Patents
Literature
906 results about "In vitro diagnostic" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
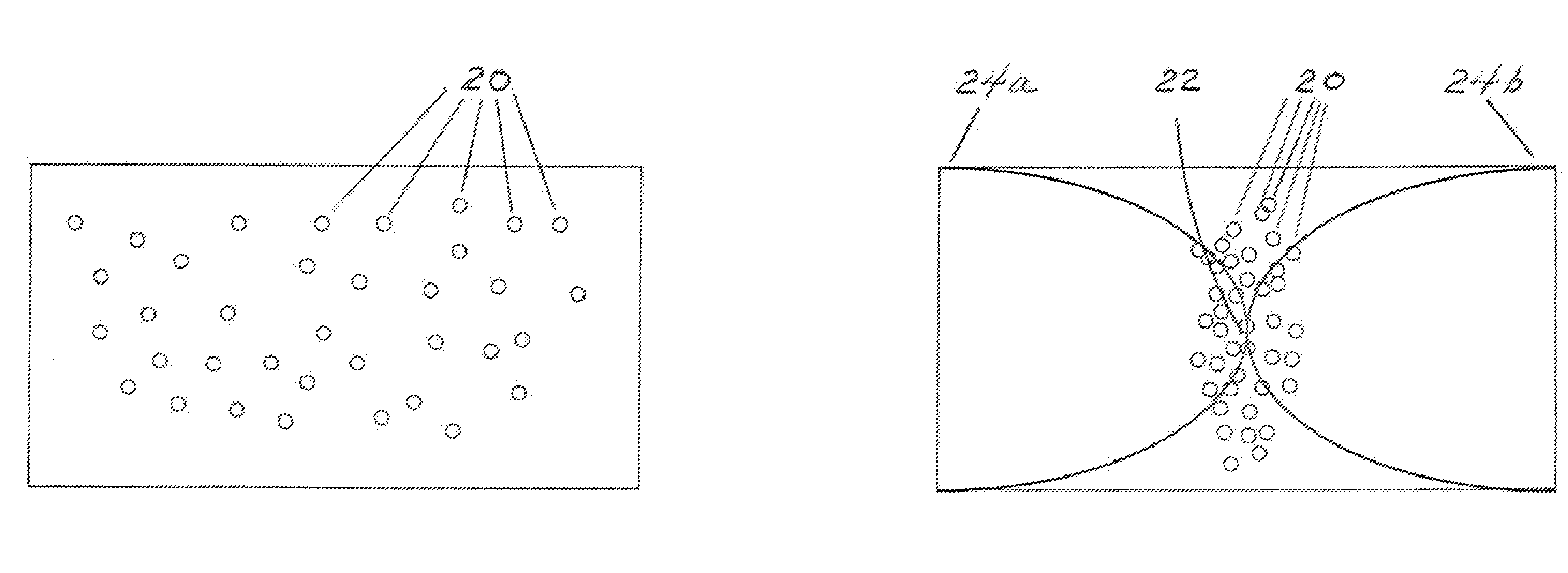
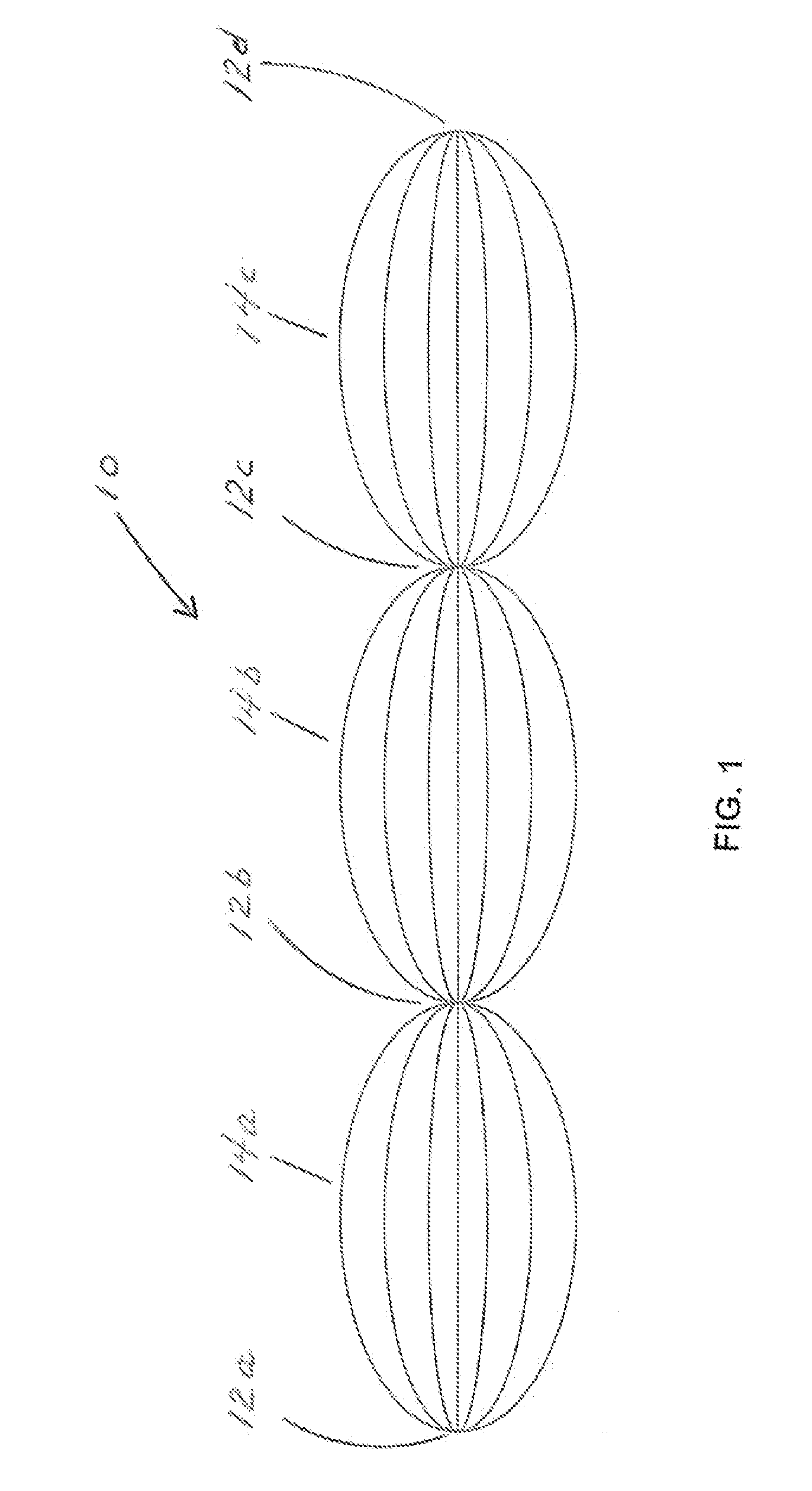
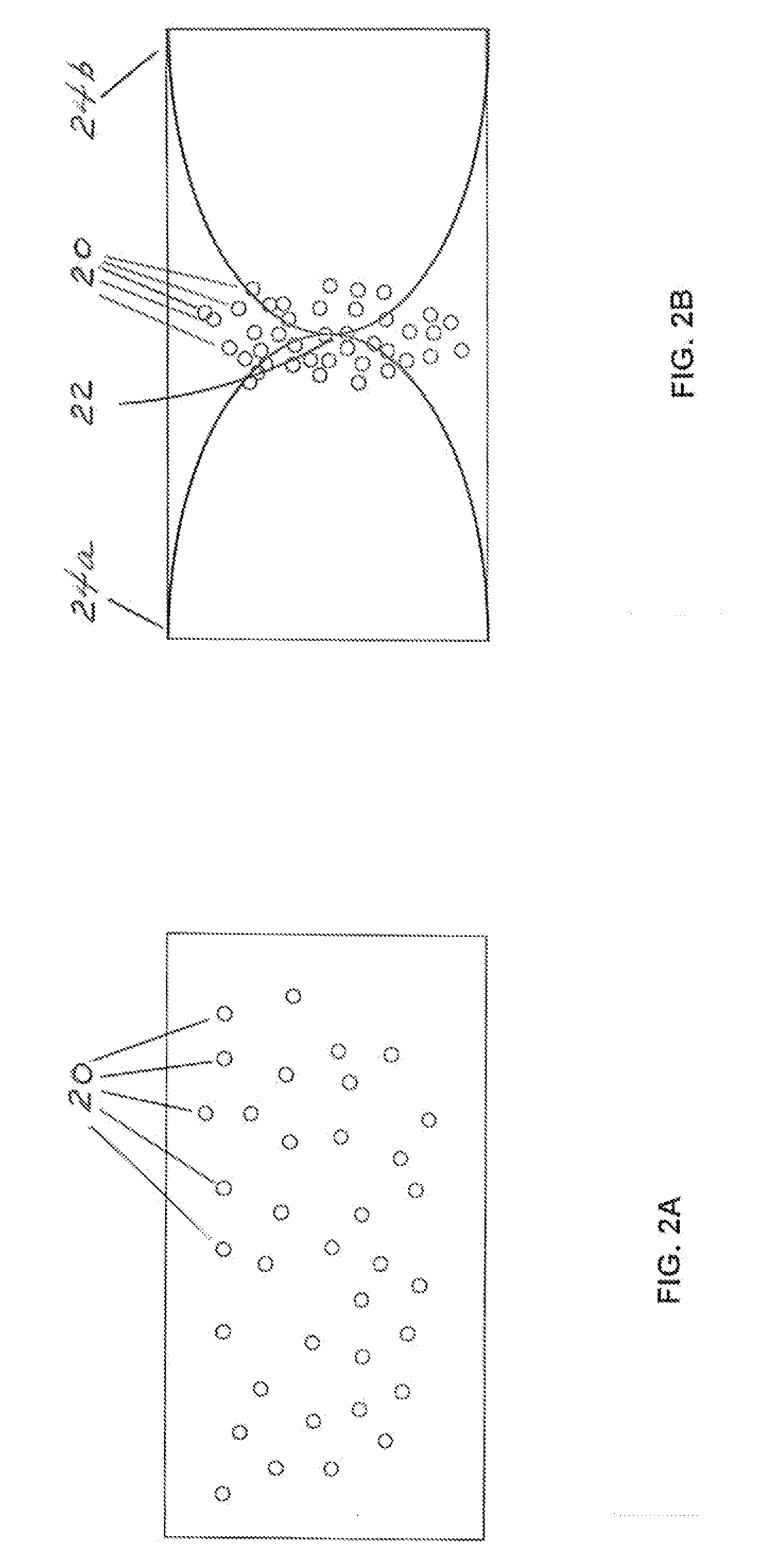
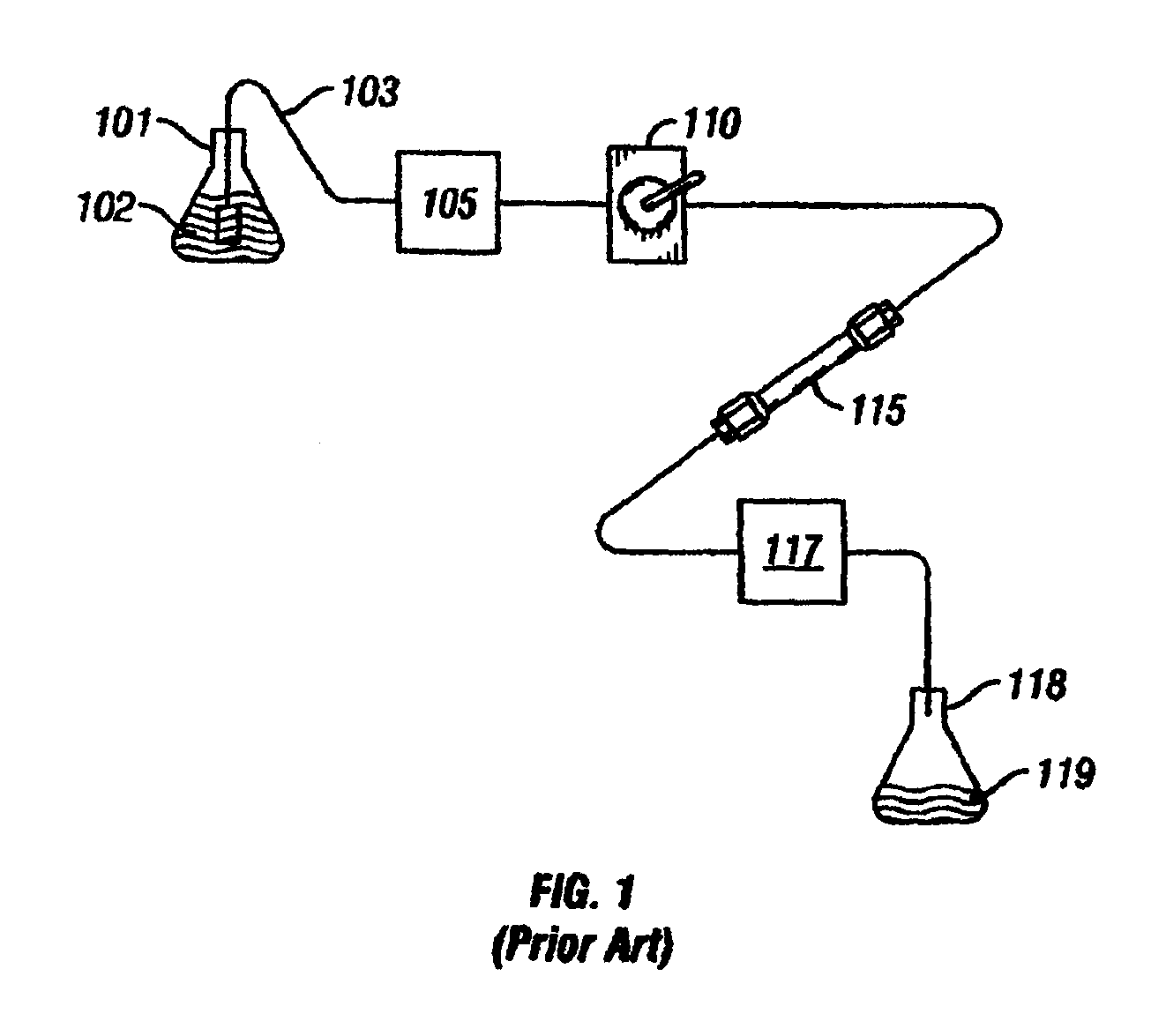
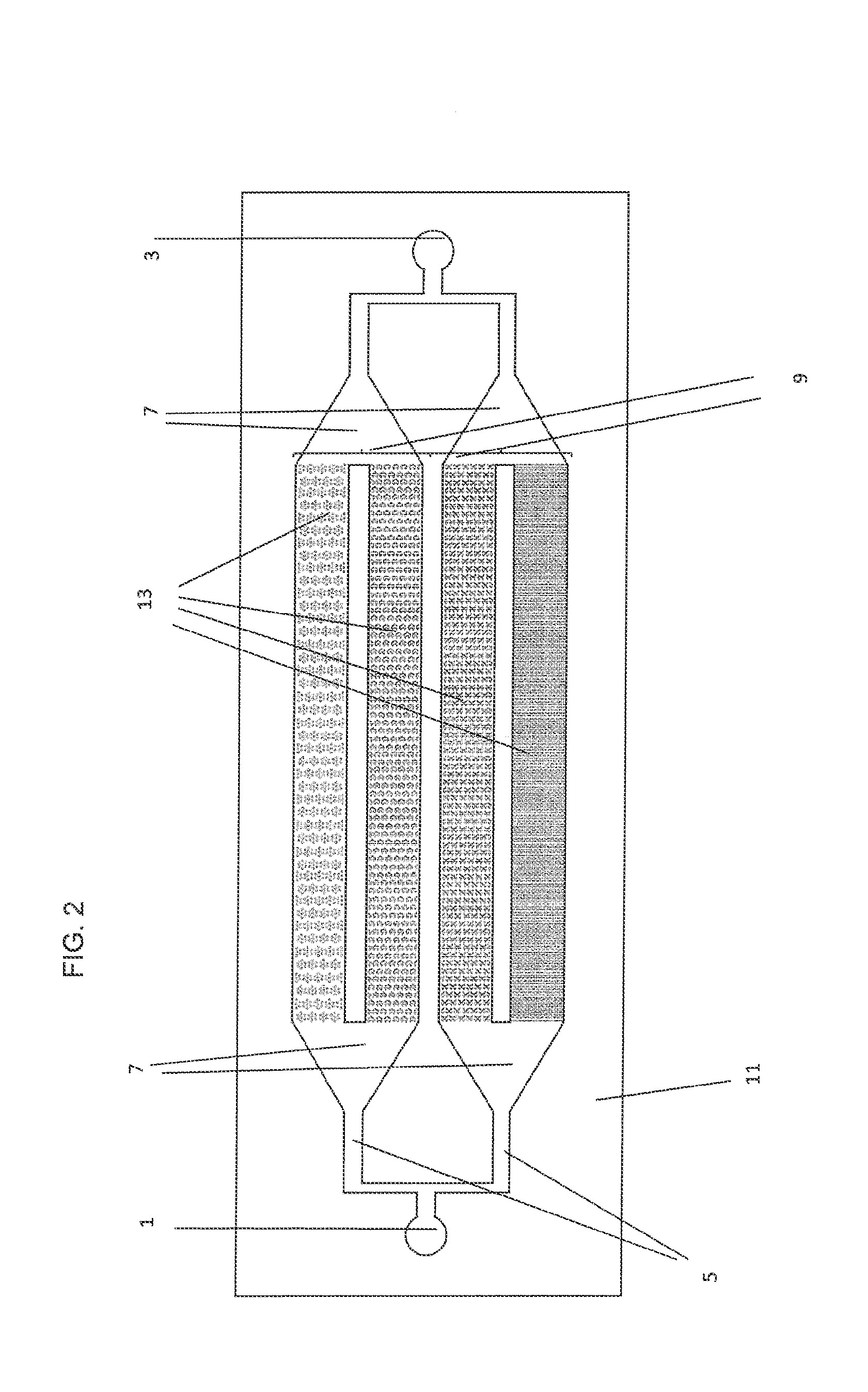
Apparatus and method for separation of particles suspended in a liquid from the liquid in which they are suspended
InactiveUS20100078384A1Improve concentrationAvoid turbulenceSemi-permeable membranesLiquid separation by electricityUltrasonic sensorCompressibility
A method for separating, or removing, particulate material, e.g., blood cells, from a sample of fluid, e.g., whole blood of a patient, in which the particulate material is suspended. In the case of separating blood cells from blood plasma or blood serum, the resulting samples of blood plasma or blood serum can be used for in vitro diagnostic applications. In normal practice, a whole blood sample of a patient are provided and then introduced into an apparatus that contains a flow channel. An acoustic field, which contains acoustic standing waves from external ultrasonic transducers, is located within the flow channel. Laminar flow is maintained in the flow channel. Blood cells and platelets are separated from blood plasma or blood serum at the end of the flow channel and collected. The method described herein allows fluid components to differentially migrate to areas of preferred acoustic interaction. The parameters that affect separation of particles are size, density, compressibility of the particles, and the fluid surrounding the particles.
Owner:ABBOTT LAB INC
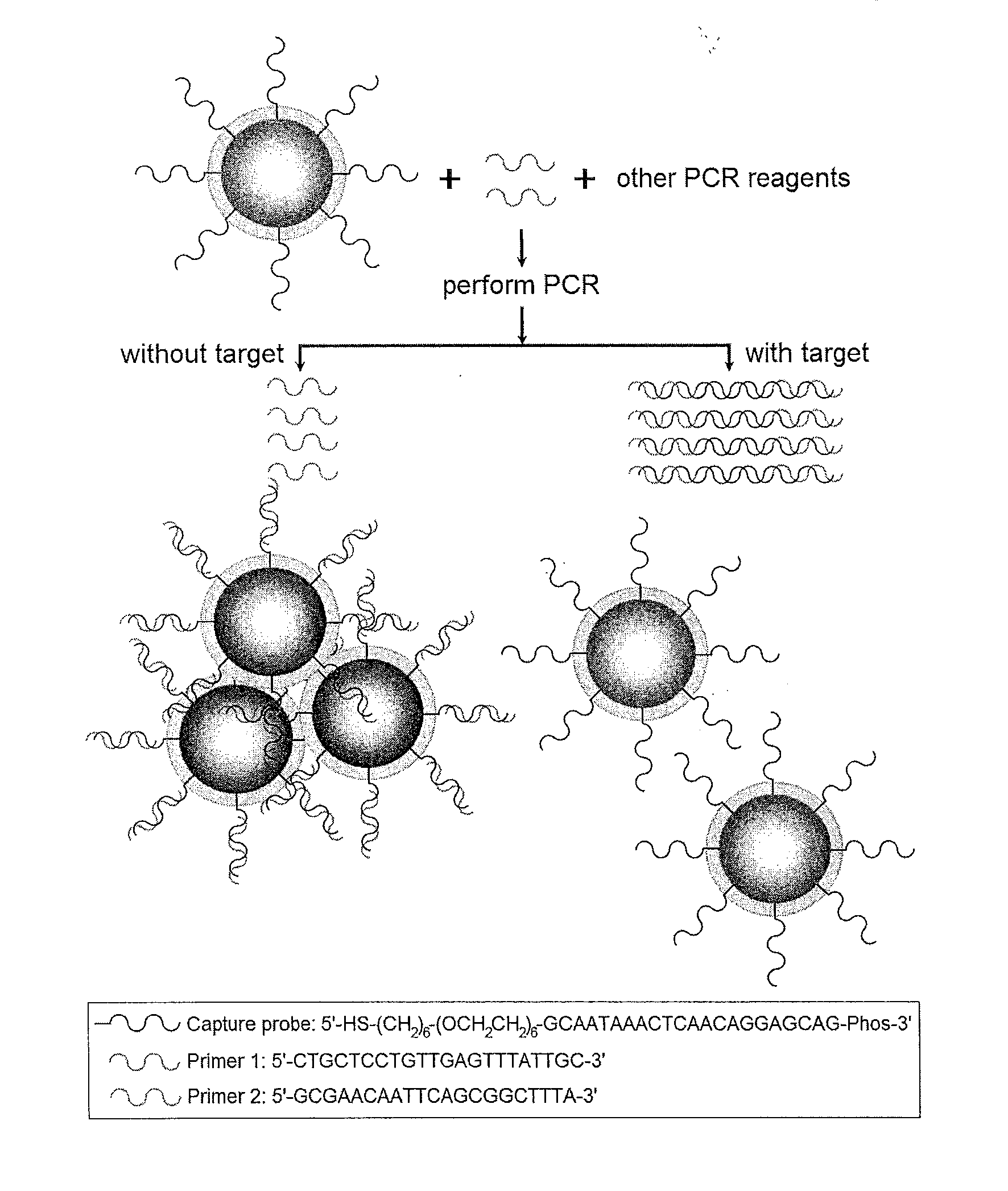
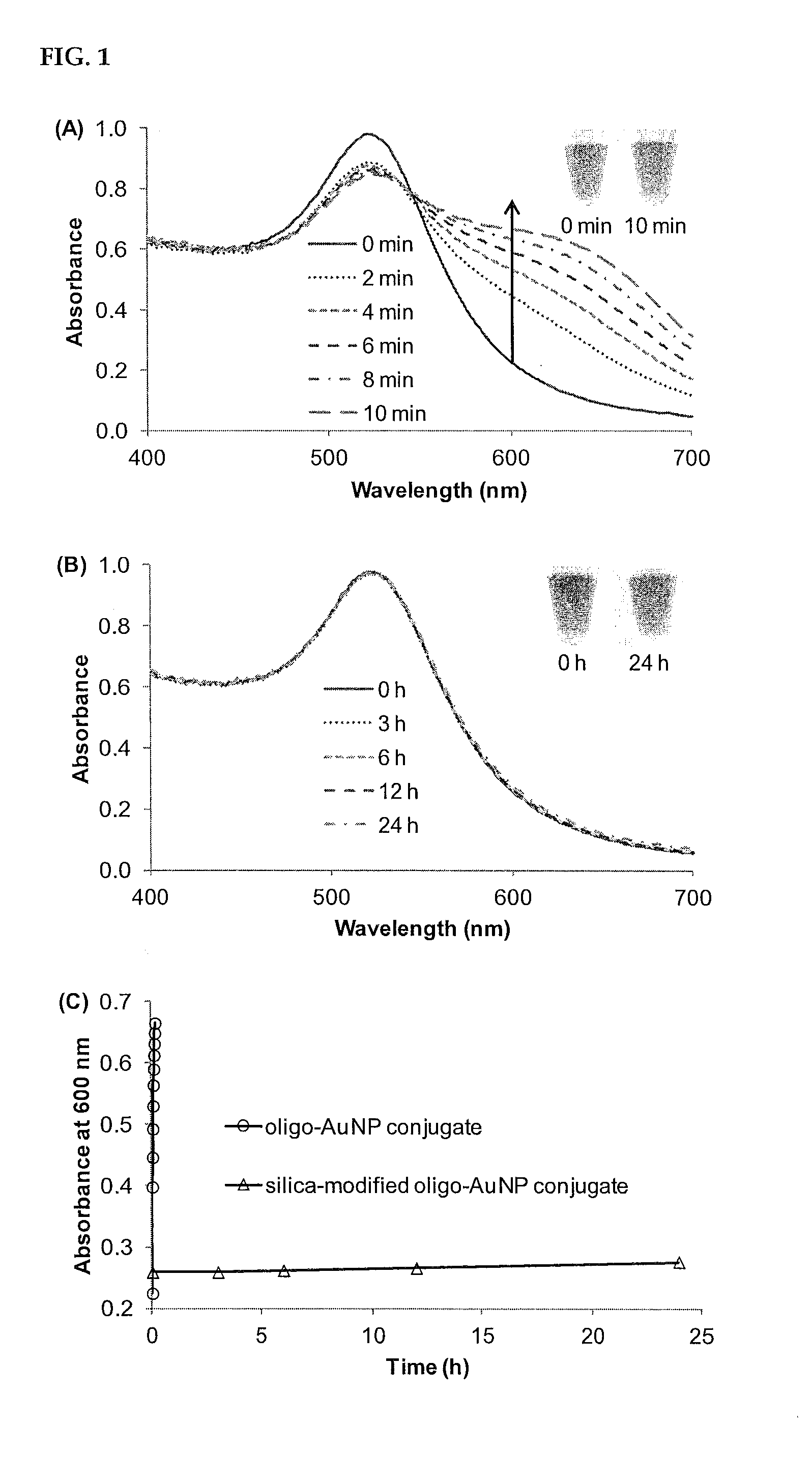
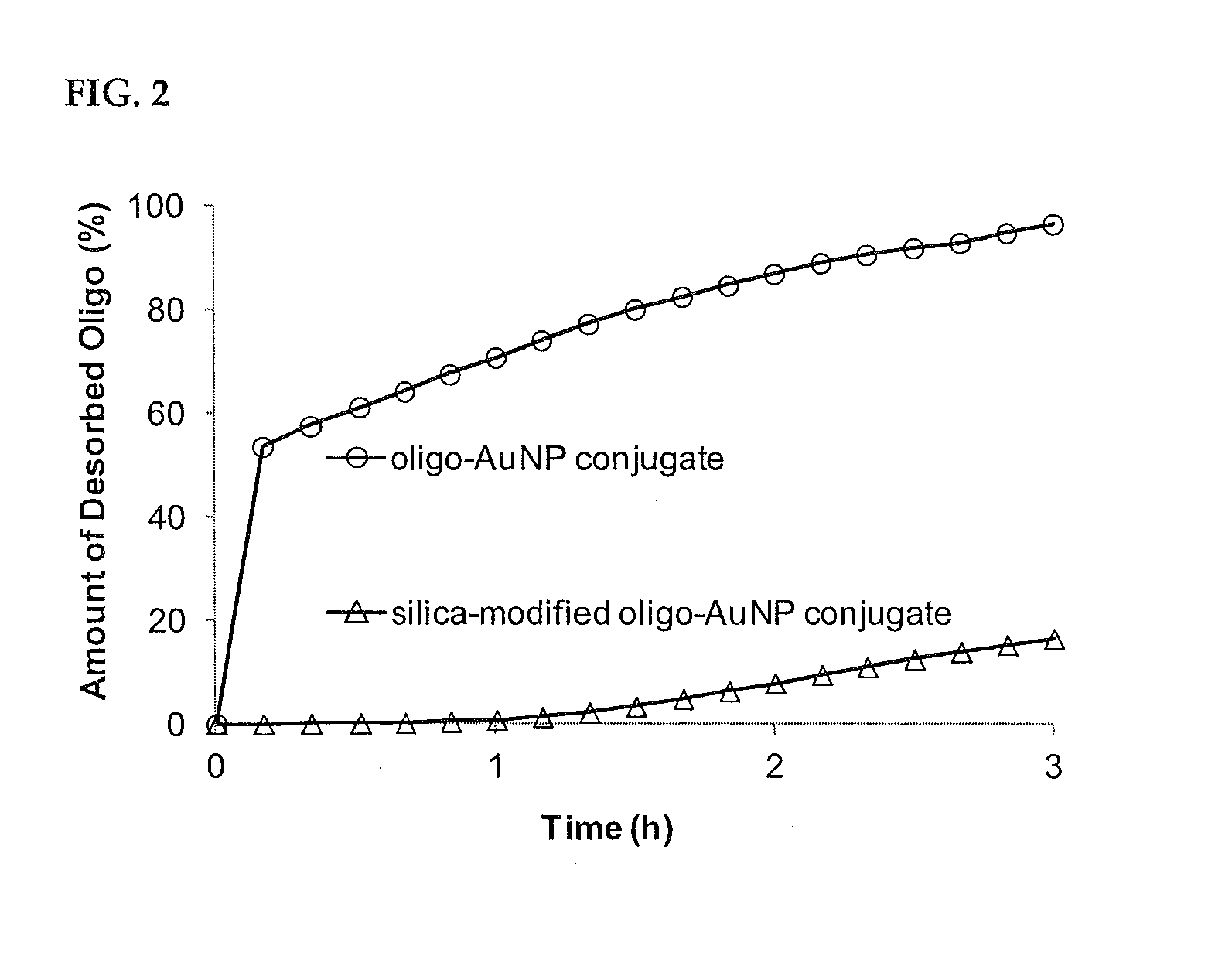
Ultra-Stable Oligonucleotide-Gold And-Silver Nanoparticle Conjugates And Method Of Their Preparation
ActiveUS20130022682A1Inexpensive and effectiveEasy to attachOrganic active ingredientsLiquid surface applicatorsIn vivoOligonucleotide
A method for stabilizing conjugates between macromolecule and nanoparticle by forming a thin reinforcement layer over the surface of a nanoparticle after macromolecule chains have attached to the surface of the nanoparticle. The stabilized conjugates can be used in a wide range of applications such as in vitro diagnostics, in vivo imaging and therapeutics, which need to be conducted under various severe or harsh conditions.
Owner:THE HONG KONG POLYTECHNIC UNIV
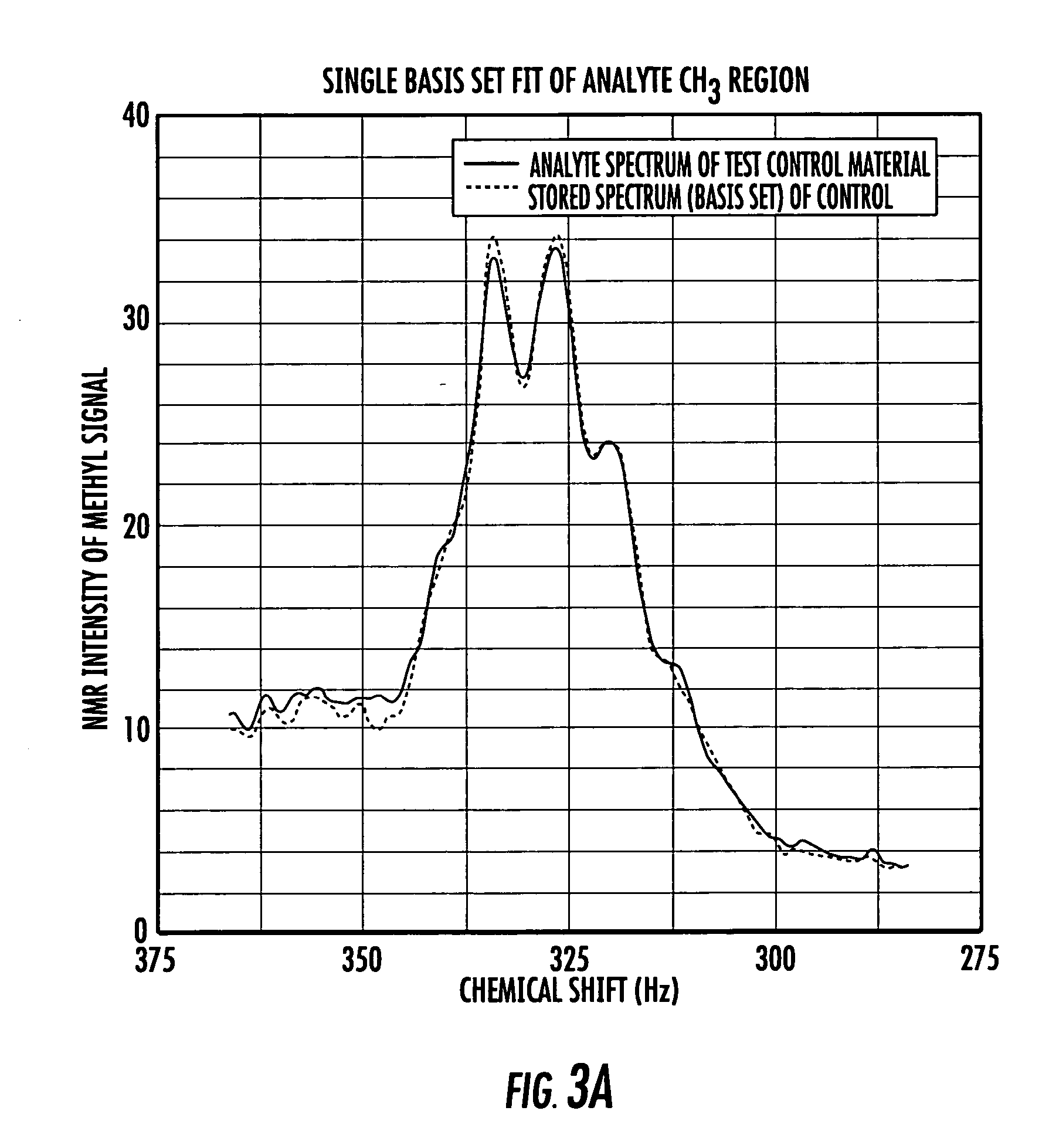
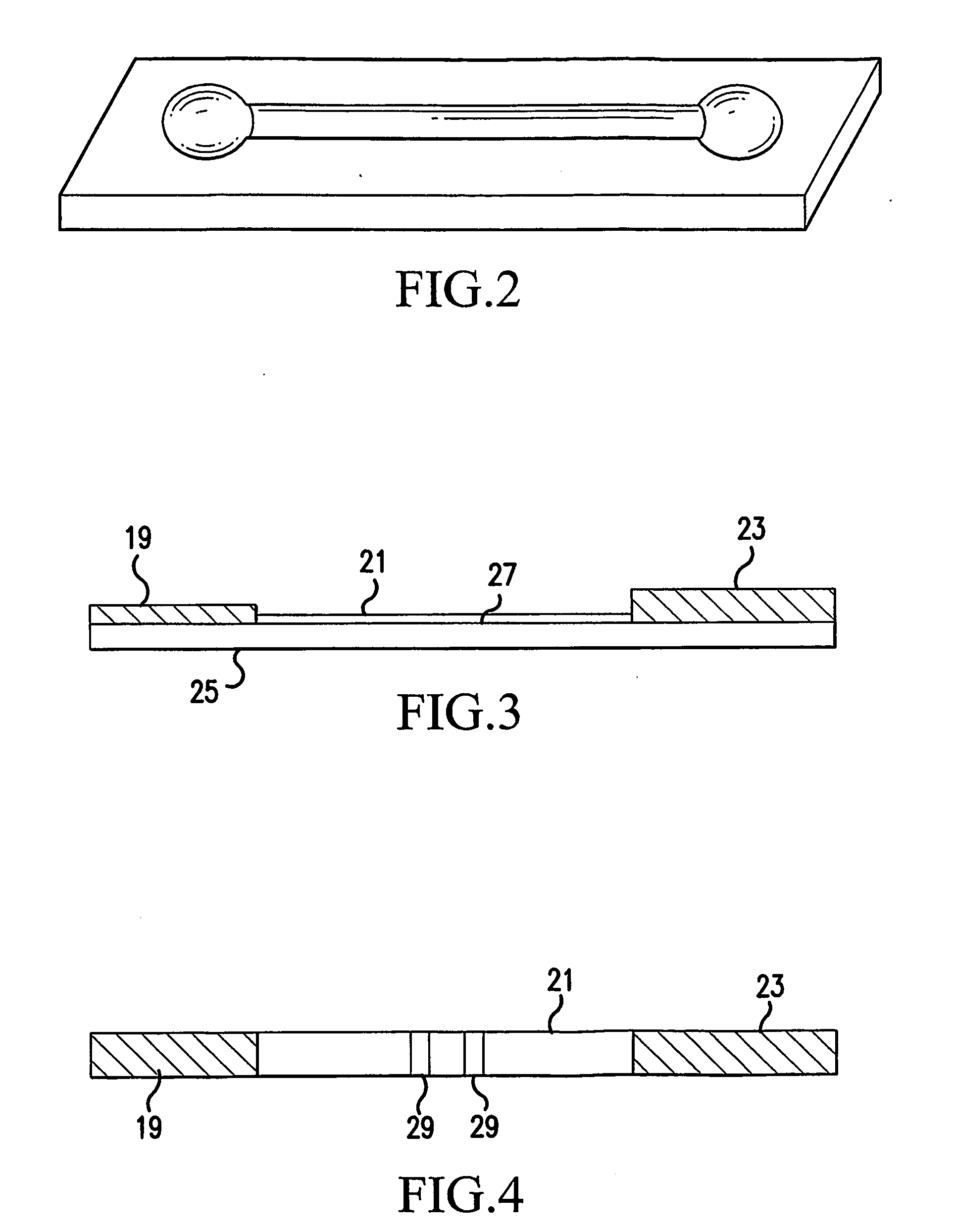
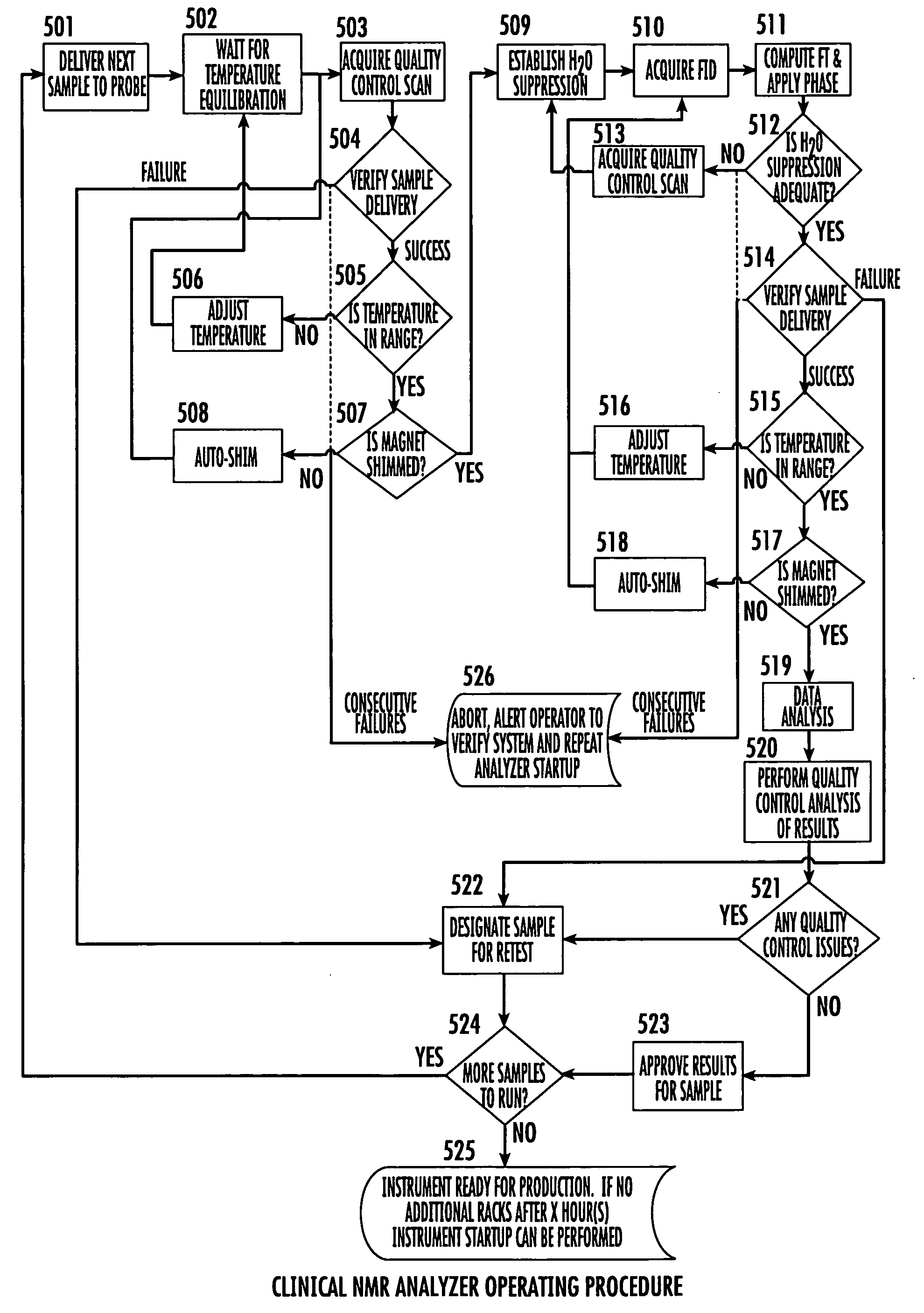
NMR clinical analyzers and related methods, systems, modules and computer program products for clinical evaluation of biosamples
ActiveUS20050222504A1Improve reliabilityAvoid downtimeMagnetic measurementsDiagnostic recording/measuringRemote systemRemote control
Methods, computer program products and apparatus automate clinical NMR in vitro diagnostic analyzers. The clinical analyzer can automatically electronically monitor selected parameters and automatically electronically adjust parameters to maintain the analyzer within desired operational ranges. The clinical NMR analyzers can be configured as a networked system with a plurality of clinical NMR analyzers located at different use sites; and at least one remote control system in communication with one or a plurality of clinical NMR analyzers, the at least one remote system configured to monitor selected local operating parameters associated with a respective clinical NMR analyzer.
Owner:LIPOSCI
Peptide sequences specific for the hepatic stages of P. falciparum bearing epitopes capable of stimulating the T lymphocytes
The present invention relates to an in vitro diagnostic method for malaria in an individual comprising placing a tissue or a biological fluid taken from an individual in contact with a molecule or polypeptide composition, wherein said molecule or polypeptide composition comprises one or more peptide sequences bearing all or part of one or more T epitopes of the proteins resulting from the infectious activity of P. falciparum, under conditions allowing an in vitro immunological reaction to occur between said composition and the antibodies that may be present in the tissue or biological fluid, and in vitro detection of the antigen-antibody complexes formed. The invention further relates to a polypeptide comprising at least one T epitope from a liver-stage specific protein produced by P. falciparum and a vaccine composition directed against malaria comprising a molecule having one or more peptide sequences bearing all or part of one or more T epitopes resulting from the infectious activity of P. falciparum in the hepatic cells.
Owner:INST PASTEUR
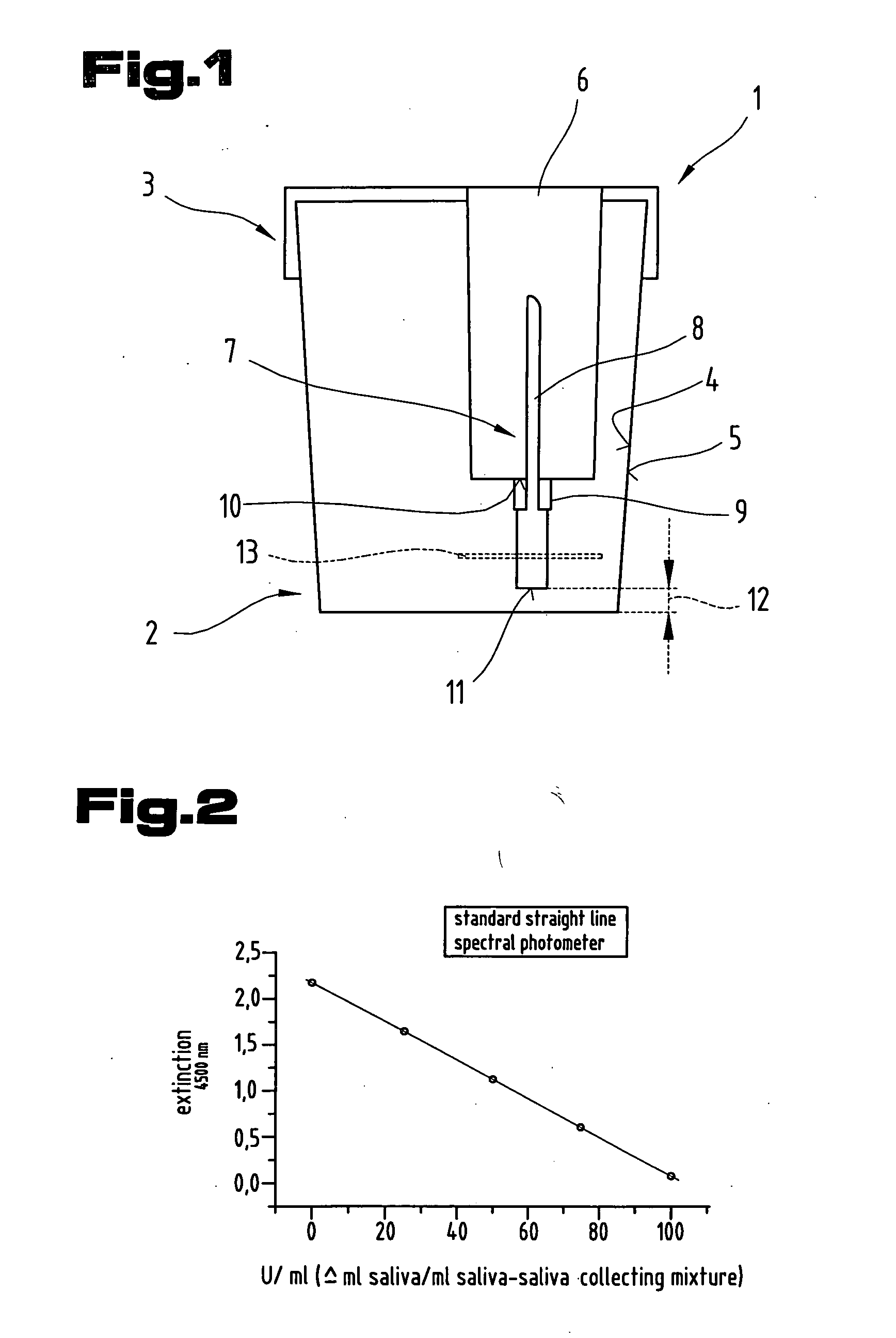
In-vitro diagnostic medical devices for determining saliva volume
InactiveUS20060073538A1Good reproducibilityImprove reliabilityMicrobiological testing/measurementVaccination/ovulation diagnosticsDentistryMedical device
The invention relates to a method of collecting saliva from the oral cavity for detecting a test substance, comprising the steps of (a) cleaning the oral cavity, (b) stimulating saliva secretion with a saliva-collecting solution, (c) removing the saliva-saliva collecting solution mixture from the oral cavity and collecting it in a container (1), (d) transferring the saliva-saliva collecting solution mixture into a sealable collection vessel.
Owner:GREINER BIO ONE GMBH
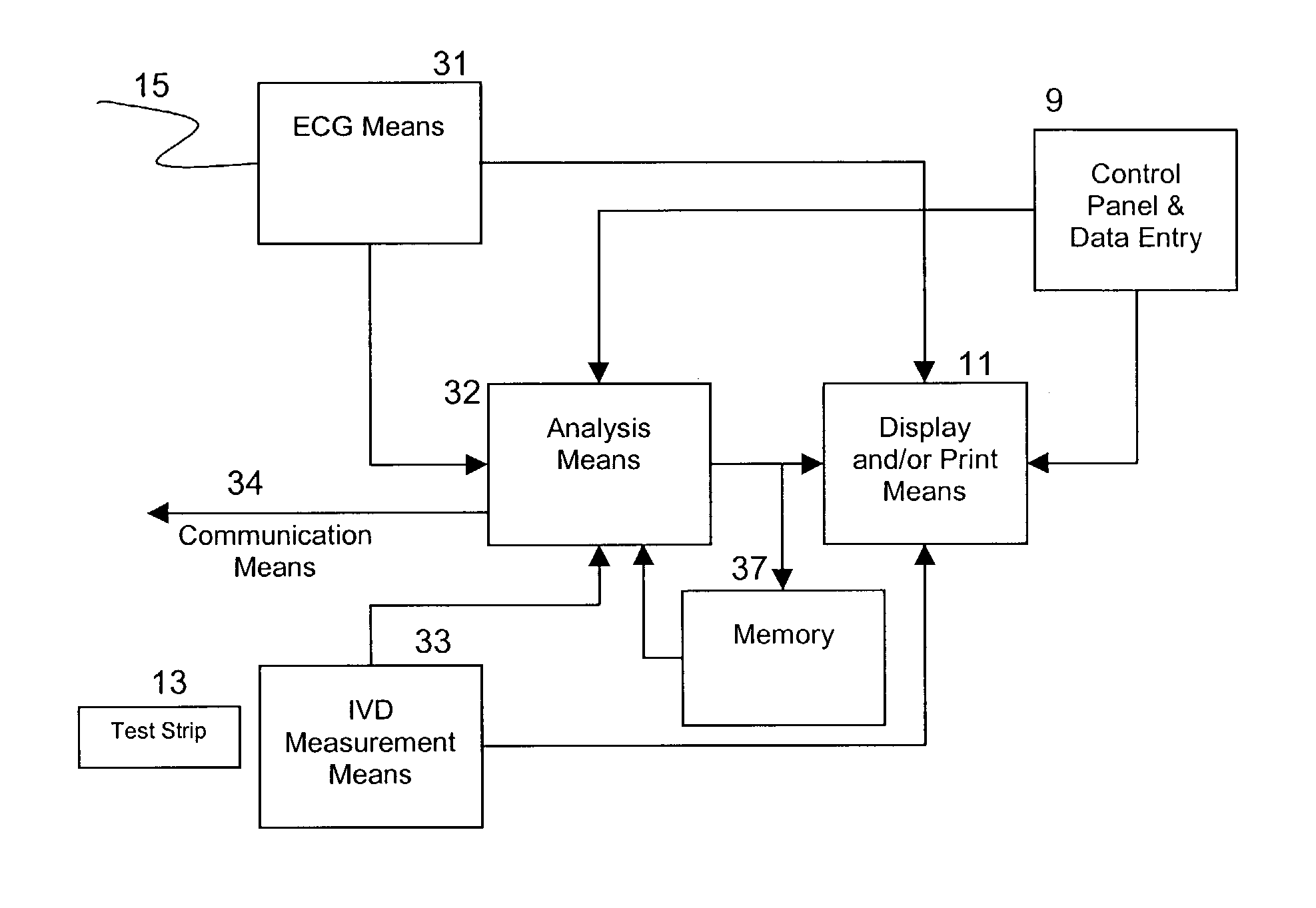
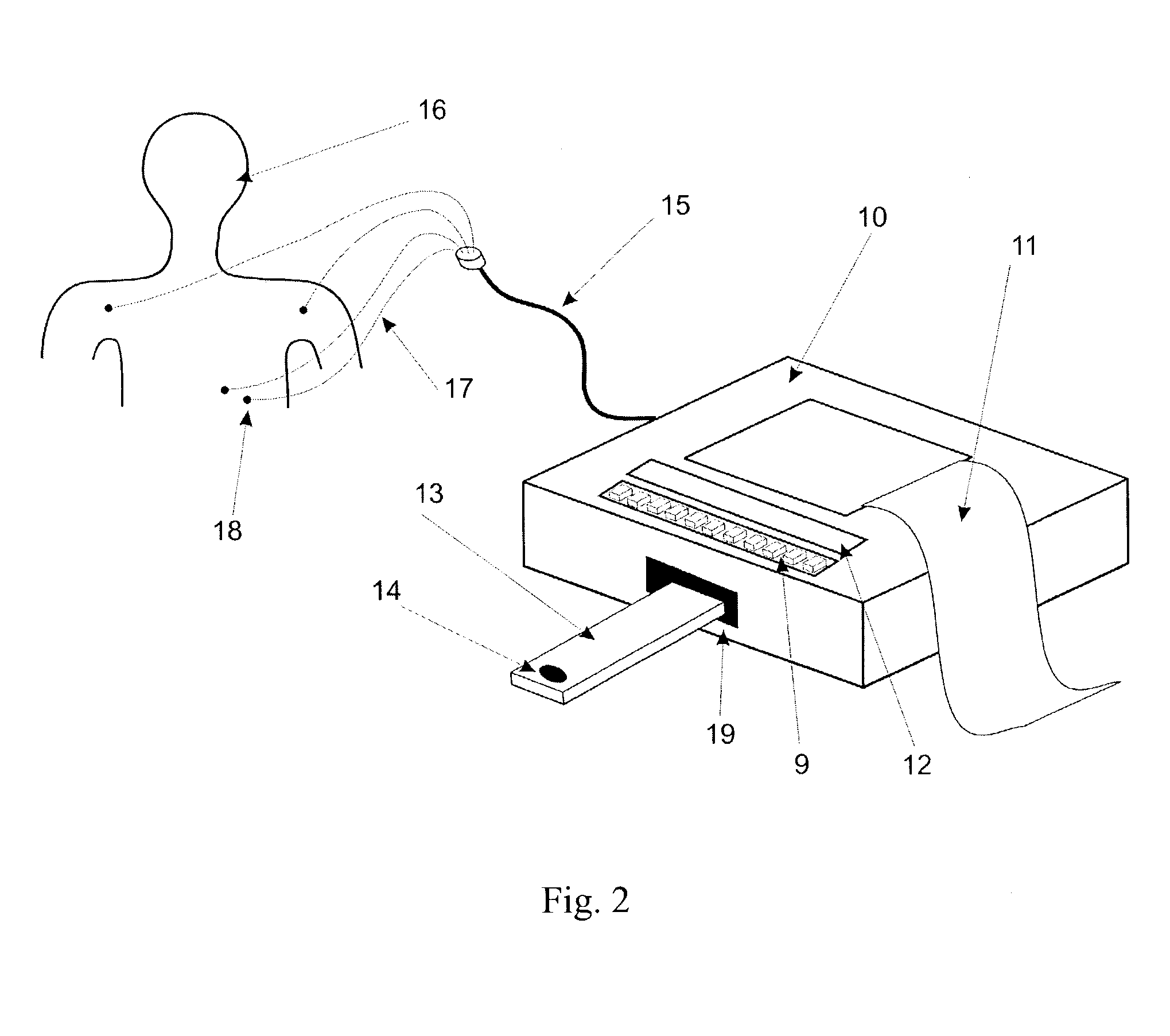
Apparatus and method for risk stratification of patients with chest pain of suspected cardiac origin
InactiveUS20050004485A1High negative predictive valueSave medical resourcesElectrotherapyElectrocardiographyACS - Acute coronary syndromeIschemia
The subject invention relates to the detection, diagnosis and risk stratification of clinical events such as acute coronary syndrome, in patients with signs and symptoms of suspected cardiac origin. In one embodiment, a clinical event in a patient is diagnosed by obtaining the patient's ECG, and at least one in vitro diagnostic assay, preferably an assay for a marker of ischemia, and optionally in vitro diagnostic assays for necrotic markers or other cardiac indicators, and combining the foregoing results in an algorithm to provide a diagnosis or a risk stratification of the clinical condition.
Owner:ISCHEMIA TECH
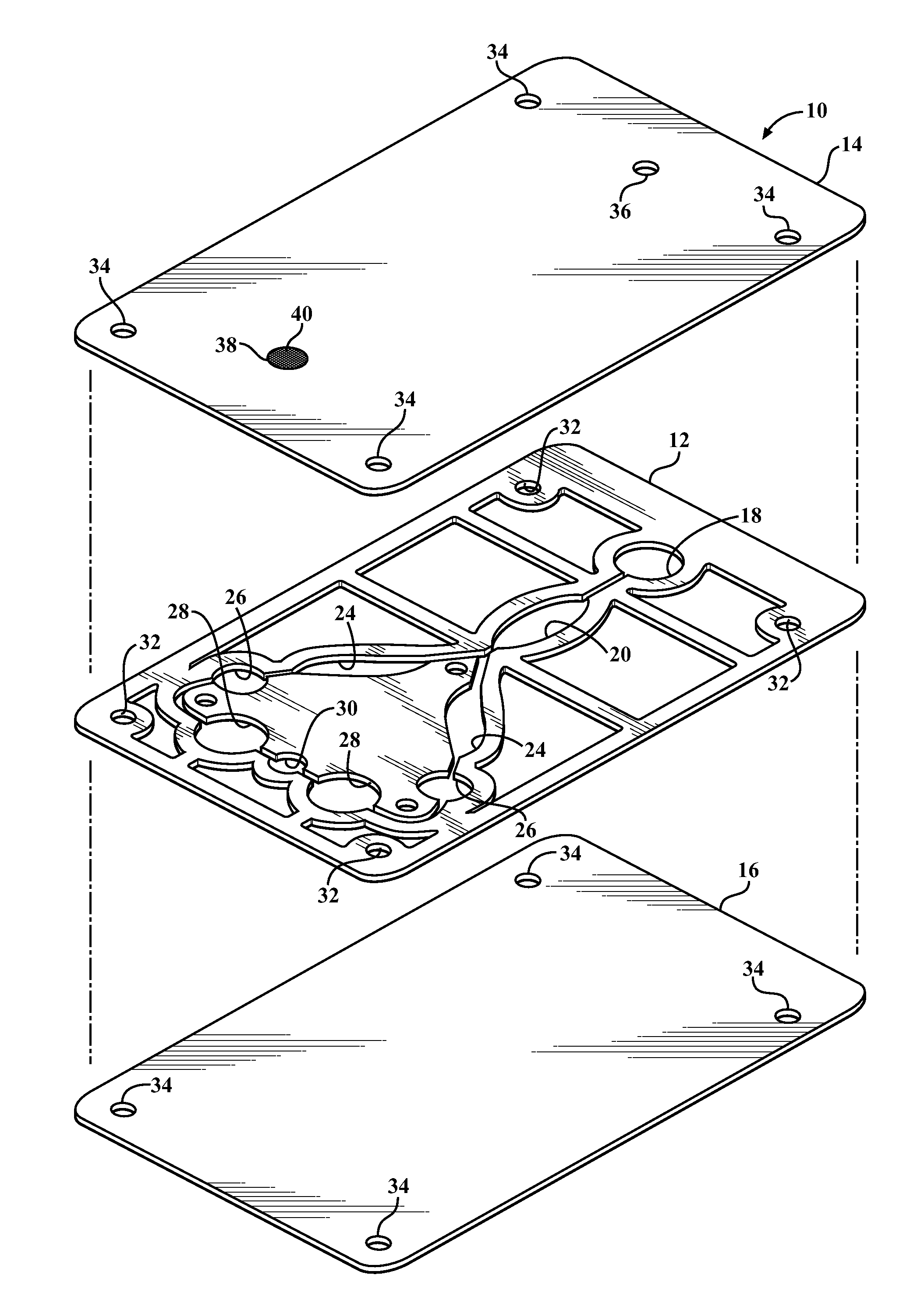
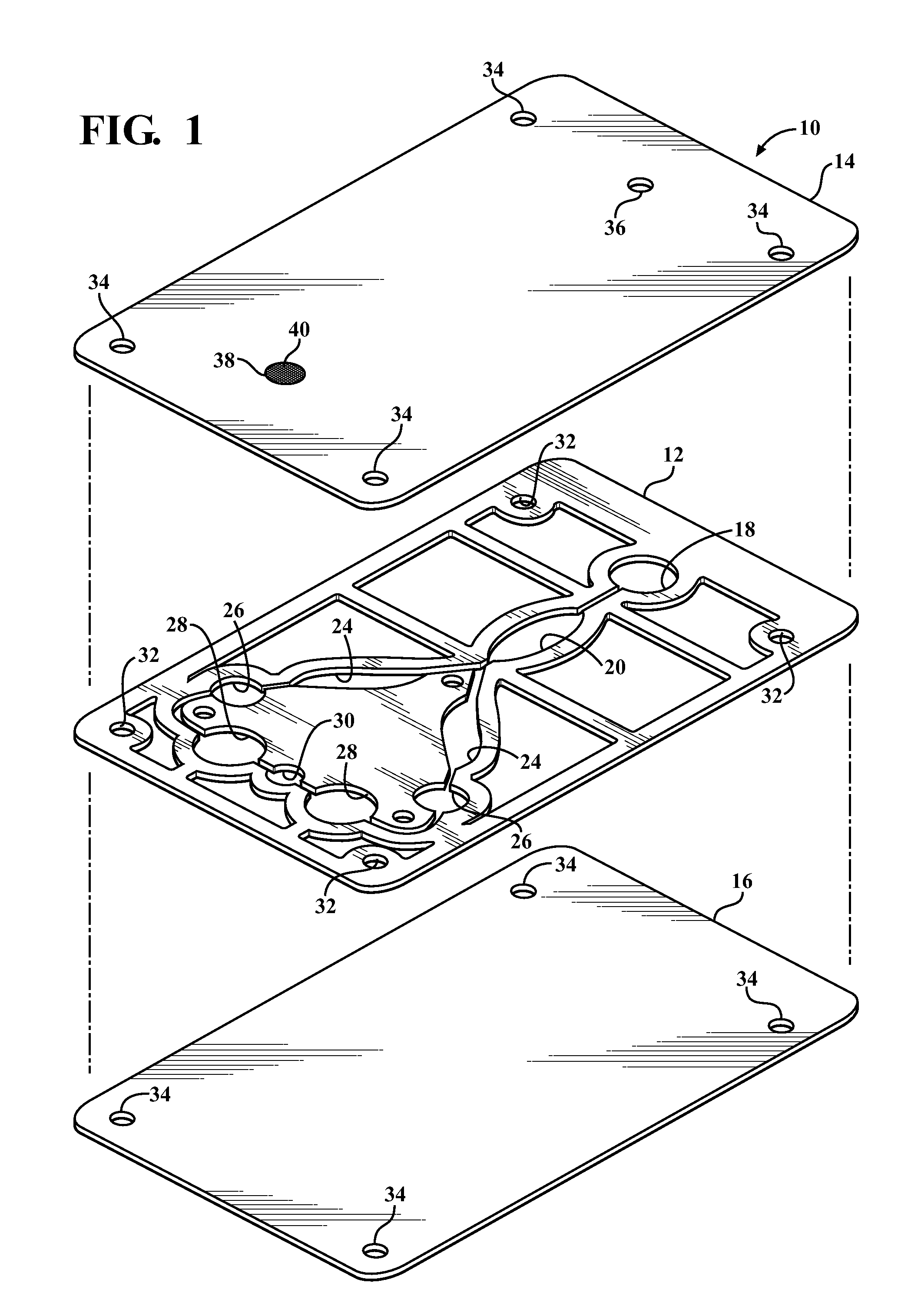
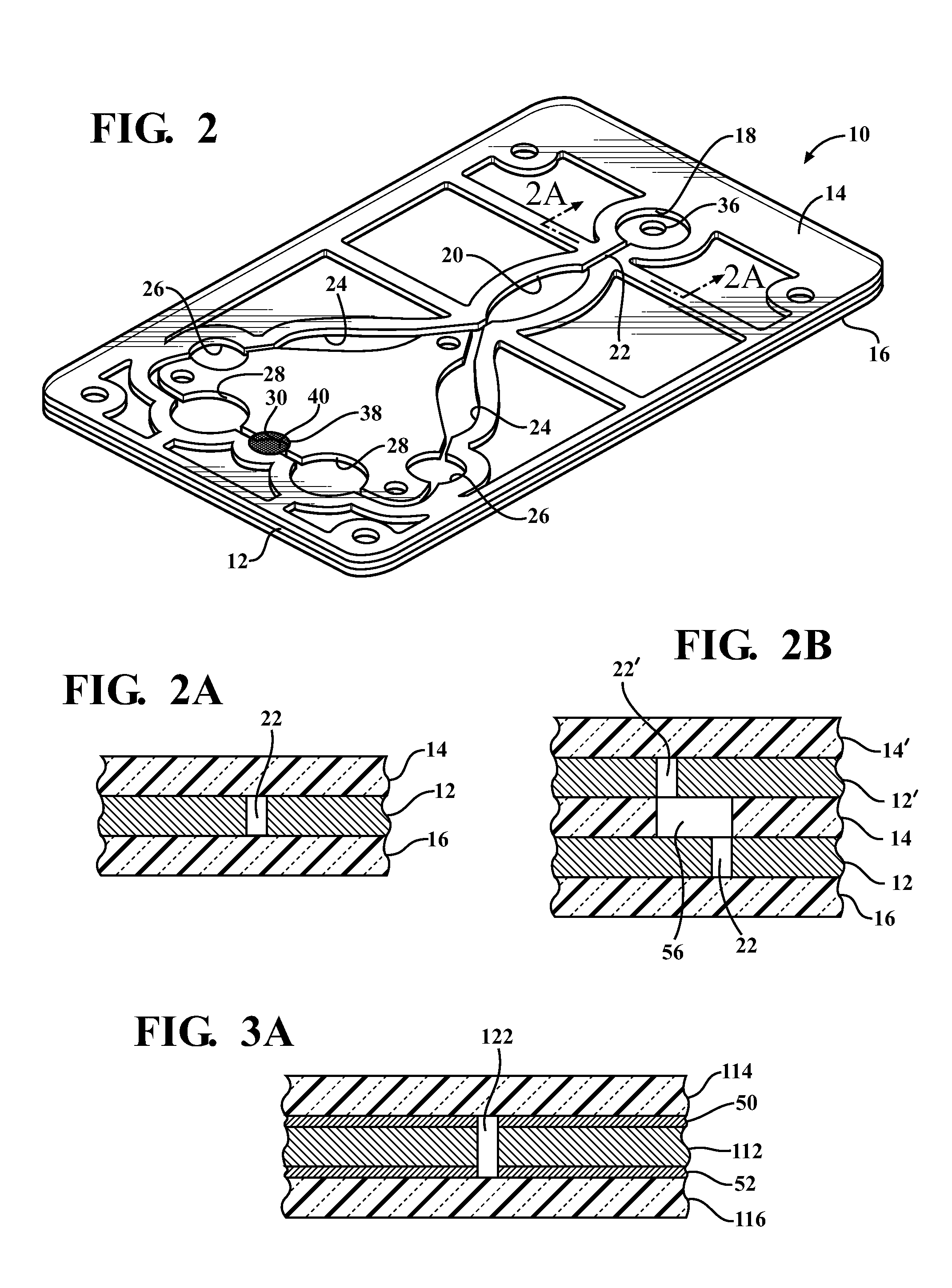
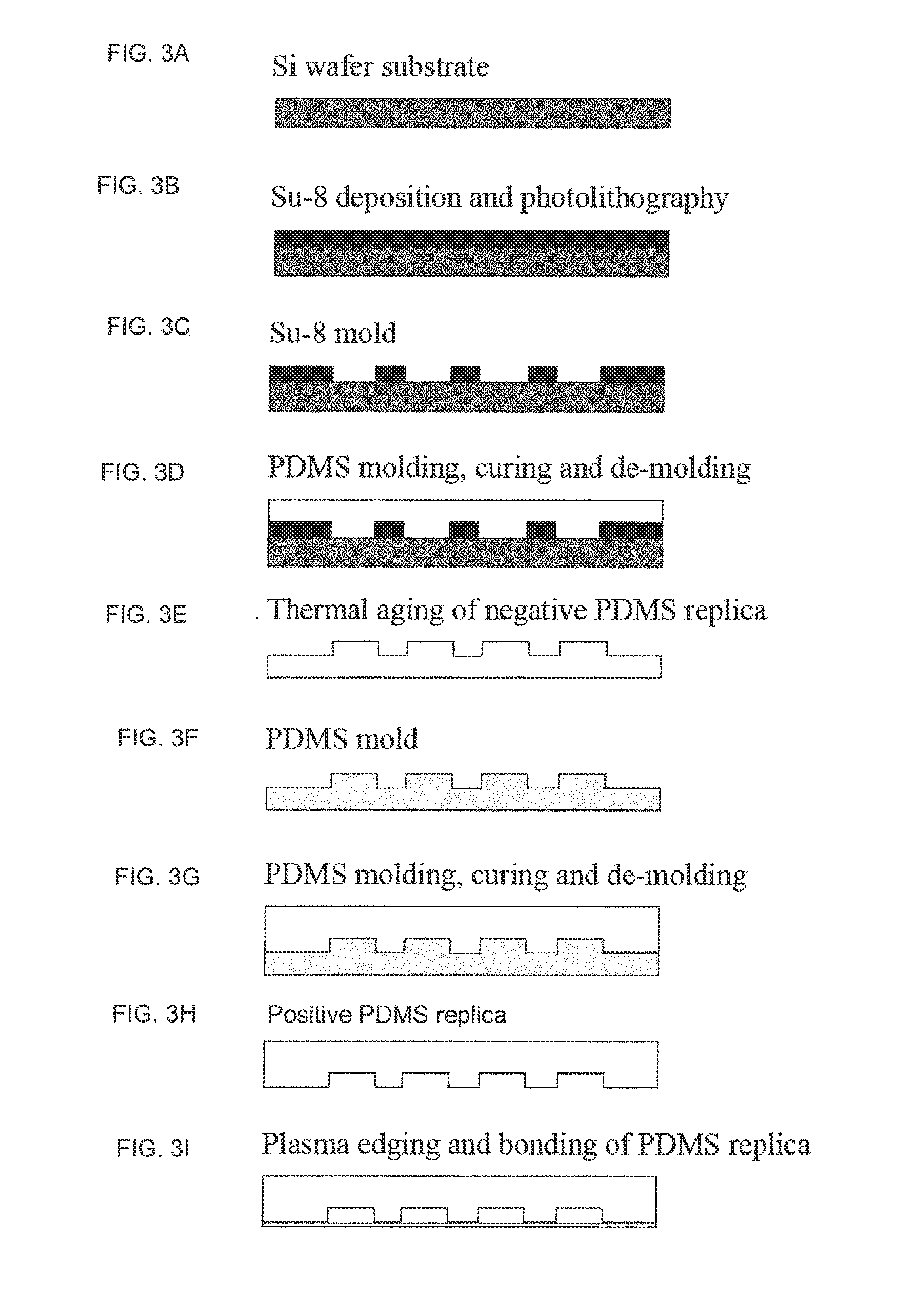
Diagnostic card with micro-fluidic channels and method of construction thereof
InactiveUS20110243813A1Lamination ancillary operationsLaminationInter layerArchitectural engineering
An in vitro diagnostic card and method of construction thereof is provided. The card includes an intermediate layer having opposite faces with at least one channel extending through the faces. A translucent first layer is fixed to one of the faces of the intermediate layer and a translucent second layer is fixed to the other of the faces of the intermediate layer opposite the translucent first layer. The channel in the intermediate layer is substantially sealed off by the first and second layers. Further, an opaque material that is absorbent to laser beam energy bonds the intermediate layer to the first and second layers.
Owner:WRIGHT INTPROP
Molecularly imprinted polymer and use thereof in diagnostic devices
InactiveUS20100068820A1AdvantageImprove accuracyMaterial analysis by observing effect on chemical indicatorPharmaceutical non-active ingredientsCross-linkFunctional monomer
An adhesive is provided containing at least one synthetic polymer with receptor sites that enable the selective capture or release of a target molecule. A polymer is synthesized by polymerizing and cross-linking a functional monomer or functional copolymers in the presence of a target or template molecule allowing for reversible interactions between the polymer and the target molecule. The target molecule may be extracted from the polymer creating receptor sites complimentary to the target molecule. Alternatively, the target molecule may remain in the polymer network and be controllably released. The molecularly imprinted polymer is formulated into an adhesive. The adhesive can be used as a component in an in-vitro diagnostic device to release template molecules or to capture target molecules in vacated receptor sites in the synthetic polymer.
Owner:ADHESIVES RES
NMR clinical analyzers and related methods, systems, modules and computer program products for clinical evaluation of biosamples
ActiveUS8013602B2Reduce inputReduce laborMagnetic measurementsDiagnostic recording/measuringControl systemRemote control
Methods, computer program products and apparatus automate clinical NMR in vitro diagnostic analyzers. The clinical analyzer can automatically electronically monitor selected parameters and automatically electronically adjust parameters to maintain the analyzer within desired operational ranges.The clinical NMR analyzers can be configured as a networked system with a plurality of clinical NMR analyzers located at different use sites; and at least one remote control system in communication with one or a plurality of clinical NMR analyzers, the at least one remote system configured to monitor selected local operating parameters associated with a respective clinical NMR analyzer.
Owner:LIPOSCI
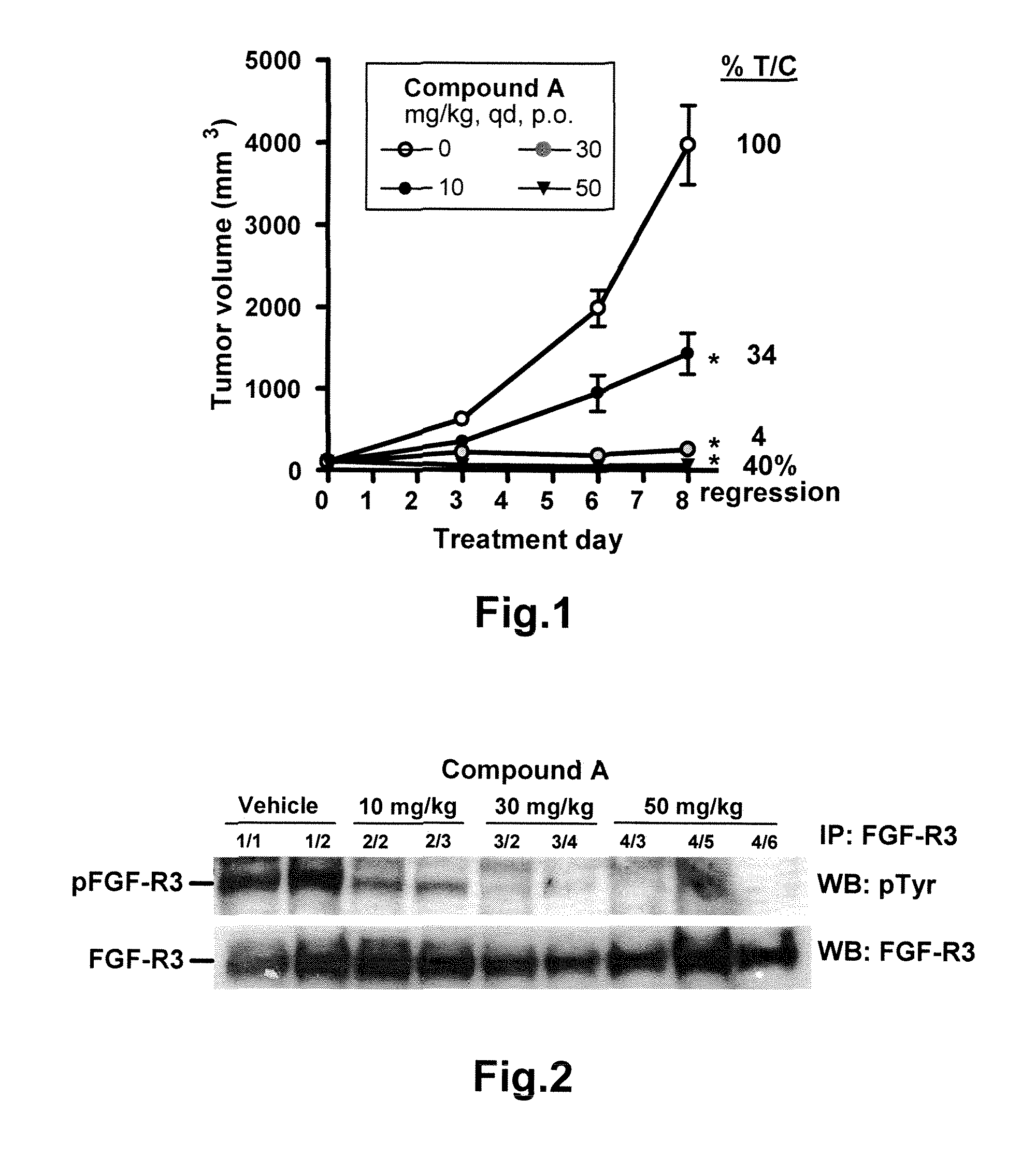
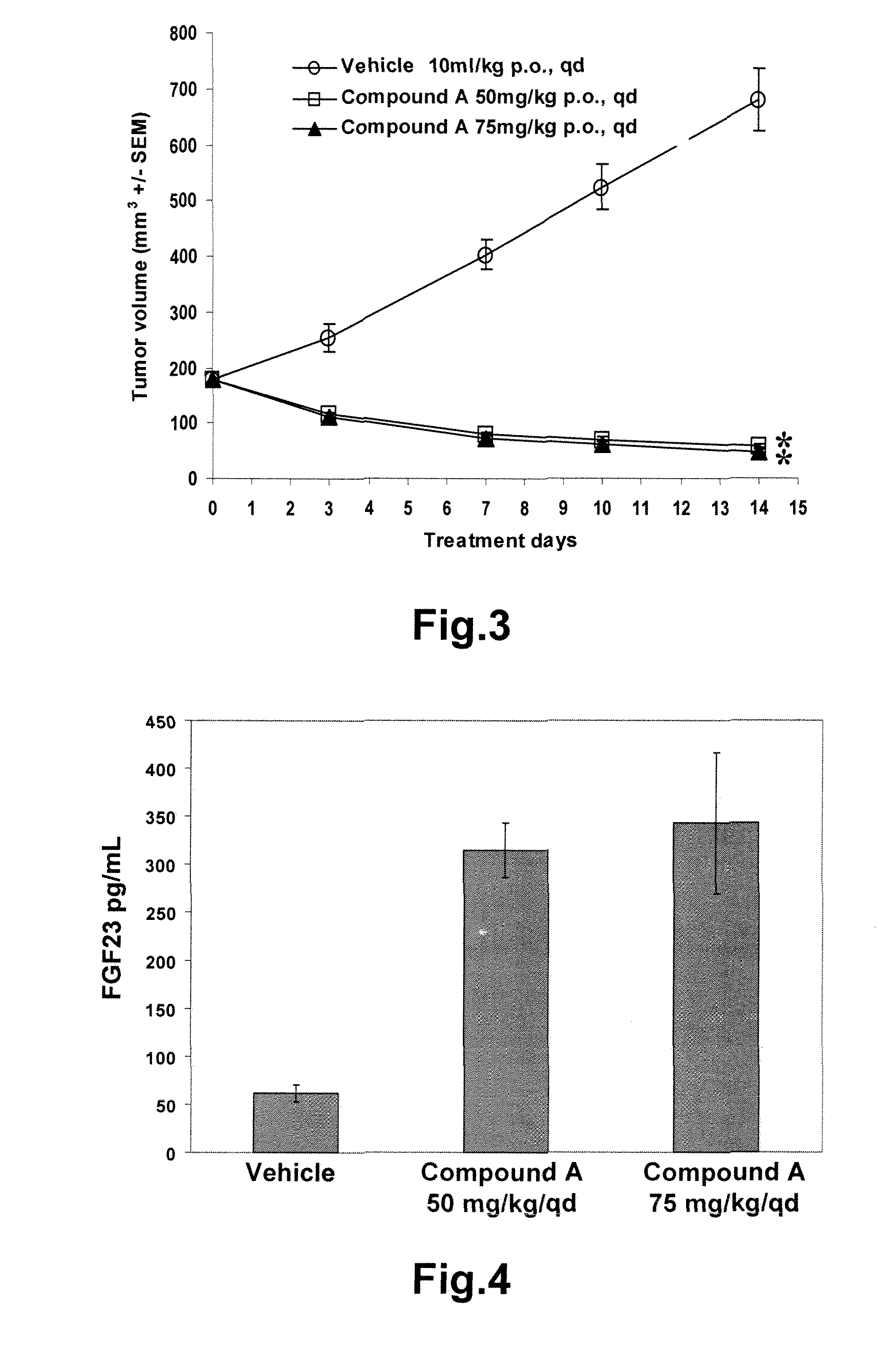
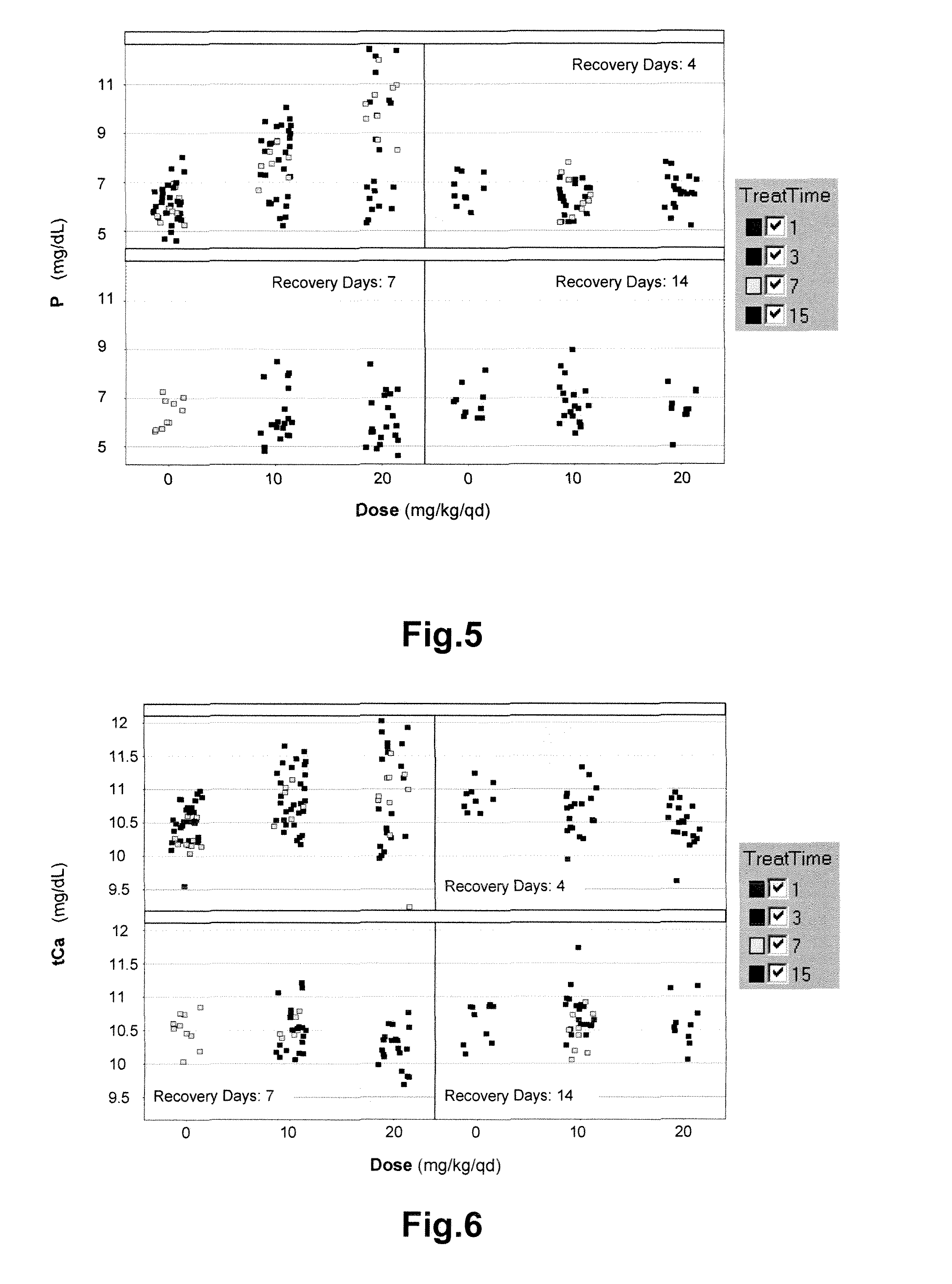
Methods of monitoring the modulation of the kinase activity of fibroblast growth factor receptor and uses of said method
InactiveUS20110045511A1Useful predictionOrganic active ingredientsBiological material analysisFibroblast growth factor receptor 2Biomarker (petroleum)
The present invention relates generally to methods of in vitro diagnostics, in particular the use of a compound selected from the group consisting of fibroblast growth factor 23 (FGF23), inorganic phosphorus (P), the product of inorganic phosphorus and total calcium (P×tCa), osteopontin (OPN) and parathyroid hormone (PTH) as biomarker. Said biomarkers can be used to monitor the modulation of fibroblast growth factor receptor (FGFR) kinase activity, in particular its inhibition, and / or the occurrence of secondary effects of FGFR inhibition. The invention further provides methods and kits relating to these uses.
Owner:NOVARTIS AG
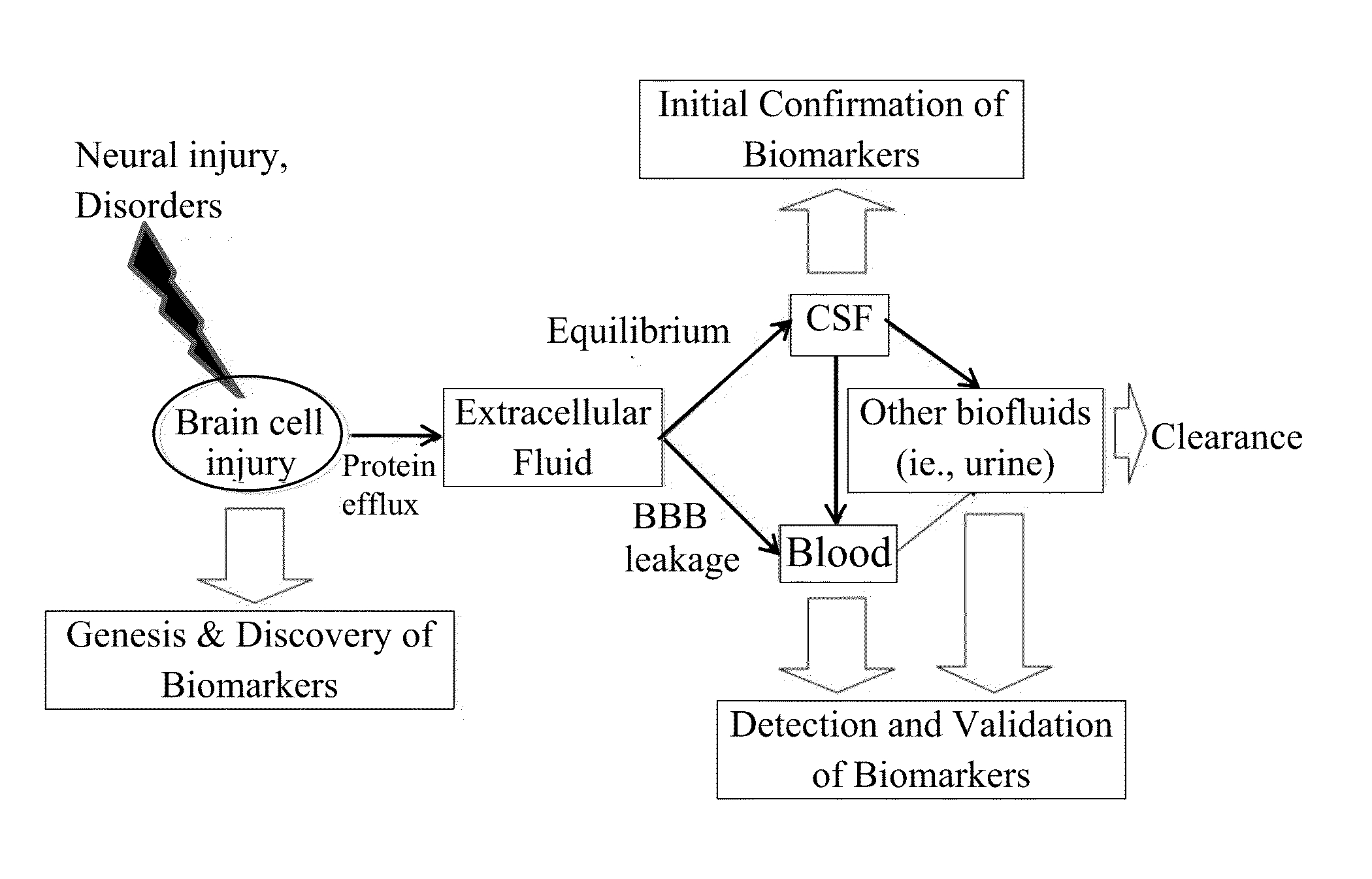
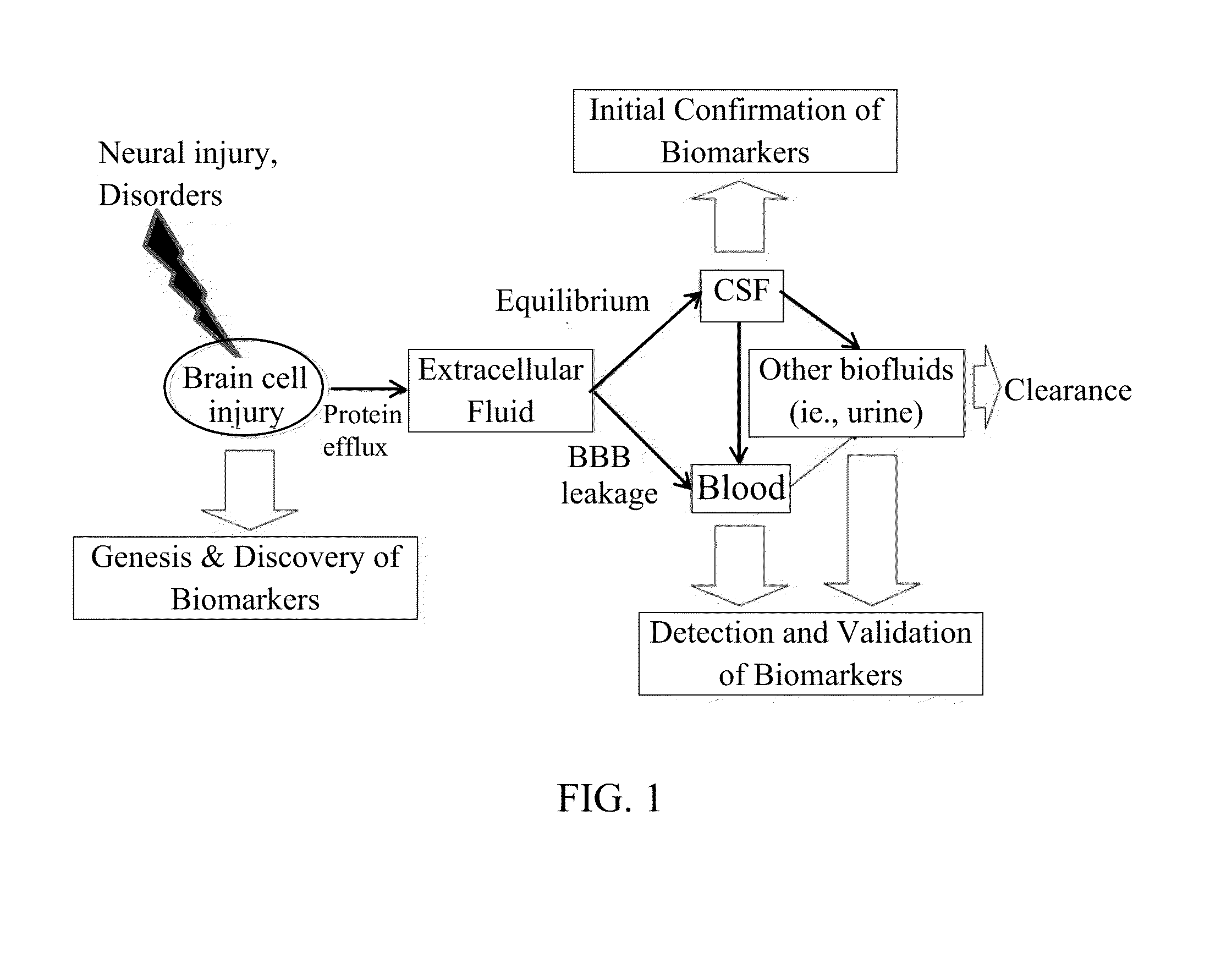
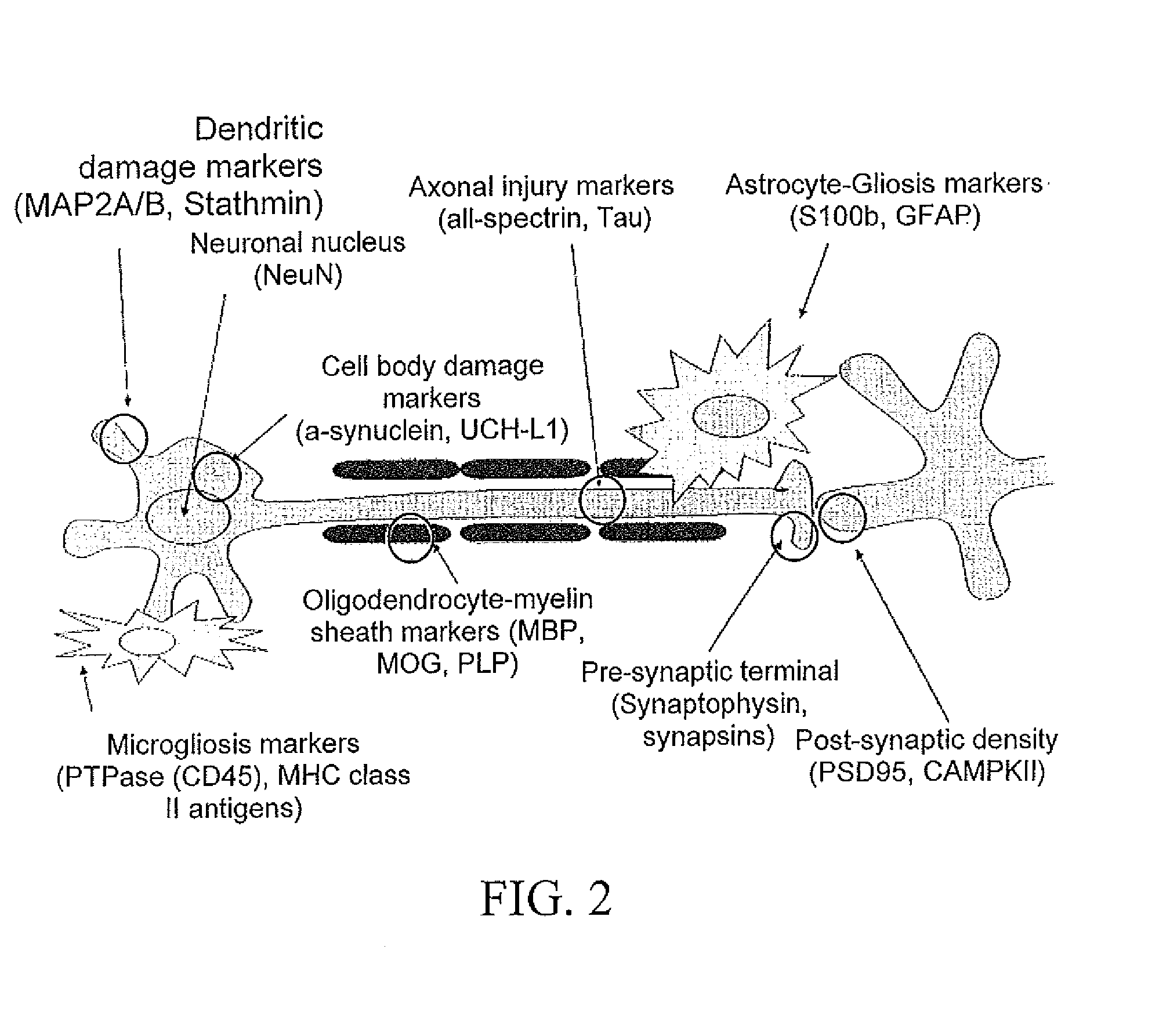
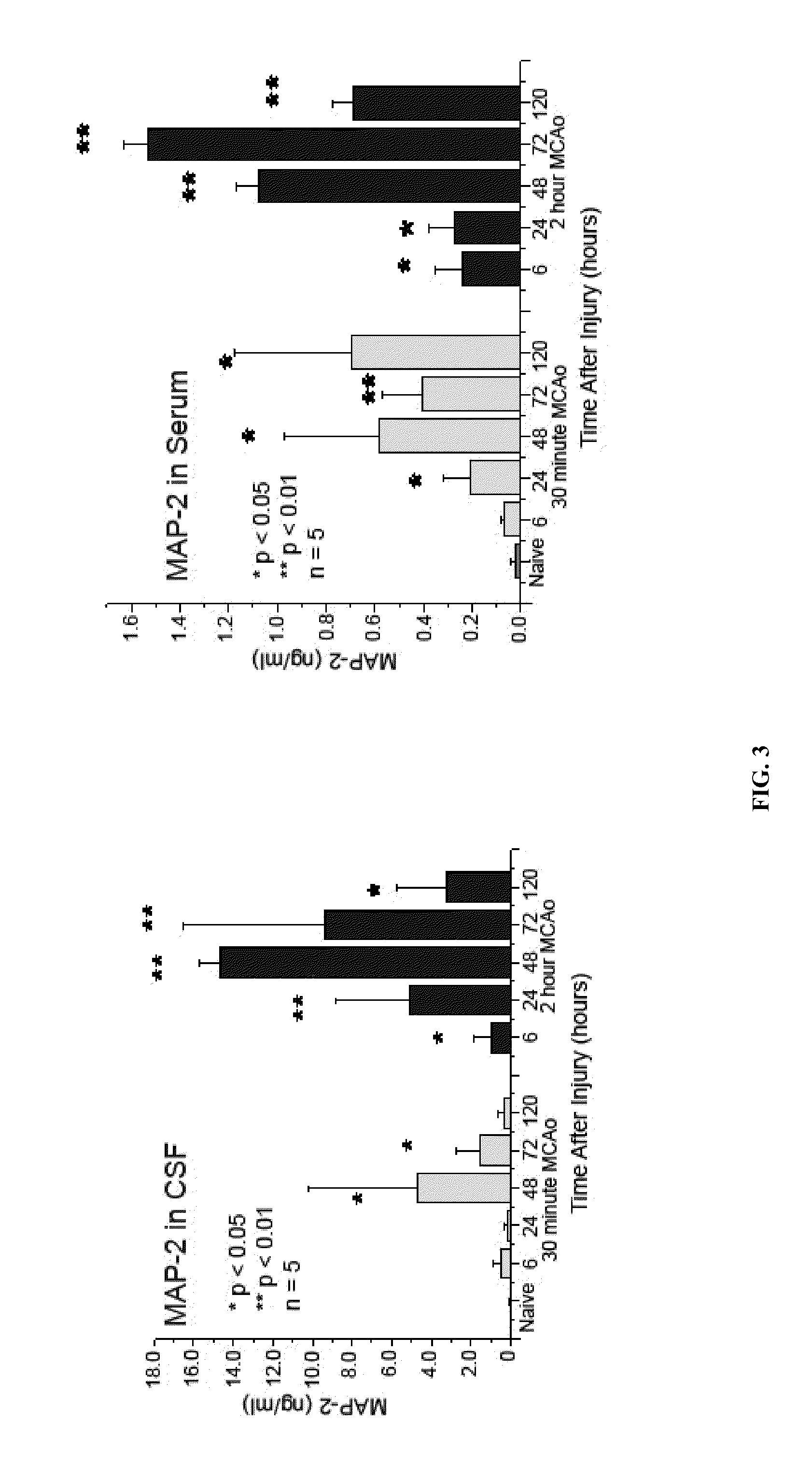
In vitro diagnostic devices for nervous system injury and other neural disorders
InactiveUS20140303041A1Auxiliary diagnosisBioreactor/fermenter combinationsPeptide librariesNervous systemDisplay device
The present invention relates to an exemplary in vitro diagnostic (IVD) device used to detect the presence of and / or severity of neural injuries or neuronal disorders in a subject. The IVD device relies on an immunoassay which identifies biomarkers that are diagnostic of neural injury and / or neuronal disorders in a biological sample, such as whole blood, plasma, serum, cerebrospinal fluid (CSF). The inventive IVD device may measure one or more of several neural specific markers in a biological sample and output the results to a machine readable format wither to a display device or to a storage device internal or external to the IVD.
Owner:BANYAN BIOMARKERS INC +1
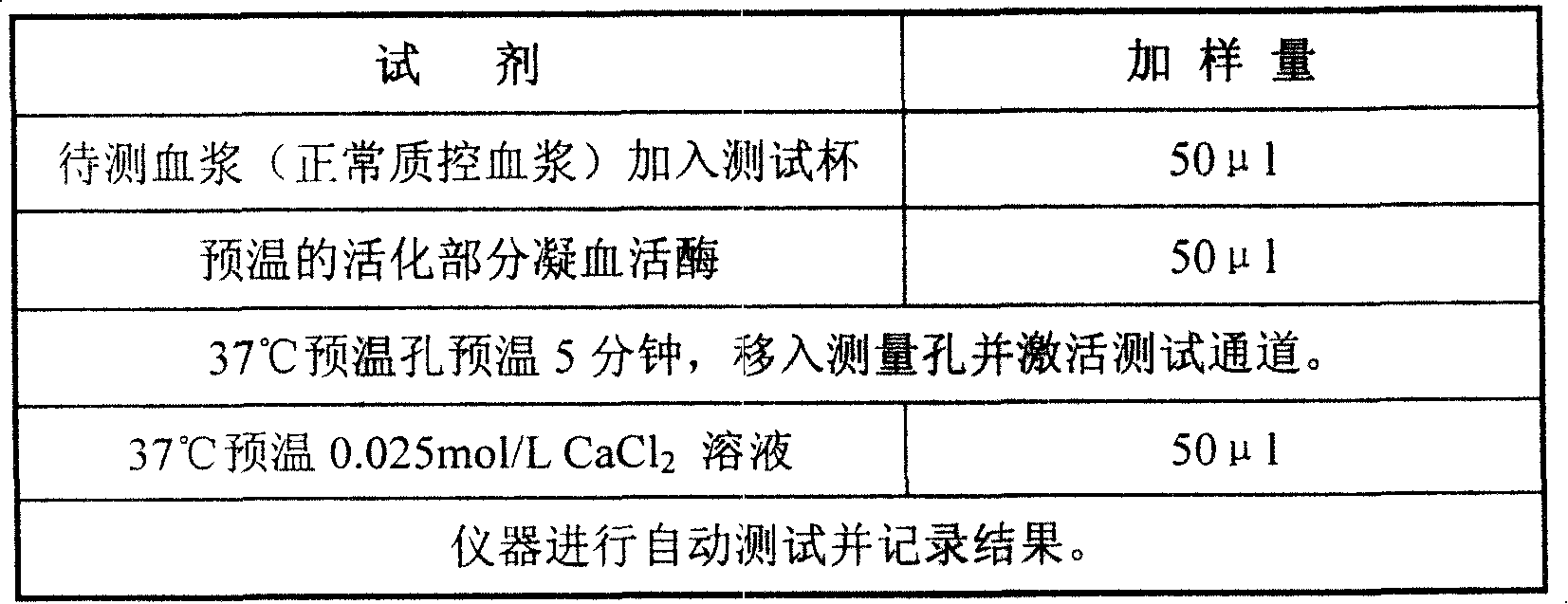
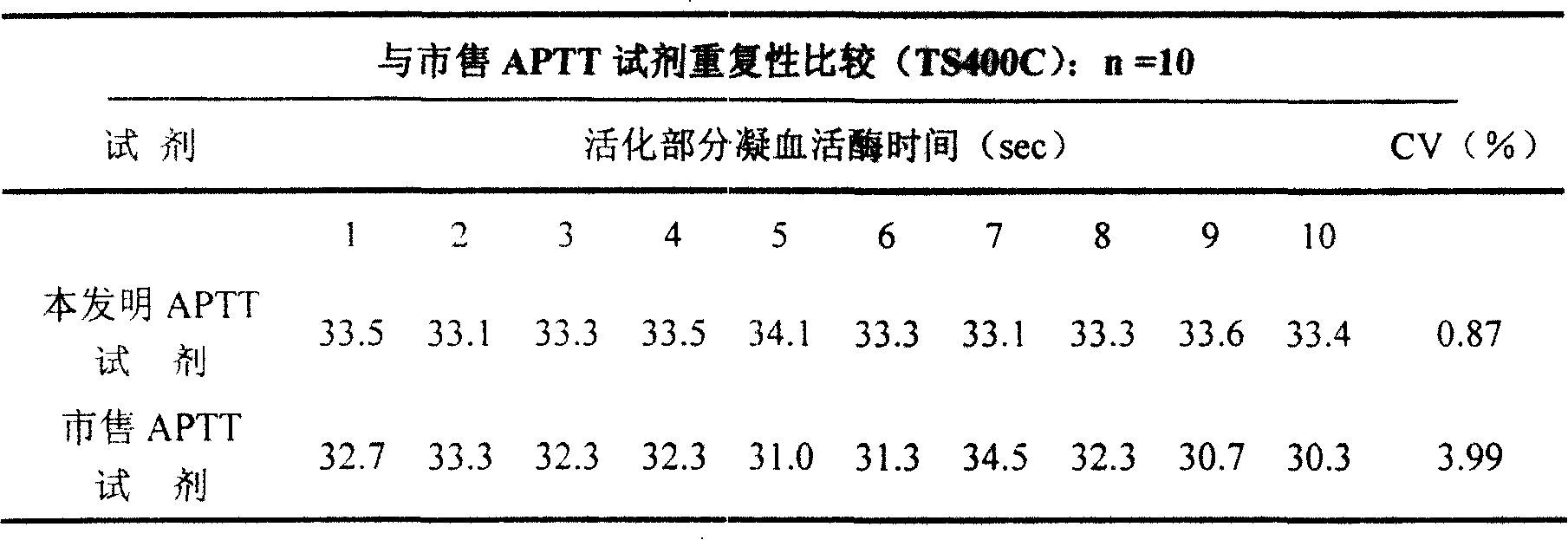
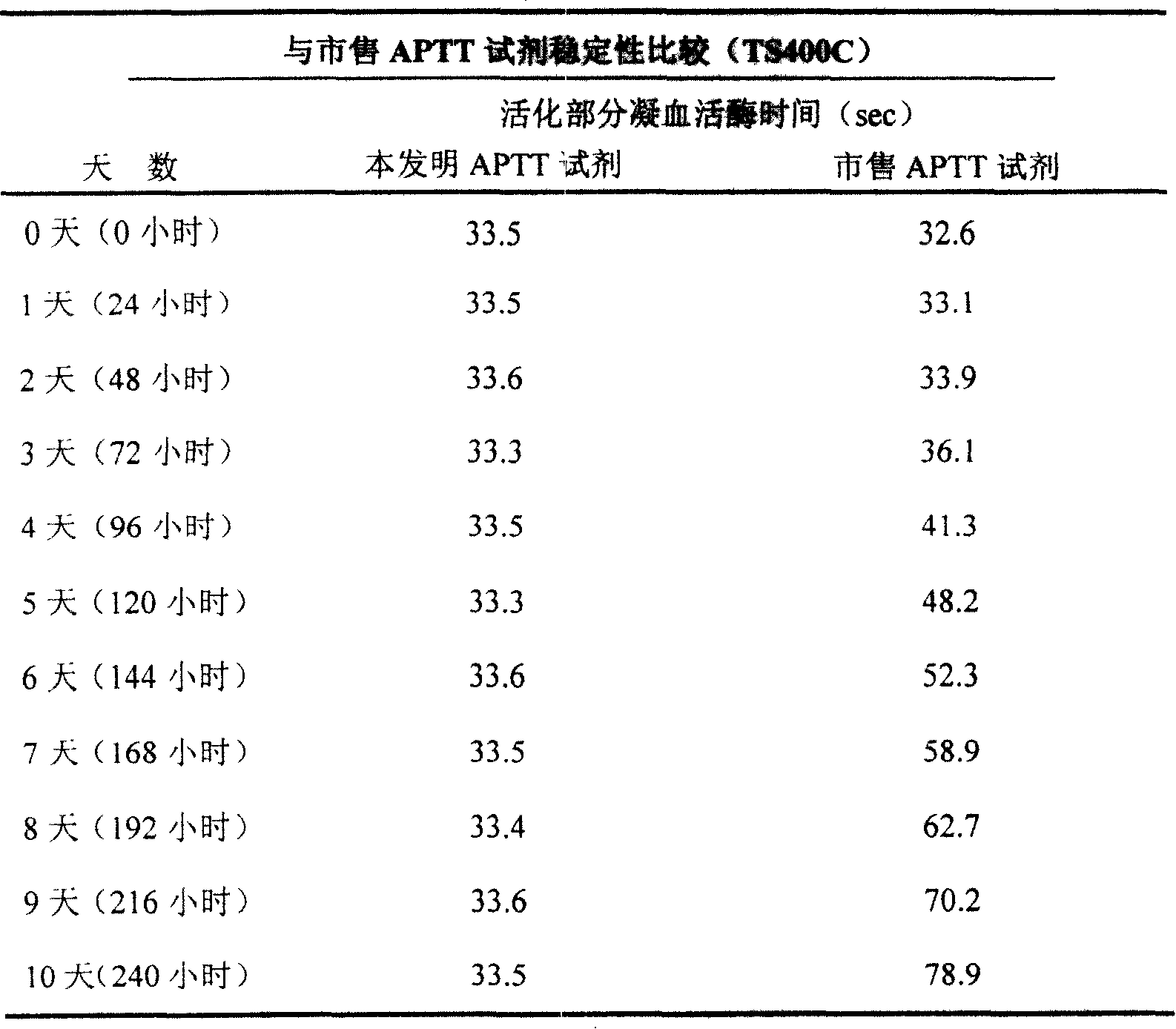
External diagnostic reagent kit used for measuring activated partial thromboplastin time
ActiveCN101221189AImprove stabilityGood repeatabilityMicrobiological testing/measurementBiological testingDisease causeAPTT - reference
The invention relates to an in vitro diagnostic kit for the determination of activated partial thromboplatin time (APTT) in clinical test. The invention consists of a partial thromboplatin reagent and a calcium chloride solution, which is used for the detection of the defects of the intrinsic coagulation pathway factors and the screening test of the related inhibitors, and the invention is also a primary means for the current coagulation factor and heparin anticoagulant treatment and the detection of lupus anticoagulant. The invention has the advantages of long stability time and good repeatability of the partial thromboplatin reagent after a re-dissolution, at the same time, the invention has better consistency of the measurement results of a blood coagulation analyzer by using an optical method and a magnetic bead method, therefore, the invention is applicable to large, medium and small hospitals, and the test results of different hospitals have comparability, therefore the invention has important meaning for implementing the one general report of test reports, provides the reliable experimental data for the clinical diagnosis and the treatment of diseases and improves the efficiency and value of the basic studies of thrombosis and hemostasis.
Owner:SHANGHAI SUNBIO TECH
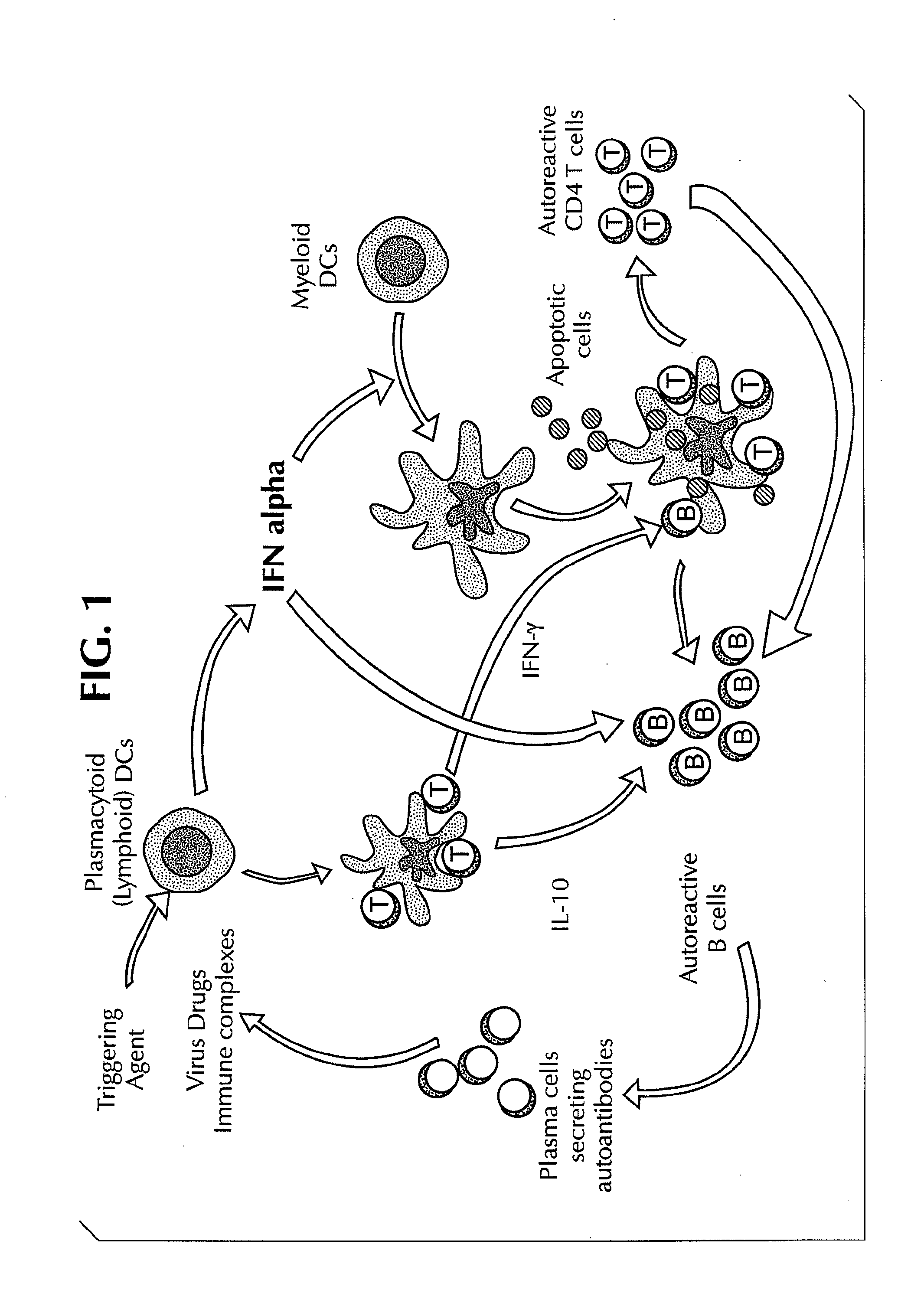
Methods for Treating Autoimmune Diseases in a Subject and In Vitro Diagnostic Assays
InactiveUS20090263474A1Organic active ingredientsPeptide/protein ingredientsDiseaseAutoimmune responses
The invention provides a method for treating an autoimmune disease in a subject by administering an interferon antagonist and a Flt3 ligand (Flt3L) antagonist. The invention also provides compositions containing one or more interferon antagonists, and one or more Flt3L antagonists, an in vitro assay for determining a subject's risk for developing an autoimmune disease, and kits for use, inter alia, with the assay.
Owner:BANCHEREAU JACQUES F +2
Dilution being capable of maintaining high stability of enzyme marker solution
ActiveCN101561432AMeet quality requirementsTo satisfy the market's needsBiological testingMatrix solutionPreservative
The invention relates to a diluent being capable of maintaining the high stability of enzyme marker solution and also relates to a method for preparing the diluent and applications thereof. The diluent comprises buffer solution, protein matrix solution, amino acid solution, sugar solution, a specific additive and a preservative. The diluent is a novel enzyme marker solution developed based on the characteristics of antibody protein and enzyme protein. With the adoption of the dilution, the stability of enzyme markers in storage and application can be effectively maintained. Tests show that the enzyme marker diluent can be stored at temperature of 37 DEG C for more than one week to fully meet the requirements for the quality of in-vitro diagnostic reagent kits. The diluent can be directly applied to the preparation of the in-vitro diagnostic reagent kits and meet the increasingly growing market demand for the in-vitro diagnostic reagent kits.
Owner:福建省洪诚生物药业有限公司
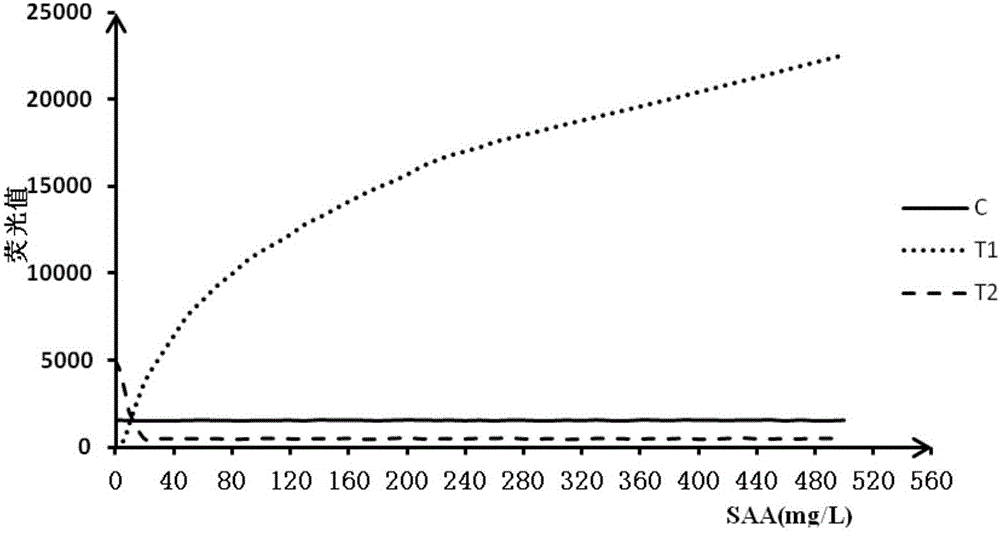
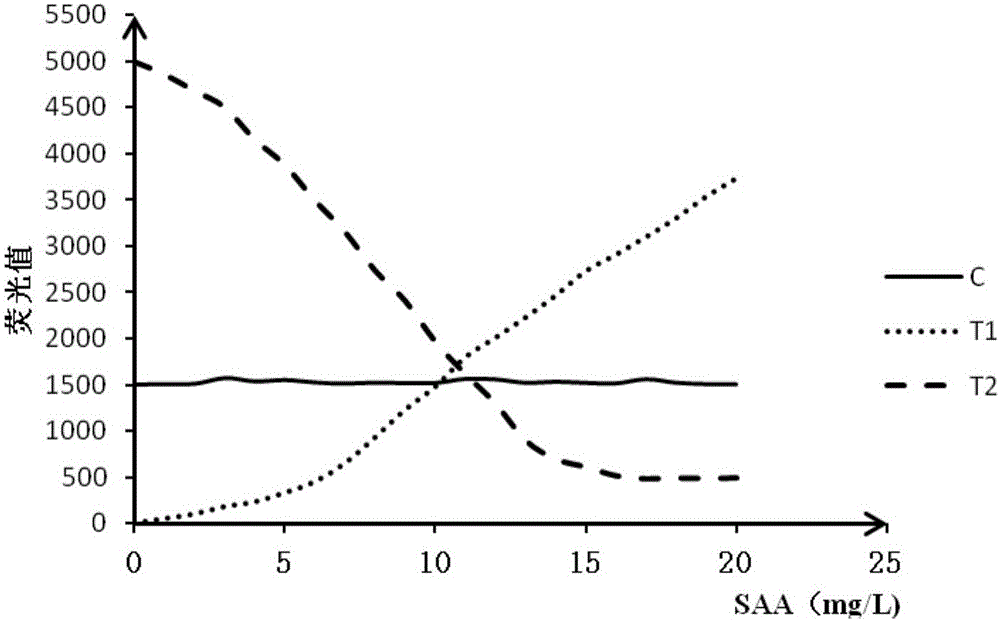
Double detection line SAA (Serum amyloid A protein) immunofluorescence chromatography quantitative detection reagent and preparation method thereof
InactiveCN105785038AExpand the scope of detectionHigh sensitivityDisease diagnosisBiological testingAntigenSAA protein
The invention discloses a double detection line SAA (Serum amyloid A protein) immunofluorescence chromatography quantitative detection reagent and a preparation method thereof. The double detection line SAA immunofluorescence chromatography quantitative detection reagent can simultaneously promote sensitivity and detection scope and can be applied to clinical detection for SAA level in patients of acute inflammation and chronic inflammation. According to the technical key points, the reagent comprises a base plate, a combining cushion, a coating film and an absorbing cushion, wherein a sample cushion is connected with the base plate; anti-SAA monoclonal antibody 1 and chick IgY are sprayed on the combining cushion and are marked with a same fluorescent microsphere; a detection line T1, a detection line T2 and a quality control C line are arranged on the coating film; anti-SAA monoclonal antibody 2 is coated with the detection line T1; antigen SAA protein is coated with the detection line T2; goat-anti-chick IgY is coated with the quality control C line. The reagent belongs to the technical field of in-vitro diagnostic reagents.
Owner:GUANGZHOU WEIMI BIOLOGICAL SCI & TECH
Stable biochemical compound calibrator and preparation method thereof
ActiveCN107843469AStable Analytical ComponentsReduce pathogenic biological hazardsPreparing sample for investigationPathogenic microorganismAntioxidant
The invention relates to a stable biochemical compound calibrator and a preparation method thereof and belongs to the technical field of biological in-vitro diagnosis. Through use of a compound protective substance composed of an excipient, an antioxidant, a surfactant, a stabilizer and a preservative, analytical components in the calibrator are stable in one month at the room temperature in dark,are stable in two years at 4 DEG C in dark and are stable in three years at -20 DEG C in dark. In preparation, a riboflavin photochemical method is used to inactivate various potential pathogenic microorganisms so that the calibrator has no potential pathogenic microorganism harmfulness. The stable biochemical compound calibrator contains more ingredients and greatly facilitates clinical use. Thepreparation method is traceable to international or domestic reference materials. The stable biochemical compound calibrator ensures clinical test result accuracy.
Owner:BIOSINO BIO TECH & SCI
Novel in-vitro diagnosis experiment method and device for allergen
The invention provides an in-vitro diagnosis experiment method and device for detecting different human antibodies (IgE and IgG4) specific to multiple allergens, and belongs to medical experiment field. The method adopts horseradish peroxidase (HRP) and alkaline phosphatase (ALP) to label anti-human IgE antibody and anti-human IgG4 antibody respectively, and combines them with solid phase carrier in the same immuno-reaction system. After incubation and washing, equal amounts of the solid phase carriers are placed in colorimetric reaction cuvettes respectively, and then chemiluminescence substrates catalyzed by different enzymes (HRP and ALP) are used to detect luminescence intensity. The method can simultaneously detect IgE antibody and IgG4 antibody of testee specific to multiple allergens. The matched device comprises immuno-bead loading structure system, washing system, and immuno-bead / micro-magnetic-bead transfer system. The device has the functions of controlling immuno-bead addition amount, transferring immuno-bead, quantitatively adding and transferring micro-magnetic-bead, etc.
Owner:SINNOWA MEDICAL SCI & TECH
Point-of-care in-vitro blood analysis system
InactiveUS6845327B2Low costReduce system costAnalysis using chemical indicatorsSamplingBlood collectionPoint of care
Devices for cost-effectively performing in-vitro diagnostic chemical analyses at multiple distributed locations within a medical institution are disclosed. One object of this invention is to provide a network of distributed sensory devices that acquire sensor signals from blood specimens and deliver those signals through a connect on to a central location for analysis by a general-purpose computer and generation of an analysis result. The analysis result is then sent to numerous locations on a network for display, including also possibly back to the location of signal acquisition. Cost-effective mobile sensing devices are also disclosed. The present system includes blood-sensor signal acquisition devices distributed throughout the hospital. The sensory signal-acquisition devices are card readers that acquire raw sensory signals from diagnostic cards inserted therein. These diagnostic cards are smart card-like devices modified for blood collection that contain sensory elements such as electrodes adapted to provide a raw sensory signal. The signal acquisition devices are modified smart card readers, which acquire the raw sensory data from an inserted smart card through a standardized contact arrangement and provide the raw data to data processing devices such as data acquisition cards. The system includes multiple remote ports for acquiring blood sensor signals. One or more card reader, when connected to a mobile general-purpose computer, can be converted into a complete mobile blood analyzer.
Owner:ABBOTT POINT CARE +1
Devices and methods for biomarker detection process and assay of neurological condition
InactiveUS20140342381A1Auxiliary diagnosisBioreactor/fermenter combinationsBiological substance pretreatmentsDisplay deviceNeuronal disease
The present invention relates to an exemplary in vitro diagnostic (IVD) device used to detect the presence of and / or severity of neural injuries or neuronal disorders in a subject. The IVD device relies on an immunoassay which identifies biomarkers that are diagnostic of neural injury and / or neuronal disorders in a biological sample, such as whole blood, plasma, serum, cerebrospinal fluid (CSF). The inventive IVD device may measure one or more of several neural specific markers in a biological sample and output the results to a machine readable format wither to a display device or to a storage device internal or external to the IVD.
Owner:BANYAN BIOMARKERS INC
Method for detecting neoplastic disorders in a solubilized body sample
ActiveUS20070190529A1Accurate informationImproved morphological evaluationSamplingMicrobiological testing/measurementReproductive tractIn vitro diagnostic
The present invention relates to a method for the early diagnosis of neoplastic disorders such as cancers as well as their precursor stages, particularly cancers of the respiratory tract, the urinary system, the reproductive tract, cancer associated with HPV infection or cancer of the anogenital tract, from solubilized body samples. The invention is also directed to test kits usable for this purpose as well as in-vitro diagnostic devices. The development of the kits and in-vitro diagnostic devices for the above purpose is also one aspect of the present invention.
Owner:VENTANA MEDICAL SYST INC
Stabilized two component system for chemiluminescent assay in immunodiagnostics
The present invention provides stabilized chemiluminescent formulations for use in in vitro diagnostics, including competitive as well as sandwich-type immunological assays. The stabilized assay system may be composed of two components, where the first component may contain a chemiluminescent organic compound, an enhancer, a homogenizing agent, and a suitable buffer with formulations having a pH range from about 7.2 to about 12, and optionally a solubilizing agent. The chemiluminescent system of the present invention is useful in immunoenzymatic analytical procedures, such as immunometric, competitive binding and sandwich type assays. In such immunoassays employing the chemiluminescent system of the present invention, the detectable light signal shows a proportional decay with time in the test samples and standards, so that the decay of the light emitted does not effect the concentration of the analyte measured over the entire analyte measurement range of the immunoassay. This allows accurate measurement of analyte concentrations in a test sample over extended periods of time.
Owner:KALRA BHANU +1
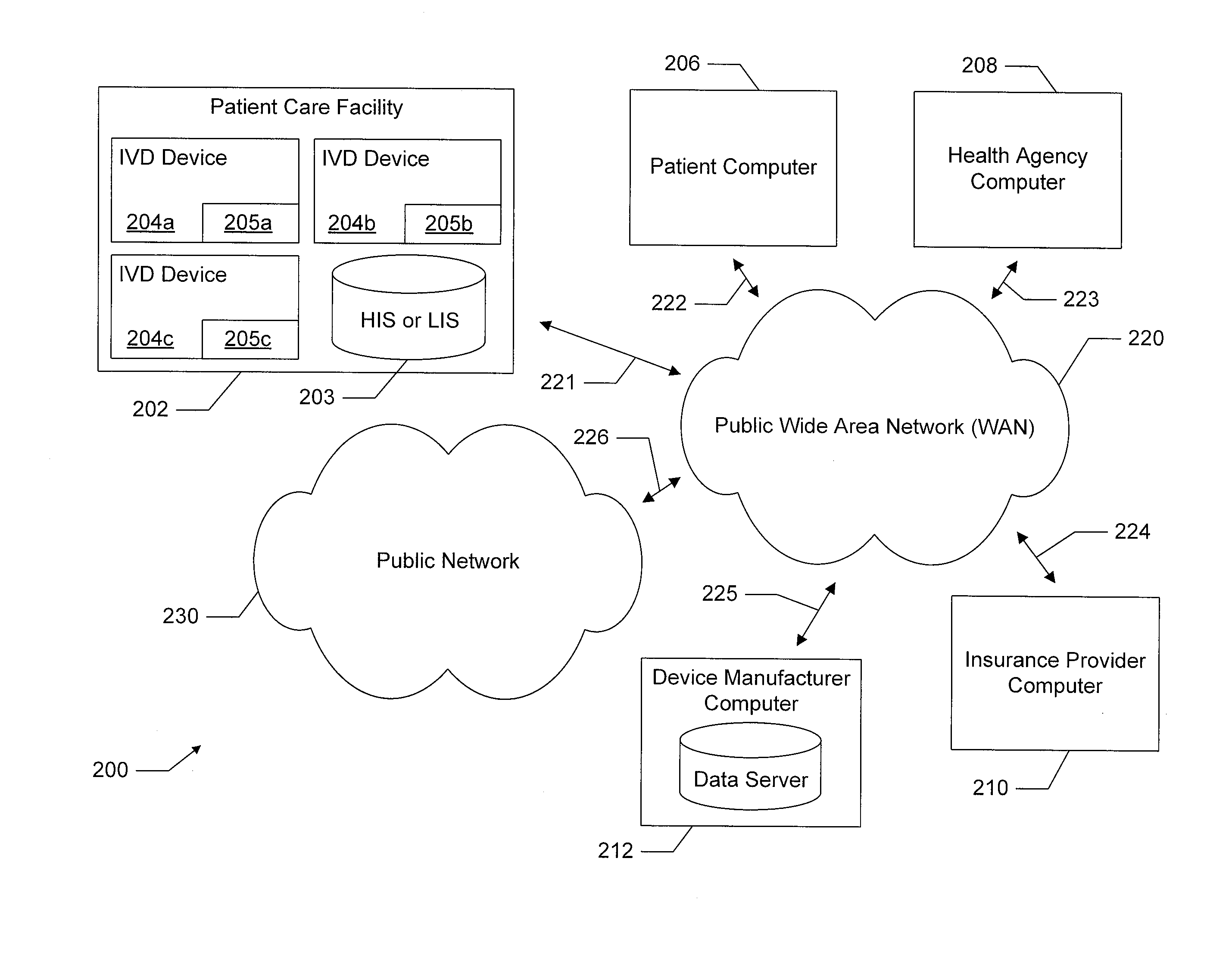
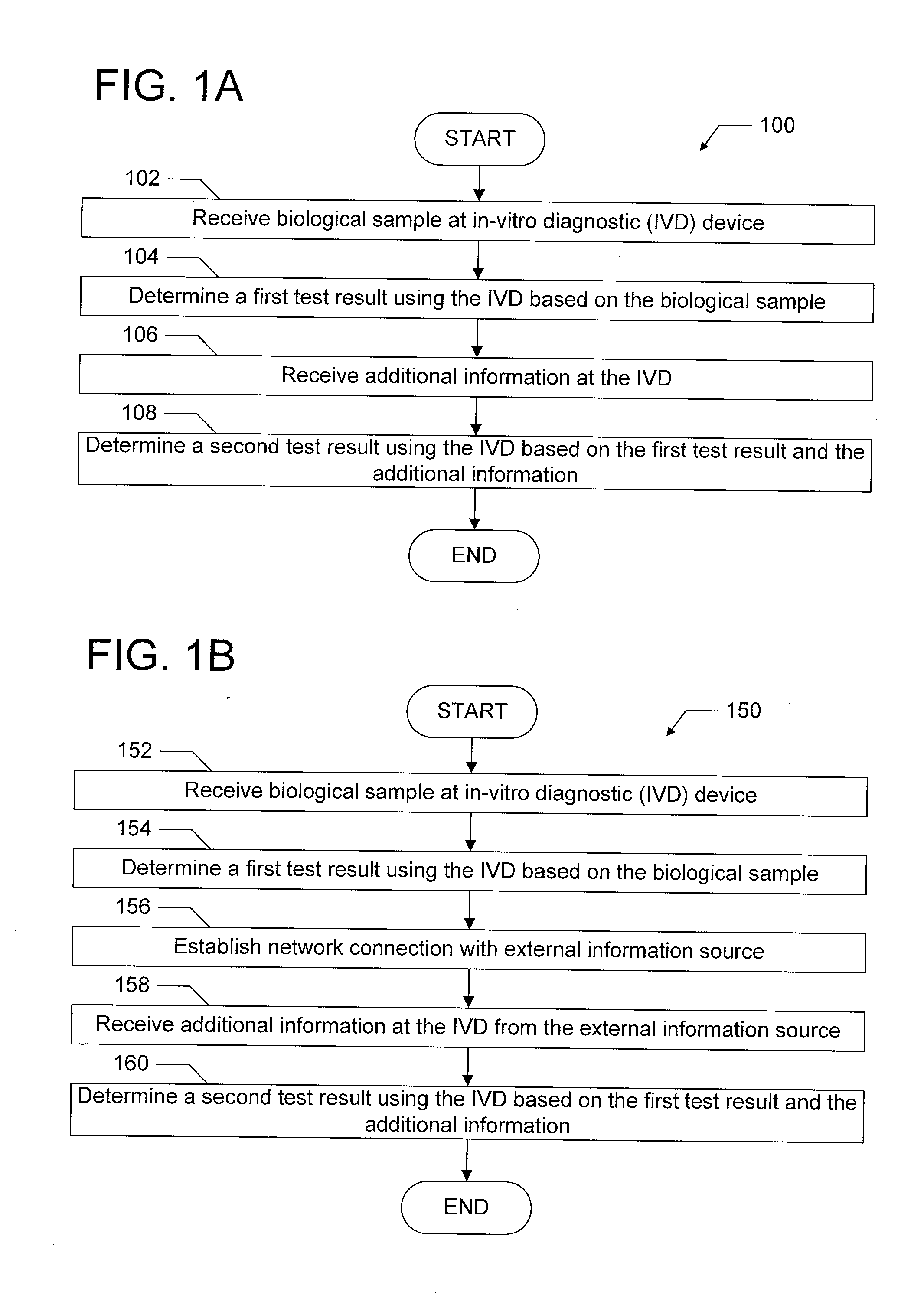
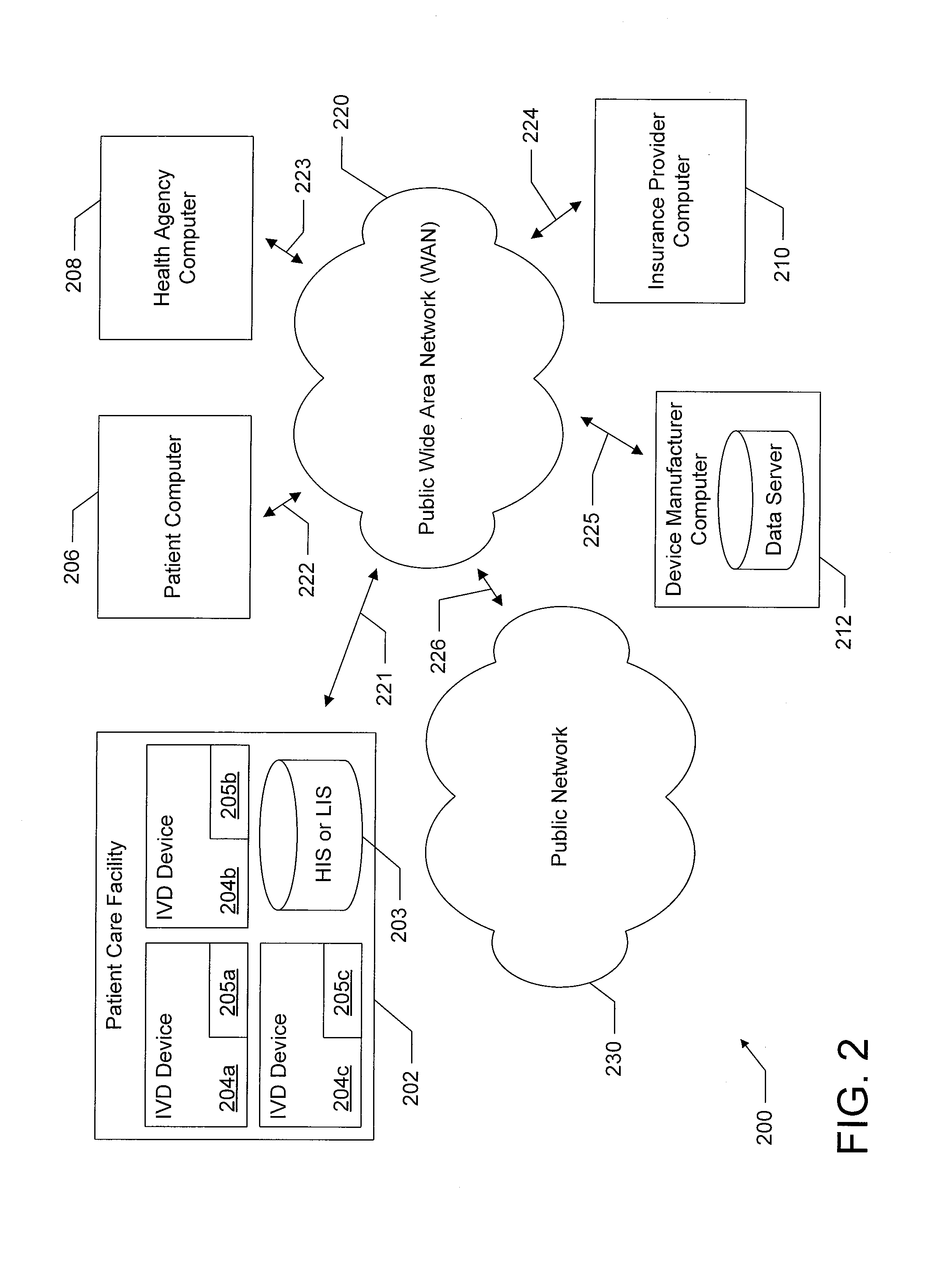
Distributed network of in-vitro diagnostic devices
ActiveUS20130066563A1Facilitate information transferFocusData visualisationComputer-assisted medical data acquisitionNetwork communicationData profiling
A system is disclosed in which a plurality of in-vitro diagnostic (IVD) devices each include a network communication device for connecting to a publicly accessible data network. For example, IVD devices are provided with a cellular modern for connecting to a public cellular network. These IVD devices connect to the data network upon completion of a diagnostic test, and upload results of the test, as well as other appropriate data, to a remote device which is also on the network. The IVD devices also download appropriate data from remote network elements. The remote network element may be a network element such as a Hospital Information System (HIS) or Laboratory Information System (LIS) database. Alternatively, the remote device may be a remote server or another IVD device. This connectivity enables the system to accumulate diagnostic test data, and to analyze, report, and / or update the IVD devices based on the accumulated data.
Owner:ALVERIX
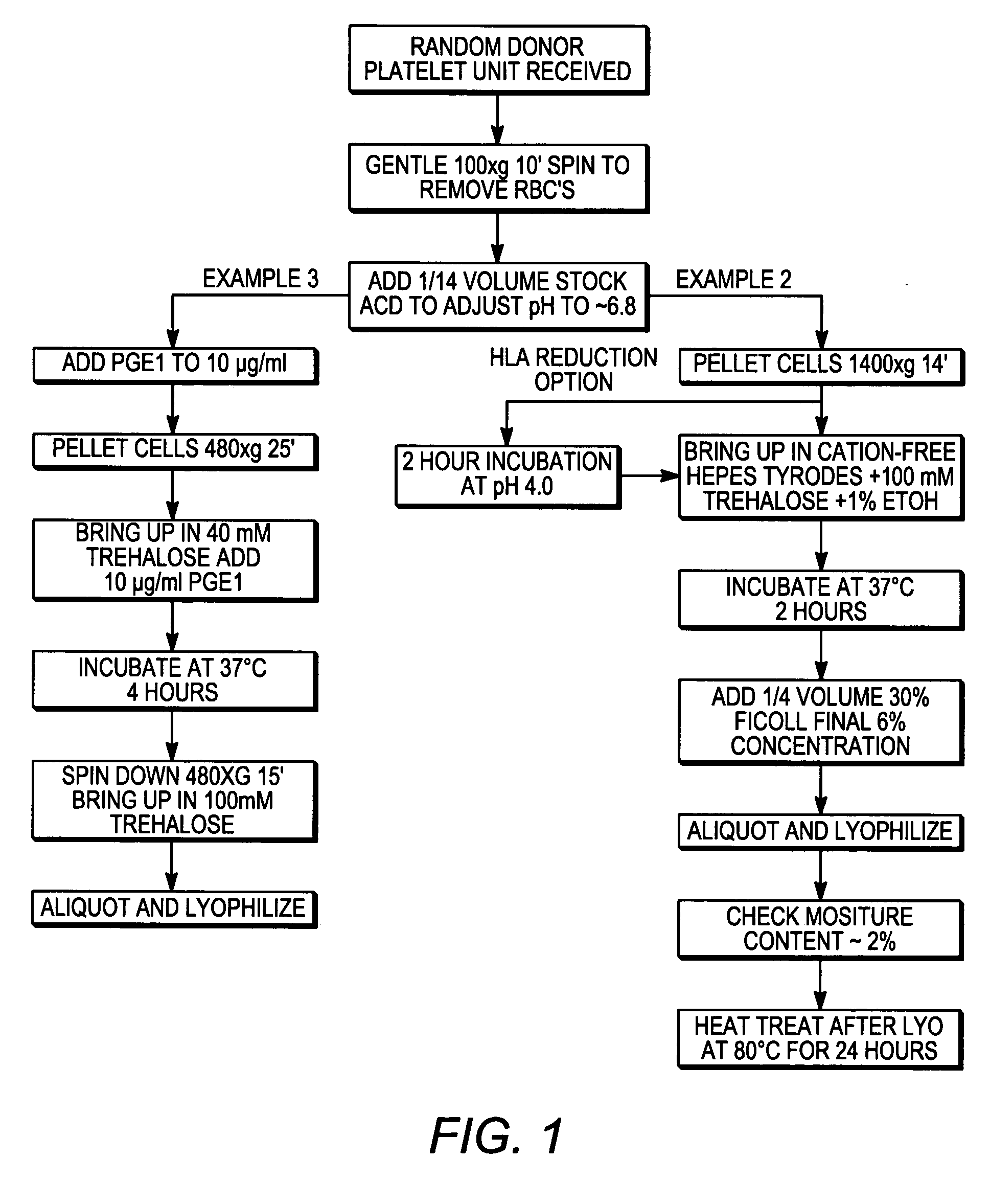
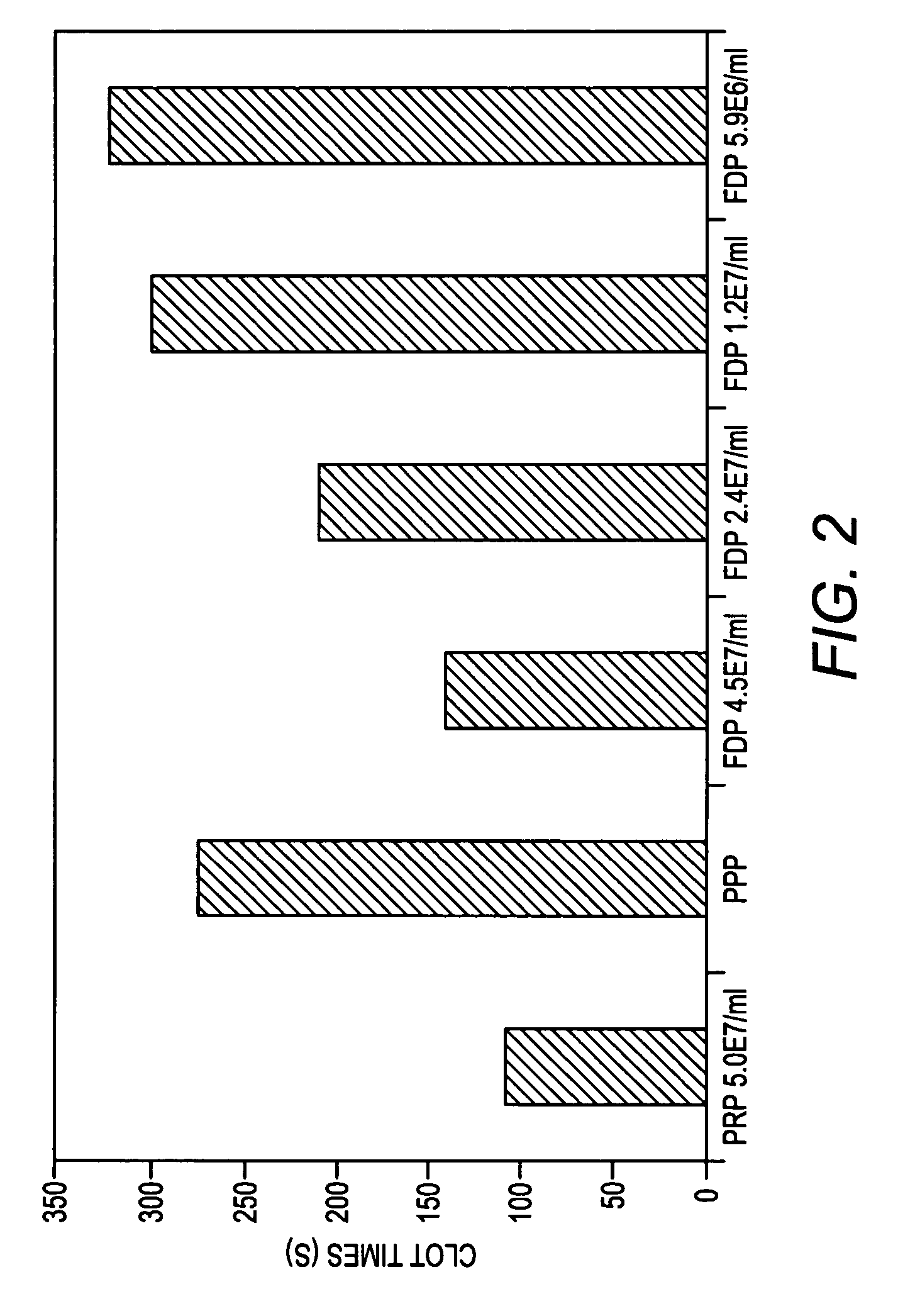
Processes for preparing lyophilized platelets
InactiveUS20060051731A1Function increaseGood curative effectDead animal preservationFreeze-dryingIn vivo
The present invention provides processes for preparing freeze-dried platelets, freeze-dried platelets made by those processes, platelets reconstituted from those freeze-dried platelets, and kits comprising those freeze-dried platelets. The freeze-dried platelets of the invention have similar characteristics to fresh platelets, have an exceptionally long shelf-life, and can be used for all standard procedures in which fresh platelets are used, including both in vitro diagnostic and research procedures and in vivo therapies.
Owner:CELLPHIRE INC
Tetramethylbenzidine (TMB) coloration solution and preparation method thereof
ActiveCN103063661AEasy to operateReduce random errorMaterial analysis by observing effect on chemical indicatorSolubilityHydrogen phosphate
The invention relates to a tetramethylbenzidine (TMB) coloration solution and a preparation method of the TMB coloration solution and belongs to the technical field of biological in-vitro diagnostic reagents. A solution and B solution are mixed in an isopyknic mode to obtain the TMB coloration solution, the A solution comprises, by molarity, citric acid 0.01-0.5 mol / L, sodium hydrogen phosphate 0.01-0.5 mol / L, hydrogen peroxide 0.01-0.5 mol / L and EDTA 0.1-1 mmol / L; the B solution comprises the TMB, hydrochloric acid and polyvinylpyrrolidone, wherein TMB 0.1-1 mmol / L, hydrochloric acid 0.01-0.5 mol / L and by weight percentage, polyvinylpyrrolidone 0.1-10%. The dissolving system of the TMB is adjusted to increase solubility of the TMB, and the polyvinylpyrrolidone enables the one-phase TMB solution to be capable of being stored for two years at the storage temperature of 2-8 DEG C.
Owner:杭州联科生物技术股份有限公司
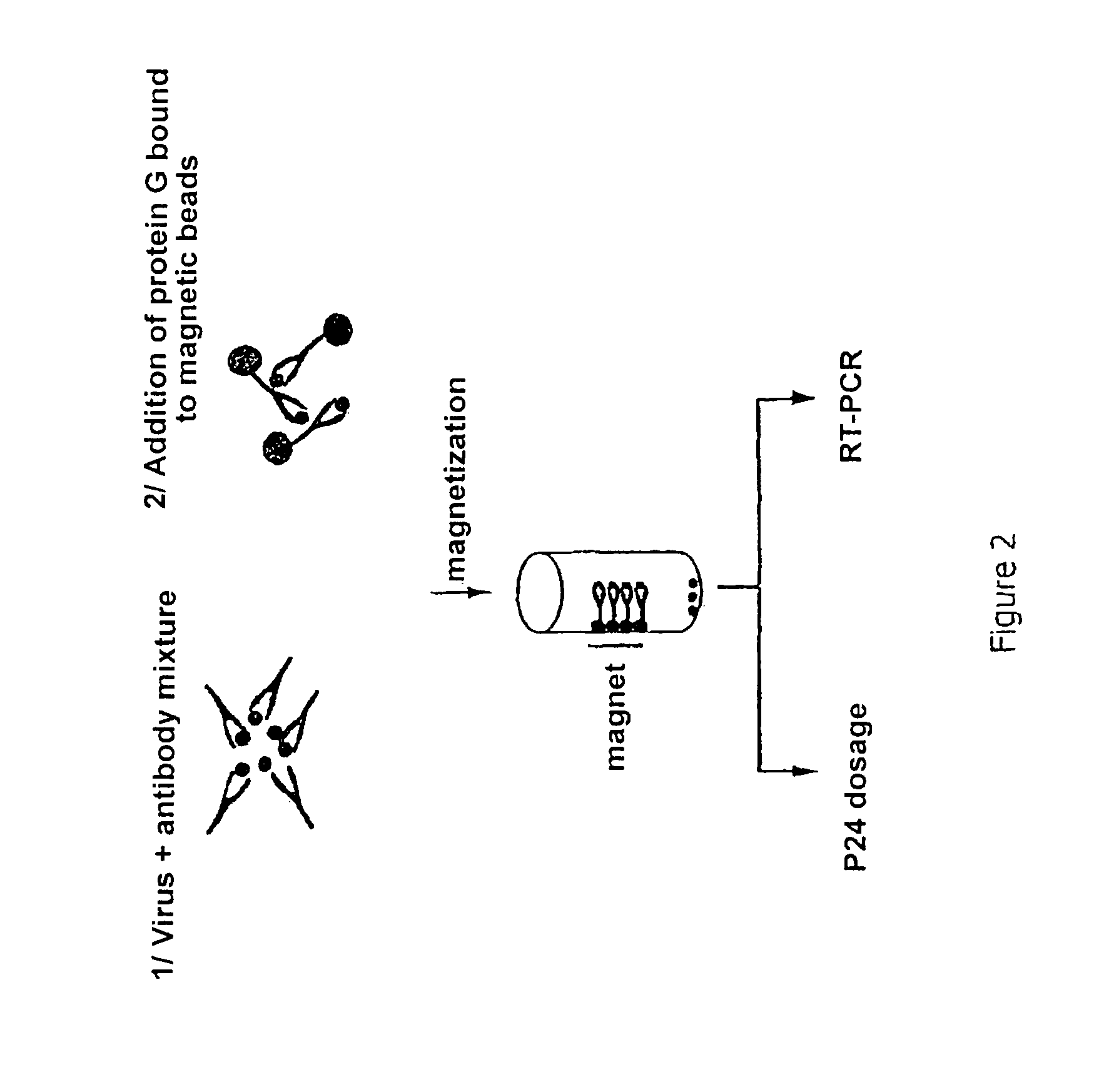
Novel application of nanotechnology and sensor technologies for ex-vivo diagnostics
InactiveUS20060040318A1Accurately and timely detectingEasy diagnosisMicrobiological testing/measurementMaterial analysis by electric/magnetic meansTarget analysisAnalyte
Systems and methods for the ex vivo diagnostic analysis of samples of bodily fluids, including exhaled breath and blood. The present invention uses nanostructure-based assemblies in combination with sensor technology to provide an efficient and accurate means for identifying the presence of a target analyte / biomarker in a sample of bodily fluid. In a preferred embodiment, the nanostructure-based assemblies of the present invention include detecting means such as RNA oligonucleotide chains or “aptamers” and releasable surrogate markers such as DMSO.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
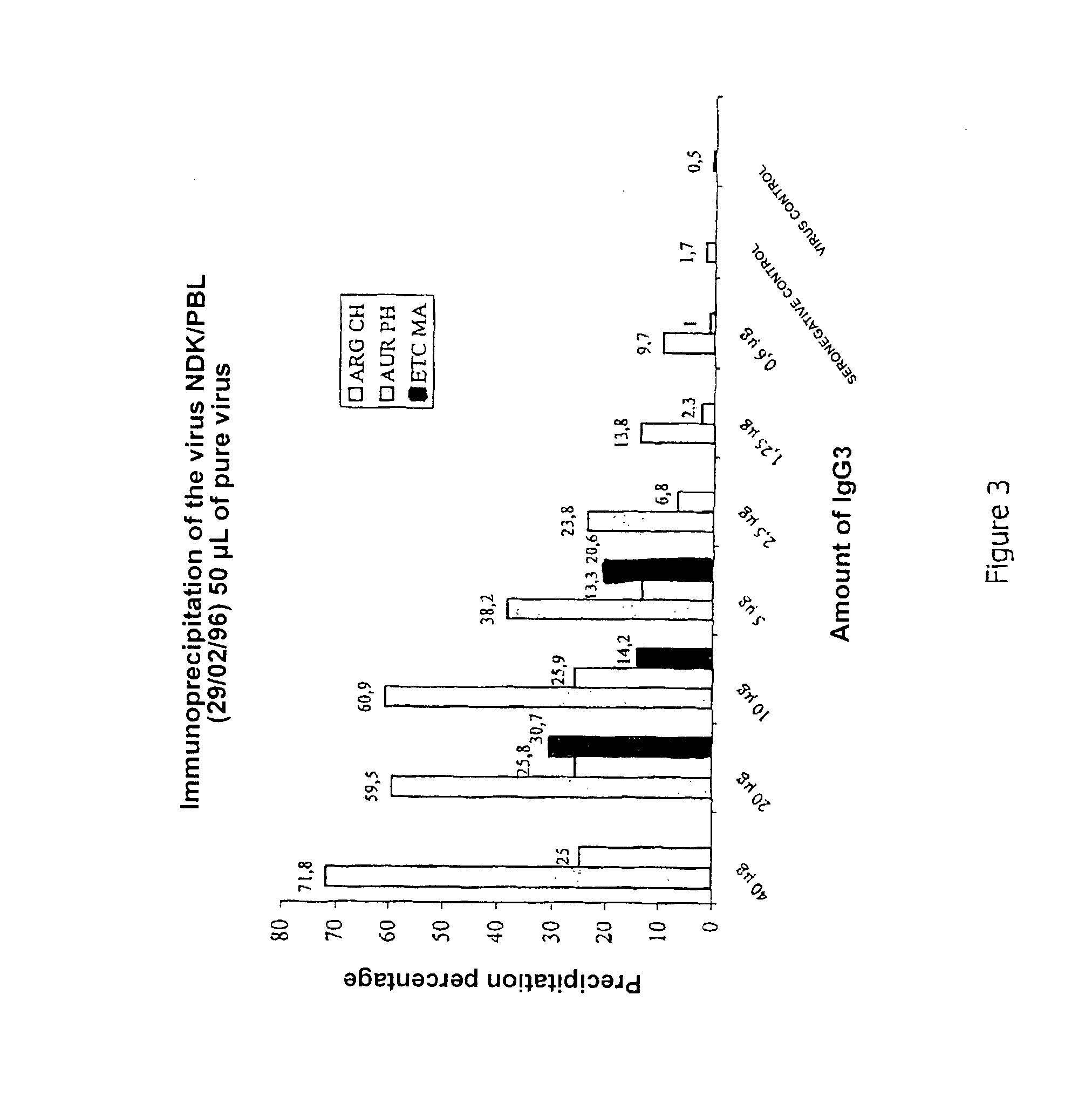
Immunoglobulin IgG3 as a marker for protecting against infectious viral diseases, and the uses of the same
The invention relates to a novel variant of isolated and / or purified immunoglobulin IgG3 which can be used as a marker for protecting against infectious viral diseases such as AIDS, as a diagnostic tool, or as a preventive and curative medicament. The invention also relates to corresponding in vitro diagnostic methods.
Owner:URRMA BIOPHARMA +1
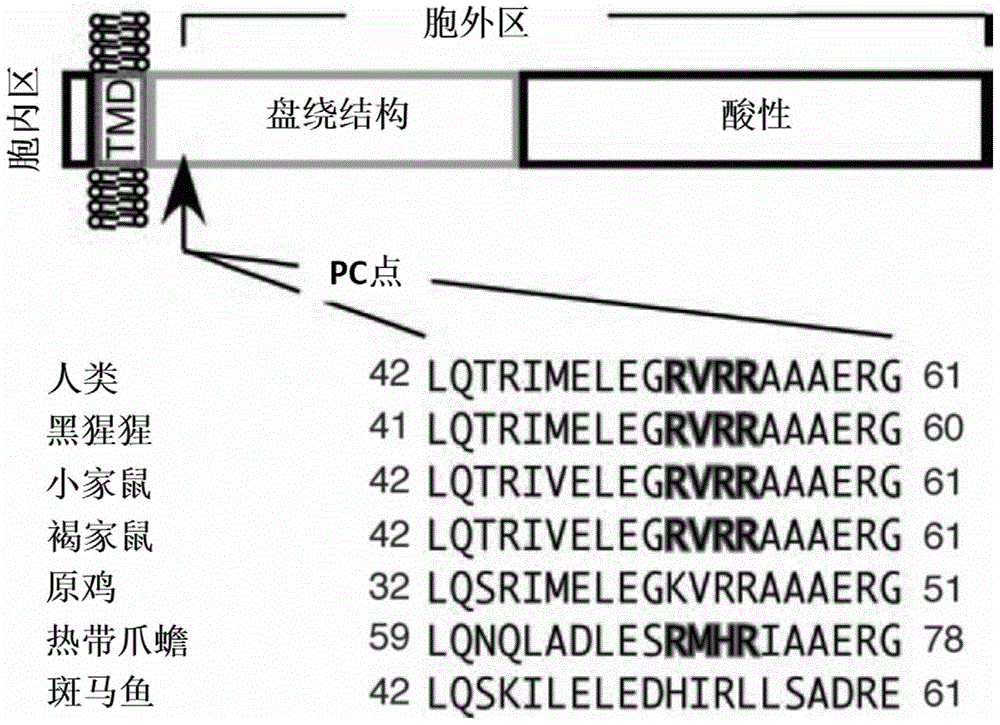
Ultra-sensitive superparamagnetic nano immunization microsphere and GP73 antigen detection method
ActiveCN105699653AThe detection process is fastStrong specificityBiological testingMicrosphereEnzyme immunoassays
The present invention relates to the technical field of diagnostic reagents, and particularly relates to a magnetic immunization in-vitro diagnostic reagent. In order to solve the technical problems of smaller capacity of an immobilized antibody, long reaction time, relatively low sensitivity and low stability of the immobilized antibody of a conventional enzyme immunoassay, the present invention provides a GP73 monoclonal antibody and a superparamagnetic nano immunization microsphere with the GP73 monoclonal antibody coupled with the surface, and a human serum or plasma GP73 antigen detection method using the superparamagnetic nano immunization microsphere by double-antibody sandwich enzyme immunoassay or chemiluminescence method. An antigen for producing the GP73 monoclonal antibody has the following amino acid sequence AAAERGAVELKK. The superparamagnetic nano immunization microsphere has the characteristics of being capable of coupling more antibodies, fast in immunoreaction speed, high in specificity, good in repeatability, low in cost, and simple in experimental condition requirements and the like.
Owner:BEIJING INST OF HEPATOLOGY +1
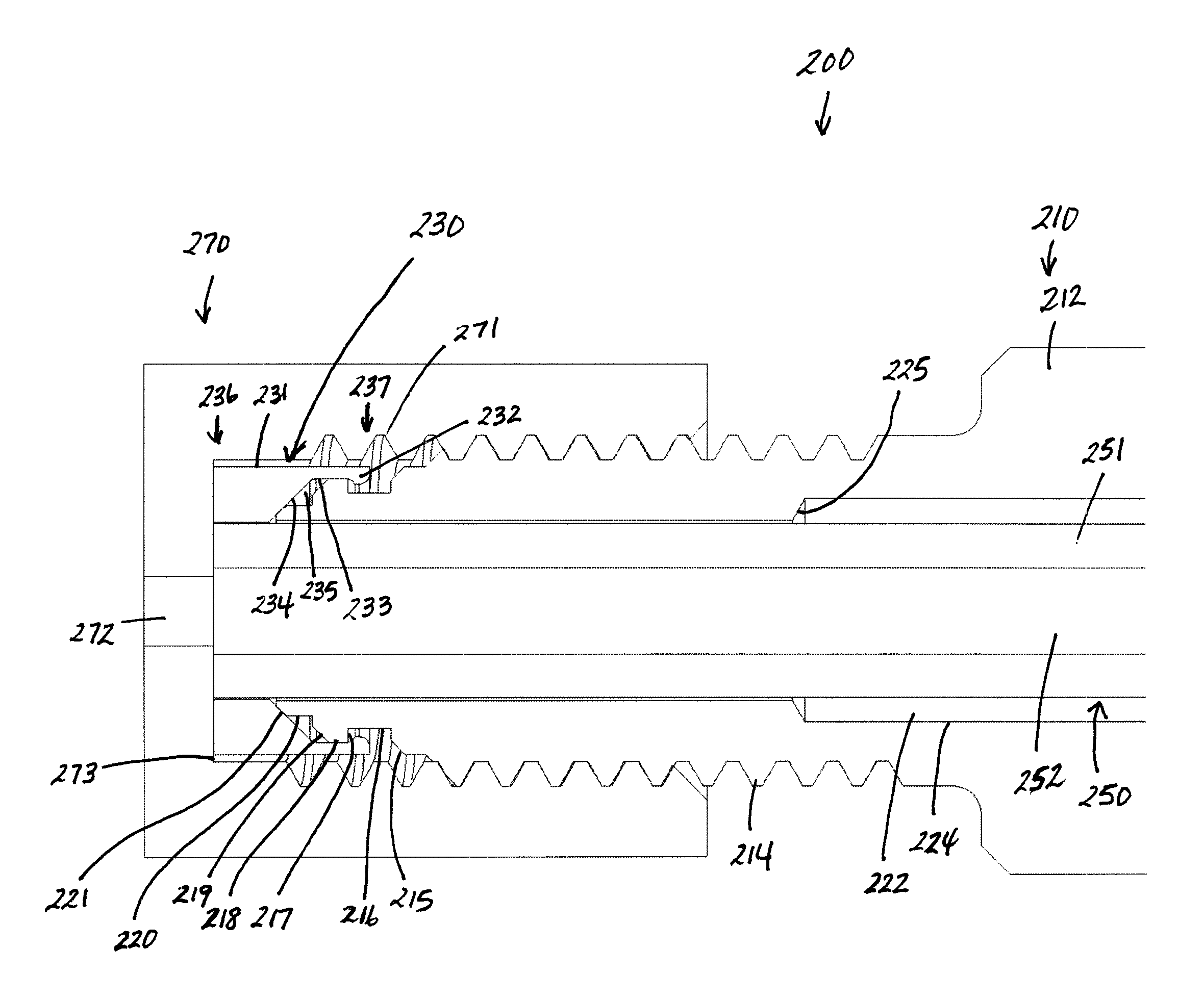
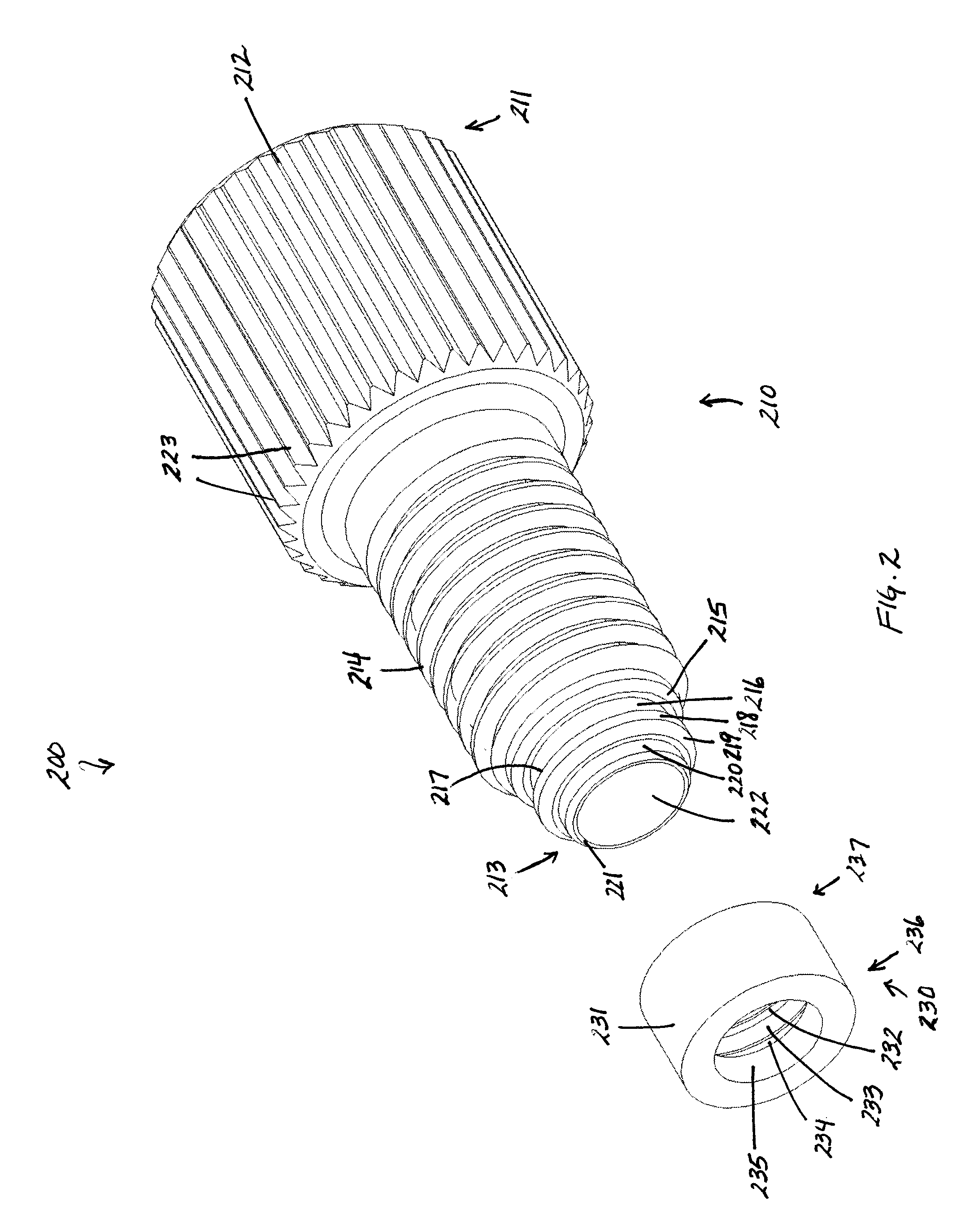
Flat bottom fitting assembly
A fitting assembly is provided having a nut and a ferrule, which in certain embodiments may be assembled by an operator. The nut and ferrule of the fitting assembly have passageways therethrough for receiving and removably holding tubing. The nut and ferrule fitting assembly may be adapted for use with a flat bottom port, such as in an analytical instrument, like liquid chromatography, gas chromatography, ion chromatography, or in in vitro diagnostic systems.
Owner:IDEX HEALTH & SCI
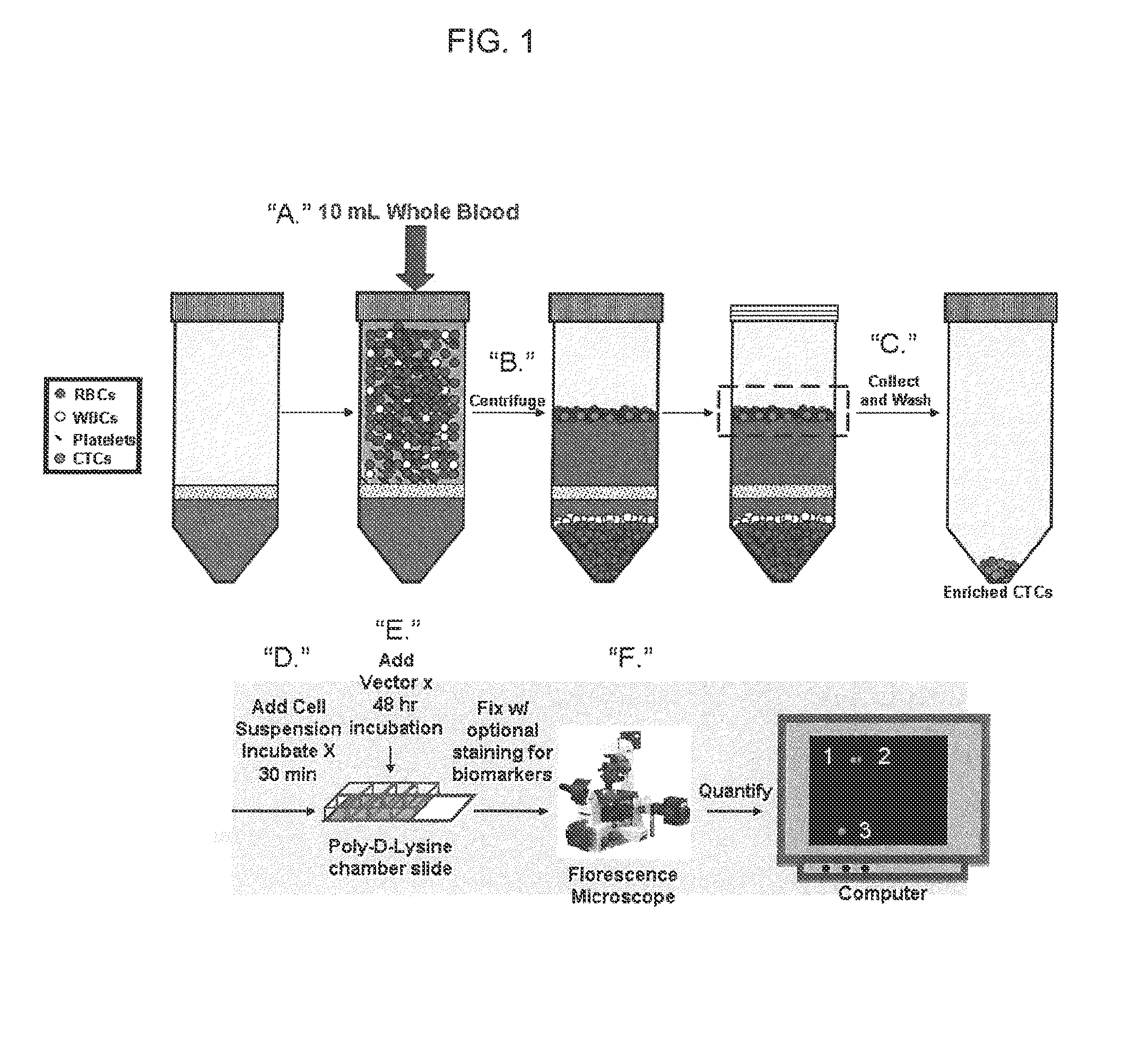
Compositions, methods and microfluidics device for telomerase based in vitro diagnostic assays for detecting circulating tumor cells (CTC)
ActiveUS20150285786A1Easy to transportNot capture smallBioreactor/fermenter combinationsBiological substance pretreatmentsTelomeraseCell culture media
A repeatable method for detecting circulating tumor cells in vitro is provided. The method involves combining a test sample from a patient suspected of having circulating tumor cells, and a non-lytic adenoviral system, and culture media for the cells. The adenoviral system utilizes (i) a first replication-defective adenoviral particle in which an expression cassette is packaged, said expression cassette comprising an adenoviral 5′ and 3′ ITRs and a tumor-specific promoter; and (ii) a coding sequence for a reporter protein which is expressed in the presence of circulating tumor cells, and an adenoviral 3′ ITR. The test sample and the non-lytic adenoviral system are incubated for a sufficient time to permit expression of the reporter protein, and measuring reporter protein expression in the test samples, whereby presence of reporter expression indicates the presence of circulating tumor cells in the sample.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Tissue dewaxing transparent agent free of benzene
InactiveCN104155160ASoft effectNot easy to shrink and deformPreparing sample for investigationLiquid base cytologyStaining
The invention relates to a tissue dewaxing transparent agent free of benzene, which is used in biological histology, histopathology or forensic science, can make a tissue transparent, and can make the tissue dewaxed. The tissue dewaxing transparent agent belongs to the technical field of in vitro diagnostic reagents. The tissue dewaxing transparent agent mainly contains 95%-100% of alicyclic hydrocarbon, and the balance of accessories; the alicyclic hydrocarbon is a monocyclic alicyclic hydrocarbon or a bicyclic alicyclic hydrocarbon. The tissue dewaxing transparent agent free of benzene has the advantages of being non-toxic, soft in effect, and not easy to cause shrinkage, deformation, hardening and embrittlement of a tissue material, capable of improving the section intact rate, insensitive to the humid environment, not easy to muddy due to absorption of moisture in the air, and the like; not only can be used for liquid based cytology, immunohistochemistry, biological histology, routine pathological diagnosis, and immunohistochemical diagnostic techniques, is also suitable for special staining method and histochemical techniques. The refractive index of used alicyclic hydrocarbon transparent agent (cyclohexane) is 1.42, the (methyl cyclohexane) refractive rate is 1.42, and is basically consistent with the tissue refractive index (1.418), and the transparent effect is better.
Owner:陆可望 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com