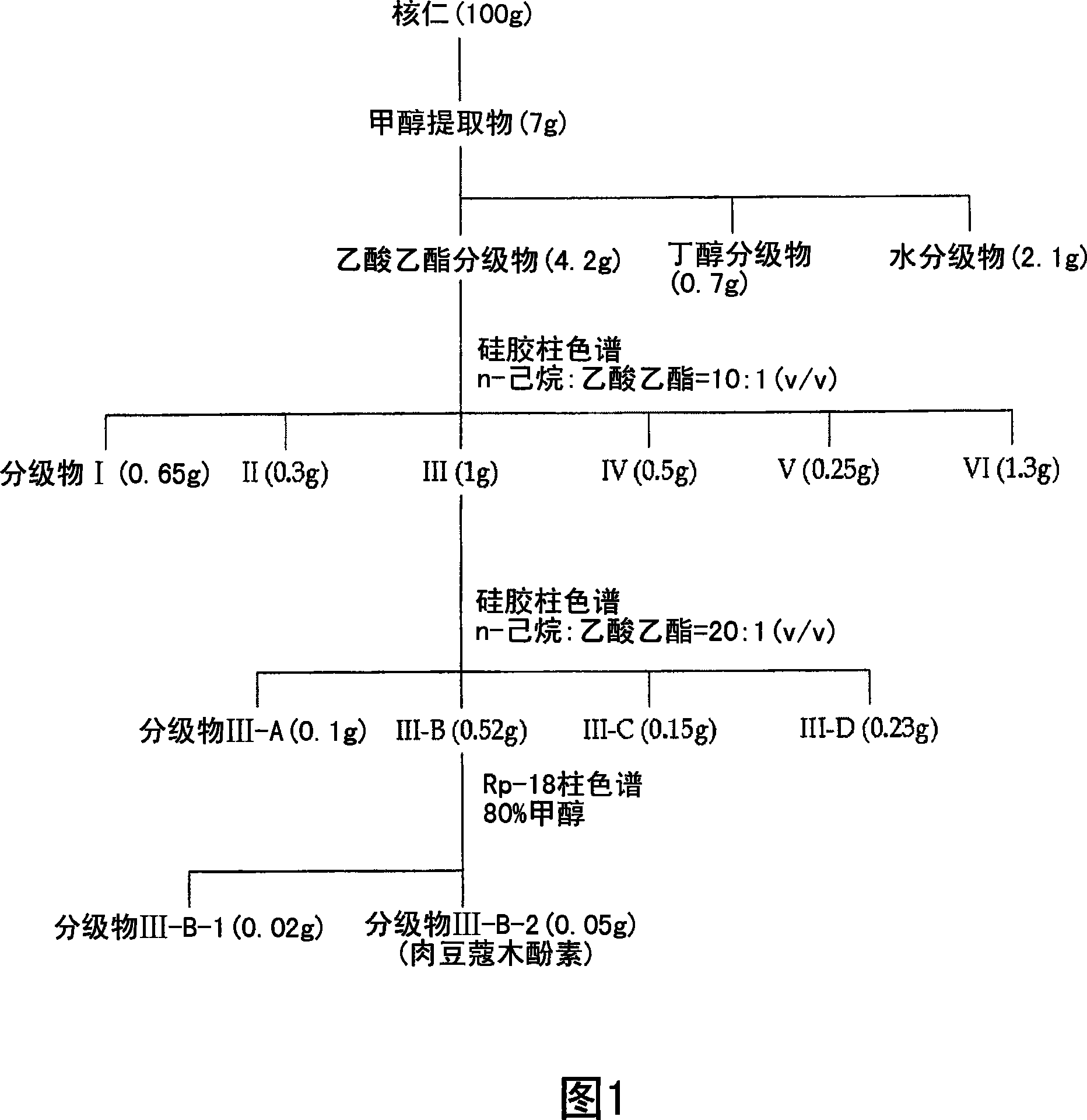
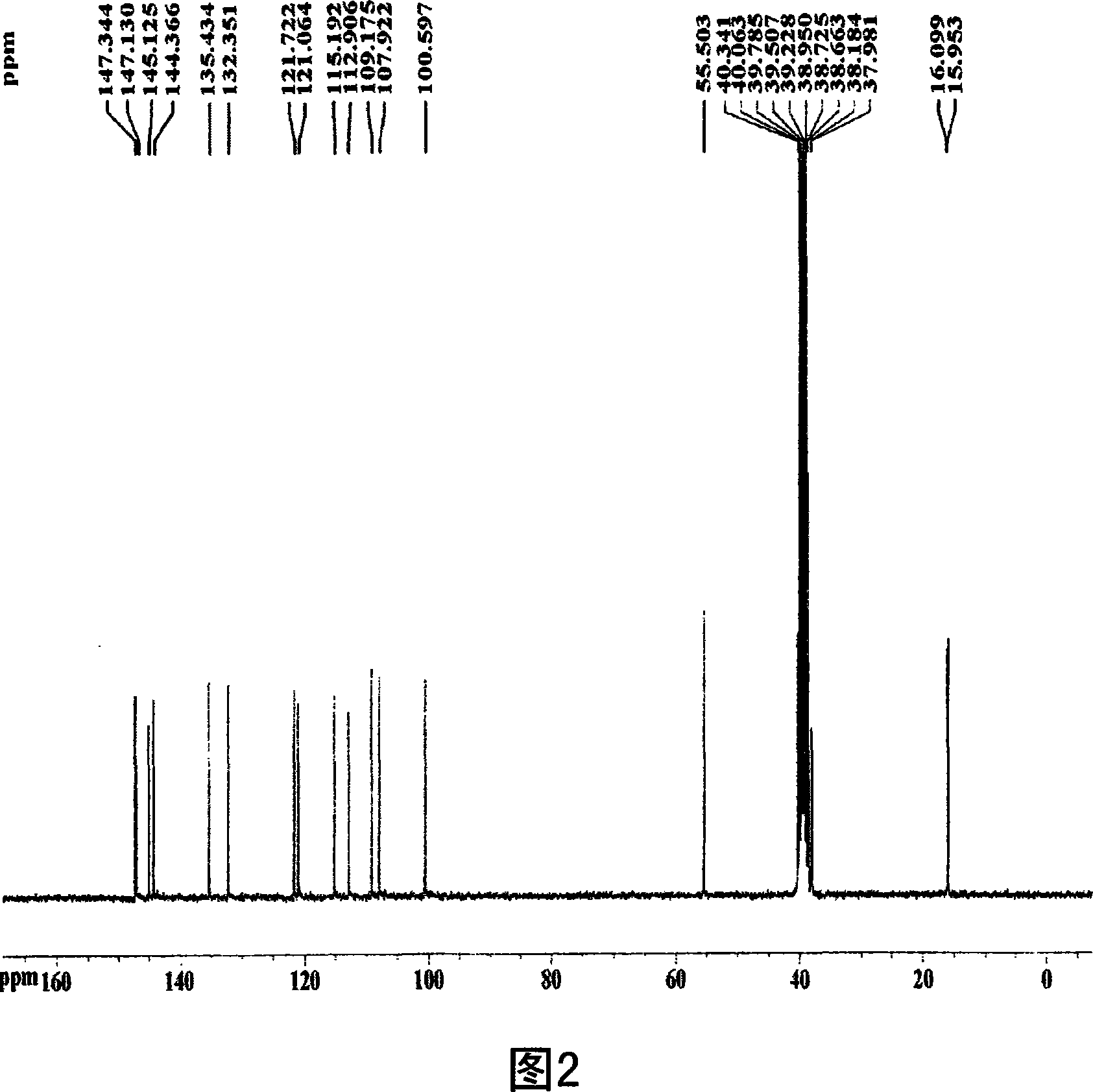
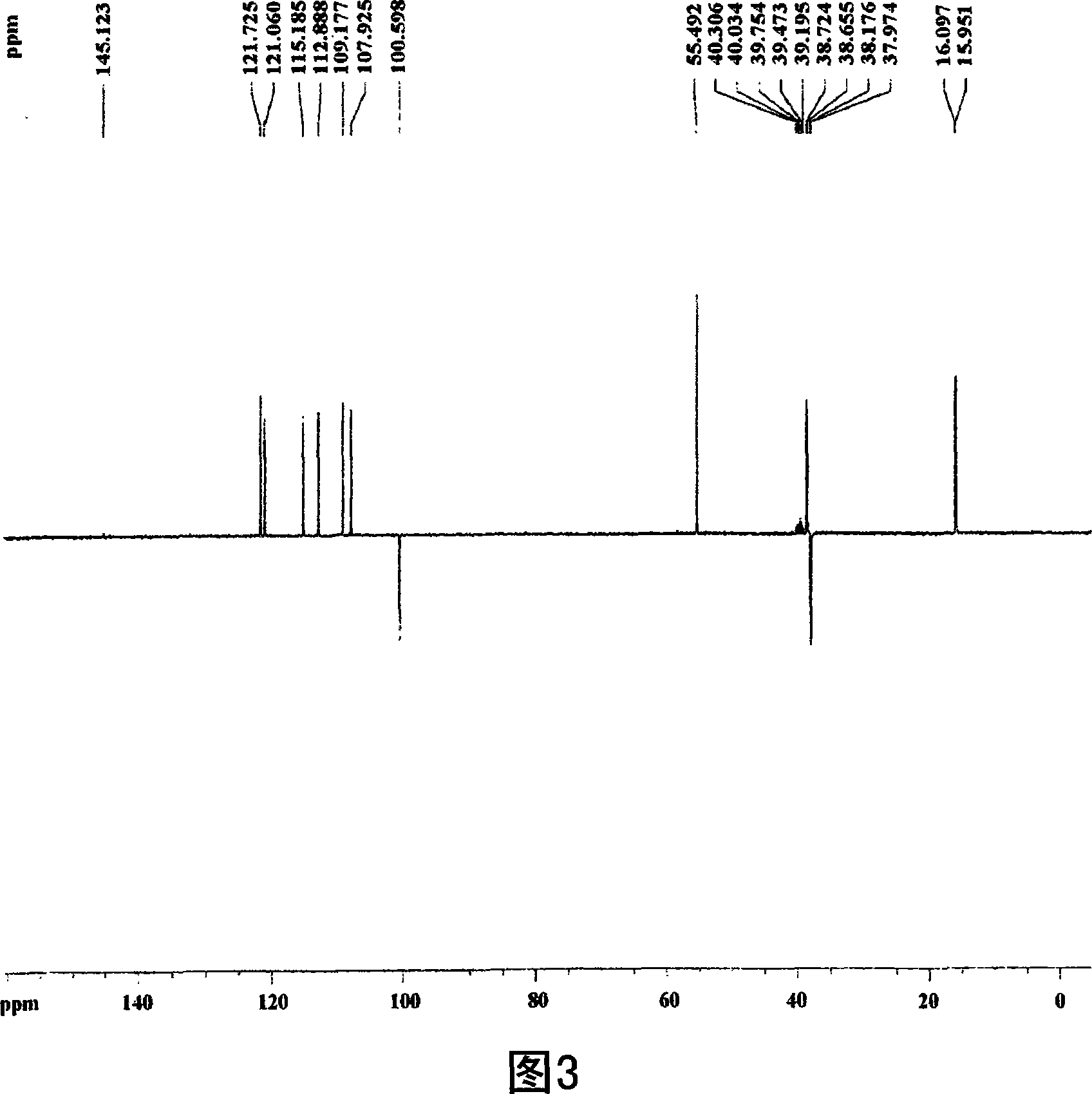
Patents
Literature
595 results about "Lignan" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
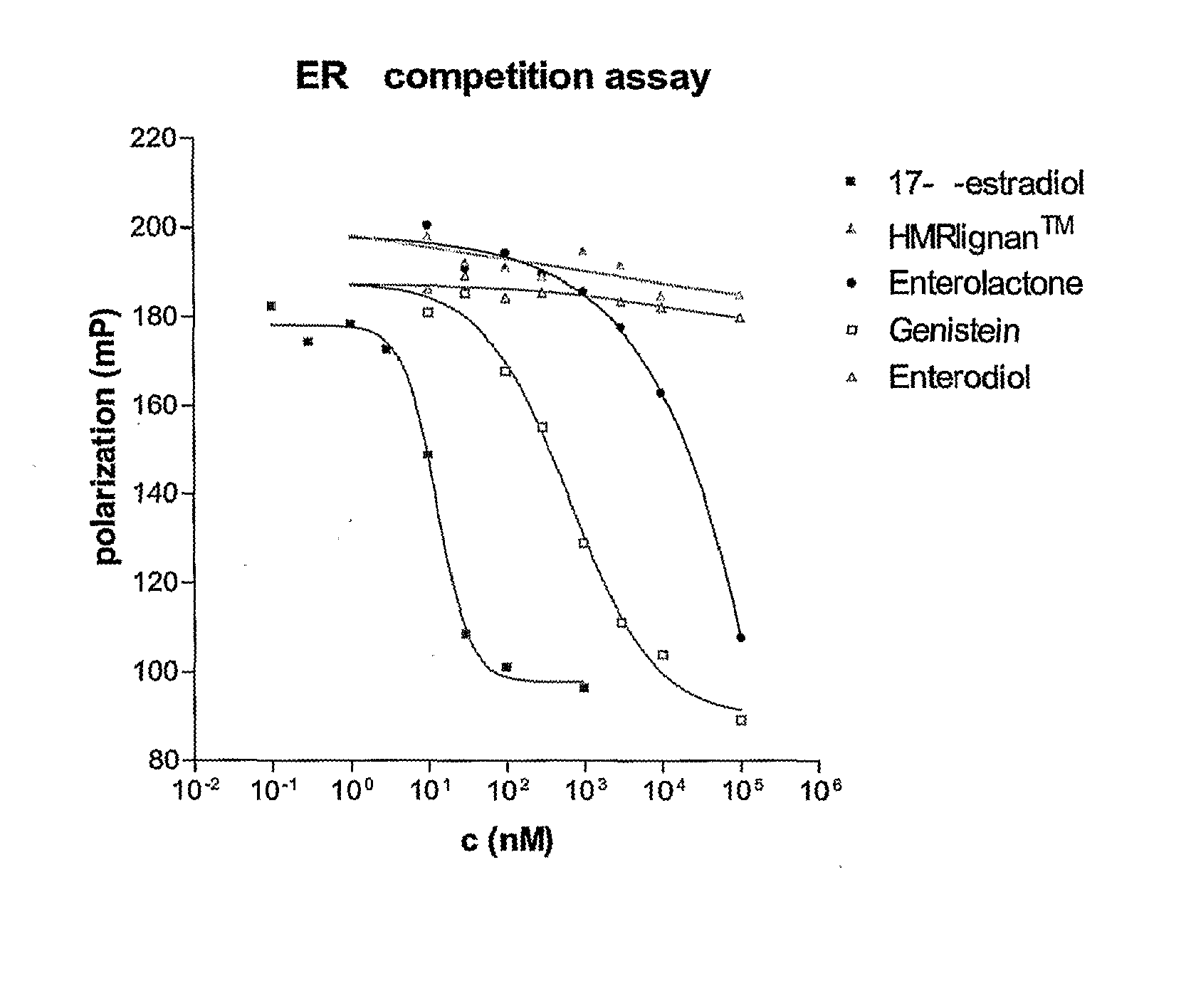
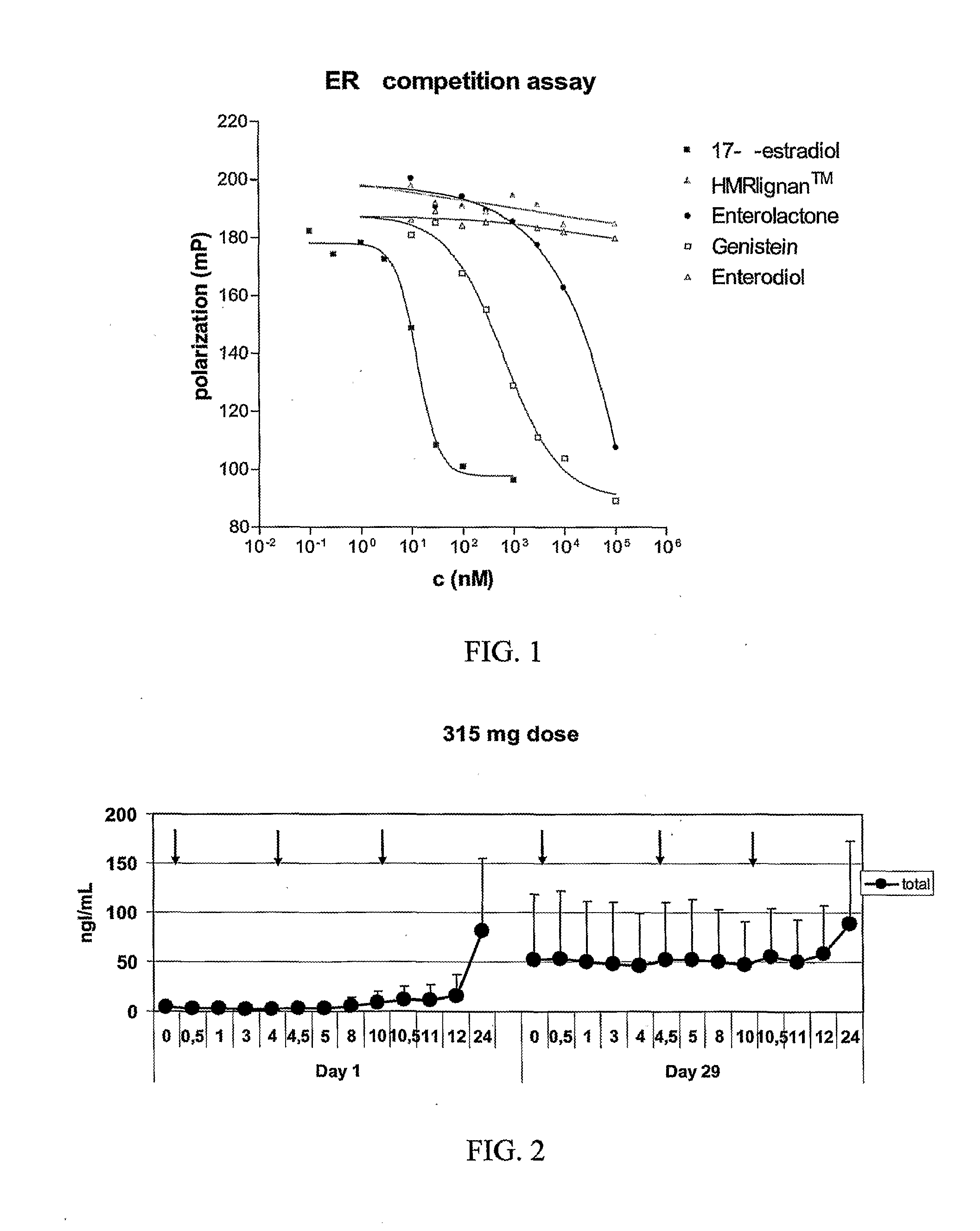
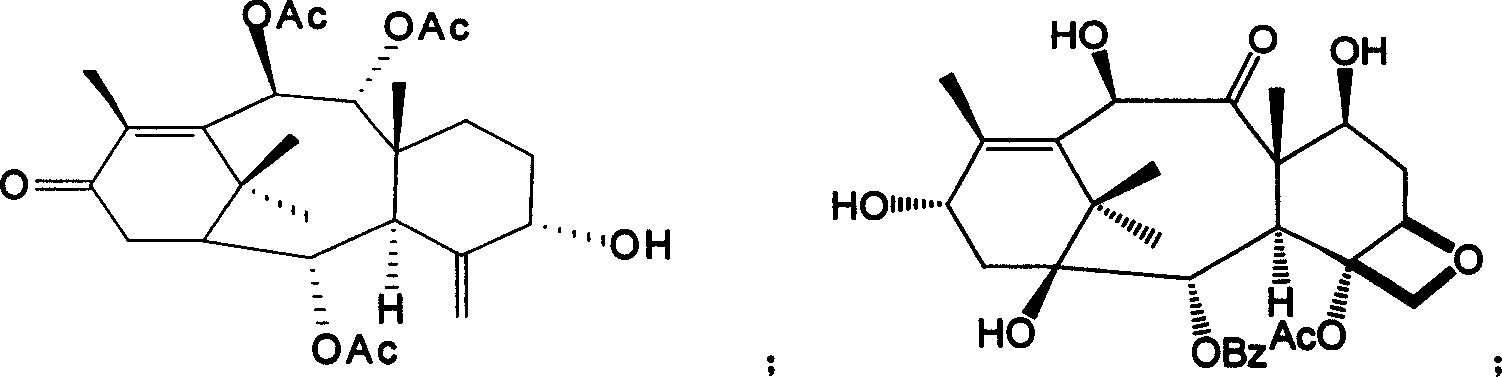
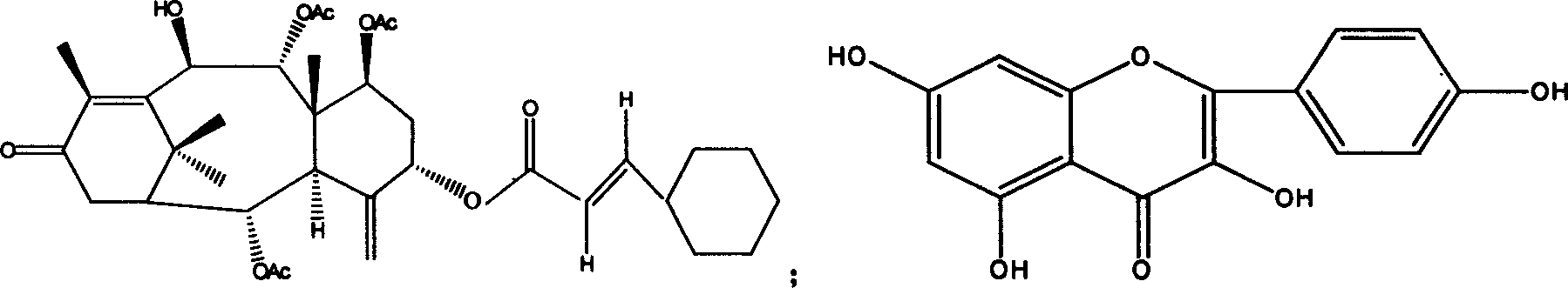
The lignans are a large group of polyphenols found in plants. Some examples of lignans are enterolignans, enterodiol and enterolactone.
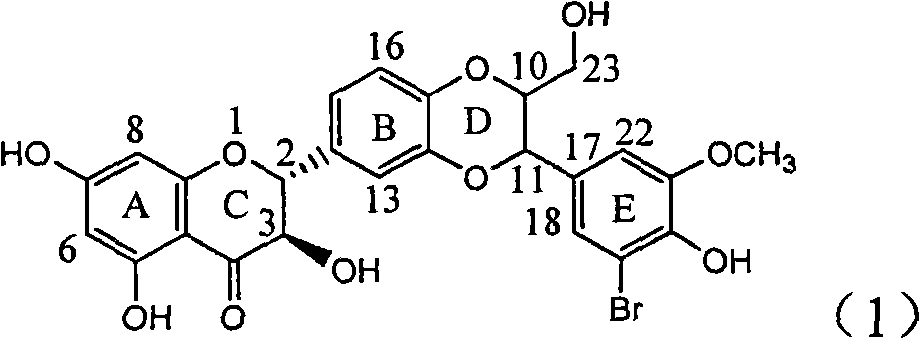
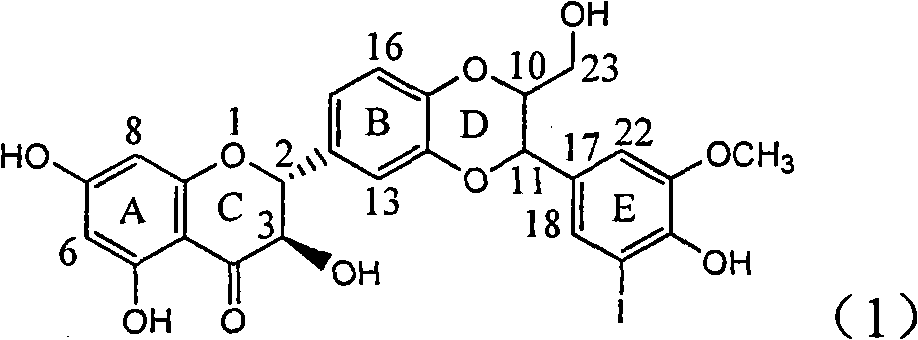
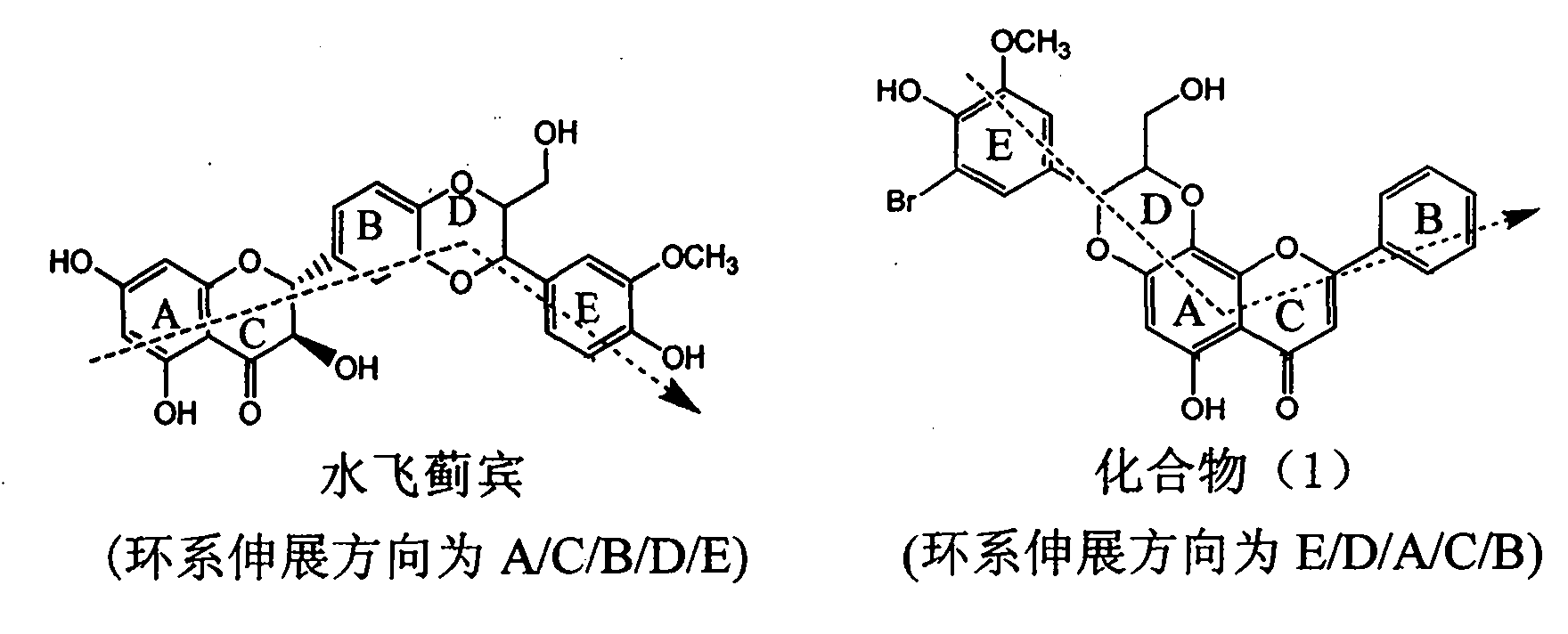
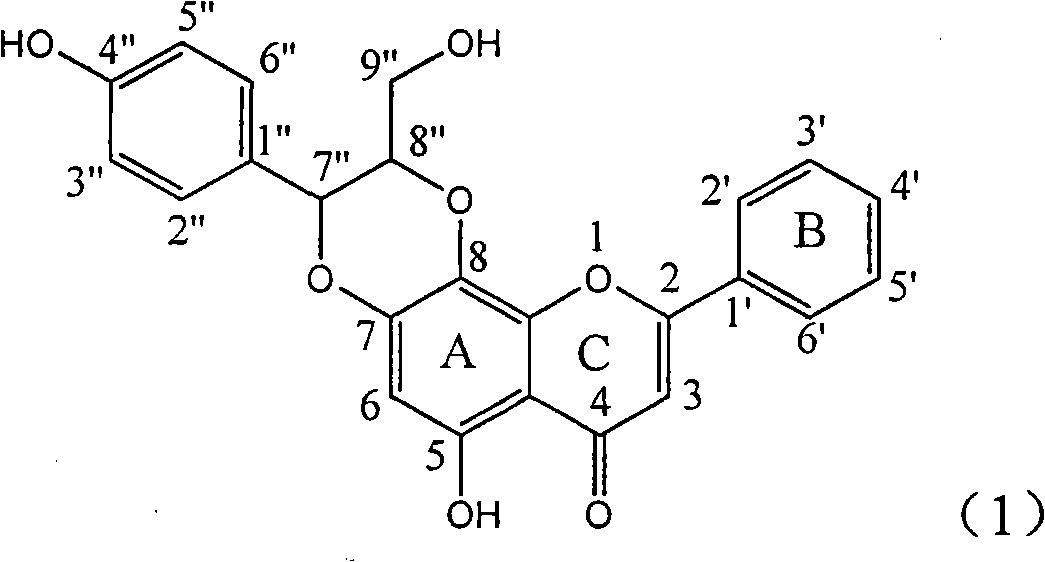
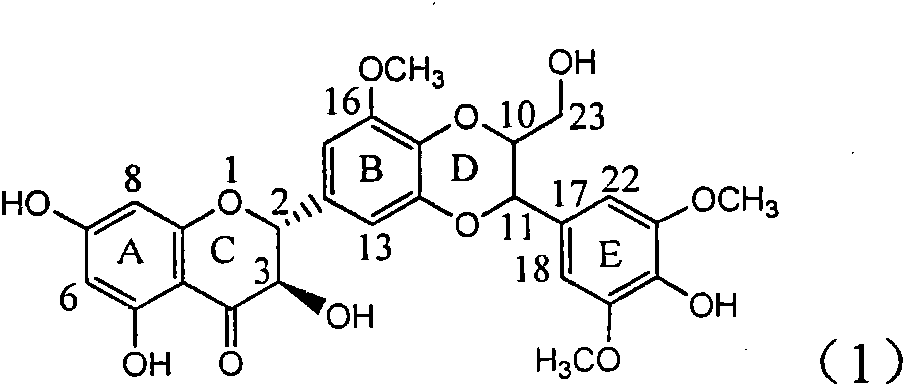
Application of ring E bromine substituted silybin in preparing medicaments for treating viral hepatitis B
InactiveCN101829094AInhibitory activityInhibition of replicative activityOrganic active ingredientsAntiviralsDiseasePositive control
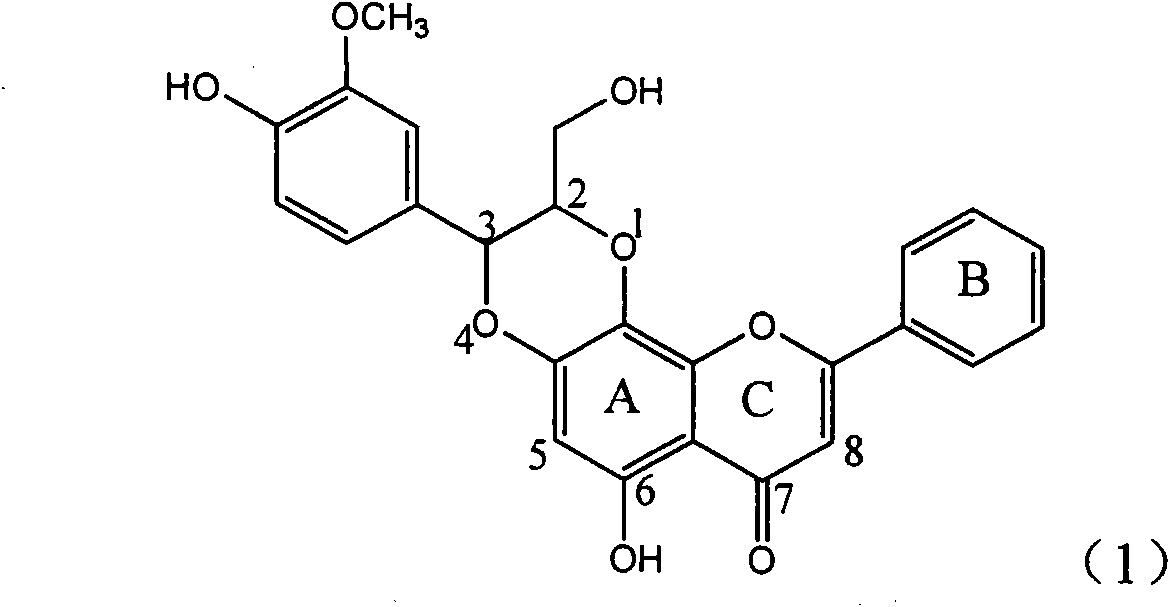
The invention relates to application of ring E bromine substituted silybin in preparing medicaments for treating viral hepatitis B, in particular to application of a compound of a formula (1) and a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away hepatitis B surface antigens (HBsAg) and hepatitis e antigens (HBeAg) and suppressing the HBV (Hepatitis B Virus) DNA replication. The compound has definite activity on suppressing the HBsAg and the HBeAg, and in the presence of a concentration of 100 micrograms / milliliter, the intensities of the compound for clearing away the HBsAg and the HBeAg are respectively 38.2 percent and 39.1 percent which are respectively 2.4 times and 2.3 times of that of a positive control medicament (10,000 units / milliliter of alpha-interferon). Meanwhile, in the presence of the concentration, the suppression ratio of the compound on the HBV DNA is 36 percent which is close to that of the alpha-interferon. Accordingly, the flavone lignan or the pharmaceutically acceptable salt thereof are indicated to be capable of being used for preparing non-nucleoside medicaments for clearing away the HBsAg and the HBeAg, suppressing the HBV DNA replication and treating HBV infection diseases.
Owner:DALI UNIV
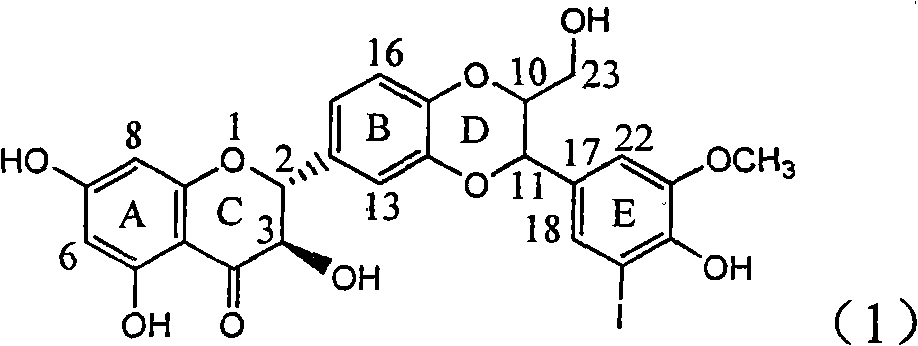
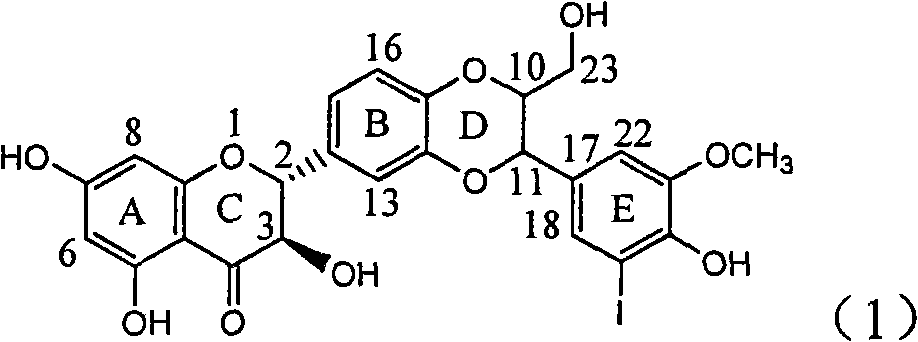
Application of ring E iodine substituted silybin in preparing medicaments for treating viral hepatitis B
InactiveCN101829096AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
The invention relates to application of ring E iodine substituted silybin in preparing medicaments for treating viral hepatitis B, in particular to application of a compound of a formula (1) and a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away hepatitis B surface antigens (HBsAg) and hepatitis e antigens (HBeAg) and suppressing the HBV (Hepatitis B Virus) DNA replication. The compound has definite activity of suppressing the HBsAg and the HBeAg, and in the presence of a concentration of 100 micrograms / milliliter, the intensities of the compound for clearing away the HBsAg and the HBeAg are respectively 20.0 percent and 29.0 percent which exceed that of a positive control medicament (10,000 units / milliliter of alpha-interferon) by 24 percent and 72 percent. Meanwhile, in the presence of the concentration, the suppression ratio of the compound on the HBV DNA is 32.6 percent which is close to that of the alpha-interferon. Accordingly, the flavone lignan or the pharmaceutically acceptable salt thereof are indicated to be capable of being used for preparing non-nucleoside medicaments for clearing away the HBsAg and the HBeAg, suppressing the HBV DNA replication and treating HBV infection diseases.
Owner:DALI UNIV
Application of flavone lignan (+/-) Scutellaprostin A in preparing medicaments for treating viral hepatitis type B
InactiveCN101953827AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseaseLignan
The invention relates to application of flavone lignan (+ / -) Scutellaprostin A in preparing medicaments for treating viral hepatitis type B, in particular to a compound with the formula (1) or pharmaceutically-acceptable salts thereof for preparing medicaments for clearing HBsAg and HBeAg and suppressing HBV (Hepatitis B Virus) DNA replication. In the invention, the intensities of the compound for clearing the HBsAg and the HBeAg under the concentration of 20 micrograms / milliliter respectively reach 81.8 percent and 81.9 percent, which are respectively 5.1 times and 4.8 times as high as the corresponding activity of alpha-interferon used as a positive contrast medicament; and what is more exciting, when the compound has the concentration, the compound performs a suppression ratio higher than 81 percent, and the value is also higher than that of both lamivudine and alpha-interferon. Accordingly, the flavone lignan or the pharmaceutically-acceptable salts can be expectably used for preparing nucleoside medicaments for clearing the HBsAg and the HBeAg, suppressing the HBV DNA replication and treating HBV infected diseases.
Owner:DALI UNIV
Antimicrobial composition with low cytotoxicity
ActiveUS20100129302A1Minimal irritationMinimal cytotoxicityBiocideCosmetic preparationsCytotoxicityLignan
A composition includes at least one antimicrobial agent; a possible solvent system with either hydrophobic or hydrophilic nature, selected according to the target of usage; as well as possible surface active agent. The compound mixture is obtained by pulverizing wood or plant material and / or by extracting the possibly pulverized wood or plant material, so that the compound mixture contains at least two different polyphenolic compounds selected from the following group: lignans, stilbenes, juvabiones and flavonoids; the compound mixture further containing oligomers of the polyphenolic compounds, so that the compound mixture preferably has the following properties: the compound mixture has an antimicrobial effect in the composition; the cytotoxicity of the polyphenols contained in the compound mixture is at least 10 times lower with respect to BHT; the compound mixture does not irritate the skin in a so-called single patch test with contents of 0.1 wt %.
Owner:GRANULA
Application of angle flavonoids lignan to preparation of medicaments for treating viral hepatitis B
InactiveCN101953828AInhibition of replicationConvenient sourceOrganic active ingredientsAntiviralsLignanInterferon alpha
The invention relates to application of an angle flavonoids lignan to preparation of medicaments for treating viral hepatitis B, in particular to application of the angle flavonoids lignan or medicinal salts thereof to preparation of medicaments for inhibiting hepatisis B virus (HBV) DNA replication and treating HBV infection diseases. The flavonoids lignan can exactly inhibit HBV DNA activity; the inhibition activity of the flavonoids lignan with high dosage (20 microgram / ml) to the HBV DNA replication is 189 percent higher than that of alpha-interferon with the maximum concentration of 10,000 unit / ml; and the flavonoids lignan belongs to a strong-effect non-nucleosides inhibition HBV natural product. The pharmacological results show that the angle flavonoids lignan or the medicinal salts thereof can be expected to be used for preparing the medicaments for inhibiting hepatisis B virus (HBV) DNA replication and treating the HBV infection diseases.
Owner:DALI UNIV
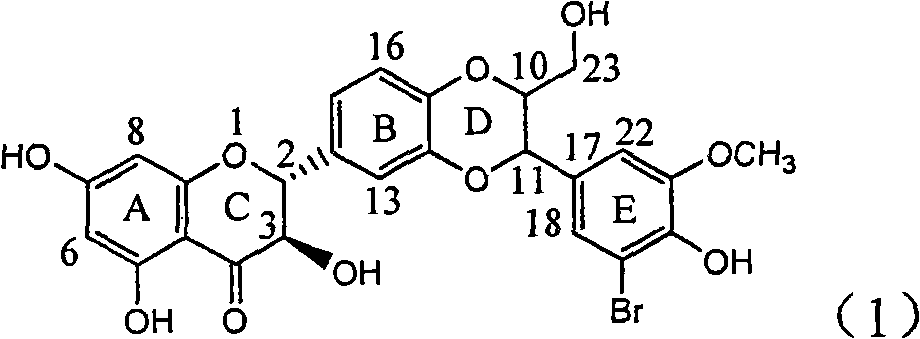
Application of ring A dioxane flavonolignan in preparing medicaments for resisting hepatitis B viruses (HBV)
InactiveCN101829085AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
The invention relates to application of ring A dioxane flavonolignan in preparing medicaments for resisting hepatitis B viruses (HBV), in particular to application of ring A dioxane coupling type flavone lignan or a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away hepatitis B e antigen (HBeAg), suppressing the HBV DNA replication and treating HBV infection diseases. The flavonolignan has certain activity on resisting the HBeAg, and the intensity of the flavonolignan for clearing away the HBeAg is higher than that of Lamivudine which is a positive control and close to that of 10,000 units / milliliter of alpha-interferon. Meanwhile, the suppression ratio of the compound to the HBV DNA replication is higher than 80 percent in the presence of a concentration of 100 micrograms / milliliter. The pharmacodynamical results indicate that the flavonolignan or the pharmaceutically acceptable salt thereof can be expected to be used for preparing the medicaments for clearing away the HBeAg, suppressing the HBV DNA replication and treating the HBV infection diseases.
Owner:DALI UNIV
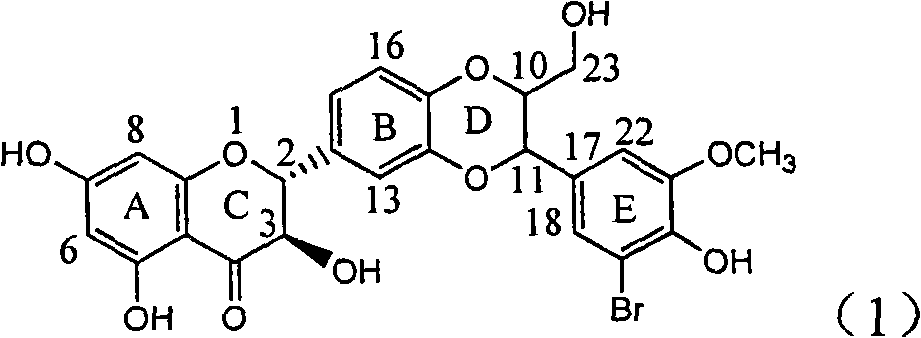
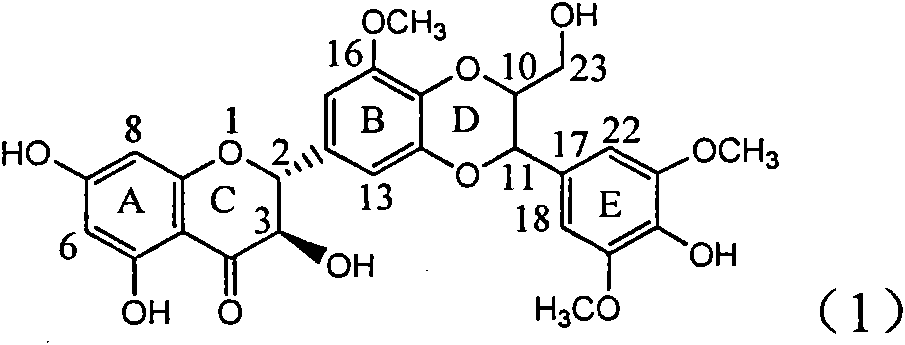
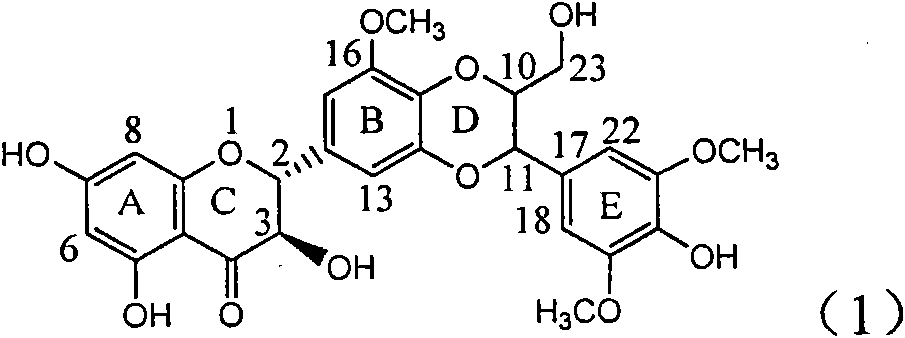
Application of B/E bi-methoxy silybin in preparing medicaments for treating viral hepatitis B
InactiveCN101829088AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
The invention relates to application of B / E bi-methoxy silybin in preparing medicaments for treating viral hepatitis B, in particular to application of silybin ester substituted by the methoxy on the ring B and the ring E or a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away HBsAg (Hepatitis B Surface Antigen) and HBeAg (Hepatitis B e Antigen) and suppressing the HBV (Hepatitis B Virus) DNA replication. The B / E bi-methoxy silybin has strong activity on suppressing the HBsAg and the HBeAg, and in the presence of a concentration of 20 micrograms / milliliter, the intensities for clearing away the HBsAg and the HBeAg are respectively 43.9 percent and 43.7 percent which are 2.7 times and 2.6 times of that of alpha-interferon which is a positive control medicament. In the presence of the concentration, the suppression ratio on the HBV DNA is 68.6 percent, and the suppression activity is 1.8 times of that of the alpha-interferon. Accordingly, the flavone lignan or the pharmaceutically acceptable salt thereof are indicated to simultaneously have the effects of strongly suppressing the HBsAG, the HBeAg and the HBV DNA and can be expected to be used for preparing the non-nucleoside medicaments for treating HBV infection diseases.
Owner:DALI UNIV
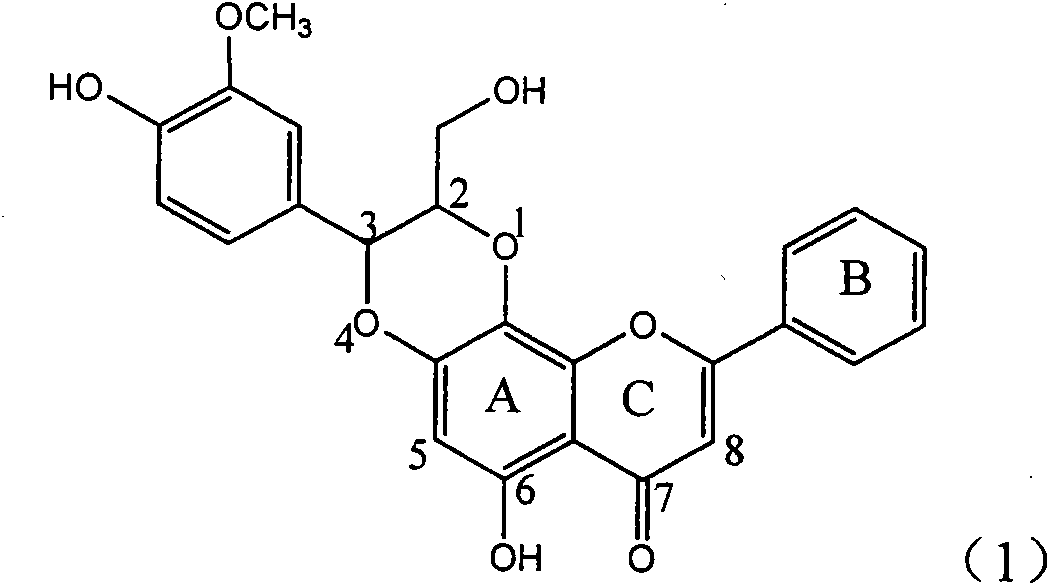
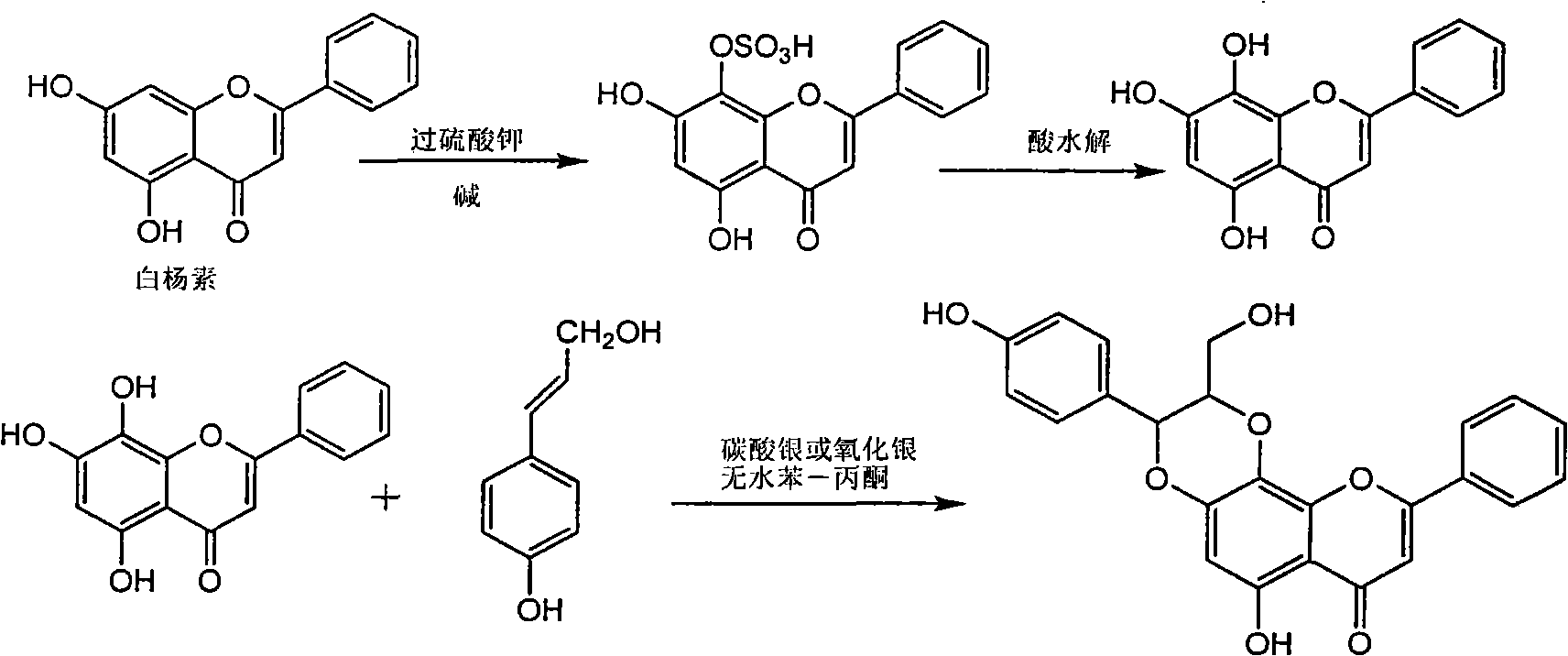
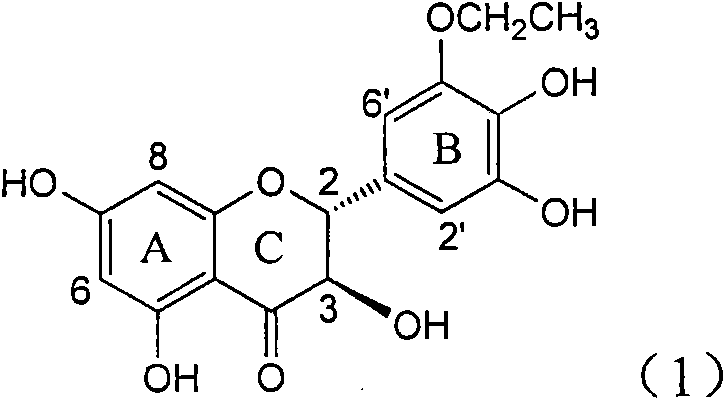
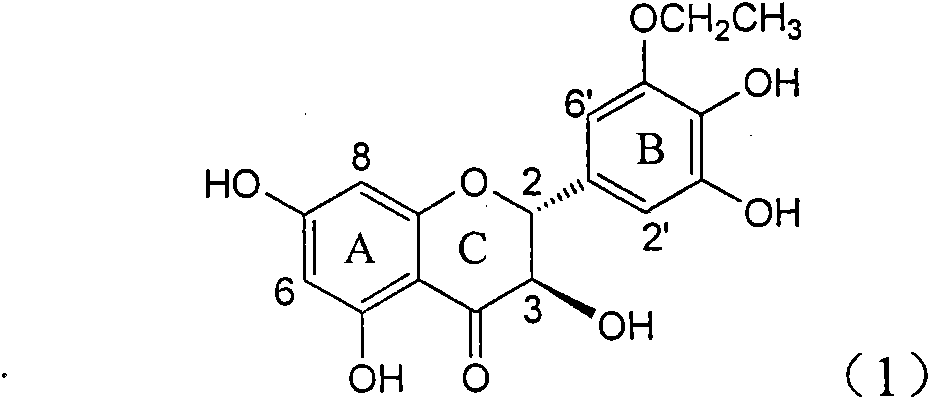
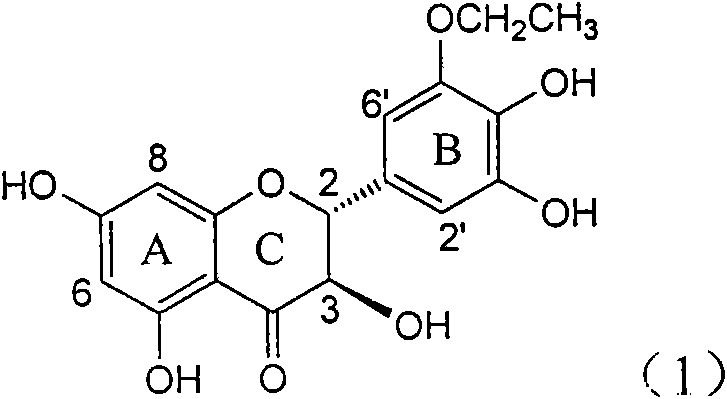
Application of B-ring ethyoxyl flavanonol in preparing medicaments for treating hepatitis B viruses
InactiveCN101822664AConvenient sourceThe source is easy to getOrganic active ingredientsOrganic chemistryDiseasePositive control
The invention relates to application of B-ring ethyoxyl flavanonol in preparing medicaments for treating hepatitis B viruses, in particular to application of a compound as shown in a formula (1) or a medicinal salt thereof in preparing medicaments for clearing away hepatitis B virus surface antigens (HBsAg) and hepatitis B e-antigen (HBeAg) and medicaments for inhibiting the duplication of hepatitis B virus desoxyribonucleic acid (HBV DNA). The compound or the medicinal salt thereof has extremely obvious activity on inhibiting the HBsAg and the HBeAg, and in the presence of a concentration of 20 microgram / milliliter, the intensities for clearing away the HBsAg and the HBeAg of the compound or the medicinal salt thereof are respectively 99.8 percent and 48.5 percent and are 6.2 times and 2.7 times of that of alpha-interferon which is a positive control medicament. More importantly, in the presence of the concentration, the inhibition ratio of the compound or the medicinal salt thereof to the HBV DNA is 64.7 percent, and the activity is 1.7 times of that of the alpha-interferon. Accordingly, the flavone lignan or the medicinal salt thereof can be expected to be used for preparing non-nucleoside medicaments for treating infectious diseases of the hepatitis B viruses.
Owner:DALI UNIV
New raw material for extracting fructus schizandrae total lignans and preparation technique and use
InactiveCN101214278AAlleviate the shortage of supplyAlleviate supply shortagesOrganic compounds purification/separation/stabilisationDigestive systemLignanSolvent
The present invention pertains to the art of medical technology and discloses a novel raw material for Schisandraceae lignans extracted substance and a preparation method thereof. The process takes Chinese medicine Schisandraceae stem (fruit stalk) and Schisandraceae leave as the raw material and adopts one or a plurality of solvent extraction method, resin absorption method, positive-phase column chromatography, reverse-phase column chromatography, etc. after grinding, so as to prepare the extracted substance with 30 to 95 percent of total lignans content. The preparation method has simple process, stable content and good repeatability. The extracted substance can be used as medicine or health food instead of Schisandraceae lignans extracted substance, which makes full use of Schisandraceae resource and releases the short supply of Schisandraceae fruit.
Owner:SHENYANG PHARMA UNIVERSITY
Process of producing sesame lignans and/or sesame flavors
InactiveUS6278005B1Great advantageFatty substance preservation using additivesEssential-oils/perfumesSesamumLignan
A process of treating sesame oil to obtain sesame lignans and flavors contained in the oil in higher purity and yield is provided. In the process, the sesame oil is subjected to a supercritical extraction.
Owner:FUJIMIYOHOEN
Use of lignan compounds for treating or preventing inflammatory disease
ActiveCN101102761AAntibacterial agentsOrganic active ingredientsInflammation mediatorsNutmeg extract
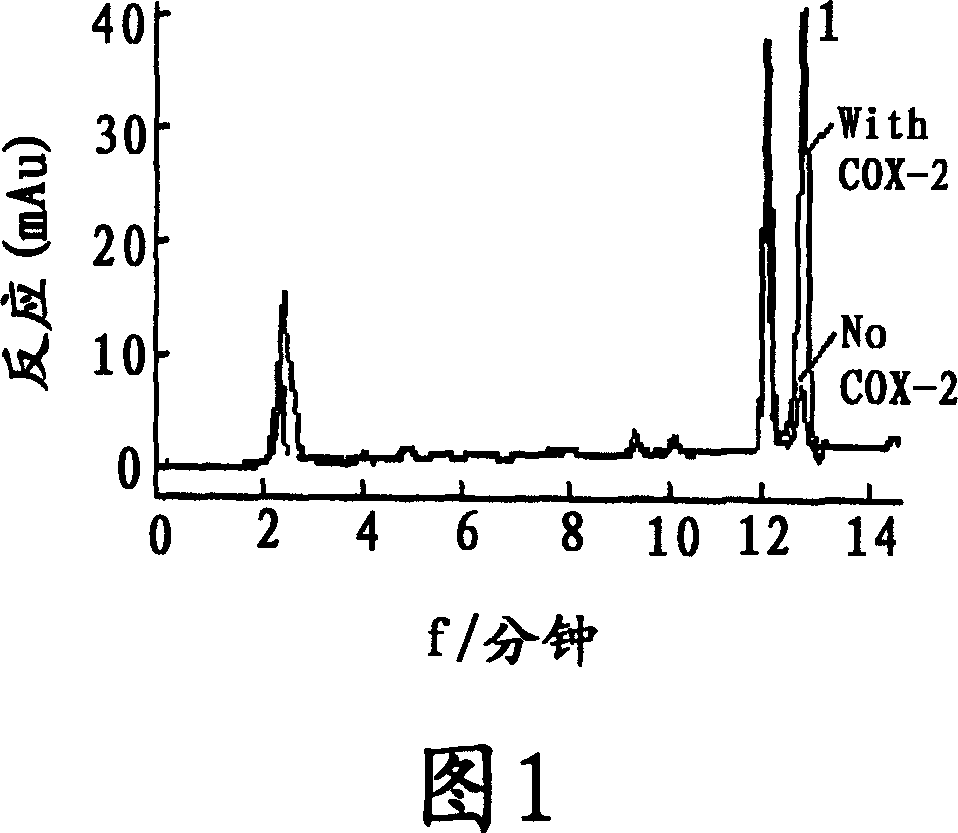
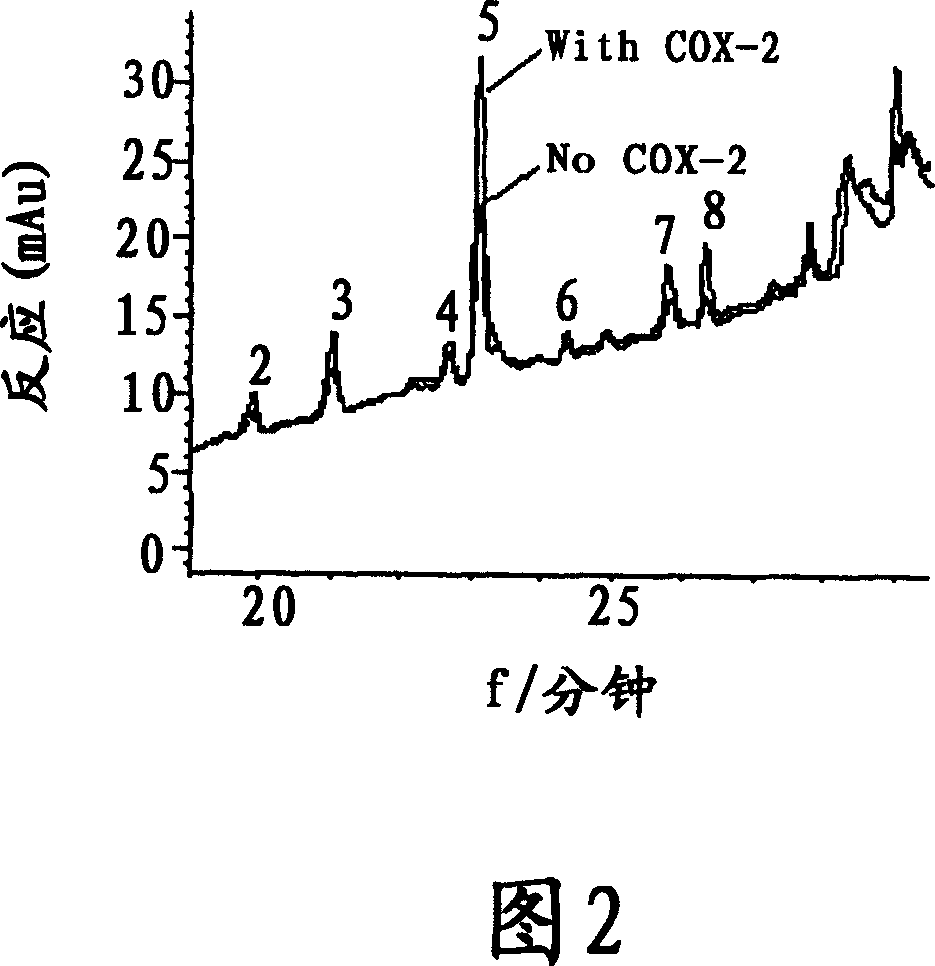
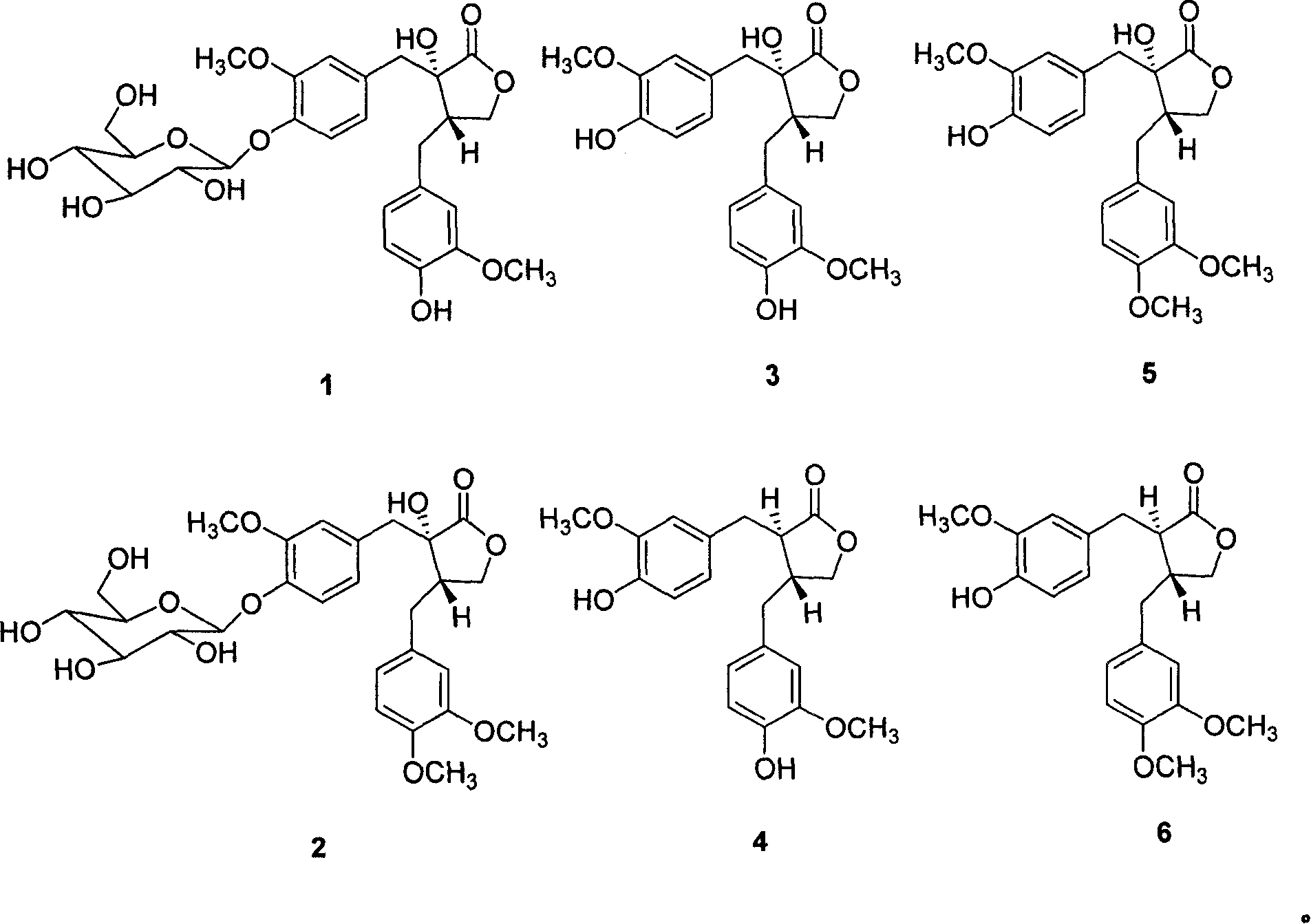
The present invention relates to the use of lignan compounds for treating or preventing an inflammatory disease. More particularly, it relates to a pharmaceutical composition for the treatment or prevention of an inflammatory disease, comprising a lignan compound represented by Formula I, as well as a treating method and the use of an inflammatory disease using the lignan compound. The lignan compound has the effect of inhibiting inflammatory reactions by inhibiting the production or expression of inflammation mediators NO, iNOS, PGE2, COX-2 and TNF-a. Accordingly, the lignan compound or a Myristica fragrans extract will be highly useful for the treatment or prevention of an inflammatory disease.
Owner:AAT 考斯泰克有限公司 +1
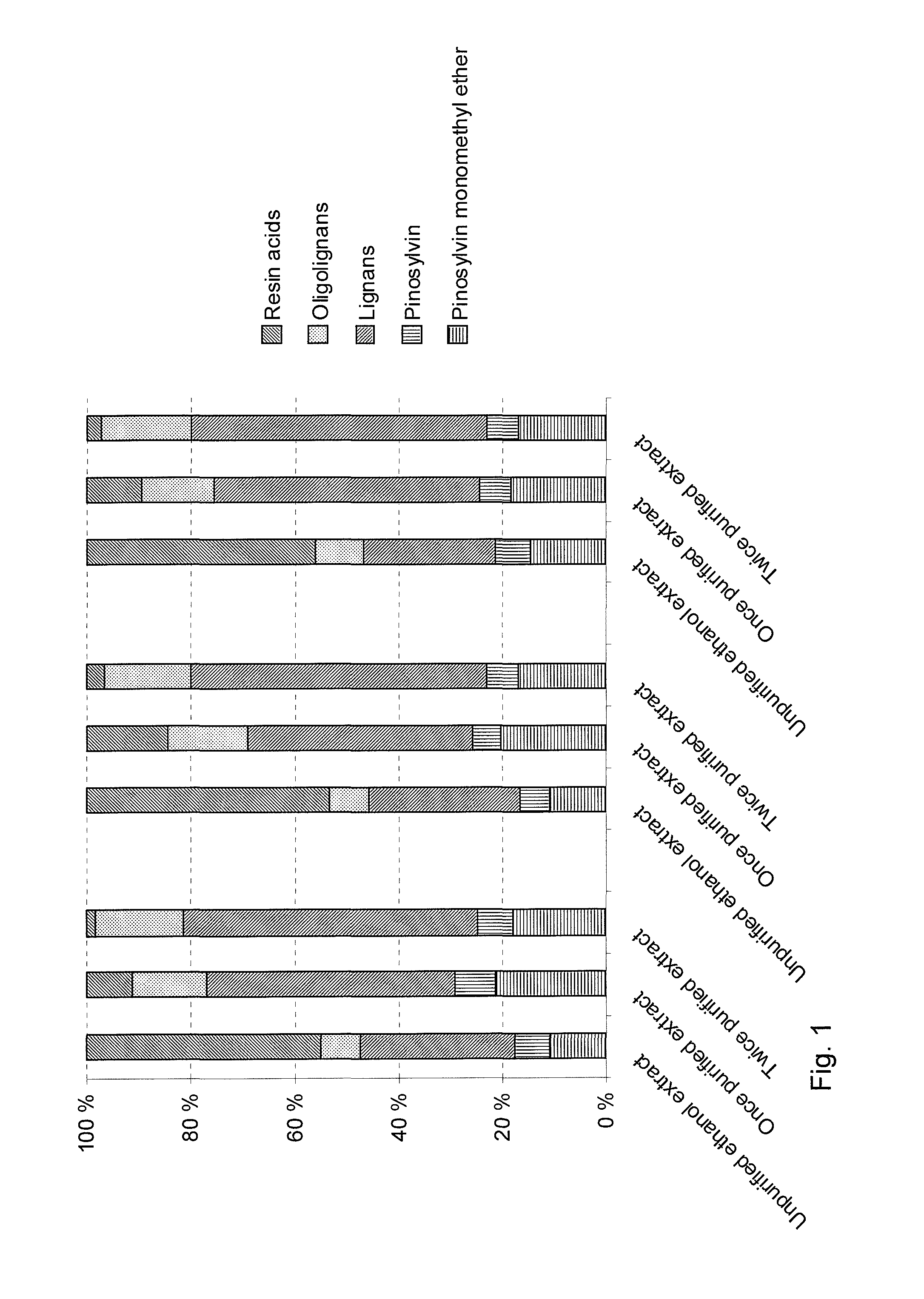
Method for the fractionation of knotwood extract and use of a liquid-liquid extraction for purification of knotwood extract
The invention relates to a method for the fractionation of knotwood extract, which has been obtained by extraction of knotwood with a hydrophilic solvent. The hydrophilic extract is extracted with a lipophilic solvent to remove lipophilic impurities. The invention also relates to the use of a liquid-liquid extraction for the purification of hydrophilic knotwood extract. The present process provides a purified knotwood extract, which contains more than 90% lignans, flavonoids and stilbenes and less than 10% impurities selected from resin acids, fatty acids, sterols, juvabiones, triglycerides and combinations thereof.
Owner:UPM-KYMMENE OYJ
Caulis trachelospermi total lignans extractive, extraction method and medicine use of the extractive and active constituent thereof
InactiveCN1919856AOrganic active ingredientsOrganic compounds purification/separation/stabilisationDiseaseLignan
The invention discloses a total lignans from caulis trachelospermi and extracting method, which is characterized by the following: possessing 30-90% lignans in the total lignans; fitting for preparing medicine to treat inflammation and or pain; making the lignan as inhibitor of epoxidised enzyme.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
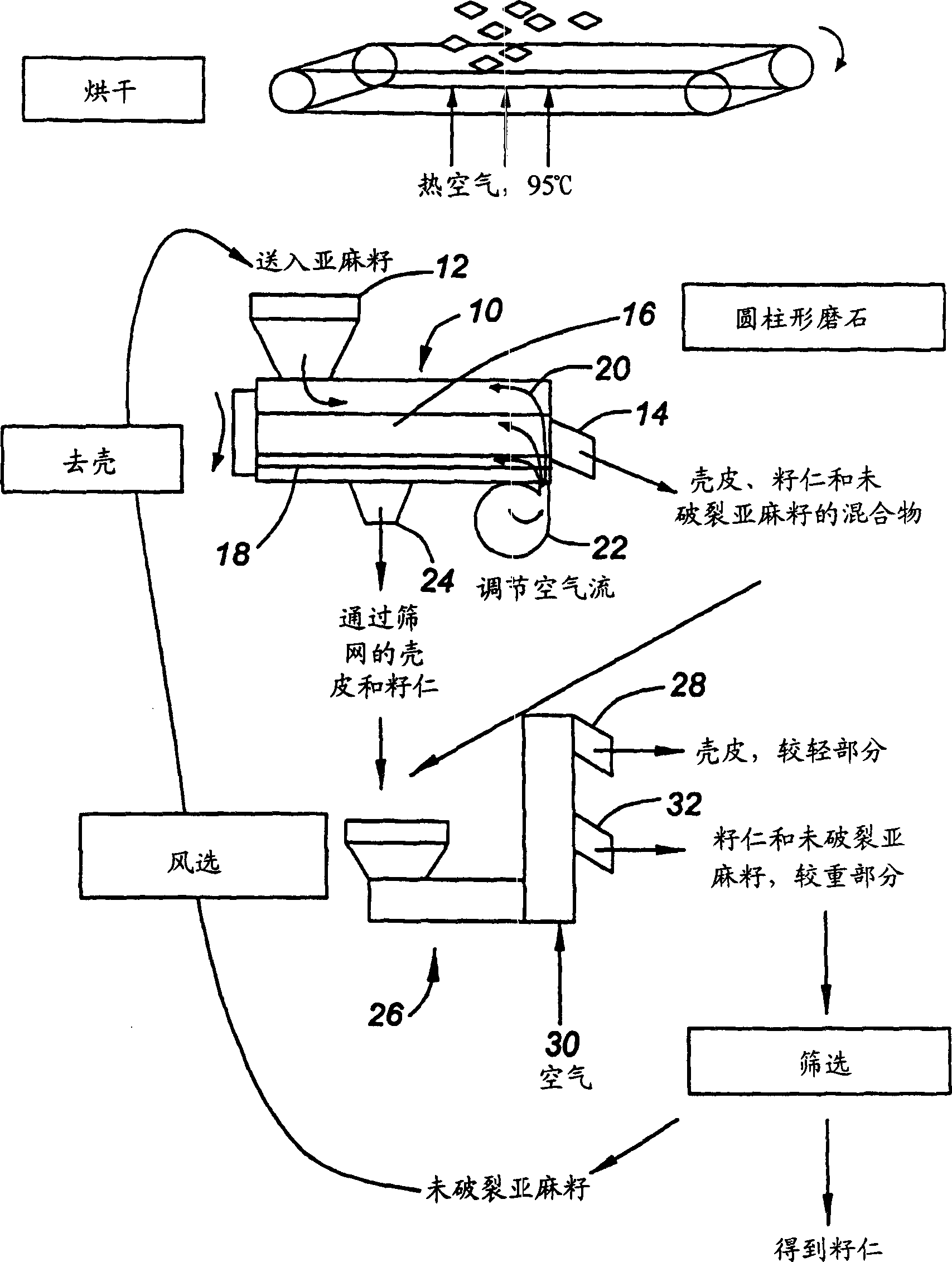
Process and apparatus for flaxseed component separation
InactiveCN1604819AEliminate or reduce adverse factorsGrain huskingGrain polishingLignanComponents of crude oil
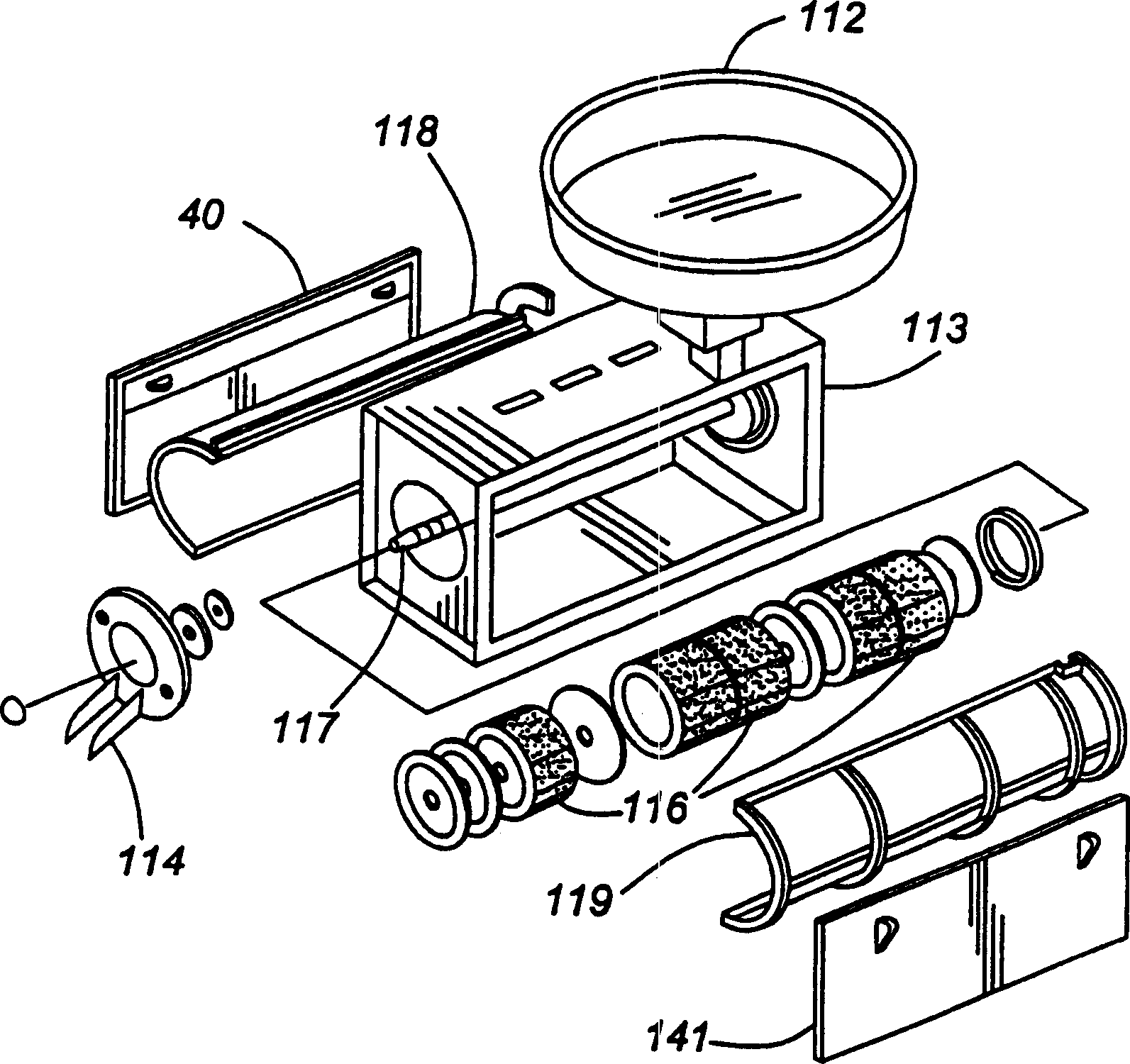
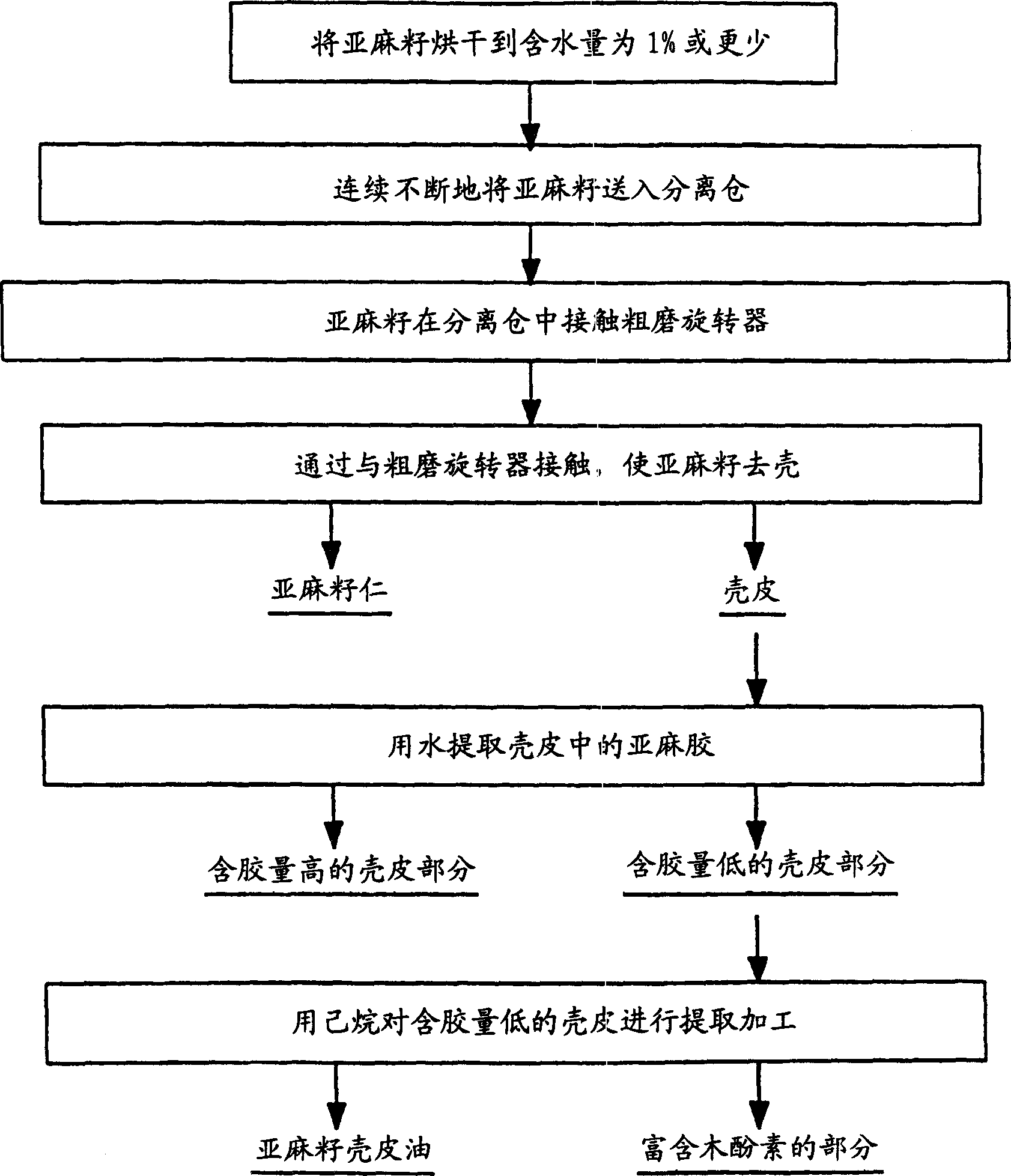
A continuous process for separating components of flaxseed is described. Flaxseed is dried to a moisture content of from about 0.5% to about 3.0%, after which is introduced into a separation chamber having an abrasive rotator therein. As the flaxseed passes over the abrasive rotator, contact with rotator separates the flaxseed components into hulls and kernels. Hulls and kernels are separated and may be used in this form or processed further. Hulls may be further processed by extraction with water and with hexane to remove flaxseed gum and oil, respectively. These two extractions may be done in any order. The process results in a lignan-rich component of flaxseed, a gum extract and oil. These separate components of flaxseed may be used in products such as feed, personal care products or nutraceuticals.
Owner:AGRI & AGRI FOOD
Nutraceutical fractions from cereal grains
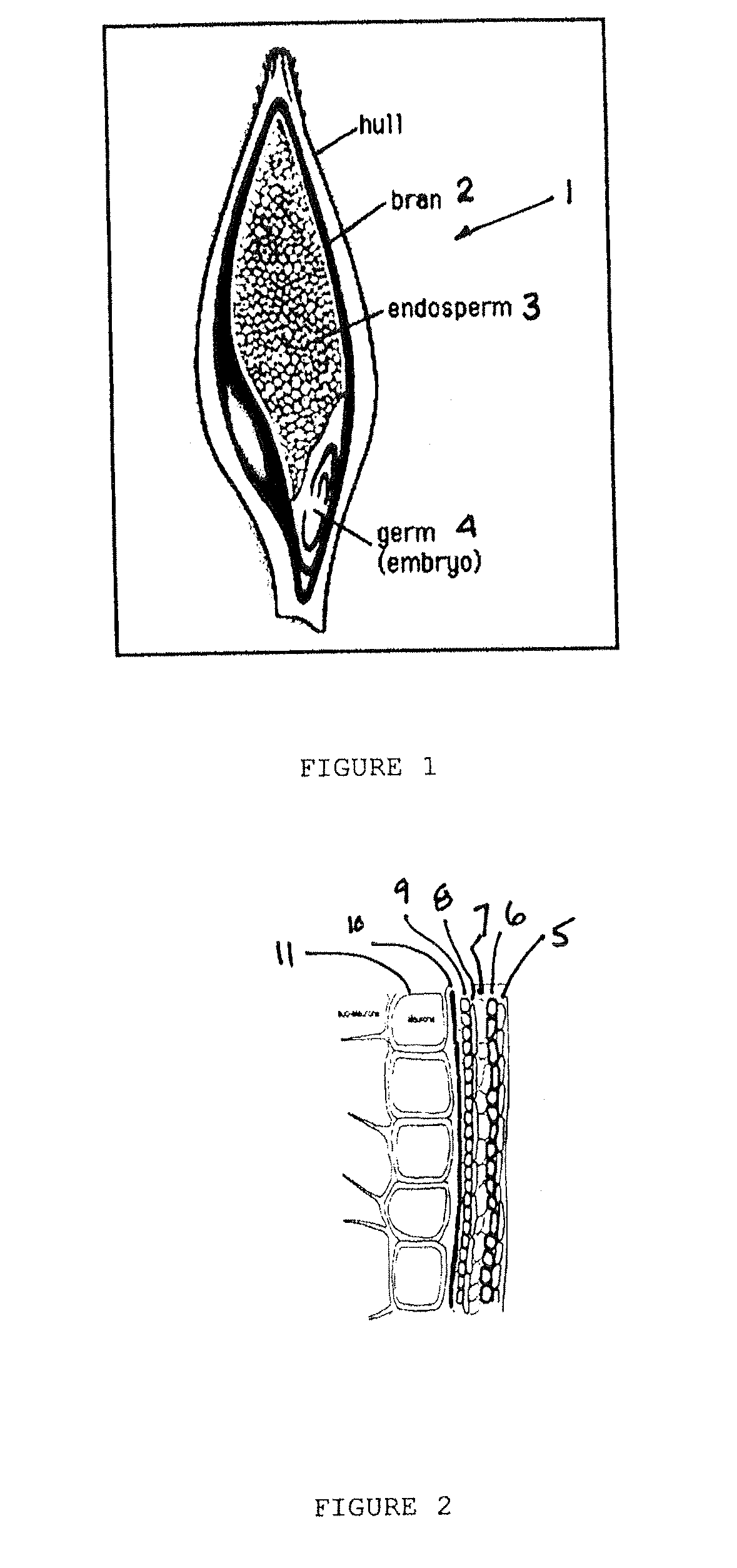
InactiveUS20090169683A1Highly solubleReduction of serum cholesterolGrain huskingGrain polishingFiberDietary supplement
The present invention is directed to isolating the valuable components of cereal grains and allowing the benefits to be more fully exploited. The concept of the present invention initially involves selection of cultivars of cereal grains such as wheat, barley, oats and rye having desired bioactive components including antioxidants, complex phenolics, lignans, flavonoids, vitamins, fiber, protein and other nutrients concentrated in one or more of the outer bran layers. Then separating the outer bran layer into three fractions, according to the desired bioactive components contained in the bran layers including antioxidants, complex phenolics, lignans, flavonoids, vitamins, fiber, protein and other nutrients. This allows the maximum benefit and value to be obtained from the bran fractions as dietary supplements, nutraceuticals, or as enriched food products.
Owner:1289620 ONTARIO
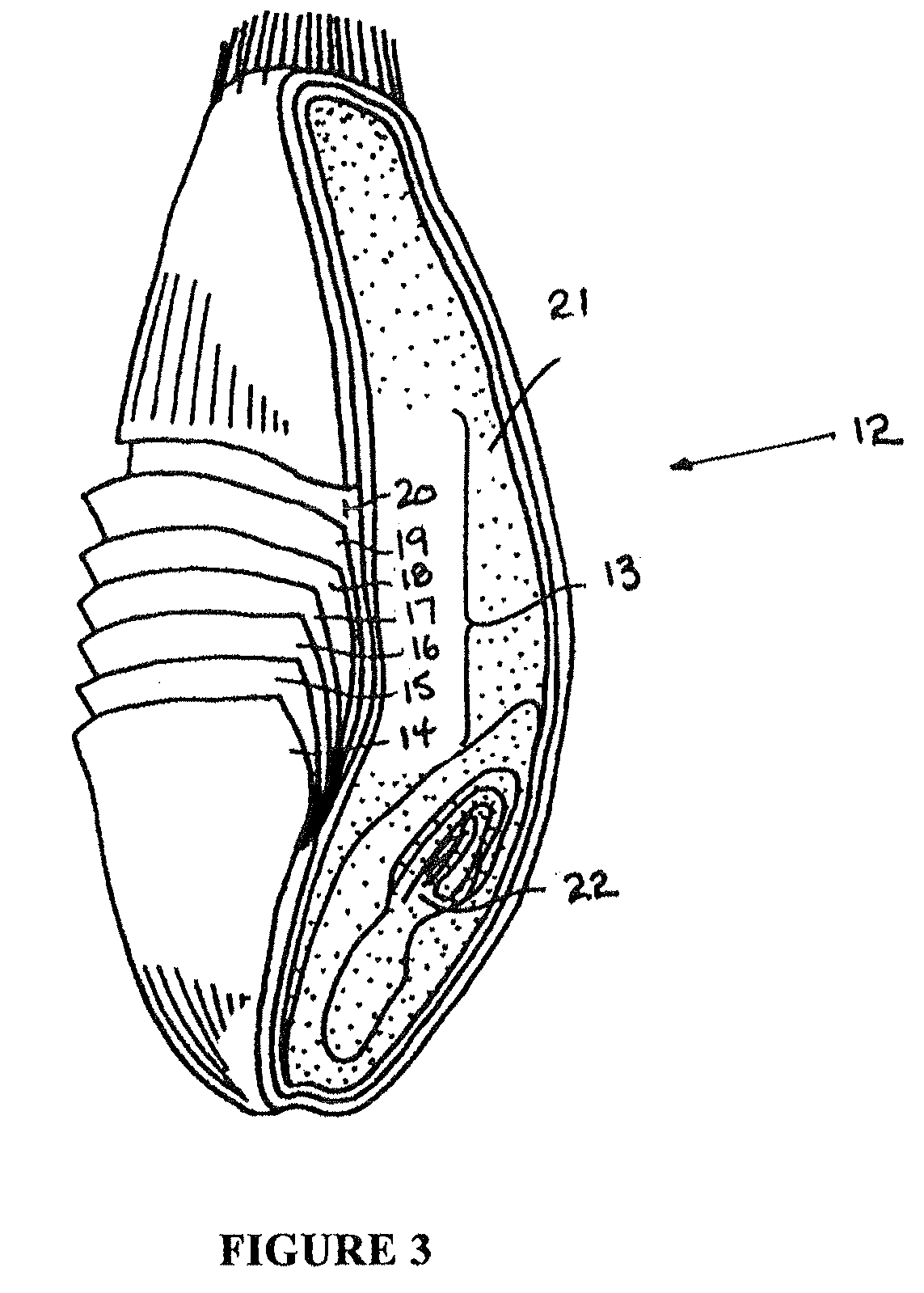
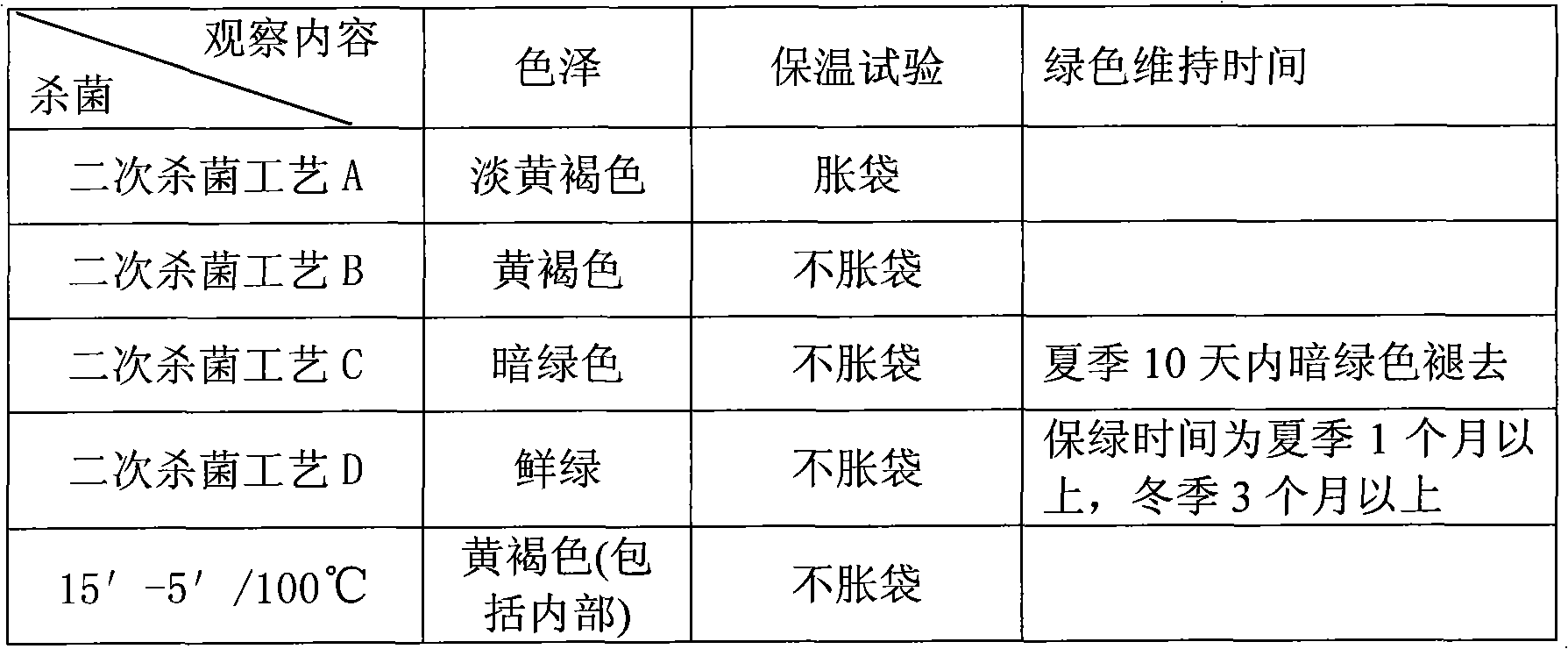
Method for frozen-storing and fresh-keeping cedrela sinensis at low temperature and method for flexibly packing cedrela sinensis
InactiveCN101779702AGood color and qualityObvious browningFruits/vegetable preservation by heatingFruits/vegetable preservation by freezing/coolingFrozen storageAdditive ingredient
The invention relates to a method for frozen-storing and fresh-keeping cedrela sinensis at a low temperature and a method for flexibly packing the cedrela sinensis. The method for frozen-storing and fresh-keeping the cedrela sinensis at the low temperature comprises the steps of the clearing up, cleaning, blanching, hardening, quick freezing, packing and frozen-storage of raw materials; and the method for flexibly packing the thecedrela sinensis comprises the steps of pretreatment, cutting up, mixing, packing and sterilizing. Through comprehensive research on the physiological and breath change and sensitivity to temperature of the cedrela sinensis in a fresh-keeping process and a color change mechanism and the like of the cedrela sinensis in machining, key industrial generic technology, such as secure color protection, color preservation and the like, at the low temperature in the machining is researched and developed and the problem of bottleneck in the fresh-keeping machining of the cedrela sinensis is solved. In the method, sesame lignan and tea polyphenol with inoxidizability and a bacteriostatic action are reasonably added into ingredients in the process so as to achieve relatively better effect on the color preservation and quality preservation of the cedrela sinensis; and after being packed and sealed, the cedrela sinensis is sterilized twice (at two different temperatures and for two different sterilization time periods) and the flexibly packed cedrela sinensis of the invention is greenish.
Owner:INST AGRO PROD PROCESSING ANHUI ACADEMY AGRI SCI
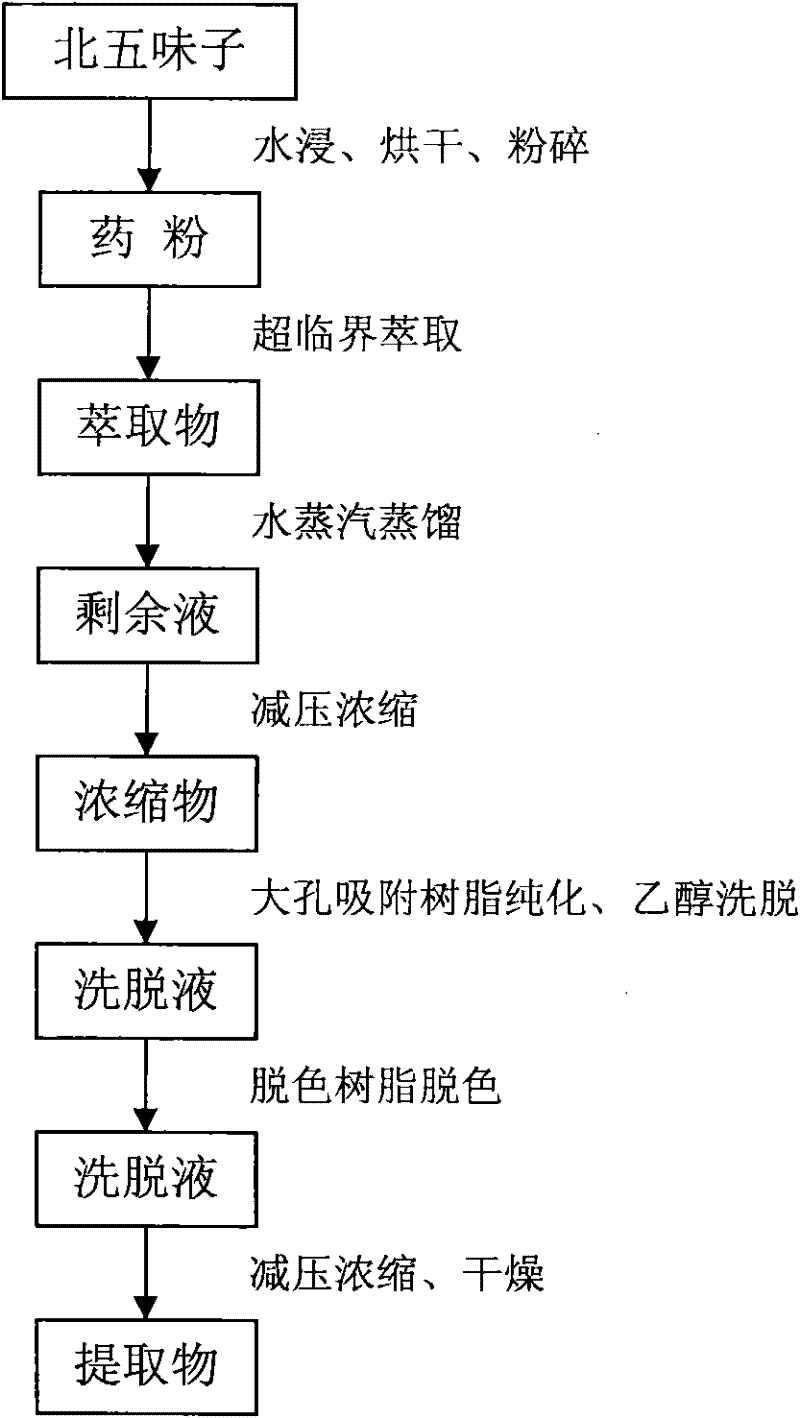
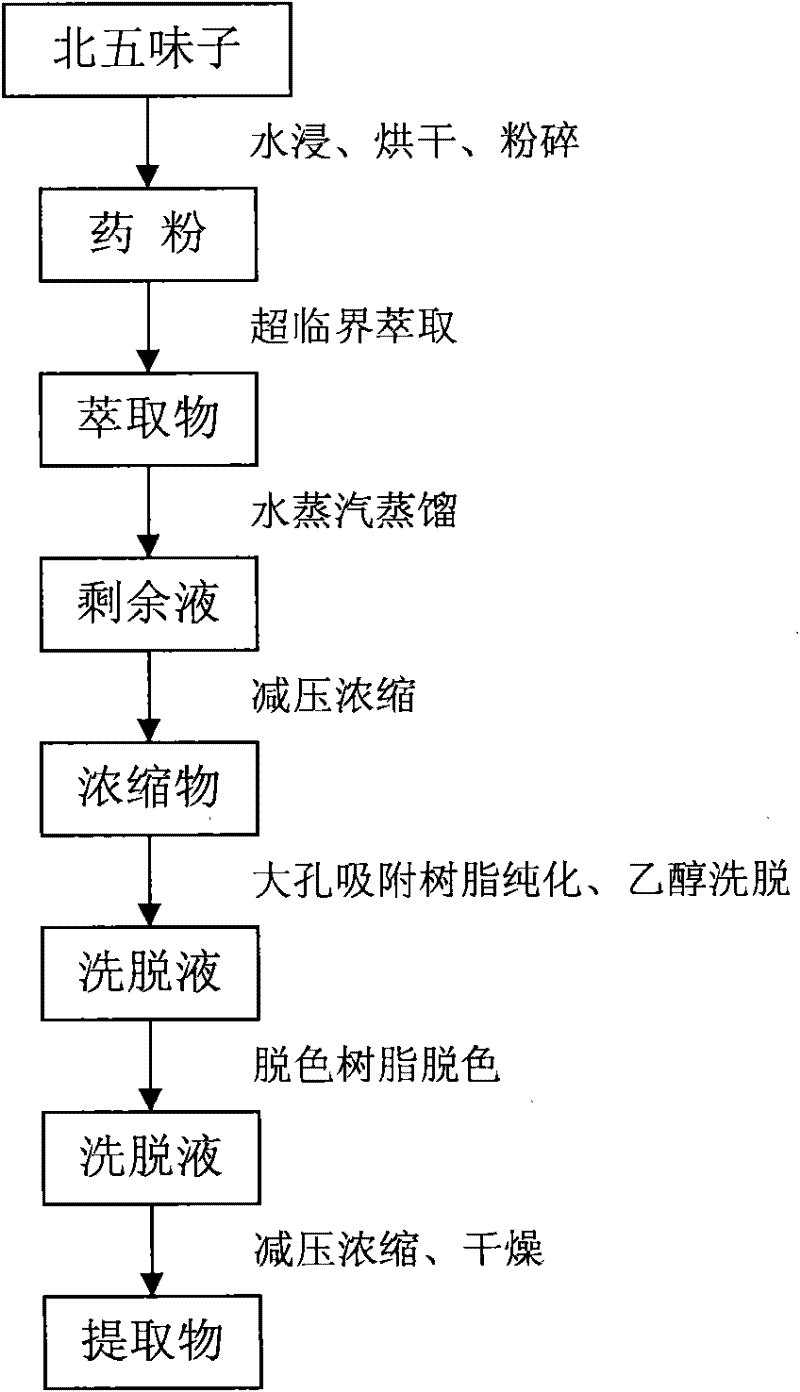
Method for extracting high-purity schisandra total lignan
The invention discloses a method for extracting high-purity schisandra total lignan. The method comprises the following steps: (1) soaking schisandra fruits in water, filtering the soaked schisandra fruits, drying and grinding filter residue for later use; (2) extracting the ground filter residue by a supercritical CO2 method, distilling the obtained extract with steam, concentrating the obtained product under reduced pressure and obtaining concentrate for later use; and (3) purifying the concentrate. The extraction method has the advantages of simple extraction process, high purity of extracted total lignan, thorough impurity removal and the like, and can extract the extract with the total lignan content of over 50 percent from the schisandra fruits.
Owner:HARBIN RENHUANG PHARMA
Process for comprehensively utilizing flax seed skin
InactiveCN103013672AIncrease added valueOvercome the hard tasteFatty oils/acids recovery from wasteSugar derivativesDietary fiberLignan
The invention relates to a process for comprehensively utilizing flax seed skin. The process comprises the following steps of: taking the flax seed skin as a raw material, degreasing the flax seed skin by solvent-extracted oil No.6, filtering, and drying flax seed skin powder; recovering the solvent-extracted oil No.6 from a filtrate to obtain flax oil; immersing the flax seed skin powder to water for degumming and then carrying out solid-liquid separation to obtain glue liquor and a solid matter such as flax dietary fiber powder; adding the glue liquor to ethanol, stirring for precipitation separation, drying and smashing the solid matter to obtain flax glue; carrying out alkaline hydrolysis on supernate, adding hydrochloric acid for neutralization, concentrating the mixture to dryness, adding the ethanol for filtering, and recovering ethanol solution to obtain a flax lignan crude product; adding the ethanol to the solid matter of the flax dietary fiber powder for standing, carrying out solid-liquid separation, air-drying the solid matter such as flax dietary fiber, and recycling the ethanol from the solution; and superfinely grinding the air-dried flax dietary fiber to obtain the superfine flax dietary fiber powder. By utilizing the process, four products can be obtained by one process, the flax seed skin is comprehensively developed and utilized furthest, and the additional value of the flax seed skin is enhanced.
Owner:RES INST OF AGRO PROD PROCESSING SHANXI ACADEMY OF AGRI SCI
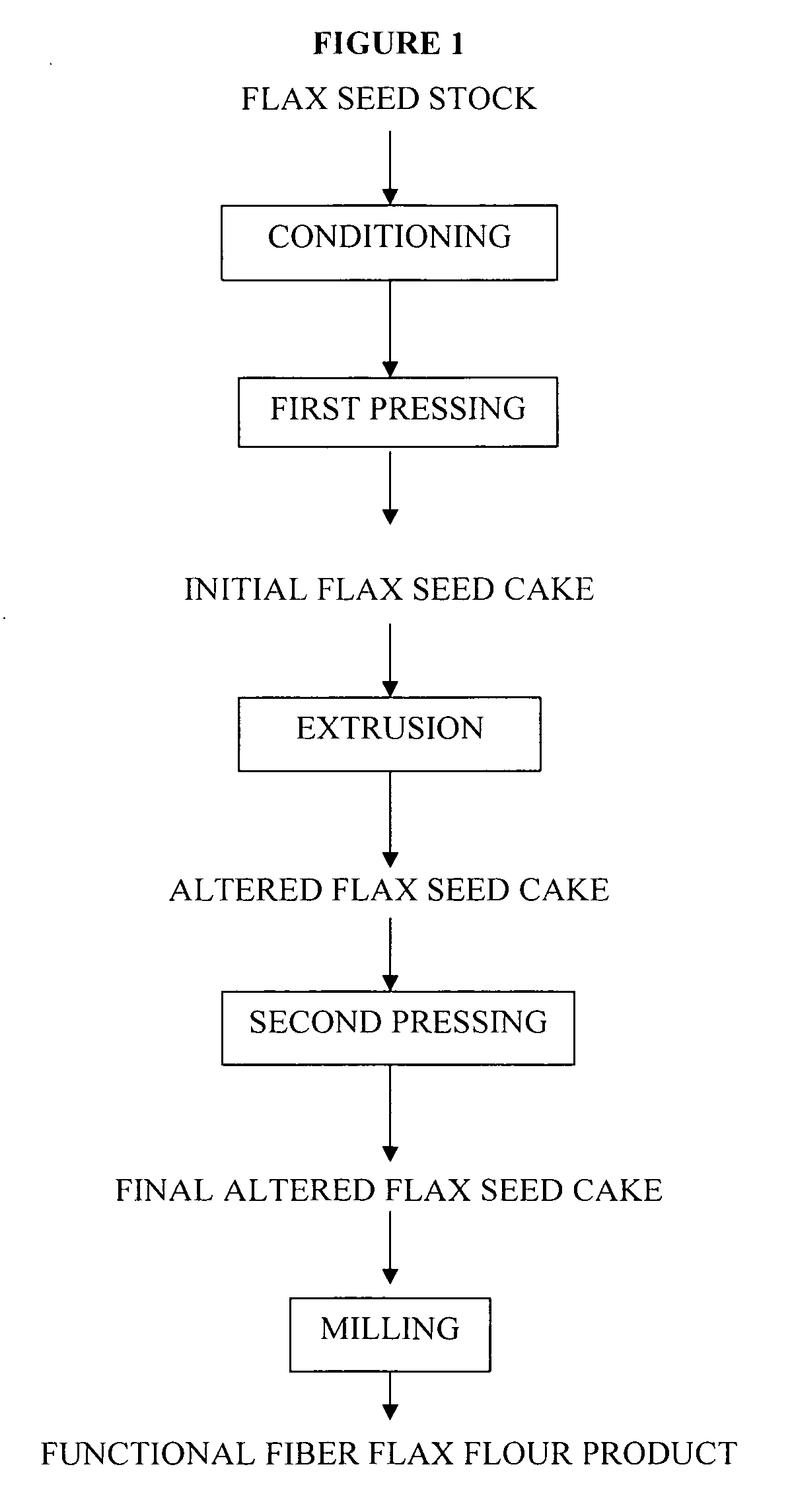
Functional fiber flour product and method for making same
InactiveUS20050249860A1Light colorReduce odorDough treatmentFood ingredientsCooking & bakingDietary supplement
The present invention relates to a functional fiber flour product for use in foods, beverages, nutritional products and dietary supplements. The invention includes a functional fiber flour product made from oilseeds and comprises soluble and insoluble dietary fibers, polyunsaturated fatty acids, monounsaturated fatty acids, protein, lignans, and low amounts of digestible carbohydrates and saturated fat. Properties of the present invention are useful in enhancing mixing, sheeting, extrusion, baking, frying and roasting characteristics of human food and beverage products and animal feed products without adversely affecting palatability or appearance attributes; properties also include considerable extended shelf life compared to prior art functional fiber products. The present invention also includes a process for making the functional fiber flour product using high pressure and high temperature mixing and extrusion equipment.
Owner:KONECSNI JEROME +4
Use of erberry and its lignanoids compound in drug for osteoporosis
InactiveCN1634025AEffective treatmentOrganic active ingredientsSkeletal disorderLignanAnti osteoporosis
The invention relates to the use of Sambucus Williamsii Hance as osteoporosis resisting medicament, and the use of lignan compounds and phenolic acid compounds as osteoporosis resisting medicament, wherein the lignan compounds and phenolic acid compounds are prepared through the steps of ethanol heating backflow of Sambucus Williamsii Hance stems and branches, extracting ethanol extract, suspending in water and extracting with chloroform, acetic acid ethyl ester, and n-butyl alcohol, disintegrating with chemical means. The invention shows no side action of estrogen samples.
Owner:CHINESE MEDICINE & NATURAL MEDICINE RES CENT SHENZHEN
Use of 8-0-4'type lignan in preparing anticomplement medicament
InactiveCN101347418AHigh activitySignificant anticomplement effectEther/acetal active ingredientsAldehyde active ingredientsHemolysisChinese traditional
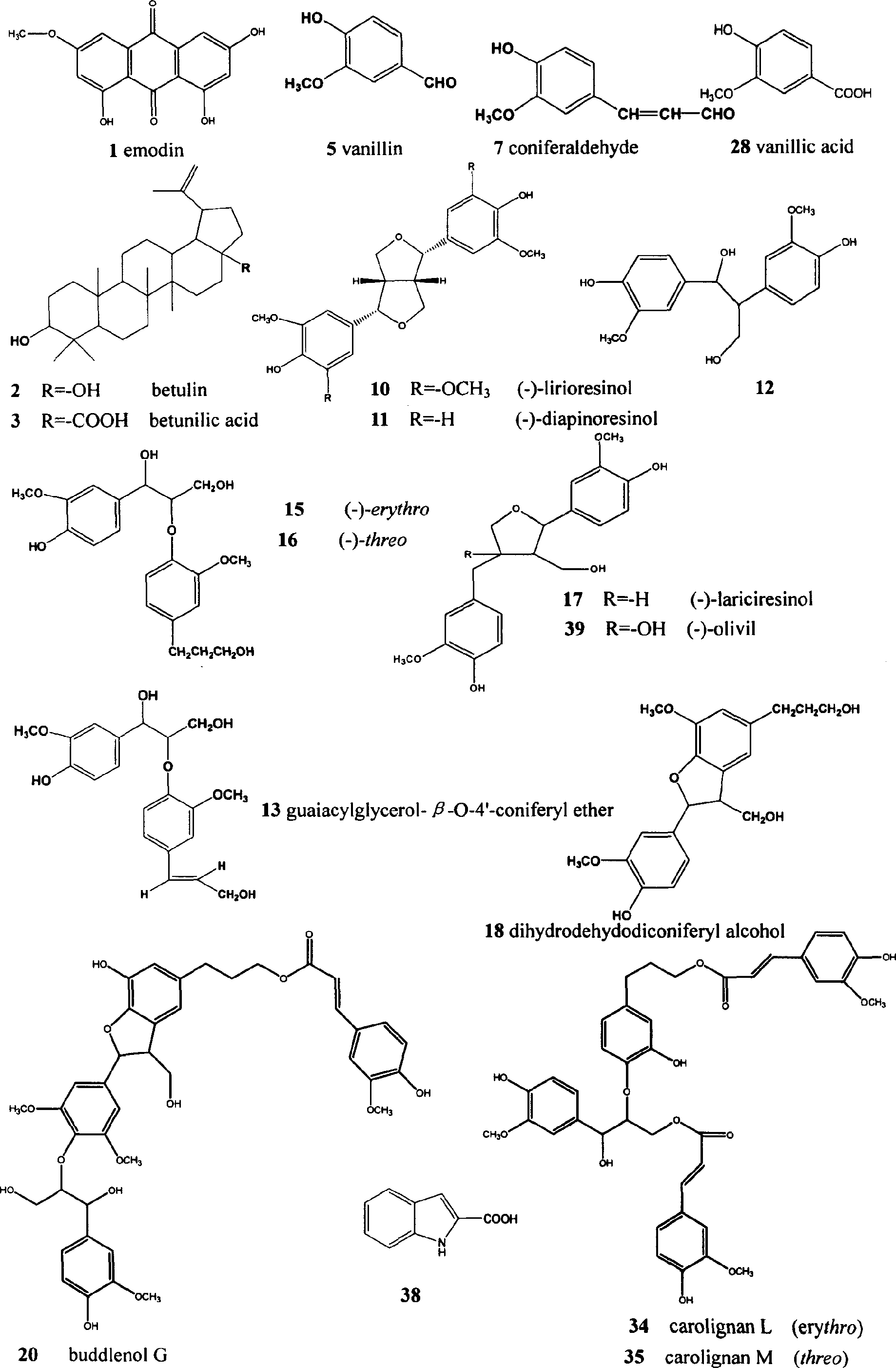
The invention belongs to the field of Chinese traditional medicines and relates to a new use of 8-0-4' lignan in preparing an anti-complement medicine. The compound 8-0-4' lignan is extracted from a Chinese medicinal herb eucommia bark, and in vitro experiments prove that the compound 8-0-4' lignan can inhibit cell hemolysis caused by the activations of classical and alternative pathways of a complement system, and has obvious inhibitory action on the activation of the classical and alternative pathways of the complement system; and the results of pharmacological tests prove that the compound 8-0-4' lignan has obvious anti-complement effect and low effective concentration. The compound 8-0-4' lignan of the invention can be further taken as an active ingredient for preparing a novel anti-complement medicine.
Owner:FUDAN UNIV
Comprehensive utilization method of peony shells
ActiveCN103042023ARealize comprehensive utilizationSugar derivativesCarbon compoundsActivated carbonLignan
The invention belongs to the technical field of application of by-products obtained after peony seed shelling, in particular to a comprehensive utilization method of peony shells. The method comprises the following steps: pretreating peony shells, and then extracting peony oil from the peony shells by a supercritical carbon dioxide extraction method, or extracting black pigment and lignan from the peony shells, or preparing the peony shells into activated carbon. The method provided by the invention has the beneficial effects that by adopting the supercritical carbon dioxide extraction method to extract the peony oil from the peony shells, the original discarded peony shells are converted into the peony oil of nutritional value or black pigment and lignan, or prepared into activated carbon, so that the waste is changed into valuable, the economic value is created, and the comprehensive utilization of the peony shells is realized.
Owner:HEZE RUIPU PENOY IND TECH DEV
Sesame oil and process for producing the same
InactiveUS8084071B2Bitterness can be substantially removedReduce contentFatty acid chemical modificationFatty-oils/fats refiningActivated carbonBiotechnology
The purpose of the present invention is to provide refined sesame oil comprising a lot of lignans that have various excellent physiological properties from sesame seeds having a high lignan content.The present invention relates to refined sesame oil having a sesamin content of 1% by weight or more and showing no bitterness; a method for the production of refined sesame oil comprising using active carbon as an absorbent in a bleaching step; a method for the production of refined sesame oil comprising controlling a bleaching temperature in a range of from 5 to 70° C. in a bleaching step with the use of activated clay active as an absorbent; and a method for the production of refined sesame oil comprising controlling a bleaching temperature in a range of from 5 to 70° C. in a bleaching step with the use of activated clay of from 0.1 to 3% by weight active as an absorbent.
Owner:J OIL MILLS INC
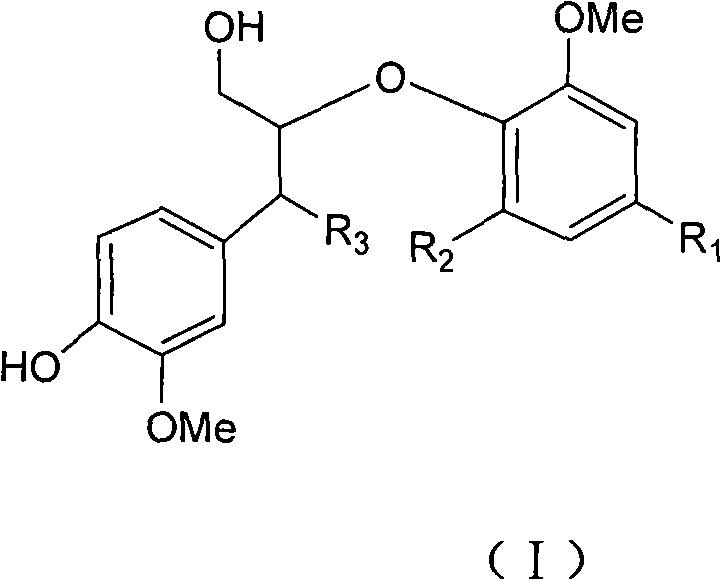
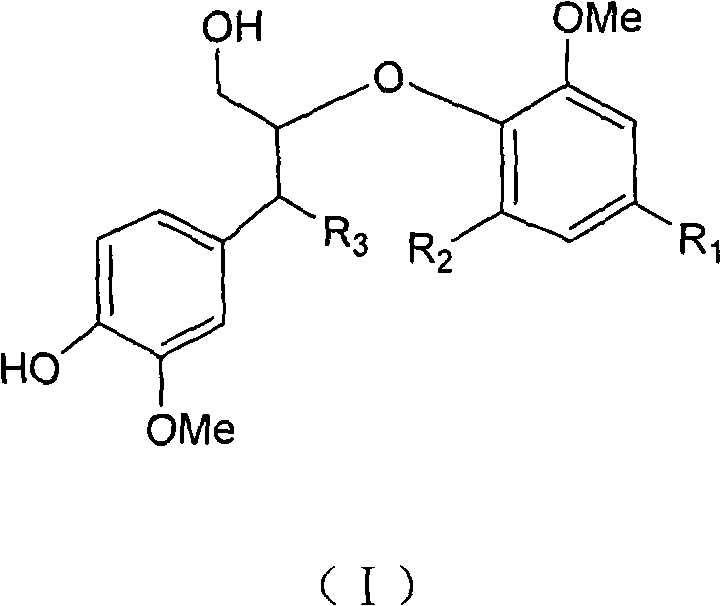
Use of a Lignan for the Manufacture of a Composition for Preventing or Alleviating of Symptoms Relating to Estrogen Deficiency
InactiveUS20080057140A1Preventing and alleviating of symptomImprove the level ofBiocideNervous disorderEnterolactoneMetabolite
The use of a lignan, which is a plant lignan, a metabolite thereof or a combination of both, for the manufacture of a composition for preventing or alleviating of symptoms relating to estrogen deficiency in an individual. Also disclosed is the use of a plant lignan capable of being a precursor for enterolactone or another metabolite of the plant lignan, for the manufacture of a composition useful for increasing the level of enterolactone or another metabolite of a plant lignan in an individual's serum, where the individual suffers from or is at risk of estrogen deficiency.
Owner:HORMOS MEDICAL OY LTD
Pedunculate herpetospermum seed extract, its dripping pill and their preparing method and application
InactiveCN1857367AStable extraction processQuality is easy to controlDigestive systemAntiviralsMedicineLignan
The present invention belongs to the field of Chinese medicine preparation technology, and is especially pedunculate herpetospermum seed extract and its dripping pill and their preparation process and applying technology. By means of pharmacodynamic research, the effective part, total pedunculate herpetospermum lignan, and the effective dosage are determined. Through modern technological process, total pedunculate herpetospermum lignan is extracted and further prepared into the total pedunculate herpetospermum lignan dripping pill used for treating viral hepatitis and tumor.
Owner:钱毓洲
Yew genus plant extract and its extraction method and application
ActiveCN1594247ASolve the problem of sustainable useIncrease profitOrganic active ingredientsOrganic compounds purification/separation/stabilisationLignanCurative effect
The invention relates to the Yew genus plant extract and its extraction method and application, wherein the extract is extracted from the stems and leaves of Northeastern yew and comprises taxone diterpenoid, total lignans, total flavone. The extract can be used as the effective composition for anti-cancer drugs.
Owner:HARBIN HONGDOUSHAN TECH DEV
Fructus schizandrae chemical composition group extract and preparation technique
ActiveCN101181354ALow toxicitySuitable for industrial productionPowder deliveryNervous disorderOrganic acidAdditive ingredient
The invention belongs to the technical field of medicine and discloses three extracts of chemical compound groups of Chinese magnolia vine and a preparation technology thereof. The invention is characterized by drug ingredients saving and less toxicity. The three extracts are respectively crude amylose extract, total organic acid extract and total Lignans extract, wherein, the weight content of the crude amylose extract is between 50 and 95 percent, the weight content of the total organic acid is between 50 and 70 percent and the weight content of the total Lignans content is at least 50 percent. The Chinese magnolia vine is crushed, screened, distilled, filtered, deposited, absorbed and eluted, and then the eluted liquid is gathered and concentrated, thus, the extract is obtained. The preparation method has simple technology and the content is stable.
Owner:SHENYANG PHARMA UNIVERSITY
Process for extracting schizandrin A from schizandra
The invention relates to a process for extracting schizandrin A from schizandra, which is characterized by preparing total lignan extracts through ultrasonic-assisted petroleum ether extraction and alkali-alcohol back extraction, carrying out mixed column chromatography on silica gel and alumina twice, eluting silica gel and alumina and recovering the solvent, and recrystallizing and drying petroleum ether-acetone or cyclohexane-ethanol, thus obtaining the schizandrin A. The process has high specificity.
Owner:NANJING ZELANG MEDICAL TECH
A method for extracting flax lignans from flaxseed cake
InactiveCN102276665AEasy to operateHigh yieldSugar derivativesSugar derivatives preparationOrganic solventLignan
The invention discloses a method for extracting linseed lignans from linseed cakes, which comprises the following steps: subcritical water extraction is performed on the linseed cakes obtained after the linseeds are pretreated to remove impurities, and the extraction conditions are as follows: The temperature is 40-200° C.; the extraction pressure is 5-15 MPa, and the extraction time is 0.1-6 hours to obtain an extract; the extract is purified with a macroporous resin, and then spray-dried to produce flax lignans. The invention uses subcritical water for extraction, which has the advantages of simple equipment, easy operation, rapidity, high yield, and cleanness. By changing the extraction temperature, the polarity of the water can be changed, so that different polarities in the sample matrix can be selectively extracted. Organic compounds, and it is extracted from pure water, without or rarely using organic solvents, so it has little or no pollution to the environment.
Owner:晨光生物科技集团天津有限公司
High lignan flaxseed product and product by process
ActiveUS7048960B2Elevated lignanImproved higher lignanFood preservationAnimal feeding stuffLignanSoluble dietary fiber
The present invention includes a high lignan concentrate (3% to 5% or greater) flax seed product, and product by process, which can be produced by 1) supplying a uniformly colored quantity of flax seeds with less than 5 percent visually darker seeds; 2) milling the selected seeds of a visually uniform color; 3) sifting the milled selected seeds into a second portion and a third portion using a preferred screen size from US# 12 up to an including US #18; 4) selecting the coarser fraction of the sifted, milled flax meal and aspirating it into a fourth lighter density portion and a fifth coarser portion, wherein the fourth lighter portion contains the valuable high lignan concentrate. As an added advantage, the high lignan flax seed meal can include approximately 40–50% insoluble dietary fiber and 50–60% soluble dietary fiber.
Owner:GLANBIA NUTRITIONALS IRELAND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com