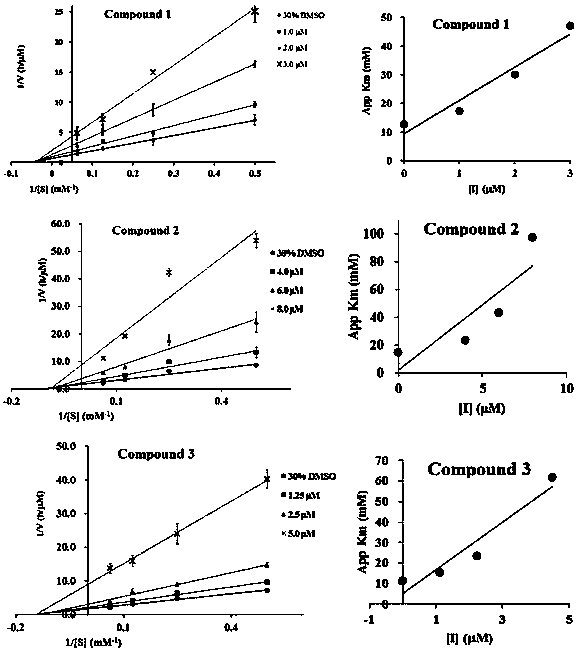
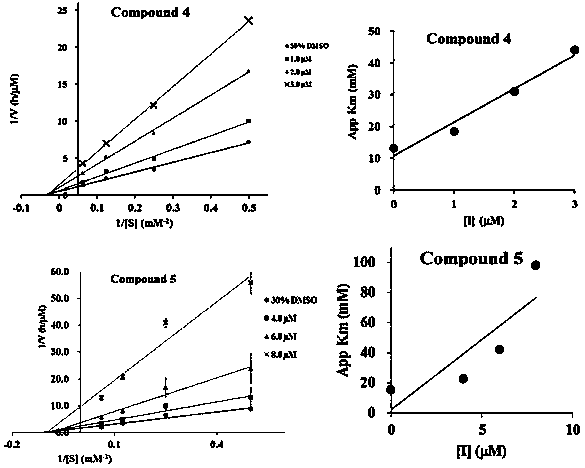
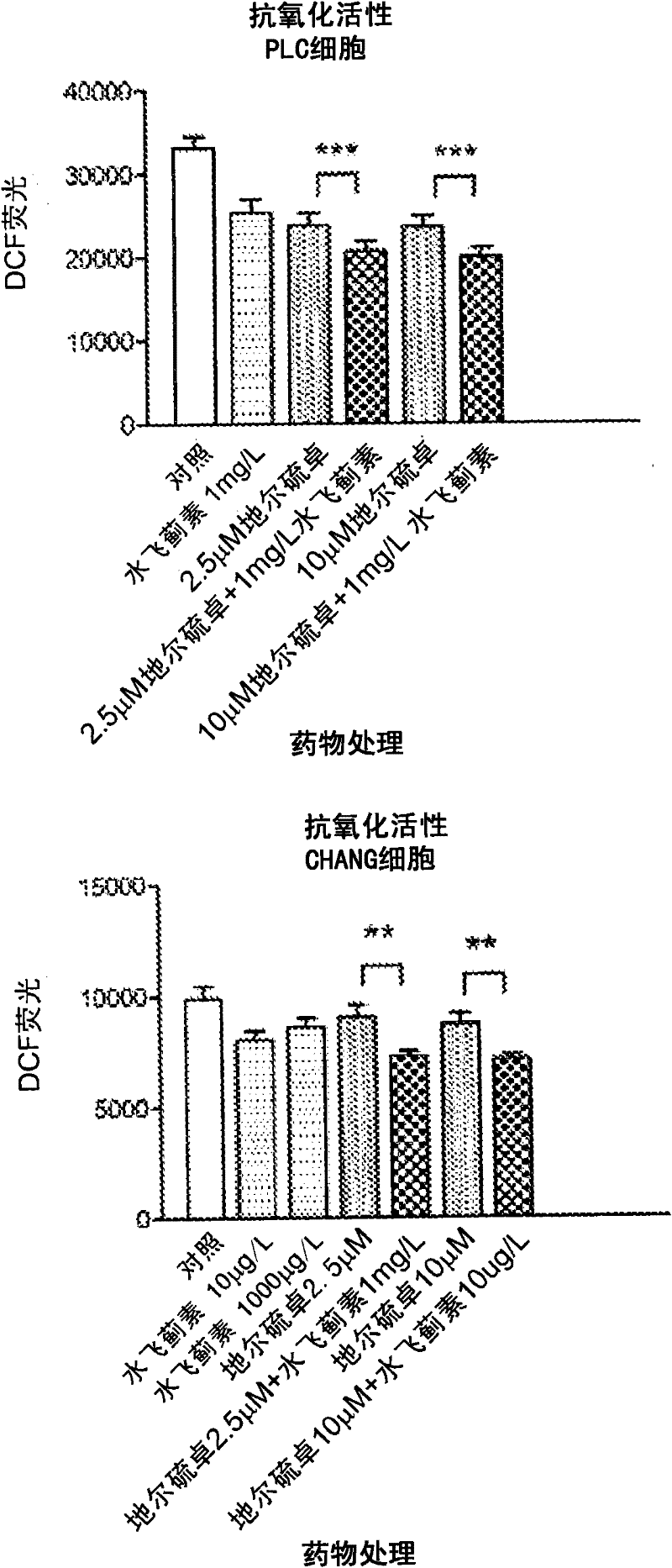
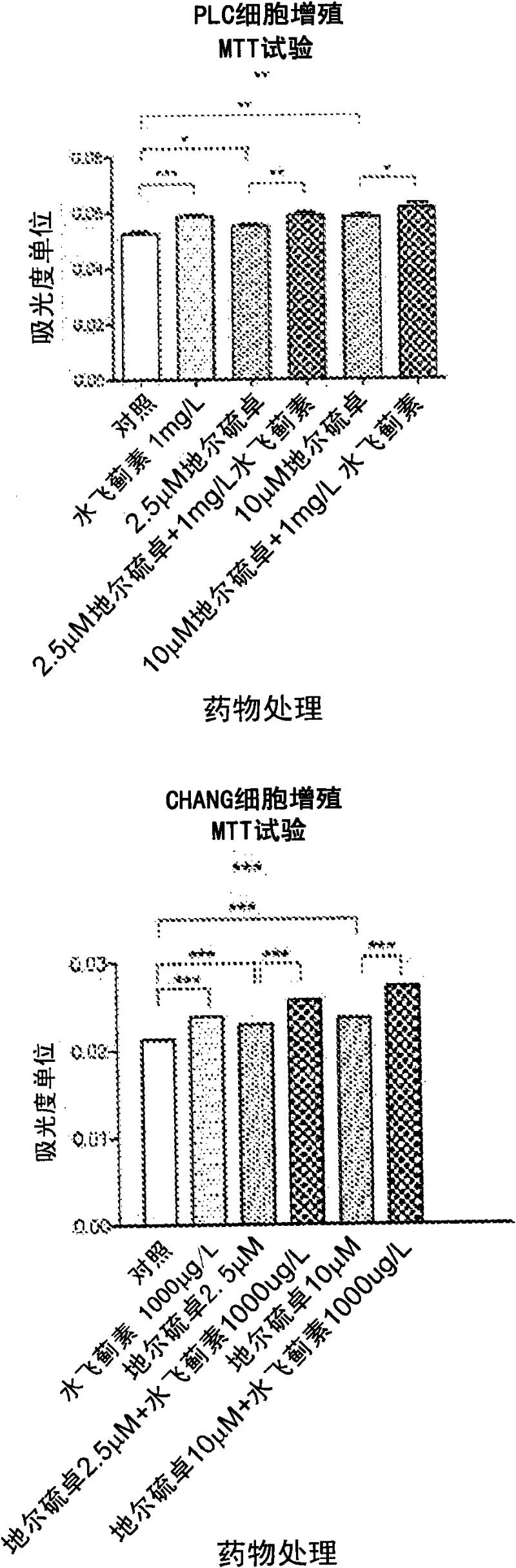
Patents
Literature
45 results about "Flavonolignan" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
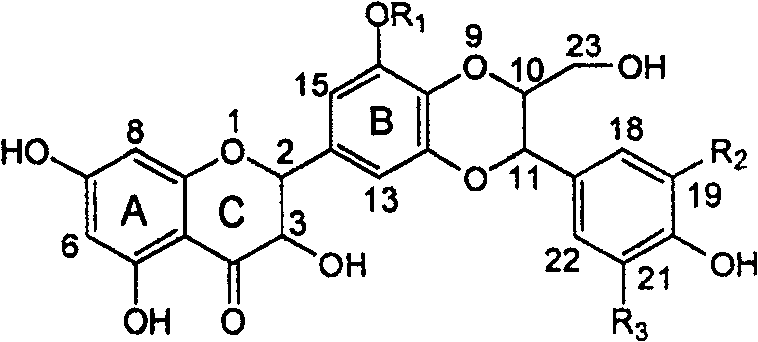
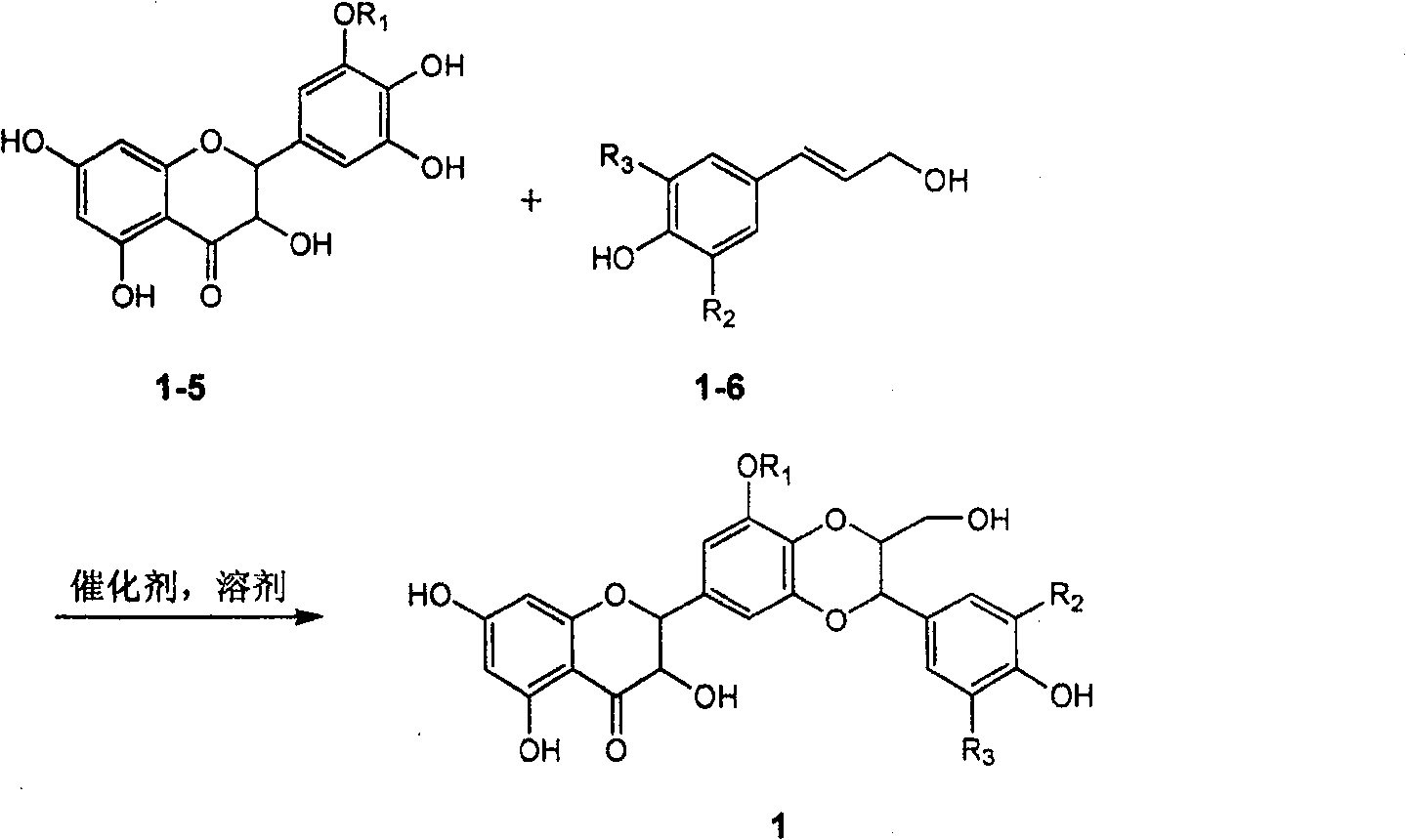
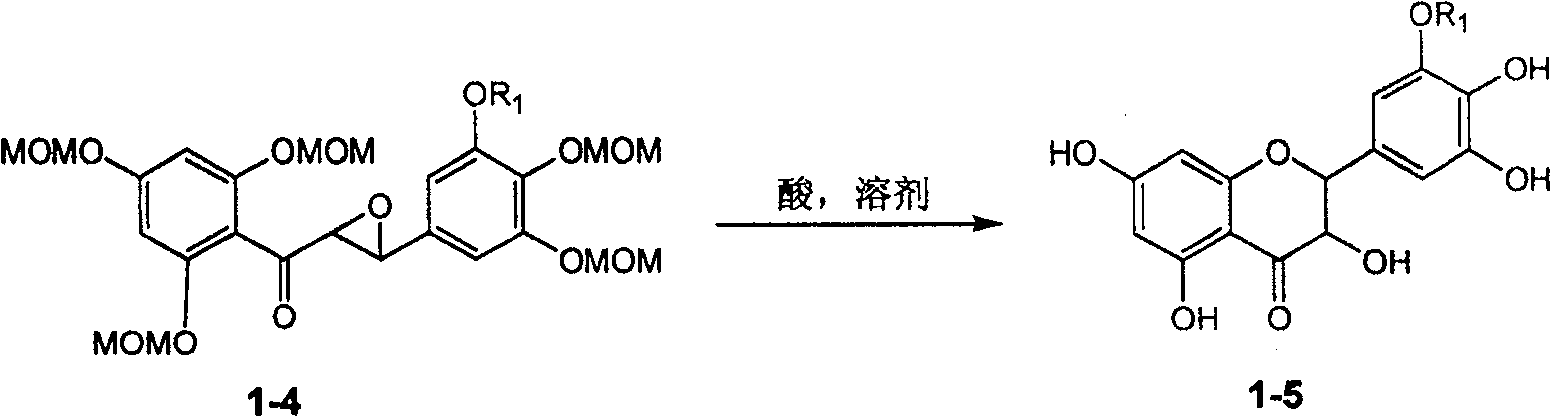
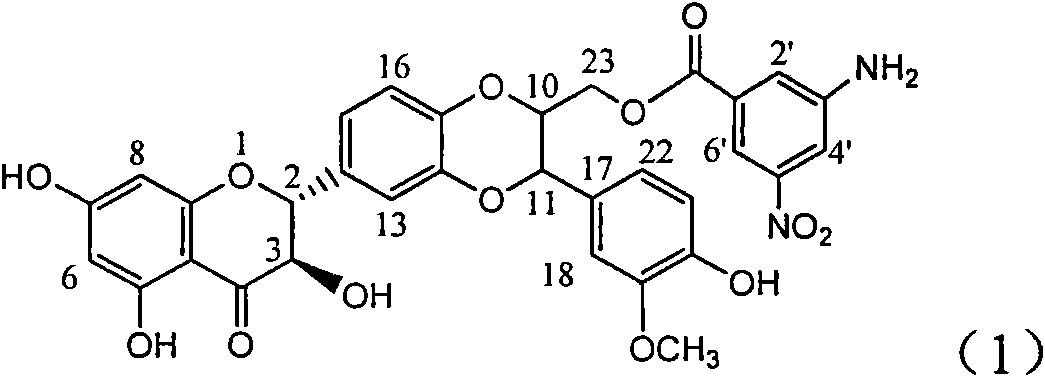
Flavonolignans are natural phenols composed of a part flavonoid and a part lignan.
Application of diamine formyl dehydrogenated silybin serving as medicament for curing viral hepatitis B
InactiveCN101829090AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
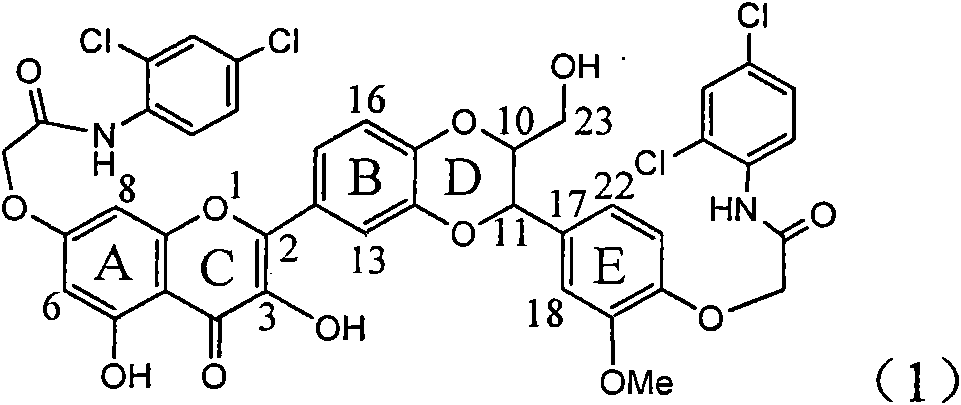
The invention relates to application of diamine formyl dehydrogenated silybin serving as a medicament for curing viral hepatitis B, in particular to application of a flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents or pharmaceutically acceptable salts thereof in preparation of a medicament for clearing HBsAg and HBeAg and a medicament for inhibiting HBV DNA replication. The flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents has extremely high HBsAg and HBeAg inhibiting activities; when the flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents is at a concentration of 20 mu g / ml, the inhibition rates of the HBsAg and the HBeAg are respectively 94.4 percent and 95.7 percent which exceed 5.9 times and 5.7 times those of a positive control alpha-interferon; and simultaneously the inhibition rate of the HBV DNA is 99.7 percent when the flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents is at the same concentration, and the inhibition activity of the flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents is higher than that of lamivudine and the alpha-interferon. In summary, the flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents or the pharmaceutically acceptable salts thereof can be expected for preparing non-nucleoside medicaments for clearing the HBsAg and the HBeAg, inhibiting the HBV DNA replication, and curing the hepatitis B virus infection diseases.
Owner:DALI UNIV
Application of aromatic carbamoyl dehydro-silibinin as medicament for treating viral hepatitis B
InactiveCN101829086AConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemDiseasePositive control
The invention relates to application of aromatic carbamoyl dehydro-silibinin as a medicament for treating viral hepatitis B, in particular to application of todehydro-silibinin flavonolignans with a ring A and a ring E which are substituted by double base aromatic carbamoyl methoxyl and pharmaceutically acceptable salt thereof for preparing medicaments for removing HBsAg and HBeAg and medicaments for inhibiting HBV DNA. The todehydro-silibinin flavonolignans has extremely obvious activity on inhibiting the HBsAG and the HBeAg, has the intensity of 46.2 percent and 68.9 percent for respectively removing the HBsAG and the HBeAg in the presence of the concentration of 100 microgram / milliliter, which is 2.9 times and 4.1 times higher than that of positive control medicament alpha-interferon, and has the inhibition ratio of 96 percent on HBV DNA in the presence of the concentration of 100 microgram / milliliter, which is higher than that of lamivudine and the alpha-interferon. Accordingly, the flavonolignans and the pharmaceutically acceptable salt thereof can be expected to be used for preparing non-nucleoside medicaments applied for removing HBsAg and HBeAg, inhibiting HBV DNA replication and treating hepatitis B virus infection diseases.
Owner:DALI UNIV
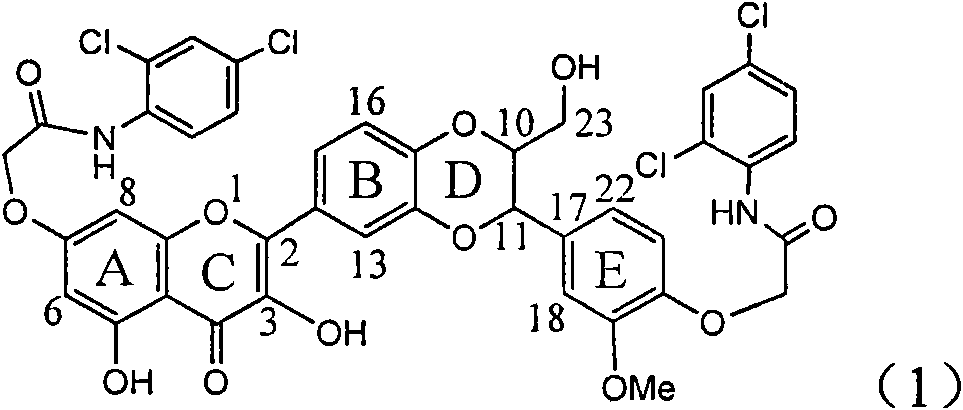
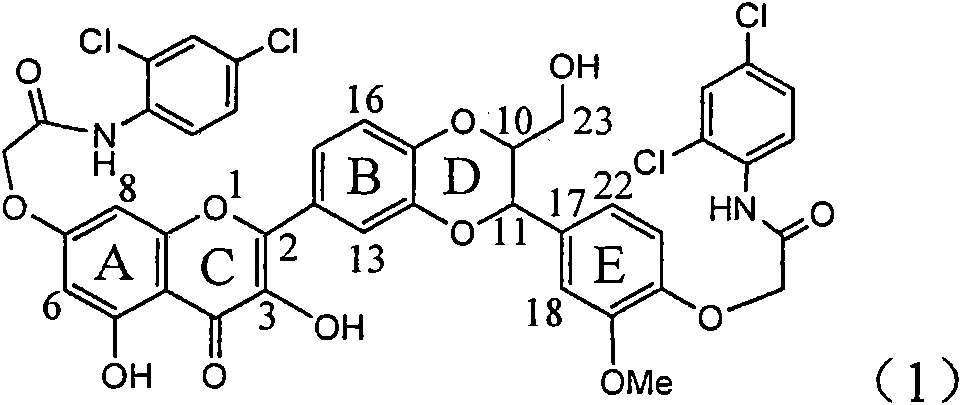
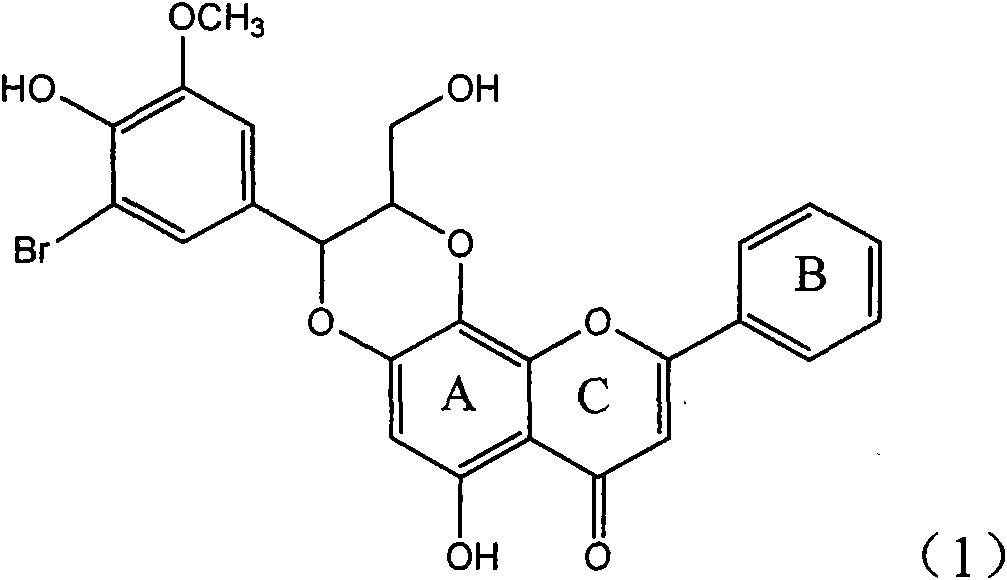
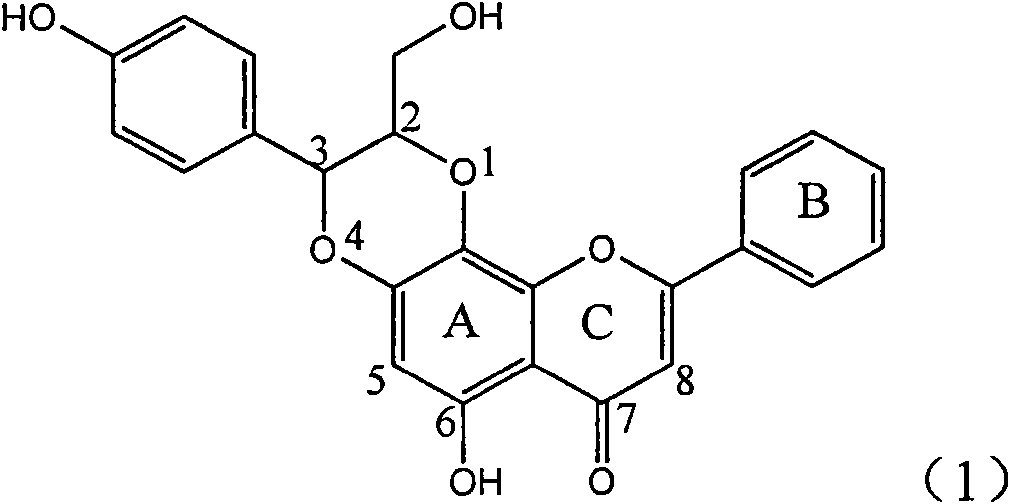
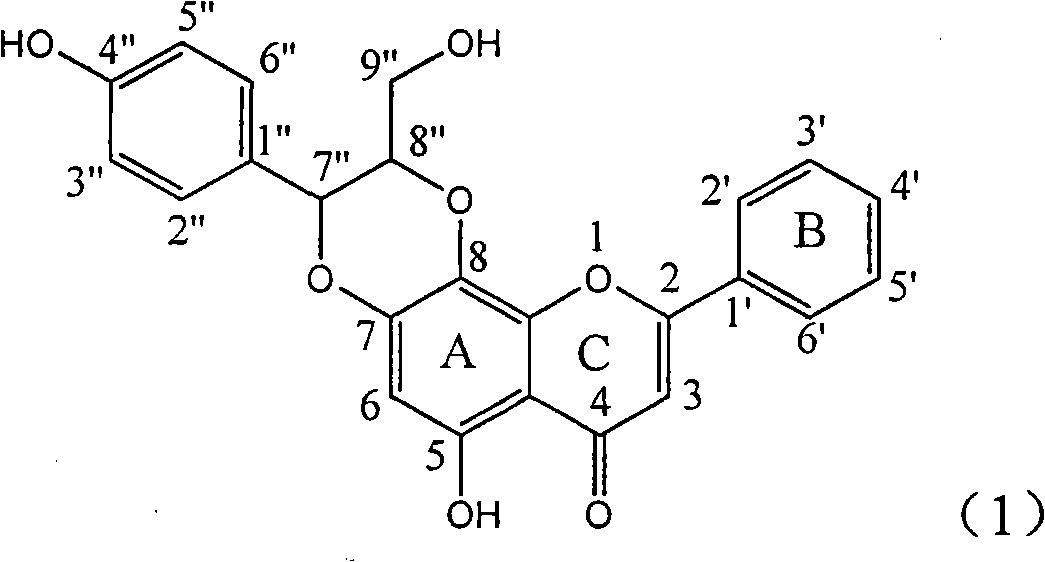
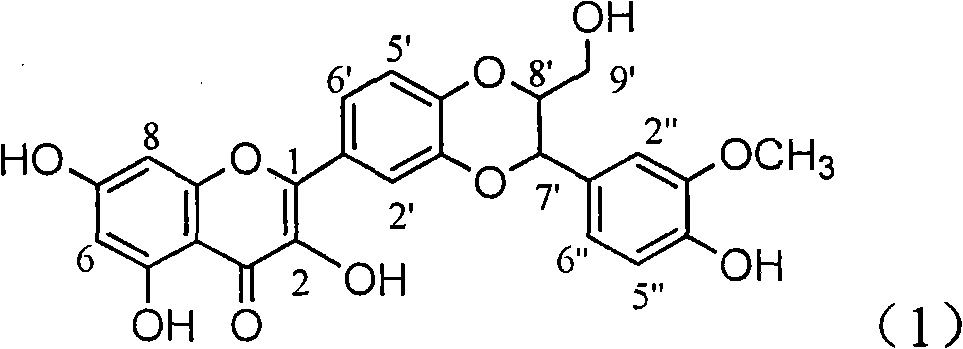
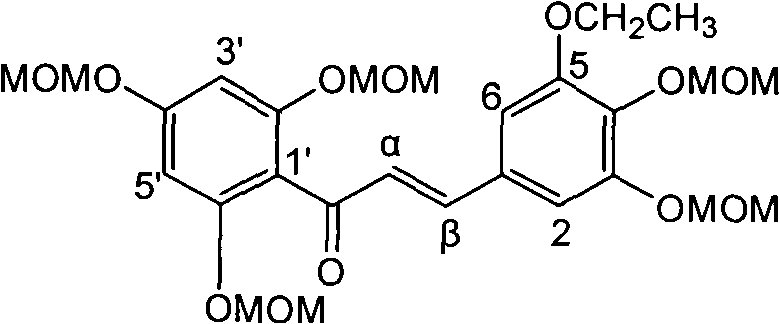
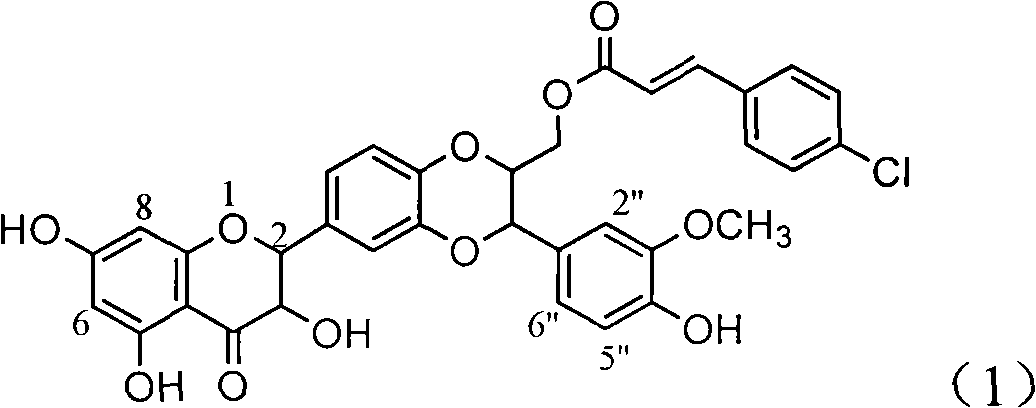
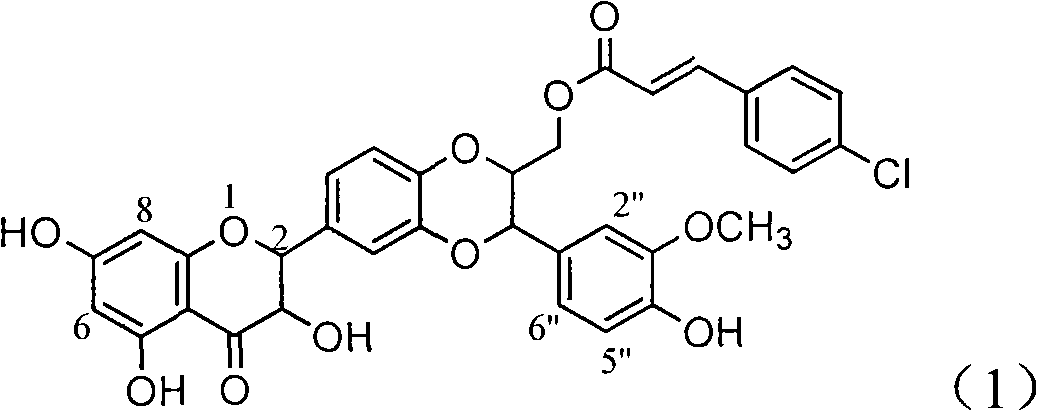
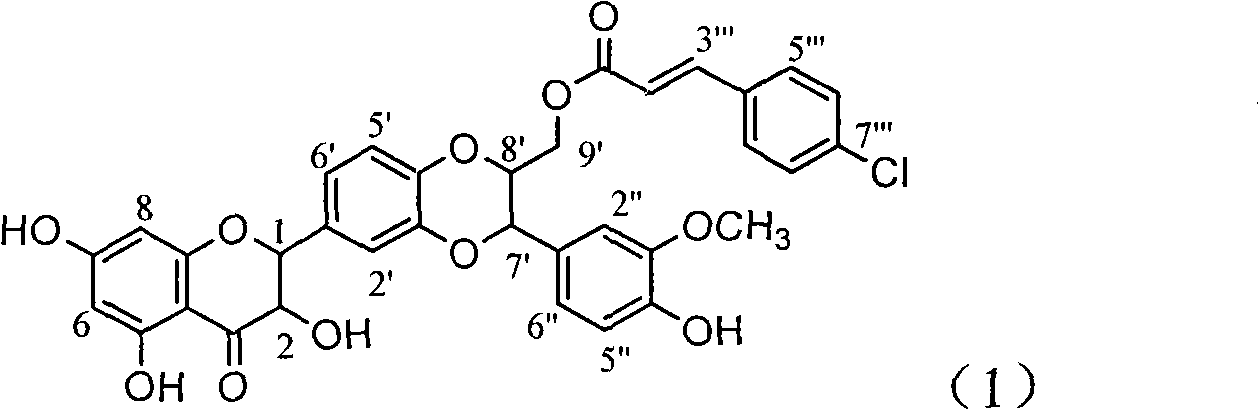
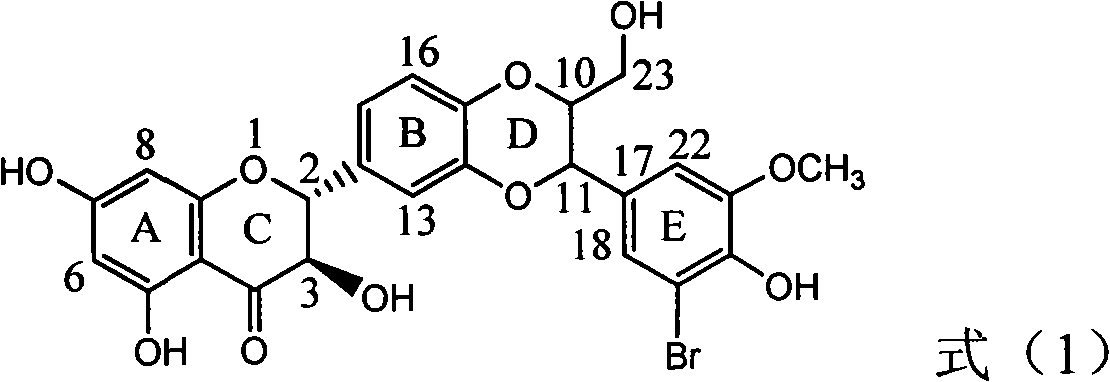
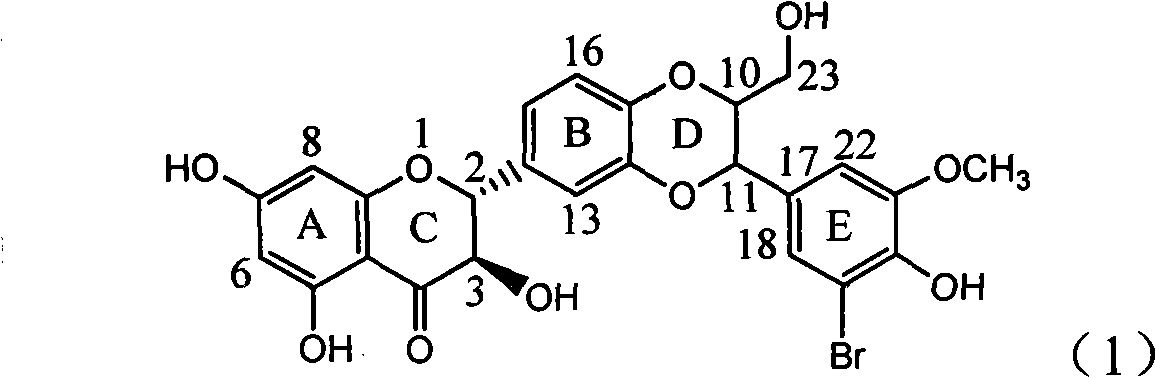
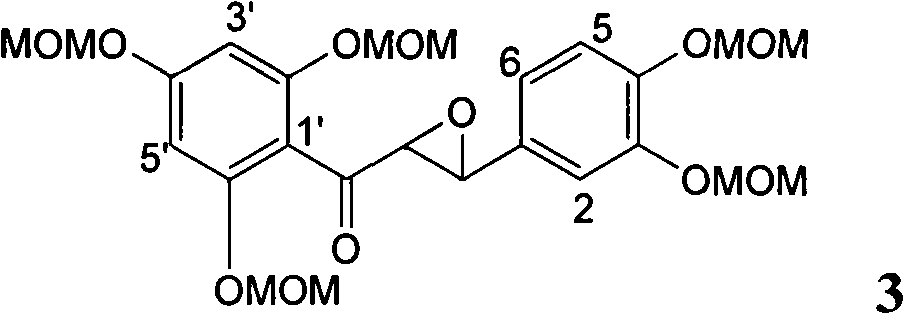
Application of ring A coupling flavonolignan in preparing medicaments for treating viral hepatitis B
InactiveCN101829104AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
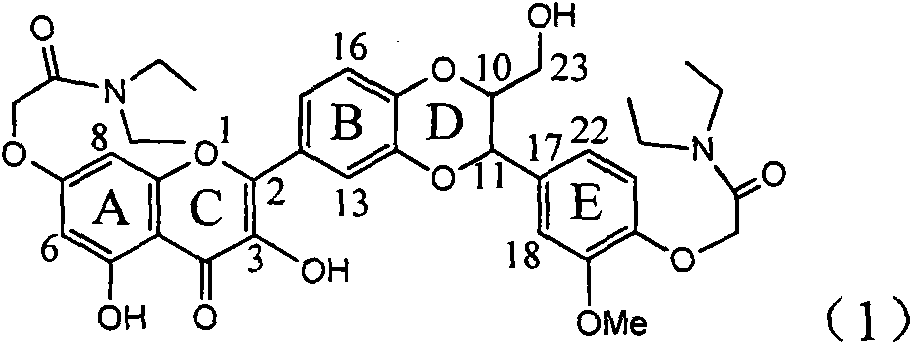
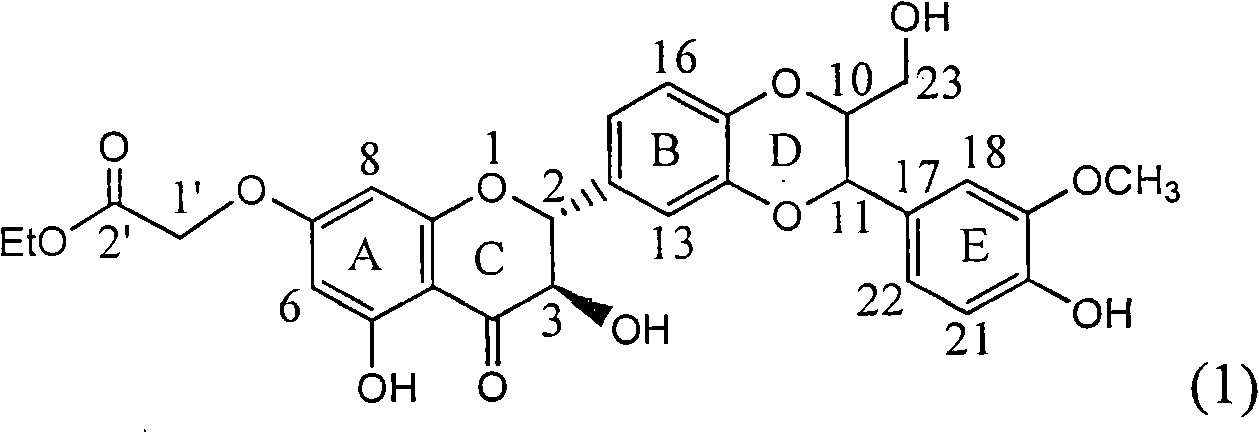
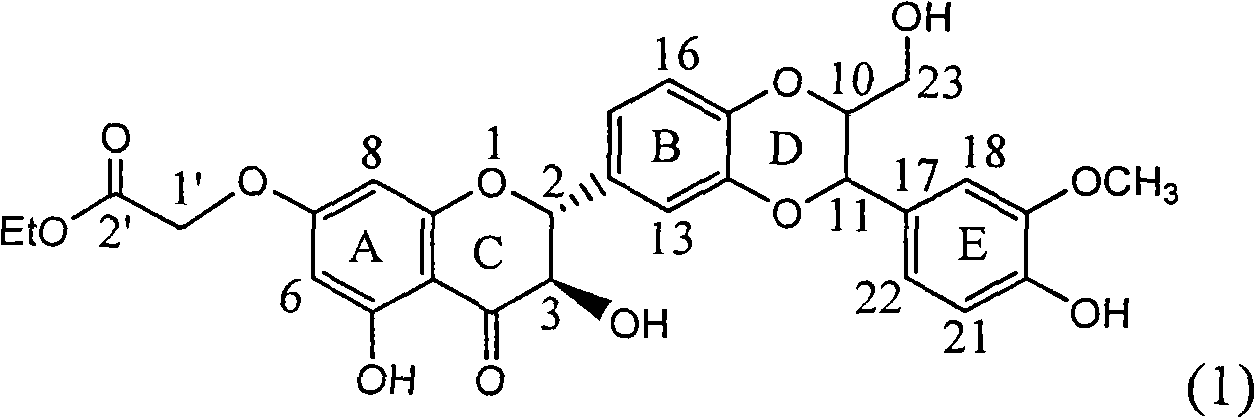
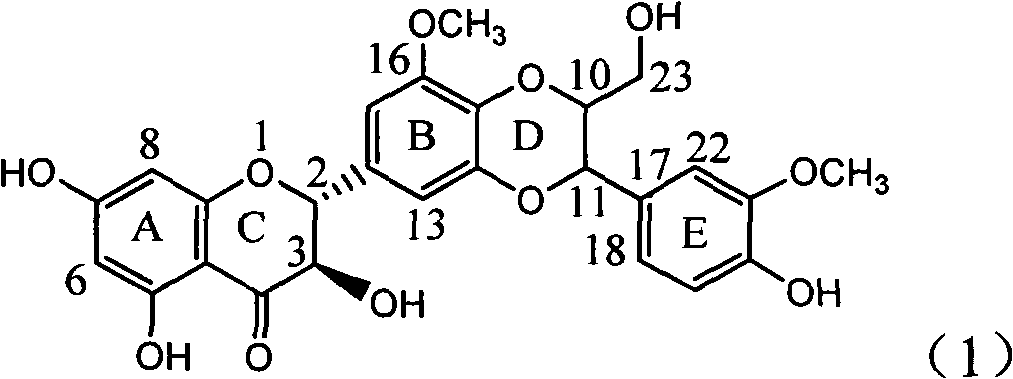
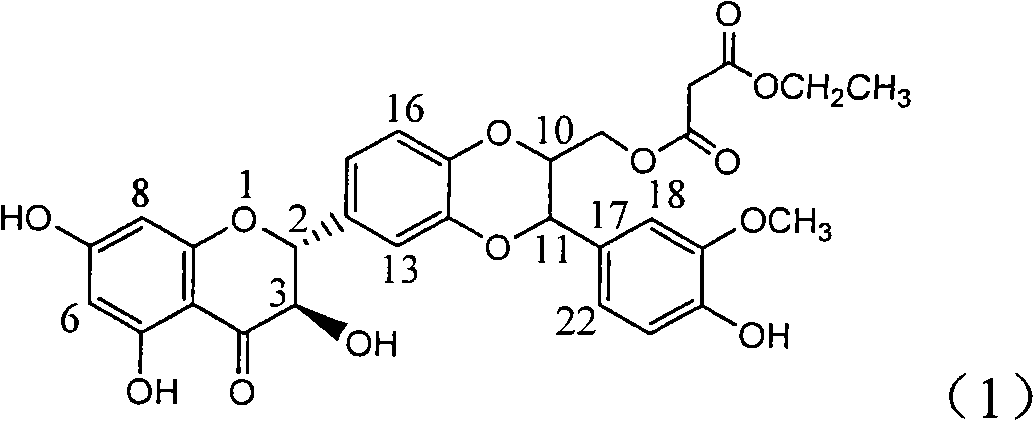
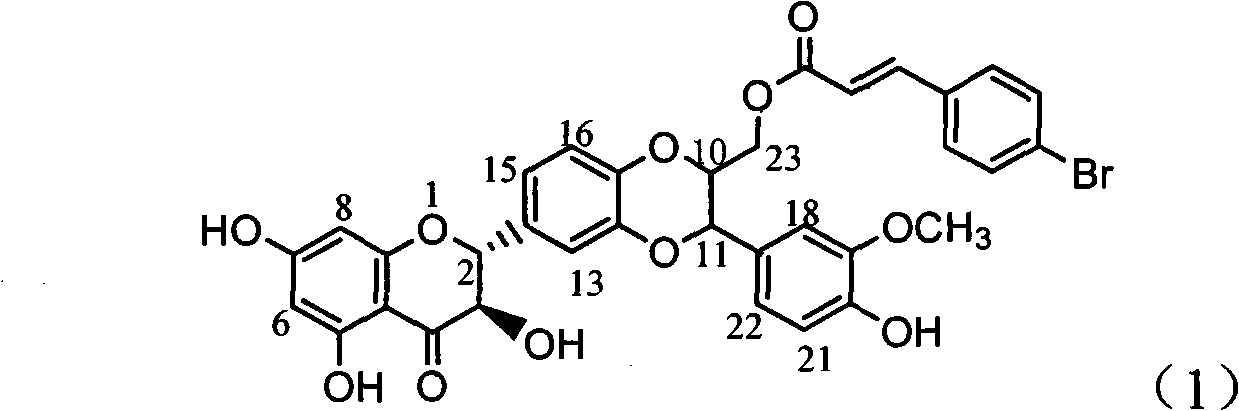
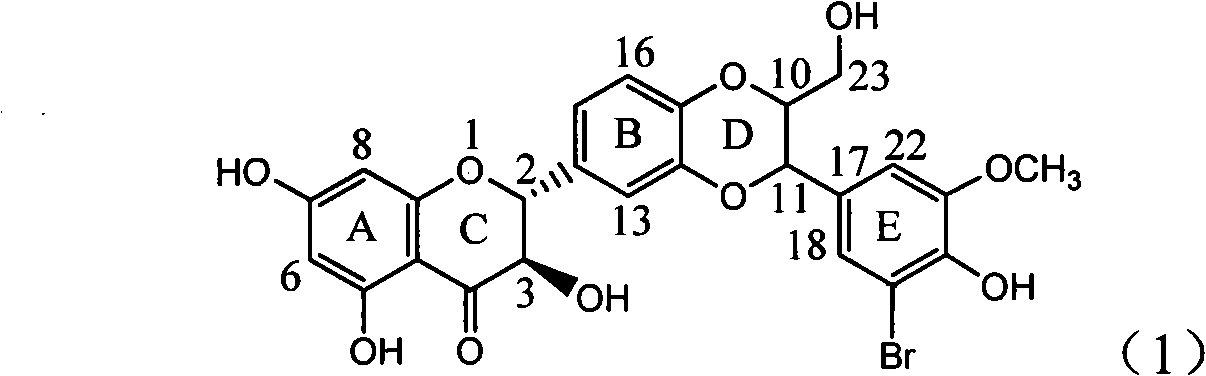
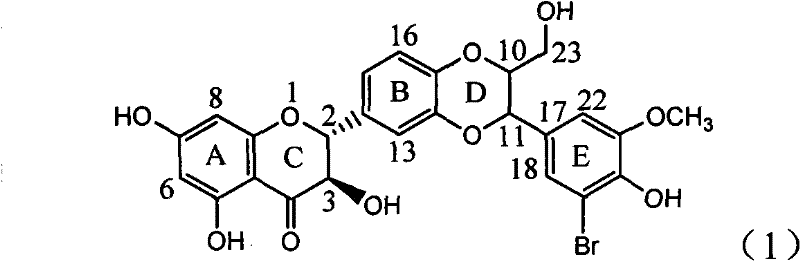
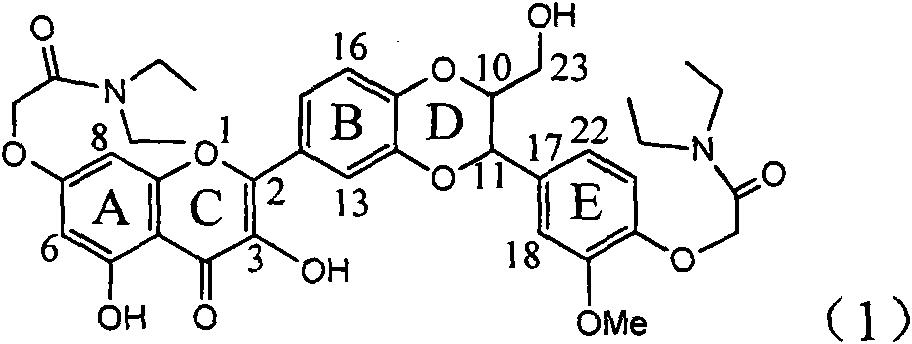
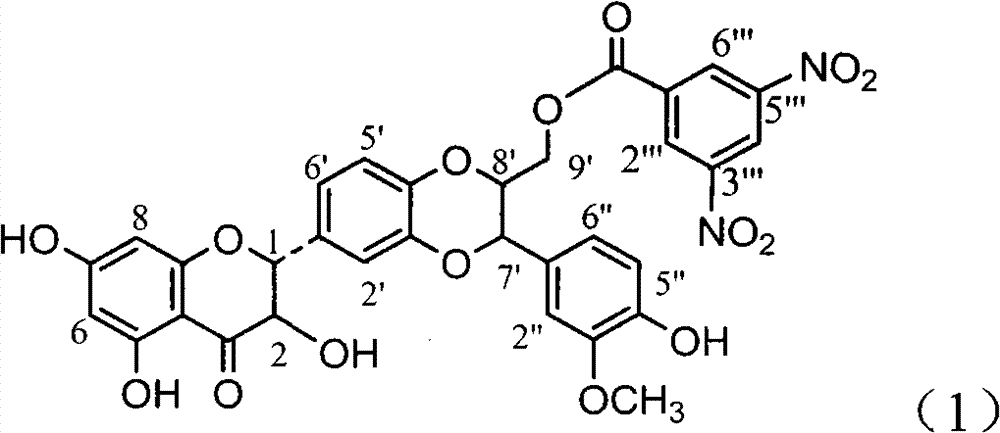
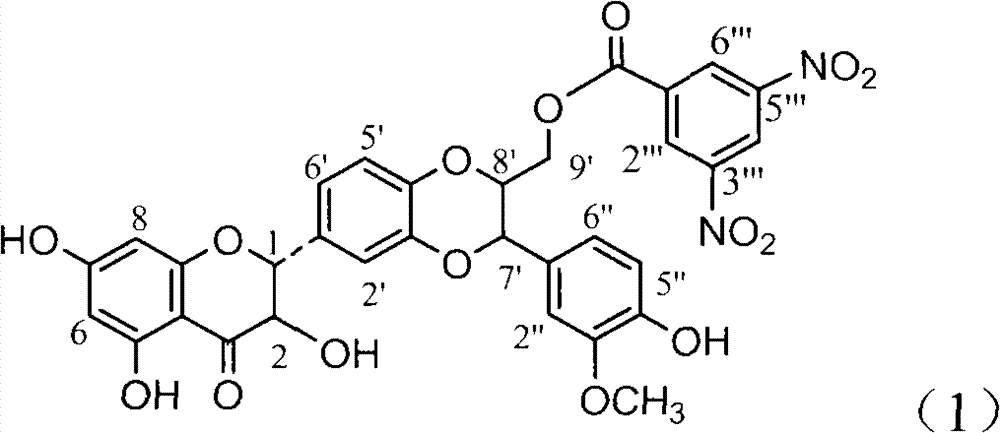
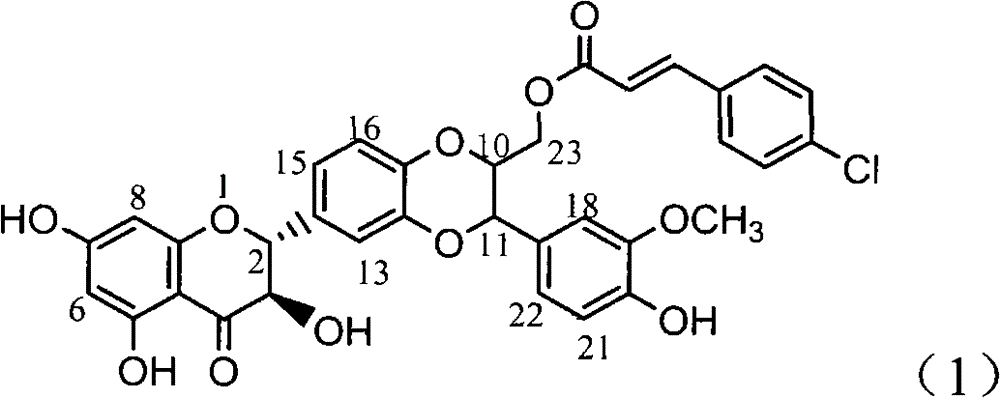
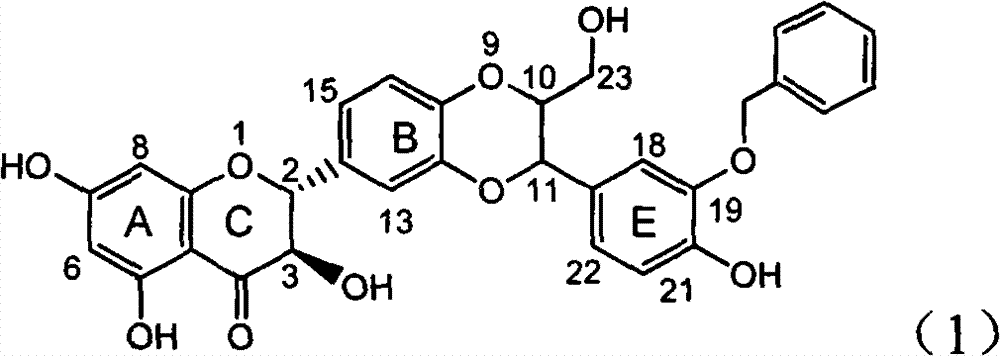
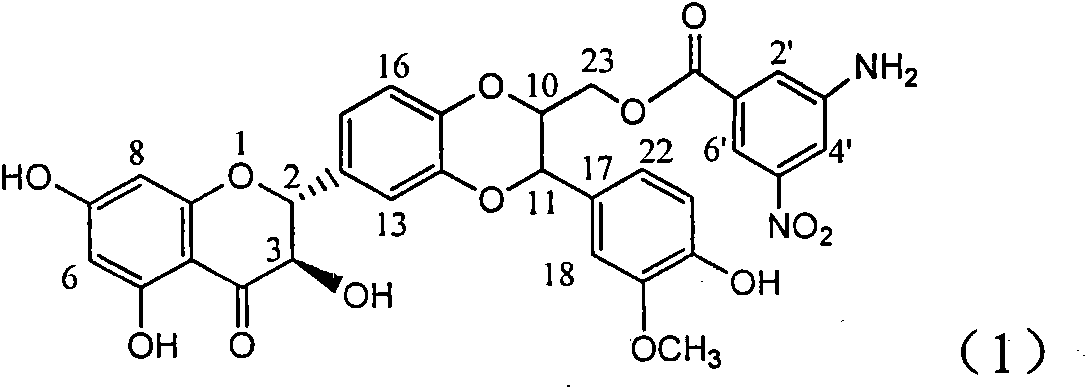
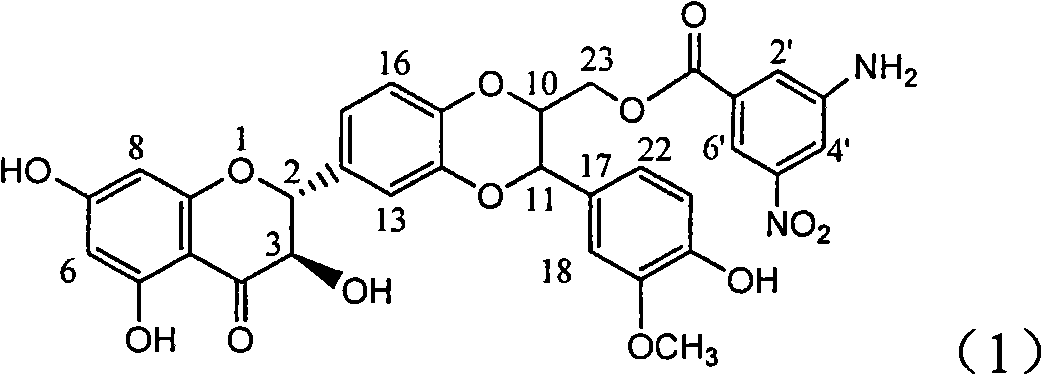
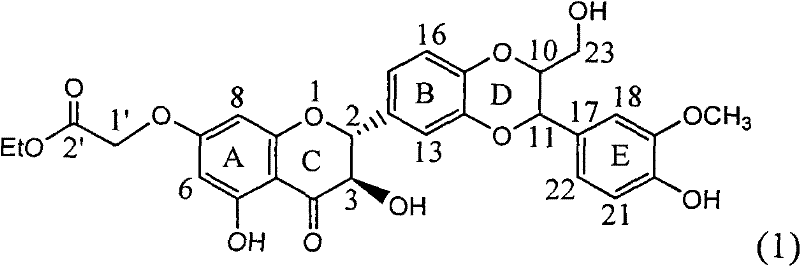
The invention relates to application of ring A coupling flavonolignan in preparing medicaments for treating viral hepatitis B, in particular to application of a compound of the formula (1) or a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away HBsAg (Hepatitis B Surface Antigen) and HBeAg (Hepatitis B e Antigen) and suppressing the HBV (Hepatitis B Virus) DNA replication. The intensities of the flavonolignan for clearing away the HBsAg and the HBeAg are respectively 29.4 percent and 29.1 percent in the presence of a concentration of 20 micrograms / milliliter, which is respectively 1.8 times and 1.7 times of the corresponding activity of a positive control medicament (10,000 units / milliliter of alpha-interferon). What is even more exciting is that in the presence of the concentration, the suppression rate of the flavonolignan to the HBV DNA is higher than 83 percent, which is higher than that of Lamivudine which is a positive control and is 2.2 times of that of the alpha-interferon to the HBV DNA. Accordingly, the flavonolignan and the pharmaceutically acceptable salt thereof are indicated to be capable of being expected to be used for preparing non-nucleoside medicaments for clearing away the HBsAg and the HBeAg, suppressing the HBV DNA replication and treating HBV infection diseases.
Owner:DALI UNIV
Application of ring B ethyoxyl silybin in preparing medicaments for treating viral hepatitis B
InactiveCN101829089AConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemDiseasePositive control
The invention relates to application of ring B ethyoxyl silybin in preparing medicaments for treating viral hepatitis B, in particular to application of ring B ethyoxyl substituted silybin ester or a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away HBsAG (Hepatitis B Surface Antigen) and HBeAg (Hepatitis B e Antigen) and suppressing the HBV (Hepatitis B Virus) DNA replication. The compound has strong activity on suppressing the HBsAG and the HBeAg, and in the presence of a concentration of 20 micrograms / milliliter, the intensities for clearing the HBsAg and the HBeAg are respectively 64.6 percent and 44.8 percent which are 4.0 times and 2.7 times of that of alpha-interferon which is a positive control medicament. In the presence of the concentration, the suppression rate of the compound on the HBV DNA is 58.1 percent which is 1.5 times of the corresponding activity of the alpha-interferon. Accordingly, the flavonolignan or the pharmaceutically acceptable salt thereof are indicated to simultaneously have strong efficacy on suppressing the HBsAg, the HBeAg and the HBV DNA and can be expected to be used for preparing non-nucleoside medicaments for treating HBV infection diseases.
Owner:DALI UNIV
Application of ring A dioxane flavonolignan in preparing medicaments for resisting hepatitis B viruses (HBV)
InactiveCN101829085AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
The invention relates to application of ring A dioxane flavonolignan in preparing medicaments for resisting hepatitis B viruses (HBV), in particular to application of ring A dioxane coupling type flavone lignan or a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away hepatitis B e antigen (HBeAg), suppressing the HBV DNA replication and treating HBV infection diseases. The flavonolignan has certain activity on resisting the HBeAg, and the intensity of the flavonolignan for clearing away the HBeAg is higher than that of Lamivudine which is a positive control and close to that of 10,000 units / milliliter of alpha-interferon. Meanwhile, the suppression ratio of the compound to the HBV DNA replication is higher than 80 percent in the presence of a concentration of 100 micrograms / milliliter. The pharmacodynamical results indicate that the flavonolignan or the pharmaceutically acceptable salt thereof can be expected to be used for preparing the medicaments for clearing away the HBeAg, suppressing the HBV DNA replication and treating the HBV infection diseases.
Owner:DALI UNIV
Application of ring A substituted silybin ester in preparing medicaments for treating viral hepatitis B
InactiveCN101829101AConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemDiseasePositive control
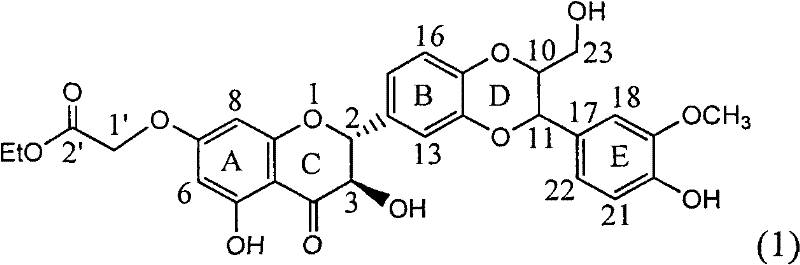
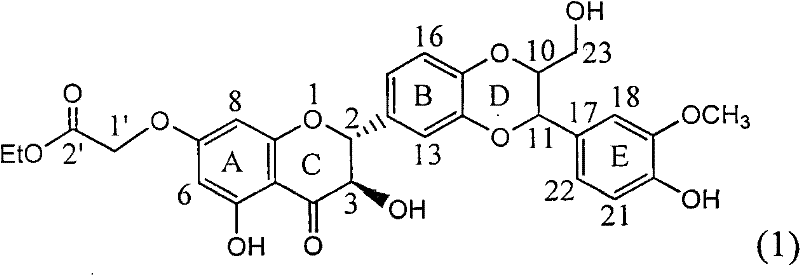
The invention relates to application of ring A substituted silybin ester in preparing medicaments for treating viral hepatitis B, in particular to application of silybin ester flavonolignan substituted by ethoxycarbonyl methyl on the ring A or a pharmaceutically acceptable salt thereof for preparing medicaments for reducing the hepatitis B virus surface antigen (HBsAg), suppressing the HBV (Hepatitis B Virus) DNA replication and treating HBV infection diseases. The flavonolignan has quite obvious activity on suppressing the HBsAg, and in the presence of a concentration of 100 micrograms / milliliter, the intensity of the flavonolignan for clearing away the HBsAG exceeds that of alpha-interferon which is a positive control medicament by 3.3 times. Meanwhile, in the presence of a concentration of 20 micrograms / milliliter, suppression ratio of the compound to the HBV DNA is close to 60 percent. The pharmacodynamical results indicate that the flavonolignan or the pharmaceutically acceptable salt thereof can be expected to be used for preparing the medicaments for treating the HBV infection diseases.
Owner:DALI UNIV
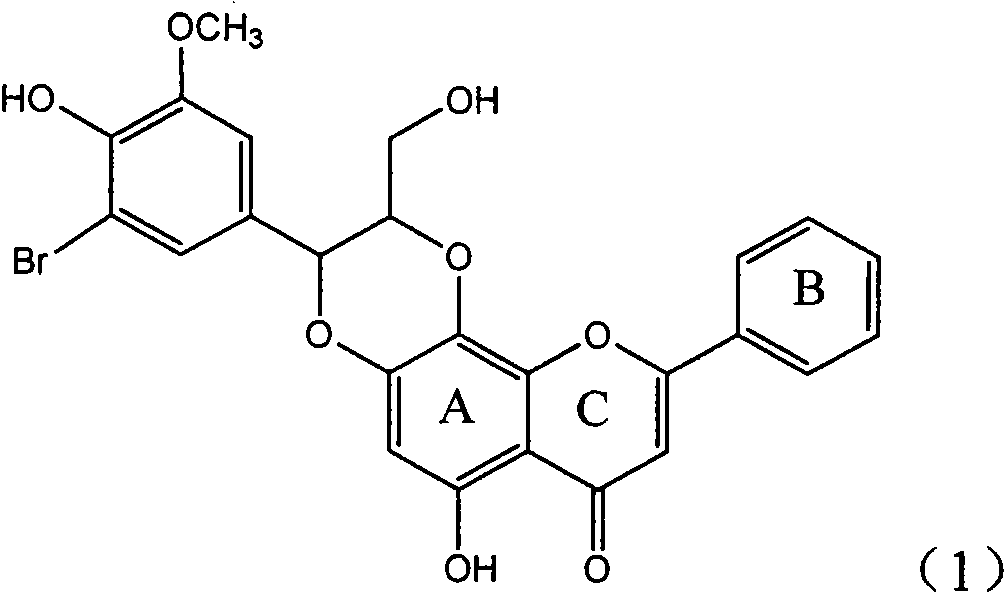
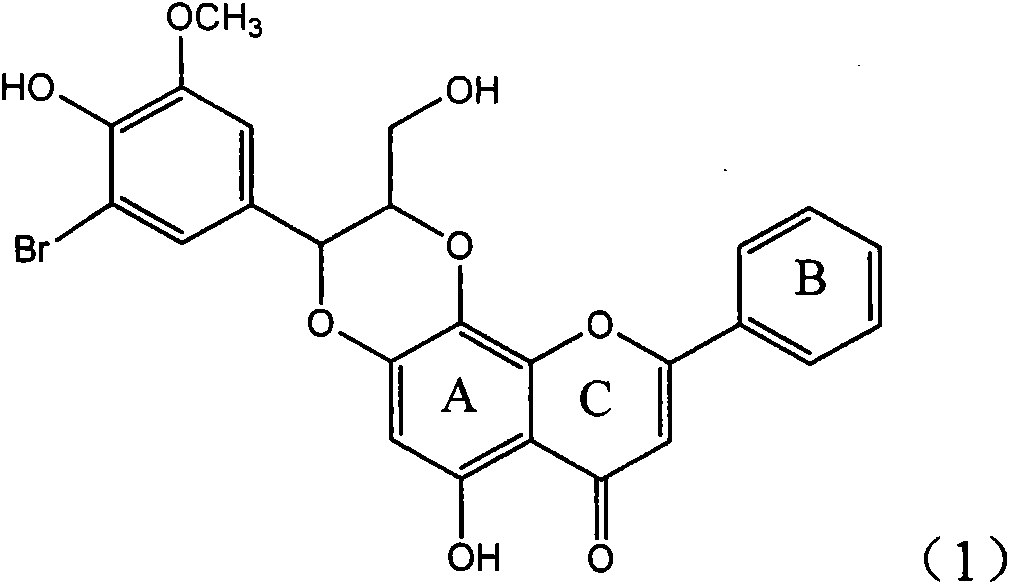
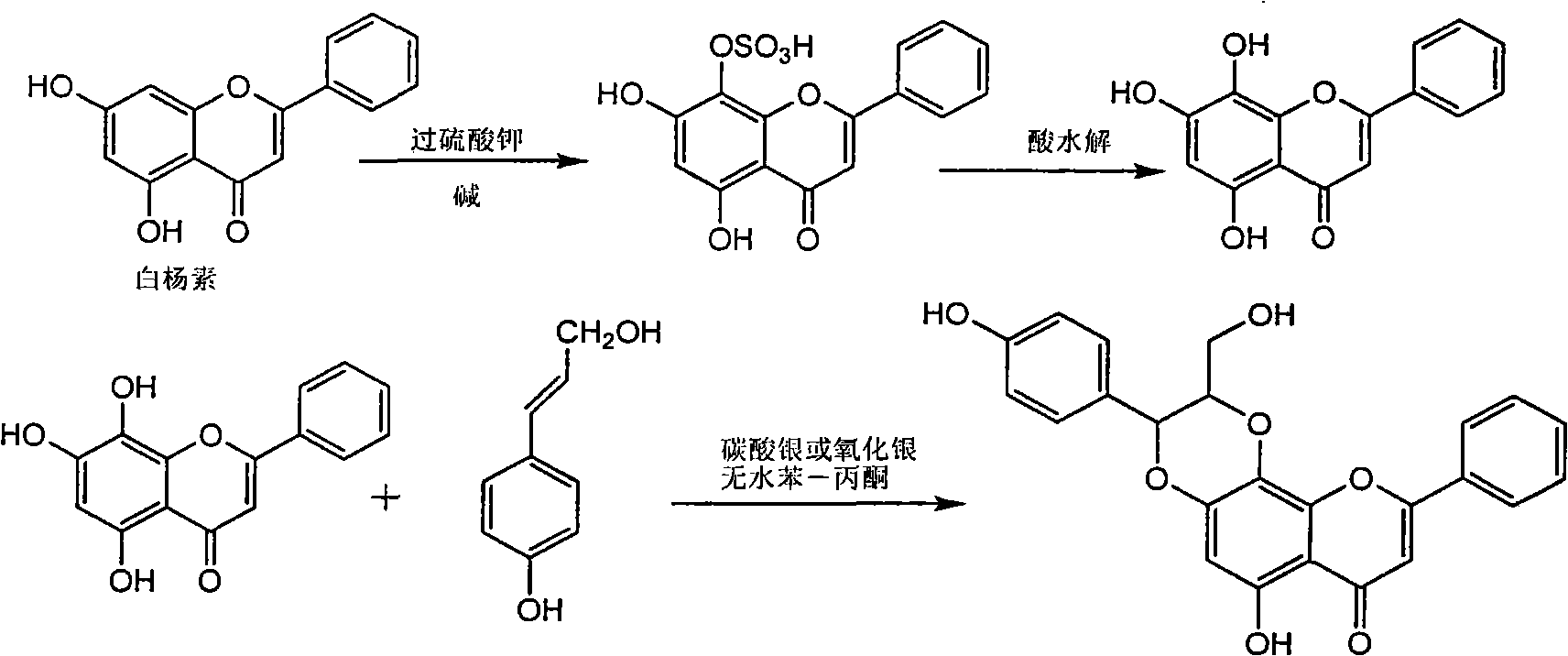
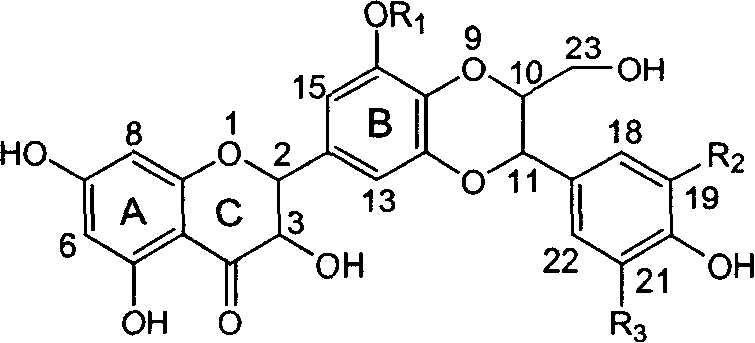
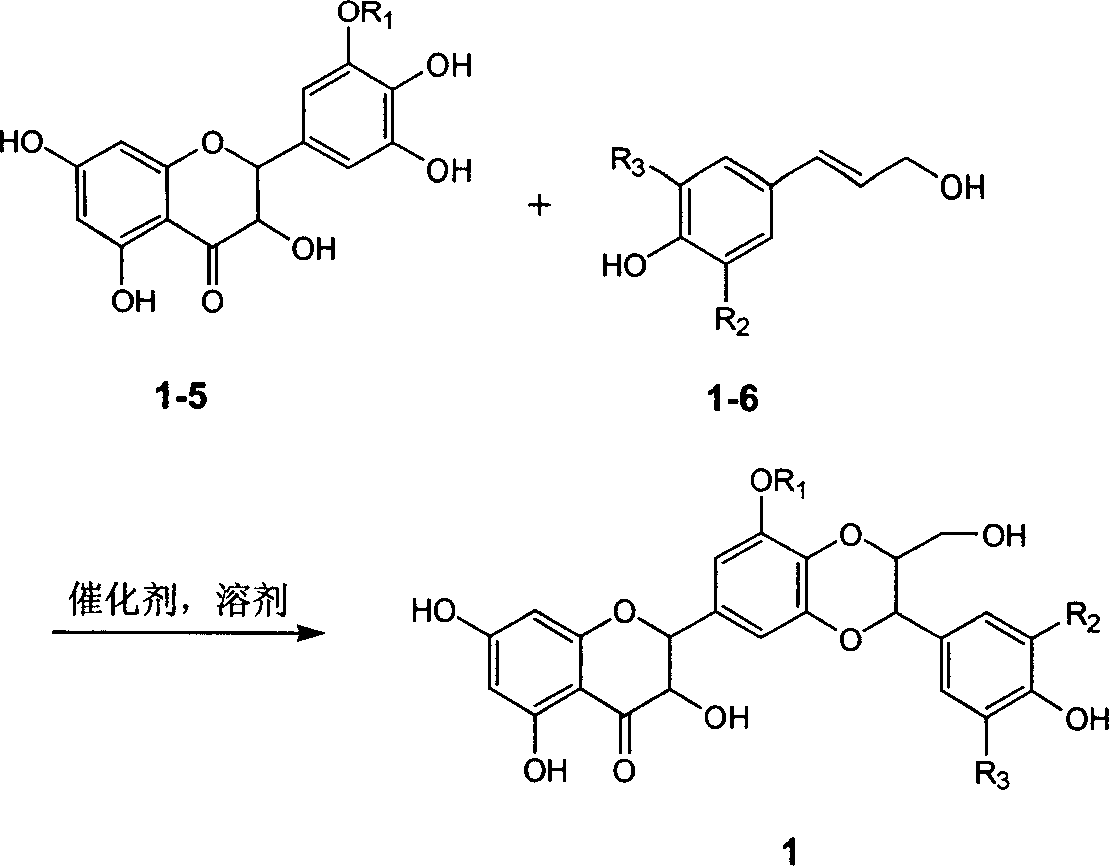
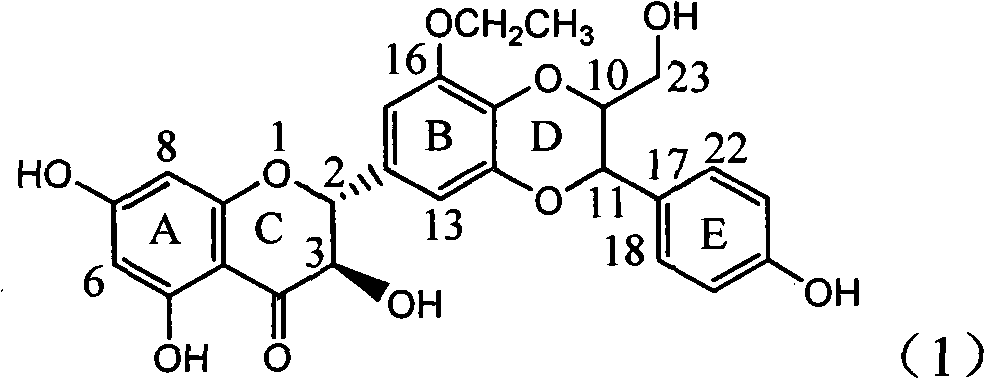
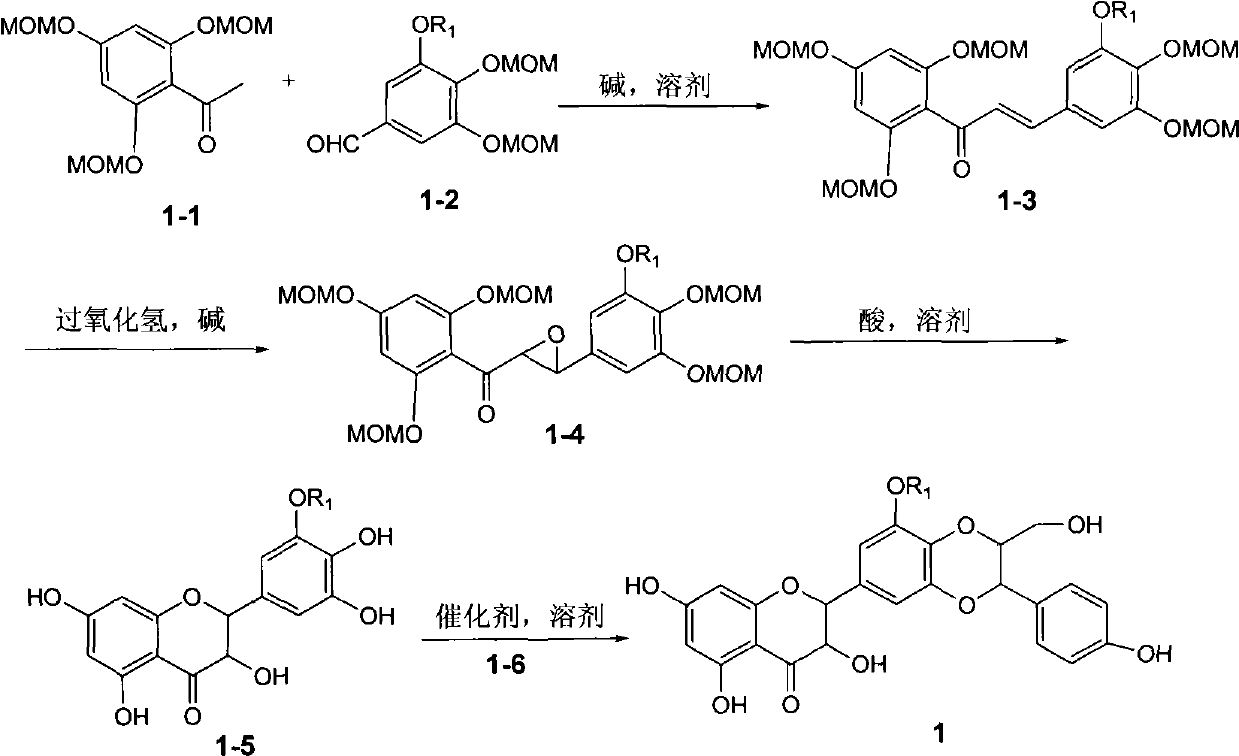
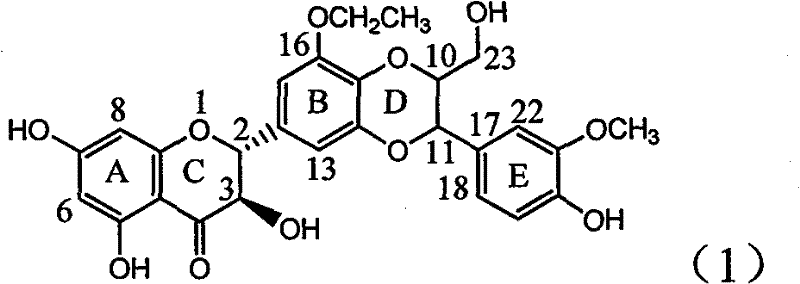
Silybin flavonolignan and their production method and use
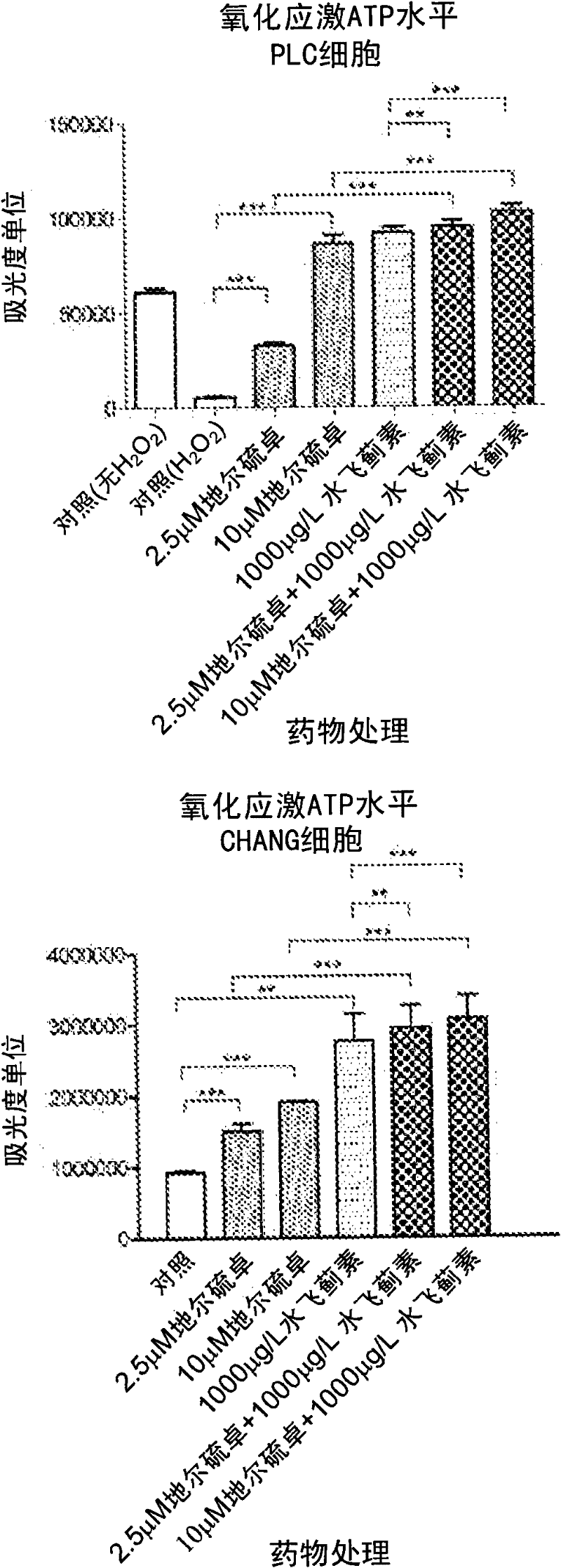
The invention relates to a silibinin flavonolignan and its medicine salt or solvates. The invention also relates to the method preparing its important mediate and its drug combination and medical application. The compound can protect primary hepatocyte from oxidative damage for SD newly born rat hepatitis virus, so it is expected to be used as drug preventing liver damage. The compound can remove superoxide anion free radical and diphenyl picryl phenylhydrazine free radical, inhibit free radical from inducing generation of fat oxidatant, protect PC 12 cell from being damaged by free radical, so it is expected to be used as drug treating diseases caused by free radical.
Owner:ZHEJIANG HISUN PHARMA CO LTD
Use of flavonoids to increase blood serum levels of dehydroepiandrosterone
InactiveUS20070160688A1Reduce undesired DHEA side effectIncreased blood serum levelBiocideCarbohydrate active ingredientsXanthonoidIsoflavonoid
A method is disclosed for increasing blood serum levels of dehydroepiandrosterone by administering the combination of one of a flavonoid, isoflavonoid, coumarin and a flavonolignan; dehydroepiandrosterone; and a plant extract.
Owner:WELLNESS
Application of dehydrogenation silybin in preparation of medicament for treating virus hepatitis B
InactiveCN101829097AConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemDiseasePositive control
The invention relates to application of dehydrogenation silybin in preparation of a medicament for treating virus hepatitis B, in particular to application of the dehydrogenation silybin or pharmaceutically acceptable salt thereof in preparation of the medicament for inhibiting HBV DNA replication. The medicament has obvious high hepatitis B virus desoxyribonucleic acid (HBV DNA) inhibiting activities and shows over 55 percent of inhibition rate for the HBV DAN at the concentration of 20 mg / ml, which is 1.5 times higher than that of a positive control medicament (10,000 unit / ml of alpha-interferon). The pharmacodynamics result shows the flavonolignan or the pharmaceutically acceptable salt thereof can be expected for preparing the non-nucleoside medicament for inhibiting HBV DNA replication and curing diseases caused by infection of hepatitis B virus.
Owner:DALI UNIV
Application of 4-cinnamoyl chloride substituted silybin to preparing glycosidase inhibitors
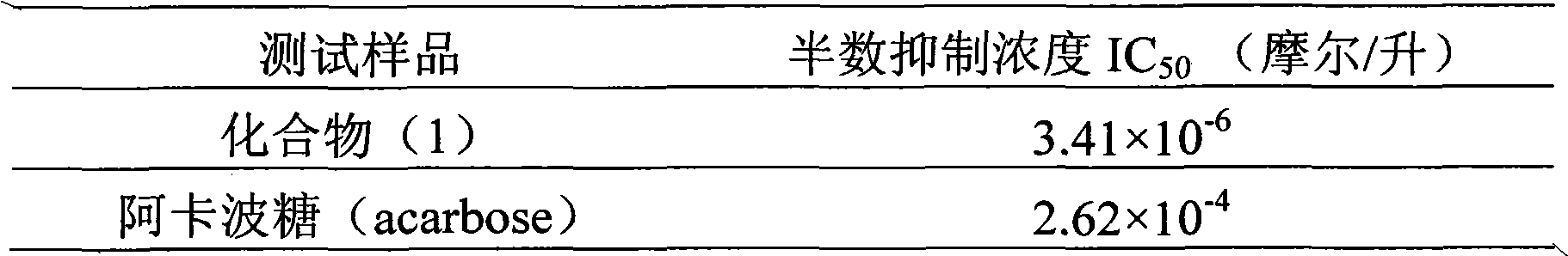
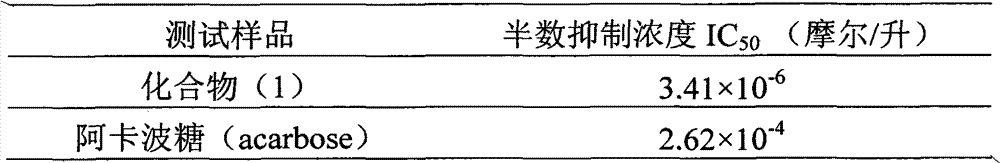
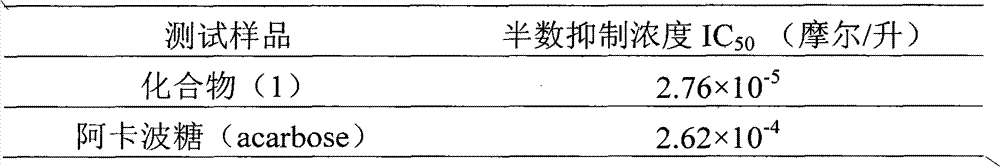
InactiveCN102000057AConvenient sourceSimple manufacturing methodOrganic active ingredientsMetabolism disorderPositive controlCinnamoyl chloride
The invention relates to application of 4-cinnamoyl chloride substituted silybin to preparing glycosidase inhibitors and in particular discloses application of 4-cinnamoyl chloride substituted silybin ester flavonolignans or pharmaceutical salt thereof to preparing drugs for inhibiting alpha-glycosidase and preventing and treating type II diabetes. The flavonolignans has extremely obvious activity for inhibiting alpha-glycosidase and the intensity of activity of the 40mcg / ml flavonolignans for inhibiting alpha-glycosidase reaches 92.1%. The measured half-inhibitory concentration of the flavonolignans shows that the intensity of activity of the flavonolignans for inhibiting alpha-glycosidase is 30 times that of the positive control drug acarbose. The pharmacodynamics result shows that the flavonolignans or pharmaceutical salt thereof can be expected to be applied to preparing glycosidase inhibitors, especially the drugs for preventing and treating type II diabetes.
Owner:DALI UNIV
Application of B ring methoxy substituted silybin to preparing glycosidase inhibitors
InactiveCN102000056AConvenient sourceSimple manufacturing methodOrganic active ingredientsMetabolism disorderAlglucerasePositive control
The invention relates to application of B ring methoxy substituted silybin to preparing glycosidase inhibitors and in particular discloses application of B ring methoxy substituted silybin ester-type flavonolignans or pharmaceutical salt thereof to preparing drugs for inhibiting alpha-glycosidase and preventing and treating type II diabetes. The flavonolignans has extremely obvious activity for inhibiting alpha-glycosidase and the intensity of activity of the 40mcg / ml flavonolignans for inhibiting alpha-glycosidase reaches 75.6%. The measured half-inhibitory concentration of the flavonolignans shows that the intensity of activity of the flavonolignans for inhibiting alpha-glycosidase is 8 times that of the positive control drug acarbose. The pharmacodynamics result shows that the flavonolignans or pharmaceutical salt thereof can be expected to be applied to preparing glycosidase inhibitors, especially the drugs for preventing and treating type II diabetes.
Owner:DALI UNIV
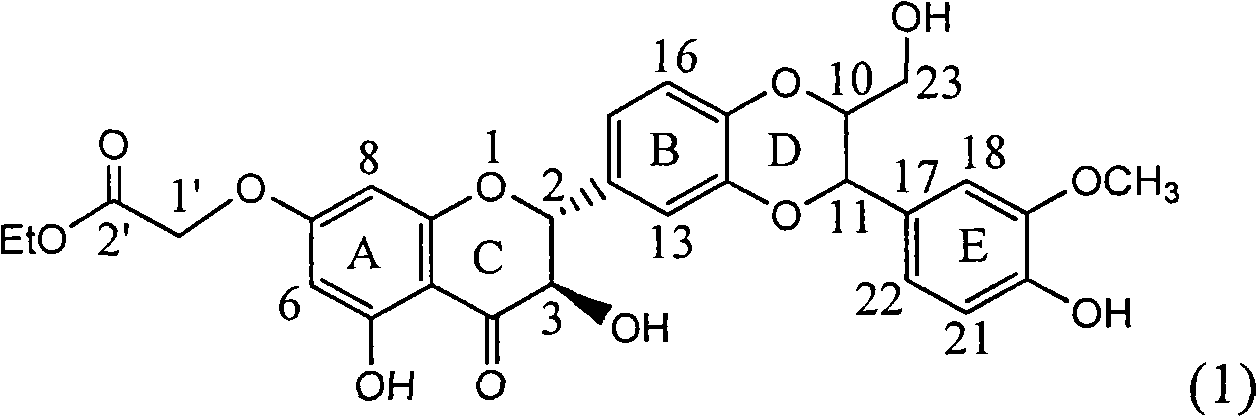
Application of substituted silybin ester in preparing medicament for treating virus hepatitis B
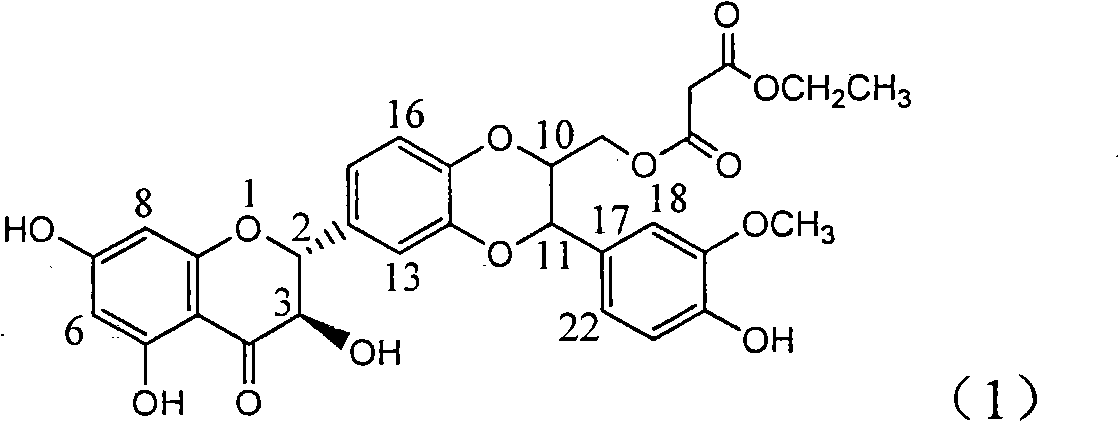
InactiveCN101829083AInhibition of replicationConvenient sourceOrganic active ingredientsAntiviralsDiseasePositive control
The invention relates to application of substituted silybin ester in preparing a medicament for treating virus hepatitis B, in particular to application of 23-position ethyl-malonyl substituted silybin ester or medicinal salt thereof in preparing a medicament for reducing hepatitis B virus surface antigen HBsAg, inhibiting HBV DNA replication and treating hepatitis B virus infected diseases. The flavonolignan has remarkable effect of inhibiting HBsAg activity, and the strength of the flavonolignan at the concentration of 100 micrograms per milliliter for clearing the HBsAg is two times and half that of a positive control medicament alpha-interferon; and meanwhile, the compound has strong inhibiting activity on the HBV DNA. Pharmacodynamical results show that the flavonolignan or the medicinal salt thereof can be expected to be used for preparing the medicament for reducing hepatitis B virus surface antigen, inhibiting HBV DNA replication and treating the hepatitis B virus infected diseases.
Owner:DALI UNIV
Application of B/E ring changed silybin to preparing glycosidase inhibitors
InactiveCN102000058AConvenient sourceSimple manufacturing methodOrganic active ingredientsMetabolism disorderAlglucerasePositive control
The invention relates to application of B / E ring changed silybin to preparing glycosidase inhibitors and in particular discloses application of B ring ethyoxyl or E ring de-methoxy substituted silybin ester-type flavonolignans or pharmaceutical salt thereof to preparing drugs for inhibiting alpha-glycosidase and preventing and treating type II diabetes. The flavonolignans has extremely obvious activity for inhibiting alpha-glycosidase and the intensity of activity of the 40mcg / ml flavonolignans for inhibiting alpha-glycosidase reaches 54.3%. The measured half-inhibitory concentration of the flavonolignans shows that the intensity of activity of the flavonolignans for inhibiting alpha-glycosidase is 2.5 times that of the positive control drug acarbose. The pharmacodynamics result shows that the flavonolignans or pharmaceutical salt thereof can be expected to be applied to preparing glycosidase inhibitors, especially the drugs for preventing and treating type II diabetes.
Owner:DALI UNIV
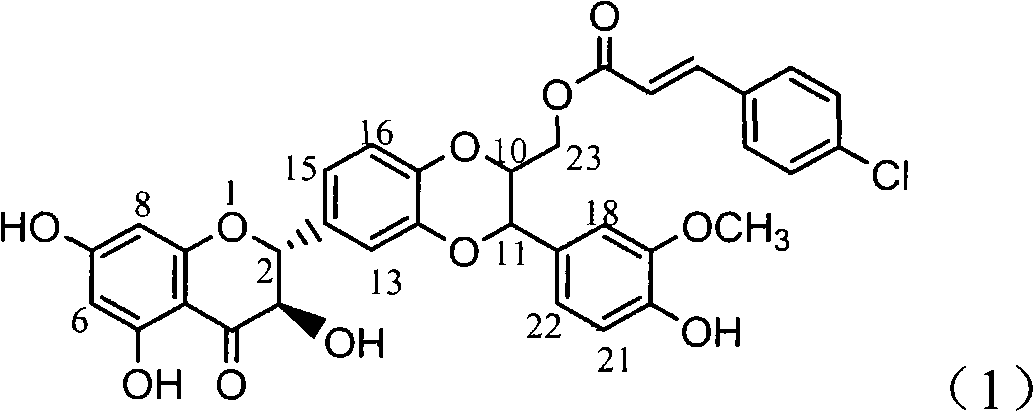
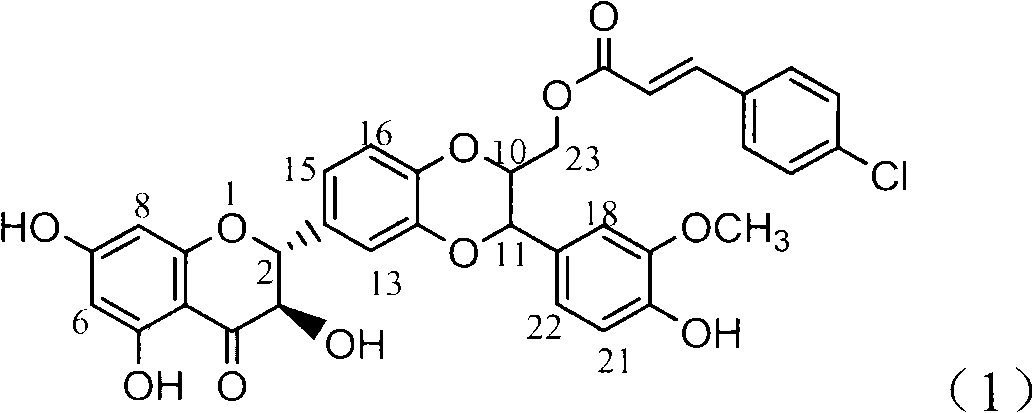
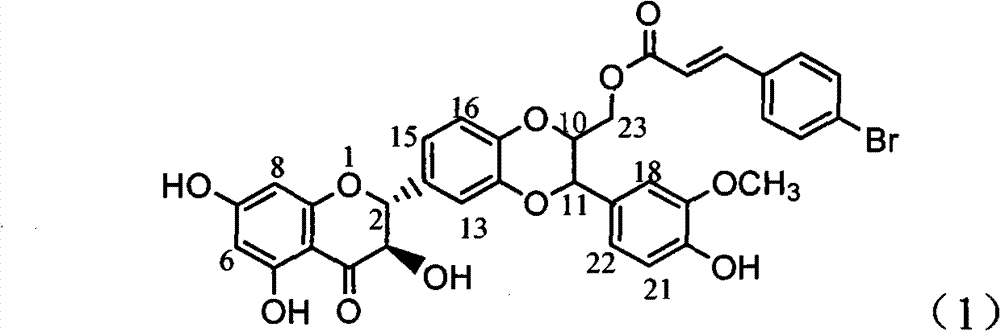
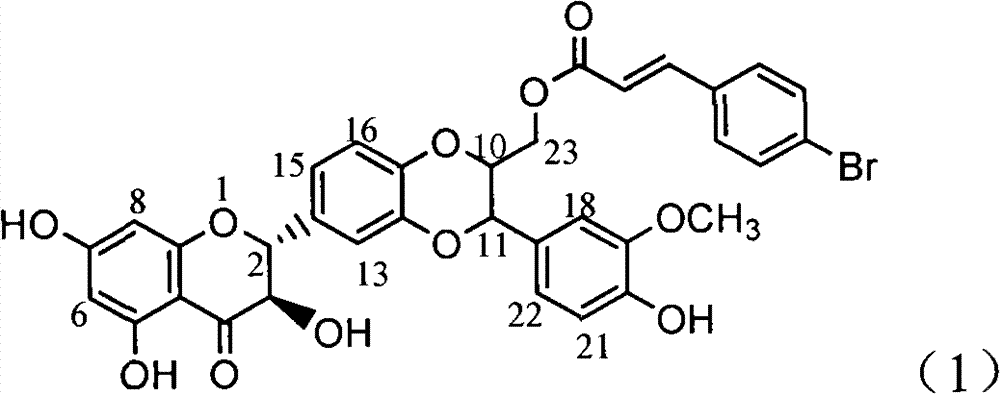
Application of 4-cinnamoyl chloride silybin in preparing medicaments for treating viral hepatitis B
InactiveCN101829099AInhibitory activityInhibition of replicationOrganic active ingredientsDigestive systemDiseaseHigh concentration
The invention relates to application of 4-cinnamoyl chloride silybin in preparing medicaments for treating viral hepatitis B, in particular to application of 4-cinnamoyl chloride substituted silybin ester type flavonolignan or a pharmaceutically acceptable salt thereof in preparing medicaments for suppressing HBV (Hepatitis B Virus) DNA replication and treating HBV infection diseases. The flavonolignan has definite activity on suppressing the HBV DNA, and the suppression activity on the replication of the HBV DNA under a high dose approaches that of alpha-interferon under a highest concentration of 10,000 units / milliliter, thus the flavonolignan belongs to effective non-nucleoside natural products for resisting the HBV. The pharmacodynamical results indicate that the flavonolignan or the pharmaceutically acceptable salt thereof can be expected to be used for preparing the medicaments for suppressing the HBV DNA replication and treating the HBV infection diseases.
Owner:DALI UNIV
Pharmaceutical application of p-bromocinnamoyl silybin to preparing glycosidase inhibitors
InactiveCN101966174AConvenient sourceSimple manufacturing methodOrganic active ingredientsMetabolism disorderAlglucerasePositive control
The invention relates to pharmaceutical application of p-bromocinnamoyl silybin to preparing glycosidase inhibitors and specifically discloses application of 23-p-bromocinnamoyl substituted silybin ester flavonolignans or medicinal salt thereof to preparing drugs for inhibiting alpha-glycosidase and preventing and treating type II diabetes. The flavonolignans has extremely obvious activity for inhibiting alpha-glycosidase and the intensity of activity of the flavonolignans for inhibiting alpha-glycosidase reaches 54.4% under the condition of 4mcg / ml concentration. The measured half-inhibitoryconcentration of the flavonolignans shows that the intensity of activity of the flavonolignans for inhibiting alpha-glycosidase is 76.8 times that of the positive control drug acarbose. The pharmacodynamics result shows that the flavonolignans or medicinal salt thereof can be expected to be applied to preparing glycosidase inhibitors, especially the drugs for preventing and treating type II diabetes.
Owner:DALI UNIV
Application of E ring bromine substituted silybin to preparing glycosidase inhibitors
InactiveCN102000062AConvenient sourceSimple manufacturing methodOrganic active ingredientsMetabolism disorderAlglucerasePositive control
The invention relates to application of E ring bromine substituted silybin to preparing glycosidase inhibitors and in particular discloses application of E ring bromine substituted silybin ester-type flavonolignans or pharmaceutical salt thereof to preparing drugs for inhibiting alpha-glycosidase and preventing and treating type II diabetes. The flavonolignans has extremely obvious activity for inhibiting alpha-glycosidase and the intensity of activity of the flavonolignans for inhibiting alpha-glycosidase reaches 77.1% under the condition of 4mcg / ml concentration. The measured half-inhibitory concentration of the flavonolignans shows that the intensity of activity of the flavonolignans for inhibiting alpha-glycosidase is 32 times that of the positive control drug acarbose. The pharmacodynamics result shows that the flavonolignans or pharmaceutical salt thereof can be expected to be applied to preparing glycosidase inhibitors, especially the drugs for preventing and treating type II diabetes.
Owner:DALI UNIV
Pharmaceutical application of p-bromocinnamoyl silybin to preparing glycosidase inhibitors
InactiveCN101966174BInhibitory activityExact originalityOrganic active ingredientsMetabolism disorderAlglucerasePositive control
The invention relates to pharmaceutical application of p-bromocinnamoyl silybin to preparing glycosidase inhibitors and specifically discloses application of 23-p-bromocinnamoyl substituted silybin ester flavonolignans or medicinal salt thereof to preparing drugs for inhibiting alpha-glycosidase and preventing and treating type II diabetes. The flavonolignans has extremely obvious activity for inhibiting alpha-glycosidase and the intensity of activity of the flavonolignans for inhibiting alpha-glycosidase reaches 54.4% under the condition of 4mcg / ml concentration. The measured half-inhibitory concentration of the flavonolignans shows that the intensity of activity of the flavonolignans for inhibiting alpha-glycosidase is 76.8 times that of the positive control drug acarbose. The pharmacodynamics result shows that the flavonolignans or medicinal salt thereof can be expected to be applied to preparing glycosidase inhibitors, especially the drugs for preventing and treating type II diabetes.
Owner:DALI UNIV
Application of E ring bromine substituted silybin to preparing glycosidase inhibitors
InactiveCN102000062BInhibitory activityExact originalityOrganic active ingredientsMetabolism disorderPositive controlPharmacodynamic Study
Owner:DALI UNIV
Application of diamine formyl dehydrogenated silybin serving as medicament for curing viral hepatitis B
InactiveCN101829090BConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsPositive controlHBsAg
The invention relates to application of diamine formyl dehydrogenated silybin serving as a medicament for curing viral hepatitis B, in particular to application of a flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents or pharmaceutically acceptable salts thereof in preparation of a medicament for clearing HBsAg and HBeAg and a medicament for inhibiting HBV DNA replication. The flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents has extremely high HBsAg and HBeAg inhibiting activities; when the flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents is at a concentration of 20 mu g / ml, the inhibition rates of the HBsAg and the HBeAg are respectively 94.4 percent and 95.7 percent which exceed 5.9 times and 5.7 times those of a positive control alpha-interferon; and simultaneously the inhibition rate of the HBV DNA is 99.7 percent when the flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents is at the same concentration, and the inhibition activity of the flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents is higher than that of lamivudine and the alpha-interferon. In summary, the flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents or the pharmaceutically acceptable salts thereof can be expected for preparing non-nucleoside medicaments for clearing the HBsAg and the HBeAg, inhibiting the HBV DNA replication, and curing the hepatitis B virus infection diseases.
Owner:DALI UNIV
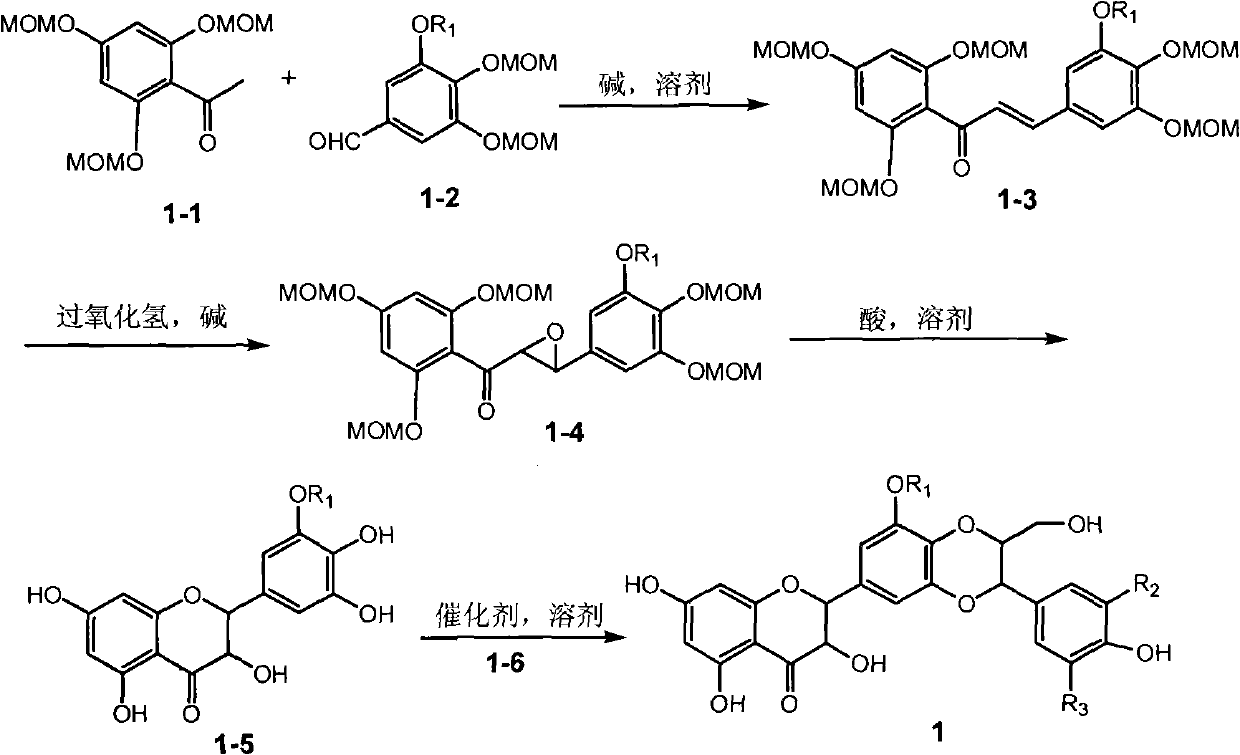
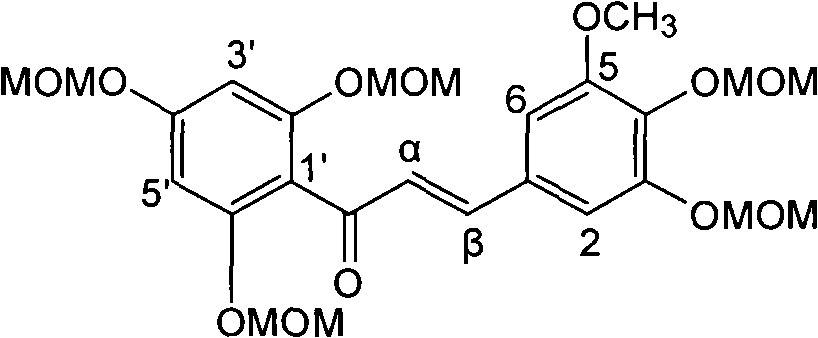
Preparation of flavonolignans in milk thistle and application thereof
The invention relates to the field of natural medicinal chemistry, a preparation of a crude extract and flavonolignans compounds in milk thistle and an application thereof, and in particular to the activity-oriented separation and purification preparation as well as antidiabetic activity of the flavonolignans compounds in traditional Chinese medicine milk thistle. The invention also discloses theisolation and preparation of the compounds, the inhibitory activity on PTP1B enzyme, the evaluation of molecular simulation docking with PTP1B enzyme, the evaluation of enzyme kinetics of PTP1B, and the selective evaluation of homologous enzymes. The test results show that the crude extract from an ethyl acetate extraction layer of milk thistle and the flavonolignan compounds play better PTP1B enzyme inhibitory activity; and the flavonolignans compounds can inhibit the activity through a hydrogen bond with strong binding to a catalytically active region of PTP1B enzyme.
Owner:SHENYANG PHARMA UNIVERSITY
Method of treatment of liver disease
InactiveCN102573864AEliminate side effectsShort half-lifeOrganic active ingredientsDigestive systemHepatic DiseasesMedicine
A method of treatment of a subject suffering from liver disease comprising the administration of (i) an oral slow-release formulation of a calcium channel blocker with antioxidant effects and (ii) at least one flavonolignan.
Owner:HOWARD J SMITH & ASSOC
Medicinal use of dinitrobenzoylsilybin for preparing glycosidase inhibitor
InactiveCN101966173BConvenient sourceEasy to prepareOrganic active ingredientsMetabolism disorderAlglucerasePositive control
Owner:DALI UNIV
Application of 4-cinnamoyl chloride substituted silybin in preparing glycosidase inhibitors
InactiveCN102000057BInhibitory activityExact originalityOrganic active ingredientsMetabolism disorderPositive controlCinnamoyl chloride
The invention relates to application of 4-cinnamoyl chloride substituted silybin to preparing glycosidase inhibitors and in particular discloses application of 4-cinnamoyl chloride substituted silybin ester flavonolignans or pharmaceutical salt thereof to preparing drugs for inhibiting alpha-glycosidase and preventing and treating type II diabetes. The flavonolignans has extremely obvious activity for inhibiting alpha-glycosidase and the intensity of activity of the 40mcg / ml flavonolignans for inhibiting alpha-glycosidase reaches 92.1%. The measured half-inhibitory concentration of the flavonolignans shows that the intensity of activity of the flavonolignans for inhibiting alpha-glycosidase is 30 times that of the positive control drug acarbose. The pharmacodynamics result shows that the flavonolignans or pharmaceutical salt thereof can be expected to be applied to preparing glycosidase inhibitors, especially the drugs for preventing and treating type II diabetes.
Owner:DALI UNIV
Application of E ring benzyloxy substituted silybin in preparing glycosidase inhibitors
InactiveCN102000063BConvenient sourceSimple manufacturing methodOrganic active ingredientsMetabolism disorderAlglucerasePositive control
Owner:DALI UNIV
Autologous fat cell in-situ targeted regeneration natural agonist and preparation method thereof
PendingCN112089820AIn situ targeted regeneration promotionHydrolysed protein ingredientsSexual disorderFlavonolignansChemical compound
The invention discloses an autologous fat cell in-situ targeted regeneration natural agonist. The autologous fat cell in-situ targeted regeneration natural agonist comprises the following main components of sika deer horn plate collagen peptide, a fructus evodiae roasted rhizoma coptidis extract and an elymus nutans extract, and the main component of the sika deer horn plate collagen peptide is polypeptide with the molecular weight of 5kDa or below. The main components of the fructus evodiae roasted coptis chinensis extract and the elymus nutans extract are flavonolignans compounds, and the natural agonist can effectively promote in-situ targeted regeneration of autologous fat cells.
Owner:湖南朗盾科技有限公司
Application of ring B ethyoxyl silybin in preparing medicaments for treating viral hepatitis B
InactiveCN101829089BConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemPositive controlInterferon alpha
The invention relates to application of ring B ethyoxyl silybin in preparing medicaments for treating viral hepatitis B, in particular to application of ring B ethyoxyl substituted silybin ester or a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away HBsAG (Hepatitis B Surface Antigen) and HBeAg (Hepatitis B e Antigen) and suppressing the HBV (Hepatitis B Virus) DNA replication. The compound has strong activity on suppressing the HBsAG and the HBeAg, and in the presence of a concentration of 20 micrograms / milliliter, the intensities for clearing the HBsAg and the HBeAg are respectively 64.6 percent and 44.8 percent which are 4.0 times and 2.7 times of that of alpha-interferon which is a positive control medicament. In the presence of the concentration, the suppression rate of the compound on the HBV DNA is 58.1 percent which is 1.5 times of the corresponding activity of the alpha-interferon. Accordingly, the flavonolignan or the pharmaceutically acceptable salt thereof are indicated to simultaneously have strong efficacy on suppressing the HBsAg, the HBeAg and the HBV DNA and can be expected to be used for preparing non-nucleoside medicaments for treating HBV infection diseases.
Owner:DALI UNIV
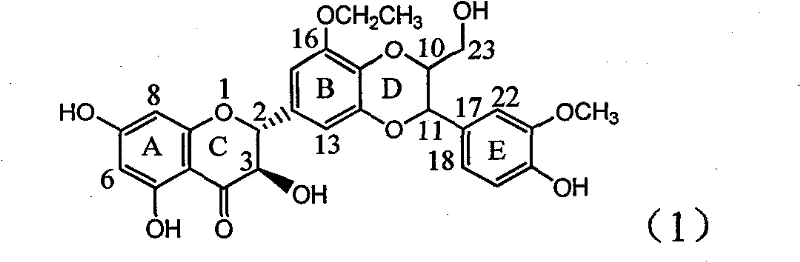
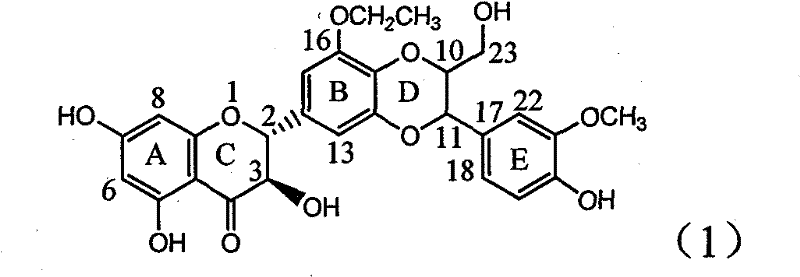
Silybin flavonolignan and their production method and use
The invention relates to a silibinin flavonoid lignan compound with anti-free radical oxidation, liver protection, prevention and treatment of senile dementia and anti-aging activities and a pharmaceutically acceptable salt or solvate thereof. The present invention also relates to the preparation method of the compound of formula (1) including its important intermediates and its pharmaceutical composition and medical application. The compound of the present invention has anti-oxidative damage protective effect on the primary cells of SD neonatal rats, and can be expected to be used as a medicine for preventing liver damage; Radicals, the activity of inhibiting the generation of lipid peroxides induced by free radicals, and the strong anti-free radical-induced damage to PC12 cells, so it can be expected to be a drug for treating various diseases caused by free radicals.
Owner:ZHEJIANG HISUN PHARMA CO LTD
Application of substituted benzoyl silybin in preparing medicament for treating virus hepatitis B
InactiveCN101829092AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseaseHigh concentration
The invention relates to application of substituted benzoyl silybin in preparing a medicament for treating virus hepatitis B, in particular to application of 3-amino-5-nitrobenzoyl substituted silybin ester type flavonolignan or medicinal salt thereof in preparing a medicament for inhibiting HBV DNA replication and treating hepatitis B virus infected diseases. The flavonolignan has the effect of definitely inhibiting HBV DNA activity, the activity of the flavonolignan in high dosage (100 micrograms per milliliter) for inhibiting the HBV DNA replication is 55 percent higher than the inhibiting activity of alpha-interferon at highest concentration of 10,000 units per milliliter, and the flavonolignan belongs to an effectual non-nucleoside natural product for inhibiting the hepatitis B virus. Pharmacodynamical results show that the flavonolignan or the medicinal salt thereof can be expected to be used for preparing the medicament for inhibiting HBV DNA replication and treating the hepatitis B virus infected diseases.
Owner:DALI UNIV
Application of ring A substituted silybin ester in preparing medicaments for treating viral hepatitis B
InactiveCN101829101BConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemPositive controlInterferon alpha
The invention relates to application of ring A substituted silybin ester in preparing medicaments for treating viral hepatitis B, in particular to application of silybin ester flavonolignan substituted by ethoxycarbonyl methyl on the ring A or a pharmaceutically acceptable salt thereof for preparing medicaments for reducing the hepatitis B virus surface antigen (HBsAg), suppressing the HBV (Hepatitis B Virus) DNA replication and treating HBV infection diseases. The flavonolignan has quite obvious activity on suppressing the HBsAg, and in the presence of a concentration of 100 micrograms / milliliter, the intensity of the flavonolignan for clearing away the HBsAG exceeds that of alpha-interferon which is a positive control medicament by 3.3 times. Meanwhile, in the presence of a concentration of 20 micrograms / milliliter, suppression ratio of the compound to the HBV DNA is close to 60 percent. The pharmacodynamical results indicate that the flavonolignan or the pharmaceutically acceptable salt thereof can be expected to be used for preparing the medicaments for treating the HBV infection diseases.
Owner:DALI UNIV
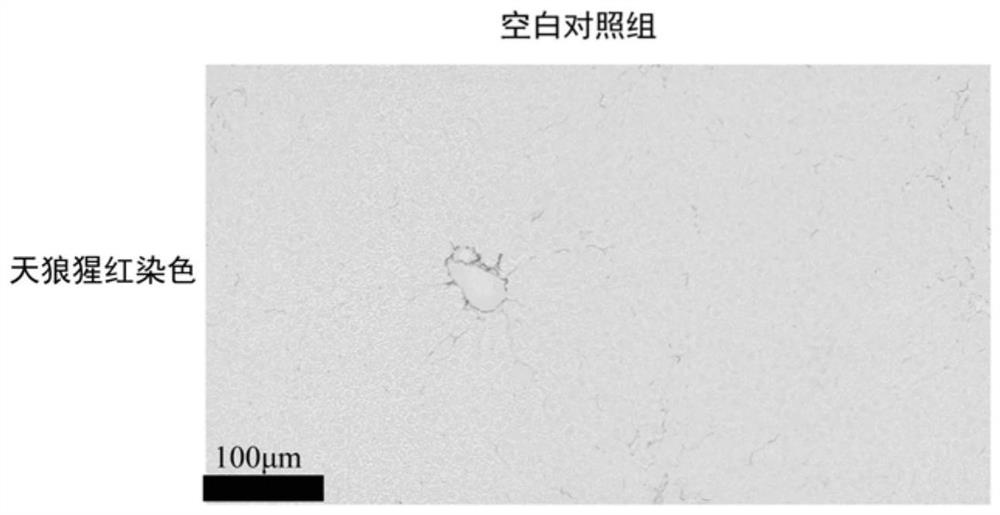
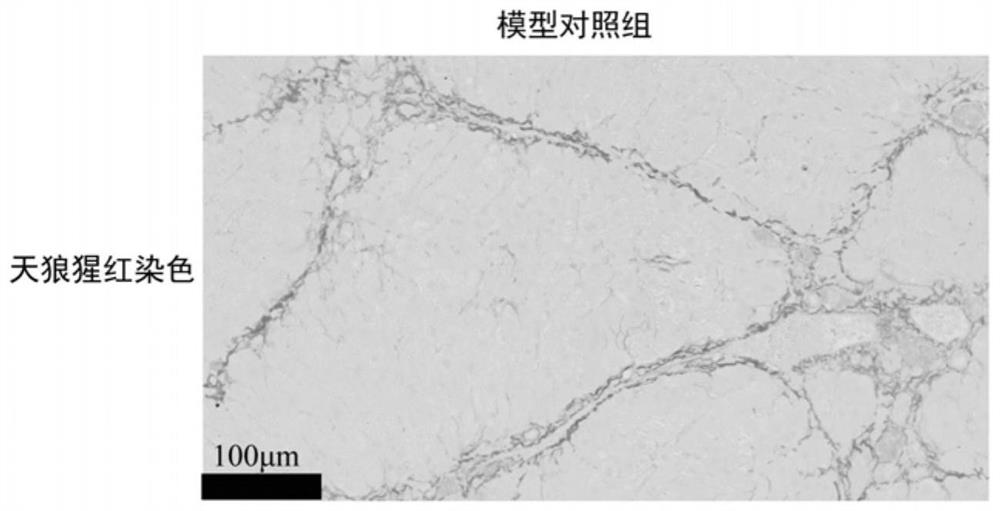
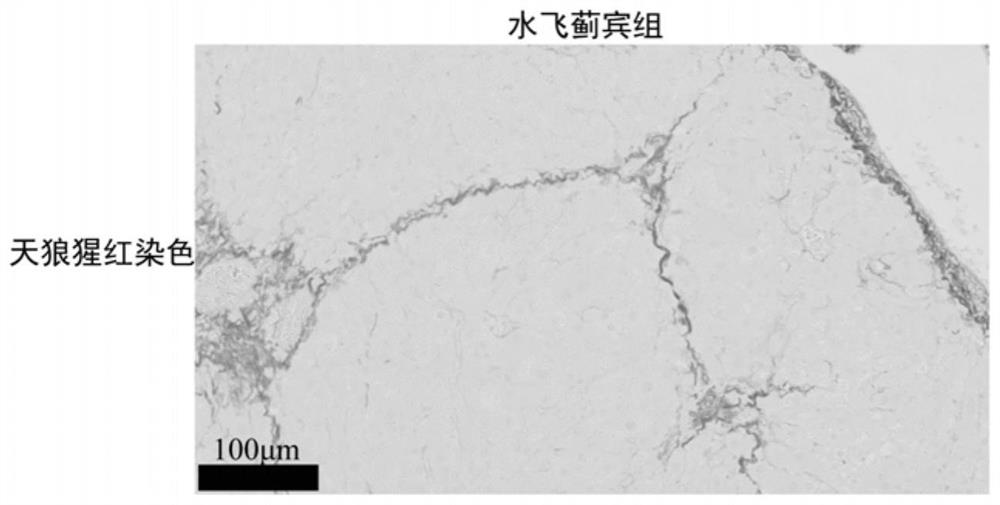
A kind of anti-hepatic fibrosis composition and its application
ActiveCN112891545BReduce liver fibrosisReverse the progression of liver fibrosisOrganic active ingredientsPeptide/protein ingredientsCyclaseAgonist
The invention discloses an anti-hepatic fibrosis composition and its application, belonging to the technical field of medicine. Composition of flavonoid lignan compound and soluble guanylate cyclase agonist can improve liver fibrosis in mice in thioacetamide liver fibrosis model. The present invention better cures liver fibrosis or provides another feasible option for treating liver fibrosis, and improves the prognosis or quality of life of patients.
Owner:CHINA PHARM UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com