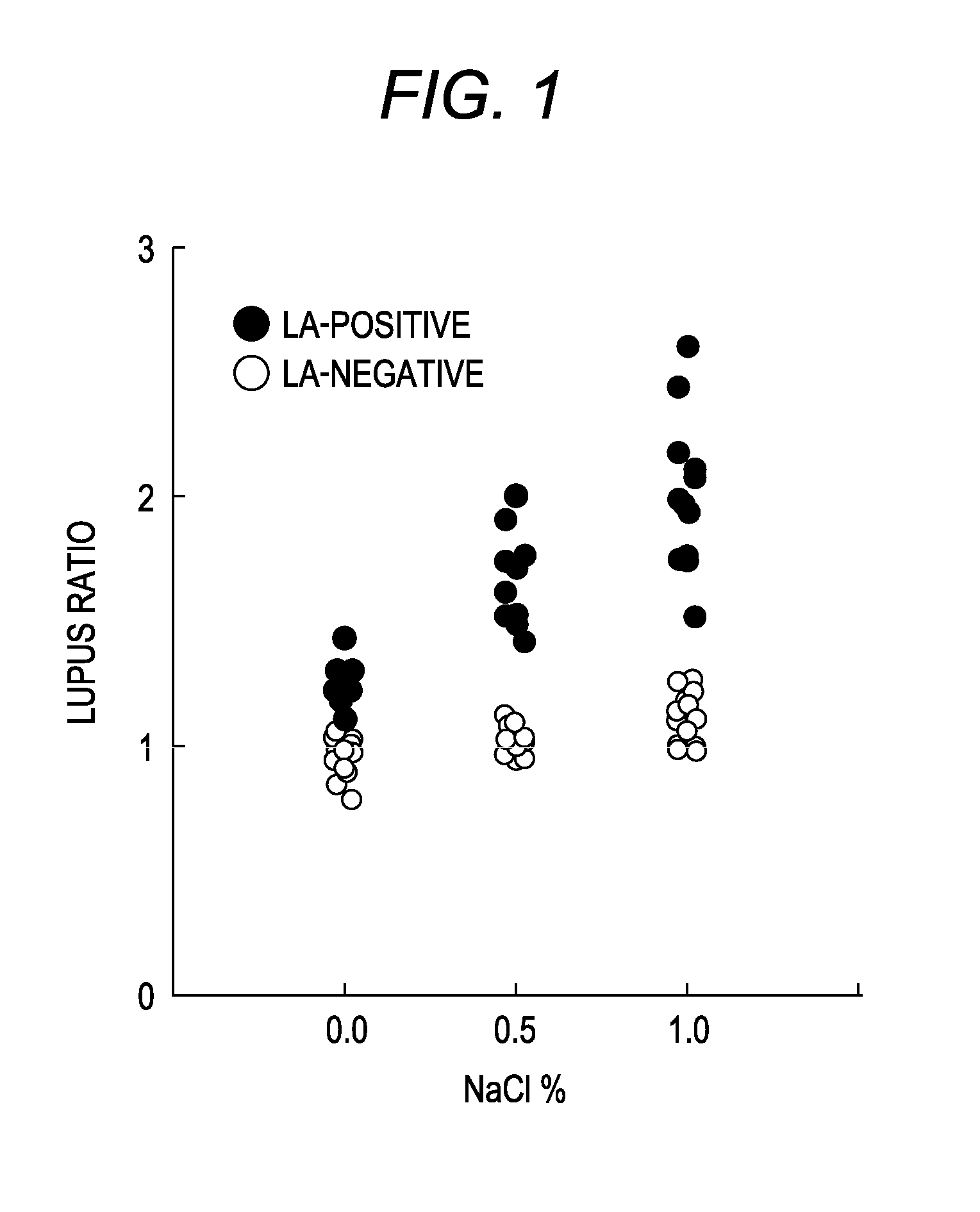
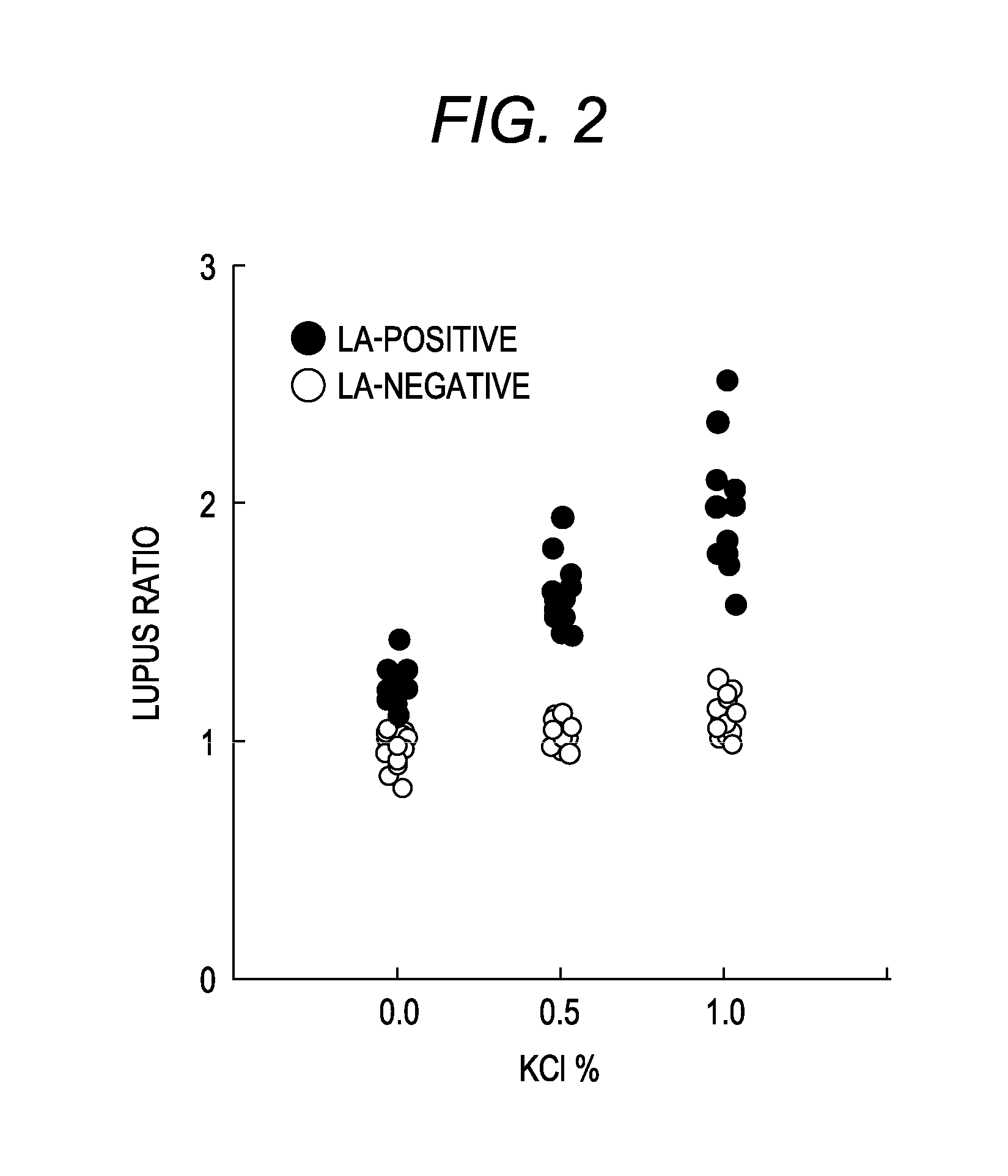
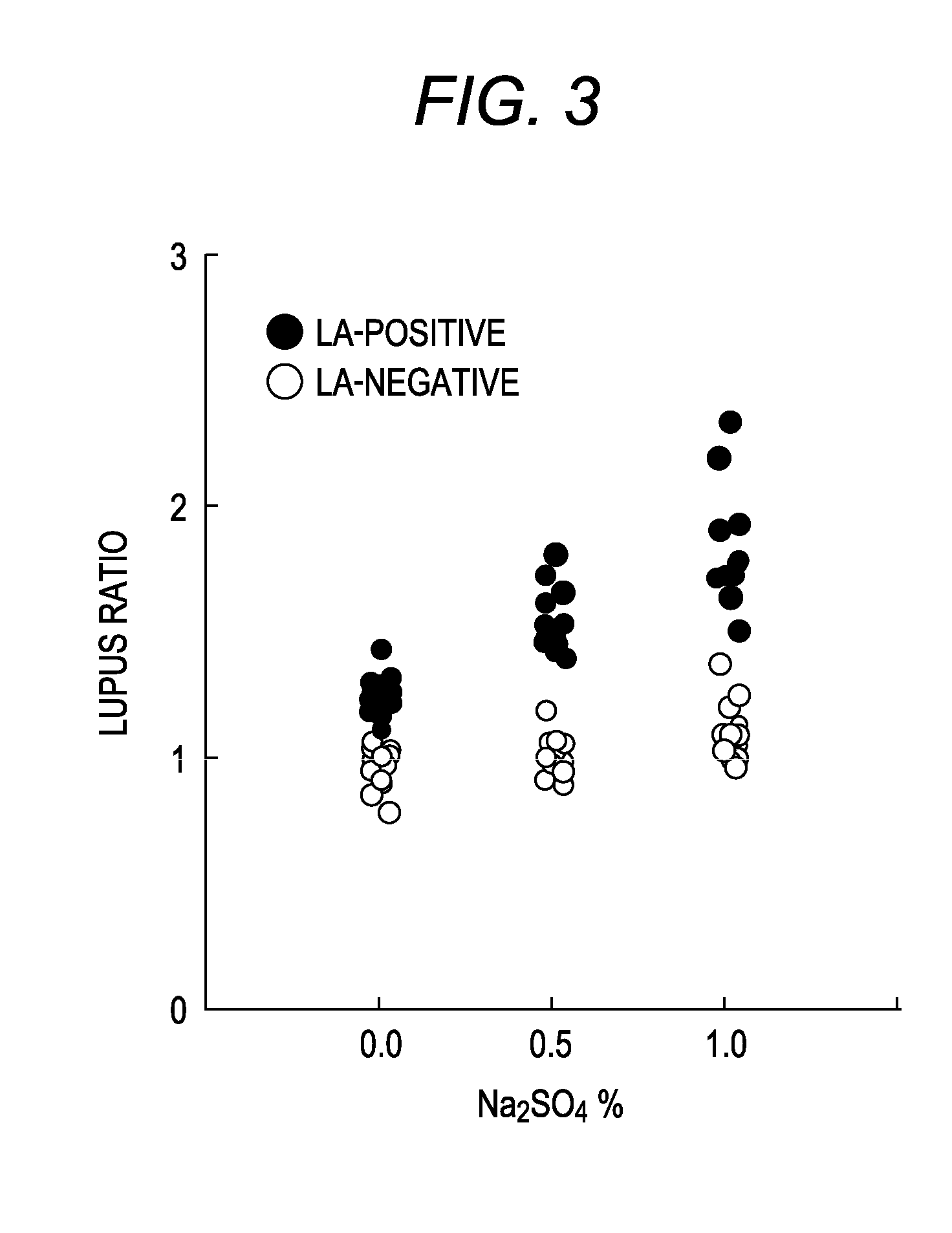
Patents
Literature
38 results about "Lupus anticoagulant" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Lupus anticoagulant is an immunoglobulin that binds to phospholipids and proteins associated with the cell membrane. Lupus anticoagulant is a misnomer, as it is actually a prothrombotic agent. Lupus anticoagulant antibodies in living systems cause an increase in inappropriate blood clotting. The name derives from their properties in vitro, as these antibodies increase laboratory coagulation tests such as the aPTT. Investigators speculate that the antibodies interfere with phospholipids used to induce in vitro coagulation. In vivo, the antibodies are thought to interact with platelet membrane phospholipids, increasing adhesion and aggregation of platelets, which accounts for the in vivo prothrombotic characteristics.
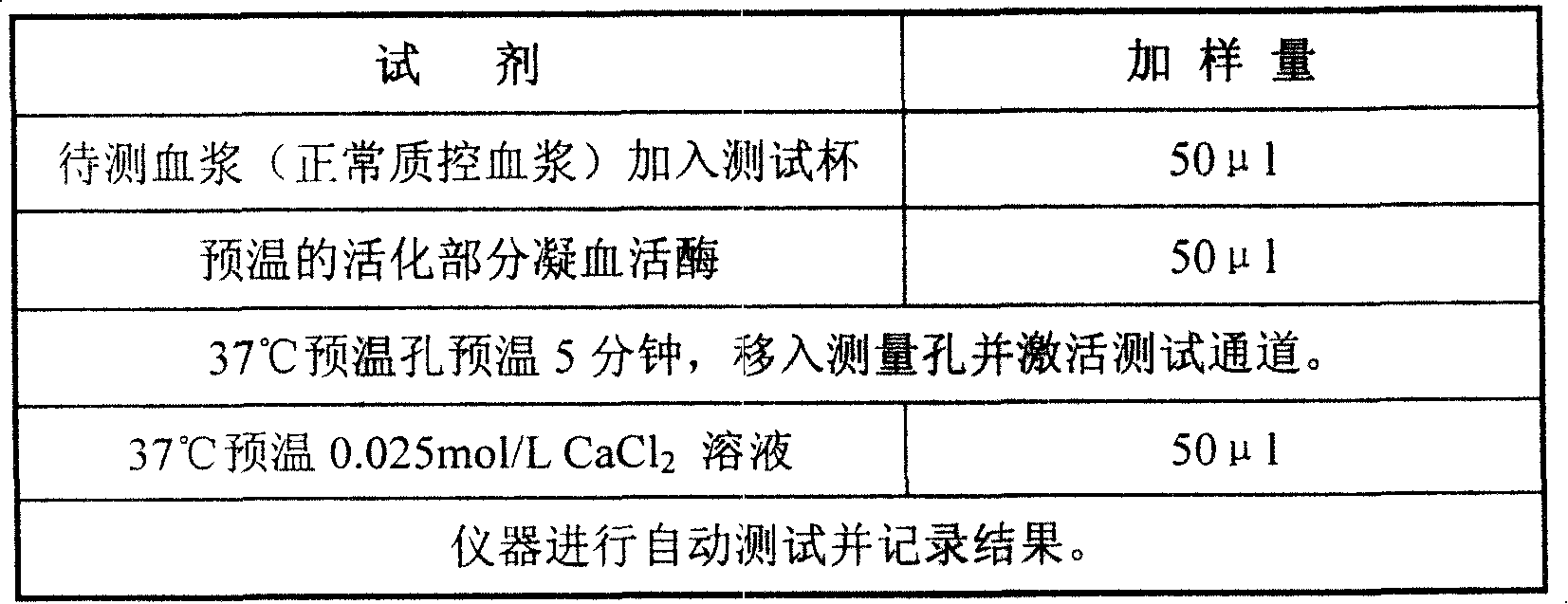
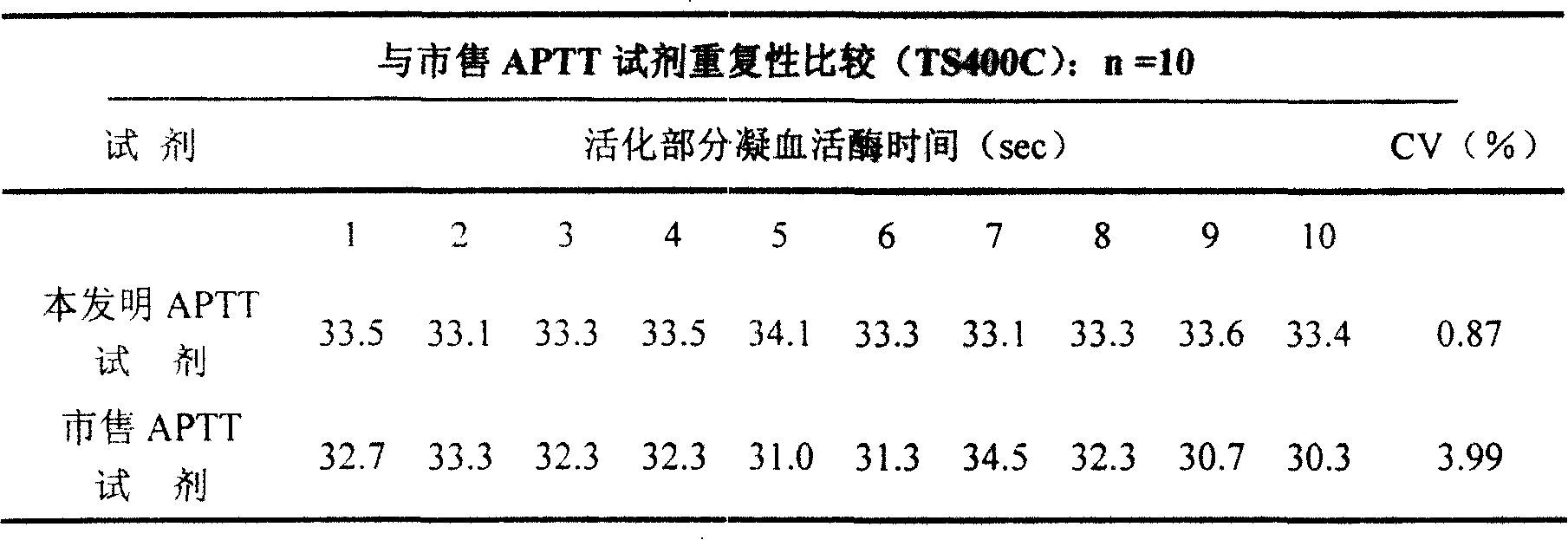
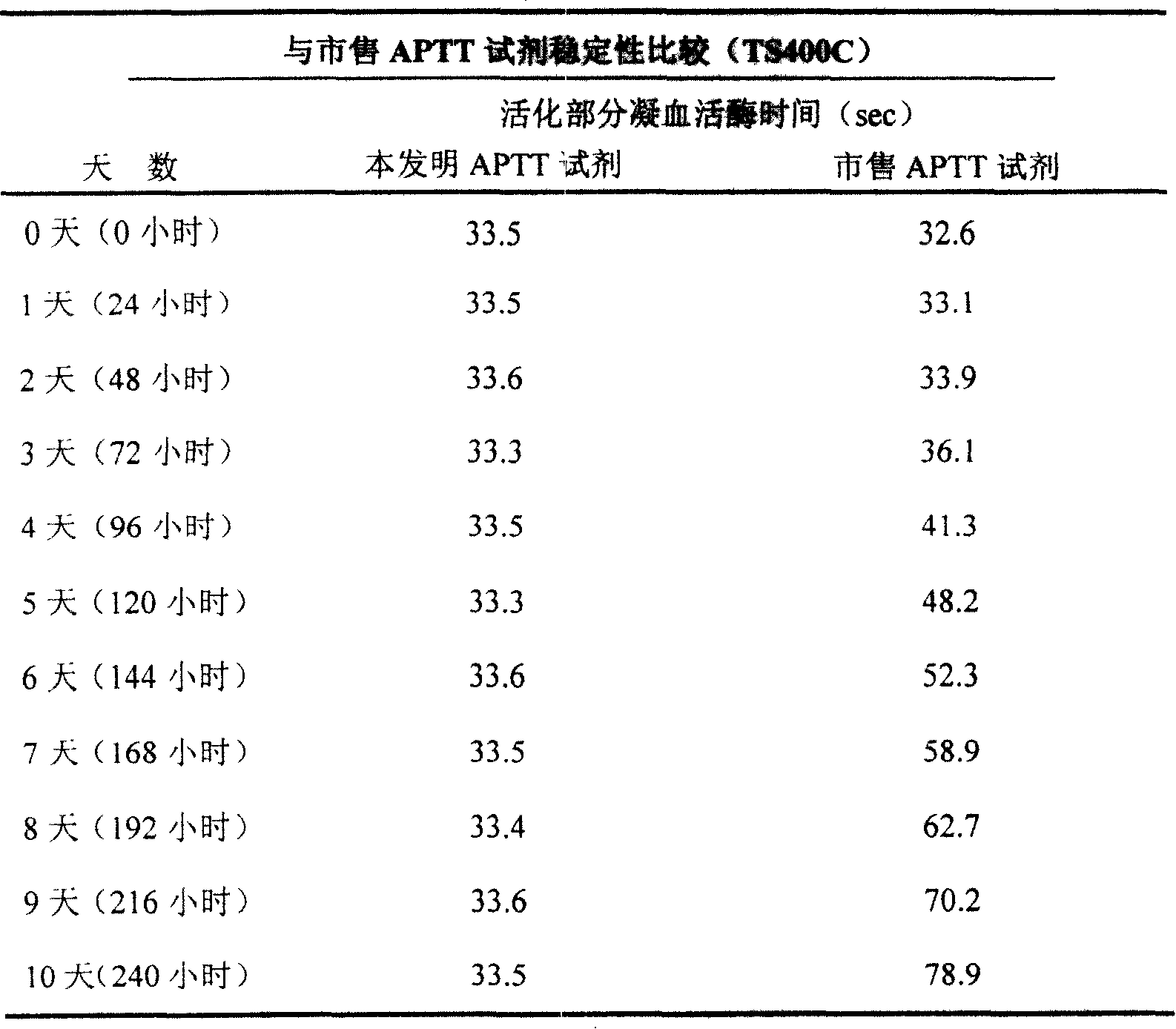
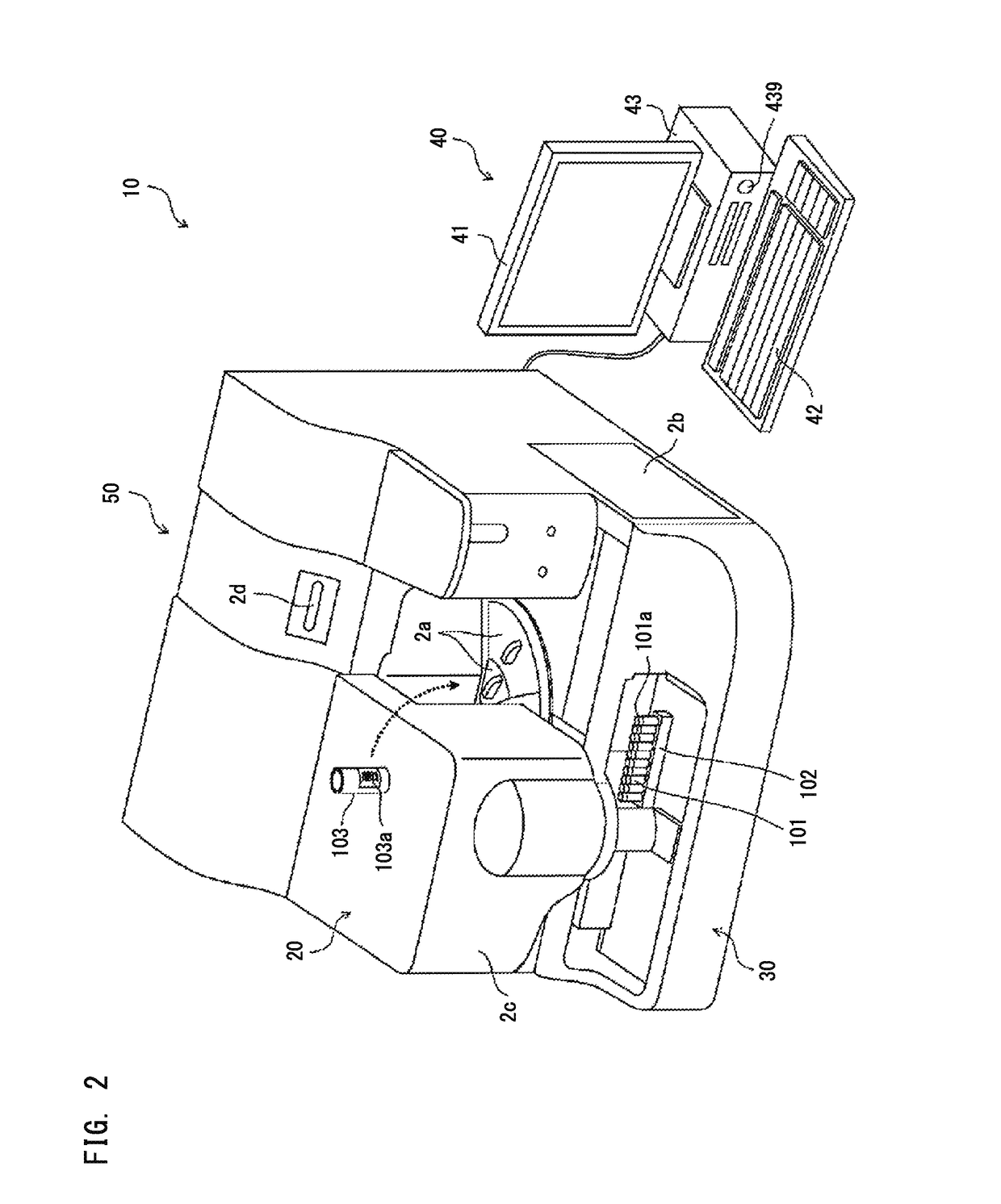
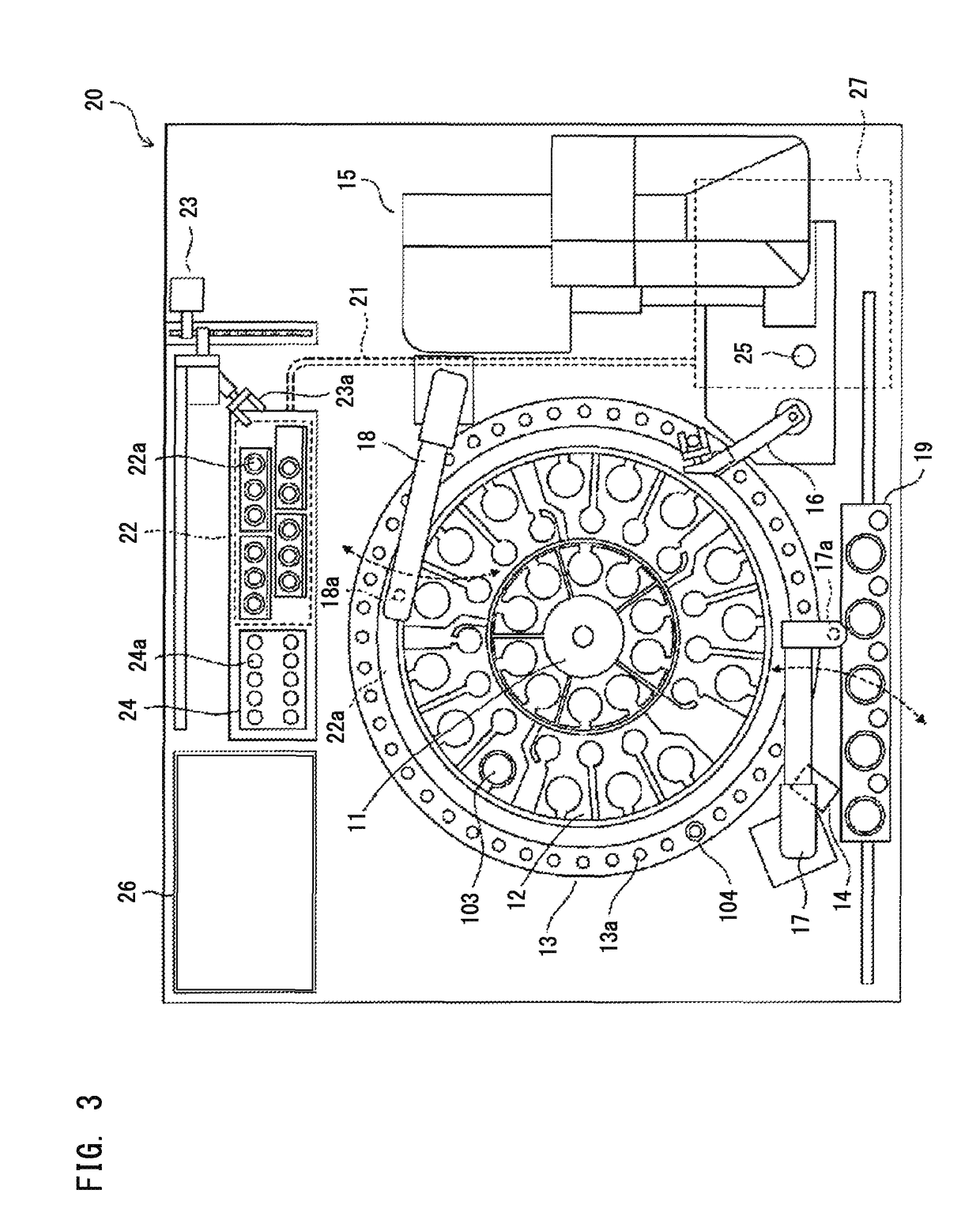
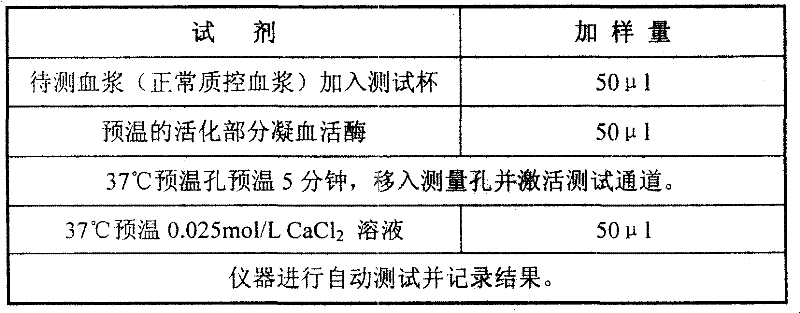
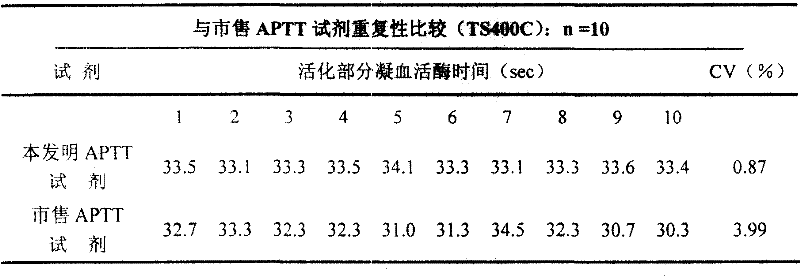
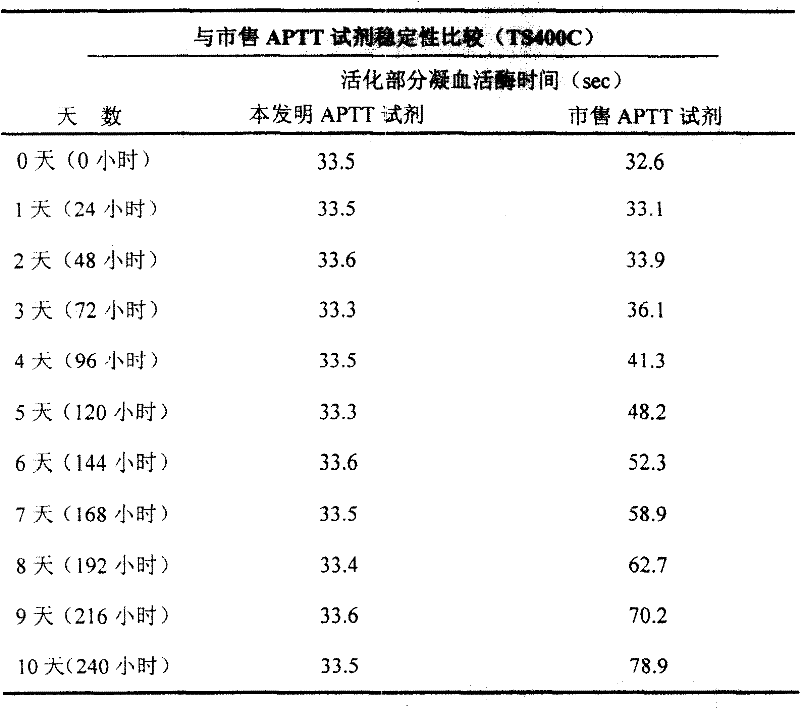
External diagnostic reagent kit used for measuring activated partial thromboplastin time
ActiveCN101221189AImprove stabilityGood repeatabilityMicrobiological testing/measurementBiological testingDisease causeAPTT - reference
The invention relates to an in vitro diagnostic kit for the determination of activated partial thromboplatin time (APTT) in clinical test. The invention consists of a partial thromboplatin reagent and a calcium chloride solution, which is used for the detection of the defects of the intrinsic coagulation pathway factors and the screening test of the related inhibitors, and the invention is also a primary means for the current coagulation factor and heparin anticoagulant treatment and the detection of lupus anticoagulant. The invention has the advantages of long stability time and good repeatability of the partial thromboplatin reagent after a re-dissolution, at the same time, the invention has better consistency of the measurement results of a blood coagulation analyzer by using an optical method and a magnetic bead method, therefore, the invention is applicable to large, medium and small hospitals, and the test results of different hospitals have comparability, therefore the invention has important meaning for implementing the one general report of test reports, provides the reliable experimental data for the clinical diagnosis and the treatment of diseases and improves the efficiency and value of the basic studies of thrombosis and hemostasis.
Owner:SHANGHAI SUNBIO TECH
Lupus anticoagulant testing
InactiveUS20070026467A1Improve matchEasy to detectSamplingDead animal preservationSystemic lupus erythematosusBlood plasma
The present invention relates generally to the field of diagnostic screening and diagnostic assays. In particular, the present invention provides an improved, rapid, and efficient method of screening for antiphospholipid antibodies, such as lupus anticoagulants (LA). The invention also relates to a kit for screening plasma levels for antiphospholipid antibodies in subjects in need thereof, such as those at risk for or suffering from, inter alia, antiphospholipid syndrome (APS) and systemic lupus erythromatosus (SLE).
Owner:BIOMEDICA DIAGNOSTICS INC
Lumbricus polypeptide having anticoagulant and thrombolytic effects, and enzymatic hydrolysis preparation method and application thereof
ActiveCN104004806AImprove bioavailabilityThe structure is simple and easy to understandPeptide preparation methodsFermentationHydrolysateHigh activity
The invention discloses a lumbricus polypeptide having anticoagulant and thrombolytic effects, and an enzymatic hydrolysis preparation method and an application thereof. The preparation method comprises the steps: cleaning and drying lumbricus dried bodies, and crushing into a powder; adding water, stirring, and centrifuging to obtain a protein precipitate; adding water to dissolve the protein, adding an alkaline protease with the ratio of the enzyme volume to the protein weight of 0.1-3 mL:100 g, and stirring; exterminating enzyme activity, adding trypsin with the ratio of the enzyme mass to the protein weight of 0.5-5 g:100 g, and stirring at the temperature of 25-60 DEG C; allowing the enzymatic hydrolysate to pass through an anion exchange column, collecting a part of eluate; and then passing through a macroporous adsorption resin, carrying out separation and purification, and collecting and drying to obtain a lumbricus anticoagulant peptide powder. A new source is added for high-activity anticoagulant active substances, the product activity is high, the preparation process is convenient and feasible, and the environment is protected; and the lumbricus polypeptide can be used as a raw material of foods for thrombosis prevention, thrombolysis and blood consistency reduction, is supplemented with lactose, mannitol and the like, and is made into a powder, a tablet, a capsule or an oral liquid.
Owner:广州速科食品有限公司
Lupus anticoagulant testing
InactiveUS7932021B2Improve matchEasy to detectSamplingDead animal preservationAntiendomysial antibodiesSystemic lupus erythematosus
Owner:BIOMEDICA DIAGNOSTICS INC
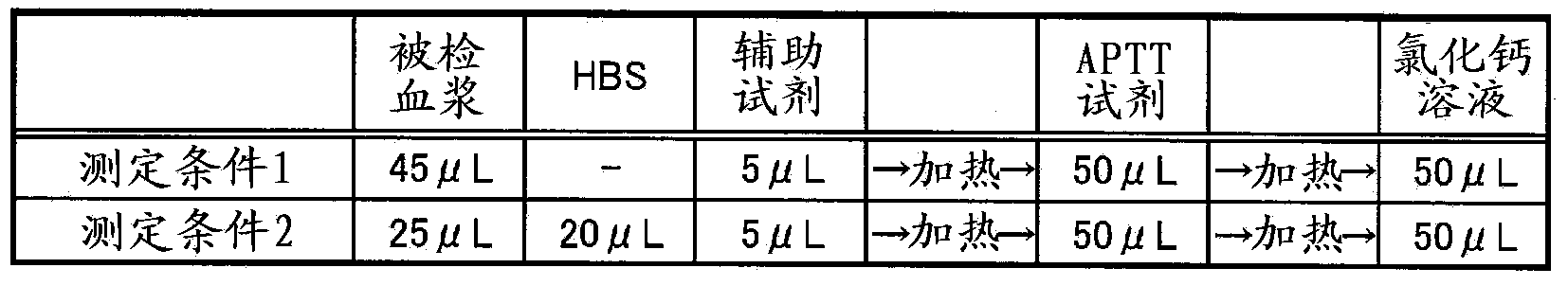
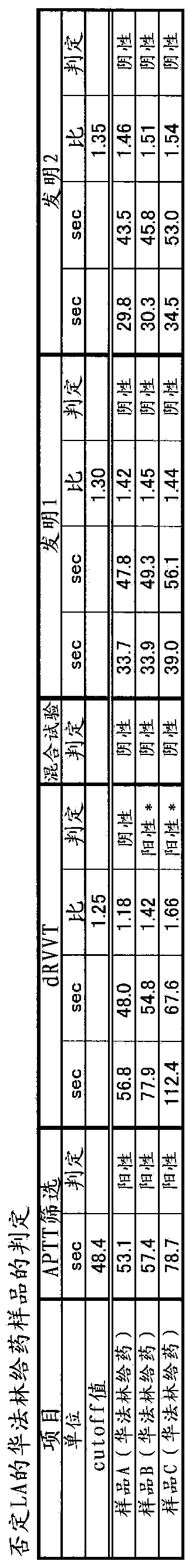
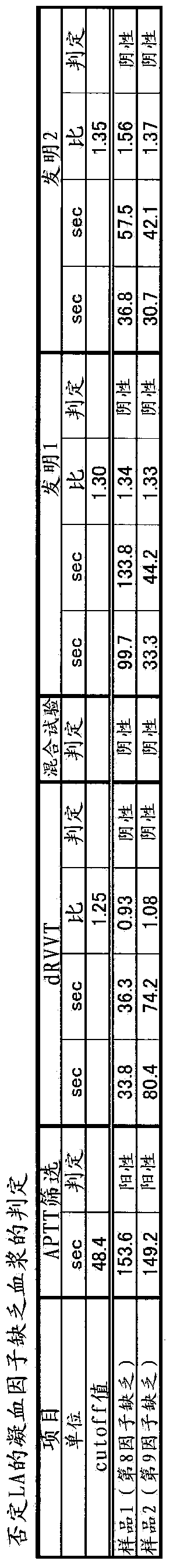
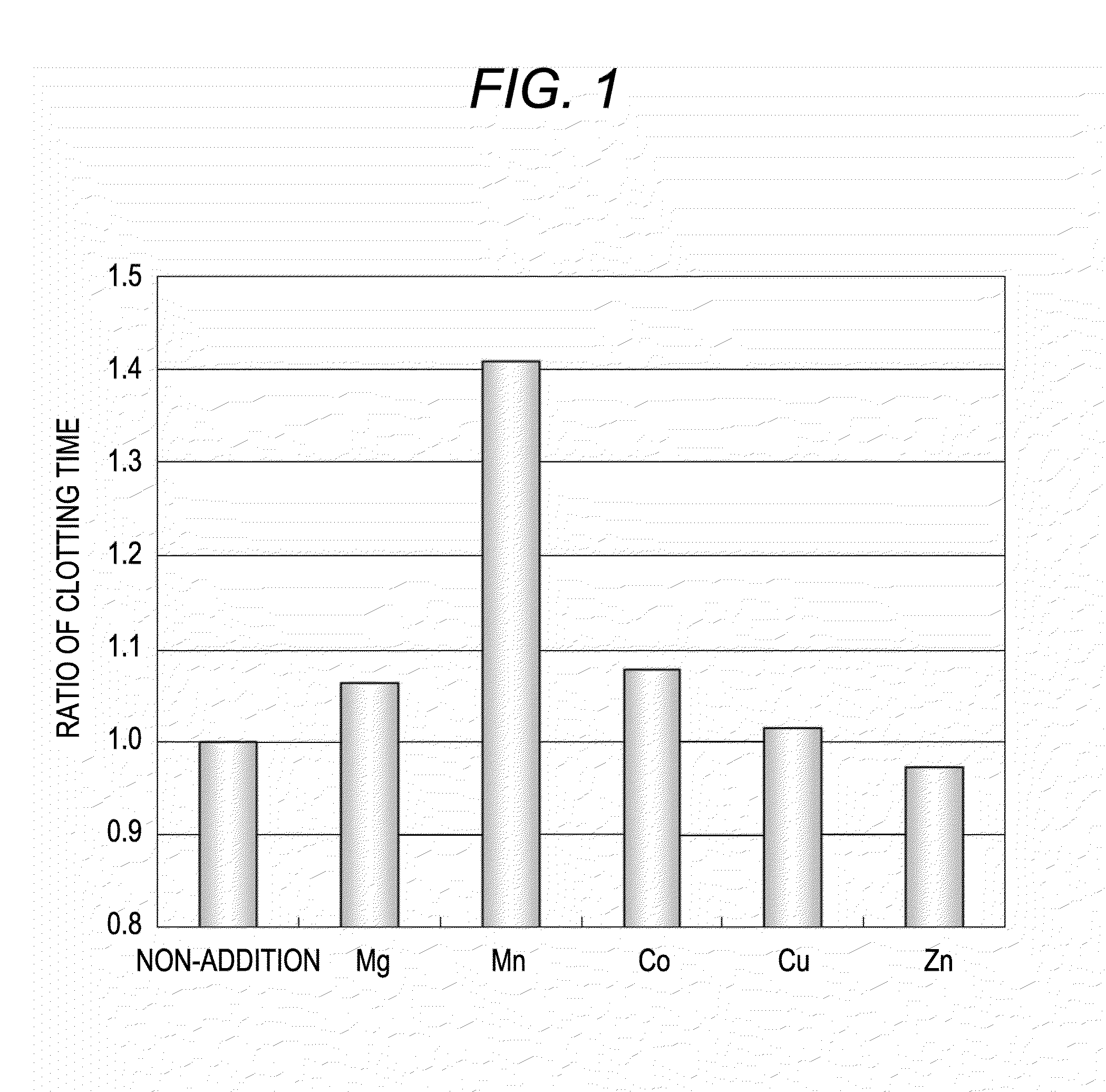
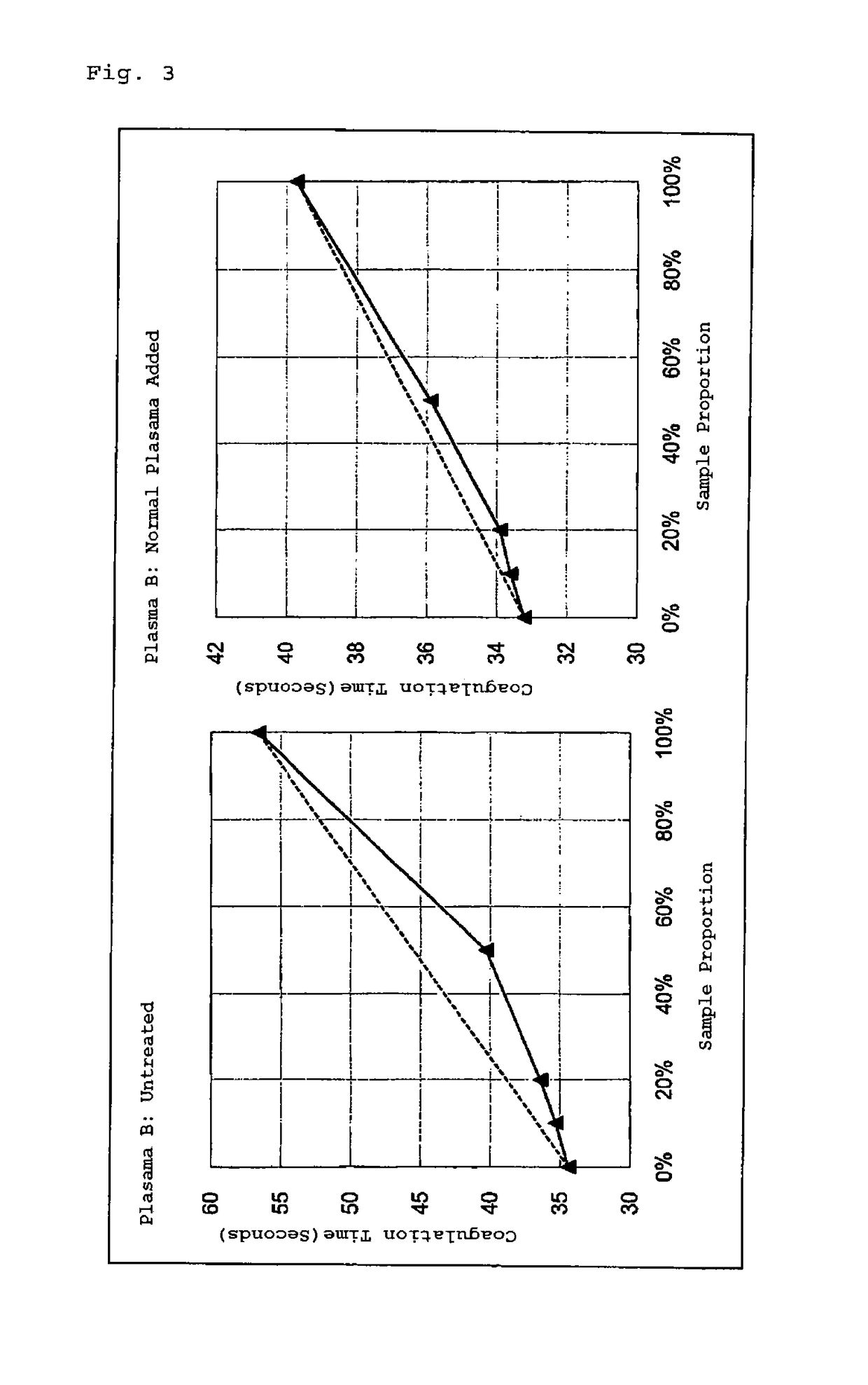
Method for measuring clotting time, method for determining presence or absence of lupus anticoagulant, and reagent kit for detecting lupus anticoagulant
Disclosed is a method for measuring clotting time including: preparing a measurement sample by mixing a blood specimen suspected to contain a lupus anticoagulant, an activator, and a divalent-metal-ion-producing compound for facilitating formation of a phospholipid-containing complex; and measuring a clotting time after adding, to the measurement sample, an aqueous solution of a calcium salt as a coagulation initiator.
Owner:SCHOOL JURIDICAL PERSON HIGASHI NIPPON GAKUEN +1
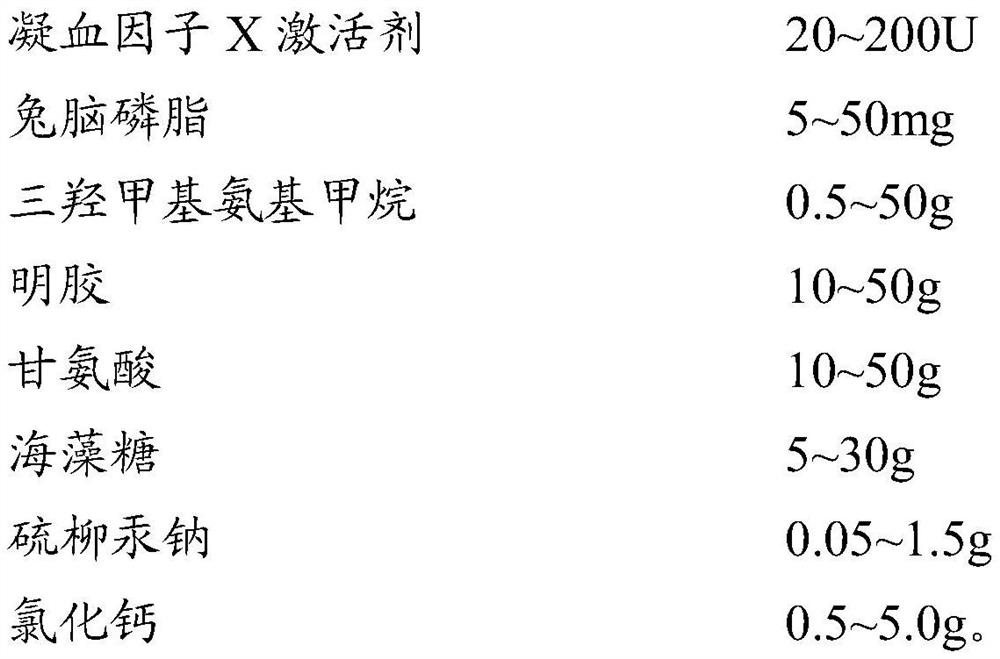
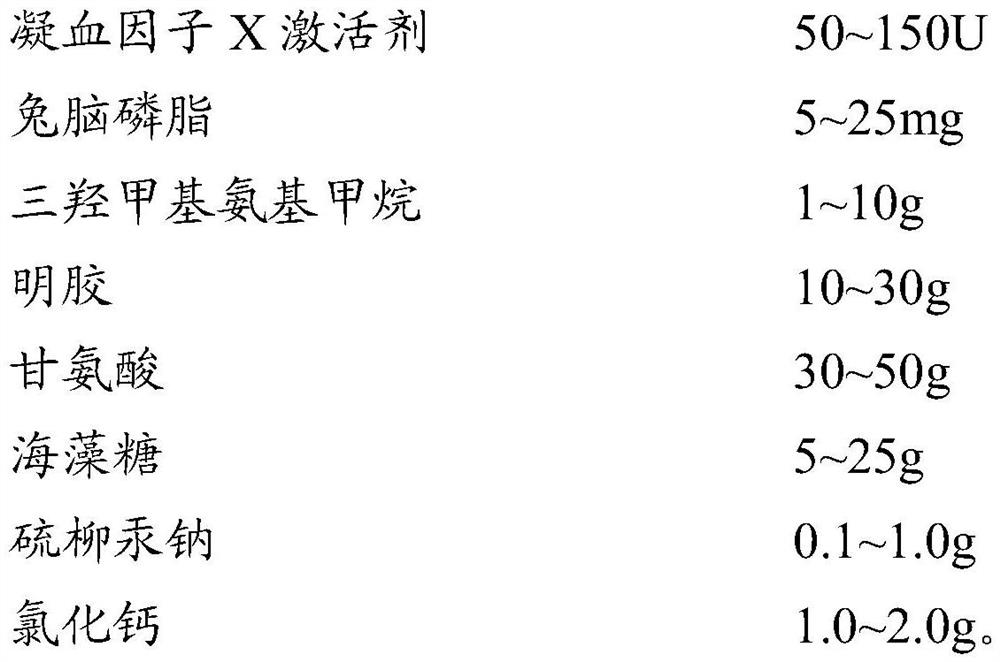
Lupus anticoagulant (LA) screening and determining reagent kit (freezing method)
The invention discloses a reagent for screening and determining lupus anticoagulant (LA) in blood plasma by a dilution viper venom test method. The LA screening and determining reagent comprises viper venom blood clotting factor X activating agents, rapeseed phosphatide and calcium ions. The rapeseed phosphatide replaces the animal cephalin in the prior art, the proper addition quantity is low, and the rapeseed phosphatide has higher specificity and sensitivity for LA. The LA screening and determining reagent has the advantages of good specificity, high sensitivity, high stability and high detection feasibility degree.
Owner:SHANGHAI SUNBIO TECH
Blood sample determination method and blood sample analyzer
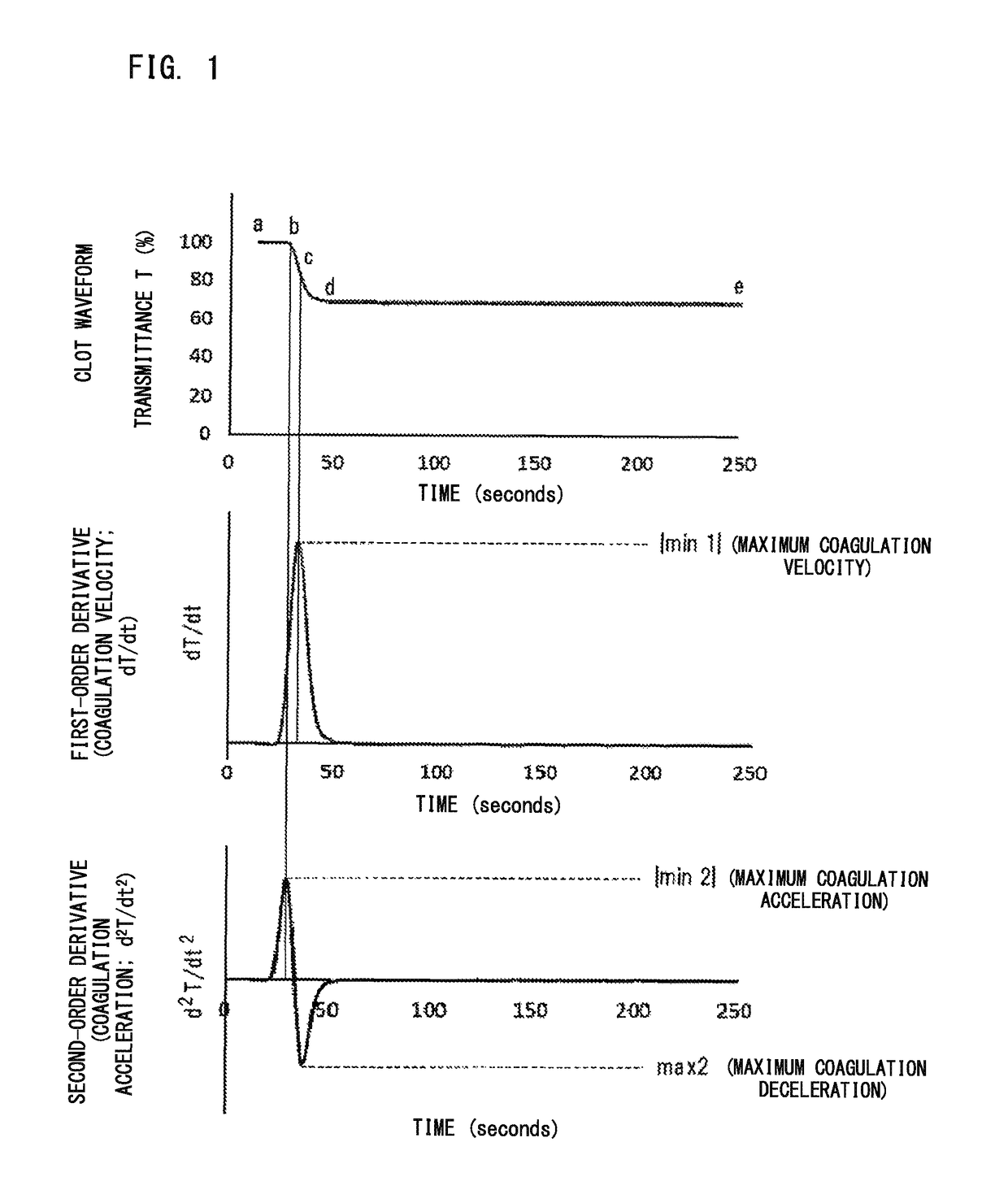
ActiveUS20160178651A1Bioreactor/fermenter combinationsBiological substance pretreatmentsLupus anticoagulantCoagulation Factor Inhibitors
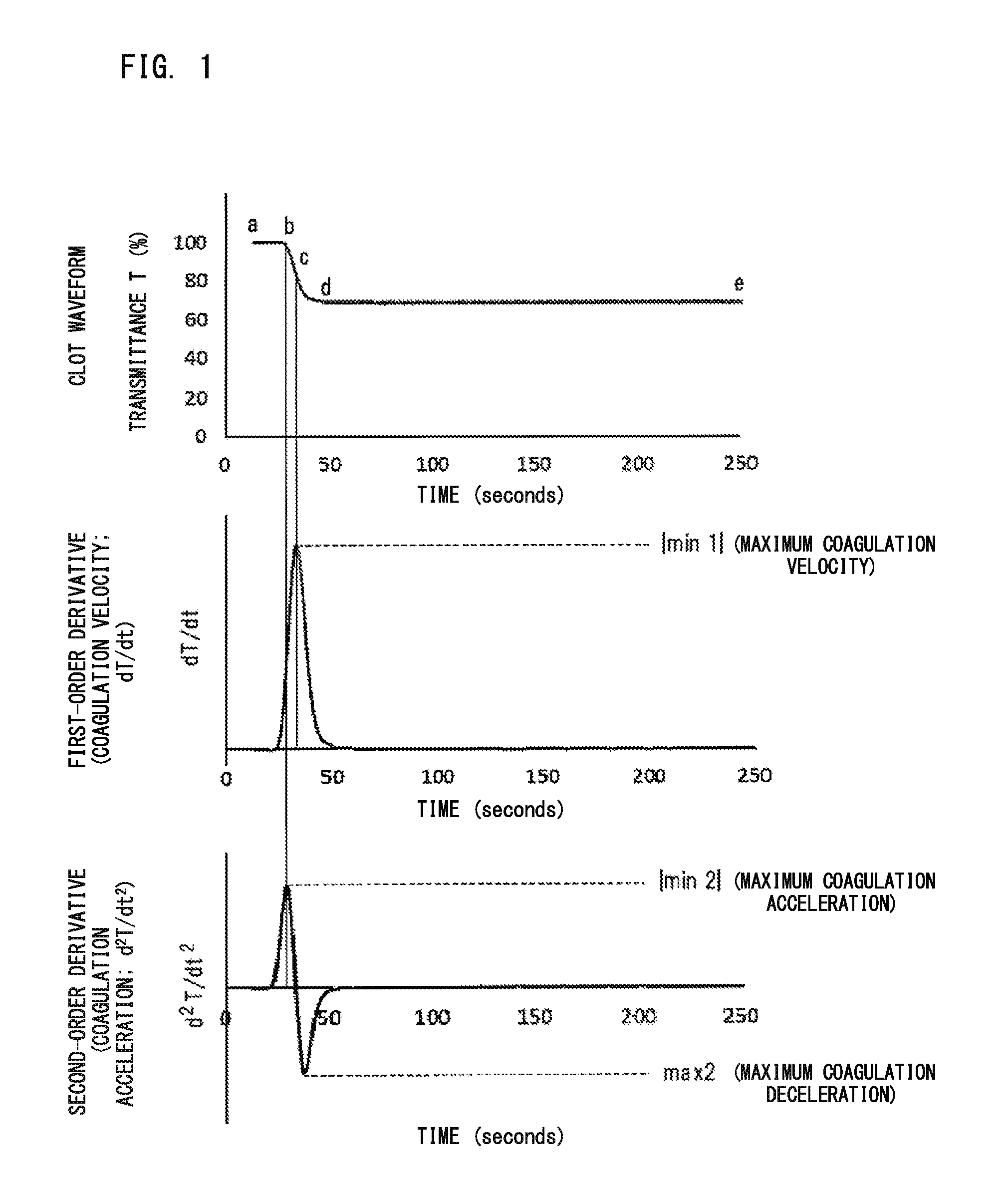
Disclosed is a blood sample determination method including: emitting light to a measurement specimen prepared by mixing a clotting time measuring reagent and a blood sample suspected to be derived from a subject having lupus anticoagulant or a coagulation factor inhibitor, to obtain optical information about an amount of light from the measurement specimen; obtaining at least one parameter regarding derivative of clot waveform, based on the obtained optical information; and determining, based on a value of the obtained parameter, whether the blood sample is suspected to be a sample derived from a subject having lupus anticoagulant or is suspected to be a sample derived from a subject having a coagulation factor inhibitor.
Owner:NARA MEDICAL UNIVERSITY +1
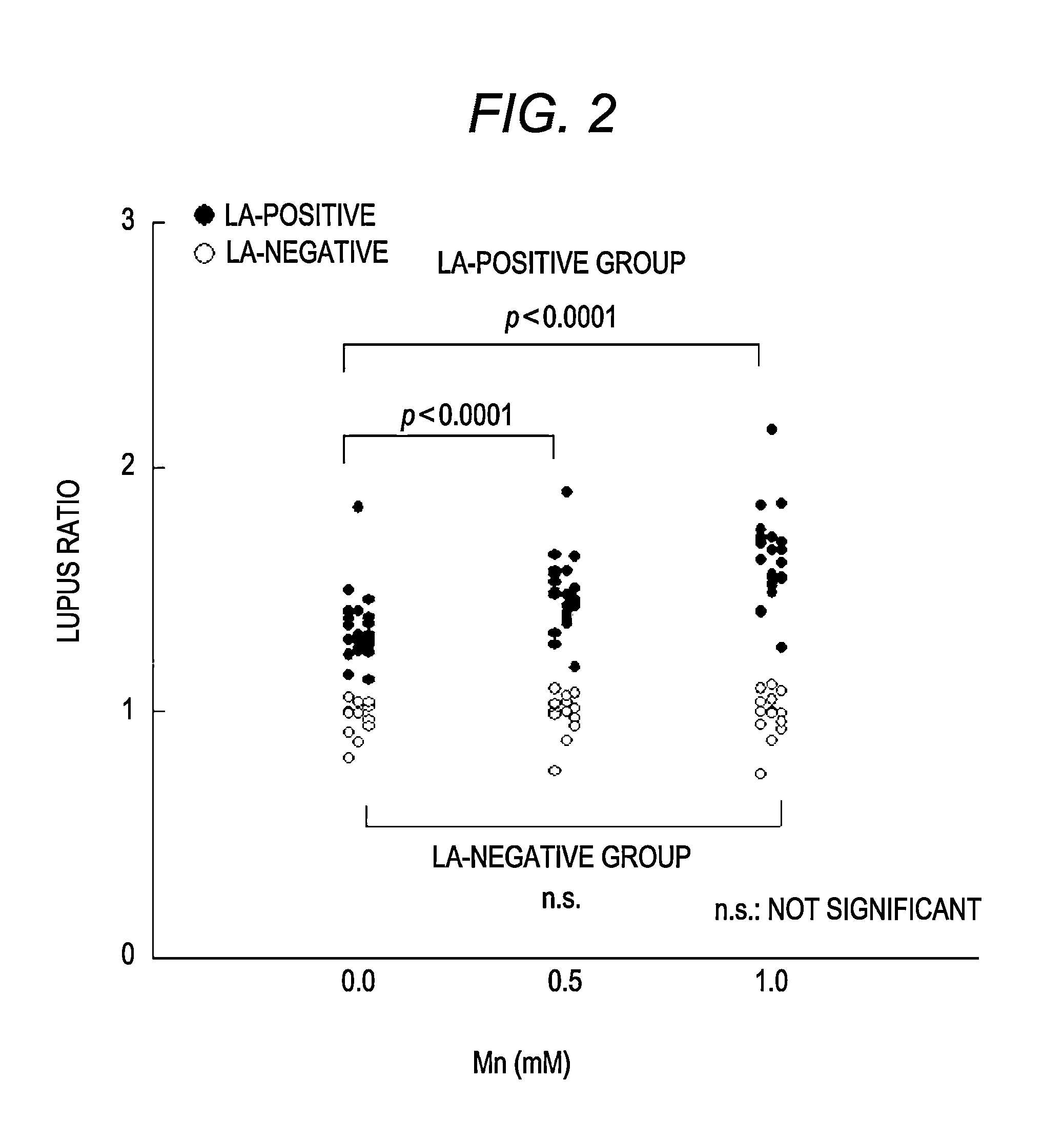
Reagent kit for detecting lupus anticoagulant and method of determining presence or absence of lupus anticoagulant
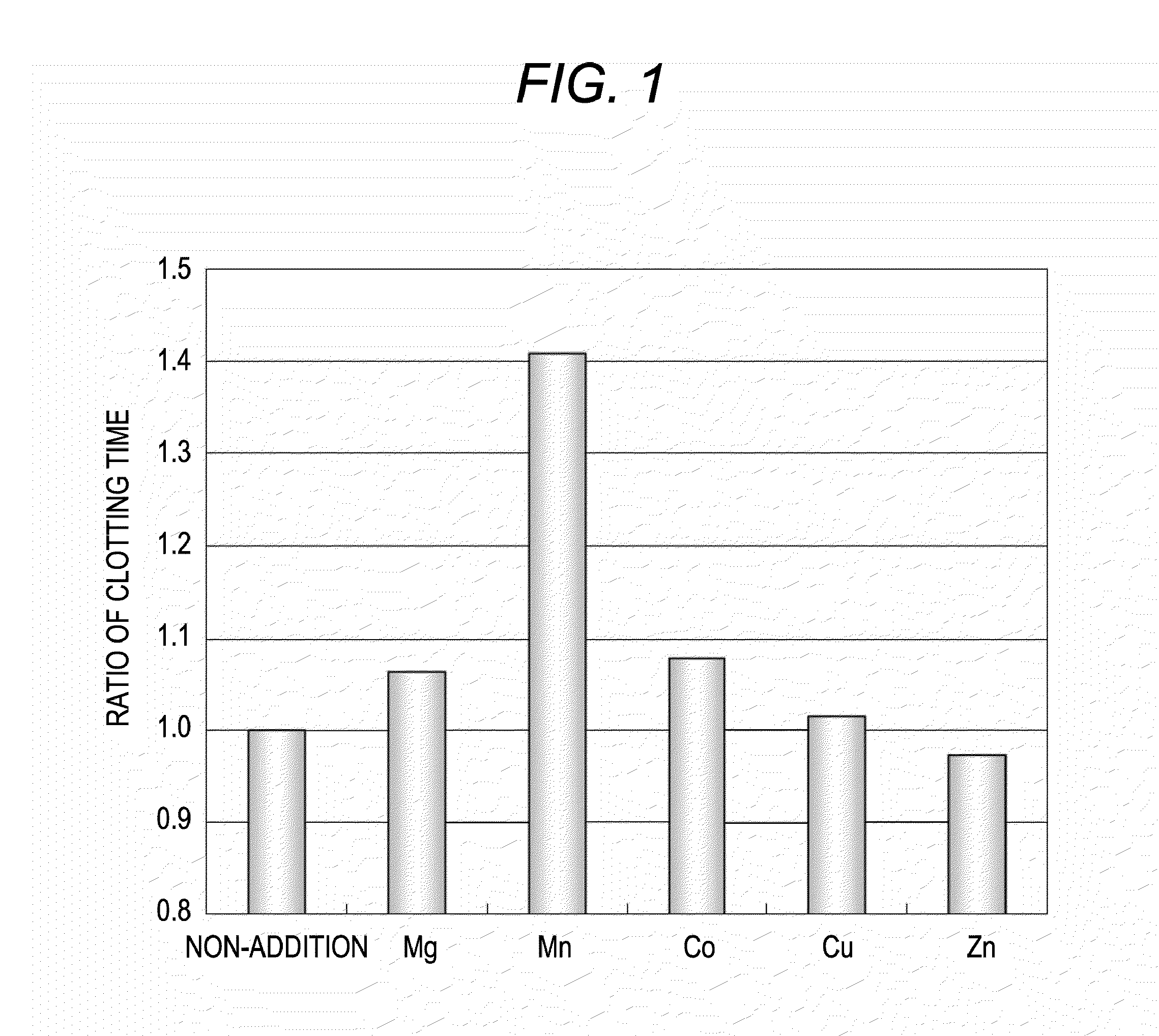
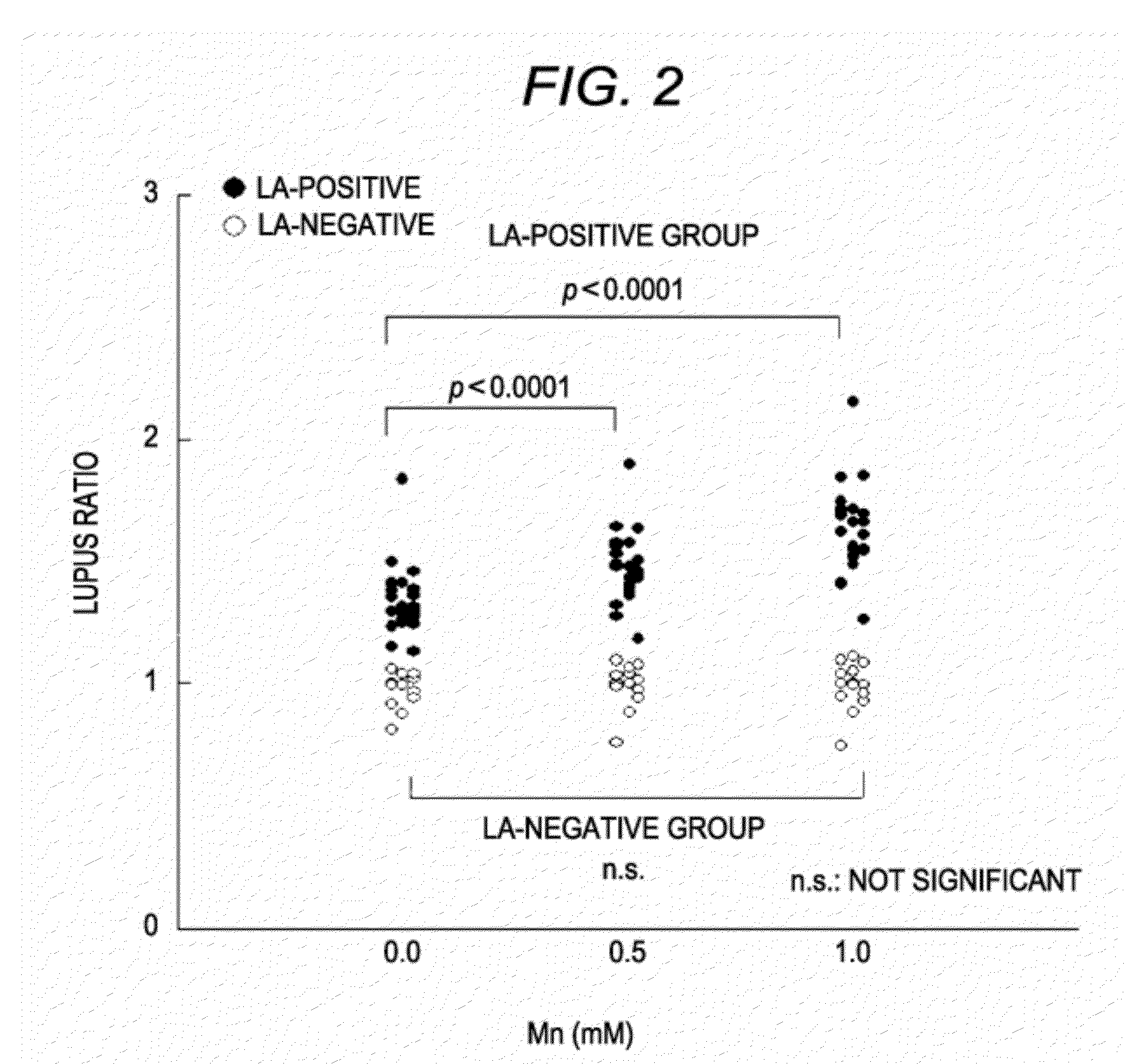
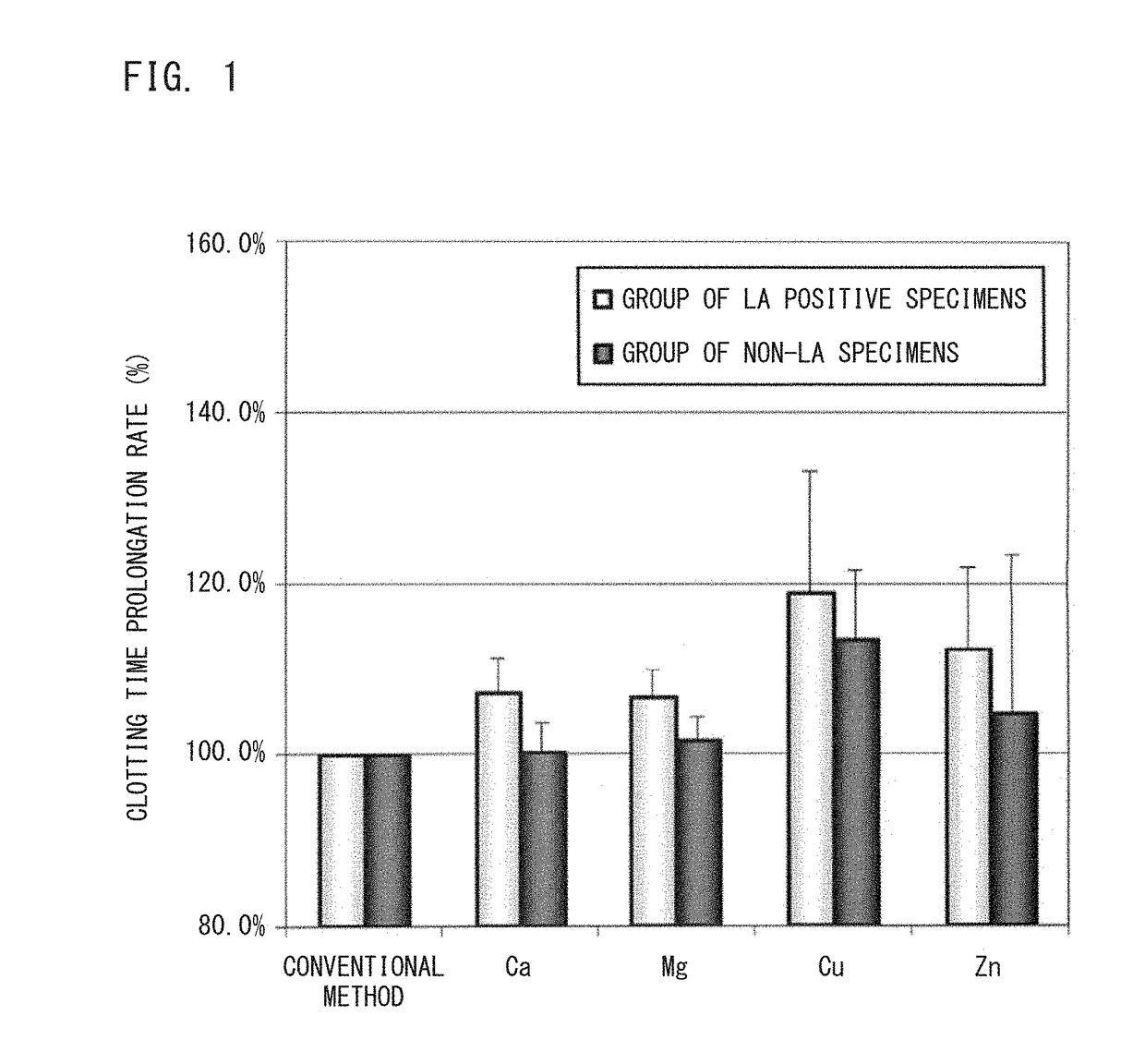
ActiveUS20120052585A1Accurately determineClear separationDisease diagnosisBiological testingManganeseLupus anticoagulant
The present invention provides a reagent kit for detecting LA which includes a first clotting time-measuring reagent containing manganese salt and a second clotting time-measuring reagent which contains manganese salt at a concentration lower than that of the first clotting time-measuring reagent or does not contain manganese salt and a method of determining the presence or absence of LA using the kit.
Owner:SYSMEX CORP
Method of measuring blood coagulation time to detect lupus anticoagulants
ActiveCN103688177AEasy and Sensitive DetectionMicrobiological testing/measurementDisease diagnosisLiver functionFactor ii
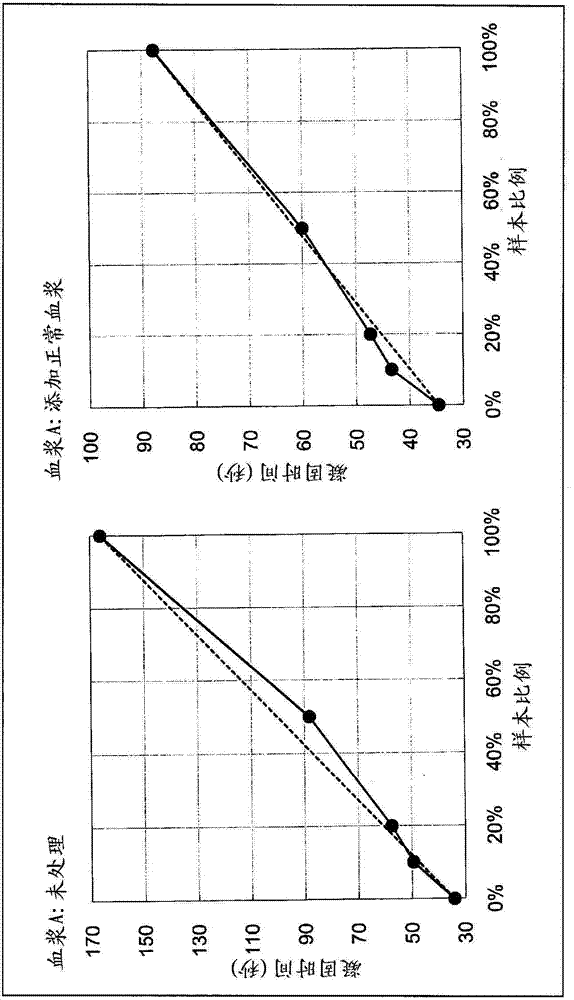
Provided is a method of measuring blood coagulation time which makes it possible to detect lupus anticoagulants more easily and with a higher degree of sensitivity in comparison to the method recommended by the International Society on Thrombosis and Haemostasis (ISTH), and which is not affected by blood-coagulation-factor deficiencies, even in blood samples from patients on warfarin, patients with vitamin K deficiencies and patients with hepatic insufficiency. The method for measuring blood coagulation time to detect lupus anticoagulants is characterized in that a buffering solution composition containing blood coagulation factors is added to a blood sample both before and during the measurement of the blood coagulation time, and the blood coagulation time is measured.
Owner:SCHOOL JURIDICAL PERSON HIGASHI NIPPON GAKUEN +1
Reagent kit for detecting lupus anticoagulant
InactiveUS20090098585A1High detection sensitivityMicrobiological testing/measurementBiological testingLupus anticoagulantPhosphatidylserine
A reagent kit capable of distinguishing between a blood sample containing lupus anticoagulant and blood samples from individuals having other anticoagulant diseases is disclosed. The reagent kit comprises two coagulation time reagents containing phosphatidylserine in a different phosphatidylserine content ratio to the total content of phospholipids from each other, threreby giving different coagulation times with use of corresponding coagulation time reagent.
Owner:SYSMEX CORP
Lupus anticoagulant detection reagent as well as preparation method and use method thereof
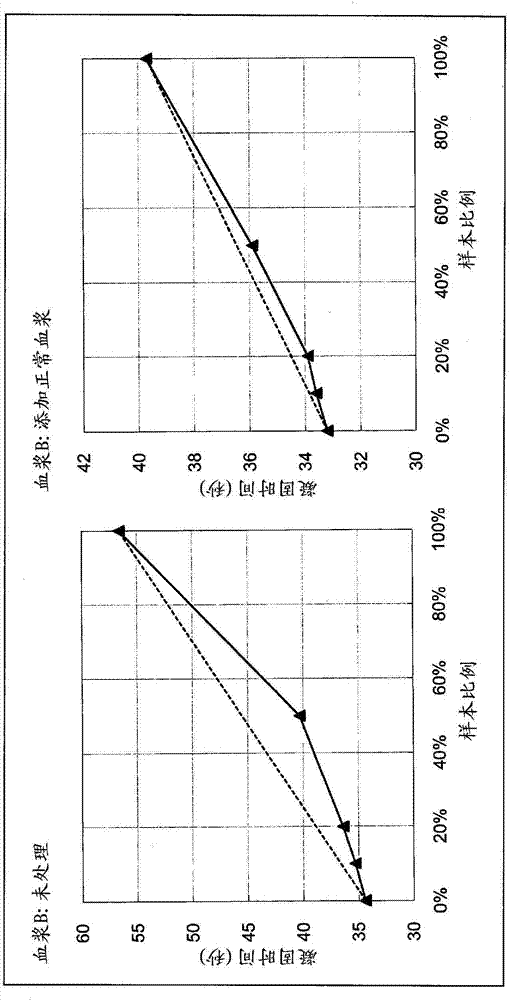
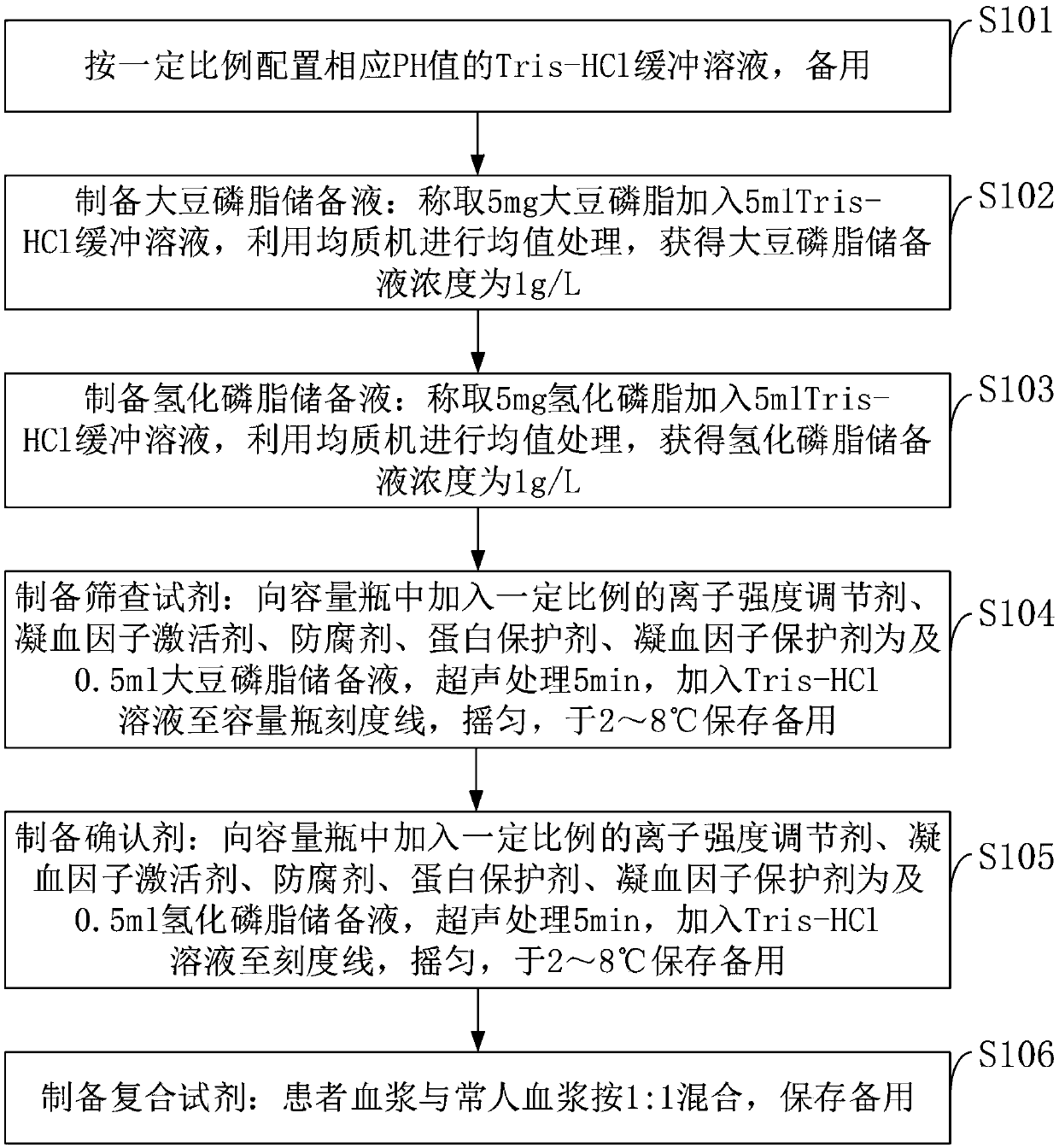
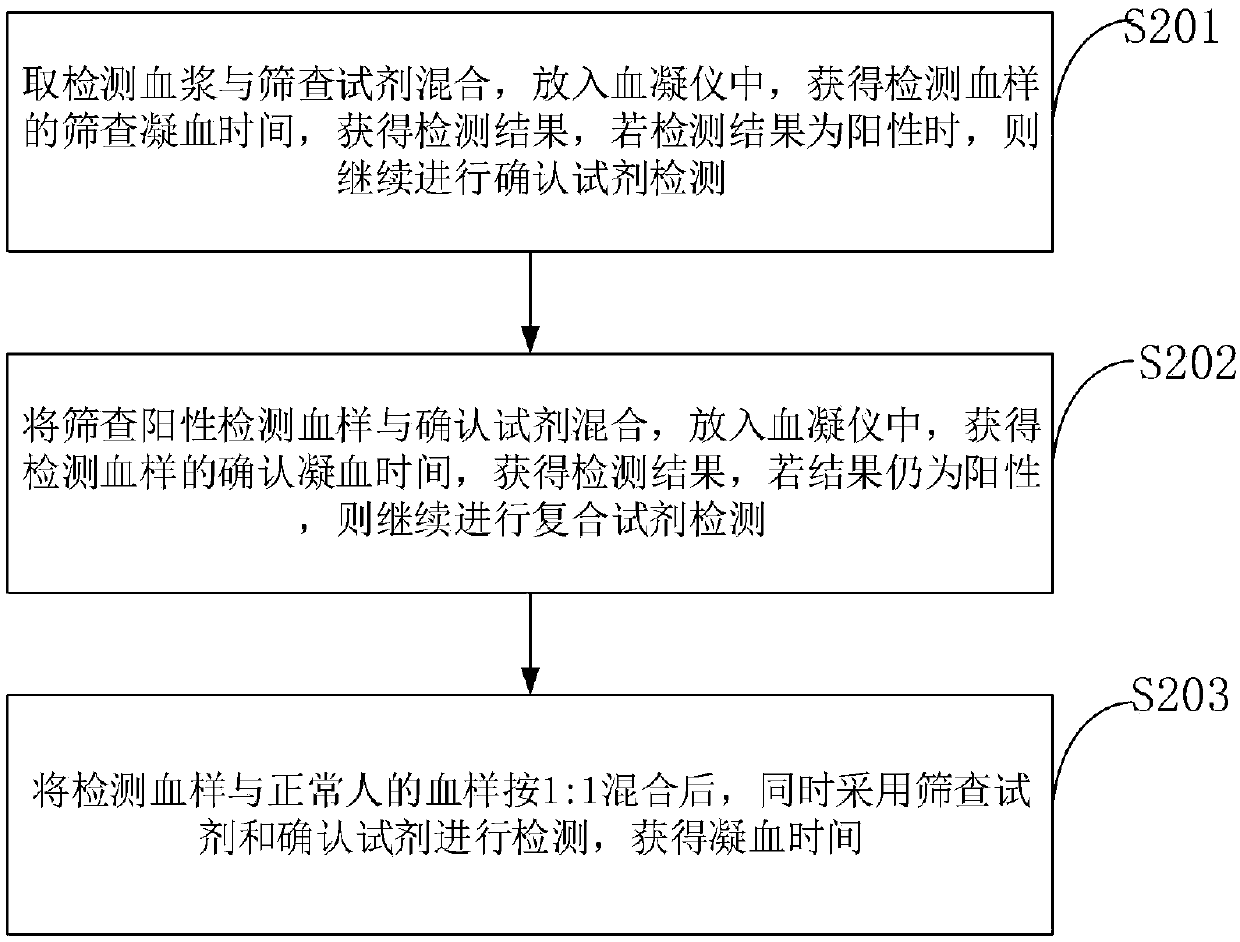
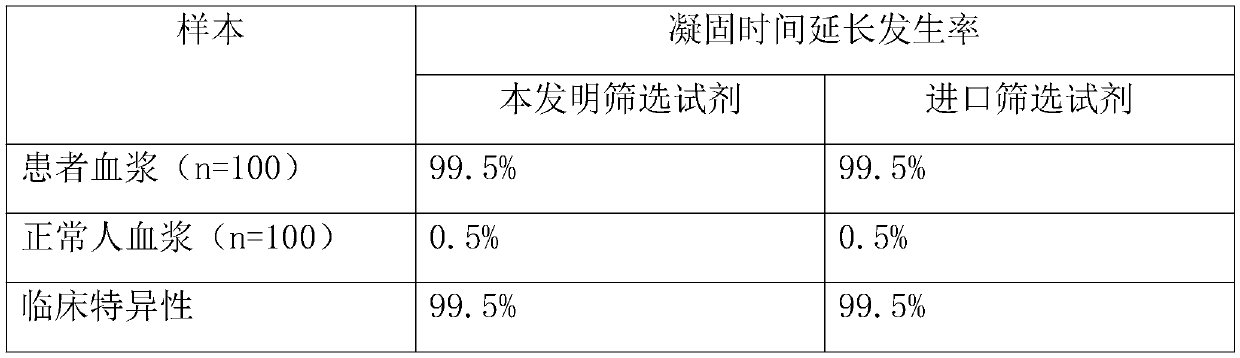
The invention belongs to the technical field of biological detection, and discloses a lupus anticoagulant detection reagent as well as a preparation method and a use method thereof. The lupus anticoagulant detection reagent mainly comprises a screening reagent, a confirming reagent and a compounding region, wherein the screening reagent is prepared from blood coagulation factor activators with thevolume percentage being 13mg / L, 65mmol / L of buffer solution with the pH being 7.5, 55mg / L of synthetic phospholipids, 75g / L of protein protection agents, 0.06 percent of preservatives, 100mmol / L of ion intensity regulators and 8g / L of blood coagulation factor protection agents; the conforming agent has high content of synthetic phospholipids. The reagent is a liquid reagent; the preparation process is simple and convenient; the cost is relatively low; through the addition of the protein protection agents, the stability of the liquid reagent is effectively ensured; the false positive rate andfalse negative rate in the detection result are effectively reduced; different synthetic phospholipids are respectively used on screening reagents and conforming reagents, so that the LA detection sensitivity is improved; the detection test accuracy is improved.
Owner:THE FIRST AFFILIATED HOSPITAL OF ARMY MEDICAL UNIV
Method for detecting lupus anticoagulants
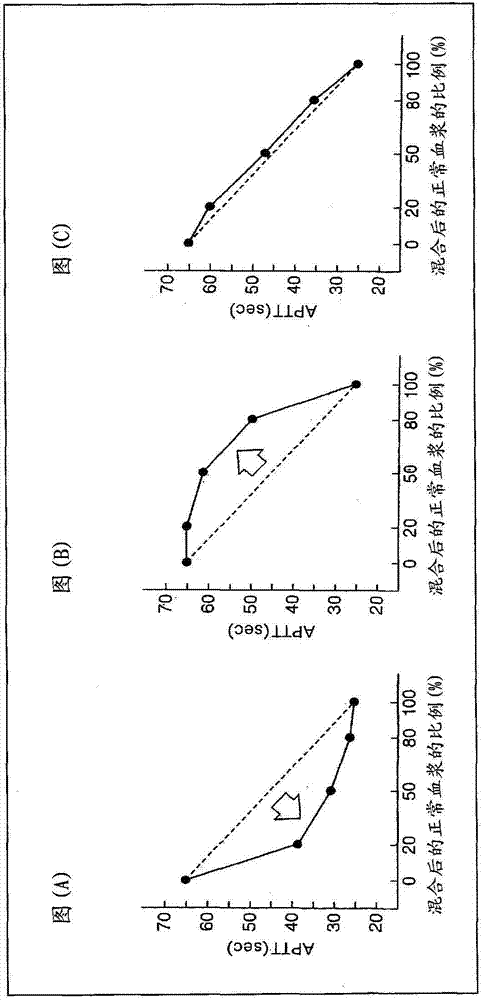
Provided is a simple method for detecting lupus anticoagulants without using blood plasma from healthy individuals, which is capable of distinguishing between blood-coagulation-factor deficiencies, even in blood samples originating from patients undergoing anticoagulant therapies using warfarin, heparin, or the like, without being affected by said anticoagulant therapies. The method for detecting the lupus anticoagulants is characterized in that it involves the following three steps, (A), (B) and (C): (A) a step in which a buffering solution composition containing blood coagulation factors is added to a blood sample and to a diluted sample of said blood sample both before and during the measurement of the blood coagulation time; (B) a step in which the blood coagulation time is measured for each of the samples from step (A); and (C) a step in which the blood coagulation times of each of the samples obtained in step (B) are compared.
Owner:SCHOOL JURIDICAL PERSON HIGASHI NIPPON GAKUEN +1
Blood sample determination method and blood sample analyzer
ActiveUS10215766B2Material analysis by observing effect on chemical indicatorScattering properties measurementsLupus anticoagulantCoagulation Factor Inhibitors
Disclosed is a blood sample determination method including: emitting light to a measurement specimen prepared by mixing a clotting time measuring reagent and a blood sample suspected to be derived from a subject having lupus anticoagulant or a coagulation factor inhibitor, to obtain optical information about an amount of light from the measurement specimen; obtaining at least one parameter regarding derivative of clot waveform, based on the obtained optical information; and determining, based on a value of the obtained parameter, whether the blood sample is suspected to be a sample derived from a subject having lupus anticoagulant or is suspected to be a sample derived from a subject having a coagulation factor inhibitor.
Owner:NARA MEDICAL UNIVERSITY +1
Blood virus RAN protective agent and blood sampling tube
ActiveCN109679946AAvoid degradationAvoid pollutionBioreactor/fermenter combinationsBiological substance pretreatmentsSodium thiocyanatePollution
The invention provides a blood virus RAN protective agent and a blood sampling tube. The blood virus RAN protective agent is prepared by dissolving the following components in DEPC (diethyl pyrocarbonate) solution 100-500mg / ml glycine, 1-5mg / ml aprotinin, 1-5mg / ml wortmannin, 10-40mg / ml glutathione, 100-500mg / ml sodium thiocyanate, 1-3mg / ml anticoagulant and 5-20mg / ml membrane protective agent. The blood virus RAN protective agent provided by the invention is capable of supplying long-term protection to virus RNA in blood under room temperature, preventing degradation and pollution of virus RNA and effectively guaranteeing accuracy and validity of blood sample.
Owner:NINGBO AJCORE BIOSCIENCES INC
Reagent kit for detecting lupus anticoagulant and method of determining presence or absence of lupus anticoagulant
ActiveUS20120220038A1Accurately determineClear separationBiological material analysisBiological testingSystemic lupus erythematosusLupus anticoagulant
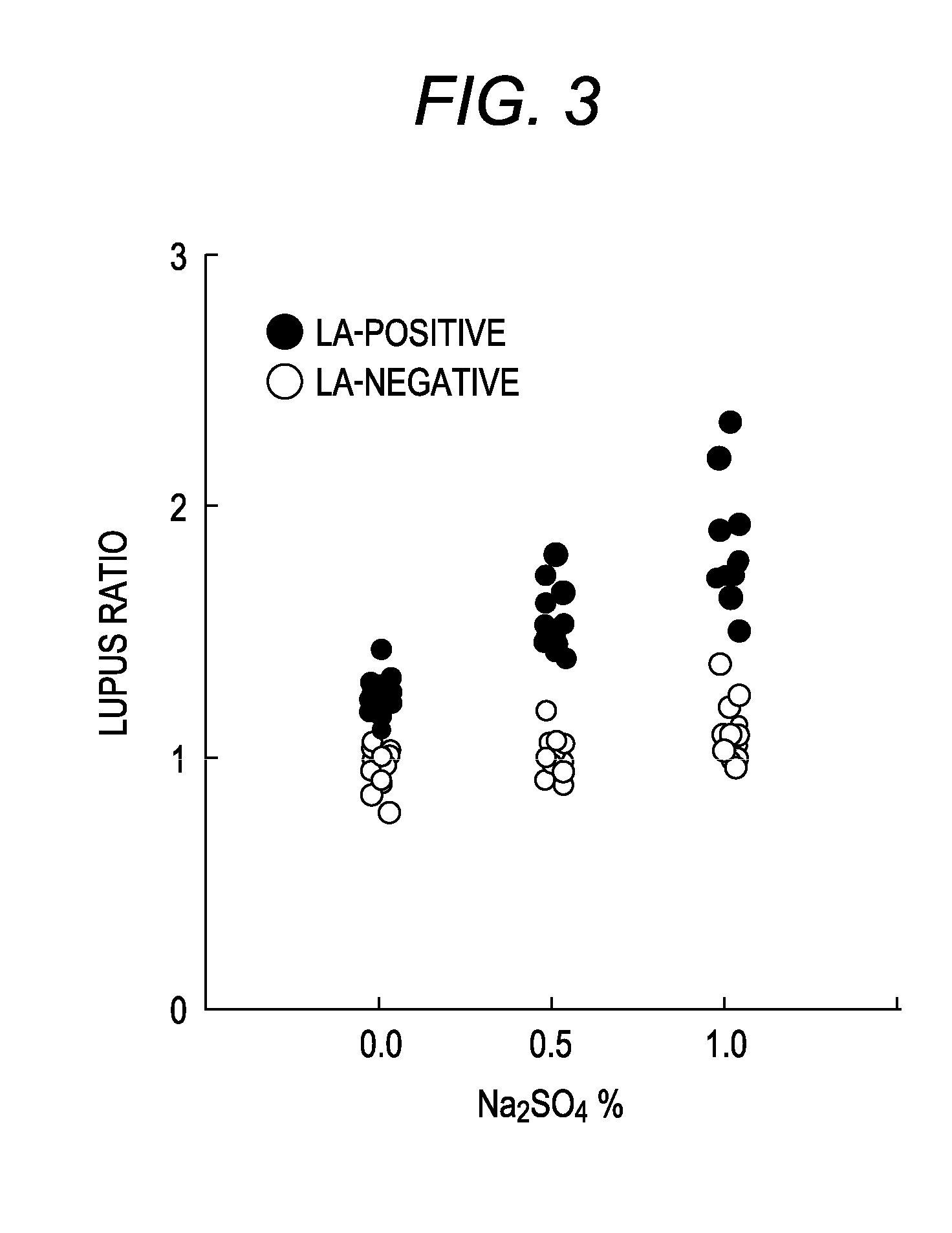
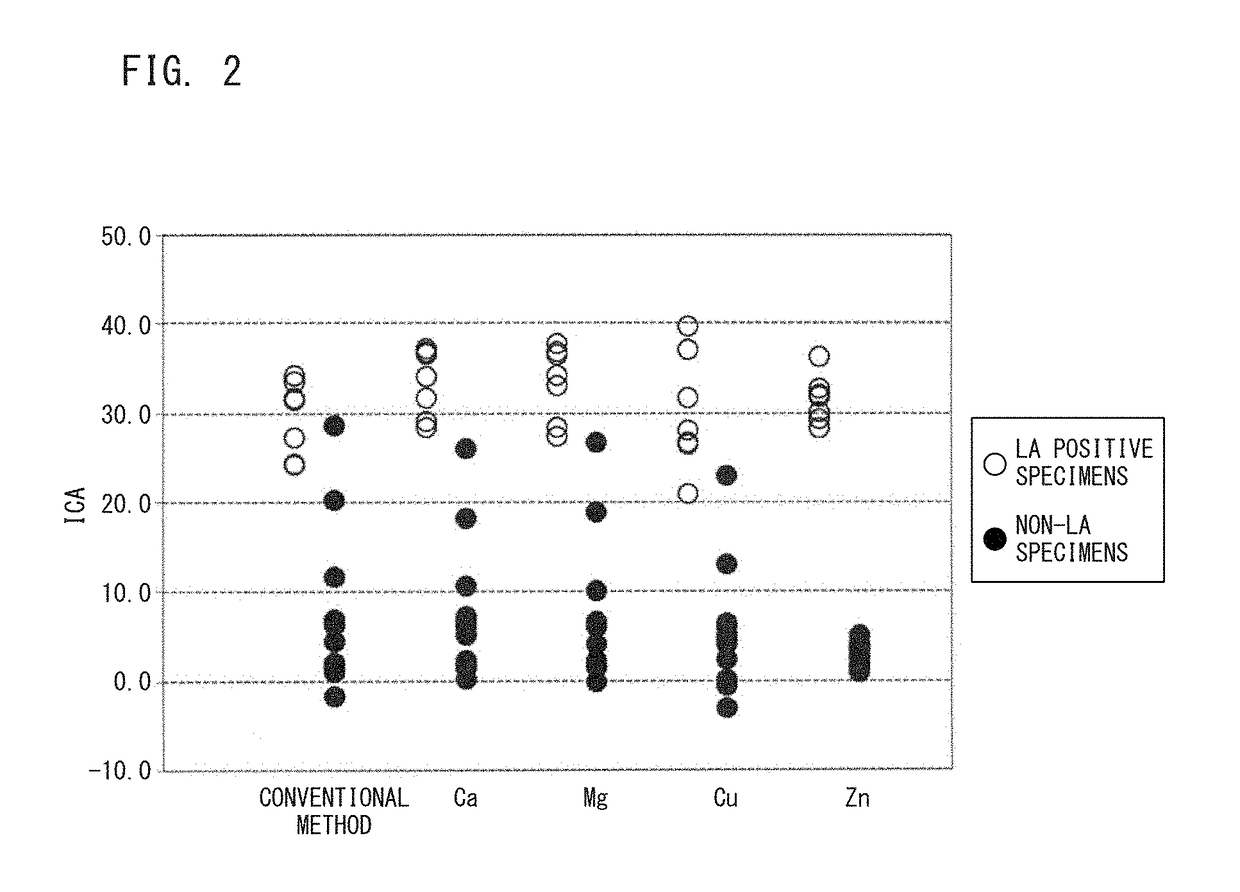
In order to provide a reagent kit for detecting LA which can clearly separate a lupus anticoagulant (LA)-positive specimen group from an LA-negative specimen group, it is configured that the reagent kit for detecting LA contains a first clotting time-measuring reagent and a second clotting time-measuring reagent and at least one of the first clotting time-measuring reagent and the second clotting time-measuring reagent contains alkali metal salt. The presence or absence of LA can be determined using the kit.
Owner:SYSMEX CORP
Reagent kit for detecting lupus anticoagulant and method of determining presence or absence of lupus anticoagulant
ActiveUS8557592B2Extension of timeAccurately determineAnalysis using chemical indicatorsMicrobiological testing/measurementLupus anticoagulantReagent
The present invention provides a reagent kit for detecting lupus anticoagulant which includes a first clotting time-measuring reagent containing manganese salt and a second clotting time-measuring reagent which contains manganese salt at a concentration lower than that of the first clotting time-measuring reagent or does not contain manganese salt and a method of determining the presence or absence of lupus anticoagulant using the kit.
Owner:SYSMEX CORP
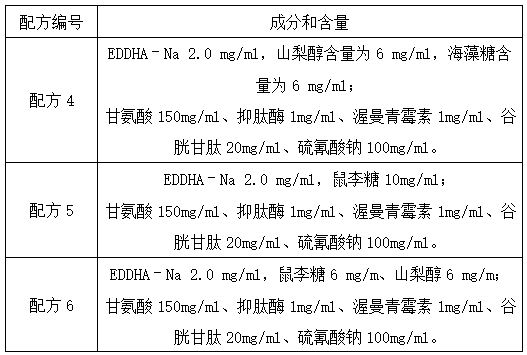
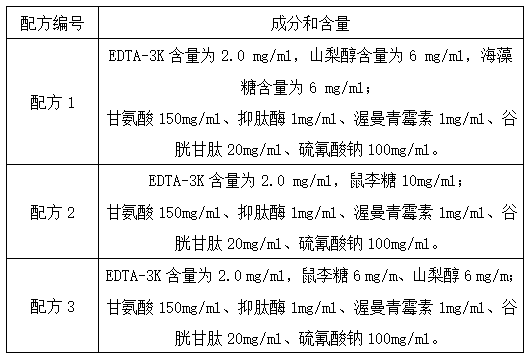
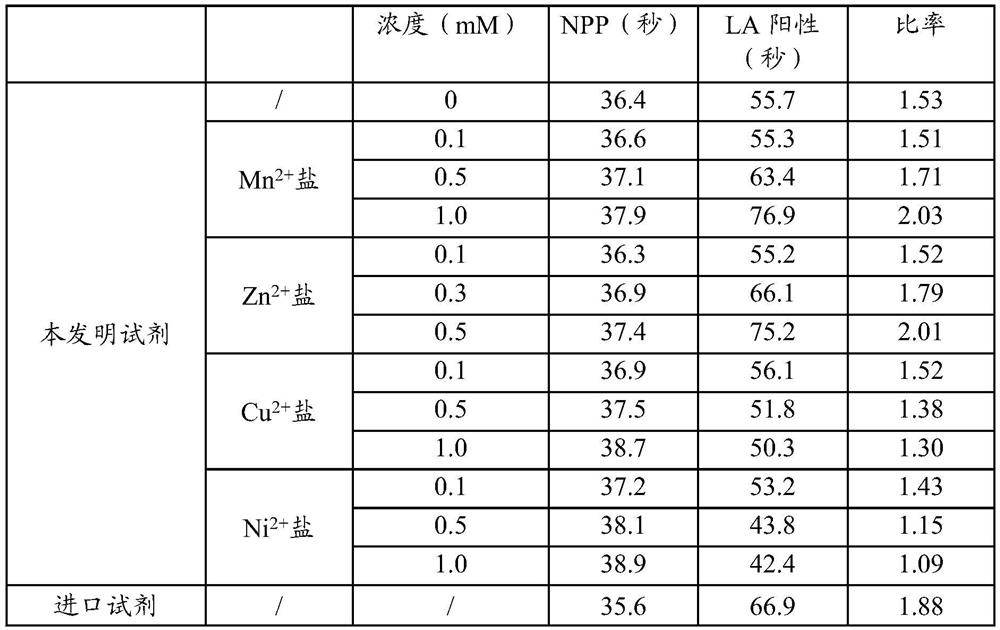
Lupus anticoagulant confirmation kit (coagulation method)
PendingCN111707837AIncreased sensitivityImprove detection accuracyBiological testingSystemic lupus erythematosusSoybean Phospholipids
The invention relates to the technical field of clinical diagnosis, and particularly discloses a lupus anticoagulant confirmation reagent based on a dRVVT method. The confirmation reagent comprises abuffer solution, soyabean lecithin, viper venom activator RVV-X, calcium, a heparin neutralizer and a stabilizer. The lupus anticoagulant confirmation reagent (kit) of the coagulation method is provided on the basis of the principle of the dRVVT method, and comprises a buffer solution, soybean phospholipid, viper venom activator RVV-X, calcium, a heparin neutralizer, a preservative, a stabilizer and Zn<2+> salt or Mn <2+> salt. By adding metal ions Zn<2+> and Mn<2+> into the confirmation reagent, the sensitivity of the confirmation reagent is improved, so that the detection accuracy of the reagent is improved.
Owner:SHANGHAI SUNBIO TECH
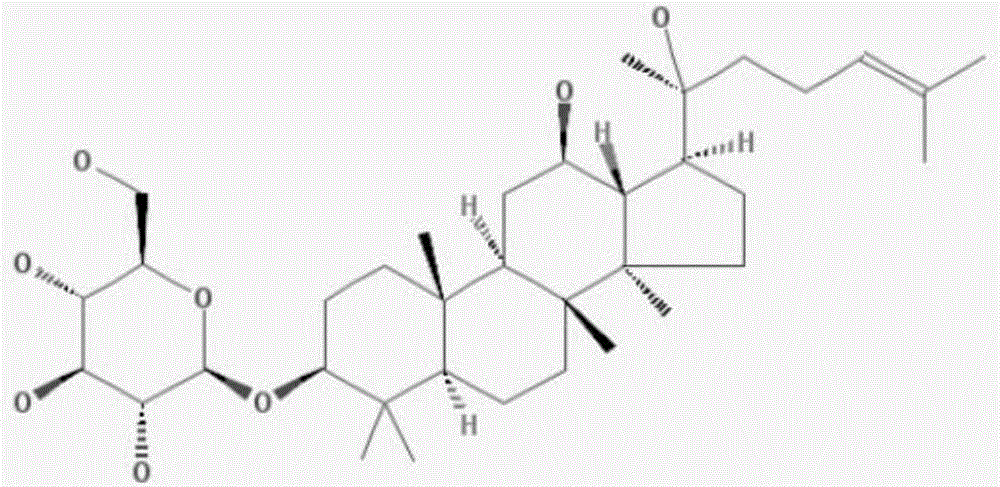
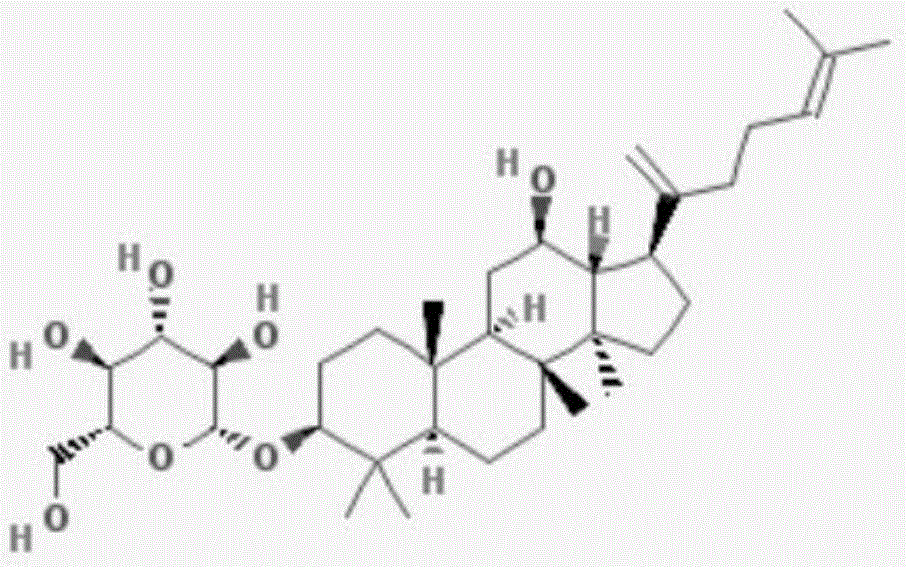
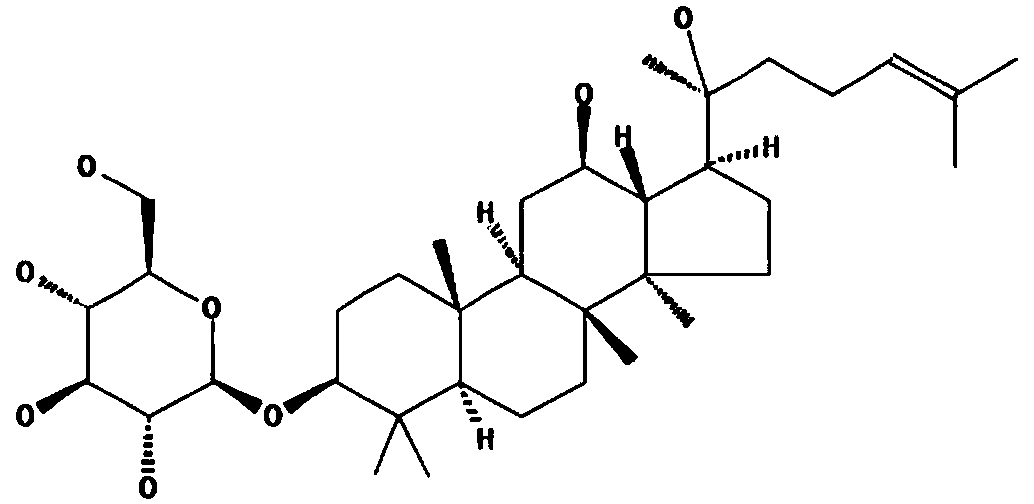
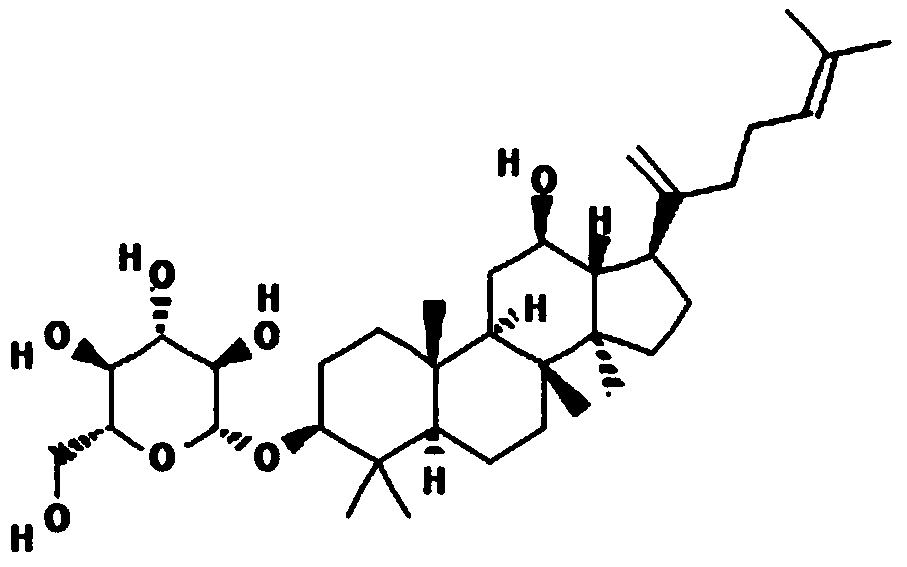
Application of ginsenoside in preparing antiphospholipid syndrome molecular targeting treatment medicine
The invention relates to application of ginsenoside in the pharmacy field, in particular to application of ginsenoside in preparing antiphospholipid syndrome molecular targeting treatment medicine. In a laboratory antiphospholipid syndrome mouse model, the compound can effectively lower the level of anticardiolipin antibodies, anti-beta2-GP1 antibodies and lupus anticoagulants in blood; meanwhile, in a pregnant mouse model, the compound effectively lower aCL activation, shortens partial thromboplastin time and reduces the blood platelet count and the abortion rate; safety study shows that the compound is high in safety, has no influence on the central nervous system, the cardiovascular system and the respiratory system and has no mutagenic action. It is indicated that the compound has high safety and a good inhibiting effect on the antiphospholipid syndrome at the test dose and can be used for treating the antiphospholipid syndrome.
Owner:延边安帝康华生物科技有限公司
Reagent kit for detecting lupus anticoagulant and method of determining presence or absence of lupus anticoagulant
ActiveUS8759108B2Extension of timeAccurately determineAnalysis using chemical indicatorsBiological material analysisSystemic lupus erythematosusLupus anticoagulant
Owner:SYSMEX CORP
Lupus anticoagulant confirmation reagent and preparation method thereof
ActiveCN112557665AImprove stabilityImprove anti-interference abilityBiological material analysisBiological testingBlood Coagulation Factor XSystemic lupus erythematosus
The invention relates to the technical field of biology, in particular to a lupus anticoagulant confirmation reagent and a preparation method thereof. The confirmation reagent comprises tris(hydroxymethyl) aminomethane, a blood coagulation factor X activator, rabbit cephalin, gelatin, glycine, trehalose, sodium merthiolate and calcium chloride. According to the lupus anticoagulant confirmation kit, by adjusting the concentration and components of the stabilizer, the lupus anticoagulant confirmation kit is good in stability and high in interference resistance, the reagent can be placed stably for 10 days after being redissolved at the temperature of 2-8 DEG C, and the freeze-dried powder can be placed stably for 2 years at the temperature of 2-8 DEG C. The stability of the reagent is superior to that of common commercially available products, reagents are fully utilized, reagent waste is reduced, and cost is well saved for hospitals.
Owner:BEIJING MDC NEW SPRING MEDICAL DEVICES
Method for measuring clotting time, method for determining presence or absence of lupus anticoagulant, and reagent kit for detecting lupus anticoagulant
Disclosed is a method for measuring clotting time including: preparing a measurement sample by mixing a blood specimen suspected to contain a lupus anticoagulant, an activator, and a divalent-metal-ion-producing compound for facilitating formation of a phospholipid-containing complex; and measuring a clotting time after adding, to the measurement sample, an aqueous solution of a calcium salt as a coagulation initiator.
Owner:SCHOOL JURIDICAL PERSON HIGASHI NIPPON GAKUEN +1
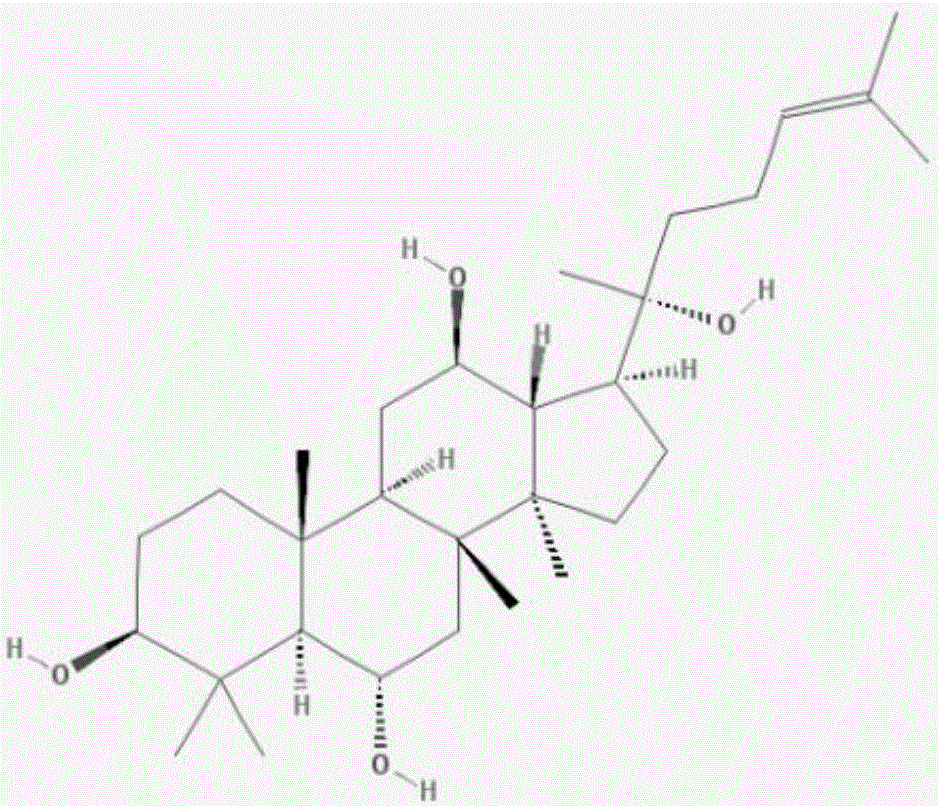
Application of Ginsenosides in the Preparation of Molecular Targeted Therapy Drugs for Antiphospholipid Syndrome
The invention relates to application of ginsenoside in the pharmacy field, in particular to application of ginsenoside in preparing antiphospholipid syndrome molecular targeting treatment medicine. In a laboratory antiphospholipid syndrome mouse model, the compound can effectively lower the level of anticardiolipin antibodies, anti-beta2-GP1 antibodies and lupus anticoagulants in blood; meanwhile, in a pregnant mouse model, the compound effectively lower aCL activation, shortens partial thromboplastin time and reduces the blood platelet count and the abortion rate; safety study shows that the compound is high in safety, has no influence on the central nervous system, the cardiovascular system and the respiratory system and has no mutagenic action. It is indicated that the compound has high safety and a good inhibiting effect on the antiphospholipid syndrome at the test dose and can be used for treating the antiphospholipid syndrome.
Owner:延边安帝康华生物科技有限公司
Plasma anticoagulant having virus inactivation function
The invention relates to a plasmid anticoagulant having a virus inactivation function. With 4% sodium citrate which is collected in a blood test as a basic anticoagulant, the virus inactivation function of the plasmid anticoagulant is achieved by adding one or more virus inactivating agents selected from sodium caprylate, formaldehyde and [beta]-propiolactone. With the application of the plasma anticoagulant having a recheck function provided by the invention, the risk of personnel infection due to exposure of positive plasma in a storing, transporting or using process after the collection of the plasma can be effectively prevented, so that the bio-safety of the plasma in an entire life cycle from the collection of the plasma can be guaranteed.
Owner:发贵科技(贵州)有限公司
A lupus anticoagulant detection reagent
ActiveCN107167618BEasy to produceSmall batch-to-batch varianceBiological testingCholesterolPhosphatidyl serine
The invention discloses a lupus anticoagulant detection reagent which includes a screening reagent and a diagnosis reagent. Both of the two reagents include a buffer solution, an activating agent silica, and synthetic phospholipid, wherein the content of the synthetic phospholipid is low in the screening reagent but is high in the diagnosis reagent. The synthetic phospholipid is composed of phosphatidyl serine, phosphatidyl choline, phosphatidyl ethanolamine, phosphatidyl glycerol and cholesterol according to the mass ratio of 5:30:40:15:10. The lupus anticoagulant detection reagent is high in sensitivity and is a liquid reagent; compared with a DRVVT method, the lupus anticoagulant detection reagent is very small in inter-batch difference and is more easy to produce.
Owner:NINGBO ACCUTECH BIOSCI LTD
Method of measuring blood coagulation time to detect lupus anticoagulants
ActiveUS9970046B2Acquisition stableEasy to detectMicrobiological testing/measurementDisease diagnosisWarfarinMedicine
Provided is a method of measuring blood coagulation time, the method being capable of LA detection easily and with high sensitivity as compared with the method recommended by the ISTH, without being affected by deficiency of blood coagulation factors even in a blood sample of a warfarin taker, a person who suffers from vitamin K deficiency, or a hepatic failure patient. Disclosed is a method of measuring the blood coagulation time to detect lupus anticoagulant, the method including adding a buffer solution composition containing blood coagulation factors to a blood sample before measurement or at the time of measurement of the blood coagulation time, and measuring the blood coagulation time.
Owner:SCHOOL JURIDICAL PERSON HIGASHI NIPPON GAKUEN +1
External diagnostic reagent kit used for measuring activated partial thromboplastin time
ActiveCN101221189BImprove stabilityGood repeatabilityMicrobiological testing/measurementBiological testingMagnetic beadDissolution
The invention relates to an in vitro diagnostic kit for the determination of activated partial thromboplatin time (APTT) in clinical test. The invention consists of a partial thromboplatin reagent and a calcium chloride solution, which is used for the detection of the defects of the intrinsic coagulation pathway factors and the screening test of the related inhibitors, and the invention is also aprimary means for the current coagulation factor and heparin anticoagulant treatment and the detection of lupus anticoagulant. The invention has the advantages of long stability time and good repeatability of the partial thromboplatin reagent after a re-dissolution, at the same time, the invention has better consistency of the measurement results of a blood coagulation analyzer by using an optical method and a magnetic bead method, therefore, the invention is applicable to large, medium and small hospitals, and the test results of different hospitals have comparability, therefore the invention has important meaning for implementing the one general report of test reports, provides the reliable experimental data for the clinical diagnosis and the treatment of diseases and improves the efficiency and value of the basic studies of thrombosis and hemostasis.
Owner:SHANGHAI SUNBIO TECH
Pharmaceutical composition containing oral anticoagulant and B vitamins and use thereof
ActiveCN104013964BLittle side effectsImprove coagulation functionOrganic active ingredientsBlood disorderVitamin b6Vitamin B12
The invention relates to a pharmaceutical composition containing a novel oral anticoagulant drug and B vitamins. One or more of the B vitamins and a pharmaceutically acceptable carrier. Among them, new oral anticoagulants mainly include dabigatran etexilate, rivaroxaban, apixaban, and edoxaban, with a content of 2.5-200mg; B vitamins mainly include folic acid compounds (preferred), vitamin B 6 , Vitamin B 12 , content is 0.1 ~ 50mg. The present invention also relates to the use of the composition in the preparation of medicines for preventing apoplexy in patients with atrial fibrillation. The medicine made from the pharmaceutical composition provided by the invention has a better effect of preventing stroke; in addition, the invention can make it convenient for patients to take medicine and increase compliance.
Owner:SHENZHEN AUSA PHARM CO LTD +2
Lupus anticoagulant detection kit
InactiveCN114814244AHigh sensitivityImprove accuracyDisease diagnosisBiological testingPhospholipinSystemic lupus erythematosus
The invention discloses a lupus anticoagulant detection kit. The lupus anticoagulant detection kit comprises an SCT-S screening reagent, an SCT-C confirmation reagent and an SCT-CaCl2 solution, the SCT-S screening reagent comprises purified silica sol, recombinant phospholipid, a first heparin neutralizer, a first buffer solution and a first auxiliary material; the SCT-C confirmation reagent comprises purified silica sol, recombinant phospholipid, a second heparin neutralizer, a second buffer solution and a second auxiliary material; the SCT-CaCl2 solution is prepared from calcium ions; the SCT-S screening reagent, the SCT-C confirmation reagent and the SCT-CaCl2 solution are in a liquid state; the content of recombinant phospholipid in the SCT-S screening reagent is lower than that of recombinant phospholipid in the SCT-C confirmation reagent; the purified silica sol and the recombinant phospholipid are matched, the reagent sensitivity is high, the positive detection rate is increased, and false negative is reduced; comprising a heparin neutralizer to eliminate the influence of heparin and improve the accuracy; the reagent is liquid, convenient to use and good in stability.
Owner:SHENZHEN DYMIND BIOTECH
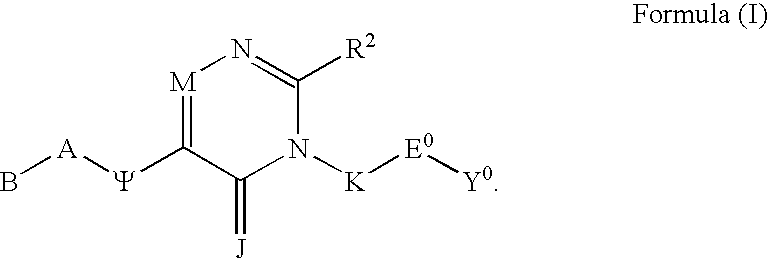
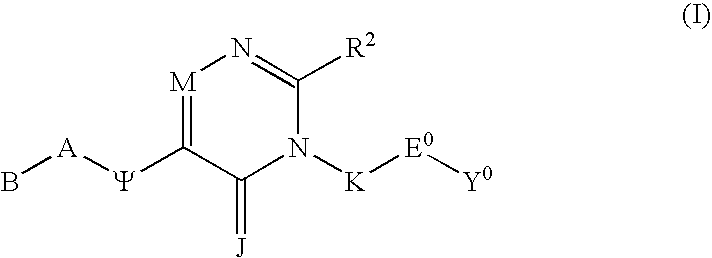
Substituted polycyclic aryl and heteroaryl 1,2,4 - triazinones useful as anticoagulants
The invention relates to substituted polycyclic aryl and heteroaryl pyrimidinone compounds useful as inhibitors of serine proteases of the coagulation cascade and compounds, compositions and methods for anticoagulant therapy for the treatment and prevention of a variety of thrombotic conditions including coronary artery and cerebrovascular diseases.
Owner:PHARMACIA CORP
Kit for removing rivaroxaban in plasma in vitro
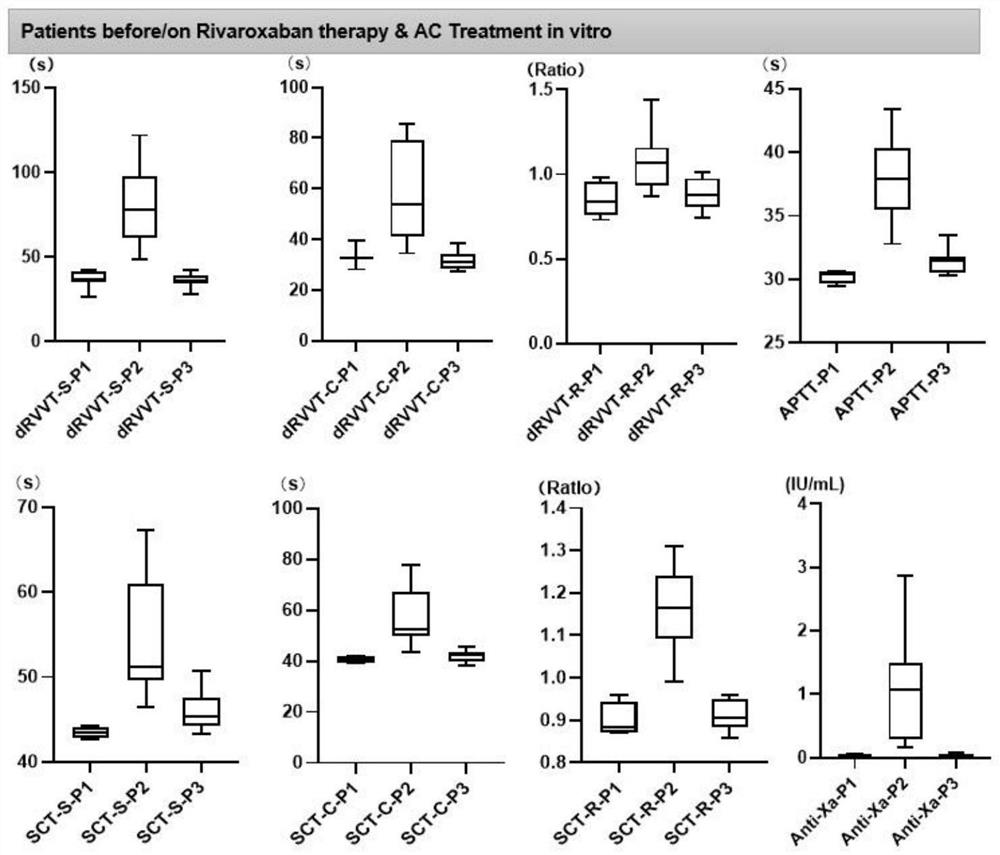
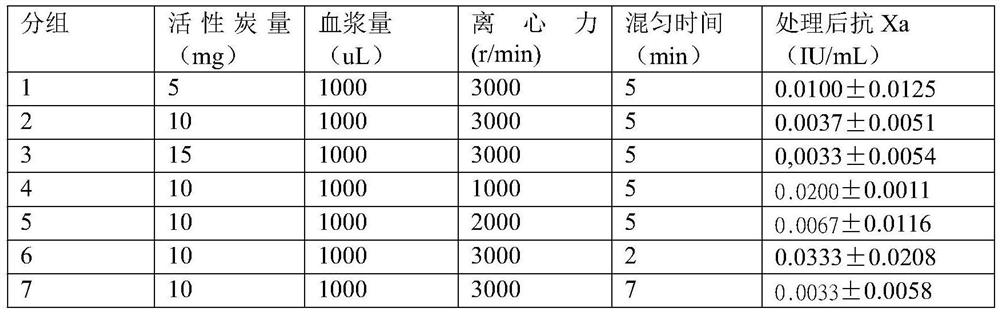
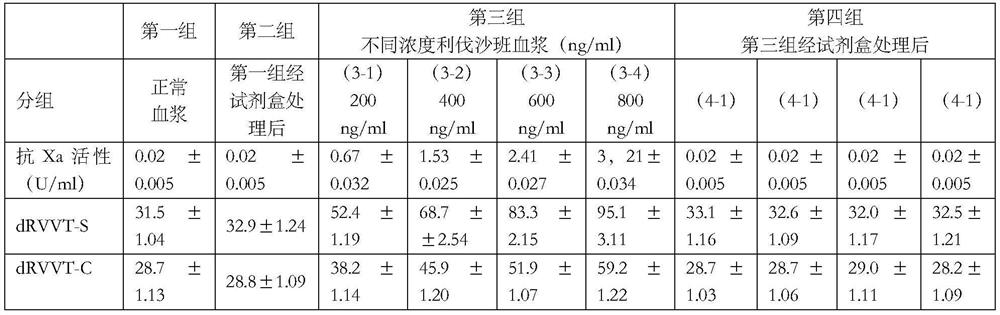
PendingCN113670692ADoes not affect coagulation factorsPreparing sample for investigationBiological testingActivated carbonMedicine
The invention discloses a kit for removing rivaroxaban in plasma, the kit comprises at least two independent test tubes, and at least one of the test tubes is filled with activated carbon. The invention provides a simple and easy-to-implement scheme, the scheme depends on an activated carbon adsorption function, rivaroxaban in detected plasma of a patient can be removed, blood coagulation factors in the plasma cannot be influenced, and lupus anticoagulant substance detection based on a coagulation method is not influenced by rivaroxaban any more.
Owner:PEOPLES HOSPITAL PEKING UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com