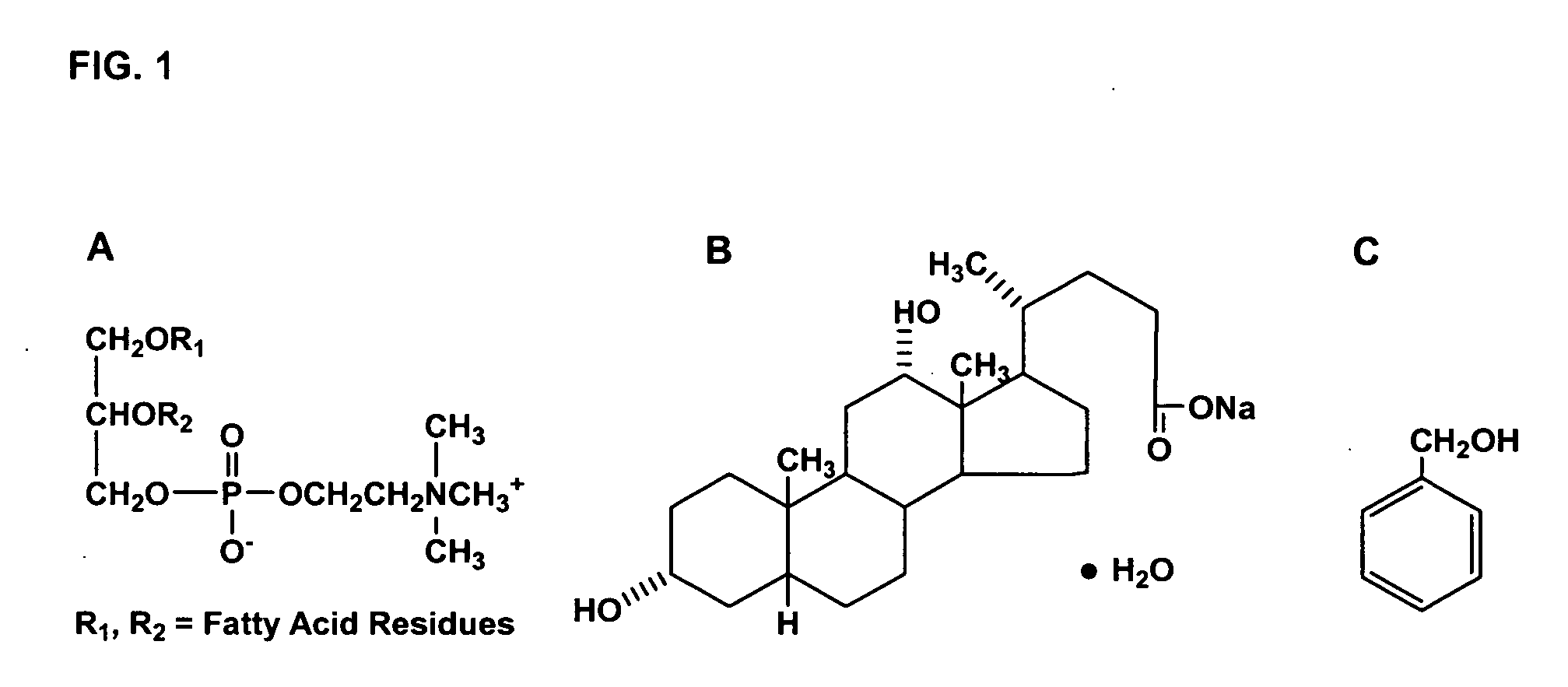
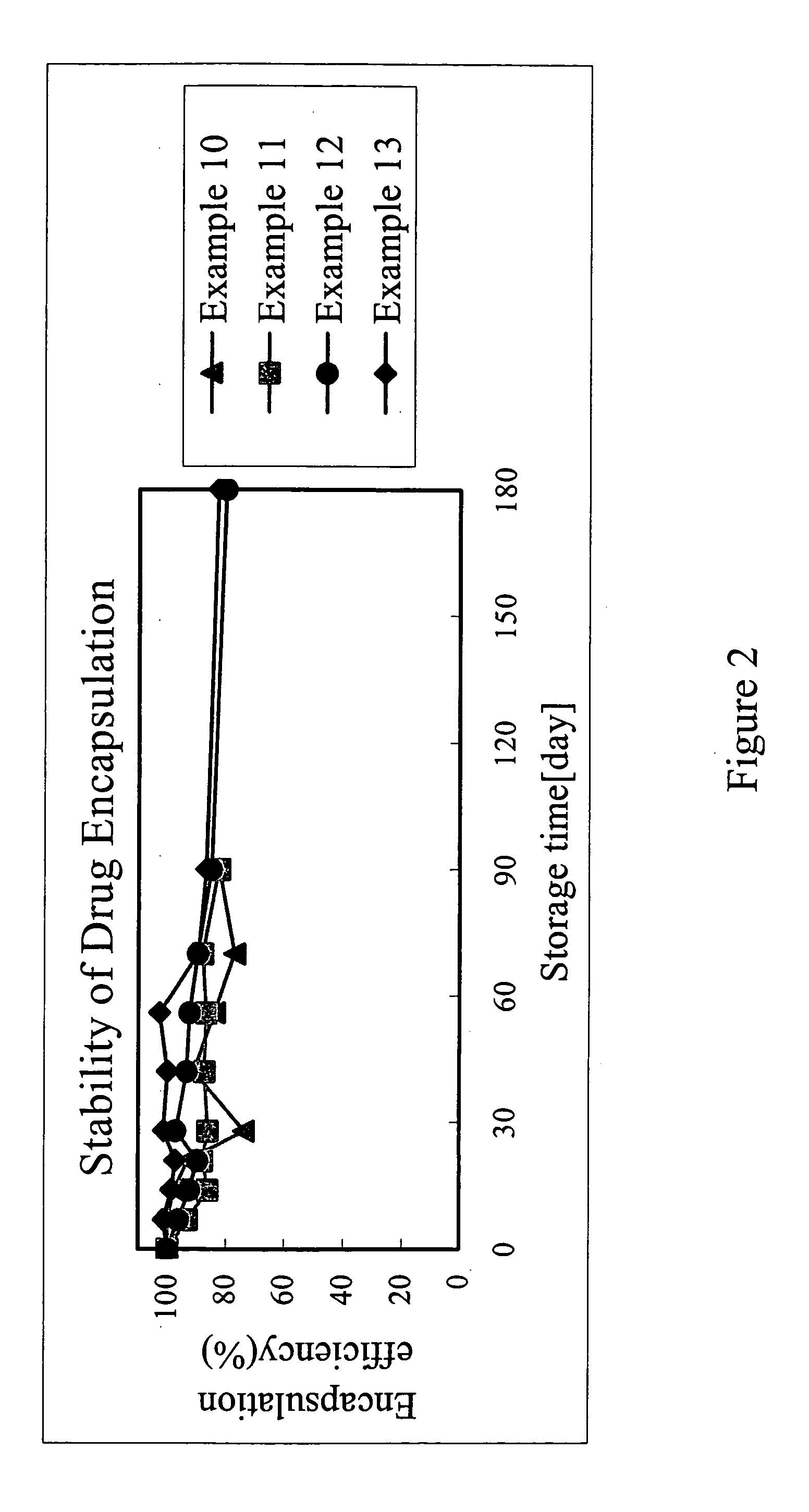
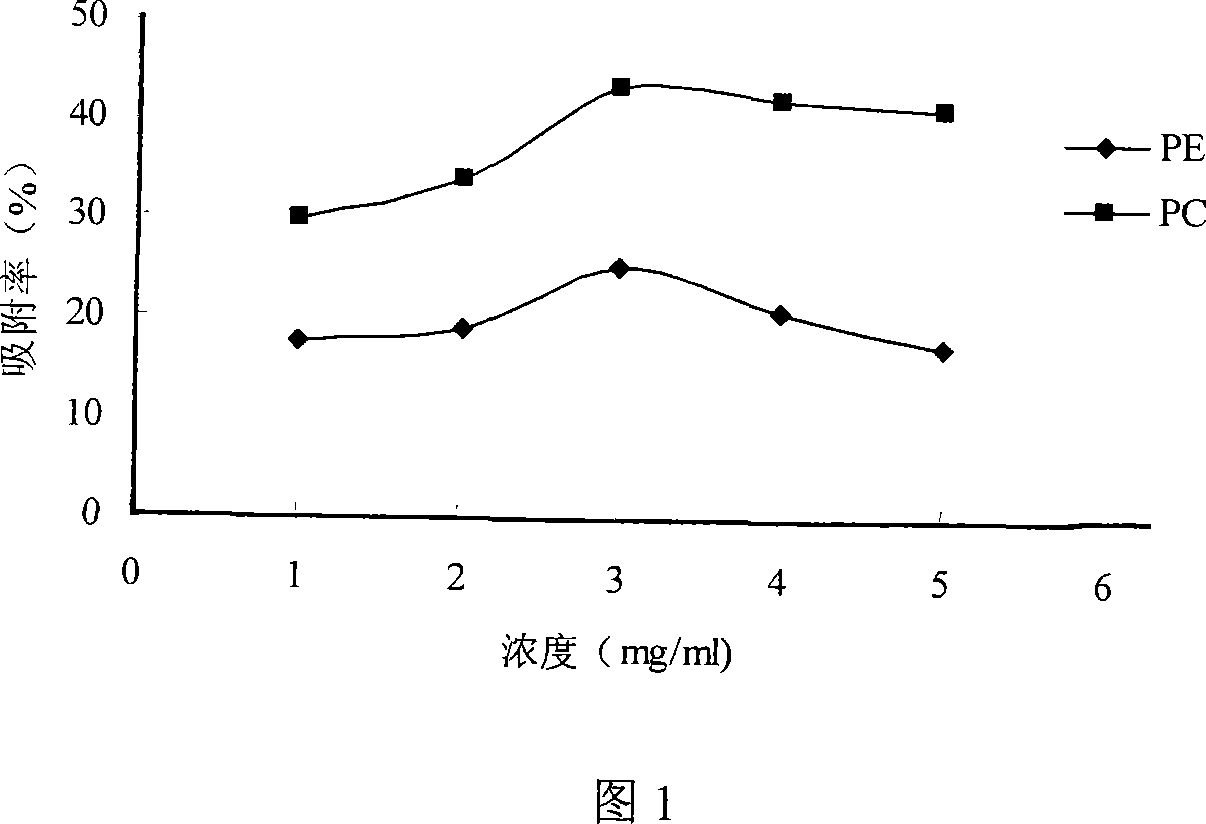
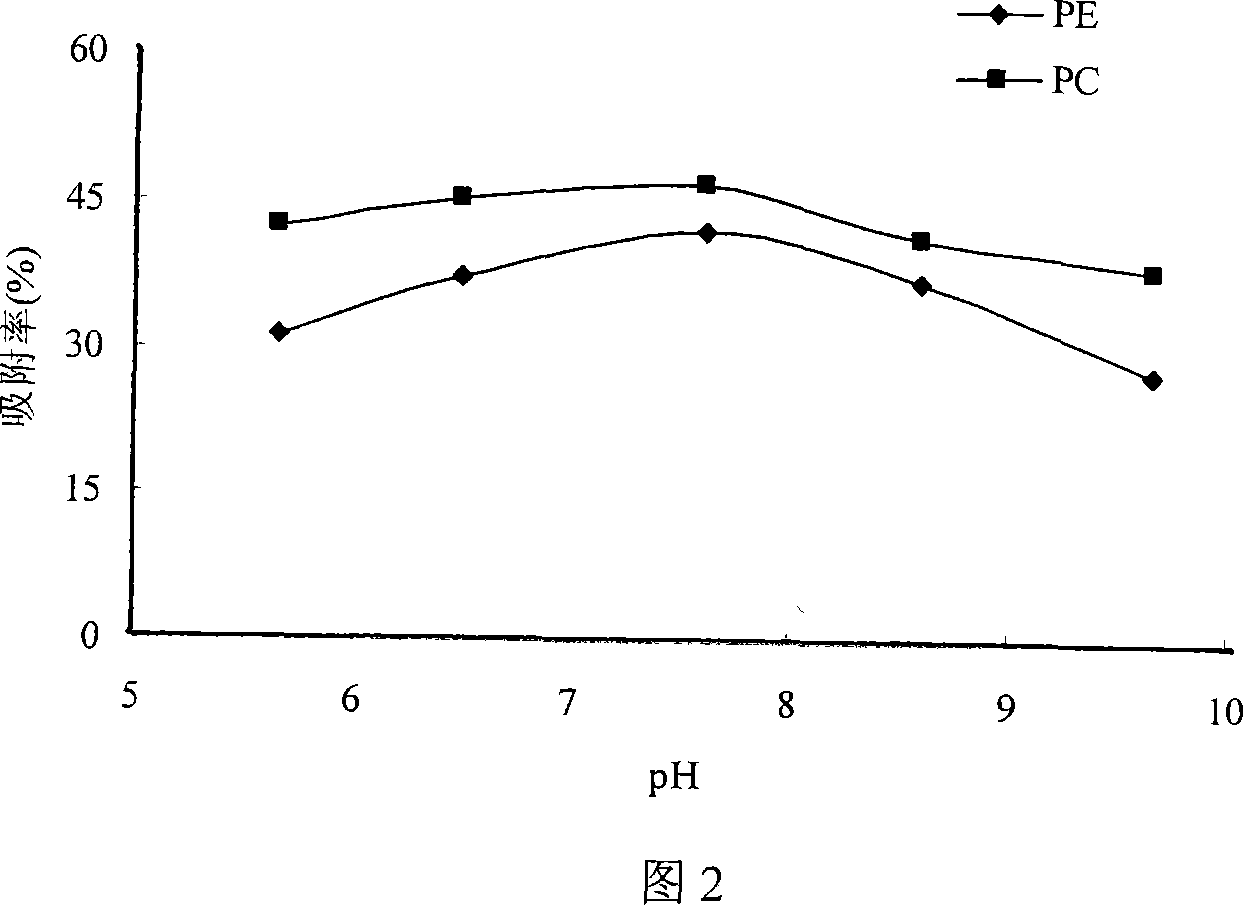
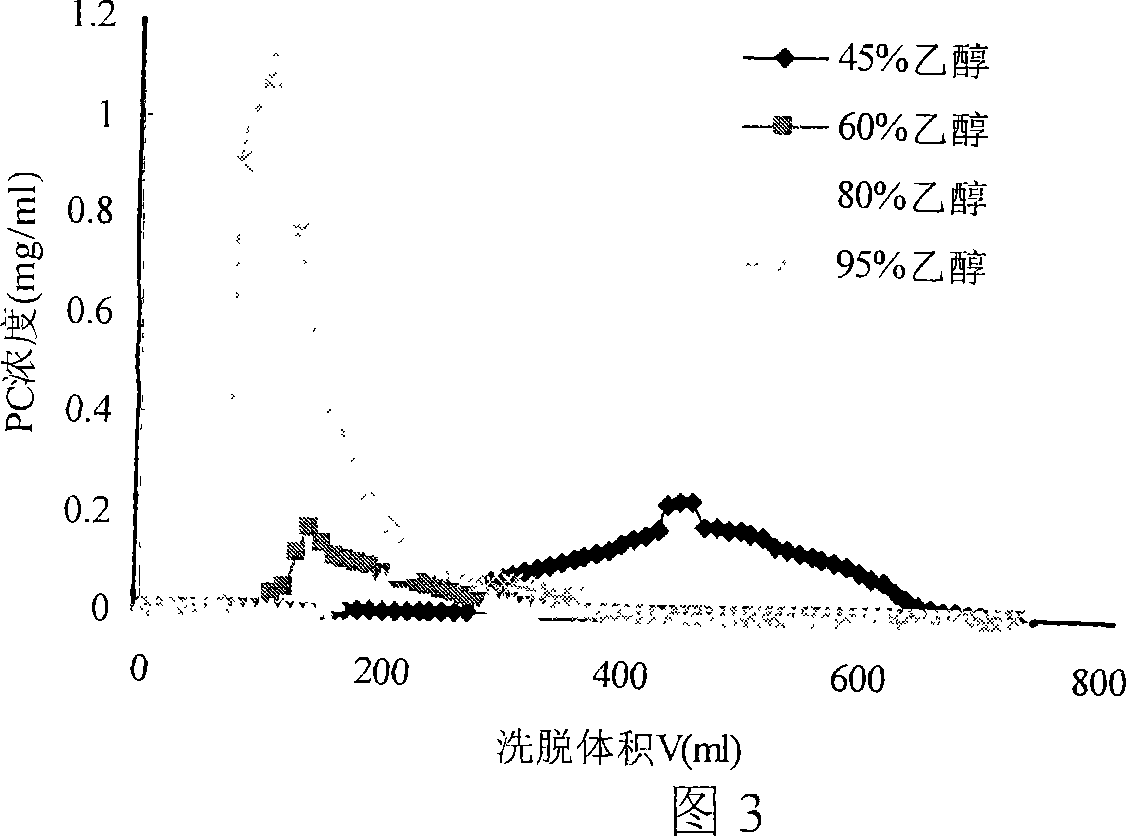
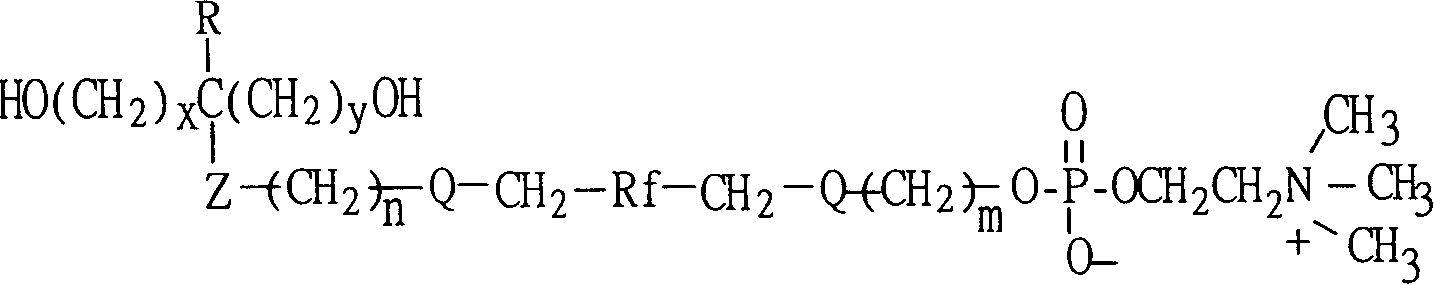Patents
Literature
166 results about "Phosphatidyl choline" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
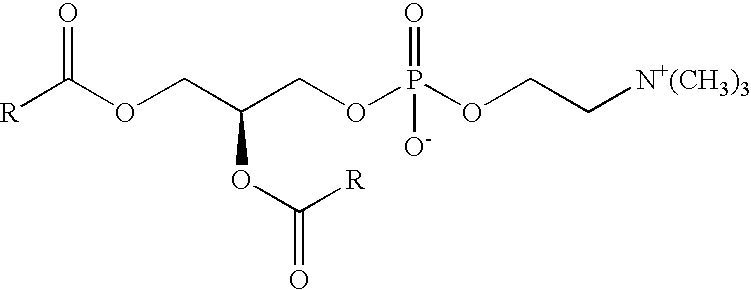
Phosphatidyl choline, more properly phosphatidycholine, is a molecule which can provide an excellent source of choline, a chemical compound which is necessary for healthy bodily function. This molecule belongs to a family of molecules known as phospholipids, all of which share the trait of having a long “tail” made...
Methods and compositions for the non-surgical removal of fat
ActiveUS20050261258A1Easy to disassembleReduces blood lossCosmetic preparationsOrganic active ingredientsSurgical removalCellulite
Compositions and methods useful in the non-surgical removal of localized fat deposits in patients in need thereof using pharmacologically active detergents are disclosed. The pharmacologically active detergent compositions can additionally include anti-inflammatory agents, analgesics, dispersion agents and pharmaceutically acceptable excipients but do not contain phosphotidylcholine. The pharmacologically active detergent compositions are useful for treating localized accumulations of fat including lower eyelid fat herniation, lipodystrophy and fat deposits associated with cellulite and do not require surgical procedures such as liposuction.
Owner:RGT UNIV OF CALIFORNIA +1
Methods and related compositions for the non-surgical removal of fat
InactiveUS20060154906A1Easy to disassembleReduces blood lossCosmetic preparationsBiocideSurgical removalCellulite
Compositions and methods useful in the non-surgical removal of localized fat deposits in patients in need thereof using pharmacologically active detergents are disclosed. The pharmacologically active detergent compositions can additionally include anti-inflammatory agents, analgesics, dispersion agents and pharmaceutically acceptable excipients but do not contain phosphotidylcholine. The pharmacologically active detergent compositions are useful for treating localized accumulations of fat including lower eyelid fat herniation, lipodystrophy and fat deposits associated with cellulite and do not require surgical procedures such as liposuction.
Owner:RGT UNIV OF CALIFORNIA +1
Acyltransferases for alteration of polyunsaturated fatty acids and oil content in oleaginous yeasts
Two acyltransferases are provided, suitable for use in the manufacture of microbial oils enriched in omega fatty acids in oleaginous yeast (e.g., Yarrowia lipolytica). Specifically, the genes encoding phophatidylcholine-diacylglycerol acyltransferase (PDAT) and diacylglycerol acyltransferase (DGAT2) have been isolated from Y. lipolytica. These genes encode enzymes that participate in the terminal step in oil biosynthesis in yeast. Each is expected to play a key role in altering the quantity of polyunsaturated fatty acids produced in oils of oleaginous yeasts.
Owner:EI DU PONT DE NEMOURS & CO
Cucurbitacin lipsome preparation method and formulation
InactiveCN1504191AHigh encapsulation efficiencyOrganic active ingredientsDigestive systemCucurbitacin BMedicine
The invention relates to a cucurbitacin liposome composition and its preparation, which has rather high encapsulation efficiency, and can be administered through vein, muscle, oral and nasal. The constituent percentage by weight of the composition are, cucurbitacin BE or cucurbitacin B 0.001-0.1%, phospholipids 0.1-10%, cholesterin 0-5%, the phospholipids can be lecithin, di-stearoyl phosphatidyl choline, di- palmityl phosphatidyl choline, di-oleoyl phosphatidyl choline, di- palmityl phosphatidyl ethanolamine, di-stearoyl phosphatidylglycerol. The preparation according to the invention can be prepared in the form of injection, oral liquid, syrup, drop and nasal spray
Owner:SHENYANG PHARMA UNIVERSITY
Process for preparing Paclitaxel liposome preparation
InactiveCN101011357ASlow down removalExtended stayOrganic active ingredientsPharmaceutical non-active ingredientsSucroseLiposome membrane
The invention relates to a method for preparing drug rehabilitation liposome agent, which comprises that using film disperse method or atomizing drying method to prepare long-circulation rehabilitation liposome, using cholesterol, distearin acyl phosphatidyl choline and myristate as stabilizers, using sucrose as freezing preservative, using chloroform and chloroform as organic solvents; and using amphipathic carbowax derivative to decorate the liposome membrane; and using compression or high-pressure homogeneity method to make the diameter of liposome smaller than 100nm and the package rate higher than 85%. The invention has less toxicity and high stability, as one novel drug slow-release target agent.
Owner:XIAN LIBANG PHARMA TECH
Small molecule metabolite map for identifying liver cancer, hepatitis or liver cirrhosis and manufacturing method thereof
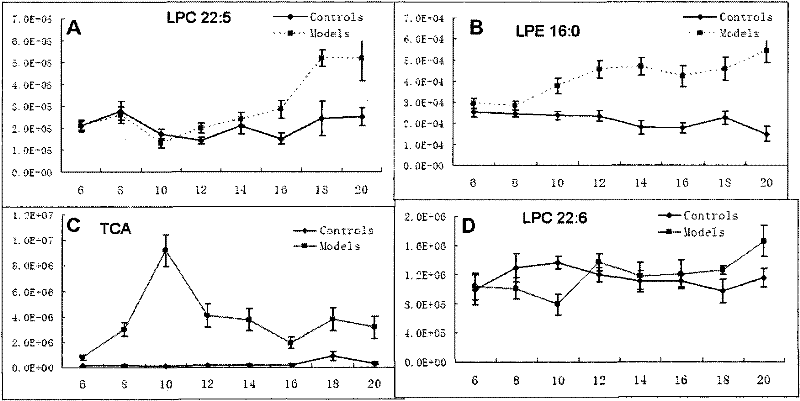
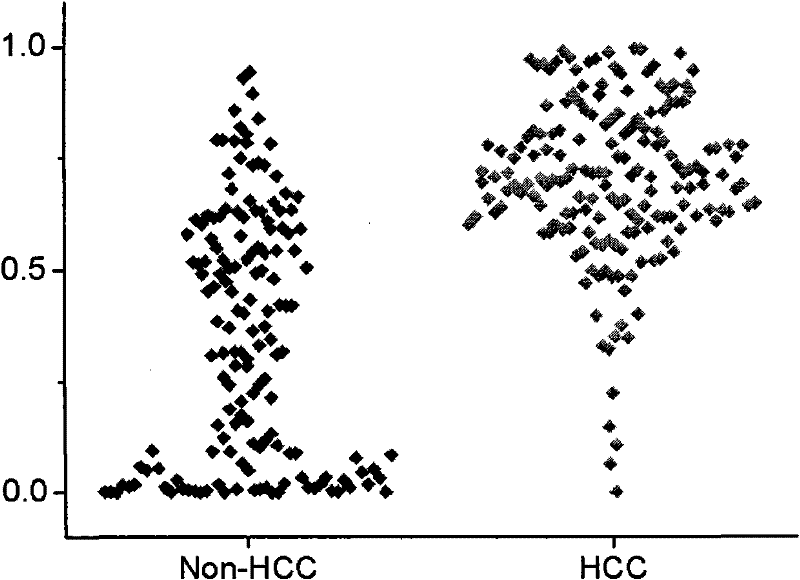
The invention relates to the technical field of medical diagnostics, in particular to a small molecule metabolite map for identifying liver cancer, hepatitis or liver cirrhosis and a manufacturing method thereof. The small molecule metabolites are four blood metabolites markers, i.e. palmitoyl hemolysis phosphoric acid ethanolamine glyceride, docosapentaenoic acyl hemolysis phosphatidyl choline, docosahexaenoic acyl hemolysis phosphatidyl choline and taurocholic acid, which respectively have the molecular weight of 453.2855, 569.3481, 567.3319 and 516.2916, and the corresponding ions of 454.2928, 570.3547, 568.3391 and 480.2776 detected through mass spectrum. Through experimentation on animals, the accuracy for determination of the liver cancer phase of a rat is 85 percent, and the accuracy for determination of the hepatitis or the liver cirrhosis is 94 percent. Clinical experiment results show that the accuracy for determination of the liver cancer is 85 percent, and the accuracy for determination of non-liver cancer is 71.9 percent. The method provided by the invention has the advantages of high sensitivity and high throughput, is superior to the existing liver cancer single diagnosis marker, and is applicable to screening and assisted diagnosis of the liver cancer.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY +1
Load small interfering RNA nanoscale lipid microbubble ultrasonic contrast agent and preparation method
InactiveCN103100093AAchieve targeted releaseRealize integrationGenetic material ingredientsEchographic/ultrasound-imaging preparationsUltrasound contrast mediaPolyethylene glycol
The invention discloses a load small interfering RNA (siRNA) nanoscale lipid microbubble ultrasonic contrast agent and a preparation method. The load siRNA lipid microbubble is formed by preparing DPPC (Dipalmitoyl Phosphatidyl Choline), DSPE (1, 2-distearoyl-sn-glycero-3-phosphoethanolamine) and DPPA (Diphenyl Phosphoryl Azide) into microbubbles (containing octafluoropropane) according to the weight part ratio of 18:1:1 and then assembling together with PEG-PLL (Polyethylene Glycol-Polylysne)-coated siRNA nanomicelle. The load siRNA nanoscale lipid is nanosclae, has an obvious ultrasonic contrast effect, and can generate obvious siRNA cell transfection efficiency under low-frequency ultrasonic irradiation, thereby further hopefully having important research values and application prospects in the fields of ultrasonic diagnosis and gene treatment.
Owner:THE THIRD AFFILIATED HOSPITAL OF SUN YAT SEN UNIV
Polyene phosphatidyl choline injection and method for preparing the same
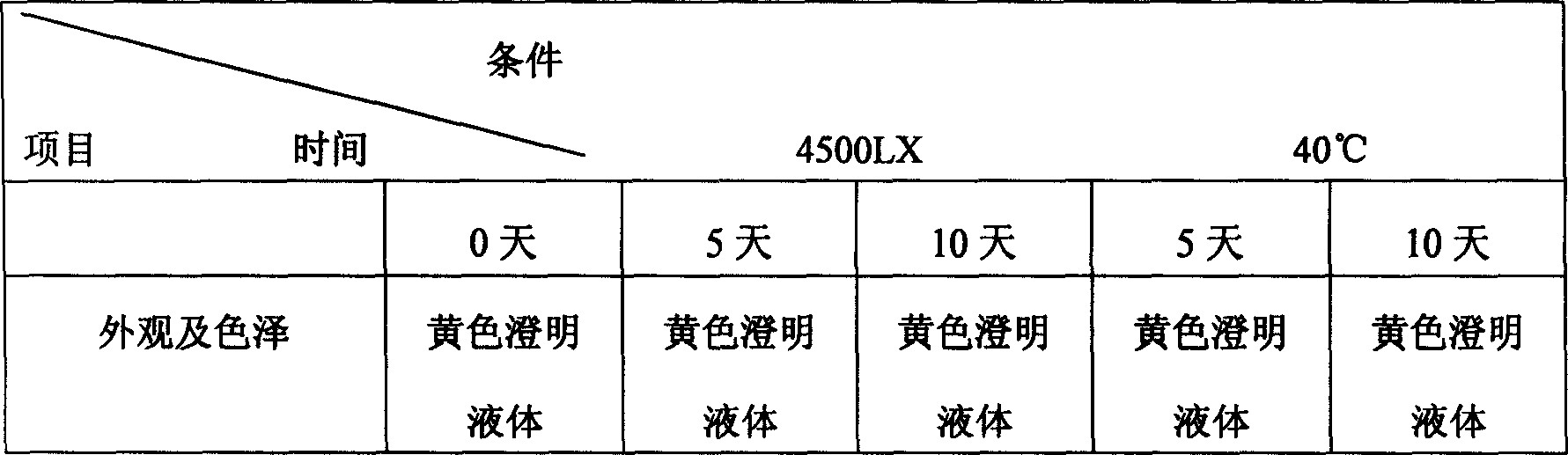
The invention discloses a polyene phosphatidyl choline injection and the preparation method. The components and the ratio (portion) of the polyene phosphatidyl choline injection of the invention is: 465 portion of polyene phosphatidyl choline injection, 88 portion of benzyl zlcohol, 50 to 800 portion of glycocholic acid, cholic acid or tween-80, 50 to 80 portion of alcohol, propanediol or glycerin, 15 to 200 portion of sodium hydroxide or sodium carbonate, 0.5 to 5 portion of 2,6-D-itert-butyl-p-cresol, 0.8 to 8 portion of Tertiary butyl-4-hydroxyl anisole, 3 to 25 portion of Vitamin E by weight. The polyene phosphatidyl choline injection of the invention has good clarity, high stability, simple preparation process and easy operation.
Owner:SICHUAN HAISCO PHARMA CO LTD +1
Method for preparing powdered phosphatidyl serine
The invention relates to a method of preparing compounds, in particular to a method of preparing powder phosphatidylserine. The invention has to overcome the problems that the prior art inputs a lot of equipment and costs a lot, and the transformation ratio of the reaction is hard to be guaranteed. The invention has the technical proposal that a method of preparing powder phosphatidylserine takes natural lecithin as the reaction substrate; and after being separated by water, the reaction substrate can be added with an inorganic system prepared by L-serine, calcium salt and buffer solution to produce rough product under the effect of phospholipase D. Concentrate can be obtained by conducting the solvent extraction to the rough product, and then can be purified by solvent to obtain phosphatidylserine. The invention is characterized in that to separate the reaction substrate needs water three to ten times (w / v) of reaction substrate; and the separating temperature is ranged from 20 DEG C to 60 DEG C. In the system, the weight of the L-serine is two to ten times of the reaction substrate, and the weight of water used in the inorganic system is three to ten times(w / v) of reaction substrate. Ph value of the inorganic system can be adjusted between 4.5 and 6.0 by the buffer solution.
Owner:杨凌萃健生物工程技术有限公司 +1
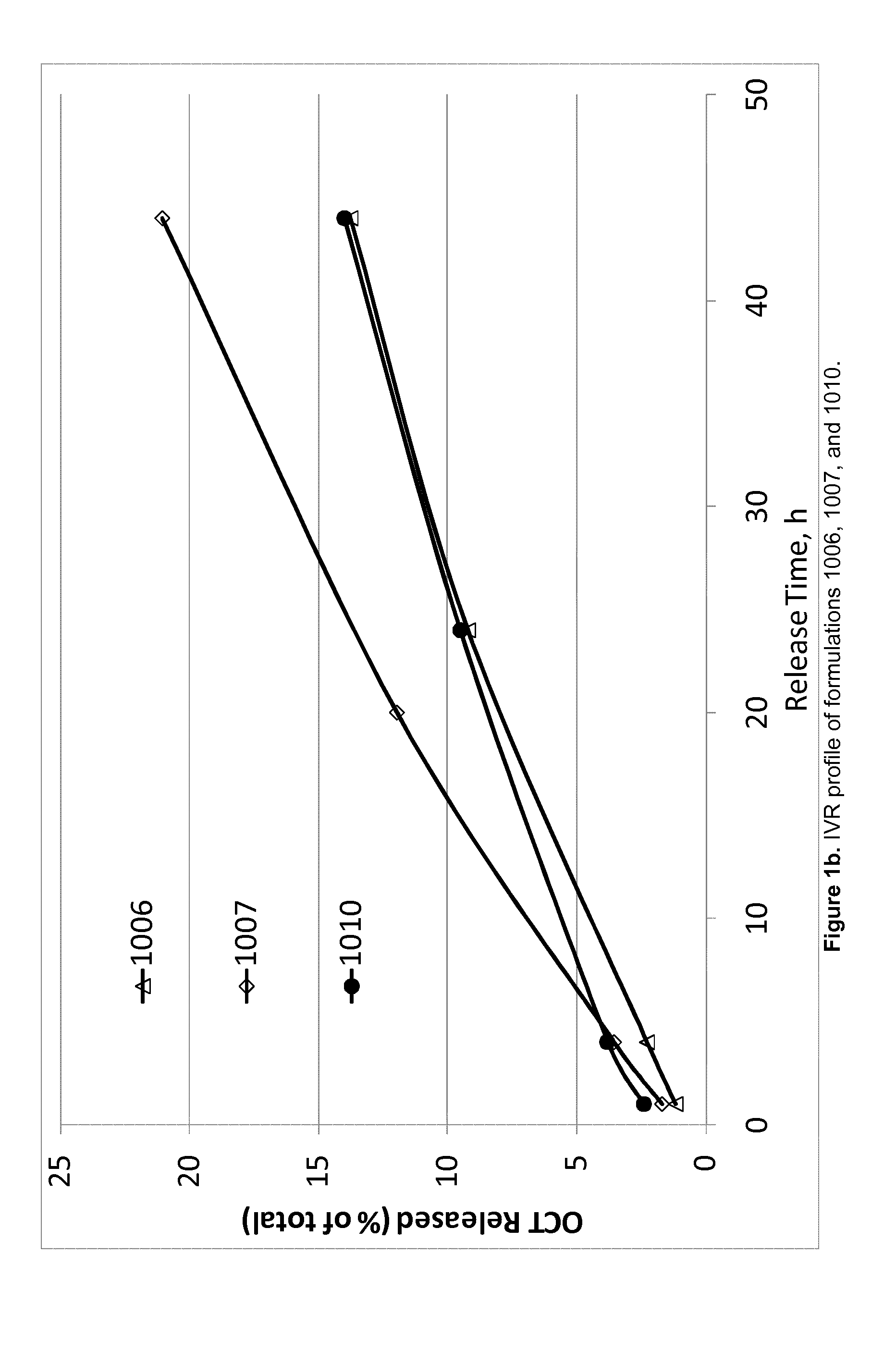
Controlled release peptide formulations
ActiveUS20140162944A1Little and no preparationLittle and preparationSenses disorderPeptide/protein ingredientsLiquid crystallineControlled release
The present invention relates to compositions forming a low viscosity mixture of: a) 20-80 wt. % of at least one diacyl glycerol and / or a tocopherol; b) 20-80 wt. % of at least one phosphatidyl choline (PC); c) 5-20 wt. % of at least one biocompatible, organic mono-alcoholic solvent; d) up to 20 wt. % polar solvent e) at least one peptide active agent; f) optionally at least one antioxidant; wherein the ratio of components a:b is in the range 40:60 to 54:46; wherein the pre-formulation forms, or is capable of forming, at least one liquid crystalline phase structure upon contact with excess aqueous fluid. The invention further relates to methods of treatment comprising administration of such compositions, and to pre-filled administration devices and kits containing the formulations.
Owner:CAMURUS AB
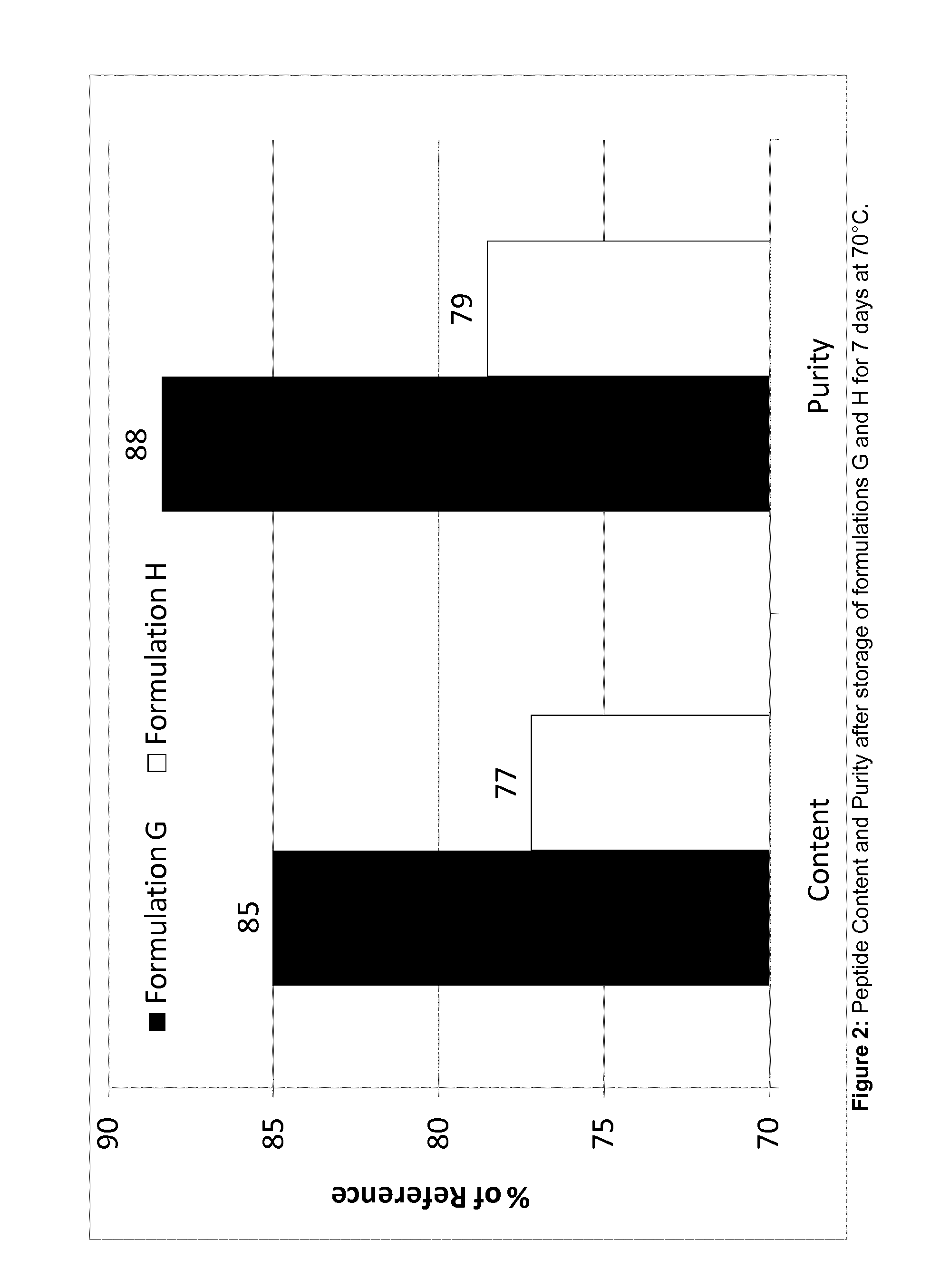
Octreotide acetate preparation and preparation method thereof
InactiveCN102525927AFull appearanceNo significant change in encapsulation efficiencyPeptide/protein ingredientsDigestive systemOctreotide preparationFreeze-drying
The invention belongs to the field of medicament preparations, and discloses an octreotide acetate lipidosome precursor and a preparation method thereof. The precursor lipidosome contains octreotide acetate, negatively-charged phospholipid and a cryoprotectant, and can contain an appropriate quantity of other lipids including phosphatidylchline and cholesterol; components such as an antioxidant, a pH regulating agent and the like can be added as required; the molar ratio of the octreotide acetate to the negatively-charged phospholipid is smaller than 1:1; and the mass ratio of the negatively-charged phospholipid to the cryoprotectant is 1:1-1:10. In the invention, a tert-butyl alcohol-water cosolvent freeze-drying method is adopted. The entrapment rate of an octreotide acetate lipidosome / micelle obtained by hydrating a freeze-dried product can be over 50 percent, an octreotide acetate lipidosome / micelle obtained by hydrating the freeze-dried product has high stability, and the problem of difficulty in entrapping a protein polypeptide medicament during preparation of a lipidosome / micelle preparation is solved. The preparation method is simple and practicable, and is suitable for industrial mass production.
Owner:SHENYANG PHARMA UNIVERSITY
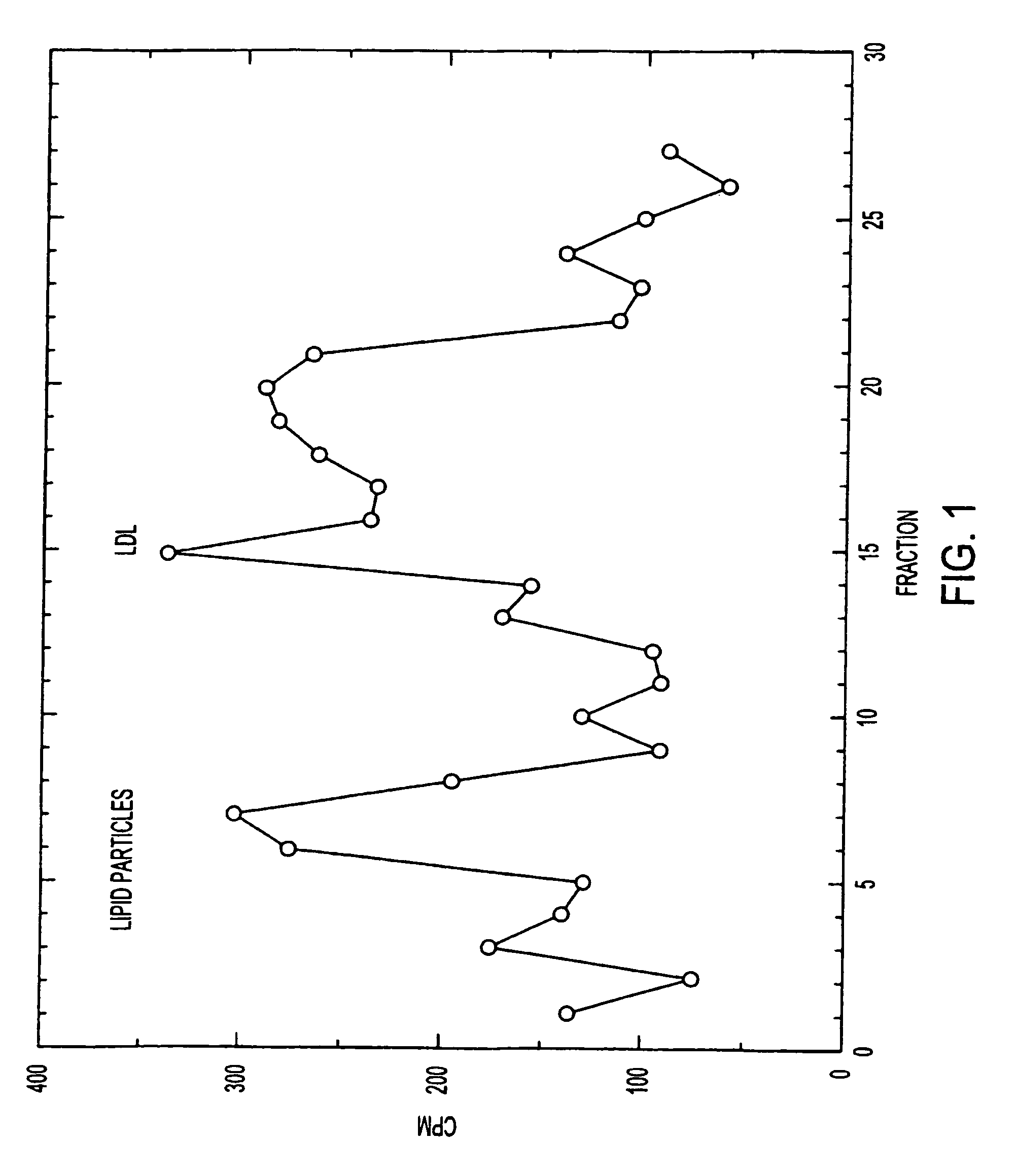
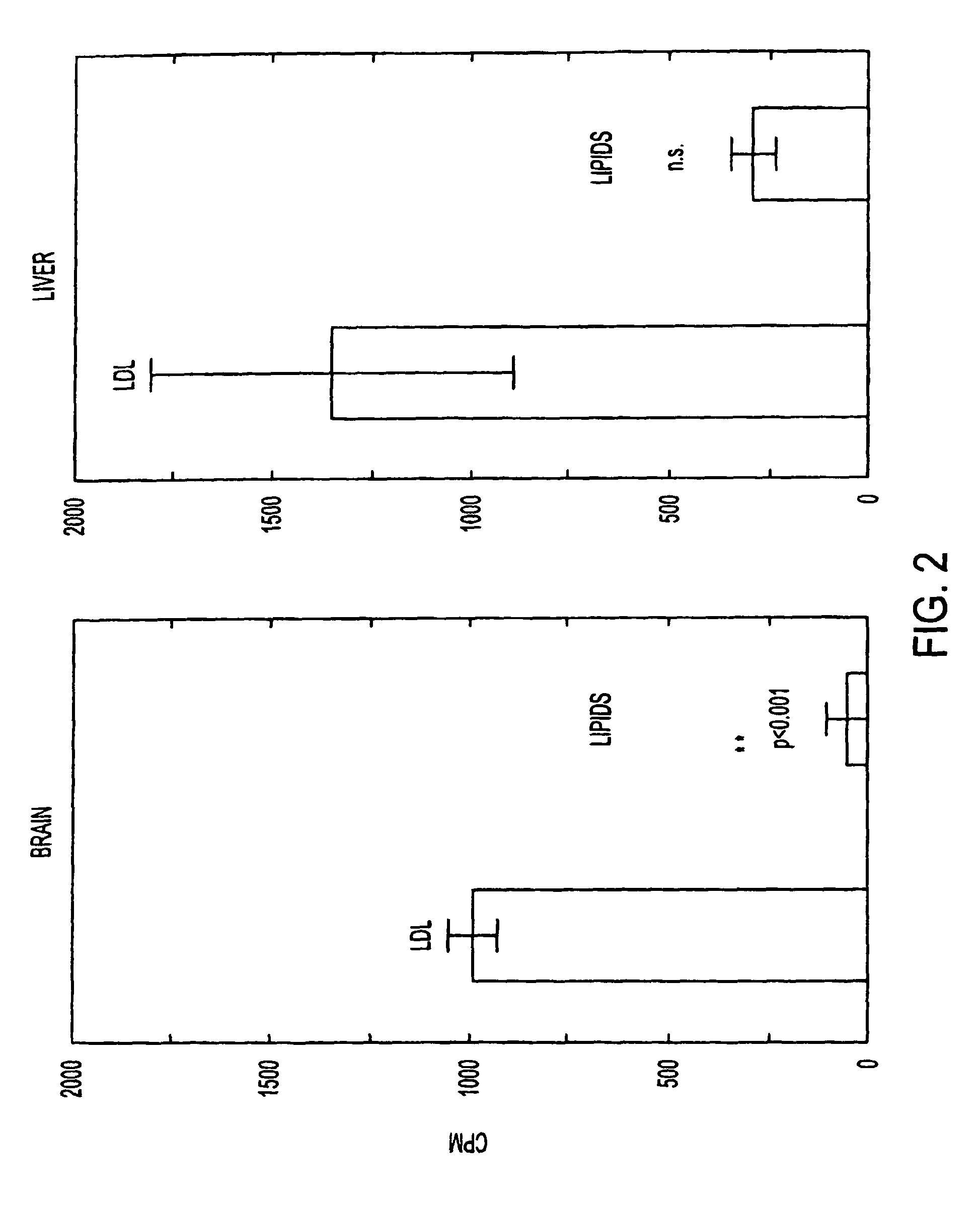
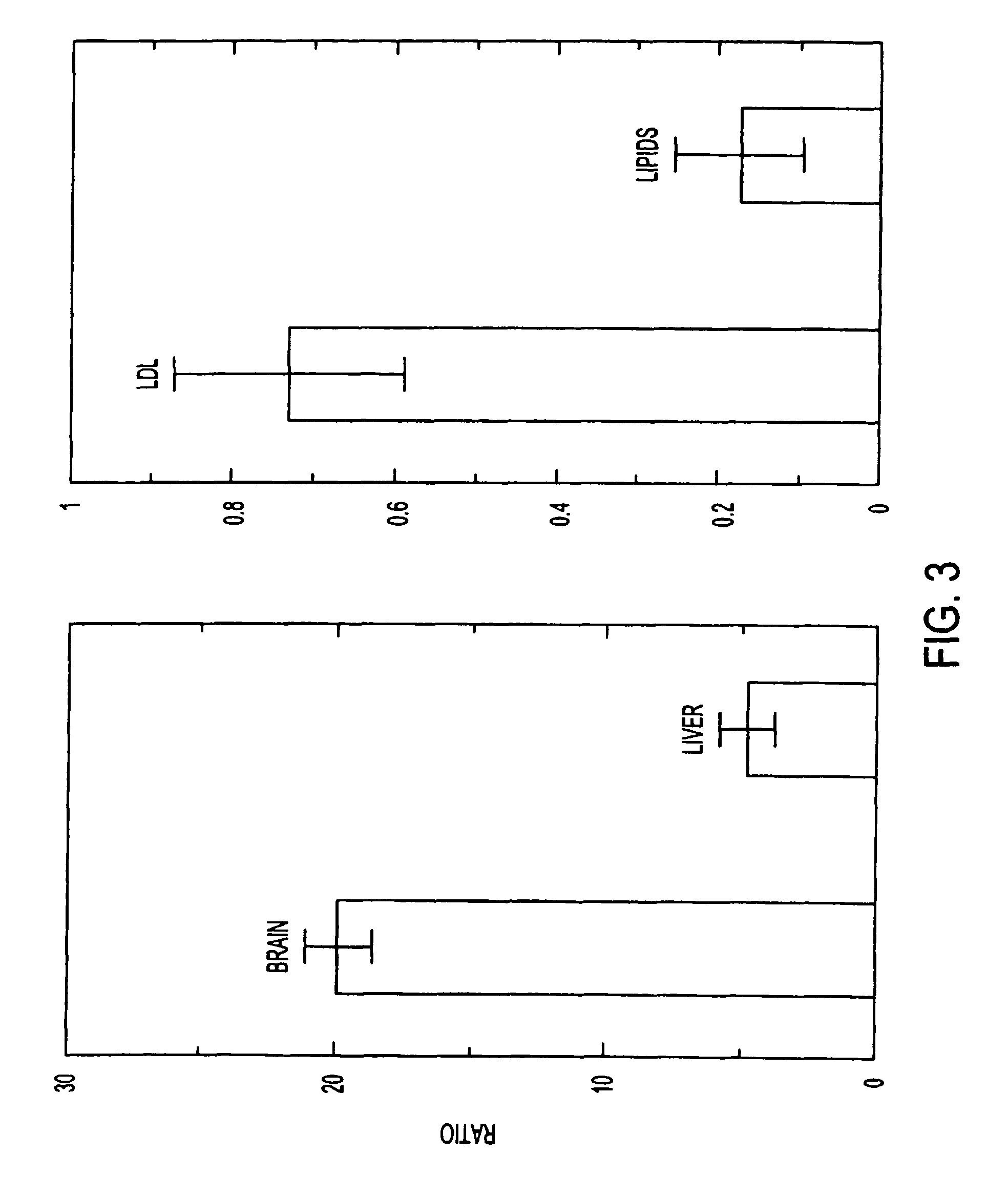
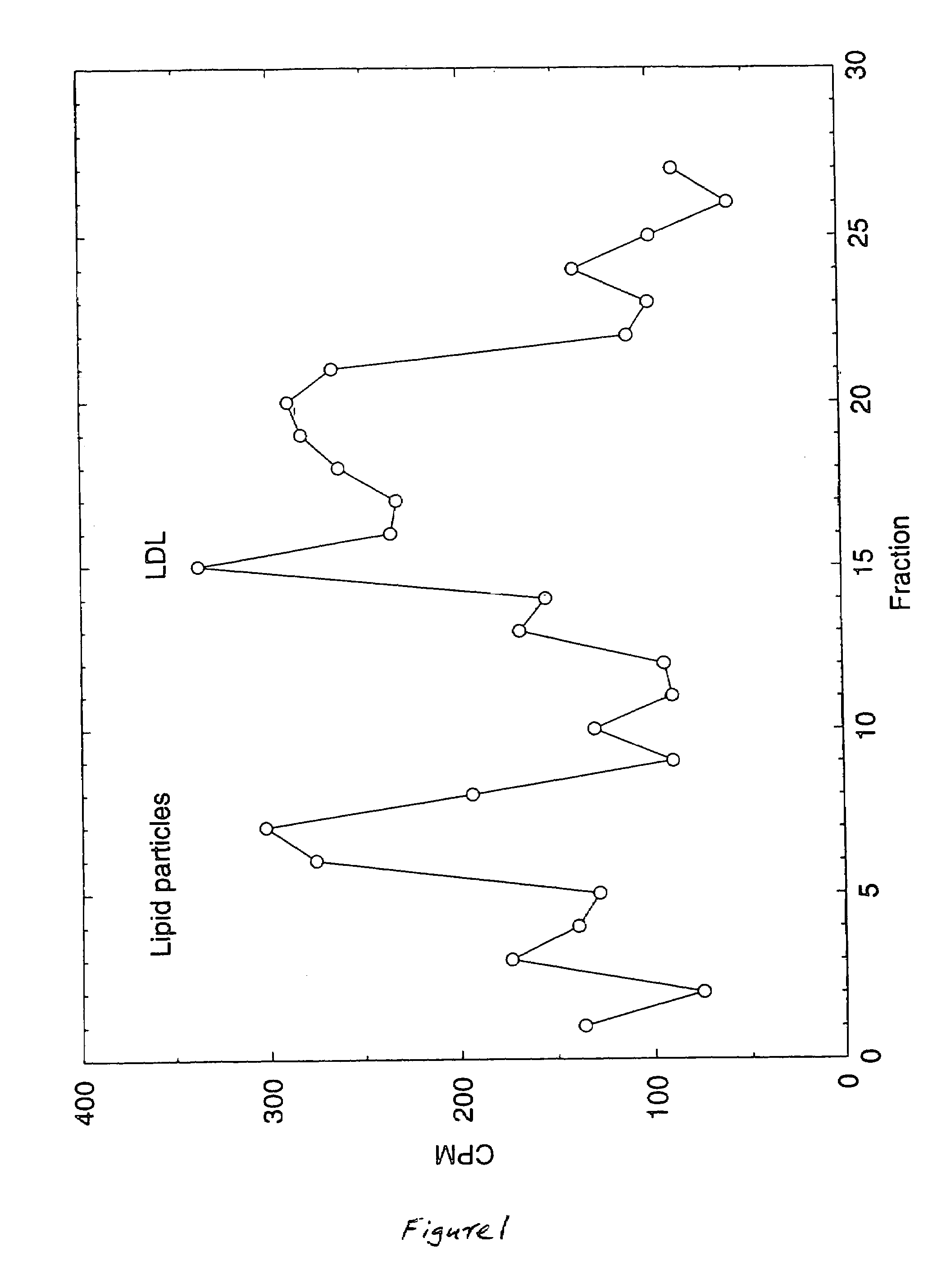
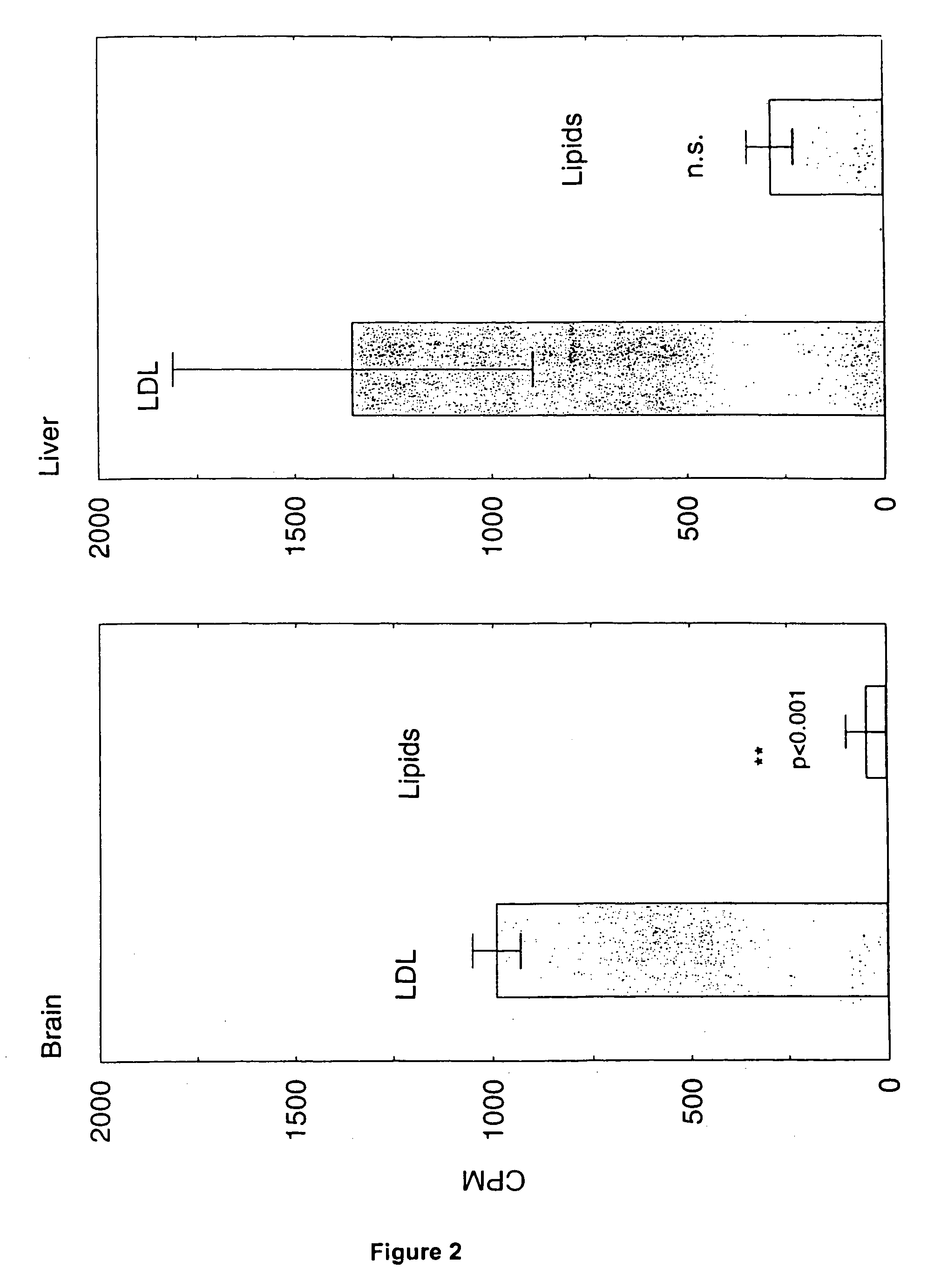
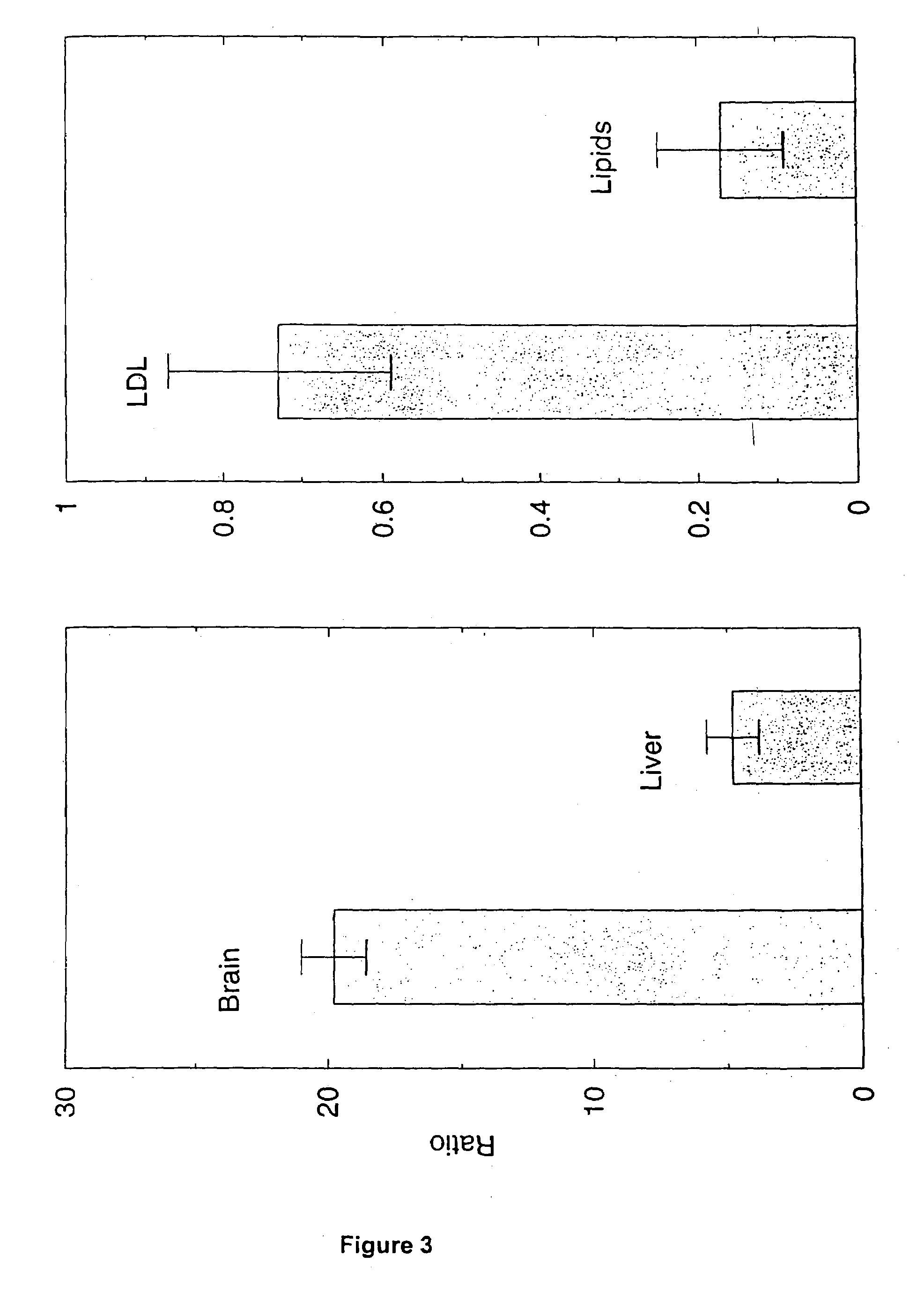
Artificial low-density lipoprotein carriers for transport of substances across the blood-brain barrier
InactiveUS7803400B2Convenience to mergeFacilitate and improve treatmentBiocidePowder deliveryDiseaseLipid formation
This invention relates to a highly efficient artificial low-density lipoprotein (LDL) carrier system for the targeted delivery therapeutic agents across the blood-brain barrier (BBB). In particular, this invention relates to artificial LDL particles comprised of three lipid elements: phosphatidyl choline, fatty-acyl-cholesterol esters, and at least one apolipoprotein. The present invention further relates to compositions, methods and kits comprising artificial LDL particles for targeting drugs to and across the BBB for the prevention and treatment of brain diseases.
Owner:WEST VIRGINIA UNIVERSITY
Liposome injection of amoxicillin sodium sulbactam sodium medicinal composition
InactiveCN101822669AImprove stabilityHigh encapsulation efficiencyAntibacterial agentsPowder deliveryDipalmitoylphosphatidylcholineFreeze-drying
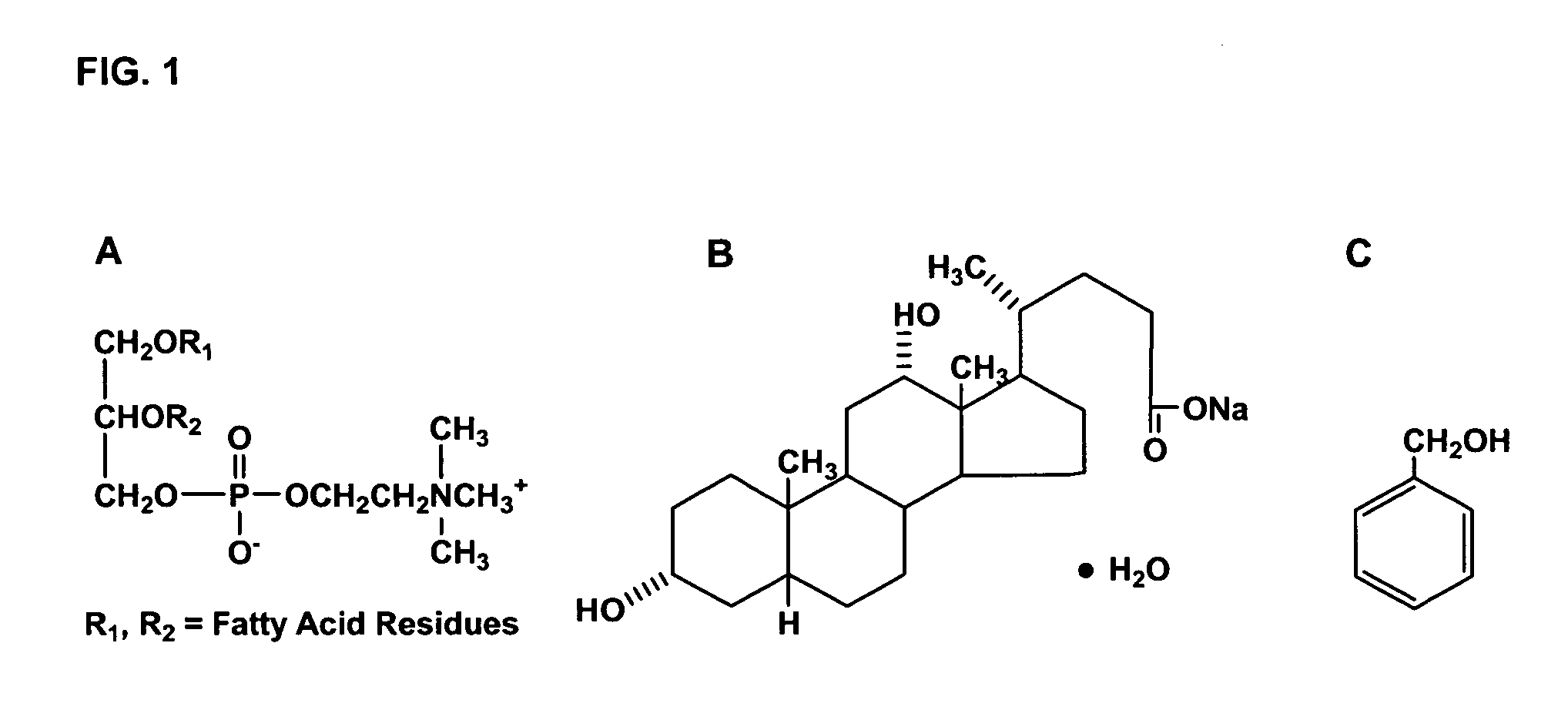
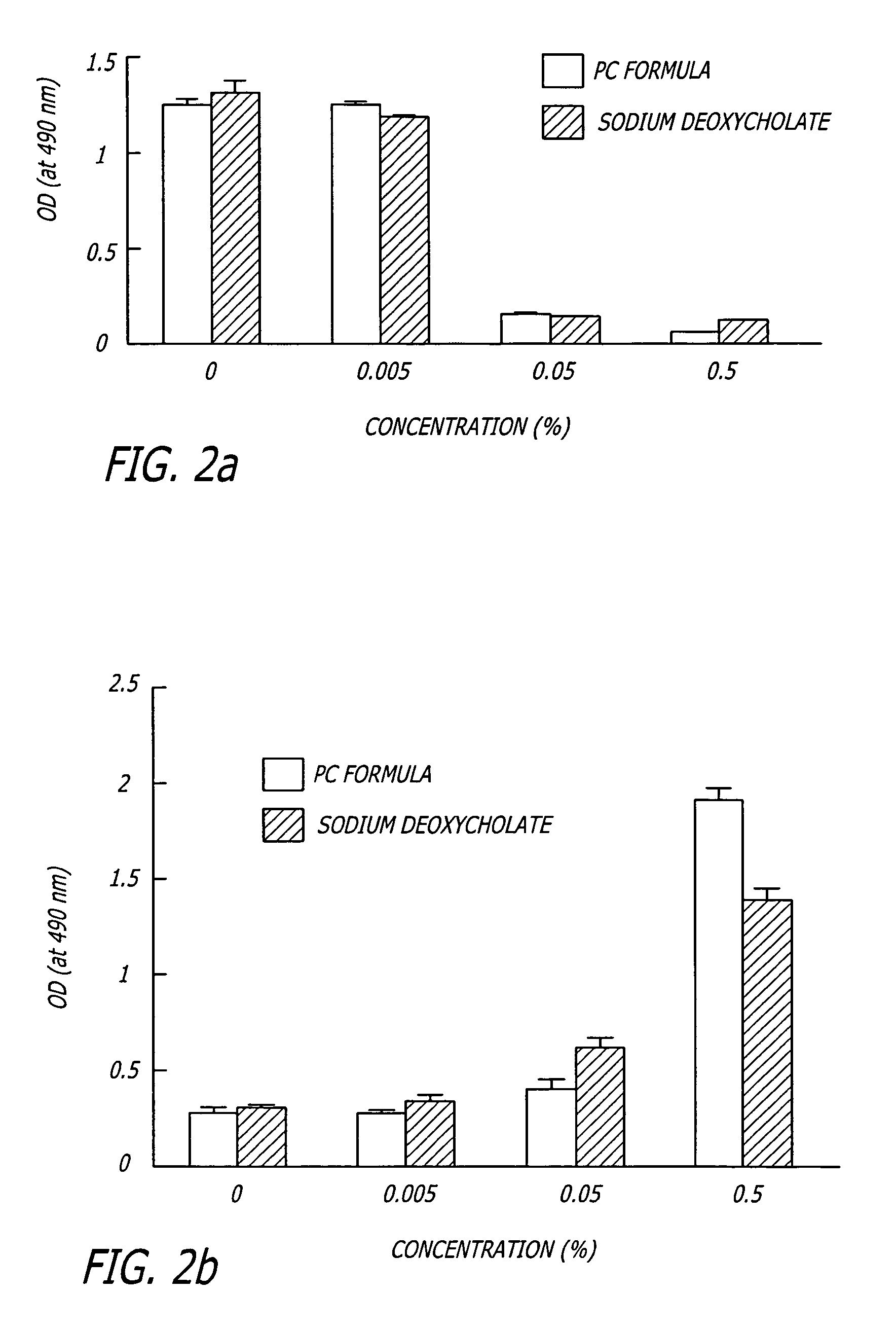
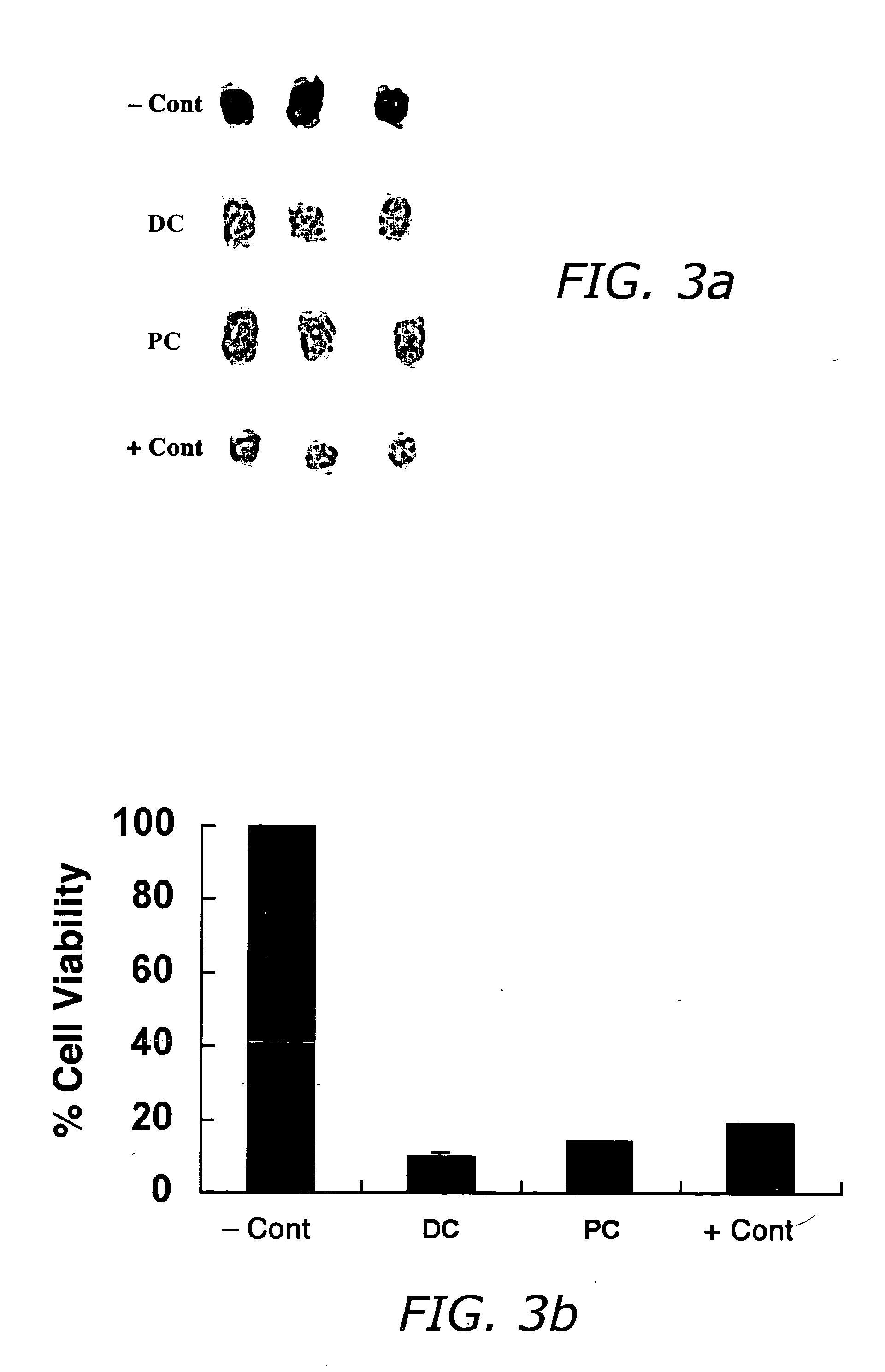
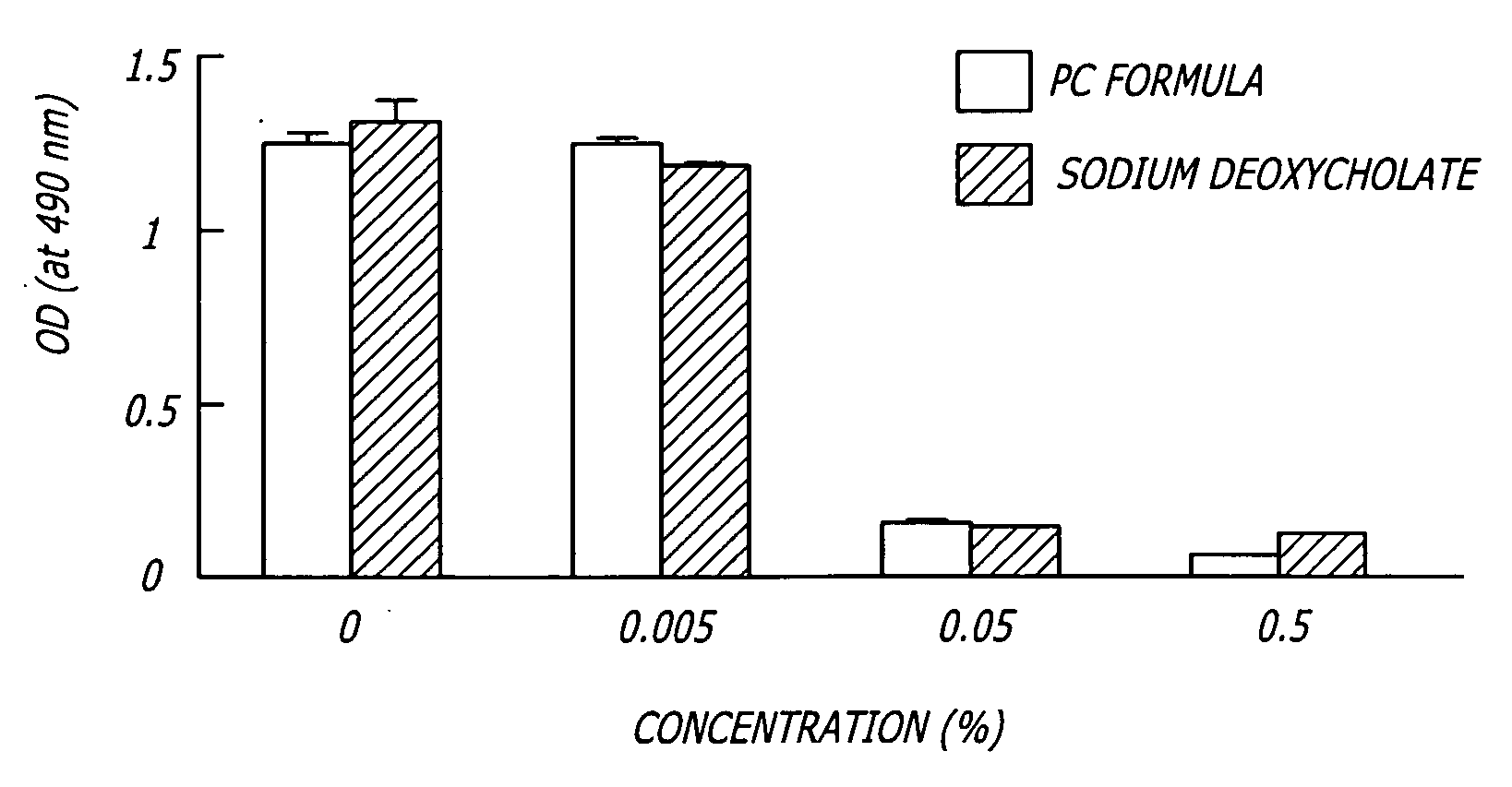
The invention provides liposome injection of an amoxicillin sodium sulbactam sodium medicinal composition. The liposome injection consists of amoxicillin sodium, sulbactam sodium, liposome carriers, freeze-drying supporting agents and optional antioxidant, wherein the liposome carriers are dipalmitoyl phosphatidyl choline and deoxysodium cholate. The liposome injection has high stability; in the freeze-drying process, a phenomenon of breaking liposome caused by dehydration, fusion, ice crystal generation and the like does not occur; and the liposome can remain good encapsulation ratio after being hydrated and redissolved.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Chewing gum including nanozome encapsulated cannabidiol
InactiveUS20170265494A1Improve bioavailabilityGood curative effectHydroxy compound active ingredientsConfectioneryCannabinoidBioavailability
Chewing gum including a liquid center, a chewy layer surrounding the liquid center, and a coating surrounding the chewy layer. The liquid center includes a nanozome encapsulated dose of a cannabinoid such as cannabidiol of approximately 1-30 mg. The nanozome is preferably a phospholipid such as a Phosphatidyl Choline. In another embodiment the nanozome is unsaturated Phosphatidyl Choline to improve bioavailability of the cannabidiol, or other cannabinoid.
Owner:INT CONSOL
Liposome and preparation method of the same
InactiveUS20050214357A1Improve solubilityReduce skin irritationCosmetic preparationsMicroencapsulation basedPolyethylene glycolLiposome
The present invention relates to a composition and a method for preparing a liposome, the liposome including a lipid bilayer and an aqueous core contains a hydrophobic or a hydrophilic drug and a component—Vitamin E derivative (d-α tocopheryl polyethylene glycol 1000 succinate; TPGS). TPGS is able to increase the encapsulation efficiency of drug in liposome as well as to enhance the stability of drug in liposomes. Such liposome is capable to increase the skin permeation of drugs. The preparation method comprises the following steps: (1) adding the drug to a Vitamin E derivative solution to form a mixture; and (2) adding at least one phosphatidyl choline to the mixture, after hydration from either sonication or homogenization.
Owner:IND TECH RES INST
Method of separating and purifying phosphatidyl choline from phospholipid by resin chromatography method
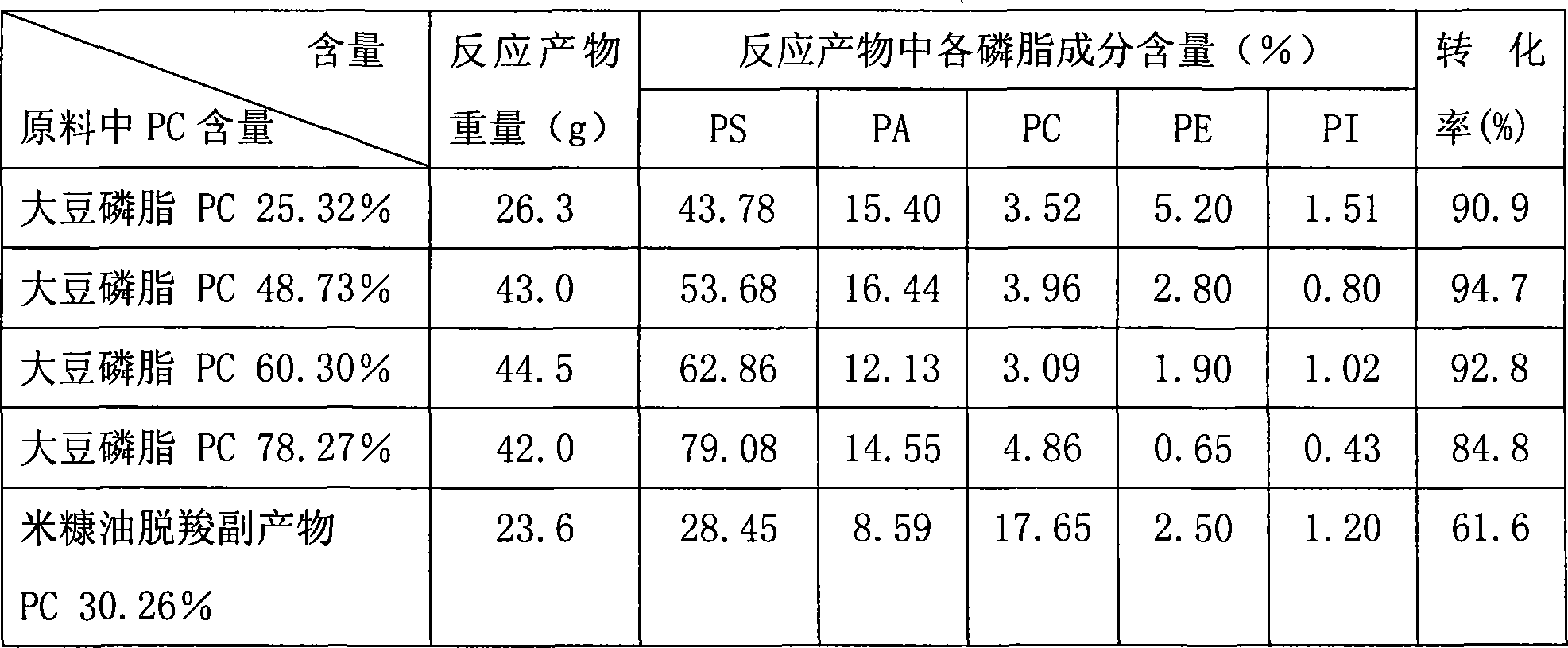
A method of using resin chromatographic analysis antigenic to purify Phosphatidyl Choline belongs to the phospholipid purification technology. The invention takes Gliadin phospholipid as raw material to prepare ethanol solution with concentration of Gliadin phospholipid 3-5mg / mL, which is add into the D113-IIIFC resin chromatographic column at the flow rate of 1mL / min, and then elutes with 95% ethanol at the rate of 0.5-1mL / min, collects the elutes, evaporates and concentrates to remove solvents, uses vacuum drying to get the pale yellow and plastic-like products of PC. In this products, the content of PC is 94.02% (wt %), and the product yield is 80.74%.
Owner:JIANGNAN UNIV
Goose fat liver sauce and its preparing method
InactiveCN1709155AFull of nutritionNutrients unique to the human body needs high-level nutrition richFood preparationAdditive ingredientUnsaturated fat
Owner:张玺
Insect cell serum-free culture medium and application thereof
ActiveCN104593316AImprove cultivation efficiencyThe components are simple and clearAnimal cellsFermentationLithium chlorideDiethylenetriamine
The invention discloses an insect cell serum-free culture medium and application thereof. The culture medium comprises amino acid, inorganic salt, vitamin and carbohydrate and further comprises 0.5-15mg / L of diethylenetriamine dioleoyl phosphatidylcholine and / or 0.1-10mg / L of distearoyl phosphatidyl choline; and particularly, further comprises 0.001-0.1mg / L of barium chloride dihydrate, 0.005-0.02mg / L of lithium chloride and 0.05-7mg / L of nickel chloride. The insect cell serum-free culture medium is simple and clear in components and low in cost and is easily prepared; by the insect cell serum-free culture medium, the insect cell culture efficiency and recombinant protein expression efficiency can be significantly increased and the large-scale culture and large-scale preparation of insect cells are well achieved.
Owner:苏州沃美生物有限公司
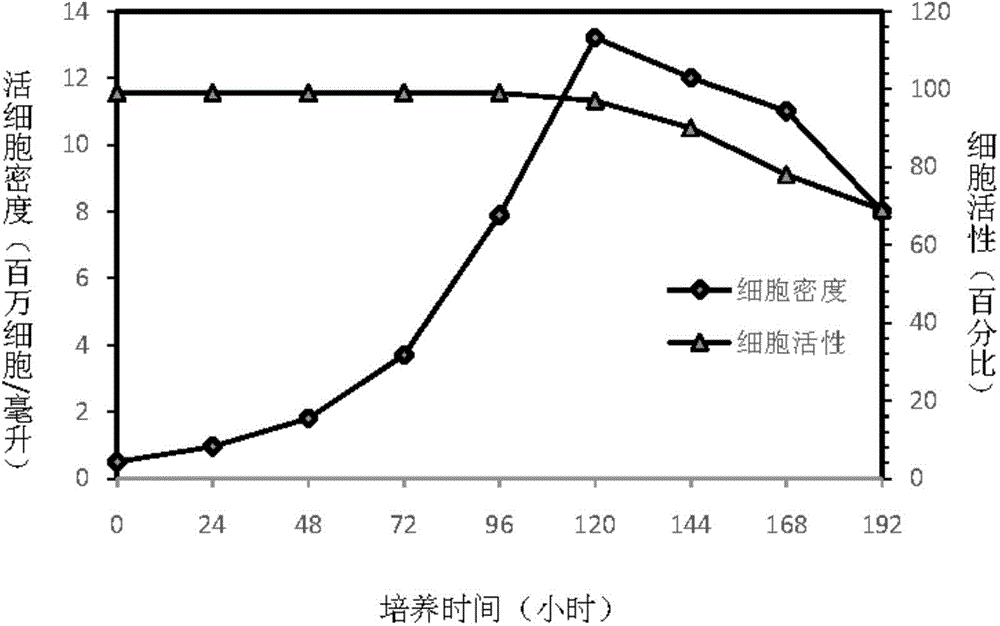
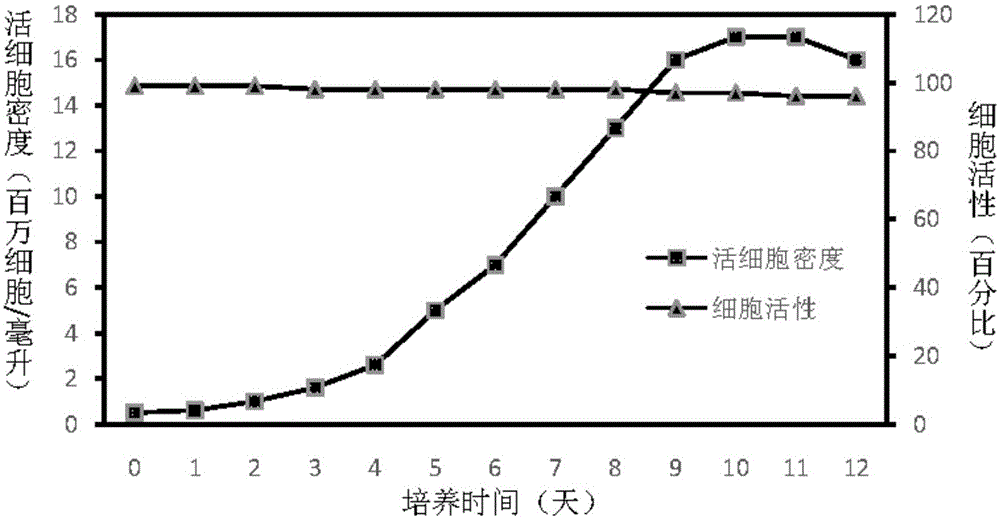
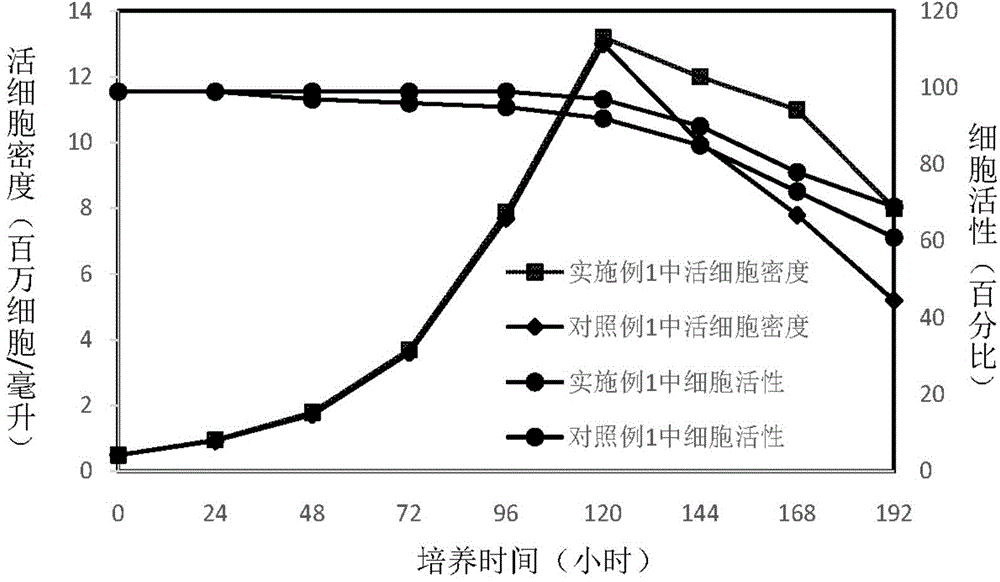
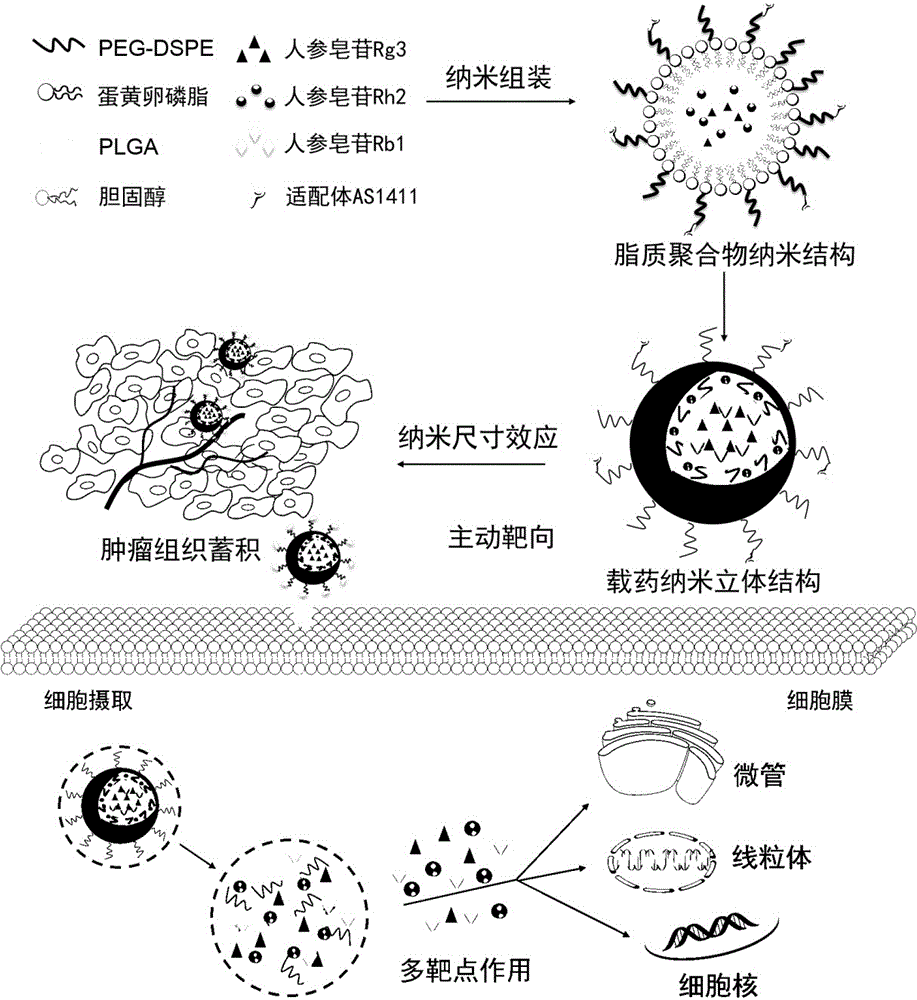
Preparing method and application of ginsenoside-multi-component jointly-loading targeting nanometer system
ActiveCN105708847AOvercoming differences in metabolic behaviorGood biocompatibilityOrganic active ingredientsPharmaceutical non-active ingredientsGinsenoside Rb1Solvent
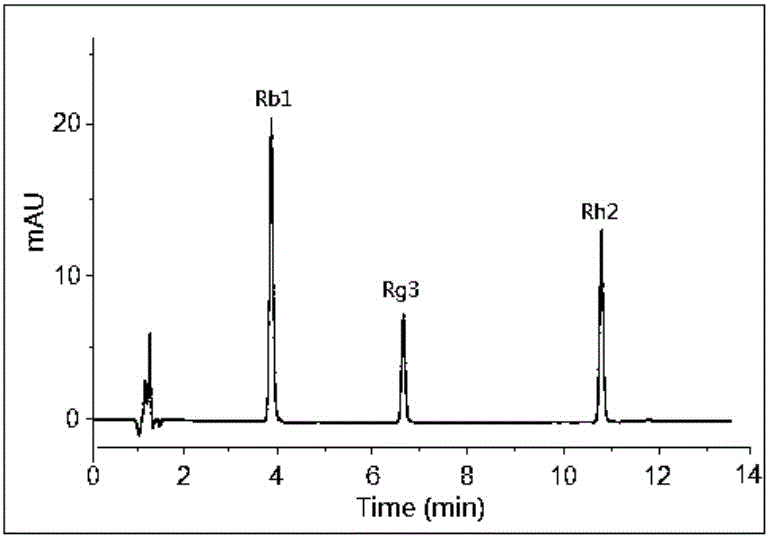
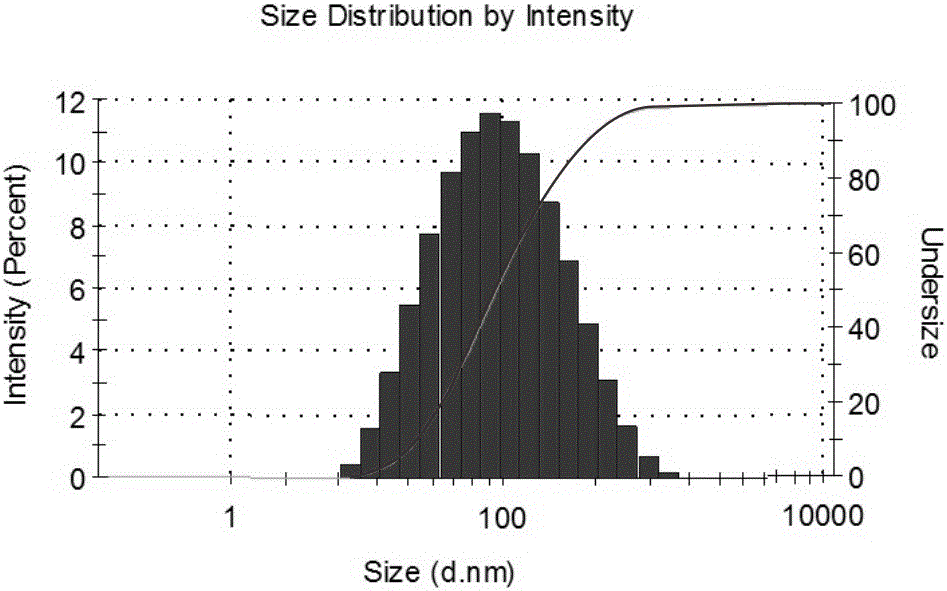
The invention discloses a preparing method of a ginsenoside-multi-component jointly-loading targeting nanometer system.The preparing method includes the steps that after egg yolk lecithin, cholesterol and polyethylene glycol-dipalmitoyl phosphatidyl choline are dissolved, warm water is slowly and dropwise added for stirring, and a lipid water solution is obtained; the multi-component active ingredients containing ginsenoside Rg3, ginsenoside Rh2 and ginsenoside Rb1 and a copolymer of polylactic acid-hydroxyacetic acid are dissolved to be slowly and dropwise added into the lipid water solution to be stirred to be even, a solvent is removed, and lipidosome nanometer particles are obtained; aptamer is dissolved to be modified to the surfaces of the lipidosome nanometer particles, and an even and opalescence-flooding nanometer system solution is obtained by filtering and sterilizing.According to the preparing method, the anti-tumor effect of medicine is improved with the nanocrystallization technology, joint transmission of a multi-component tumor tissue can be achieved in the mode that the three ginsenoside ingredients are jointly loaded to the nanometer system, and therefore the problems that the metabolic behaviors and the tumor-cell entering capacity of different ingredients are different are solved.
Owner:CHENGDU UNIV
Polyene phosphatidyl choline lyophilized powdered injection
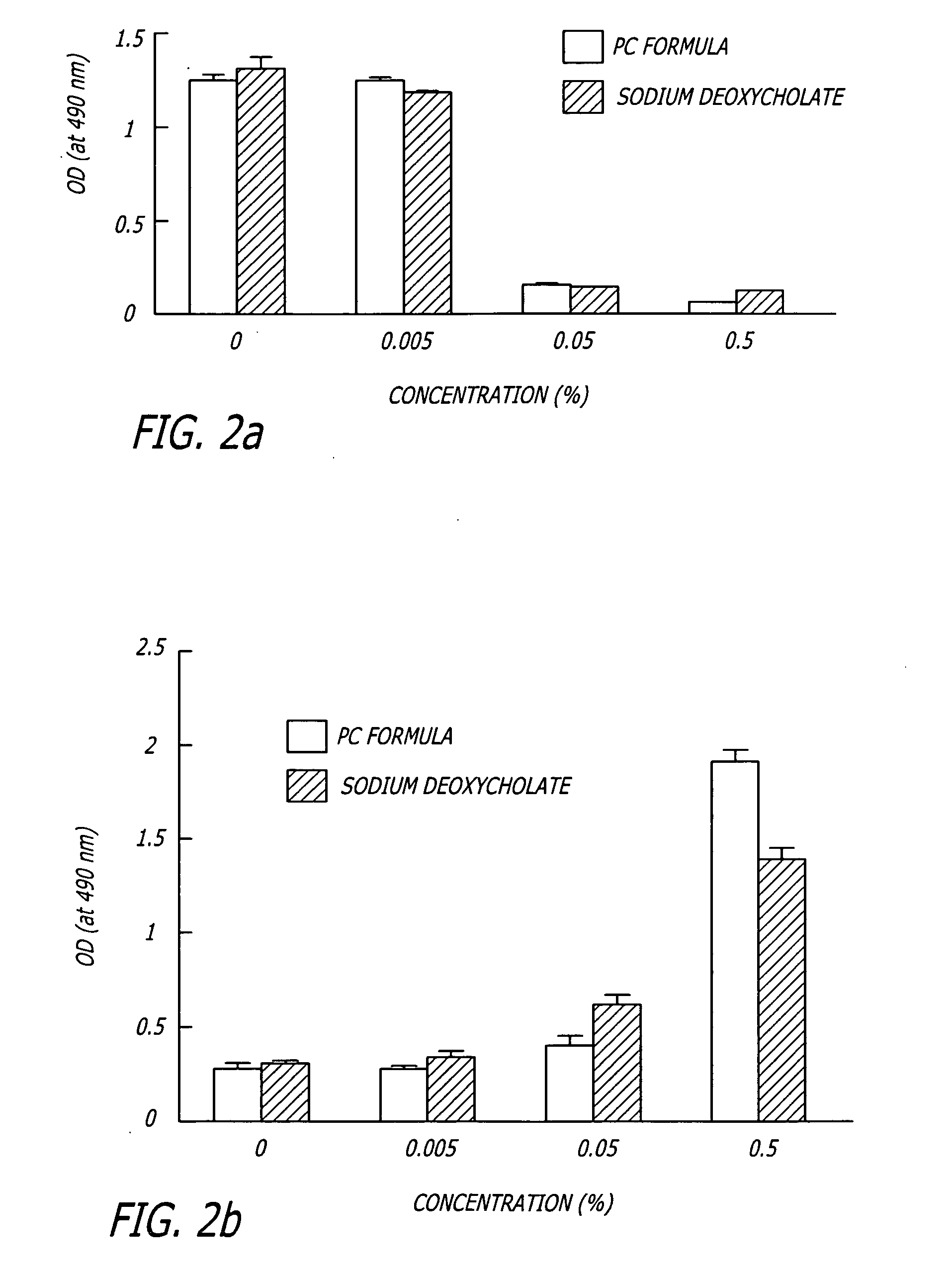
The invention discloses a polyene phosphatidyl choline freeze dried injection for the treatment of hepatic diseases, which comprises polyene phosphatidyl choline as the main ingredients and pharmaceutically acceptable carrying agents, wherein the carrying agent can be auxiliary solvent selected from sodium cholate, sodium deoxycholate, sodium deoxycholate, hydroxypropyl-beta-cyclodextrin, hydroxyethyl-beta-cyclodextrin and / or methyl cyclodextrin. The injection has higher stability.
Owner:GUANGDONG XIANQIANG PHARMA
Artificial low-density lipoprotein carriers for transport of substances across the blood-brain barrier
ActiveUS7220833B2Convenience to mergeFacilitate and improve treatmentAntibacterial agentsOrganic active ingredientsLipid formationDisease
This invention relates to a highly efficient artificial low-density lipoprotein (LDL) carrier system for the targeted delivery therapeutic agents across the blood-brain barrier (BBB). In particular, this invention relates to artificial LDL particles comprised of three lipid elements: phosphatidyl choline, fatty-acyl-cholesterol esters, and at least one apolipoprotein. The present invention further relates to compositions, methods and kits comprising artificial LDL particles for targeting drugs to and across the BBB for the prevention and treatment of brain diseases.
Owner:WEST VIRGINIA UNIVERSITY
Process for preparing high purity soy phoshatidylcholine without lysophosphatide
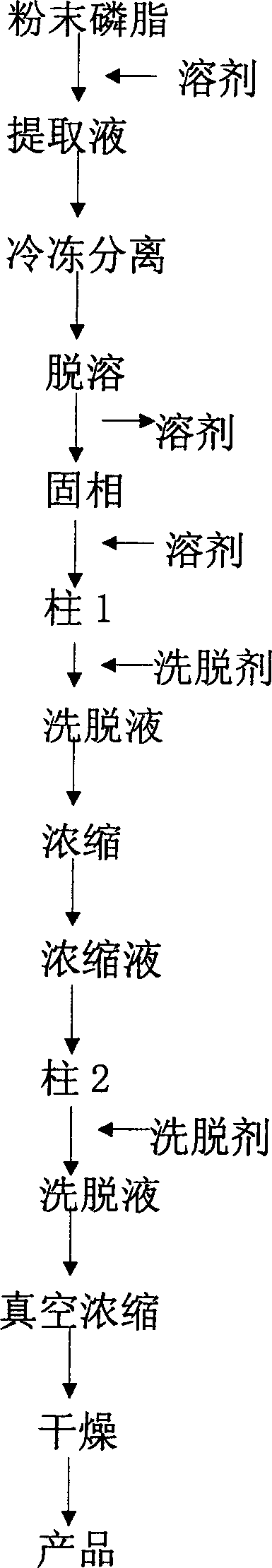
InactiveCN1837222AEasy to manufactureWide range of usesPhosphatide foodstuff compositionsSolventSoybean Phospholipids
The invention discloses a high-purity soya bean phosphatidyl choline without lysophosphatide preparing method in the phosphatide technique domain, which comprises the following steps: using soybean podwer phosphatide for raw material; extracting by dissolvant; freezing raffinate; treating liquor by dual column chromatogram; eluting for treating column 1; dislodging phosphatidyl ethanolamine; eluting and treating column2; dislodging lysophosphatide, wherein eluent of dissolvant,column 1 and column 2 for extracting is common single solvent; the production has no lysophosphatide; the bilineurine content of soya bean lecithin is more than 90%.
Owner:JIANGNAN UNIV
Preparation process of polyene phosphatidylcholine injection
InactiveCN1602878ANo obvious degradationDigestive systemPhosphorous compound active ingredientsPolyene phosphatidylcholineActivated carbon
The invention discloses a method for preparing polyene phosphatidyl choline injection, including mixing polyene phosphatidyl choline, solubilizer, and injection water in proportion, stirring them into a colloidal dispersive system, adding in activated carbon to absorb, and adding proper antiseptic. It is a low-cost, simple-technique preparing method.
Owner:北京瑞伊人科技发展有限公司 +1
Oral solid preparation containing polyene phosphatidyl choline and preparation method thereof
InactiveCN101756907AImprove yield and quality accuracyConducive to intermediate quantitative controlOrganic active ingredientsDigestive systemPolyeneQuality control
The invention relates to an oral solid preparation containing polyene phosphatidyl choline and a preparation method thereof. The oral solid preparation comprises polyene phosphatidyl choline as a raw material, antioxidants and related accessories necessary for corresponding forming of the preparation. The procedure is as follows: cryogenically comminuting and sieving the polyene phosphatidyl choline, and then pelletizing, granulating and drying under reduced pressure; then adding accessories for the corresponding preparation, thereby obtaining the corresponding oral solid preparation according to the procedure for corresponding forming of the preparation. The finished preparation is convenient for clinical using and has good material liquidity for production and preparation, which leads to an easy operated forming process of corresponding preparation formation with good quality control.
Owner:北京瑞伊人科技发展有限公司 +1
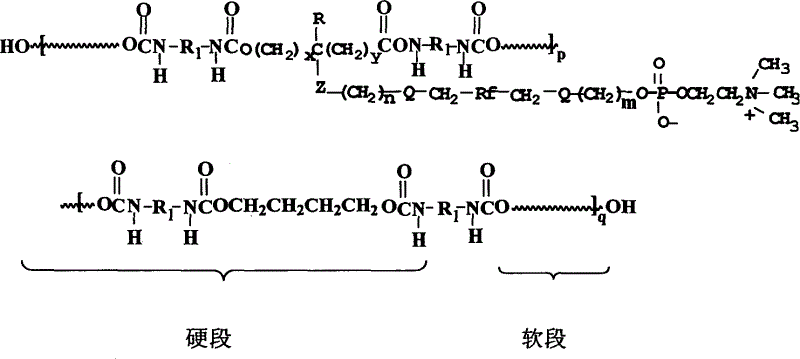
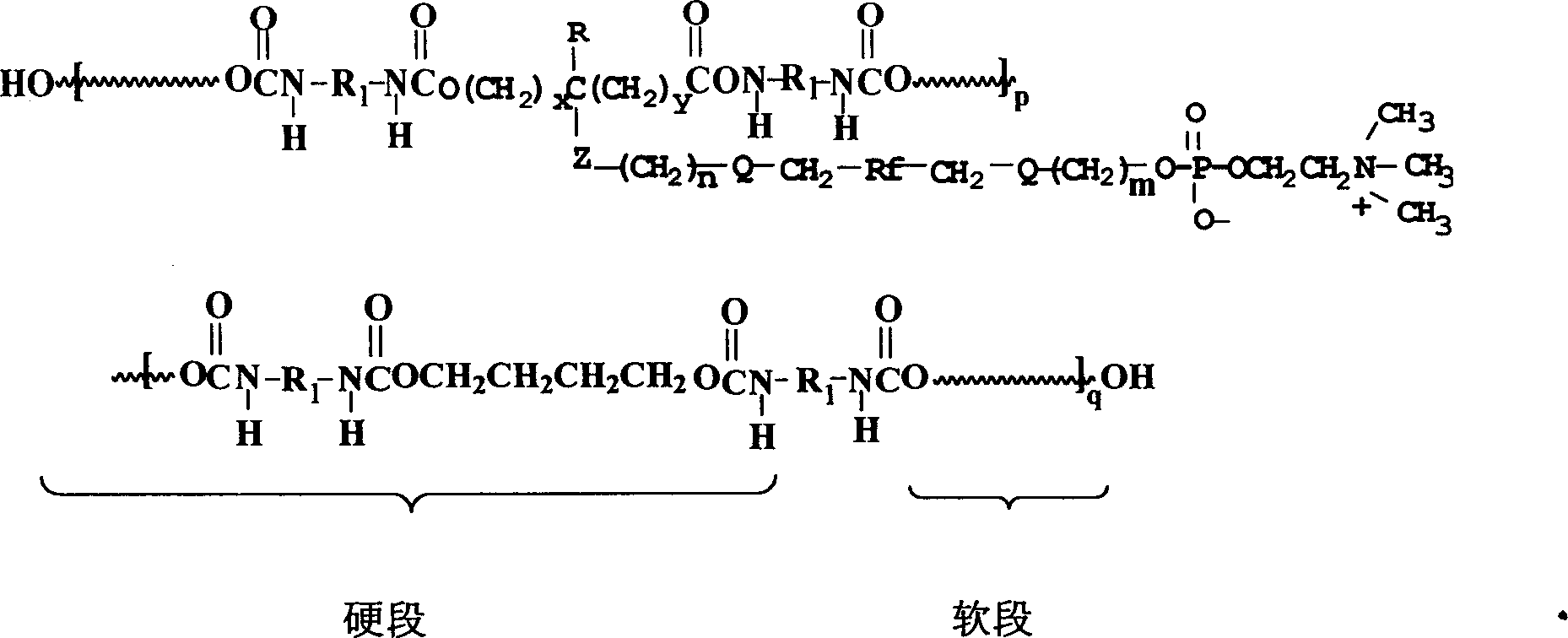
Polyurethane material with side chain possessing fluorophosphatidylcholine and its preparation method
The invention provides a polyurethane material with side chain possessing fluorophosphatidylcholine and its preparation method, which is prepared through alternating copolymerization of a soft segment composed of polyether dihydroxy alcohol and / or polycarbonate diatomic alcohol and a rigid chain segment formed by diisocyanate and chain expanding agent, characterized in that the copolymer comprises the following repetition structural elements, wherein the partial rigid chain segment side chain contains fluorine-containing phosphatidyl choline groups, whose weight average molecular weight is 30000-60000.
Owner:SICHUAN UNIV
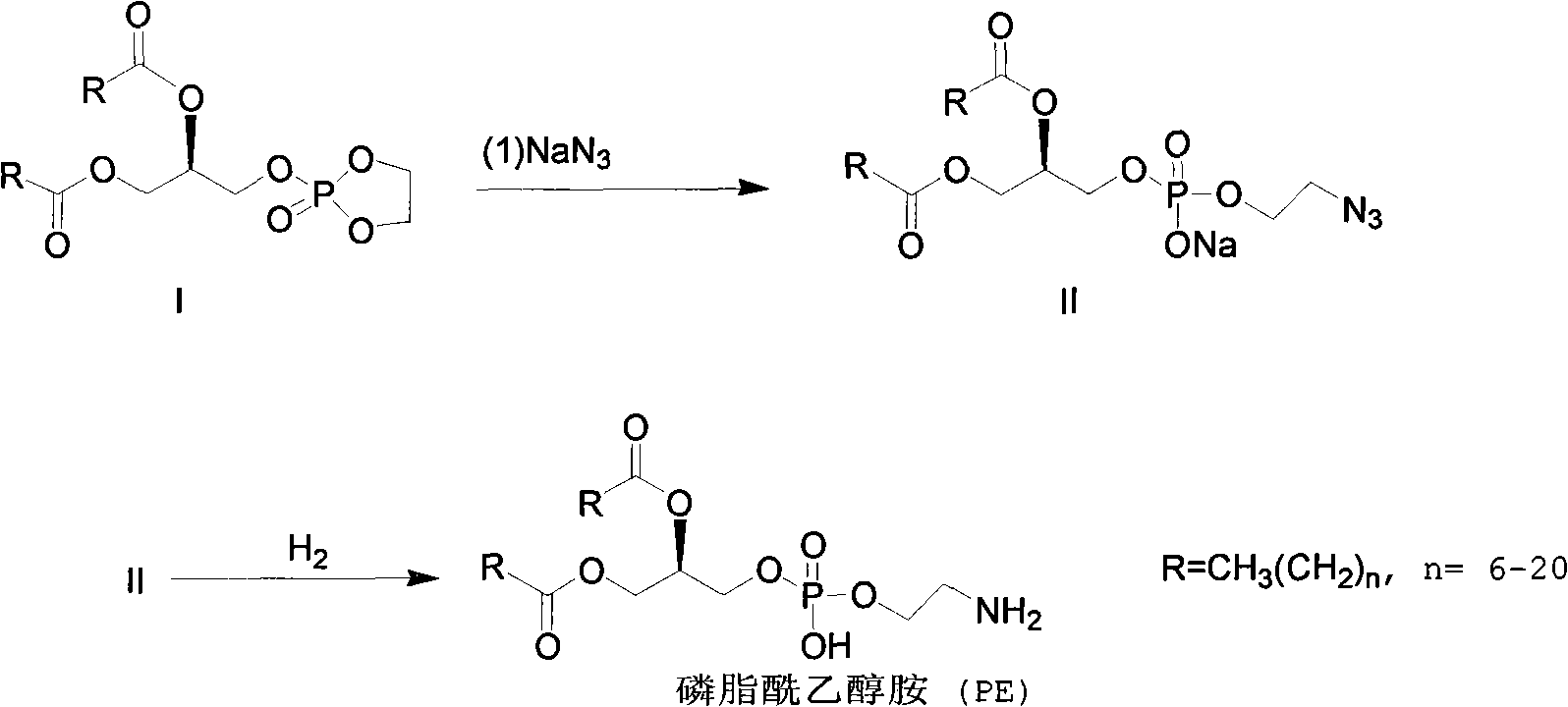
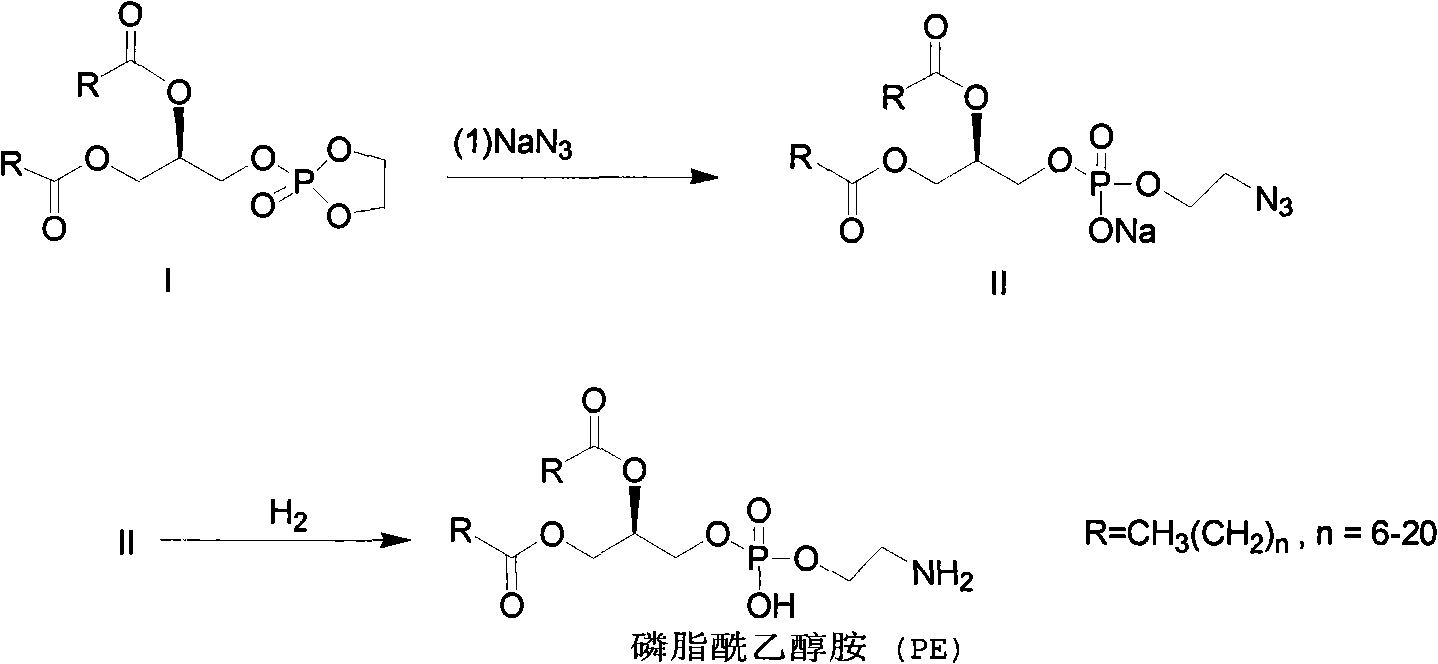
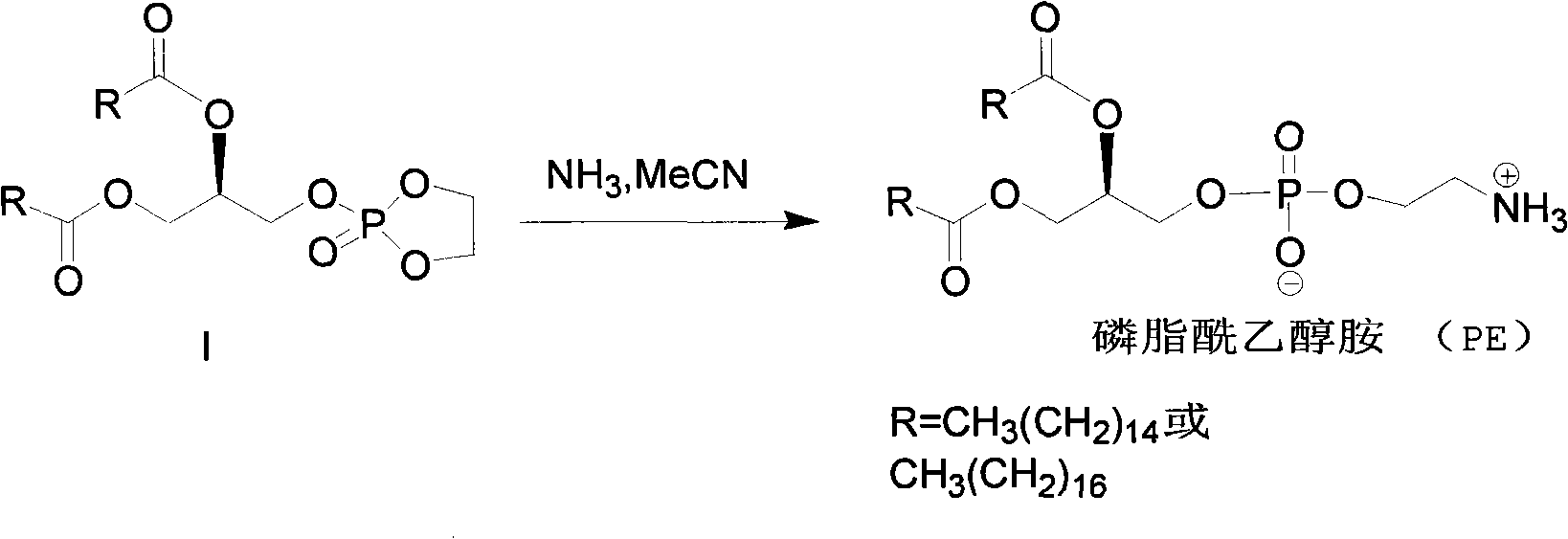
Method for synthesizing PE (Phosphatidyl Ethanolamine)
ActiveCN101805367AShort synthetic routeHigh yieldPhosphorus organic compoundsCombinatorial chemistrySodium azide
The invention provides a method for synthesizing PE (Phosphatidyl Ethanolamine), which comprises the following steps of: (a) carrying out a reaction of a compound I and sodium azide to obtain a compound II; and (b) hydrogenating the compound II to obtain the PE. The invention has short synthesis path, simple and convenient operation, low cost, high yield and easy industrial production.
Owner:EAST CHINA NORMAL UNIVERSITY +2
Coated calcium particulates for use in beverage products
Coated particulate material used to enable the incorporation of calcium materials in a beverage product (especially those having a low pH) preserved with sodium hexametaphosphate (SHMP) are disclosed. The particulates are made up of a substrate material, such as a calcium salt, such as calcium phosphate. The substrate material can, in preferred embodiments, be incorporated into a prill which utilizes a sterol as the prilling material. The substrate material, preferably in the form of a prill, is then coated with a phospholipid coating, such as hydrogenated phosphatidyl choline, such that the final coated particulate product includes from about 70% to about 200% (by weight of the substrate) of the phospholipid coating. Beverage compositions which include these coated particulates are also disclosed.
Owner:SUNNY DELIGHT BEVERAGES
Preparation method of targeted temperature-controlled water-loading silibinin temperature-sensitive lipidosome-microbubble complex drug delivery system
InactiveCN104367546AGood temperature control characteristicsEfficient releaseOrganic active ingredientsAntineoplastic agentsUltrasound contrast mediaPolyethylene glycol
The invention provides a preparation method of a targeted temperature-controlled water-loading silibinin temperature-sensitive lipidosome-microbubble complex drug delivery system. The preparation method comprises the following steps of with single-palmitoyl phosphatidyl choline, dipalmitoyl phosphatidyl choline, distearoyl phosphatidyl ethanolamine-polyethylene glycol-amino and silibinin as raw materials, preparing water-loading silibinin temperature-sensitive lipidosome by virtue of a membrane rotary evaporation method; and coupling the water-loading silibinin temperature-sensitive lipidosome with an ultrasonic contrast agent, namely SonoVue microbubble, so as to prepare the targeted temperature-controlled water-loading silibinin temperature-sensitive lipidosome-microbubble complex drug delivery system. According to the preparation method, a new drug delivery system is established by combining the temperature-sensitive lipidosome with the microbubble; the efficient release of local tissues of a drug at a sub-high temperature field are achieved by virtue of the targeted explosion of the microbubble and the local temperature control effect of the temperature-sensitive lipidosome, so that the bioavailability of the drug is improved, and furthermore, residual tumors during thermal ablation treatment are reduced.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Pharmaceutical compositions and methods for lowering blood pressure and pulse rate
InactiveUS20050043274A1Lower blood pressureLowering pulse rateBiocidePeptide/protein ingredientsMedicinePulse rate
A pharmaceutical composition comprising (1) a compound that is converted to a glycosaminoglycan in a patient; (2) a primary antioxidant component; (3) at least one amino acid component; and (4) one or more of lecithin, phosphatidyl choline, or choline and methods for lowering blood pressure and / or pulse rate in a patient by administering to the patient the pharmaceutical composition.
Owner:MURAD HOWARD
Somatostatin receptor agonist formulations
The present invention relates to compositions forming a low viscosity mixture of: a) 20-50 wt.% of at least one diacyl glycerol; b) 20-54 wt.% of at least one phosphatidyl choline (PC); c) 5-15wt.% of at least one biocompatible, organic mono-alcoholic solvent; d) 1 to 20 wt.% polar solvent e) 5 to 150 mg / ml of at least one peptide somatostatin receptor agonist comprising pasireotide; f) optionally at least one antioxidant; wherein the ratio of components a:b is in the range 40:60 to 54:46; wherein the pre-formulation forms, or is capable of forming, at least one liquid crystalline phase structure upon contact with excess aqueous fluid. The invention further relates to methods of treatment comprising administration of such compositions, and to pre-filled administration devices and kits containing the formulations.
Owner:CAMURUS AB
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com