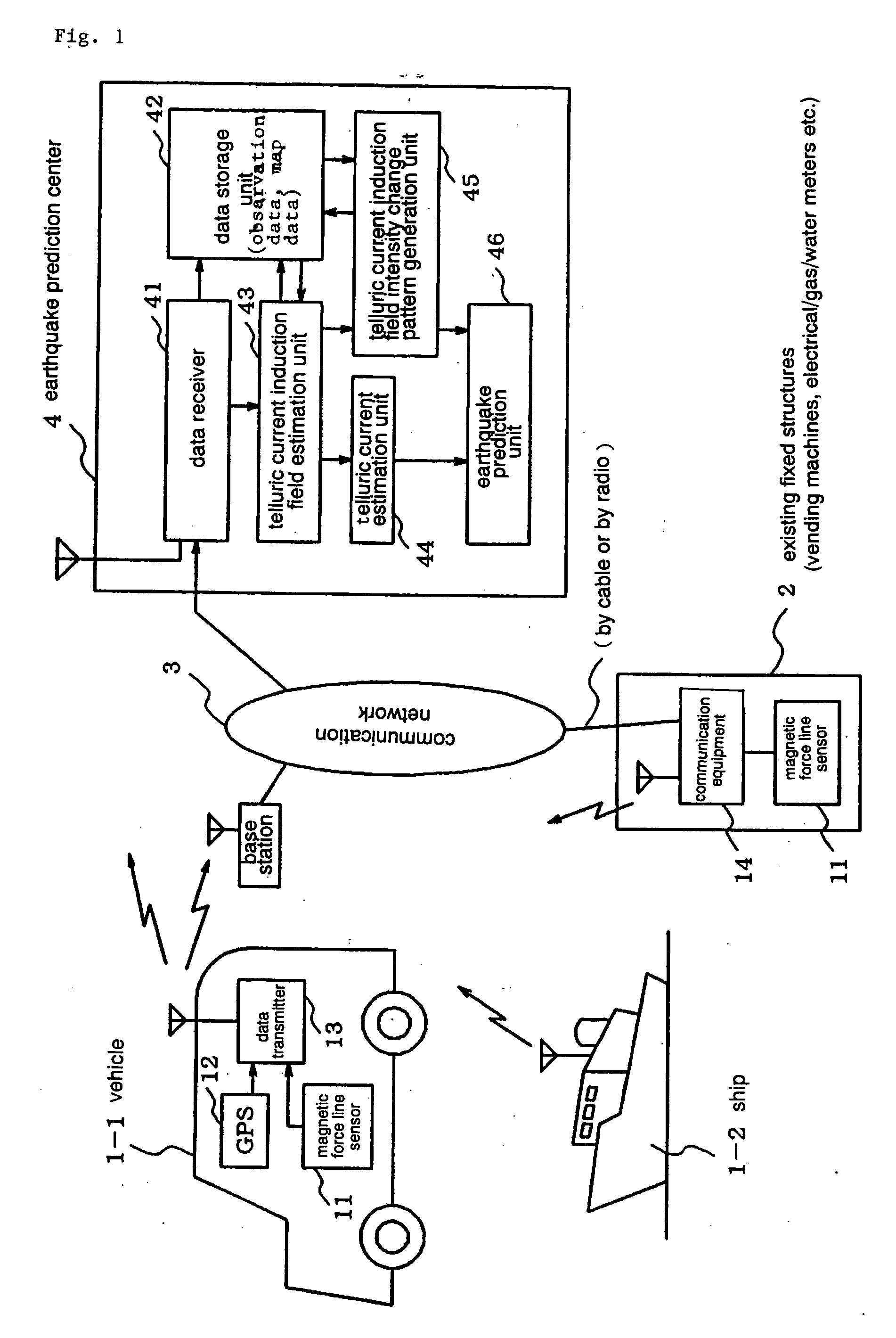
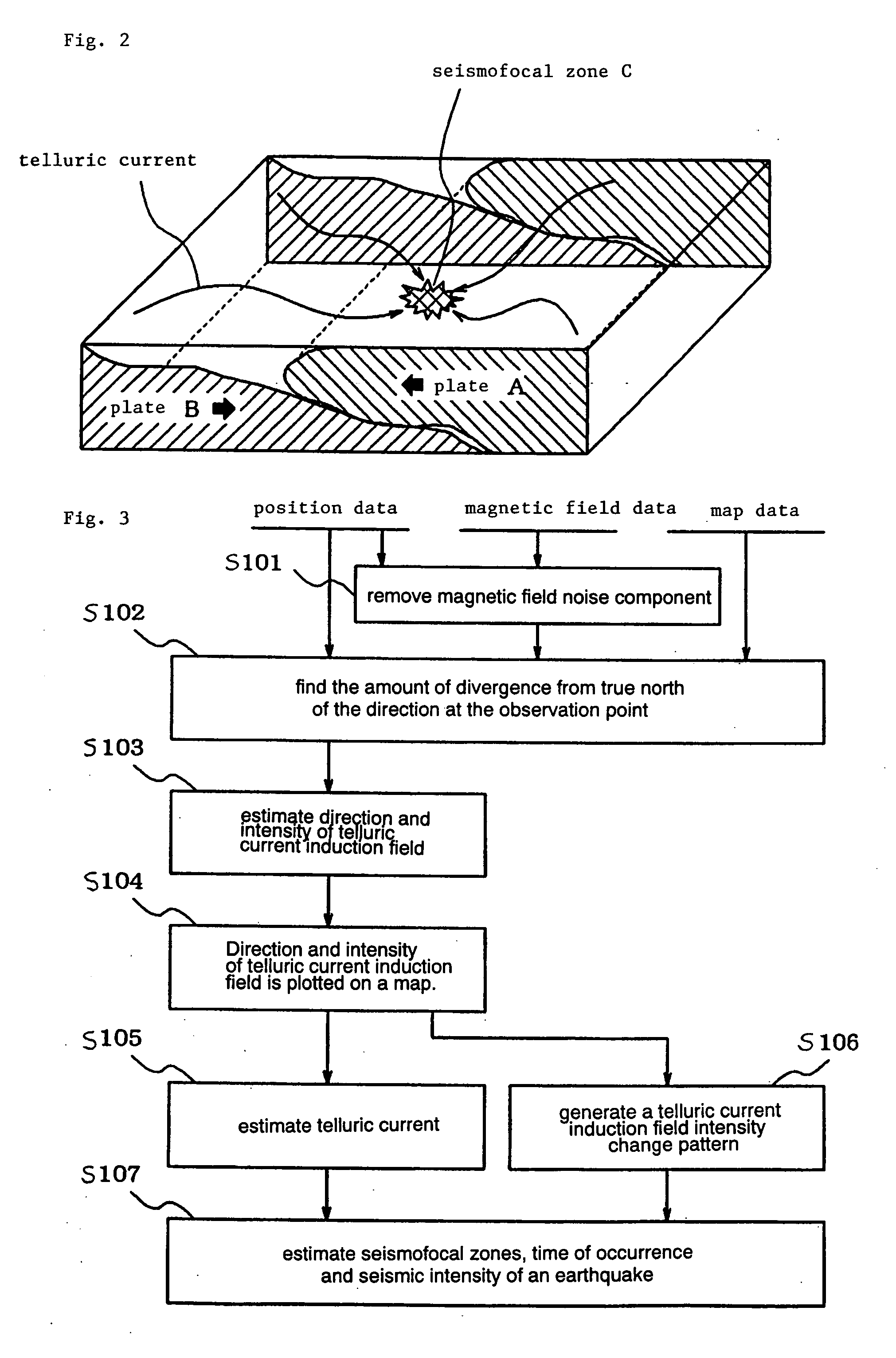
Earthquarke prediction method and system thereof
InactiveUS20050280421A1Low costAccurate predictionEarthquake measurementSeismic signal processingEarthquake predictionTransmitter
Vehicles (1-1) or ships (1-2) each carry a magnetic force line sensor (11), a GPS position detector (12), and a data transmitter (13) and travel within an observation area transmitting magnetic field data and position data of each point to an earthquake prediction center (4). A telluric current induction field estimation unit (43) of the earthquake prediction center (4) estimates telluric current induction fields based on the observation data that it receives and collects. A telluric current estimation unit (44) estimates telluric currents based on the results of estimating the telluric current induction fields. A telluric current induction field intensity change pattern generation unit (45) generates patterns that indicate the change over time of the intensity of telluric current induction fields. An earthquake prediction unit (46) analyzes the state of distribution of the telluric currents and the patterns of change in the intensities of the telluric current induction fields and estimates a seismofocal zone, seismic intensity, and time of occurrence of a seismic event.
Owner:NEC MOBILING LTD
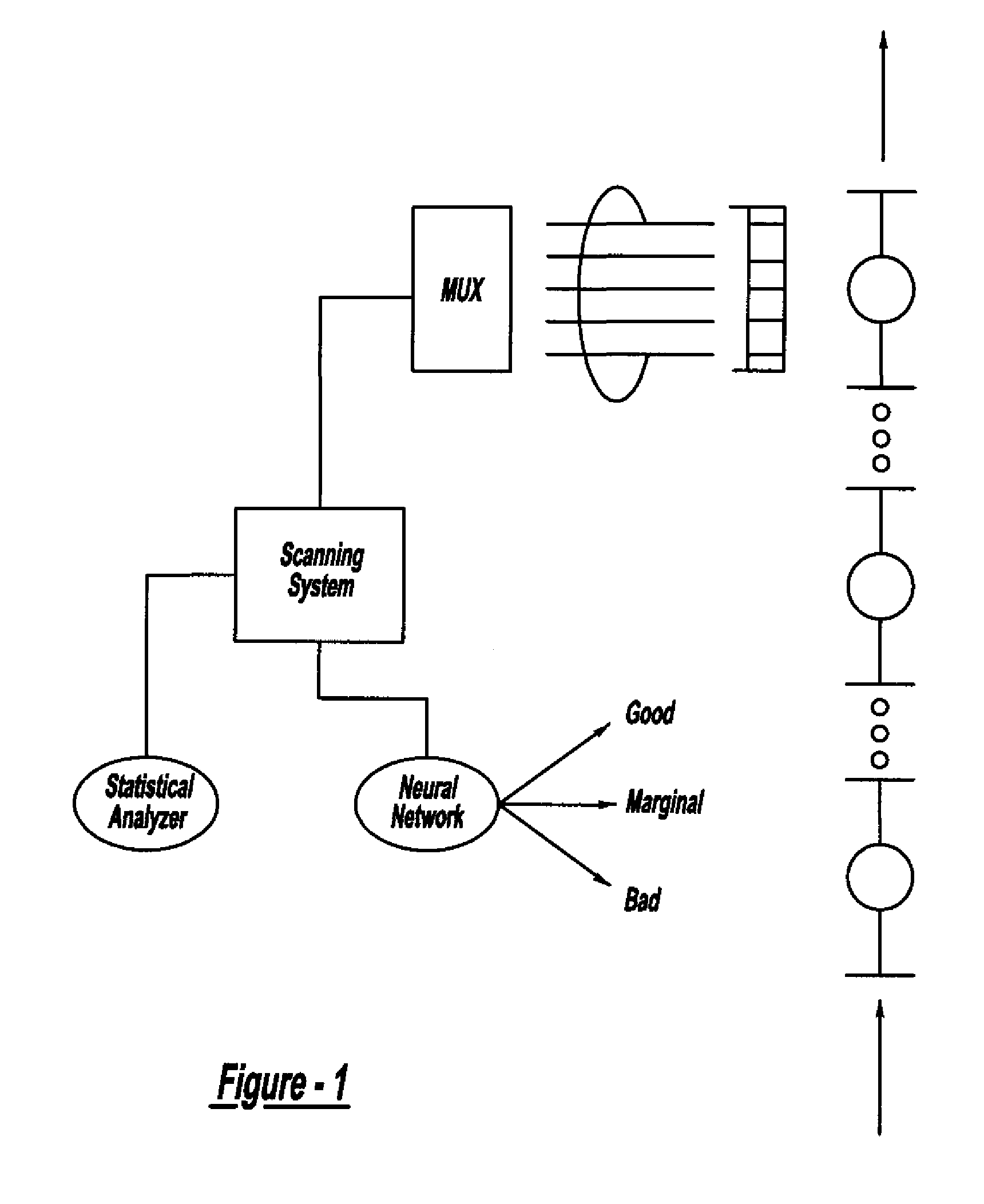
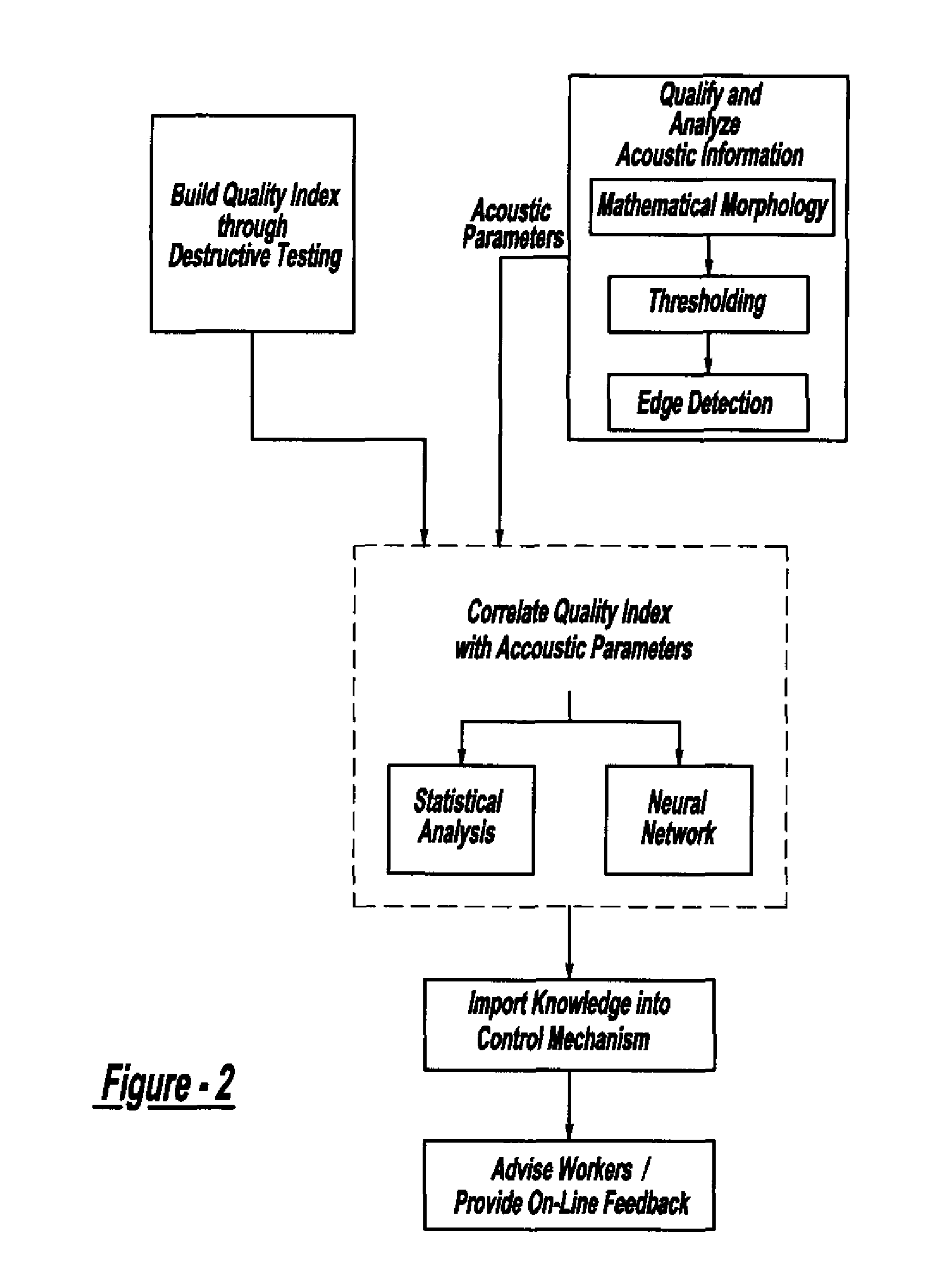
Method and system for assessing quality of spot welds
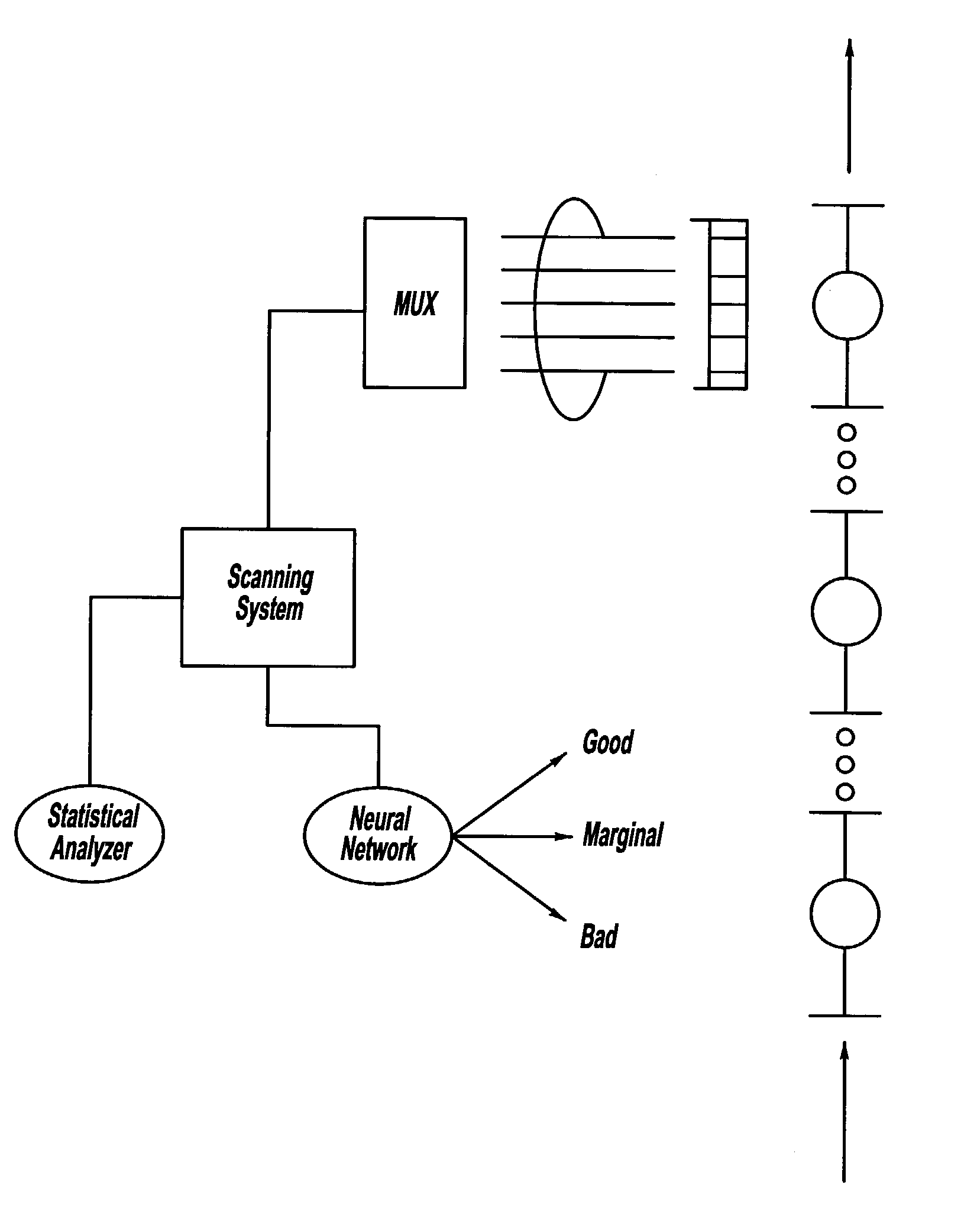
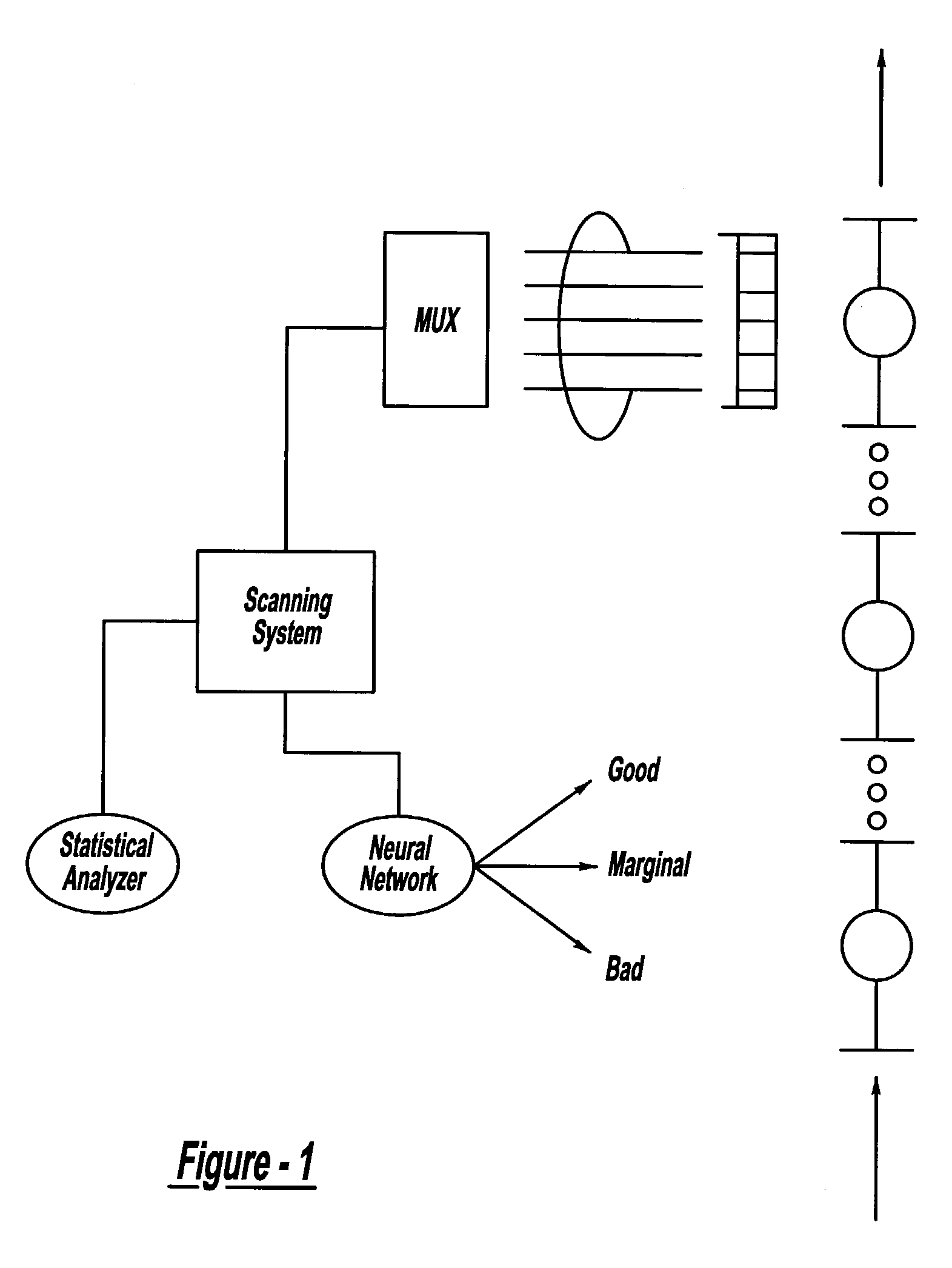
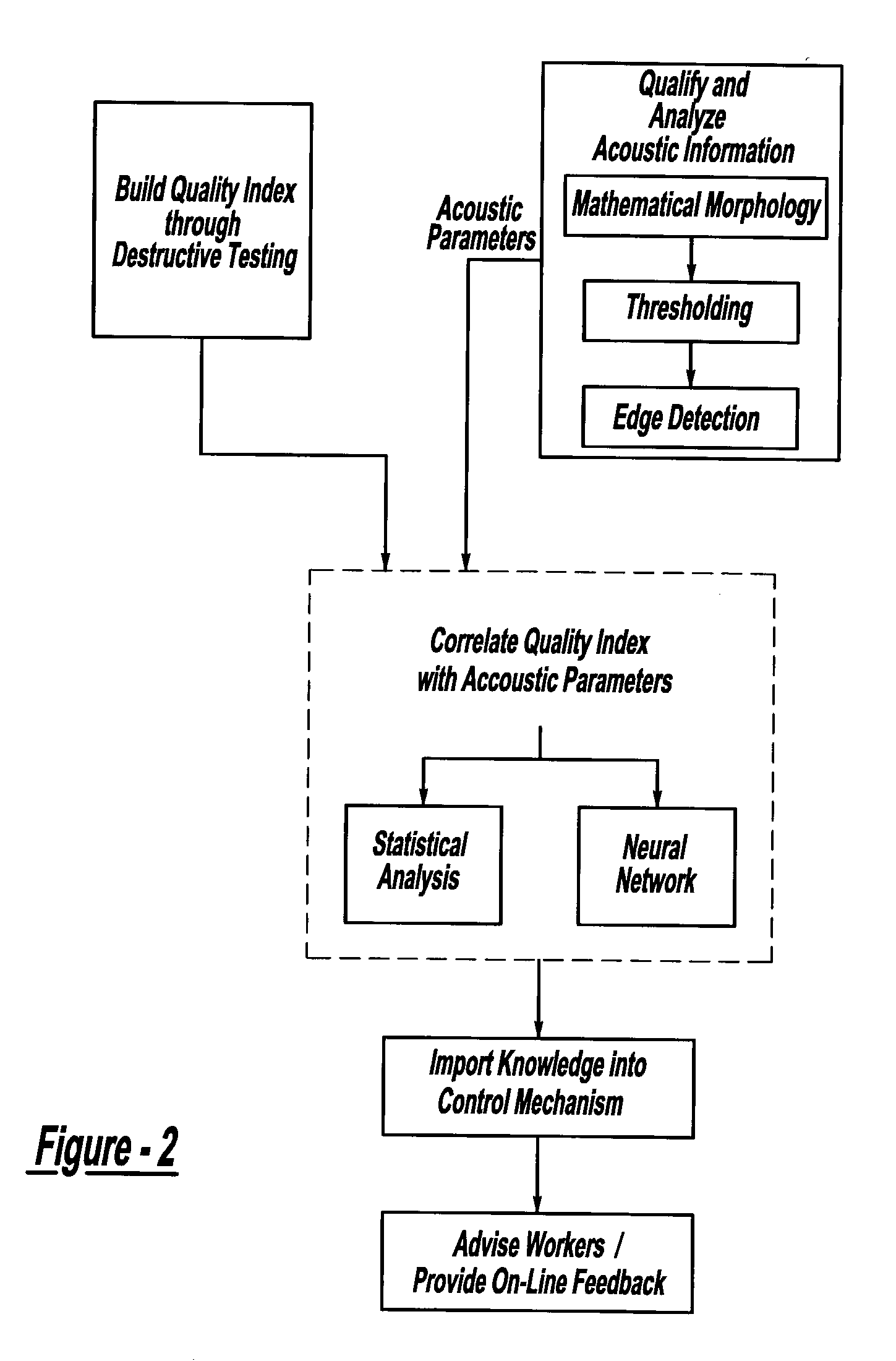
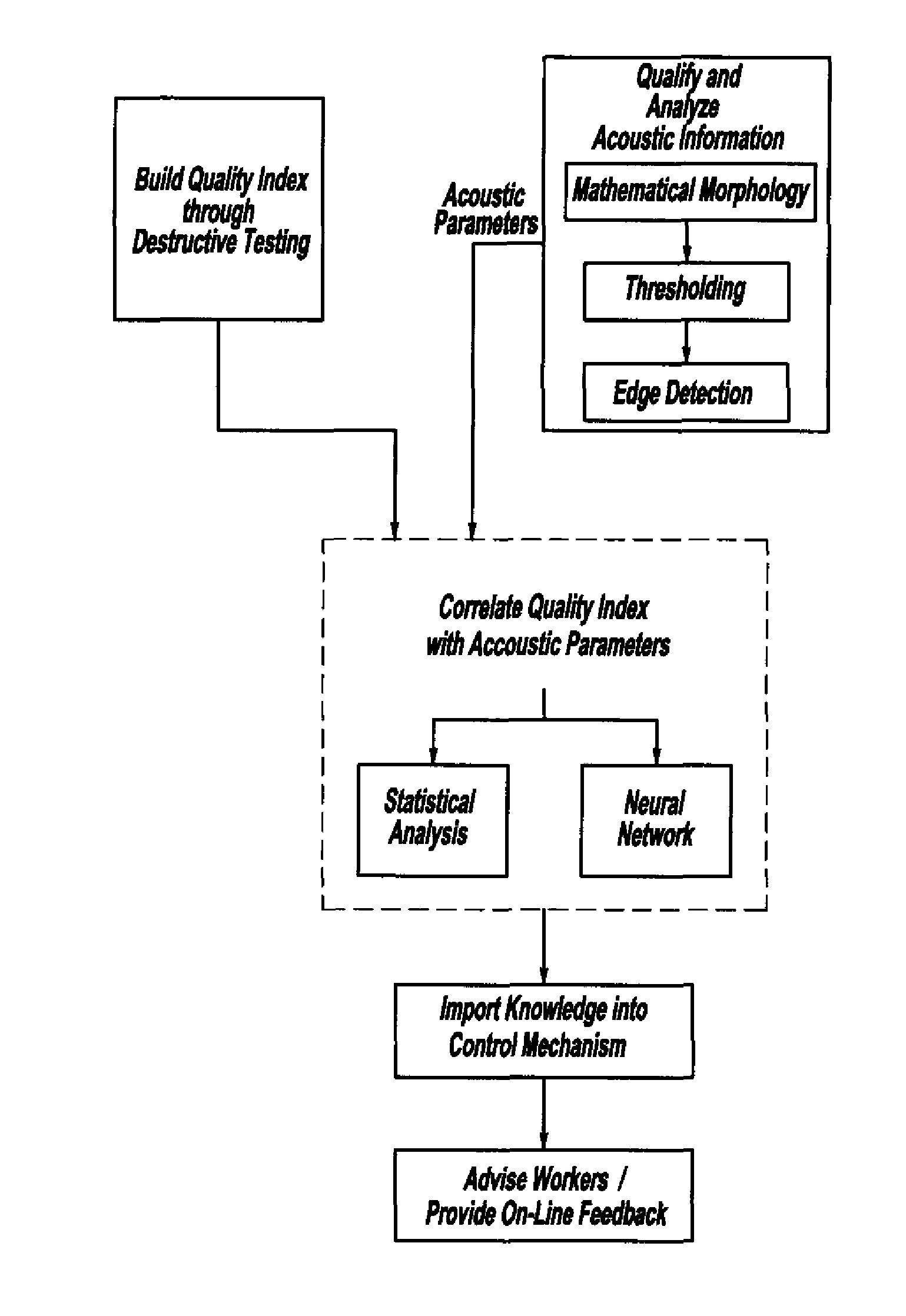
ActiveUS7132617B2Reduce in quantityReduce manufacturing costAnalysing solids using sonic/ultrasonic/infrasonic wavesProcessing detected response signalDigital dataElectricity
A system and method for assessing the quality of spot weld joints between pieces of metal includes an ultrasound transducer probing a spot weld joint. The ultrasound transducer transmits ultrasonic radiation into the spot weld joint, receives corresponding echoes, and transforms the echoes into electrical signals. An image reconstructor connected to the ultrasound transducer transforms the electrical signals into numerical data representing an ultrasound image. A neural network connected to the image reconstructor analyzes the numerical data and an output system presents information representing the quality of the spot weld joint. The system is trained to assess the quality of spot weld joints by scanning a spot weld joint with an ultrasound transducer to produce the data set representing the joint; then physically deconstructing the joint to assess the joint quality.
Owner:FCA US
Method And System For Assessing Quality Of Spot Welds
InactiveUS20070038400A1Reduce in quantityReduce manufacturing costMultiple-port networksMagnetic property measurementsDigital dataSonification
A system and method for assessing the quality of spot weld joints between pieces of metal includes an ultrasound transducer probing a spot weld joint. The ultrasound transducer transmits ultrasonic radiation into the spot weld joint, receives corresponding echoes, and transforms the echoes into electrical signals. An image reconstructor connected to the ultrasound transducer transforms the electrical signals into numerical data representing an ultrasound image. A neural network connected to the image reconstructor analyzes the numerical data and an output system presents information representing the quality of the spot weld joint. The system is trained to assess the quality of spot weld joints by scanning a spot weld joint with an ultrasound transducer to produce the data set representing the joint; then physically deconstructing the joint to assess the joint quality.
Owner:FCA US
Systems and methods for monitoring behavior informatics
InactiveUS7269516B2Improve abilitiesIncrease powerDrug and medicationsCharacter and pattern recognitionBehavioral neurologyDiagnosis laboratory
A system and method used to assess animal behavior includes a module having sensors that collects a variety of physical and biological data from a test subject. Interpretation of the data is provided to assess the test subject's behavior, neurology, biochemistry and physiology. The module is useful in observing the effects of a drug on the test animal and providing information on the drug's signature. Another advantage is the module's portability that allows it to be used in standard laboratory cages. This portability allows the animal to be tested in its own habitat, that can reduce any erroneous data due to stressing the animal when removed to a test cage. Additionally, the module's design allows for parallel data collection and interpretation from several laboratory animals undergoing different experiments. Multi-dimensional modeling of the test subject based the system's interpretation of the data allows pattern recognition of the drug signature, and predictive drug analysis.
Owner:CARNEGIE MELLON UNIV +1
Apparatus and method for predicting collision
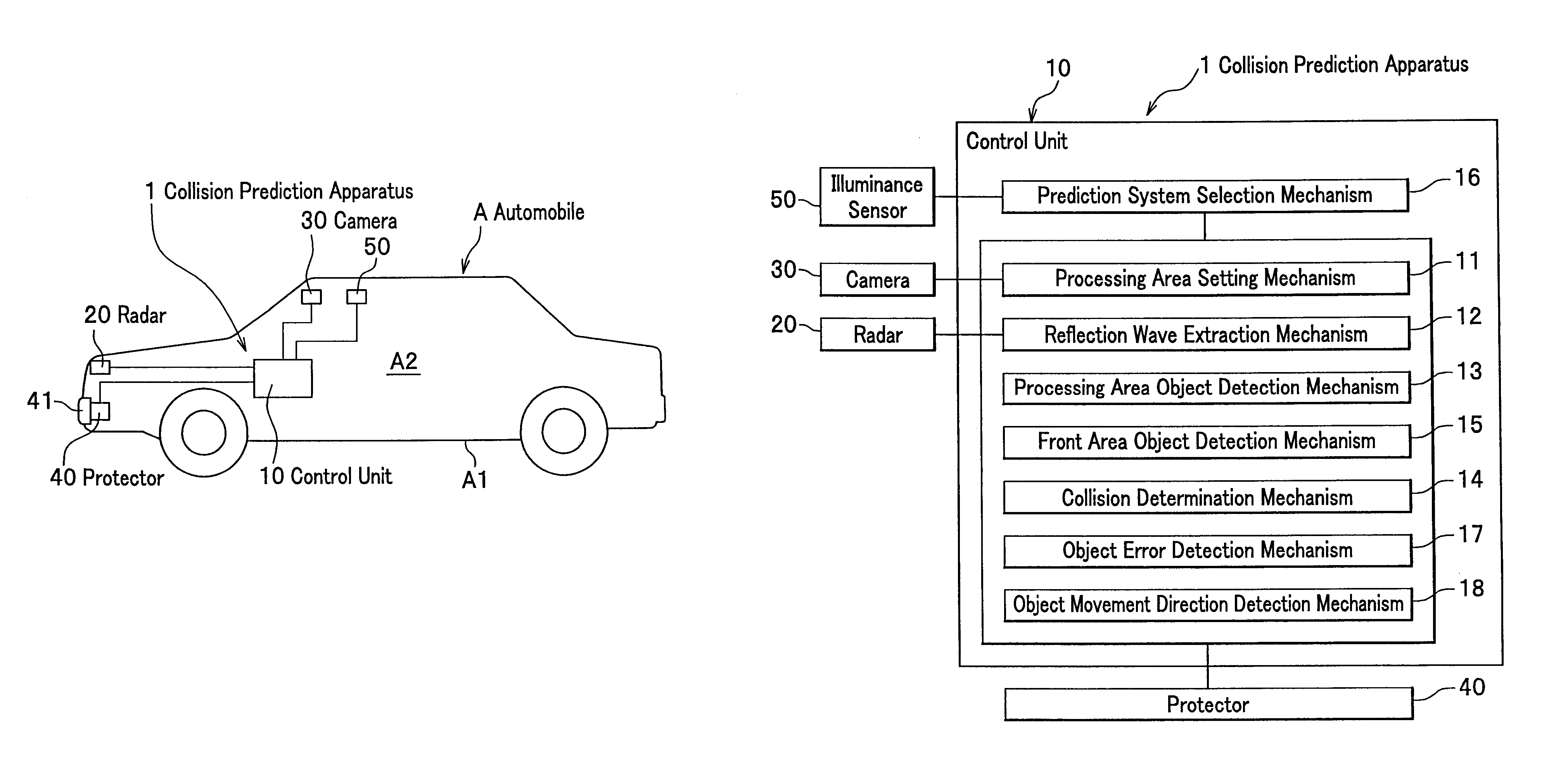
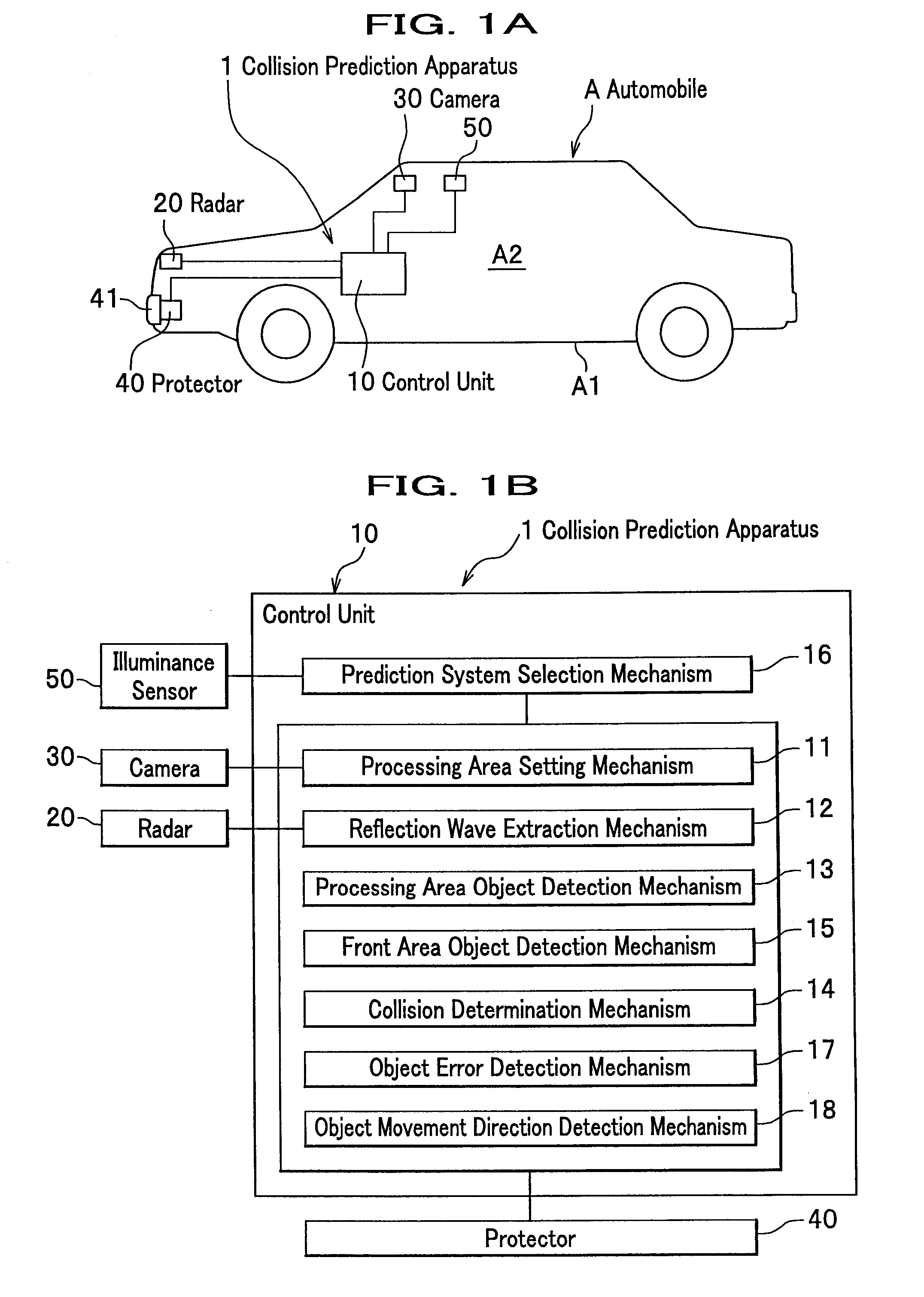
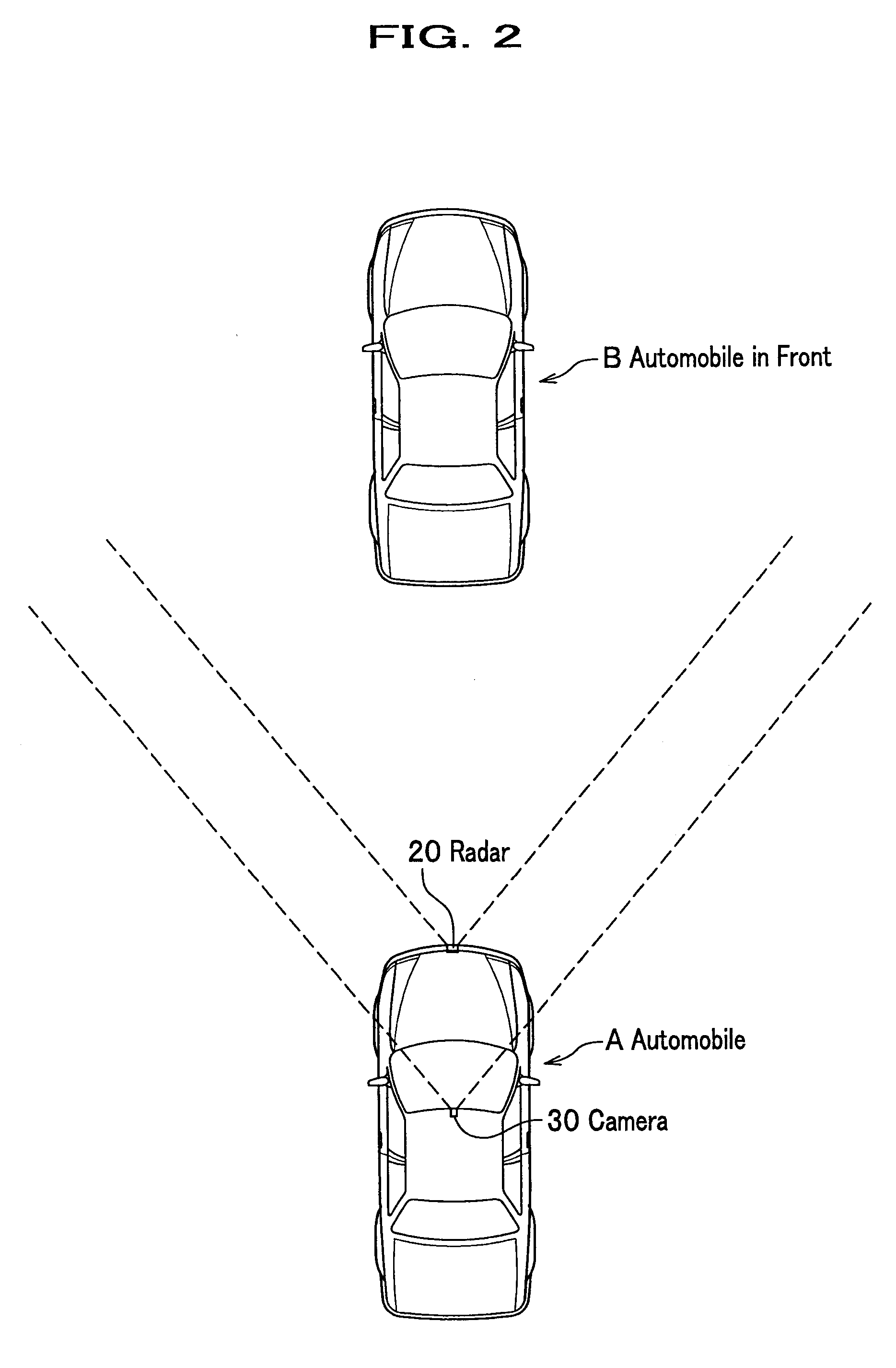
InactiveUS8068134B2Accurate detectionAccurately collisionTelevision system detailsDigital data processing detailsRadarEngineering
The present invention is a collision prediction apparatus that comprises a plurality of sensors for detecting an object in front of a vehicle by different mechanism and a control unit for selecting a sensor adequate for an input condition and predicting a collision between the vehicle and the object, based on information obtained from the selected sensor, wherein the plurality of the sensors comprise a radar for scanning front of the vehicle and a camera for taking an image of the front of the vehicle, and when a condition that it is lighter outside the vehicle than a predetermined value is input, it is enabled to configure a control unit so as to predict a collision between the vehicle and the object.
Owner:HONDA MOTOR CO LTD
Non-destructive inspection, testing and evaluation systems for intact aircraft and components and method therefore
InactiveUS6637266B1Accurate predictionEasy to useVibration measurement in solidsAnalysing solids using sonic/ultrasonic/infrasonic wavesRadiographic ExamNon destructive
Owner:FROOM DOUGLAS ALLEN
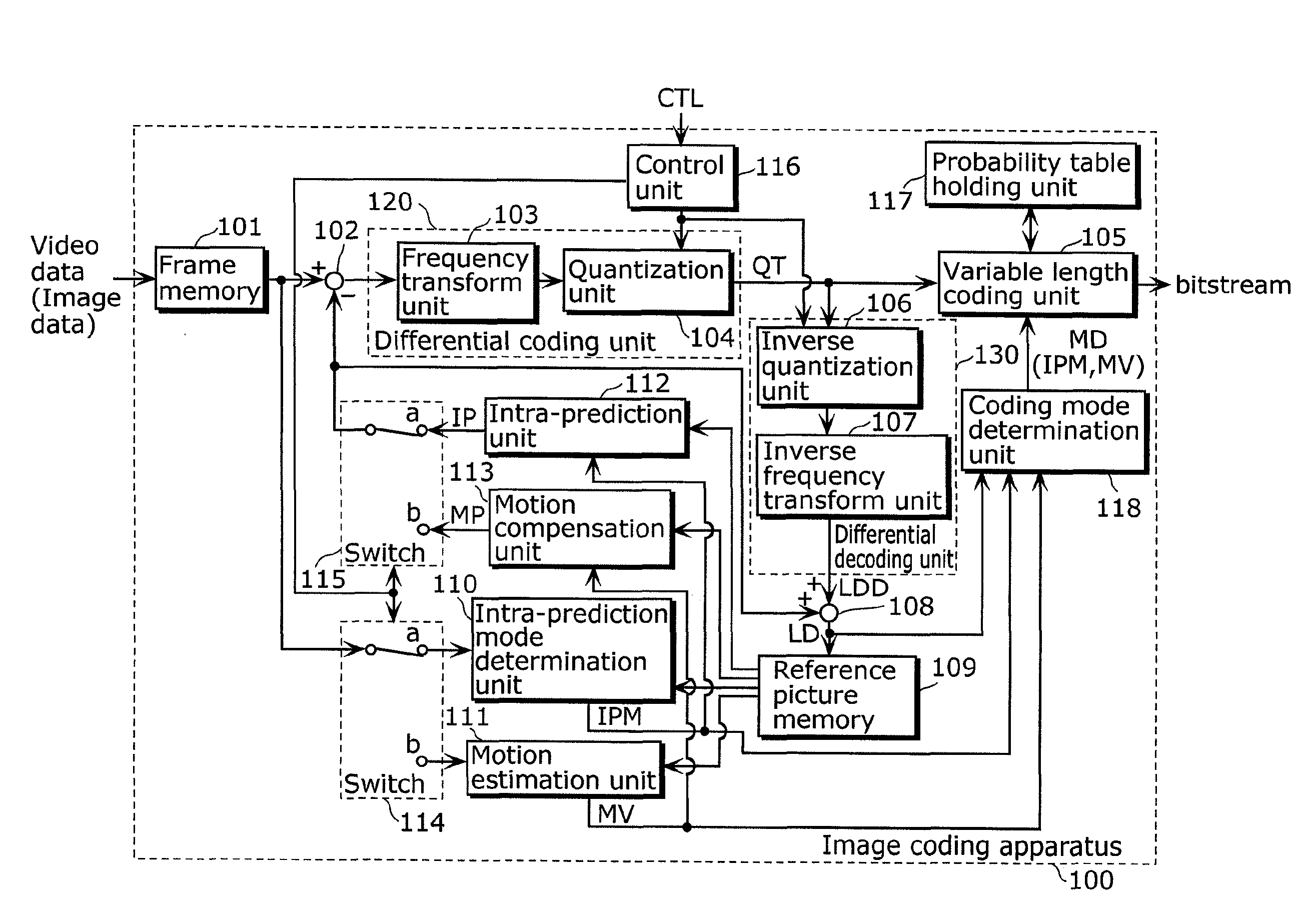
Image coding method and image decoding method
ActiveUS20100128995A1Accurate predictionReduce distortion problemsCharacter and pattern recognitionDigital video signal modificationAlgorithmCoding decoding
Owner:SUN PATENT TRUST
Systems and methods for predicting healthcare related financial risk
ActiveUS20060129428A1Reduce medical costsPrevent and mitigate occurrenceMedical simulationMedical data miningRisk levelRisk model
A system for predicting healthcare financial risk including the process of accessing patient data associated with one or more patents, accessing geographic and healthcare system data, filtering the patient data, geographic data, and healthcare system data into clean data, and applying a predictive risk model to the clean data to generate patient profile data and to identify a portion of the patients associated with a level of predicted financial risk.
Owner:HEALTH DIALOG SERVICES CORP
Methods for obtaining and using haplotype data
InactiveUS7058517B1Shorten the timeReduce effortMicrobiological testing/measurementProteomicsPopulationS genotyping
Methods, computer program(s) and database(s) to analyze and make use of gene haplotype information. These include methods, program, and database to find and measure the frequency of haplotypes in the general population; methods, program, and database to find correlation's between an individual's haplotypes or genotypes and a clinical outcome; methods, program, and database to predict an individual's haplotypes from the individual's genotype for a gene; and methods, program, and database to predict an individual's clinical response to a treatment based on the individual's genotype or haplotype.
Owner:GENAISSANCE PHARMA INC
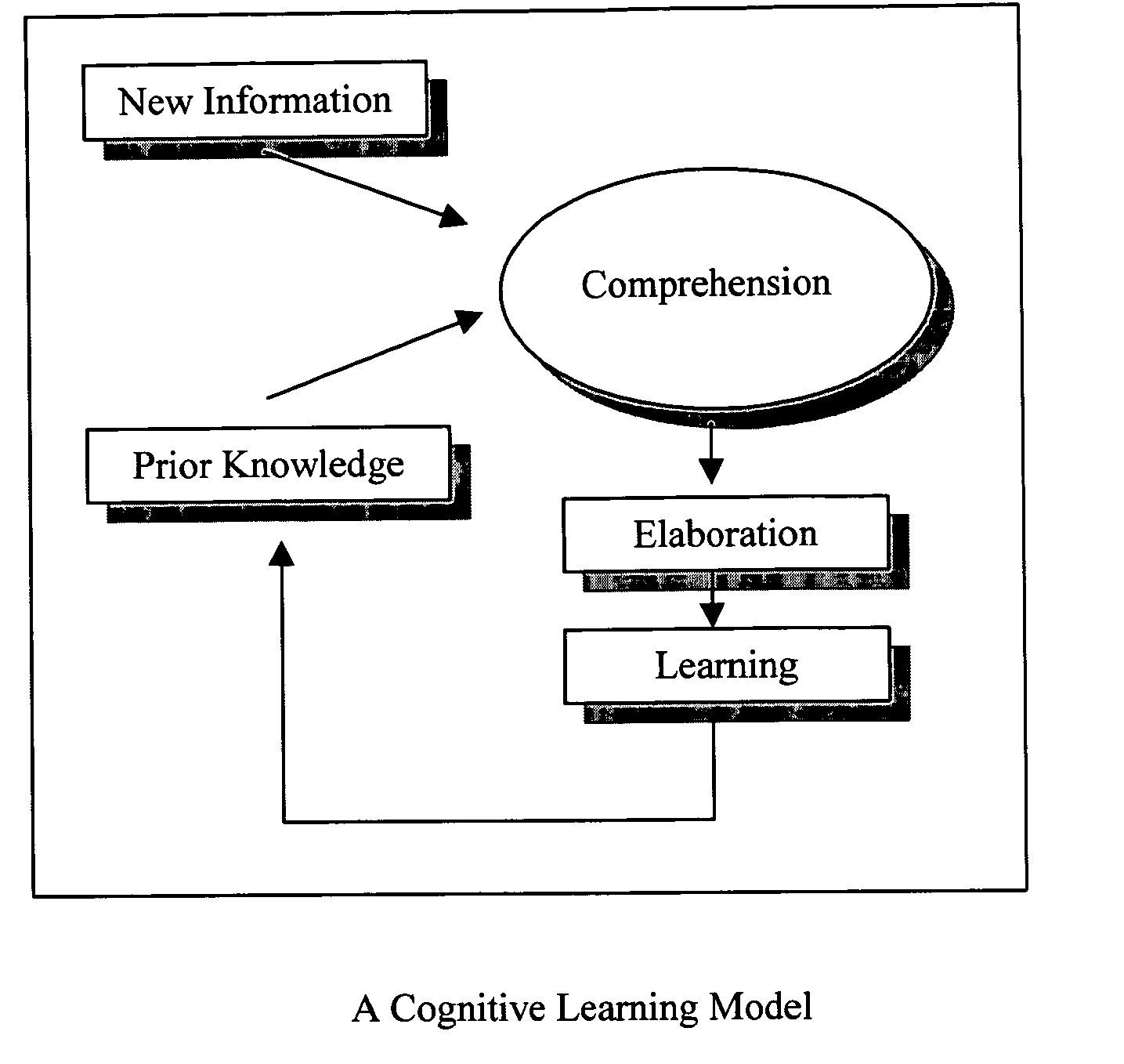
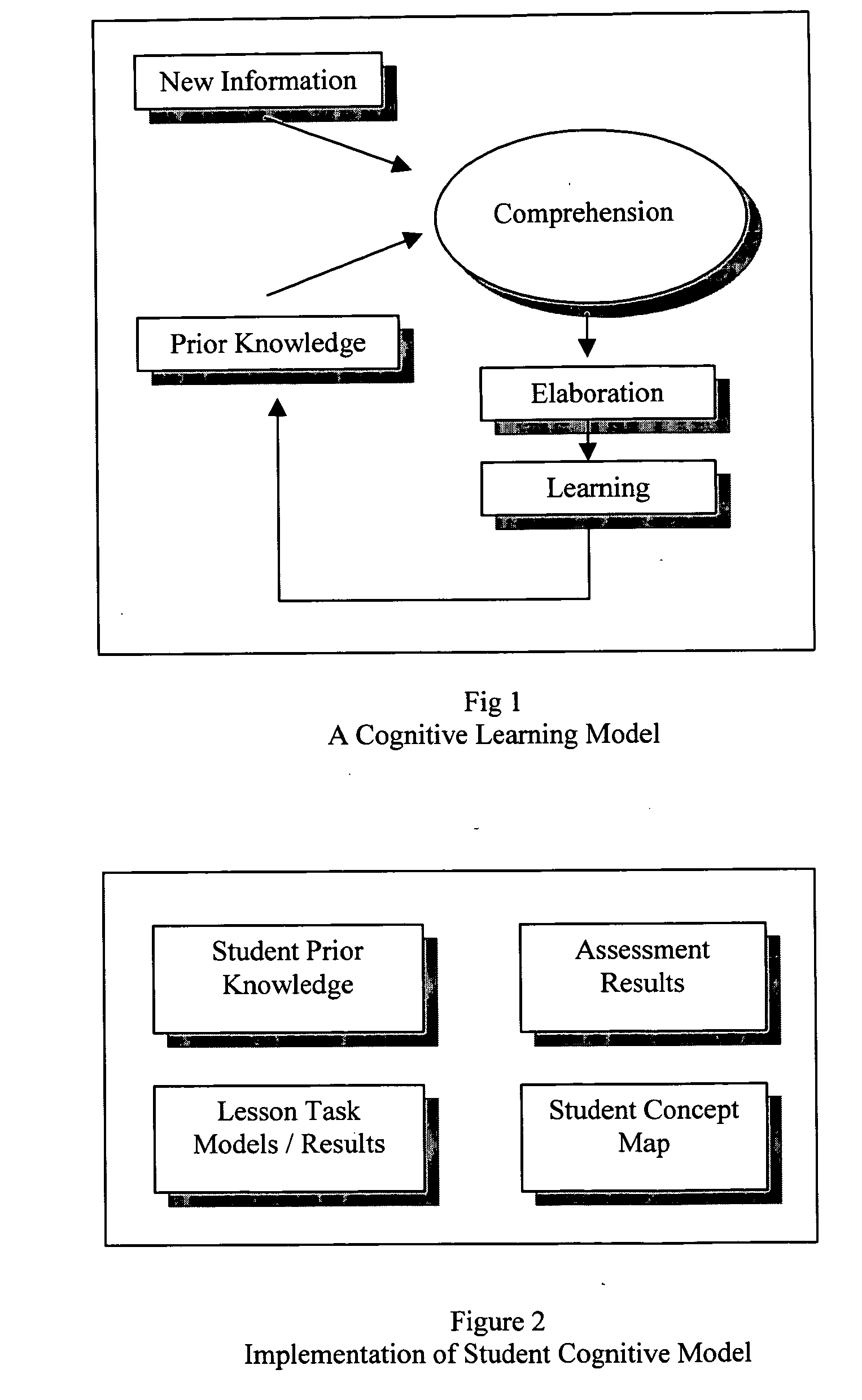
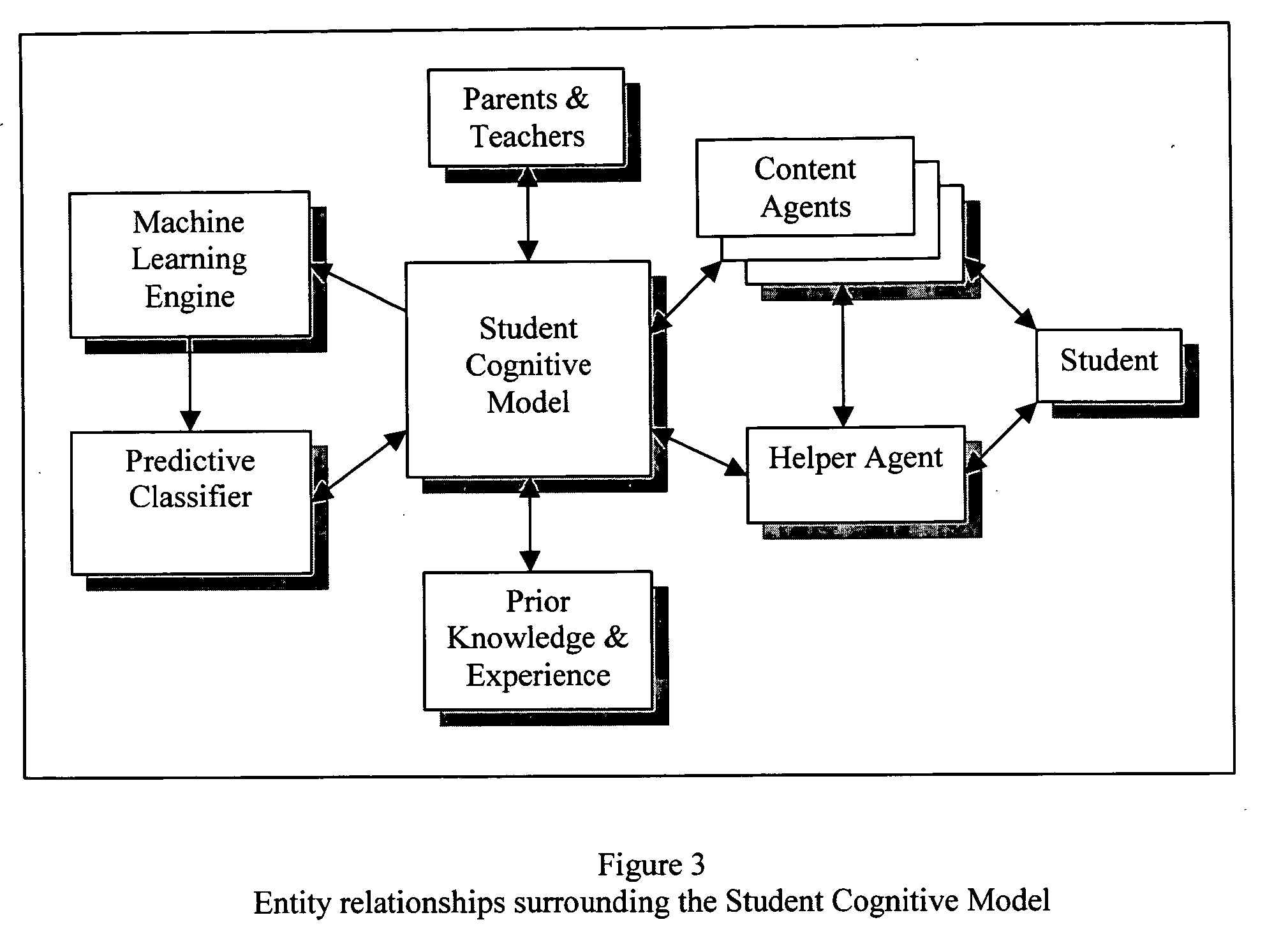
Predictive artificial intelligence and pedagogical agent modeling in the cognitive imprinting of knowledge and skill domains
InactiveUS20060166174A1Accurate predictionNew informationReadingElectrical appliancesPredictive learningAnimation
Owner:ROWE T PETER +2
Advanced bi-directional predictive coding of interlaced video
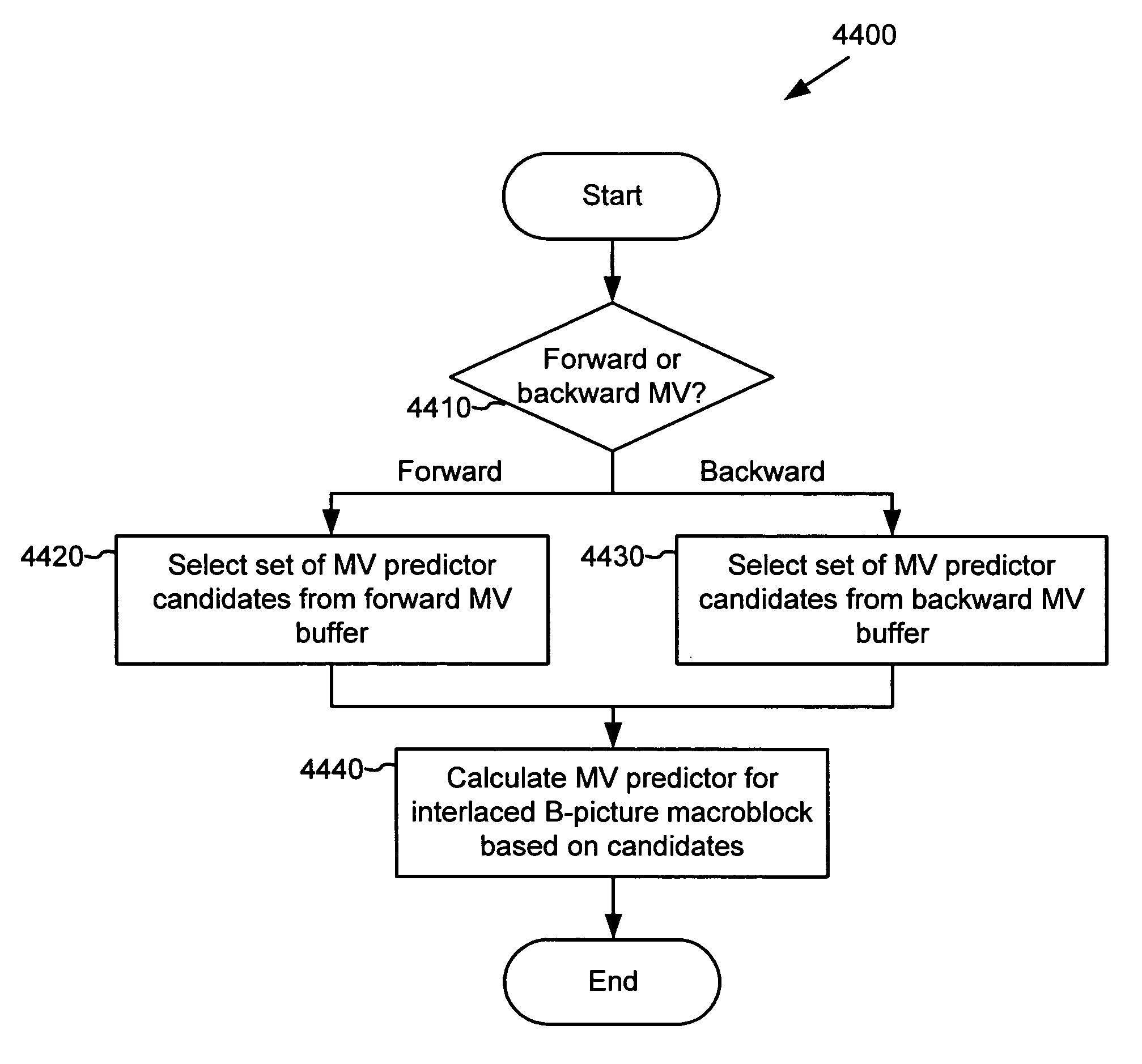
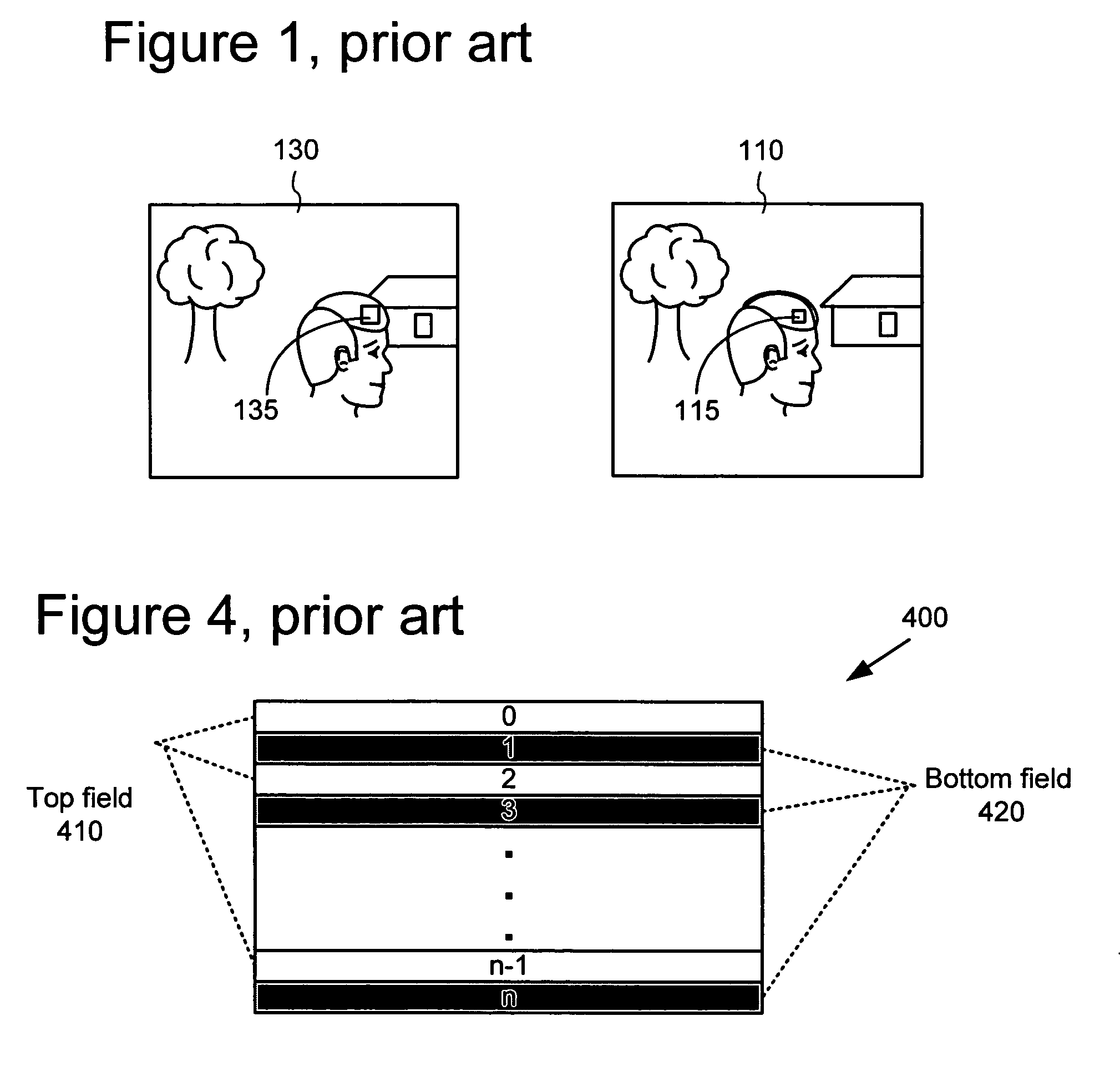
ActiveUS20050053292A1Accurate compensationReduce encoding overheadPicture reproducers using cathode ray tubesCode conversionInterlaced videoMotion vector
For interlaced B-fields or interlaced B-frames, forward motion vectors are predicted by an encoder / decoder using forward motion vectors from a forward motion vector buffer, and backward motion vectors are predicted using backward motion vectors from a backward motion vector buffer. The resulting motion vectors are added to the corresponding buffer. Holes in motion vector buffers can be filled in with estimated motion vector values. An encoder / decoder switches prediction modes between fields in a field-coded macroblock of an interlaced B-frame. For interlaced B-frames and interlaced B-fields, an encoder / decoder computes direct mode motion vectors. For interlaced B-fields or interlaced B-frames, an encoder / decoder uses 4 MV coding. An encoder / decoder uses “self-referencing” B-frames. An encoder sends binary information indicating whether a prediction mode is forward or not-forward for one or more macroblocks in an interlaced B-field. An encoder / decoder uses intra-coded B-fields [“BI-fields”].
Owner:MICROSOFT TECH LICENSING LLC
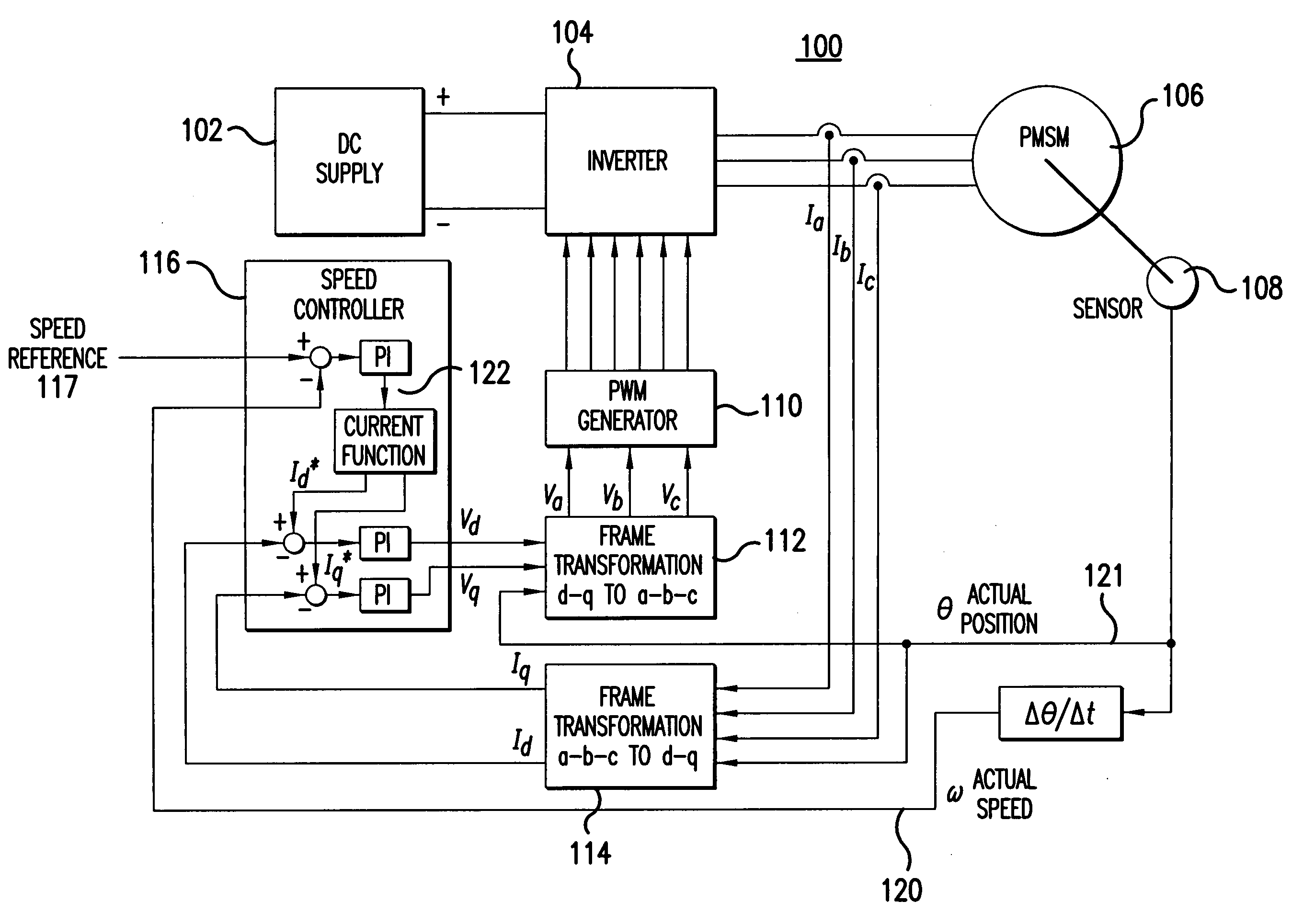
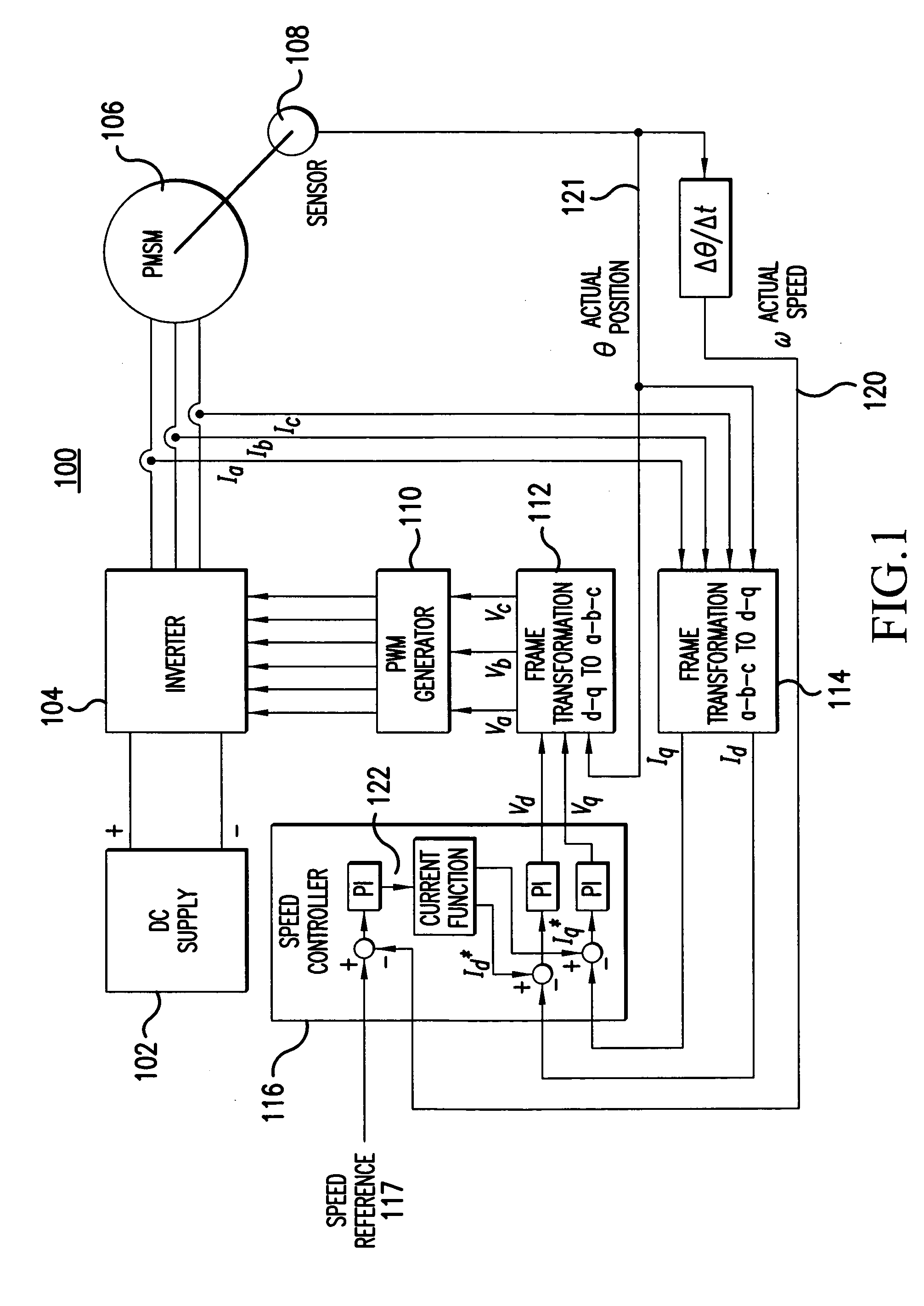
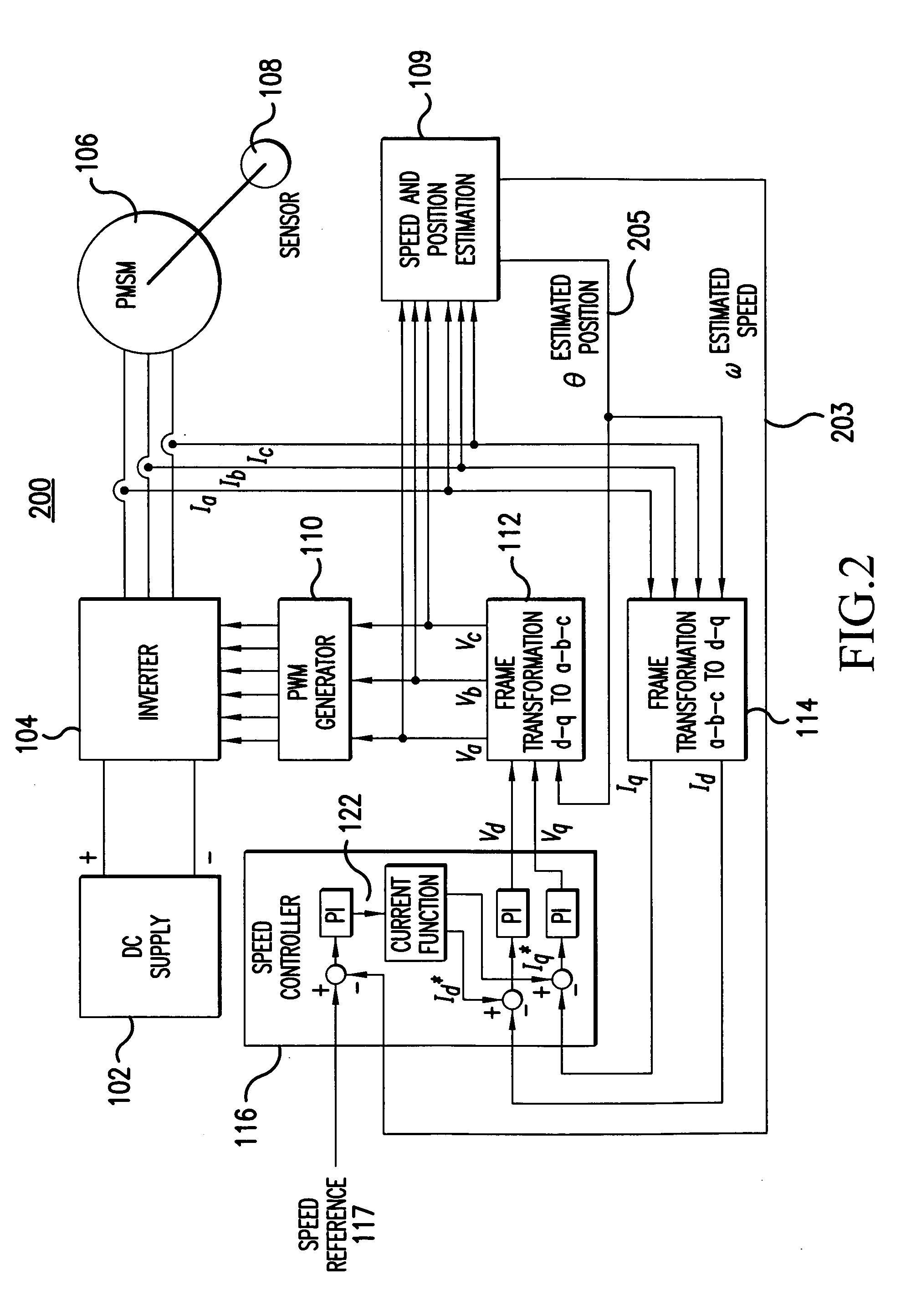
Sensorless control method and apparatus for a motor drive system
InactiveUS20050007044A1Quick calculationAccurate predictionDC motor speed/torque controlAC motor controlKaiman filterControl power
A method and apparatus provide a state observer control system 600 for a motor 106 that uses an extended Kalman filter 330 to predict initial rotor position and afterwards accurately predict rotor position and / or speed under variable types of loading conditions. A control system model 300 is generated that allows variable setting of an initial rotor position to generate estimated rotor position and speed as outputs. The control system model 300 includes an EKF (extended Kalman filter) estimator 330, speed controller 322, a current controller 324, and a variable load component 310. During operation, EKF estimator 330 estimates rotor speed 327 and position 333 based on reference voltages 402, 404 and currents 1325 generated by speed and current controllers 322, 324 and input from frame transformers 326, 328. Additionally, the reference currents and voltages 402, 404, 1325 are frame-transformed to be used as feedback signals 418, 346 in the system 600 and as drive signals to control power to be applied to a motor load 602.
Owner:HONEYWELL INT INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com