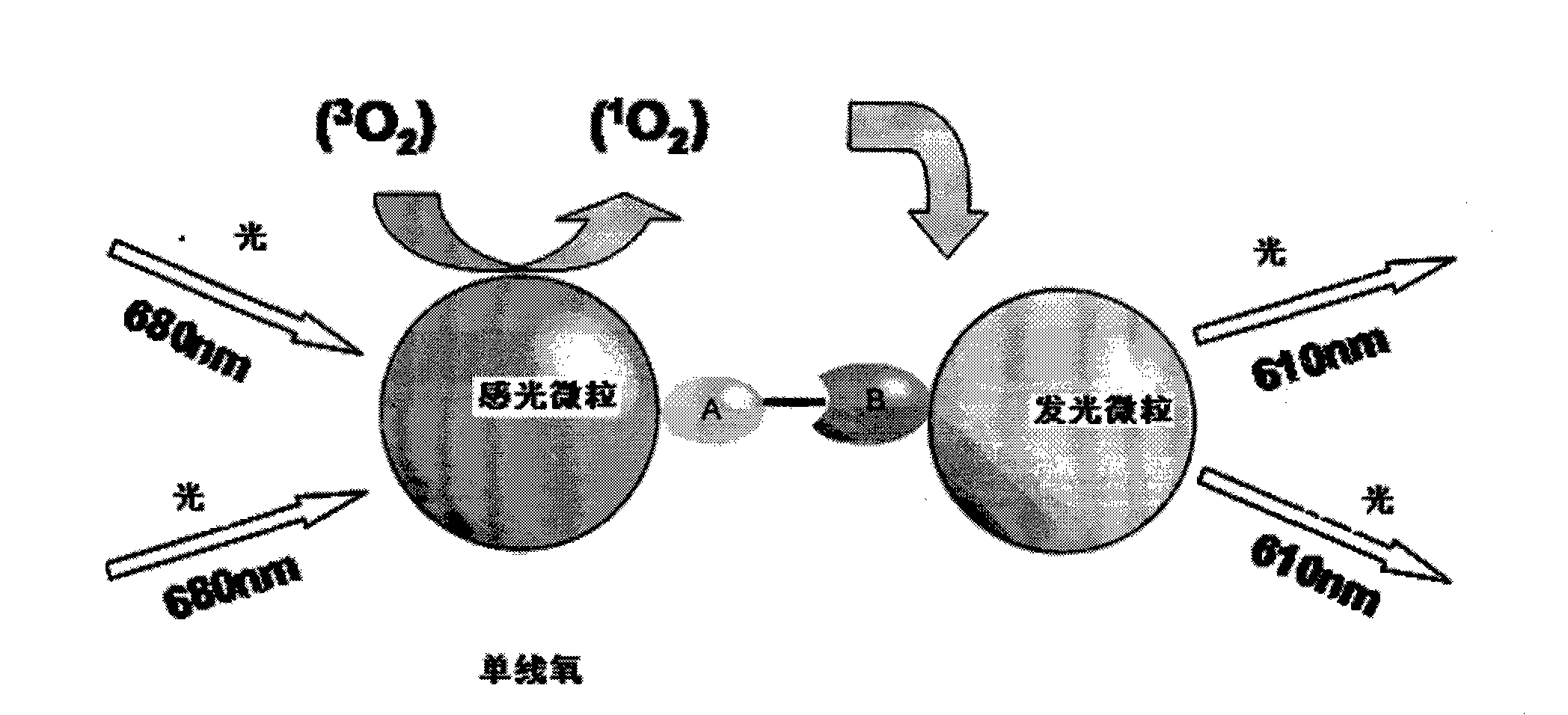
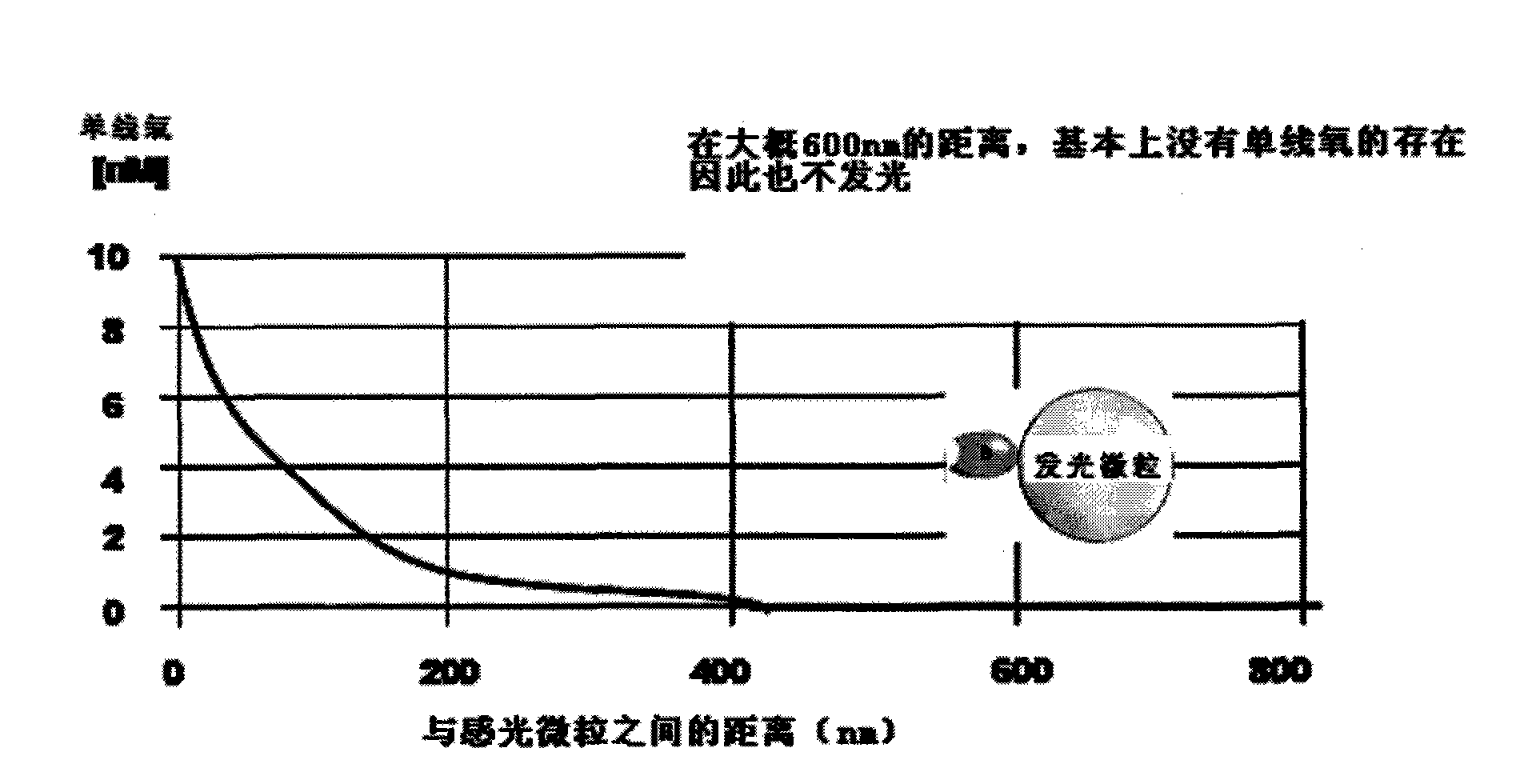
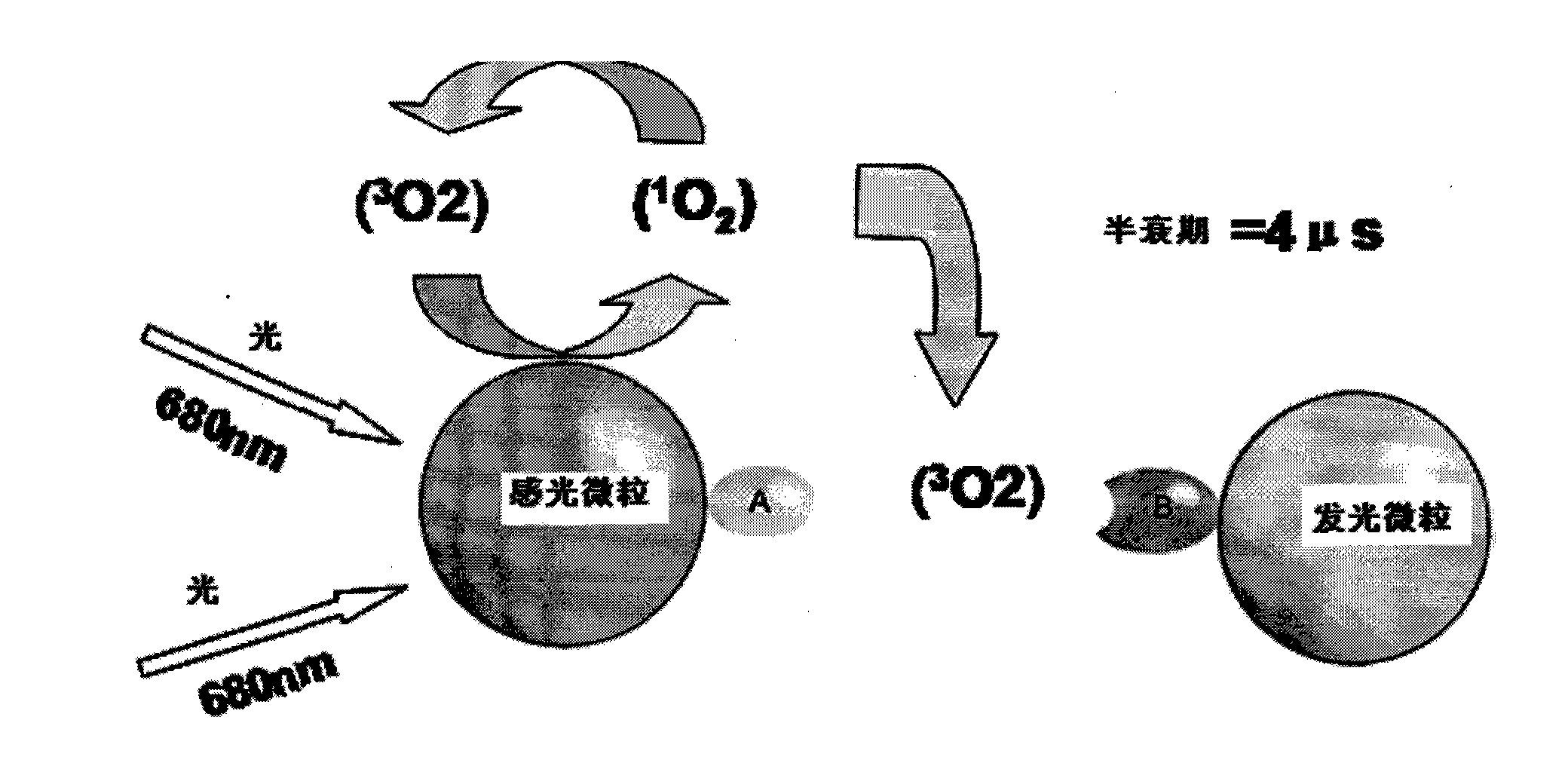Hepatitis B virus surface antigen detection particles, preparation thereof and use thereof
A hepatitis B and surface antigen technology, applied in the field of hepatitis B virus surface antigen detection particles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Example 1 Preparation of Surface Antibody-Coated Luminescent Particles
[0081] Preparation:
[0082] 1) Suspension treatment of luminescent particles: absorb a certain amount of luminescent particles and centrifuge in a high-speed refrigerated centrifuge, discard the supernatant, add a certain amount of MES buffer, ultrasonically break until the particles are resuspended, add MES buffer to adjust the concentration of luminescent particles to 100mg / ml.
[0083] 2) Antibody treatment: HBsAb was dialyzed against 0.05M MES buffer at pH 6.0 (hereinafter referred to as MES buffer). After the dialysis, the concentration was measured and adjusted to 8 mg / ml.
[0084] 3) MES buffer, 100mg / ml luminescent particle suspension (MES buffer) and 8mg / ml anti-HBe (MES buffer) and mixed at a volume ratio of 1:2:5, and mixed quickly , to obtain the reaction solution.
[0085] 4) Prepare 25mg / ml NaBH with MES buffer 3 CN solution was added at a volume ratio of 1:25 to the reaction solut...
Embodiment 2
[0123] Example 2 Preparation of biotin-labeled antibody
[0124] Preparation:
[0125] 1) Antibody treatment: dialyze HBsAb in 0.1M NaHCO 3 solution, the antibody concentration was measured and adjusted to 1 mg / ml.
[0126] 2) Prepare 16.17mg / ml Biotin solution with DMSO.
[0127] 3) Labeling: Take the processed 1 mg / ml HBsAb labeled antibody and the prepared Biotin solution, mix them according to the volume ratio of 72:10000, and mix them uniformly quickly. Stand at 4°C for 12-16 hours.
[0128] 4) Dialysis: Dialyze the reacted biotin-labeled antibody against biotin-labeled dialysis buffer (pH 8.00).
[0129] 5) Aspirate the dialyzed biotinylated antibody and transfer it to a clean centrifuge tube, and take a sample to determine the antibody concentration. Adjust the concentration of qualified biotin-labeled antibody to 0.5mg / ml.
[0130] React antibodies with Biotin in different ratios and detect:
[0131] Optical signal detection method:
[0132] 25 μl of samples we...
Embodiment 3
[0136] Example 3 Preparation of Photosensitive Microparticles Coated with Avidin
[0137]Photosensitive particles: use photosensitive particles with a particle size of 220±40nm (PentaTek, USA)
[0138] Preparation:
[0139] a. Treatment of photosensitive particle suspension: absorb a certain amount of photosensitive particles and centrifuge in a high-speed refrigerated centrifuge, discard the supernatant, add a certain amount of MES buffer, ultrasonically sonicate on the ultrasonic cell disruptor until the particles are resuspended, and then add MES buffer Adjust the photosensitive particle concentration to 100mg / ml.
[0140] b. Preparation of avidin solution: weigh a certain amount of Avidin, add MES buffer to dissolve to 8mg / ml.
[0141] c. Mixing: Mix the treated photosensitive microparticle suspension, 8 mg / ml Avidin and MES buffer at a volume ratio of 2:5:1, and mix quickly to obtain a reaction solution.
[0142] d. Reaction: Prepare 25mg / ml NaBH in MES buffer 3 CN so...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| power | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com