Patents
Literature
1025 results about "Diagnosis laboratory" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Laboratory diagnosis. a diagnosis arrived at after study of secretions, excretions, or tissue through chemical, microscopic, or bacteriological means or by biopsy.
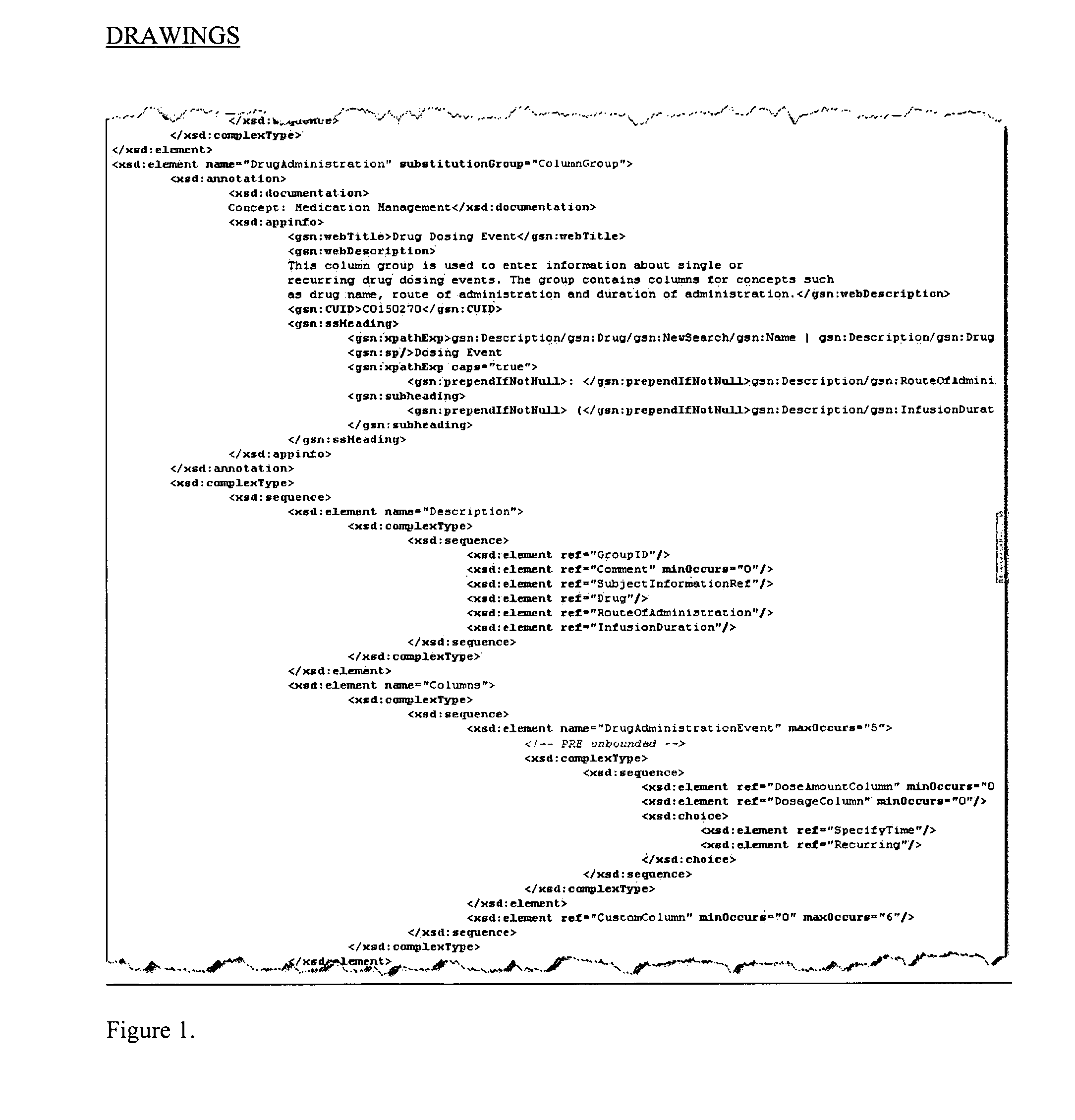
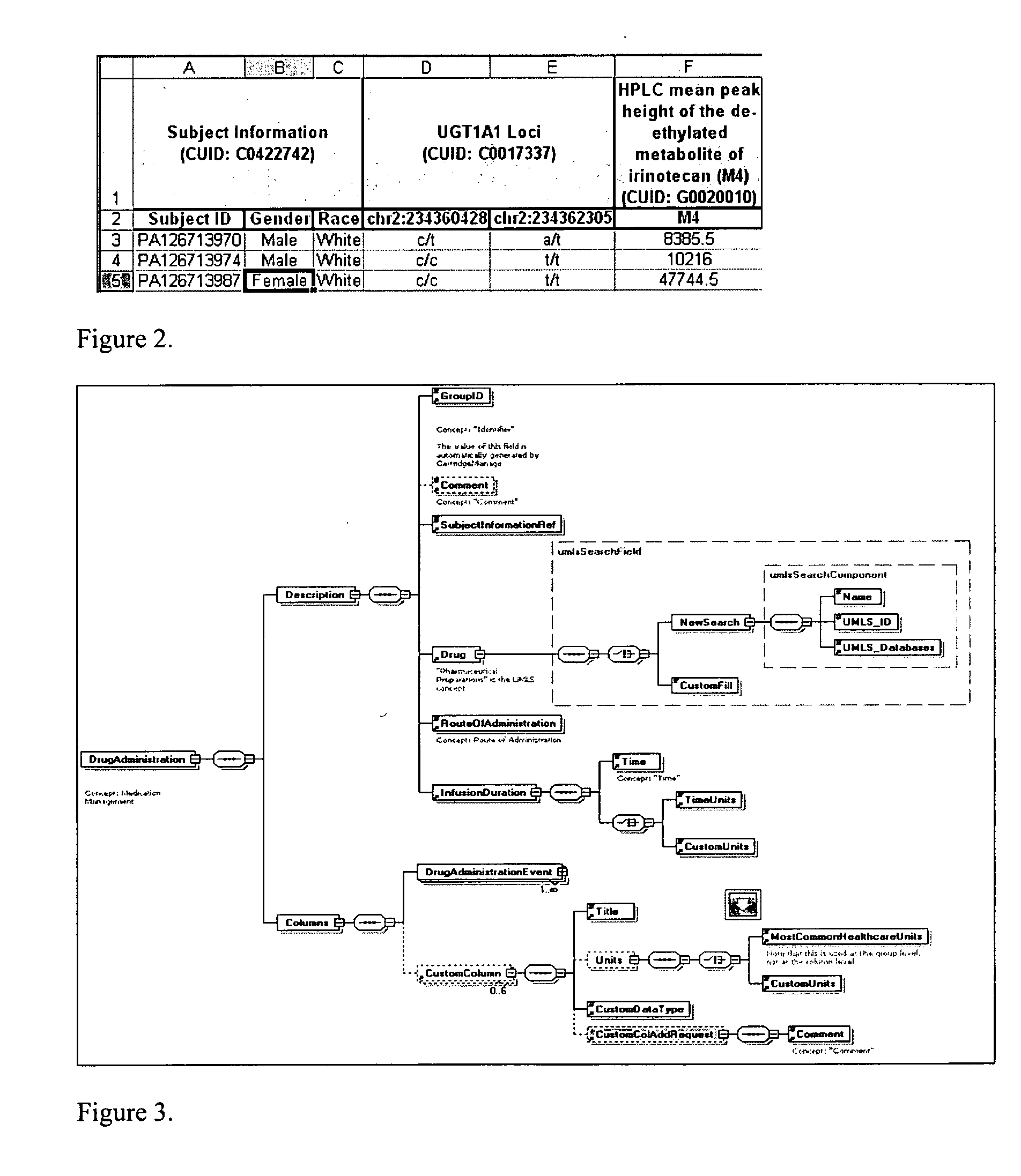
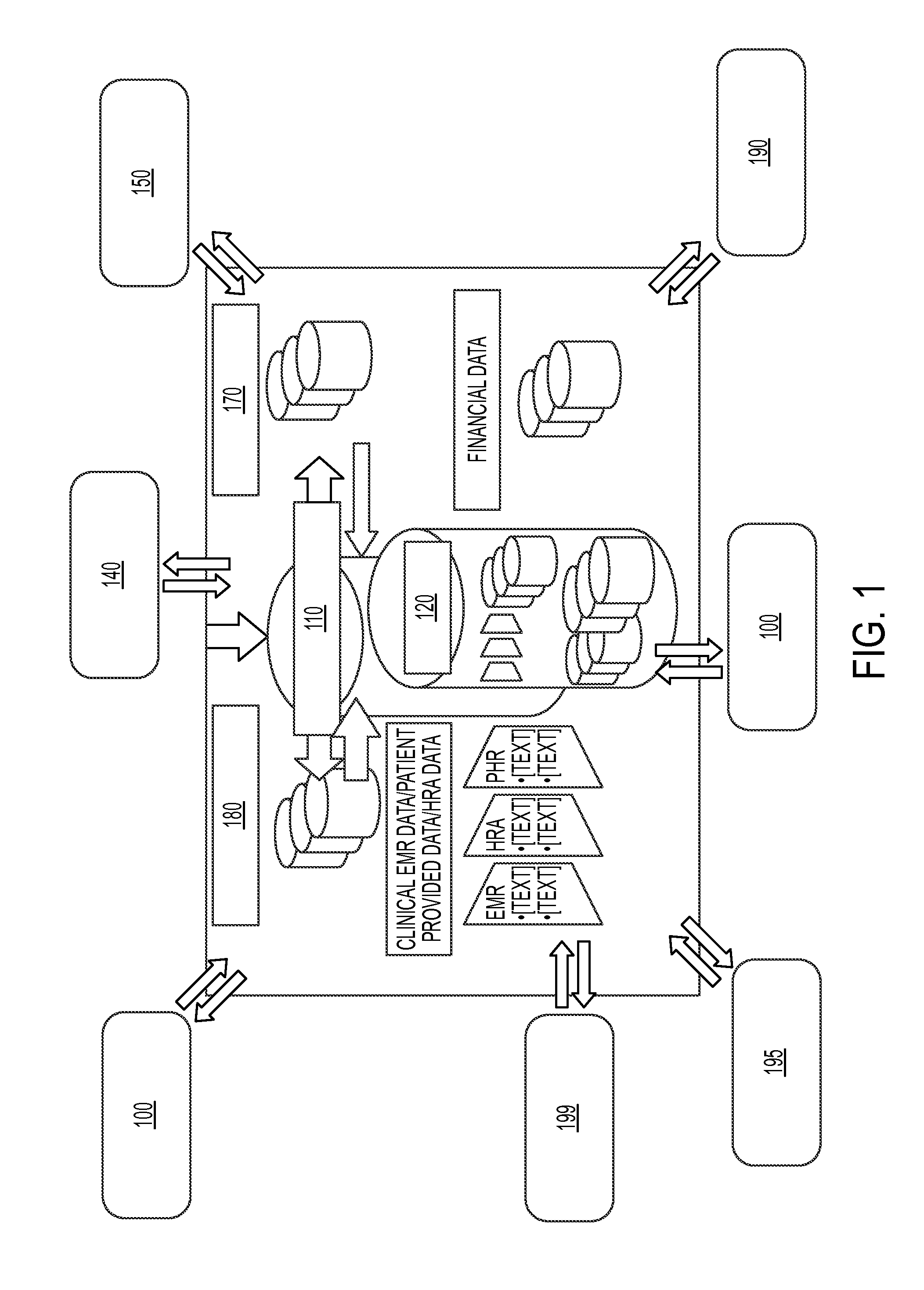
System and method for integrating and validating genotypic, phenotypic and medical information into a database according to a standardized ontology
InactiveUS20070178501A1Safest and most effective treatmentGood decisionData processing applicationsMicrobiological testing/measurementData validationMedical record
The system described herein enables clinicians and researchers to use aggregated genetic and phenotypic data from clinical trials and medical records to make the safest, most effective treatment decisions for each patient. This involves (i) the creation of a standardized ontology for genetic, phenotypic, clinical, pharmacokinetic, pharmacodynamic and other data sets, (ii) the creation of a translation engine to integrate heterogeneous data sets into a database using the standardized ontology, and (iii) the development of statistical methods to perform data validation and outcome prediction with the integrated data. The system is designed to interface with patient electronic medical records (EMRs) in hospitals and laboratories to extract a particular patient's relevant data. The system may also be used in the context of generating phenotypic predictions and enhanced medical laboratory reports for treating clinicians. The system may also be used in the context of leveraging the huge amount of data created in medical and pharmaceutical clinical trials. The ontology and validation rules are designed to be flexible so as to accommodate a disparate set of clients. The system is also designed to be flexible so that it can change to accommodate scientific progress and remain optimally configured.
Owner:NATERA
Immunoassay that provides for both collection of saliva and assay of saliva for one or more analytes with visual readout
InactiveUS6248598B1Eliminate riskBioreactor/fermenter combinationsBiological substance pretreatmentsAnalyteSaliva sample
A device that provides for both the collection of saliva and detection of at least one analyte therein, e.g., a drug, is provided. This device provides for rapid analysis of saliva samples, while also providing a convenient assay method that does not require the addition of extraneous reagents, or other materials. Thereby, this device can be used by non-laboratory personnel without risk of user introduced errors.
Owner:BOGEMA STUART C
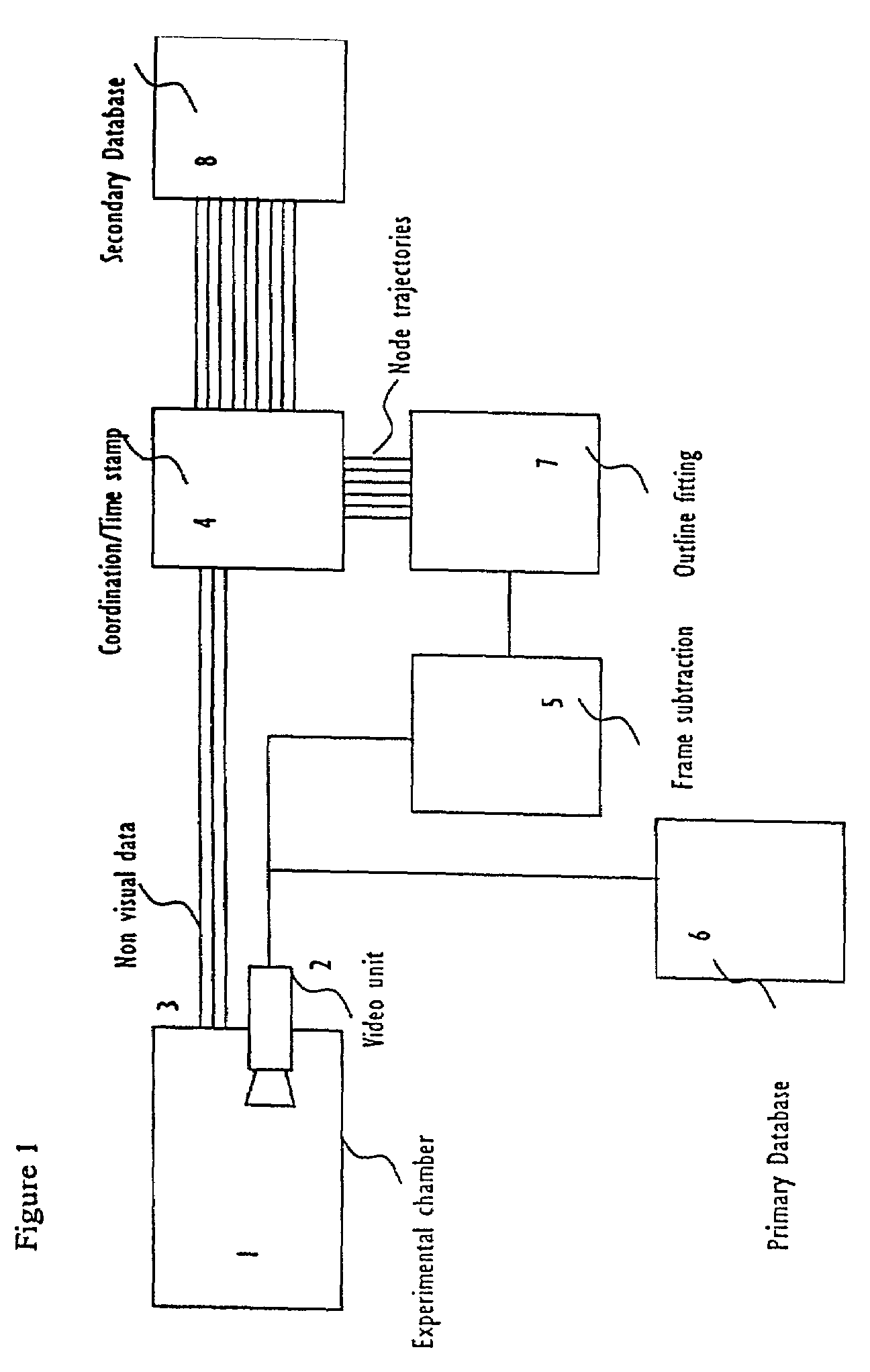
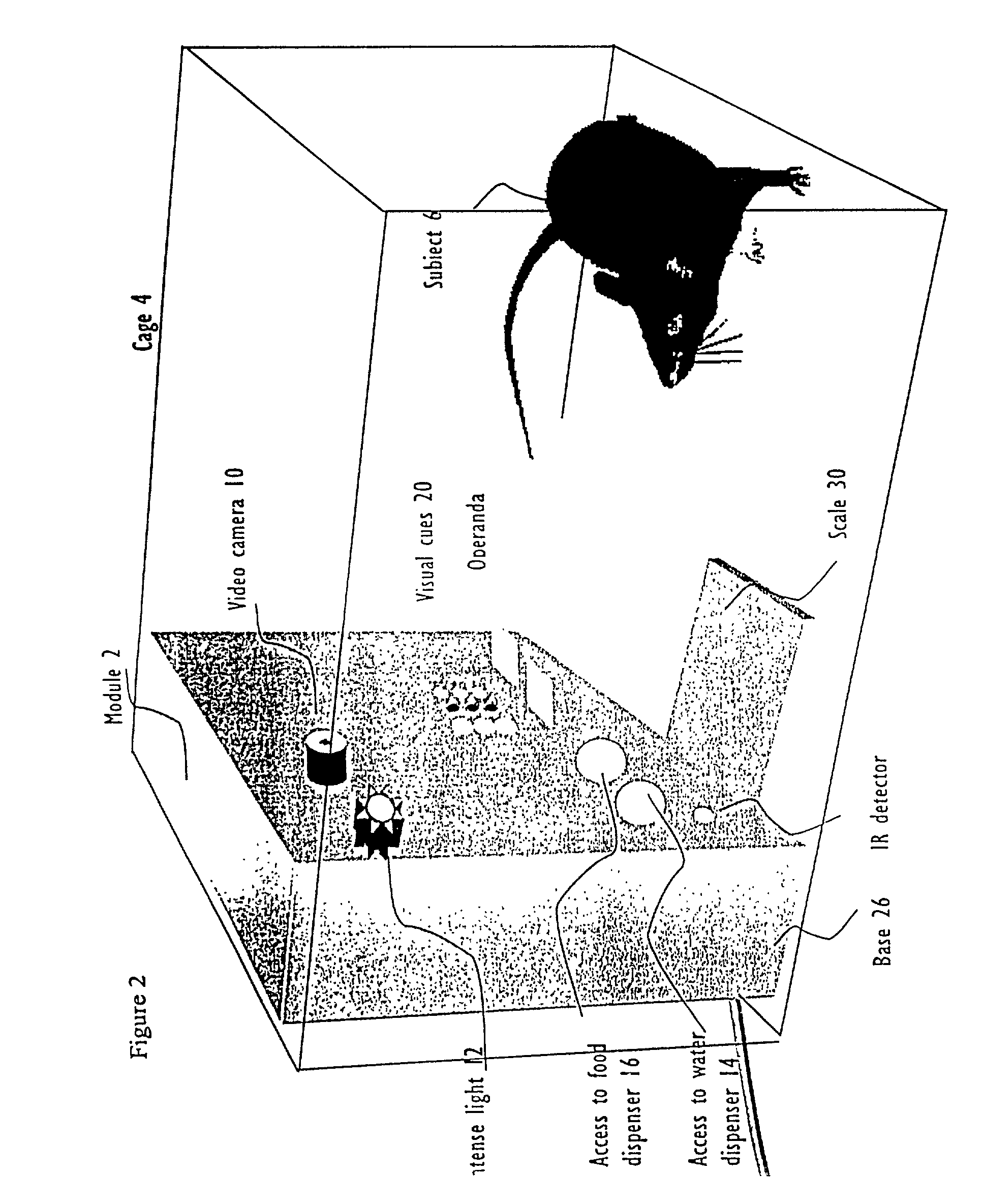
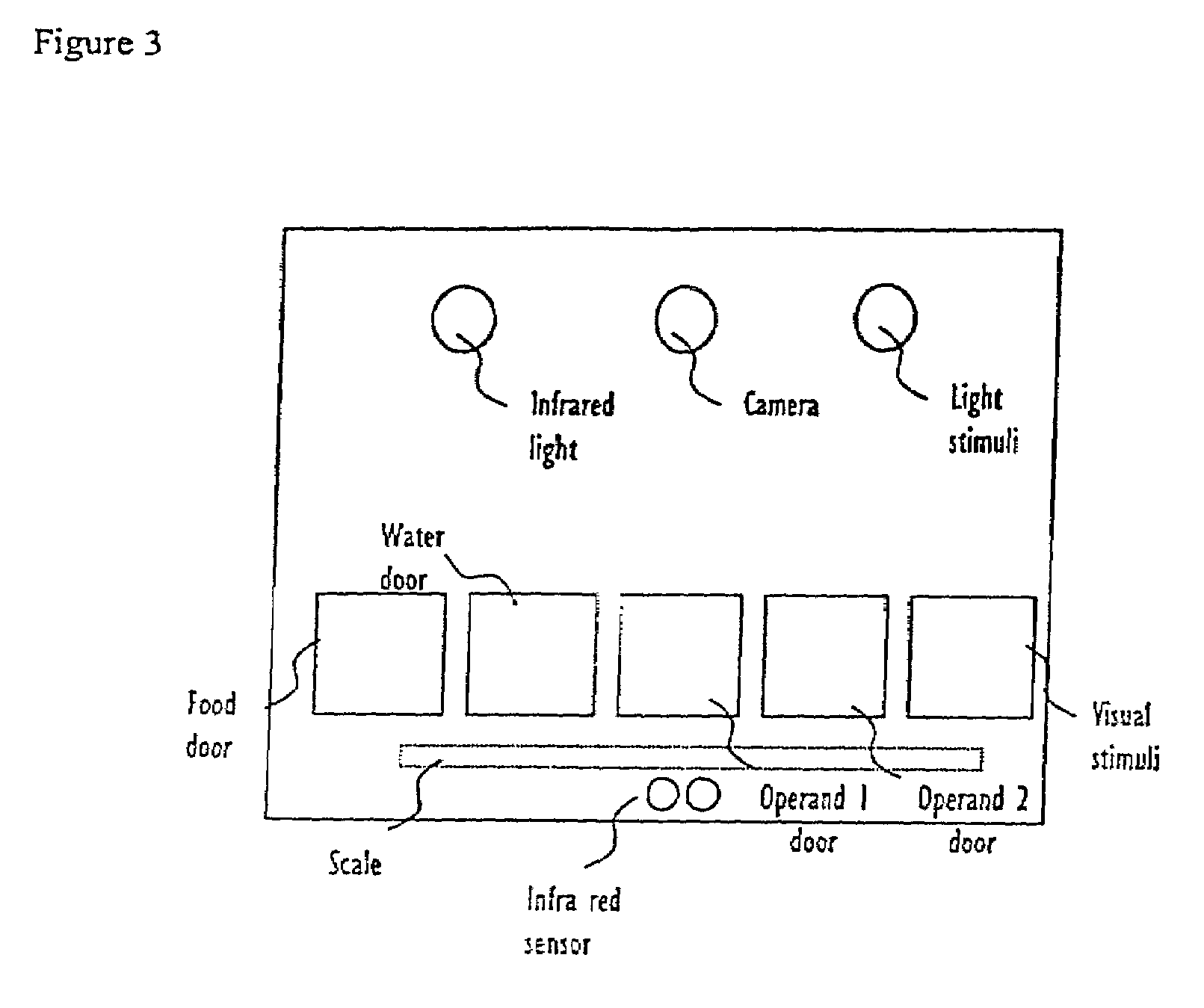
Systems and methods for monitoring behavior informatics
InactiveUS7269516B2Improve abilitiesIncrease powerDrug and medicationsCharacter and pattern recognitionBehavioral neurologyDiagnosis laboratory
A system and method used to assess animal behavior includes a module having sensors that collects a variety of physical and biological data from a test subject. Interpretation of the data is provided to assess the test subject's behavior, neurology, biochemistry and physiology. The module is useful in observing the effects of a drug on the test animal and providing information on the drug's signature. Another advantage is the module's portability that allows it to be used in standard laboratory cages. This portability allows the animal to be tested in its own habitat, that can reduce any erroneous data due to stressing the animal when removed to a test cage. Additionally, the module's design allows for parallel data collection and interpretation from several laboratory animals undergoing different experiments. Multi-dimensional modeling of the test subject based the system's interpretation of the data allows pattern recognition of the drug signature, and predictive drug analysis.
Owner:CARNEGIE MELLON UNIV +1
Systems and Methods for Collecting and Transmitting Assay Results
ActiveUS20140057255A1Quality improvementLittle variabilityMicrobiological testing/measurementMaterial analysis by optical meansDiseaseChemical reaction
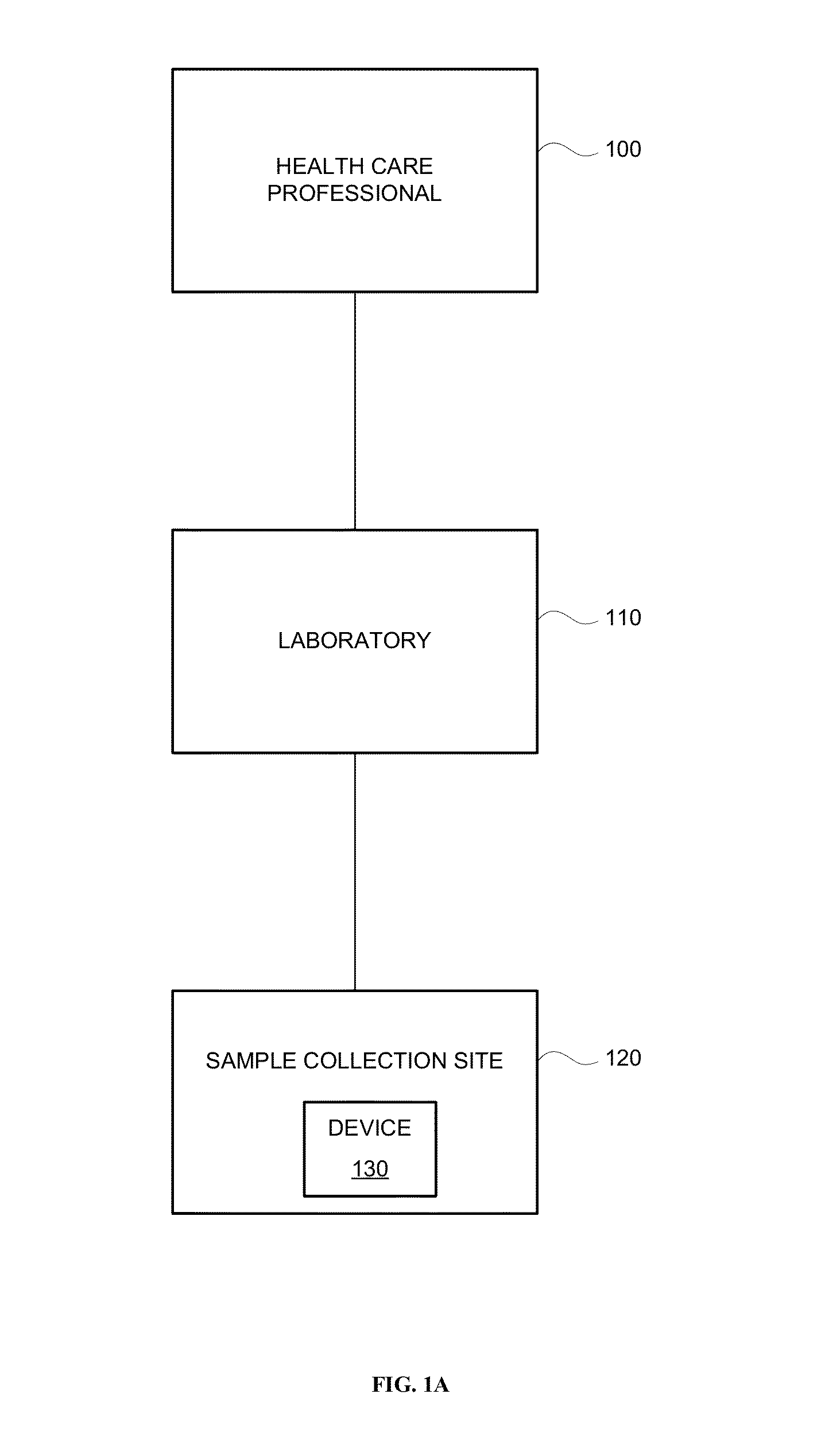
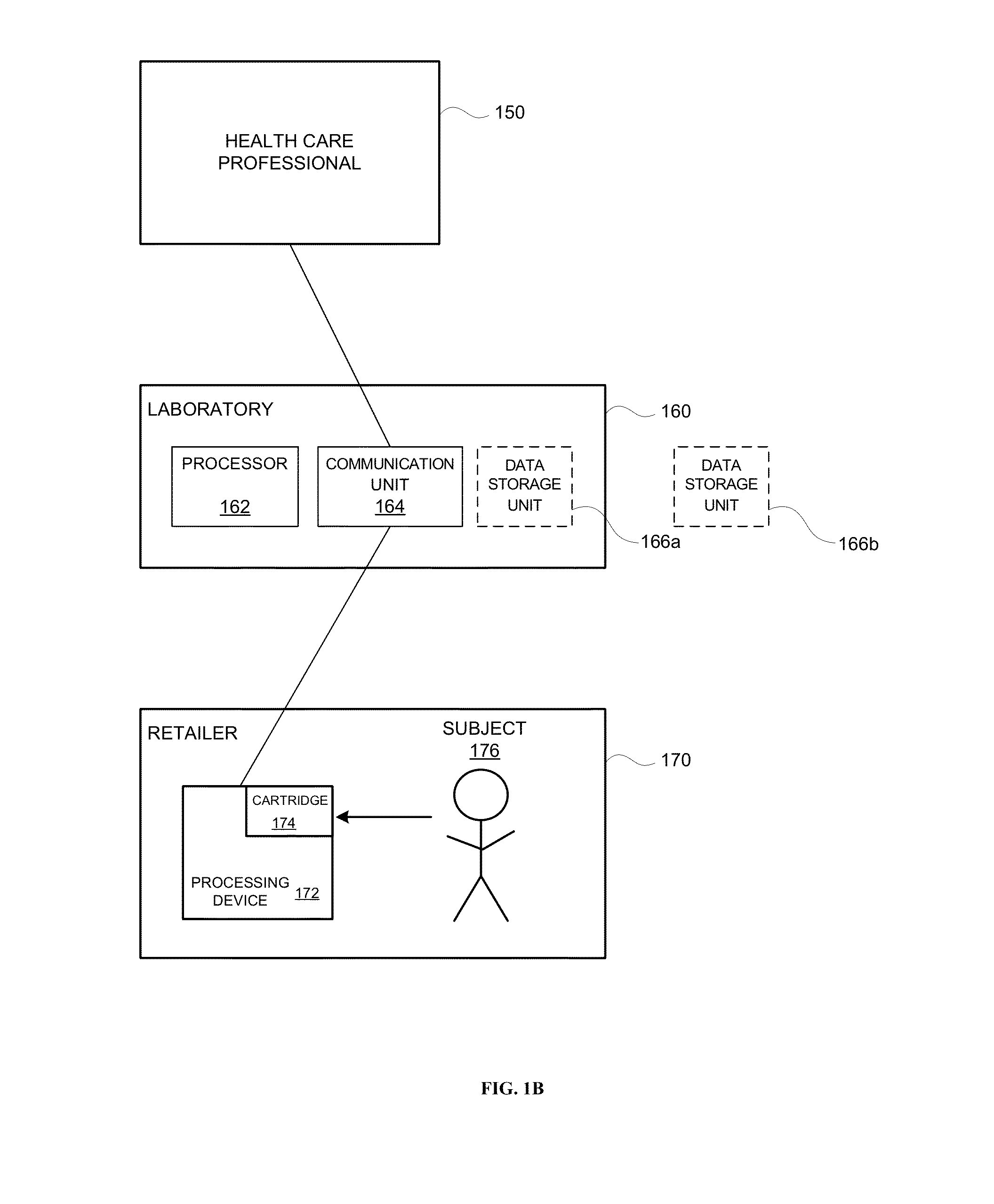
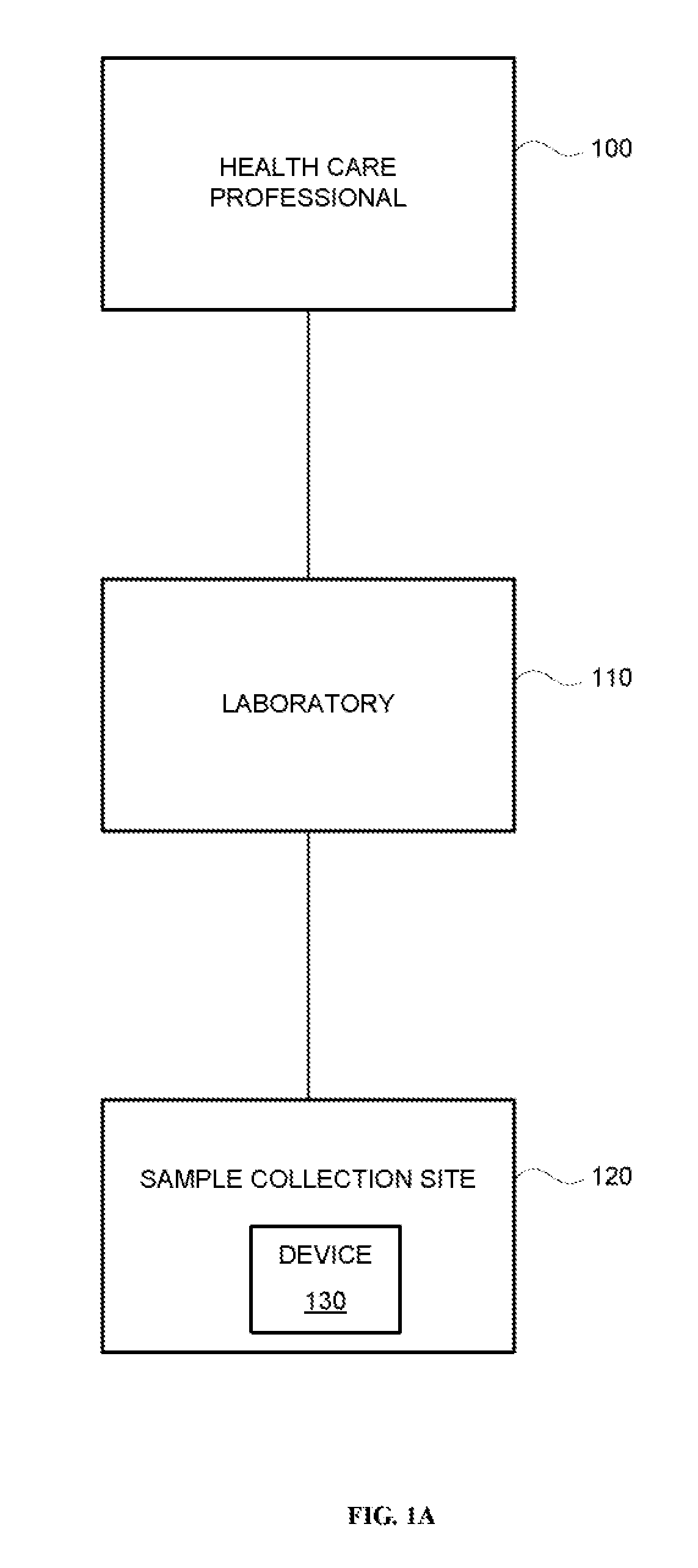
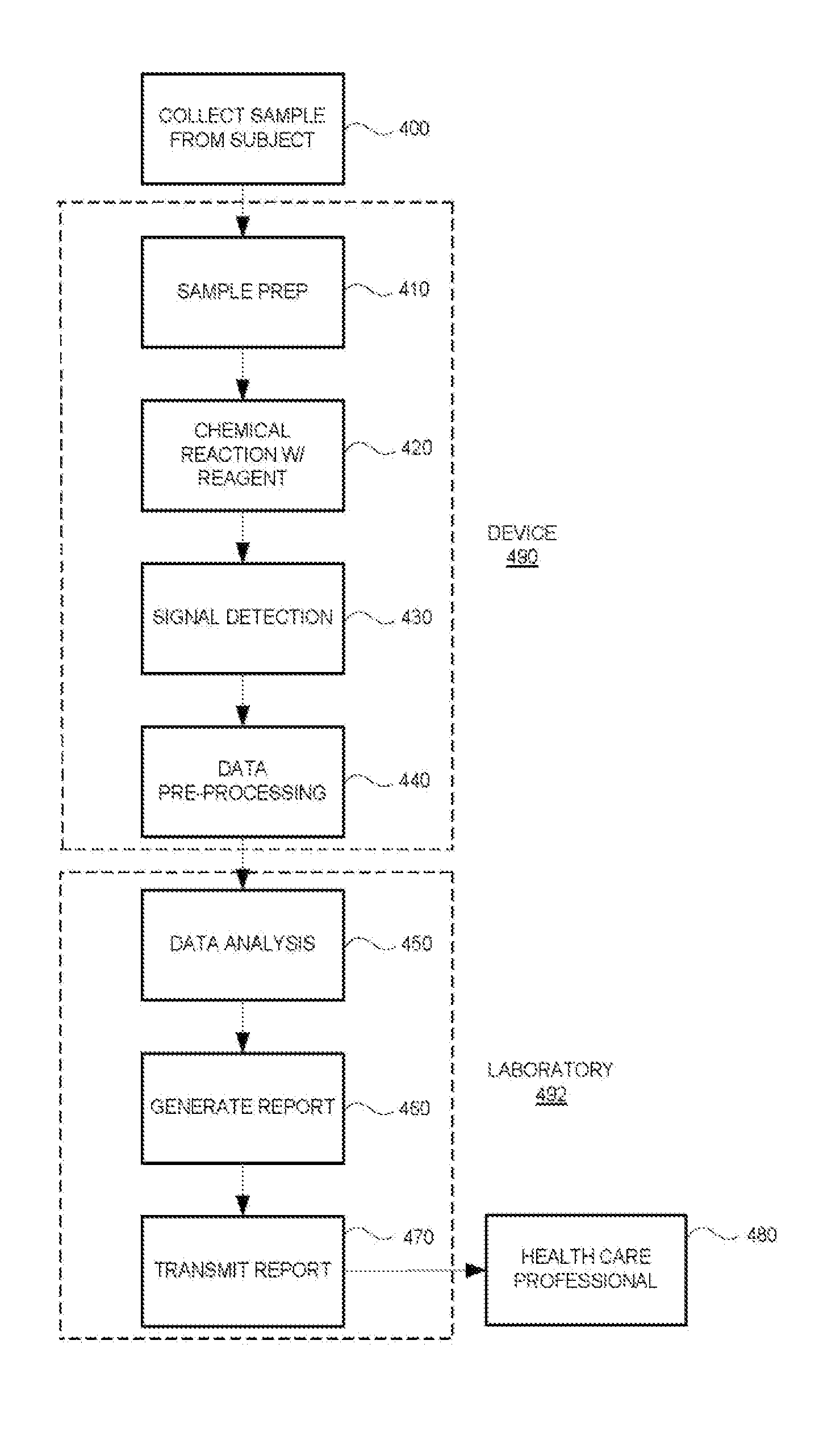
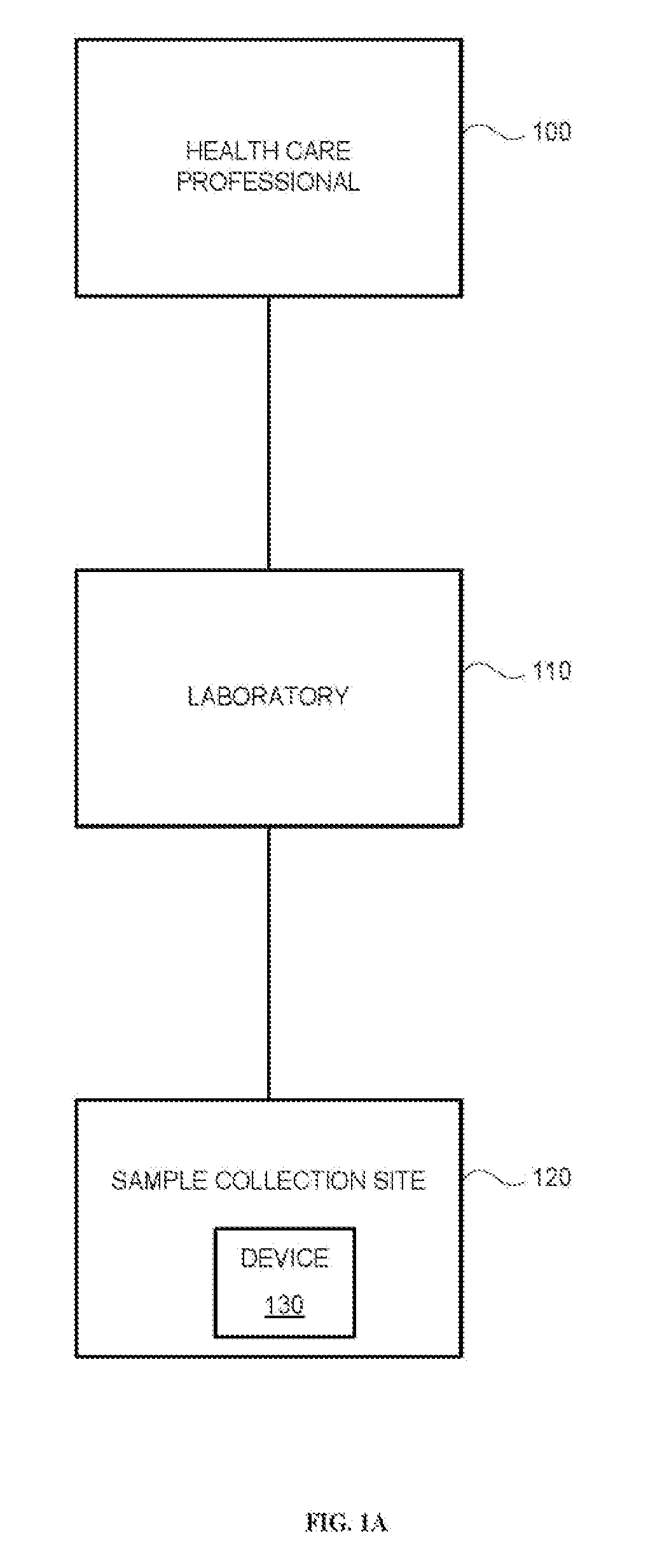
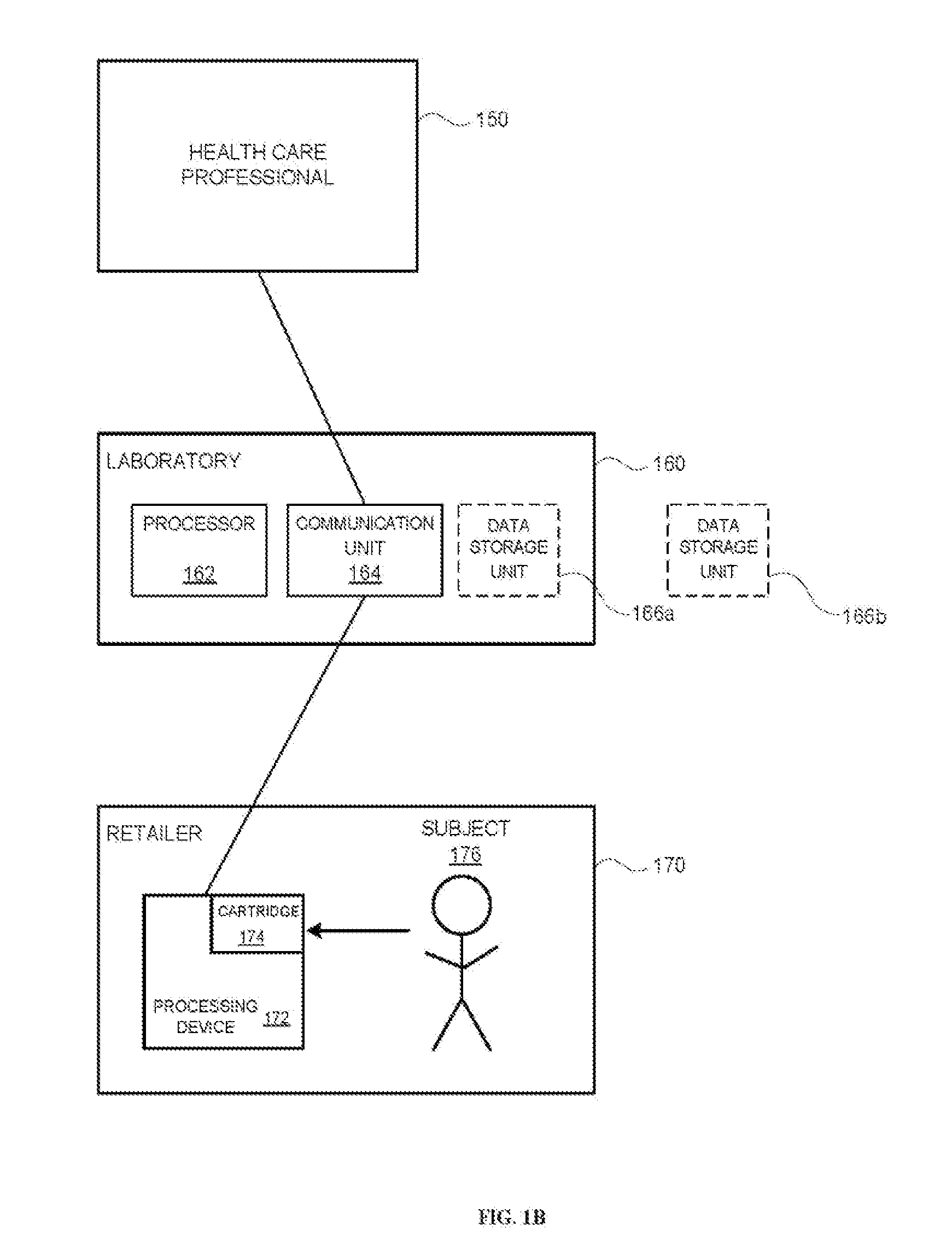
Systems and methods are provided for collecting, preparing, and / or analyzing a biological sample. A sample collection site may be utilized with one or more sample processing device. The sample processing device may be configured to accept a sample from a subject. The sample processing device may perform one or more sample preparation step and / or chemical reaction involving the sample. Data related to the sample may be sent from the device to a laboratory. The laboratory may be a certified laboratory that may generate a report that is transmitted to a health care professional. The health care professional may rely on the report for diagnosing, treating, and / or preventing a disease in the subject.
Owner:LABRADOR DIAGNOSTICS LLC
Method and apparatus to diagnose the metastatic or progressive potential of cancer, fibrosis and other diseases
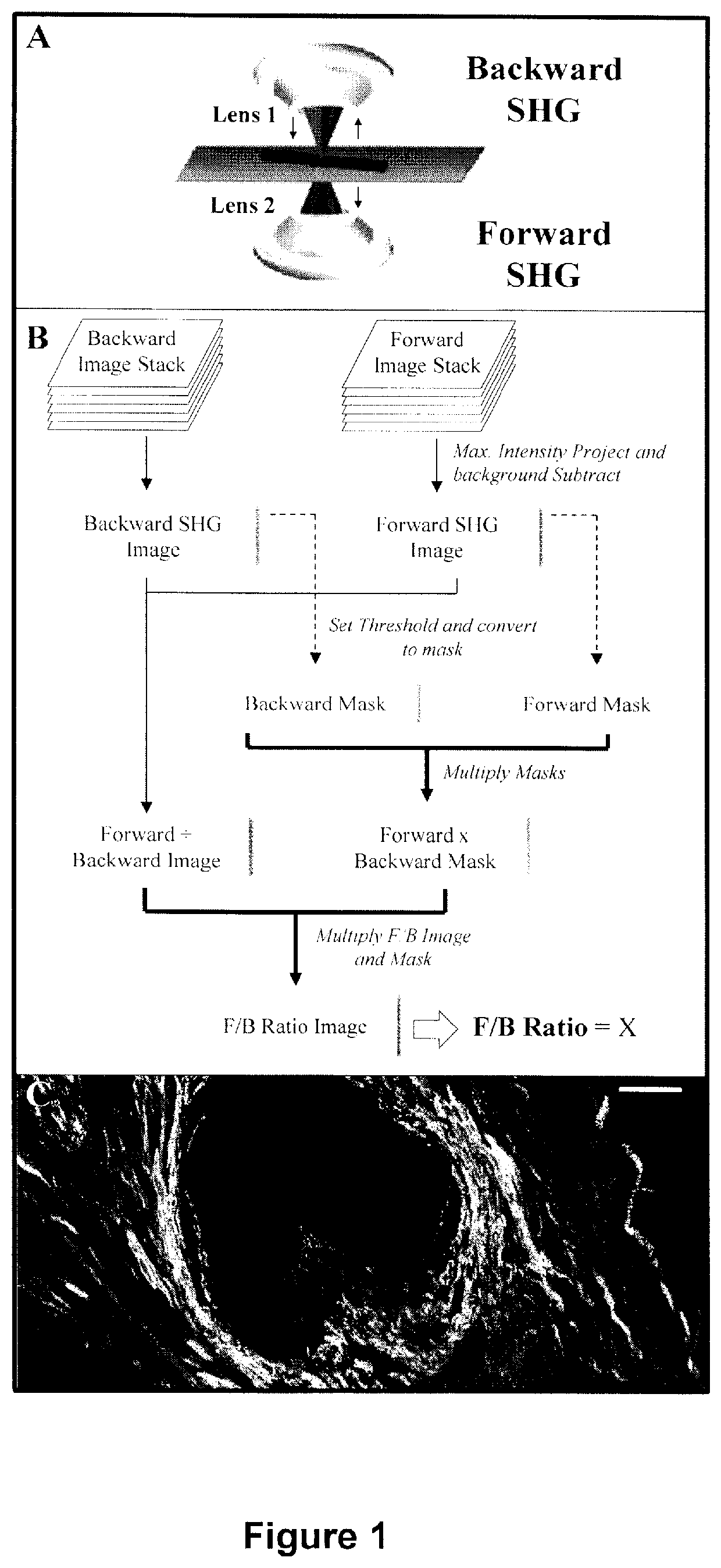
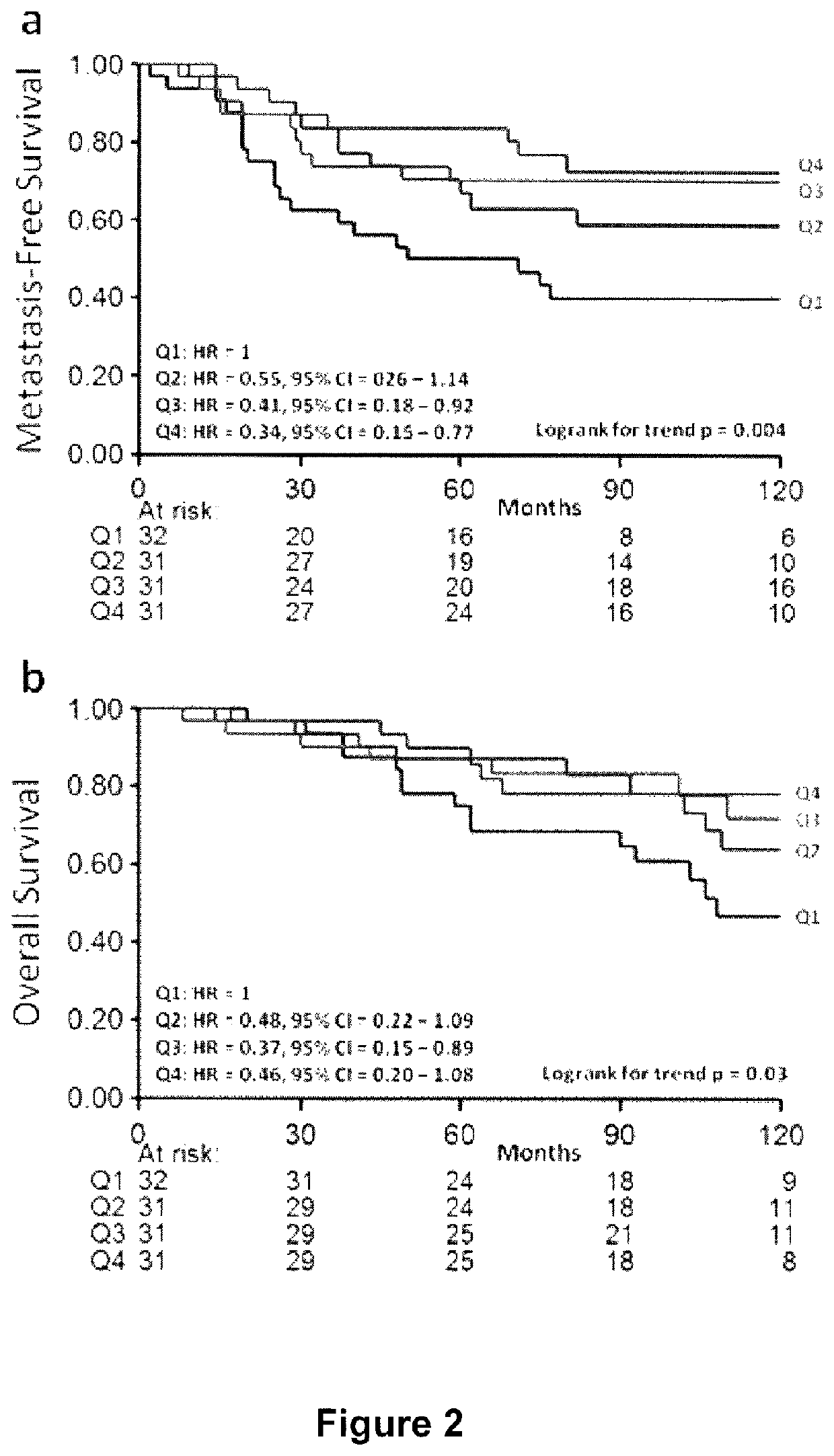
A method and apparatus for determining the progressive potential of a disease is disclosed. The forward to backward propagating second harmonic generation signal derived from a second harmonic generation instrument is used to assess the collagen microstructure of imaged body tissue by way of numerical values that are in turn used to determine the progressive or metastatic potential of the disease. The disease may, for example, be a cancer such as breast cancer, lung fibrosis, colorectal adenocarcinoma, or the like. The apparatus may include in vivo instruments or laboratory diagnostic instruments with methods disclosed herein.
Owner:UNIVERSITY OF ROCHESTER
Multiple purpose, portable apparatus for measurement, analysis and diagnosis
InactiveUS20050203353A1Satisfies needAccurately detecting colorBioreactor/fermenter combinationsBiological substance pretreatmentsData displayPoint of care
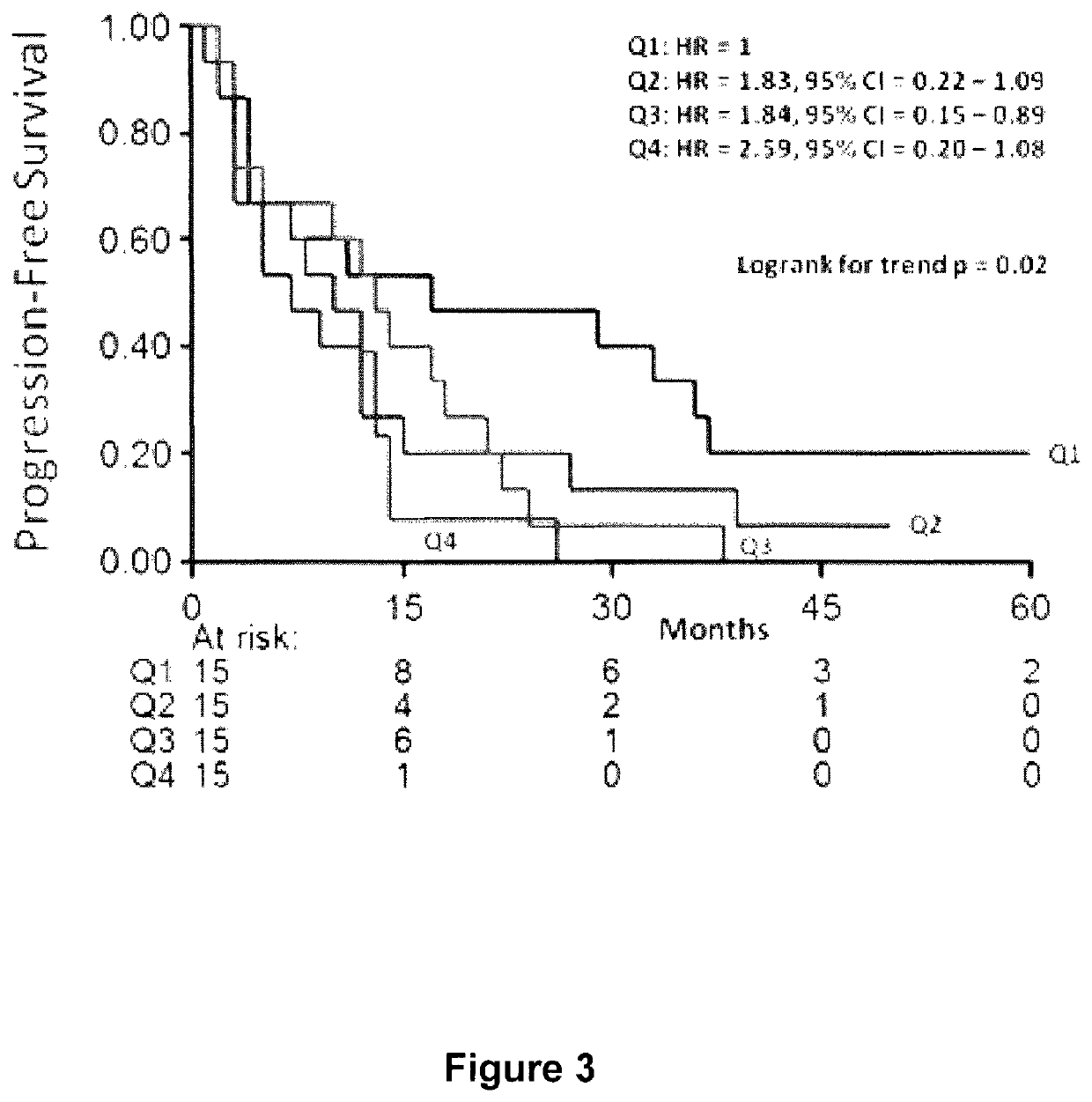
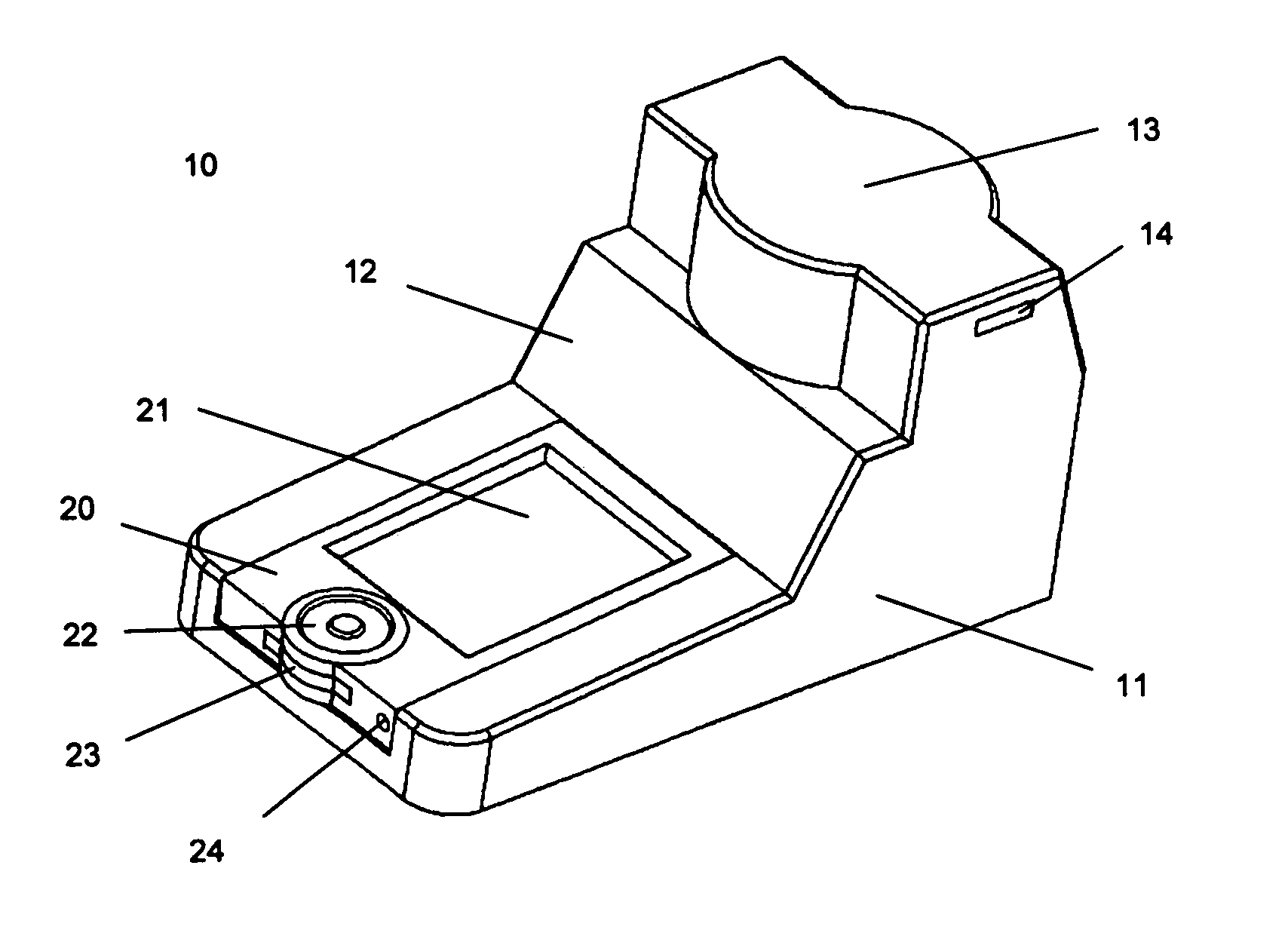
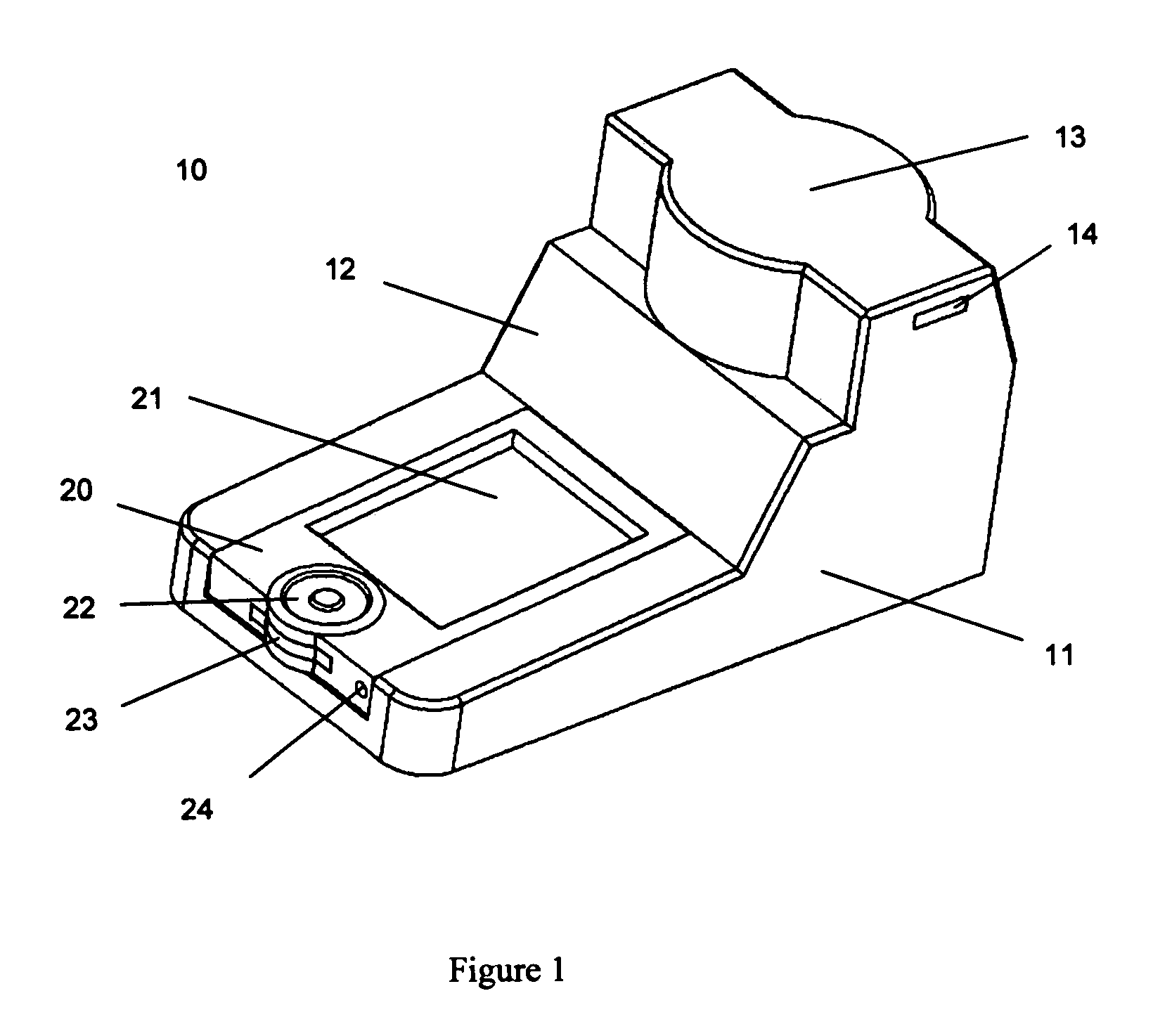
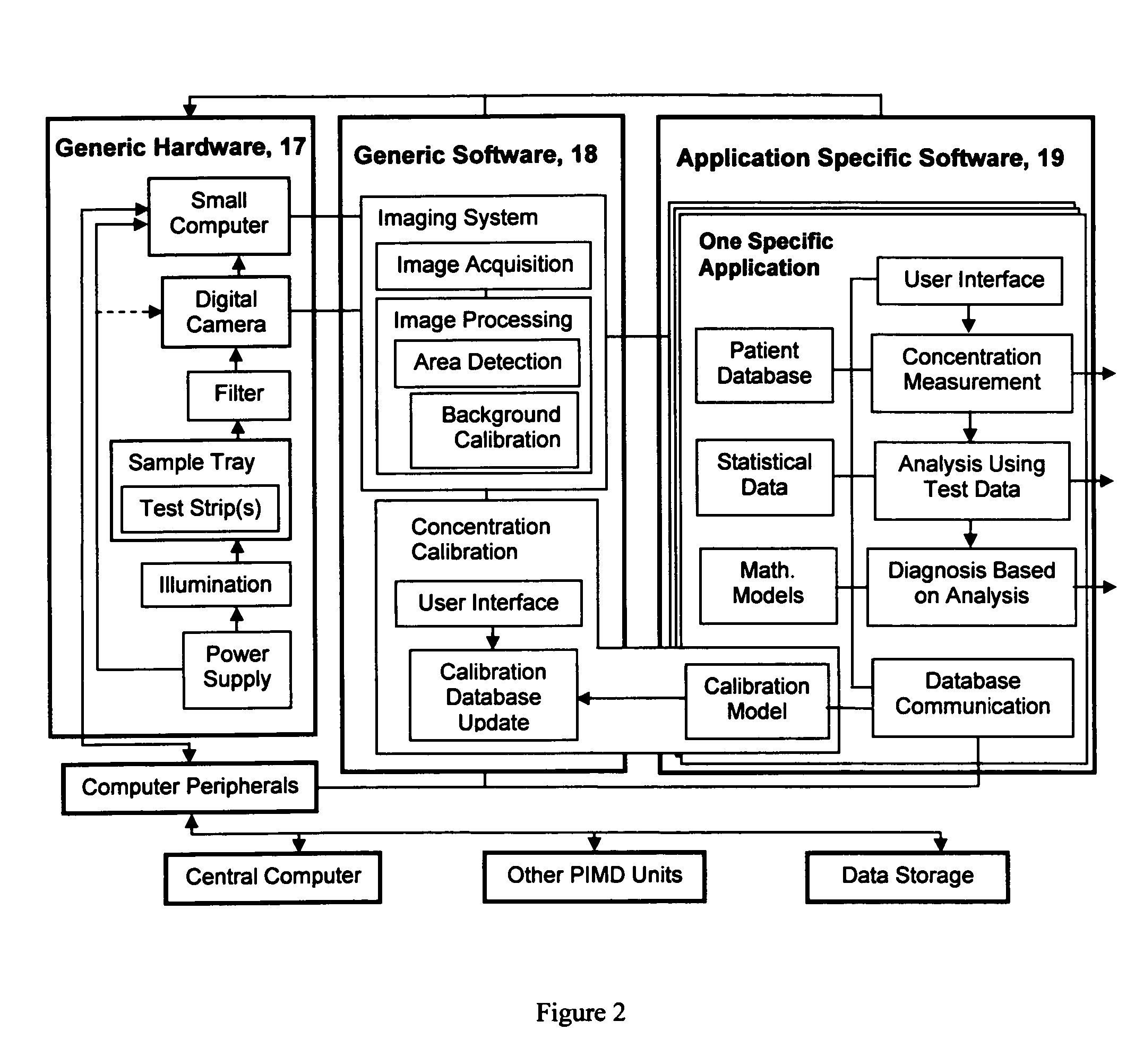
The present invention pertains to a portable apparatus for quantitatively measuring the concentration of specific substances in test samples of a lateral flow or microplate assay in medical, biomedical and chemical applications, and for making subsequent analysis and diagnosis. The portable apparatus includes a sample tray for carrying and aligning the test sample in the apparatus; a enclosure that may also serves as the frame of the apparatus; a digital image acquisition system that is used to obtain the digital image of the test sample on the sample tray; and a data display, processing, and analysis unit that is a general purpose or dedicated computer, such as a handheld computer (HHC), a pocket personal computer (PPC), a personal digital assistant (PDA), a palm-top computer, a laptop computer, or a dedicated microprocessor and associated hardware, for measuring the concentration of specific substances in the test sample, and making subsequent analysis and diagnosis, based on the measurement, statistical data, prior knowledge and mathematical model. The stated enclosure and frame, the digital image acquisition system, and the data display, processing and analysis unit are integrated to form the portable apparatus for various applications. The integrated apparatus of this invention, with a possible name—Portable Intelligent Multi-Diagnoser (PIMD), thus forms a portable and multiple-purpose tool for measuring the concentration of specific substances in test samples, and making subsequent analysis and diagnosis in a variety of settings, such as a mobile site, point of care or near patient care, and small laboratories.
Owner:MA JIE +1
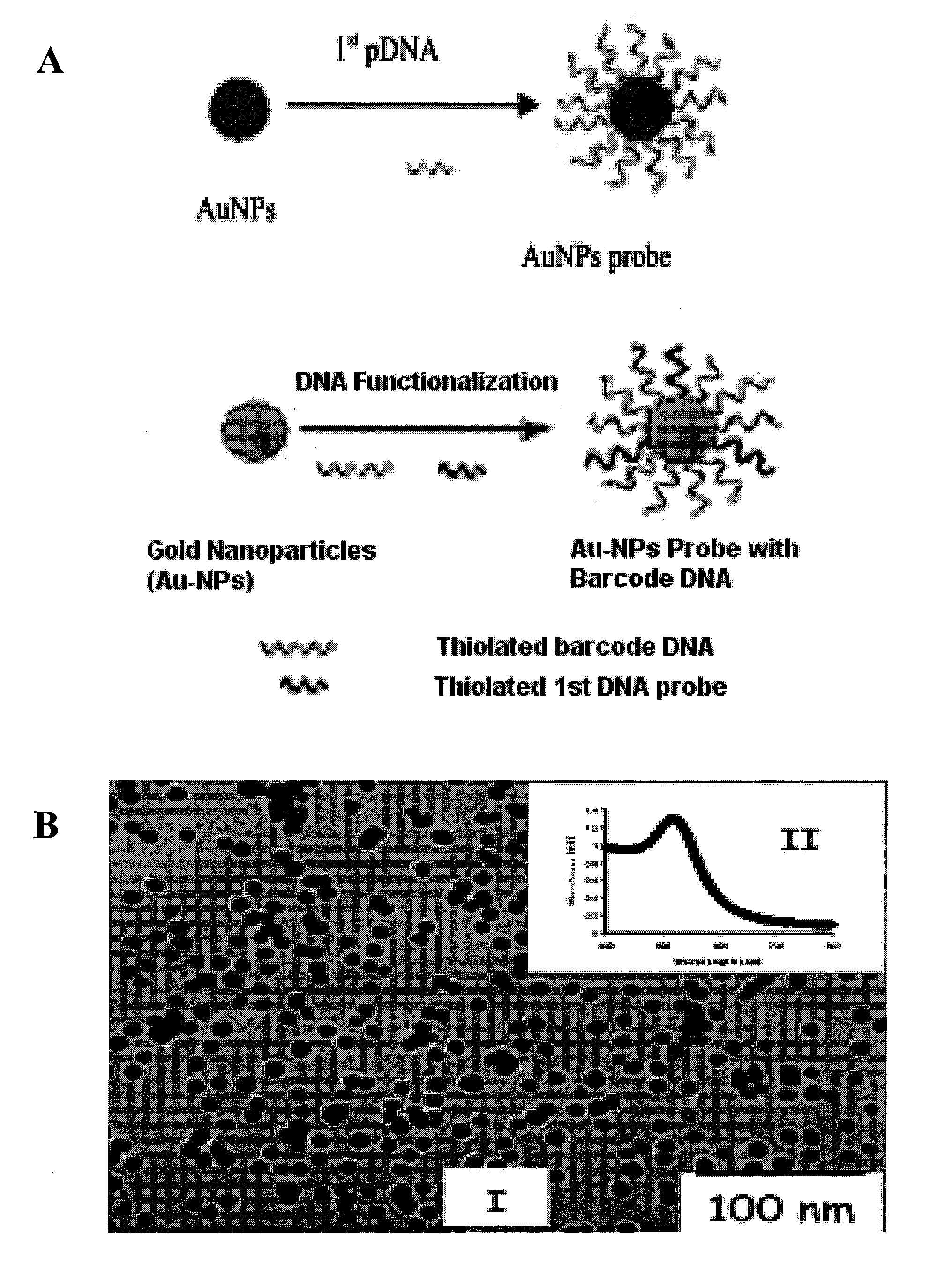
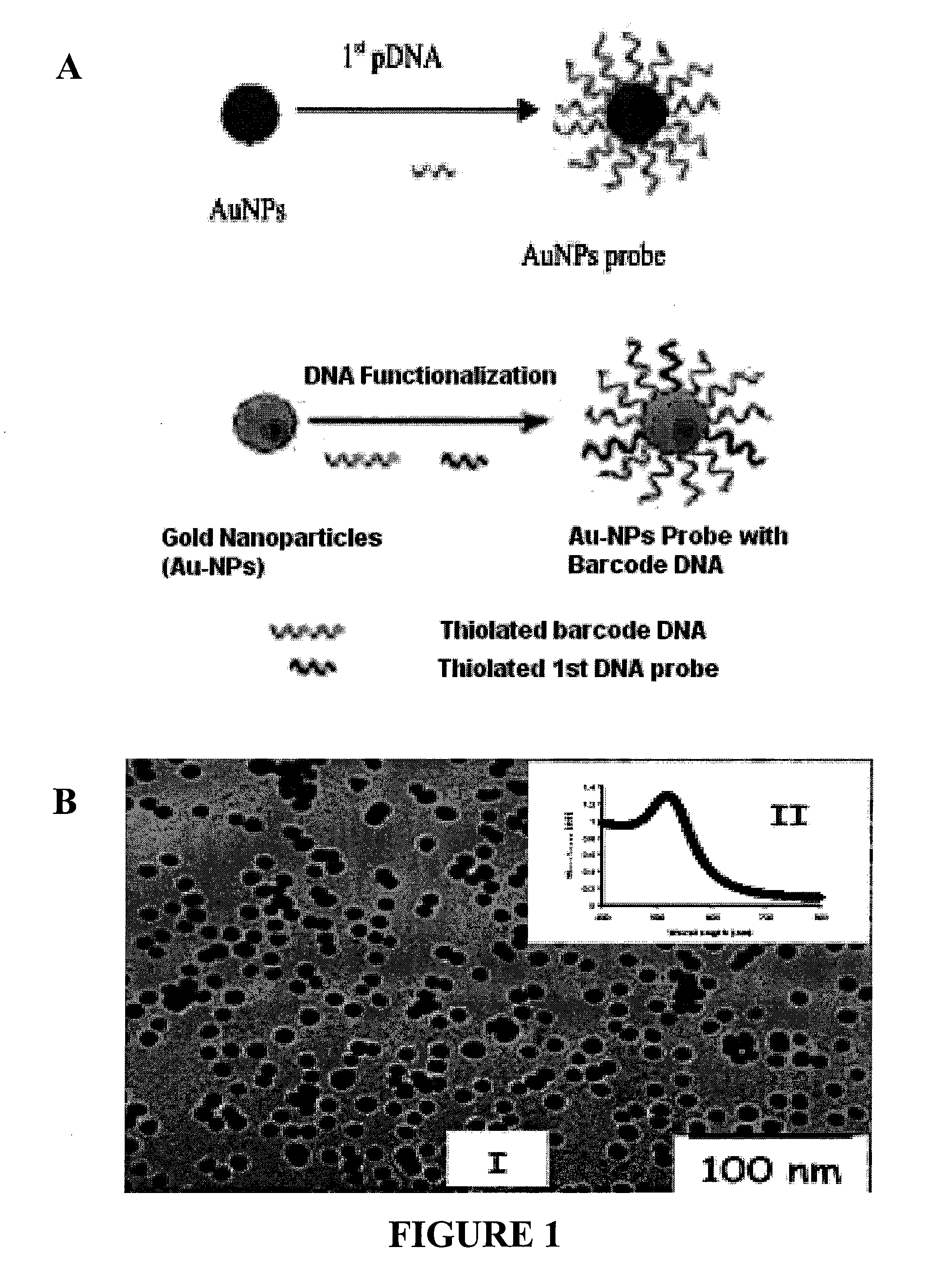
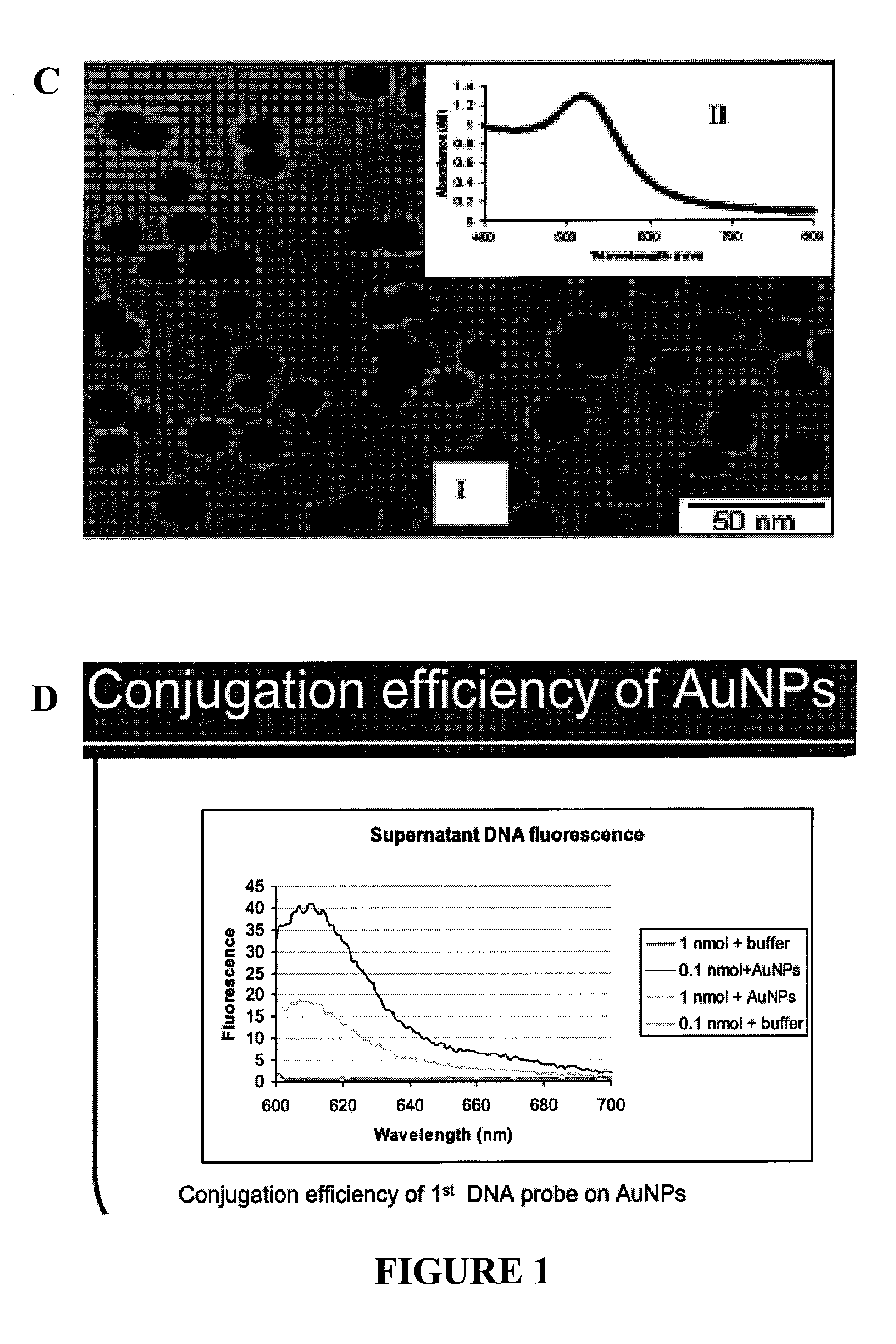
Nanoparticle tracer-based electrochemical DNA sensor for detection of pathogens-amplification by a universal nano-tracer (AUNT)
InactiveUS20110171749A1Rapid and sensitive detectionRapid and sensitive and valid identificationMaterial nanotechnologyNanomedicineSalmonella entericaEscherichia coli
The present invention relates to methods and compositions for identifying a pathogen. The inventions provide an antibody-based biosensor probe comprising (AUNT) in combination with a polymer-coated magnetic nanoparticle. In particular, a nanoparticle-based biosensor was developed for detection of Escherichia coli O157:H7 bacterium in food products. Further described are biosensors for detecting pathogens at low concentrations in samples. Even further, a gold nanoparticle-based electrochemical biosensor detection and amplification method for identifying the insertion element gene of Salmonella enterica Serovar Enteritidis is described. The present invention provides compositions and methods for providing a handheld potentiostat system for detecting pathogens outside of the laboratory. The AUNT biosensor system has applications detecting pathogens in food, water, beverages, clinical samples, and environmental samples.
Owner:BOARD OF TRUSTEES OPERATING MICHIGAN STATE UNIV
Device for performing a blood, cell, and/or pathogen count and methods for use thereof
ActiveUS20130273524A1Rapid and accurate and affordable laboratory-quality blood testingEliminate replacementBioreactor/fermenter combinationsBiological substance pretreatmentsPoint of careBlood test
Devices and methods for performing a point of care blood, cell, and / or pathogen count or a similar blood test. Disclosed herein are systems that can be used to provide rapid, accurate, affordable laboratory-quality testing at the point of care. The systems described herein are capable of imaging and counting individual cells in a prepared cell sample (e.g., a peripheral blood smear or a blood sample prepared in a microfluidic device) or another prepared cell-containing sample without the need for a microscope or other expensive and cumbersome optics. The systems described herein are designed to eliminate or replace expensive, centralized clinical testing equipment and technical personnel. Such systems may include automated data reporting and decision support.
Owner:I CALQ
Systems and methods for sample processing and analysis
InactiveUS20130080071A1Quality improvementLittle variabilityComputer-assisted medical data acquisitionBiological testingDiseaseChemical reaction
Systems and methods are provided for collecting, preparing, and / or analyzing a biological sample. A sample collection site may be utilized with one or more sample processing device. The sample processing device may be configured to accept a sample from a subject. The sample processing device may perform one or more sample preparation step and / or chemical reaction involving the sample. Data related to the sample may be sent from the device to a laboratory. The laboratory may be a certified laboratory that may generate a report that is transmitted to a health care professional. The health care professional may rely on the report for diagnosing, treating, and / or preventing a disease in the subject.
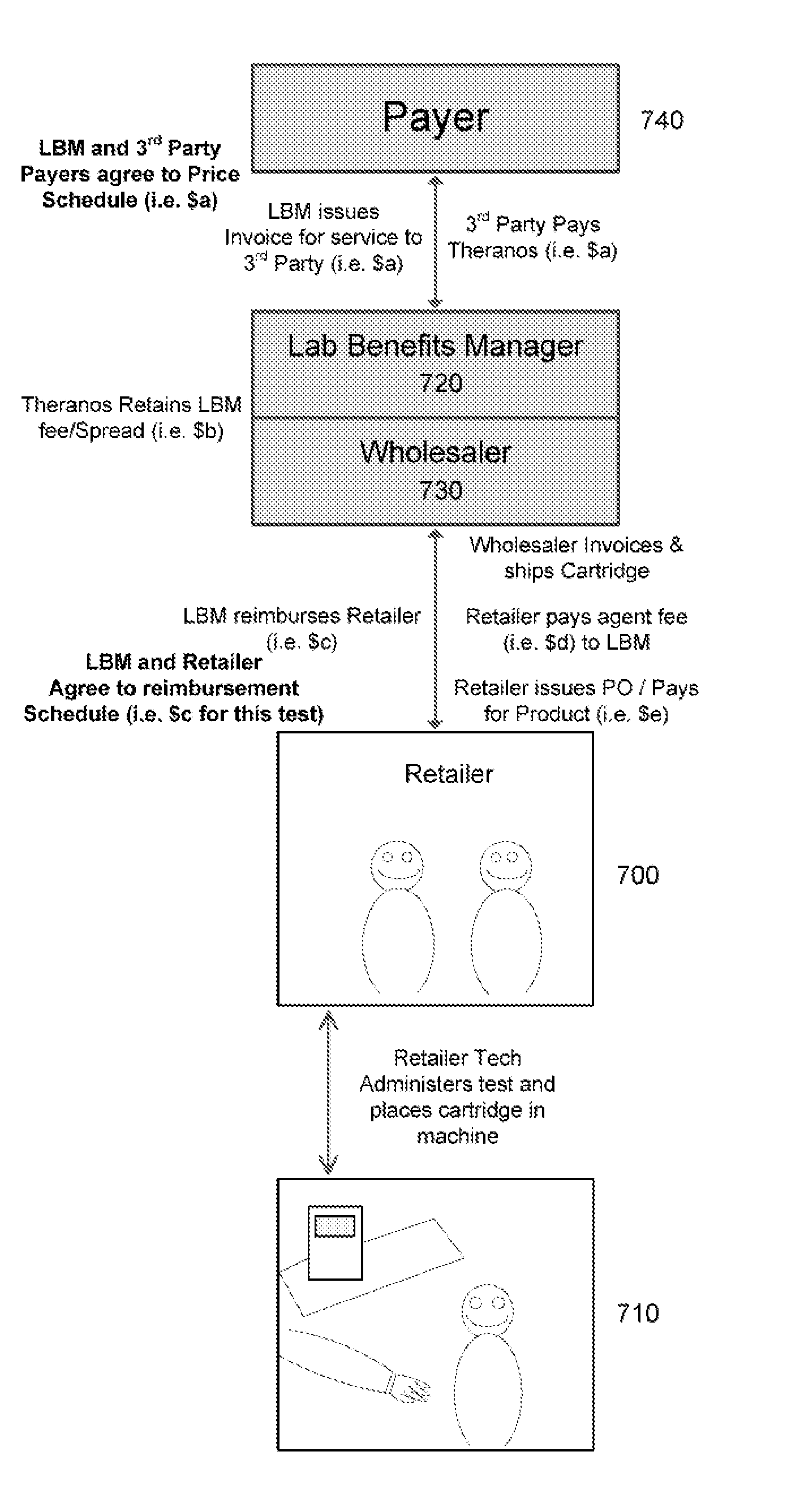
Owner:THERANOS
Infection control management and workflow system
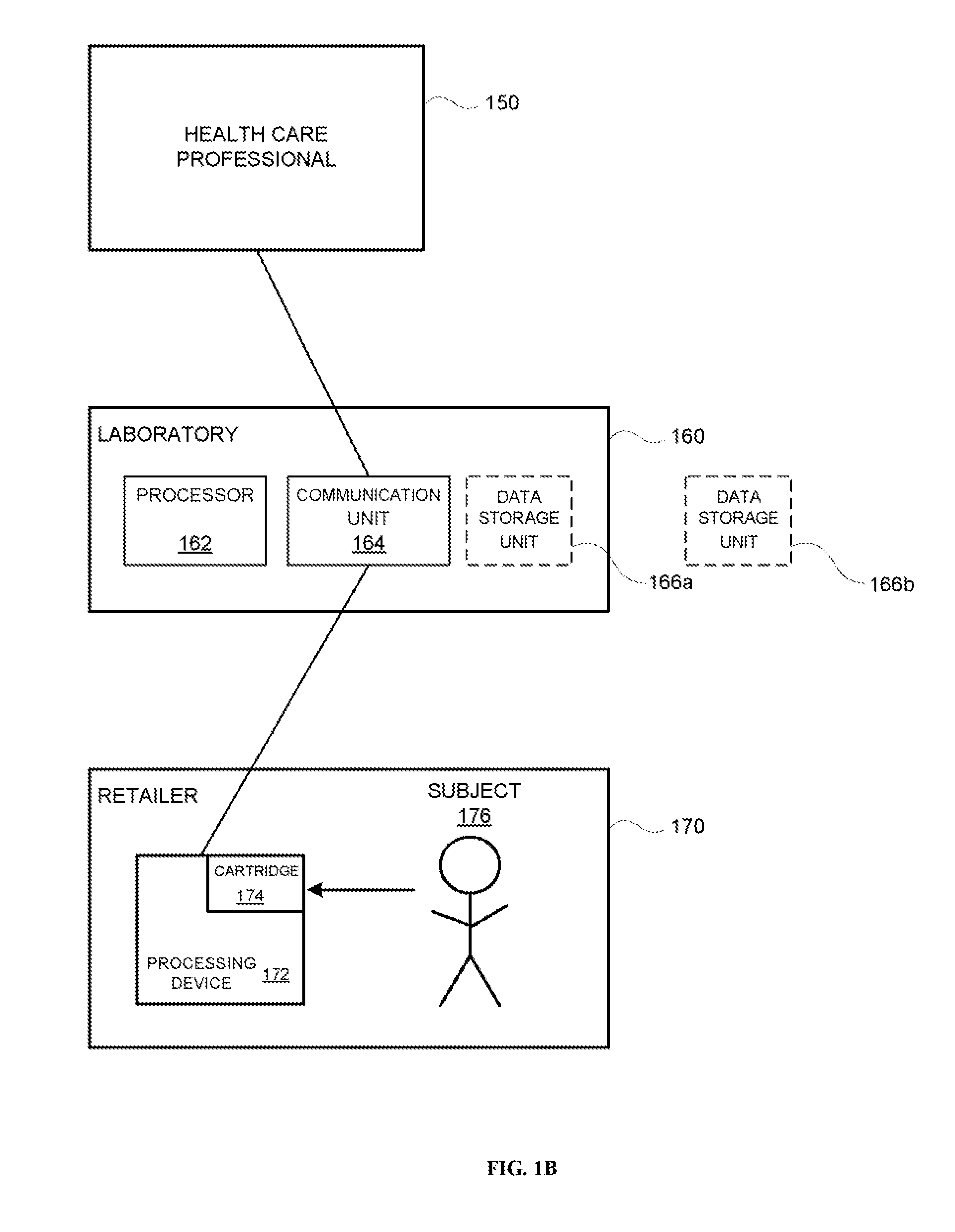
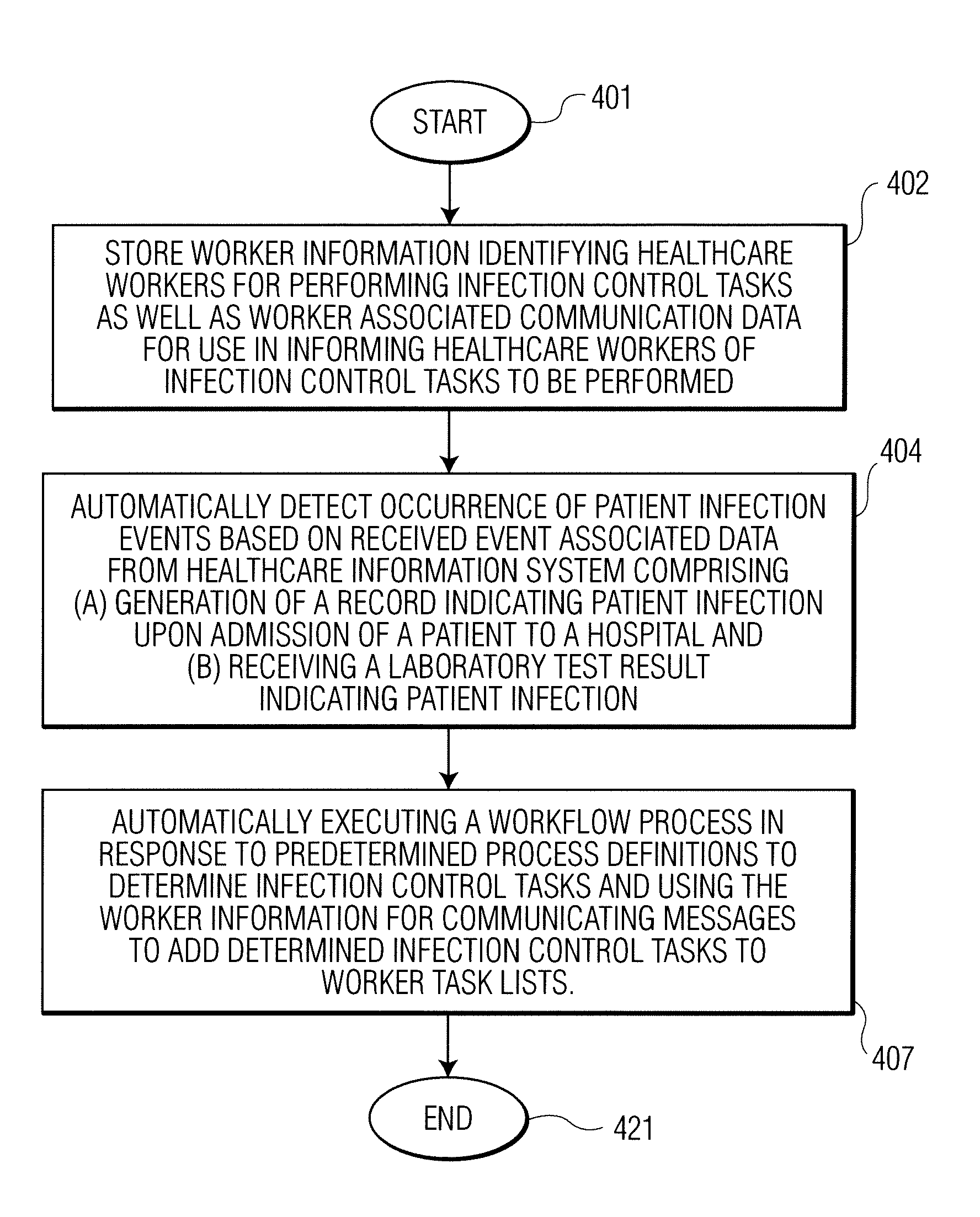
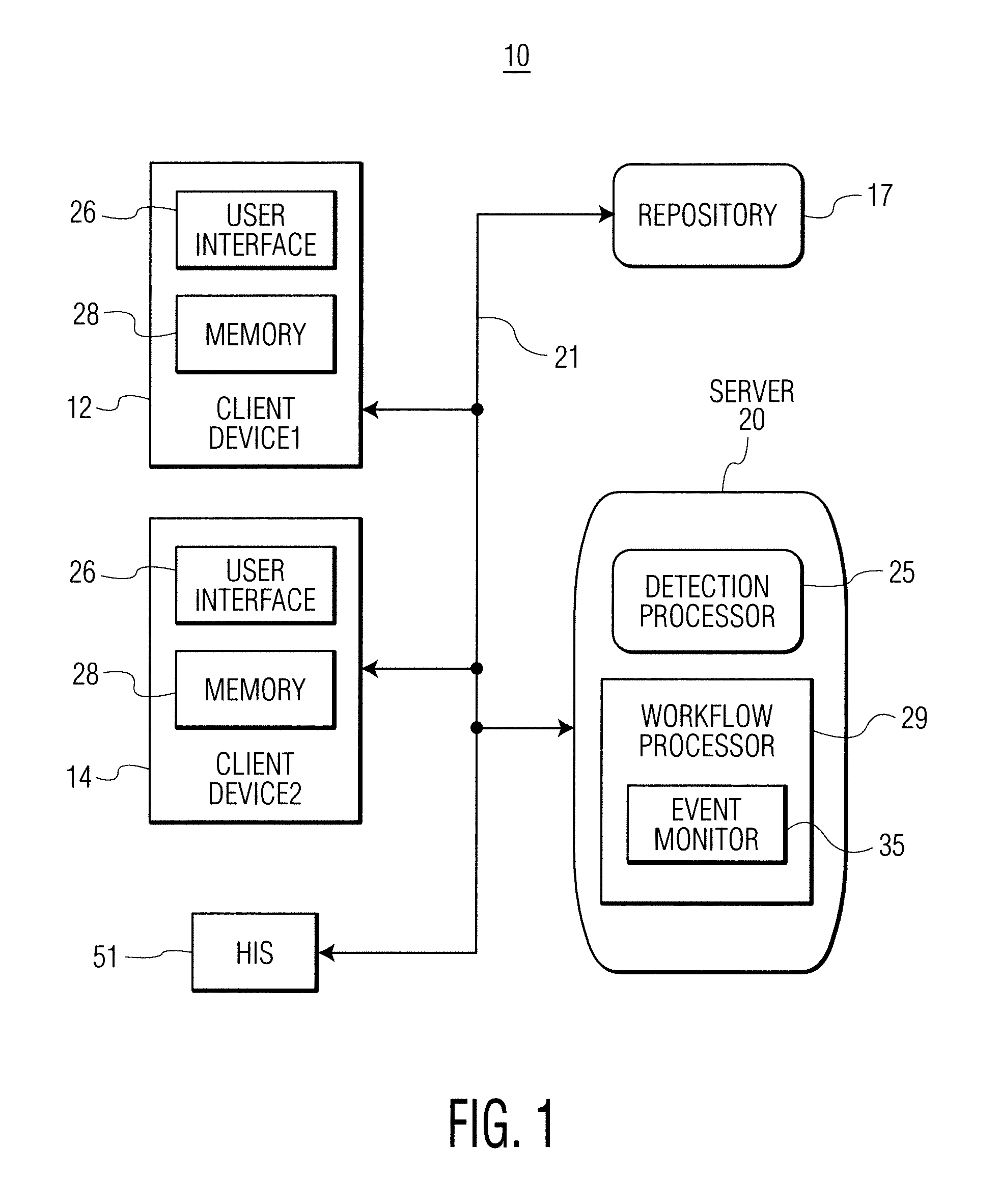
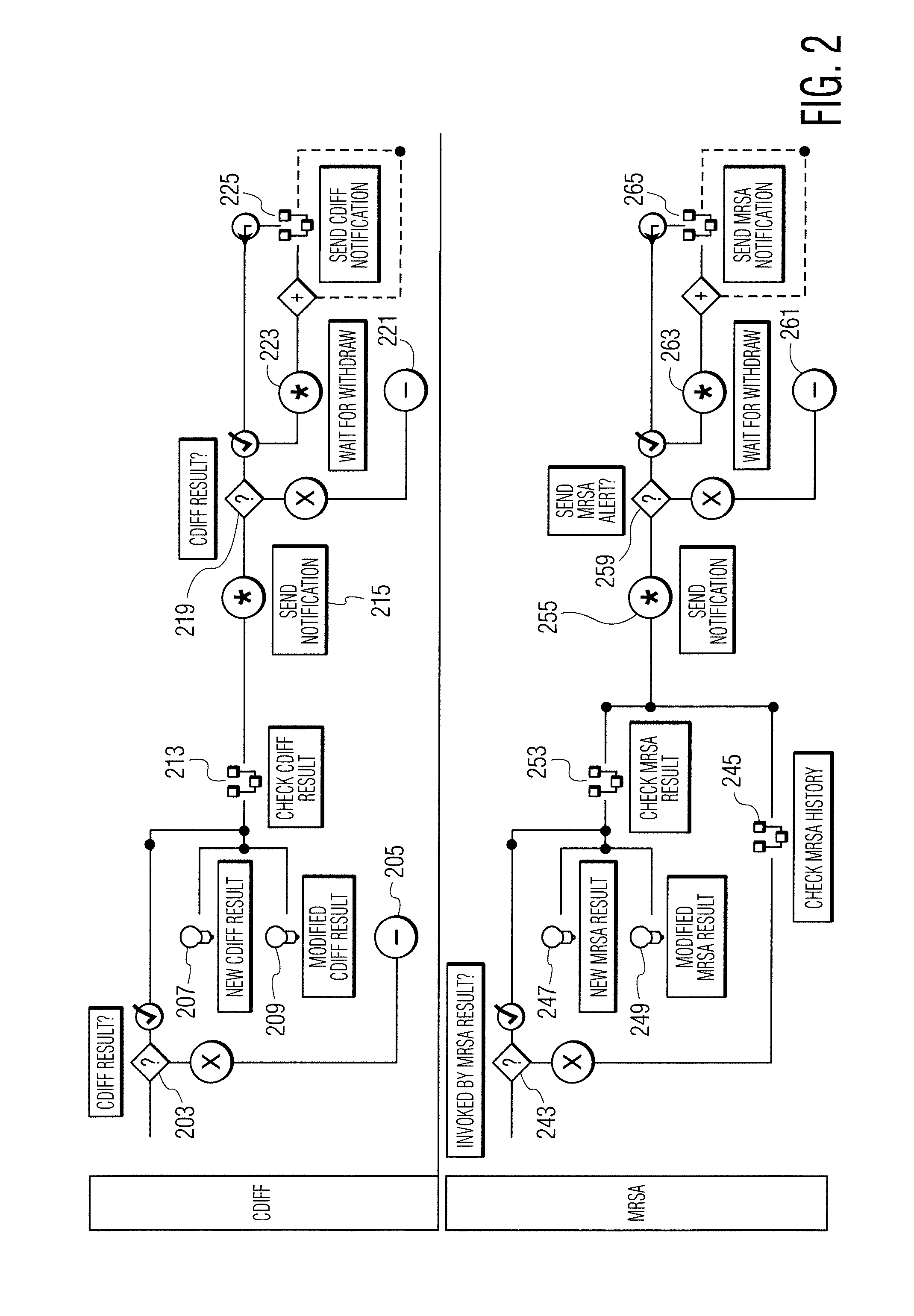
A system identifies multiple medical conditions, observations, and laboratory test results using active sensors and predetermined rules to identify infected patients. An infection control and workflow management system includes a repository of worker information identifying healthcare workers for performing infection control tasks as well as worker associated communication data for use in informing healthcare workers of infection control tasks to be performed. A detection processor automatically detects infection in patients from multiple different sources including from at least one of, (a) a medical record evaluated upon admission of a patient to a hospital and (b) a laboratory test result. A workflow processor uses the worker information for automatically communicating a message to inform a healthcare worker of a task to be performed to initiate infection control tasks using communication data in response to detection of an infected patient.
Owner:CERNER INNOVATION
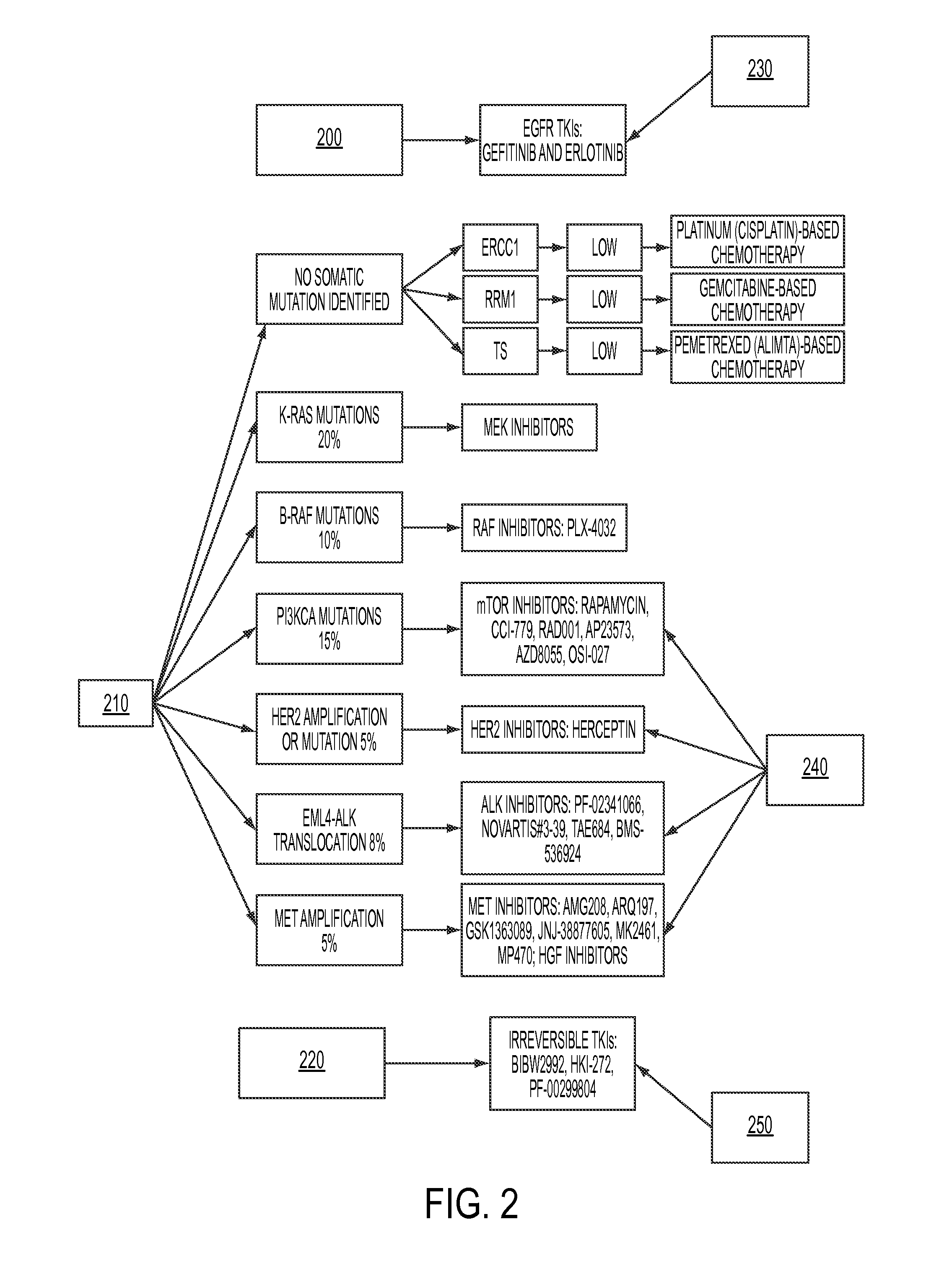
Personalized medical management system, networks, and methods
InactiveUS20120231959A1Low costReduce inefficienciesBioreactor/fermenter combinationsBiological substance pretreatmentsPersonalizationDisease
Disclosed herein are systems and methods for the assignment of therapeutic pathways to members of a network of oncology. The systems and methods allow for storage of disparate information in a database and determine a uniform semantics for all of the stored information. In addition, the systems and methods allow for the calculation of treatment pathways based on patient information as well as publicly-available information relating to particular diseases, and for the refinement of those treatment pathways as new information is added. Robot-assisted genomic labs permit automated genetic testing, which is integrated with the system.
Owner:KEW GROUP
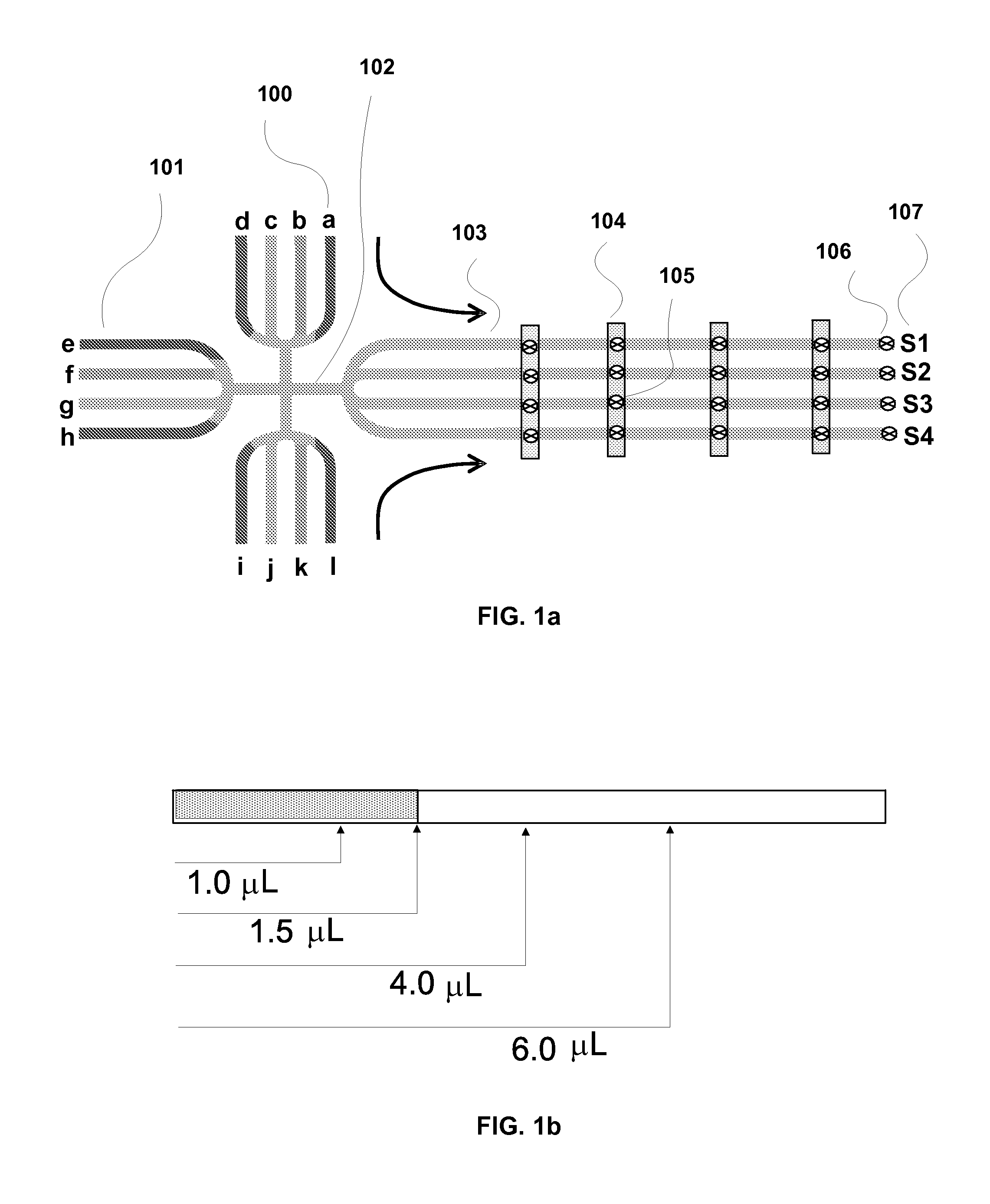
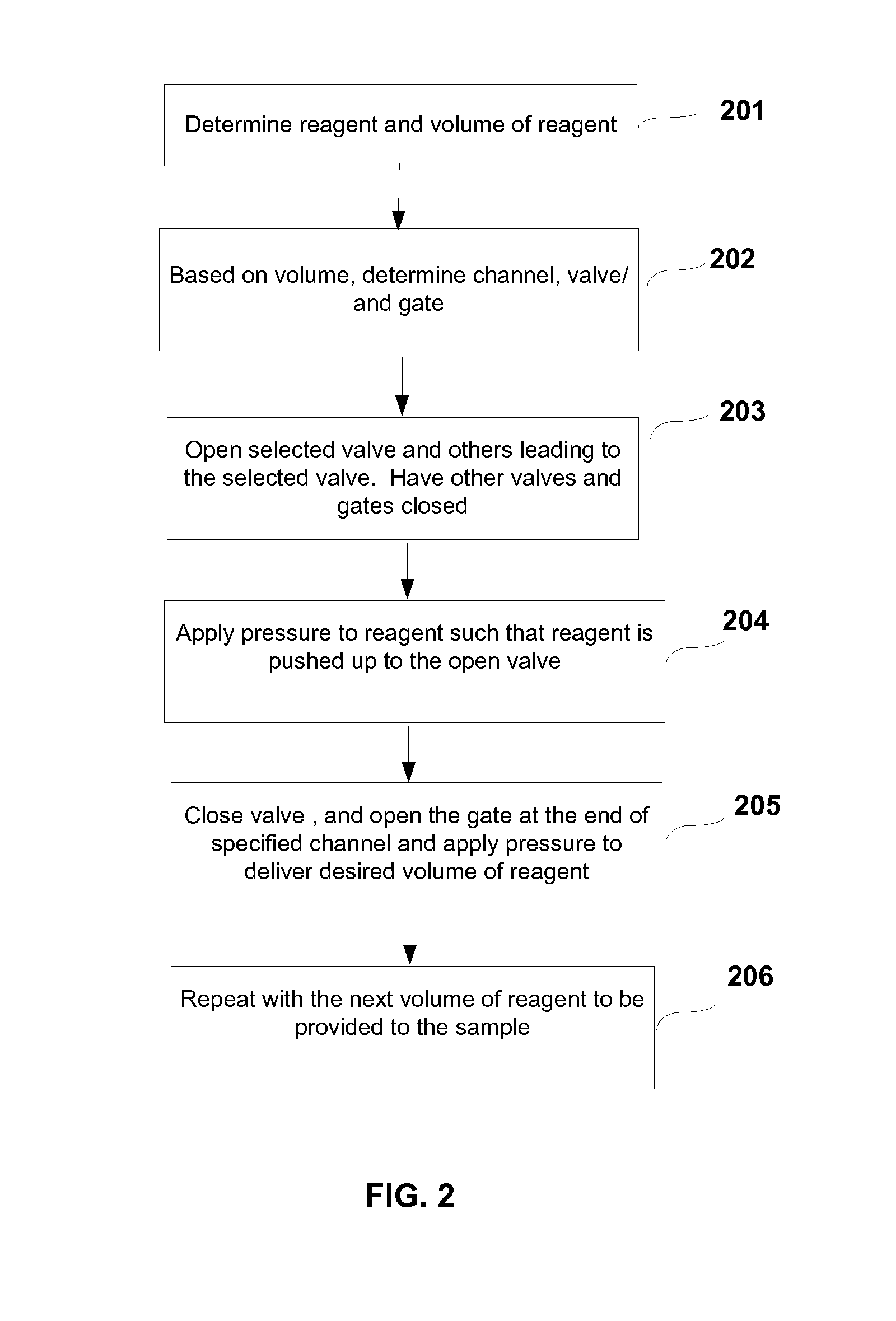
System and method for multiplex liquid handling
InactiveUS20080311585A1Bioreactor/fermenter combinationsHeating or cooling apparatusChemical reactionEngineering
The present invention generally relates to microfabricated devices for carrying out and controlling chemical reactions and analysis. In particular, the present invention provides systems, methods, devices and computer software products related to multiplex liquid handling systems utilizing lab cards related to biological assays.
Owner:AFFYMETRIX INC
Fluid sampling, analysis and delivery system
InactiveUS20050228313A1Small volumeMinimal amountMedical devicesPressure infusionMedication monitoringPancreatic hormone
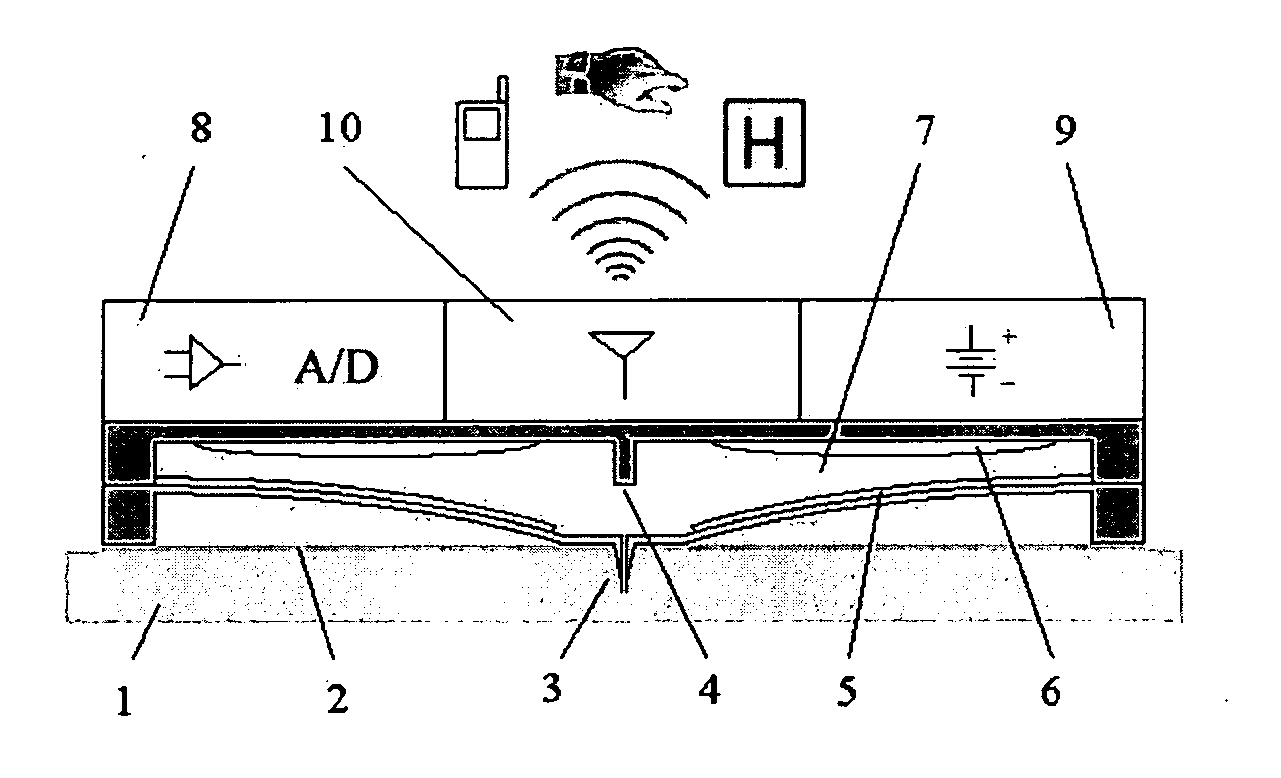
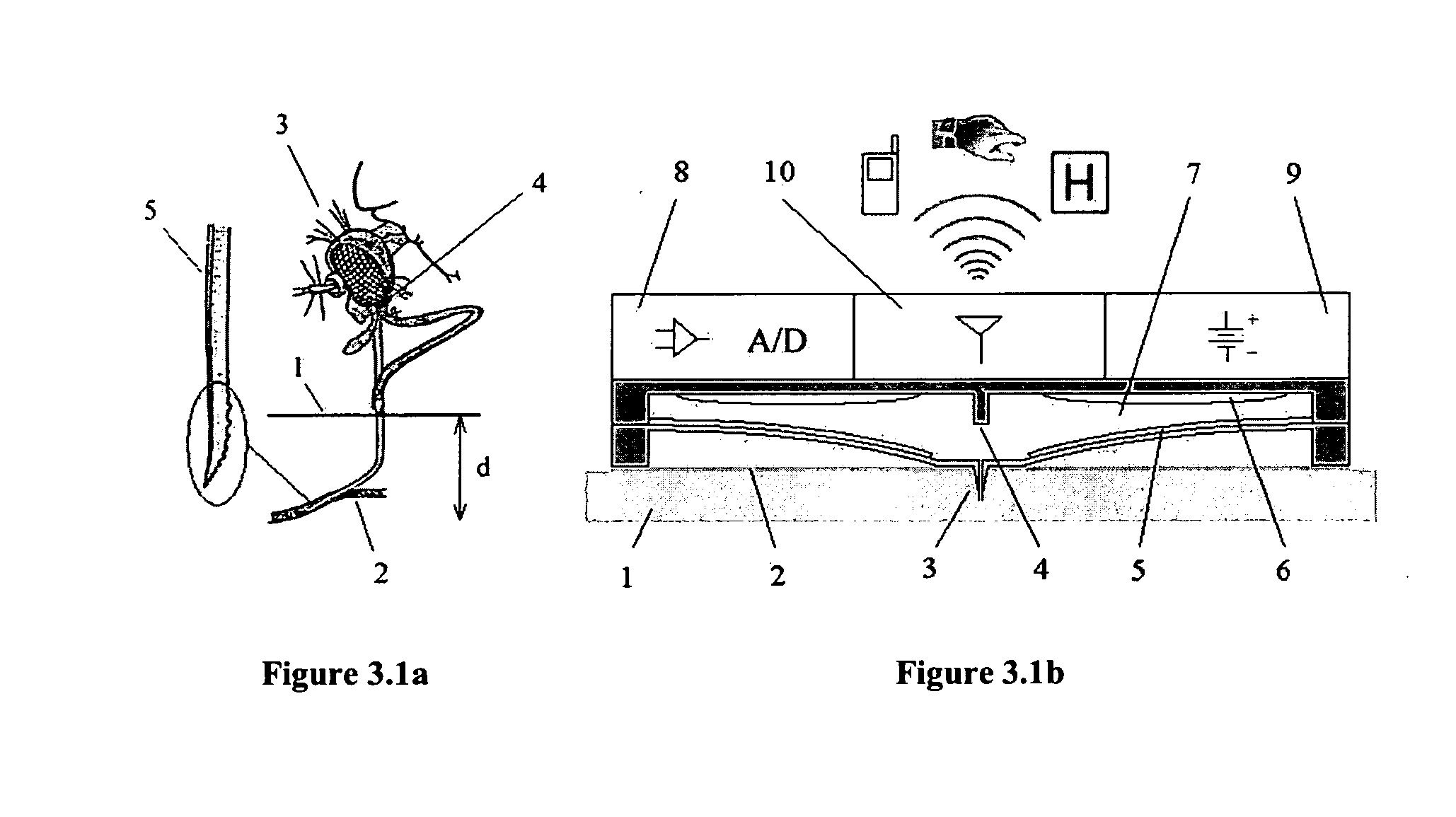
The lack of safe, reliable, automated and clinically acceptable blood sampling has been the main problem precluding the development of real-time systems for blood analysis and subsequent closed-loop physiological function control. While the analysis of a static blood sample in laboratory conditions has been rapidly advancing in reliability and blood volume reduction, non-invasive real-time blood analysis performed in vivo (while the blood is circulating in the body) has been elusive and unreliable. In this study we propose an innovative idea for semi-invasive blood sampling and analysis, which resembles the operation of a mosquito. At a miniature scale the proposed system does penetrate the skin to extract a static blood sample for further analysis, but the extent of this penetration, and the fact that it can be made painless, is particularly attractive for such applications as automated glucose analysis for closed-loop control of insulin infusion (artificial pancreas), continuous drug monitoring, or even periodic DNA analysis for security and identification purposes. These design aspects are described, and a specific implementation, applying MEMS (Micro Electro Mechanical Systems) technology, is suggested. The proposed microsystem is a matrix of individually controllable e-Mosquito™ cells, packaged in a disposable patch and attached to the skin, could be an avenue for real-time semi-invasive blood analysis and diagnostics.
Owner:UNIV TECH INT
Systems and methods for early disease detection and real-time disease monitoring
InactiveUS20160007893A1Convenient and smoothEasy accessSensorsBlood characterising devicesDisease monitoringSkin exposure
Devices, systems and methods are provided for the real-time detection of disease through constant or periodic monitoring of biomolecules in an individual without laboratory sampling. Biomolecule detector devices may be worn in contact with skin, or be implanted in fluid communication with a biological fluid to provide information and identity of disease-related circulating biomolecules in an individual.
Owner:FREENOME LTD
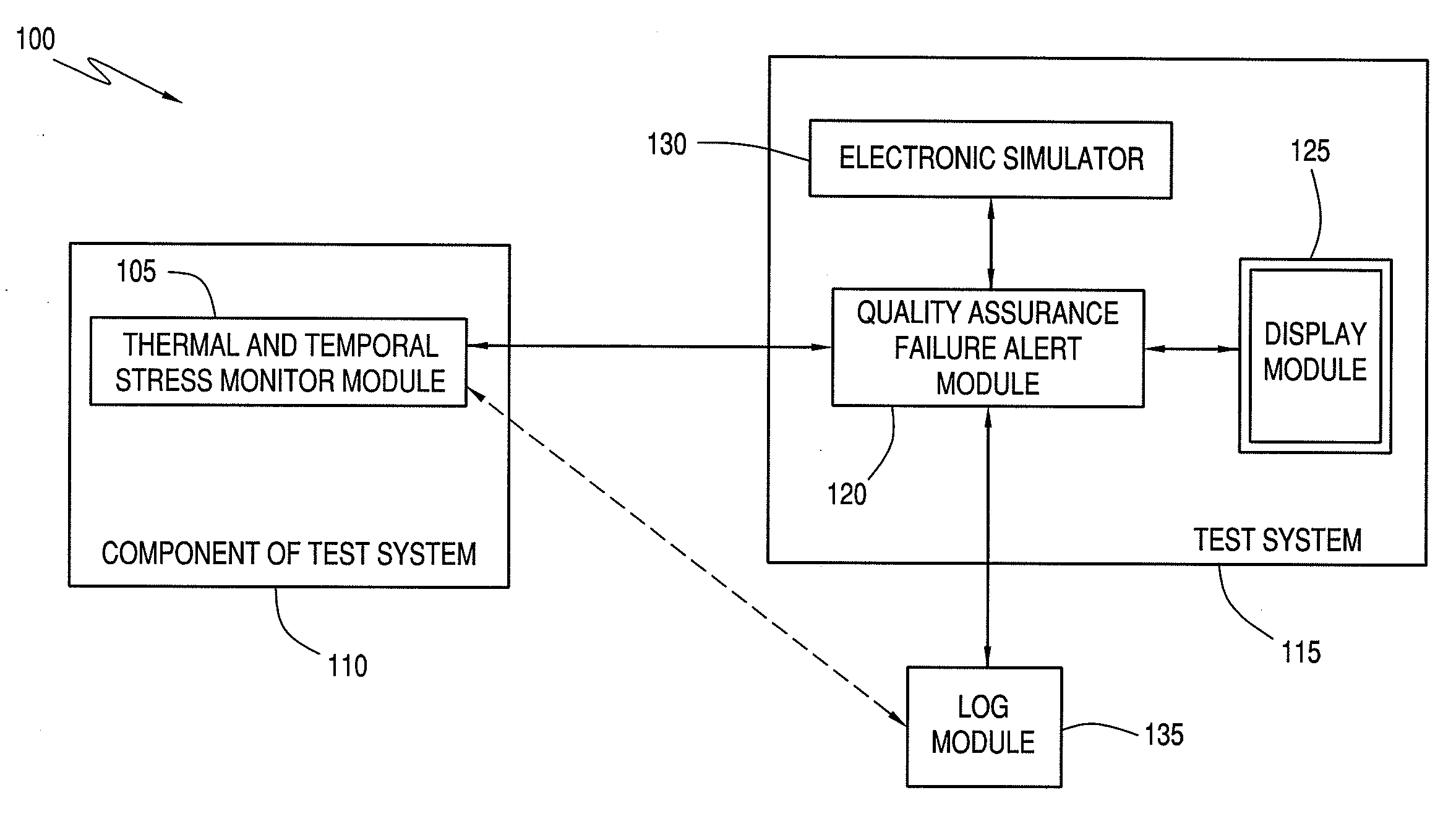
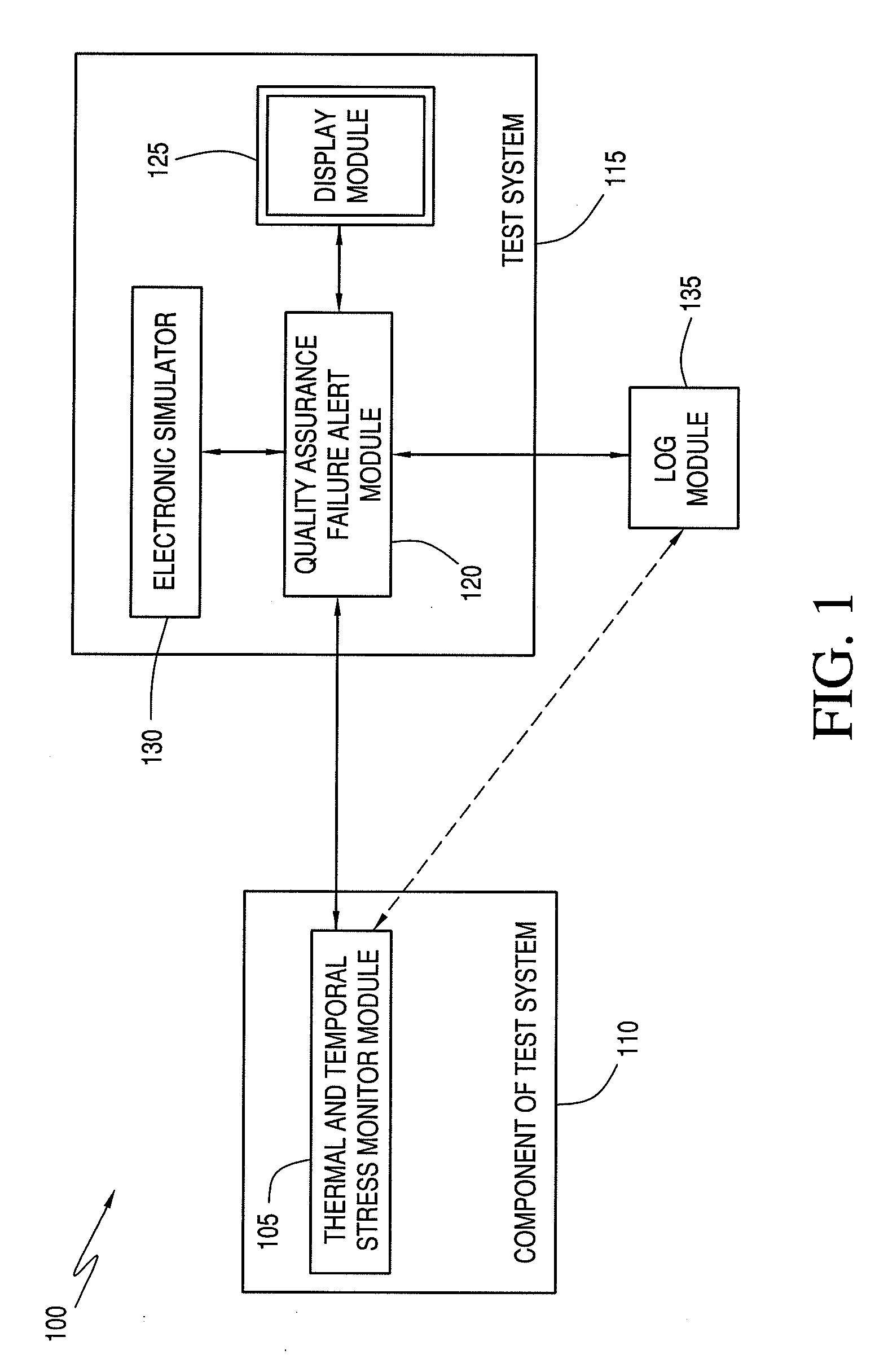
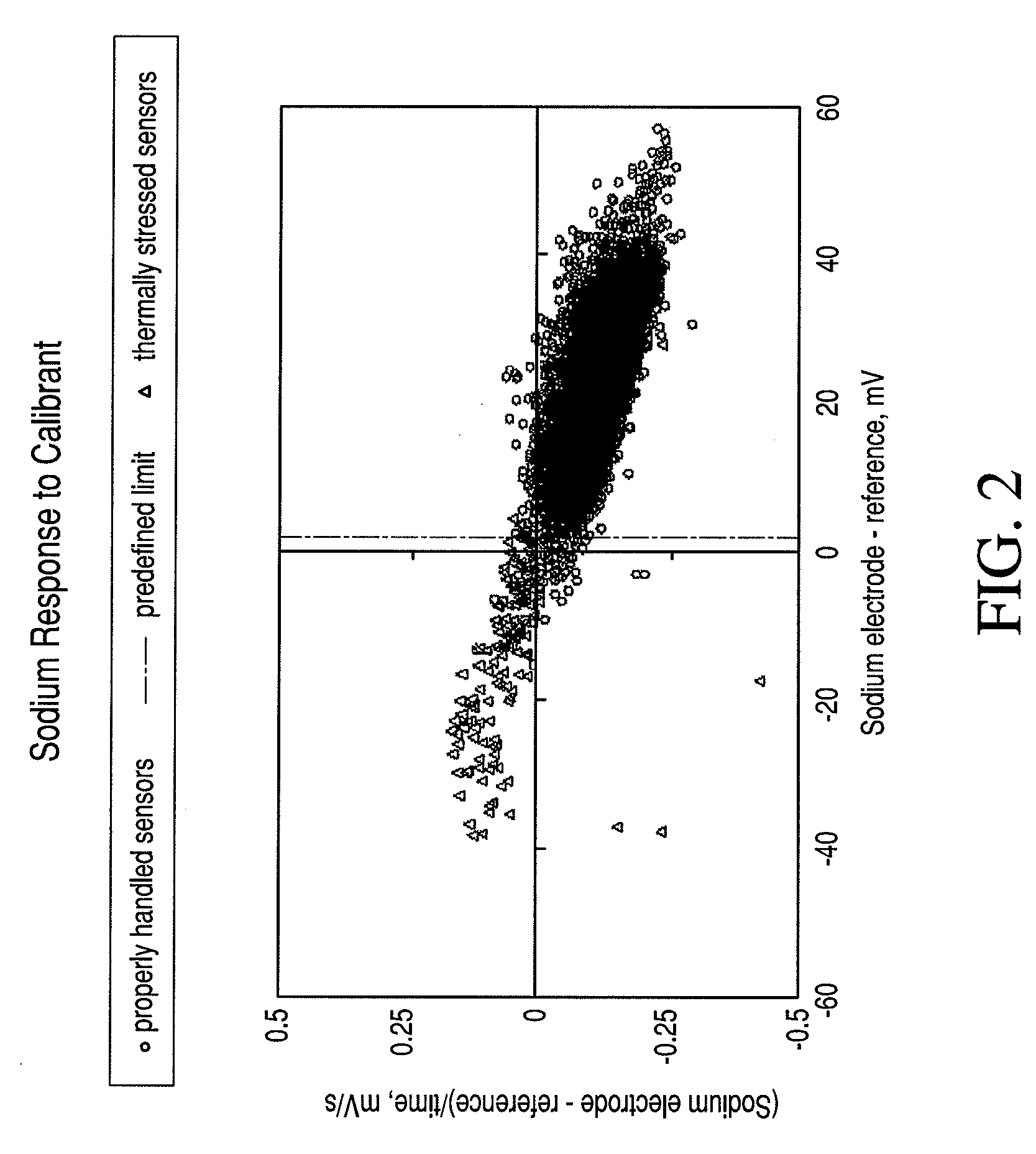
Quality assurance system and method for point-of-care testing
ActiveUS20090119047A1Quality failureThermometer detailsThermometers using mean/integrated valuesQuality assurancePatient care
An improved quality assurance system and method for point-of-care testing are disclosed. The present invention provides quality assurance for laboratory quality tests performed by a blood analysis system or the like at the point of patient care without the need for running liquid-based quality control materials on the analysis system. Quality assurance of a quantitative physiological sample test system is performed without using a quality control sample by monitoring the thermal and temporal stress of a component used with the test system. Alert information is generated that indicates that the component has failed quality assurance when the thermal and temporal stress exceeds a predetermined thermal-temporal stress threshold. Alternatively, the present invention provides quality assurance for laboratory quality tests performed by a blood analysis system or the like at the point of patient care by minimizing the need for running liquid-based quality control materials on the analysis system.
Owner:ABBOTT POINT CARE
Cap-pap test
InactiveUS6143512ABioreactor/fermenter combinationsBiological substance pretreatmentsCervical Pap TestAcid phosphatase
The CAP-PAP Test is a double-staining, single-slide microscopic method. An in vitro diagnostic medical device for manual and automatic staining and interpreting of the Pap smear for cervical cancer screening, cervical dysplasia and for follow-up therapy can be developed using this double-staining, single-slide microscopic method. Abnormal cervical cells are labeled with an intracellular acid phosphatase derived pigment (azo-dye) to improve visibility of abnormal cervical cells on conventionally stained Pap smears. The enzyme marker improves human perception and / or sensitivity of automatic instruments when distinguishing cell a abnormality and interpretation of Pap smears. Increased accuracy of CAP-PAP-vs-Pap test is expected to reduce false negative readings of the conventional Pap test. A rapid manual version of the test that is low cost, does not require additional personnel training and is instantly applicable in all cytopathology laboratories is provided. The invention further provides a diagnostic kit, an automatic stainer and an automatic evaluation device for performing the double-staining, single-slide microscopic method.
Owner:MARKOVIC NENAD +1
Intelligent decision-making assisting system based on multi-modal ultrasomics
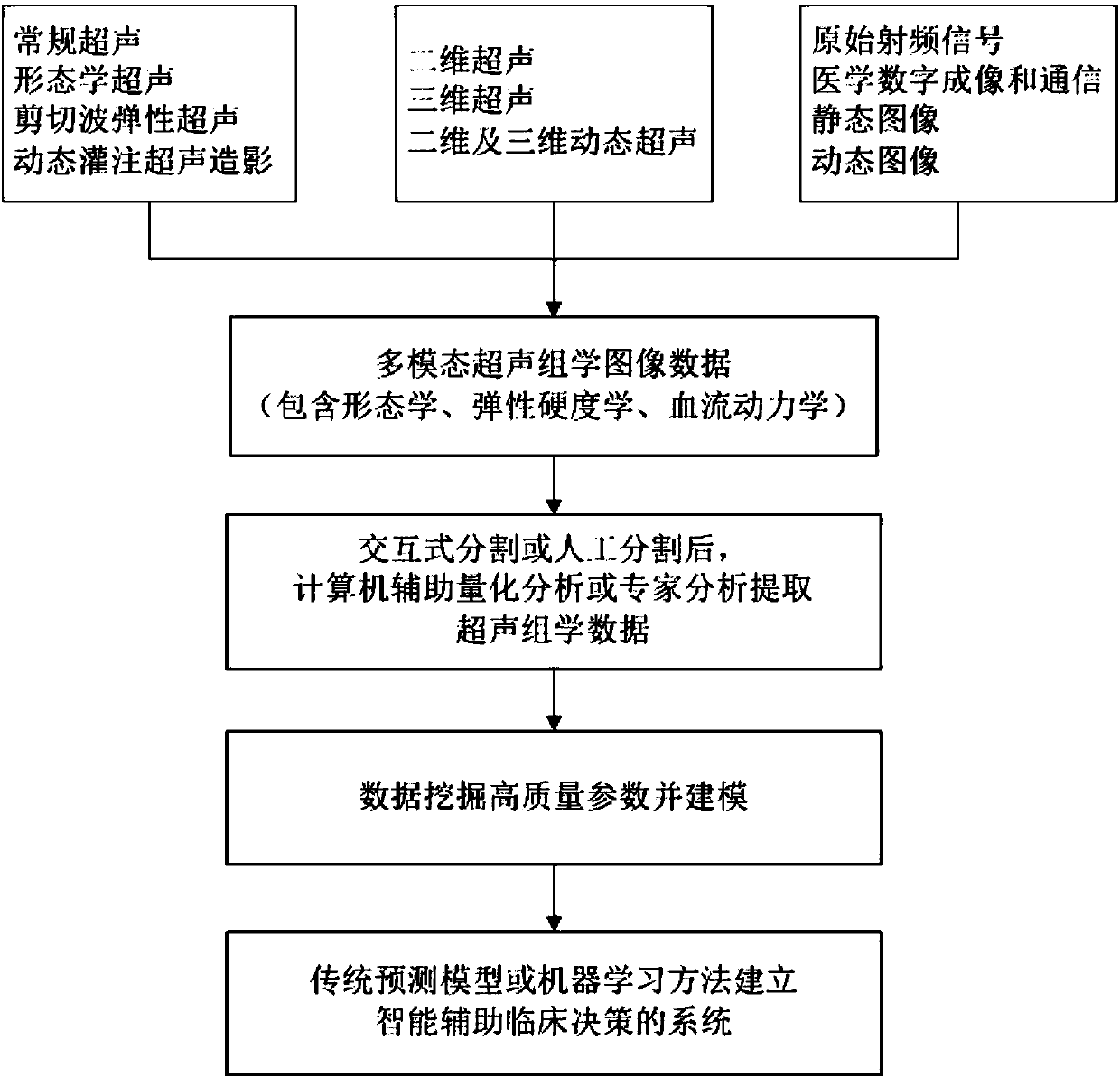
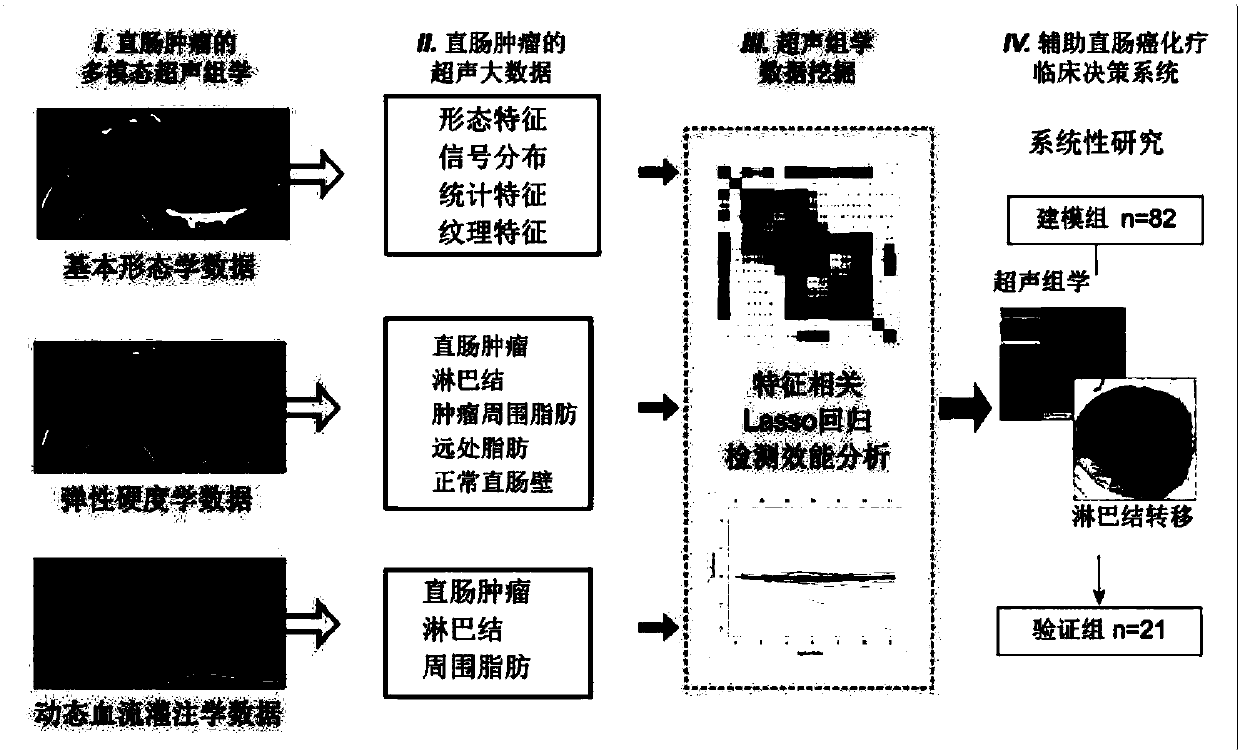
InactiveCN107582097AGood segmentation resultComplete individualized precision medical adviceBlood flow measurement devicesOrgan movement/changes detectionDiseaseGenomics
The invention discloses an intelligent decision-making assisting system based on multi-modal ULTRASOMICS. Firstly, ultrasonic data from different signal sources of an instrument is acquired, an interactive or manual dividing method is adopted to locate an interested area in an ultrasonic image, data of morphology, blood perfusion and hardness science of diseases is obtained, and a multi-modal ultrasonic characteristic database is constructed; through the combination of relevant clinical information, laboratory checking information and gene chip information, ultrasomics data is mined, a machinelearning method is utilized to construct different models, the recurrence rate and the survival rate are predicted, the curative effect and the radiotherapy and chemotherapy sensitivity are judged inthe early stage, and the correlation of ultrasomics, genomics, proteomics, transcriptomics and metabonomics is analyzed. As macroscopic ultrasomics, the intelligent decision-making assisting system provides a repeatable evaluation means for noninvasive individualized precise medicine.
Owner:THE FIRST AFFILIATED HOSPITAL OF SUN YAT SEN UNIV
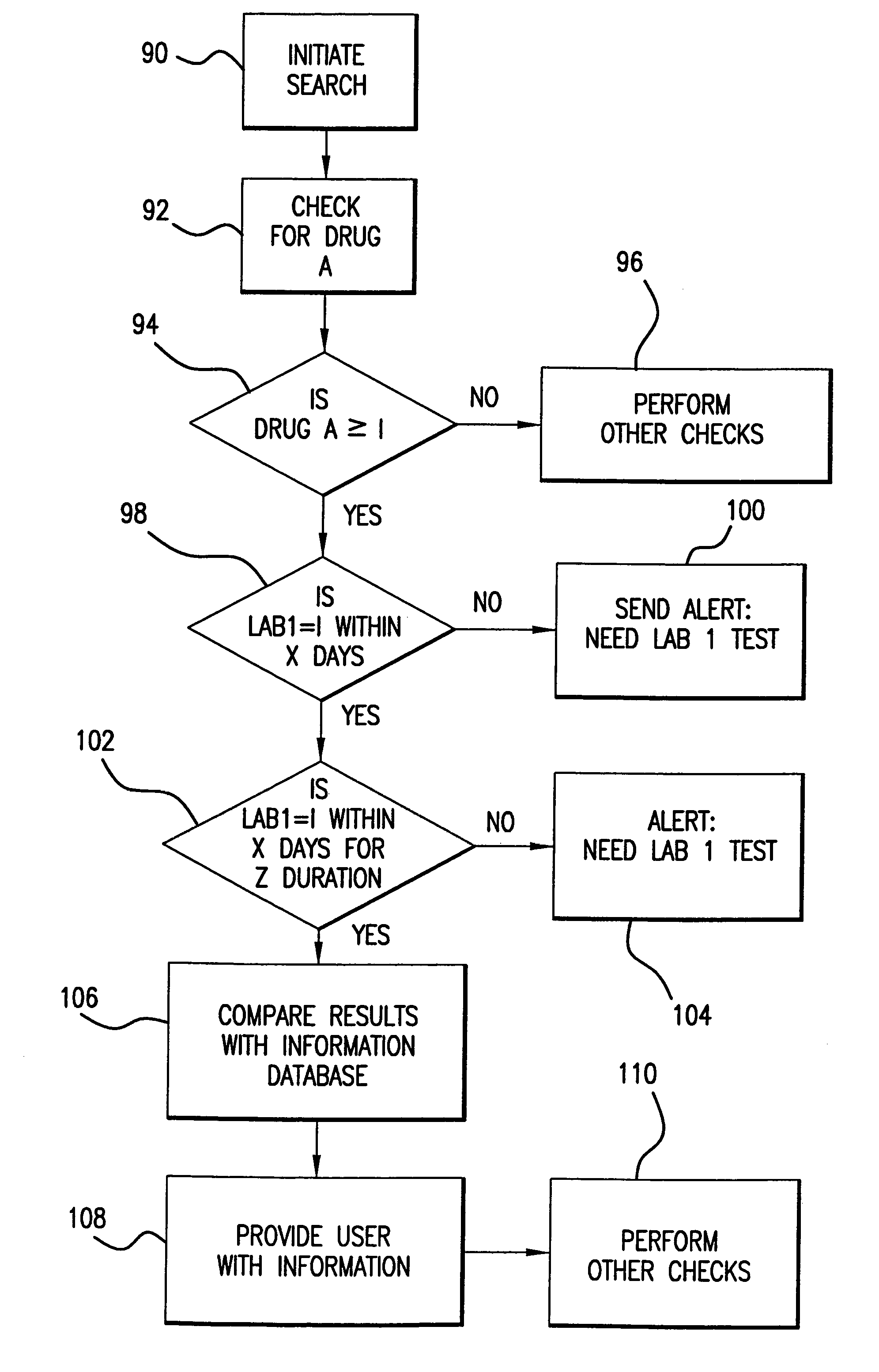
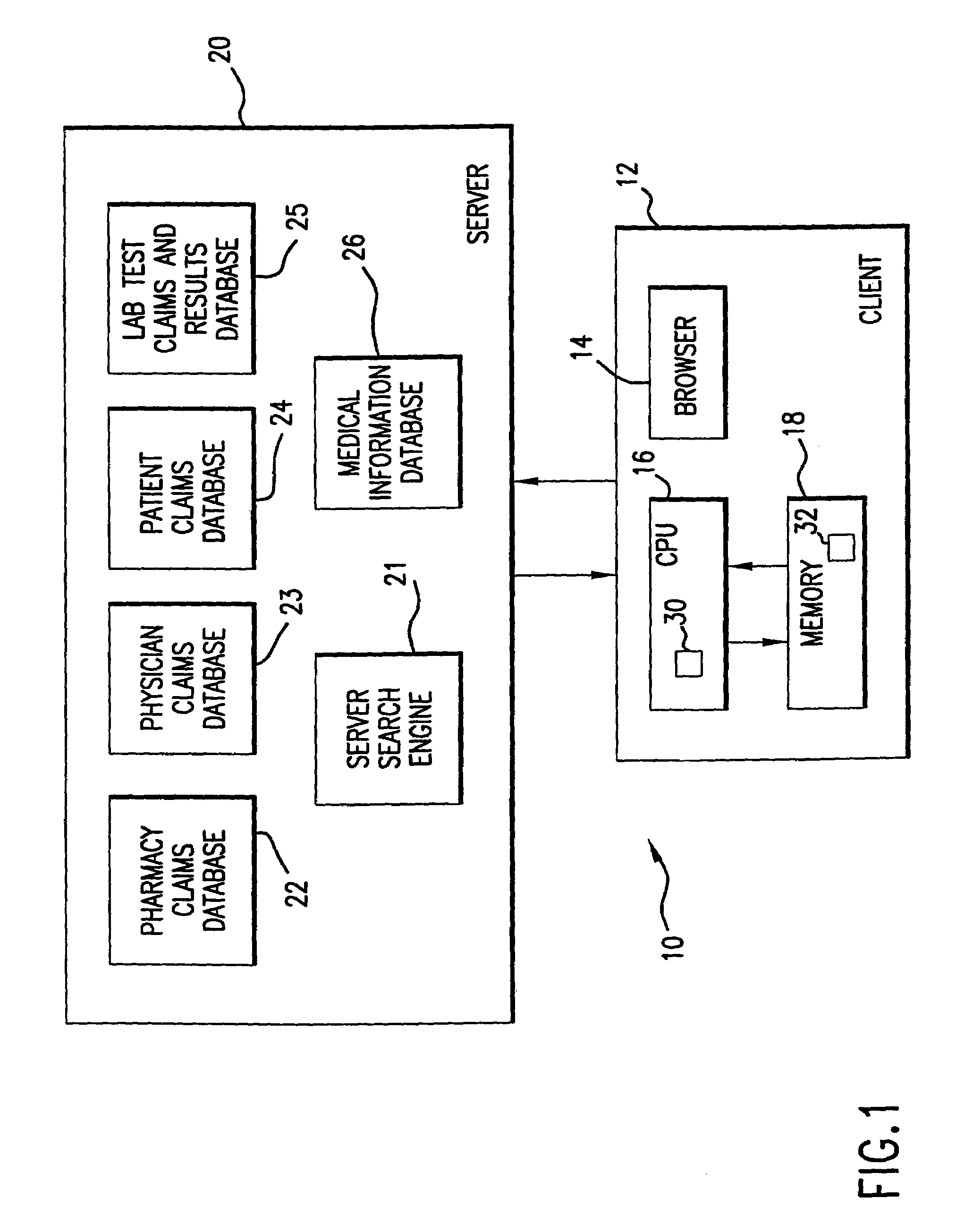
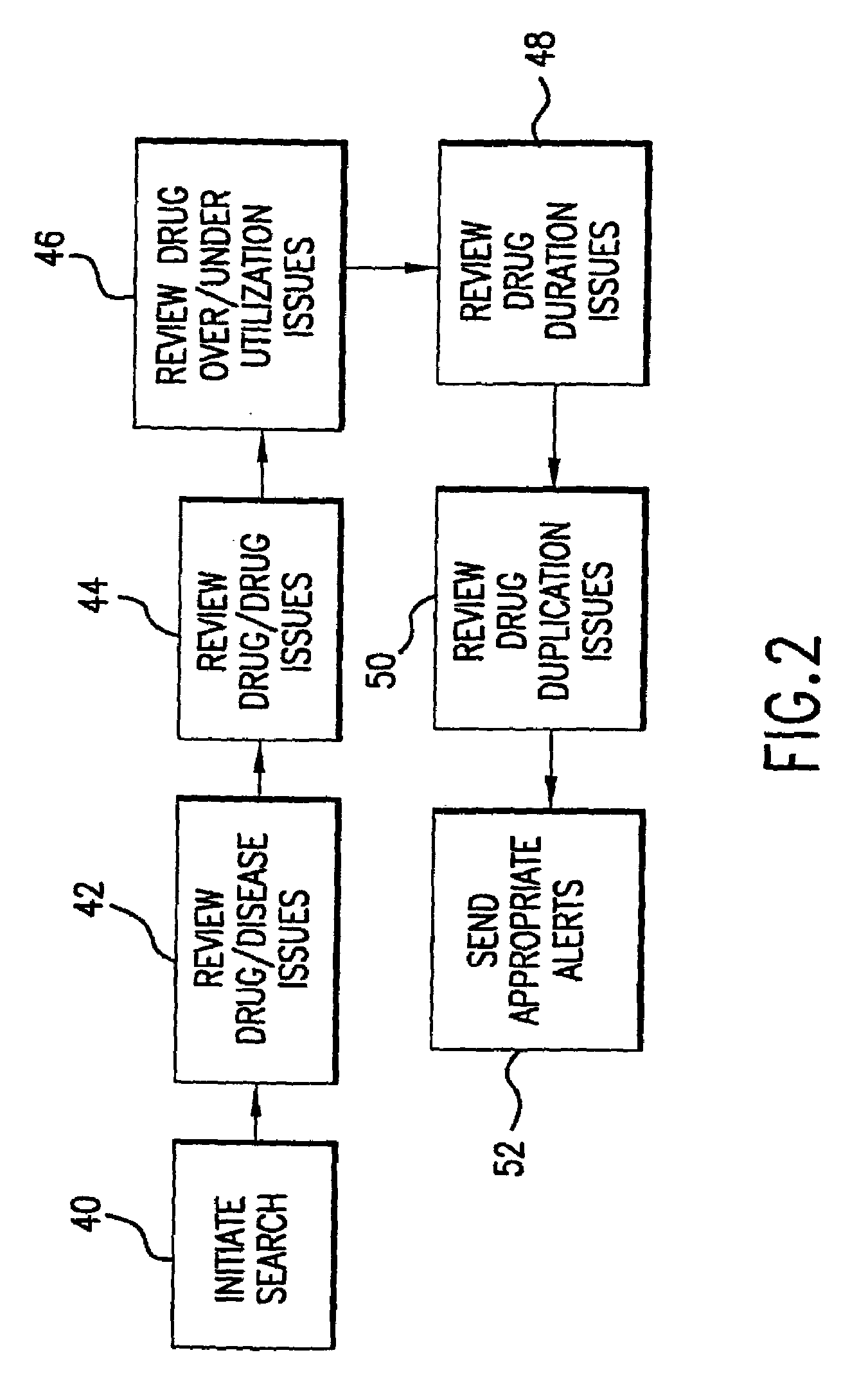
System for monitoring regulation of pharmaceuticals from data structure of medical and labortory records
InactiveUS7124031B1Strong specificityHigh strengthPhysical therapies and activitiesDigital data processing detailsDiseaseLaboratory Test Result
A system is provided that integrates of records of clinical laboratory services into the assessment and optimization of patient health care and, in particular, regulation of the use of pharmaceuticals. Laboratory test result records are used in conjunction with other health care benefits records to monitor regulation of use of pharmaceuticals by patients. The incorporation of laboratory tests and results into such a utilization system allows improvement in the management of a patient's therapy based on a more precise picture of the patient's level of illness as revealed by the laboratory test results. The system of the present invention also allows optimization of the selection of laboratory tests to be performed, and also provides an outcome assessment of the risk of hospitalization due to pharmaceutical treatments resulting in physician intervention, leading to a change in physician prescribing behavior and, accordingly, a decrease in drug induced hospitalizations and improved quality of patient care and savings of health care costs.
Owner:PROVANTAGE INC +1
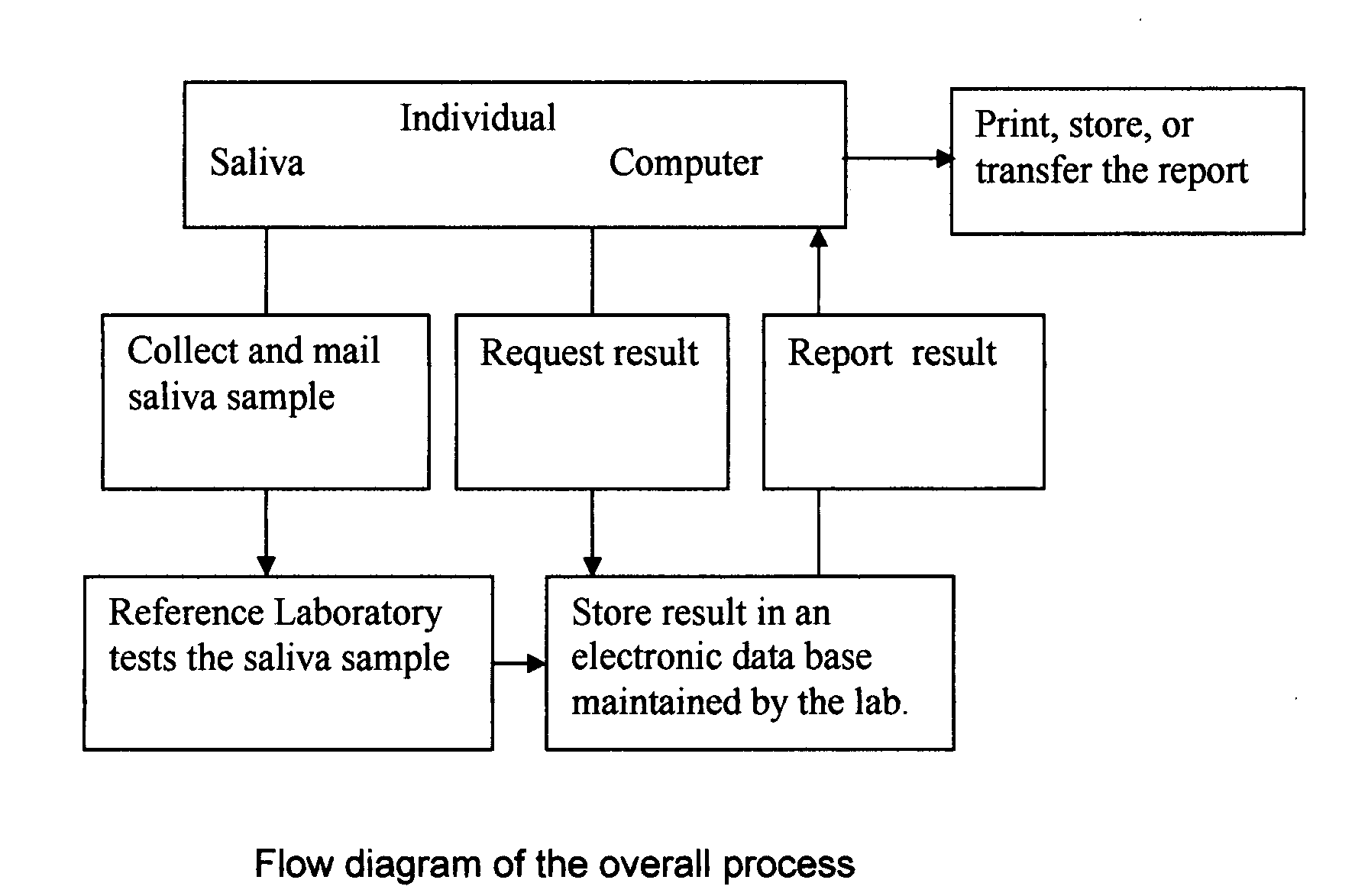
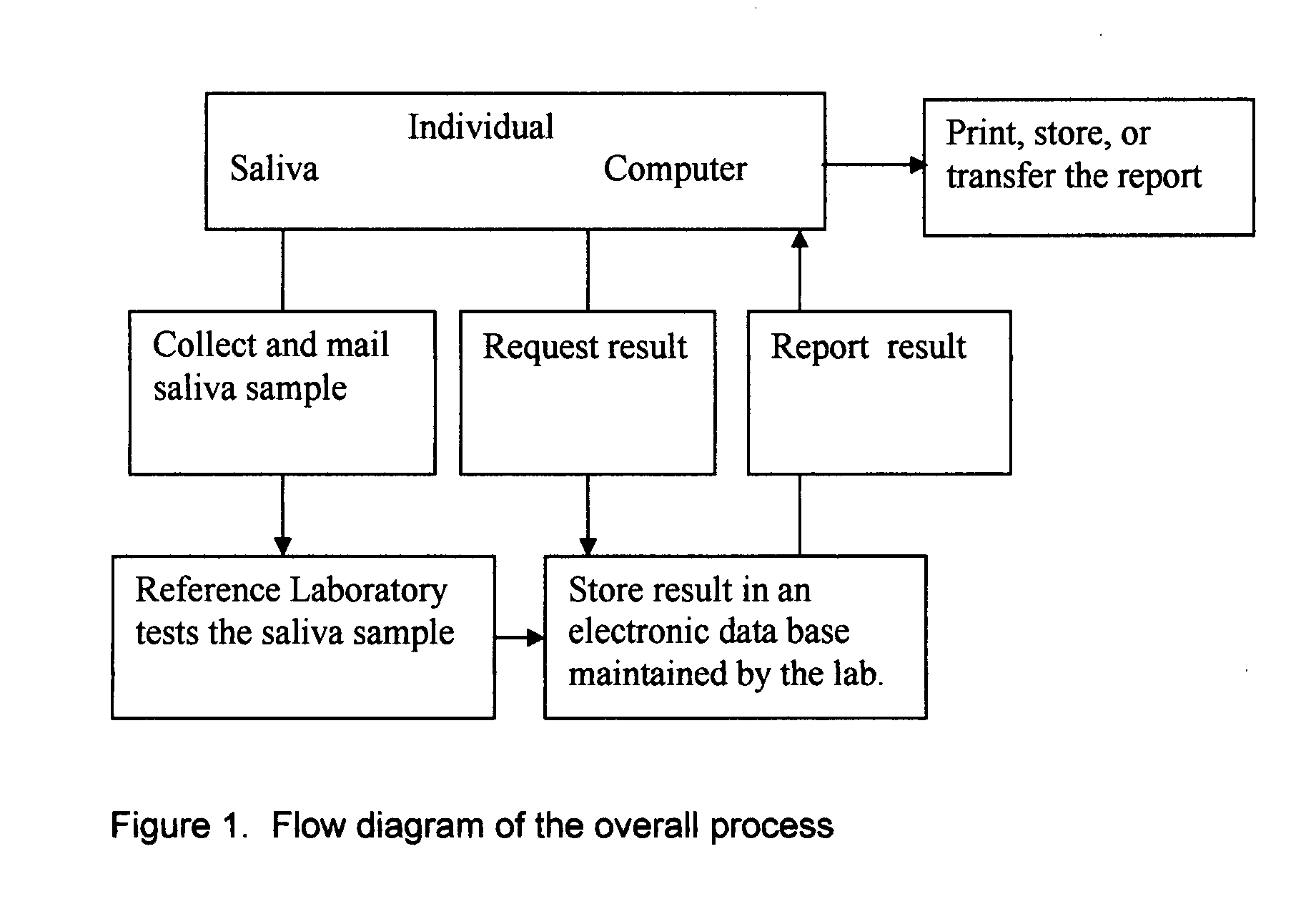
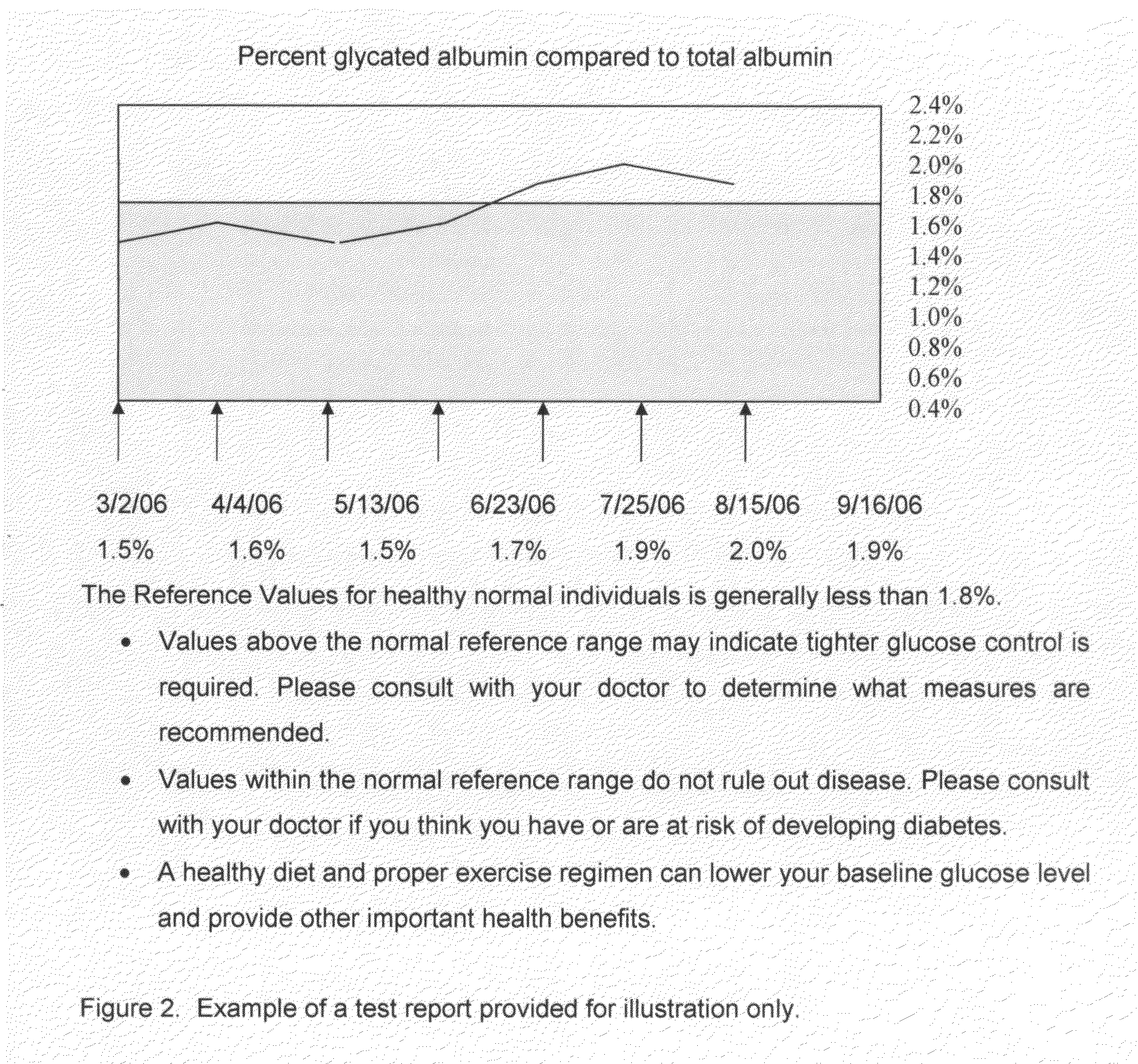
Home test for glycated albumin in saliva
InactiveUS20080227210A1Withdrawing sample devicesVaccination/ovulation diagnosticsSaliva sampleSaliva collection
A home test for measuring glycated albumin levels in saliva. The saliva sample is collected at home using a standardized saliva collection kit and mailed to a testing laboratory that performs the test and reports the result directly back to the customer via the internet. The home test can be used to monitor glucose control in diabetics and in healthy individuals. It may also be used as a diagnostic aid in identifying individuals with diabetes, or who are at risk of developing diabetes.
Owner:SMITH HENRY
Device for performing a blood, cell, and/or pathogen count and methods for use thereof
ActiveUS9322767B2Rapid and accurate and affordable laboratory-quality blood testingEliminate equipmentBioreactor/fermenter combinationsBiological substance pretreatmentsPoint of careDiagnosis laboratory
Devices and methods for performing a point of care blood, cell, and / or pathogen count or a similar blood test. Disclosed herein are systems that can be used to provide rapid, accurate, affordable laboratory-quality testing at the point of care. The systems described herein are capable of imaging and counting individual cells in a prepared cell sample (e.g., a peripheral blood smear or a blood sample prepared in a microfluidic device) or another prepared cell-containing sample without the need for a microscope or other expensive and cumbersome optics. The systems described herein are designed to eliminate or replace expensive, centralized clinical testing equipment and technical personnel. Such systems may include automated data reporting and decision support.
Owner:I CALQ
Biological sample detection vehicle and vehicular detection system thereof
InactiveCN102795146ARealize security protectionAchieve protectionBioreactor/fermenter combinationsBiological substance pretreatmentsRural areaPublic health
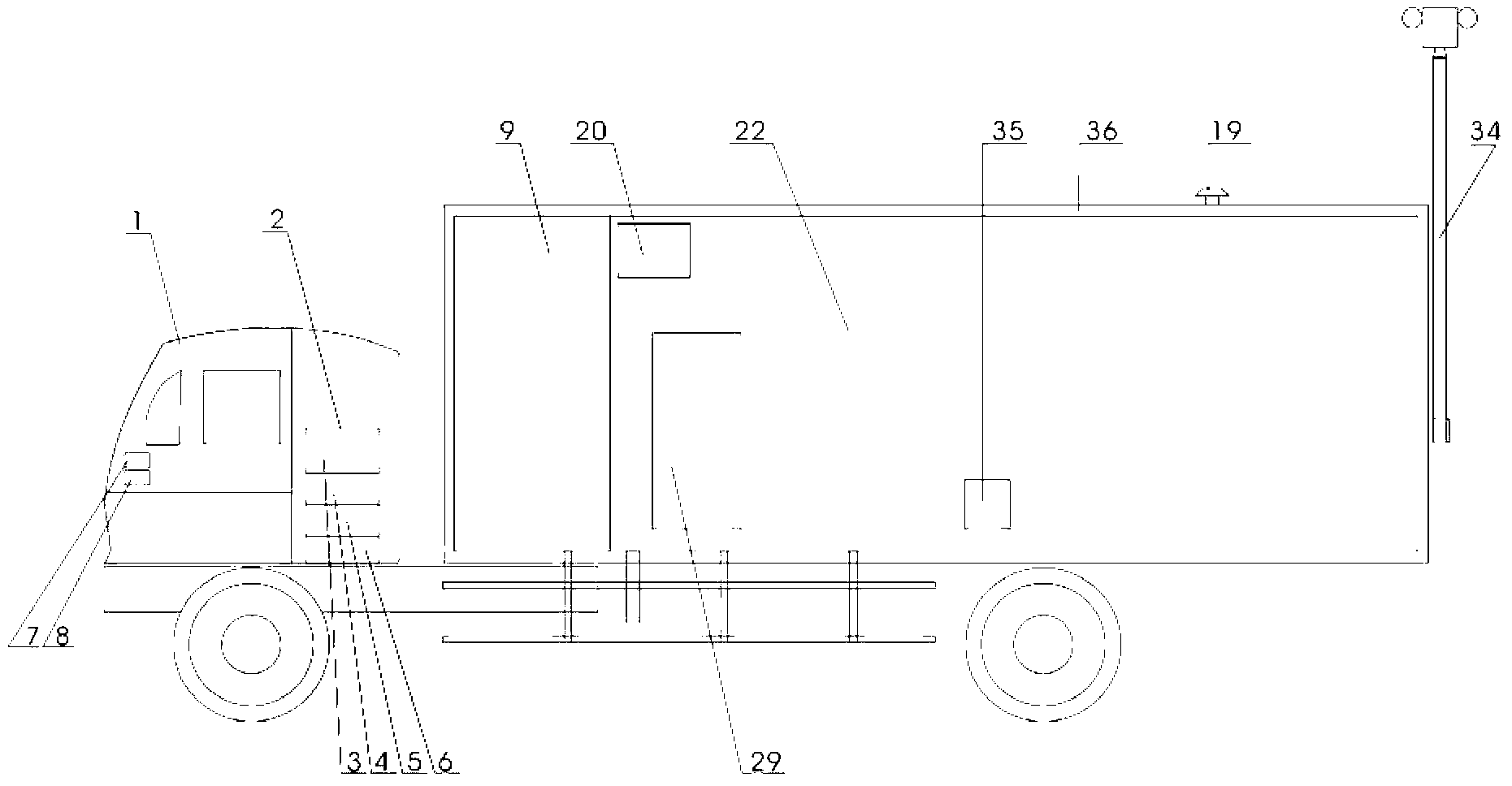
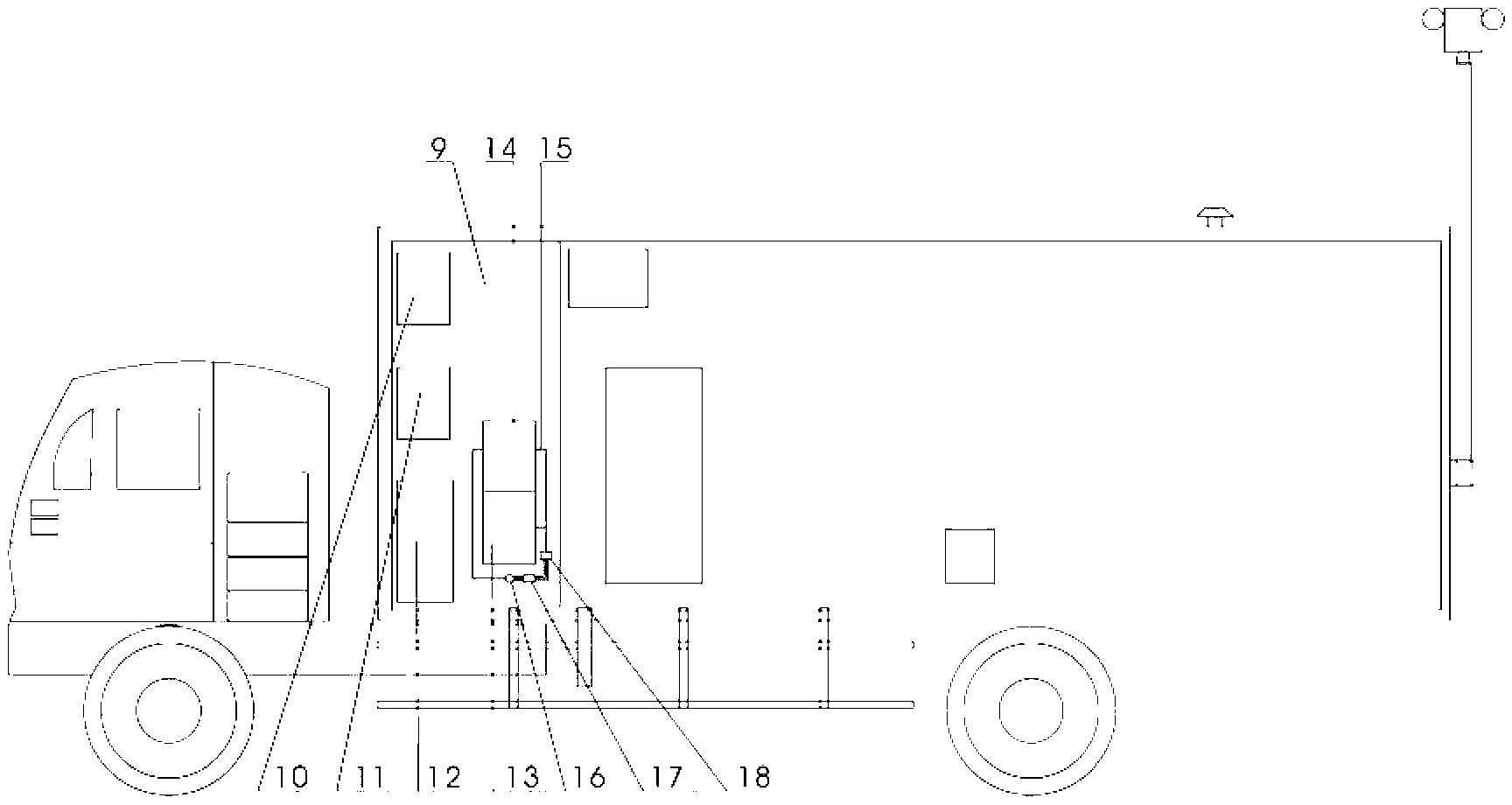
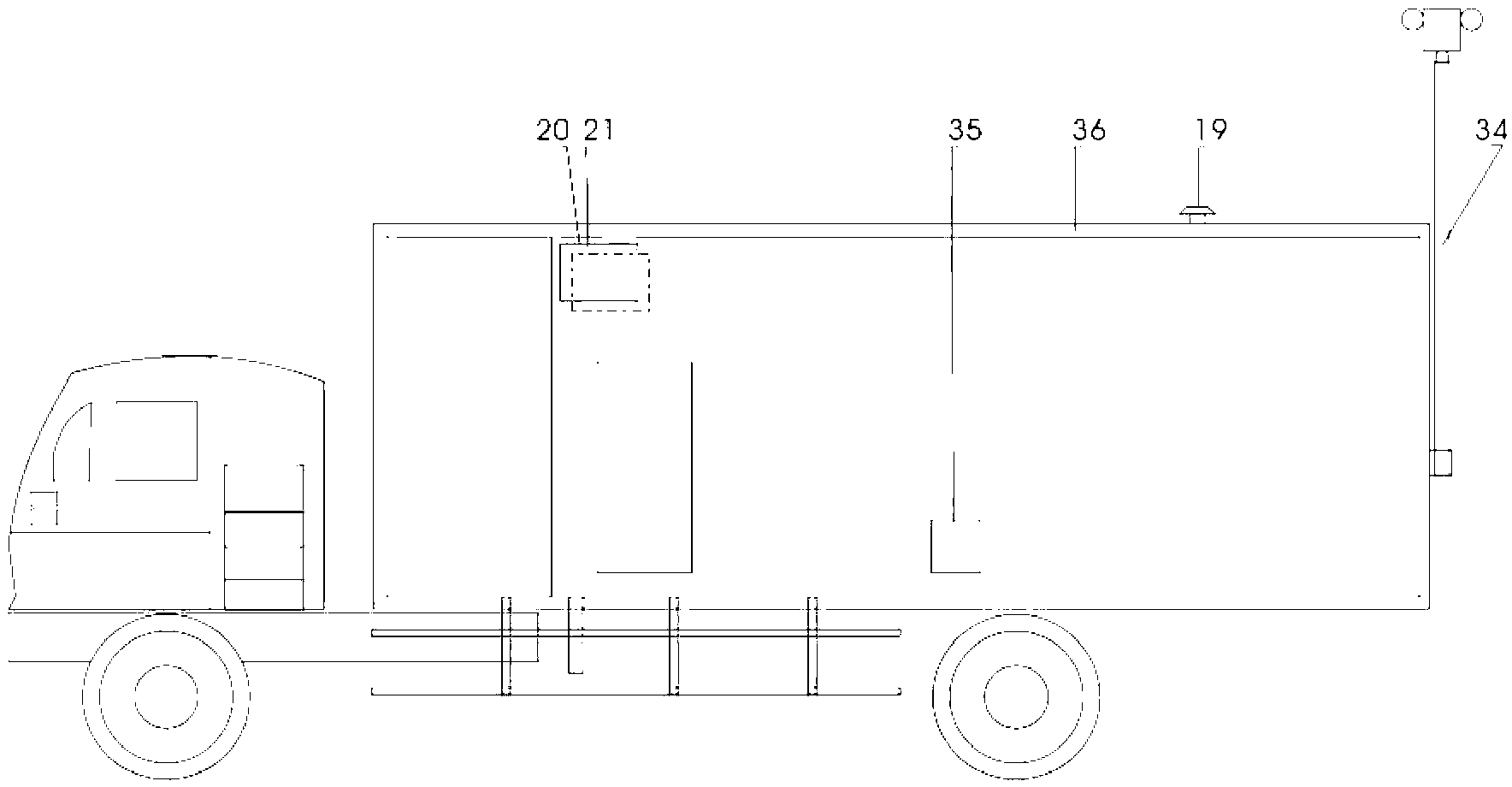
The invention relates to a biological sample detection vehicle and a vehicular detection system thereof. At present, a mobile lab achieving biosafety level-3 protection level is developed at home and abroad, which is called 'P3 vehicle' commonly, conventional home and abroad P3 vehicle is formed by grouping more than 2 box-type vehicles, and has large size and complicated operation. The invention aims at providing a miniature biological sample detection vehicle which has excellent maneuvering characteristic and biosafety protection function, adapts to epidemic situations or public health incidents and consists of a cockpit (1), a front cabin (9), a rear cabin (22) and an atmosphere sampler (19), wherein the front cabin is an equipment cabin, and the rear cabin is an experimental cabin. The biological sample detection vehicle integrates biological sampling, air pre-alarming and quick detection into a whole, has good maneuvering property and matched functions, has the function of carrying out various biological sampling and pathogenic microorganism detection in the field of epidemic situation in remote rural areas or urban roadways, and can be used for executing a 'bio-control' quick detection task in contingency medical rescue with high efficiency.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Early death risk assessment model establishing method and device based on ensemble learning
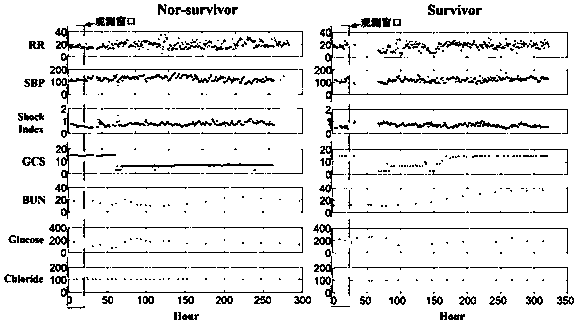
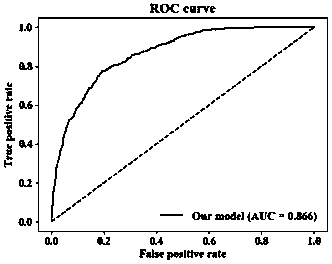
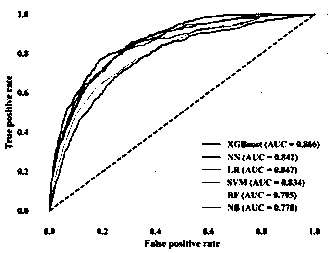
InactiveCN110827993AEarly interventionEarly treatmentMedical data miningEnsemble learningMedical intensive care unitEmergency medicine
The invention provides an early death risk assessment model establishing method and device based on ensemble learning. The method and device are used for assessing the death risk of an old multi-organfailure patient during hospitalization based on diagnosis and treatment data of the first day when the old multi-organ failure patient enters an intensive care unit. The method comprises the steps ofdata set construction, data processing and model construction and evaluation. The method comprises the following steps: acquiring three pieces of demographic information, five pieces of vital sign information, five laboratory examination indexes and two clinical indexes of a patient on the first day during living in an intensive care unit; inputting the data into a risk assessment device, and carrying out internal data preprocessing, feature calculation and model operation. Finally, the risk of adverse outcomes in the hospital of the patient can be predicted early, and doctors are helped to treat the patient as soon as possible.
Owner:BEIHANG UNIV +1
Device for detecting a medical condition or disease
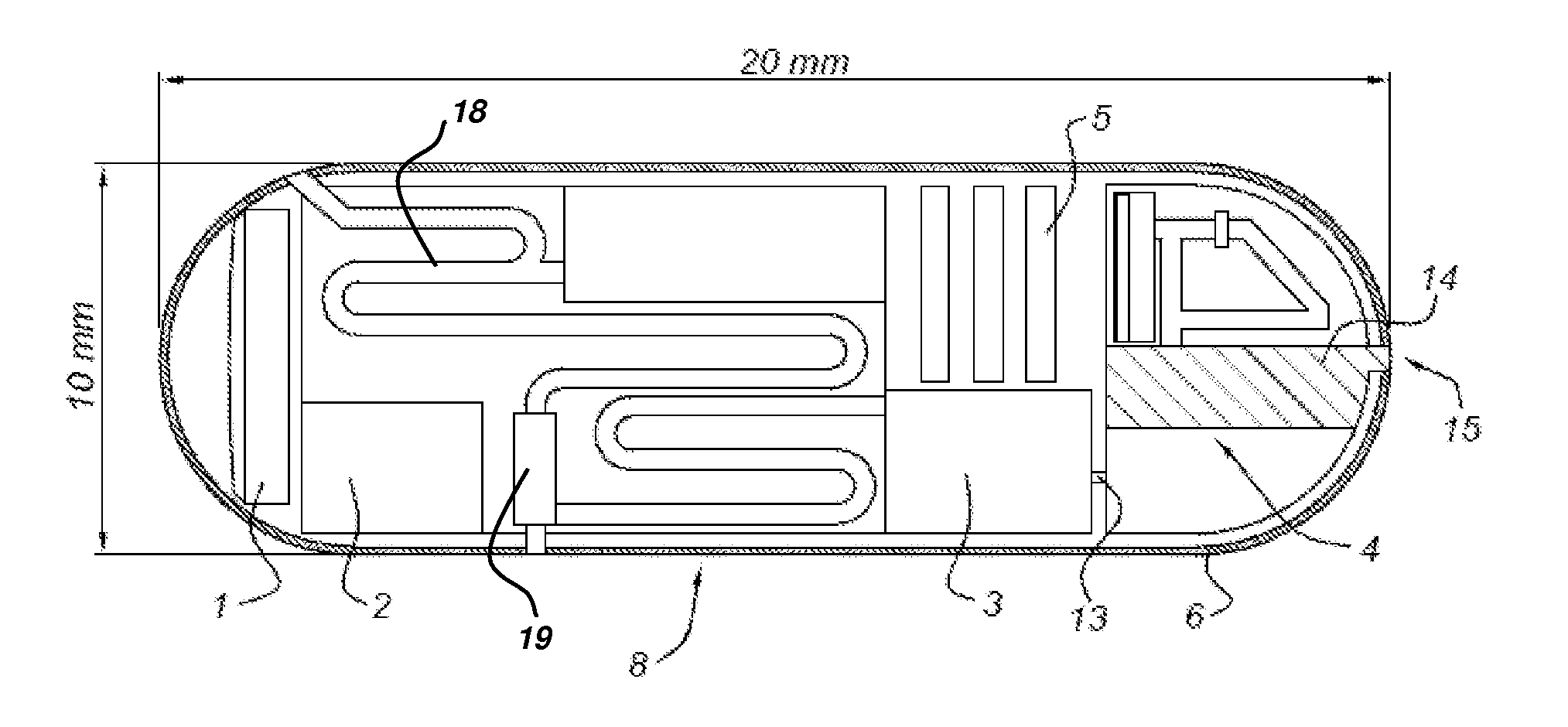
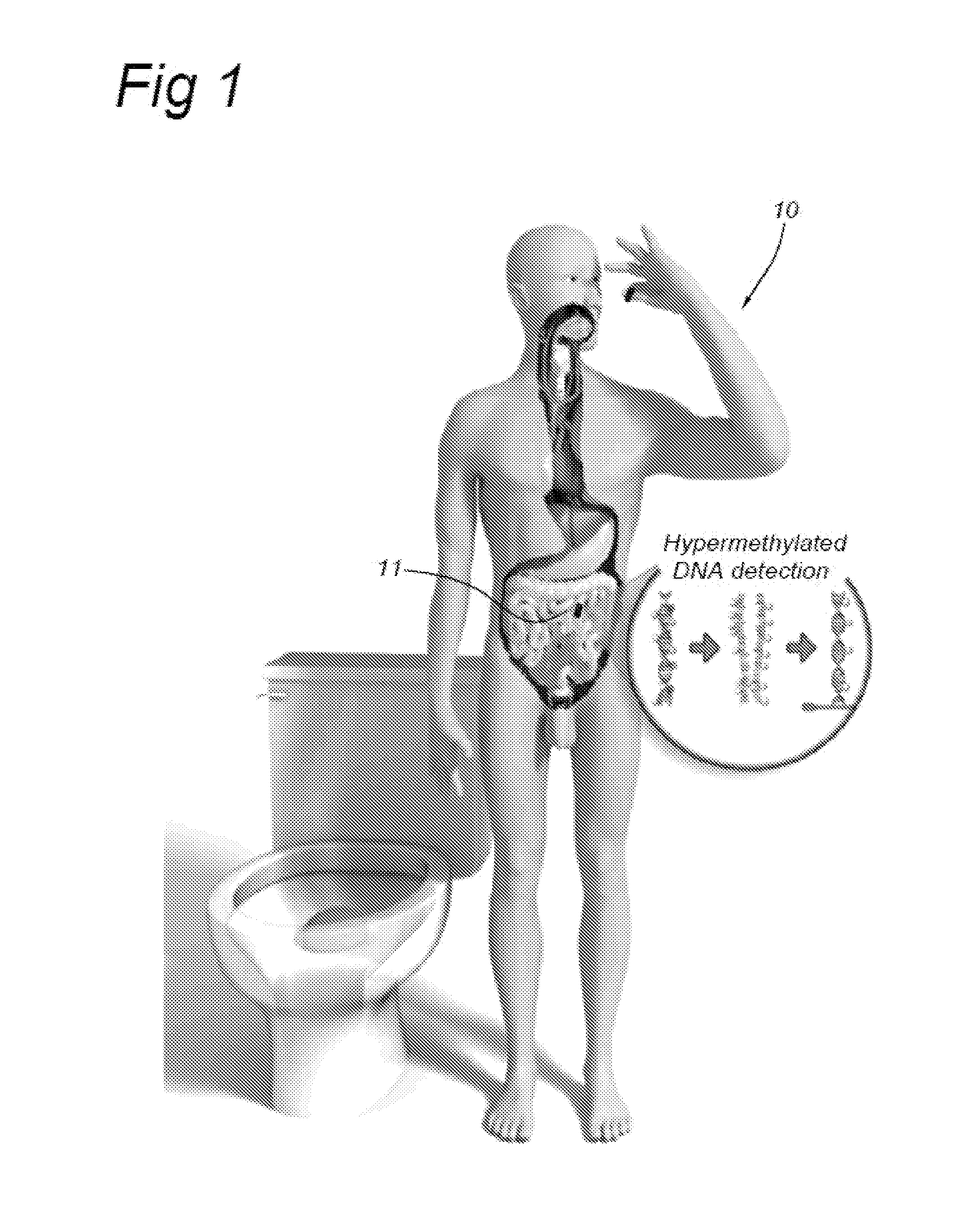
ActiveUS20110046458A1Effective treatmentVaccination/ovulation diagnosticsEndoradiosondesDiseaseFeces
The invention relates to a capsule or chip or sensor comprising a marker / detector and signalling device / method associated with the development of a medical condition / disease and to its use. This In Situ Lab On a Chip Signalling device (ISLOGS device) is used for detecting a medical condition / disease in situ or in vivo (inside the body), for example by swallowing it for testing in the digestive tract or by putting it into the vagina, mouth or nose. All reactions takes place in the body and when the test result is positive the device will notify the subject by colorizing the tested body fluid or biomaterial (for example faeces, urine or saliva) or the device itself. The detection is made outside of the body when the biomaterial or ISLOCS device has left the body.
Owner:NANOMED DIAGNOSTICS BV
Systems, methods, and computer program products for facilitating communications, workflow, and task assignments in medical practices and clinics
InactiveUS20070185736A1Eliminate needShorten the timeLocal control/monitoringDrug and medicationsDiagnostic testVaccination
A method of facilitating patient examinations in a medical office includes the following steps performed via a handheld device: identifying an examination location of a patient; identifying one or more medical procedures to be performed, wherein the one or more medical procedures are selected from the group consisting of: laboratory analysis of biological samples obtained from the patient, diagnostic tests on the patient, patient vaccinations and / or injections, and preparing a medical prescription; displaying a patient examination summary, wherein the displayed summary includes patient identification information, patient location information, patient health status information, and a list of identified medical procedures; and communicating wirelessly the patient examination summary to a nurse station computer within the medical office.
Owner:EFLAG PROFESSIONAL SOLUTIONS
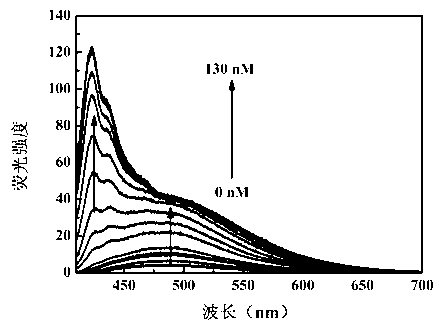
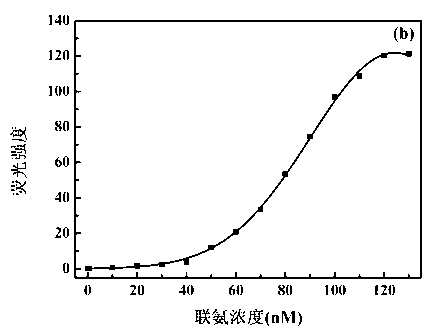
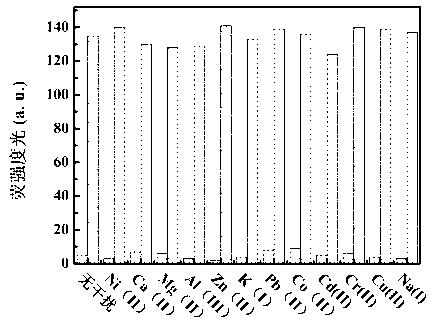
Benzothiazole-cyanophenyl compound serving as hydrazine fluorescence probe as well as preparation method and application method of benzothiazole-cyanophenyl compound
ActiveCN103214428ASimple preparation processConjugate plane largeOrganic chemistryFluorescence/phosphorescenceIndustrial waste waterStructural formula
The invention discloses a benzothiazole-cyanophenyl compound serving as a hydrazine fluorescence probe. The benzothiazole-cyanophenyl compound has a structural formula as shown in (I); the compound is prepared by performing cyclodehydration with bromobenzaldehyde and 2-amino-4-chloro thiophenol serving as the raw materials, then performing coupled reaction in order to connect with a bromobenzaldehyde derivate, and finally performing Knoevenagel reaction with malononitrile. The benzothiazole-cyanophenyl compound has the advantages that the raw materials are low in price and easy to gain, the synthetic route is simple, and the yield is relatively high; rigid structures such as benzothiazole and phenylacetylene groups are introduced into such a fluorescence probe, thus high fluorescence quantum efficiency is realized, and relatively high thermal stability and dissolubility are brought. The probe adopts the photoinduced charge transfer mechanism and the conjugate passivation mechanism, therefore, a response range respect to hydrazine can be expanded; the probe has the characteristics of being fast in response, high in sensitivity and high in selectivity, is suitable for being applied to safety detection of foods as well as safety detection of a laboratory, in particular applied to industrial wastewater monitor; and the probe has a wide application prospect in environment monitoring, ecological protection, disease diagnosis, industrial production and pollution discharge inspection.
Owner:浙江富昇科技有限公司
Systems and methods for collecting and transmitting assay results
ActiveUS20130156286A1Quality improvementLittle variabilityImage analysisCharacter and pattern recognitionDiseaseChemical reaction
Owner:LABRADOR DIAGNOSTICS LLC
Bacterial colony number sample for verifying microbiological capacity of food and its prepn process
InactiveCN1706964AThe result will not affectAccelerate the pace of internationalization of certification and accreditationMicrobiological testing/measurementEscherichia coliColony number
The bacterial colony number sample for verifying food microbiological capacity relates to quality control technology in microbiological detection. The sample is matrix with added Bacillus cereus as leading bacteria and background bacteria. The matrix is practical food, and the background bacteria include one or several kinds of serratia marcescens, Citrobacter freundii, enteroaerogen and escherichia coli. The sample of the present invention has homogeneous colony number content, high finishing rate, and high stability, and may be used in test capacity verification of domestic and international labs. In the same time, the capacity verifying sample may be used in verification of detection method, calibration of test instrument, quality control and check of test result and other aspects.
Owner:卢行安 +2
Polynucleotides and polypeptides of Anaplasma phagocytophilum and methods of using the same
InactiveUS20050142557A1Rapid diagnosisEnhance specificityBacteriaPeptide/protein ingredientsPhagocyteWhole blood units
We have successfully sequenced and cloned the gene expressing the Major Surface Protein 5 (MSP5) of A. phagocytophilum. The recombinant MSP5 (rMSP5) protein has been tested using sera from humans and dogs infected with A. phacytophilum. The polypeptide has been found to be immunogenic and useful as a diagnostic test antigen. The polypeptide antigen of the subject invention can provide the basis of a diagnostic assay that would allow the rapid, in-house, laboratory diagnosis of infection with A. phagocytophilum using a sample (e.g., serum, plasma, or whole blood) from an infected human or animal. Additionally, the subject invention provides methods of detecting the presence of A. phagocytophilum in biological or environmental samples utilizing antibodies provided by the subject invention. Furthermore, the use of the single antigen in the diagnosis of this important disease offers many advantages including enhanced test specificity, ease of testing and consistency of results using synthetically produced test antigens instead of cultured, whole organisms.
Owner:UNIV OF FLORIDA RES FOUNDATION INC +1
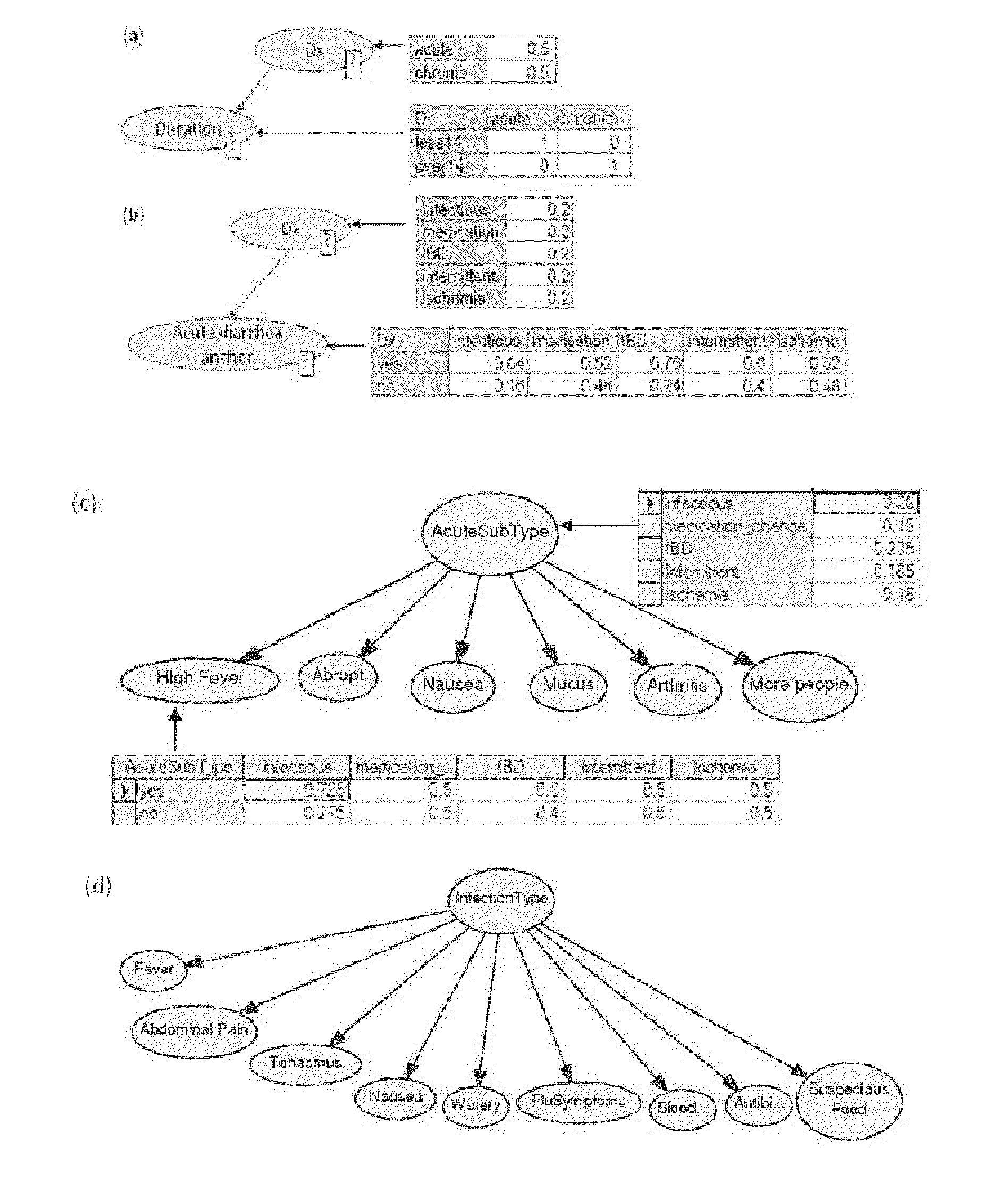
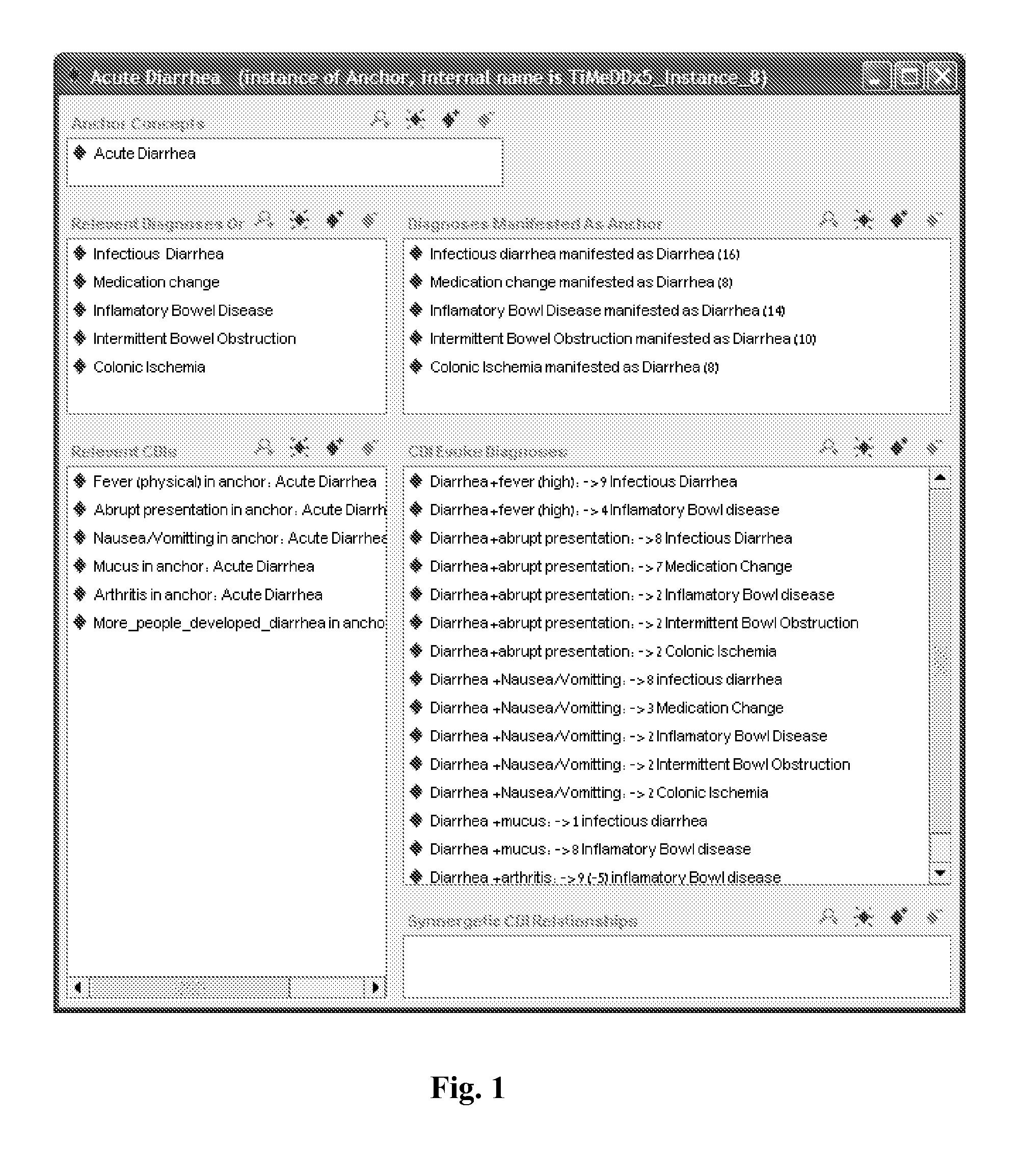
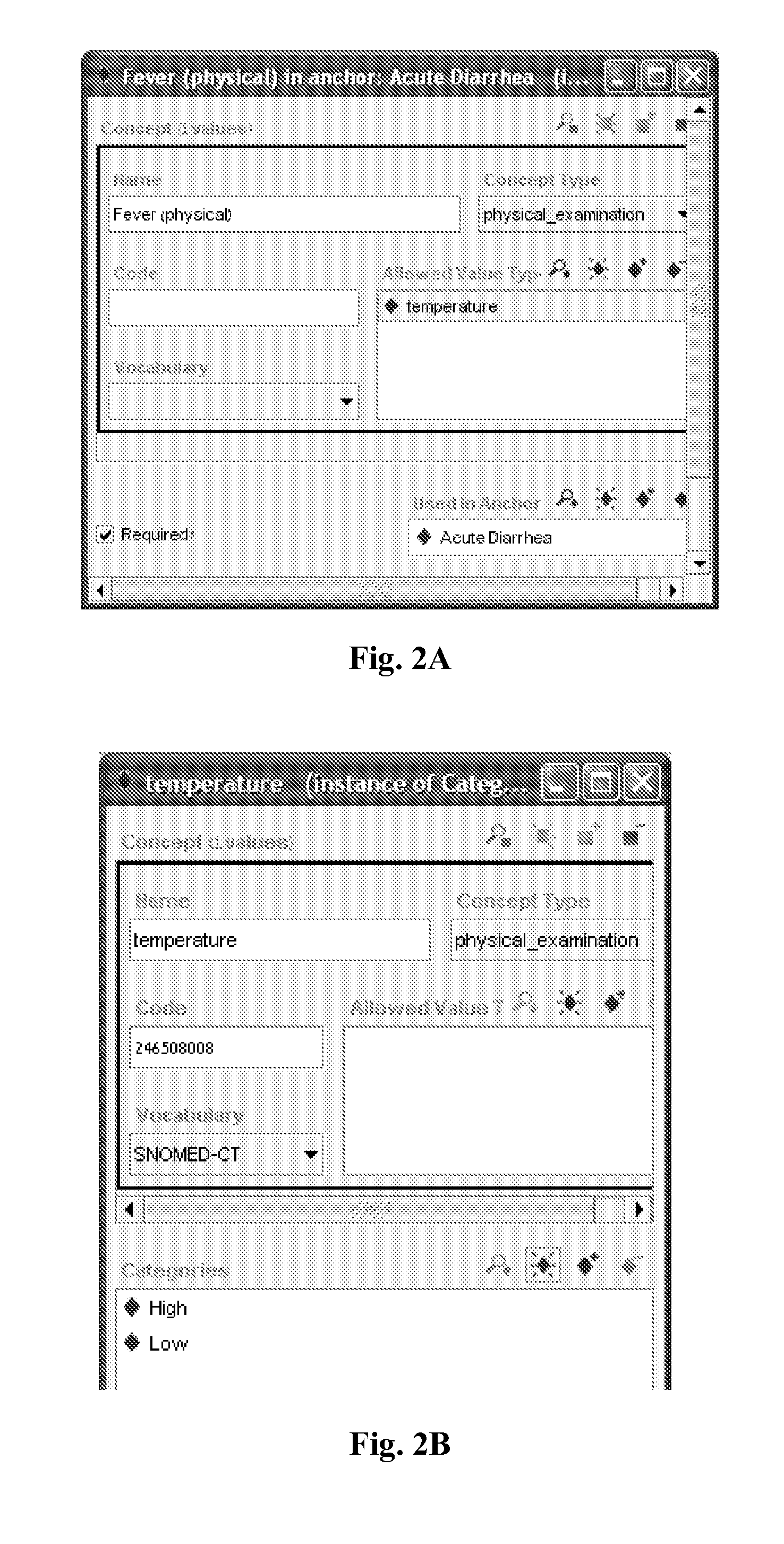
Multi-phase anchor-based diagnostic decision-support method and system
InactiveUS20110257988A1Efficient conductionData processing applicationsHealth-index calculationLaboratory Test ResultHealth professionals
A medical diagnosis decision support system for assisting a health professional to diagnose a medical condition. The system is first provided with an anchor condition that can be a symptom, a sign, a laboratory test result, or an imaging test results or any combination thereof The system then guides users in a series of predetermined phases regarding abstract or concrete diagnosis groups that should be considered and appropriate data that should be collected during the clinical investigation process. The system suggests history and physical examination clinical data items, laboratory, and imaging tests that should be collected in order to differentiate among alternative diagnoses. In each phase, possible diagnoses are listed and ranked.
Owner:MOR RES APPL LTD +1
A real-time isothermal recombinase-polymerase amplification detection kit for African swine fever viruses
ActiveCN106521027AQuick checkRapid diagnosisMicrobiological testing/measurementMicroorganism based processesForward primerAfrican swine fever
A real-time isothermal recombinase-polymerase amplification detection kit for African swine fever viruses is disclosed. A forward primer sequence for detecting the African swine fever viruses through a method provided by the kit is shown as SEQ ID NO:1, a reverse primer sequence is shown as SEQ ID NO:2 and a probe sequence is shown as SEQ ID NO:3. A real-time isothermal recombinase-polymerase amplification method provided by the invention for ASFV detection is simple and convenient in operation, rapid in reaction and low in detection cost, can be used for ASFV detection in a laboratory and on site, particularly ASFV detection in quarantine ports, airports and epidemic disease outbreak sites, and provides a novel and reliable technique support for ASF control in China.
Owner:HANGZHOU ZHONGCE BIO SCI&TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com