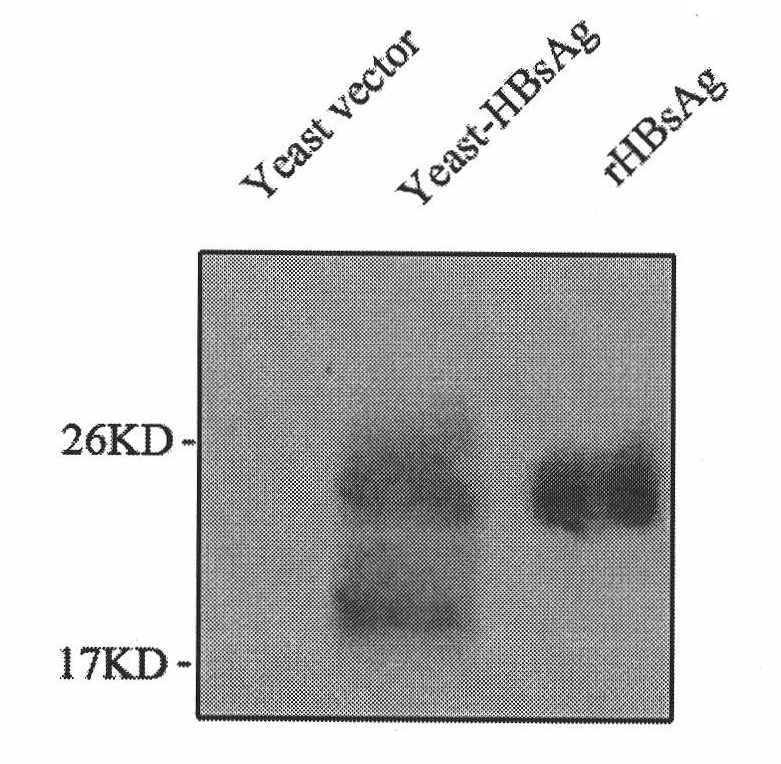
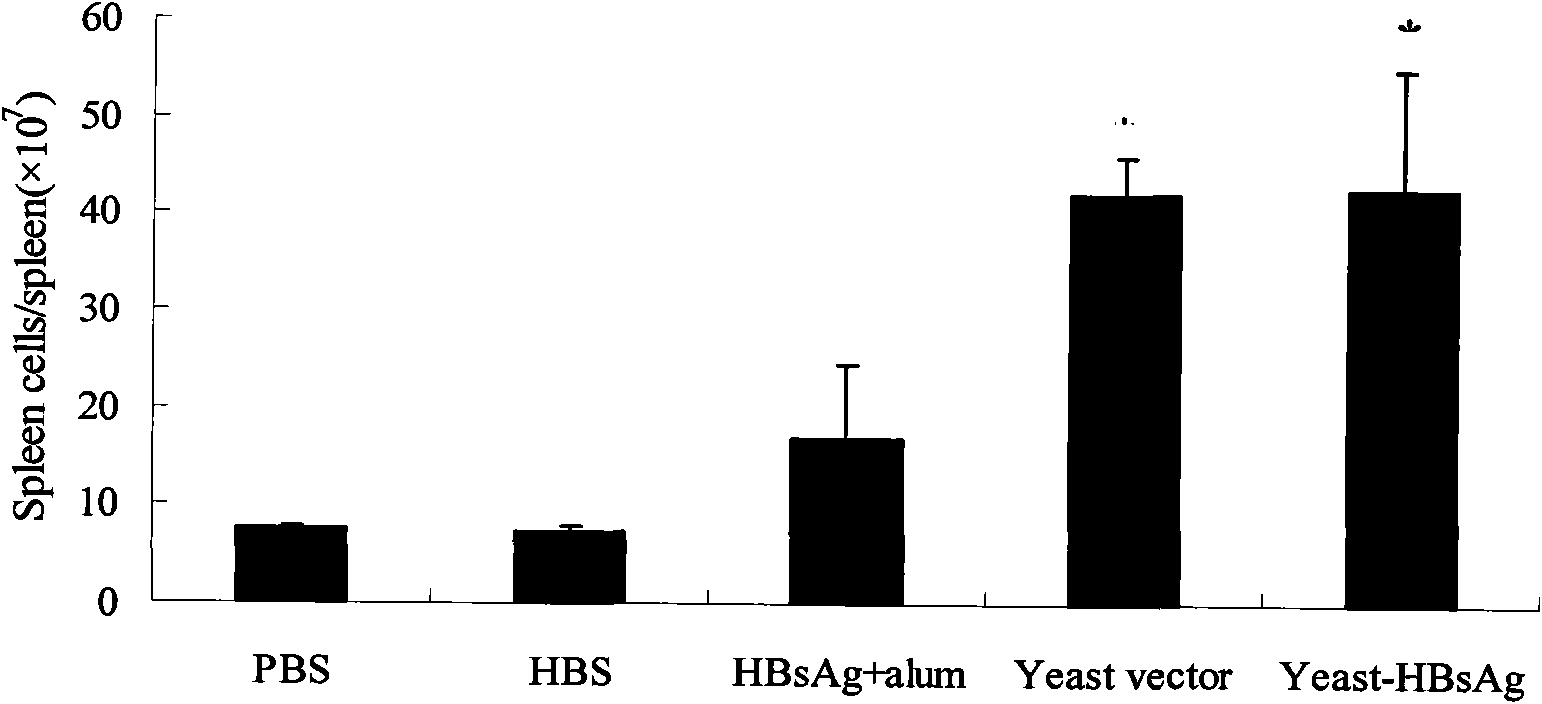
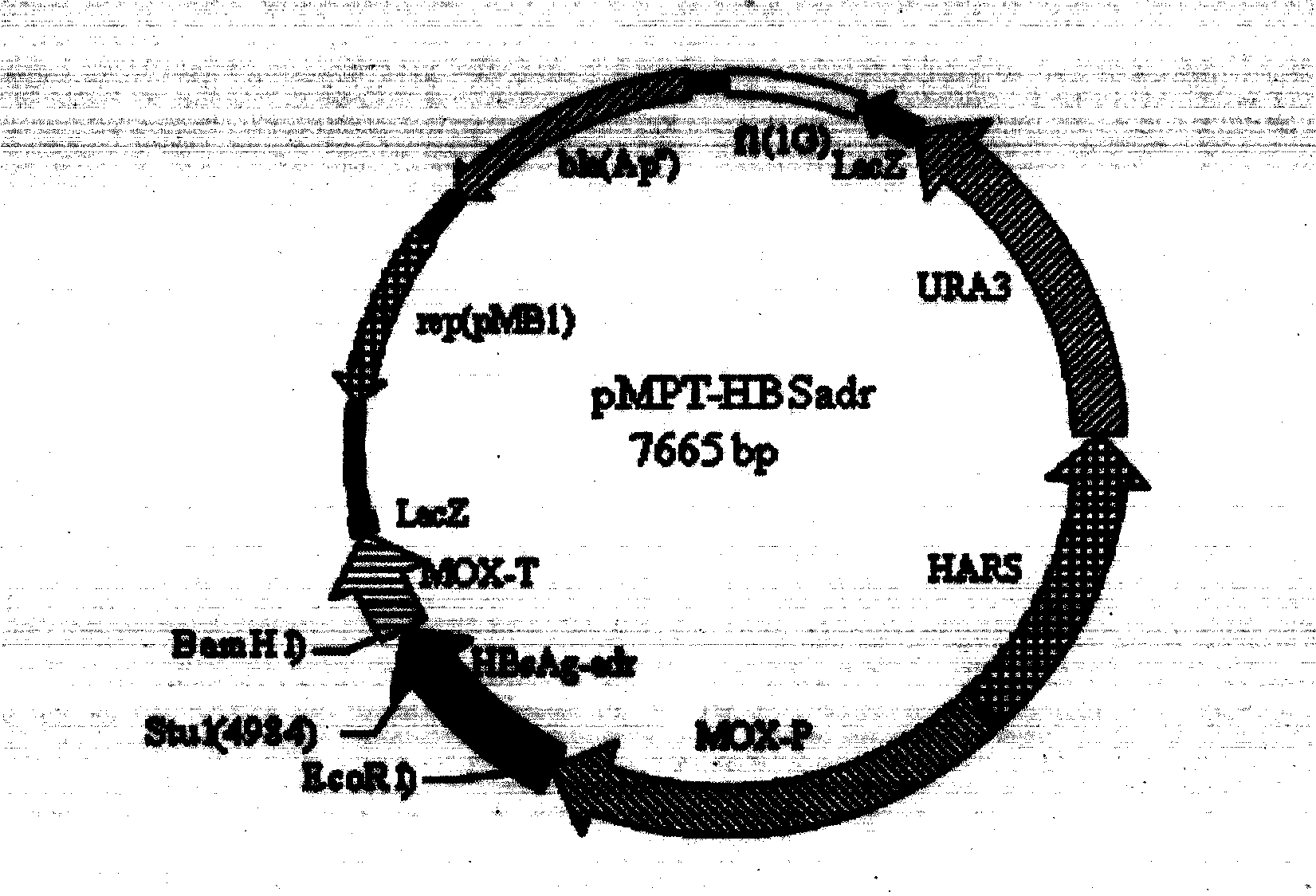
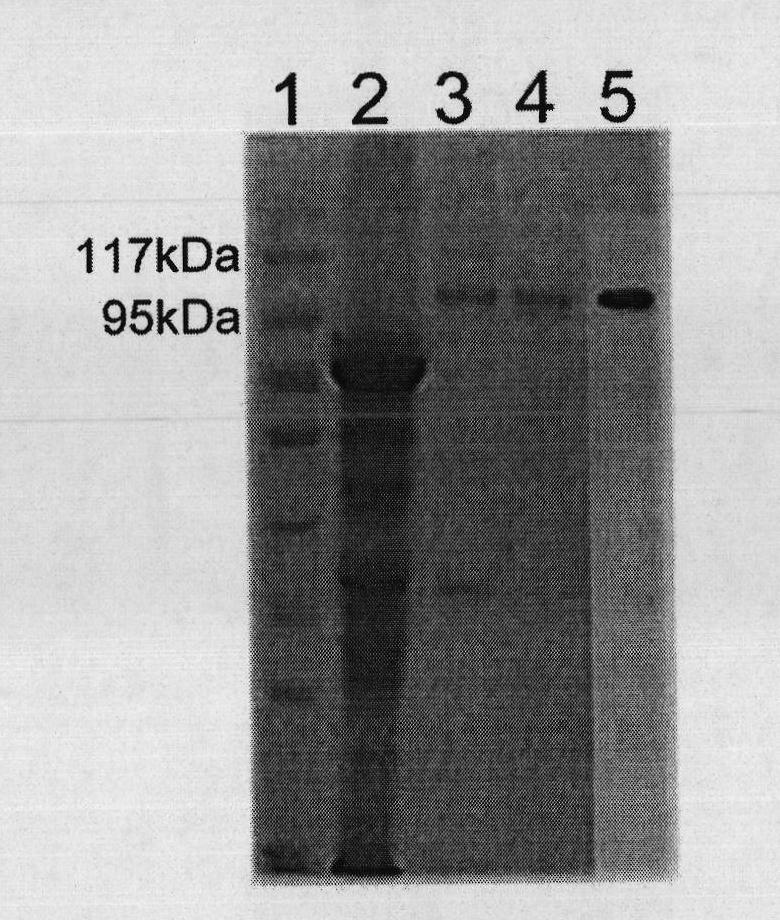
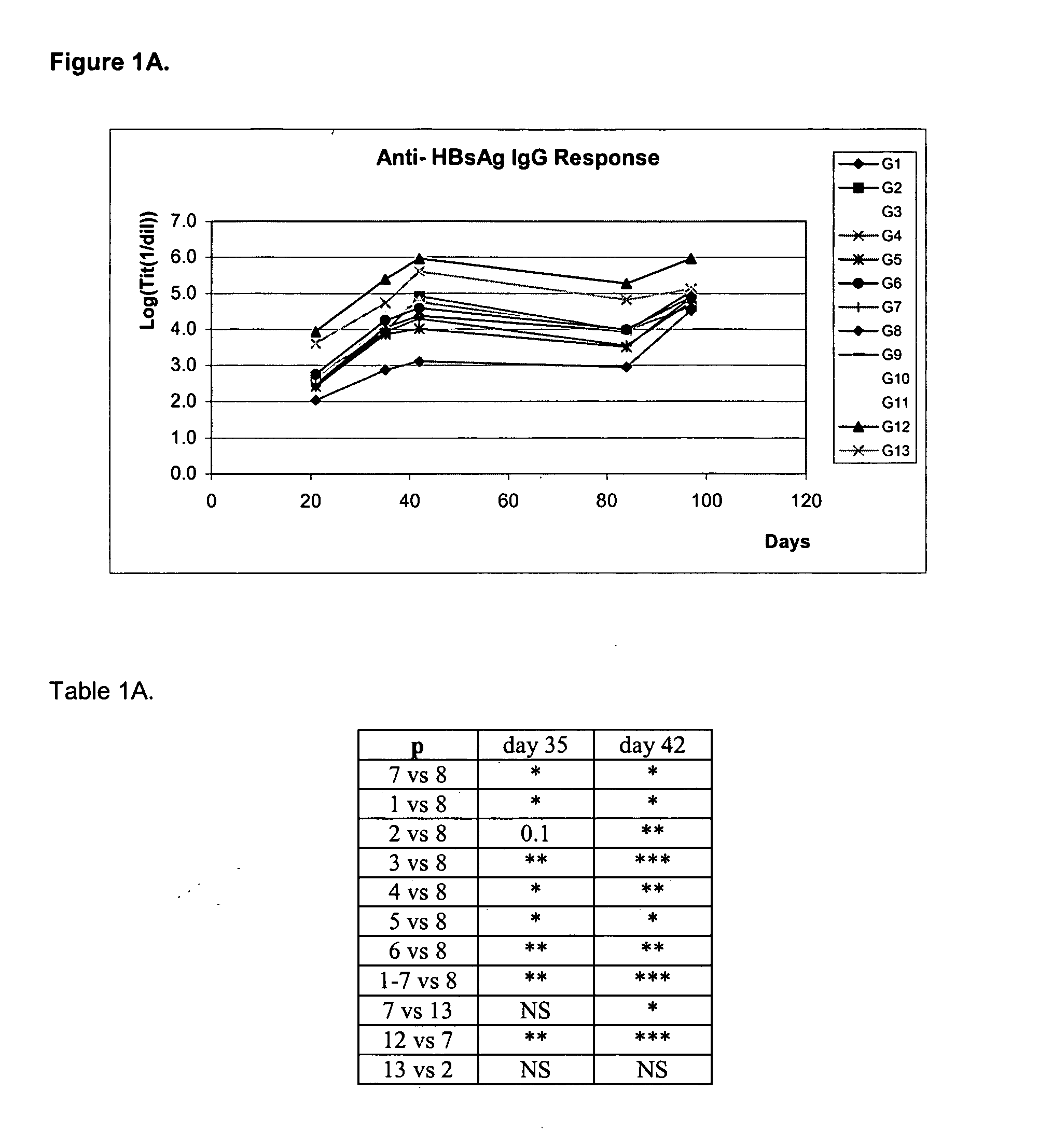
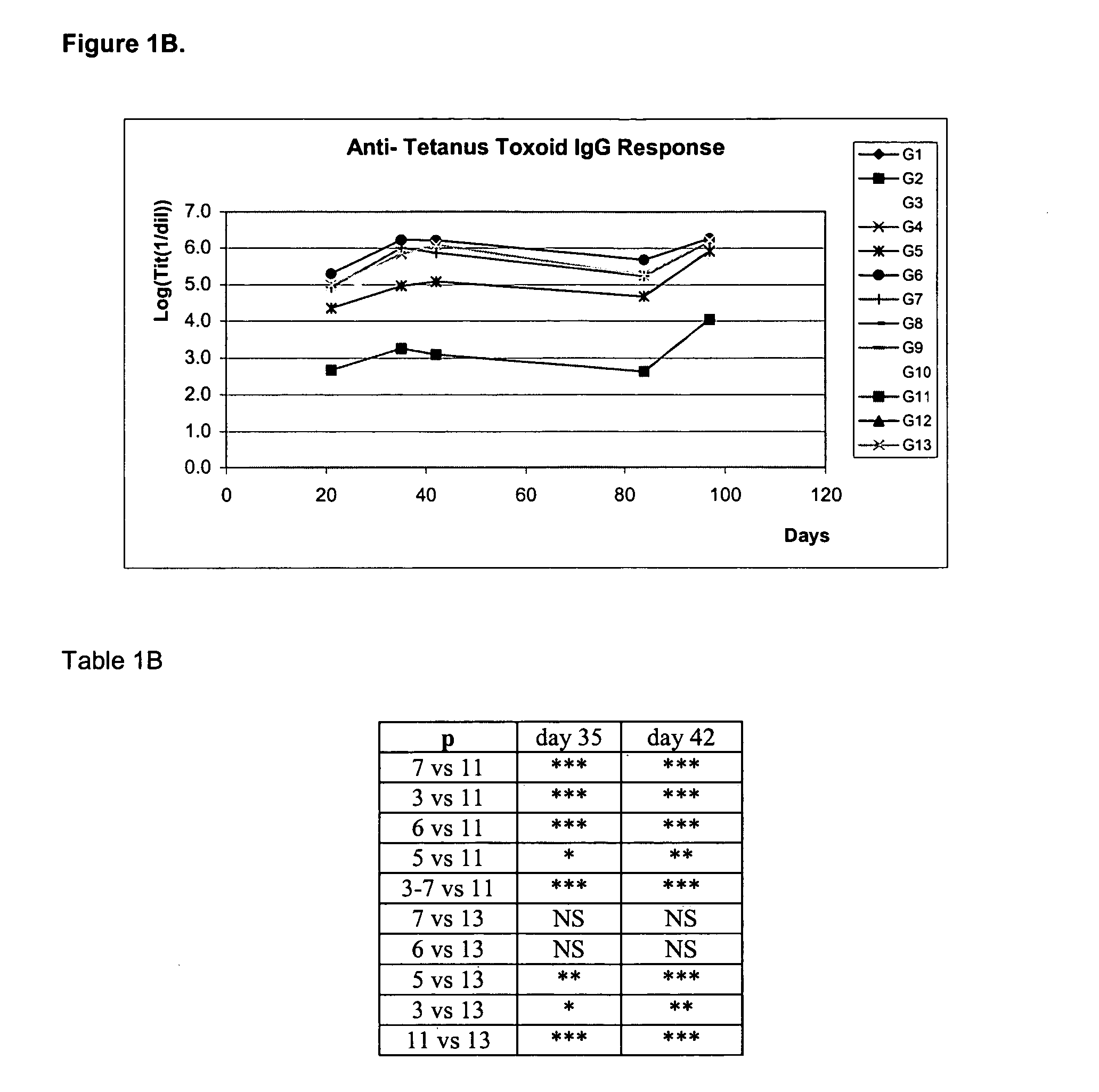
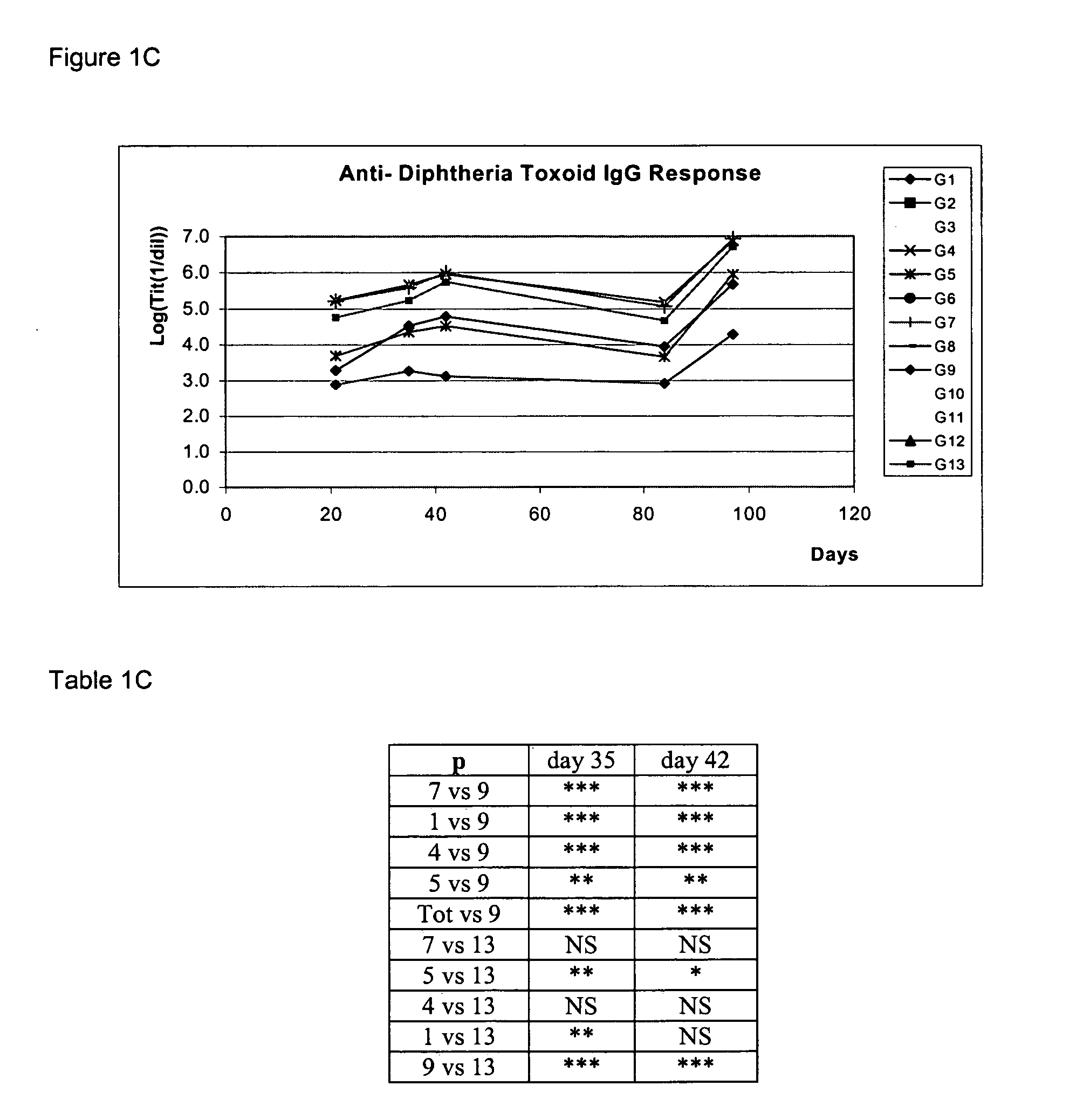
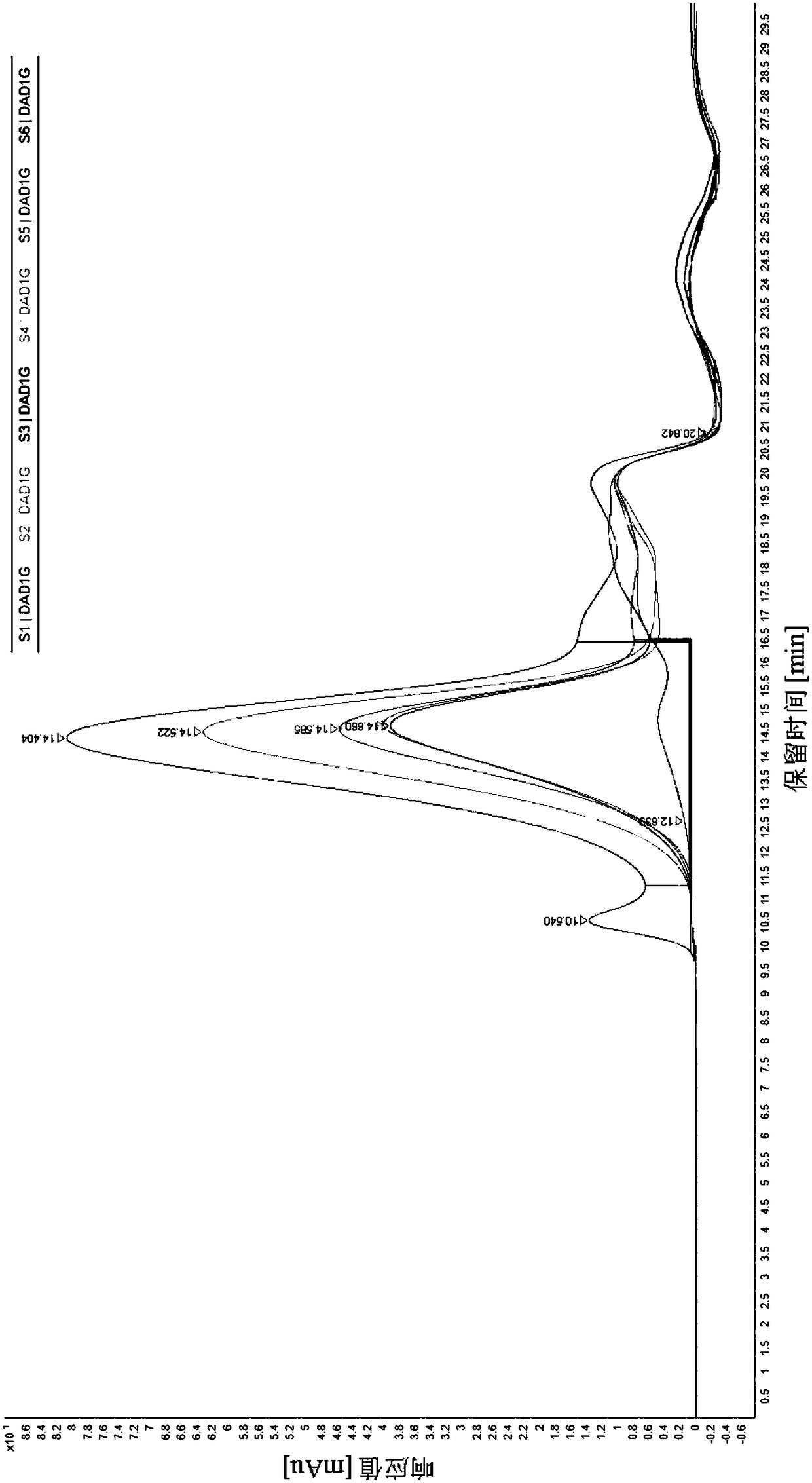
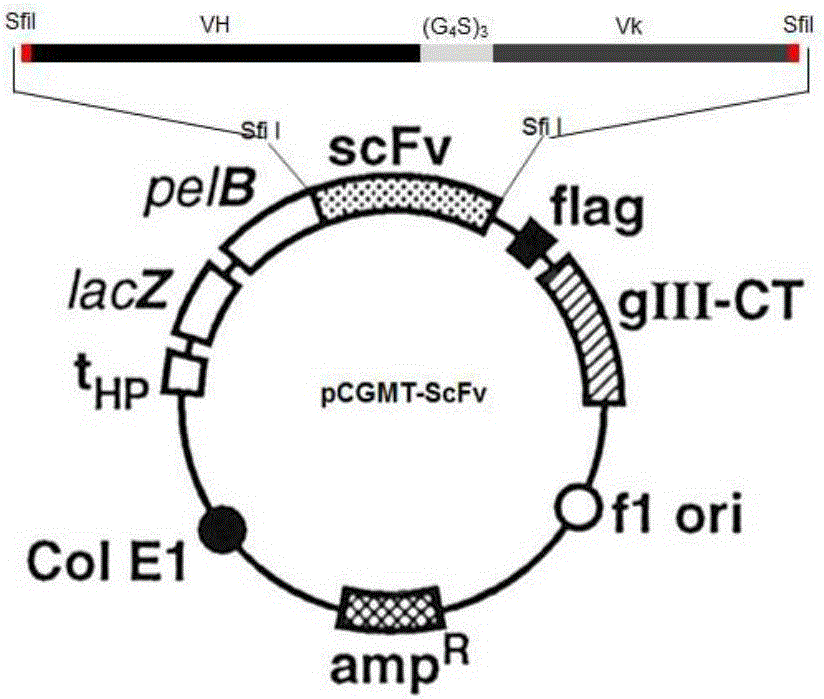
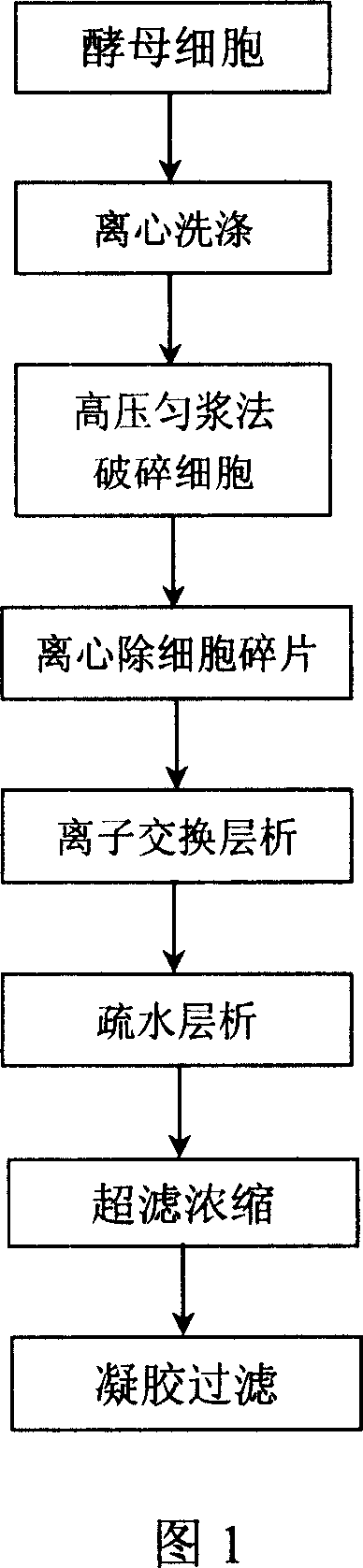
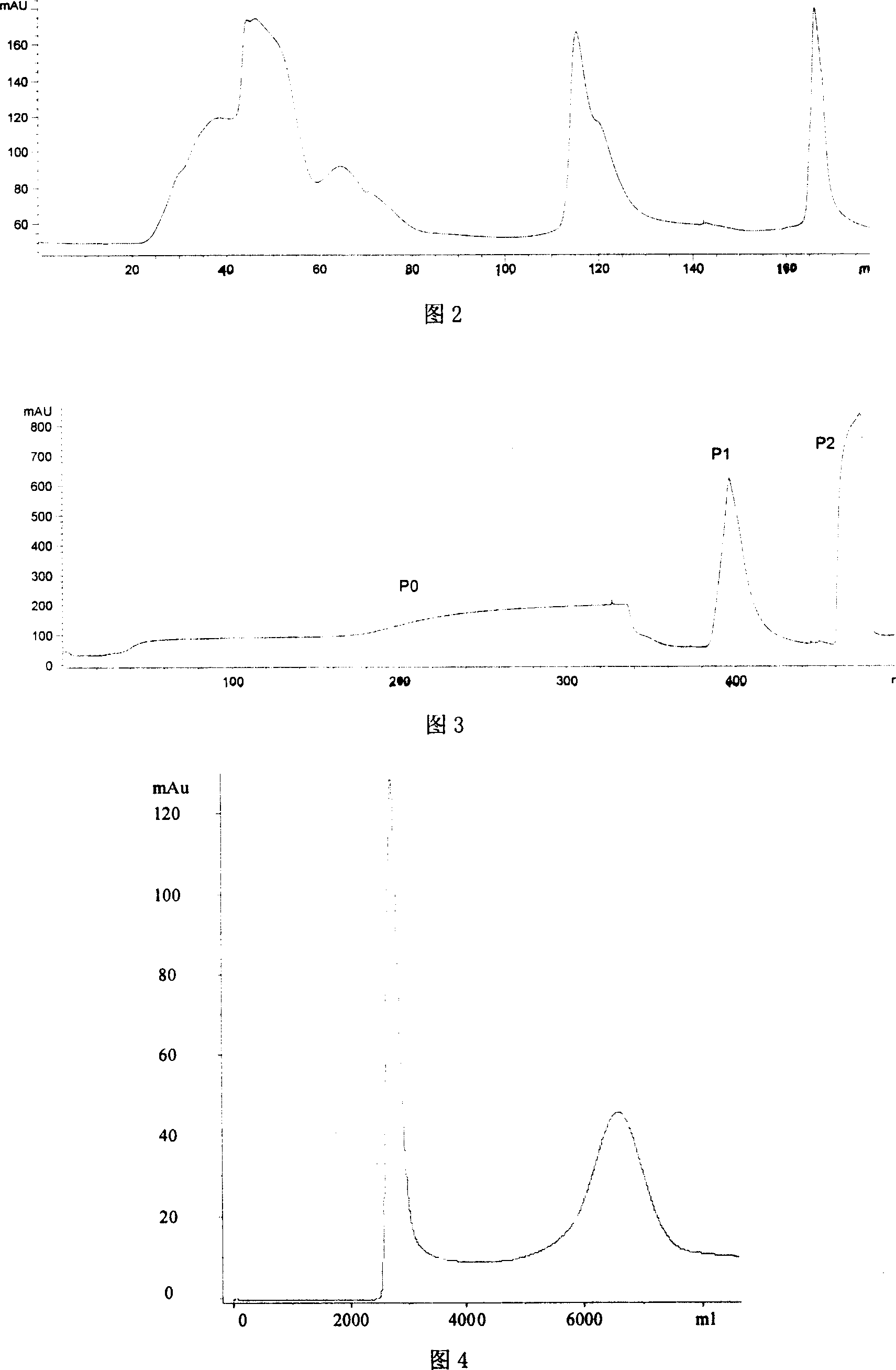
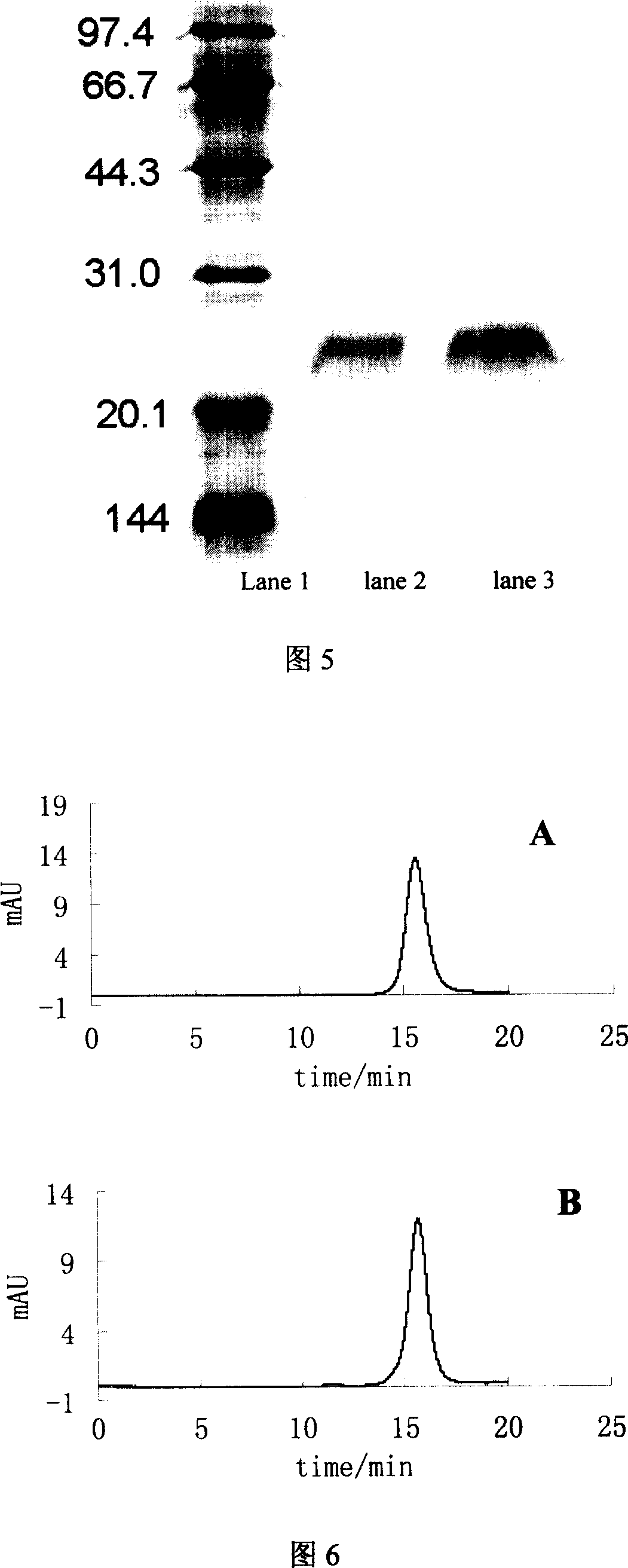
Patents
Literature
157 results about "Hepatitis B Surface Antigens" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
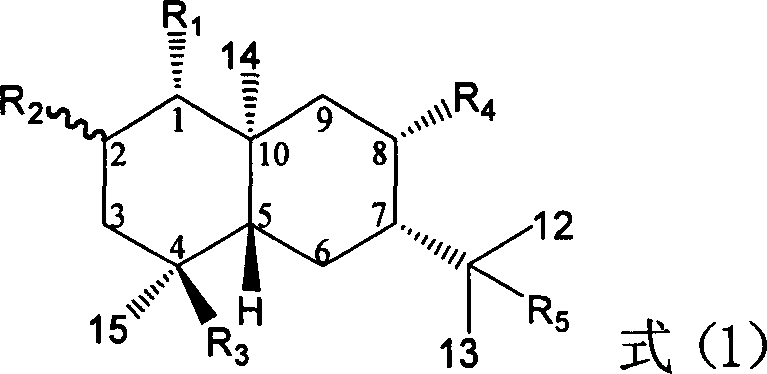
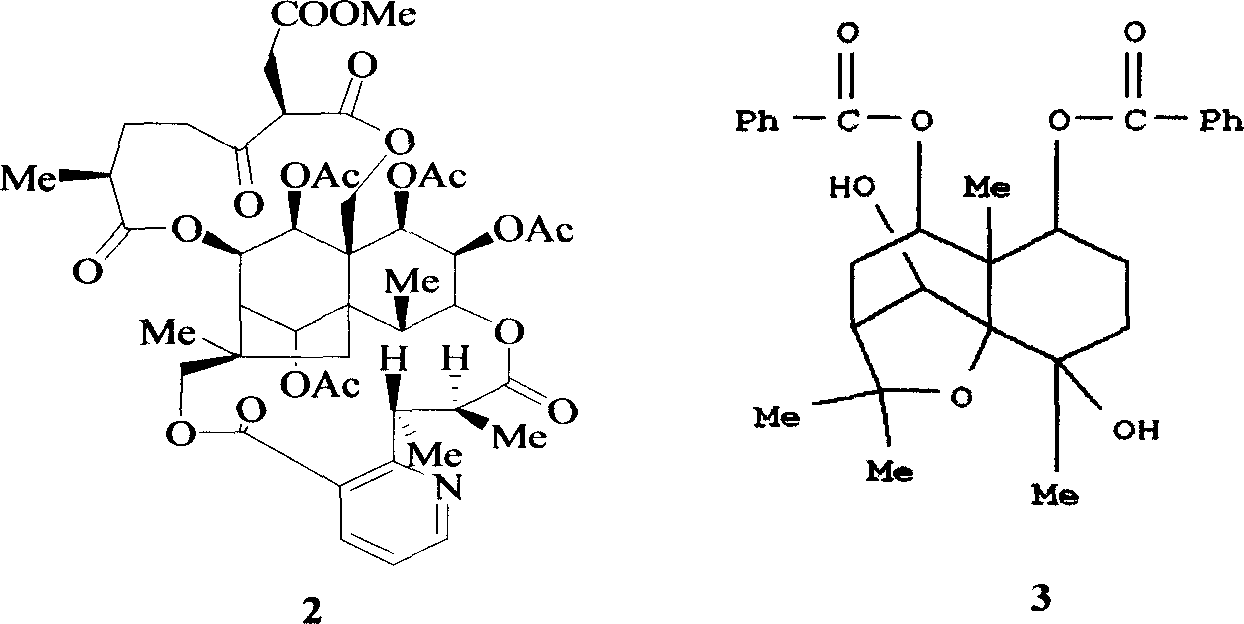
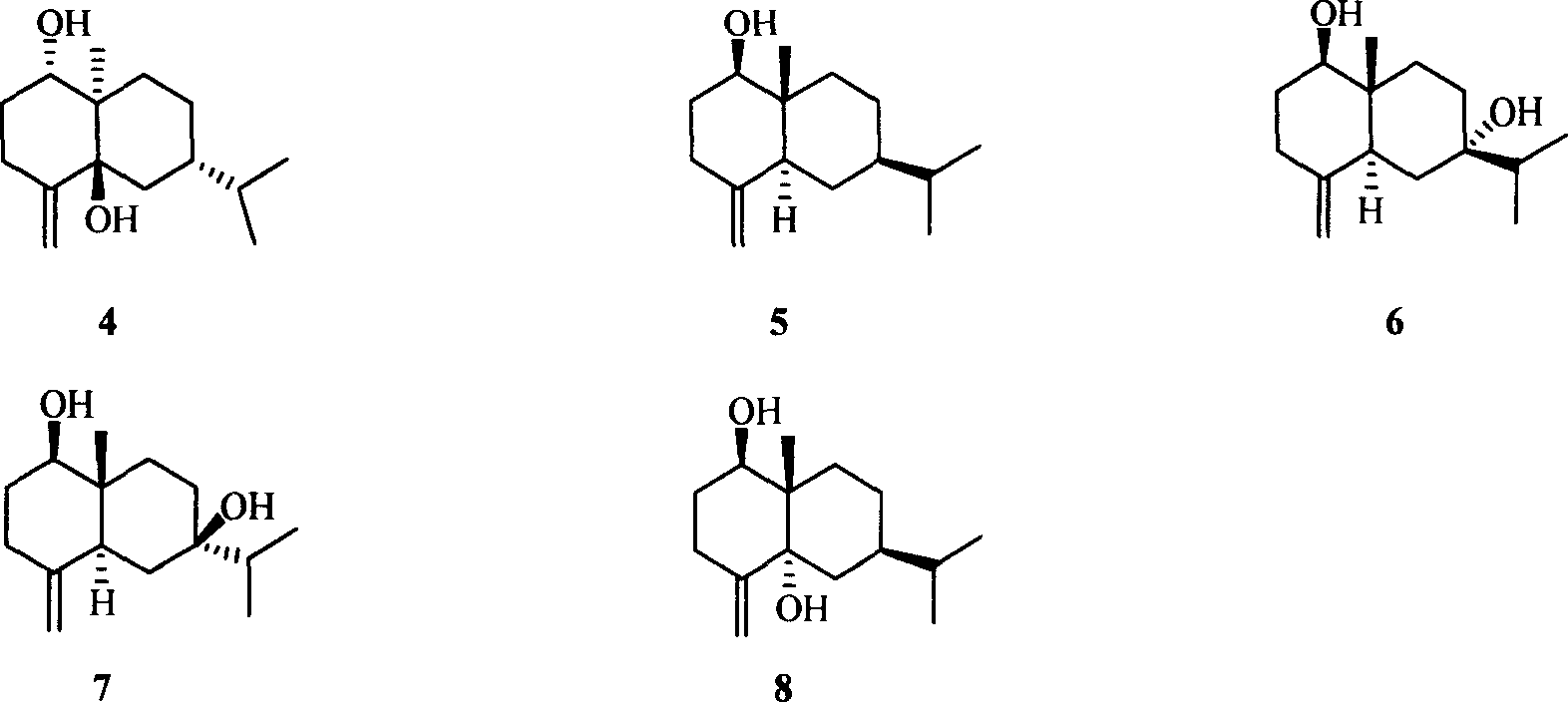
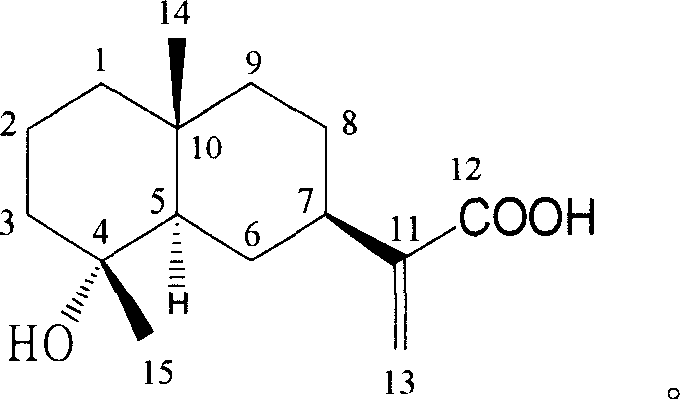
Pharmaceutical use of ent-eudesmane alcohol type sesquiterpene for inhibiting hepatitis virus
InactiveCN1935762APrevention and treatment of viral hepatitis BHBsAg reductionSugar derivativesHydroxy compound active ingredientsDiseaseSolvent
The invention relates to an enantiomorphic amine alkyl sesquiterpene alcohol and glucoside and the medicated salt or solvent thereof, as well as the effect and activity of the composed medicine combination, mainly relating to the medical use in reducing HBV-DNA replication activity. And it has considerably strong inhibiting effect on HBsAG screted by HepG2.2.15 and HBV-DNA replication as compared with positive contrast Lamivudine; and it has obvious inhibition activity to HBV-DNA replication at large dosage (100 mug / mL) and medium dosage(20 mug / mL) as contrasted with Lamivudine, and can be expected to apply to preparing medicines for curing HB virus infection disease.
Owner:赵昱
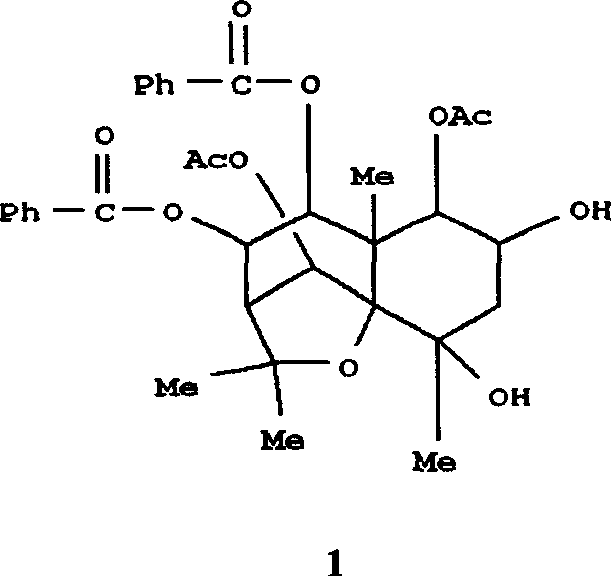
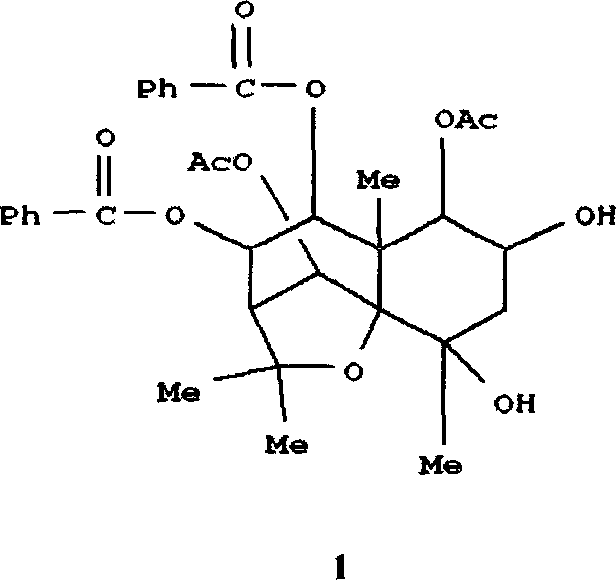
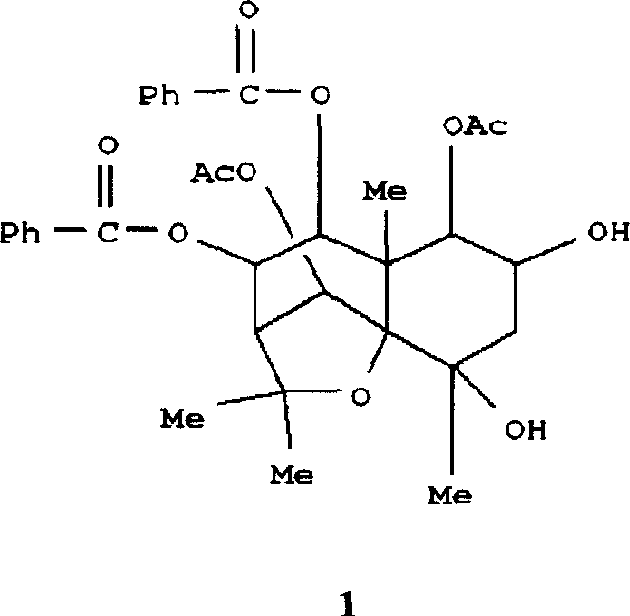
Medical use of 2 alpha, 3 beta-dihydroxy-5, 11(13)- diallyl eudesmane-12 acid for inhibiting hepatitis B virus
InactiveCN1923188APrevention and treatment of viral hepatitis BHBsAg reductionOrganic active ingredientsDigestive systemDiseaseDrug compound
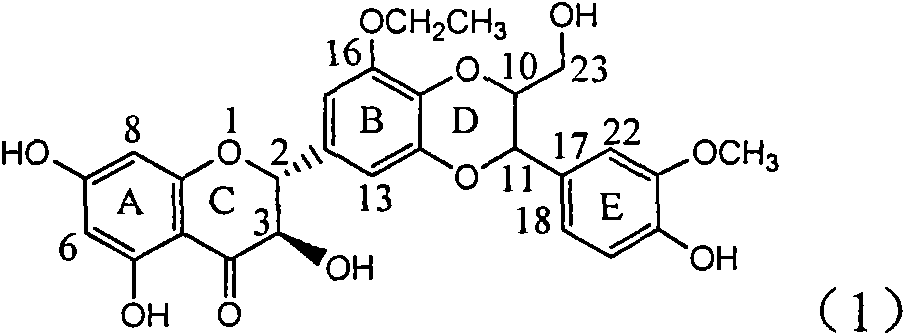
The invention relates to a sesterterpane as formula (1) and relative medical drug or solvent, and relative drug compound, which can reduce hepatitis B surface antigen and restrain hepatitis B HBVDNA copy activity. Wherein, said invention has strong restrain on hepatitis B surface antigen (HBsAg) generated by HepG2.2.15 cell and the copy of hepatitis B deoxyribonucleic acid (HBV-DNA), while it restrain ability is higher than positive contrast difuradin; and the copy restrain activity at large amount (100 mug / mL) and middle amount (20 mug / mL) on the hepatitis B HBV-DNA are both higher then difuradin.
Owner:赵昱
Eudesmane type sesquiterpenes acid and application thereof
InactiveCN1923787AMedication safetyInhibitory effect is not obviousOrganic chemistryDigestive systemAntigenCompound name
The invention discloses a eudesmane typed sesquiterpene acid compound named 5alphaH-eudesmane-11 (13)-alkylene-12 acid, which is characterized by the following: inhibiting the replication of HBsAg and HBV-DNA; fitting for preparing prevention drug of virus B hepatitis; reducing the application in the hepatitis B surface antigen drug.
Owner:ZHEJIANG UNIV
Enantiomorphous eremophilanic acid and its medical use for inhibiting hepatitis B surface antigen
The invention relates to the medicine technical field and concretely relates to a mixture formed by two enantiomorphous eremophilane acids separated from murrey ligularia and the officinal salt as well as the medicine combined material. The enantiomorphous eremophilane acid can be used for preparing the medicine curing hepatitis B virus infectious diseases due to the ability of suppressing the antigenic activity on the surface of hepatitis B virus.
Owner:WENZHOU MEDICAL UNIV
Use of acetamide dehydrogenation silibinin as medicament for treating viral hepatitis B
InactiveCN101829091APowerful removalInhibitory activityOrganic active ingredientsDigestive systemAntigenDisease
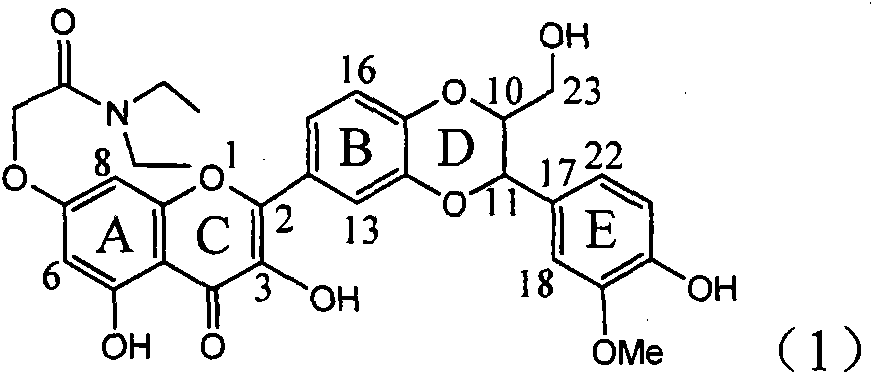
The invention relates to the use of acetamide dehydrogenation silibinin as a medicament for treating viral hepatitis B, in particular to the use of dehydrogenation silibinin esters flavonoid lignanoid replaced by A ring methoxy formyl amine or pharmaceutically acceptable salt as the medicament for eliminating HBsAg (hepatitis B surface antigen) and HBeAg (hepatitis Be antigen) and restraining copy of HBV DNA. The cetamide dehydrogenation silibinin can obviously restrain the HBsAg and HBeAg activity, and the strengths for eliminating the HBsAg and HBeAg are 90.5% and 63.6% at the concentration of 20 microgramme / milliter and are 5.6 times and 3.8 times more than positive contrast medicament alpha-interferon. Meanwhile, the restraining rate to the HBV DNA is 90.4% at the concentration, is 12% higher than lamivudine, and is 2.4 times more than a- interferon. Therefore, the flavonoid lignanoid or the pharmaceutically acceptable salt can be expected for treating hepatitis B virus infection as the non-nucleoside medicament.
Owner:DALI UNIV
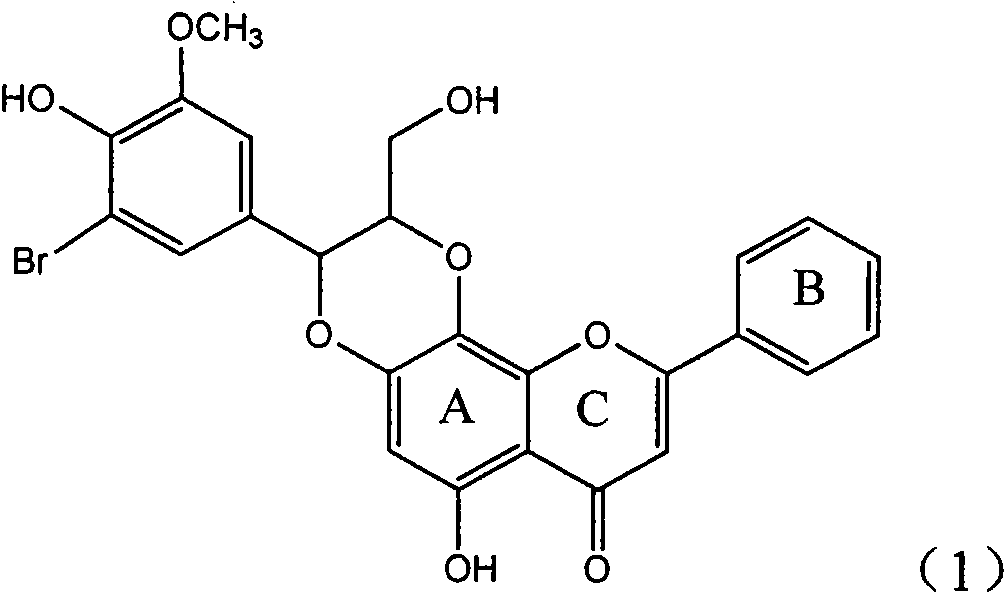
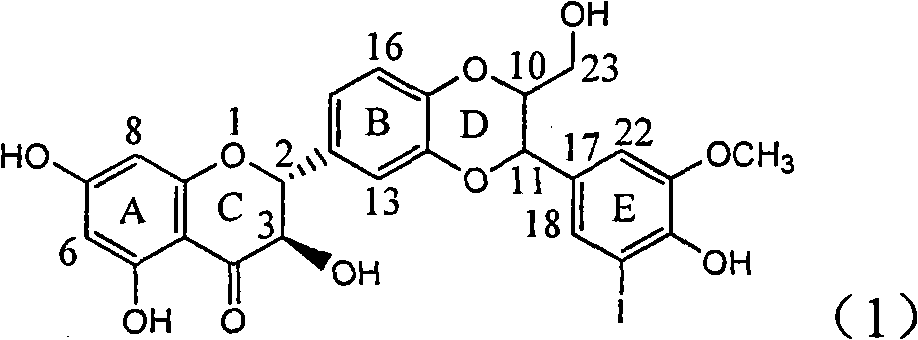
Application of ring E bromine substituted silybin in preparing medicaments for treating viral hepatitis B
InactiveCN101829094AInhibitory activityInhibition of replicative activityOrganic active ingredientsAntiviralsDiseasePositive control
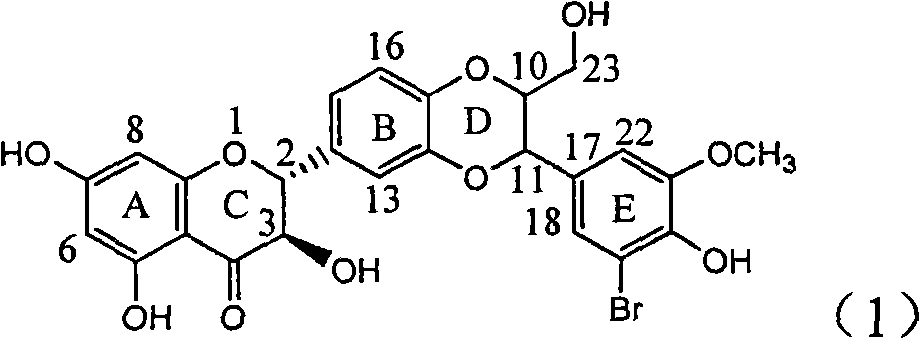
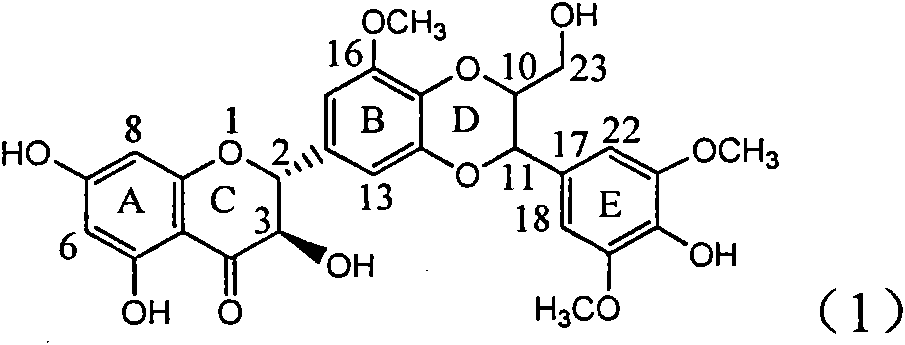
The invention relates to application of ring E bromine substituted silybin in preparing medicaments for treating viral hepatitis B, in particular to application of a compound of a formula (1) and a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away hepatitis B surface antigens (HBsAg) and hepatitis e antigens (HBeAg) and suppressing the HBV (Hepatitis B Virus) DNA replication. The compound has definite activity on suppressing the HBsAg and the HBeAg, and in the presence of a concentration of 100 micrograms / milliliter, the intensities of the compound for clearing away the HBsAg and the HBeAg are respectively 38.2 percent and 39.1 percent which are respectively 2.4 times and 2.3 times of that of a positive control medicament (10,000 units / milliliter of alpha-interferon). Meanwhile, in the presence of the concentration, the suppression ratio of the compound on the HBV DNA is 36 percent which is close to that of the alpha-interferon. Accordingly, the flavone lignan or the pharmaceutically acceptable salt thereof are indicated to be capable of being used for preparing non-nucleoside medicaments for clearing away the HBsAg and the HBeAg, suppressing the HBV DNA replication and treating HBV infection diseases.
Owner:DALI UNIV
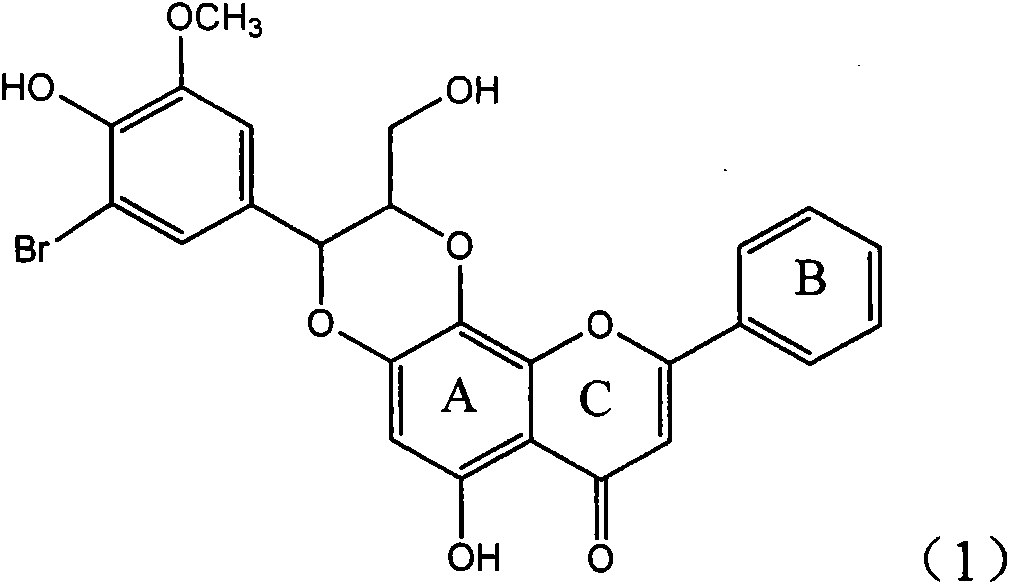
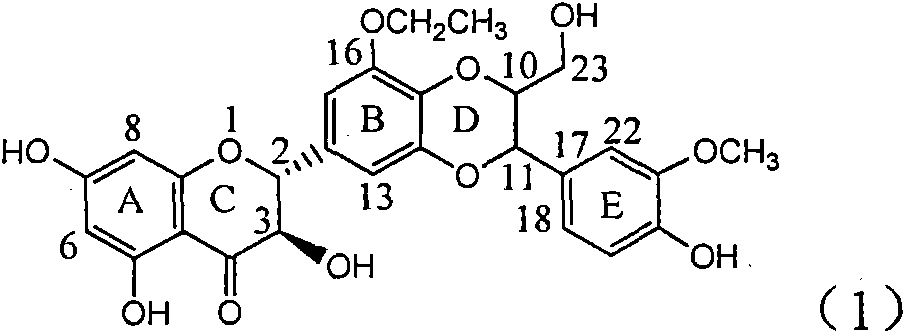
Application of ring A coupling flavonolignan in preparing medicaments for treating viral hepatitis B
InactiveCN101829104AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
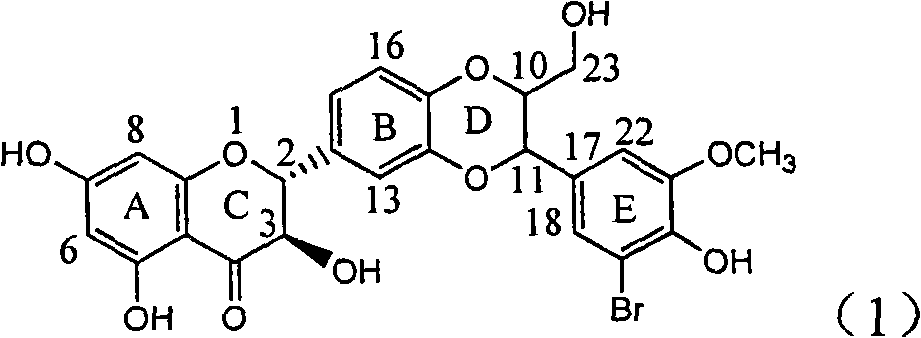
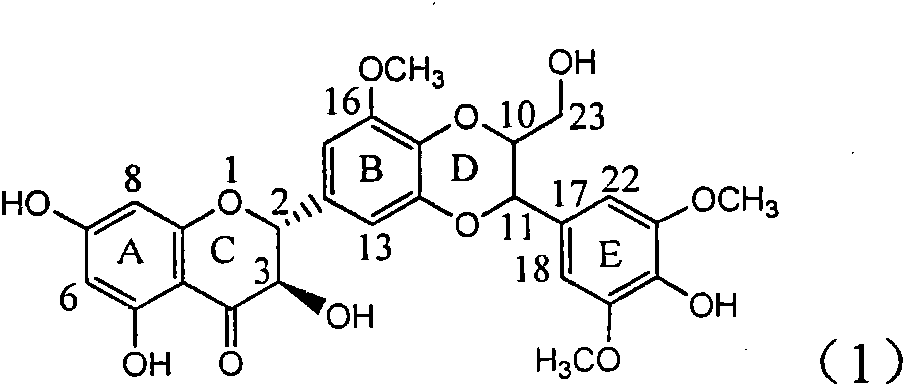
The invention relates to application of ring A coupling flavonolignan in preparing medicaments for treating viral hepatitis B, in particular to application of a compound of the formula (1) or a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away HBsAg (Hepatitis B Surface Antigen) and HBeAg (Hepatitis B e Antigen) and suppressing the HBV (Hepatitis B Virus) DNA replication. The intensities of the flavonolignan for clearing away the HBsAg and the HBeAg are respectively 29.4 percent and 29.1 percent in the presence of a concentration of 20 micrograms / milliliter, which is respectively 1.8 times and 1.7 times of the corresponding activity of a positive control medicament (10,000 units / milliliter of alpha-interferon). What is even more exciting is that in the presence of the concentration, the suppression rate of the flavonolignan to the HBV DNA is higher than 83 percent, which is higher than that of Lamivudine which is a positive control and is 2.2 times of that of the alpha-interferon to the HBV DNA. Accordingly, the flavonolignan and the pharmaceutically acceptable salt thereof are indicated to be capable of being expected to be used for preparing non-nucleoside medicaments for clearing away the HBsAg and the HBeAg, suppressing the HBV DNA replication and treating HBV infection diseases.
Owner:DALI UNIV
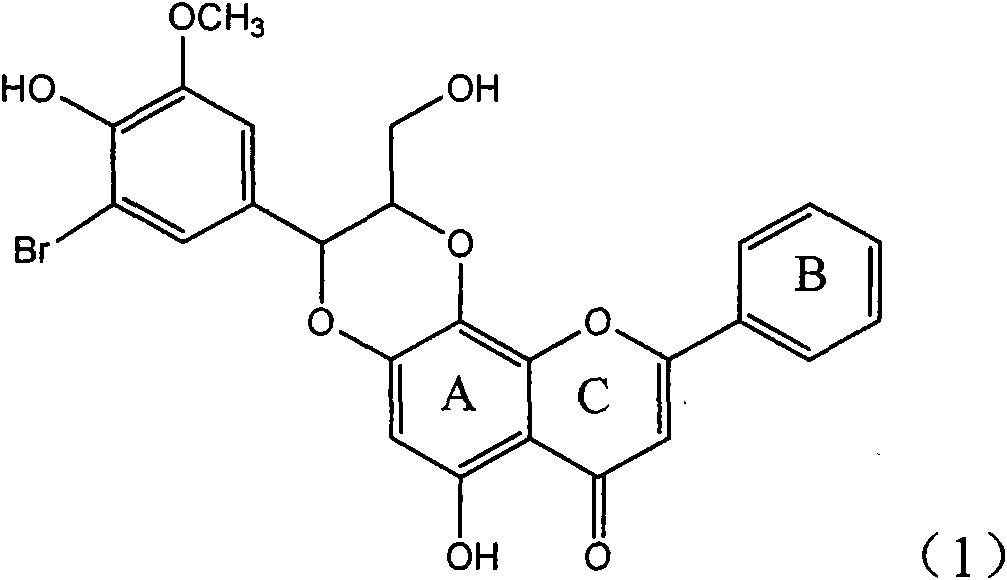
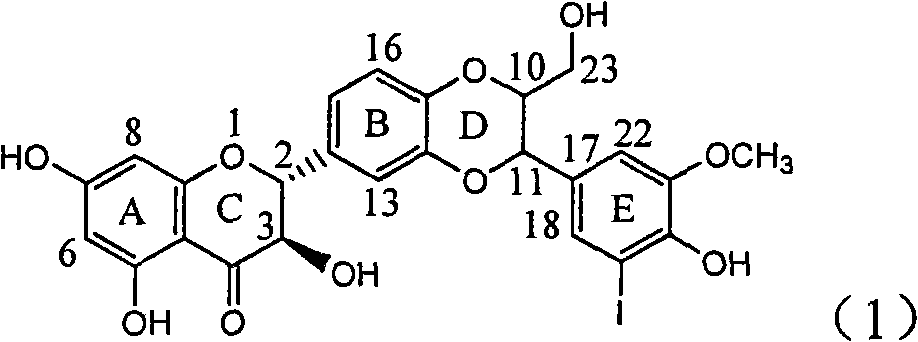
Application of ring E iodine substituted silybin in preparing medicaments for treating viral hepatitis B
InactiveCN101829096AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
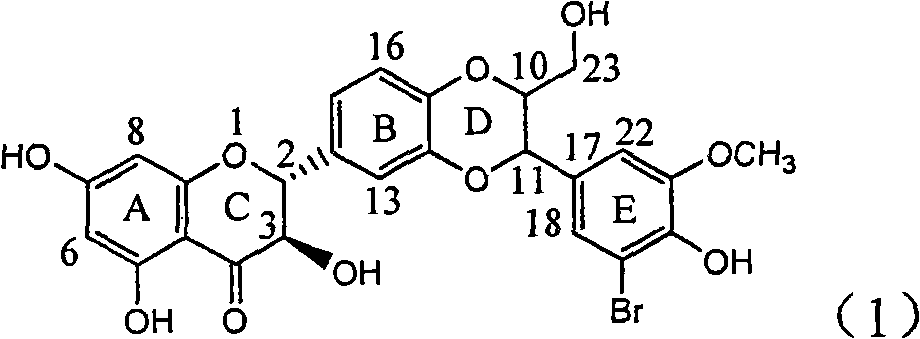
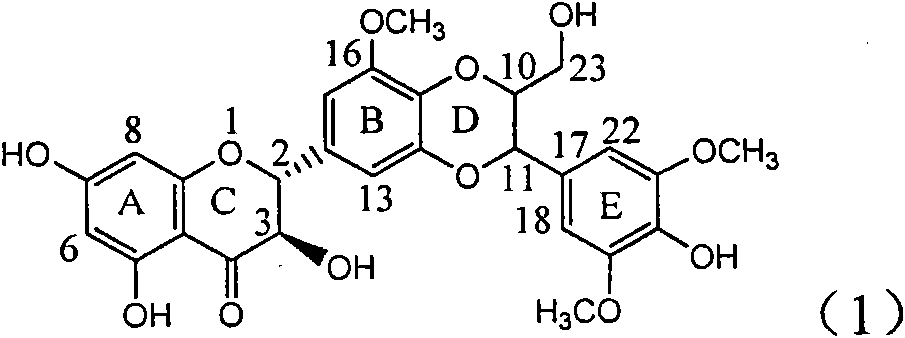
The invention relates to application of ring E iodine substituted silybin in preparing medicaments for treating viral hepatitis B, in particular to application of a compound of a formula (1) and a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away hepatitis B surface antigens (HBsAg) and hepatitis e antigens (HBeAg) and suppressing the HBV (Hepatitis B Virus) DNA replication. The compound has definite activity of suppressing the HBsAg and the HBeAg, and in the presence of a concentration of 100 micrograms / milliliter, the intensities of the compound for clearing away the HBsAg and the HBeAg are respectively 20.0 percent and 29.0 percent which exceed that of a positive control medicament (10,000 units / milliliter of alpha-interferon) by 24 percent and 72 percent. Meanwhile, in the presence of the concentration, the suppression ratio of the compound on the HBV DNA is 32.6 percent which is close to that of the alpha-interferon. Accordingly, the flavone lignan or the pharmaceutically acceptable salt thereof are indicated to be capable of being used for preparing non-nucleoside medicaments for clearing away the HBsAg and the HBeAg, suppressing the HBV DNA replication and treating HBV infection diseases.
Owner:DALI UNIV
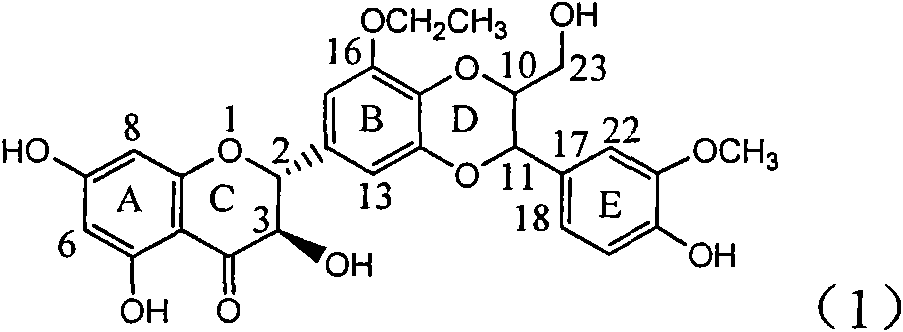
Application of ring B ethyoxyl silybin in preparing medicaments for treating viral hepatitis B
InactiveCN101829089AConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemDiseasePositive control
The invention relates to application of ring B ethyoxyl silybin in preparing medicaments for treating viral hepatitis B, in particular to application of ring B ethyoxyl substituted silybin ester or a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away HBsAG (Hepatitis B Surface Antigen) and HBeAg (Hepatitis B e Antigen) and suppressing the HBV (Hepatitis B Virus) DNA replication. The compound has strong activity on suppressing the HBsAG and the HBeAg, and in the presence of a concentration of 20 micrograms / milliliter, the intensities for clearing the HBsAg and the HBeAg are respectively 64.6 percent and 44.8 percent which are 4.0 times and 2.7 times of that of alpha-interferon which is a positive control medicament. In the presence of the concentration, the suppression rate of the compound on the HBV DNA is 58.1 percent which is 1.5 times of the corresponding activity of the alpha-interferon. Accordingly, the flavonolignan or the pharmaceutically acceptable salt thereof are indicated to simultaneously have strong efficacy on suppressing the HBsAg, the HBeAg and the HBV DNA and can be expected to be used for preparing non-nucleoside medicaments for treating HBV infection diseases.
Owner:DALI UNIV
Application of B/E bi-methoxy silybin in preparing medicaments for treating viral hepatitis B
InactiveCN101829088AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
The invention relates to application of B / E bi-methoxy silybin in preparing medicaments for treating viral hepatitis B, in particular to application of silybin ester substituted by the methoxy on the ring B and the ring E or a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away HBsAg (Hepatitis B Surface Antigen) and HBeAg (Hepatitis B e Antigen) and suppressing the HBV (Hepatitis B Virus) DNA replication. The B / E bi-methoxy silybin has strong activity on suppressing the HBsAg and the HBeAg, and in the presence of a concentration of 20 micrograms / milliliter, the intensities for clearing away the HBsAg and the HBeAg are respectively 43.9 percent and 43.7 percent which are 2.7 times and 2.6 times of that of alpha-interferon which is a positive control medicament. In the presence of the concentration, the suppression ratio on the HBV DNA is 68.6 percent, and the suppression activity is 1.8 times of that of the alpha-interferon. Accordingly, the flavone lignan or the pharmaceutically acceptable salt thereof are indicated to simultaneously have the effects of strongly suppressing the HBsAG, the HBeAg and the HBV DNA and can be expected to be used for preparing the non-nucleoside medicaments for treating HBV infection diseases.
Owner:DALI UNIV
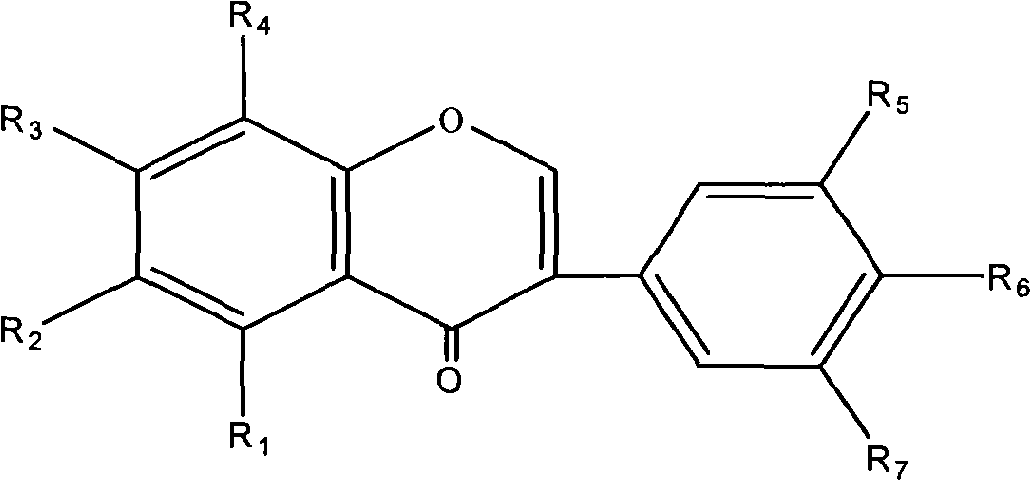
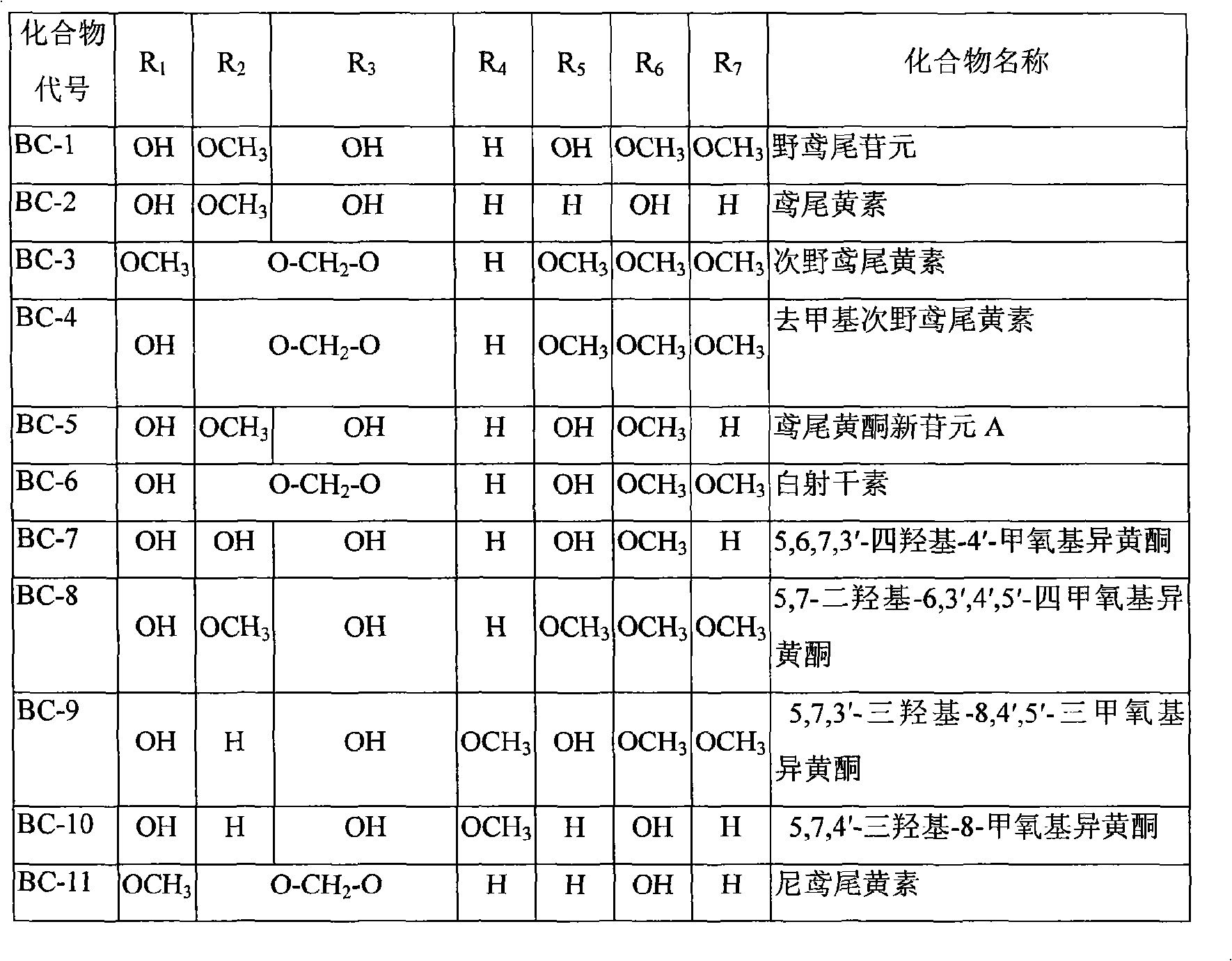
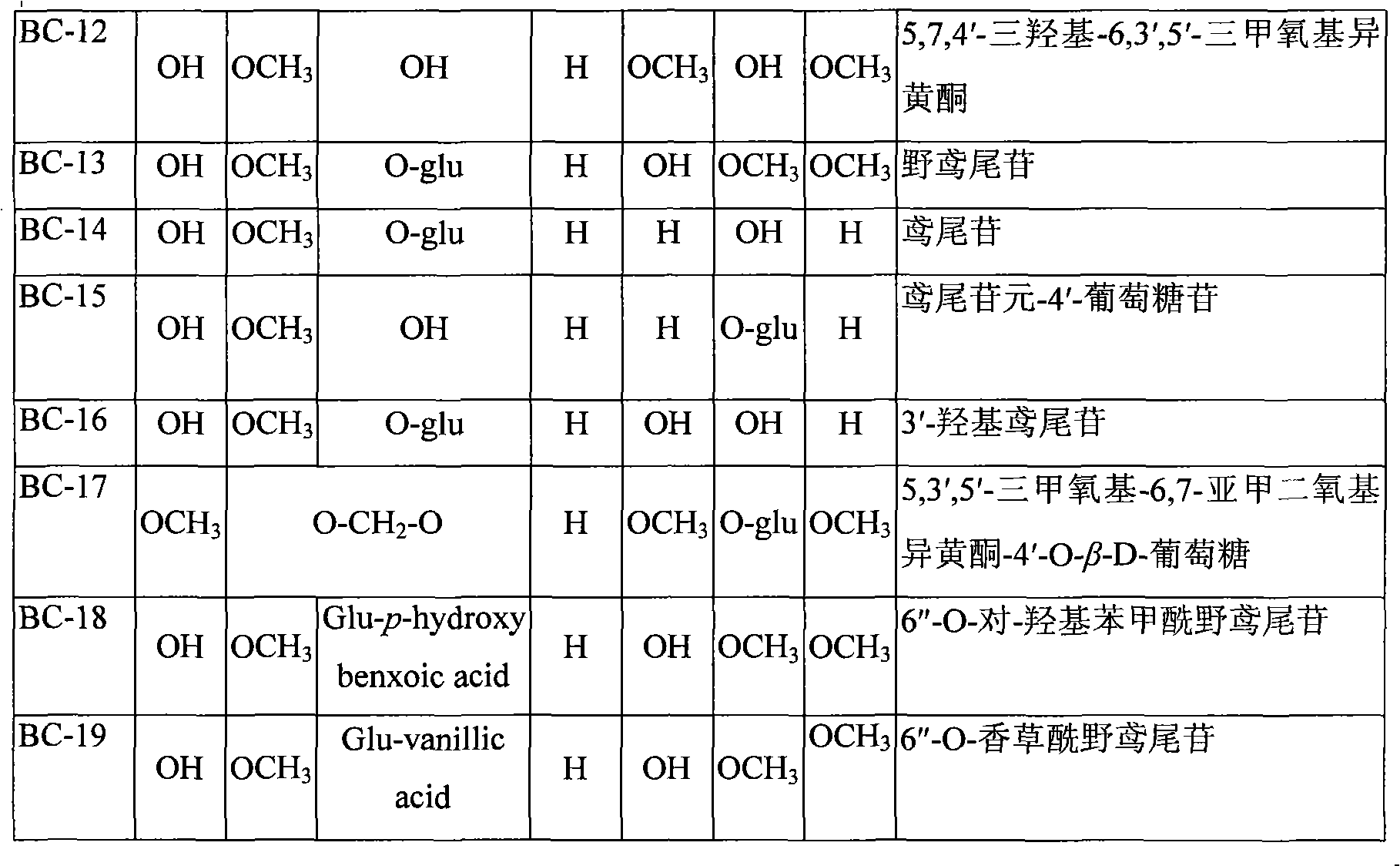
Use of composition of isoflavonoids from Belamcanda chinensis in preparing anti-hepatitis medicament
InactiveCN101301287ASignificant anti-HBV effectOrganic active ingredientsDigestive systemAntigenBelamcanda chinensis
The invention belongs to the medical technical field, in particular relating to an application of isoflavonoids from Belamcanda chinensis in the preparation of anti-hepatitis virus drugs. A Hepatitis B surface antigen (HBsAg) detection reagent test and a hepatitis B core antigen (HBeAg) detection reagent test are performed, and the results indicate that the isoflavonoids from the Belamcanda chinensis can remarkably suppress hepatitis B surface antigen (HBsAg) and hepatitis B core antigen (HBeAg) and has remarkable effect on preventing and treating hepatitis B viruses. Therefore, the isoflavonoids from the Belamcanda chinensis can be used to prepare medicines or health-care food used to prevent and treat the hepatitis viruses.
Owner:上海双科医药科技有限公司
Method of Storing a Vaccine Containing an Aluminum Adjuvant
ActiveUS20120091026A1Reduce adsorptionDecrease and eliminate desorptionCapsDecorative coversAntigenAdjuvant
The invention relates to a method for loading and storing a vaccine composition, containing the antigen adsorbed on the aluminum adjuvant which (a) comprises (i) loading the composition into a container; and (ii) closing the container with a device in particular acting as a stopper, the surface of the device getting into contact with the composition being coated with a fluoropolymer such as Teflon™; and / or (b) loading the composition into a container wherein the inner surface of which is coated with polymerized silicone. The use of fluoropolymer or polymerized silicone optimizes the adsorbed antigen stability upon storage. In a particular embodiment, the antigen is the hepatitis B surface antigen and the aluminum adjuvant is aluminum oxy hydroxide.
Owner:SANOFI PASTEUR SA
Vaccine for controlling persistent infection of hepatitis B virus
InactiveCN102038948AExcellent adjuvant effectPromote maturityAntiviralsAntibody medical ingredientsAdjuvantHepatitis B Surface Antigens
The invention belongs to the biotechnology field, and relates to a vaccine for controlling the persistent infection of hepatitis B virus. According to the invention, a Hansenula polymorpha cell that is heat inactivated and expresses hepatitis B surface antigen is adopted as the hepatitis B vaccine, wherein the HBsAg is an antigen part of the vaccine and the Hansenula polymorpha cell is an adjuvant part of the vaccine. Animal immunity experiment shows that: the vaccine can induce the aggregation of immune cells to the spleen, promote the maturation of DCs, and both induce Th1 immune response and enhance Th2 immune response in mice, including total IgG, IgG1, IgG2a, specific CTL activity, and the production ability of antigen-specific IFN-gama. The invention can make up for the deficiency of the impossibility of inducing Th1 immune response for traditional vaccines that take aluminium hydroxide as an adjuvant, and is helpful to the control of the persistent infection of hepatitis B virus.
Owner:FUDAN UNIV
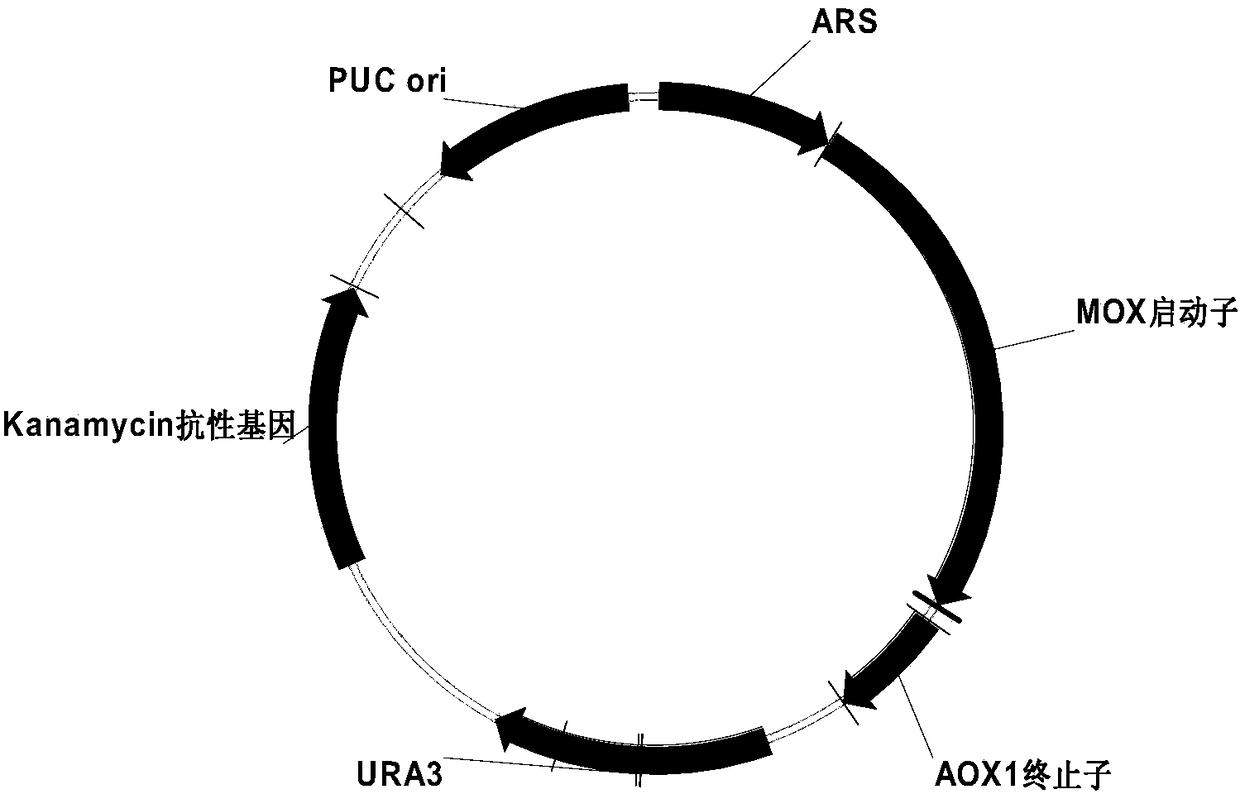
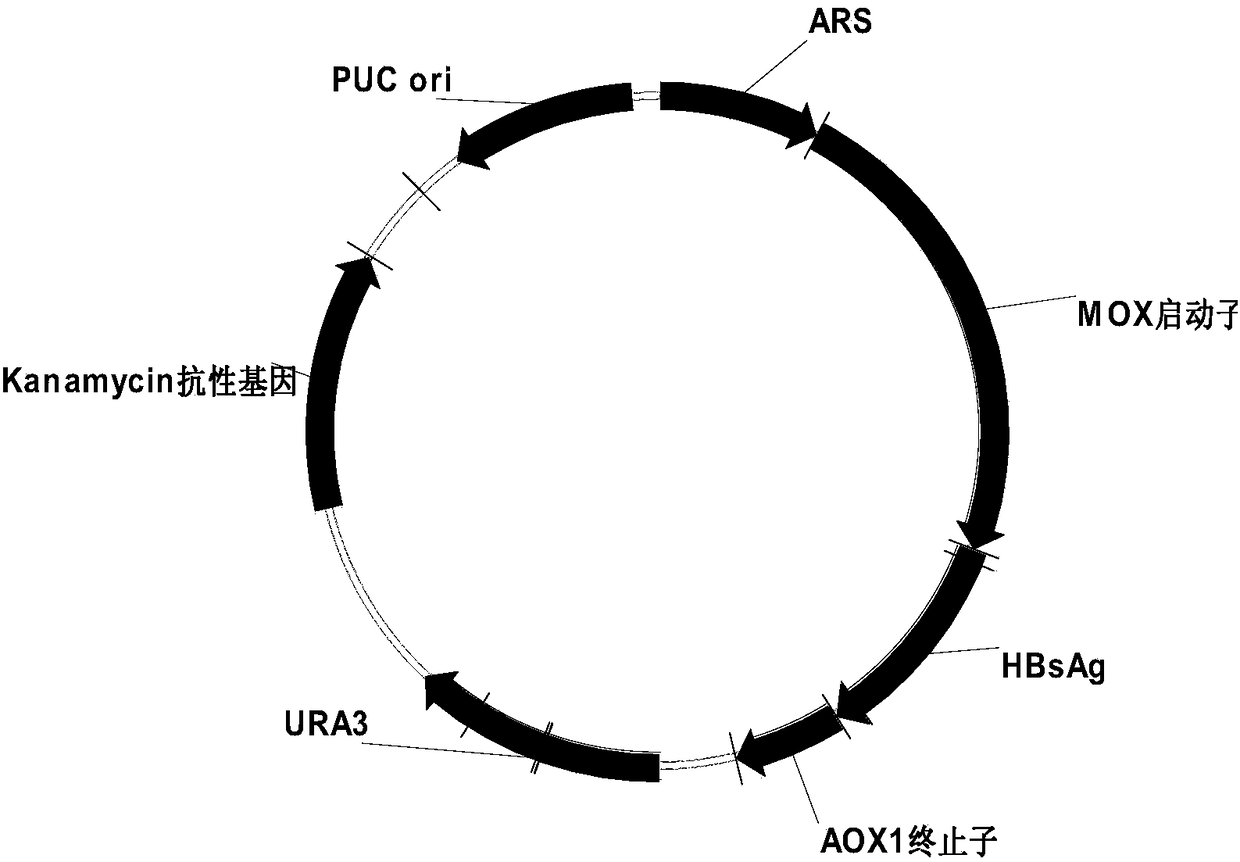
Recombinant DNA sequence, hansenula polymorpha, preparation method for hepatitis B surface antigen, and hepatitis B vaccine
InactiveCN104232661AImprove expression levelSplit genetic stability is highFungiMicroorganism based processesChemical synthesisHigh cell
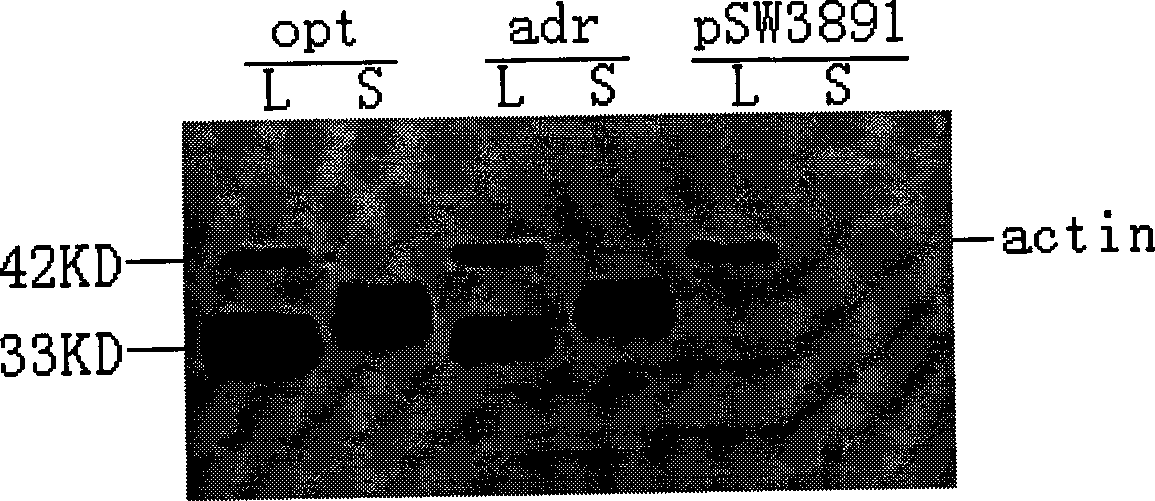
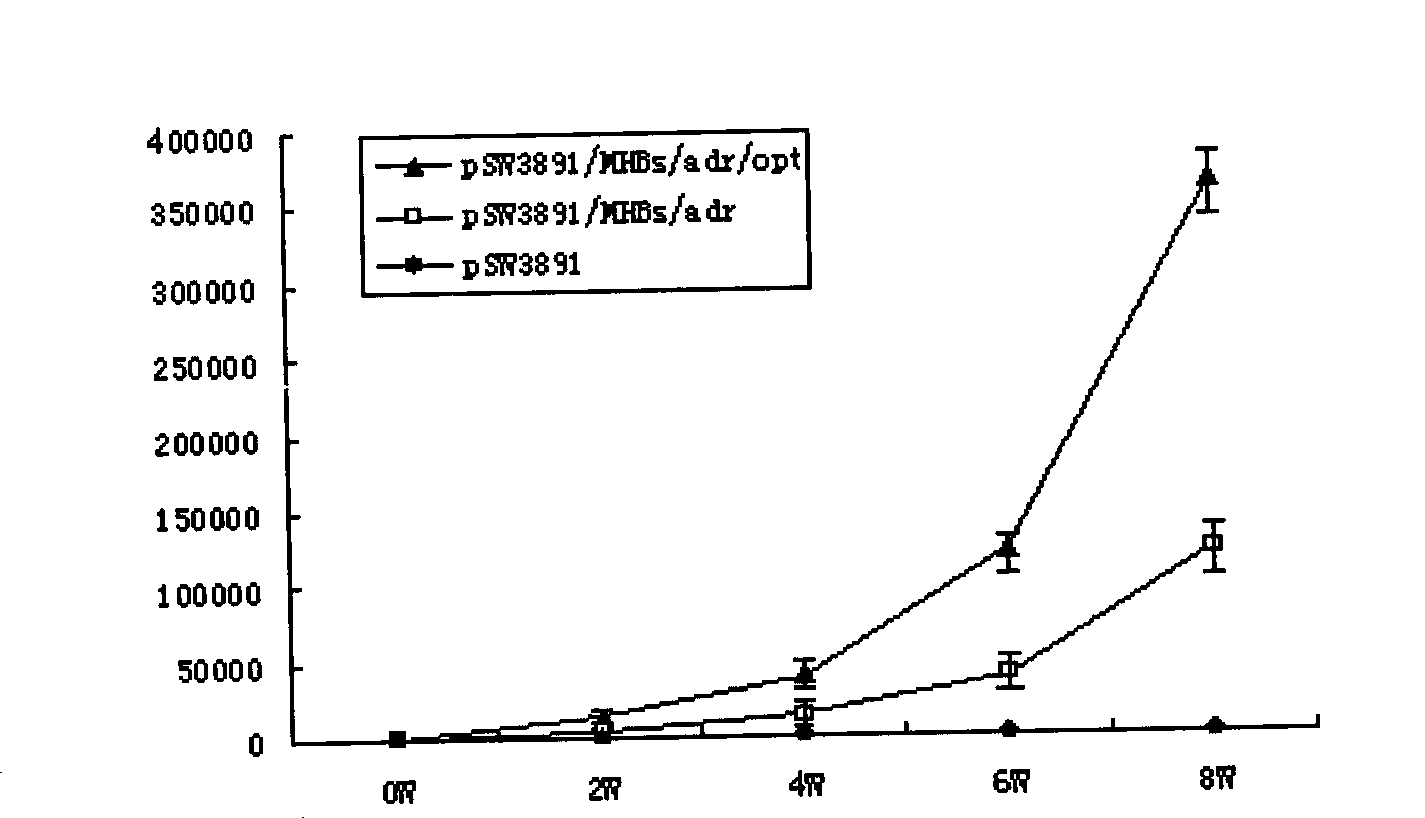
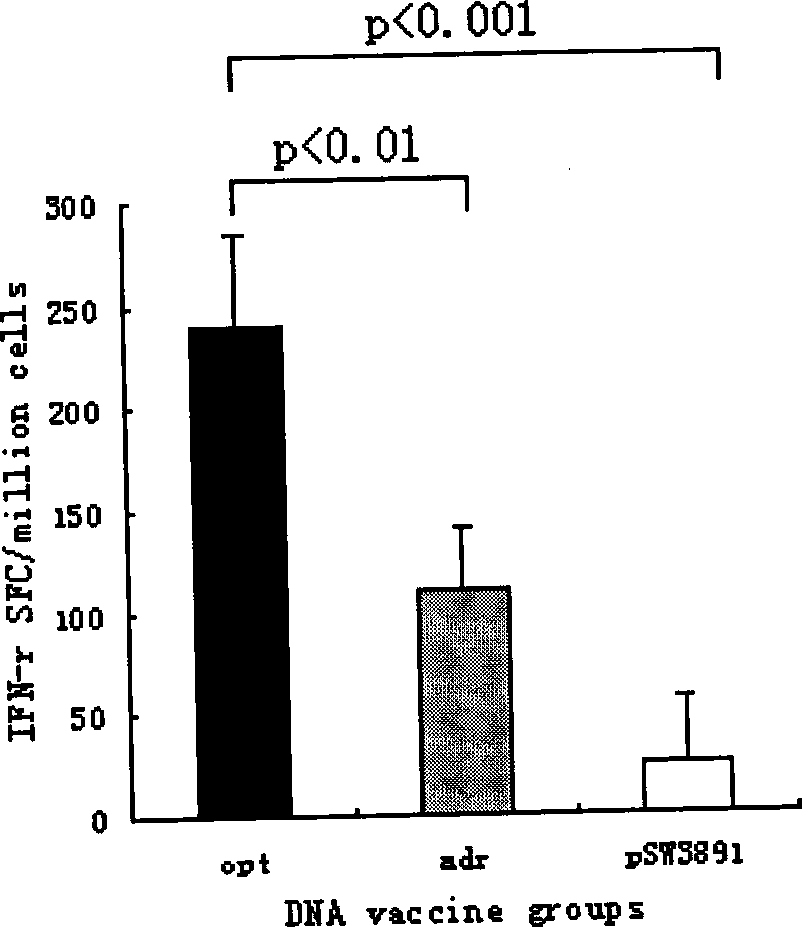
The invention provides a recombinant DNA sequence, hansenula polymorpha, a preparation method for hepatitis B surface antigen, and a hepatitis B vaccine. The recombinant DNA sequence is obtained by codon optimization of coding genes of the hepatitis B virus surface antigen according to codon usage frequency of the hansenula polymorpha. The invention also provides the hansenula polymorpha comprising the recombinant DNA sequence, a method for preparing adr sub-type hepatitis B surface antigen by using the recombinant DNA sequence, and the hepatitis B vaccine. The adr sub-type hepatitis B surface antigen has high expression level of the recombinant DNA sequence. The recombinant hansenula polymorpha is fast in growth speed, has high HBsAg yield, can be fermented in high cell density by using a cheap chemically synthetic medium, has low fermentation contamination rate and is beneficial to large-scale production; and HBsAg adr vaccine provided by the invention has high trend of Th1 and Th2 type cellullar immunologic response.
Owner:北京天坛生物制品股份有限公司
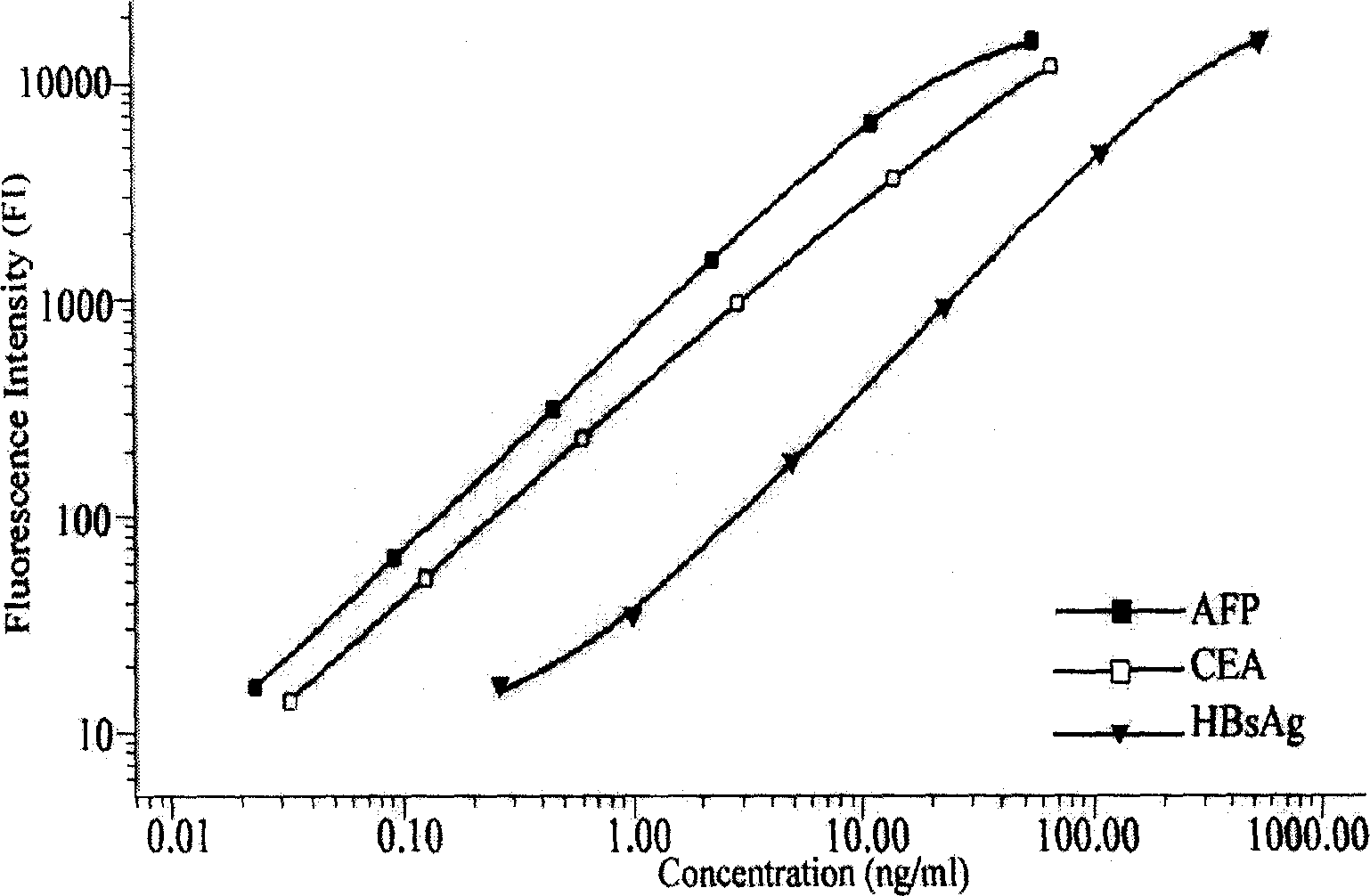
Preparation method of liquid phase protein chip
InactiveCN101144815AEarly detectionEarly treatmentMaterial analysisHigh risk populationsFluorescence
The present invention relates to the biologic technology field, and discloses a liquid phase albumen chip and the preparation and the usage method thereof which can simultaneously test human serum carcinoma embryonic antigen (CEA), Alpha fetoprotein (AFP), and hepatitis B surface antigen (HBsAg). The present invention couples the specificity antibody of CEA, AFP, and HBsAg on different fluorescence micro-spheres, and uses the test antibody marked by biotin or phycoerythrin to determine the nature quickly and quantitatively analyze the above three indexes with the double antibody sandwich method. The present invention uses the filtering membrane board when testing, and washes the board 3 times after each reaction is finished to increase the signal and improve the sensitivity. The present invention has high sensitivity, strong specificity, stable result, excellent repeatability, and simple operation; 1 micro liter serum sample can test three indexed simultaneously. The present invention is applicable to the health test and the general examination as well as the clinic test of the high risk population, and can facilitate the early diagnosis and the early treatment of the knub.
Owner:GUANGZHOU DARUI BIOTECH
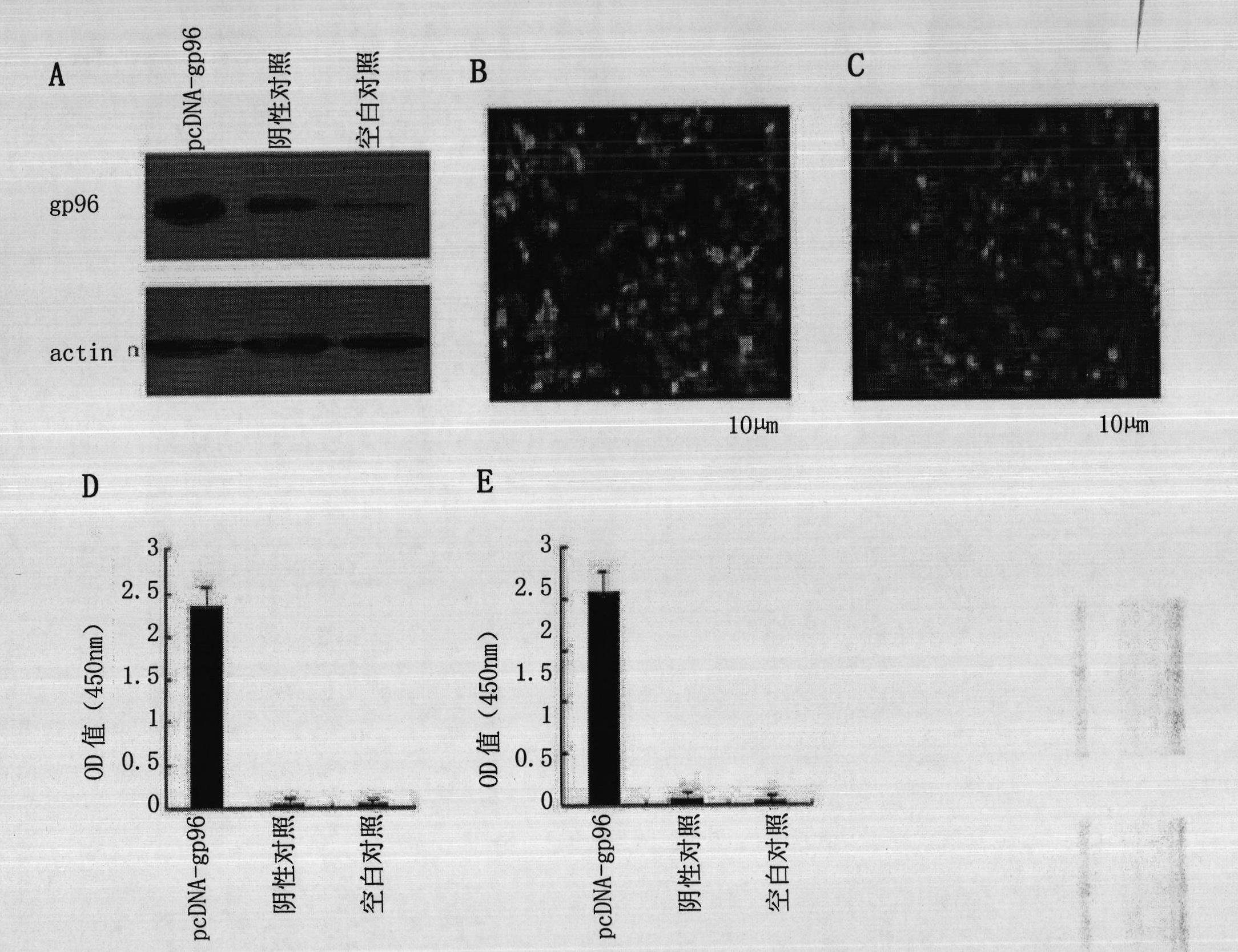
Therapeutic hepatitis B vaccine
ActiveCN102462840AInhibition of replicationDigestive systemAntiviralsActive componentHepatitis B Surface Antigens
The invention discloses a therapeutic hepatitis B vaccine. Active components of the therapeutic hepatitis B vaccine comprise a protein gp96, a hepatitis B surface antigen and a hepatitis B core protein. The protein gp96 has a sequence shown in the sequence 1 of the sequence table. The hepatitis B surface antigen has a sequence shown in the sequence 5 of the sequence table. The hepatitis B core protein has a sequence shown in the sequence 3 of the sequence table. The active components also comprise a plasmid pcDNA-gp96 containing a coding gene of the protein gp96, a plasmid pcDNA-HB containing a coding gene of the hepatitis B surface antigen and a plasmid pcDNA-HBc containing a coding gene of the hepatitis B core protein. The therapeutic hepatitis B vaccine provided by the invention can effectively inhibit hepatitis B virus (HBV) replication, can eliminate viruses infecting the liver and has a very important value to hepatitis B prevention and treatment.
Owner:北京热休生物技术有限公司
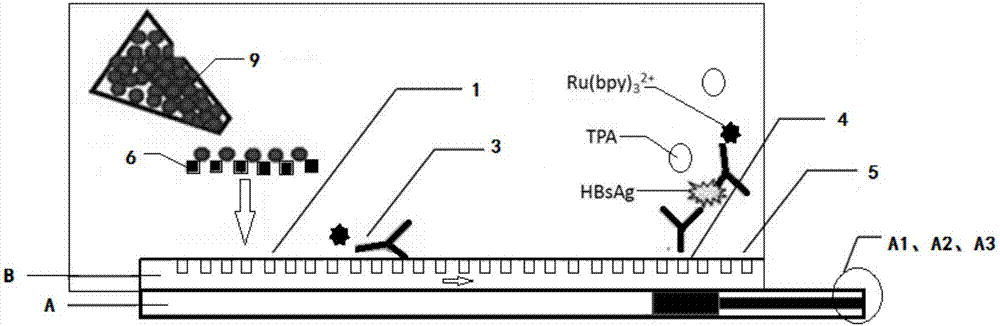
Kit for detecting hepatitis B surface antigen and detection method and application of kit
ActiveCN104698172AHigh sensitivityAvoid the problem of losing antigenBiological material analysisAnalysis by material excitationMicrosphereHook effect
The invention discloses a kit for detecting a hepatitis B surface antigen and a detection method and application of the kit, belonging to the technical field of in vitro diagnosis and detection. The kit comprises the following components: (1) a magnetic micro-spherical system which comprises magnetic microspheres directly connected or indirectly connected with an antiBsAg antibody 1, (2) a first marker system which comprises an anti-HBsAg antibody 2 directly or indirectly connected with a first tracer or a second tracer, and (3) a second marker system which comprises an anti-HBsAg antibody 2 directly or indirectly connected with the first tracer or the second tracer or an anti-HBsAg antibody 3 directly or indirectly connected with the first tracer or the second tracer. The kit and the detection method can be used for detecting the hepatitis B surface antigen and have the advantages of high sensitivity and no HOOK effect.
Owner:SHENZHEN NEW INDS BIOMEDICAL ENG
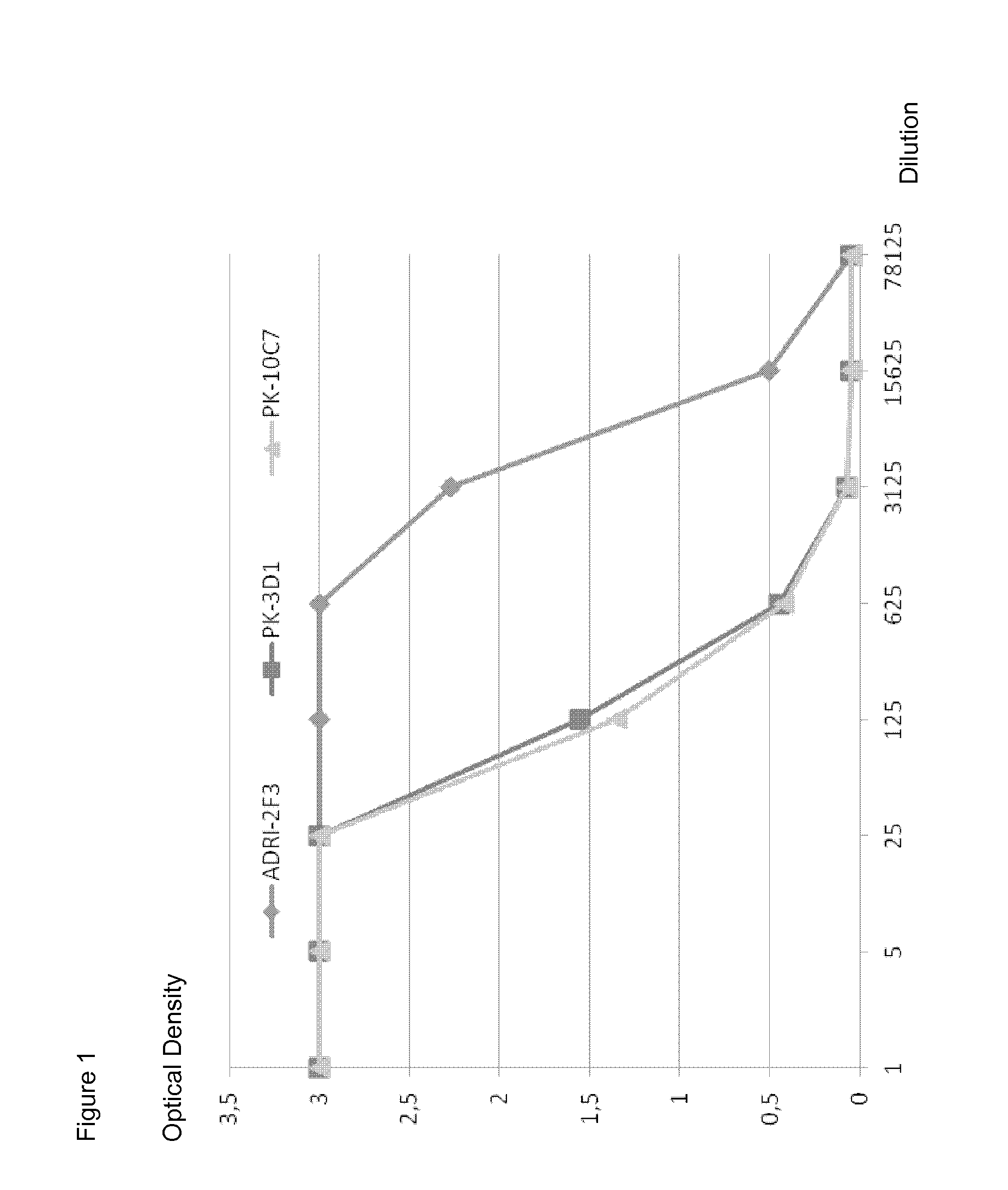
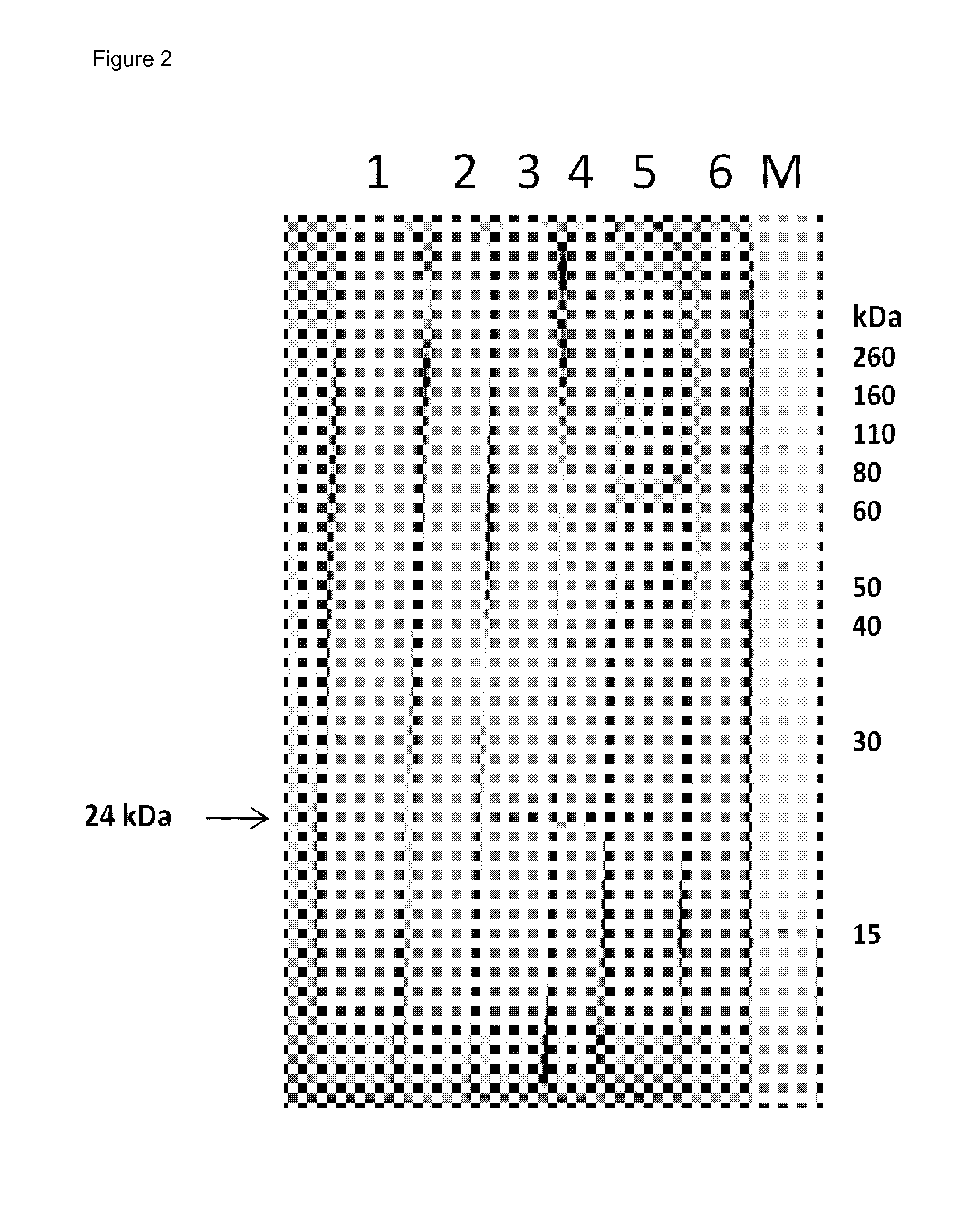
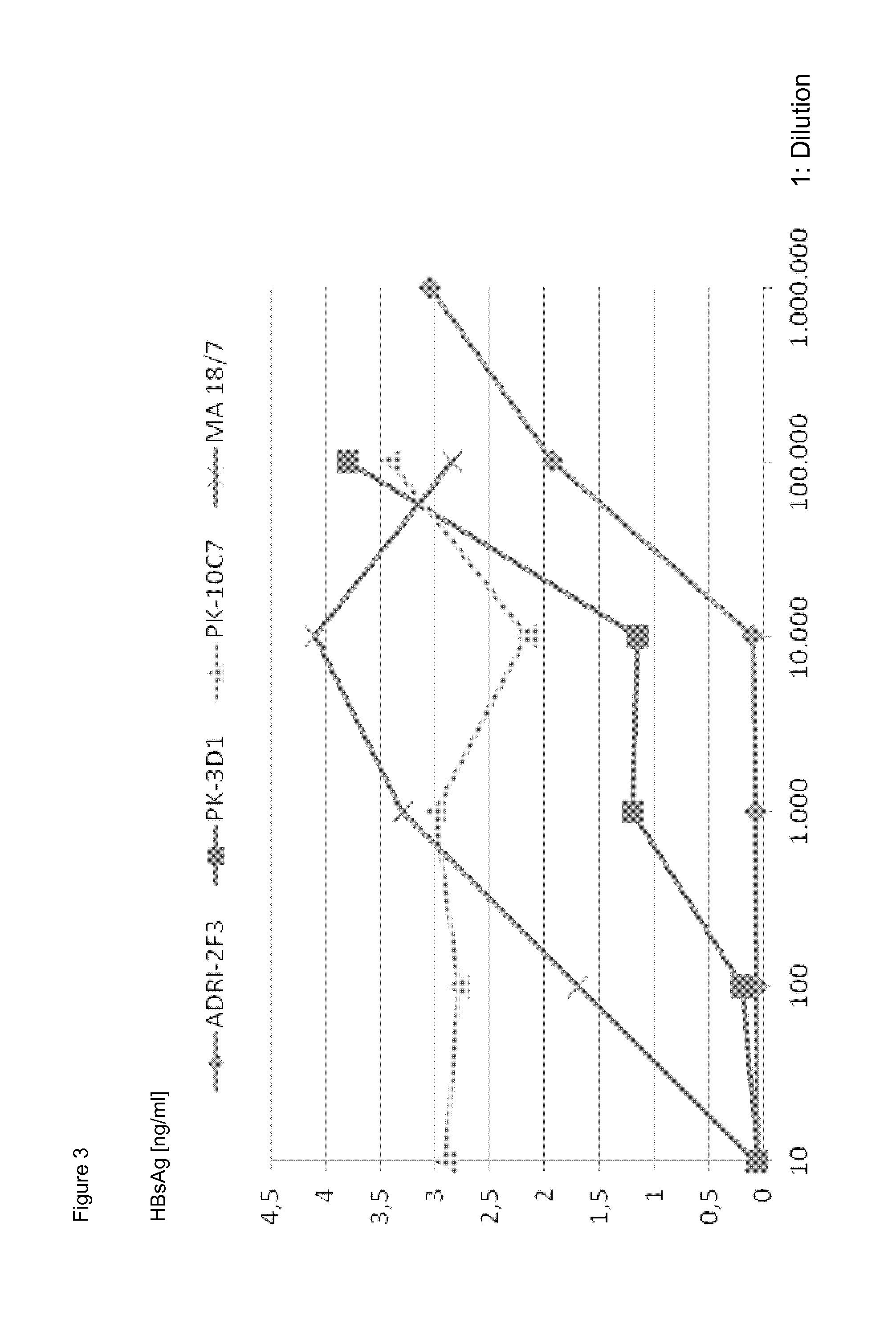
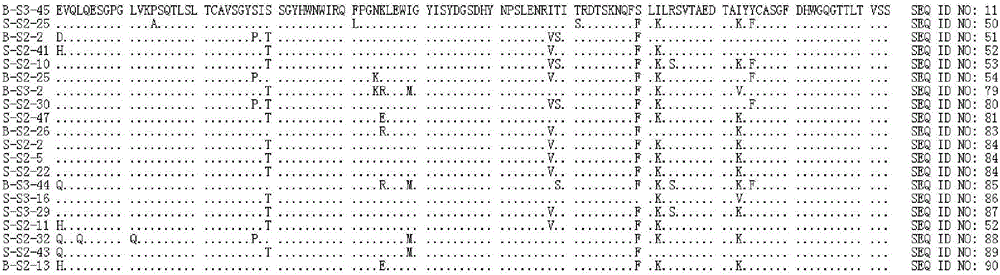
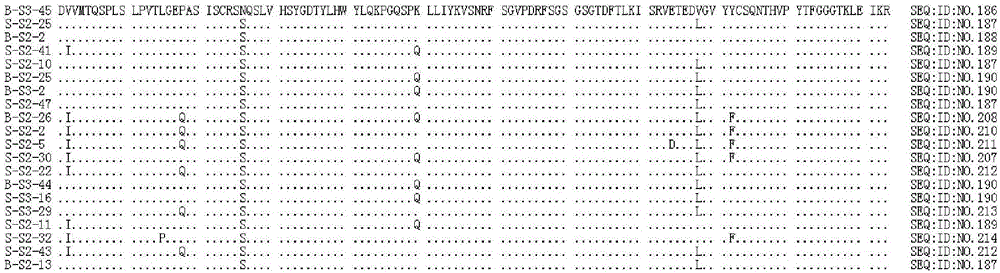
Neutralizing human monoclonal antibodies against hepatitis b virus surface antigen
ActiveUS20160326233A1Efficient and effectiveImmunoglobulins against virusesAntiviralsHeavy chainHepatitis B Surface Antigens
The invention is in the field of medical treatment and prevention. The invention provides an antibody or a part thereof capable of specifically binding to the Hepatitis B surface antigen (HBsAg) and having Hepatitis B Virus (HBV) neutralizing activity, wherein said antibody or fragment thereof comprises a light chain CDR1 region comprising the amino acid sequence of SEQ ID NO: 5, a light chain CDR2 region comprising the amino acid sequence of SEQ ID NO: 6, a light chain CDR3 region comprising the amino acid sequence of SEQ ID NO: 7, a heavy chain CDR1 region comprising the amino acid sequence of SEQ ID NO: 8, a heavy chain CDR2 region comprising the amino acid sequence of SEQ ID NO: 9 and a heavy chain CDR3 region comprising the amino acid sequence of SEQ ID NO: 10.
Owner:MONDELLI MARIO UMBERTO FRANCESCO
Chemiluminescence method for qualitative and quantitative detection of hepatitis B virus
InactiveCN1963512AAccurate calculationAccurate back calculationChemiluminescene/bioluminescenceHepatitis B Virus AntigenTest agent
This invention relates to hepatitis B virus chemical light qualitative and quantitative test method, which tests hepatitis B surface antigen through its HBsAg and tests hepatitis B surface antibody through antibody HBs chemical test agent case and tests hepatitis B virus antigen through hepatitis antigen HBeAg test agent case and tests hepatitis virus e antibody through virus e antibody chemical property and tests hepatitis virus core antibody through virus core antibody chemical property.
Owner:AUTOBIO DIAGNOSTICS CO LTD
Hepatitis b virus surface antigen as a mucosal immunostimulator and the resulting formulations
InactiveUS20050025780A1Enhance immune responseViral antigen ingredientsReverse transcribing DNA virusesHeterologous AntigensAdjuvant
The invention relates to a mucosal surface antigen which is used to promote and increase in the immune response against co-administered antigens in the formulations out line in the invention. Said novel formulations are obtained from the dual use of the surface antigen as an immunostimulatory agent and, at the same time, as a vaccine antigen. In this way it is possible to obtain multiple formulations of the hepatitis B surface antigen and heterologous antigens, with immunogenicity levels similar to those obtained following parenteral administration and with a reduction in components that can dispense with the use of nasal adjuvants, thereby converting same antigens into elements that can promote an increase in the response to the other co-administered antigens. Said novel use of the hepatitis B virus surface antigen and the resulting antigen formulations can be used in the pharmaceutical industry as therapeutic and preventive vaccine formulations.
Owner:CENT DE ING GENETICA & BIOTECNOLOGIA
Method for producing recombinant hepatitis B surface antigen
ActiveCN108330145AHigh purityHigh recovery rateVirus peptidesMicroorganism based processesAntigenUltrafiltration
The invention relates to a method for producing a recombinant hepatitis B surface antigen. According to the invention, having undergone bidirectional pressurization screening, a bacterial strain acquired by using the method is high in copy number and genetically stable; by controlling the concentration of methanol, high-density fermentation process is stable and the level of expression is high; and the recombinant hepatitis B surface antigen with high purity and high recovery rate can be obtained by subjecting yeast cells to fragmentation, clarification, silica gel adsorption, ion exchange, ultrafiltration, concentration, density gradient centrifugation and molecular sieve chromatography after fermentation, so the method has the characteristics of short process period, high antigen purity,uniform antigen particles and the like.
Owner:JIANGSU THERAVAC BIO PHARMA
hepatitis B surface antigen resistant antibody and application thereof
ActiveCN106565840ALow serum levelsEfficient removalImmunoglobulins against virusesAntiviralsSerum igeHepatitis B Surface Antigens
The invention relates to a hepatitis B surface antigen (HBsAg) resistant antibody (in particular to a humanized antibody), nucleic acid molecules for coding the antibody, a method for preparing the antibody and a pharmaceutical composition containing the antibody. In addition, the invention relates to application of the antibody and the pharmaceutical composition. The antibody and the pharmaceutical composition can be used for preventing and / or treating HBV infection and diseases (such as hepatitis B) related to the HBV infection, used for neutralizing the HBV virulence in the body of a subject (such as a person), or used for reducing the HBV DNA and / or HBsAg serum level in the body of the subject.
Owner:XIAMEN UNIV +1
Method for separating and purifying recombined hepatitis b surface antigen expressed by Hansenula yeast
InactiveCN101003564AHigh recovery of purification activityReduce stepsVirus peptidesPeptide preparation methodsAntigenFiltration
This invention relates to a method for separating and purifying recombinant hepatitis B virus surface antigen expressed in Hansenula polymorpha. The method comprises: (1) crushing culture solution of recombinant B virus surface antigen expressed in Hansenula polymorpha till 50-90% cells are crushed; (2) centrifuging to remove cell debris, adjusting pH value and electrical conductivity, performing anion exchange chromatography, and collecting the eluates with activity higher than 20%; (3) incorporating the eluates, adjusting the pH value and electrical conductivity, and performing hydrophobic chromatography; (4) concentrating the eluate with ultrafiltration membrane; (5) separating by a gel filtration column to obtain recombinant B virus surface antigen expressed in Hansenula polymorpha with purity higher than 99%. The method has such advantages as high recovery rate, high product purity, few steps, and short time.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
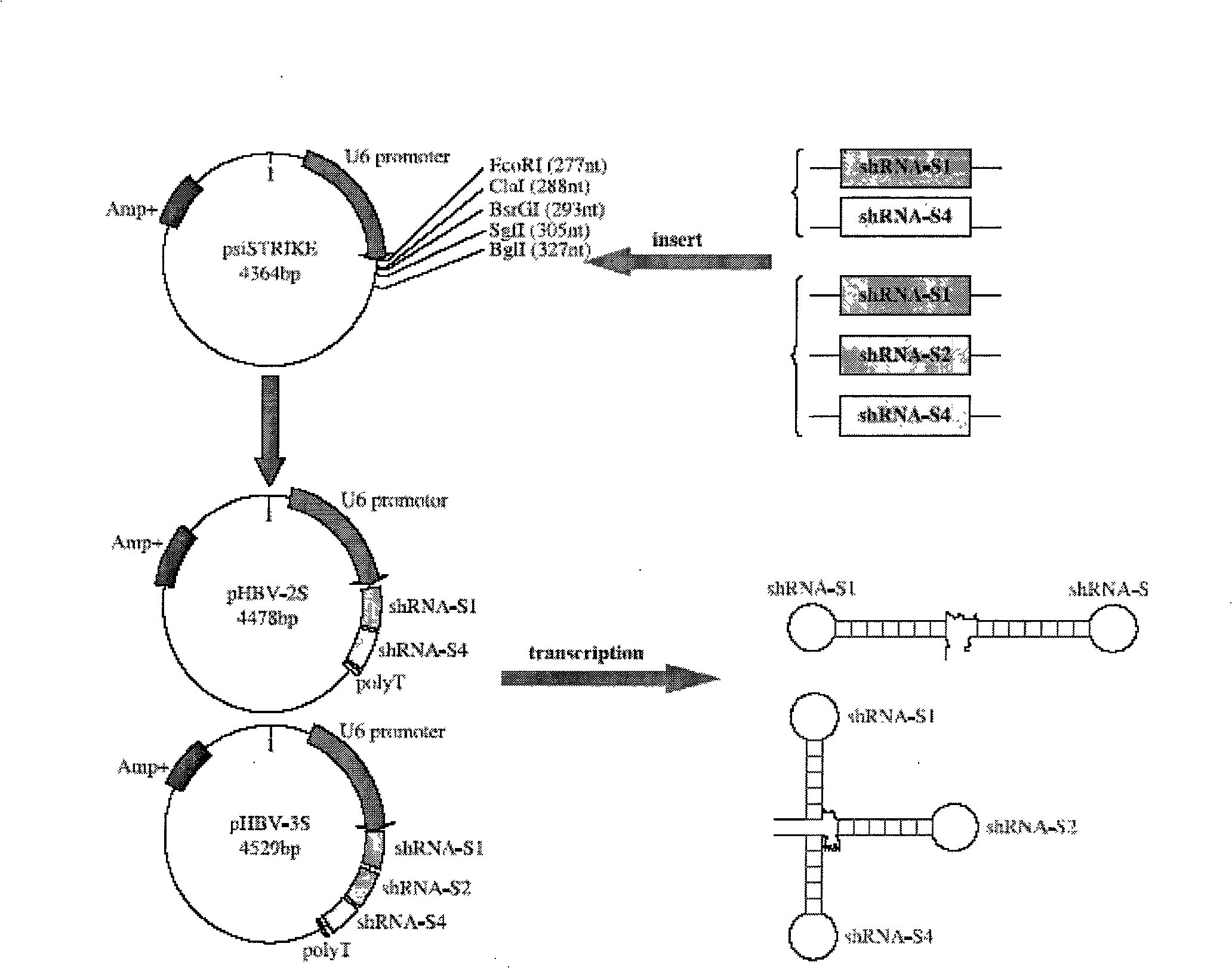
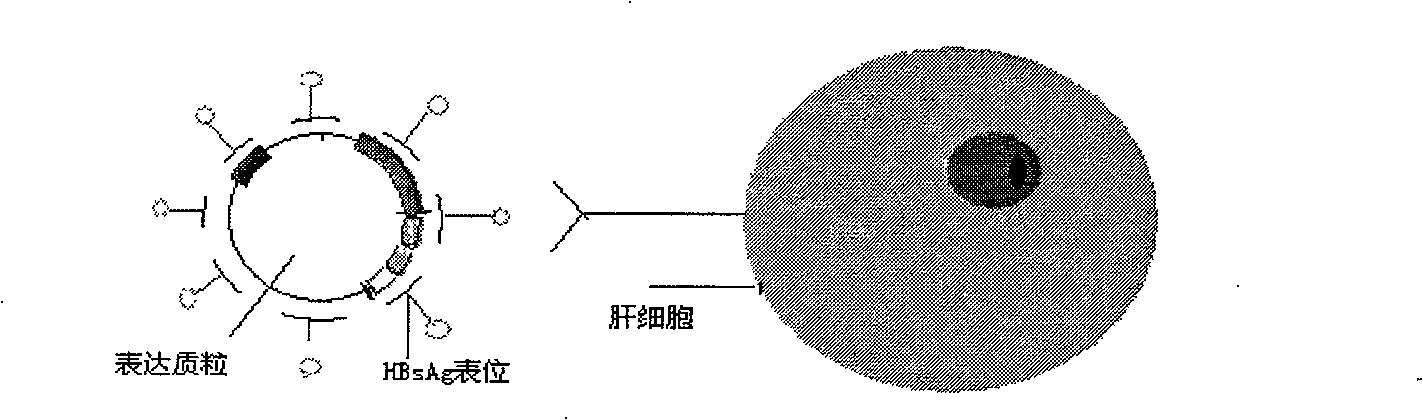
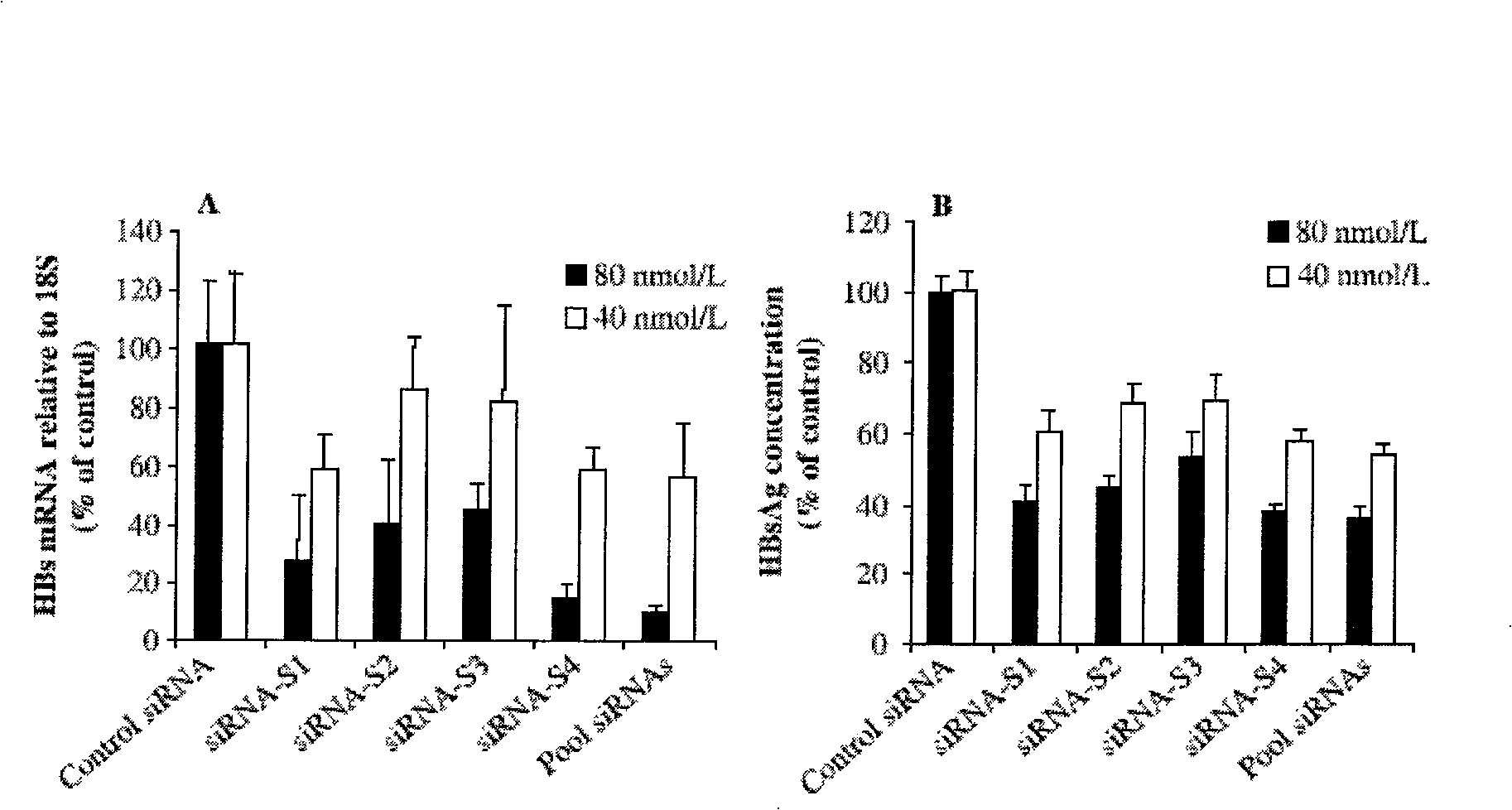
Anti-hepatitis b virus gene medicament based on siRNA pool interference and mediated by complex carrier
InactiveCN101322847AGood compatibilityIncrease targeting effectGenetic material ingredientsDigestive systemReceptor degradationHepatocyte
The invention discloses a siRNA interference-based anti-HBV gene-based drug mediated by complex carriers. Based on the characteristic of HBV and expression products thereof, active epitope protein in HBsAg expressed by HBV is biologically linked with cationic liposome and specially binds with hepatic cell surface receptors with the help of the epitope protein in the HBsAg to induce RNAi to enter hepatic cells; meanwhile, the meltability of bilayers of the cationic liposome and the cytolipin is used for increasing the efficiency of an siRNA expressed carrier entering into the hepatic cells. The drug of the invention can improve the effectiveness of the siRNA, biological stability in the human body, safety and effective targeting delivery and can be used in the clinical treatment.
Owner:淮安市第四人民医院
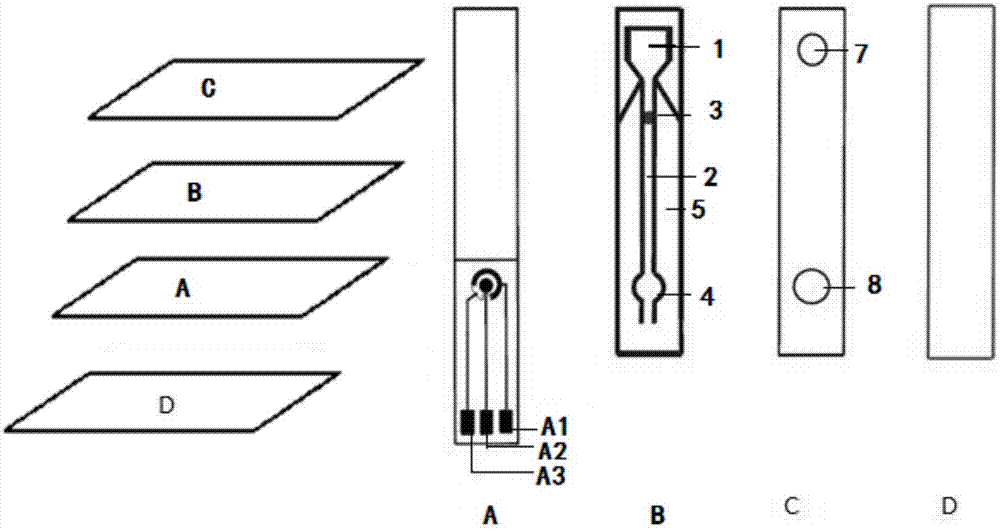
Integrated electrochemical light emitting paper micro-fluidic chip and preparation method and application thereof
InactiveCN106996929AVersatileHigh detection sensitivityChemiluminescene/bioluminescenceHepatitis B Surface AntigensElectrochemiluminescence
The invention discloses an integrated electrochemical light emitting paper micro-fluidic chip, a preparation method and a method using the chip to detect hepatitis B surface antigen. The preparation method of the paper micro-fluidic chip comprises the following steps of preparing an electrode layer; preparing a paper channel layer on paper, immobilizing antibodies in an antibody embedding area and a detection area, and covering the surface of a sample area with a blood filtering film, so as to functionalize the paper channel layer; using a hot press technique to prepare the electrochemical light emitting paper micro-fluidic chip which comprises an upper sealing layer, the paper channel layer, the electrode layer and a lower sealing layer from top to bottom. The method using the chip to detect the hepatitis B surface antigen comprises the following steps of dripping a full blood sample into a sample adding area, enabling the sample to generate immune reaction in the flowing process, cleaning, and putting into an electrochemical light emitting detector for detection. The integrated electrochemical light emitting paper micro-fluidic chip has the advantages that the functions are rich, the detection sensitivity is high, the use is simple and convenient, and the cost is low; the sample pretreatment, mixing, reaction and detection are integrated, and the chip can be widely applied to the field of medical detection.
Owner:GUANGZHOU GENERAL HOSPITAL OF GUANGZHOU MILITARY COMMAND
Methods for the treatment of hepatitis b and hepatitis d infections
ActiveUS20140065102A1BiocideOrganic active ingredientsHepatitis D InfectionHepatitis B Surface Antigens
It is disclosed a method for the treatment of HBV infection or HBV / HDV co-infection, the method comprising administering to a subject in need of treatment a first pharmaceutically acceptable agent that removes the hepatitis B surface antigen from the blood and a second pharmaceutically acceptable agent which stimulates immune function.
Owner:REPLICOR INC
Hepatitis B nucleic acid vaccine with optimized codon
InactiveCN101502650AHigh protein expressionImprove responseDigestive systemAntiviralsMammalDigestion
The invention relates to a hepatitis B virus nucleic acid vaccine optimized by codon. In the invention, hepatitis B surface antigen (HBs) gene order (adr subtype) is analyzed to find codon locus which tells the differences between the codon preferences of the gene order and the codon preferences of the mammal; the codon of the HBs gene order is replaced to obtain the surface antigen gene; the gene order is combined and expanded to obtain MHBs, Pst I, BamH I, double digestion MHBs gene and carrier pSW3891 plasmid optimized by the codon; 10ul connection system is configured to obtain a middle protein gene. The nucleic acid vaccine of the invention overcomes the defects that the differences between prokaryote and eukaryote in terms of codon preferences cause that the foreign gene can not be expressed effectively in mammal reservoir and can not generate relatively good immune sheltering effect; in addition, the invention remarkably improve protein expression of the foreign gene in the mammal reservoir, effectively stimulates immune system of the reservoir to generate relatively good immunological reaction of human body fluids and cellular immune response.
Owner:邢益平 +1
Hepatitis B virus surface antigen detection particles, preparation thereof and use thereof
InactiveCN101666801AReduce sensitivityHigh sensitivityChemiluminescene/bioluminescenceBiological testingSerum igeAntigen testing
The invention relates to a reagent for the diagnosis of hepatitis B and discloses hepatitis B surface antigen detection particles, which are luminous particles coated by a hepatitis B surface antigen.The invention also discloses the preparation and use of the hepatitis B surface antigen detection particles. In addition, the invention also discloses an in-vitro diagnostic kit for determining the hepatitis B surface antigen in a sample and also discloses the using method of the kit. The kit of the invention can be used in combination with other serums and clinic information to diagnose the acute or chronic hepatitis B infection condition of an individual and can also be used for screening hepatitis B in perinatal females to judge the hepatitis B infection risk of the newborns.
Owner:BEYOND DIAGNOSTICS (SHANGHAI) CO LTD
Application of imperatorin in preparing medicament for preventing and treating hepatitis or liver injury
ActiveCN101904839AEasy to manufactureWide variety of sourcesDigestive systemAntiviralsHepatitis B Surface AntigensVirus
The invention relates to the technical field of medicament, providing a novel application of coumarin compound imperatorin in preparing a medicament for preventing and treating virus hepatitis and resisting immunological liver injury or chemical liver injury. Pharmacological tests prove that the imperatorin has stronger activity of inhibiting hepatitis B surface antigen (HbsAg) and hepatitis B virus e antigen (HbeAg) and resisting the immunological liver injury or the chemical liver injury, so the imperatorin can be used for preparing the medicament for preventing and treating the virus hepatitis and resisting the immunological liver injury or the chemical liver injury. The imperatorin has the advantages of convenient preparation, wide sources, low price and high safety. The invention provides the novel application of the imperatorin, and provides a new medicinal source for preventing and treating the virus hepatitis and resisting the immunological liver injury or the chemical liver injury.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Combined reagent in colloidal gold method for detecting syphilis antibody and hepatitis b surface antigen
InactiveCN1627073AThe experimental results are simple and clearConvenient, quick and accurate detection methodMaterial analysisNitrocellulose filterAntigen
This method can test if a patient has been infected by syphilis or hepatitis B virus with a drop of blood, which combines two test measures for testing diseases of syphilis antibody and hepatitis B surface antigen on a same test paper, utilizes immune chromatographic technology and automatic micro-spraying equipment to fix the pured syphilis recombination antigen and anti-HBS single-clone antibody on a qualified nitrocellulose filter then to mix them labelled with colloidal gold in certain proportion to prepare a golden combined pad attached by proper sample process pads to form test paper slip, which quickly tests the syphilis antibody or hepatitis B surface antigen in a person blood, serum or plasma by double-antigen sandwich and double antibody sandwich methods.
Owner:深圳益生堂生物企业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com