Application of 4-cinnamoyl chloride substituted silybin to preparing glycosidase inhibitors
A technology of silibinin ester and chlorinated meat, which is applied in the field of pharmaceutical application of 4-chlorocinnamoyl silibinin for the preparation of glycosidase inhibitors, can solve the problems of diabetes without glycosidase inhibition, and achieve industrialization Clear prospect, large-scale production of energy saving and emission reduction, strong inhibitory effect of α-glucosidase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
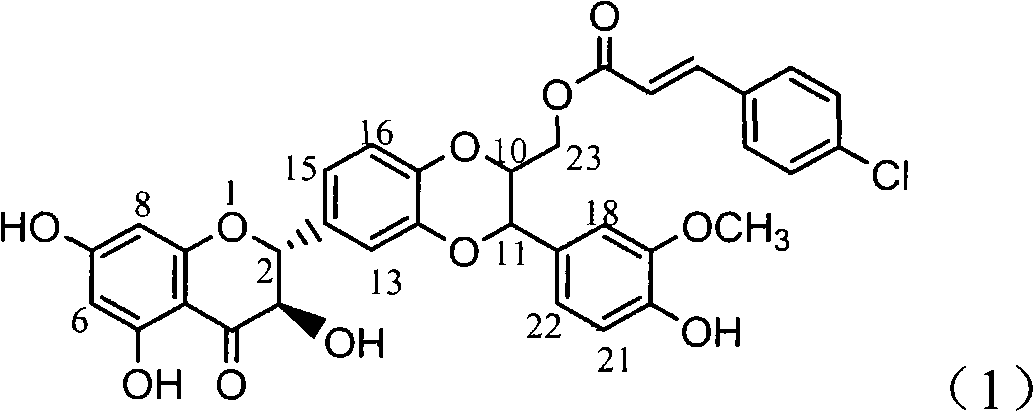
[0019] Example 1 : the preparation of formula (1) compound
[0020] 1.1 Instruments and reagents:
[0021] The ultraviolet spectrum was measured with a Shimadzu UV-240 ultraviolet spectrophotometer; the hydrogen nuclear magnetic resonance spectrum 1 H-NMR is measured by INOVA type superconducting nuclear magnetic resonance spectrometer (VARIAN INOVA-400MHz) (tetramethylsilyl ether TMS is internal standard); Electrospray mass spectrometry ESI-MS is measured by Bruker Esquire3000+mass spectrometer, column chromatography uses silica gel ( 100-200, 200-300 and 300-400 mesh) and silica gel GF254 (10-40 mesh) for thin-layer chromatography are produced by Qingdao Ocean Chemical Factory; all reagents used are analytically pure; preparative thin-layer chromatography (PTLC) Aluminum foil silica gel plates from Merck were used; Sephadex LH-20 for column chromatography was produced by Amersham Pharmacia Biotech AB in Sweden; reverse-phase silica gel RP-18 was produced by Chromatorex fro...
Embodiment 2
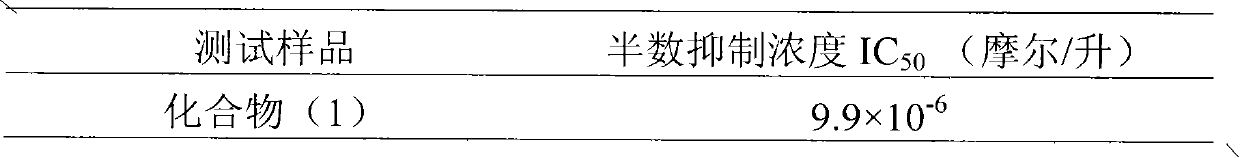
[0026] Example 2 : The inhibitory activity detection of formula (1) compound to α-glucosidase
[0027] 2.1 Instruments and reagents
[0028] 2.1.1 Experimental Instruments
[0029] Microplate reader: ELISA plate reader (Bio-Tek Instruments, USA)
[0030] 2.1.2 Reagents
[0031] α-glucosidase is α-D-glucosidase (Sigma, 500U / ml); 4-nitrophenol-α-D-glucopyranoside (PNPG, Merck), reduced glutathione (Shanghai Shenggong) , Acarbose is Baitangping (Bayer Healthcare Co., Ltd., Beijing).
[0032] 2.2 Test method
[0033] The inhibitory effect of compounds on α-glucosidase was determined by colorimetric method. Add phosphate buffer (67 mmol / L, pH6.8, 170 microliters), reduced glutathione (1 mg / ml, 5 microliters), α-D-glucosidase (diluted with phosphate buffer into 0.2U / ml, 25 microliters), compound (1) was dissolved in dimethyl sulfoxide, diluted with phosphate buffer, 25 microliters per well, so that the final concentration was 0.04 mg / ml, 0.004 mg / ml, 0.0004 mg / ml, finally a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com