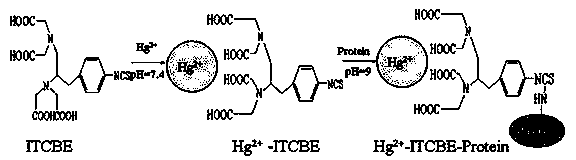
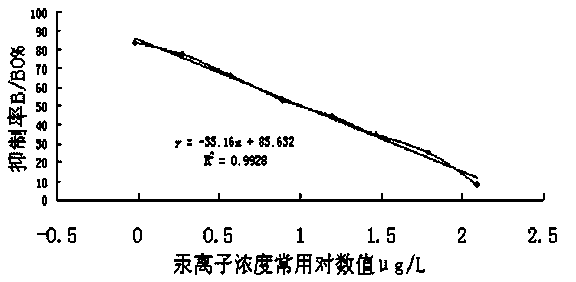
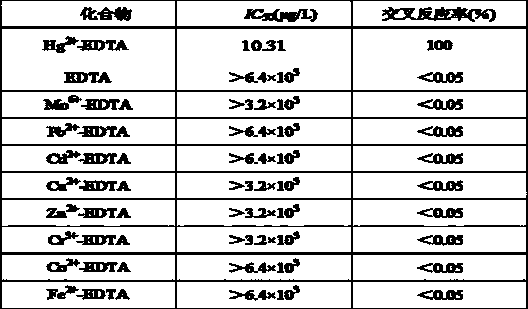
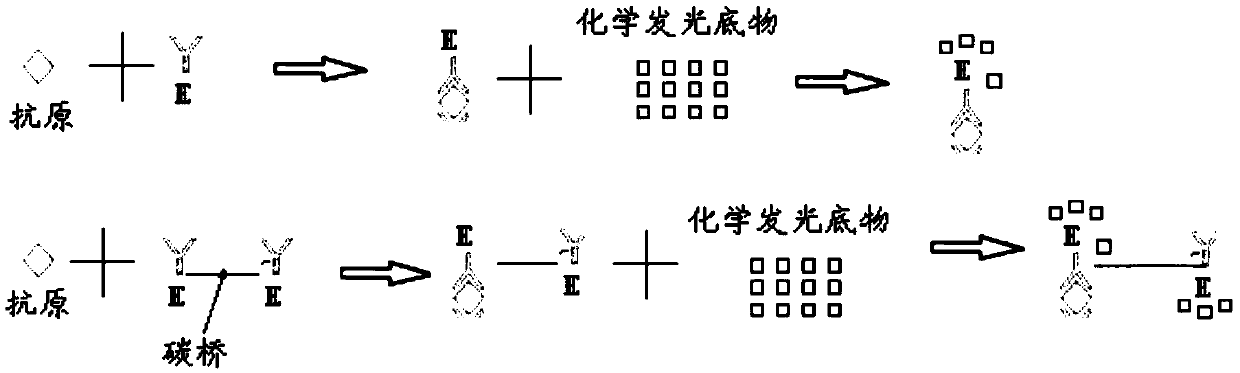
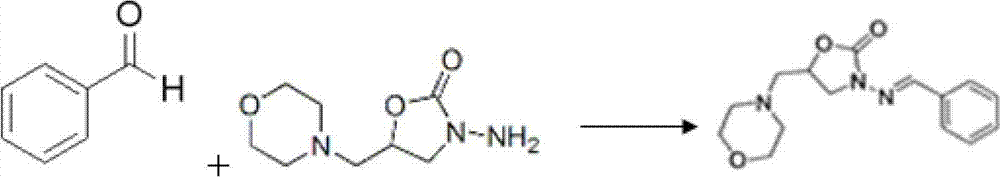
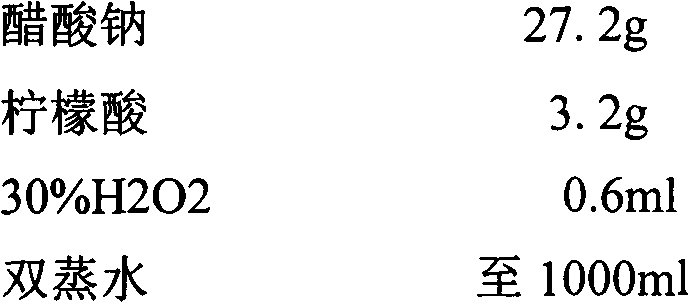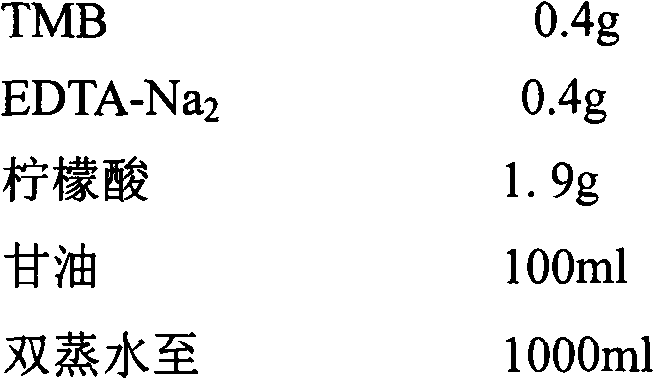Patents
Literature
236 results about "Abzyme" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An abzyme (from antibody and enzyme), also called catmab (from catalytic monoclonal antibody), and most often called catalytic antibody, is a monoclonal antibody with catalytic activity. Abzymes are usually raised in lab animals immunized against synthetic haptens, but some natural abzymes can be found in normal humans (anti-vasoactive intestinal peptide autoantibodies) and in patients with autoimmune diseases such as systemic lupus erythematosus, where they can bind to and hydrolyze DNA. To date abzymes display only weak, modest catalytic activity and have not proved to be of any practical use. They are, however, subjects of considerable academic interest. Studying them has yielded important insights into reaction mechanisms, enzyme structure and function, catalysis, and the immune system itself.
Catalytic monoclonal antibodies with protease activity for selective lysis of protein component of plaques aggregates pathological conditions
InactiveUS6387674B1Reduce usageUseful in treatmentNervous disorderPeptide/protein ingredientsAbzymeDisease
Catalytic monoclonal antibodies (abzymes) with selective protease activity in the pathologies characterized by the presence of plaques and fibrillar aggregates with protein component; methods for the preparation thereof and the use thereof as medicaments in the treatment of pathologies such as Alzheimer's disease, amyloidosis, atherosclerosis, prions diseases.
Owner:ABIOGEN PHARMA SPA
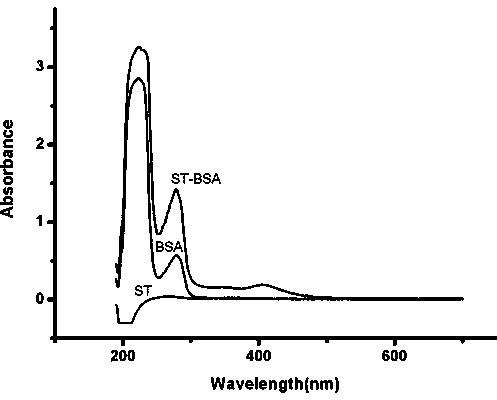
Hybridoma cell strain ST03, anti-aflatoxin biosynthesis precursor ST monoclonal antibody and application thereof
ActiveCN103849604ANo cross reactionHigh sensitivityTissue cultureImmunoglobulins against fungi/algae/lichensAbzymeAflatoxin biosynthesis
The invention relates to a hybridoma cell strain ST03, an anti-aflatoxin biosynthesis precursor ST monoclonal antibody and application thereof. The hybridoma cell strain ST03 with the preservation number of CCTCCNO.C2013187 can be used for preparing high-valence anti-aflatoxin biosynthesis precursor ST monoclonal antibody, wherein valence measured by an enzyme linked immunosorbent assay (ELISA) method of the anti-aflatoxin biosynthesis precursor ST mouse ascites antibody can reach 6.4*10<5>. The anti-aflatoxin biosynthesis precursor ST monoclonal antibody is high in sensitivity, has 50% inhibition concentration IC50 of 0.36 ng / mL on the aflatoxin biosynthesis precursor ST, has no cross reaction with the aflatoxin B1, aflatoxin B2, aflatoxin G1 and G2, and can be applied to measuring content of the aflatoxin biosynthesis precursor ST.
Owner:OIL CROPS RES INST CHINESE ACAD OF AGRI SCI
Hybridoma cell line 1C11 and anti-aflatoxin general monoclonal antibody generated by same as well as applications thereof
ActiveCN101993855AHigh sensitivityHigh practical application valueMicroorganism based processesTissue cultureCell strainHybridoma cell
The invention provides a hybridoma cell line 1C11 and an anti-aflatoxin general monoclonal antibody secreted by the same as well as the applications thereof. The hybridoma cell line 1C11 can be used for preparing a high-titer aflatoxin antibody, and a mouse hydroperitoneum antibody is measured to reach 5.12*106 by using an ELISA (Enzyme-Linked Immunosorbent Assay). The anti-aflatoxin general monoclonal antibody has high sensitivity, respectively reaches the IC50 (50% inhibiting concentration) of aflatoxin B1, B2, G1 and G2 to be 1.2, 1.3, 2.2 and 18.0 pg / mL, is the antibody with highest sensitivity among currently reported four aflatoxin antibodies, is used for measuring the total aflatoxin amounts, i.e. the total amounts of the aflatoxin B1, B2, G1 and G2 and has great practical application values.
Owner:INST OF OIL CROPS RES CHINESE ACAD OF AGRI SCI
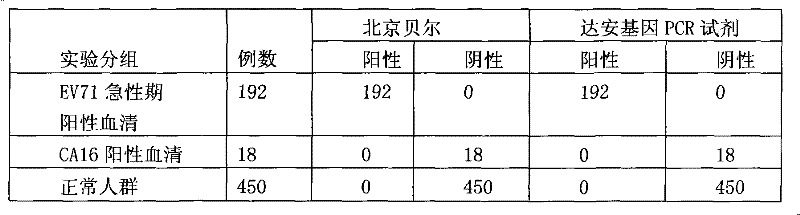
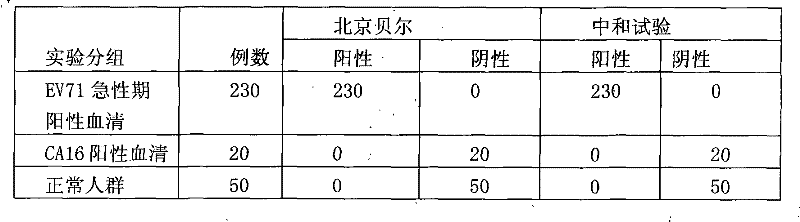
Diagnostic reagent kit (enzyme-linked immunosorbent assay (ELISA)) for enterovirus (EV) 71-type antibody (immune globulin M (IgM))
InactiveCN102243232AEasy to operateAvoid distractionsColor/spectral properties measurementsEnterovirusAbzyme
The invention relates to the field of biomedicine, in particular to an enzyme-linked immunization diagnostic reagent kit for detecting an enterovirus (EV) 71-type antibody (immune globulin M (IgM)), and a preparation method and application of the diagnostic reagent kit. The probability of hand-foot-and-mouth disease and severe infection (viral encephalitis, viral cerebrospinal meningitis and pulmonary edema) caused by EV71 type is relatively higher, and case fatality rate is relatively higher and can be 10 to 25 percent. The enzyme-linked immunization diagnostic reagent kit of the EV71-IgM antibody can be used for diagnosing the infection of the EV71 type. According to related documents about the detection of the EV71-IgM, EV71 virus cultures serving as indirect enzyme-linked immuno sorbent assay (ELISA) of envelope antigens has defects in such aspects as specificity, sensitivity and stability, and due to high cultivation cost and low efficiency, a large amount of virus cannot be supplied to the market. In order to overcome the defects, the invention provides the reagent kit which is used for detecting the EV71-IgM in human blood serum, required by clinical examination, simple and convenient to operate and applicable to all medical disease control departments, and the preparation method and the application of the reagent kit. The invention has the technical scheme that: firstly, the human blood serum is added into a micro-pore plate, wherein the IgM antibody is obtained by an anti-mu chain which is pre-enveloped on the micro-pore plate, and other uncombined components are washed and removed; secondly, an enzyme labeling object is added, the EV71-IgM in the obtained IgM can be combined with the specificity of an EV71 recombinant antigen which is labeled by horse radish peroxidase (HRP), and after washing, the HRP can react with substrates which are added subsequently; and finally, the aim of detecting the EV71-IgM antibody is fulfilled.
Owner:BEIJING BEIER BIOENG
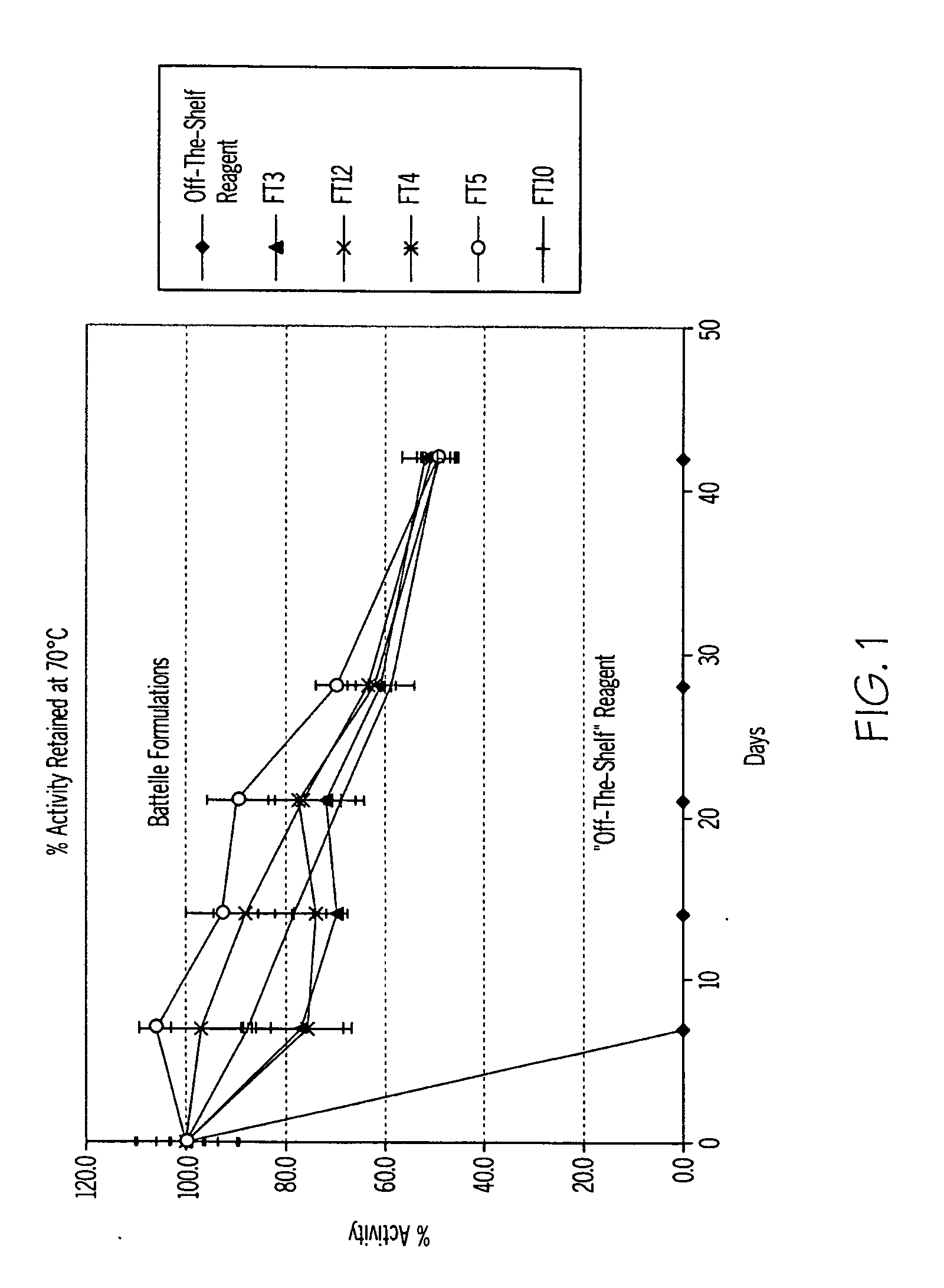
Protein stabilization
Owner:BATTELLE MEMORIAL INST
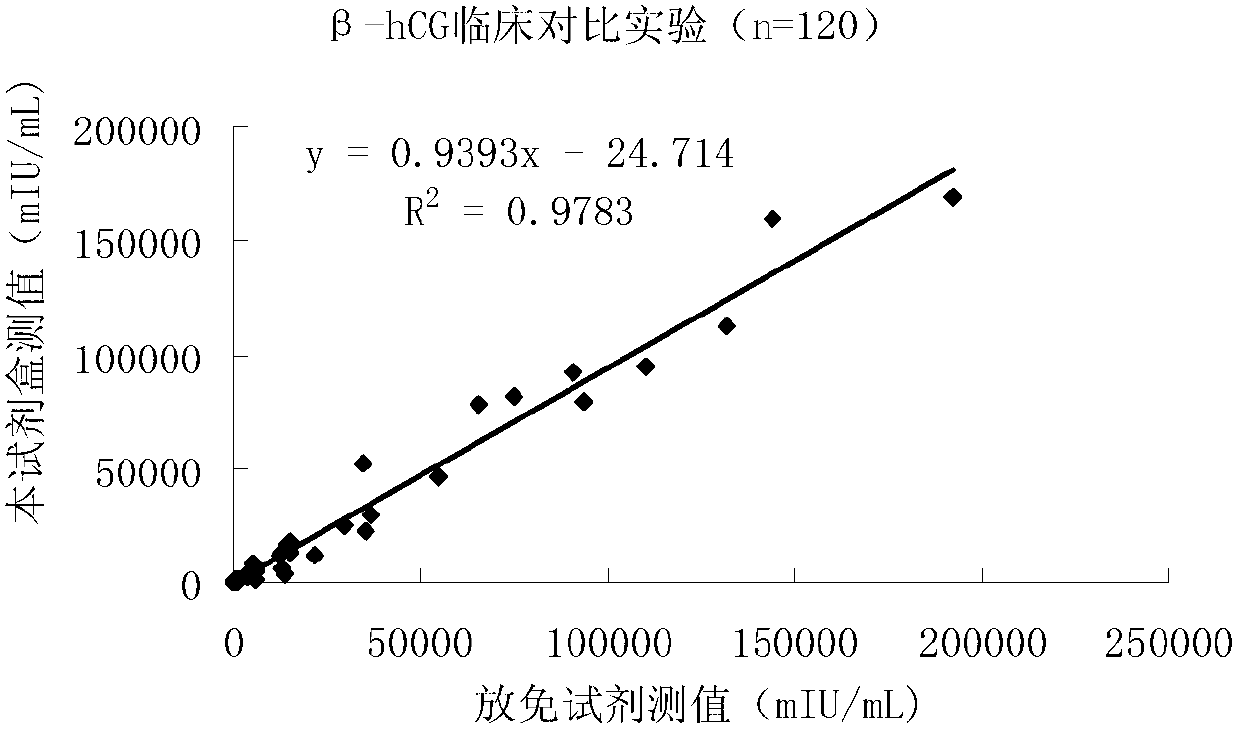
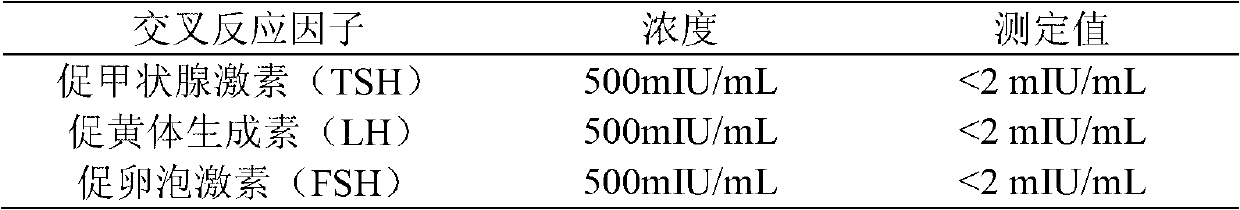
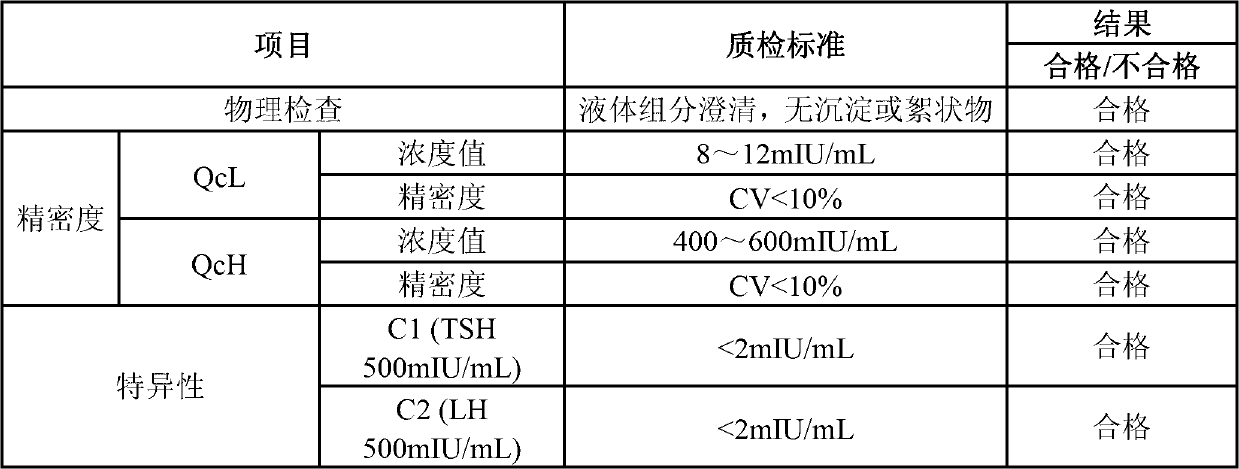
Quantitative detection kit combining magnetic particles with chemiluminescence immunoassay for beta human chorionic gonadotropin (beta-hCG), and preparation method of kit
ActiveCN102998467AImprove performanceLow cross-reaction coefficientBiological testingBiotin-streptavidin complexAbzyme
The invention discloses a quantitative detection kit combining magnetic particles with chemiluminescence immunoassay for beta human chorionic gonadotropin (beta-hCG). The kit comprises beta-hCG calibrators, magnetic particle suspension coupled with streptavidin, a beta-hCG antibody labeled with biotin, a beta-hCG abzyme combination, a beta-hCG quality controller, chemiluminescence liquor A, chemiluminescence liquor B, 20 times concentrated washing liquor, and a reaction tube, wherein enzyme adopted by the beta-hCG abzyme combination is horse radish peroxidase with the purity RZ being more than or equal to 3.0 and the activity being more than or equal to 250U / ml. The invention also discloses a preparation method of the kit. Compared with the conventional kit, the quantitative detection kit is simple and convenient to operate, is safe, does not cause environment pollution, and also has the advantages of wide concentration range, low cost, good stability and the like of detection samples.
Owner:BIOSCIENCE (TIANJIN) DIAGNOSTIC TECH CO LTD
Enzyme-linked immunosorbent assay (ELISA) kit for duck hepatitis virus type-I serum antibody, test method and application thereof
InactiveCN101839917AStrong specificityIncreased sensitivityDepsipeptidesFermentationAntigenDuck hepatitis A virus
The invention relates to an enzyme-linked immunosorbent assay (ELISA) kit for duck hepatitis virus type-I serum antibody and relates to a test method and application of the kit. The kit comprises an enzyme label plate coated by the recombinant VP1 (virus protein) protein, a rabbit anti-duck IgY antibody marked by horseradish peroxidase, a TMB substrate colour reagent, a positive serum, a negative serum and a kit specification. In the invention, by adopting the polymerase chain reaction, the VP1 genes are amplified from the DHV-1genome and the VP1 gene-containing recombinant expression plasmid pET32a-VP1 is constructed; the plasmid is transferred to host cells BL21 (DE3), and the in-vitro expression VP1 protein is purified by a nickel column and then used as the antigen; the enzyme-linked immunosorbent assay kit is established; the positive serum is the standard positive serum of duck hepatitis virus type-I and the negative control is the standard negative serum of duck. The test kit has the advantages of strong specificity, high sensitivity, simple operation, easy large-scale popularization and application, very important application value in diagnosis of duck hepatitis virus type-I, survey of epidemiology and immunization survey and the like.
Owner:HENAN UNIV OF SCI & TECH
Magnetic particle chemiluminescence immunoassay kit and assay method for human thyroglobulin antibodies (TGAb)
InactiveCN102901812AEasy to separateAdequate and prompt responseChemiluminescene/bioluminescenceAbzymeImmune complex deposition
The invention relates to a magnetic particle chemiluminescence immunoassay method for human thyroglobulin antibodies (TGAb) and belongs to the technical field of immunoassay. TG antigens marked by fluorescein isothiocyanate (FITC) and human IgG antibodies marked by alkaline phosphatase are combined to form an antigen-antibody-second enzyme-labeled antibody sandwich immune complex with a sandwich-like structure. Then, magnetic particles connected with FITC antibodies are added, the antigen-antibody complex is connected to the magnetic particles through specific combination of the FITC antibodies and the FITC and directly deposited in an external magnetic field, and the complex formed by immune reaction can be separated from the other non-combined substances without centrifuging. A kit combines chemiluminescence with the magnetic particles to provide a reaction system close to a homogeneous phase. Compared with the prior art, the kit has the advantages of higher sensitivity, wide linear range, quickness and the like; the cost of a product is greatly reduced; and the kit has a broad application prospect on the aspects of clinical examination and the like.
Owner:JIANGSU ZECEN BIOTECH CO LTD
Preparation of soluble human selenium-containing single-chain abzyme
InactiveCN101280015AEasy to prepareIncrease vitalityImmunoglobulins against animals/humansPeptide preparation methodsBiotechnologyEscherichia coli
The invention discloses a method to prepare soluble human selenium-containing catalytic single-chain antibodies, belonging to biotechnology field. The method includes the following steps: with glutathione derivatives as target antigen, filtrating the recombinant phage display human single-chain antibody library through immune affinity selection method to obtain single-chain antibodies B3 and D8; assembling the single-chain antibodies to secretory procaryon or eucaryon expression vector; translating colon bacillus or yeast cell; expressing and purifying the single-chain antibody proteins in procaryon or eucaryon; introducing GPX catalytic group SeCys at the substrate combining sites of the antibodies through chemical mutation method or directly expressing the soluble single-chain antibody proteins which contain GPX catalytic groups at the substrate combining sites with auxotrophic colon bacillus through genetic mutation techniques to endue the antibodies with GPX catalytic activity. The method of the invention is simple and the human selenium-containing catalytic single-chain antibodies prepared with the method are of high catalytic activity; the proteins expressed by the antibodies are soluble; therefore the method is good for large-scale production and is of broad application prospect in biological pharmacy.
Owner:JILIN UNIV
Hybridoma cell strain 2C9, anti-aflatoxin M1 monoclonal antibody produced by hybridoma cell strain 2C9 and application thereof
ActiveCN102220286AHigh sensitivityStrong specificityTissue cultureImmunoglobulins against fungi/algae/lichensAbzymeAssay
The invention provides a hybridoma cell strain 2C9, an anti-aflatoxin M1 monoclonal antibody produced by the secretion of the hybridoma cell strain 2C9 and application thereof. The hybridoma cell strain 2C9 is preserved in the China Center for Type Culture Collection, and the preservation number is CCTCC NO.C201018. The hybridoma cell strain 2C9 can be used for preparing high-titer anti-aflatoxin M1 monoclonal antibody, and a method of rat ascites antibody ELISA (Enzyma-linked Immunosorbent Assay) is adopted to detect the titer which can reach 4.26*106. The ant-aflatoxin M1 monoclonal antibody has high sensitivity, the 50% of inhibition concentration IC50 aflatoxin M1 caused by the anti-aflatoxin M1 monoclonal antibody is 67pg / ml, and the cross reaction rates between the anti-aflatoxin M1 monoclonal antibody and aflatoxin B1, between the anti-aflatoxin M1 monoclonal antibody and aflatoxin B2, between the anti-aflatoxin M1 monoclonal antibody and aflatoxin G1 and between the anti-aflatoxin M1 monoclonal antibody and aflatoxin G2 are respectively less than 0.1%. The anti-aflatoxin M1 monoclonal antibody can be used for quick detection of aflatoxin M1.
Owner:OIL CROPS RES INST CHINESE ACAD OF AGRI SCI
Sorghum mosaic virus tri-anti sandwich enzyme-linked immuno sorbent assay kit
InactiveCN101852807AHigh detection sensitivityStrong specificitySerum immunoglobulinsImmunoglobulins against virusesElisa kitPositive control
The invention discloses a sorghum mosaic virus tri-anti sandwich enzyme-linked immuno sorbent assay (TAS-ELISA) kit which comprises positive control, negative control, a sorghum mosaic virus polyclonal antibody, a sorghum mosaic virus monoclonal antibody, an enzyme labeled antibody and other materials and drugs, wherein the sorghum mosaic virus polyclonal antibody is obtained through rabbit immune separation serum after separating and purifying a sorghum mosaic virus sugarcane separator; and the sorghum mosaic virus monoclonal antibody is obtained by mouse immune, cell fusion and clone purification. The TAS-ELISA kit prepared by the invention has high detection sensitivity, strong specificity, high repeatability and high economic usefulness.
Owner:云南省农业科学院生物技术与种质资源研究所
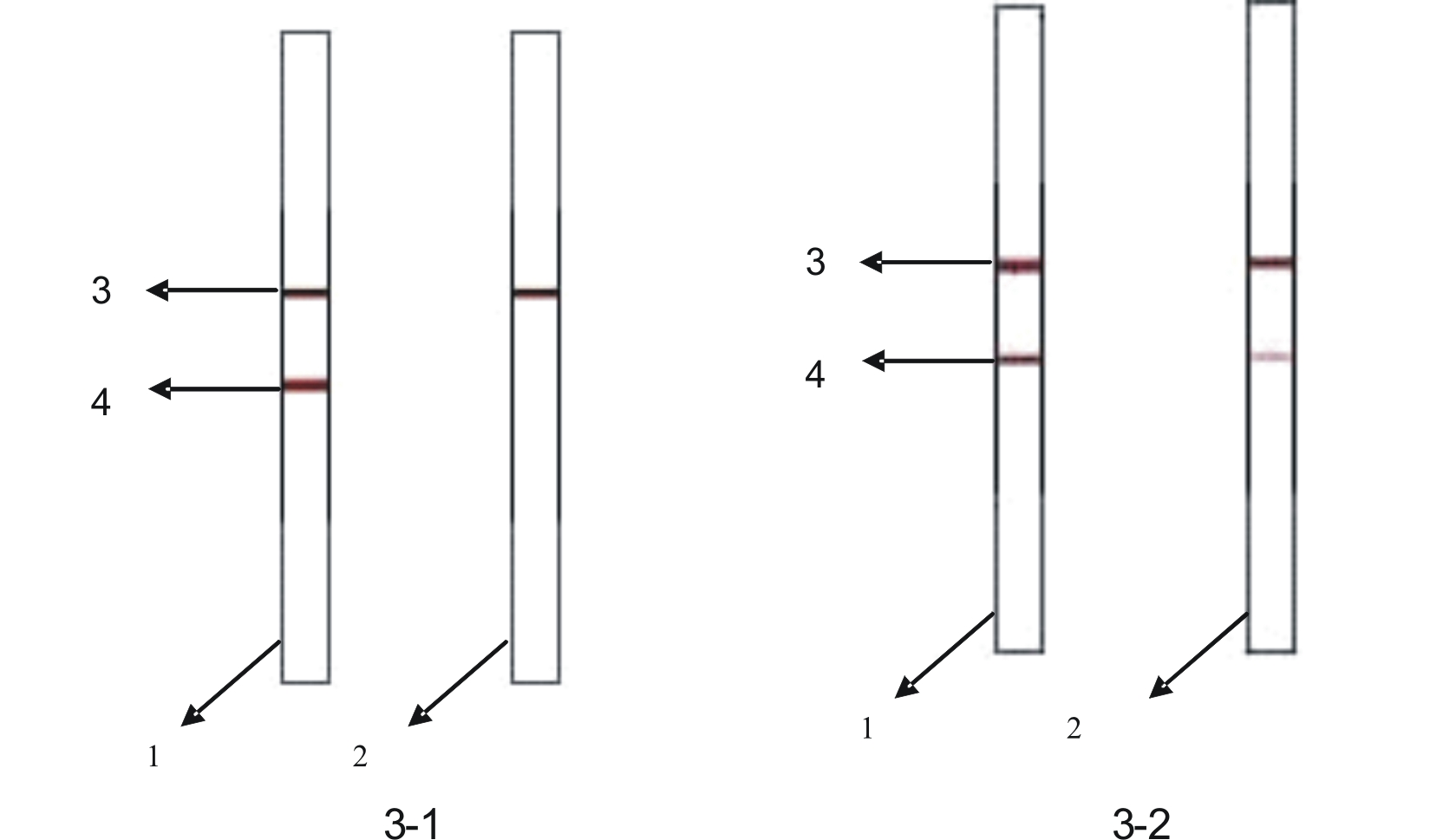
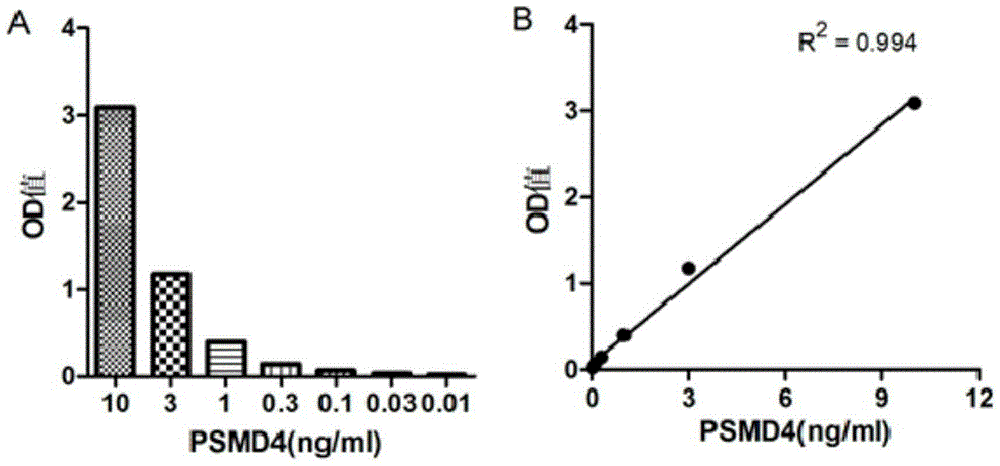
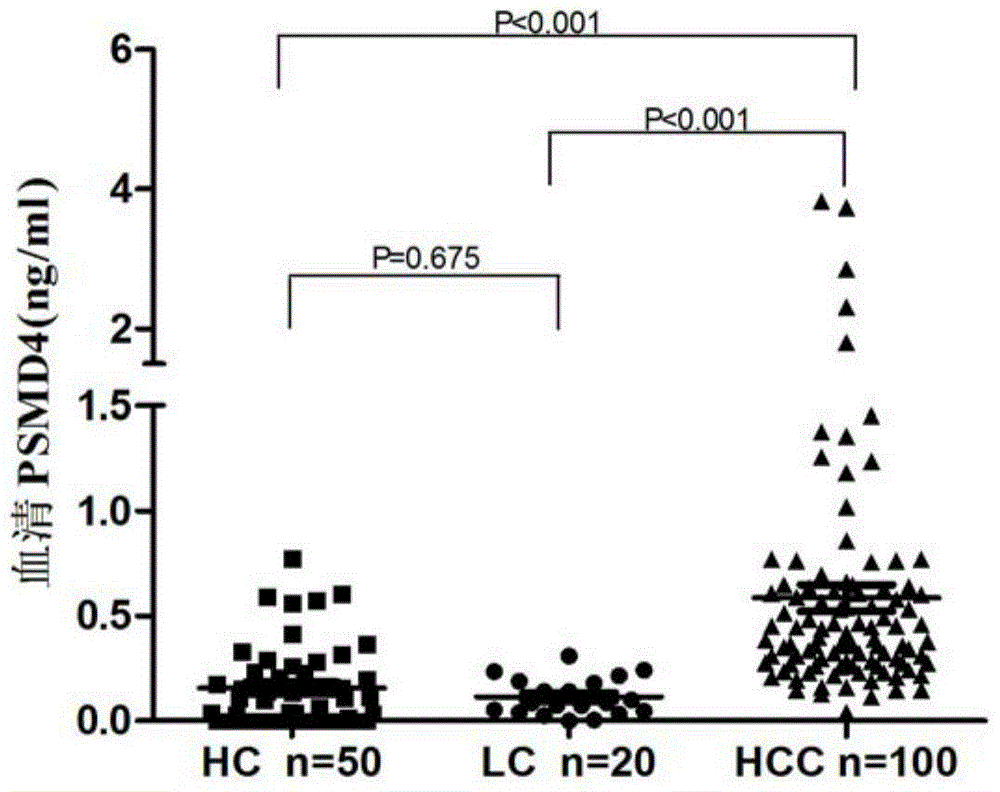
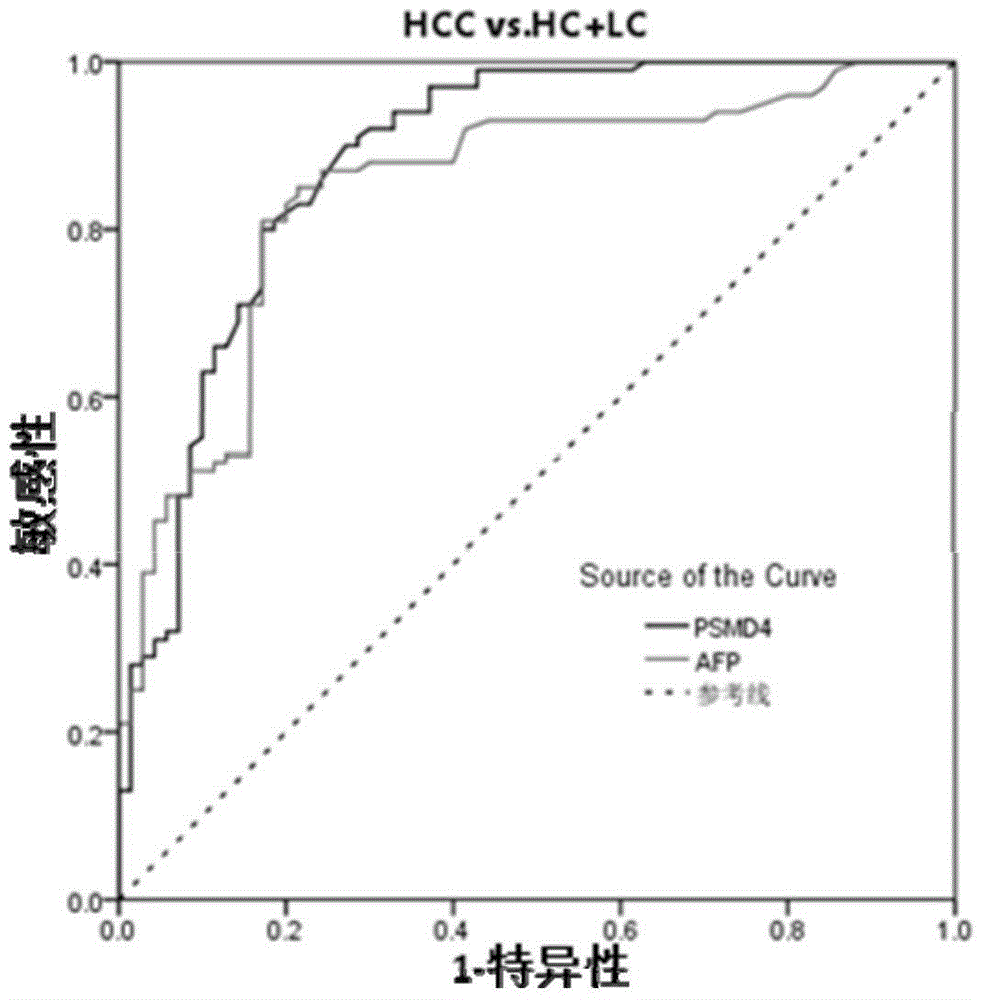
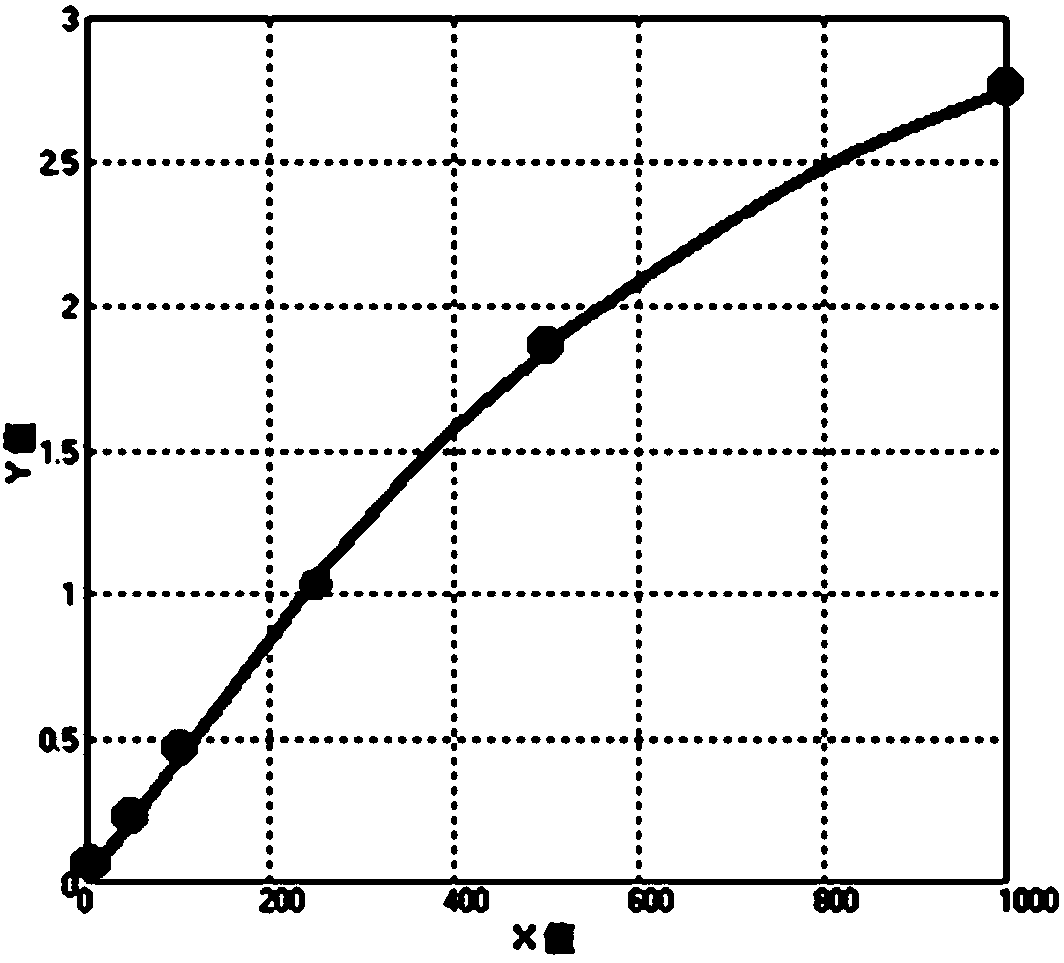
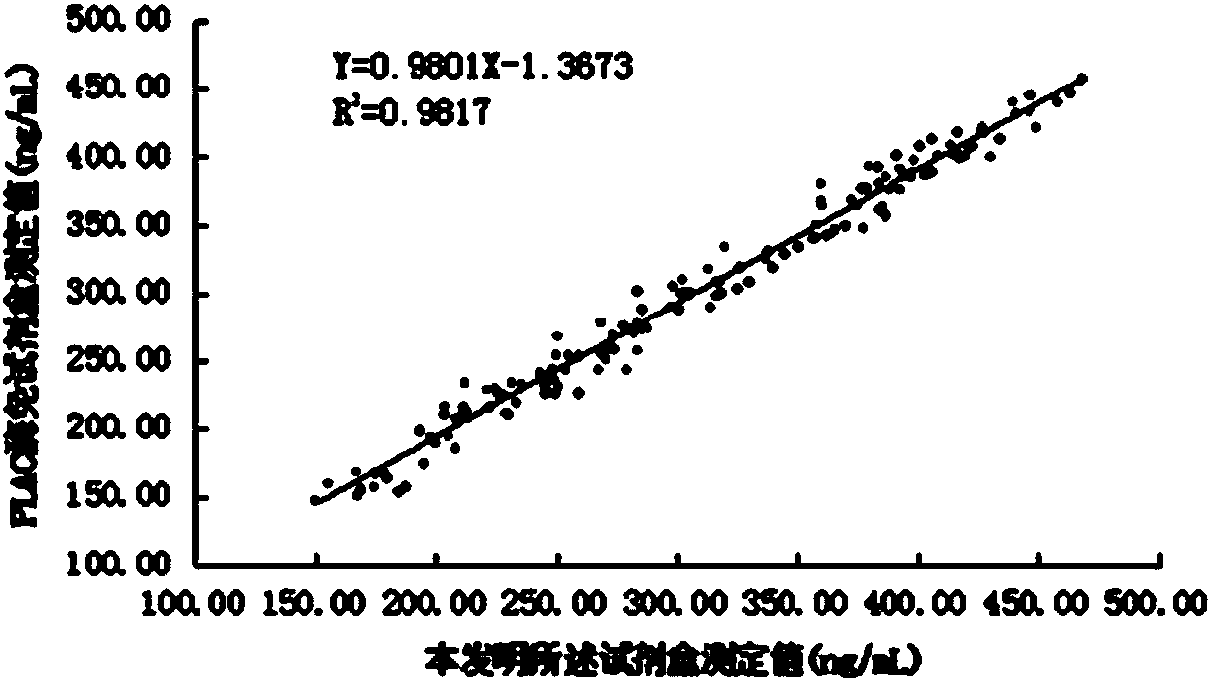
PSMD4 protein ELISA detection kit as well as detection method and application thereof
The invention belongs to the fields of immunology and biotechnology, and particularly relates to a PSMD4 protein ELISA detection kit as well as a detection method and application to serological diagnosis for liver cancer of the kit. According to extensive and deep research of the inventor, the expression level of PSMD4 protein in the serum of liver cancer patients can be detected according to an enzyme-linked immunosorbent assay (ELISA) method, and the PSMD4 protein is proved for the first time in the serum of the liver cancer patients. The kit comprises an ELISA plate coated with a PSMD4 antibody, a PSMD4 antigen detection antibody, an ELIAS secondary antibody, standard protein and the like. The invention further provides the detection method and application of the kit. The kit is simple and convenient to operate, can detect the content of the PSMD4 protein in the serum of a liver cancer patient correctly and highly sensitively, and provides a novel method for clinical examination and basic research.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Lipoprotein-related phospholipase A2 enzyme-linked immunosorbent assay (ELISA) kit and preparation method thereof
InactiveCN104007260AHigh sensitivityWide detection rangeBiological material analysisAbzymePhospholipase
The invention discloses a lipoprotein-related phospholipase A2 enzyme-linked immunosorbent assay (ELISA) kit and a preparation method of the lipoprotein-related phospholipase A2 ELISA kit. The lipoprotein-related phospholipase A2 ELISA kit comprises standard substances, quality control substances, a coating carrier, an enzyme marker, a color-substrate solution, an analysis buffer solution and a concentrated cleaning solution. The preparation method of the lipoprotein-related phospholipase A2 ELISA kit includes the first step of preparing the lipoprotein-related phospholipase A2 standard substances and the lipoprotein-related phospholipase A2 quality control substances, the second step of preparing the carrier wrapped by lipoprotein-related phospholipase A2 antibodies, the third step of preparing the enzyme marker of the lipoprotein-related phospholipase A2 antibodies, the fourth step of preparing the analysis buffer solution, the fifth step of preparing the concentrated cleaning solution, and the sixth step of assembling the lipoprotein-related phospholipase A2 ELISA kit. The lipoprotein-related phospholipase A2 ELISA kit can be used for replacing other kits for quantitative detection of lipoprotein-related phospholipase A2, and is low in cost, easy and convenient to operate, high in sensitivity and capable of being widely popularized and used clinically on a large scale.
Owner:TIANJIN KANGERKE BIOSCI
Hepatitis b virus surface antigen chemiluminescence immune assay determination kit and method for preparing same
InactiveCN101363861AGuaranteed SensitivityEasy to produce and operateChemiluminescene/bioluminescencePolyclonal antibodiesBiotin-binding proteins
The invention particularly relates to a kit for determining the surface antigen of the hepatitis B virus, and a preparation method thereof, which belongs to the medical field of immunological analysis. The kit comprises (1) a hepatits B virus surface antigen calibration material; (2) an avidin-coated carrier; (3) the polyclonal antibody or monoclonal antibody of a biotinylated hepatitis B virus surface antibody; (4) the polyclonal antibody or monoclonal antibody enzyme-labeled of the surface antibody; and (5) a chemiluminescent primer. Furthermore, the preparation method comprises the following steps of (1) preparing a calibration material from the pure surface antigen; (2) coating the carrier with avidin; (3) performing biotinylation of the polyclonal antibody or monoclonal antibody of the surface antibody; labeling the polyclonal antibody or monoclonal antibody of the surface body with an enzyme; (4) sub-packaging the calibration material and the chemiluminescent primer; and (5) assembling. The kit has the advantages of simpliness, rapidness, sensitiveness, stability and the like.
Owner:北京科美东雅生物技术有限公司
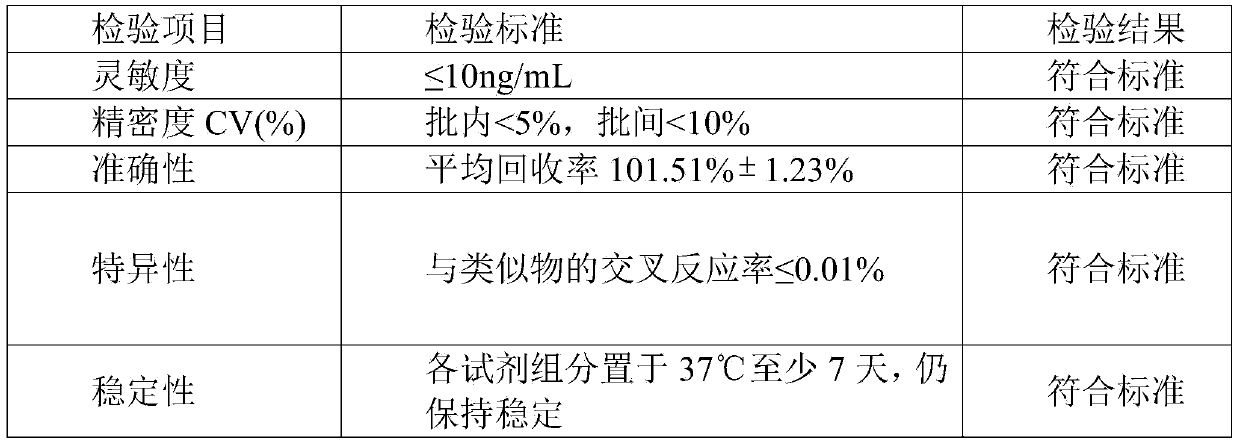
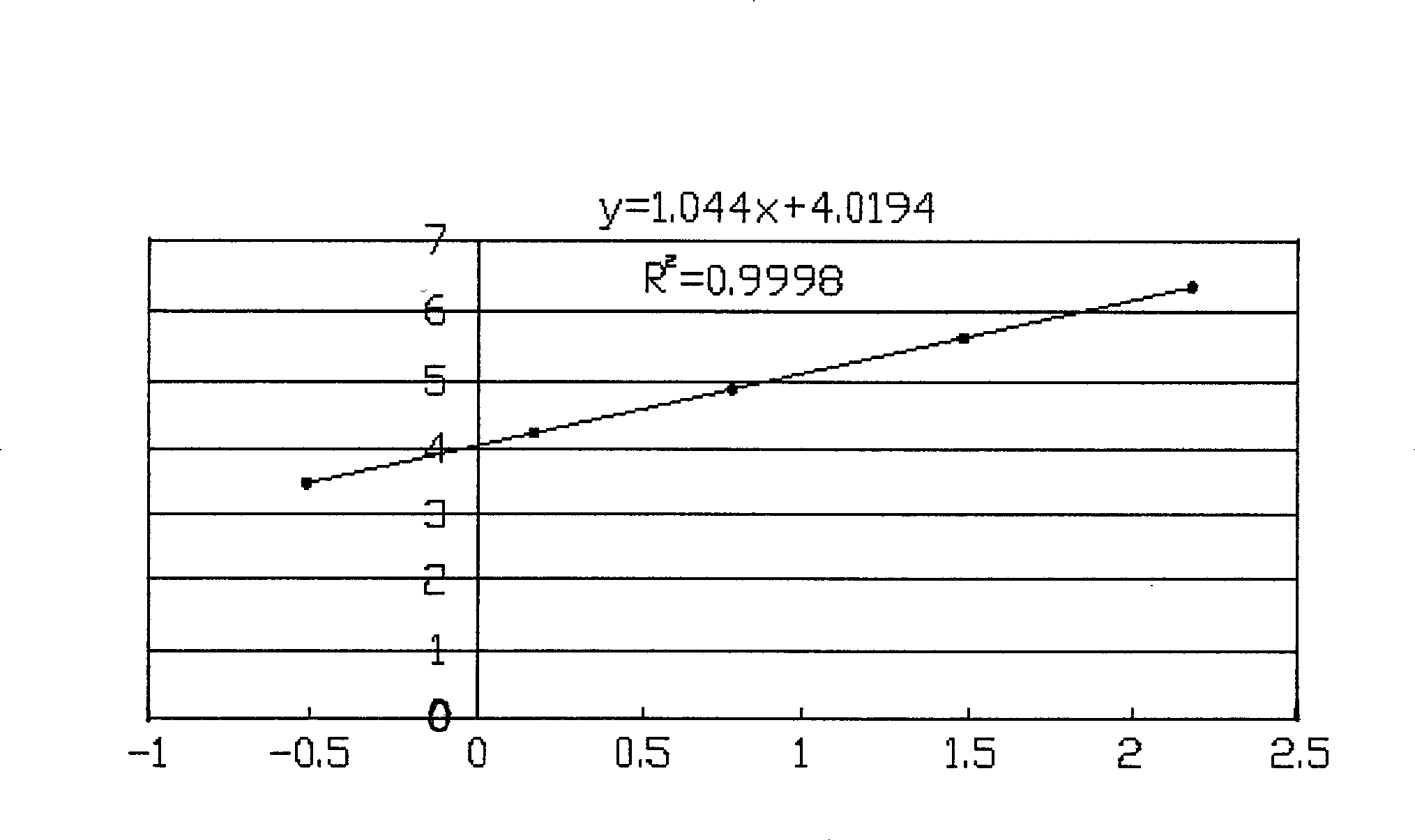
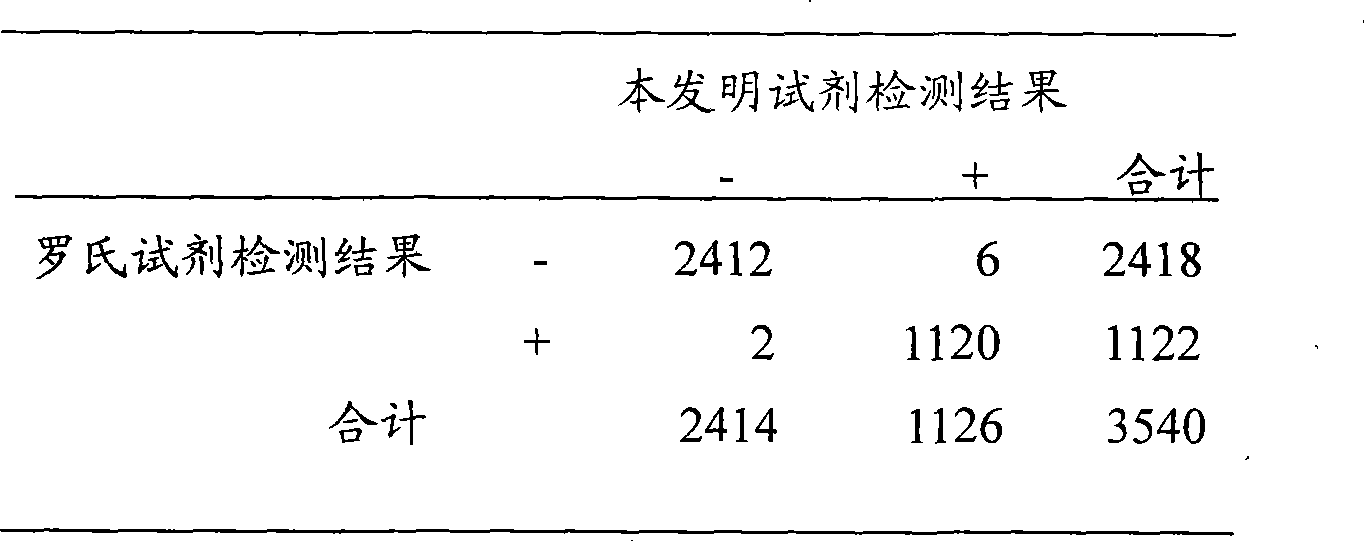
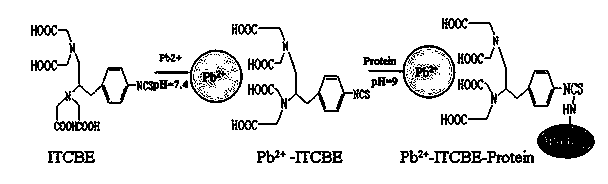
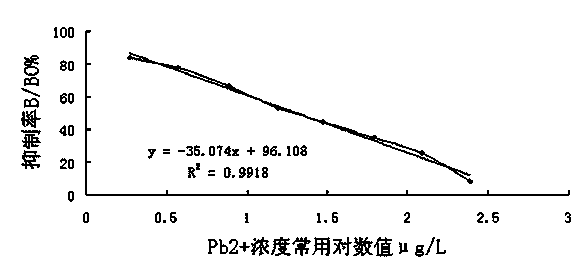
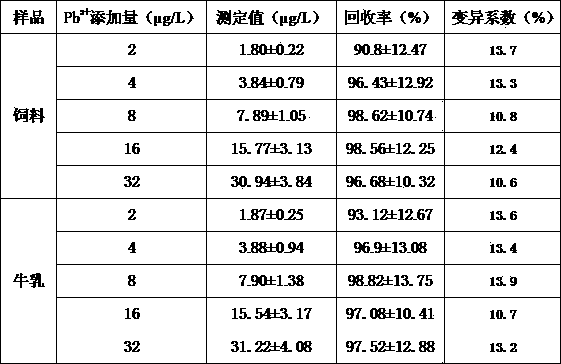
Indirect competition enzyme linked immunoreagent kit for detecting lead ions and manufacturing method thereof
InactiveCN103472230AResolving the immunogen
<u>sex</u>
questionHigh potencyMaterial analysisAbzymeAntigen
The invention relates to an indirect competition enzyme linked immunoreagent kit for detecting lead ions and a manufacturing method of the kit. An elisa plate enveloped by lead ion envelope antigens, lead-ion monoclonal antibodies, elisa second antibodies, a substrate color developing solution, a stop solution, a lead ion standard solution, a washing liquor concentrated solution and a sample treating liquid are arranged in the kit. The indirect competition enzyme linked immunoreagent kit can detect the lead irons in a trace mode, is used for detecting the lead iron contamination residual in the environment, the soil, water, foods and containers, and has the advantages of being rapid, easy and convenient to use, sensitive, peculiar, economical and the like. The kit is few in detection step and high in timeliness, saves detection time, reduces operation errors and can perform field detection. The kit not only can be used for screening samples in large batch, but also can perform rapid detection on samples in small batch, and provides effective means for food import and export inspection, food inspection, monitoring and evaluation of environmental pollution and the like.
Owner:HENAN INST OF SCI & TECH
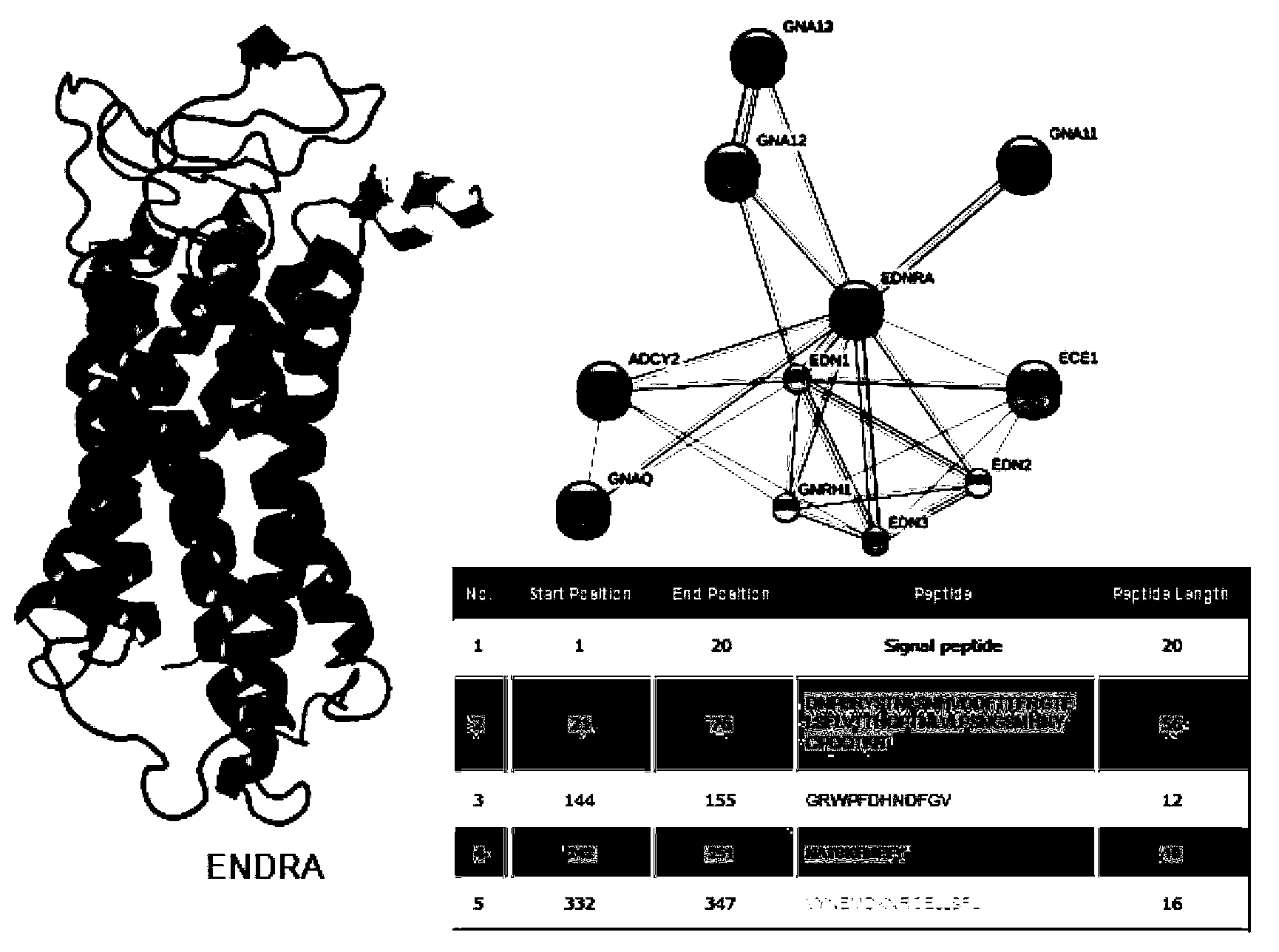
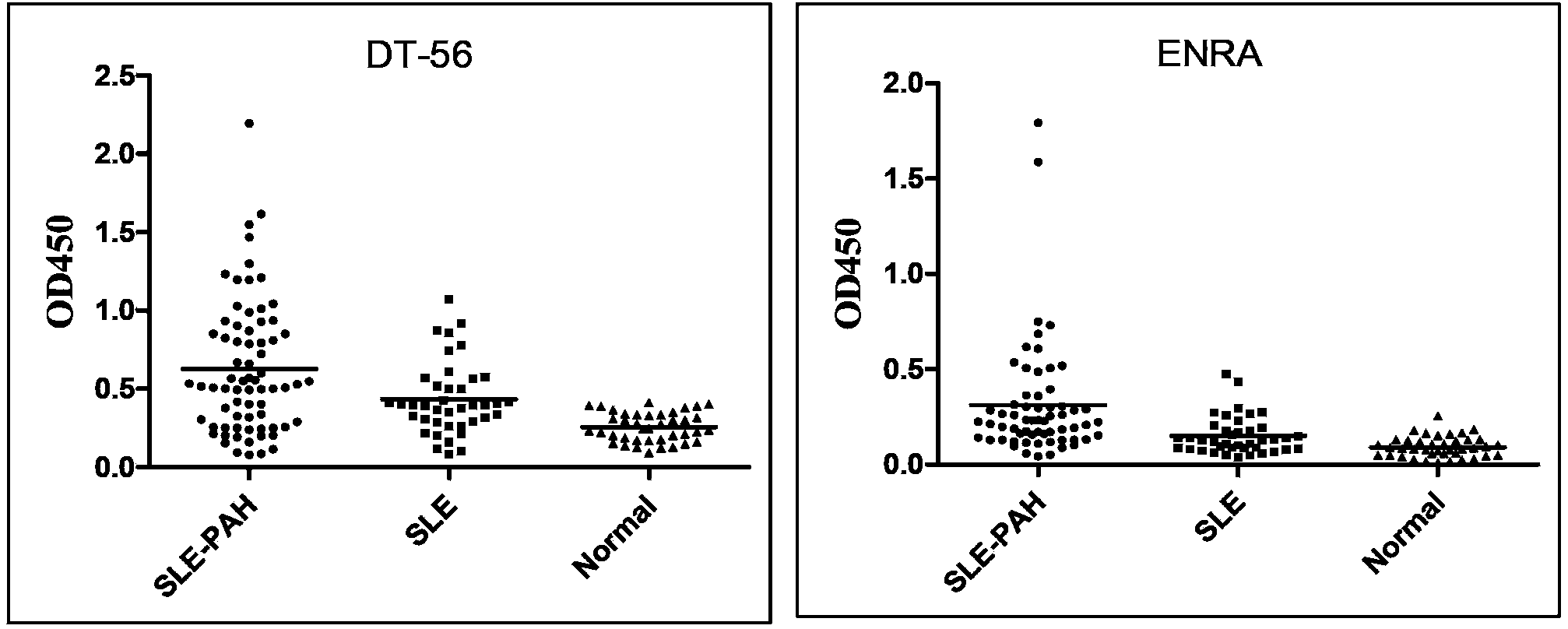
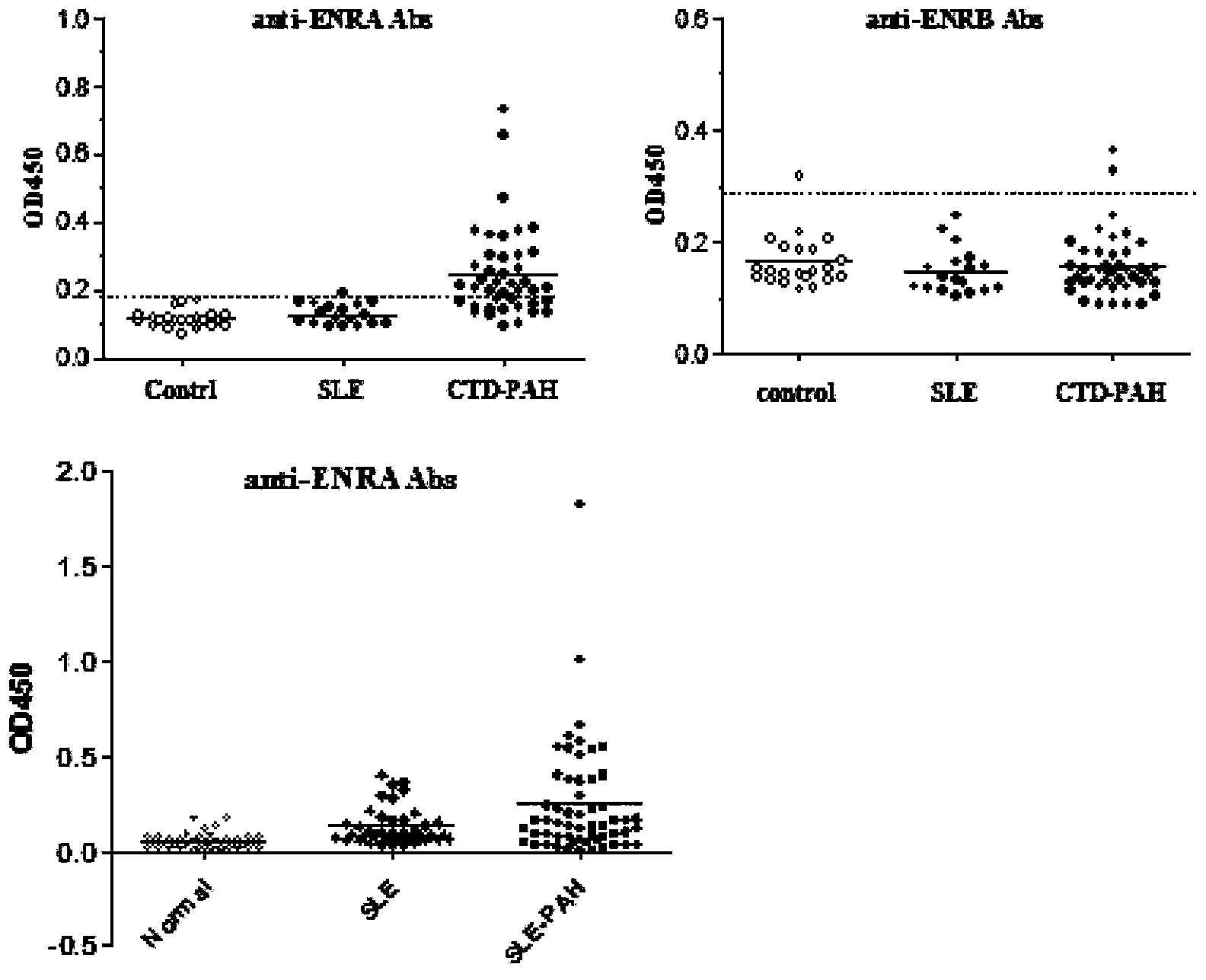
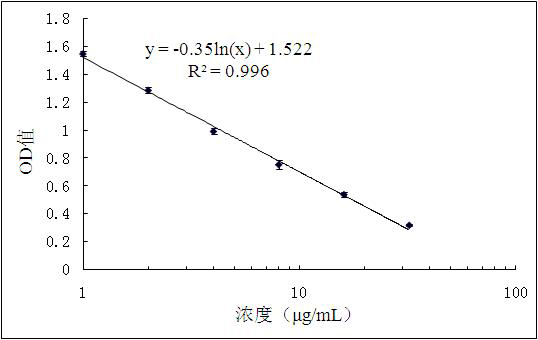
Enzyme-linked immunosorbent assay (ELISA) based on anti-ENRA (anti-endothelin receptor A) antibody of epitope antigen peptide and application thereof in CTD-PAH (connective tissue diseases-pulmonary arterial hypertension)
InactiveCN103728454ALow costImproving the practicality of clinical testingDisease diagnosisBiological testingAmino acidPulmonary hypertension
The invention relates to an enzyme-linked immunosorbent assay (ELISA) based on anti-ENRA (anti-endothelin receptor A) antibody of epitope antigen peptide; the enzyme-linked immunosorbent assay (ELISA) can be used in clinical tests of CTD-PAH (connective tissue diseases-pulmonary arterial hypertension); four extracellular peptide fragments with different lengths are synthesized in vitro, an epitope peptide fragment in good consistency with full-length ENRA is screened, the epitope peptide fragment is artificially synthesized to use as an antigen peptide package board to establish the enzyme-linked immunosorbent assay (ELISA) based on the anti-ENRA (anti-endothelin receptor A) antibody of the epitope antigen peptide; the epitope antigen peptide is a peptide fragment comprising the following amino acid sequence: DNPERYSTNLSNHVDDFTTFRGTELSFLVTTHQPTNLVLPSNGSMHNYCPQQTKIT; the enzyme-linked immunosorbent assay reduces the cost using full-length eukaryotic-expression endothelin receptor as a substrate, improves the clinical detection practicality, becomes a biomarker of CTD-PAH (especially SLE(systemic lupus erythematosus)-PAH), and provides valuable information for clinical diagnosis and treatment decisions.
Owner:吴庄民
Bird's-nest enzyme linked immunosorbent assay kit
InactiveCN102621329AIn favor of legitimate rights and interestsImprove accuracyBiological testingAbzymeElisa kit
The invention provides a bird's-nest enzyme linked immunosorbent assay kit, which comprises sialoglycoprotein standard liquid, a sialoglycoprotein antibody, an enzyme-labeled secondary antibody, an ELISA plate and substrate color development liquid. The bird's-nest enzyme linked immunosorbent assay kit can be used for quantitatively testing bird's-nests and relevant products of the bird's-nest, and is wide in application range, fine in specificity, high in sensitivity and fine in matrix interference resistant effect, accuracy of detection results and repeatability and low in detection cost, and field detection and screening of large quantities of samples can be carried out conveniently.
Owner:SHENZHEN ACAD OF METROLOGY & QUALITY INSPECTION
Indirect competition enzyme linked immunoreagent kit for detecting mercury ions and manufacturing method thereof
The invention relates to an indirect competition enzyme linked immunoreagent kit for detecting mercury ions and a manufacturing method of the kit. An elisa plate enveloped by mercury ion envelope antigens, mercury-ion-resisting monoclonal antibodies, elisa second antibodies, a substrate color developing solution, a stop solution, a mercury ion standard solution, a washing liquor concentrated solution and a sample treating liquid are arranged in the kit. The kit can detect the mercury irons in a trace mode, is used for detecting the mercury irons in the environment, the soil, water, foods, medicines, cosmetics and the like, and has the advantages of being rapid, easy and convenient to use, sensitive, peculiar, economical and the like. The kit is few in detection step and high in timeliness, saves detection time, reduces operation errors and can perform field detection. The kit is low in requirement for pretreating samples, simple in treatment process, not only can be used for screening the samples in large batch, but also can perform rapid detection on the samples in small batch, and not only provides technical support for environment and food safety, but also provides effective technological means for food import and export inspection, food inspection, monitoring and evaluation of environmental pollution and the like.
Owner:ZHENGZHOU UNIV
Detection kit and preparation method thereof and detection method of troponin T
ActiveCN109596835AImprove coating efficiencyDoes not affect activityBiological testingBiotin-streptavidin complexAntigen
The invention belongs to the technical field of in-vitro detection, and particularly relates to a detection kit and a preparation method and application thereof. The detection kit comprises a magneticseparation reagent and an enzyme-labeled reagent; the magnetic separation reagent is prepared from troponin T antibody-coated gold magnetic particles or prepared from streptavidin-coated gold magnetic particles and a biotin-labeled troponin T antibody; and the enzyme-labeled reagent is prepared from an enzyme-labeled antibody polymer, wherein the enzyme-labeled antibody polymer is prepared from at least two enzyme-labeled antibodies and carbon bridges; the carbon bridges connect any two or more, adjacent or non-adjacent enzyme-labeled antibodies, and each carbon bridge has at least two connecting loci for connecting the enzyme-labeled antibodies; and each enzyme-labeled antibody is prepared from a detection antibody and a labeling enzyme coupled to the detection antibody, and the connecting loci are connected with the detection antibodies and / or the labeling enzymes. When chemiluminescence detection is conducted, a reaction signal value of a chemiluminescence method is amplified, thesensitivity of an antigen captured by the detection reagent can be enhanced, and the detection sensitivity is improved.
Owner:深圳天辰医疗科技有限公司
Anti-African swine fever P30 protein single-domain antibody and ELISA kit for detecting African swine fever virus
ActiveCN111072774AQuick checkAccurate detectionBiological material analysisImmunoglobulins against virusesAbzymeElisa kit
The invention, which belongs to the technical field of virus detection, provides an anti-African swine fever P30 protein single-domain antibody and an ELISA kit for detecting the African swine fever virus. The amino acid sequence of the single-domain antibody p30-17 for resisting the African swine fever virus P30 protein is as shown in SEQ ID No. 1. In addition, the invention also relates to an ELISA kit for detecting African swine fever virus based on a double-antibody sandwich method. The ELISA kit comprises an elisa plate coated with a capture antibody being a single-domain antibody p30-17,an enzyme-labeled detection antibody being a single-domain antibody p30-30 of the anti-African swine fever virus p30 protein with an amino acid sequence shown as SEQ ID No.2, a standard positive reference substance, a serum diluent, a substrate developing solution A, a substrate developing solution B, a stop solution and a concentrated washing solution. The kit can realize rapid and accurate detection and has good detection specificity.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Encapsulation method of bacterial lipopolysaccharide, reagent kit and method of detecting specific lipopolysaccharide
InactiveCN1374526AConvenient researchEasy to detectMicrobiological testing/measurementBiological testingDiluentMonoclonal antibody
The encapsulation process of bacterial lipopolysaccharide (LPS) includes dissolving LPS in 0.1 mol / L carbonate buffering liquid of pH 9.6 and containing 0.02-2 % trichloroacetic acid and adding into enzyme labelling detecting hole irradiated with ultraviolet ray in advance, treating in 35-39 deg.c warming bath; setting at 4 deg.c, spin drying and confining in 35-39 deg.c warming bath by adding defatted milk powder or albumin as confining liquid; and spin drying, washing and maintaining at 4 deg.c. Furthermore, reagent kit for detecting specific bacterial LPS in the the said method and the fast detection method are also provided. The reagent kit includes: bacterial LPS monoclonal antibody; second enzyme labelling antibody; standard LPS; diluent liquid; and encapsulated enzyme labelling detecting strip. The present invention has the advantages of being simple and convenient.
Owner:SOUTH CHINA SEA INST OF OCEANOLOGY - CHINESE ACAD OF SCI
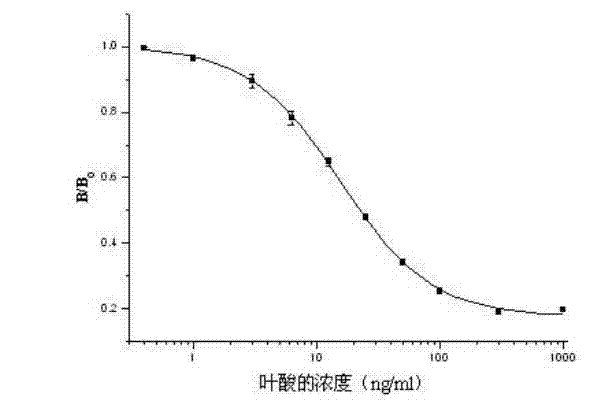
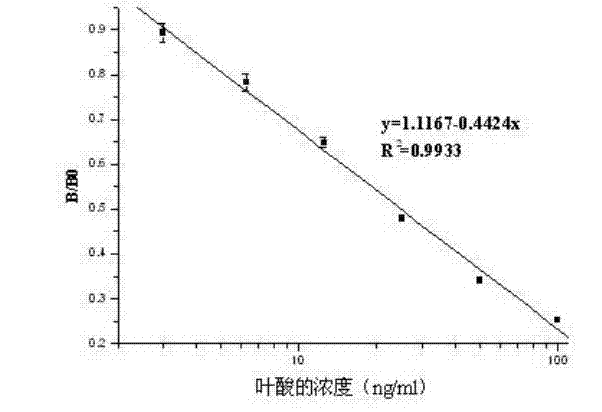
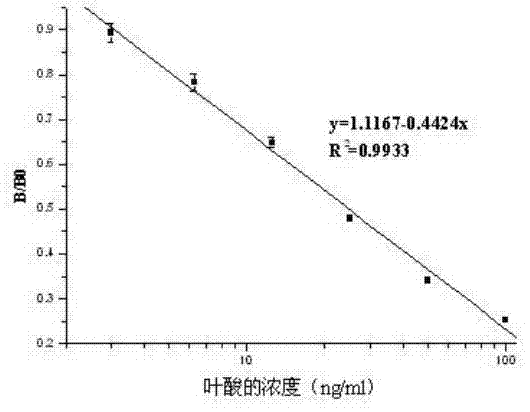
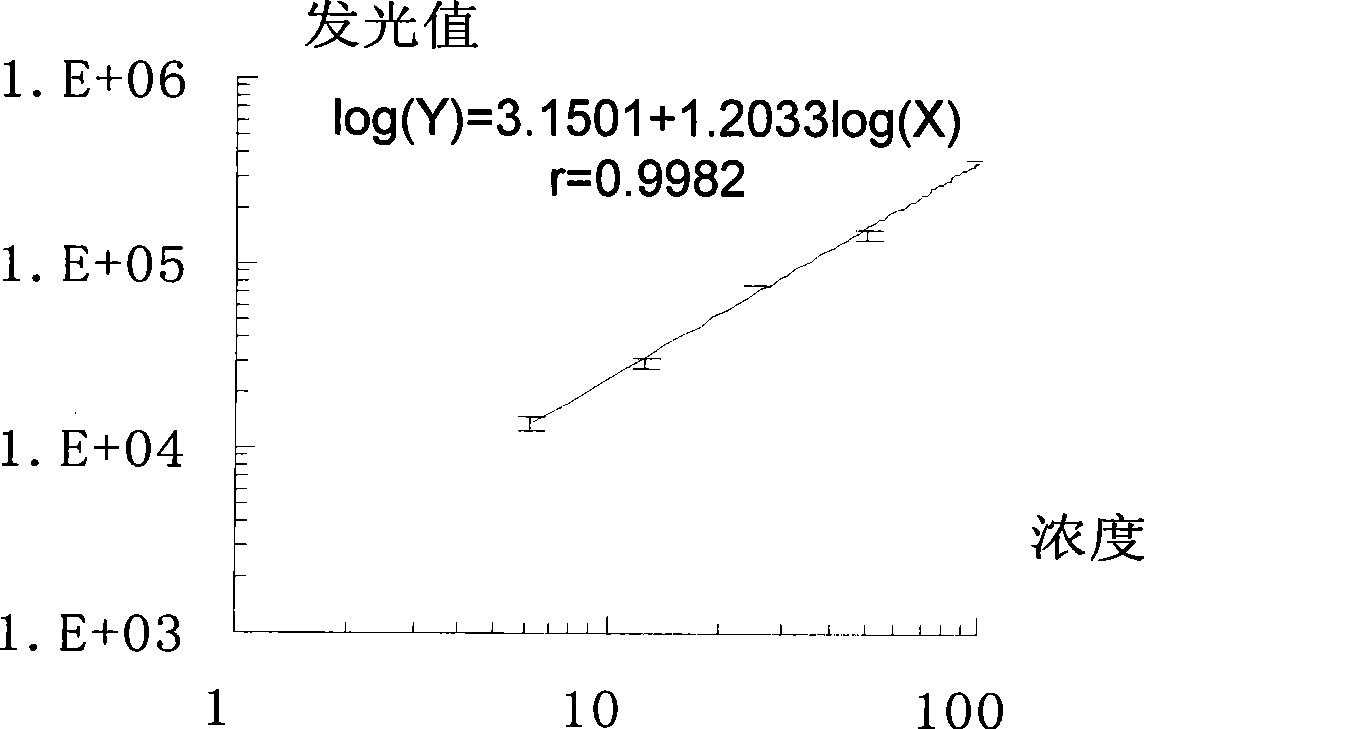
ELISA (enzyme linked immunosorbent assay) kit of folic acid
An ELISA (enzyme linked immunosorbent assay) kit of folic acid belongs to the technical field of enzyme linked immunosorbent assay. The reagent components in the kit comprise: an enzyme label plate coated with a folic acid coating antigen (the antigen is prepared by coupling folic acid and ovalbumin); a folic acid polyclonal antibody; a goat anti-rabbit antibody marked by enzyme-labelled bi-antibody which is horseradish peroxidase; a folic acid standard solution; a concentrated phosphate buffer; a concentrated cleaning solution; a substrate colour developing solution A; a substrate colour developing solution B and a stop solution. The ELISA kit provided by the invention performs ELISA by using a polyclonal antibody, wherein IC50=15.5ng / ml. The lowest detecting limit in milk products is 11.9ng / ml; the coefficients of variation between batches and in batches are 11.8%; and the recovery is 89.1-110.3%. The ELISA kit provided by the invention has the characteristics of simple structure, fast operations, accurate detection result and high sensitivity; and the kit can be used for fast detecting folic acids contained in foods, feeds and vitamin products.
Owner:NANKAI UNIV
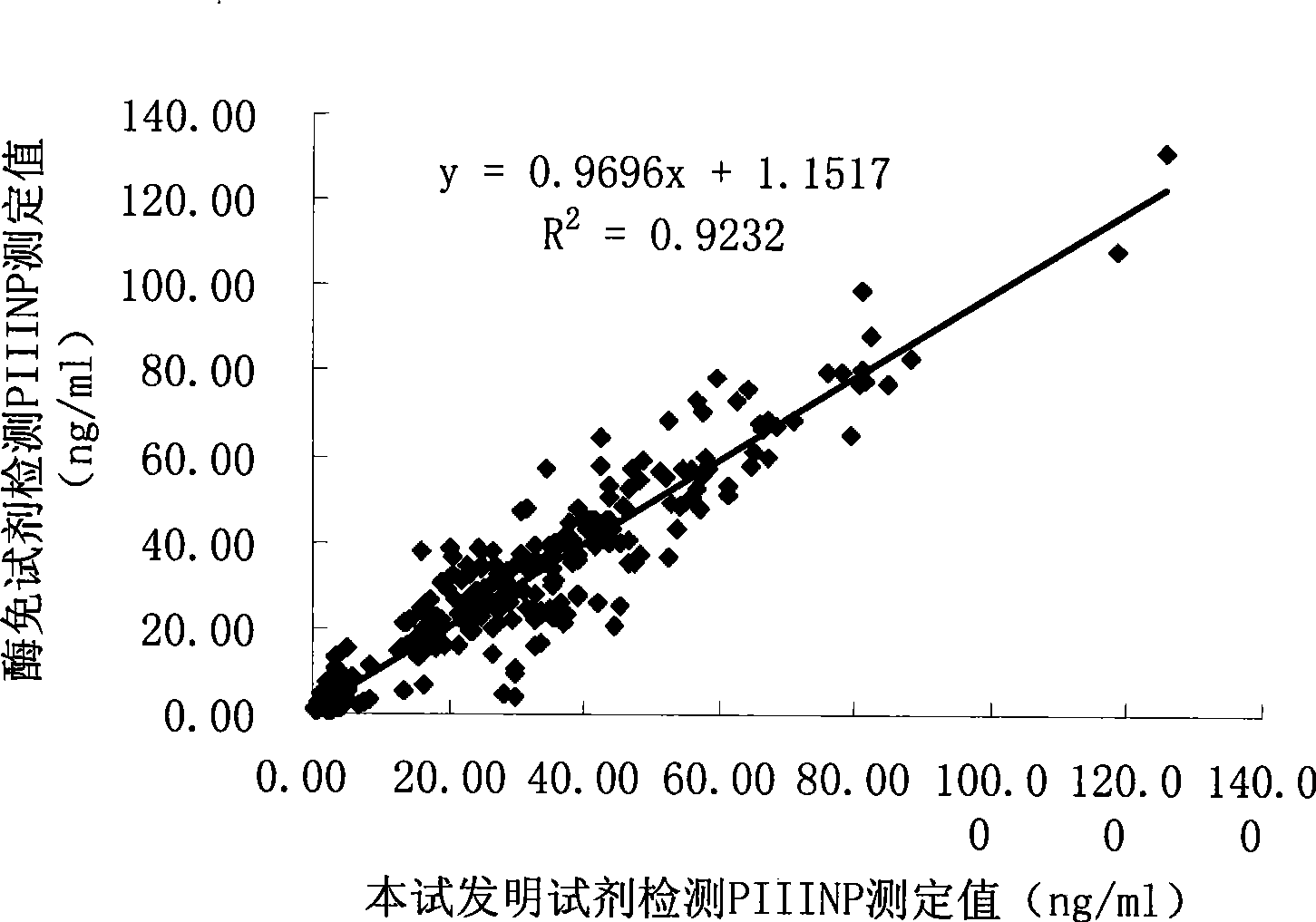
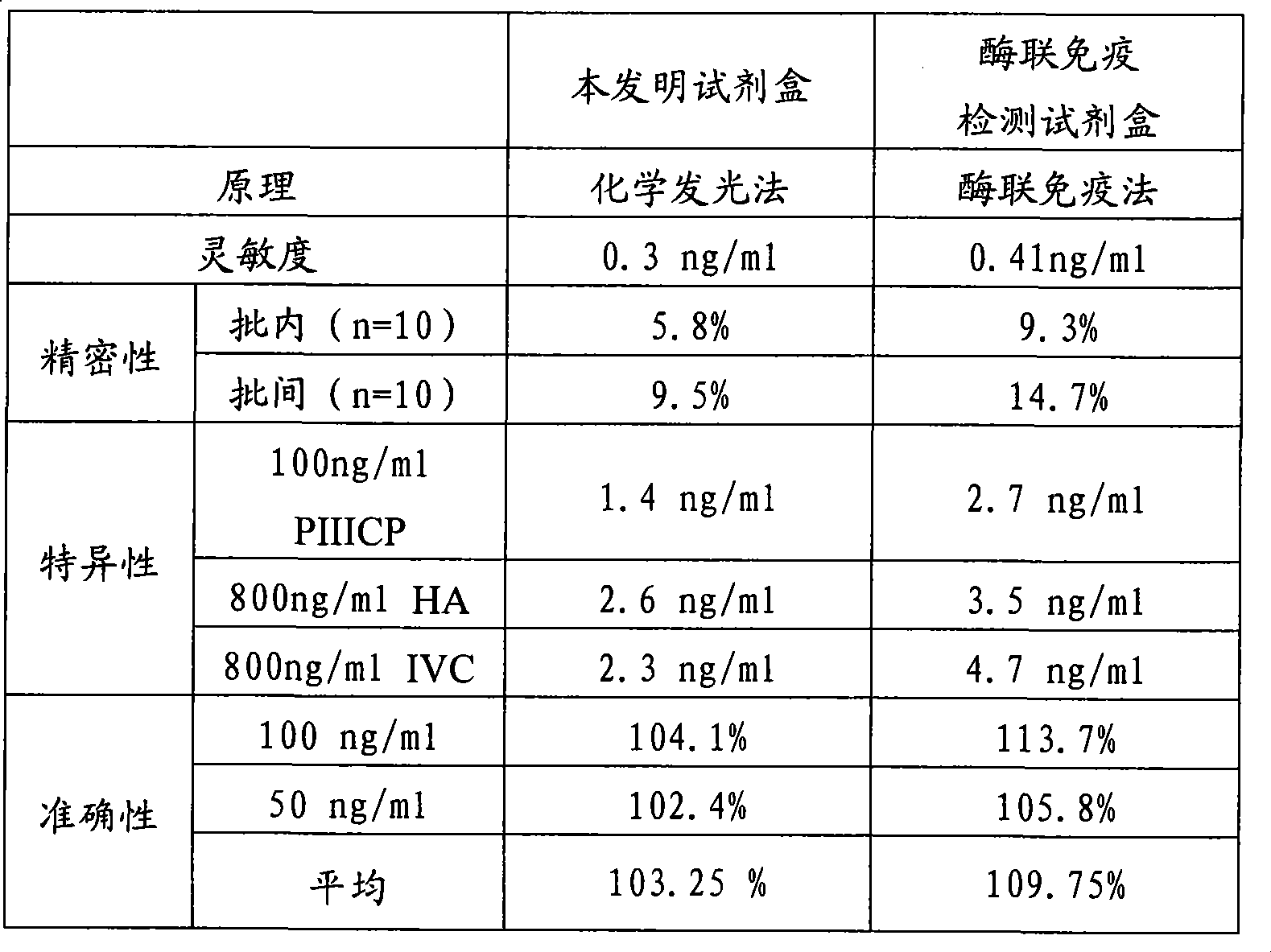
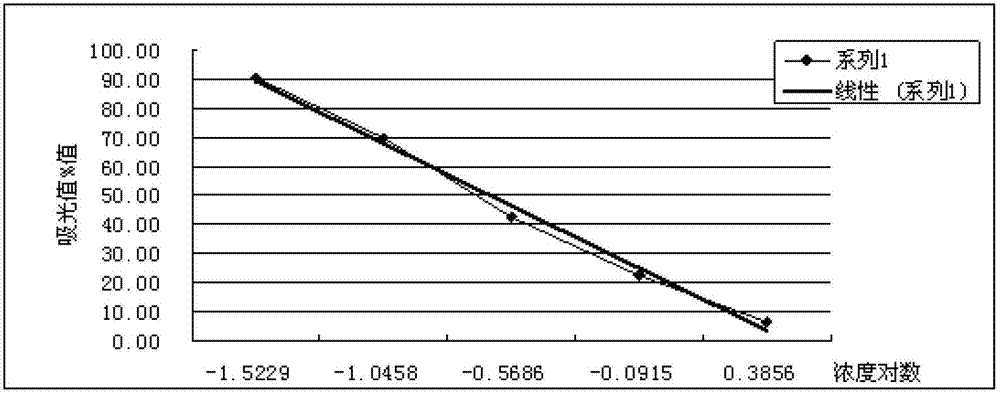
III type precollagen N end peptide chemiluminescence immune analysis quantitative determination reagent kit and preparing method thereof
InactiveCN101377509AGuaranteed sensitivityEasy to operateChemiluminescene/bioluminescenceBiological testingQuantitative determinationMonoclonal antibody
The invention relates to the medical field of immunoassay, more specially, the invention provides a chemiluminescent immunoassay quantitative detection kit for III-type procollagen N-terminal peptide (PIIINP) and a preparation method thereof. The kit of the invention comprises 1) III-type procollagen N-terminal peptide calibrators, 2) solid-phase vectors which are coated with III-type procollagen N-terminal peptide monoclonal antibodies, 3) enzyme markers for III-type procollagen N-terminal peptide monoclonal antibodies, 4) chemiluminescent substrates and 5) concentrated washing solution. Further, the preparation method of the kit according to the invention comprises the following steps: 1) preparing the III-type procollagen N-terminal peptide calibrators with pure III-type procollagen N-terminal peptide, 2) coating the vectors with III-type procollagen N-terminal peptide monoclonal antibodies, 3) marking the III-type procollagen N-terminal peptide monoclonal antibodies with the enzyme, 4) preparing the chemiluminescent substrates, 5) preparing the concentrated washing solution, 6) packaging the III-type procollagen N-terminal peptide calibrators, the enzyme markers, the chemiluminescent substrates and the concentrated washing solution and 7) assembling finished products. The kit of the invention has the advantages of simplicity, convenience, rapidness, sensitivity, stability and the like.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
Monoclonal antibody, enzyme-linked immunosorbent assay method and kit for detecting furaltadone residue marker AMOZ
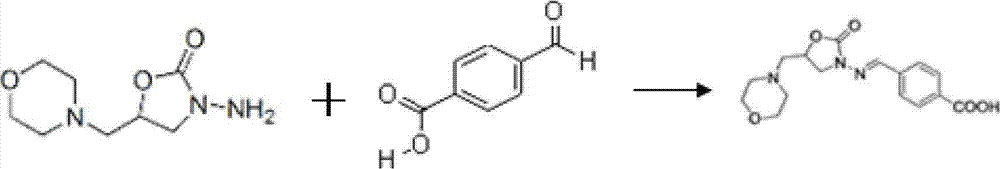
ActiveCN102766213AImprove detection efficiencyHigh precisionMicroorganism based processesTissue cultureAbzymeFuran
The invention discloses a monoclonal antibody capable of identifying a furaltadone residue marker 3-amino-5-morpholinomethyl-2-oxazolidinone (AMOZ). The monoclonal antibody is secreted by a hybridoma cell AMOZ; and the hybridoma is preserved in China Center for Type Culture Collection, and has a preservation number of CCTCC NO:C201188. The invention also discloses an enzyme immunoassay method and a reagent kit for detecting furaltadone marker residue marker AMOZ, and application thereof to detection of the furanketone residue marker AMOZ. Compared with prior art, the monoclonal antibody prepared by the invention, the ELISA Kit and the ELISA method have high detection sensitivity, high precision and good accuracy; a hapten synthesis process is simple, and has high synthesis efficiency; and a derivatization reagent for sample pretreatment is benzene formaldehyde, which has advantage of small toxicity compared with other commonly used derivative reagent like o-nitrobenzaldehyde.
Owner:WUHAN SHANGCHENG BIOTECH
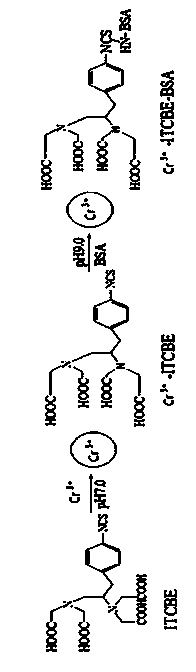
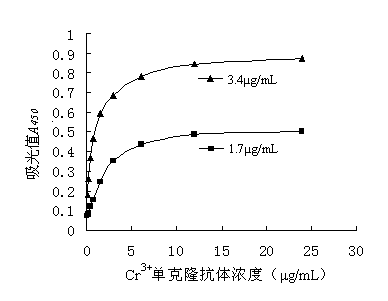
Indirect competitive enzyme linked immunosorbent assay kit for detecting chromium ions as well as preparation and detection methods thereof
The invention relates to an indirect competitive enzyme linked immunosorbent assay (elisa) kit for detecting chromium ions as well as preparation and detection methods thereof. A trivalent chromic ion envelope antigen-enveloped elisa plate, a trivalent chromic ion-resistant monoclonal antibody, a second enzyme-labeled antibody, a substrate color-developing solution, a stop solution, a trivalent chromic ion standard solution, a lotion concentrated solution and a sample processing solution are arranged in the kit. Samples are processed before detection so that Cr3<+> in the samples forms Cr<3+>-EDTA (ethylene diamine tetraacetic acid), and then the chromium ions are detected by the kit. The chromium ion enzyme linked immunosorbent assay kit can be used for detecting the trace amount of chromium ions, has the Cr<3+> detection sensitivity of 2ng / ml, has the characteristics of quickness, simplicity and convenience, sensitivity, specificity, economy and the like, has few detection steps, saves the detection time, and reduces the operation error; the detection cost is less than 1 / 20 of that of a physical and chemical analysis method, the timeliness is high, the field detection can be performed, and the kit is mainly used for screening large-batch samples of chromium ion pollution residues in environments, soil, water and foods.
Owner:HENAN INST OF SCI & TECH
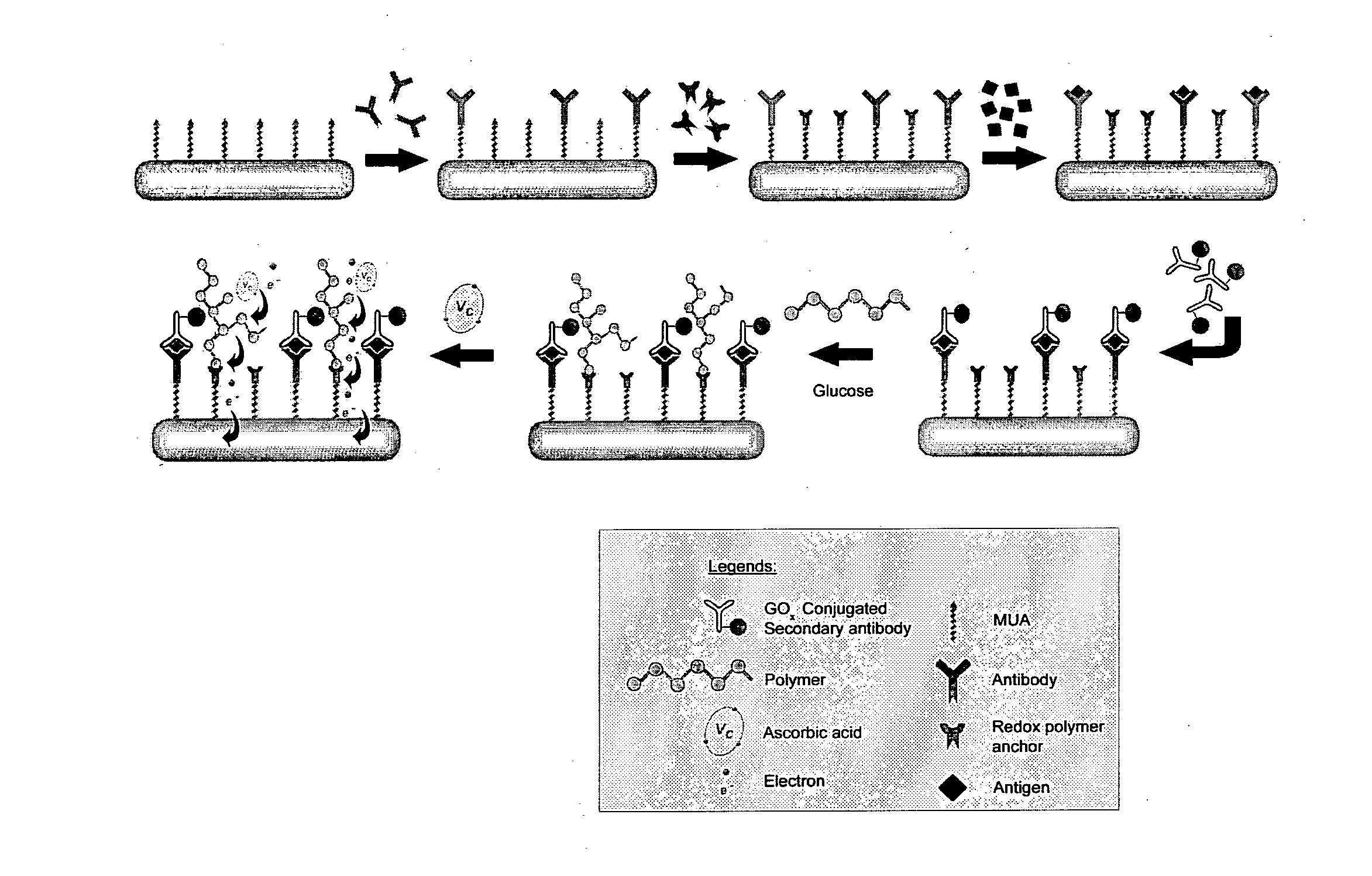
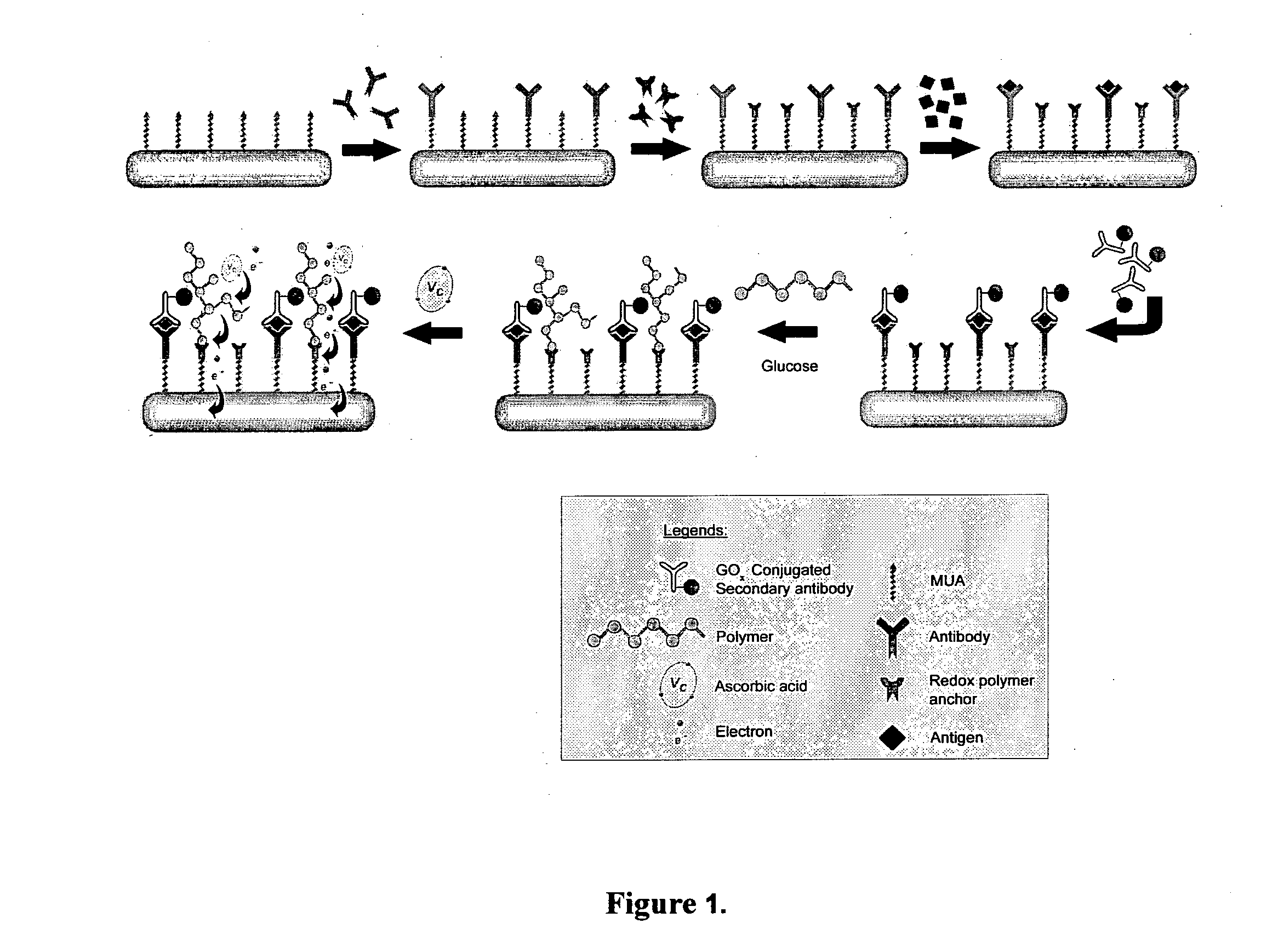
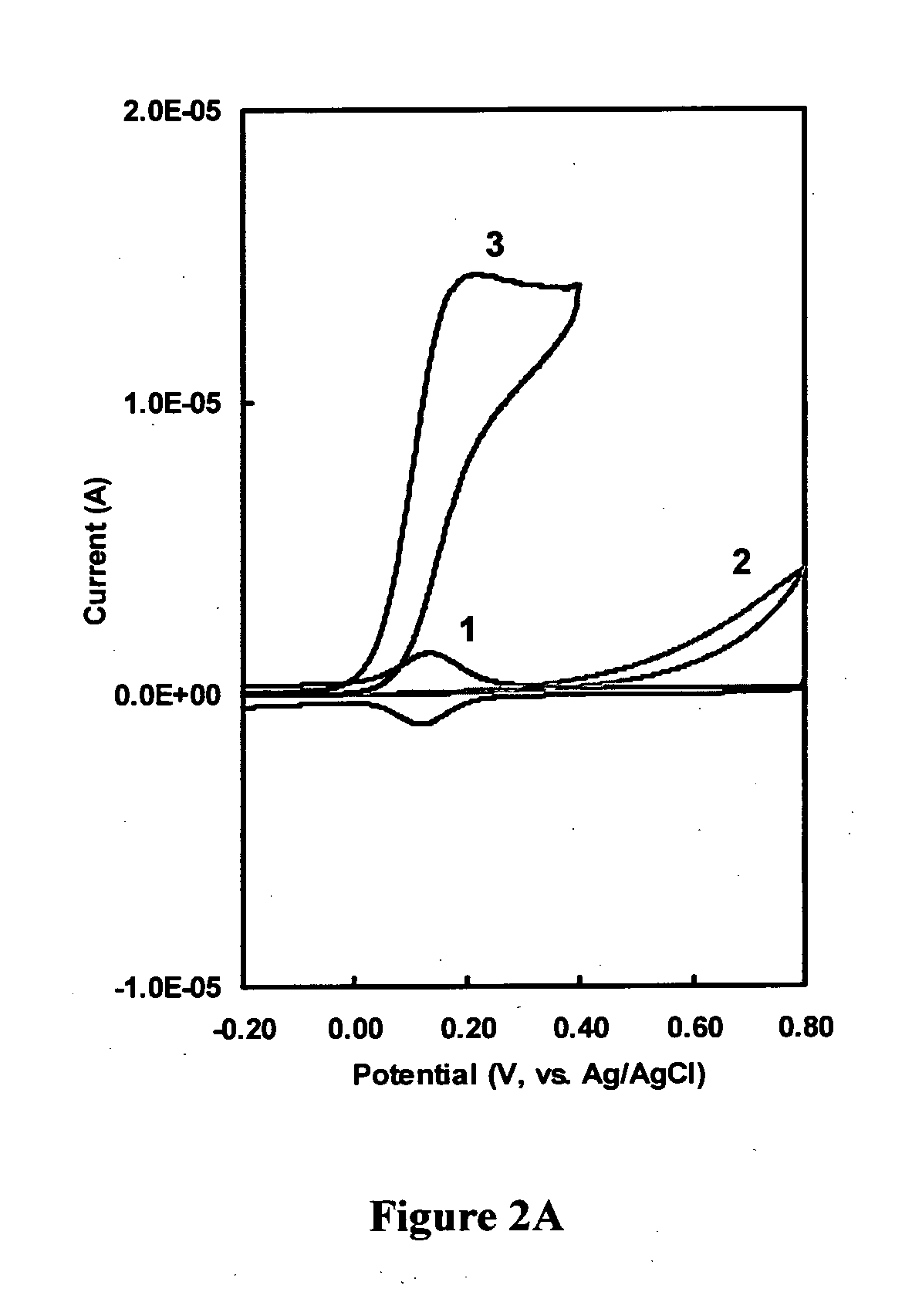
Immunoassay
InactiveUS20110189705A1Bioreactor/fermenter combinationsBiological substance pretreatmentsRedox enzymesAntigen binding
The invention provides a highly sensitive immunoassay for detection of a biological species. The immunoassay comprises exposing an electrode to an analyte liquid putatively containing the biological species so as to couple the biological species, if present in the analyte liquid, to a binding antibody on the electrode. The electrode comprises a binding antibody and an anchor group, each being coupled to an electrically conductive substrate, said binding antibody being capable of binding to the biological species and said anchor group being capable of binding to a redox polymer. The electrode is then exposed to an antibody-enzyme liquid comprising an antibody-enzyme species, said antibody-enzyme species comprising a detection antibody capable of binding to the biological species, said detection antibody being coupled to a redox enzyme, whereby, if the analyte liquid comprises the biological species, the redox enzyme couples to the electrode by means of the coupling of both the detection antibody and the binding antibody to the biological species. The electrode is then exposed to a polymer solution comprising the redox polymer and to an enzyme substrate, whereby if the redox enzyme is coupled to the anchor group on the electrode the redox polymer is reduced and couples to the anchor group on the electrode. A voltage is then applied between the electrode and a reference electrode and the electrode is exposed to an oxidisable species, whereby a magnitude of an electric current between said electrode and a reference electrode is indicative of the presence or absence of the biological species.
Owner:AGENCY FOR SCI TECH & RES
EV71 (human enterovirus 71) antigen enzyme-linked reaction detection kit and its preparation method
The invention relates to the biotechnical field, concretely relates to a virus antigen content detection kit and its preparation method, and more concretely relates to an enzyme-linked reaction kit for qualitatively and quantitatively detecting the EV71 virus antigen content, and its preparation method. The double-antibody enzyme-linked reaction detection kit adopted in the invention and its preparation method have the characteristics of good specificity, high sensitivity, convenience, fastness, economy and the like.
Owner:BEIJING HUAWEI BRAVOBIO
Rabies virus antibody (IgG) enzyme-linked immunoassay kit and detection method thereof
The invention provides a rabies virus antibody (IgG) enzyme-linked immunoassay kit and a detection method thereof. The detection kit is composed of a purified rabies virus antigen coated microporous plate, enzyme labeled SPA and other reagents. The detection method adopts an indirect method principle to detect the rabies virus IgG antibody in human or animal serum or blood plasma, and is suitable for rabies vaccine immunized serology effect evaluation and epidemiology investigation. An enzyme labeled antibody applied in the invention is a Staphylococal protein A (SPA), and the SPA can be combined with an Fc fragment in IgG molecules in human or mammal serum, so the kit provided by the invention has all the characteristics of an ELISA kit, can be used for human rabies virus antibody detection, and can also be used for detecting the immune effect of various species of animals.
Owner:成大生物(本溪)有限公司
Quantitative determination kit for neutralizing antibodies of virus and application thereof
The invention belongs to the field of a biotechnology and particularly relates to a quantitative determination kit for neutralizing antibodies of virus and application of the quantitative determination kit to detection of the neutralizing antibodies of virus of severe fever with thrombocytopenia syndrome. Virus NP proteins of the severe fever with thrombocytopenia syndrome are subjected to prokaryotic expression to prepare a rat source monoclonal antibody or polyclonal antibody aiming at NP proteins. The tissue cell half infection amount (TCIF50) of determined viruses is measured by detecting the NP proteins through an enzyme linked immunosorbent assay. A specimen to be detected and the equal amount of virus liquid are mixed and inoculated with cells. The monoclonal antibody or the polyclonal antibody of the NP proteins is monoclonal antibody; a specimen neutralizing antibody valence is detected by a double-antibody enzyme linked immunosorbent assay. The quantitative determination kit can be applied to the aspects of clinical immune effect evaluation of severe fever with thrombocytopenia syndrome vaccines, blood serum epidemiologic studies of the severe fever with thrombocytopenia syndrome of crowds or faunas, in-vitro valence determination and estimation of the manually-prepared virus-neutralizing antibodies of the severe fever with thrombocytopenia syndrome. The method provided by the invention has the characteristics of sensitivity, rapidness, specificity, high flux and the like.
Owner:JIANGSU PROVINCIAL CENT FOR DISEASE PREVENTION & CONTROL
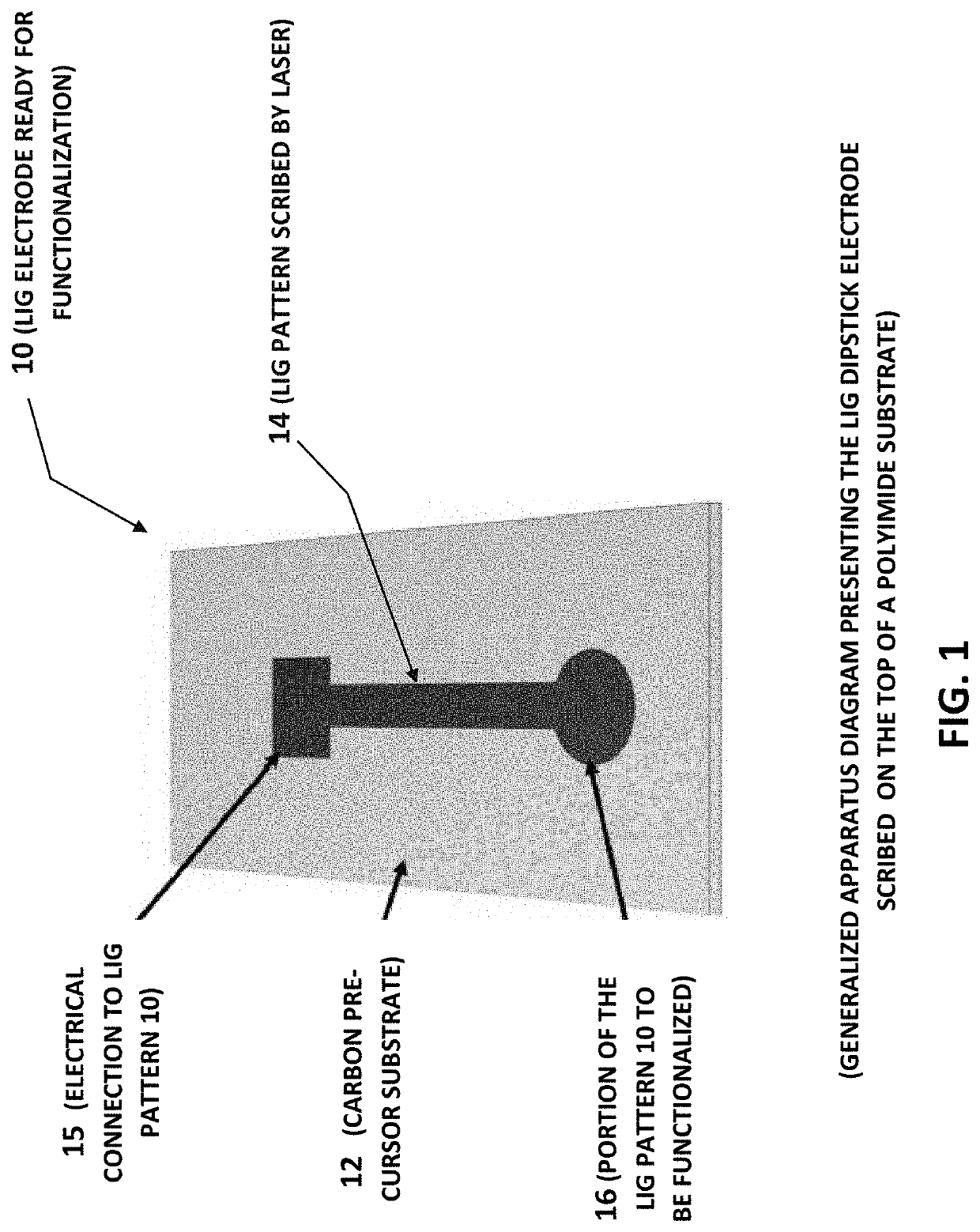
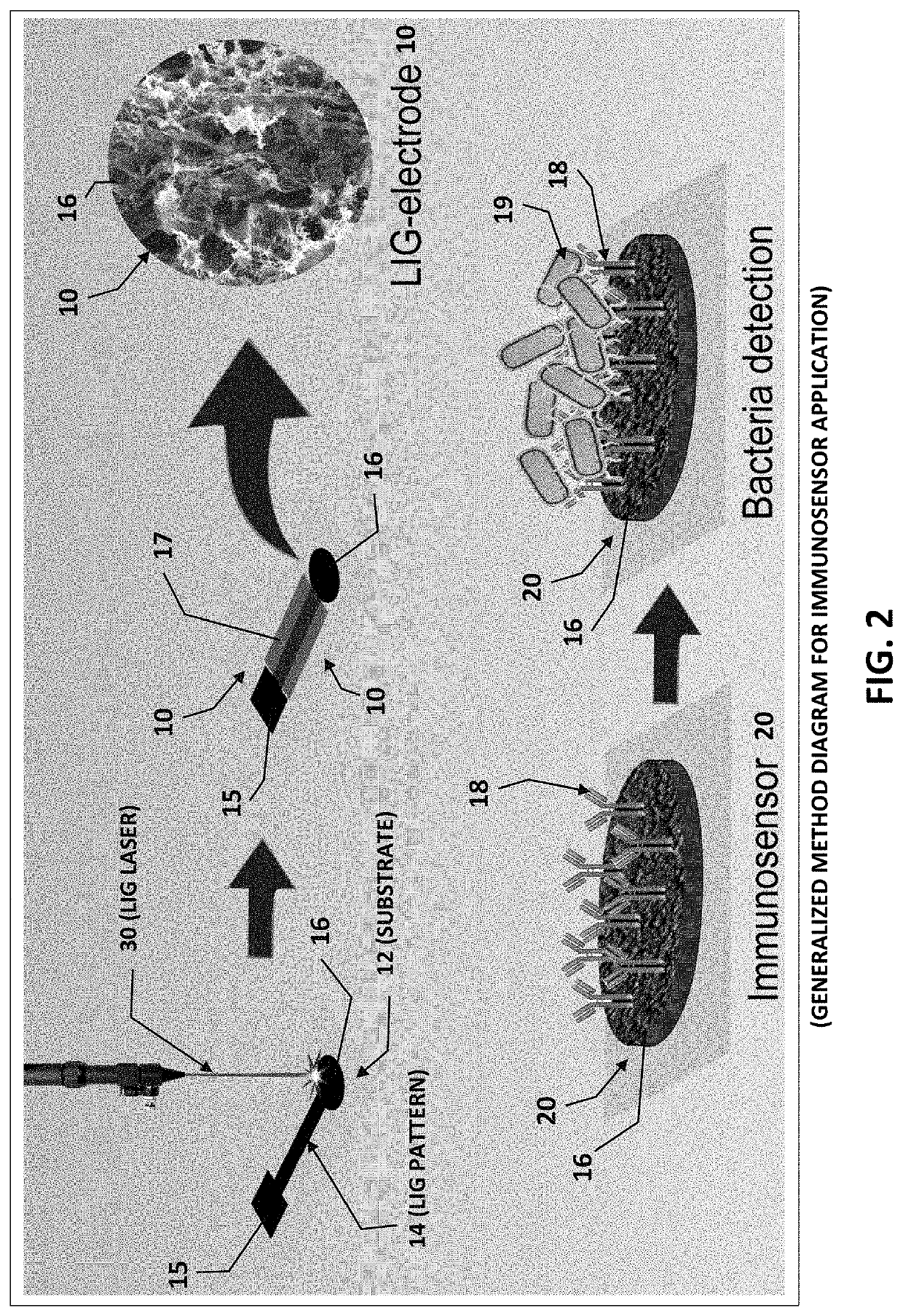
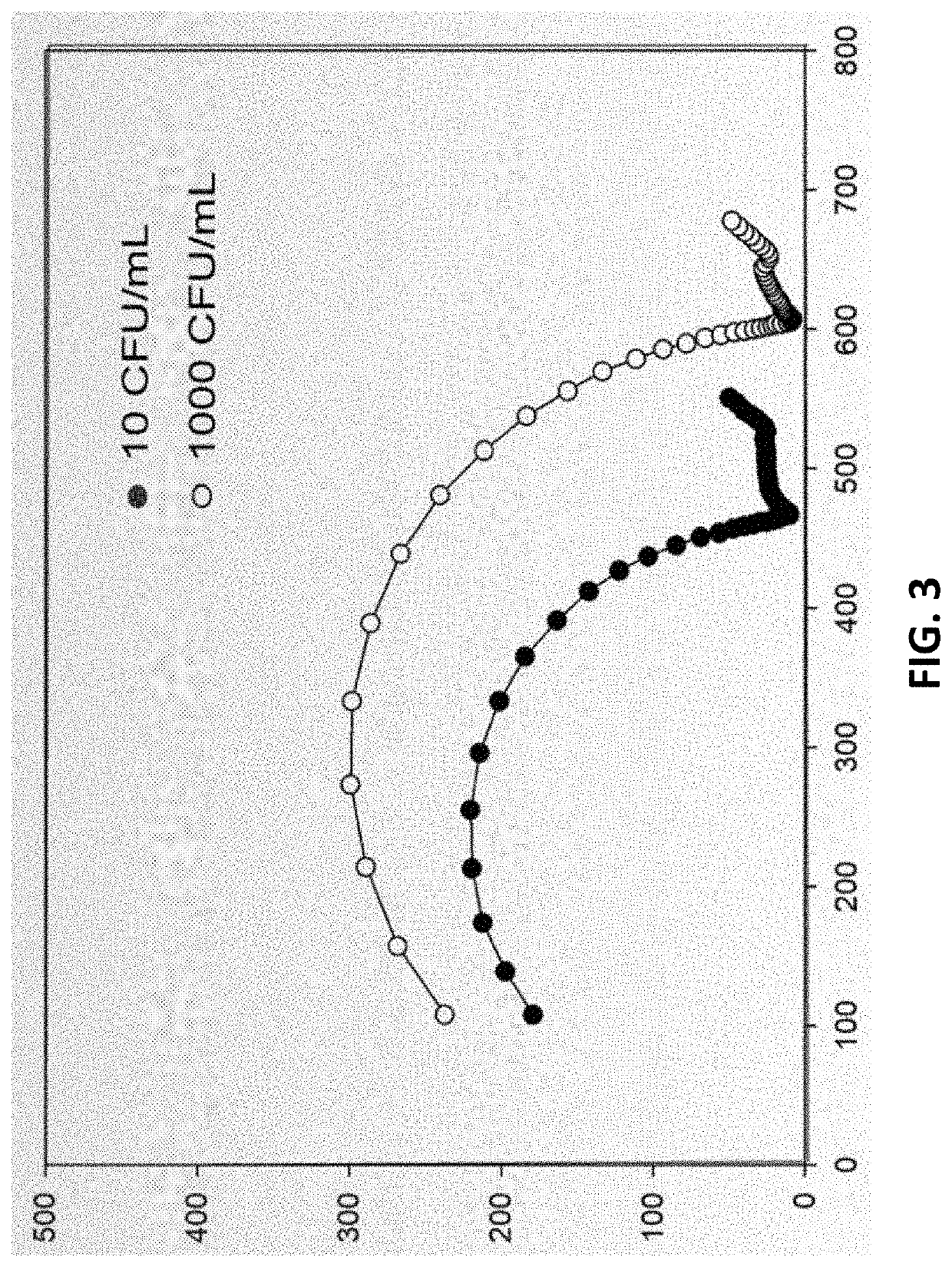
Laser-induced graphene electrodes adaptable for electrochemical sensing and catalysis
PendingUS20210332489A1Improve electrochemical signal sensitivityShort response timeBiological material analysisGrapheneAbzymePtru catalyst
Apparatus and methods of fabrication and use of highly effective laser-induced graphene (LIG) electrodes including for electrochemical sensing and catalysis. One example is a sensitive and label-free laser-induced graphene (LIG) electrode functionalized for a specific application. One example of functionalization with antibodies, an enzyme, or an ionophore to electrochemically quantify a target species The LIG electrodes were produced by laser induction on film having a carbon precursor (e.g. polyimide) in ambient conditions, and hence circumvent the need for high-temperature, vacuum environment, and metal seed catalysts commonly associated with graphene-based electrodes fabricated via chemical vapor deposition processes. These results demonstrate how LIG-based electrodes can be used for electrochemical sensing in general. Other examples of applications include, but are not limited to, ion-sensing, pesticide monitoring and detection, and water splitting, using the LIG-based electrode(s) adapted for those purposes.
Owner:IOWA STATE UNIV RES FOUND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com