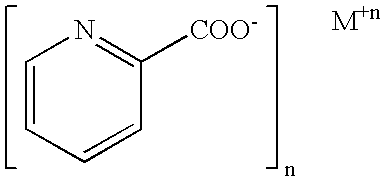
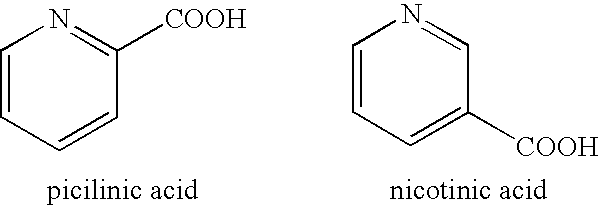
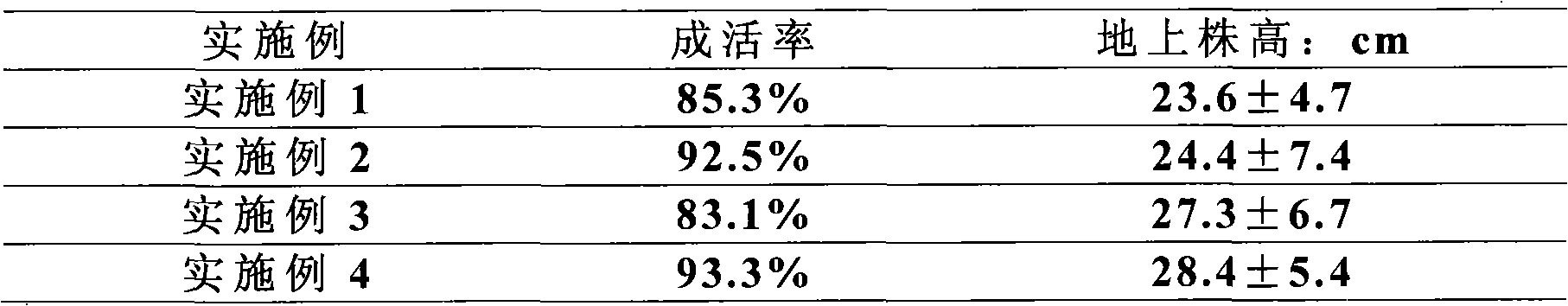
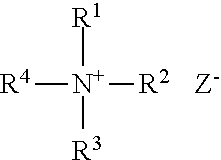Patents
Literature
1254 results about "Microgram" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In the metric system, a microgram or microgramme is a unit of mass equal to one millionth (1×10⁻⁶) of a gram. The unit symbol is μg according to the International System of Units; the recommended symbol in the United States when communicating medical information is mcg. In μg the prefix symbol for micro- is the Greek letter μ (Mu).
Compositions and methods for inhibiting expression of a target gene
ActiveUS7829693B2Inhibit expression of target geneOrganic active ingredientsNervous disorderDiseaseNucleotide
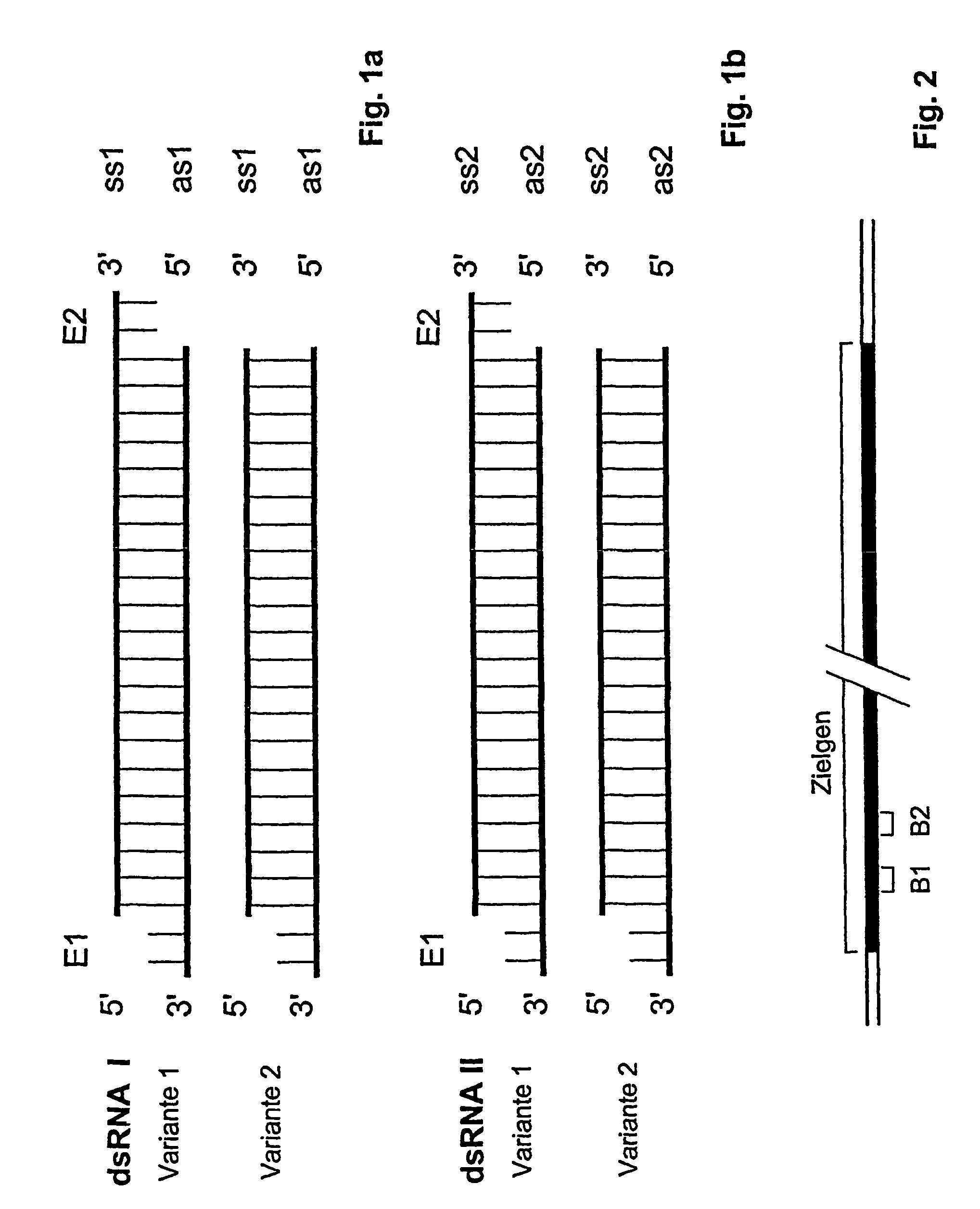
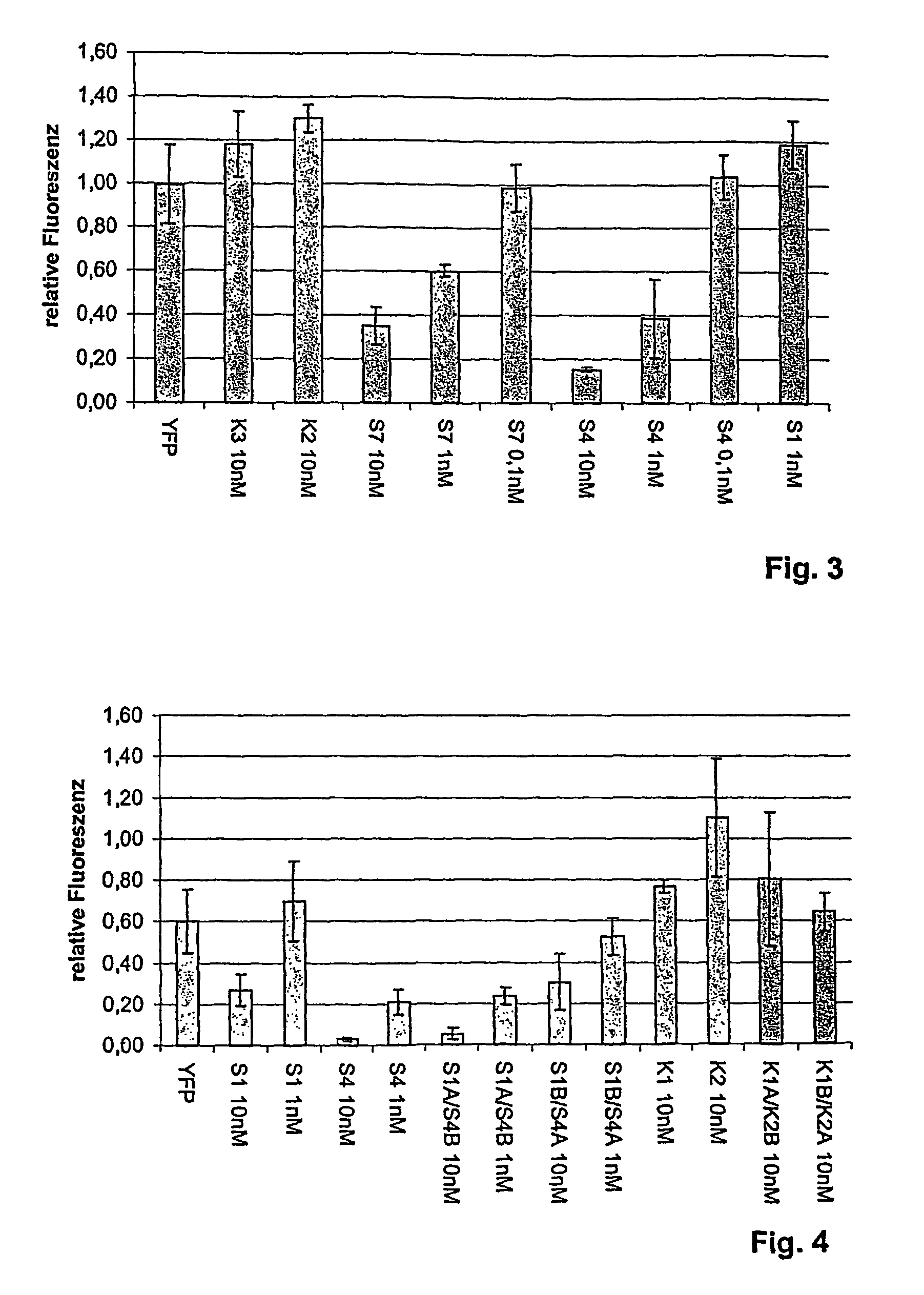
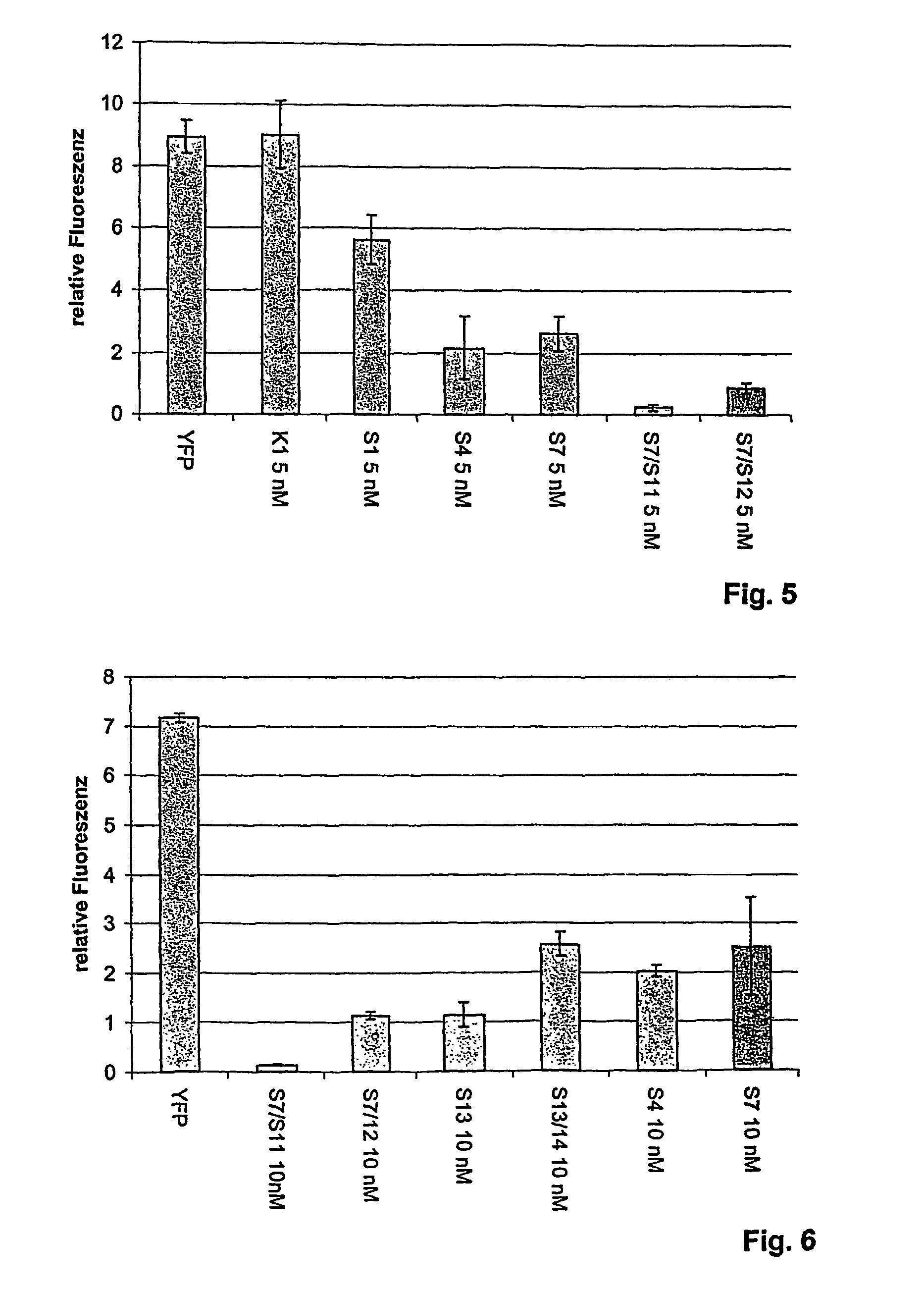
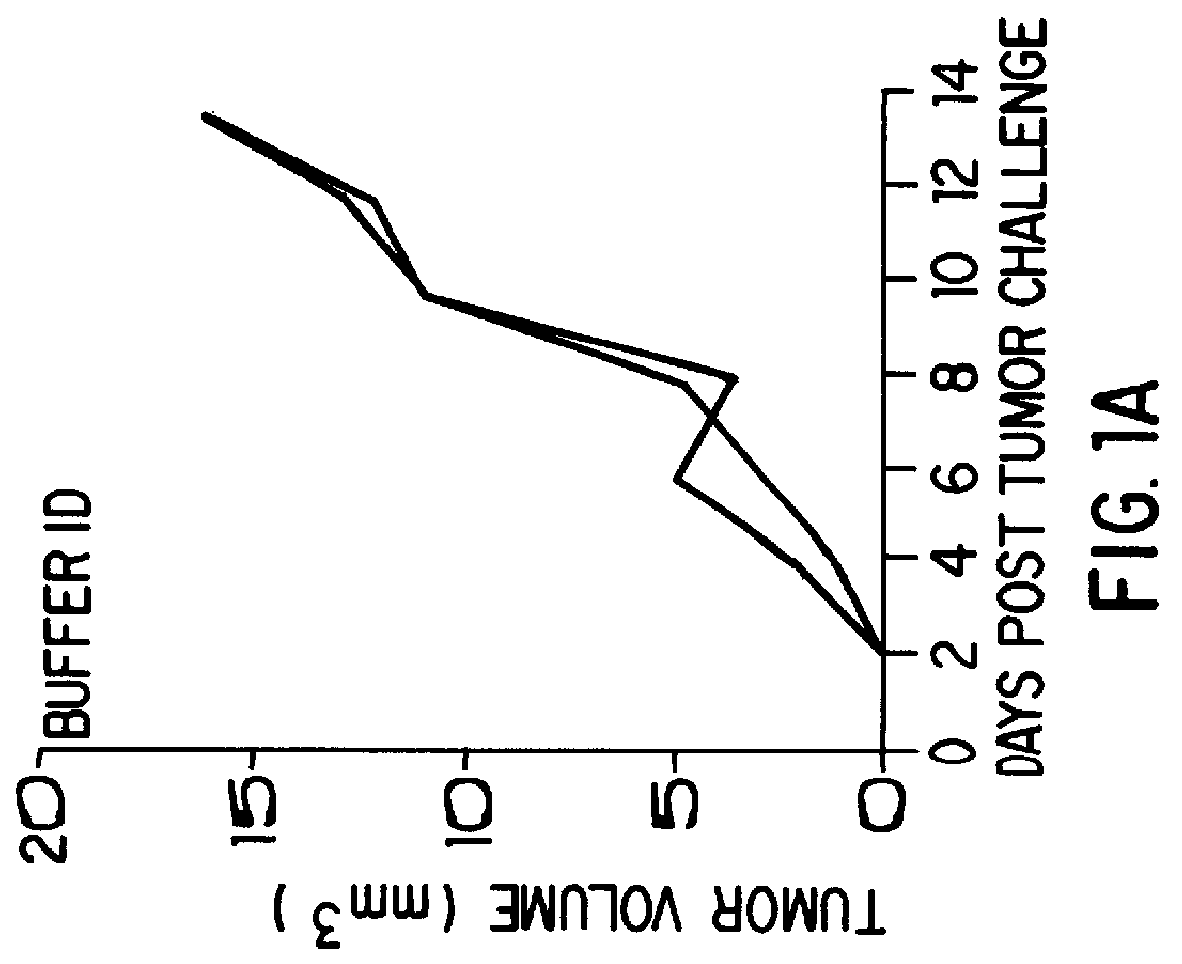
The present invention relates to a double-stranded ribonucleic acid (dsRNA) having a nucleotide sequence which is substantially identical to at least a part of a target gene and which is no more than 49, preferably less than 25, nucleotides in length, and which comprises a complementary (antisense) RNA strand having a 1 to 4 nucleotide overhang at the 3′-end and a blunt 5′-end. The invention further relates to a pharmaceutical composition comprising the dsRNA and a pharmaceutically acceptable carrier. The pharmaceutical compositions are useful for inhibiting the expression of a target gene, as well as for treating diseases caused by expression of the target gene, at low dosages (i.e., less than 5 milligrams, preferably less than 25 micrograms, per kg body weight per day). The invention also relates to methods for inhibiting the expression of a target gene, as well as methods for treating diseases caused by the expression of the gene.
Owner:ALNYLAM PHARM INC
Prevention and treatment of primary and metastatic neoplastic diseases and infectious diseases with heat shock/stress protein-peptide complexes
InactiveUS6017540AEnhancing host 's immunocompetenceHigh activityBiocidePeptide/protein ingredientsStress ProteinsIn vivo
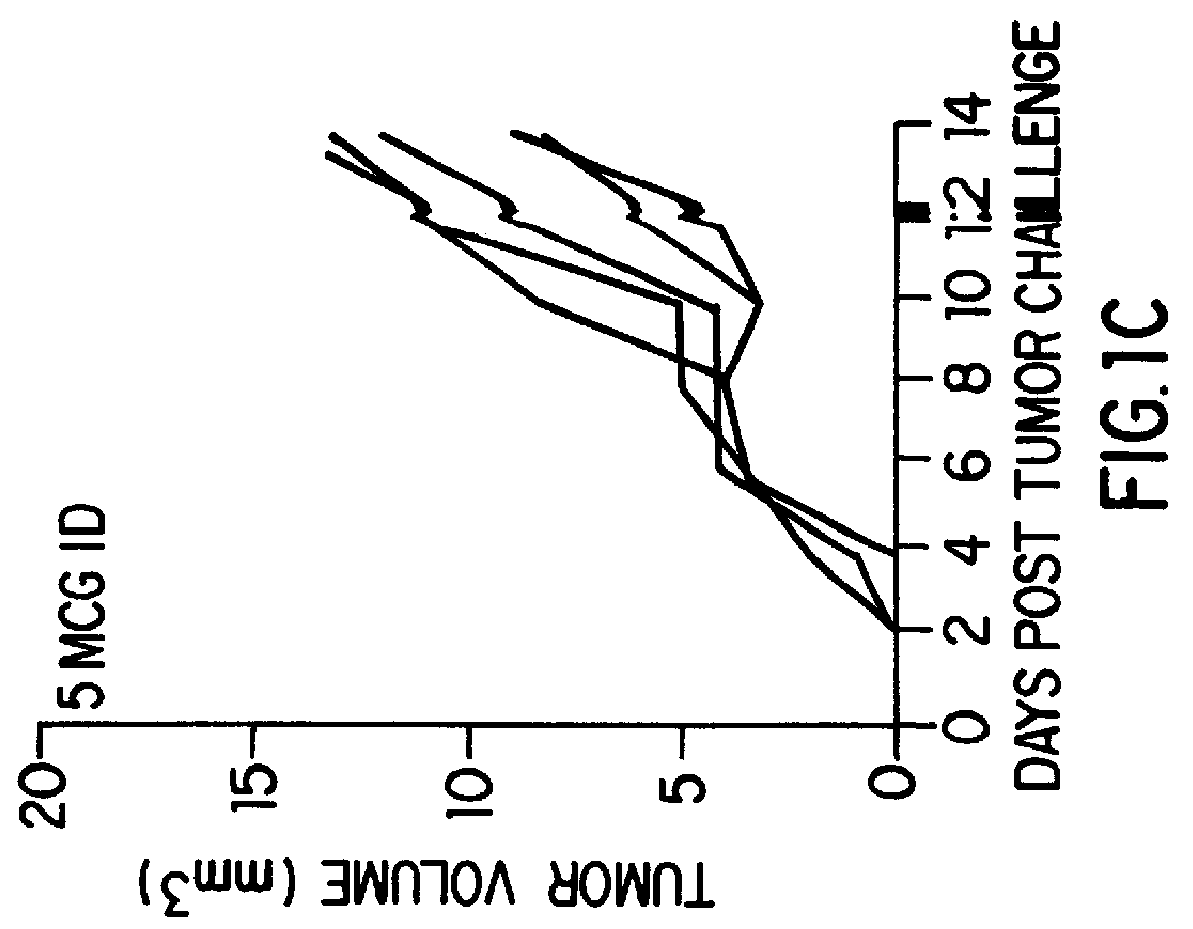
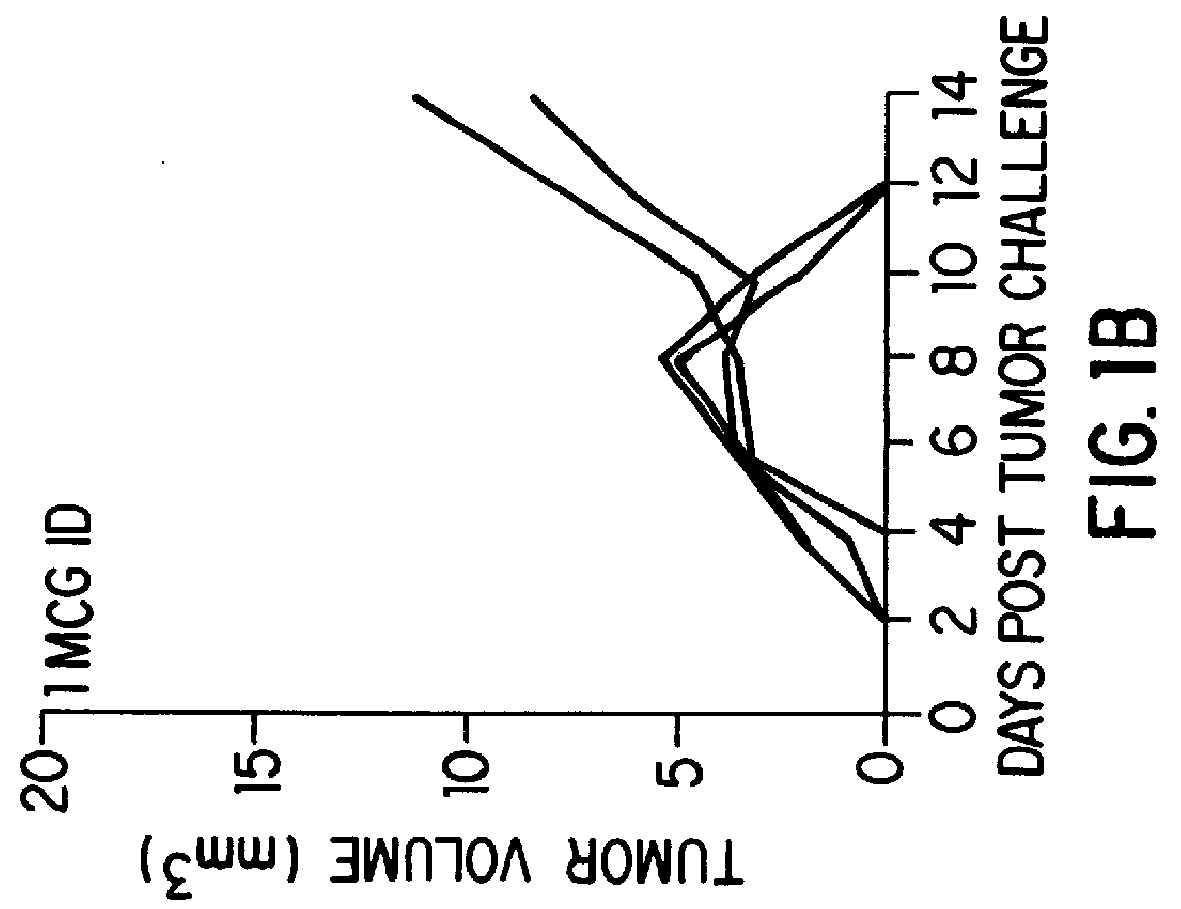
The present invention relates to methods and compositions for eliciting an immune response and the prevention and treatment of primary and metastatic neoplastic diseases and infectious diseases. The methods of the invention comprise administering a composition comprising an effective amount of a complex, in which the complex consists essentially of a heat shock protein (hsp) noncovalently bound to an antigenic molecule. Optionally, the methods further comprise administering antigen presenting cells sensitized with complexes of hsps noncovalently bound to an antigenic molecule. "Antigenic molecule" as used herein refers to the peptides with which the hsps are endogenously associated in vivo as well as exogenous antigens / immunogens (i.e., with which the hsps are not complexed in vivo) or antigenic / immunogenic fragments and derivatives thereof. In a preferred embodiment, the complex is autologous to the individual. In a specific embodiment, the effective amounts of the complex are in the range of 0.1 to 9.0 micrograms for complexes comprising hsp70, 5 to 49 micrograms for hsp90, and 0.1 to 9.0 micrograms for gp96.
Owner:FORDHAM UNIVERSITY
Hydrogenation catalyst composition, preparation method and use thereof
ActiveCN101306374AHigh activityMetal/metal-oxides/metal-hydroxide catalystsRefining to eliminate hetero atomsGramSulfur
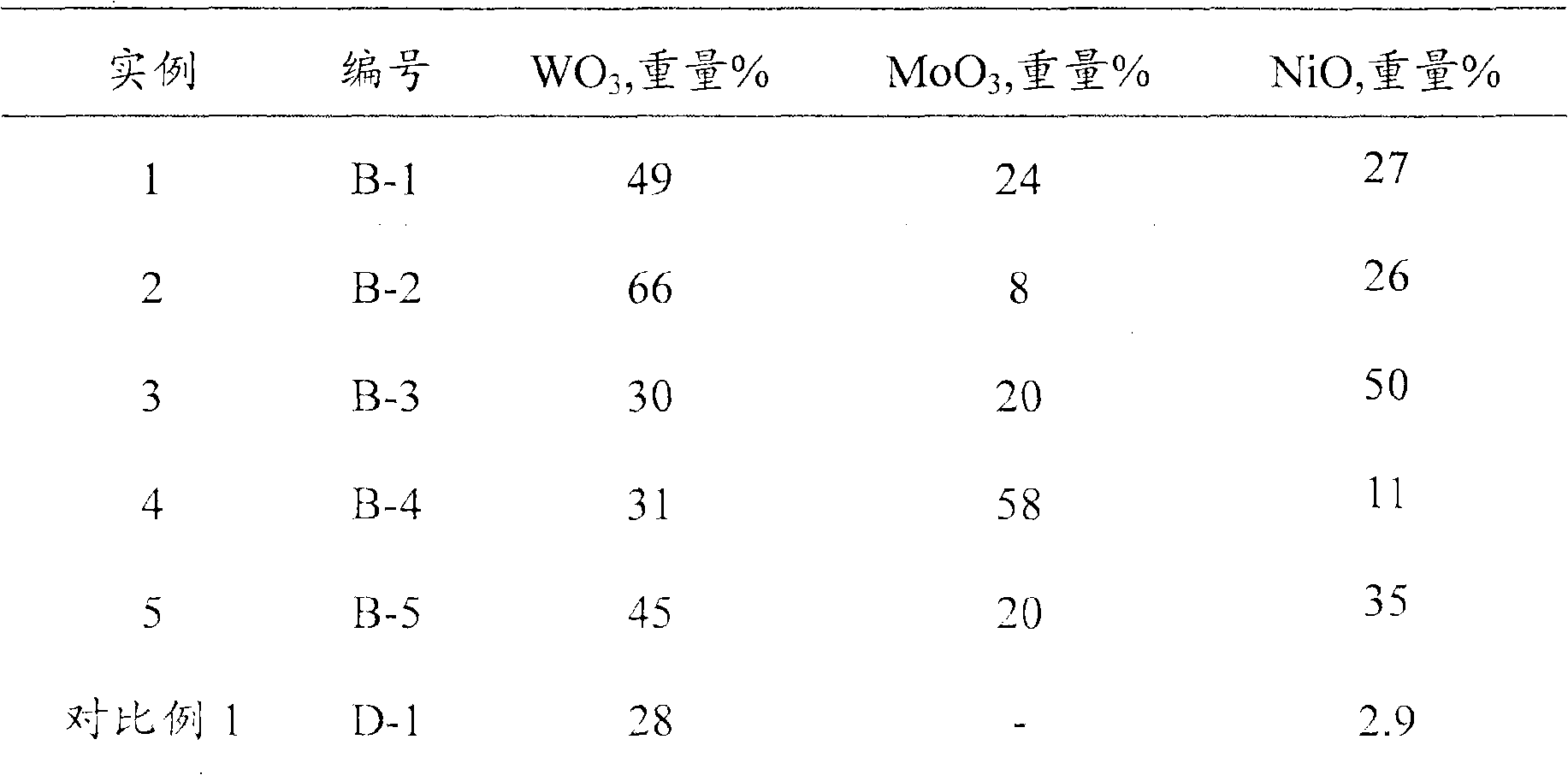
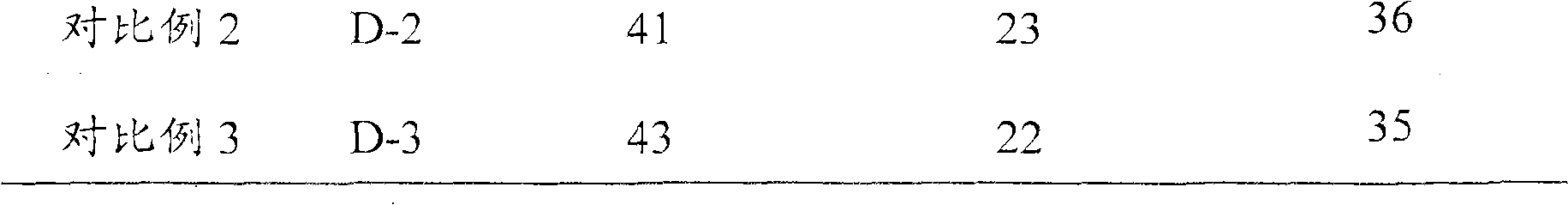
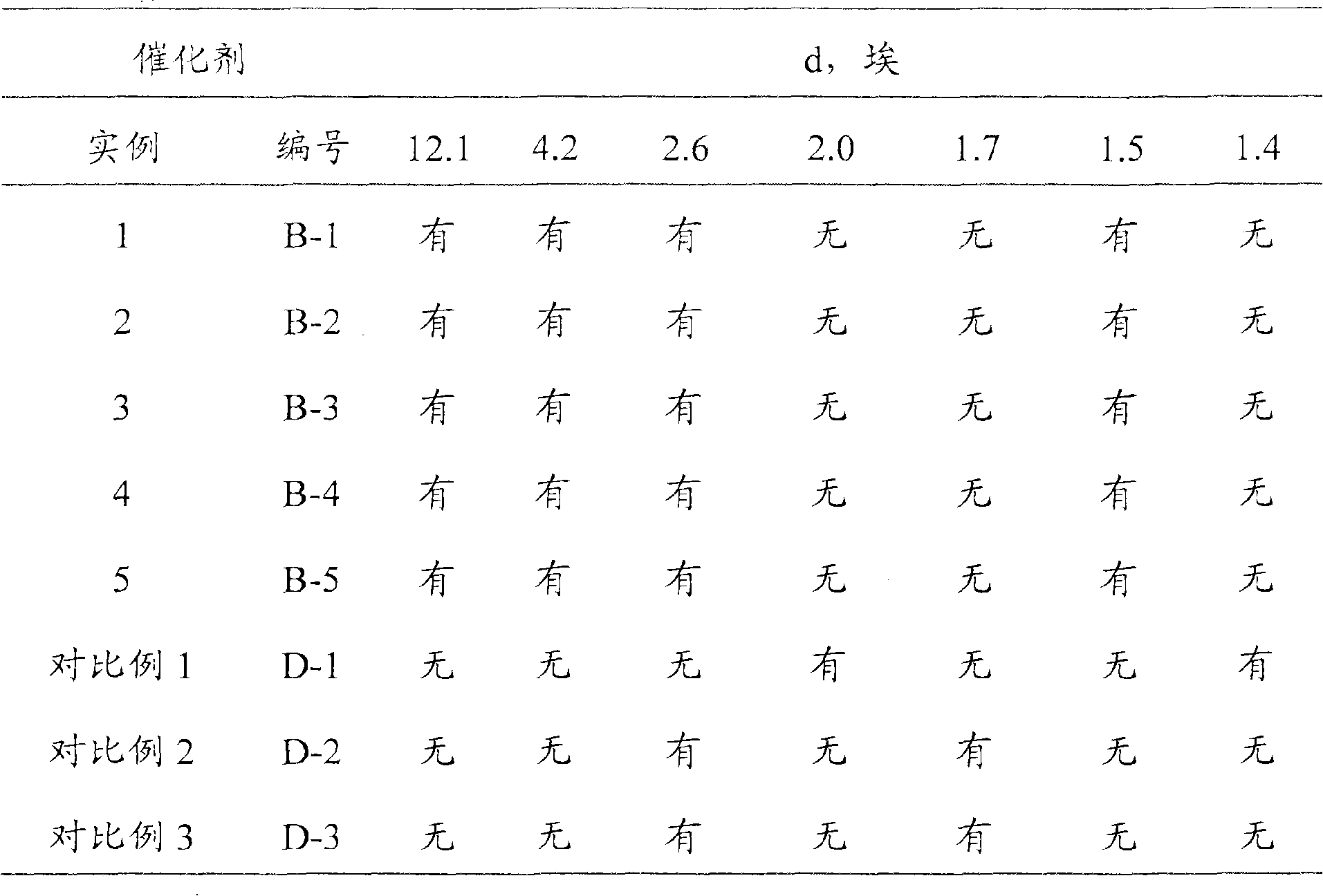
The invention discloses a hydrogenation catalyst combination, as well as the preparation and the application thereof. The catalyst comprises a catalytic agent containing at least one VIII group metal component, at least two VIB group metal components and an organic addition agent, X-ray is used for diffracting the characterization, and the catalyst has diffraction peaks when d is equal to 9.8 A-12.8 A, d is equal to 3.65 A-4.65 A, d is equal to 2.1 A-3.1 A and d is equal to 1.03 A- 2.03 A. Compared with the prior art, the catalyst of the invention has higher hydrogenation activity. When the invention is used for hydro-treating hydrocarbon oil, the reaction can be carried out under milder reaction conditions. If the hydrocarbon oil hydro-treating method provided by the invention is adopted, clean diesel oil which has sulfur of lower than 10 microgram / gram and accords with the European No. V discharge standard can be directly produced under the pressure of 6.4 MPa and the temperature of 340 DEG C.
Owner:CHINA PETROLEUM & CHEM CORP +1
Compositions and methods using complexes of heat shock protein 90 and antigenic molecules for the treatment and prevention of neoplastic diseases
The present invention relates to methods and compositions for eliciting an immune response and the prevention and treatment of primary and metastatic neoplastic diseases and infectious diseases. The methods of the invention comprise administering a composition comprising an effective amount of a complex, in which the complex consists essentially of a heat shock protein (hsp) noncovalently bound to an antigenic molecule. "Antigenic molecule" as used herein refers to the peptides with which the hsps are endogenously associated in vivo as well as exogenous antigens / immunogens (i.e., with which the hsps are not complexed in vivo) or antigenic / immunogenic fragments and derivatives thereof. In a preferred embodiment, the complex is autologous to the individual. The effective amounts of the complex are in the range of 10-600 micrograms for complexes comprising hsp7o, 50-1000 micrograms for hsp9o, and 10-600 micrograms for gp96. The invention also provides a method for measuring tumor rejection in viva in an individual, preferably a human, comprising measuring the generation by the individual of MHC Class I-restricted CD8+ cytotoxic T lymphocytes specific to the tumor. Methods of purifying hsp7o-peptide complexes are also provided.
Owner:FORDHAM UNIVERSITY
Chromium compositions and methods for using the same for inhibiting drug-induced insulin resistance
InactiveUS20050214384A1Avoid seizuresAvoid developmentBiocideHeavy metal active ingredientsDietary ChromiumInsulin resistance
A method for inhibiting drug-induced insulin resistance is provided which includes administering a dietary chromium complex to an individual receiving a contemporaneous dose of a drug that induces insulin resistance, wherein the amount of chromium complex administered is an amount effective to inhibit the development of insulin resistance. Advantageously, the amount of chromium complex administered per day is between about 300 and 1,000 micrograms per day. Compositions including a drug which induces insulin resistance in combination with a chromium complex are similarly described.
Owner:N21 ACQUISITION HLDG
Method of achieving improved hair feel
A method of achieving improved hair feel. The method comprises applying to hair a composition comprising: (a) a specific cationic guar polymer; (b) a specific cationic copolymer; (c) an anti-dandruff active; (d) a cosmetically acceptable carrier; (e) a surfactant; wherein the weight ratio of (a):(b) is from about 1000:1 to about 3.5:1; and wherein the sum of (a)+(b) is an amount of from about 0.0001% to about 0.7%, by total weight of the composition. The composition forms coacervate particles upon dilution of the composition with water and the coacervate particles have a squeeze flow viscosity of from about 1 Pa·s to about 100 Pa·s. The percentage of coacervate particles with a floc size of greater than about 20 micron is from about 1% to about 60% and the on-scalp deposition of the anti-dandruff active is at least about 1 microgram / cm2.
Owner:PROCTER & GAMBLE CO
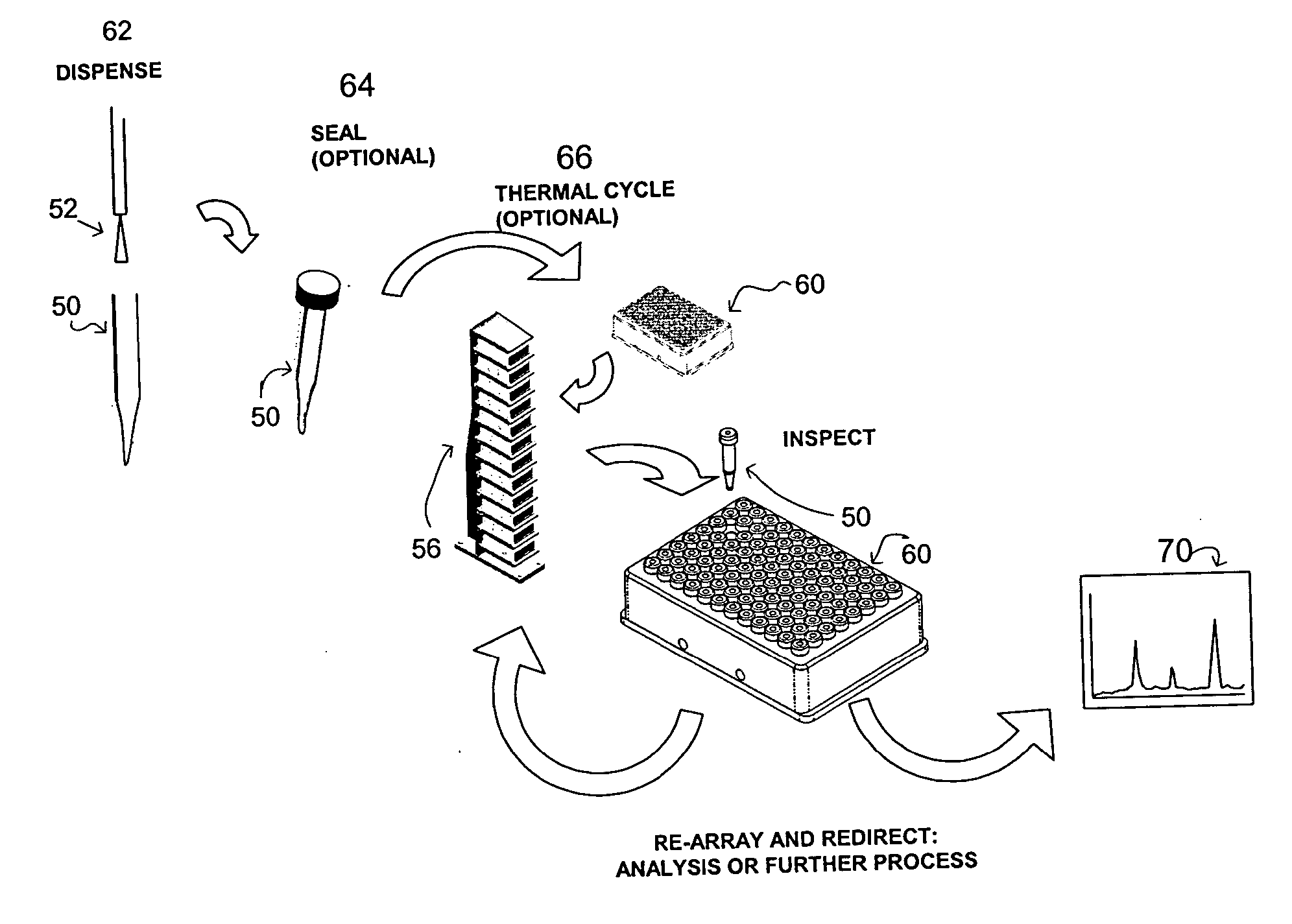
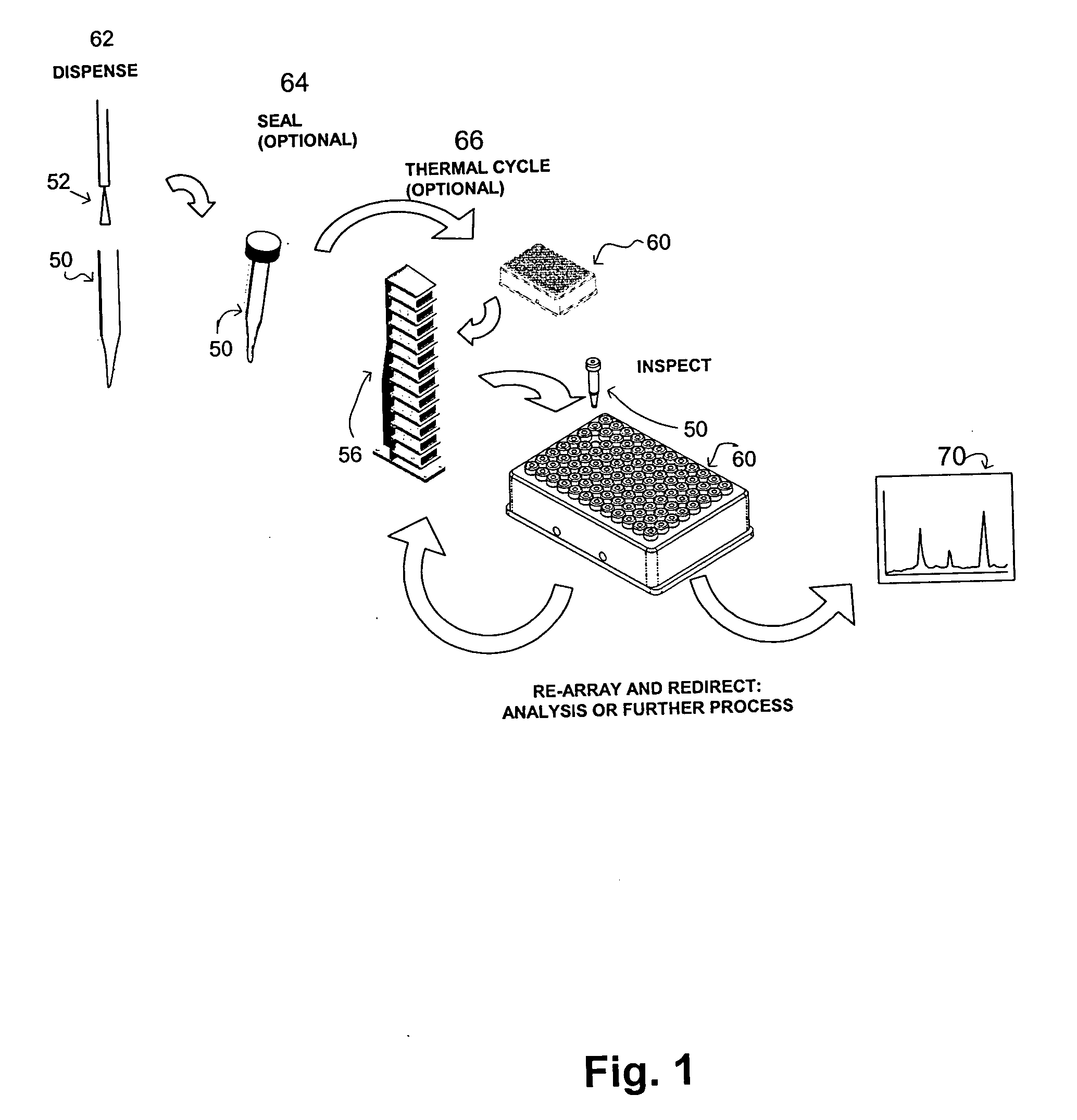
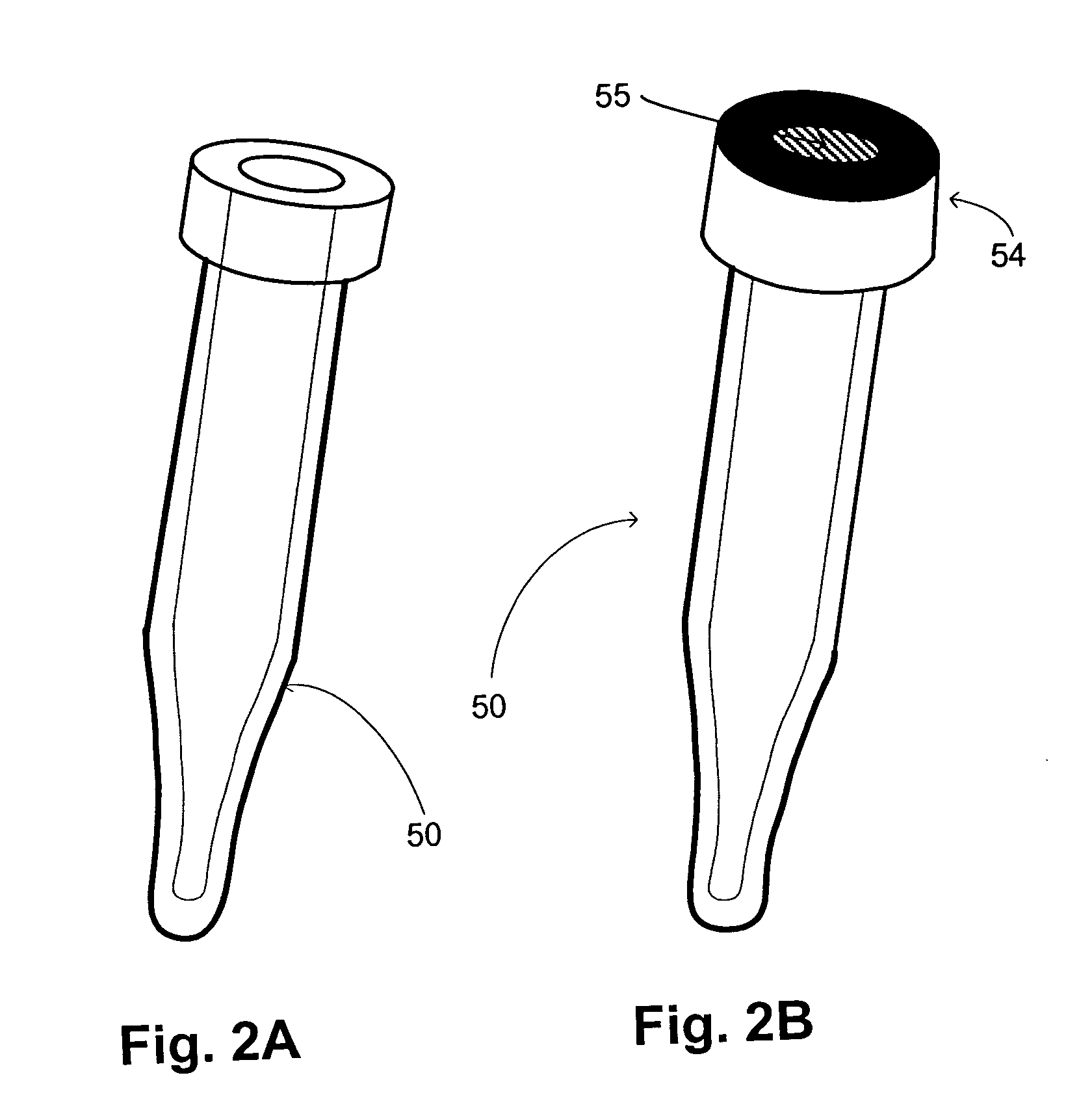
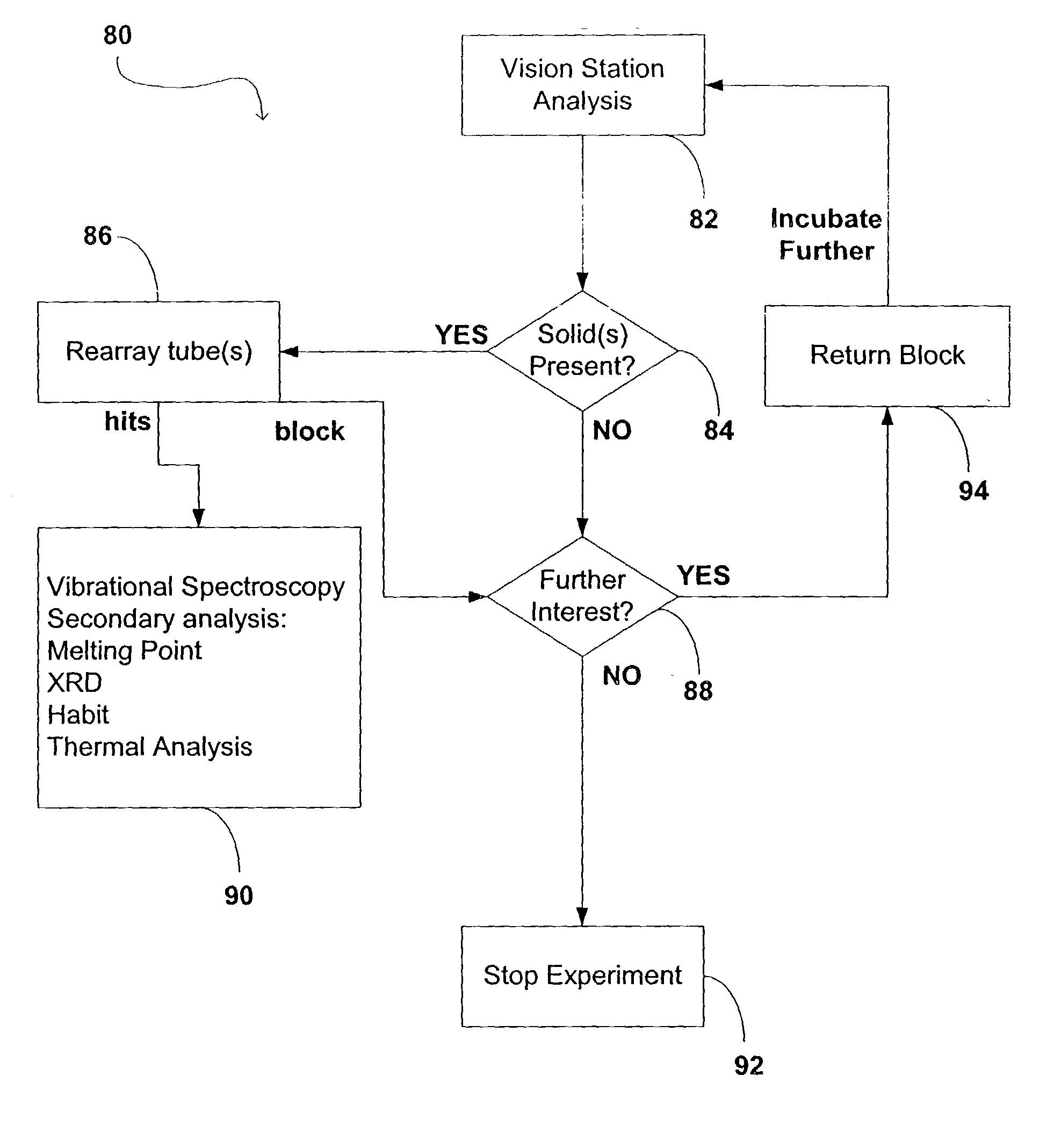
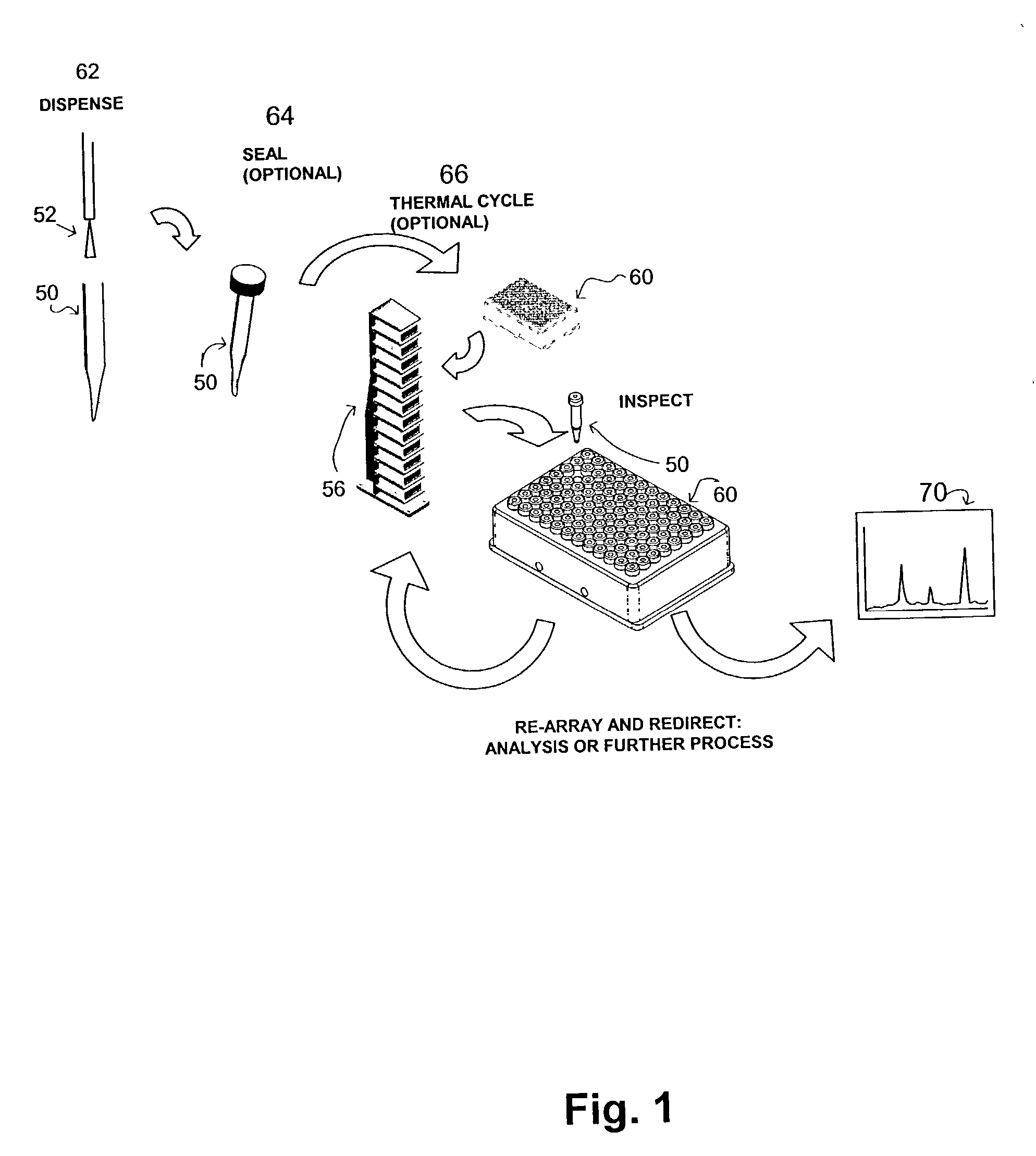
Apparatus and method for high-throughput preparation and spectroscopic classification and characterization of compositions
InactiveUS20040252299A9Optical radiation measurementBioreactor/fermenter combinationsComputer scienceMicrogram
Systems and methods are described that allow the high-throughput preparation, processing, and study of arrays of samples, each of which comprises at least one compound. Particular embodiments of the invention allow a large number of experiments to be performed in parallel on samples that comprised of one or more compounds on the milligram or microgram quantities of compounds. Other embodiments of the invention encompass methods and devices for the rapid screening of the results of such experiments, as well as methods and devices for rapidly determining whether or not similarities exist among groups of samples in an array. Particular embodiments of the invention encompass methods and devices for the high-throughput preparation of different forms of compounds (e.g., different crystalline forms), for the discovery of new forms of old compounds, and for the discovery of new methods of producing such forms. Embodiments of the invention also allow for the high-throughput determination of how specific compounds or forms of compounds behave when exposed to other chemicals or environmental conditions.
Owner:TRANSFORM PHARMACEUTICALS INC
Apparatus and method for high-throughput preparation, visualization and screening of compositions
InactiveUS20030106492A1Sequential/parallel process reactionsHeating or cooling apparatusComputer scienceMicrogram
Systems and methods are described that allow the high-throughput preparation, processing, and study of arrays of samples, each of which comprises at least one compound. Particular embodiments of the invention allow a large number of experiments to be performed in parallel on samples that comprised of one or more compounds on the milligram or microgram quantities of compounds. Other embodiments of the invention encompass methods and devices for the rapid screening of the results of such experiments, as well as methods and devices for rapidly determining whether or not similarities exist among groups of samples in an array. Particular embodiments of the invention encompass methods and devices for the high-throughput preparation of different forms of compounds (e.g., different crystalline forms), for the discovery of new forms of old compounds, and for the discovery of new methods of producing such forms. Embodiments of the invention also allow for the high-throughput determination of how specific compounds or forms of compounds behave when exposed to other chemicals or environmental conditions.
Owner:TRANSFORM PHARMACEUTICALS INC
Compositions and methods for inhibiting expression of a target gene
Owner:ALNYLAM PHARMA INC
Natural menaquinone 7 compositions
InactiveUS20050123603A1Decreasing bone turnoverBiocidePeptide/protein ingredientsNutrition supplementationMedicine
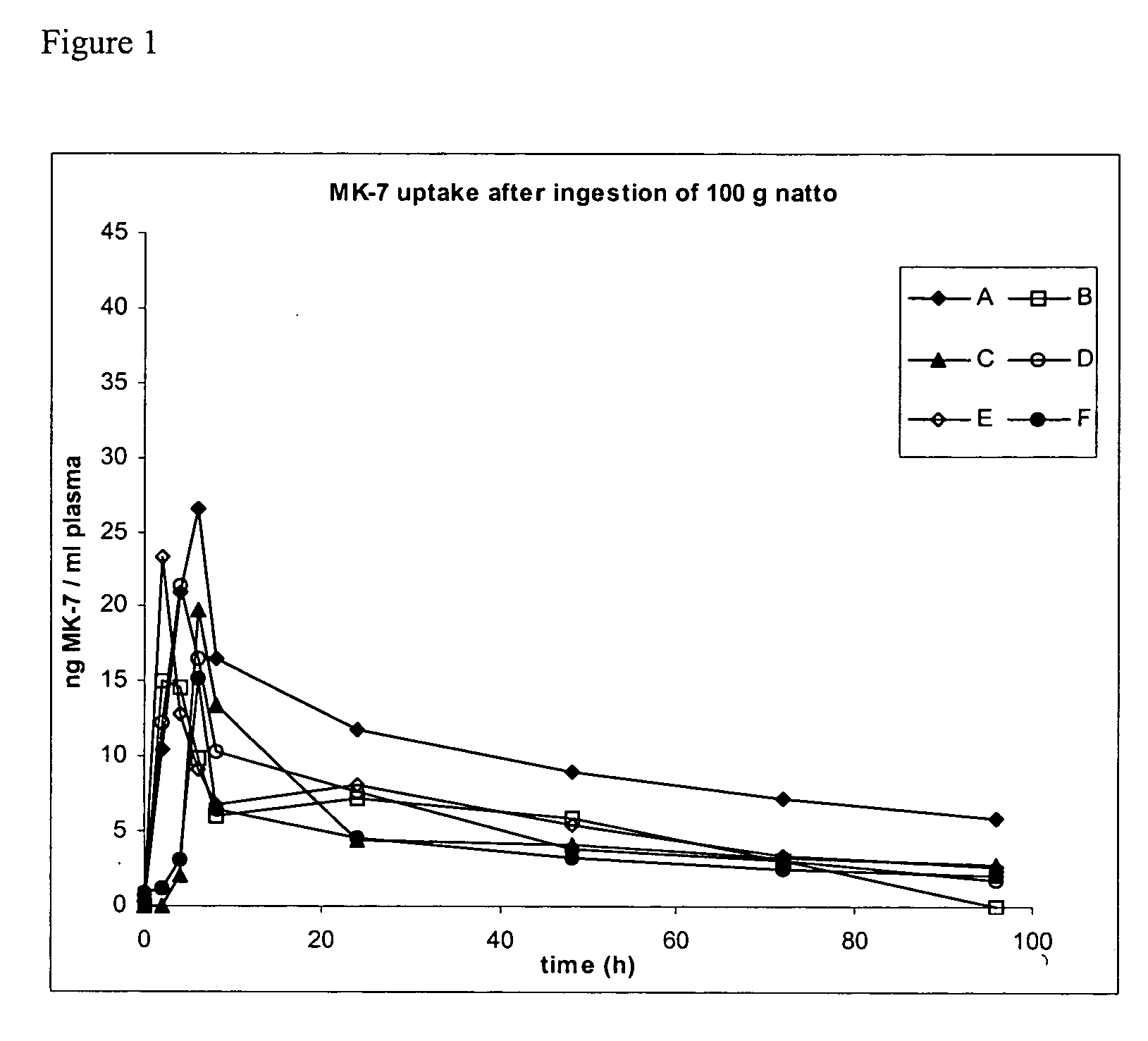
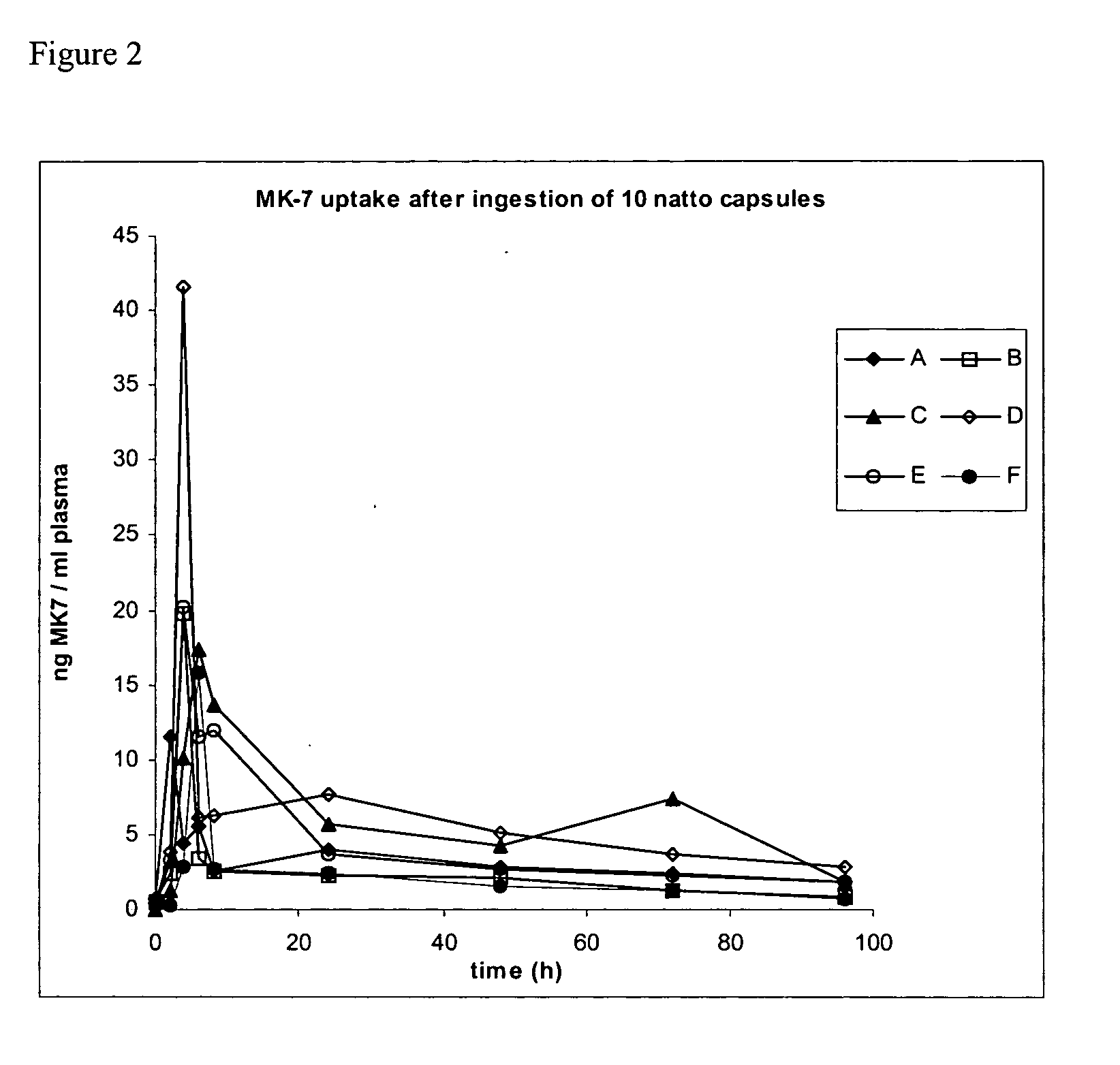
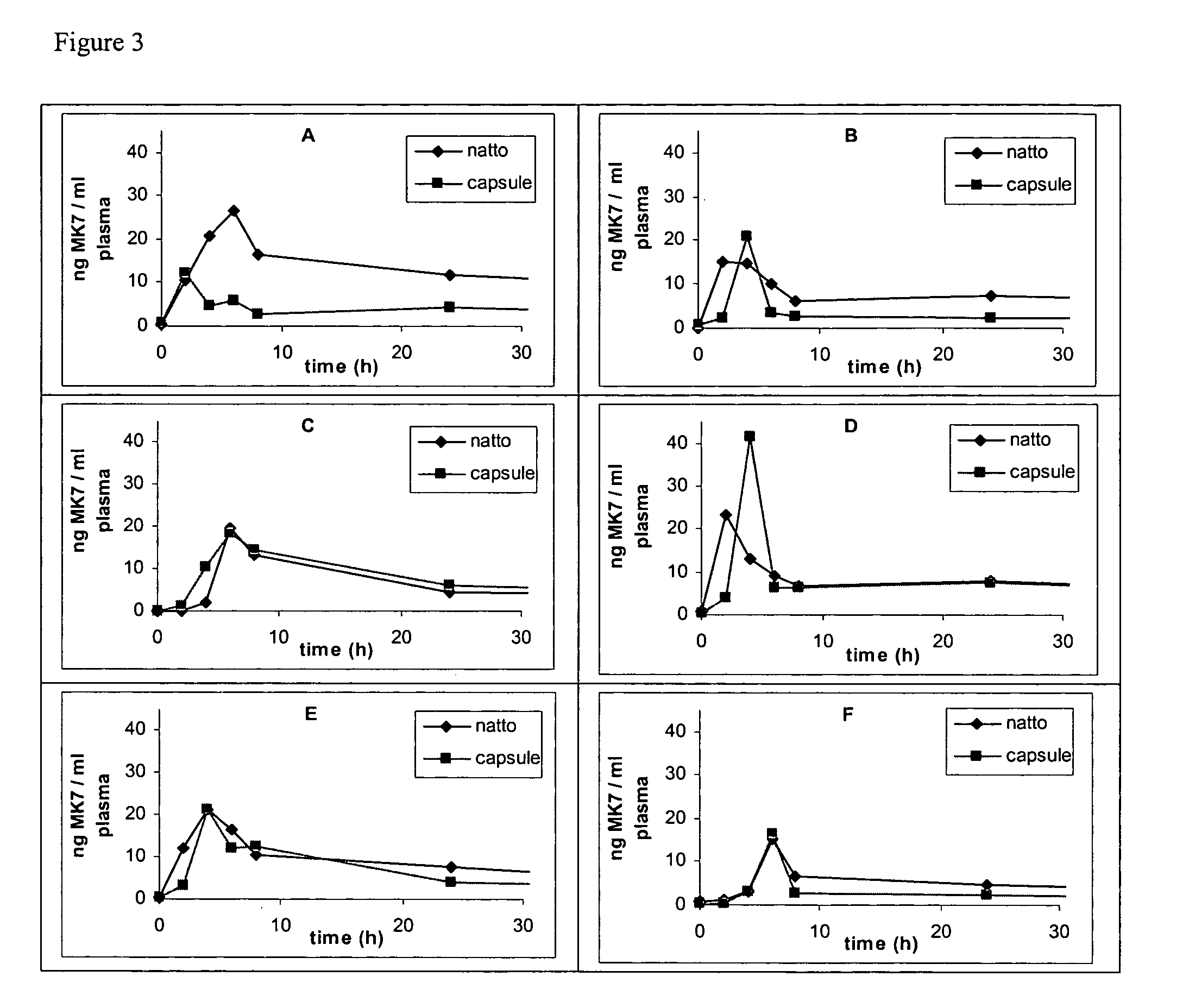
The present invention relates to the field of human nutrition, and in particular to pharmaceutical compositions and nutritional supplements comprising an effective daily dose of menaquinone 7. In particular, the present invention provides pharmaceutical compositions, nutritional supplements and food products that comprise from about 10 to about 200 micrograms of menaquinone 7. In some embodiments, the menaquinone 7 is provided in a natto extract.
Owner:AKER BIOMARINE ASA
Method for preparation of microarrays for screening of crystal growth conditions
InactiveUS7244396B2Slow evaporation rateMinimize controlFrom normal temperature solutionsHydrolasesCrystal growthMicrogram
A method of screening solution conditions suitable for the crystallization of macromolecules with a picogram to a microgram of protein using picoliter or nanoliter volumes is provided. A preferred method comprises preparing a plurality of recipe solutions in milliliter volumes, aspirating protein and recipe solutions, respectively, from a plurality of wells, and dispensing the protein and recipe solutions into a plurality of wells, covering the combined protein and recipe solutions with oil, and maintaining the solution wells until crystallization or precipitation occur therein.
Owner:UAB RES FOUND
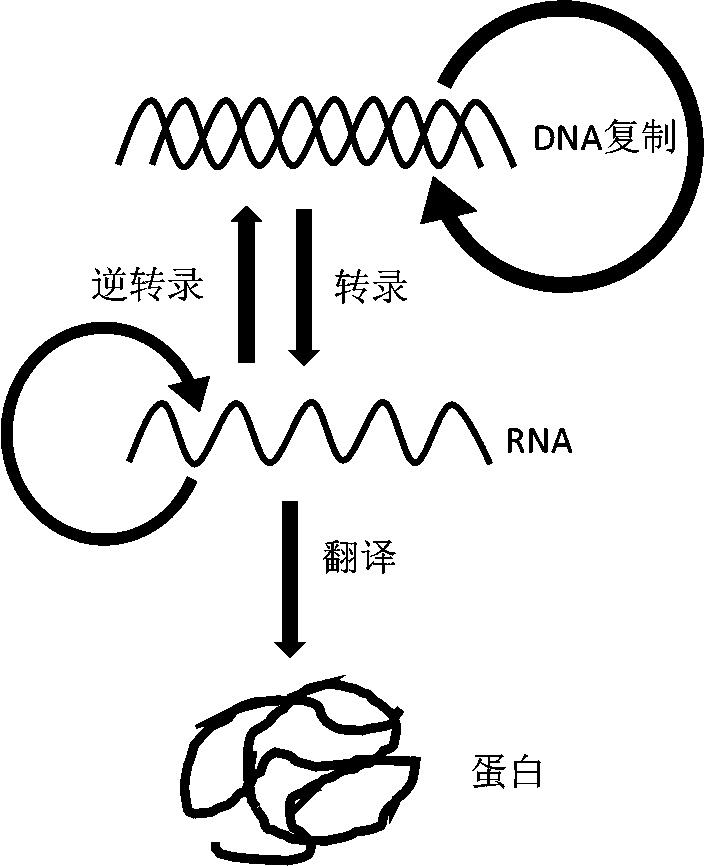
Synthesis system, preparation, kit and preparation method of in-vitro DNA-to-Protein (D2P)
The invention provides a theoretical design and technical design of cell-free protein synthesis for DNA replication, transcription and translation coupling, a preparation, a kit and a preparation method. Specifically, with the application of the in-vitro cell-free synthesis system provided by the invention, complex protein can be synthesized, and moreover, DNA and mRNA can be synthesized; and effective, high-throughput and quite convenient protein synthesis can be completed with the use of a DNA template by a minute quantity (nanogram-microgram).
Owner:KANGMA SHANGHAI BIOTECH LTD
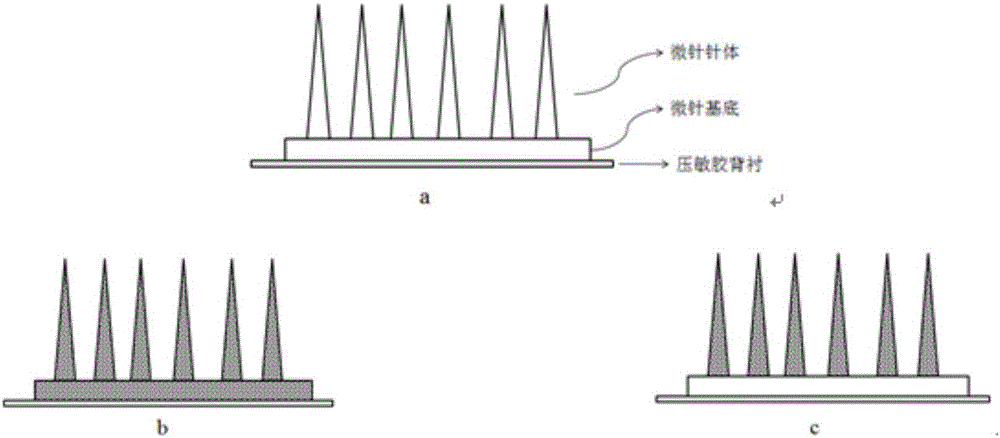
Flexible slow-release micro-needle patch and preparation method thereof
InactiveCN106422045AGood dispersionGood sustained releaseOrganic active ingredientsMicroneedlesSolubilityMedicine
The invention discloses a flexible slow-release micro-needle patch and a preparation method thereof. The micro needle patch comprises a micro-needle, a substrate and a back lining, wherein the micro-needle and the substrate form a micro-needle array; a base material of the micro-needle or a base material of the micro-needle array contains a drug in a crystal form; the solubility of the drug in the crystal form in water is less than 100 microgram / ml. The micro-needle patch has the characteristics of simple process, high preparation safety, low process cost and high drug loading capacity.
Owner:BEIJING CAS MICRONEEDLE TECH LTD
Treatment of depression and pharmaceutical preparations therefor
InactiveUS6191133B1Good curative effectEliminate side effectsBiocideAmine active ingredientsNorepinephrine reuptake inhibitorNoradrenaline reuptake
It has been found that the treatment of depression using known serotonin reuptake inhibitors (SRIs) and noradrenaline reuptake inhibitors (NRIs) may be improved by the administration therewith of folic acid or a precursor which produces folate in the patient. The daily dose of NRI or SRI is as prescribed for treatment of depression in the usual way. The daily dose of the folic acid or precursor should be such as to provide a folate dosage of 300-5000 micrograms / day.
Owner:COPPEN ALEC JAMES
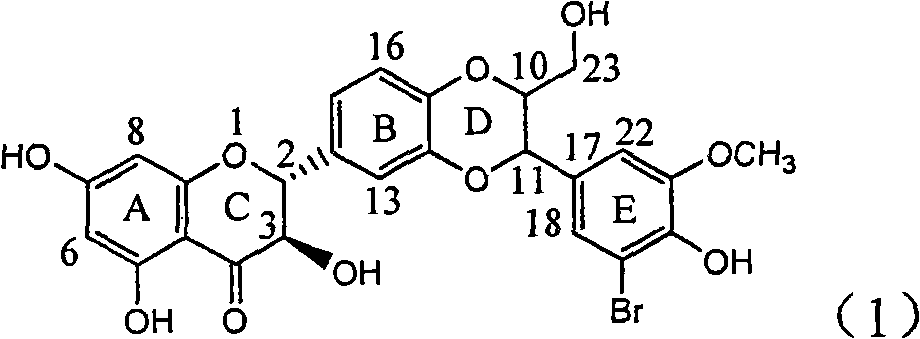
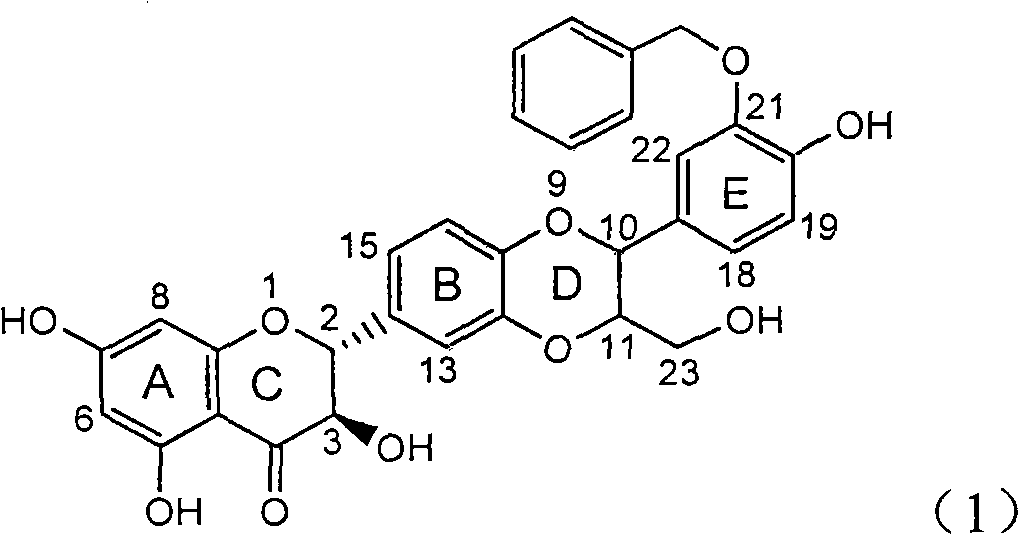
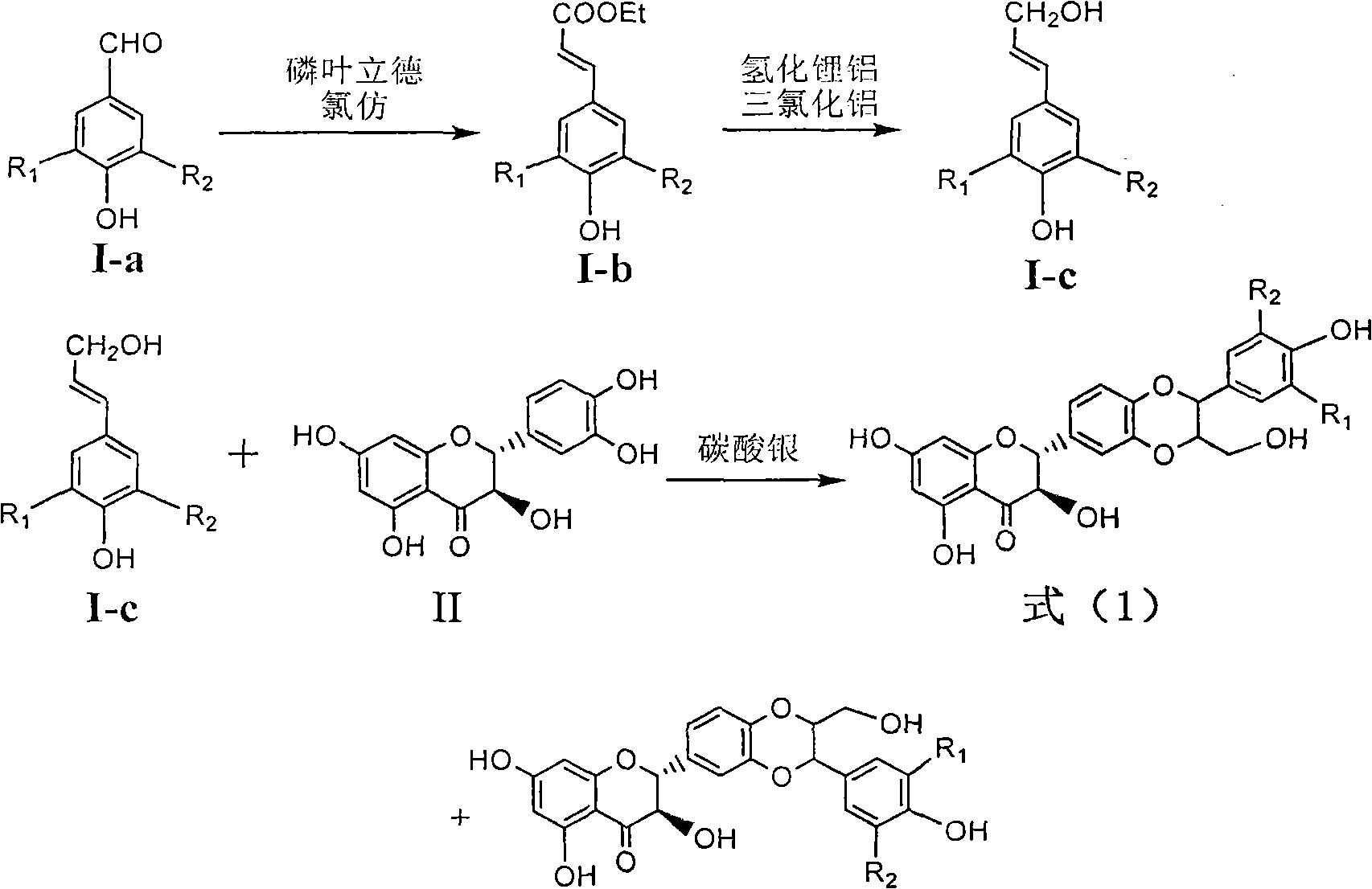
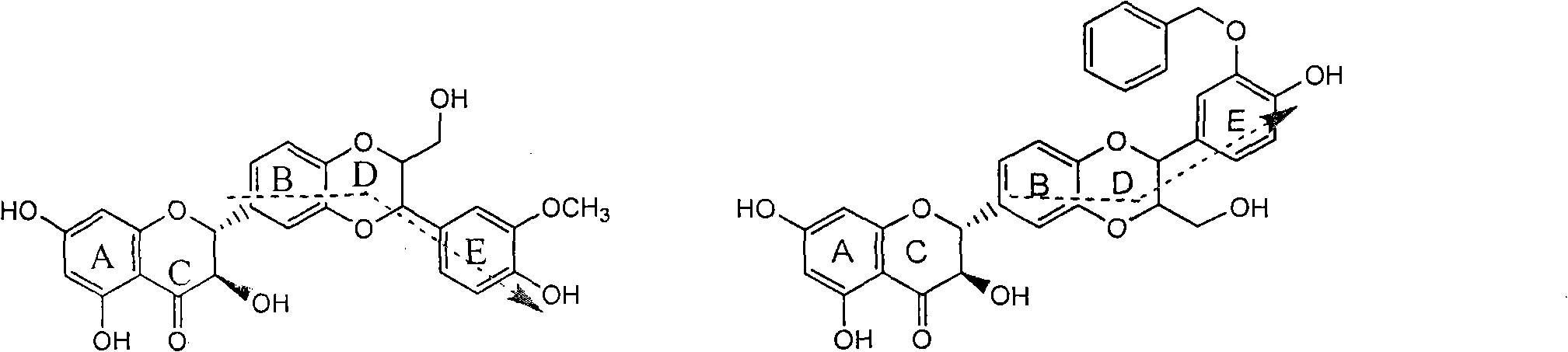
Preparation of brominated flavanonollignan and application in medicine for treating viral hepatitis B
InactiveCN101955478AConvenient sourceThe source is easy to getOrganic active ingredientsOrganic chemistryPositive controlInterferon alpha
The invention relates to the preparation of brominated flavanonollignan and an application in medicines for treating viral hepatitis B, in particular to a B cyclo-dioxane flavanonollignan compound and a preparation method thereof as well as the application of the compound or pharmaceutically acceptable salts thereof in the preparation of medicines for eliminating hepatitis B surface antigens (HBsAg) and hepatitis B e antigens (HBeAg) and medicines for inhibiting HBV DNA replication. The compound has obvious activity of inhibiting HBsAg and HBeAg, and the intensities of the compound for eliminating HBsAg and HBeAg under the concentration of 20 microgram / millimeter are respectively 2.1 times and 1.2 times larger than the corresponding activity of a positive control medicine alpha-interferon; meanwhile, the compound displays high inhibition ratio more than 57% on HBV DNA at the concentration. The results show that the favonolignan or pharmaceutically acceptable salts thereof can be expected to be used for preparing non-nucleoside type medicines for eliminating HBsAg and HBeAg, inhibiting HBV DNA replication and treating HBV infected diseases.
Owner:DALI UNIV
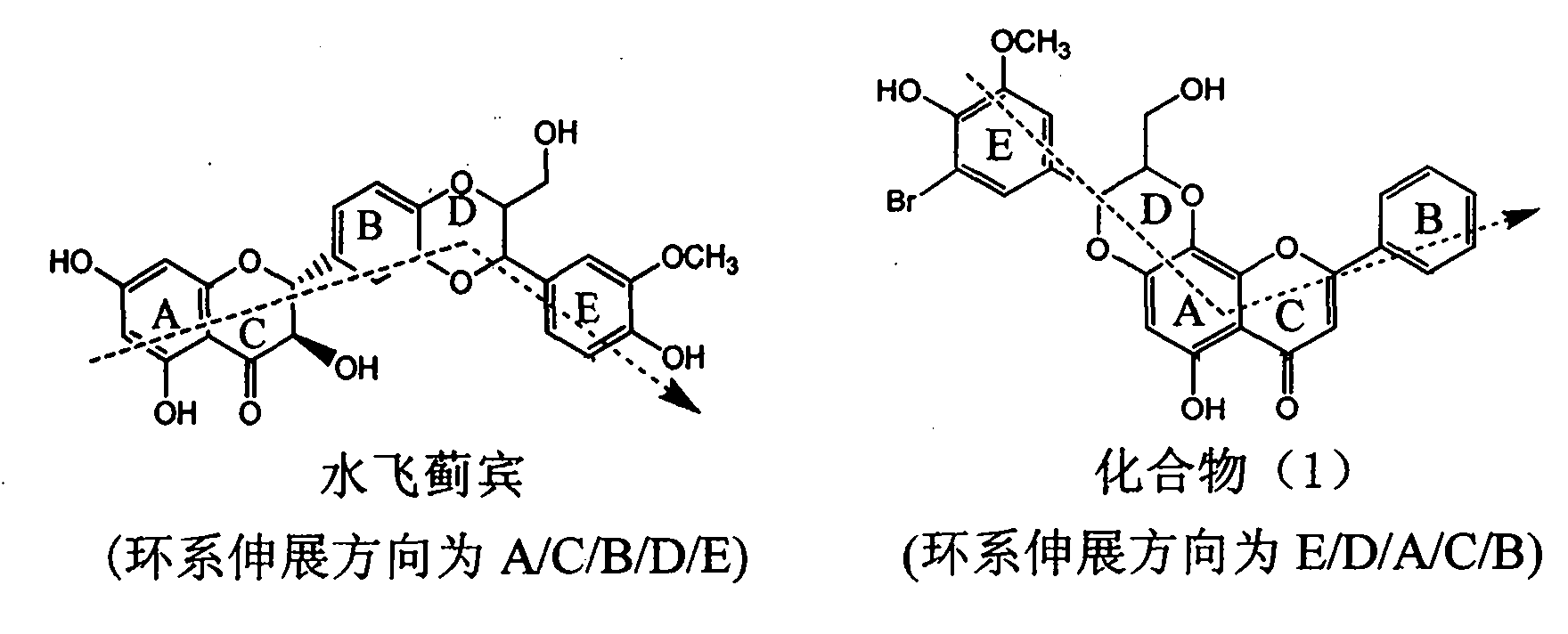
Application of aromatic carbamoyl dehydro-silibinin as medicament for treating viral hepatitis B
InactiveCN101829086AConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemDiseasePositive control
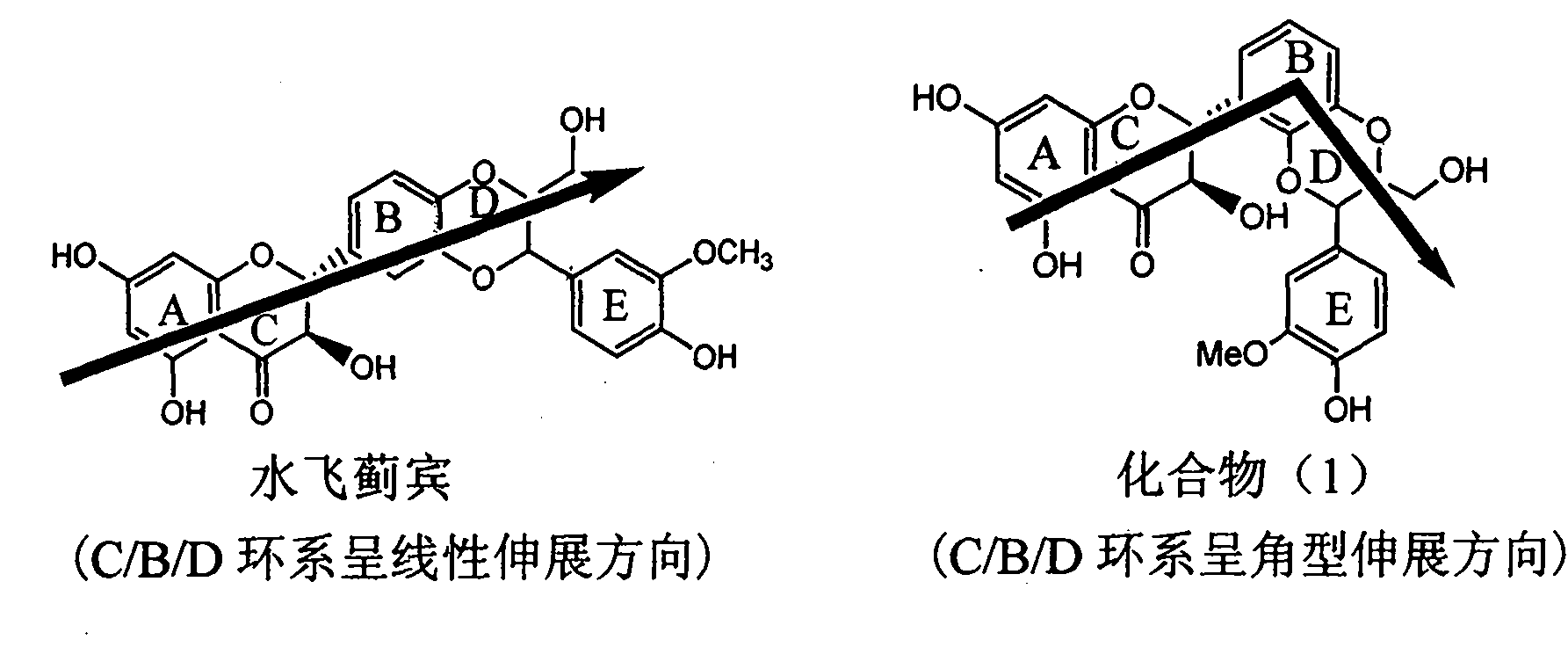
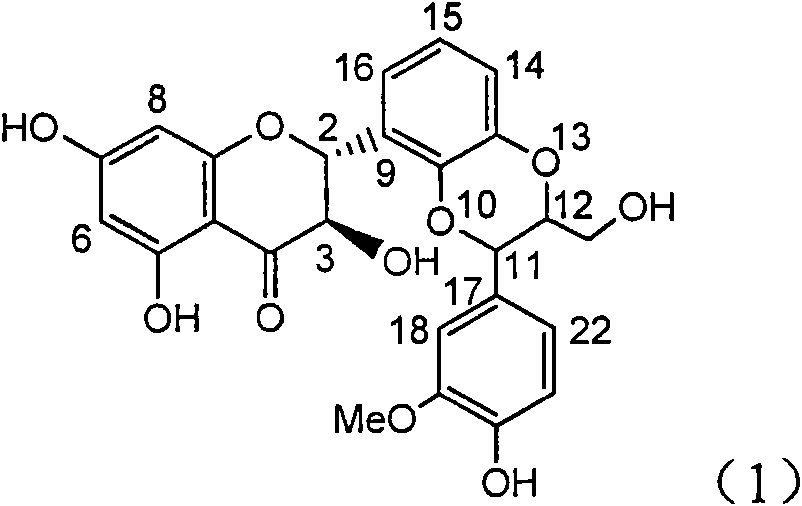
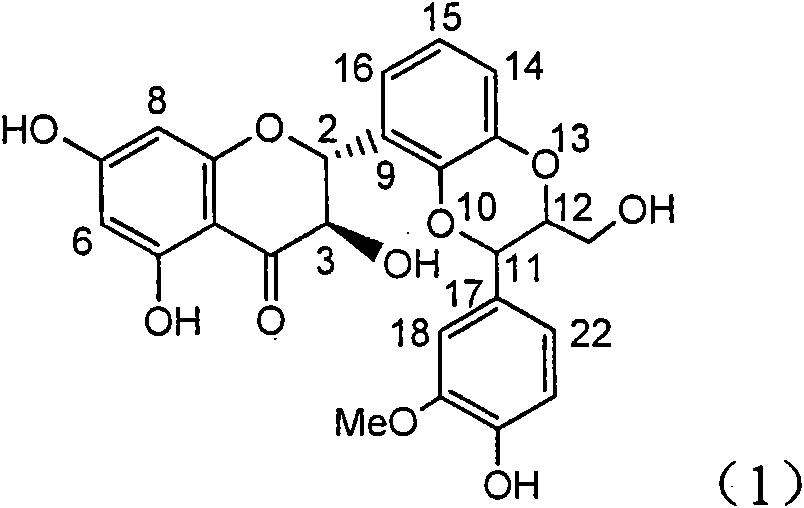
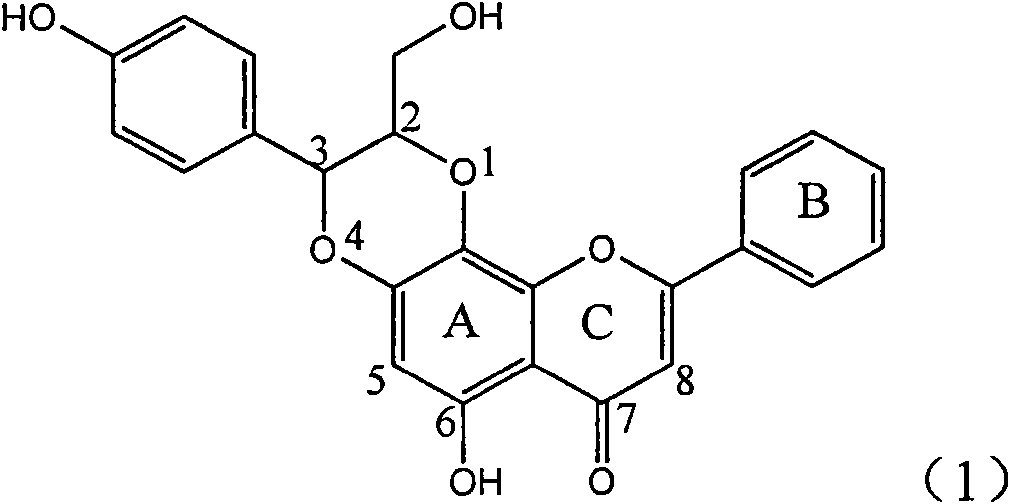
The invention relates to application of aromatic carbamoyl dehydro-silibinin as a medicament for treating viral hepatitis B, in particular to application of todehydro-silibinin flavonolignans with a ring A and a ring E which are substituted by double base aromatic carbamoyl methoxyl and pharmaceutically acceptable salt thereof for preparing medicaments for removing HBsAg and HBeAg and medicaments for inhibiting HBV DNA. The todehydro-silibinin flavonolignans has extremely obvious activity on inhibiting the HBsAG and the HBeAg, has the intensity of 46.2 percent and 68.9 percent for respectively removing the HBsAG and the HBeAg in the presence of the concentration of 100 microgram / milliliter, which is 2.9 times and 4.1 times higher than that of positive control medicament alpha-interferon, and has the inhibition ratio of 96 percent on HBV DNA in the presence of the concentration of 100 microgram / milliliter, which is higher than that of lamivudine and the alpha-interferon. Accordingly, the flavonolignans and the pharmaceutically acceptable salt thereof can be expected to be used for preparing non-nucleoside medicaments applied for removing HBsAg and HBeAg, inhibiting HBV DNA replication and treating hepatitis B virus infection diseases.
Owner:DALI UNIV
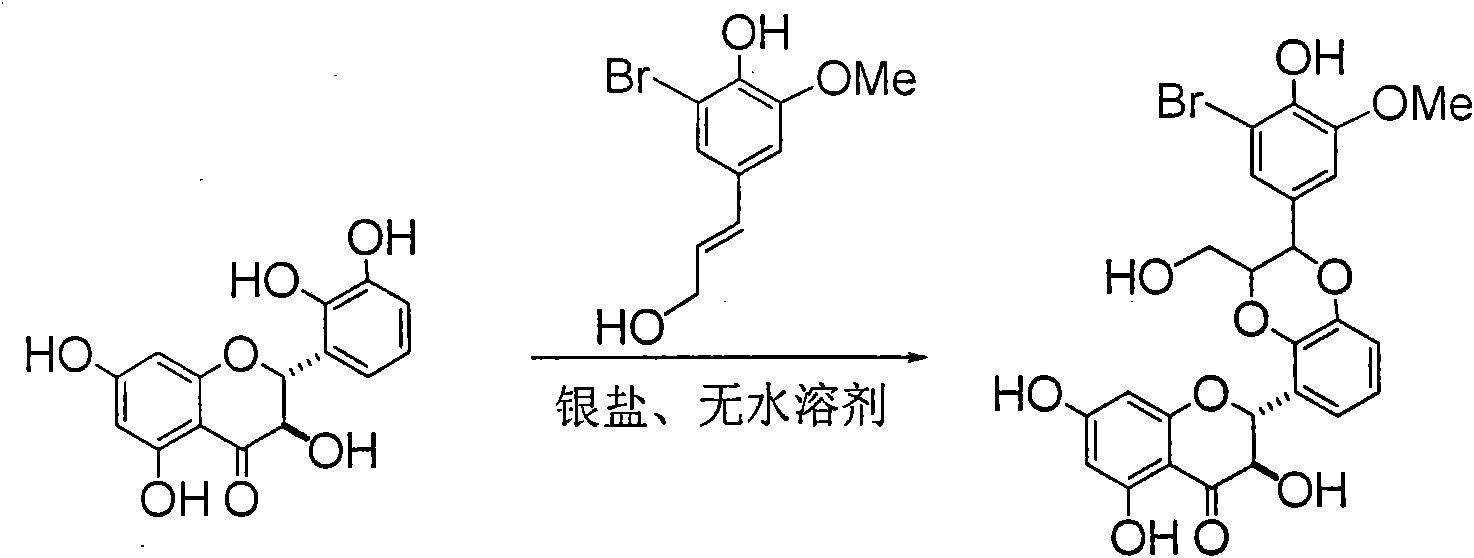
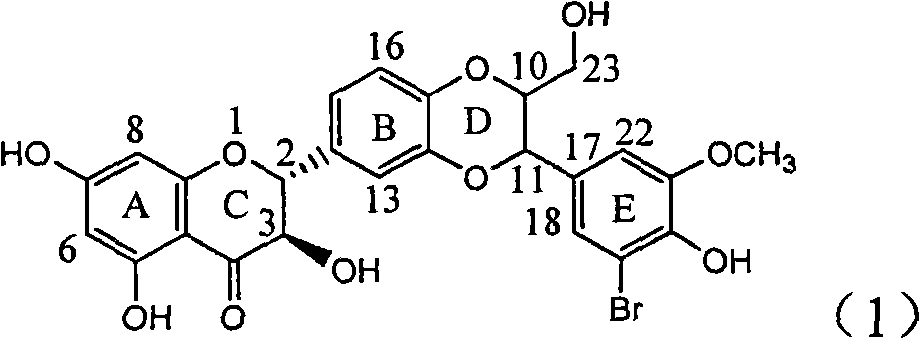
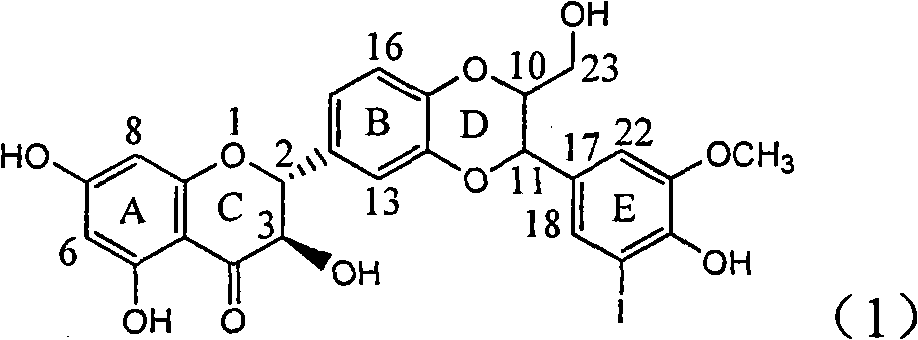
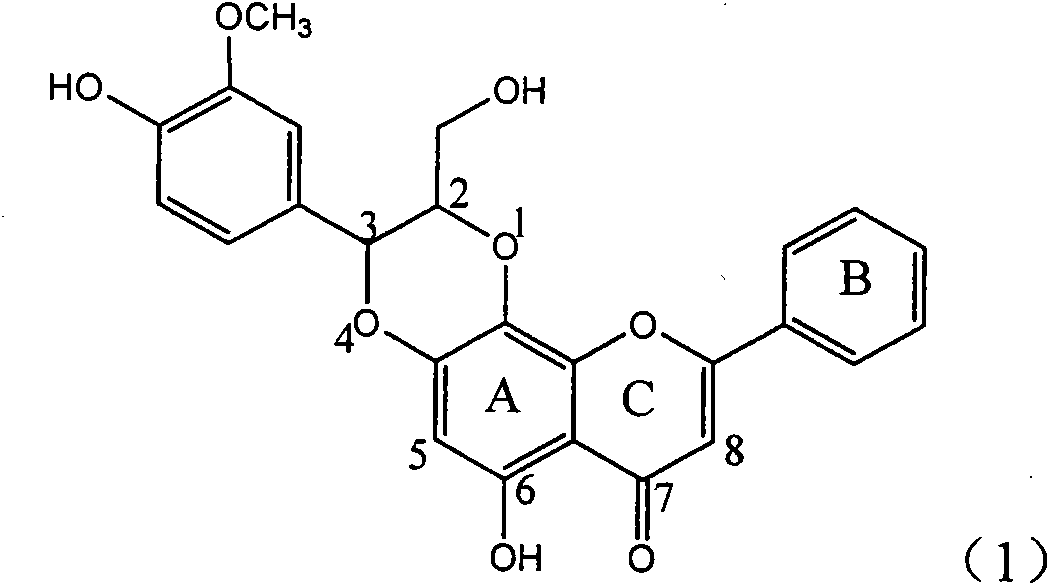
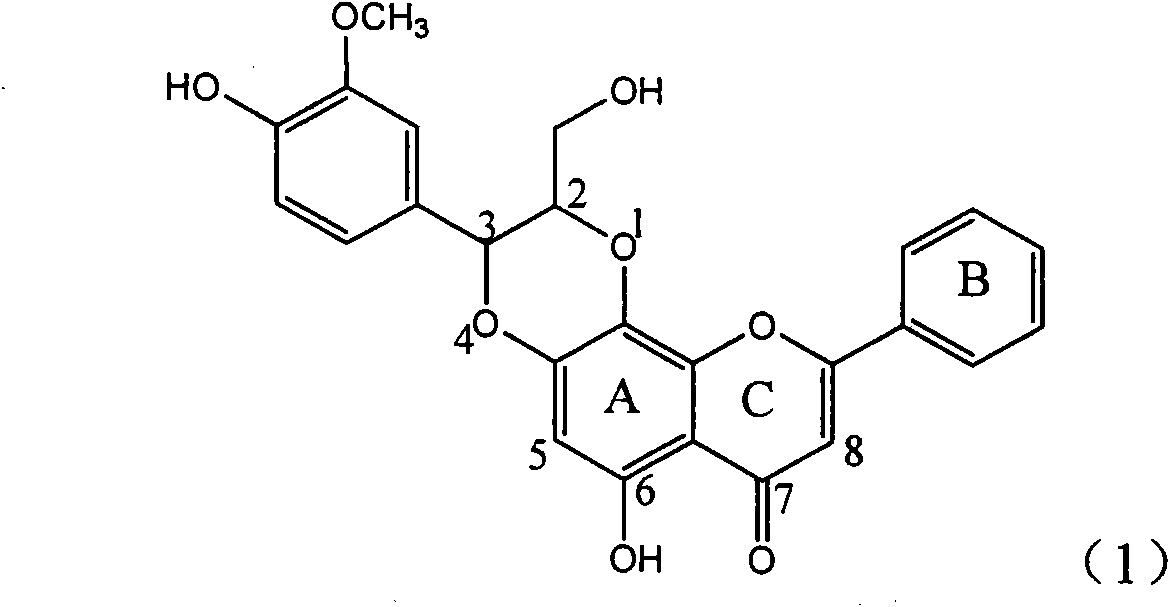
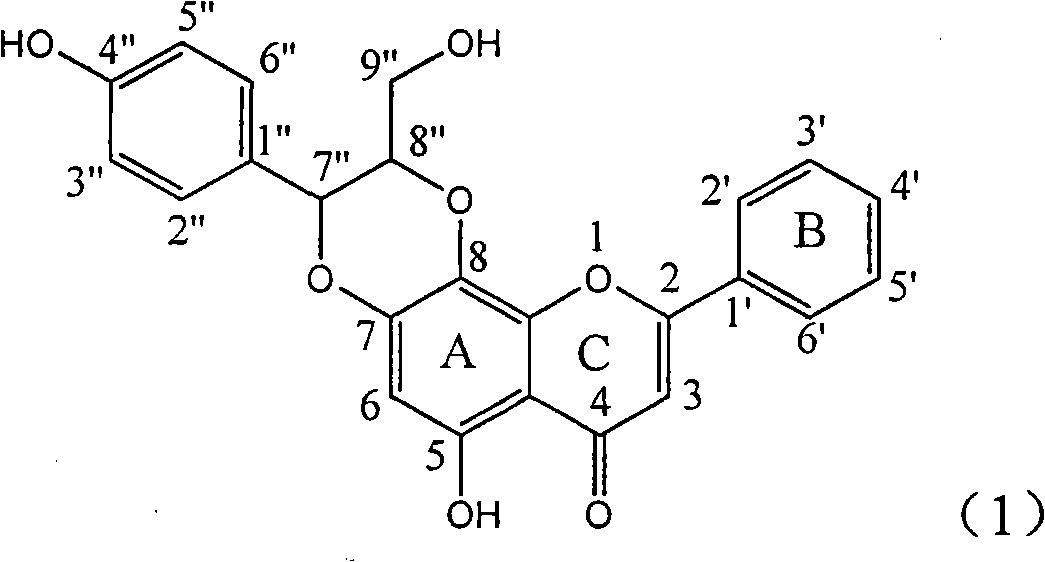
Application of ring E bromine substituted silybin in preparing medicaments for treating viral hepatitis B
InactiveCN101829094AInhibitory activityInhibition of replicative activityOrganic active ingredientsAntiviralsDiseasePositive control
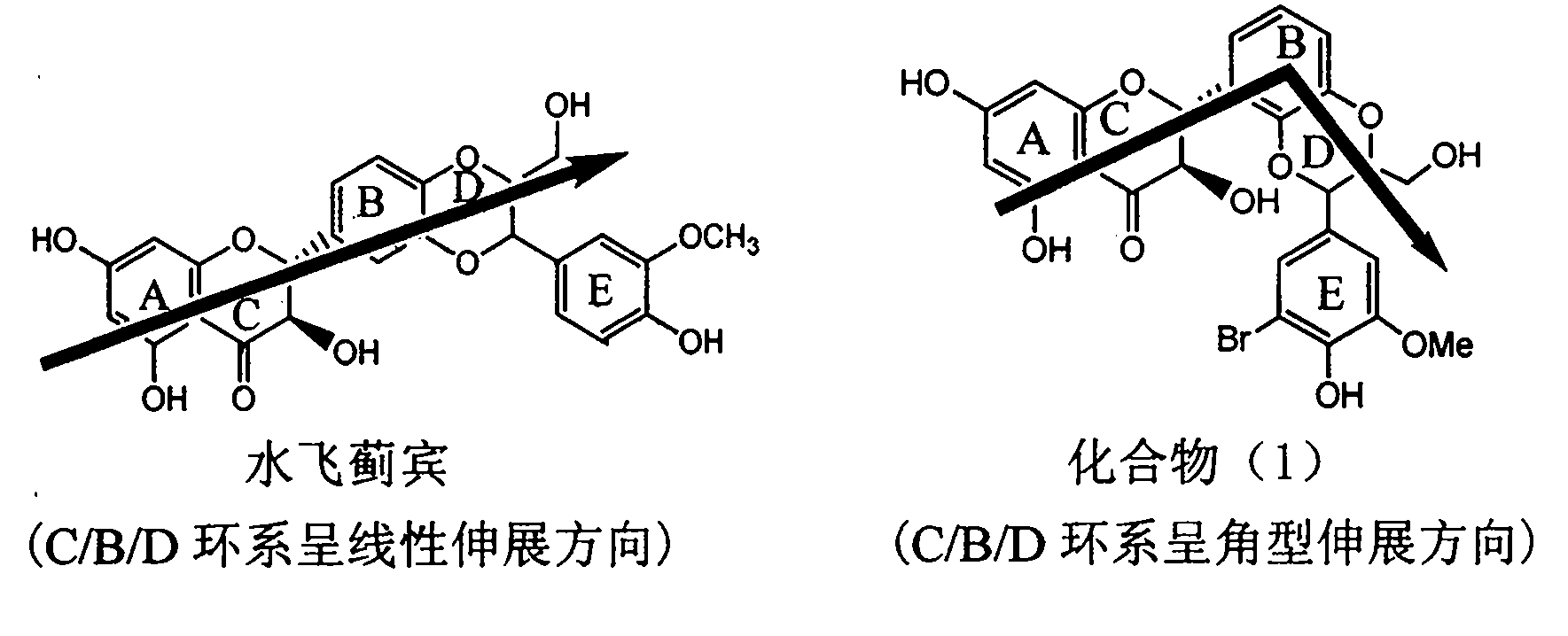
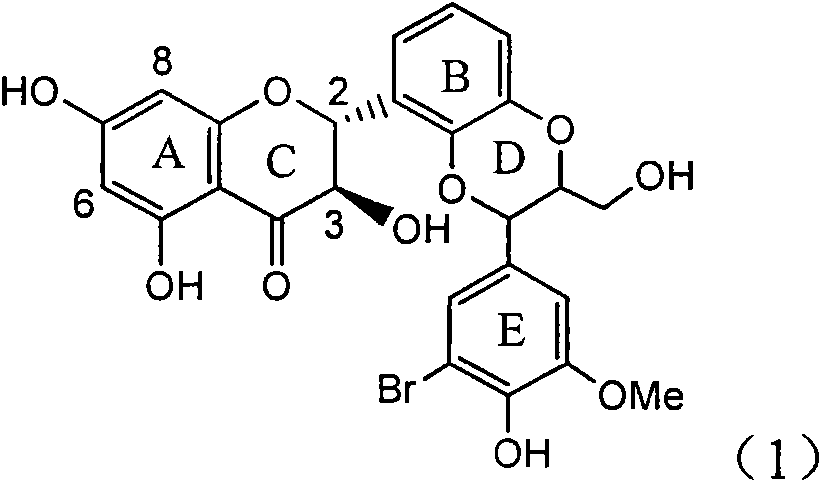
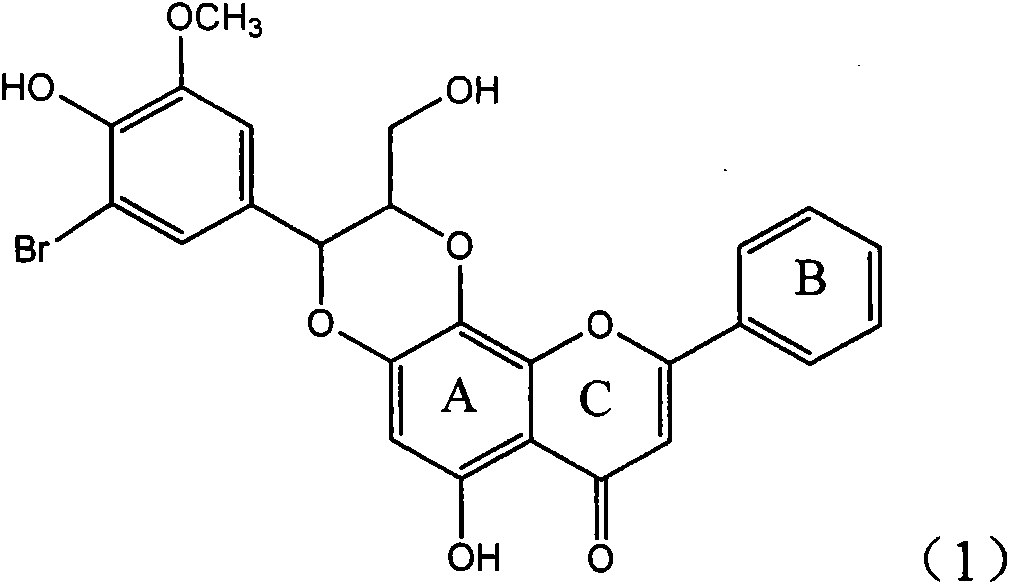
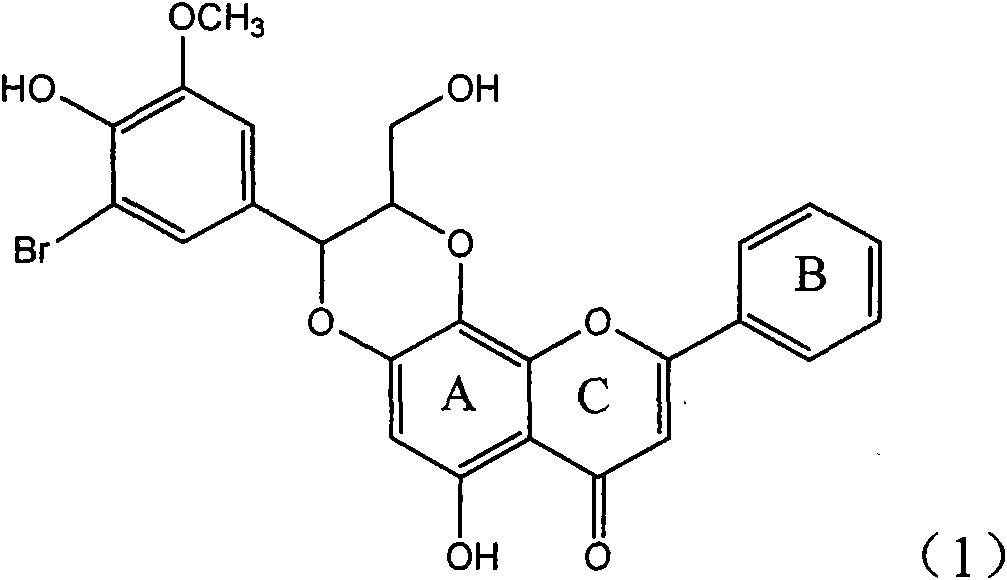
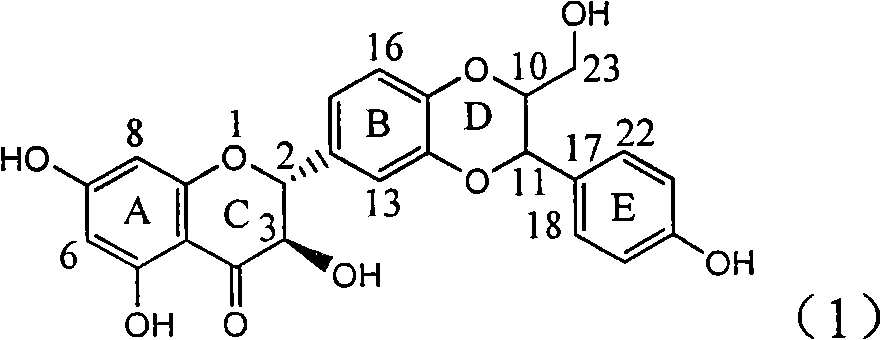
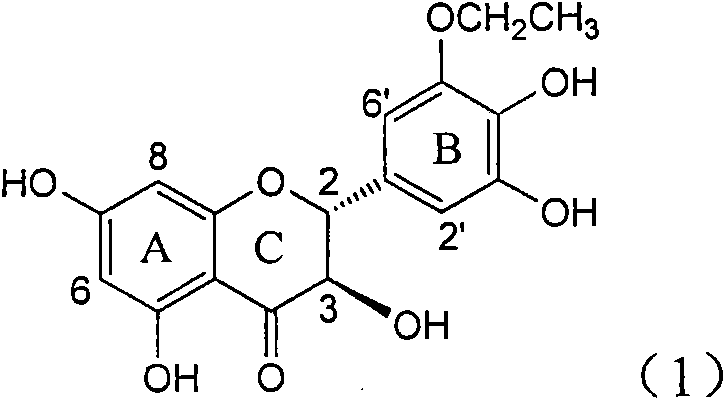
The invention relates to application of ring E bromine substituted silybin in preparing medicaments for treating viral hepatitis B, in particular to application of a compound of a formula (1) and a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away hepatitis B surface antigens (HBsAg) and hepatitis e antigens (HBeAg) and suppressing the HBV (Hepatitis B Virus) DNA replication. The compound has definite activity on suppressing the HBsAg and the HBeAg, and in the presence of a concentration of 100 micrograms / milliliter, the intensities of the compound for clearing away the HBsAg and the HBeAg are respectively 38.2 percent and 39.1 percent which are respectively 2.4 times and 2.3 times of that of a positive control medicament (10,000 units / milliliter of alpha-interferon). Meanwhile, in the presence of the concentration, the suppression ratio of the compound on the HBV DNA is 36 percent which is close to that of the alpha-interferon. Accordingly, the flavone lignan or the pharmaceutically acceptable salt thereof are indicated to be capable of being used for preparing non-nucleoside medicaments for clearing away the HBsAg and the HBeAg, suppressing the HBV DNA replication and treating HBV infection diseases.
Owner:DALI UNIV
Walnut rich in selenium and organic fertilizer rich in selenium for walnut rich in selenium
ActiveCN103918520ASave resourcesEasy to produceFertilising methodsCultivating equipmentsOrganic fertilizerMicrogram
The invention belongs to the field of agricultural technologies and particularly relates to a walnut rich in selenium and organic fertilizer rich in selenium for the walnut rich in selenium. The walnut and the organic fertilizer are mainly used for solving the technical problems that the fertilization amount of existing fertilizer for producing subsidiary agricultural products rich in selenium is difficult to control, the environment is polluted, dependence of crops is prone to generation, land is barren, and cost is high. According to the technical scheme, the walnut rich in selenium is obtained through fertilization of the self-made organic fertilizer rich in selenium, the selenium content of a walnut kernel ranges from 200 microgram per kilogram to 800 microgram per kilogram, and the walnut and the organic fertilizer have the advantages of being environmentally friendly, free of pollution and low in cost.
Owner:汾州裕源土特产品有限公司
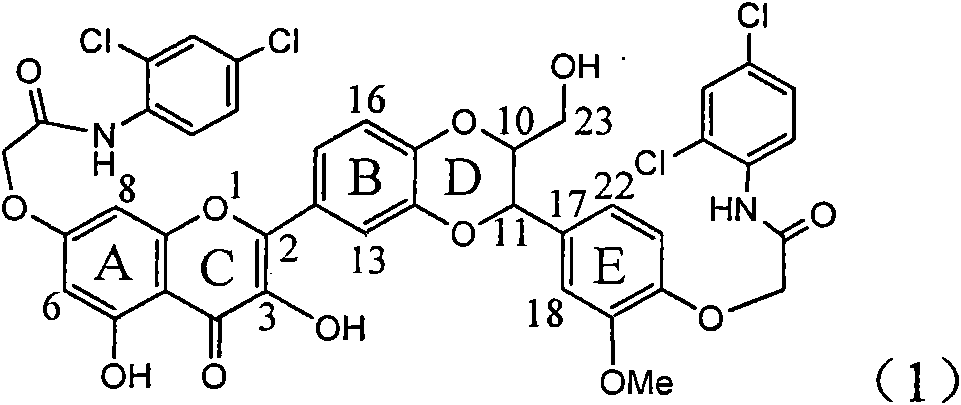
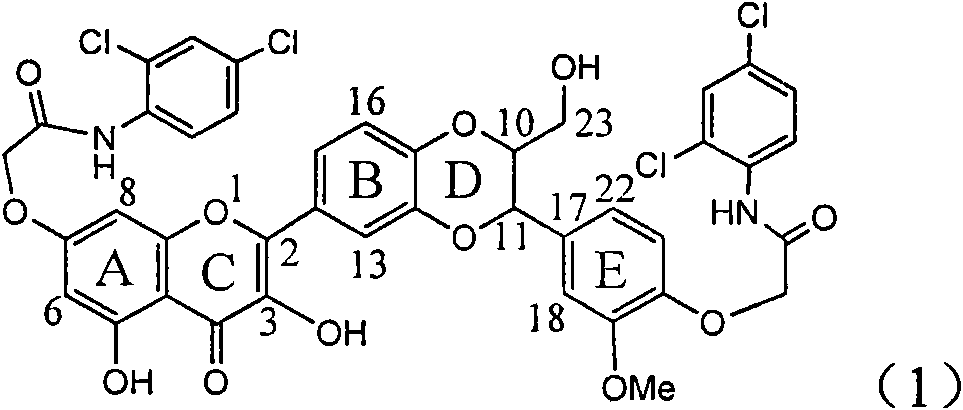
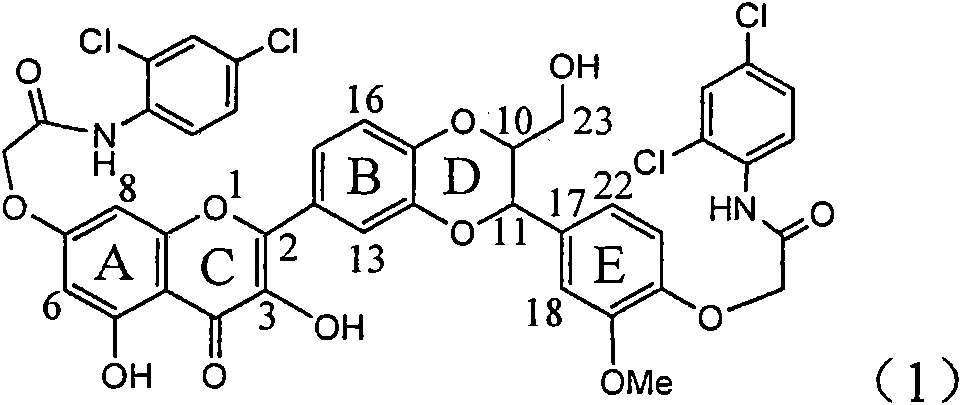
Application of ring A coupling flavonolignan in preparing medicaments for treating viral hepatitis B
InactiveCN101829104AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
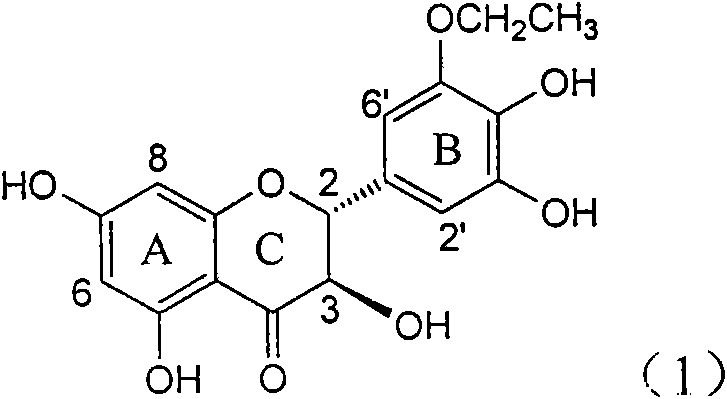
The invention relates to application of ring A coupling flavonolignan in preparing medicaments for treating viral hepatitis B, in particular to application of a compound of the formula (1) or a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away HBsAg (Hepatitis B Surface Antigen) and HBeAg (Hepatitis B e Antigen) and suppressing the HBV (Hepatitis B Virus) DNA replication. The intensities of the flavonolignan for clearing away the HBsAg and the HBeAg are respectively 29.4 percent and 29.1 percent in the presence of a concentration of 20 micrograms / milliliter, which is respectively 1.8 times and 1.7 times of the corresponding activity of a positive control medicament (10,000 units / milliliter of alpha-interferon). What is even more exciting is that in the presence of the concentration, the suppression rate of the flavonolignan to the HBV DNA is higher than 83 percent, which is higher than that of Lamivudine which is a positive control and is 2.2 times of that of the alpha-interferon to the HBV DNA. Accordingly, the flavonolignan and the pharmaceutically acceptable salt thereof are indicated to be capable of being expected to be used for preparing non-nucleoside medicaments for clearing away the HBsAg and the HBeAg, suppressing the HBV DNA replication and treating HBV infection diseases.
Owner:DALI UNIV
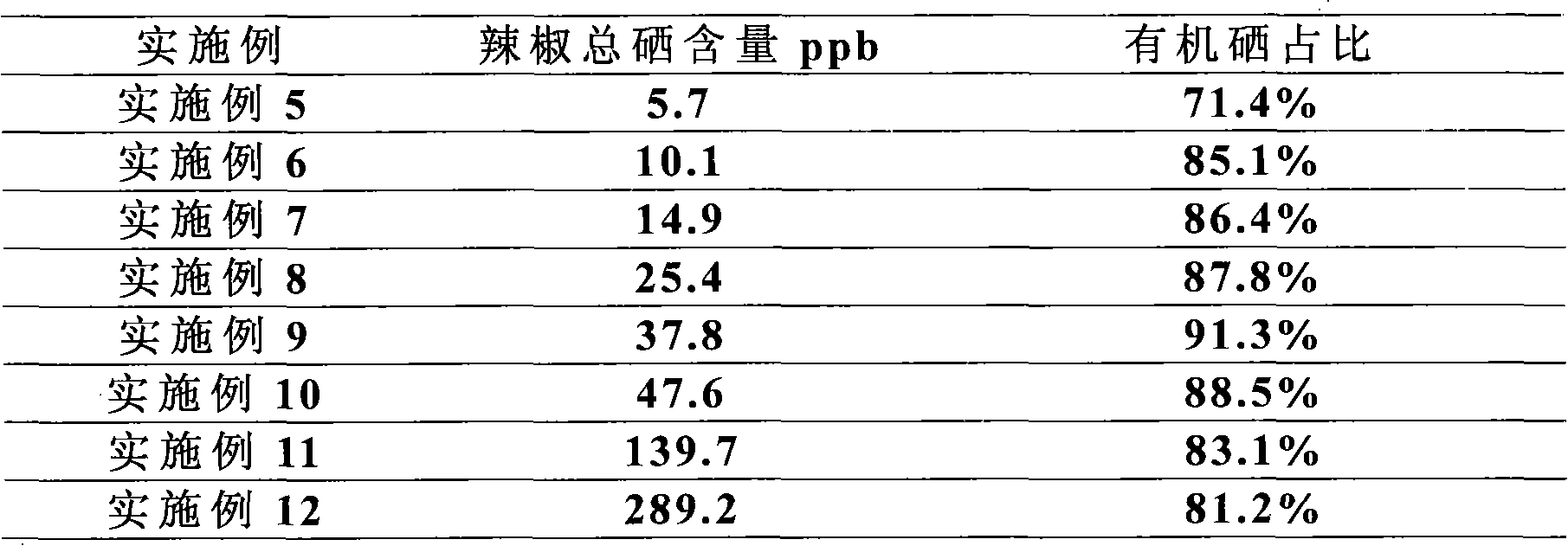
Hot pepper rich in organic selenium and production method thereof
InactiveCN102150531AGood for targeted absorptionFacilitated releaseFertilising methodsHorticultureSludgePlant nutrition
The invention discloses a hot pepper rich in organic selenium and a production method thereof. The total selenium content in the hot pepper rich in organic selenium is 10-300 micrograms per kilogram and the content of the organic selenium is equal to or more than 80% of the total selenium content. The method comprises the following steps: using a selenium-enriched seedling culture matrix of hot pepper to culture the seedlings of the hot pepper rich in organic selenium; transplanting the seedlings together with soil into a land; and finally harvesting the hot pepper rich in organic selenium, wherein a selenium-enriched seedling culture matrix of hot pepper contains nanometer selenium plant nutrient agent, garden soil, organic fertilizer, pond sludge and plant ash, and the mass ratio of the nanometer selenium plant nutrient agent to the garden soil to the organic fertilizer to the pond sludge to the plant ash is (0.001-0.1): (2-8):(1-4): (1-4): (0.5-1). The hot pepper is rich in selenium element and the whole content is uniform. By using the method, the proportion of organic selenium is increased, the problem of the prior art of the stability of selenium content in the hot peppers grown in different batches is solved, the uniform selenium content in the hot pepper is ensured, and the standard mass production can be realized.
Owner:SUZHOU SETEK
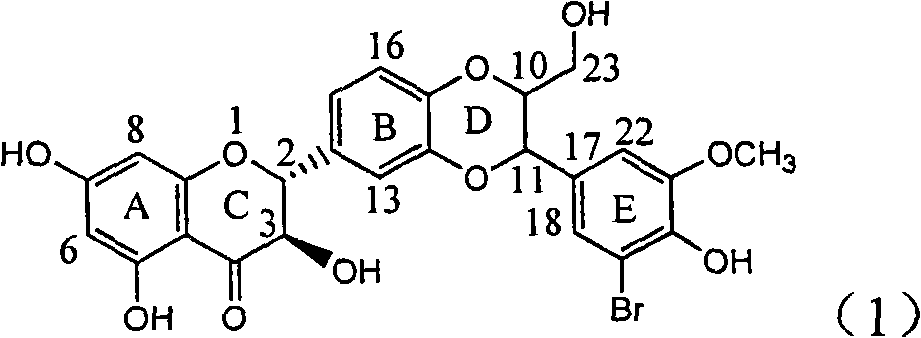
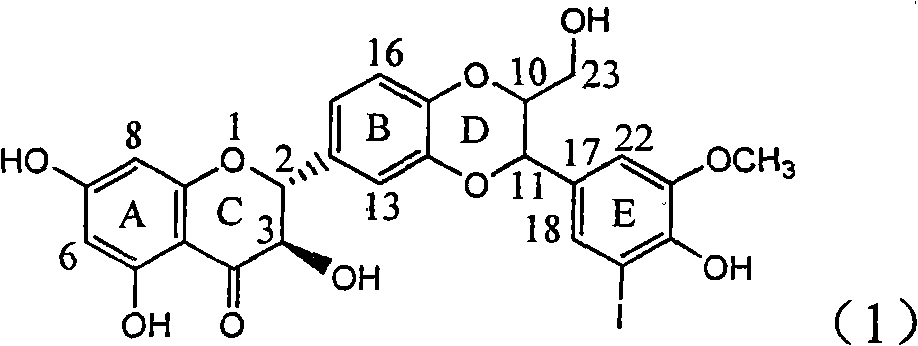
Application of ring E iodine substituted silybin in preparing medicaments for treating viral hepatitis B
InactiveCN101829096AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
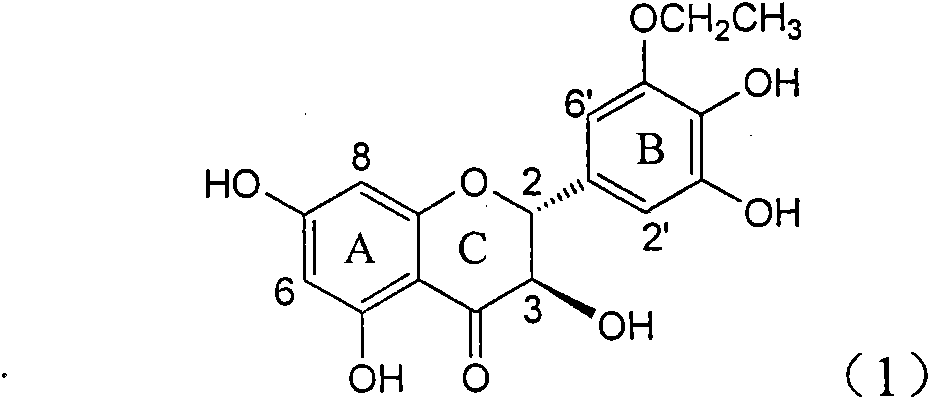
The invention relates to application of ring E iodine substituted silybin in preparing medicaments for treating viral hepatitis B, in particular to application of a compound of a formula (1) and a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away hepatitis B surface antigens (HBsAg) and hepatitis e antigens (HBeAg) and suppressing the HBV (Hepatitis B Virus) DNA replication. The compound has definite activity of suppressing the HBsAg and the HBeAg, and in the presence of a concentration of 100 micrograms / milliliter, the intensities of the compound for clearing away the HBsAg and the HBeAg are respectively 20.0 percent and 29.0 percent which exceed that of a positive control medicament (10,000 units / milliliter of alpha-interferon) by 24 percent and 72 percent. Meanwhile, in the presence of the concentration, the suppression ratio of the compound on the HBV DNA is 32.6 percent which is close to that of the alpha-interferon. Accordingly, the flavone lignan or the pharmaceutically acceptable salt thereof are indicated to be capable of being used for preparing non-nucleoside medicaments for clearing away the HBsAg and the HBeAg, suppressing the HBV DNA replication and treating HBV infection diseases.
Owner:DALI UNIV
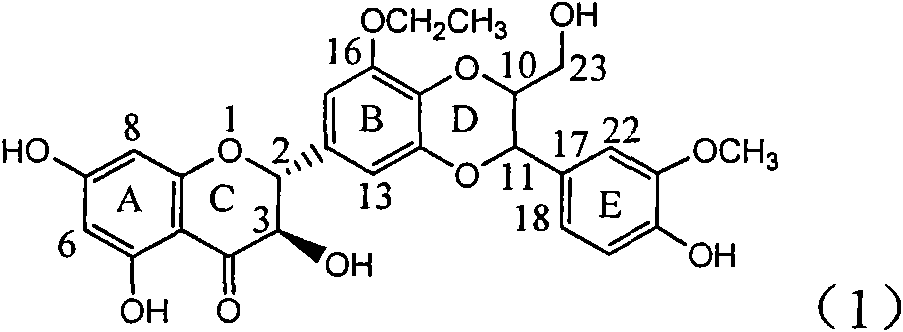
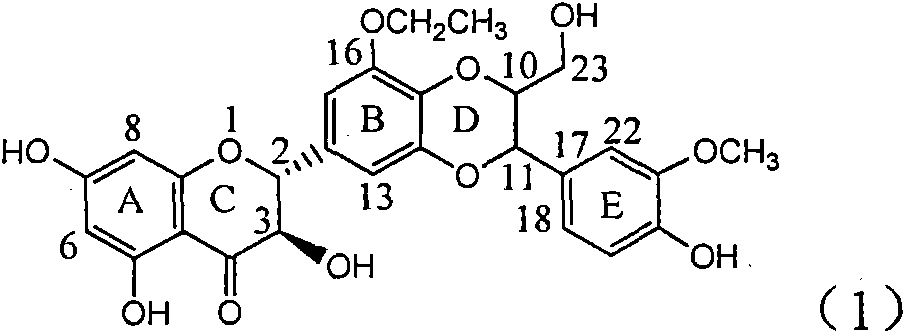
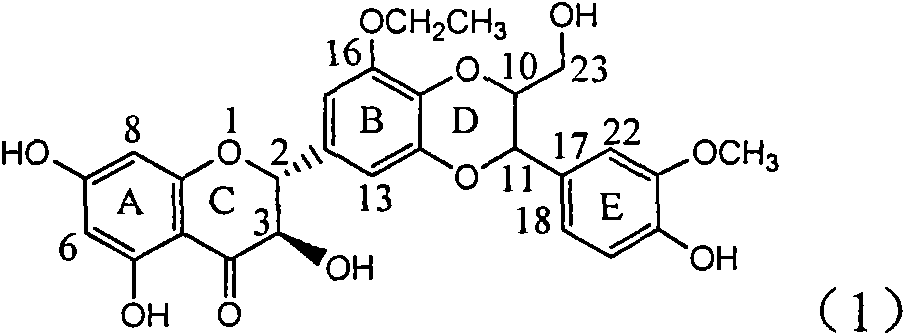
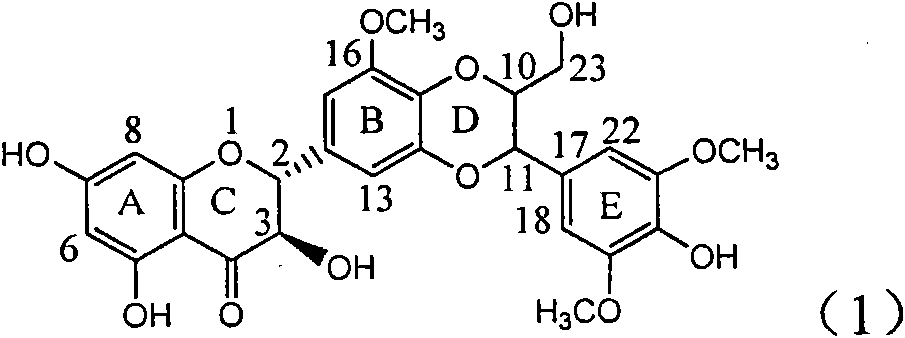
Application of ring B ethyoxyl silybin in preparing medicaments for treating viral hepatitis B
InactiveCN101829089AConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemDiseasePositive control
The invention relates to application of ring B ethyoxyl silybin in preparing medicaments for treating viral hepatitis B, in particular to application of ring B ethyoxyl substituted silybin ester or a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away HBsAG (Hepatitis B Surface Antigen) and HBeAg (Hepatitis B e Antigen) and suppressing the HBV (Hepatitis B Virus) DNA replication. The compound has strong activity on suppressing the HBsAG and the HBeAg, and in the presence of a concentration of 20 micrograms / milliliter, the intensities for clearing the HBsAg and the HBeAg are respectively 64.6 percent and 44.8 percent which are 4.0 times and 2.7 times of that of alpha-interferon which is a positive control medicament. In the presence of the concentration, the suppression rate of the compound on the HBV DNA is 58.1 percent which is 1.5 times of the corresponding activity of the alpha-interferon. Accordingly, the flavonolignan or the pharmaceutically acceptable salt thereof are indicated to simultaneously have strong efficacy on suppressing the HBsAg, the HBeAg and the HBV DNA and can be expected to be used for preparing non-nucleoside medicaments for treating HBV infection diseases.
Owner:DALI UNIV
Application of substituted isosilybin in preparing medicament for treating virus hepatitis B
InactiveCN101829098AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
The invention relates to application of substituted isosilybin in preparing a medicament for treating virus hepatitis B, in particular to application of E-ring substituted isosilybin or medicinal salt thereof in preparing a medicament for clearing hepatitis B e antigen, inhibiting HBV DNA replication and treating hepatitis B virus infected diseases. The E-ring substituted isosilybin has strong effect of inhibiting HBeAg activity, and the strength of the E-ring substituted isosilybin at the concentration of 100 micrograms per milliliter for clearing the HBeAg is 3.5 times that of a positive control front-line medicament (10,000 units per milliliter of alpha-interferon); and moreover, the compound at the concentration of 100 micrograms per milliliter has strong inhibiting rate (97.7 percent) on the HBV DNA. Pharmacodynamical results show that the E-ring substituted isosilybin or the medicinal salt thereof can be expected to be used for preparing the medicament for treating the hepatitis B virus infected diseases.
Owner:DALI UNIV
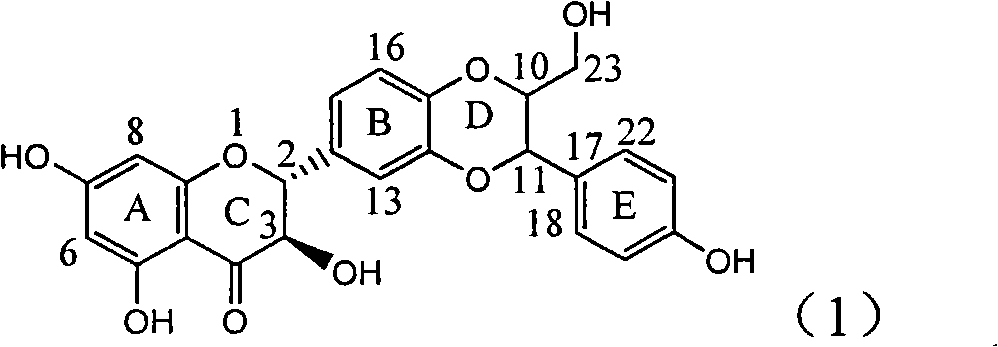
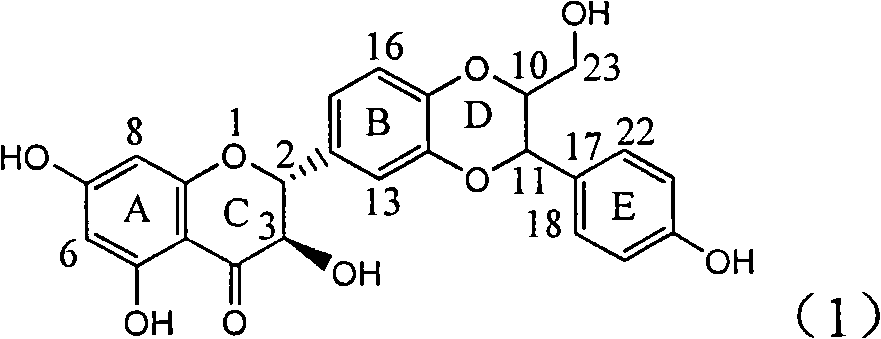
Application of flavone lignan (+/-) Scutellaprostin A in preparing medicaments for treating viral hepatitis type B
InactiveCN101953827AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseaseLignan
The invention relates to application of flavone lignan (+ / -) Scutellaprostin A in preparing medicaments for treating viral hepatitis type B, in particular to a compound with the formula (1) or pharmaceutically-acceptable salts thereof for preparing medicaments for clearing HBsAg and HBeAg and suppressing HBV (Hepatitis B Virus) DNA replication. In the invention, the intensities of the compound for clearing the HBsAg and the HBeAg under the concentration of 20 micrograms / milliliter respectively reach 81.8 percent and 81.9 percent, which are respectively 5.1 times and 4.8 times as high as the corresponding activity of alpha-interferon used as a positive contrast medicament; and what is more exciting, when the compound has the concentration, the compound performs a suppression ratio higher than 81 percent, and the value is also higher than that of both lamivudine and alpha-interferon. Accordingly, the flavone lignan or the pharmaceutically-acceptable salts can be expectably used for preparing nucleoside medicaments for clearing the HBsAg and the HBeAg, suppressing the HBV DNA replication and treating HBV infected diseases.
Owner:DALI UNIV
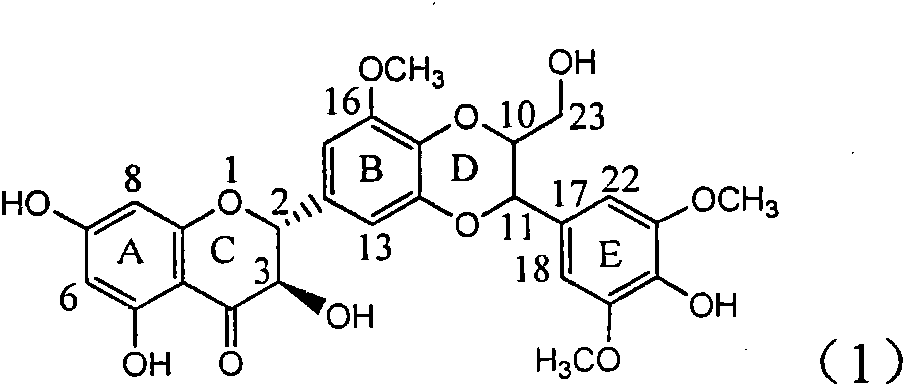
Application of angle flavonoids lignan to preparation of medicaments for treating viral hepatitis B
InactiveCN101953828AInhibition of replicationConvenient sourceOrganic active ingredientsAntiviralsLignanInterferon alpha
The invention relates to application of an angle flavonoids lignan to preparation of medicaments for treating viral hepatitis B, in particular to application of the angle flavonoids lignan or medicinal salts thereof to preparation of medicaments for inhibiting hepatisis B virus (HBV) DNA replication and treating HBV infection diseases. The flavonoids lignan can exactly inhibit HBV DNA activity; the inhibition activity of the flavonoids lignan with high dosage (20 microgram / ml) to the HBV DNA replication is 189 percent higher than that of alpha-interferon with the maximum concentration of 10,000 unit / ml; and the flavonoids lignan belongs to a strong-effect non-nucleosides inhibition HBV natural product. The pharmacological results show that the angle flavonoids lignan or the medicinal salts thereof can be expected to be used for preparing the medicaments for inhibiting hepatisis B virus (HBV) DNA replication and treating the HBV infection diseases.
Owner:DALI UNIV
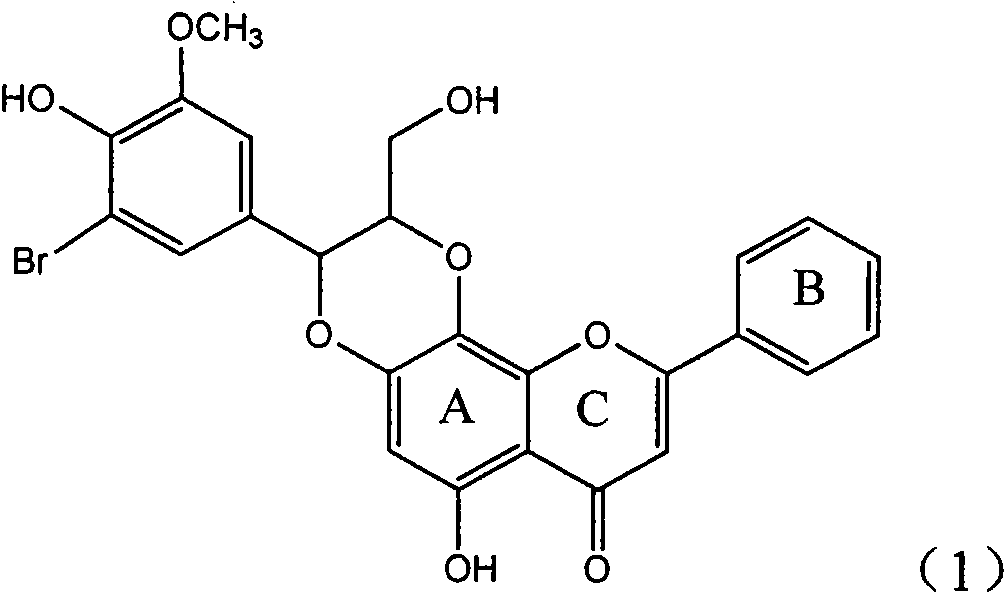
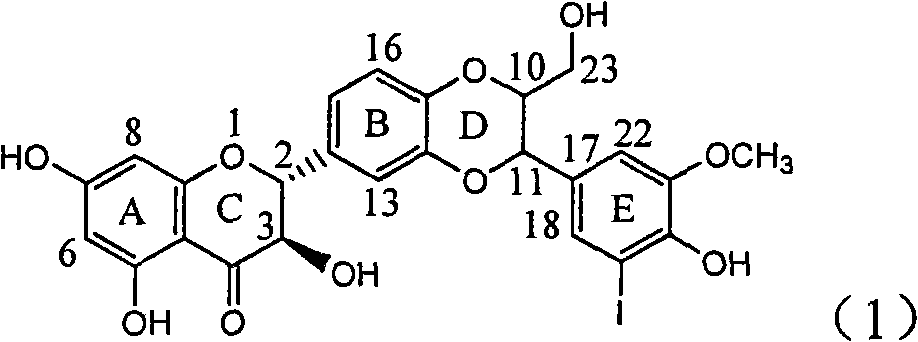
Application of ring A dioxane flavonolignan in preparing medicaments for resisting hepatitis B viruses (HBV)
InactiveCN101829085AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
The invention relates to application of ring A dioxane flavonolignan in preparing medicaments for resisting hepatitis B viruses (HBV), in particular to application of ring A dioxane coupling type flavone lignan or a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away hepatitis B e antigen (HBeAg), suppressing the HBV DNA replication and treating HBV infection diseases. The flavonolignan has certain activity on resisting the HBeAg, and the intensity of the flavonolignan for clearing away the HBeAg is higher than that of Lamivudine which is a positive control and close to that of 10,000 units / milliliter of alpha-interferon. Meanwhile, the suppression ratio of the compound to the HBV DNA replication is higher than 80 percent in the presence of a concentration of 100 micrograms / milliliter. The pharmacodynamical results indicate that the flavonolignan or the pharmaceutically acceptable salt thereof can be expected to be used for preparing the medicaments for clearing away the HBeAg, suppressing the HBV DNA replication and treating the HBV infection diseases.
Owner:DALI UNIV
Application of E-ring demethoxy-silibinin for preparing medicament for treating viral hepatitis B
InactiveCN101912383AConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemDiseasePositive control
The invention relates to application of E-ring demethoxy-silibinin for preparing medicaments for treating viral hepatitis B, and particularly to application of compound in formula (1) and pharmaceutically acceptable salt thereof for preparing medicaments for clearing HBsAg and HBeAg and suppressing HBV DNA replication. The invention has extremely superactive activity for suppressing the HBsAg and HBeAg; in the presence of the concentration of 20 microgram per millilitre, the intensities for clearing the HBsAg and HBeAg are 95.0% and 34.4% respectively, which are 5.9 and 2.0 times corresponding activity of a positive control medicament alpha-interferon; and it should be noticed that the suppression ratio of the medicament for HBV DNA at the concentration is about 91.5%, which is 13% higher than lamivudine and 2.4 times alpha-interferon suppression activity. In summary, the flavonolignans or pharmaceutically acceptable salt thereof can be prospectively used for preparing non-nucleoside medicaments for clearing the HBsAg and HBeAg, suppressing HBV DNA replication and treating hepatitis B virus infection disease.
Owner:DALI UNIV
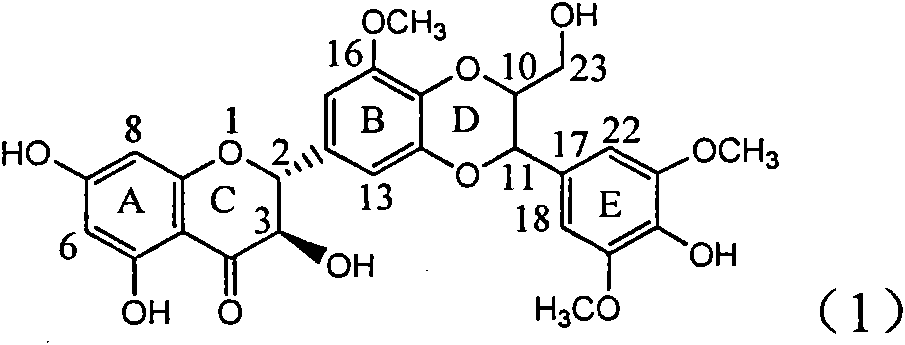
Application of B/E bi-methoxy silybin in preparing medicaments for treating viral hepatitis B
InactiveCN101829088AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
The invention relates to application of B / E bi-methoxy silybin in preparing medicaments for treating viral hepatitis B, in particular to application of silybin ester substituted by the methoxy on the ring B and the ring E or a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away HBsAg (Hepatitis B Surface Antigen) and HBeAg (Hepatitis B e Antigen) and suppressing the HBV (Hepatitis B Virus) DNA replication. The B / E bi-methoxy silybin has strong activity on suppressing the HBsAg and the HBeAg, and in the presence of a concentration of 20 micrograms / milliliter, the intensities for clearing away the HBsAg and the HBeAg are respectively 43.9 percent and 43.7 percent which are 2.7 times and 2.6 times of that of alpha-interferon which is a positive control medicament. In the presence of the concentration, the suppression ratio on the HBV DNA is 68.6 percent, and the suppression activity is 1.8 times of that of the alpha-interferon. Accordingly, the flavone lignan or the pharmaceutically acceptable salt thereof are indicated to simultaneously have the effects of strongly suppressing the HBsAG, the HBeAg and the HBV DNA and can be expected to be used for preparing the non-nucleoside medicaments for treating HBV infection diseases.
Owner:DALI UNIV
Application of B-ring ethyoxyl flavanonol in preparing medicaments for treating hepatitis B viruses
InactiveCN101822664AConvenient sourceThe source is easy to getOrganic active ingredientsOrganic chemistryDiseasePositive control
The invention relates to application of B-ring ethyoxyl flavanonol in preparing medicaments for treating hepatitis B viruses, in particular to application of a compound as shown in a formula (1) or a medicinal salt thereof in preparing medicaments for clearing away hepatitis B virus surface antigens (HBsAg) and hepatitis B e-antigen (HBeAg) and medicaments for inhibiting the duplication of hepatitis B virus desoxyribonucleic acid (HBV DNA). The compound or the medicinal salt thereof has extremely obvious activity on inhibiting the HBsAg and the HBeAg, and in the presence of a concentration of 20 microgram / milliliter, the intensities for clearing away the HBsAg and the HBeAg of the compound or the medicinal salt thereof are respectively 99.8 percent and 48.5 percent and are 6.2 times and 2.7 times of that of alpha-interferon which is a positive control medicament. More importantly, in the presence of the concentration, the inhibition ratio of the compound or the medicinal salt thereof to the HBV DNA is 64.7 percent, and the activity is 1.7 times of that of the alpha-interferon. Accordingly, the flavone lignan or the medicinal salt thereof can be expected to be used for preparing non-nucleoside medicaments for treating infectious diseases of the hepatitis B viruses.
Owner:DALI UNIV
Compositions and methods using complexes of heat shock protein gp96 and antigenic molecules for the treatment and prevention of infectious diseases
InactiveUS6143299AElicit immune responseOrganic active ingredientsPeptide/protein ingredientsMHC class IAntigenic valence
The present invention relates to methods and compositions for eliciting an immune response and the prevention and treatment of primary and metastatic neoplastic diseases and infectious diseases. The methods of the invention comprise administering a composition comprising an effective amount of a complex, in which the complex consists essentially of a heat shock protein (hsp) noncovalently bound to an antigenic molecule. "Antigenic molecule" as used herein refers to the peptides with which the hsps are endogenously associated in vivo as well as exogenous antigens / immunogens (i.e., with which the hsps are not complexed in vivo) or antigenic / immunogenic fragments and derivatives thereof. In a preferred embodiment, the complex is autologous to the individual. The effective amounts of the complex are in the range of 10-600 micrograms for complexes comprising hsp70, 50-1000 micrograms for hsp90, and 10-600 micrograms for gp96. The invention also provides a method for measuring tumor rejection in vivo in an individual, preferably a human, comprising measuring the generation by the individual of MHC Class I-restricted CD8+ cytotoxic T lymphocytes specific to the tumor. Methods of purifying hsp70-peptide complexes are also provided.
Owner:FORDHAM UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com