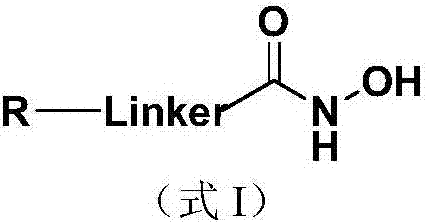
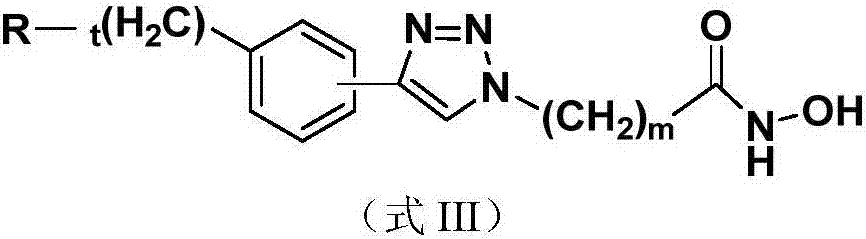
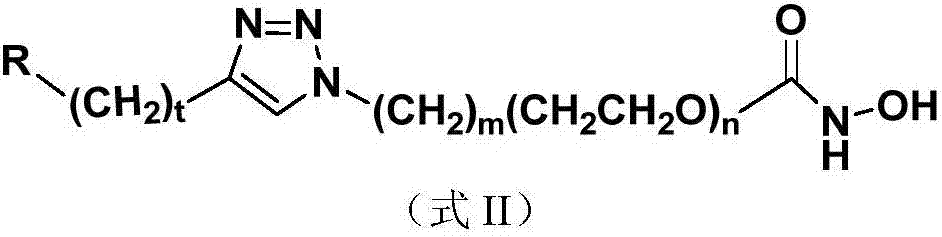Nucleoside and base hydroxamic acid derived compound and preparation method and applications thereof
A nucleoside base hydroxamic and hydroxamic acid functional technology, applied in the field of medicine, can solve the problems of drug resistance of tumor cells, toxic side effects, and reduced therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] 6-(4-((4-Amino-2-oxopyrimidin-1(2H)-yl)methyl)-1H-1,2,3-triazol-1-yl)-N-hydroxyhexanamide
[0102]
Embodiment 1A
[0104] Ethyl 6-Azidohexanoate
[0105]
[0106] Ethyl 6-bromohexanoate (1eq) was dissolved in DMF, sodium azide (3eq) was added, the temperature was slowly raised from room temperature to 80°C and reacted at this temperature overnight. Ethyl acetate was then added, excess sodium azide was removed by filtration, water was added and extracted with ethyl acetate. The organic phase was washed with water and saturated brine and dried over anhydrous sodium sulfate. Filtration and spin off the solvent on a rotary evaporator afforded the title compound. 1H NMR (400MHz, DMSO-d6) δ4.05 (q, J = 7.1Hz, 2H), 3.31 (t, J = 6.8Hz, 2H), 2.29 (t, J = 7.3Hz, 2H), 1.60–1.47 (m,4H),1.38–1.27(m,2H),1.18(t,J=7.1Hz,3H). 13 CNMR (101MHz, DMSO) δ173.26, 60.18, 51.05, 33.91, 28.46, 26.16, 24.52, 14.64.
Embodiment 1B
[0108] 4-Amino-1-(prop-2-yn-1-alk)pyrimidin-2(1H)-one
[0109]
[0110] Dissolve cytosine (1eq) and 3-bromopropyne (3eq) in anhydrous DMF, add anhydrous cesium carbonate (2eq), heat to 80°C and react overnight. After the reaction was completed, water was added and extracted with ethyl acetate. The organic phase was washed with water and saturated brine, and dried over anhydrous magnesium sulfate. The title compound was obtained by column separation after removing the solvent of the organic phase. 1 H NMR (400MHz, DMSO-d6) δ7.52(d, J=7.7Hz, 1H), 6.64(d, J=7.8Hz, 1H), 4.77(d, J=2.5Hz, 2H), 3.49(t ,J=2.5Hz,1H). 13 C NMR (101MHz, DMSO) δ166.55, 155.61, 145.26, 94.61, 79.96, 75.79, 37.84.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com