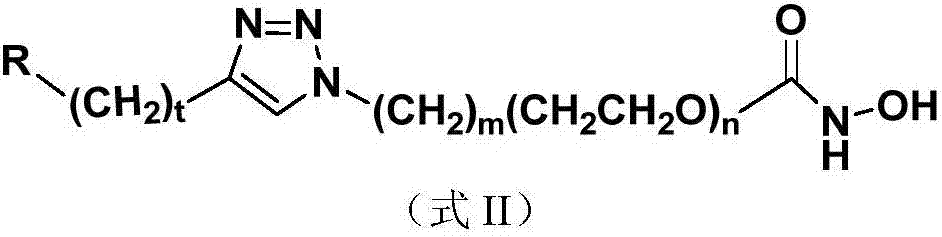Patents
Literature
32 results about "Proliferation assay" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
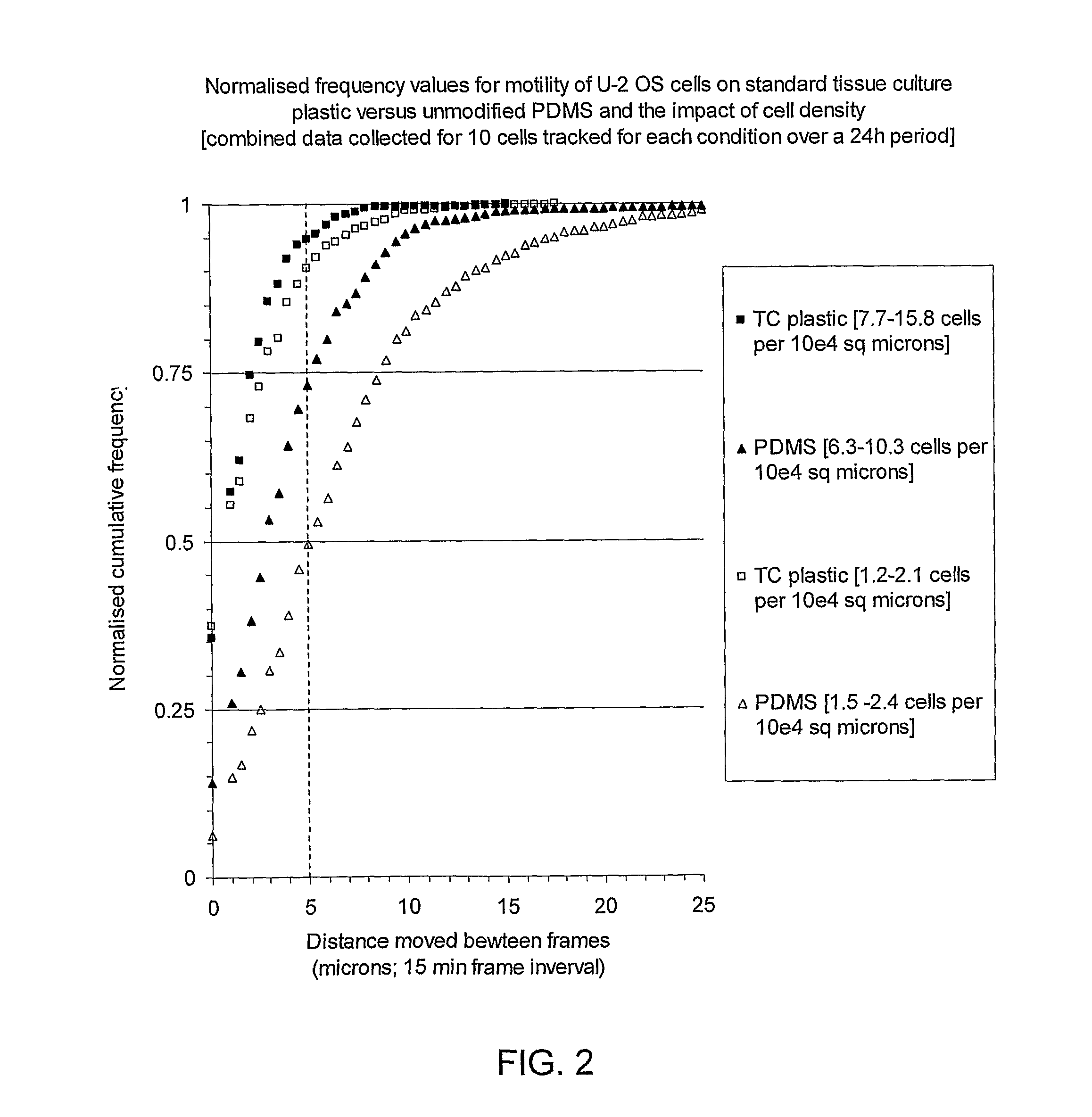
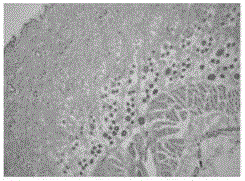
The Proliferation Assay allows to determine the number of cells that are growing in the absence or presence of certain proliferation affecting agents, e.g. TNF-alpha or anti-Fas antibody (IPO-4).
Agonist antibody to human thrombopoietin receptor
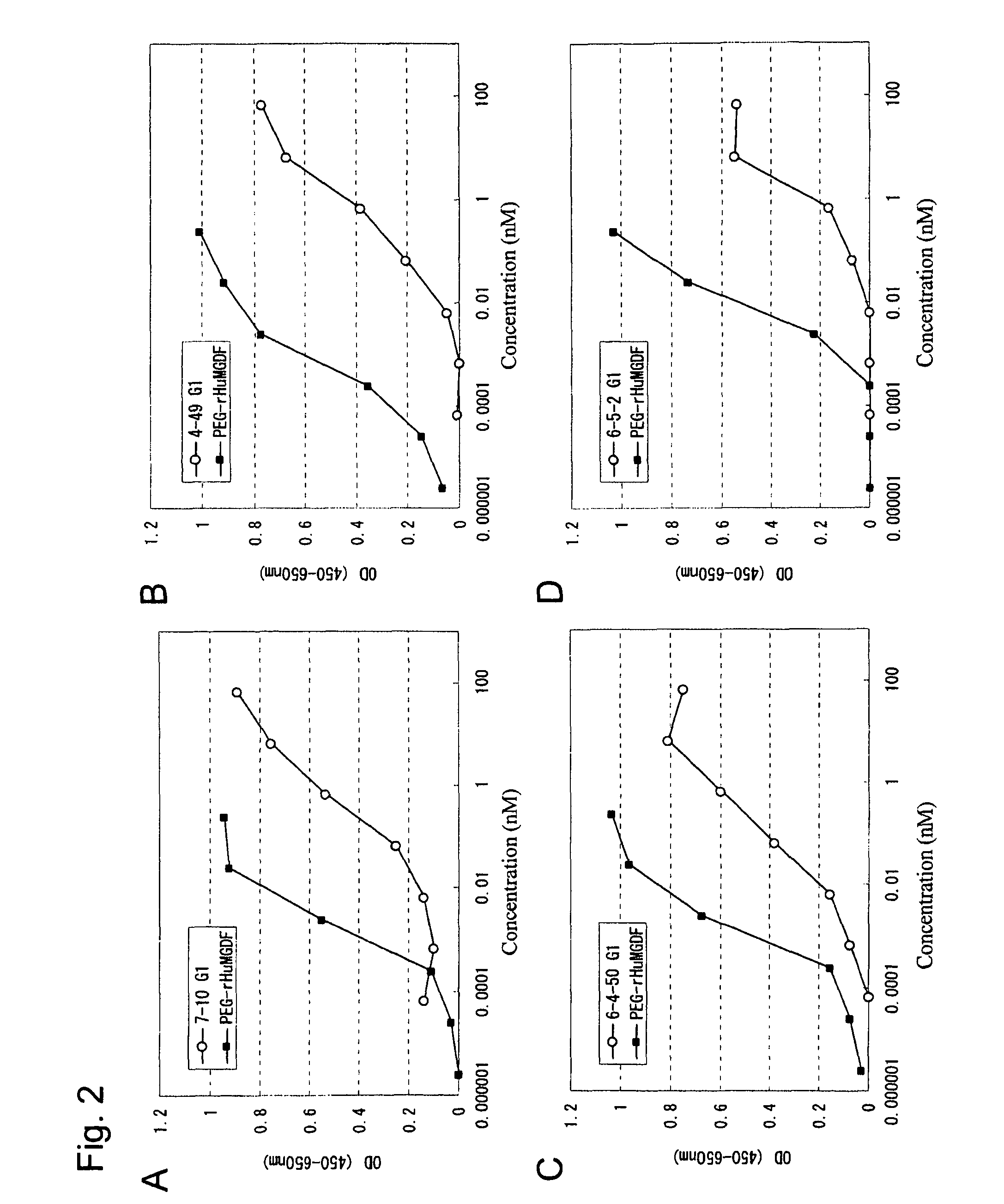
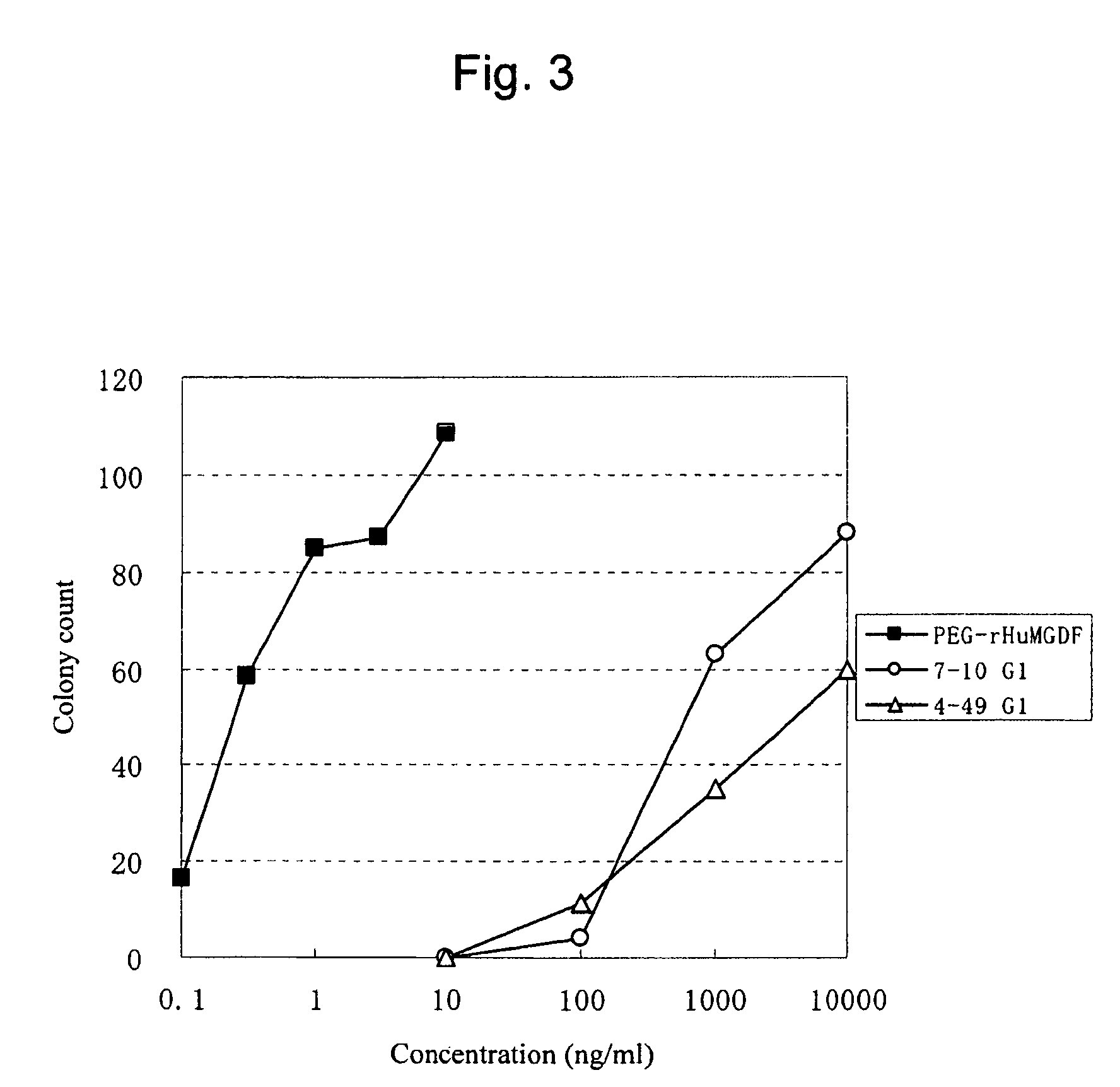
InactiveUS20100004429A1High activityLow antigenicityThrombopoietinHybrid immunoglobulinsHuman plateletUmbilical cord
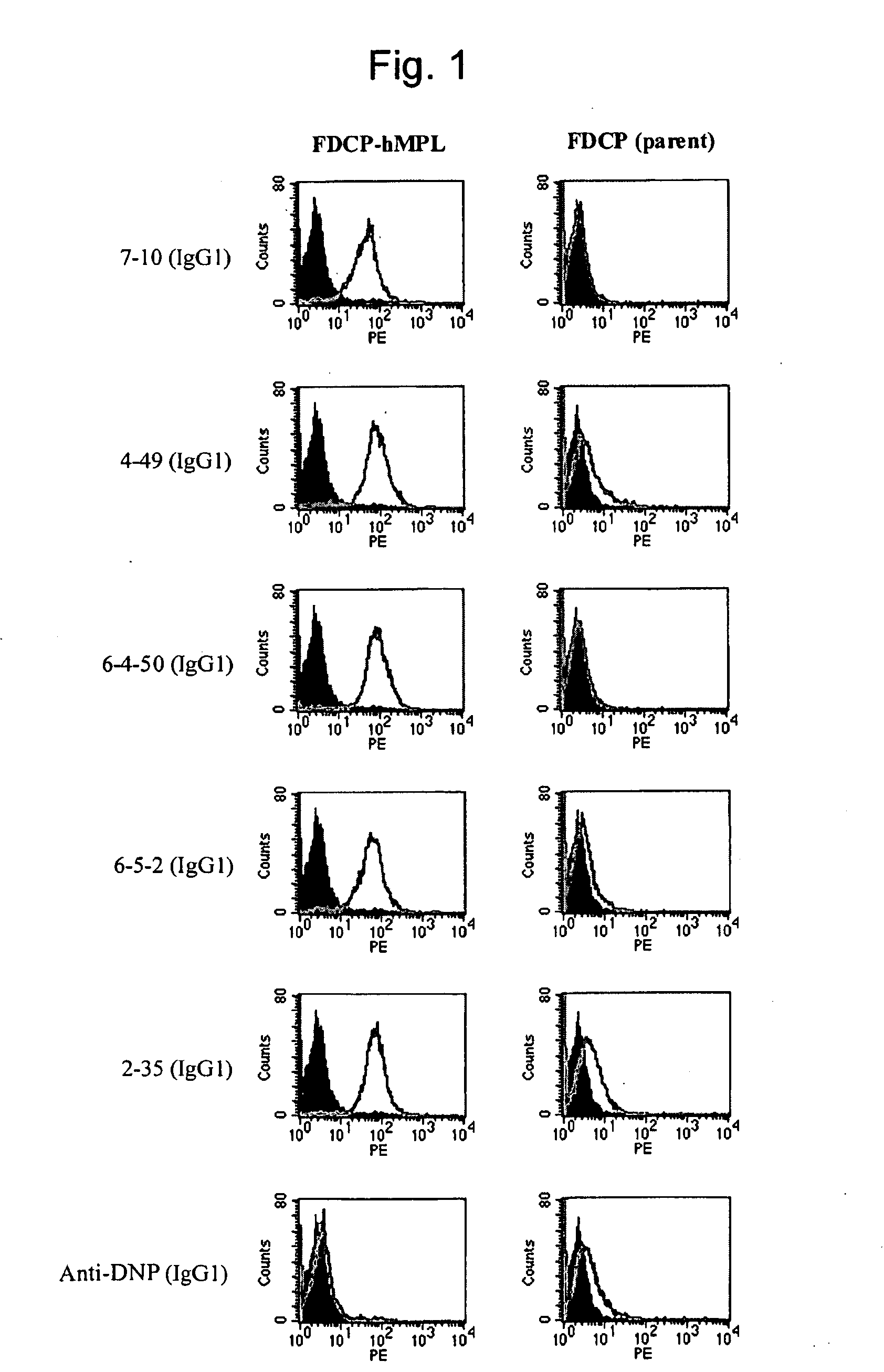
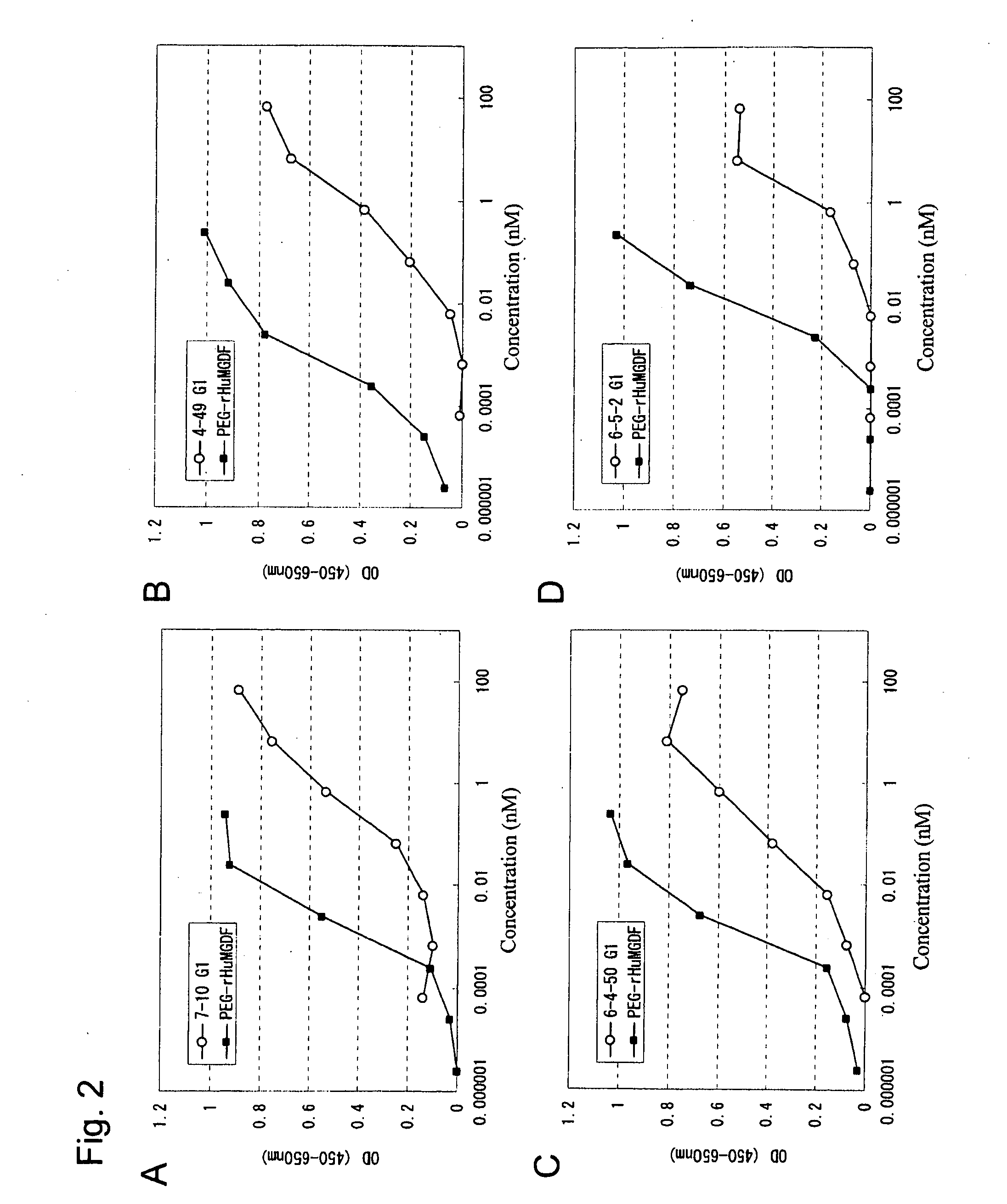
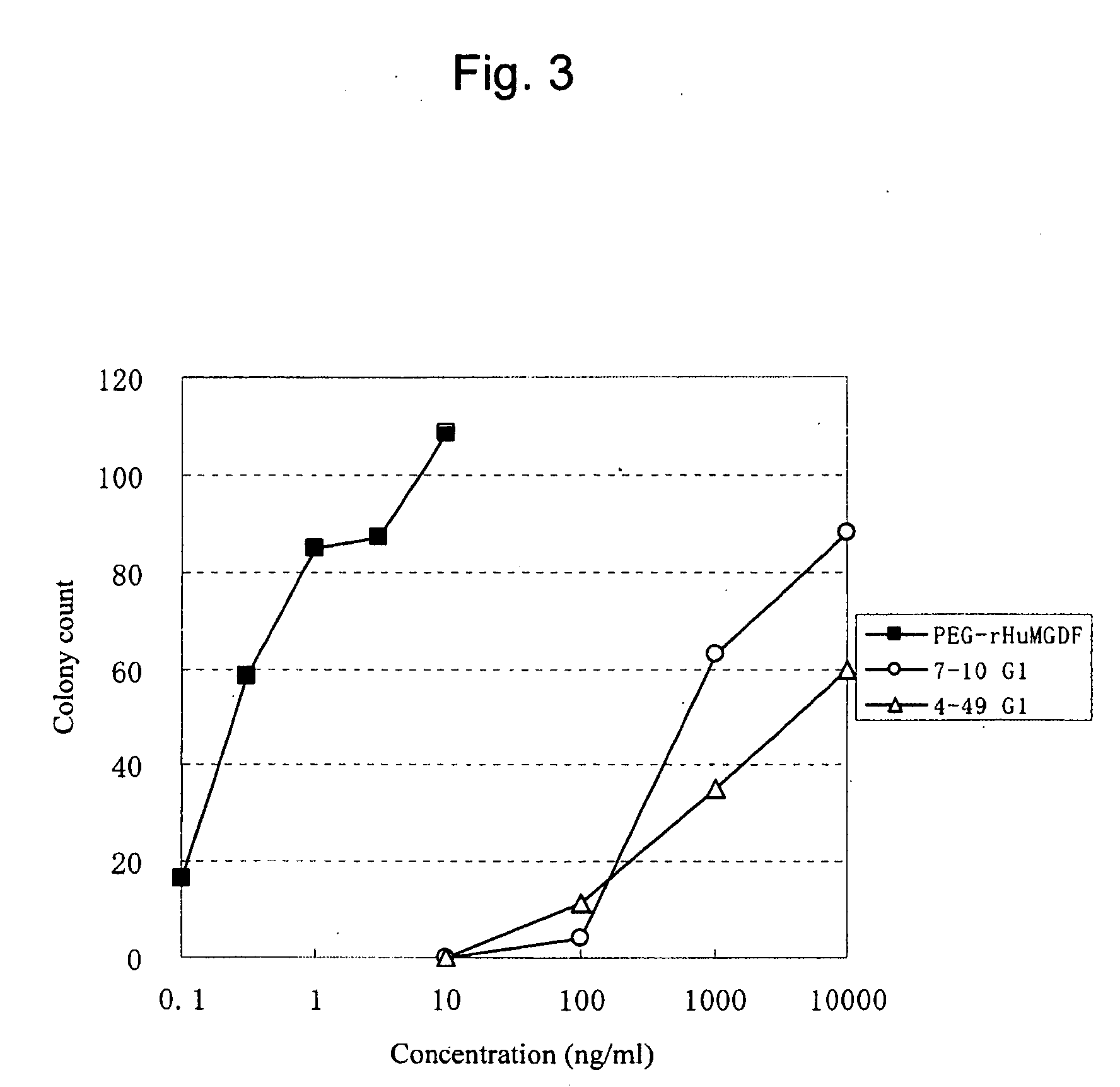
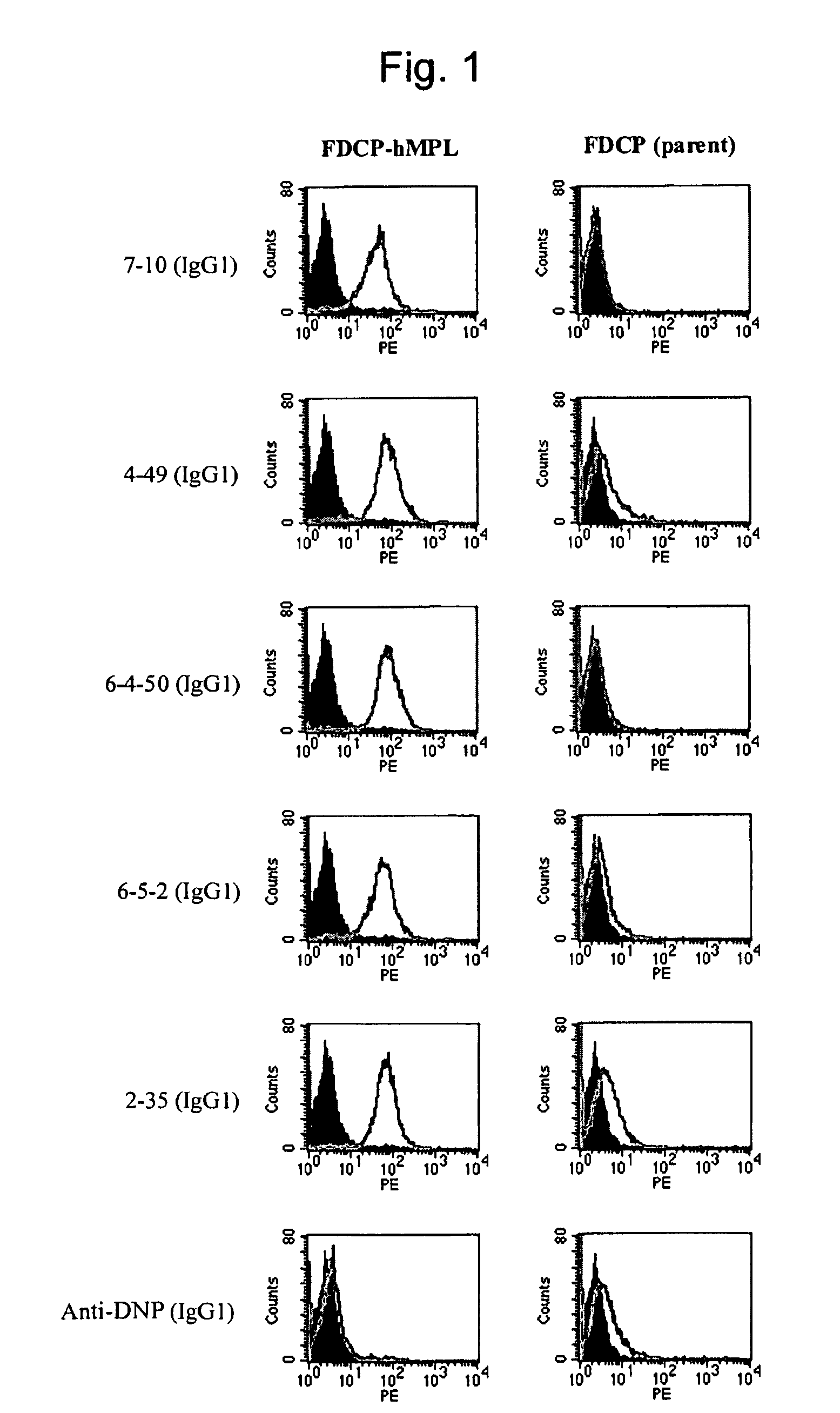
This invention provides an agonist antibody to a human thrombopoietin receptor (alias: human c-Mpl). More particularly, this invention provides an agonist antibody to a human thrombopoietin receptor, wherein the agonist antibody comprises: antibody constant regions comprising (1) amino acid sequences in a heavy chain constant region and a light chain constant region of a human antibody, (2) an amino acid sequence of a heavy chain constant region with a domain substituted between human antibody subclasses, and an amino acid sequence of a light chain constant region of a human antibody, or (3) amino acid sequences comprising a deletion(s), substitution(s), addition(s), or insertion(s) of one or several amino acid residues in the amino acid sequences of (1) or (2) above; and antibody variable regions capable of binding to and activating a human thrombopoietin receptor; and wherein the agonist antibody has the properties: (a) that the antibody induces colony formation at a concentration of 10,000 ng / ml or lower as determined by the CFU-MK colony formation assay using human umbilical-cord-blood-derived CD34+ cells; and (b) that the antibody has a maximal activity at least 50% higher than that of PEG-rHuMGDF and an 50% effective concentration (EC50) of 100 nM or less in the cell proliferation assay using UT7 / TPO cell. Also provided is a pharmaceutical composition for treating thrombocytopenia comprising said antibody.
Owner:KYOWA HAKKO KIRIN CO LTD
Agonist antibody to human thrombopoietin receptor
This invention provides an agonist antibody to a human thrombopoietin receptor (alias: human c-Mpl). More particularly, this invention provides an agonist antibody to a human thrombopoietin receptor, wherein the agonist antibody comprises: antibody constant regions comprising (1) amino acid sequences in a heavy chain constant region and a light chain constant region of a human antibody, (2) an amino acid sequence of a heavy chain constant region with a domain substituted between human antibody subclasses, and an amino acid sequence of a light chain constant region of a human antibody, or (3) amino acid sequences comprising a deletion(s), substitution(s), addition(s), or insertion(s) of one or several amino acid residues in the amino acid sequences of (1) or (2) above; and antibody variable regions capable of binding to and activating a human thrombopoietin receptor; and wherein the agonist antibody has the properties: (a) that the antibody induces colony formation at a concentration of 10,000 ng / ml or lower as determined by the CFU-MK colony formation assay using human umbilical-cord-blood-derived CD34+ cells; and (b) that the antibody has a maximal activity at least 50% higher than that of PEG-rHuMGDF and an 50% effective concentration (EC50) of 100 nM or less in the cell proliferation assay using UT7 / TPO cell. Also provided is a pharmaceutical composition for treating thrombocytopenia comprising said antibody.
Owner:KYOWA HAKKO KIRIN CO LTD
Microtrench and tumour proliferation assay
InactiveUS20110171663A1Achieve traceabilityReduce swelling volumeBioreactor/fermenter combinationsBiological substance pretreatmentsCell based assaysCell type
There is provided a cell culture microtrench being defined on or in a surface of a substrate, wherein the ratio of the width of the microtrench to the maximum length of the short axis of a cell type of interest is about 6 or preferably less, the length of the short axis of the cell type being measured when a cell is in detached or suspended form. There is also provided an array comprising such a microtrench and uses of such microtrenches, including cell-based assays.
Owner:UNIV COLLEGE CARDIFF CONSULTANTS LTD
Preparation of cells, cell aggregates and tissue fragments
ActiveUS8871159B1Withdrawing sample devicesPreparing sample for investigationAngiogenesis growth factorCell clustering
This invention provides compositions and methods useful for the processing of a tissue sample and the preparation of cells, cell aggregates and / or tissue fragments. The invention provides two-stage filer devices and two-membrane devices. Cell aggregates and / or tissue fragments prepared using such devices and according to methods of the present invention can be used in a variety of assay systems, including, but not limited to, drug validation assays, drug screening assays, proliferation assays, metabolic assays, metastasis assays, angiogenesis assays, binding assays, biochemical assays, cellular assays, genetic assays, and the like.
Owner:APFEL CHRISTIAN
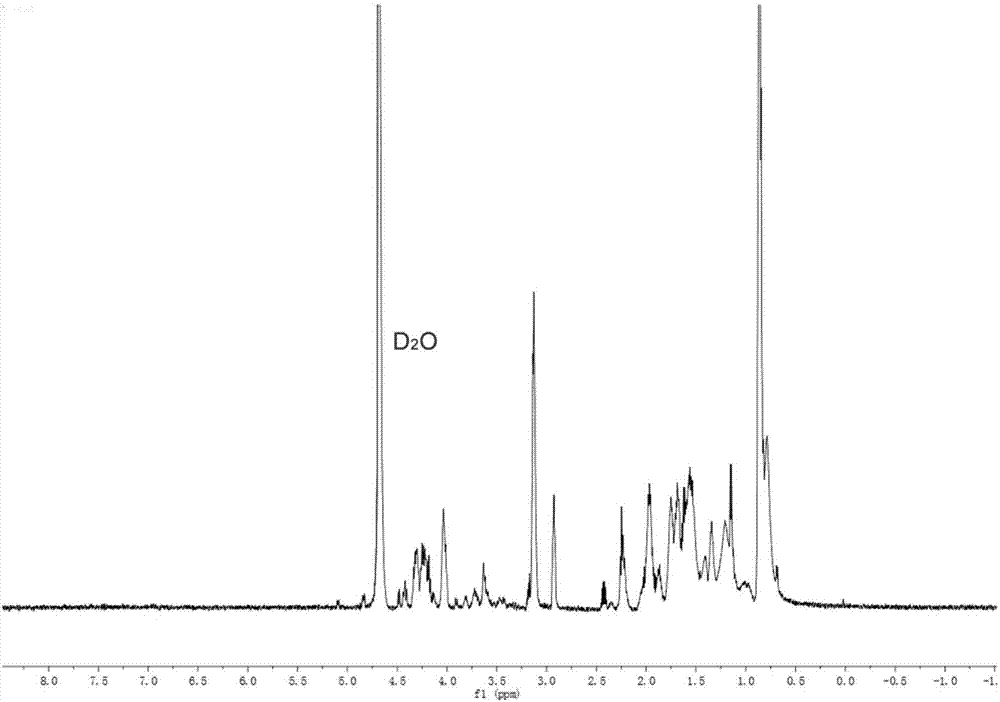
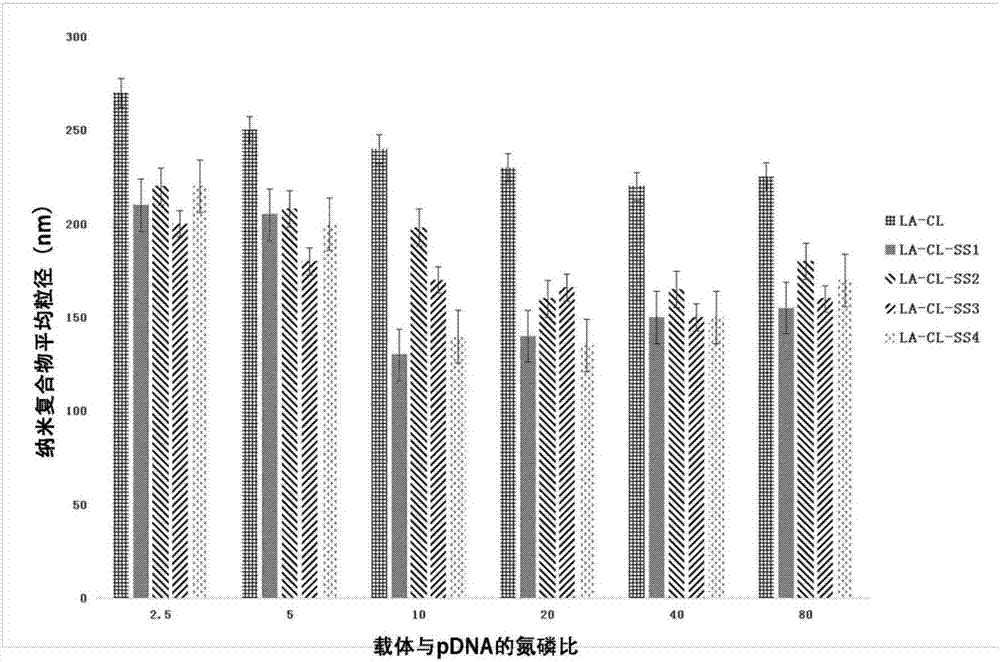
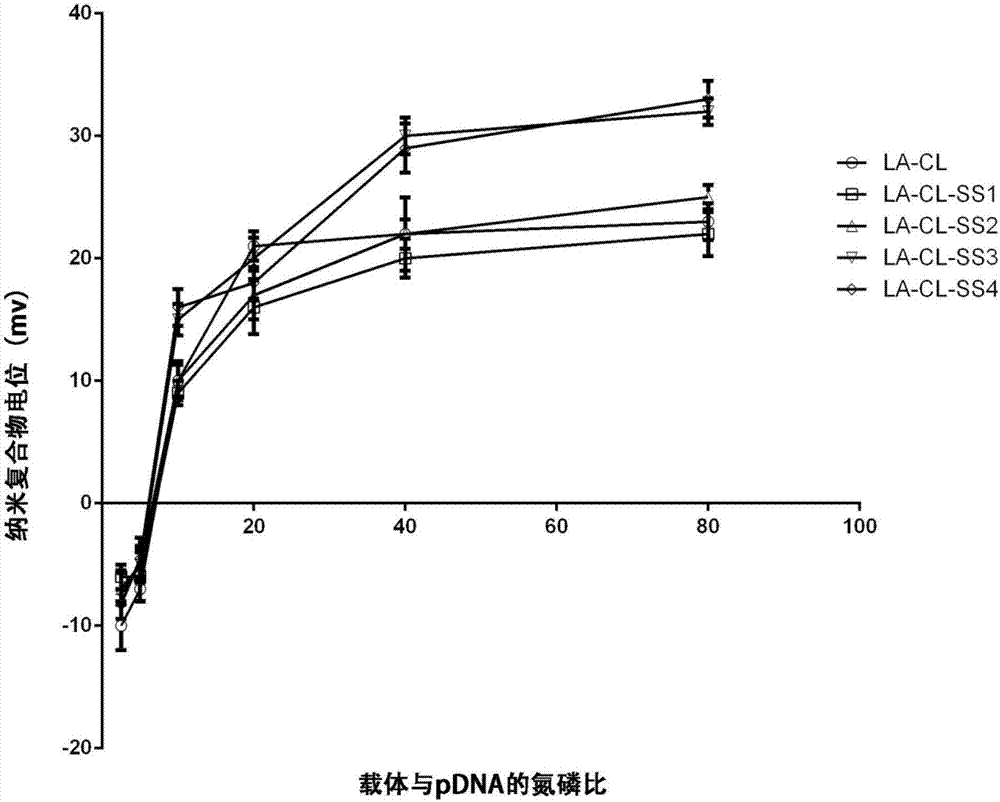
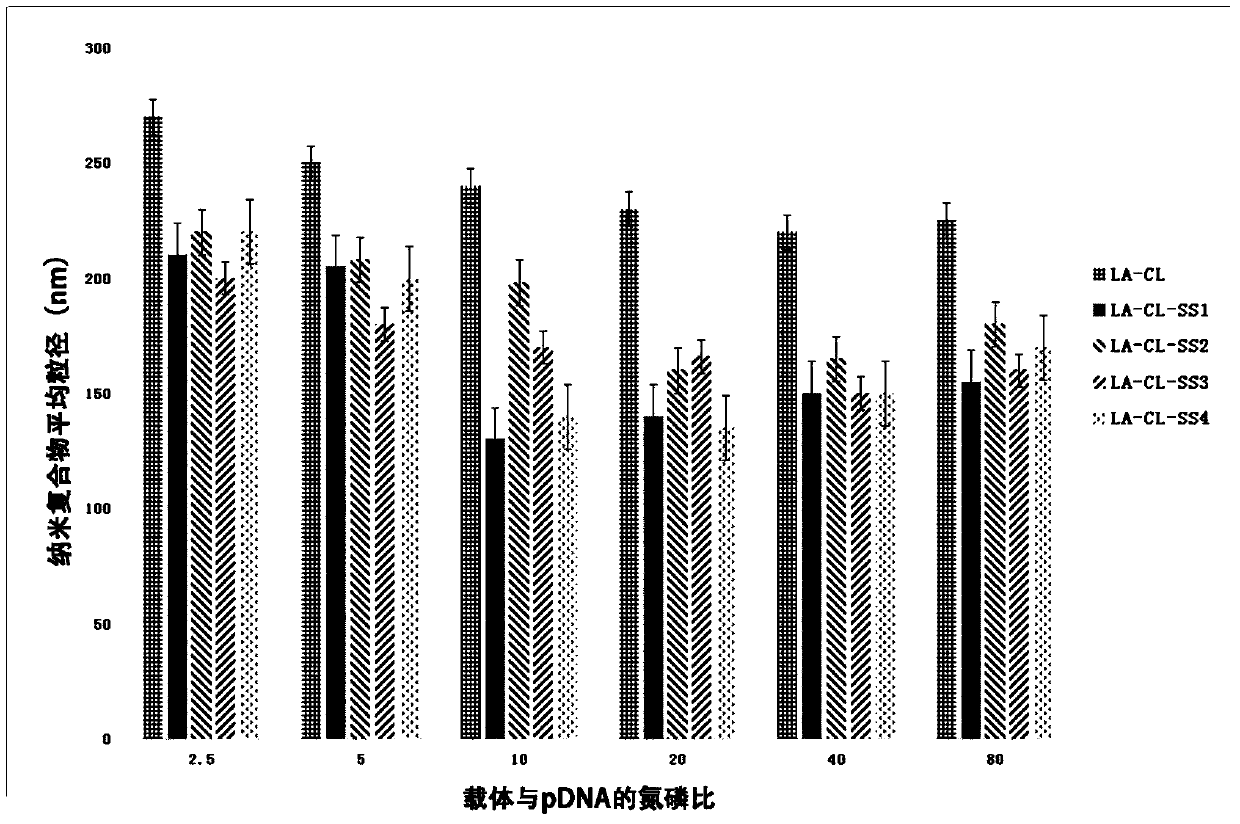
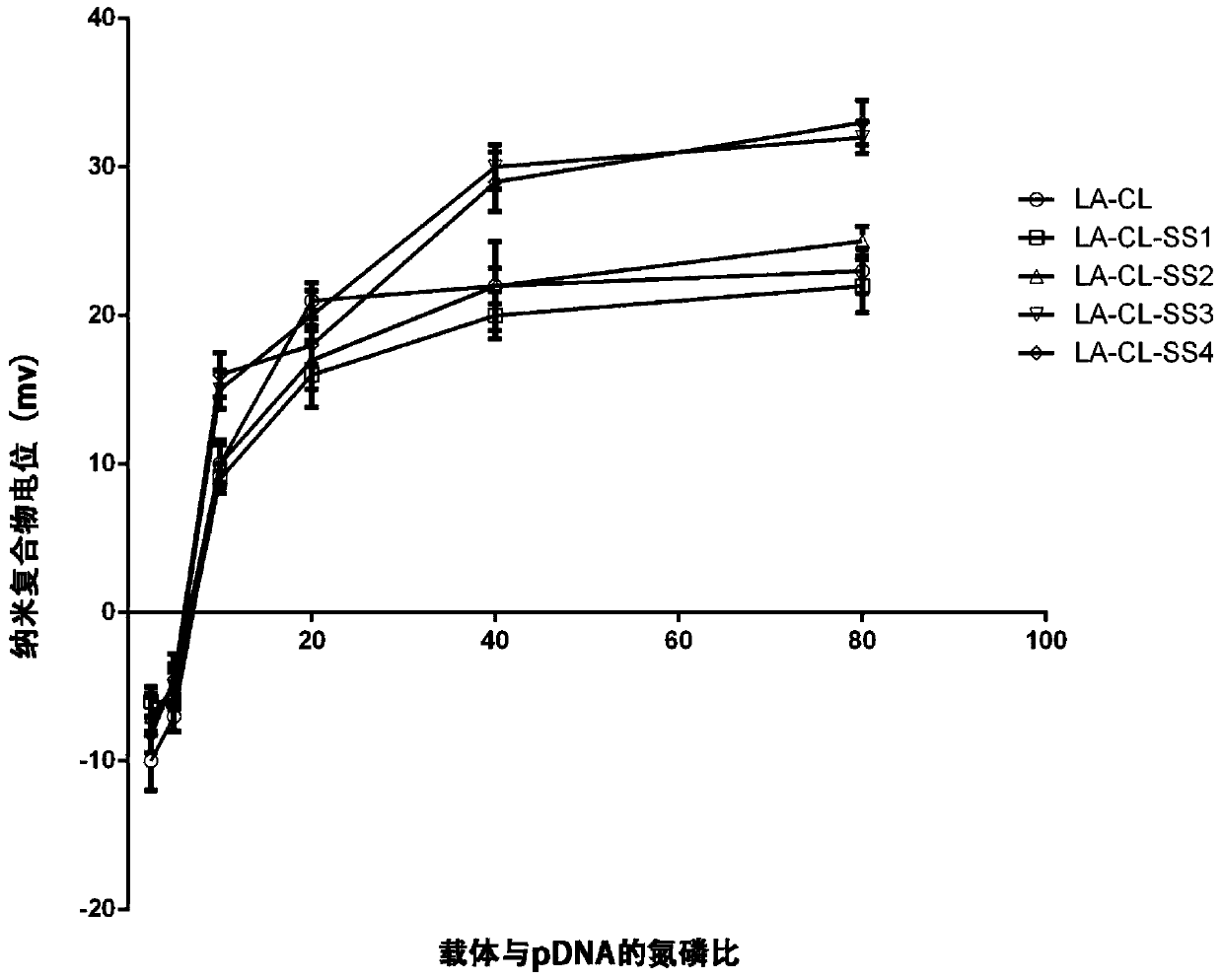
Lipoic acid modified intrinsically disordered protein nano-carrier, preparation method thereof and application of nano-carrier
ActiveCN107129522ALow cytotoxicityPromote degradationOrganic active ingredientsGenetic material ingredientsDisulfide bondingCancer cell
The invention relates to the technical field of medicines, in particular to a lipoic acid modified intrinsically disordered protein nano-carrier, a preparation method thereof and an application of the nano-carrier. The nano-carrier is crosslinked by disulfide bonds of lipoic acid, formed polypeptide polymer can be rapidly degraded in cells and cannot be accumulated in the cells, and amino acid forming a polypeptide carrier exists in vivo and has no toxic and side effects on the cells and a human body. CCK-8 cell proliferation assay indicates that the prepared nano-carrier has low cell toxicity and good gene and chemotherapeutic drug co-carrier capacity, can successfully deliver sensitivity of autophagic specificity enhanced cancer cells to chemotherapeutic drugs caused by siRNA suppressive chemotherapeutic drugs in breast cancer therapy, promotes breast cancer cell apoptosis and accordingly becomes a targeted, efficient and low-toxicity nano-scale delivery system in breast cancer therapy.
Owner:SHANGHAI WEI ER BIOPHARM TECH CO LTD +3
Novel antiangiogenic peptides
The present invention provides an antiangiogenic polypeptide having the amino acid sequence set forth in SEQ ID NO: 1, or a fragment thereof, which is effective to inhibit endothelial cell proliferation as determined by the capillary EC proliferation assay. Preferably, the fragment has at least 50% inhibition of bFGF-stimulated EC proliferation at 5 μg / ml-20 μg / ml, more preferably 75% inhibition, most preferably 95% inhibition.
Owner:CHILDRENS MEDICAL CENT CORP
Nucleoside and base hydroxamic acid derived compound and preparation method and applications thereof
ActiveCN106883217ARaw materials are easy to getEasy to prepareOrganic chemistryAntineoplastic agentsHistone deacetylaseHydroxamic acid
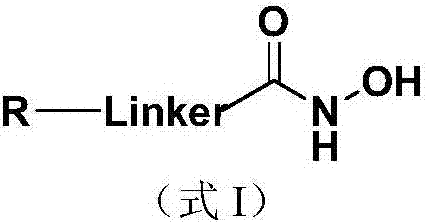
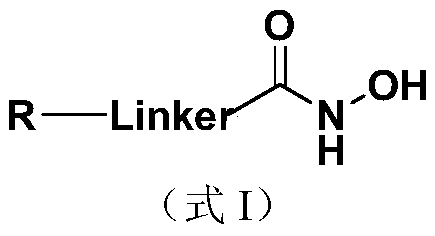
The invention discloses a nucleoside and base hydroxamic acid compound with DNA transmethylase and / or histone deacetylase inhibition activity, and a preparation method and applications thereof. The structural formula of the nucleoside and base hydroxamic acid compound is shown as the formula I, wherein R is nucleoside and base, nucleoside and base with substituent group or nucleoside and base analogue in DNA and / or RNA; Linker is a connecting chain connecting R with hydroxamic acid function group, the connecting chain comprises but is not limited within alkyl chain, alkyl chain with heteroatom, alkyl chain with aromatic ring, and alkyl chain with heterocycle. In-vitro cell proliferation assay show that the compound shown in the formula I can well inhibit the proliferation of leukemia cell K562 and histocyte lymphoma cell U937. Transmethylase and histone deacetylase inhibition assay shows that the compound shown in the formula I is a compound having inhibition activity on the DNA transmethylase and / or histone deacetylase. The formula I is as shown in the specification.
Owner:SHENZHEN GRADUATE SCHOOL TSINGHUA UNIV
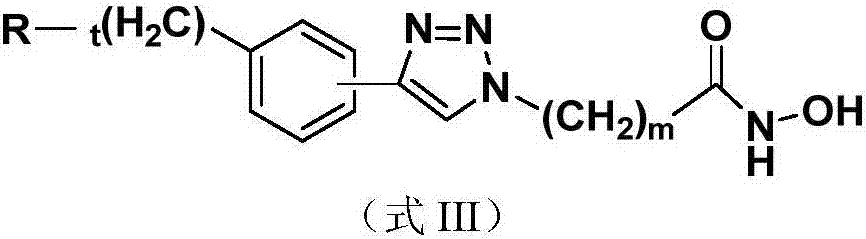
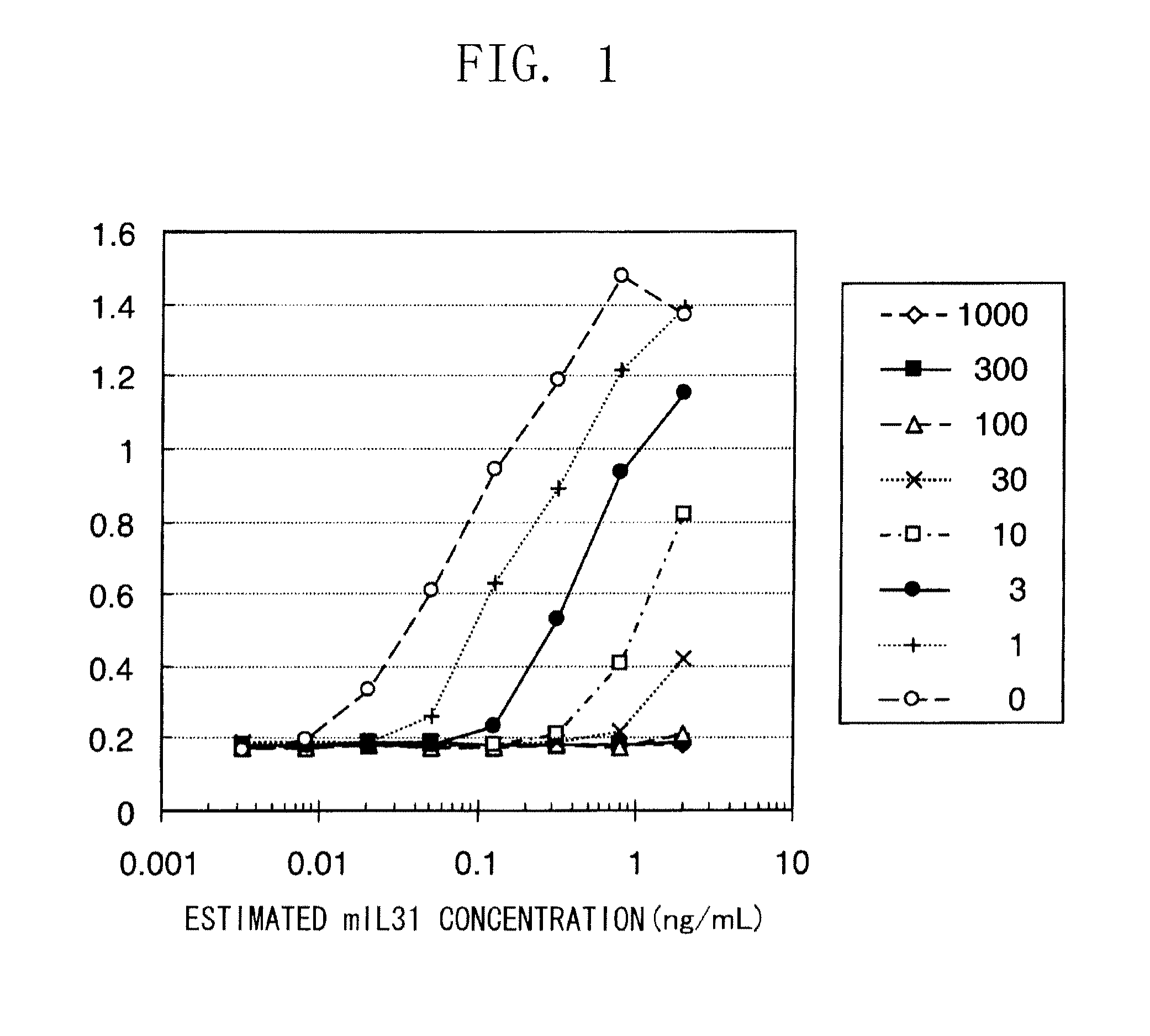
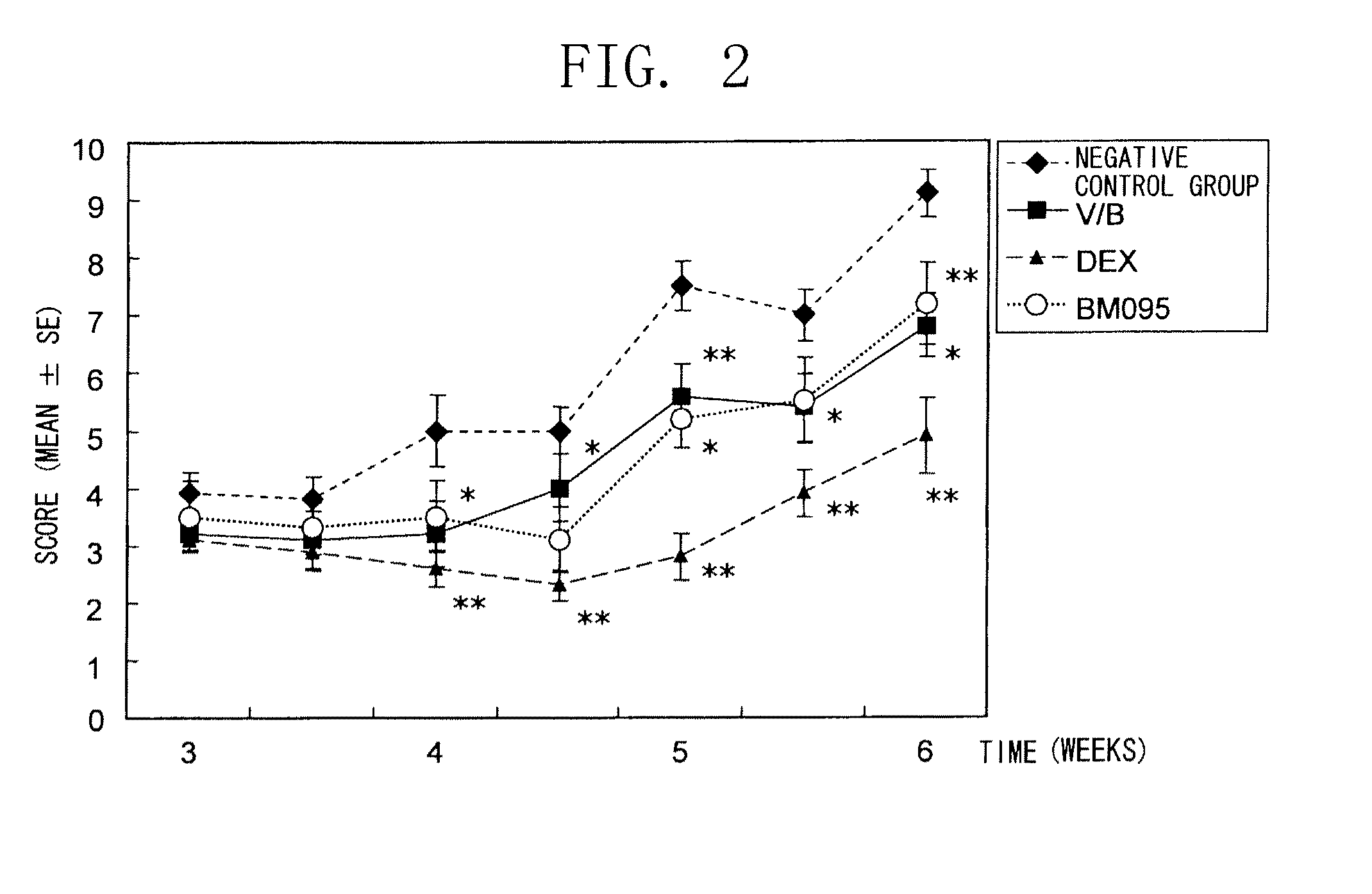
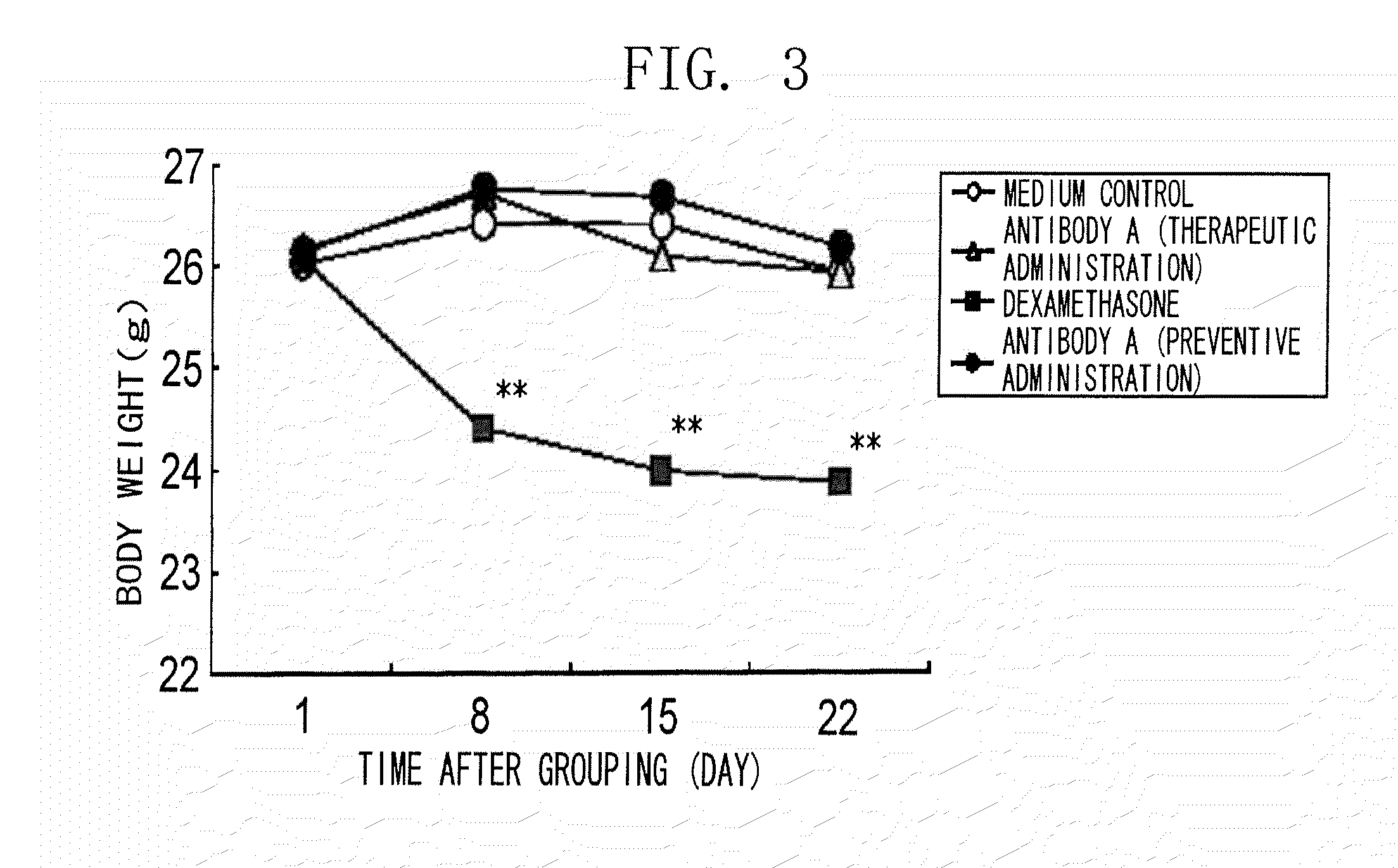
Method of treating an inflammatory disease comprising administering an NR 10 antibody antagonist
The present inventors obtained, from a phage library of human antibodies, an anti-mouse NR 10 neutralizing antibody-expressing BM095 clone that shows a strong proliferation-suppressing activity in an IL-31-dependent Ba / F3 cell proliferation assay system. When this anti-mouse NR 10 neutralizing antibody was administered to NC / Nga mice, a model of atopic dermatitis which is a mouse model of chronic dermatitis that arises as a result of repeated applications of picryl chloride, a mouse model of rheumatoid arthritis, and a mouse model of osteoarthritis, a significant effect of symptom suppression was observed. This revealed that the anti-NR 10 neutralizing antibody is indeed effective as a therapeutic agent for inflammatory diseases. In addition, the present inventors successfully obtained an anti-human NR 10 neutralizing antibody, providing extremely useful therapeutic agents with practical clinical applications.
Owner:CHUGAI PHARMA CO LTD
Antiangiogenic peptides
The present invention provides an antiangiogenic polypeptide having the amino acid sequence set forth in SEQ ID NO: 1, or a fragment thereof, which is effective to inhibit endothelial cell proliferation as determined by the capillary EC proliferation assay. Preferably, the fragment has at least 50% inhibition of bFGF-stimulated EC proliferation at 5 μg / ml-20 μg / ml, more preferably 75% inhibition, most preferably 95% inhibition.
Owner:CHILDRENS MEDICAL CENT CORP
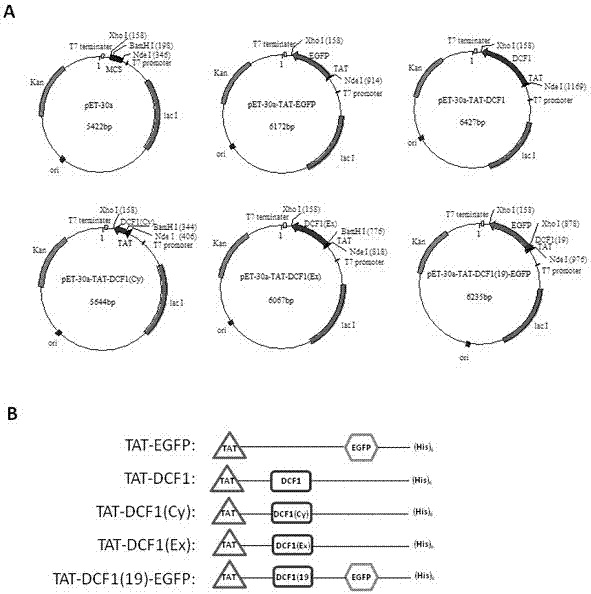
Novel application of fusion protein TAT-DCF1
InactiveCN106913864AReduced activityReduce ratePeptide/protein ingredientsPharmaceutical non-active ingredientsTumour volumeFhit gene
The invention relates to a novel application of fusion protein TAT-DCF1. By judging dcf1-induced U251 cell line apoptosis by virtue of such methods as immuno-electron microscope, atomic force microscope, CCK8 proliferation assay, JC-1 dyeing, nude mouse tumor heterotopic transplantation and the like, results show that dcf1, which is positioned in mitochondria, can cause pathological change of the mitochondria structure and result in drop in membrane potential, so that expression amounts of related genes are changed, and the apoptosis is finally induced. Nude mouse experiments show that them dcf1 can obviously diminish tumor volume of glioma, so that tumor growth is inhibited.
Owner:SHANGHAI UNIV
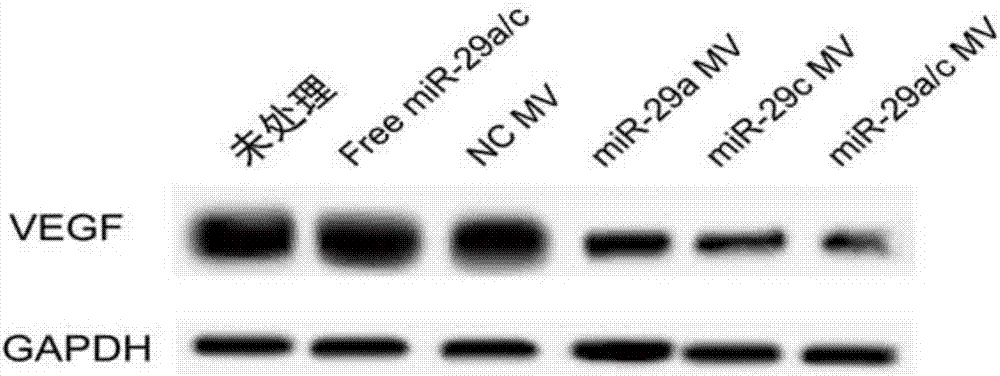
Method for inhibiting gastric cancer angiogenesis by using micro vesicles as miRNA (Micro Ribonucleic Acid) transport carriers
InactiveCN107422108ASecretion level inhibitionPrevent proliferationCompounds screening/testingOrganic active ingredientsHEK 293 cellsVascular endothelium
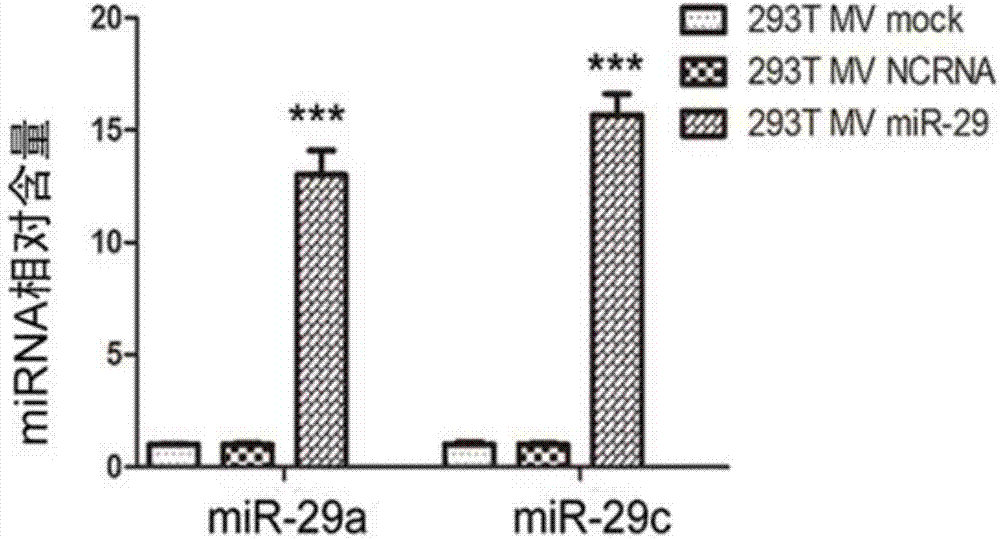
The invention discloses a method for inhibiting gastric cancer angiogenesis by using micro vesicles as miRNA (Micro Ribonucleic Acid) transport carriers. Through extraction and identification of micro vesicles, quantitative analysis of miRNA, protein extraction, Western blot, ELISA (Enzyme-linked Immuno Sorbent Assay) and EdU (5-Ethynyl-2'-deoxyuridine) proliferation assays, an in-vitro angiogenesis assay, a tumorigenesis assay and a tail intravenous injection micro vesicle assay, the situation that the quantitative analysis of the content of corresponding miRNA in artificially modified micro vesicles shows that the content of miR-29 is obviously increased by being compared with a negative control is discovered, the VEGF (Vascular Endothelial Growth Factor) protein secretion level of gastric cancer cells is obviously inhibited after the gastric cancer cells are hatched by the micro vesicles containing the miR-29, the proliferation of vascular endothelial cells co-cultured by the gastric cancer cells treated by the micro vesicles secreted by HEK (Human Embryonic Kidney)-293 cells treated by high-expression miR-29a and miR-29c is obviously inhibited, ring formation of the vascular endothelial cells co-cultured by the gastric cancer cells treated by the micro vesicles secreted by the HEK-293 cells treated by the high-expression miR-29a and the miR-29c is obviously inhibited, and the tumor volume in a mouse containing the micro vesicles which are subjected to the tail intravenous injection and high-expression miR-29a / c treatment is obviously smaller than the tumor volume in a mouse treated by the corresponding negative control.
Owner:TIANJIN TUMOR HOSPITAL
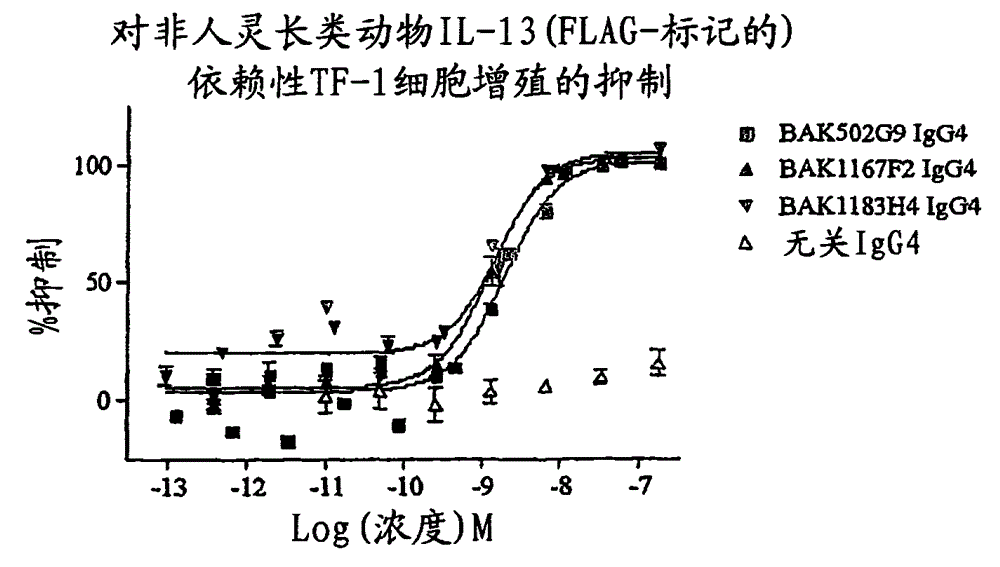
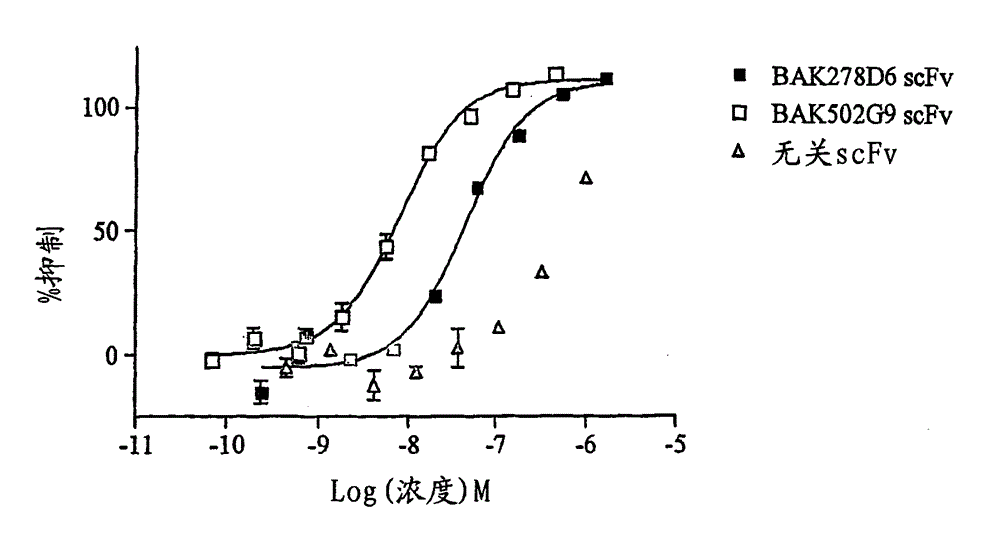
Human antibody molecules for IL-13
There are described recombinant VH and VL domains of human antibodies that bind IL-13. A series of proteins characterised by three CDR in each VH / VL are described. Preferably the molecules are single chain Fv or antibody molecules. Nucleic acids expressing the VH and VL domains are selected from phage display libraries. Neutralization potency of the resulting scFv's was tested in TF proliferation assays. The fragments and antibodies showed efficacy for IL-13 binding in murine models of pulmonary inflammation, the primate (cynomolgus) model of dermal allergy, IgE release from B cells and histamine potentiation in Ca2+ signalling in smooth muscle. Mice transgenic for human IL-13 antibodies and epitope mapping studies are also described. It is envisaged that the anti 11-13 reagents may be used to treat asthma, atopic dermatitis, allergic rhinitis, fibrosis, IBS, or Hodgkin's lymphoma.
Owner:MEDIMMUNE LTD
Stimulated cell standards
InactiveUS20110183372A1Easy to refactorImproves Structural IntegrityPreparing sample for investigationBiological testingFreeze-dryingCarboxyfluorescein succinimidyl ester
Methods for producing stimulated, positive and negative control reference standard for monitoring intracellular cytokine levels and cytokine release in test samples by stimulating cells to produce cytokines in the presence of a cytokine release inhibitor, fixing the stimulated cells with a fixative such as paraformaldehyde, washing to remove excess fixatives and freeze-drying the stimulated, fixed cells. Methods for producing labeled reference standards for cell proliferation assays are also disclosed, in which proliferation-competent mammalian cells, isolated from a human or animal body are labeled with a label, such as a dye, that is divided between daughter cells during cell proliferation (e.g., carboxyfluorescein succinimidyl ester), the cells are stimulated to proliferate, the proliferated cells are fixed by addition of a fixative and then preserved by freeze drying or cryopreservation.
Owner:THE UK SEC FOR HEALTH & HER BRITANNIC MAJESTYS GOVERNMENT OF THE UK OF GREAT BRITAIN & NORTHERN IRELAND
Methods for enhancing survival and/or proliferation of neural stem cells and neurite extension enhancers therefor pharmaceutical compositions containing neural stem cells assay methods and screening methods
InactiveUS7785596B2Slow proliferationHigh proliferation rateNervous disorderPeptide/protein ingredientsLiquid mediumAssay
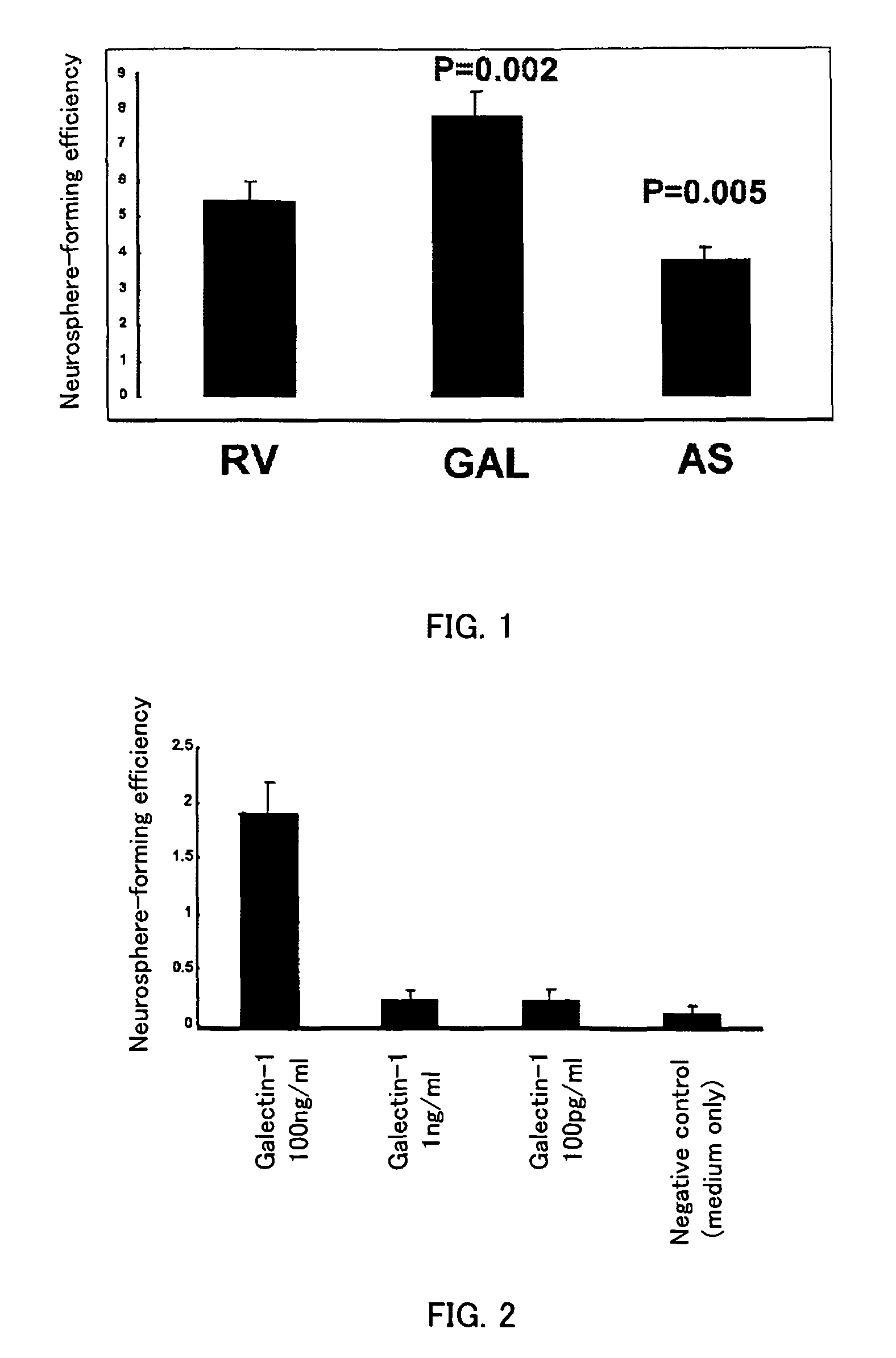
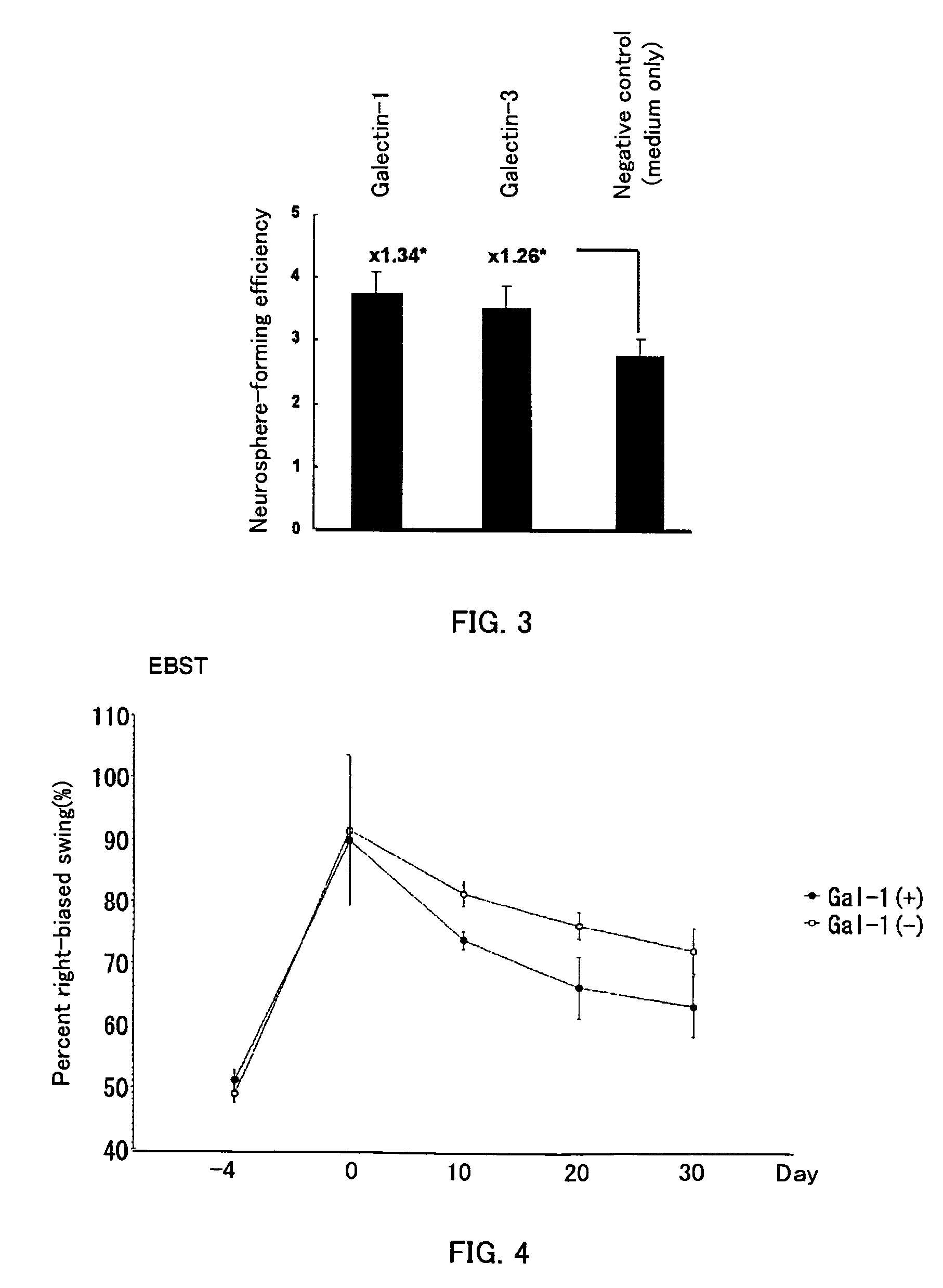
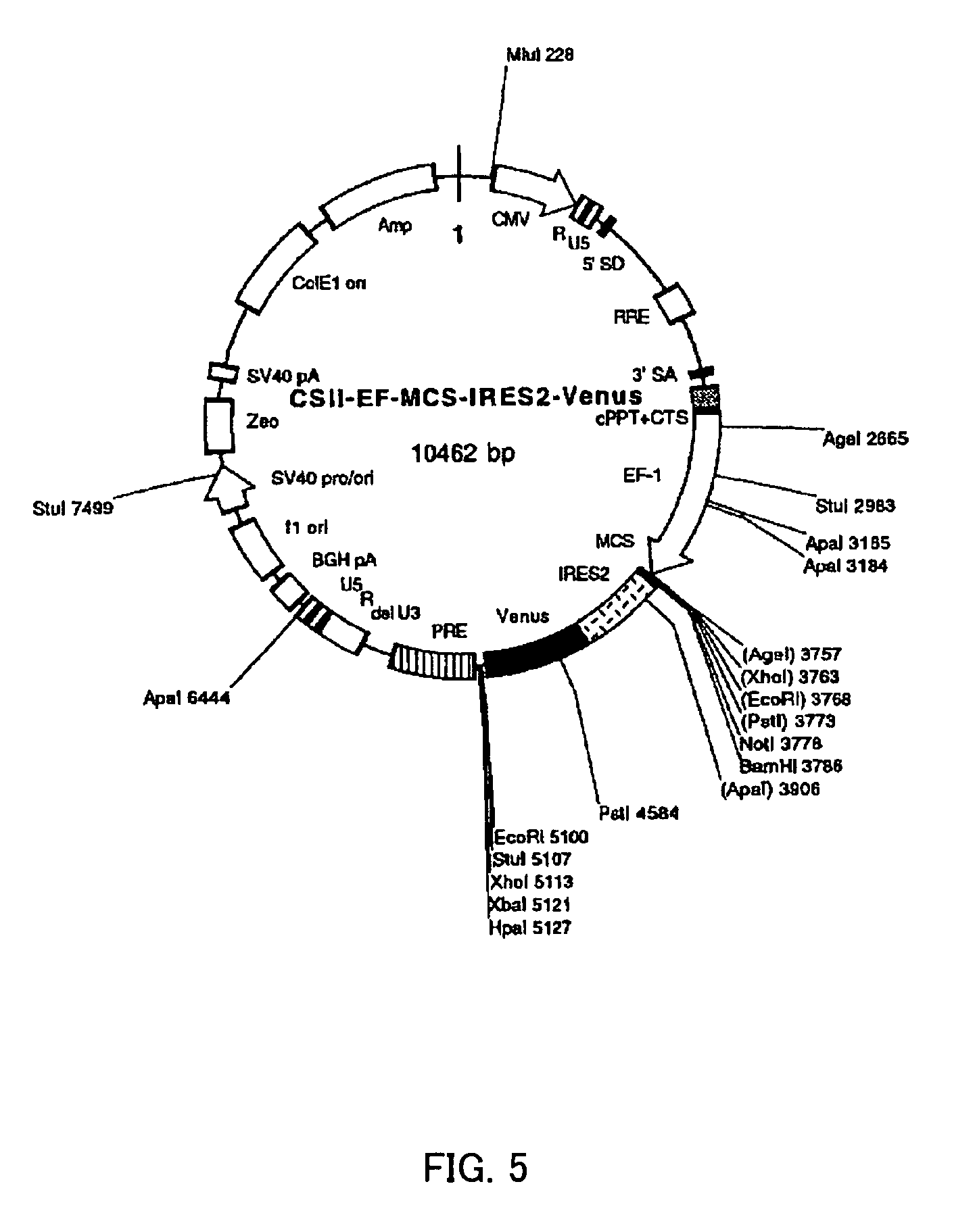
Methods for enhancing survival and / or proliferation of neural stem cells and pharmaceutical compositions containing neural stem cells prepared by such methods, together with methods for assaying factors enhancing survival and / or proliferation of neural stem cells and methods for screening for such factors.Either Galectin-1 is overexpressed in neural stem cells or neural stem cells are cultured in a liquid medium containing Galectin-1. Pharmaceutical compositions containing Galectin-1-overexpressing neural stem cells and pharmaceutical composition containing Galectin-1, prepared by the aforementioned methods, improve higher cerebral functions damaged by cerebral ischemia. Further, by seeding neural stem cells at clonal concentrations and determining whether the seeded neural stem cells are capable of proliferating in an assay medium to be assayed, whether the factor enhances survival and / or proliferation of neural stem cells is assayed and a factor enhancing survival and / or proliferation of neural stem cells are identified using this assay method.
Owner:KEIO UNIV
Method of synthesizing antagonist peptides for cell growth
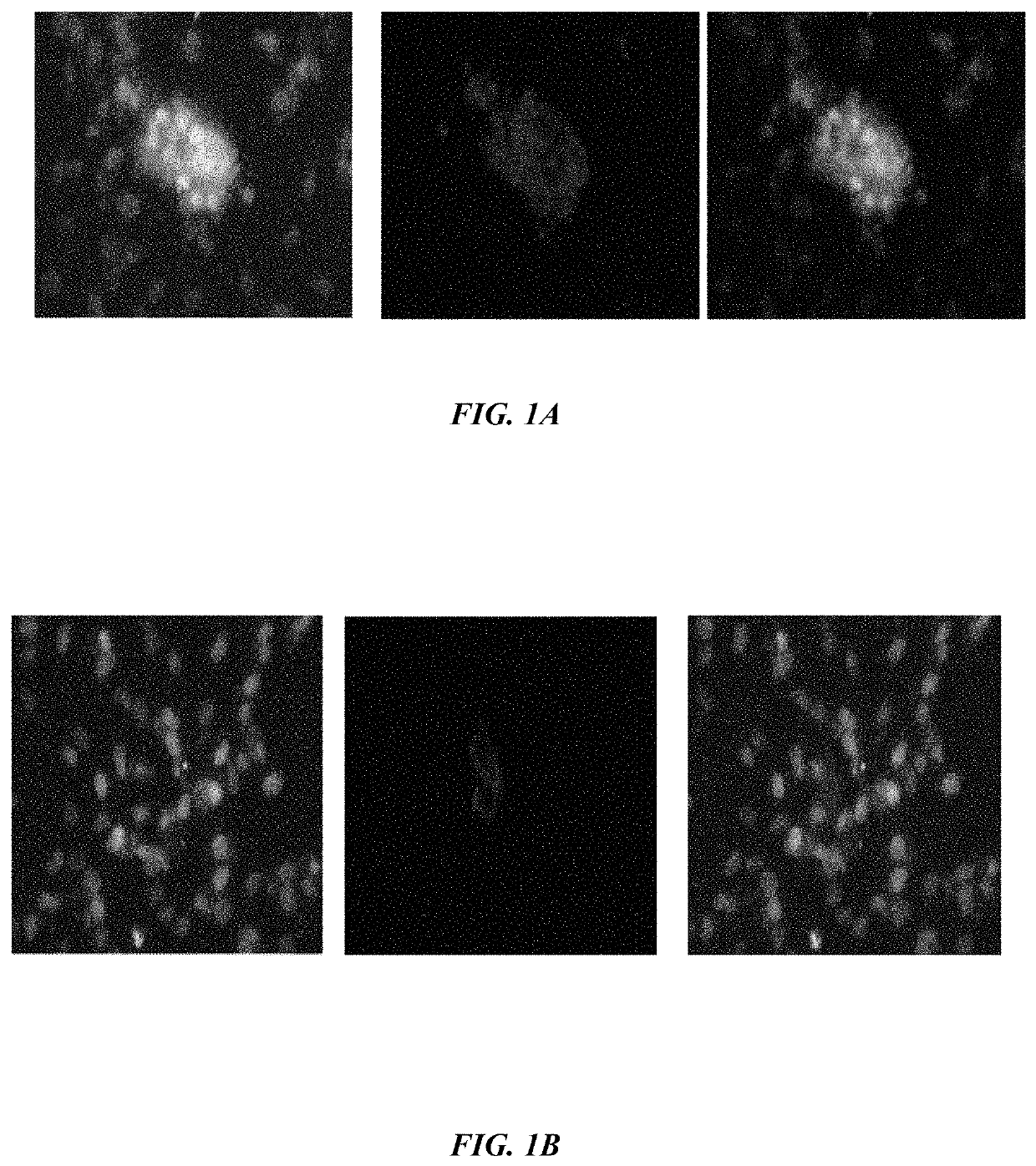
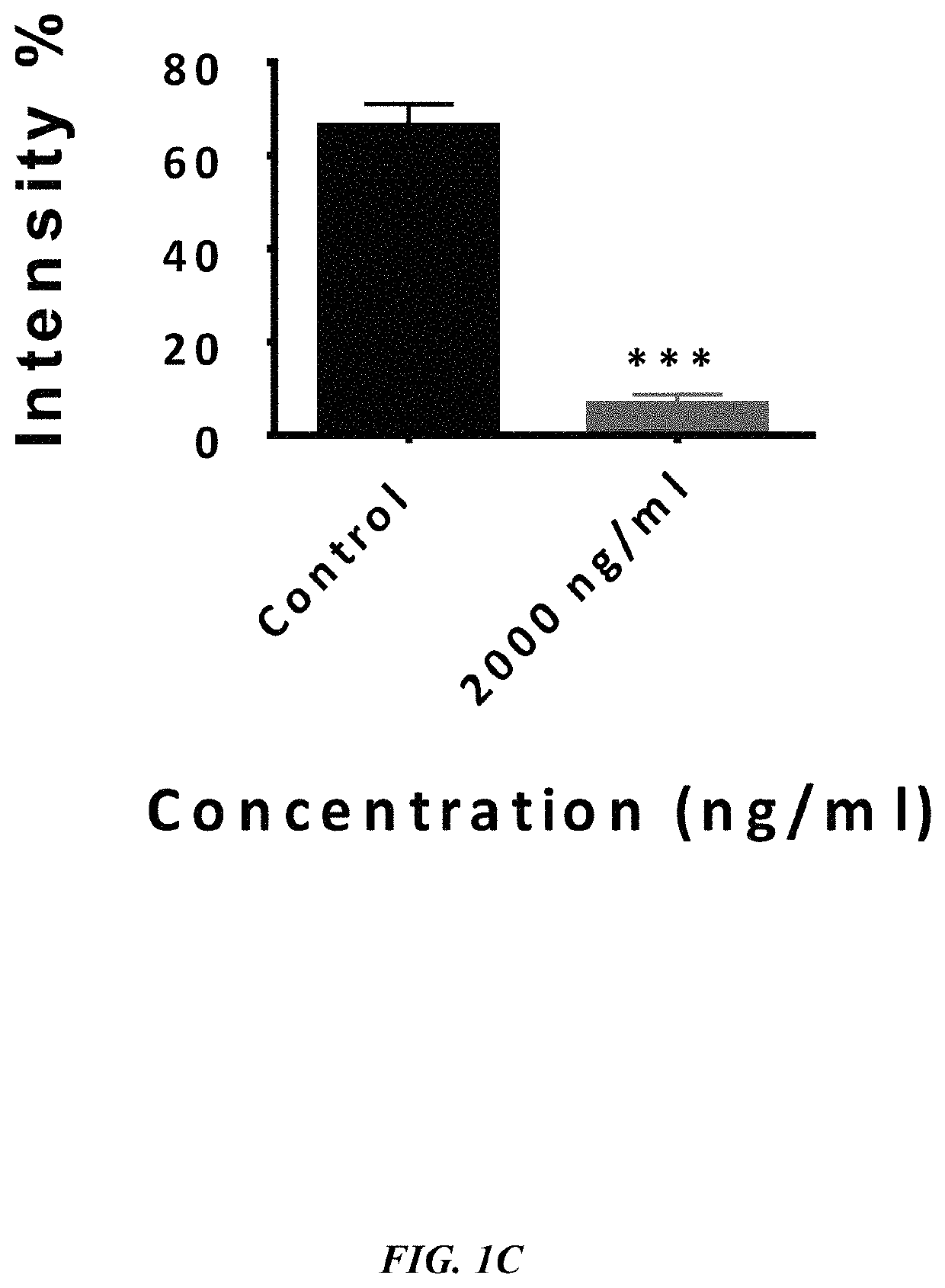
ActiveUS20190233490A1Avoid blindingPromote blood vessel formationPeptide/protein ingredientsBiological material analysisCompetitive bindingStaining
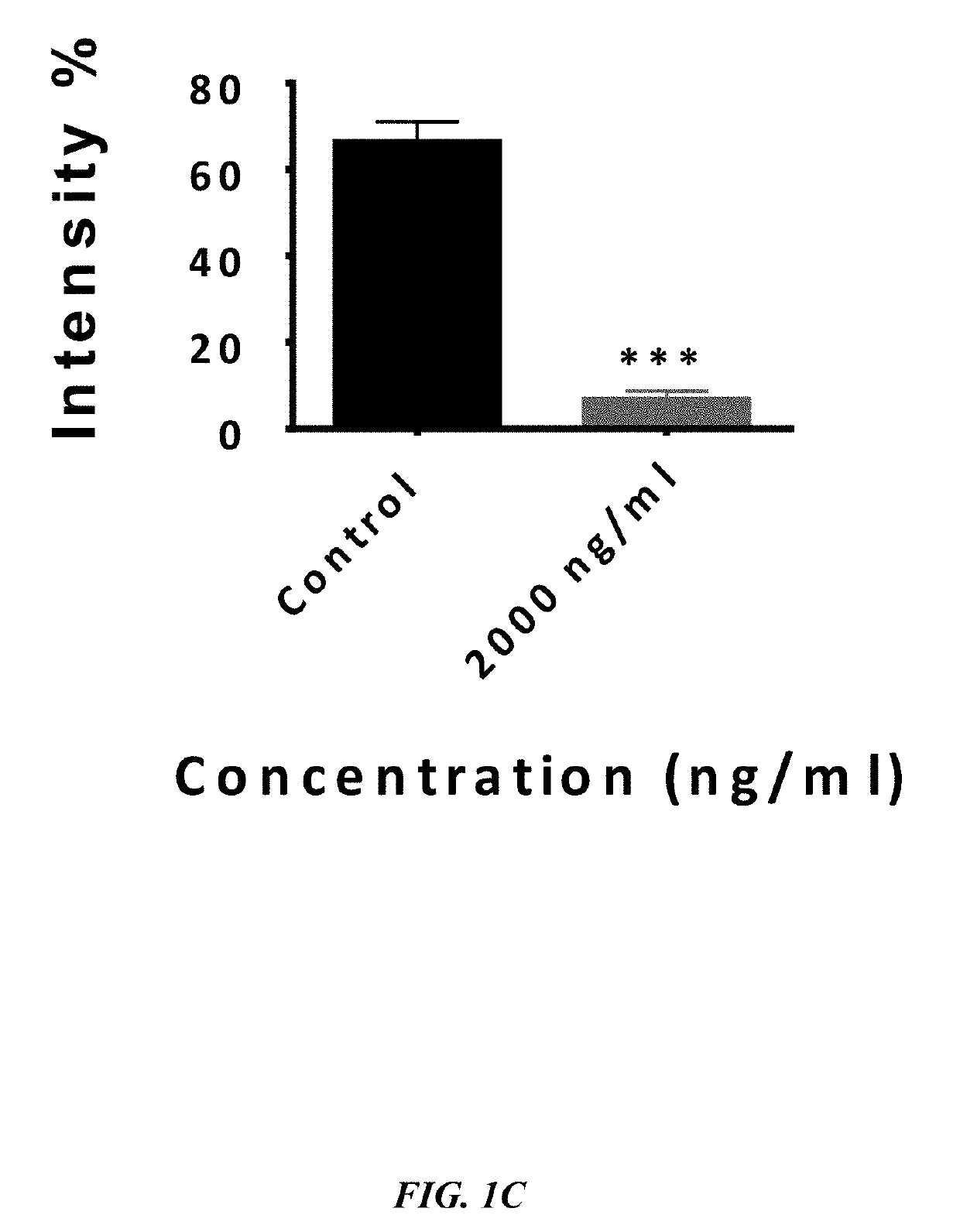
The embodiments herein disclose a method for synthesizing antagonistic peptide VEGF and bFGF. The method comprises synthesizing the antagonistic peptide for VEGF and bFGF and analyzing the purity of peptides. The quality of antagonistic peptide for VEGF and bFGF is analyzed by HPLC chromatogram and Mass spectrometry analysis. The biochemical activity of the antagonistic peptide for VEGF and bFGF is analyzed by competitive binding assay, cell proliferation assay, Matrigel assay for anti-angiogenic activity analysis, histopathological staining and Western blot analysis. The competitive binding assay of antagonistic peptide for VEGF and bFGF illustrate that peptides binds with cell receptors at a concentration of 2000 ng / ml. The cell proliferation assay illustrates that cell growth is arrested when antagonistic peptide for VEGF and bFGF are at a concentration of 2000 ng / ml. The anti-angiogenic activity analysis illustrates that angiogenesis is arrested when the concentration of antagonistic peptide for VEGF and bFGF is 2000 ng / ml.
Owner:ASGHARI SEYED MOHSEN +1
Compositions and methods for diagnosing and treating autoimmune diseases
Disclosed are compositions and methods for detecting, isolating, and / or characterizing a T cell or autoantibody associated with type I diabetes. The composition and methods comprise the use of a hybrid insulin peptide having an N-terminal amino acid sequence taken from the human insulin peptide and a C-terminal amino acid sequence taken from a secretory granule protein that are joined through a peptide bond to form an autoimmune antigen. The detecting, isolating and characterization step further includes performing an immunoassay and / or T cell proliferation assay with the disclosed hybrid insulin peptides, where preferably, the immunoassay is an ELISPOT assay.
Owner:UNIV OF COLORADO THE REGENTS OF
Protein kinase C inhibitor for treating triple-negative breast cancer
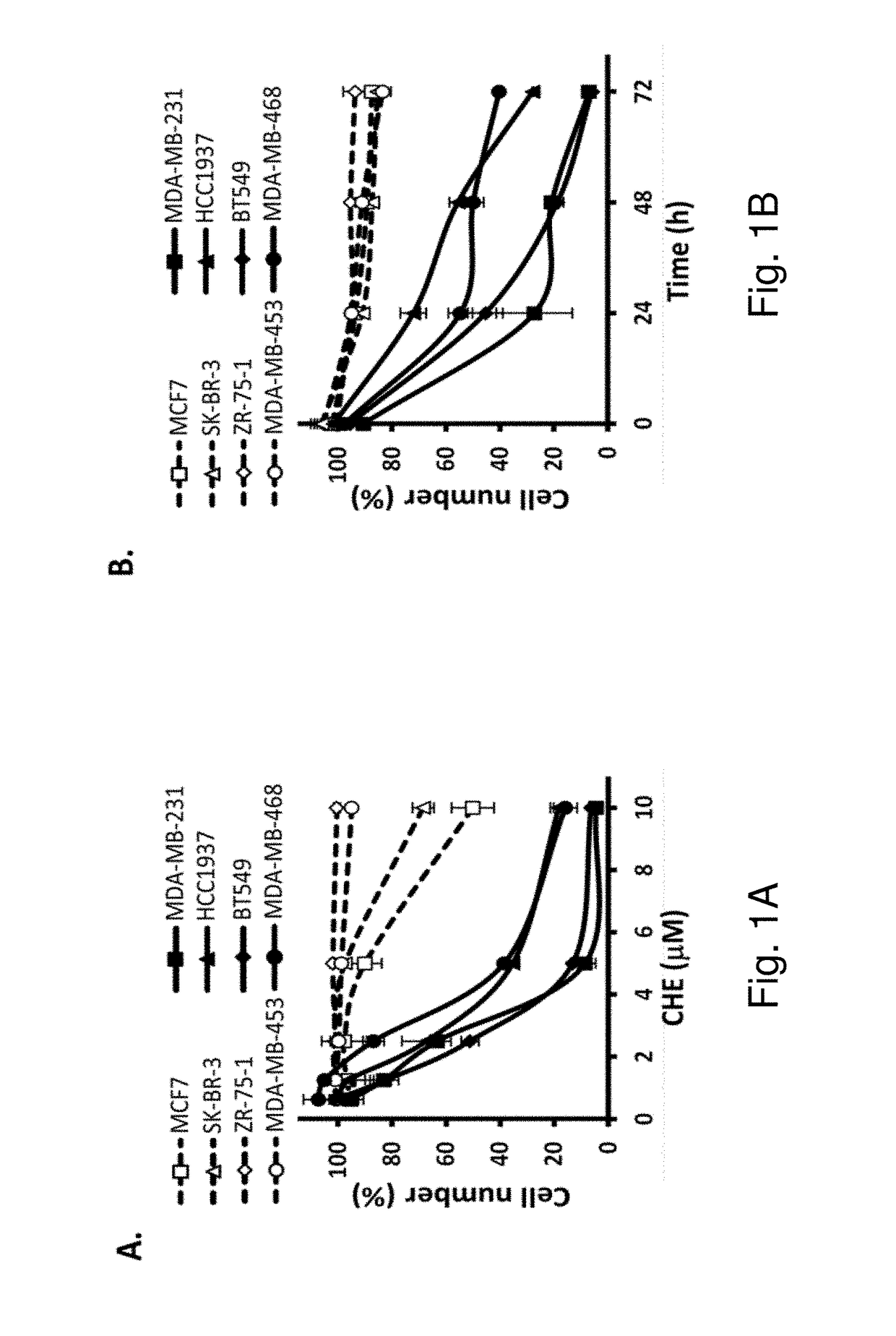
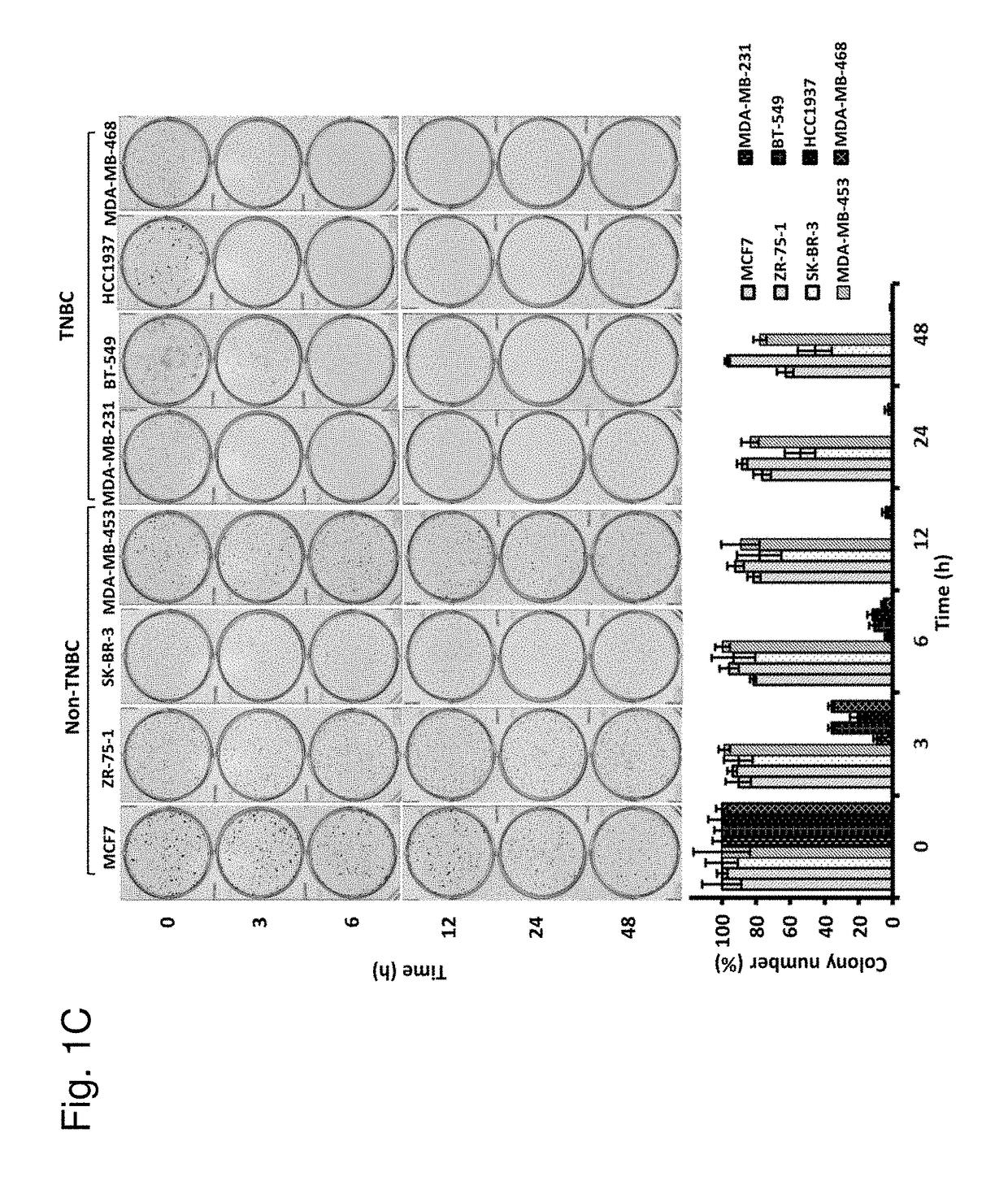
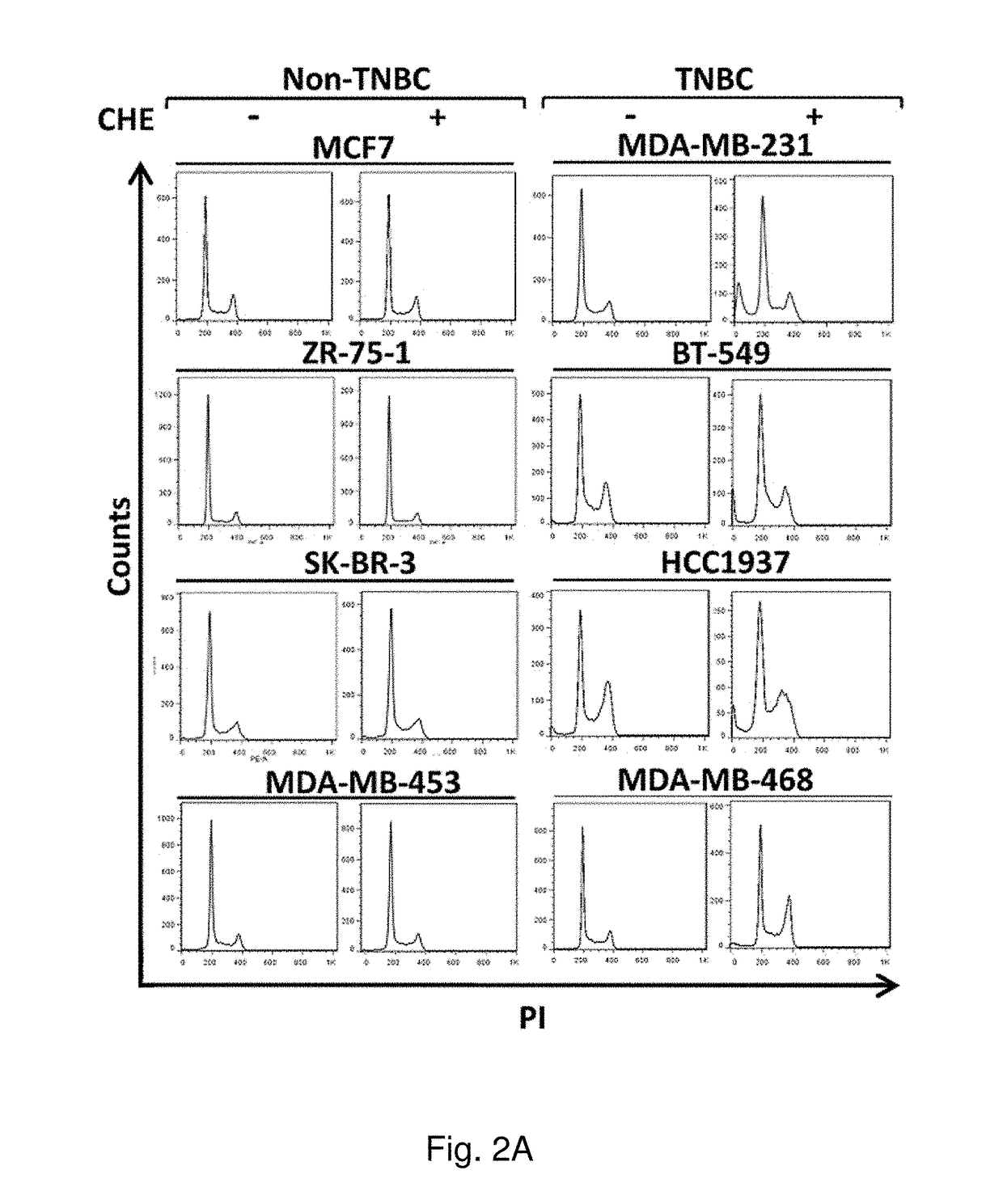
Protein kinase C plays important roles in TNBC development and could be a specific target. The in vitro anti-proliferative activity of PKC inhibitor chelerythrine on a panel of breast cancer cell lines was evaluated. Chelerythrine selectively inhibited the growth of TNBC cell lines over non-TNBC cell lines as demonstrated by cell proliferation assay and colony formation assay. The selective anti-proliferative effect of chelerythrine was associated with the differential apoptosis induction ability on breast cancer cell lines and induction of cell cycle arrest in some TNBC cell lines. By analysis of the expression levels of different PKC subtypes, the inventors found multiple PKC isozymes may mediate the selective activity of chelerythrine on TNBC cells. Finally, combination of chelerythrine and chemotherapy reagent taxol showed synergistic / additive effect on TNBC cell lines.
Owner:MACAU UNIV OF SCI & TECH
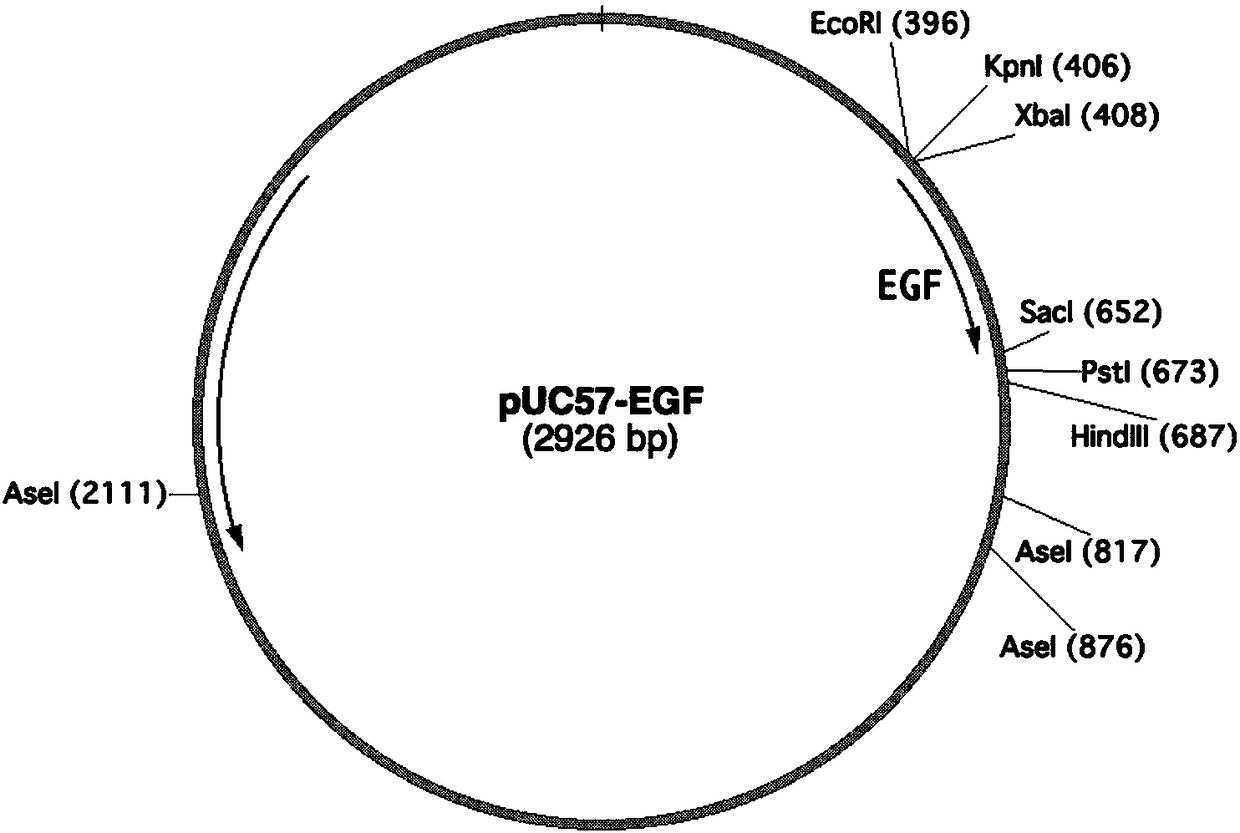
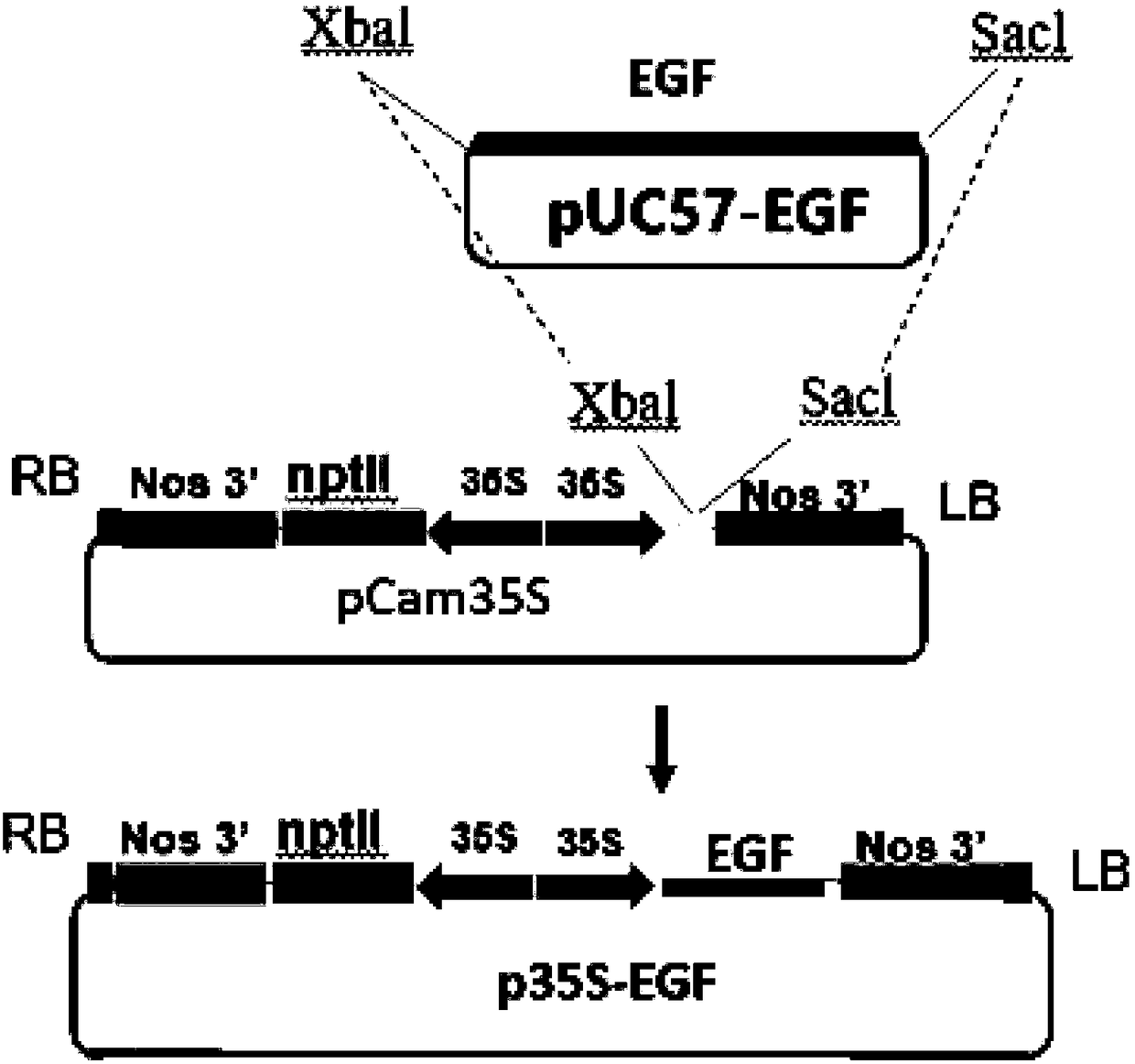
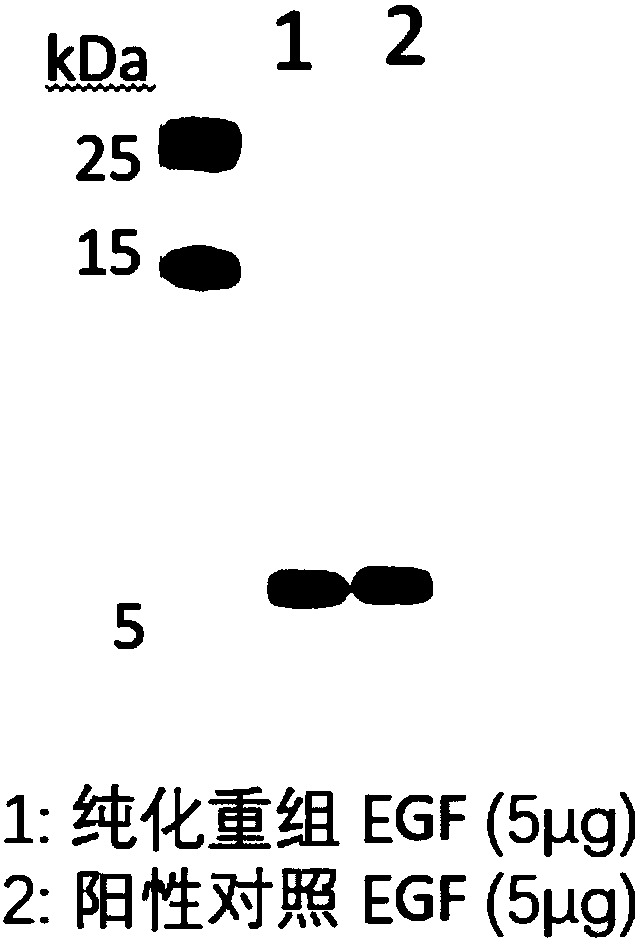
Application of plant serving as host in expressing epidermal growth factor
The invention relates to the technical field of biology, in particular to a plant, in particular to an application of a plant serving as a host in expressing an epidermal growth factor. The human epidermal growth factor (abbreviated as EGF) can be expressed by using a recombinant vector as well as an agrobacterium-mediated vacuum permeation method. The expression system determines that the plant foreign proteins can be collected after being impregnated with agrobacteria for four days. The successful expression of the recombinant EGF is determined by using an SDS-PAGE method and a western method. The cell proliferation assay proves that EGF produced by the plant and particularly by lettuce has bioactivity. The method provided in the invention is low in cost, convenient, and capable of producing a great amount of active recombinant human EGF.
Owner:山东益华生物科技有限公司
Method for testing proliferative response of drug to tumor cell derived from patient
PendingCN110029141AReliable simulationPredicted responseCompound screeningApoptosis detectionDrug testing methodsCell-Extracellular Matrix
The invention relates to use of a 3D cell culture technology in an in-vitro drug test method, and more particularly to a method for testing a proliferative response of a drug to tumor cell derived from a patient, which can reliably screen the candidate drugs by predictive proliferation assay for efficacy and / or mechanism of action. The method comprises the steps of: (1) obtaining cells from a biopsy or tumor resection material; (2) culturing the obtained cells on a 3D extracellular matrix; (3) treating the tumor cells cultured on the 3D extracellular matrix with drug treatment; (4) performinghigh-content imaging on the processed tumor cells; and (5) evaluating the high-content imaging of the processed tumor cells, and testing the proliferative response of the drug to the tumor cells derived from the patient.
Owner:SHANGHAI BIODURO BIOLOGICS
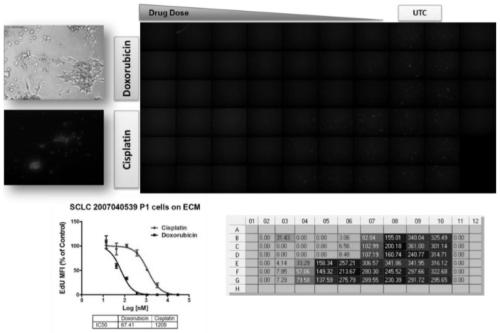
Vector for assaying in-vitro cell proliferation and dynamic in-vitro cell proliferation assay method
ActiveCN107916270AStrong fluorescent brightnessGood light stabilityMicrobiological testing/measurementFermentationFluorescenceCell strain
The invention relates to a vector for assaying in-vitro cell proliferation and a dynamic in-vitro cell proliferation assay method. The vector for dynamically assaying in-vitro cell proliferation is characterized in that the vector consists of a PLKO.1 sequence, a CMV sequence, an eGFP sequence, an IRES sequence and a Puro sequence which are sequentially connected. Compared with the similar cell lines, a stably transfected cell line constructed by the invention has the advantages of high fluorescent brightness, good photostability and low background signals, and does not have significant influence on the physiology of cells. The assay method comprises the following steps: three vectors, i.e. the vector disclosed by the invention, the psPAX2 vector and the PMD2.G vector, are used for packaging slow virus; the slow virus obtained in step (1) is used for transfecting target cells, and puromycin screening and flow cytometry sorting are carried out, so that the stably transfected cell line is obtained; an appropriate number of cells are chosen to be added into a cell culture plate, and after the cells are attached to the wall, an image is acquired by a high-content cell imaging system; data are processed, and a cell proliferation curve is drawn. The vector and the method disclosed by the invention have the advantages of high automation degree, little error, good repeatability and high sensitivity.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Method for researching reprogramming process regulated by DNA methylation
PendingCN113278686AEasy to operateExperimental ideas are clearMicrobiological testing/measurementPreparing sample for investigationAlkaline phosphatase stainingDNA methylation
The invention relates to a method for researching reprogramming process regulated by DNA methylation. The method includes the following steps: step (1), adding a DNA methylase inhibitor 5'aza based on the induction of Yamanaka factors Oct4, Sox2, Klf4 and c-Myc, inducing somatic cells according to an inducing method provided by Shinya Yamanaka, and observing cellular morphology at different inducing days; step (2), when cell clone is observed, collecting cells to perform fluorescence quantitative detection, indirect immunofluorescence detection, alkaline phosphatase staining, karyotype analysis, and edu proliferation assay; and step (3), after determining an effect of the 5'aza, respectively combining four factors and the 5'aza to treat the somatic cells to perform induction, detecting the formation of ips clone, and determining the most effective combination form. Through the induction of different combinations of the Yamanaka factors and the DNA methylase inhibitor 5'aza, an effect of DNA methylation for inducing the reprogramming of the somatic cells can be verified.
Owner:YANGZHOU UNIV
Application of plant serving as host in expressing insulin-like growth factor
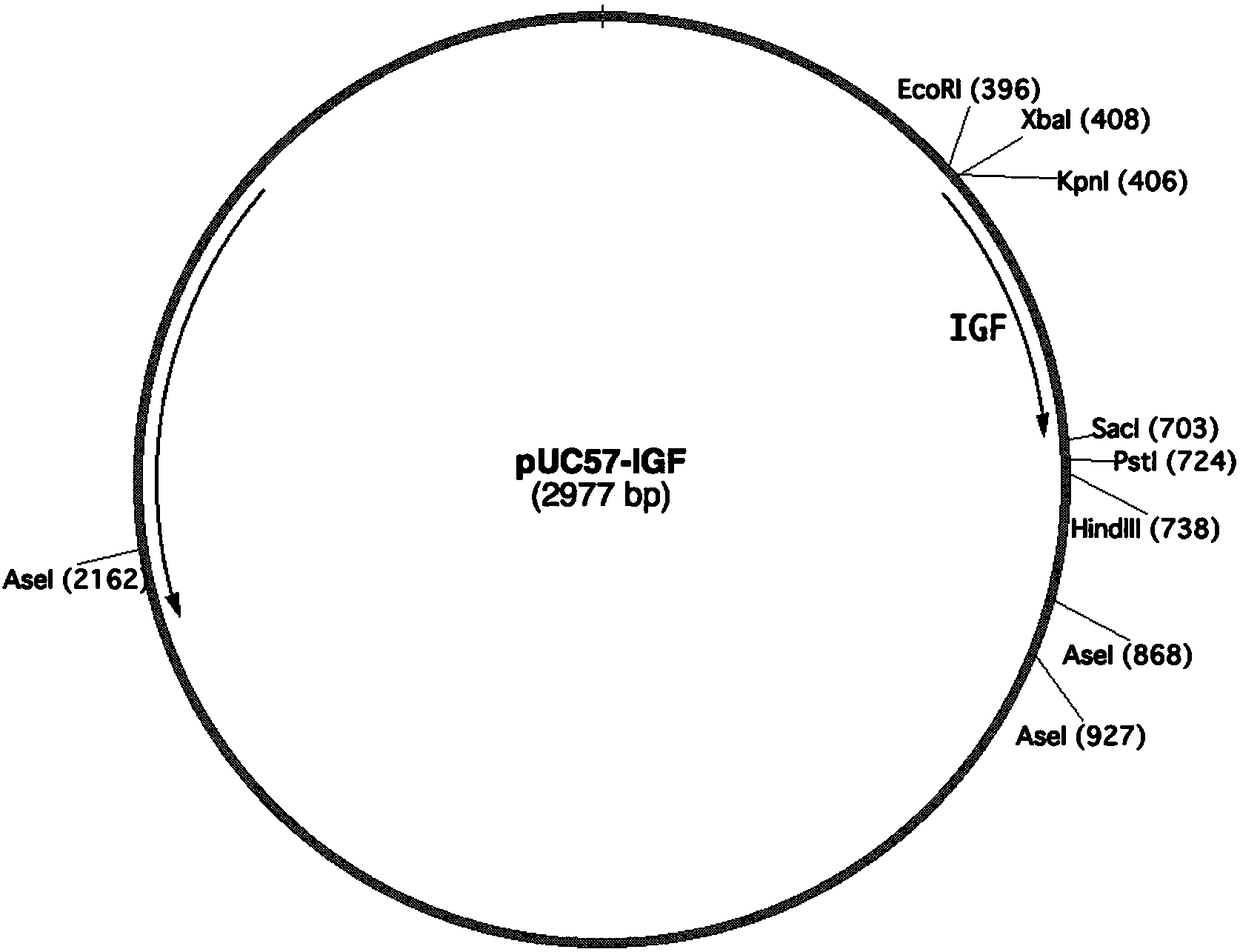
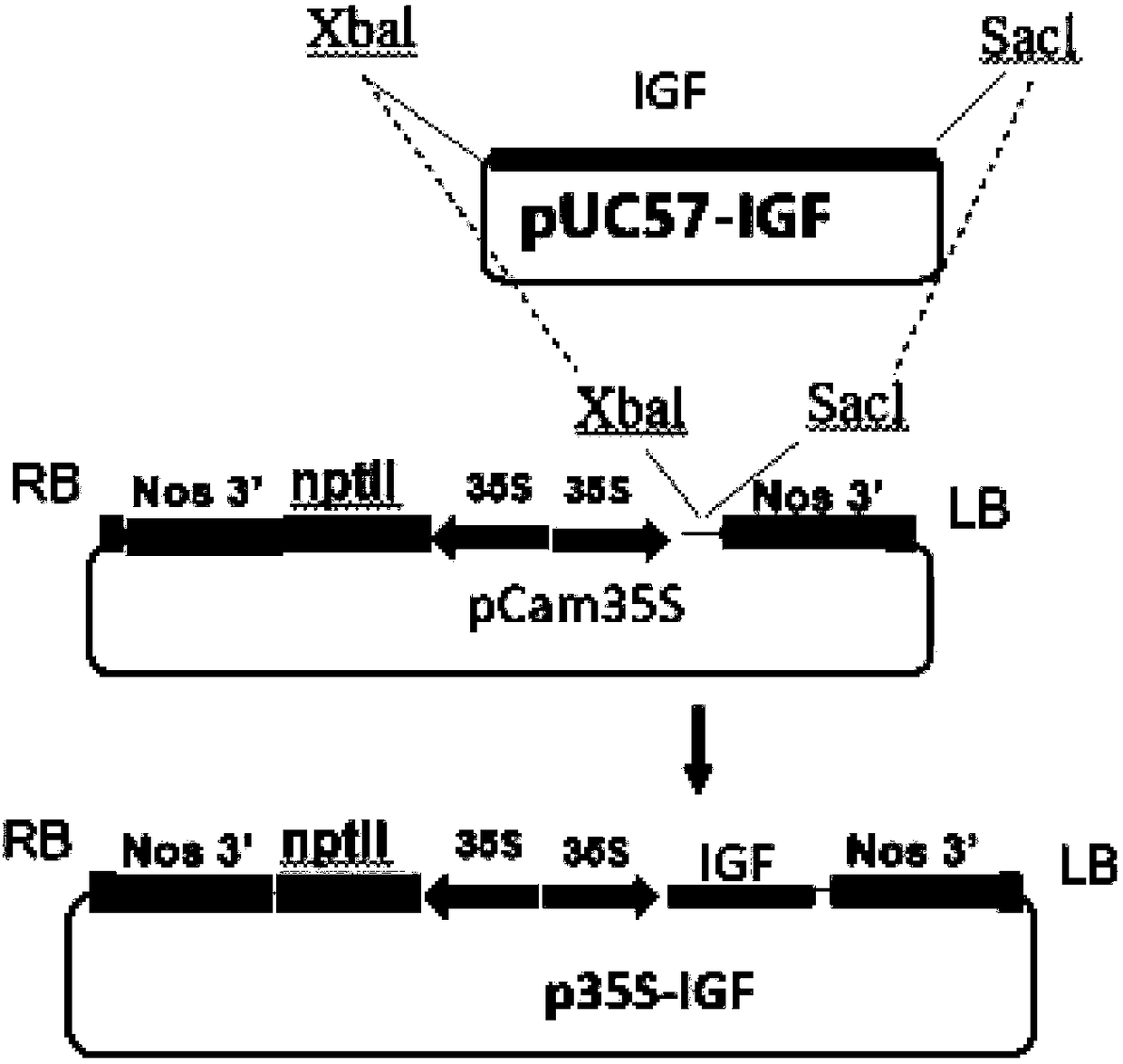
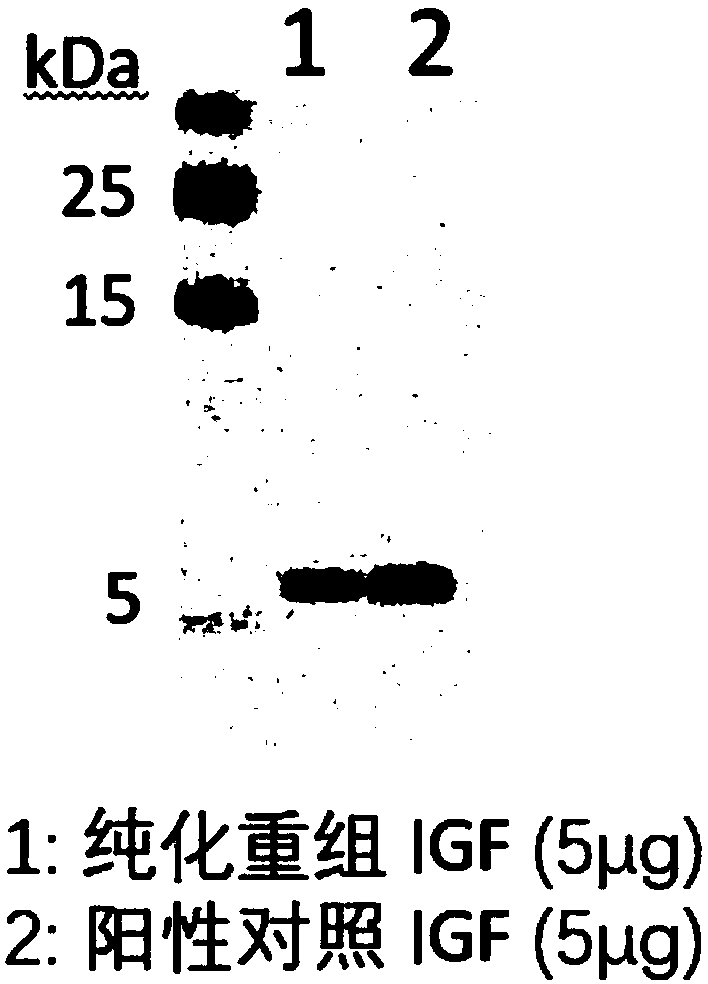
PendingCN109456397AVector-based foreign material introductionGrowth factors/regulatorsInsulin-like growth factorForeign protein
The invention relates to the technical field of biology, in particular to a plant, in particular to an application of a plant serving as a host in expressing an insulin-like growth factor. The human insulin-like growth factor (abbreviated as IGF) can be expressed by using a recombinant vector as well as an agrobacterium-mediated vacuum permeation method. The expression system determines that the plant foreign proteins can be collected after being impregnated with agrobacteria for four days. The successful expression of the recombinant IGF is determined by using an SDS-PAGE method and a westernmethod. The cell proliferation assay proves that IGF produced by the plant and particularly by lettuce has bioactivity. The method provided in the invention is low in cost, convenient, and capable ofproducing a great amount of active recombinant human KGF.
Owner:SAGACITY FAITHFUL CONVERGENCE HEALTH TECH LTD
A kind of anti-tumor protein and its application
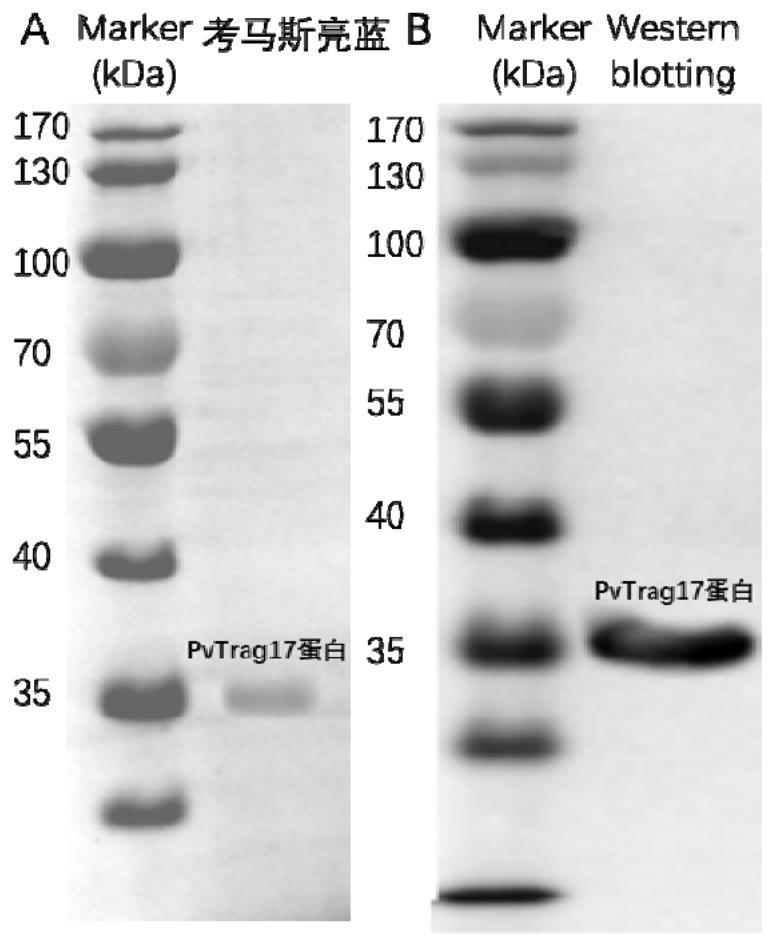
ActiveCN109182341BPrevent proliferationAbility to inhibit migrationPeptide/protein ingredientsDepsipeptidesCancer cellBULK ACTIVE INGREDIENT
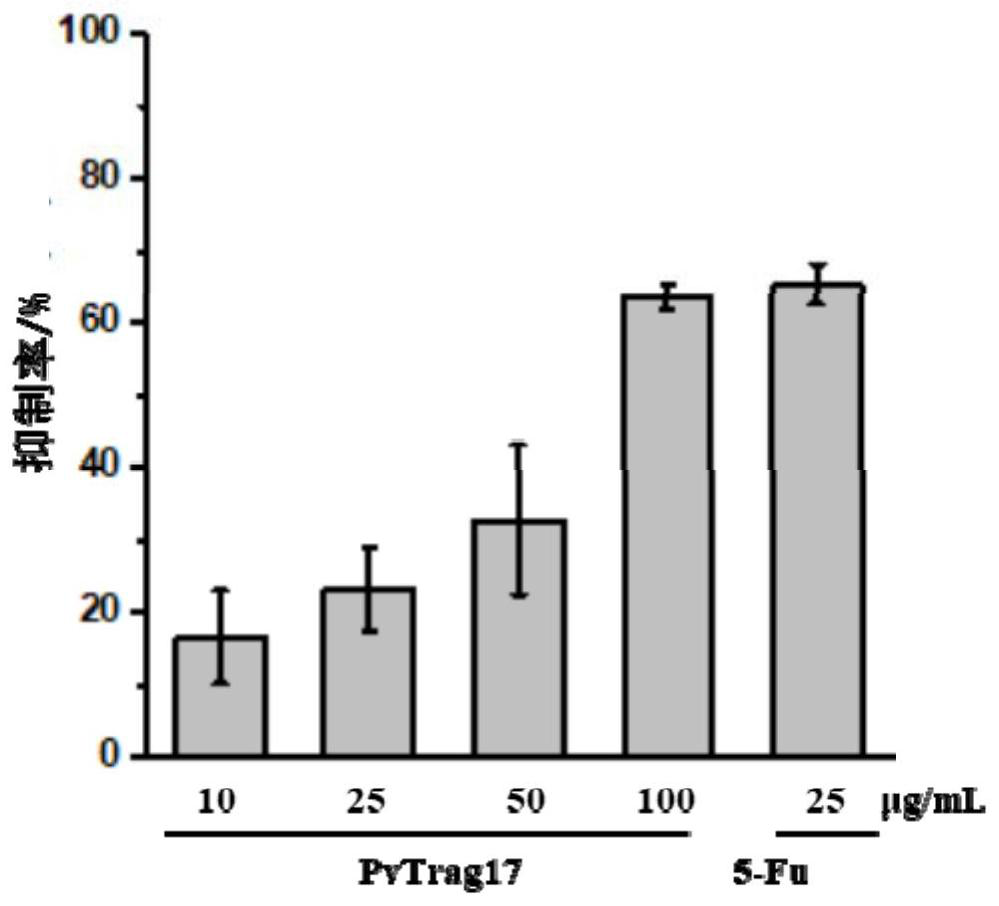
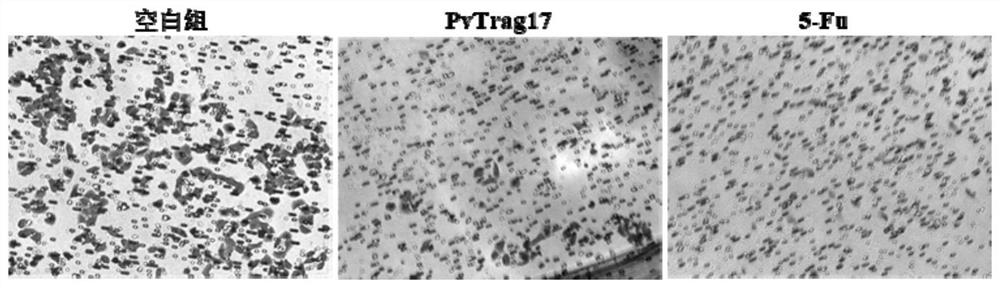
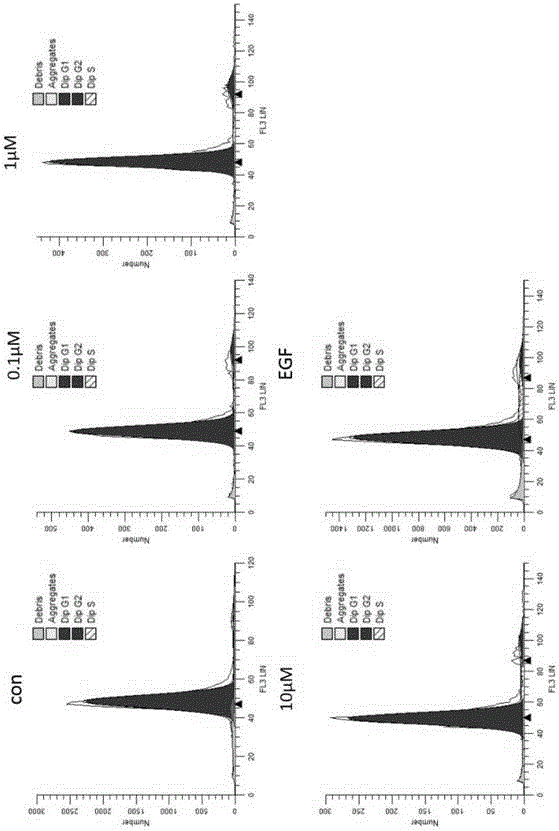
The invention discloses an anti-tumor protein and an application thereof, belonging to the technical field of biomedicine. The invention firstly screens and verifies the application of PvTrag17 protein shown in SEQ ID NO. 1 in anti-tumor field, The results of CCK8 proliferation assay showed that PvTrag17 protein inhibited the proliferation of HepG2 cells by 63.1% and almost completely inhibited the migration of HepG2 cells. The PvTrag17 protein of the invention can be used as an antineoplastic drug of active ingredient or raw material, and has wide application prospect.
Owner:JIANGNAN UNIV
Peptide and application thereof
InactiveCN106800588APromote proliferationExact aging preventionCosmetic preparationsToilet preparationsSide effectTherapeutic effect
The invention relates to the protein field, in particular to a peptide and application thereof. According to the invention, tripeptide Arg-Arg-Tyr acts on human skin fibroblasts to perform CCK-8 proliferation assay, cell proliferation detection assay by Edu process, and cell cycle detection assay by flow cytometry on human skin fibroblasts. The assays prove that the tripeptide Arg-Arg-Tyr can effectively promote the proliferation of human skin fibroblasts and increase the proportion of S+G2M phase, has definite therapeutic effect on prevention or treatment of skin aging, and has small side effect, thus having important practical significance.
Owner:INFINITUS (CHINA) CO LTD
A kind of nucleoside base hydroxamic acid derivative compound and its preparation method and application
ActiveCN106883217BRaw materials are easy to getEasy to prepareOrganic chemistryAntineoplastic agentsHistone deacetylaseHydroxamic acid
The invention discloses a nucleoside and base hydroxamic acid compound with DNA transmethylase and / or histone deacetylase inhibition activity, and a preparation method and applications thereof. The structural formula of the nucleoside and base hydroxamic acid compound is shown as the formula I, wherein R is nucleoside and base, nucleoside and base with substituent group or nucleoside and base analogue in DNA and / or RNA; Linker is a connecting chain connecting R with hydroxamic acid function group, the connecting chain comprises but is not limited within alkyl chain, alkyl chain with heteroatom, alkyl chain with aromatic ring, and alkyl chain with heterocycle. In-vitro cell proliferation assay show that the compound shown in the formula I can well inhibit the proliferation of leukemia cell K562 and histocyte lymphoma cell U937. Transmethylase and histone deacetylase inhibition assay shows that the compound shown in the formula I is a compound having inhibition activity on the DNA transmethylase and / or histone deacetylase. The formula I is as shown in the specification.
Owner:SHENZHEN GRADUATE SCHOOL TSINGHUA UNIV
Method of synthesizing antagonist peptides for cell growth
ActiveUS10745454B2Improve purification effectImprove stabilityPeptide/protein ingredientsBiological material analysisMatrigelCompetitive binding
The embodiments herein disclose a method for synthesizing antagonistic peptide VEGF and bFGF. The method comprises synthesizing the antagonistic peptide for VEGF and bFGF and analyzing the purity of peptides. The quality of antagonistic peptide for VEGF and bFGF is analyzed by HPLC chromatogram and Mass spectrometry analysis. The biochemical activity of the antagonistic peptide for VEGF and bFGF is analyzed by competitive binding assay, cell proliferation assay, Matrigel assay for anti-angiogenic activity analysis, histopathological staining and Western blot analysis. The competitive binding assay of antagonistic peptide for VEGF and bFGF illustrate that peptides binds with cell receptors at a concentration of 2000 ng / ml. The cell proliferation assay illustrates that cell growth is arrested when antagonistic peptide for VEGF and bFGF are at a concentration of 2000 ng / ml. The anti-angiogenic activity analysis illustrates that angiogenesis is arrested when the concentration of antagonistic peptide for VEGF and bFGF is 2000 ng / ml.
Owner:ASGHARI SEYED MOHSEN +1
A lipoic acid-modified inherently disordered protein nanocarrier and its preparation method and application
ActiveCN107129522BLow cytotoxicityPromote degradationOrganic active ingredientsGenetic material ingredientsDisulfide bondingCancer cell
The invention relates to the technical field of medicines, in particular to a lipoic acid modified intrinsically disordered protein nano-carrier, a preparation method thereof and an application of the nano-carrier. The nano-carrier is crosslinked by disulfide bonds of lipoic acid, formed polypeptide polymer can be rapidly degraded in cells and cannot be accumulated in the cells, and amino acid forming a polypeptide carrier exists in vivo and has no toxic and side effects on the cells and a human body. CCK-8 cell proliferation assay indicates that the prepared nano-carrier has low cell toxicity and good gene and chemotherapeutic drug co-carrier capacity, can successfully deliver sensitivity of autophagic specificity enhanced cancer cells to chemotherapeutic drugs caused by siRNA suppressive chemotherapeutic drugs in breast cancer therapy, promotes breast cancer cell apoptosis and accordingly becomes a targeted, efficient and low-toxicity nano-scale delivery system in breast cancer therapy.
Owner:SHANGHAI WEI ER BIOPHARM TECH CO LTD +3
SgRNA screening for DJ-1 gene editing as well as vector and application of sgRNA
The invention discloses an sgRNA for a DJ-1 gene. A prokaryotic evaluation system such as a white-to-blue clone formation assay confirms the editing efficiency of the sequence, and proves that the sequence provided by the invention is excellent in editing efficiency and provides a reference for a future DJ-1 gene therapy; cell proliferation assays show that cell proliferation of cell lines slows down after the DJ-1 gene is knocked out; and at the same time, an effective cell tool is provided for a further DJ-1 mechanism research.
Owner:ZHANGJIAGANG INST OF IND TECH SOOCHOW UNIV +1
Enteric capsule shell and preparation method thereof
InactiveCN106361723ALow pricePromote growthCapsule deliveryMacromolecular non-active ingredientsBiocompatibility TestingSericin
The invention relates to an enteric capsule shell. The enteric capsule shell is prepared from, by mass, 1%-45% of sericin protein, 35%-70% of silk fibroin, 1%-15% of formamide, 2%-30% of ethylene glycol and 2%-25% of proline; natural silk protein good in biocompatibility serves as a raw material, small molecules are added to improve the crystallization property of the material, the natural silk protein can be used for replacing gelatin to serve as the capsule shell material, and the problems caused by capsule shells prepared from the gelatin are solved. It is proved through cell proliferation assays that the obtained capsule shell material can support good growth of cells and is good in cell compatibility and harmless to human body. The silk protein is low in price, capable of obtaining the raw materials easily and easy to purify, cannot carry pathogens common to mammals and is suitable for pharmaceutical materials.
Owner:NANTONG TEXTILE & SILK IND TECH RES INST
Stimulated cell standards
InactiveUS9267956B2Improves Structural IntegrityPreparing sample for investigationBiological testingCarboxyfluorescein succinimidyl esterFreeze-drying
Methods for producing stimulated, positive and negative control reference standard for monitoring intracellular cytokine levels and cytokine release in test samples by stimulating cells to produce cytokines in the presence of a cytokine release inhibitor, fixing the stimulated cells with a fixative such as paraformaldehyde, washing to remove excess fixatives and freeze-drying the stimulated, fixed cells. Methods for producing labeled reference standards for cell proliferation assays are also disclosed, in which proliferation-competent mammalian cells, isolated from a human or animal body are labeled with a label, such as a dye, that is divided between daughter cells during cell proliferation (e.g., carboxyfluorescein succinimidyl ester), the cells are stimulated to proliferate, the proliferated cells are fixed by addition of a fixative and then preserved by freeze drying or cryopreservation.
Owner:THE UK SEC FOR HEALTH & HER BRITANNIC MAJESTYS GOVERNMENT OF THE UK OF GREAT BRITAIN & NORTHERN IRELAND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com