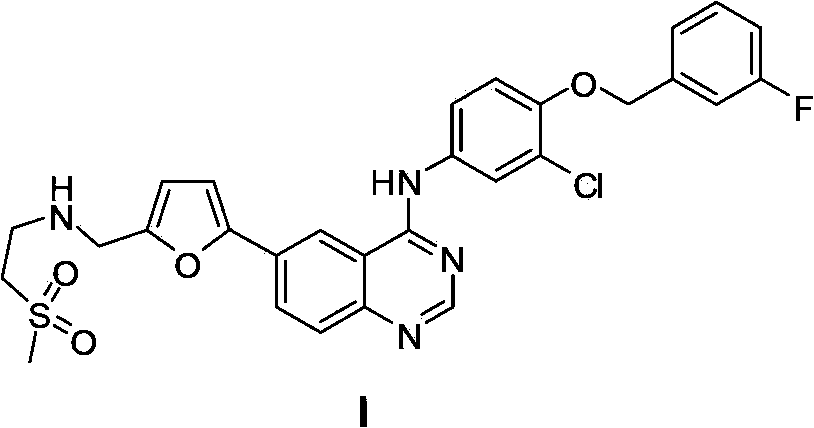
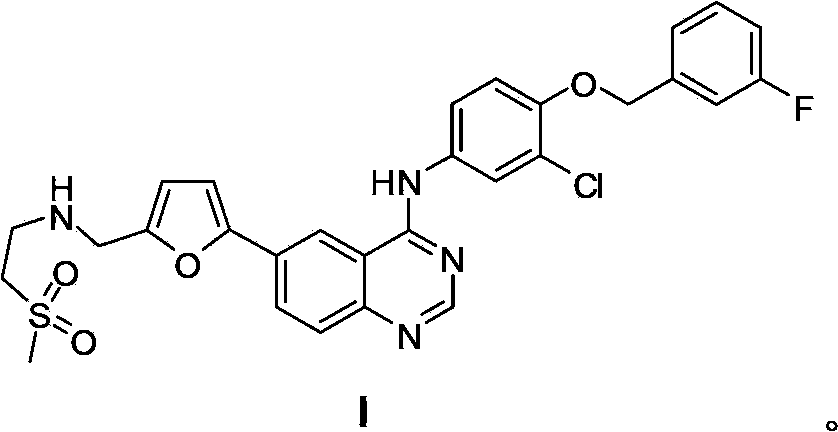
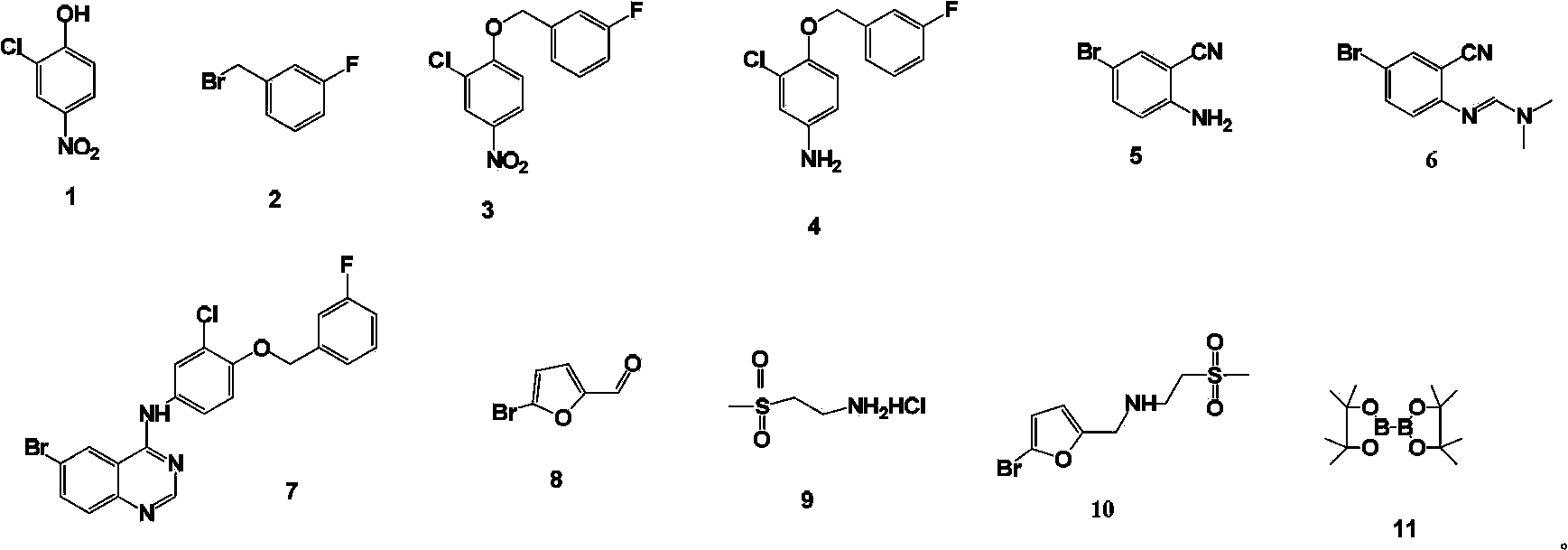
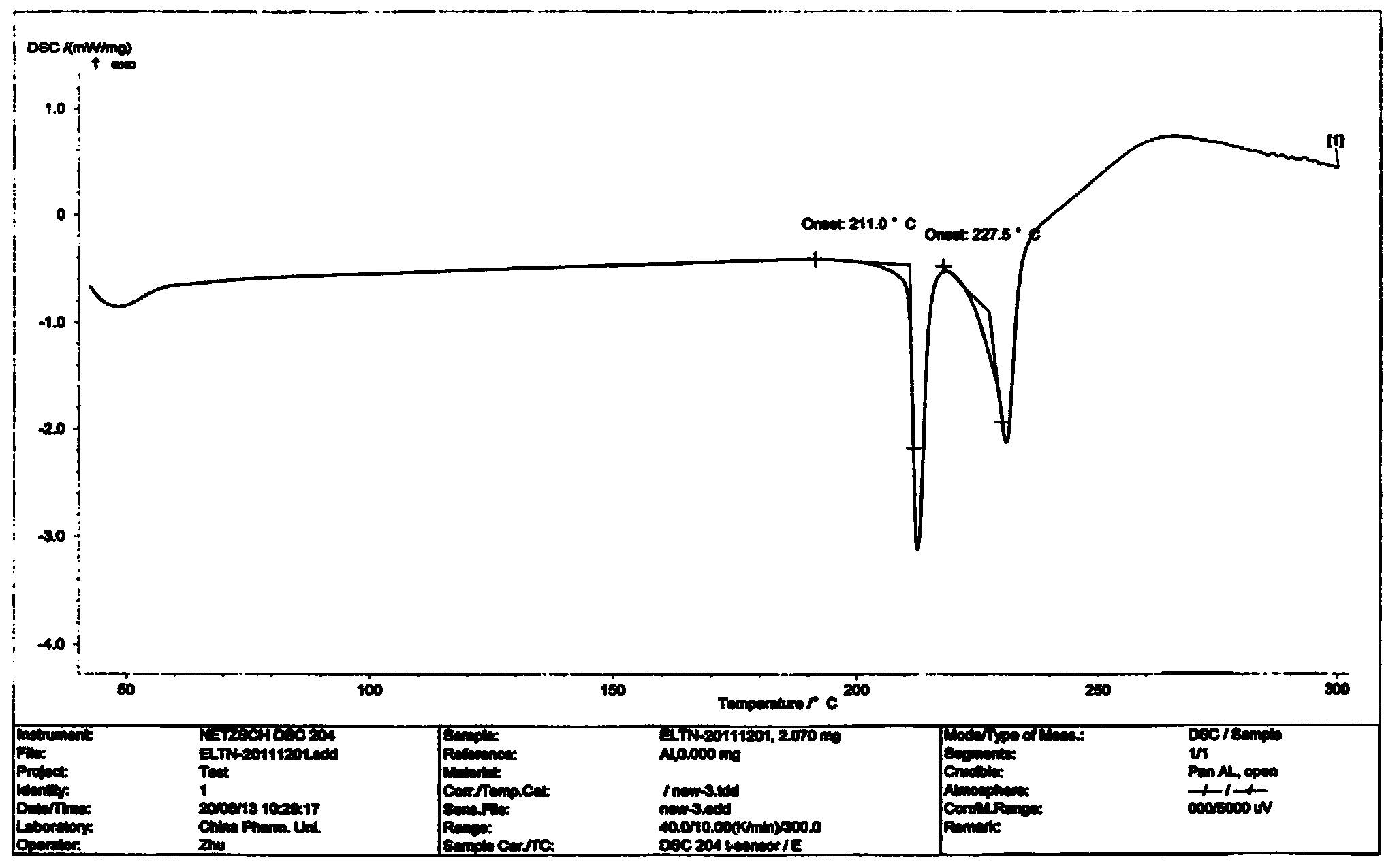
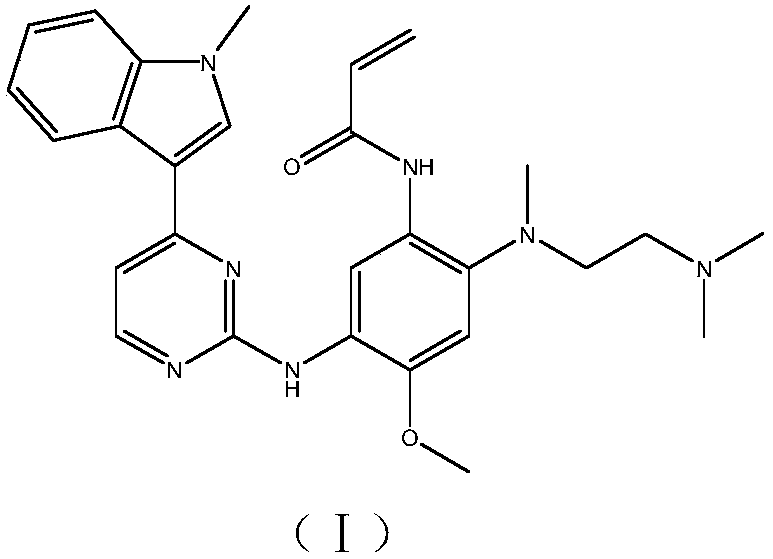
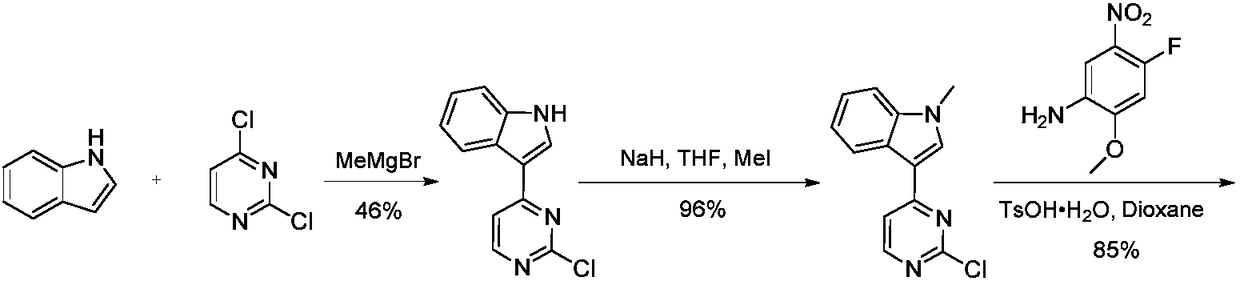
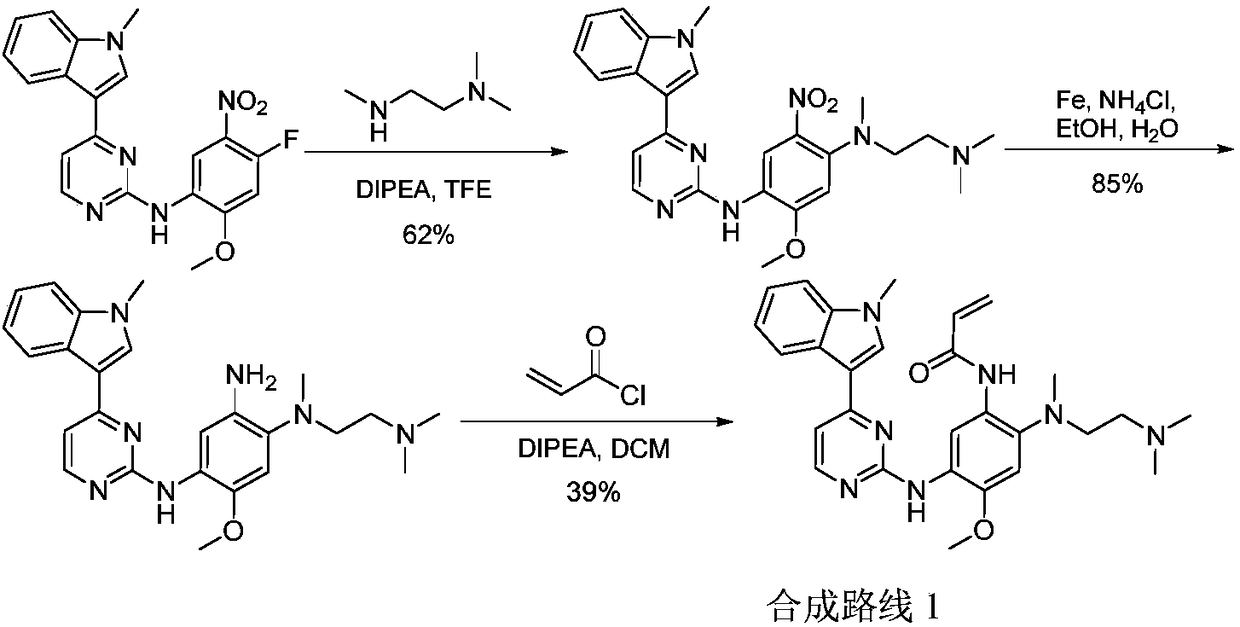
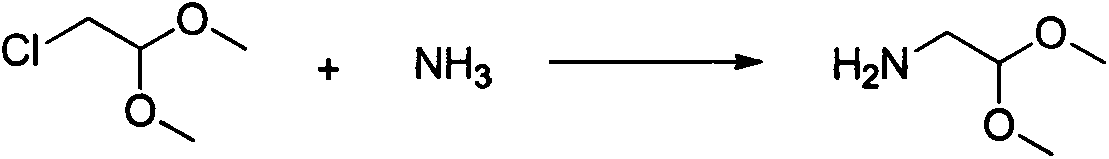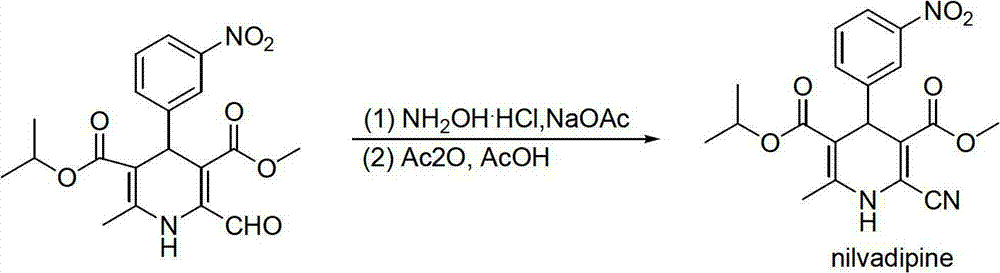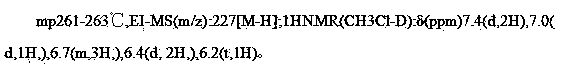Patents
Literature
262 results about "Dimethyl acetal" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Dimethyl acetals can be prepared from carbonyl compounds with excess methanol catalyzed by a Brønsted (i.e. protic) acid or Lewis acid (e.g. BF3) together with a dehydrating agent or other means of water removal that will drive the equilibrium in the following reaction to the right.
Clean reclaiming process for byproduct chloromethane of glyphosate acid
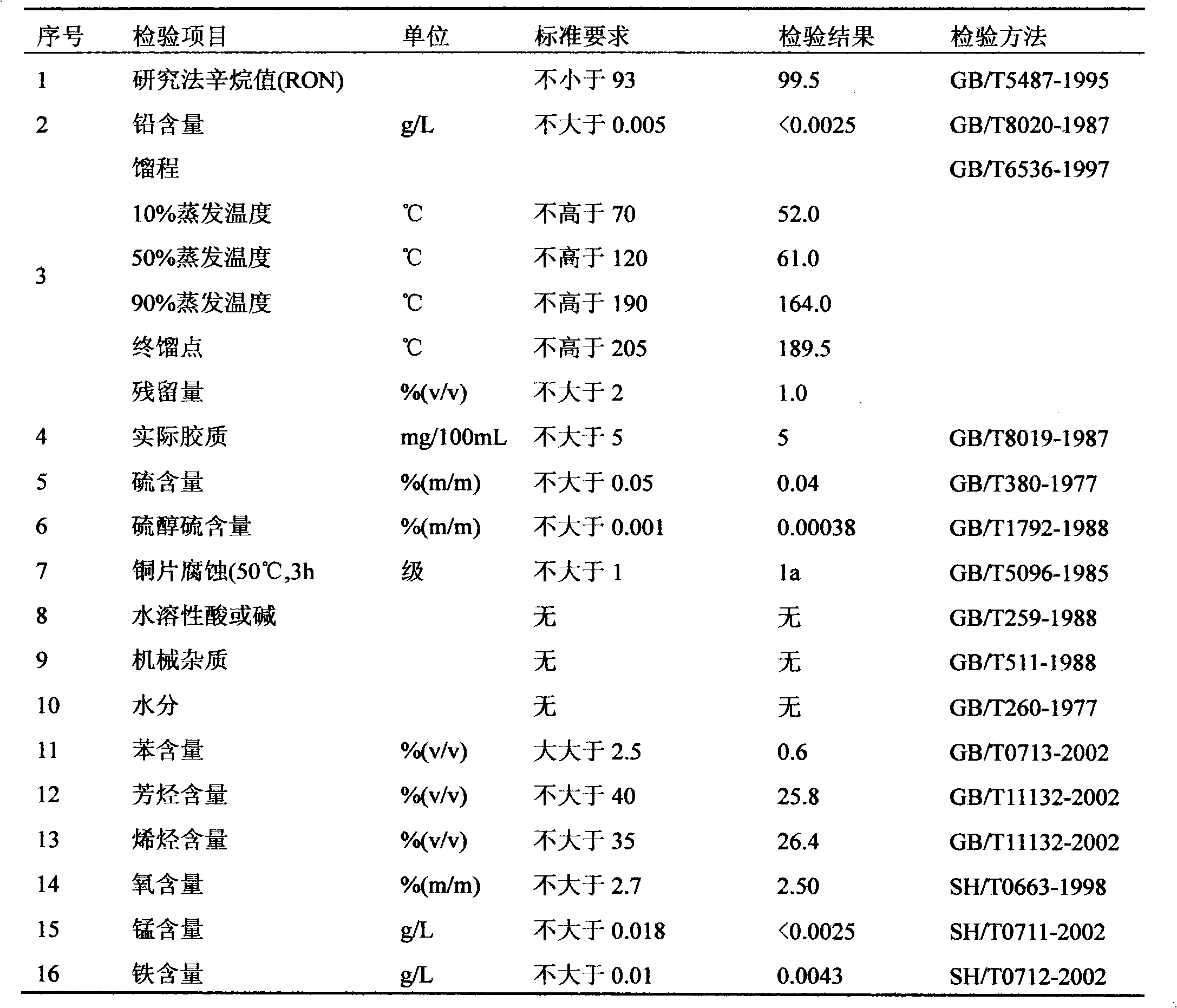
InactiveCN1629112ARealize cleaner productionEasy to operateGroup 5/15 element organic compoundsHalogenated hydrocarbon separation/purificationPhosphorous acidForeign matter
The discharged exhaust gas during the glyphosate acid production process through phosphorous acid alkyl acid includes 20-90% of methyl chloride, methanol, formaldehyde dimethyl acetal, water, hydrogen chloride and other foreign matter, by solvent absorption of foreign matter, condensation impurity or charging part of the foreign matter such as methanol, formaldehyde dimethyl acetal into hydrochloride-containing liquid and converting it into methyl chloride, high purity of methyl chloride can be obtained. The process not only solves the problem of environmental pollution in the glyphosate acid production, the production cost can also be lowered substantially.
Owner:ZHEJIANG XINAN CHEM INDAL GROUP
Clean reclaiming process for byproduct chloromethane of glyphosate acid
InactiveCN1629111ARealize cleaner productionEasy to operateGroup 5/15 element organic compoundsHalogenated hydrocarbon separation/purificationPhosphorous acidForeign matter
The discharged exhaust gas during the glyphosate acid production process through phosphorous acid alkyl acid includes 20-90% of methyl chloride, methanol, formaldehyde dimethyl acetal, water, hydrogen chloride and other foreign matter, by solvent absorption of foreign matter, condensation impurity or charging part of the foreign matter such as methanol, formaldehyde dimethyl acetal into hydrochloride-containing liquid and converting it into methyl chloride, high purity of methyl chloride can be obtained. The process not only solves the problem of environmental pollution in the glyphosate acid production, the production cost can also be lowered substantially.
Owner:ZHEJIANG XINAN CHEM INDAL GROUP
Method for preparing praziquantel
InactiveCN103739601AThe synthesis process is simpleImproved post-treatment processOrganic chemistryDimethyl acetalCyclohexanecarboxylic acid
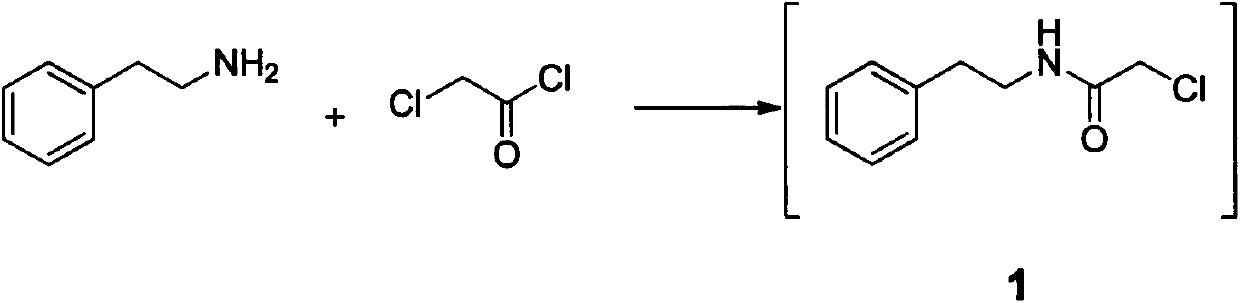
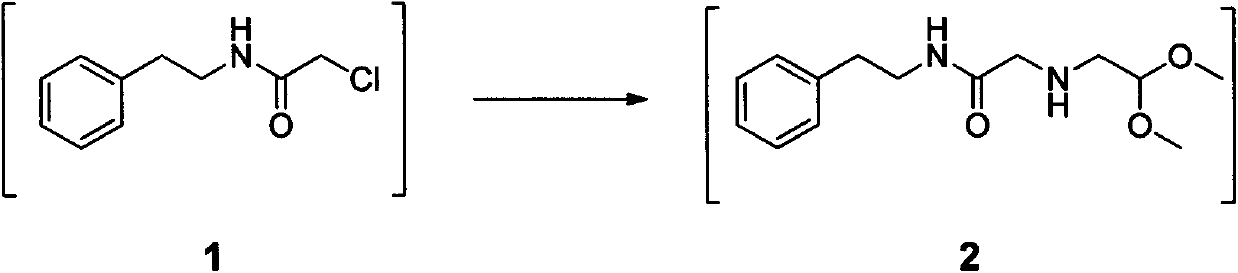
The invention relates to a method for preparing praziquantel, which is a one-pot method and comprises the following steps: performing an ammonolysis reaction of chloroacetaldehyde dimethyl acetal and an ammonia aqueous solution to generate aminoacetaldehyde dimethyl acetal; performing a condensation reaction of beta-phenylethylamine and chloroacetyl chloride in an organic solvent in alkaline environment to generate an intermediate 1; performing a condensation reaction of the intermediate 1 and the aminoacetaldehyde dimethyl acetal in an organic solvent to generate an intermediate 2; performing cyclization of the intermediate 2 in the presence of an acidic catalyst to generate an intermediate 3; performing a reaction of the intermediate 3 and cyclohexanecarboxylic acid chloride in an organic solvent in alkaline environment, and performing solvent crystallization to obtain the target product of praziquantel.
Owner:JIANGSU CHENGXIN PHARMA
Chiral porous organic polymer material and preparation method
InactiveCN105801815ASave raw materialsReduce manufacturing costOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsCross-linkPhosphate
The invention discloses a chiral porous organic polymer material and a preparation method, and belongs to the technical field of chemical and novel materials.The preparation method comprises the steps that a 3,3'-substitued binaphthol phosphate compound and / or an aromatic ring compound and / or a condensed ring compound and / or a heterocyclic ring compound are subjected to a Friedel-Crafts alkylation reaction at the temperature of 30 DEG C-120 DEG C under the action of a lewis acid catalyst in the presence of a formaldehyde dimethyl acetal cross-linking agent, and then the chiral porous organic polymer material with the specific surface area of 3-3000 m<2> / g.According to the chiral porous organic polymer material and the preparation method, by changing the components serving as reaction monomers, the specific surface area and pore parameters of the obtained chiral porous organic polymer material and the variety and content of binaphthol can be adjusted, and the product can be applied to the heterogeneous asymmetric catalyzing field by serving as a heterogeneous catalyst, has the advantages of being easy to operate, mild in reaction condition and the like, is low in cost of the raw materials and production cost and has the wide industrial application prospect.
Owner:JILIN UNIV
Gasoline additive for environmental protection and energy-saving vehicle
InactiveCN101870891ALow running costImprove powerLiquid carbonaceous fuelsDimethyl acetalButylated hydroxytoluene
The invention discloses a gasoline additive for an environmental protection and energy-saving vehicle. The gasoline additive comprises the following components in parts by weight: 12-18 parts of nitromethane, 5-8 parts of boron, 2-5 parts of pyridine, 6-8 parts of didodecyldimethylammonium, 3-6 parts of carbon fluoride, 1-3 parts of magnesium perchlorate, 2-4 of BHT (butylated hydroxytoluene), 1-3 parts of light calcium carbonate, 4-7 parts of dimethyl acetal, 4-7 parts of 3-methyl-1-butylene, 16-21 parts of methyl tert-butyl ether, 6-9 parts of 2,2,3-triptane, 1-3 parts of cerium oxide, 1-3 parts of beryllium carbide, 5-8 parts of N,N-dimethylaniline and 2-4 parts of amino acid palladium complex. The additive can effectively improve the gasoline quality, increase the gasoline octane number, enhance the fire performance of fuels, can be completely burned together with the fuels without generating precipitation or residues, reduce the tail gas exhaust pollution of motor vehicles, reduce the running cost of the motor vehicles, and has the obvious oil saving effect.
Owner:孙勇
Preparation method of gefitinib
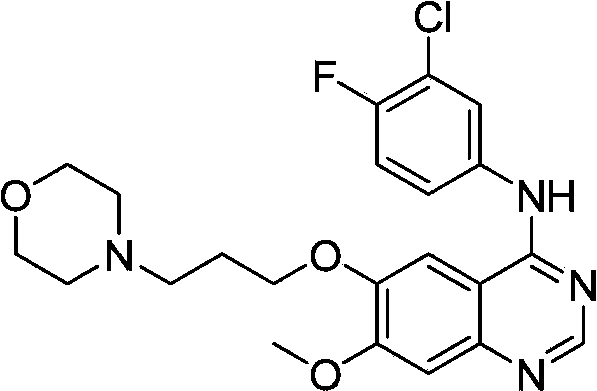
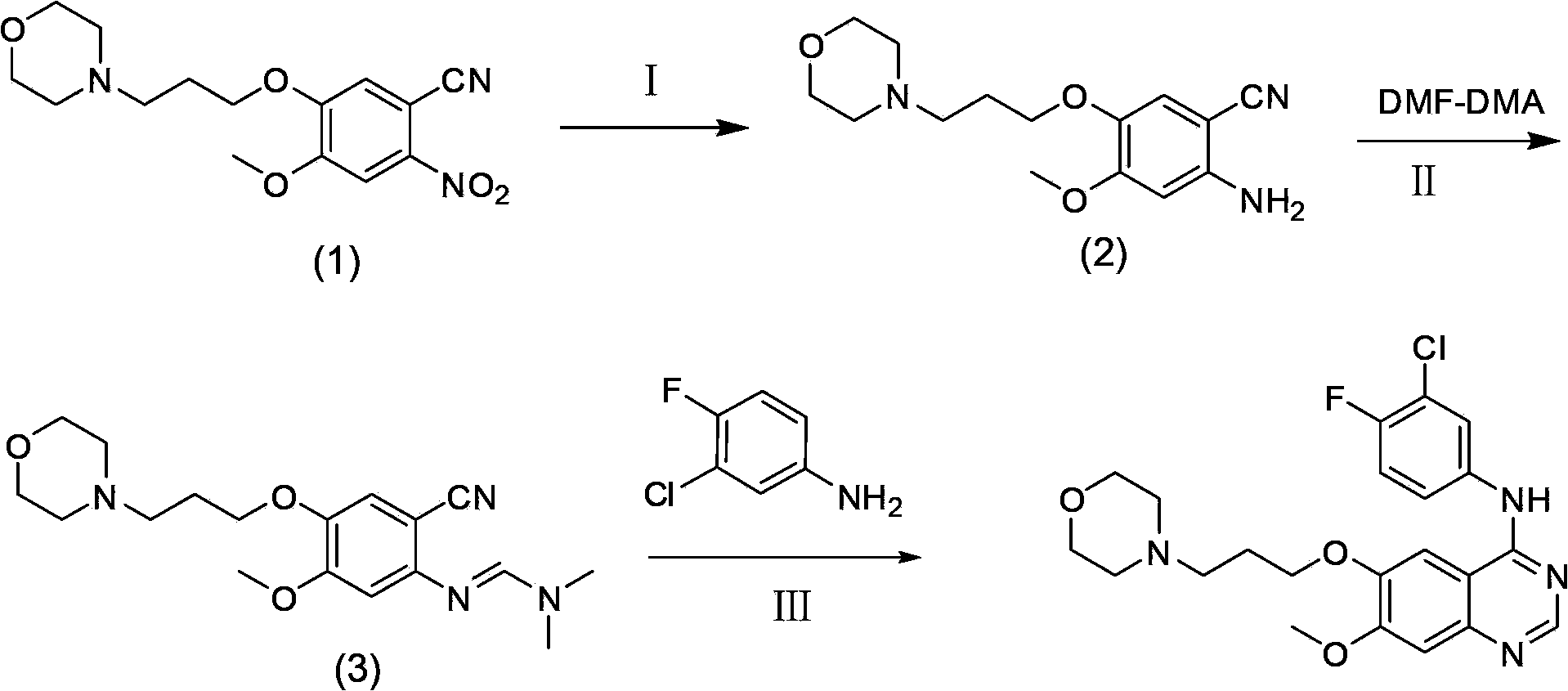
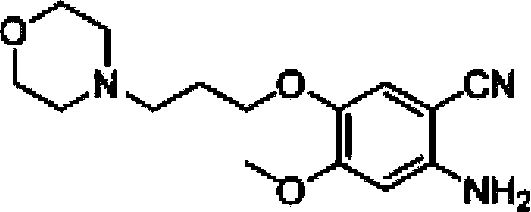
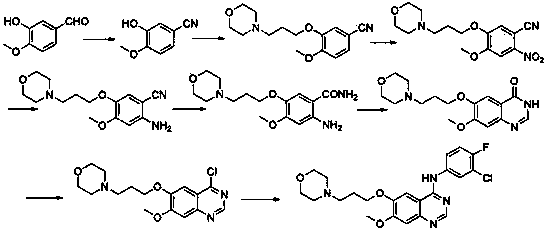
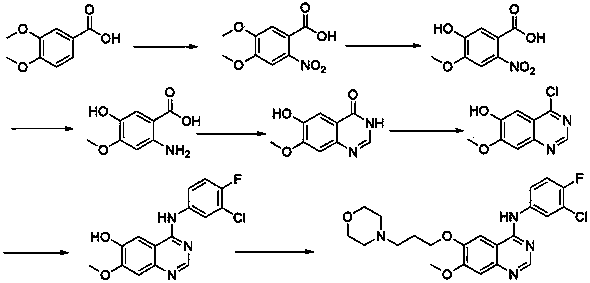
ActiveCN103570633AReduce usageFor the purpose of purificationOrganic chemistryPurification methodsMorpholine
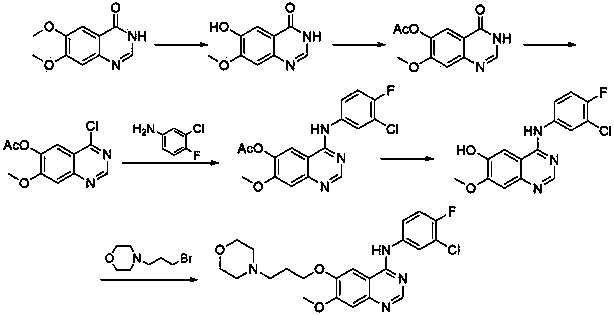
The invention discloses a preparation method of gefitinib. The preparation method takes 4-methoxyl-5-(3-morpholine propoxyl)-2-nitrobenzonitrile as a raw material, then subjecting the raw material to treatments of reduction and salt forming reactions so as to obtain an intermediate 2-amino-4-methoxyl-5-(3-morpholine propoxyl) benzonitrile hydrochloride, then directly subjecting the intermediate to react with N,N-dimethyl formamide dimethyl acetal so as to obtain N'-(2-cyano-5-methoxyl-4-(3-morpholinyl propoxyl)benzyl)-N,N-dimethyl formamidine, and finally subjecting the formamidine intermediate to carry out rearrangement reactions with 3-chloro-4-fluoroaniline so as to obtain the gefitinib. The preparation method has the advantages of mild reaction conditions, convenient intermediate purification method, and suitability for industrial production.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Porous organic polymer framework material and preparation method and application thereof
ActiveCN106496530AEasy to prepareMild reaction conditionsOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsCross-linkDimethyl acetal
The invention discloses a porous organic polymer framework material and a preparation method and application thereof, and belongs to the technical field of chemical and new materials. The porous organic polymer framework material connected by using methylene is obtained under the catalysis of a lewis acid catalyst by using 2,7-di(nitrogen-carbazolyl)-9 fluorenone as a structural unit and formaldehyde dimethyl acetal as a cross-linking agent, and the porous organic polymer framework material can be applied to selective imine synthesis from photocatalysis of organic amine or selective sulfoxide synthesis from photocatalysis of thioether. The porous organic polymer framework material has the characteristics of simple operation, mild reaction conditions, wide applicability and the like.
Owner:JILIN UNIV
Method for manufacturing novel dendritic fluorescent chemosensor and application
InactiveCN102020985ASensing shortcutSensitiveOrganic chemistryChemiluminescene/bioluminescenceEthylenediamineFluorescence
The invention discloses a method for manufacturing a dendritic fluorescent chemosensor and the method for detecting Fe<3+> in a sample. The method for manufacturing the dendritic fluorescent chemosensor is mainly technically characterized by comprising the following steps of: performing reaction on aminoacetaldehyde dimethyl acetal, methyl acrylate and ethylenediamine in a methanol medium under the protection of argon at the temperature of 55 to 65 DEG C for 24 to 30 h to obtain a dendritic compound; and reacting in darkness the dendritic compound with rhodamine B hydrazide in the methanol medium under the protection of the argon at room temperature for 48 to 58 h by taking 4-hydroxybenzaldehyde as a crosslinker, and performing filtration and washing to obtain the dendritic fluorescent chemosensor. In the detection method, the mixed solution of ethanol and water serves as the medium, and the Fe<3+> content of various samples is determined by utilizing a fluorophotometer in Tris-HCl buffer solution with the pH value of 6.8 to 7.5. The dendritic fluorescent chemosensor has the linear range of 0 to 10.0 mu g.mL<-1>, the detection limit of 0.026 mu g.mL<-1>, and sensitivity and selectivity which are valuable in analytical chemistry.
Owner:UNIV OF JINAN
Honeysuckle quality evaluation method
ActiveCN106248817AEasy to handleEasy processing conditionsComponent separationChlorogenic acidDimethyl acetal
The invention discloses a honeysuckle quality evaluation method. According to the honeysuckle quality evaluation method, chlorogenic acid, galuteolin and six iridoid glycoside ingredients in honeysuckle are determined, wherein the six iridoid glycoside ingredients are loganic acid, morroniside, loganin, chiratin, secoxyloganin and secologanin dimethyl acetal. The honeysuckle quality evaluation method disclosed by the invention has the advantages that the iridoid glycoside ingredients in the honeysuckle are included in a honeysuckle quality evaluation system for the first time, six representative iridoid glycoside ingredients in the honeysuckle are selected and determined at the same time for guaranteeing reliability and effectiveness of the quality evaluation method, and a dispersive solid phase extraction-high performance liquid chromatography (DSPE-HPLC) is established for the first time and used for detecting the six iridoid glycoside ingredients of different structures, so that reliability and effectiveness of honeysuckle quality evaluation are obviously improved, quality control level of traditional Chinese medicine honeysuckle and related patent medicines is further improved, and the honeysuckle quality evaluation method disclosed by the invention is significant on guidance of clinical application.
Owner:SHANDONG ANALYSIS & TEST CENT
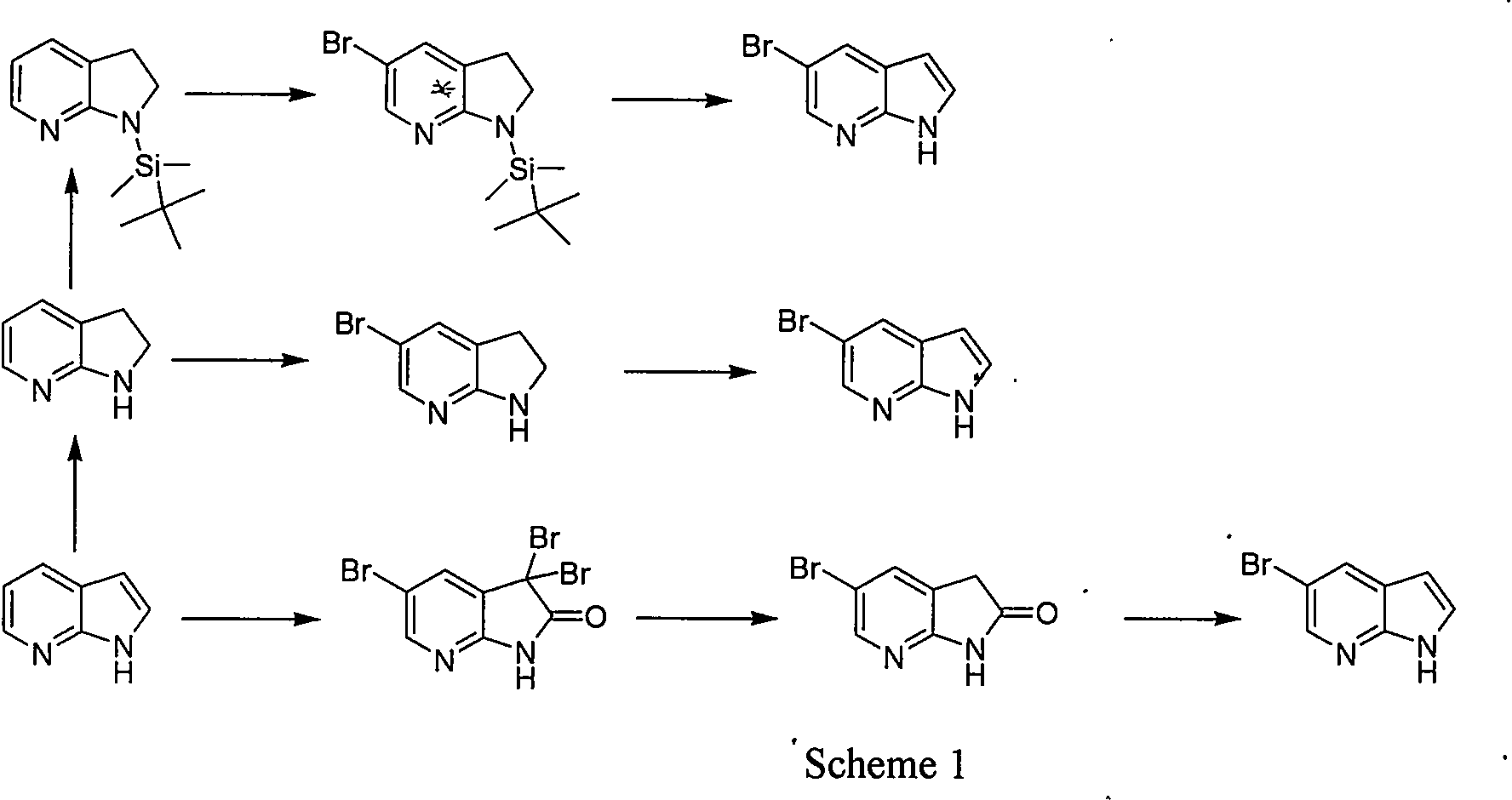
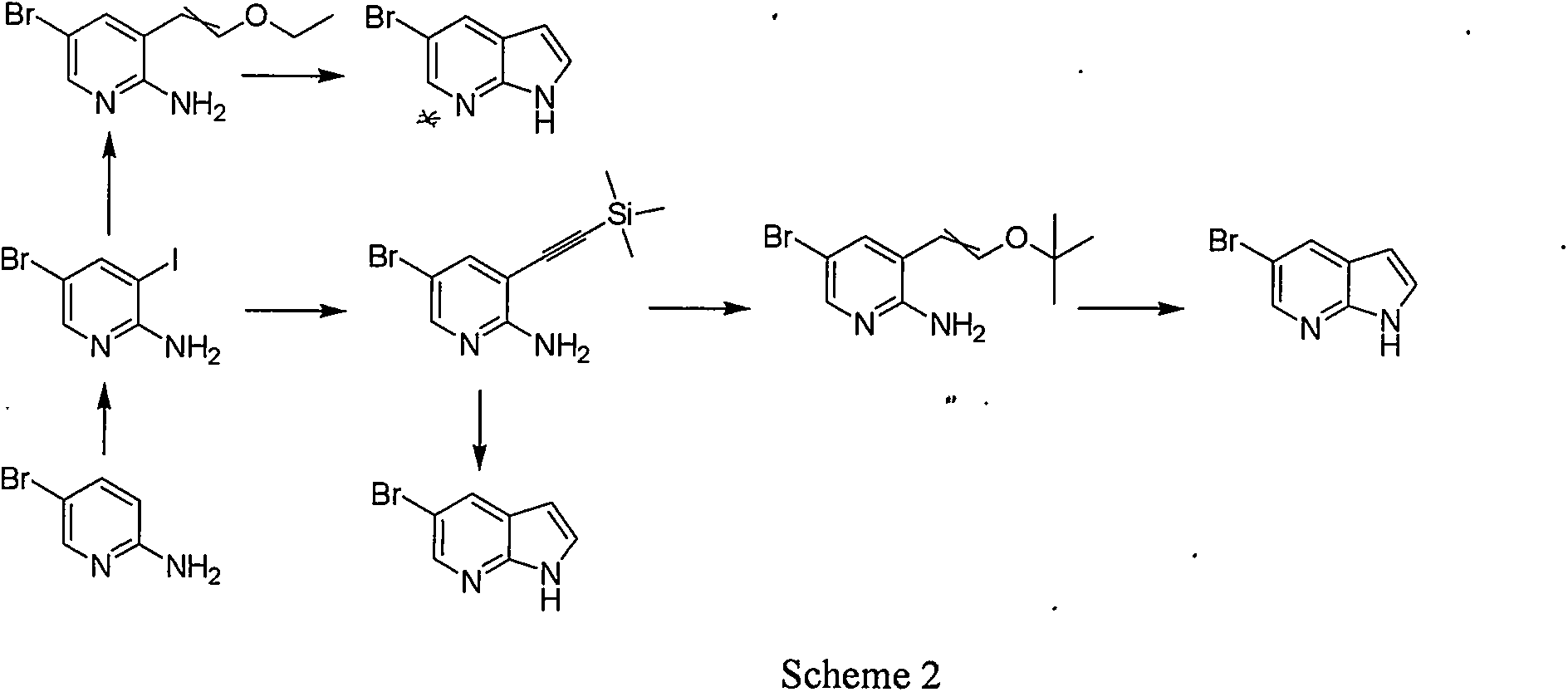
Synthetic process of 5-bromo-7-azaindole
InactiveCN104387384ARaw materials are easy to getShort reaction stepsOrganic chemistryN dimethylformamideDimethyl acetal
The invention relates to a synthetic process of 5-bromo-7-azaindole which is an important medical intermediate. The synthetic process comprises the steps of generating 2-nitro-3-methyl-5-bromopyridine from 2-amino-3-methyl-5-bromopyridine by virtue of the oxidation of caro acid, and generating an intermediate 3 under the actions of tetrahydropyrrole and N,N-dimethylformamide dimethyl acetal (DMF-DMA), and finally carrying out reduction and loop closing under the action of a raney nickel / 85% hydrazine hydrate system or other low valence metal so as to generate 5-bromo-7-azaindole. The synthetic process is suitable for industrial production and has the advantages that the raw material cost is low, initial raw materials are easily available, and the operation is easy.
Owner:SHANGHAI HUMAN BIOTECH CO LTD
Erlotinib preparation method suitable for industrial production
The invention relates to a preparation method of erlotinib and hydrochloride thereof. The preparation method comprises the following steps: 1, reacting 4,5-di(2-methoxyethoxy)-2-nitrophenylacetonitrile with sodium hydrosulfite to obtain 4,5-di(2-methoxyethoxy)-2-aminophenylacetonitrile: controlling the reaction temperature to 45-55DEG C, adding sodium hydrosulfite in batches, adjusting the pH value to 8-9 by using ammonia water after the reaction ends, and directly filtering to obtain 4,5-di(2-methoxyethoxy)-2-aminophenylacetonitrile; and 2, reacting 4,5-di(2-methoxyethoxy)-2-aminophenylacetonitrile with N,N-dimethylformamide dimethyl acetal to obtain a product, and directly reacting the product with m-aminophenylacetylene without purification to obtain erlotinib; and also comprises: carrying out re-crystallization purification treatment on crude erlotinib obtained in step 2 in order to obtain finished erlotinib with the single impurity content being smaller than 0.1%.
Owner:海南卓泰制药有限公司
New preparation method of lapatinib
ActiveCN103483324AEfficient manufacturingHigh purityOrganic chemistryN dimethylformamideDimethyl acetal
The present invention provides a preparation method of lapatinib. The method comprises contacting a compound shown as a formula 1 with a compound shown as a formula 2 to produce a compound shown as a formula 3; reducing the compound shown as the formula 3 to produce a compound shown as a formula 4; contacting a compound shown as a formula 5 with N,N-dimethylformamide dimethyl acetal to produce a compound shown as a formula 6; contacting the compound shown as the formula 6 with the compound shown as the formula 4 to produce a compound shown as a formula 7; in the presence of an acid, an alkali and NaNH(OAc)3, contacting a compound shown as a formula 8 with a compound shown as a formula 9 to produce a compound shown as a formula 10; in the presence of a catalyst and an alkali, contacting the compound shown as the formula 10 with a compound shown as a formula 11 to produce a transition intermediate, and contacting the transition intermediate with the compound shown as the formula 7 and p-toluenesulfonic acid to produce a compound shown as a formula I; through use of the method, the lapatinib can be effectively prepared.
Owner:HUBEI BIO PHARMA IND TECHCAL INST
Process for preparation of imatinib base
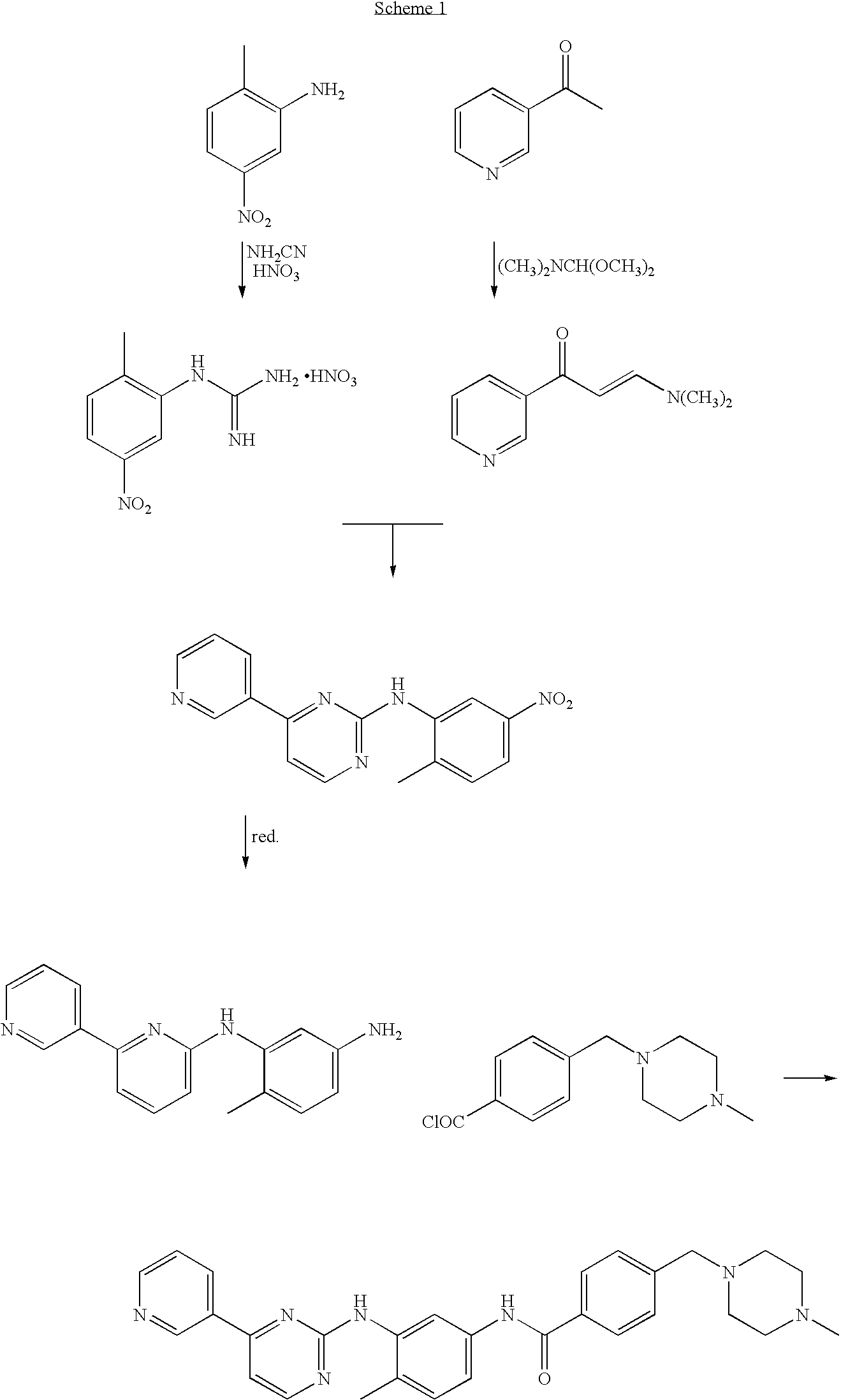
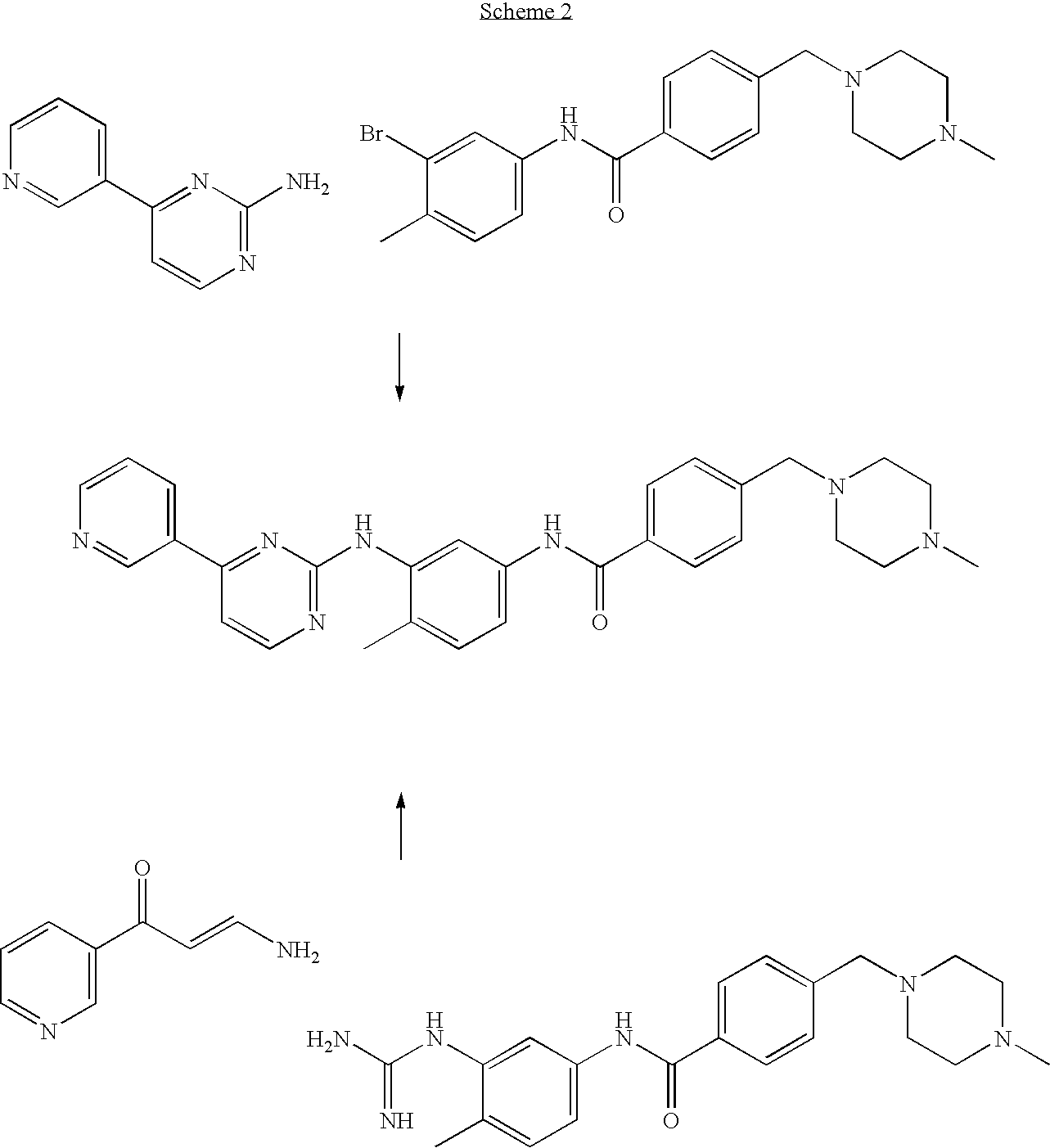
An improved process for the preparation of imatinib base and its pharmaceutically acceptable acid addition salts by (a) reacting 2-methyl-5-nitroaniline with cyanamide in the presence of hydrochloric acid to obtain 1-(2-methyl-5-nitrophenyl)guanidine hydrochloride; (b) converting 1-(2-methyl-5-nitrophenyl)guanidine hydrochloride to 1-(2-methyl-5-nitrophenyl)guanidine nitrate; (c) condensing 3-acetylpyridine with N,N-dimethylformamide dimethyl acetal to obtain 3-(dimethylamino)-1-(3-pyridinyl)-prop-2-en-1-one; (d) reacting 3-(dimethylamino)-1-(3-pyridinyl)-prop-2-en-1-one with 1-(2-methyl-5-nitrophenyl)guanidine nitrate to obtain N-(5-nitro-2-methylphenyl)-4-(3-pyridinyl)-2-pyrimidineamine; (e) reducing N-(5-nitro-2-methylphenyl)-4-(3-pyridinyl)-2-pyrimidineamine using hydrazine in the presence of Raney nickel to obtain N-(5-amino-2-methylphenyl)-4-(3-pyridinyl)-2-pyrimidine-amine; (f) condensing N-(5-amino-2-methylphenyl)-4-(3-pyridinyl)-2-pyrimidine-amine with 4-chloromethylbenzoyl chloride in the presence of an inorganic base to obtain 4-(chloromethyl)-N-(4-methyl-3-(4-(pyridin-3-yl)pyrimidin-2-ylamino)phenyl)benzamide; and (g) condensing 4-(chloromethyl)-N-(4-methyl-3-(4-(pyridin-3-yl)pyrimidin-2-ylamino)phenyl)benzamide with an excess of N-methylpiperazine to obtain imatinib base; and adding water or a mixture of water and an organic solvent; and isolating said imatinib base. The process allows for using simple starting materials, while simultaneously avoiding a laborious isolation and purification of intermediates and the final product, thereby facilitating scale-up.
Owner:INSTITUT FARMACEUTYCZNY
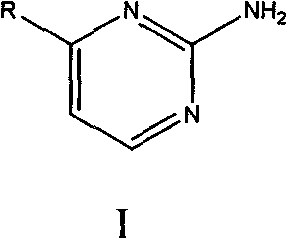
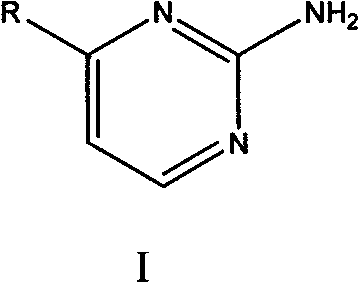
4-substituent-2-amido pyrimidine compound and preparation method thereof
InactiveCN101602757AThe reaction process is simpleMild conditionsOrganic chemistrySodium methoxideAlcohol
The invention relates to a 4-substituent-2-amido pyrimidine compound and a preparation method thereof. The invention adopts a technical scheme of having a structure shown as general formula (I). The preparation method comprises the following steps that: an aromatic ring or heterocyclic ring compound with acetyl radicals is put into a container together with N, N-dimethyl formamide dimethyl acetal, and the mixture is evenly stirred, is heated until refluxing and reacts for 3 to 5 hours, the stirring is stopped, and the mixture is cooled to room temperature, filtered and dried to obtain an intermediate product; sodium ethylate or sodium methoxide is dissolved in absolute alcohol, the mixture is evenly stirred, guanidine hydrochloride is added into the mixture, the obtained mixture is stirred for 0.5 to 1.5 hours at room temperature, and A liquid is obtained; the intermediate product is dissolved in the absolute alcohol to obtain B liquid; and the B liquid is slowly dropped into the A liquid, the reaction system is heated until refluxing, the reaction is carried out for 5 to 7 hours, the stirring is stopped, and the obtained mixture is cooled to room temperature, stands overnight, is filtered, washed and dried. The invention has high yield, high purity, simple technology and universality.
Owner:丹东恒悦新材料有限公司
Natural emulsion composite slurry and preparation method thereof, protection gloves and preparation method thereof
ActiveCN108299689ASave materialReduce manufacturing costGlovesDomestic articlesEmulsionPolypropylene glycol
The invention relates to natural emulsion composite slurry. The natural emulsion composite slurry is prepared from natural emulsion and a heat sensitizing agent, wherein the natural emulsion is subjected to pre-sulfurization, and the heat sensitizing agent is diluted by cold water and is a composition comprising one or more of polyvinylmethyl ether, polyether polyformaldehyde dimethyl acetal and polypropylene glycol. The invention further relates to natural emulsion thermosensitive embossed protection gloves prepared from the natural emulsion composite slurry. The natural emulsion composite slurry can not only prevent the gloves from glue penetration during impregnation, avoid damage to the human body, save energy and protect the environment but also prevent liquid from entering the gloves, so that the prepared gloves are soft in texture, light, sensitive and good in breathability, people does not easily feel tired when wearing the gloves for a long time, and therefore the prepared gloves are particularly suitable for mechanical operation; besides, the prepared gloves can have good composite performance of abrasion resistance, cutting resistance, tearing resistance, stabbing resistance, aging resistance and the like, so that influence on the latter aging resistance performance of glove products and blooming are avoided.
Owner:SHANDONG XINGYU GLOVES
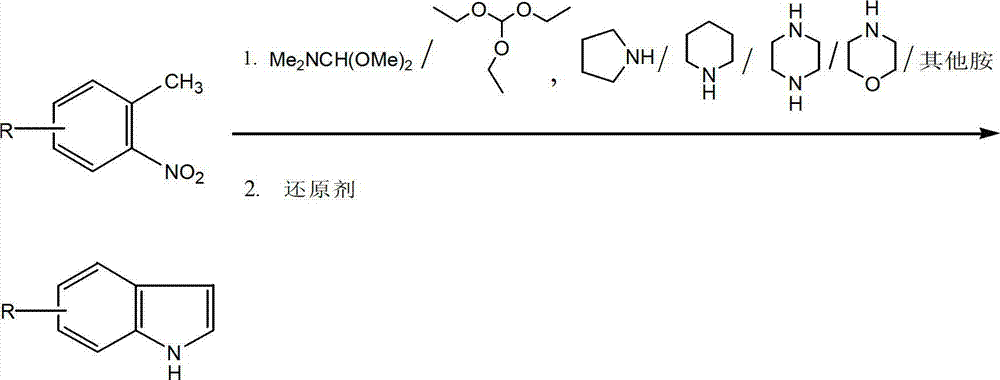
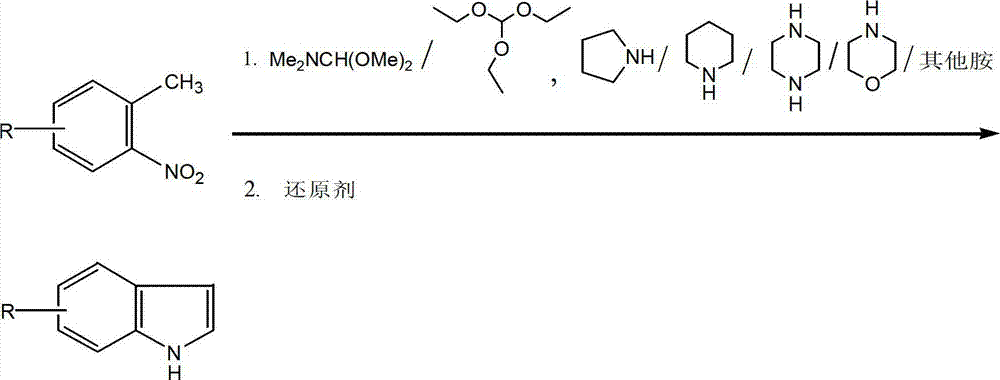
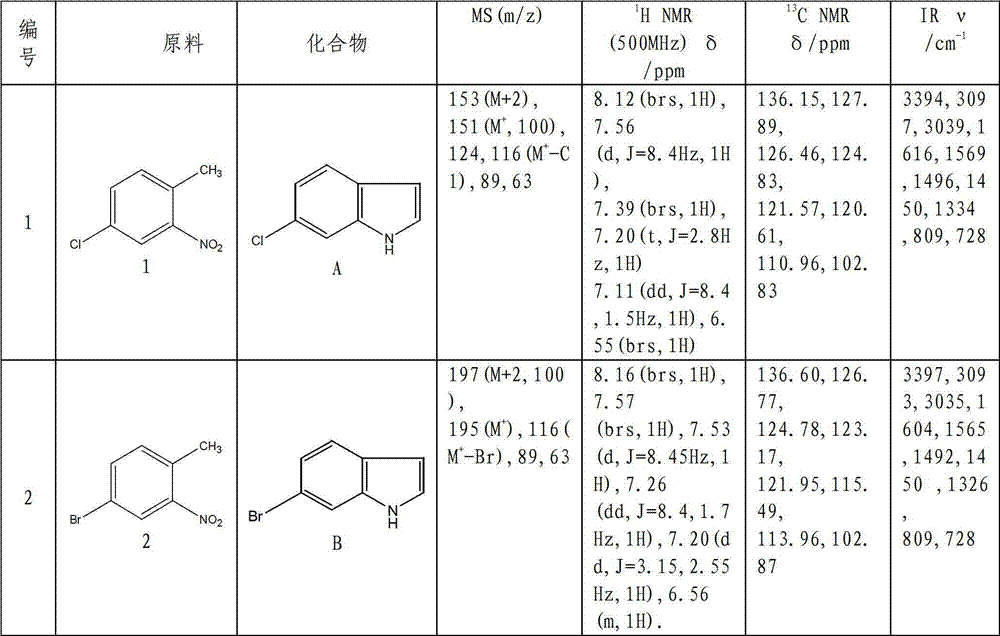
Method for synthesizing substituted indole compounds through one-pot method
The invention relates to a synthesis method of substituted indole compounds, and particularly relates to a method for synthesizing substituted indole compounds through a one-pot method. The method comprises the following steps: under alkaline and anaerobic conditions, reacting ortho-nitrotoluene derivatives and N,N-dimethylformamide dimethyl acetal or triethyl orthoformate used as raw materials in an organic solvent; and then, adding a reducer, and performing reduction and cyclization reaction to obtain indole derivatives, wherein R is a monosubstitution or polysubstitution located on site 4, 5, 6 or 7; and the R substituent is hydrogen, alkyl, substituted alkyl, alkoxy, amino or halogen atom. According to the invention, a one-pot method is adopted; the conventional and readily accessible ortho-nitrotoluene compounds are directly used as raw materials for reaction; separation and purification of intermediate compounds are not required; and the indole derivatives can be synthesized through the one-pot method by effectively controlling the reaction conditions, the charging sequence and the charging ratio. According to the invention, the technological operation procedure is simplified, the reaction time is shortened, the cost is saved, the total yield is improved, and better production and practical values can be achieved.
Owner:YANTAI INST OF COASTAL ZONE RES CHINESE ACAD OF SCI
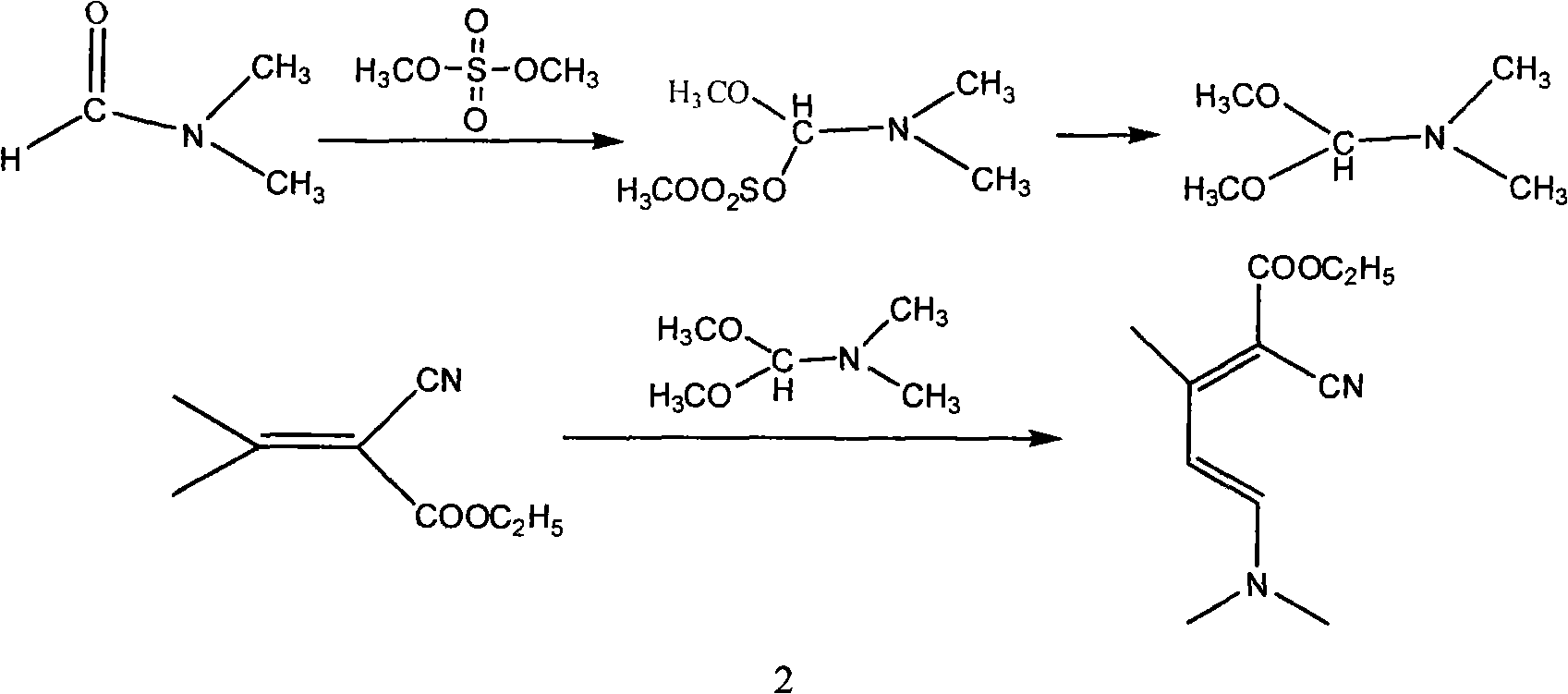
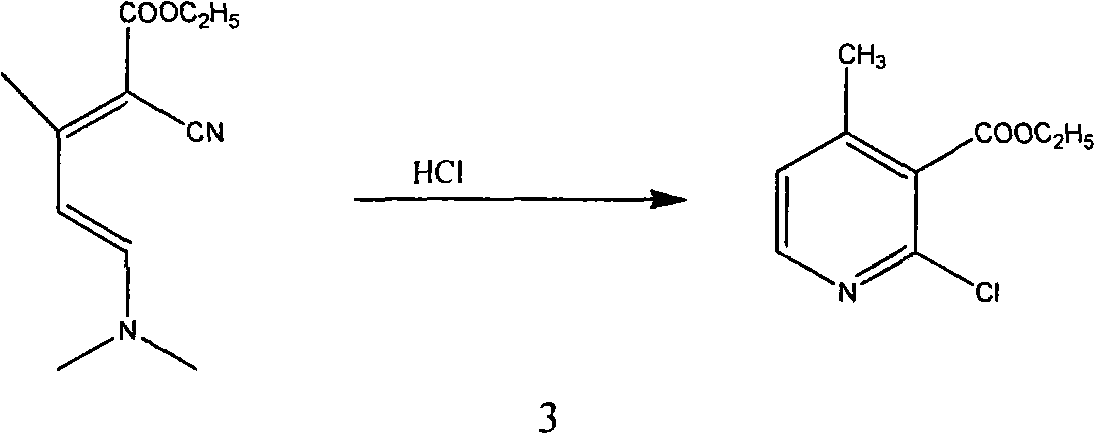
Method for synthesizing 2-chloro-3-amino-4-methylpyridine by ethyl cyanoacetate and acetone
InactiveCN101565399AComplicated operationFew stepsOrganic chemistrySodium methoxideOrganic synthesis
The invention relates to a method for synthesizing an important intermediate 2-chloro-3-amino-4-methylpyridine for an anti-AIDS medicament Nevirapine, and belongs to the technical field of organic synthesis. The method comprises the following process steps that: ethyl cyanoacetate and acetone are dehydrated and condensed to generate a condensation compound I under the action of a catalyst; dimethyl formamide, dimethyl sulfate and sodium methoxide solution react to generate N,N-dimethylformamiade dimethyl acetal (N,N-dimethyl formamide A), and then the N,N-dimethylformamiade dimethyl acetal reacts with the condensation compound I to generate conjugated enamine, namely a condensation compound II; the condensation compound II is cyclized by hydrochloric acid and ethanol to form a cyclic compound 2-chloro-4-methyl-ethyl nicotinate; the 2-chloro-4-methyl-ethyl nicotinate is ammonolyzed by ammonia gas to form 2-chloro-4-methyl-niacinamide; and the 2-chloro-4-methyl-niacinamide is subjected to Hofmann degradation reaction to form the 2-chloro-3-amino-4-methylpyridine. Compared with the prior synthesizing method, the method of the invention has the remarkable characteristic of reducing the reaction steps, and is suitable for large-scale industrialized production; the molar total yield of the five-step reaction is improved to 27 percent from the prior 24 percent; and the purity of the product reaches over 99 percent.
Owner:江苏鼎昊医药科技有限公司
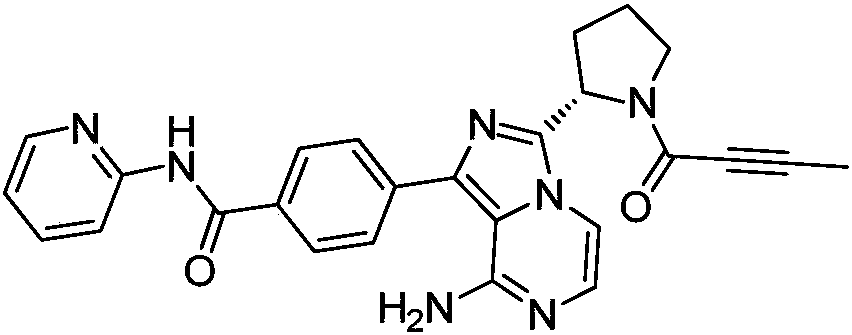
Acalabrutinib and synthesizing method for intermediate thereof
ActiveCN108250186AReduce heavy metal residuesReduce usageOrganic chemistryBulk chemical productionDimethyl acetalChloroacetaldehyde
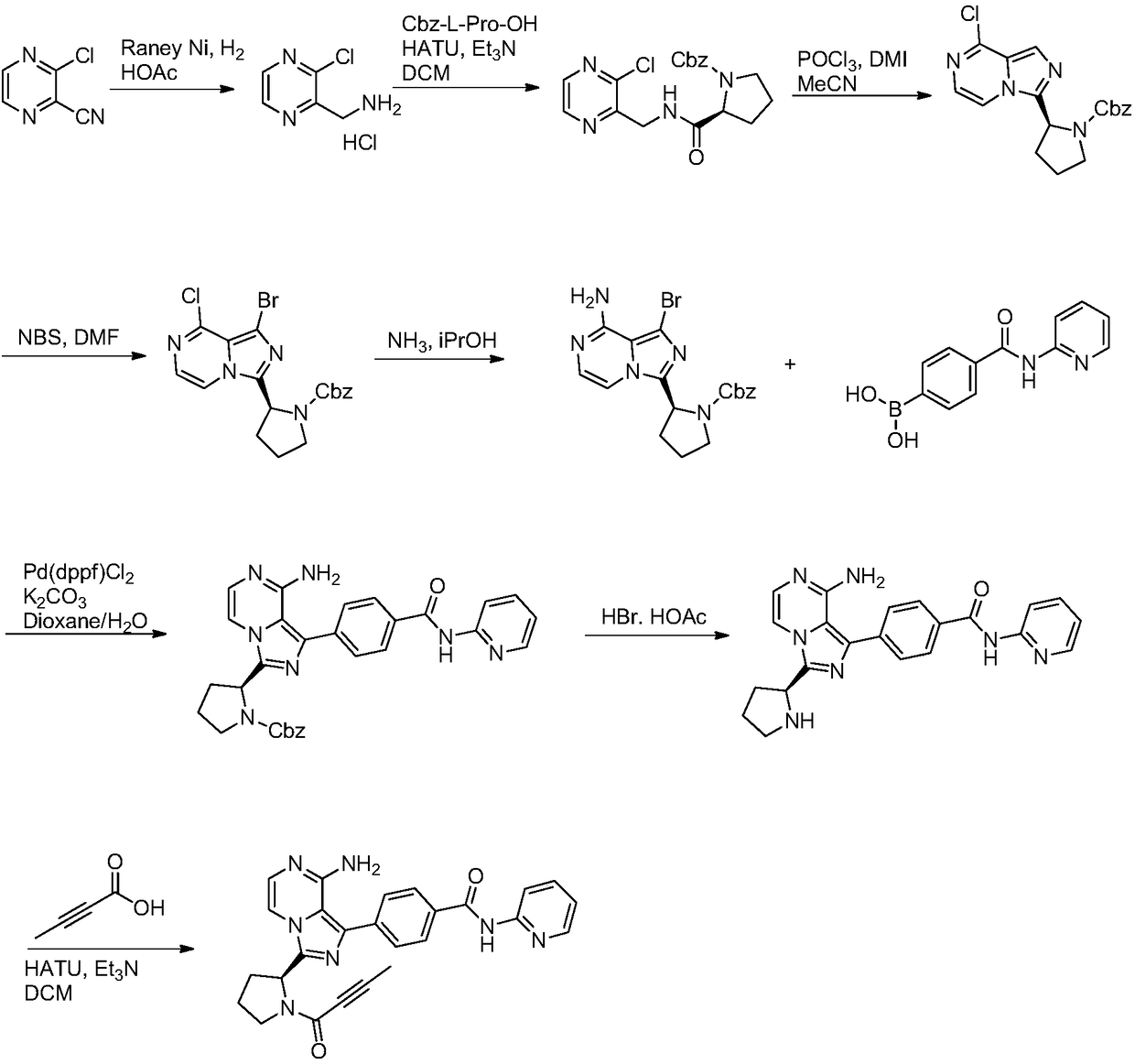
The invention discloses an Acalabrutinib synthesizing method which comprises the steps: utilizing L-proline derivative compound shown in a formula 1 and 2-chloro-2-formyl acetonitrile as beginning rawmaterials to directly condense and cyclize to obtain a compound in a formula 2; after bromination, reacting with cheap chloroacetaldehyde dimethyl acetal to obtain intermediate compound in a formula3; obtaining key intermediate compound shown in a formula 6 through hydrolysis and cyclization reaction; then performing Suzuki coupling with borate intermediate compound shown in a formula 8; then de-protecting and salifying to obtain compound shown in a formula 10; finally, performing condensation with tetrolic acid to obtain a final product Acalabrutinib shown in a formula 11. The path has theadvantages of simpleness in operation, higher total yield and suitability for enlarged production, and a purity of the obtained product is also higher.
Owner:HANGZHOU CHEMINSPIRE TECH CO LTD
Process for synthesizing norfloxacin
ActiveCN101481350ASimple processing methodMild reaction conditionsAntibacterial agentsOrganic chemistryN dimethylformamideDimethyl acetal
The invention discloses a synthetic method of norfloxacin. The method comprises the following steps: taking toluene as a solvent, causing methyl carbonate to react with 2,4-dichloroo-5-fluoroacetophenone in the presence of a catalyst at the temperature of 70-90 DEG C to obtain 2,4-dichloro-5-fluorobenzenepropionic acid methyl ester, reflux reacting with N,N-dimethylformamide dimethyl acetal in toluene solution for 2-3h to obtain 3-ethylamino-2-(2,4-dichloro-5-fluorobenzoyl) methyl acrylate, cyclizing to obtain 1-ethyl-7-chloro-6-fluo-1,4-dihydro-4-oxoquinoline-3-carboxylic acid methyl ester, reacting with a chelating agent at the temperature of 80-110 DEG C for 2-3h to obtain 1-ethyl-7-chloro-6-fluo-1,4-dihydro-4-oxoquinoline-3-carboxylic acid methyl ester trifluoracetic acid anhydride bononized chelate, reacting with piperazine at the temperature of 20-40 DEG C for 10-24h to obtain the norfloxacin. The method has the advantages of simple process and mild reaction condition, avoids production of reverse ring in the traditional process, provides high-purity product, and is applicable to industrialized production.
Owner:ZHEJIANG LEPU PHARMA CO LTD
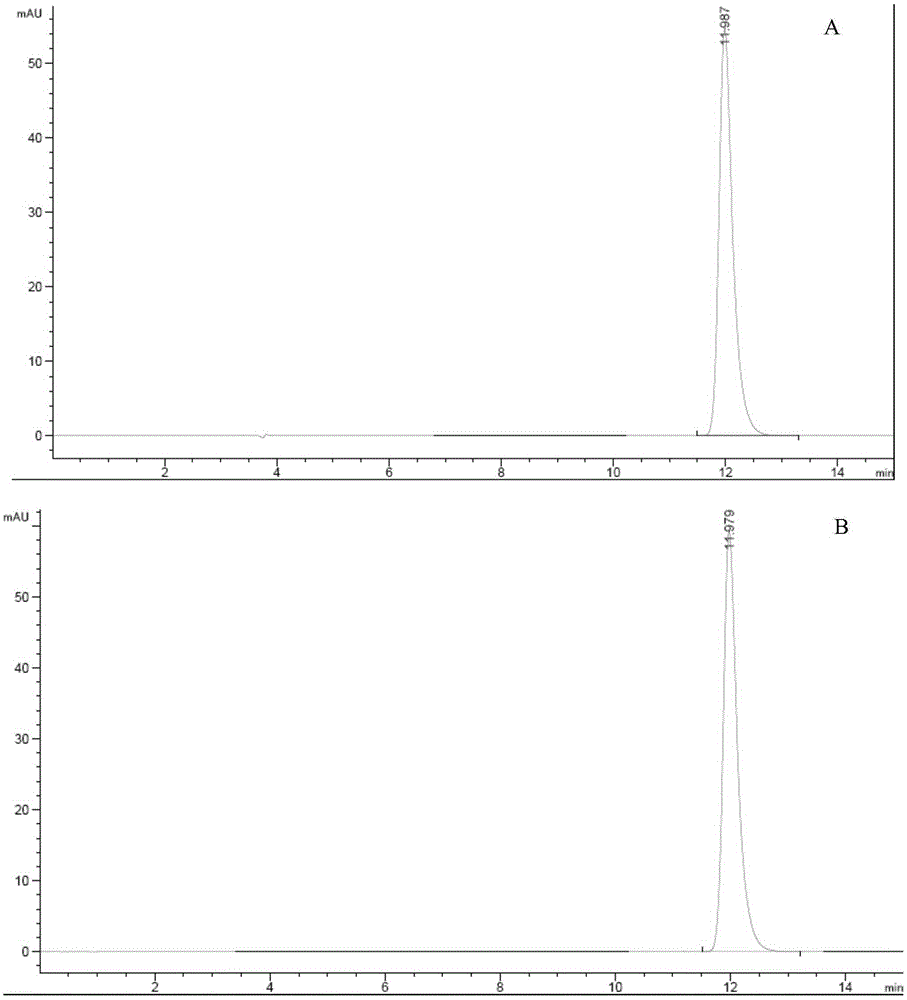
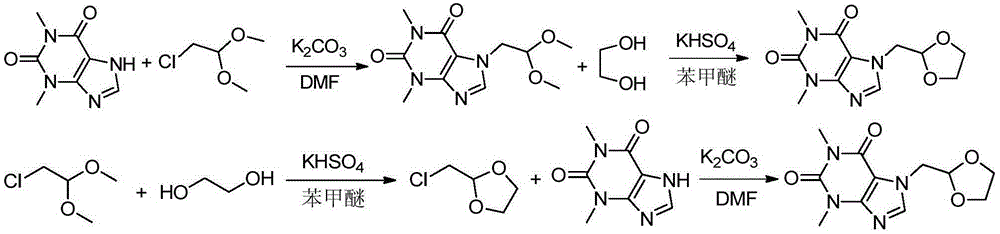
Preparation methods of doxofylline
The invention discloses preparation methods of doxofylline. The preparation methods include the steps: under the action of an acid-binding agent, carrying out a condensation reaction of theophylline and halogenated acetaldehyde dimethyl acetal in a polar solvent, to obtain an intermediate 7-(2,2-dimethoxy ethyl)theophylline; and then, with soluble hydrosulfate as a catalyst, carrying out a condensation cyclization reaction of the intermediate 7-(2,2-dimethoxy ethyl)theophylline with ethylene glycol in a solvent, to obtain doxofylline; or, firstly, carrying out a condensation cyclization reaction of halogenated acetaldehyde dimethyl acetal and ethylene glycol to obtain halogenated acetaldehyde ethylene acetal, and carrying out a condensation reaction with theophylline, to obtain doxofylline. The soluble hydrosulfate is used as the catalyst and replaces conventional p-toluenesulfonic acid, moreover, and anisole and other solvents are used for replacing methylbenzene used in a conventional reaction route, so that under a condition of ensuring high yield of the product, the toxicity of the solution in the synthesis reaction is reduced, and the difficult problem that residual toxicity easily exists in the finished product is solved.
Owner:浙江北生药业汉生制药有限公司
Preparing method of anticancer medicine
The invention discloses a novel synthesis method of an anticancer medicine gefitinib. The preparing method includes subjecting 4-methoxy-5-(3-morpholinopropoxy)-2-nitrobenzonitrile that is adopted as a raw material to reduction by sodium hydrosulfite, salifying with hydrochloric acid, reacting with N,N-dimethylformamide dimethyl acetal to obtain a condensation product, subjecting the condensation product and 3-chloro-4-fluoroaniline to cyclization to obtain the gefitinib. The initial raw material adopted by the preparing method is cheap and easily available. The synthesis route is simplified. The raw material utilization rate and the total yield are largely increased. Reaction intermediates are mostly purified by a recrystallization method or are directly used for a next reaction. The method has characteristics of high yield, less three-waste in reaction processes and low cost, and is suitable for industrial production.
Owner:GUANGZHOU BAIYUNSHAN PHARMA HLDG CO LTD BAIYUNSHAN PHARMA GENERAL FACTORY
Anticorrosion and antibacterial aluminium alloy surface treating agent
InactiveCN104099021AImprove anti-corrosion and anti-bacterial abilityGood acid and alkali resistanceAntifouling/underwater paintsPaints with biocidesSodium Lauryl SarcosinateMeth-
The invention discloses an anticorrosion and antibacterial aluminium alloy surface treating agent which comprises a component A and a component B, wherein the component A comprises the following materials in parts: 0.5-1 part of benzalkonium bromide, 0.4-1 part of flaxseed gum, 0.4-1 part of sodium N-lauroylsarcosinate, 2-4 parts of diethylene glycol, 3-4 parts of sodium phosphate, 0.9-1.5 parts of 3-methoxybutyraldehyde dimethyl acetal, 2-3 parts of glycerinum, 2-3 parts of silane coupler KH-858 and 40-50 parts of deionized water; the component B comprises the following materials in parts: 0.4-1 part of 1-methyl-2-pyrrolidinone, 1-2 parts of potassium iodide, 0.3-1 part of bone black, 1-2 parts of ammonium polyphosphate, 0.3-0.6 part of magnesium fluoride, 2-4 parts of a coalescing agent, 10-12 parts of a silane coupling agent KH560 and 70-90 parts of deionized water. According to the invention, a film formed on the surface of aluminium alloy by the surface treating agent is excellent in corrosion resistance, antibacterial property, acid-base resistance, salt frog resistance and high in rust resisting property; the film layer is firm, excellent in compactness, uniform in shape and high in adhesive force.
Owner:铜陵创能动力机械有限公司
Preparation method of 1,4,7,10-tetraazadodecane
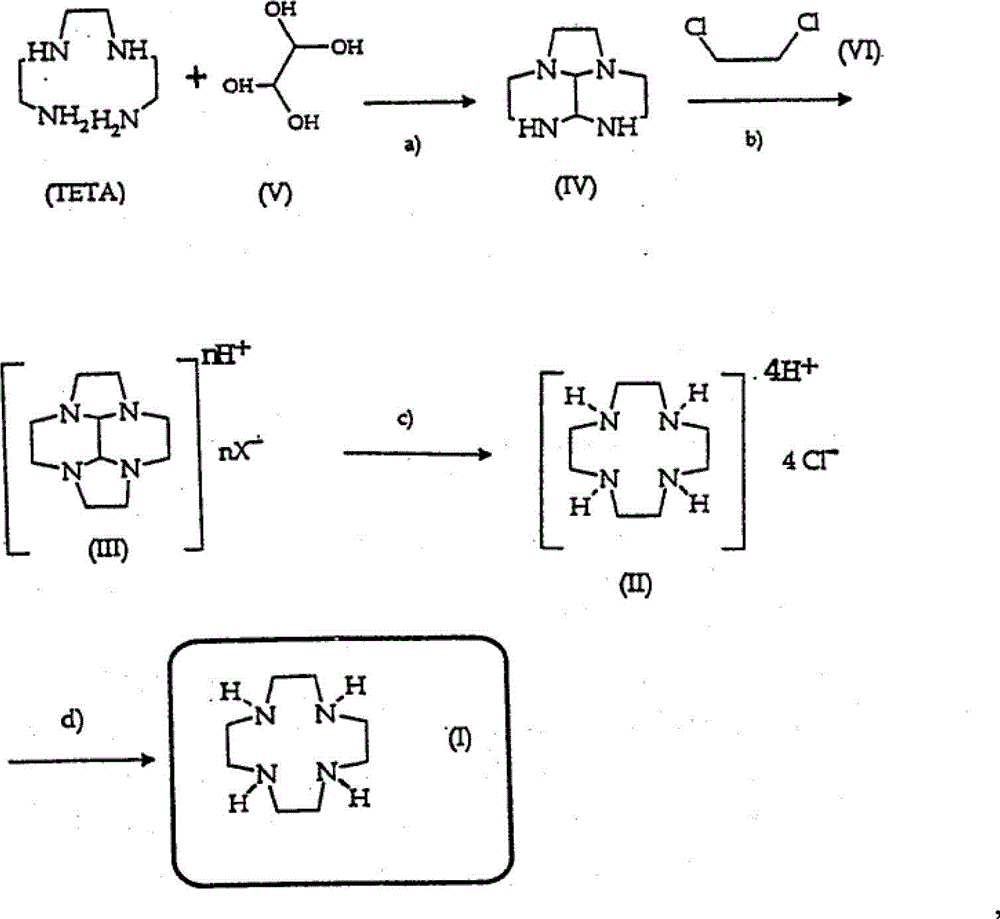
The invention relates to a preparation method of 1,4,7,10-tetraazadodecane, which comprises the following steps: (1) reacting triethylenetetramine and N,N-dimethylformamide dimethyl acetal in a solvent benzene at 75-85 DEG C to generate diimidazoline; (2) in the presence of potassium carbonate, reacting the diimidazoline with 1,2-ethylene dibromide in a solvent ethanol to prepare the amber semisolid bromine salt intermediate; and (3) reacting the bromine salt intermediate with sodium hydroxide in a solvent water to generate the 1,4,7,10-tetraazadodecane. The technical process is simple and convenient to operate; the total yield of the target product exceeds 60%; and thus, the invention is suitable for industrial large-scale production.
Owner:太仓市茜泾化工有限公司
Preparation method of chloroacetaldehyde dimethyl acetal
PendingCN107954869AReduce generationHigh selectivityPreparation by ester-hydroxy reactionOrganic compound preparationDimethyl acetalChloroacetaldehyde
The invention discloses a preparation method of chloroacetaldehyde dimethyl acetal. The method adopts vinyl acetate, anhydrous methanol and chlorine as reaction raw materials, adopts a step-by-step feeding one-pot boiling method and also adds a polymerization inhibition inducing agent, so that the reaction raw materials can maximally participate in the reaction to generate a target product, the generation of side products can be reduced, and the prepared product is high in yield and high in purity. The preparation method of the invention is controllable in conditions, simple and easy in product separation method and convenient in industrialized operation.
Owner:临沂优盛新材料科技有限公司
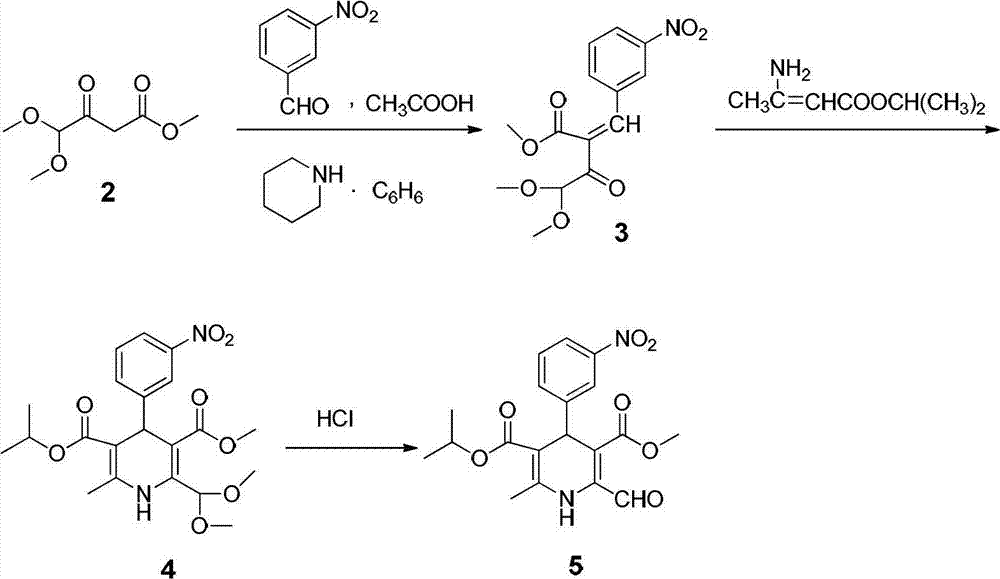
Preparation method of nilvadipine intermediate
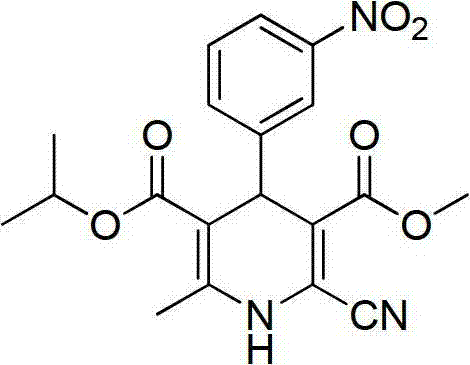
InactiveCN102816110AHigh purityAvoid purification processOrganic chemistryDimethyl acetalNitrobenzene
The invention provides a preparation method of 3-methoxycarbonyl-6-methyl-4-(3-nitrophenyl)-2-formyl-1, 4-dihydropyridine-5-isopropyl carbonate. The method comprises the following steps that condensation and amination reactions between pyruvic aldehyde dimethyl acetal and dimethyl carbonate are carried out under the catalysis of a strong base to obtain 3-amino-4, 4-dimethoxyl-methyl crotonate; dehydration condensation reaction between isopropyl acetoacetate and nitrobenzaldehyde is carried out under the catalysis of a weak base to obtain 2-(3-nitrobenzylidene)-isopropyl acetoacetate; dehydration condensation and hydrolysis reactions between 3-amino-4, 4-dimethoxyl-methyl crotonate and 2-(3-nitrobenzylidene)-isopropyl acetoacetate are carried out to obtain 3-methoxycarbonyl-6-methyl-4-(3-nitrophenyl)-2-formyl-1, 4-dihydropyridine-5-isopropyl carbonate. The method provided by the invention solves the technical problems in the prior art including complex preparation process, low purity and less yield during the preparation of nilvadipine intermediate 3-methoxycarbonyl-6-methyl-4-(3-nitrophenyl)-2-formyl-1, 4-dihydropyridine-5-isopropyl carbonate.
Owner:HUNAN FANGSHENG PHARMACEUTICAL CO LTD
Rose essence used for perfume
The invention discloses rose essence used for perfume, which is characterized by comprising the following components by weight percent: 4.8-5.0 percent of rose absolute, 0.8-1.0 percent of coldly ground ginger oil, 10.0-12.0 percent of phenethyl alcohol, 3.5-4.0 percent of rose, 18.0-22.0 percent of geraniol, 10.0-11.0 percent of santalwood, 20.0-24.0 percent of citronellol, 5.0-6.0 percent of cedar wood oil, 0.7-0.9 percent of phenylacetaldehyde dimethyl acetal, 4.0-5.0 percent of isolongitolanone, 4.0-5.0 percent of ionone, 0.1-0.2 percent of civetta paste, 0.1-0.2 percent of rose oxide, 0.1-0.3 percent of musk tincture and 0.3-0.5 percent of damascenone. The rose essence used for the perfume in the formula is suitable for the perfumed products of white cream, emulsion, fragrant sirup and the like, does not have the defect of discoloration and has the advantages of pure and abundant fragrance, strong permanence, very little use quantity and the like.
Owner:张彬
Preparation method of erlotinib hydrochloride crystal form A
ActiveCN103396371AAvoid adjusting pHAvoid the extraction processOrganic chemistryChemical recyclingDimethyl acetalPharmaceutical Substances
The invention provides a preparation method of an erlotinib hydrochloride crystal form A, which belongs to the technical field of the preparation of a drug compound. The preparation method comprises the following steps: enabling 2-amino-4,5-di(2-methoxy ethyoxyl) cyanophenyl and N, N-dimethyl amide dimethyl acetal to react; re-crystallizing and purifying the obtained Schiff base intermediate, and then synthesizing with aminophenylacetylene to obtain erlotinib free alkali; and adding a hydrochloric acid solution, and recrystallizing to obtain the erlotinib hydrochloride crystal form A. According to the scheme of the invention, the process route is short, the product purity is high, the repeatability is good, and the operation is simple and easy to implement, and therefore, the preparation method is suitable for large-scale industrial production.
Owner:NANJING YOUKE BIOLOGICAL MEDICAL RES
Preparation method of osimertinib intermediate
ActiveCN109485638AEmission reductionImprove protectionOrganic chemistryN dimethylformamideDimethyl acetal
The invention relates to a preparation method of an osimertinib intermediate, comprising: subjecting ortho-nitrotoluene and 3,3-dialkoxypropanenitrile as raw materials to alkali-catalyzed nucleophilicaddition to obtain 1-(2-nitro)phenyl-4,4-dialkoxy-2-butanone, subjecting 1-(2-nitro)phenyl-4,4-dialkoxy-2-butanone and N,N-dimethylformamide dimethyl acetal to thermal condensation to obtain 1-dimethylamino-2-(2-nitro)phenyl-5,5-dialkoxy-3-n-pentanone, subjecting the attained reaction liquid to direct catalytic hydrogenation to obtain 3-(3,3-dialkoxy)propionylindole, subjecting 3-(3,3-dialkoxy)propionylindole to reaction with a methylation reagent under alkaline conditions to generate 3-(3,3-dialkoxy)propionyl-N-methylindole, and subjecting 3-(3,3-dialkoxy)propionyl-N-methylindole and 2-methoxy-4-fluoro-5-nitrophenylguanidine to exocondensation to obtain the osimertinib intermediate. The materials herein are low in price and easy to attain, the route is short, and the preparation method is environmentally friendly and high in yield.
Owner:XINFA PHARMA
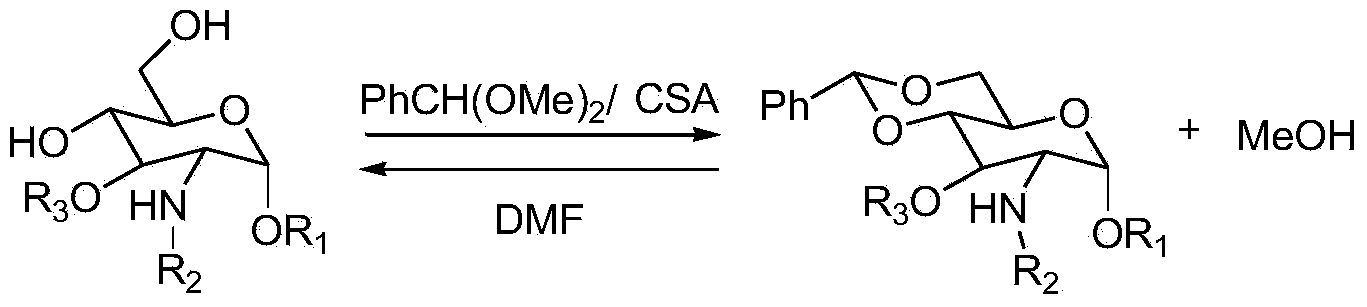
Method for protecting D-glucosamine derivative by using benzaldehyde dimethyl acetal
ActiveCN103588825AHigh reaction conversion rateHigh puritySugar derivativesSugar derivatives preparationRefluxOrganic solvent
The invention discloses a method for protecting a D-glucosamine derivative by using benzaldehyde dimethyl acetal, and belongs to the chemical field of organic chemical glycosylation. According to the method, D-glucosamine and the derivative of D-glucosamine are taken as substrates, D-glucosamine, the derivate of D-glucosamine and benzaldehyde dimethyl acetal perform reflux in an organic solvent to generate a benzylidene glucose derivative under the catalysis of CSA (camphorsulfonic acid); methyl alcohol generated by reaction is continuously removed during reaction, so that the equilibrium rightward movement is promoted, and the reaction conversion rate is increased; and the removed organic solvent can be recycled and reused after treated, and the reaction economy is improved further.
Owner:内蒙古佳瑞米精细化工有限公司
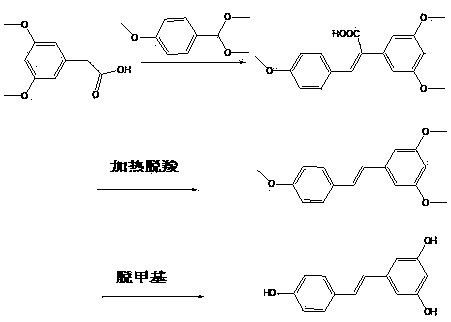
Preparation method for resveratrol
ActiveCN103664537AShort processEasy to operateOrganic chemistryOrganic compound preparationPhenyl acetic acidAcetic acid
The invention provides a preparation method for resveratrol. The method comprises the following steps: with (3,5-dimethoxyphenyl)acetic acid as a raw material, subjecting (3,5-dimethoxyphenyl)acetic acid and methoxybenzaldehyde dimethyl acetal to a one-pot reaction so as to obtain a crude resveratrol product; and then carrying out neutralizing with alkali lye, decoloring in alcohol and recrystallization so as to prepare a refined resveratrol product. Compared with the prior art, the preparation method provided by the invention has the advantages of short process flow, simple operation and suitability for industrial production, and the prepared refined resveratrol product has yield of more than 93%, a melting point of 261 to 263 DEG C, HPLC of greater than 99%, a whitish color, good quality and low preparation cost.
Owner:HUNAN KEYUAN BIO PRODS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com