Patents
Literature
94 results about "Surgical removal" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Surgical removal. During surgical removal, the skin is numbed with an injection of a local anesthetic. The tattoo is removed with a scalpel, and the edges of skin are stitched back together. After the procedure, antibacterial ointment helps promote healing.
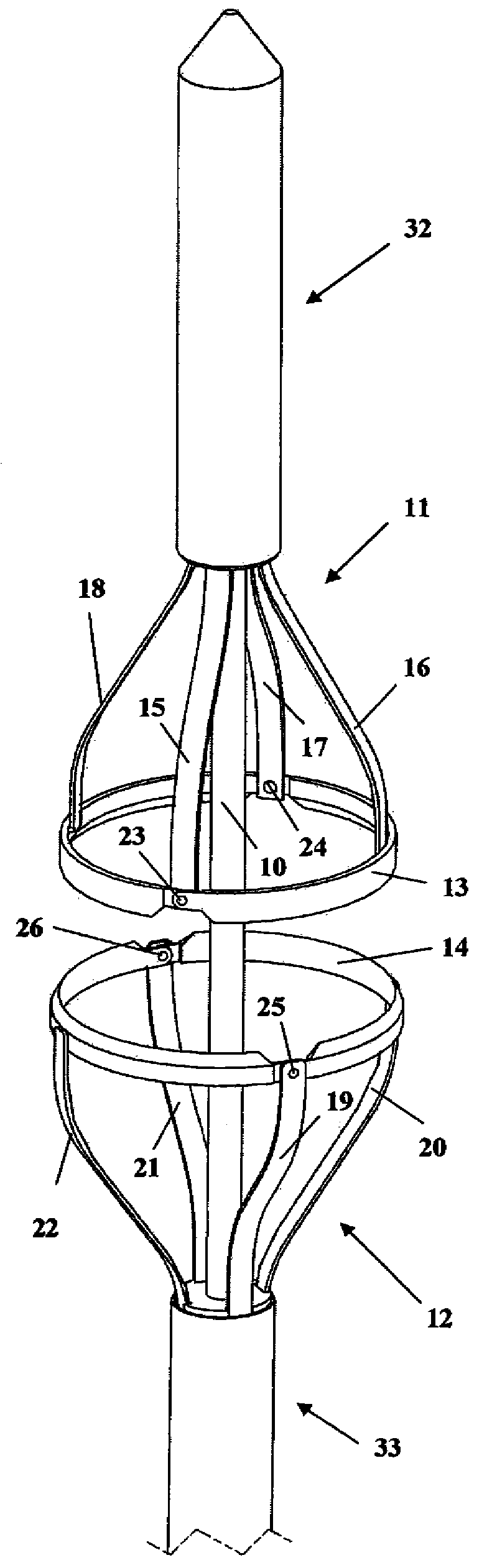
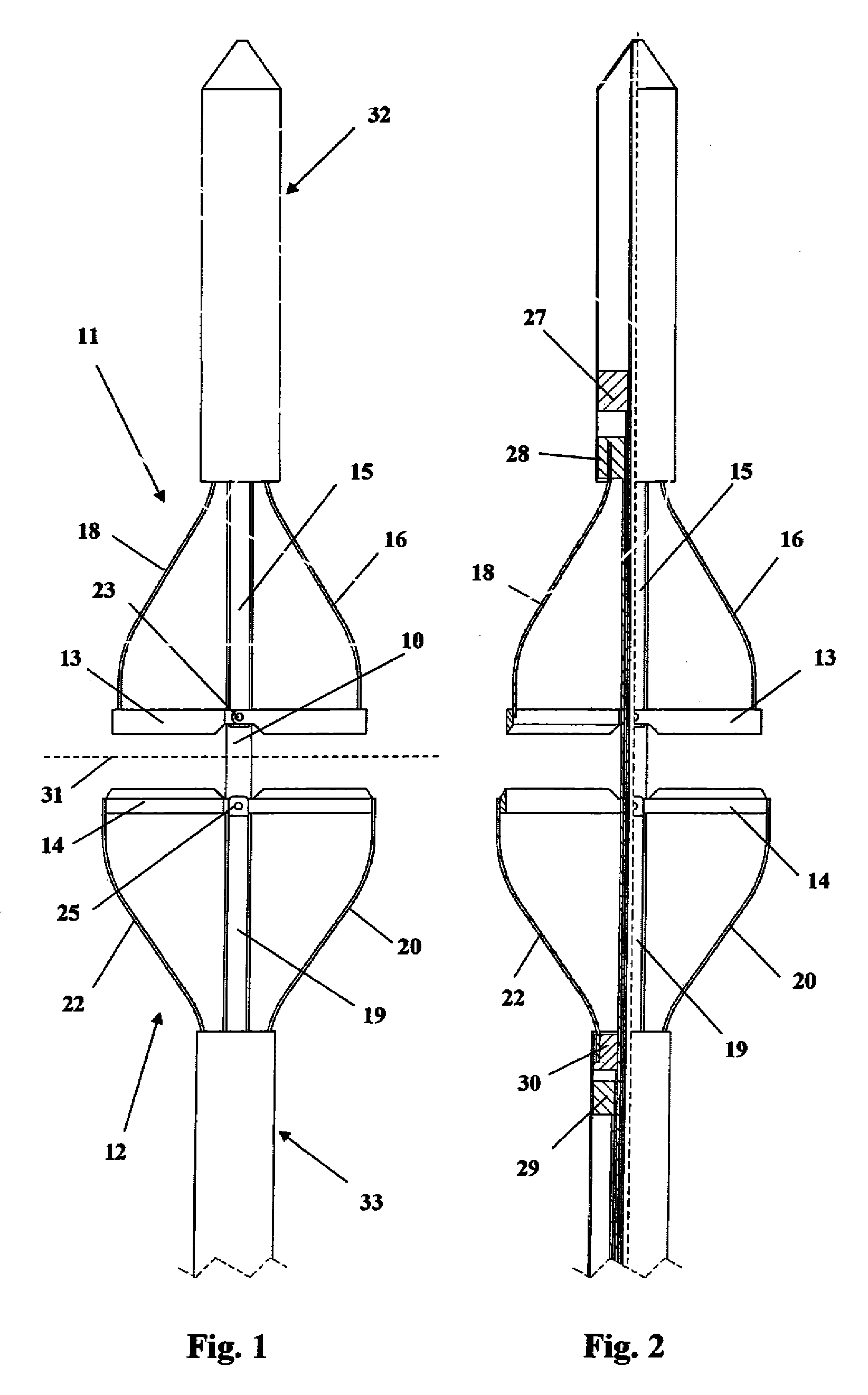
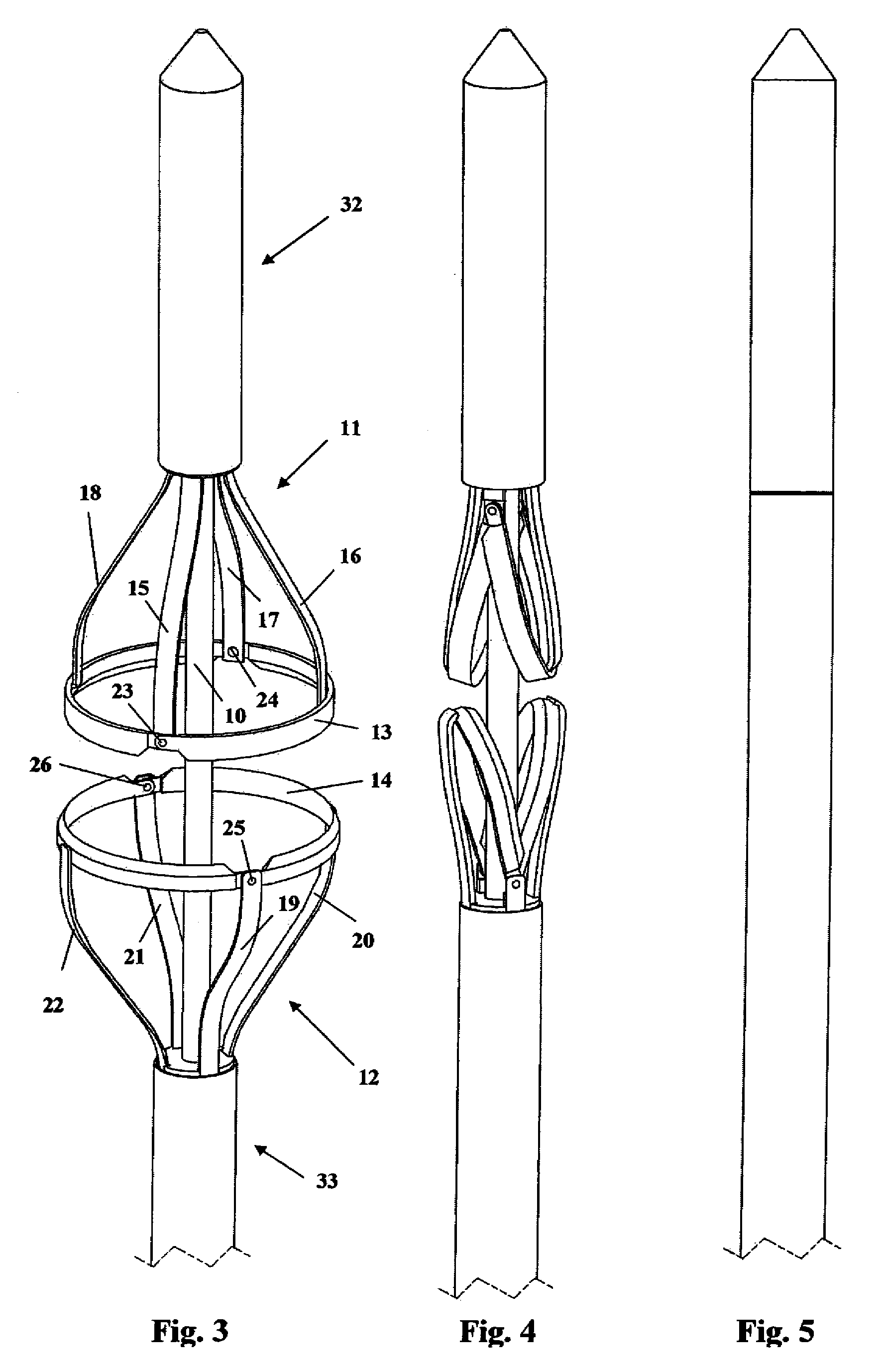
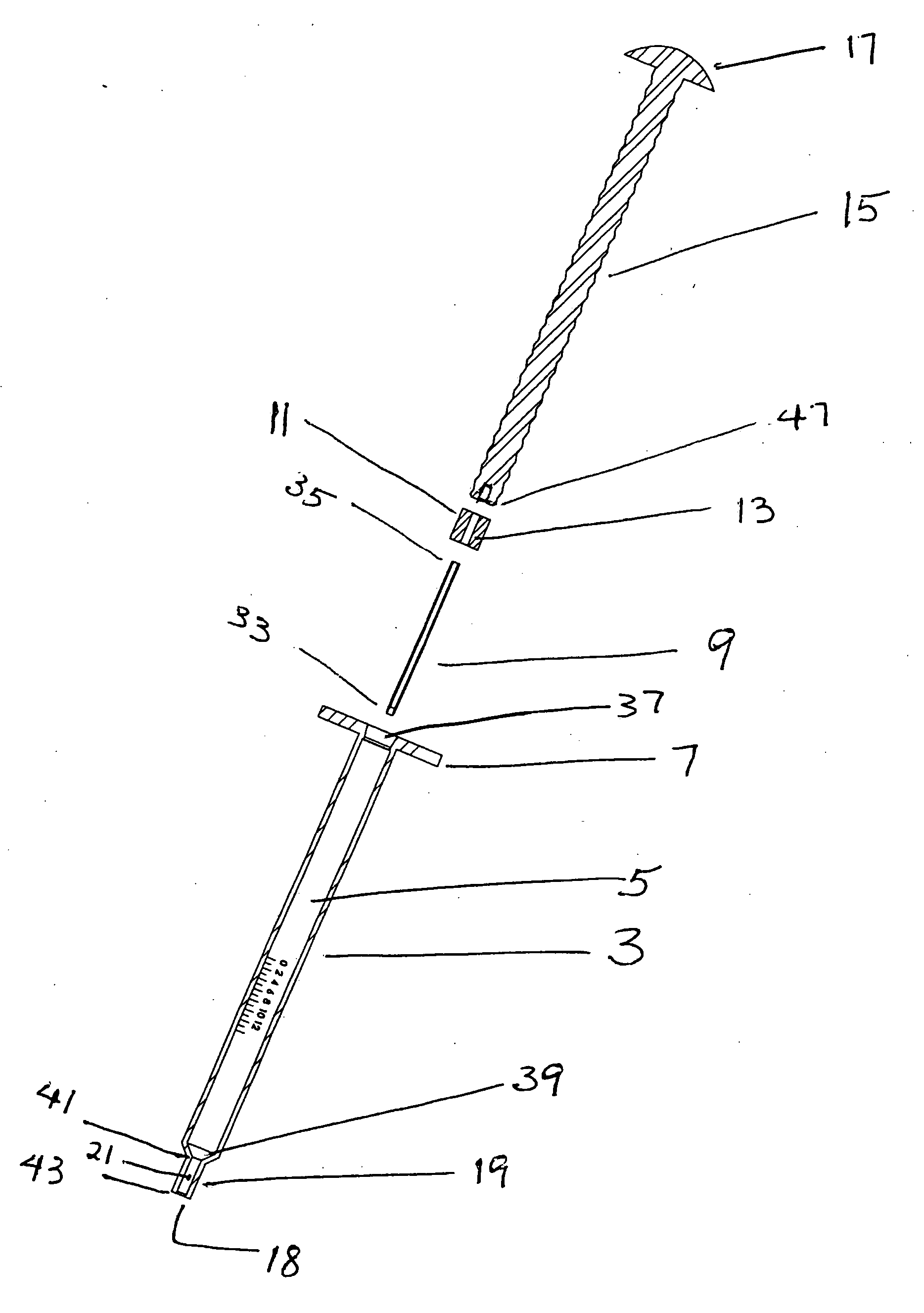
Devices and methods for selective surgical removal of tissue
ActiveUS7738969B2Improve securityAvoid injuryCannulasAnti-incontinence devicesSurgical removalVascular structure
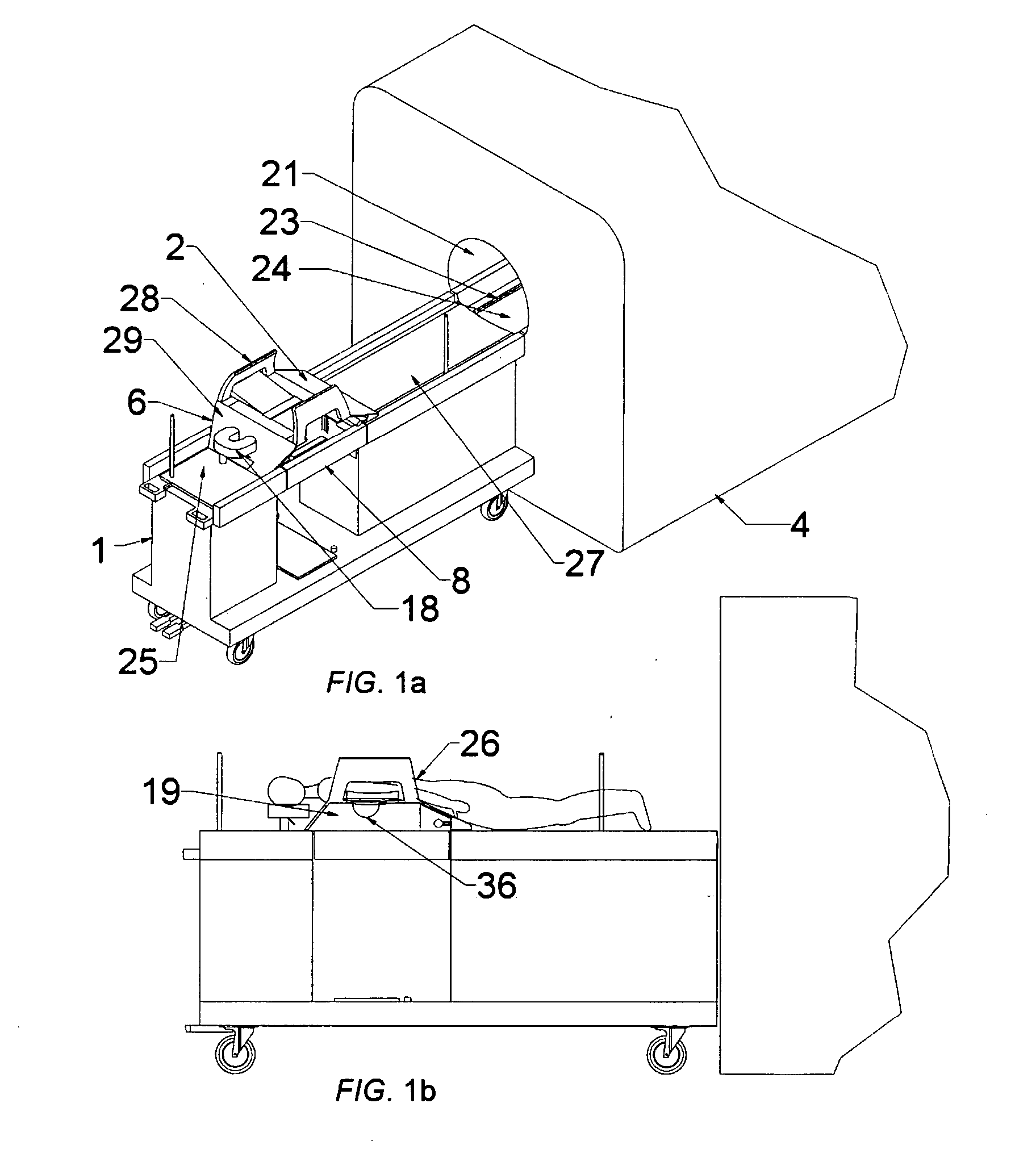
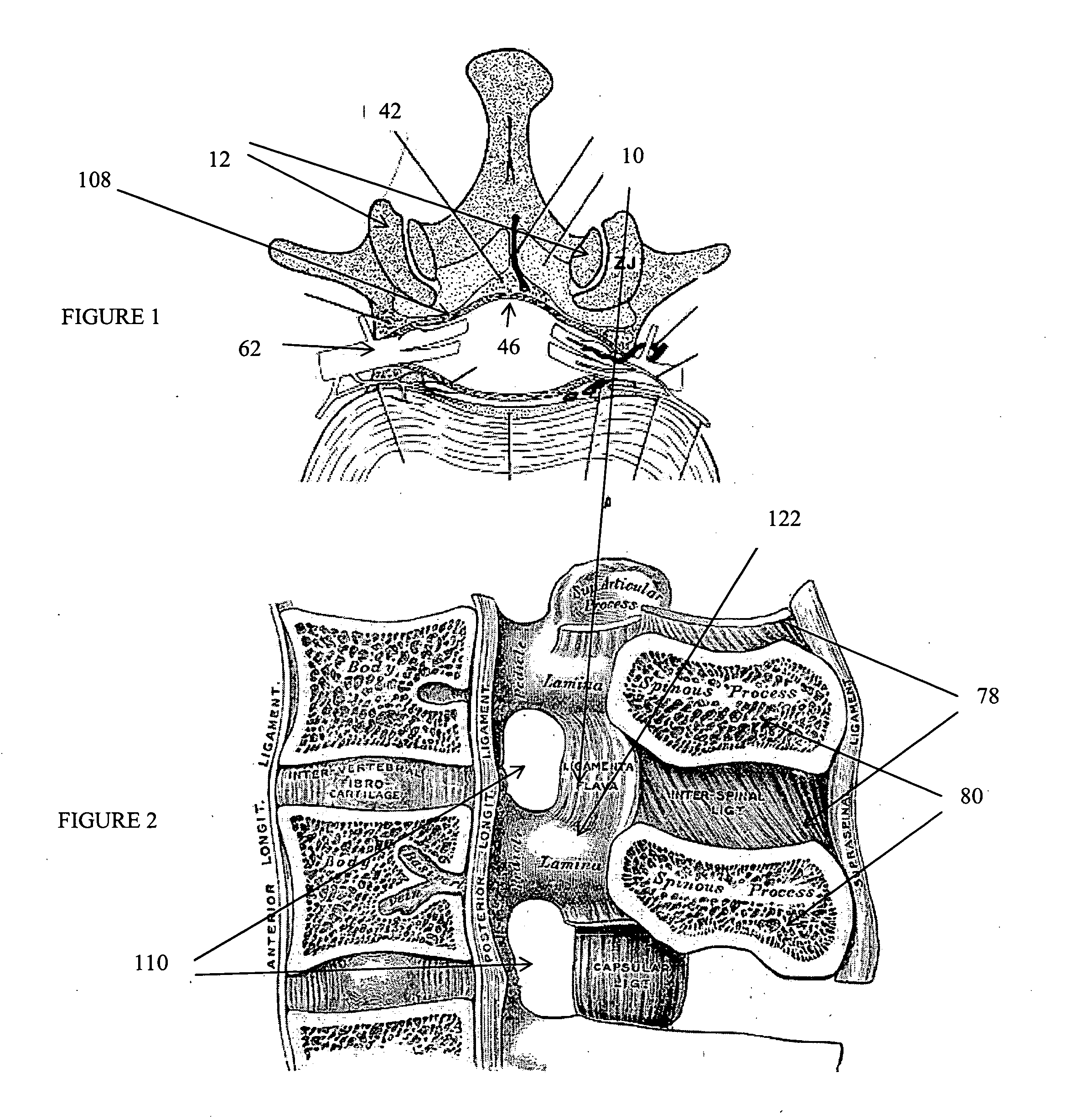
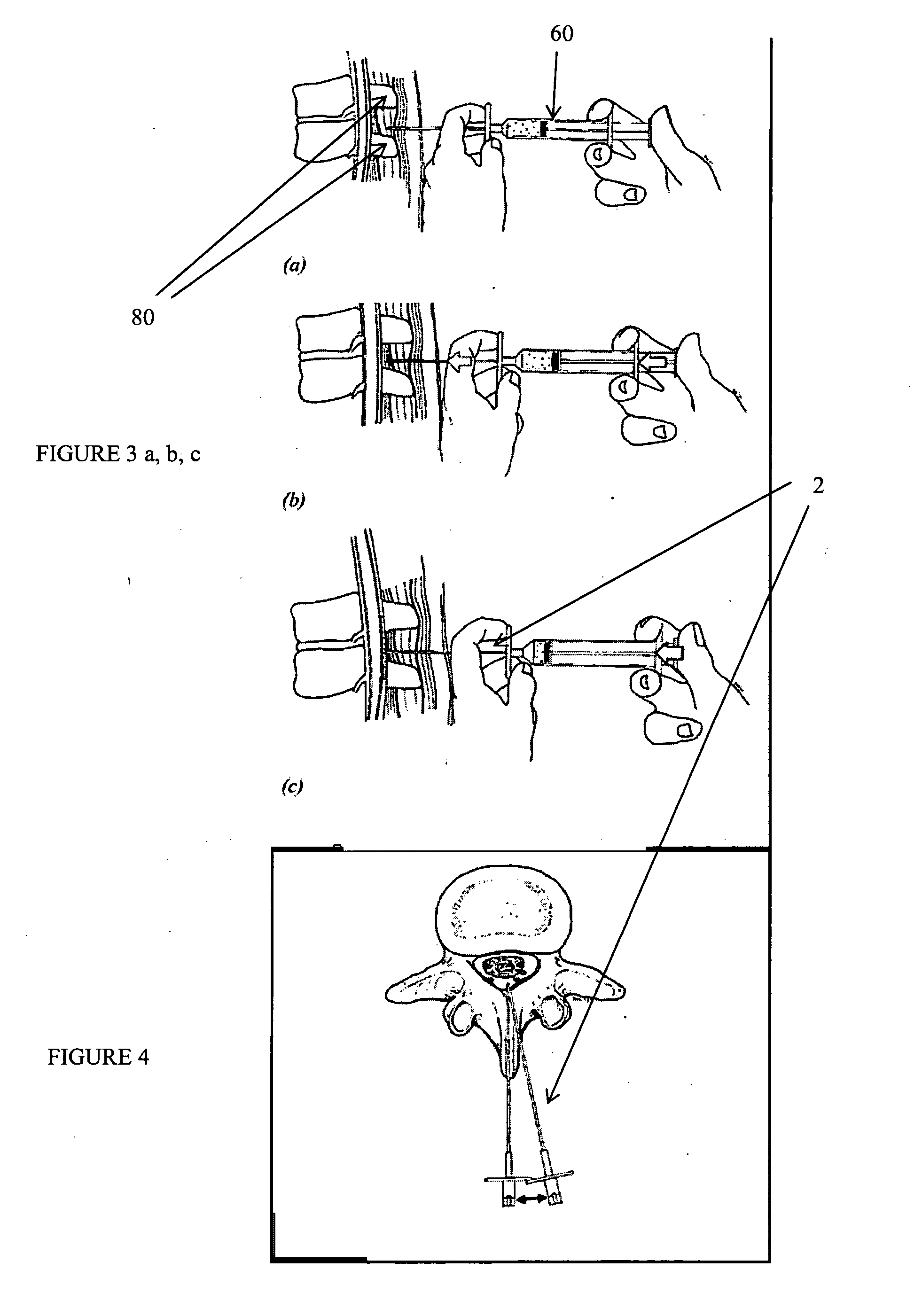
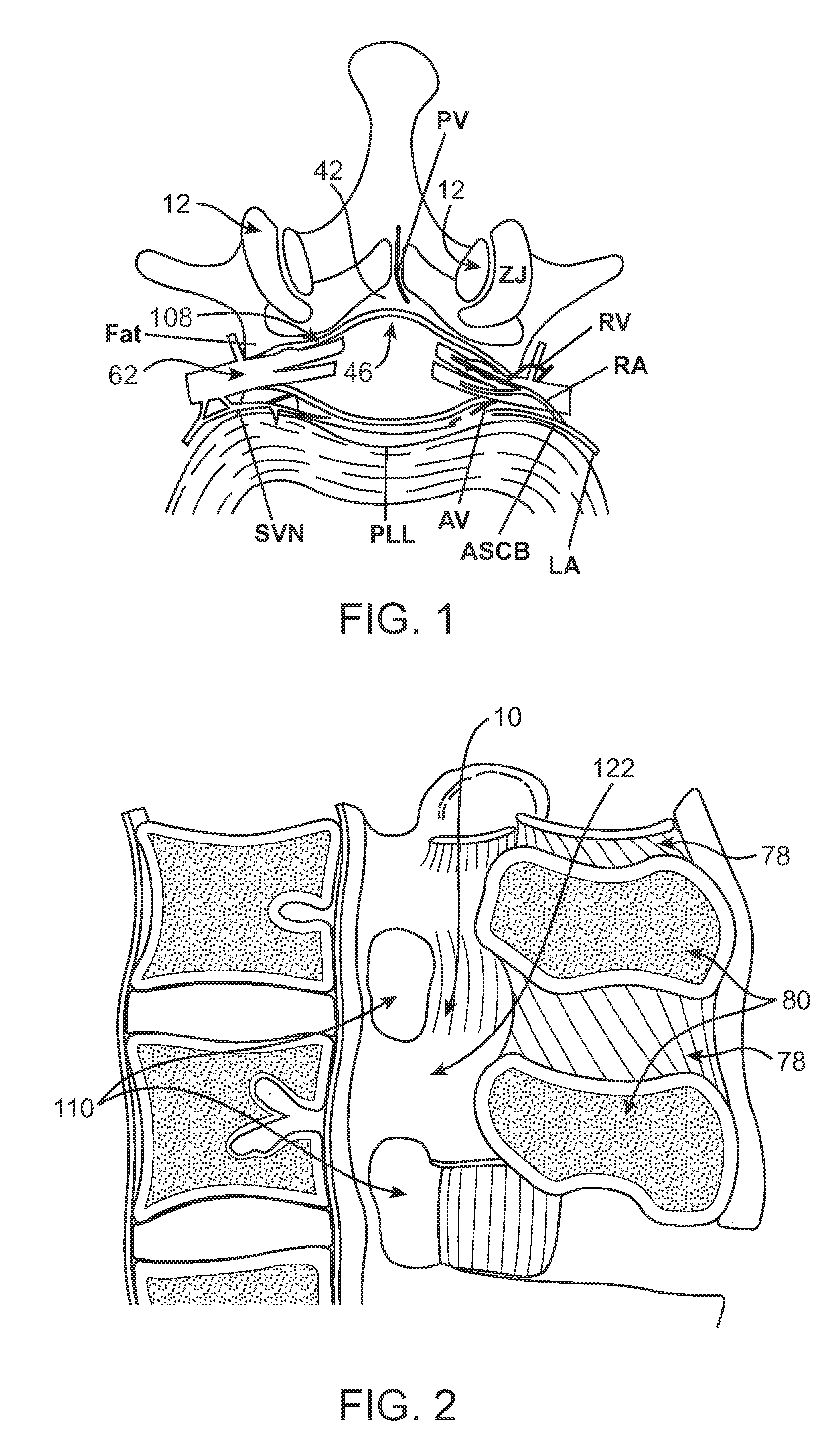
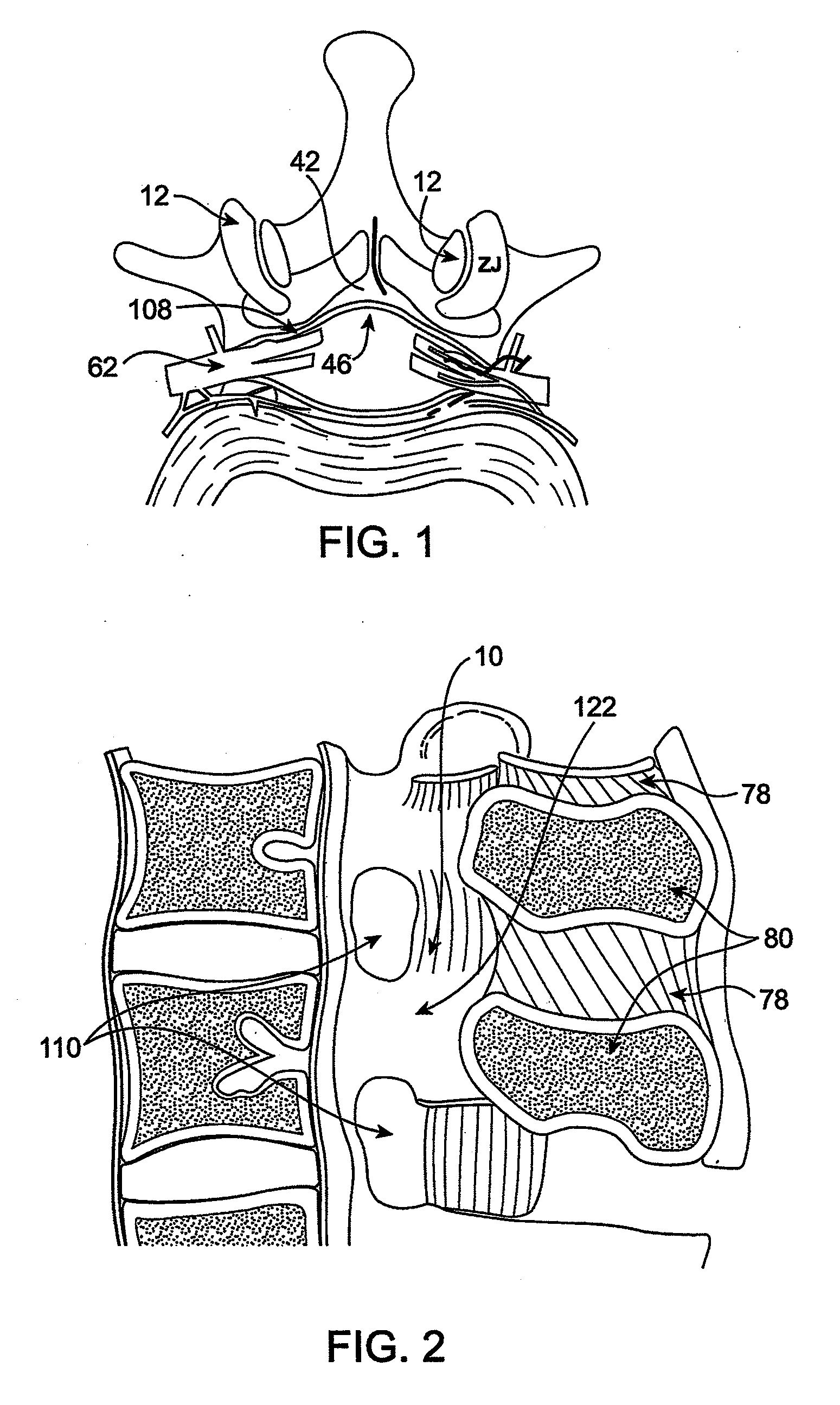
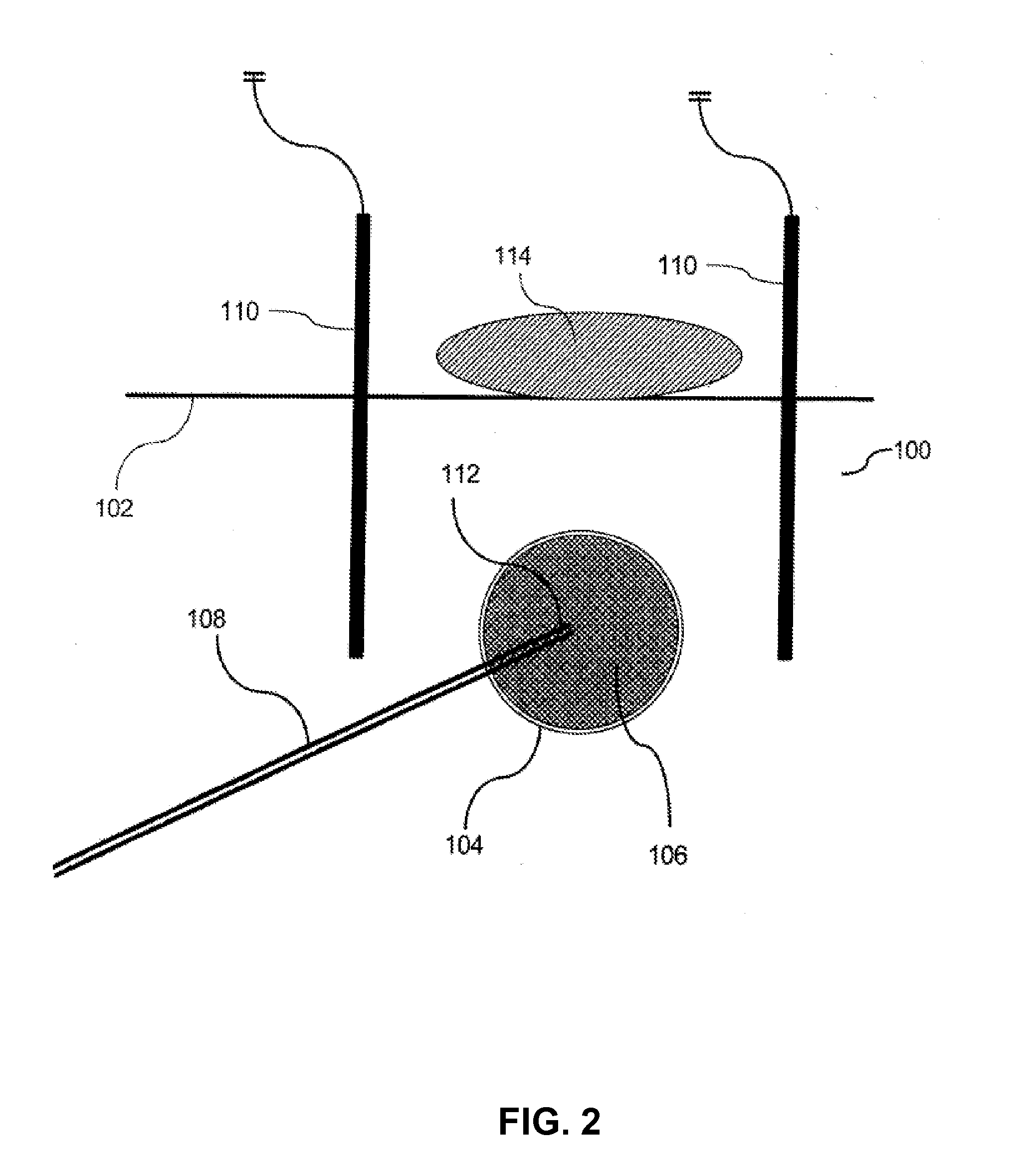
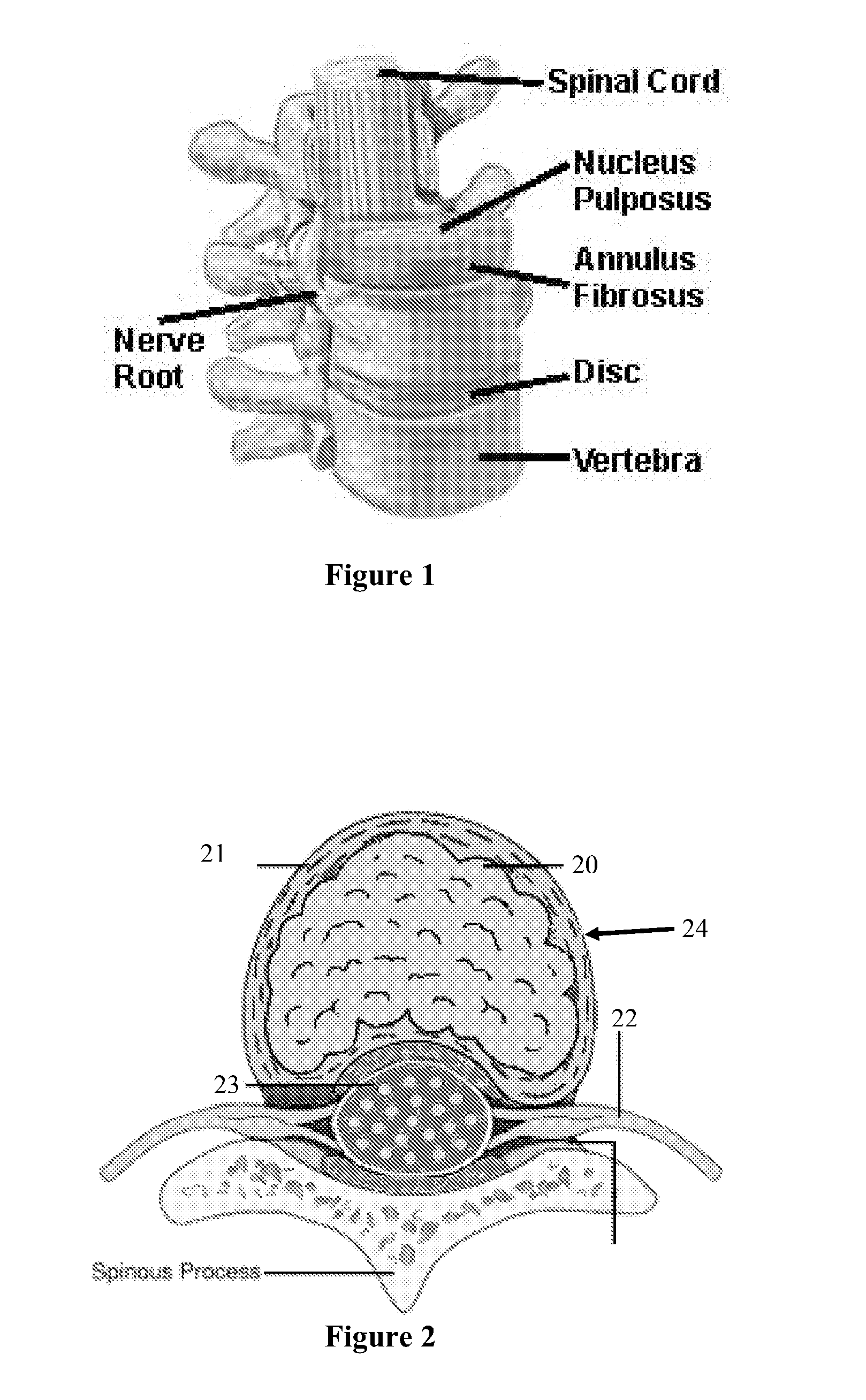
Methods and apparatus are provided for selective surgical removal of tissue. In one variation, tissue may be ablated, resected, removed, or otherwise remodeled by standard small endoscopic tools delivered into the epidural space through an epidural needle. The sharp tip of the needle in the epidural space, can be converted to a blunt tipped instrument for further safe advancement. The current invention includes specific tools that enable safe tissue modification in the epidural space, including a barrier that separates the area where tissue modification will take place from adjacent vulnerable neural and vascular structures. A nerve stimulator may be provided to reduce a risk of inadvertent neural abrasion.
Owner:MIS IP HLDG LLC +1
Hybrid imaging method to monitor medical device delivery and patient support for use in the method
ActiveUS20050080333A1Easy procedureConvenient treatmentSurgical needlesStretcherLiver and kidneySurgical removal
This invention discloses a method and apparatus to deliver medical devices to targeted locations within human tissues using imaging data. The method enables the target location to be obtained from one imaging system, followed by the use of a second imaging system to verify the final position of the device. In particular, the invention discloses a method based on the initial identification of tissue targets using MR imaging, followed by the use of ultrasound imaging to verify and monitor accurate needle positioning. The invention can be used for acquiring biopsy samples to determine the grade and stage of cancer in various tissues including the brain, breast, abdomen, spine, liver, and kidney. The method is also useful for delivery of markers to a specific site to facilitate surgical removal of diseased tissue, or for the targeted delivery of applicators that destroy diseased tissues in-situ.
Owner:INVIVO CORP
Devices and methods for tissue access
ActiveUS20060089633A1Easy to disassembleEliminate needCannulasAnti-incontinence devicesSurgical departmentNerve stimulation
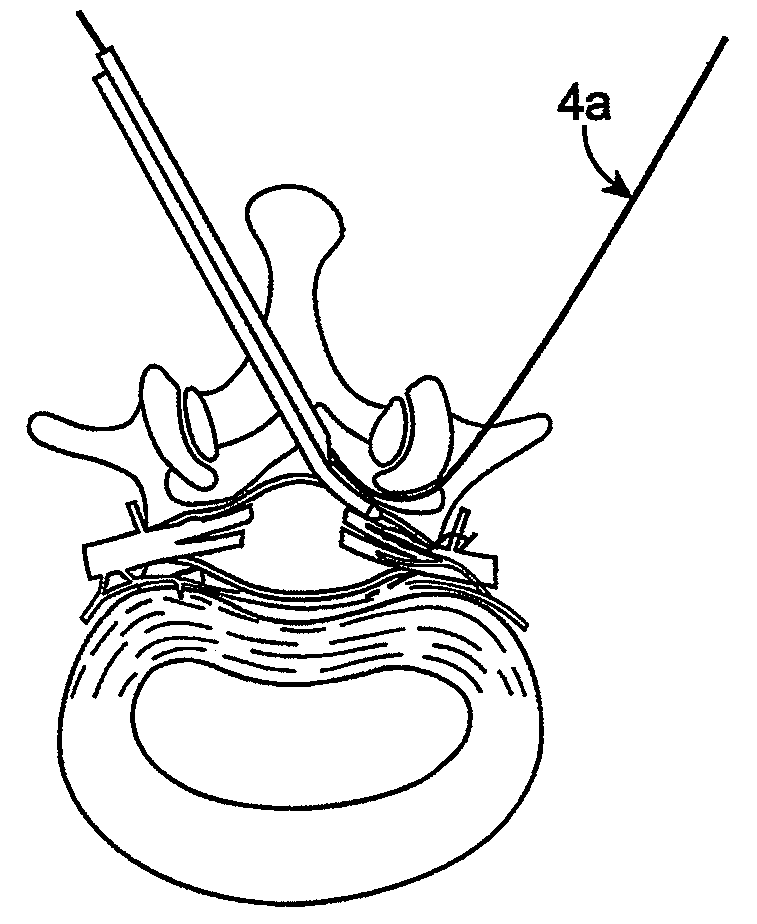
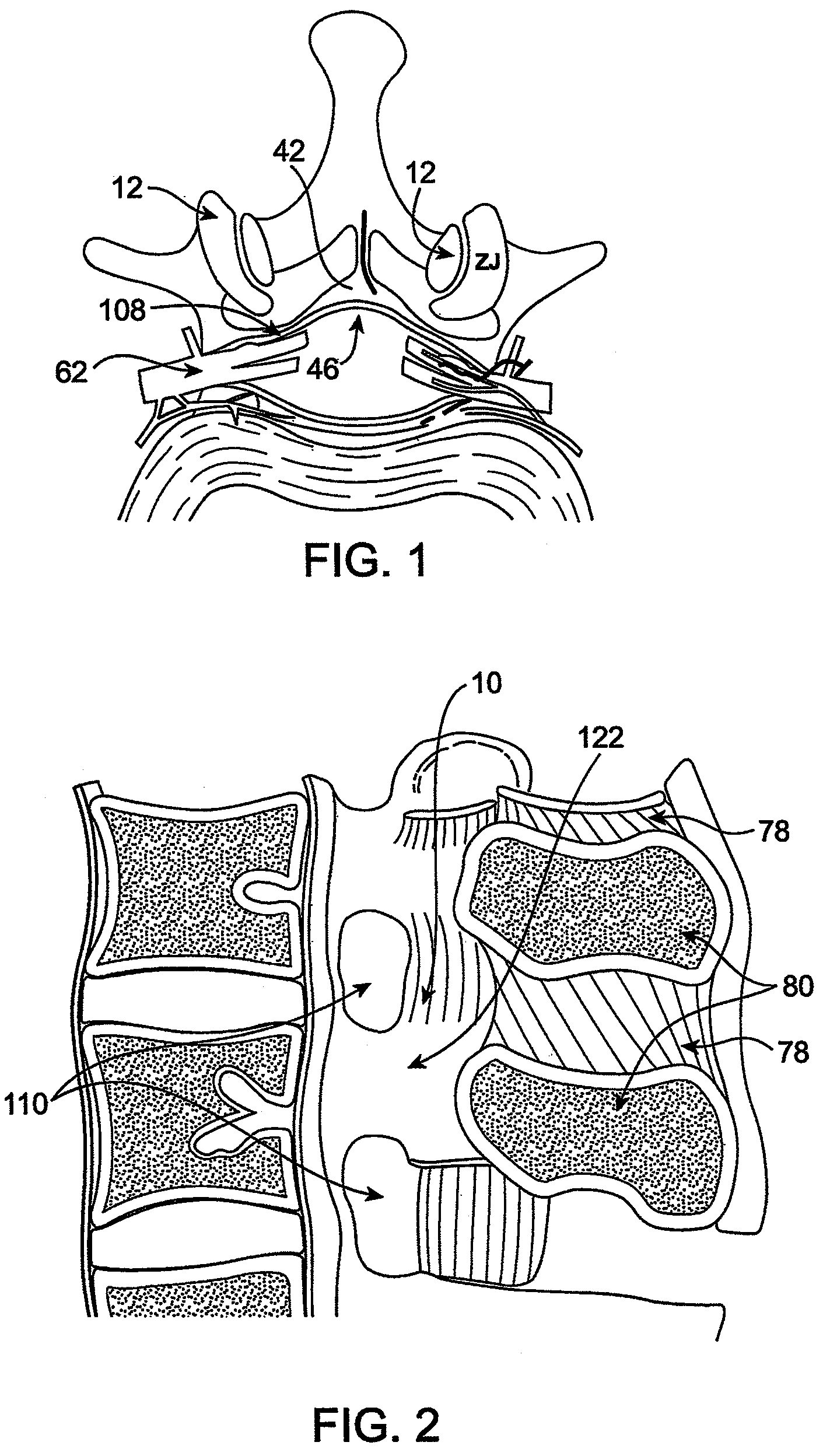
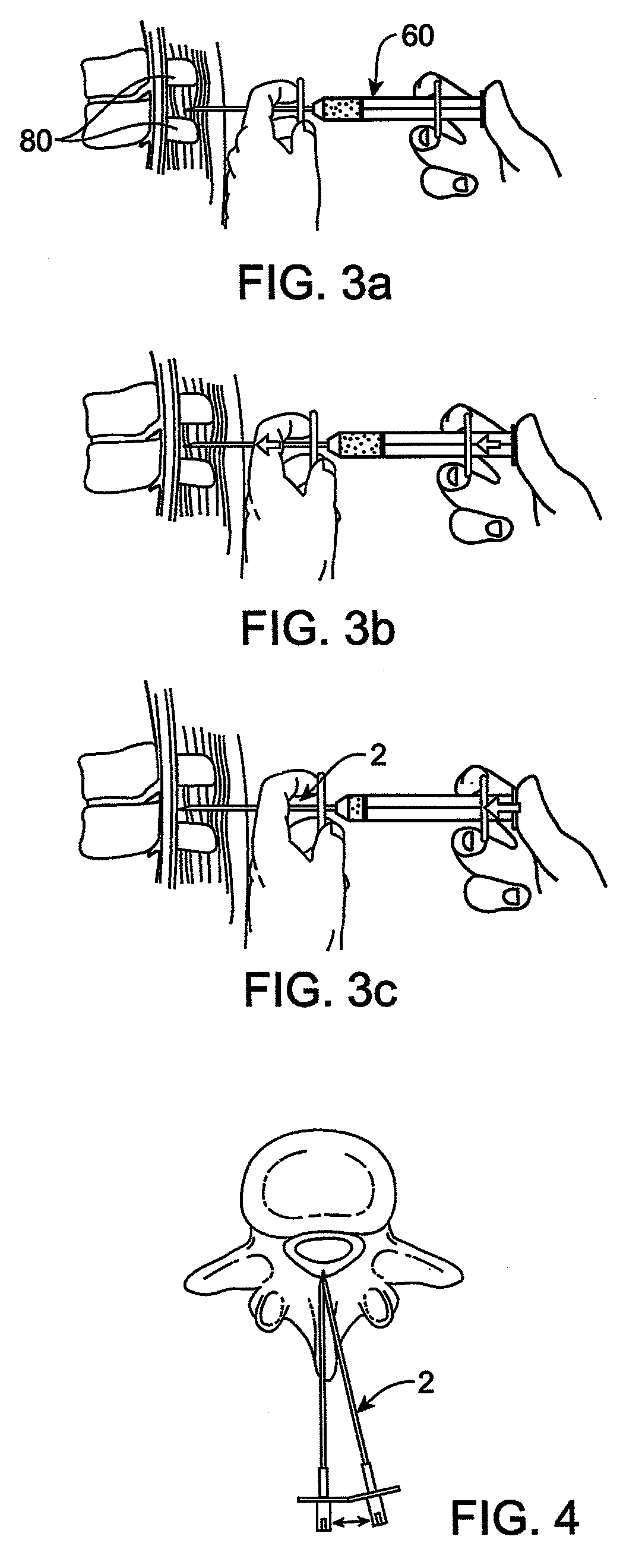
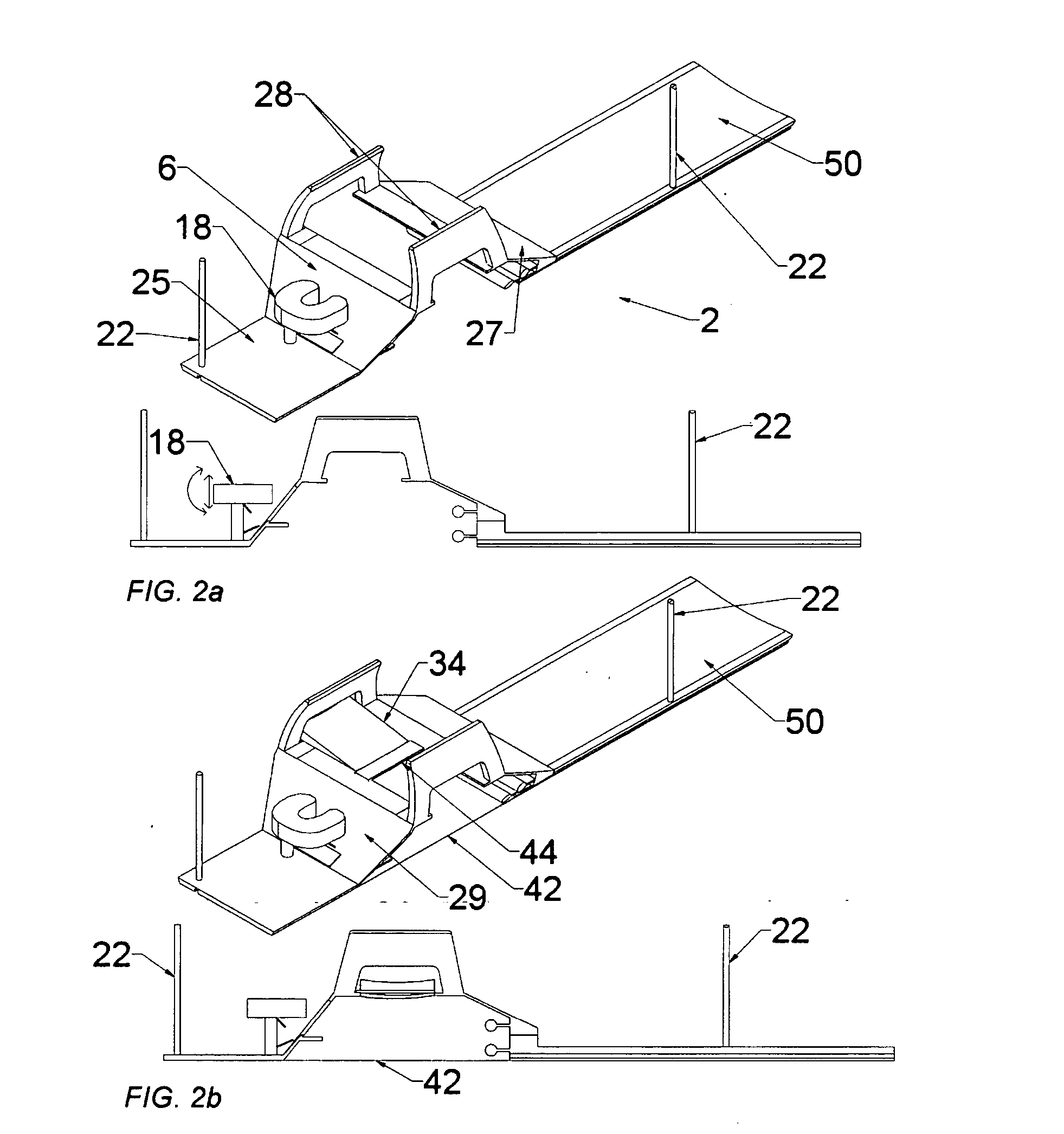
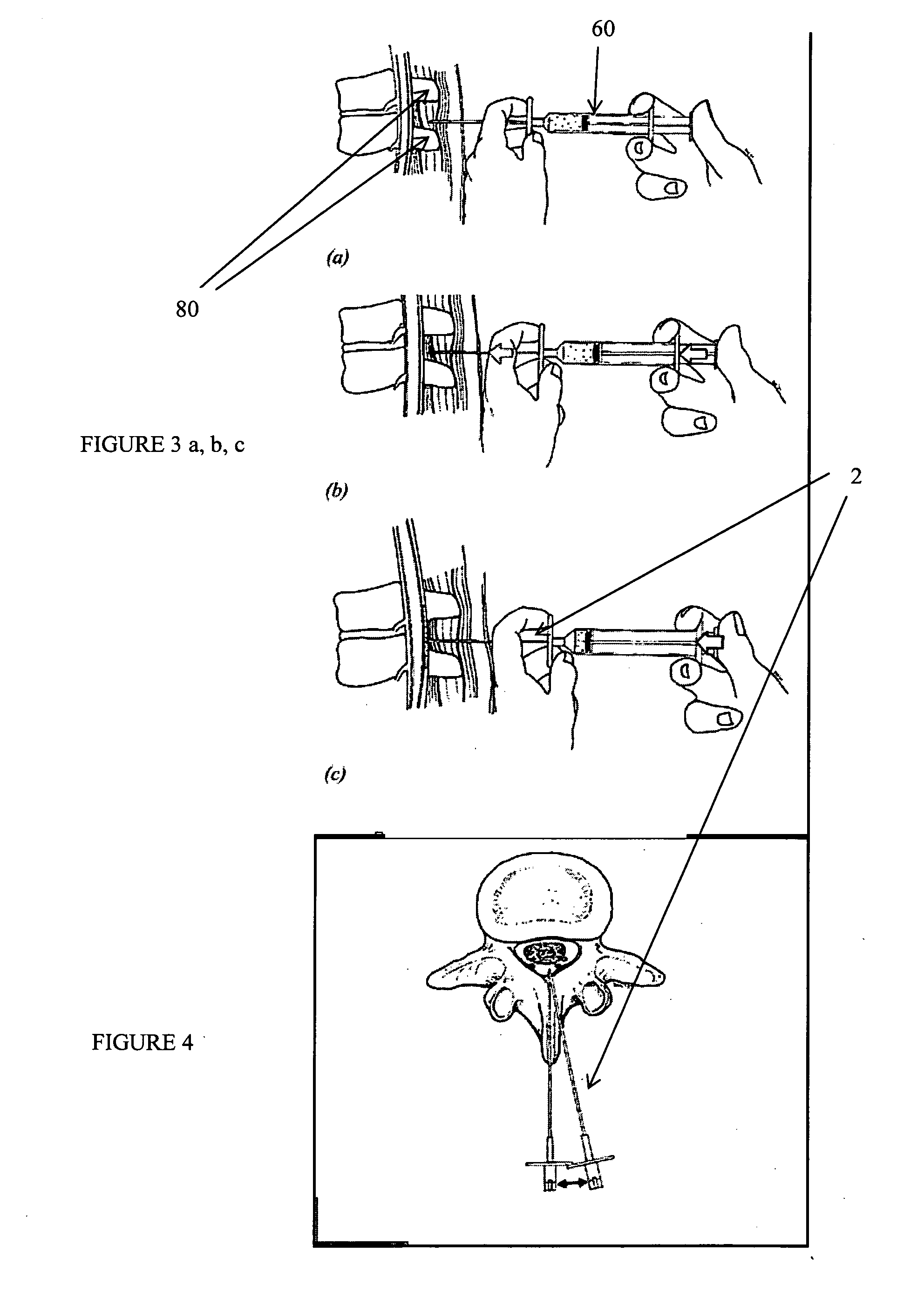
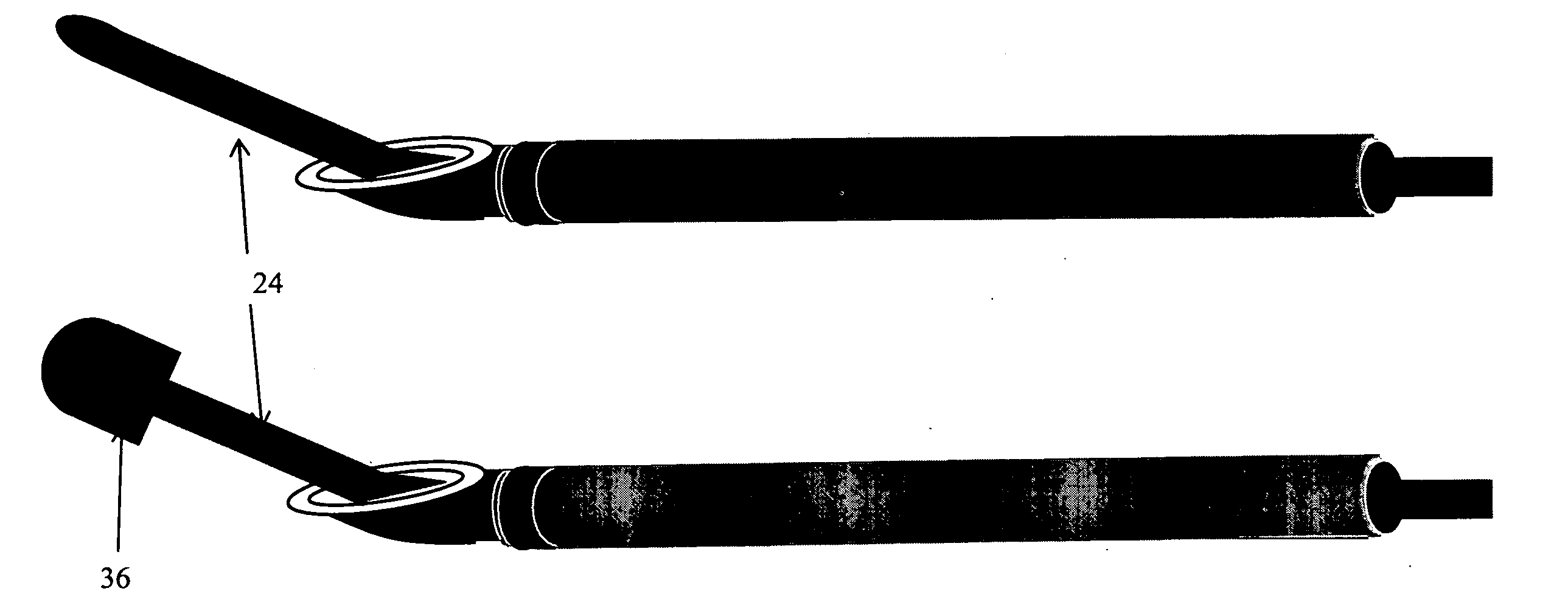
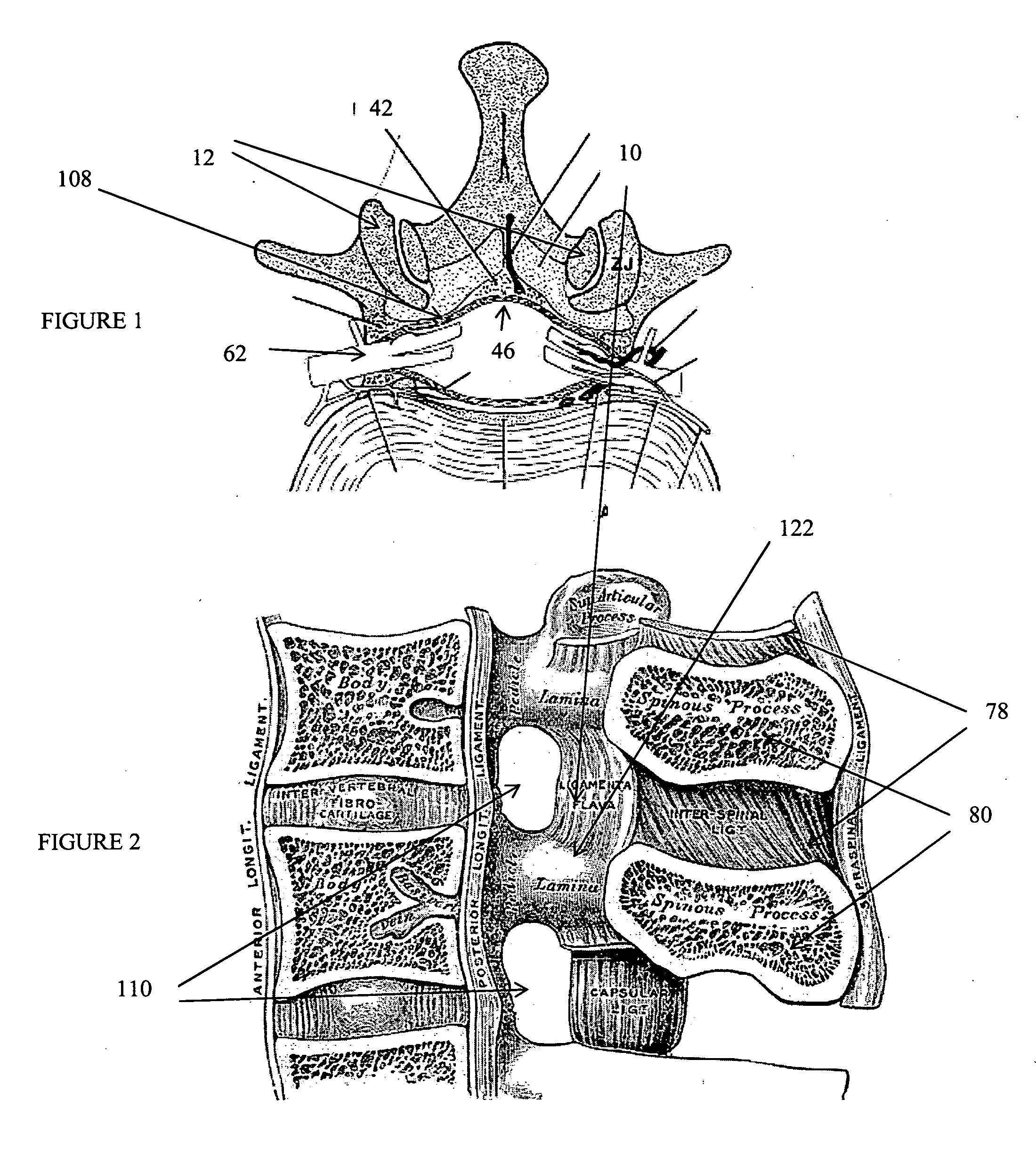
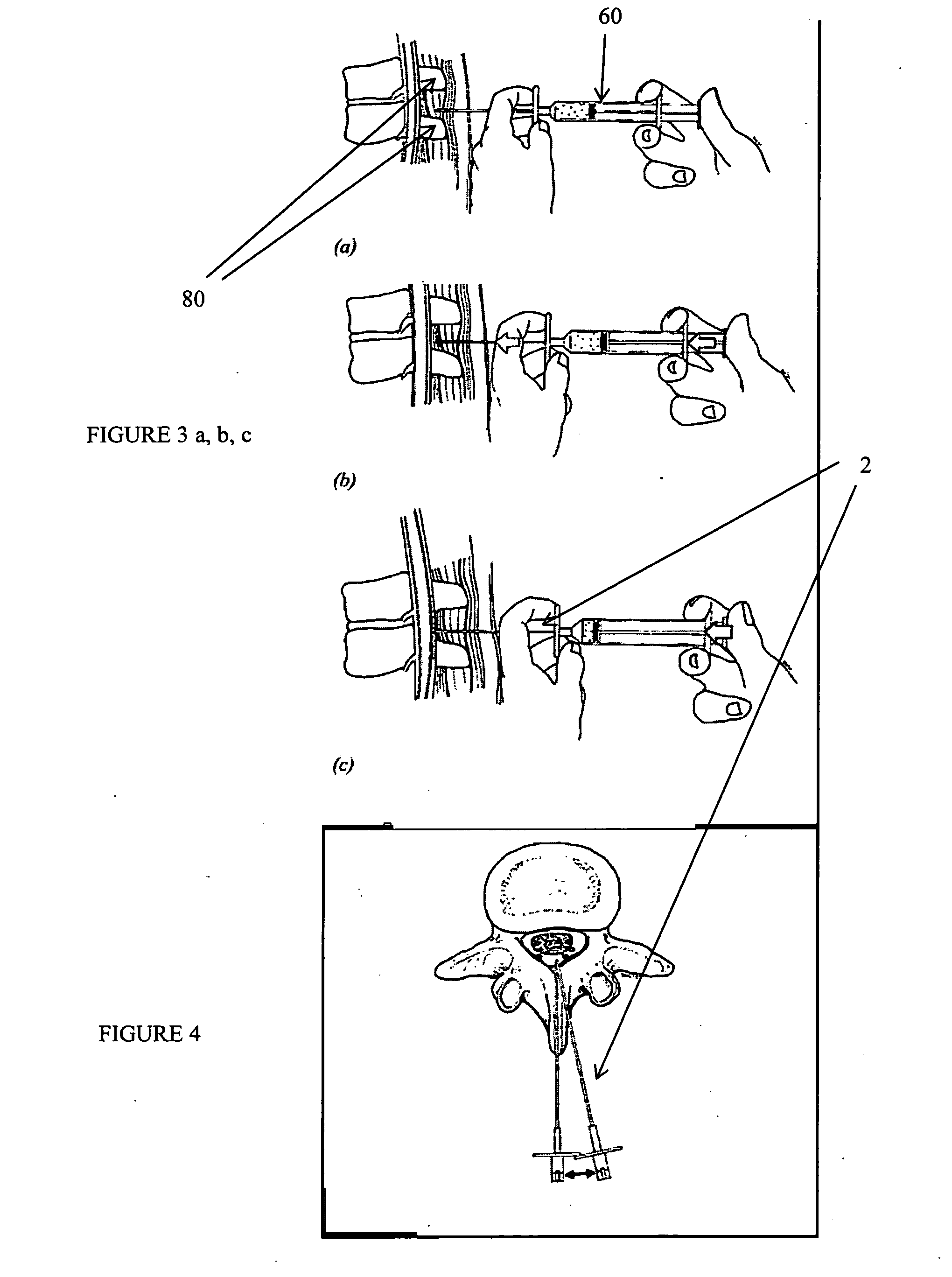
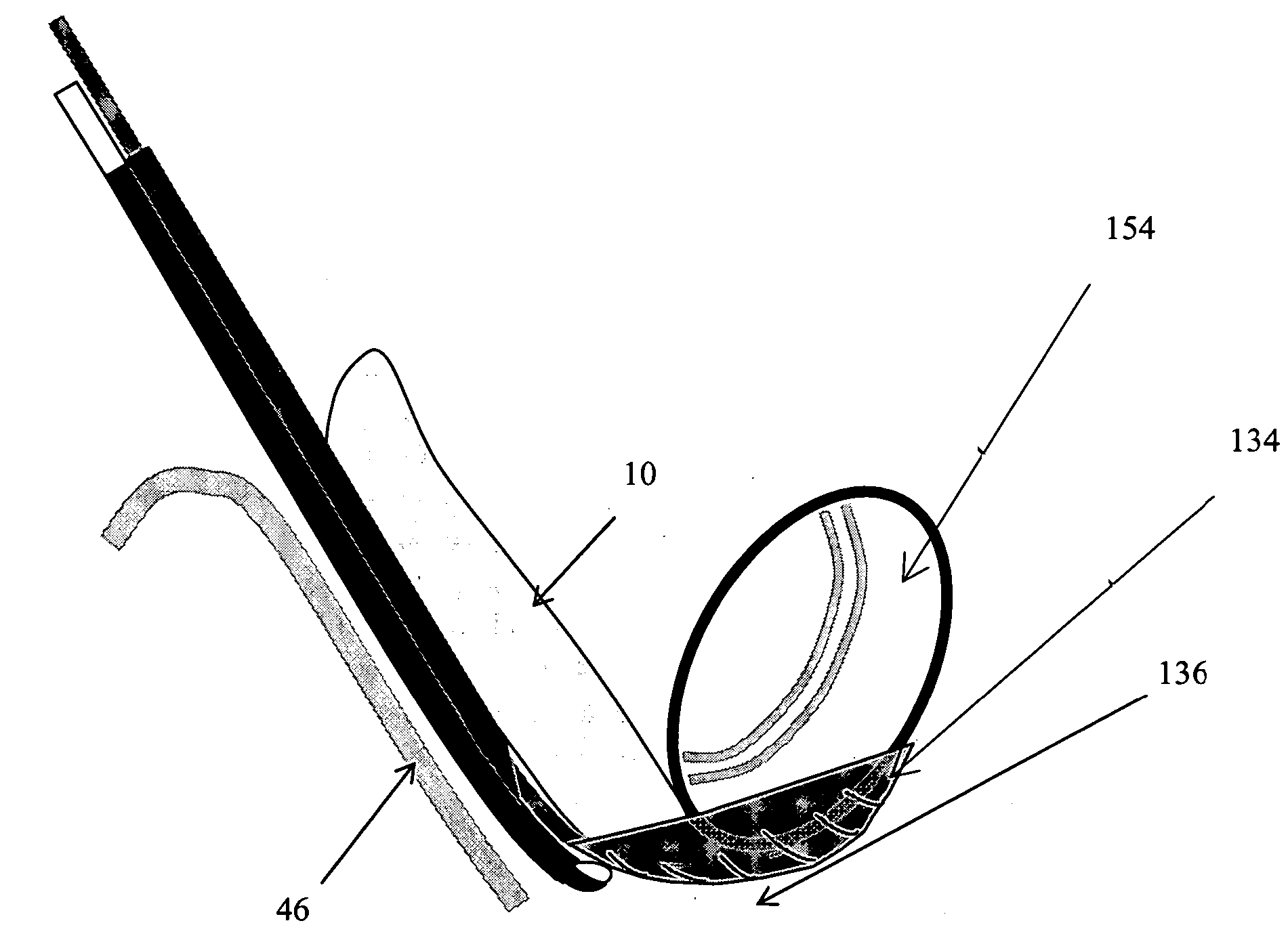
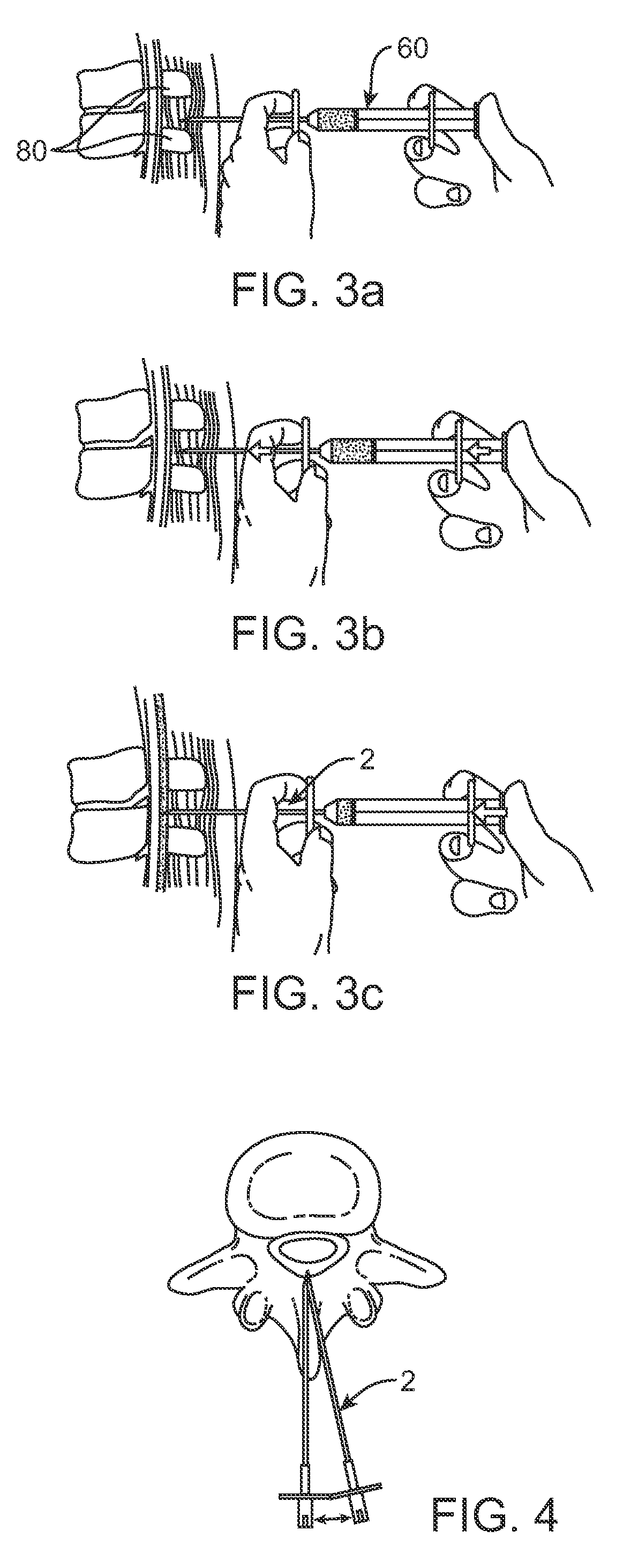
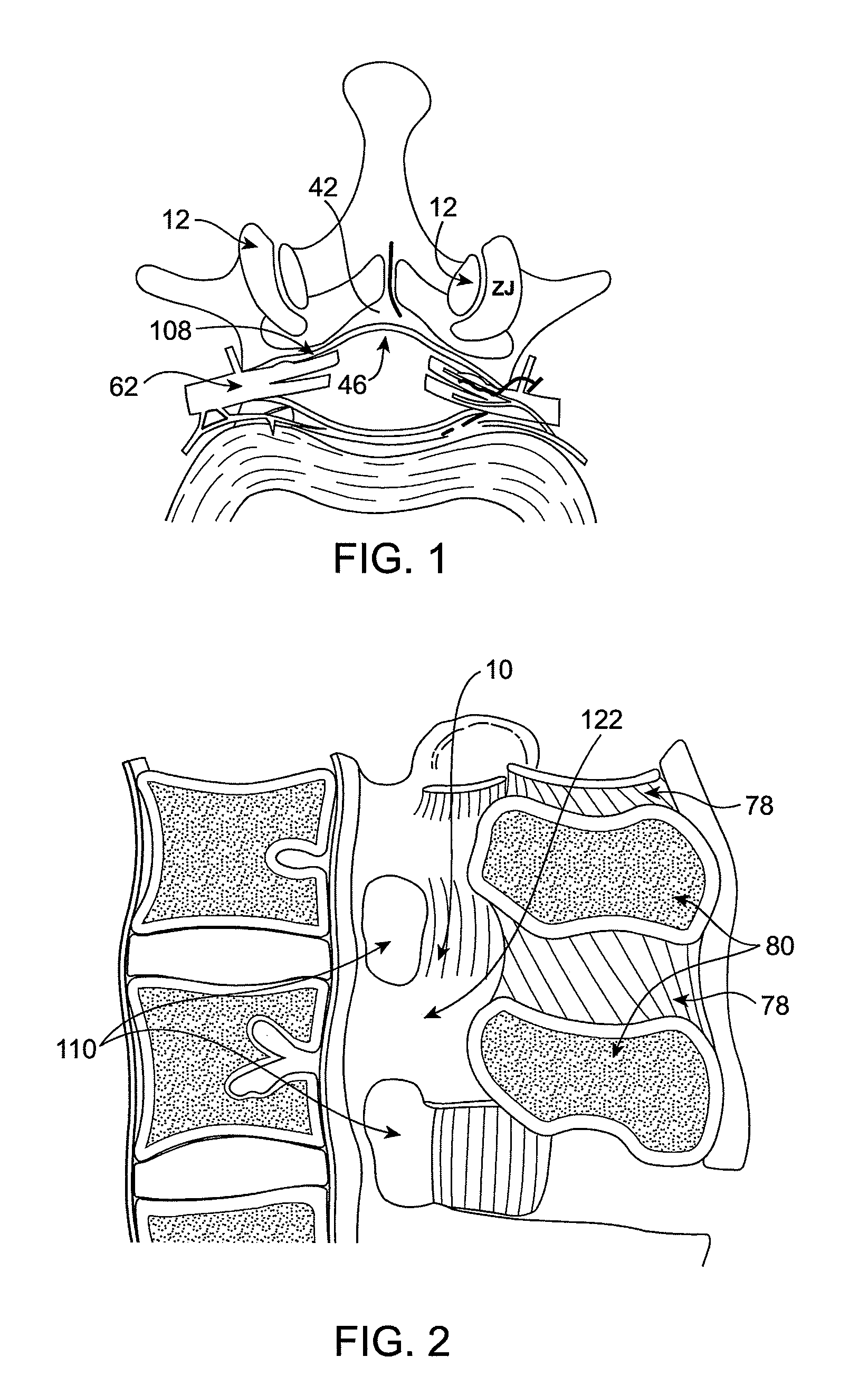
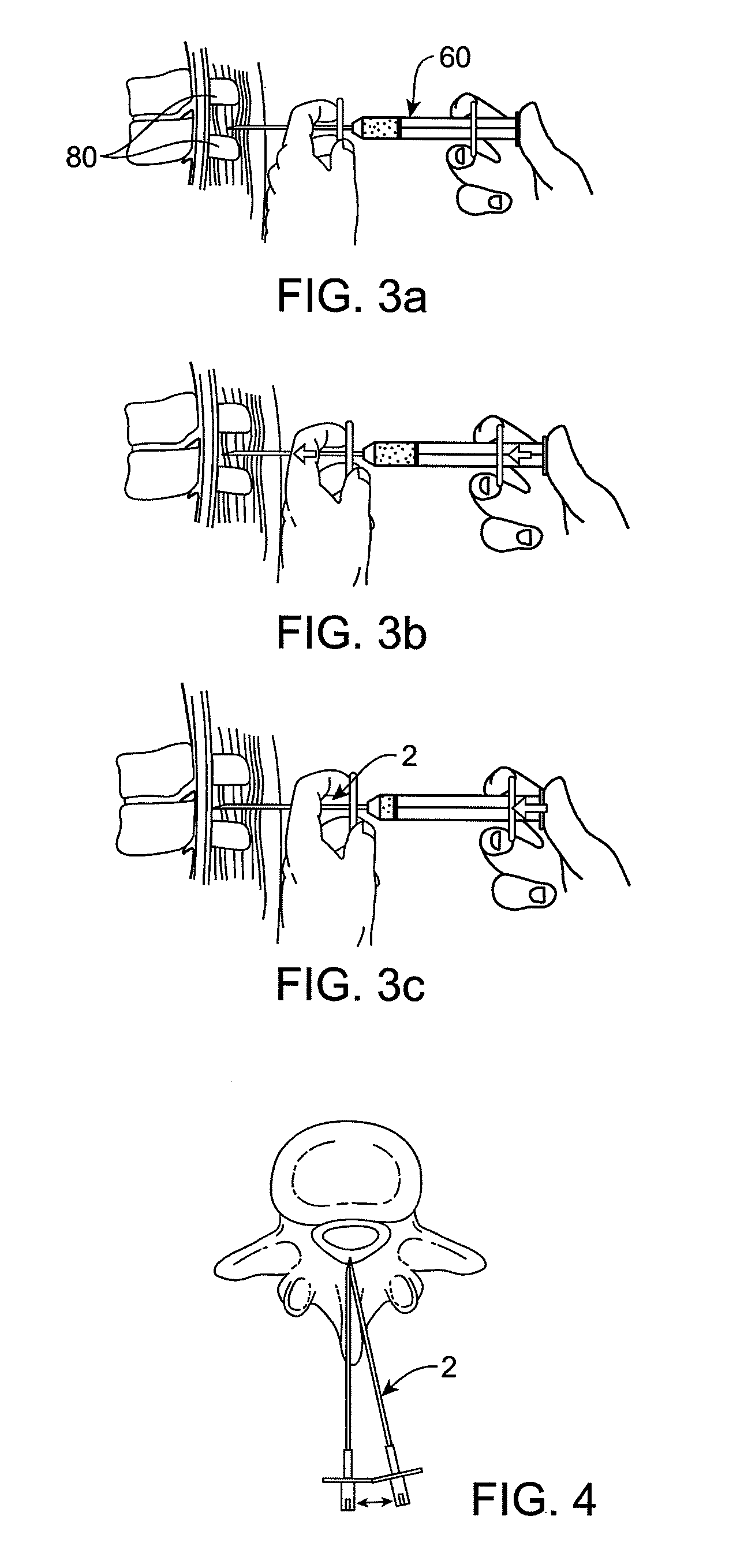
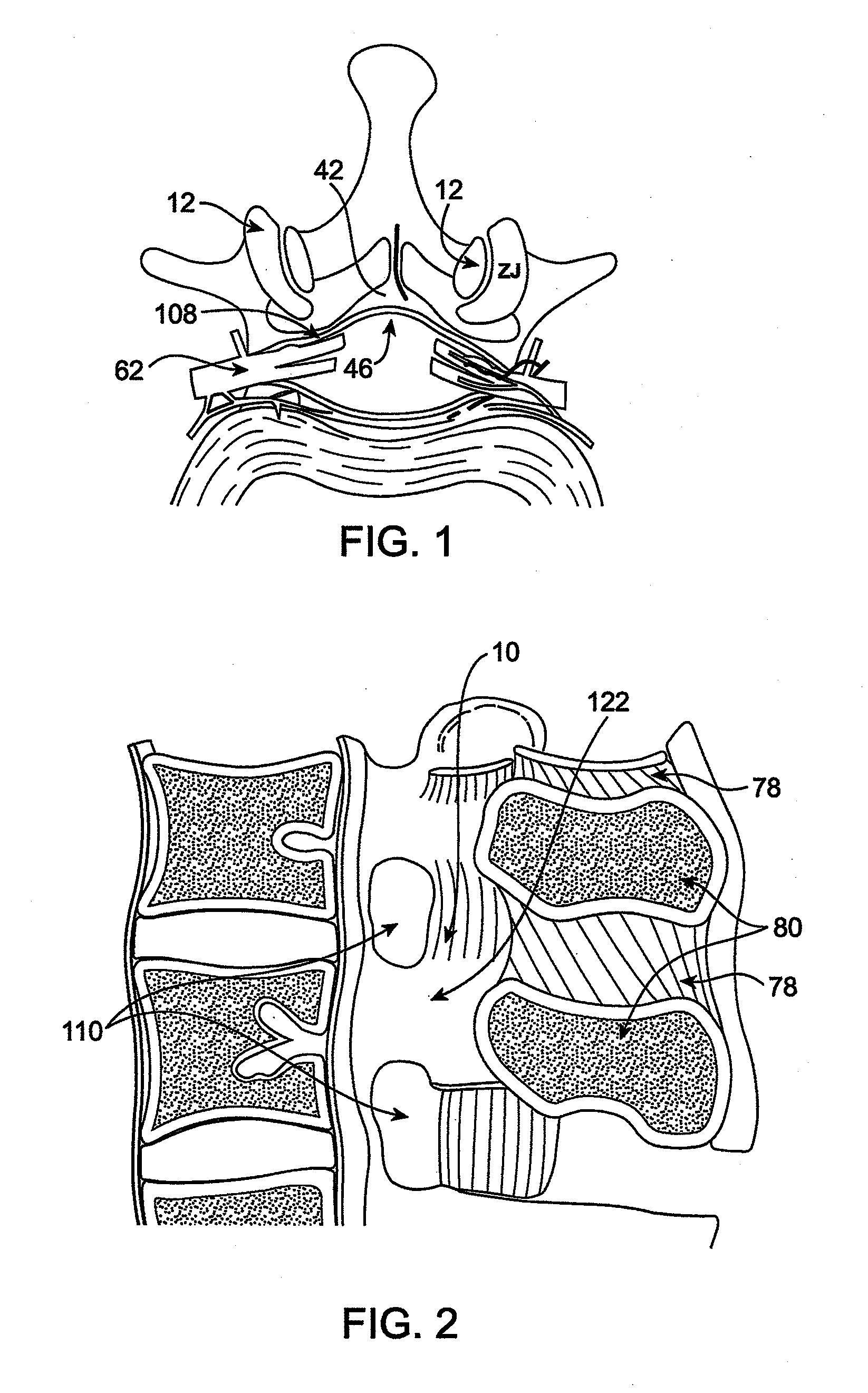
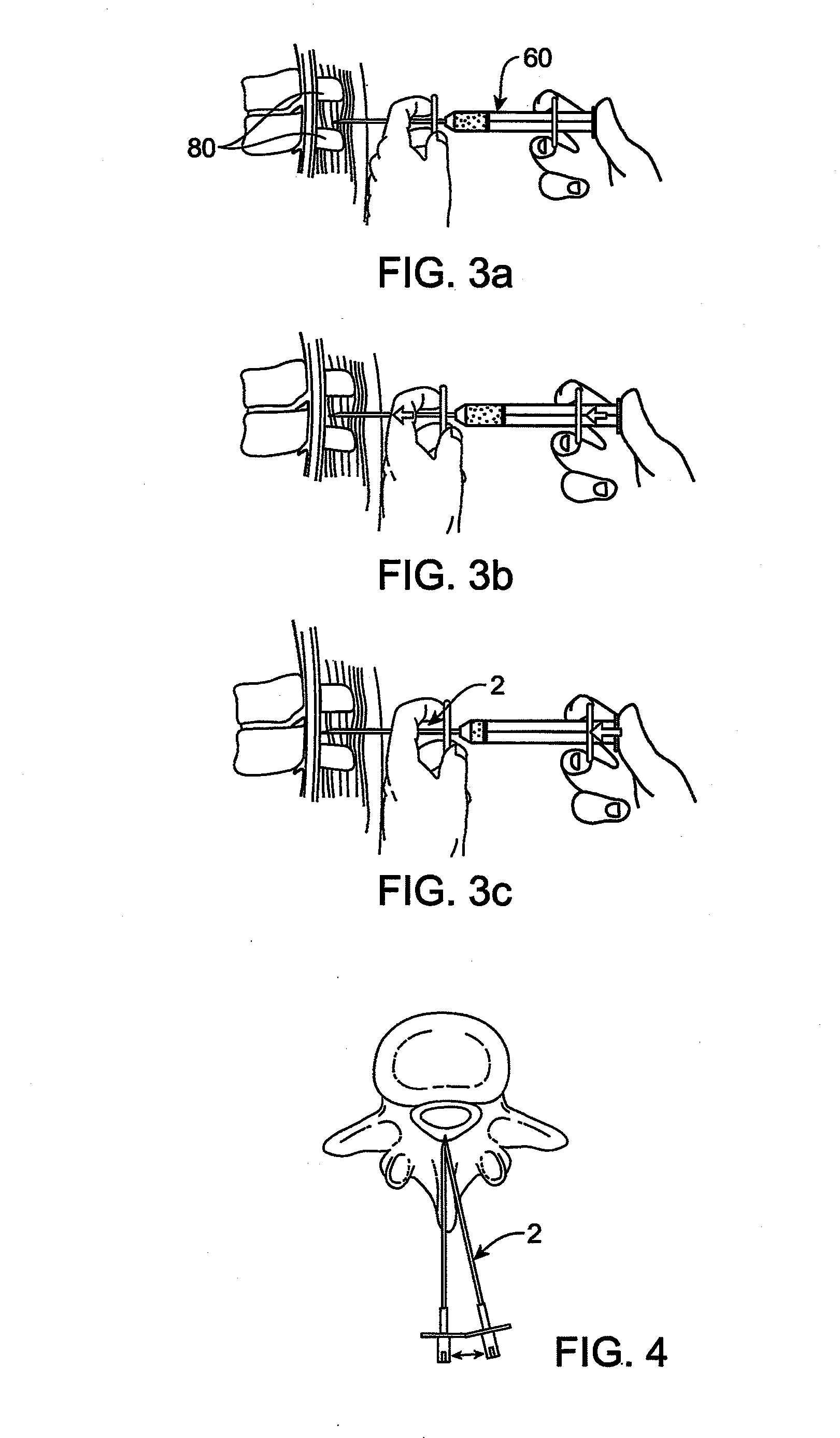
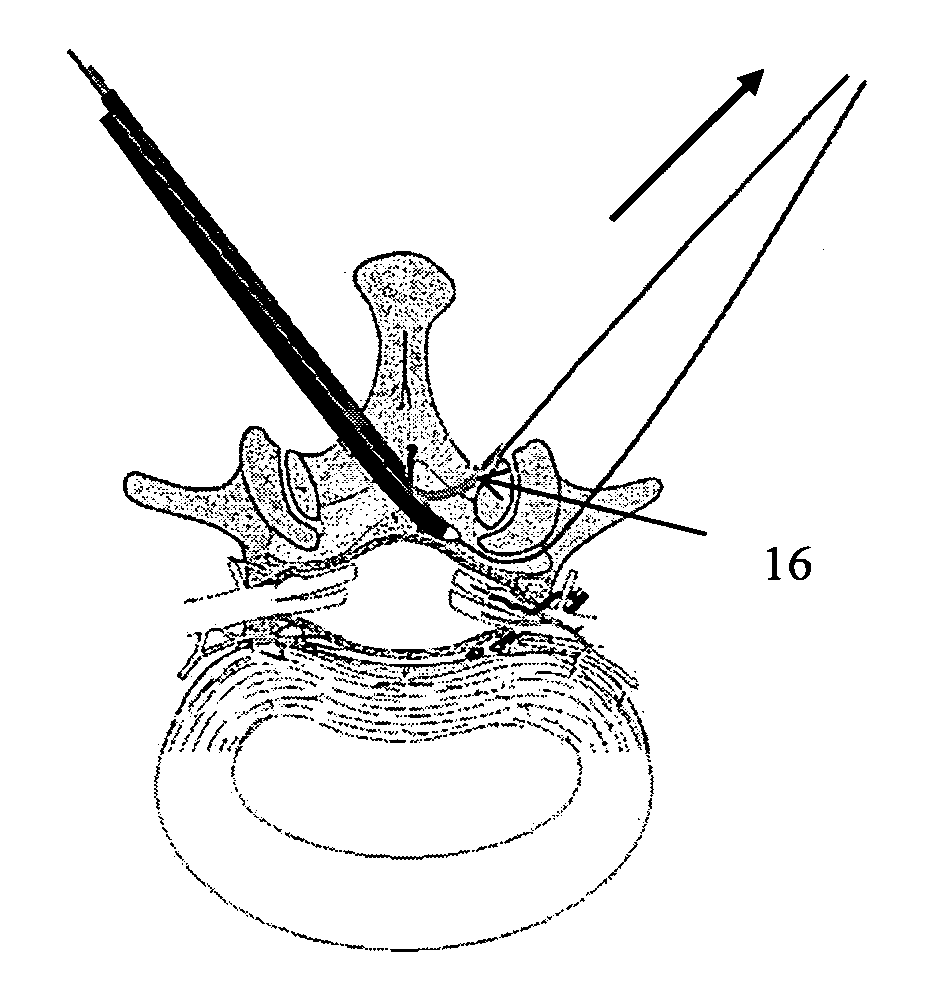
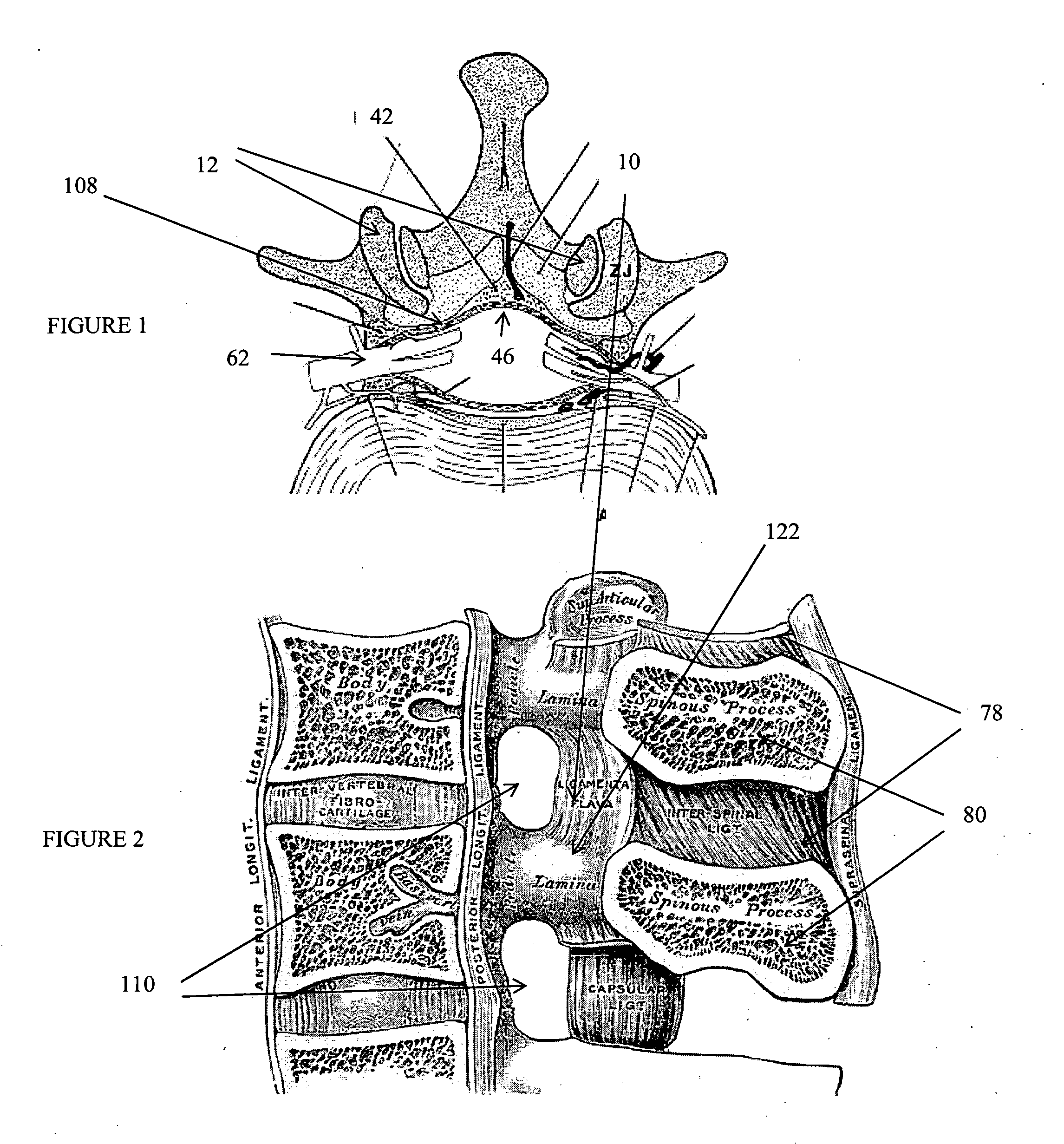
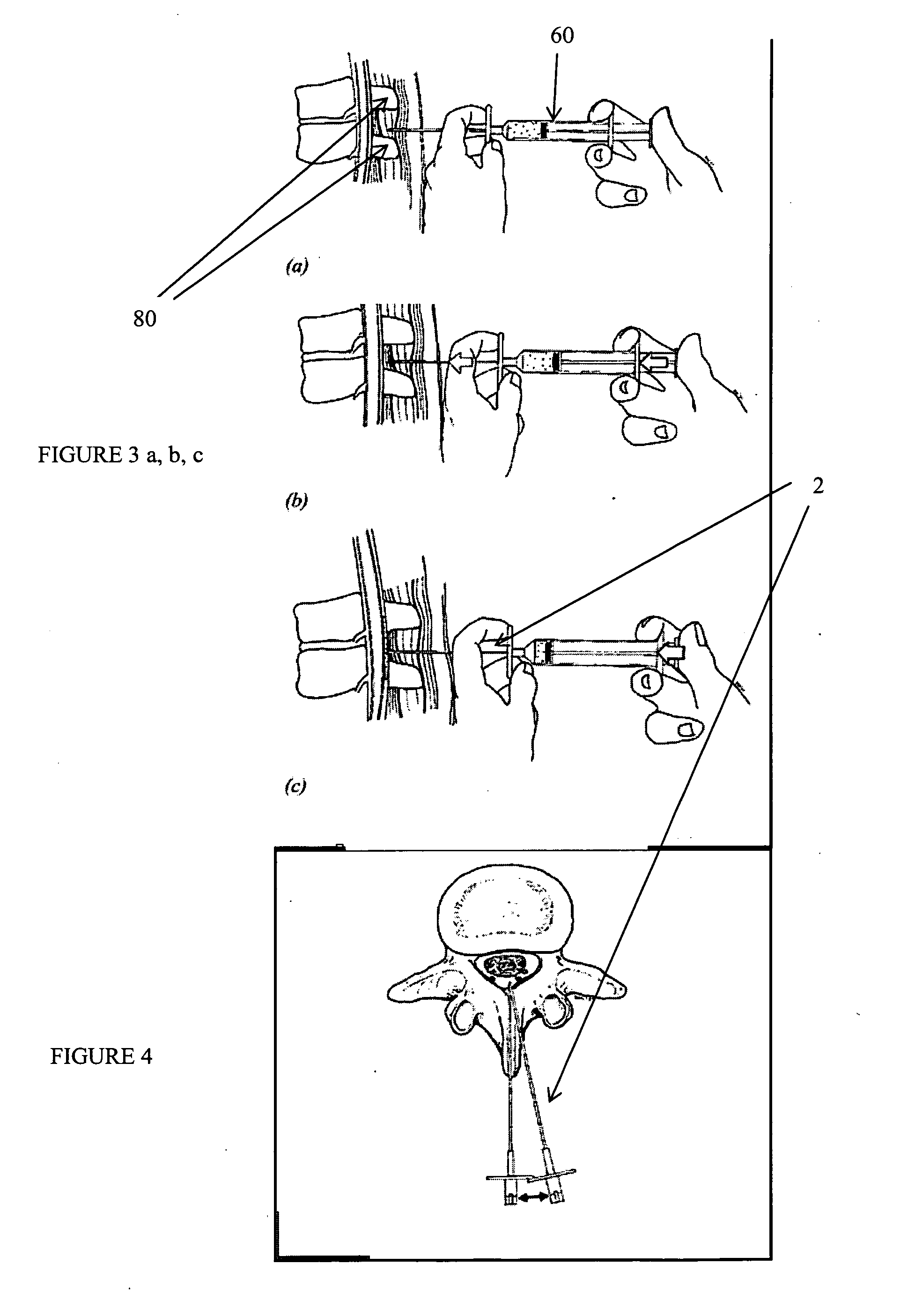
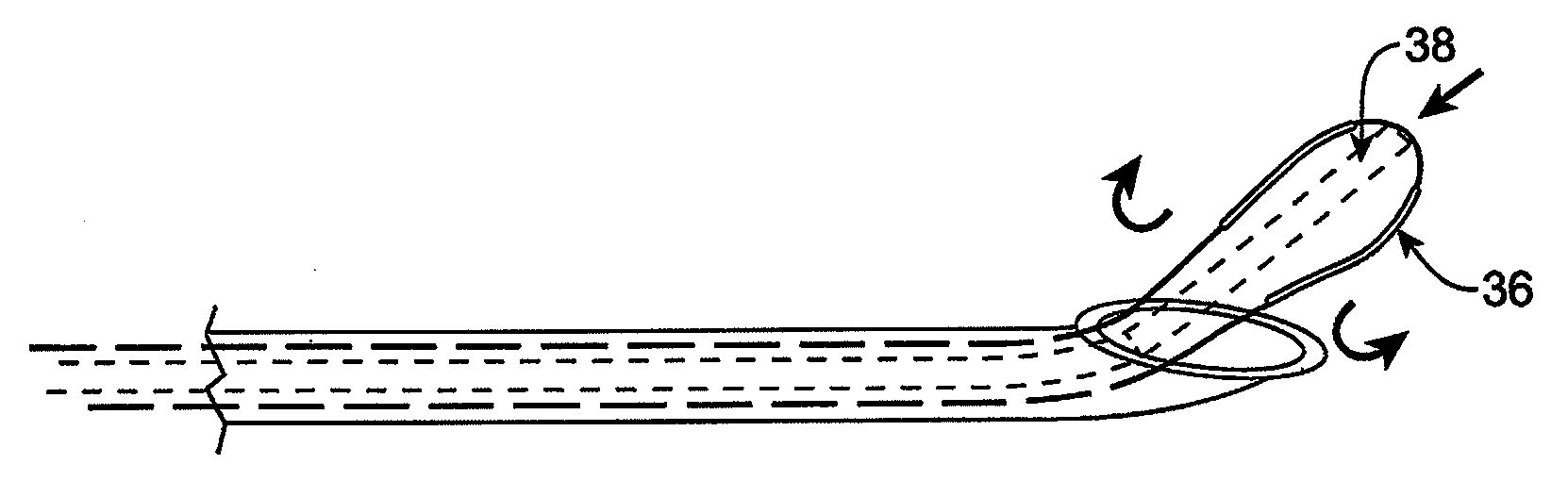
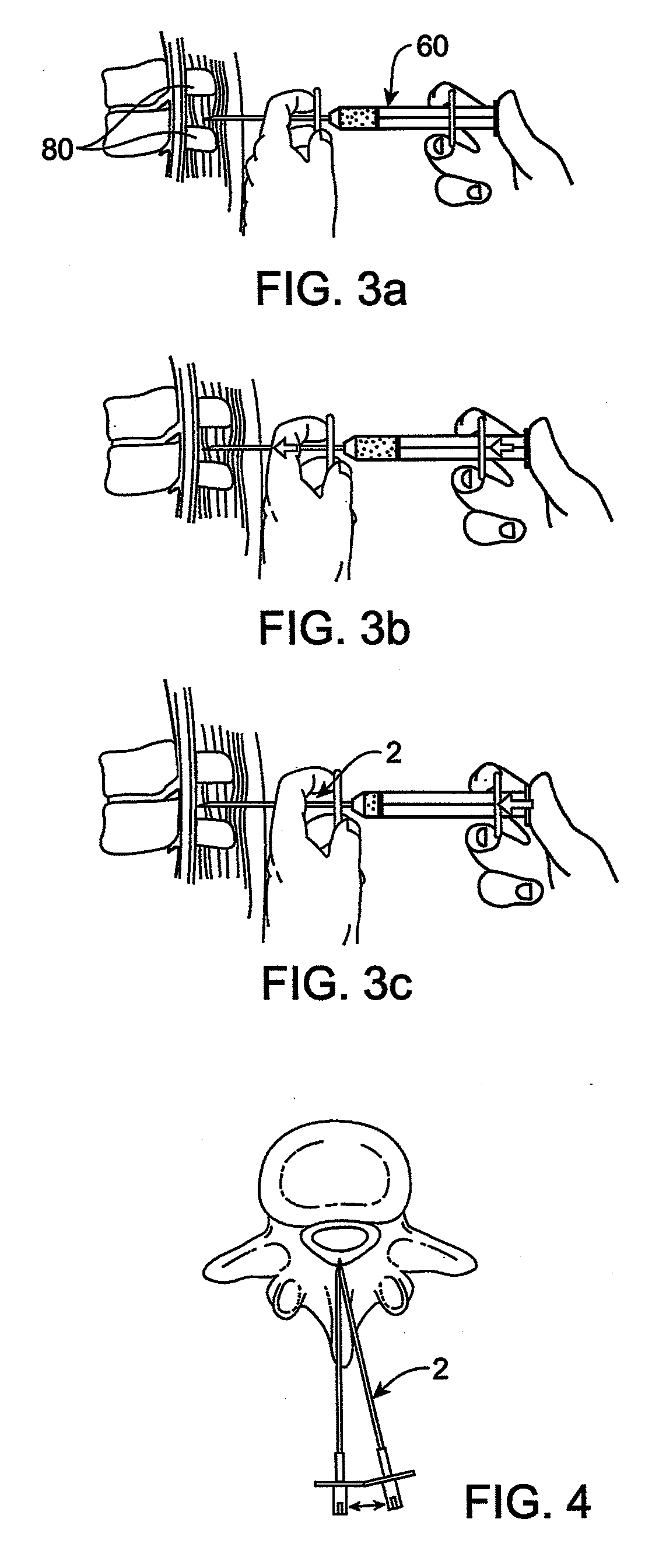
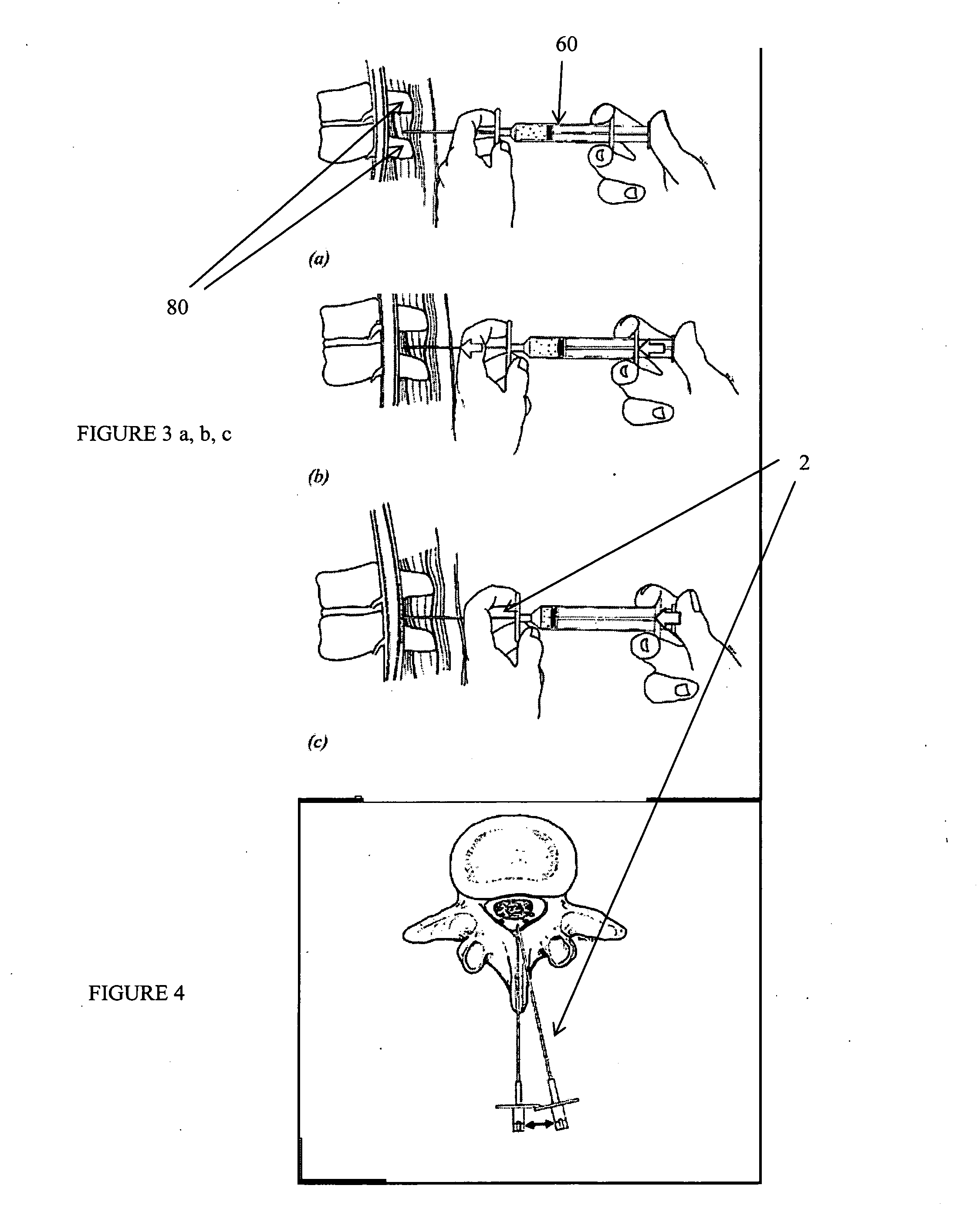
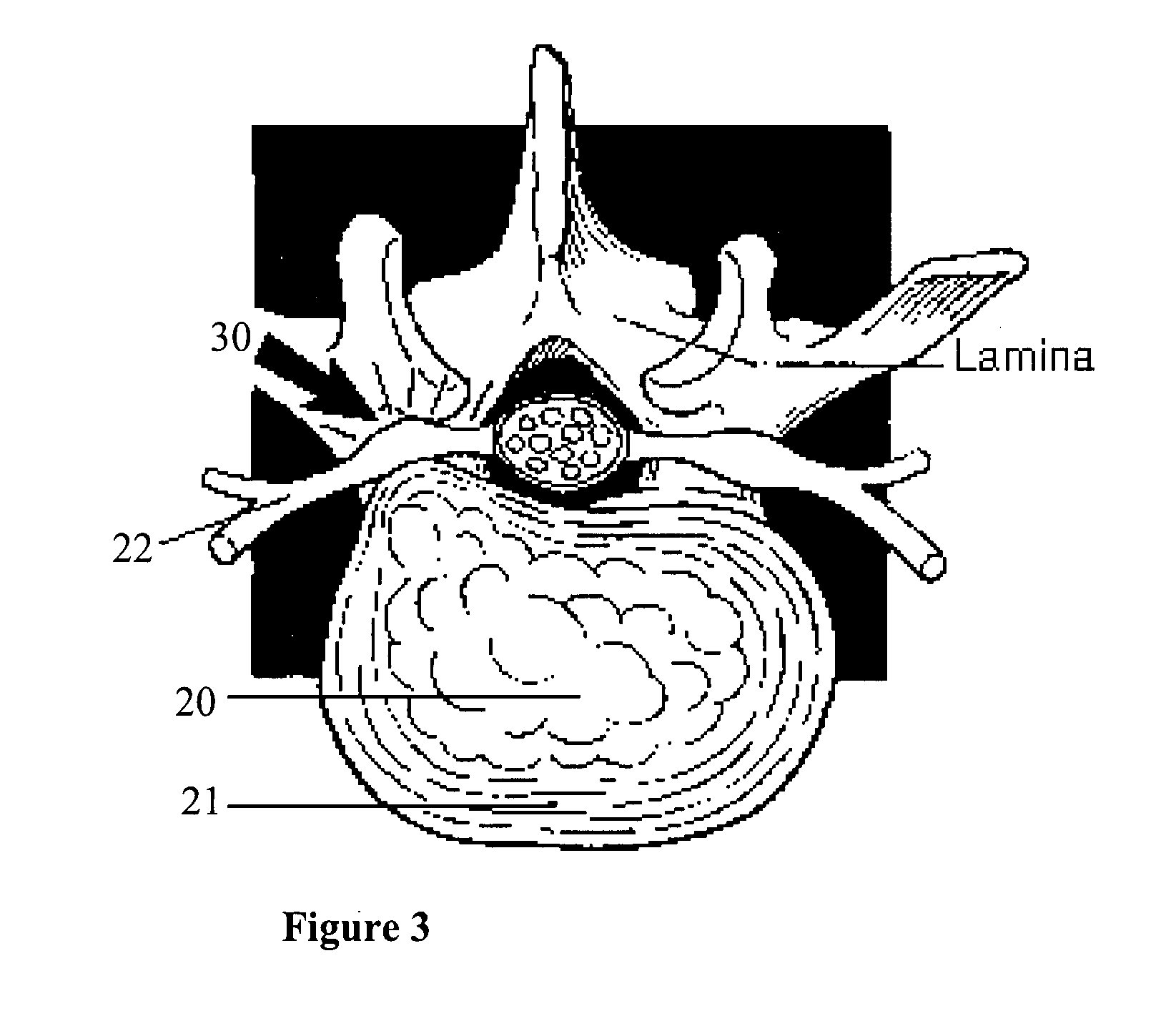
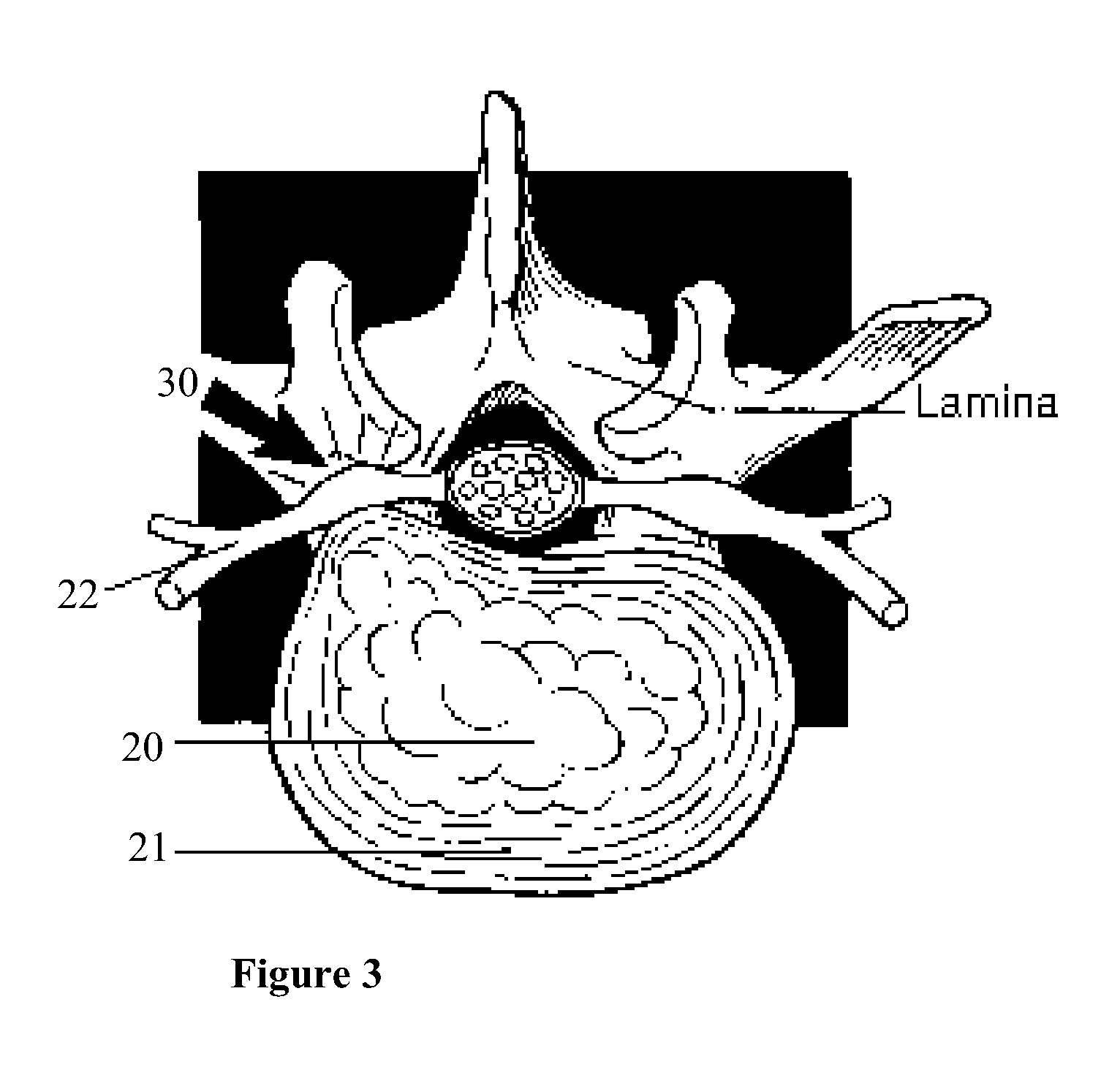
Methods and apparatus are provided for selective surgical removal of tissue, e.g., for enlargement of diseased spinal structures, such as impinged lateral recesses and pathologically narrowed neural foramen. In one variation, tissue may be ablated, resected, removed, or otherwise remodeled by standard small endoscopic tools delivered into the epidural space through an epidural needle. Once the sharp tip of the needle is in the epidural space, it is converted to a blunt tipped instrument for further safe advancement. A specially designed epidural catheter that is used to cover the previously sharp needle tip may also contain a fiberoptic cable. Further embodiments of the current invention include a double barreled epidural needle or other means for placement of a working channel for the placement of tools within the epidural space, beside the epidural instrument. The current invention includes specific tools that enable safe tissue modification in the epidural space, including a barrier that separates the area where tissue modification will take place from adjacent vulnerable neural and vascular structures. In one variation, a tissue abrasion device is provided including a thin belt or ribbon with an abrasive cutting surface. The device may be placed through the neural foramina of the spine and around the anterior border of a facet joint. Once properly positioned, a medical practitioner may enlarge the lateral recess and neural foramina via frictional abrasion, i.e., by sliding the abrasive surface of the ribbon across impinging tissues. A nerve stimulator optionally may be provided to reduce a risk of inadvertent neural abrasion. Additionally, safe epidural placement of the working barrier and epidural tissue modification tools may be further improved with the use of electrical nerve stimulation capabilities within the invention that, when combined with neural stimulation monitors, provide neural localization capabilities to the surgeon. The device optionally may be placed within a protective sheath that exposes the abrasive surface of the ribbon only in the area where tissue removal is desired. Furthermore, an endoscope may be incorporated into the device in order to monitor safe tissue removal. Finally, tissue remodeling within the epidural space may be ensured through the placement of compression dressings against remodeled tissue surfaces, or through the placement of tissue retention straps, belts or cables that are wrapped around and pull under tension aspects of the impinging soft tissue and bone in the posterior spinal canal.
Owner:SPINAL ELEMENTS INC +1
Devices and methods for tissue access
InactiveUS20060095028A1Improve securityAvoid injuryEar treatmentCannulasAccess methodSurgical removal
Methods and apparatus are provided for selective surgical removal of tissue. In one variation, tissue may be ablated, resected, removed, or otherwise remodeled by standard small endoscopic tools delivered into the epidural space through an epidural needle. The sharp tip of the needle in the epidural space, can be converted to a blunt tipped instrument for further safe advancement. The current invention includes specific tools that enable safe tissue modification in the epidural space, including a barrier that separates the area where tissue modification will take place from adjacent vulnerable neural and vascular structures. A nerve stimulator may be provided to reduce a risk of inadvertent neural abrasion.
Owner:BAXANO
Devices and methods for tissue access
InactiveUS20060122458A1Enabling symptomatic reliefApproach can be quite invasiveCannulasDiagnosticsSurgical departmentNerve stimulation
Methods and apparatus are provided for selective surgical removal of tissue, e.g., for enlargement of diseased spinal structures, such as impinged lateral recesses and pathologically narrowed neural foramen. In one variation, tissue may be ablated, resected, removed, or otherwise remodeled by standard small endoscopic tools delivered into the epidural space through an epidural needle. Once the sharp tip of the needle is in the epidural space, it is converted to a blunt tipped instrument for further safe advancement. A specially designed epidural catheter that is used to cover the previously sharp needle tip may also contain a fiberoptic cable. Further embodiments of the current invention include a double barreled epidural needle or other means for placement of a working channel for the placement of tools within the epidural space, beside the epidural instrument. The current invention includes specific tools that enable safe tissue modification in the epidural space, including a barrier that separates the area where tissue modification will take place from adjacent vulnerable neural and vascular structures. In one variation, a tissue removal device is provided including a thin belt or ribbon with an abrasive cutting surface. The device may be placed through the neural foramina of the spine and around the anterior border of a facet joint. Once properly positioned, a medical practitioner may enlarge the lateral recess and neural foramina via frictional abrasion, i.e., by sliding the tissue removal surface of the ribbon across impinging tissues. A nerve stimulator optionally may be provided to reduce a risk of inadvertent neural abrasion. Additionally, safe epidural placement of the working barrier and epidural tissue modification tools may be further improved with the use of electrical nerve stimulation capabilities within the invention that, when combined with neural stimulation monitors, provide neural localization capabilities to the surgeon. The device optionally may be placed within a protective sheath that exposes the abrasive surface of the ribbon only in the area where tissue removal is desired. Furthermore, an endoscope may be incorporated into the device in order to monitor safe tissue removal. Finally, tissue remodeling within the epidural space may be ensured through the placement of compression dressings against remodeled tissue surfaces, or through the placement of tissue retention straps, belts or cables that are wrapped around and pull under tension aspects of the impinging soft tissue and bone in the posterior spinal canal.
Owner:BAXANO
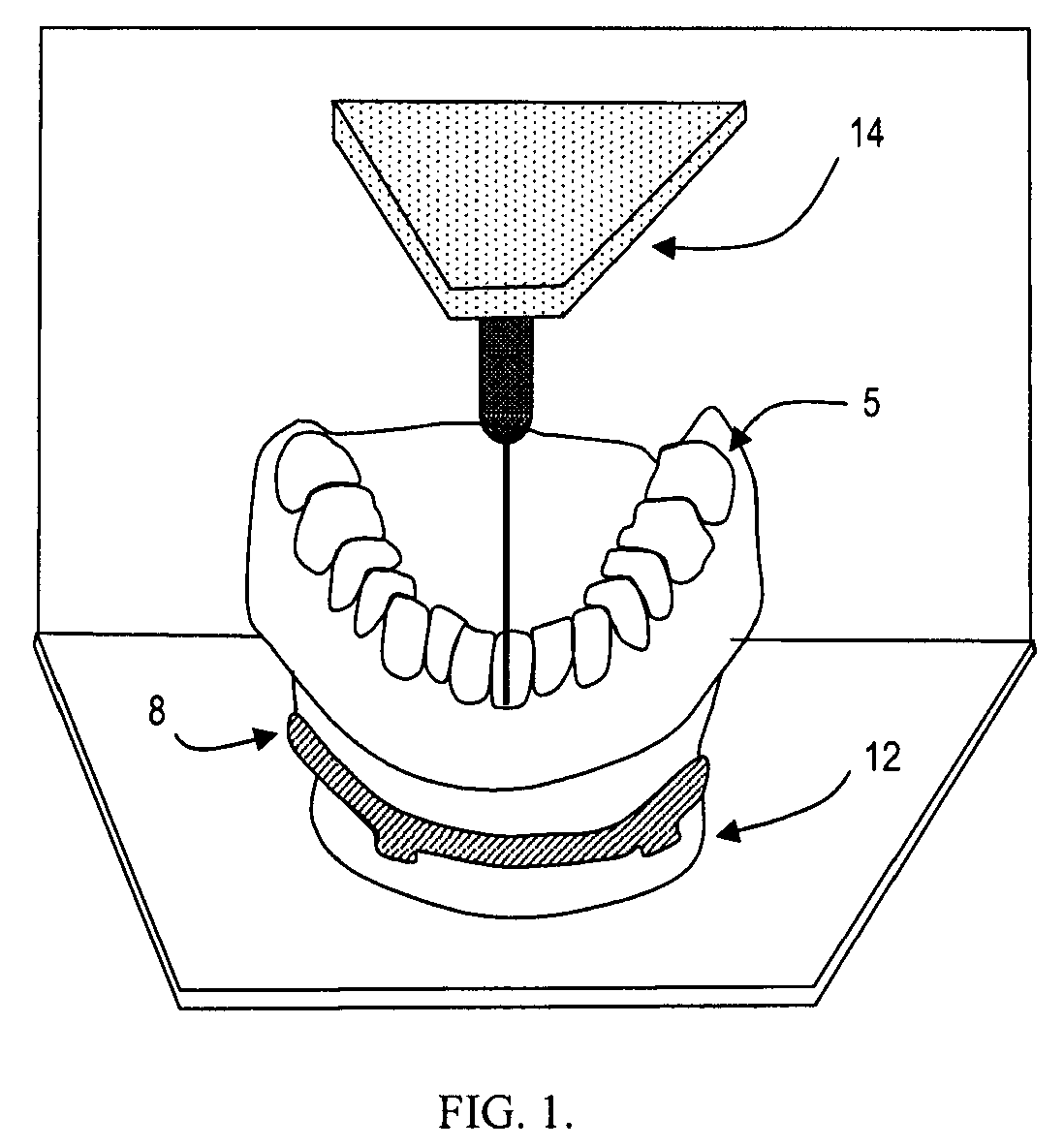
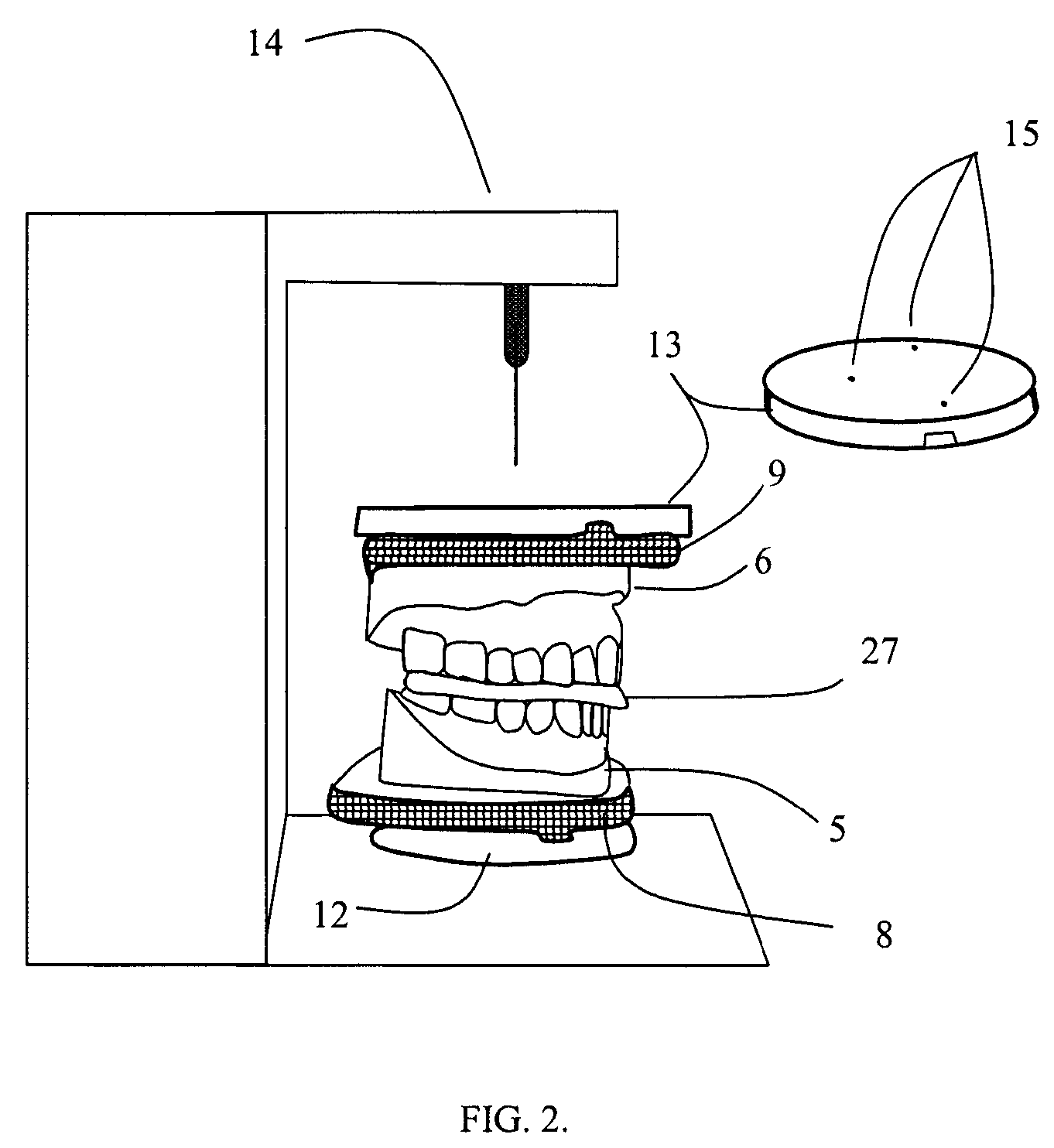
Surgical guides and methods for positioning artificial teeth and dental implants
InactiveUS20080085489A1Eliminate radiographic scatterMedical simulationDental implantsDigital dataNatural tooth
A method is set forth for making a computer model of patient's jaws on the basis of digital information. Digital data about the jaws, teeth, soft tissues and artificial teeth is joined in computer space to create aesthetic and functional plans for the removal of teeth, shaping of supporting bone and placement of dental implants. Artificial teeth and pre-manufactured prosthetic devices are made and attached to the dental implants at the time of surgery. The aesthetic and functional position of artificial teeth is determined prior to surgical removal of natural teeth and the ideal position of implants and the proper form of the remaining bone are determined prior to surgery. Surgical guides used to shape bone, record occlusal orientation and position dental implants are manufactured using computer milling or layered manufacturing.
Owner:VOXELOGIX CORP
Devices and methods for tissue modification
ActiveUS20060095059A1Easy to disassembleEliminate needCannulasAnti-incontinence devicesSurgical departmentNerve stimulation
Owner:MIS IP HLDG LLC +1
Hybrid imaging method to monitor medical device delivery and patient support for use in the method
ActiveUS7379769B2Easy procedureConvenient treatmentSurgical needlesStretcherLiver and kidneySurgical removal
Owner:INVIVO CORP
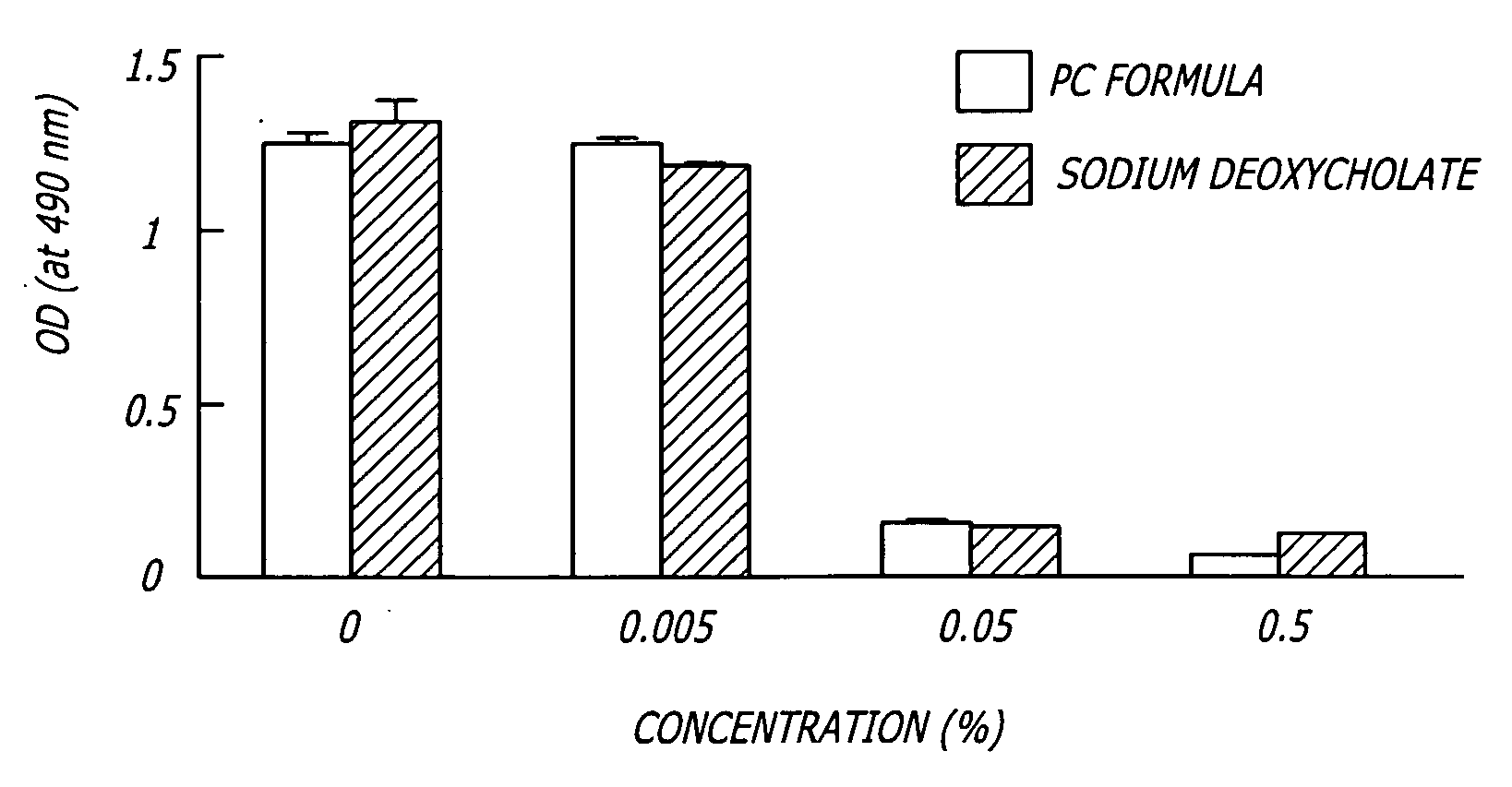
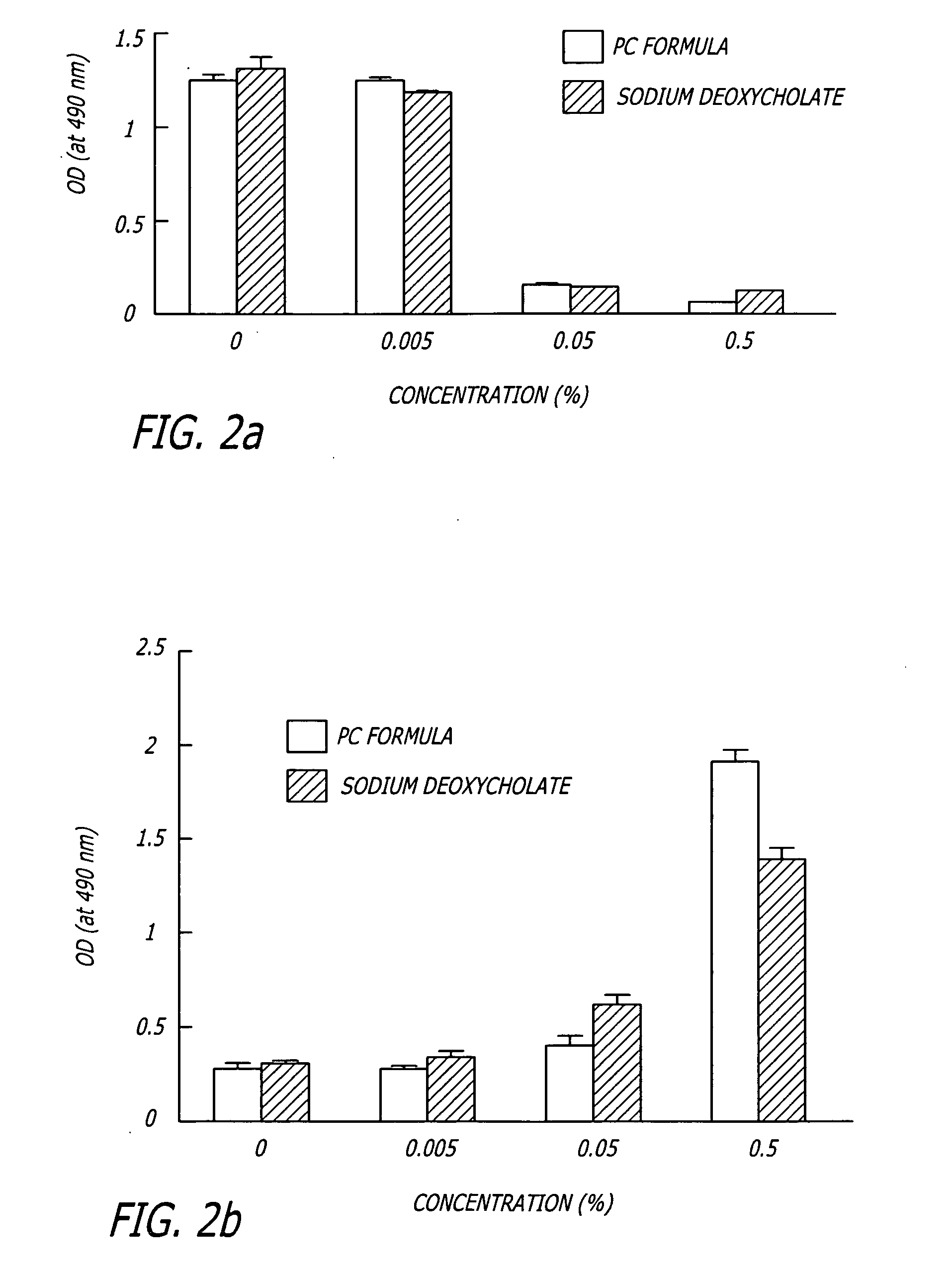
Methods and compositions for the non-surgical removal of fat
ActiveUS20050261258A1Easy to disassembleReduces blood lossCosmetic preparationsOrganic active ingredientsSurgical removalCellulite
Compositions and methods useful in the non-surgical removal of localized fat deposits in patients in need thereof using pharmacologically active detergents are disclosed. The pharmacologically active detergent compositions can additionally include anti-inflammatory agents, analgesics, dispersion agents and pharmaceutically acceptable excipients but do not contain phosphotidylcholine. The pharmacologically active detergent compositions are useful for treating localized accumulations of fat including lower eyelid fat herniation, lipodystrophy and fat deposits associated with cellulite and do not require surgical procedures such as liposuction.
Owner:RGT UNIV OF CALIFORNIA +1
Devices and methods for tissue modification
ActiveUS20060089609A1Enabling symptomatic reliefApproach can be quite invasiveCannulasAnti-incontinence devicesSurgical removalEpidural needles
Methods and apparatus are provided for selective surgical removal of tissue. In one variation, tissue may be ablated, resected, removed, or otherwise remodeled by standard small endoscopic tools delivered into the epidural space through an epidural needle. The sharp tip of the needle in the epidural space, can be converted to a blunt tipped instrument for further safe advancement. The current invention includes specific tools that enable safe tissue modification in the epidural space, including a barrier that separates the area where tissue modification will take place from adjacent vulnerable neural and vascular structures. A nerve stimulator may be provided to reduce a risk of inadvertent neural abrasion.
Owner:MIS IP HLDG LLC +1
Devices and methods for tissue access
ActiveUS20110160731A1Easy to disassembleEliminate needElectrotherapyCannulasAccess methodLateral recess
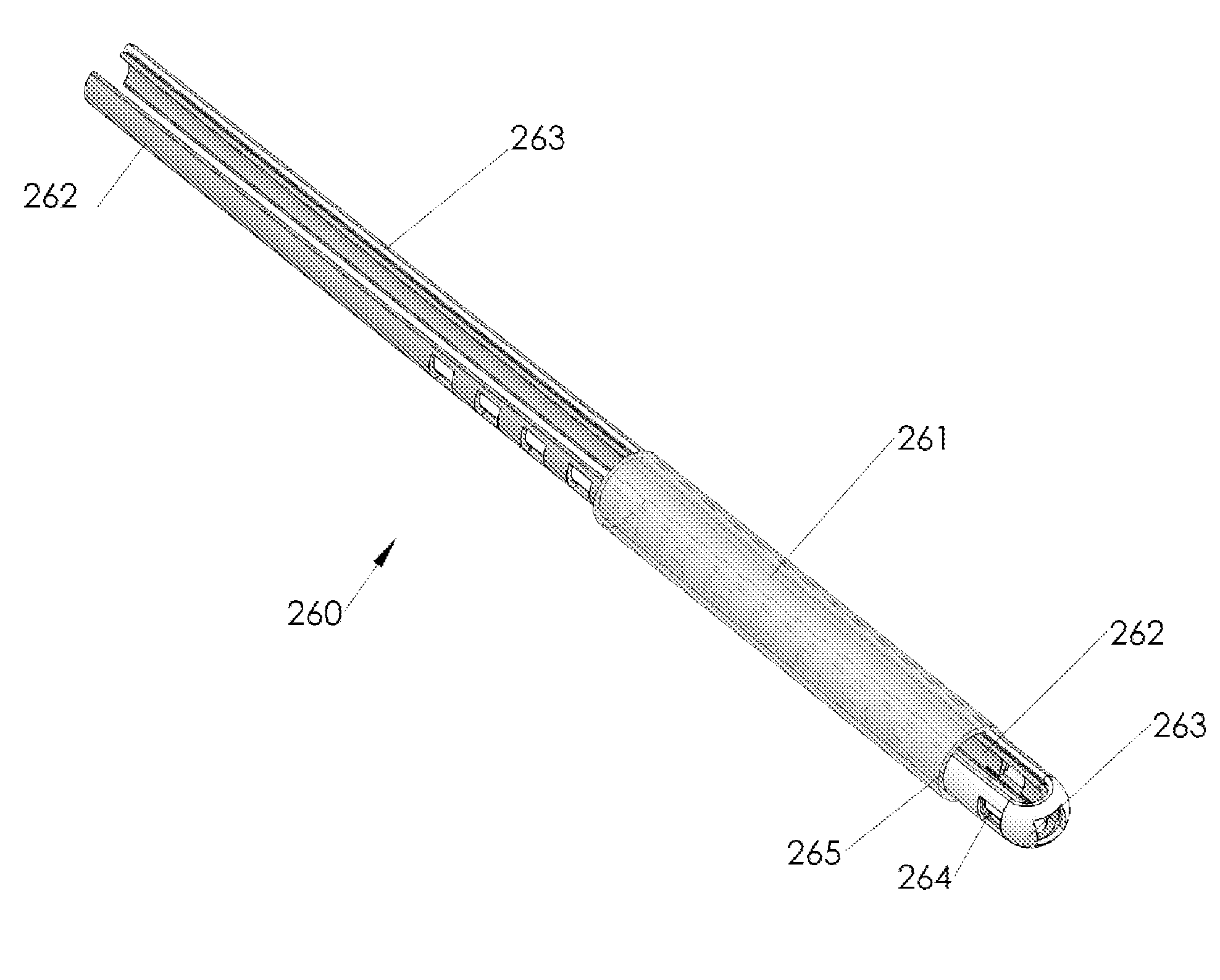
Methods and apparatus are provided for selective surgical removal of tissue, e.g., for enlargement of diseased spinal structures, such as impinged lateral recesses and pathologically narrowed neural foramen. In some embodiments, a surgical tissue removal device includes a flexible elongate body that is adapted to conform with the target anatomy and a guidewire connector at the distal end region of the flexible elongate body configured to removably connect to the end of a guidewire so that the guidewire and flexible elongate body can be pulled distally. The body may have at least one blade edge, and the flexible elongate body may be a thin, flat, ribbon shaped flexible body that comprises a profile having a width that is substantially greater than a height.
Owner:SPINAL ELEMENTS INC
Devices and methods for tissue modification
InactiveUS20100094231A1Improve securityAvoid injuryCannulasDiagnosticsSurgical removalEpidural needles
Owner:BAXANO
Methods and related compositions for the non-surgical removal of fat
InactiveUS20060154906A1Easy to disassembleReduces blood lossCosmetic preparationsBiocideSurgical removalCellulite
Compositions and methods useful in the non-surgical removal of localized fat deposits in patients in need thereof using pharmacologically active detergents are disclosed. The pharmacologically active detergent compositions can additionally include anti-inflammatory agents, analgesics, dispersion agents and pharmaceutically acceptable excipients but do not contain phosphotidylcholine. The pharmacologically active detergent compositions are useful for treating localized accumulations of fat including lower eyelid fat herniation, lipodystrophy and fat deposits associated with cellulite and do not require surgical procedures such as liposuction.
Owner:RGT UNIV OF CALIFORNIA +1
Devices and methods for tissue modification
ActiveUS20090204119A1Enabling symptomatic reliefApproach can be quite invasiveSpinal electrodesCannulasSurgical removalVascular structure
Methods and apparatus are provided for selective surgical removal of tissue. In one variation, tissue may be ablated, resected, removed, or otherwise remodeled by standard small endoscopic tools delivered into the epidural space through an epidural needle. The sharp tip of the needle in the epidural space, can be converted to a blunt tipped instrument for further safe advancement. The current invention includes specific tools that enable safe tissue modification in the epidural space, including a barrier that separates the area where tissue modification will take place from adjacent vulnerable neural and vascular structures. A nerve stimulator may be provided to reduce a risk of inadvertent neural abrasion.
Owner:MIS IP HLDG LLC +1
Devices and methods for selective surgical removal of tissue
ActiveUS20060094976A1Improve securityAvoid injuryCannulasAnti-incontinence devicesTissue remodelingLateral recess
Methods and apparatus are provided for selective surgical removal of tissue, e.g., for enlargement of diseased spinal structures, such as impinged lateral recesses and pathologically narrowed neural foramen. In one variation, tissue may be ablated, resected, removed, or otherwise remodeled by standard small endoscopic tools delivered into the epidural space through an epidural needle. Once the sharp tip of the needle is in the epidural space, it is converted to a blunt tipped instrument for further safe advancement. A specially designed epidural catheter that is used to cover the previously sharp needle tip may also contain a fiberoptic cable. Further embodiments of the current invention include a double barreled epidural needle or other means for placement of a working channel for the placement of tools within the epidural space, beside the epidural instrument. The current invention includes specific tools that enable safe tissue modification in the epidural space, including a barrier that separates the area where tissue modification will take place from adjacent vulnerable neural and vascular structures. In one variation, a tissue removal device is provided including a thin belt or ribbon with an abrasive cutting surface. The device may be placed through the neural foramina of the spine and around the anterior border of a facet joint. Once properly positioned, a medical practitioner may enlarge the lateral recess and neural foramina via frictional abrasion, i.e., by sliding the tissue removal surface of the ribbon across impinging tissues. A nerve stimulator optionally may be provided to reduce a risk of inadvertent neural abrasion. Additionally, safe epidural placement of the working barrier and epidural tissue modification tools may be further improved with the use of electrical nerve stimulation capabilities within the invention that, when combined with neural stimulation monitors, provide neural localization capabilities to the surgeon. The device optionally may be placed within a protective sheath that exposes the abrasive surface of the ribbon only in the area where tissue removal is desired. Furthermore, an endoscope may be incorporated into the device in order to monitor safe tissue removal. Finally, tissue remodeling within the epidural space may be ensured through the placement of compression dressings against remodeled tissue surfaces, or through the placement of tissue retention straps, belts or cables that are wrapped around and pull under tension aspects of the impinging soft tissue and bone in the posterior spinal canal.
Owner:SPINAL ELEMENTS INC +1
Implantable products comprising nanoparticles
The present disclosure relates to nanoparticle-containing implantable and preferably biodegradable medical products and their use for the thermotherapeutic after-treatment after surgical removal of tumors and cancerous ulcers.
Owner:MAGFORCE AG
Methods and compositions for the non-surgical removal of fat
ActiveUS7622130B2Easy to disassembleReduces blood lossOrganic active ingredientsCosmetic preparationsSurgical removalCellulite
Compositions and methods useful in the non-surgical removal of localized fat deposits in patients in need thereof using pharmacologically active detergents are disclosed. The pharmacologically active detergent compositions can additionally include anti-inflammatory agents, analgesics, dispersion agents and pharmaceutically acceptable excipients but do not contain phosphotidylcholine. The pharmacologically active detergent compositions are useful for treating localized accumulations of fat including lower eyelid fat herniation, lipodystrophy and fat deposits associated with cellulite and do not require surgical procedures such as liposuction.
Owner:RGT UNIV OF CALIFORNIA +1
Devices and methods for selective surgical removal of tissue
ActiveUS20090125036A1Improve securityAvoid injuryCannulasAnti-incontinence devicesSurgical removalVascular structure
Methods and apparatus are provided for selective surgical removal of tissue. In one variation, tissue may be ablated, resected, removed, or otherwise remodeled by standard small endoscopic tools delivered into the epidural space through an epidural needle. The sharp tip of the needle in the epidural space, can be converted to a blunt tipped instrument for further safe advancement. The current invention includes specific tools that enable safe tissue modification in the epidural space, including a barrier that separates the area where tissue modification will take place from adjacent vulnerable neural and vascular structures. A nerve stimulator may be provided to reduce a risk of inadvertent neural abrasion.
Owner:MIS IP HLDG LLC +1
Devices and methods for tissue access
InactiveUS20060100651A1Improve securityAvoid injuryEar treatmentCannulasTissue remodelingSpinal column
Methods and apparatus are provided for selective surgical removal of tissue, e.g., for enlargement of diseased spinal structures, such as impinged lateral recesses and pathologically narrowed neural foramen. In one variation, tissue may be ablated, resected, removed, or otherwise remodeled by standard small endoscopic tools delivered into the epidural space through an epidural needle. Once the sharp tip of the needle is in the epidural space, it is converted to a blunt tipped instrument for further safe advancement. A specially designed epidural catheter that is used to cover the previously sharp needle tip may also contain a fiberoptic cable. Further embodiments of the current invention include a double barreled epidural needle or other means for placement of a working channel for the placement of tools within the epidural space, beside the epidural instrument. The current invention includes specific tools that enable safe tissue modification in the epidural space, including a barrier that separates the area where tissue modification will take place from adjacent vulnerable neural and vascular structures. In one variation, a tissue removal device is provided including a thin belt or ribbon with an abrasive cutting surface. The device may be placed through the neural foramina of the spine and around the anterior border of a facet joint. Once properly positioned, a medical practitioner may enlarge the lateral recess and neural foramina via frictional abrasion, i.e., by sliding the tissue removal surface of the ribbon across impinging tissues. A nerve stimulator optionally may be provided to reduce a risk of inadvertent neural abrasion. Additionally, safe epidural placement of the working barrier and epidural tissue modification tools may be further improved with the use of electrical nerve stimulation capabilities within the invention that, when combined with neural stimulation monitors, provide neural localization capabilities to the surgeon. The device optionally may be placed within a protective sheath that exposes the abrasive surface of the ribbon only in the area where tissue removal is desired. Furthermore, an endoscope may be incorporated into the device in order to monitor safe tissue removal. Finally, tissue remodeling within the epidural space may be ensured through the placement of compression dressings against remodeled tissue surfaces, or through the placement of tissue retention straps, belts or cables that are wrapped around and pull under tension aspects of the impinging soft tissue and bone in the posterior spinal canal.
Owner:SPINAL ELEMENTS INC +1
Devices and methods for tissue modification
ActiveUS20060089640A1Improve securityAvoid injuryCannulasAnti-incontinence devicesSurgical removalEpidural needles
Methods and apparatus are provided for selective surgical removal of tissue. In one variation, tissue may be ablated, resected, removed, or otherwise remodeled by standard small endoscopic tools delivered into the epidural space through an epidural needle. The sharp tip of the needle in the epidural space, can be converted to a blunt tipped instrument for further safe advancement. The current invention includes specific tools that enable safe tissue modification in the epidural space, including a barrier that separates the area where tissue modification will take place from adjacent vulnerable neural and vascular structures. A nerve stimulator may be provided to reduce a risk of inadvertent neural abrasion.
Owner:MIS IP HLDG LLC +1
High temperature thermal therapy of breast cancer
A method is described for preventing breast cancer recurrence after surgical removal of a breast cancer mass. Specifically, the method is thermal treatment of breast tissue surrounding a cavity after lumpectomy for breast cancer. Delivery of a heated fluid is through a balloon catheter to the cavity generated after lumpectomy with the goal of ablating the surrounding tissue, including transformed cells that were not removed through surgery. A balloon and controller were designed for this application.
Owner:THERMATOME
Intraocular lens optic
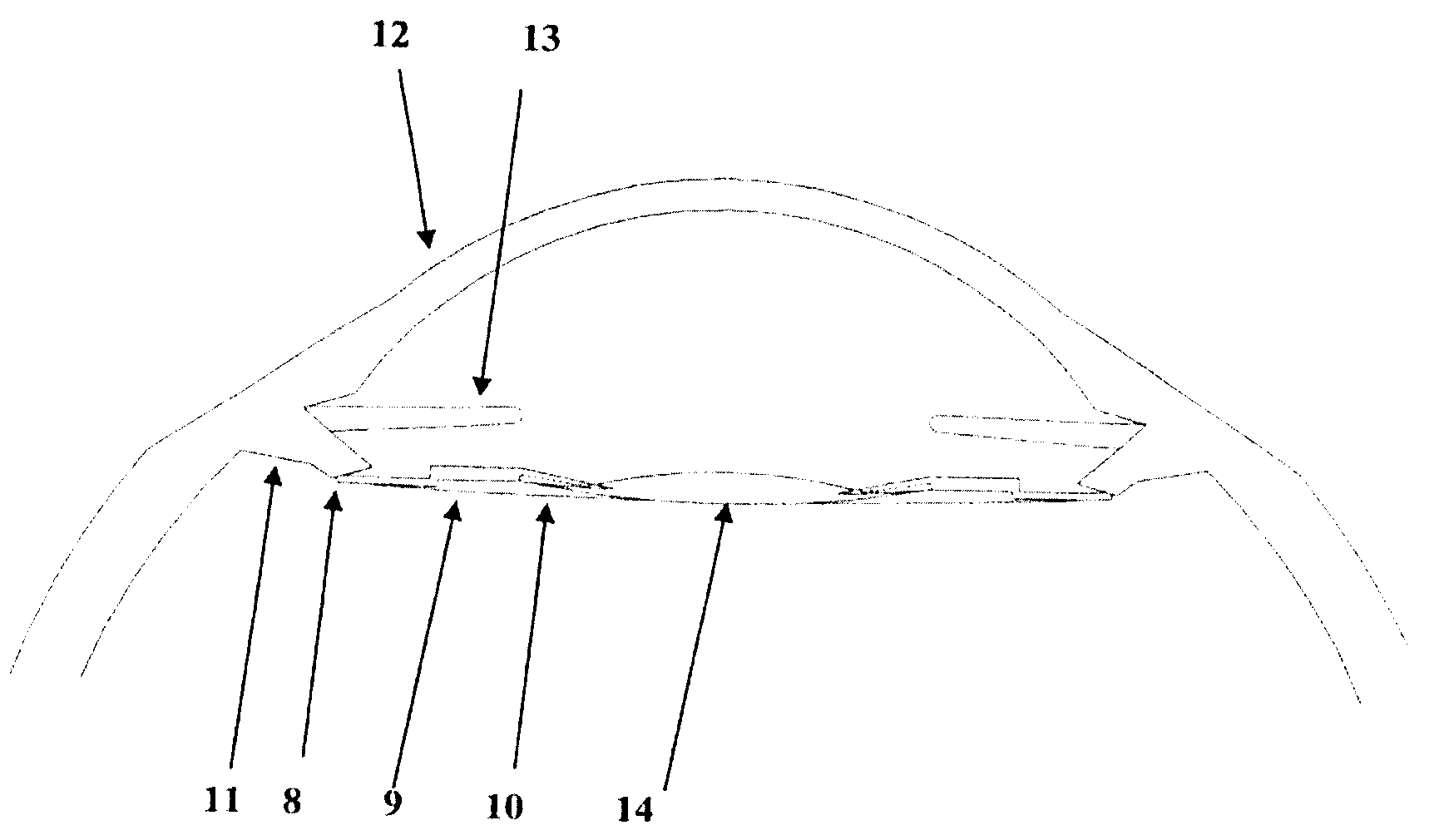
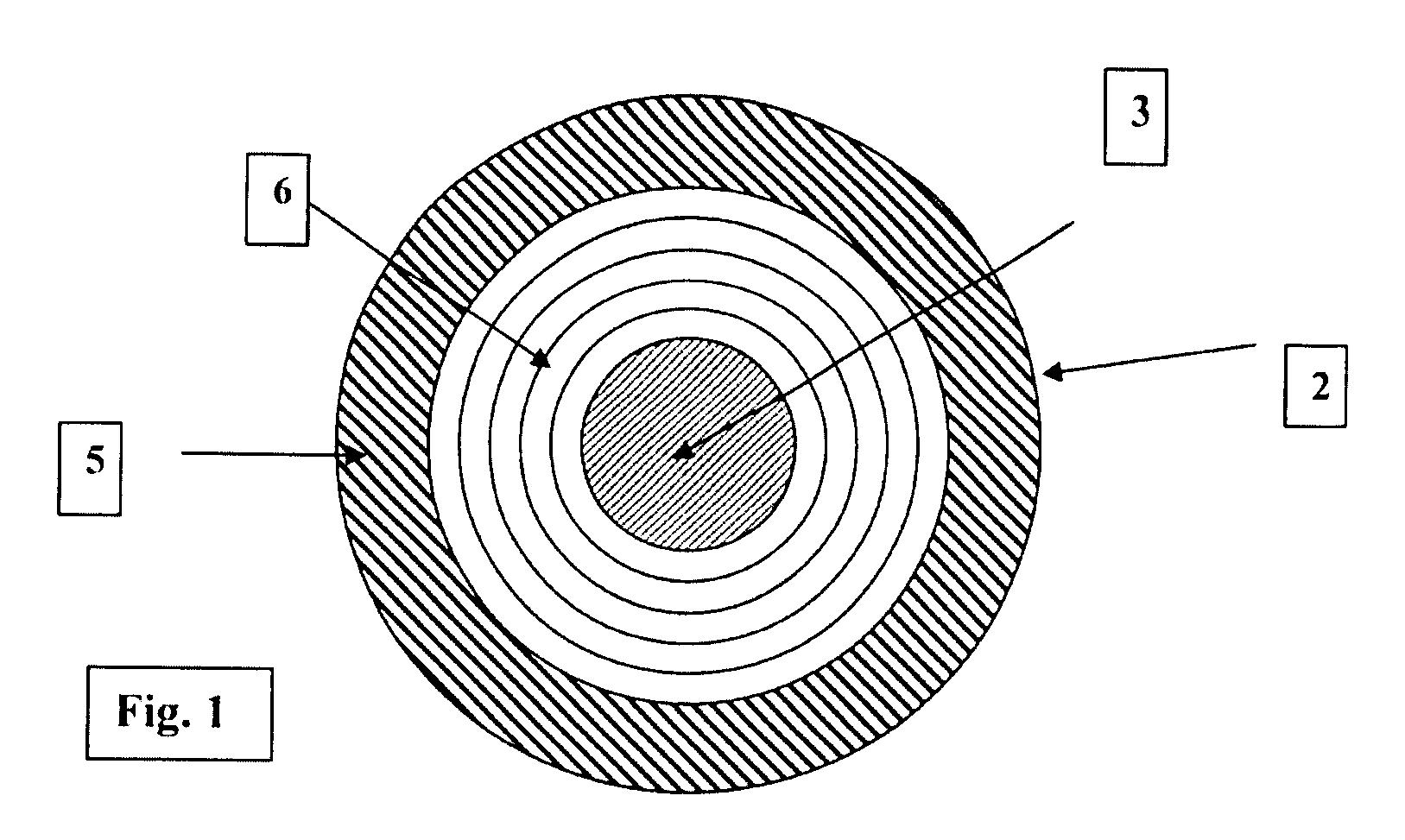
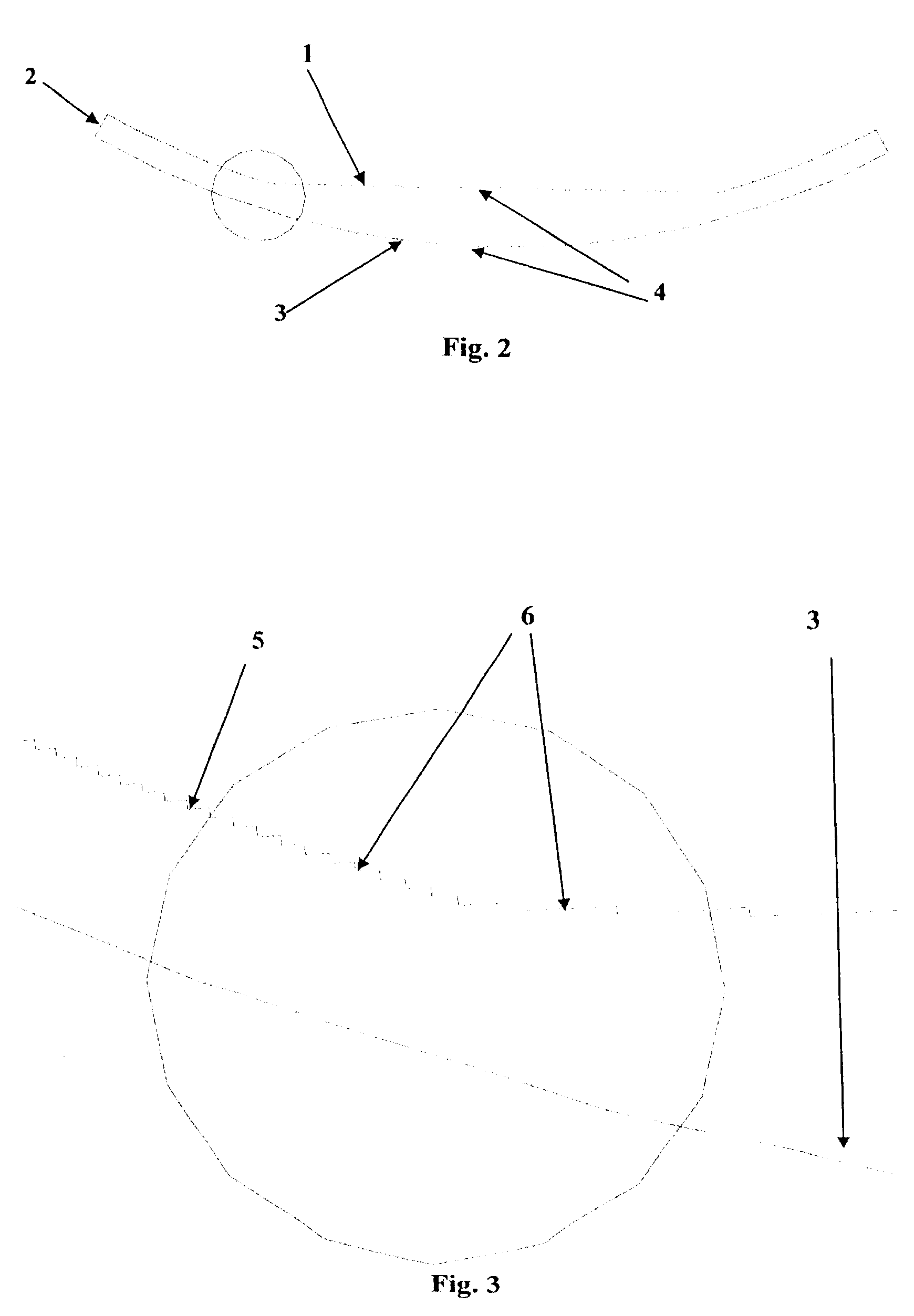
An intraocular lens optic (e.g. FIG. 1) having a maximum thickness of 500 microns (3) and a diameter of 6 millimeters, with concentric rings on the anterior surface of the lens. The lens, coupled with suitable haptic designs, is to be implanted within the lens capsule (19) of the eye after surgical removal of the natural crystalline lens. The anterior surface of the lens (1) has concentric rings (6) with steps of approximately 10 microns (5) that can be concave, convex or piano, with the edge of the step parallel in each case to the light rays traversing the lens at that point. The posterior surface of the lens (3) is aspherical and smooth. The concentric rings focus 95% or better of light at a specific target point on the retina, thus making a monofocal lens, with focal flexibility provided through haptic design providing movement of the lens forward in the posterior chamber in response to contraction and expansion of the ciliary body and concomitant repositioning of the zonules. The inventive lens is a unitarily formed, seamless body comprised preferably of hydrophilic acrylates or acrylates and silicone blends. Other possible materials include hydrophobic acrylates, polymethylmethacrylate (such as for example PMMA) or acrylic blends. The inventive lens, being less than 500 microns thick, provides greater transfer of light through the lens, thus more closely replicating the function of a natural, emmotropic lens, while the thinness, making the lens lightweight, allows the ciliary body to move the lens with less effort, thus facilitating comfort in the presbyopic eye.
Owner:ANEW IOL TECH
Instrument for the surgical removal of a defective heart valve
An instrument for the surgical removal of a defective heart valve. The instrument has elements that can be collapsed, in which the instrument cutting elements are collapsed when the instrument is introduced into the surgical field and are covered by means of contact protective sheaths. The instrument cutting elements are formed as cutting ring halves that can fold in around a hinge axis, these cutting ring halves being elastically deformable during the folding-in process, whereby their deformation is achieved by fish joints that also serve to aid in mutually moving the contact protective sheaths onto each of two body members.
Owner:ENDOSMART GES FUR INNOVATIVE MEDIZINTECHN
Enhanced follicular extraction punch and method
Provided herein is an apparatus and method useful for surgical removal of mammalian tissue at specific depths and specific angles.
Owner:COLE JOHN P
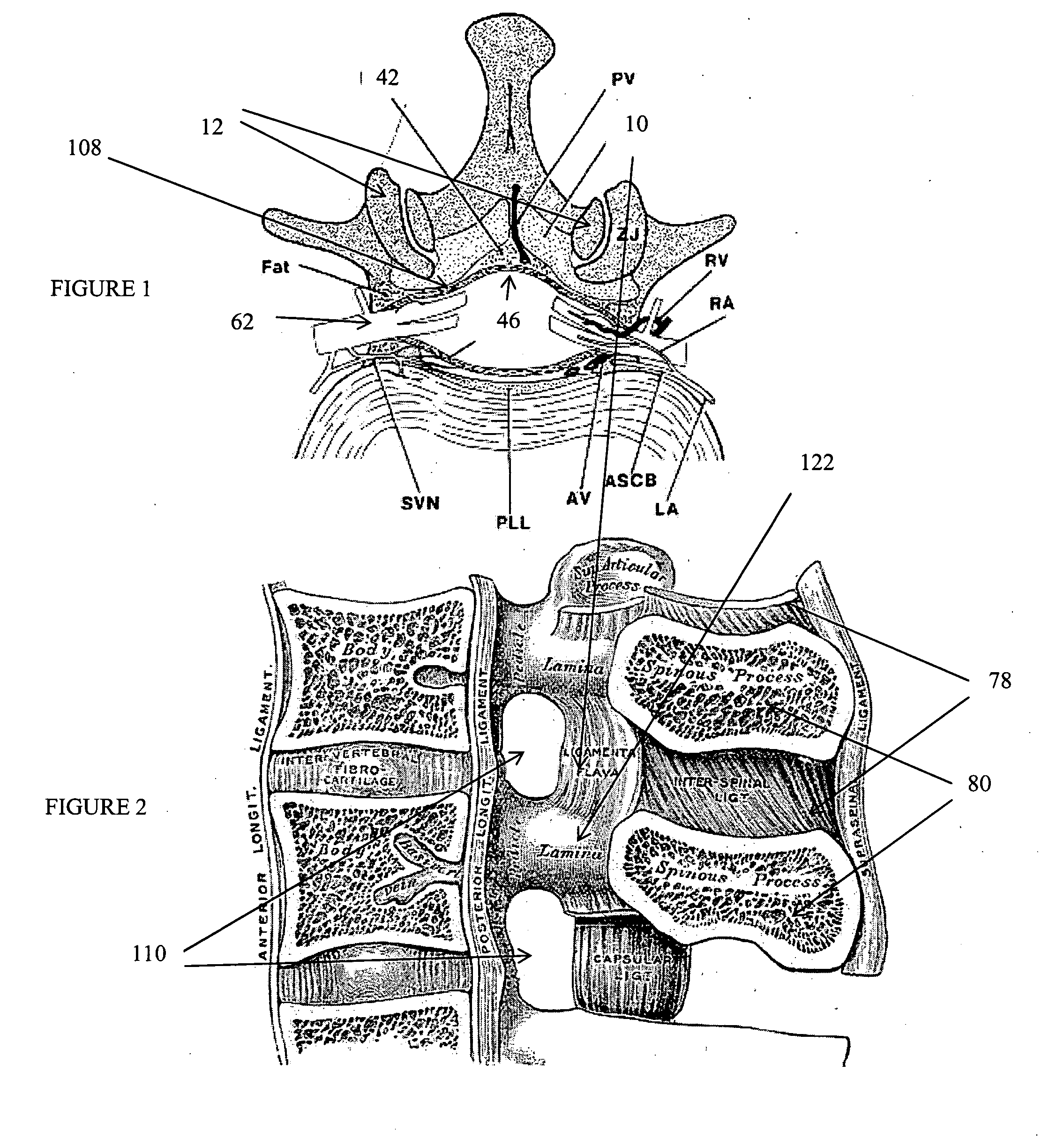
Implement and method to extract nucleus from spine intervertebral disc
InactiveUS20070265633A1Reduce riskMinimally invasiveVaccination/ovulation diagnosticsExcision instrumentsLamina terminalisSurgical removal
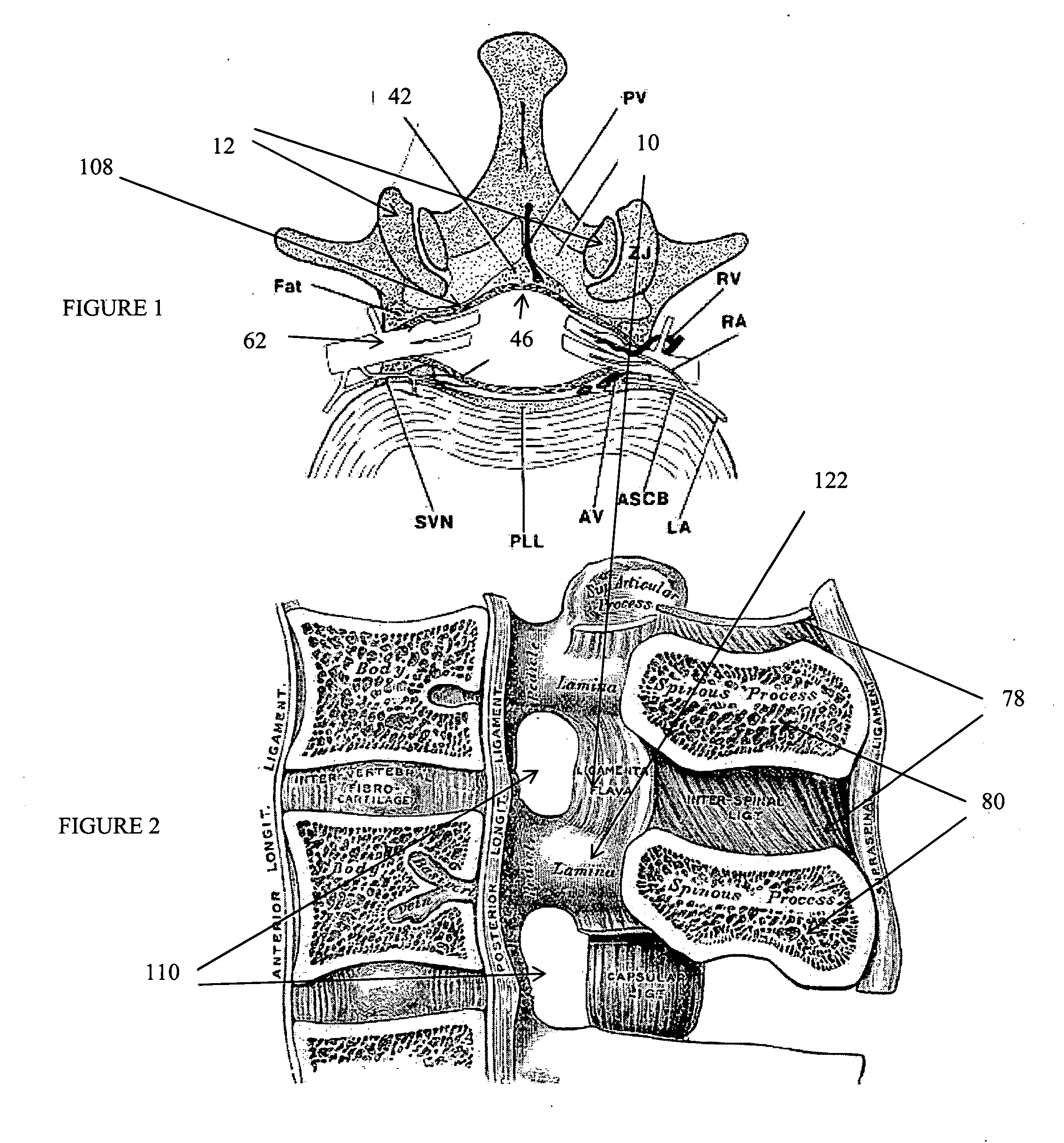
This invention proposes a device directed to rapid surgical removal of the nucleus pulposus from the spine intervertebral space. The invention is manipulated within the intervertebral space to engage and dislodge small pieces of nucleus material that are mobilized proximally for disposal. Aspects of the invention are included to protect the endplate tissue of vertebrae and limit damage to the integrity of the disc annulus.
Owner:DEVICIX
Nucleus Extraction from Spine Intervertebral Disc
InactiveUS20060241566A1Reduce riskMinimally invasiveExcision instrumentsSurgical pincettesSpinal columnLamina terminalis
This invention proposes devices and methods directed to providing rapid and complete surgical removal of the nucleus from the spine intervertebral space. In addition, the invention protects the endplate tissue of vertebrae containing the disc and limits damage to the integrity of the disc annulus.
Owner:DEVICIX
Rapid closing surgical closure device
A surgical or wound closure device utilizes a slide fastener for rapidly closing a surgical incision or wound with precise apposition of the sides of the incision. The surgical closure devices are configured for linear incisions, shaped incisions, such as used for wedge biopsy or excisional biopsy, and long incisions, such as used for laparotomy or surgical removal of redundant skin. The surgical closure device may be adhered to the patient's skin prior to making an incision and is subsequently used for closing the incision.
Owner:ZIPLINE MEDICAL
Antipyretic compositions and methods
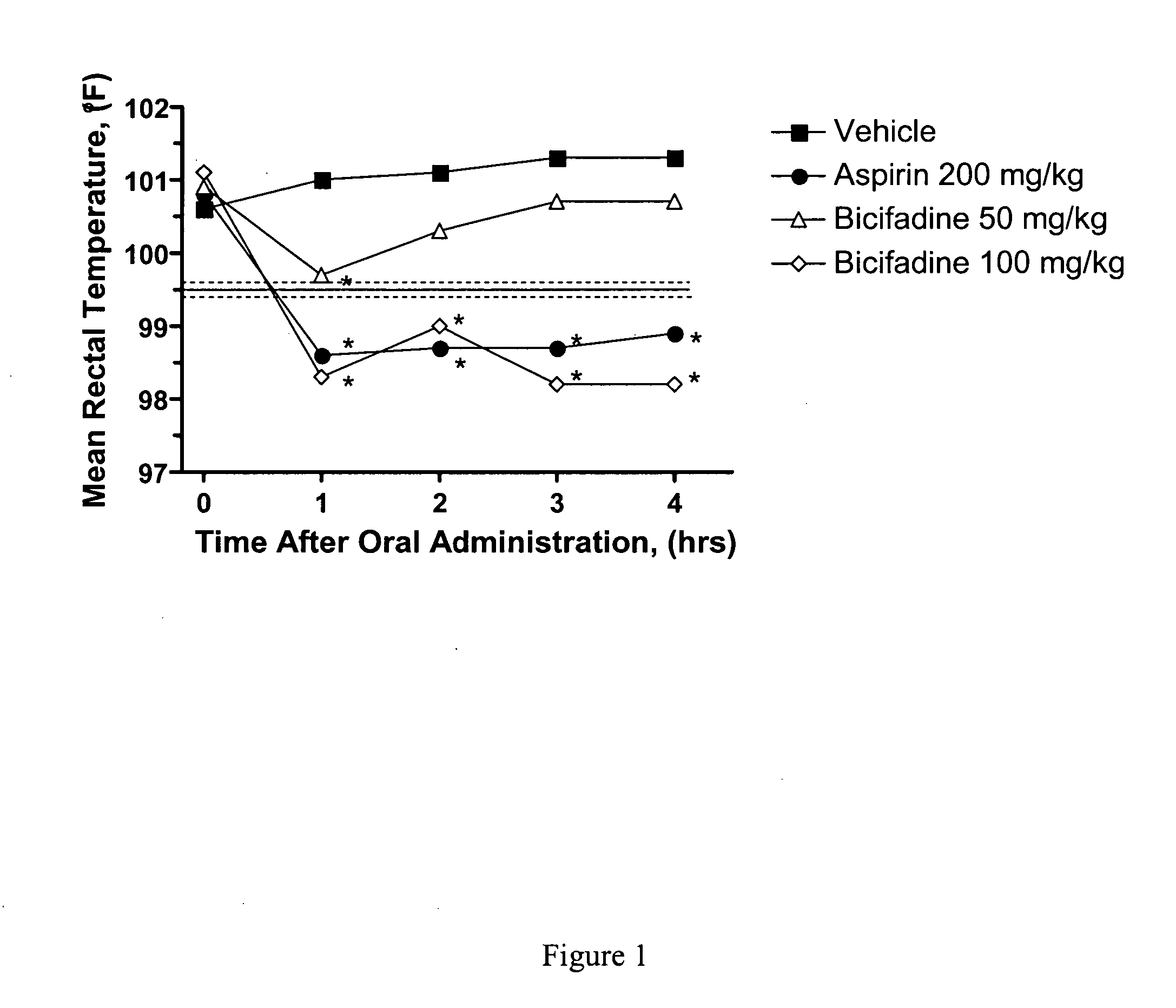
InactiveUS20060100263A1Prevent hyperthermiaReducing elevated body temperatureBiocideNervous disorderSurgical removalFebrile seizure
Methods and compositions containing bicifadine are provided for the treatment and prevention of hyperthermia in mammalian subjects. The methods and compositions may be used to prevent or treat fever, pyresis, menopausal hot flashes; peri menopausal hot flashes, postmenopausal hot flashes, hot flashes caused by anti-estrogen therapy, hot flashes secondary to surgical removal of estrogen producing tissue, hot flashes caused by radiation therapy, malignant hyperthermia, serotonin syndrome, heat stroke, febrile seizures, and neuroleptic malignant syndrome, among other conditions. Additional compositions and methods are provided employing bicifadine to treat pyresis and simultaneously elicit an analgesic response in mammalian subjects. Yet additional compositions and methods are provided which employ bicifadine in combination with a second antipyretic agent, a second analgesic agent, or a different therapeutic agent to yield more effective antipyretic treatment tools, and / or dual activity therapeutic methods and formulations useful to prevent or reduce hyperthermia and one or more additional symptoms (e.g., pain, or depression) in mammalian subjects.
Owner:DOV PHARMA
Surgical guides and methods for positioning artificial teeth and dental implants
InactiveUS7835811B2Eliminate radiographic scatterMedical simulationDental implantsDigital dataNatural tooth
A method is set forth for making a computer model of patient's jaws on the basis of digital information. Digital data about the jaws, teeth, soft tissues and artificial teeth is joined in computer space to create aesthetic and functional plans for the removal of teeth, shaping of supporting bone and placement of dental implants. Artificial teeth and pre-manufactured prosthetic devices are made and attached to the dental implants at the time of surgery. The aesthetic and functional position of artificial teeth is determined prior to surgical removal of natural teeth and the ideal position of implants and the proper form of the remaining bone are determined prior to surgery. Surgical guides used to shape bone, record occlusal orientation and position dental implants are manufactured using computer milling or layered manufacturing.
Owner:VOXELOGIX CORP
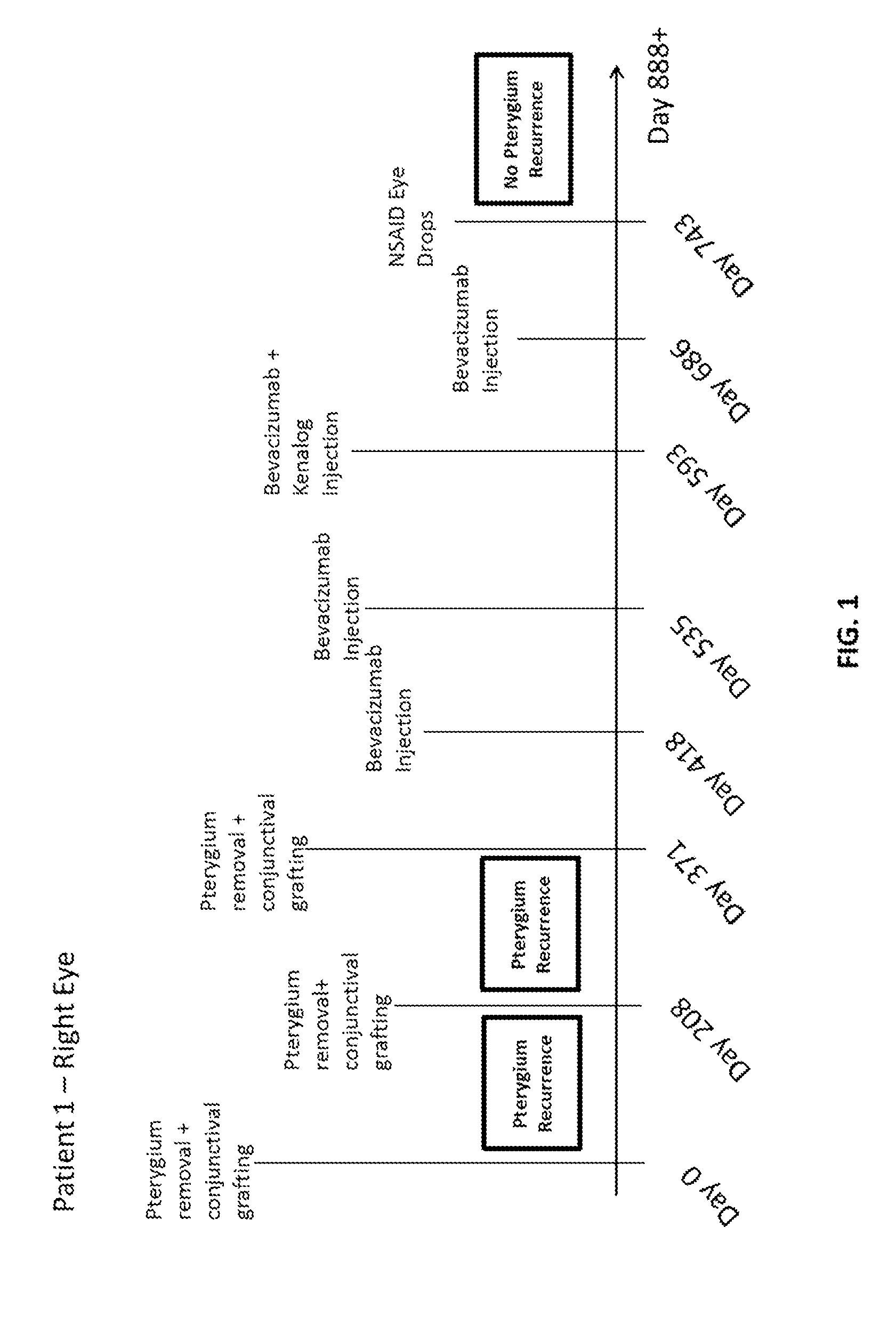
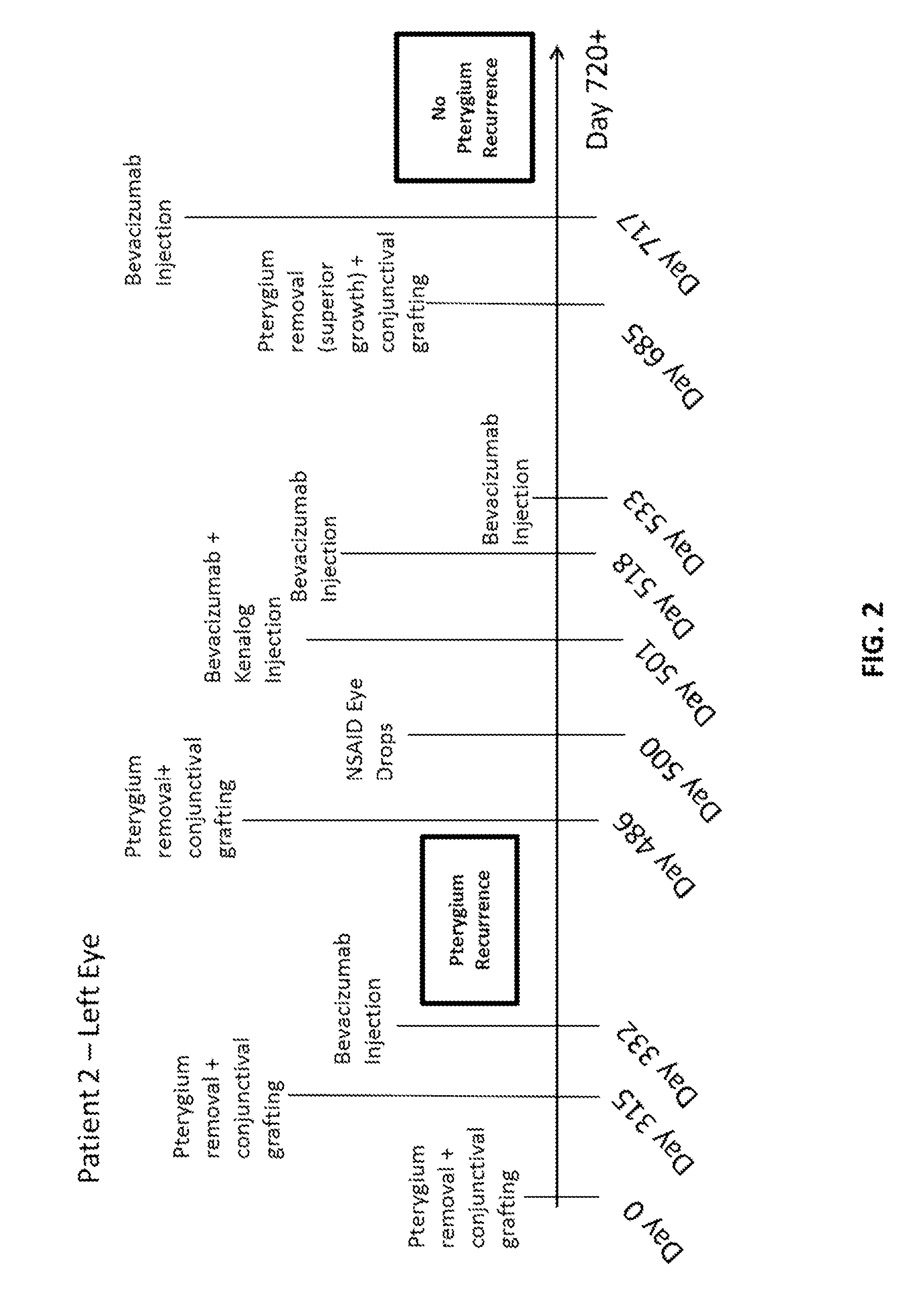
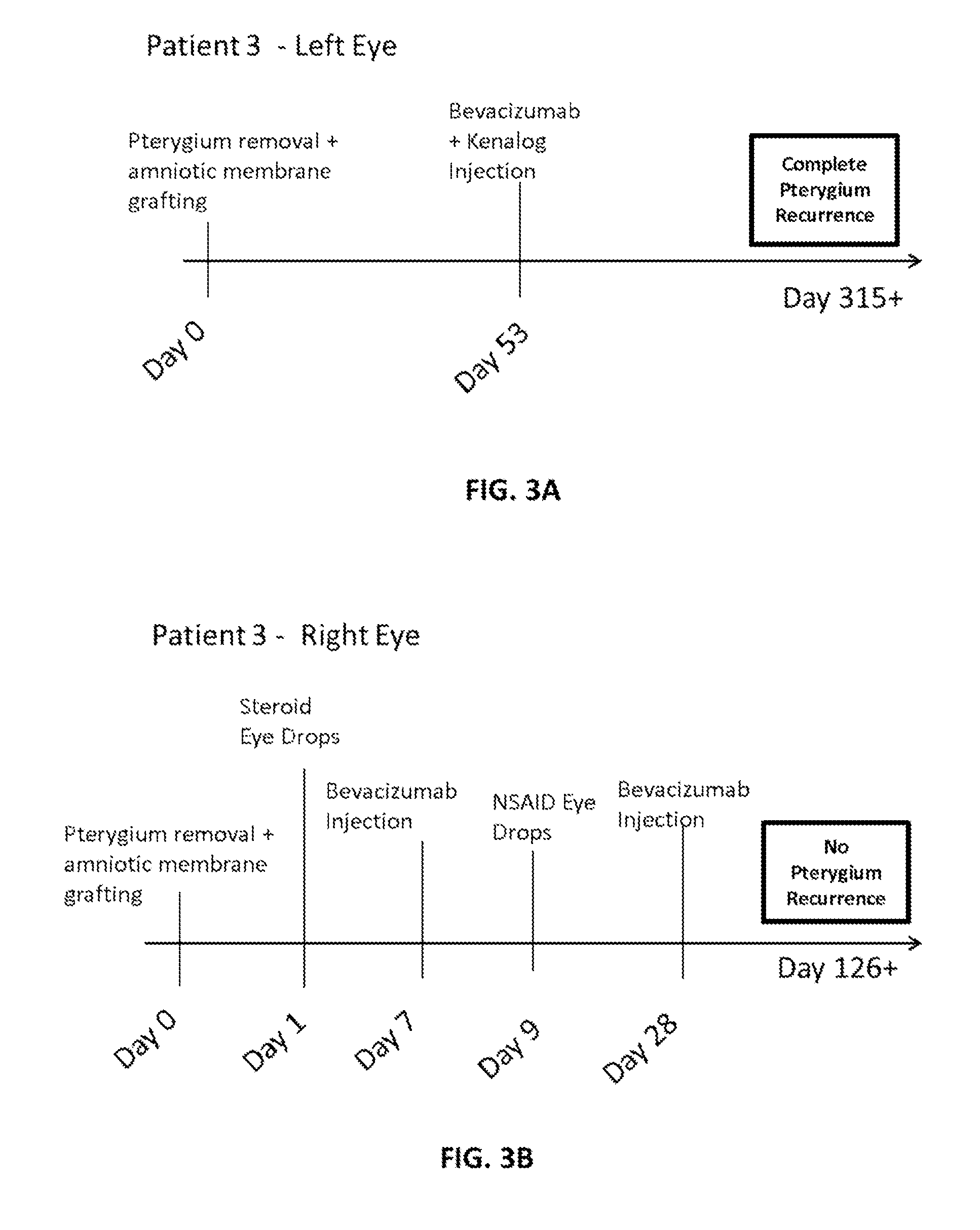
Methods of treating pterygium
Methods for treating pterygium recurrence following pterygiectomy, and for treating keloid recurrence, following surgical removal of the keloid, are disclosed. The methods include administering an anti-VEGF agent (e.g., antibody (e.g., bevacizumab) or small molecule inhibitor of VEGF signaling), or a combination therapy that includes co-administering an anti-VEGF agent, with an anti-inflammatory steroid and / or a non-steroidal anti-inflammatory drug (NSAID) to a subject.
Owner:PHAM RANDAL TANH HOANG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com