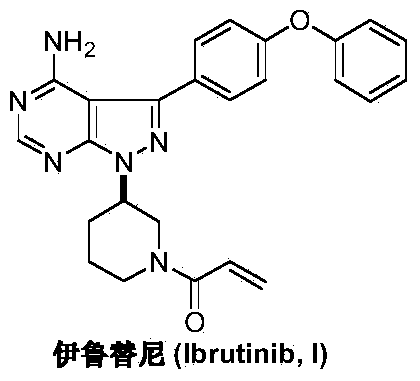
Preparation method of Ibrutinib
A technology of ibrutinib and cyclization reaction, which is applied in the field of preparation of ibrutinib, can solve the problems of manufacturing cost and unsatisfactory reaction conditions, and achieve the effects of promoting development, easy availability of raw materials, and environmentally friendly and economical process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
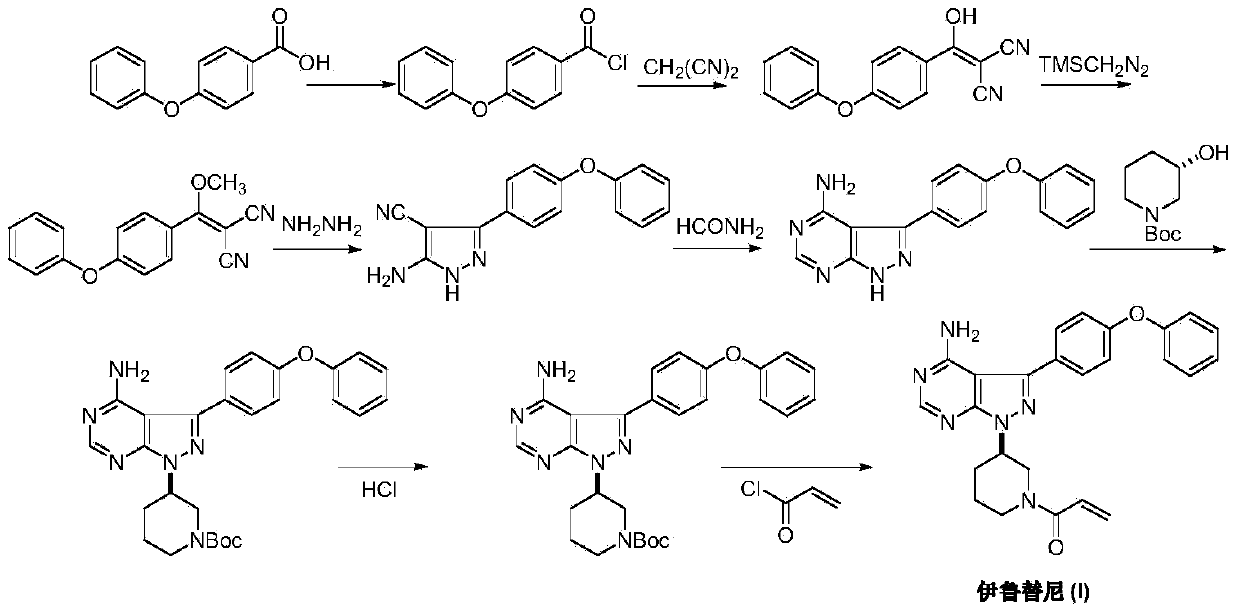
[0025] Add malononitrile (6.6g, 0.1mol), sodium hydride (4.8g, 0.2mol, 80% dispersed in paraffin) and freshly treated anhydrous tetrahydrofuran 100mL in a three-necked reaction flask, and add 4- A solution of phenoxybenzoyl chloride (II) (23.2 g, 0.1 mol) in 50 mL tetrahydrofuran. After keeping the reaction at room temperature for 2 hours, 250 mL of 1M dilute hydrochloric acid was added, stirred and reacted for 30 minutes, and extracted three times with ethyl acetate. The organic phases were combined, dried over anhydrous magnesium sulfate, and the resulting solid was concentrated and dissolved in 150 mL of dioxane and 50 mL of saturated sodium bicarbonate solution. Dimethyl sulfate (37.8 g, 0.3 mol) was added, the temperature was raised to 80-90° C., the reaction was stirred for 3 hours, and the reaction was detected by TLC. Add 400 mL of deionized solution, extract 3 times with methyl tert-butyl ether, combine the organic phases, and dry over anhydrous sodium sulfate. The ...
Embodiment 2
[0027] Add 4-phenoxyphenyl (methoxy)vinylidene dicyanomethane (III) (13.8g, 50mmol), 1-(3R-hydrazino-1-piperidinyl)- 2-propen-1-one (IV) (8.5g, 50mmol) and ethanol 200mL were added dropwise with triethylamine (5g, 50mmol) while stirring. The temperature was raised to reflux, and the reaction was stirred for 5 hours, and the reaction was detected by TLC. Concentrate under reduced pressure, add deionized water to the residue, stir and crystallize at room temperature. Filtration, the obtained solid ethanol and water (1:1) were recrystallized to obtain off-white solid 1-[(3R)-[3-(4-phenoxyphenyl)-4-cyano-5-amino-1H- Pyrazolyl]-1-piperidinyl]-2-propen-1-one (V) 16.6 g, yield 80.4%.
Embodiment 3
[0029] Add 1-[(3R)-[3-(4-phenoxyphenyl)-4-cyano-5-amino-1H-pyrazolyl]-1-piperidinyl]-2 to the three-necked reaction flask -propen-1-one (V) (4.13g, 10mmol), N,N-dimethylformamide dimethyl acetal (1.19g, 15mmol) and toluene 50mL, add acetic acid 3mL under stirring, heat up to 105- 110°C, use an oil-water separator to separate the produced methanol, keep it warm for 3 hours, and detect the reaction by TLC. Concentrate under reduced pressure to remove toluene, add 30% concentrated ammonia water to the residue, and a solid precipitates out. The temperature was raised to reflux under stirring, and the reaction was detected by TLC after 3 hours. When the temperature was lowered to room temperature, solids were precipitated, and crystallized under slow stirring for 12 hours. After filtration, the crude product was recrystallized from ethanol to obtain 3.2 g of ibrutinib (I), with a yield of 72.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com