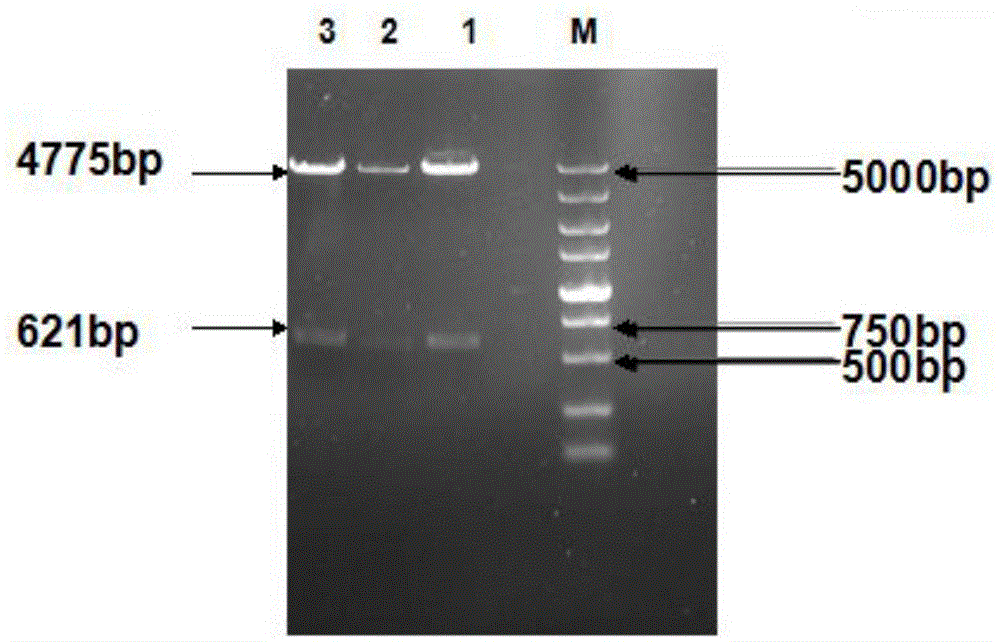
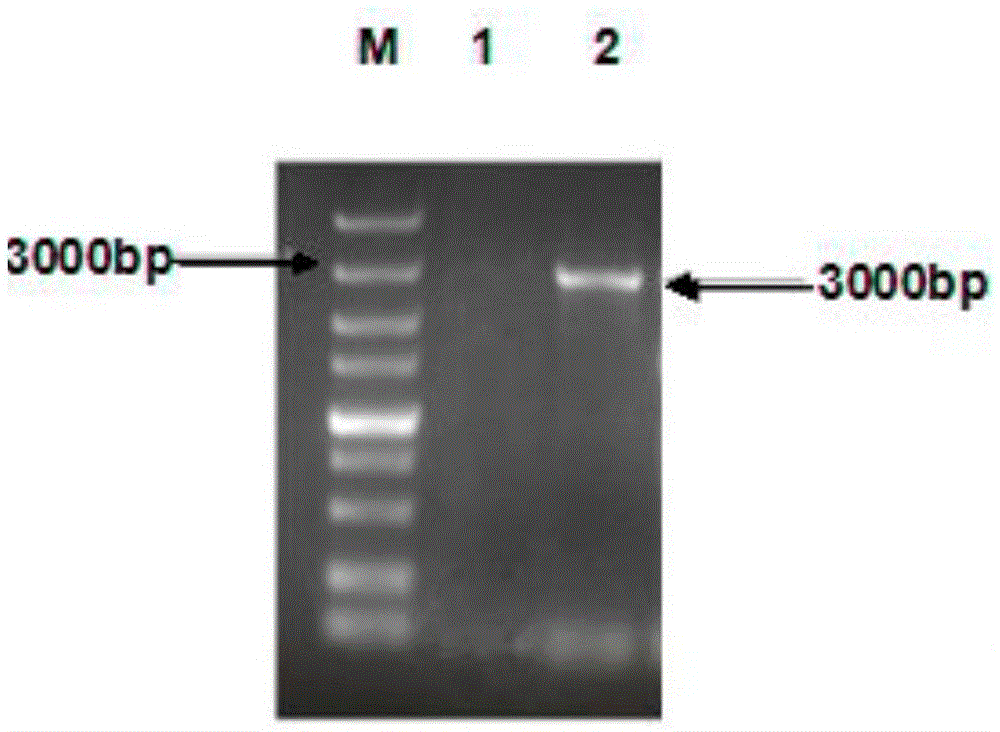
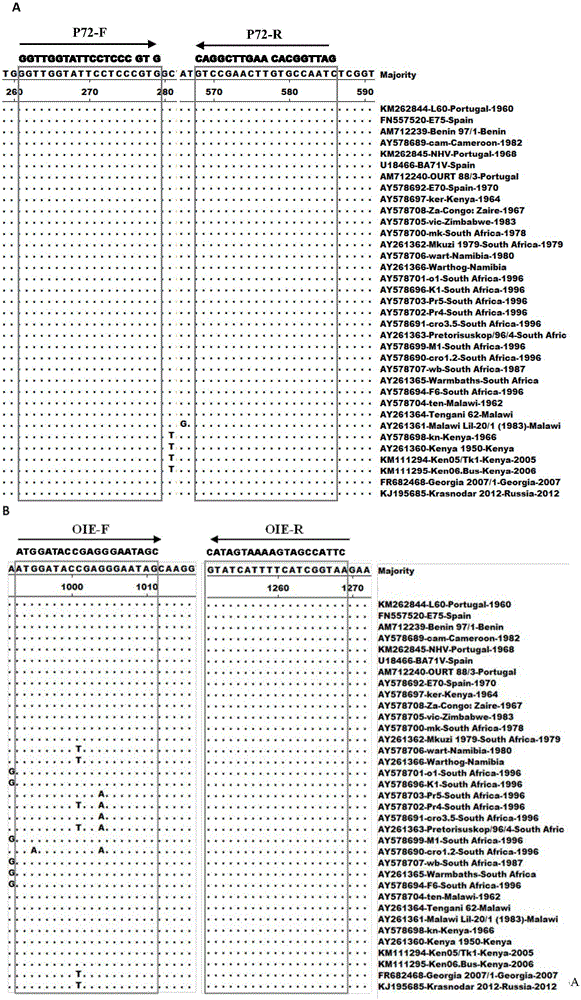
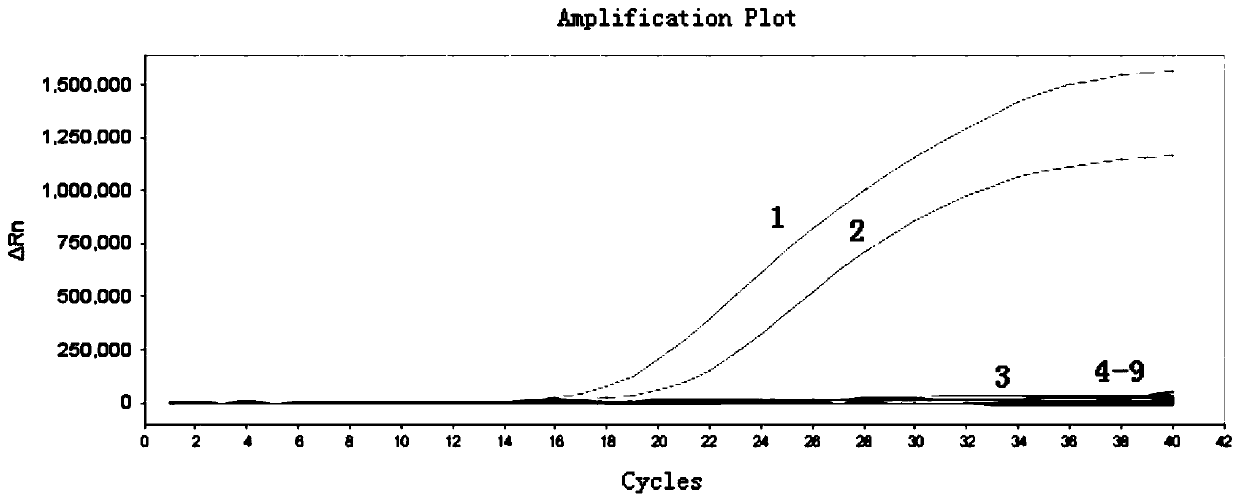
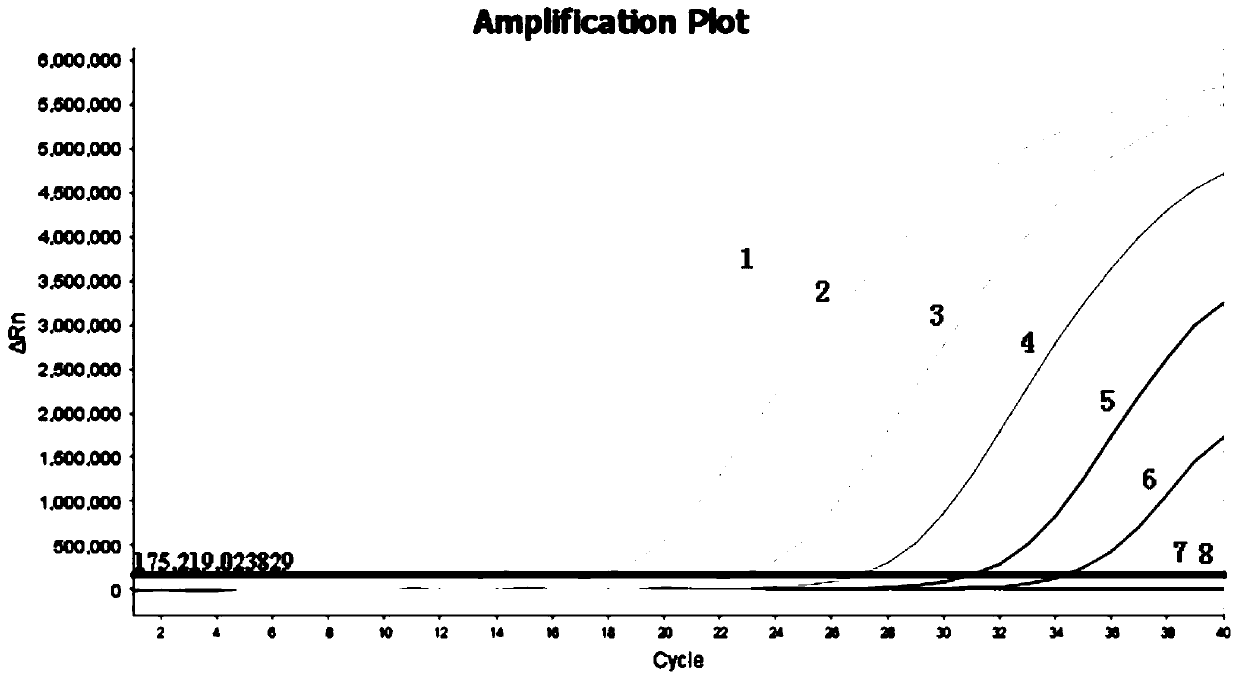
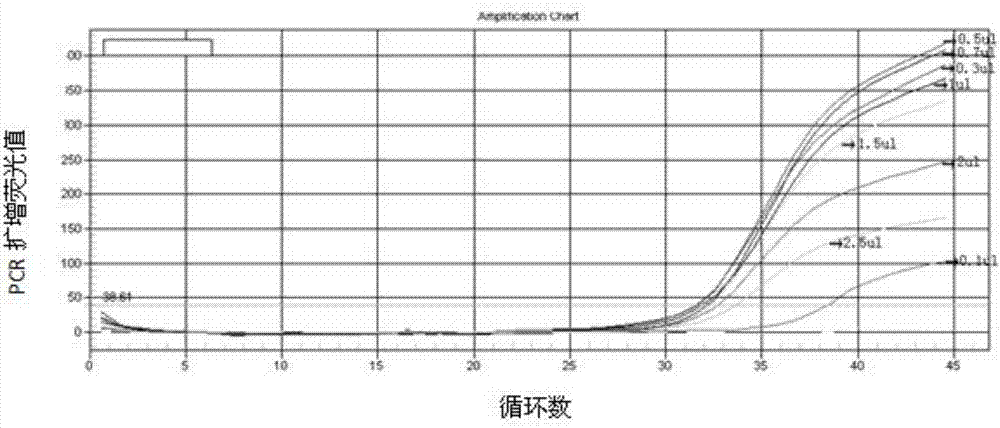
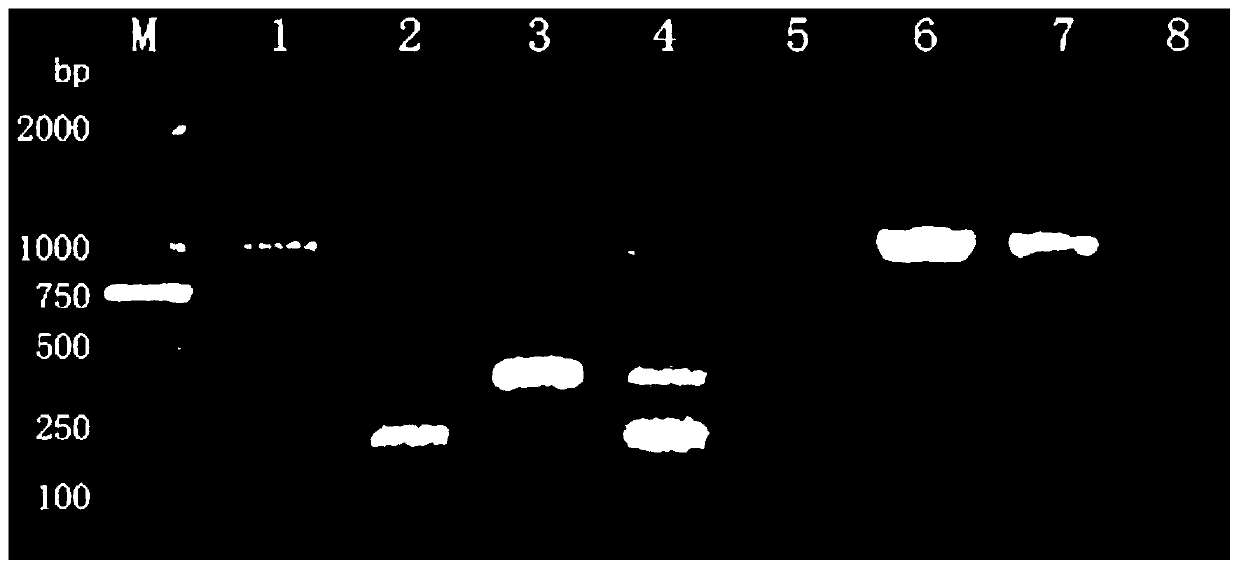
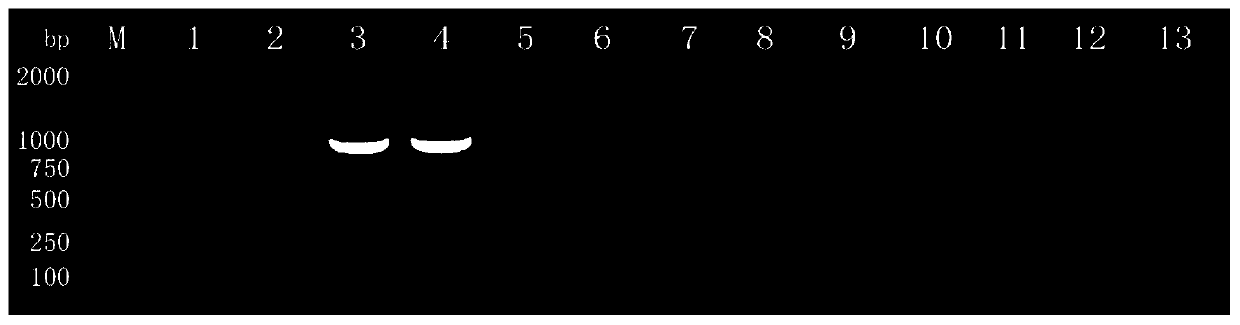
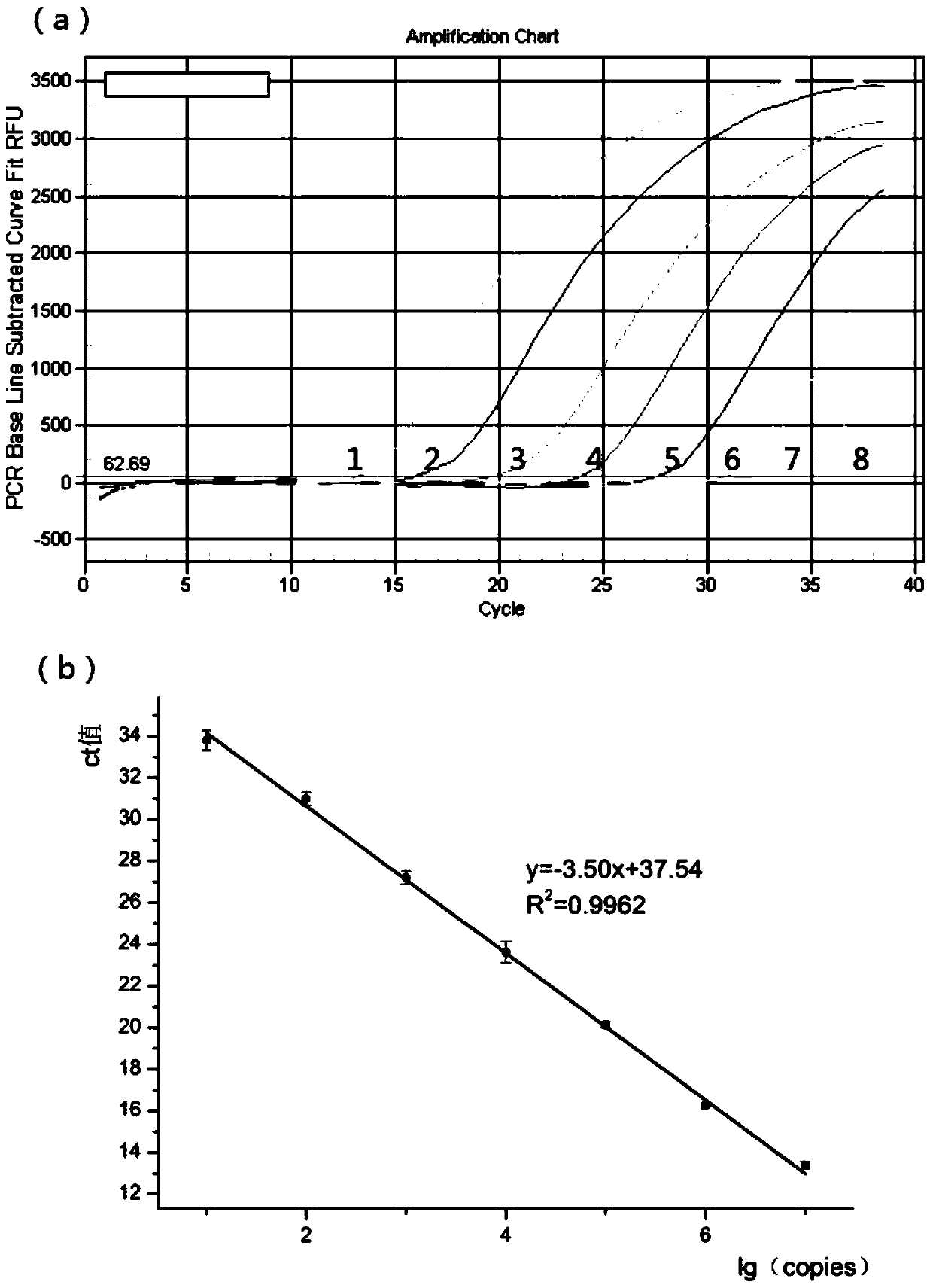
Patents
Literature
370 results about "African swine fever" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
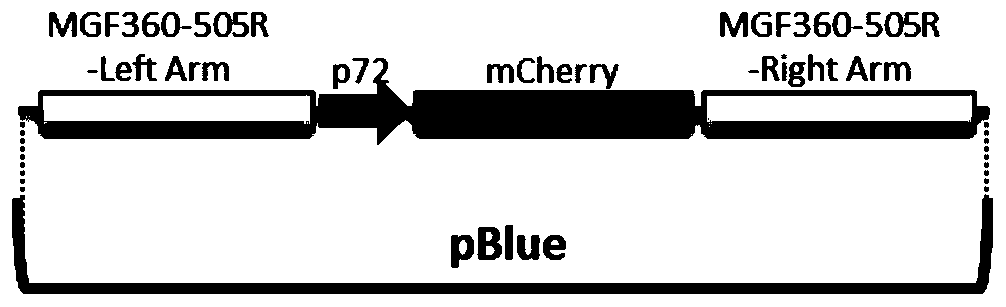
Gene deletion attenuated African swine fever virus and application thereof as vaccine
ActiveCN110093324AGood immune protectionFull Poison Attack ProtectionViral antigen ingredientsVirus peptidesAfrican swine feverGenetic engineering
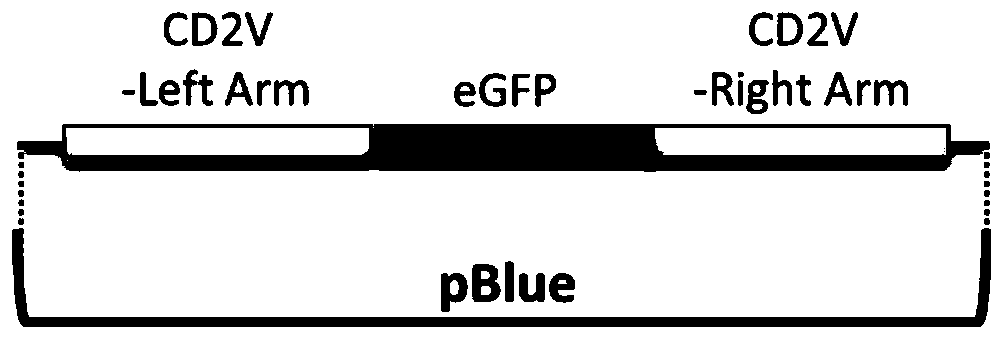
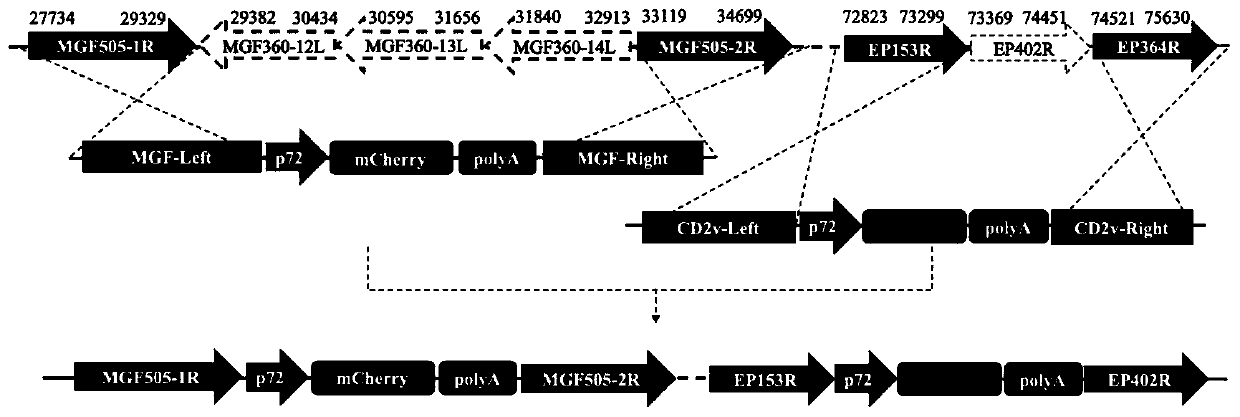
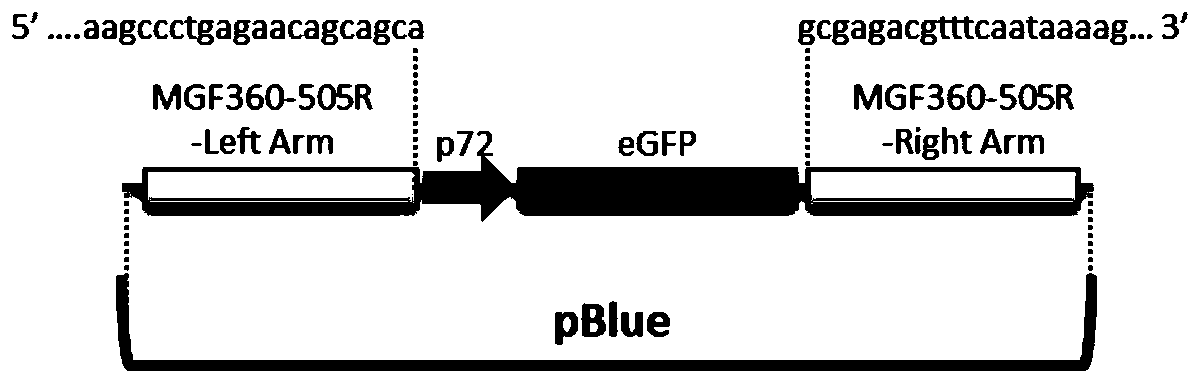
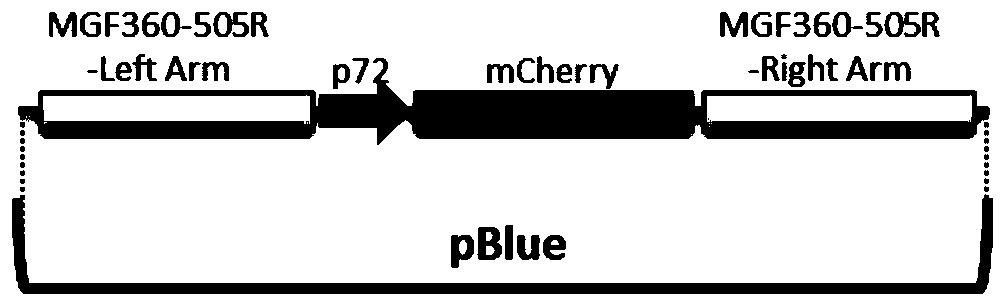
The invention relates to a gene deletion attenuated African swine fever virus as a vaccine and the vaccine, and a construction method thereof. An African swine fever Chinese epidemic strain Pig / CN / HLJ / 2018 is adopted, a virulence gene of the African swine fever virus is deleted by a genetic engineering technology, and the gene deletion virus of MGF360-505R deletion and joint deletion of CD2V and MGF360-505R is obtained. Experiments show that the two virus strains can provide 100% immune protection against the African swine fever Chinese epidemic virulent strains, can be used as vaccines for safe and effective prevention and control of African swine fever in China, and have great social value.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Four-gene-deletion weak-toxin strain for African swine fever viruses and application of four-gene-deletion weak-toxin strain
InactiveCN110551695AEasy to solveViral antigen ingredientsMicrobiological testing/measurementAfrican swine feverToxin
The invention discloses a four-gene-deletion weak-toxin strain for African swine fever viruses. The weak-toxin strain is the four-gene-deletion weak-toxin strain for an African swine fever virus SY18separation strain, and the following gene function protein is deleted: CD2v gene coding products and three multigene family genes( MGF360-12L, MGF360-13L and MGF360-14L ) coding products. The invention further discloses an application of the weak-toxin strain of the African swine fever viruses to preparation of vaccines for preventing or treating African swine fever. The weak-toxin strain of the African swine fever viruses can provide complete immunoprotection effect on attack of ASFV parent toxin strains, is high in safety, and is suitable for being used as vaccine candidate strains for preventing the African swine fever.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
CHO (Chinese hamster ovary) cell strain with high-efficiency expression of CD2V protein of African swine fever (ASF)
ActiveCN110078801AHigh expressionEasy to purifyVirus peptidesMicroorganism based processesAfrican swine feverChinese hamster
The invention provides CD2V protein of African swine fever (ASF) capable of being expressed with high-efficiency in a CHO (Chinese hamster ovary) cell strain. The amino acid sequence of the CD2V protein is shown in SEQ ID NO:4; a recombinant plasmid constructed by the invention is used for expressing the CD2V protein of an African swine fever virus in CHO cells; the invention further provides a recombinant CHO cell strain prepared by transfecting the CHO cells through the recombinant plasmid, the recombinant CHO cell strain can be used to prepare the CD2V protein, and the prepared protein canbe used for differential diagnosis of the African swine fever. According to the cell strain with the expression of the CD2V protein of the African swine fever, the expression quantity is high, purification is easy, the cell strain can be used for the differential diagnosis, and a solid foundation is laid for the production of subunit vaccines and diagnostic reagents of the African swine fever.
Owner:YEBIO BIOENG OF QINGDAO
Targeted genetic engineering vaccine for African swine fever immune system
ActiveCN110760006APrevent Dependency (ADE) EffectsGood immune effectAntibody mimetics/scaffoldsVirus peptidesImmune effectsAfrican swine fever
The invention belongs to the technical field of vaccines, and particularly relates to a targeted genetic engineering vaccine for an African swine fever immune system. The main raw material of the African swine fever immune system targeted genetic engineering vaccine is African swine fever fusion protein. The African swine fever fusion protein comprises a fragment selected from p72 protein, a fragment selected from p54 protein and a fragment selected from p30 protein. Wherein the fragment selected from the p72 protein at least comprises a sequence as shown in SEQ ID NO.1, the fragment selectedfrom the p54 protein at least comprises a sequence as shown in SEQ ID NO.2, and the fragment selected from the p30 protein at least comprises a sequence as shown in SEQ ID NO.3. The African swine fever fusion protein has the advantages of good immune effect and the like.
Owner:河南省生物工程技术研究中心 +1
A real-time isothermal recombinase-polymerase amplification detection kit for African swine fever viruses
ActiveCN106521027AQuick checkRapid diagnosisMicrobiological testing/measurementMicroorganism based processesForward primerAfrican swine fever
A real-time isothermal recombinase-polymerase amplification detection kit for African swine fever viruses is disclosed. A forward primer sequence for detecting the African swine fever viruses through a method provided by the kit is shown as SEQ ID NO:1, a reverse primer sequence is shown as SEQ ID NO:2 and a probe sequence is shown as SEQ ID NO:3. A real-time isothermal recombinase-polymerase amplification method provided by the invention for ASFV detection is simple and convenient in operation, rapid in reaction and low in detection cost, can be used for ASFV detection in a laboratory and on site, particularly ASFV detection in quarantine ports, airports and epidemic disease outbreak sites, and provides a novel and reliable technique support for ASF control in China.
Owner:HANGZHOU ZHONGCE BIO SCI&TECH CO LTD
African swine fever B and T cell tandem epitope fusion vaccine
InactiveCN111018995AGood immune effectAvoid the risk of accelerated viral infectionAntibody mimetics/scaffoldsViral antigen ingredientsAfrican swine feverTGE VACCINE
The invention, which belongs to the technical field of vaccines, particularly relates to an African swine fever B and T cell tandem epitope fusion vaccine. The main component of the African swine fever B and T cell tandem epitope fusion vaccine is African swine fever tandem epitope fusion protein. The African swine fever tandem epitope fusion protein comprises a B cell neutralizing epitope peptidefragment and a T cell epitope; and the B cell neutralizing epitope peptide comprises the following fragments: at least one neutralizing epitope peptide of each of p72, p54, p30 proteins. When the African swine fever tandem epitope fusion protein is used as a vaccine, the immune effect is good; and the antibody level significantly higher than that of a control group can be detected after one immunization. Since the non-neutralizing epitope is reduced as much as possible in the fusion protein, the risk of accelerating virus infection (ADE effect and antibody dependence enhancement effect) by anon-neutralizing antibody after immunization can be avoided.
Owner:河南省生物工程技术研究中心 +1
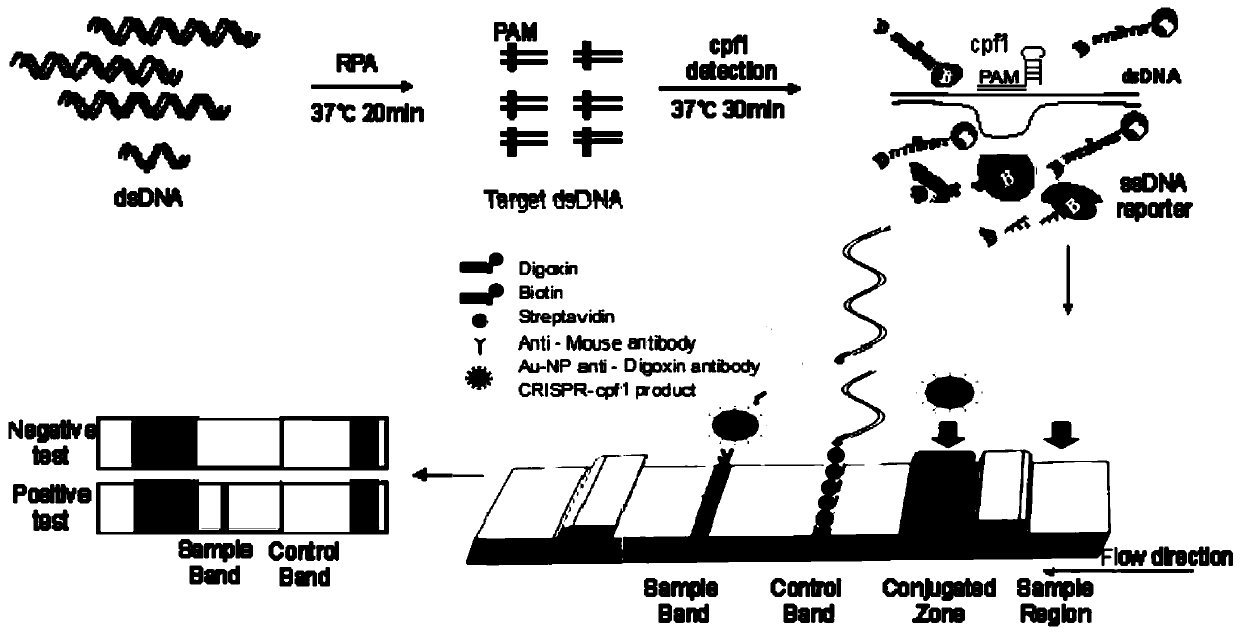
Cpf1 reagent kit and detection method for quickly detecting nucleic acid of African swine fever virus
ActiveCN110551846AHigh sensitivityStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationAfrican swine feverFluorescence
The invention discloses a Cpf1 reagent kit for quickly detecting nucleic acid of an African swine fever virus. The Cpf1 reagent kit comprises a Cpf1 detection system suitable for quickly detecting theAfrican swine fever virus, and an immune colloidal gold test strip, wherein the Cpf1 detection system comprises specific crRNA protein, specific Cpf1 protein and a single-chain DNA(ssDNA) reporting system in accordance with a p72 gene of the African swine fever virus, the specific crRNA is one or more of crRNAs from ASFV P72 crRNA1 to ASFV P72 crRNA10, and the sequence of the specific crRNA is SEQ NO.4 to SEQ NO.13; and the single-chain DNA(ssDNA) reporting system comprises ssDNA FQreporter for fluorescence detection of a microplate reader and / or ssDNA DB reporter for detecting the immune colloidal gold test strip. According to the Cpf1 reagent kit disclosed by the invention, for the first time, the Cpf1 is used for detecting the African swine fever virus, and has the advantages of beinghigh in sensitivity, high in specificity, short in time consumption, high in flux, independent of large-scale experiment equipment and the like. The advantages enable a detection method based on the immune colloidal gold test strip developed by the invention to be conveniently used in basic laboratories and breeding enterprises to be used for performing detection, identification and diagnosis on basic quick detection of the African swine fever.
Owner:SHANGHAI TECH UNIV
African swine fever P30 protein recombinant baculovirus expression vector and preparation method thereof
The invention provides African swine fever P30 protein recombinant baculovirus expression vector and a preparation method thereof. The method comprises: amplifying in plasmid PCR-4TOPO-P30 of ASFV (African swine fever virus) P30 full-length gene to obtain P39 gene, linking the amplified P30 gene to a baculovirus vector pFastBac 1 to construct recombinant baculovirus vector pFastBac1-ASFV-P30, converting into competent Escherichia coli cells DH10Bac to obtain recombinant shuttle bacmid rBacmid-ASFV-P30, transfecting to insect cells Sf9 after verification is correct to obtain recombinant baculovirus, and passage amplifying the recombinant baculovirus, linking baculovirus high in titer and containing ASFV P30 gene to High Five insect cells for eukaryotic expression of ASFV P30. The African swine fever P30 protein recombinant baculovirus expression vector is constructed by using the method, a recombinant baculovirus expression system is used to express African swine fever P30 protein in insect cells, and basis is laid for African swine fever ELISA (enzyme-linked-immunosorbent serologic assay) detections.
Owner:QINGDAO AGRI UNIV
PCR primer for detecting African swine fever virus, kit and application thereof
ActiveCN105695634AAccurate diagnosisEasy to distinguishMicrobiological testing/measurementDNA/RNA fragmentationAfrican swine feverNucleotide sequencing
The invention discloses a PCR primer for detecting an African swine fever virus, a kit and application thereof, and belongs to the detection field of the African swine fever virus. According to a conserved region of an ASFV p72 gene on the GenBank, four pairs of primers are designed, the primer with strong specificity and high sensitivity is screened out from the four pairs of primers, and the primer is composed of nucleotide sequences as shown in SEQ ID No.1 and SEQ ID No.2. The invention further discloses a kit prepared from the primer and used for detecting the African swine fever virus, and a corresponding PCR detection method is established. The detection method established by the invention is more specific and sensitive in comparison with two African swine fever virus PCR detection methods recommended by OIE; and the clinical sample detection result shows that the PCR detection method disclosed by the invention is simple in operation, low in cost, good in specificity and high in sensitivity, and can be effectively applied to the screening and fast diagnosis of the African swine fever.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Amphimorphic FQ-PCR detection reagent kit for identifying African swine fever and swine fever virus wild strains
PendingCN110184390ARapid identificationImprove throughputMicrobiological testing/measurementDNA/RNA fragmentationTonsilAfrican swine fever
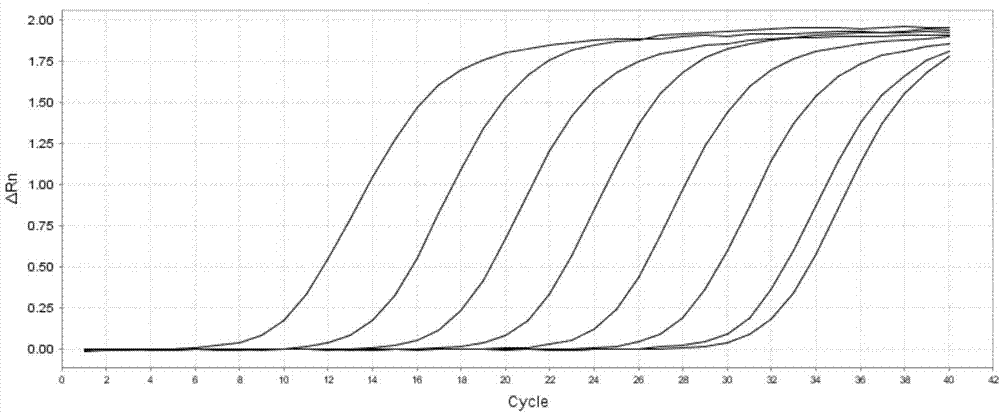
The invention provides an amphimorphic FQ-PCR detection reagent kit for identifying African swine fever and swine fever virus wild strains. A P72 gene of ASFV and a 5'UTR noncoding region of CSFV arerespectively used as an amplification target area, a pair of specific primers and a TaqMan MGB probe (SEQ ID NO:1-6) are designed, a real-time fluorescent quantitation PCR(FQ-PCR) technique is used, and identification and detection of ASFV and CSFV are realized. The detection reagent kit provided by the invention is suitable for detecting viral nucleic acid in samples of serum, spleen, lymph nodes, tonsil, kidney and the like of suspected ASFV or CSFV infected pigs, the sensitivity can reach 1.0*10<1>copy / [mu]L, and the detection reagent kit does not have any cross reactions with other pathogens which are liable to be in mixed infection with the ASFV and the CSFV or of which the infection symptoms are similar such as PRRSV, PRV, PCV2, PPV, JEV and HPS.
Owner:HENAN CENT FOR ANIMAL DISEASE CONTROL & PREVENTION
Attenuated African swine fever virus with gene deletion and its application as a vaccine
ActiveCN110093324BGood immune protectionFull Poison Attack ProtectionViral antigen ingredientsVirus peptidesAfrican swine feverGenetic engineering
The invention relates to a gene deletion attenuated African swine fever virus as a vaccine and the vaccine, and a construction method thereof. An African swine fever Chinese epidemic strain Pig / CN / HLJ / 2018 is adopted, a virulence gene of the African swine fever virus is deleted by a genetic engineering technology, and the gene deletion virus of MGF360-505R deletion and joint deletion of CD2V and MGF360-505R is obtained. Experiments show that the two virus strains can provide 100% immune protection against the African swine fever Chinese epidemic virulent strains, can be used as vaccines for safe and effective prevention and control of African swine fever in China, and have great social value.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
African swine fever fluorescent PCR (polymerase chain reaction) assay reagent, African swine fever fluorescent PCR assay kit and application thereof
ActiveCN106957927AIncreased sensitivityHigh homologyMicrobiological testing/measurementMicroorganism based processesFluorescenceAfrican swine fever
The invention relates to an African swine fever fluorescent PCR (polymerase chain reaction) assay reagent, an African swine fever fluorescent PCR assay kit and application thereof, which belong to the field of bioengineering. With the p72 gene as a reference, a pair of specific PCR primers and a TaqMan fluorescent probe are designed and optimized. Moreover, the primers are designed with the full length of the p72 gene to amplify the p72 gene, and the PCR product is cloned into a pGEM-T vector. Standard curves are drawn with a positive plasmid as a standard product, and the assay range is 10 to 1.0*10<7> copies. In the whole process of assaying African swine fever by an African swine fever fluorescent PCR method disclosed by the invention, only 2.5 to 3 hours are taken from DNA extraction to the appearance of an assay result, manual operation is greatly reduced, and time is greatly shortened. Furthermore, a plurality of samples can be assayed each time, and particularly assay of a large batch of samples can be realized.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Triple PCR detection primer and kit for rapidly distinguishing African swine fever virus wild strains from gene deletion strains
ActiveCN110551853AReduce testing costsShorten detection timeMicrobiological testing/measurementMicroorganism based processesAgricultural scienceAfrican swine fever
The invention discloses a triple PCR detection primer set for rapidly distinguishing African swine fever virus wild strains from CD2V and / or 360-505R gene deletion strains. Nucleotide sequences of three pairs of detection primers are as shown in SEQ ID NO: 1-6. Three pairs of primers are utilized to amplify three genes of African swine fever viruses CD2V, P72 and 360-505R at a time, so that the detection cost for identifying different genes is reduced, and the detection time for identifying different genes is shortened; and moreover, three genes with different lengths can be amplified only byone-time PCR reaction, and whether gene deletion exists in the strains or not can be distinguished. The three genes can be identified by one-time PCR amplification only, the cost is reduced by about 2 / 3 for a traditional method for respectively amplifying and detecting the samples of the three genes, and the triple PCR detection primer set for rapidly distinguishing the African swine fever virus wild strains from the CD2V and / or 360-505R gene deletion strains has wide market prospect.
Owner:SOUTH CHINA AGRI UNIV +1
Triple real-time fluorescent quantitative PCR kit for detecting African swine fever wild strains and gene deletion strain
InactiveCN111020062AHigh detection sensitivityStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationClassical swine fever virus CSFVAfrican swine fever
The invention provides a triple real-time fluorescent quantitative PCR detection primer for detecting an African swine fever wild strain and a gene deletion strain, and a kit and a detection method thereof. The triple fluorescent quantitative detection kit is developed and researched for three genes CD2V, VP72 and MGF-360 14L of the African swine fever virus by utilizing a multiple fluorescent PCRtest means, and whether a sample is infected with the African swine fever virus and whether gene deletion exists in the infected virus or not can be determined at the same time. The method can detecta large number of samples at the same time, provides an effective tool for scientifically and reasonably preventing and controlling African swine fever, guarantees the healthy development of the pigindustry, and has the advantages of convenience in operation, high sensitivity, strong specificity, short detection time and the like.
Owner:INST OF ANIMAL SCI & VETERINARY HUBEI ACADEMY OF AGRI SCI
Triple fluorescent RT-PCR (Reverse Transcription-Polymerase Chain Reaction) detection reagent for African swine fever viruses, swine fever viruses and respiratory syndrome viruses and preparation method and application thereof
InactiveCN104745731AMicrobiological testing/measurementMicroorganism based processesPositive controlAfrican swine fever
The invention discloses a triple fluorescent RT-PCR (Reverse Transcription-Polymerase Chain Reaction) detection reagent for African swine fever viruses, swine fever viruses and respiratory syndrome viruses and a preparation method and application thereof. Three sets of specific primers and Taqman probes as well as positive controls specific to African swine fever virus CP530R genes, swine fever virus 5 minute-UTR genes and swine respiratory syndrome virus NSP2 genes respectively are designed and synthesized, and a rapid, easy and convenient triple fluorescent RT-PCR detection system with high specificity and high sensitivity is established by using the three sets of primers and probes, so that nucleic acids of the African swine fever viruses, the swine fever viruses and the respiratory syndrome viruses can be detected synchronously from a detected sample within 3-4 hours in a rapid, accurate, specific, safe, easy and convenient way. The detection reagent can be applied to synchronous detection of the nucleic acids of trace African swine fever viruses, swine fever viruses and respiratory syndrome viruses in hogs and relevant samples thereof.
Owner:ANIMAL & PLANT & FOOD INSPECTION CENT OF TIANJIN ENTRY EXIT INSPECTION & QUARANTINE BUREAU
African swine fever polymerase chain reaction (PCR) detection method and oligonucleotide primer pair
ActiveCN103320536AStrong specificityGuaranteed to be correctMicrobiological testing/measurementMicroorganism based processesPig farmsDisease
The invention belongs to the technical field of biological detection and relates to an African swine fever polymerase chain reaction (PCR) detection method and an oligonucleotide primer pair. The method comprises the following steps: designing a pair of specific primers by referring to a complete genome sequence of 23 ASFVP72 protein strains recorded in GenBank, exploring the optimal reaction system and reaction conditions according to the designed primers, performing sensitivity test, specificity test and reproducibility test on the established PCR reaction method, and performing PCR method detection on clinical samples of sows suffered from reproductive disturbance or piglets with respiratory disturbance symptoms and aborted fetuses from 14 pig farms. The test results prove that the established PCR method can sensitively and rapidly detect the African swine fever virus. The PCR detection method established in the research has high applicability and can be used for detecting the African swine fever disease.
Owner:QINGDAO AGRI UNIV
African swine fever neutralizing epitope subunit vaccine
PendingCN111018996AImprove securityAvoid the risk of infectionViral antigen ingredientsVirus peptidesAfrican swine feverNeutralising antibody
The invention, which belongs to the technical field of vaccines, particularly relates to an African swine fever neutralizing epitope subunit vaccine that is mainly composed of African swine fever neutralizing epitope fusion protein. The African swine fever neutralizing epitope fusion protein mainly comprises a B cell neutralizing epitope peptide fragment including at least one neutralizing epitopepeptide of p72 protein, at least one neutralizing epitope peptide of p54 protein and at least one neutralizing epitope peptide of p30 protein. With the African swine fever neutralizing epitope fusionprotein, the risk of accelerating virus infection possibly caused by non-neutralizing antibodies can be effectively eliminated and the immune safety of the fusion protein can be effectively improved.Besides, when the African swine fever neutralizing epitope fusion protein is used for immunizing pigs, the antibody level obviously higher than that of a control group can be generated after one immunization.
Owner:河南省生物工程技术研究中心 +1
African swine fever virus vaccine and preparation method thereof
ActiveCN112876570AAntibody mimetics/scaffoldsVirus peptidesAntiendomysial antibodiesAfrican swine fever
The invention discloses an African swine fever vaccine and a preparation method thereof. According to the invention, the structural proteins P72, P30, P54 or CD2v-AC of the African swine fever virus are respectively displayed on the surface of the cage-shaped structure of the self-assembled ferritin, so that the humoral immune efficacy and width of the vaccine are improved, and the immunogenicity of the structural proteins of the African swine fever virus is improved. Structural proteins P30, P54 and CD2v are recombined to obtain recombinant proteins, and the recombinant proteins are connected with ubiquitin to obtain two recombinant proteins, so that the cellular immune effect of the structural proteins of the African swine fever virus is further improved, and better immune protection is provided for animals. The invention also provides a method for preparing the recombinant protein or the African swine fever vaccine. The African swine fever vaccine provided by the invention can initiate a wide neutralizing anti-African swine fever antibody, not only improves the immune efficacy, but also expands the immune range, provides effective immune protection for virulent infection, and has the potential of becoming a universal safe vaccine with multiple protection effects.
Owner:THE INST OF BIOTECHNOLOGY OF THE CHINESE ACAD OF AGRI SCI
Method for fast detecting loop-mediated isothermal amplification of African swine fever viruses
InactiveCN105463135AStrong specificityHigh sensitivityMicrobiological testing/measurementWater bathsAfrican swine fever
The invention discloses a method for fast detecting loop-mediated isothermal amplification of African swine fever viruses. An LAMP optimal reaction system is prepared from 25 microliters of matter which comprises l microliter of 8U Bst DNA polymerase, 2.5 microliters of a 10*ThermoPol buffer solution, 6 microliters of 2.5 mM each dNTPs, 4 microliters of 25 mM MgCl2, 1 microliter of outer primers F3 and B3 (5 micrometers), 1 microliter of inner primers FIP and BIP (50 micrometers), 1 microliter of a DNA template, 2.5 microliters of 10M bataine, and the balance ddH2O. An LAMP reaction is performed in a constant-temperature water bath kettle, reaction temperature is 63 DEG C, and reaction time is 45 min. The method is extremely high in specificity and sensitivity, fast, efficient, low in cost, easy and convenient to operate, capable of being effectively applied to fast diagnosis of African swine fever viruses, and applicable to field operation of an animal quarantine institution and a livestock farm.
Owner:SICHUAN AGRI UNIV
Real-time fluorescent PCR primer probe combination and kit for detecting African swine fever virus wild strains
ActiveCN110760617AEasy to controlImprove purification effectMicrobiological testing/measurementMicroorganism based processesAnimal virusAfrican swine fever
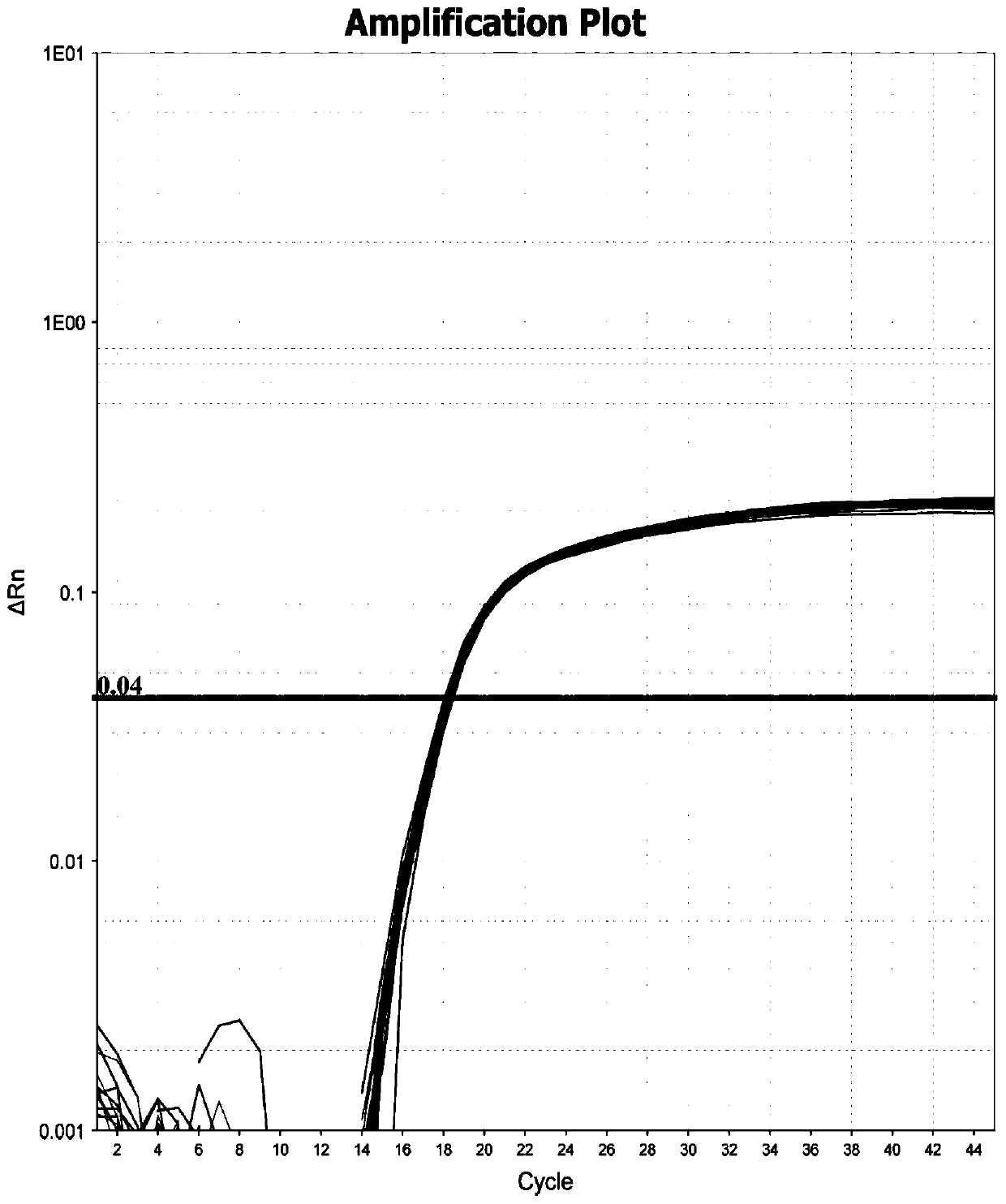
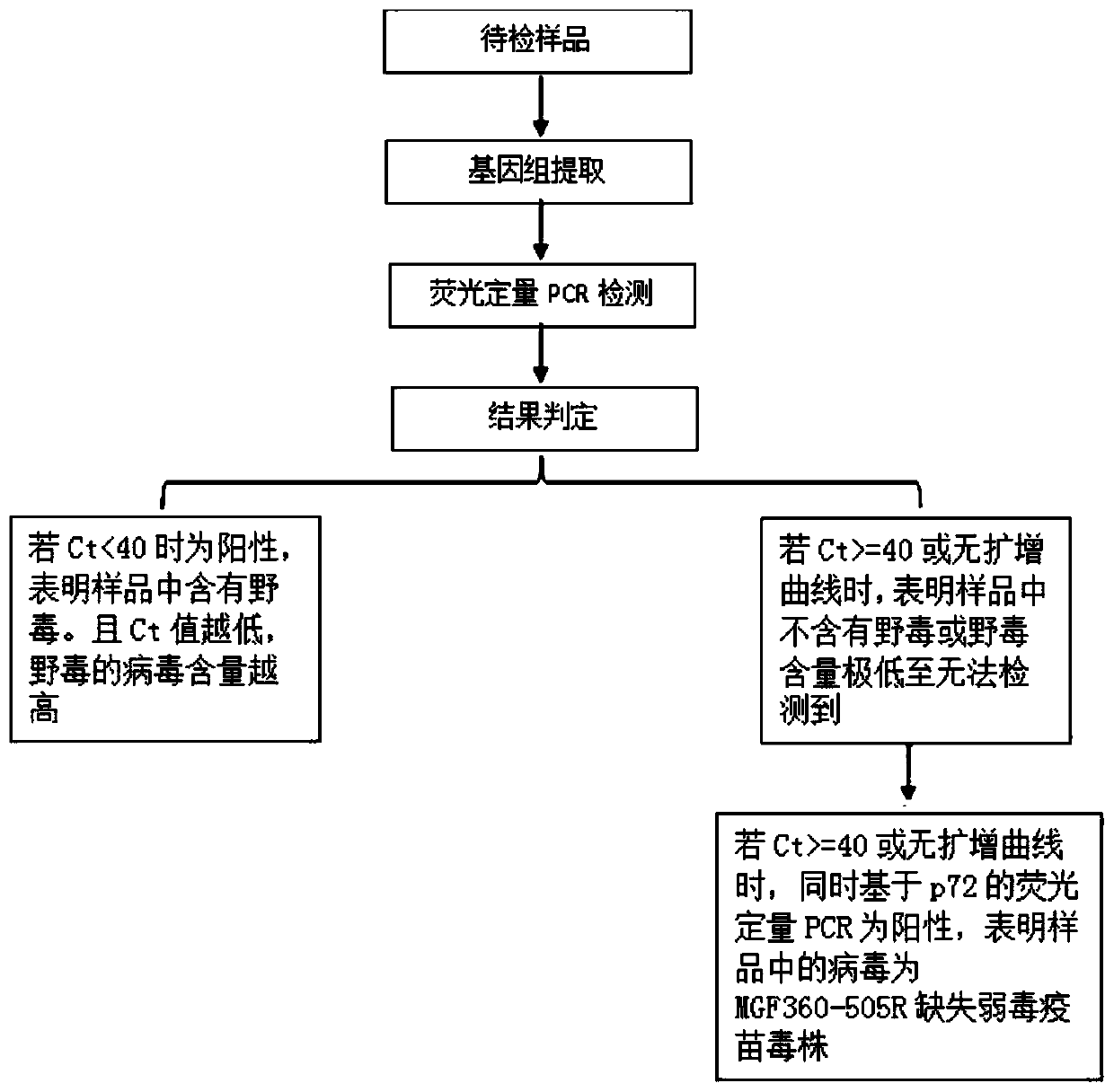
The invention relates to the technical field of animal virus detection in the field of veterinarians, and particularly discloses a real-time fluorescent PCR primer probe combination and a kit for detecting African swine fever virus wild strains. A PCR method based on the kit can not only specifically detect the African swine fever virus wild strains, but also serve as a method for distinguishing between the African swine fever virus wild strains and MGF360-505R loss attenuated vaccine virus strains when in use in combination with current p72 fluorescent PCR, therefore, important tools are provided for detecting the African swine fever virus wild strains and animals infected by the African swine fever virus wild strains, and control over African swine fevers and purification of the Africanswine fevers are promoted.
Owner:广纳达康(广州)生物科技有限公司
African swine fever cytotoxic T lymphocyte (CTL) epitope polypeptide and application thereof
ActiveCN110862435AImprove securityVerify securityVirus peptidesAntiviralsAfrican swine feverT lymphocyte
The invention provides an African swine fever cytotoxic T lymphocyte (CTL) epitope polypeptide and an application thereof, and belongs to the technical field of preparation of polypeptide vaccines. According to the invention, polypeptides which are derived from African swine fever virus (ASFV) and can be stably bound by major histocompatibility complex class I (MHC I) are screened through a bioinformatic method and a method for identifying binding motifs of MHC I, and the screened polypeptide has the capability of starting CD8+T cells to generate immune response and stimulating organisms to specifically generate CTL, wherein an amino acid sequence of the polypeptide is shown in any one of from SEQ ID NO.1 to SEQ ID NO.18. The polypeptide provided by the invention can be used for preparingAfrican swine fever specific epitope vaccines or polypeptide vaccines, has high safety and good specificity, and has a potential application value in prevention and control work of African swine fever.
Owner:CHINA AGRI UNIV
Condon optimized African swine fever virus P54 gene, nucleic acid vaccine and application thereof
InactiveCN103805615ATo solve the immune prevention and controlSame immune effectViral antigen ingredientsGenetic material ingredientsImmune effectsAfrican swine fever
The invention relates to a condon optimized African swine fever virus P54 gene and a nucleic acid vaccine based on the gene, wherein the nucleic acid vaccine consists of an eukaryotic expression vector and the condon optimized African swine fever virus P54 gene. The nucleic acid vaccine is constructed through the following steps of optimizing the condon of P54protein, designing a specific primer, transferring sequence of the artificially synthesized African swine fever virus P54 gene into a cloning vector and recombining the purified P54 gene into the eukaryotic expression vector. The nucleic acid vaccine can be used for preventing the occurrence of African swine fever and is a powerful tool for solving the immune prevention and control of the African swine fever. Compared with the traditional vaccines, the nucleic acid vaccine has the advantages of small quantity, security, long-term effect, stability and convenience in operation when reaching same immune effect.
Owner:孙洁
Primer group for simultaneously identifying wild strain and gene deletion strain of African swine fever based on multiple qPCR technology and test kit
InactiveCN111996191AReduce pollutionShorten the timeMicrobiological testing/measurementDNA/RNA fragmentationAfrican swine feverViral test
The invention provides a primer group for simultaneously identifying a wild strain and a gene deletion strain of African swine fever based on a multiple qPCR technology and a test kit, and belongs tothe technical field of virus detection. The primer probe group for simultaneously identifying the wild strain and the gene deletion strain of the African swine fever based on the multiple qPCR technology comprises a p72 gene specific primer probe, a CD2v gene specific primer probe and an MGF gene specific primer probe. The test kit comprises the primer probe group. Multiple qPCR detection is performed by adopting the test kit; p72 gene specific primers of a conserved gene of ASFV can amplify the wild strain and the gene deletion vaccine strain; specific primers of a CD2v gene and an MGF gene can only amplify the wild strain; and the three pairs of primers are combined for use, so that the wild strain and the gene deletion vaccine strain of the ASFV can be simultaneously identified. The primer group and the test kit have the advantages of accurate detection, sensitivity, high efficiency, low cost and the like, and have relatively high practical value for diagnosis of clinical samples and the breeding industry.
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI
Cell line capable of stably expressing protein P54 of African swine fever virus as well as preparation and application of cell line
ActiveCN107937349AStrong specificityGood passage stabilityVirus peptidesAntiviralsSerum igeAfrican swine fever
The invention provides a Vero cell line capable of stably expressing protein P54 of an African swine fever virus. The collection number of the cell line is CGMCC No.14316. The cell line not only can identify a polyclonal antibody of African swine fever, but also can generate specific reaction with positive serum of an African swine fever-infected pig. The monoclonal Vero cell line of an African swine fever P4 antigen can be used for stably expressing the protein P54 of the African swine fever virus, and can be used as a cell model or a virus infection model; the cell transmission stability ishigh, the growing speed is high, and culture conditions are simple; a large number of cell lines can be provided; the cell line has a good application prospect for researches on detection and pathogenesis mechanisms of the African swine fever.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Fluorescence quantitative PCR detection reagent for Asf virus and preparation method and use thereof
InactiveCN1840698AThe detection method is accurateFast detection methodMicrobiological testing/measurementFluorescence/phosphorescenceClassical swine fever virus CSFVAfrican swine fever
The related bio-reagent is designed by: with conservative African swine fever virus VP73 gene fragment as target object, designing and composing carefully for large quantities of primers and probes, taking optimal pairing screen test on different conditions to obtain the most proper primer and probe. The fluorescence quantitative RT-PCR testing reagent includes one couple of specific primers and one specific fluorescent probe, and the amplification fragment length is 66bp. This invention is fit to fast testing for African swine fever.
Owner:CHECKOUT & QUARANTINE TECH CENT YUNNAN ENTRY &EXIT CHECKOUT & QUARANTINE BUR
Recombinant African swine fever virus CD2V subunit protein as well as preparation method and application thereof
PendingCN111471089ABatch-to-batch stabilityImprove controllabilityViral antigen ingredientsVirus peptidesAdjuvantAfrican swine fever
The invention discloses a recombinant African swine fever virus CD2V subunit protein as well as a preparation method and application thereof. The protein comprises an extracellular region and an intracellular region of African swine fever virus surface envelope protein, and the amino acid sequence of the protein is shown as SEQ ID NO.3. The preparation method comprises the following steps: 1) cloning a codon-optimized gene sequence shown as SEQ ID NO.1 into an eukaryotic expression vector; 2) transfecting a recombinant expression vector containing the African swine fever virus subunit proteincoding gene into CHO cells; 3) culturing, screening and domesticating a CHO cell strain in the step 2) to obtain a highly-expressed cell strain; 4) fermenting and culturing the cell strain in the step3), and performing purifying to obtain the African swine fever virus CD2V subunit protein; and 5) mixing the CD2V protein with a pharmaceutically acceptable adjuvant to obtain a subunit vaccine. Theinvention can provide the African swine fever surface CD2V subunit protein which can be industrially produced on a large scale, the preparation method is simple and low in cost, and the prepared vaccine can reach the existing national standard.
Owner:NOVO BIOTECH CORP
Anti-African swine fever P30 protein single-domain antibody and ELISA kit for detecting African swine fever virus
ActiveCN111072774AQuick checkAccurate detectionBiological material analysisImmunoglobulins against virusesAbzymeElisa kit
The invention, which belongs to the technical field of virus detection, provides an anti-African swine fever P30 protein single-domain antibody and an ELISA kit for detecting the African swine fever virus. The amino acid sequence of the single-domain antibody p30-17 for resisting the African swine fever virus P30 protein is as shown in SEQ ID No. 1. In addition, the invention also relates to an ELISA kit for detecting African swine fever virus based on a double-antibody sandwich method. The ELISA kit comprises an elisa plate coated with a capture antibody being a single-domain antibody p30-17,an enzyme-labeled detection antibody being a single-domain antibody p30-30 of the anti-African swine fever virus p30 protein with an amino acid sequence shown as SEQ ID No.2, a standard positive reference substance, a serum diluent, a substrate developing solution A, a substrate developing solution B, a stop solution and a concentrated washing solution. The kit can realize rapid and accurate detection and has good detection specificity.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Rationally developed african swine fever attenuated virus strain protects against challenge with parental virus georgia 2007 isolate
ActiveUS9808520B1Viral antigen ingredientsMicrobiological testing/measurementAfrican swine feverDomestic pig
African swine fever virus (ASFV) is the etiological agent of a contagious, often lethal viral disease of domestic pigs. The control of African Swine Fever (ASF) has been hampered by the unavailability of vaccines. Experimental vaccines have been derived from naturally occurring, cell culture-adapted, or genetically modified live attenuated ASFVs; however, these vaccines are only successful when protecting against homologous viruses. We have constructed a recombinant Δ9GL / ΔUK virus derived from the highly virulent ASFV Georgia 2007 (ASFV-G) isolate by deleting the specific virulence-associated 9GL (B119L) and the UK (DP96R) genes. In vivo, ASFV-G Δ9GL / ΔUK administered intramuscularly to swine even at relatively high doses (106 HAD50) does not induce disease. Importantly, animals infected with 104 or 106 HAD50 are solidly protected against the presentation of clinical disease when challenged at 28 days post infection with the virulent parental strain Georgia 2007.
Owner:UNIV OF CONNECTICUT +1
CD2 deficient african swine fever virus as live attenuated or subsequently inactivated vaccine against african swine fever in mammals
InactiveUS20150165018A1Potential damageEliminate side effectsViral antigen ingredientsInactivation/attenuationHylochoerus meinertzhageniDomestic pig
The present invention is directed to a preferably live attenuated or subsequently inactivated African swine fever virus (ASFV), comprising a non-functional genomic CD2 gene, wherein such ASFV is non deficient in its replication, as well as to corresponding compositions or immunogenic compositions or vaccines, methods of production and uses for treating and / or preventing African swine fever in mammals, preferably of the family Suidae, for instance pigs, more preferably domestic pigs (Sus scrofa domesticus), wild pigs (Sus scrofa scrofa), warthogs (Potamochoerus porcus), bushpigs (Potamochoerus larvatus), giant forest hogs (Hylochoerus meinertzhageni) as well as feral pigs.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Polypeptides from African Swine Fever virus as vaccines for preventive and therapeutic use
InactiveUS20080131449A1Effective preventionEffective treatmentPeptide/protein ingredientsViral antigen ingredientsMammalAfrican swine fever
The present invention generally relates to the use of selected polypeptides from African Swine Fever virus for the prevention and therapy of African Swine Fever infections as well as other infections, including immune deficiencies in mammals and humans.
Owner:RATH MATTHIAS +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com