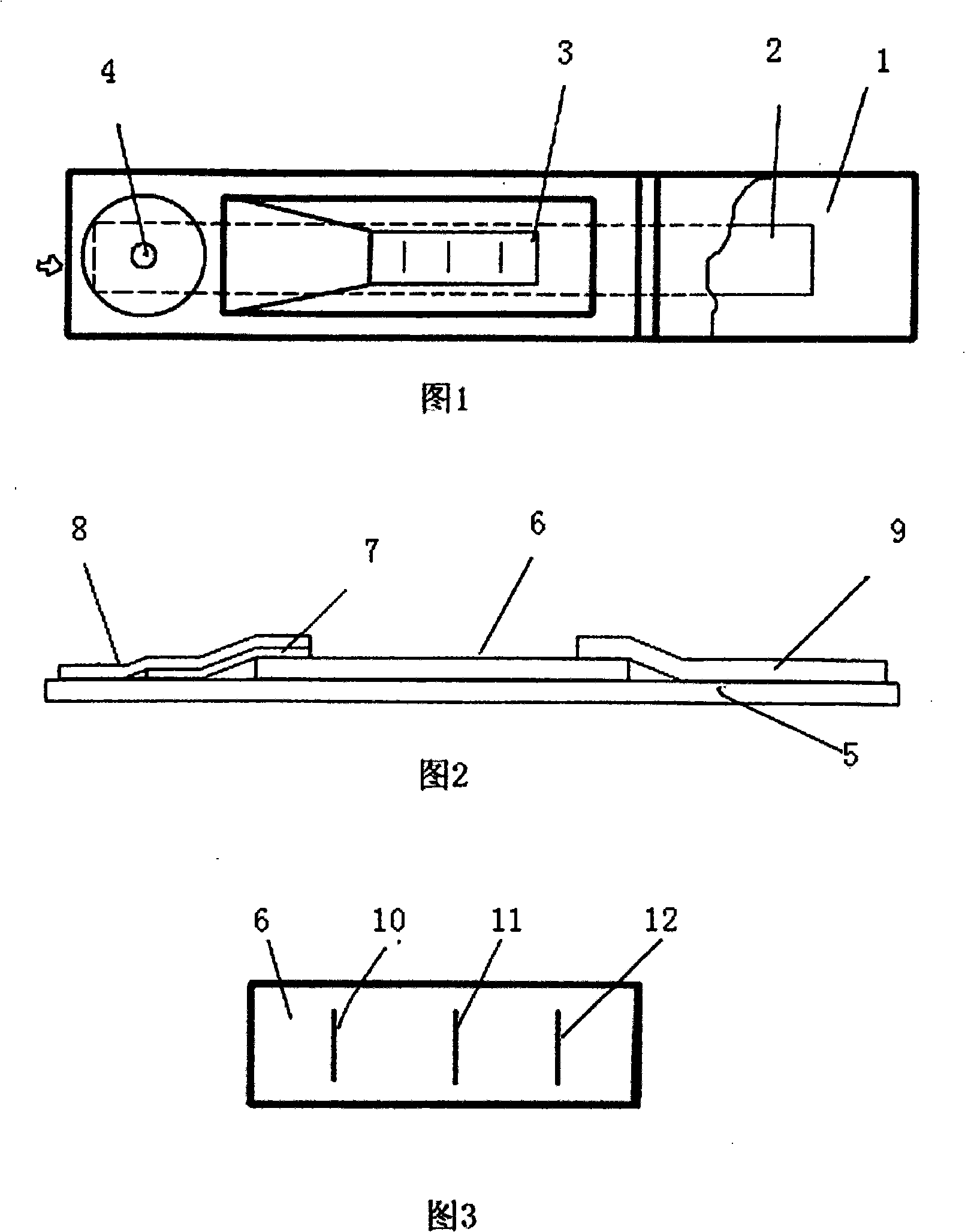
Patents
Literature
238 results about "Rabbit Antibody" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A rabbit antibody is an antibody derived from a rabbit. Such antibodies are commercially prepared by laboratories which specialize in the production of antibodies and a range of other products derived from animals.
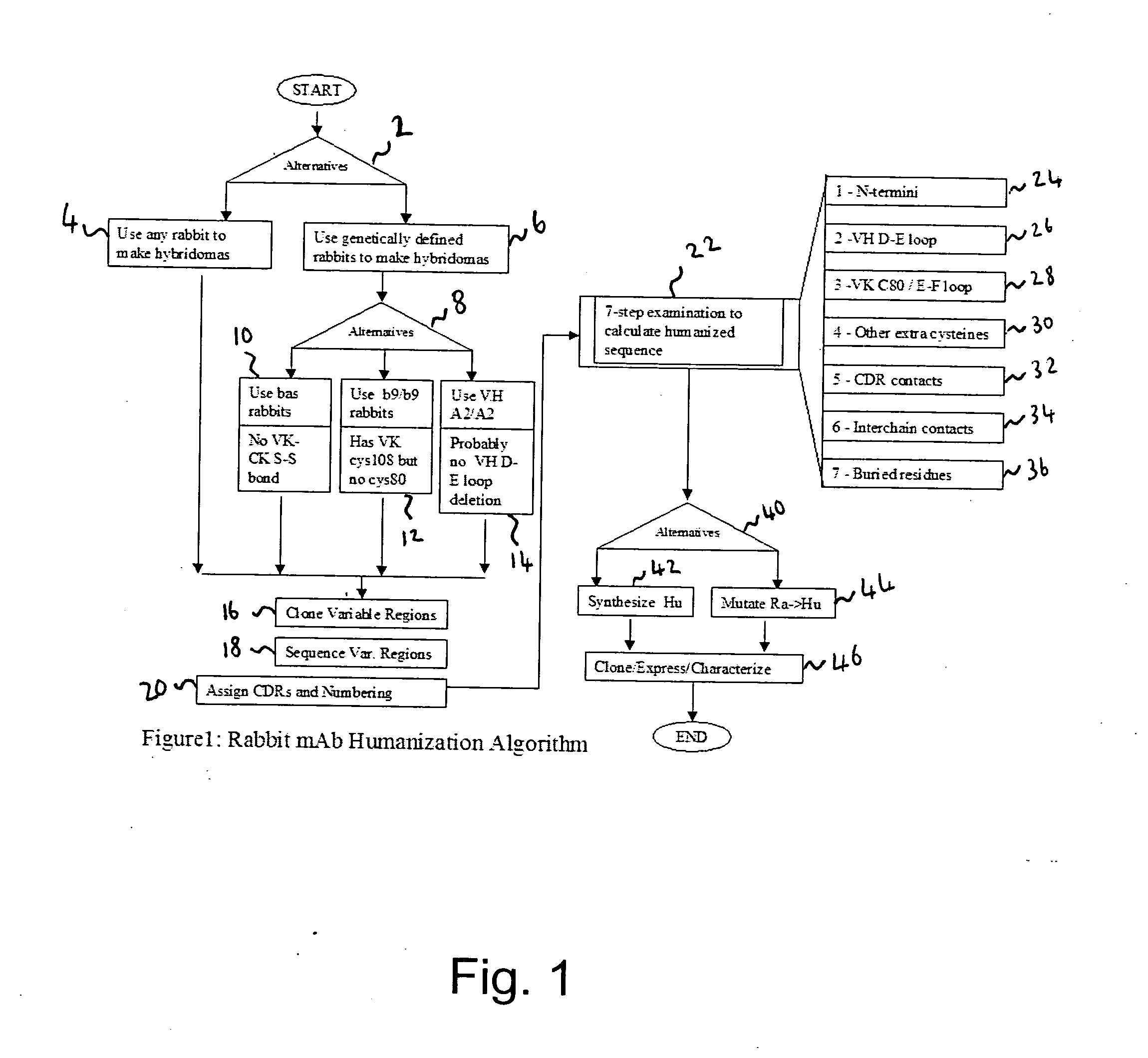
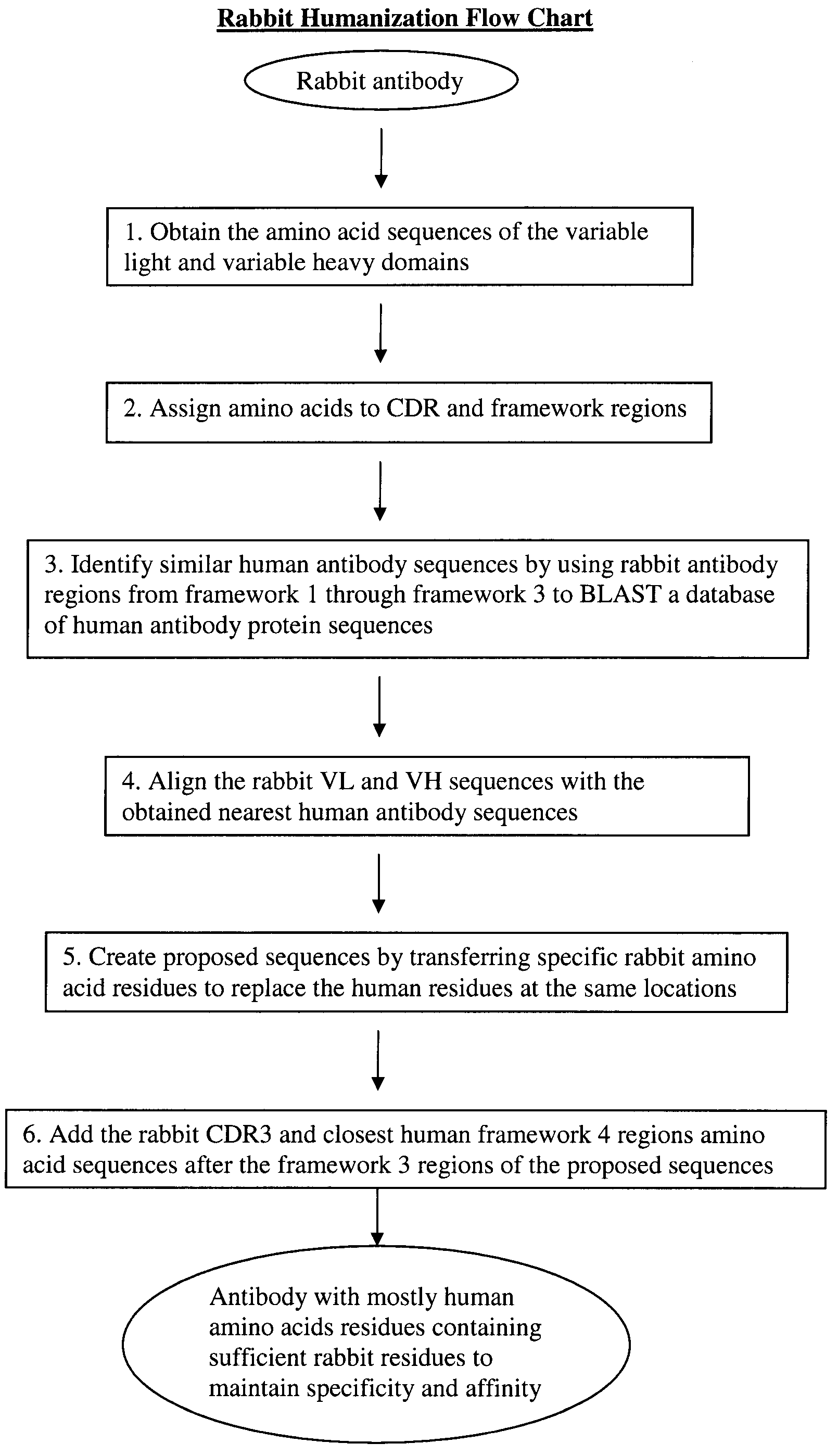
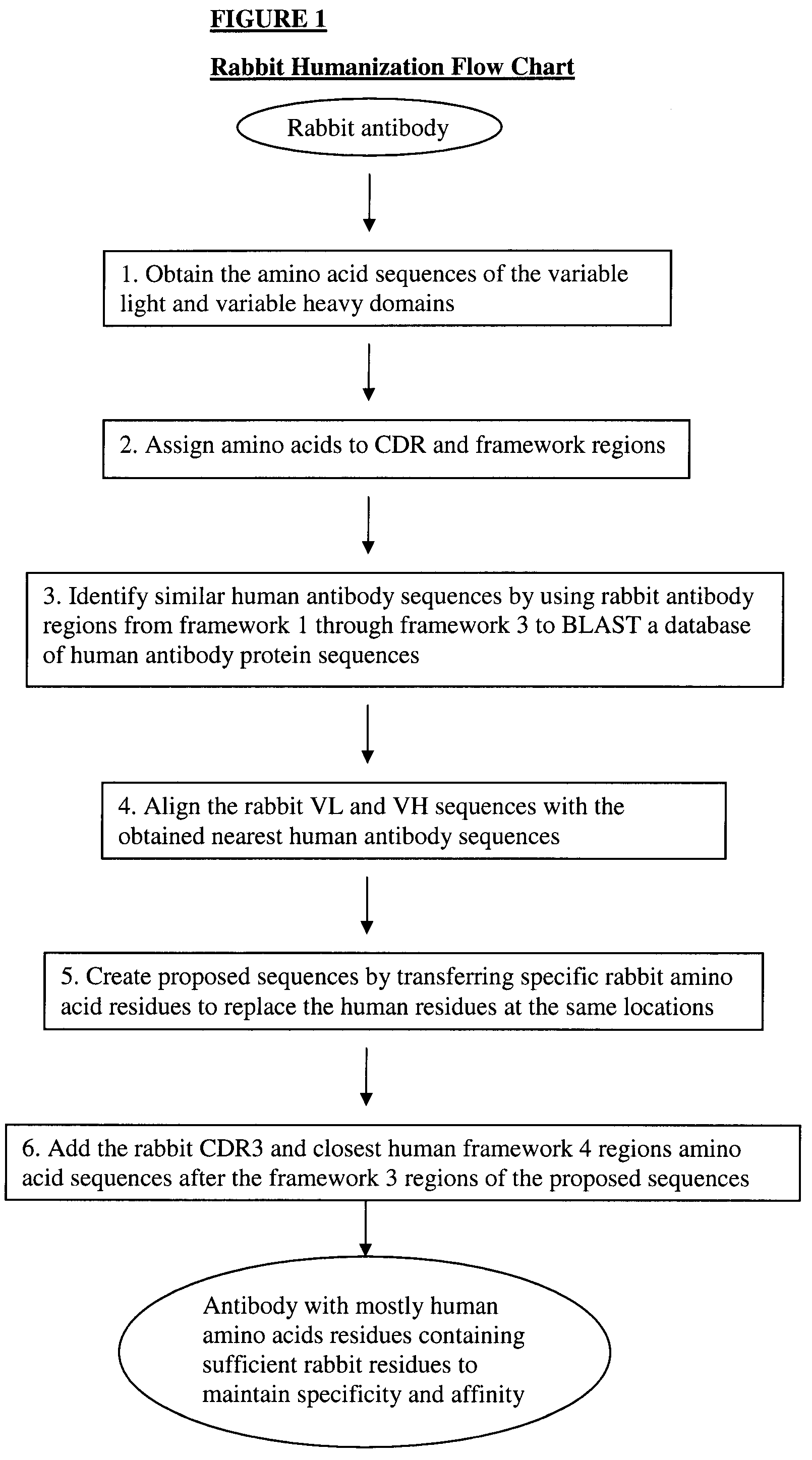
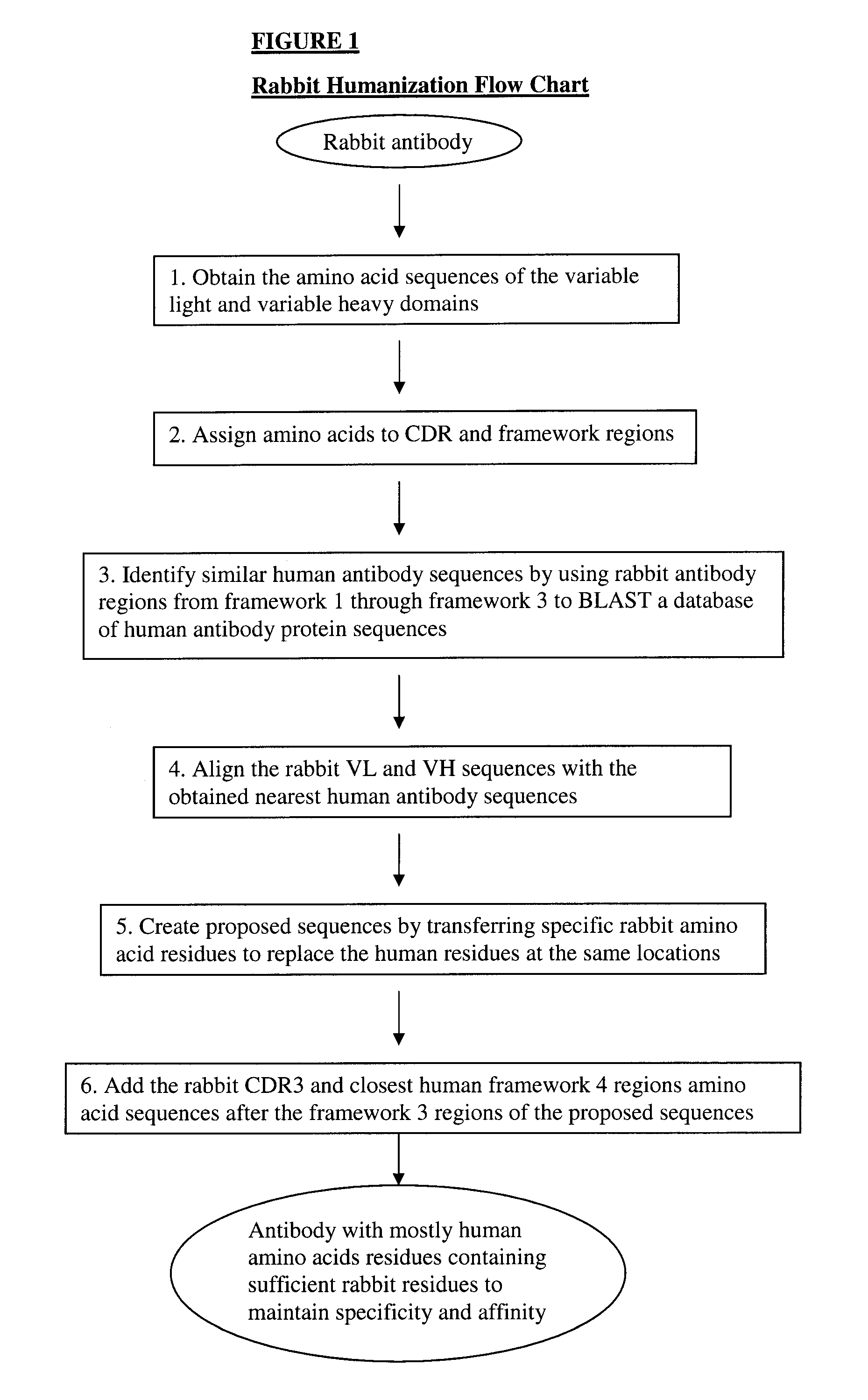
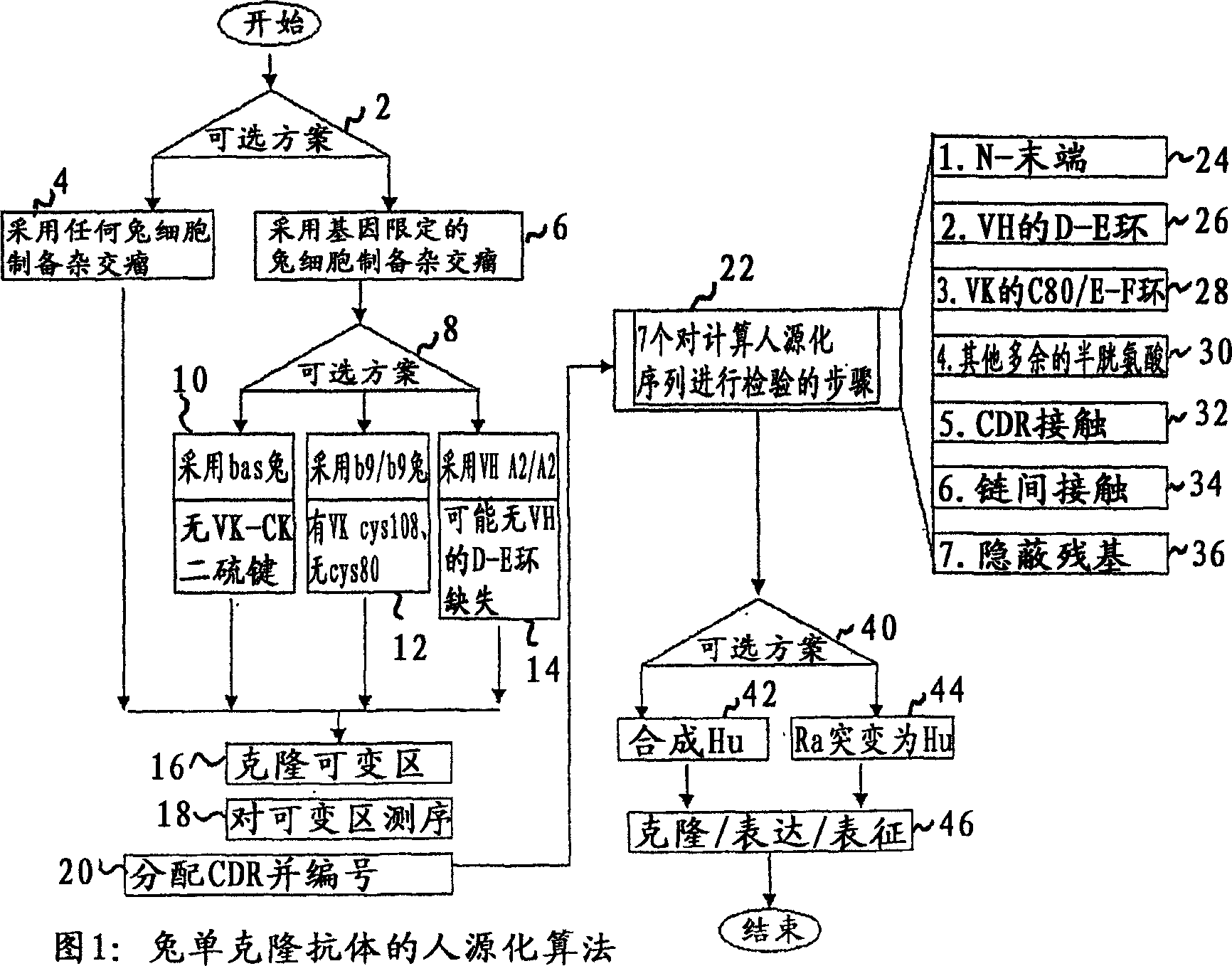
Methods for humanizing rabbit monoclonal antibodies
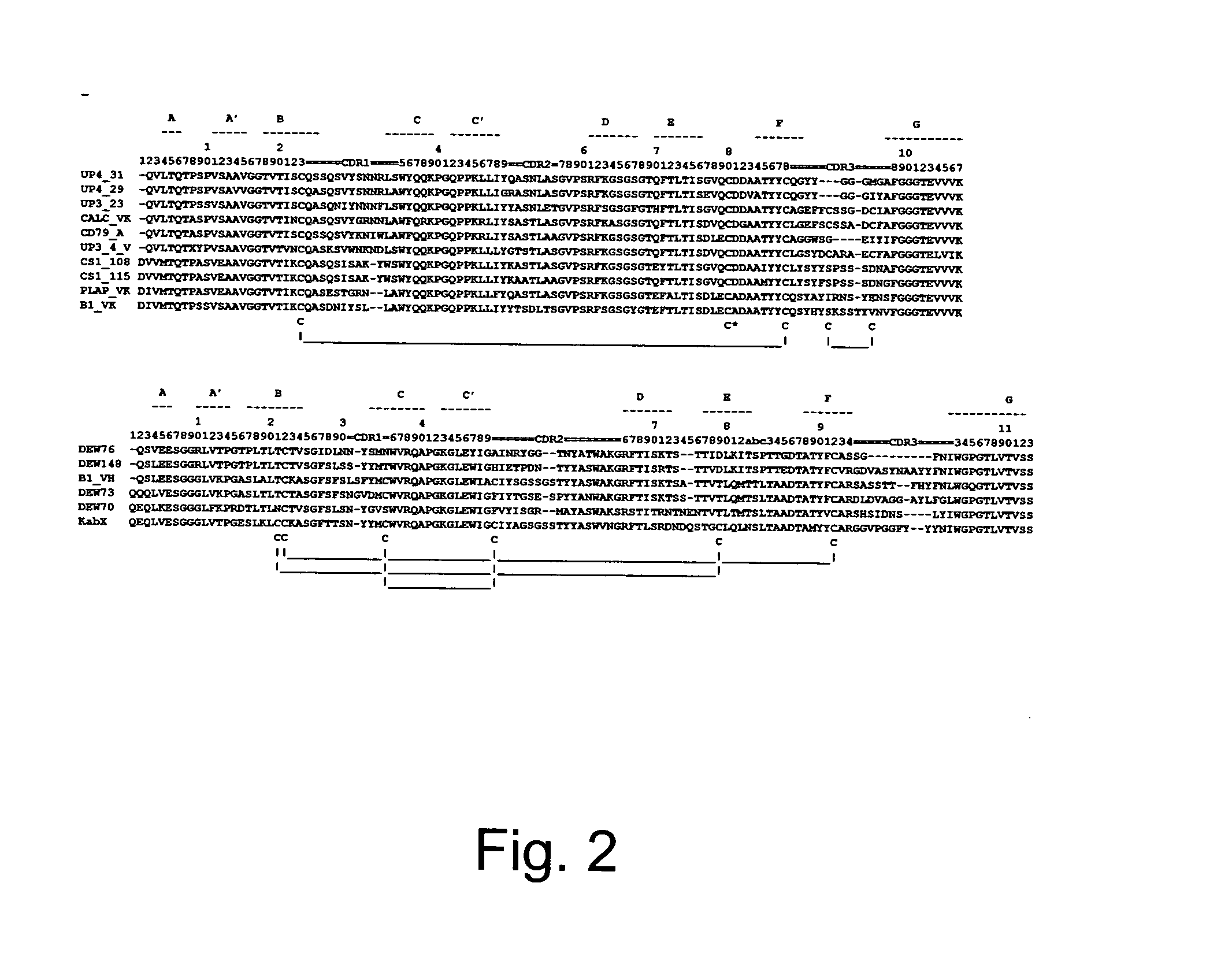
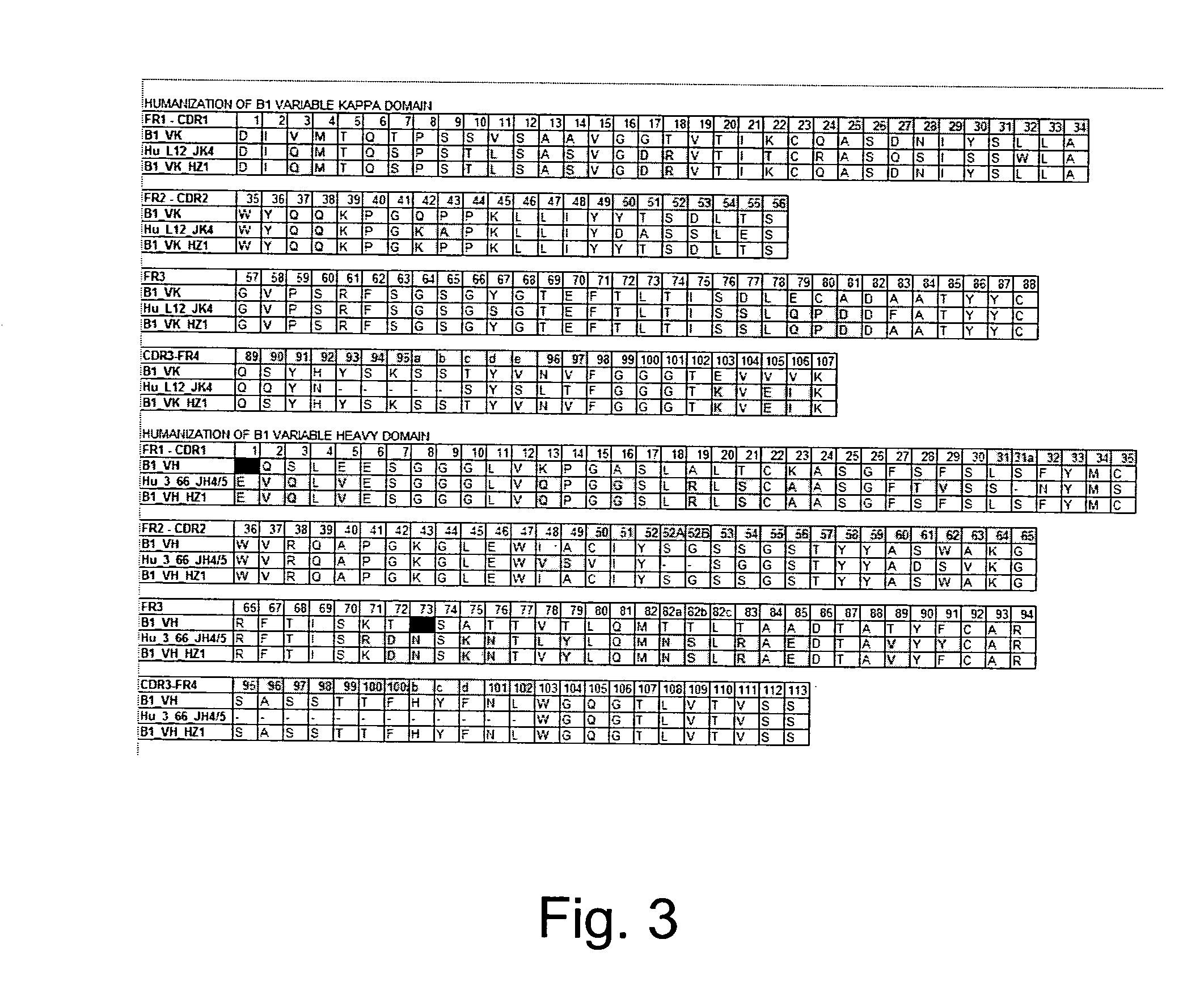
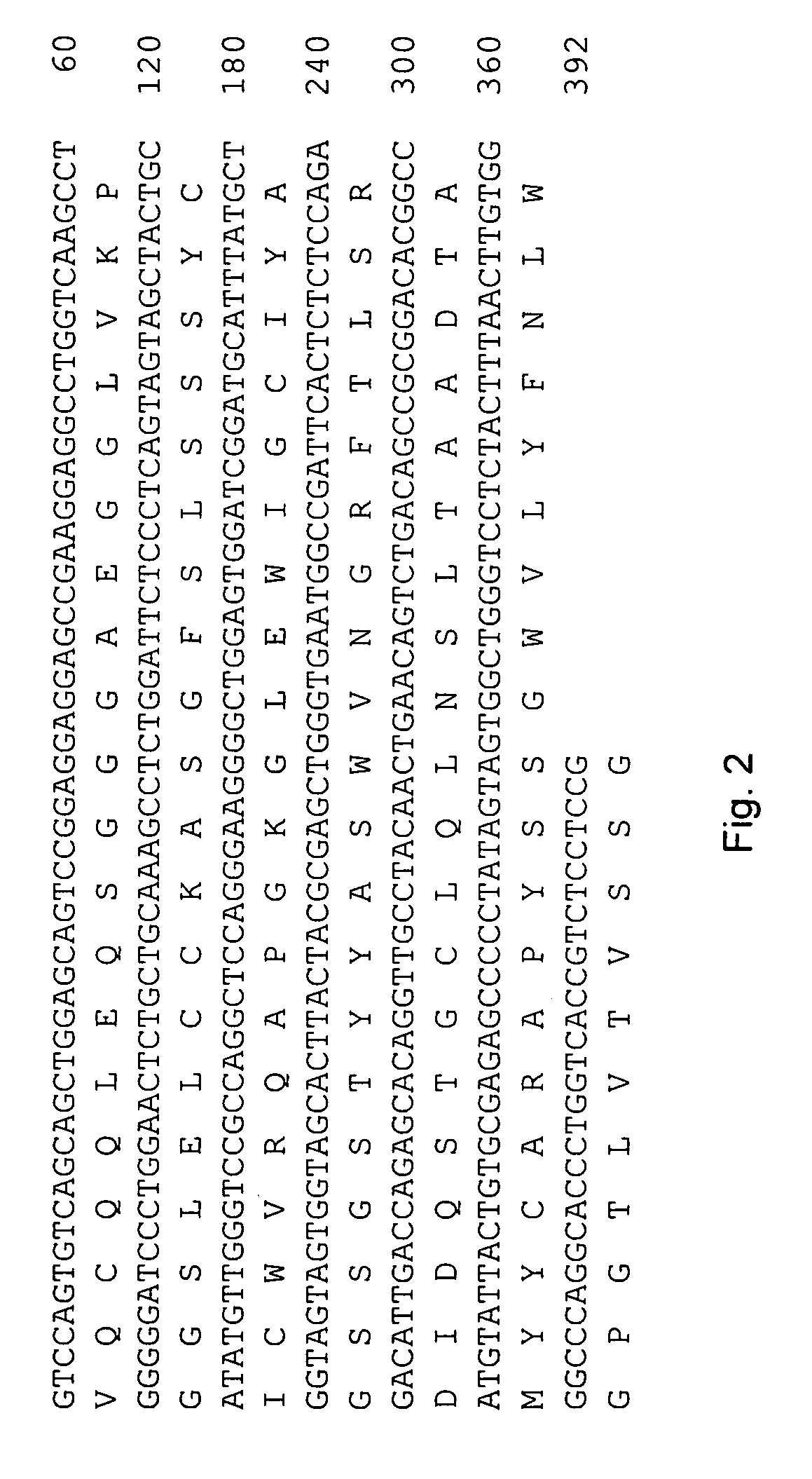
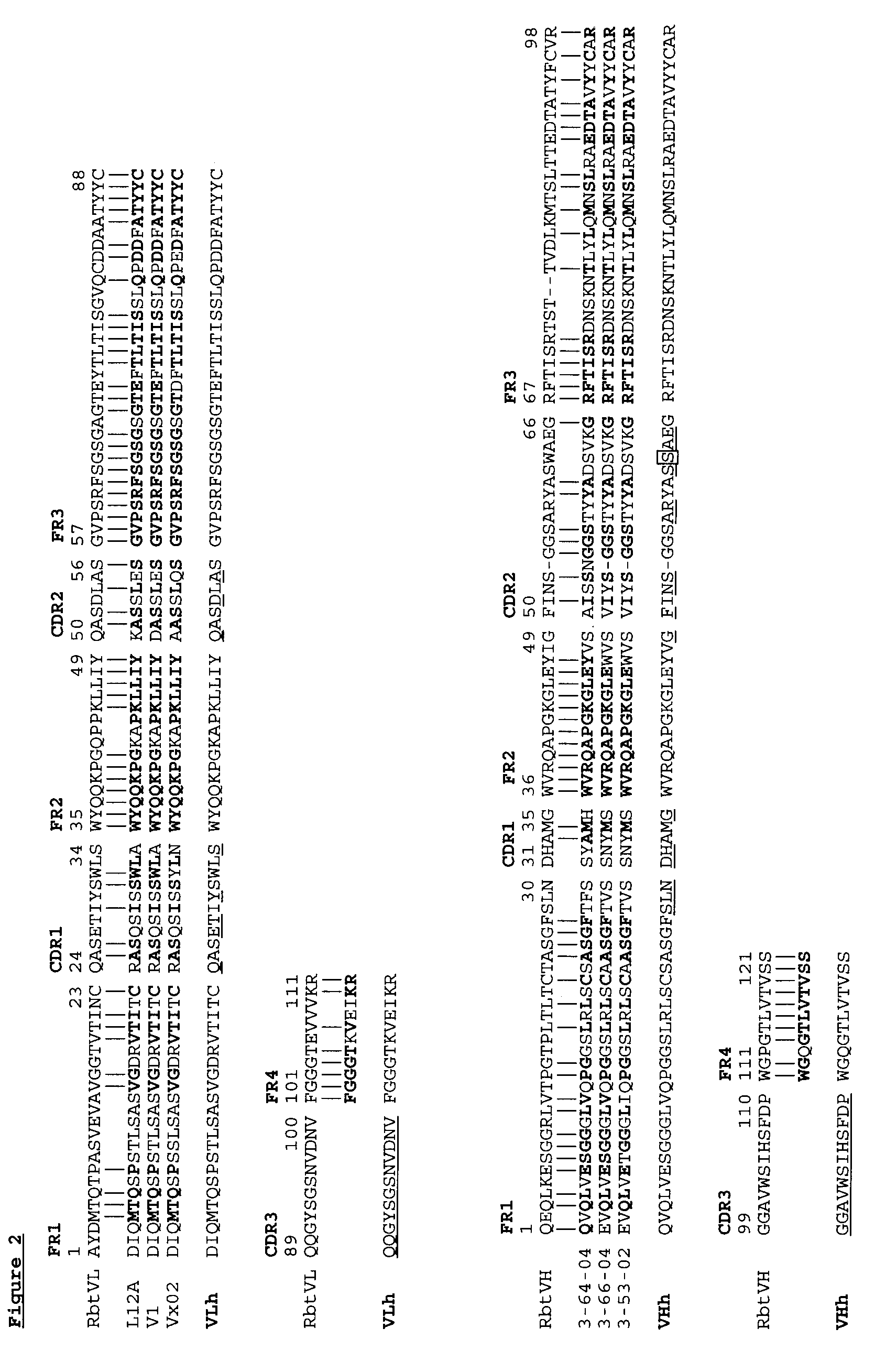
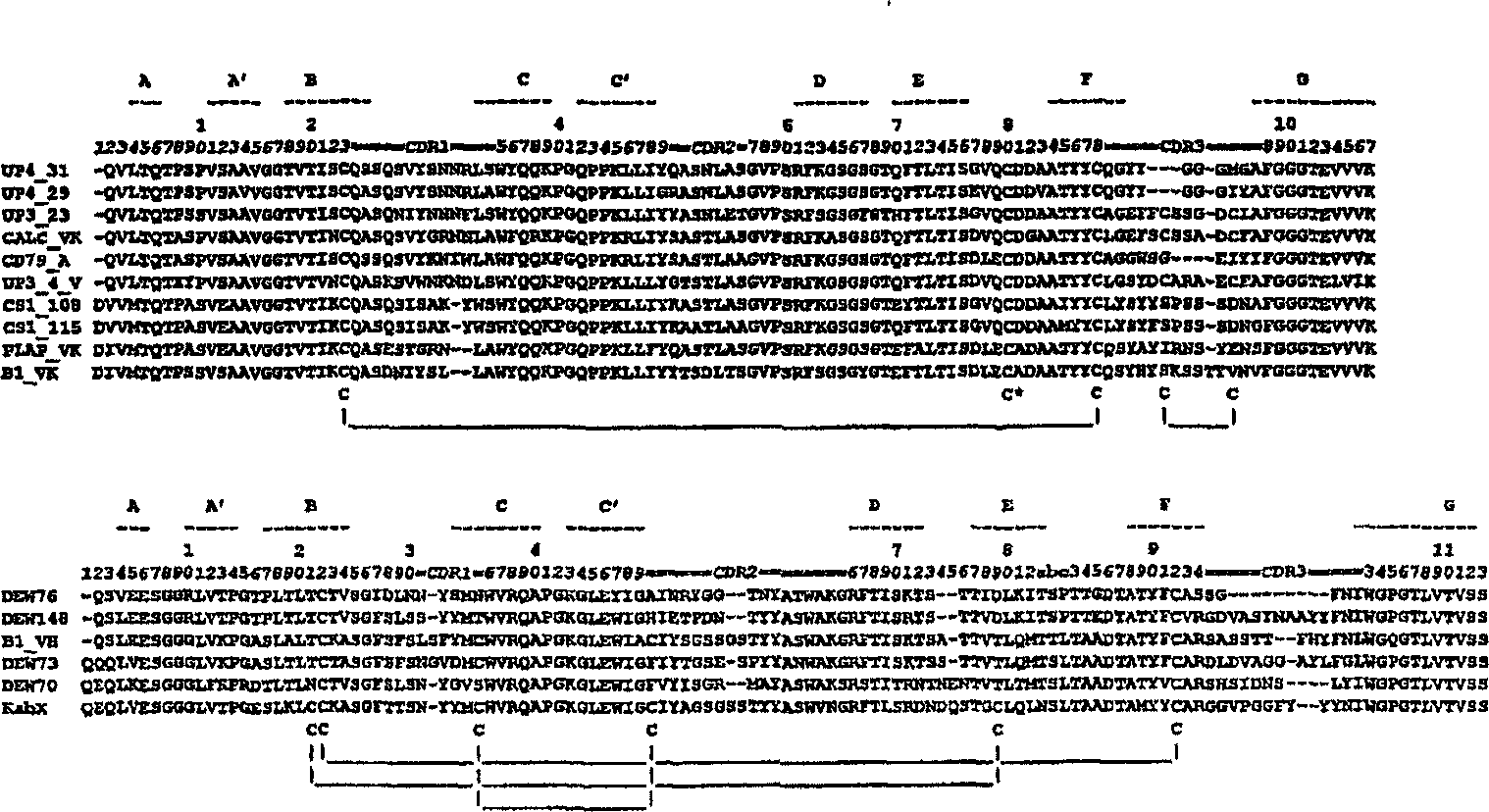
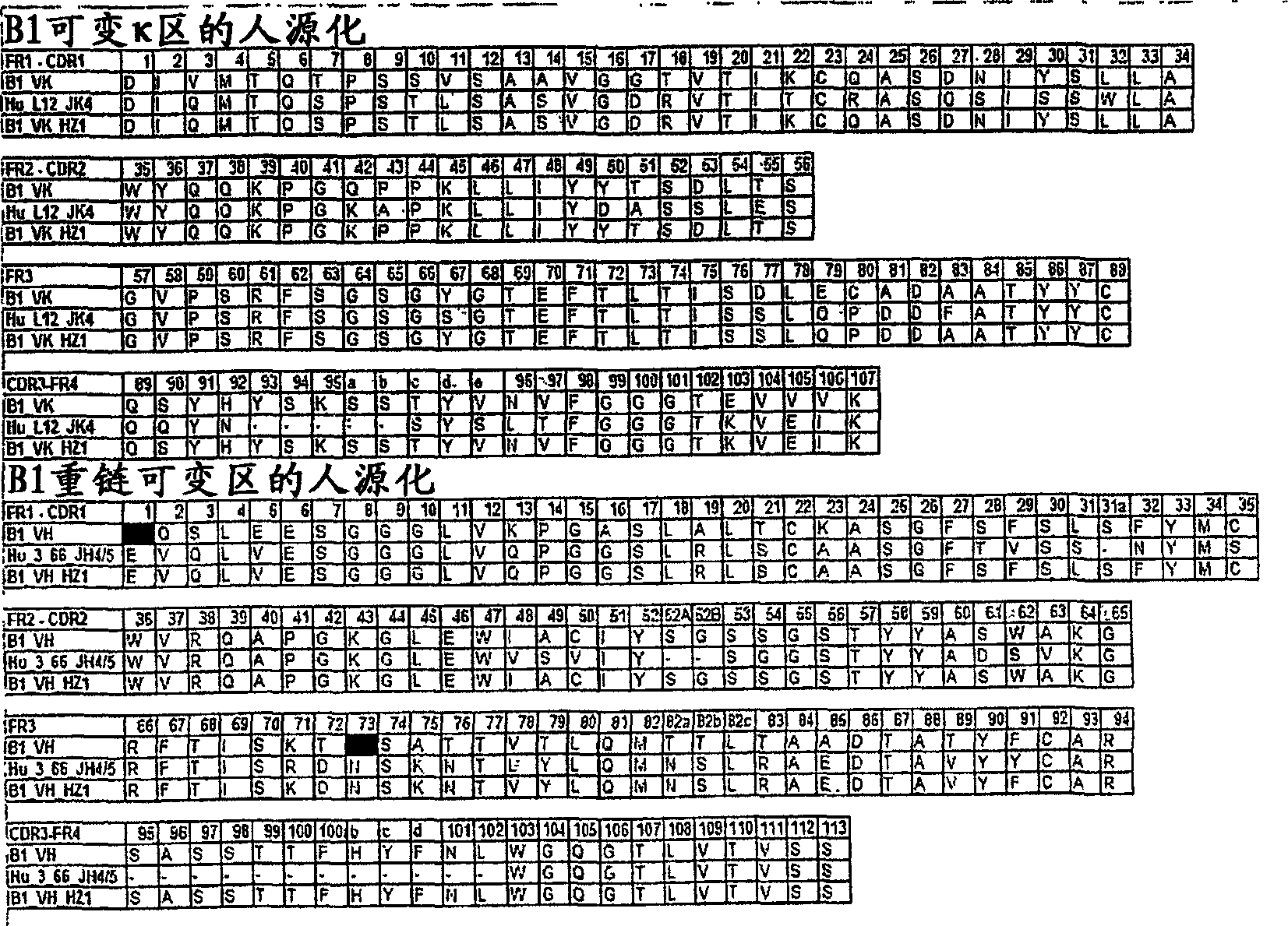
The invention provides a method for humanizing a rabbit monoclonal antibody. In general, the method involves comparing an amino acid sequences of a parent rabbit antibody to the amino acid sequences of a similar human antibody, and altering the amino acid sequence of the parent rabbit antibody such its framework regions are more similar in sequence to the equivalent framework regions of the similar human antibody. In many embodiments, amino acids in the parent rabbit antibody that are not CDR contact residues, interchain contact residues, or buried residues, are not modified. The invention further provides nucleic acids encoding the subject antibodies, as well as vectors and host cells comprising the nucleic acids and methods for producing a subject antibody. The subject antibodies, nucleic acid compositions and kits find use in a variety of applications, including diagnostics and therapeutic treatment and research of conditions and diseases.
Owner:EPITOMICS INC
Fusion partner for production of monoclonal rabbit antibodies
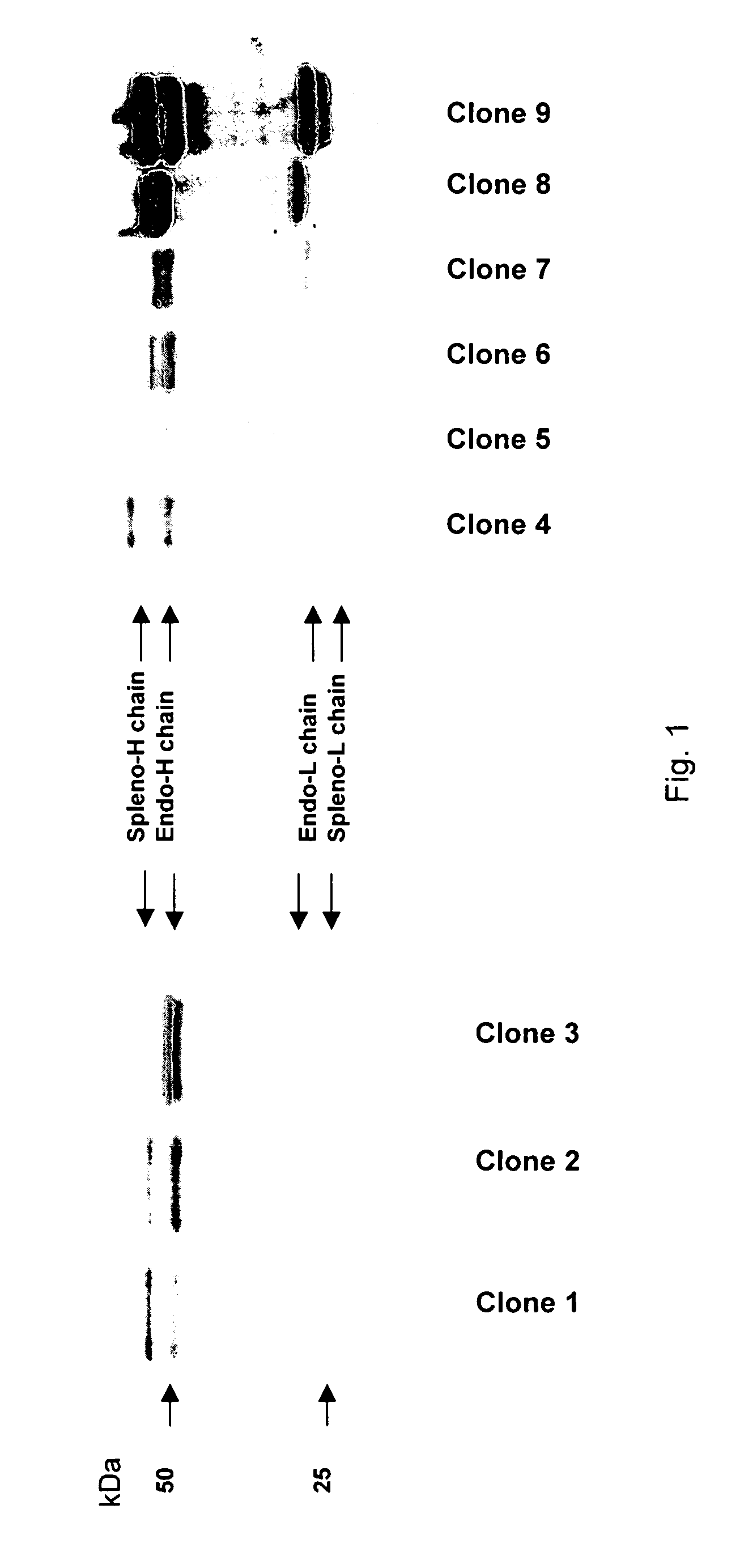
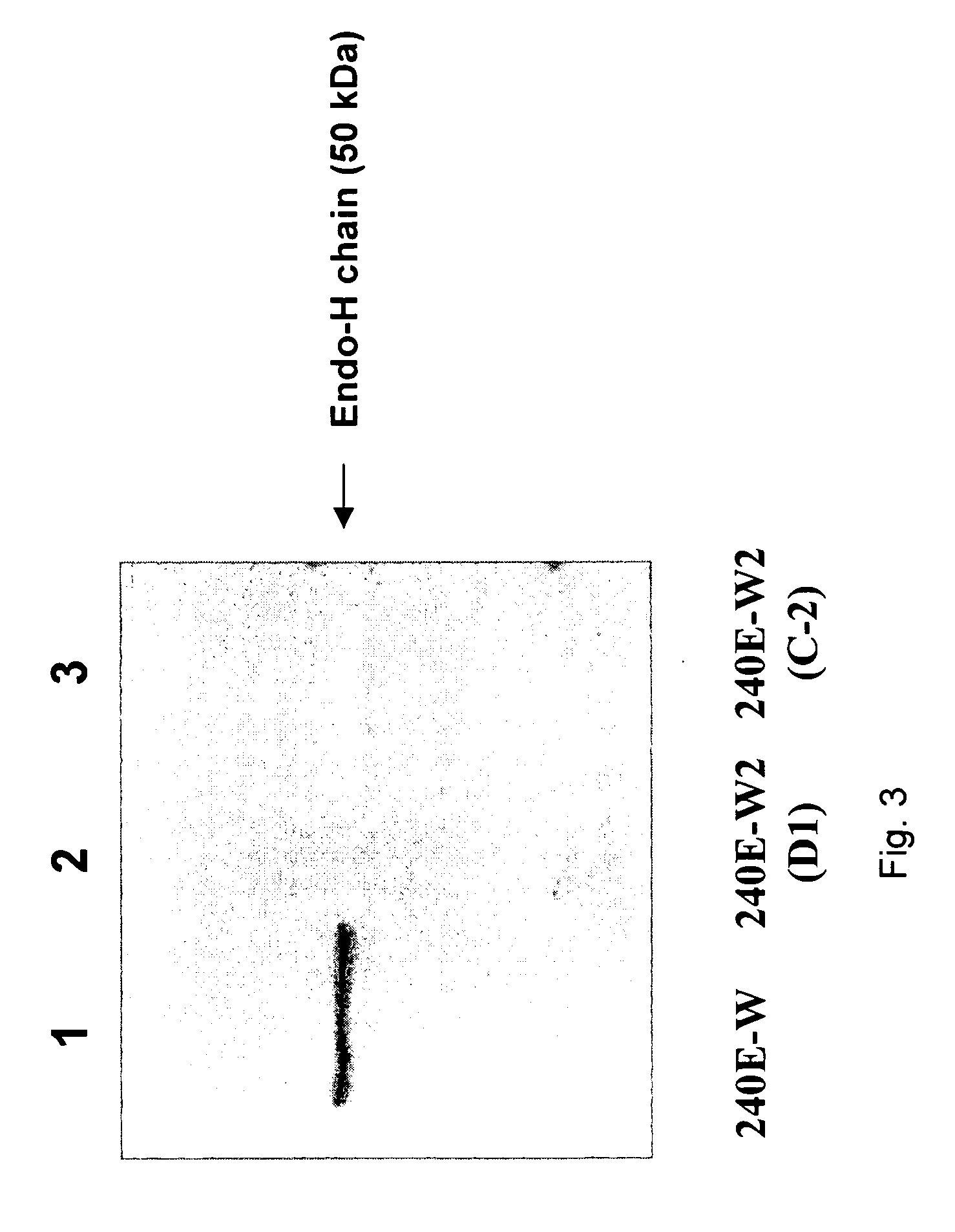
The invention provides a rabbit-derived immortal B-lymphocyte capable of fusion with a rabbit splenocyte to produce a hybrid cell that produces an antibody. The immortal B-lymphocyte does not detectably express endogenous immunoglobulin heavy chain and may contain, in certain embodiments, an altered immunoglobulin heavy chain-encoding gene. A hybridoma resulting from fusion between the subject immortal B-lymphocyte and a rabbit antibody-producing cell is provided, as is a method of using that hybridoma to produce an antibody. The subject invention finds use in a variety of different diagnostic, therapeutic and research applications.
Owner:EPITOMICS INC
Humanization of rabbit antibodies using a universal antibody framework
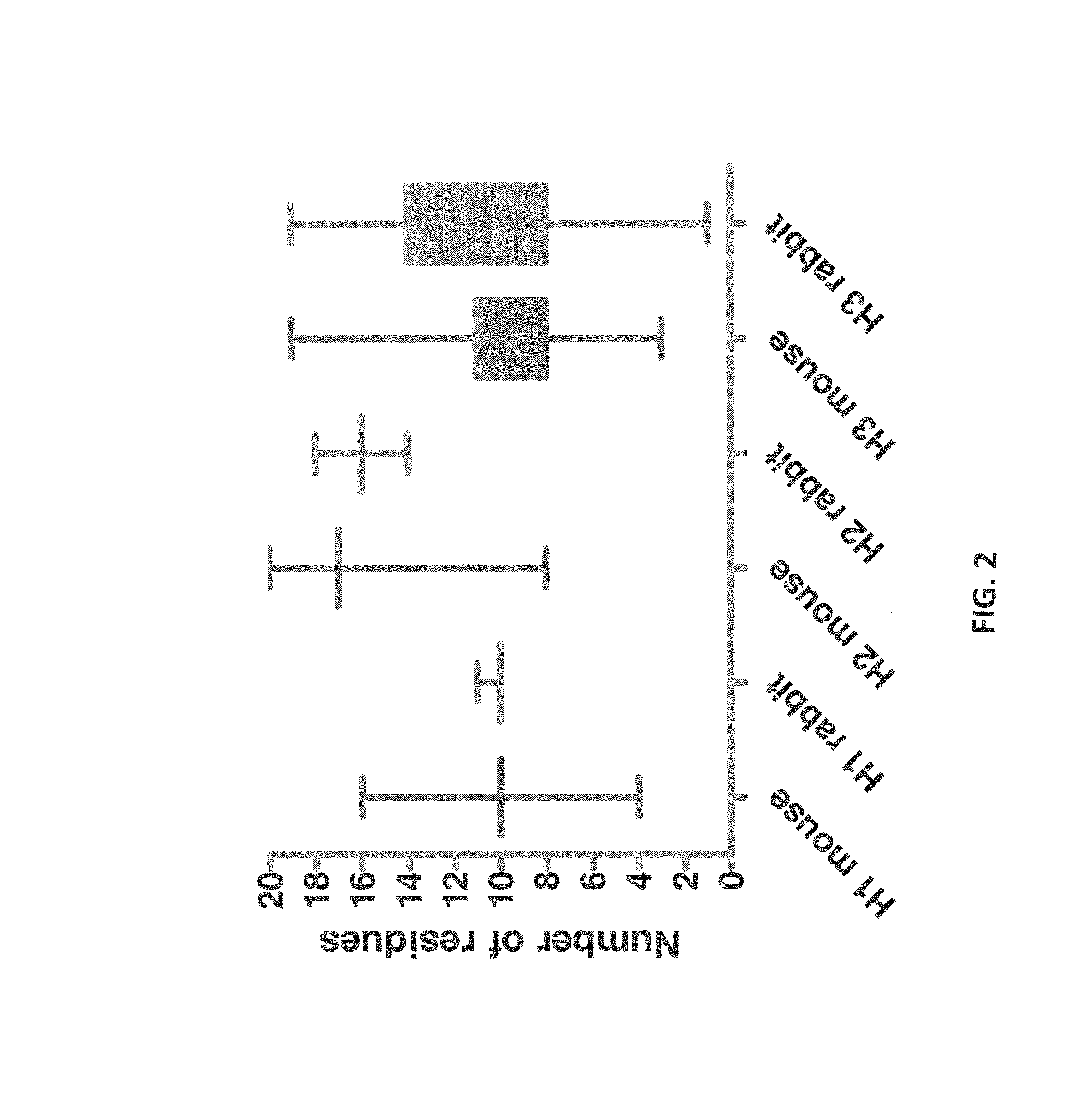
ActiveUS8293235B2Reliably retain the spatial orientation of the rabbit antibodiesImprove bindingSugar derivativesAntibody mimetics/scaffoldsAntibody receptorRabbit Antibody
The present invention relates to an universal antibody acceptor framework and to methods for grafting non-human antibodies, e.g., rabbit antibodies, using a universal antibody acceptor framework. Antibodies generated by the methods of the invention are useful in a variety of diagnostic and therapeutic applications.
Owner:NOVARTIS AG
Novel Rabbit Antibody Humanization Methods and Humanized Rabbit Antibodies
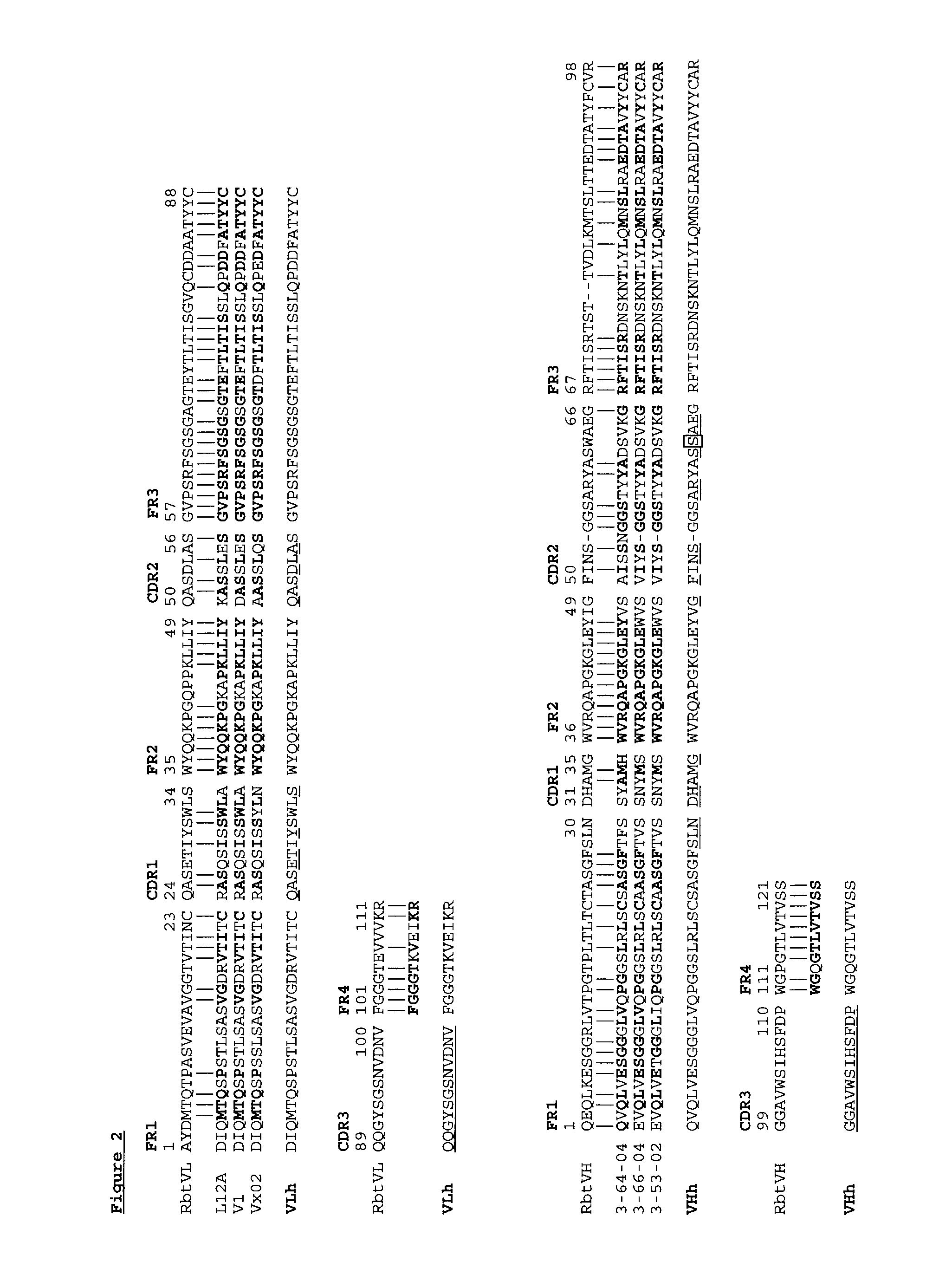
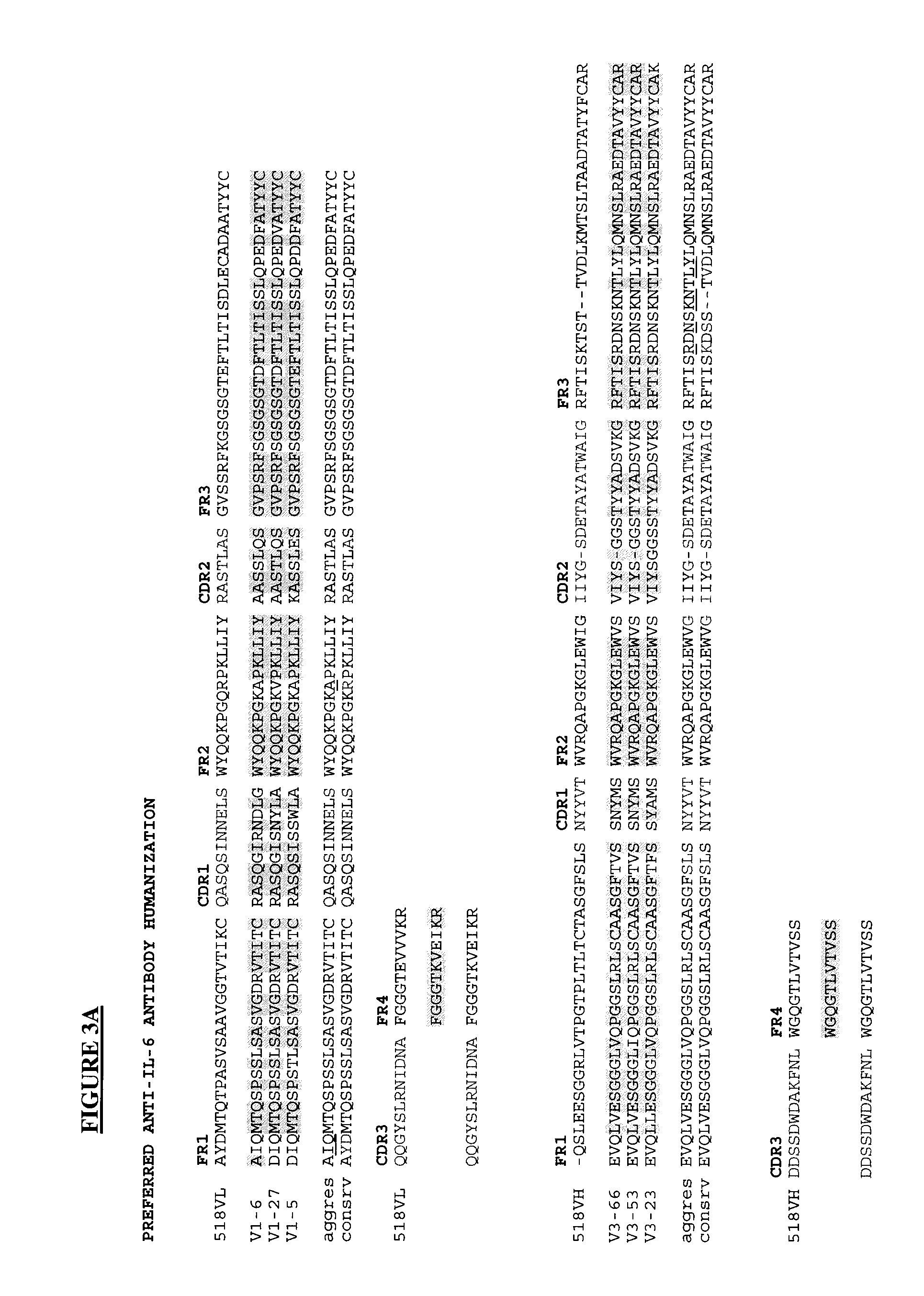
The present invention is directed to novel and improved methods for humanizing rabbit heavy and light variable regions. The resulting humanized rabbit heavy and light chains and antibodies and antibody fragments containing are well suited for use in immunotherapy and immunodiagnosis as they retain the antigen binding affinity of the parent antibody and based on their very high level of sequence identity to human antibody sequences should be essentially non-immunogenic in humans. The invention exemplifies the protocol for the manufacture of therapeutic humanized anti-human TNF-alpha and anti-human IL-6 antibodies.
Owner:ALDERBIO HLDG LLC
Novel rabbit antibody humanization methods and humanized rabbit antibodies
The present invention is directed to novel and improved methods for humanizing rabbit heavy and light variable regions. The resulting humanized rabbit heavy and light chains and antibodies and antibody fragments containing are well suited for use in immunotherapy and immunodiagnosis as they retain the antigen binding affinity of the parent antibody and based on their very high level of sequence identity to human antibody sequences should be essentially non-immunogenic in humans. The invention exemplifies the protocol for the manufacture of therapeutic humanized anti-human TNF-alpha and anti-human IL-6 antibodies.
Owner:ALDERBIO HLDG LLC
Acceptor framework for CDR grafting
ActiveUS8399625B1Improve bindingSuperior biophysical propertySugar derivativesImmunoglobulins against animals/humansCdr graftingReceptor for activated C kinase 1
The present invention relates to an antibody acceptor framework and to methods for grafting non-human antibodies, e.g., rabbit antibodies, using a particularly well suited antibody acceptor framework. Antibodies generated by the methods of the invention are useful in a variety of diagnostic and therapeutic applications.
Owner:CELL MEDICA INC
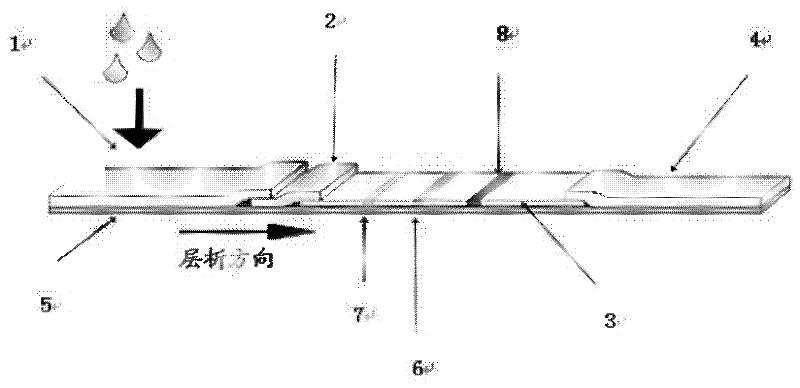
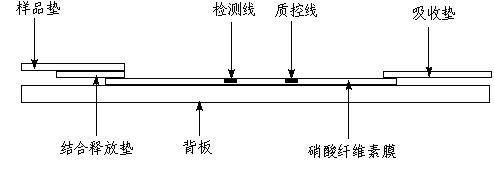
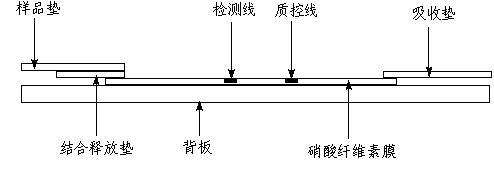
Fluorescent microsphere immunochromatographic testing card for testing five indexes of hepatitis b and method for preparing same
InactiveCN101726596ASimple and fast operationHigh sensitivityBiological testingLuminescent compositionsCelluloseHepatitis B virus
The invention discloses a fluorescent microsphere immunochromatographic testing card for testing five indexes of hepatitis b and a method for preparing the same. The testing card comprises a hepatitis b surface antigen test paper strip, a hepatitis b e surface antigen test paper strip, a hepatitis b surface antibody test paper strip, a hepatitis b e surface antibody test paper strip, and a hepatitis b core antibody test paper strip. Each test paper strip is formed by overlapping and bonding filter paper, a sample pad, a glass fiber film spray-coated with fluorescent microspheres, a cellulose nitrate film and water absorption paper on a bottom plate by glue in sequence, wherein the cellulose nitrate film is coated with antigens serving as a testing area and anti-rabbit antibodies serving as a quality control area; and during a test, after emitted fluorescent light passes a filter, the emitted spectrum is collected, accumulated and multiplied by the CCD scanning technology and then converted into a numerical signal, the numerical signal is multiplied by a correction factor, and the strength of the corrected fluorescent light is substituted in a standard curve of a fluorescence analyzer, so that the concentrations of the five indexes of hepatitis b of the sample can be automatically worked out. The test of hepatitis b viruses by the testing card has the characteristics of specificity, sensitivity, simpleness and accuracy.
Owner:WUXI ZODOLABS BIOTECH
Clenobuterol hydrochloride, salbutamol and paylean three joint inspection card and method for processing detecting sample
The invention relates to a clenobuterol hydrochloride, salbutamol and paylean three joint inspection card and a method for processing a detecting sample, and belongs to the technical field of detection of a beta-receptor stimulating agent, wherein a test strip is arranged inside a shell of the three joint inspection card, and the clenobuterol hydrochloride, salbutamol and paylean three joint inspection card is formed by the adhesion of a sample gasket, a colloidal gold membrane, a cellulose nitrate membrane and a water absorbing membrane to a bearing backboard in turn; the colloidal gold membrane is a glass fiber membrane of a colloidal gold marker containing a clenobuterol hydrochloride antibody, a salbutamol antibody and a paylean antibody; three detection strips are arranged on the cellulose nitrate membrane and contain clenobuterol hydrochloride protein conjugate, salbutamol protein conjugate and paylean protein conjugate respectively; and a quality control strip containing an anti-rabbit antibody or an anti-mouse antibody is arranged. The clenobuterol hydrochloride, salbutamol and paylean three joint inspection card has the advantages of simultaneously detecting the clenobuterol hydrochloride, the salbutamol and the paylean in urine or feed, animal tissue, meat and liver. The inspection card is easy to prepare and quick and convenient to use, saves the detection cost, and has accurate result.
Owner:无锡安迪生物工程有限公司
Test strip for detecting HIV antibodies in spittle and preparation method thereof
The invention discloses a test strip for detecting HIV antibodies in spittle and a preparation method thereof. The test strip comprises a sample pad, a fiberglass film, a nitrocellulose film and absorbent paper, wherein the fiberglass film is tightly connected with one end of the sample pad and contains colloidal gold particle markers; the nitrocellulose film is tightly connected with the other end of the fiberglass film; the absorbent paper is tightly connected with the other end of the nitrocellulose film; the sample pad, the fiberglass film, the nitrocellulose film and the absorbent paper are all arranged on a base plate; the nitrocellulose film comprises a detection area coated with HIV recombinant antigens and a control area coated with goat anti rabbit antibodies; and the colloidal gold particle marker comprises a colloidal gold particle-avidin-biotin-antihuman IgG antibody composite micro-signal amplification system, and colloidal gold marking rabbie IgG antibodies. Due to the adoption of the avidin-biotin micro-signal amplification system, the test strip amplifies signals of the target antibodies, improves detection sensitivity and avoids false negative or detection omission caused by too weak signals.
Owner:GUANGZHOU WONDFO BIOTECH
Reagent box for detecting clenbuterol hydrochloride and its detection method
InactiveCN1793927ASimple structureEasy to useChemiluminescene/bioluminescenceTime resolved fluorescence immunoassayBiology
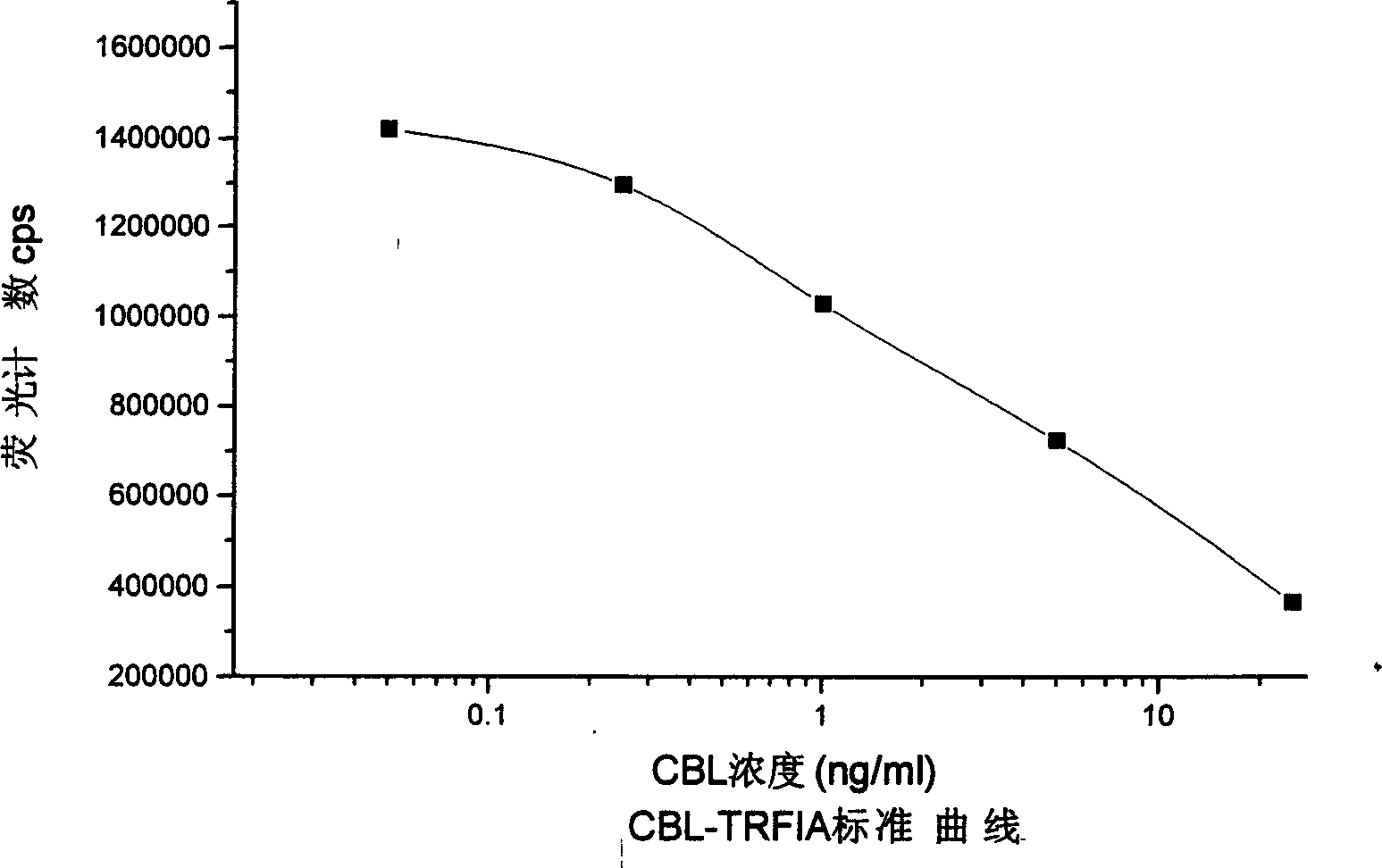
A method for detecting CBL includes competing CBL antibody by tree CBL with CBL ¿C OVA on micro hole board, washing off unconnected CBL antibody, adding EU3+ - sheep resisting rabbit antibody and washing off unconnected EU3+ - sheep resisting rabbit antibody, adding intensified liquid and using time ¿C identification luminoscope to determine its fluorescence intensity, presenting fluorescence intensity to CBL concentration in sample to be inverse ratio, comparing with standard curve to obtain CBL content in sample. The reagent kit for realizing said method is also disclosed.
Owner:JIANGNAN UNIV
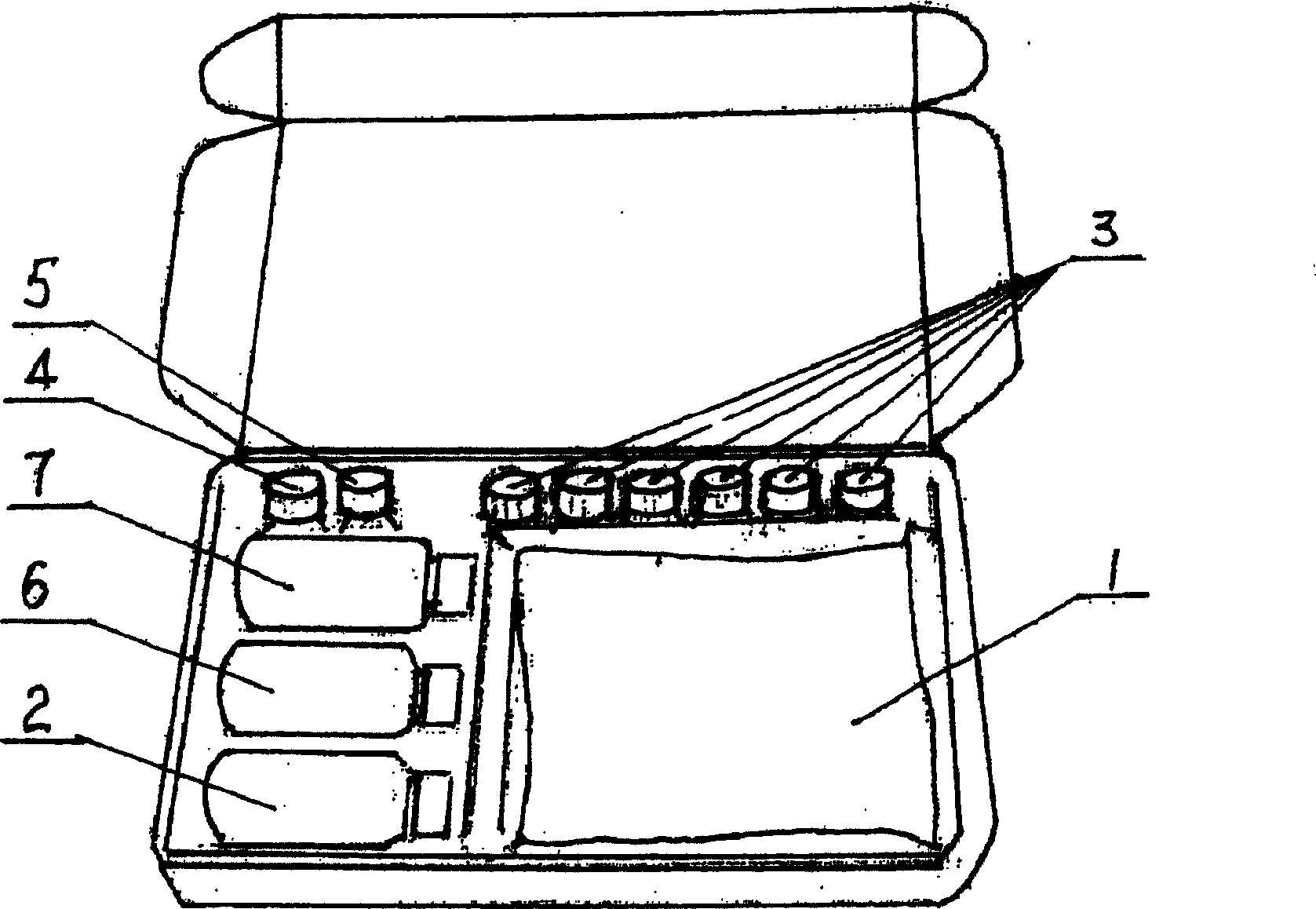
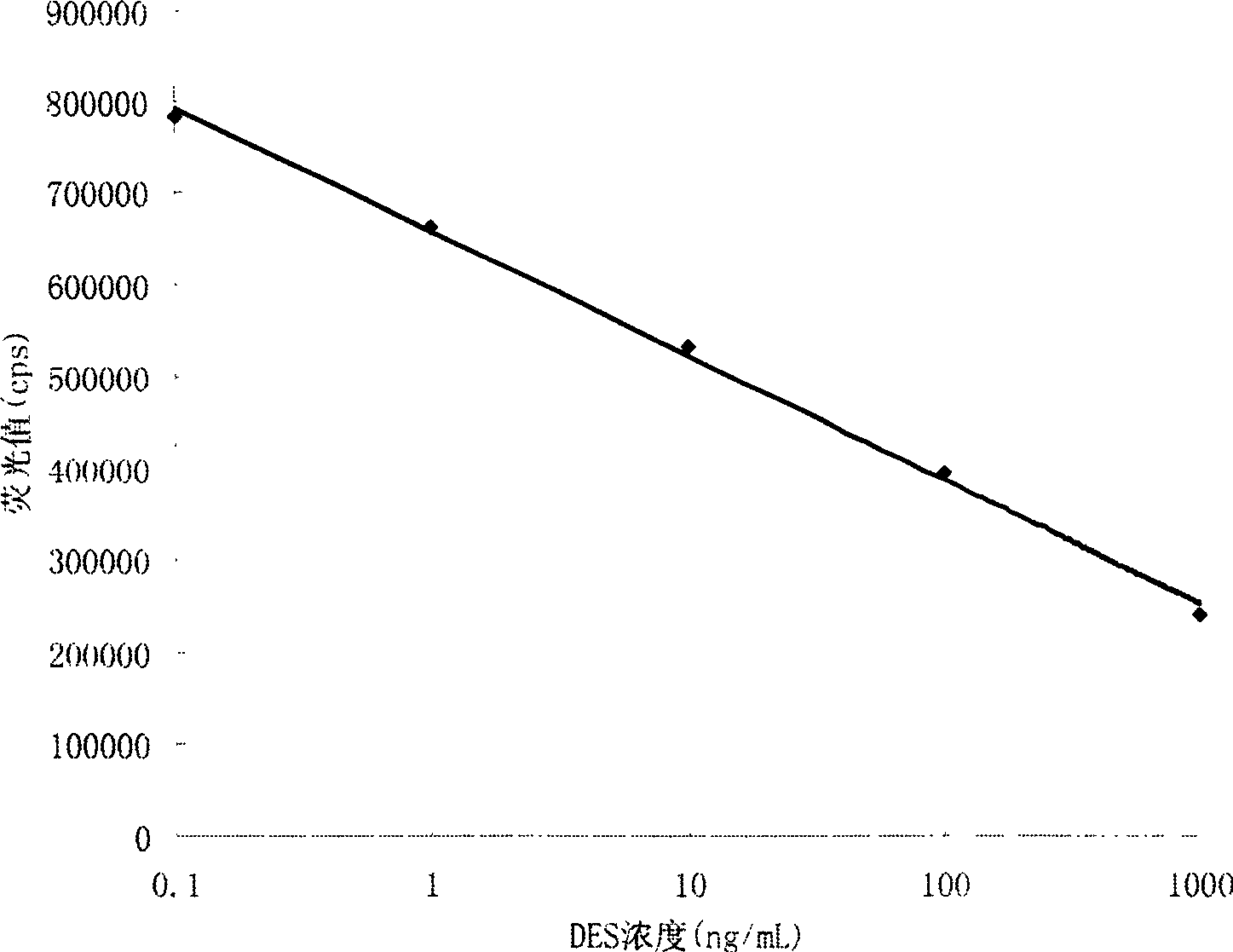
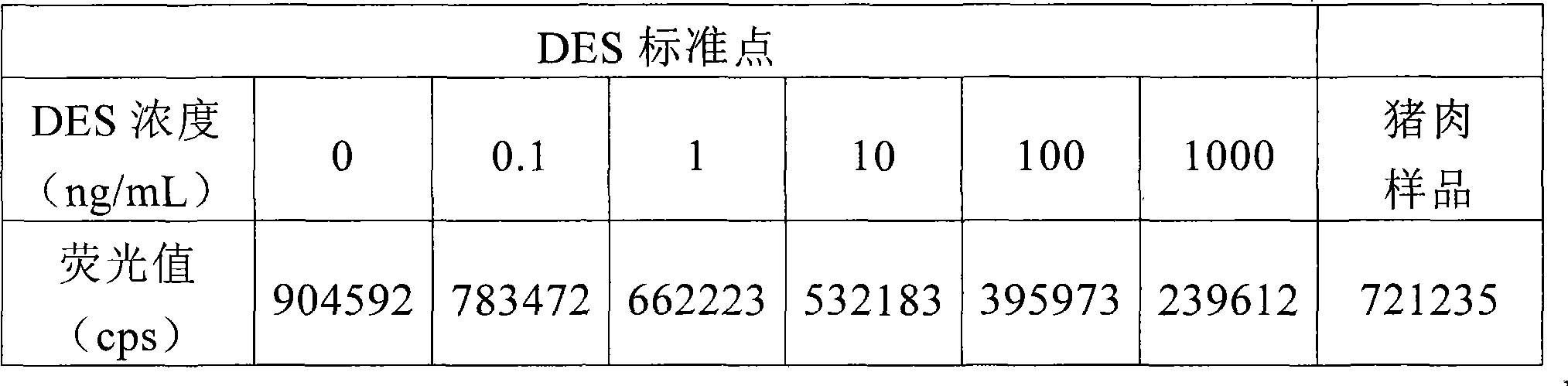
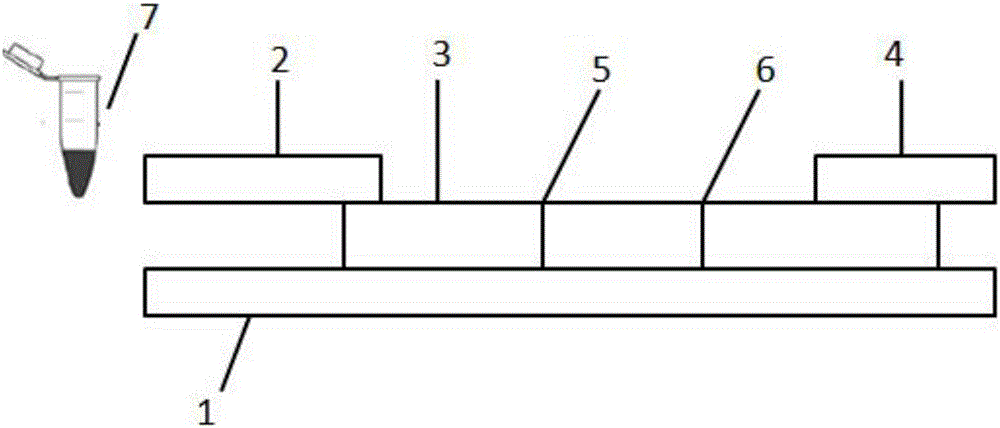
Detection reagent kit and detection method for diethyl stilbestrol
InactiveCN101482562ASimple structureEasy to useMaterial analysisTime resolved fluorescence immunoassayCarrier protein
The invention provides a kit for detecting diethylstilbertrol and detection method thereof, belonging the the time resolution fluoroimmunoassay (TRFIA) technical field, capable of detecting the content of diethylstilbestrol (DES) in animal-source food, blood, urine and feedstuff. The kit uses the TRFIA to detect the DES and the mark immune response is the base of the detection. A micropore plate is coated by DES-carrier protein and added with DES standard or sample and then added with DES antibody. The free DES and the DES-carrier protein on the micropore plate complete the DES antibody and the DES antibody which is not bonded with the free DES or the DES-carrier protein is cleared away and added with the EU3+-goat anti-rabbit antibody, after labeling the immune reaction, the non-bonded EU3+-goat anti-rabbit antibody is cleared away. After adding the reinforcement liquid, the fluorescence intensity cps is detected using a time resolution fluorescence instrument and the fluorescence intensity is inversely proportional to the DES concentration in sample and the content of DES in sample can be determined according to the standard curve. The DES detection kit has features of simple structure, convenient use, cheapness, high sensitivity up to 1ng / mL.
Owner:JIANSGU INST OF MICROBIOLOGY
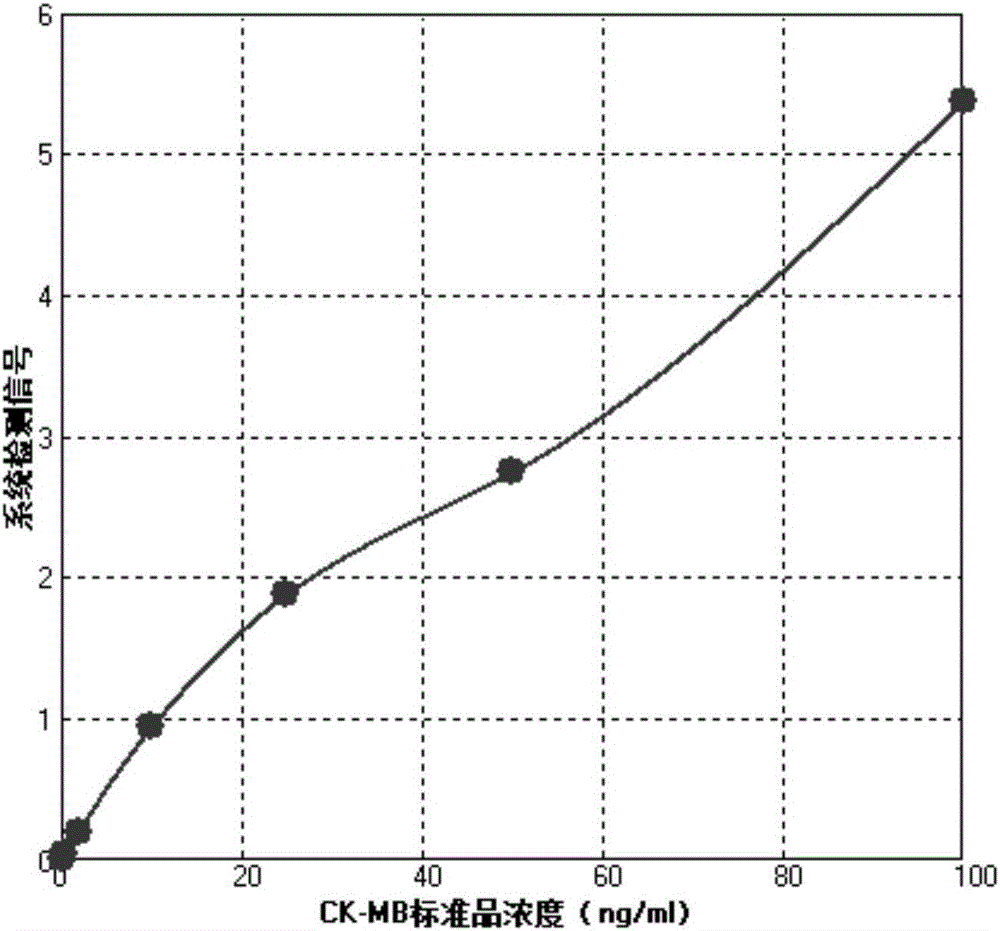
Time-resolved fluorescence immunochromatography reagent used for rapid quantitative detection of CK-MB, and preparation method thereof
InactiveCN106248927AHigh densityImprove accuracyFluorescence/phosphorescenceMonoclonal antibodyQuality control
The invention discloses a time-resolved fluorescence immunochromatography reagent used for rapid quantitative detection of CK-MB, and a preparation method thereof, and belongs to the field of clinical diagnose. The time-resolved fluorescence immunochromatography reagent is composed of a test paper strip and a fluorescent liquid; the test paper strip comprises a base plate, Fusion5, a nitrocellulose membrane, and a water absorption pad; Fusion5, the nitrocellulose membrane, and the water absorption pad are connected in sequence in the horizontal direction, and are fixedly arranged on the base plate; the nitrocellulose membrane is coated with a CK-MB monoclonal antibody 1 detection line and a quality control line composed of rabbit IgG antibodies; and the fluorescent liquid comprises CK-MB monoclonal antibody 2 labelled fluorescent microspheres, and goat anti-rabbit antibody labeled fluorescent microspheres. According to the time-resolved fluorescence immunochromatography reagent, time-resolved fluorescent microspheres are used for increasing fluorescence intensity, reducing background signals, and realizing quantitative detection of CK-MB content of whole blood, serum, or blood plasma at the same time; and only 10 to 20ml of a sample is needed. Application of the time-resolved fluorescence immunochromatography reagent is convenient and rapid; operation is simple; detection time is short; specificity and sensitivity are high; detection results are more accurate; and the time-resolved fluorescence immunochromatography reagent is suitable for clinical POCT rapid diagnosis.
Owner:SHANGHAI UPPER BIO TECH PHARMA
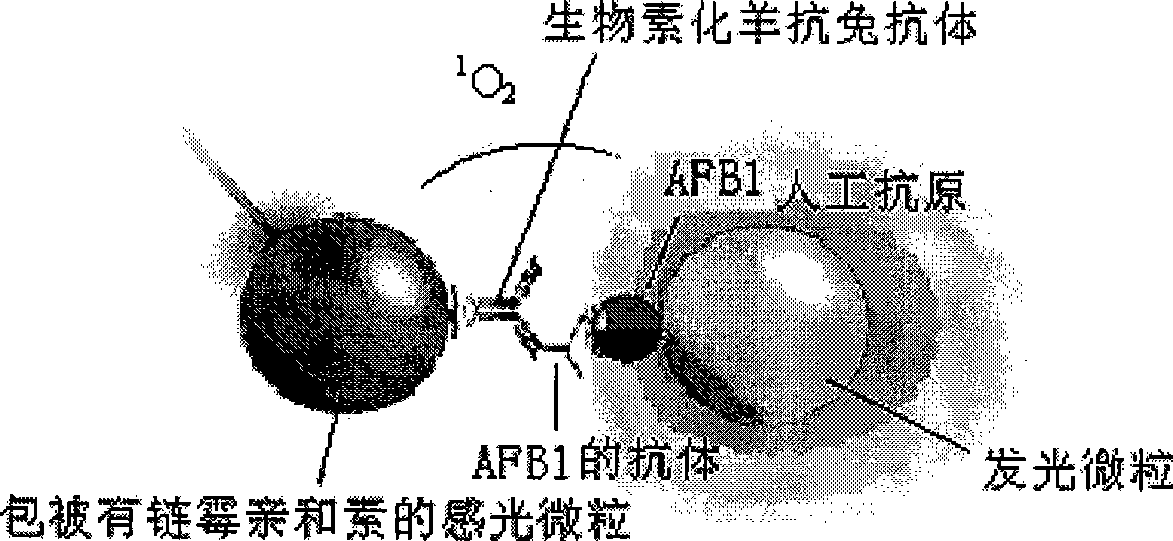
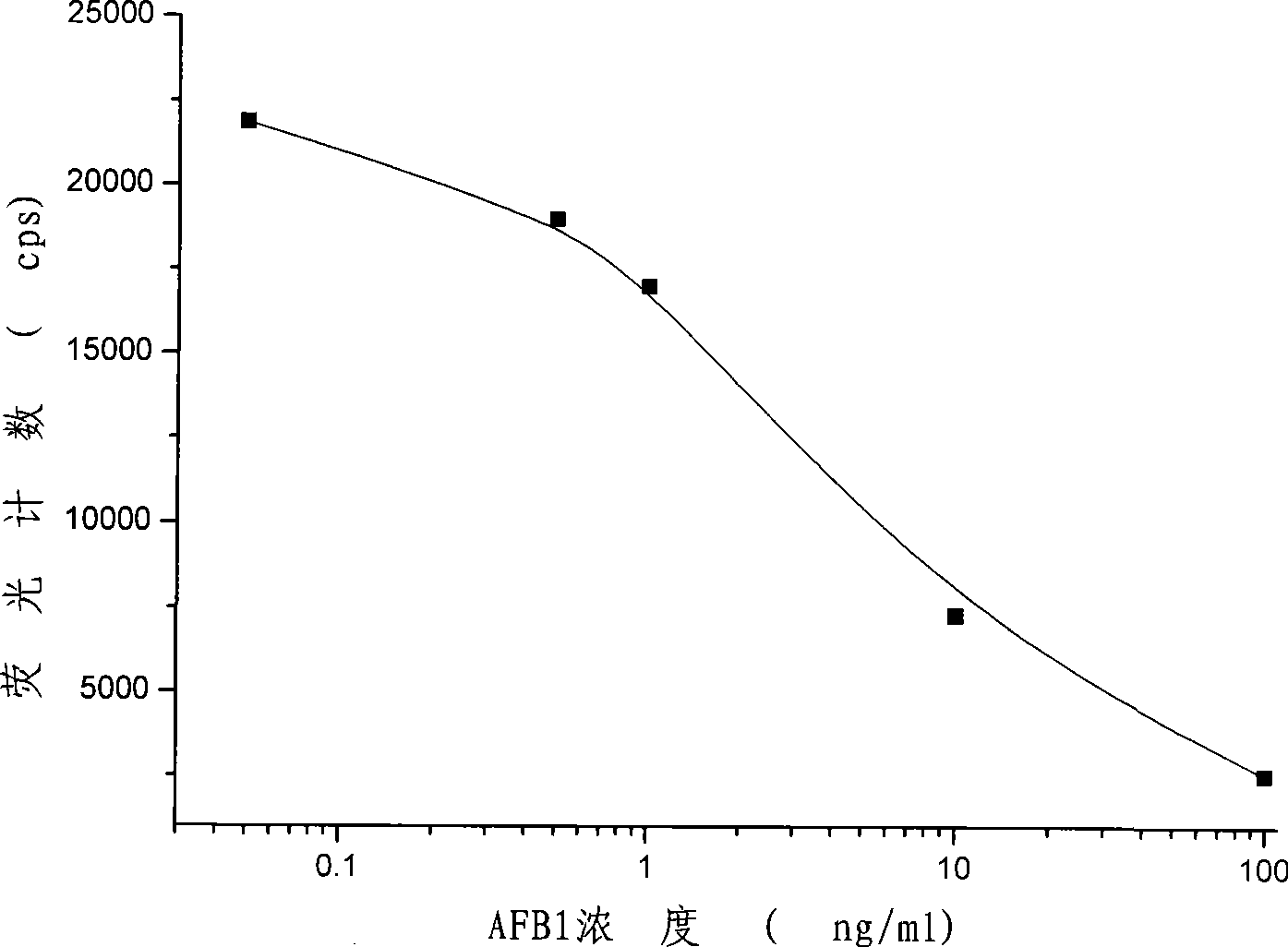
Light induced chemiluminescent immunoassay kit of aflatoxin B1 and detecting method thereof
InactiveCN101393208ASimple structureSimple and fast operationChemiluminescene/bioluminescenceFluorescence/phosphorescenceBiotin-streptavidin complexFluorescence
The invention discloses a light induced chemiluminescent immunoassay reagent kit for aflatoxin B1and a detection method thereof, which belong to the technical field of light induced chemiluminescent immunoassay. A luminescent particle enveloped with AFB1-BSA is added into a microporosity plate, a AFB1 standard or sample, a rabbit anti-AFB1 antibody, a biotin goat anti-rabbit antibody are sequentially added for a photophobic reaction, then a photosensitive particle enveloped with streptavidin is photophobically added, and the mixture is incubated and then is detected. The AFB1-BSA enveloped on the luminescent particle competes with the AFB1 in the standard or sample for connecting to the AFB1 antibody to form a complex with the biotin goat anti-rabbit antibody and the photosensitive particle enveloped with the streptavidin, the energy is transferred to the luminescent particle to produce fluorescence by producing and transferring singlet ionic oxygen under optical excitation, a light induced chemiluminescent detector is used to detect, the intensity of optical signals is inversely proportional to the concentration of the AFB1, and the content of the AFB1 in the measured sample is determined by contrasting with the standard curve. The invention is used to detect the content of the AFB1 in foodstuff, feedstuff and products of the foodstuff and the feedstuff; and the reagent kit has the advantages of simple structure, simple and convenient operation, low cost, short detection time, and high sensitivity.
Owner:JIANGSU INST OF NUCLEAR MEDICINE
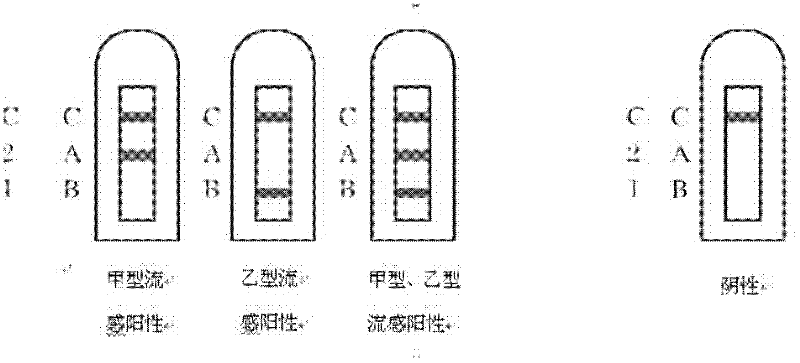
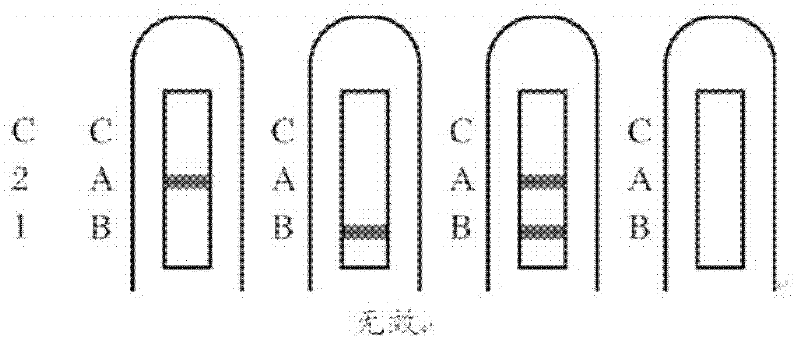
Combined detection test paper of influenza A virus antigen and influenza B virus antigen and preparation method thereof
InactiveCN102445537AEnhanced signalHigh detection sensitivityMaterial analysisInfluenza B virus antigenPHA granule
The invention discloses combined detection test paper of influenza A virus antigen and influenza B virus antigen and a preparation method thereof. The test paper comprises a sample pad, a fiberglass membrane containing a colloidal gold particle label, a nitrocellulose membrane and water absorbing paper, wherein the nitrocellulose membrane comprises a detection area which is coated with an influenza A virus antibody, a detection area which is coated with an influenza B virus antibody and a control area which is coated with an goat anti-rabbit antibody; the colloidal gold particle label comprises a micro signal amplification system and a colloidal gold labeled rabbit IgG antibody; and the micro signal amplification system is a colloidal gold particle-avidin-biotin-influenza A / B virus antibody. According to the invention, an avidin-biotin microsignal amplification system is added in a double-antibody sandwich detection system, the signal of a target antibody is enlarged, the detection sensitivity is increased, false negative or detection omission due to weak signals can be avoided, simultaneously combined detection can be carried out on the influenza A and B virus antigens, and the detection time, sample and cost can be saved.
Owner:GUANGZHOU WONDFO BIOTECH
Immune-fluorescence test strip component for rapidly detecting C-reactive protein quantitatively, detection card component produced by same and method for preparing same
ActiveCN102680702AHigh sensitivityImprove signal-to-noise ratioBiological testingFluorescence/phosphorescencePorphyrinIMMUNE FLUORESCENCE
The invention discloses an immune-fluorescence test strip component for rapidly detecting C-reactive protein quantitatively, a detection card component produced by the same and a method for preparing the same. The test strip component comprises a test strip and a platinum porphyrin marked specific antibody independently packed. The test strip comprises a bottom lining, a water absorption pad, coating analysis film and a sample pad. The coating analysis film is provided with a detection line and a quality control line, a specific antibody coated by the detection line is a C-reactive protein resistance monoclonal antibody, and a specific antibody coated by the quality control line is a rabbit intravenous gamma globulin (IgG) antibody; the detection card component comprises a test strip, a card box composed of a cover plate and a back plate and a latinum porphyrin marked specific antibody independently packed. The test strip component for detecting C-reactive protein in body fluid has the advantages of being simple in operation, rapid, sensitive, good in specificity and the like, and having good clinical application prospect.
Owner:GUANGZHOU HONGQI OPTICAL INSTR TECH
Method for quickly immunologically detecting beta-lactamase in milk
InactiveCN101710122AStrong specificityImprove accuracyMaterial analysisMonoclonal antibodyHorseradish peroxidase
The invention provides a method for quickly immunologically detecting a beta-lactamase in milk, and belongs to the technical field of immunological detection. The method is characterized in that: a three-antibody sandwich method reagent consists of a rat anti-beta-lactamase monoclonal antibody coated on an elisa plate, a rabbit anti-beta-lactamase polyclonal antibody and a horseradish peroxidase labelled sheep and rabbit antibody; and immunological detection is performed to detect the beta-lactamase in a raw milk by a three-antibody sandwich method. Compared with the prior art, the method canaccurately, specially and quickly immunologically detect the beta-lactamase illegally added into the milk so as to timely monitor and control the quality of the raw milk from the source and guaranteethe safety of the milk and the health of human, and is good in social and economic efficiency.
Owner:BIOLOGY INST OF HEBEI ACAD OF SCI
Multi-in-one combined detection kit for drug and preparation process and enclosed reagent thereof
InactiveCN1632583AAppropriate detection sensitivityHigh precisionMaterial analysisTest agentBiochemical engineering
It is a poison multiple and combination test agent box and its process method, which comprise plastic shell and test paper bar. The plastic shell comprises upper cover and down cover, wherein, the upper cover comprises specimen adding holes, test result display hole and fix mark hole; the down cover is set with flute. The test paper comprises test specimen area, test result display area and water absorption area, wherein, the test specimen area comprises glue gold and filter paper; the test result display area comprises pyroxylin film, C line control area with sheep and rabbit polyclonal antibody, test display area with heroin antigen and test display area with methyl amphetamine antigen. The water absorption area is set with absorption paper and fix mark.
Owner:梅里埃(上海)生物制品有限公司
Methods for humanizing rabbit monoclonal antibodies
InactiveCN1839144AHybrid immunoglobulinsBacteriaComplementarity determining regionTherapeutic treatment
The invention provides a method for humanizing a rabbit monoclonal antibody. In general, the method involves comparing an amino acid sequences of a parent rabbit antibody to the amino acid sequences of a similar human antibody, and altering the amino acid sequence of the parent rabbit antibody such its framework regions are more similar in sequence to the equivalent framework regions of the similar human antibody. In many embodiments, amino acids in the parent rabbit antibody that are not CDR contact residues, interchain contact residues, or buried residues, are not modified. The invention further provides nucleic acids encoding the subject antibodies, as well as vectors and host cells comprising the nucleic acids and methods for producing a subject antibody. The subject antibodies, nucleic acid compositions and kits find use in a variety of applications, including diagnostics and therapeutic treatment and research of conditions and diseases.
Owner:EPITOMICS INC
Monoclonal antibody for detection and classification of cervical cancer and application thereof
ActiveCN105131113AStrong specificityHigh affinityImmunoglobulins against animals/humansAntibody ingredientsCervical lesionCervical tissue
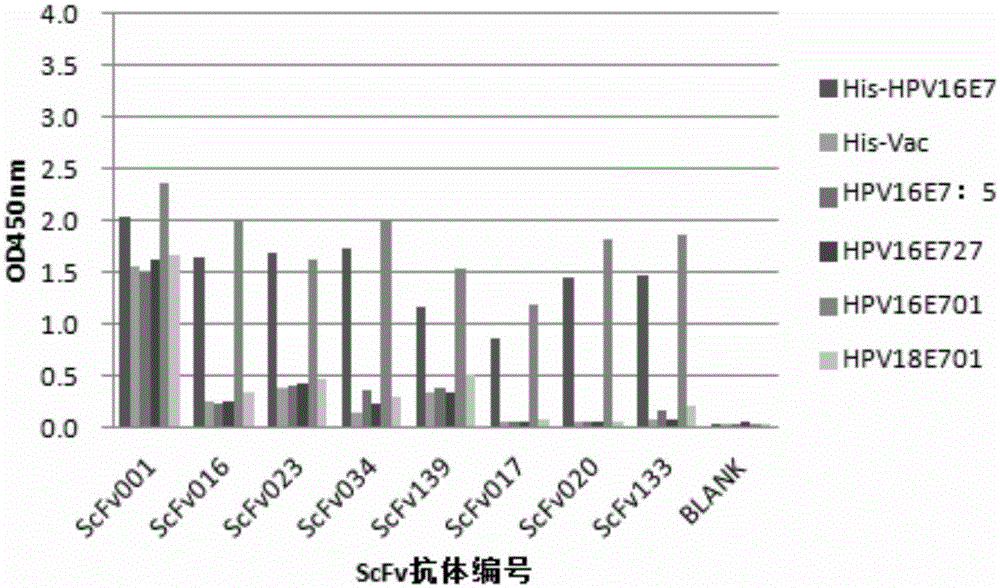
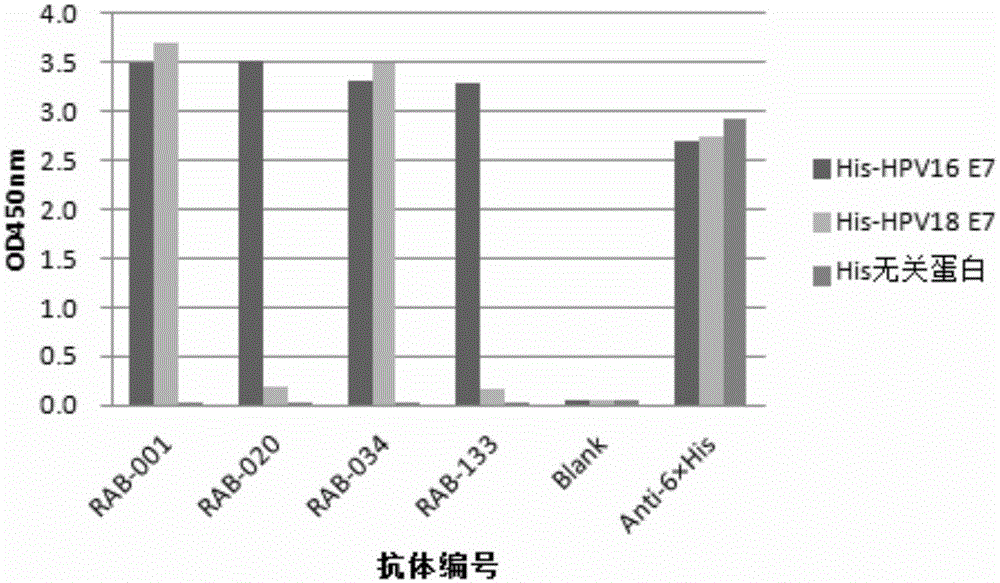
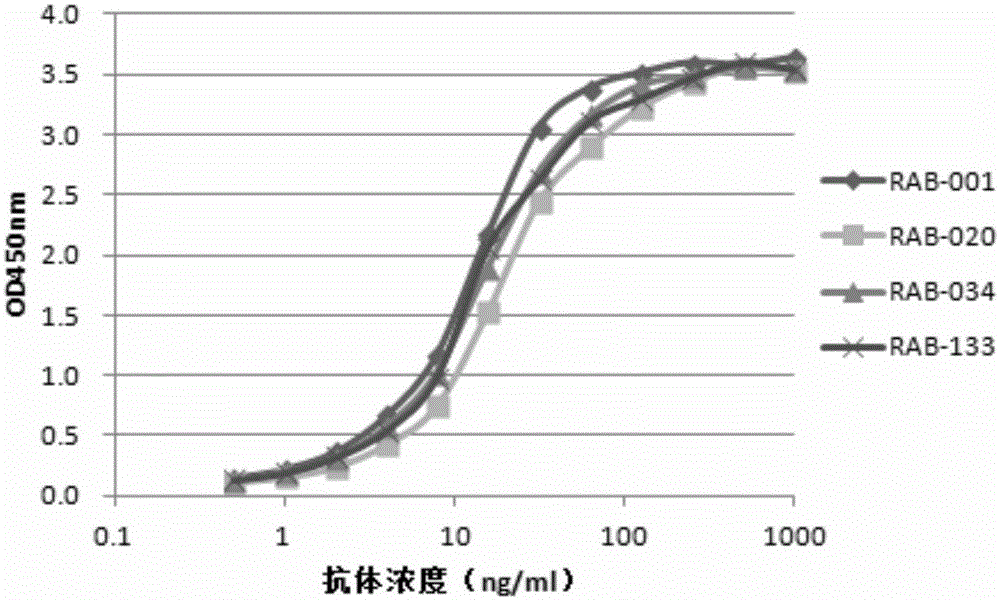
The invention provides a monoclonal rabbit antibody for identifying HPV16 positive cervical tissues. The antibody can be used for specifically detecting a biomarker HPV16E7 protein in tissues including cervical cancer and cervical lesions, so that HPV persistent infection related cervical cancer tissues from abnormal or noncancerous cervical epithelial tissues, cervical cancer caused by high-risk HPV infection can be accurately diagnosed, and risk early-warning can be especially performed for whether early cervical lesions cancerate or not, so that the misdiagnosed rate of cervical lesions can be effectively reduced, and injuries and resource waste caused by over treatment for patients can be improved.
Owner:ATTOGEN BIOMEDICAL SUZHOU INC
Benzopyrene rapid detection card
A benzopyrene rapid detection card belongs to the technical field of food detection. A test strip is arranged in the casing of the benzopyrene rapid detection card; the test strip is formed by sticking a sample pad, a colloidal gold film, a nitrocellulose film and a water absorbent film on a test strip support back plate in turn, wherein the colloidal gold film is a fiberglass membrane containing the anti-benzopyrene antibody colloidal gold marker; and the nitrocellulose film is provided with two display belts, one is a detection belt containing benzopyrene-protein conjugate, the other is a quality control containing anti-rabbit antibody or anti-rat antibody. The invention has the advantage that the immunological method is used to directly detect the content of benzopyrene in samples such as edible oil and fried food. The benzopyrene rapid detection card is easy to prepare and convenient and fast to use, and the detection result is accurate.
Owner:无锡安迪生物工程有限公司
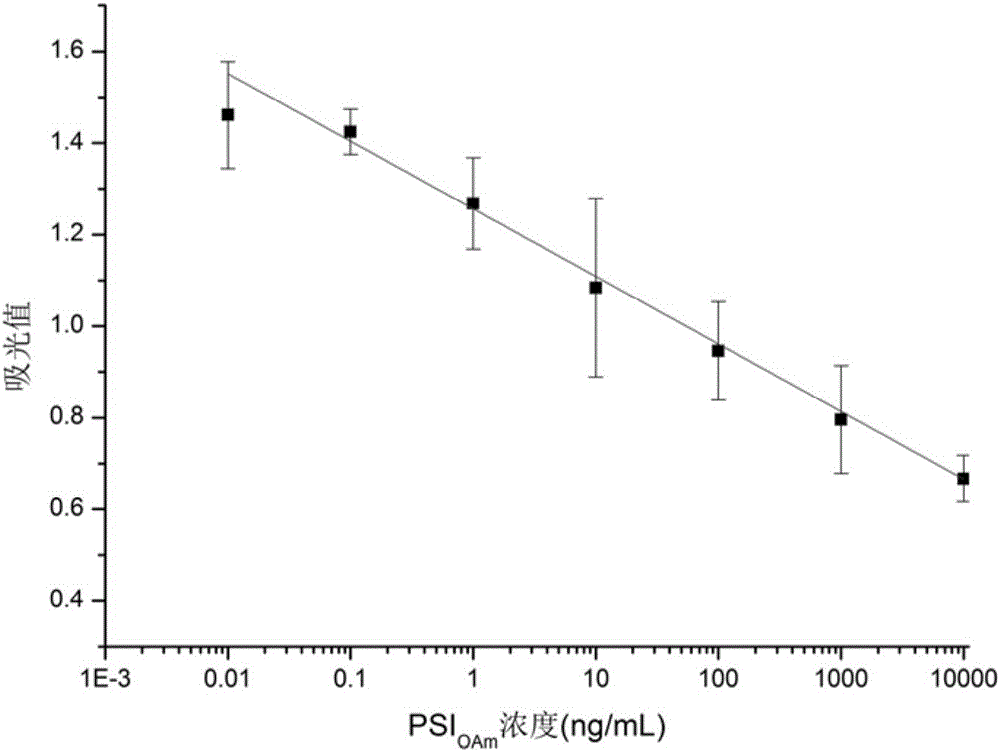
Method for quantitatively detecting oleylamine grafted polysuccinimide macromolecule nanometer drug carrier based on indirect competitive enzyme-linked immunosorbent assay
The invention discloses a method for quantitatively detectingan oleylamine grafted polysuccinimide(PSIOAm) macromolecule nanometer drug carrier based on an indirect competitive enzyme-linked immunosorbent assay. A secondary antibody is utilized, so that a detection signal is amplified, and the sensitivity of experimental analysis is improved; the aim of quantitatively detecting the PSIOAm is achieved through measuring an optical signal of a compound of a PSIOAm envelope antigen, a PSIOAm antibody which is the primary antibody and an HRP-marked goat anti-rabbit antibody which is the secondary antibody. The method is simple to operate, high in feasibility, high in sensitivity, low in detection limit, and capable of realizing high throughput detection.
Owner:ANHUI NORMAL UNIV
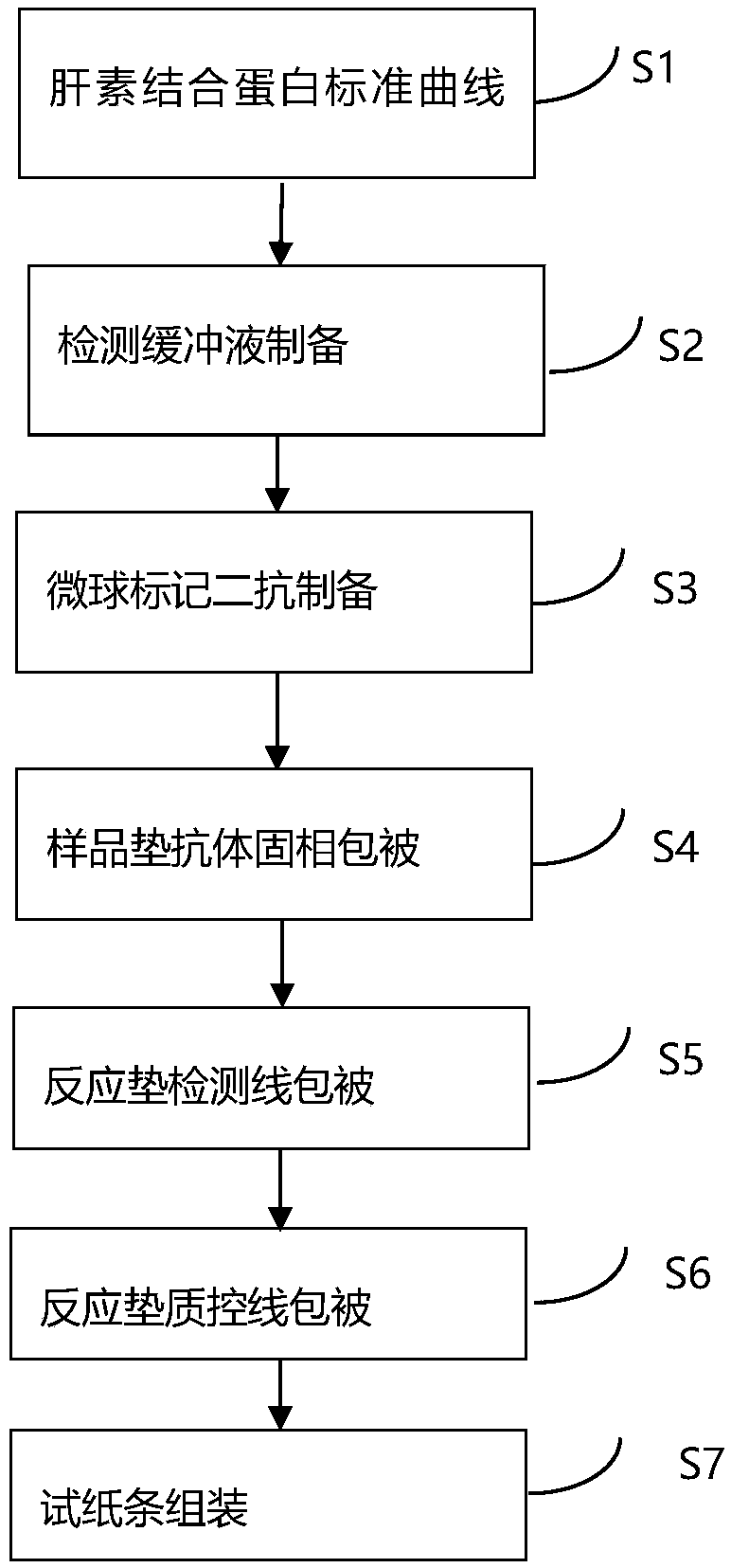
Heparin binding protein detection test paper for immunomicrosphere chromatography detection
InactiveCN108267592ASimple clinical operationHigh sensitivityBiological testingMicrosphereFluorescence
The invention relates to the technical field of clinical infectious disease marker detection, in particular to heparin binding protein detection test paper for immunomicrosphere chromatography detection. A method includes the following steps that a calibration curve is prepared; a detection buffer solution containing rabbit anti-human heparin binding protein antibody is prepared, fluorescent or colorful latex microsphere-marked chicken anti-rabbit antibody is prepared, a sample pad coats a solid phase, a detection line coated with a mouse anti-human heparin binding protein antibody and a reaction pad coated with a goat anti-rabbit antibody quality control line are prepared, a separation membrane is assembled, a test paper strip is assembled, a clinical sample is detected, and the dried test paper strip is placed in an aluminum foil bag. The heparin binding protein detection test paper for fluorescence chromatography detection has high cost effectiveness, and the level of heparin binding protein in body fluids of patients is quantitatively detected and evaluated. The method is used for a POCT detection system and screening or detection of changes of heparin binding protein in the body fluids of the patients to determine a detection method of infectious disease markers so that the heparin binding protein detection technology can become a clinical conventional detection project.
Owner:PRO MED BEIJING TECH
Citrus yellow shoot candidatus liberibacter asiaticus detection test paper
The invention belongs to the plant protection subject of the agricultural category, and particularly relates to citrus yellow shoot candidatus liberibacter asiaticus detection test paper. The detection test paper is prepared through the following steps that pathogenic bacteria antigen is extracted and purifed from catharanthus roseus host with citrus yellow shoot candidatus liberibacter asiaticus antigen and used for injecting immunized rabbits to obtain yellow shoot candidatus liberibacter asiaticus specific antiserum, the yellow shoot candidatus liberibacter asiaticus specific antiserum is purified to obtain the citrus yellow shoot candidatus liberibacter asiaticus antigen, immune colloidal gold is prepared, a combined pad which is soaked with the immune colloidal gold and a chromatography membrane which is coated with a detection line T-citrus yellow shoot candidatus liberibacter asiaticus antigen and a quality control line C-goat anti-rabbit antibody are further prepared, and finally a detection test paper product is assembled. Against for the requirement of the detection of a great amount of citrus yellow shoot samples, the citrus yellow shoot candidatus liberibacter asiaticus detection test paper which is convenient, rapid and high in specificity is provided. The test paper has following advantages that 1, convenience in use and simpleness in operation are realized; 2, a detection result can be obtained within a short period of time; 3, the stability is good; and 4, the cost is low, and the test paper is particularly suitable for the discrimination of citrus yellow shoot plants of a fruit garden.
Owner:INST OF PLANT PROTECTION FAAS
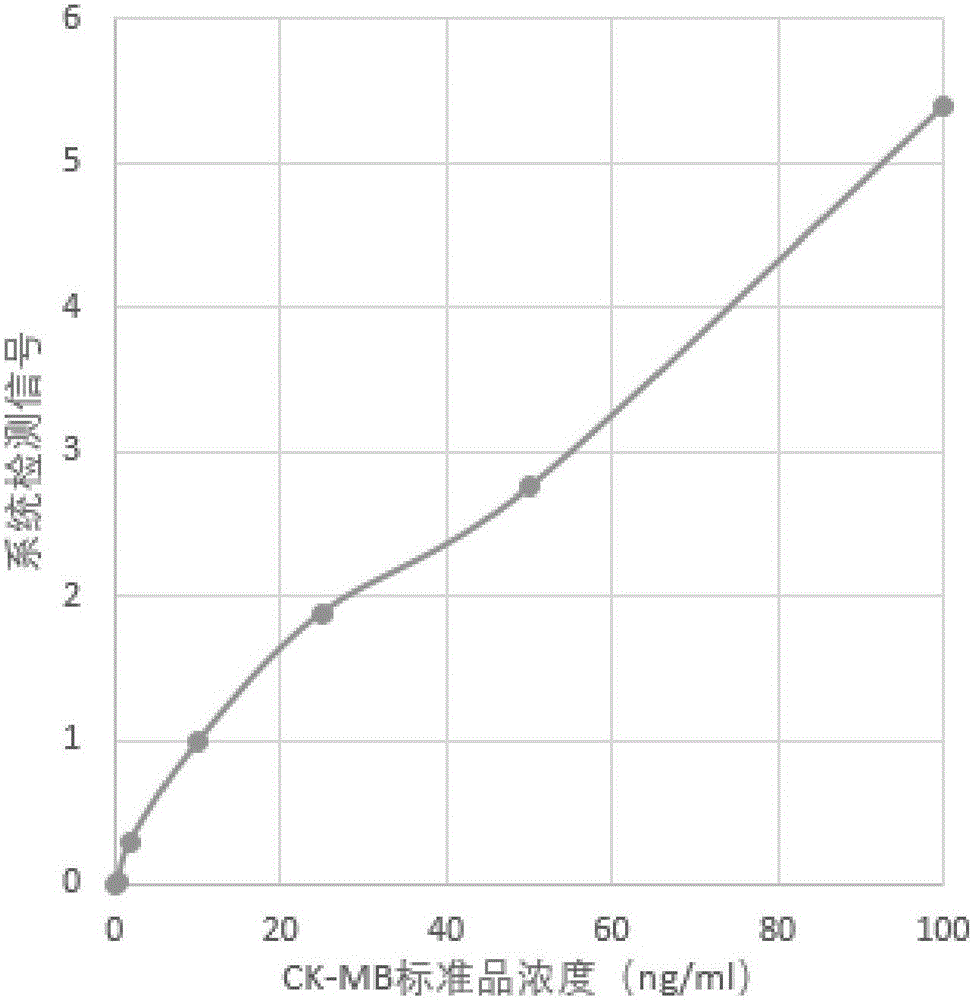
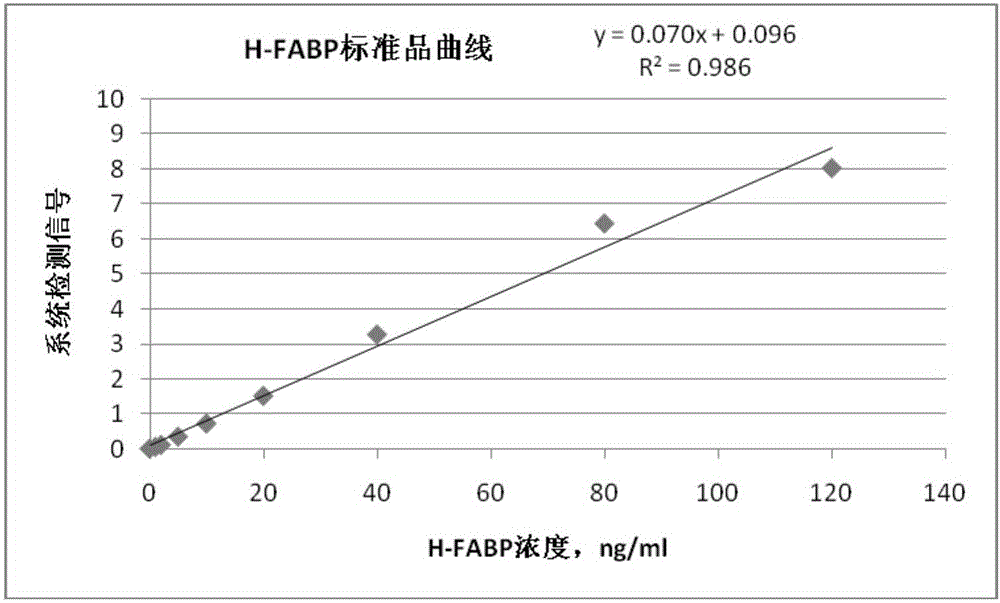
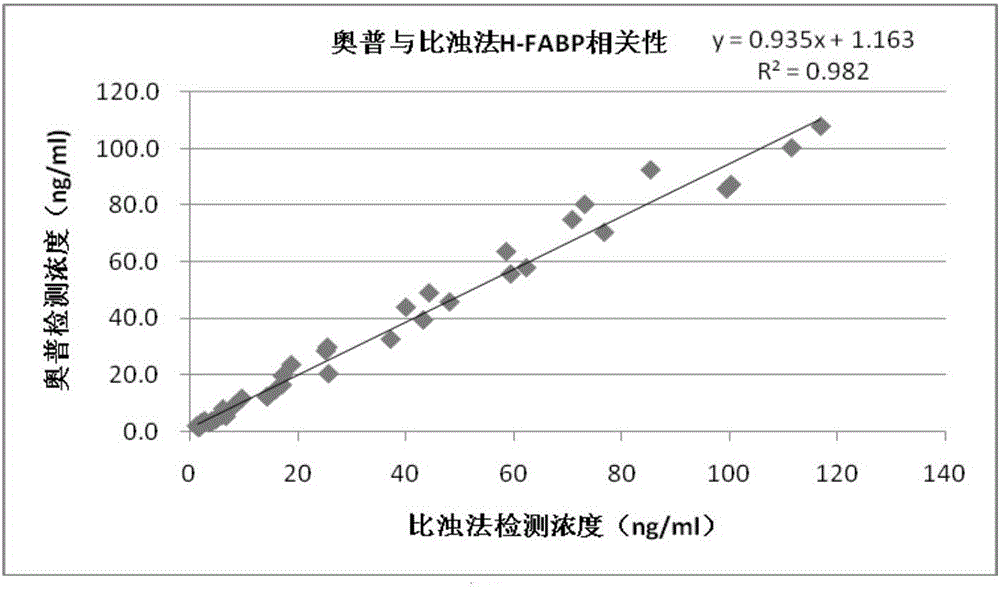
TRF (time-resolved fluorescence) immunochromatography reagent for rapidly and quantitatively detecting H-FABP (heart fatty acid-binding protein) and preparation method
InactiveCN105891508AHigh densityImprove accuracyDisease diagnosisBiological testingMicrospherePoint-of-care testing
The invention discloses a TRF (time-resolved fluorescence) immunochromatography reagent for rapidly and quantitatively detecting H-FABP (heart fatty acid-binding protein) and a preparation method and belongs to the field of clinical medical diagnosis. The reagent comprises two parts including a test strip and a fluorescent liquid, wherein the test strip comprises a bottom plate, Fusion5, a nitrocellulose membrane and a water absorbent pad; the Fusion5, the nitrocellulose membrane and the water absorbent pad are horizontally and sequentially connected and fixed onto the bottom plate; the nitrocellulose membrane is coated with a detection line for H-FABP monoclonal antibodies 1 and a quality control line comprising rabbit IgG (immunoglobulin G) antibodies; the fluorescent liquid contains TRF microspheres labeled by H-FABP monoclonal antibodies 2 and TRF microspheres labeled by goat anti-rabbit antibodies. According to the reagent, the fluorescence intensity is improved by the aid of the TRF microspheres, background signals are reduced, meanwhile, the content of the H-FABP in whole blood, serum or plasma is quantitatively detected, and only 10-20 microliters of samples are required. The test strip is convenient, rapid, simple to operate, short in detection time, high in specialty, high in sensitivity, more accurate in detection result and applicable to rapid diagnosis for clinical POCT (point-of-care testing).
Owner:SHANGHAI UPPER BIO TECH PHARMA
Paraquat-glyphosate dual detecting card and processing method of test sample thereof
InactiveCN101493458AEasy to manufactureLow detection costPreparing sample for investigationGlass fiberParaquat
The invention discloses a paraquat-glyphosate bigeminal detection card; the internal part of an outer shell has a test strip which is pasted with a nitrocellulose membrane in the middle of a supporting back plate; the two end parts are respectively pasted with an absorbent membrane and a sample pad; the inner ends are respectively overlapped with one end of the nitrocellulose membrane; a section of colloidal gold film which is a glass fiber membrane containing anti-paraquat and anti-glyphosate monoclonal antibody or polyclonal antibody colloidal gold marker is clamped between the overlapped part of the sample pad and the nitrocellulose membrane; the nitrocellulose membrane has 2 test strips which respectively contain a paraquat protein conjugate and a glyphosate protein conjugate; and a matrix pore area contains an anti-rabbit antibody or anti-mouse antibody. The sample pad and the nitrocellulose membrane respectively face a sampling hole and a detection window hole. The invention has the advantages that the paraquat and glyphosate contained in fruits, vegetables, and cereals can be simultaneously detected; the preparation is easy, the detection cost is saved, the use is convenient, the detection is rapid, the sensitivity is high and the result is accurate.
Owner:周坚
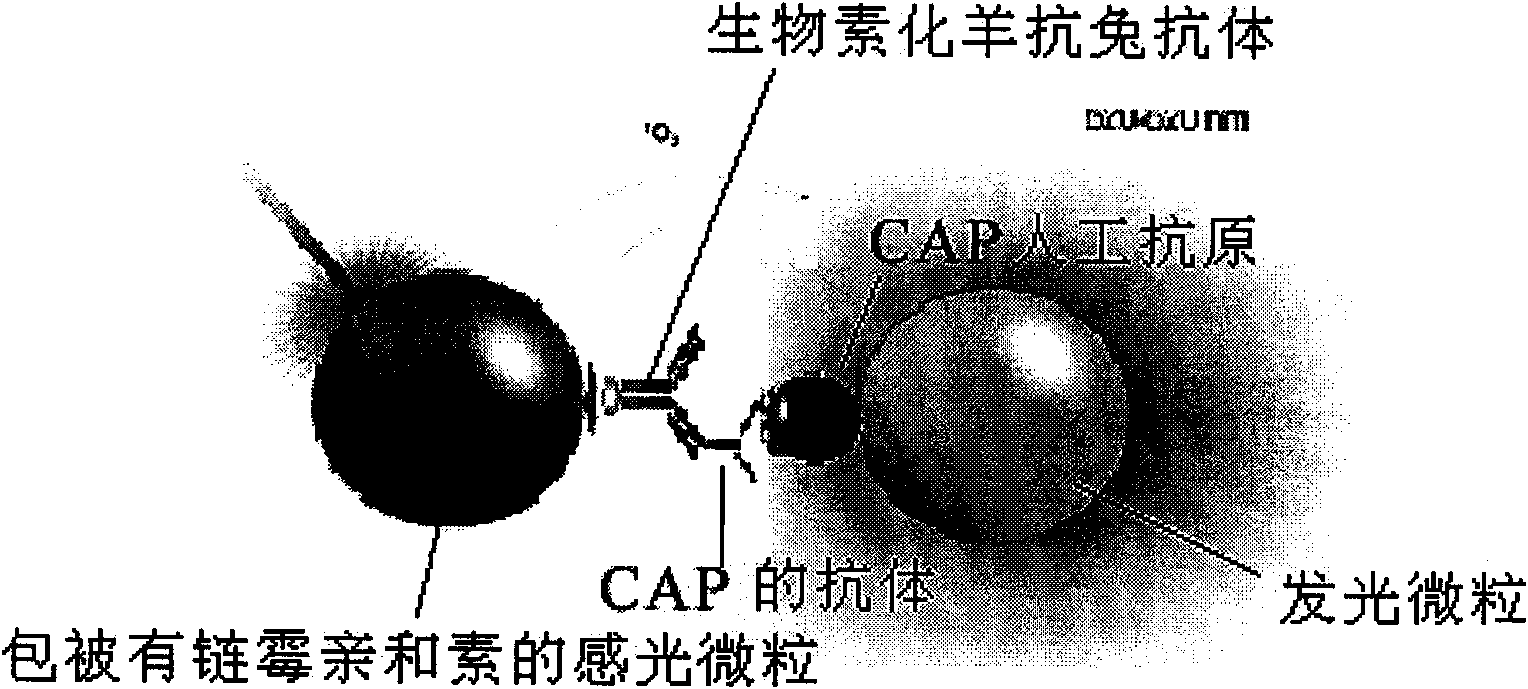
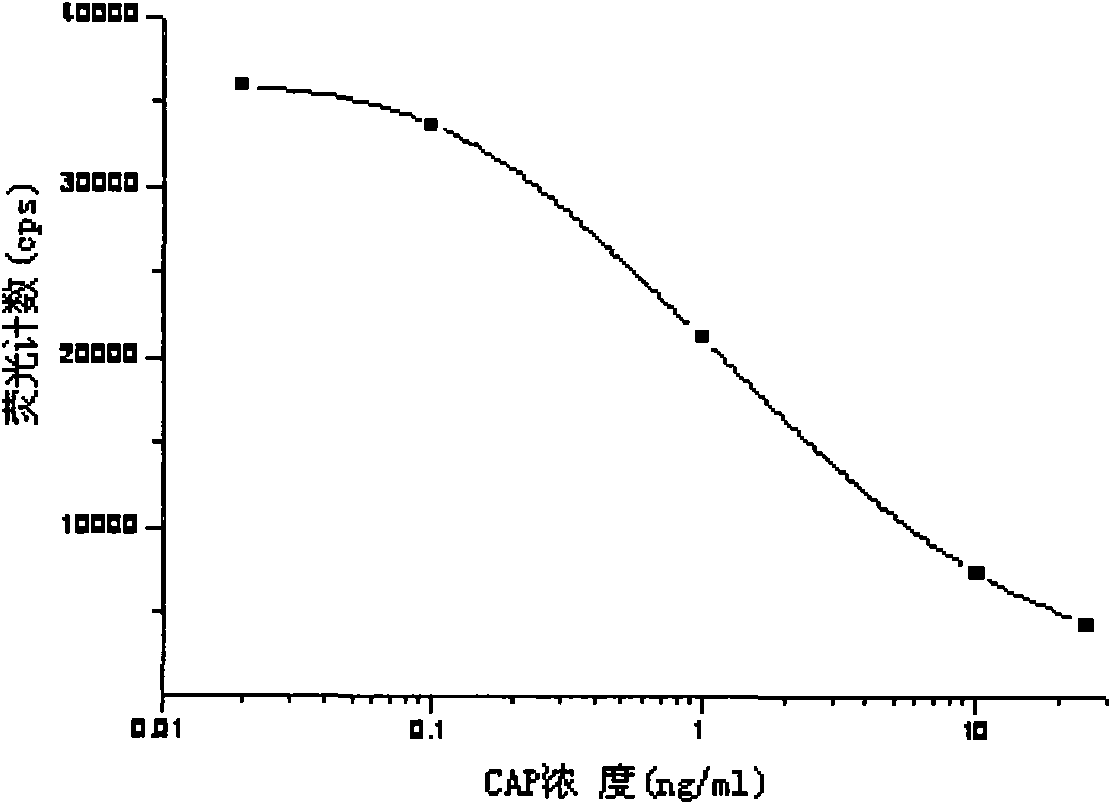
Light-induced chemiluminescent immunoassay kit and test method for chloramphenicol
InactiveCN101603961ASimple structureSimple and fast operationPreparing sample for investigationBiological testingBiotin-streptavidin complexFluorescence
The invention discloses a light-induced chemiluminescent immunoassay kit and a test method for chloramphenicol (CAP) and belongs to the technical field of light-induced chemiluminescent immunoassay technology. A nontransparent white microporous plate is sequentially added with CAP-OVA coated luminous particles, a CAP standard substance or a sample to be tested, a rabbit anti-CAP antibody and a biotin goat anti-rabbit antibody for reaction without light and then is added with streptavidin-coated light sensitive particles for after-incubation test. The CAP-OVA on the luminous particles and free CAP compete to be connected to the CAP antibody to form a compound body with the biotin goat anti-rabbit antibody and the streptavidin-coated light sensitive particles. Under the excitation of red light, the compound body transfers energy to the luminous particles through the generation and transmission of singlet ionized oxygen for generating fluorescence. A light induced chemiluminescent detector is used to detect the intensity of an optical signal, and the CAP content of the sample can be determined by referring to a standard curve according on the basis that the intensity of the optical signal is in inverse proportion to the CAP concentration of the sample. The method is used for determining the CAP content of foods such as honey, milk and eggs. The kit is simple in structure, short in determination time, high in sensitivity and simple and convenient in operation.
Owner:JIANGSU INST OF NUCLEAR MEDICINE
Immune colloidal gold test strip for detecting organic phosphorus pesticide and preparation method thereof
InactiveCN104034886AEasy to operateEasy to detectMaterial analysisPesticide residuePolyvinyl chloride
The invention discloses an immune colloidal gold test strip for detecting organic phosphorus pesticide and a preparation method of the test strip, and belongs to the technical field of pesticide detection. The test strip comprises a PVC (Polyvinyl Chloride) backing, a sample pad, a colloidal gold combining pad, a detection line, a quality control line, a nitrocellulose membrane and a water absorbing pad, wherein the colloidal gold combining pad is wrapped by a universal organic phosphorous pesticide antibody marked by colloidal gold; conjugate of universal organic phosphorous pesticide hapten and carrier protein is sprayed on the detection line; an anti-mouse or anti-rabbit IgG antibody is sprayed on the quality control line. The test strip has the characteristics of high specificity, high sensitivity, good repeatability and the like, and can be popularized and applied to organic phosphorus pesticide residue detection on vegetables, fruits and the like.
Owner:GUANGXI DOCTOR HAIYI INFORMATION TECH
Kit for detecting heparin binding protein through immunofluorescence chromatography and preparation method of kit
The invention relates to the technical field of medical supplies, and particularly discloses a kit for detecting heparin binding protein through immunofluorescence chromatography and a preparation method of the kit. The kit comprises a first buffer solution, a second buffer solution and a reagent card. The first buffer solution is a phosphate buffer solution containing rabbit anti-human heparin binding protein antibodies, wherein the concentration of the rabbit anti-human heparin binding protein antibodies is 0.83-2 nanograms per microliter. The second buffer solution is a phosphate buffer solution containing fluorescently-labeled affinipure chicken anti-rabbit, wherein the concentration of fluorescently-labeled affinipure chicken anti-rabbit is 0.05-0.2 microgram per microliter. The reagent card comprises a plastic cushion plate. A nitrocellulose membrane is arranged on the plastic cushion plate. A detection line and a quality control line are arranged on the nitrocellulose membrane in a spaced and left-right mode, a glass cellulose membrane is arranged on the portion, on the left side of the detection line, of the nitrocellulose membrane. A piece of water absorption paper is arranged on the portion, on the right side of the quality control line, of the nitrocellulose membrane. A blood filter membrane is arranged on the glass cellulose membrane. The detection line is wrapped by rabbit anti-human heparin binding protein antibodies. The quality control line is wrapped by goat anti-rabbit antibodies. The kit has a good linear range and is high in flexibility and accuracy.
Owner:河南生生医疗器械有限公司
Test paper strip for rapidly detecting traces of chlorothalonil and preparation method thereof
The invention relates to a test paper strip for rapidly detecting traces of chlorothalonil and a preparation method thereof. The test paper strip is characterized in that a supporting layer is used as the bottom layer, an adsorption layer is used as the intermediate layer, a protective layer is fixed on the adsorption layer, the adsorption layer comprises an adsorption fibrous layer, a gold-labeled antibody fibrous layer, a cellulose film and an absorbent material layer at the handle end in order from a test terminal, wherein the cellulose film is provided with detection blots printed by using a chlorothalonil coupling carrier protein solution and control blots printed by using a goat anti-mouse IgG or rabbit anti-mouse IgG (or goat anti-rabbit IgG) antibody solution; the gold-labeled antibody is a colloidal gold labeled chlorothalonil monoclonal antibody or polyclonal antibody; and the chlorothalonil coupling carrier protein is bovine serum albumin, chicken ovalbumin or haemocyanin. The test paper strip disclosed herein has the advantages of strong specificity, high sensitivity, simple, and accurate detection, low cost, wide application scope, and easiness in popularization and application.
Owner:HENAN ACAD OF AGRI SCI
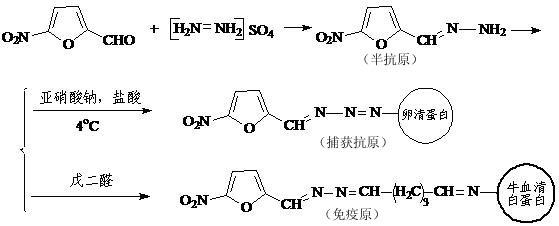
Immune nano gold test strip for quickly detecting four nitrofuran medicaments
The invention relates to an immune nano gold test strip for quickly detecting four nitrofuran medicaments. The immune nano gold test strip is prepared by the following steps of: synthesizing general hapten, immunogen and captured antigen aiming at the four nitrofuran medicaments comprising nitrofurazone, furaltadone, nitrofurantoin and furazolidone by using 5-nitrofurfural as a raw material, immunizing a domestic rabbit serving as an experimental animal to acquire polyclonal IgG antibody, preparing gold labeled antibody by using nano gold and the polyclonal IgG antibody, coating the gold labeled antibody solution on a Glass33 glass cellulose membrane, namely a combined release pad, coating the captured antigen and goat anti-rabbit antibody on a detection line and a quality control line of an AE99 nitrocellulose membrane respectively, sequentially sticking the AE99 nitrocellulose membrane, the combined release pad, namely the Glass33 glass cellulose membrane, an absorption pad Cotton linters 2668 and a sample pad Glass33 on a polyvinyl chloride (PVC) back board, and finally, cutting the back board into 4*50mm test strips. The test strip can simultaneously and quickly detect the four medicaments comprising nitrofurazone, furaltadone, nitrofurantoin and furazolidone in feed; and the cost of the test strip is less than 5 yuan, so the test strip is suitable to be widely applied in feed detection or food animal detection.
Owner:河北省兽药监察所
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com