Methods and related compositions for the non-surgical removal of fat
a composition and fat technology, applied in the direction of drug compositions, biocides, metabolic disorders, etc., can solve the problems of shock or fluid accumulation that must be drained, perforation injury to vital organs, friction burns or other damage to skin or nerves,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Sodium Deoxycholate and Phosphatidylcholine Formulations
[0059] Phosphatidylcholine bile salt formulation (PBF) (5.0% highly purified soy derived PC, 4.75% sodium deoxycholate, and 0.9% benzyl alcohol, in sterile water, Table 1) was obtained from Hopewell Pharmacy, Hopewell, N.J. Sodium deoxycholate and Triton® X-100 detergent (Triton®, alkylaryl polyether alcohol) were obtained from Sigma-Aldrich Corp. (St. Louis, Mo.). Empigen® BB detergent (Empigen®, lauryldimethylbetaine, Calbiochem, Biosciences, Inc., La Jolla, Calif.). Stock reagents (5% dilutions) were prepared in PBS buffer.
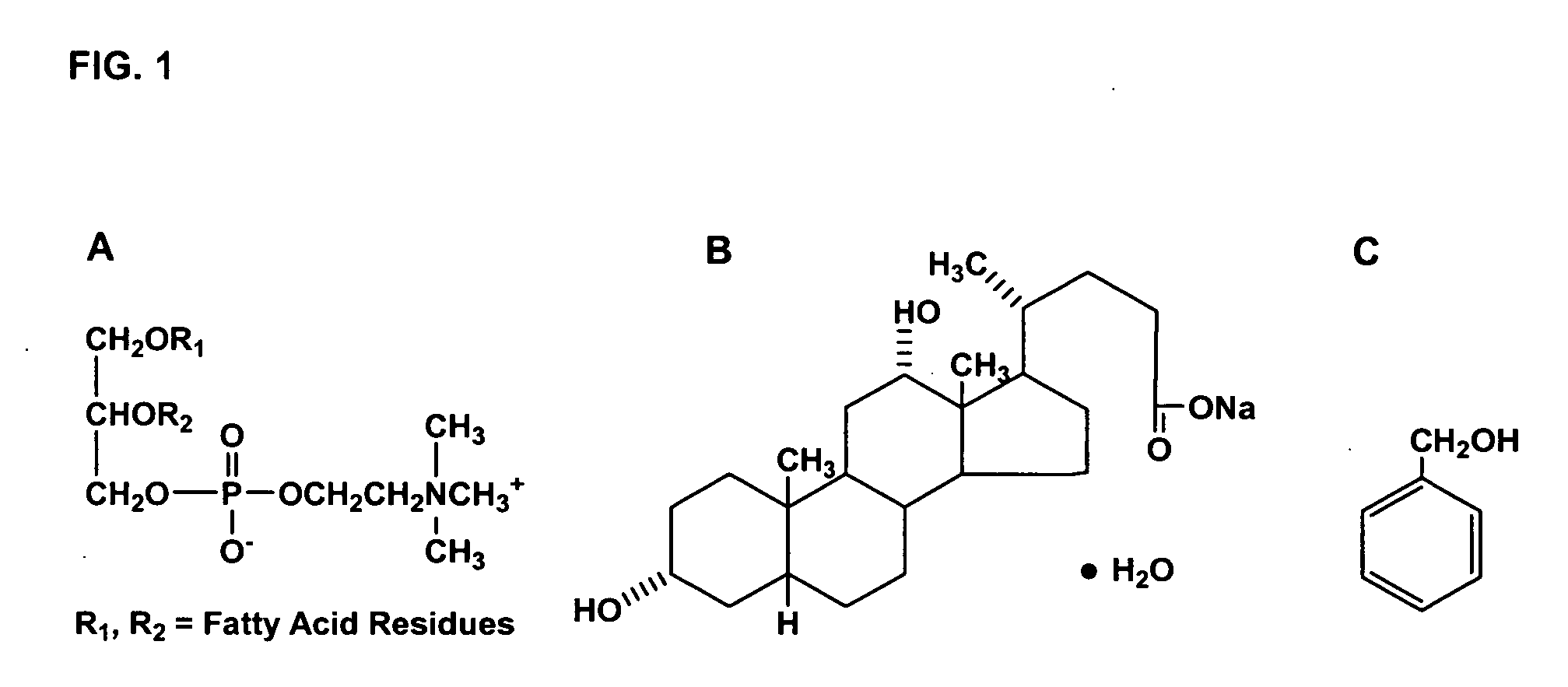
[0060] The molecular structure of (a) phosphatidylcholine, (b) sodium deoxycholate and (c) benzyl alcohol are depicted in FIG. 1.
TABLE 1Injectable PBFPhosphatidylcholine5.00%(w / v)Sodium deoxycholate4.75%Benzyl alcohol0.90%Water100mL
example 2
Effects of Sodium Deoxycholate and Phosphatidylcholine Solutions in Cultured Cells
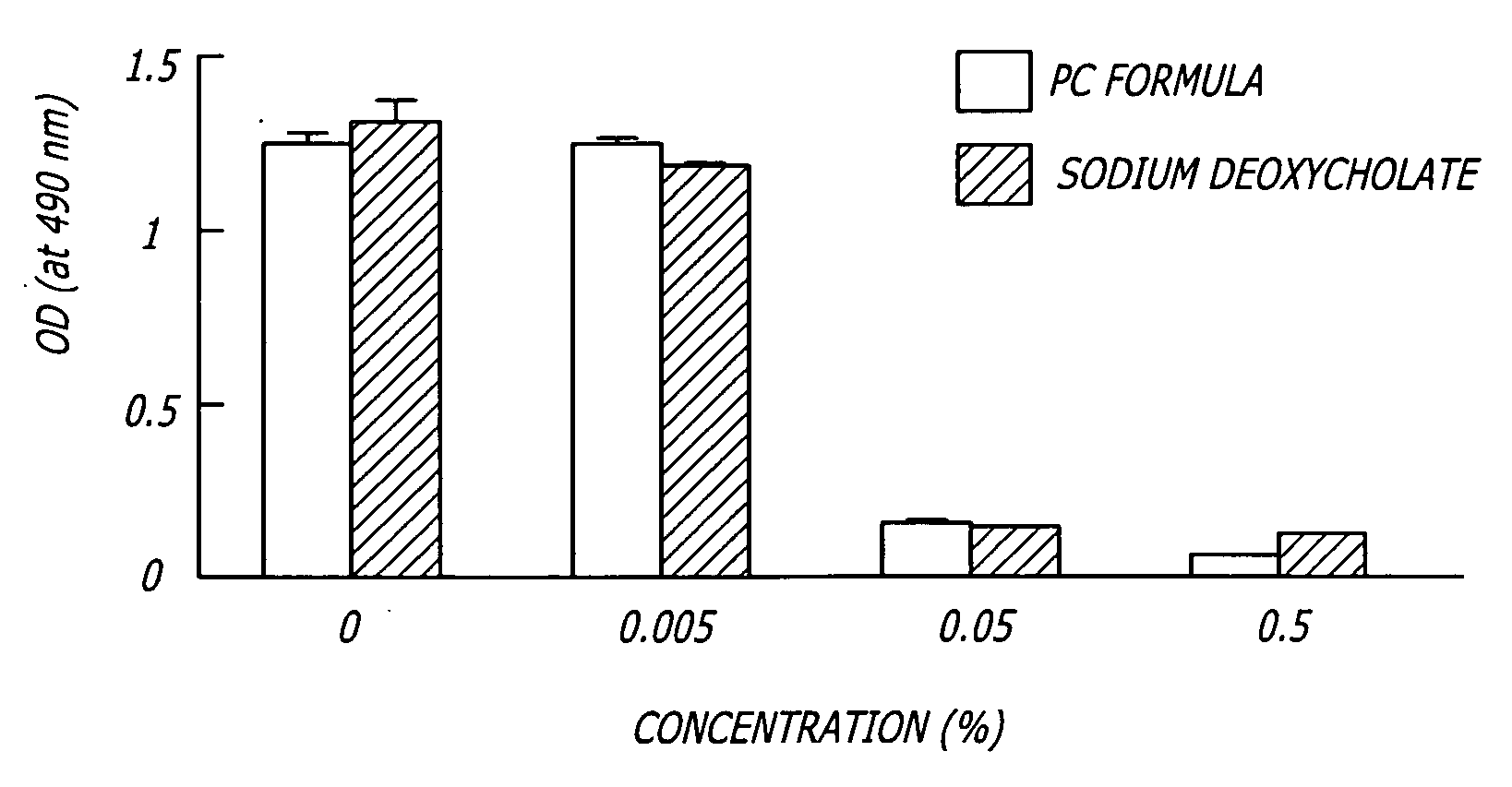
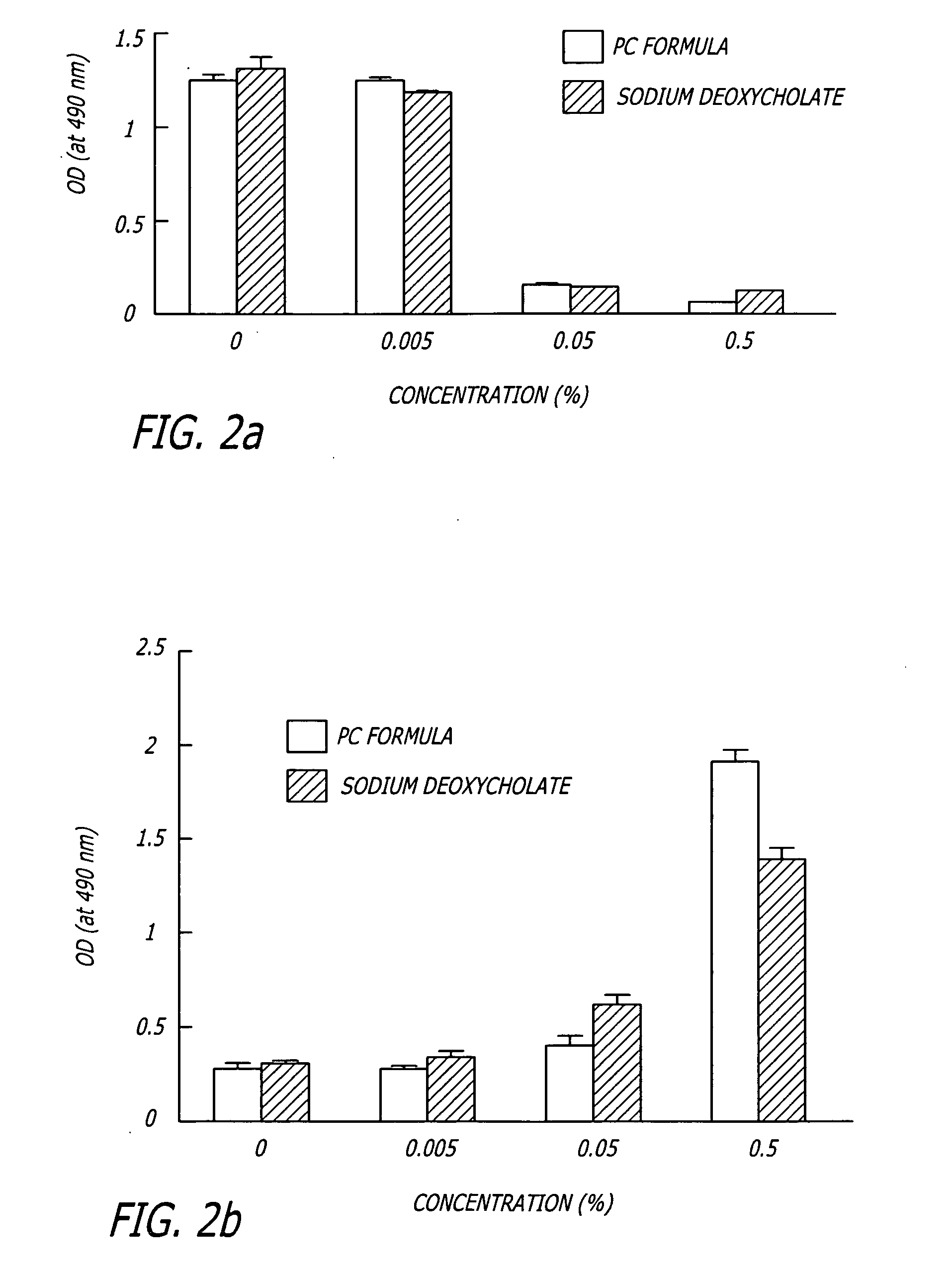
[0061] To measure cell viability after detergent treatment, HaCaT human keratinocyte cells were cultured in DMEM (Dulbecco's modified Eagle's medium) supplemented with 10% fetal calf serum, penicillin, and streptomycin. HaCaT cells were cultured in 6 well plates and incubated with 0%, 0.005%, 0.050% or 0.500% PBF (PC Formula) or sodium deoxycholate for 30 min at 37° C. prior to determination of cell viability using the MTS assay, which uses a tetrazolium compound that produces a color change when bioreduced by metabolically active cells (CellTiter 96® AQueous Non-Radioactive Cell Proliferation Assay, Promega, Corp. Madison, Wis.). Cell viability was determined by an absorbance spectrophotometer (at 490 nm) after a 4 hour incubation with the assay at 37° C. To determine cell viability in fresh tissue, fat specimens were incubated for 4 hours in 24 well plates with stock reagents and the MTS assay. Tiss...
example 3
Effects of Sodium Deoxycholate and Phosphatidylcholine Solutions in Porcine Tissue
[0063] Porcine tissue was obtained immediately after sacrifice, shaved, and placed on ice for a maximum of four hours before use. Fat specimens were obtained by removing the epidermis and dermis of a punch biopsy with a scalpel and trimmed. Fat specimens were loaded with calcein dye by incubating 1 hour at 37° C. with Calcein-AM (Sigma). Stock reagents were added to the fat specimens and incubated for 30 min at 37° C. with gentle agitation. Calcein retention was determined by tissue fluorescence using purple (411 nm) light and visually observing the emitted green (500 nm) light using an emission filters.
[0064] Histology was performed by injecting stock reagent solutions (0.5 mL) into full thickness porcine skin at various levels (epidermis, dermis, and subcutaneous tissue) with 1.0 mL syringes and 30-gauge, 0.5 inch needles. Needle depth was visualized along the margin of the porcine tissue with the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery time | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| medical composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com